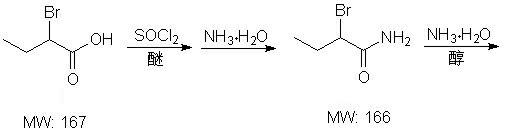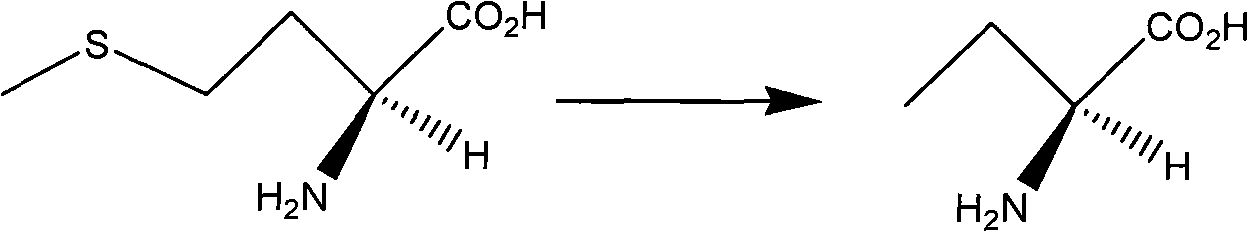Patents
Literature
175 results about "Levetiracetam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
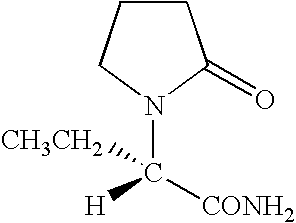
Levetiracetam is used with other medications to treat seizures (epilepsy).
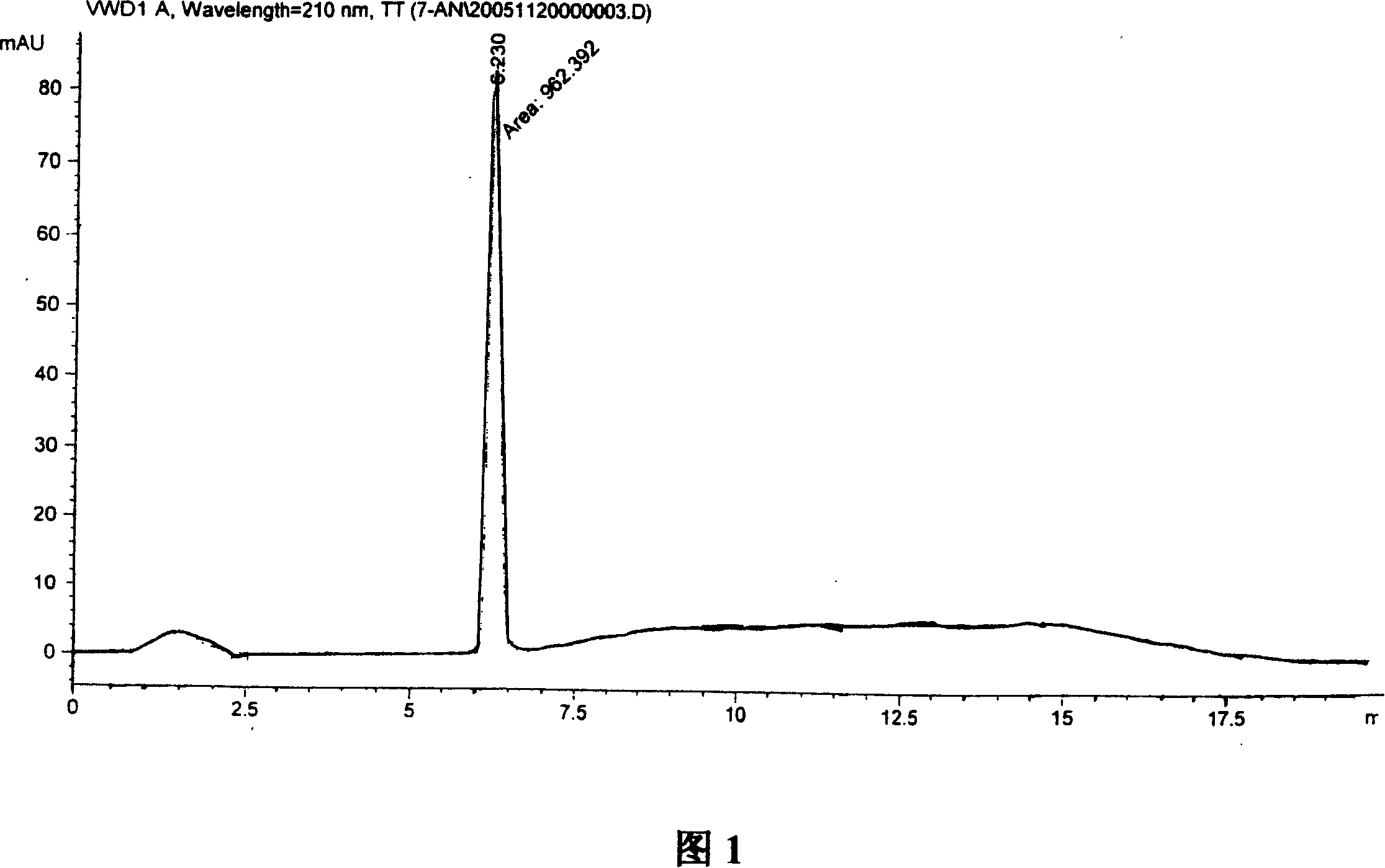
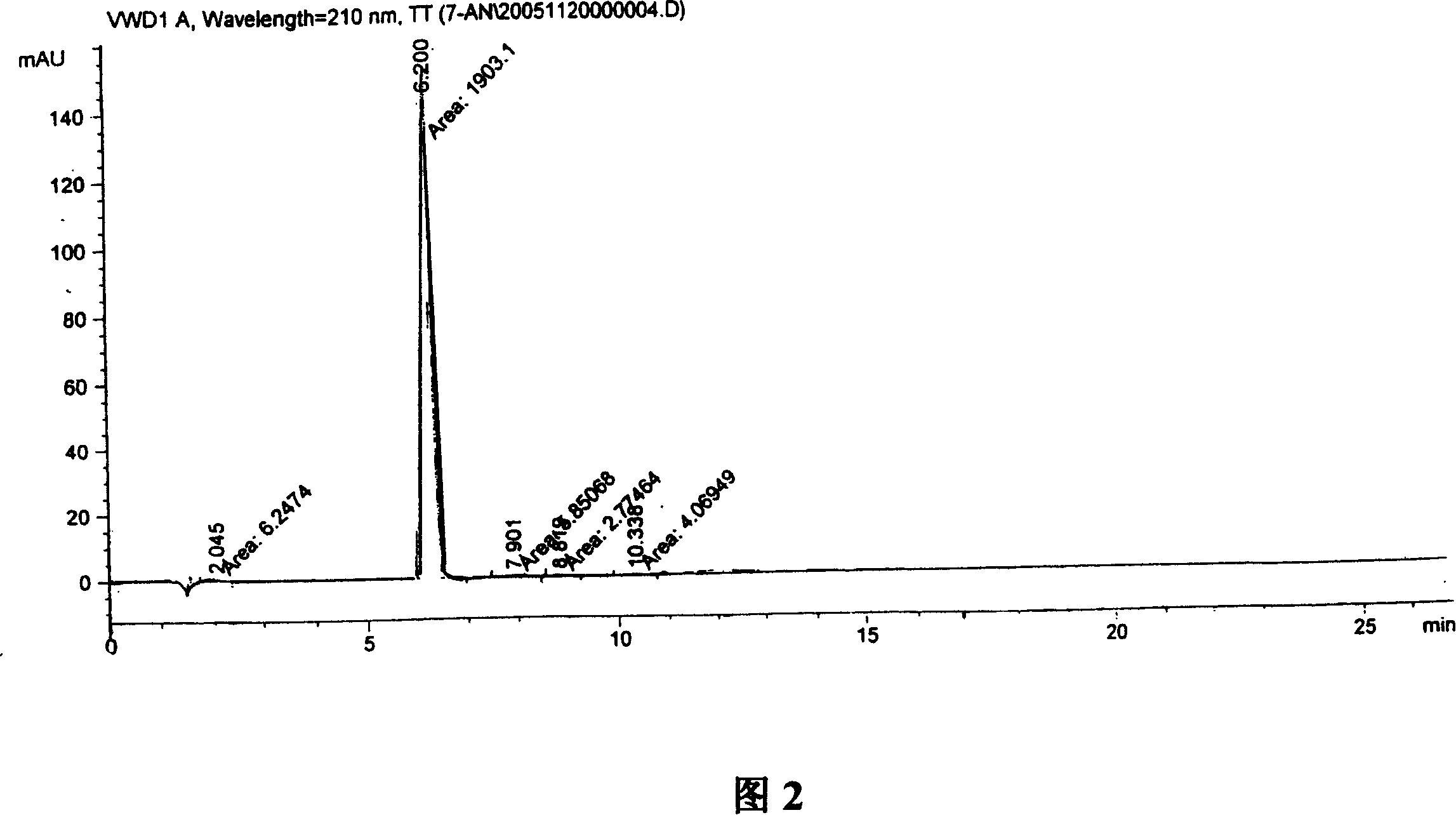
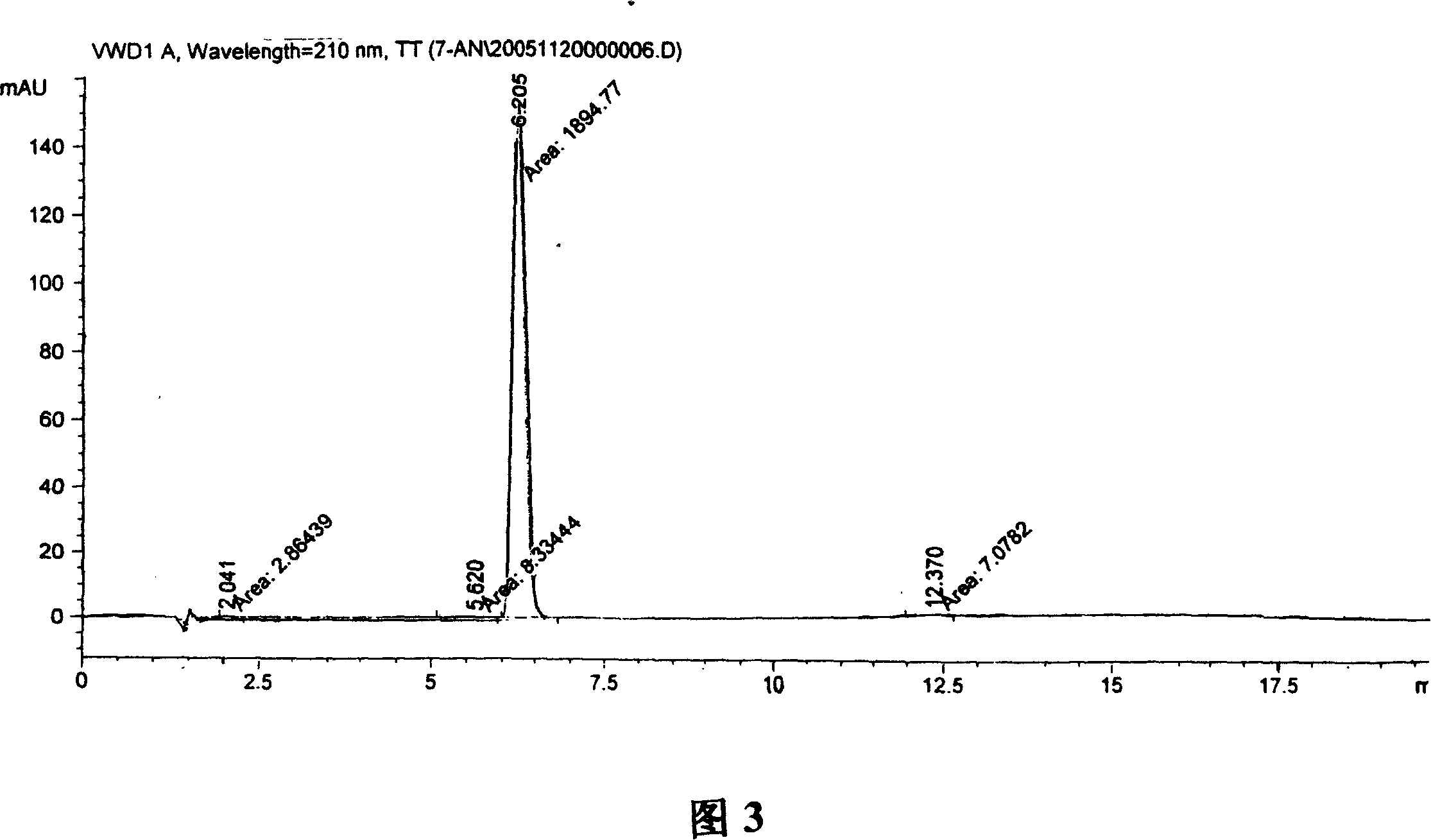
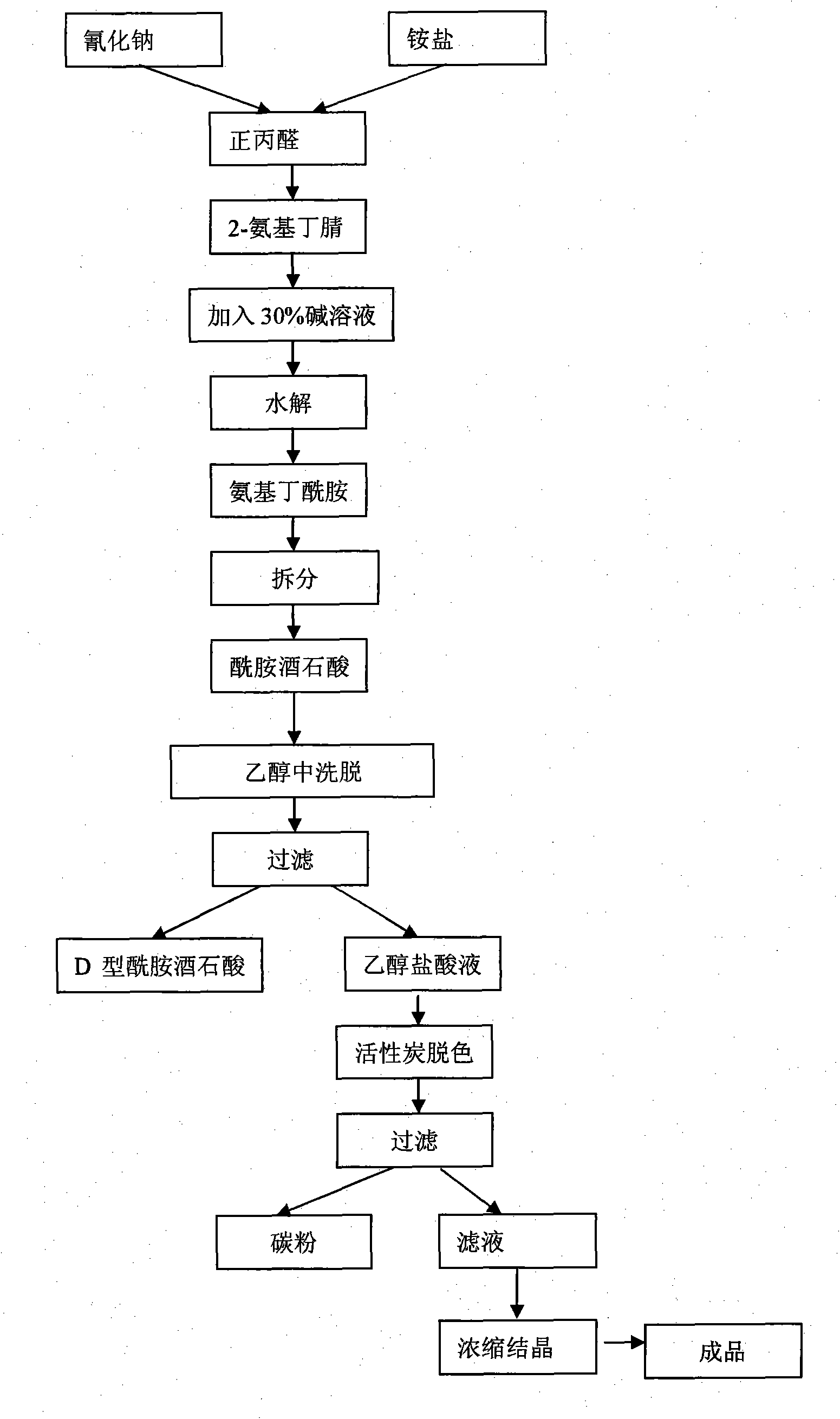
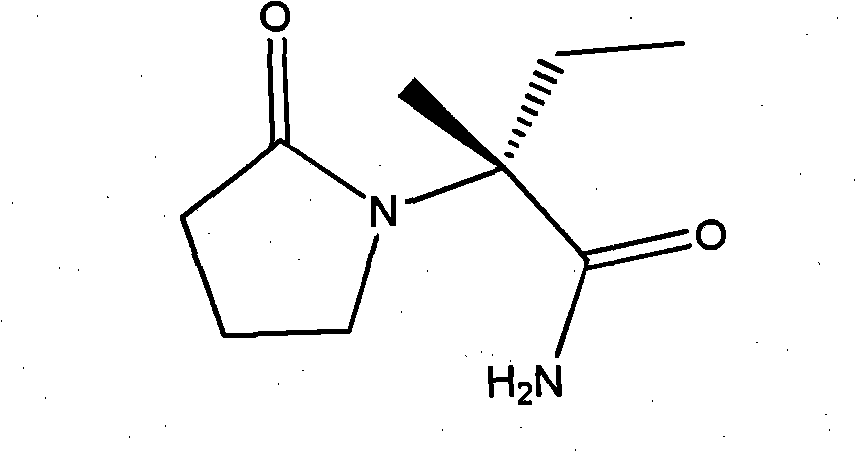
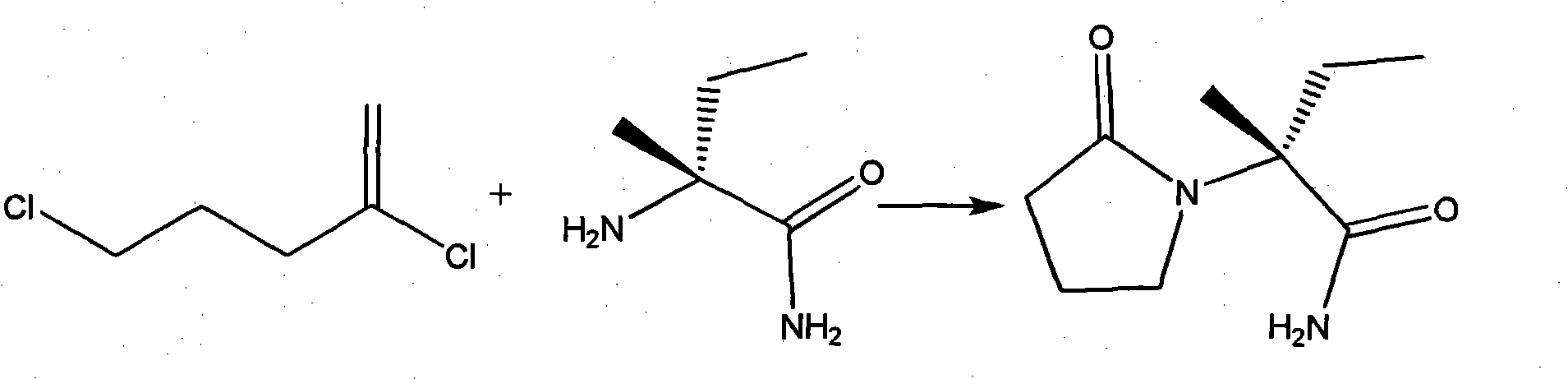
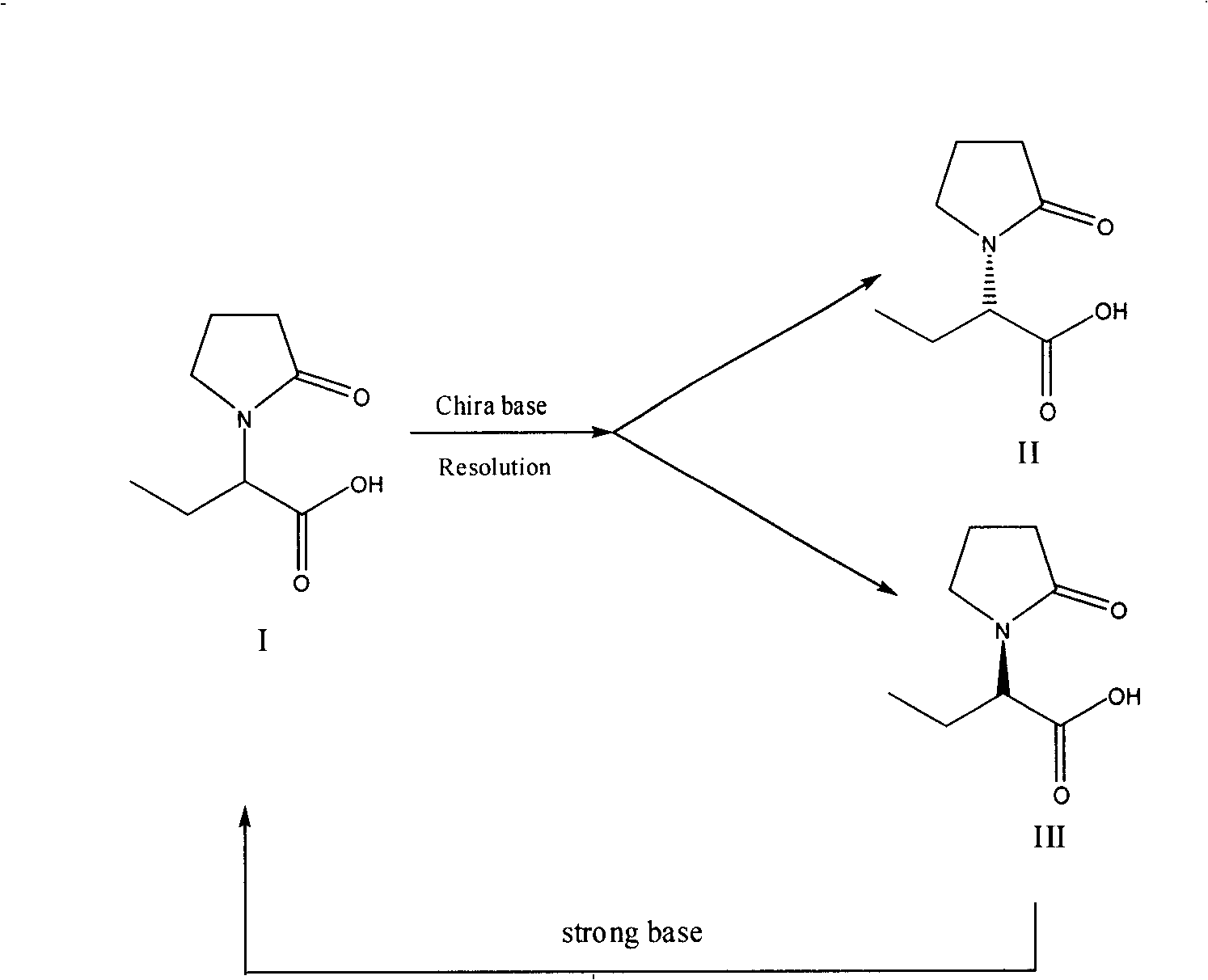
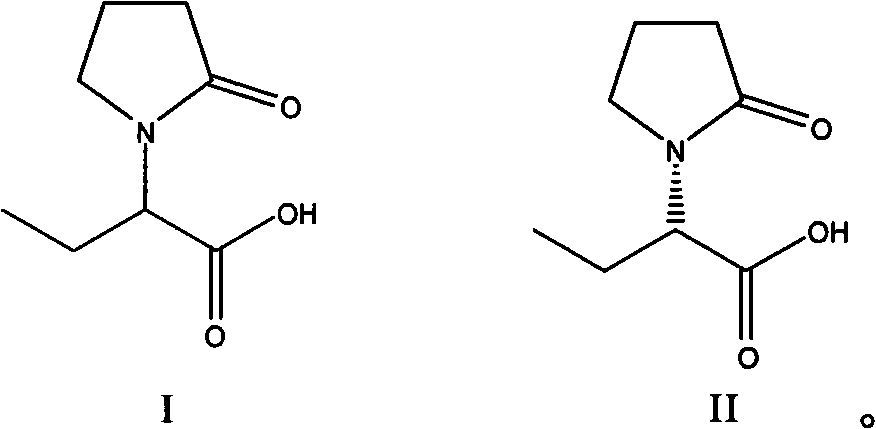
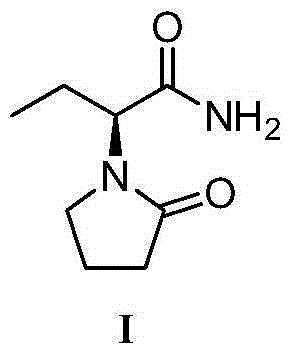
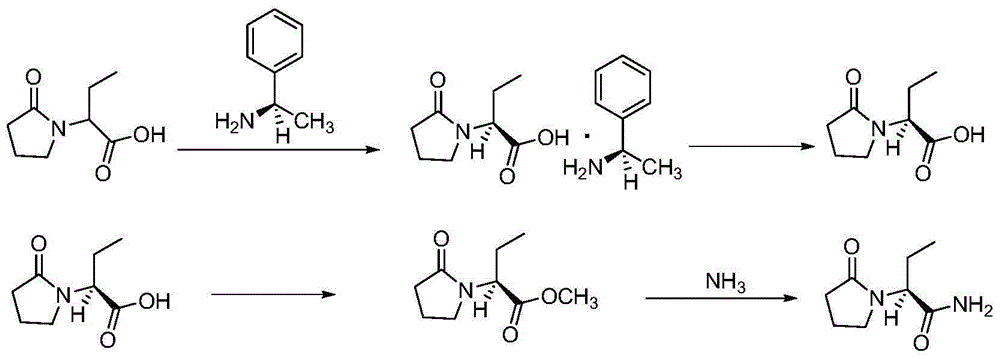
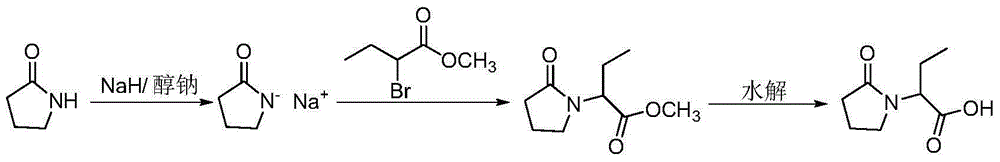
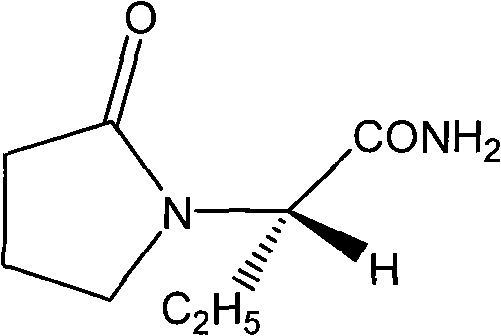
Synthesis, split and racemization of chirality medicament levetiracetam midbody (S)-(+)-2-amido butyramide hydrochlorate
ActiveCN101130504AReduce dosageLow costOrganic compound preparationCarboxylic acid amides optical isomer preparationButyramidePyrrolidine
