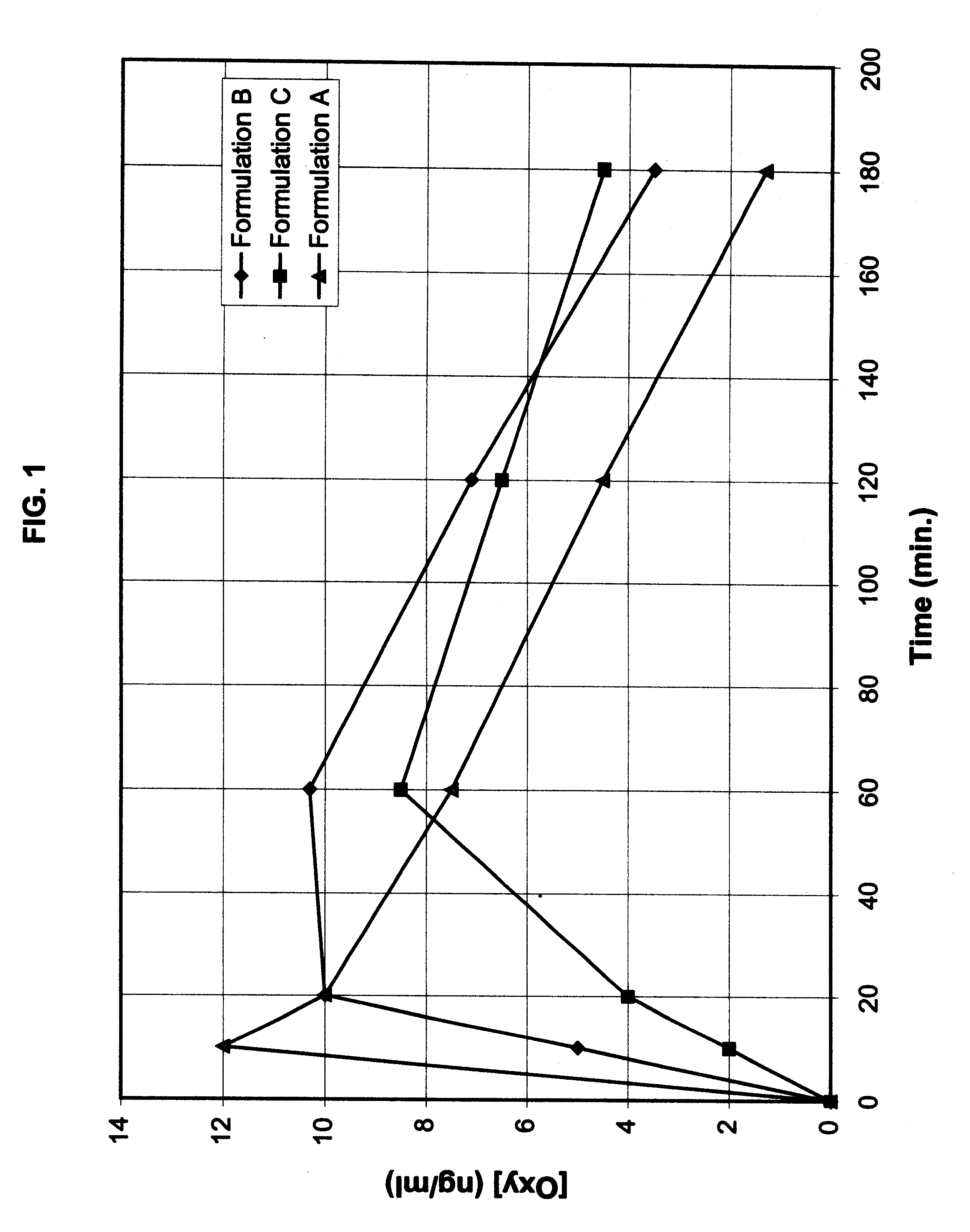
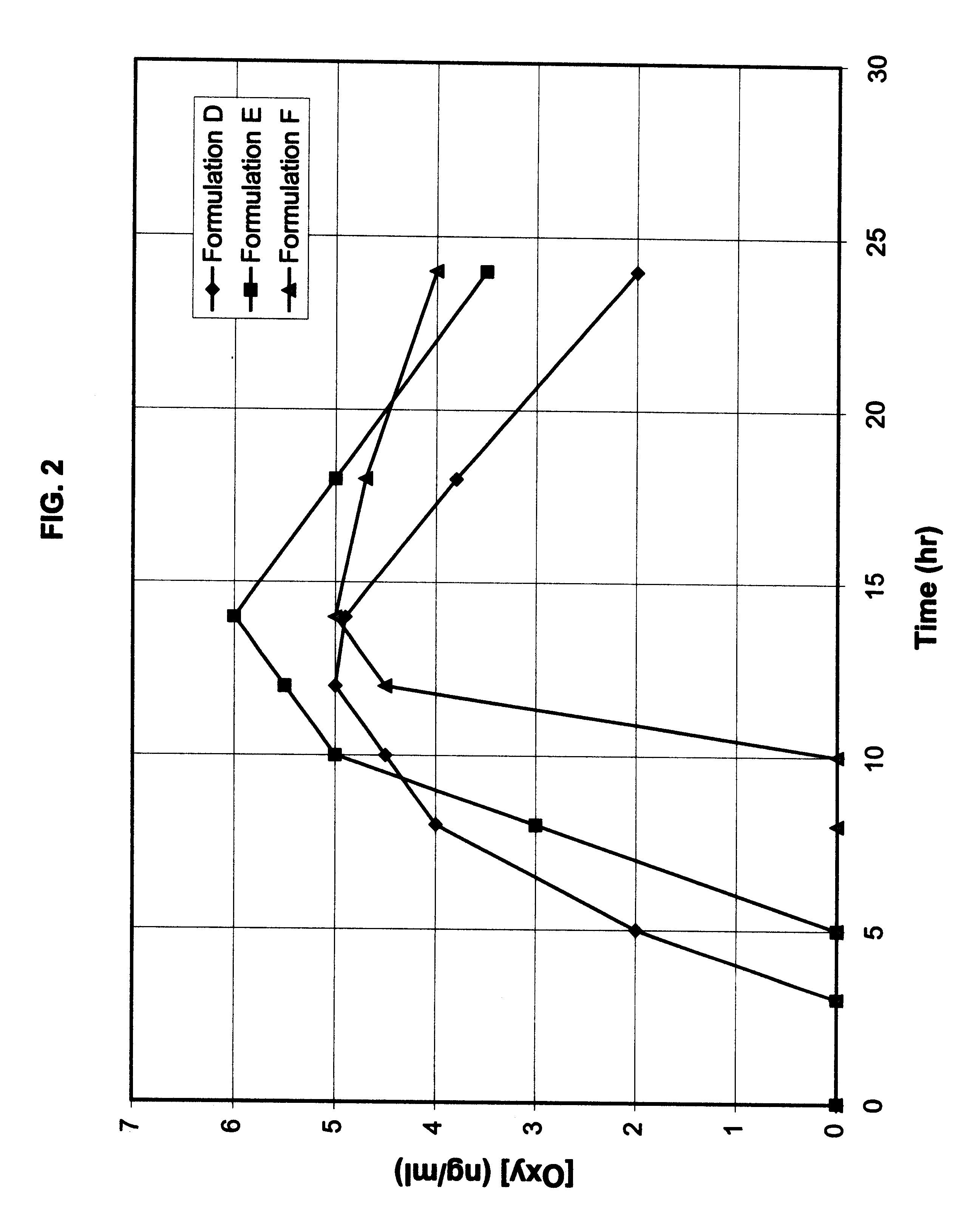
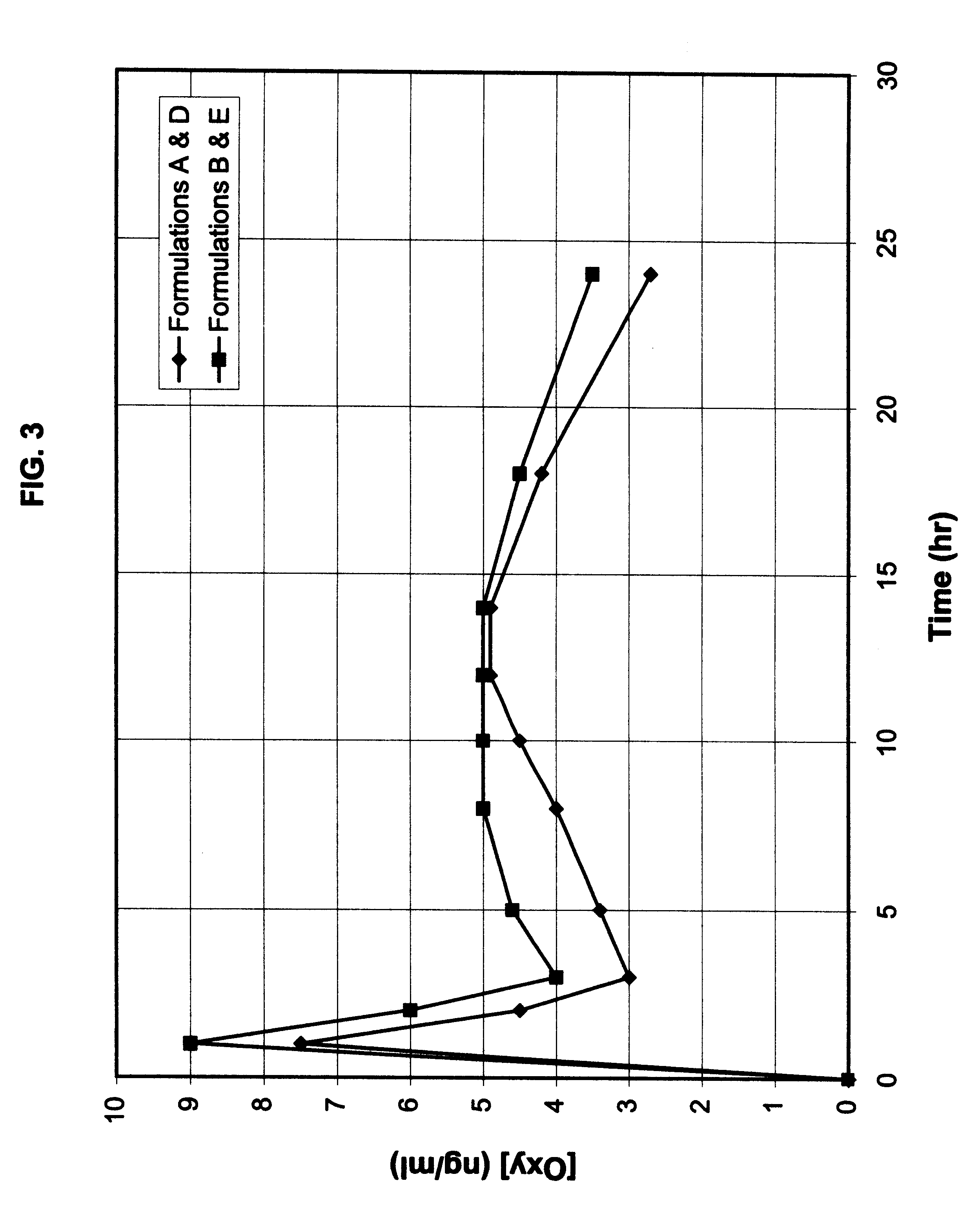
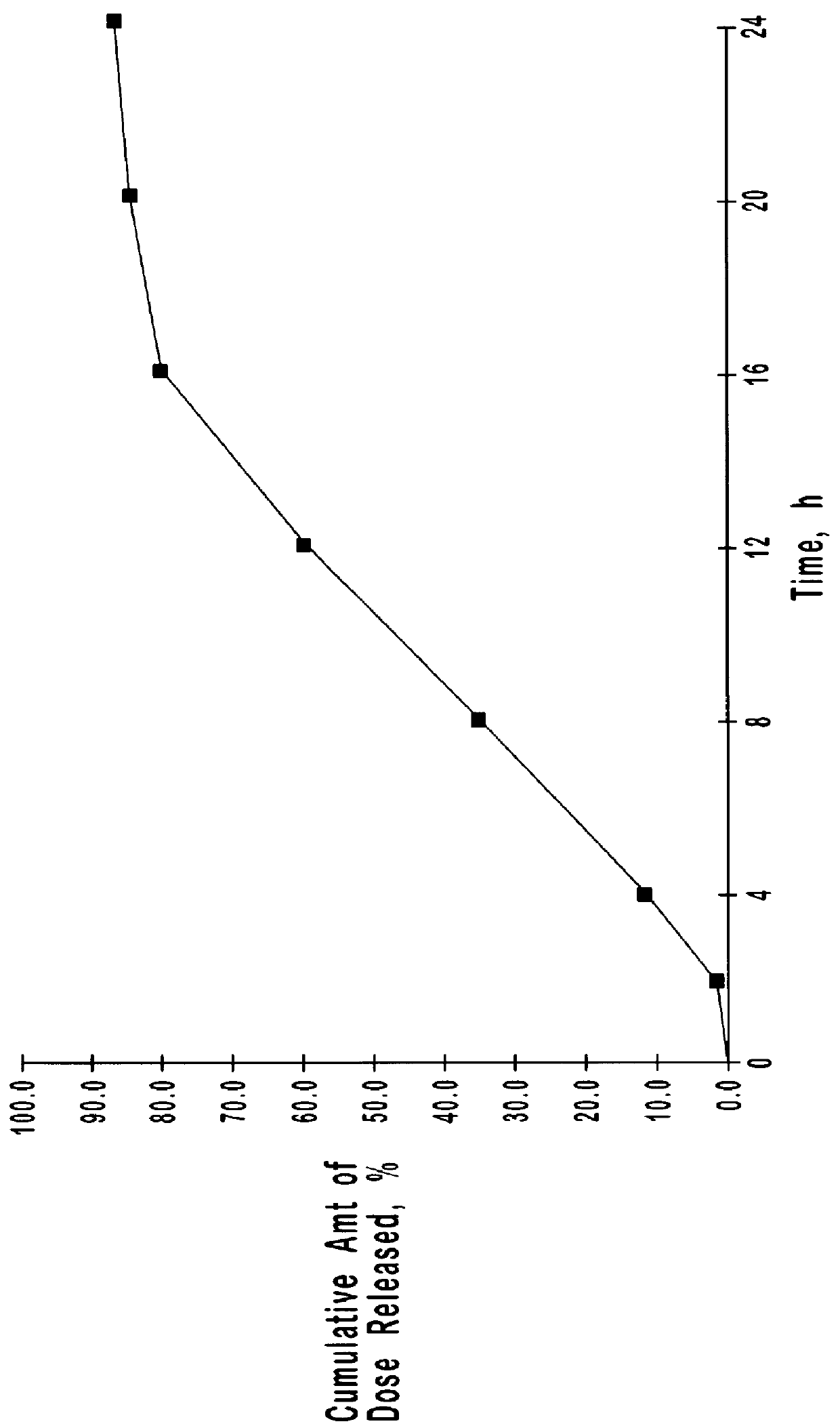
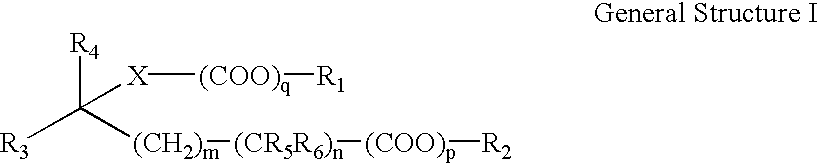Patents
Literature
52 results about "Oxybutynin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oxybutynin is used to treat an overactive bladder.
Multi-tablet oxybutynin system for treating incontinence
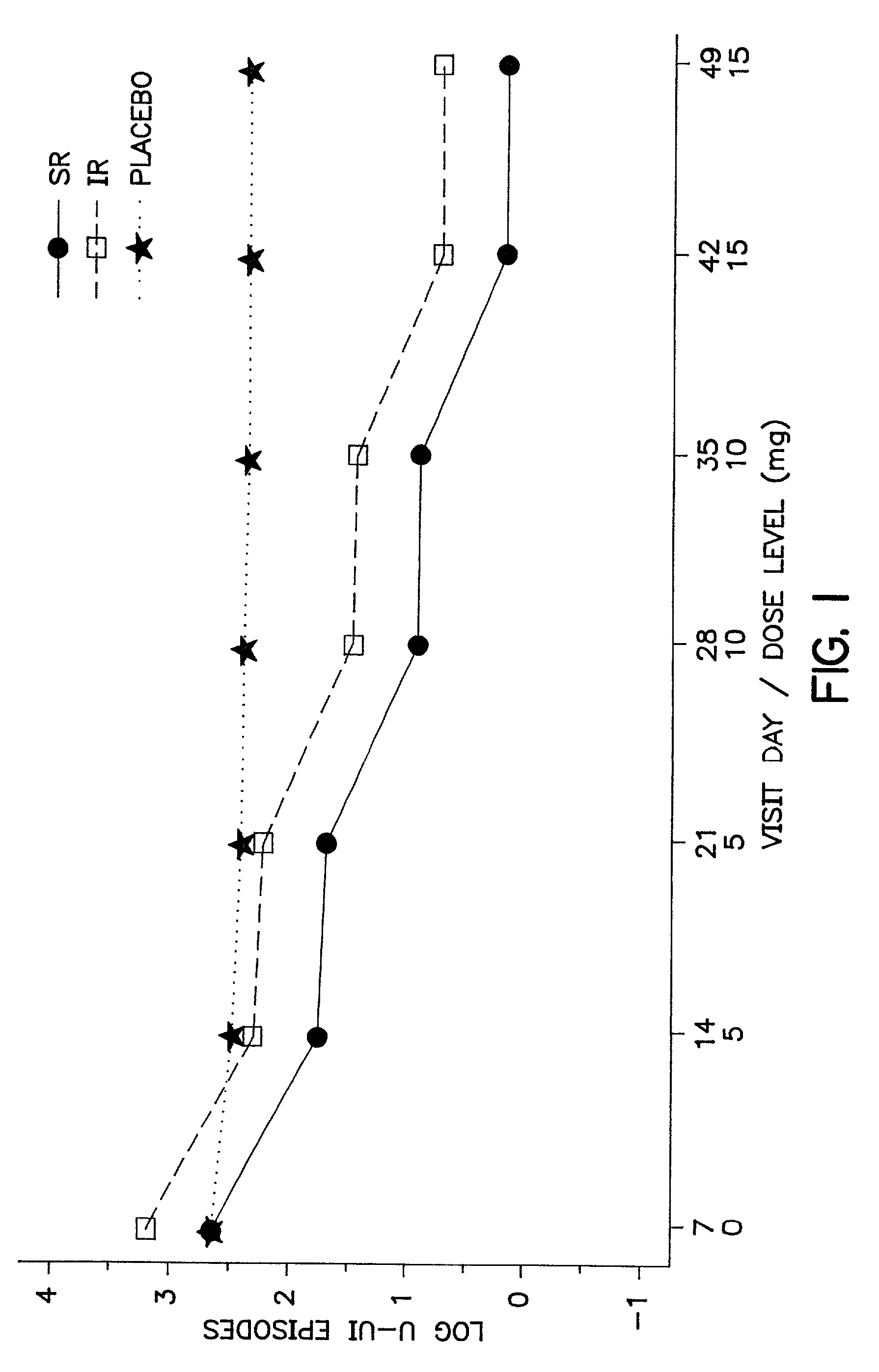
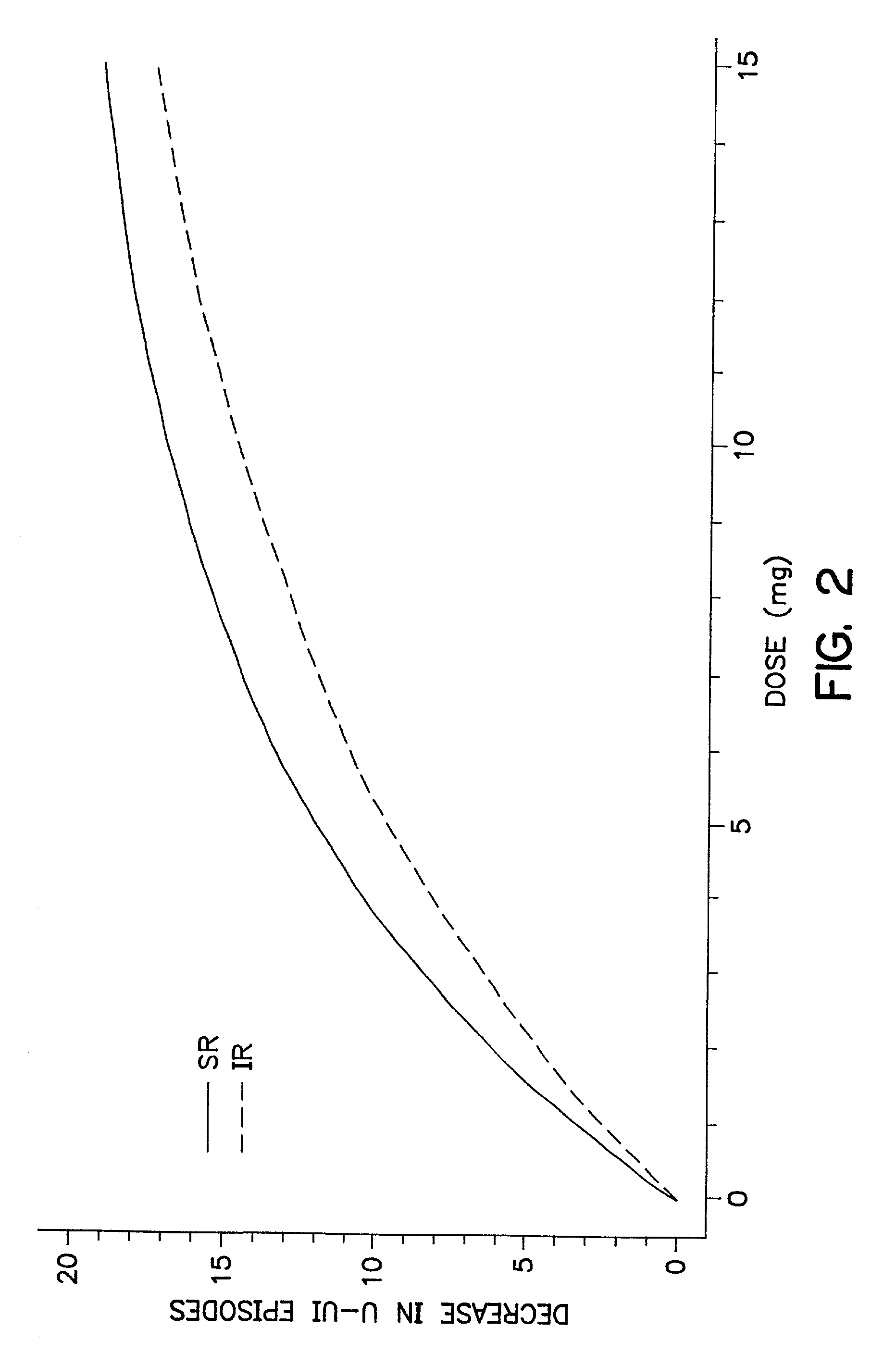
The present invention provides a simple multi-tablet system for the treatment of urinary incontinence with oxybutynin. Particular embodiments of the invention provide a first tablet that releases oxybutynin over a short period of time, e.g. less than six hours, and a second tablet that releases oxybutynin over an extended period of time, e.g., eighteen to twenty-four hours, to maintain therapeutically effective levels oxybutynin in the mammal for a period of about twenty four hours. Unlike other systems, this system is easily adaptable to compensate for patient to patient variability in response to oxybutynin therapy. The invention also provides a method of treating urinary incontinence with the above system and a kit comprising various first and second tablets to rapidly develop a patient's preferred dosing regimen, i.e., the dosing regimen which provides the greatest therapeutic benefit and / or least amount or severity of side effects.
