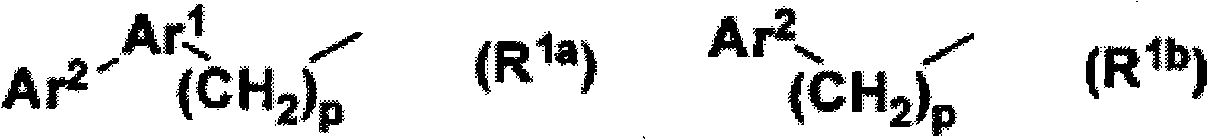Patents
Literature
33 results about "Hypoparathyroidism" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A condition in which there are abnormally low levels of parathyroid hormone.
PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS
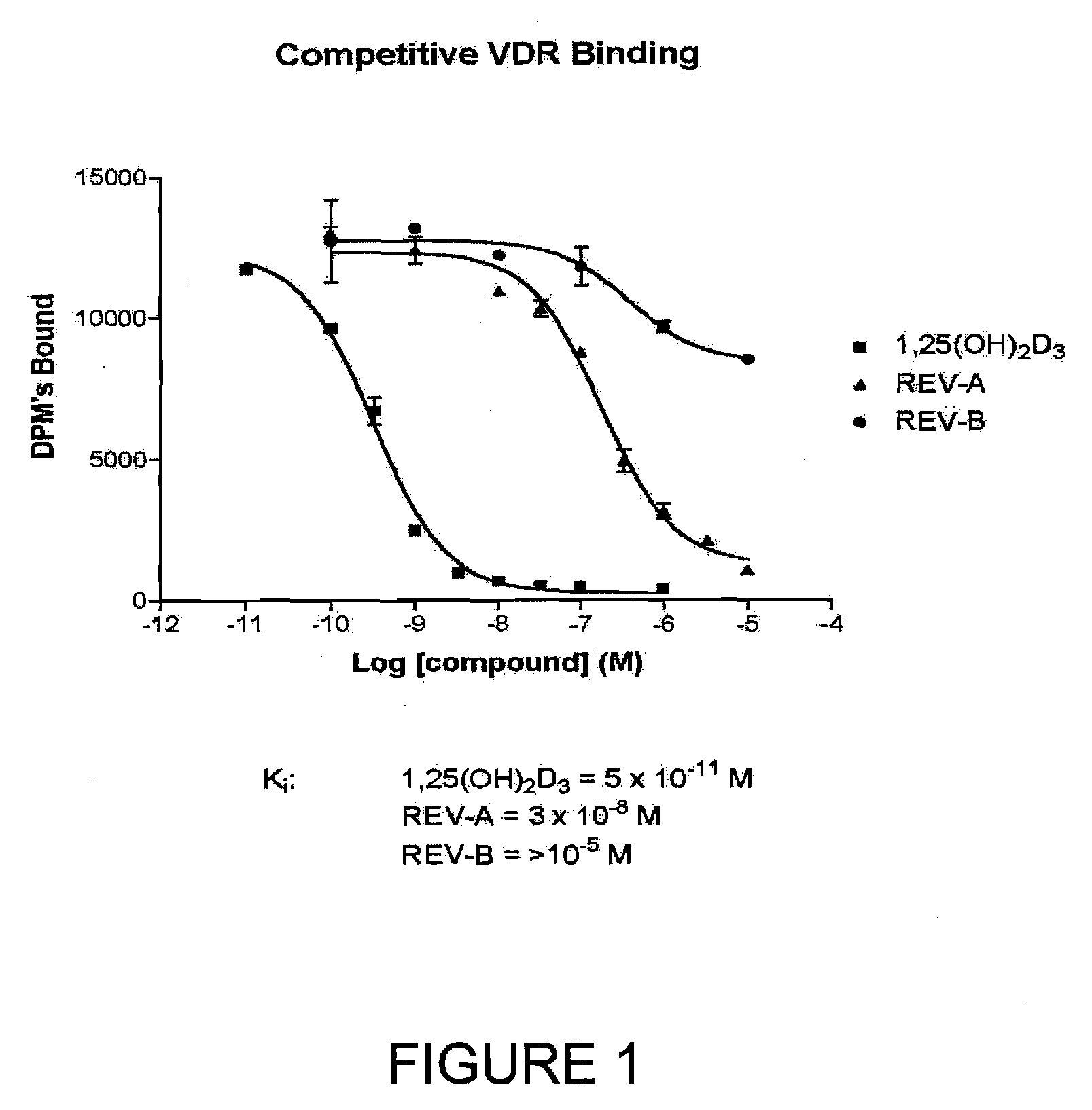
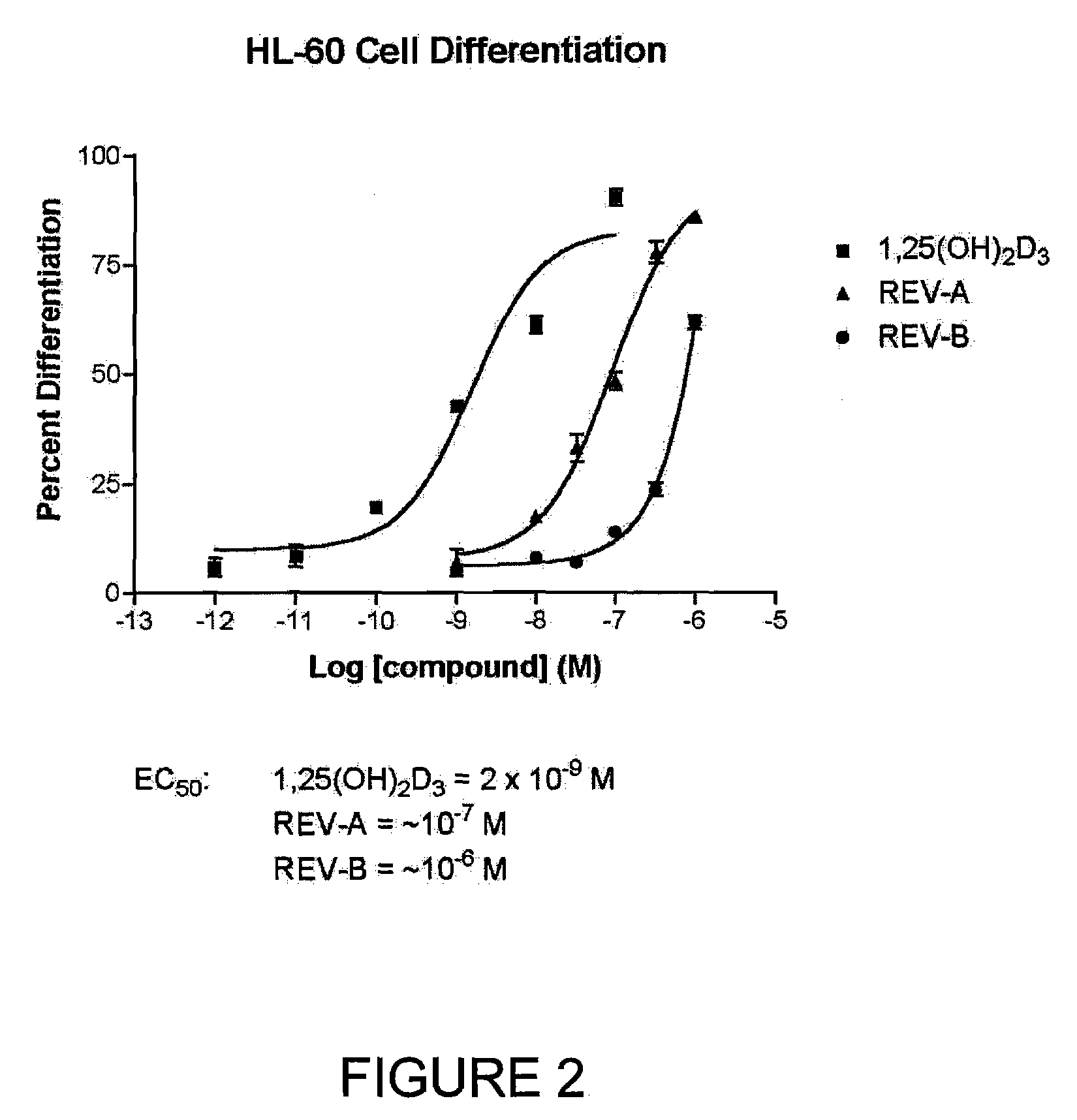
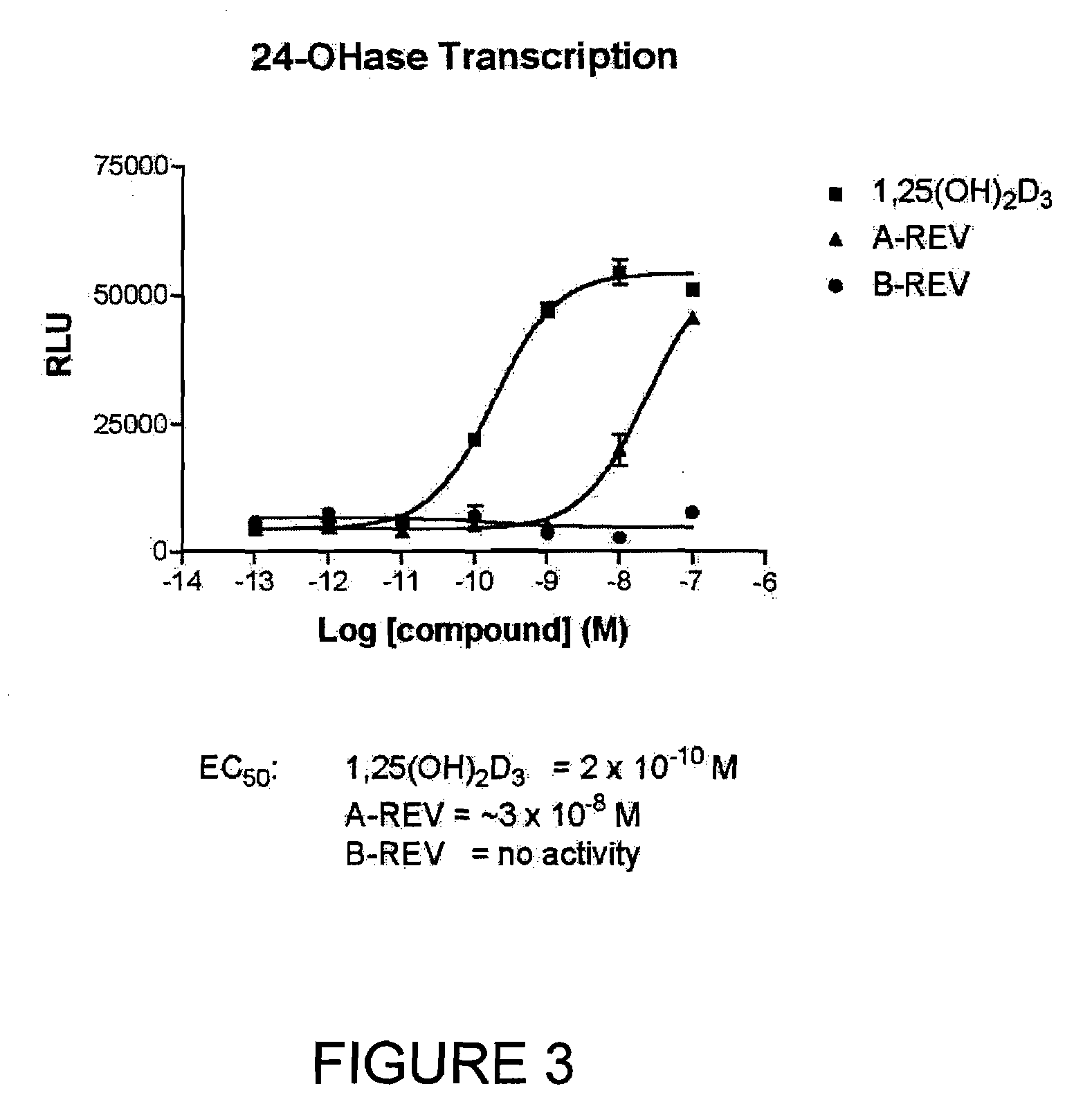
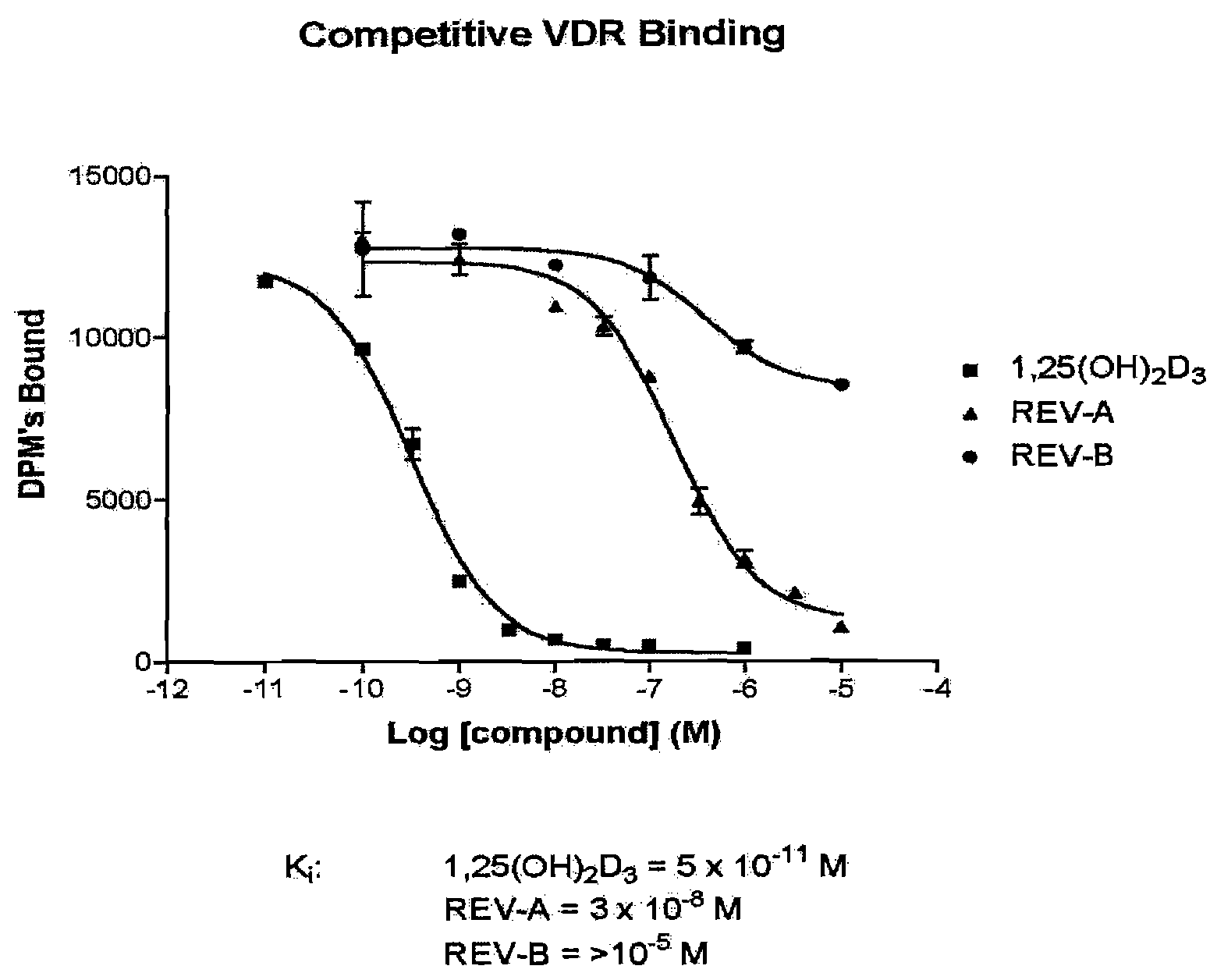
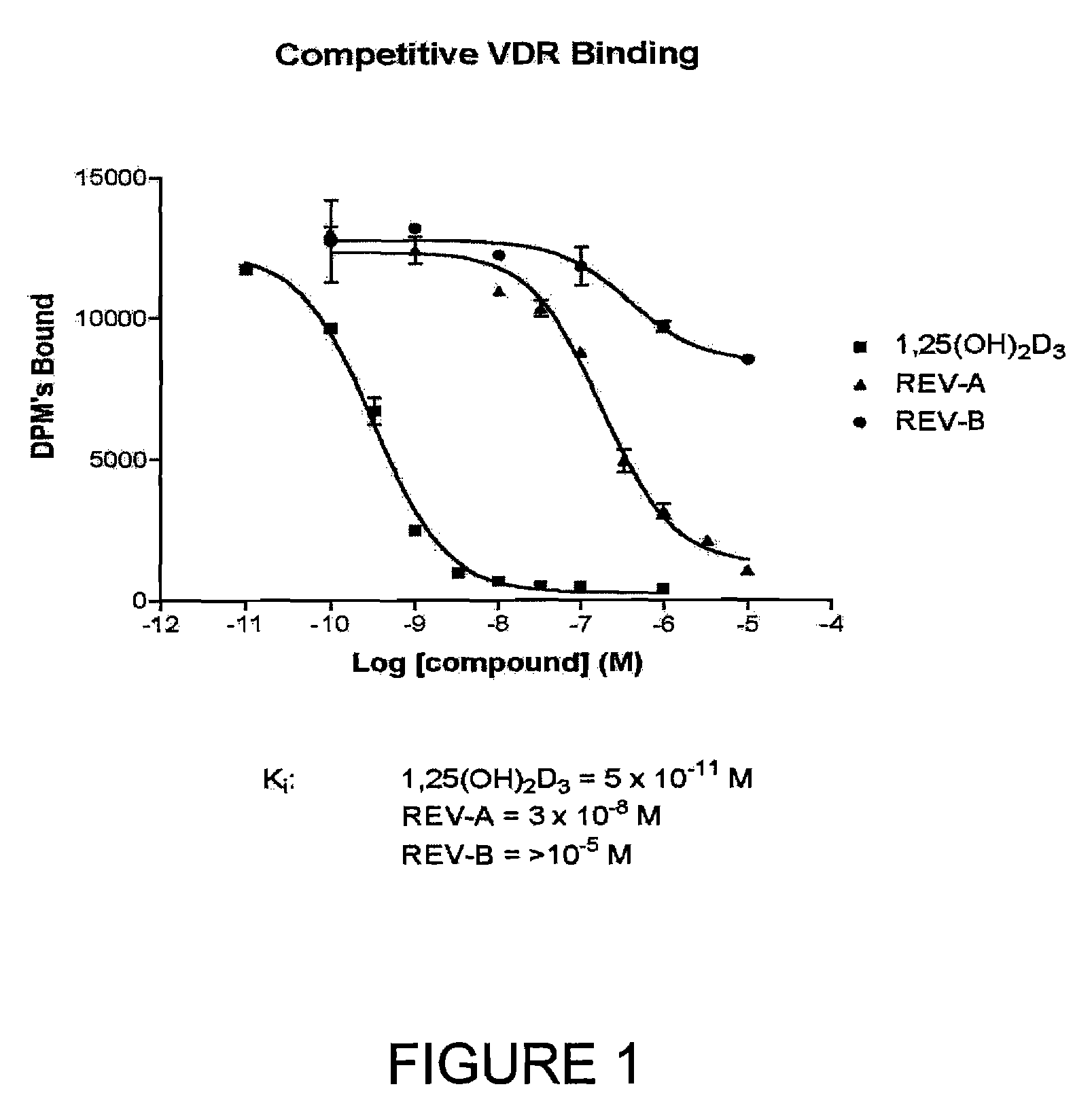
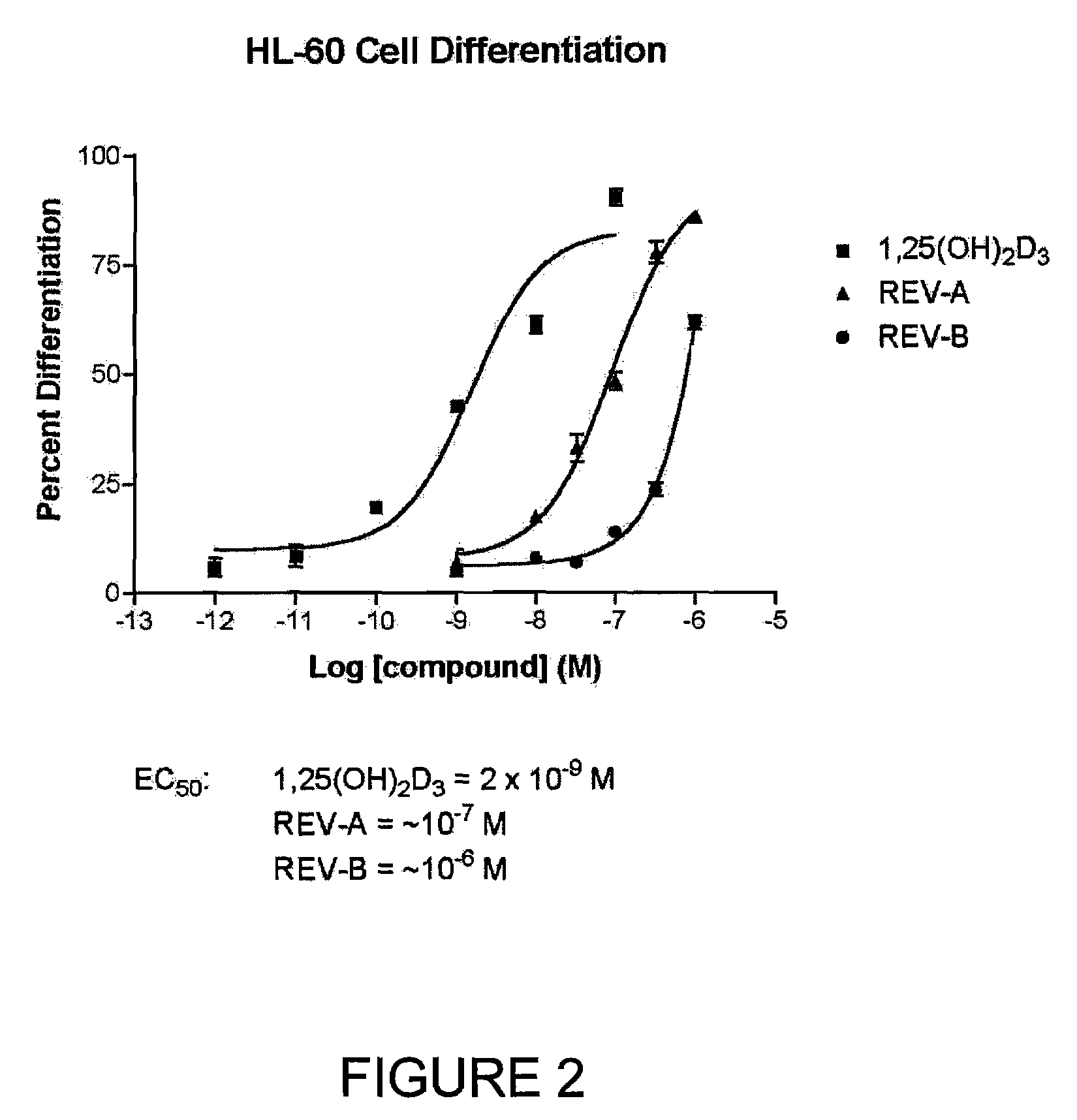
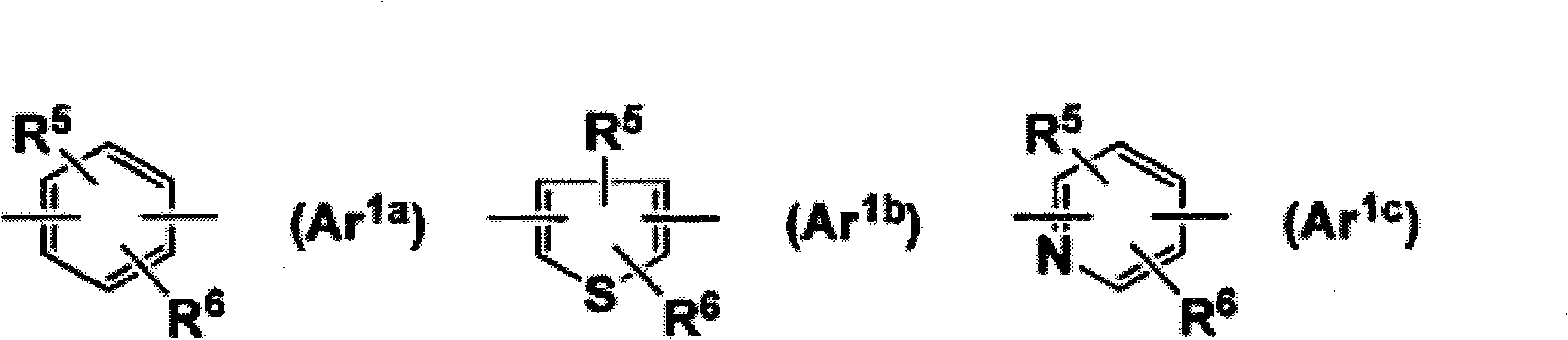
The present invention is directed to novel pyrido[4,3-d]pyrimidin-4(3H)-one derivatives and pharmaceutically acceptable salts thereof of structural formula I wherein the variables R1, R2, R3, R4 and R5 are as described herein. Also provided are pharmaceutical compositions comprising the compounds of formula I as well as methods of treatment employing compounds of formula I to treat a disease or disorder characterized by abnormal bone or mineral homeostasis such as hypoparathyroidism, osteoporosis, osteopenia, periodontal disease, Paget's disease, bone fracture, osteoarthritis, rheumatoid arthritis, and humoral hypercalcemia of malignancy.
