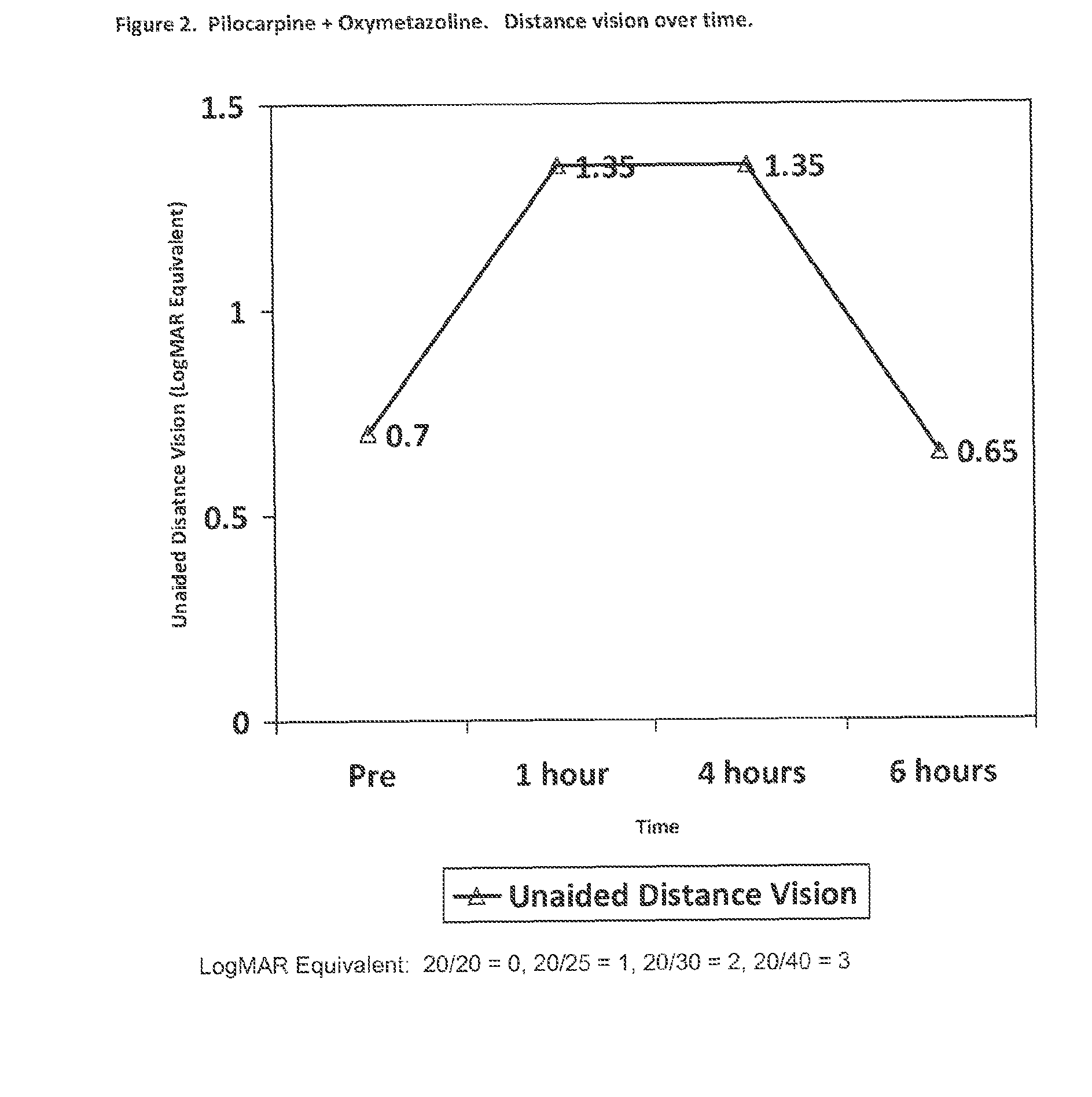
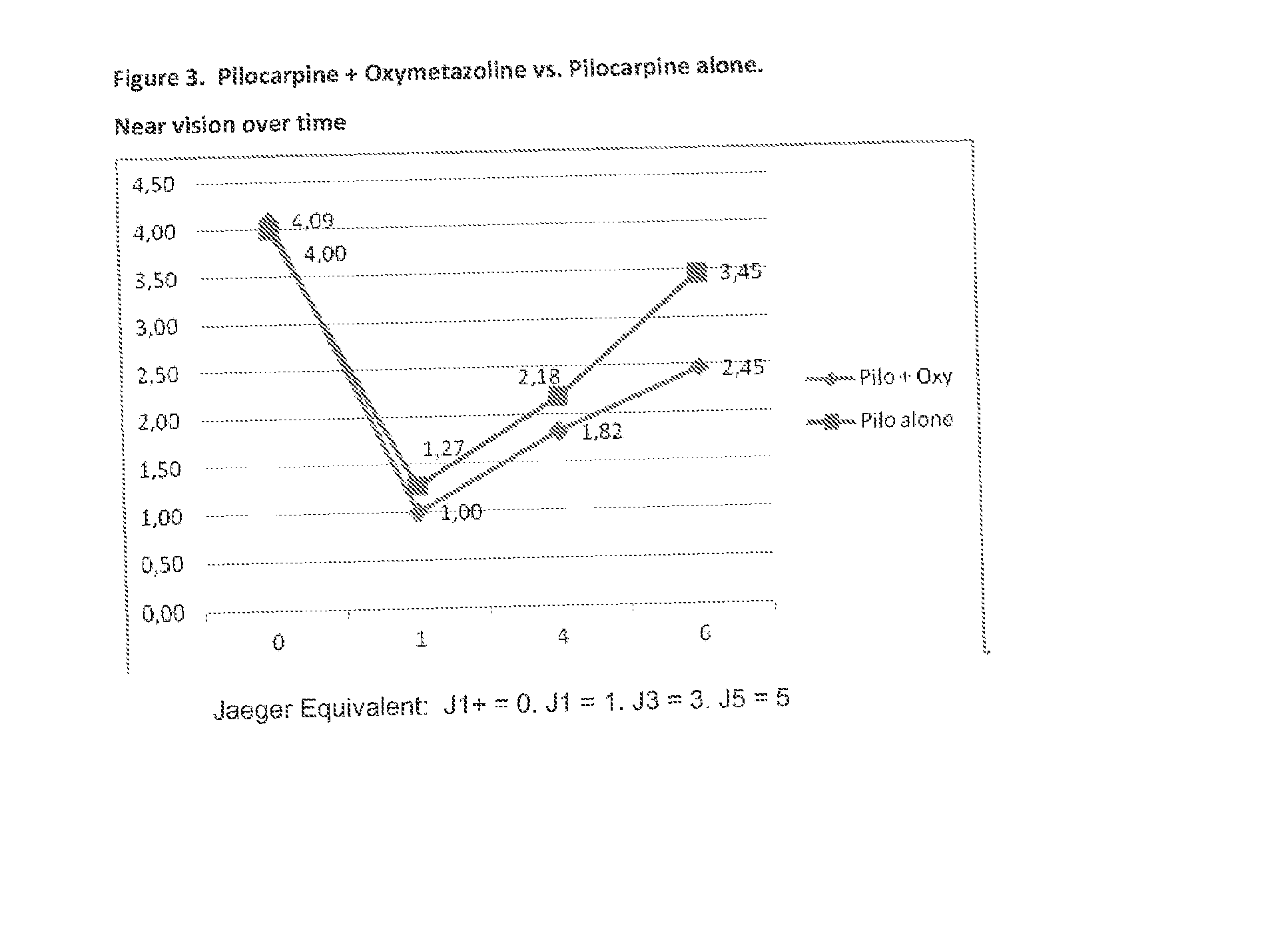
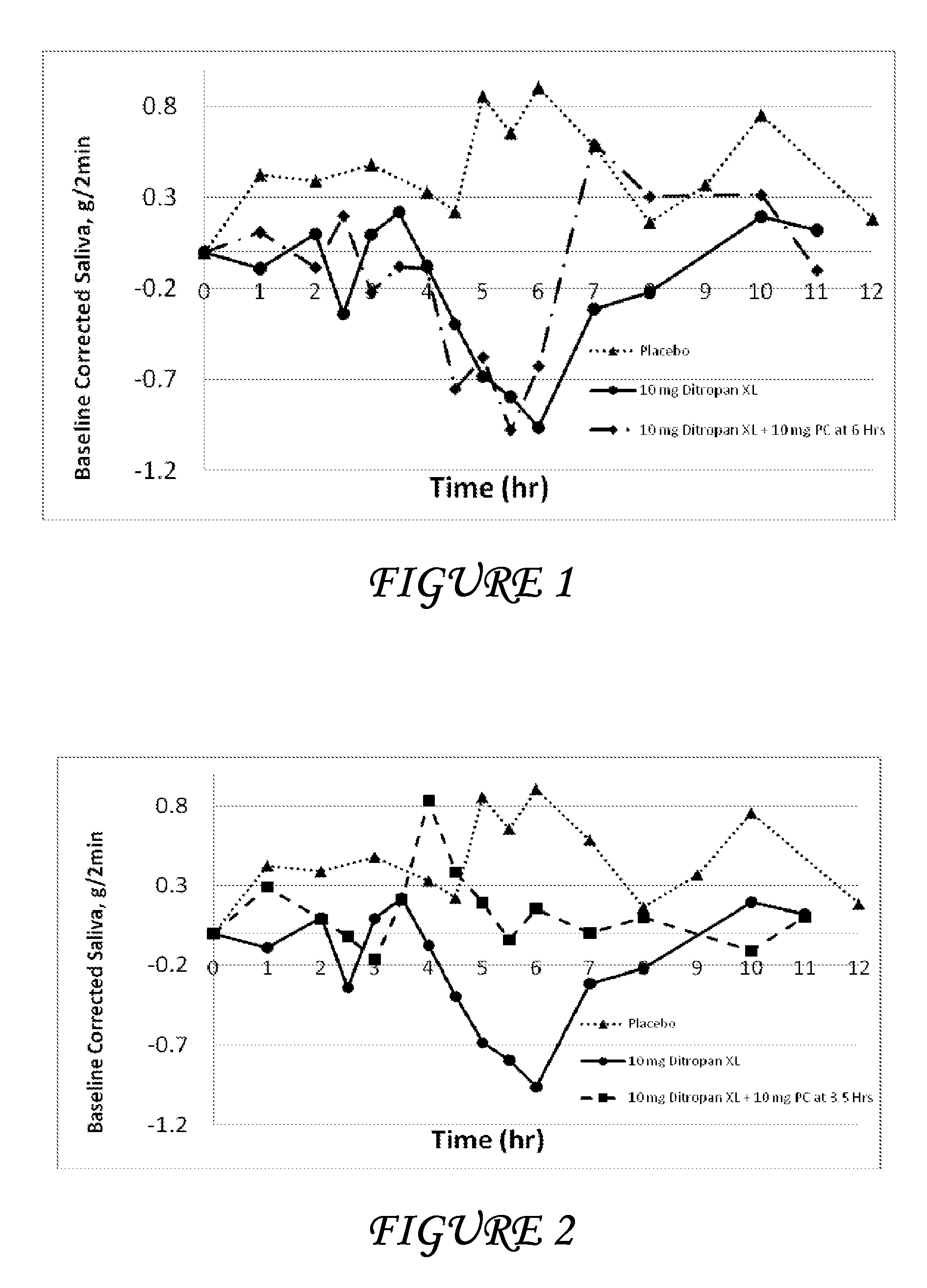
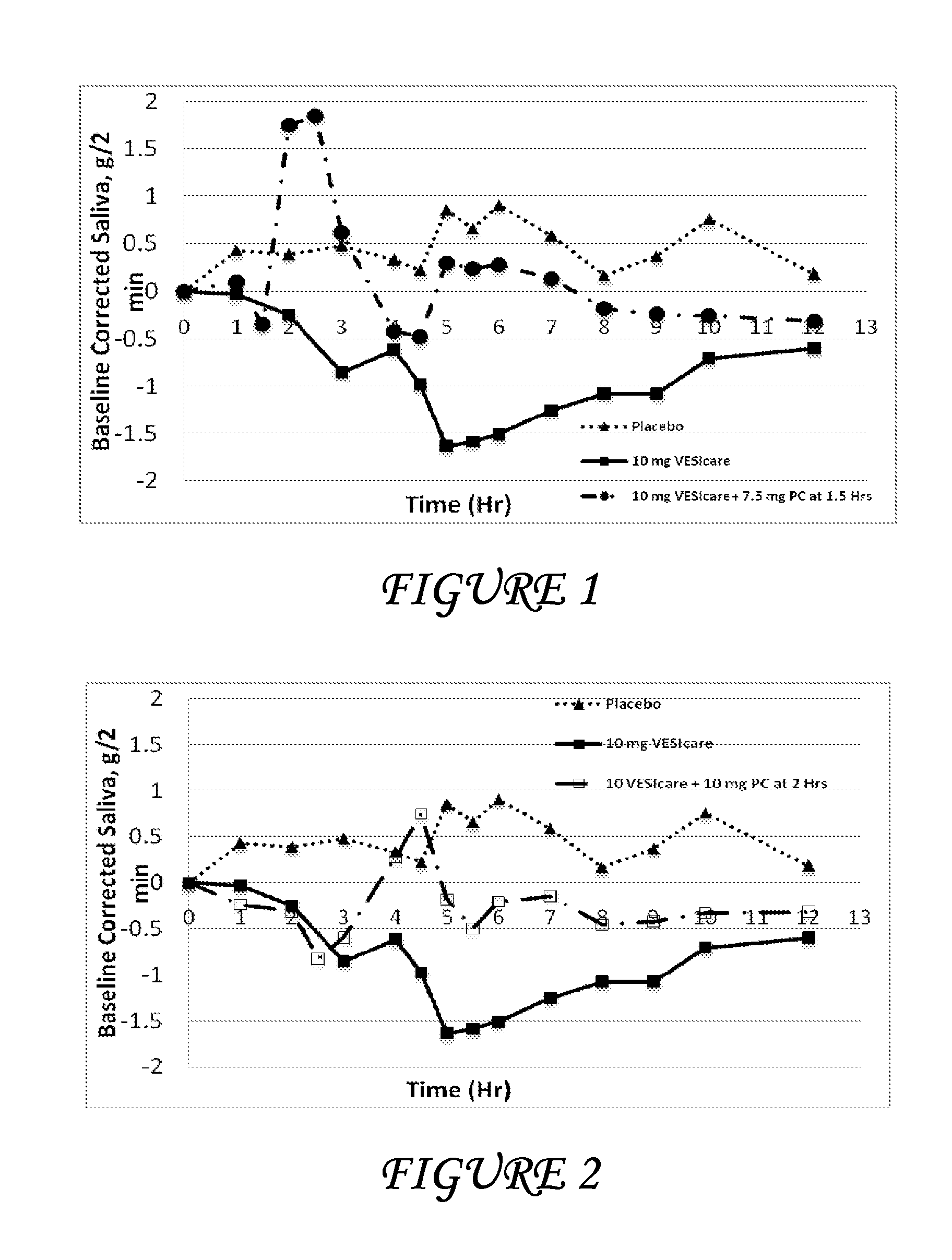
Patents
Literature
65 results about "Pilocarpine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used alone or with other medications to treat high pressure inside the eye due to glaucoma or other eye diseases (e.g., ocular hypertension).
