Patents
Literature
44 results about "Elevated serum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hyperalbuminemia is the medical terminology used to describe elevated levels of serum albumin. Chief factors responsible for an increase in the albumin level are: Severe infections. Congenital disorders. Severe dehydration. Hepatitis. Malnourishment. Chronic inflammatory diseases.
Use of anti-TNF antibodies as drugs for the treatment of disorders with an elevated serum level of interleukin-6
The invention relates to the use of TNF antagonists for producing drugs for the treatment of disorders characterized by elevated serum levels of interleukin-6.
Owner:BASF SE +1
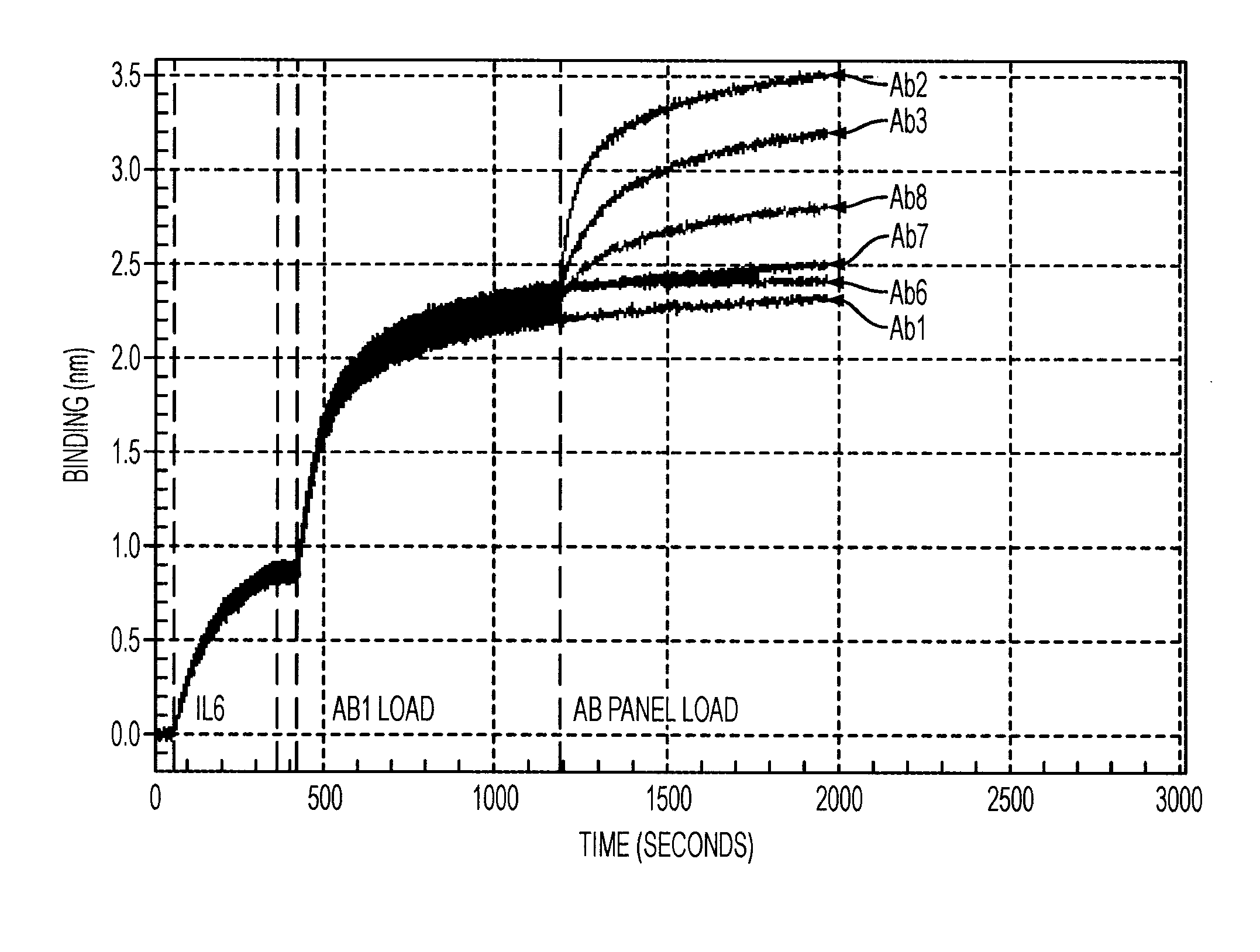
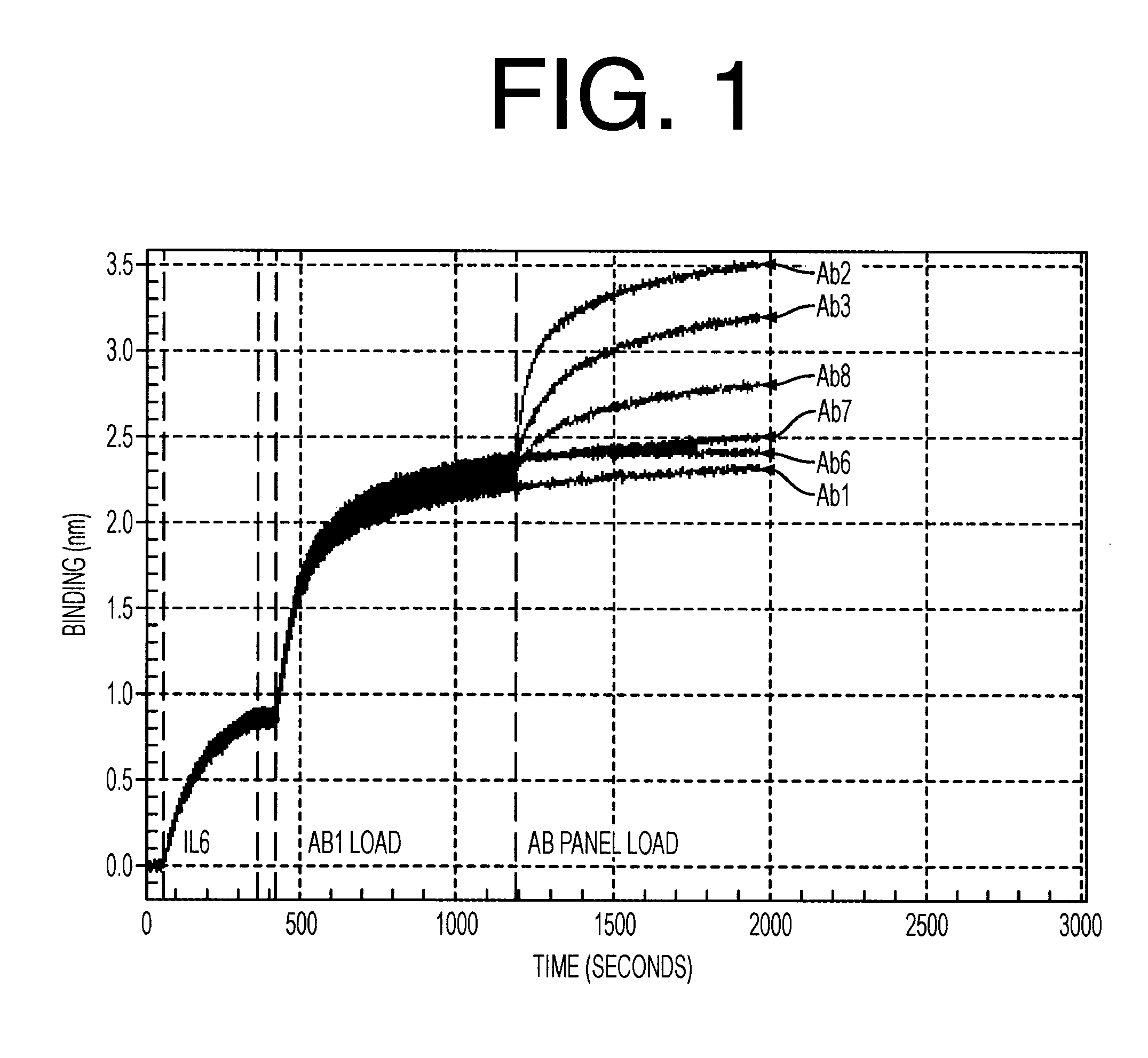
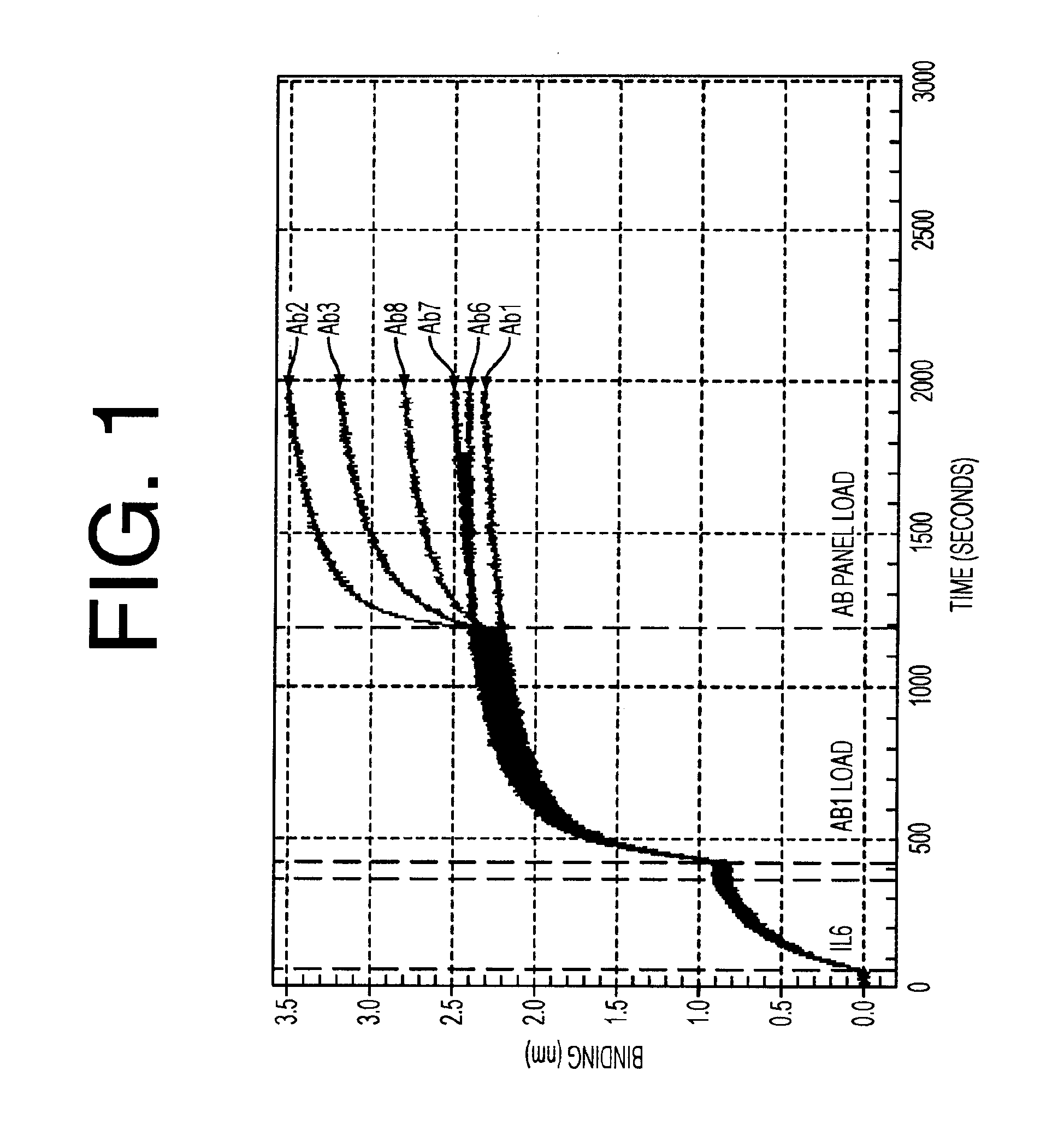
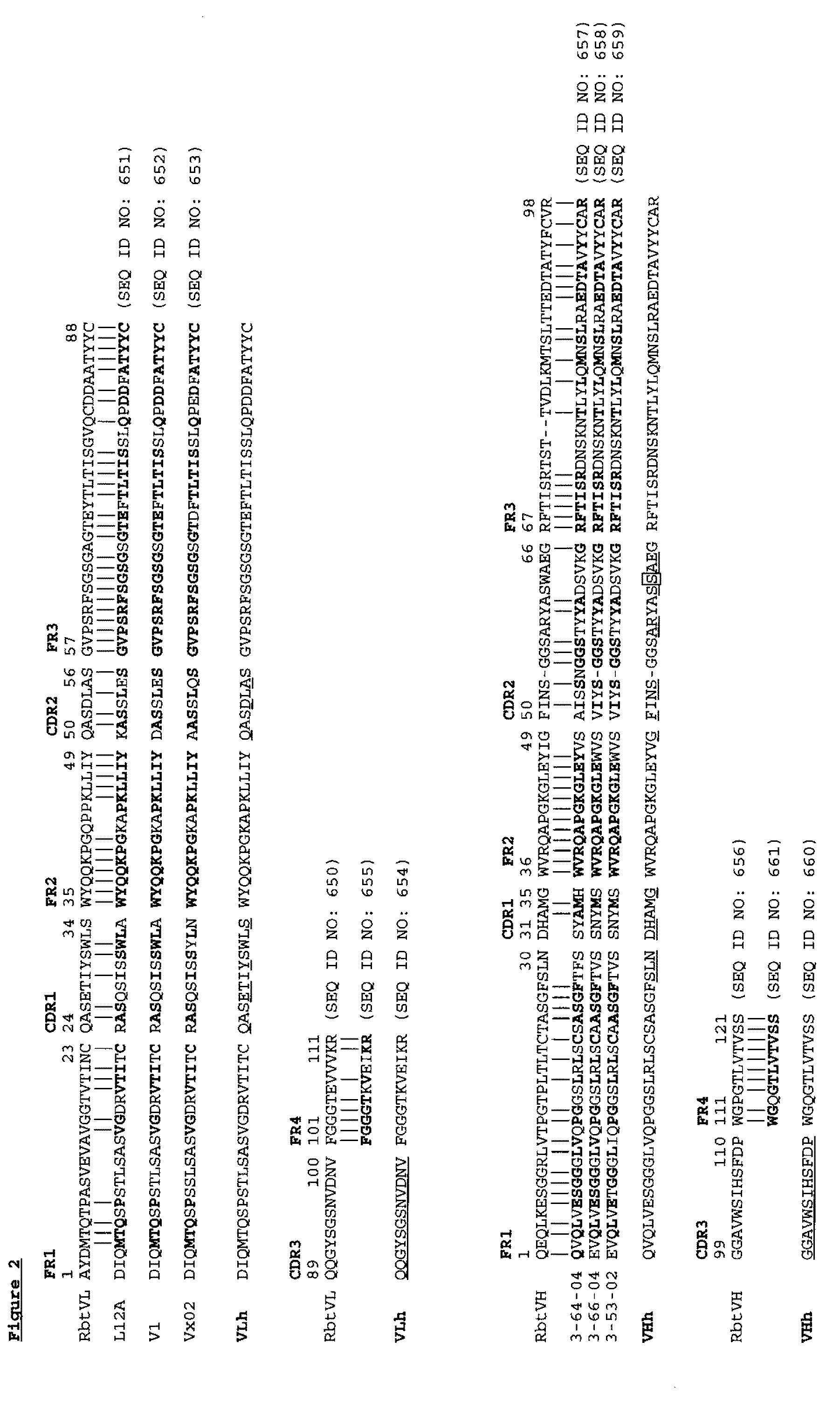
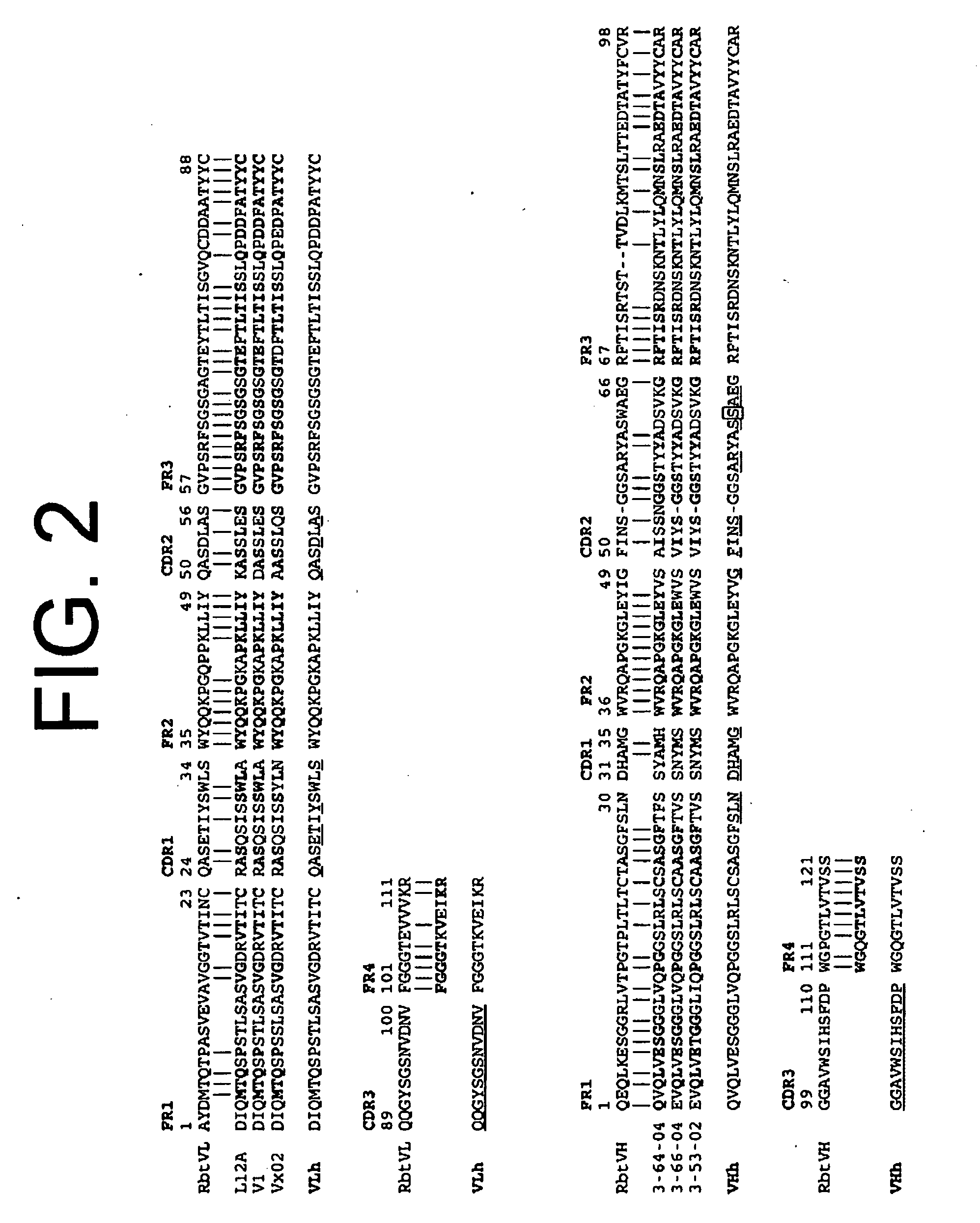
Antibodies to il-6 and use thereof
ActiveUS20100129357A1Eliminate—the risk of thrombosisPrevent thrombosisPeptide/protein ingredientsImmunoglobulins against animals/humansDiseaseAntibody fragments
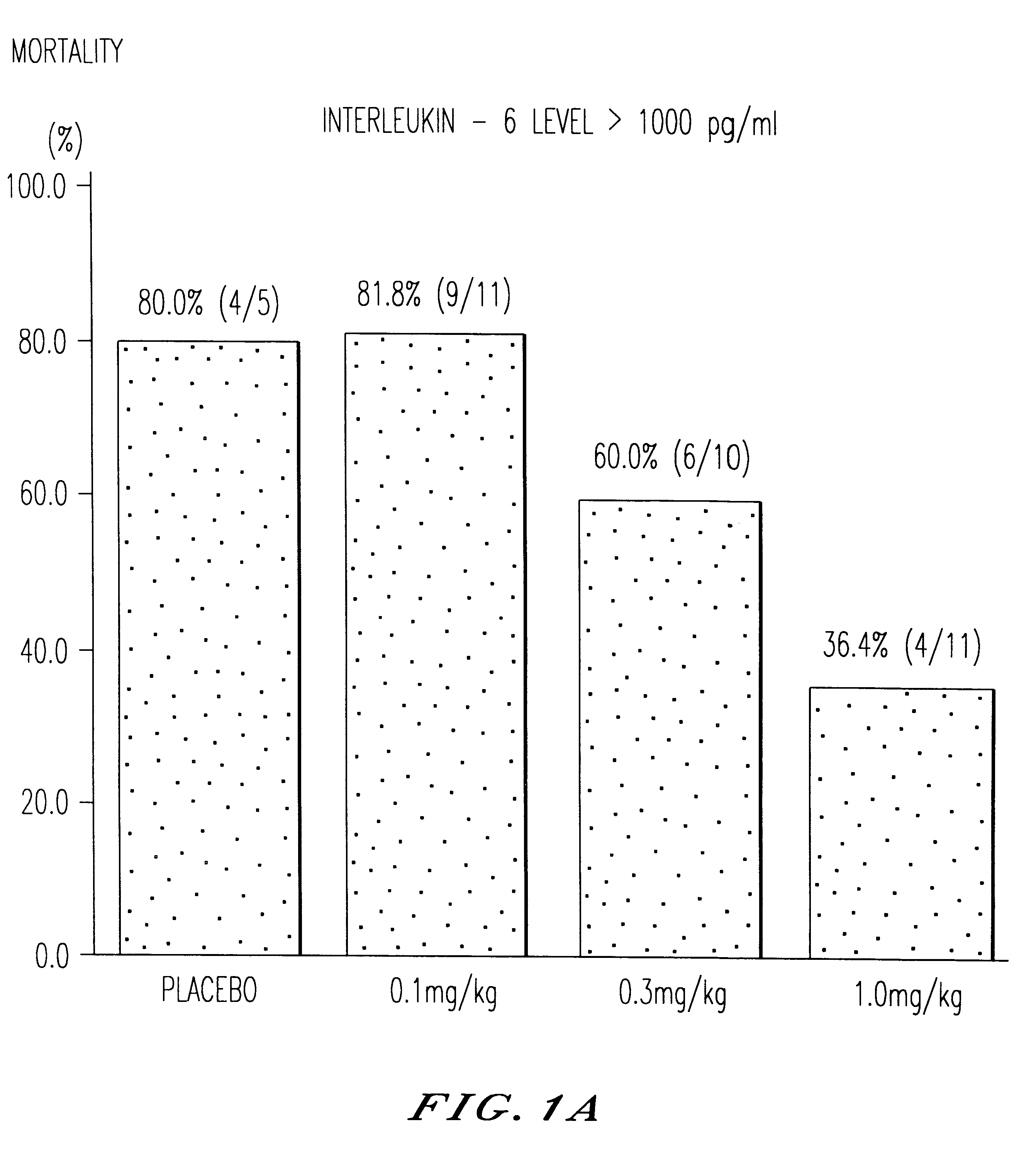
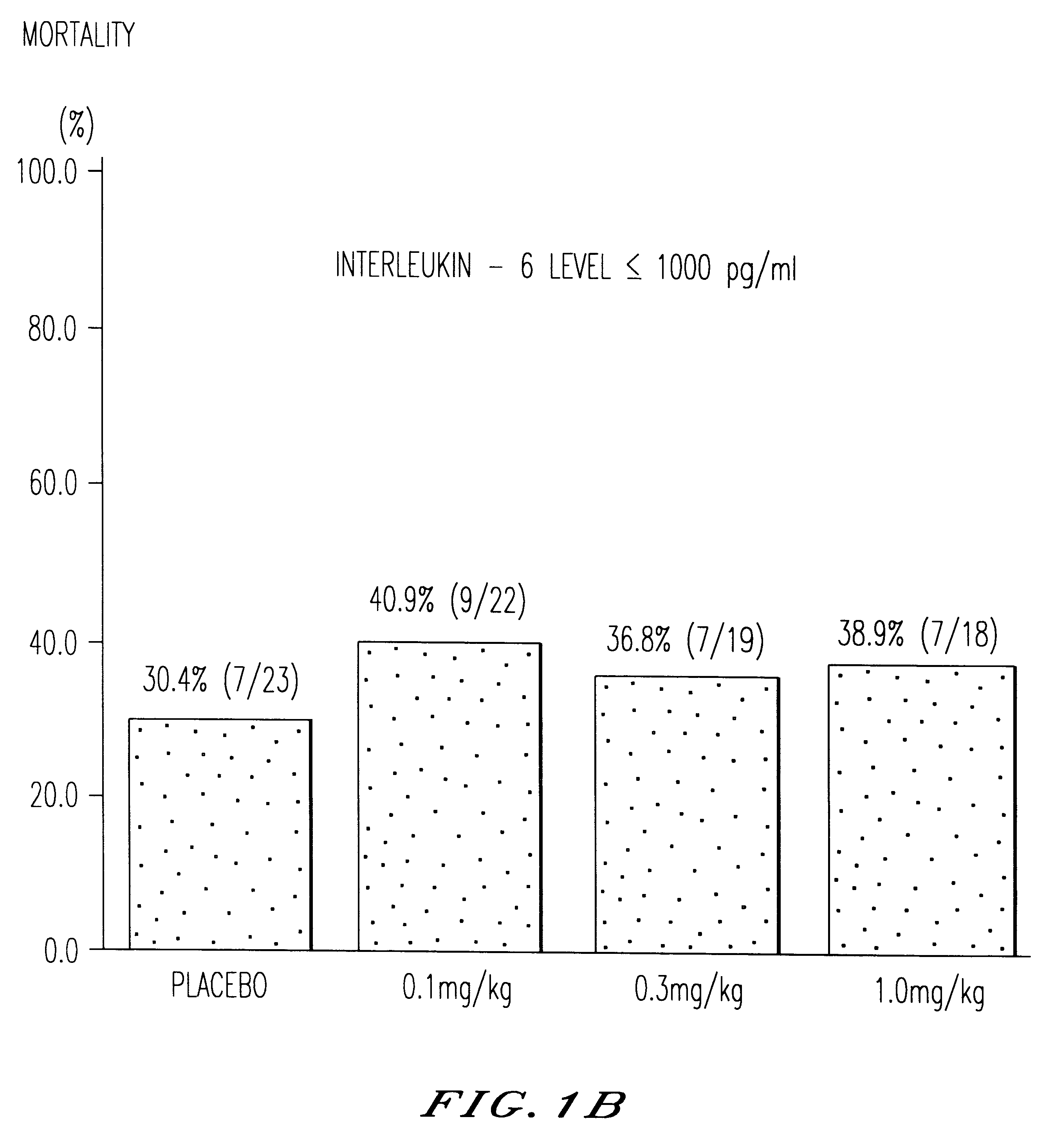
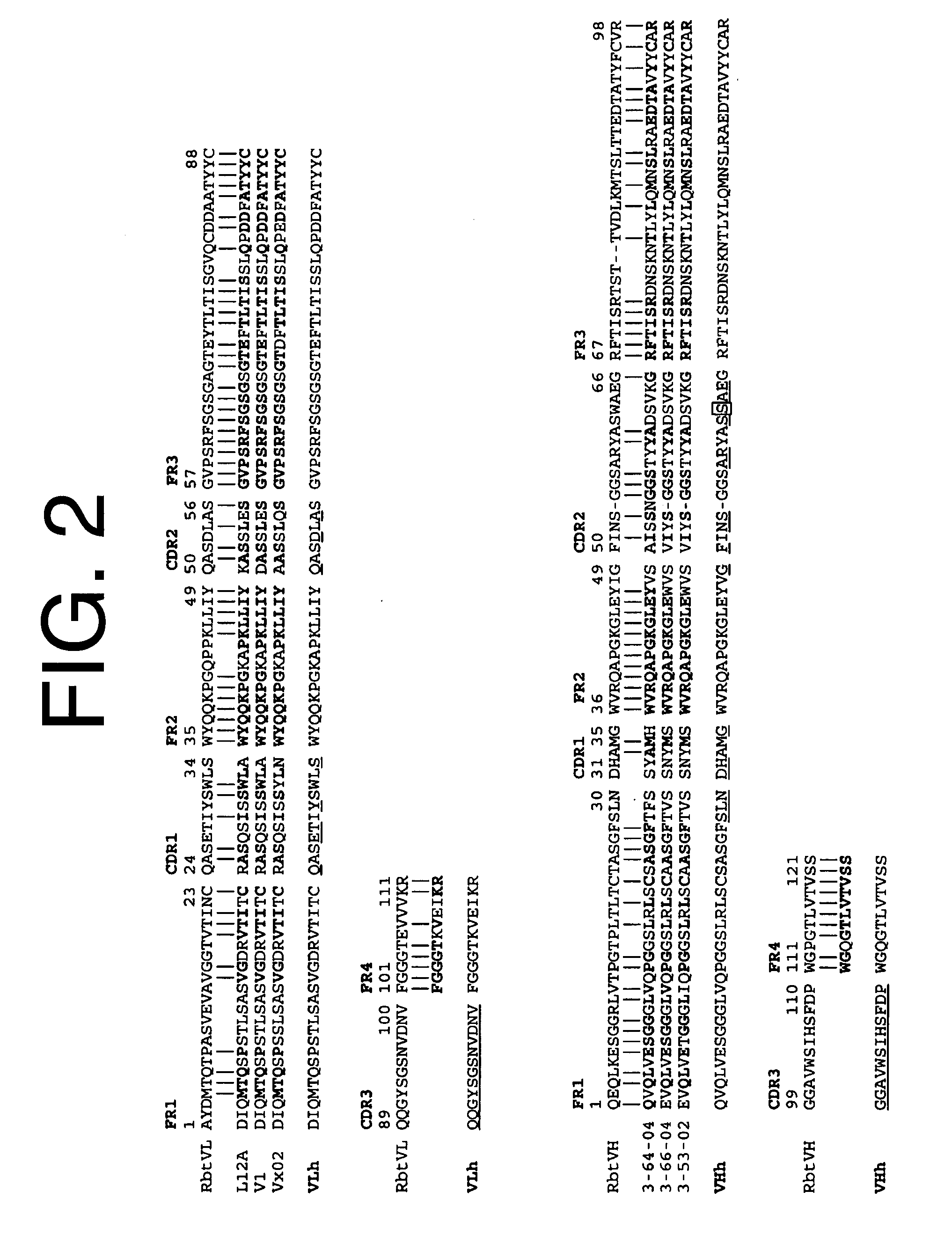
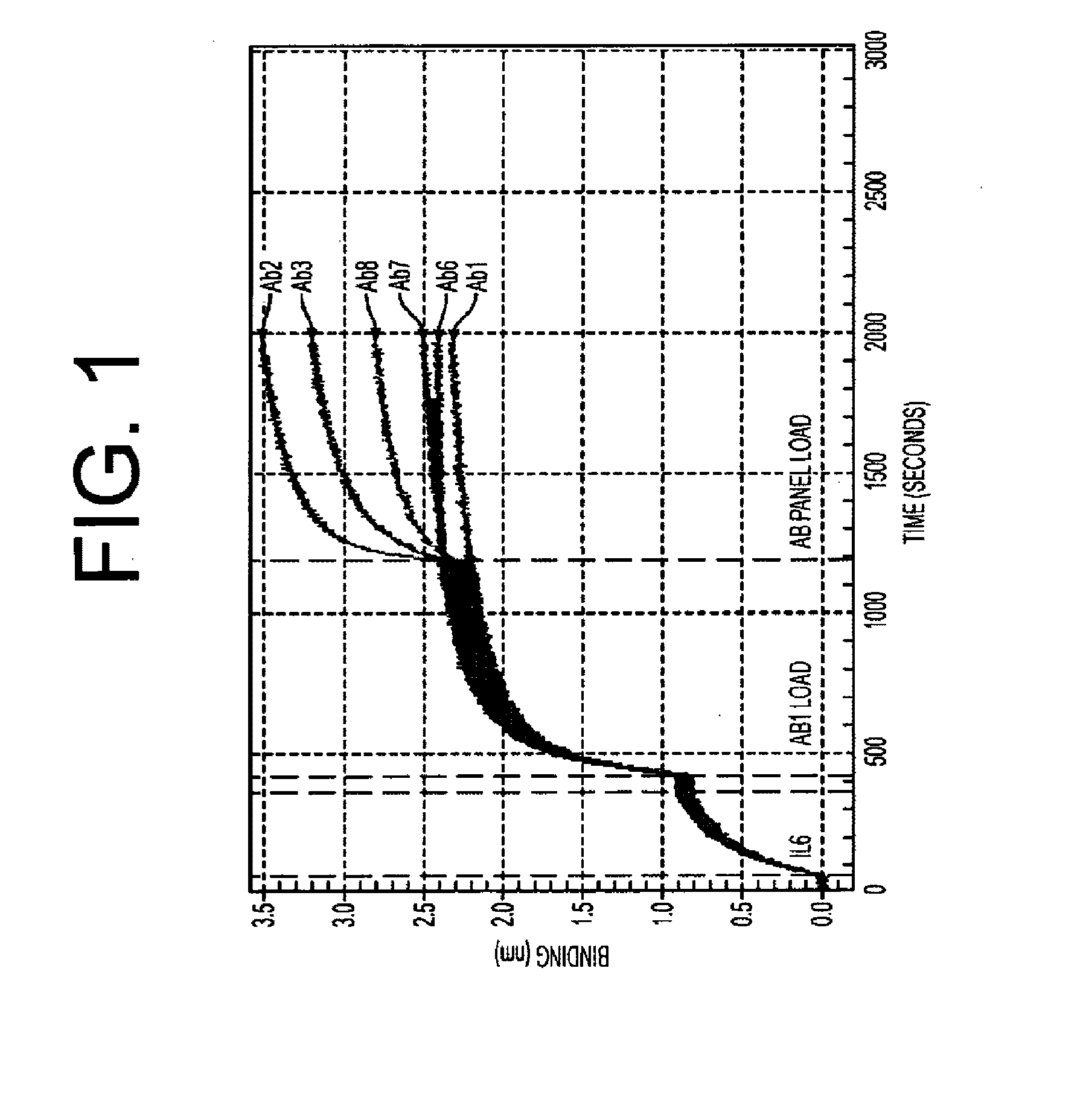
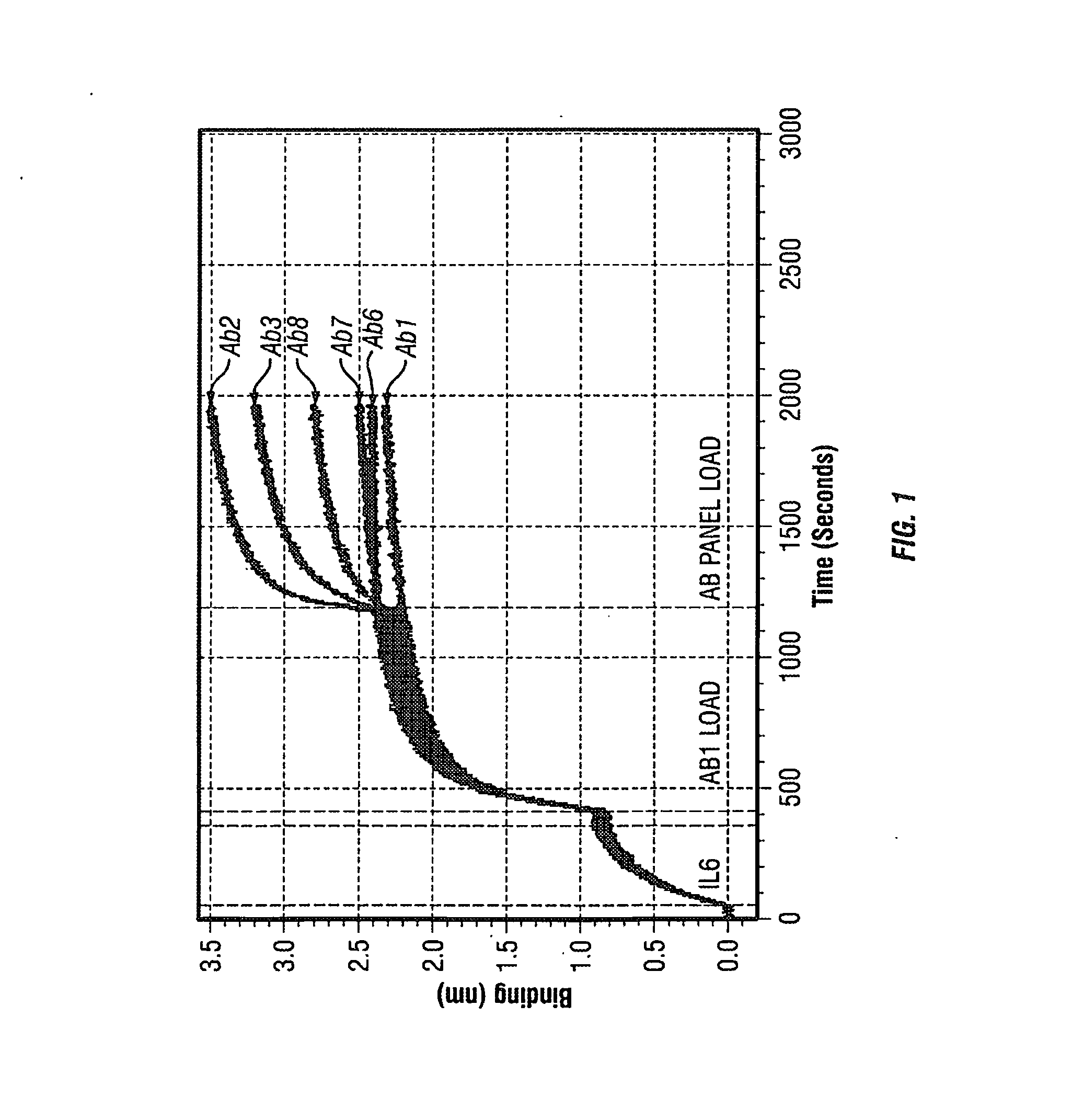
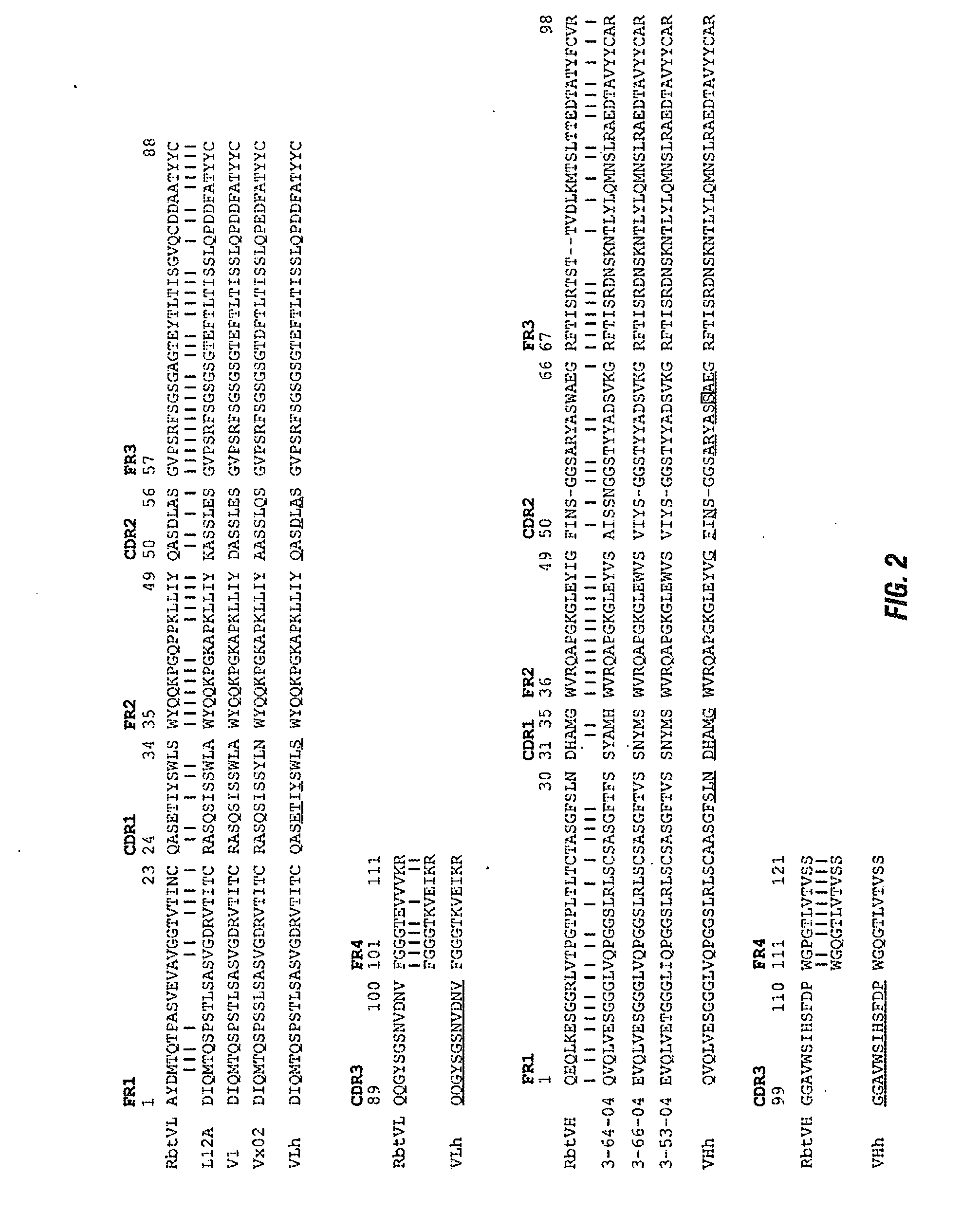
The present invention is directed to therapeutic methods using IL-6 antagonists such as an Ab1 antibody or antibody fragment having binding specificity for IL-6 to prevent or treat disease or to improve survivability or quality of life of a patient in need thereof. In preferred embodiments these patients will comprise those exhibiting (or at risk of developing) an