Owner:PFIZER INC
Pyrido[4,3-d]pyrimidin-4(3H)-one derivatives as calcium receptor antagonists
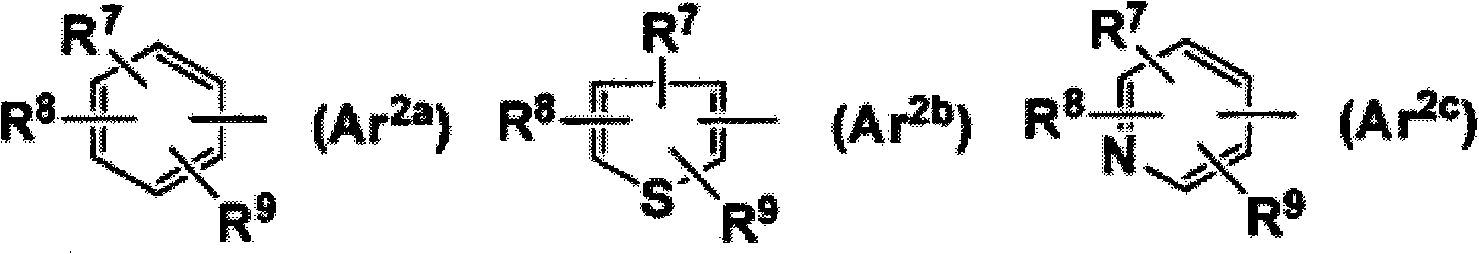
The present invention is directed to novel pyrido[4,3-d]pyrimidin-4(3H)-one derivatives and pharmaceutically acceptable salts thereof of structural formula Iwherein the variables R1, R2, R3, R4 and R5 are as described herein. Also provided are pharmaceutical compositions comprising the compounds of formula I as well as methods of treatment employing compounds of formula I to treat a disease or disorder characterized by abnormal bone or mineral homeostasis such as hypoparathyroidism, osteoporosis, osteopenia, periodontal disease, Paget's disease, bone fracture, osteoarthritis, rheumatoid arthritis, and humoral hypercalcemia of malignancy.
Owner:PFIZER INC
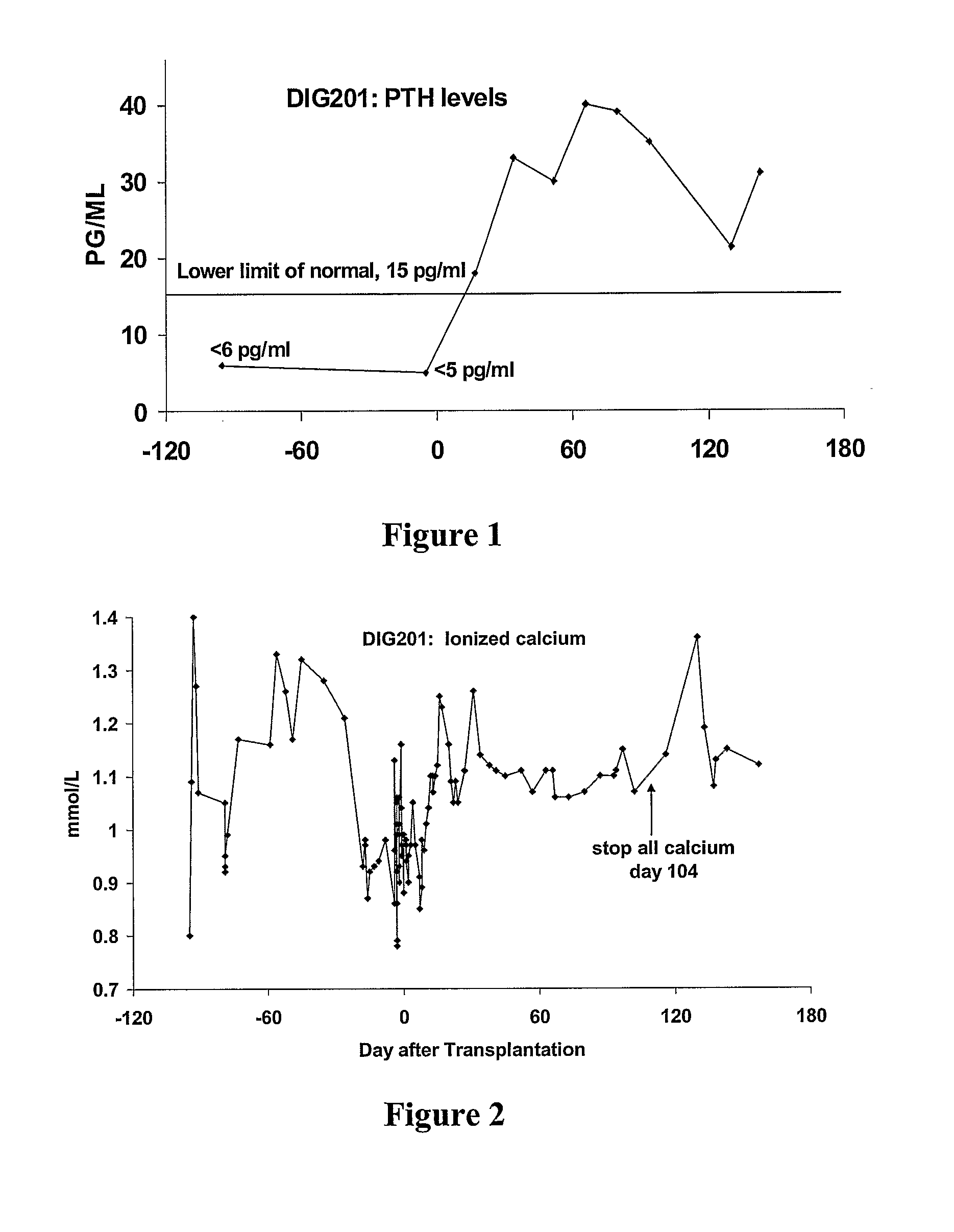
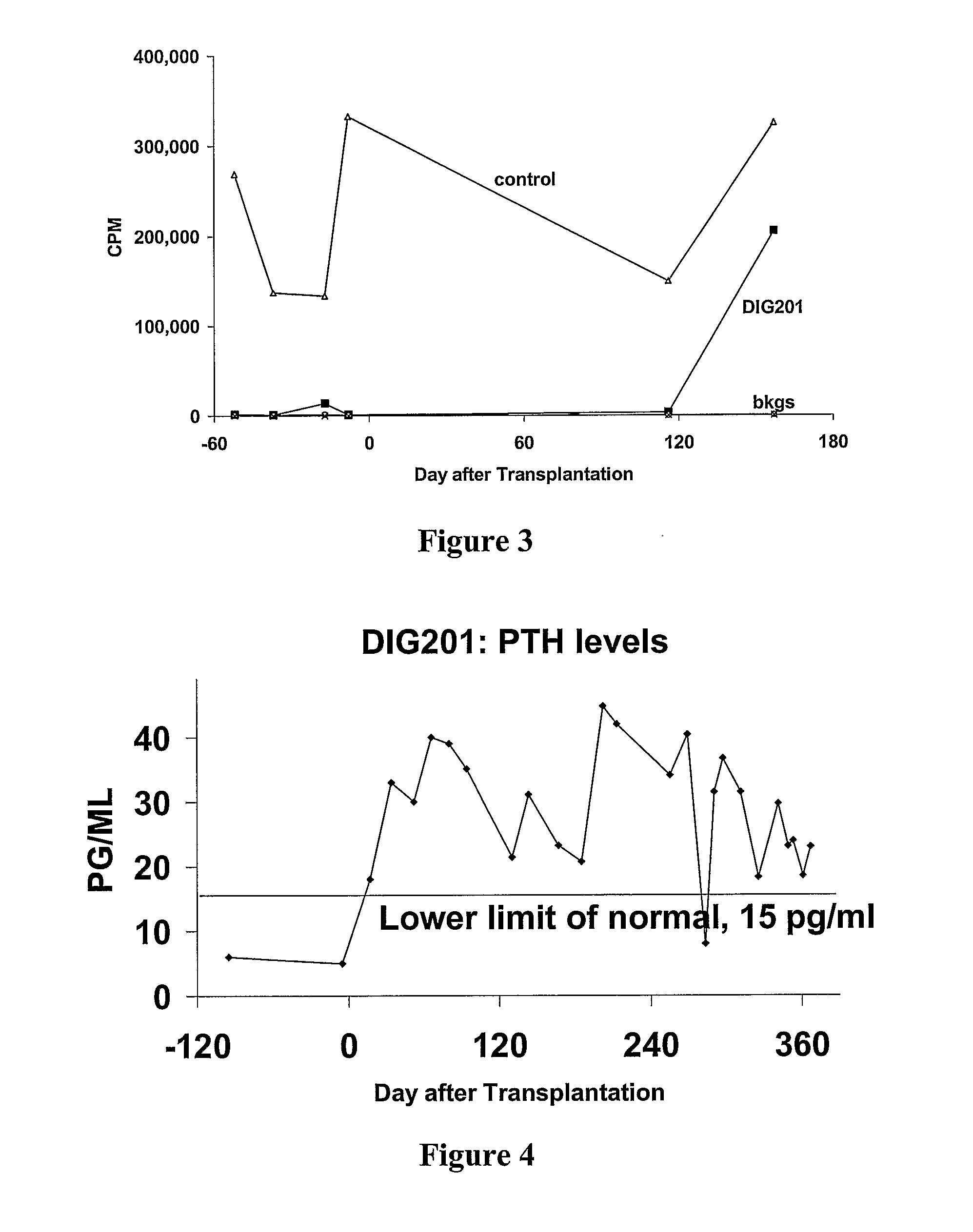
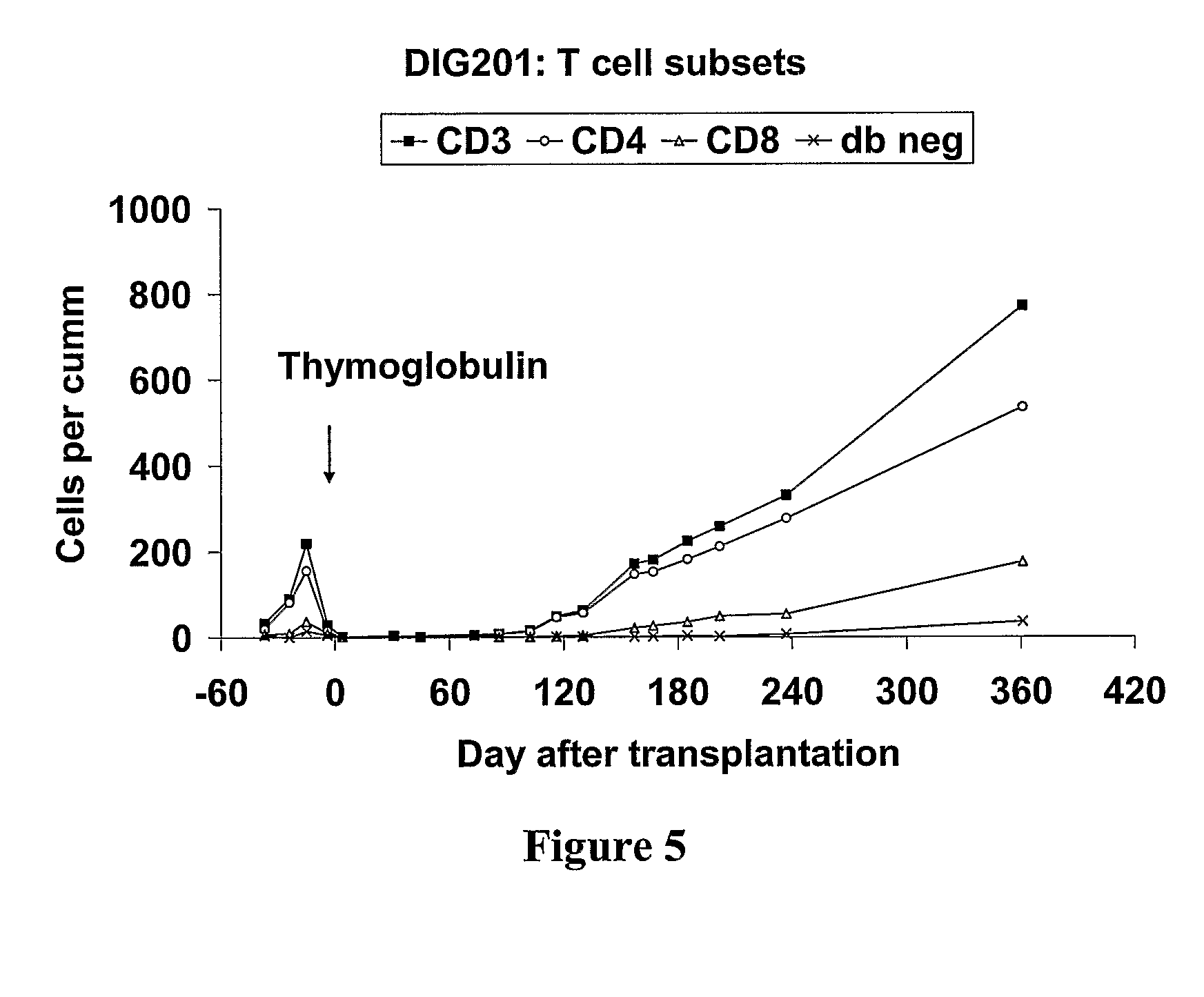
Parathyroid and thymus transplantation in digeorge syndrome subjects
A method of treating hypoparathyroidism in a human DiGeorge syndrome subject comprises (a) implanting thymus tissue into the subject in an amount effective to treat the DiGeorge syndrome; and (b) implanting, preferably concurrently implanting, parathyroid tissue into the subject in an amount effective to treat the hypoparathyroidism.
Owner:DUKE UNIV
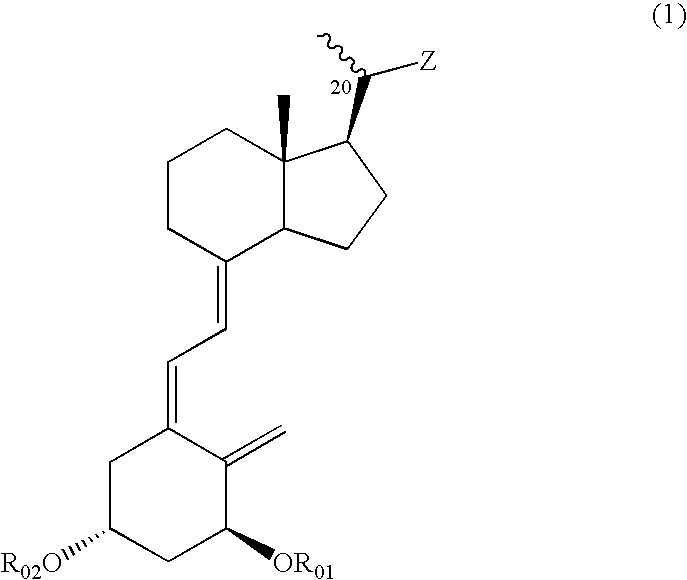
Vitamin D3 derivative and treating agent for inflammatory respiratory disease using same
InactiveUS6867313B2Effective as treating agentsEliminate the effects ofOrganic active ingredientsBiocideRespiratory diseaseBULK ACTIVE INGREDIENT
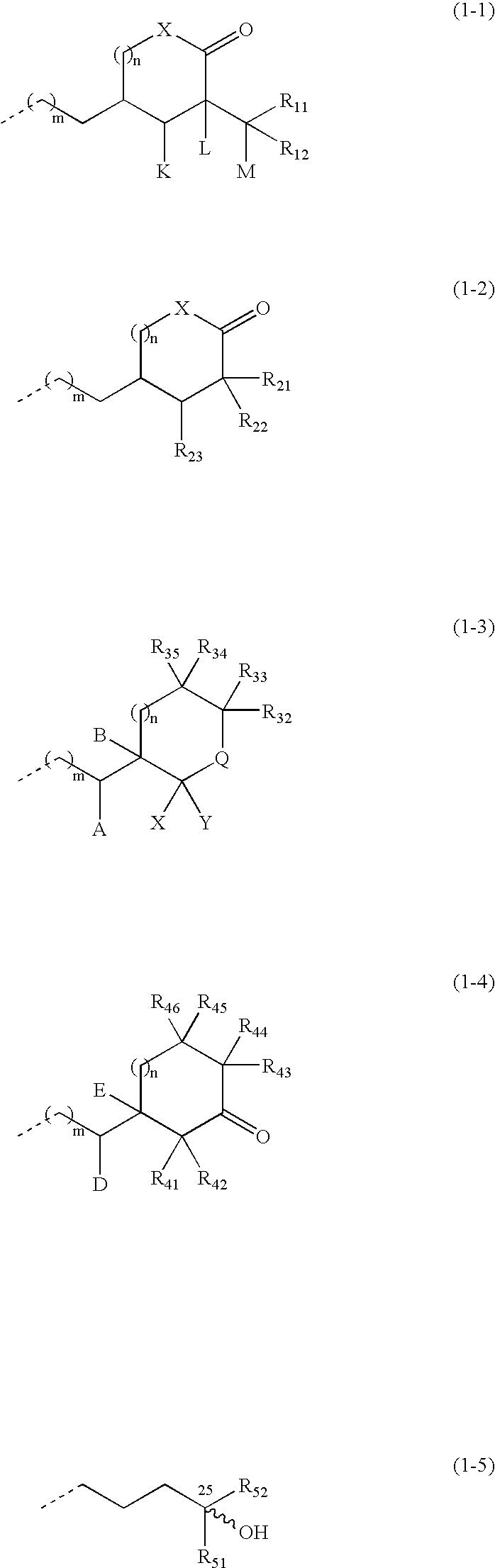
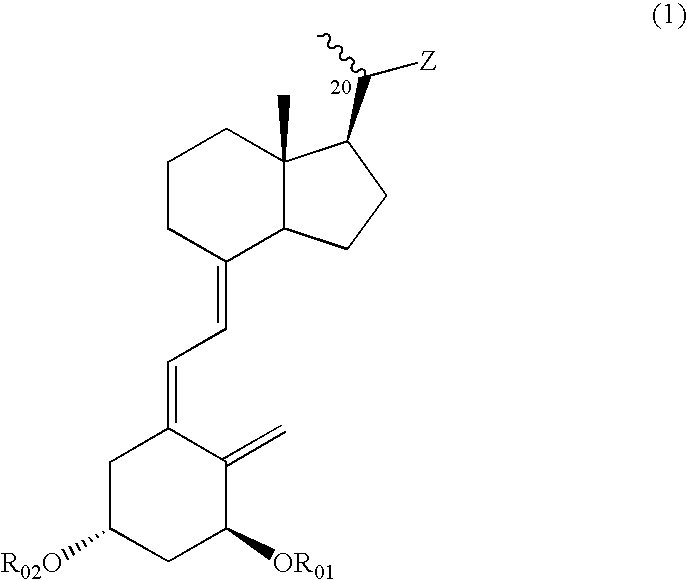
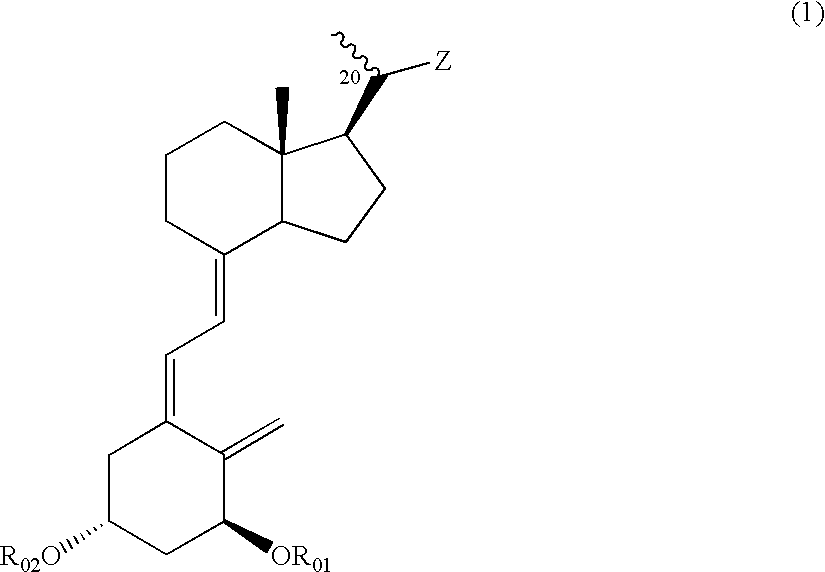
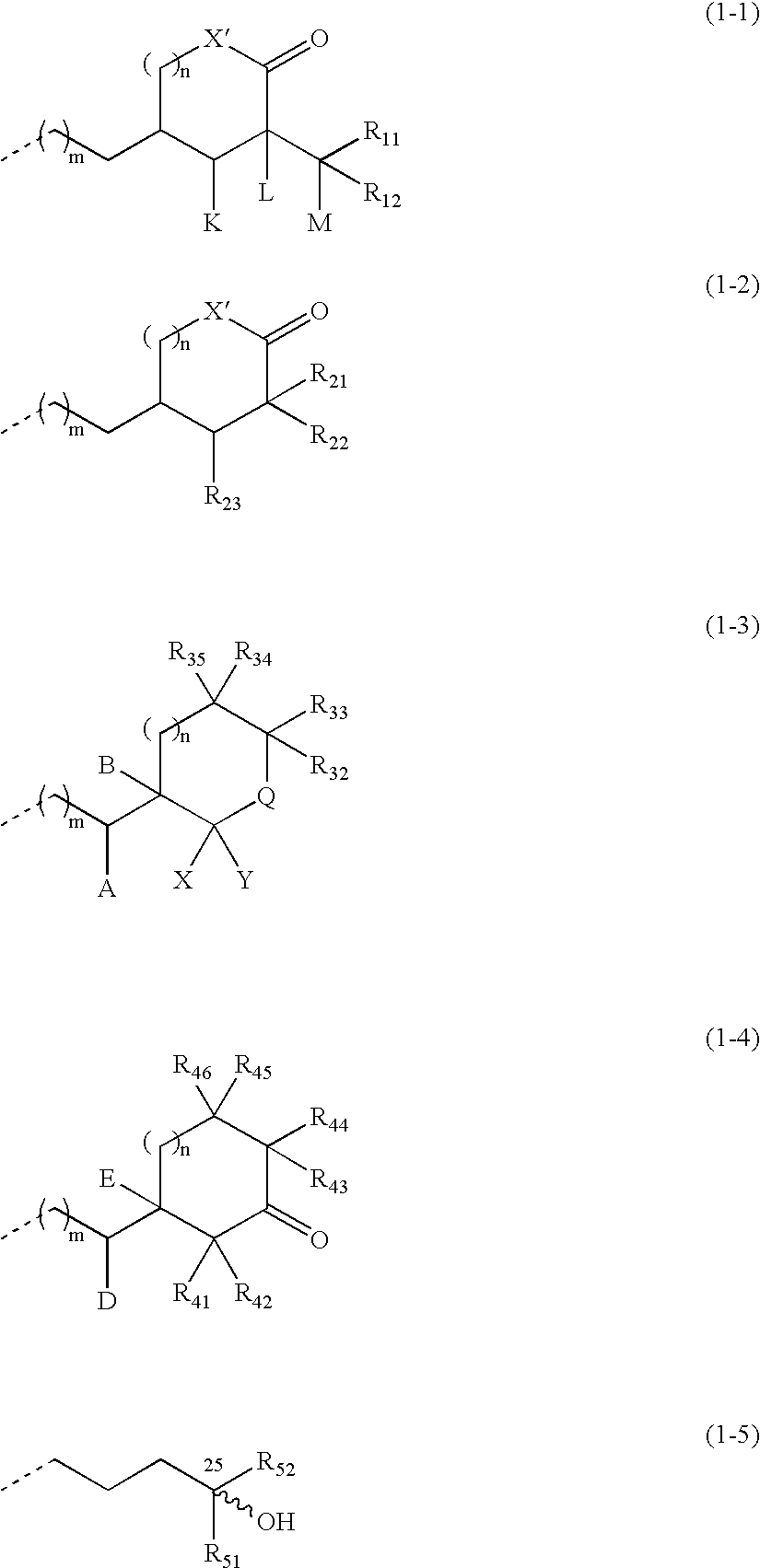
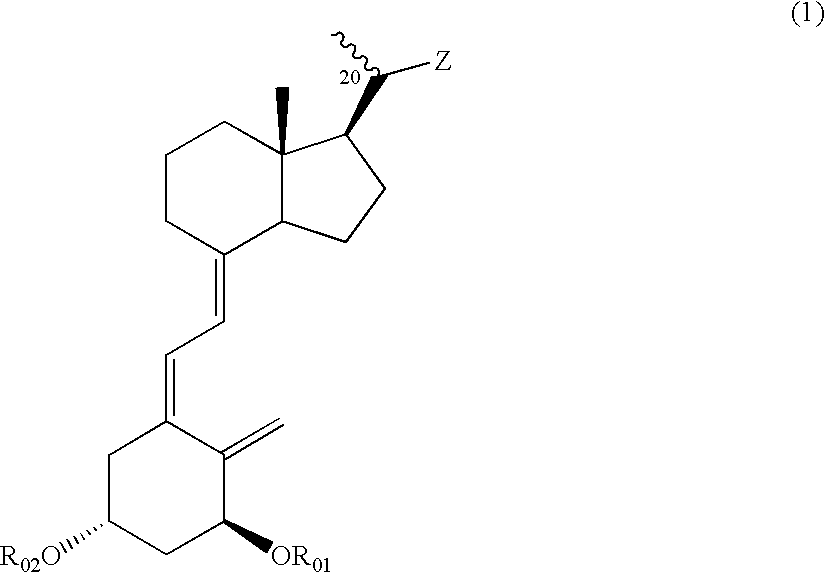
Compounds expressed by the following general formula (1), [wherein, R01 and R02 are each independently a hydrogen atom or a protecting group for a hydroxyl group; Z is one out of the following formulae (1-1) to (1-5)]. The compounds can be used as active ingredients of treating agents for inflammatory respiratory diseases, malignant tumors, rheumatoid arthritis, osteoporosis, diabetes mellitus, hypertension, alopecia, acne, psoriasis, dermatitis, hypercalcemia, hypoparathyroidism and metabolic disorder of cartilage.