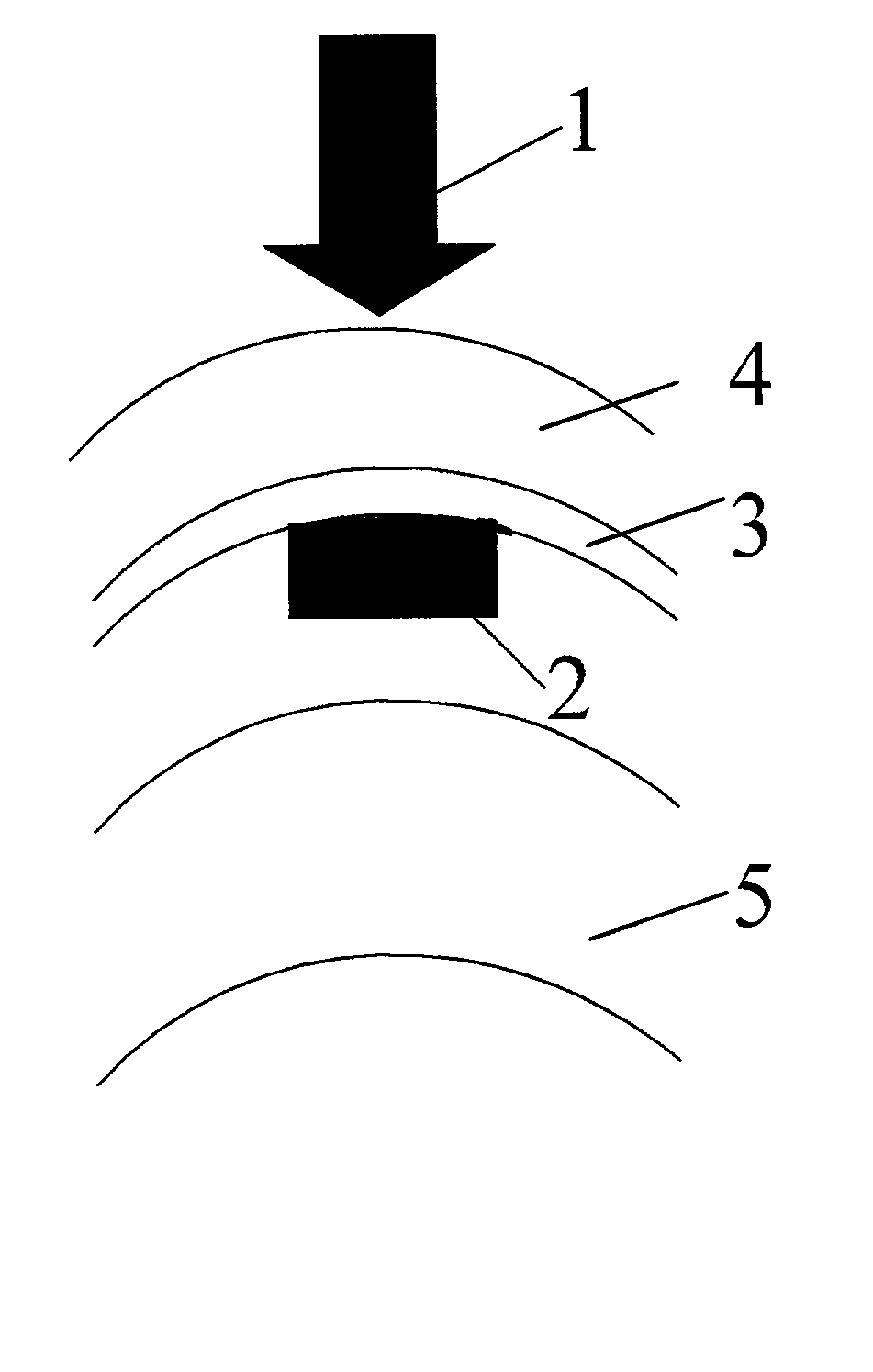
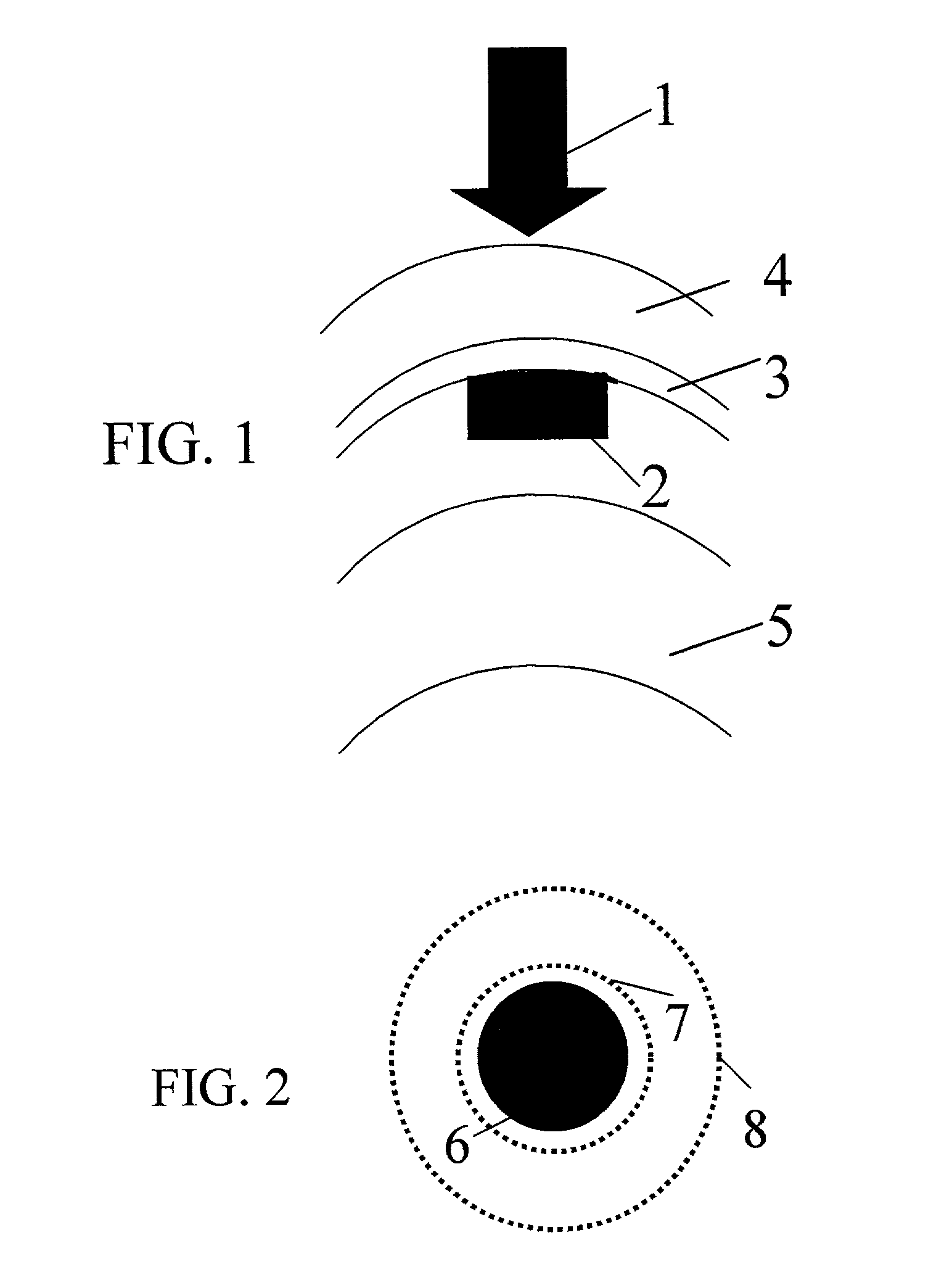
Method and apparatus for treatment of presbyopia by lens relaxation and anterior shift
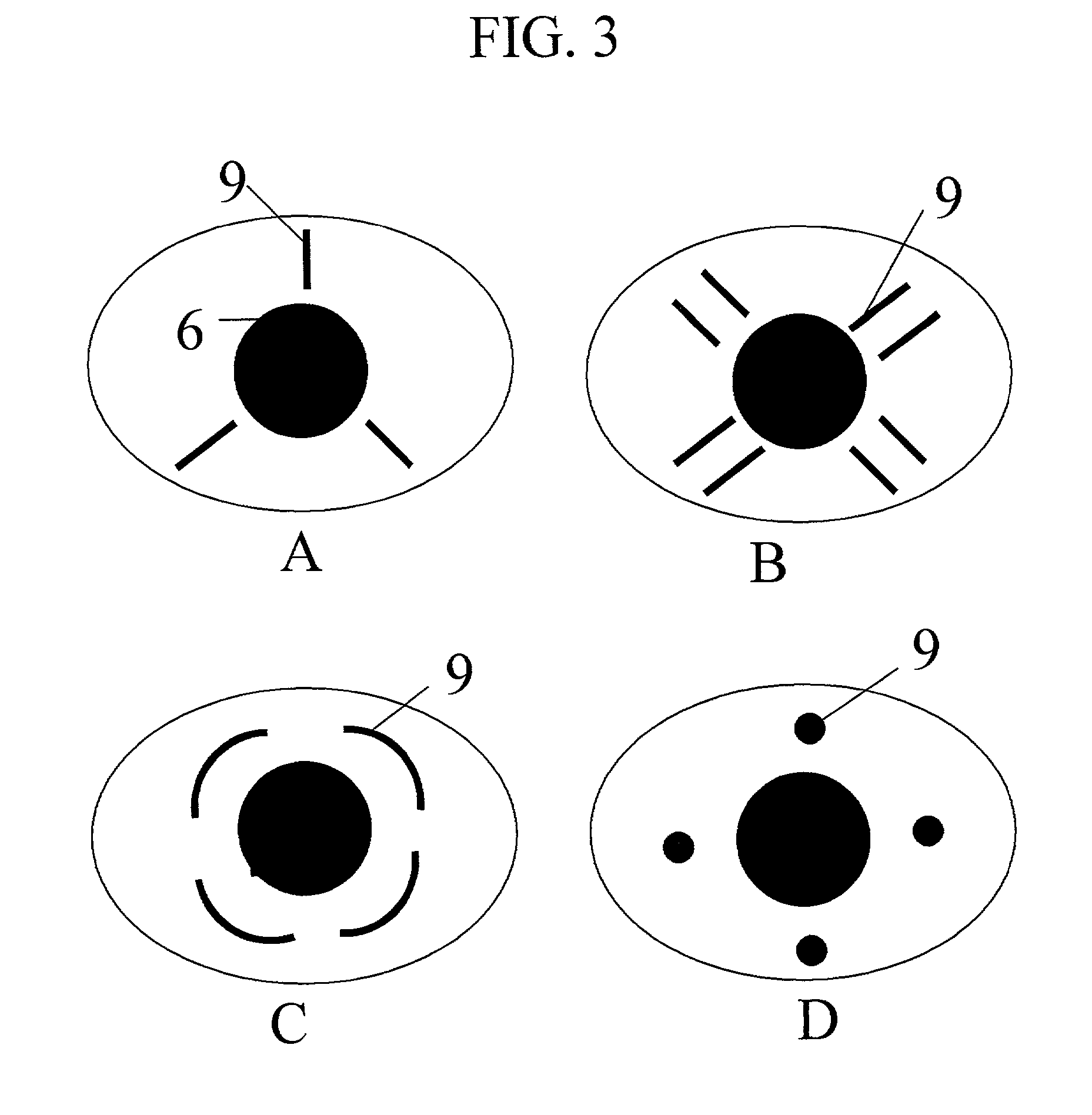
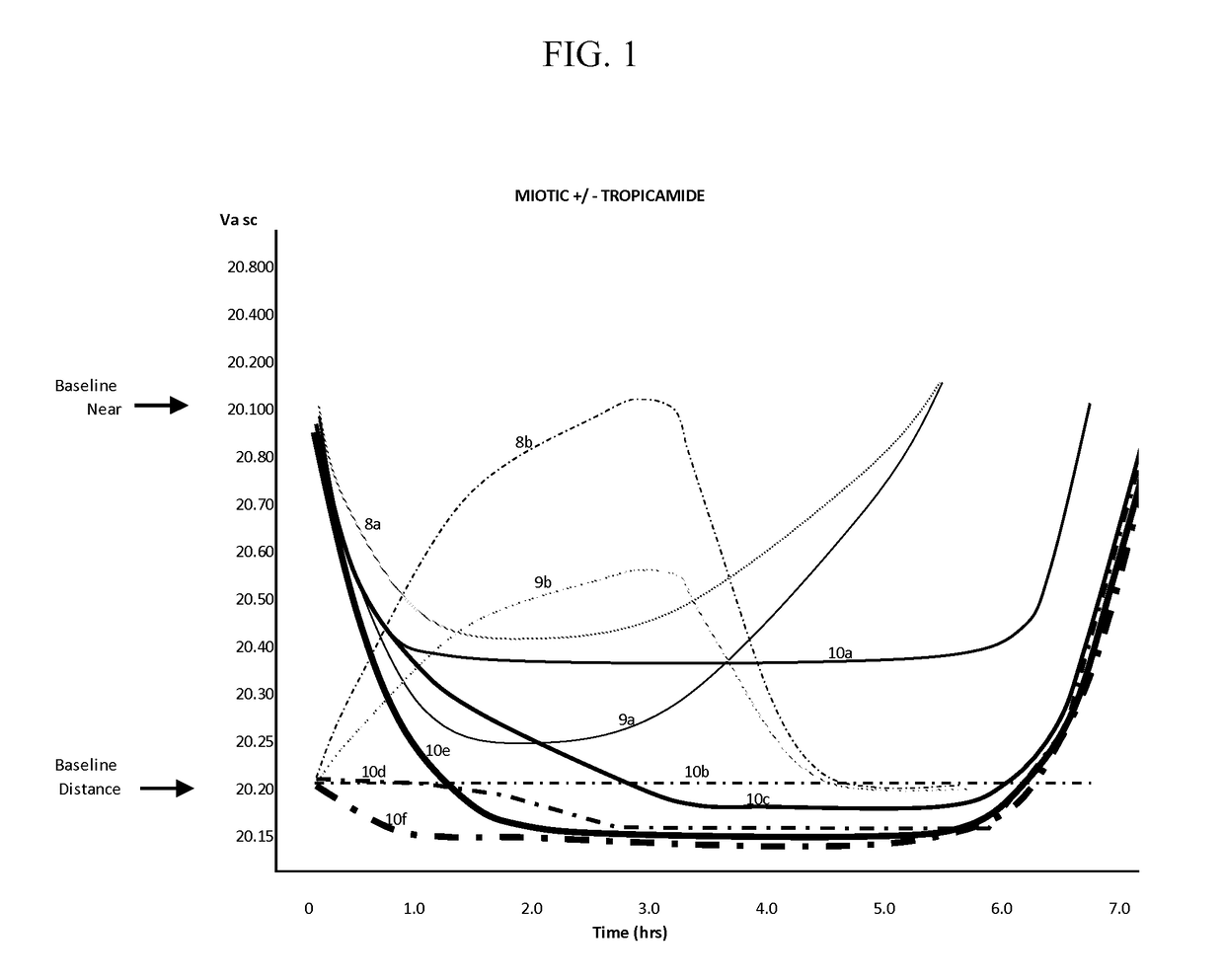
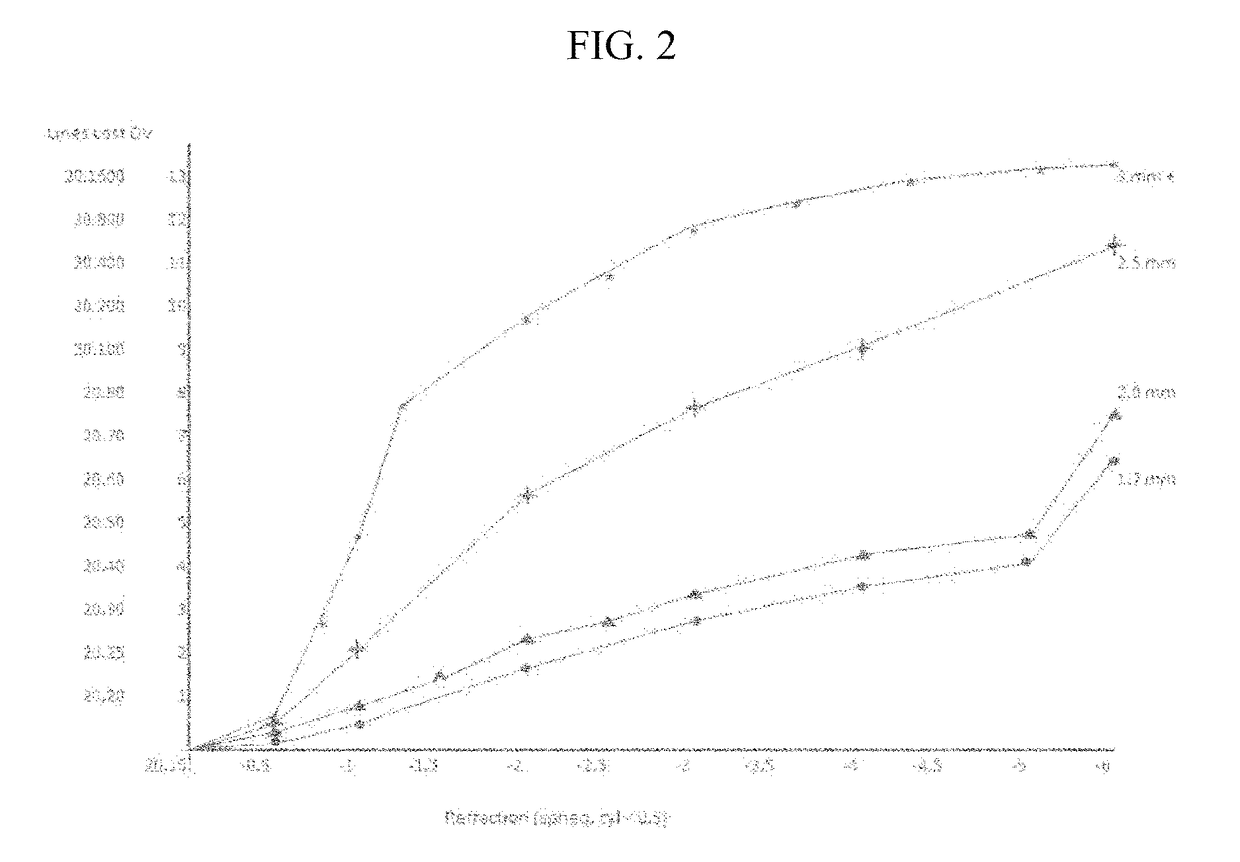
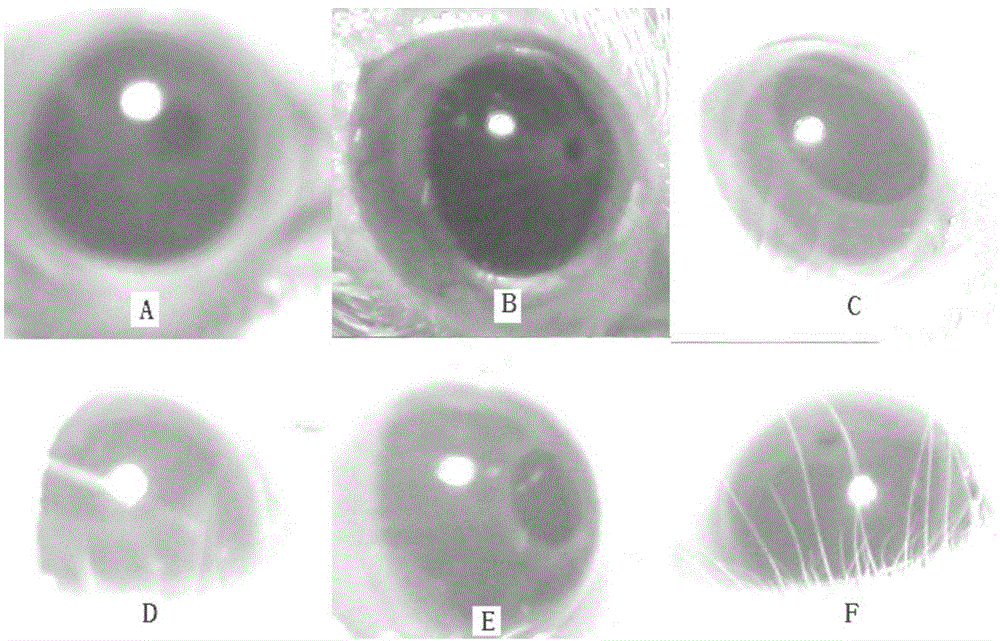
A surgical method and apparatus for presbyopia correction removal of the sclera tissue are disclosed. Mechanisms based on sub-conjunctiva filled-in of the sclera area and cause the sclera-ciliary-body and zonule "complex" become more flexible (or less rigidity) are proposed. Total accommodation based a lens relaxation and lanes anterior shift is calculated and proposed as the guidance of the parameters for device design and clinical outcomes The preferred embodiments for the ablation patterns include radial lines, curved lines, ring dots or any non-specific shapes in a symmetric geometry. The surgery apparatus includes non-laser device of radio frequency wave, electrode device, bipolar device and plasma assisted device. Another preferred embodiment is to use post-operation medication such as pilocarpine (1%-10%) or medicines with similar nature which may cause ciliary body contraction for more stable and enhancement after the treatment.
