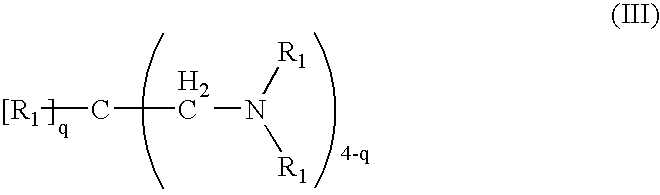
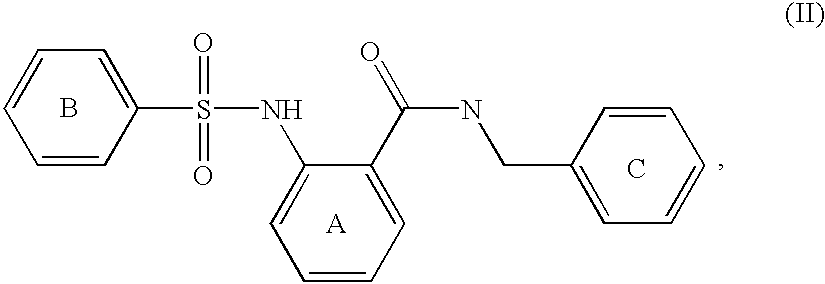
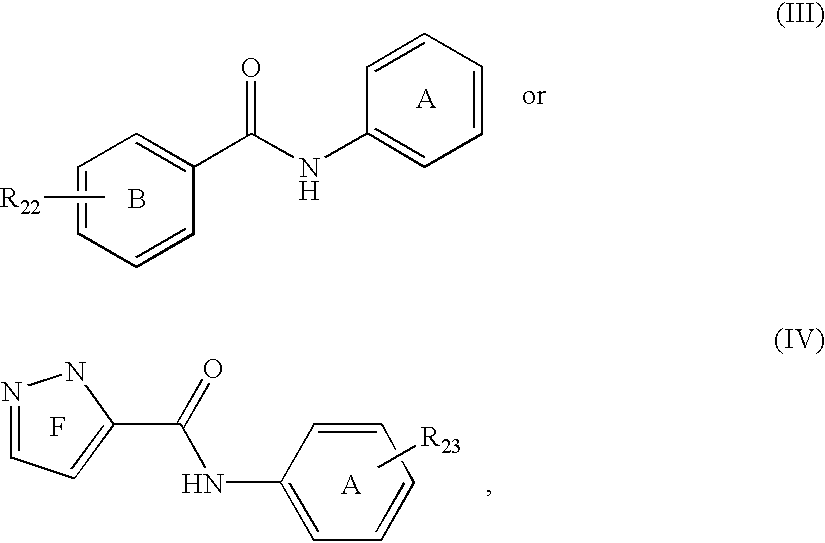
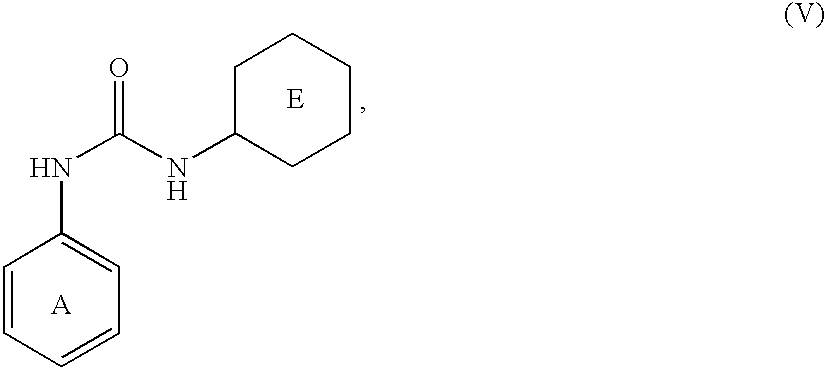
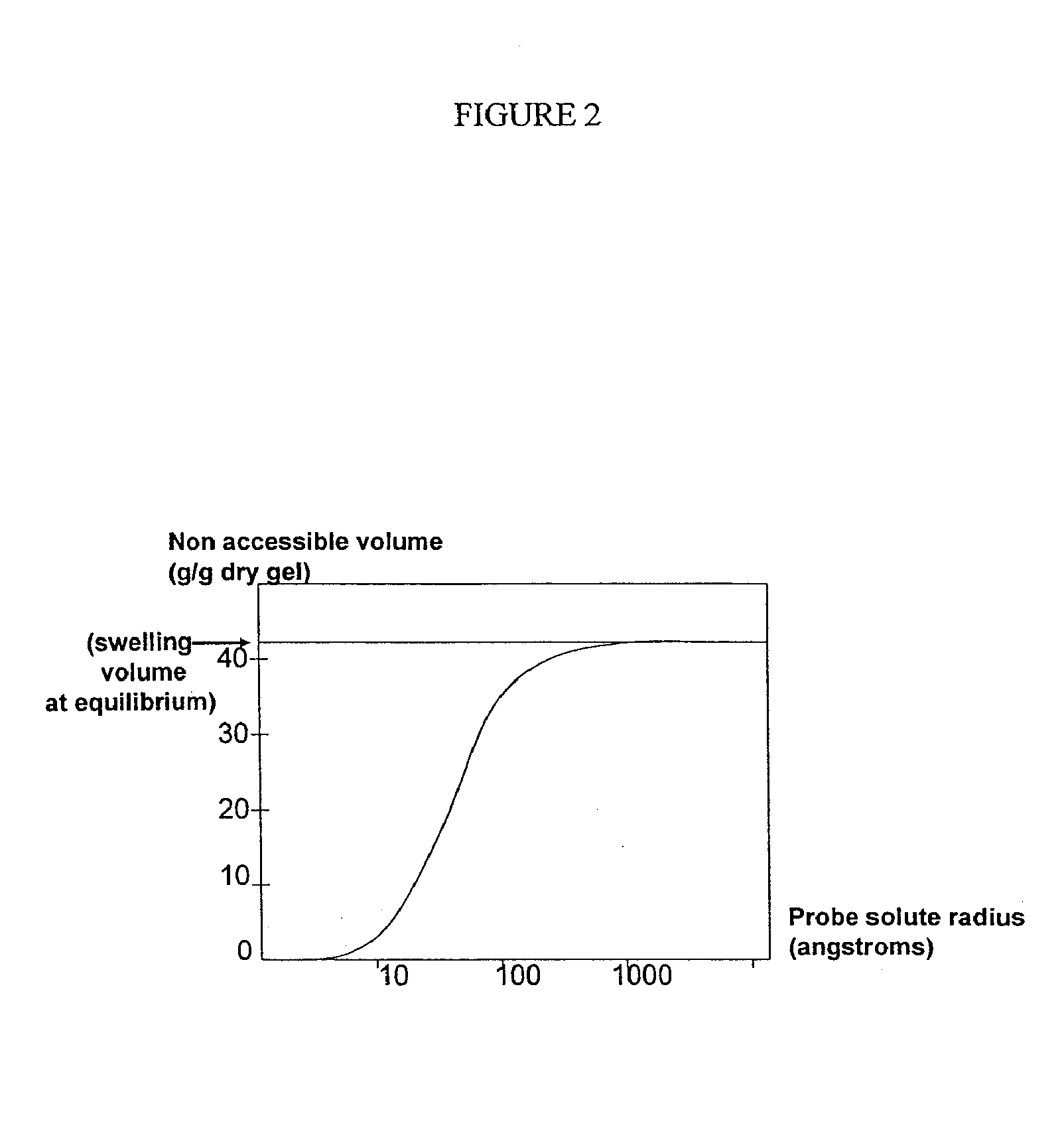
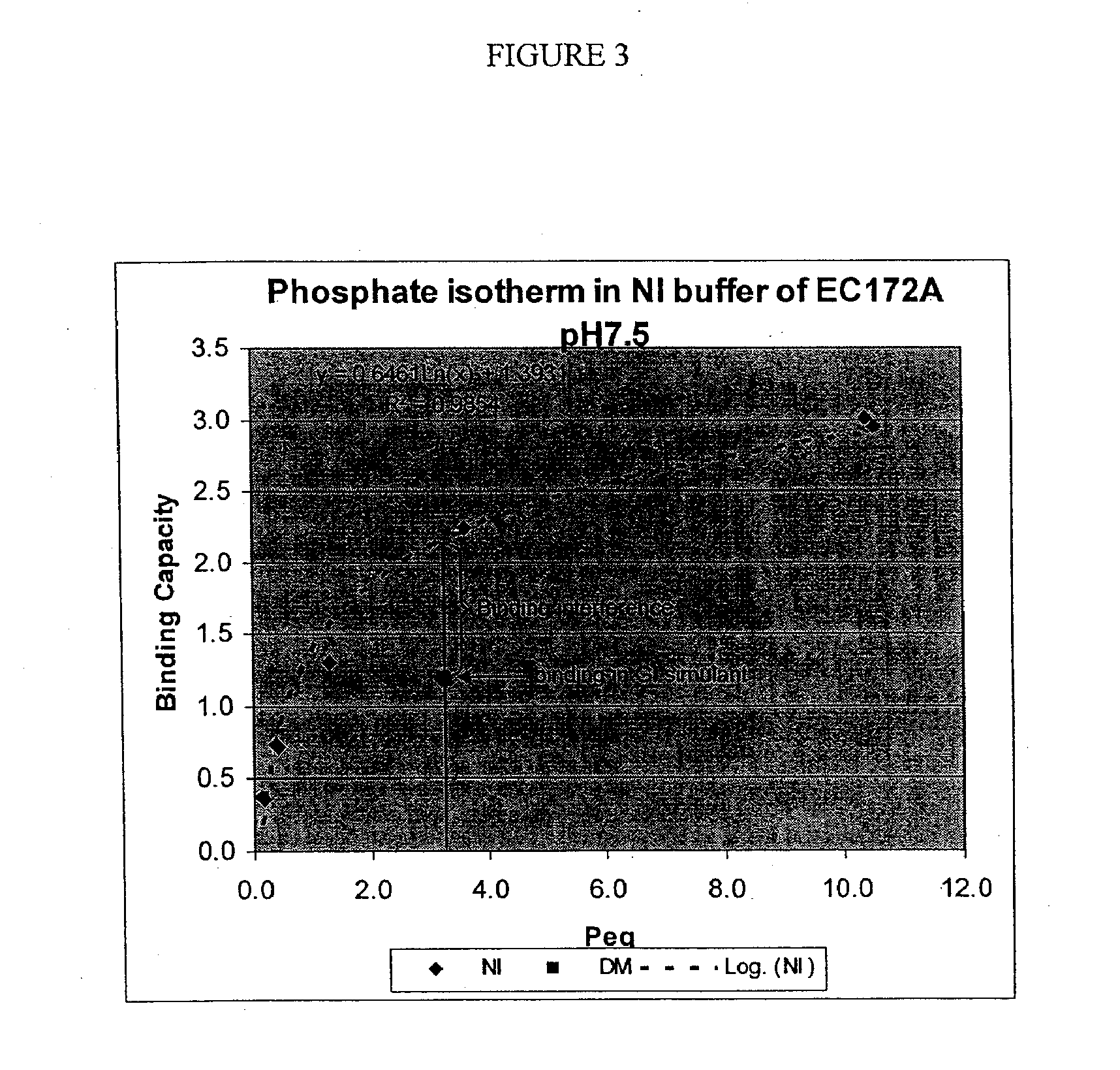
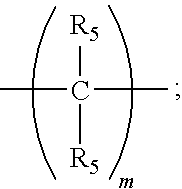
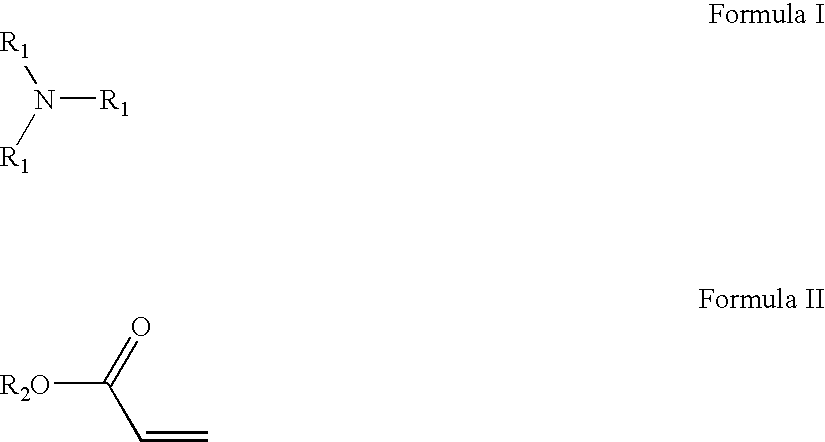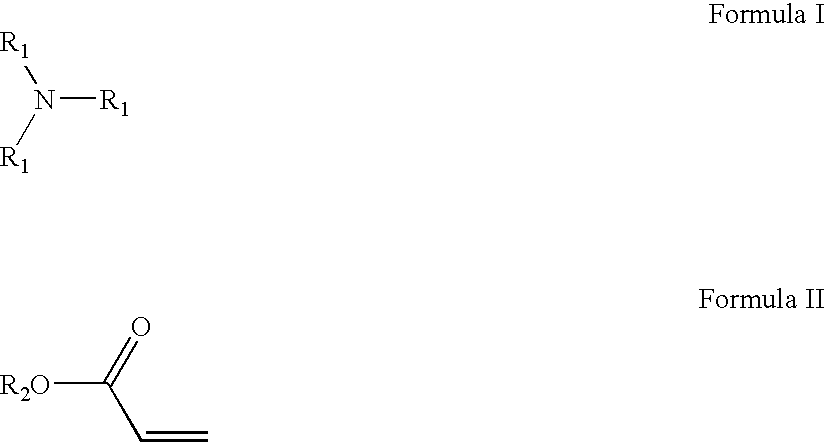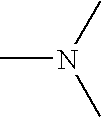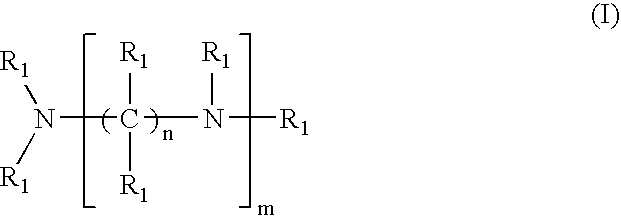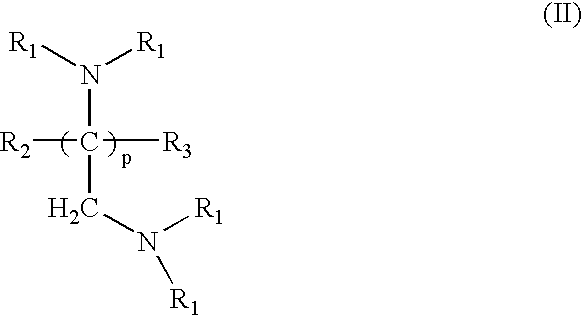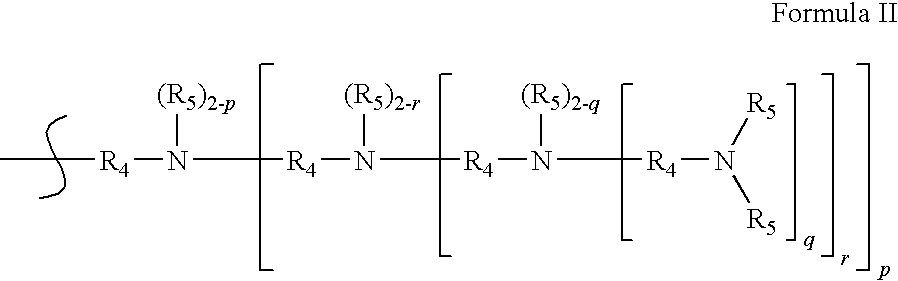Patents
Literature
140 results about "Hyperphosphatemia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hyperphosphatemia is an electrolyte disorder in which there is an elevated level of phosphate in the blood. Most people have no symptoms while others develop calcium deposits in the soft tissue. Often there is also low calcium levels which can result in muscle spasms.