Owner:TEIJIN LTD
PTH-like peptides
InactiveUS20060019902A1Improve biological activityImprove stabilityPeptide/protein ingredientsSkeletal disorderDiseaseDysostosis
The present invention relates to peptides that are parathyroid hormone (PTH) analogs, useful for the treatment of hypoparathyroidism and diseases characterized by bone mass reduction, such as osteoporosis, and for stimulating bone repair or favoring the engraftment of a bone implant; to the pharmaceutical compositions comprising these PTH-like peptides and use thereof.
Owner:ABIOGEN PHARMA SPA
Parathyroid hormone-like polypeptides
InactiveUS20070117157A1Peptide/protein ingredientsSkeletal disorderAbnormal calciumDrug biological activity
Novel parathyroid hormone polypeptides and biologically active fragments thereof are disclosed along with nucleic acid molecules encoding same. In particular, parathyroid hormone polypeptides and biologically active fragments (and encoding nucleic acid molecules) derived from fish species (eg Japanese pufferfish (Fugu rubripes)) are disclosed. Such polypeptides and fragments may be used for treatment of diseases associated with abnormal calcium homeostasis (eg osteoporosis, osteopenia, Paget's disease, bone cancer, hyperparathyroidism, hypoparathyroidism, hypercalcemia, psoriasis and other skin-related conditions).
Owner:TEELEOSTIN LTD
Parathyroid hormone analogs and uses thereof
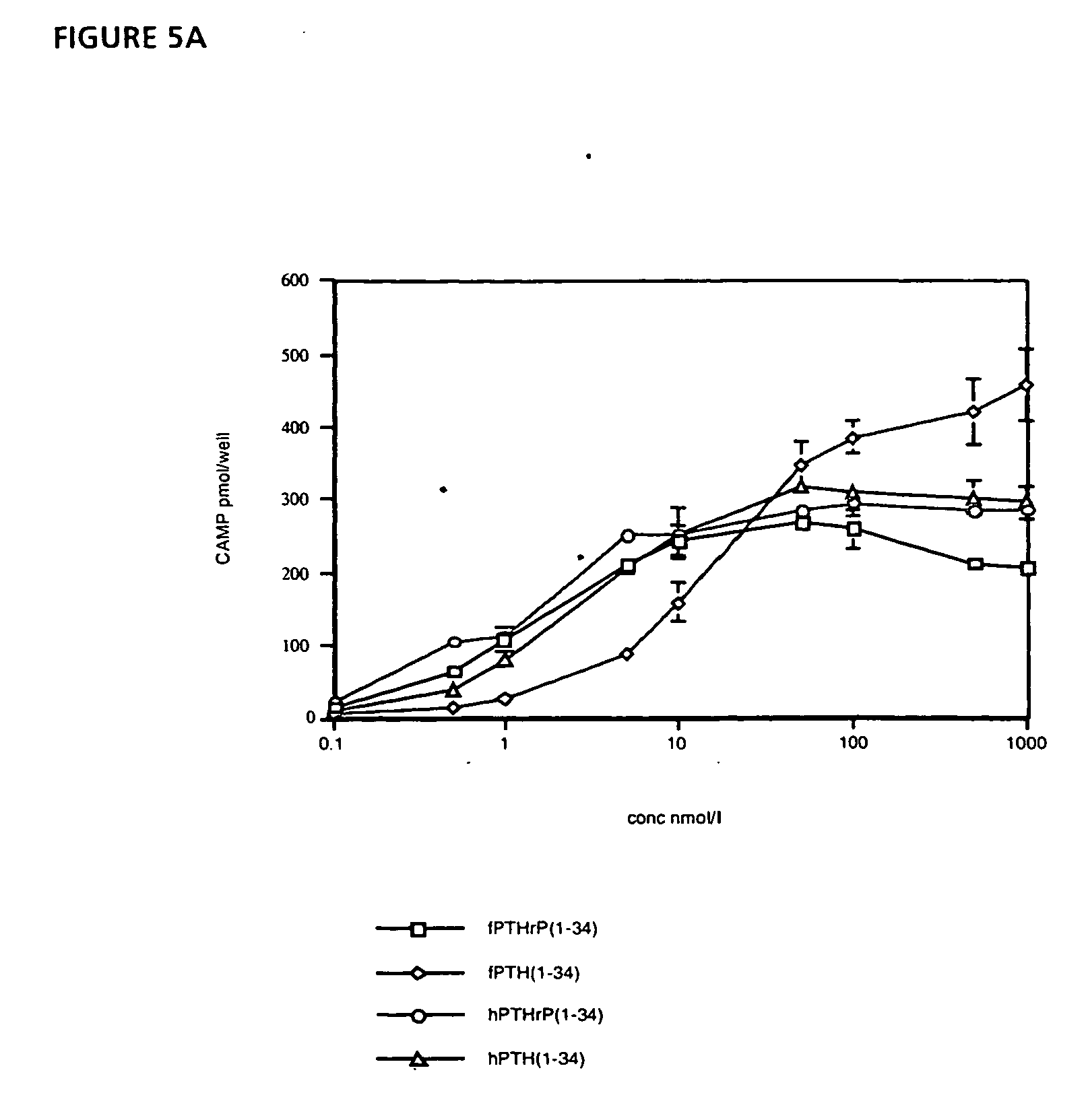
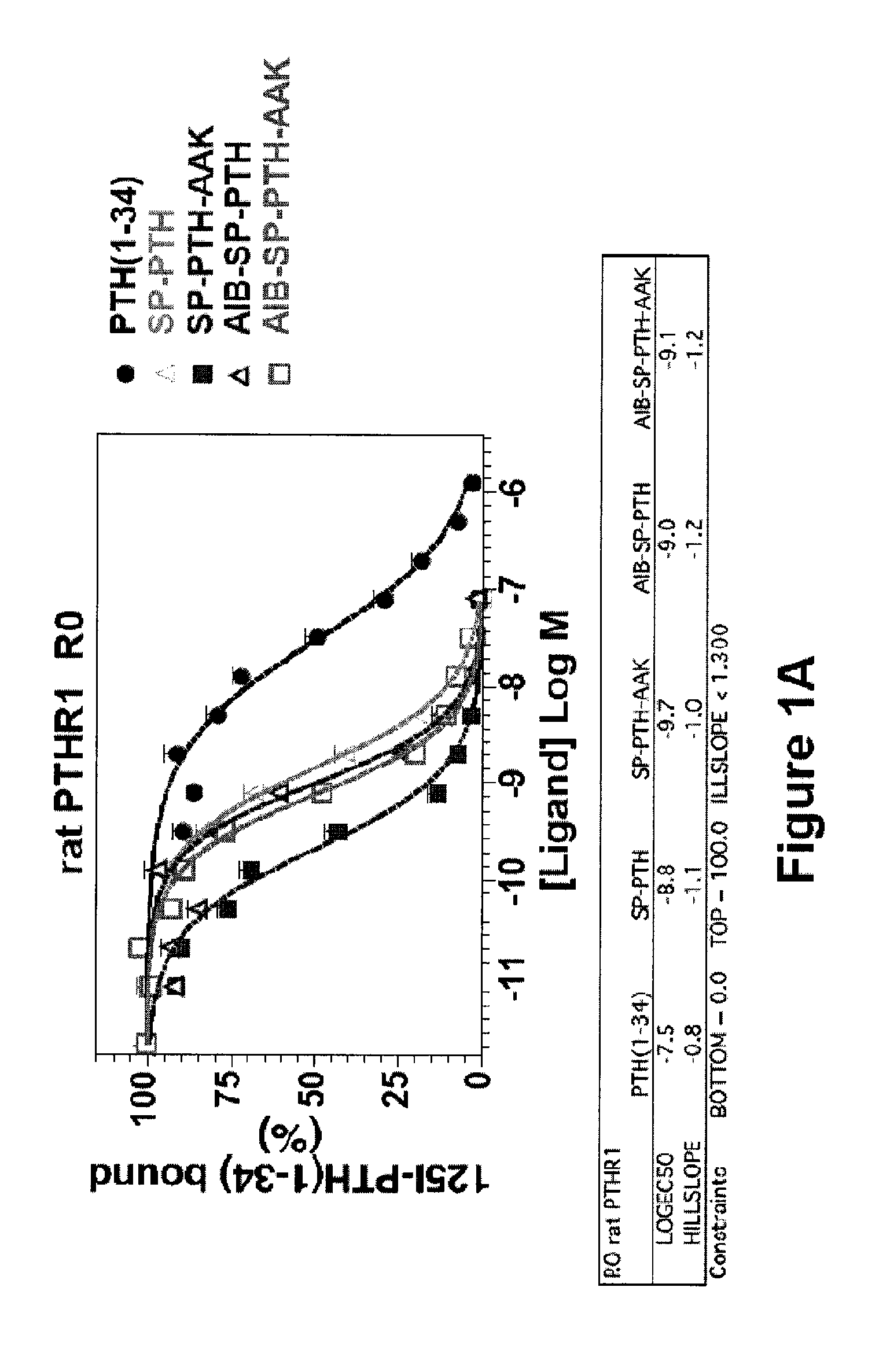
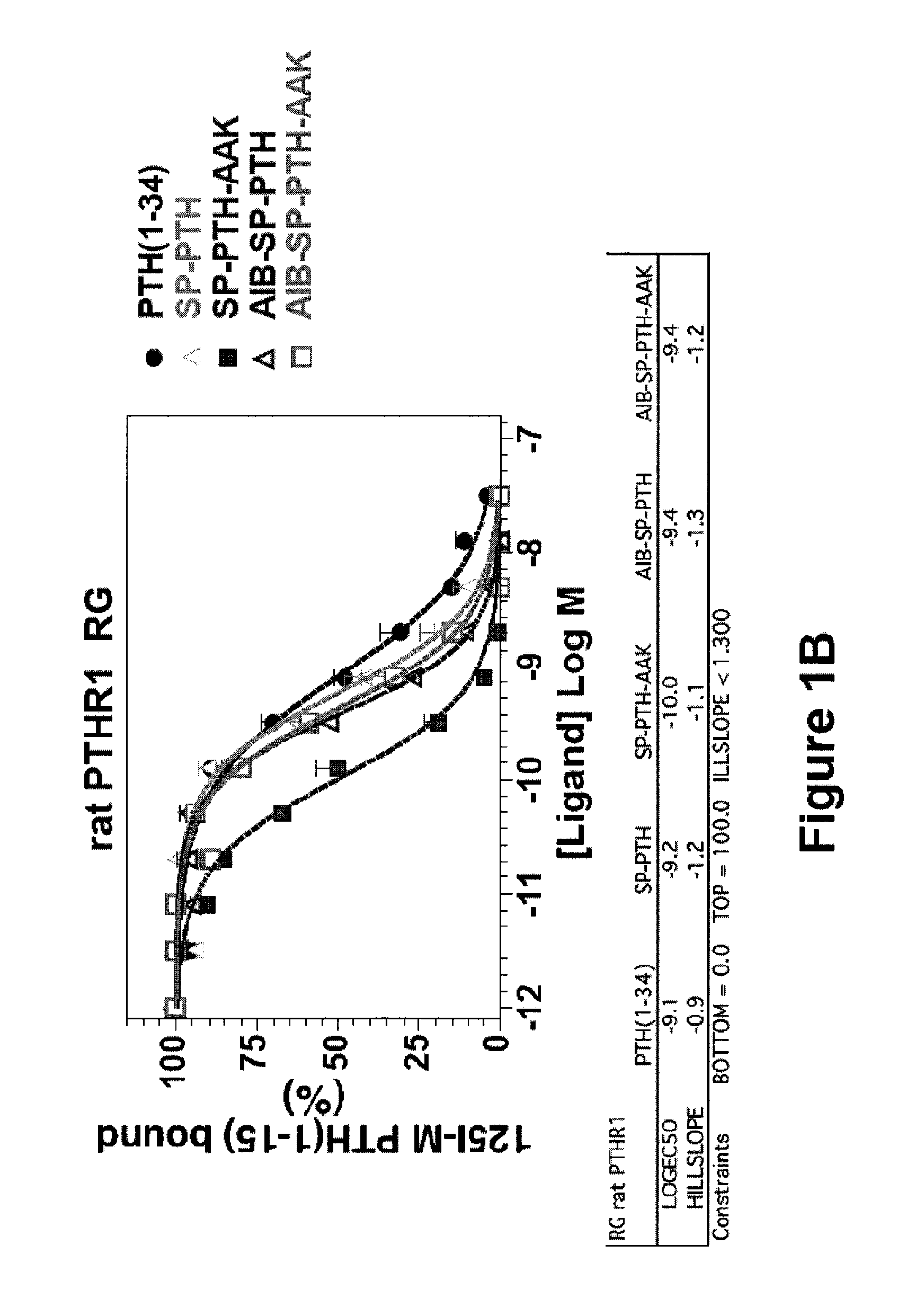
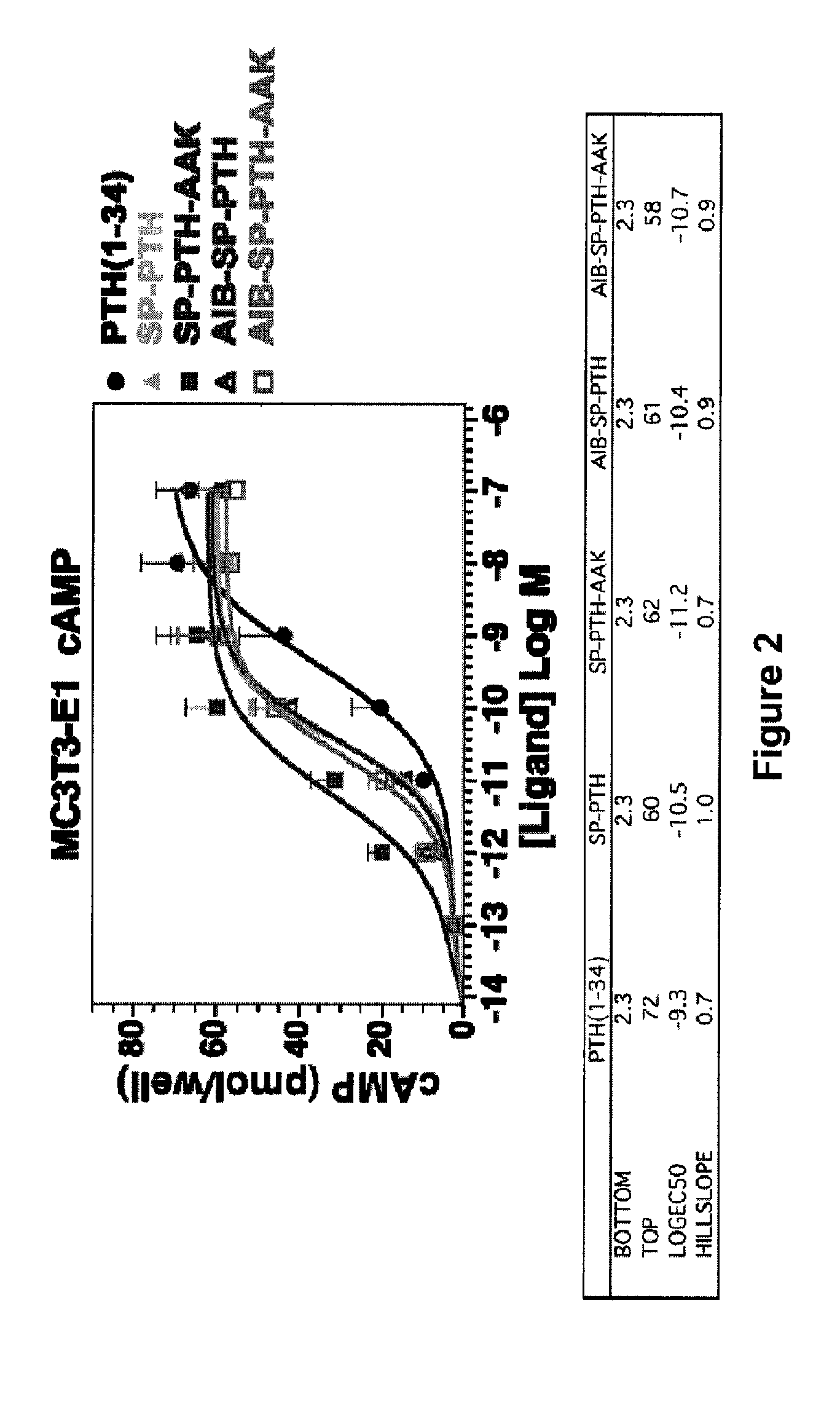
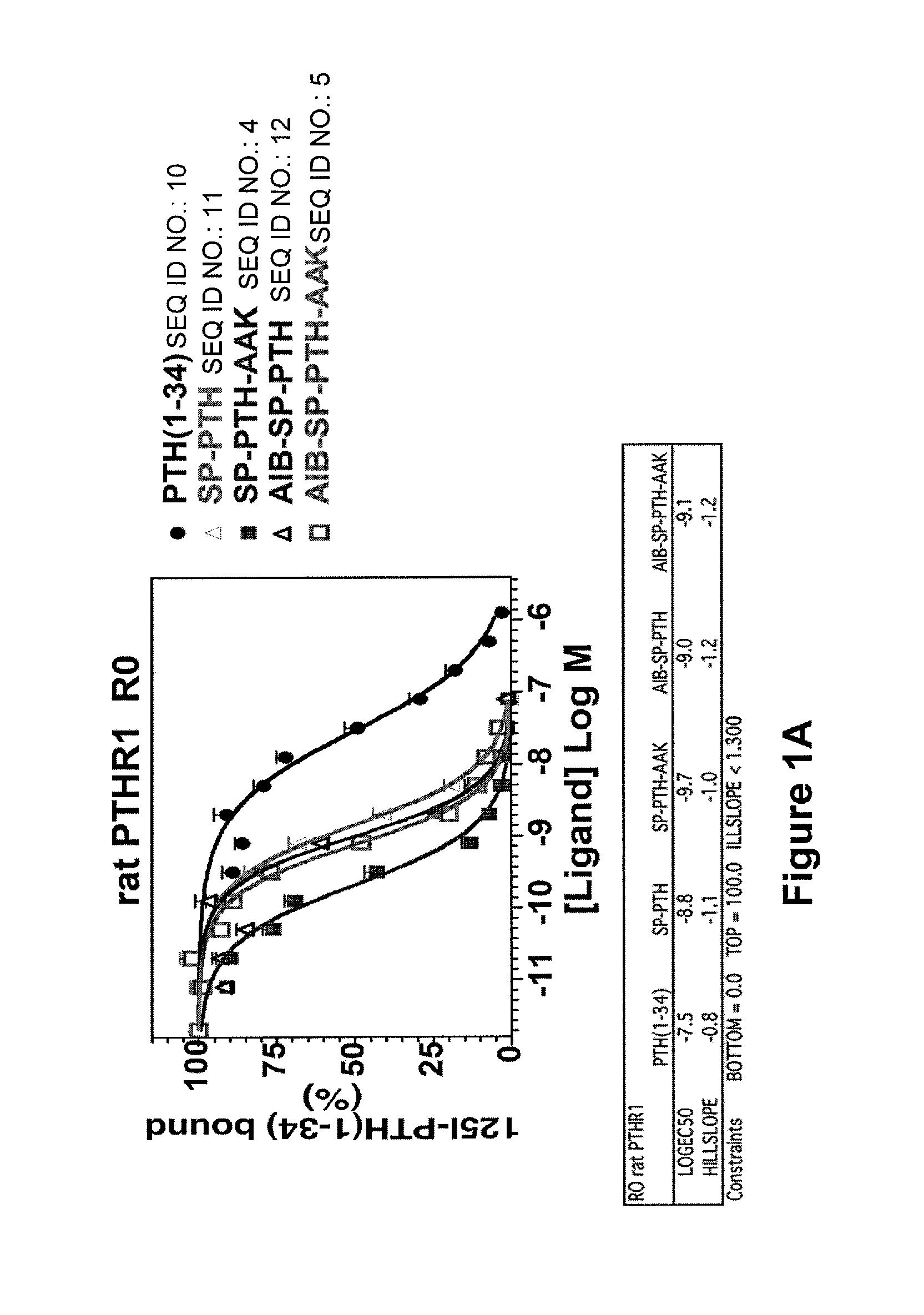
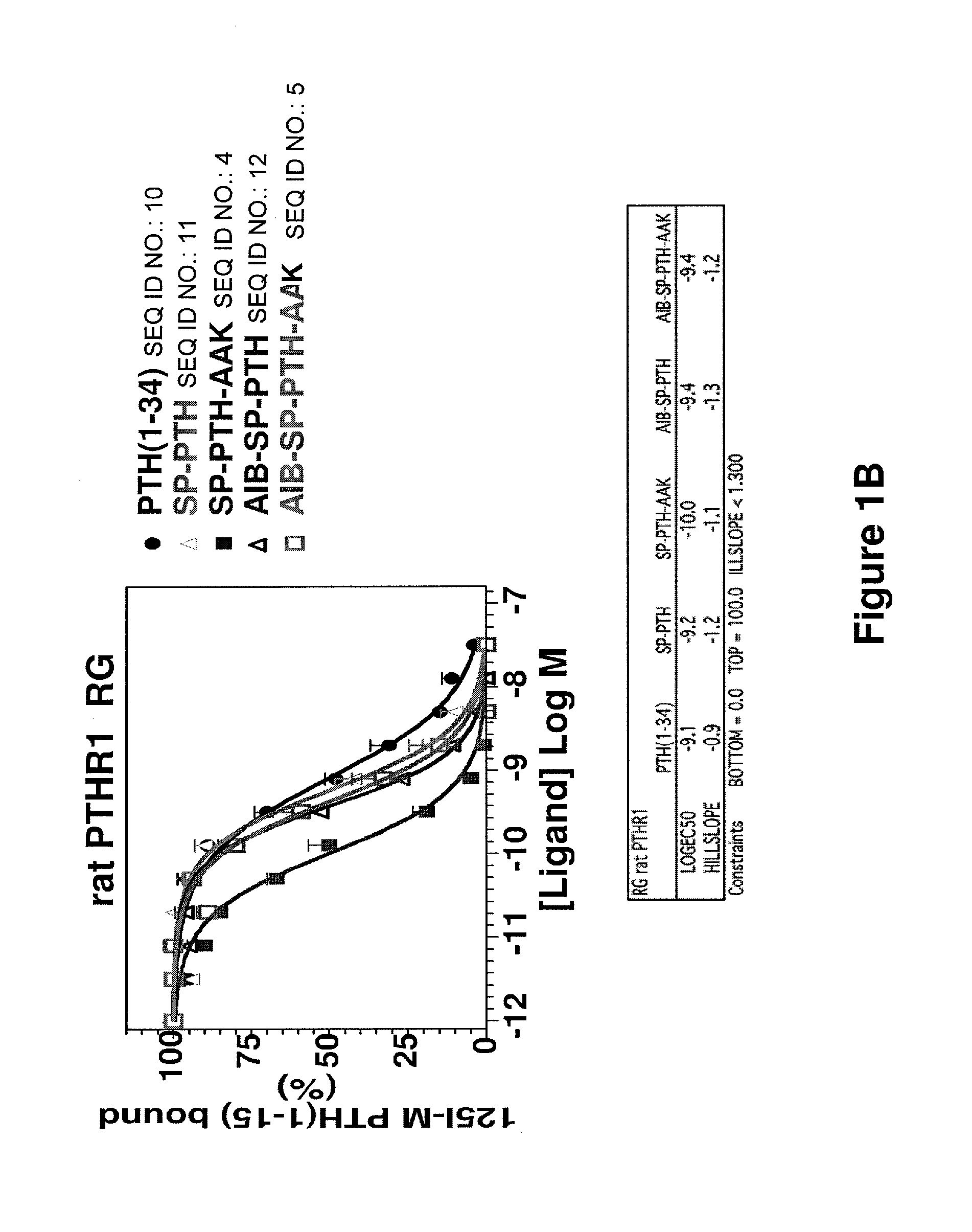
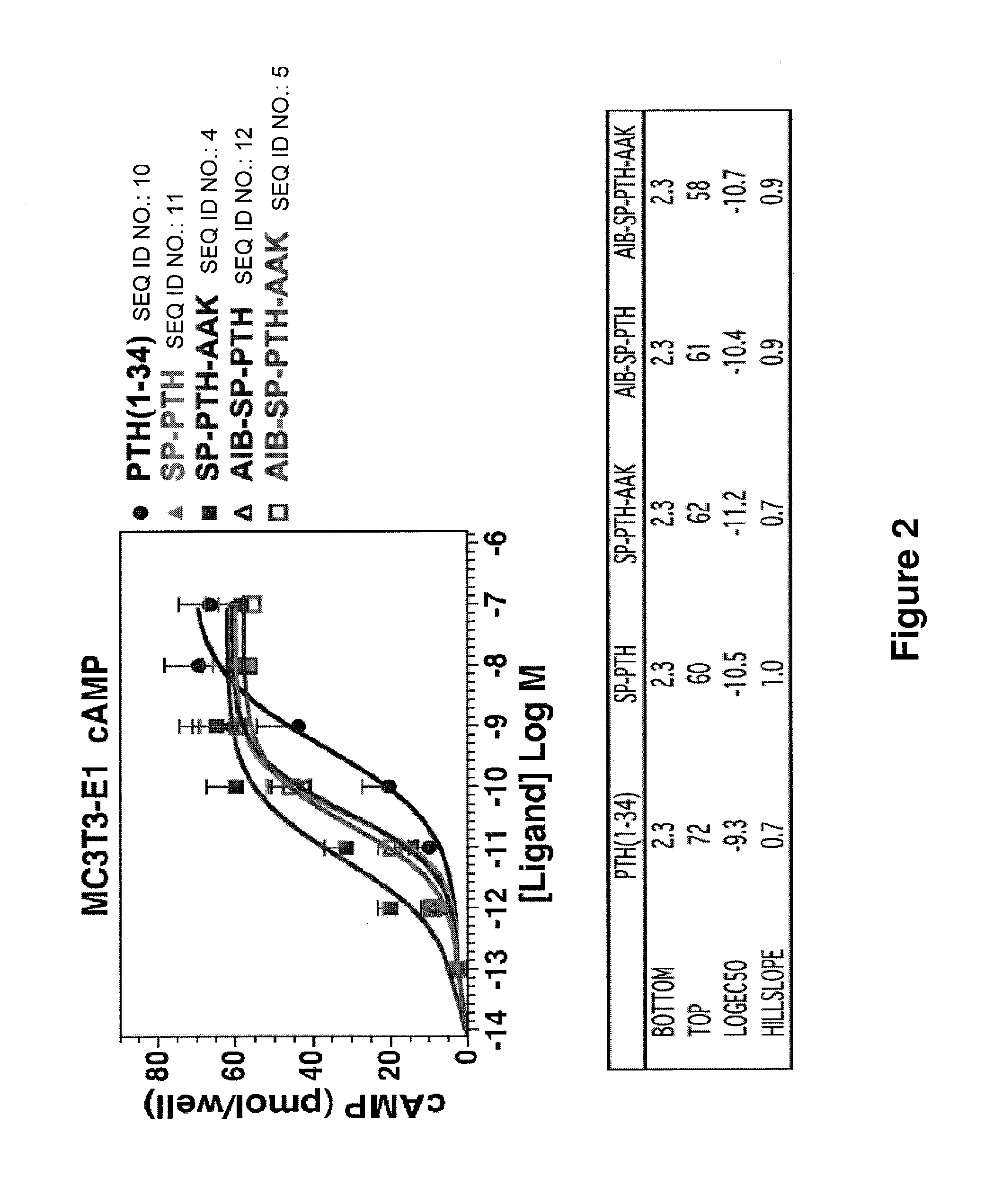
Disclosed are two PTH analog ligands, SP-PTH-AAK and Aib-SP-PTH-AAK, that have long-acting activity at the PTH receptor, as demonstrated both in vitro and in vivo. These polypeptides are thus particularly useful in the treatment of diseases, such as hypoparathyroidism, in which long-acting activity is desired. The method of making the analog polypeptides is also disclosed.
