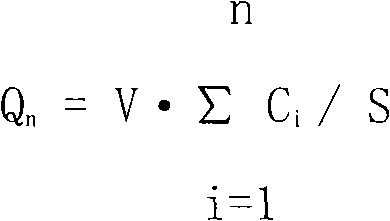Tolterodine gel preparation and preparation method thereof
A preparation and gel matrix technology, applied in pharmaceutical formulations, urinary system diseases, drug combinations, etc., can solve the problems of many adverse reactions and low bioavailability, and achieve smooth gel, good air permeability, and improved dispersibility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
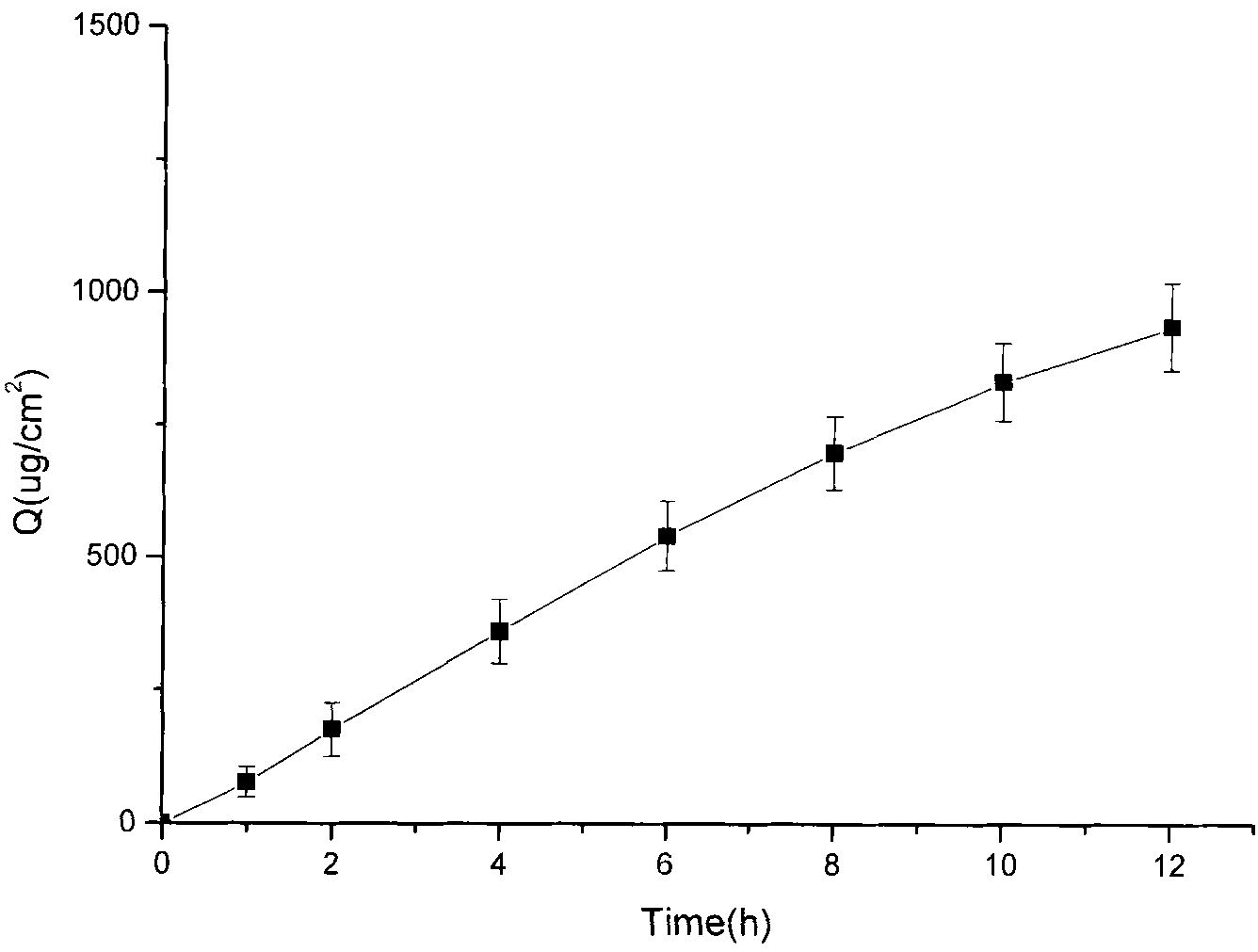
[0028] Tolterodine Gel Preparation
[0029] Prepare the gel (according to 100g) according to the following prescription:
[0030] Free base tolterodine 1g, carbomer 980 1.5g, azone 5g, glycerin 10g, sodium hydroxide 2.5g, ethanol 30ml, and distilled water as the balance.
[0031] Add the free base tolterodine into ethanol, vortex to fully dissolve it, then add glycerin, azone and distilled water to make a solution; take the prescribed amount of Carbomer 980, sprinkle it into the above solution, Stir mechanically at a speed of 100rpm for 24h until it swells completely, add sodium hydroxide to adjust the pH value to 7, and obtain the product.
