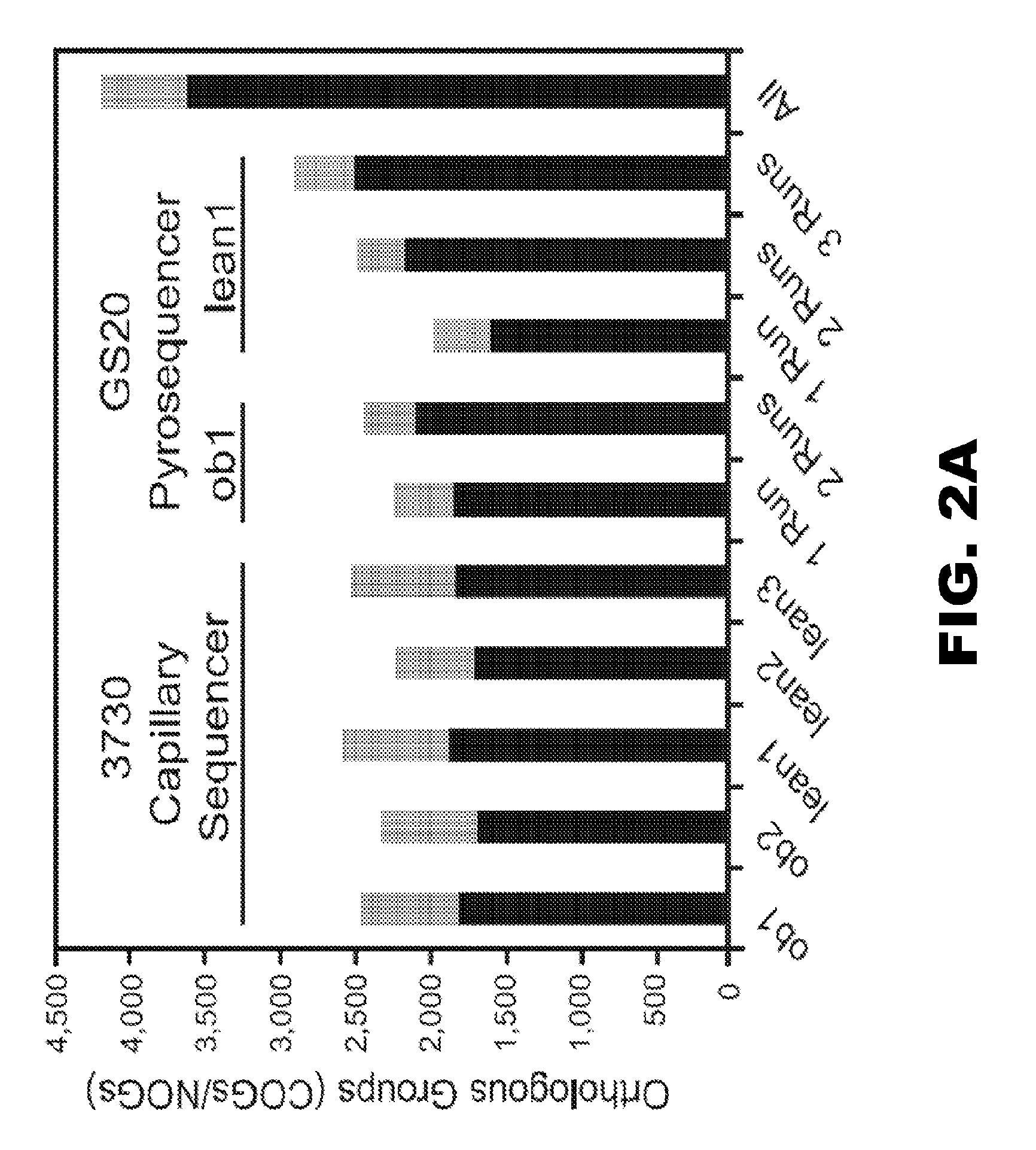
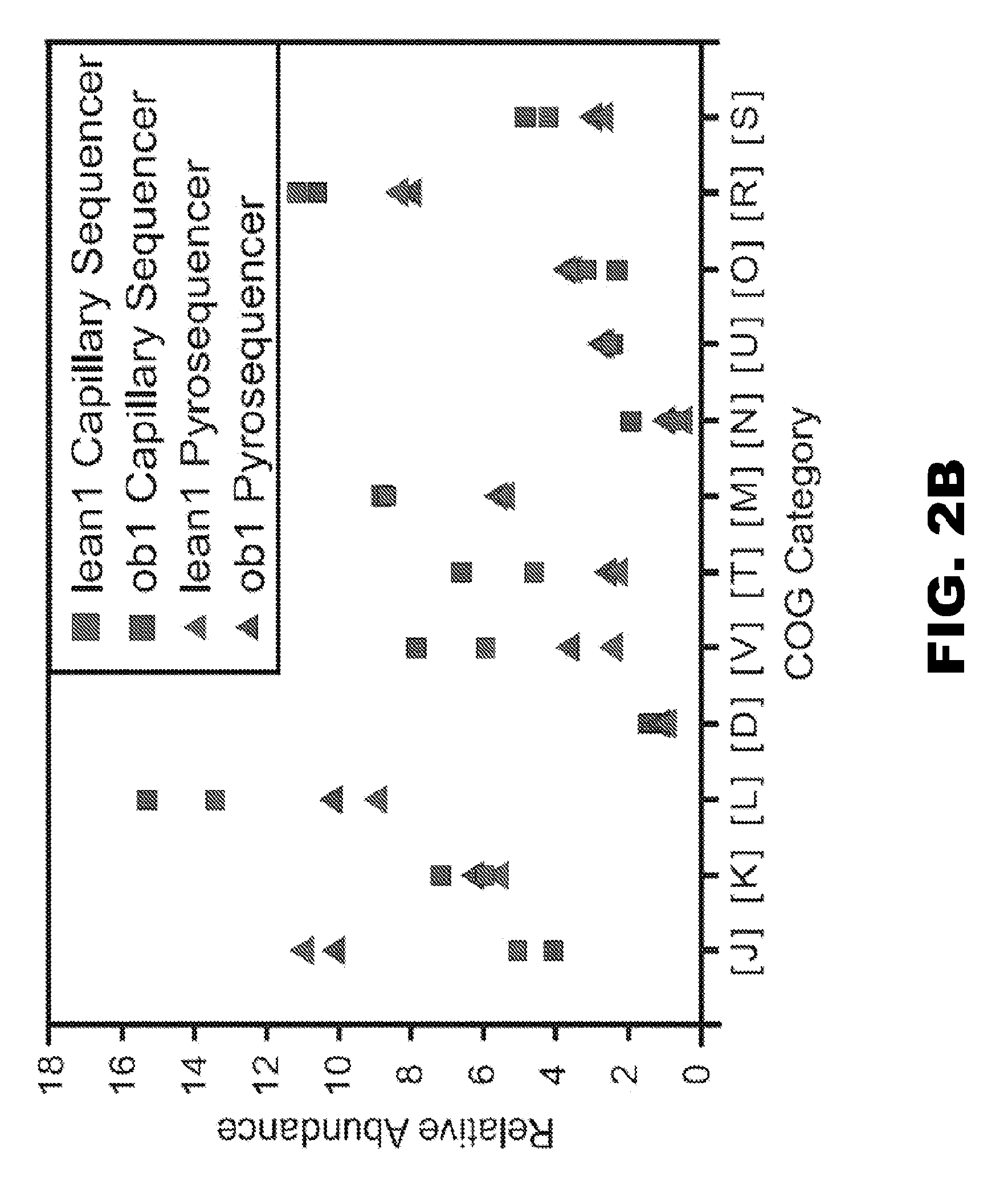
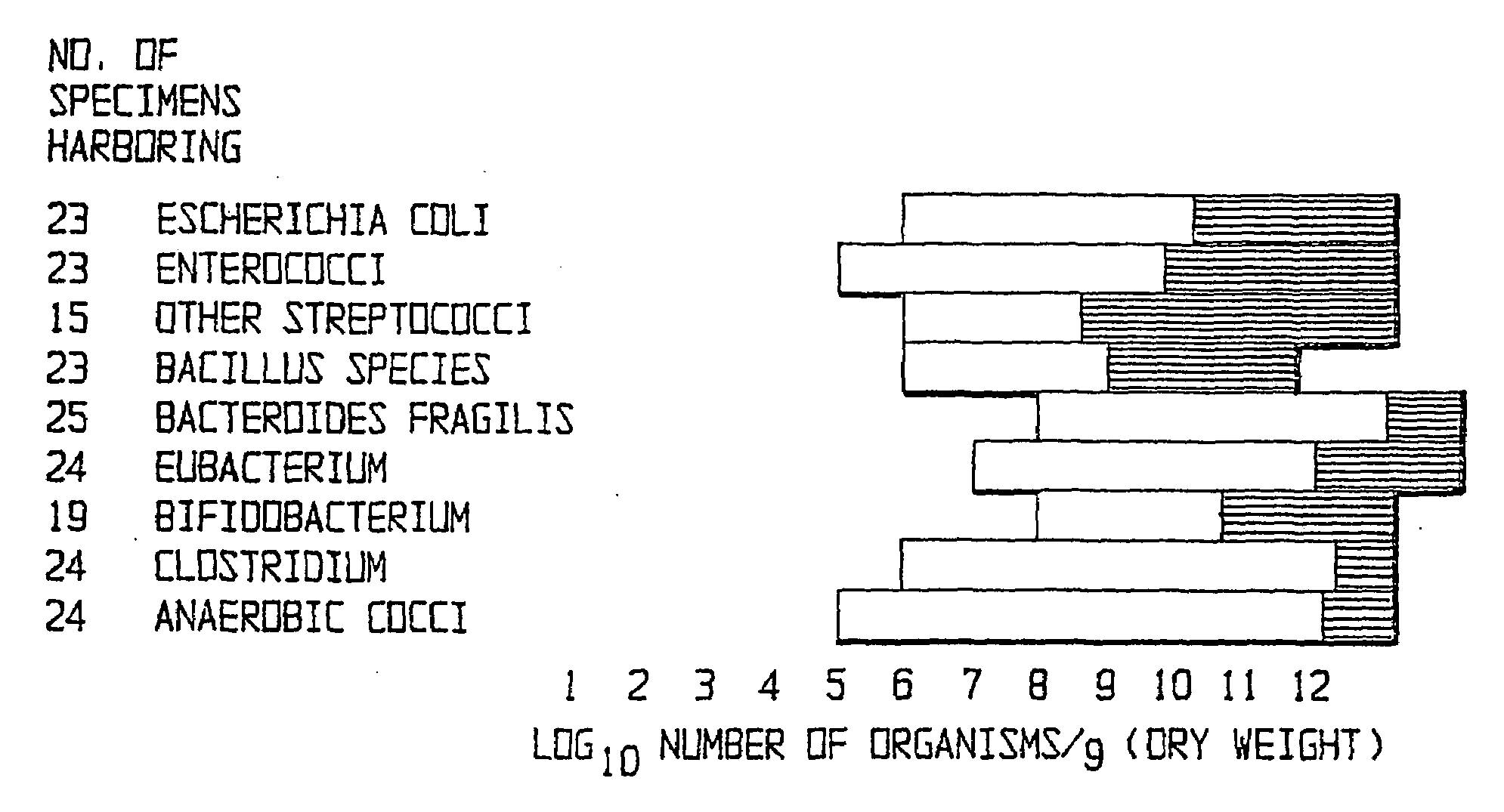
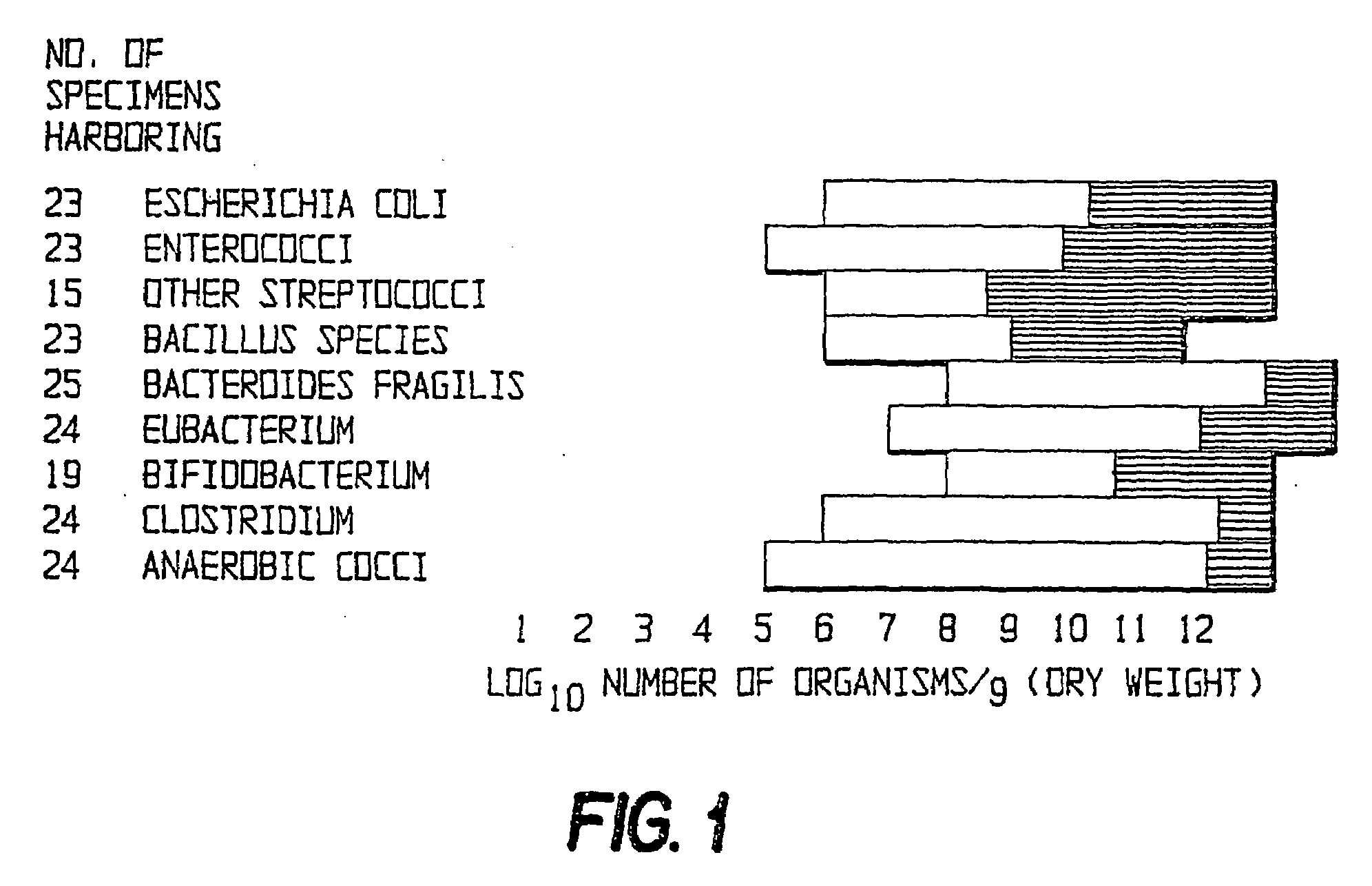
Patents
Literature
4634results about "Bacteria material medical ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Probiotic recolonisation therapy
The present invention relates to pharmaceutical compositions suitable for the treatment of chronic diseases associated with the presence of abnormal or an abnormal distribution of microflora in the gastrointestinal tract of a mammalian host, which compositions comprise viable non-pathogenic or attenuated pathogenic Clostridia. The compositions further comprise one or more additional viable non-pathogenic or attenuated pathogenic microorganisms selected from the group consisting of Bacteroides, Eubacteria, Fusobacteria, Propionibacteria, Lactobacilli, anaerobic cocci, Ruminococcus, E.Coli, Gemmiger, Desullomonas, Peptostreptococcus, and fungi. The present invention also provides pharmaceutical compositions suitable for the treatment of the same chronic diseases comprising viable non-pathogenic or attenuated pathogenic Escherichia coli, at least one strain of viable non-pathogenic or attenuated pathoenic Bacteroides and at least one strain of viable non-pathogenic or attenuated pathogenic microorganism.
