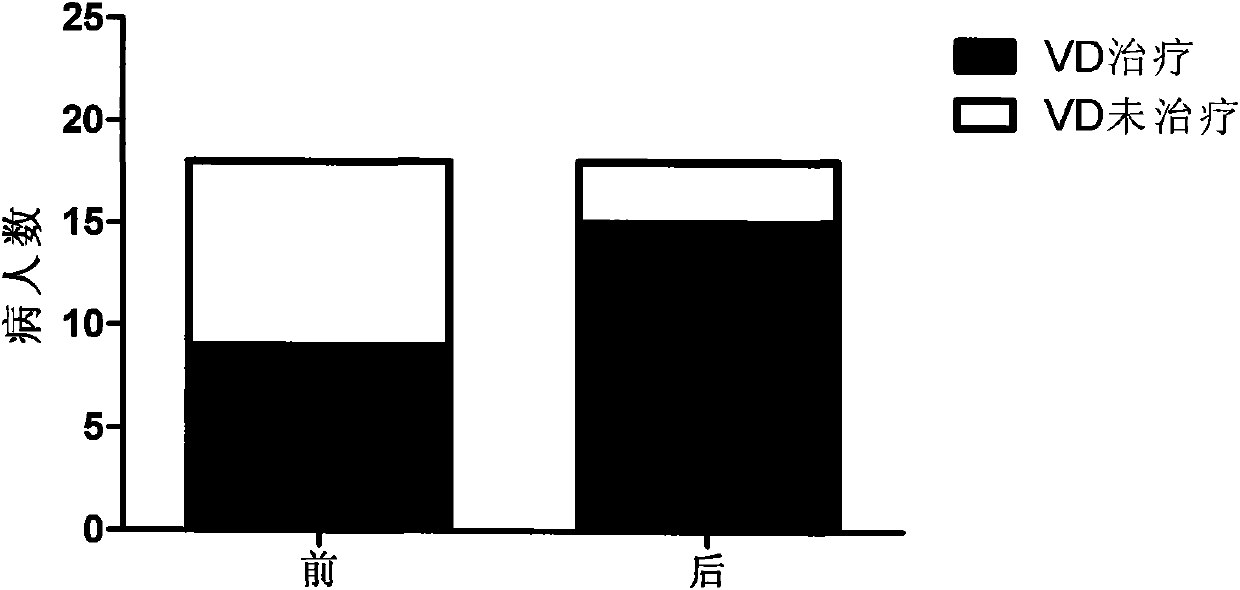
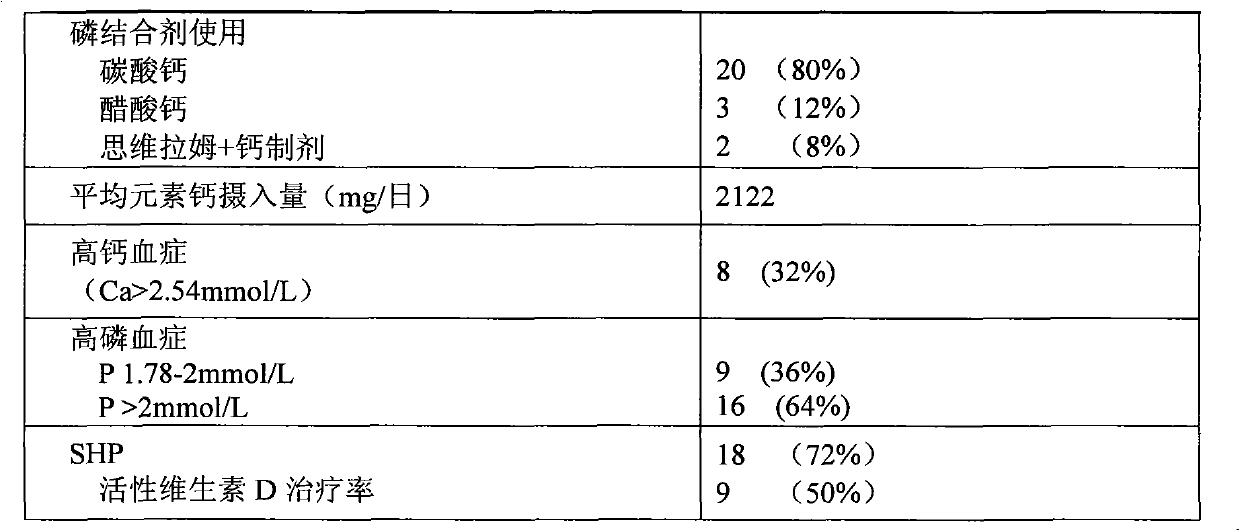
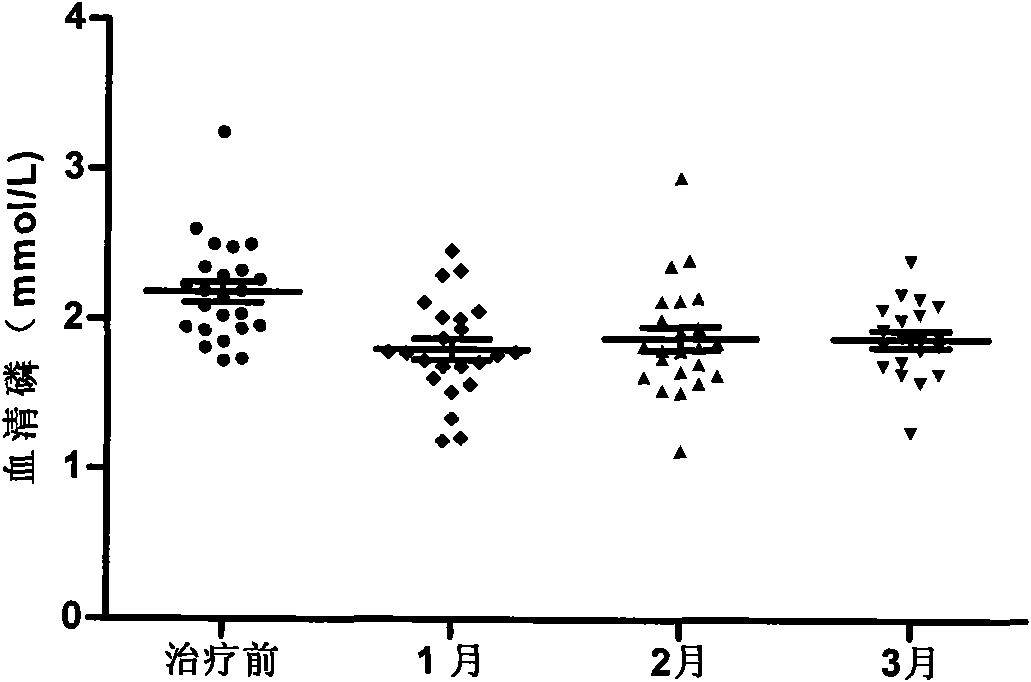
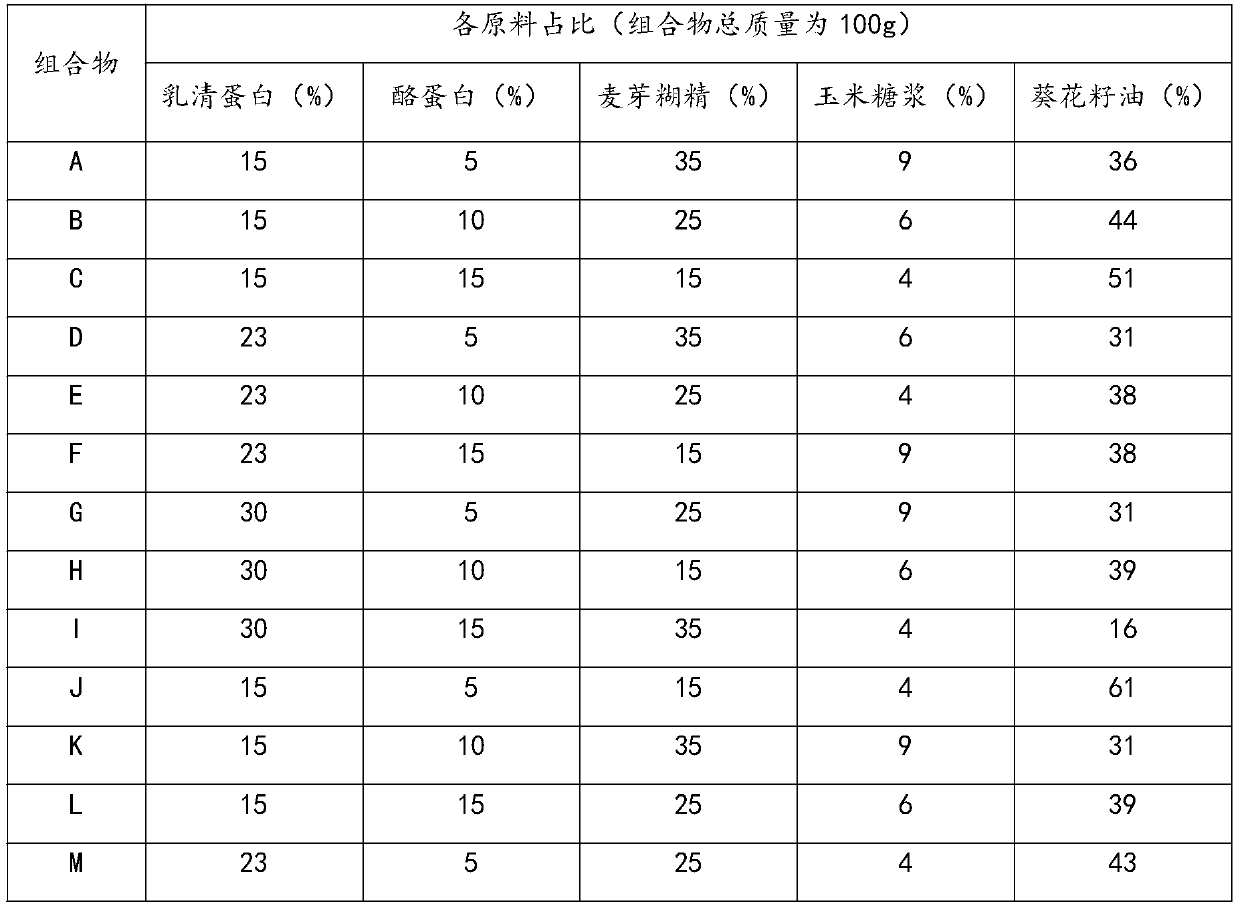
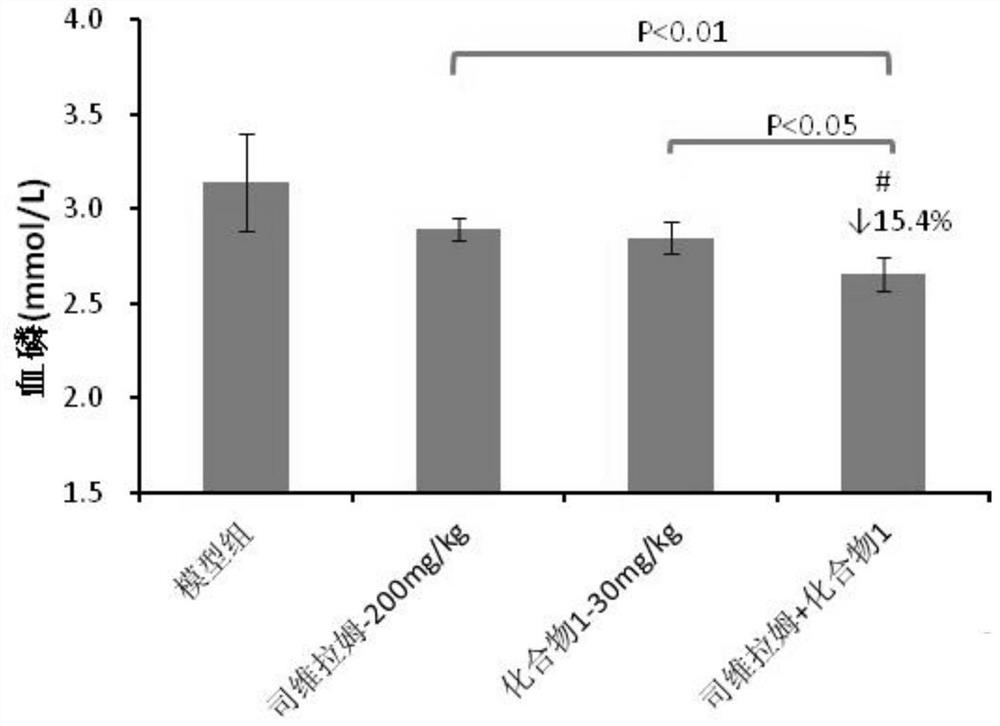
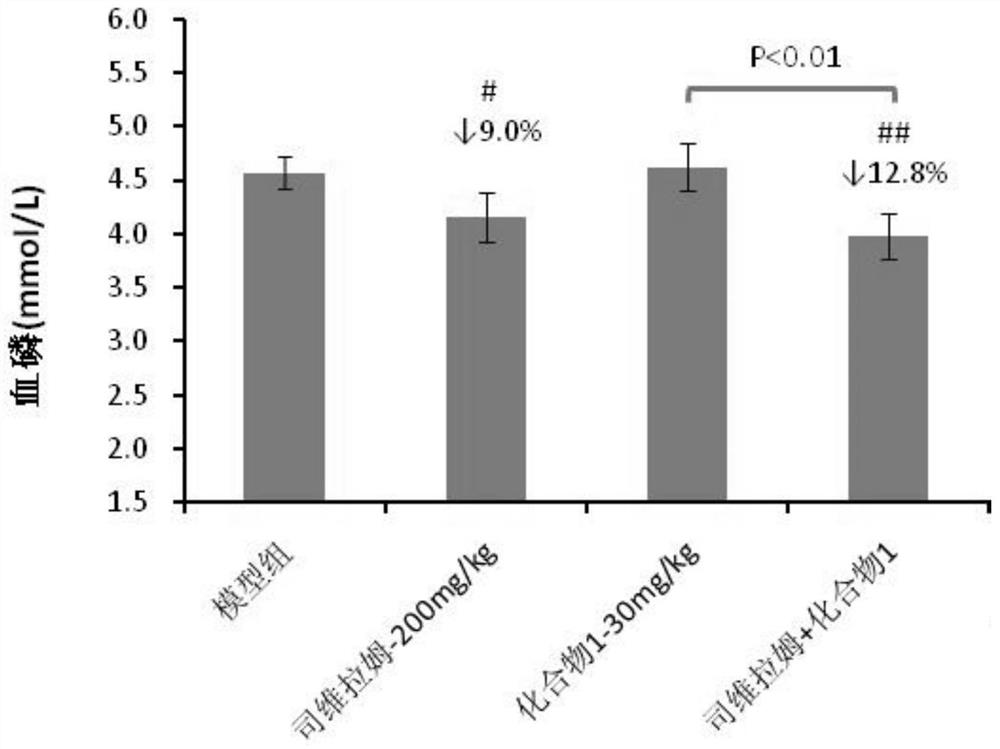
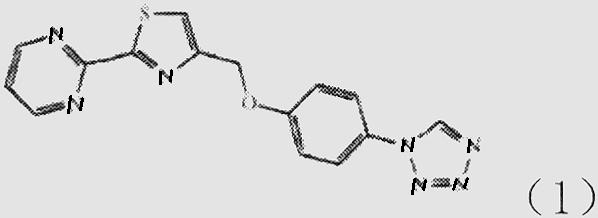
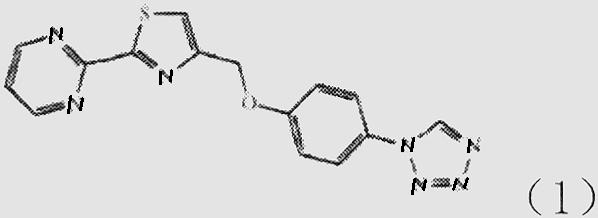
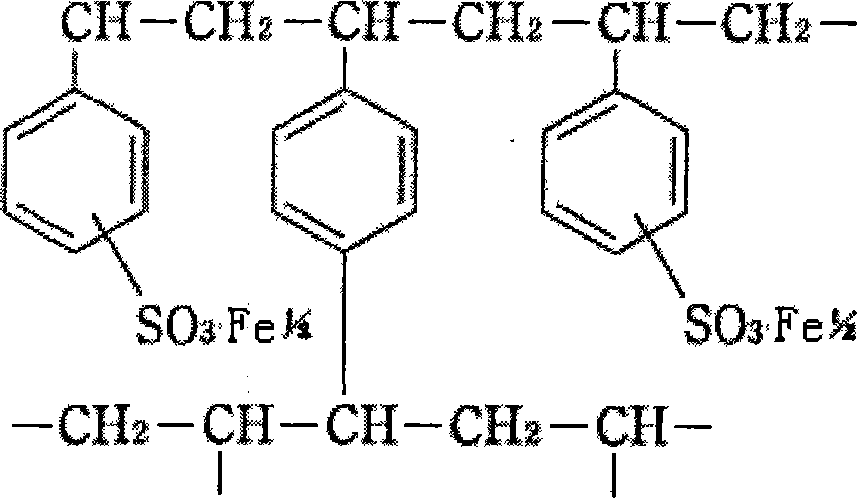Patents
Literature
46 results about "Hyperphosphoremia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
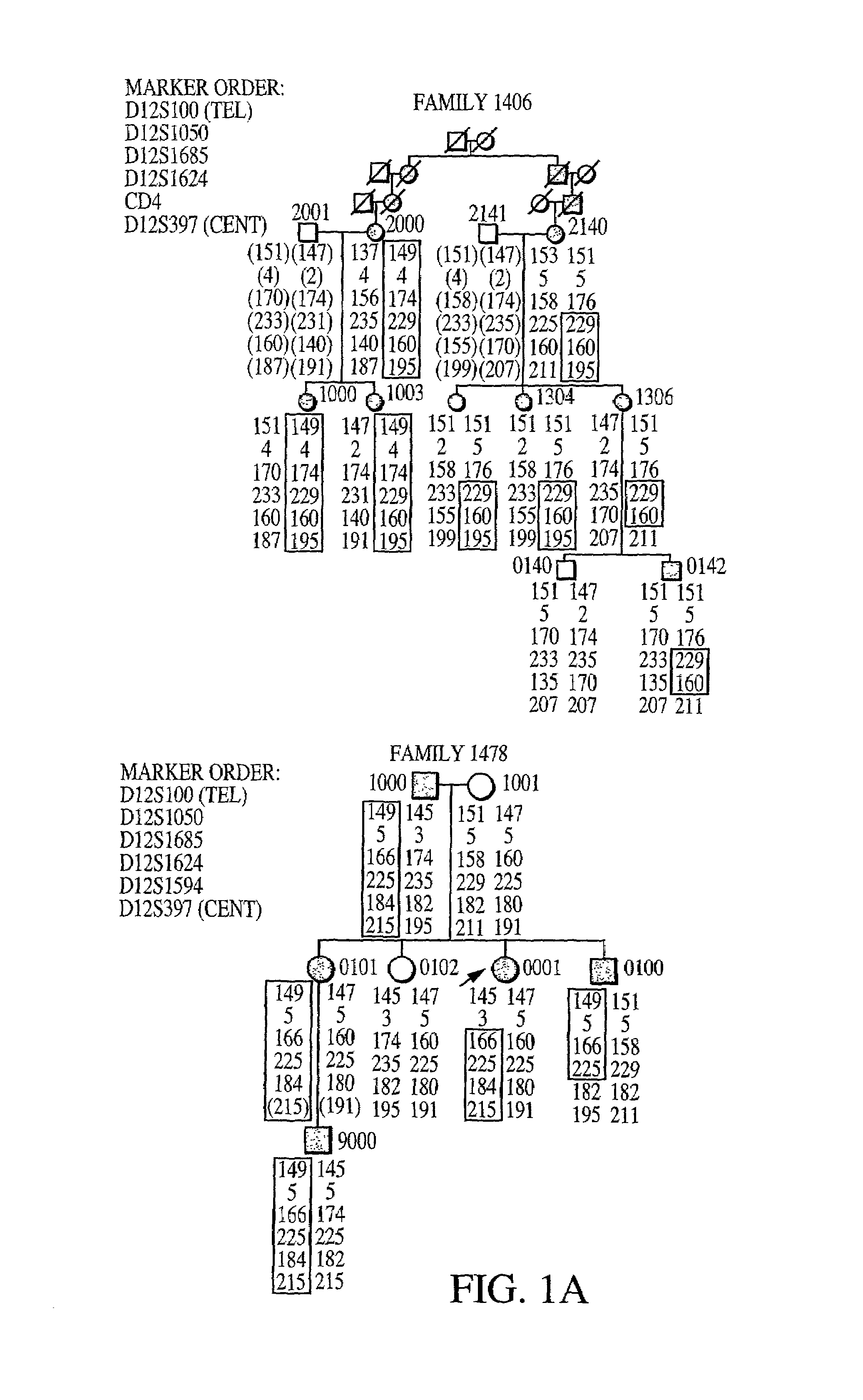
Fibroblast growth factor (FGF23) nucleic acids
InactiveUS7223563B2Increase stabilityImprove stabilityOrganic active ingredientsFungiHypophosphatemiaOsteoporosis
The invention relates to novel nucleic acids encoding a fibroblast growth factor-23(FGF23) and proteins encoded thereby, mutations in which are associated with autosomal dominant rickets (ADHR). The invention further relates to methods of diagnosing and treating hypophosphatemic and hyperphosphatemic disorders comprising inhibiting or stimulating, respectively, the biological activity of FGF23 in a patient. The invention also relates to methods of treating osteoporosis, dermatomyositis, and coronary artery disease comprising stimulating the biological activity of FGF23 in a patient.