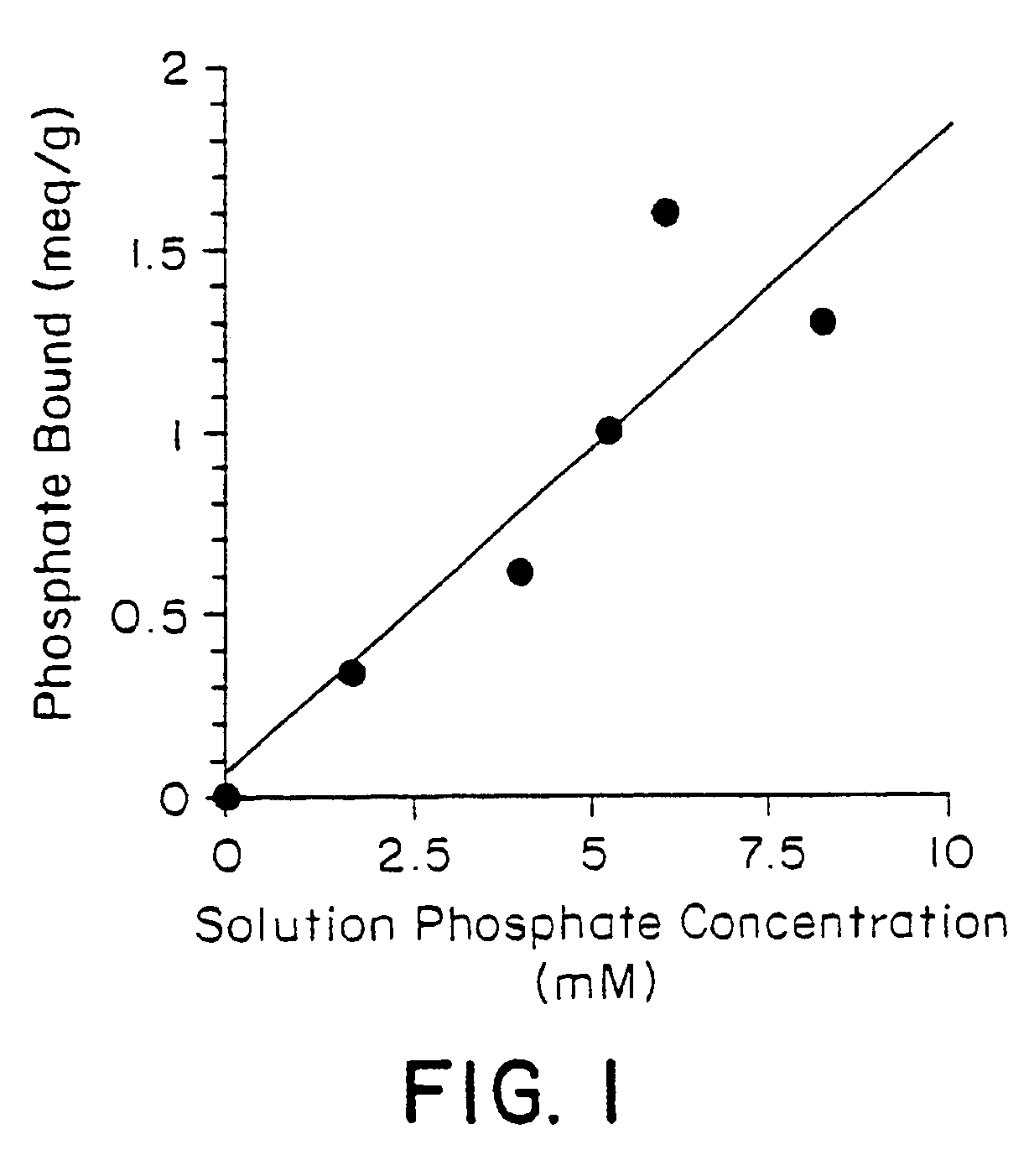
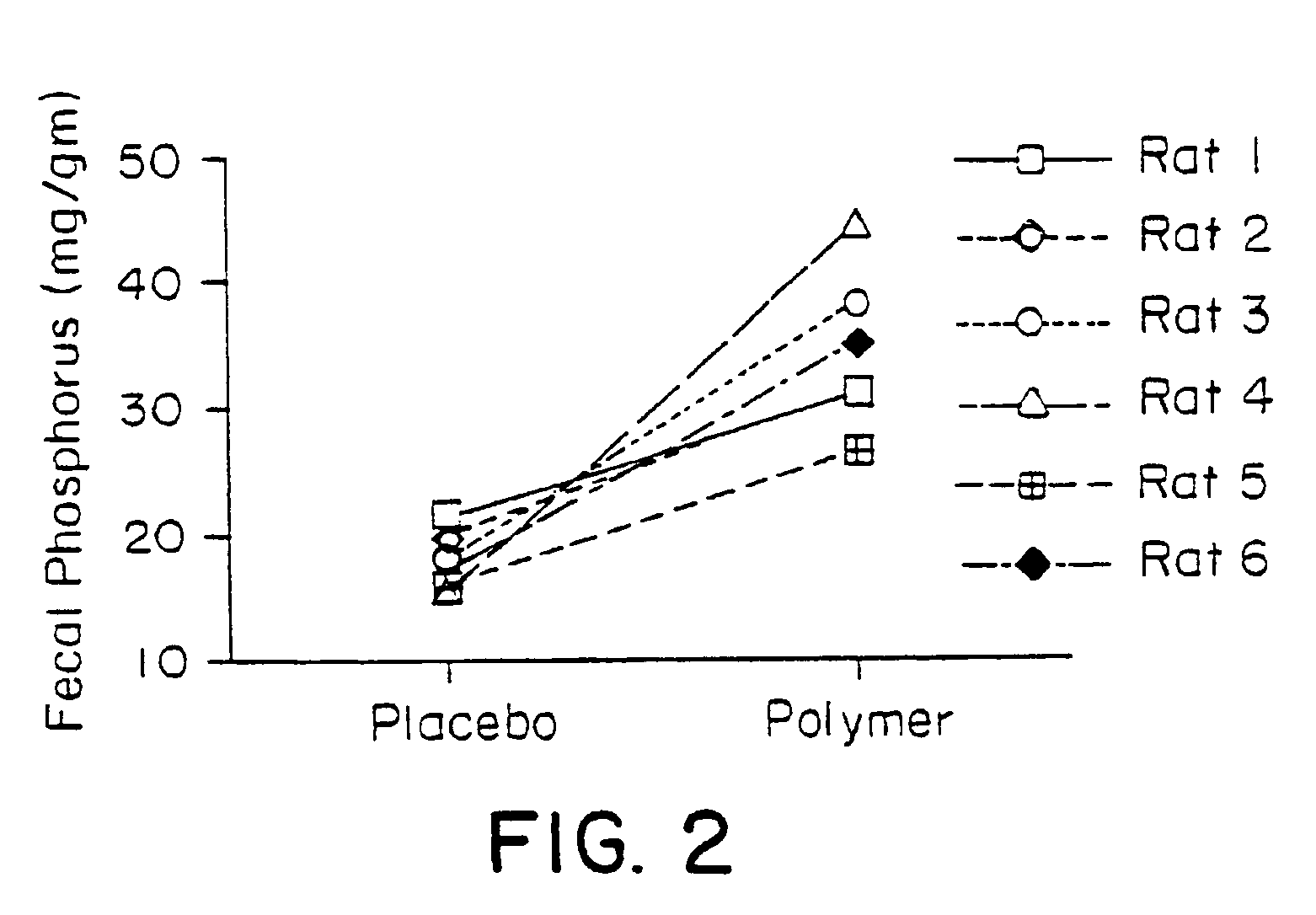
Method of making phosphate-binding polymers for oral administration
InactiveUS6858203B2Low serum levelsPromote absorptionPowder deliveryMetabolism disorderOral medicationBuccal administration
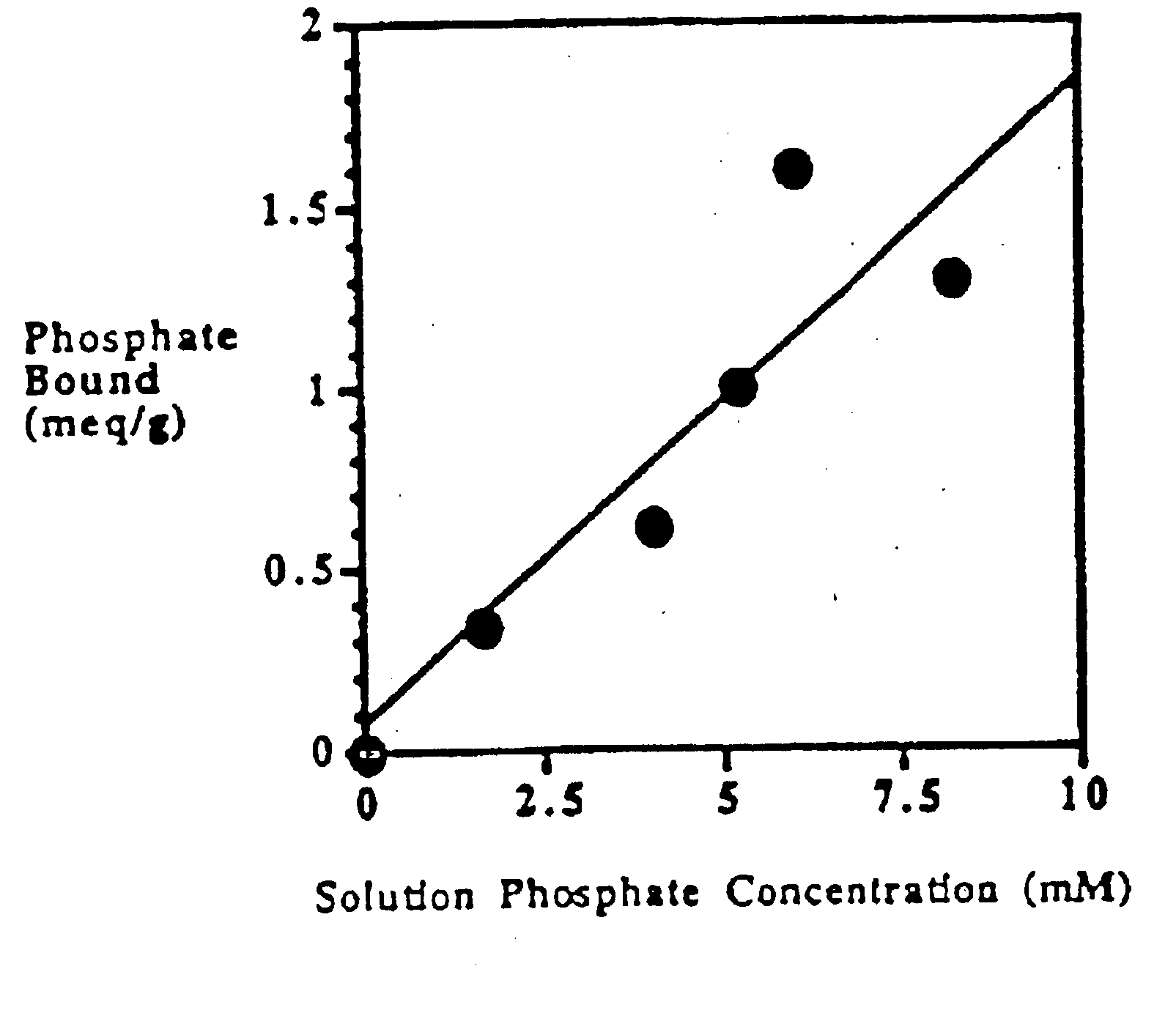
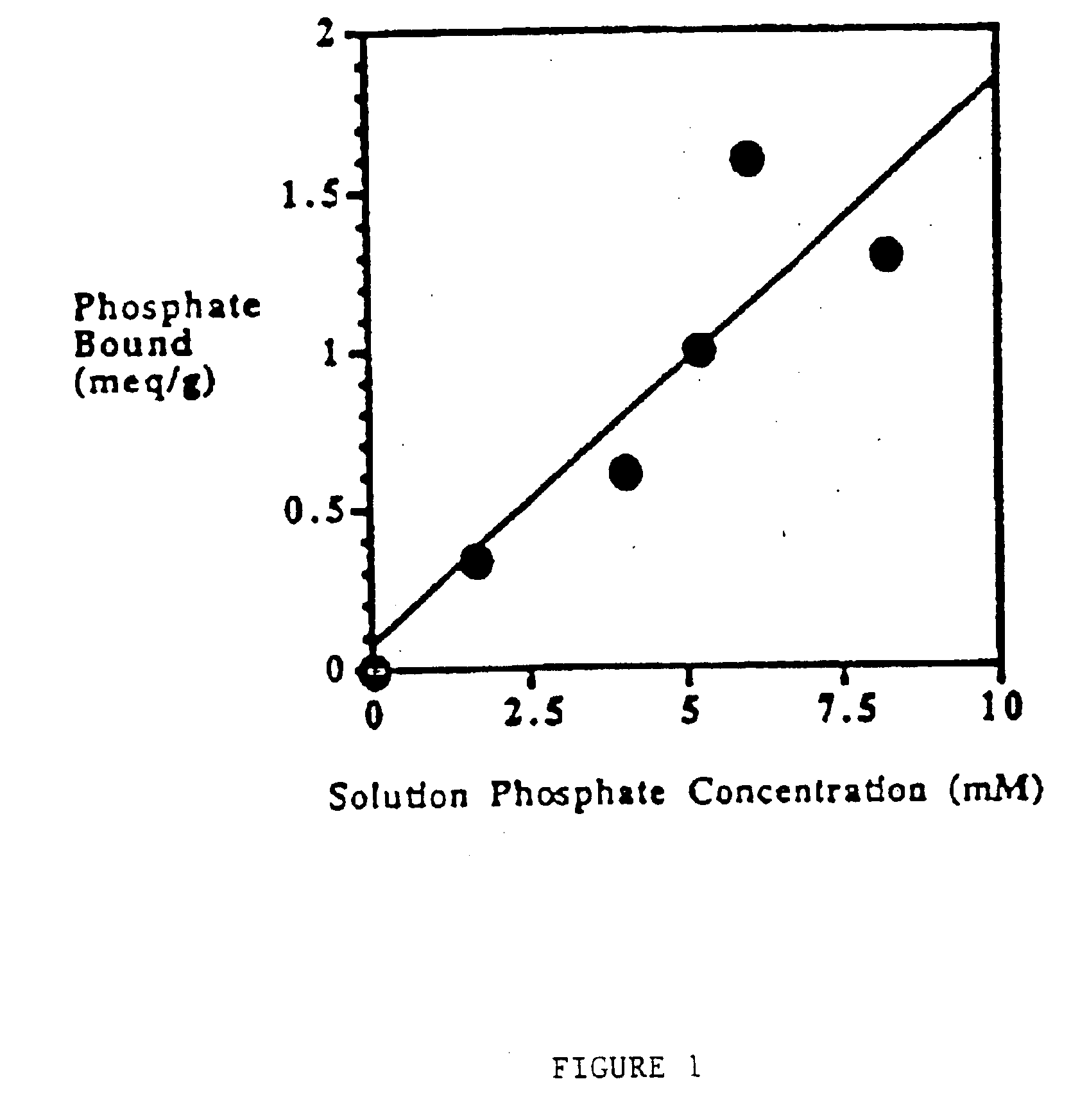
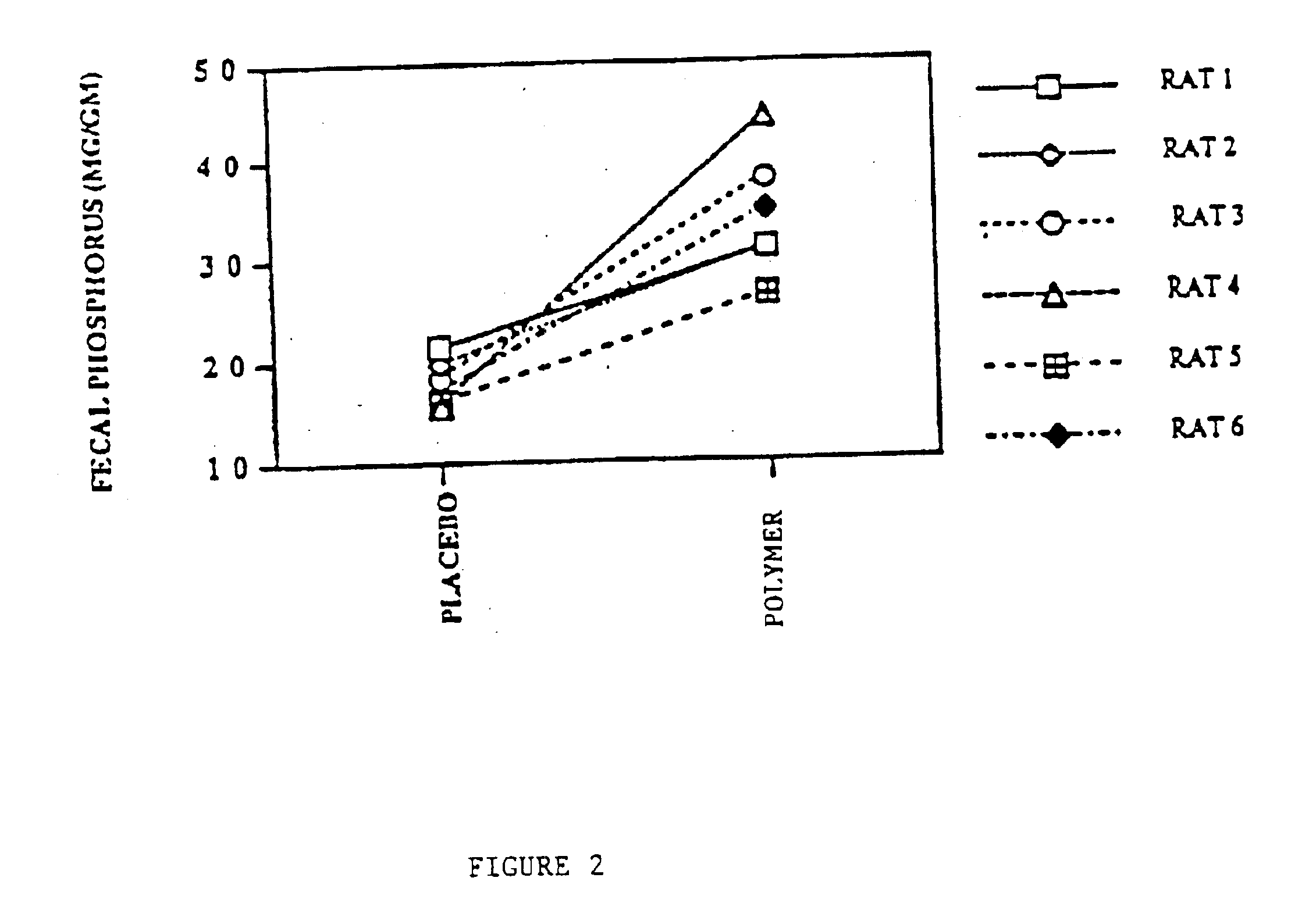
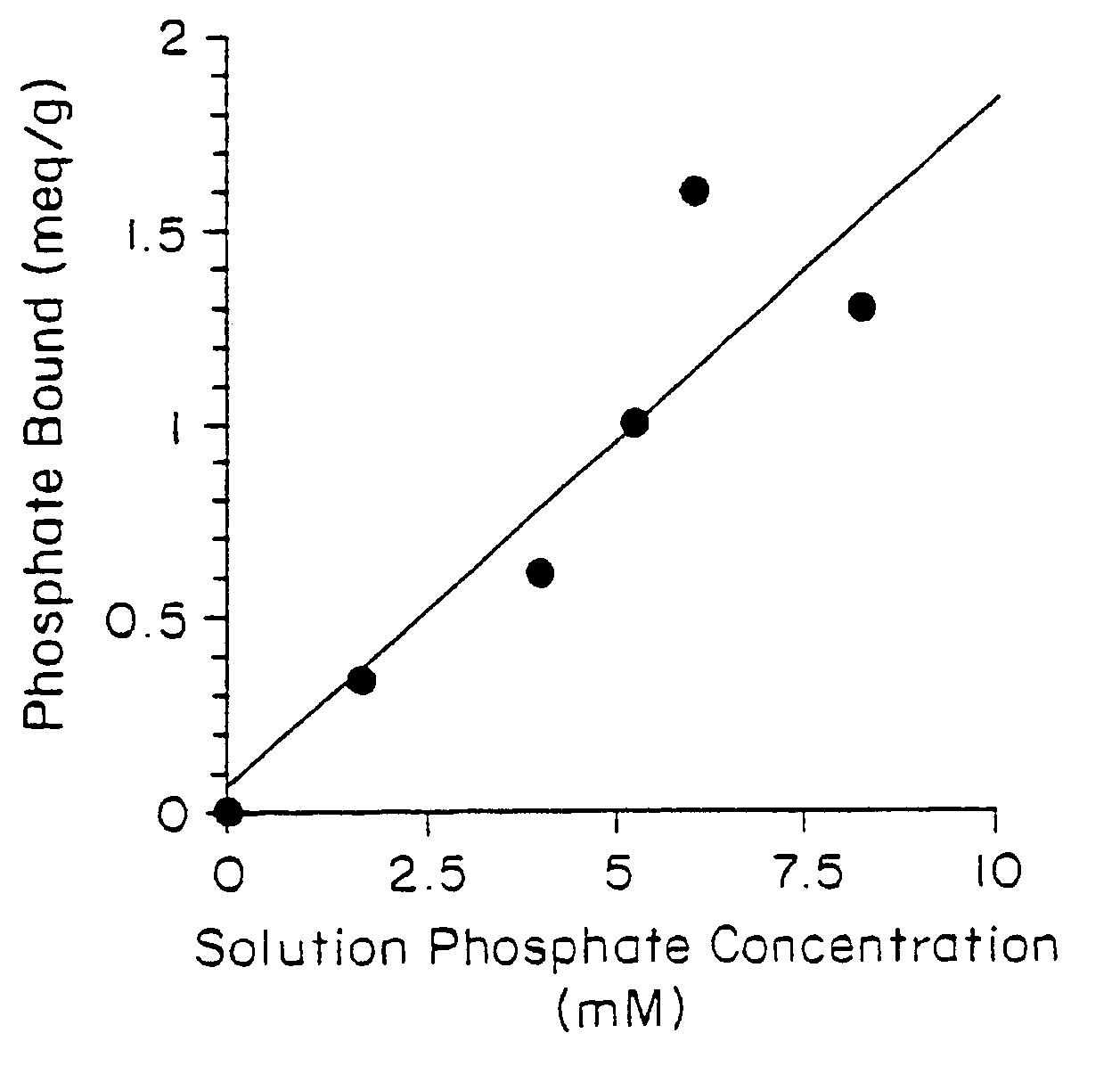
Phosphate-binding polymers are provided for removing phosphate from the gastrointestinal tract. The polymers are orally administered, and are useful for the treatment of hyperphosphatemia.
Owner:GENZYME CORP
Phosphate-binding polymers for oral administration
InactiveUS7014846B2Low serum levelsPromote absorptionPowder deliveryMetabolism disorderOral medicationBuccal administration
