A method for preparing oral rapidly disintegrating tablets for the treatment of hyperphosphatemia by 3D printing technology
An oral fast disintegrating tablet, 3D printing technology, applied in the field of medicine, can solve the problems of lanthanum carbonate 3D printing drug formula printing process of hyperphosphatemia, which has not been reported, the production process is cumbersome, and it is difficult to take, etc., and achieves high market application prospects. , the preparation process is simple, the effect of high drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
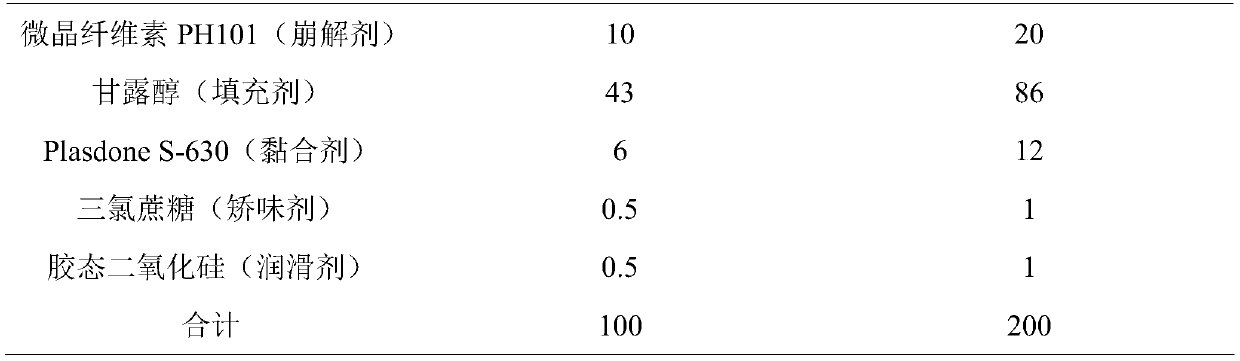
[0034] (1) Prescription (tablet)
[0035]
[0036]
[0037] Note: The amount of each component added in the prescription is based on the current amount ± 0.1g.
[0038] (2) 3D printing process:
[0039] 1) Pass lanthanum carbonate raw material (micronized lanthanum carbonate raw material) and mannitol through a 150-mesh sieve before weighing, and dry the viscous powder (mainly lanthanum carbonate) in an oven at 50°C before sieving 15 minutes, it is convenient to mix the viscous powder;
[0040] 2) Mix the prepared powder, stir and mix thoroughly to obtain the drug powder, ready to print;
[0041] 3) Preparation of adhesive (spray solution): 10% ethanol+(1.0% glycerol, 0.5% polysorbate 80)+2% Plasdone S-630 as adhesive; that is, adhesive is 10% ethanol by volume percentage The aqueous solution is used as a solvent, and glycerin, polysorbate 80, and Plasdone S-630 are dissolved therein, and the mass fractions of glycerin, polysorbate 80, and Plasdone S-630 in the adhesi...
Embodiment 2
[0051] (1) Prescription (tablet)
[0052]
[0053] Note: The amount of each component added in the prescription is based on the current amount ± 0.1g.
[0054] (2) 3D printing process
[0055] Condition is identical with embodiment 1;
[0056] After inspection: the weight of the tablet is 1.08g, the hardness is 3.48kg, and the disintegration time limit is 3-7 seconds.
Embodiment 3
[0058] (1) Prescription (tablet)
[0059]
[0060] Note: The amount of each component added in the prescription is based on the current amount ± 0.1g.
[0061] (2) 3D printing process
[0062] Except following conditions, other is identical with embodiment 1:
[0063] ①Single-layer liquid spraying twice, the number of layers is 61;
[0064] After inspection: the tablet weighs 1.62g, the hardness is 4.71kg, and the disintegration time limit is 4-5 seconds.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com