Owner:FINCH THERAPEUTICS HLDG LLC
Treatment of gastro-intestinal disorders
InactiveUS6645530B1Dampen bacterial inactivationAcid secretion in the stomach could also be pharmacologically suppressedBiocideMilk preparationEscherichia coliDisease
A method of treating chronic disorders associated with the presence of abnormal microflora or an abnormal distribution of microflora in the gastrointestinal tract involves removing the host's existing enteric microflora and substitution of feces from a disease screener donor or composition comprising microorganism selected from the group consisting of Bacteroides and E. coli.
Owner:CRESTOVO LLC
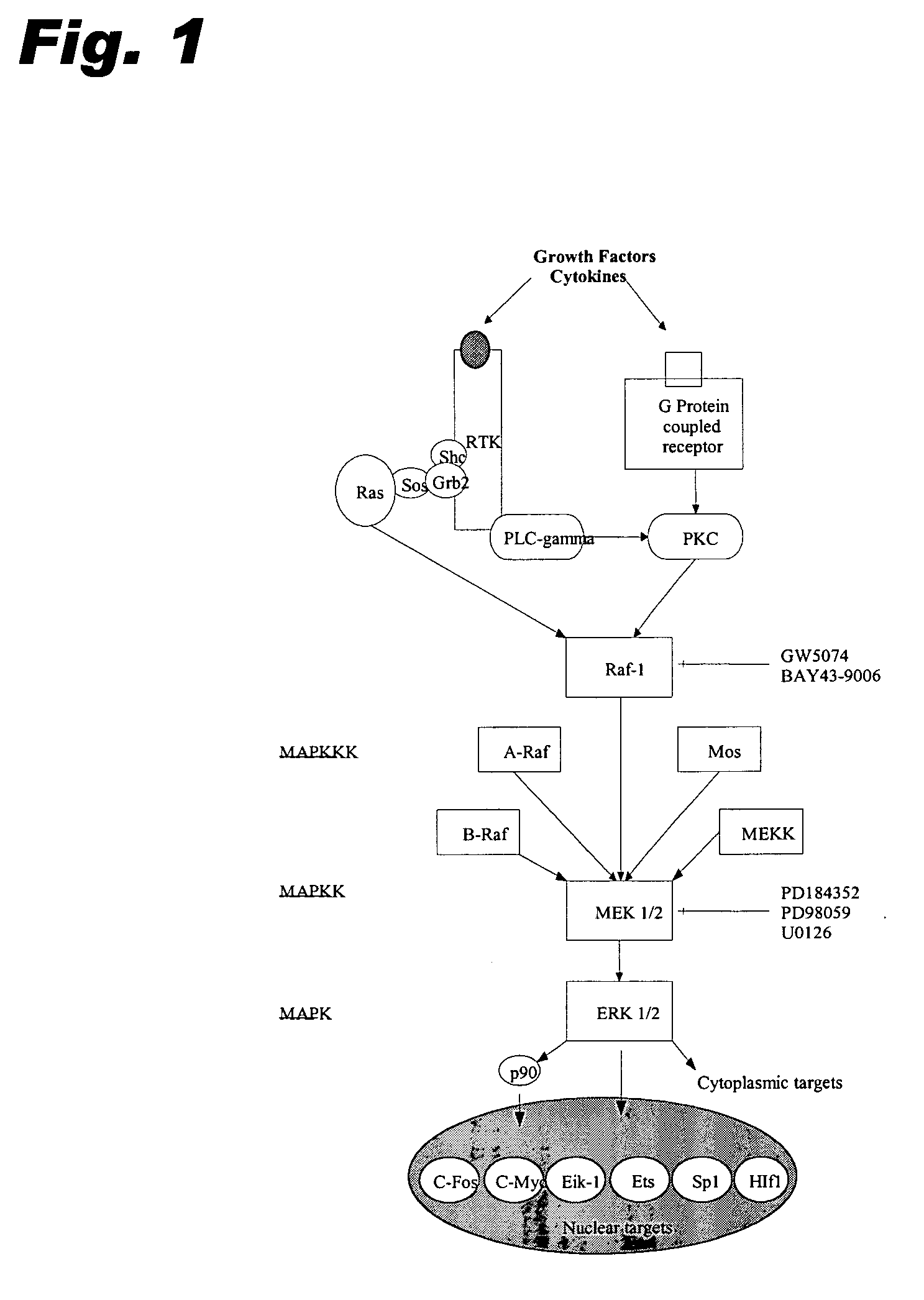
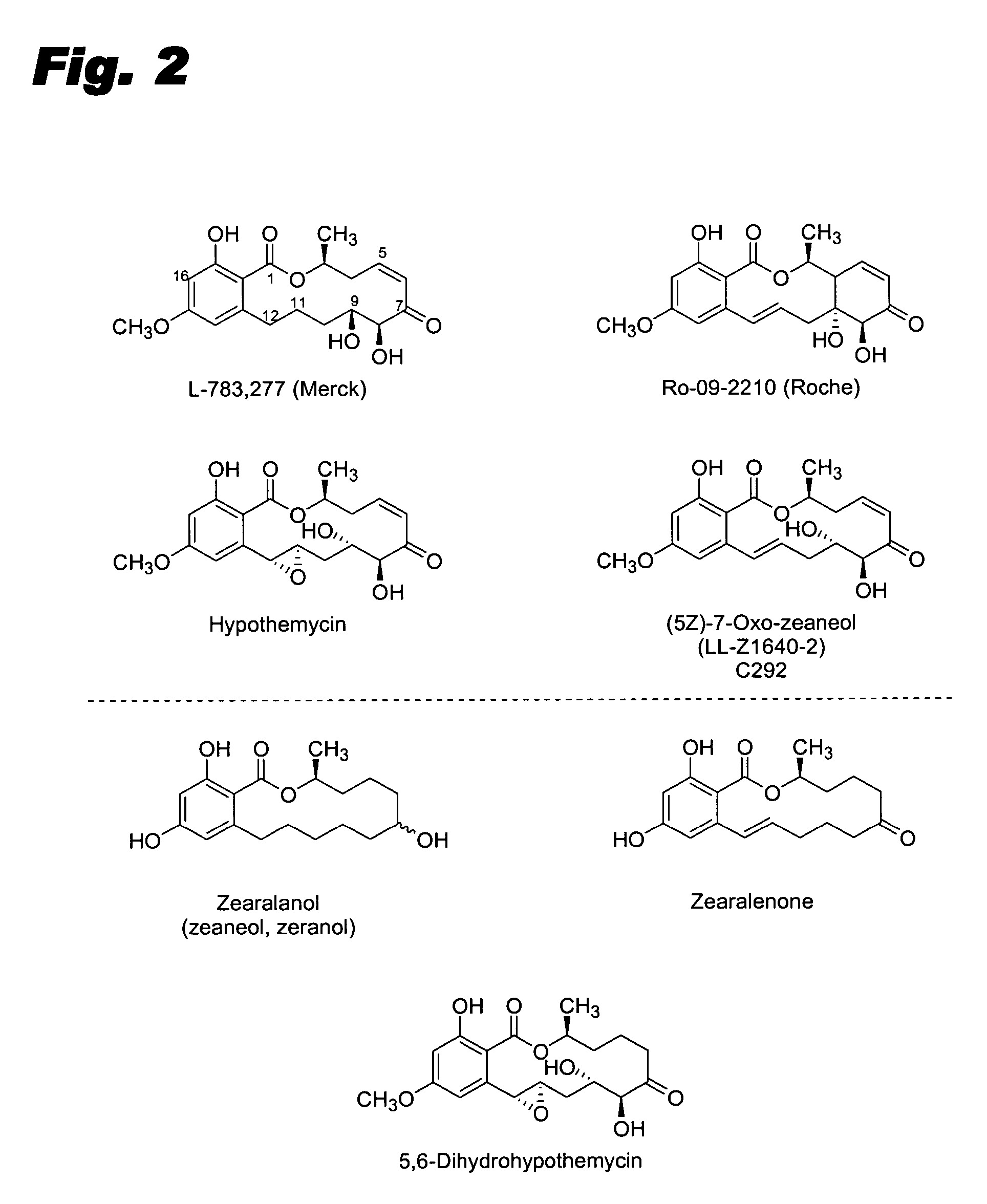
Specific kinase inhibitors
Resorcylic acid lactones having a C5-C6 cis double bond and a ketone at C7 and other compounds capable of Michael adduct formation are potent and stable inhibitors of a subset of protein kinases having a specific cysteine residue in the ATP binding site.