Phosphate-binding polymers are provided for removing phosphate from the gastrointestinal tract. The polymers are orally administered, and are useful for the treatment of hyperphosphatemia.
Owner:GENZYME CORP
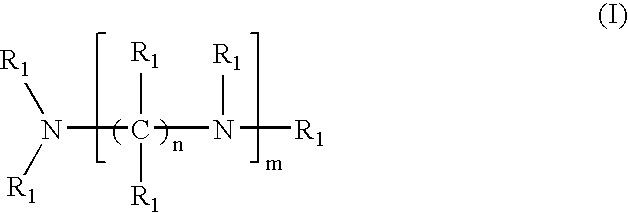
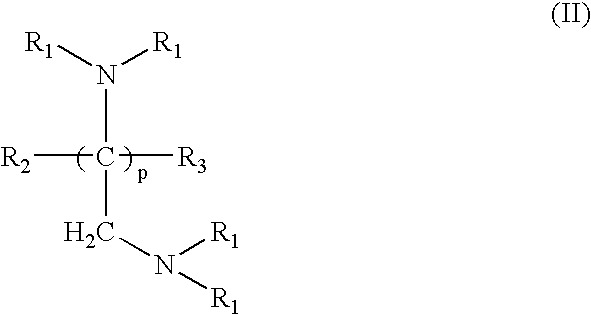
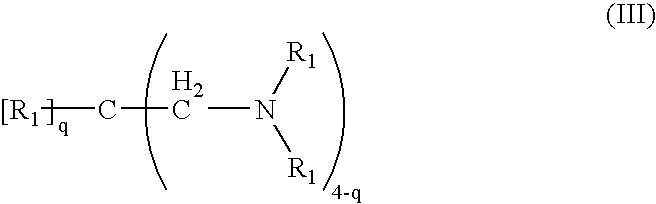
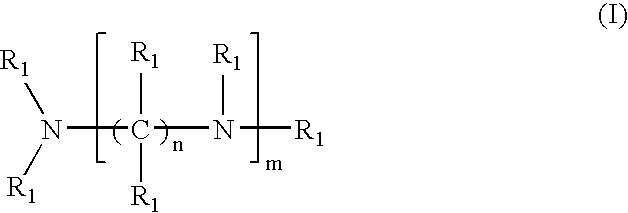
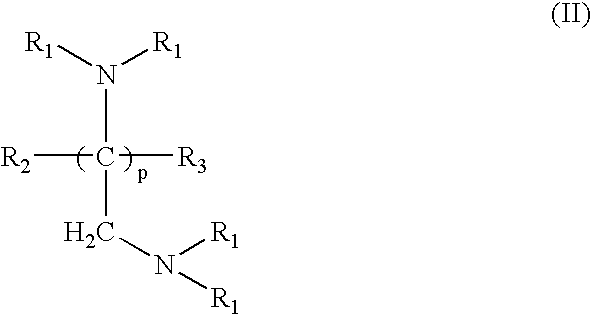
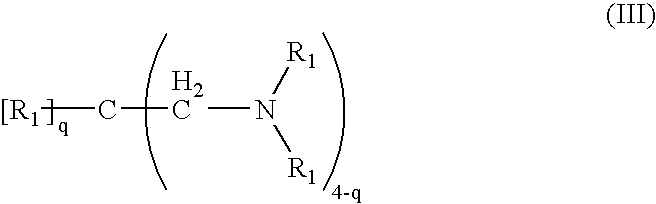
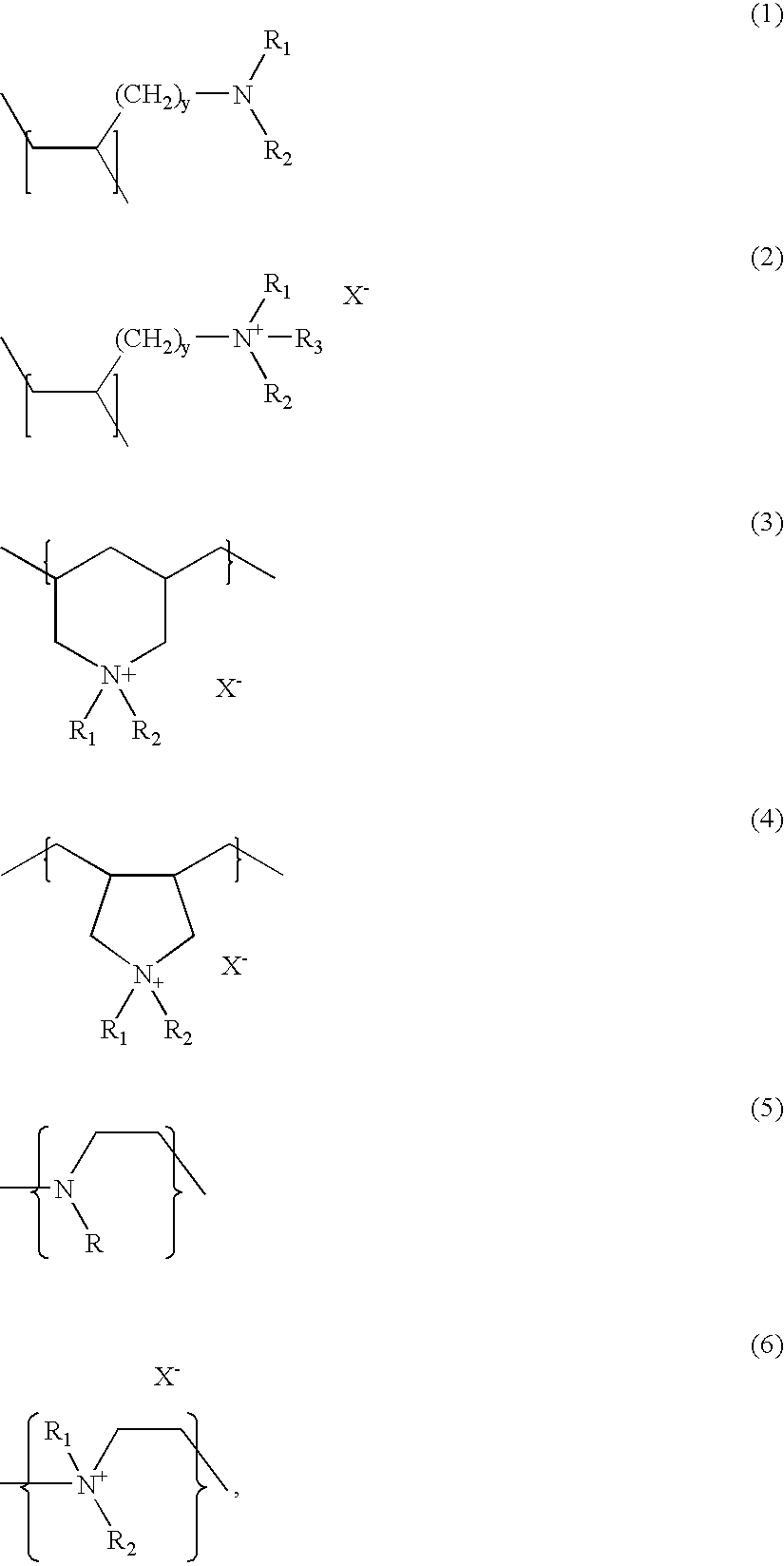
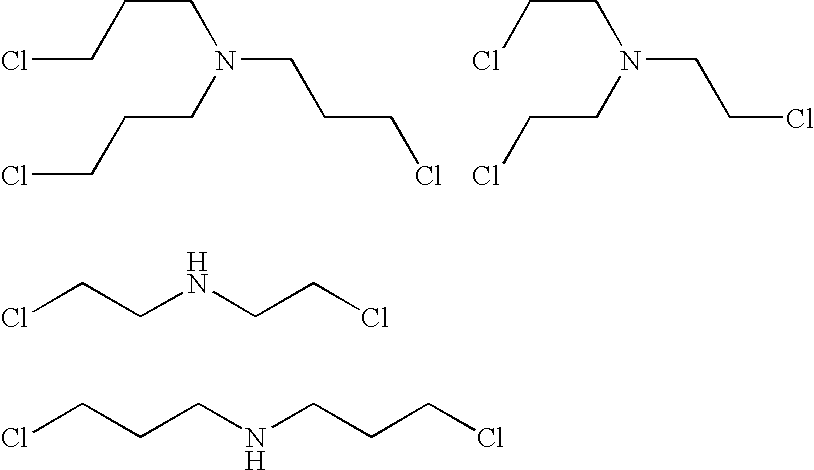
Crosslinked amine polymers
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides polymeric and pharmaceutical compositions comprising crosslinked amine polymers. Methods of use of the polymeric and pharmaceutical compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of renal diseases and hyperphosphatemia.