Owner:OSMOTICA CORP
Method for the management of incontinence
InactiveUS6919092B2Reduce conversionLessen the circulating desoxy metaboliteBiocideHydroxy compound active ingredientsOxybutyninDrug indicated
A composition and a dosage form are disclosed comprising oxybutynin alone / or accompanied by another drug indicated for therapy. A method is disclosed for administering oxybutynin alone / or accompanied by a different drug or for administering oxybutynin and a different drug according to a therapeutic program for the management of incontinence alone, and for other therapy.
Owner:ALZA CORP
Methods for the Treatment of Hyperhidrosis
The present invention relates in general to methods of treating sweating disorders and in particular to topical compositions for the treatment of hyperhidrosis. The methods of the present invention relate to the topical application of a composition comprising a therapeutically effective amount of oxybutynin, tolterodine or a substituted benzamide such as sulpiride.
Owner:ZINGER MENNI MENASHE
Oxybutynin therapy
InactiveUS6124355AReducing and eliminating unwanted influenceAdministration limitationBiocidePowder deliveryOxybutyninDosage form
A composition comprising oxybutynin, a dosage form comprising oxybutynin, and a method for administering oxybutynin are disclosed for oxybutynin therapy.
Owner:ALZA CORP
Transdermal compositions for Anti-cholinergic agents
InactiveUS20140037713A1Reduce morbidityAvoids undesirable odor and irritation effectsCosmetic preparationsOrganic active ingredientsOxybutyninSide effect
The present invention relates generally to compositions or formulations for transdermal or transmucosal administration of anti-cholinergic agents such as oxybutynin. The invention utilizes a novel delivery vehicle and is a substantially malodorous-free and irritation free transdermal formulation which is substantially live of long chain fatty alcohols, long-chain fatty acids, and long-chain fatty esters. A method is disclosed for treating a subject for hyperhidrosis with these formulations while reducing the incidences of peak concentrations of drug and undesirable side effects associated with oral anti-cholinergics.
Owner:ANTARES PHARMA IPL
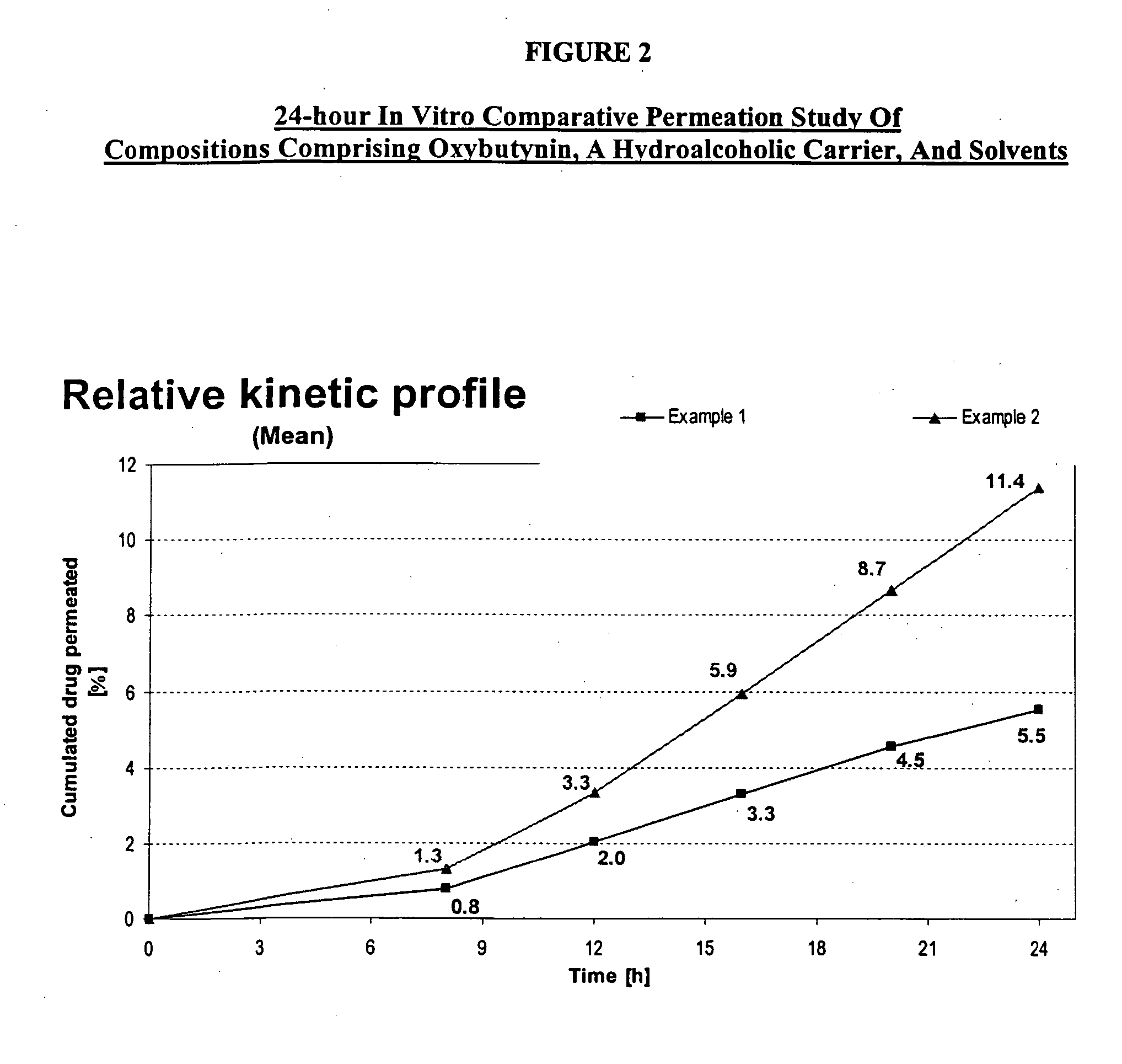
Permeation enhancing compositions for anticholinergic agents
InactiveUS7425340B2Improve permeabilityIncrease permeationBiocideNervous disorderOxybutyninSide effect
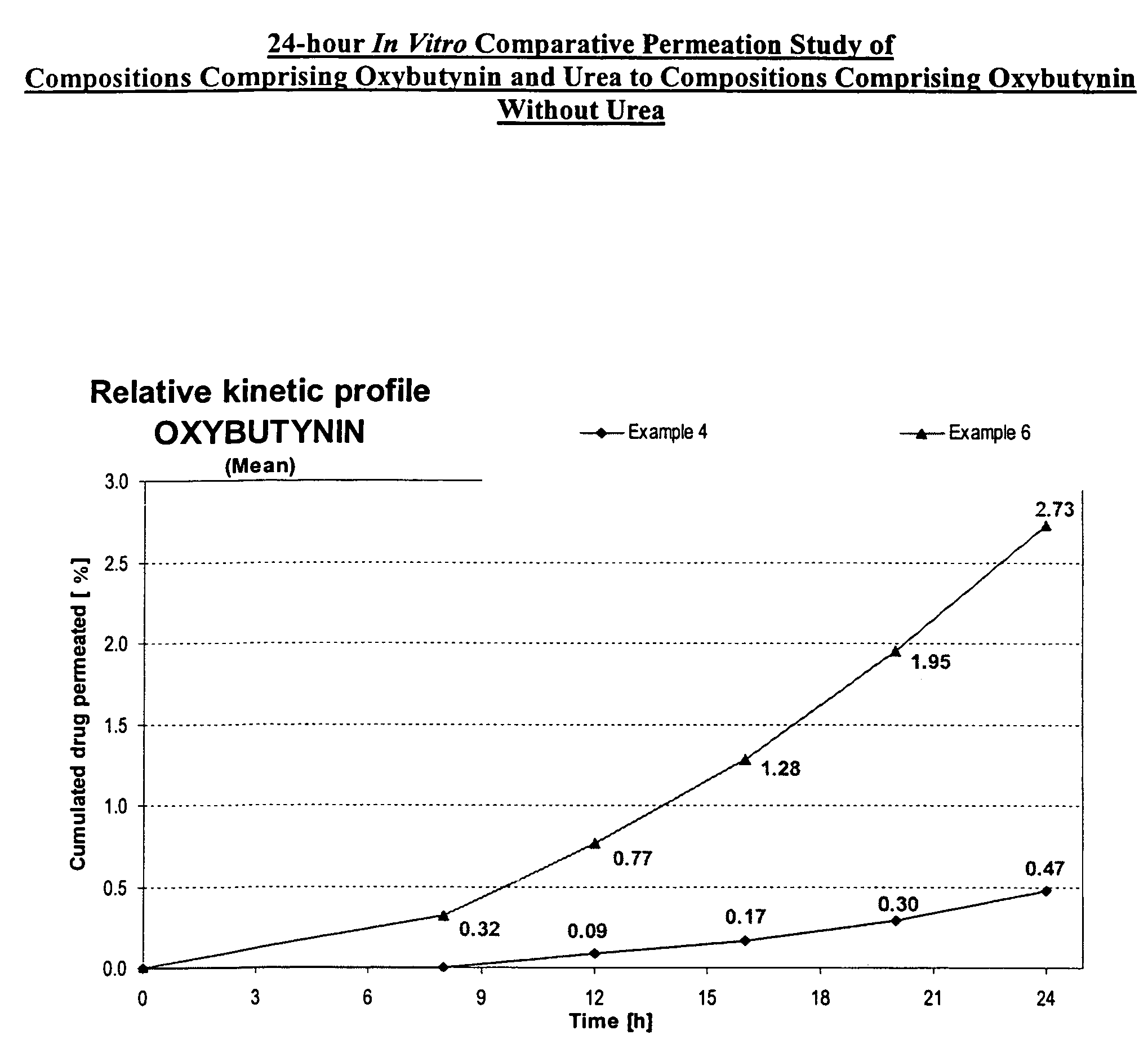
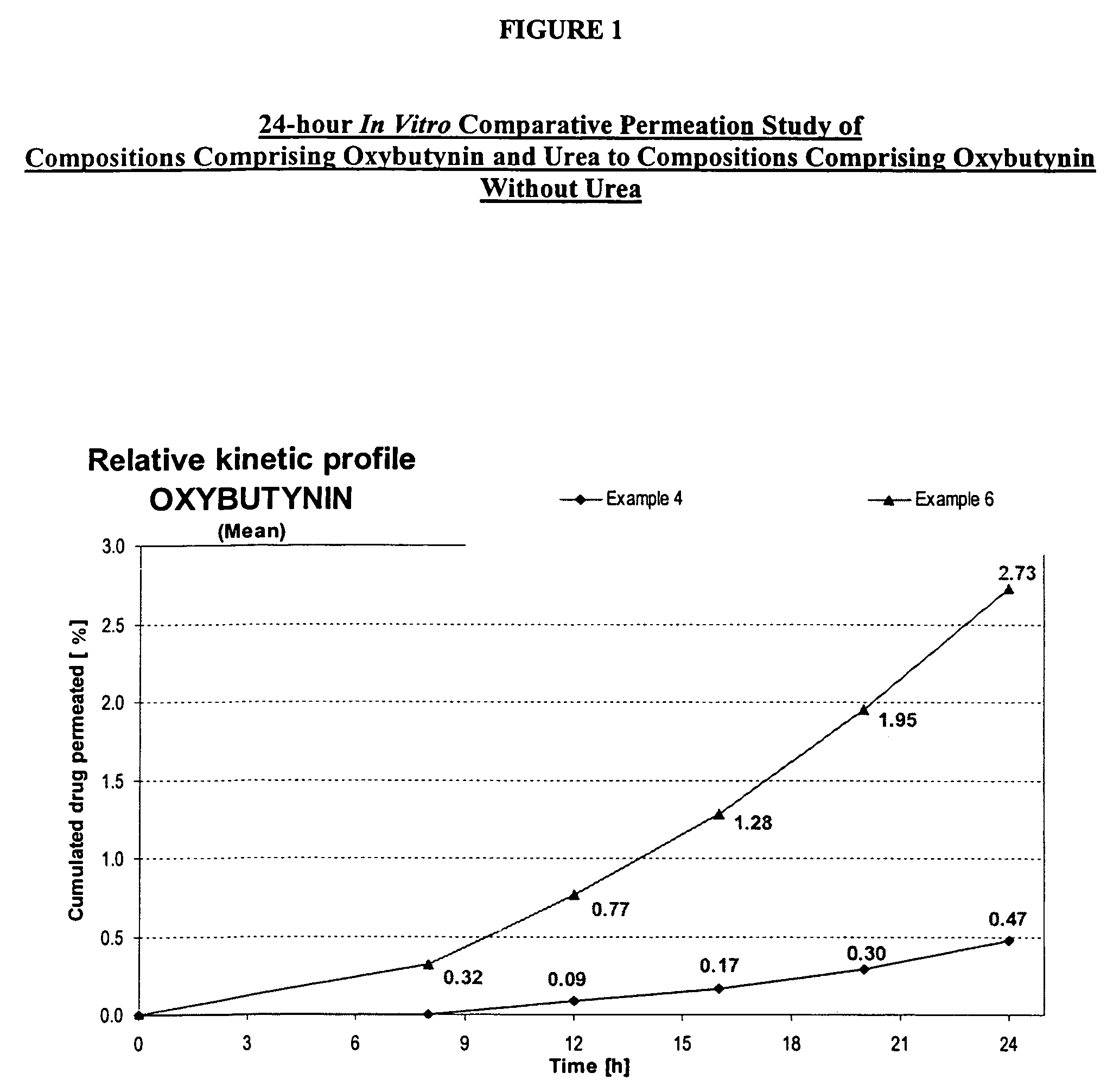
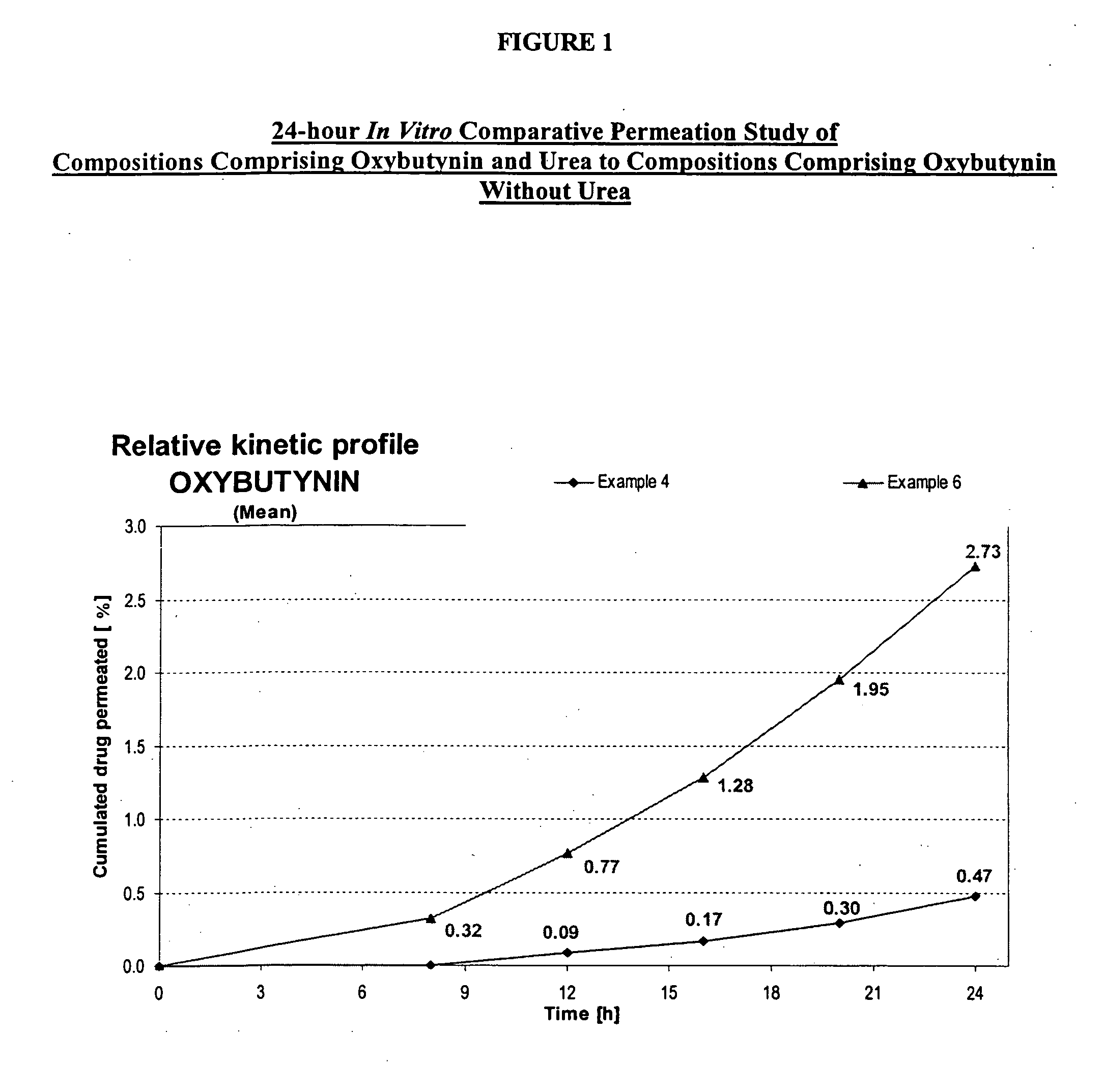
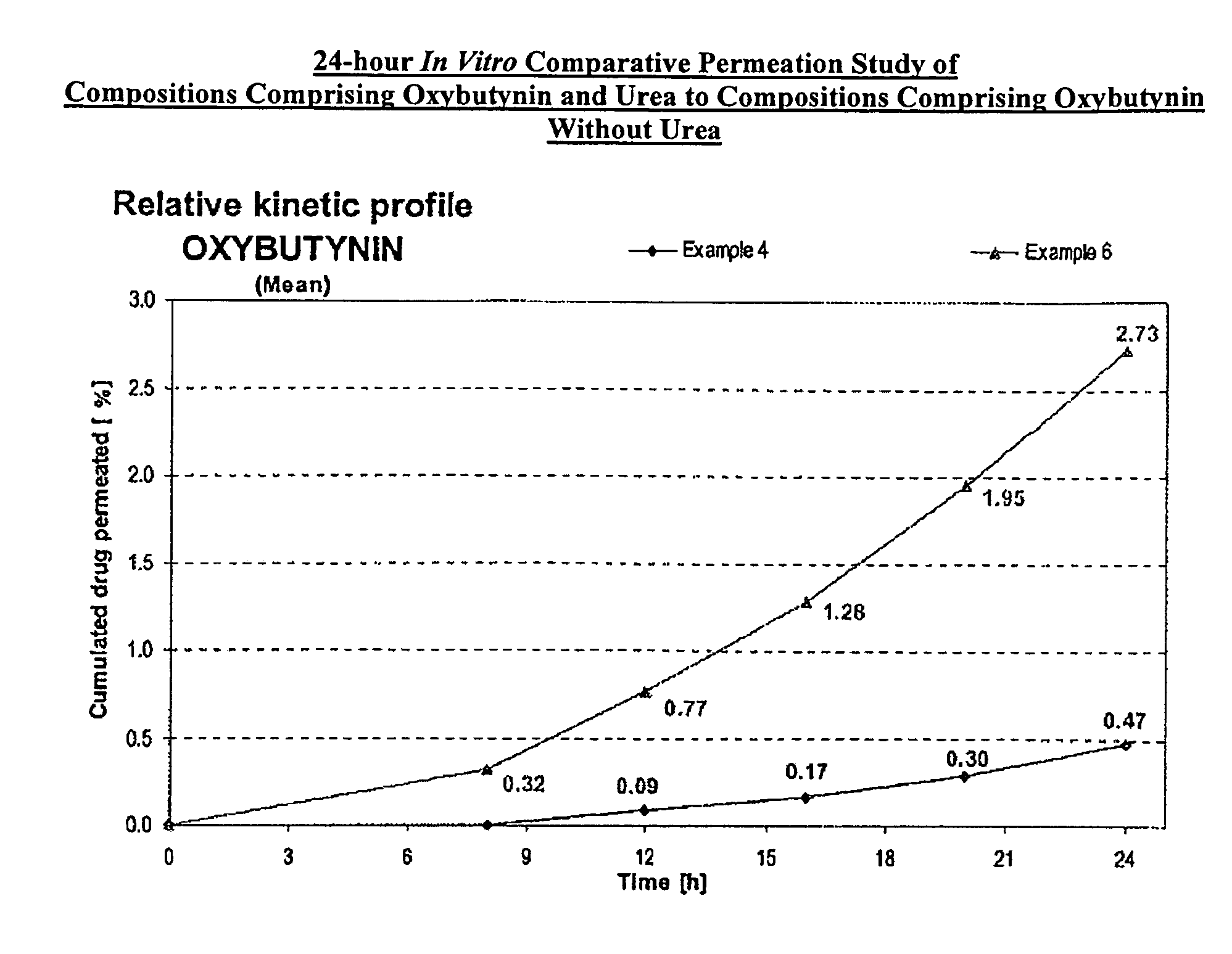
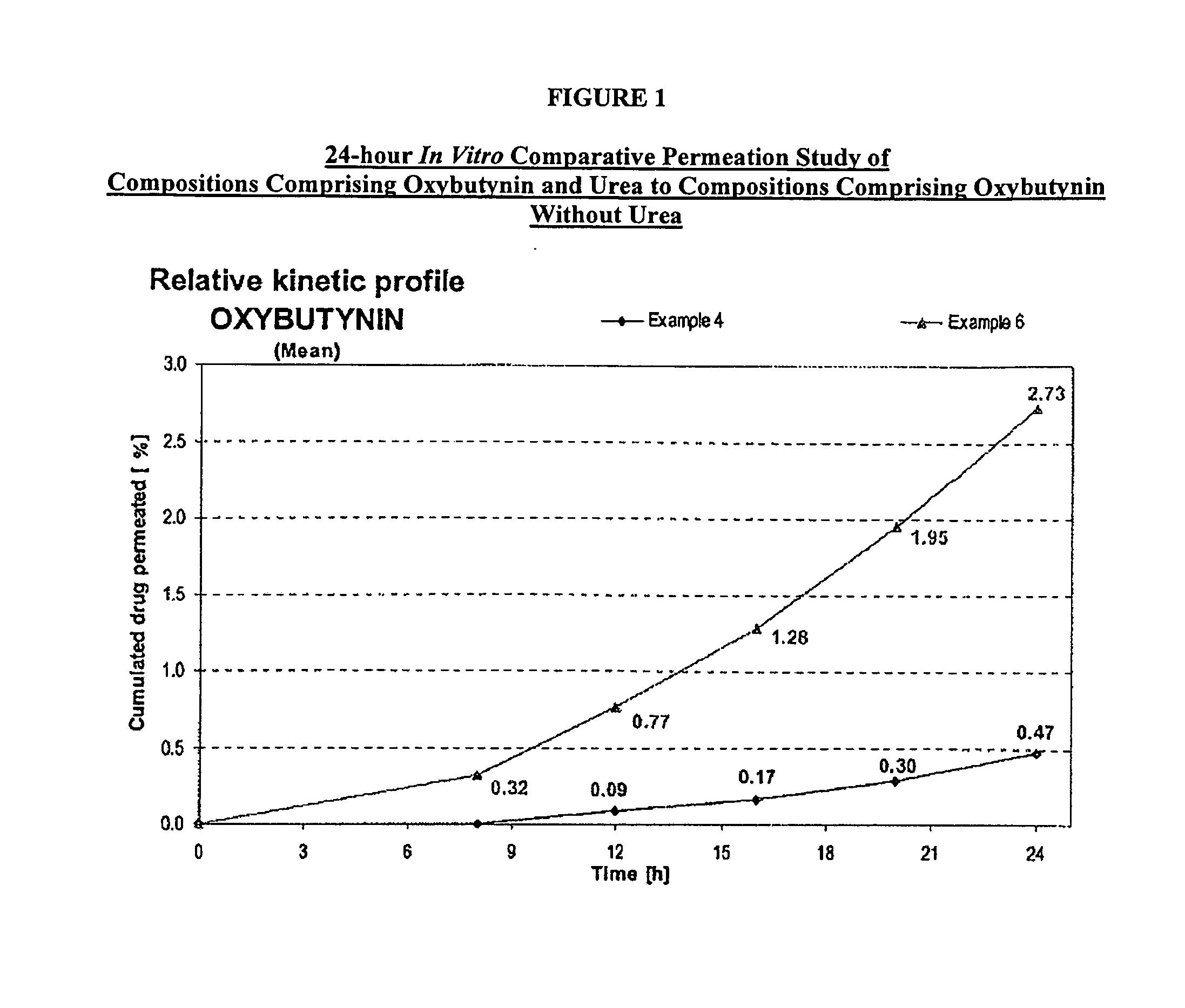
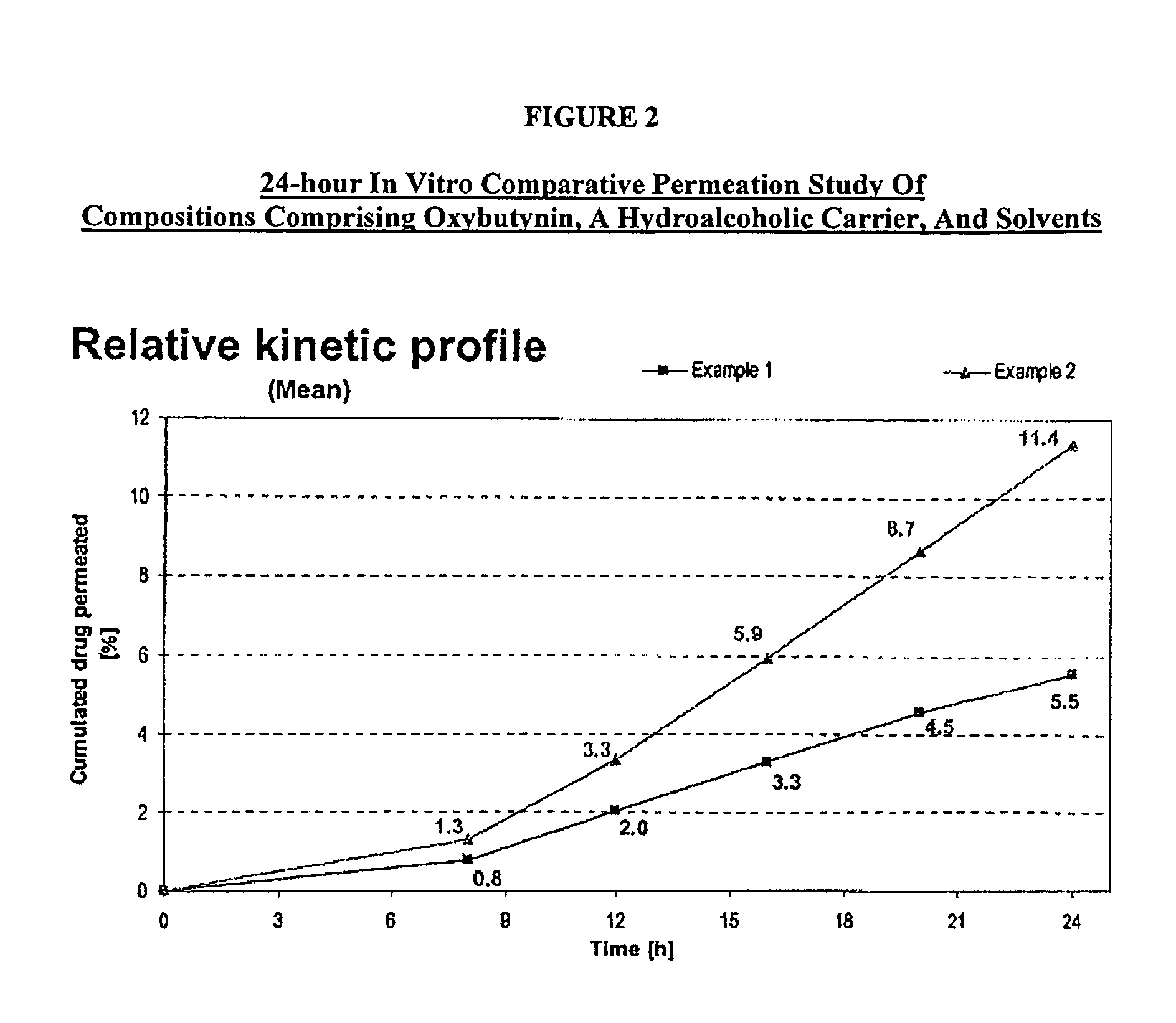
A transdermal or topical composition including anticholinergic agents, such as oxybutynin, a urea-containing compound and a carrier system. A method is disclosed for treating a subject for urinary incontinence while reducing the incidences of peak concentrations of drug and undesirable side effects.
