Spongy asenapine sublingual film agent with micropores and preparation method thereof
A kind of asenapine and sponge-like technology, applied in the field of asenapine preparations, can solve problems such as unfavorable production, transportation, storage and use, failure to achieve rapid onset of effect, unfavorable film formation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
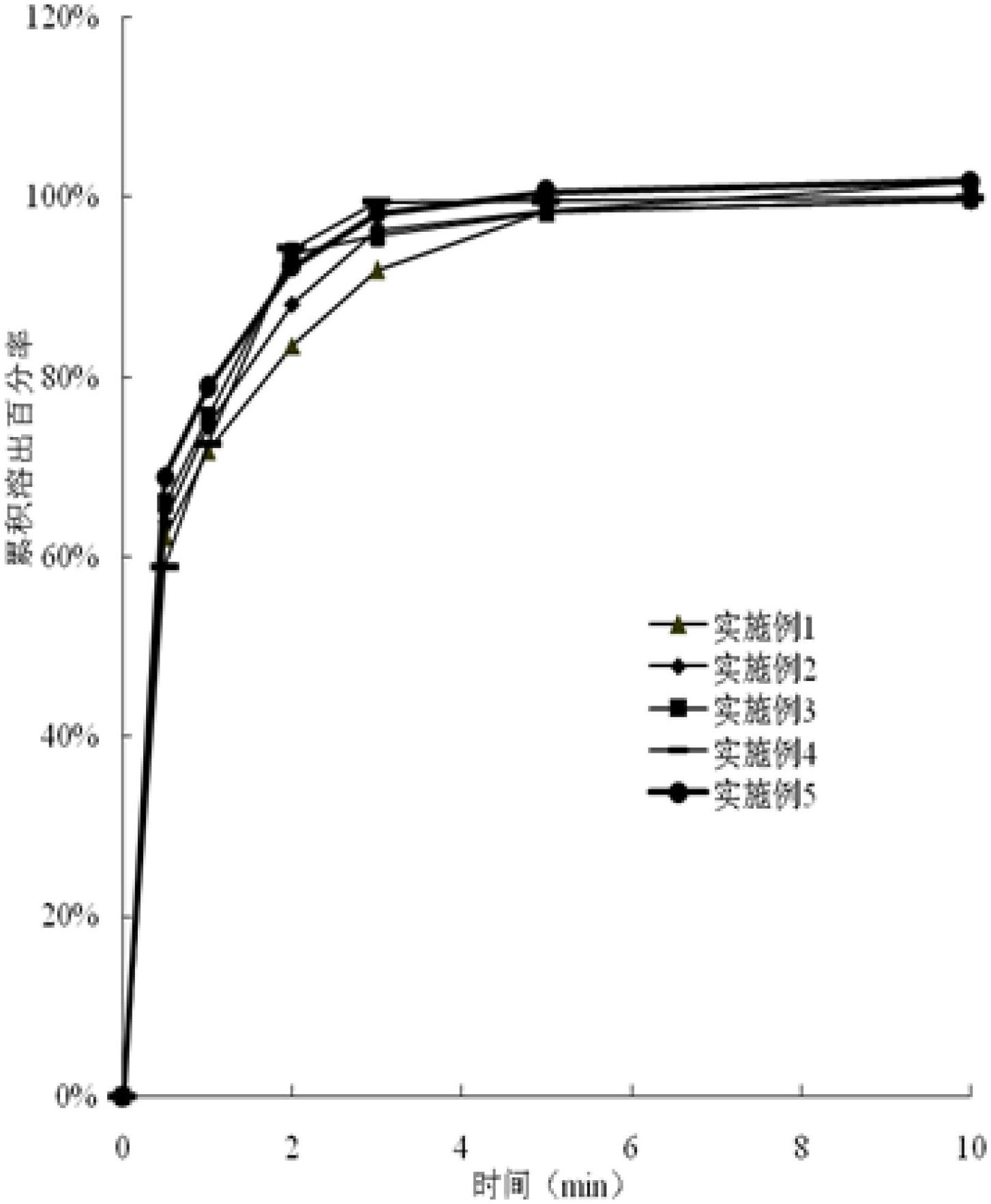
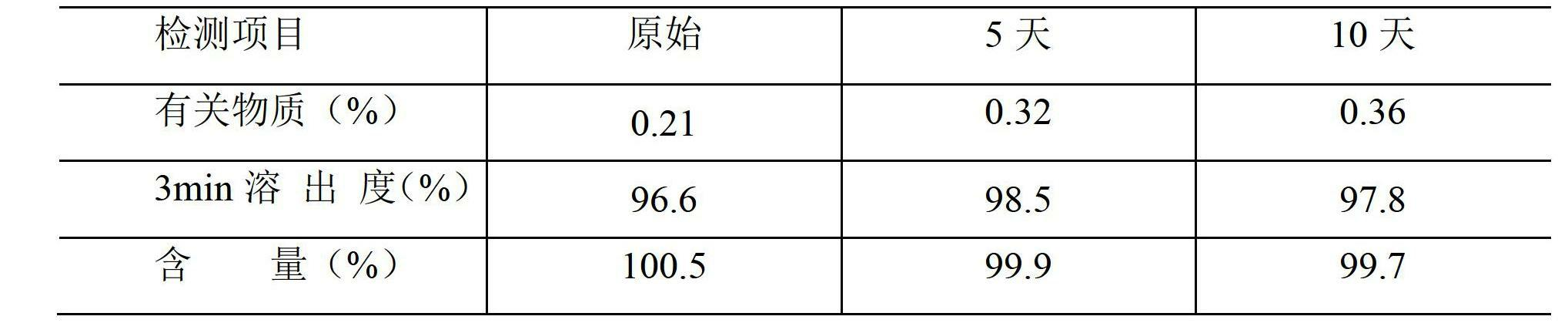
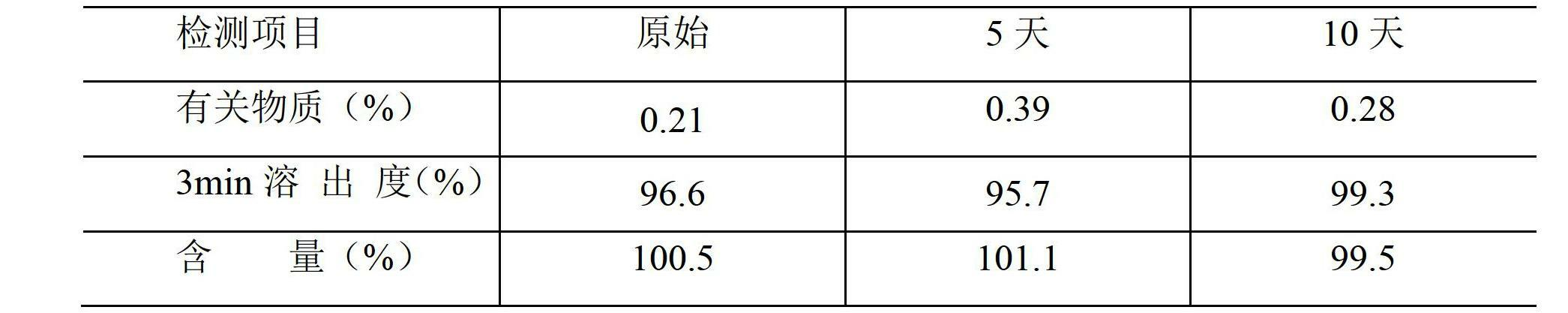
Image
Examples
Embodiment 1
[0056] 80 g of PVA with a molecular weight of 130,000 Daltons and 20 g of HPC with a molecular weight of 600,000 Daltons were dissolved in 500 g of water to form a water slurry.
[0057] Add 100g of pore forming agent, 10g of asenapine, 2g of aspartame, 5g of glycerin, 0.1g of antioxidant BHA (tert-butyl p-hydroxyanisole) and 3g of titanium dioxide into the above water slurry, stir and mix.
[0058] The components of the pore-forming agent are chitin with a particle size of 0.5 μm and acetone adsorbed thereon, and the parts by weight of chitin and the parts by weight of the solvent are:
[0059] Chitosan 100 parts
[0060] Acetone 400 parts
[0061] (2) Then use the method reported in the literature of the study of nitroglycerin membranes (Chinese Journal of Pharmaceutical Industry, 1977, 12 (5)) to coat and form membranes to obtain thin film precursors;
[0062] (3) Dry the thin film precursor obtained in step (2) at 80°C to obtain the thin film for drug film preparation wi...
Embodiment 2
[0070] 60 g of PVP with a molecular weight of 10,000 Daltons and 30 g of HPC with a molecular weight of 1,000,000 Daltons were dissolved in 400 g of water to form a water slurry.