Owner:LUDWIG MAXIMILIANS UNIV MUNCHEN ABETEILUNG MEDIZINISCHE GENETIK +1
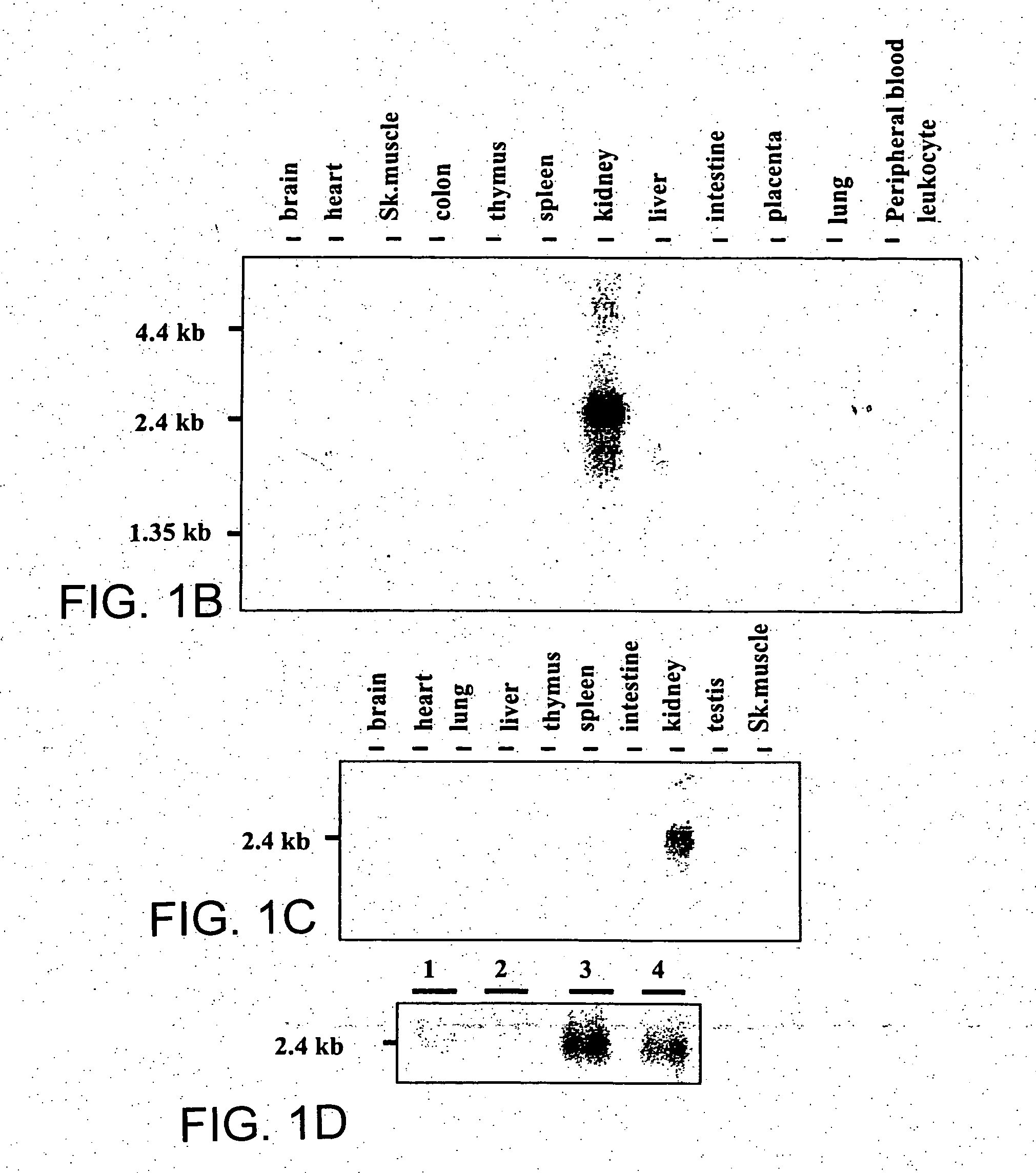
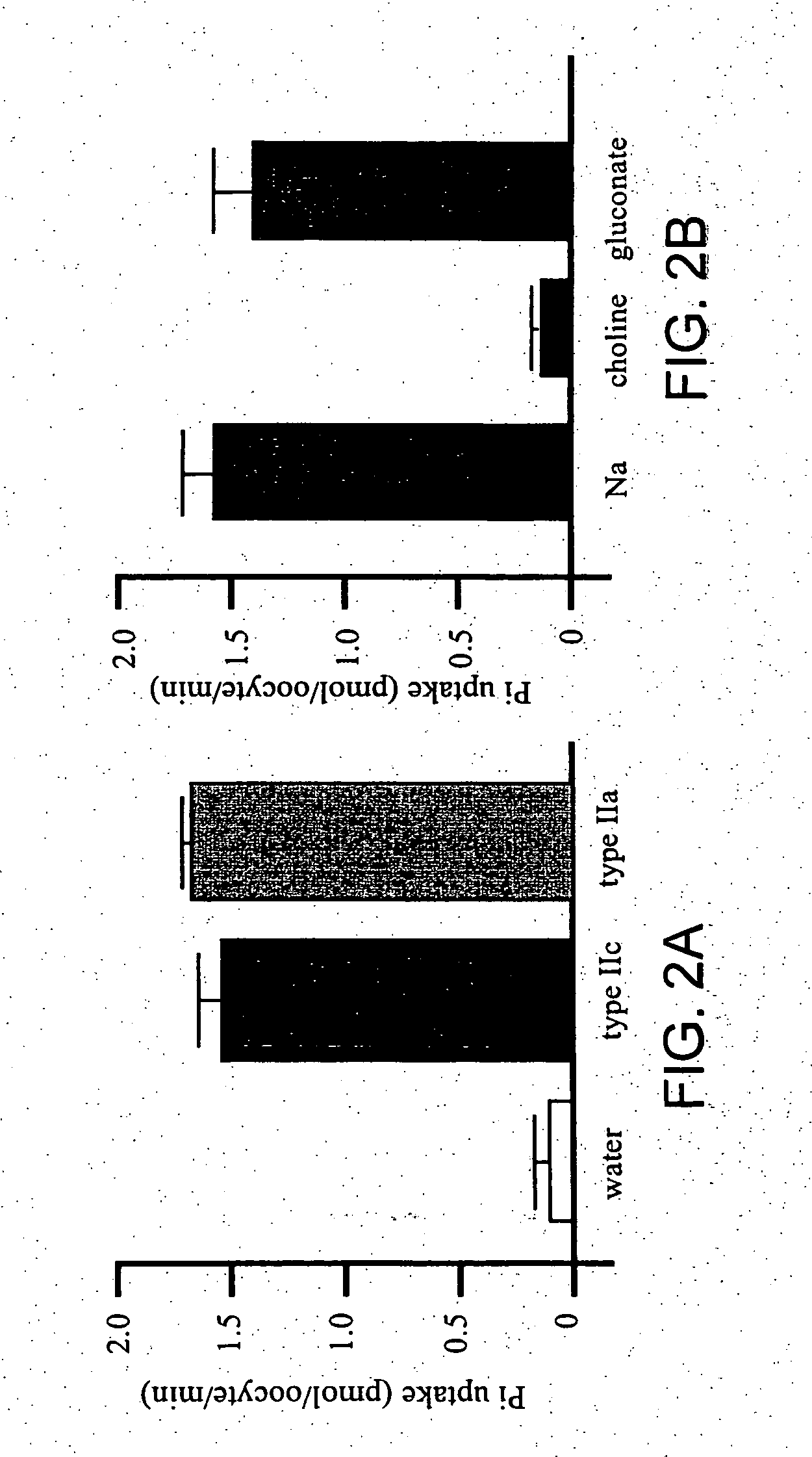
Novel type II Na/Pi cotransporters and type II Na/Pi cotransporter expression regulatory factors
InactiveUS20050004348A1Inhibit expressionInhibition of reabsorptionAntibacterial agentsOrganic active ingredientsAdult stageHypophosphatemia
The present invention provides novel type IIc Na / Pi cotransporters. These cotransporters are important Pi transporters that are highly expressed during the growth period from the weaning stage to the adult stage. Furthermore, the present invention provides FGF23 and mutants thereof as factors that regulate the expression of type II Na / Pi cotransporters. FGF23 suppresses Pi reabsorption through suppression of type II Na / Pi cotransporter expression in kidneys. Therefore, FGF23 can be used as a target substance for regulating Pi reabsorption in kidneys. The present invention provides important factors for the development of preventive and therapeutic agents for hyperphosphatemia or hypophosphatemia.