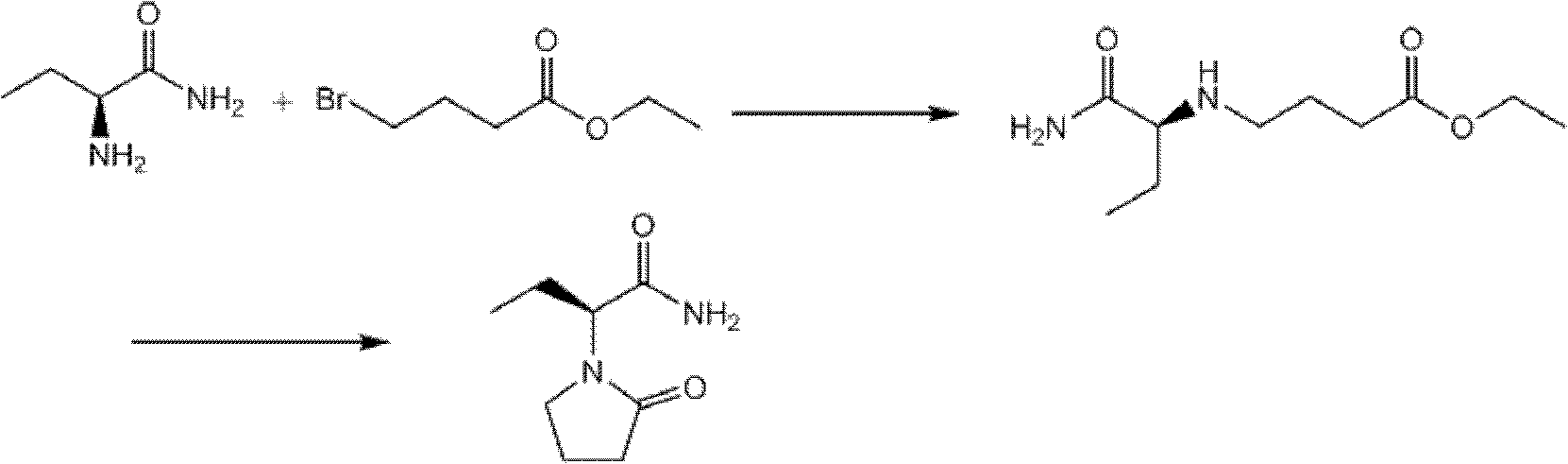
The invention discloses a new synthesizing technique of chiral drug (S)-alpha-ethyl-2-oxo-1-pyrrolidine acetamide ( left Piracetam) intermediate (S)-(+)-2-aminobutanamide hydrochlorate, which comprises the following steps: adopting 2-brobutyrate as initial raw material; aminating; esterifying; ammonolyzing; detaching; looping; obtaining the object compound; making mixed rotary free alkaline (+-)-2-aminobutanamide; adopting half-quantum resolution method to connect chemical detaching salt to evolve salt; removing the detaching agent through alkalization; obtaining the (S)-(+)-2-aminobutanamide hydrochlorate with optical activity; using the mother liquor to make the product. The invention improves the receiving rate and saves the cost of raw material with simply technical operation and low cost, which resolves the resolved mother liquor after racemic action again to reduce the pollution of environment, therefore fitting for industrialized manufacturing.