Owner:LIN J T
Devices for integrated, repeated, prolonged, and/or reliable sweat stimulation and biosensing
ActiveUS20150112164A1Effective simulationMitigate interference of stimulatingElectrotherapySurgerySkin surfaceIrritation
A sweat sensing device includes a plurality of sweat collection pads communicating with a sensor. Each of the pads is activated by a timing circuit which allows one or more of the pads to be activated at a selected time and subsequent deactivated after a defined period of time. This allows for selective collection of sweat from a plurality of pads over a prolonged period of time. An impedance measuring circuit can be employed to determine if one or more of the pads becomes disconnected, in order to avoid irritation. Further, the devices can use a common microfluidic device which both transports sweat activating substance, such as pilocarpine, to the surface of the skin and directs sweat away from the skin to a sensing device.
Owner:UNIVERSITY OF CINCINNATI
Method and apparatus for the treatment of presbyopia and glaucoma by ciliary body ablation
InactiveUS20050279369A1Improve postoperative resultsPromote resultsLaser surgeryDiagnosticsRadio frequencyNon specific
Laser and non-laser means to remove a portion of the ciliary body tissue for the treatment of presbyopia and glaucoma are disclosed. Mechanisms based on elasticity increase the sclera-ciliary-body and zonule “complex” is proposed. Total accommodation based a lens relaxation and lanes anterior shift is calculated. The preferred embodiments for the ablation patterns include radial lines, curved lines, ring dots or any non-specific shapes in a symmetric geometry. The surgery apparatus includes lasers in UV (0.19 to 0.35 micron) and IR (2.8 to 3.2) micron, and non-laser device of radio frequency wave, electrode device, bipolar device and plasma-assisted device. Post-operation medication such as pilocarpine (0.5%-5%) or medicines with similar to reduce postoperative regression or enhance the accommodation is presented. A much deeper, about (0.8-1.4) mm, ablation depth supraciliary body is proposed for (50% / -200%) greater accommodation than the prior arts based on superficial scleral ablation or expansion.
Owner:NEW VISION
Compositions and methods for treating presbyopia, mild hyperopia, and irregular astigmatism
ActiveUS20140113946A1Reduce actionEliminate side effectsBiocideSenses disorderSide effectMuscarinic receptor site
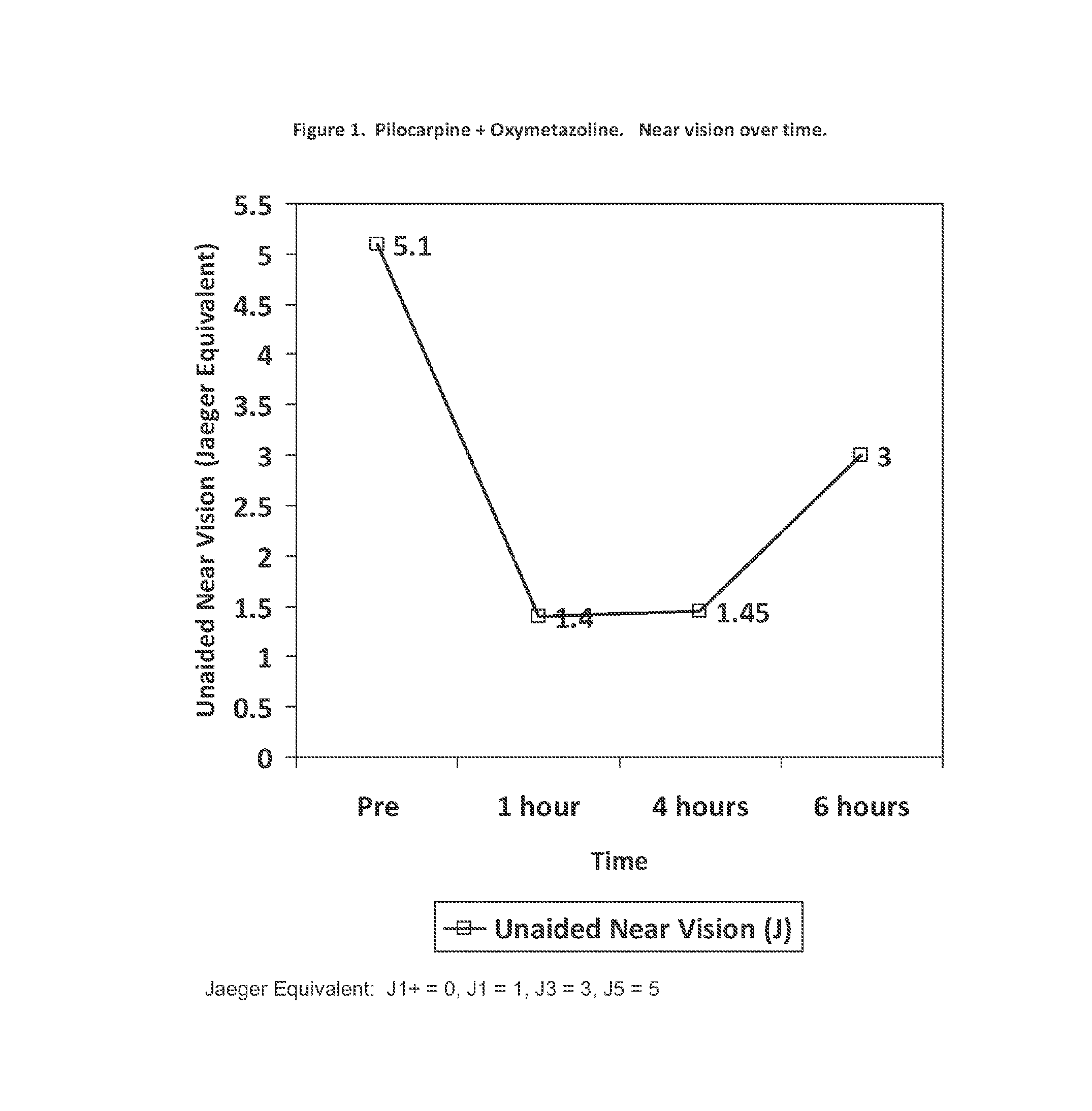
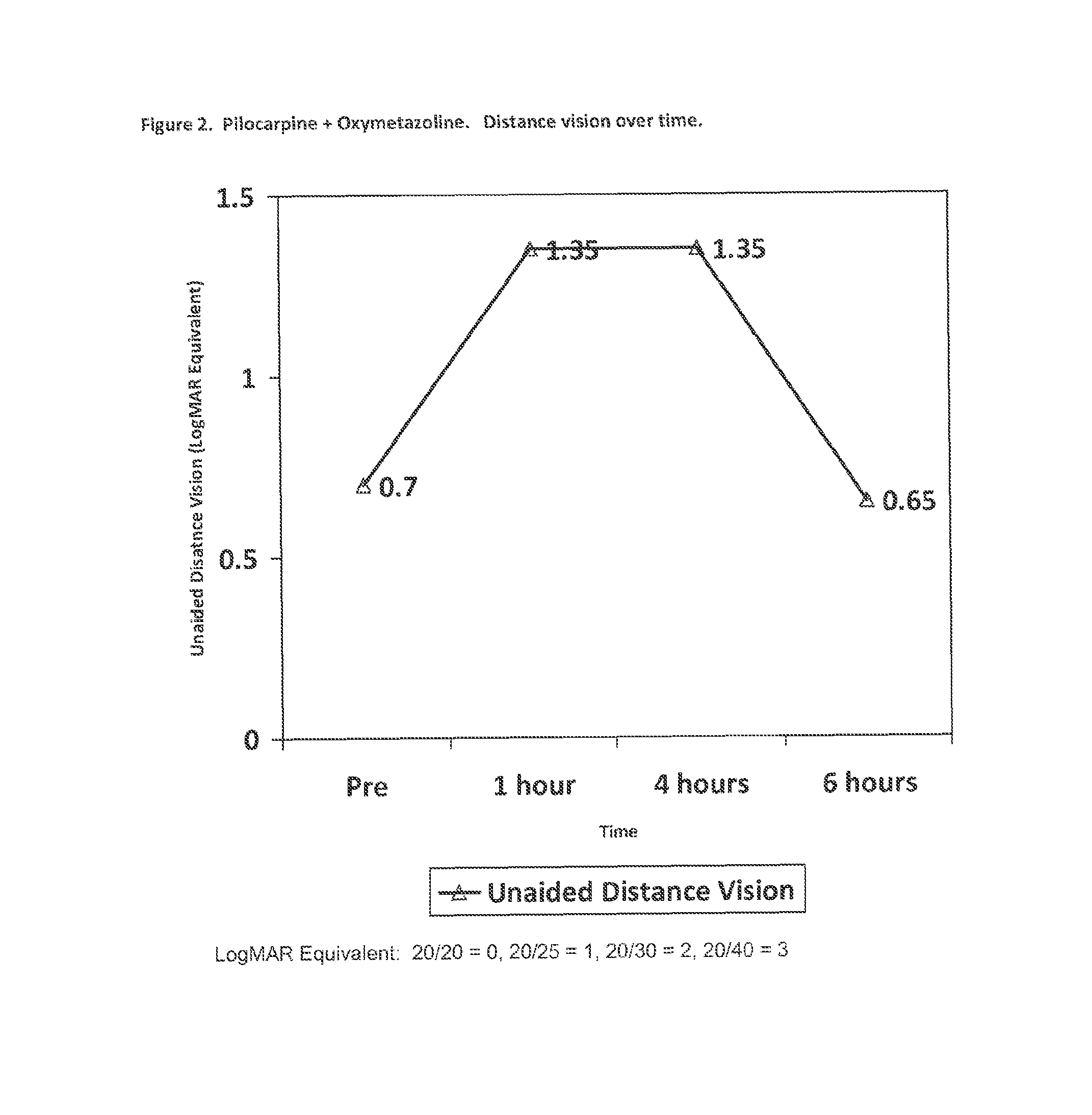
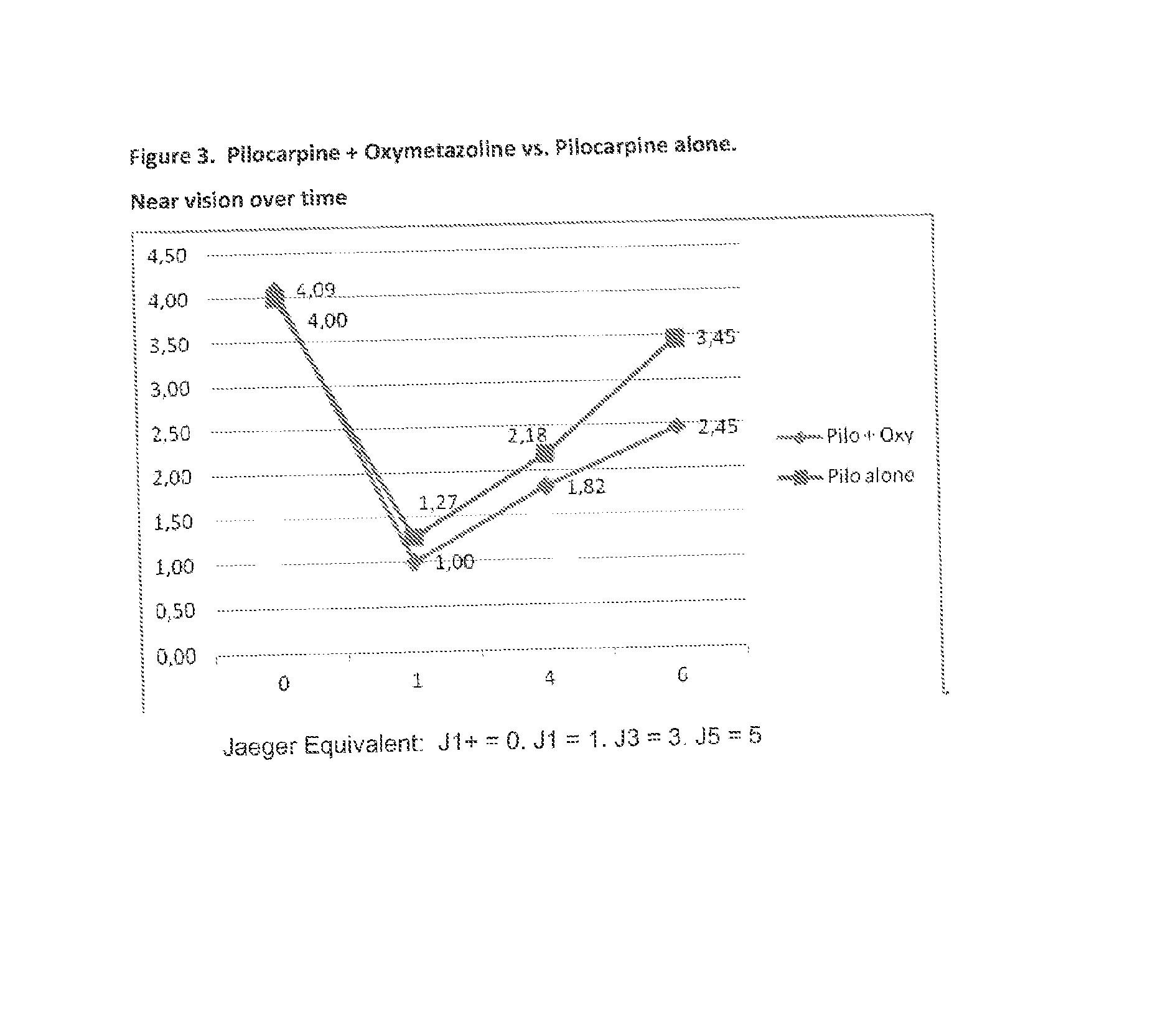
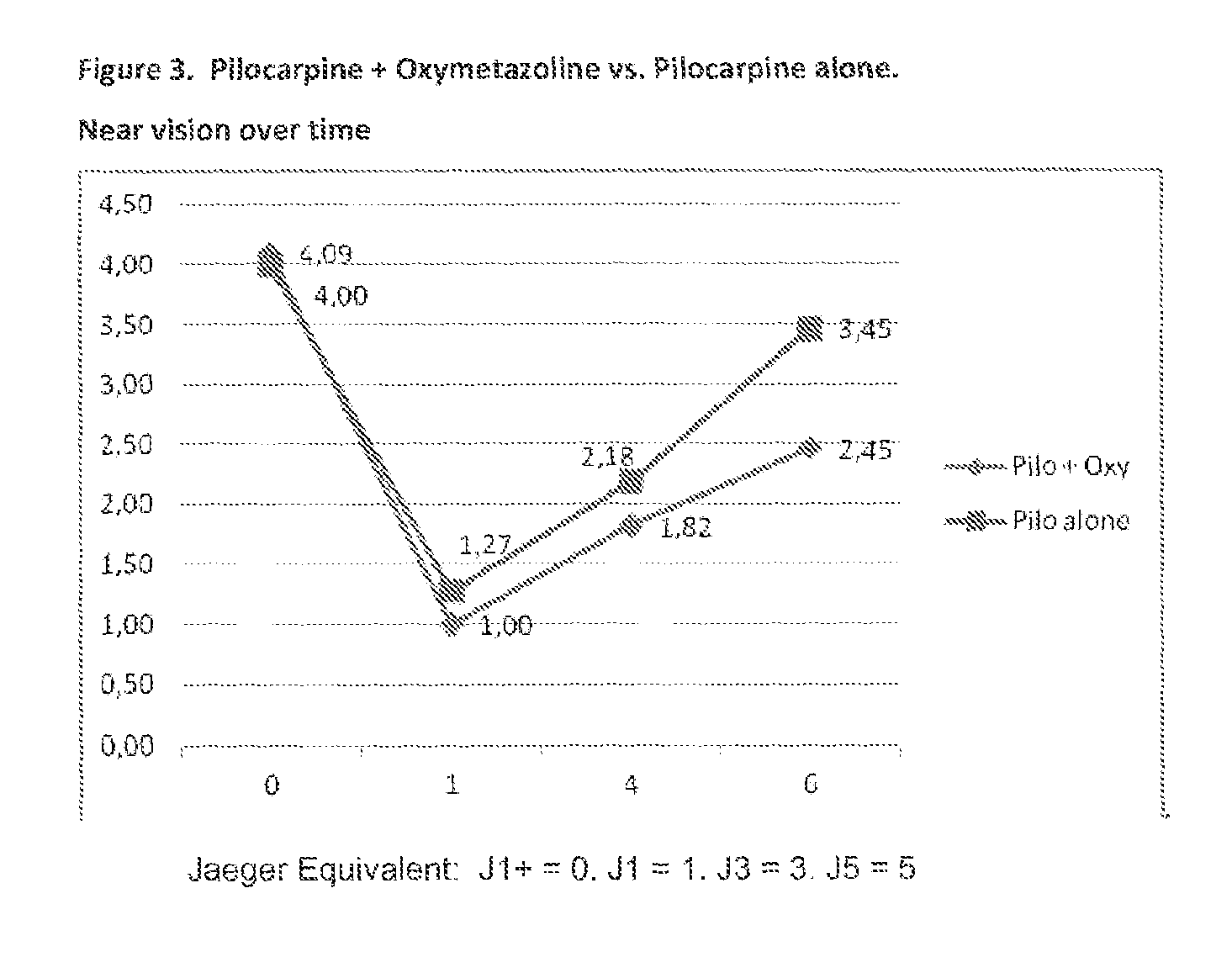
The present invention is directed to compositions and methods for treating presbyopia, mild hyperopia, and irregular astigmatism. The compositions include a cholinergic agent, such as a muscarinic acetylcholine receptor M3 agonist, and an alpha agonist having an imidazoline group or a non-steroidal anti-inflammatory agent (NSAID) having COX-2 selectivity. It has been found that an alpha agonist having an imidazoline group or non-steroidal anti-inflammatory agent (NSAID) having COX-2 selectivity in combination with a cholinergic agent, such as pilocarpine, act synergistically to improve the accommodative and focusing ability of the eye while minimizing the side effects from each compound.