Owner:CHUGAI PHARMA CO LTD
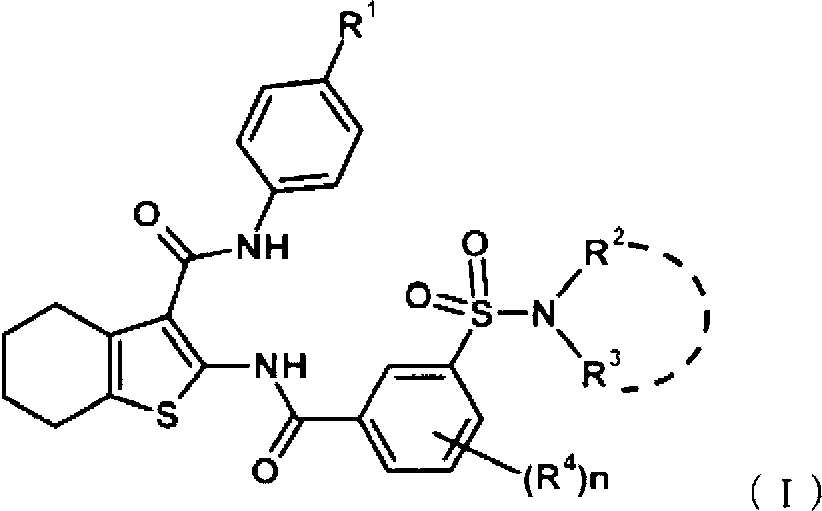
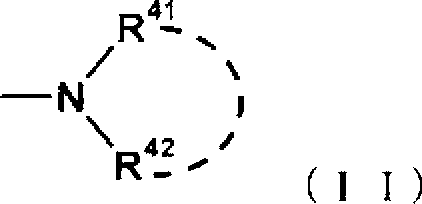
Tetrahydrobenzothiophene compound
Disclosed is a compound which has an intestinal phosphate transporter (NPT-IIb) inhibition activity and is useful as an active ingredient for a therapeutic agent and / or a prophylactic agent for hyperphosphatemia. A tetrahydrobenzothiophene compound represented by formula (I) has an NPT-IIb inhibition activity and can be used as a prophylactic agent and / or a therapeutic agent for hyperphosphatemia. (In the formula, R1 represents -O-(lower alkyl), -(lower alkylene)-phenyl, or the like; R2 and R3 are same as or different from each other and independently represent H, a lower alkyl group, a cycloalkyl group, an aryl group, a heteroaryl group, or the like, or R2, R3 and a nitrogen atom to which R2 and R3 are bound together may form a 5- to 7-membered saturated cyclic amino group; R4's are same as or different from each other and independently represent a halogen atom, a lower alkyl group, or the like; and n represents 0 to 2.)
Owner:ASTELLAS PHARMA INC
Oral Compositions for Absorption of Phosphorus Compounds
InactiveUS20080125394A1Convenient treatmentEasy to controlBiocideOrganic active ingredientsHyperphosphoremiaPhosphorus serum level
The present invention provides medicaments useful for reducing phosphorus serum level, especially in those subjects affected from hyperphosphatemia. More specifically, the present invention relates to pharmaceutical compositions to be administered by oral route in fasting periods, in order to absorb phosphorus compounds from fluids of the enteric tract, especially from saliva.
Owner:CM&D PHARMA
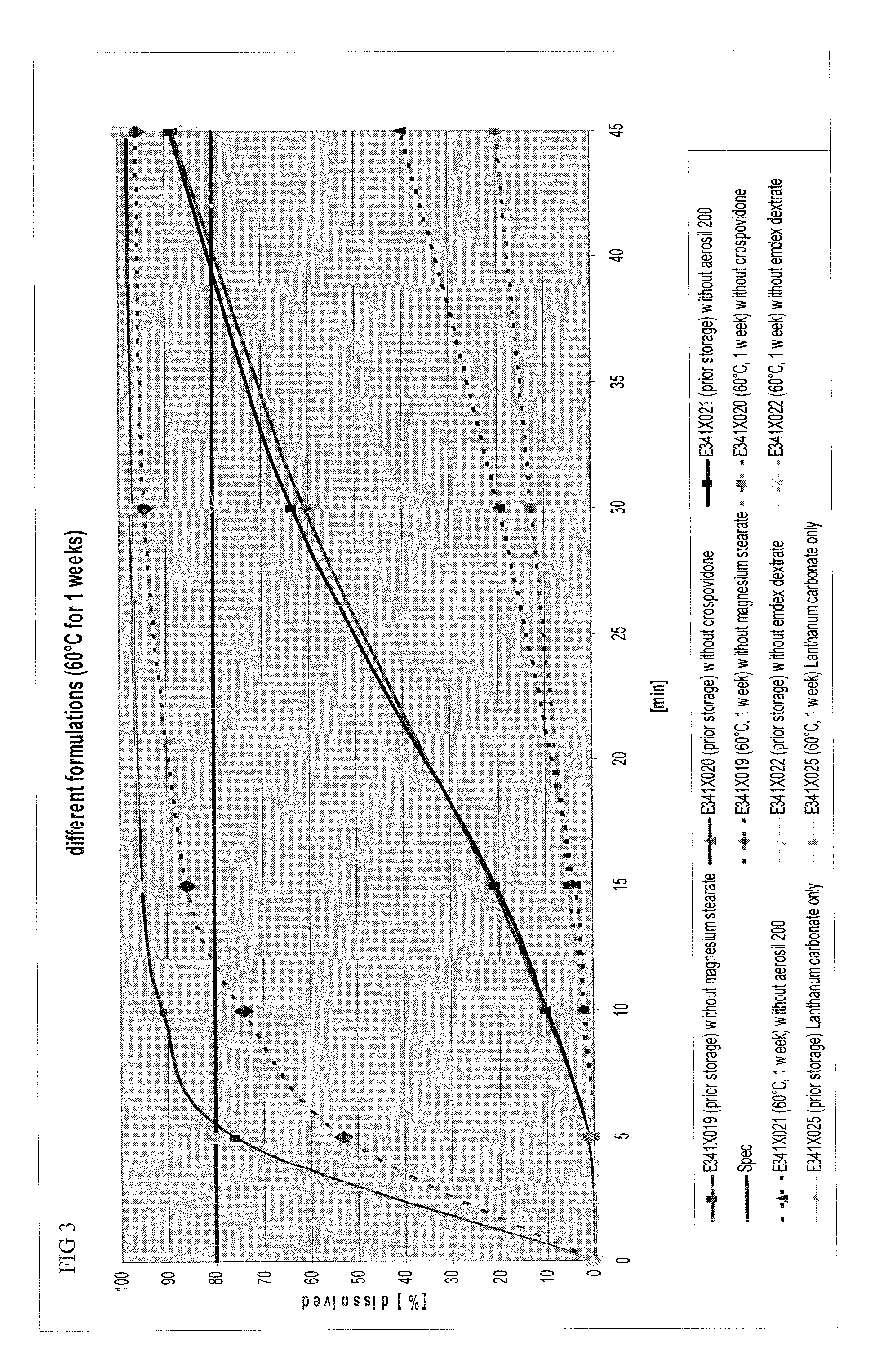
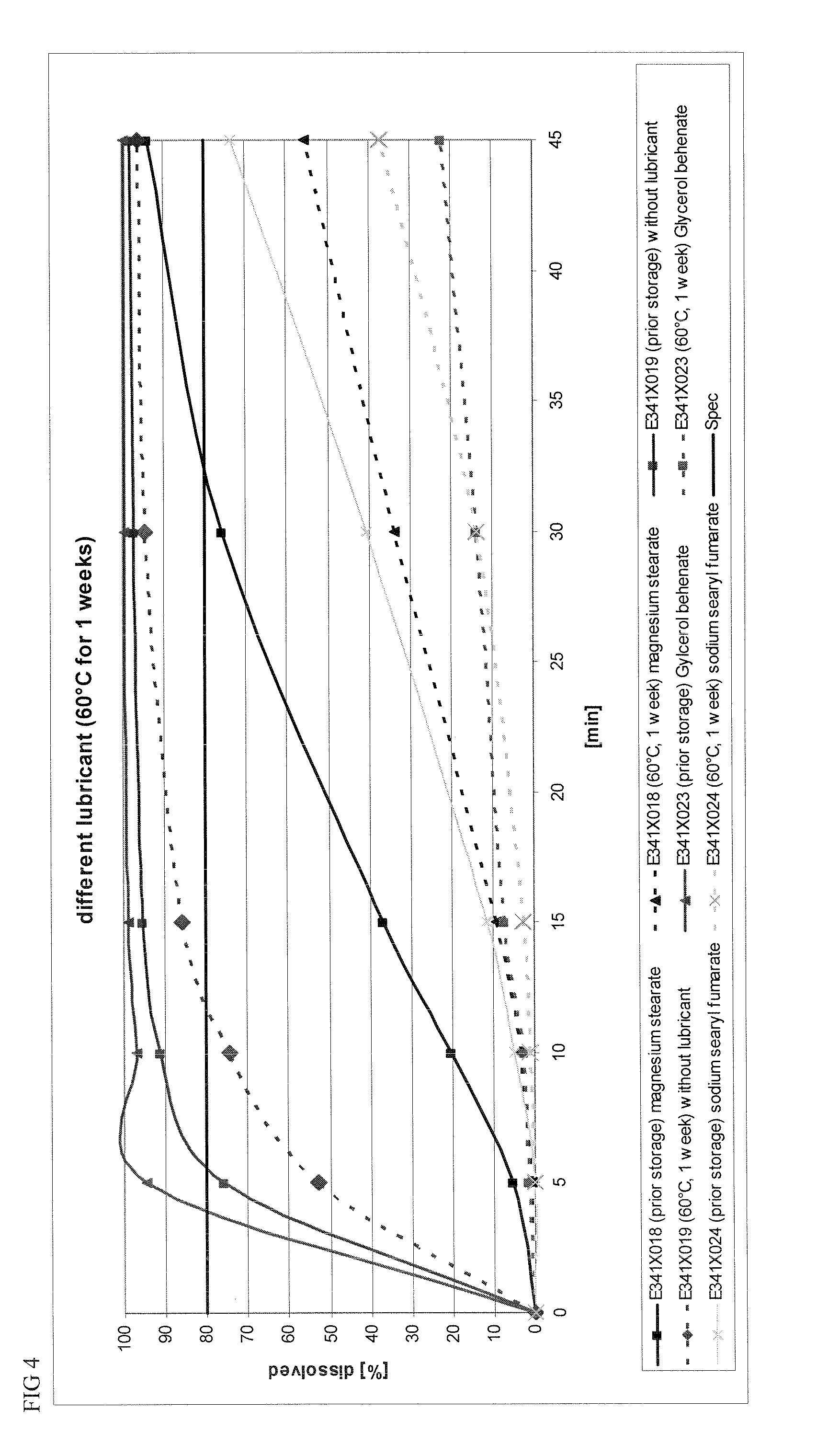
Capsule and powder formulations containing lanthanum compounds
The present invention includes an oral pharmaceutical capsule comprising a shell, lanthanum carbonate or lanthanum carbonate hydrate, and a lubricant such as talc, wherein the shell encapsulates the lanthanum carbonate or its hydrate and the lubricant. Capsule shells comprise, for example, gelatin. The present invention also includes an oral pharmaceutical powder comprising lanthanum carbonate or lanthanum carbonate hydrate and a pharmaceutically acceptable excipient. The oral pharmaceutical capsules and powders of the present invention can be administered to treat a patient at risk of or suffering from hyperphosphatemia, at risk of or suffering from chronic kidney disease (CKD), at risk of or suffering from soft tissue calcification associated with CKD, or at risk of or suffering from secondary hyperparathyroidism.
Owner:TAKEDA PHARMACEUTICALS CO LTD
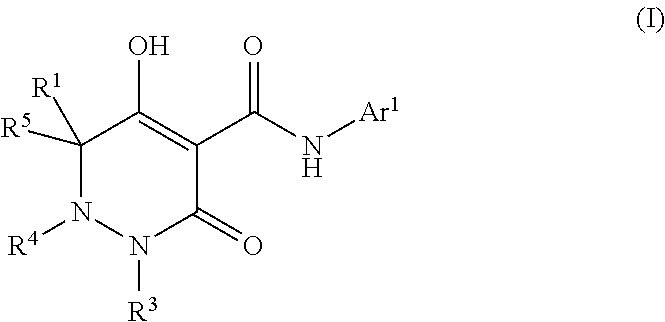
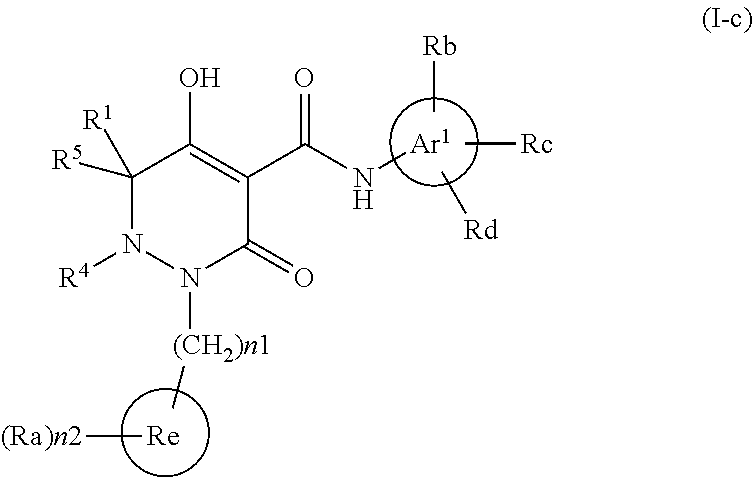
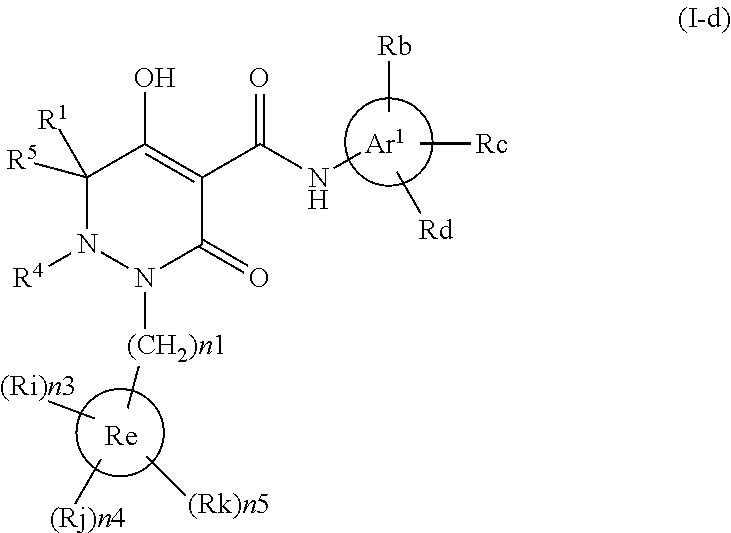
Dihydropyridazine-3,5-dione derivative and pharmaceuticals containing the same
ActiveUS20160002251A1Enhanced inhibitory effectHeavy metal active ingredientsOrganic chemistryChronic kidney failurePyridazine
The present invention provides a dihydropyridazine-3,5-dione derivative or a salt thereof, or a solvate of the compound or the salt, a pharmaceutical drug, a pharmaceutical composition, a sodium-dependent phosphate transporter inhibitor, and a preventive and / or therapeutic agent for hyperphosphatemia, secondary hyperparathyroidism, chronic renal failure, chronic kidney disease, and arteriosclerosis associated with vascular calcification comprising the compound as an active ingredient, and a method for prevention and / or treatment.