Owner:ABA CHEM CORP
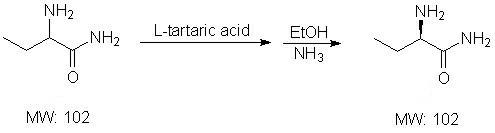
Process method for producing L-2-aminobutanamide hydrochloride serving as intermediate of levetiracetam
InactiveCN101928229ALow costShort synthesis cycleOrganic compound preparationCarboxylic acid amides preparationSodium cyanideHydrolysis
The invention discloses a process method for producing L-2-aminobutanamide hydrochloride serving as an intermediate of levetiracetam, which solves the problem that the conventional produced product has excessively high impurity content or production cost is too high for an enterprise to bear and the like. The method is characterized by comprising the following steps of: reacting propionaldehyde with ammonia water, ammonium chloride and sodium cyanide to obtain 2-amino butyronitrile; hydrolyzing the 2-amino butyronitrile under an alkali condition to obtain 2-aminobutanamide; and splitting the 2-aminobutanamide with L-tartaric acid to obtain the L-2-aminobutanamide hydrochloride. An L-2-amino amides product of which the content is over 99.5 percent is obtained by removing a byproduct, namely, sodium chloride by recrystallization so as to meet the use requirement of foreign customers. The process has the advantages of high yield, high safety and low cost and can be widely suitable for industrialized production of medium-sized and small enterprises.
Owner:HUANGGANG HUAYANG PHARMA
Method for synthesizing intermediate L-2-aminobutyrylamide hydrochloride of chiral drug levetiracetam
InactiveCN102020584AMild reaction conditionsLow costCarboxylic acid amides optical isomer preparationSynthesis methodsUnit operation
The invention belongs to the field of the preparation of chiral drug intermediates and particularly relates to a method for synthesizing a intermediate L-2-aminobutyrylamide hydrochloride of chiral drug levetiracetam. The method is characterized by taking 2-bromobutyric acid as a starting raw material and comprising the steps of: firstly, preparing a 2-bromobutyric amide intermediate, reacting the 2-bromobutyric amide intermediate to generate DL-2-aminobutyrylamide, and finally, splitting and salifying the DL-2-aminobutyrylamide to synthesize the L-2-aminobutyrylamide hydrochloride. Compared with the traditional synthesis method, the method has the advantages of mild reaction conditions, low cost, higher yield, greatly improved process safety, cheap and easily-obtained raw materials, simple unit operations and low requirements on equipment and is suitable for industrial large-scale production, amidation reaction is carried out at normal temperature and normal pressure, and the reaction is easy to control.
Owner:浙江沙星医药化工有限公司
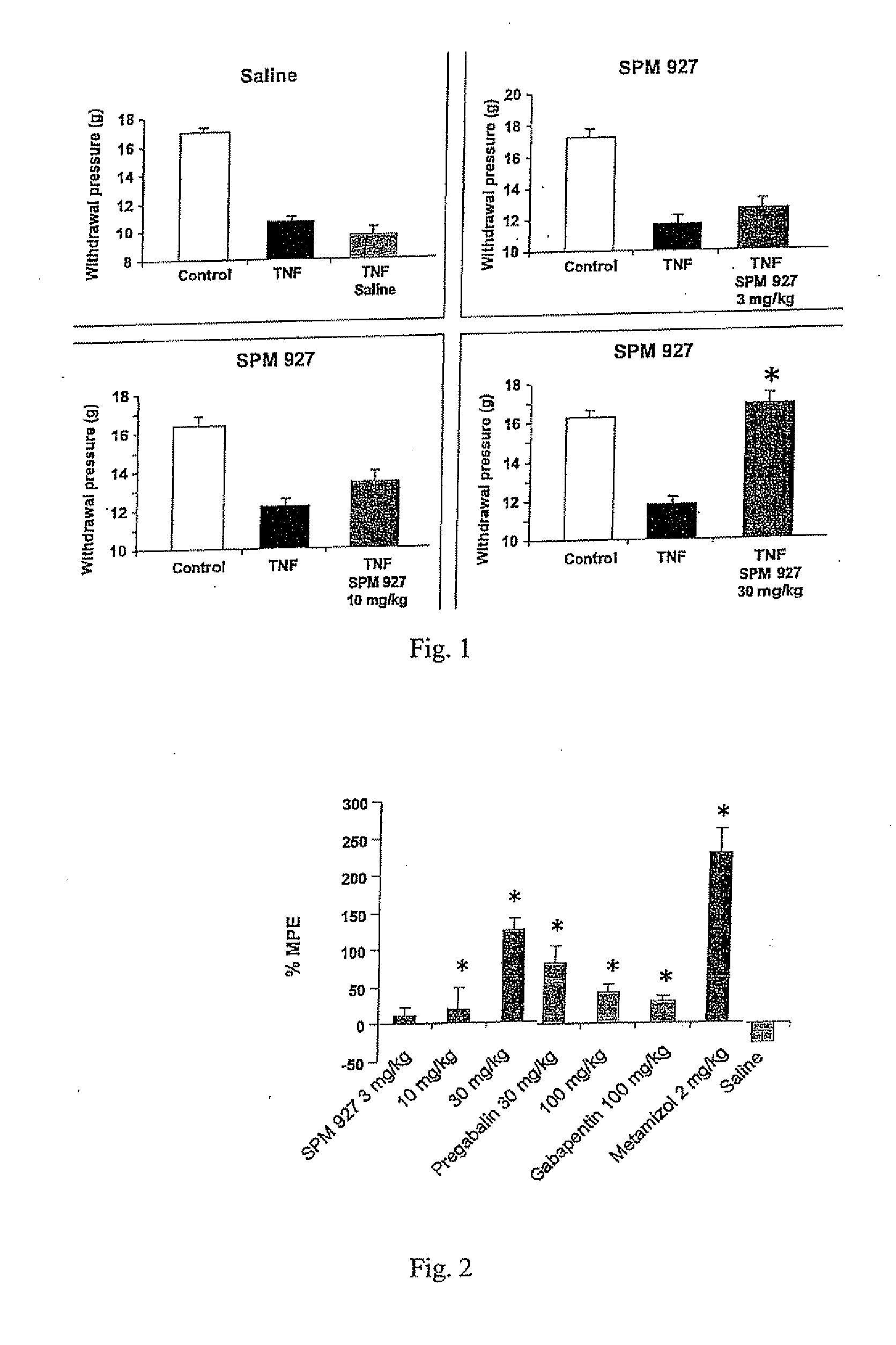
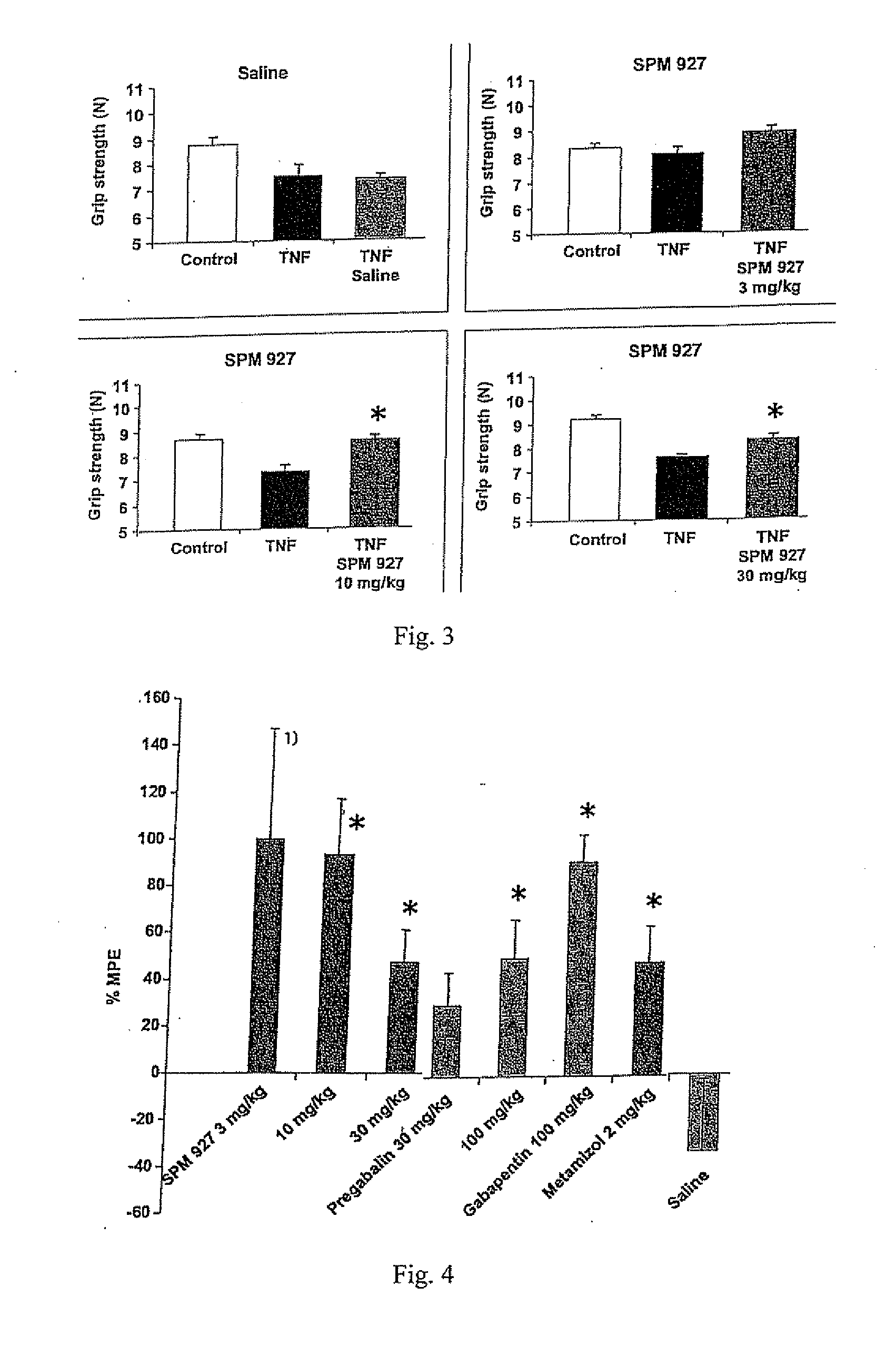
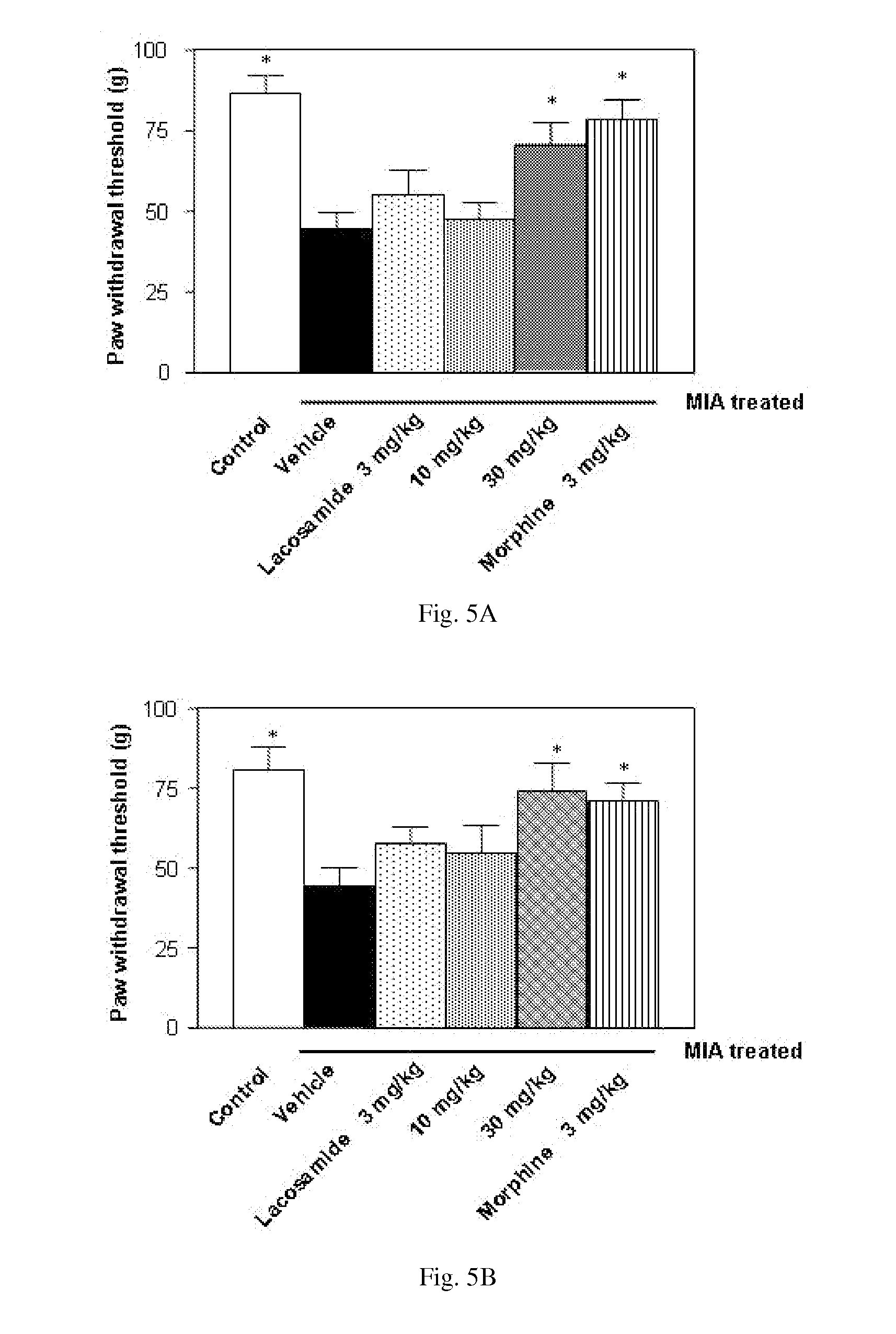
Therapeutic combination for painful medical conditions
An embodiment of the present invention relates to a therapeutic combination including (a) lacosamide and / or a pharmaceutically acceptable salt thereof; and (b) levetiracetam and / or a pharmaceutically acceptable salt thereof. The combination can be provided in a single dosage form or separate dosage forms.