Owner:ANTARES PHARMA IPL
Transdermal compositions for anticholinergic agents
InactiveUS20100216880A1Avoiding undesirable peak in drug concentrationReduce morbidityAntibacterial agentsBiocideOxybutyninLong chain fatty acid
The present invention relates generally to compositions or formulations for transdermal or transmucosal administration of anticholinergic agents such as oxybutynin. The invention utilizes a novel delivery vehicle and is a substantially malodorous-free and irritation free transdermal formulation which is substantially free of long chain fatty alcohols, long-chain fatty acids, and long-chain fatty esters. A method is disclosed for treating a subject for urinary incontinence with these formulations while reducing the incidences of peak concentrations of drug and undesirable side effects associated with oral anticholinergics.
Owner:ANTARES PHARMA IPL
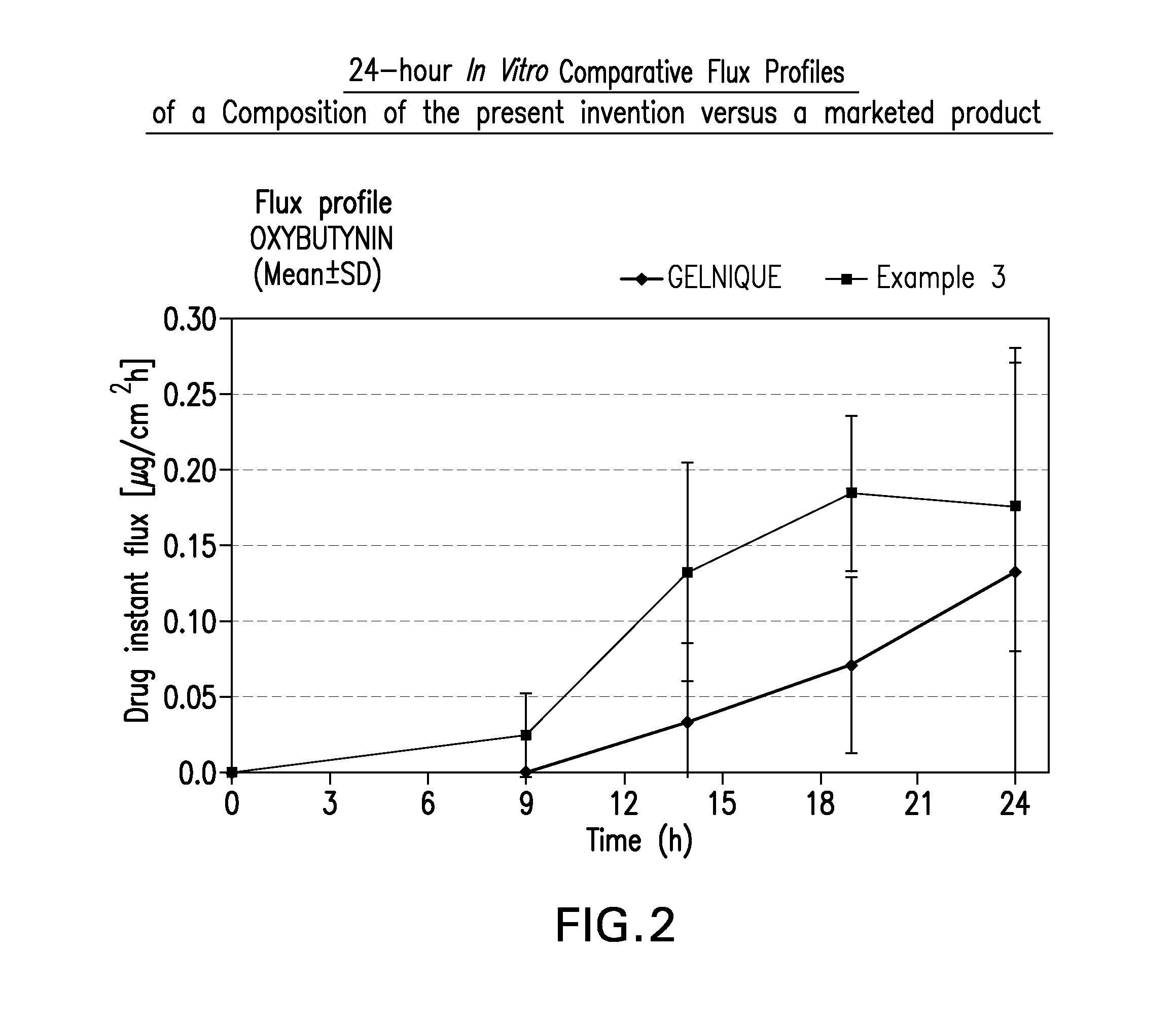
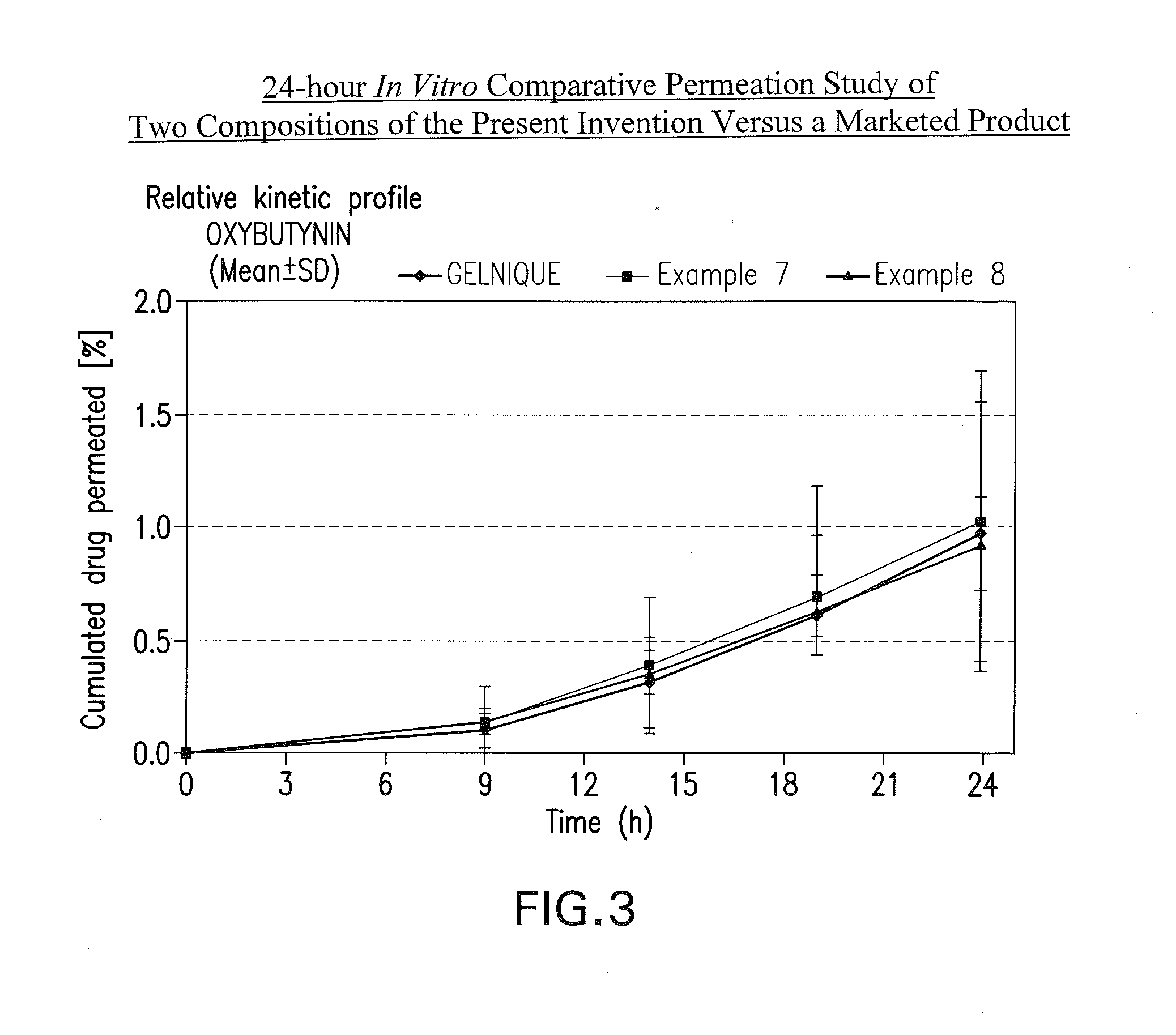
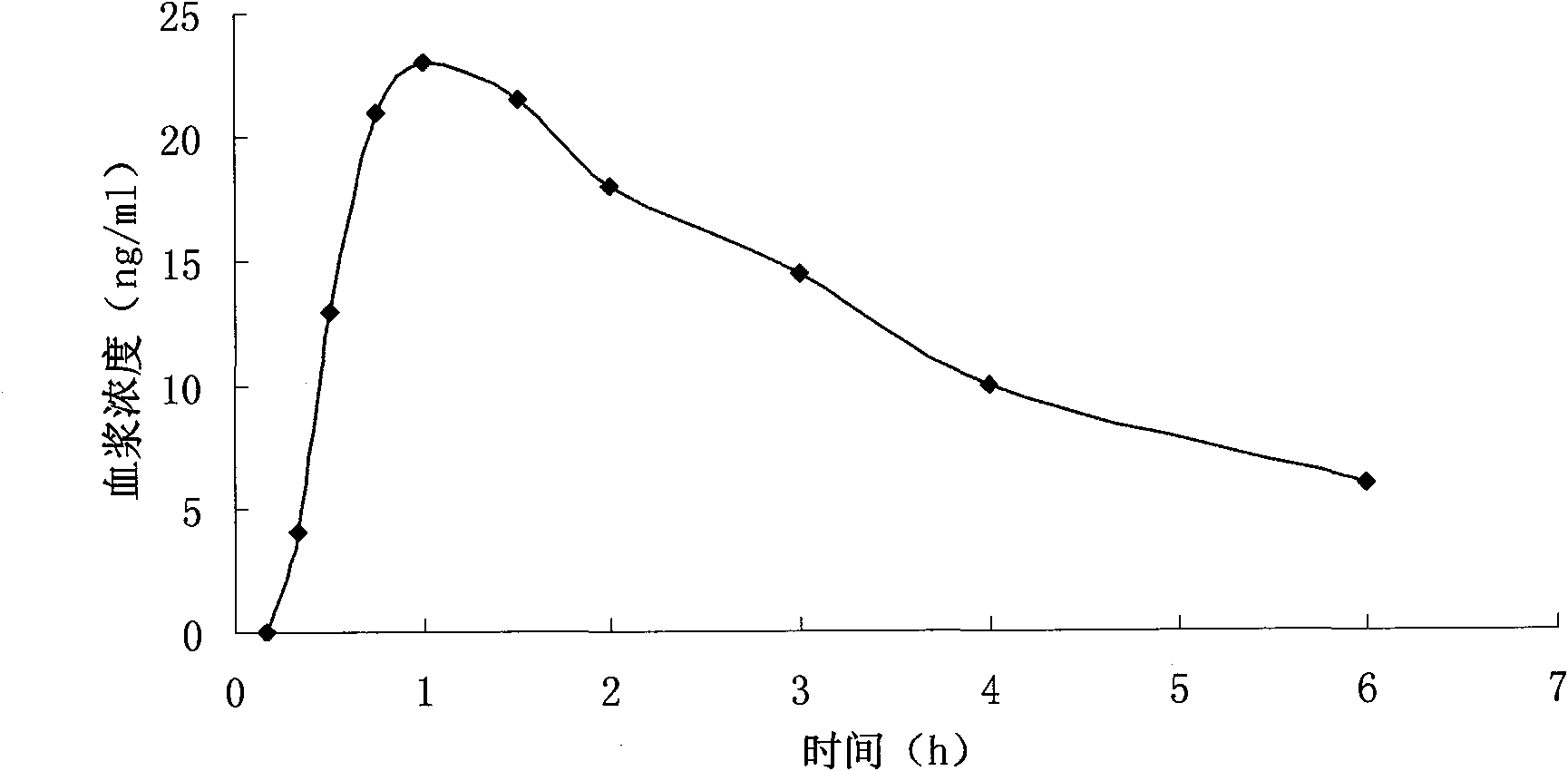
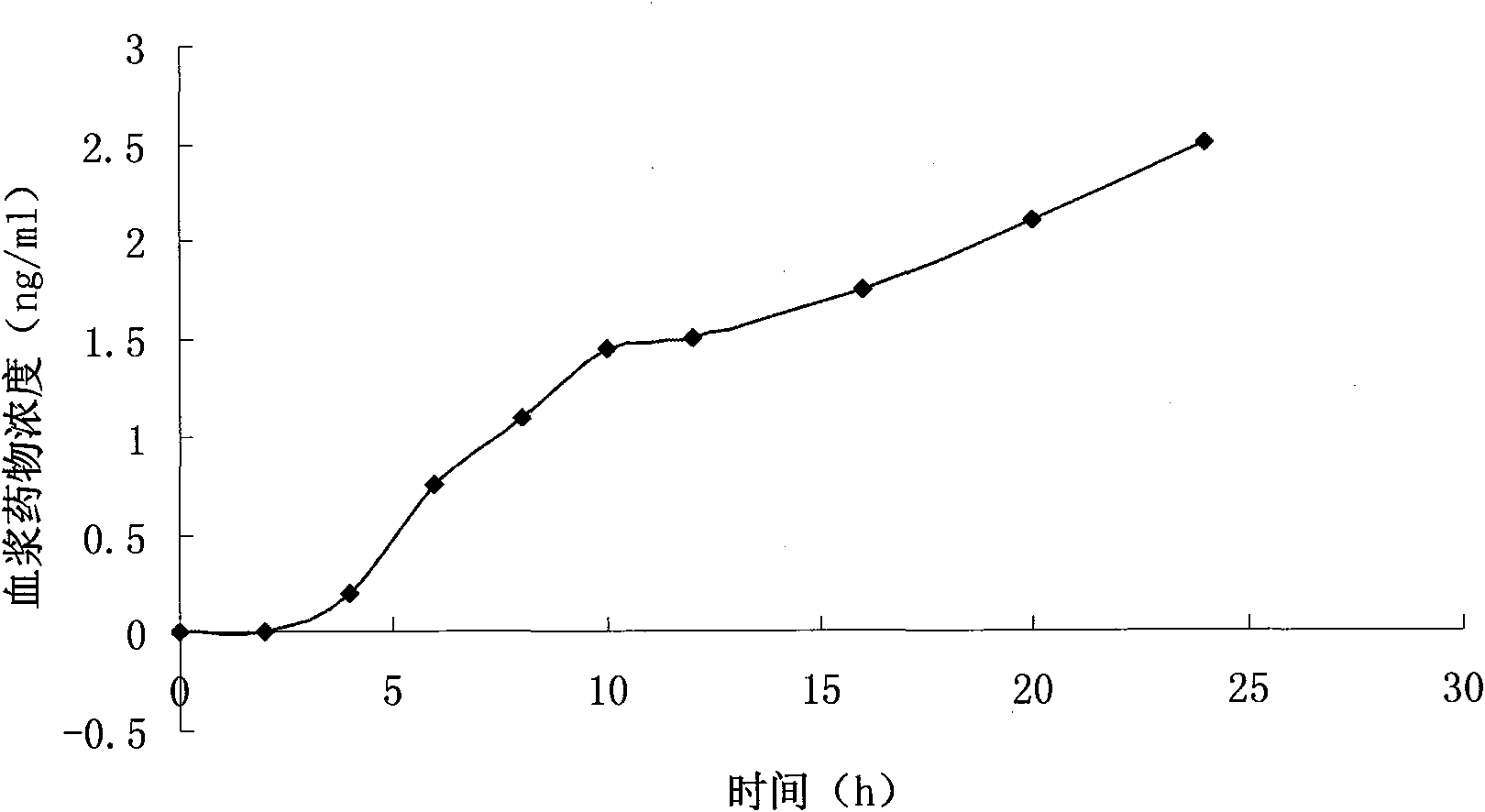
Transdermal absorption preparation of oxybutynin as well as preparation method and medication application thereof
The invention provides a transdermal absorption preparation containing oxybutynin, which comprises gels, ointment and cream and aims at reducing the adverse reaction rate of the oxybutynin and lightening the serious adverse reaction degree, wherein when the content of the oxybutynin in the transdermal absorption preparation is 0.1-30 percent by weight, the content of the oxybutynin in the transdermal absorption preparation is preferentially to be 8-12 percent by weight. The invention also discloses the requirements of the transdermal absorption preparation in the aspects of formula compatibility and proportioning, as well as a preparation method and medication application of the transdermal absorption preparation.