Owner:THE GENERAL HOSPITAL CORP
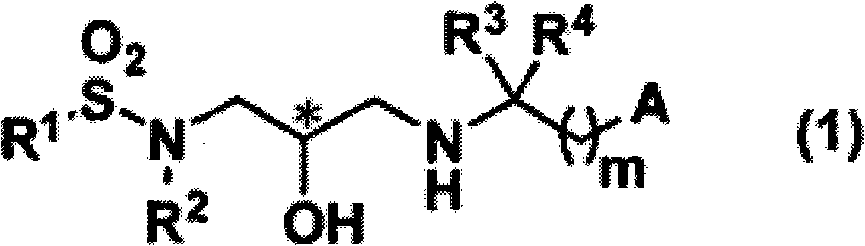
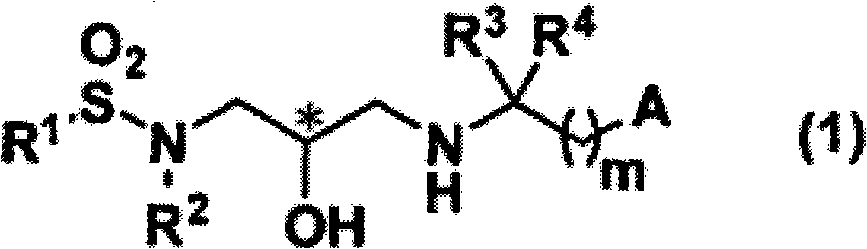
Sulfonamide compound and application thereof
InactiveCN102056897AStrong PTH secretion promoting effectPromote regenerationOrganic chemistryPeptide/protein ingredientsDysostosisMedicine
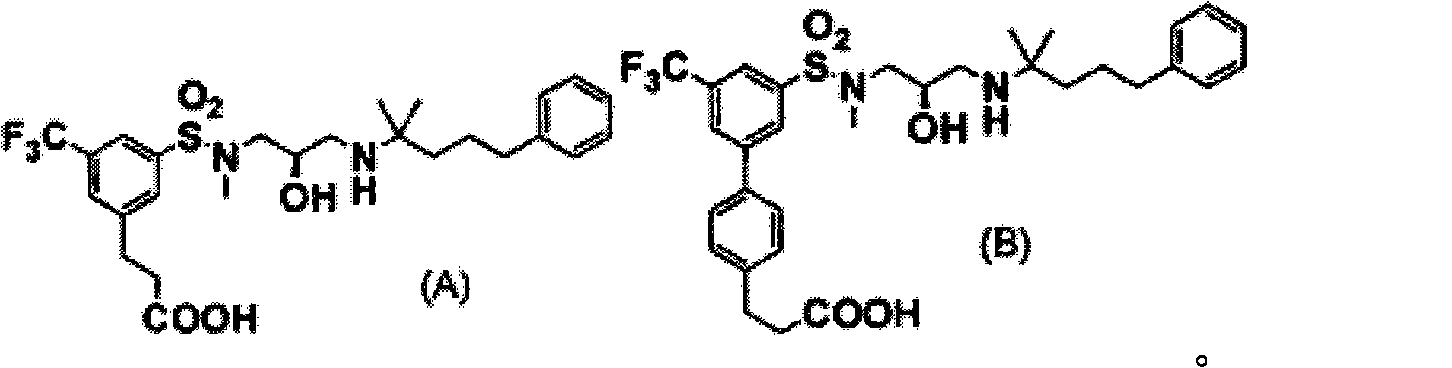
Disclosed is a sulfonamide compound represented by general formula (1) that can be used as an effective component in a CaSR antagonist that is extremely effective in preventing and / or treating bone diseases, such as osteoporosis. Said compound has excellent PTH secretion promoting effects. Said compound is useful as a medically effective component for preventing and / or treating osteoporosis or bone fractures, hypoparathyroidism, or other bone diseases. The general formula (1) is shown in the description.
Owner:ASAHI KASEI PHARMA
Vitamin D3 derivative and treating agent for inflammatory respiratory disease using same
InactiveUS6960573B2Eliminate the effects ofBiocideGroup 4/14 element organic compoundsRespiratory tract diseaseCartilage metabolism
Compounds expressed by the following general formula (1), [wherein, R01 and R02 are each independently a hydrogen atom or a protecting group for a hydroxyl group; Z is one out of the following formulae (1-1), (1-2), (1-3), (1-4) and (1-5)]. The compounds can be used as active ingredients of treating agents for inflammatory respiratory diseases, malignant tumors, rheumatoid arthritis, osteoporosis, diabetes mellitus, hypertension, alopecia, acne, psoriasis, dermatitis, hypercalcemia, hypoparathyroidism and metabolic disorder of cartilage.
Owner:TEIJIN LTD
19-Nor-Vitamin D Analogs with 1,2 or 3,2 Heterocyclic Ring
InactiveUS20070238712A1Preventing and treating obesityInhibits fat cell differentiationOrganic active ingredientsBiocideRenal osteodystrophyVitamin D Analog
19-nor-vitamin D analogs having an additional heterocyclic ring connecting the 3β-oxygen and carbon-2 or the 1α-oxygen and carbon-2 of the A-ring of the analog, and pharmaceutical uses therefore, are described. These compounds exhibit significant activity in mobilization of bone, making them therapeutic agents for the treatment or prophylaxis of osteoporosis, osteomalacia, osteopenia, renal osteodystrophy and hypoparathyroidism.
Owner:WISCONSIN ALUMNI RES FOUND
19-nor-vitamin D analogs with 1,2 or 3,2 heterocyclic ring
19-nor-vitamin D analogs having an additional heterocyclic ring connecting the 3β-oxygen and carbon-2 or the 1α-oxygen and carbon-2 of the A-ring of the analog, and pharmaceutical uses therefore, are described. These compounds exhibit significant activity in mobilization of bone, making them therapeutic agents for the treatment or prophylaxis of osteoporosis, osteomalacia, osteopenia, renal osteodystrophy and hypoparathyroidism.
Owner:WISCONSIN ALUMNI RES FOUND
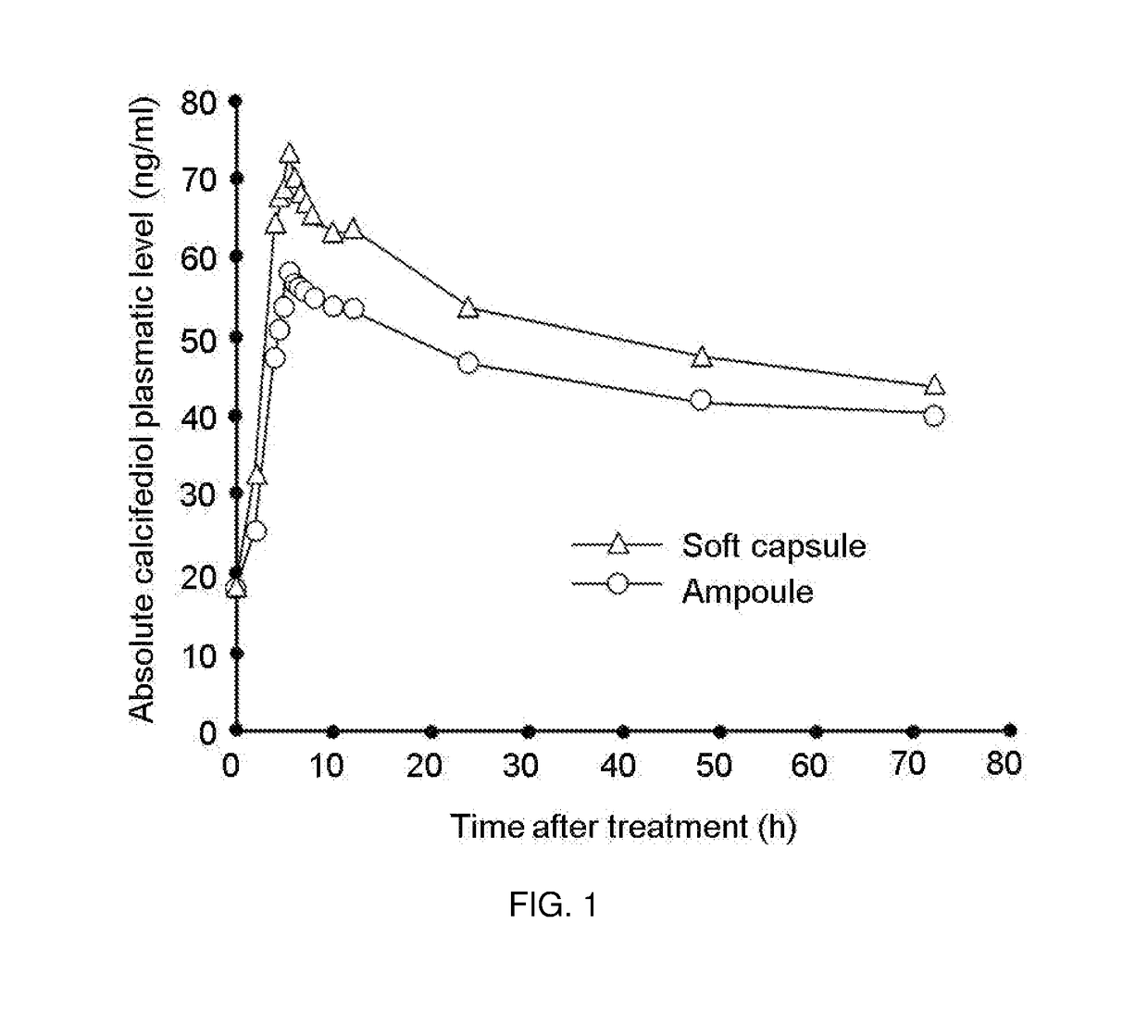
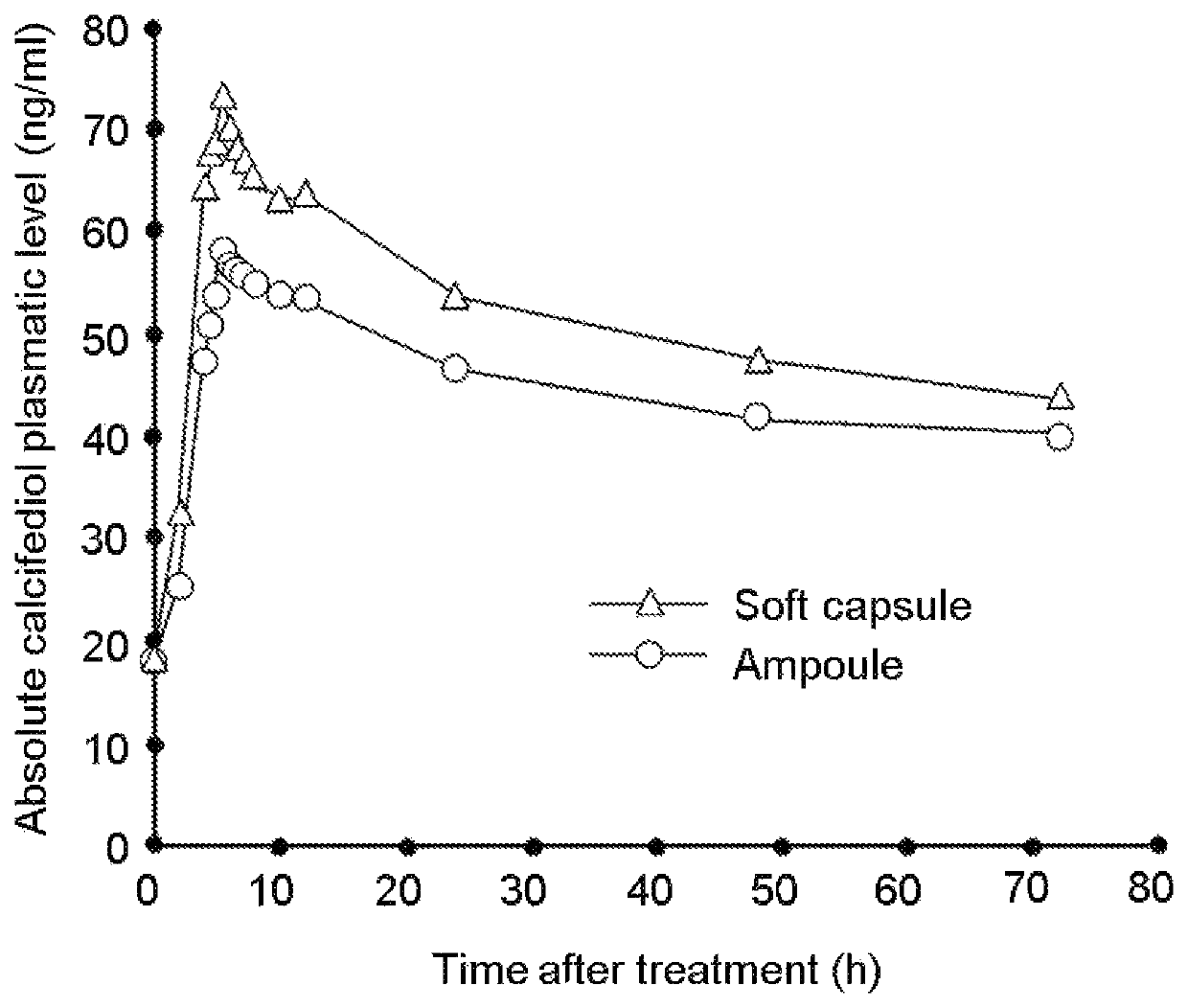
Calcifediol soft capsules
ActiveUS20170348249A1Improve bioavailabilityHydroxy compound active ingredientsAntipyreticRenal osteodystrophyHypophosphatemia
The present invention relates to calcifediol soft capsules, to their use in the treatment or prevention of diseases related to vitamin D deficiency, such as vitamin D deficiency, demineralization such as hypocalcemia and hypophosphatemia, renal osteodystrophy, rickets, osteoporosis, osteopenia, osteoarthritis, osteoarthrosis, osteomalacia, hypoparathyroidism, and inflammatory bowel disease, and to their process of manufacture.