Owner:UCB SA
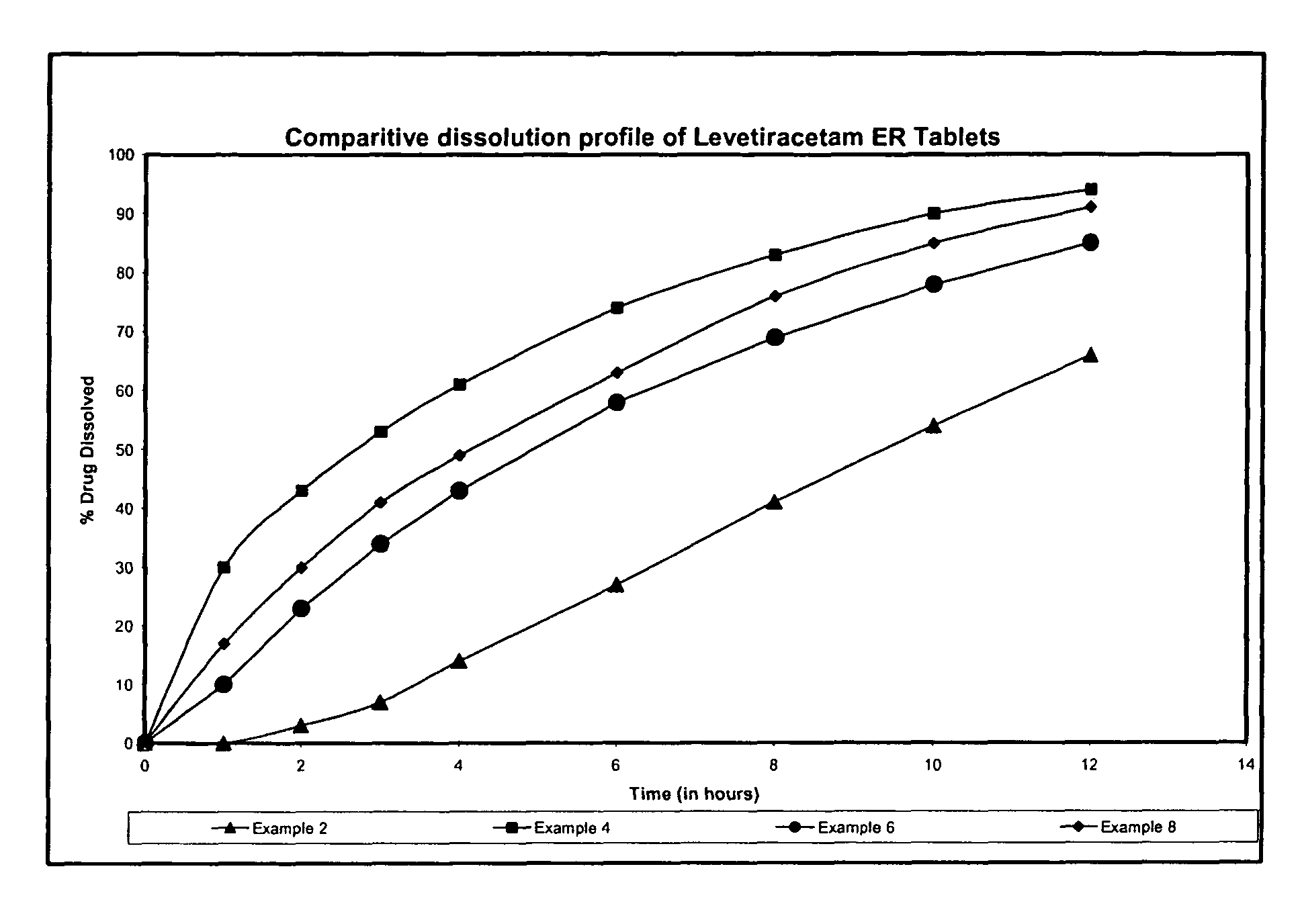
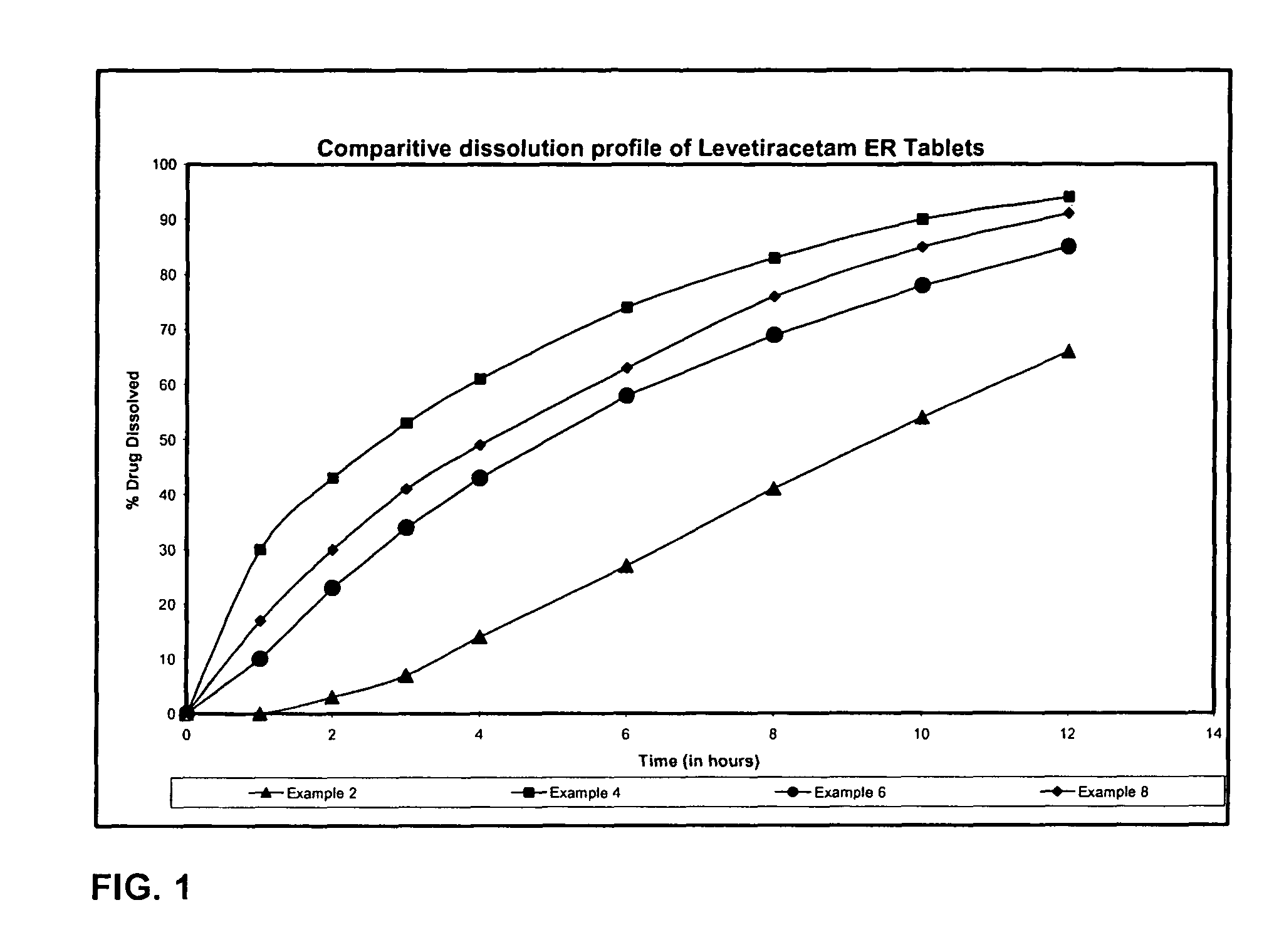
Extended release formulation of Levetiracetam
ActiveUS7863316B2Reduced inter subject variabilityBiocideNervous disorderWater dispersibleFOOD EFFECT
Owner:UCB PHARMA SA
Levetiracetam oral disintegrating tablet and preparation method thereof
ActiveCN102085194AOrganic active ingredientsNervous disorderOrally disintegrating tabletAdditive ingredient
The invention provides a levetiracetam oral disintegrating tablet and a preparation method thereof. The levetiracetam oral disintegrating tablet is prepared by adopting a freeze drying process and contains levetiracetam as an active constituent, polyethylene glycol 6000 as a solid dispersing agent, maltodextrin as a freeze-drying protective agent and hydrolyzed gelatin as an adhesive. The levetiracetam is a novel antiepileptic drug, and the levetiracetam oral disintegrating tablet provided by the invention has the advantage of improving the medication compliance of patients and the curative effect.
Owner:BEIJING YILING BIOENG
Stable levetiracetam compositions and methods
InactiveUS20080045583A1Effective amountImprove stabilityBiocideNervous disorderPreservative freePreservative
Stable, liquid compositions of levetiracetam that are substantially or entirely free of preservatives, particularly parabens, and / or sugars, such as natural sugars and sugar alcohols. The liquid compositions preferably include oral solutions or suspensions, and may include pharmaceutically acceptable excipients.
Owner:WOCKHARDT EU OPERATIONS SWISS
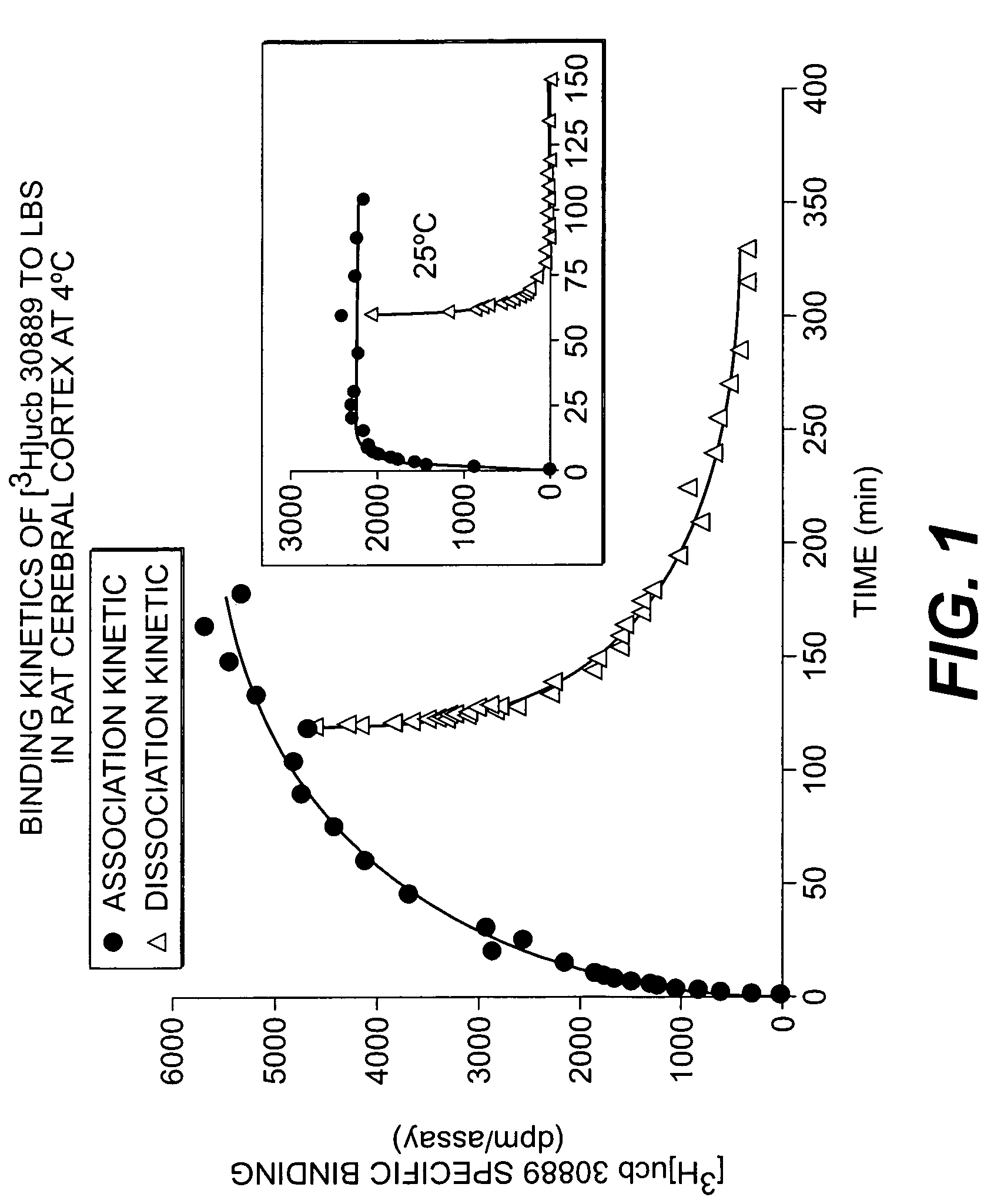
Methods for the identification of agents for the treatment of seizures, neurological diseases, endocrinopathies and hormonal diseases
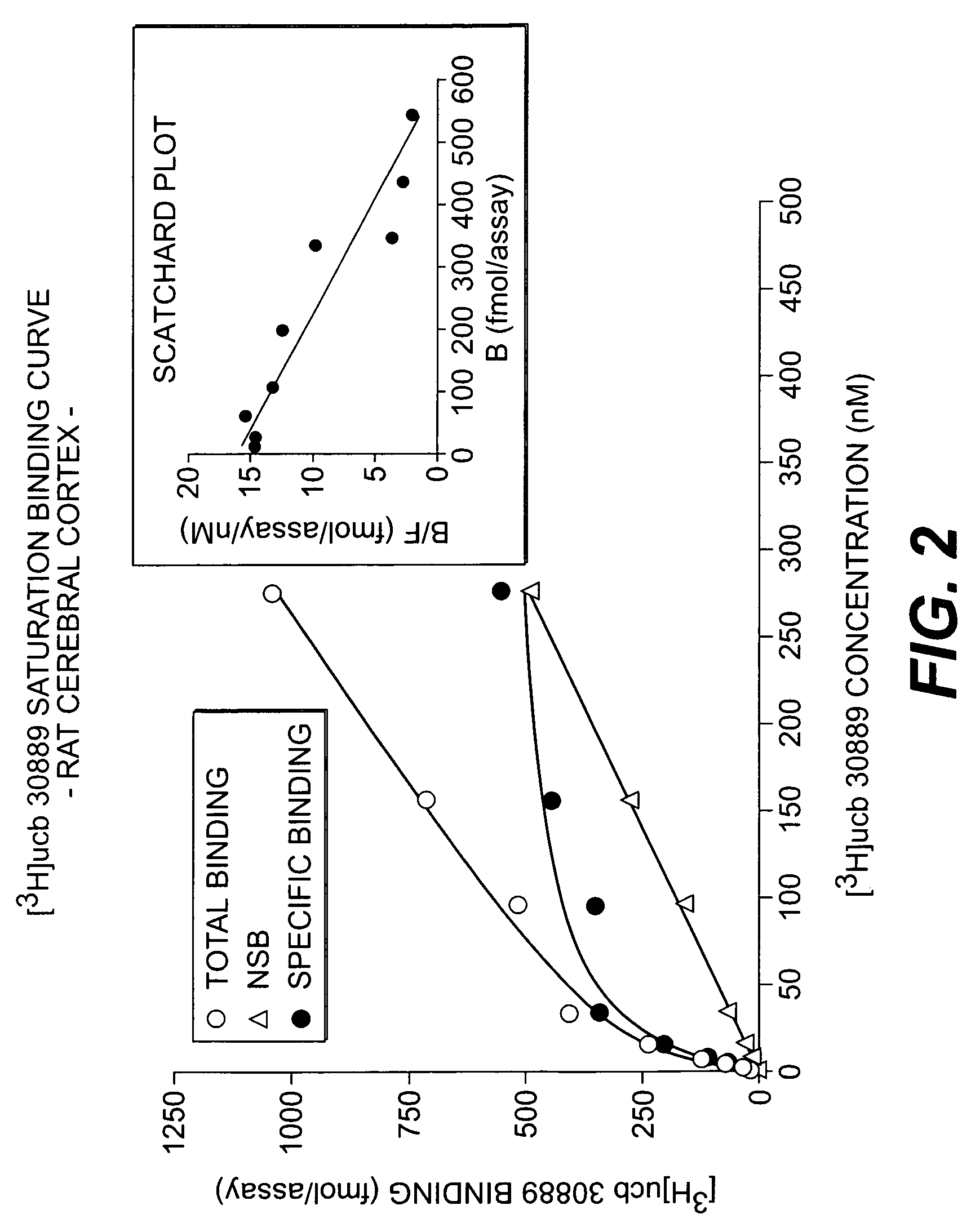
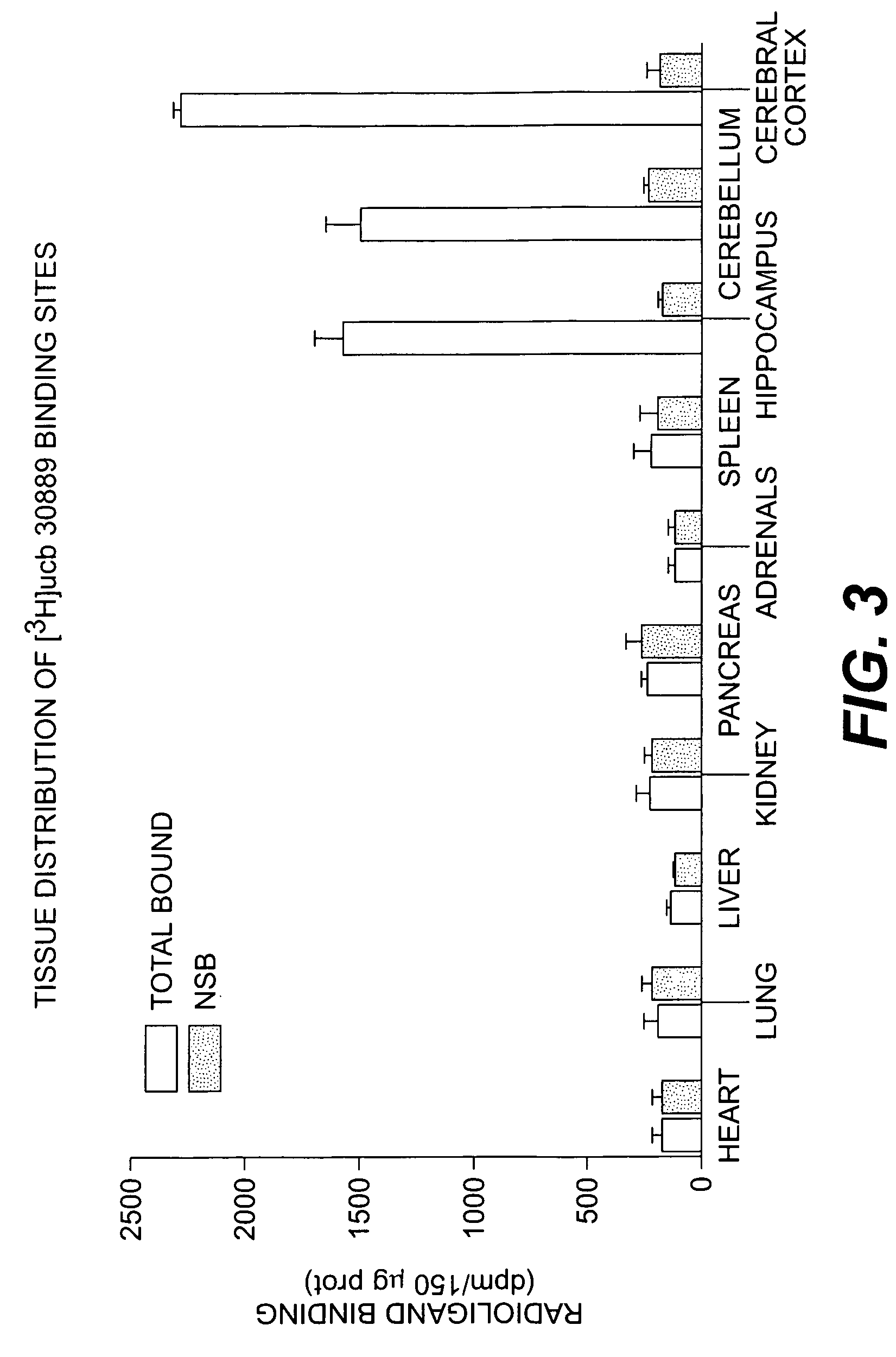
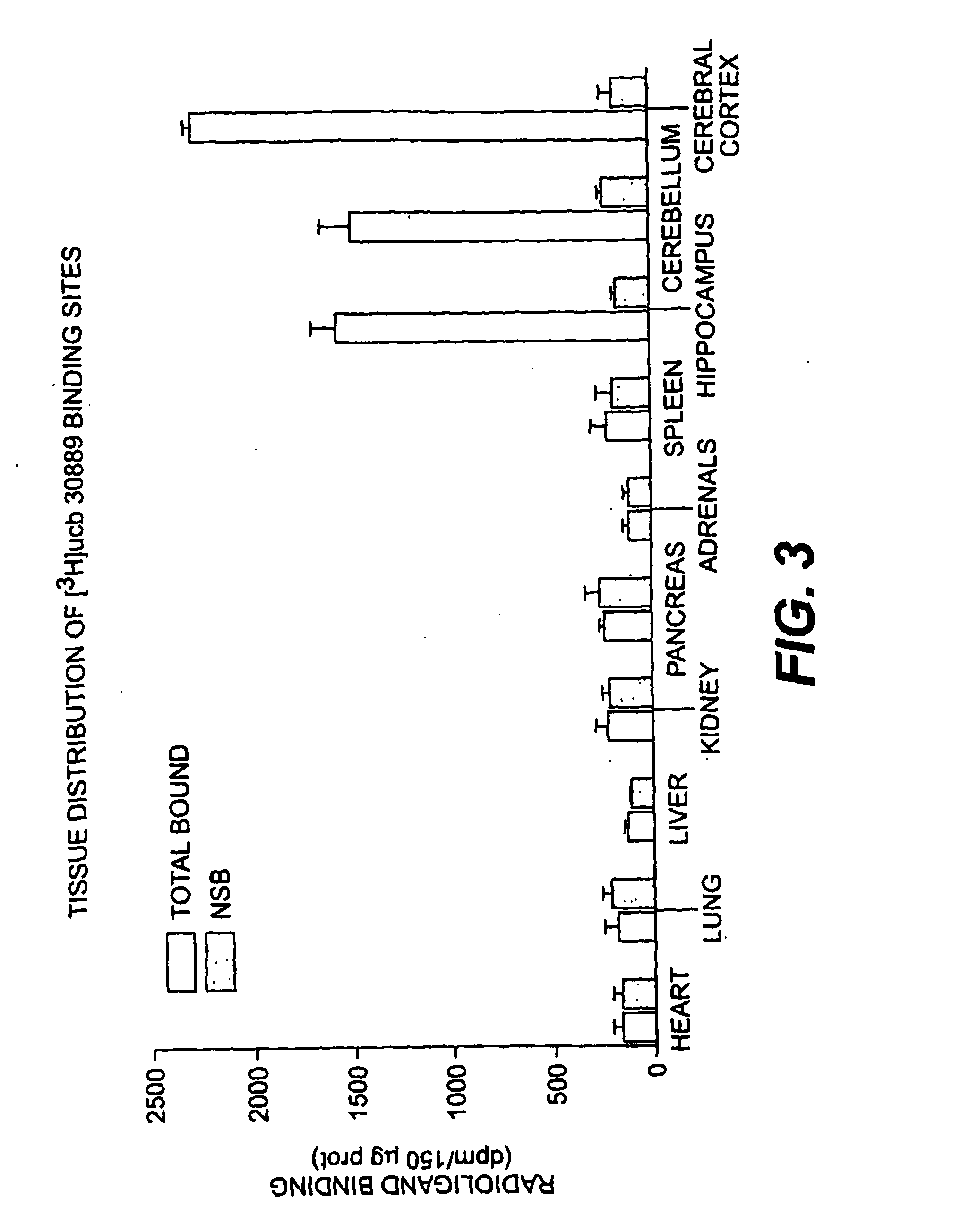
The present invention is drawn to methods of characterization of the properties and functions of SV2 proteins. The invention further includes methods of identifying compounds or agents which modulate the activity of SV2 proteins. Included in these methods is the identification of compounds or agents which modulate the binding of levetiracetam to SV2 proteins, including SV2A. Additionally, the present invention provides biotinylated ligands as a tool to screen chemical libraries and characterize the SV2 proteins. Further, the present invention provides a method of solubilizing and purifying functionally active membrane associated proteins, such as SV2.