Owner:陕西麦科奥特生物科技有限公司
Therapy for the treatment of disease
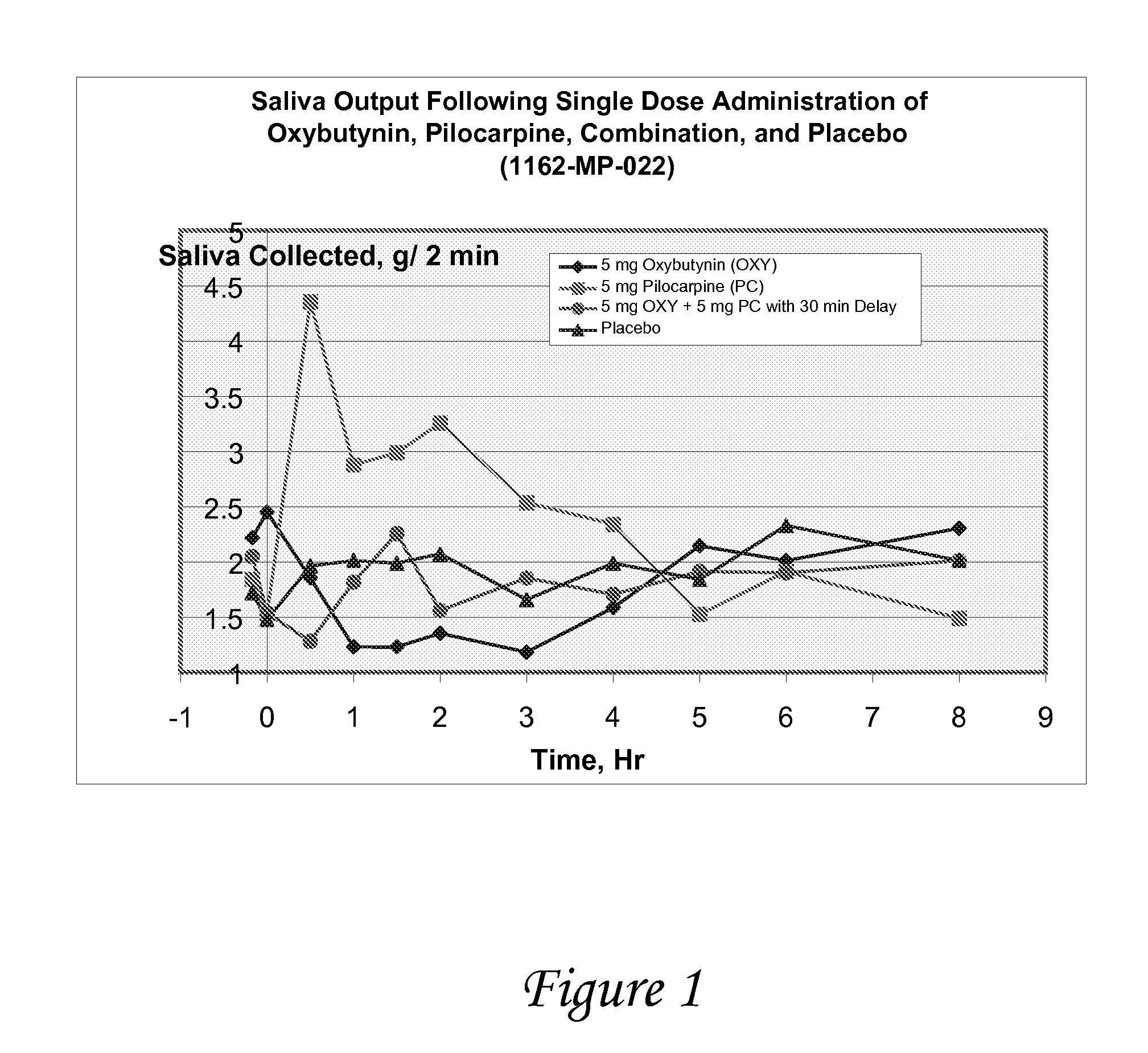
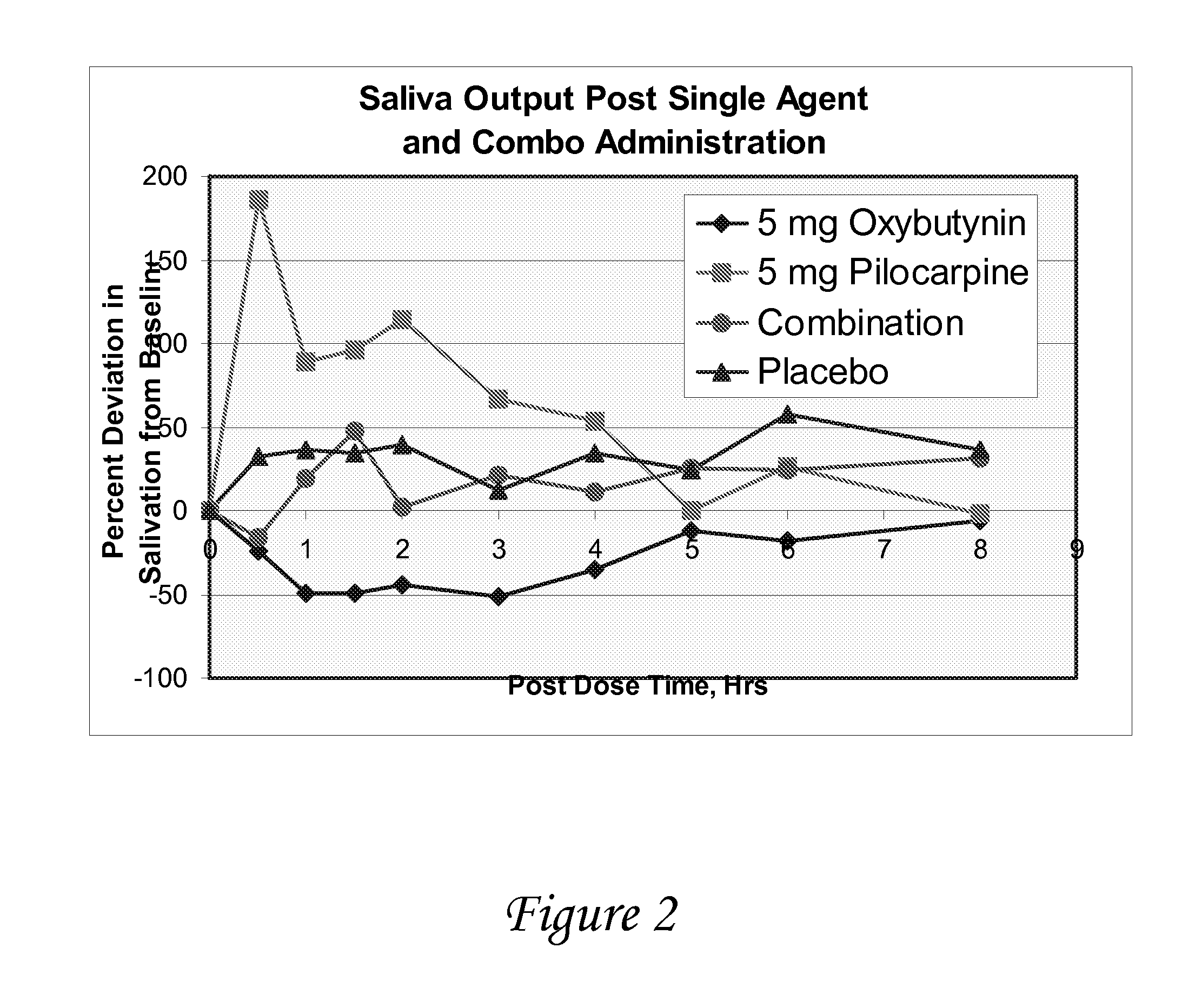
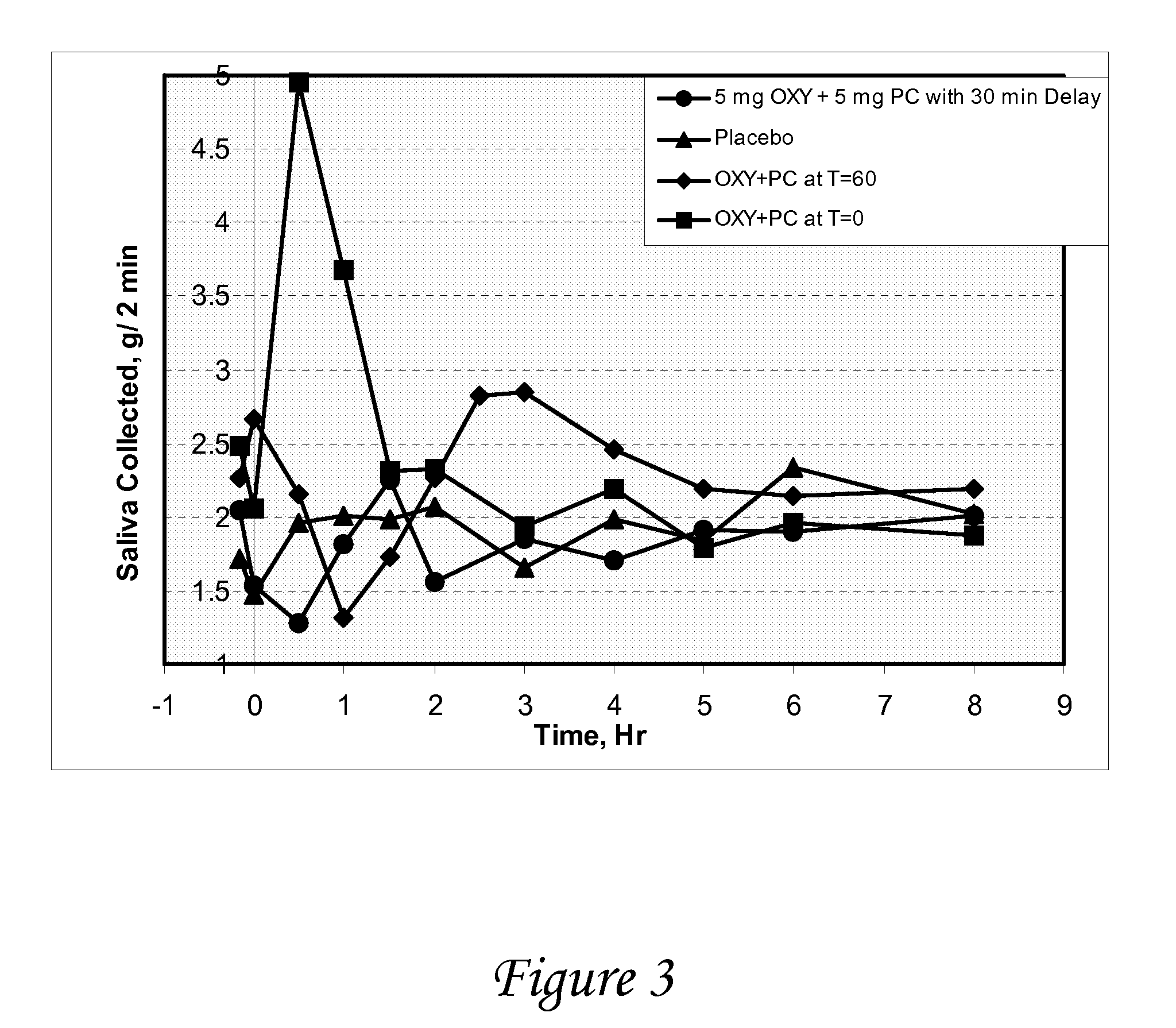
Disclosed herein are pharmaceutical compositions comprising oxybutynin, or a free base thereof or a pharmaceutically acceptable salt thereof, and pilocarpine, or a free base thereof or a pharmaceutically acceptable salt thereof. Also disclosed are methods of treating a patient suffering from overactive bladder comprising administering to the patient the above pharmaceutical composition.
Owner:THERAVIDA INC
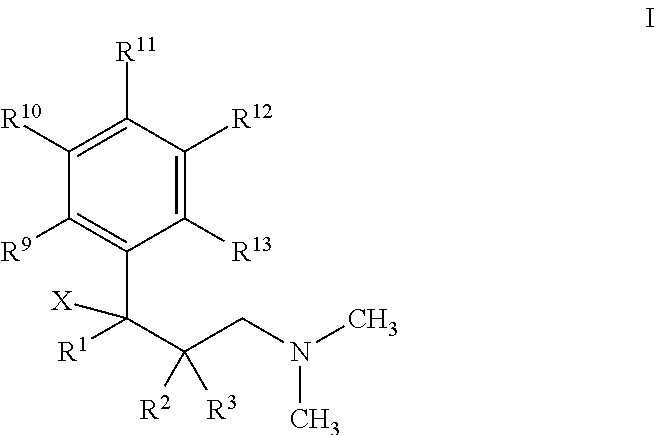
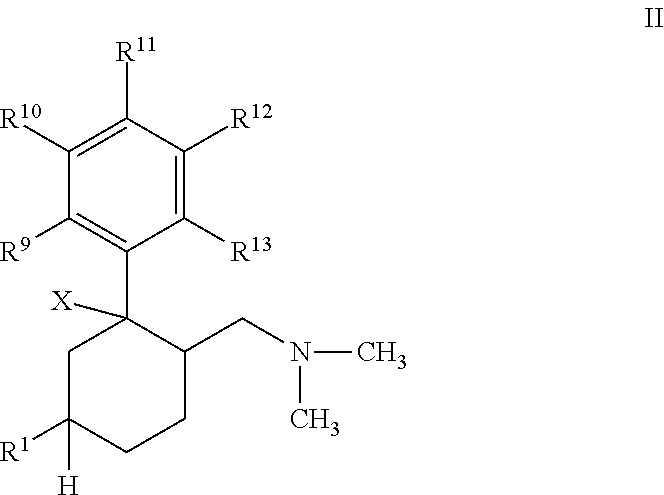
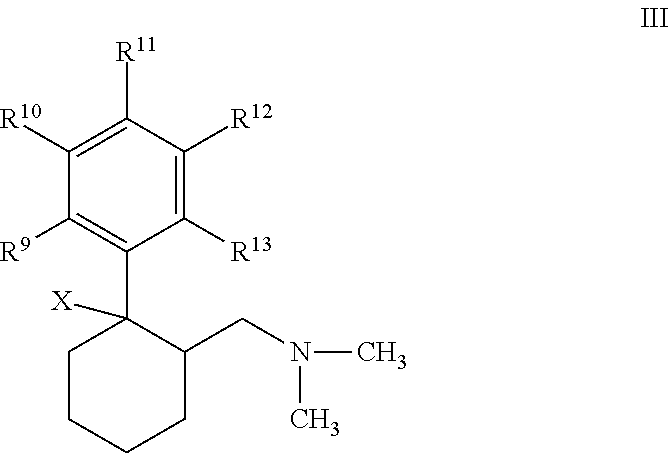
Novel anticholinergic compounds and methods of use
In a preferred embodiment, the subject invention concerns novel analogs of oxybutynin. The present invention also concerns methods for synthesizing the oxybutynin analogs of the present invention. The invention also pertains to methods for treating patients suffering from incontinence and other conditions.
Owner:ARYX THERAPEUTICS
Pharmaceutical combinations
Pharmaceutical combinations comprising a beta-3 adrenergic receptor agonist and a muscarinic receptor antagonist, and methods for their use are disclosed. Disclosed combinations include solabegron and oxybutynin. Methods of using the pharmaceutical combinations for the treatment of one or more symptoms associated with overactive bladder, for example, frequency of urgency, nocturia, and urinary incontinence, are also disclosed.