Owner:ILYPSA
Phosphate transport inhibitors
Disclosed are compounds which have been identified as inhibitors of phosphate transport. Many of the compounds are represented by Structural Formula (I):Ar1—W—X—Y—Ar2;or a pharmaceutically acceptable salt thereof. Ar1 and Ar2 are independently a substituted or unsubstituted aryl group or an optionally substituted five membered or six membered non-aromatic heterocylic group fused to an optionally substituted monocylic aryl group. W and Y are independently a covalent bond or a C1–C3 substituted or unsubstituted alkylene group. X is a heteroatom-containing functional group, an aromatic heterocyclic group, substituted aromatic heterocyclic group, non-aromatic heterocyclic group, substituted non-aromatic heterocyclic group, an olefin group or a substituted olefin group. Also disclosed are methods of treating a subject with a disease associated with hyperphosphatemia, as well as a disease mediated by phosphate-transport function. The methods comprise the step of administering an effective amount of the one of the compounds described above.
Owner:GENZYME CORP
Crosslinked amine polymers
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides polymeric and pharmaceutical compositions comprising crosslinked amine polymers. Methods of use of the polymeric and pharmaceutical compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of renal diseases and hyperphosphatemia.
Owner:ILYPSA
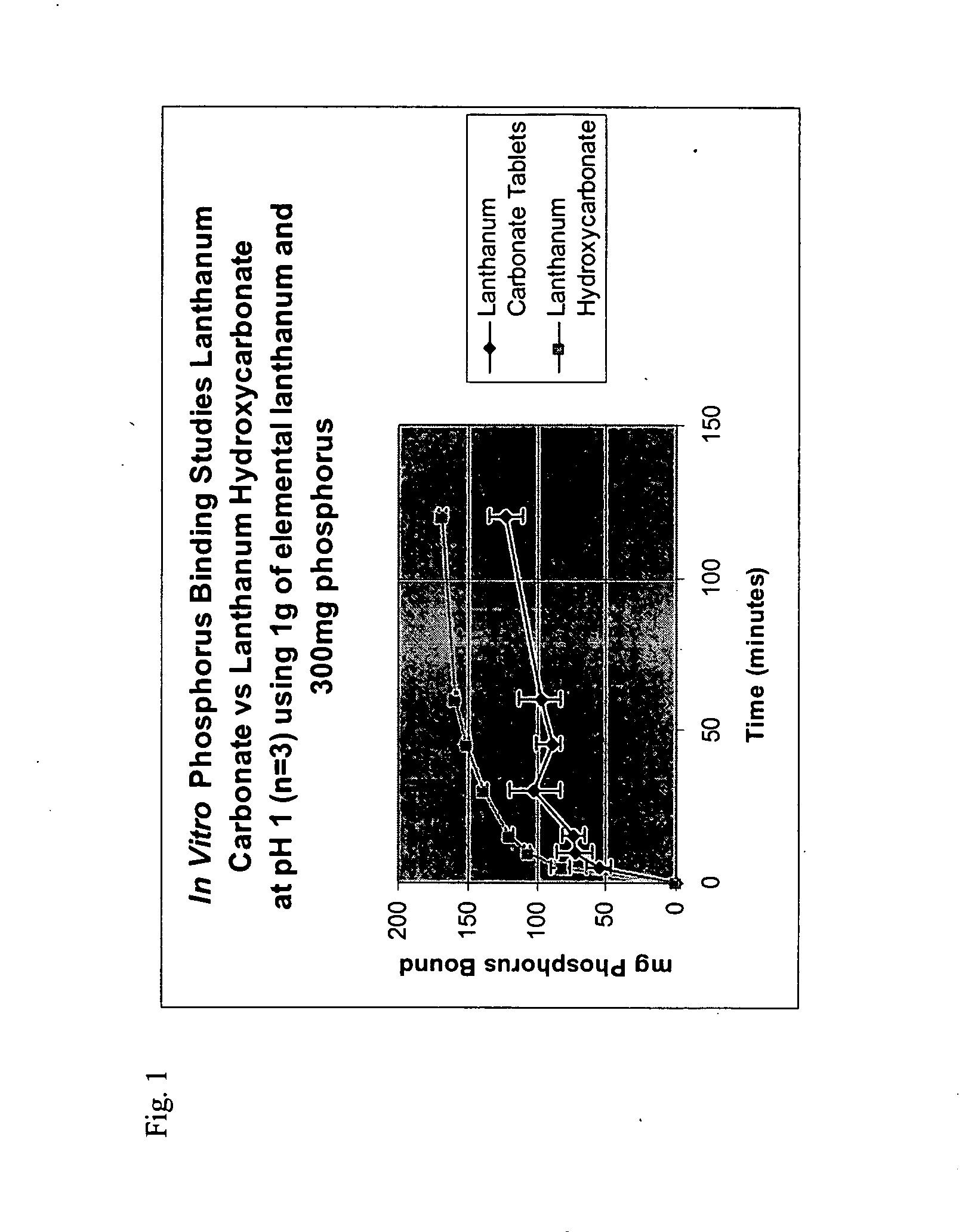
Method of treating hyperphosphataemia using lanthanum hydroxycarbonate
InactiveUS20060153932A1Preventing and reducing and abolishing incidenceMaintain standardHeavy metal active ingredientsBiocideCalcificationHyperparathyroidism
This disclosure relates to the treatment of subjects at risk for chronic kidney disease (CKD), having stage one to five CKD, having hyperphosphataemia, susceptible to or suffering from soft tissue calcification associated with CKD, or susceptible to or suffering from hyperparathyroidism, by orally administering a pharmaceutical composition containing a therapeutically effective amount of lanthanum hydroxycarbonate.
Owner:SHIRE PHARMA INC +1
Crosslinked amine polymers
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides polymeric and pharmaceutical compositions comprising crosslinked amine polymers. Methods of use of the polymeric and pharmaceutical compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of renal diseases and hyperphosphatemia.
Owner:ILYPSA
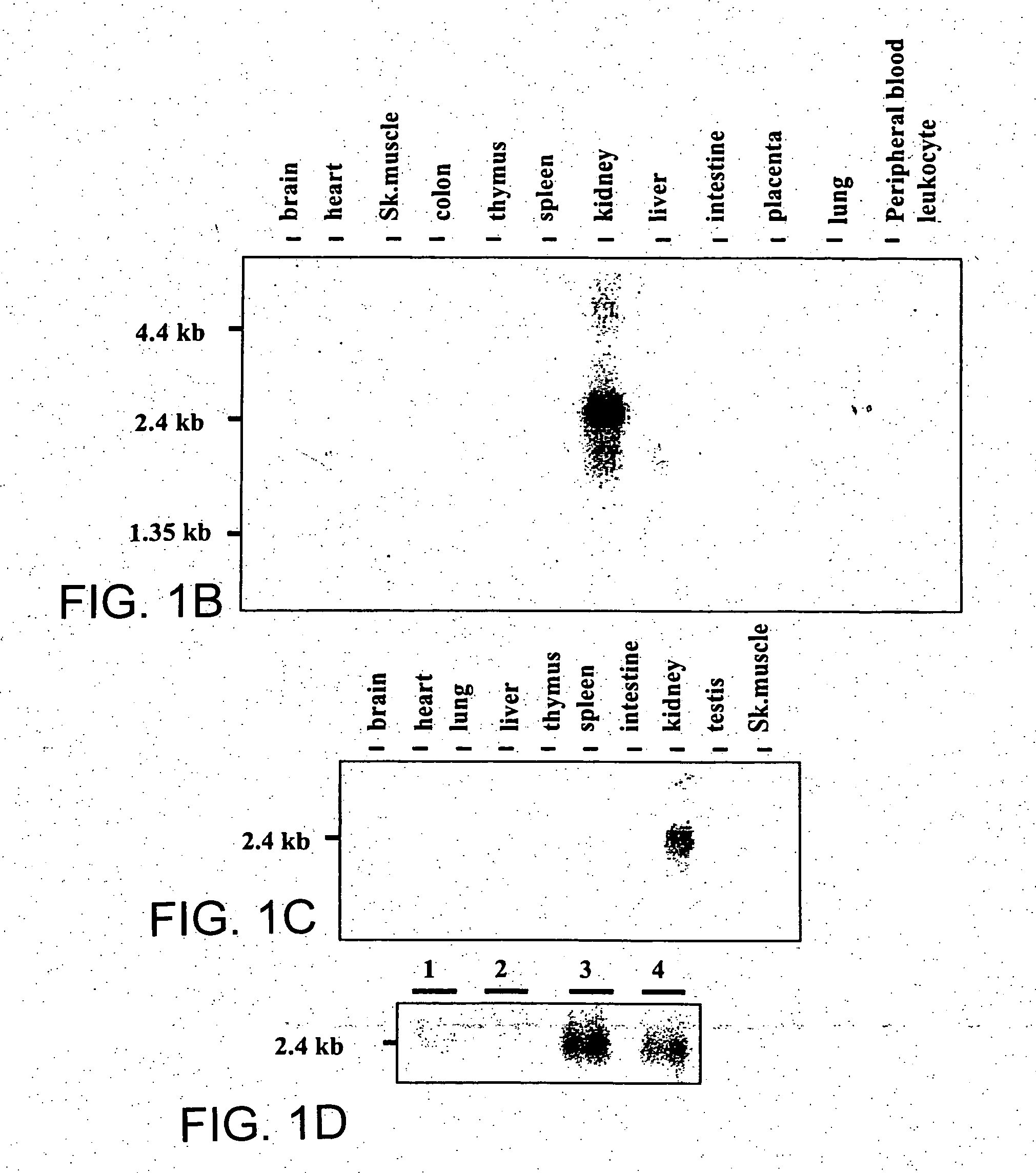
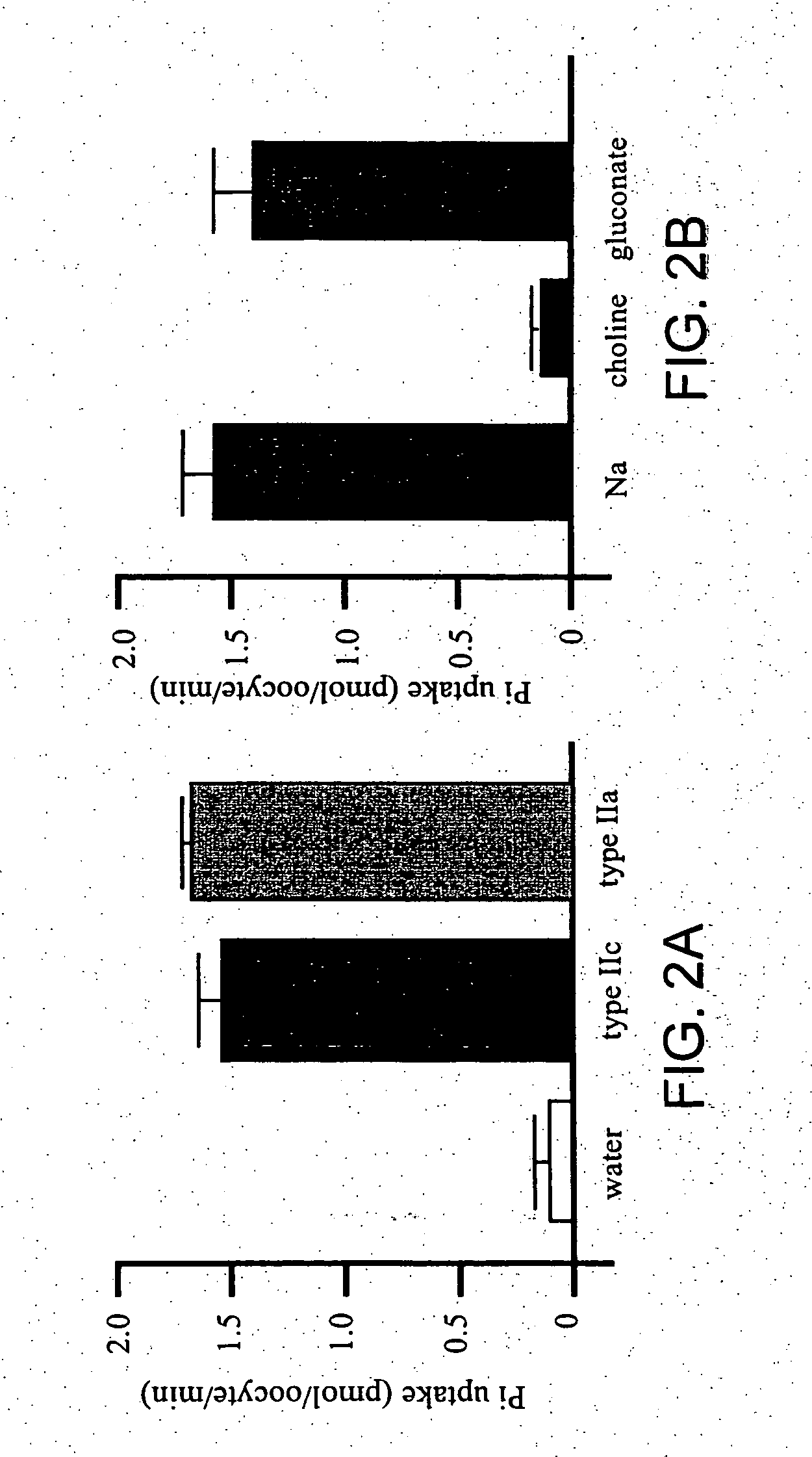
Novel type II Na/Pi cotransporters and type II Na/Pi cotransporter expression regulatory factors
InactiveUS20050004348A1Inhibit expressionInhibition of reabsorptionAntibacterial agentsOrganic active ingredientsAdult stageHypophosphatemia
The present invention provides novel type IIc Na / Pi cotransporters. These cotransporters are important Pi transporters that are highly expressed during the growth period from the weaning stage to the adult stage. Furthermore, the present invention provides FGF23 and mutants thereof as factors that regulate the expression of type II Na / Pi cotransporters. FGF23 suppresses Pi reabsorption through suppression of type II Na / Pi cotransporter expression in kidneys. Therefore, FGF23 can be used as a target substance for regulating Pi reabsorption in kidneys. The present invention provides important factors for the development of preventive and therapeutic agents for hyperphosphatemia or hypophosphatemia.