elevated serum C-reactive protein level, reduced serum albumin level, elevated D-dimer or other cogulation cascade related protein(s), cachexia, fever, weakness and / or fatigue prior to treatment. The subject therapies also may include the administration of other actives such as chemotherapeutics, anti-coagulants, statins, and others.
Owner:VITAERIS INC +1
Method for decreasing low density lipoprotein
The present invention relates to a method for decreasing elevated serum / plasma LDL-cholesterol levels or LDL-cholesterol levels and CRP levels in a mammal in need thereof. The methods comprises administering an effective amount of a tetracycline formulation. In one embodiment, the tetracycline formulation is a non-antibacterial tetracycline. In another embodiment, the tetracycline formulation is an antibacterial tetracycline at a sub-antibacterial amount.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +2
Antagonists of il-6 to prevent or treat cachexia, weakness, fatigue, and/or fever
ActiveUS20110217303A1Easy to keepImprove the quality of lifeMetabolism disorderMuscular disorderSerum reactionProtein level
The present invention is directed to therapeutic methods using antibodies and fragments thereof having binding specificity for IL-6 to prevent or treat cachexia, fever, weakness and / or fatigue in a patient in need thereof. In preferred embodiments these patients will comprise those exhibiting (or at risk of developing) an elevated serum C-reactive protein level. In another preferred embodiment, the patient's survivability or quality of life will preferably be improved.
Owner:VITAERIS INC +1
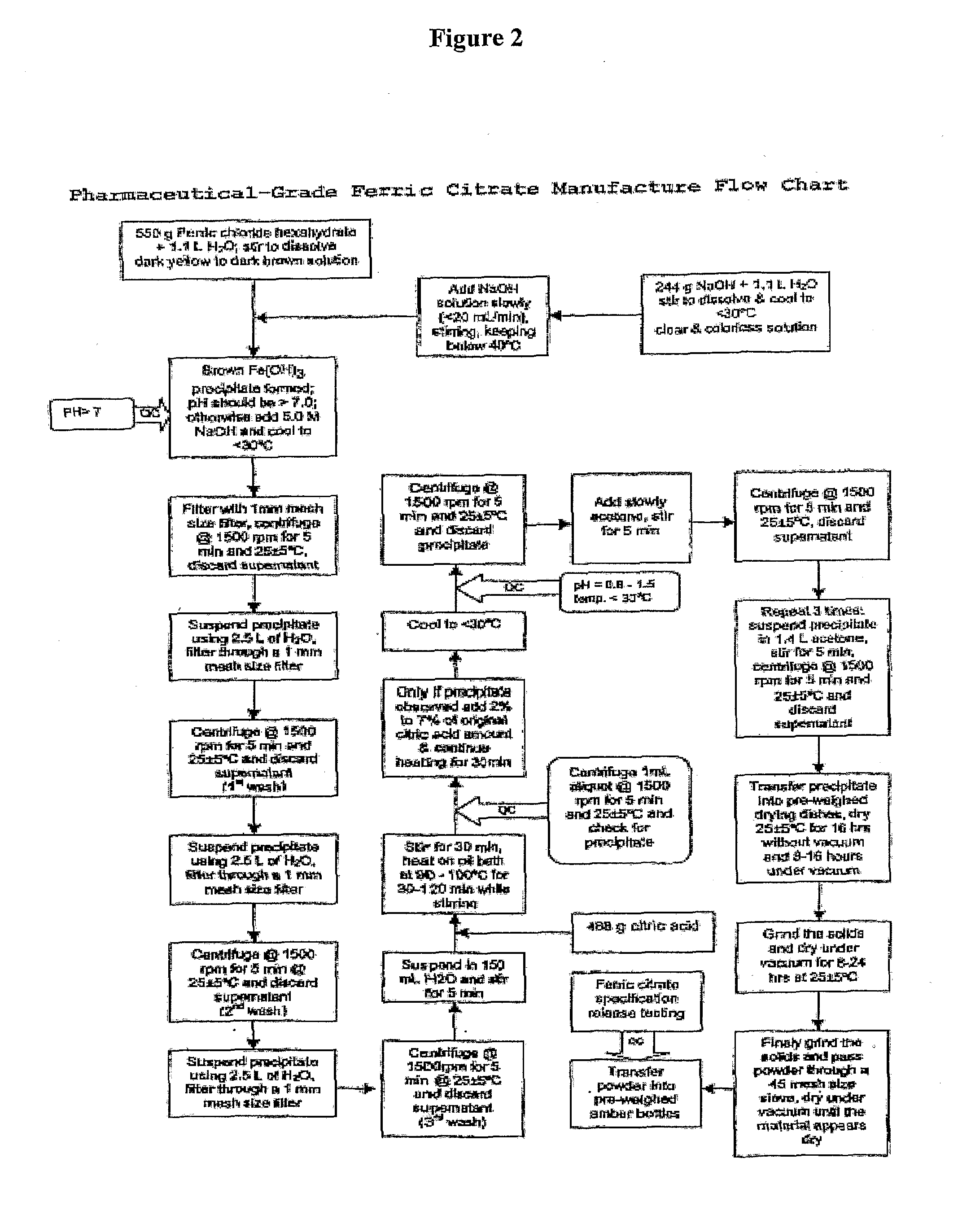
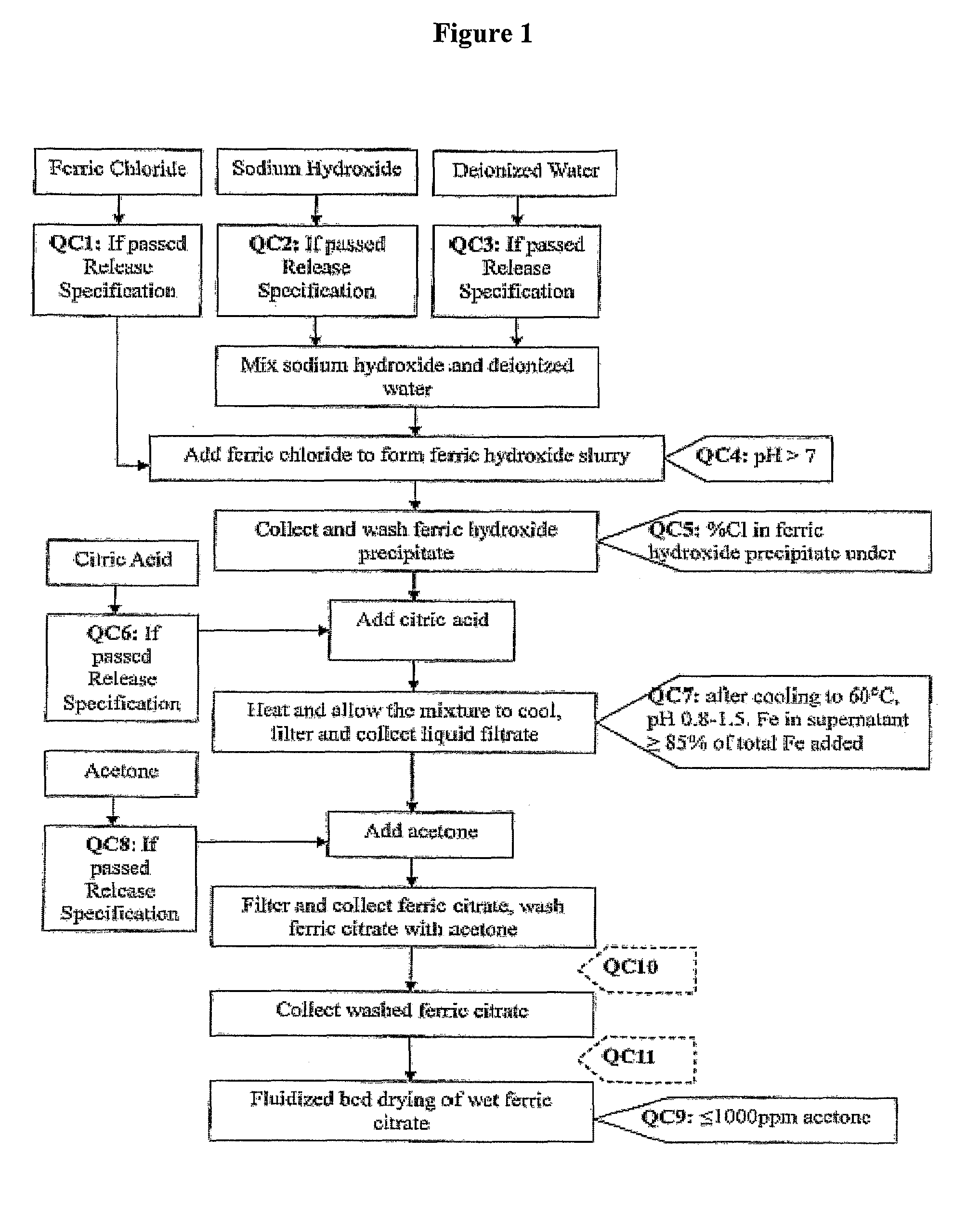