Owner:B3AR THERAPEUTICS INC
Permeation enhancing compositions for anticholinergic agents
InactiveUS20050287194A1Improve permeabilityIncrease permeationBiocideNervous disorderOxybutyninSide effect
A transdermal or topical composition including anticholinergic agents, such as oxybutynin, a urea-containing compound and a carrier system. A method is disclosed for treating a subject for urinary incontinence while reducing the incidences of peak concentrations of drug and undesirable side effects.
Owner:ANTARES PHARMA IPL
Methods and compositions for administration of oxybutynin
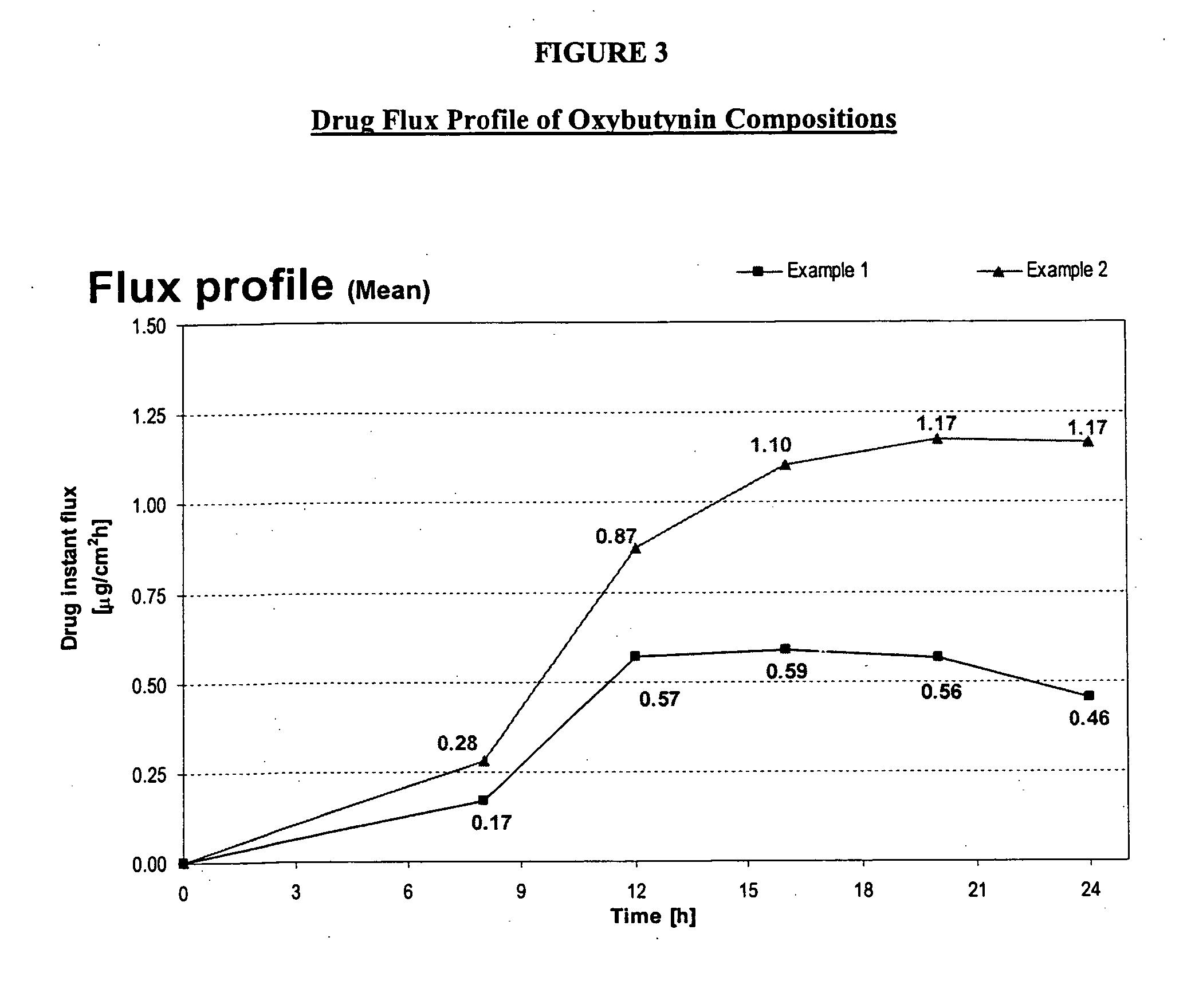
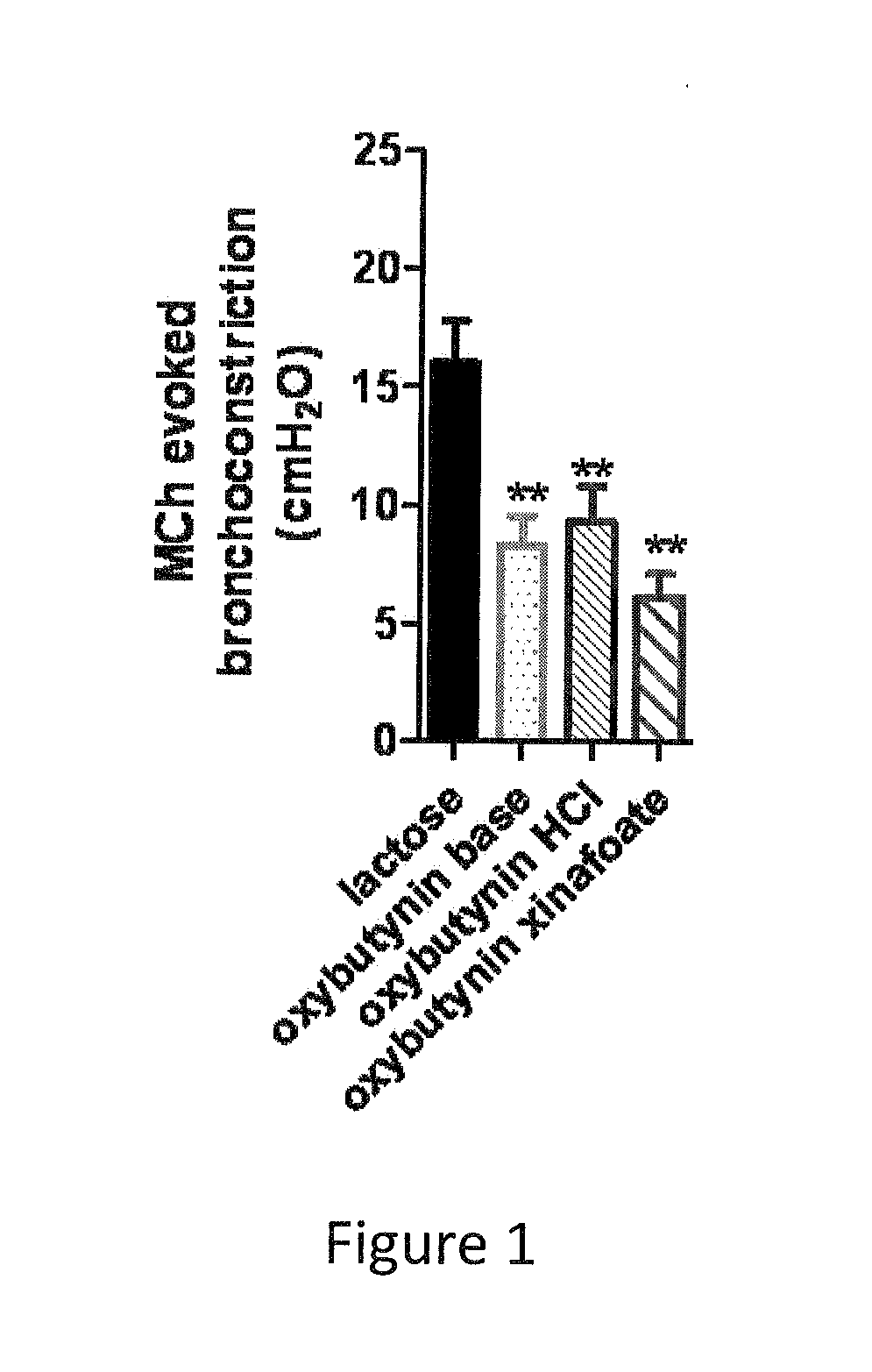
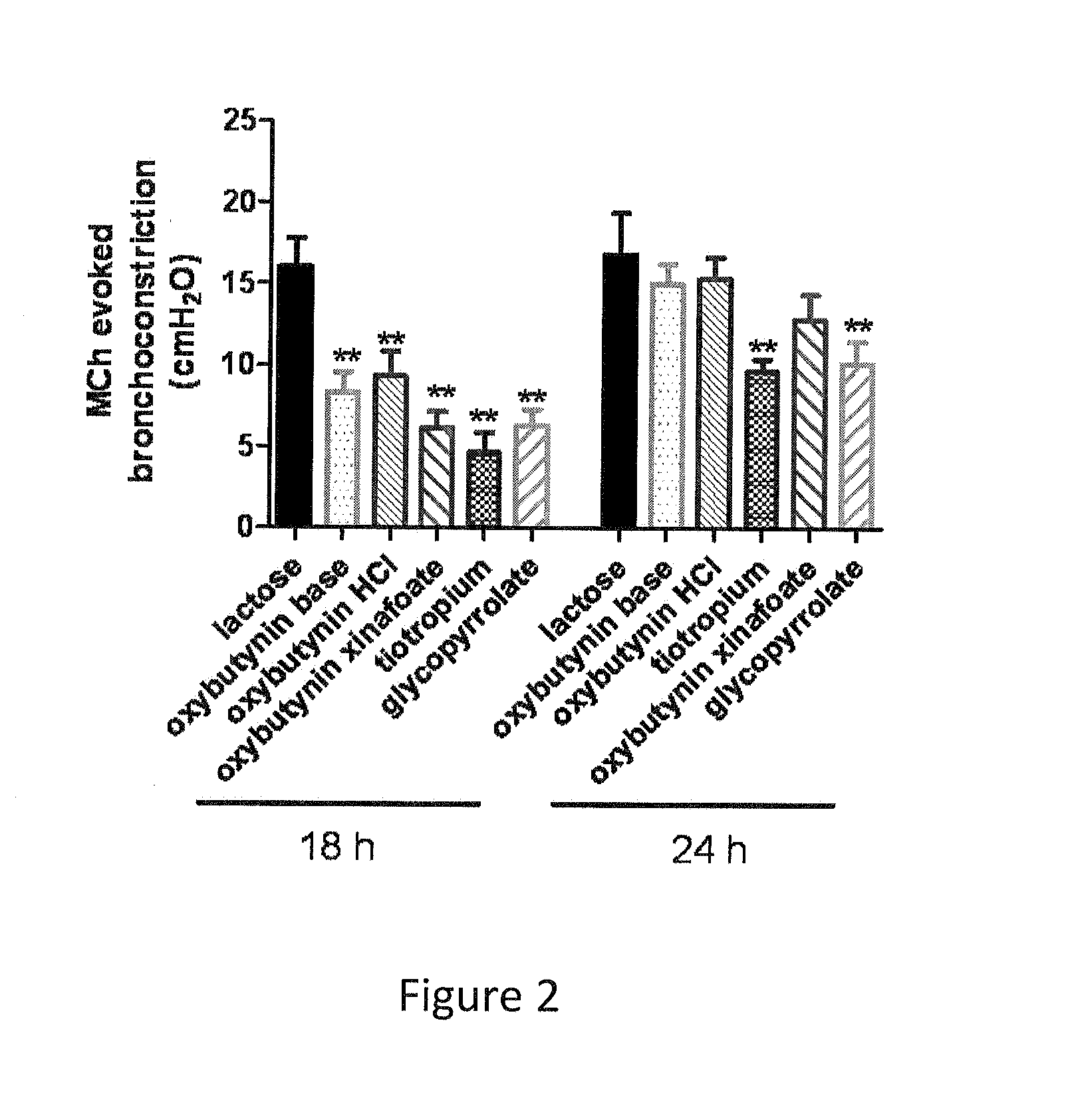
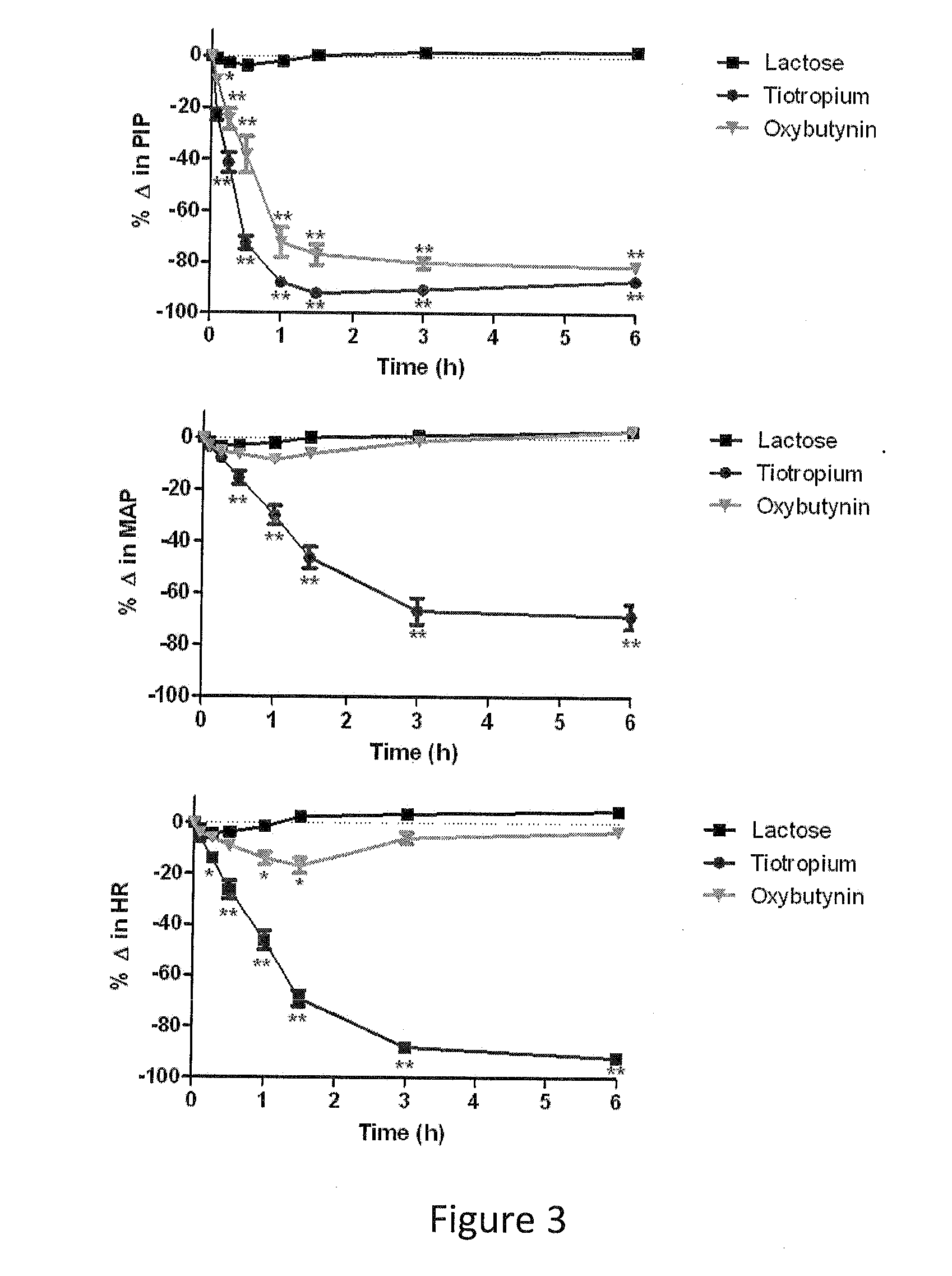
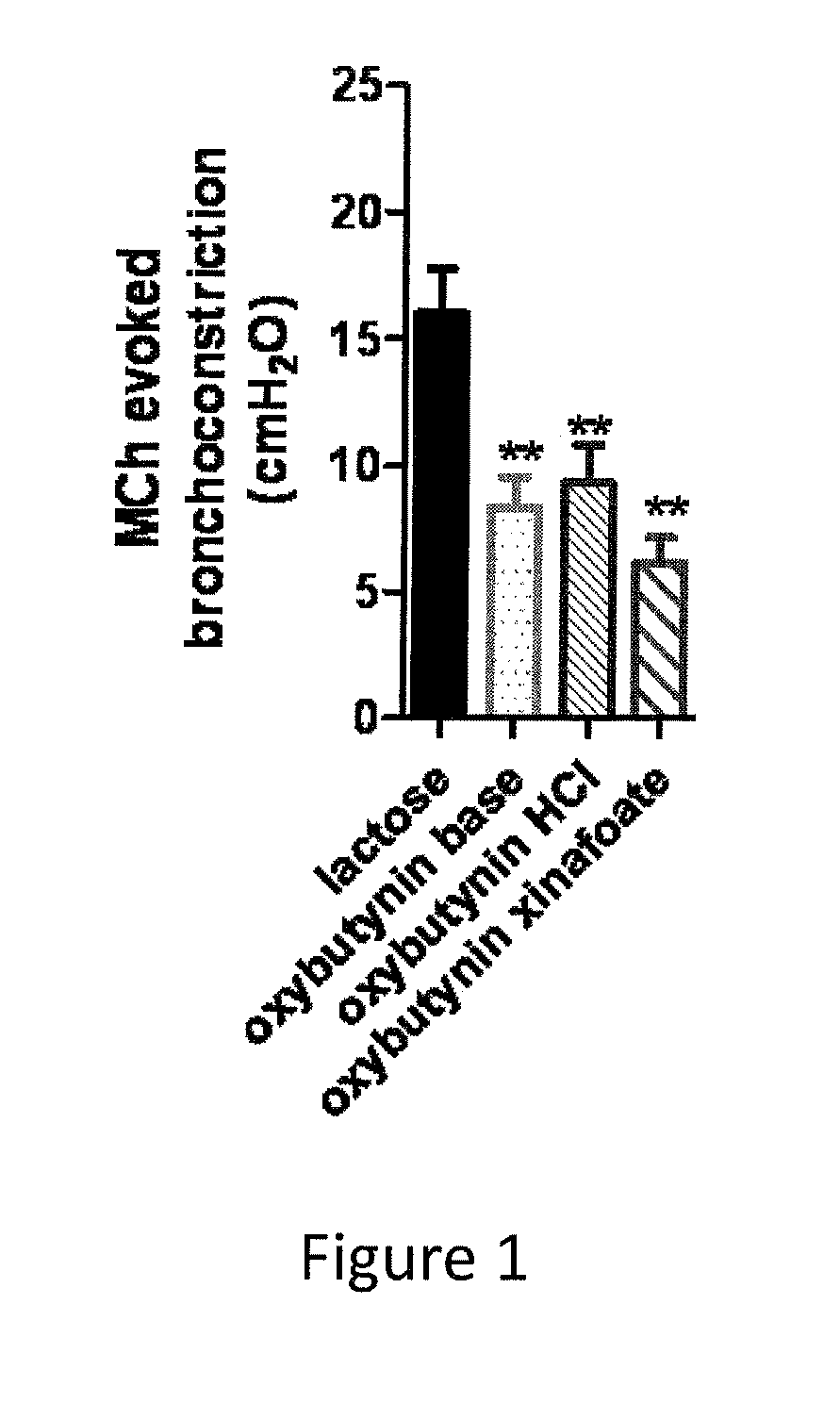
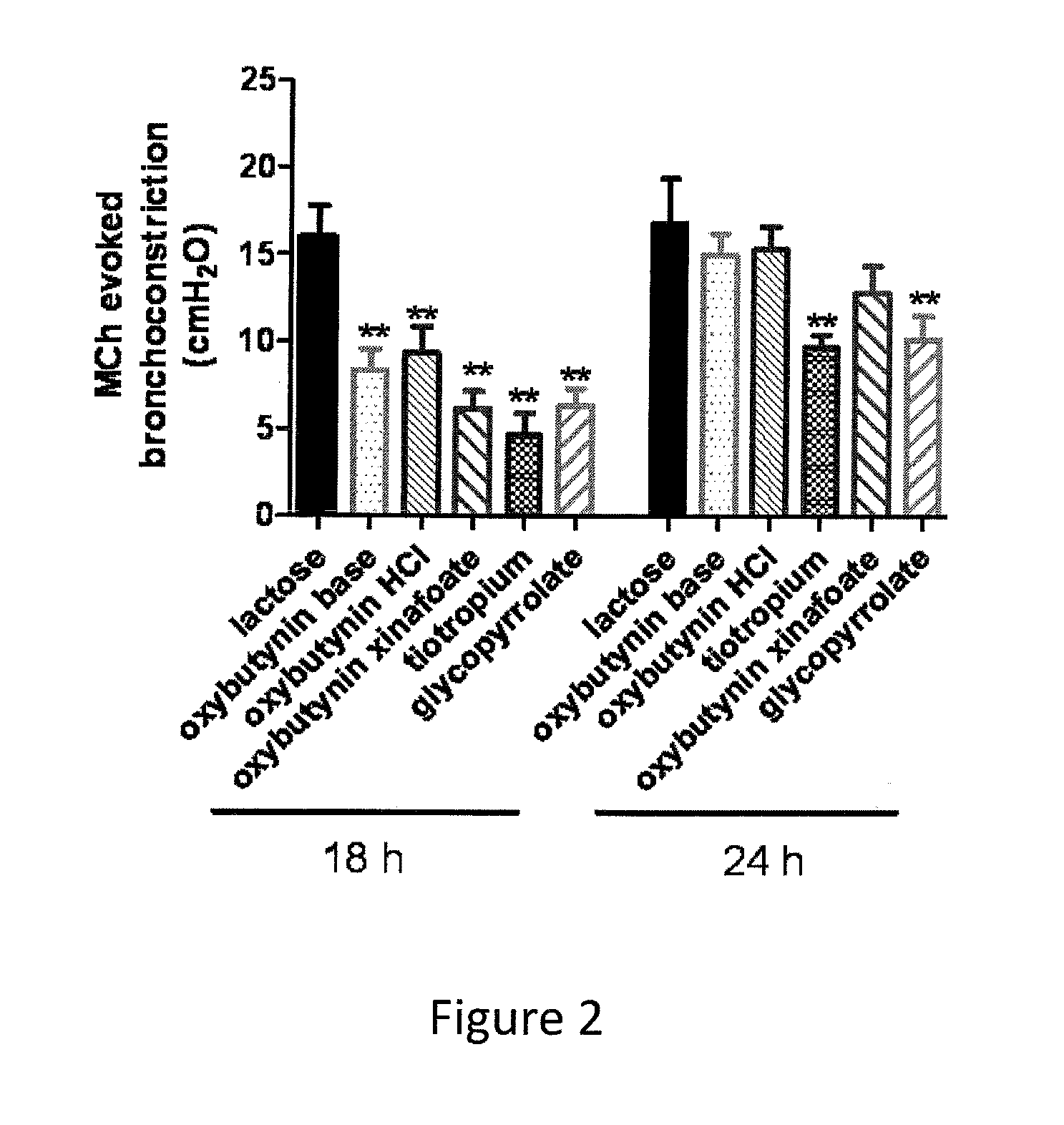
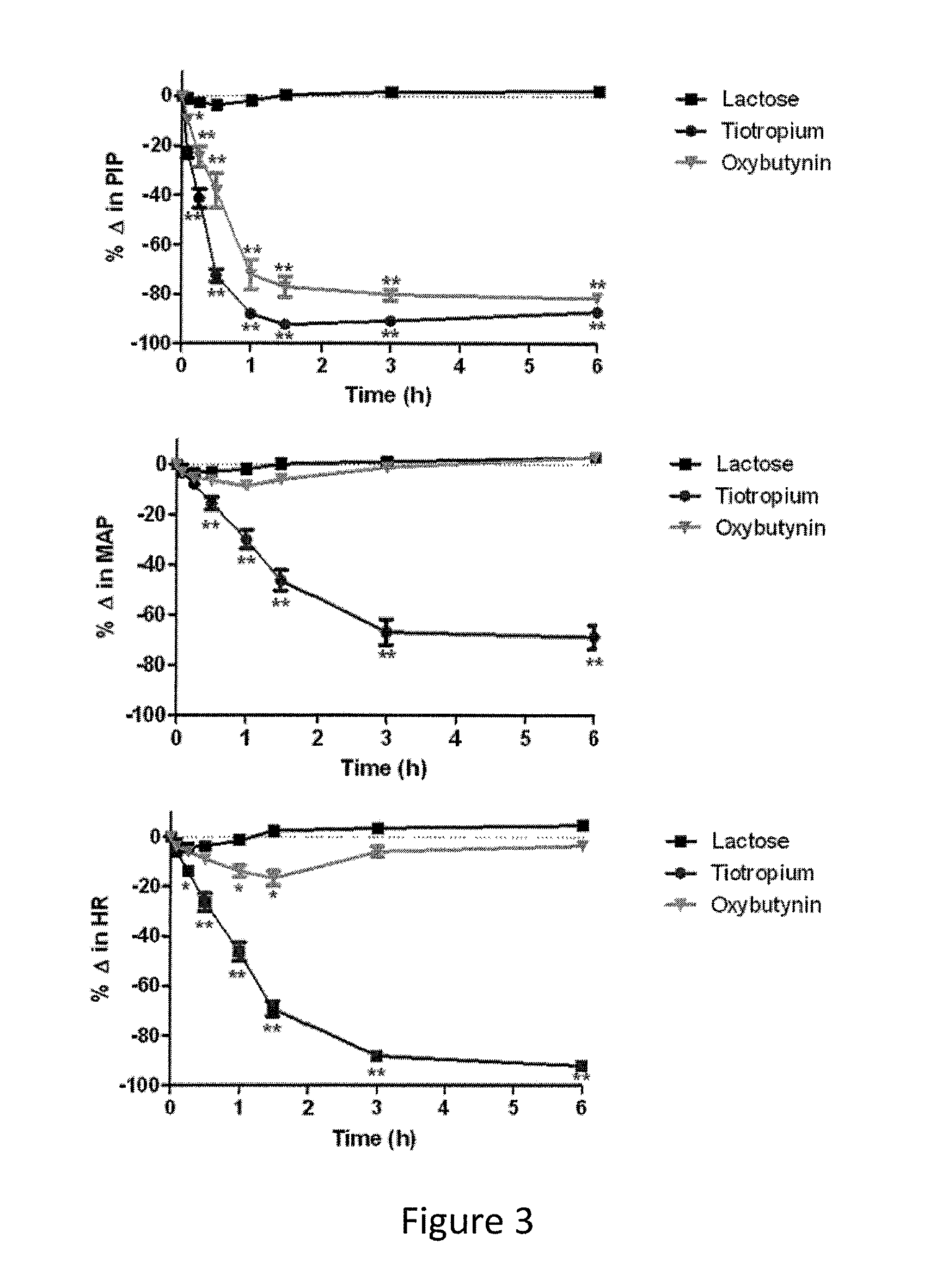
The present invention is directed to methods and compositions for treating pulmonary disease comprising delivering directly to a patient's lungs a therapeutically effective amount of oxybutynin in combination with one or more pharmaceutically effective agents. Oxybutynin may be selected from the group consisting of, but not limited to, a xinafoate salt, a palmitate salt, a pamoic salt, a resonate salt, a laurate salt and other salts. The pharmaceutically effective agents comprise bronchodilators, antiinflammatories, corticosteroids, corticosteroid reversal agent or alveolar growth agents or other agents selected from proteinase or protease inhibitors.
