A kind of preparation method of asenapine
A technology of asenapine maleate and tetrahydrofuran, applied in the field of organic synthesis, can solve problems such as easy combustion or explosion, potential safety hazards, and violent reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
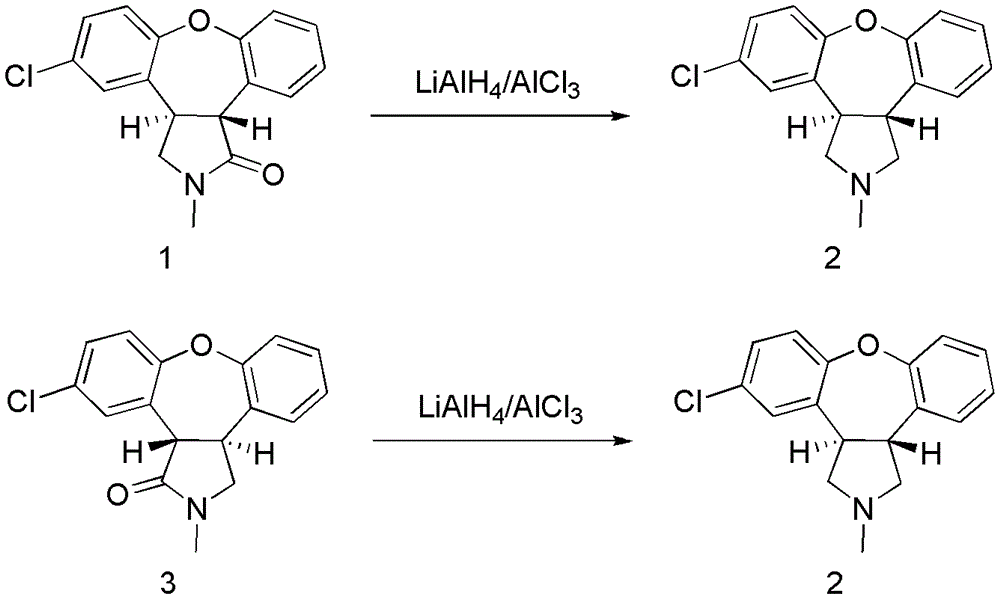
[0021] Example 1 Trans-5-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxa And [4,5-C]pyrrol-1-one (compound 1) to prepare asenapine
[0022] 0℃, under nitrogen protection and stirring, add 11.2g (83.8mmol) of AlCl 3 , Put into dry 240mL tetrahydrofuran, drip 70% red aluminum toluene solution and tetrahydrofuran mixed solution, of which the red aluminum toluene solution and tetrahydrofuran were 52mL and 60ml; after the dripping, add trans-5- Chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxa[4,5-C]pyrrol-1-one (compound 1,20g, 66.8mmol) dissolved in 160mL of tetrahydrofuran, after the dripping, heat and stir for 30min, warm up to 66℃, stir for 5h, use petroleum ether: ethyl acetate=1: 2 as the developing solvent, TLC (GF254 ) The detection of the raw material point disappeared; cooling to room temperature, adding 300 mL of ethyl acetate to the reaction solution, stirring for 30 minutes, and then adding dropwise 250 mL of 1 mol / L sodium hydroxide solution to ...
Embodiment 2
[0025] Example two trans-11-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxa And [4,5-C]pyrrol-1-one (compound 3) to prepare asenapine
[0026] 0℃, under nitrogen protection and stirring, the AlCl 3 (8.2g, 61.7mmol) into 240mL of dry tetrahydrofuran, add dropwise a mixed solution of 70% red aluminum toluene solution (38.3mL, 133.6mmol) and tetrahydrofuran (50mL), control the rate of dropping to make the temperature below 5℃, After the dripping, add trans-5-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxa[ 4,5-C]pyrrol-1-one (compound 1, 20g, 66.8mmol) in tetrahydrofuran (160mL), after the dripping, keep stirring for 30min, warm to 66℃, stir and react for 5h, use petroleum ether: acetic acid Ethyl is the developing solvent, and the volume ratio of petroleum ether: ethyl acetate is 1:2, the raw material point detected by TLC (GF254) disappears, cool to room temperature, add 300 mL ethyl acetate to the reaction solution, stir for 30 min, and then add to the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com