Owner:UCB SA
Levetiracetam tablet and preparation method
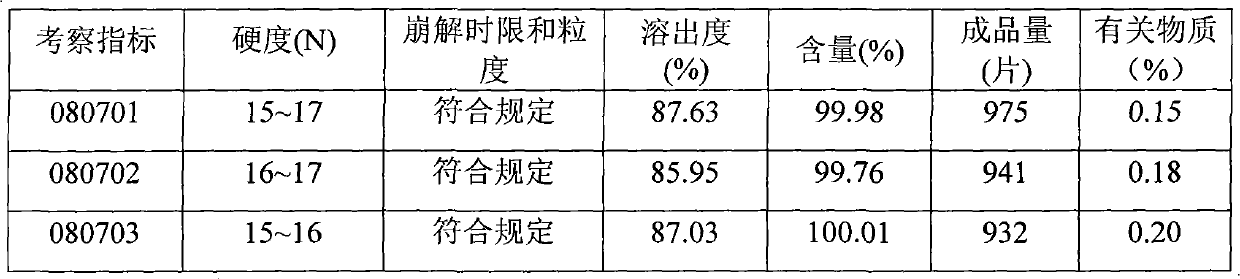
InactiveCN101584673AHigh hardnessPromote dissolutionNervous disorderPharmaceutical non-active ingredientsMedicinePlasticizer
The invention discloses a Levetiracetam tablet and preparation method thereof. Levetiracetam is a bolus injection medicine with bad compressibility and proportion of supplementary materials which can be added is small. Quality of Levetiracetam is unstable and it is hard to produce Levetiracetam industrially. The invention comprises components of weight percentage content: 70% to 80% of Levetiracetam as active component, 1.5% to 8.0% of plasticizer, 9% to 17% of disintegrant, 3% to 14% of filler, 0.1% to 0.5% of bond and 0.1% to 3% of fluidizer. Addition of plasticizer is added by inner way of wet granulation. Levetiracetam as active component has bad mechanic compressibility. The invention adds plasticizer to solve tablet compression difficulty in industrial embodiment thoroughly, which realizes the purpose of being suitable for coating with stable quality and easy industrial application.
Owner:ZHEJIANG JINGXIN PHARMA
Levetiracetam sustained-release tablets and preparation method thereof
InactiveCN102068414AStable blood concentrationProlong the action timeOrganic active ingredientsNervous disorderSide effectOral medication
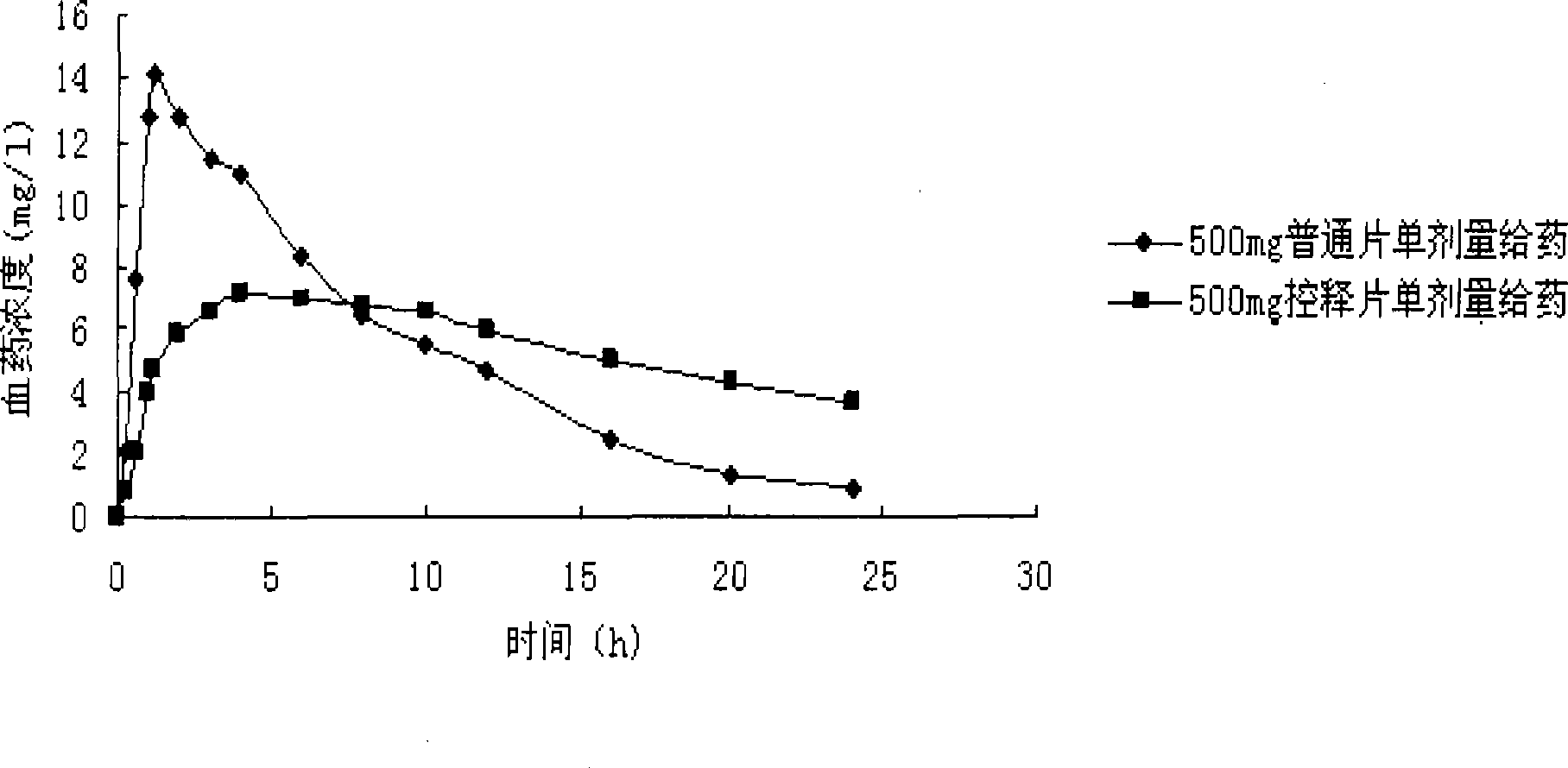
The invention discloses levetiracetam sustained-release tablets and a preparation method thereof. The levetiracetam sustained-release tablets consist of levetiracetam, a filler, a sustained-release agent, an adhesion agent and a lubricant according to the weight ratio of 100: 30: (3-60): (30-70): 1. The preparation method comprises the following steps of: pelletizing by the wet method, drying the granules, arranging the granules and adding a proper quantity of lubricant, uniformly mixing and pressing to obtain the levetiracetam sustained-release tablets. Compared with the common preparation, the levetiracetam sustained-release tablets prepared by the preparation method have the advantages of small blood concentration fluctuation range, reduction in toxin and side effect and increase in biddability of patients by oral administration once every day. The sustained-release preparation will be clinically applied to additional treatment on partial seizure of epilepsia patients.