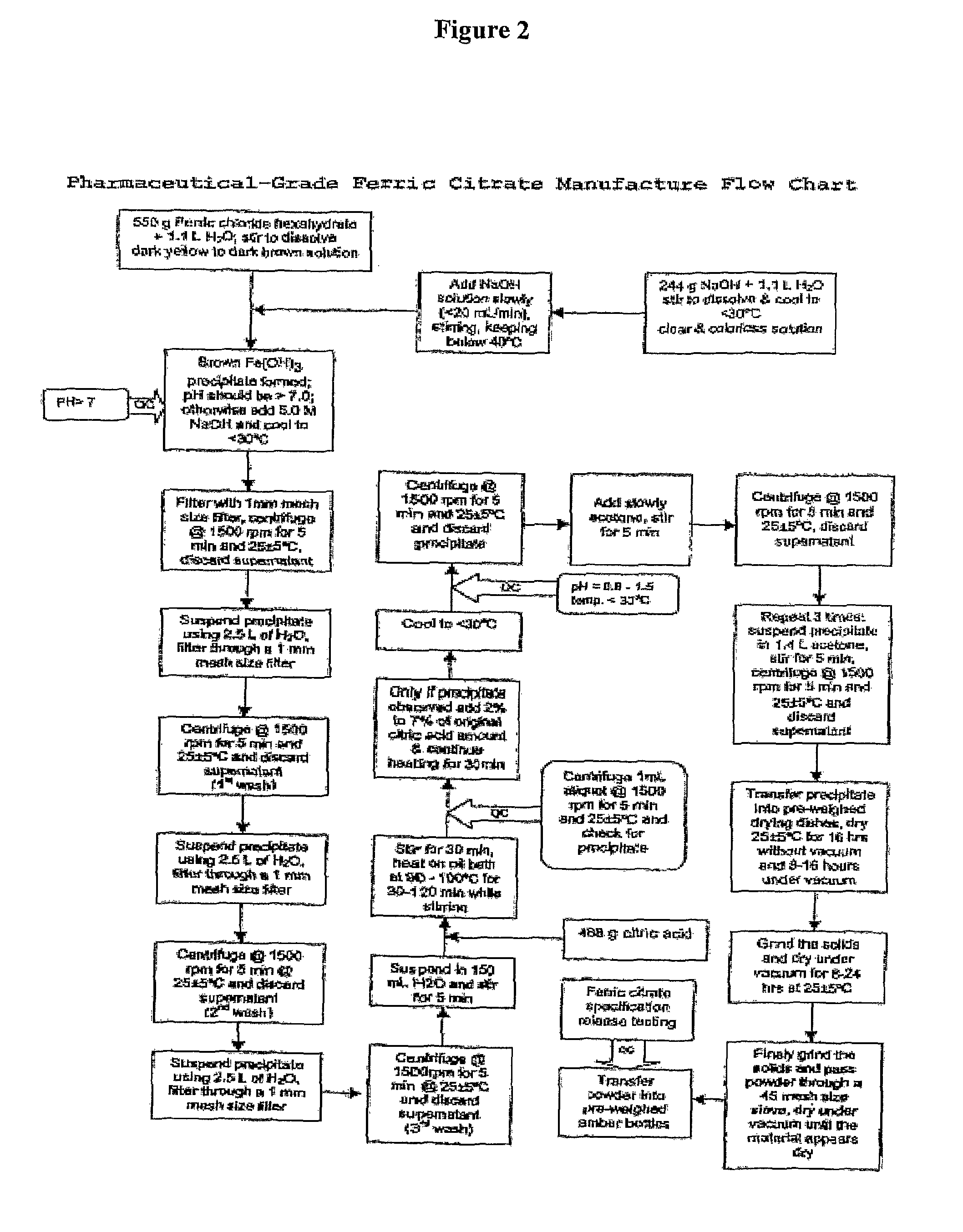
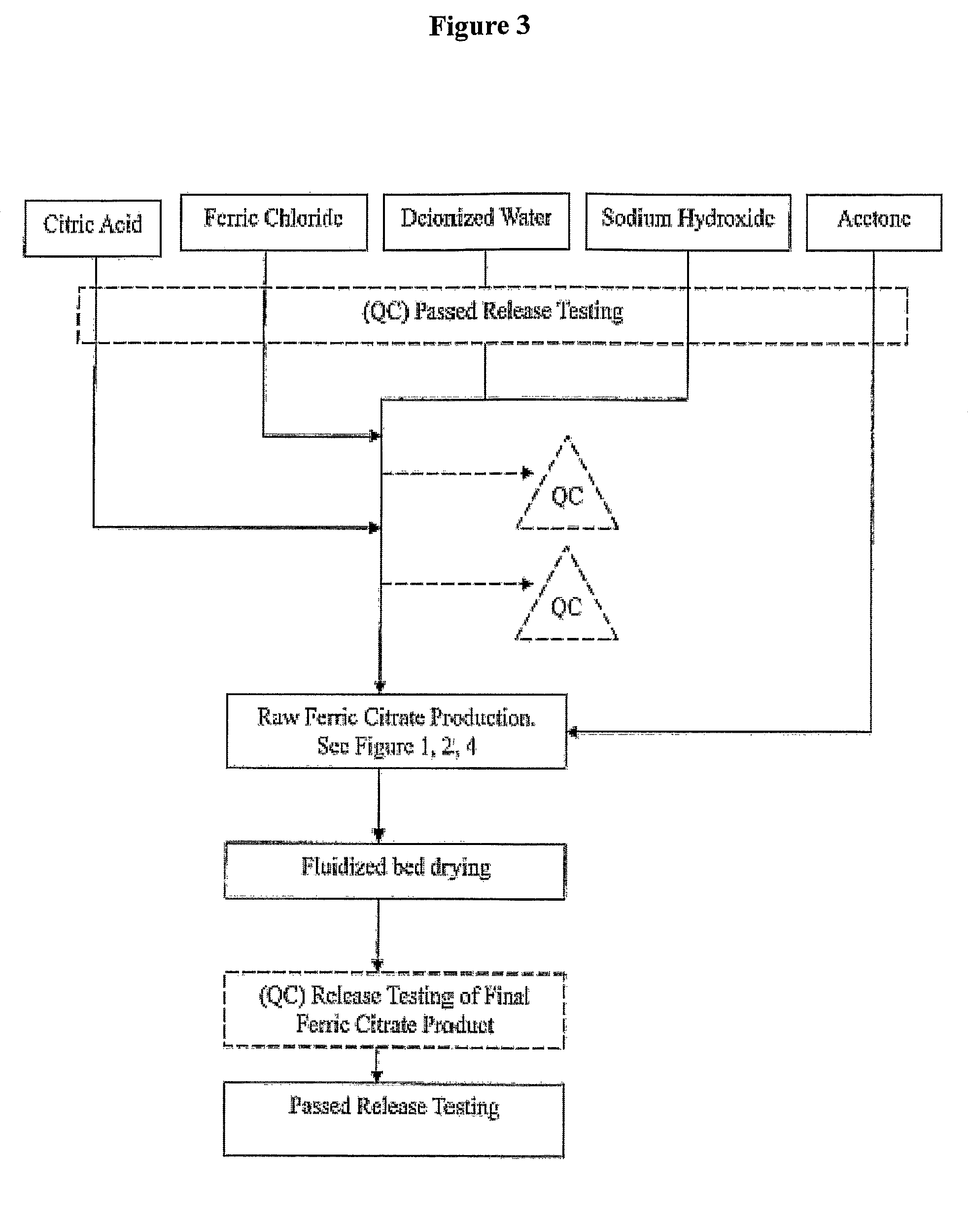
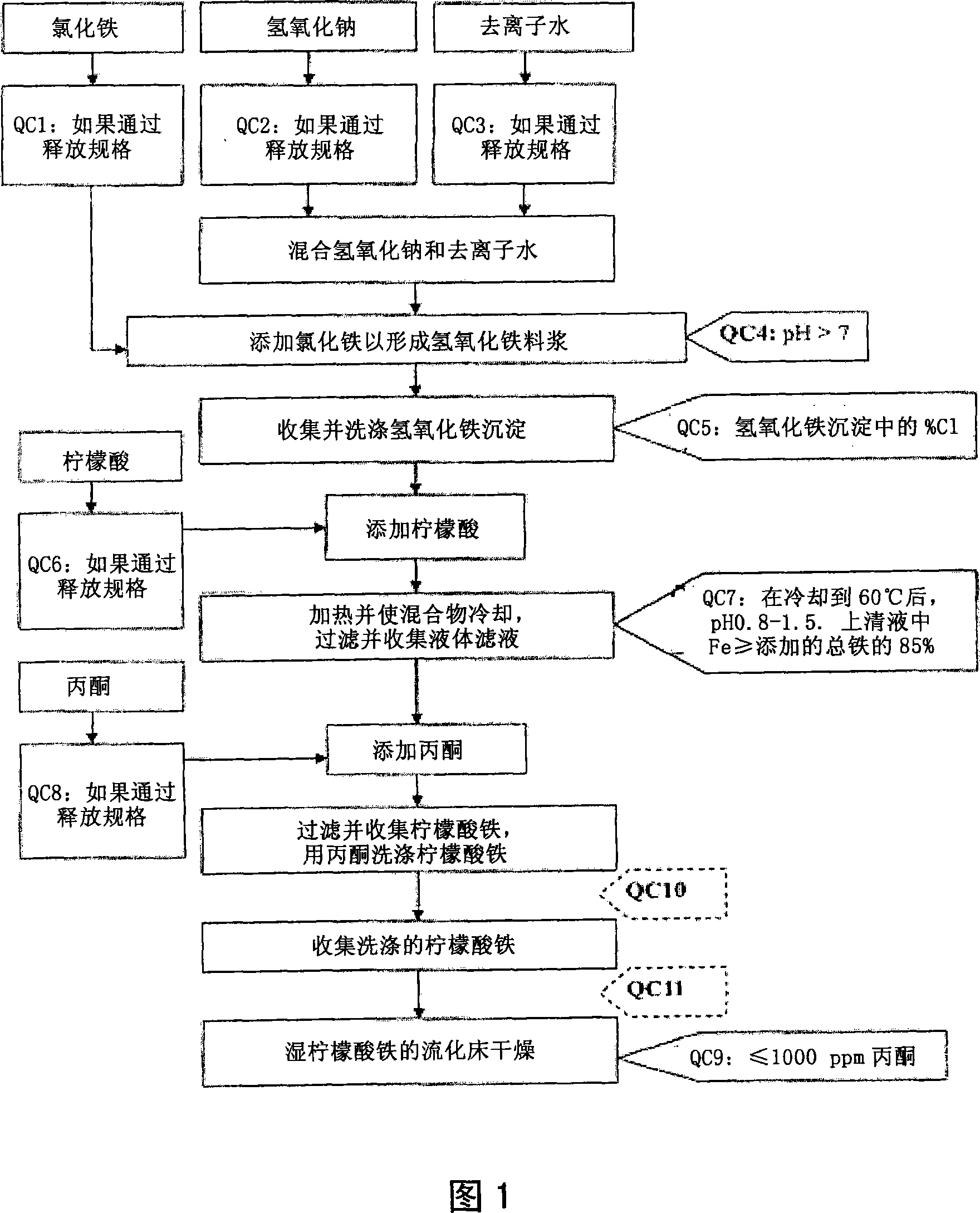
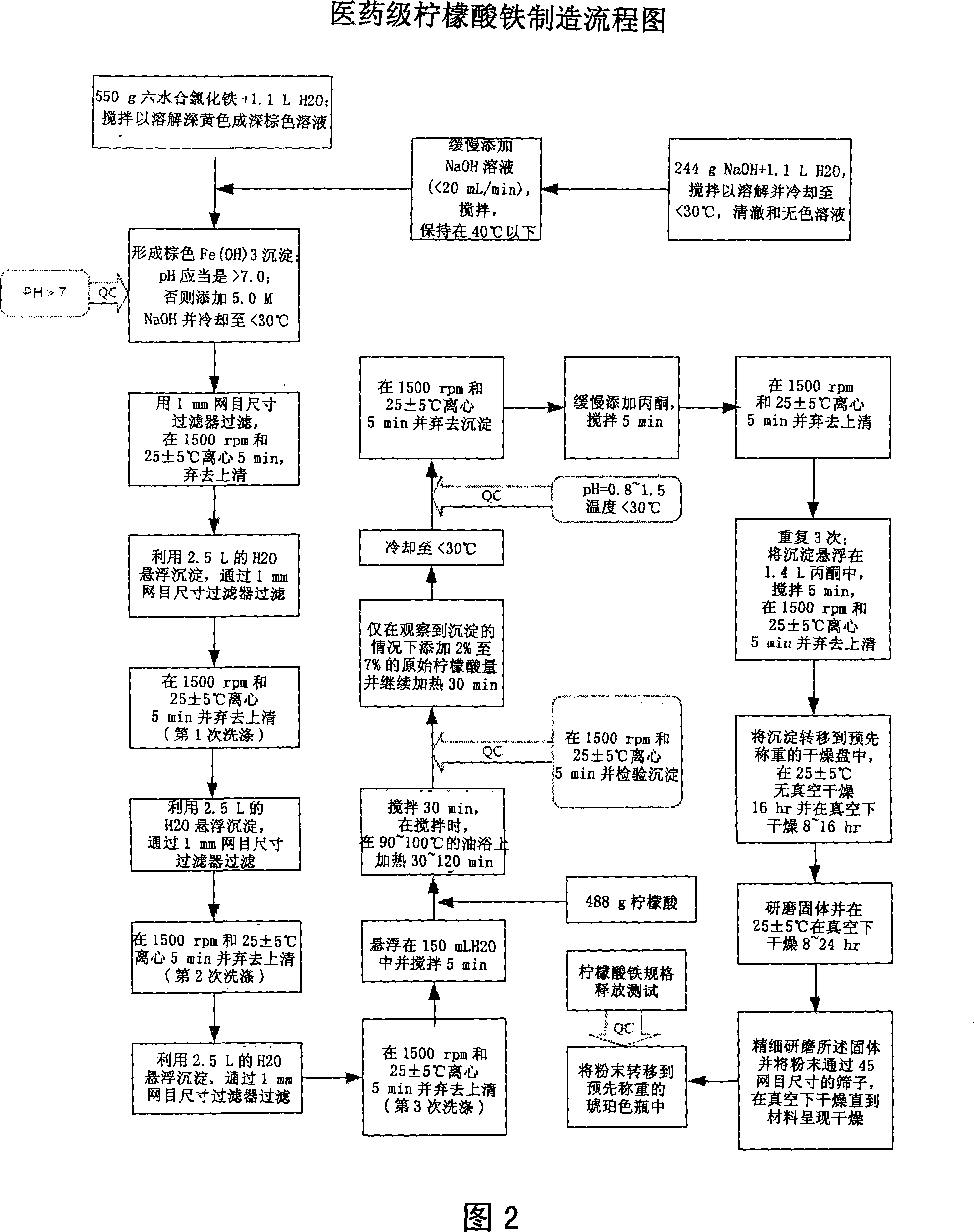
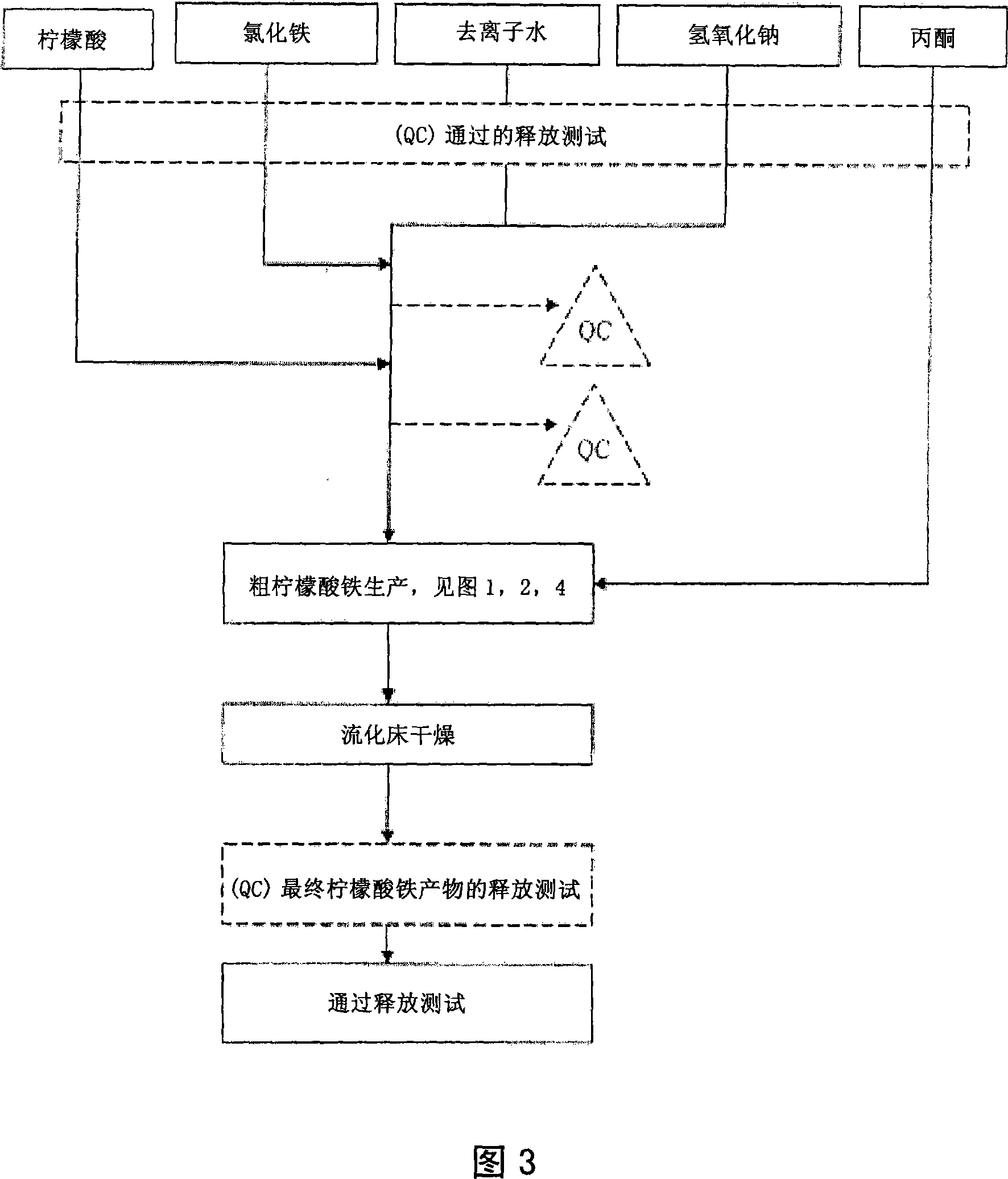
Pharmaceutical-Grade Ferric Organic Compounds, Uses Thereof and Method of Making Same
ActiveUS20080274210A1Long-term administrationHeavy metal active ingredientsOrganic active ingredientsDiseaseSerum phosphate
Owner:PANION & BF BIOTECH INC
Antibodies to IL-6 and use thereof
ActiveUS8323649B2Eliminate—the risk of thrombosisPrevent thrombosisPeptide/protein ingredientsImmunoglobulins against animals/humansSurvivabilityAntibody fragments
Owner:VITAERIS INC +1
Antagonists of il-6 to prevent or treat cachexia, weakness, fatigue and/or fever
InactiveUS20130028860A1Improve the quality of lifeAntibacterial agentsCompounds screening/testingVicrivirocVein
Owner:VITAERIS INC +1
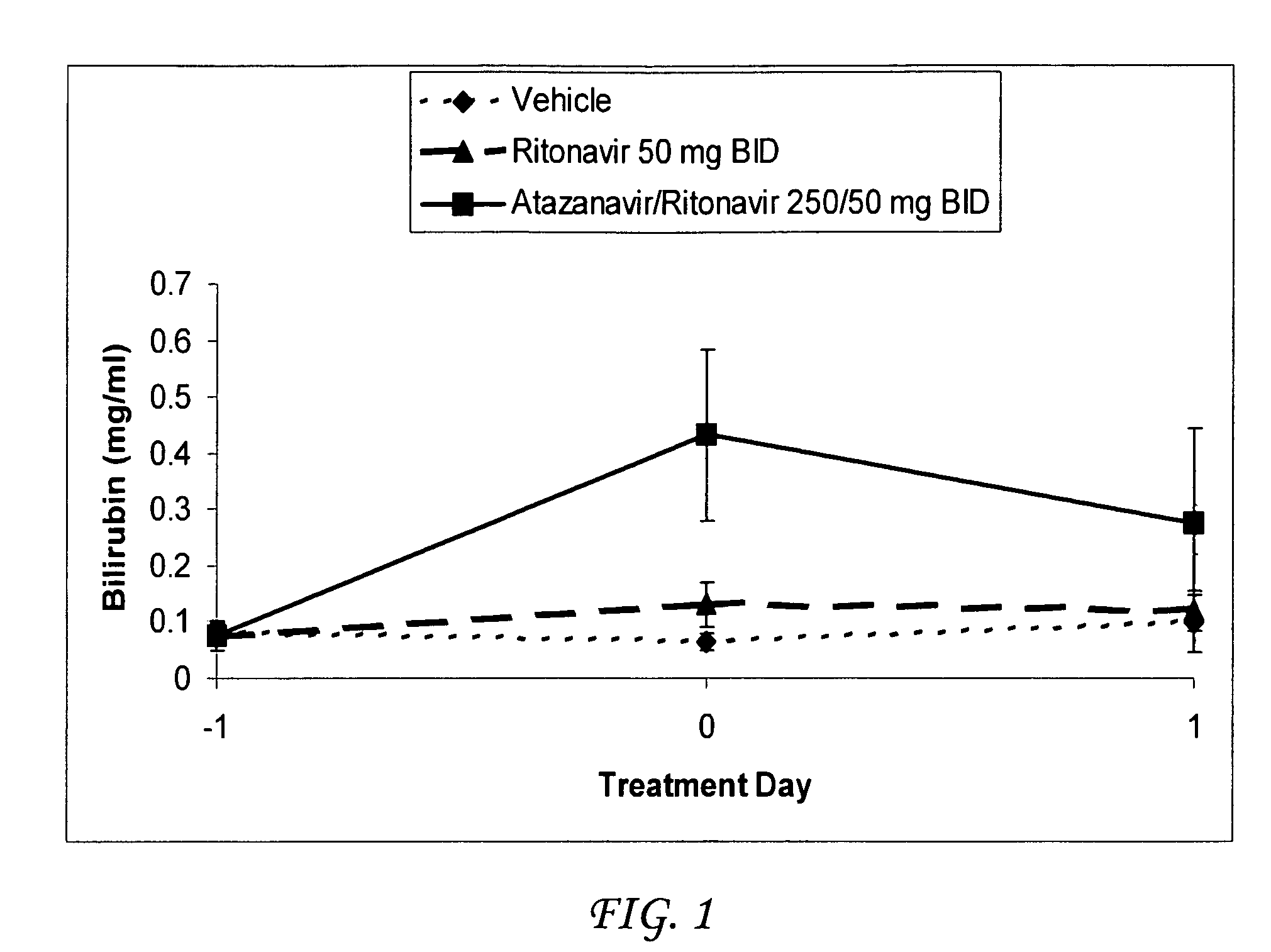
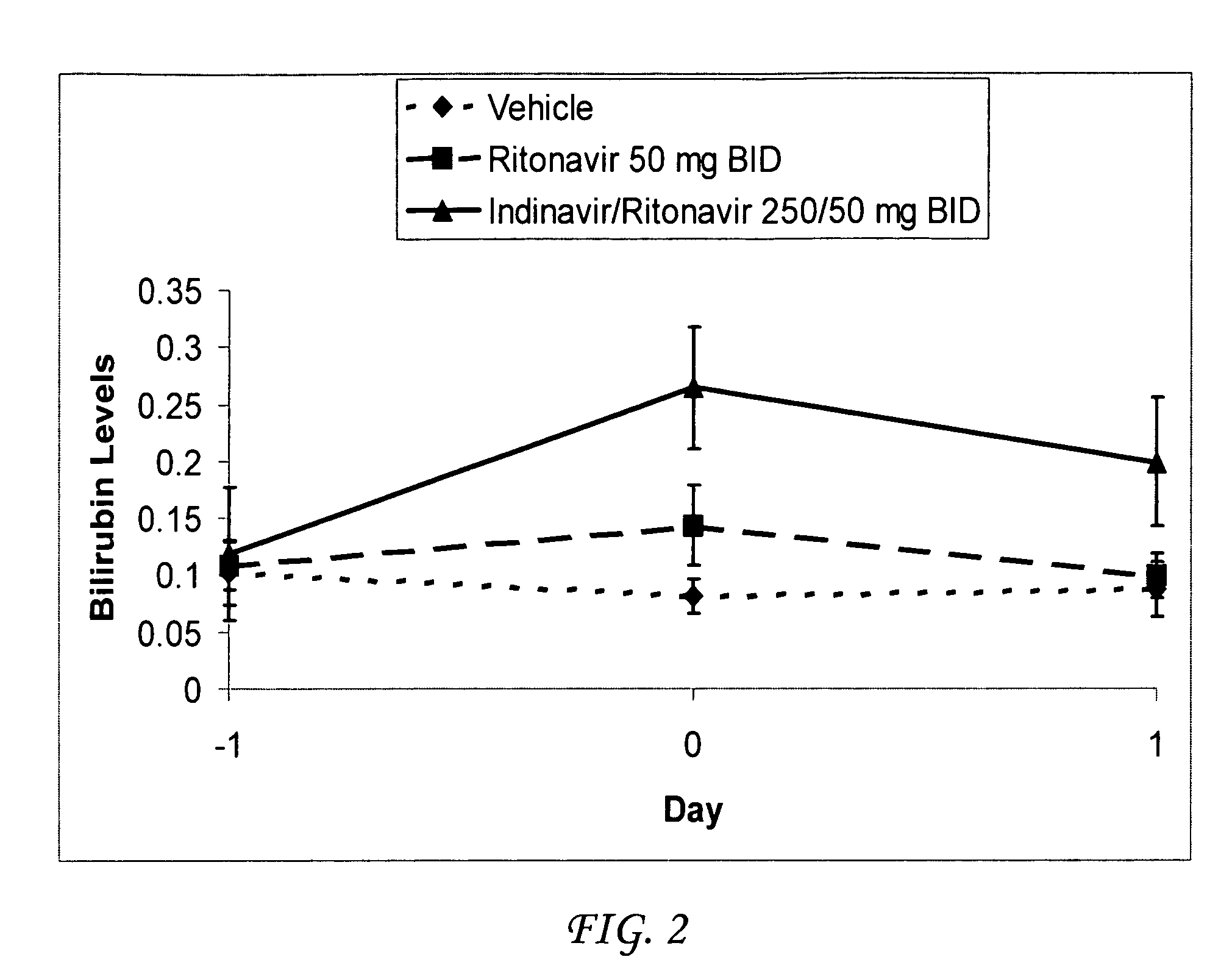
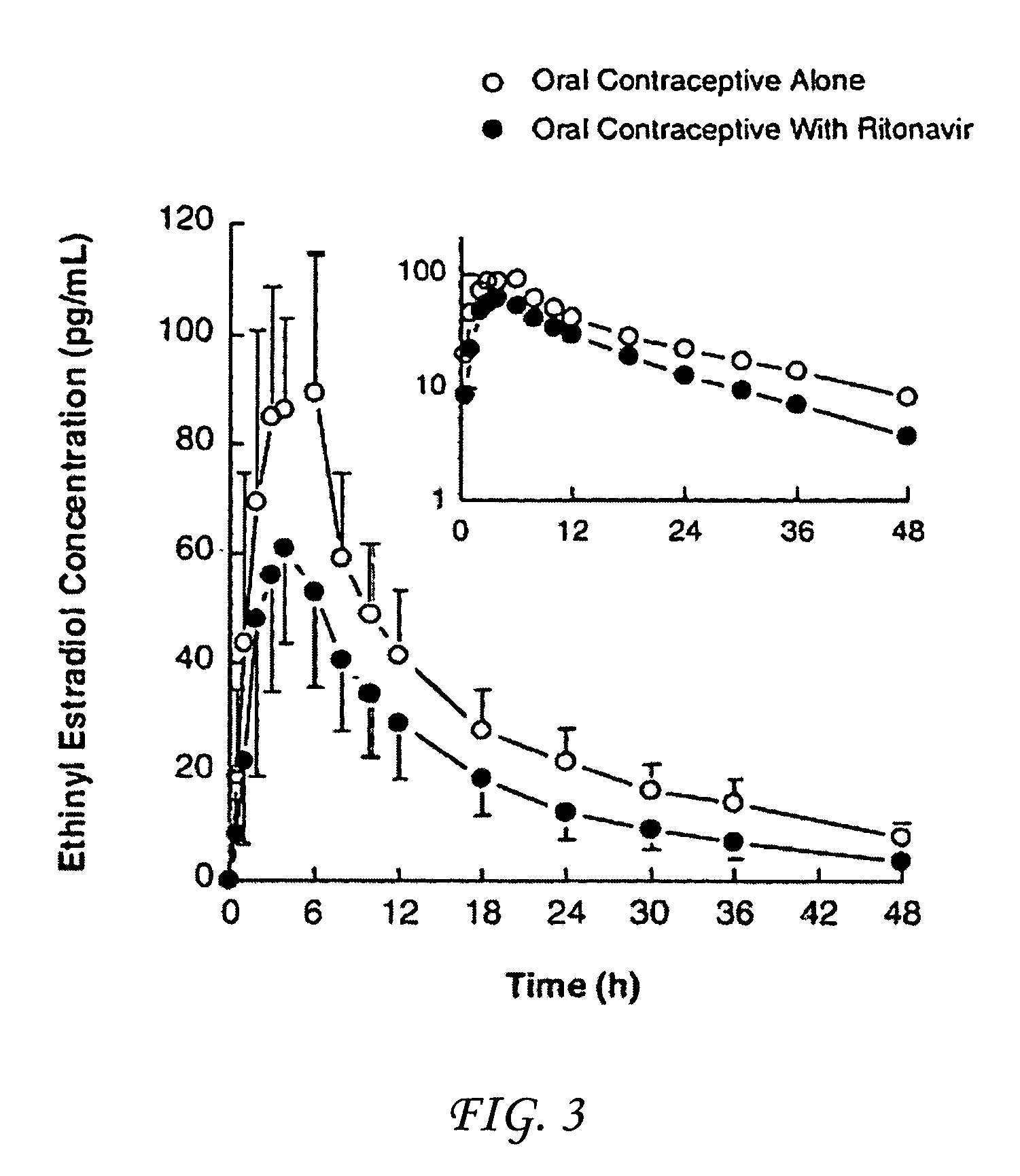
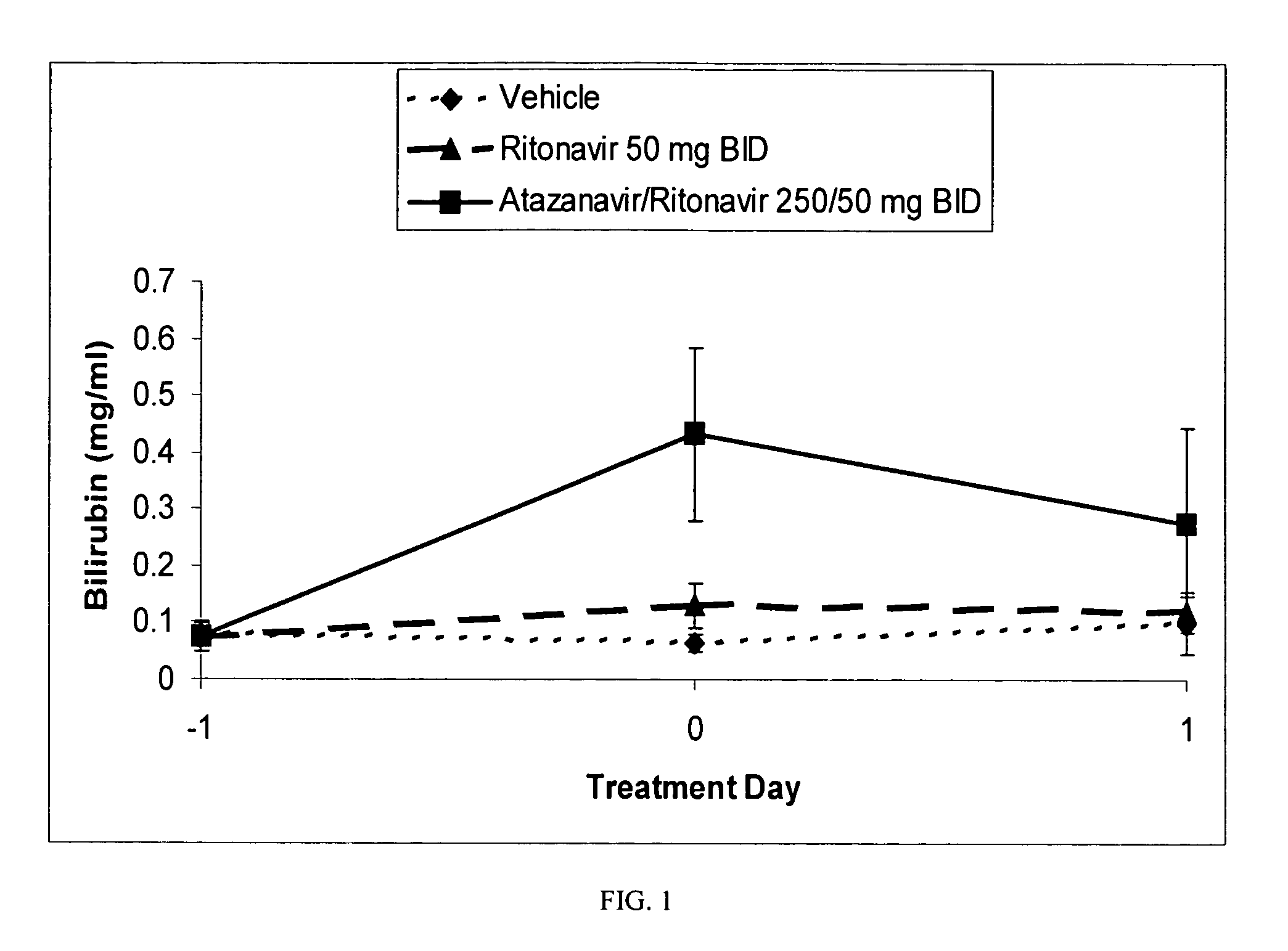
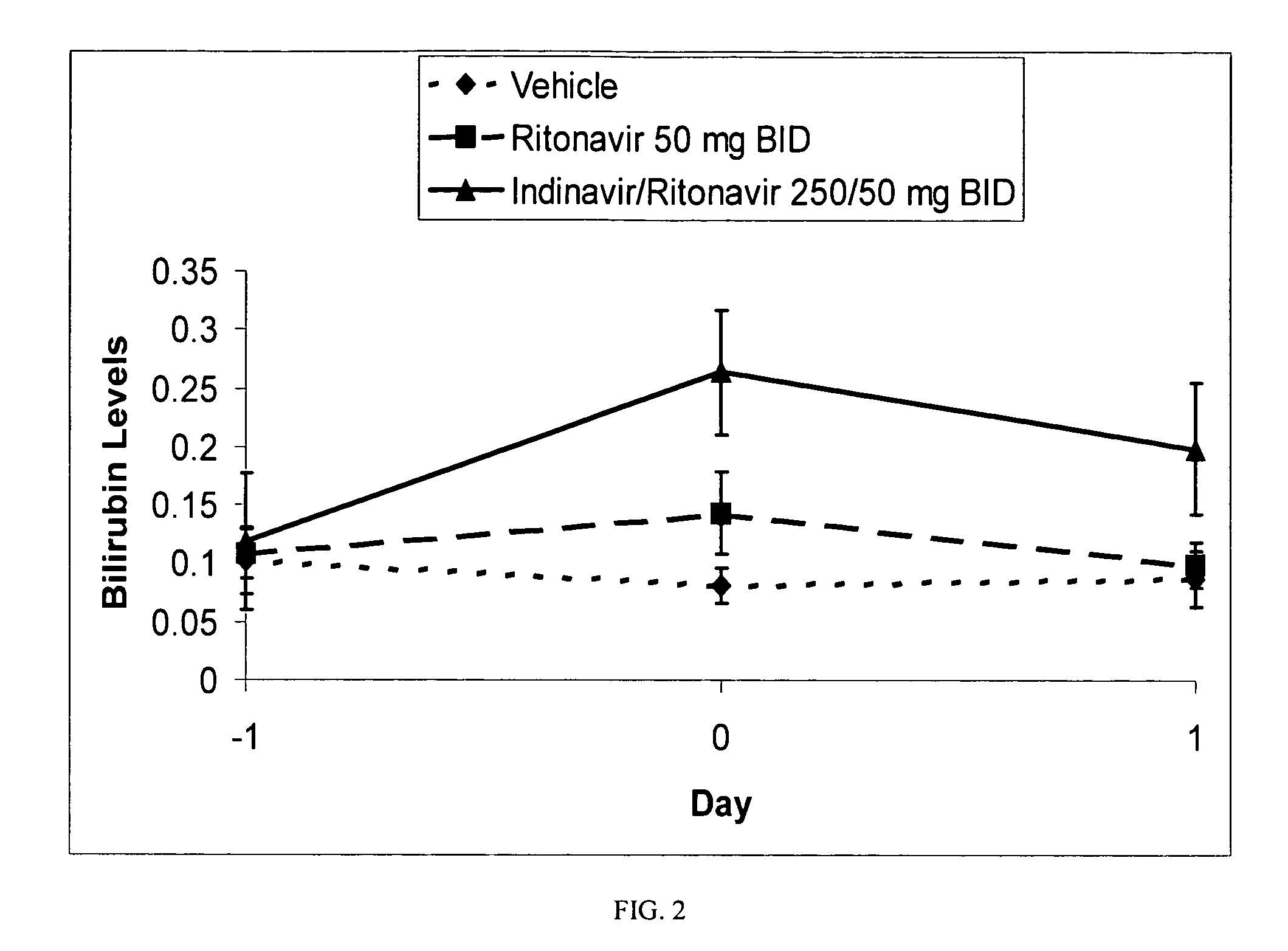
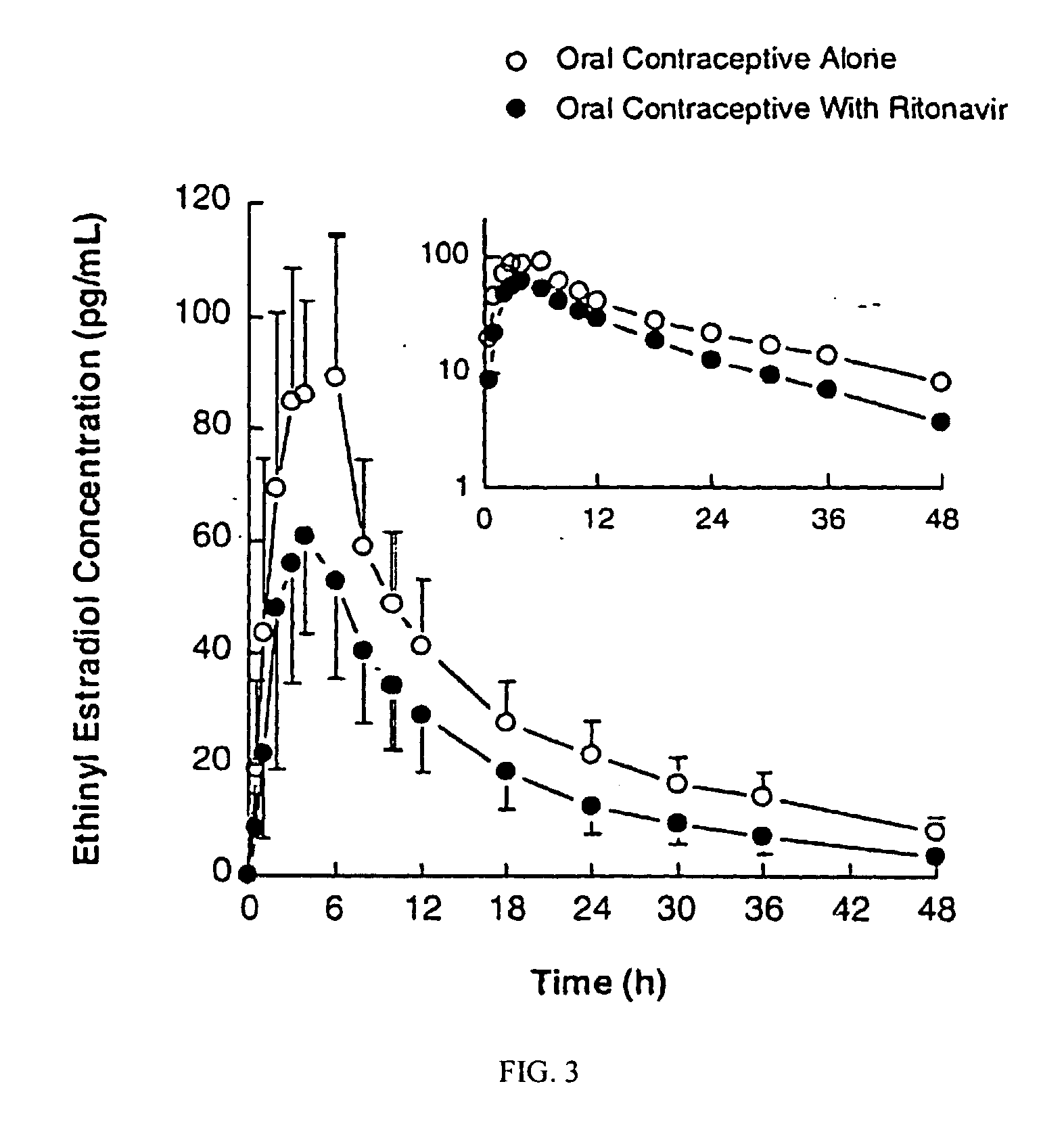
Method for treating a disease, disorder or adverse effect caused by an elevated serum concentration of an UGT1A1 substrate
The present invention is directed to a method for inducing UGT1A1 isoform expression for treatment of a disease, disorder or adverse effect caused by an elevated serum concentration of an UGT1A1 substrate comprising the step of administering to a subject an effective amount of ritonavir. In particular, the present invention is directed to a method of treating unconjugated hyperbilirubinemia by UGT1A1 induction comprising the step of administering to a subject an effective amount of ritonavir.