Owner:MICRODOSE THERAPEUTX INC
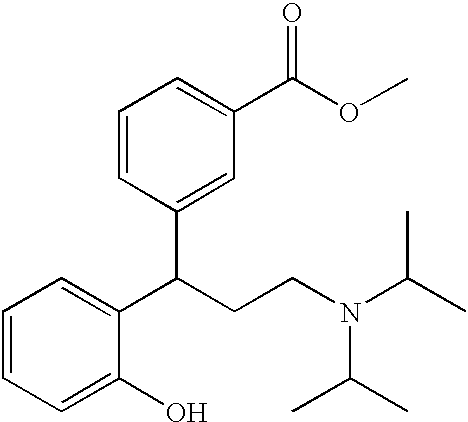
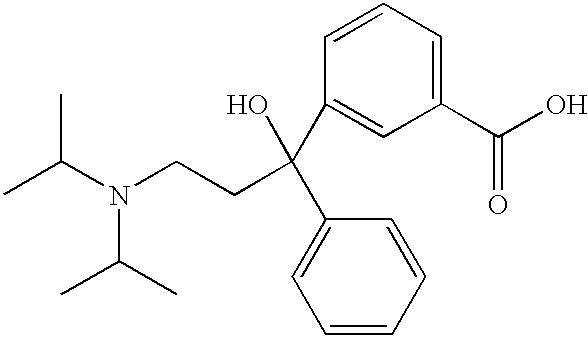
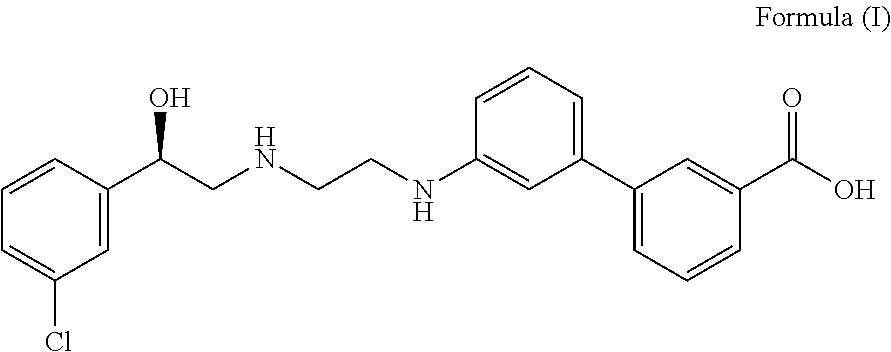
Derivatives of 3,3-diphenylpropylamines
Owner:UCB SA
Derivatives of 3,3-diphenylpropylamines
InactiveUS7230030B2Reduce absorptionUnfavourable metabolismBiocideNervous disorderSmooth muscleOxybutynin
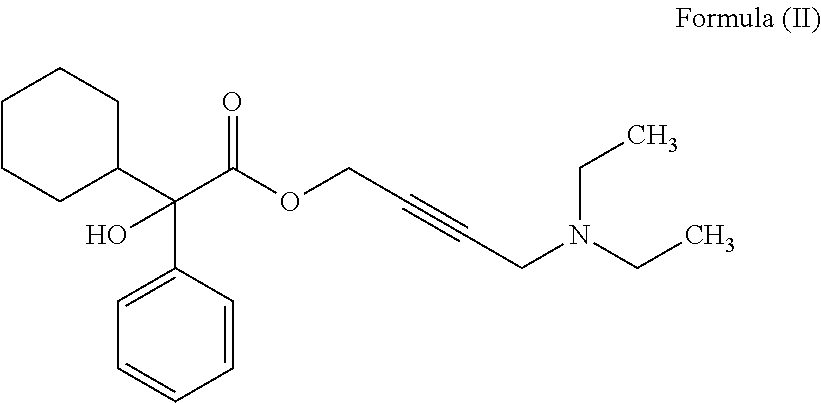
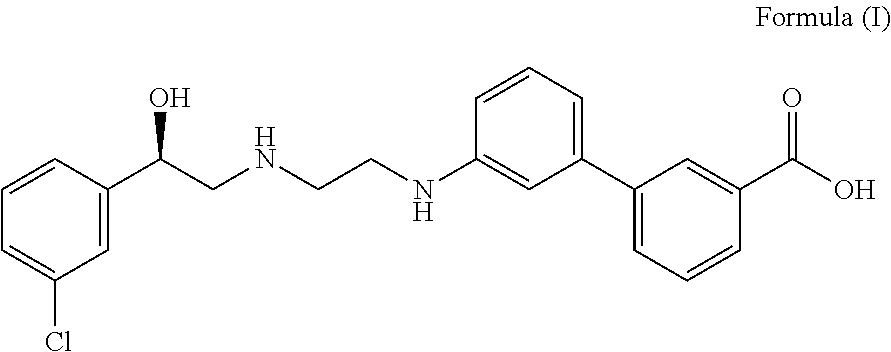
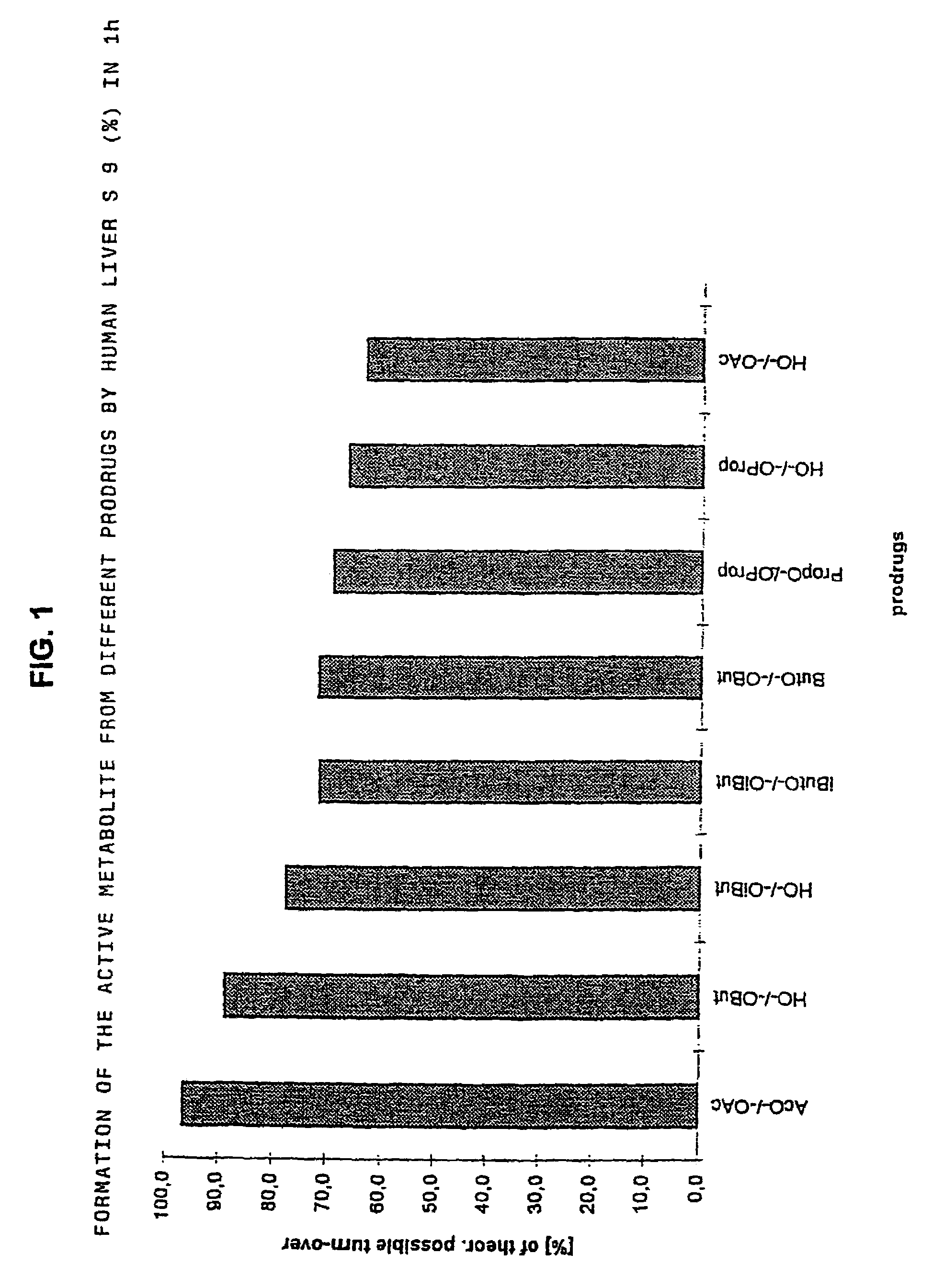
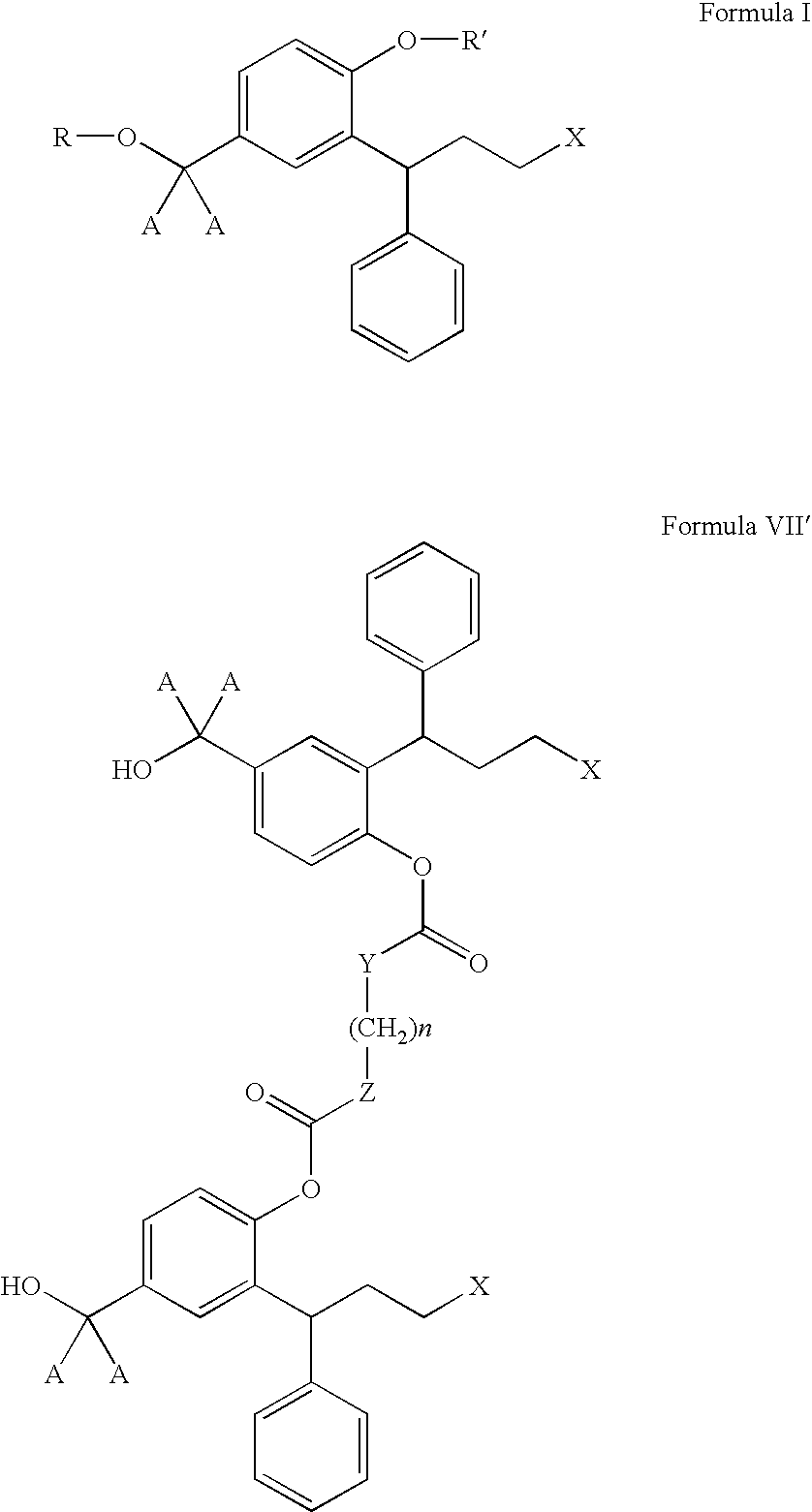
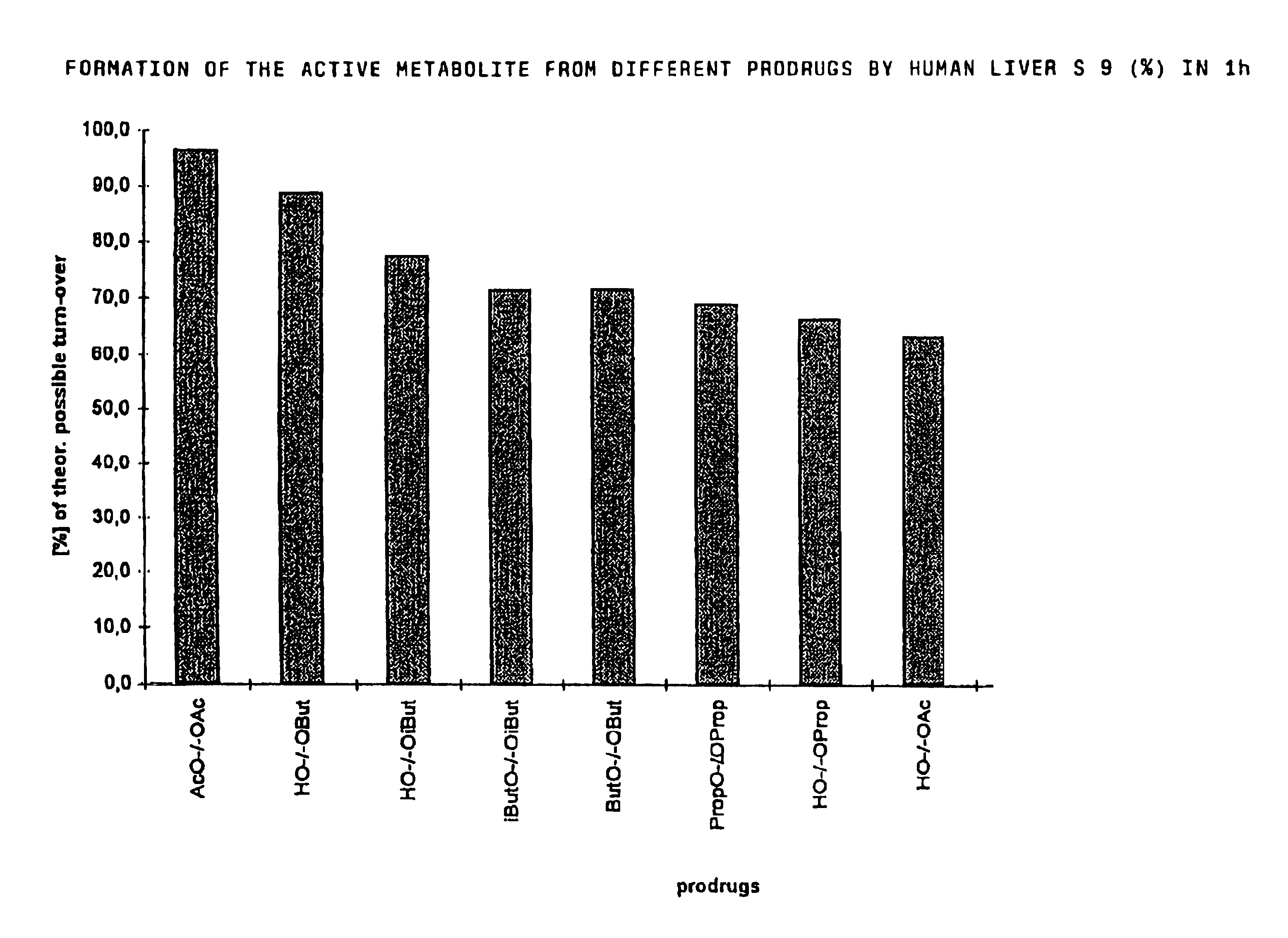
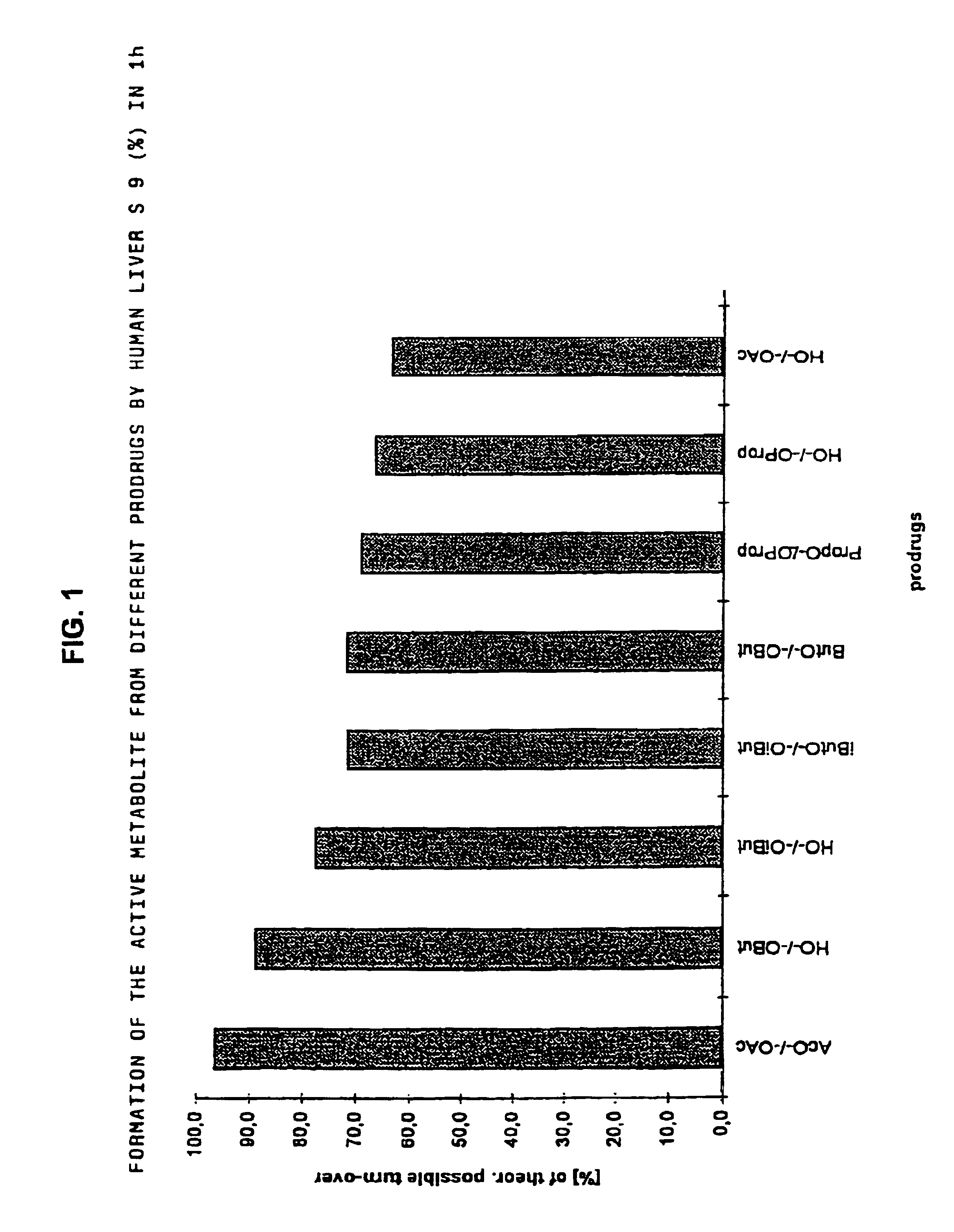
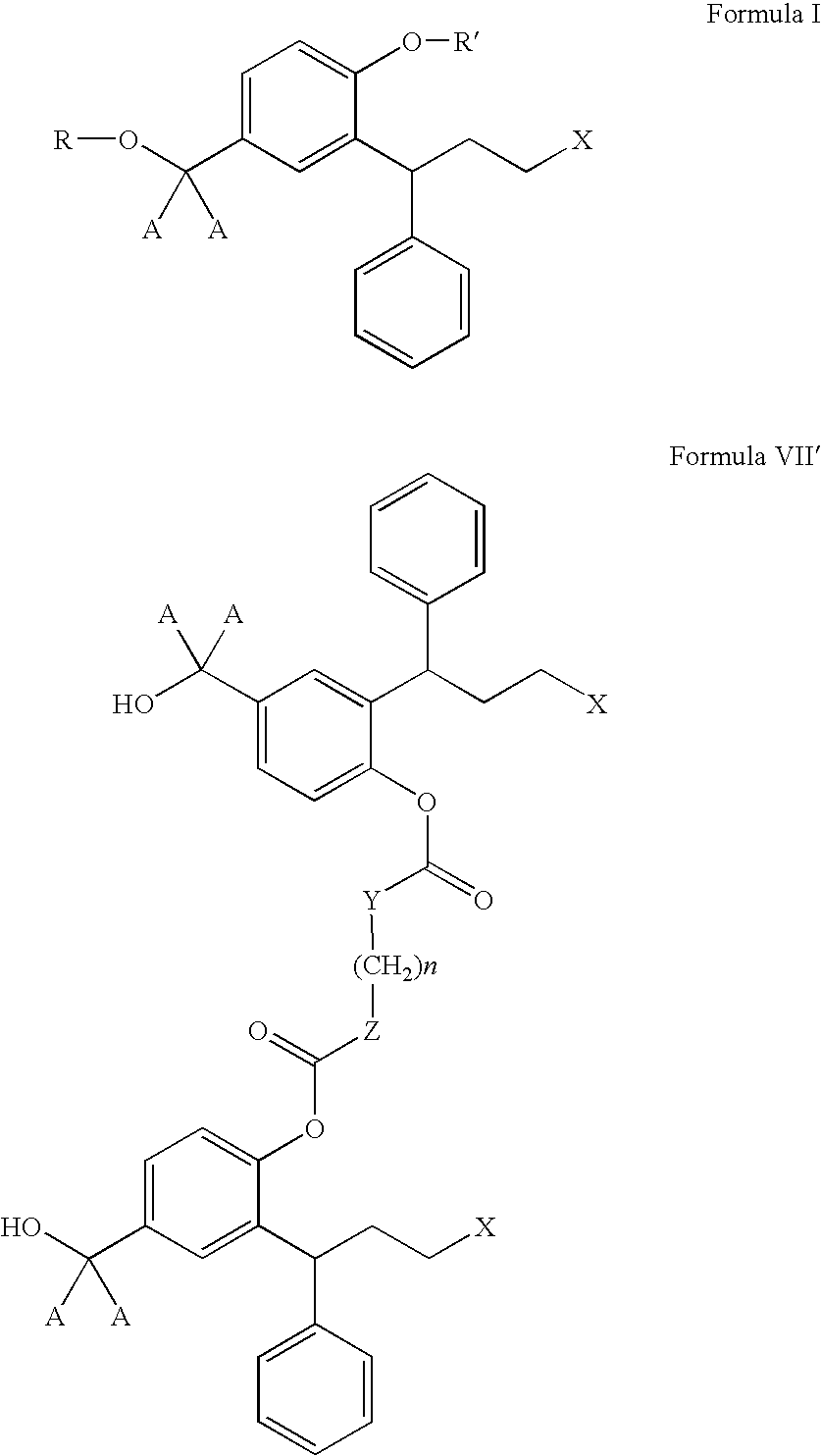
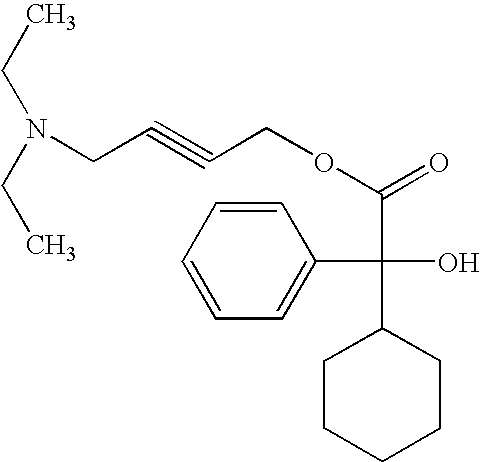
The invention concerns novel derivatives of 3,3-diphenylpropylamines, methods for their preparation, pharmaceutical compositions containing the novel compounds, and the use of the compounds for preparing drugs. More particularly, the invention relates to novel prodrugs of antimuscarinic agents with superior pharmacokinetic properties compared to existing drugs such as oxybutynin and tolterodine, methods for their preparation, pharmaceutical compositions containing them, a method of using said compounds and compositions for the treatment of urinary incontinence, gastrointestinal hyperactivity (irritable bowel syndrome) and other smooth muscle contractile conditions.
Owner:UCB SA
Methods and compositions for administration of oxybutynin
InactiveUS20080299207A1Fast absorptionImprove bioavailabilityBiocidePowder deliveryDiseaseOxybutynin
Administration of Oxybutynin in nebulized dry powder form directly to a patient's lungs for treating urinary incontinence or respiratory disease.
