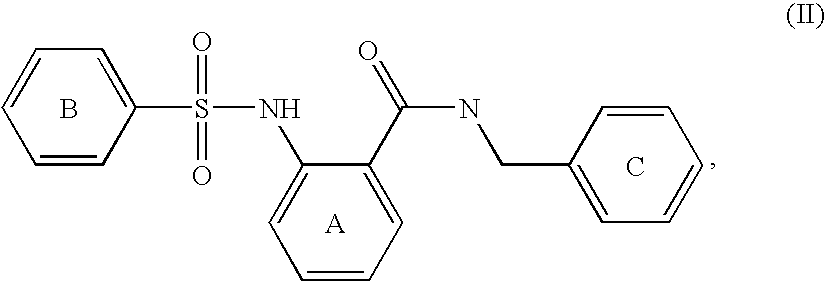
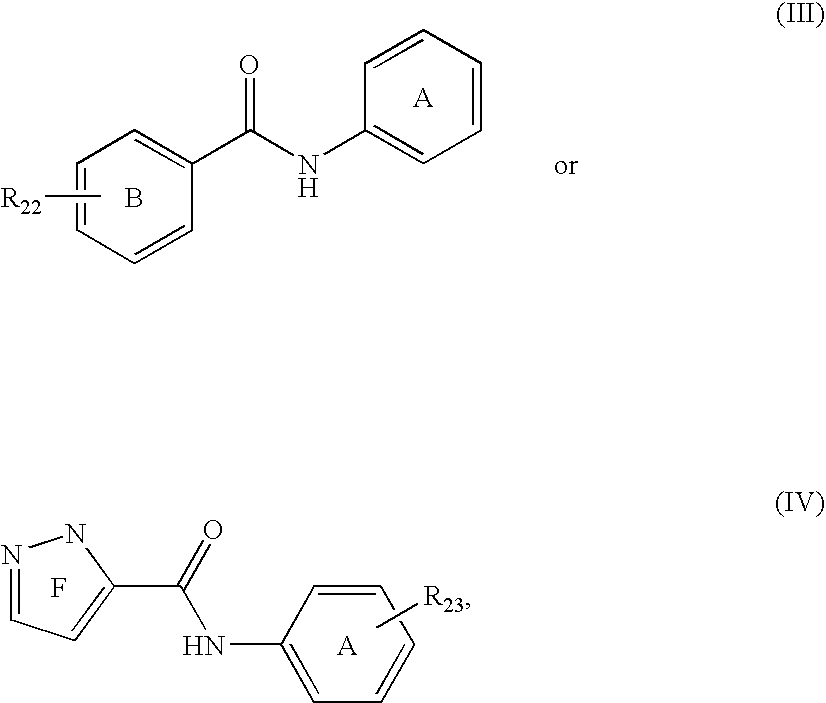
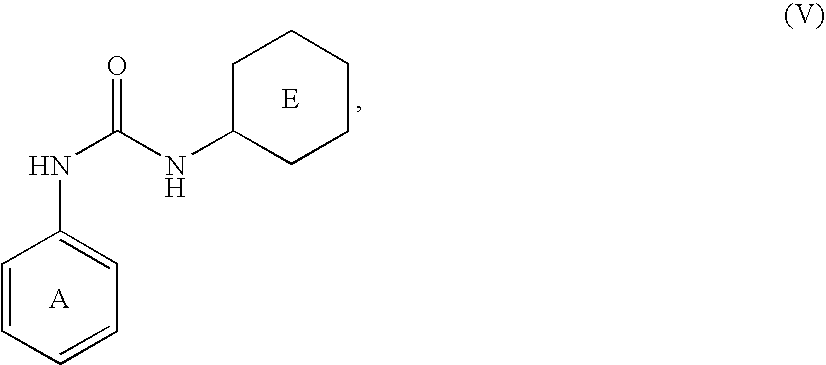
Phosphate transport inhibitors
a phosphate transport and inhibitor technology, applied in drug compositions, metabolic disorders, cardiovascular disorders, etc., can solve the problems of increased risk of cardiovascular events, serious side effects, and progression of renal failure, and achieve the effect of effective inhibitors of phosphate transpor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Methyl 5-bromo-2-[(4′-trifluoromethoxyphenyl)sulfonyl]amino benzoate
[0086]
[0087] Methyl 2-amino-5-bromo benzoate (2.78 g, 0.012 mol) was dissolved in pyridine (3 mL). The mixture was cooled in an ice bath below 5° C. and 4-trifluoromethoxy benzene sulfonyl chloride (3.5 g, 0.013 mol, 1.1 equivalents) was added slowly to the mixture. The mixture was warmed to room temperature, and stirred for 1 hour. The solvent was removed and the resulting solid was suspended in HCl (1M, 40 mL) for 30 minutes. The mixture was filtered, the solid suspended in hexane (40 mL) and stirred for 30 minutes. The mixture was filtered and the solid was washed with hexane (2×20 mL). Solid was dried in a vacuum oven at 40° C. Recovery=4.63 g (85% yield).
example 2
5-Bromo-2-[(4′-trifluoromethoxyphenyl)sulfonyl]amino benzoic acid
[0088]
[0089] Methyl 5-bromo-2-[(4′-trifluoromethoxyphenyl)sulfonyl]amino benzoate (0.854 g, 0.019 mmol) was dissolved in a mixture of tetrahydrofuran (20 mL) / water (10 mL). A solution of sodium hydroxide (50%, 5 mL) was added and the mixture was heated to reflux for 2-3 hours. The tetrahydrofuran was removed by rotary evaporation and the mixture was acidified with HCl (2 M). The mixture was extracted with ethyl acetate (20 mL). The organic solvent was removed to dryness and then dissolved in ethyl acetate (3×15 mL), and solvent removed. The oil was triturated in hexane (20 mL) until a solid formed. The mixture was filtered and the solid was dried in a vacuum oven at 40° C. Recovery=0.691 g (83% yield). M / Z of (M-H)−=432, 1H NMR (DMSO) δ=7.42-7.48 (1H), 7.55-7.6 (2H), 7.74-7.8 (1H), 7.92-8.0 (3H), 11.2 (br, 1H).
example 3
5-Bromo-2-[(4′-trifluoromethoxyphenyl)sulfonyl]amino benzoyl chloride
[0090]
[0091] 5-bromo-2-[(4′-trifluoromethoxyphenyl)sulfonyl]amino benzoic acid (3.27 g, 7.4 mmol) was suspended in thionyl chloride (30 mL) and the mixture was heated to reflux for 4 hours. The solvent was removed to dryness and the residue was dissolved in anhydrous ethyl acetate (2×20 mL), and solvent removed. The solid was dried at 40° C. in vacuum oven overnight.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com