Owner:ABBVIE INC
Antagonists of IL-6 to raise albumin and/or lower crp
ActiveUS20130183264A1Organic active ingredientsNervous disorderSerum reactionAntiendomysial antibodies
The present invention is directed to therapeutic methods using IL-6 antagonists such as antibodies and fragments thereof having binding specificity for IL-6 to improve survivability or quality of life of a patient in need thereof. In preferred embodiments, the anti-IL-6 antibodies will be humanized and / or will be aglycosylated. Also, in preferred embodiments these patients will comprise those exhibiting (or at risk of developing) an elevated serum C-reactive protein level or a reduced serum albumin level prior to treatment. In another preferred embodiment, the patient's Glasgow Prognostic Score will be increased and survivability will preferably be improved.
Owner:VITAERIS INC +1
Antagonists of IL-6 to prevent or treat Cachexia, weakness, fatigue, and/or fever
ActiveUS20090291077A1Easy to keepImprove the quality of lifeMetabolism disorderMuscular disorderSurvivabilityWeakness
The present invention is directed to therapeutic methods using antibodies and fragments thereof having binding specificity for IL-6 to prevent or treat cachexia, fever, weakness and / or fatigue in a patient in need thereof. In preferred embodiments these patients will comprise those exhibiting (or at risk of developing) an elevated serum C-reactive protein level. In another preferred embodiment, the patient's survivability or quality of life will preferably be improved.
Owner:VITAERIS INC +1
Antagonists of IL-6 to raise albumin and/or lower CRP