Owner:CHUGAI PHARMA CO LTD
Low-phosphorus whey protein powder suitable for patients with chronic nephrosis, and preparation method of low-phosphorus whey protein powder
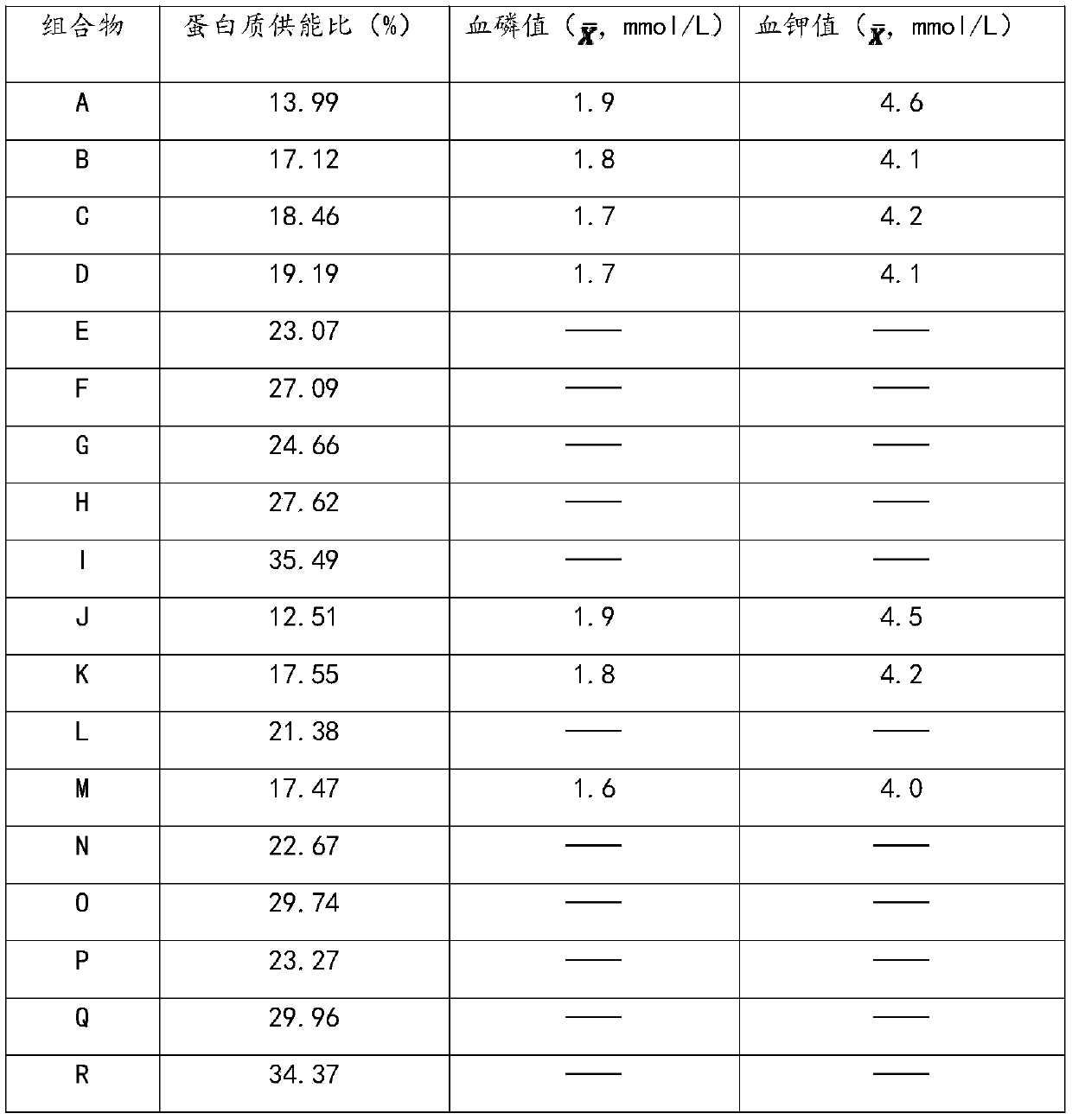
The invention provides low-phosphorus whey protein powder suitable for patients with chronic nephrosis. After the technical scheme is adopted, a brand new protein powder formula is designed aiming at the constitutional characteristics of the patients with the chronic nephrosis, so that the contents of phosphorus and potassium elements in a protein source are improved on one hand, and appropriate microelements are matched for the situation of nutritional disorder of the patient with the chronic nephrosis and especially for patients accepting dialysis therapy on the other hand; the low-phosphorus whey protein powder aims at avoiding the occurrence of hyperphosphatemia on the basis of replenishing high quality protein for the patients with the nephrosis, and improves the nutritional status of the patients with the nephrosis and promotes the physical recovery of the patients with the nephrosis at the same time. The product provided by the invention can help to improve the tolerance of the patients during treatment, reduce morbidity and mortality, improve the living quality of the patients, and the like. On this basis, a preparation method of the low-phosphorus whey protein powder is designed according to the characteristics of the formula materials; the method is carried out in a way of dry blending, and reduces the energy consumption by limiting the feeding sequence and the feeding amount; the obtained product is light in weight, good in water solubility and complete in nutrient retention.
Owner:中恩(天津)医药科技有限公司
Methods of reducing phosphate absorption
ActiveUS20100166760A1Eliminate side effectsReduce hyperphosphatemiaMetabolism disorderImmunoglobulins against cell receptors/antigens/surface-determinantsSerum phosphateHuman animal
A method for reducing phosphate absorption in a human or non-human animal subject at risk of developing or having developed hyperphosphatemia is disclosed. The method includes the step of administering orally to the subject an anti-intestinal sodium phosphate cotransporter type 2B (Npt2B) antibody in an amount effective to reduce or maintain the serum phosphate concentration in the subject.
Owner:CYCTOCHROMA INC +1
Application of medicinal carbon in preparation of medicaments for curing hyperphosphatemia
InactiveCN101904868AReduce deathReduce productionMetabolism disorderCarbon active ingredientsPeritoneal dialysisSecondary hyperparathyroidism
The invention relates to novel medicinal application of medical carbon, in particular to the application of the medicinal carbon in the preparation of medicaments for curing hyperphosphatemia. In the invention, clinical tests prove that the medicinal carbon, when taken orally, can reduce the serium inorganic phosphorus level and the product of calcium and phosphorus of patients who have received hemodialysis and peritoneal dialysis but do not have the hyperphosphatemia controlled after receiving the treatment by the calcium-containing phosphate binder, and provides a voltage detection (VD) therapy opportunity for patients with secondary hyperparathyroidism which cannot be treated by the VD therapy because of over-high product of calcium and phosphorus. The medical carbon is favorable for reducing and preventing angiocardiopathy development death, caused by chronic kidney disease-mineral and bone disorder (CKD-MBD), of the dialysis patients. Thus, the medicinal carbon can be used as novel medicaments for curing the hyperphosphatemia of patients with dialysis.
Owner:河北长天药业有限公司
Method for preparing lanthanum carbonate by reacting supercritical carbon dioxide and lanthanum oxide
InactiveCN103145170ACause some damagesSimple and fast operationRare earth metal compoundsBulk chemical productionHyperphosphoremiaLanthanum
The invention discloses a method for preparing lanthanum carbonate by reacting supercritical carbon dioxide and lanthanum oxide, which comprises the following steps: filling 50-99.999 wt% lanthanum oxide rare-earth raw material which has passed a 10-200-mesh sieve into an extraction kettle of a supercritical carbon dioxide extraction instrument, and reacting for 1-24 hours to obtain the lanthanum carbonate in the extraction kettle, wherein in the reaction process, the temperature of the extraction kettle is controlled at 32-100 DEG C, the pressure is 7.5-50 MPa, and the carbon dioxide with the mass percent of higher than 99.5% is subjected to forced circulation. The method is simple to operate, and has the advantages of no byproduct, low cost, high product purity and no environmental pollution. The lanthanum carbonate is a novel medicine for treating hyperphosphatemia, and has wide market prospects.
Owner:JISHOU UNIVERSITY
Phosphate-binding chitosan and uses thereof
The present invention provides compositions and methods for removing phosphate from a subject using chitosan. The present invention also provides compositions and methods for treating hyperphosphatemia based on phosphate-binding chitosan.
Owner:CYPRESS PHARMA
Method for preparing lanthanum carbonate by reacting subcritical carbon dioxide and lanthanum oxide
InactiveCN103145169ACause some damagesSimple and fast operationRare earth metal compoundsRare earthRadiochemistry
The invention discloses a method for preparing lanthanum carbonate by reacting subcritical carbon dioxide and lanthanum oxide, which comprises the following steps: filling 80-99.999 wt% lanthanum oxide rare-earth raw material which has passed a 40-300-mesh sieve into a reaction kettle, and reacting for 20-60 hours to obtain the lanthanum carbonate in the reaction kettle, wherein in the reaction process, the temperature of the extraction kettle is controlled at 25-31 DEG C, the pressure is 5-7.4 MPa, and the carbon dioxide with the mass percent of higher than 99.5% is subjected to forced circulation. The method is simple to operate, and has the advantages of no byproduct, low cost, high product purity and no environmental pollution. The lanthanum carbonate is a novel medicine for treating hyperphosphatemia, and has wide market prospects.
Owner:JISHOU UNIVERSITY
Composition with effect of preventing and treating complications of dialysis patient, and preparation method and application of composition
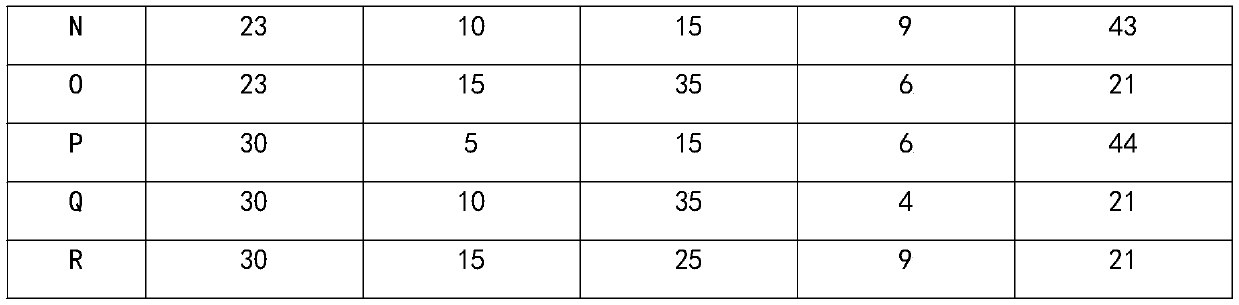
ActiveCN110538315AHelps correct negative nitrogen balanceImprove protein nutritional statusOrganic active ingredientsPeptide/protein ingredientsPatient compliancePotassium
The invention provides a composition with the effect of preventing and treating complications of a dialysis patient, and a preparation method and application of the composition. The composition is prepared from the components in parts by mass: 5-33 parts of whey proteins, 1-20 parts of casein or salts of the casein, 1-50 parts of maltodextrin, 1-10 parts of corn syrup and 5-75 parts of sunflower seed oil. The blood potassium level can be controlled to be lower than 5.0 mmol / L, the blood phosphorus level is controlled to be lower than 1.78 mmol / L, the complications such as protein malnutrition,hyperphosphatemia, hyperkalemia and hypertension of the dialysis patient are comprehensively relieved, the dialysis interval time of the dialysis patient is prolonged, and the harm of dialysis to thebody is reduced. In addition, the composition in each unit dose is brewed with 60 mL of water, the liquid intake burden is relieved and is equivalent to the osmotic pressure of the human body, gastrointestinal tolerance is better, and patient compliance is higher.
Owner:麦孚营养科技(北京)有限公司 +1
Coated pharmaceutical compositions
InactiveUS20150283170A1Granular deliverySynthetic polymeric active ingredientsEnd stage renal diseasePolymer
The present invention relates to polycarbophil coated crosslinked amine polymers and / or pharmaceutical compositions comprising polycarbophil coated crosslinked amine polymers. The polycarbophil coated crosslinked amine polymers have several therapeutic applications, including, but not limited to, hyperphosphatemia, chronic kidney disease and End-Stage Renal Disease.
Owner:GENZYME CORP
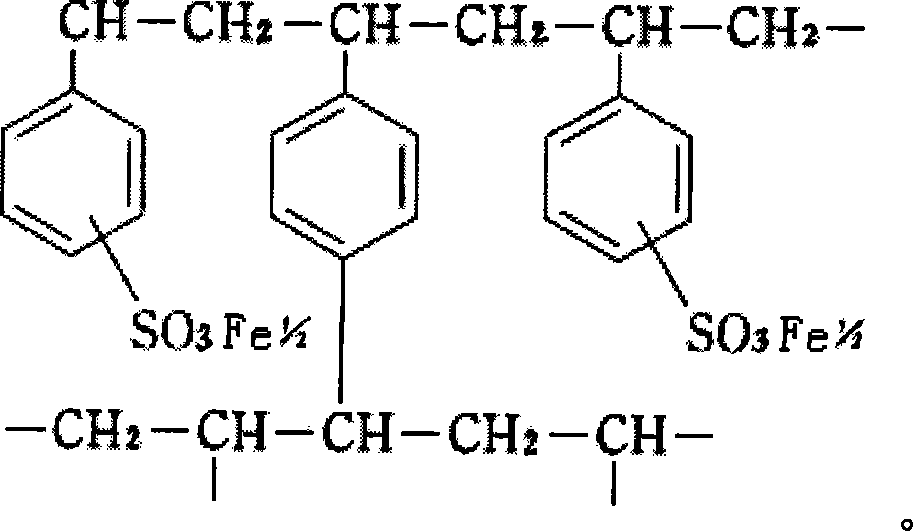
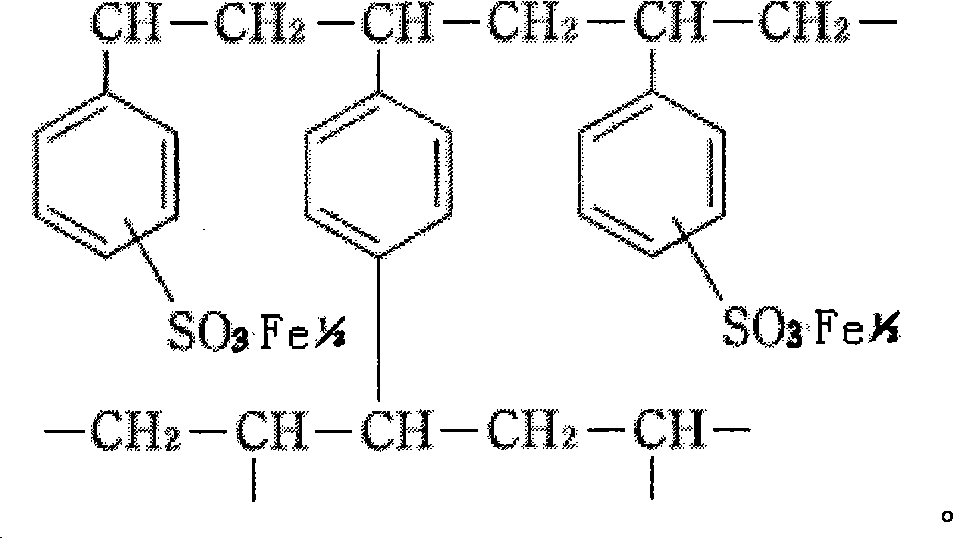
A medicine for treating hyperphosphatemia disease and iron deficiency anemia disease
ActiveCN101156869APhosphate reductionTo achieve the purpose of treating hyperphosphatemiaOrganic active ingredientsPowder deliveryDiseasePhysiology
The invention relates to medicine treating hyperphospheremia and iron deficiency anemia. Polystyrene sulfoacid ferrous iron resin is used as the effective component of the medicine, the medicine is produced by either adding medical auxiliary material or not, administration is performed by passing through gastrointestinal tract, the effective component and phosphate in the alimentary tract of a patient are combined together to be an insoluble substance which is excreted to the outside of the body through the alimentary tract, thereby the phosphate in the body of a chronic renal failure patient is reduced, and the aim of treating the hyperphospheremia is achieved. Simultaneously, residual ferrous ions of the polystyrene sulfoacid ferrous iron resin are dissociated, and are absorbed by the human body to supplement the ferrous ions in the body, thereby achieving the aim of treating the iron deficiency anemia of the chronic renal failure patient. The medicine can also be used for the treatment of the hyperphospheremia of the chronic renal failure patient and used as the preventive agent of the iron deficiency anemia.