Owner:FAES FARMA SA
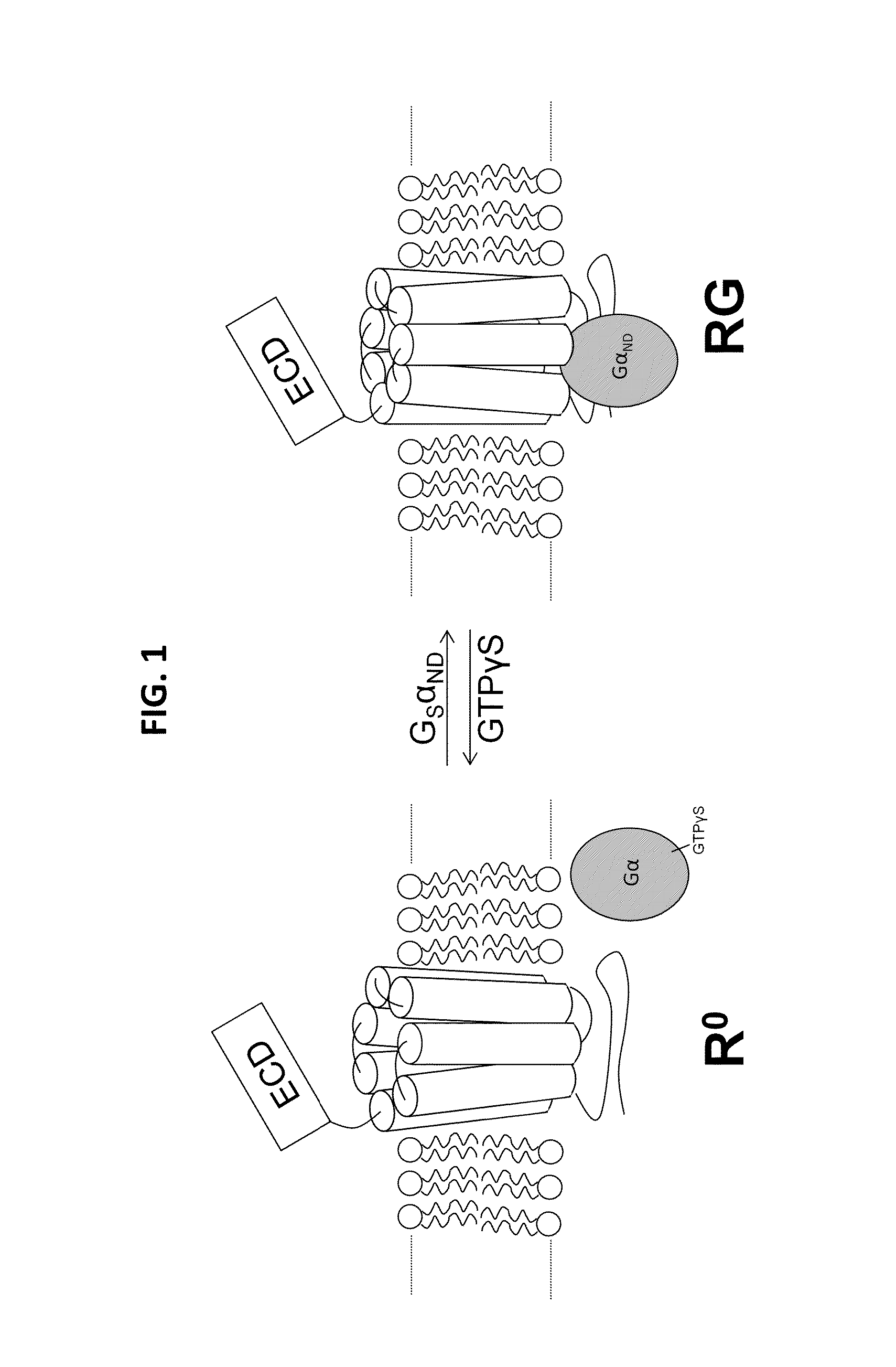
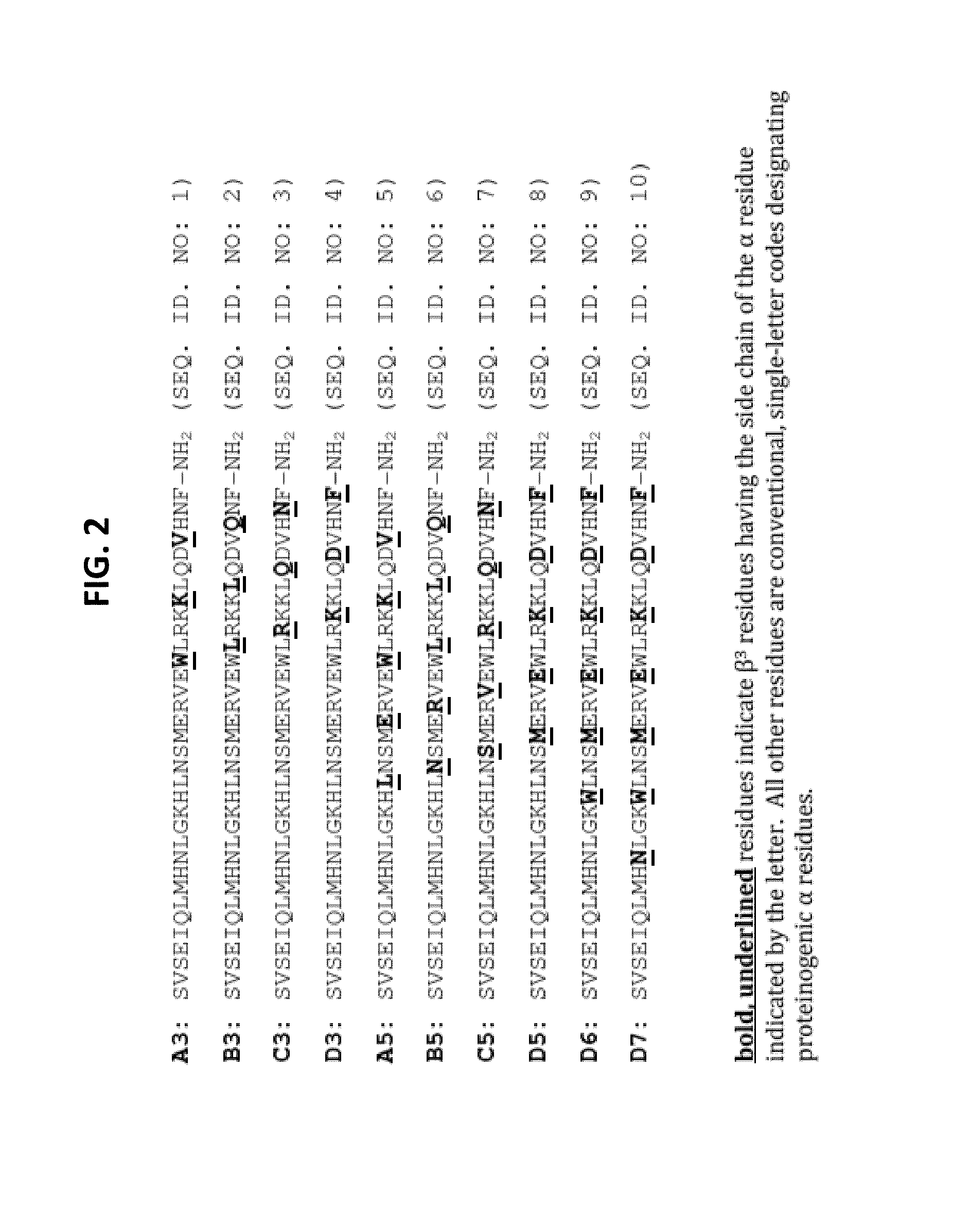
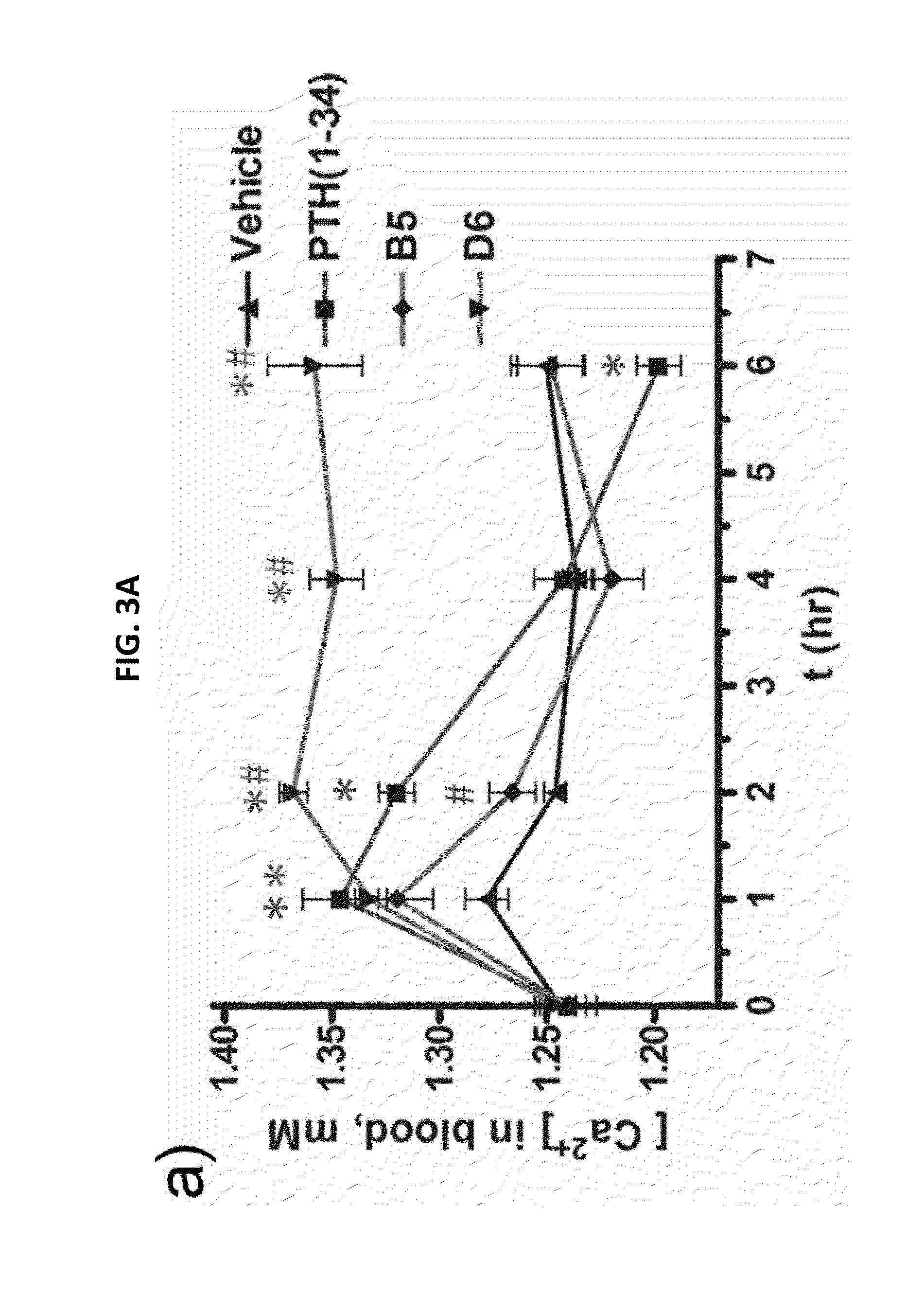
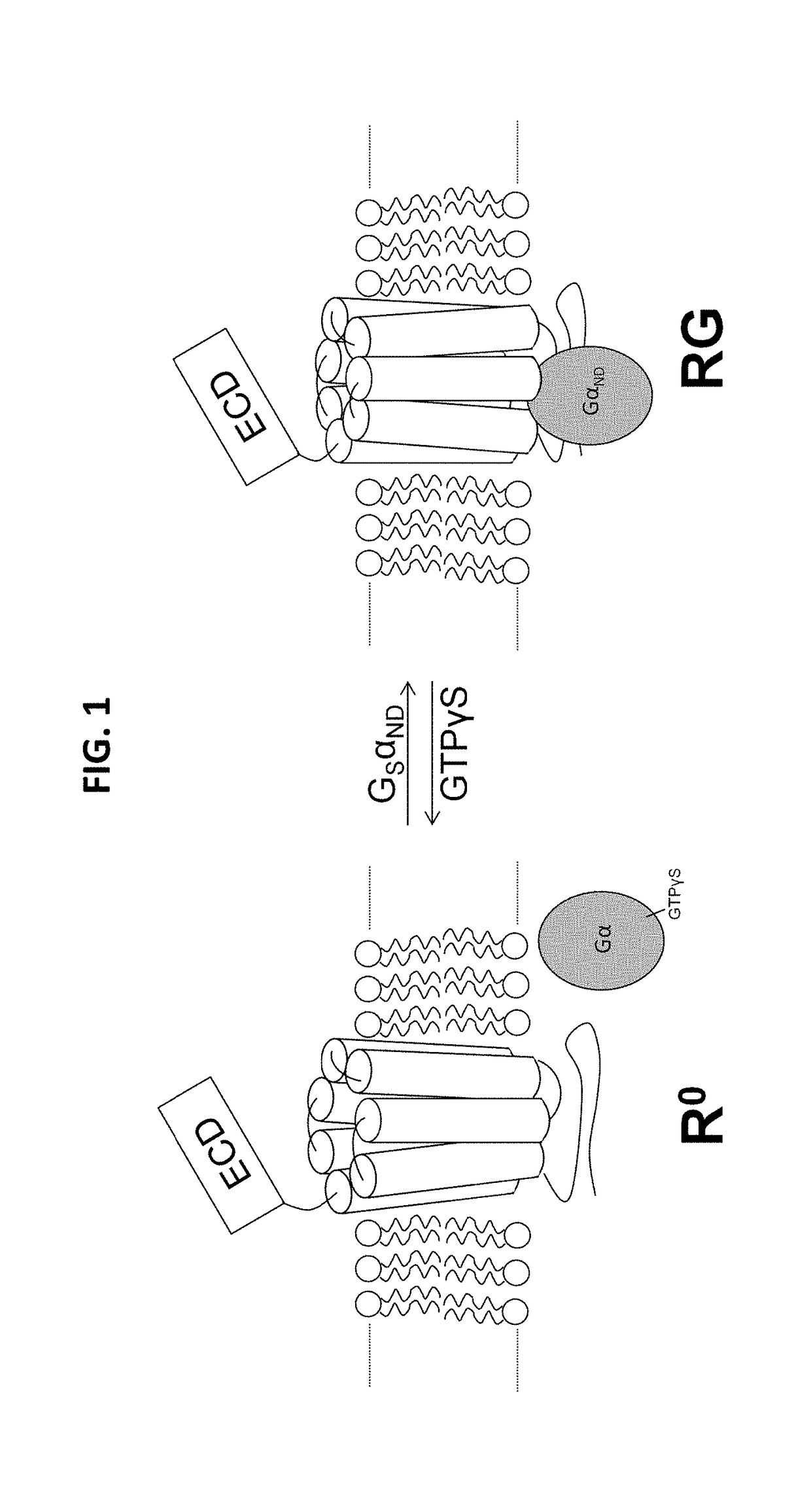
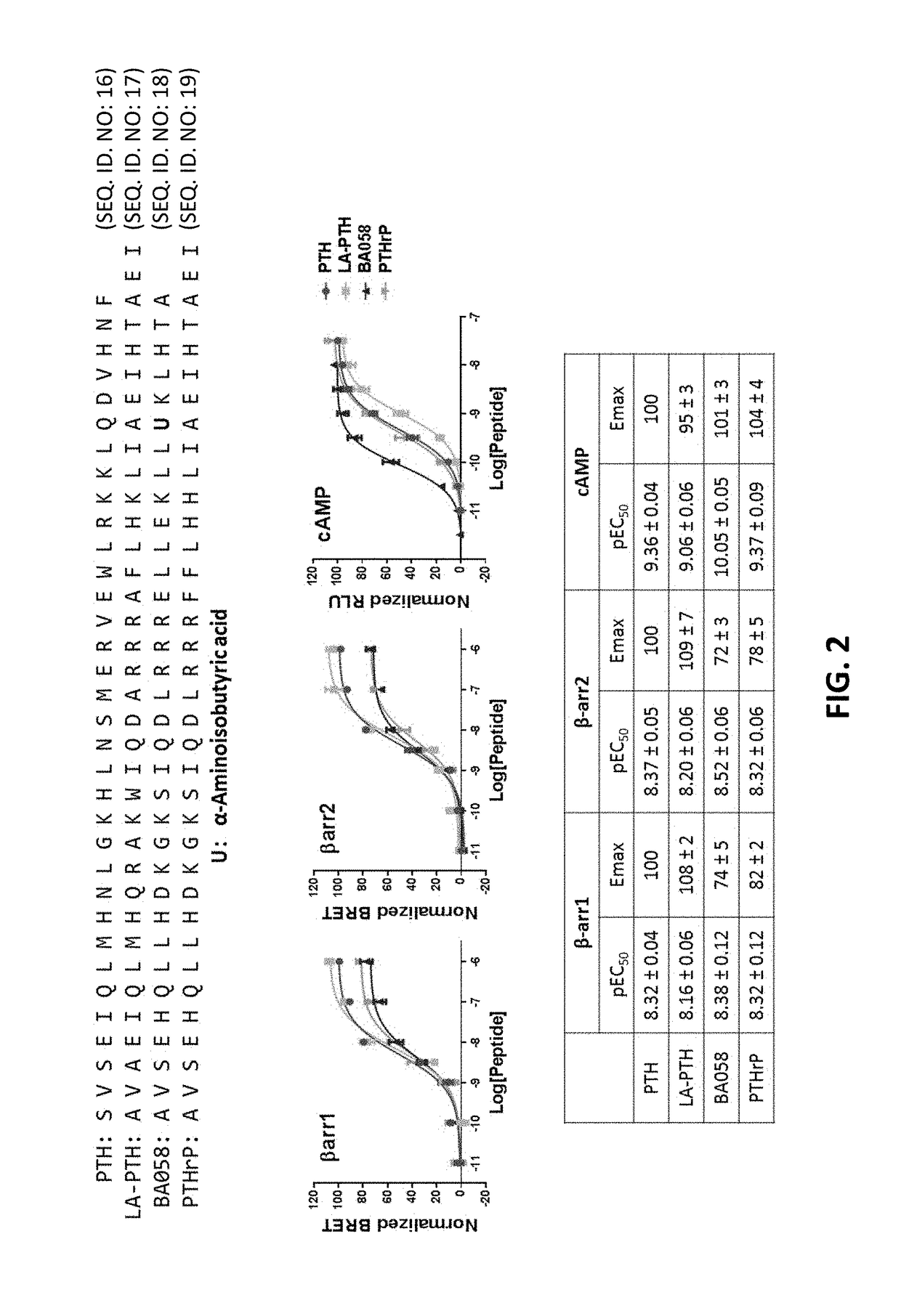
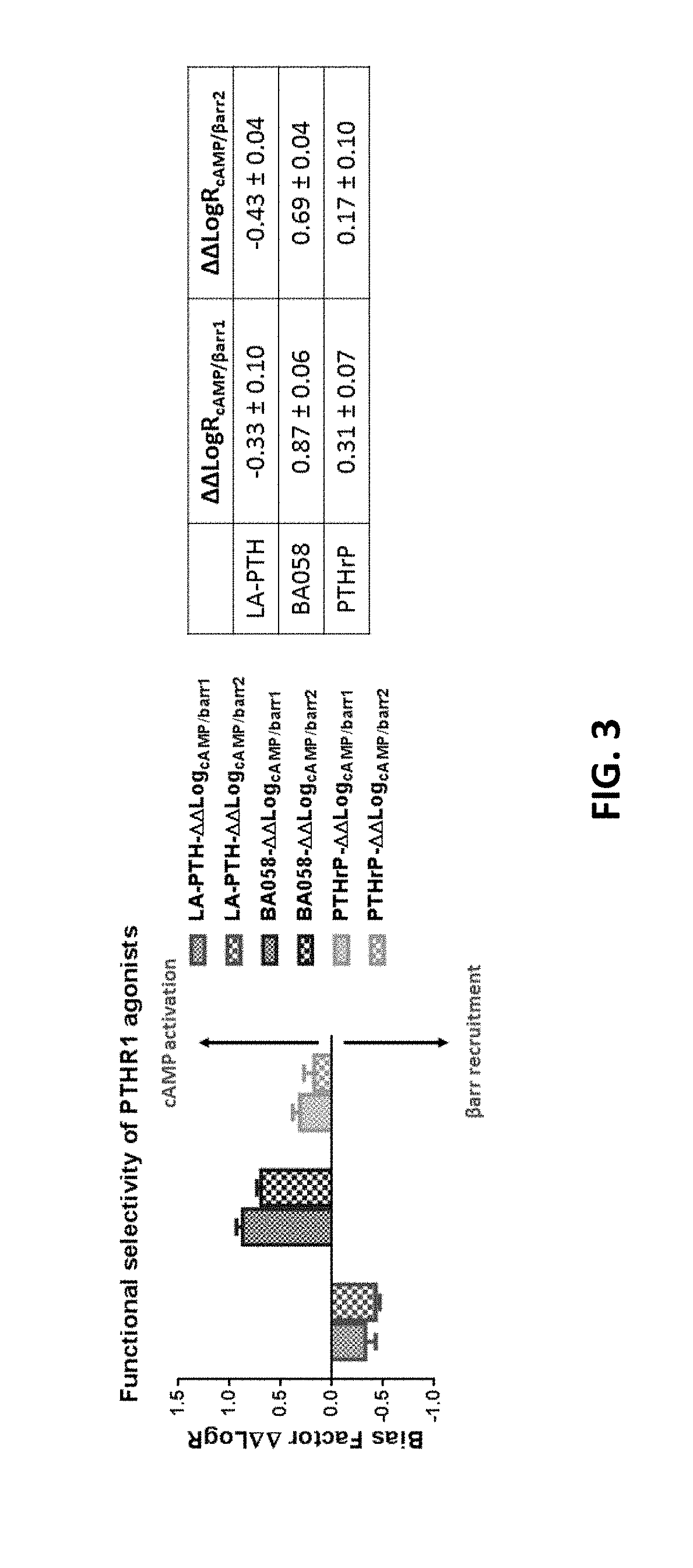
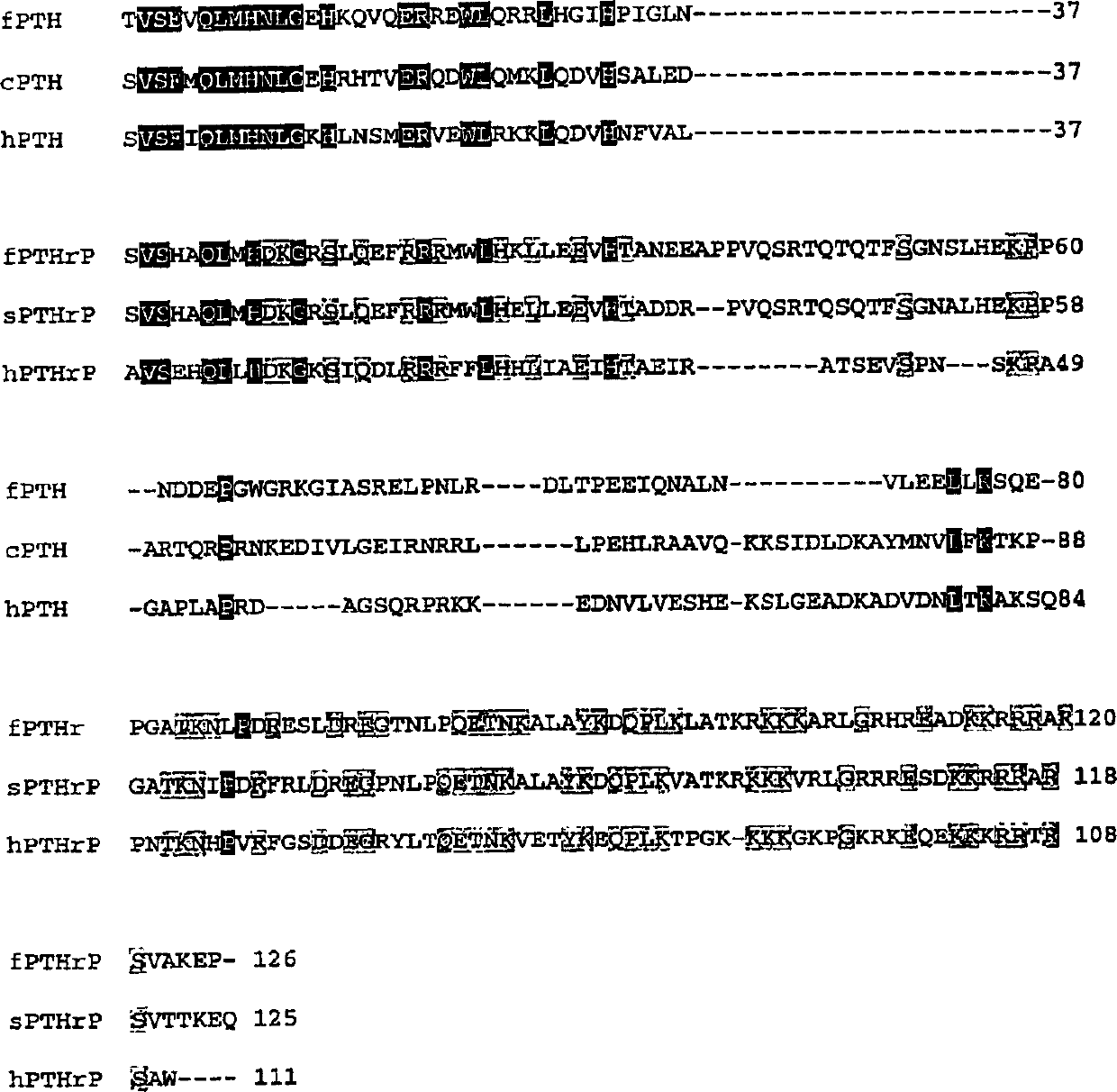
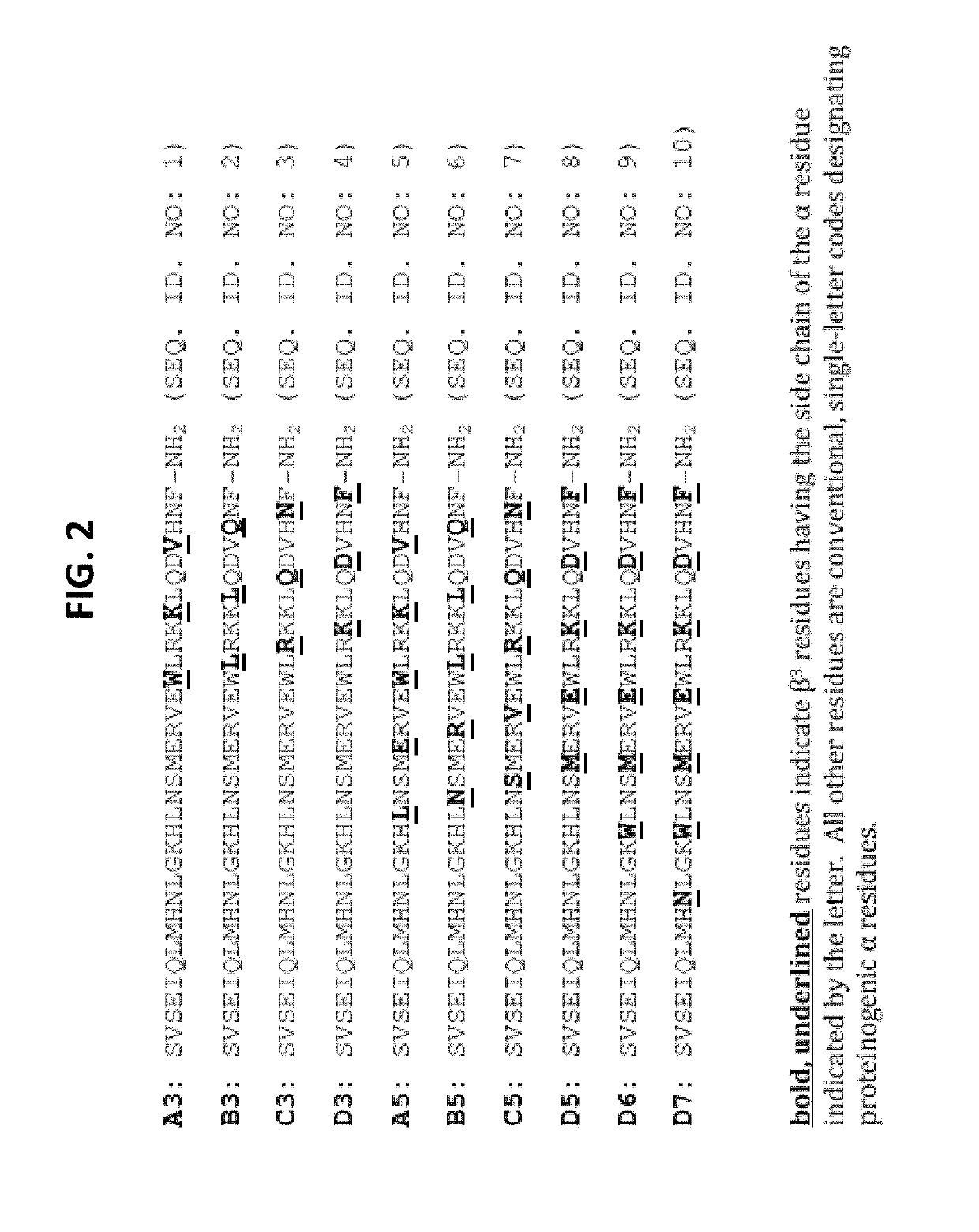
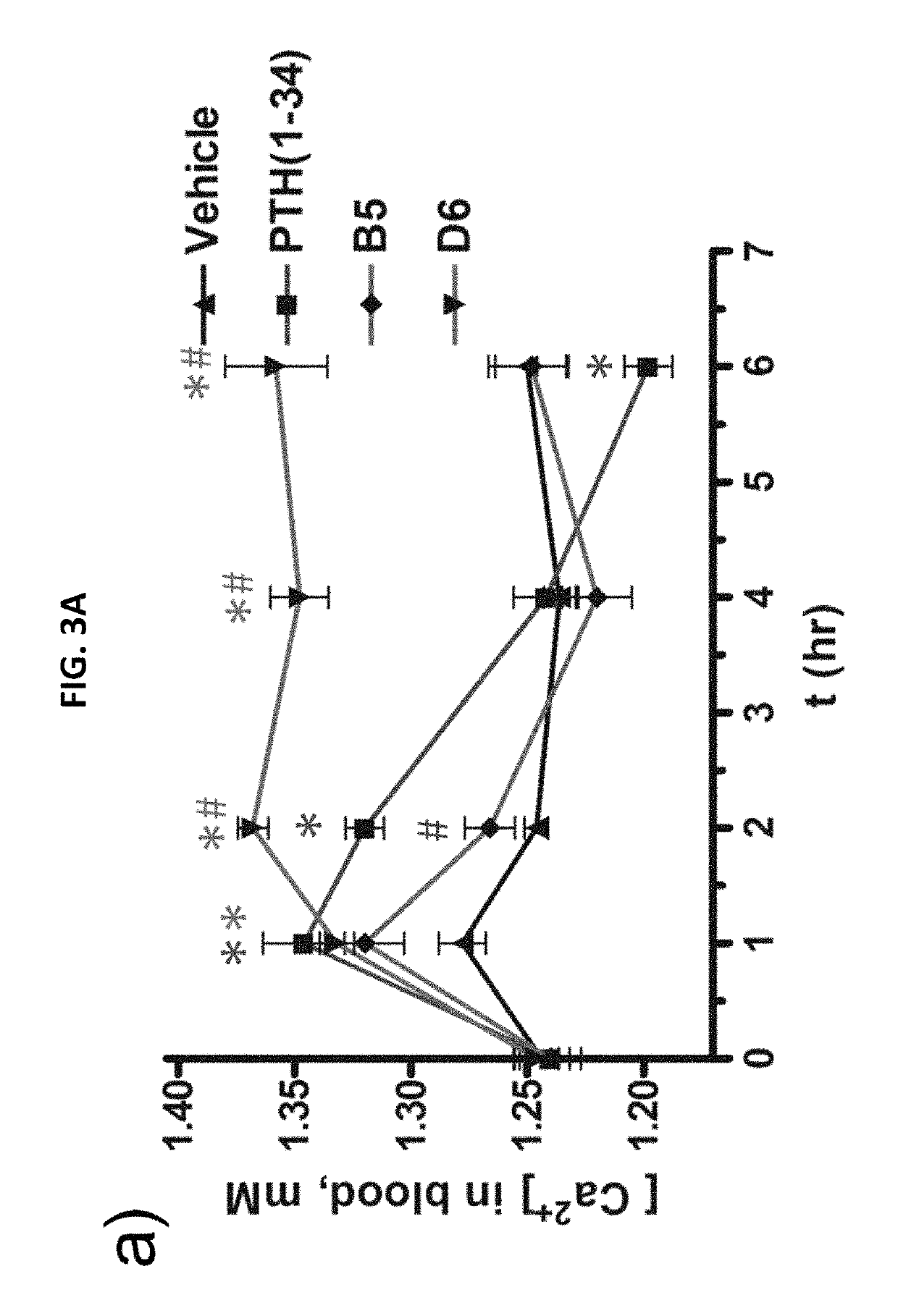
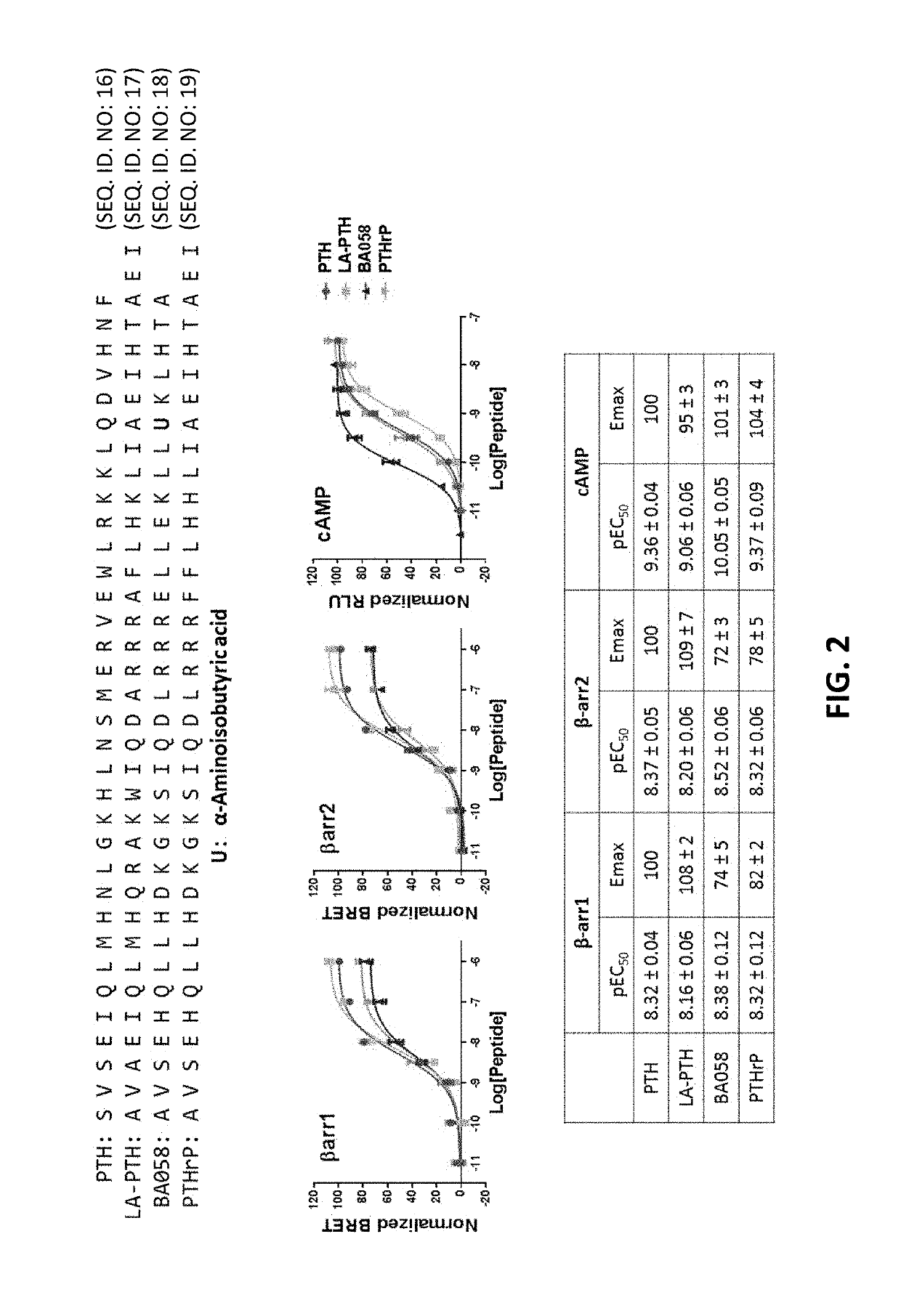
Alpha-/beta-polypeptide analogs of parathyroid hormone (PTH) and method of using same
ActiveUS20140378382A1Altered selectivityPeptide/protein ingredientsDepsipeptidesParathyroid hormoneHypoparathyroidism
Described are polypeptide analogs of parathyroid hormone (PTH) that include at least two non-adjacent β-amino acid residues in place of a naturally occurring α-amino acid residues. Also described are pharmaceutical compositions useful for treating hypoparathyroidism that contain the analogs and methods of using the analogs to treat hypoparathyroidism.