ActiveUS8404235B2Quality improvementImprove survivabilityPeptide/protein ingredientsAntipyreticSerum igeSurvivability
The present invention is directed to therapeutic methods using IL-6 antagonists such as antibodies and fragments thereof having binding specificity for IL-6 to improve survivability or quality of life of a patient in need thereof. In preferred embodiments these patients will comprise those exhibiting (or at risk of developing) an elevated serum C-reactive protein level or a reduced serum albumin level prior to treatment. In another preferred embodiment, the patient's Glasgow Prognostic Score will be increased and survivability will preferably be improved.
Owner:VITAERIS INC +1
Antagonists of IL-6 to raise Albumin and/or lower CRP
ActiveUS20090291082A1Quality improvementImprove survivabilityPeptide/protein ingredientsAntipyreticSurvivabilityQuality of life
The present invention is directed to therapeutic methods using IL-6 antagonists such as antibodies and fragments thereof having binding specificity for IL-6 to improve survivability or quality of life of a patient in need thereof. In preferred embodiments these patients will comprise those exhibiting (or at risk of developing) an elevated serum C-reactive protein level or a reduced serum albumin level prior to treatment. In another preferred embodiment, the patient's Glasgow Prognostic Score will be increased and survivability will preferably be improved.
Owner:VITAERIS INC +1
Diagnosis and treatment of human dormancy-related sequellae
Owner:POWELL CO LTD
METHODS FOR REDUCING LIPOPROTEIN(a) LEVELS BY ADMINISTERING AN INHIBITOR OF PROPROTEIN CONVERTASE SUBTILISIN KEXIN-9 (PCSK9)