Owner:KOSAN BIOSCI
Gut microbiome as a biomarker and therapeutic target for treating obesity or an obesity related disorder
InactiveUS20100172874A1Decreasing energy harvestingGood for weight lossBiocideMetabolism disorderDiseaseMicroorganism
The present invention relates to the gut microbiome as a biomarker and therapeutic target for energy harvesting, weight loss or gain, and / or obesity in a subject. In particular, the invention provides methods of altering and monitoring the relative abundance of Bacteroides and Firmicutes in the gut microbiome of a subject.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Probiotic recolonisation therapy
The present invention relates to pharmaceutical compositions suitable for the treatment of chronic diseases associated with the presence of abnormal or an abnormal distribution of microflora in the gastrointestinal tract of a mammalian host, which compositions comprise viable non-pathogenic or attenuated pathogenic Clostridia. The compositions further comprise one or more additional viable non-pathogenic or attenuated pathogenic microorganisms selected from the group consisting of Bacteroides, Eubacteria, Fusobacteria, Propionibacteria, Lactobacilli, anaerobic cocci, Ruminococcus, E.Coli, Gemmiger, Desullomonas, Peptostreptococcus, and fungi. The present invention also provides pharmaceutical compositions suitable for the treatment of the same chronic diseases comprising viable non-pathogenic or attenuated pathogenic Escherichia coli, at least one strain of viable non-pathogenic or attenuated pathoenic Bacteroides and at least one strain of viable non-pathogenic or attenuated pathogenic microorganism.
Owner:FINCH THERAPEUTICS HLDG LLC
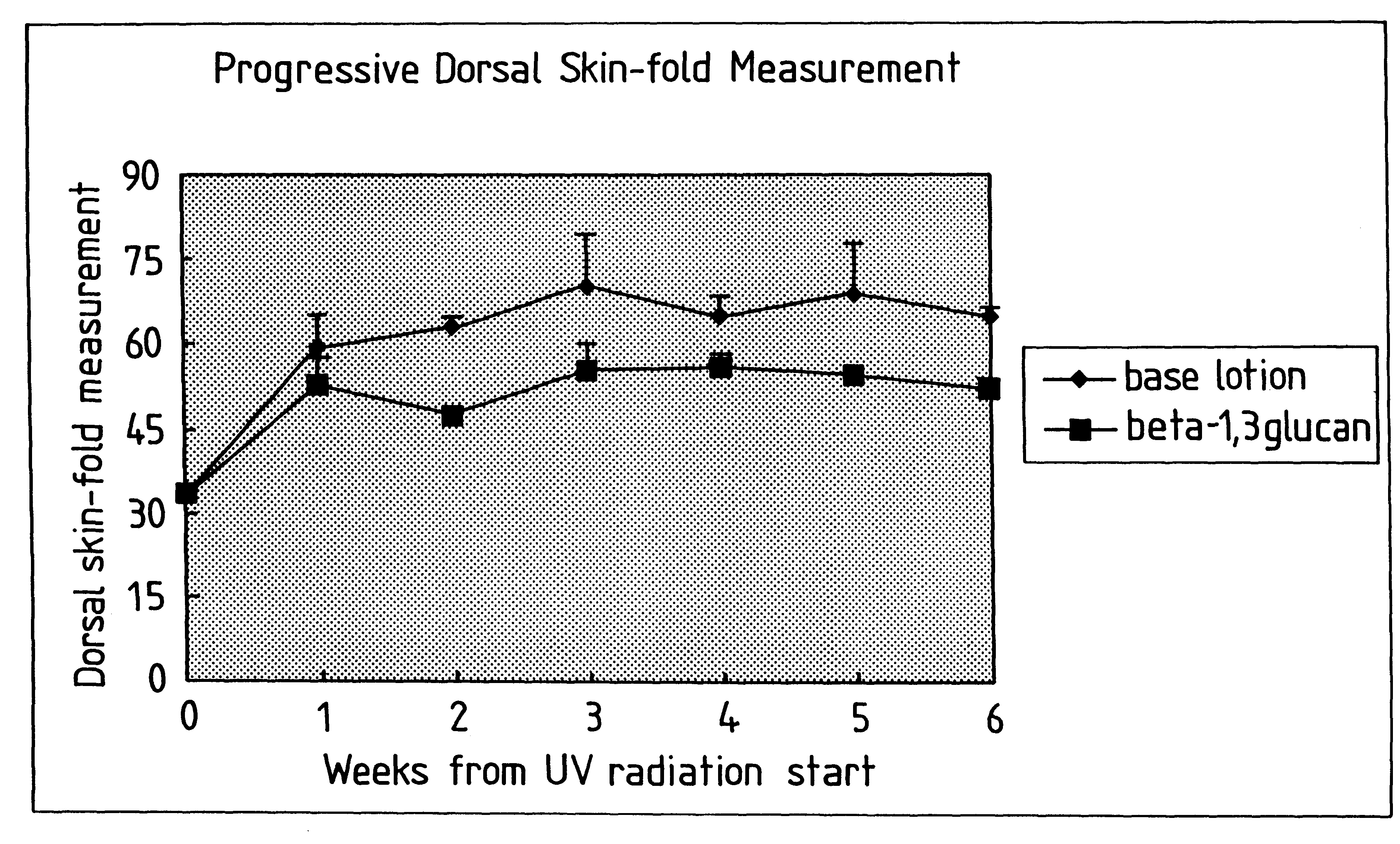
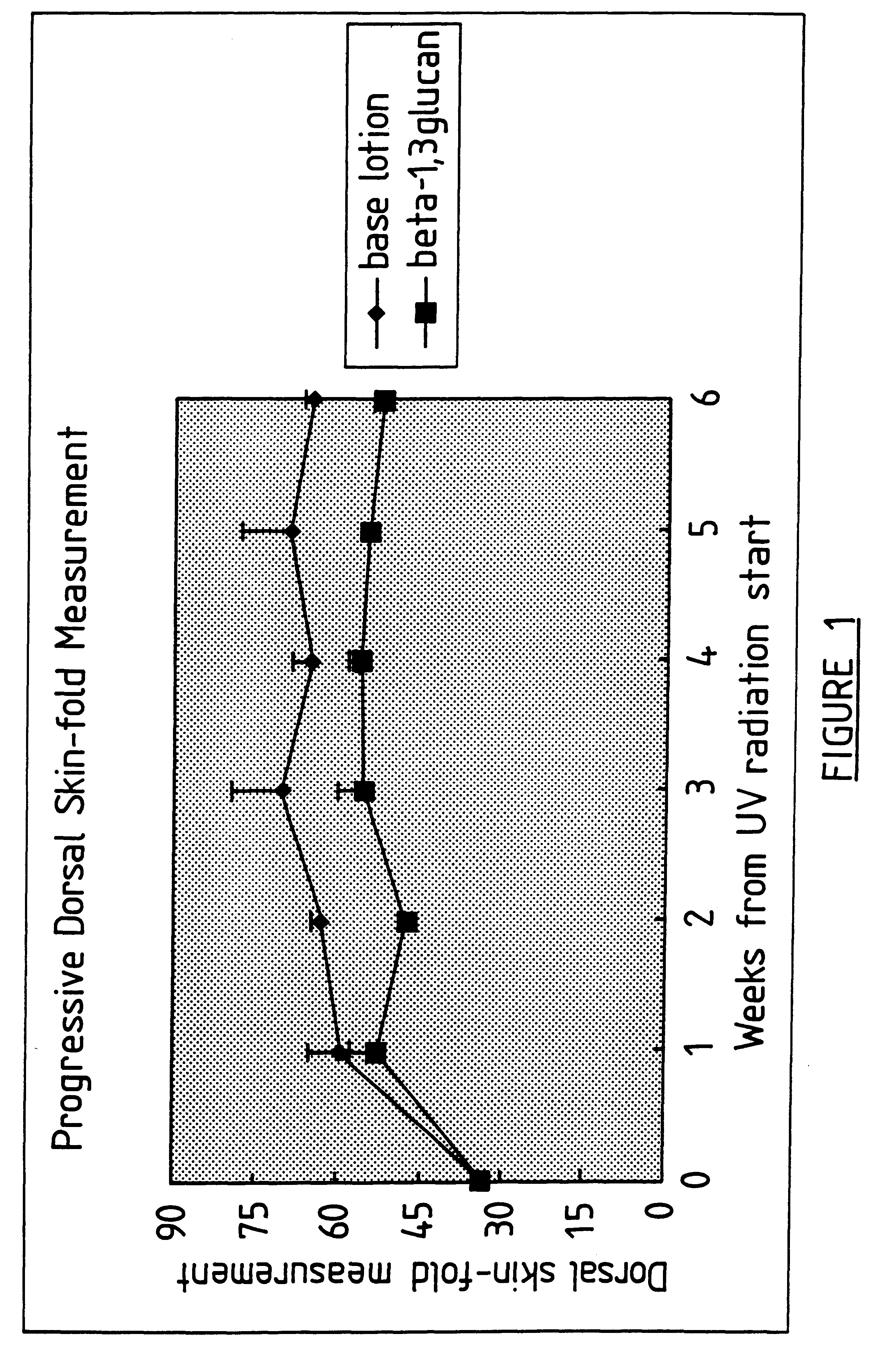
Process for glucan preparation and therapeutic uses of glucan
InactiveUS6242594B1Low costSuitable solubility characteristicAntibacterial agentsOrganic active ingredientsOrganic solventMicroparticle
A process for the production of beta-3-(1,3)(1,6) glucan from a glucan containing cellular source is described, together with compositions and uses / methods of treatment involving glucan. The process of the invention comprises the steps of: (a) extracting glucan containing cells with alkali and heat, in order to remove alkali soluble components; (b) acid extracting the cells of step (a) with an acid and heat to form a suspension; (c) extracting the suspension obtained of step (b) or recovered hydrolyzed cells with an organic solvent which is non-miscible with water and which has a density greater than that of water separating the resultant aqueous phase, solvent containing phase and interface so that substantially only the aqueous phase comprising beta-(1,3)(1,6) glucan particulate material remains; wherein the extraction with said organic solvent provides separation of glucan subgroups comprising branched beta-(1,3)(1,6)-glucan, and essentially unbranched beta-(1,3) glucan which is associated with residual non-glucan contaminents; and (d) drying the glucan material from step (c) to give microparticulate glucan.