Owner:THE GENERAL HOSPITAL CORP +1
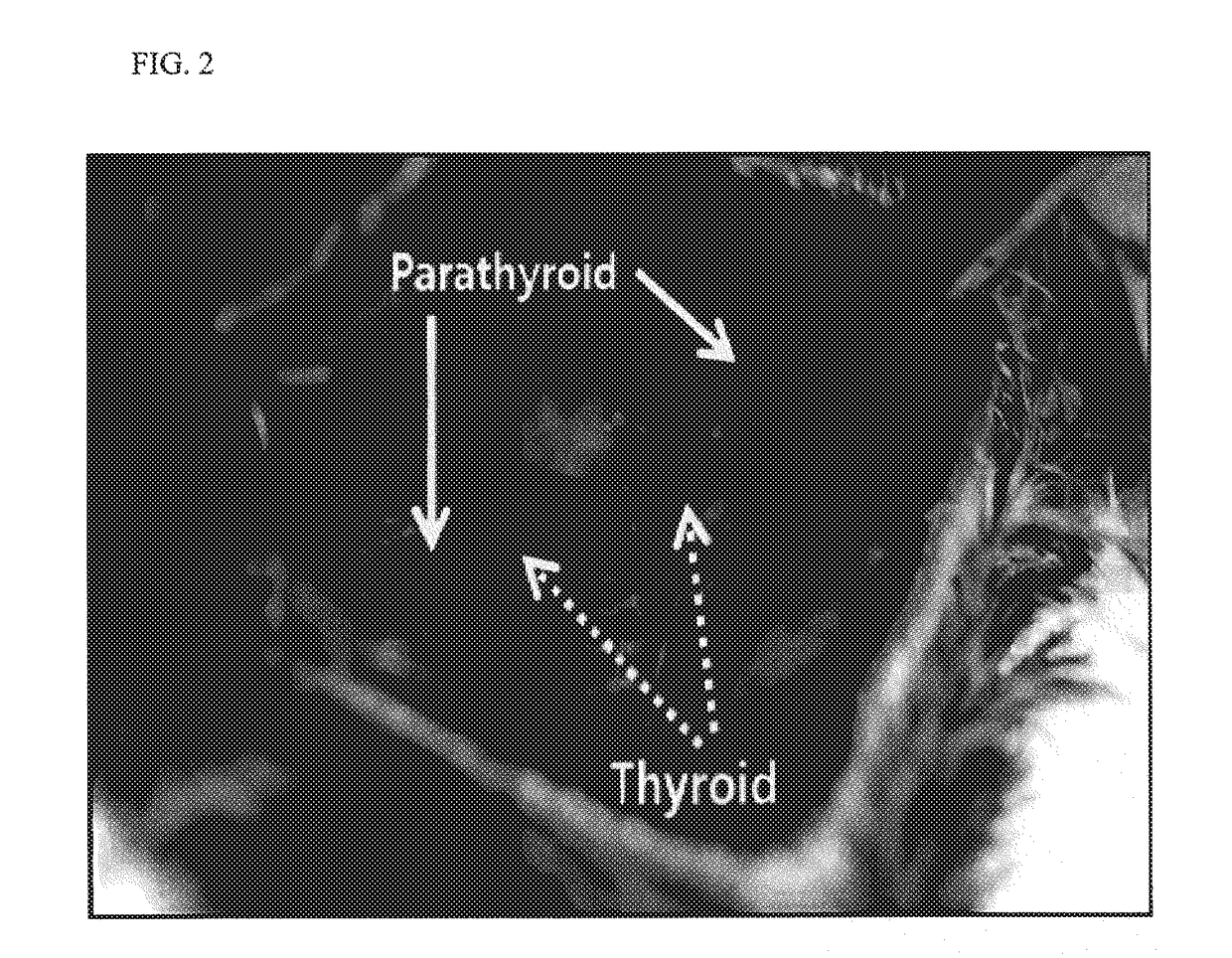
Traditional Chinese medicine composition for treating blood stasis and lusterless face type hypoparathyroidism
InactiveCN104800564ASafe and effective treatmentNo significant differenceEndocrine system disorderPlant ingredientsLimoniumLudwigia repens
The invention discloses a traditional Chinese medicine composition for treating blood stasis and lusterless face type hypoparathyroidism. The traditional Chinese medicine composition is characterized by being prepared from the following medicinal materials in parts by weight: 20 parts of southern Yunnan satyrium nepalense, 22 parts of oreas artina, 12 parts of caulis polygoni multiflori, 12 parts of arillus longan, 30 parts of mucuna birdwoodiana, 20 parts of melodinus hemsleyanus root, 25 parts of limonium bicolor, 8 parts of colla corii asini, 6 parts of calathodes oxycarpa sprague, 8 parts of herba aristolochiae, 12 parts of gerbera piloselloides cass, 6 parts of valeriana officinalis, 10 parts of broadleaf sedge root, 24 parts of ludwigia repens, 10 parts of marrubium vulgare, 12 parts of balanophora polyandra griff and 20 parts of stellaria yunnanensis. The traditional Chinese medicine composition provided by the invention is used for treating blood stasis and lusterless face type hypoparathyroidism, is high in effective rate, reliable in curative effect and has no recrudescence after the hypoparathyroidism is cured.
Owner:GEN HOSPITAL OF JINAN MILITARY REGION PLA
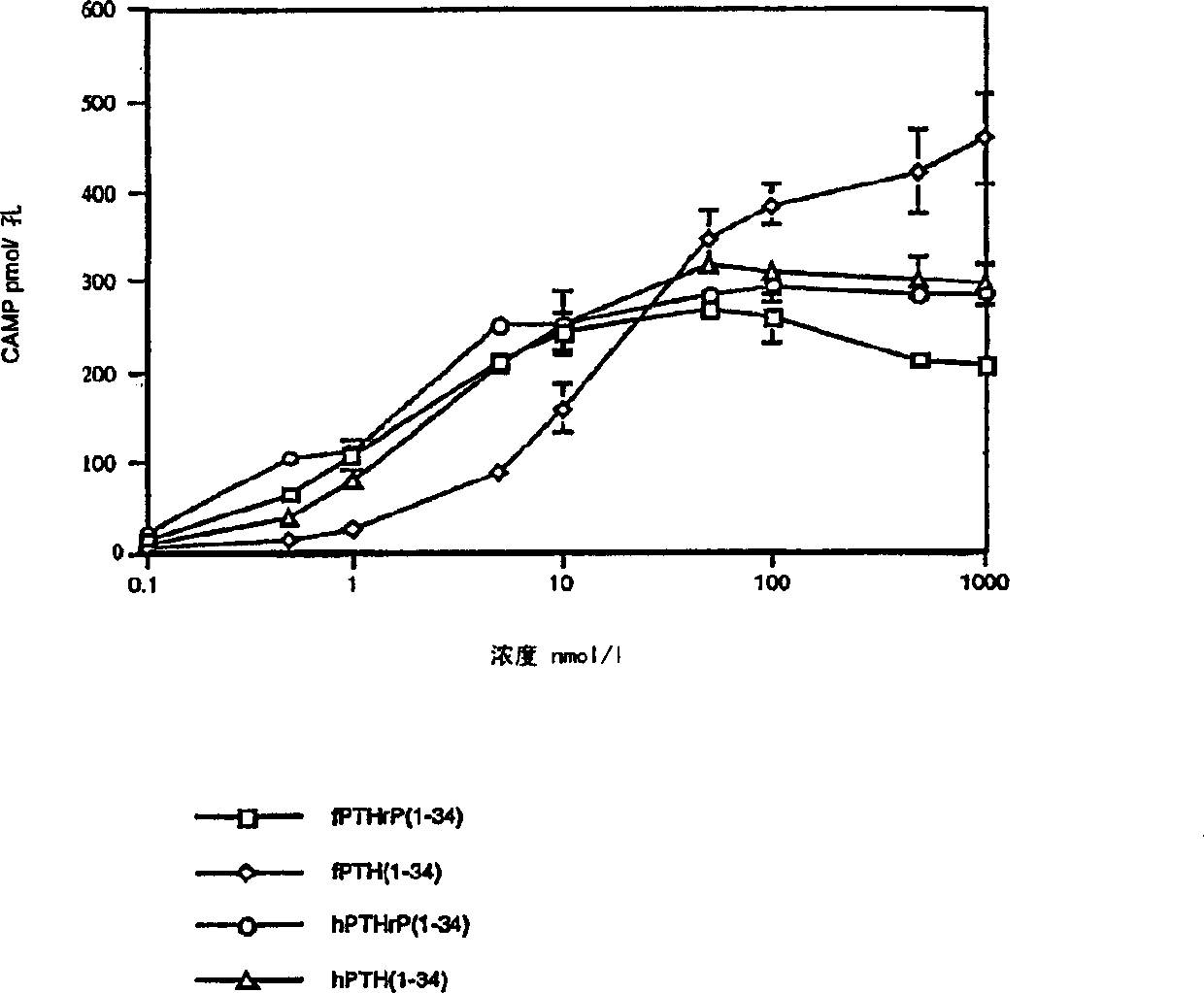
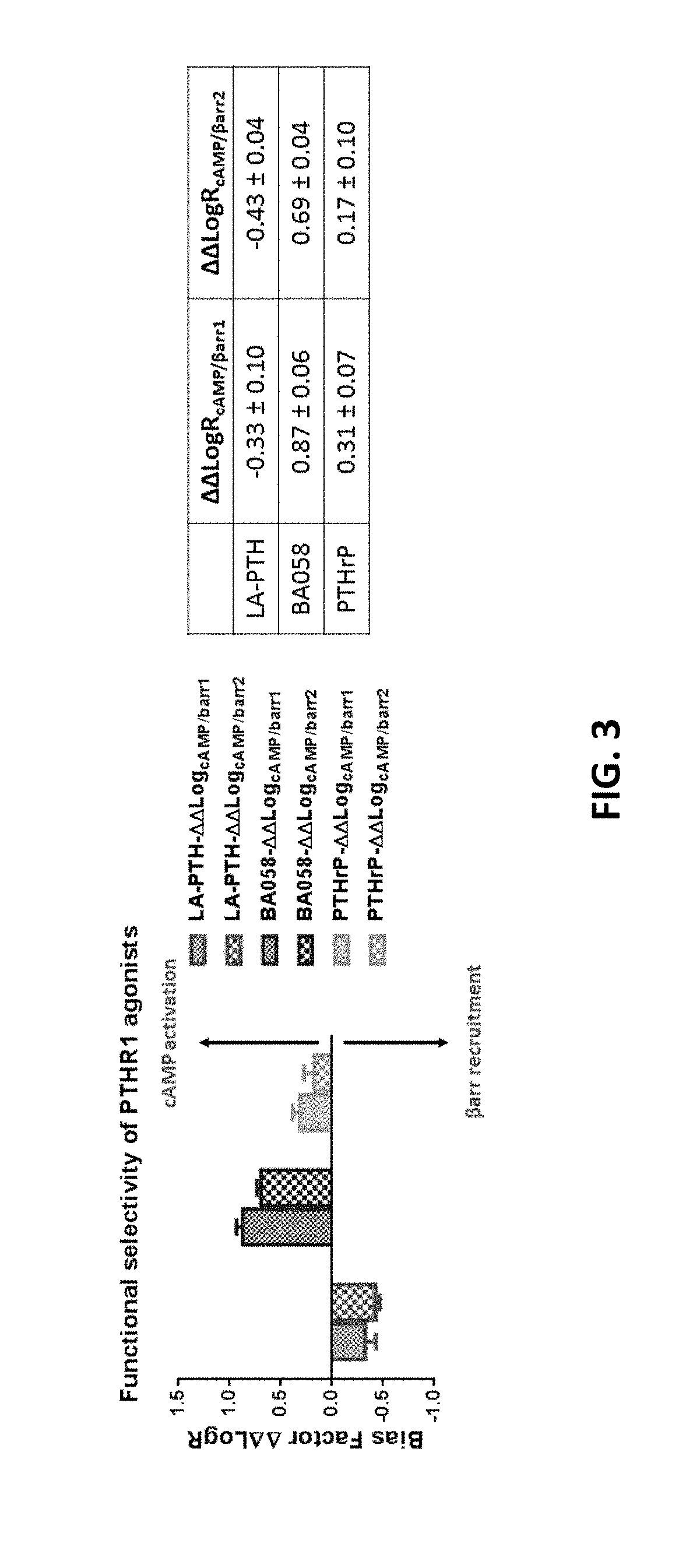
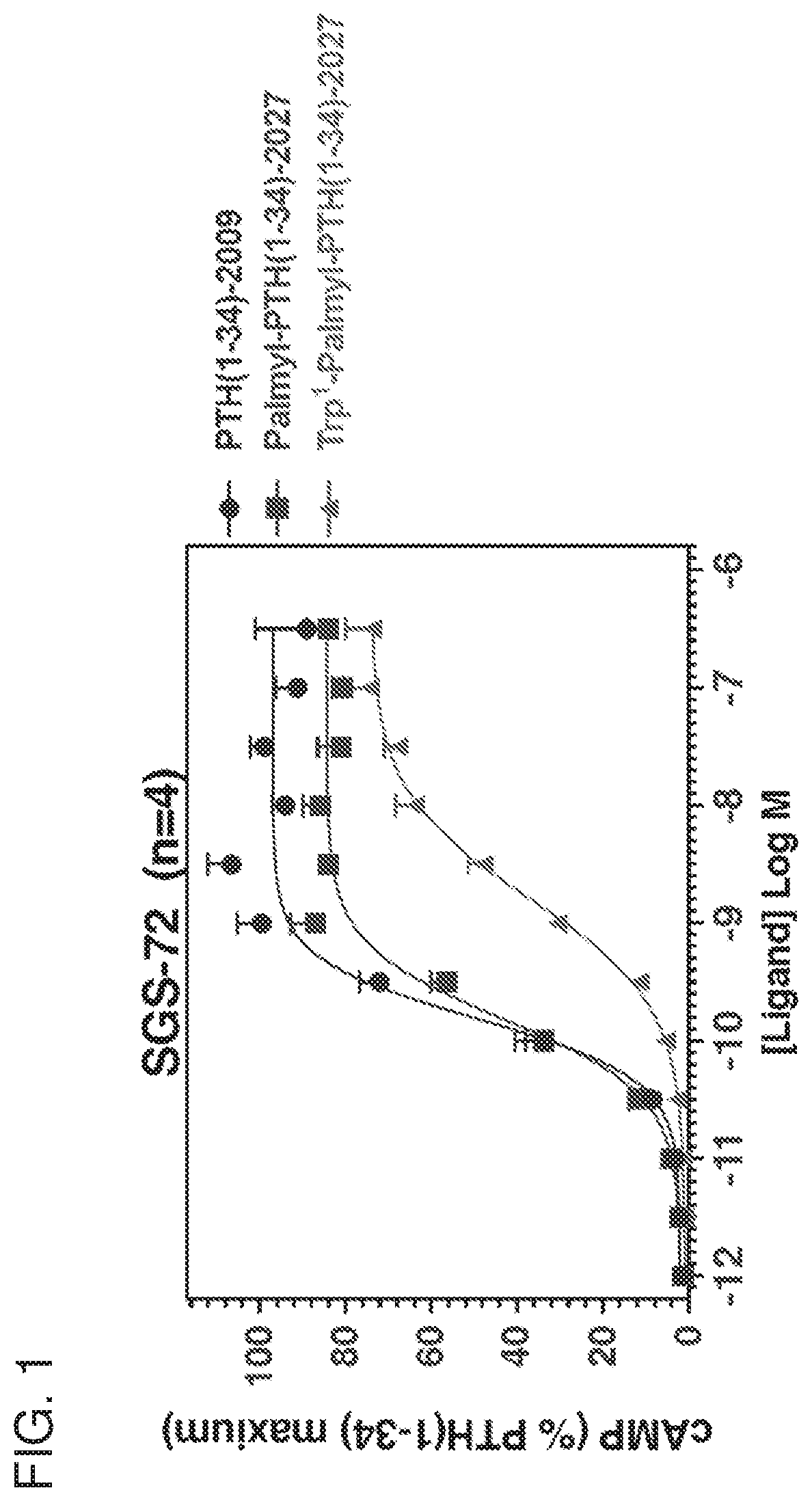
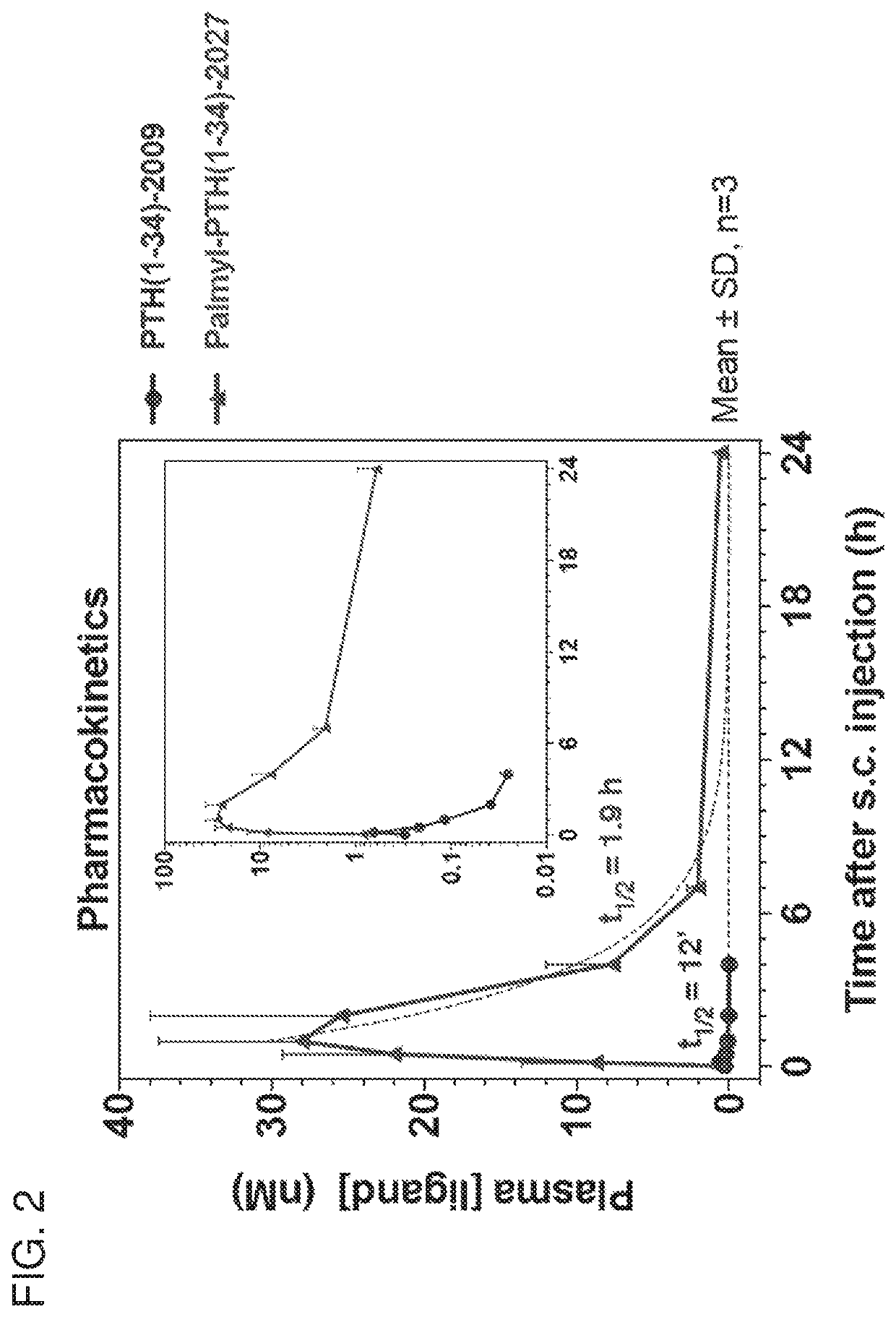
Analogues of parathyroid hormone (1-34) that function as agonists of the parathyroid hormone receptor-1 and display modified activity profiles
InactiveUS20190062397A1Peptide/protein ingredientsParathyroid hormonesAmino acid substitutionAgonist
Described are polypeptide analogs of parathyroid hormone (PTH) that include an unnatural amino acid substitution at positions 7 or 8 from the N-terminus of the polypeptide. Also described are pharmaceutical compositions useful for treating hypoparathyroidism that contain the analogs and methods of using the analogs to treat hypoparathyroidism.