ActiveUS20130243784A1Lowering serum Lp(a) levelIncreased riskSenses disorderMetabolism disorderLipoprotein(a)Proprotein Convertase Subtilisin/Kexin 9
The present invention provides methods for reducing lipoprotein(a) (Lp(a)) in patients. The methods of the present invention comprise selecting a patient who exhibits elevated serum Lp(a), and administering to the patient a pharmaceutical composition comprising a PCSK9 inhibitor. In certain embodiments, the PCSK9 inhibitor is an anti-PCSK9 antibody such as the exemplary antibody referred to herein as mAb316P.
Owner:REGENERON PHARM INC
Diagnosis and treatment of human dormancy-related sequellae
InactiveUS20060052278A1Effective treatmentReduce doseBiocideSnake antigen ingredientsAntigenAutoimmune condition
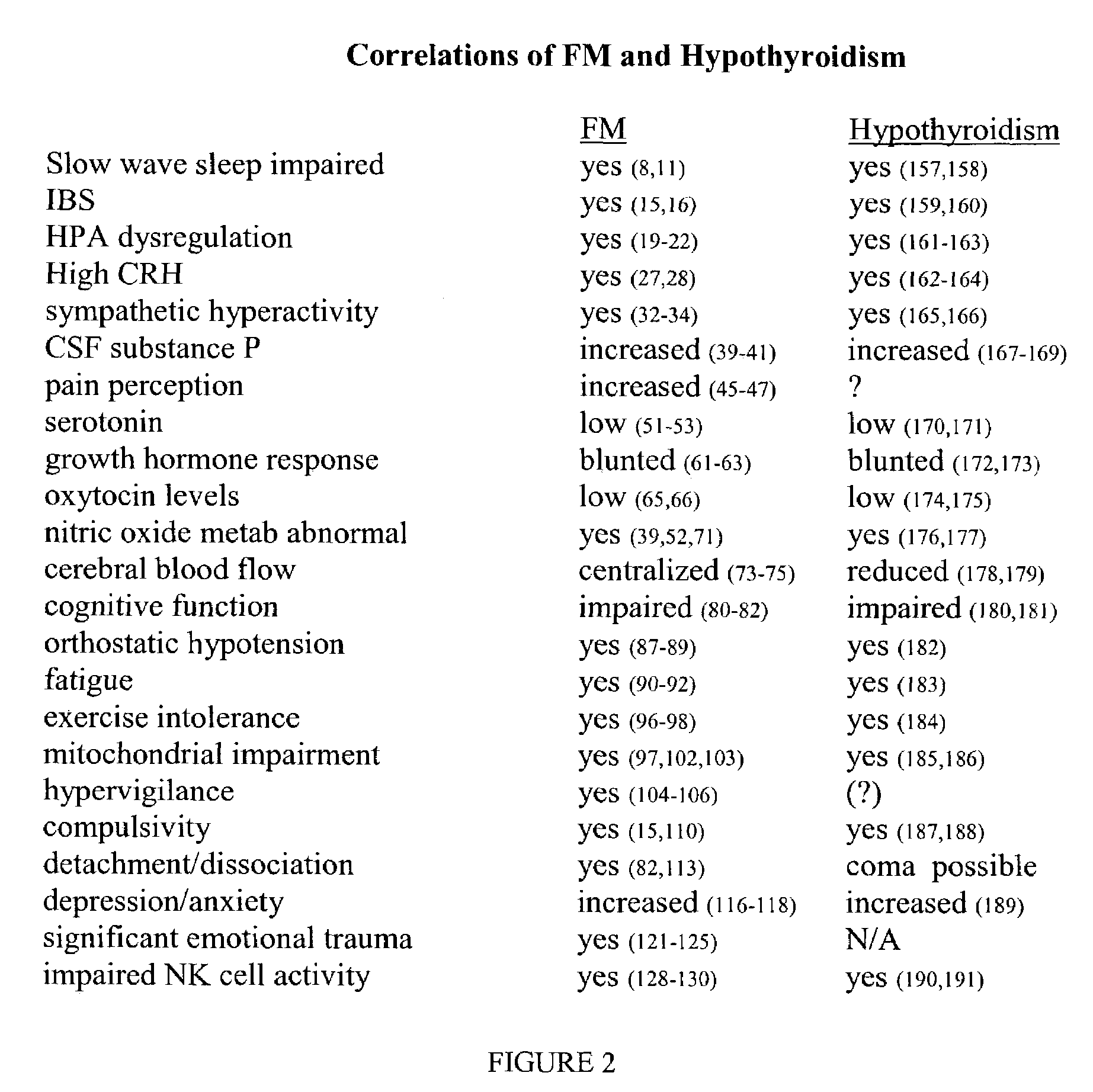
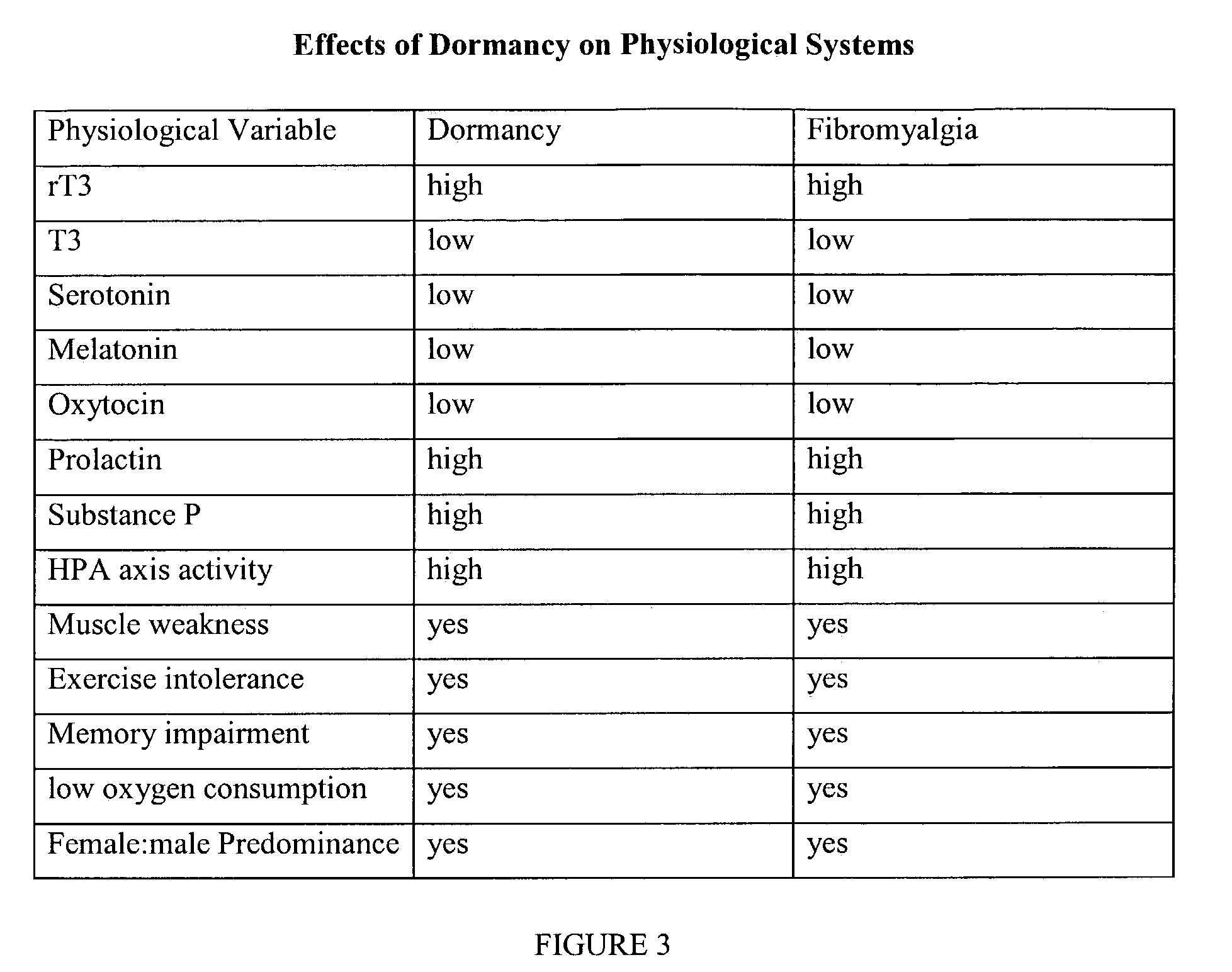
New methods for diagnosis and treatment of human dormancy syndrome-related sequellae are provided. Human dormancy syndrome (HDS) is characterized by elevated serum ratio of rT3 / fT3 compared to a population of normal subjects. HDS includes fibromyalgia, chronic fatigue, cancer, autoimmune disease, obesity and related dormancy conditions. Dormancy and HDS-related sequellae are imposed on humans by infection with lipopolysaccharide (LPS; or endotoxin)-producing organisms, especially those that are intracellular and those that create antigens that stimulate the TLR pathways. In such instances, the elimination or neutralization of the LPS signal along with the infectious source is required to impact the sequellae of HDS. Treatment includes use of novel and non-obvious doses of antibiotics, optionally including agents that decrease the adverse effects of endotoxin.
