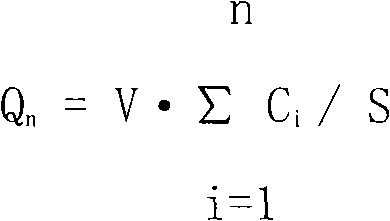Patents
Literature
61 results about "Tolterodine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
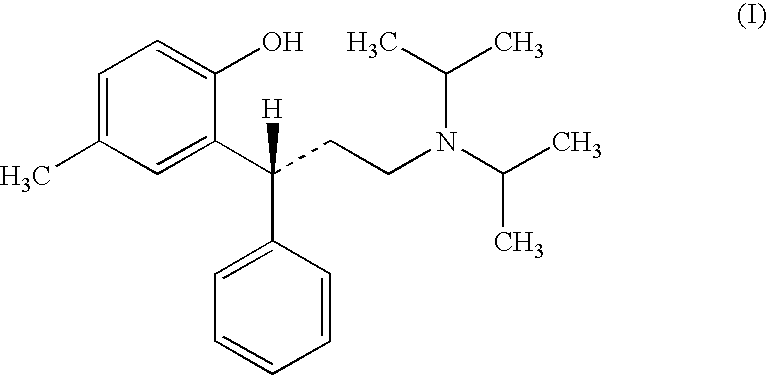
This medication is used to treat an overactive bladder.
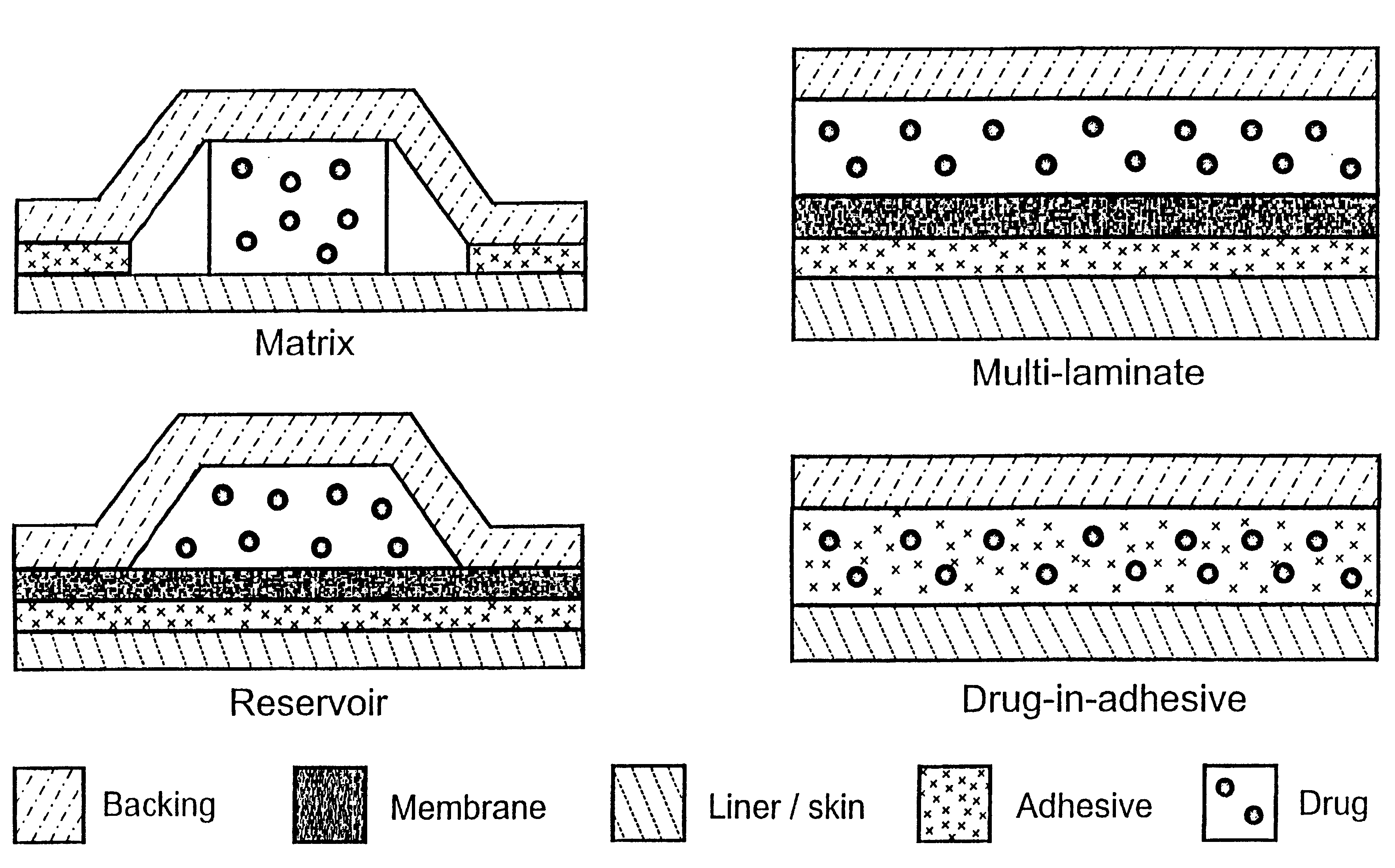
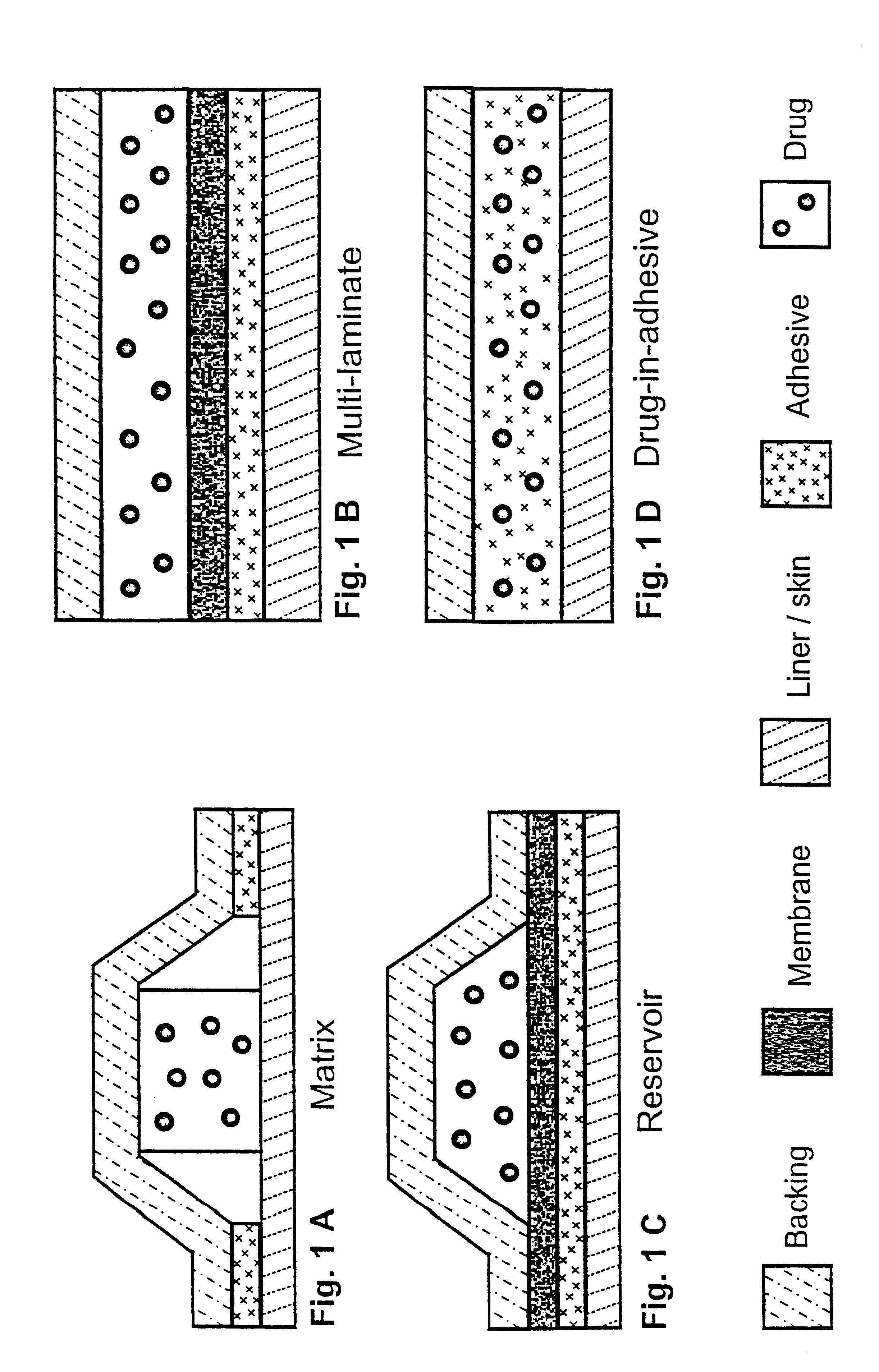
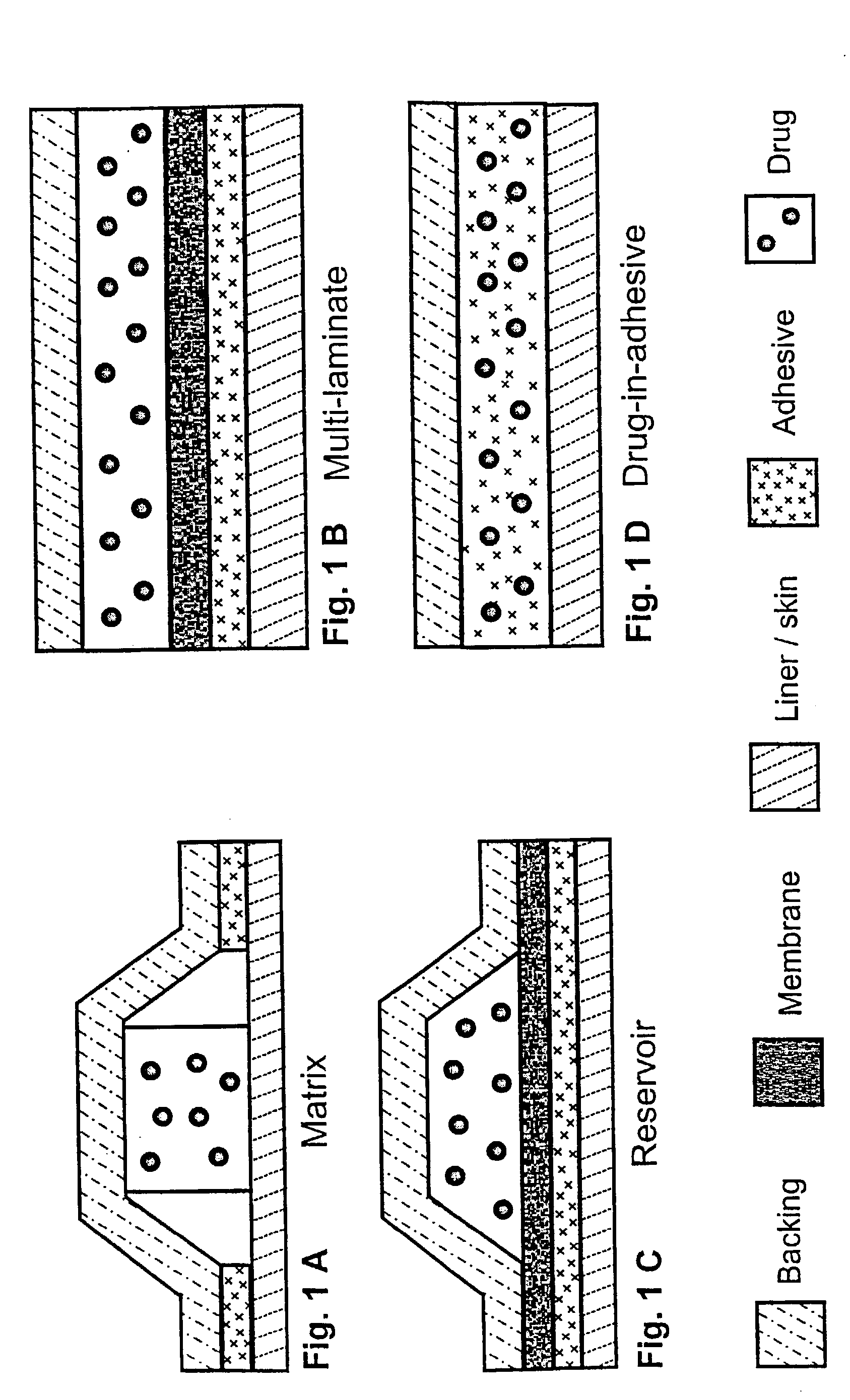
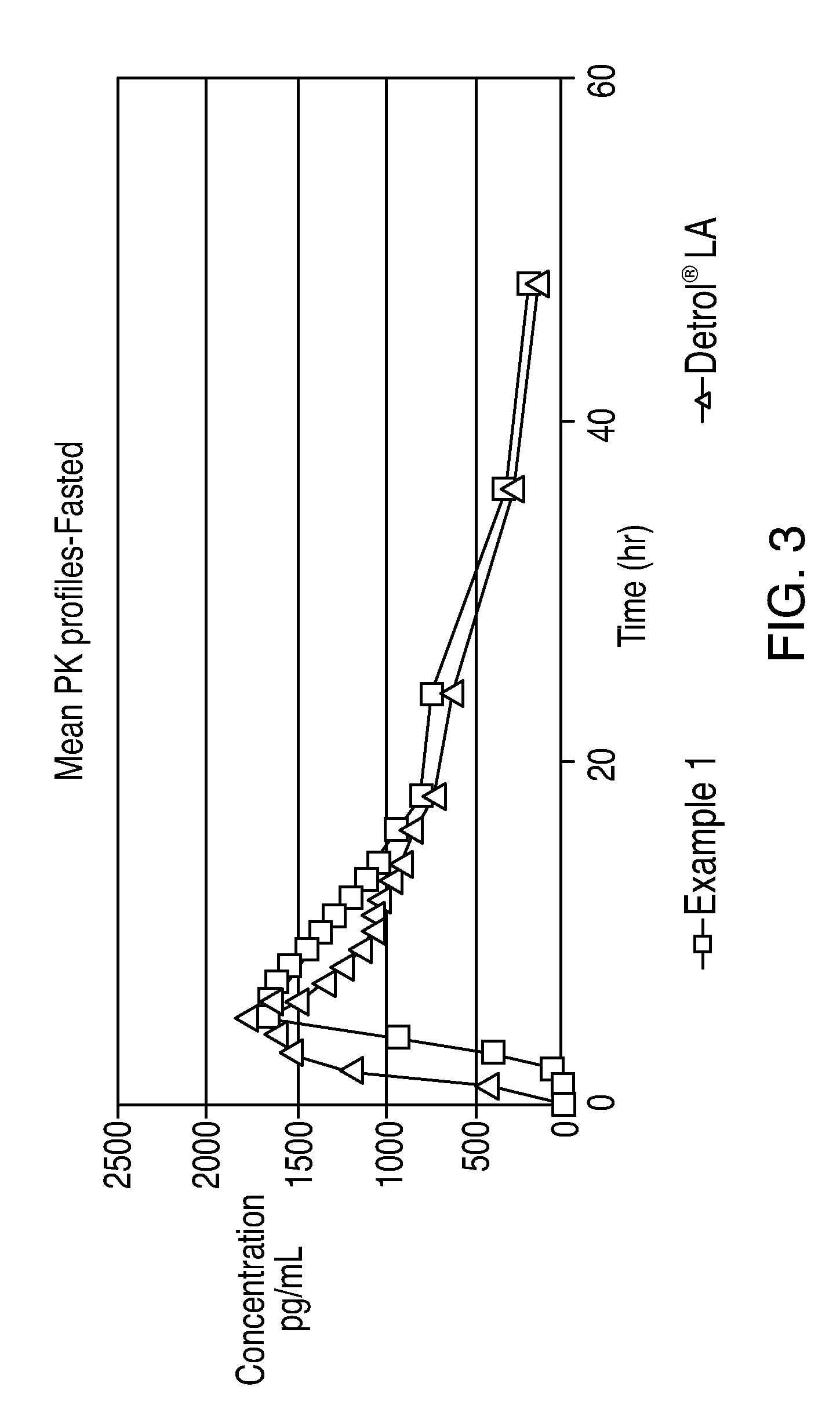
Transdermally administered tolterodine as anti-muscarinic agent for the treatment of overactive bladder
InactiveUS6517864B1Achieve effectClinical efficacyOrganic active ingredientsAerosol deliveryMuscarinic antagonistMetabolite
Device for transdermal administration of tolterodine, optionally encompassing salts, prodrugs and metabolites thereof, optionally together with pharmaceutically acceptable carrier(s) to a human being or an animal in order to achieve an effect against overactive bladder. Use of a compound having an effect against overactive bladder comprising tolterodine, optionally encompassing salts, prodrugs and metabolites thereof, and optionally together with pharmaceutically acceptable carrier(s), for the manufacture of a composition to be administered transdermally for achieving an effect against overactive bladder. Method for achieving an effect against overactive bladder in a living body by transdermal administration of a compound comprising tolterodine, optionally encompassing salts, prodrugs and metabolites thereof, and optionally together with pharmaceutically acceptable carrier(s).