Owner:WISCONSIN ALUMNI RES FOUND
Parathyroid hormone-like polypeptides
Novel parathyroid hormone polypeptides and biologically active fragments thereof are disclosed along with nucleic acid molecules encoding same. In particular, parathyroid hormone polypeptides and biologically active fragments (and encoding nucleic acid molecules) derived from fish species (eg Japanese pufferfish (Fugu rubripes)) are disclosed. Such polypeptides and fragments may be used for treatment of diseases associated with abnormal calcium homeostasis (eg osteoporosis, osteopenia, Paget's disease, bone cancer, hyperparathyroidism, hypoparathyroidism, hypercalcemia, psoriasis and other skin-related conditions).
Owner:TEELEOSTIN LTD
Calcifediol soft capsules
ActiveUS10525018B2Improve bioavailabilityHydroxy compound active ingredientsAntipyreticJoint diseaseOsteo arthritis
The present invention relates to calcifediol soft capsules, to their use in the treatment or prevention of diseases related to vitamin D deficiency, such as vitamin D deficiency, demineralization such as hypocalcemia and hypophosphatemia, renal osteodystrophy, rickets, osteoporosis, osteopenia, osteoarthritis, osteoarthrosis, osteomalacia, hypoparathyroidism, and inflammatory bowel disease, and to their process of manufacture.
Owner:FAES FARMA SA
Alpha-/beta-polypeptide analogs of parathyroid hormone (PTH) and method of using same
Described are polypeptide analogs of parathyroid hormone (PTH) that include at least two non-adjacent β-amino acid residues in place of a naturally occurring α-amino acid residues. Also described are pharmaceutical compositions useful for treating hypoparathyroidism that contain the analogs and methods of using the analogs to treat hypoparathyroidism.
Owner:THE GENERAL HOSPITAL CORP +1
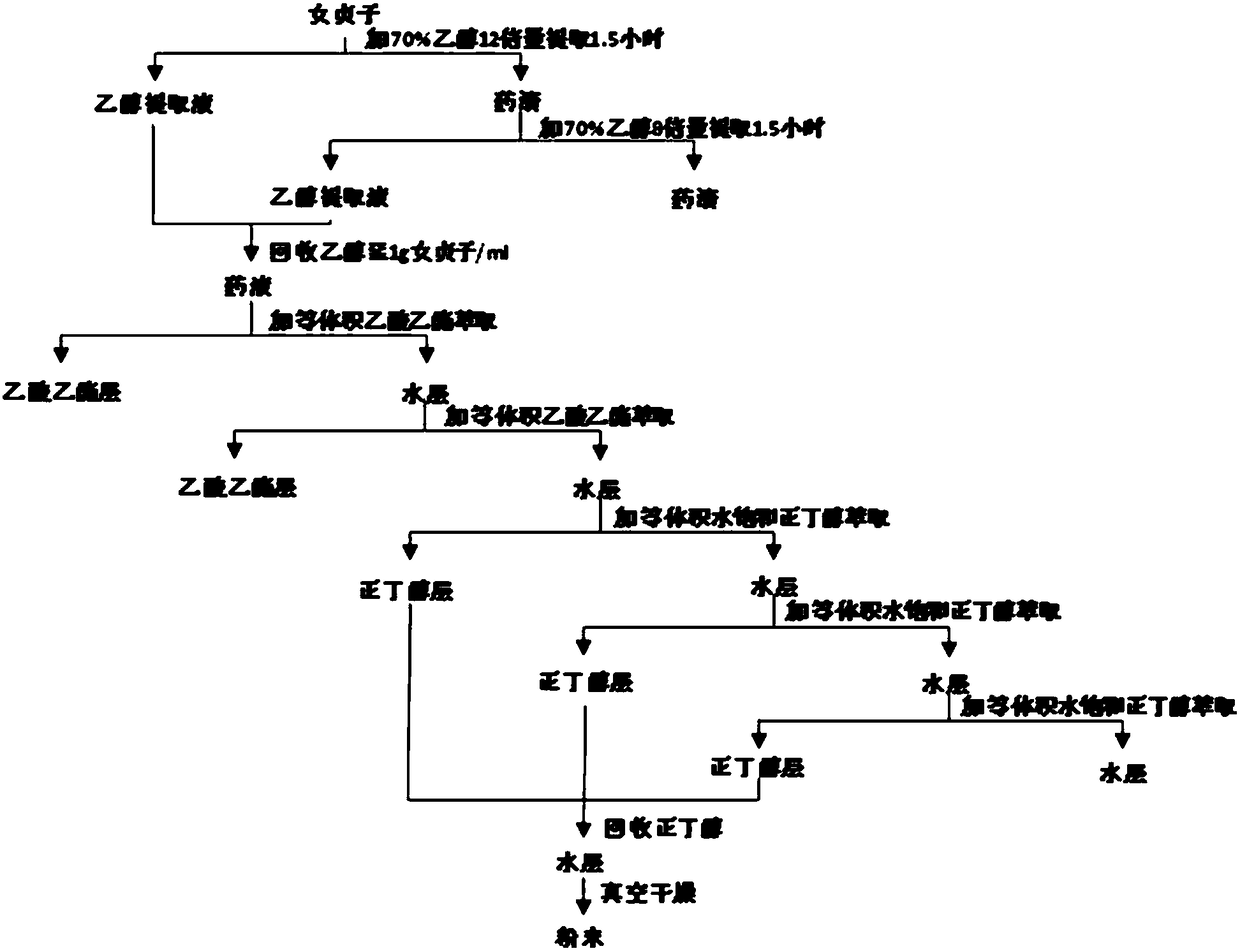
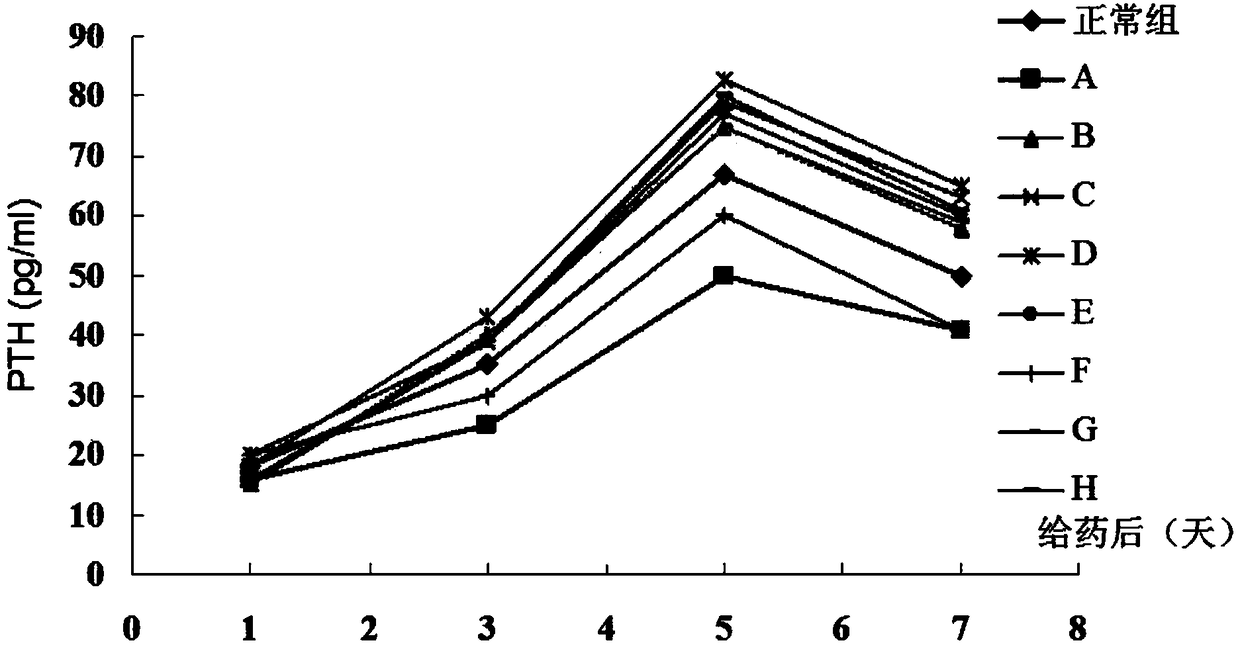
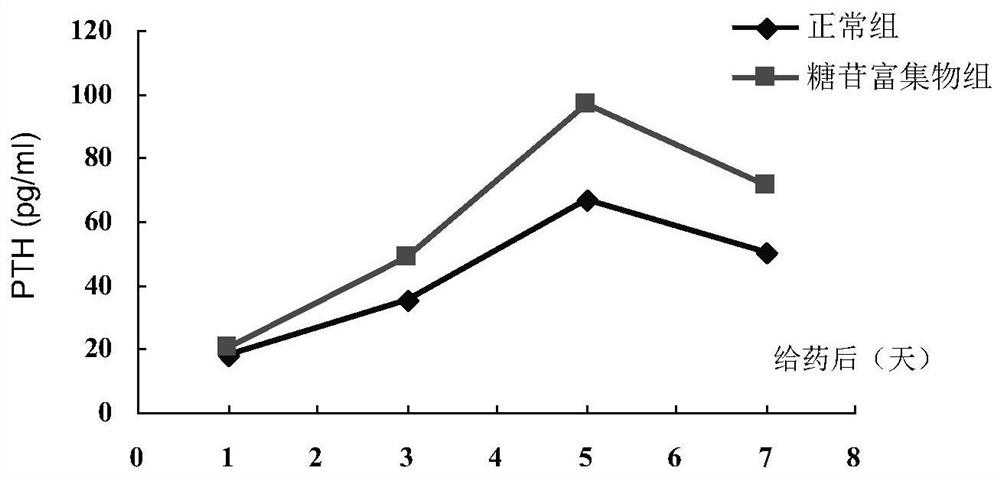
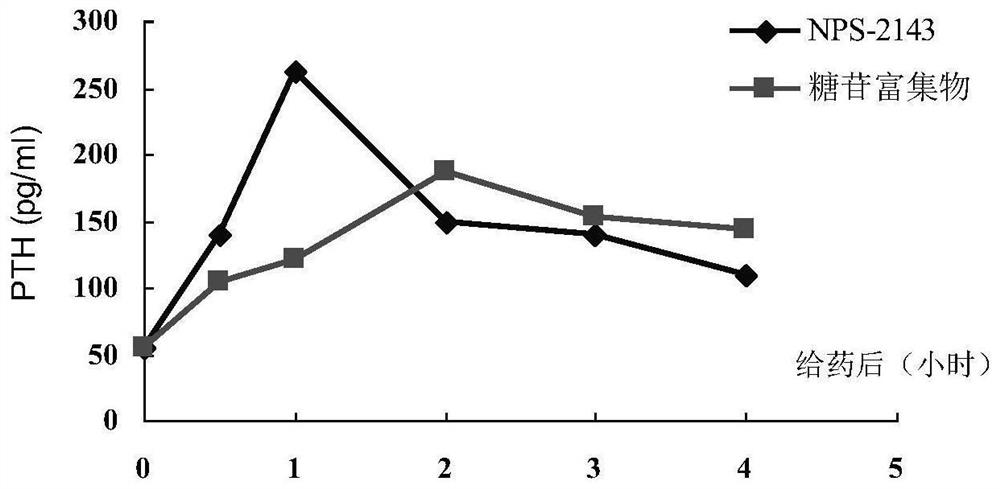
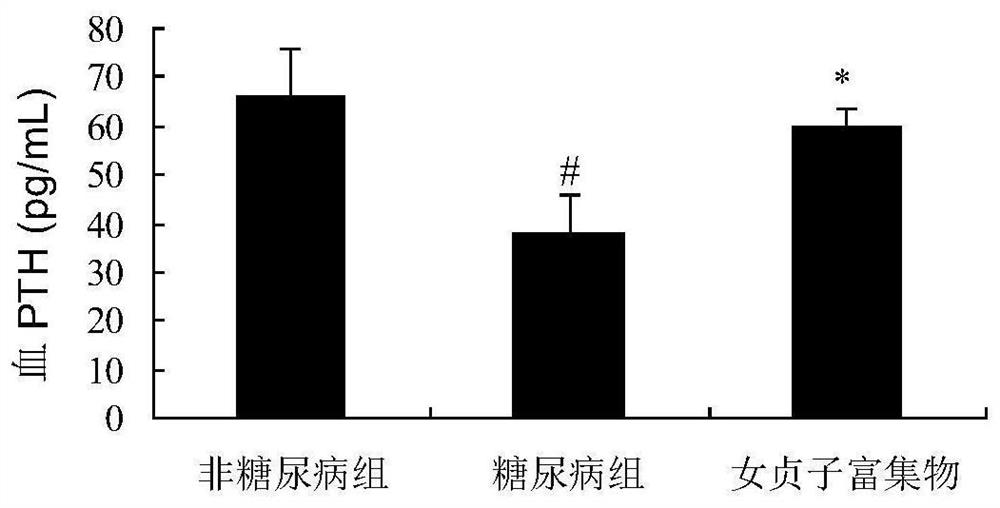
Ligustrum lucidum glycoside concentrate and application thereof
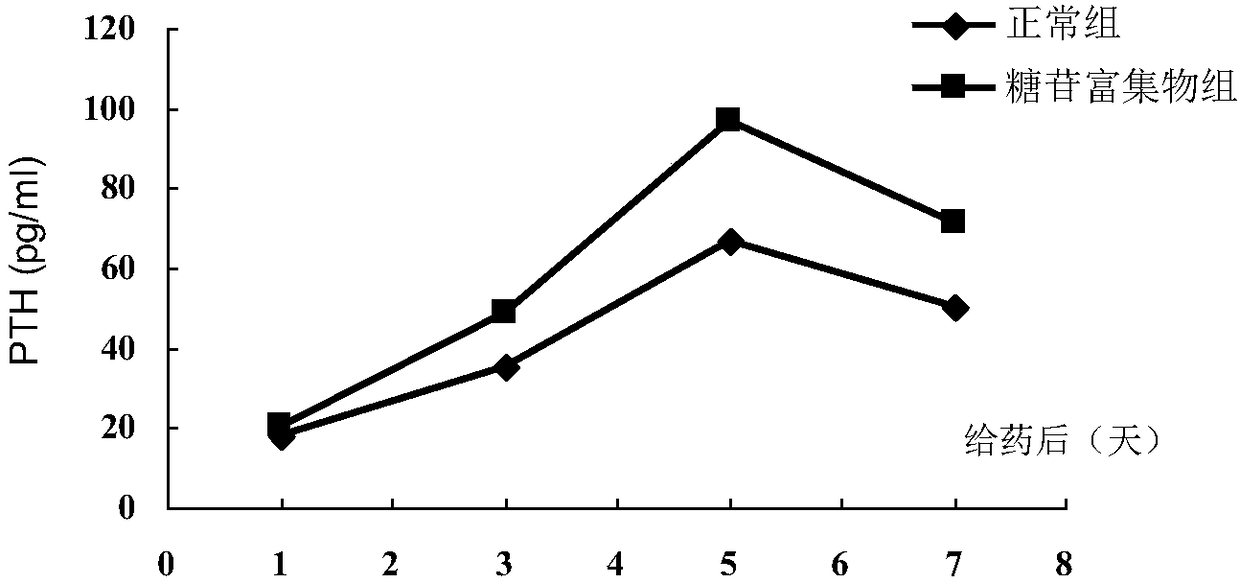
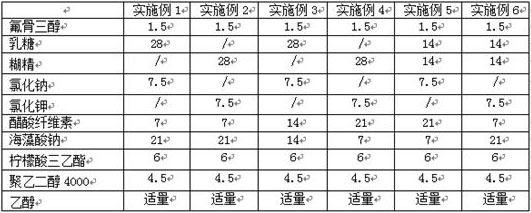
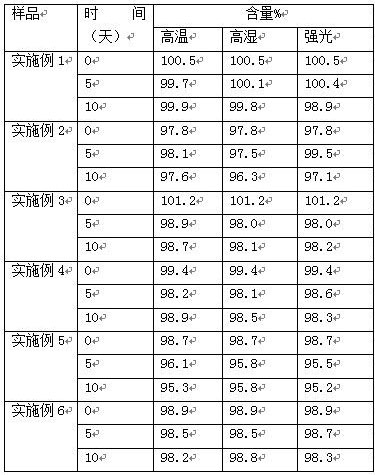
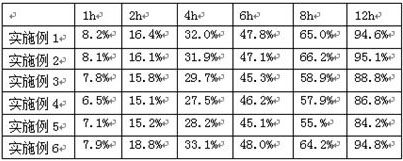
ActiveCN109276598AIncrease serum PTH levelsIncrease secretionEndocrine system disorderPlant ingredientsReflux extractionHypoparathyroidism
The invention discloses a ligustrum lucidum glucoside concentrate and an application thereof. A preparation method of the ligustrum lucidum glucoside concentrate has the following steps: adding 10-14times amount of ethanol having a volume concentration of 50-80% into ligustrum lucidum, performing reflux extraction for 1-3 h, filtering, adding 8 times amount of the ethanol having a volume concentration of 50-80% into medicine residues, performing reflux extraction for 1-3 h, filtering, combining two filtrates, recovering solvents, adding appropriate amount of ethyl acetate for extraction, discharging an ethyl acetate layer, adding water-saturated n-butanol into a water layer for extraction, combining a water-saturated n-butanol layer, condensing, and performing vacuum drying to prepare theligustrum lucidum glucoside concentrate. The invention also discloses an application of the ligustrum lucidum glucoside concentrate in preparation of medicine for treating hypoparathyroidism.
Owner:LONGHUA HOSPITAL SHANGHAI UNIV OF TRADITIONAL CHINESE MEDICINE
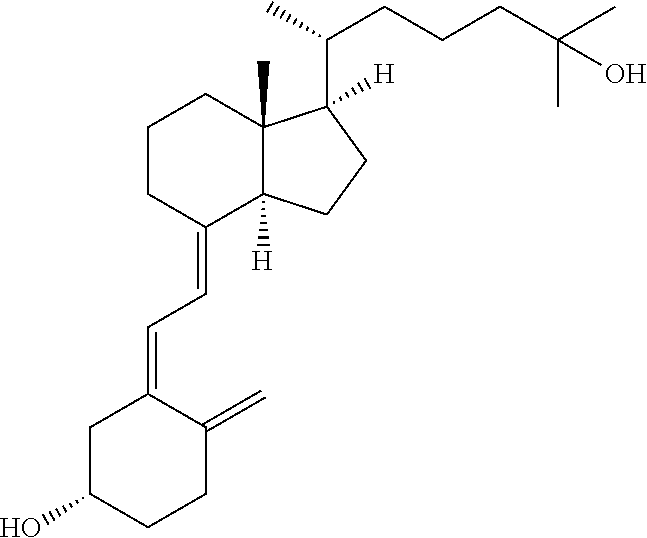
Falecalcitriol controlled-release tablet
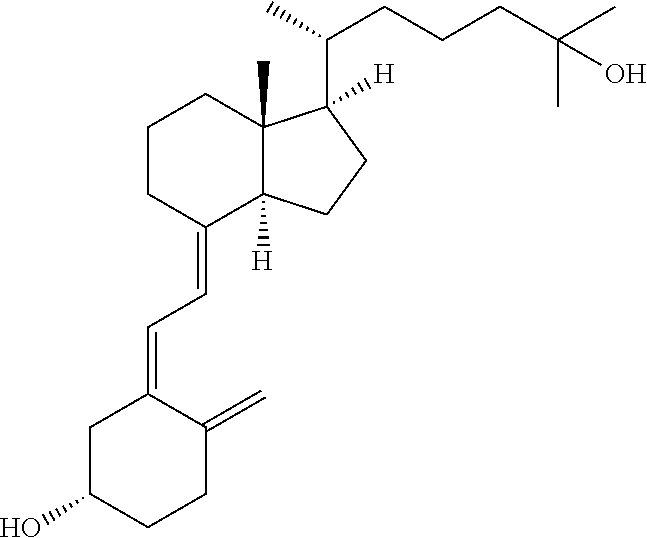
InactiveCN111821275ASmooth releaseLittle side effectsOrganic active ingredientsMetabolism disorderCalcium metabolismHypofunctions
The invention provides a preparation method of a falecalcitriol controlled-release tablet, and belongs to the technical field of medicines. The falecalcitriol controlled-release preparation in the invention is composed of a tablet containing core and a coating film wrapping the tablet containing core; the tablet containing core comprises falecalcitriol, a filler and sodium chloride; the coating film comprises a controlled-release material, a plasticizer and a pore-foaming agent; when preparation, the tablet containing core is wrapped by the coating film, wherein the main component, namely falecalcitriol, belongs to the vitamin D3 analogue, can adjust calcium metabolism and resist rickets, and can be used for treating hypoparathyroidism. The drug in the invention can be released slowly anduniformly; the purposes of being long to act and increasing the curative effect are achieved; the dosage also can be reduced when the same drug effect is kept; therefore, side effects of taking drugsto patients are reduced; furthermore, the preparation process is simple; and the obtained product is steady in quality and suitable for large-scale production and application.
Owner:CP PHARMA QINGDAO CO LTD
Parathyroid hormone analogs and uses thereof
Disclosed are two PTH analog ligands, SP-PTH-AAK and Aib-SP-PTH-AAK, that have long-acting activity at the PTH receptor, as demonstrated both in vitro and in vivo. These polypeptides are thus particularly useful in the treatment of diseases, such as hypoparathyroidism, in which long-acting activity is desired. The method of making the analog polypeptides is also disclosed.