Owner:ALLERGAN INC
Therapy for the treatment of disease
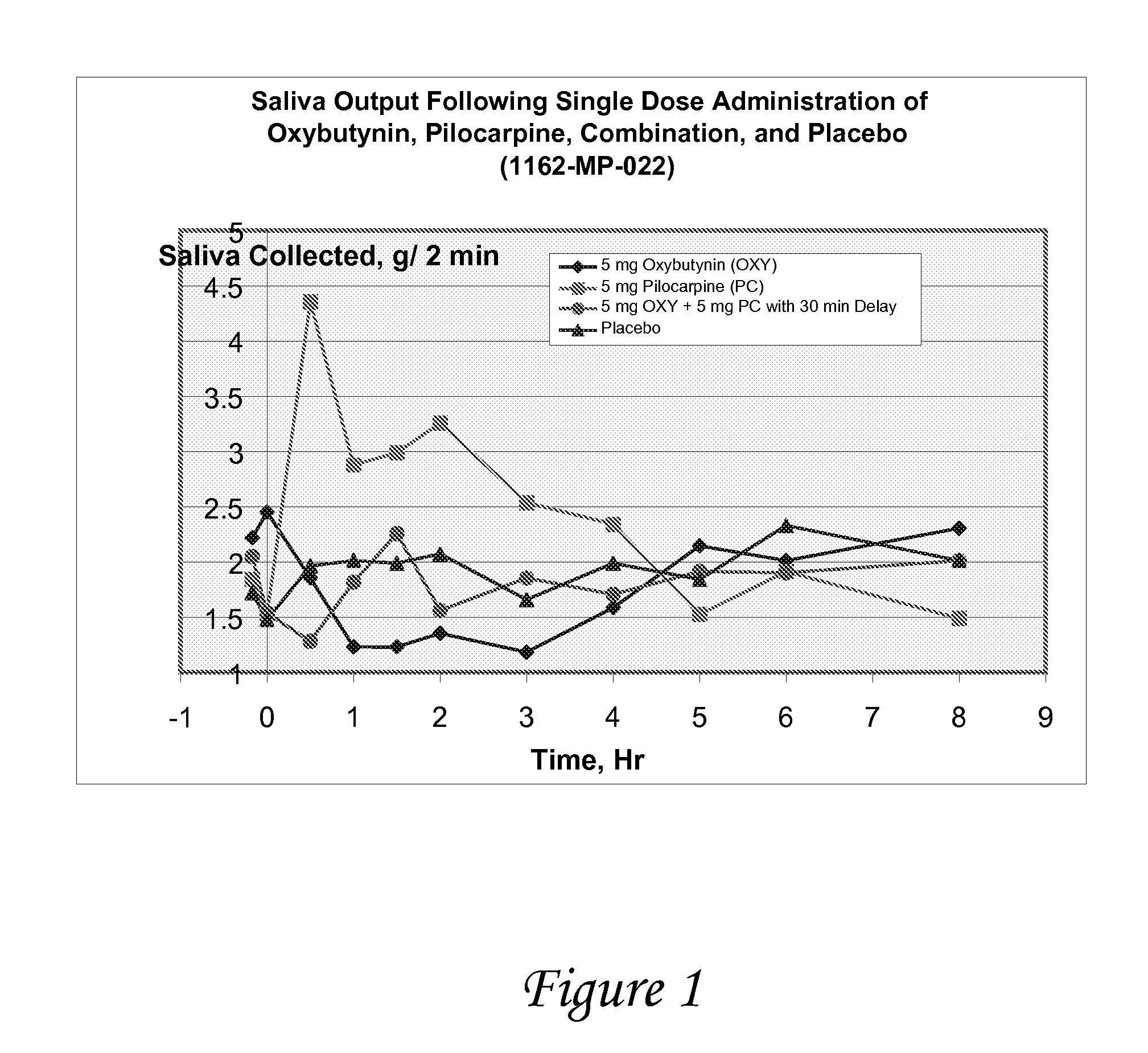
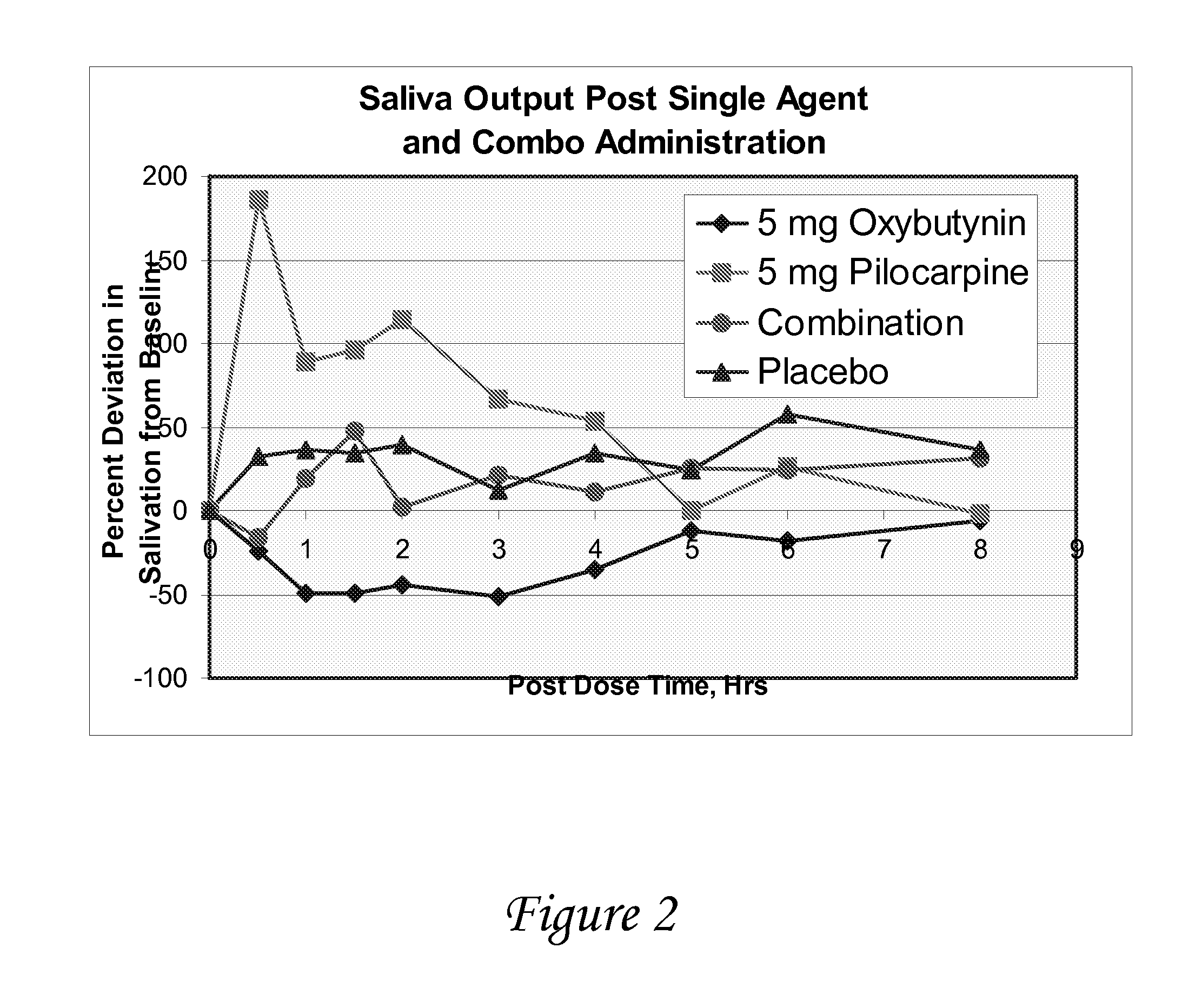
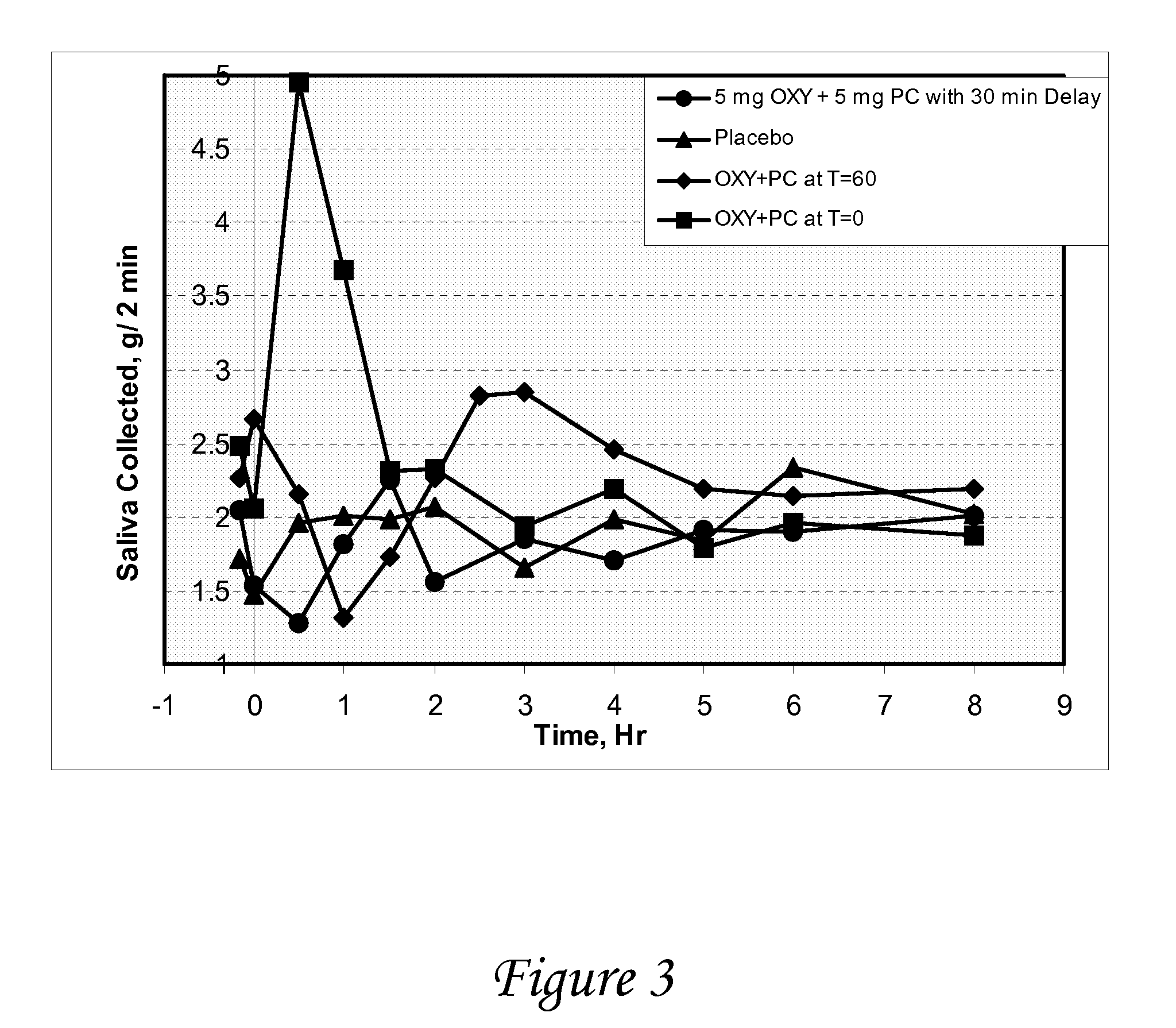
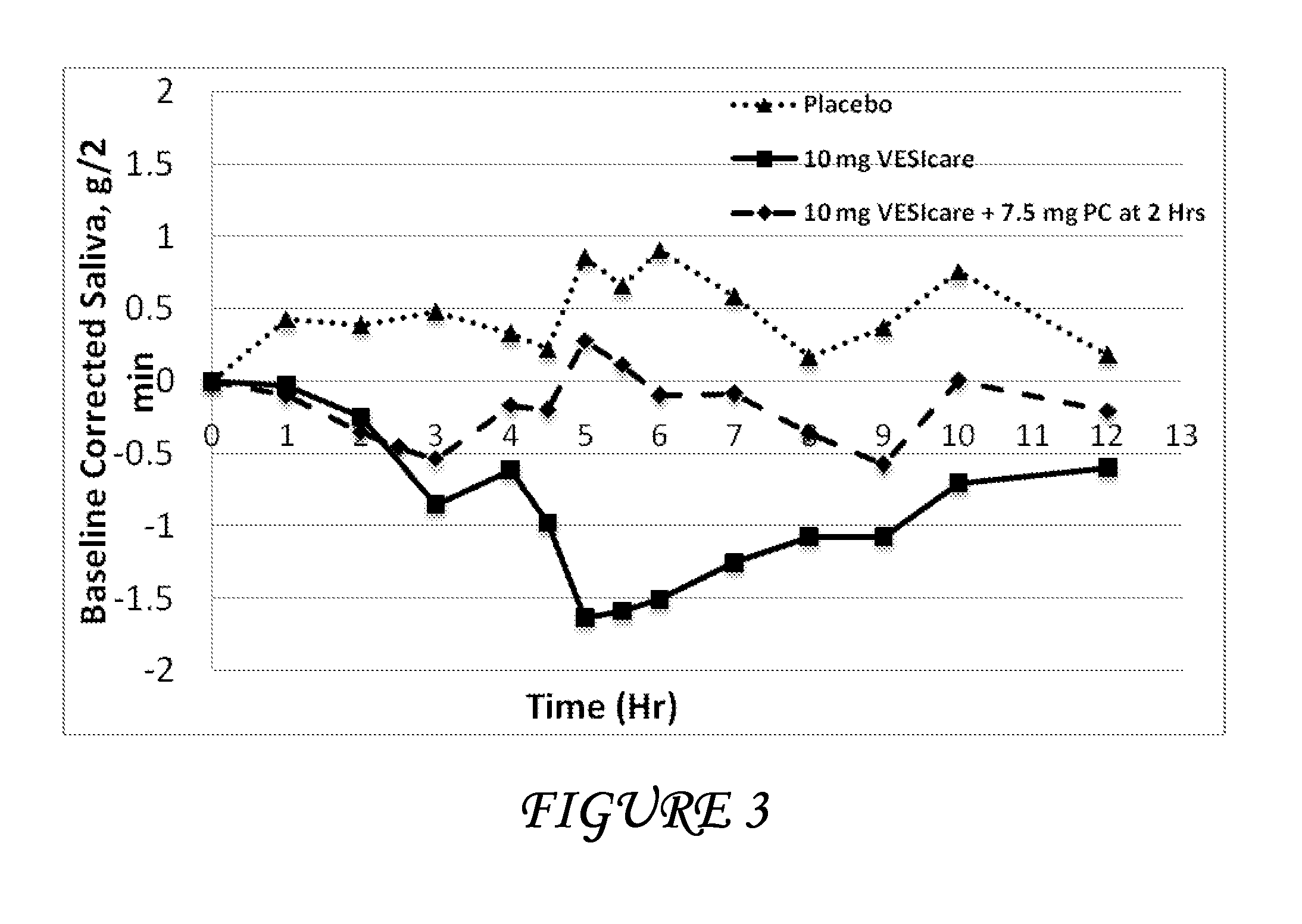
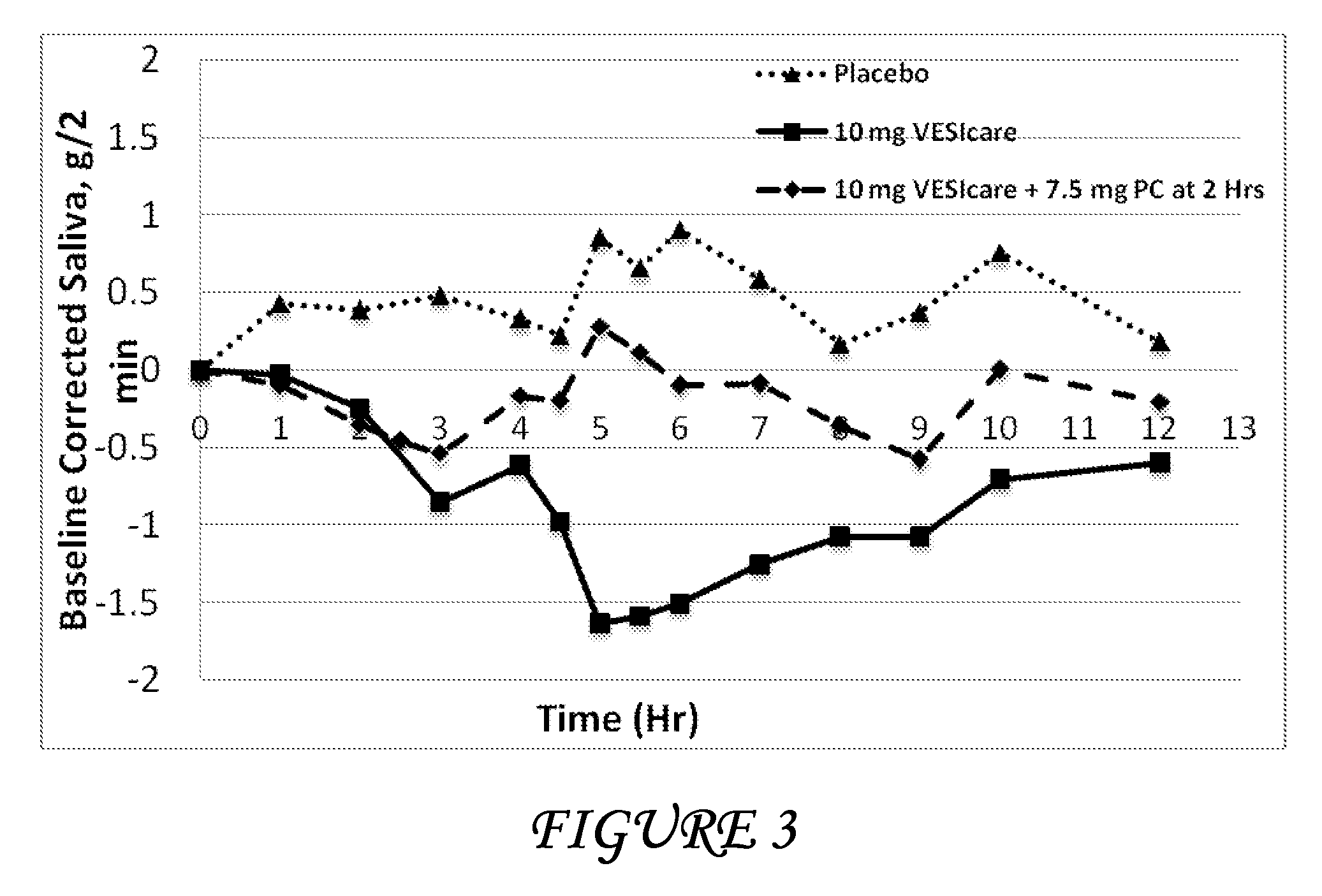
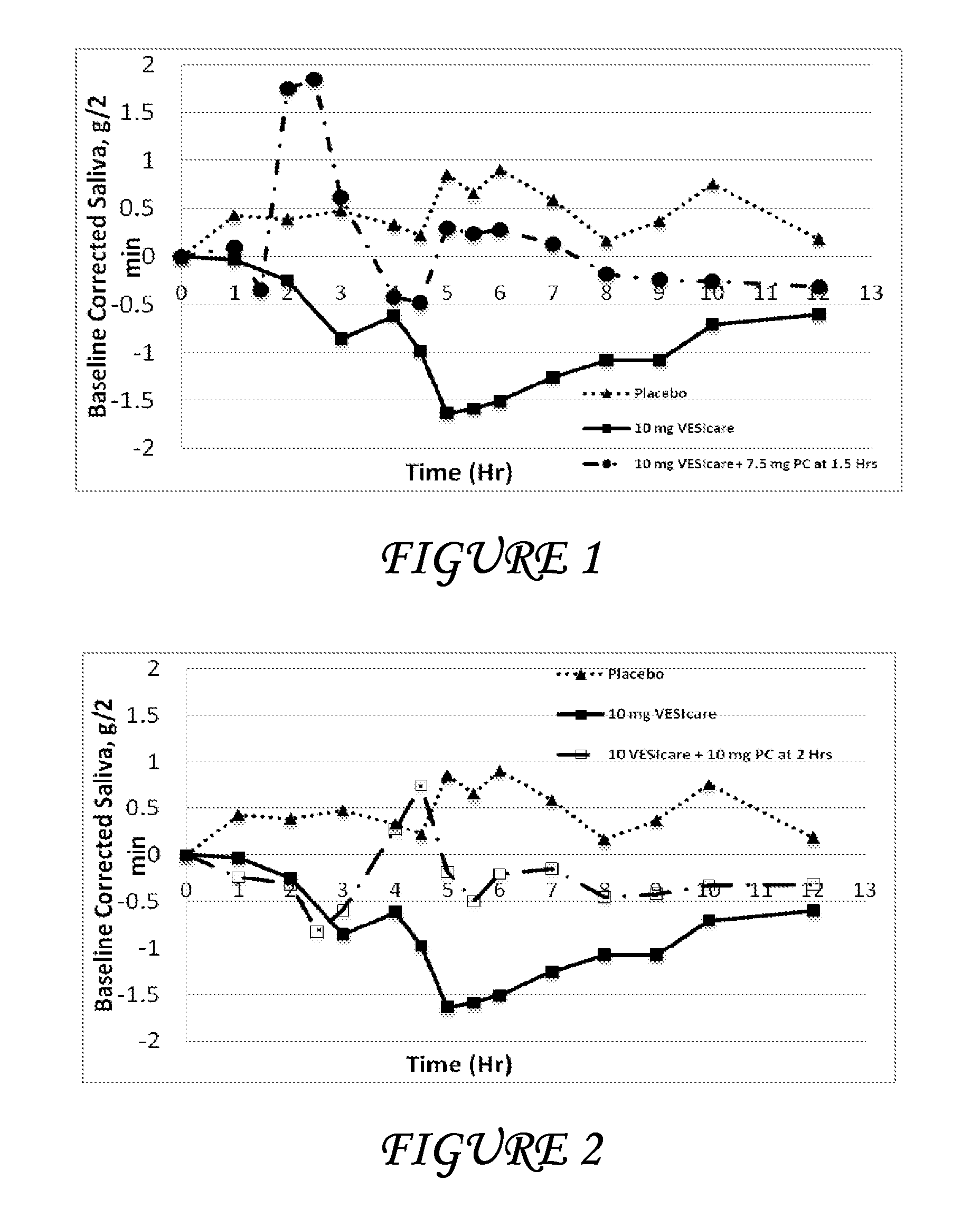
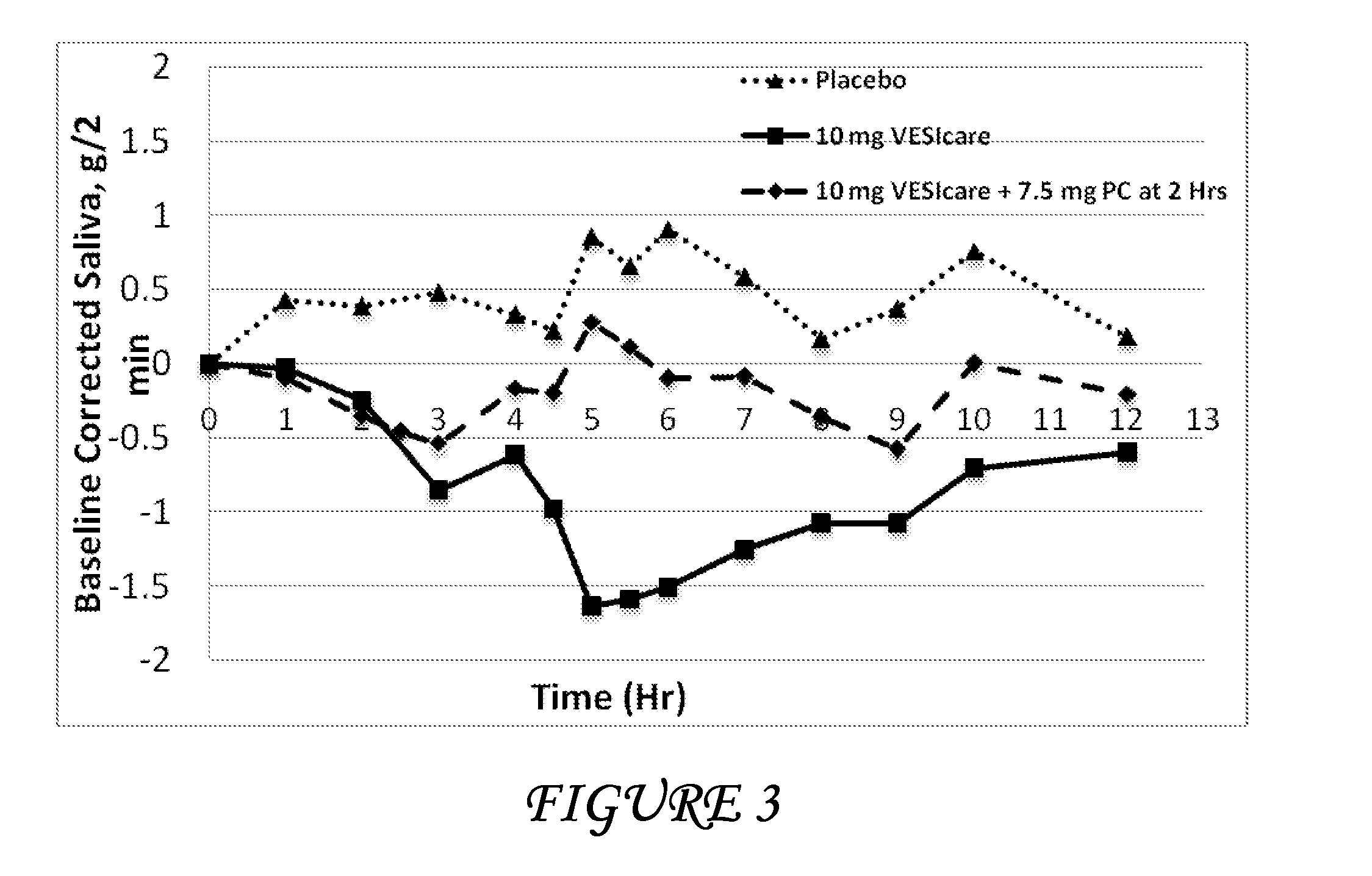
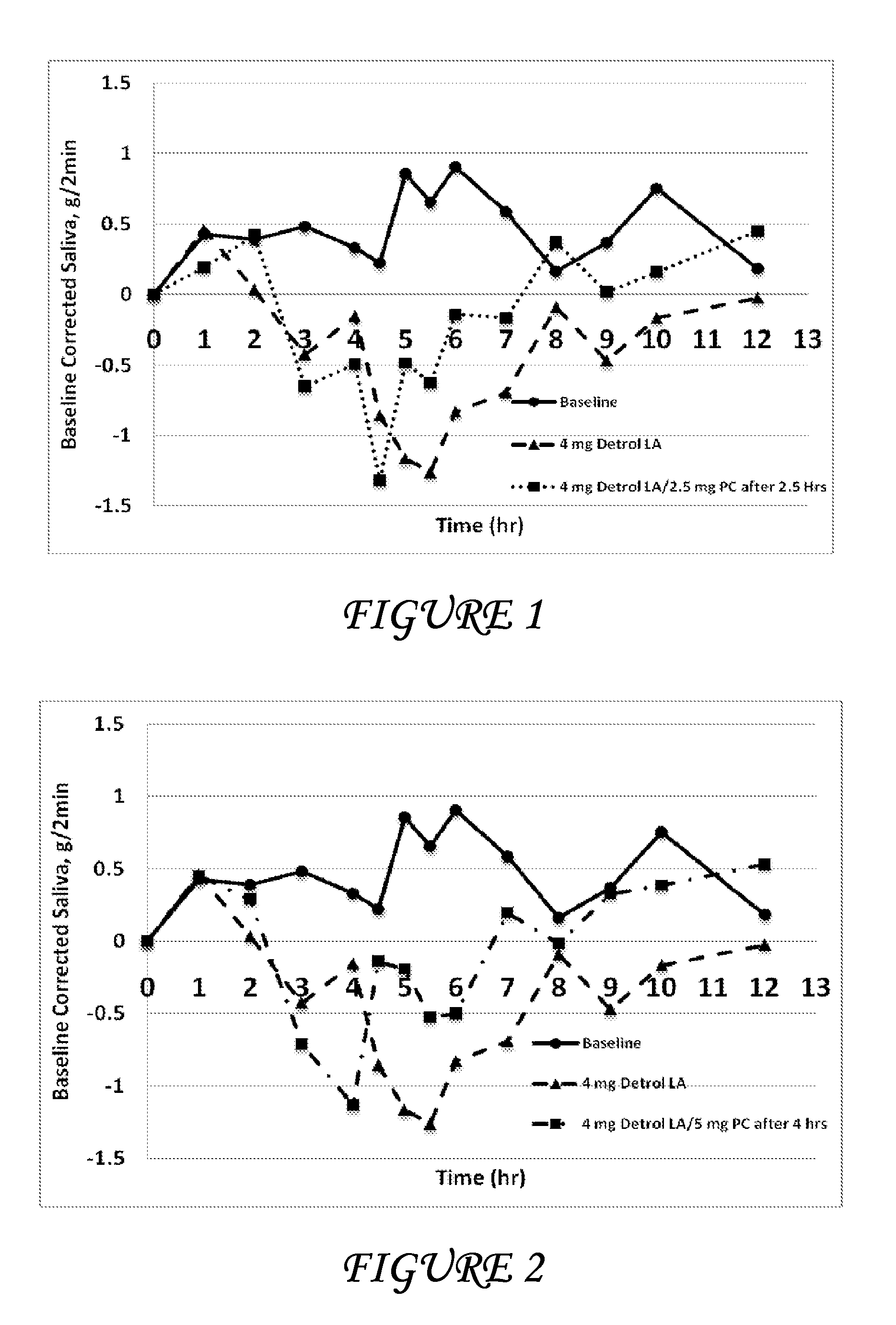
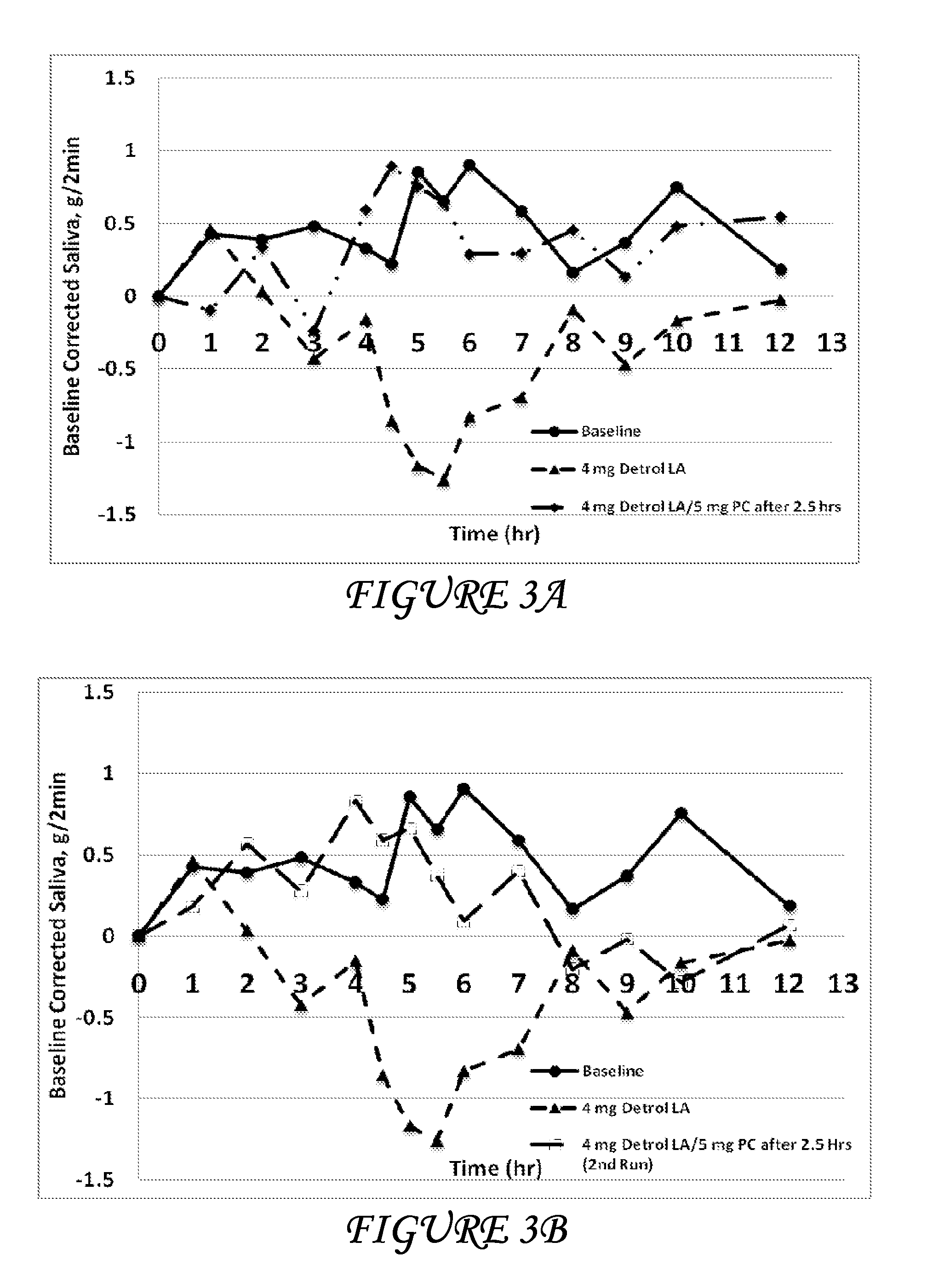