Owner:MCNEIL AB +1
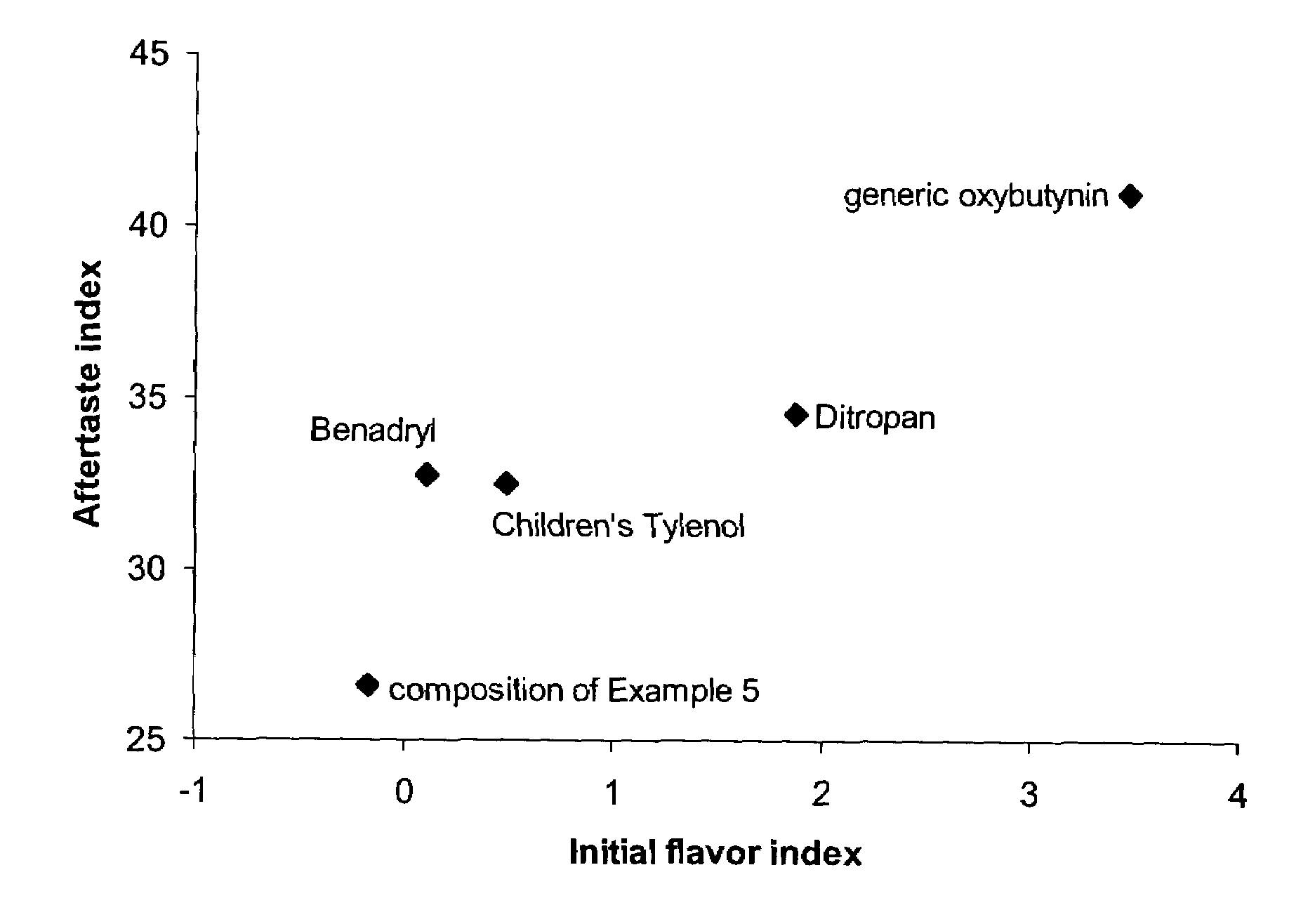
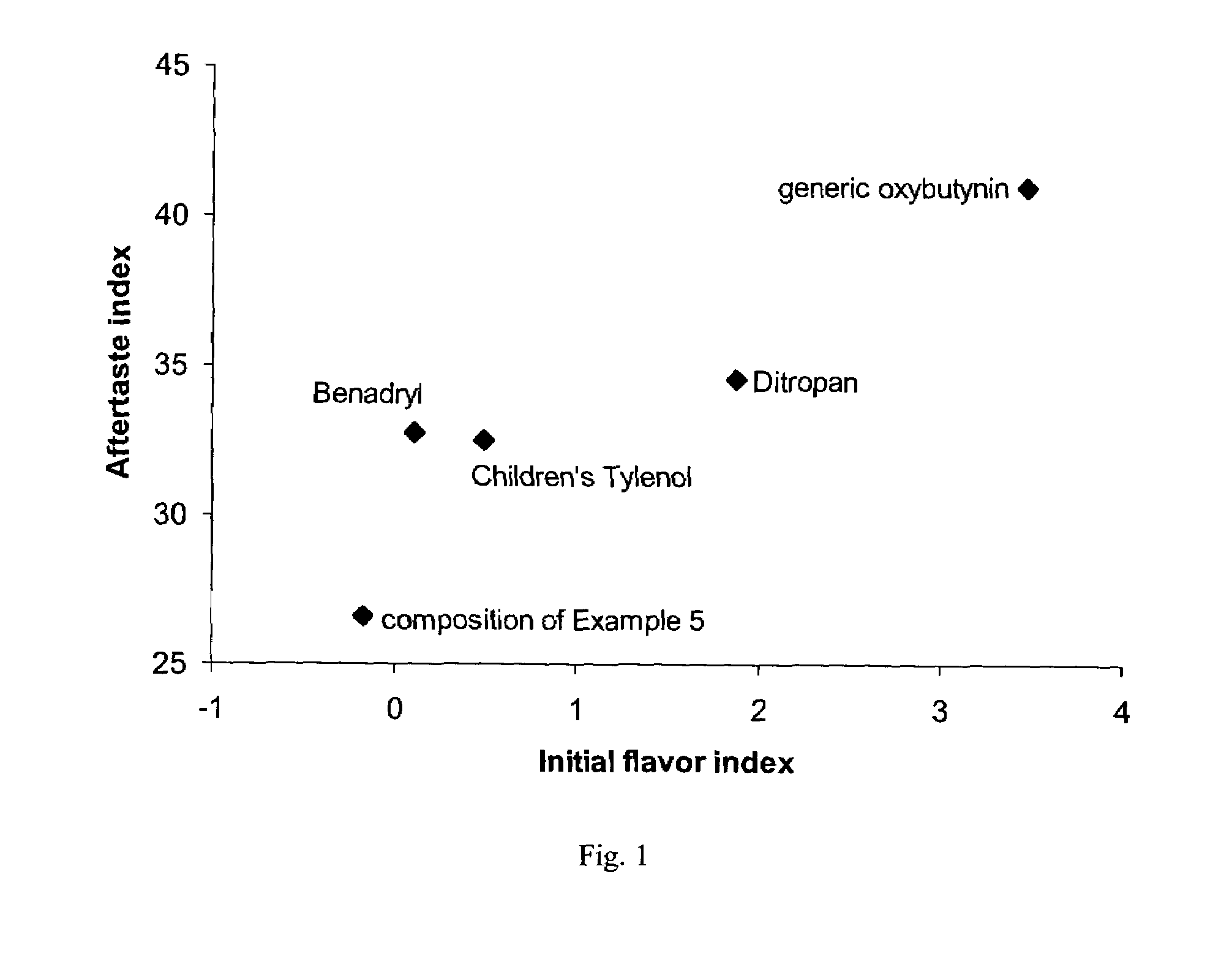
Methods for the Treatment of Hyperhidrosis
The present invention relates in general to methods of treating sweating disorders and in particular to topical compositions for the treatment of hyperhidrosis. The methods of the present invention relate to the topical application of a composition comprising a therapeutically effective amount of oxybutynin, tolterodine or a substituted benzamide such as sulpiride.
Owner:ZINGER MENNI MENASHE
Transdermally administered tolterodine as anti-muscarinic agent for the treatment of overactive bladder
InactiveUS20030124179A1Clinical efficacyReduce riskBiocideOrganic active ingredientsMuscarinic antagonistNasal cavity
The present invention is drawn to set of formulations of at least one compound selected from tolterodine, salts thereof, prodrugs thereof and / or metabolites thereof, wherein in the set of formulations contains at least one device for transdermal administration and at least one formulation for oral, sublingual, buccal, nasal, pulmonary, rectal and / or other transmucosal administration, in order to achieve an effect against overactive bladder and / or symptoms associated with this condition. The present invention is further drawn to methods of treating an overactive bladder with the formulations.
Owner:MCNEIL AB
Oral liquid tolterodine composition
A pharmaceutical composition in a form of an orally deliverable liquid comprises water having in solution therein a pharmaceutically acceptable water-soluble salt of a tolterodine related compound at a therapeutically effective concentration in the composition. The composition has a pH of about 2 to about 6 and further comprises a sweetening agent and an antimicrobial agent at a concentration that is antimicrobially effective at the pH of the composition. The composition is useful for treating a muscarinic receptor mediated disorder, more particularly overactive bladder, in a subject by orally administering to the subject a therapeutically effective amount of the composition.
Owner:PHARMACIA CORP
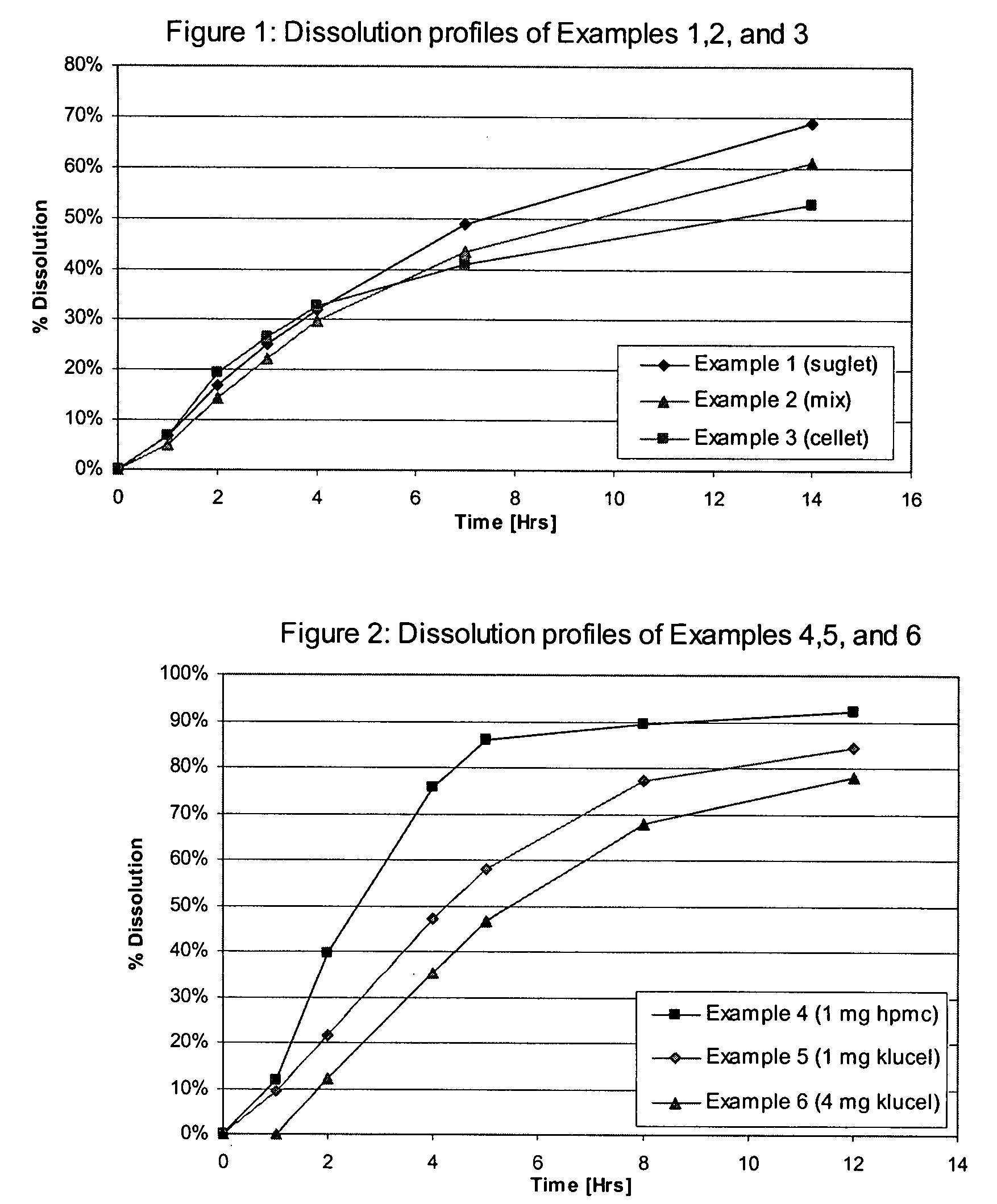
Tolterodine bead
A controlled release tolterodine bead is formed having a microcrystalline cellulose core, a PVP-containing water soluble coating, a tolterodine drug layer, and a controlled release layer.
Owner:SYNTHON BV
Pharmaceutical formulations for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a plurality of first beads each comprising: a core; a first layer comprising pilocarpine or a pharmaceutically acceptable salt thereof; and a second layer comprising a first polymer. Also disclosed are pharmaceutical compositions comprising a plurality of second beads each comprising: a core; and a first layer comprising tolterodine or a pharmaceutically acceptable salt thereof. Further disclosed are pharmaceutical formulations comprising: a) a plurality of the first beads; b) a plurality of the second beads; or c) a plurality of the first beads and a plurality of the second beads.