Owner:TR THERAPEUTICS
Use of neurotoxin therapy for treatment of urologic and related disorders
The present invention relates to methods for treating neurological-urological conditions. This is accomplished by administration of at least one neurotoxin.
Owner:ALLERGAN INC
Clostridial toxin derivatives able to modify peripheral sensory afferent functions
InactiveUS6395513B1Pain reliefReduce and preferably prevent transmissionNervous disorderPeptide/protein ingredientsClostridial toxinProjection neuron
The invention relates to an agent specific for peripheral sensory afferents. The agent may inhibit the transmission of signals between a primary sensory afferent and a projection neuron by controlling the release of at least one neurotransmitter or neuromodulator from the primary sensory afferent. The agent may be used in or as a pharmaceutical for the treatment of pain, particularly chronic pain.
Owner:HEALTH PROTECTION AGENCY +1
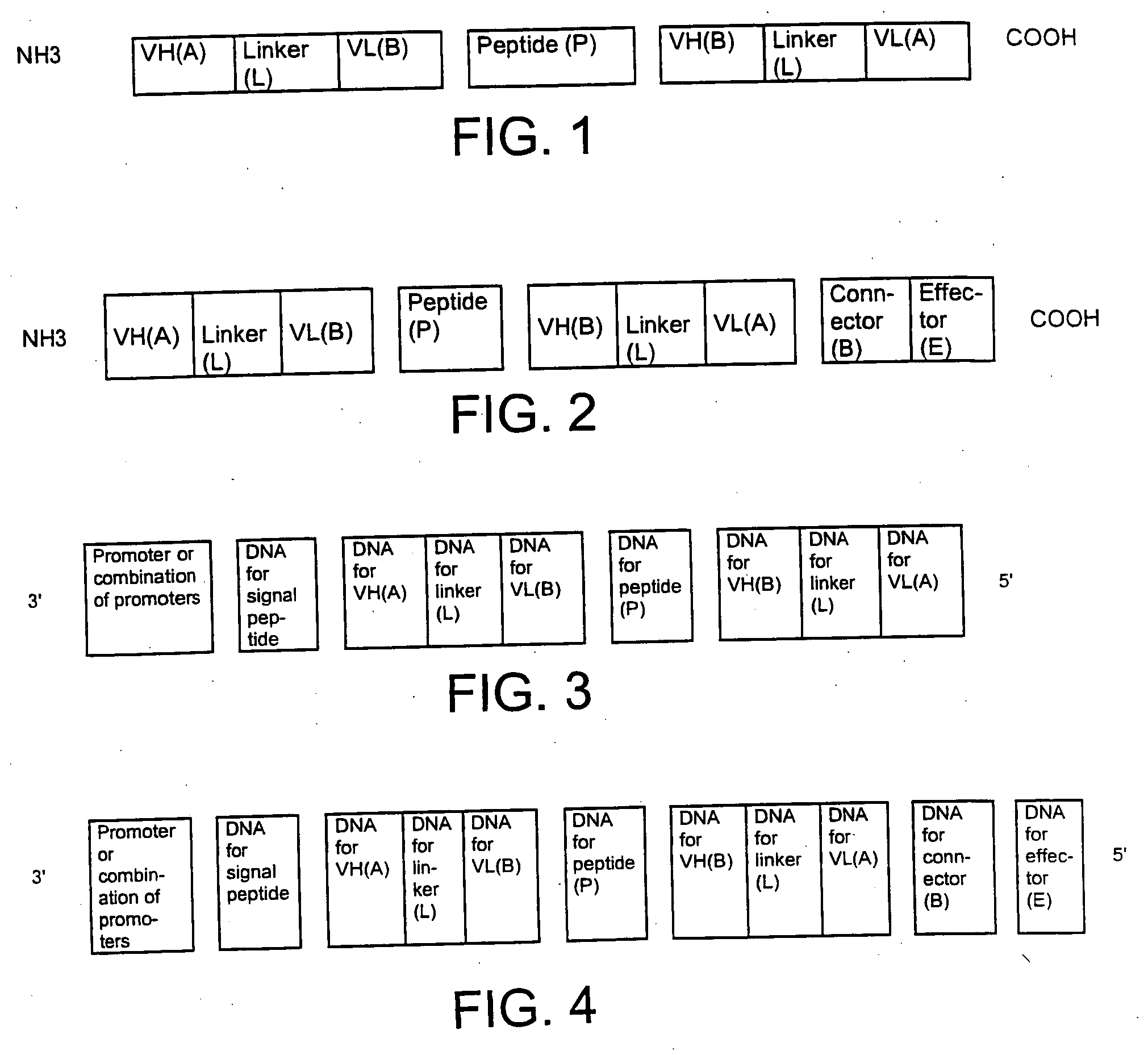
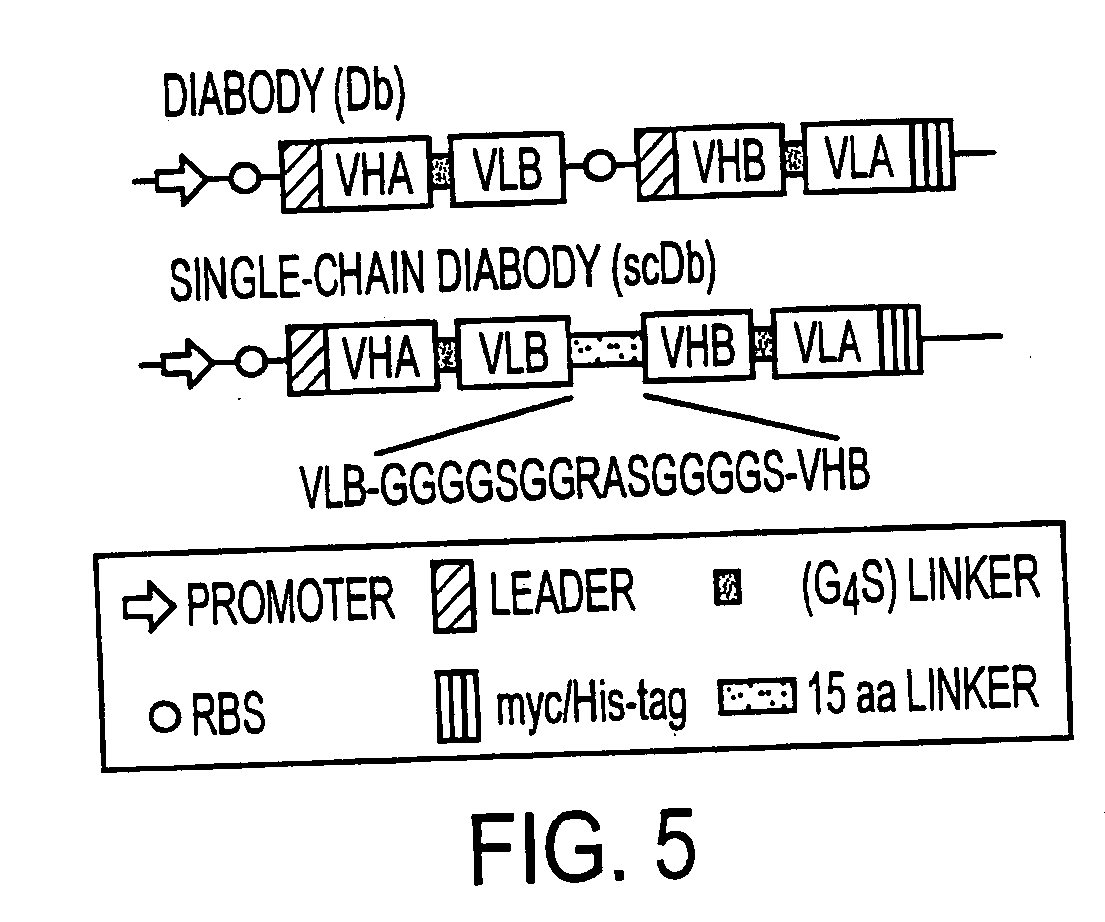
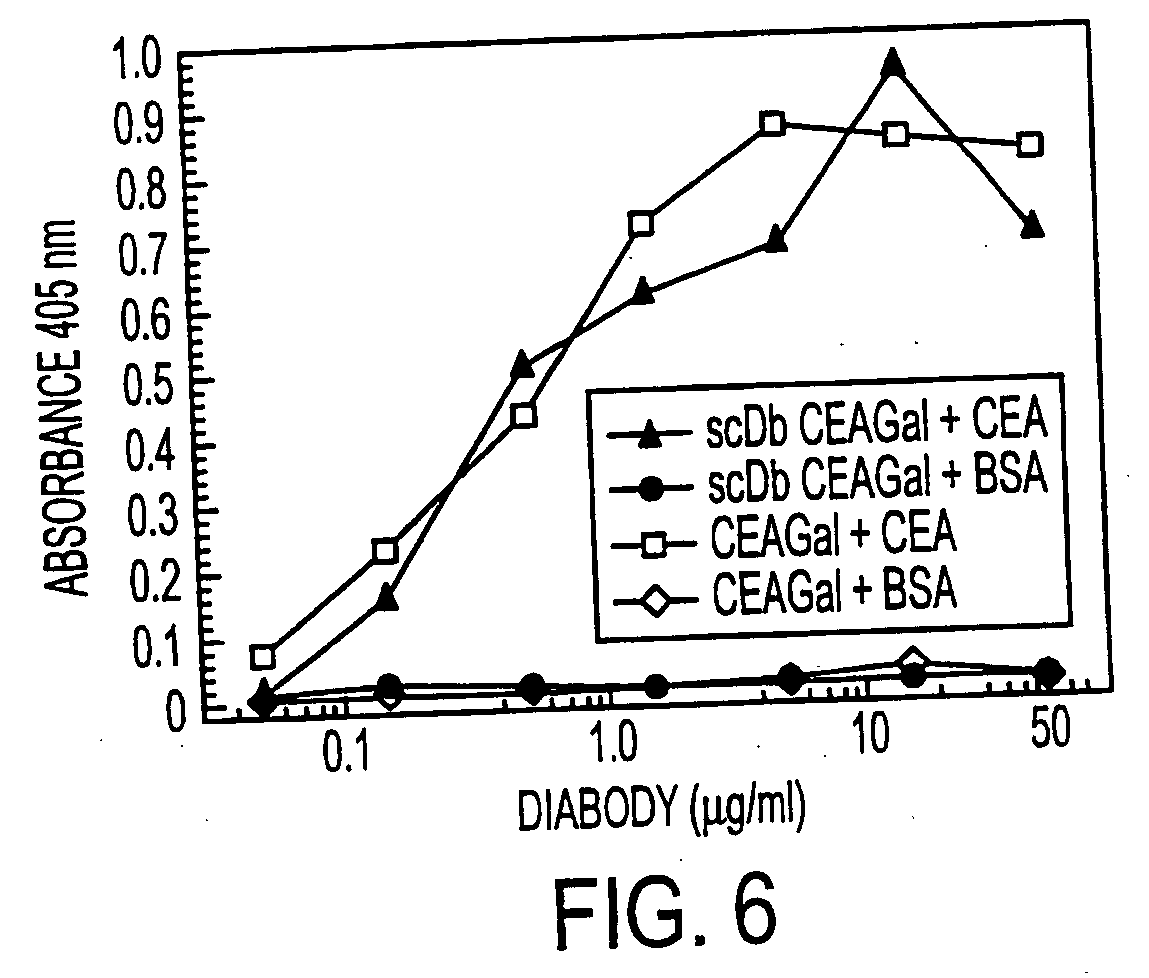
Single-chain multiple antigen-binding molecule, its preparation and use
InactiveUS20050004352A1Reduce dissociationLess complexOrganic active ingredientsFungiAntigen bindingVariable domain
The present invention relates to a single-chain, multiple antigen-binding molecule with diverse variable domains of a heavy and of a light chain of an immunoglobulin, which are connected in the form of a VH-VL construct, which are in turn connected together via a peptide, and to the preparation and use thereof as pharmaceutical or diagnostic aid.