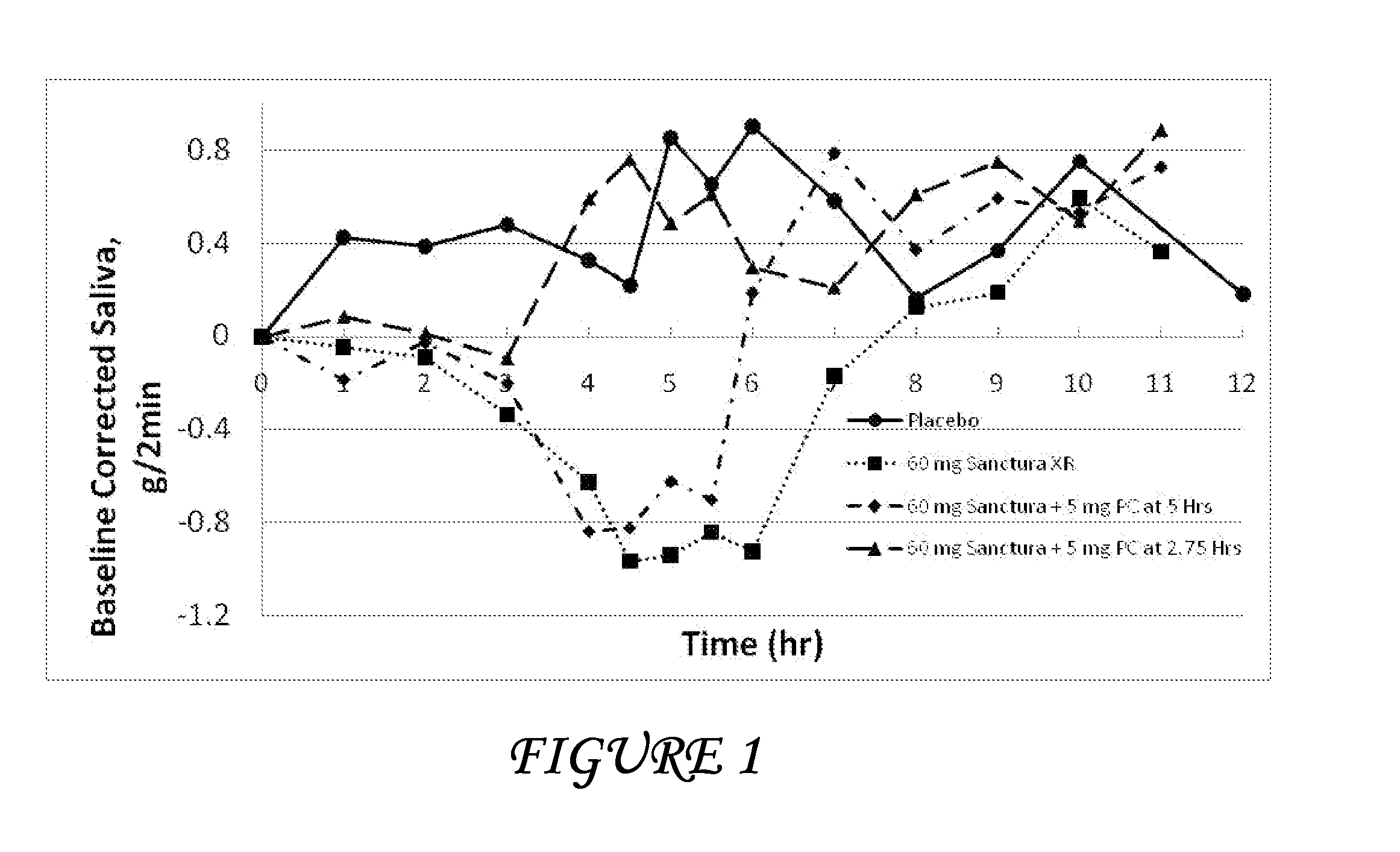
Disclosed herein are pharmaceutical compositions comprising oxybutynin, or a free base thereof or a pharmaceutically acceptable salt thereof, and pilocarpine, or a free base thereof or a pharmaceutically acceptable salt thereof. Also disclosed are methods of treating a patient suffering from overactive bladder comprising administering to the patient the above pharmaceutical composition.
