Methods for the identification of agents for the treatment of seizures, neurological diseases, endocrinopathies and hormonal diseases
a technology for neurodegenerative disorders and agents, applied in the field of drug discovery in neurological disorders, endocrinopathies and hormonal diseases, can solve the problems of disease condition, adverse effects of synaptic vesicle function on neurotransmission in general, and control of neurotransmitter releas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Development of a Levetiracetam Analog for Binding Studies
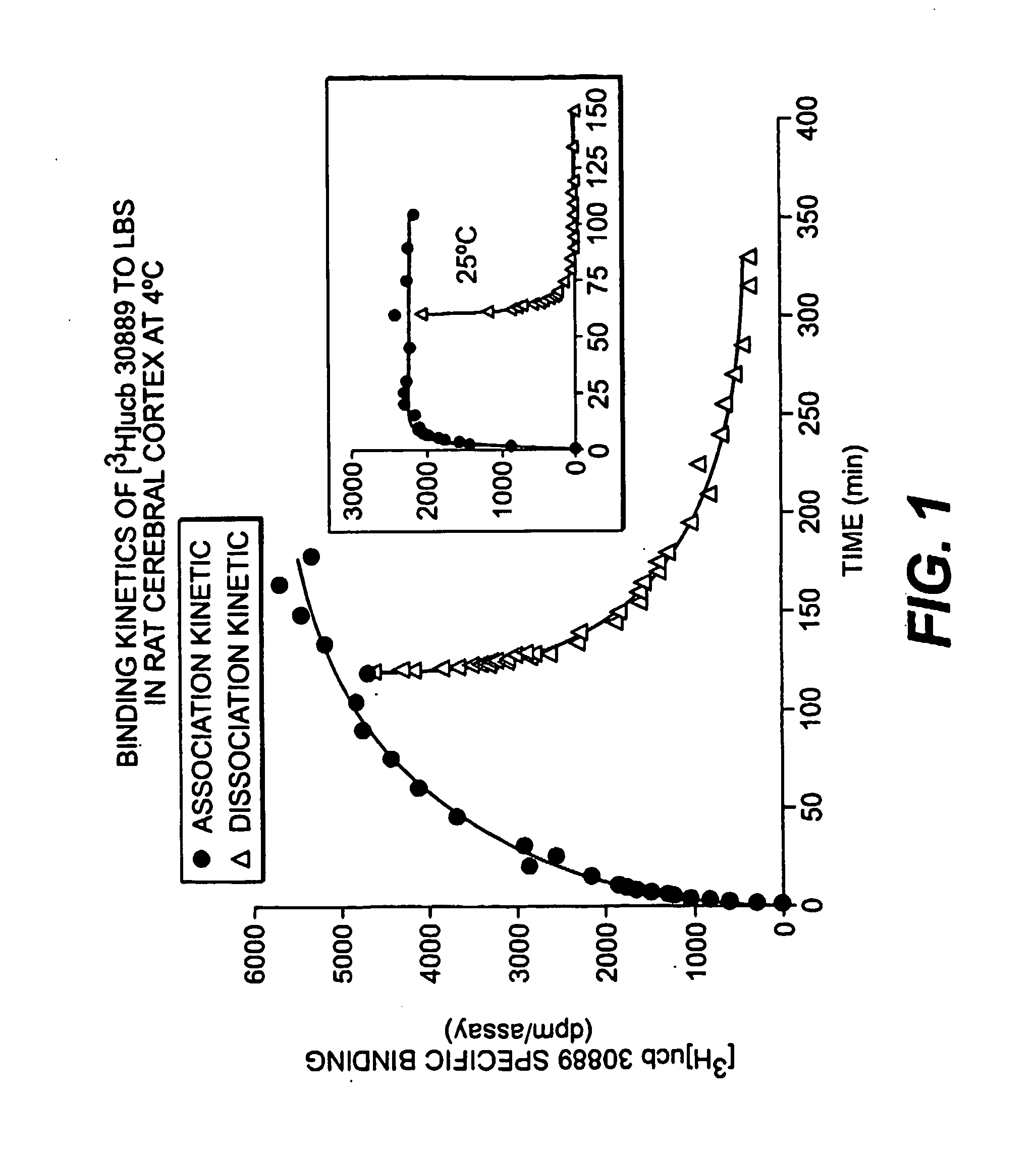
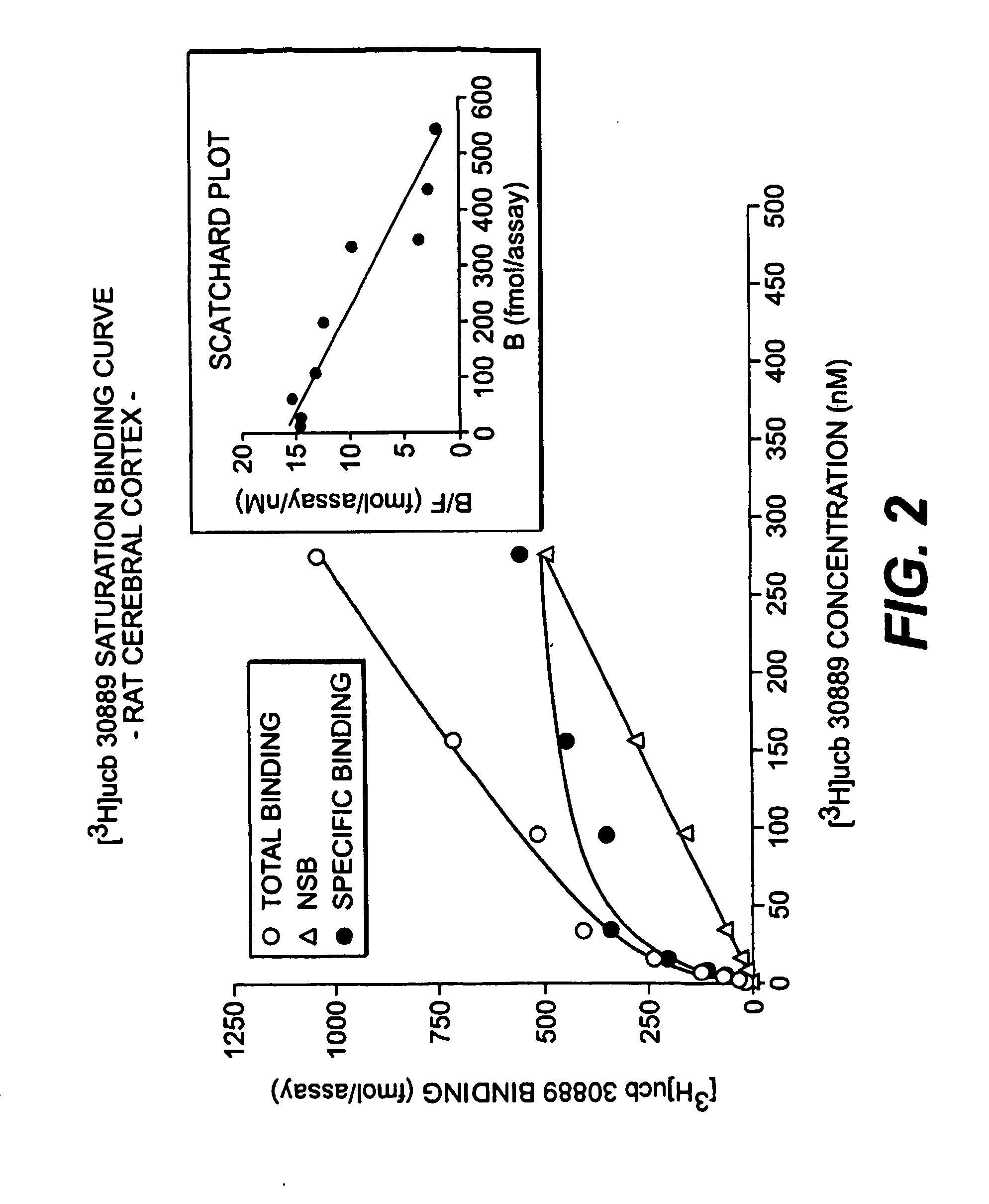
[0181] LEV has been shown to bind to a specific binding site located preferentially in the brain (levetiracetam binding site or LBS : Noyer et al., Euro. J. Pharmacol. 286:137-146. (1995); Gillard et al. 2003)). However, [3H]LEV displayed only micromolar affinity for this site, making it unsuitable for in depth characterization. This example describes the binding properties of [3H]ucb 30889, (2S)-2-[4-(3-azidophenyl)-2-oxopyrrolidin-1-yl]butanamide, an analogue of levetiracetam. Binding experiments were conducted on crude rat brain membranes at 4° C. as described in Noyer et al. (Euro. J. Pharmacol. 286:137-146 (1995)). Incubation time for equilibrium studies was 120 min. For kinetic and competition studies, [3H]ucb 30889 (30 Ci / mmol) was used at a concentration of 1.3 nM in 0.5 ml of a Tris-HCl (pH 7.4) buffer containing 2 mM Mg2+. Localization of the LBS in brain substructures was assessed by autoradiography on 25 μm thick ...