Owner:AFFITECH RESEARCH AS
Method for treatment of disorders of the gastrointestinal system
There are provided novel synthetic stool preparations comprising bacteria isolated from a fecal sample from a healthy donor. The synthetic stool preparations are used for treating disorders of the gastrointestinal tract, including dysbiosis, Clostridium difficile infection and recurrent Clostridium difficile infection, prevention of recurrence of Clostridium difficile infection, treatment of Crohn's disease, ulcerative colitis, irritable bowel syndrome, inflammatory bowel disease, and diverticular disease, and treatment of food poisoning such as salmonella. Methods of preparation and methods of use of the synthetic stool preparations are also provided.
Owner:UNIVERSITY OF GUELPH +2
Methods for using tetanus toxin for beneficial purposes in animals (mammals)
Methods of using tetanus toxin to modulate or control neural functions or nonneural cellular activities at selected sites in animals, particularly in mammals, and more particularly in humans, are provided. Pharmaceutical formulations to modulate neural functions or non-neural cellular activities of an animal at selected sites in animals, particularly in mammals, and more particularly in humans are also provided. Uses of tetanus toxin in preparation of medicaments for methods of treating clinical disorders or symptoms of animals, particularly mammals and more particularly humans are also provided.
Owner:SANDERS
Health supplement
InactiveUS20090110674A1Avoid quantityMaximizing synergistic interactionPeptide/protein ingredientsBacteria material medical ingredientsBody systemAdditive ingredient
A health supplement consisting of numerous ingredients from several general groups including: anti-aging, anti-oxidant, vitamins, minerals, and elemental substances including metals. The supplement is specifically formulated for maximizing synergistic interaction of the ingredients while eliminating harmful interaction among the ingredients. A holistic approach to health is followed with ingredients to aid all systems of the human body. By addressing all human body systems, the supplement acts as a precursor to anti-aging and, when body systems are detoxified and oxidation is controlled, the effect of aging may be reversed.
Owner:AGA AB
Method for treating neuromuscular disorders and conditions with botulinum toxin types A and B