Owner:THERAVIDA INC
Compositions and methods for treating presbyopia, mild hyperopia, and irregular astigmatism
ActiveUS20140200211A1Eliminate side effectsEasy to useBiocideSenses disorderSide effectMuscarinic receptor site
The present invention is directed to compositions and methods for treating presbyopia, mild hyperopia, and irregular astigmatism. The compositions include a cholinergic agent, such as a muscarinic acetylcholine receptor M3 agonist, and an alpha agonist having an imidazoline group or a non-steroidal anti-inflammatory agent (NSAID) having COX-2 selectivity. It has been found that an alpha agonist having an imidazoline group or non-steroidal anti-inflammatory agent (NSAID) having COX-2 selectivity in combination with a cholinergic agent, such as pilocarpine, act synergistically to improve the accommodative and focusing ability of the eye while minimizing the side effects from each compound.
Owner:ALLERGAN INC
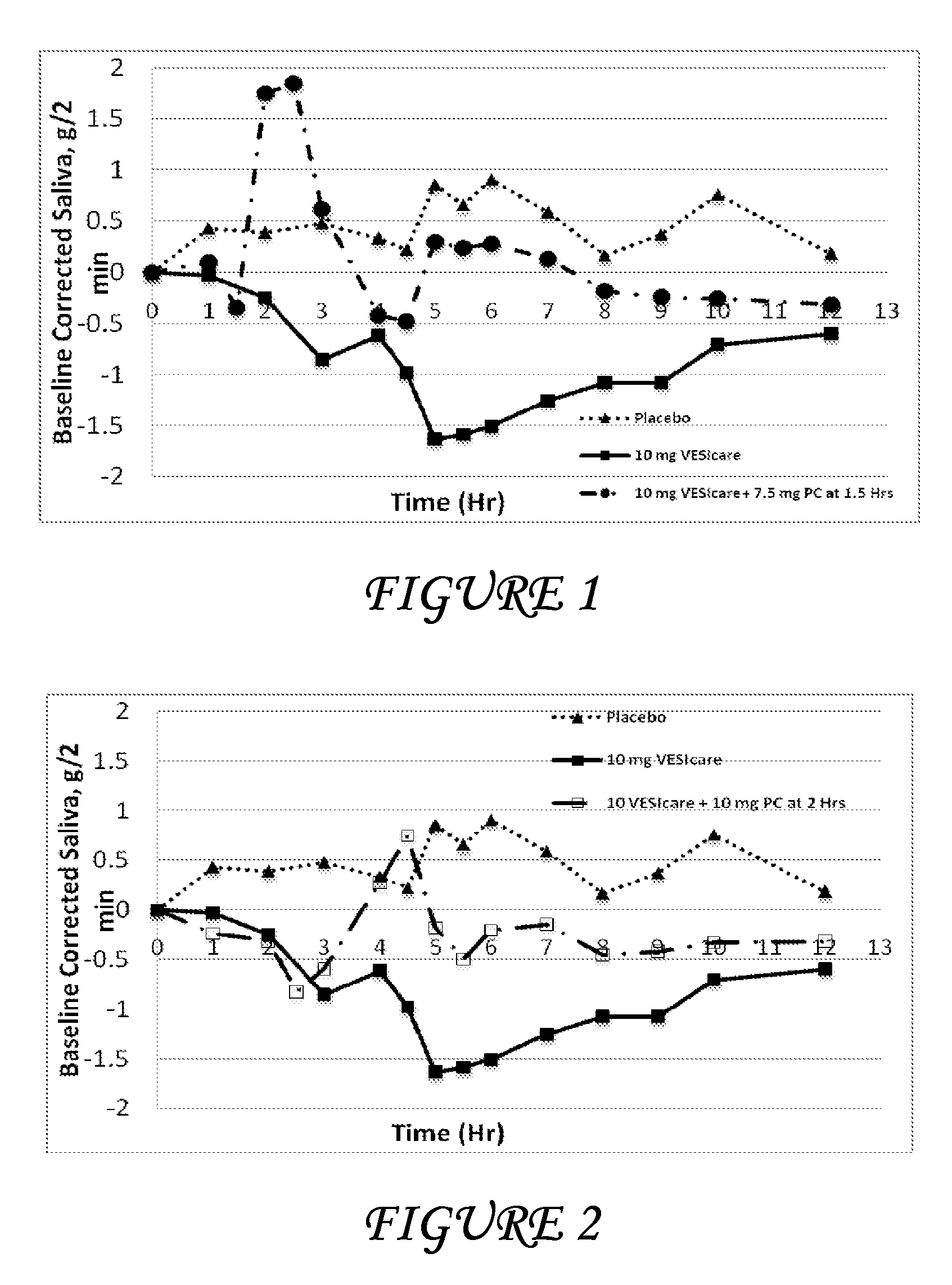
Pharmaceutical formulations for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a plurality of first beads each comprising: a core; a first layer comprising pilocarpine or a pharmaceutically acceptable salt thereof; and a second layer comprising a first polymer. Also disclosed are pharmaceutical compositions comprising a plurality of second beads each comprising: a core; and a first layer comprising tolterodine or a pharmaceutically acceptable salt thereof. Further disclosed are pharmaceutical formulations comprising: a) a plurality of the first beads; b) a plurality of the second beads; or c) a plurality of the first beads and a plurality of the second beads.
Owner:THERAVIDA INC
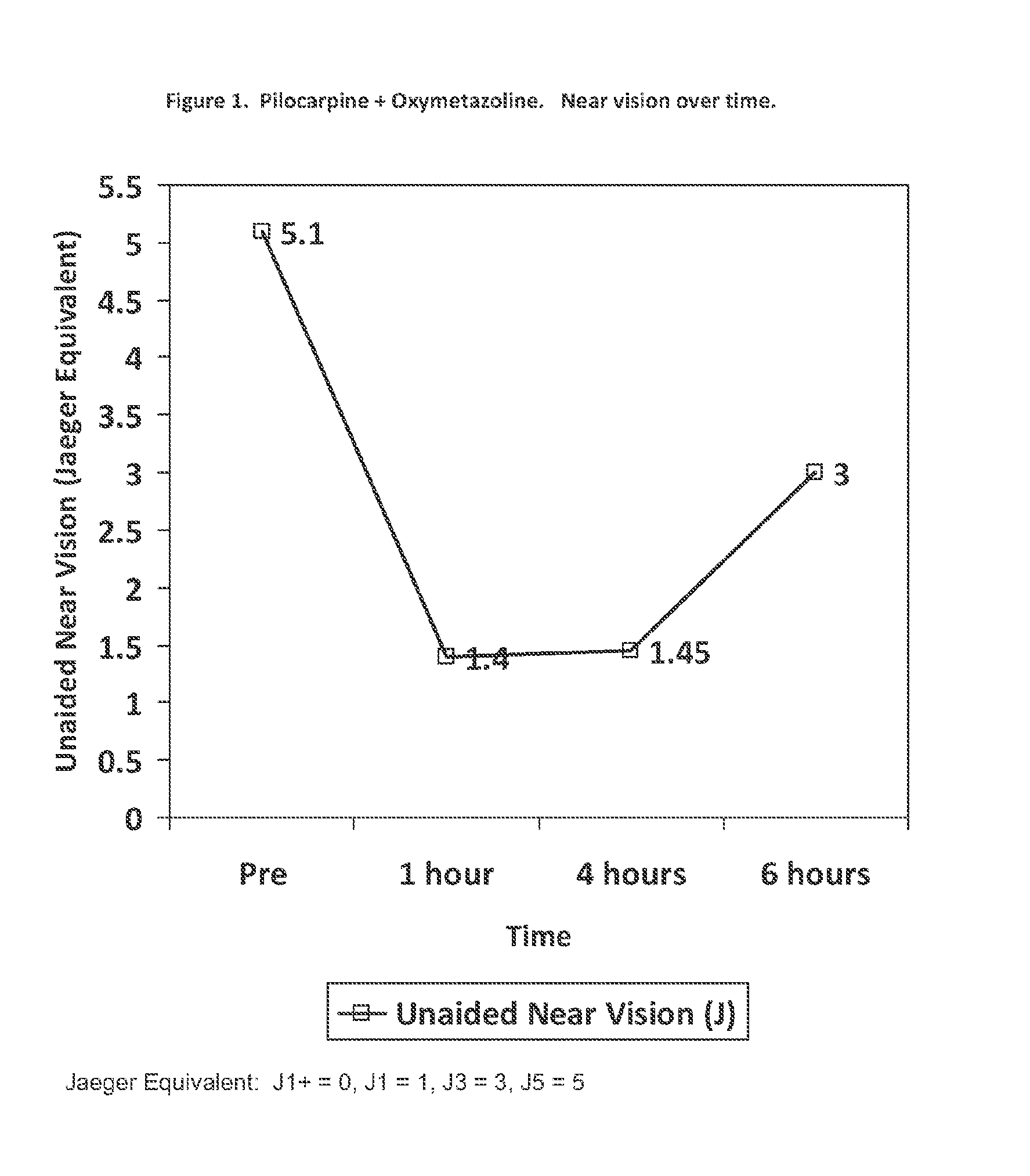
Ophthalmic formulation and method for ameliorating presbyopia
An ophthalmic formulation having an effective amount of a parasympathomimetic agent comprising pilocarpine, or a pharmaceutically acceptable salt thereof, and one or more α1 adrenergic agonists or antagonists is disclosed. The ophthalmic formulation may enable treatment of conditions adversely affecting the visual acuity of a patient, including presbyopia. A method of using the disclosed ophthalmic formulation to treat or ameliorate symptoms of presbyopia is also disclosed.
Owner:VEJARANO RESTREPO LUIS FELIPE
Combinations of oxybutynin and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release oxybutynin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release oxybutynin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release oxybutynin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Pharmaceutical formulations of pilocarpine
Disclosed herein are pharmaceutical compositions comprising at least one minitablet, where the minitablet comprises a core, comprising pilocarpine, or a pharmaceutically acceptable salt thereof; and a coating layer comprising a coating polymer.
Owner:THERAVIDA INC
Ophthalmic Composition for Correcting Presbyopia
Ophthalmic composition for the correction of presbyopia. Composition consisting of 2% Pilocarpine, characterized by being diluted to 1% with sterile Balanced Salt Solution (BSS), obtaining 1% pilocarpine (2.5 ml)+bromfenac, 1.8 mgrs of bromfenac (2.5 ml)+sterile Balanced Salt Solution (BSS) (2.5 ml), finally obtaining eye drops of 7.5 ml. It is also possible to obtain a 1.5% and 2% pilocarpine.
Owner:EUROCANARIAS OFTALMOLOGICA
Combinations of solifenacin and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Combinations of solifenacin and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Pharmaceutical formulations for the treatment of overactive bladder
Owner:THERAVIDA INC
Combinations of solifenacin and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Compositions and methods for the treatment of presbyopia
The invention provides compositions and methods for the treatment of presbyopia. In a preferred embodiment correction of presbyopia occurs without reduction in distance vision acuity. The compositions of the invention preferably contain pilocarpine and tropicamide.
Owner:LENZ THERAPEUTICS INC
Compositions for the treatment of presbyopia
InactiveUS20190008832A1Promote contractionEliminate stiffnessSenses disorderPharmaceutical delivery mechanismStimulantStimulant/agonist
The present invention relates to a composition comprising (a) pilocarpine or pharmaceutically acceptable salts thereof, (b) at least one alpha-stimulant agonist or pharmaceutically acceptable salts thereof and / or (c) at least one nonsteroidal anti-inflammatory agent (NSAID) or pharmaceutically acceptable salts thereof wherein (a) is present in a percentage by weight lower than 0.40%, (b) and / or (c) is present in a percentage by weight lower than 0.090% based on the total volume of the composition.