Owner:GUANGDONG BANGMIN PHARMA
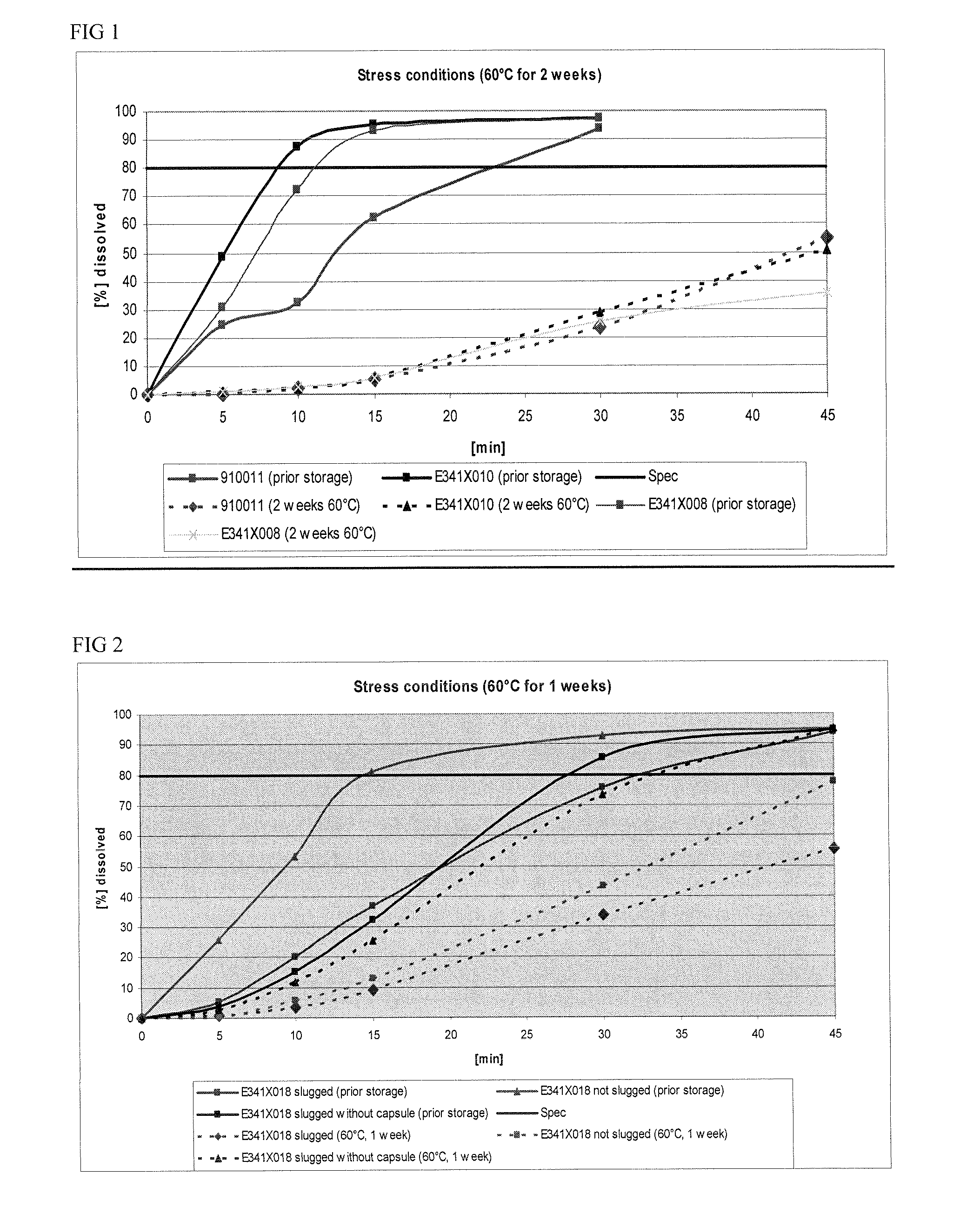
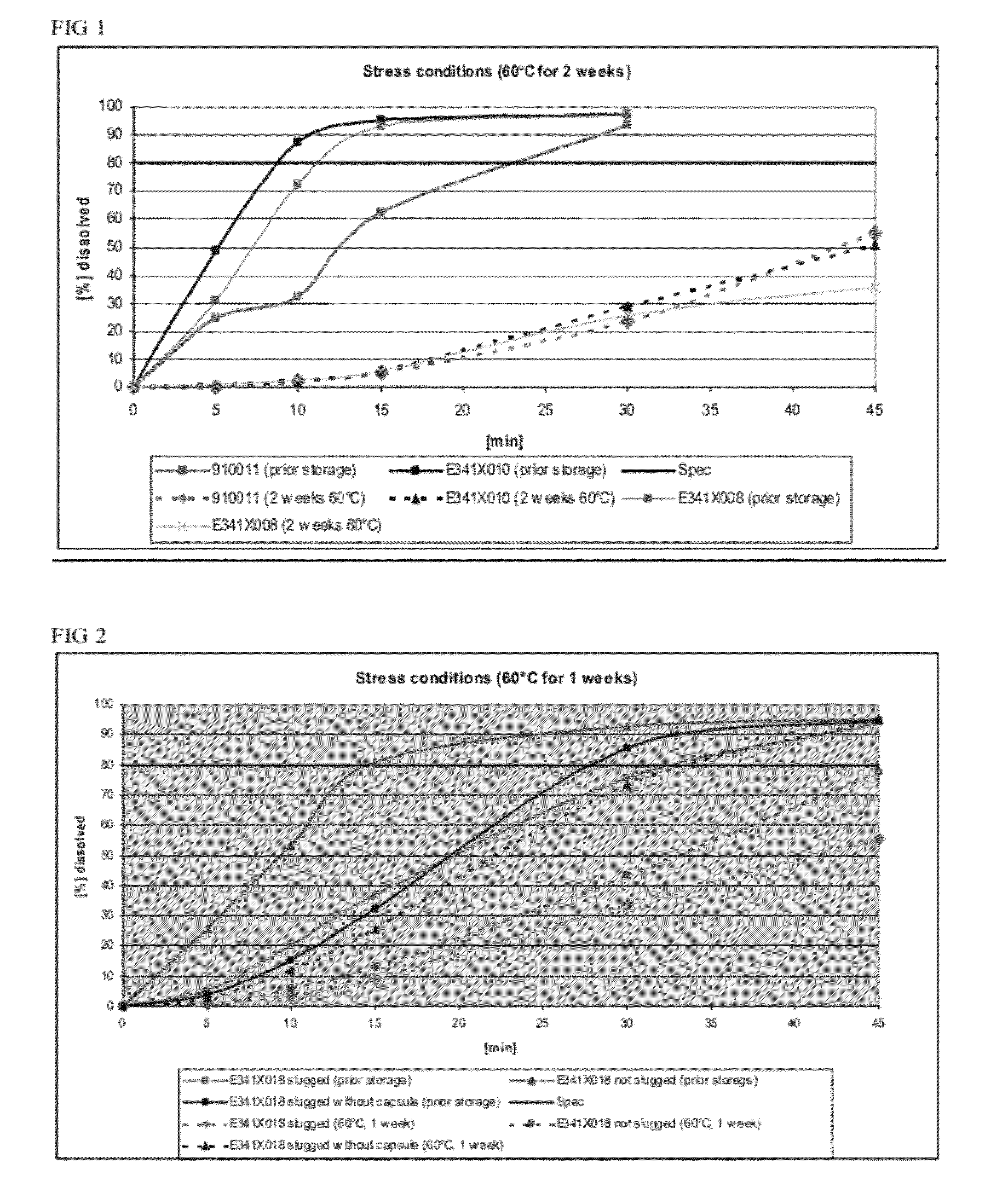
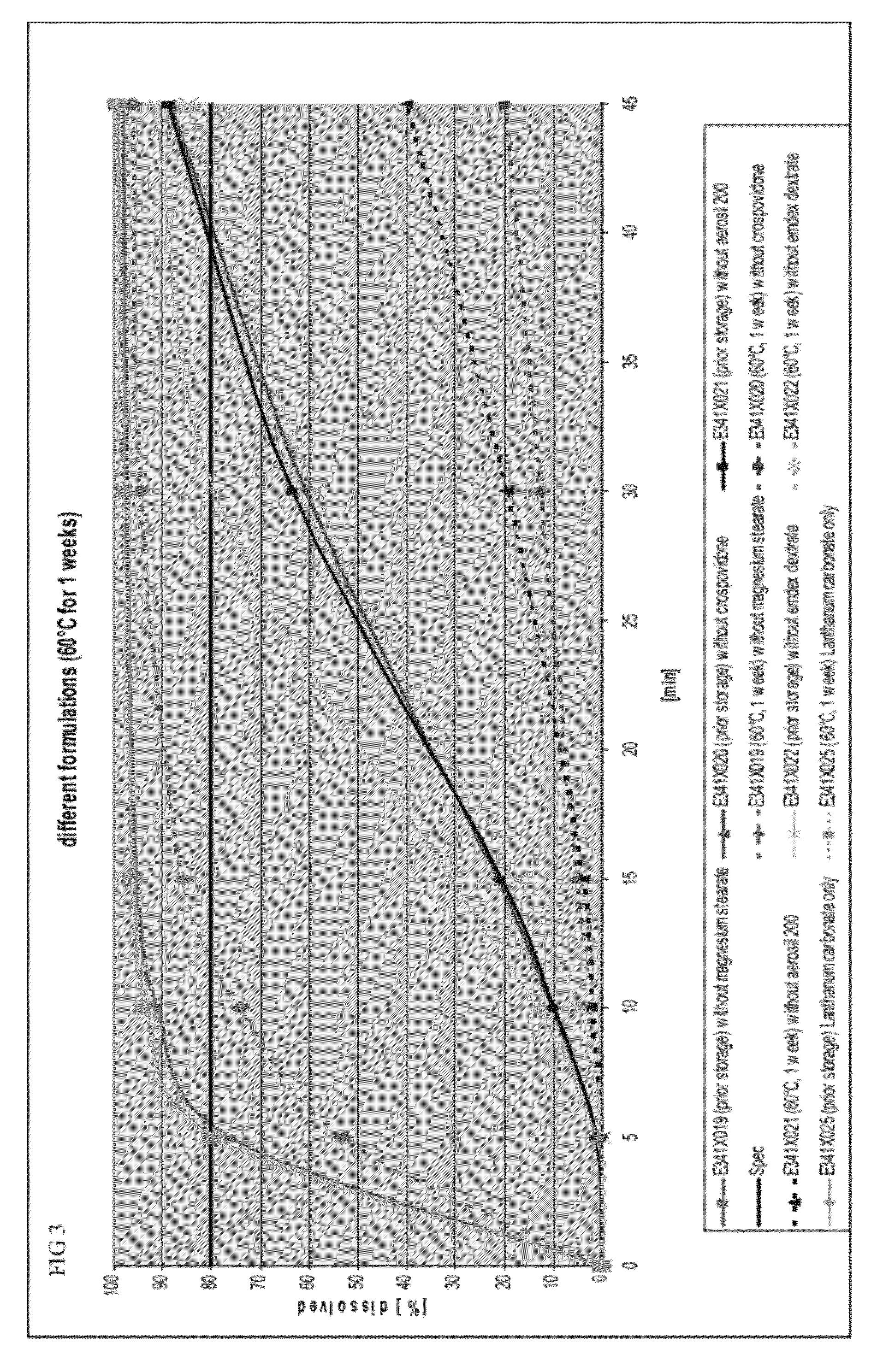
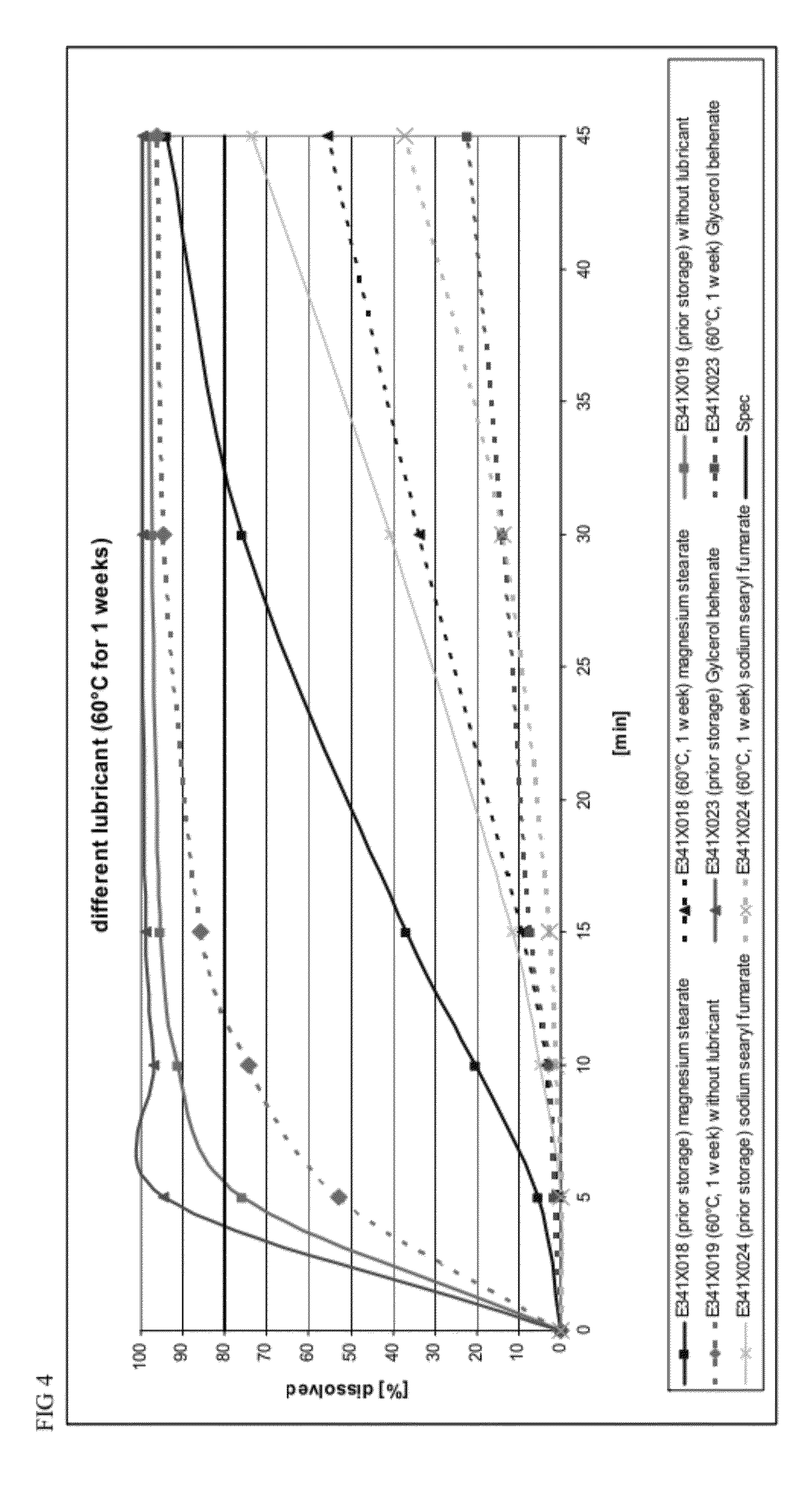
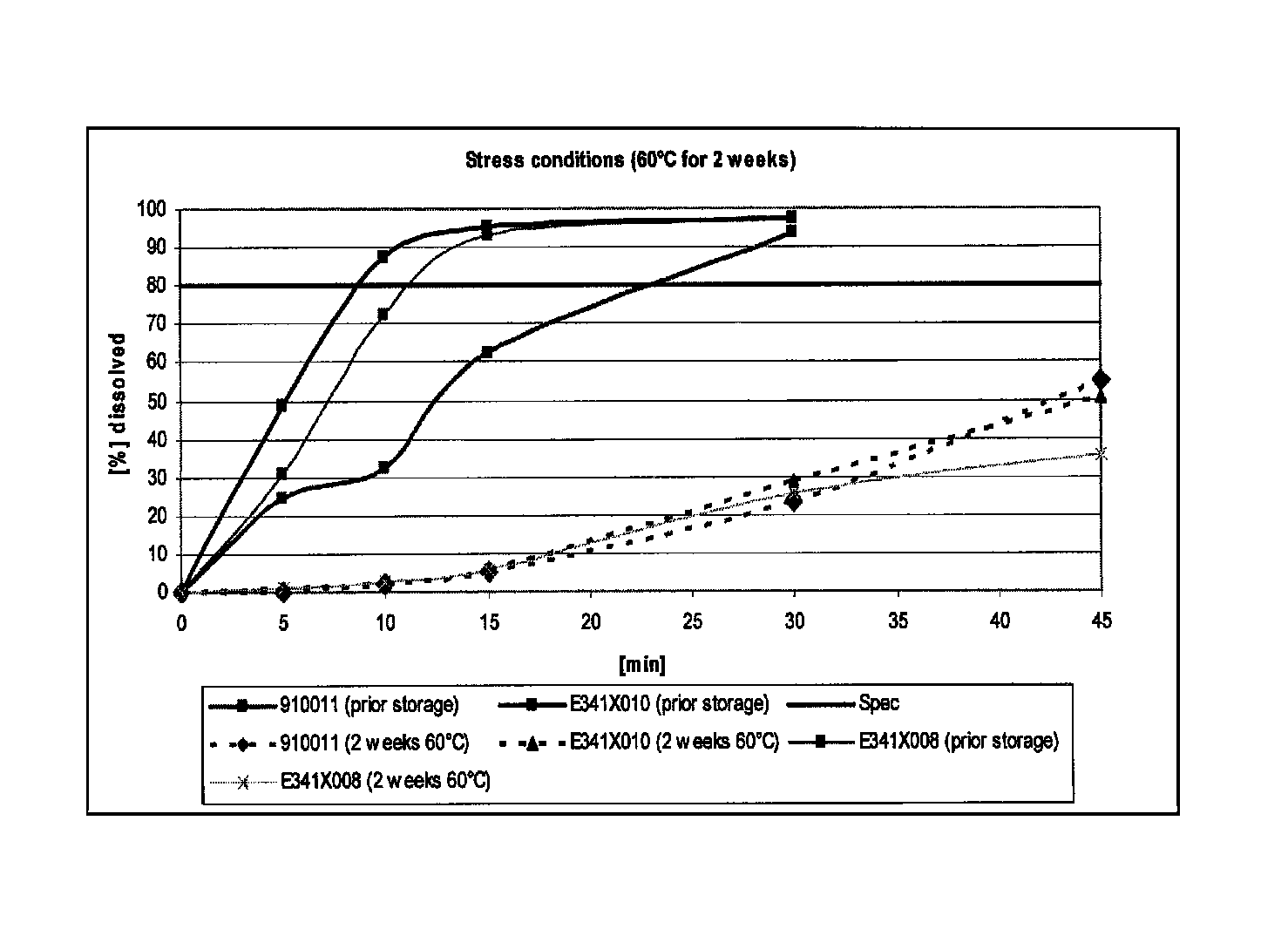
Capsule formulations containing lanthanum compounds
InactiveUS20120141580A1Powder deliveryHeavy metal active ingredientsSecondary hyperparathyroidismHyperphosphoremia
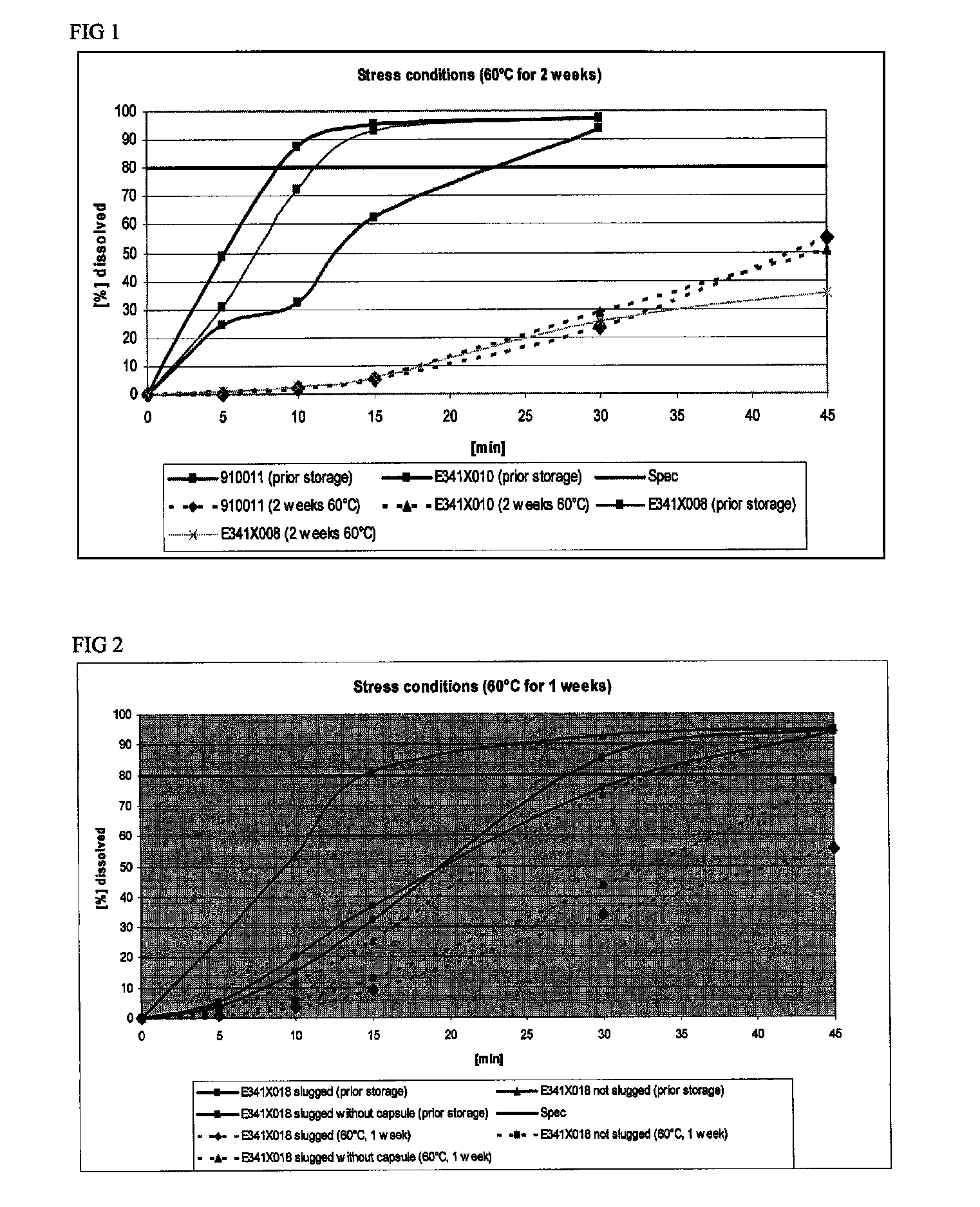
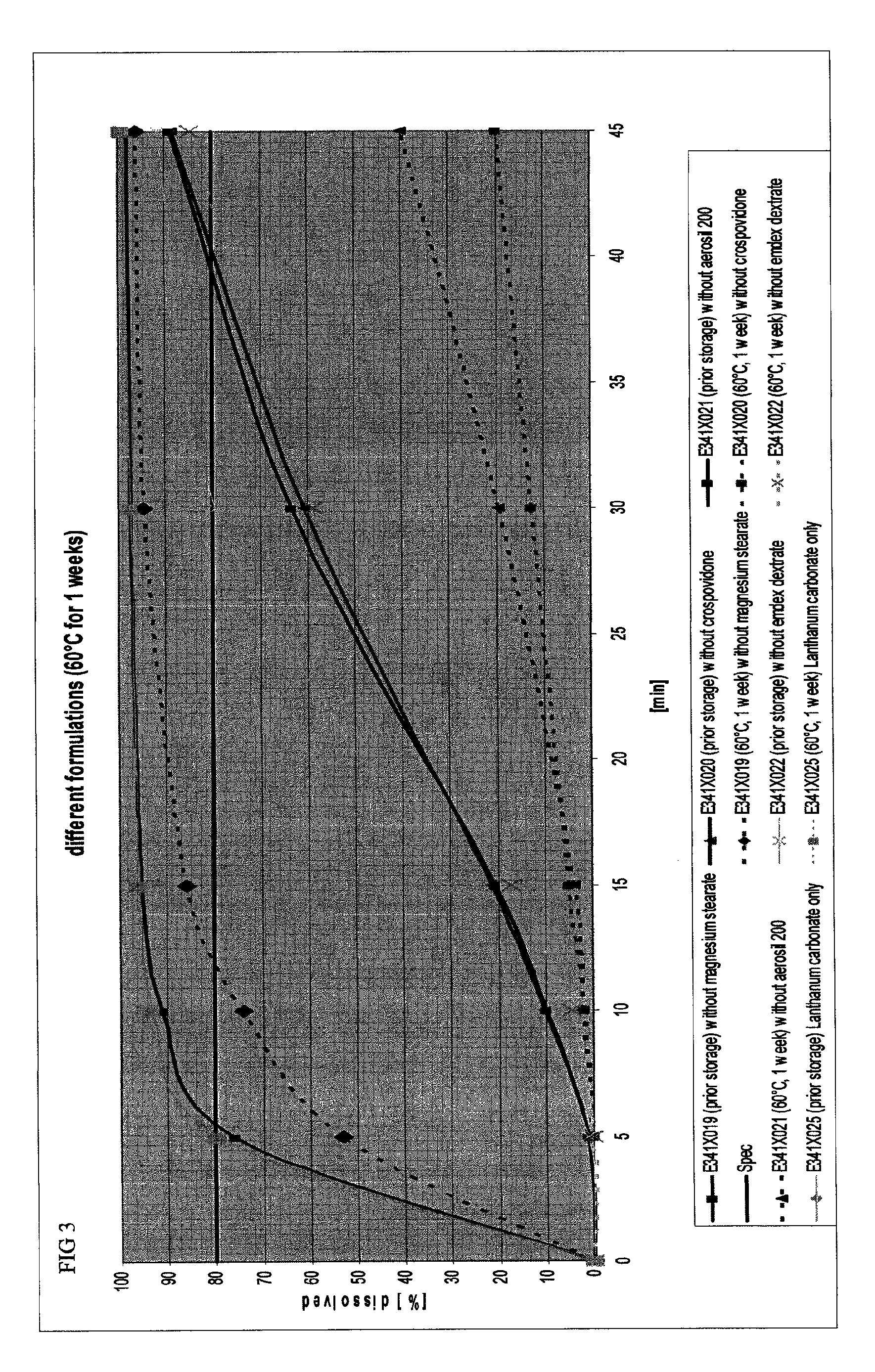
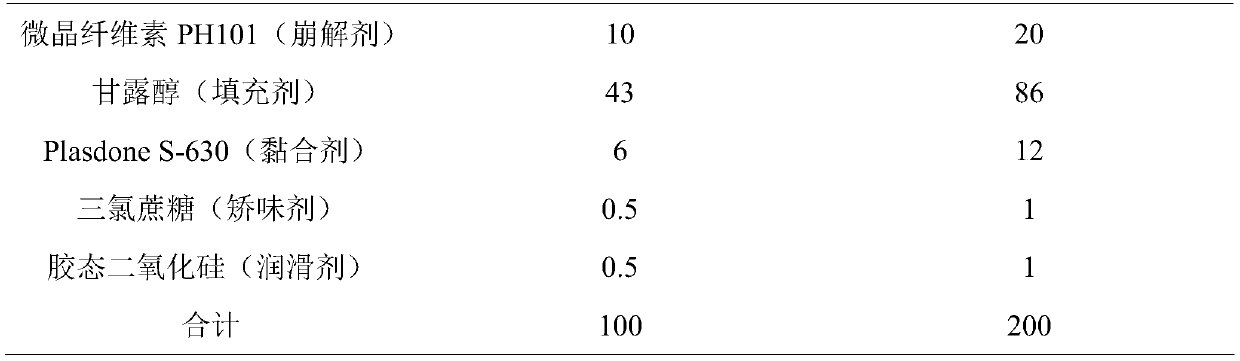
The present invention includes an oral pharmaceutical capsule comprising a shell, lanthanum carbonate or lanthanum carbonate hydrate, and a lubricant such as talc, wherein the shell encapsulates the lanthanum carbonate or lanthanum hydrate and the lubricant. Capsule shells comprise, for example, gelatin. The capsules of the present invention dissolve at a similar rate before and after storage. The oral pharmaceutical capsules of the