Owner:HUZHOU LLISSY BIOLOGY TECH +1
Pharmaceutical composition containing a pyrrolidone anticonvulsant agent and method for the preparation thereof
InactiveUS8187635B2Solve the lack of hardnessSufficient friabilityOrganic active ingredientsNervous disorderCalcium biphosphateDiluent
The present invention relates to a pharmaceutical formulation of solid dosage forms comprising a therapeutically effective amount of a pyrrolidone anticonvulsant agent, and in particular Levetiracetam or a pharmaceutical acceptable salt or derivative thereof, in combination with an effective diluent, such as Dibasic Calcium Phosphate, and additional pharmaceutical excipients, and a process for the preparation thereof by wet granulation.
Owner:PHARMATHEN
Method for preparing levetiracetam intermediate
ActiveCN101333180AHigh split efficiencyLow toxicityOptically-active compound separationOrganic racemisationAcetic acidAlcohol
The invention discloses a preparation method for a levetiracetam intermediate (S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid, which takes (RS)-alpha-ethyl-2-oxo-1- pyrrolidine acetic acid as the starting material and takes (R)-alpha-methyl benzylamine as resolving agent to get the product through resolving in resolving solvent. The invention is characterized in that the resolving solvent can be C1-C7 ketones, C1-C7 alcohols, C1-C4 esters or the mixture of the solvents. In addition, the by-product (R)-alpha-ethyl -2 - oxo -1 - pyrrolidine acetic acid can be recovered through racemization under the effect of alkali so as to regain the (RS)-alpha-ethyl -2-oxo-1- pyrrolidine acetic acid. Compared with the existing invention, the preparation method of the invention has the advantages that during resolving, the solvent selected for the compound (RS)-alpha-ethyl -2-oxo -1 - pyrrolidine acetic acid has high resolving efficiency and small toxicity; the resolving salt only needs to be refined once, so that the optical purity of (S)-alpha-ethyl -2-oxo -1 - pyrrolidine acetic acid can meet the requirement (alpha20D is equal to -25.0 plus or minus 1 degree (C is equal to 1, acetone)). In addition, the racemic recovery of the compound (R)-alpha-ethyl -2-oxo-1-pyrrolidine acetic acid can reduce environmental pollution and decrease the cost of the product.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Methods for the identification of agents for the treatment of seizures, neurological diseases, endocrinopathies and hormonal diseases
The present invention is drawn to methods of characterization of the properties and functions of SV2 proteins. The invention further includes methods of identifying compounds or agents which modulate the activity of SV2 proteins. Included in these methods is the identification of compounds or agents which modulate the binding of levetiracetam SV2 proteins, including SV2A.
Owner:UCB SA
Levetiracetam slow release pellet capsule preparation and preparation method thereof
InactiveCN101647789AImprove liquidityReduce or eliminate irritationNervous disorderPharmaceutical delivery mechanismMass compositionSide effect
The invention relates to a levetiracetam slow release pellet capsule preparation and a preparation method thereof. The levetiracetam slow release pellet capsule preparation comprises the following components in percentage by mass: 50-70 percent of levetiracetam, 15-30 percent of blank pellet, 5-10 percent of hydroxypropylmethyl cellulose or polyvidone, 7-15 percent of ethylcellulose or acrylic resin, 1-8 percent of talc powder and 0.5-2 percent of cataloid. The preparation method comprises the following steps: coating levetiracetam fine powder on the blank pellet by a binding agent to be madeinto a medicine-contained pellet; coating an isolating layer on the medicine-contained pellet; coating a slow release coating film on the medicine-contained pellet coated with the isolating layer to prepare a slow release pellet; and mixing the slow release pellet, the talc powder and the cataloid and filling the mixture into a capsule. The levetiracetam is made into the slow release capsule preparation by a pellet and slow release technology, and the slow release preparation can stabilize blood medicine concentration, reduce the generating frequency and degree of the side effect and fundamentally solve the problems of great influence on a tablet by gastric pyloric sphincter and big differences of gastric emptying individuals.
Owner:天津药物研究院药业有限责任公司
Levetiracetam medicinal composition and preparation method thereof
The invention discloses a levetiracetam-containing medicinal composition for injection and a preparation method thereof. The medicinal composition comprises an active component, namely levetiracetam and other excipient components. The medicinal composition is mainly used for the treatment of epilepsy.
Owner:AVENTIS PHARMA HAINAN
Pharmaceutical Composition
The present invention relates to an oral controlled release pharmaceutical composition in the form of a unit dosage form comprising:(a) a highly soluble high dose active ingredient consisting essentially of therapeutically effective amount of levetiracetam or a pharmaceutically acceptable derivative thereof, and(b) a rate controlling means comprising a rate-controlling agent and / or a coating selected from (i) a active ingredient permeable coating surrounding the unit dosage form, and (ii) an active ingredient impermeable coating covering one or more surfaces but not all the surfaces of the unit dosage form,wherein the composition is in the form of a compact tablet and the levetiracetam or a pharmaceutically acceptable derivative thereof is present in an amount ranging from about 55% to about 90% by weight of the tablet.
Owner:SUN PHARMA INDS
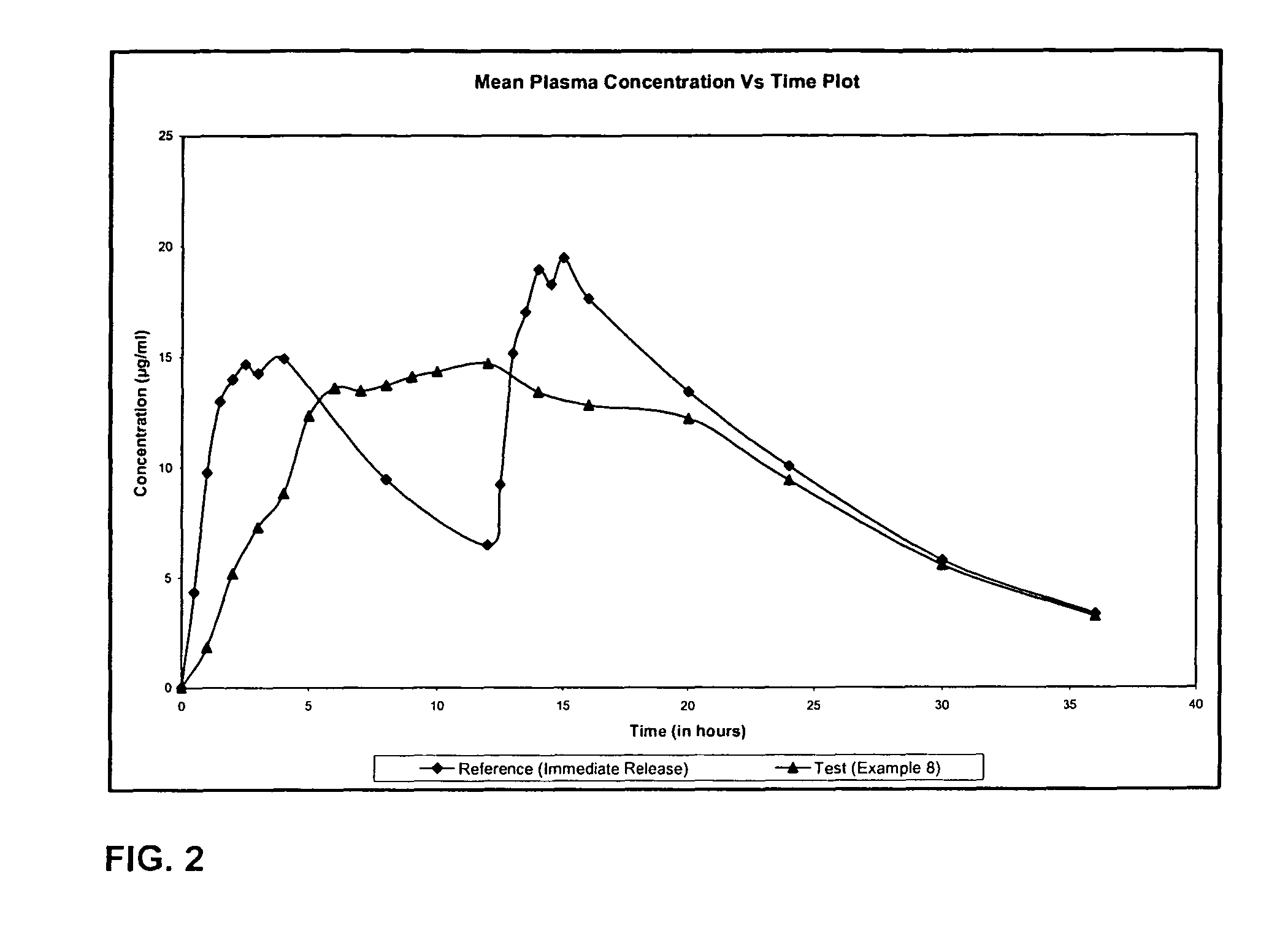
Extended release formulation of levetiracetam
ActiveUS20070092569A1Reduced inter subject variabilityBiocideNervous disorderWater dispersibleExtended release tablets
The present invention relates to extended release pharmaceutical compositions of Levetiracetam and processes for preparing the same. The extended release tablet of Levetiracetam is with a core comprising of Levetiracetam and water dispersible rate controlling polymer, and the tablet core is optionally functional coated comprising a combination of water non-dispersible and / or water dispersible polymer. It provides extended therapeutically effective plasma levels over a twenty four hour period with diminished incidences of neuropsychiatric adverse events by eliminating the troughs and peaks of drug concentration in a patient's blood plasma. The composition also exhibits no food effect.
Owner:UCB PHARMA SA
Preparation method of levetiracetam
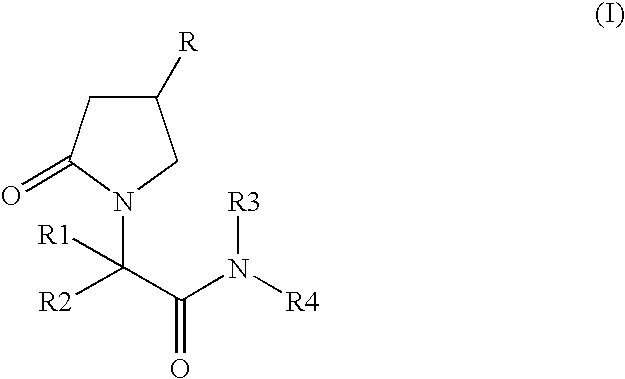
The invention provides a preparation method of levetiracetam (I). The method comprises the steps that racemization 2-halogenated butyric acid methyl ester which is in low in cost and easy to obtained is used as a raw material, a biological enzyme method is adopted to synthesize key chiral intermediate (R)-2-halogenated butyric acid methyl ester, the intermediate is subjected to ammonolysis and cyclized with 4-chlorobutyryl chloride, and finally the levetiracetam is obtained. The chiral center is built by the adoption of the enzyme asymmetric catalytic technology, a reaction path is directly reduced to three steps, the comprehensive yield is high, and the cost is low. Compared with a chemical catalyst, a biological enzyme catalyst has the advantages of being high in enantioselectivity and regioselectivity and low in energy consumption, producing few by-products and the three wastes, and the like.