Owner:POWELL CO LTD
Antagonists of IL-6 to raise albumin and/or lower CRP
ActiveUS20120014955A1Quality improvementImprove survivabilityPeptide/protein ingredientsAntipyreticSerum igeSurvivability
The present invention is directed to therapeutic methods using IL-6 antagonists such as antibodies and fragments thereof having binding specificity for IL-6 to improve survivability or quality of life of a patient in need thereof. In preferred embodiments these patients will comprise those exhibiting (or at risk of developing) an elevated serum C-reactive protein level or a reduced serum albumin level prior to treatment. In another preferred embodiment, the patient's Glasgow Prognostic Score will be increased and survivability will preferably be improved.
Owner:ALDERBIO HLDG LLC +1
Methods for diagnosis and treatment of crohn's disease
The inventors have discovered an elevated serum response to CBir1 flagellin in Crohn's disease patients. The present invention relates to methods for diagnosis and treatment of Crohn's disease and / or subtypes of Crohn's disease. Diagnosis is accomplished by determining the presence of the anti-CBir1 expression or determining the presence of anti-CBir1 expression and detection of the presence of pANCA. Treatment methods include antigen-directed therapy targeting CBir1 flagellin and manipulating the bacteria in the colon and / or small intestine.
Owner:CEDARS SINAI MEDICAL CENT
Pharmaceutical-grade ferric organic compounds, uses thereof and method of making same
ActiveUS8093423B2Improve scalabilityImprove the preparation effectHeavy metal active ingredientsBiocideSerum phosphateSimple Organic Compounds
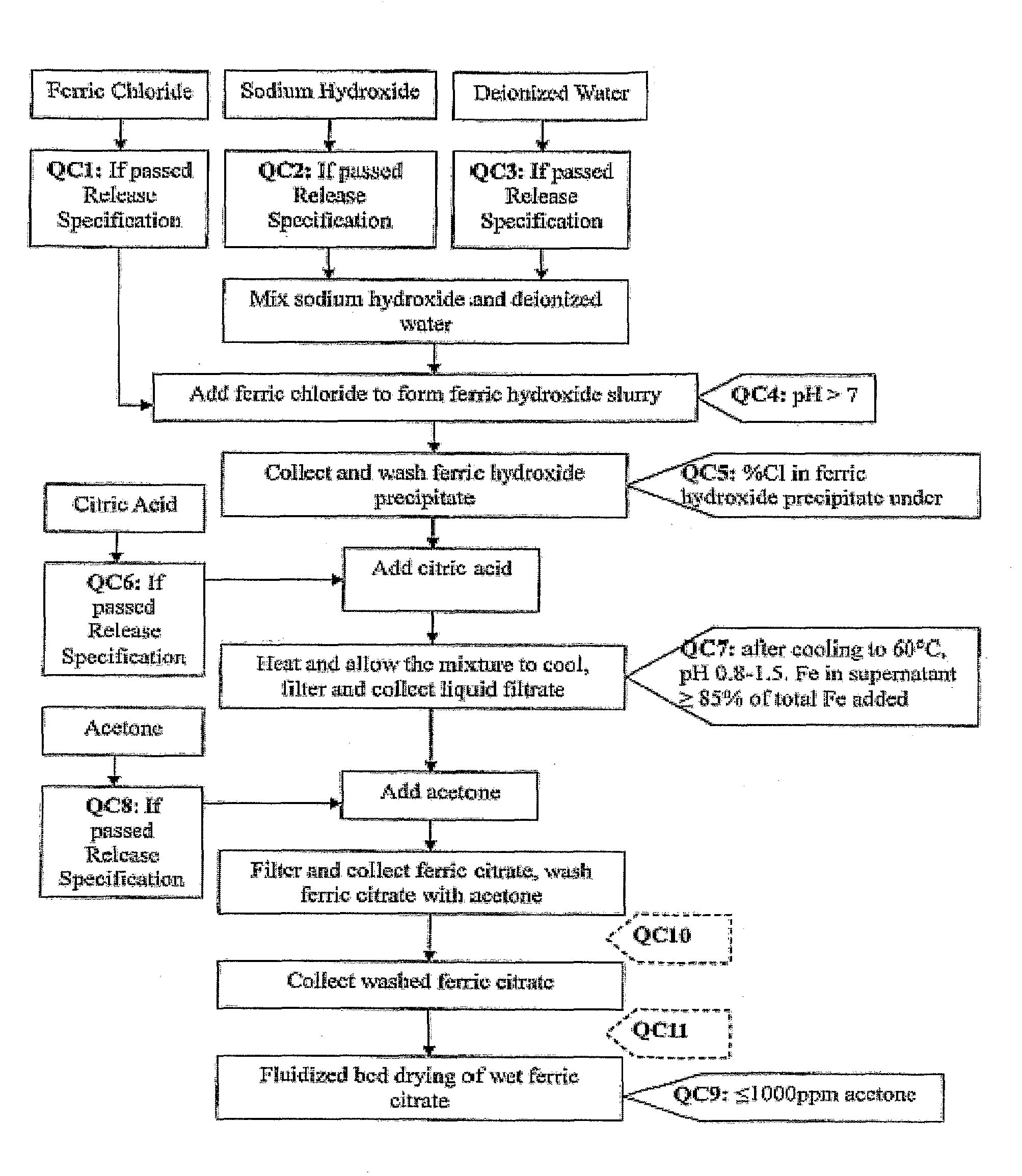
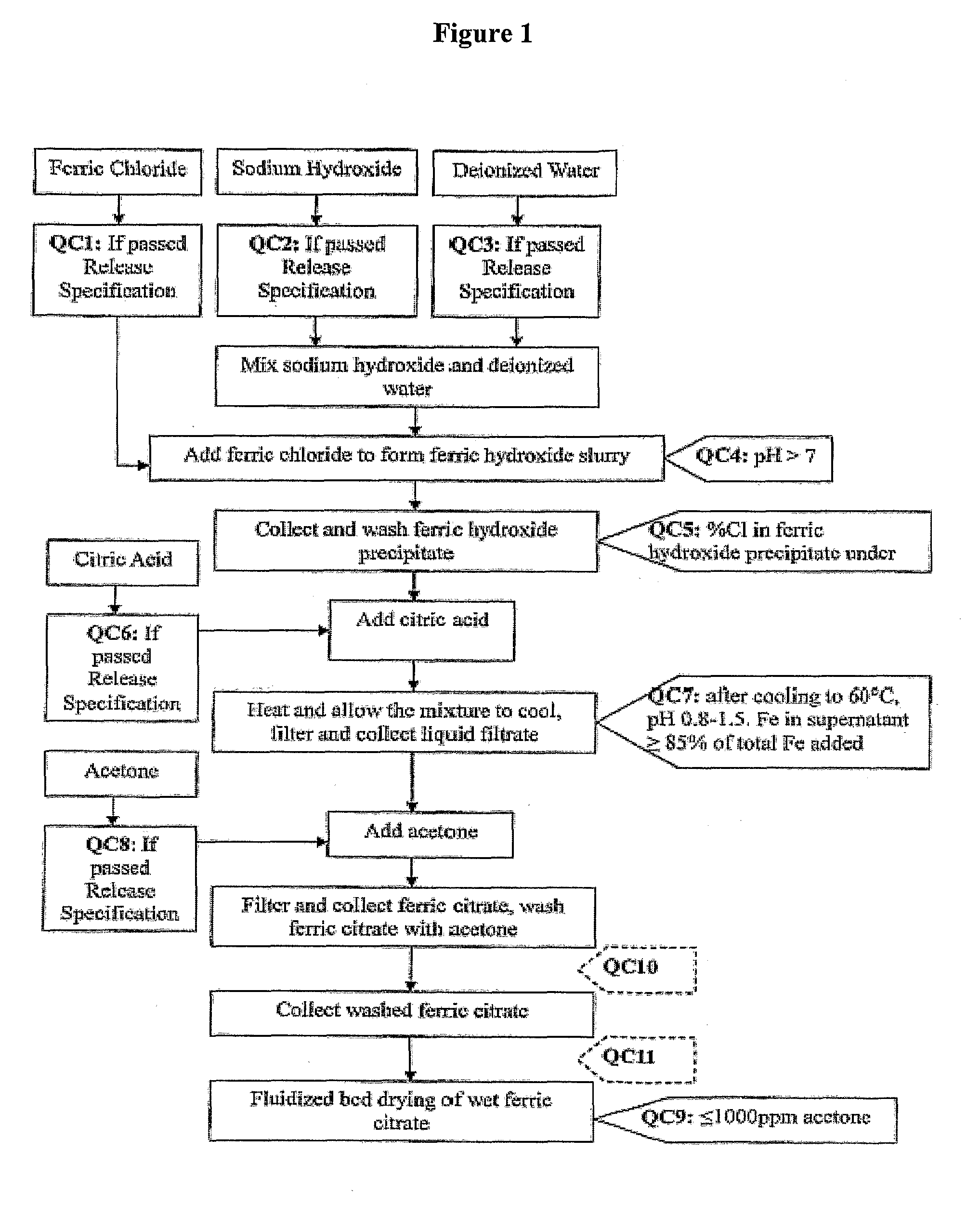
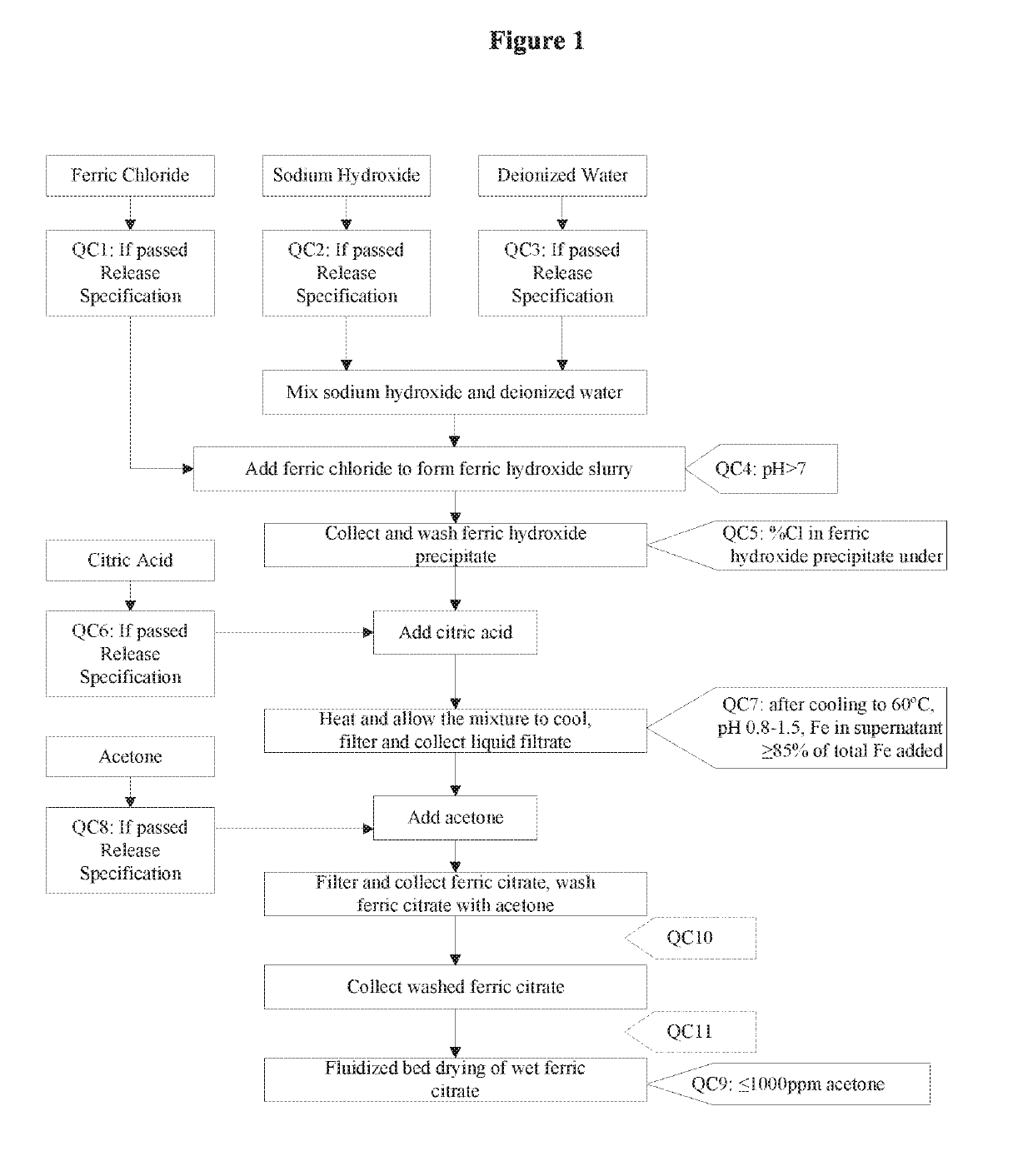
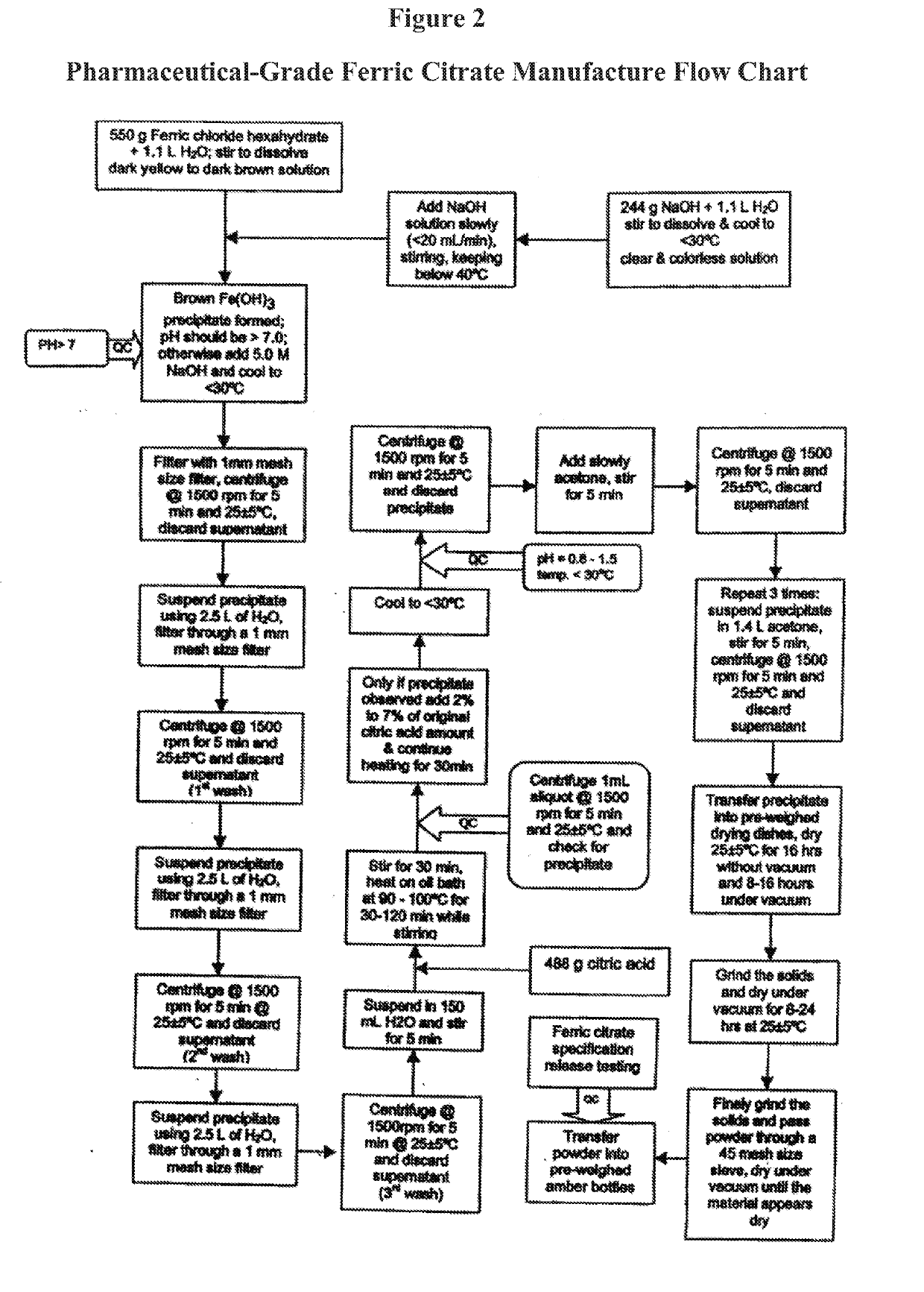
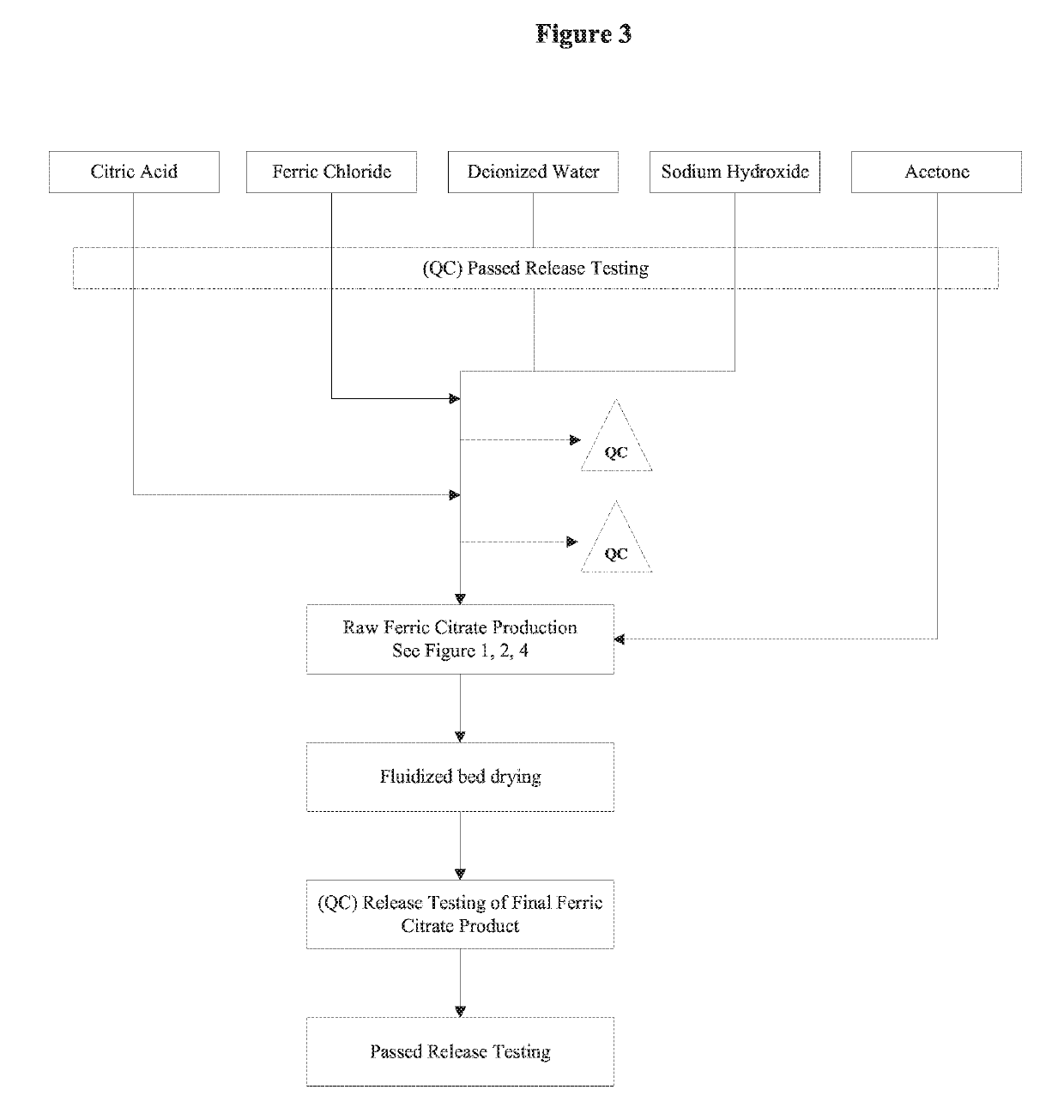
The present invention discloses a pharmaceutical-grade ferric organic compounds, including ferric citrate, which are soluble over a wider range of pH, and which have a large active surface area. A manufacturing and quality control process for making a pharmaceutical-grade ferric citrate that consistently complies with the established Manufacture Release Specification is also disclosed. The pharmaceutical-grade ferric organic compounds are suitable for treating disorders characterized by elevated serum phosphate levels.
