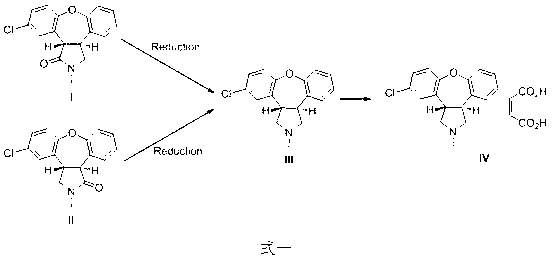
Preparation method of asenapine
A compound, red aluminum technology, applied in the field of preparation of schizophrenia drug Asenapine (Asenapine), can solve the problems of dangerous operation, high cost, high risk, etc., and achieve high yield, low production cost and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
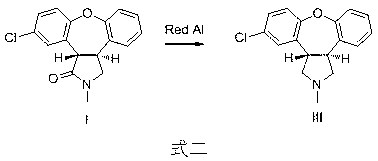
[0011] Embodiment one : In the synthetic route shown in formula 2, wherein compound I is used as a raw material, the preparation of compound III: add 30 g of compound I to a 500 mL three-necked flask, dissolve it with 300 mL of toluene, and add 70% red Aluminum solution 144g. The reaction mixture was heated to 40°C to 50°C under constant stirring for 3 hours. Under cooling in an ice-water bath, add 100 mL of methanol dropwise to quench the reaction, add 100 mL of water and 200 mL of 10% sodium hydroxide solution, and stir for a while. The organic phase was separated, and the aqueous phase was extracted with 100 mL of dichloromethane. The organic phases were combined, dried, and concentrated under reduced pressure to recover the solvent to obtain 26 g of compound III with a yield of 71%.
Embodiment 2
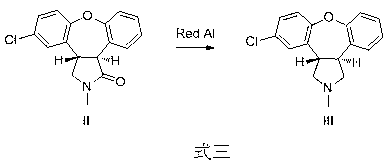
[0012] Embodiment two : In the synthetic route shown in formula three, wherein compound II is used as a raw material, 30 g of compound I is added in a 500 mL three-necked flask, dissolved with 300 mL of toluene, and 144 g of 70% red aluminum solution is added dropwise at room temperature. The reaction mixture was heated to 40°C to 50°C under constant stirring for 3 hours. Under cooling in an ice-water bath, add 100 mL of methanol dropwise to quench the reaction, add 100 mL of water and 200 mL of 10% sodium hydroxide solution, and stir for a while. The organic phase was separated, and the aqueous phase was extracted with 100 mL of dichloromethane. The organic phases were combined, dried, and concentrated under reduced pressure to recover the solvent to obtain 25.5 g of compound III with a yield of 69%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com