Owner:ZHEJIANG CHANGMING PHARMA
Method for preparing levetiracetam
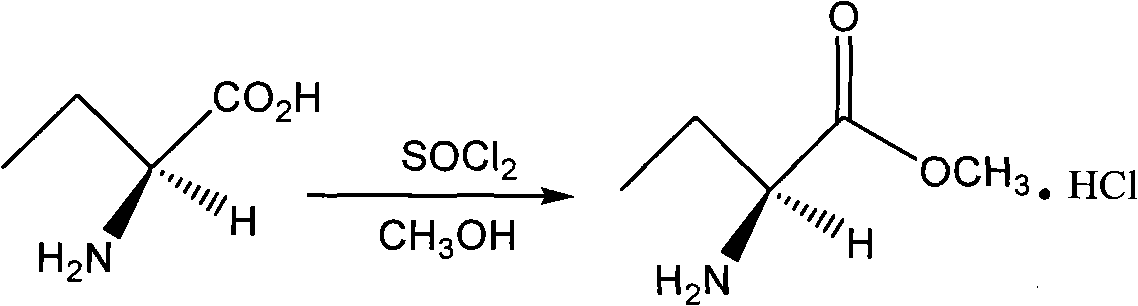
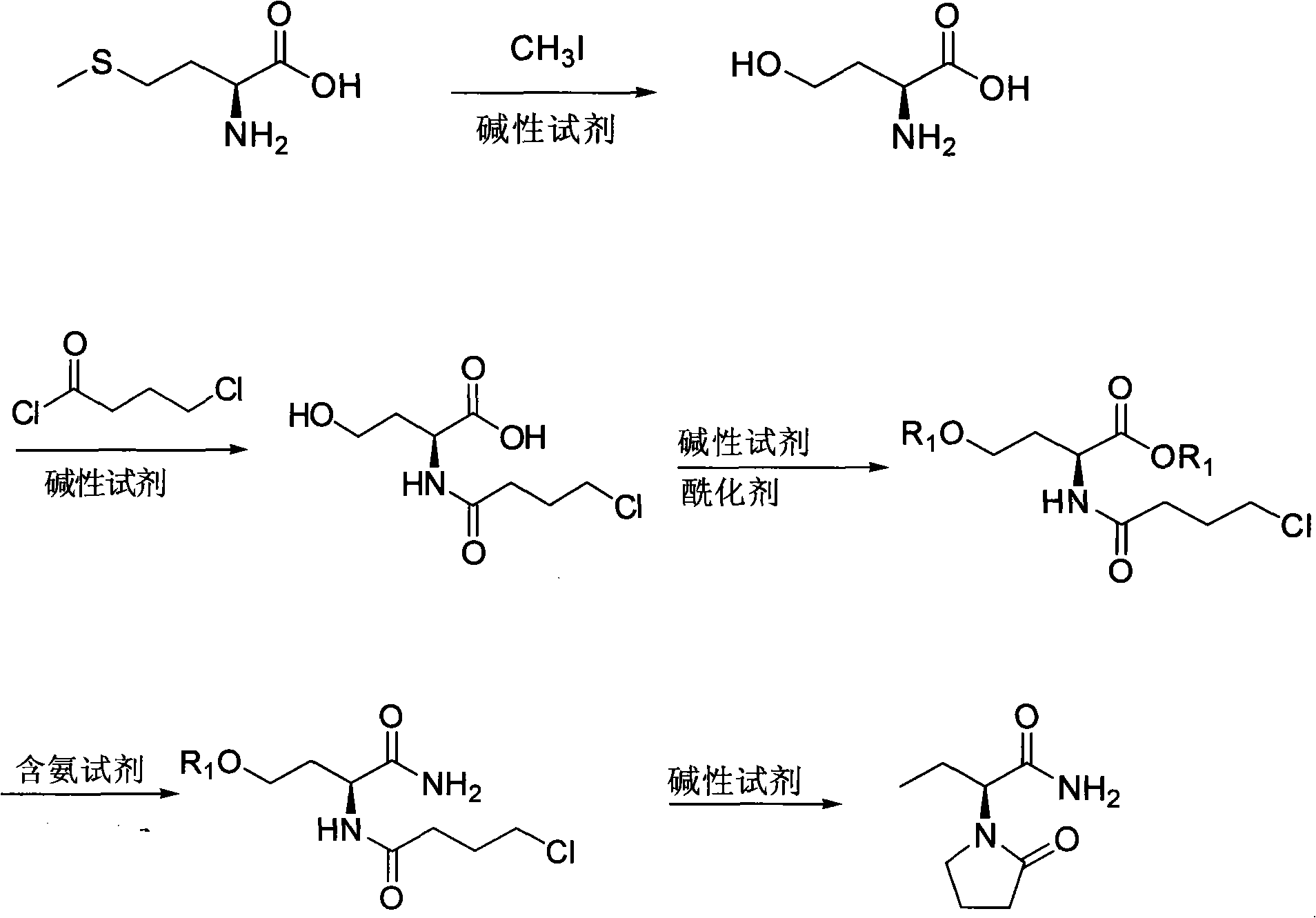
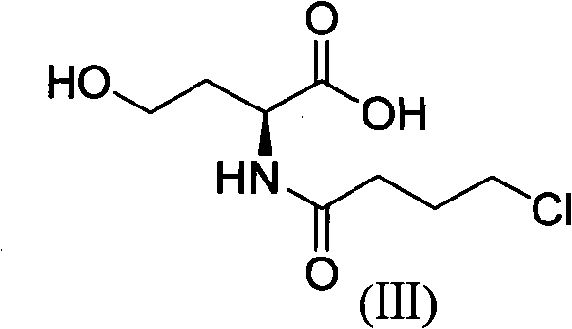
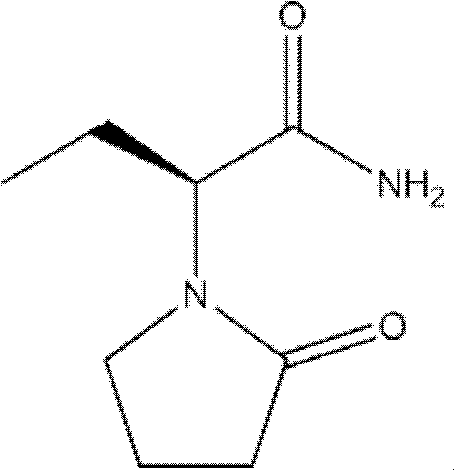
InactiveCN101624362ASuitable for industrial productionSuitable for process productionOrganic chemistryMethionine biosynthesisSolvent
The invention discloses a method for preparing levetiracetam, which is characterized by using methionine as a raw material to prepare levetiracetam through the steps of desulfurization, methylation, esterification, ammonolysis, amidation and intramolecular condensation cyclic reaction. The technical steps of the method are suitable for technologized production, and the improved technology has no strict reaction conditions. The raw material is easy to obtain, the operation is simple, the yield is high, and methanol and other solvents in preparation process can be recycled without generating the three wastes.
Owner:BENGBU BBCA MEDICINE SCI DEV
Tsukamurella-tyrosinosolvens and application thereof in catalysis preparation of (S) -alpha - ethyl -2-oxo-1-pyrrolidine acetic acid prepared by catalysis
ActiveCN101748087AHigh stereoselectivityBacteriaMicroorganism based processesTsukamurellaBioconversion
The present invention provides a novel strain, tsukamurella-tyrosinosolvens E105, and an application thereof in the chiral biocatalysis preparation of (S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid. The tsukamurella-tyrosinosolvens E105 is stored in China Center for Type Culture Collection (CCTCC) at Wuhan University of Science and Technology (postal code: 430072) on Dec.16th, 2009. The preservation series number is CCTCC NO:M209306. The present invention adopts a microorganism preparation method of (S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid for the first time and provides a novel strain of high stereoselectivity and capability of preparing (S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid of high optical purity. The present invention can make the optical purity of the target product (S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid prepared by the chiral biocatalysis of tsukamurella-tyrosinosolvens E105 cells reach 99.4%, and make productivity reach 48.1%. The novel strain obtained by screening provides helpful references in the aspects of research in the microorganism preparation of the key chiral intermediate of levetiracetam and the technique optimization of bioconversion.
Owner:ZHEJIANG UNIV OF TECH
Levetiracetam slow release medicinal composite and preparation method thereof
ActiveCN102379857ASimple preparation processPromote reproductionOrganic active ingredientsNervous disorderControl releaseBiocompatibility Testing
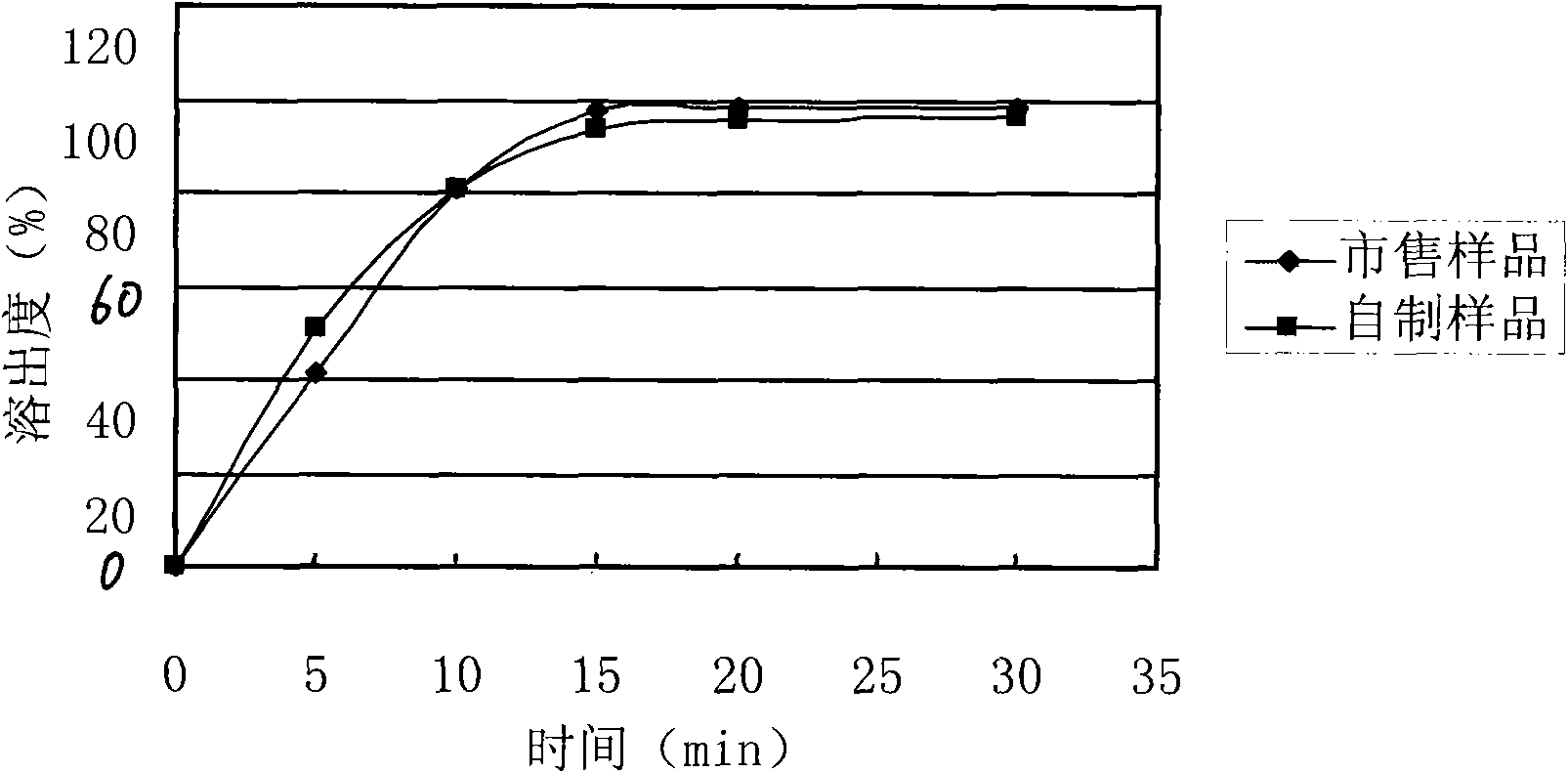
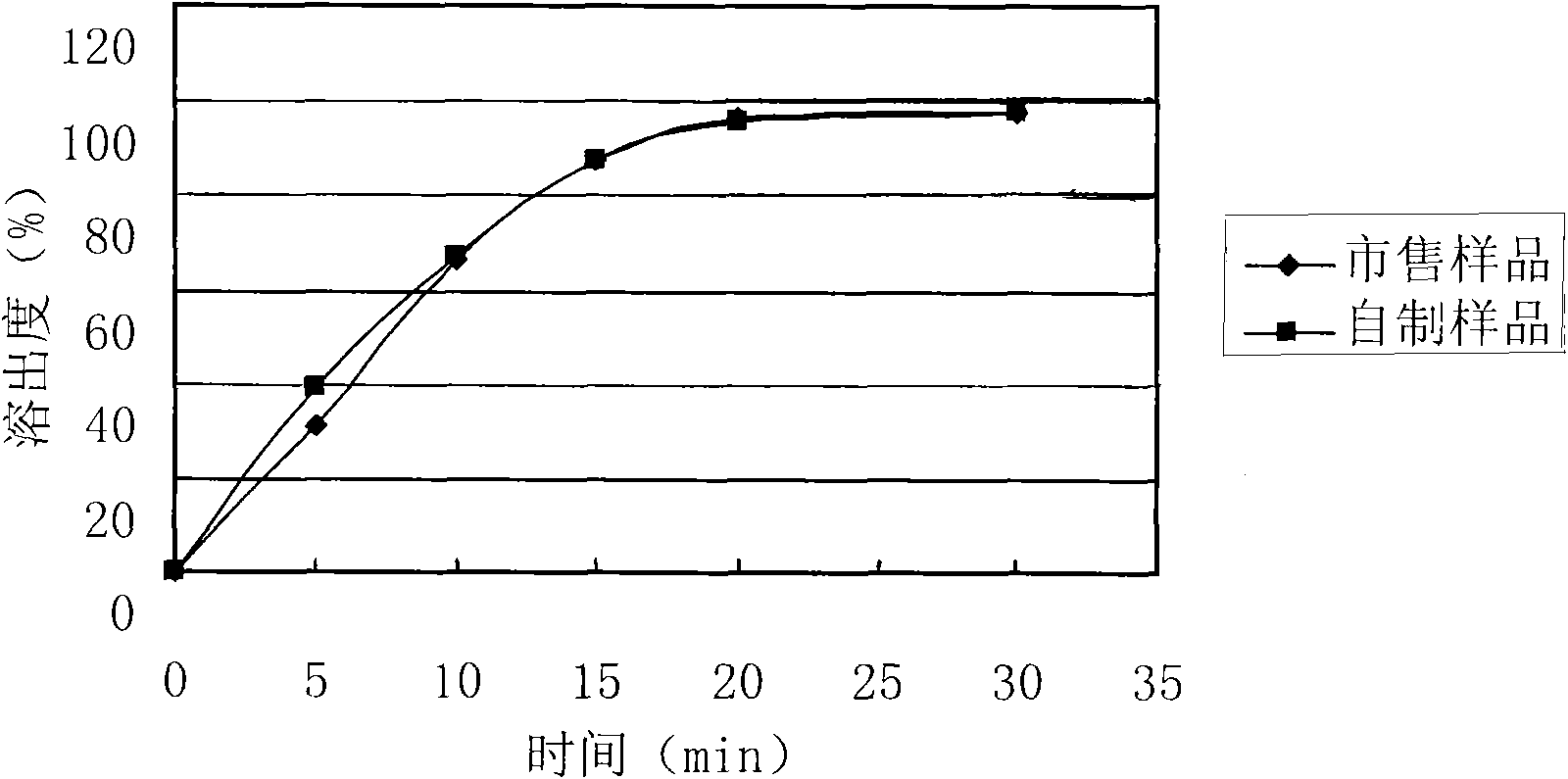
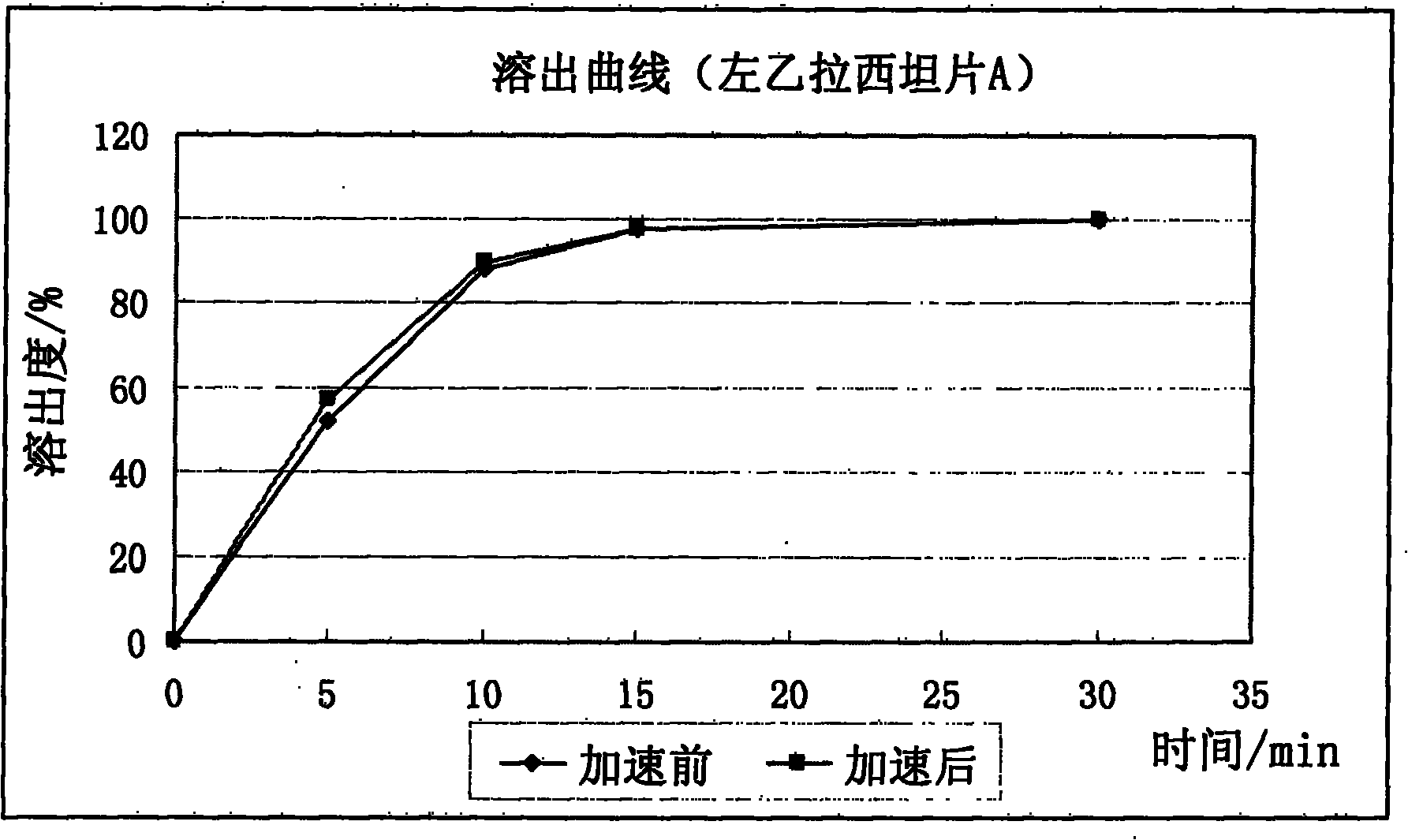
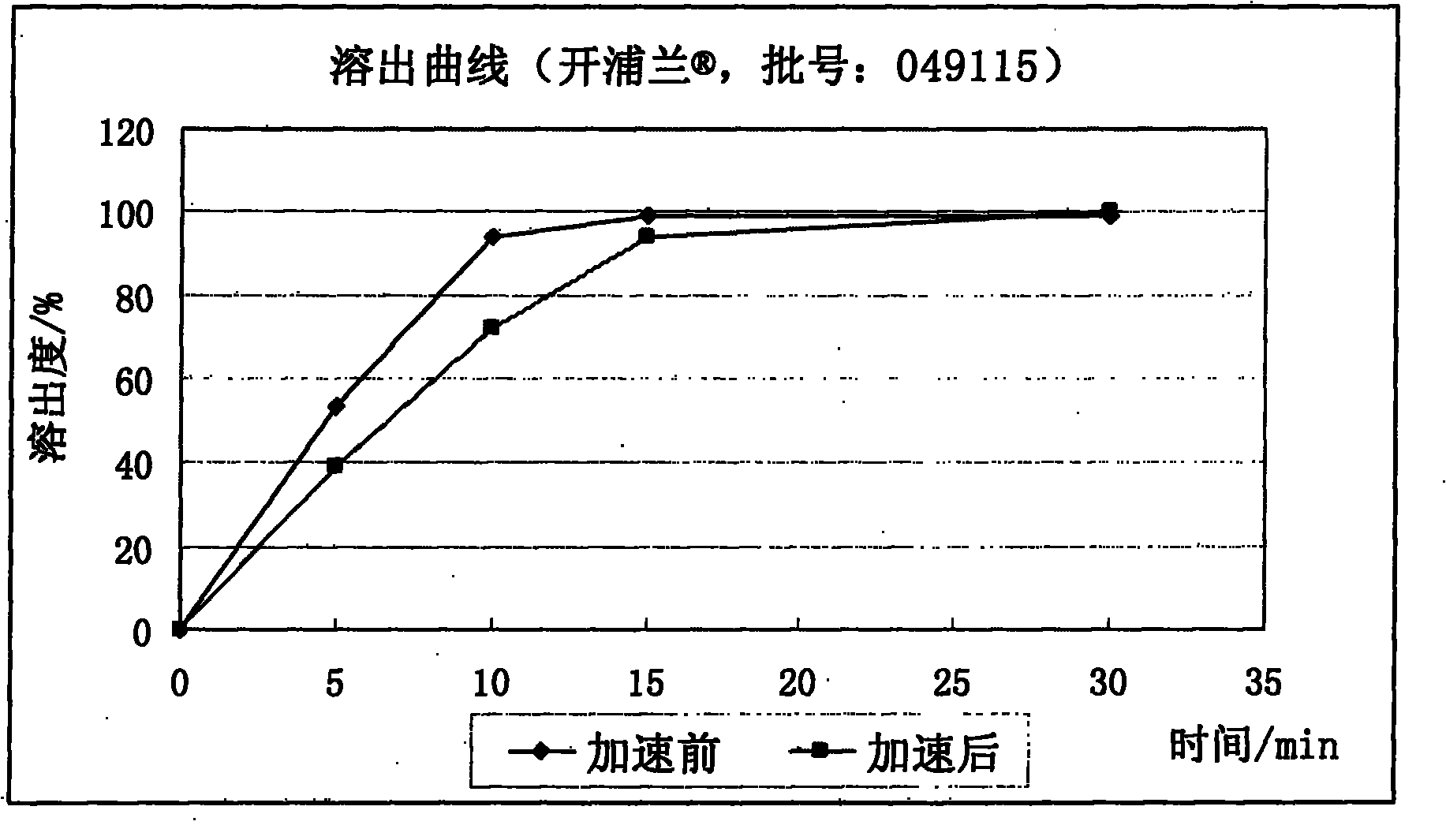
The invention relates to a levetiracetam slow release medicinal composite and a preparation method thereof. The tablet core of the medicinal composite is mainly composed by 55-65% of levetiracetam in weight percentage and 35-45% of non hydrophilia auxiliary material with the slow release function in weight percentage, the non hydrophilia auxiliary material with the slow release function is mixed by one, two or three of ethyl cellulose, methyl cellulose or behenic acid glyceride, and the slow release medicinal composite does not need a functional controlled release coating to adjust the drug release. The levetiracetam medicinal composite has stable drug release performance, good biocompatibility and dissolution performance; and the preparation technology is simple, is easy to reproduce, and is applicable to industrialization production.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Levetiracetam tablet and preparation method thereof
ActiveCN102038657AEliminate impact on product qualitySimple process controlOrganic active ingredientsNervous disorderActive componentPharmacology
The invention relates to a levetiracetam tablet. The levetiracetam tablet at least comprises levetiracetam used as an active component and a binder accounting for more than 6% of the total weight of the tablet. The invention further relates to a preparation method of the levetiracetam tablet. The improved levetiracetam tablet is simple in production technology and low in cost, and is suitable for commercial production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Synthesis method of levetiracetam
ActiveCN102558012AAvoid prescriptive requirementsMeet production requirementsOrganic chemistrySynthesis methodsPollution
The invention relates to a synthesis method of levetiracetam and belongs to the technical field of medicine synthesis. The invention provides a synthesis method of levetiracetam for the purpose of solving the technical problems that in the prior art, a process in which thionyl chloride is used as a raw material or benzene is used for splitting is large in environment pollution, complicated in splitting and is disadvantageous to production. The method comprises the following steps of: carrying out alkylation reaction on (S)-2-reanal which is used as the raw material and 4-chlorobutyryl chloride; carrying out acylation reaction on a product obtained from the former step and an acylation agent; and then carrying out cyclization reaction through ammonolysis in the presence of a phase transfer catalyst to obtain the levetiracetam. The invention provides a bran-new synthesis method of levetiracetam. In the method, a splitting process is omitted so as to avoid the problem existing when benzene is used as a splitting agent; a thionyl chloride reagent is not used, so as to reduce human damage and environment pollution; and the yield and the quality of a product are high, the total molar yield of the product is more than 81%, the HPLC (high performance liquid chromatography) purity of the product is more than 98%, and the optical purity of the product is more than 99.0%.
Owner:江苏八巨药业有限公司
Method for preparing Levetiracetam