Owner:CHUGAI PHARMA CO LTD
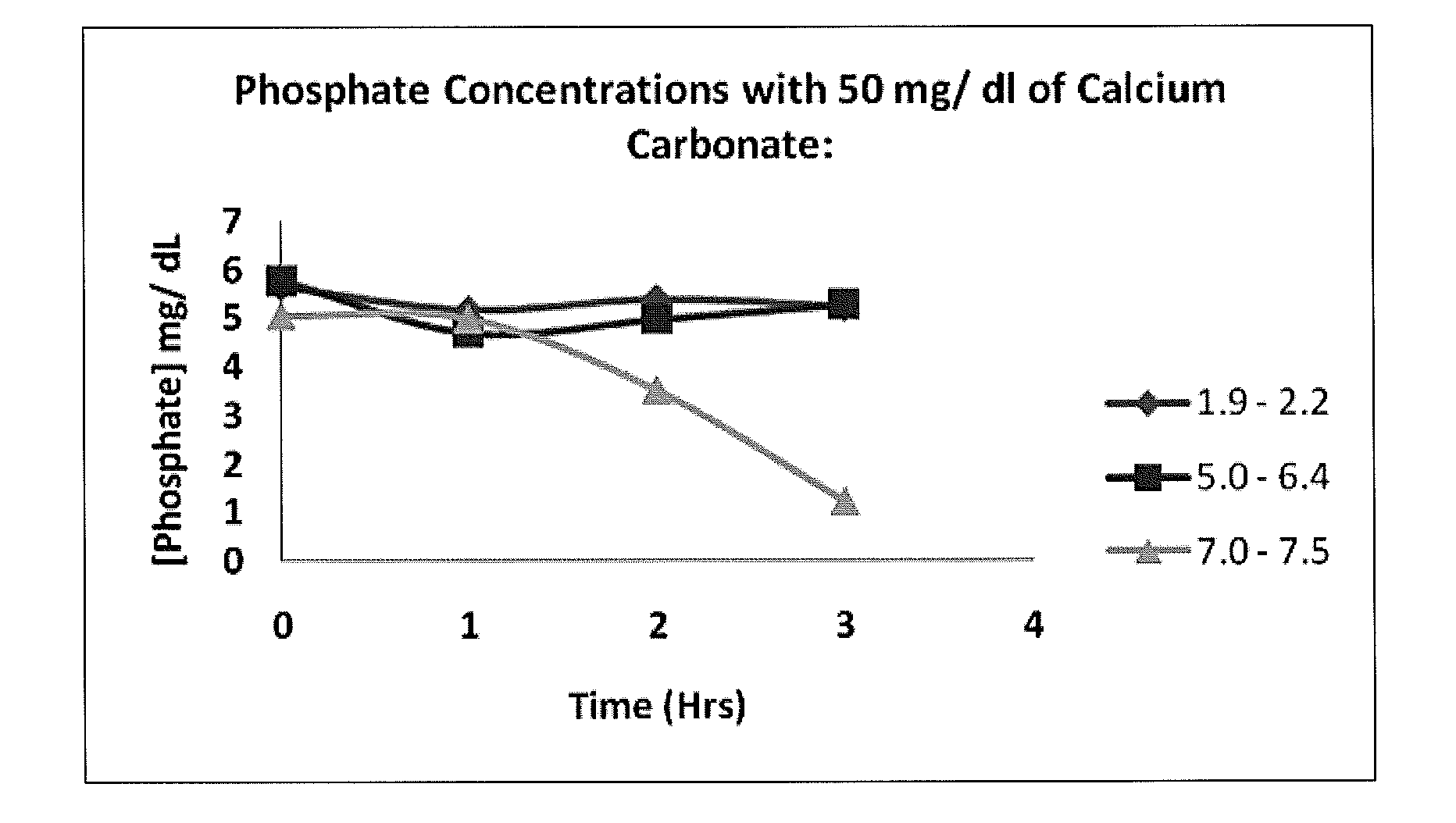
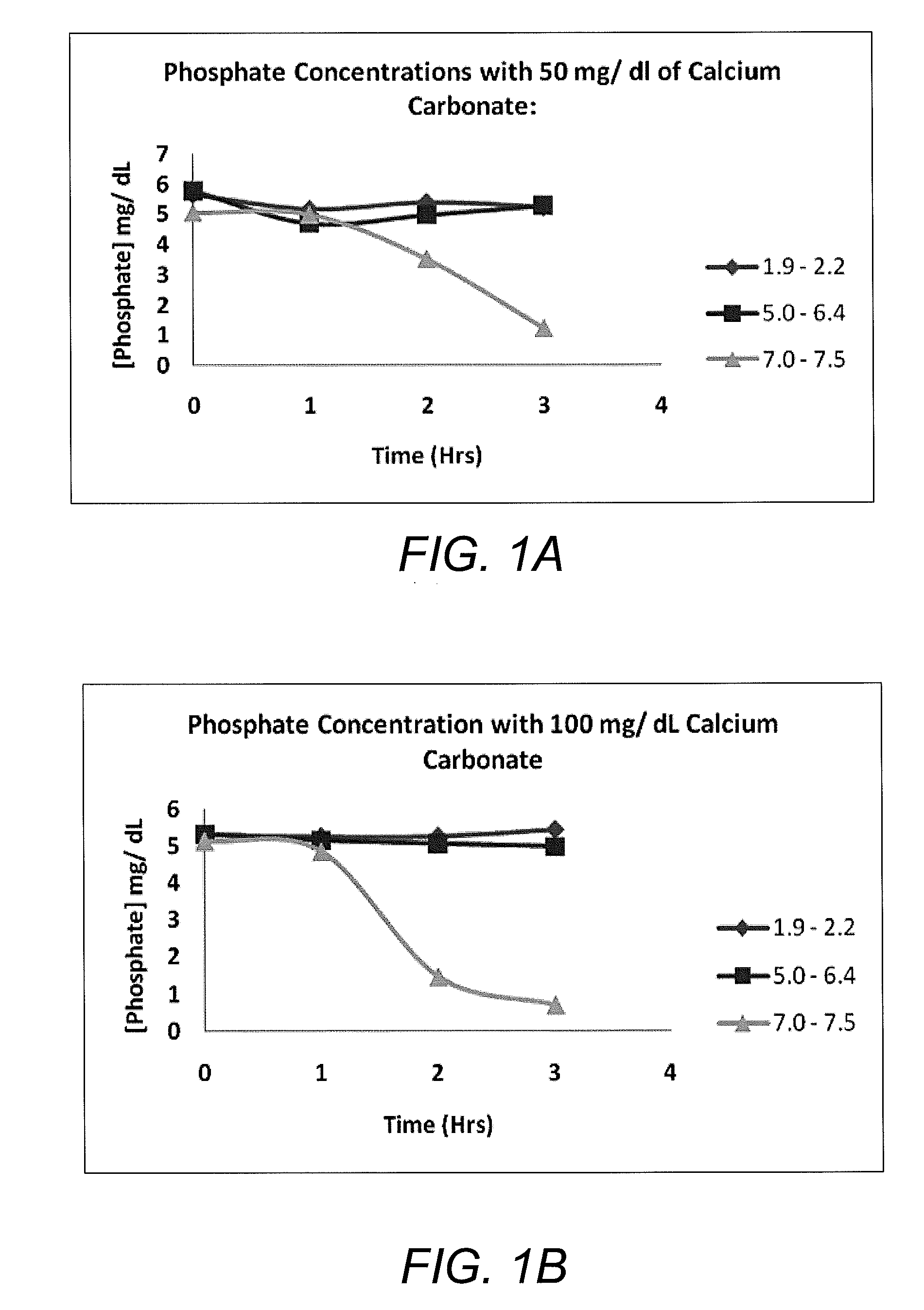
Calcium carbonate compositions for preventing or treating hyperphosphatemia
Compositions and methods for inhibiting gastrointestinal absorption of phosphate in a subject are provided. Such compositions are composed of enteric-coated, sustained-release calcium carbonate, which find application in the prevention or treatment of hyperphosphatemia.
Owner:KIBOW BIOTECH
Phosphate-binding polymers for oral administration
InactiveUS20060171916A1Low serum levelsPromote absorptionPowder deliveryMetabolism disorderOral medicationPolymer
Phosphate-binding polymers are provided for removing phosphate from the gastrointestinal tract. The polymers are orally administered, and are useful for the treatment of hyperphosphatemia.