Owner:THERAVIDA INC
Skin irritation suppressant and transdermal preparation
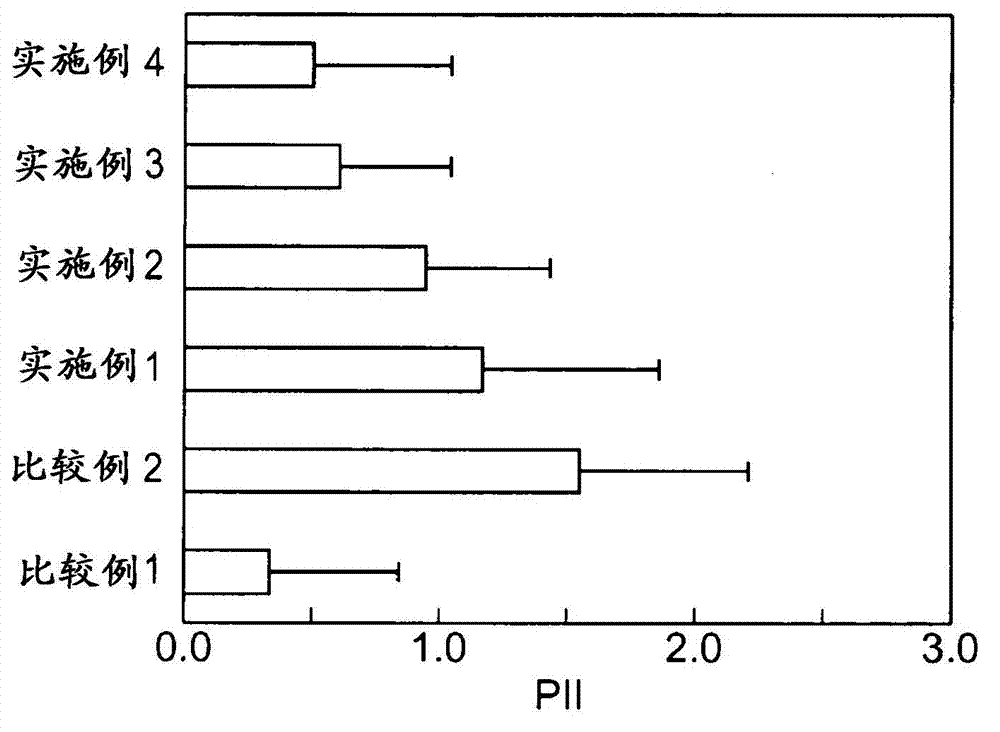
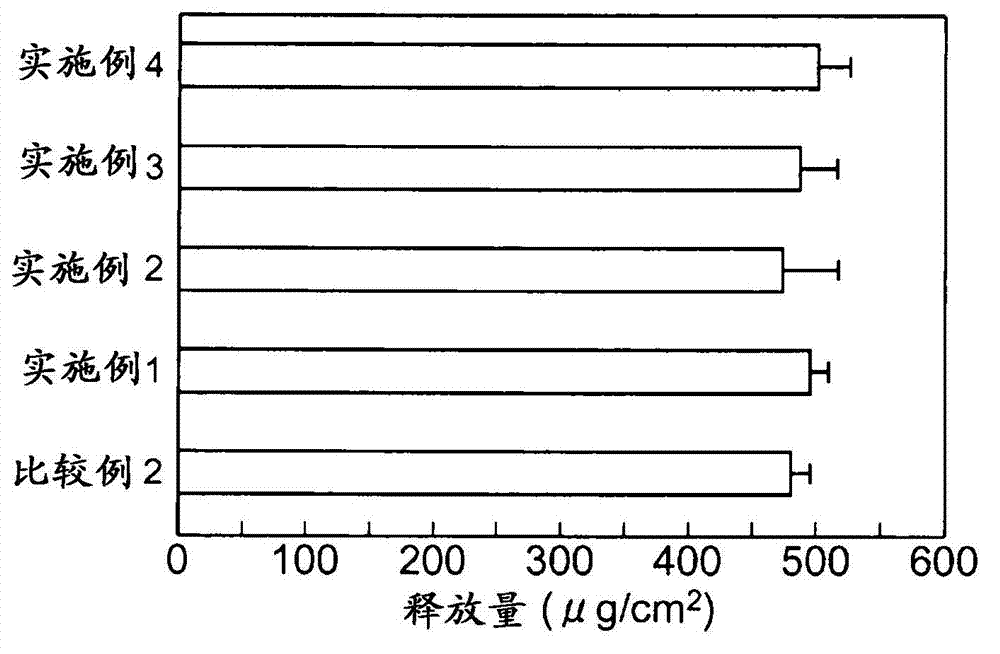
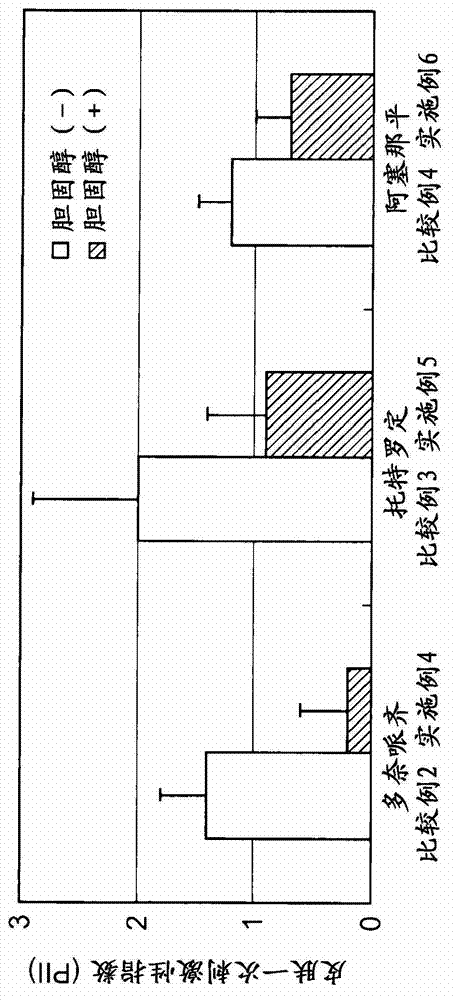
InactiveUS20130053357A1Sufficient reduction effectReduce skin effectOrganic active ingredientsBiocideCholesterol derivativeCitalopram
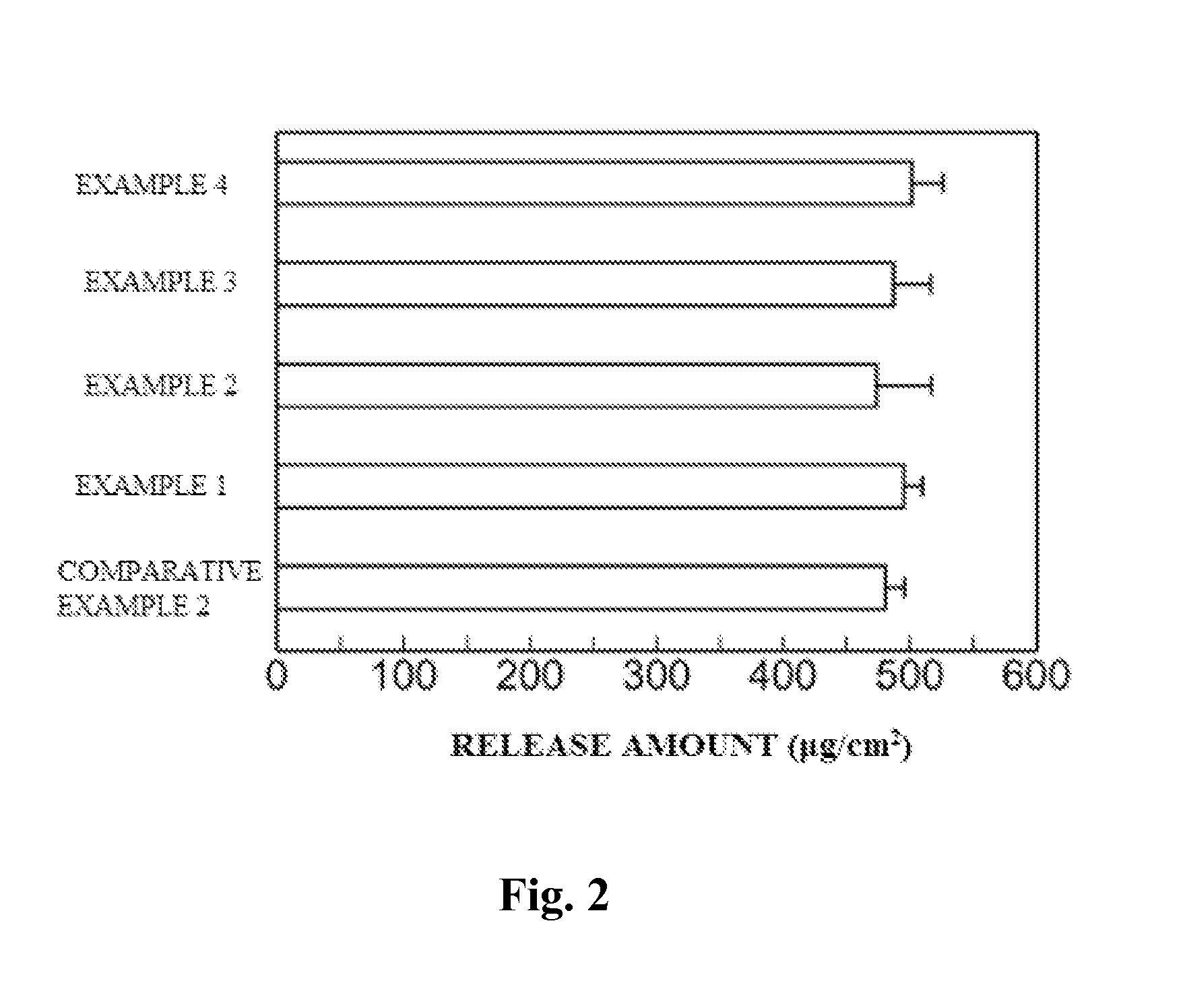
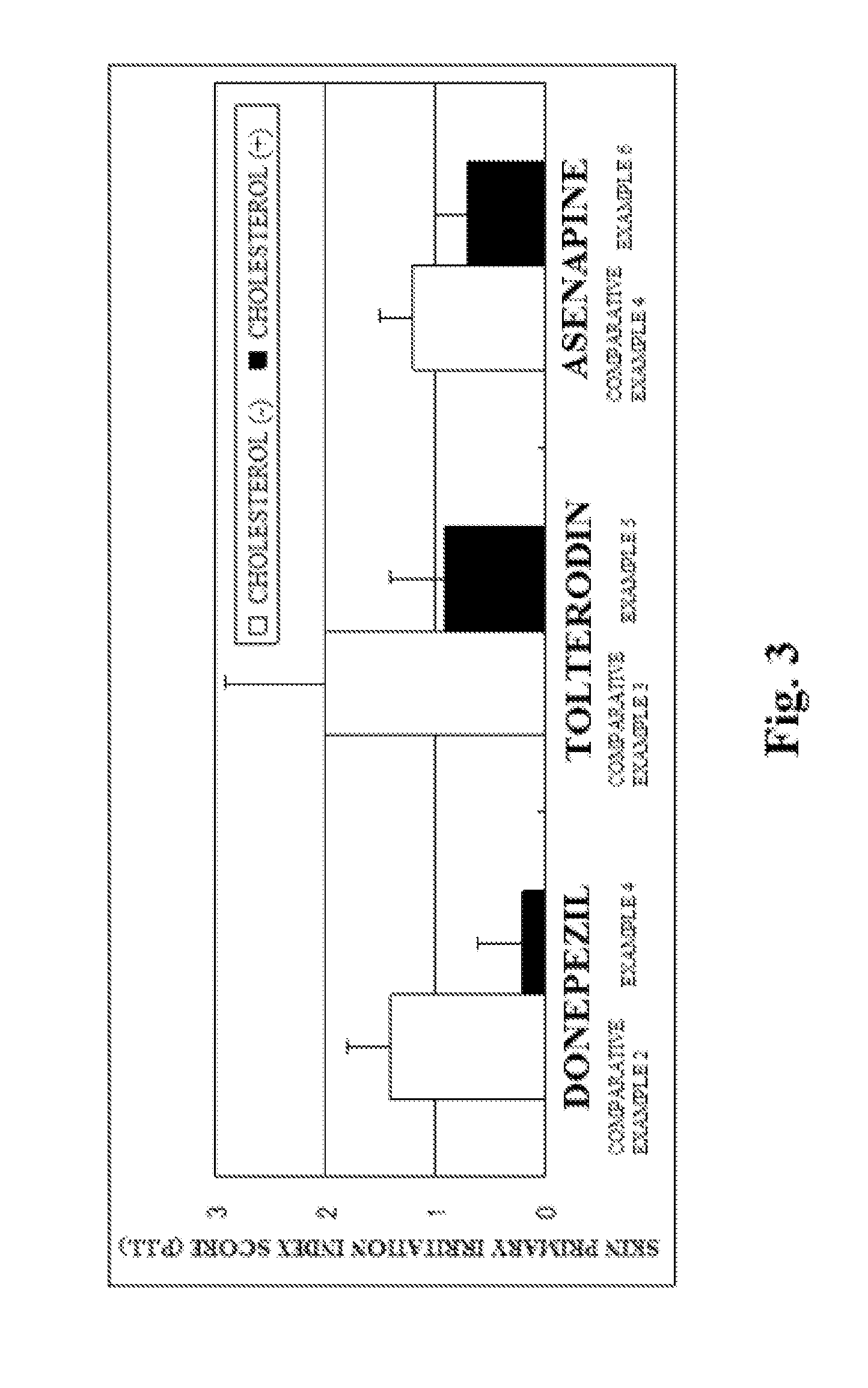
Provided is a skin irritation suppressant for transdermal preparations, having a sufficient reduction effect of skin irritation due to a drug. Also provided is a transdermal preparation comprising the skin irritation suppressant. One embodiment of the invention is a skin irritation suppressant for suppressing the skin irritation due to a drug and a pharmaceutical ingredient to be used in a transdermal preparation other than the drug, the skin irritation suppressant comprising a sterol compound selected from the group consisting of cholesterol, cholesterol derivatives and cholesterol analogs, and the drug is one or more basic drugs selected from the group consisting of tolterodine, asenapine, bisoprolol, risperidone, nicotine and citalopram, and their pharmaceutically acceptable salts.
Owner:HISAMITSU PHARM CO INC
Method for treating urinary disorders
The present invention relates to a method, preferable an oral method, for treating urinary disorders, such as unstable or overactive bladder, while minimizing the occurrences of dry mouth, dyspepsia and reduced stream of tears. The methods of the present invention comprise orally administering to a mammal, preferably a human, a pharmaceutically effective dose of an antimuscarinic agent, such as tolterodine, when needed, whereby a symptomatic relief of urgency and / or frequency is achieved.
