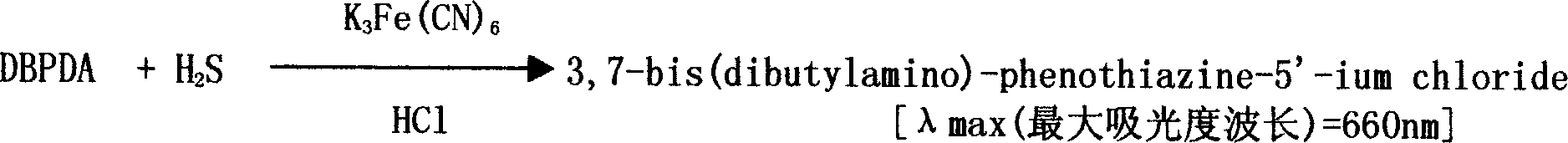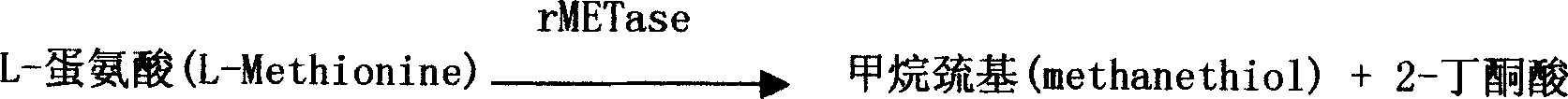Method and kit for investigating humotype semi-cystinol by enzyme biochemical reaction
A technology of homocysteine and chemical reaction, applied in the field of determination of homocysteine content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] 1. HCY determination
[0037] 1) Reduction and enzymatic conversion:
[0038] Add 0.025ml 0-1000μM L-isotype to 0.25ml reaction solution (50mM Tris-HCl pH 7.5, containing 20μM pyridoxal phosphate (PLP for short), 0.05% Triton X-100, 1mM TCEP and 0.1U / ml rMETase) Cystine, placed in a 37°C water bath for 30 minutes.
[0039] 2) Determination of hydrogen sulfide:
[0040] Add 0.025ml color developing solution (1N HCl containing 20mM DMPD 2HCl and 30mM FeCl 3 ), and stand at room temperature for 10 minutes after mixing. Using distilled water as a reference, read the absorbance at 670nm.
[0041] 3) Results:
[0042] L-homocysteine linear range 3-1000μM
[0043] 2. Homocysteine (HCY) Biochemical Kit
[0044] Product Name: Homocysteine (HCY) Biochemical Kit
[0045] Model: Biochemical Reagent
[0046] Product code: DC-HCY
[0047] Specifications: Reagent I 25ml, Reagent II 2.5ml, HCY standard 1 bottle, HCY normal serum control 1 bottle, HCY elevated serum cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com