present invention can be administered to treat a patient at risk for or suffering from hyperphosphatemia, at risk for or suffering from chronic kidney disease (CKD), at risk for or suffering from soft tissue calcification associated with CKD, or at risk for or suffering from secondary hyperparathyroidism.
Owner:SHIRE PLC
Lanthanum hydroxide therapeutical effects on hyperphosphatemia and relative diseases
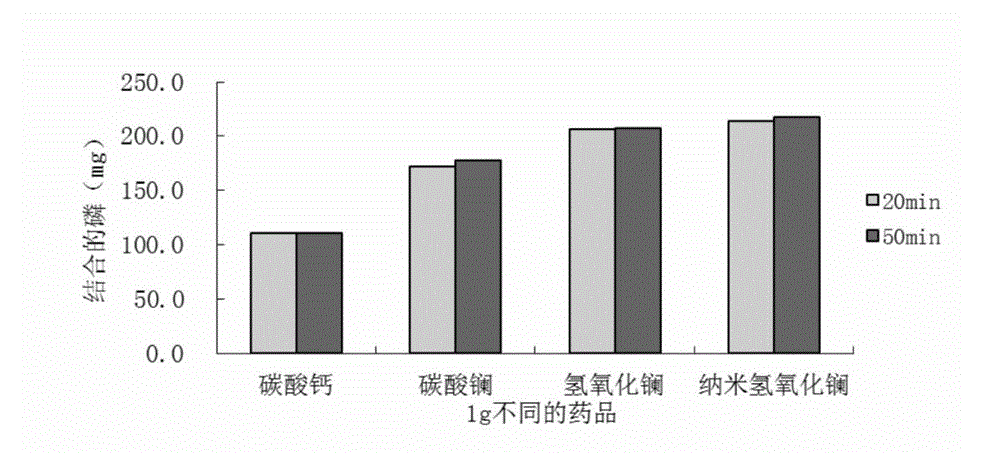
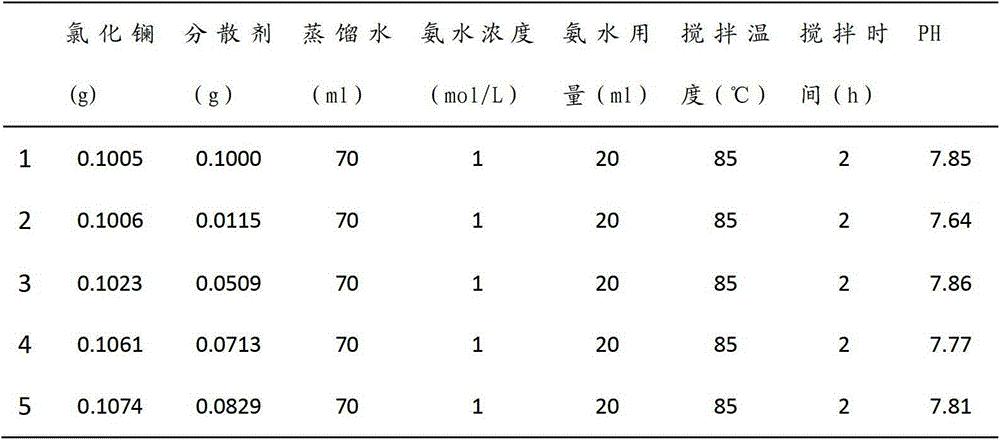
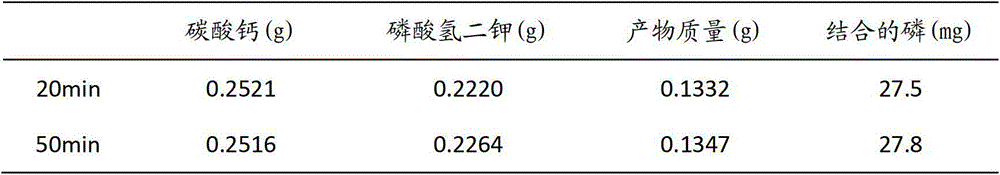
The invention relates to lanthanum hydroxide which has the functions of preparing and curing chronic renal failure (CRF), hyperphosphatemia or / and hyperparathyroidism (SHPT), vitamin D metabolic block, renal osteopathy and cardiovascular system calcification drugs, wherein the lanthanum hydroxide is nanometer lanthanum hydroxide, and daily dose of the lanthanum hydroxide or the nanometer lanthanum hydroxide is 600mg to 7800mg..