Owner:MICRODOSE THERAPEUTX INC
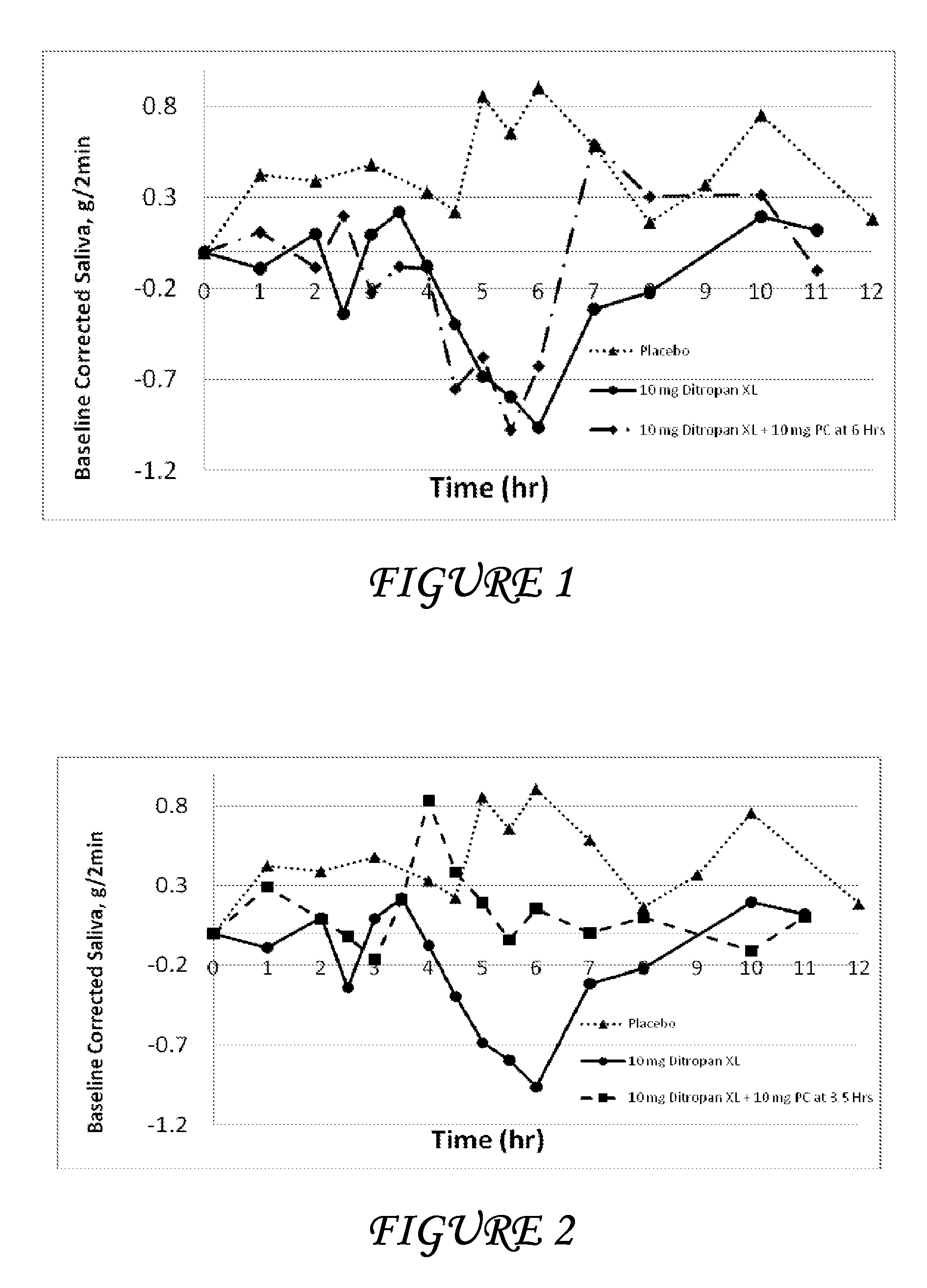
Combinations of oxybutynin and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release oxybutynin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release oxybutynin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release oxybutynin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Methods and compositions for administration of oxybutynin
Administration of Oxybutynin directly to a patient's lungs for treating urinary incontinence, respiratory disease or IBD.
Owner:MICRODOSE THERAPEUTX INC
Permeation enhancing compositions for anticholinergic agents
InactiveUS20080260842A1Avoiding undesirable peak in drug concentrationReduce morbidityBiocideAerosol deliveryOxybutyninAnticholinergic agents
A transdermal or topical skin-friendly composition including anticholinergic agents, such as oxybutynin, a urea-containing compound and a carrier system. A method is disclosed for treating a subject for urinary incontinence while reducing the incidences of peak concentrations of drug and undesirable side effects associated with oral anticholinergics.
Owner:ANTARES PHARMA IPL
Transdermal delivery of oxybutynin in gel formulations
InactiveUS20050064037A1Minimize impactPowder deliveryOrganic active ingredientsOxybutyninSide effect
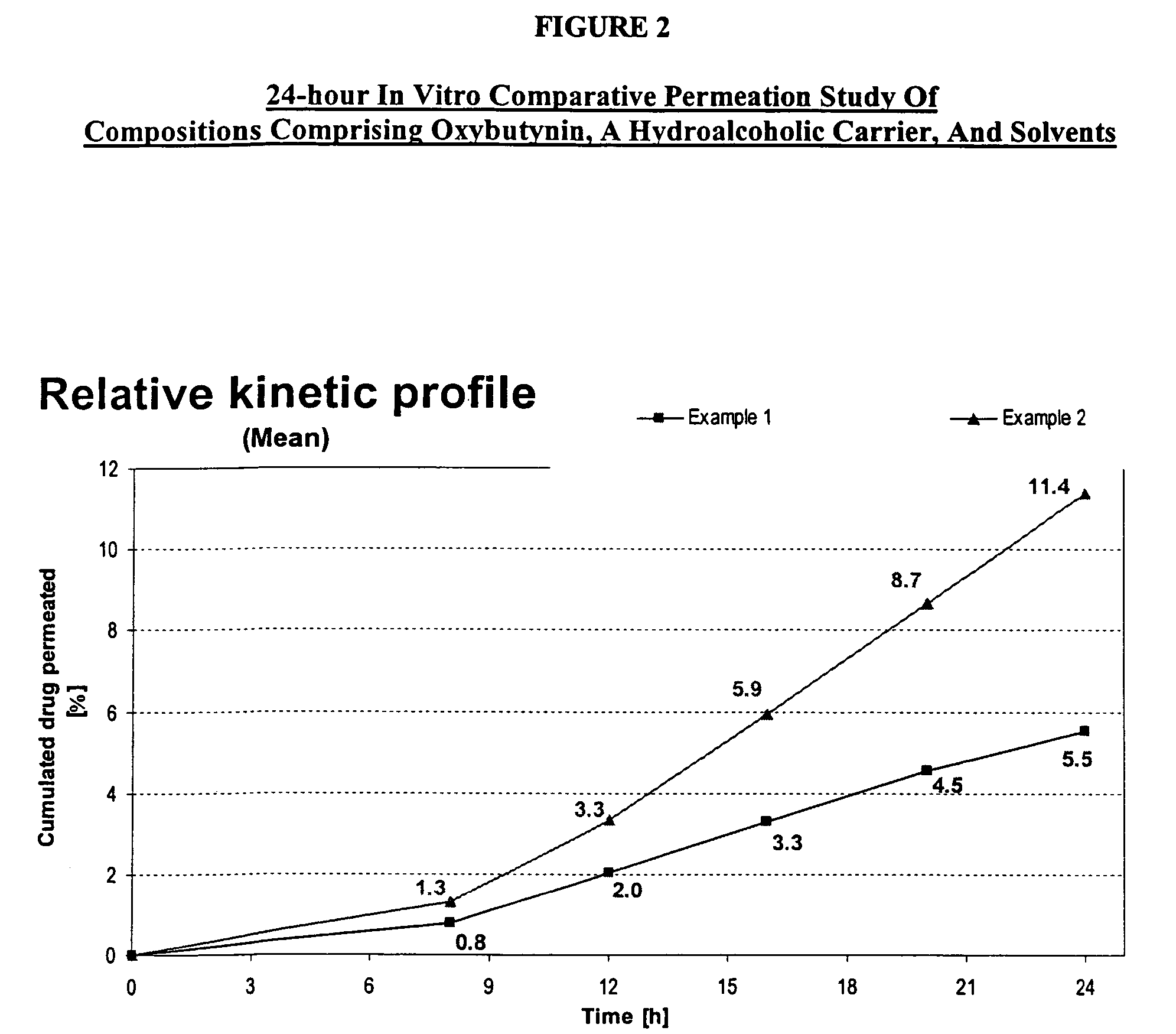
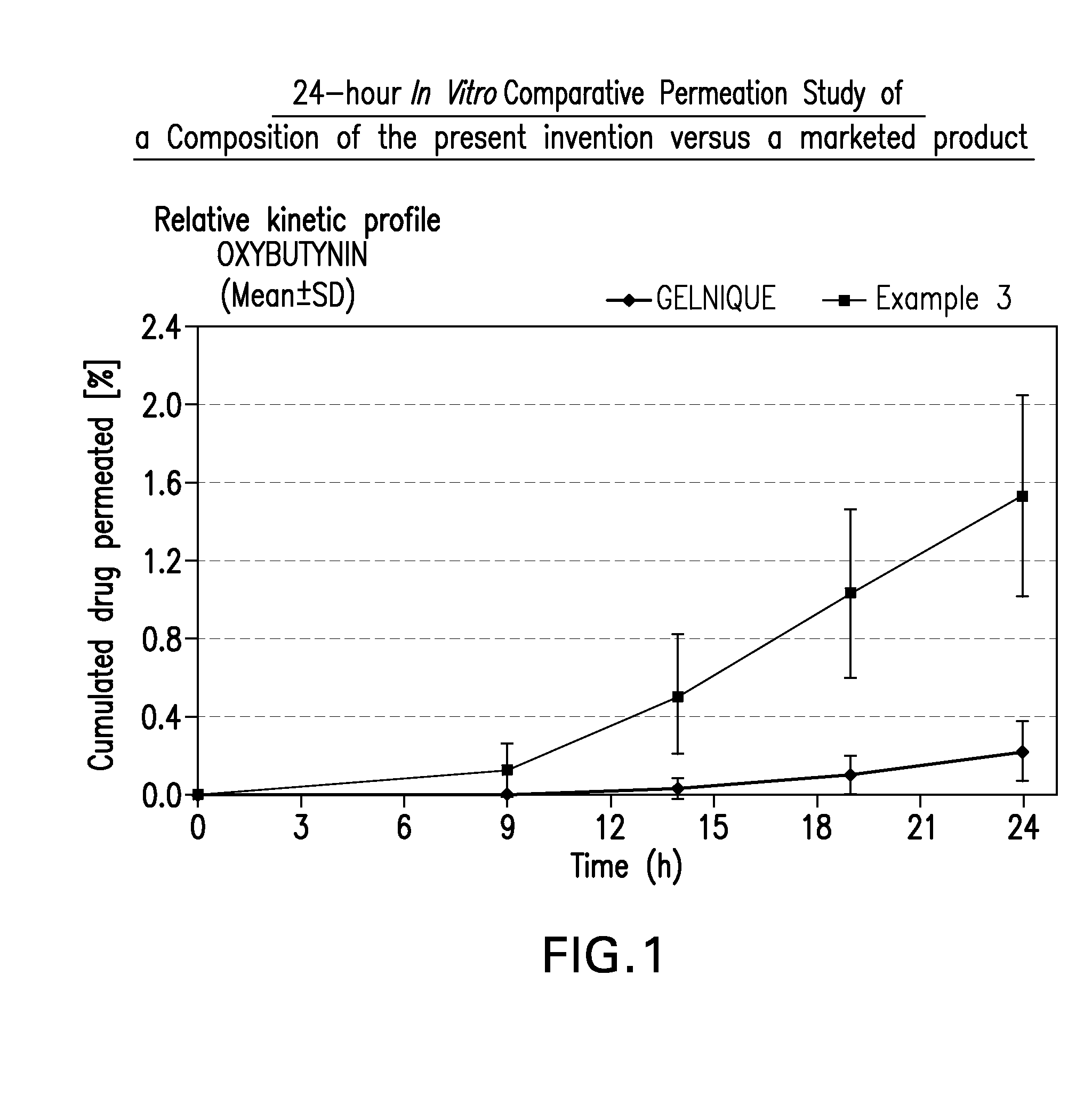
A topical gel formulation of oxybutynin is provided that exhibits enhanced bioavailability, minimized adverse side effects and skin irritations, and improved patient compliance compared to the existing oral and transdermal formulations of oxybutynin.
Owner:ORIENT EUROPHARMA
Oxybutynin-containing transdermal absorption preparation
InactiveUS20140271796A1Reduce skin irritationOrganic active ingredientsBiocideOxybutyninCholesterol derivative
A transdermal absorption preparation containing at least one drug selected from oxybutynin and pharmaceutically acceptable salts thereof and 0.05% by mass or more of a sterol selected from cholesterols, cholesterol derivatives and cholesterol analogs, relative to a total amount of the transdermal absorption preparation.
Owner:HISAMITSU PHARM CO INC
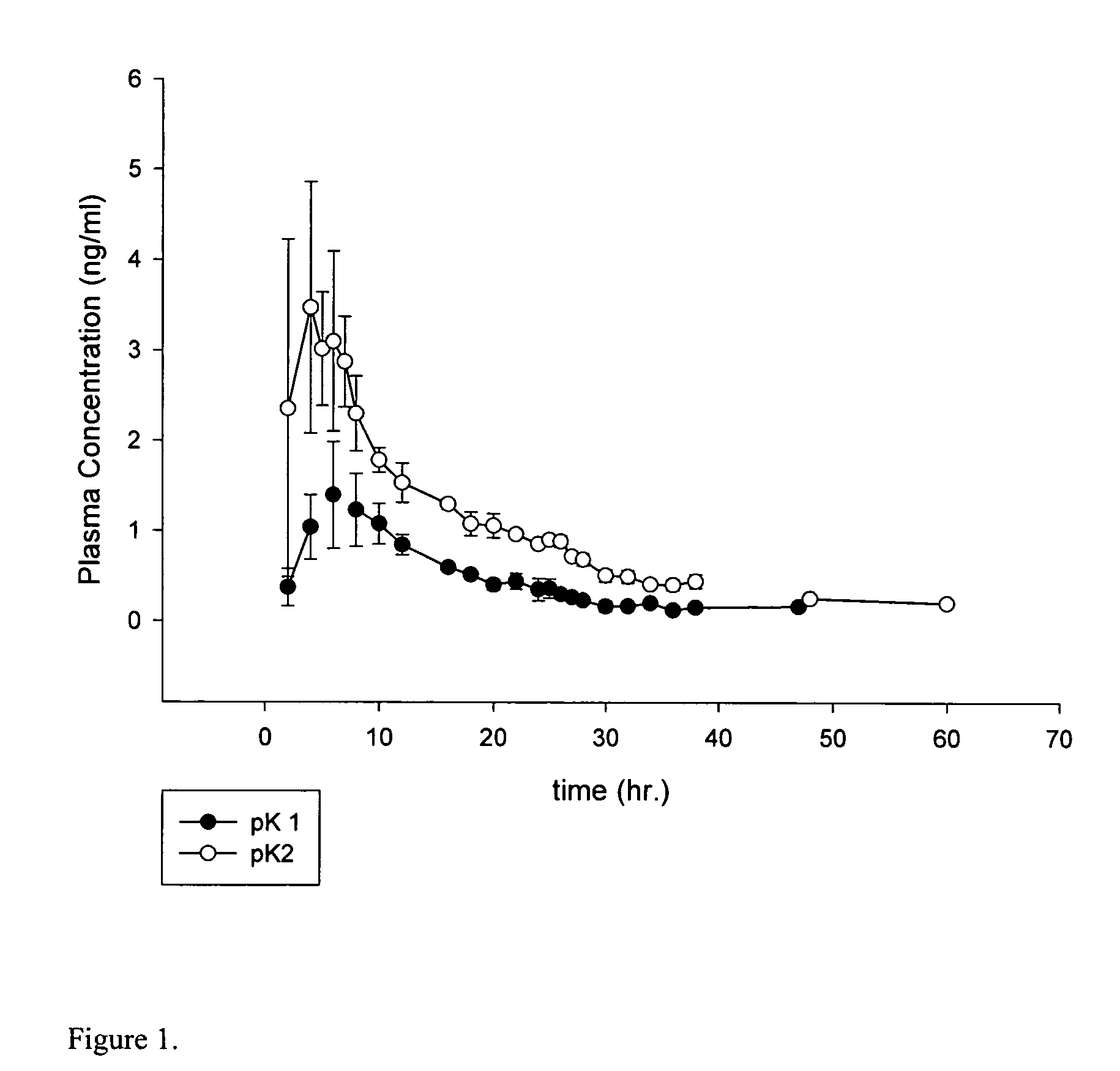
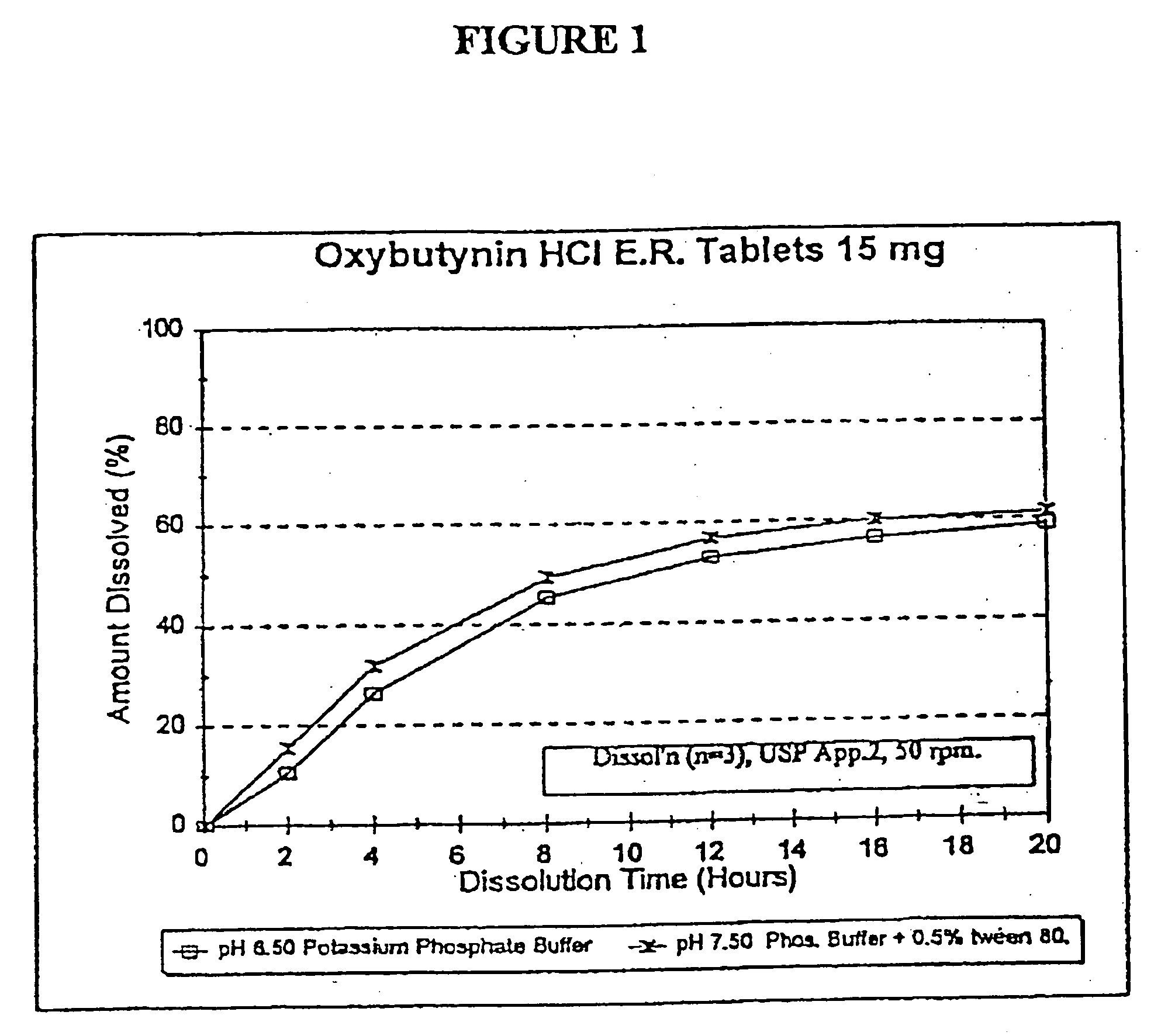
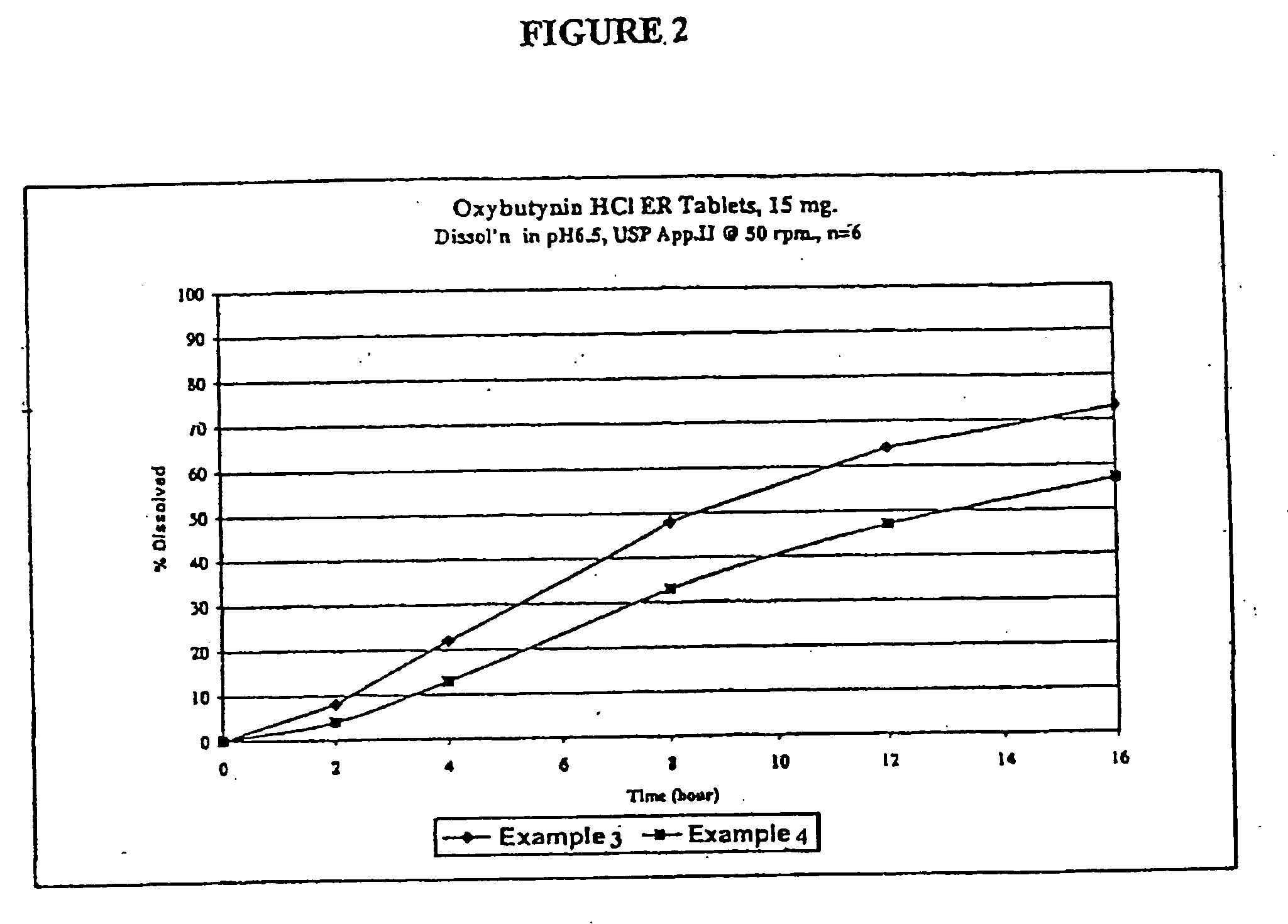
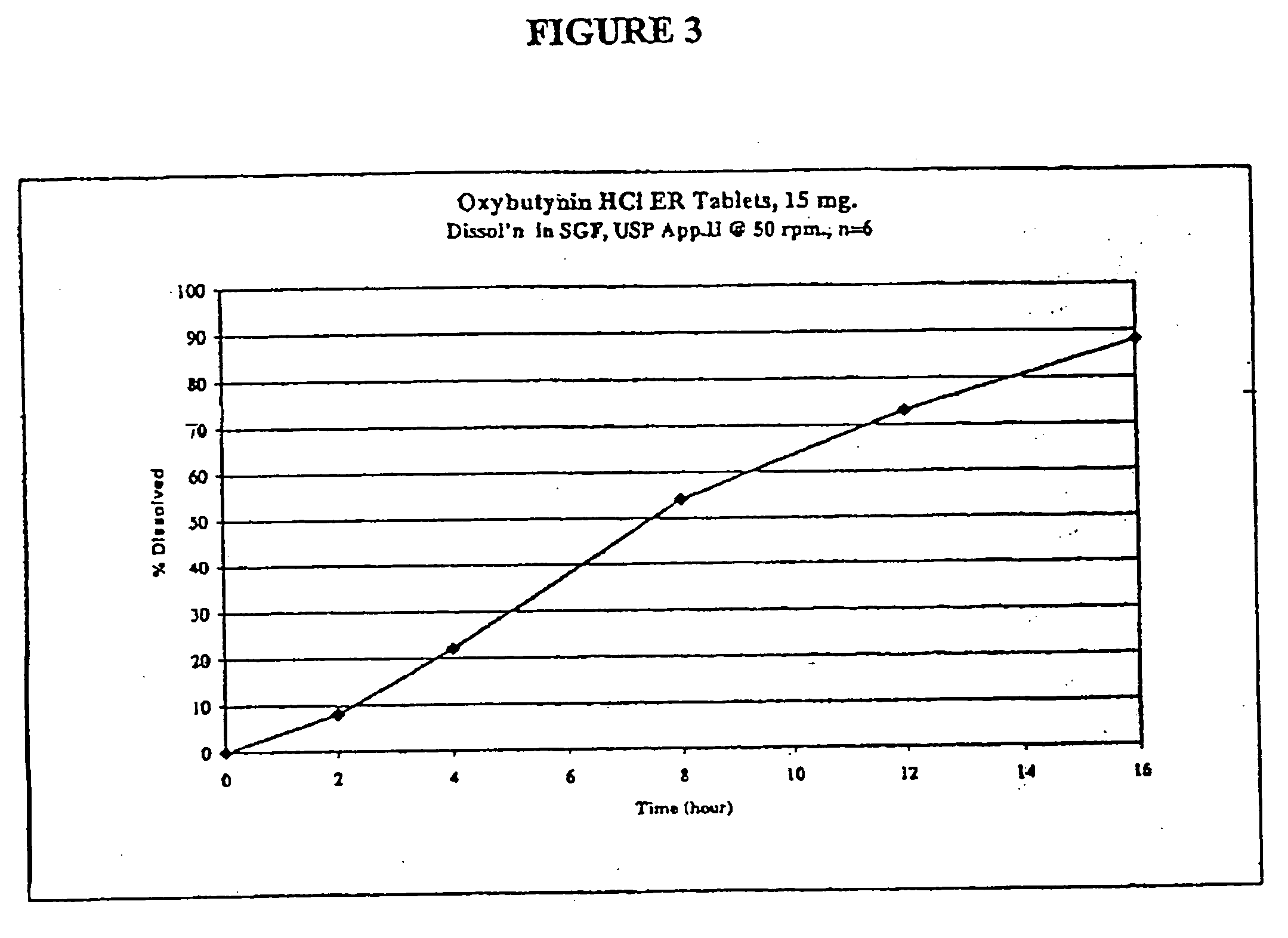
Extended release oxybutynin formulation
A controlled release oxybutynin tablet that employs a homogeneous core with less than 50% of a water swellable polymer and a semi-permeable membrane that surrounds the homogeneous core.
