Preparation method of asenapine
A technology of tetrahydrofuran and red aluminum, applied in the direction of organic chemistry, can solve the problems of potential safety hazards, restrictions on the large-scale production of asenapine, and the inability to use it in large quantities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
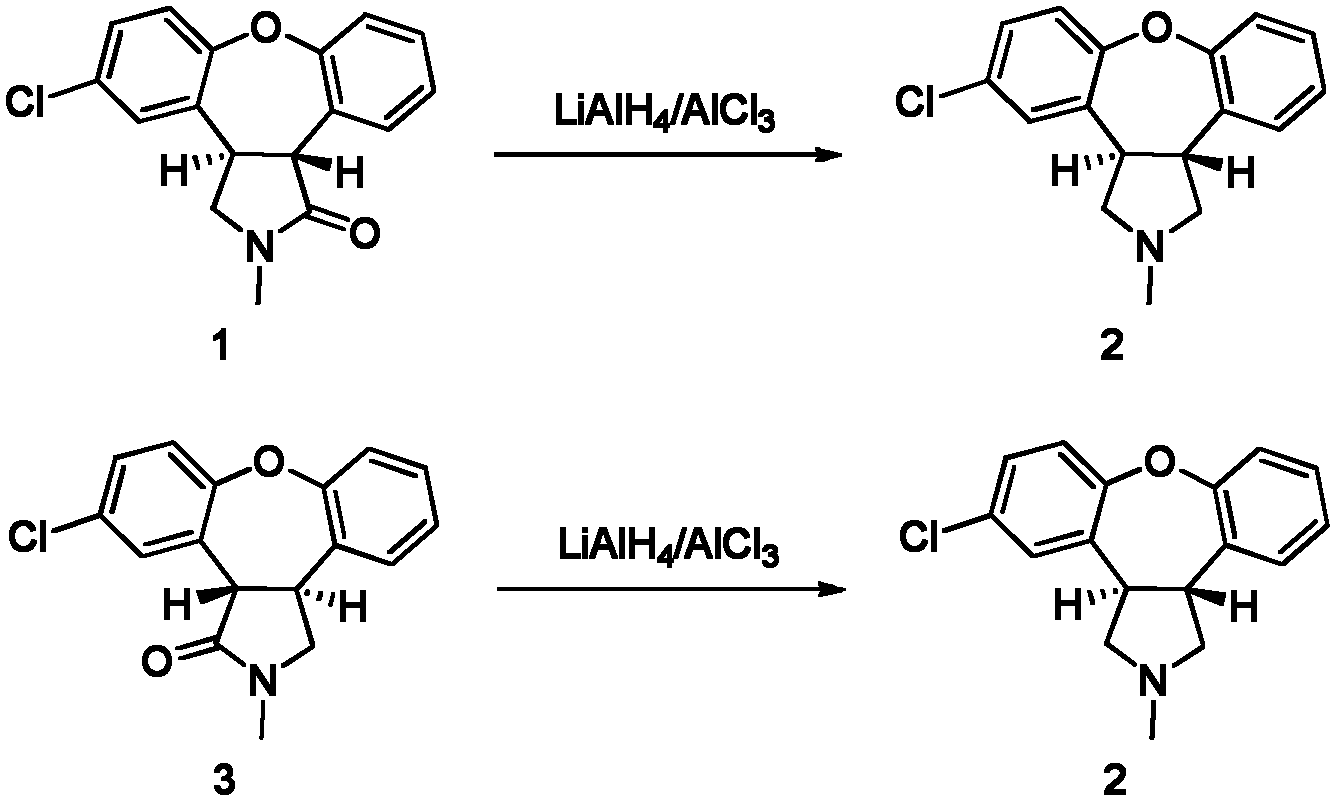
[0021] Example 1 Trans-5-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxa The method for preparing asenapine from [4,5-C]pyrrol-1-one (compound 1)
[0022] 0°C, under nitrogen protection and stirring, 11.2g (83.8mmol) of AlCl 3 , put into dry 240mL tetrahydrofuran, add dropwise a mixed solution of 70% red aluminum toluene solution and tetrahydrofuran, wherein the red aluminum toluene solution and tetrahydrofuran are 52mL and 60ml respectively; after the dropwise addition, add trans-5- Chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxa[4,5-C]pyrrol-1-one (compound 1, 20g, 66.8mmol) dissolved in 160mL of tetrahydrofuran, after the dropwise addition, keep stirring for 30min, heat up to 66°C, stir for 5h, use petroleum ether: ethyl acetate = 1:2 as the developer, TLC (GF254 ) to detect the disappearance of the raw material point; cool to room temperature, add 300 mL of ethyl acetate to the reaction solution, stir for 30 min, then add 250 mL of 1 mol / L sodi...
Embodiment 2
[0025] Example 2 Trans-11-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxa The method for preparing asenapine from [4,5-C]pyrrol-1-one (compound 3)
[0026] 0°C, under nitrogen protection and stirring, the AlCl 3(8.2g, 61.7mmol) was dropped into 240mL dry tetrahydrofuran, and a mixed solution of 70% red aluminum toluene solution (38.3mL, 133.6mmol) and tetrahydrofuran (50mL) was added dropwise, and the rate of addition was controlled so that the temperature was lower than 5°C. After the dropwise addition, trans-5-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxa[ 4,5-C]pyrrol-1-one (compound 1, 20g, 66.8mmol) in tetrahydrofuran (160mL), after the dropwise addition, keep stirring for 30min, heat up to 66°C, stir and react for 5h, add petroleum ether: acetic acid Ethyl is used as a developer, and the volume ratio of petroleum ether: ethyl acetate is 1:2. TLC (GF254) detects that the raw material point disappears. After cooling to room temperature, 300 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com