Owner:PHARMACIA CORP
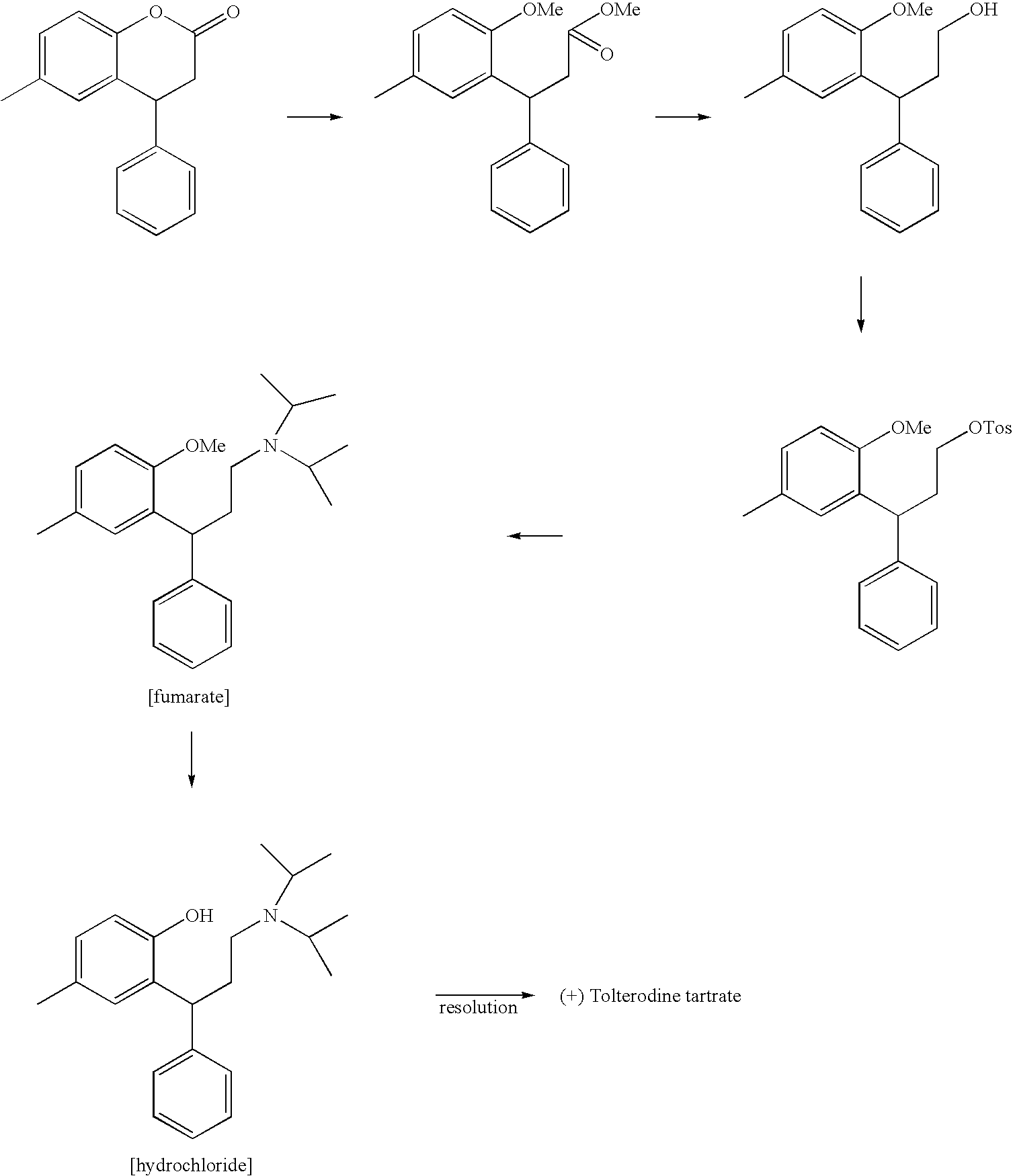
Method for preparing Tolterodine and tartrate
ActiveCN1626504AGood curative effectMild reaction conditionsOrganic compound preparationAmino-hyroxy compound preparationAlcoholOrganic solvent
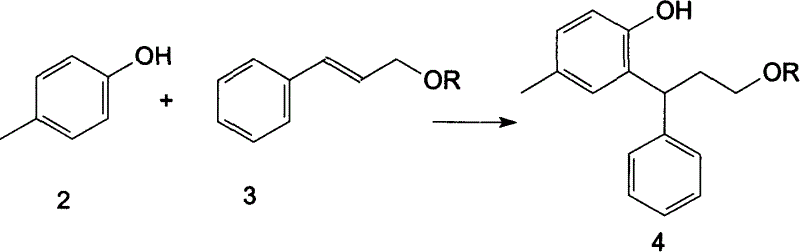
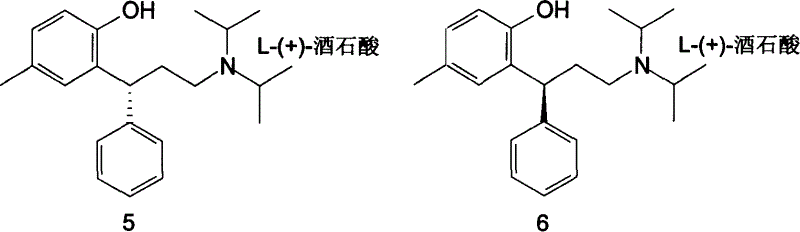
A process for preparing Tuoteluoding and its tartrate includes condensation reaction between anti-styryl alcohol ester and methylphenol, direct alkalizing, extracting in organic solvent, vacuum distilling to remove solvent, purifying to obtain Tuoteluoding, condensation reaction on diisopropylamine, reacting on L-(+) tartaric acid to become salt, splitting it into (L-(+)-tartrate-R-Tuoteluoding) and (L-(+)-tartrate-S-Tuoteluoding), reaction of L-(+)-tartrate-S-Tuoteluoding on alkali, butyl lithium, or magnesium isopropyl bromide, and reacting no tartaric acid to obtain L-(+)-tartrate-R-Tuoteluoding.
Owner:LUNAN PHARMA GROUP CORPORATION
Derivatives of 3,3-diphenylpropylamines
Owner:UCB SA
Derivatives of 3,3-diphenylpropylamines
InactiveUS7230030B2Reduce absorptionUnfavourable metabolismBiocideNervous disorderSmooth muscleOxybutynin
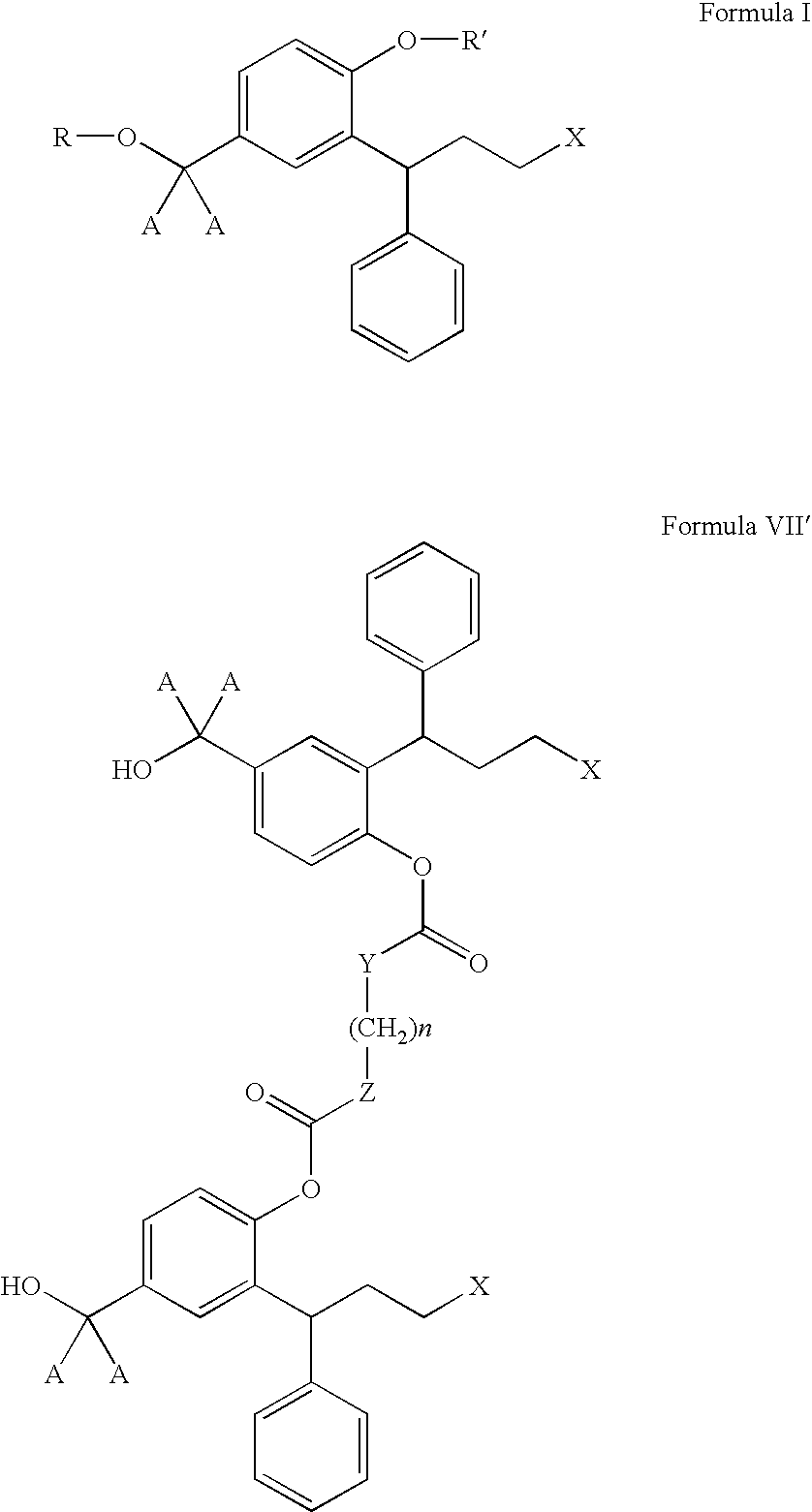
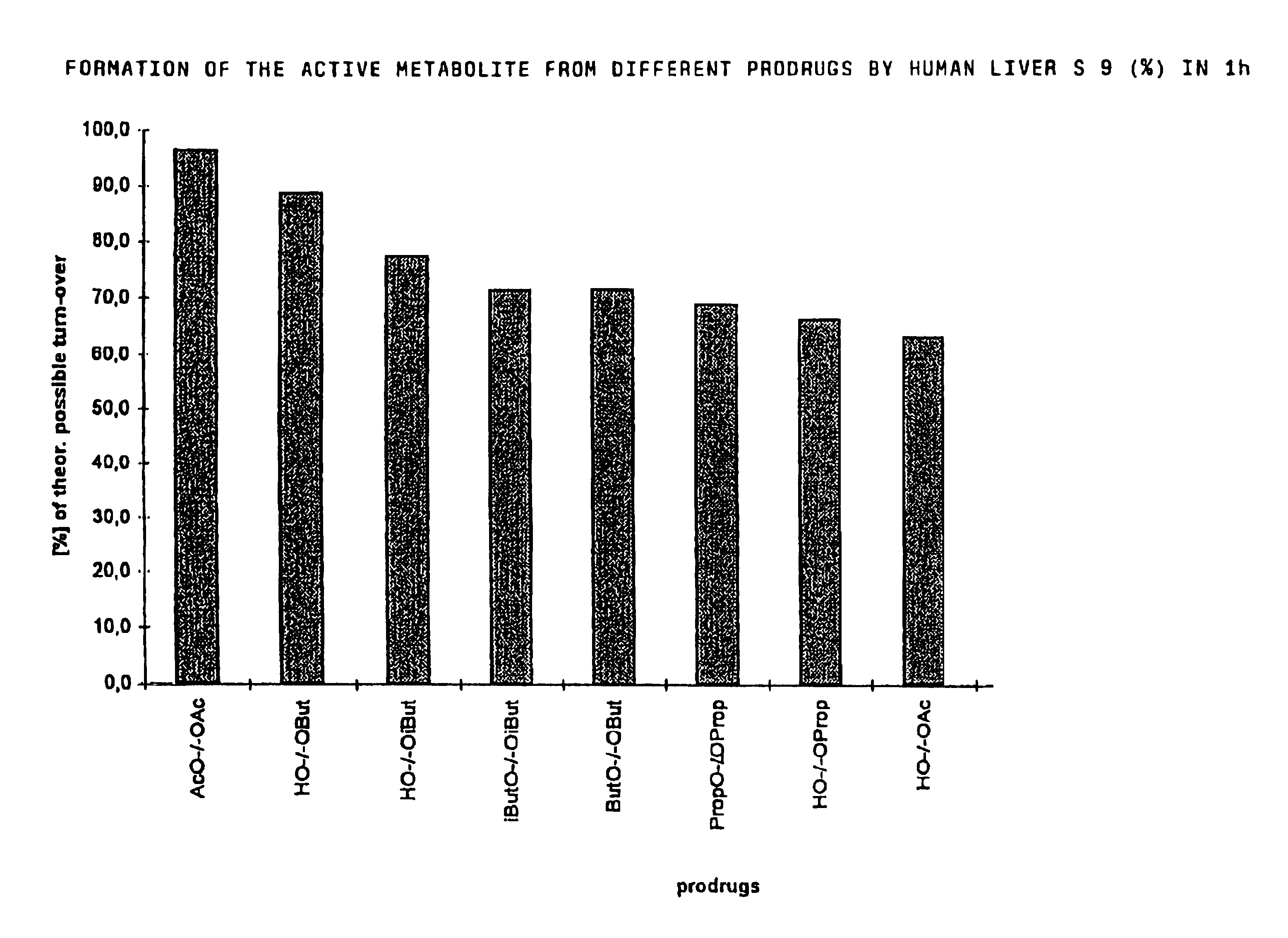
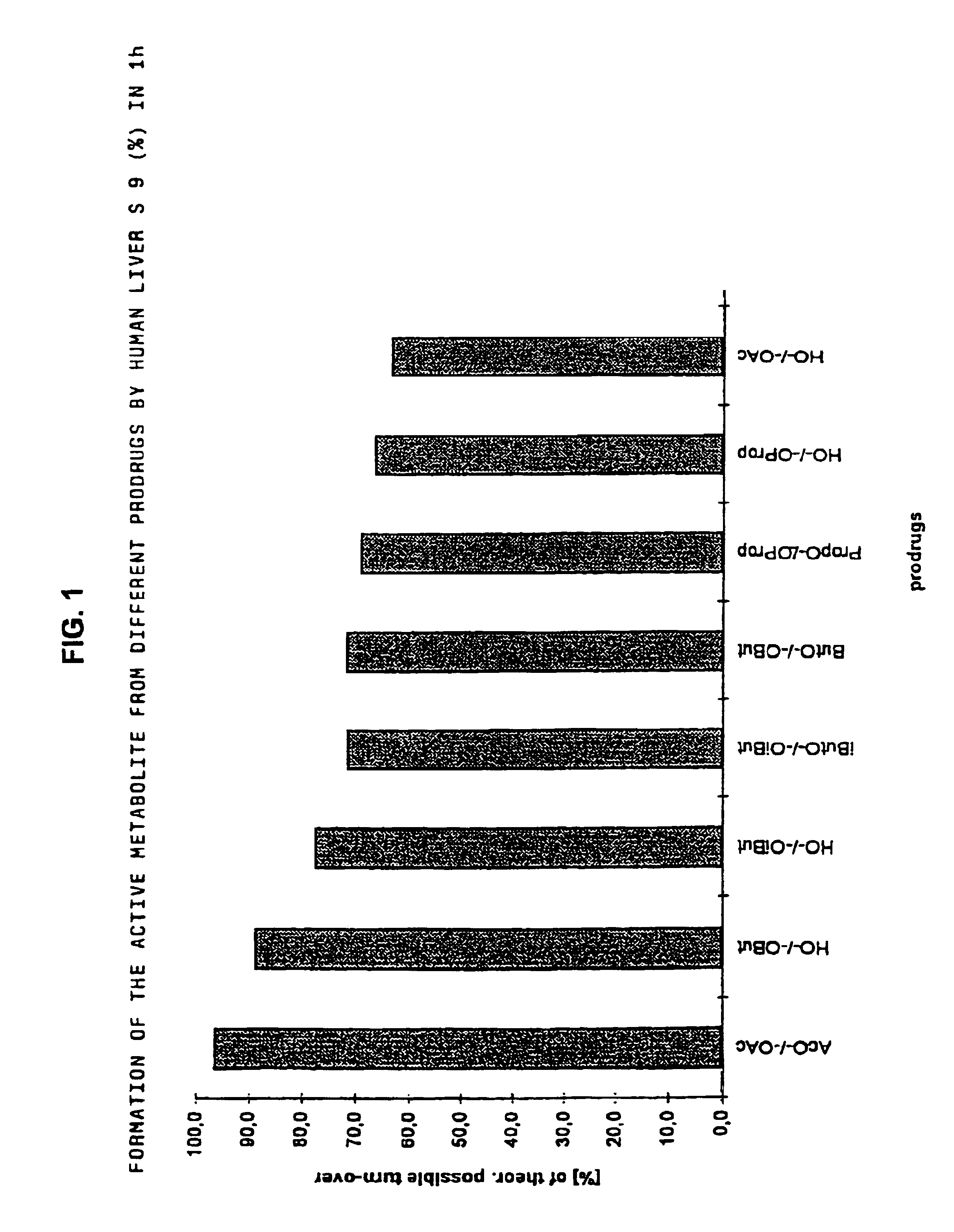
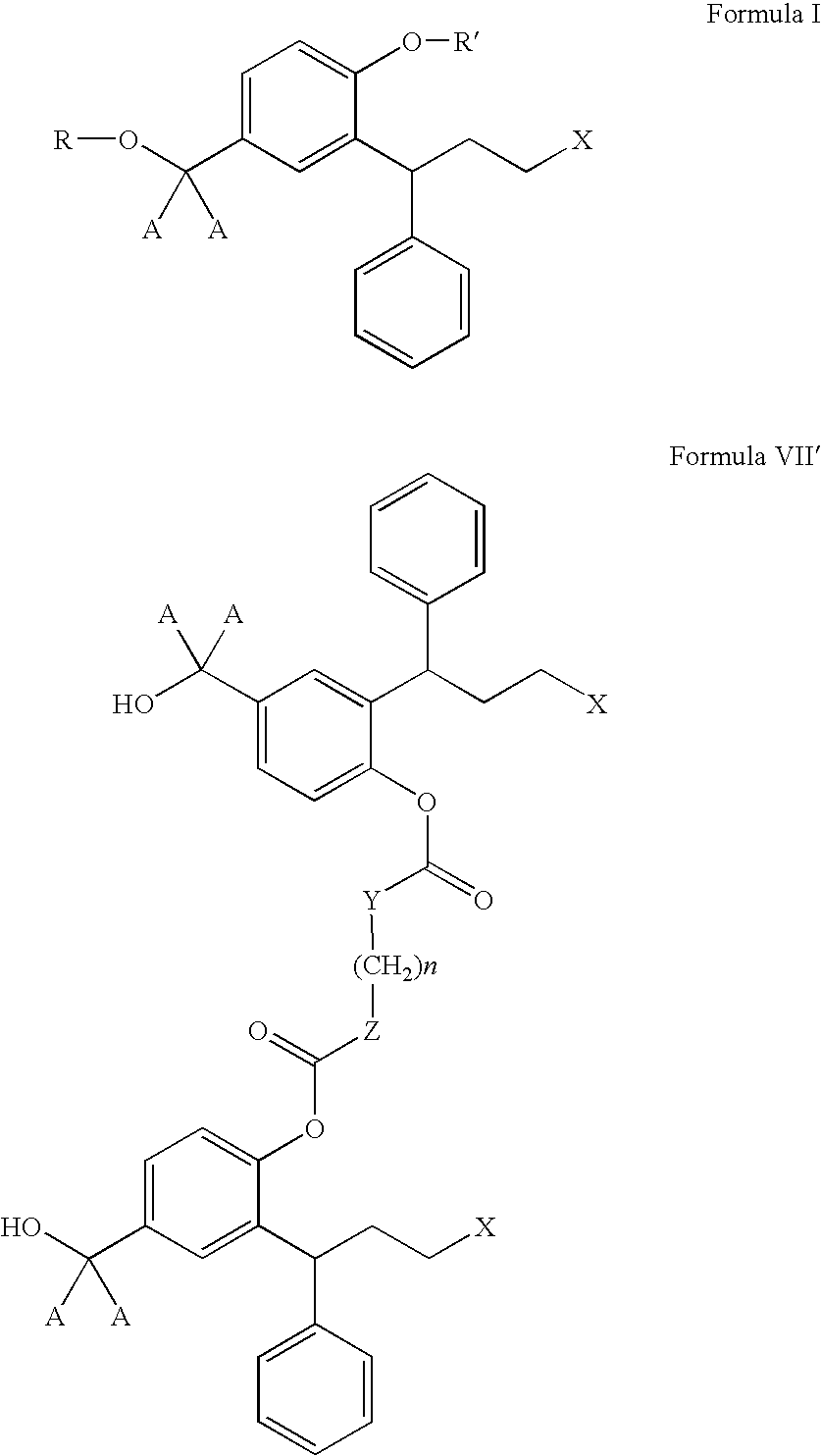
The invention concerns novel derivatives of 3,3-diphenylpropylamines, methods for their preparation, pharmaceutical compositions containing the novel compounds, and the use of the compounds for preparing drugs. More particularly, the invention relates to novel prodrugs of antimuscarinic agents with superior pharmacokinetic properties compared to existing drugs such as oxybutynin and tolterodine, methods for their preparation, pharmaceutical compositions containing them, a method of using said compounds and compositions for the treatment of urinary incontinence, gastrointestinal hyperactivity (irritable bowel syndrome) and other smooth muscle contractile conditions.