A method of treating a patient suffering from a disease, disorder or condition includes the administration to the patient of a therapeutically effective amount of botulinum toxin of a selected serotype until the patient experiences loss of clinical response to the administered botulinum toxin and thereafter administering to the patient a therapeutically effective amount of another botulinum toxin of a different serotype.
Owner:SOLSTICE NEUROSCI
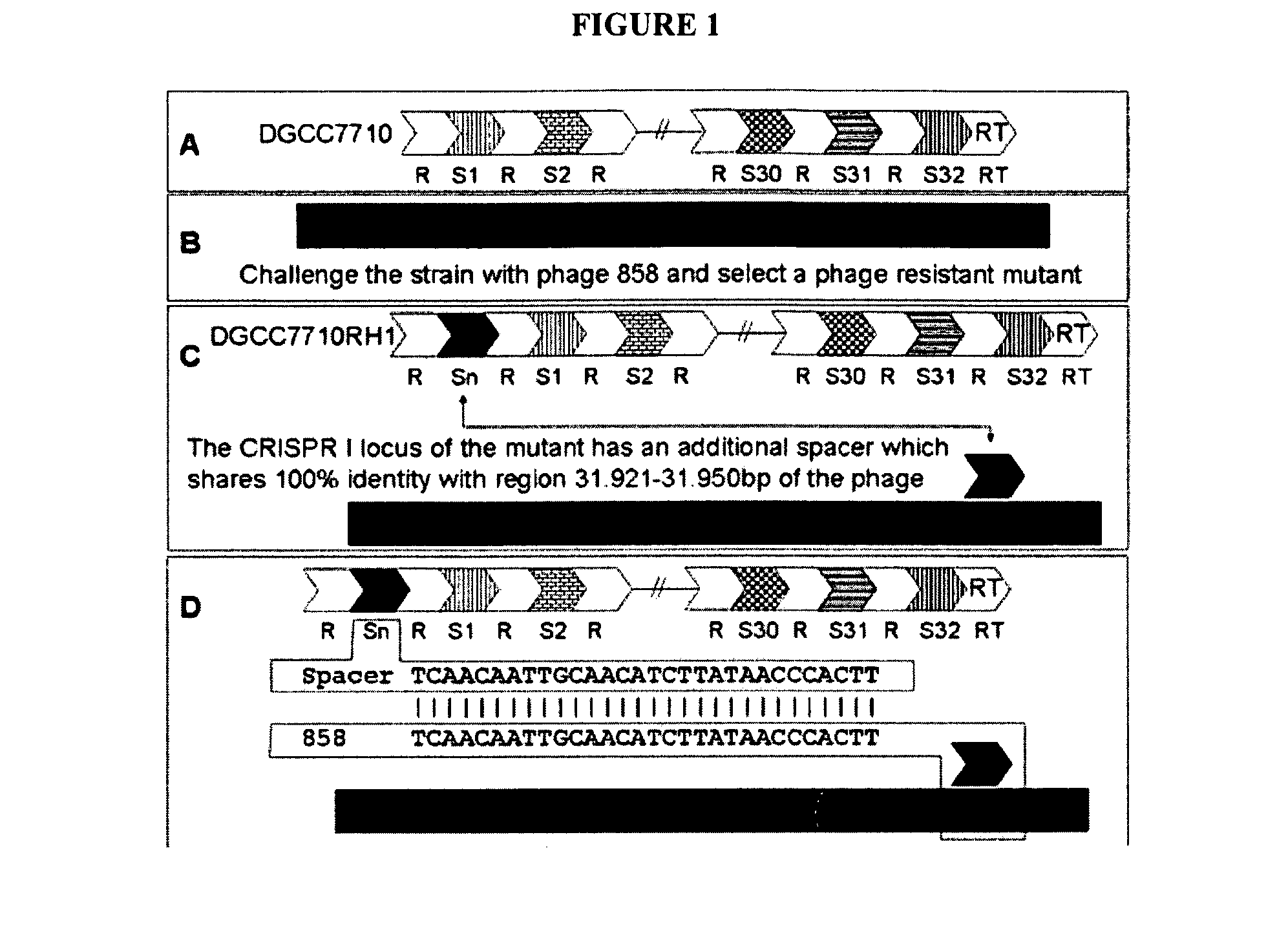
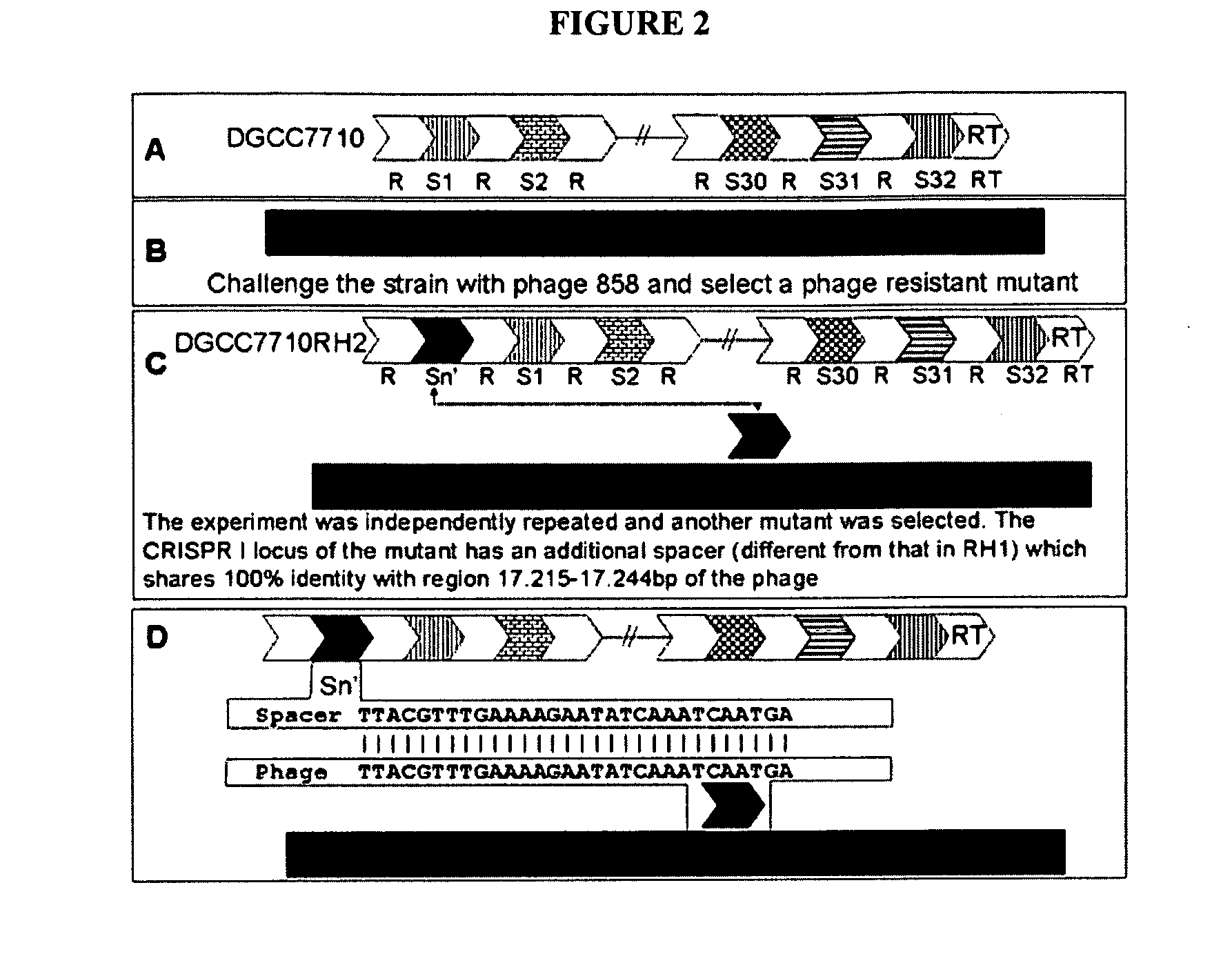
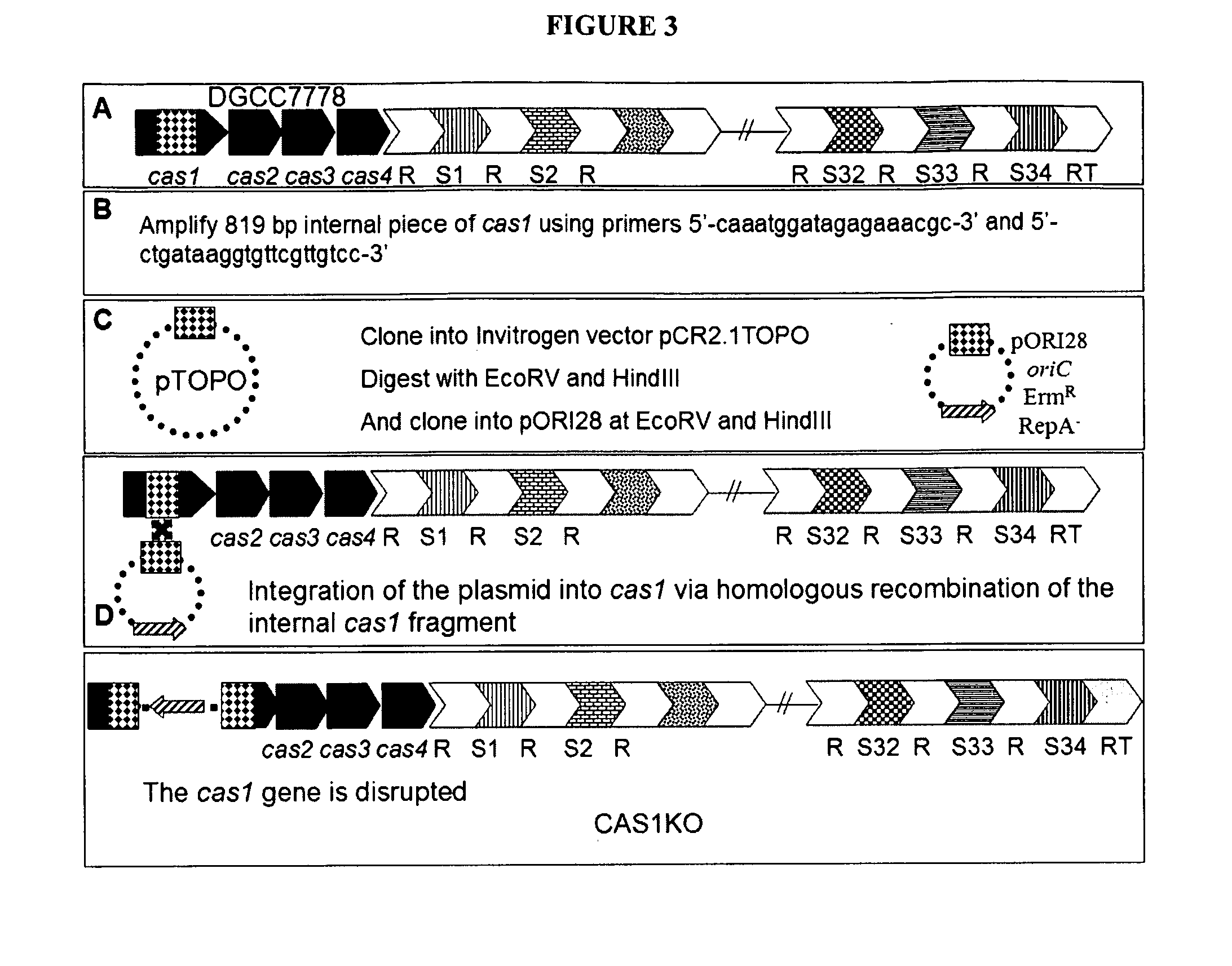
Cultures with Improved Phage Resistance
InactiveUS20110002889A1Reduced degree of homologyReduce decreaseBiocideBacteriaVirulent characteristicsBacteriophage
The present invention provides methods and compositions related to modulating the resistance of a cell against a target nucleic acid or a transcription product thereof. In some preferred embodiments, the present invention provides compositions and methods for the use of one or more cas genes or proteins for modulating the resistance of a cell against a target nucleic acid or a transcription product thereof. In some embodiments, the present invention provides methods and compositions that find use in the development and use of strain combinations and starter culture rotations. In additional embodiments, the present invention provides methods for labelling and / or identifying bacteria. In some preferred embodiments, the present invention provides methods for the use of CRISPR loci to determine the potential virulence of a phage against a cell and the use of CRISPR-cas to modulate the genetic sequence of a phage for increased virulence level. In still further embodiments, the present invention provides means and compositions for the development and use of phages as biocontrol agents.
Owner:DUPONT NUTRITION BIOSCIENCES APS
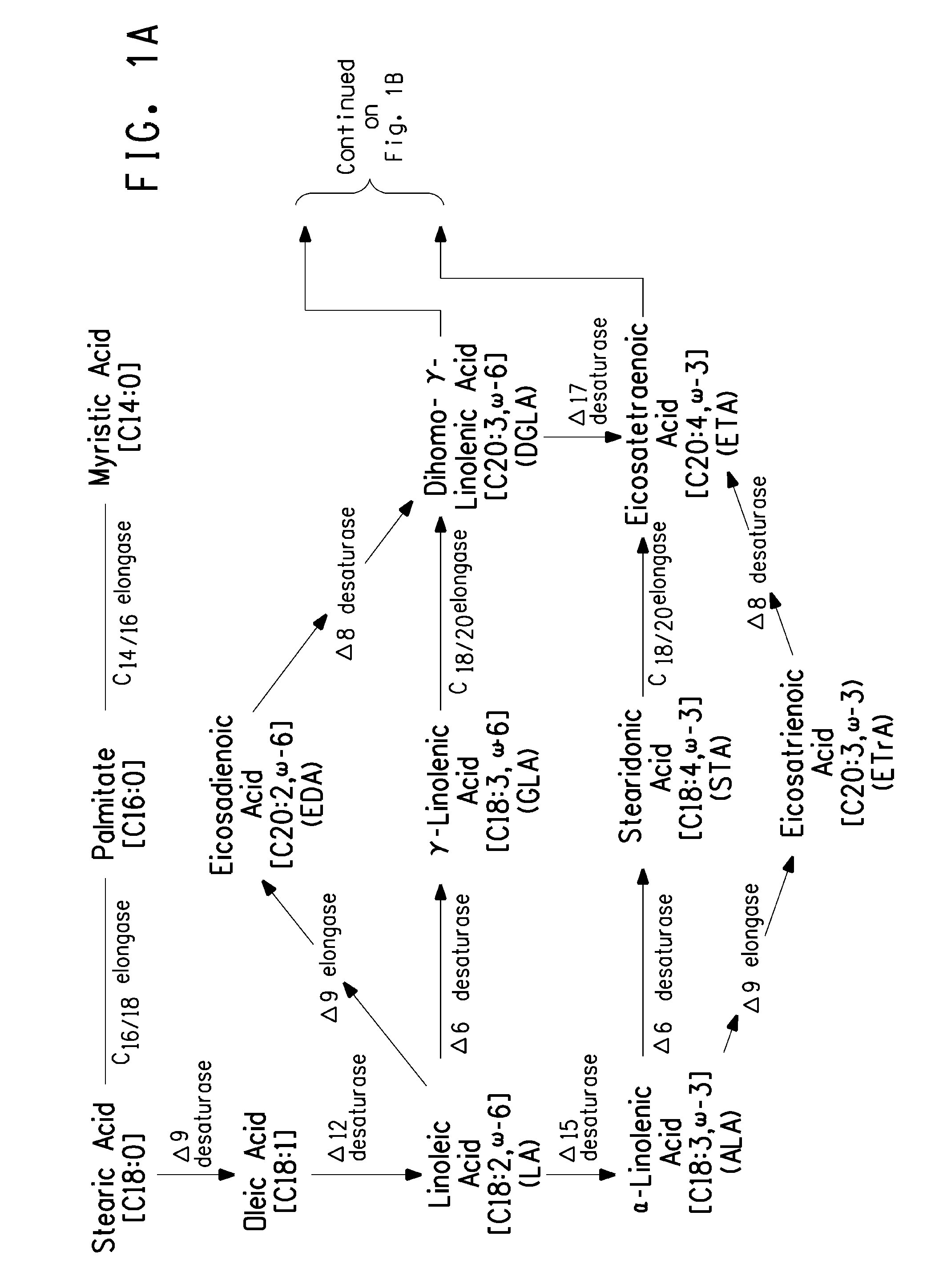
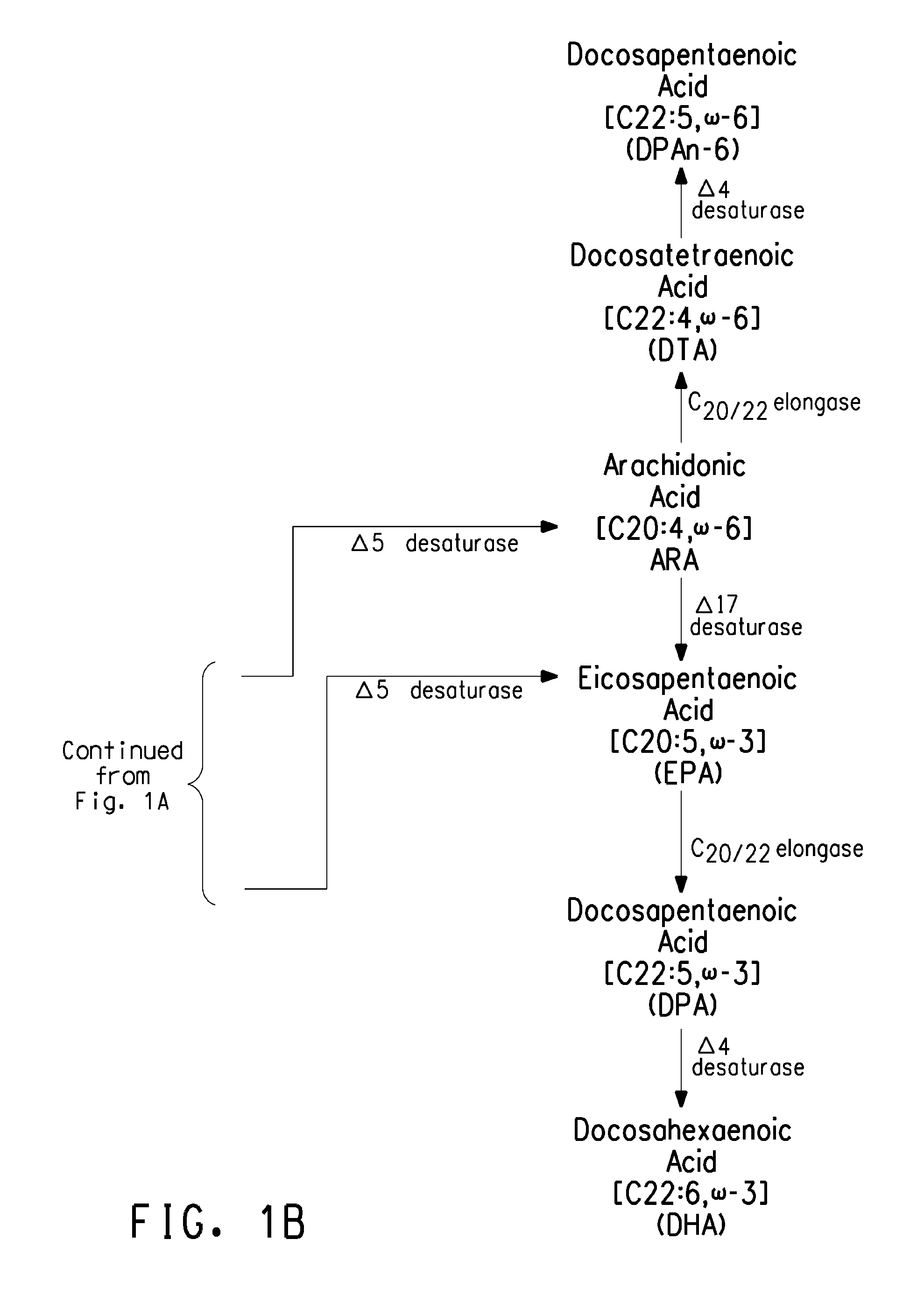
Optimized strains of yarrowia lipolytica for high eicosapentaenoic acid production
Engineered strains of the oleaginous yeast Yarrowia lipolytica capable of producing greater than 50 weight percent of eicosapentaenoic acid [“EPA”], an ω-3 polyunsaturated fatty acid, in the total oil fraction are described. These strains over-express heterologous Δ9 elongases, Δ8 desaturases, Δ5 desaturases, Δ17 desaturases, Δ12 desaturases and C16 / 18 elongases, and optionally over-express diacylglycerol cholinephosphotransferases. Preferred gene knockouts are also described. Production host cells, methods for producing EPA within said host cells, and products comprising EPA from the optimized Yarrowia lipolytica strains are claimed.