Owner:INNER MONGOLIA MEDICAL UNIV
Capsule and powder formulations containing lanthanum compounds
The present invention includes an oral pharmaceutical capsule comprising a shell, lanthanum carbonate or lanthanum carbonate hydrate, and a lubricant such as talc, wherein the shell encapsulates the lanthanum carbonate or its hydrate and the lubricant. Capsule shells comprise, for example, gelatin. The present invention also includes an oral pharmaceutical powder comprising lanthanum carbonate or lanthanum carbonate hydrate and a pharmaceutically acceptable excipient. The oral pharmaceutical capsules and powders of the present invention can be administered to treat a patient at risk of or suffering from hyperphosphatemia, at risk of or suffering from chronic kidney disease (CKD), at risk of or suffering from soft tissue calcification associated with CKD, or at risk of or suffering from secondary hyperparathyroidism.
Owner:TAKEDA PHARMA CO LTD
A method for preparing oral rapidly disintegrating tablets for the treatment of hyperphosphatemia by 3D printing technology
ActiveCN107213126BGuaranteed hardnessSimple preparation processAdditive manufacturing apparatusMetabolism disorderPatient complianceGlycerol
The invention provides a method for preparing oral rapidly disintegrating tablets for the treatment of hyperphosphatemia by 3D printing technology: fully mix the raw powder of lanthanum carbonate pharmaceutical grade and auxiliary materials, and use ethanol aqueous solution, glycerin, polysorbate, and copovidone The mixed solution of S-630 is made into tablets by powder-liquid 3D printing technology. The tablet prepared by the invention has a large drug loading capacity, is easy to disintegrate, can improve patient compliance, and is suitable for treating hyperphosphatemia with precise doses for patients with chronic renal failure.
Owner:XIAN DI JIA BIOTECH CO LTD
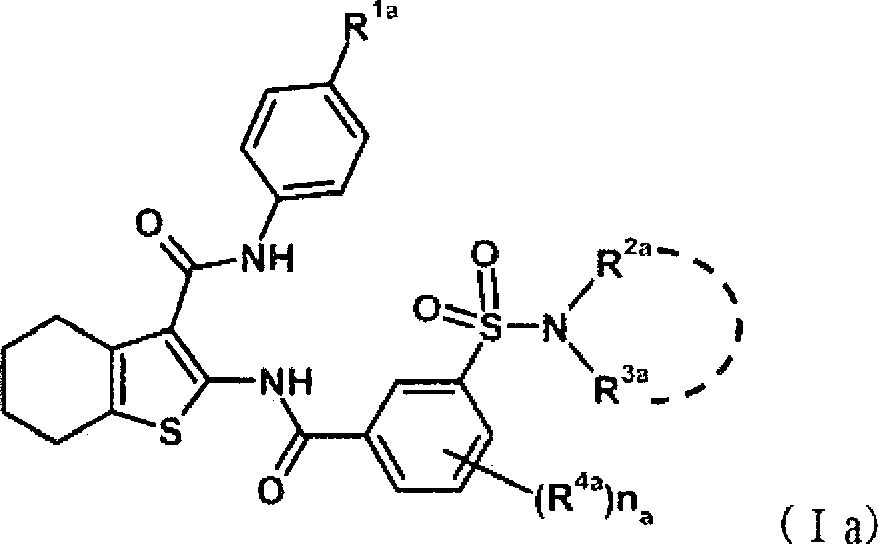
Benzamide derivatives, their preparation method and their application in medicine
The invention relates to a benzamide derivative as well as a preparation method and medicinal application thereof. Specifically, the invention relates to a benzamide derivative shown by a general formula (I), a preparation method thereof and a pharmaceutical composition containing the benzamide derivative, and an application of the benzamide derivative as a therapeutic agent and particularly as an intestinal 2B-type sodium phosphate cotransporter (Npt2b) inhibitor and an application thereof in preparing a medicine for treating and / or preventing hyperphosphatemia, wherein the definition of each substituent in the general formula (I) is the same as that in the specification.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Nutrition conveying method of low-protein type total nutrient
PendingCN111685315ADelivery scienceEffective absorptionInorganic compound food ingredientsFood ingredient functionsBiotechnologyGlycolipid metabolism
The invention provides a nutrition conveying method of a low-protein type total nutrient. According to the method for conveying the nutrient according to the intestinal gas amount of a microbiota, theintestinal gas amount of the microbiota related to a patient is monitored; the nutrient delivery amount delivered to the patient is controlled and / or the nutrient delivery rate is adjusted based on the relative change of the microbial community intestinal tract gas relative to the baseline value, so that the nutrient delivery is more scientific, and the absorption is more efficient. Besides, thelow-protein nutrient disclosed by the invention is scientific in formula, not only can reduce the intake of dietary protein to delay the loss of the residual kidney function of the patient, but also is beneficial to reducing the urine protein level of the patient and improving hyperphosphatemia, glycolipid metabolism and hypertension.
Owner:JIANG TENG MEDICAL SCI & TECH CO LTD
Methods for reducing phosphate absorption
InactiveUS20100143385A1Reducing phosphate absorptionReduce and maintain serum phosphate concentrationAntibacterial agentsEgg immunoglobulinsSerum phosphateHuman animal
It is disclosed here a method for reducing phosphate absorption in a human or non-human animal subject wherein the subject consumes a diet containing phytic acid or phytate and either has or is at risk of developing hyperphosphatemia. The method includes the step of administering orally to the subject an anti-phytic acid (C6H6[OPO(OH)2]o) antibody or an anti-phytate antibody in an amount effective to reduce or maintain the serum phosphate concentration in the subject.
Owner:CYCTOCHROMA INC +1
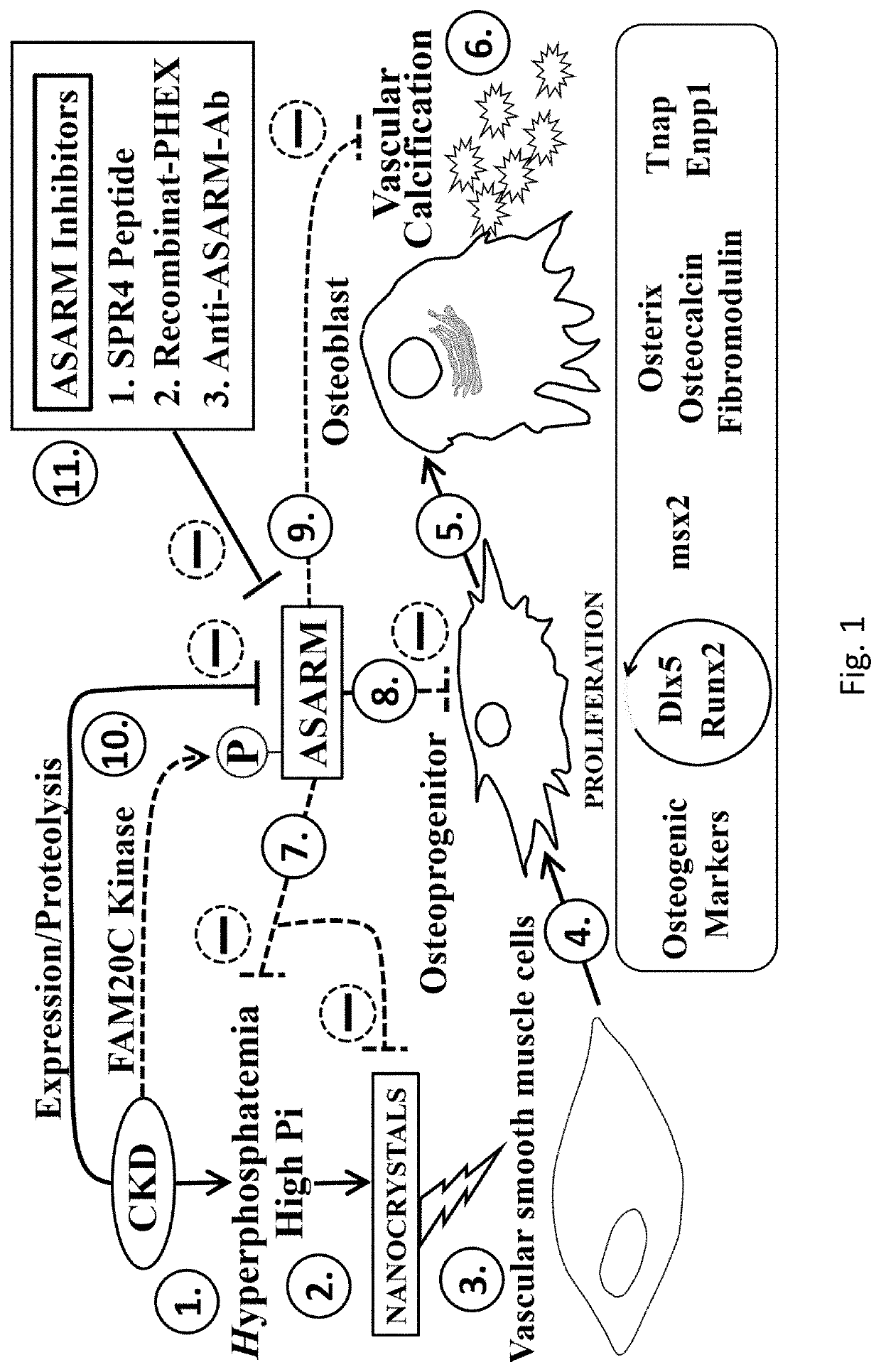
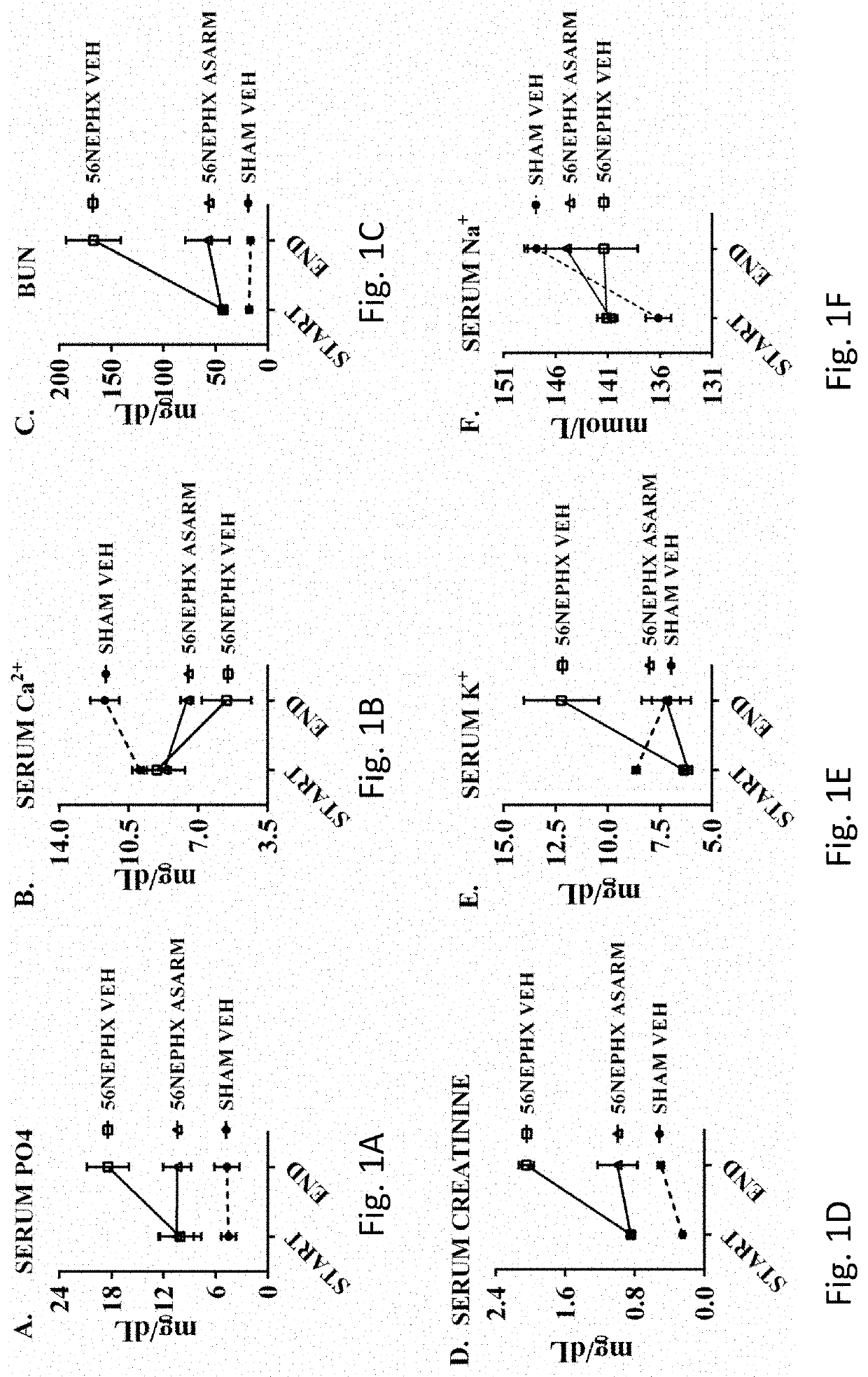
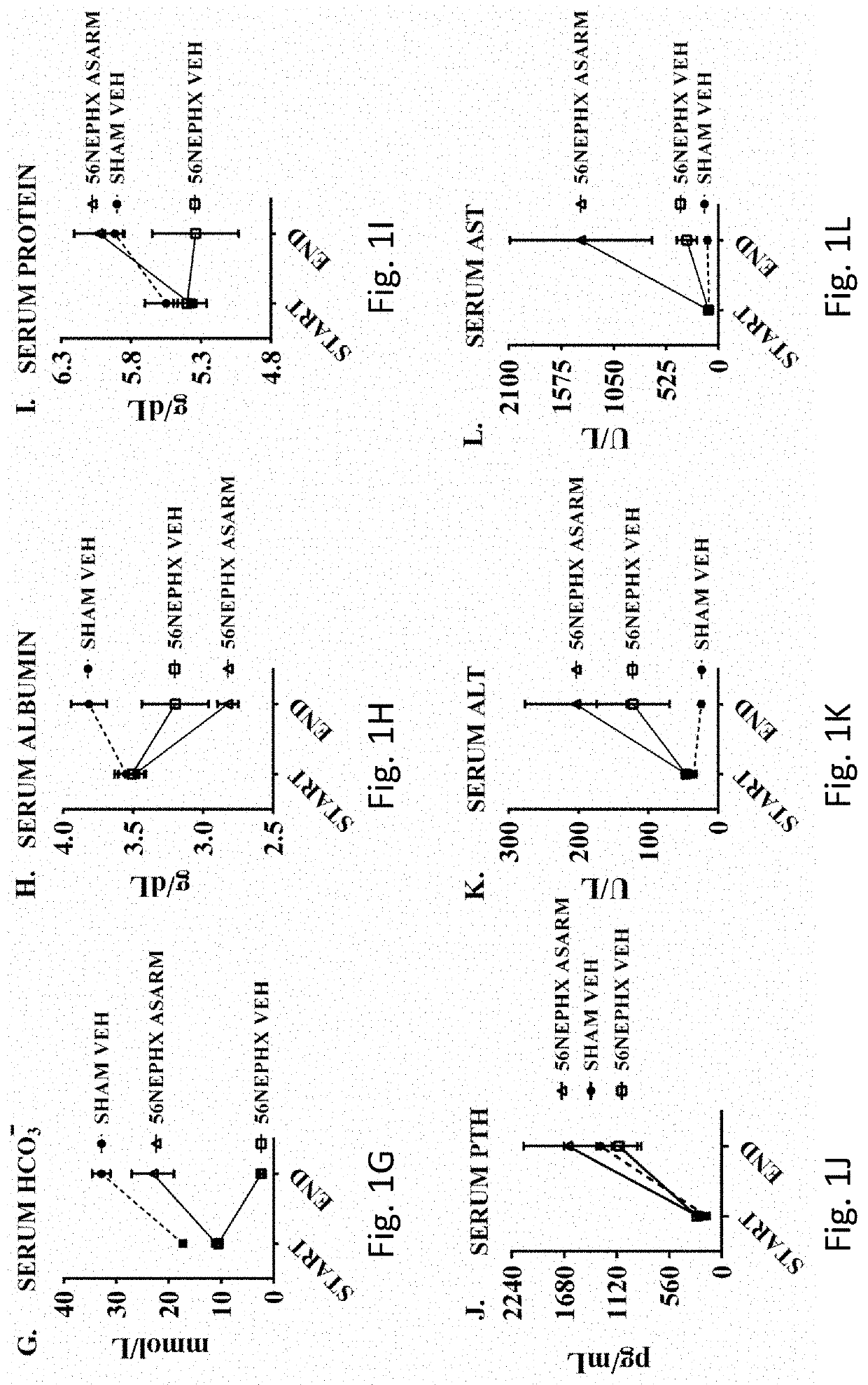
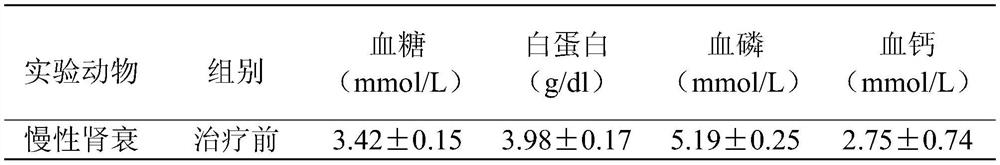
Asarm treatment for kidney and mineralization disorders and hyperphosphatemia
PendingUS20220105151A1Reducing serum phosphate levelAvoid absorptionPeptide/protein ingredientsUrinary disorderFibrosisHyperphosphoremia
A method of treating or inhibiting a kidney disorder can include administering a therapeutically effective amount of the ASARM peptide to provide a treatment for the kidney disorder (treat disease) and / or inhibit development of the kidney disorder (prophylactic). The kidney disorder can be selected from the group consisting of chronic kidney disease mineral bone disorder (CKD-MBD), calciphylaxis, nephrogenic systemic fibrosis (NSF), end stage renal disease, and combinations thereof. The therapy can include inhibiting vascular calcification (VC), hard tissue calcification, soft tissue calcification, mineralization, or combinations thereof in the subject with the ASARM peptide. A method of inhibiting mineralization can include administering a therapeutically effective amount of the ASARM peptide to provide a treatment for inhibit mineralization in the subject. A method of treating hyperphosphatemia can include administering a therapeutically effective amount of the ASARM peptide to a subject to provide a treatment for hyperphosphatemia.