Owner:UCB SA
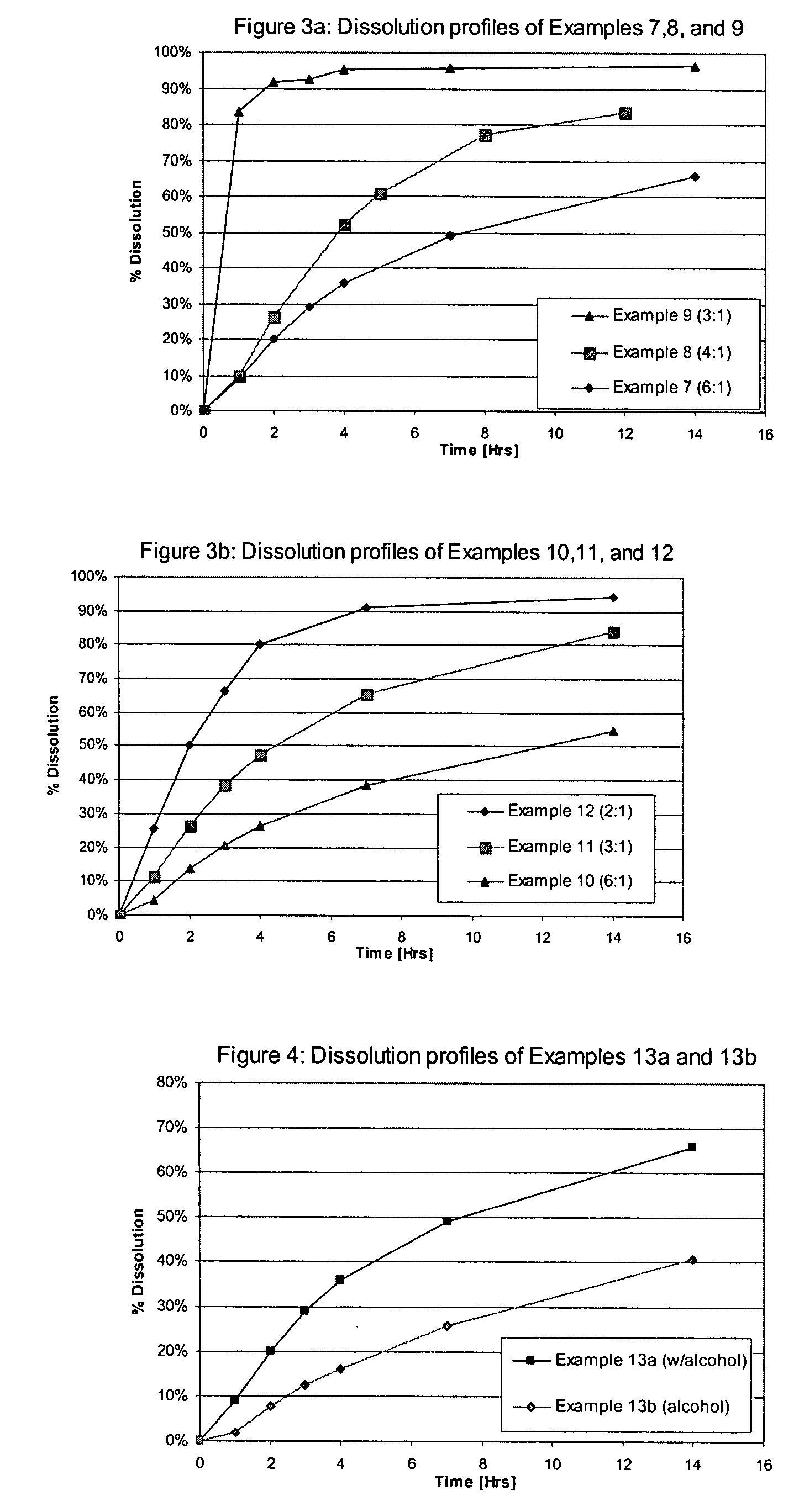
Tolterodine beads
Controlled release tolterodine beads are formed of a sugar core, an innermost sealcoat layer comprising HPMC, a tolterodine drug layer, and an outer control release layer. The thickness of the hydrophilic HPMC sealcoat layer can be used help modulate the release of the tolterodine drug.
Owner:SYNTHON BV
Skin irritation suppressant and transdermal preparation
InactiveCN102858372ALess irritatingOrganic active ingredientsNervous disorderCholesterol derivativePharmaceutical Adjuvants
Owner:HISAMITSU PHARM CO INC
Process for preparing tolterodine
InactiveUS20060094904A1Reduce the number of stepsLow costOrganic compound preparationDiaryl/thriaryl methane dyesPolymer scienceTolterodine
Owner:DR REDDYS LAB LTD +1
Pharmaceutical formulations for the treatment of overactive bladder
Owner:THERAVIDA INC
Oral liquid tolterodine composition
InactiveUS20050032905A1Sufficient solubilityEfficient ConcentrationAntibacterial agentsBiocideWater solubleTolterodine
A pharmaceutical composition in a form of an orally deliverable liquid comprises water having in solution therein a pharmaceutically acceptable water-soluble salt of a tolterodine related compound at a therapeutically effective concentration in the composition. The composition has a pH of about 2 to about 6 and further comprises a sweetening agent and an antimicrobial agent