Owner:GENZYME CORP
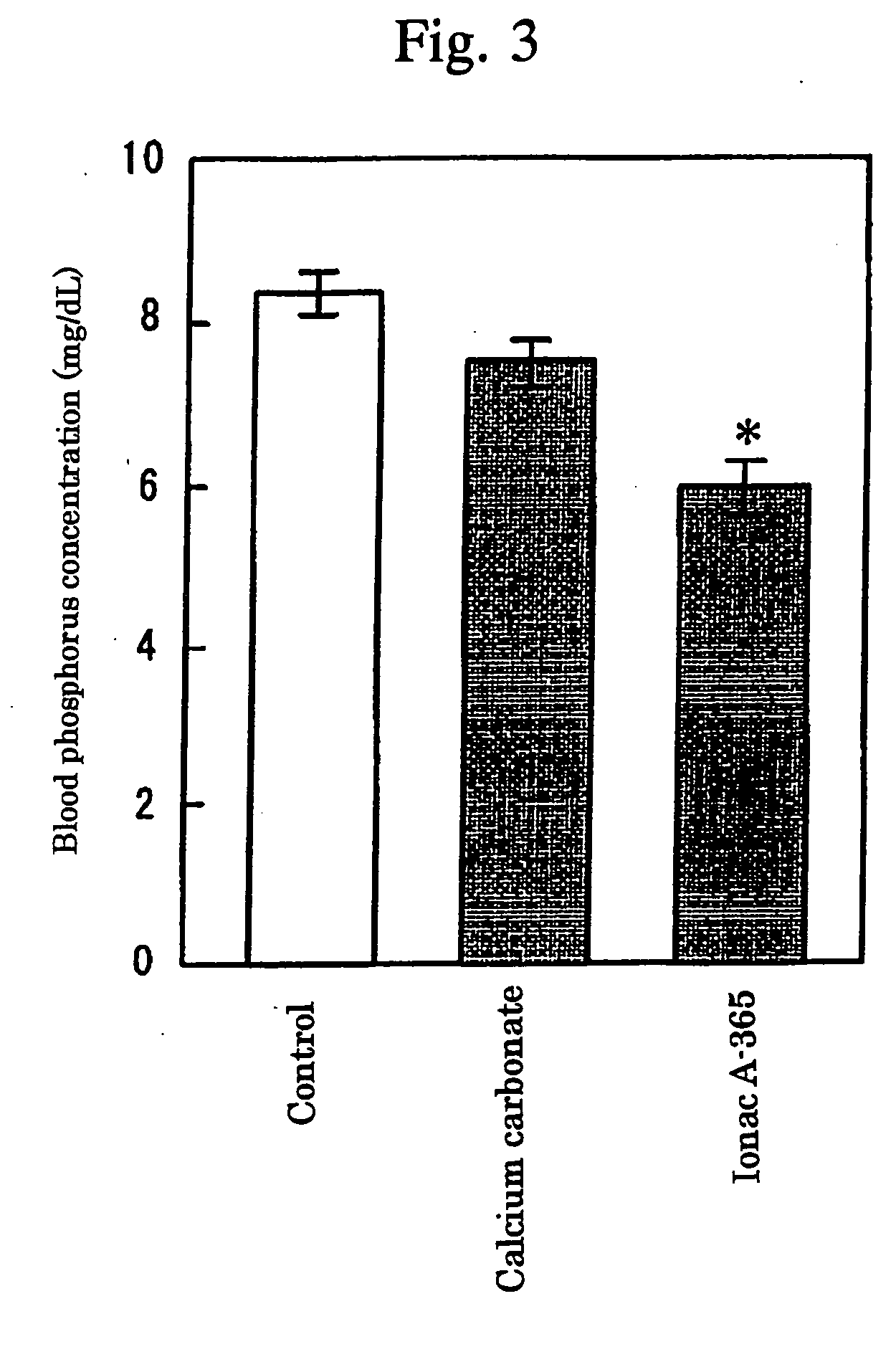
Preventives and/or remedies for hyperphosphatemia
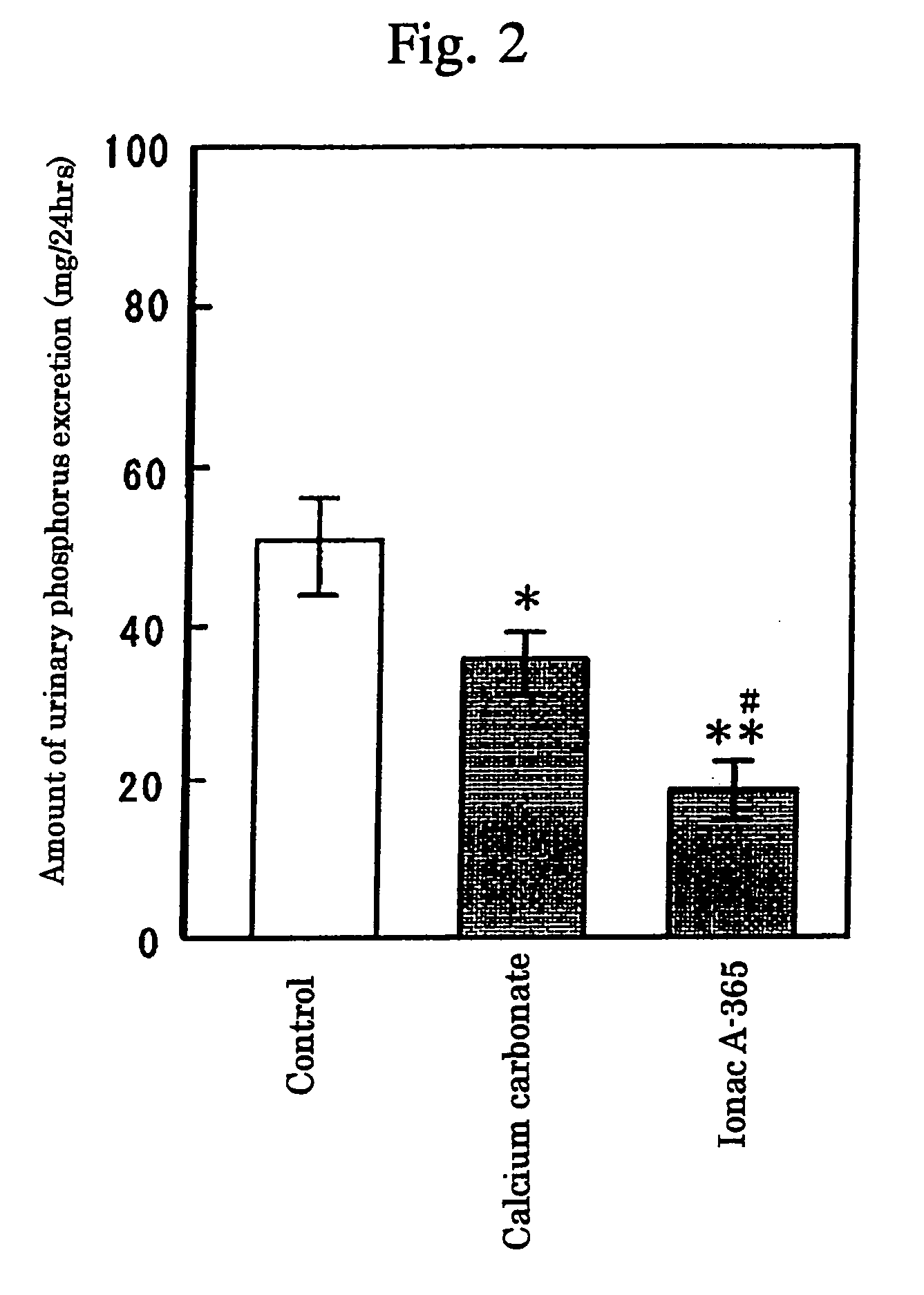
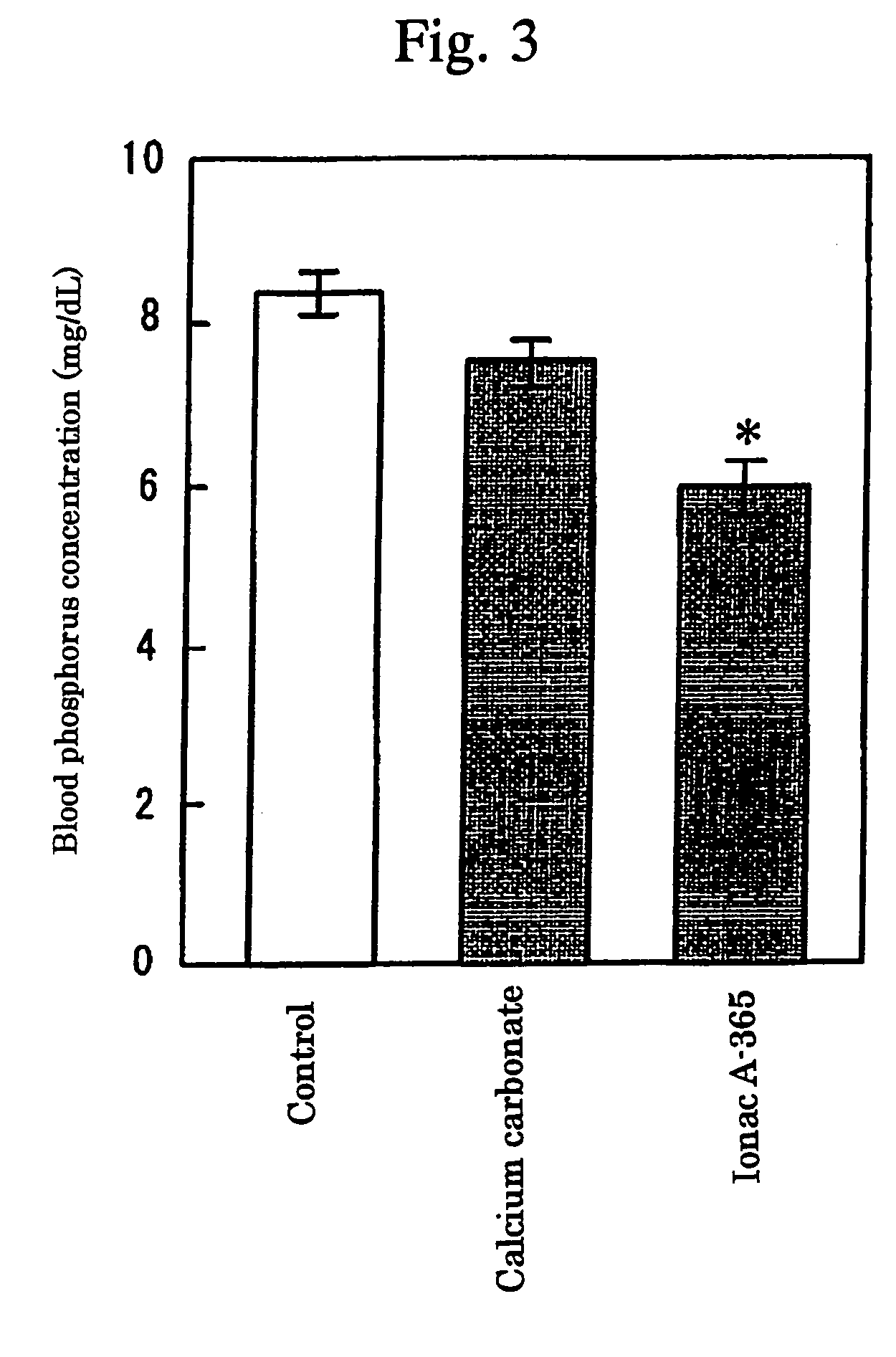
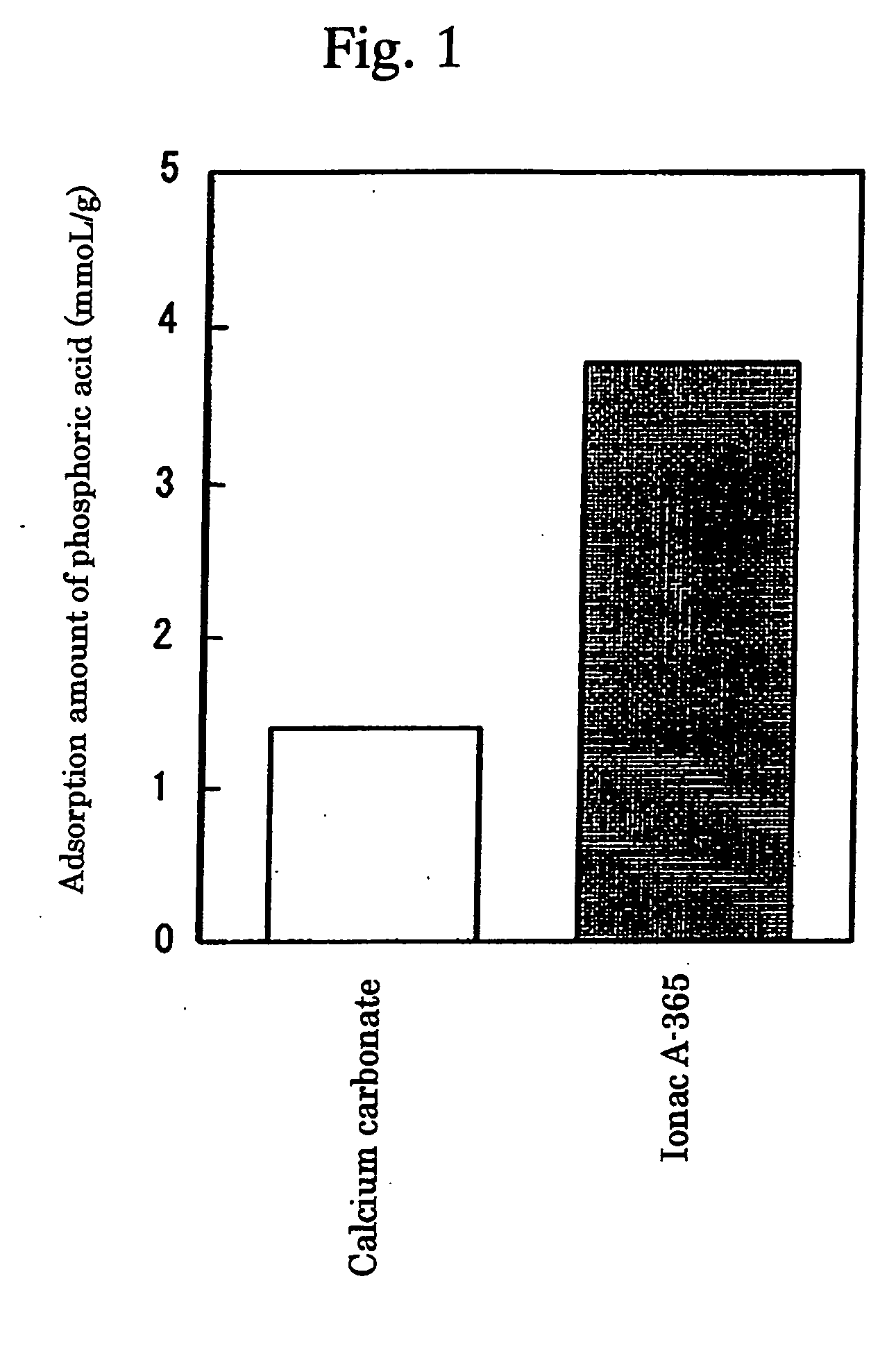
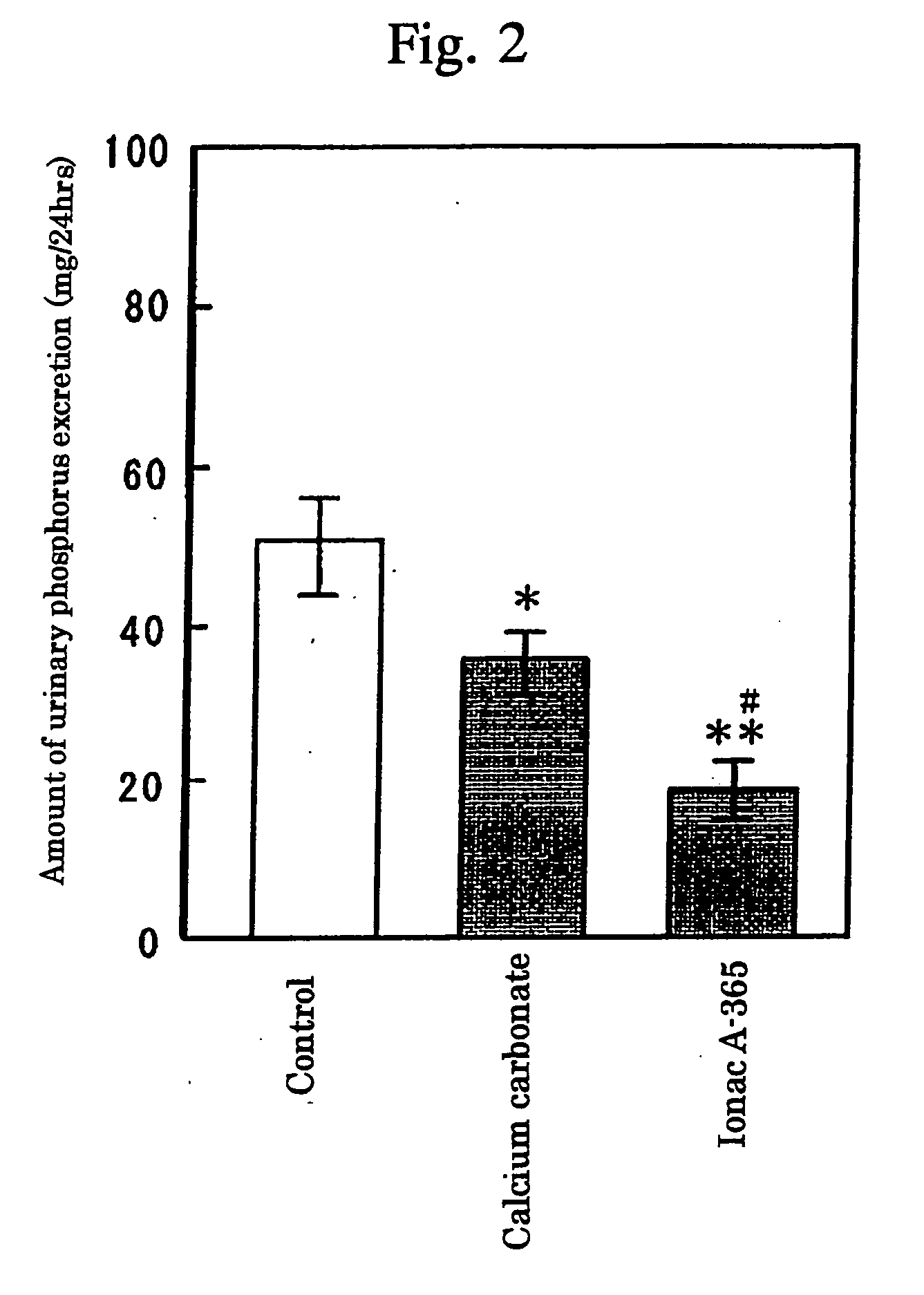
InactiveUS7087223B2High selectivityAvoid high concentrationsMetabolism disorderUrinary disorderBlood phosphorusPhosphate ion
Provided in a phosphate ion adsorbent containing a weakly basic anion exchange resin as an active ingredient which aims at providing preventives and / or remedies for hyperphosphatemia having a high selectivity for the adsorption of phosphate ion and showing an effect of lowering blood phosphorus level and another effect of suppressing phosphorus excretion into the urine.