The invention discloses a method for preparing Levetiracetam. In the method, L-methionine is used as a raw material to prepare the Levetiracetam through the steps including hydroxylation of methylthio, acidylation of amido, amidation of carboxylic acid as well as cyclization. The raw material used by the method for preparing Levetiracetam is natural amino acid-L-methionine which has broad sources and low cost. Moreover, the method for preparing Levetiracetam has high total yield coefficient; the optical purity of the obtained product is high; the conditions needed for the reaction and the reaction process are simple; and an internal compensation product obtained by a complex splitting method is avoided, and a new choice is provided for preparing and producing the Levetiracetam.
Owner:WUXI SUNFU PHARMA
Preparation method of levetiracetam
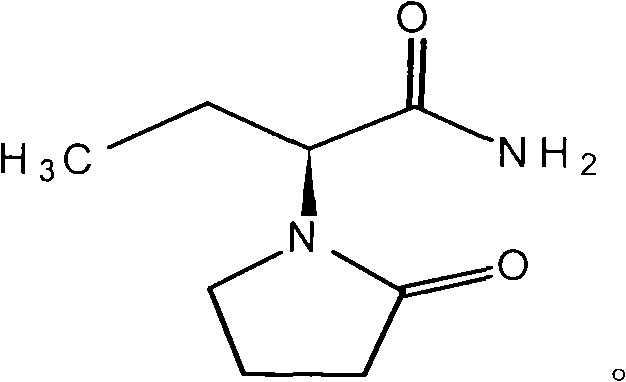
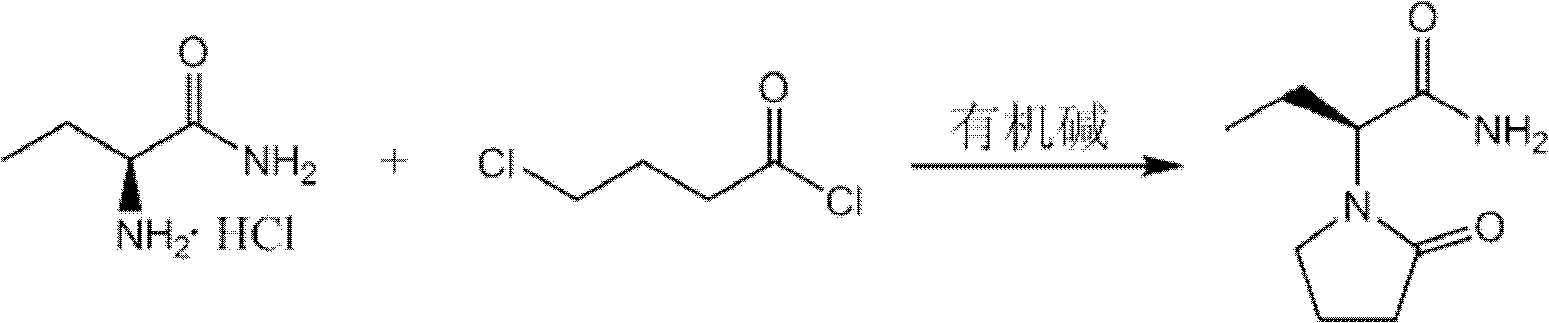
The invention provides a preparation method of levetiracetam. The method comprises the steps that: (1) in an inert solvent, an organic alkali is adopted as a catalyst, and (S)-2-amino butanamide hydrochloride and 4-chloroprene chloride are subjected to a reaciton, such that levetiracetam is prepared. The reaction formula (I) is shown below. In the reaction formula (I), the organic alkali is selected from trimethylamine, triethylamine, tripropylamine and diisopropylethylamine ethylamine. Compared with prior arts, the method provided by the invention has the advantages that: according to the invention, the applications of a large amount of inorganic strong alkali and water absorbent are avoided, such that the production of a large amount of strong-alkaline solid waste is avoided; the method has the advantages of short reaction time, easy-to-control operation process, reduced raw material cost, and suitability for large-scale industrial production; with the method, levetiracetam yield is improved, and the obtained levetiracetam has ideal optical purity.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
Levetiracetam osmotic pump controlled release tablet and preparation method thereof
InactiveCN101422442ASmooth and sustained releaseReduce toxic and side effectsNervous disorderPharmaceutical delivery mechanismSide effectActive matter
The invention belongs to the technical field of medicament and provides a Levetiracetam osmotic pump controlled-release tablet and a preparation method thereof. The invention consists of the accessory of the Levetiracetam playing the effect of release control and a semi-transparent membrane; in the invention, proper accessory and medicament are mixed to press a tablet core at first; then a layer of semi-transparent membrane is coated outside the tablet core; then at least one small hole is punched on the semi-transparent membrane so as to lead active matters to be released from the semi-transparent membrane, thereby controlling the release of the medicament. Compared with a common preparation, the controlled-release preparation prepared by the invention has the advantages of small wave range of the blood medicine concentration, reducing toxic and side effect, being taken once in one day and improving the compliance of sufferers. The controlled-release preparation is applied on the adjunctive therapy for the partial seizure of epileptics in clinic.
Owner:SHENYANG PHARMA UNIVERSITY
Combined-step process for pharmaceutical compositions
The invention relates to a process for the solid oral pharmaceutical formulation of a pharmaceutically active ingredient such as levetiracetam, comprising a wet granulation of the pharmaceutically active ingredient and simultaneous fluid bed drying such that, as the pharmaceutical blend granulates it is simultaneously dried thus preventing it from becoming a paste. The invention therein thus provides a novel formulation preparation process characterized by a “combined” Granulation and Fluid Bed Drying process step.
Owner:MYLAN PHARMA ULC
Rapid Disperse Dosage Form Containing Levetiracetam
ActiveUS20140271862A1Easy to swallowFacilitating administrationBiocidePowder deliveryDiseaseHigh doses
A high dose rapidly dispersing three-dimensionally printed dosage form comprising a high dose of levetiracetam in a porous matrix that disperses in water within a period of less than about 10 seconds is disclosed. Also disclosed are methods of preparing the dosage form and of treating a condition, disease or disorder that is therapeutically responsive to levetiracetam.
Owner:APRECIA PHARMA LLC
Levetiracetam sustained release tablet as well as preparation method thereof
ActiveCN104586806ASuitable particle size distributionModerate hardnessOrganic active ingredientsPharmaceutical non-active ingredientsSustained Release TabletOrganic solvent
The invention relates to a levetiracetam sustained release tablet as well as a preparation method thereof in the field of pharmaceutical preparations. The sustained release tablet comprises a tablet core and a coating, wherein the table core is prepared from levetiracetam, a framework material, a flow aid, a binder and a lubricant. The sustained release tablet is prepared from the following components in percentage by weight: 55-80% of levetiracetam, 15-35% of the framework material, 0.05-0.20% of the binder, 0.8-2% of the flow aid, 2-7% of the lubricant and 2-4% of the coating. The preparation method comprises the following steps: (1) granulating; (2) mixing; (3) tabletting; (4) preparing a coating solution; and (5) coating. The product is simple in prescription, good in dissolution effect and good in stability. The preparation method is free of organic solvents such as ethanol and the like and has the characteristics of simple and convenient preparation process, easily operation and low production cost. The product is particularly suitable for industrial large-scaled production and can be widely applied to the field of pharmaceutical preparations.
Owner:SHENYANG NO 1 PHARMA FACTORY DONGBEI PHARMA GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com