at a concentration that is antimicrobially effective at the pH of the composition. The composition is useful for treating a muscarinic receptor mediated disorder, more particularly overactive bladder, in a subject by orally administering to the subject a therapeutically effective amount of the composition.
Owner:PHARMACIA CORP
Process for preparing tolterodine
InactiveUS7355077B2Reduce the number of stepsLow costOrganic compound preparationDiaryl/thriaryl methane dyesPolymer scienceTolterodine
Owner:DR REDDYS LAB LTD +1
Controlled release formulation of tolterodine
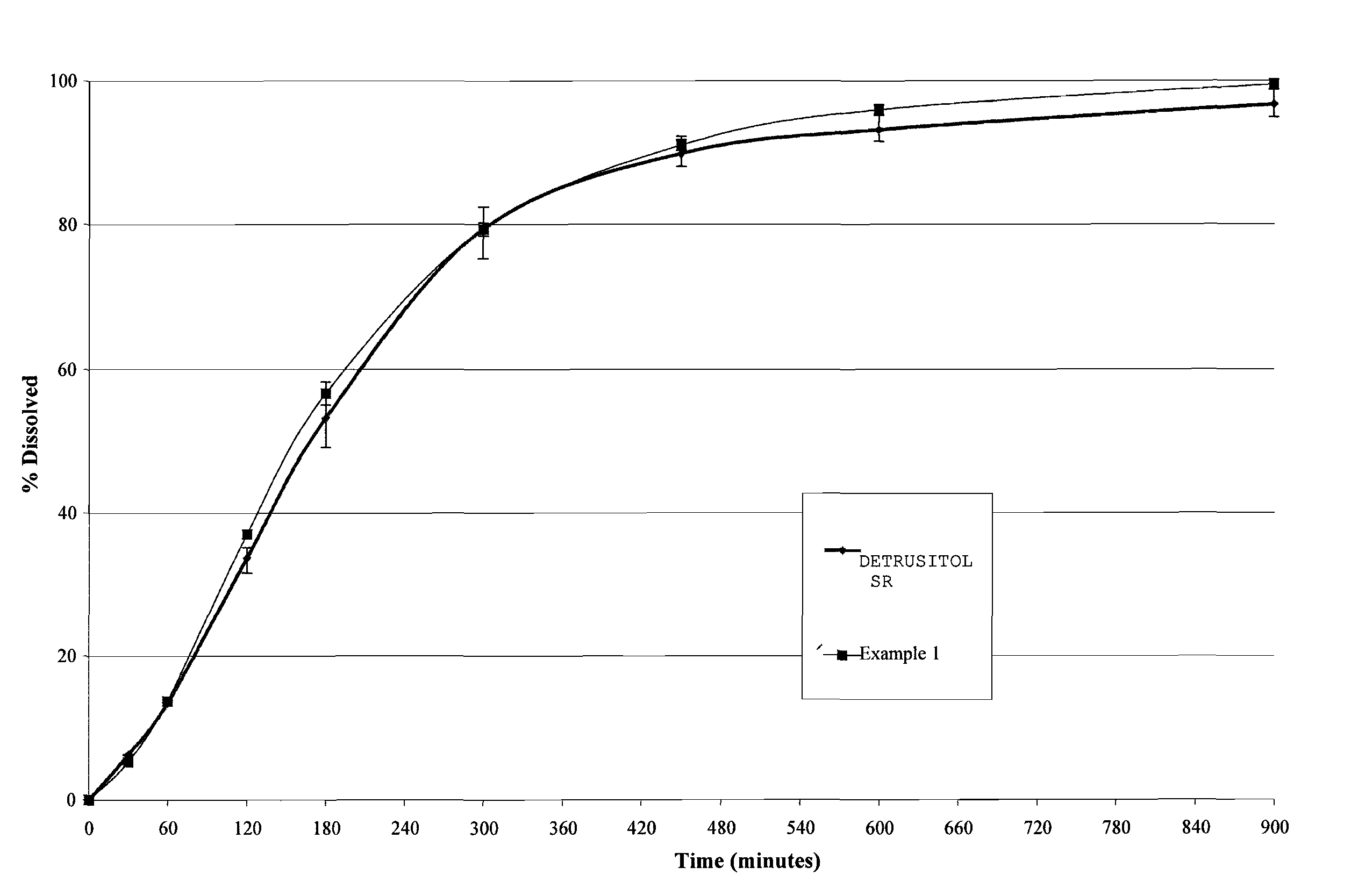
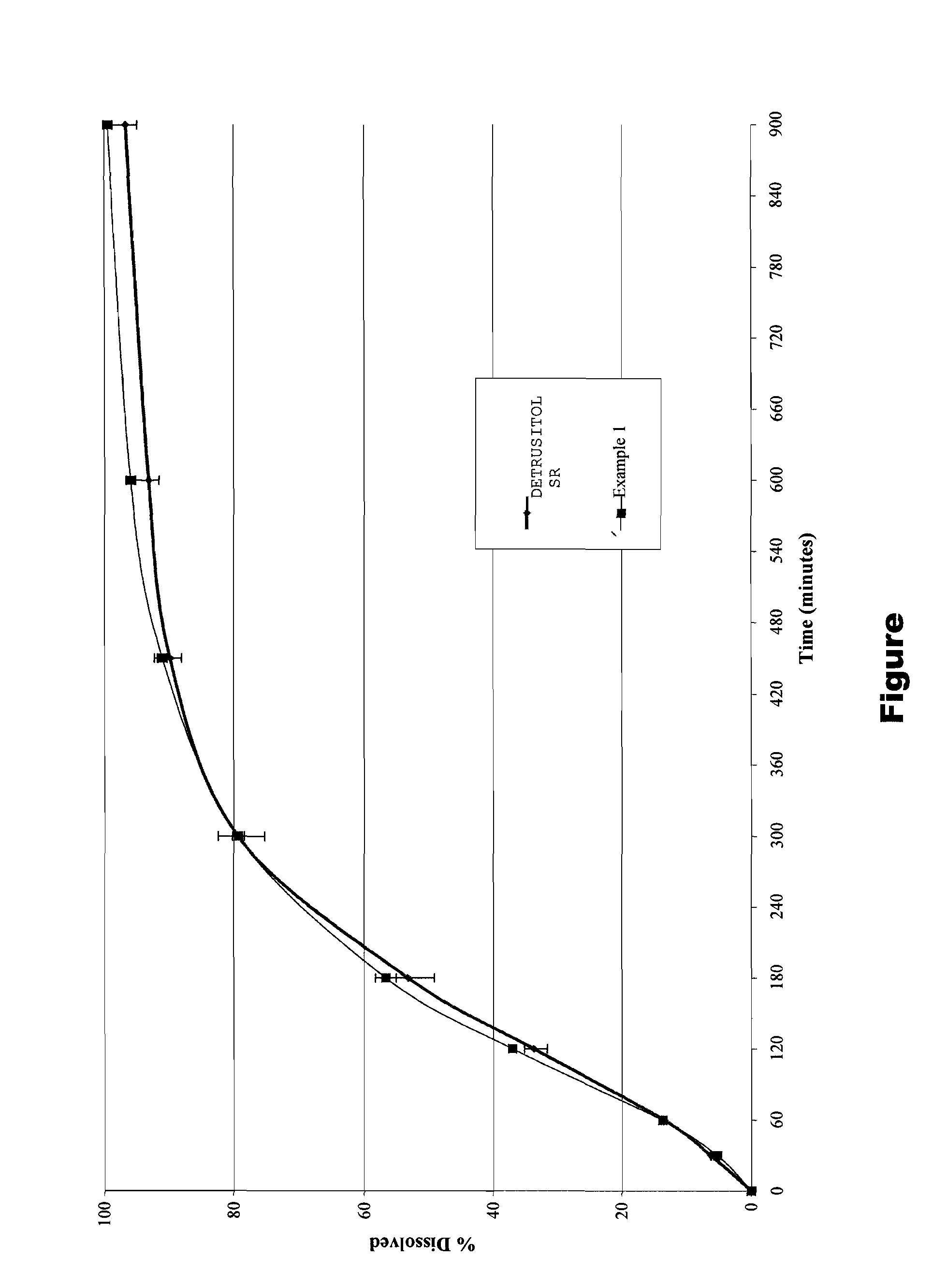
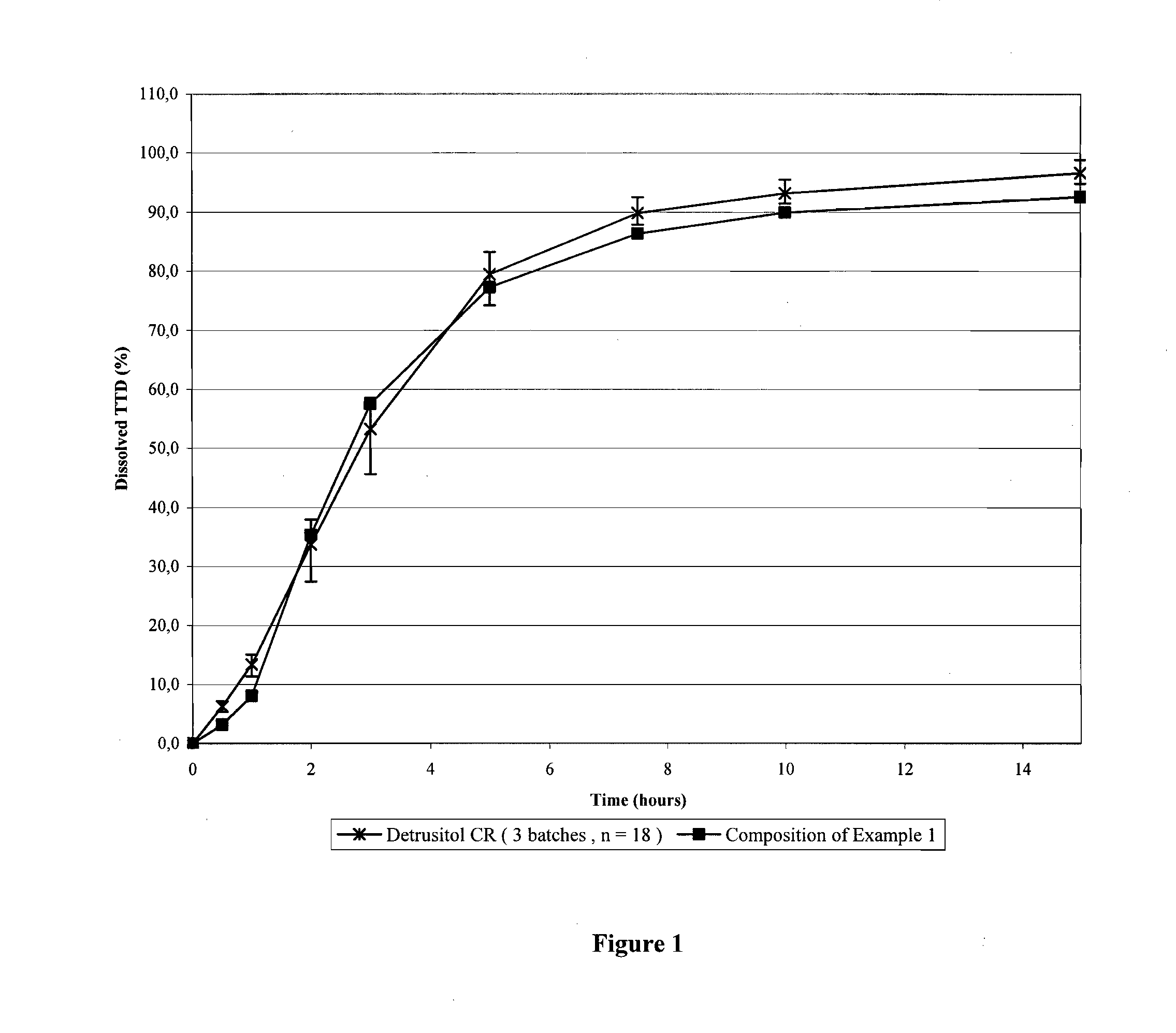
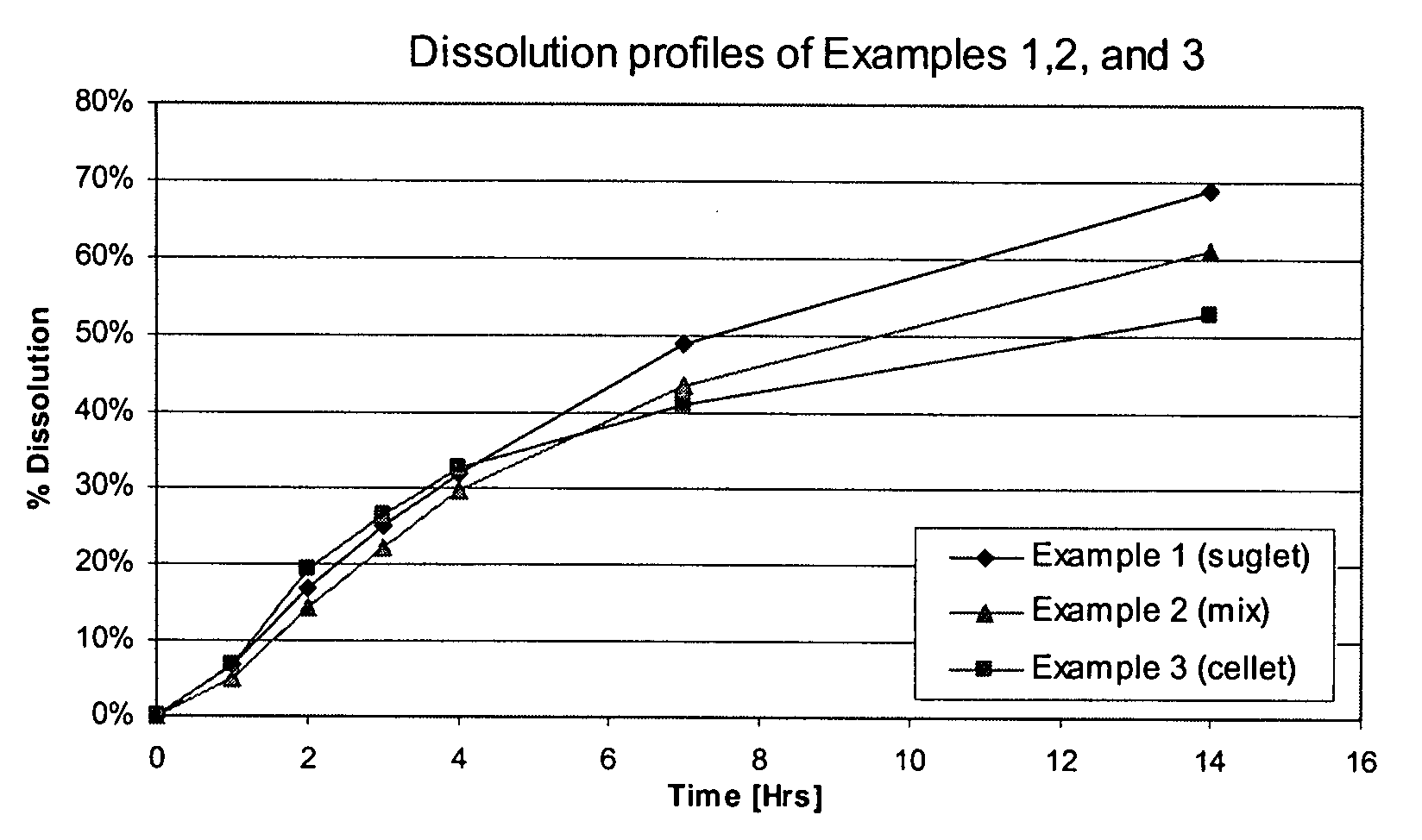
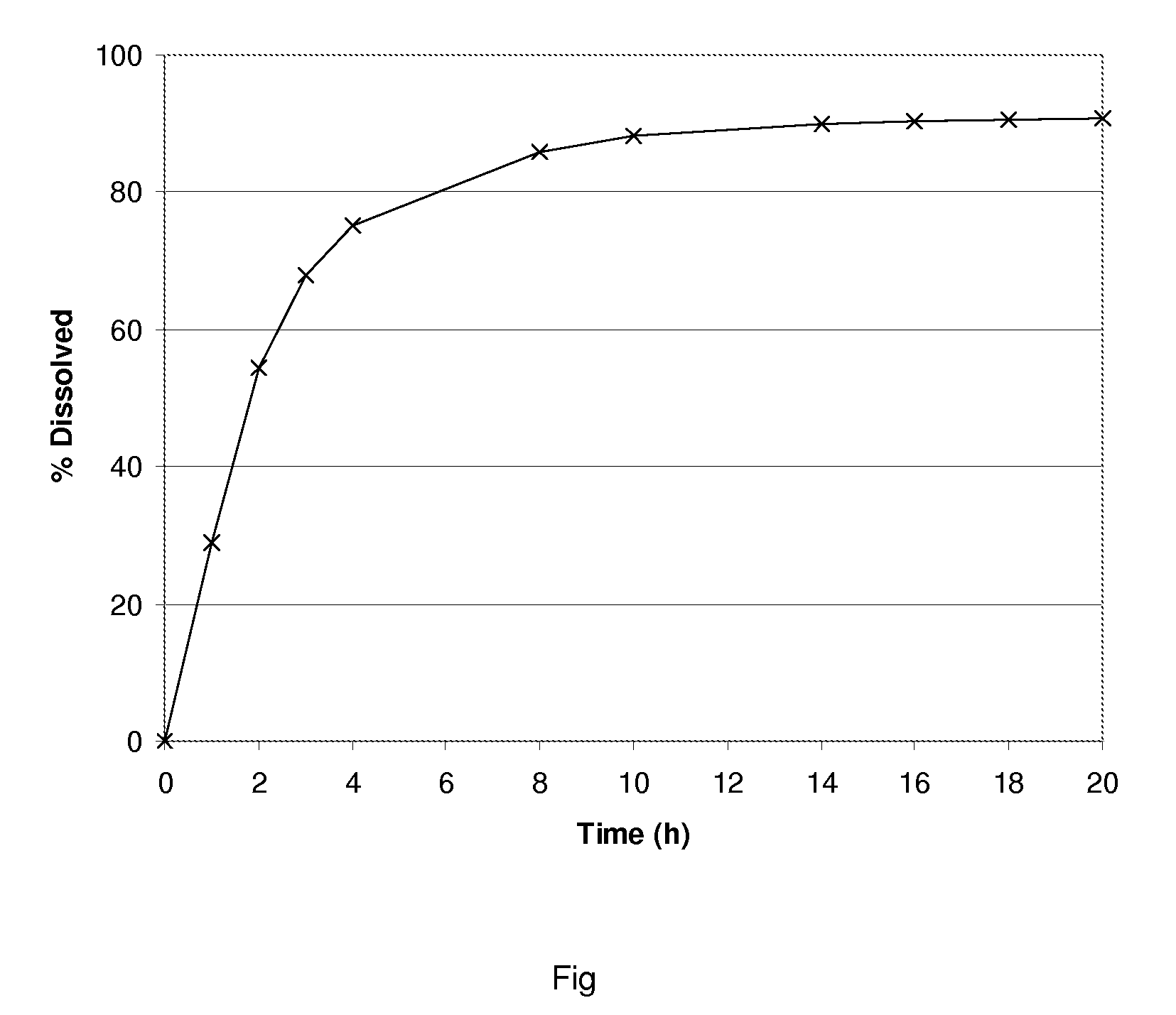
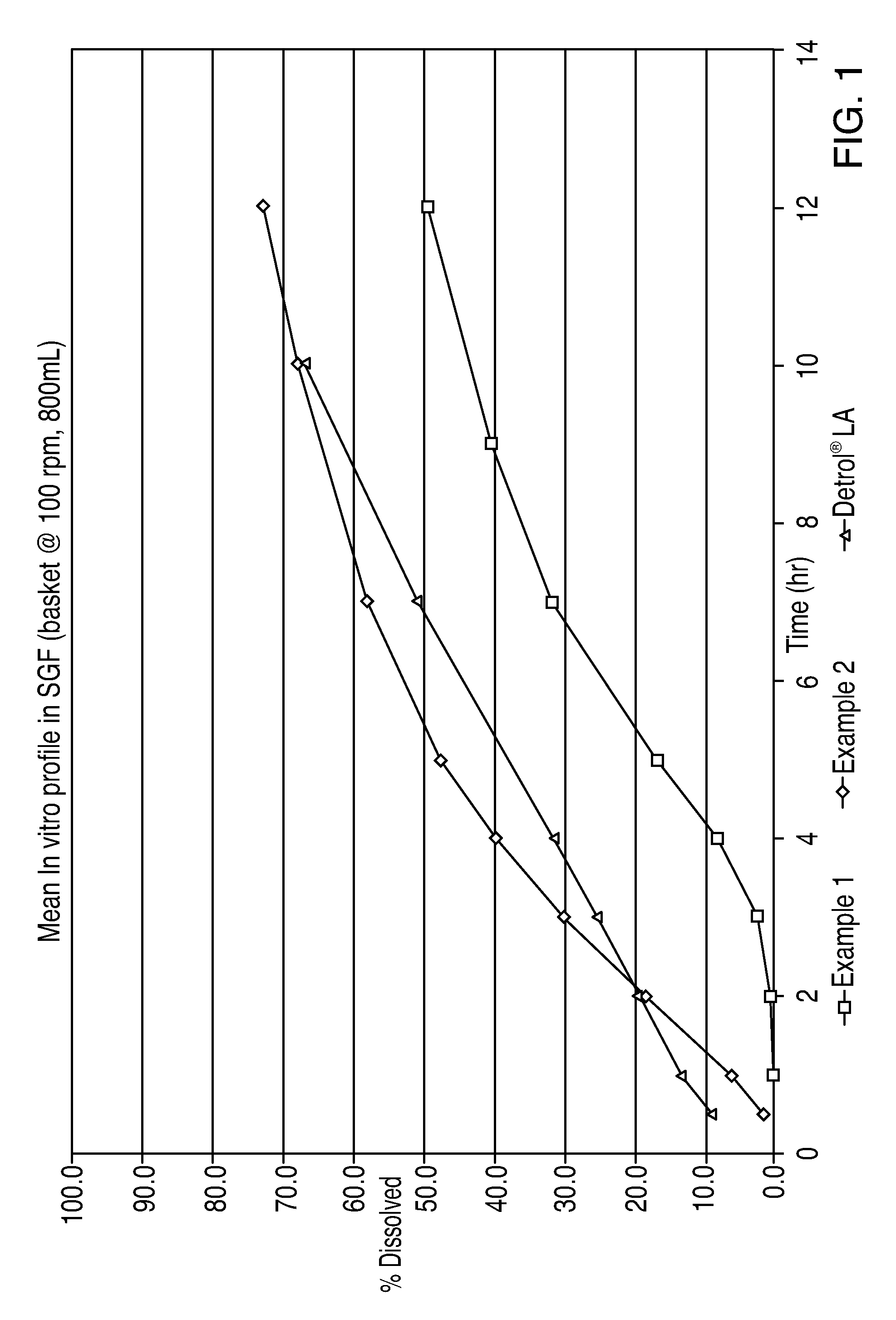
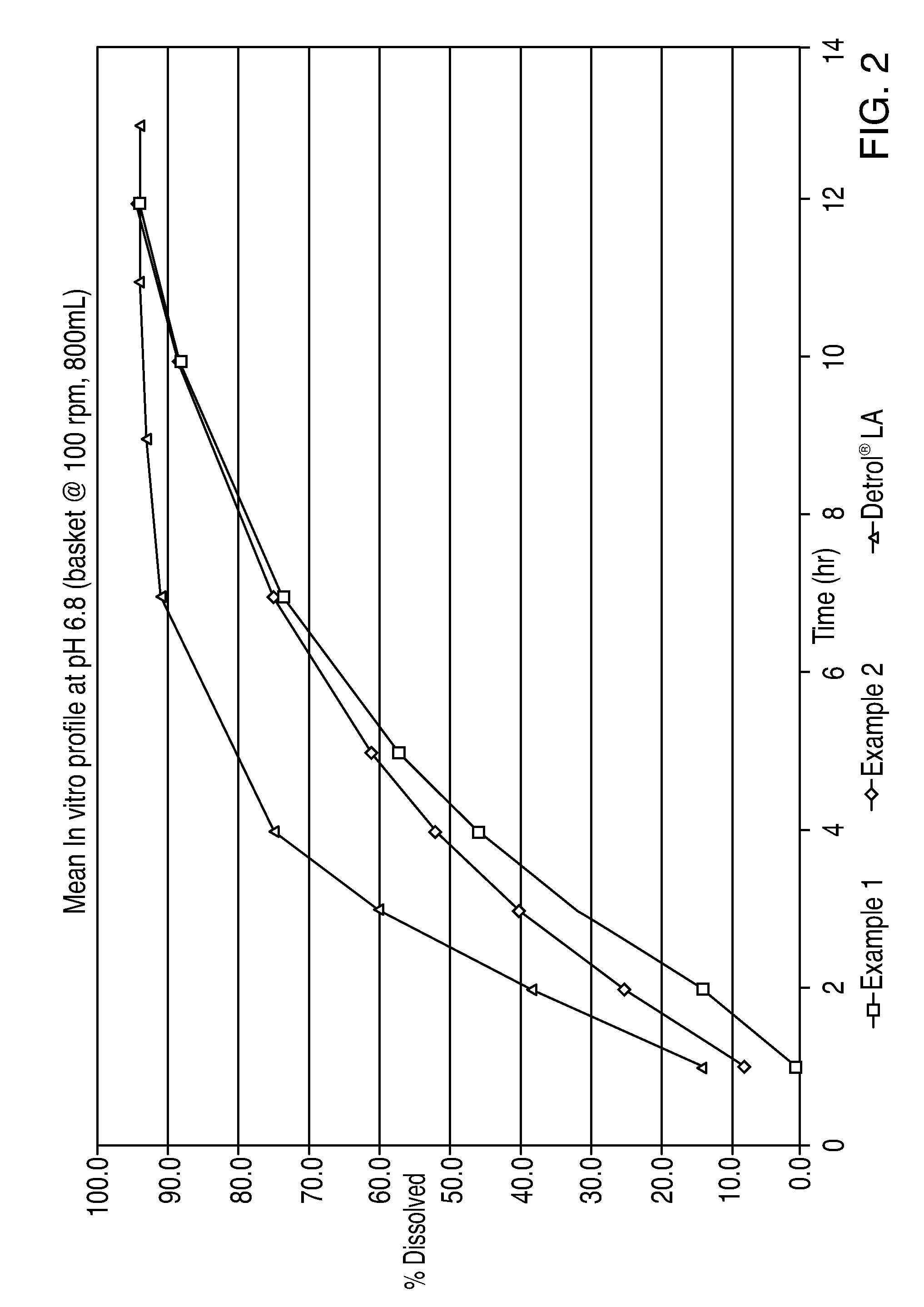
The invention encompasses stable multiparticulate pharmaceutical compositions of tolterodine having at least one pharmaceutically acceptable excipient and at least two populations of multiparticulates each population having tolterodine or a salt thereof and the ratio of the populations is from 90:10 to 10:90 by weight, wherein after storage for 1 month at 40° C. and 75% relative humidity the difference between the dissolution profile at 4 hours is no more than about 5% when compared to the dissolution profile at the time of manufacture.