Owner:PINELLI ROBERTO
Establishment method of cerebral-palsy-based temporal lobe epilepsy animal model
ActiveCN110269046AEasy to set upImprove survival rateAnimal husbandryShort-term memoryCognitive impairment
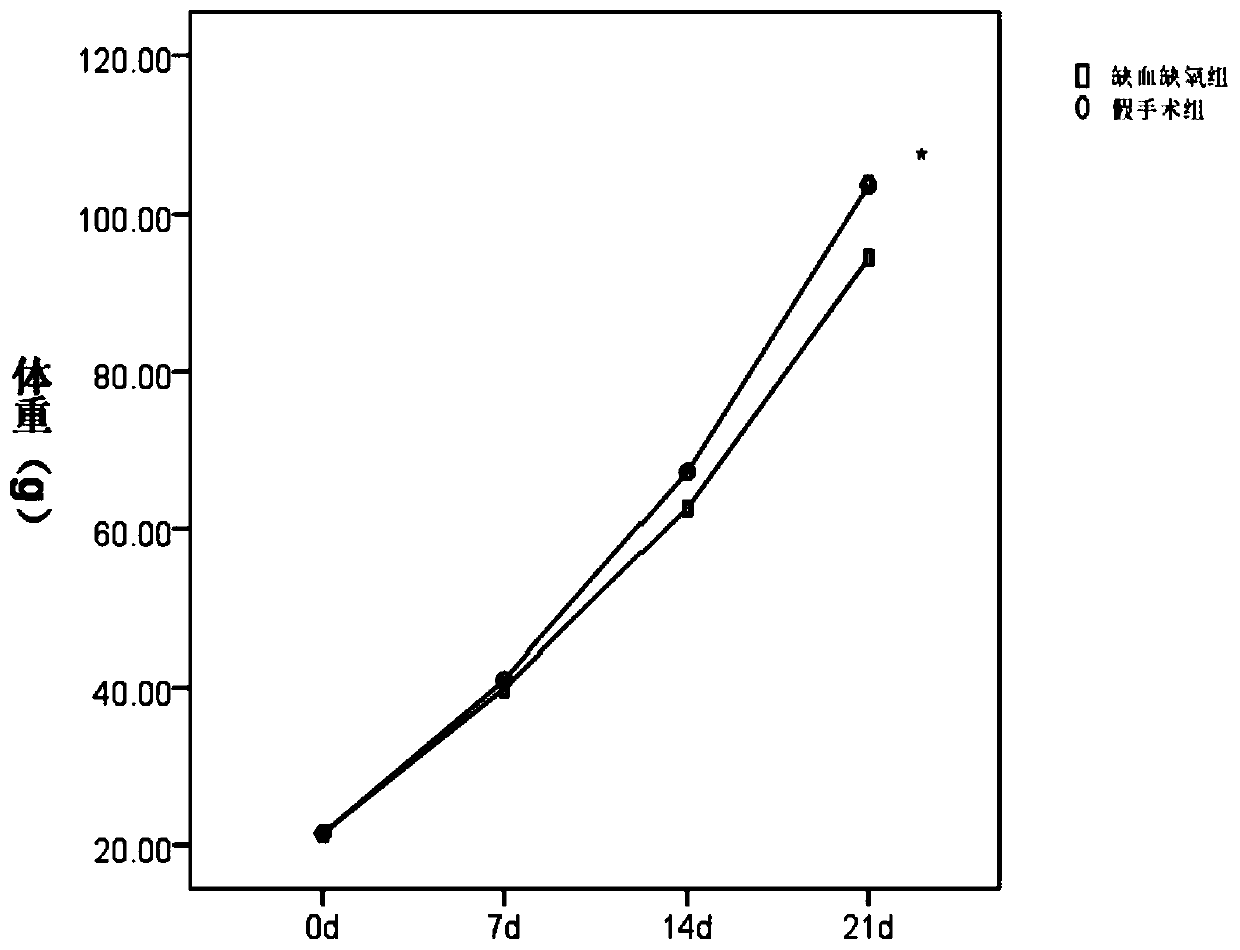
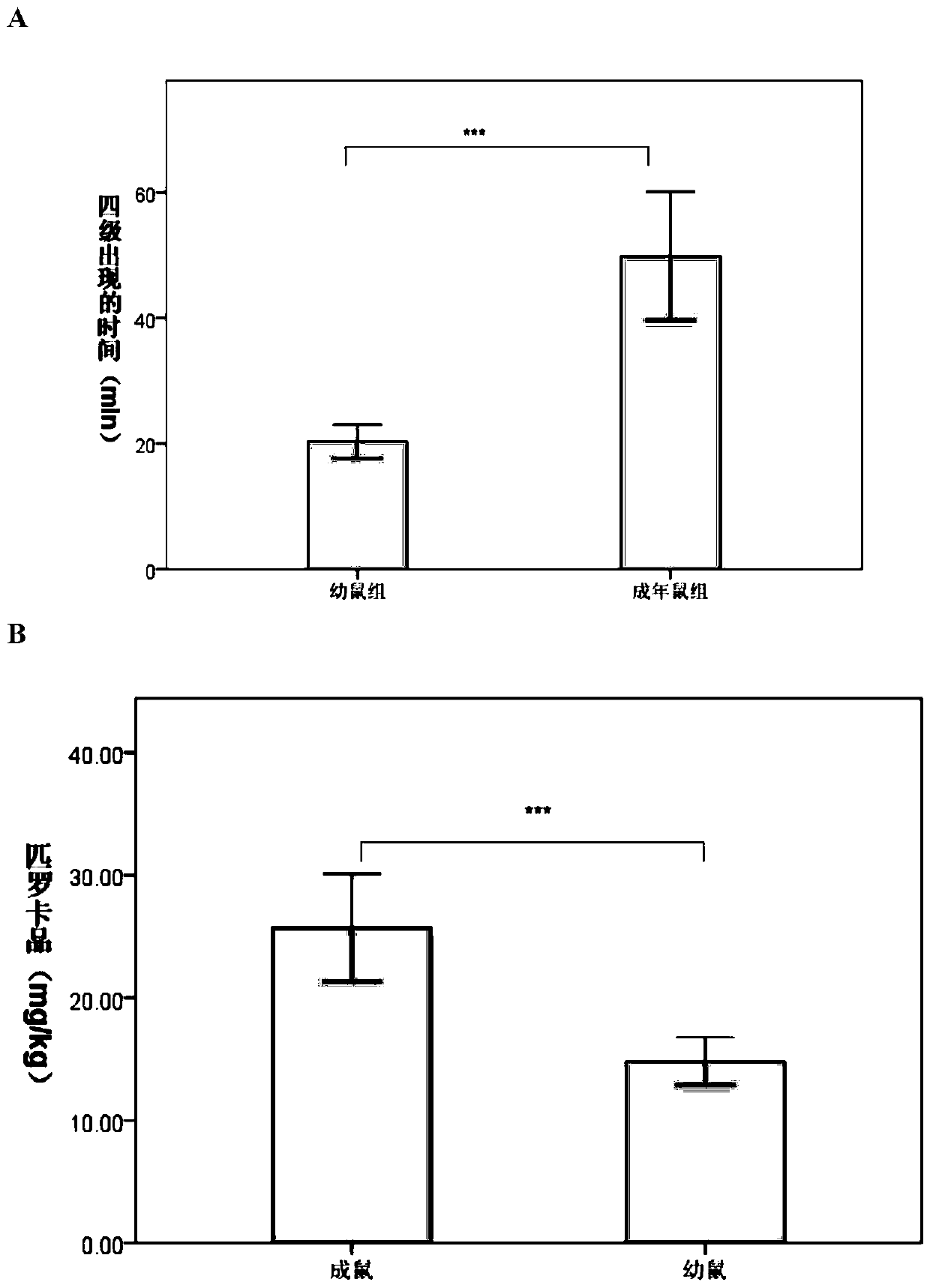
The invention provides a cerebral-palsy-based temporal lobe epilepsy animal model and a preparation method thereof. The method includes the steps that after a single-side-neck total arterial ligation operation is carried out on a young animal and hypoxia is conducted for 2-3.5 hours, the animal returns to a female mouse and bred for 3-5 w, pilocarpine is injected into the abdominal cavity of the survival animal to induce a Racine standard epilepsy of the fourth stage or above, and the cerebral-palsy-based temporal lobe epilepsy animal model is obtained. The animal model is simple in establishment method and high in survival rate, the survival rate is 70% or above, and the model animal is damaged in learning and long-term memory function and is free of damage of short-term memory. Electroencephalograms can be used for clinic attack epilepsies besides finding abnormal situations of the electric attack, namely the brain waves, without clinical attack, can be widely applied to treatment of curative effects of different treatment methods on cerebral palsy child patients with the temporal lobe epilepsy, and especially can be used for evaluating the curative effects of temporal lobe epilepsy child patients with cerebral palsy which causes cognitive impairment.
Owner:FIRST AFFILIATED HOSPITAL OF DALIAN MEDICAL UNIV
Pharmaceutical formulations
Owner:THERAVIDA INC
Ophthalmic composition for correcting presbyopia
Ophthalmic composition for the correction of presbyopia. Composition consisting of 2% Pilocarpine, characterized by being diluted to 1% with sterile Balanced Salt Solution (BSS), obtaining 1% pilocarpine (2.5 ml)+bromfenac, 1.8 mgrs of bromfenac (2.5 ml)+sterile Balanced Salt Solution (BSS) (2.5 ml), finally obtaining eye drops of 7.5 ml. It is also possible to obtain a 1.5% and 2% pilocarpine.
Owner:EUROCANARIAS OFTALMOLOGICA
Compositions and methods for treating presbyopia, mild hyperopia, and irregular astigmatism
ActiveUS9301933B2Eliminate side effectsEasy to useSenses disorderPharmaceutical delivery mechanismFar-sightednessSide effect
The present invention is directed to compositions and methods for treating presbyopia, mild hyperopia, and irregular astigmatism. The compositions include a cholinergic agent, such as a muscarinic acetylcholine receptor M3 agonist, and an alpha agonist having an imidazoline group or a non-steroidal anti-inflammatory agent (NSAID) having COX-2 selectivity. It has been found that an alpha agonist having an imidazoline group or non-steroidal anti-inflammatory agent (NSAID) having COX-2 selectivity in combination with a cholinergic agent, such as pilocarpine, act synergistically to improve the accommodative and focusing ability of the eye while minimizing the side effects from each compound.
Owner:ALLERGAN INC
Procedure for screening of neuroactive substance and the associated neural plasticity
InactiveUS20050144654A1Simple and inexpensive and rapid methodRapid and simple and inexpensiveCompounds screening/testingBiological testingDrug withdrawalNeuroactive substances
The invention relates to a procedure for screening of neuroactive substance and the associated neural plasticity by treating fruitfly Drosophila melanogaster adult males with fly medium containing either of the neuroactive compounds strychnine, pentylenetetrazol, pilocarpine hydrochloride, tetraethylammonium chloride, lithium carbonate and ethosuximide, subjecting flies to negative geotaxis and horizontal locomotor assays, observing the height climbed and distance walked by and the climbing speed of the flies wherein an altered height climbed by flies under drug treatment and a long-lasting alteration in climbing speed of flies after drug withdrawal is most characteristic of the neuractive compounds.
Owner:COUNCIL OF SCI & IND RES
Ophthalmic formulation and method for ameliorating presbyopia
ActiveUS10507245B2Organic active ingredientsSenses disorderAdrenergic receptor agonistsParasympathomimetic Agents
An ophthalmic formulation having an effective amount of a parasympathomimetic agent comprising pilocarpine, or a pharmaceutically acceptable salt thereof, and one or more α1 adrenergic agonists or antagonists is disclosed. The ophthalmic formulation may enable treatment of conditions adversely affecting the visual acuity of a patient, including presbyopia. A method of using the disclosed ophthalmic formulation to treat or ameliorate symptoms of presbyopia is also disclosed.
Owner:VEJARANO RESTREPO LUIS FELIPE
Cation-modified pilocarpine hydrochloride flexible nano-liposome eye-drops preparation and preparation method thereof
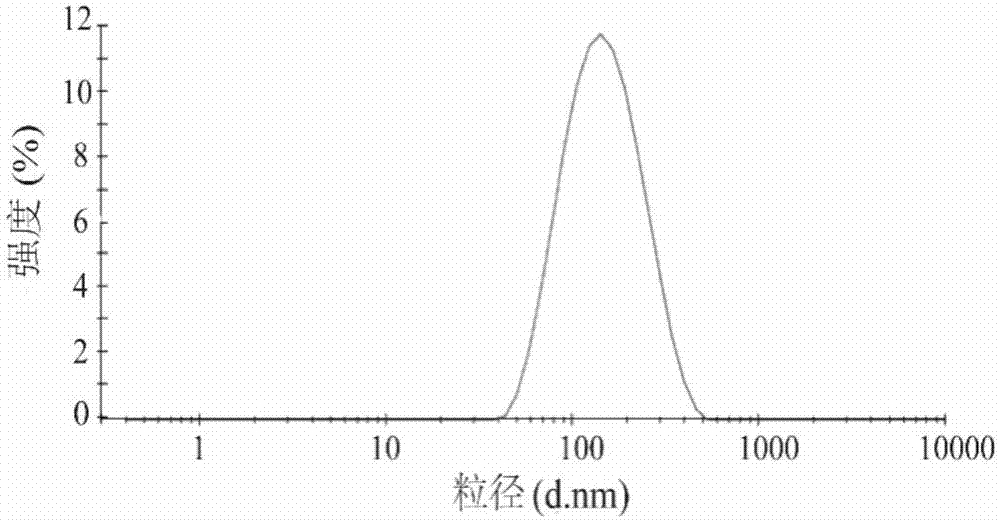
ActiveCN107982219AHuge membrane surface areaImprove adhesionOrganic active ingredientsSenses disorderCholesterolBiocompatibility Testing
The invention discloses a cation-modified pilocarpine hydrochloride flexible nano-liposome eye-drops preparation and a preparation method thereof. The preparation method comprises the following steps:(1) adding lecithin and cholesterol into chloroform, and performing ultrasonic treatment to obtain suspension; and dissolving pilocarpine hydrochloride and a penetration enhancer in methanol to obtain a solution I; (2) uniformly mixing the suspension and the solution I, and removing an organic solvent to obtain a uniform lipid membrane; (3) dissolving a softener into purified water to obtain a solution II, and dissolving the lipid membrane into the solution II so as to obtain primary mixed emulsion; (4) performing ultrasonic treatment on the primary mixed emulsion, and adding into a warm bathso as to obtain a pilocarpine hydrochloride flexible nano-liposome preparation; and (5) dissolving a cationic material into the purified water to obtain a solution III, stirring the pilocarpine hydrochloride flexible nano-liposome preparation, dropping the solution III, and stirring, thereby obtaining the eye-drops preparation in the invention. The preparation disclosed by the invention has hugemembrane surface area and high adhesion, and the drug penetration is increased. The eye-drops preparation has excellent biocompatibility and low eye irritation.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
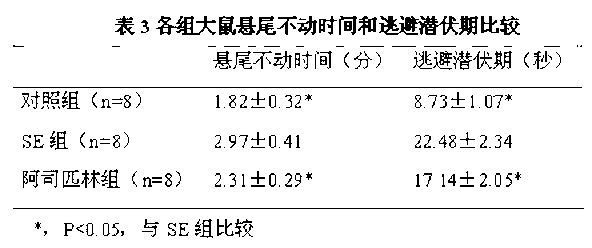
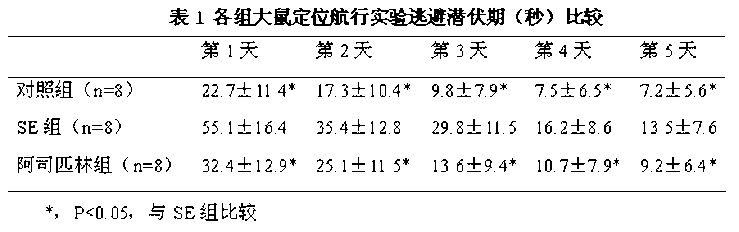
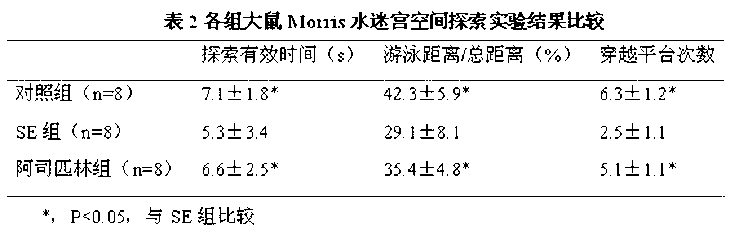
Application of aspirin in treatment of cognitive and behavioral disorders after epilepsy
The invention discloses an application of aspirin in treatment of cognitive and behavioral disorders after epilepsy. The inventor creates a lithium chloride-pilocarpine induced rat model of chronic epilepsy, detects the spatial learning and memory of the rat through a Morris water maze, detects the behavior depression of the rat through a tail suspension test, and finds that the cognitive function and behavior disorder of the epileptic rat are obviously perfected when being dosed with the aspirin at the chronic epilepsy attack stage.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
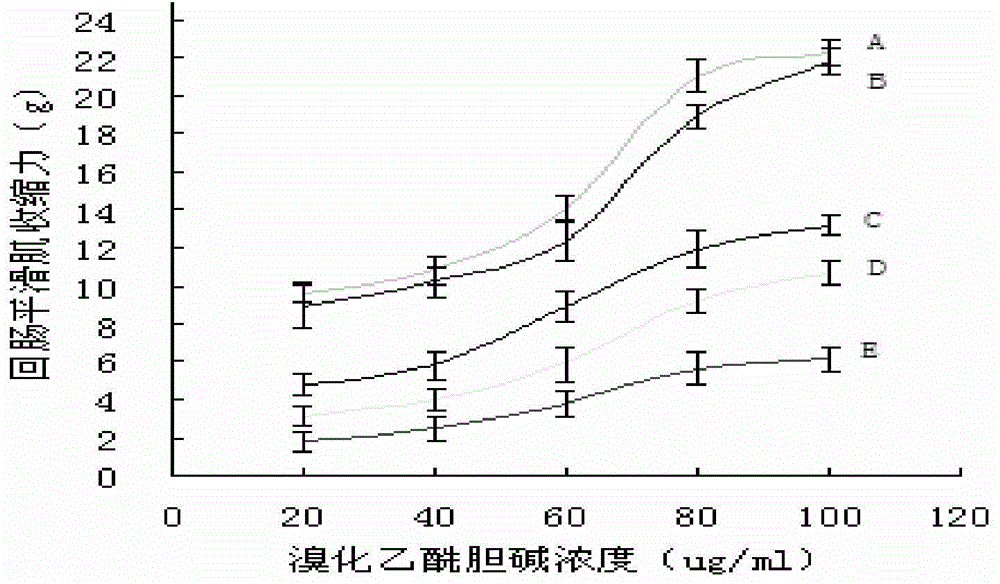
Application of mussaenda erythrophylla glucoside U in preparation of antiacetyl-choline medicine
The invention discloses an application of mussaenda erythrophylla glucoside U in preparation of an antiacetyl-choline medicine. An inventor researches influences of mussaenda erythrophylla glucoside U to contractility of ileum smooth muscles of in-vitro guinea pig caused by acetylcholine bromide and the influences to mouse pupil diameter, sialaden secretion quantity and small intestine carbon powder promotion percentage of an M cholinergic neuron excitation model caused by pilocarpine, and the results prove that the of mussaenda erythrophylla glucoside U is the antiacetyl-choline medicine. By performing a mouse acute toxicity test, the half lethal dose of the mussaenda erythrophylla glucoside U for intravenous injection is obtained, and a proper dose of the mussaenda erythrophylla glucoside U for antiacetyl-choline intravenous injection can be obtained by means of calculation based on a mouse in-vivo test dose. Therefore, after a proper dose of the mussaenda erythrophylla glucoside U is taken by a person by intravenous injection or by other methods, a pharmacodynamic effect the same as that of atropine can be played. Thus, the mussaenda erythrophylla glucoside U and a medicine excipient are used for preparing an antiacetyl-choline medicine preparation which can be used for treating clinical patients with organophosphorus nerve agent poisoning, acute microcirculation disturbance, stomach and intestine cramp and biliary tract or urethral canal spasmodic pain.