Owner:UNIVERSITY OF KANSAS
A nanocomposite material for adsorbing phosphorus and its preparation and application
ActiveCN107412166BImprove adsorption capacityNot easy to catchOrganic active ingredientsMetabolism disorderSerum phosphateSevelamer
The invention relates to a phosphate adsorption composite nanomaterial as well as preparation and an application thereof. The composite nanomaterial comprises a nano-carrier and Sevelamer carbonate in the mass ratio being (3-12):1, wherein the nano-carrier comprises lipidosome, and Sevelamer carbonate is polymer carbonate of 2-propen-1-amine and epoxy chloropropane. The phosphate adsorption nano-composite material is used for preparing a medicine for reducing serum phosphate. Compared with the prior art, the excellent adsorption performance of polymer carbonate of 2-propen-1-amine and epoxy chloropropane is sufficiently utilized, the advantages that the long-circulation nano-carrier is non-toxic, stable and not easily captured by a phagocytic system in a body are played, and the high-biocompatibility lipidosome / Sevelamer composite nanomaterial easy to excrete is prepared. The method adopts a simple preparation process and easily controllable conditions and is thus suitable for clinic treatment of intractable hyperphosphatemia.
Owner:TONGJI UNIV
A gel-based layered inorganic phosphorus binder and its preparation method and application
ActiveCN109528764BEffective protectionEasy accessHeavy metal active ingredientsMetabolism disorderPhosphoric acidCross linker
The invention discloses a gel-based layered inorganic phosphorus binder and its preparation method and application. The preparation method is to take the layered inorganic matter, add water and stir at room temperature to make it fully swell; add a gelling agent, and continue stirring until Completely dissolve, add the above mixture dropwise into the cross-linking agent solution to obtain a composite gel ball, wash repeatedly with deionized water several times until the washing liquid does not contain the cross-linking agent, and then freeze-dry to obtain a gel Grass-based inorganic phosphorus binder. The invention also includes the application of the gel-based layered inorganic phosphorus binder in the preparation of medicines for treating hyperphosphatemia or chronic renal failure. The present invention introduces a gel system, which can effectively protect the layered inorganic matter, enable sufficient phosphorus binder to reach the intestines and stomach, and improve the efficiency of combining with phosphate.
Owner:北京市中关村医院 +1
Intestinal dialysis composition for pet dogs and cats and preparation method of intestinal dialysis composition
ActiveCN110812391AReduce synthetic ureaReduce absorptionUrinary disorderAluminium/calcium/magnesium active ingredientsEthylic acidHyperphosphoremia
The invention discloses an intestinal dialysis composition for pet dogs and cats and a preparation method of the intestinal dialysis composition and belongs to the field of veterinary care. The dialysis composition comprises the following components: 10-20g of unprocessed rehmannia roots, 10-20g of chitosan, 10-20g of calcium butyrate, 50-100g of modified zeolite and 200-300ml of acetic acid witha volume percentage of 4%. The preparation method comprises the following steps: sufficiently dissolving the unprocessed rehmannia roots, the chitosan and the calcium butyrate into the acetic acid, adding the modified zeolite, blending the components into a paste, performing sufficient infiltration, performing pelletizing by using a pelletizer, and performing drying at 60 DEG C for 4 hours, so asto obtain a finished product of which the particle size is 0.8-1.2mm. Components of the intestinal dialysis composition for pet dogs and cats take synergetic effects into play, uremia toxins such as urea and decomposition products, namely substances such as ammonia, phosphate and creatinine, of the uremia toxins, in intestines, can be adsorbed, small molecule substances such as the uremia toxins such as urea in blood can be promoted to be discharged to enterocoels, then the concentration of the small molecule substances in blood can be reduced, hyperazotemic and hyperphosphatemia caused by kidney failure can be relieved, and recovery of kidney failure can be facilitated. The composition is economic and practical and convenient to operate.
Owner:JIANGSU AGRI ANIMAL HUSBANDRY VOCATIONAL COLLEGE
Hyperphosphatemia treatment agent
PendingCN111225674ALower serum phosphorus concentrationQuality improvementOrganic active ingredientsMetabolism disorderSerum phosphorusCrosslinked polymers
The present invention addresses the problem of providing a low-dose hyperphosphatemia treatment agent that can substantially reduce serum phosphorus concentration. The present invention provides a hyperphosphatemia treatment agent that contains, as an active ingredient, particles that include a prescribed crosslinked polymer (preferably crosslinked polyallylamine).
Owner:FUJIFILM CORP +1
Intestinal dialysis composition for pet dogs and cats and preparation method thereof
ActiveCN110812391BReduce synthetic ureaReduce absorptionUrinary disorderAluminium/calcium/magnesium active ingredientsHyperphosphoremiaCompanion animal
Owner:陕西琢真小动物健康产业研发中心有限公司
Gel-based layered inorganic phosphorus binding agent and preparation method and application thereof
ActiveCN109528764AEffective protectionEasy accessHeavy metal active ingredientsMetabolism disorderGel basedFreeze-drying
The invention discloses a gel-based layered inorganic phosphorus binding agent and a preparation method and an application thereof. The preparation method includes: preparing a layered inorganic substance, adding water and stirring the mixture at room temperature to fully swell the substance; adding a gelatinizer, continuously stirring the mixture to complete dissolution, and dropwise adding the mixture to a crosslinker solution to prepare composite gel spheres; repeatedly washing the composite gel spheres for several times with deionized water until the washing liquid does not contain the crosslinker; freeze-drying the mixture to obtain the gel-based layered inorganic phosphorus binding agent. The invention also provides an application of the gel-based layered inorganic phosphorus bindingagent in preparation of medicines for treating hyperphosphatemia or chronic renal failure. By introducing the gel system, the layered inorganic substance is protected well, and a phosphorus binding agent can arrive at intestines and stomach with full dose, thus improving the binding efficiency between the phosphorus binding agent and phosphate.
Owner:北京市中关村医院 +1
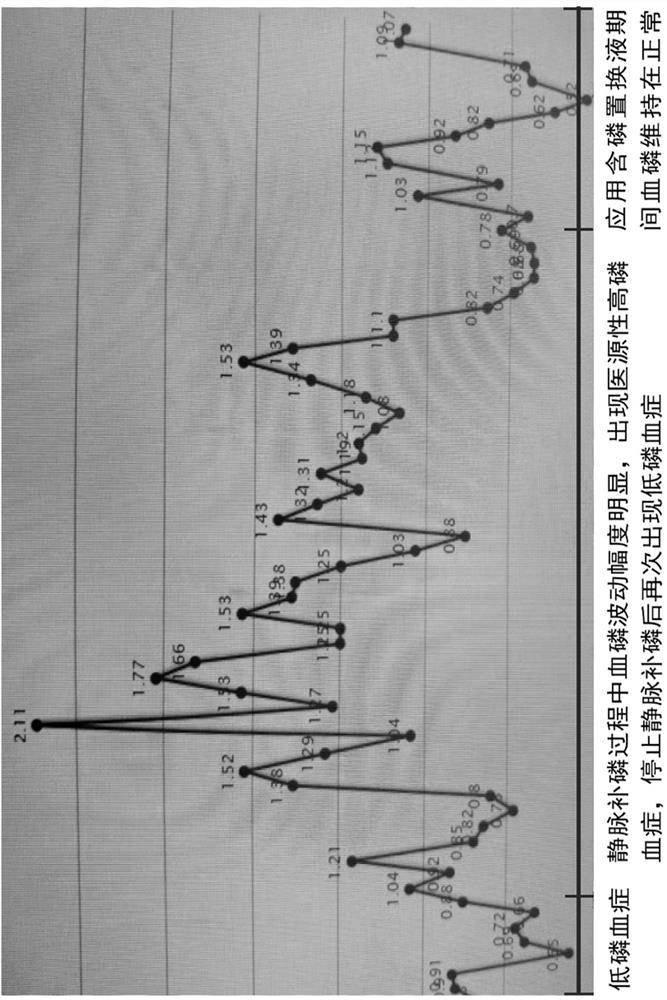
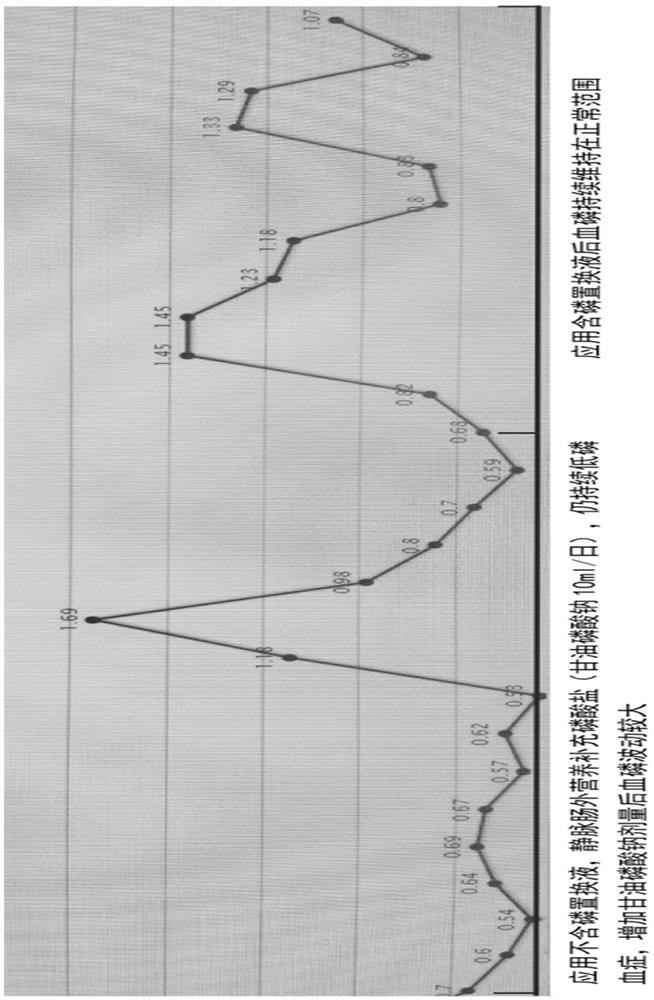
Preparation method and application of blood filtration displacement liquid containing organic phosphate
PendingCN114848655AReduce the incidence of hyperphosphatemiaRealize individualized treatment of phosphorus concentration in replacement fluidOrganic active ingredientsMetabolism disorderSodium phosphatesNutrition
The invention provides a preparation method and application of a blood filtration displacement liquid containing organic phosphate. Sodium glycerophosphate is used as organic phosphate and has good mixing compatibility with a calcium-containing solution, and a sodium glycerophosphate injection is clinically used as an intravenous nutrition additive or a component of a mixed electrolyte solution. Sodium glycerophosphate is catalyzed by alkaline phosphatase in vivo and hydrolyzed into inorganic phosphate and glycerol which are metabolized and utilized by the organism. The sodium glycerophosphate injection can be used as a source of phosphorus-containing displacement liquid phosphate. The plasma phosphorus concentration is 1.0-1.5 mmol / L, but due to the fact that part of phosphorus is combined with plasma protein to form a compound, the filterable ionic state phosphorus concentration is actually 0.9-1.0 mmol / L. Therefore, in 2021, the replacement liquid with the phosphorus concentration being 0.7-1.0 mmol / L and the phosphorus concentration being 1.0 mmol / L recommended by China Standard Operation Regulations for Blood Purification can effectively prevent and treat the CRRT critical symptom hypophosphatemia, and the replacement liquid with the phosphorus concentration being 0.7-1.0 mmol / L and the phosphorus concentration being 1.0 mmol / L can be used for preventing and treating the CRRT critical symptom hypophosphatemia. Meanwhile, iatrogenic hyperphosphatemia is avoided as far as possible.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com