Owner:TEVA PHARM USA INC
Tolterodine, compositions and uses thereof, and preparation of the same
Racemic tolterodine free base in crystalline form, tolterodine with improved purity, compositions and uses thereof, and processes of preparing the same.
Owner:CIPLA LTD
Coated formulations for tolterodine
A sustained release pharmaceutical composition comprising coating comprising at least one water-insoluble permeable polymer and at least one water soluble polymer and homogenous cores containing only tolterodine or a salt thereof and microcrystalline cellulose is described.
Owner:LEK PHARMA D D
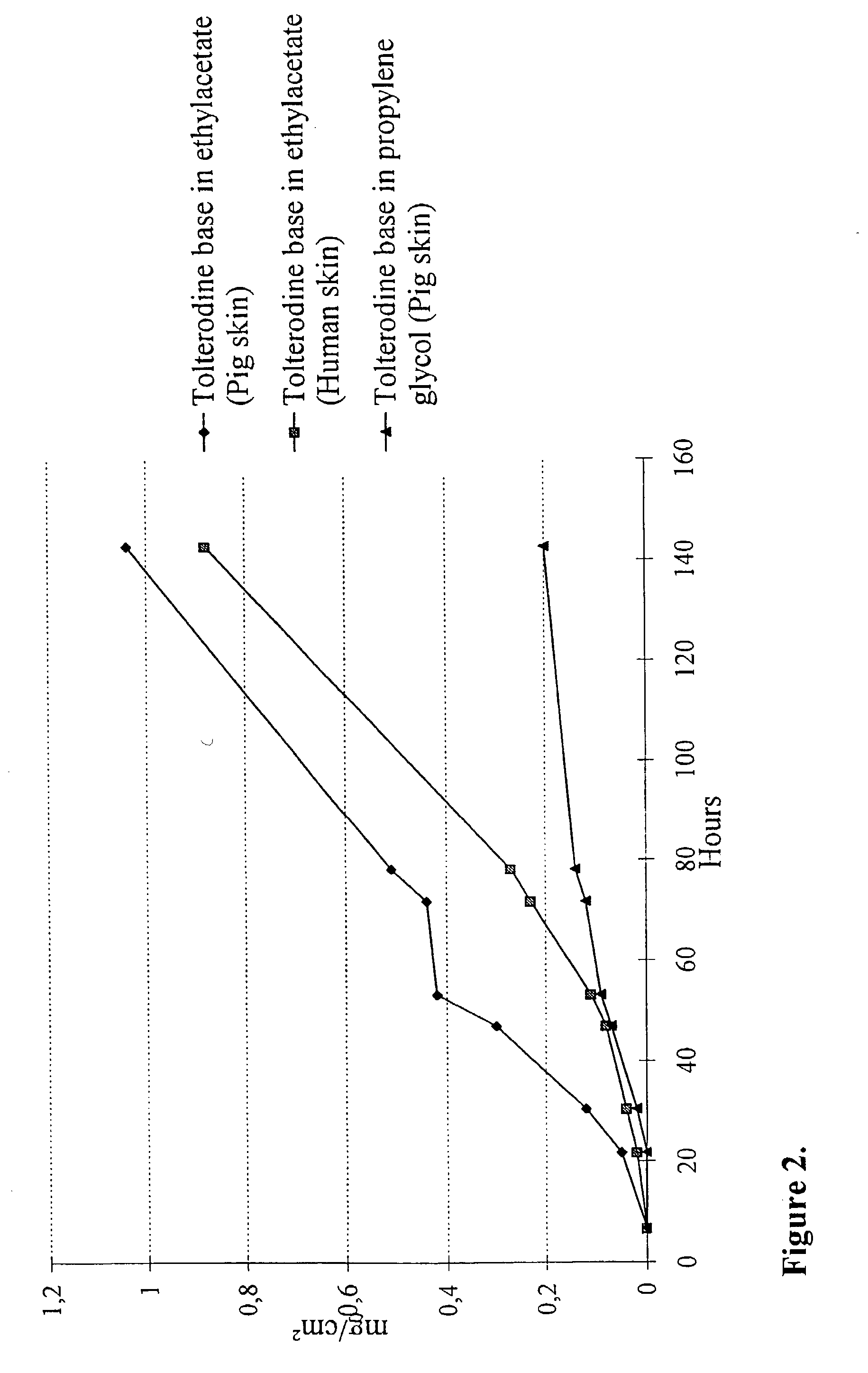
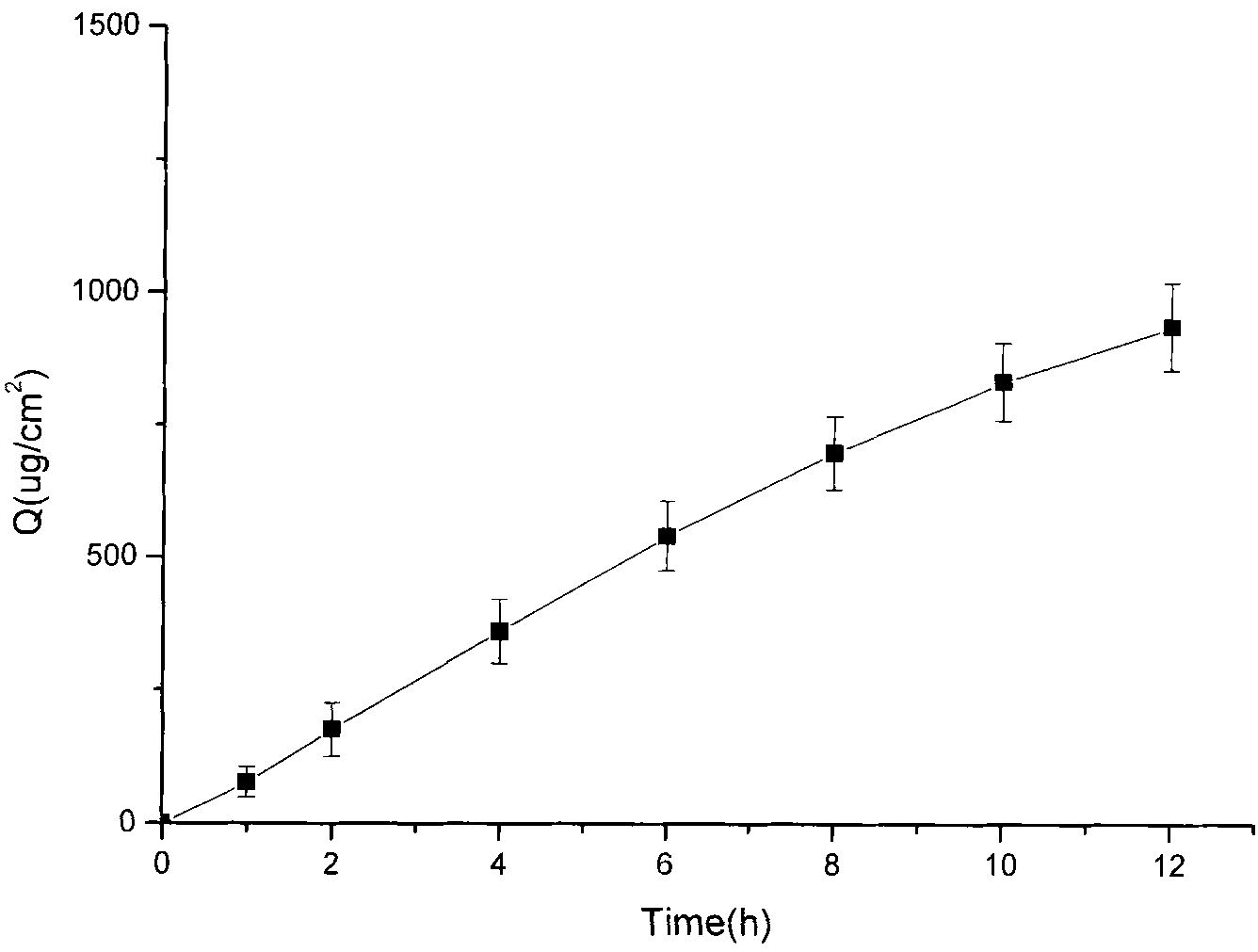
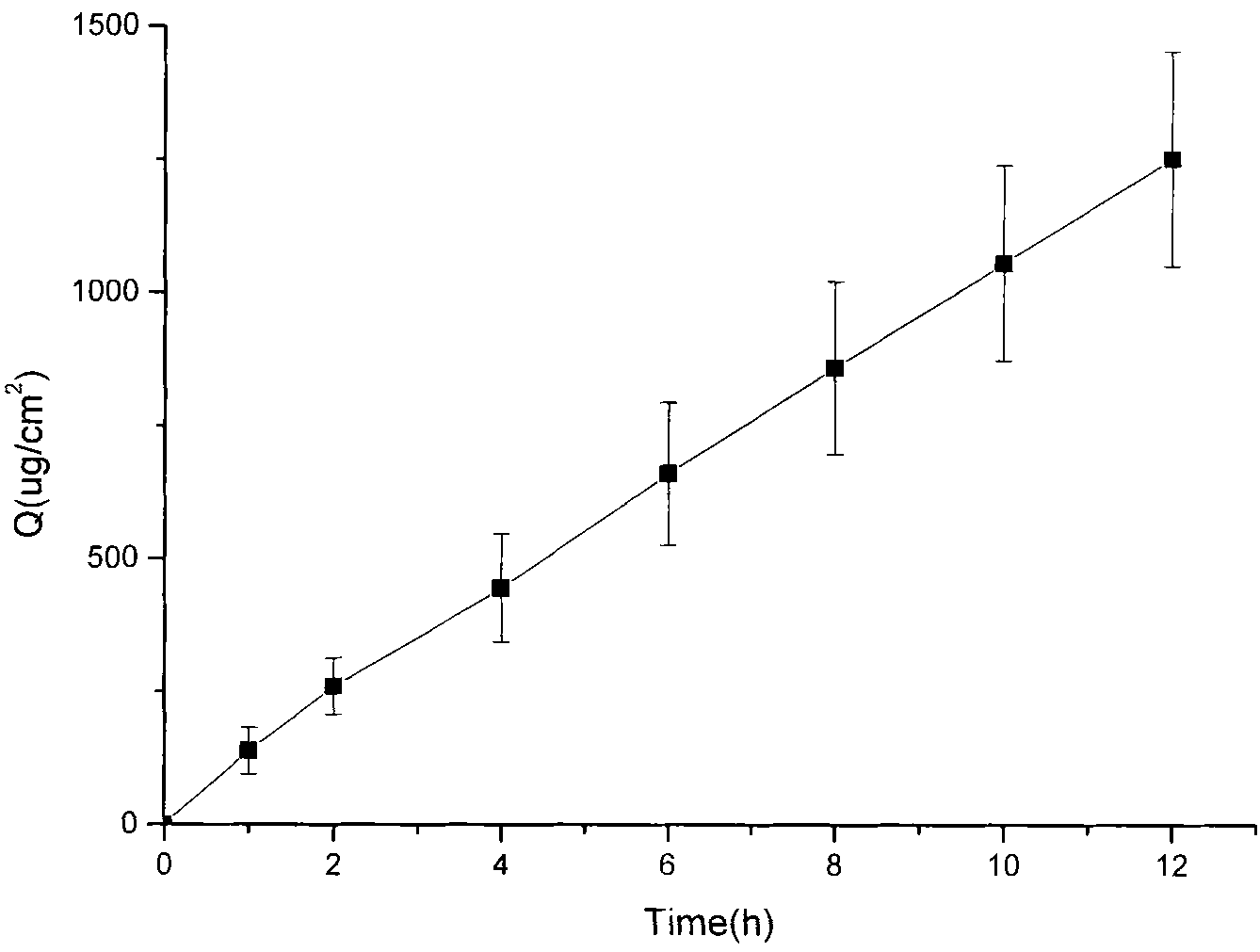
Transdermal composition comprising tolterodine
InactiveUS20120123162A1Keep for a long timeLong storageOrganic active ingredientsOrganic chemistryPharmacologyTolterodine
A transdermal composition comprising tolterodine having improved storage stability is disclosed. The transdermal composition comprising tolterodine contains an antioxidant to stabilize tolterodine and can be stored for a long period of time.