[0071] Add 90g pore-forming agent, 20g asenapine, 5g sucralose, 3g citric acid, 0.1g chelating agent EDTA (disodium ethylenediaminetetraacetic acid), 0.1g orange flavor and 2g titanium dioxide to the above water slurry In, stir and mix.
[0072] The components of the pore-forming agent include silicon dioxide micropowder with a particle size of 10 μm and acetone adsorbed thereon, the parts by weight of the microsilica powder and the parts by weight of the solvent are:
[0073] Silica powder 100 parts
[0074] Acetone 900 parts
[0075] Others are the same as embodiment 1.
[0076] The weight ratio of each component:
[0077] Low molecular weight water-soluble polymer material: high molecular weight water-soluble polymer material=2:1;
[0078] Water-soluble polymer material: water-insoluble micr...
Embodiment 3
[0082] Dissolve 60 g of sodium alginate with a molecular weight of 32,000 Daltons and 120 g of CMC-Na with a molecular weight of 700,000 Daltons in 700 g of water to form a water slurry.
[0083] Add 90g pore-forming agent, 50g asenapine, 5g cyclamate, 3g maleic acid, 0.1g antioxidant BHT (tert-butyl hydroxytoluene), 2g PEG400 and 3g titanium dioxide to the above water slurry, stir and mix .
[0084] The components of the pore-forming agent include CaCO3 with a particle size of 1 μm and ethanol adsorbed thereon, and the parts by weight of CaCO3 and the parts by weight of the solvent are:
[0085] CaCO3 100 parts
[0086] Ethanol 100 parts
[0087] Others are the same as embodiment 1.
[0088] The weight ratio of each component:
[0089] Low molecular weight water-soluble polymer material: high molecular weight water-soluble polymer material=1:2;
[0090] Water-soluble polymer material: water-insoluble micropowder = 1:0.25;
[0091] The diameter of the micropores is 10 nm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com