Owner:THE GENERAL HOSPITAL CORP
Parathyroid hormone variants
PendingCN113840625APolypeptide with localisation/targeting motifPeptide/protein ingredientsSerum Calcium LevelPharmaceutical drug
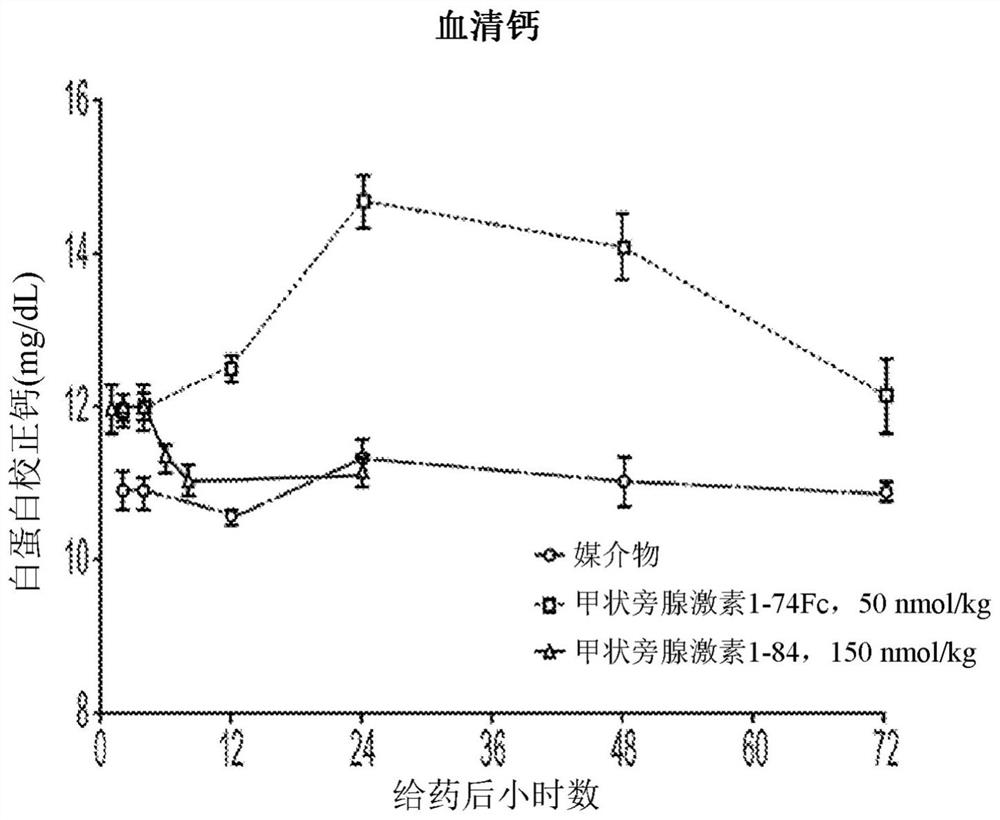
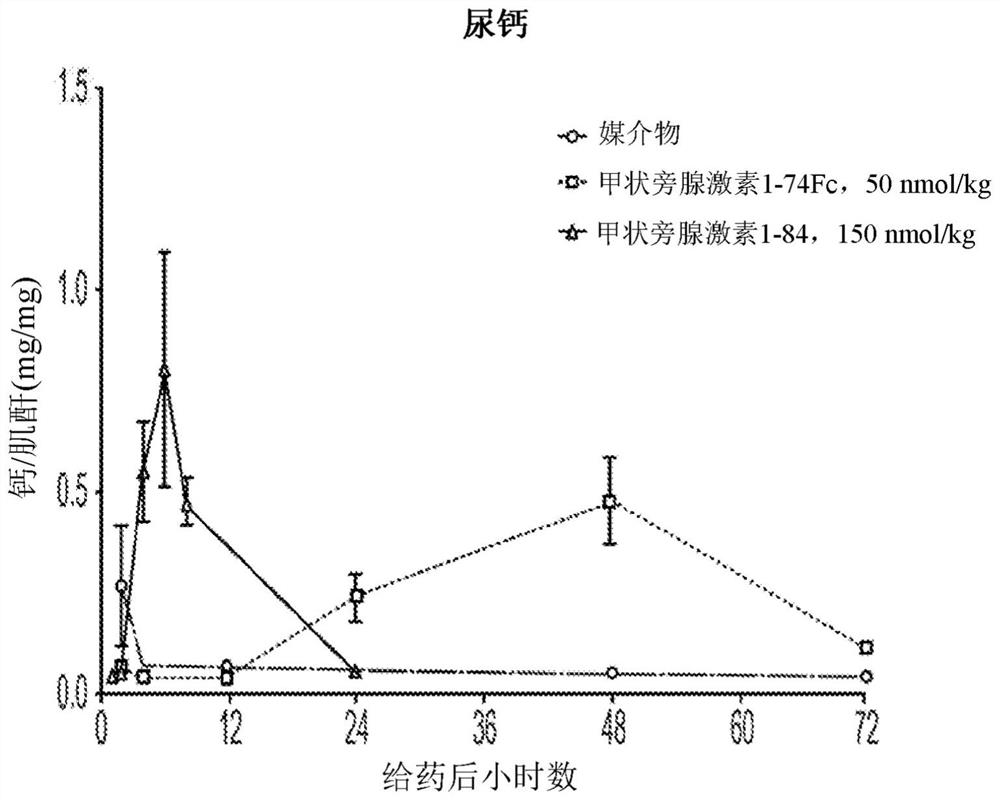
The invention relates to parathyroid hormone (PTH) variants and pharmaceutical compositions comprising same. The invention further relates to PTH compositions with improved serum half-life and peak-trough ratios, and methods of controlling serum calcium levels with the PTH variants and compositions of the invention. The invention further relates to methods of treating hypoparathyroidism and / or hypocalcemia due to hypoparathyroidism with the PTH variants and compositions of the invention.
Owner:TAKEDA PHARMA CO LTD
Animal model having hypoparathyroidism and method for producing the same
InactiveUS20180125042A1Economical and efficientOptimal calcium contentGenetic engineeringMaterial analysisBiotechnologyAnimal science
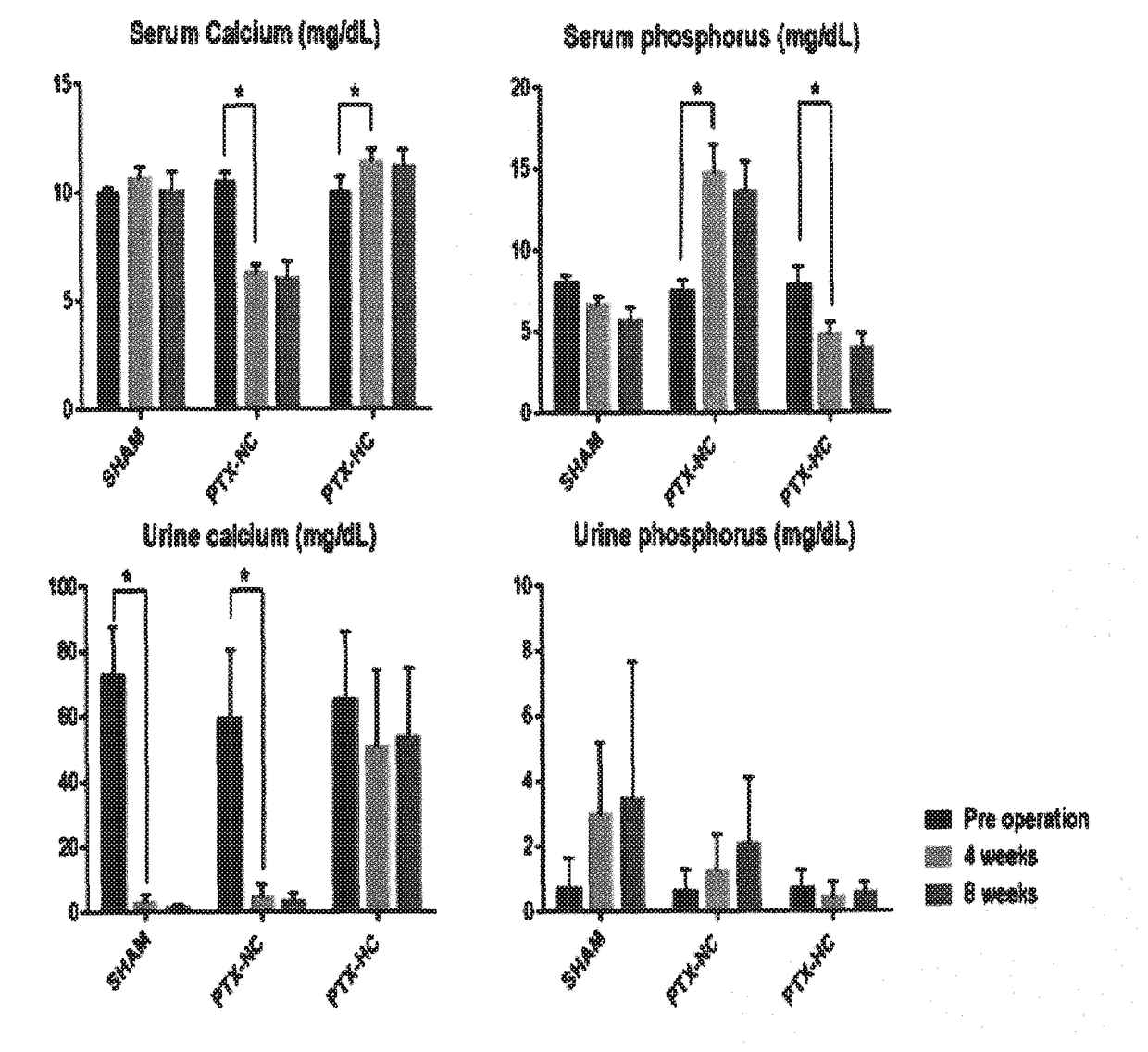
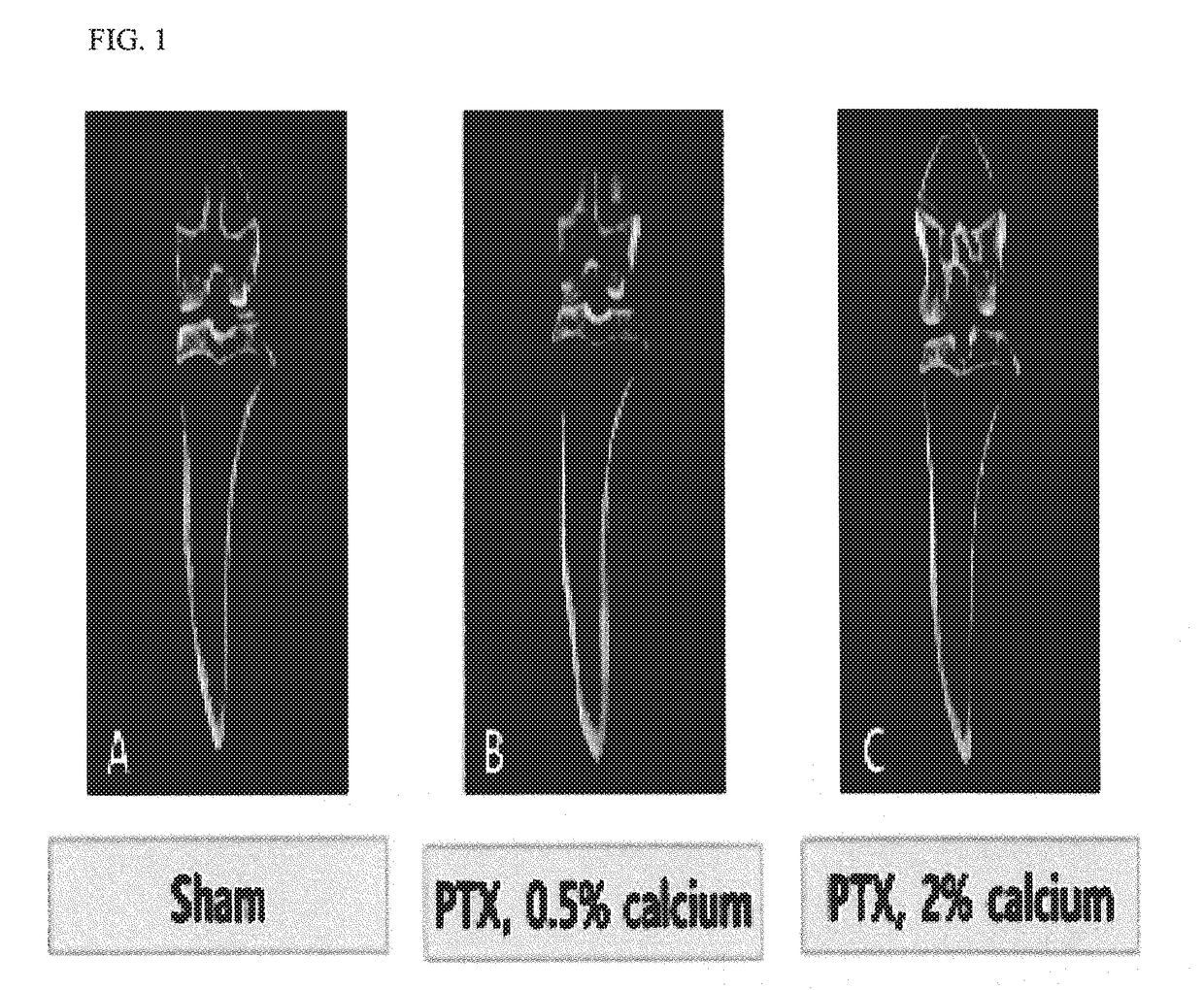
The present invention relates to an animal model having hypoparathyroidism and a method for producing the same. According to the present invention, the animal model having hypoparathyroidism is economical and efficient in that it can demonstrate a pathophysiology of hypoparathyroidism and maintain a survival of animals without any distortion of the pathophysiology by supplying an optimal calcium content diet.
Owner:EWHA UNIV IND COLLABORATION FOUND
Ligustrum lucidum glucoside enrichment and its application
ActiveCN109276598BIncrease serum PTH levelsIncrease secretionEndocrine system disorderPlant ingredientsGlycosideMedicine
The invention discloses a glycoside enrichment of Ligustrum lucidum, the preparation method of which comprises the following steps: adding 10-14 times of ethanol with a volume concentration of 50-80% to Ligustrum lucidum, refluxing and extracting for 1-3h, filtering, and Add 8 times the amount of ethanol with a volume concentration of 50-80% to the residue, reflux for extraction for 1-3 hours, filter, combine the two filtrates, recover the solvent, add an appropriate amount of ethyl acetate for extraction, discard the ethyl acetate layer, and add water to the water layer to saturate Extract with n-butanol, combine the water-saturated n-butanol layers, concentrate, and vacuum-dry to obtain the glycoside enrichment of Ligustrum lucidum. The invention also discloses the application of the Ligustrum lucidum glucoside enrichment in the preparation of medicine for treating hypoparathyroidism.
Owner:LONGHUA HOSPITAL SHANGHAI UNIV OF TRADITIONAL CHINESE MEDICINE
Sulfonamide compound and application thereof
InactiveCN102056897BOrganic chemistryPeptide/protein ingredientsPharmaceutical SubstancesOsteoporosis
Owner:ASAHI KASEI PHARMA
PTH-like peptides
InactiveUS7132394B2High activityImprove stabilityPeptide/protein ingredientsSkeletal disorderDiseaseBone implant
The present invention relates to peptides that are parathyroid hormone (PTH) analogs, useful for the treatment of hypoparathyroidism and diseases characterized by bone mass reduction, such as osteoporosis, and for stimulating bone repair or favoring the engraftment of a bone implant; to the pharmaceutical compositions comprising these PTH-like peptides and use thereof.
Owner:ABIOGEN PHARMA SPA
Analogues of parathyroid hormone (1-34) that function as agonists of the parathyroid hormone receptor-1 and display modified activity profiles
InactiveUS20200354428A9Peptide/protein ingredientsParathyroid hormonesAmino acid substitutionPharmaceutical drug
Described are polypeptide analogs of parathyroid hormone (PTH) that include an unnatural amino acid substitution at positions 7 or 8 from the N-terminus of the polypeptide. Also described are pharmaceutical compositions useful for treating hypoparathyroidism that contain the analogs and methods of using the analogs to treat hypoparathyroidism.
Owner:WISCONSIN ALUMNI RES FOUND
Parathyroid hormone polypeptide conjugates and methods of their use
PendingUS20200405820A1Reducing constitutive activityFacilitated releasePeptide/protein ingredientsPharmaceutical non-active ingredientsArthritisHyperphosphoremia
Disclosed are peptide-fatty acid conjugates, pharmaceutical compositions containing them, and methods of their medical use in the treatment of, e.g., a disease or condition associated with the PTHR1 signaling overactivity (e.g., hypercalcemia, hypophosphatemia, hyperparathyroidism, or Jansen's chondrodysplasia) or deficiency (e.g., hypoparathyroidism, hyperphosphatemia, osteoporosis, fracture repair, osteomalacia, arthritis, or thrombocytopenia).
Owner:THE GENERAL HOSPITAL CORP
Human parathyroid hormone eukaryotic expression recombinant plasmid vector and construction method thereof
PendingCN112143755AImprove securityImprove performancePeptide/protein ingredientsAntibody mimetics/scaffoldsEnzyme digestionGenetics
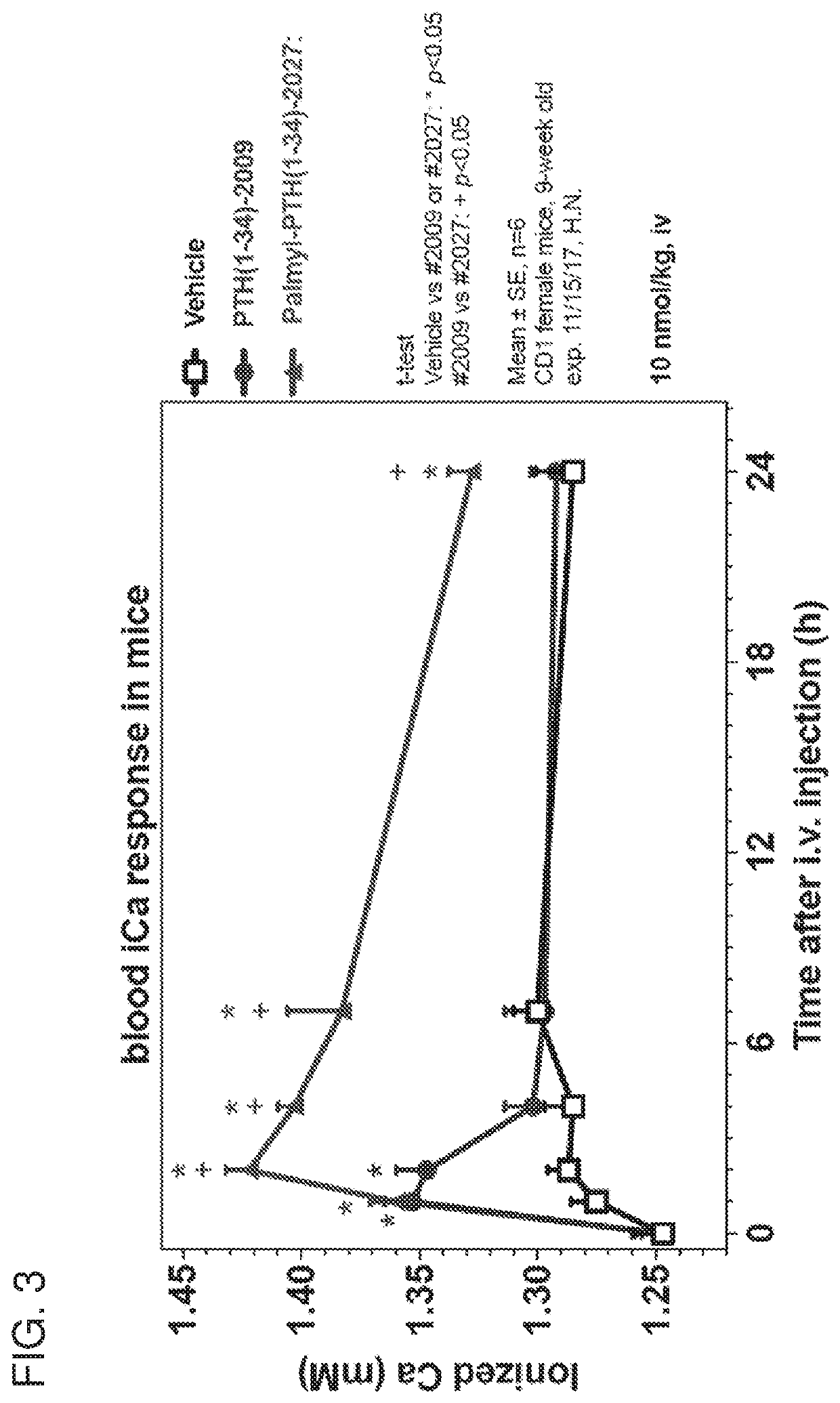
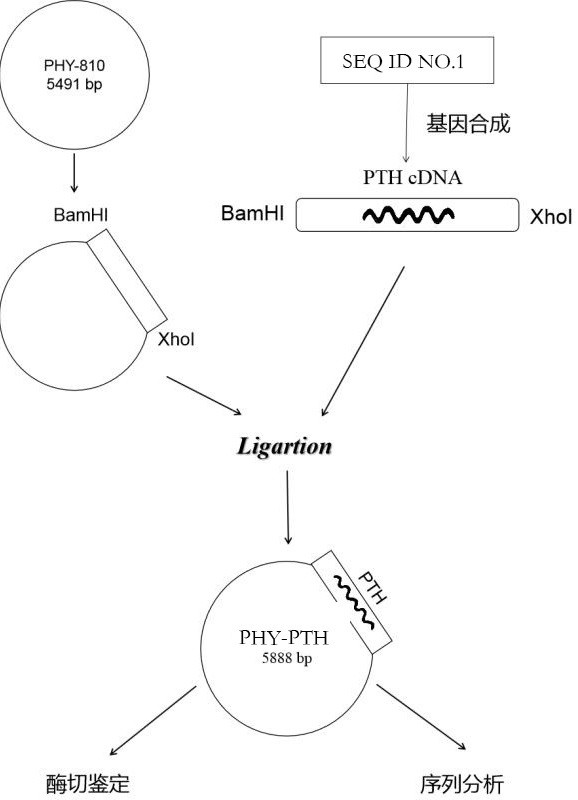
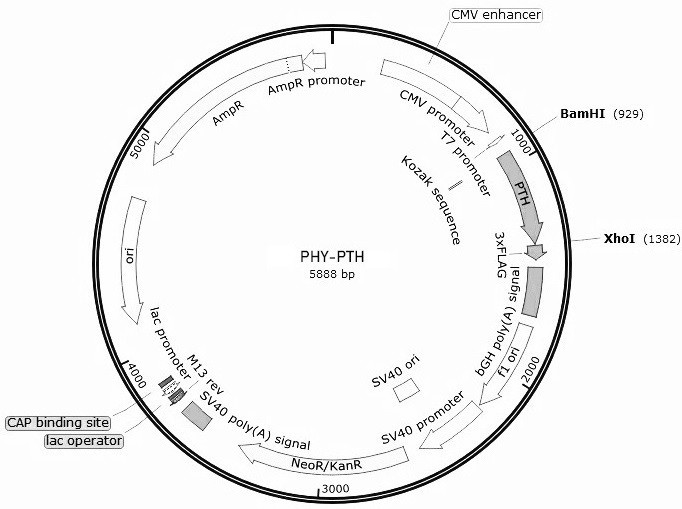
The invention relates to the field of gene medicines, and particularly provides a human parathyroid hormone eukaryotic expression recombinant plasmid vector and a construction method thereof. The construction method comprises the following steps of artificially synthesizing a parathyroid hormone (hPTH1-84aa) gene (SEQ ID NO.1), carrying out PCR amplification to obtain parathyroid hormone gene segments, carrying out double enzyme digestion on a PCR product by using restriction enzymes BamHI and XhoI, connecting the product with an eukaryotic expression vector plasmid PHY-810 subjected to the same enzyme digestion, converting the connected expression vector into competent bacteria, and carrying out coating and screening to obtain the human PTH gene eukaryotic expression recombinant plasmid vector (figure 1: PHY-PTH). The eukaryotic expression recombinant plasmid vector constructed by the method enables cells to highly express PTH, and can be used for gene therapy of hypoparathyroidism and osteoporosis.
Owner:深圳市瑞普逊科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS](https://images-eureka.patsnap.com/patent_img/34f9b00a-7f53-4816-814c-6dbb659ca913/US20080085887A1-20080410-D00000.png)
![PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS](https://images-eureka.patsnap.com/patent_img/34f9b00a-7f53-4816-814c-6dbb659ca913/US20080085887A1-20080410-D00001.png)
![PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS](https://images-eureka.patsnap.com/patent_img/34f9b00a-7f53-4816-814c-6dbb659ca913/US20080085887A1-20080410-C00001.png)
![Pyrido[4,3-<i>d</i>]pyrimidin-4(3<i>H</i>)-one derivatives as calcium receptor antagonists Pyrido[4,3-<i>d</i>]pyrimidin-4(3<i>H</i>)-one derivatives as calcium receptor antagonists](https://images-eureka.patsnap.com/patent_img/3c78f7e7-7476-48aa-a8c5-3978546d5356/US07829572-20101109-D00001.png)
![Pyrido[4,3-<i>d</i>]pyrimidin-4(3<i>H</i>)-one derivatives as calcium receptor antagonists Pyrido[4,3-<i>d</i>]pyrimidin-4(3<i>H</i>)-one derivatives as calcium receptor antagonists](https://images-eureka.patsnap.com/patent_img/3c78f7e7-7476-48aa-a8c5-3978546d5356/US07829572-20101109-C00001.png)
![Pyrido[4,3-<i>d</i>]pyrimidin-4(3<i>H</i>)-one derivatives as calcium receptor antagonists Pyrido[4,3-<i>d</i>]pyrimidin-4(3<i>H</i>)-one derivatives as calcium receptor antagonists](https://images-eureka.patsnap.com/patent_img/3c78f7e7-7476-48aa-a8c5-3978546d5356/US07829572-20101109-C00002.png)