Owner:GUANGXI INST OF CHINESE MEDICINE & PHARMA SCI
Combinations of tolterodine and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release tolterodine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release tolterodine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release tolterodine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Pilocarpine compositions and methods of use thereof
InactiveUS20080254101A1Improve stabilityRapidly and efficiently absorbed by the oral mucosaBiocideOrganic active ingredientsDry mouthBuccal mucosa
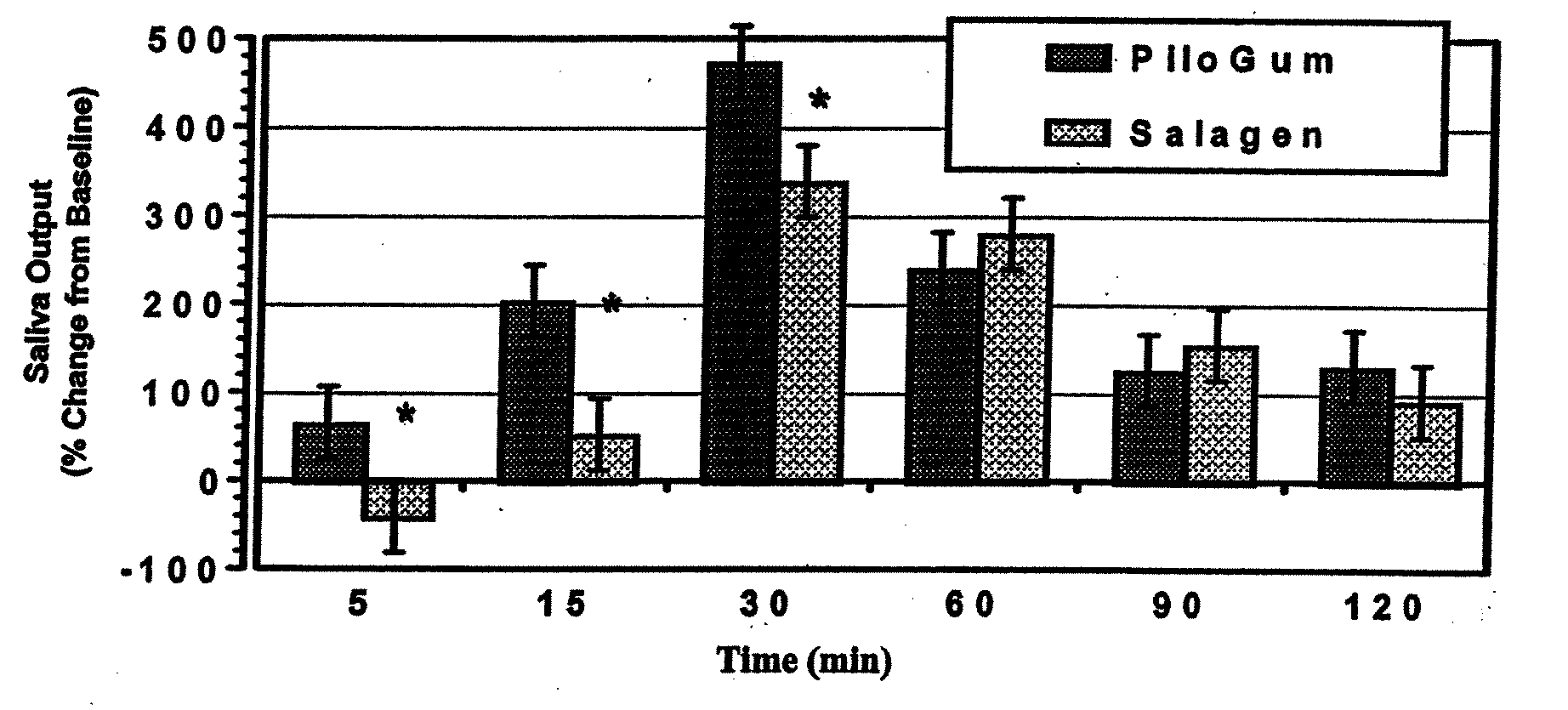
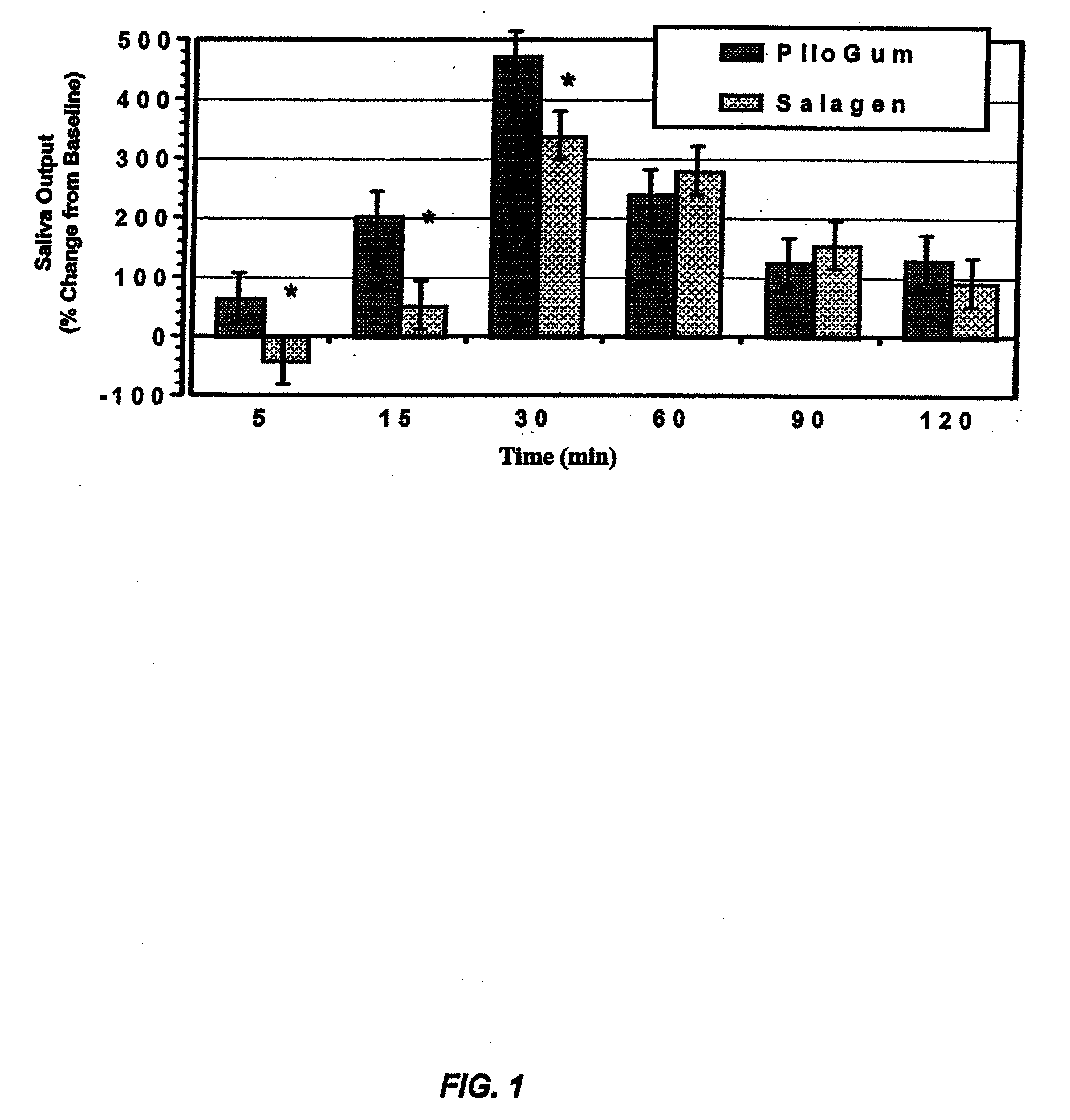
The present invention provides novel compositions for the delivery of pilocarpine or a pharmaceutically acceptable salt thereof across the oral mucosa, preferably across the buccal mucosa. In particular, the buffer systems in the compositions of the present invention contain an amount of a strong base that is less than the amount of a weak base, thereby increasing the stability of compositions such as chewing gum compositions and raising the pH of saliva to a pH greater than about 7.5 to facilitate the substantially complete conversion of pilocarpine from its ionized to its un-ionized form. Methods for using the compositions of the present invention for treating conditions such as dry mouth are also provided.
Owner:TRANSCEPT PHARMA
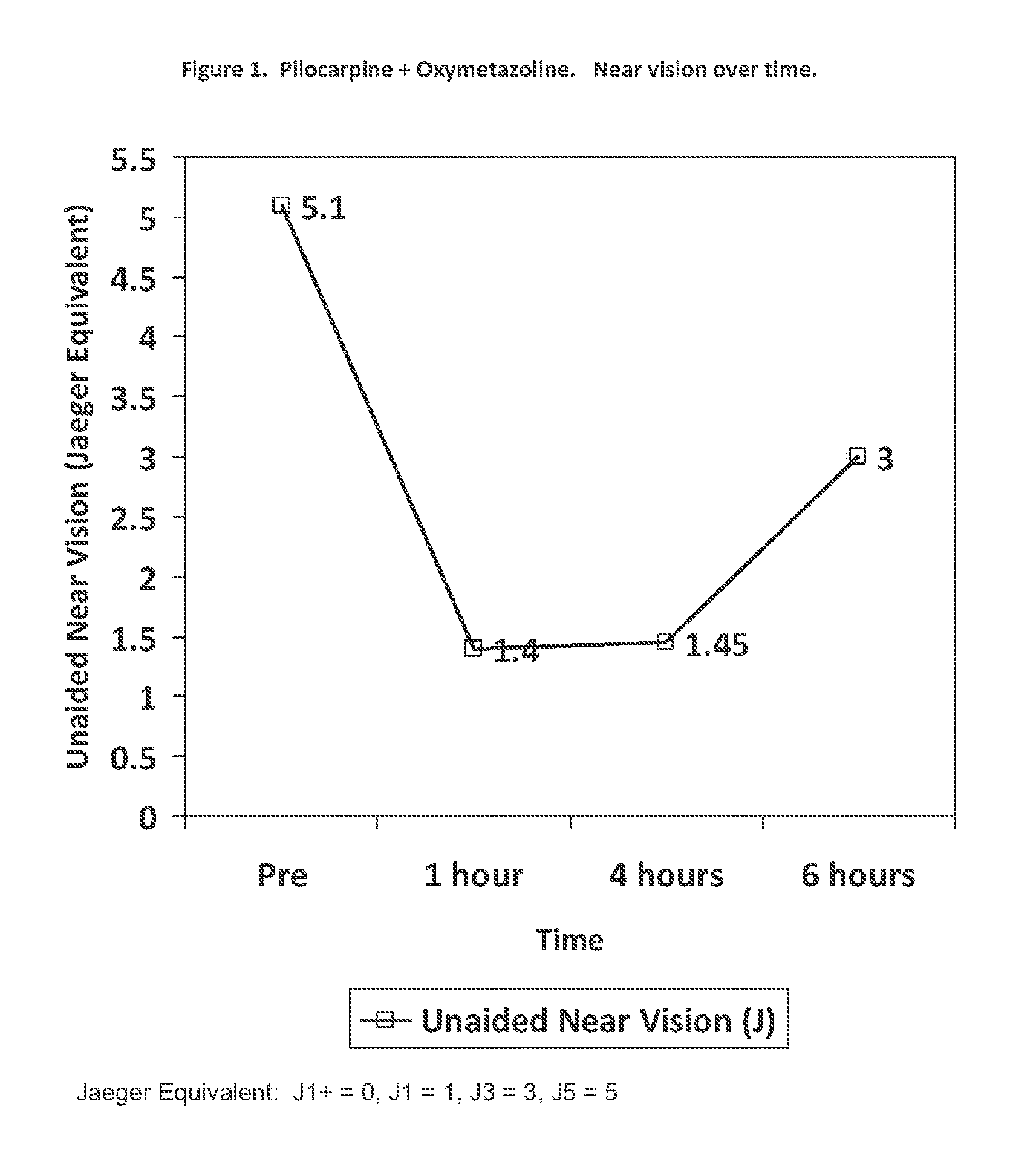
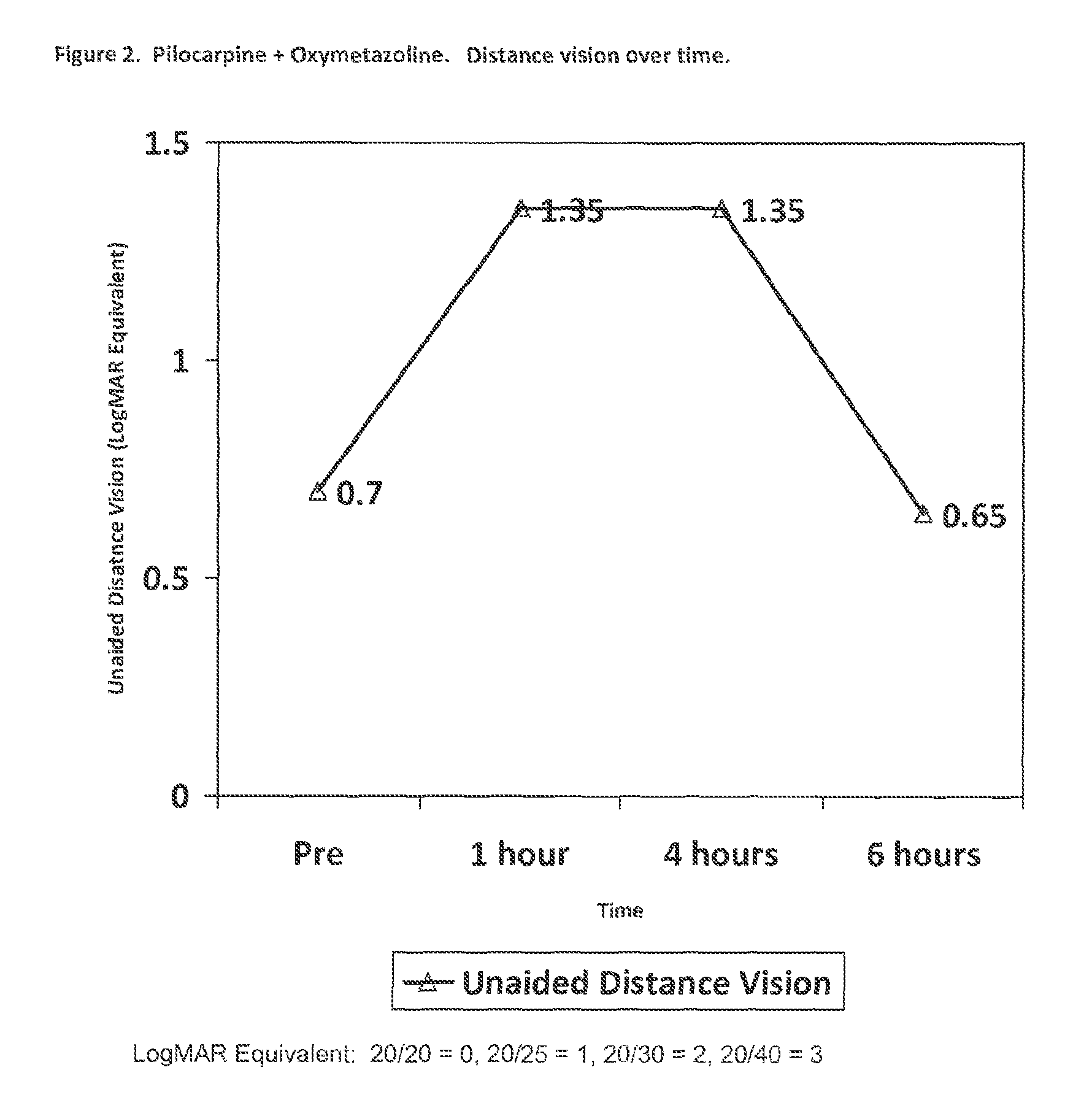
Methods and devices for delivering pilocarpine to the eye as a micro-dose stream of droplets
The present disclosure provides methods and devices for delivering pilocarpine to the eye. In certain aspects, the disclosure provides method for delivering a composition comprising pilocarpine to an eye of a subject in need thereof as a micro-dose stream of droplets. In certain embodiments, the method treats or alleviates one or more symptoms of presbyopia, in particular presbyopia in adults 40 years of age or older, in the subject in need thereof. In certain embodiments, the present disclosure utilizes relatively high concentrations of pilocarpine in the solution to be administered and as a single active agent.
Owner:EYENOVIA
Combinations of trospium and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release trospium, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release trospium, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release trospium, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com