Owner:DUPONT US HLDG LLC
Method of treating gastrointestinal diseases associated with species of genus clostridium
The invention includes a method of treating gastrointestinal diseases associated with species of genus Clostridium such as clostridium deficit in human patients with gastrointestinal disorders having an etiological component such as a microbial agent producing a toxin where treated with an antimicrobial composition an amount effective to inhibit or eliminate the microbial agent. The antimicrobial composition in a form of probiotic mixture can be administrated alone or in combination with an antimicrobial agent, such as a bacteriophage which is specific for a bacterium producing toxin or antibiotics which are then used to eliminate or inhibit the clostridial species overgrown in a patient's gastrointestinal tract. Disorders that can be treated by the method of the invention include diarrhea or inflammatory bowel diseases such as colitis or Crohn's disease.
Owner:UNITED STATES OF AMERICA
Microparticles for Oral Delivery
The invention provides microbeads containing oil-associated biologically active compounds and methods for their manufacture and use. The microbeads consist of a soluble complex of non-digestible polymer and emulsifier with oil-associated biologically active compounds embedded in a matrix of digestible polymer. The disclosed microbead complex protects the biologically active compounds, such as vitamins, fish oil and carotenoids, from oxidation, taste and odor degradation. The disclosed microbeads also provide protection from the stomach digestive distraction, and allows for the delivery of the biologically active compounds in the intestine.
Owner:INTERVET INC
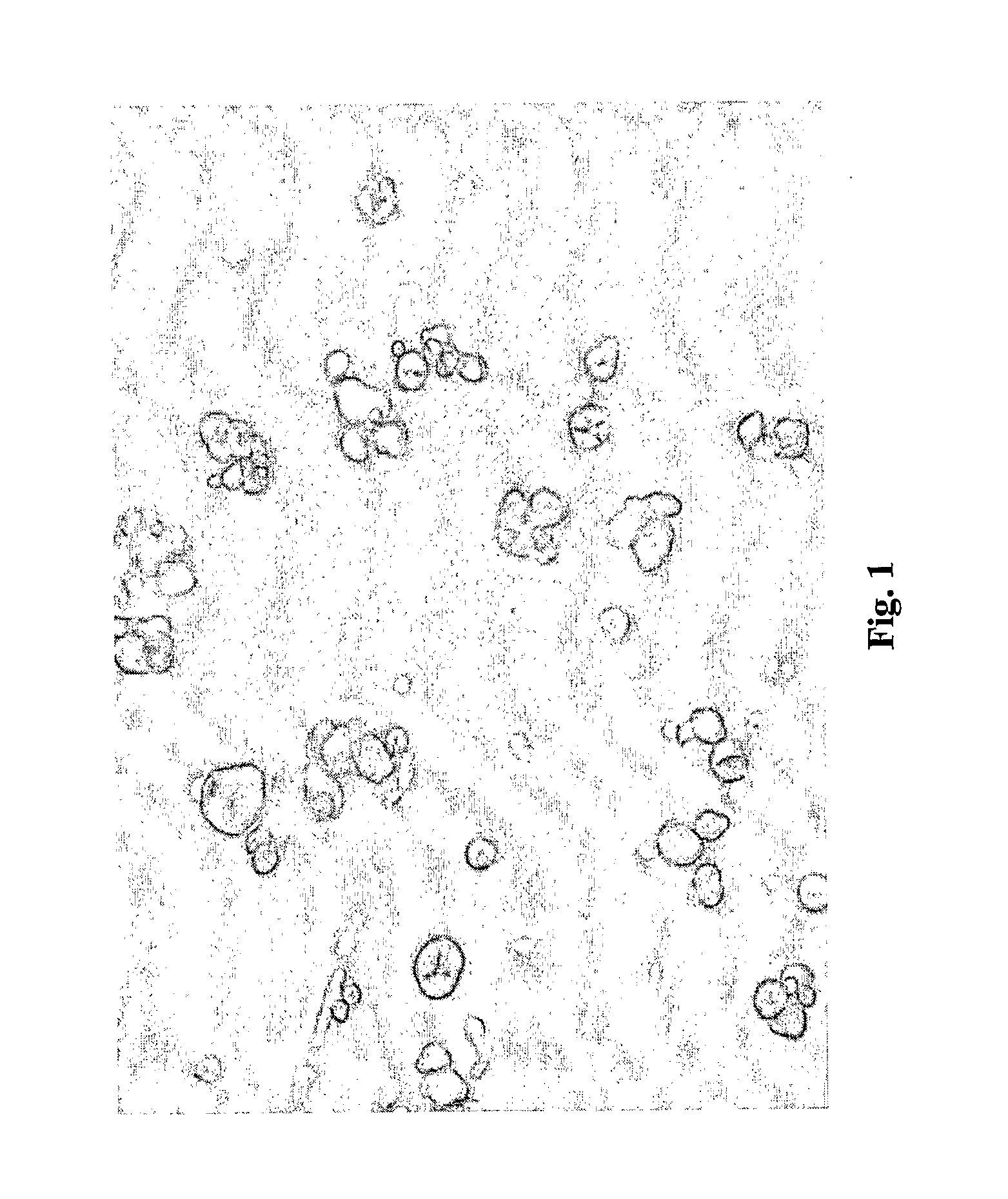
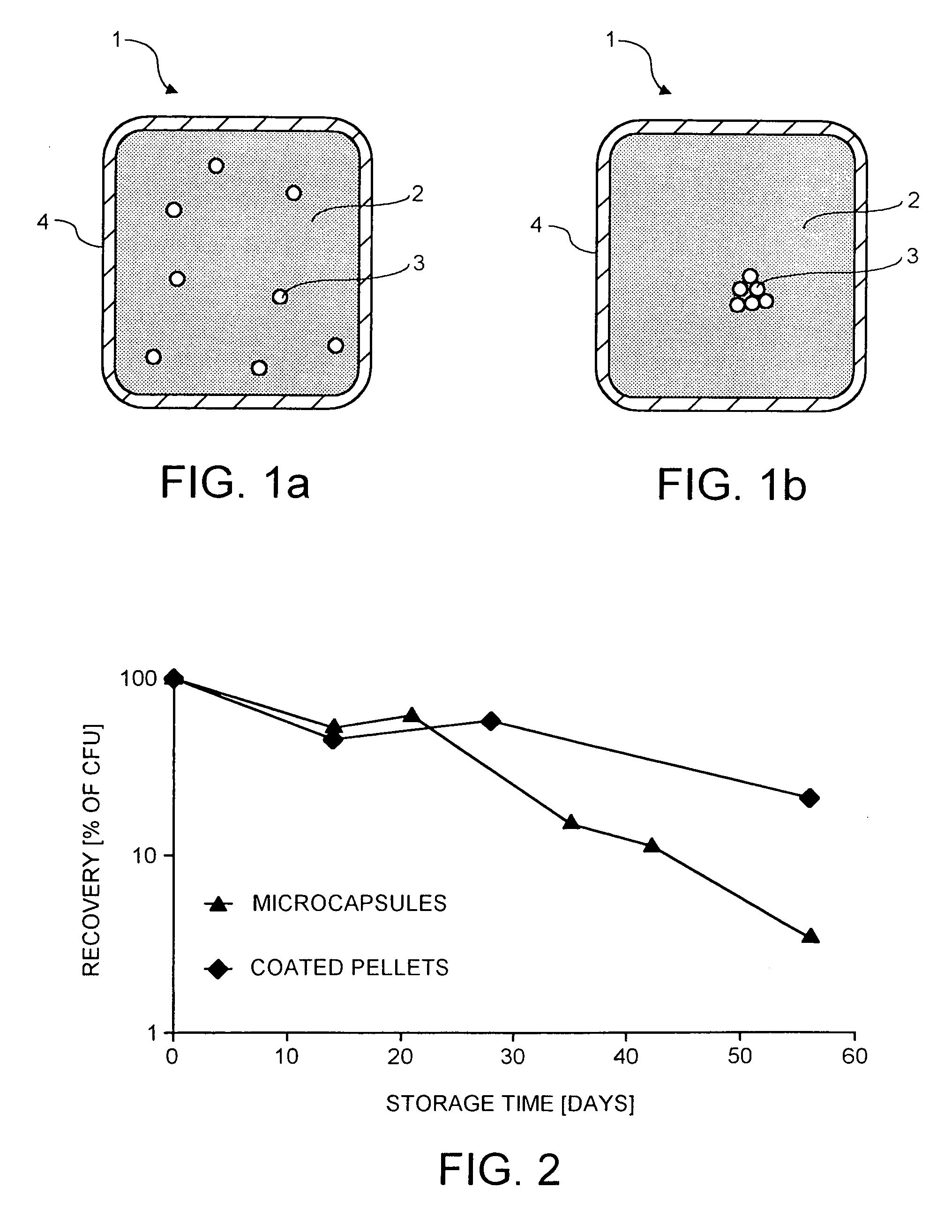
Probiotic delivery system
InactiveUS20050153018A1Improve stabilityProcessing is easy and straightforwardFood ingredient as barrier agentMicroorganism preservationMicroorganismBiotechnology
The present invention relates to a probiotic delivery system that is preferably added to a food product. In particular, the invention shows that compacted pellets having a volume of at least 0.02 cm3, that comprise, besides viable micro-organisms, arbitrary or eligible components, such as fillers, binder, plasticizer, other functional ingredients and a coating may be added to semi-moist, moist or semi-dry products. The micro-organisms remain viable for a longer time than commercially obtainable preparations of probiotics.
