Levetiracetam sustained release tablet as well as preparation method thereof
A sustained-release tablet and tablet core technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients. and other problems, to achieve the effect of simple product prescription, low production cost and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
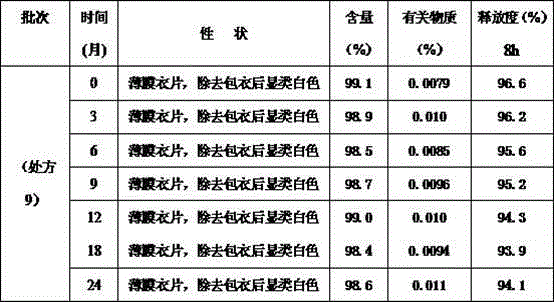
[0020] Table 1 Recipes using two different framework materials
[0021]
[0022] Preparation:
[0023] (1) Granulation: Weigh the prescribed amount of levetiracetam, add 25g of low-viscosity hypromellose (HPMC E50) 3% w / w aqueous solution to make soft material, granulate with a 20-mesh sieve, boil and dry, 10 mesh sieve;
[0024] The parameters of boiling drying are: fan power: 30-50Hz; blowback interval: 3-20 seconds; inlet air temperature: 40-60°C; material temperature: 30-40°C, measure the moisture of the mixture, and control the moisture by 0.5-1.5%;
[0025] (2) Mixing: Weigh the prescribed amount of levetiracetam granules, skeleton material and glidant and mix for 15-20 minutes, then add lubricant and mix for 2-3 minutes;
[0026] (3) Tablet compression: compress the mixed materials into tablet cores with a tablet hardness of 13-15kg;
[0027] (4) Preparation of coating solution: disperse 20g of Opadry 295F in 113g of water, stir for 40-50 minutes, and prepare a 15% ...
Embodiment 2
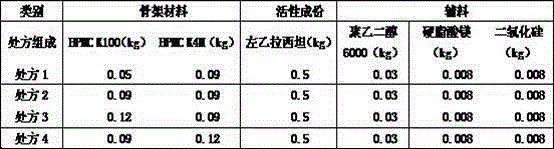
[0031] Table 2 Recipe using a separate framework material
[0032]
[0033] Preparation:
[0034] (1) Granulation: Weigh the prescribed amount of levetiracetam, add 25g of low-viscosity hypromellose (HPMC E50) 3% w / w aqueous solution to make soft material, granulate with a 30-mesh sieve, boil and dry, 20 mesh sieve;
[0035] The parameters of boiling drying are: fan power: 30-50Hz; blowback interval: 3-20 seconds; inlet air temperature: 40-60°C; material temperature: 30-40°C, measure the moisture of the mixture, and control the moisture by 0.5-1.5%;
[0036] (2) Mixing: Weigh the prescribed amount of levetiracetam granules, skeleton material and glidant and mix for 15-20 minutes, then add lubricant and mix for 2-3 minutes;
[0037] (3) Tablet compression: compress the mixed materials into tablet cores with a tablet hardness of 13-15kg;
[0038] (4) Preparation of coating solution: disperse 22g of Opadry 295F in 115g of water, stir for 40-50 minutes, and prepare a 16% coa...
Embodiment 3
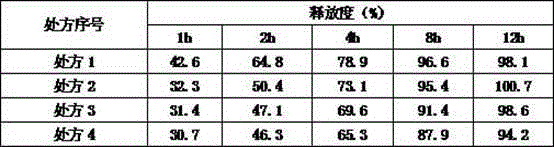
[0041] Table 3 Prescriptions using different amounts of lubricants
[0042]
[0043] Preparation:
[0044] (1) Granulation: Weigh the prescribed amount of levetiracetam, add 25g of low-viscosity hypromellose (HPMC E50) 3% w / w aqueous solution to make a soft material, granulate with a 20-mesh sieve, boil and dry, 20 mesh sieve;
[0045] The parameters of boiling drying are: fan power: 30-50Hz; blowback interval: 3-20 seconds; inlet air temperature: 40-60°C; material temperature: 30-40°C, measure the moisture of the mixture, and control the moisture by 0.5-1.5%;
[0046] (2) Mixing: Weigh the prescribed amount of levetiracetam granules, skeleton material and glidant and mix for 15-20 minutes, then add lubricant and mix for 2-3 minutes;
[0047] (3) Tablet compression: compress the mixed materials into tablet cores with a tablet hardness of 13-15kg;
[0048] (4) Preparation of coating solution: disperse 20g of Opadry 295F in 91g of water, stir for 40-50 minutes, and prepare...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com