Owner:HISAMITSU PHARM CO INC
Preventives and/or remedies for hyperphosphatemia
InactiveUS20050084476A1High selectivityAvoid high concentrationsMetabolism disorderUrinary disorderBlood phosphorusPhosphate ion
Provided in a phosphate ion adsorbent containing a weakly basic anion exchange resin as an active ingredient which aims at providing preventives and / or remedies for hyperphosphatemia having a high selectivity for the adsorption of phosphate ion and showing an effect of lowering blood phosphorus level and another effect of suppressing phosphorus excretion into the urine.
Owner:HISAMITSU PHARM CO INC
Phosphate transport inhibitors
Disclosed are compounds which have been identified as inhibitors of phosphate transport. Many of the compounds are represented by Structural Formula (I): Ar1—W—X—Y—Ar2; or a pharmaceutically acceptable salt thereof. Ar1 and Ar2 are independently a substituted or unsubstituted aryl group or an optionally substituted five membered or six membered non-aromatic heterocylic group fused to an optionally substituted monocylic aryl group. W and Y are independently a covalent bond or a C1-C3 substituted or unsubstituted alkylene group. X is a heteroatom-containing functional group, an aromatic heterocyclic group, substituted aromatic heterocyclic group, non-aromatic heterocyclic group, substituted non-aromatic heterocyclic group, an olefin group or a substituted olefin group. Also disclosed are methods of treating a subject with a disease associated with hyperphosphatemia, as well as a disease mediated by phosphate-transport function. The methods comprise the step of administering an effective amount of the one of the compounds described above.
Owner:JOZEFIAK THOMAS +3
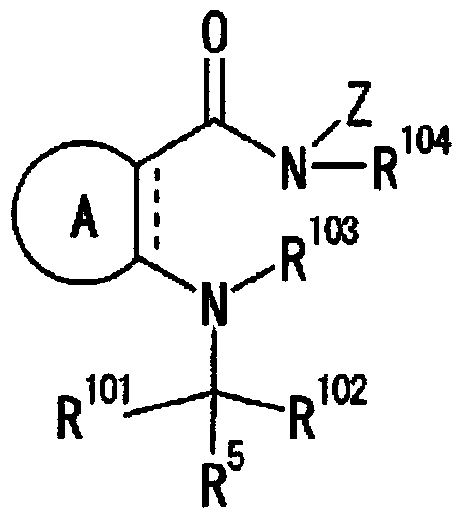
Tetrahydrobenzothiophene compound
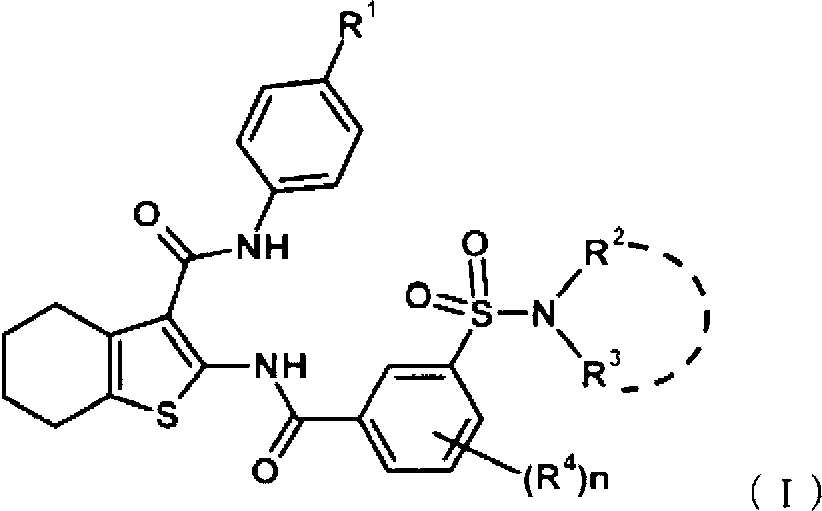
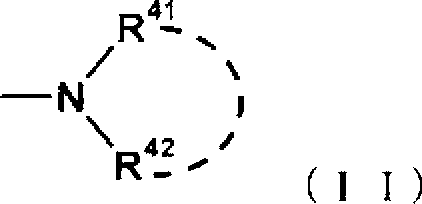
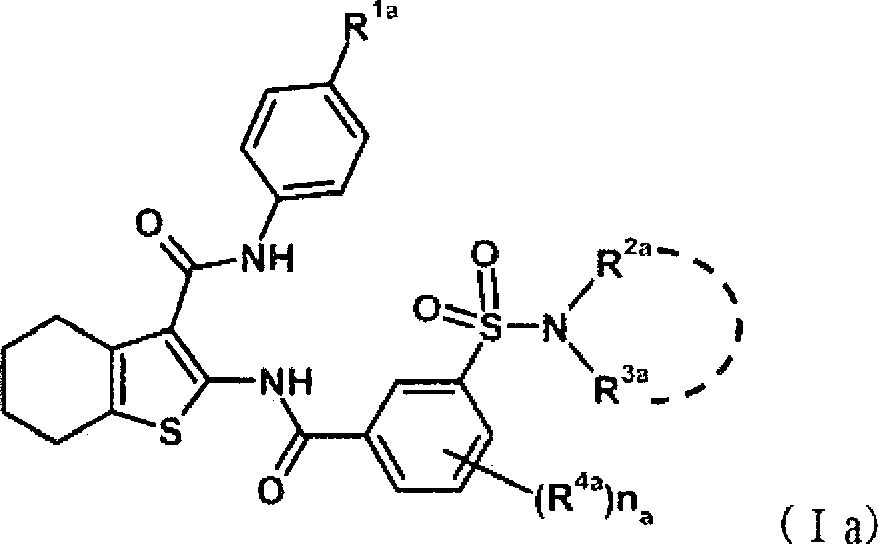
Disclosed is a compound which has an intestinal phosphate transporter (NPT-IIb) inhibition activity and is useful as an active ingredient for a therapeutic agent and / or a prophylactic agent for hyperphosphatemia. A tetrahydrobenzothiophene compound represented by formula (I) has an NPT-IIb inhibition activity and can be used as a prophylactic agent and / or a therapeutic agent for hyperphosphatemia. (In the formula, R1 represents -O-(lower alkyl), -(lower alkylene)-phenyl, or the like; R2 and R3 are same as or different from each other and independently represent H, a lower alkyl group, a cycloalkyl group, an aryl group, a heteroaryl group, or the like, or R2, R3 and a nitrogen atom to which R2 and R3 are bound together may form a 5- to 7-membered saturated cyclic amino group; R4's are same as or different from each other and independently represent a halogen atom, a lower alkyl group, or the like; and n represents 0 to 2.)
Owner:ASTELLAS PHARMA INC
Pharmaceutical compositions
InactiveUS20090155368A1Good acid stabilityPowder deliveryMetabolism disorderCrystallographyPolyamine
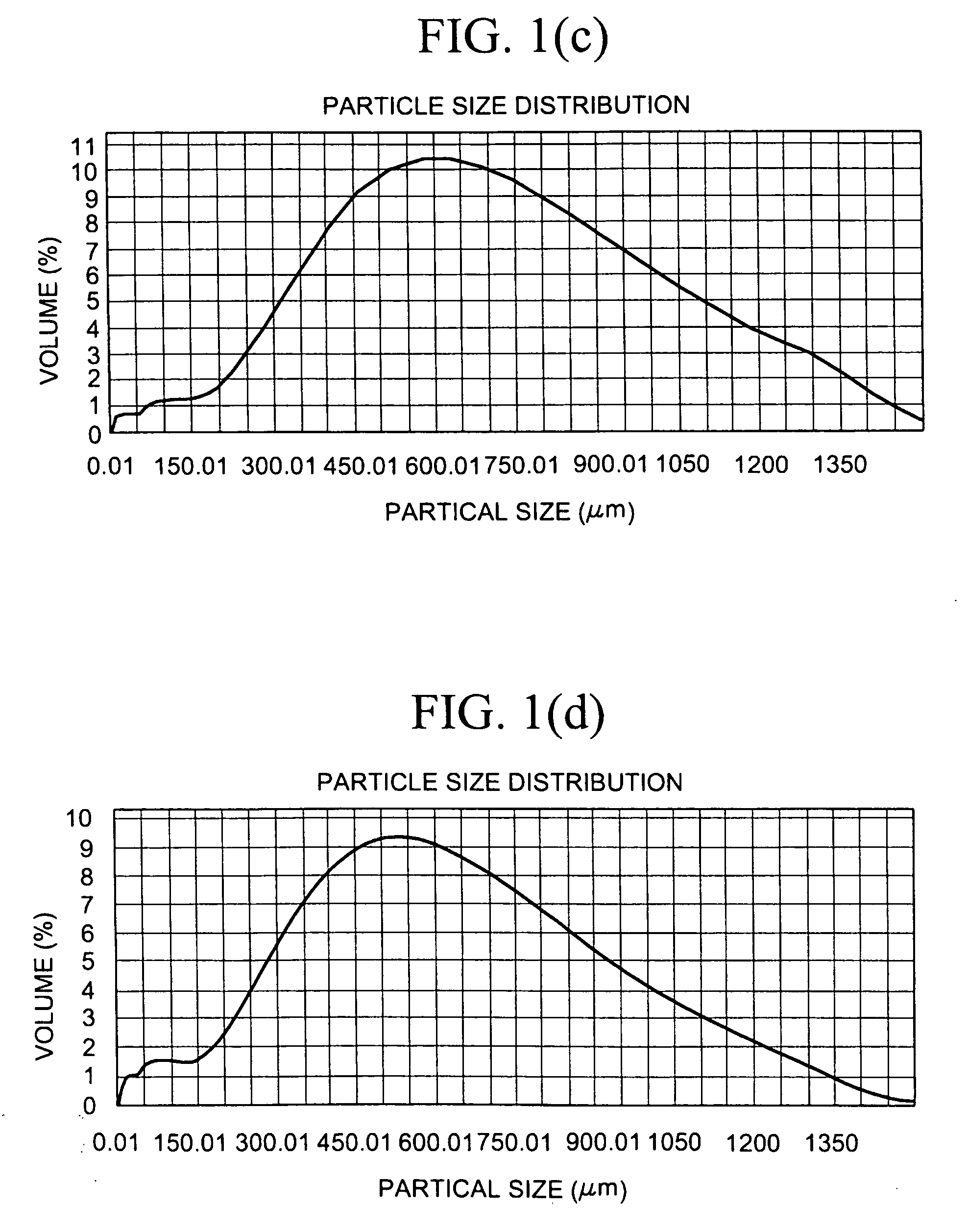
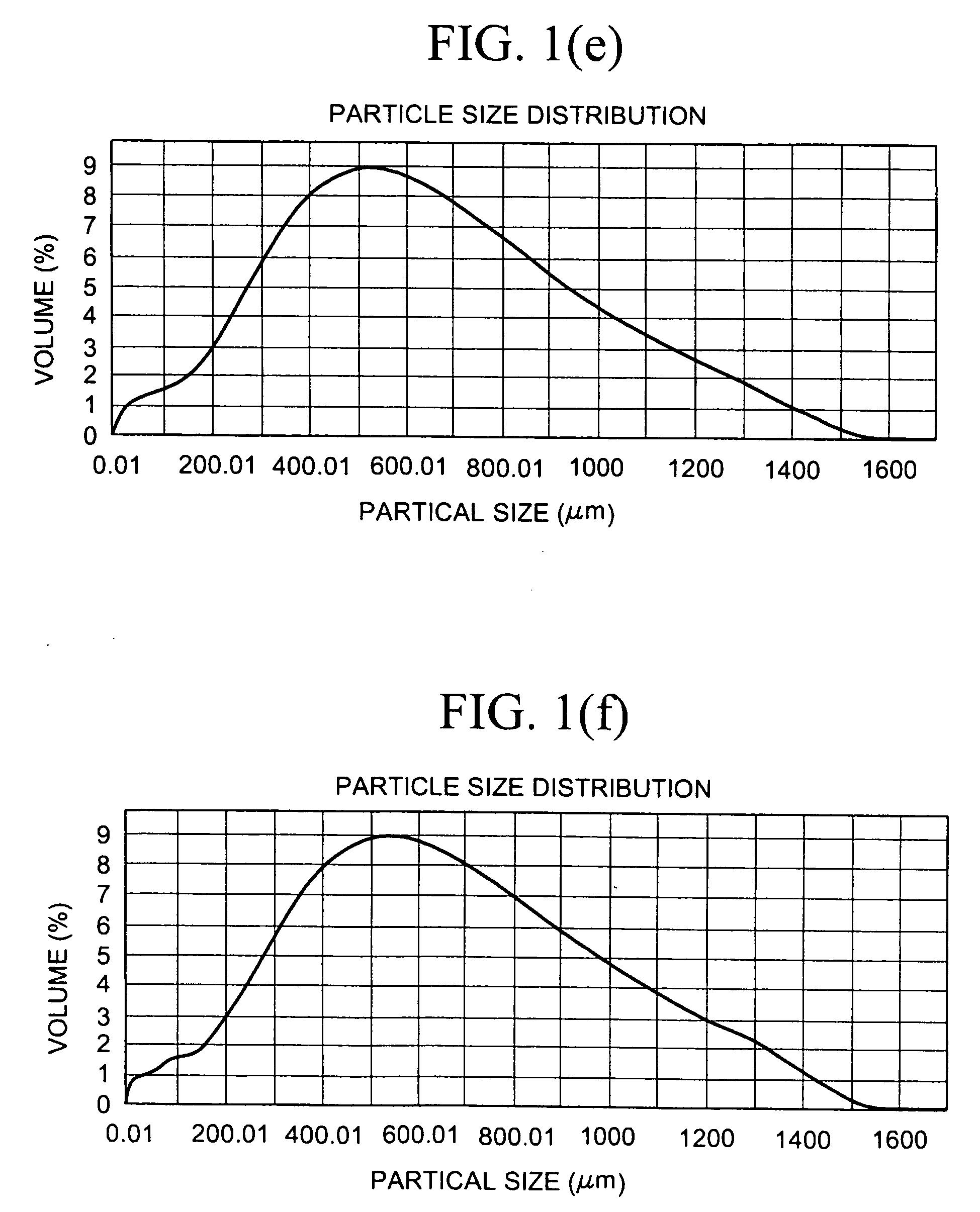
The present invention relates to crosslinked polyamine particles and / or pharmaceutical compositions comprising, at least in part, crosslinked polyamine particles and aggregates of such particles (including cured aggregates of crosslinked polyamine particles). The compositions may be in the form of tablets comprising, for example, particles larger than 500 μm, and used for treating patients, for example, patients with hyperphosphatemia.