example 2
Cellular and Subcellular Distribution of the LBS
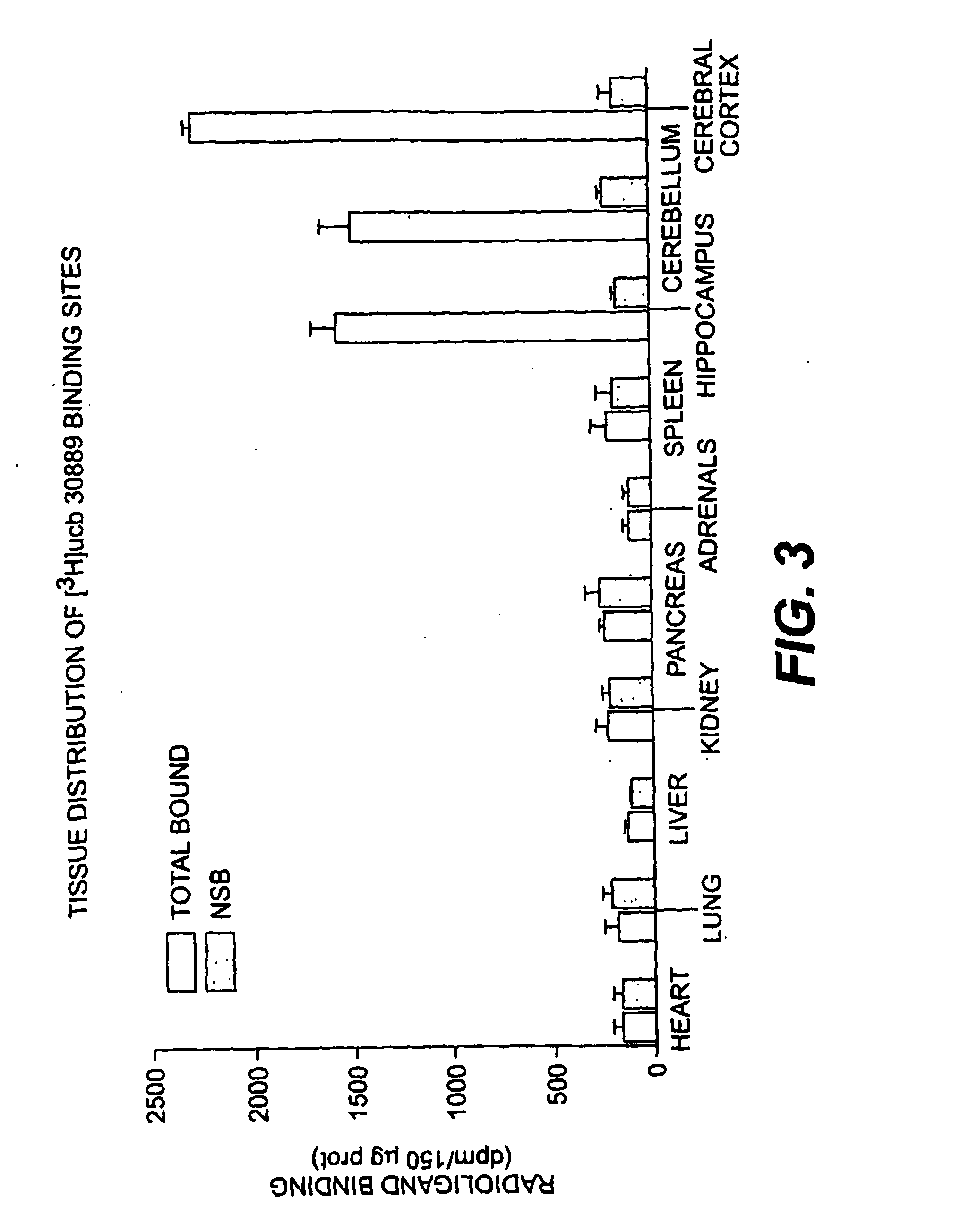
[0188] To identify and characterize the LBS in situ, [3H]ucb 30889 was used to map the LBS within the brain and to study both its cellular and subcellular distribution. For rat brain autoradiography, 25 μm slices were incubated with 1.3 nM [3H]ucb 30889 for 120 min at 4° C. in 50 mM Tris-HCl buffer (pH 7.4). Binding assays with rat brain membranes and various neuronal cell lines were performed under similar conditions. Non-specific binding was determined by the inclusion of 1 mM levetiracetam in the assay. For photolabeling, membranes were incubated with 40 nM [3H]ucb 30889 for 120 min at 4° C. in the same buffer, followed by irradiation with UV-light for 30 min (Fuks et al., Eur. J. Pharmacol. 478:11-19 (2003)).
[0189] For rat brain autoradiography, 25 μm slices were incubated with 1.3 nM [3H]ucb 30889 for 120 min at 4° C. in 50 mM Tris-HCl buffer (pH 7.4). FIG. 7 shows that ucb 30889 binding sites are heterogeneously distributed in ...
example 3
The LBS is on SV2A
[0195] In this example, the biochemical characterization of LBS in rat brain led to studies to identify potential candidate LBS proteins for cloning and binding characterization. Based on the integral membrane nature of the protein, brain specific expression, apparent size, and synaptic vesicle localization, the SV2 protein family was analyzed as a candidate for localization of the LBS. Accordingly, SV2 proteins were cloned and assayed for binding of LBS ligands.
[0196] Materials: Levetiracetam and derivatives were synthesized at UCB Pharma (Braine-l'Alleud, Belgium). [3H]ucb 30889, (2S)-2-[4-(3-azidophenyl)-2-oxopyrrolidin-1-yl]butanamide (32 Ci / mmol), was custom labelled by Amersham Biosciences (Roosendaal, The Netherlands). The monoclonal antibody against SV2 proteins developed by Buckley and Kelly (Buckley et al., J. Cell. Biol., 100, 1284-94 (1985)) was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and mainta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com