Embodiment 2
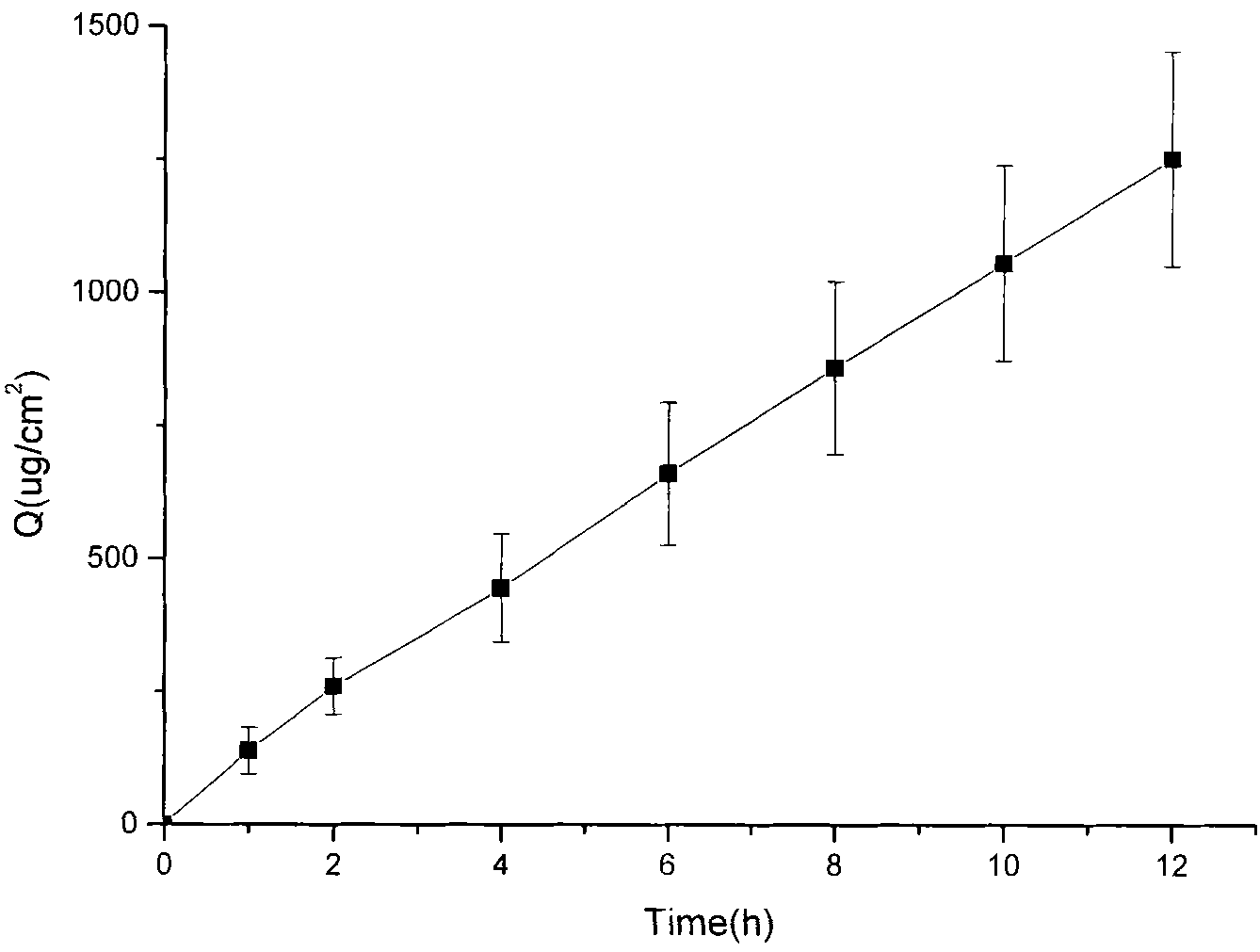
[0033] Tolterodine Gel Preparation
[0034] Prepare the gel (according to 100g) according to the following prescription:
[0035] Free base tolterodine 3g, hydroxypropyl cellulose 3g, linoleic acid 5g, propylene glycol 10g, ethanol 10ml, the balance is distilled water.
[0036] Add the free base tolterodine into ethanol, vortex to dissolve it fully, then add propylene glycol, linoleic acid and distilled water to make a solution; take the prescribed amount of hydroxypropyl cellulose and sprinkle it into the above solution , mechanically stirred at 100rpm for 24h until it swelled completely.
Embodiment 3
[0038] Tolterodine Gel Preparation
[0039] Prepare the gel (according to 100g) according to the following prescription:
[0040] Free base tolterodine 3g, hydroxypropyl cellulose 1.5g, carbomer 980 1g, silicone elastomer (Pharmat, Guangzhou Biaomei Pharmaceutical Excipients Co., Ltd.) 0.5g, N-methylpyrrolidone 5g, glycerin 10g , 2 g of triethanolamine, 10 ml of ethanol, and the balance is distilled water.
[0041] Add the free base tolterodine into ethanol, vortex to dissolve it fully, then add glycerin, N-methylpyrrolidone and distilled water to make a solution; weigh the prescribed amount of hydroxypropyl cellulose, carbomer 980 and silicone elastomer, mix well and sprinkle into the above solution together, mechanically stir at 100rpm for 24h until completely swollen, add triethanolamine dropwise to adjust the pH value, and obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com