Owner:GENZYME CORP
Composition, methods and reagents for the synthesis of a soluble form of human PHEX
InactiveUS7427498B2Easy to prepareEasy to purifyImmobilised enzymesBioreactor/fermenter combinationsPhosphoric acidMutant
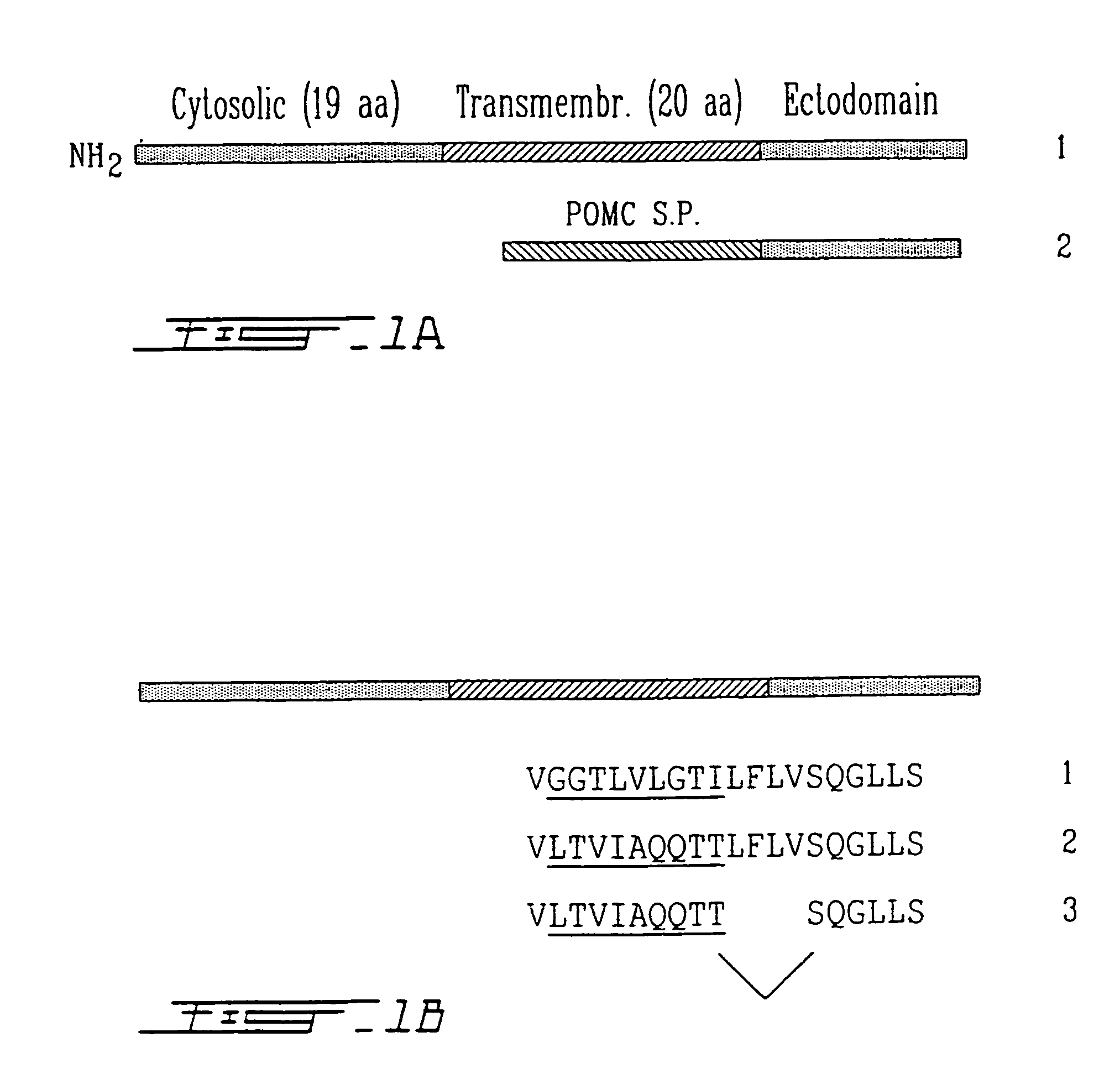
This invention relates to a soluble form of PHEX, PHEX being a type II integral membrane glycoprotein. This enzyme is the gene product of a phosphate-regulating gene with homologies to endopeptidases on the X chromosome. To produce a soluble form of PHEX, the transmembrane anchor domain has been modified to encode a signal peptidase coding sequence. The soluble PHEX therefore comprises the active ectodomain. An inactive mutant of PHEX is also an object of this invention. Both soluble and inactive mutant forms of PHEX can be used to screen ligands to PHEX. These ligands can also be used as substrates or inhibitors of PHEX. PHEX being phosphaturic, an inhibitor thereof will be used to treat phosphaturia and / or hypophosphatemia. On the opposite, a substrate for PHEX or PHEX itself can be used to treat hyperphosphatemia.
Owner:ALEXION PHARMA INC
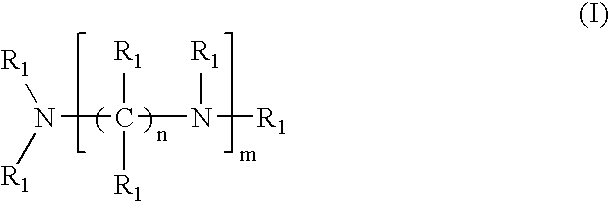
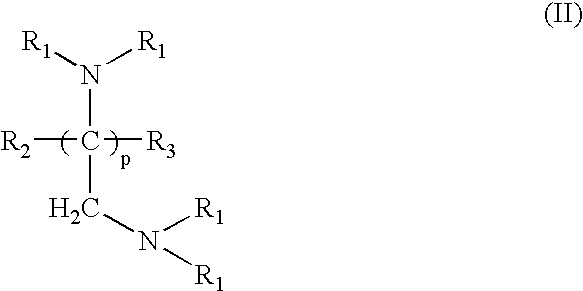
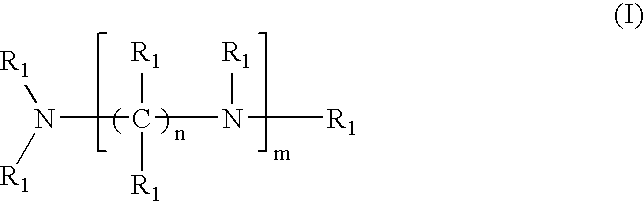
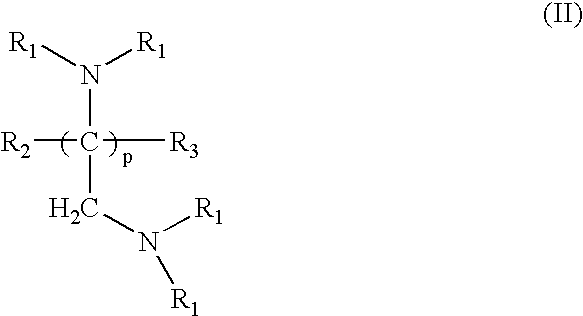
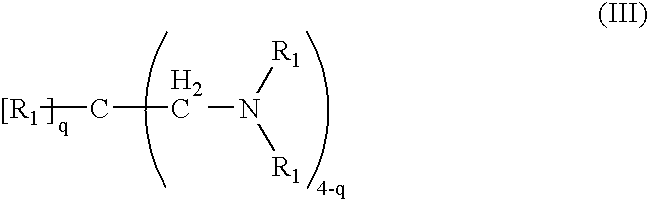
Amido-amine polymer compositions
Compounds, polymers, crosslinked polymers and pharmaceutical compositions comprising the same may be derived from multi-amine monomers and multi-functional monomers having two or more amine reactive groups. Such compounds, polymers, crosslinked polymers and compositions may be used to treat hyperphosphatemia or to remove ions from the gastrointestinal tract of animals, including humans.
Owner:GENZYME CORP
Crosslinked amine polymers
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides polymeric and pharmaceutical compositions comprising crosslinked amine polymers. Methods of use of the polymeric and pharmaceutical compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of renal diseases and hyperphosphatemia.
Owner:ILYPSA
Phosphorus binder composition for treatment of hyperphosphatemia
ActiveUS8759398B2Avoid absorptionReducing serum phosphorus concentrationBiocideOrganic chemistryGastrointestinal tractGastroenterology
The present invention relates to oral pharmaceutical products which are useful for binding phosphorus in ingesta, and inhibiting absorption of phosphorus from the gastrointestinal tract of subjects. A method for binding phosphorus in ingesta and inhibiting its absorption from the gastrointestinal tract is also provided. The pharmaceutical products and methods of the present invention are particularly useful in the treatment of hyperphosphatemia of chronic uremia and reducing serum phosphorus levels in patients requiring such therapy.
Owner:BIOLINK LIFE SCI
Treatment of hyperphosphatemia using crosslinked small molecule amine polymers
InactiveUS20070110706A1Improving therapeutic property and suitabilityInterference of resultingPowder deliveryMetabolism disorderIon bindingPolymer
Owner:ILYPSA
Coated Pharmaceutical Compositions
The present invention relates to polycarbophil coated crosslinked amine polymers and / or pharmaceutical compositions comprising polycarbophil coated crosslinked amine polymers. The polycarbophil coated crosslinked amine polymers have several therapeutic applications, including, but not limited to, hyperphosphatemia, chronic kidney disease and End-Stage Renal Disease.
Owner:GENZYME CORP
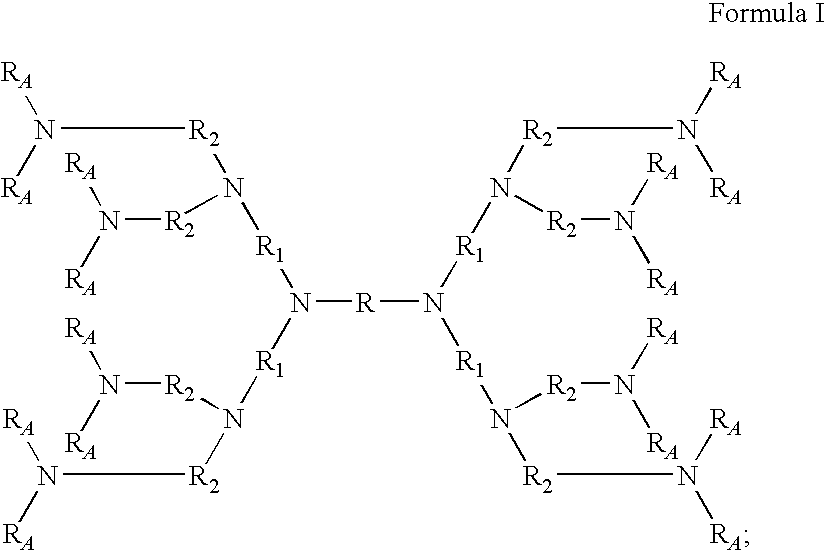
Dendrimer Compositions
Amine compounds, amine polymers, crosslinked amine polymers and pharmaceutical compositions comprising the same may include polyhydroxy-containing cores that may be substituted with amine groups and may be used to treat hyperphosphatemia or to remove ions from the gastrointestinal tract of animals, including humans.
Owner:GENZYME CORP
Zinc-containing treatments for hyperphosphatemia
InactiveUS20080014288A1Reduce urinary phosphate levelLower Level RequirementsBiocideOrganic active ingredientsHMG-CoA reductaseIron salts
A method of treating hyperphosphatemia in a subject comprises the step of administering to the subject an effective amount of a pharmaceutically acceptable zinc salt. A pharmaceutical composition comprises a pharmaceutically acceptable carrier; a pharmaceutically acceptable zinc salt; and a phosphate sequestrant. In one embodiment, the phosphate sequestrant is selected from a pharmaceutically acceptable lanthanum, calcium, magnesium and iron salt. In another embodiment, the phosphate sequestrant is an amine polymer, wherein the molar ratio of a zinc ion of the zinc salt to amine nitrogen atoms in the amine polymer is 0.1-3.0. The invention also includes a pharmaceutical composition comprising a pharmaceutically acceptable carrier; a pharmaceutically acceptable zinc salt; and an agent selected from the group consisting of a phosphate transport inhibitor, an HMG-CoA reductase inhibitor and an alkaline phosphatase inhibitor.
Owner:GENZYME CORP
Amine dendrimers
Ion binding compounds and compositions may include compounds, polymers and compositions that include amine moieties. Ion binding polymers may be crosslinked amine polymers and may be used to remove ions, such as phosphate ions, from the gastrointestinal tract of animals, such as humans. Such compounds, polymers and compositions may be used therapeutically to treat a variety of medical conditions, such as hyperphosphatemia.
Owner:GENZYME CORP
Phosphorus binder for treatment of kidney disease
ActiveUS20060228424A1Reduce the amount requiredPrevent hyperphosphatemiaHeavy metal active ingredientsBiocideDietary supplementGastroenterology
The present invention relates to oral compositions which are useful for binding phosphorus in ingesta, and inhibiting absorption of phosphorus from the gastrointestinal tract of subjects. A method for binding phosphorus in ingesta and inhibiting its absorption from the gastrointestinal tract is also provided. The dietary supplements and pharmaceutical products and methods of the present invention are particularly useful in the treatment of hyperphosphatemia of chronic uremia and reducing serum phosphorus levels in patients requiring such therapy.
Owner:BIOLINK LIFE SCI
Amine polymer compositions
A pharmaceutical composition for treating hyperphosphatemia can include polymers derived from multi-amine monomers and multifunctional monomers, where the multifunctional monomer includes more than one electrophilic group.
Owner:GENZYME CORP
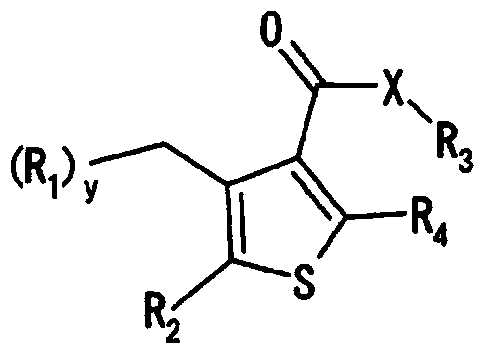
Aminoalkyl-substituted n-thienyl benzamide derivative
The objective of the present invention is to provide a compound having inhibitory activity against an intestinal phosphate transporter (NPT-IIb), and useful as an active ingredient in a hyperphosphatemia therapeutic agent and / or preventive. A thiophene compound represented by formula (I) may be used as a hyperphosphatemia therapeutic agent and / or preventive having NPT-IIb inhibitory activity. (In the formula, R1-6 each represent H or a lower alkyl or the like, L1-4 each represent a lower alkyl or the like, X1 represents CH2 or O, X2-3 each represent CH or N, k is 1-3, and n is 0-1.)
Owner:ASTELLAS PHARMA INC
Phosphate-binding magnesium salts and uses thereof
InactiveUS20090269399A1Reduce doseEffective phosphate-bindingBiocideMetabolism disorderMagnesium saltPharmacology
The present invention provides, among other things, compositions and methods suitable for the treatment of hyperphosphatemia based on phosphate-binding magnesium salts. In some embodiments, the present invention provides compositions and methods suitable for the treatment of hyperphosphatemia based on the combination of phosphate-binding magnesium and calcium salts.
Owner:CURRAX PHARMA LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com