Owner:PANION & BF BIOTECH INC
Ferric organic compounds, uses thereof and methods of making same
The present invention discloses a pharmaceutical-grade ferric organic compounds, including ferric citrate, which are soluble over a wider range of pH, and which have a large active surface area. A manufacturing and quality control process for making a pharmaceutical -grade ferric citrate that consistently complies with the established Manufacture Release Specification is also disclosed. The pharmaceutical-grade ferric organic compounds are suitable for treating disorders characterized by elevated serum phosphate levels.
Owner:GLOBOASIA
Methods and compositions for the treatment of diseases or conditions associated with increased C-reactive protein, interleukin-6, or interferon-gamma levels
InactiveUS20080003213A1Reduce IL- levelLower Level RequirementsBiocidePeptide/protein ingredientsInterleukin 6Adenosine
The invention features methods and compositions for reducing the serum C-reactive protein (CRP), IL-6, and / or IFN-γ levels in a patient in need thereof, and for treating diseases and conditions associated with an increased serum CRP, IL-6, and / or IFN-γ levels. The invention also features methods and compositions for treating a patient diagnosed with, or at risk of developing, periodontal disease by administering a corticosteroid or an analog thereof and / or a tetra-substituted pyrimidopyrimidine or an adenosine analog upregulator.
Owner:COMBINATORX
METHODS FOR REDUCING LIPOPROTEIN(a) LEVELS BY ADMINISTERING AN INHIBITOR OF PROPROTEIN CONVERTASE SUBTILISIN KEXIN-9 (PCSK9)
Owner:REGENERON PHARM INC
A kind of Lactobacillus reuteri and its application
ActiveCN107523526BImprove the level ofImprove oral glucose toleranceMilk preparationNervous disorderLactobacillus reuteriIrritable bowel syndrome
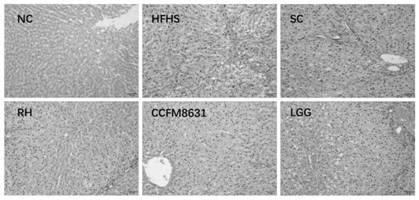
The invention relates to the technical field of microorganisms, and discloses a lactobacillus reuteri and its application. The preservation number of Lactobacillus reuteri CCFM8631 of the present invention is CGMCC No.14394, which can significantly improve the level of rat peripheral blood neurotransmitter 5-serotonin; restore the rat peripheral blood testosterone hormone level caused by a high-sugar and high-fat diet and the abnormal abundance of Blautia, Turicibacter, Oscillospira and Bifidobacterium in the intestinal flora; tolerance to simulated gastrointestinal fluid, rapid colonization in the intestinal tract, significantly improved the liver of rats with metabolic syndrome caused by high-sugar and high-fat diet , pathological damage of the duodenum and elevated levels of triglyceride and total cholesterol in serum, used to prevent, slow down or treat metabolic disorders such as metabolic syndrome, irritable bowel syndrome and anxiety and depression related to metabolic syndrome and other mental illness.
Owner:INFINITUS (CHINA) CO LTD
Diagnosis and treatment of human dormancy syndrome
InactiveUS7288257B2Good effectReduce formationPeptide/protein ingredientsMicrobiological testing/measurementElevated serumPopulation
New methods for diagnosis of human dormancy syndrome are provided. Human dormancy syndrome is characterized by elevated serum ratio of rT3 / fT3 compared to a population of normal subjects from which subjects suffering from fibromyalgia, chronic fatigue, obesity, dementias including Alzheimer's Disease and related dormancy conditions are excluded, and the presence of one or more findings related to reduced activity including torpor, chronic fatigue, insulin resistance, dementias, obesity and the like. Treatment of human dormancy syndrome is directed toward increasing fT3 levels or decreasing rT3 levels, or both, using pharmaceutical and / or behavioral methods. Other conditions that are associated with HDS can also be treated using T3 therapy, with or without specific psychological, behavioral or pharmaceutical therapies.
Owner:POWELL CO LTD
Pharmaceutical-grade ferric organic compounds, uses thereof and methods of making same
InactiveUS20190269722A1Long-term administrationImprove scalabilityOrganic active ingredientsBiocideSerum phosphatePhosphate level
The present invention discloses pharmaceutical-grade ferric organic compounds, including ferric citrate, which are soluble over a wider range of pH, and which have a large active surface area. A manufacturing and quality control process for making a pharmaceutical-grade ferric citrate that consistently complies with the established Manufacture Release Specification is also disclosed. The pharmaceutical-grade ferric organic compounds are suitable for treating disorders characterized by elevated serum phosphate levels.
Owner:PANION & BF BIOTECH INC
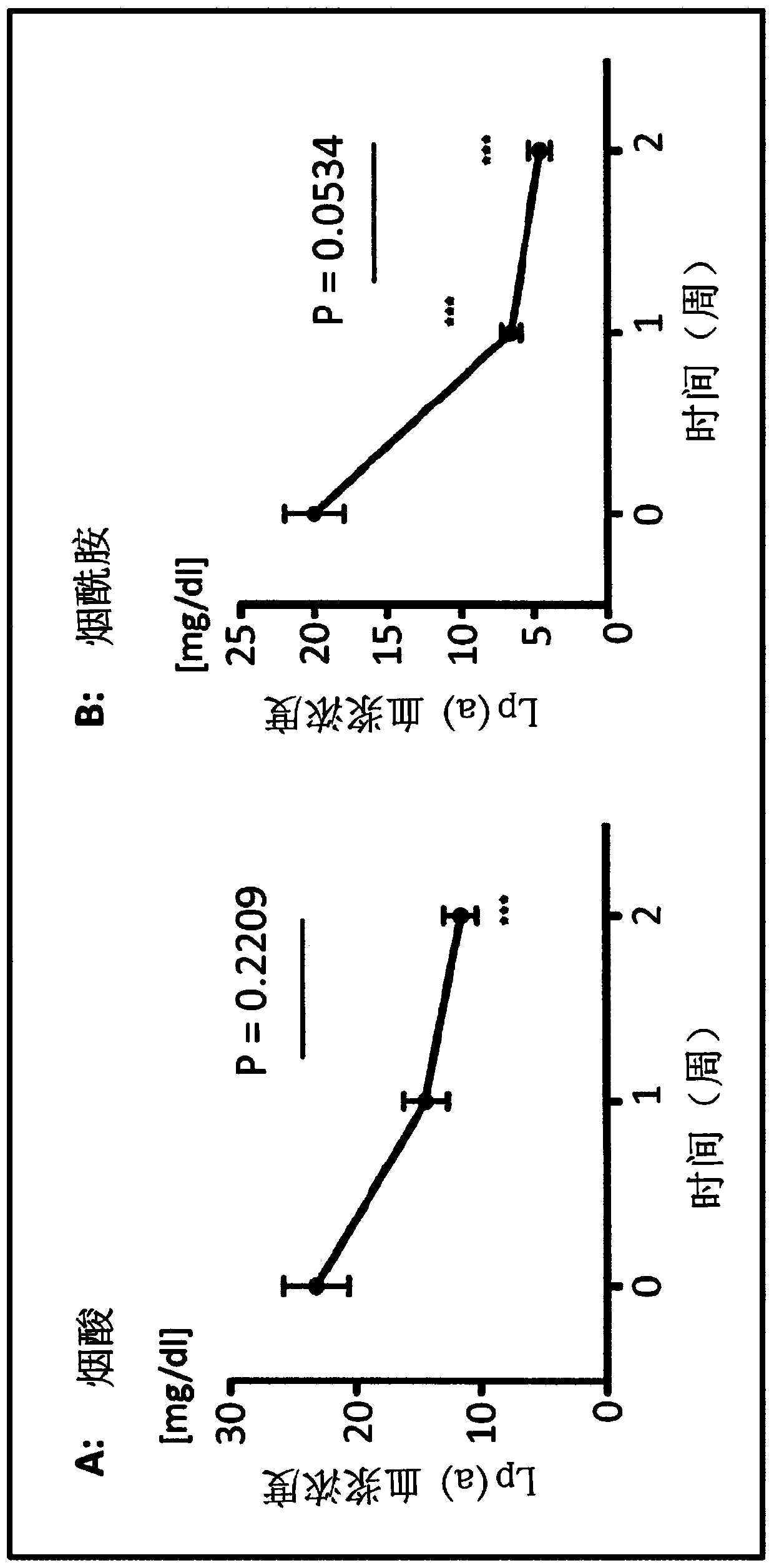
Nicotinamide for treating dyslipidemia
InactiveCN110869019AAddress dyslipidemiaDyslipidemia prevention and/or treatmentOrganic active ingredientsHeavy metal active ingredientsDyslipidemiaRenal Failures
The present invention relates to a pharmaceutical preparation comprising nicotinamide for use in a method of preventing and / or treating dyslipidemia, particularly resulting from renal failure, and a pharmaceutical preparation comprising nicotinamide for use in a method of preventing and / or treating elevated serum phosphate levels (hyperphosphatemia) and dyslipidemia, both particularly resulting from renal failure.
Owner:SALMON PHARMA
Method for assessing the risk of cardiovascular disease
InactiveUS20050064497A1Enhanced protection against cardiovascular diseaseImprove protectionMicrobiological testing/measurementBiological testingCholesterolBlood plasma
The present invention is directed to a method identifying a condition in an individual in which elevation of serum or plasma HDL concentration or HDL cholesterol concentration provides enhanced protection against cardiovascular disease, the method comprising the step of testing the individual for a disorder that detrimentally affects the protective effect of HDL, whereby absence of such a disorder is an indication of enhanced protection against cardiovascular disease when said individual exhibits elevated serum or plasma HDL or HDL cholesterol concentration.
Owner:JURILAB
Method for treating a disease, disorder or adverse effect caused by an elevated serum concentration of an ugtiai substrate
The present invention is directed to a method for inducing UGT1A1 isoform expression for treatment of a disease, disorder or adverse effect caused by an elevated serum concentration of an UGT1A1 substrate comprising the step of administering to a subject an effective amount of ritonavir. In particular, the present invention is directed to a method of treating unconjugated hyperbilirubinemia by UGT1A1 induction comprising the step of administering to a subject an effective amount of ritonavir.
Owner:ABBVIE INC
Kenrel oil composite extracted from plant kernel and its extraction process and medicinal preparation
InactiveCN1204238CEnhance immune functionElevated serum proteinUnknown materialsCapsule deliveryIntestinal structureTotal protein
The invention relates to a walnut kernel oil composition extracted from walnut kernels and a pharmaceutical preparation thereof. In every 100ml of the emulsion of the present invention, the walnut kernel oil composition for injection is 5-30g; the emulsifier is 0.5-3.0g; the isotonic agent is 0.5-3.5g; the remainder is water for injection. In addition to supplementing the body's nutritional energy, anti-fatigue, hypoxia resistance, significantly improving human immune function, and increasing serum total protein, it also has a certain inhibitory effect on animal transplanted tumor Lewis lung cancer and mouse liver cancer HAC. It also has the functions of nourishing the kidney, warming the lung and moistening the intestines of the raw material drug, and is cheap. It is another energy-type emulsion with multiple functions.
Owner:李大鹏
New application of supporting and brain-promoting medicinal composition to treatment of elevated serum lipid
InactiveCN102526453AHas therapeutic effectAnthropod material medical ingredientsMetabolism disorderLipid formationOral medication
The invention discloses application of a supporting and brain-promoting medicinal composition to the preparation of medicaments for treating elevated serum lipid. Compared with a control group, the invention has the advantage that supporting and brain-promoting capsules for oral administration can regulate serum lipid.
Owner:HENAN LINGRUI PHARMA
Mitochondrial modulation to improve metabolic syndrome during aging
PendingUS20220281902A1High blood levelGood blood pressureOrganic active ingredientsMetabolism disorderPancreatic hormoneIncrease blood pressure
Compounds, compositions and methods are provided for mitochondrial modulation. The subject mitochondrial modulator compounds generally include a head group linked to a charged moiety. In certain cases, the head group is a heterocyclic or a heteroaryl group. Aspects of the subject methods include a method of modulating mitochondria. Aspects of the subject methods include treating a subject having a metabolic syndrome-related disease or a symptom thereof by administering to the subject a therapeutically effective amount of a subject compound. In certain cases, the disease is selected from hyperlipidemia, type 2 diabetes, fatty liver disease, obesity, cardiovascular disease and stroke. In certain cases, the symptom is selected from abdominal obesity, insulin resistance, hyperinsulinemia, high levels of blood fats, increased blood pressure, and elevated serum lipids.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com