Owner:ANDRX LABS
Oxybutynin therapy
InactiveUS20010009995A1Reduce morbidityDecrease dry-mouthOrganic active ingredientsPharmaceutical delivery mechanismOxybutyninUrology
A composition comprising oxybutynin, a device comprising oxybutynin, and a method for administering oxybutynin are disclosed for oxybutynin therapy.
Owner:GUPTA SUNEEL K +2
Pasting agent
InactiveUS20060165763A1Improve the level ofOrganic active ingredientsAdhesive dressingsOxybutyninPolymer science
A patch comprising a backing layer and an adhesive layer disposed on the backing layer and compounded with an adhesive agent and oxybutynin and / or a pharmaceutically acceptable salt thereof, wherein the adhesive agent comprises an acrylic polymer substantially free of both carboxyl group and hydroxyl group and a rubber polymer, in which weight ratio of content of the acrylic polymer to content of the rubber polymer is from 1:4 to 1:19.
Owner:HISAMITSU PHARM CO INC
Transvaginal Delivery of Drugs
InactiveUS20110003000A1Reduce systemic levelEliminate side effectsPowder deliveryBiocideOxybutyninDisease
Drug delivery compositions which are suitable for transvaginal administration for the treatment of diseases and disorders of the urogenital tract are described. The drug delivery compositions are administered directly to the vagina using a convenient transvaginal application that deposits a very small volume of drug at the desired site for delivery. This method of administration reduces the systemic levels of the drugs and decreases the side effects which are associated with systemic administration. In the preferred embodiment, the compositions are in the form of a gel. The formulation is administered in volumes of less than or equal to 1 milliliter. In the preferred embodiment, the composition contains an antimuscarinc drug, such as oxybutynin.
Owner:FEMMEPHARMA HLDG CO INC
Oxybutynin transdermal therapeutic system combination
ActiveUS20160243070A1Useful in treatmentLarge doseNervous disorderEster active ingredientsOxybutyninCholinesterase inhibition
There is described a pharmaceutical combination comprising oxybutynin or a pharmaceutically acceptable addition salt thereof, in a transdermal therapeutic system, and an acetylcholinesterase inhibitor, useful for safely treating hypocholinergic disorders of the central nervous system such as Alzheimer type dementia. In this combination, the acetylcholinesterase inhibitor (AChEI) is present at a dose that is higher than the maximal recommended dose, per unit form. In particular, the transdermal therapeutic system comprising oxybutynin is in combination with rivastigmine in a transdermal formulation or oral form.
Owner:CHASE PHARMA CORP
Triacetin as a transdermal penetration enhancer
A composition and method for enhancing transdermal penetration of a basic drug are described. The composition comprises a matrix patch comprising an effective amount of a basic drug, preferably having a pKa of about 8.0 or greater, an effective amount of penetration enhancer consisting essentially of triacetin, and a polymer later preferably comprising a pressure-sensitive adhesive. A preferred basic drug is oxybutynin and acid addition salts thereof. The method for enhancing transdermal penetration comprises applying the matrix patch to a selected area of skin.
Owner:WATSON LAB INC
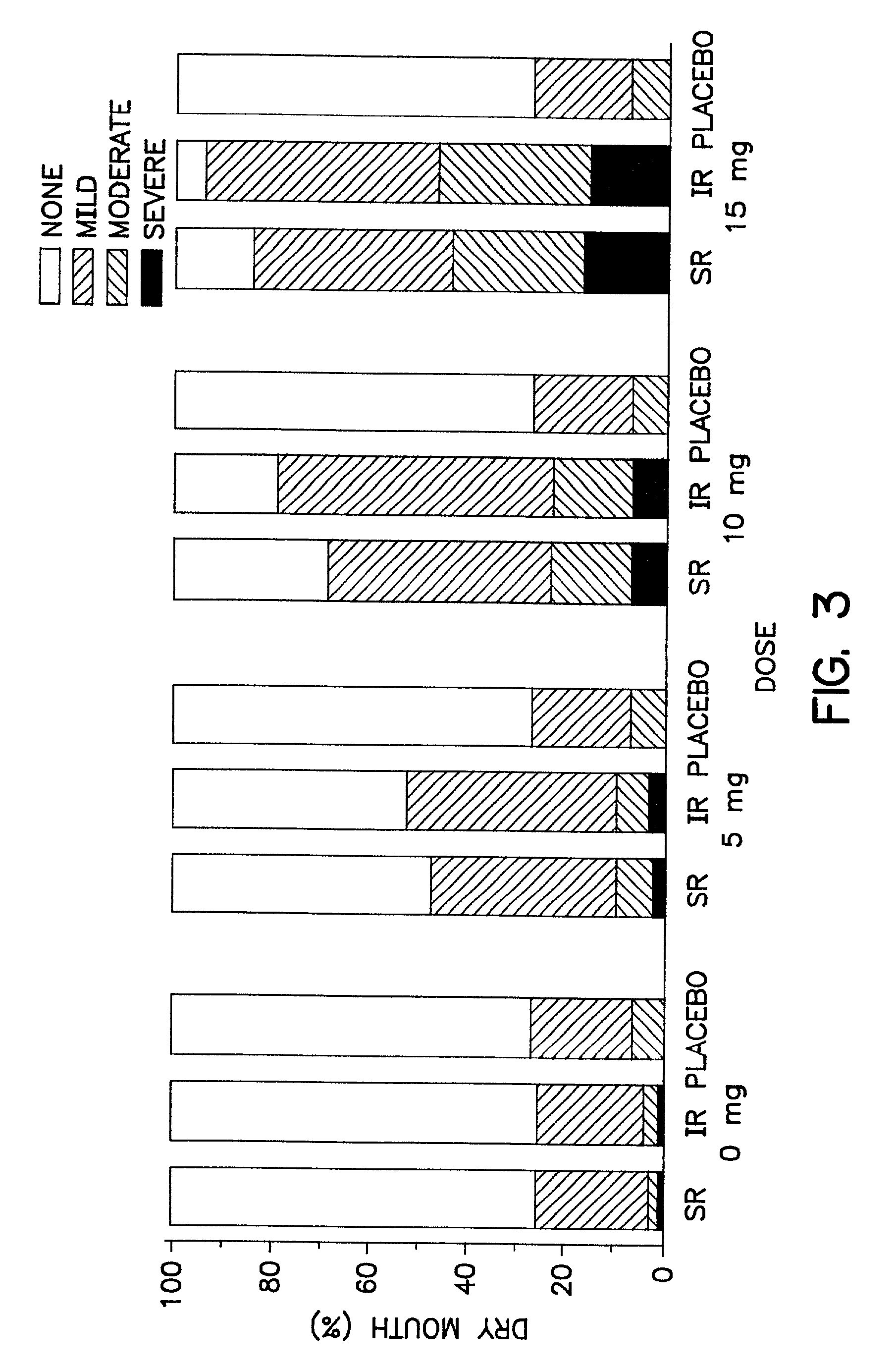
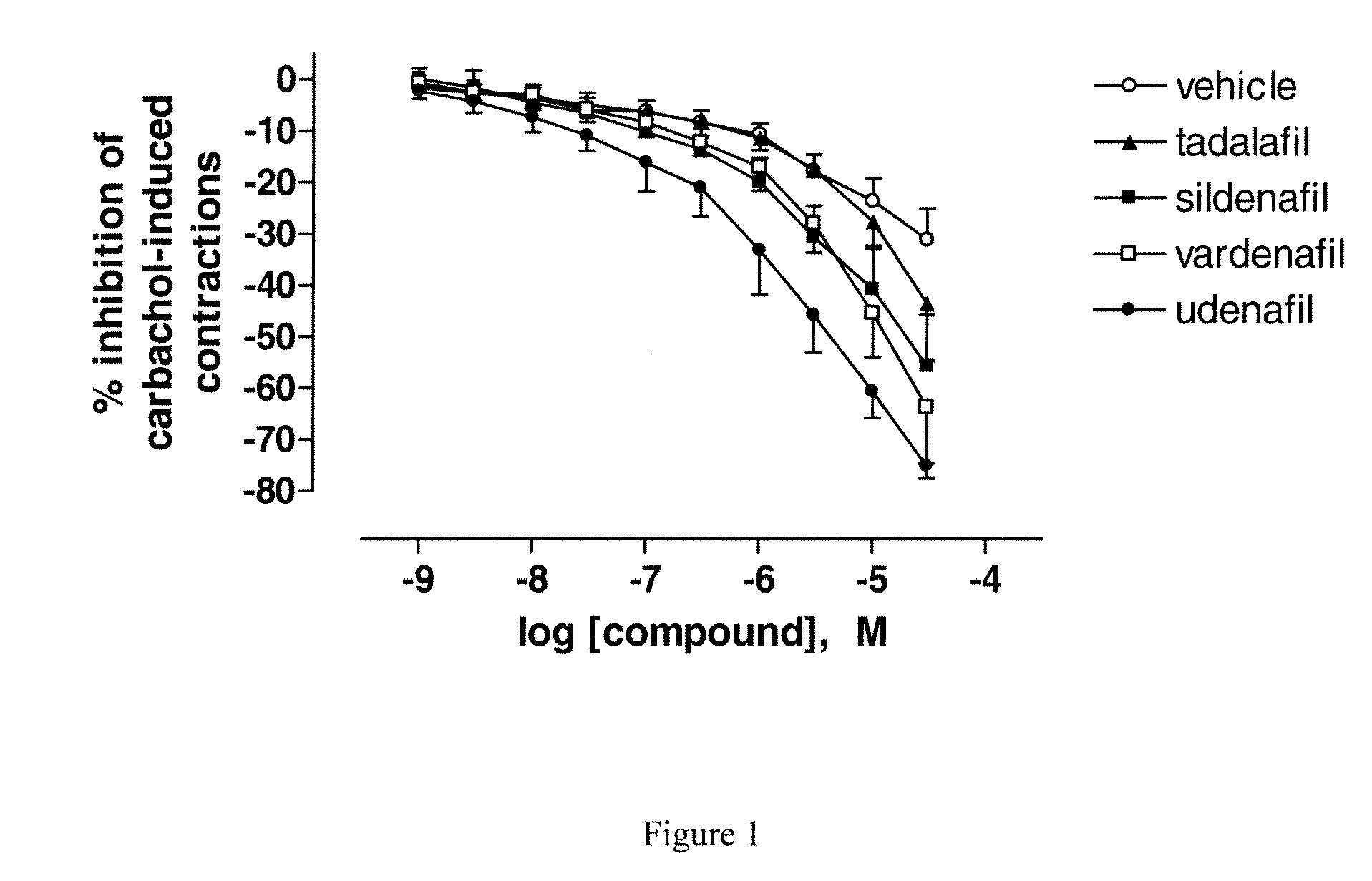
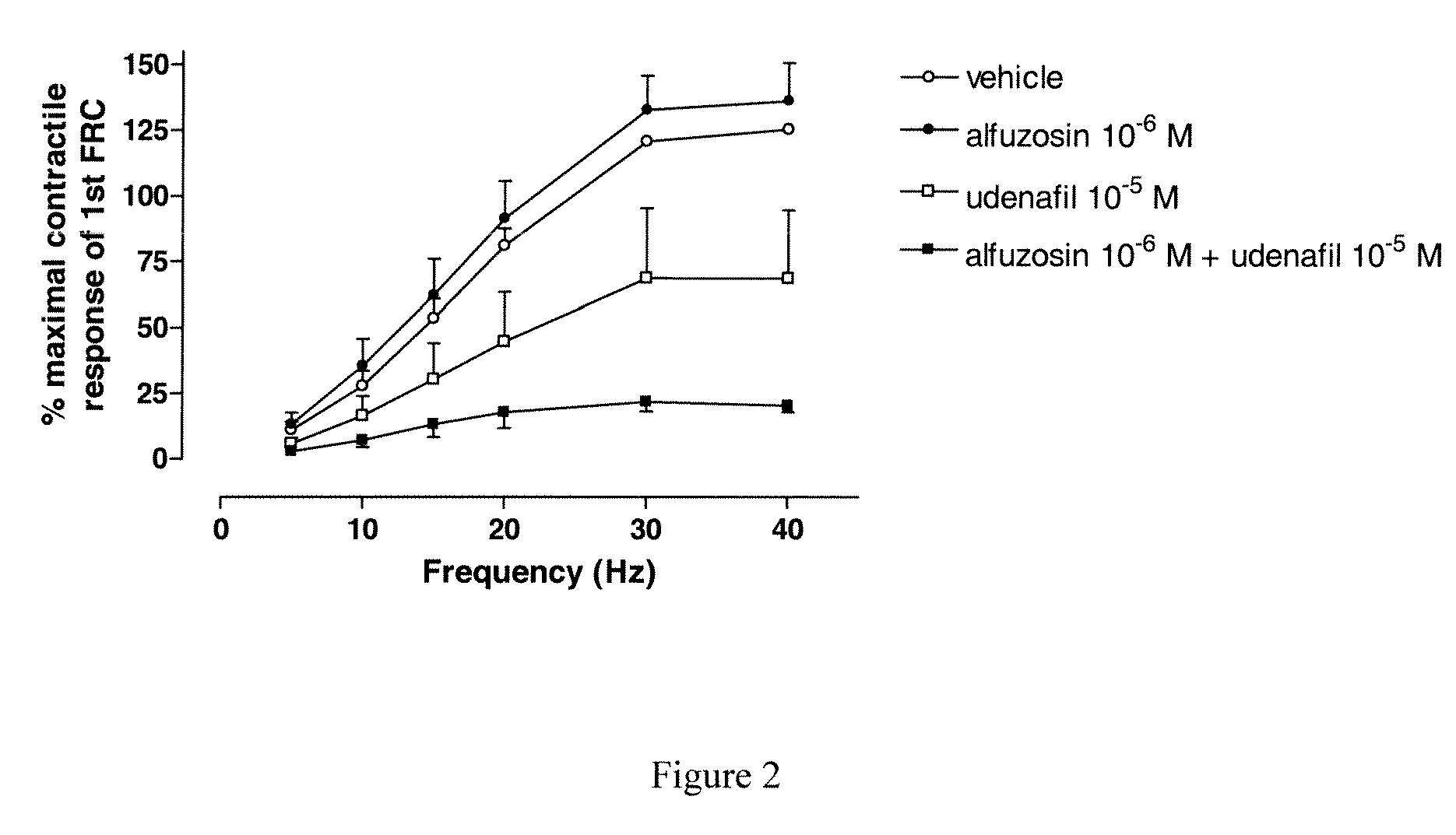
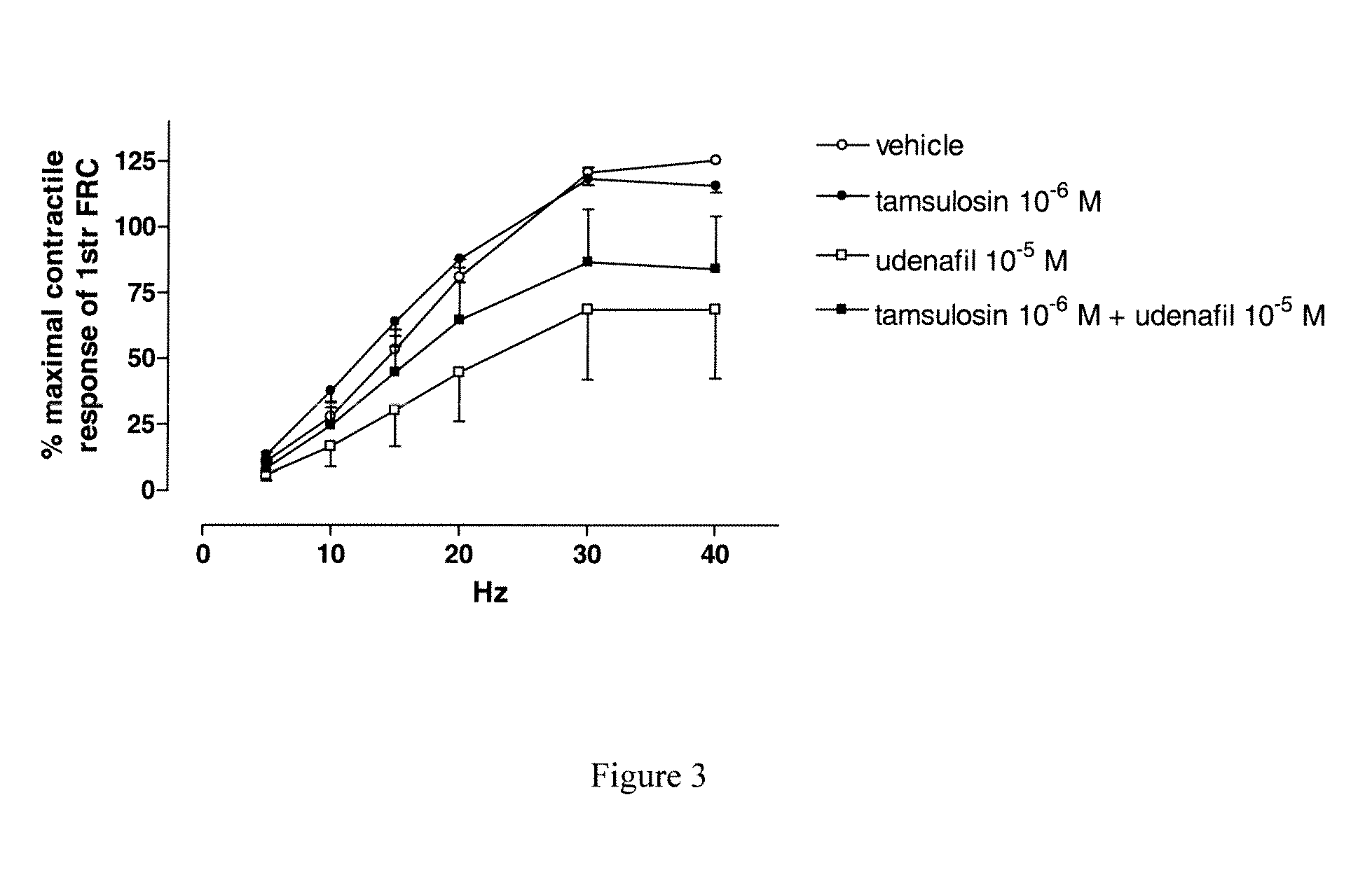
Use of a Combination of Udenafil and Alfuzosin or Oxybutynin for the Treatment of Overactive Bladder
The invention relates to a specific combination of two active agents: udenafil and one of alfuzosin and oxybutynin and its use for the treatment of overactive bladder.
Owner:PELVIPHARM
Combination of Selected Opioids with Muscarine Antagonists for Treating Urinary Incontinence
Active compound combinations of compounds of group A, particularly opioids such as (+)-(2R,3R)-1-dimethylamino-3-(3-methoxy-phenyl)-2-methyl-pentan-3-ol or a salt thereof with a physiologically tolerated acid, and compounds of group B, particularly anti-muscarine agents such as oxybutynin or a salt thereof with a physiologically tolerated acid suitable for treatment of an increased urge to urinate or urinary incontinence. Related pharmaceutical formulations and methods of treatment of an increased urge to urinate or urinary incontinence are also provided.
Owner:GRUNENTHAL GMBH
Method for the Management of Incontinence
InactiveUS20080021102A1Reduce conversionLessen the circulating desoxy metaboliteBiocidePill deliveryOxybutyninDrug indicated
A composition and a dosage form are disclosed comprising oxybutynin alone / or accompanied by another drug indicated for therapy. A method is disclosed for administering oxybutynin alone / or accompanied by a different drug or for administering oxybutynin and a different drug according to a therapeutic program for the management of incontinence alone, and for other therapy.
Owner:ALZA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com