Owner:SK CHEM CO LTD
Controlled Release Muscarinic Receptor Antagonist Formulation
InactiveUS20090214665A1Improve bioavailabilityOrganic active ingredientsBiocideMuscarinic antagonistControlled release
An oral controlled release pharmaceutical composition for muscarinic receptor antagonist, preferably tolterodine, that employs a drug core, a rapidly disintegrating or rapidly dissolving coating applied to the drug core and a controlled release coating.
Owner:IMPAX LAB INC
Transdermal patch and method for augmenting adhesive strength thereof
ActiveCN103153294AExcellent adhesionOrganic active ingredientsPharmaceutical non-active ingredientsTransdermal patchMedicine
A transdermal patch provided with a backing and a pressure-sensitive adhesive layer, wherein said pressure-sensitive adhesive layer includes at least one component selected from the group consisting of tolterodine acetate and salts thereof.
Owner:HISAMITSU PHARM CO INC
Tolterodine gel preparation and preparation method thereof
InactiveCN101810565AAvoid or reduce adverse reactionsAvoid the disadvantages of taking awayOrganic active ingredientsPharmaceutical delivery mechanismGel preparationWear resistant
The invention discloses a tolterodine gel preparation, which is mainly prepared from free alkali tolterodine, gel matrix, film-forming matrix, organosilicon elastic body, percutaneous penetration accelerators, pH regulators, humectants, and the like. During use, the gel preparation contains the film-forming matrix which can form a flexible film after being dried, and can be externally coated on the thigh, the belly, the upper arm or shoulder, and other parts; flexible and wear-resistant thin film can be rapidly formed in the selected skin region; the gel is prevented from being taken away by the clothes; and meanwhile, the application site is in an unsealed state and has good air permeability. The contained organosilicon elastic body can obviously improve the dispersibility, flexibility, wettability, glossiness, and other performances of the gel system. The prepared gel is smooth, crystal clear and fine, has no foreign body sensation on the skin after use, durable medicament and slow release, good percutaneous absorption effect, convenient use, and improves the patient compliance.
Owner:李又欣 +2
Process for the preparation of tolterodine
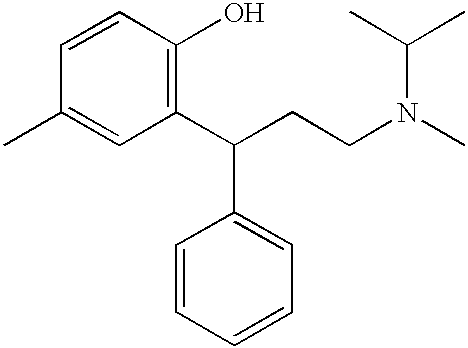
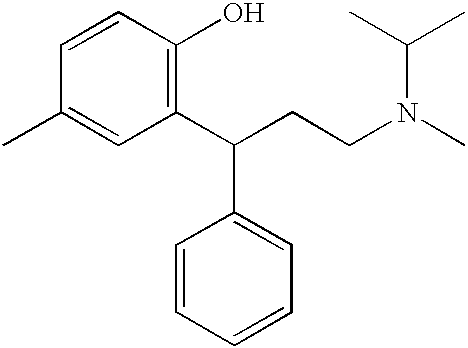
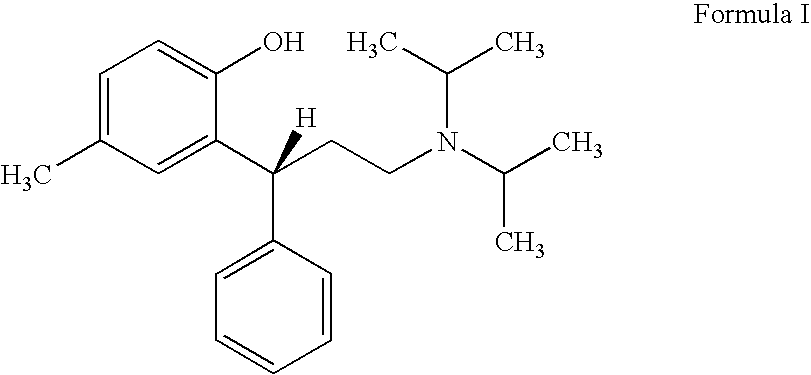
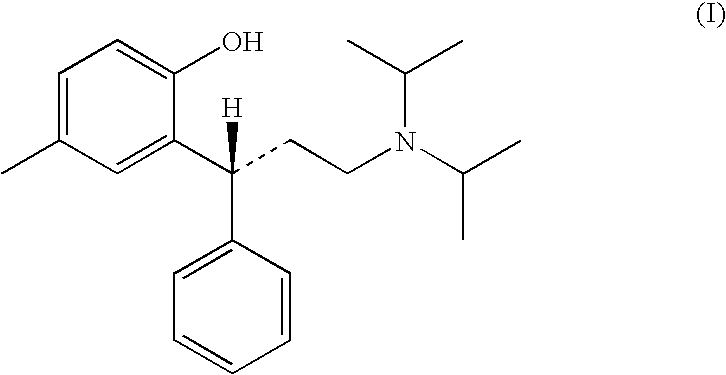
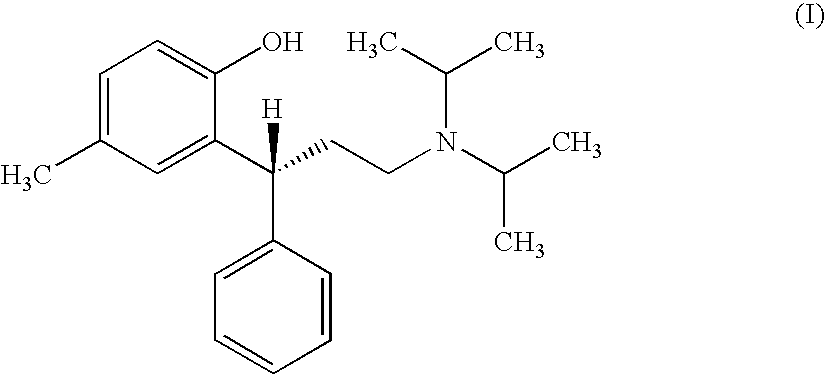
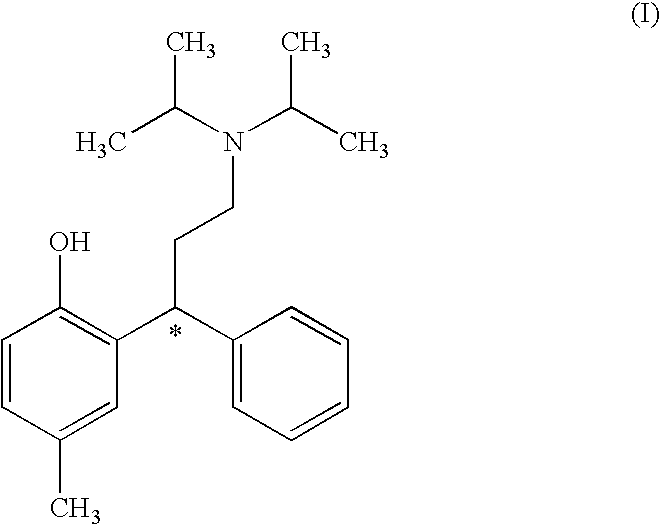
A novel process prepares tolterodine, i.e. (R)-N,N-diisopropyl-3-(2-hydroxy-5-methylphenyl)-3-phenylpropanamine, in the racemic form, as well as intermediates useful for its preparation. Tolterodine free base may be prepared by reacting diisopropyl-(3-phenyl-3-p-tolyloxy-propyl)-amine and 60% aqueous sulfuric acid for three hours under stirring at room temperature. The reaction mixture is then poured over ice / water and then alkalized with 50% NaOH.
Owner:DIPHARMA SPA
Composition with low skin irritation for transdermal delivery of tolterodine
Disclosed is a composition for transdermal administration containing tolterodine. The composition comprises at least one essential oil as an abirritant to minimize skin irritation caused by tolterodine. The composition relieves skin irritation caused by tolterodine. Therefore, a successful commercial application of a tolterodine-containing transdermal preparation based on the composition can be expected.
Owner:SK CHEM CO LTD
Combinations of tolterodine and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release tolterodine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release tolterodine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release tolterodine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Tolterodine fumarate oral solid preparation and preparation method thereof
The invention discloses a Tolterodine fumarate oral solid preparation and a preparation method thereof. The oral solid preparation is prepared from Tolterodine fumarate, starch, microcrystalline cellulose, calcium hydrogen carbonate or calcium carbonate, binder, and lubricant. The preparation method comprises the steps of: grinding raw materials, mixing, adding binder, making into damp mass, drying, grading, granulating, sub-packaging by a conventional method to obtain granules, adding lubricant, and making into capsules or tablets. The preparation method can solve content uniformity and disintegrating problems, and enable the indices of the preparation to fulfil the requirements in the Pharmacopoeia.
Owner:SICHUAN KELUN PHARMA CO LTD
Preparation method and oral liquid of holothuria scabra extract
InactiveCN108578438AEffective in treating urinary incontinenceHigh cure efficiencyDispersion deliveryUrinary disorderCentrifugationTraditional medicine
The invention provides a preparation method of a holothuria scabra extract. Holothurian scabra is subjected to water extraction to obtain the holothuria scabra extract. The method comprises the following steps that the internal organs of holothuria scabra are removed, and holothuria scabra is washed; holothuria scabra, processed in the last step, with the mass M1 is taken, water with the mass M2 is added to holothuria scabra, homogenate, sterilization, filtering and centrifugation are conducted, and supernatant is collected, wherein the mass ratio of M1 to M2 is 1:(8-12). Furthermore, the invention provides the holothuria scabra extract obtained by the preparation method and application of the holothuria scabra extract in preparation of medicine for treating incontinence of urine. According to the preparation method, it is discovered that the holothuria scabra extract has the function of treating incontinence of urine, the effect of the extract for treating incontinence of urine is superior to the effect of tolterodine for treating incontinence of urine, and the healing effective rate is high. Furthermore, oral liquid with the holothuria scabra extract is prepared. The oral liquidcan be applied to treatment of incontinence of urine.
Owner:GUANGDONG MEDICAL UNIV
Process for Obtaining Tolterodine
ActiveUS20070254959A1High yieldShort reaction timeOrganic active ingredientsBiocideEnantiomerDiisopropylamine
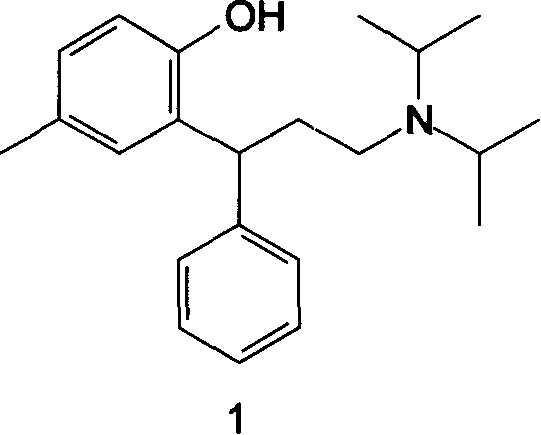
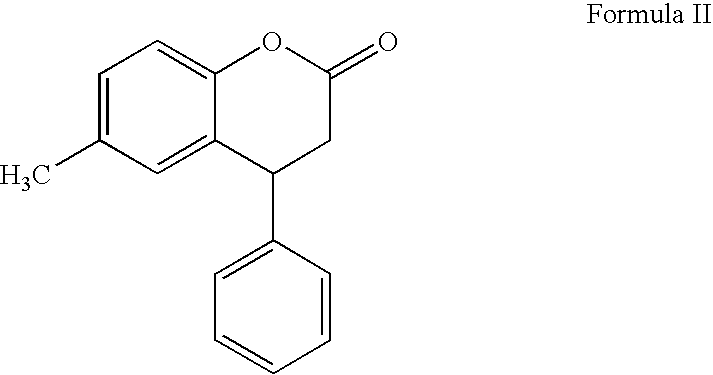
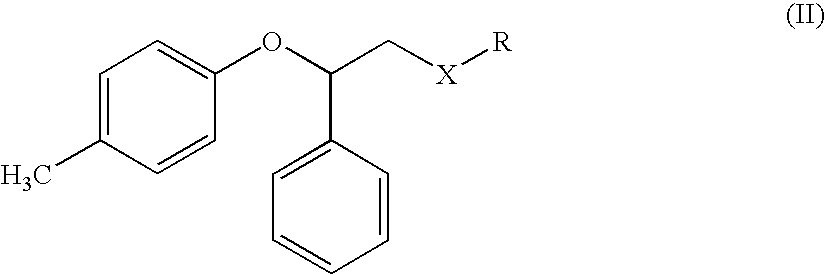
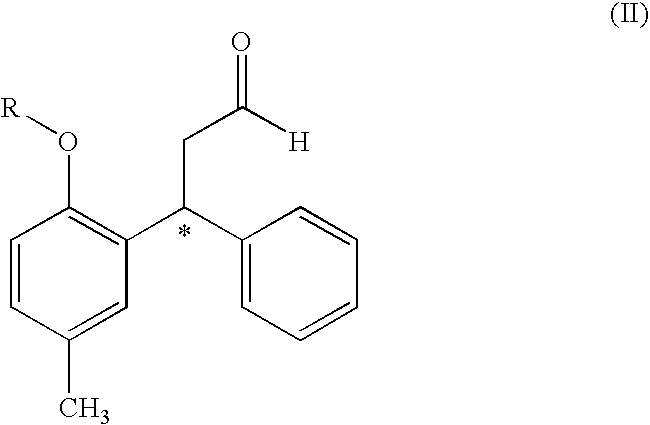
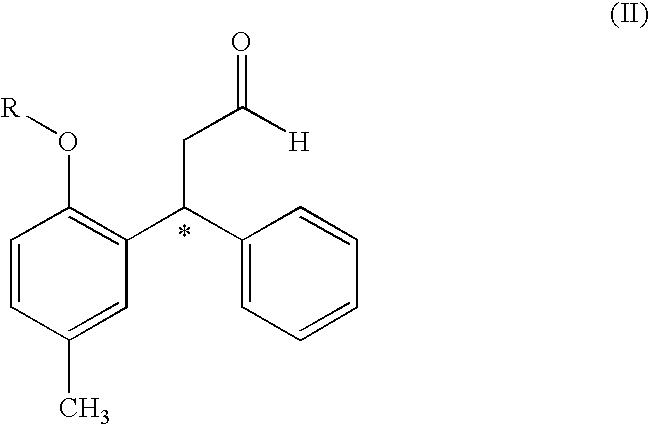
The process comprises reacting a compound of formula (II), where R is a hydroxyl protecting group, and the asterisk indicates an asymmetric carbon atom, with diisopropylamine in the presence of a reducing agent; optionally converting the resulting intermediate into a salt and, if so desired, isolating it; removing the hydroxyl protecting group; and if so desired, separating the desired (R) or (S) enantiomer, or the mixture of enantiomers and / or converting the obtained compound into a pharmaceutically acceptable salt thereof. Tolterodine is a muscarinic receptor antagonist useful in treating urinary incontinence and other symptoms of urinary bladder hyperactivity.
Owner:RAGACTIVES SL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com