Owner:NESTEC SA
Storage stable compositions of biological materials
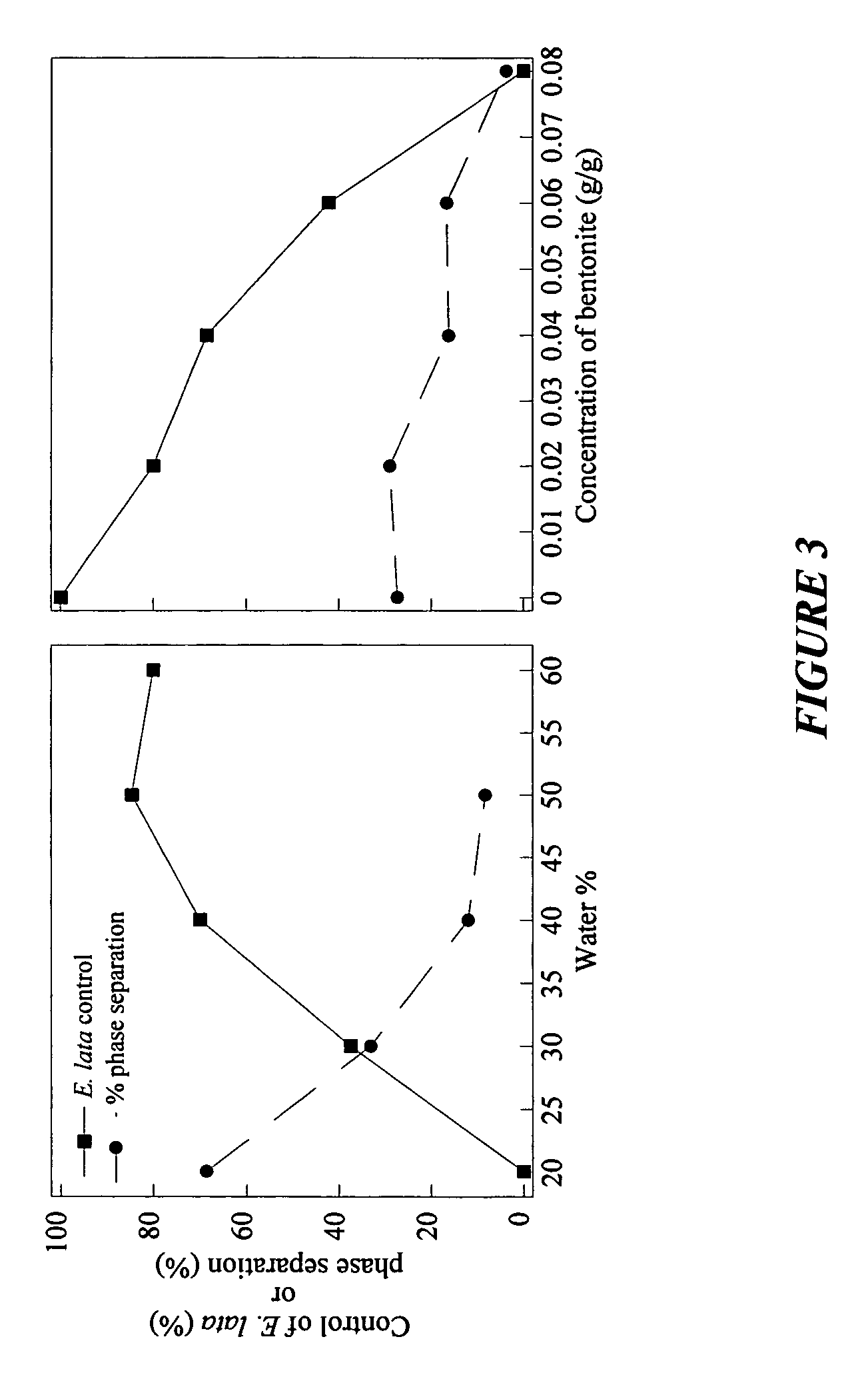
Storage stable compositions of biological materials, including bioactive biological materials are provided in the form of a water-in-oil emulsion, comprising:(a) cellular material selected from living and / or dormant prokaryotic and / or eukaryotic cells and tissues, the cellular material being compatible with water-in-oil emulsions;(b) one or more oils selected from vegetable oils and fish oils;(c) an oil-soluble nonionic polymeric surfactant having a molecular weight of from about 2500 to about 15000; and(d) water.The compositions may also contain a thickener such as a hydrophobic fumed silica or bentonite.Compositions may be used for various purposes, depending on the contained biological material. Specific examples include compositions containing Fusarium lateritium control of Eutypa lata in plant wounds made by cutting or pruning, and compositions containing Lagenidium giganteum for control of mosquitoes.
Owner:RGT UNIV OF CALIFORNIA
Use of a composition made of mineral nutrients and optionally acetogenic and/or butyrogenic bacteria in order to avoid or reduce the formation of gas in the large intestine of a mammal and the resulting abdominal problems
InactiveUS20100247489A1Raise countSufficient supplyHeavy metal active ingredientsBiocideAcetic acidMammal
The present invention relates to a composition comprising one or more minerals selected from the group consisting of selenium, molybdenum or tungsten, which is carried out galenically or chemically in a way that the mineral or minerals are released completely or in part, just before, during or shortly after arrival at the large intestine, and their use in the manufacture of a medicament for administering to a mammal for the prevention or reduction of gas formation in the colon thus conditioned abdominal complaints, particularly bloatings, meteorism or abdominal cramps. Furthermore, the invention relates to a procedure for the isolation of acetogenic and butyrogenic bacterial strains that are suitable for therapeutic purposes outlined above.
Owner:SAUR BROSCH ROLAND
Method of reducing ecologically adverse changes of the gastro intestinal microbial flora in patients under treatment with medicaments
InactiveUS20020022019A1Reduce generationBiocideOrganic active ingredientsMicroorganismPresent method
A method for reducing ecologically adverse changes of the gastrointestinal micro-flora in patients under treatment with medicaments (which may also be referred to herein as the therapeutic compounds or medications) such as gastric acid reducing medicaments or antibiotics. A pharmaceutical product useful in the present method comprising a medicament and a probiotically active organism as a combined preparation presented in a commercial package unit.
Owner:CHR HANSEN AS
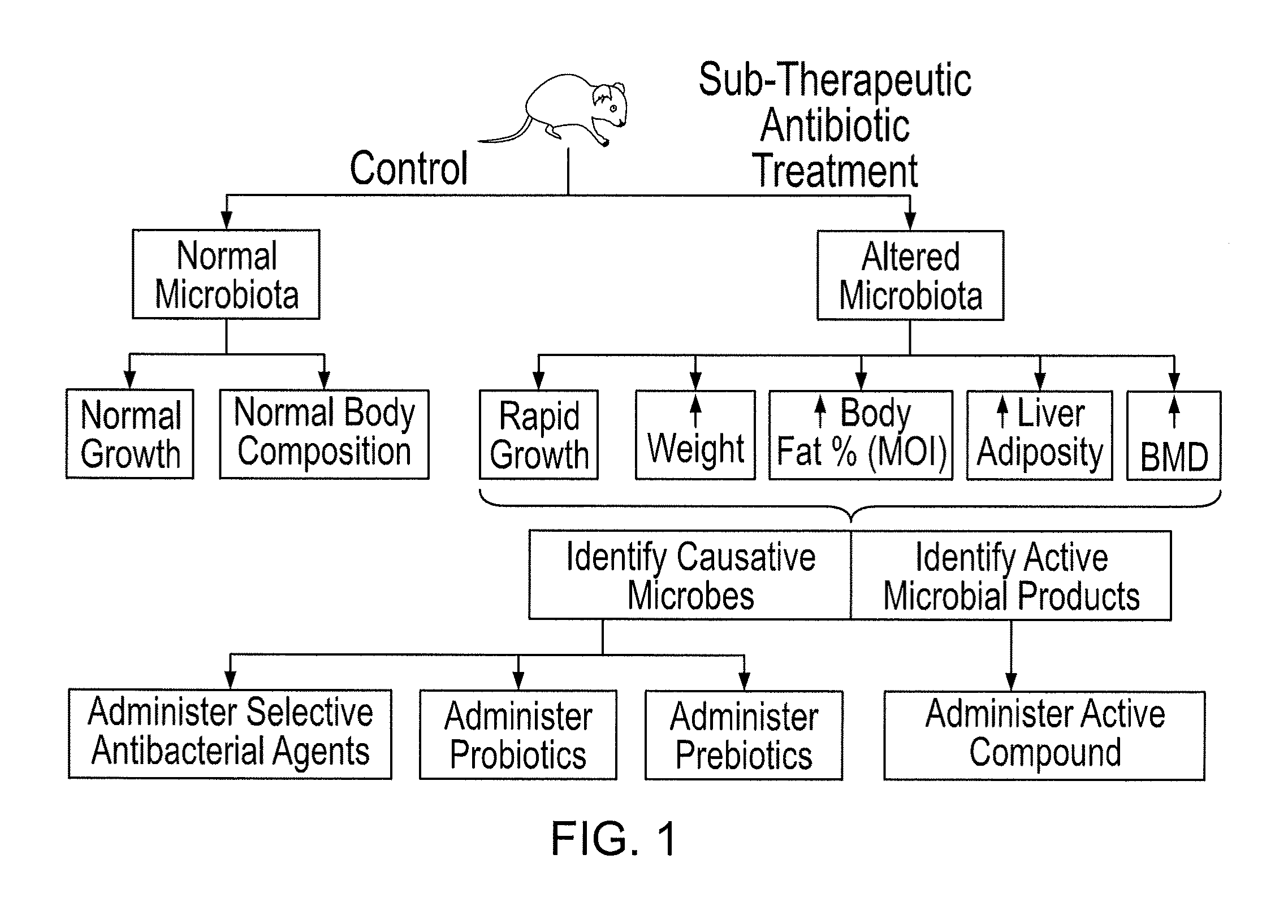
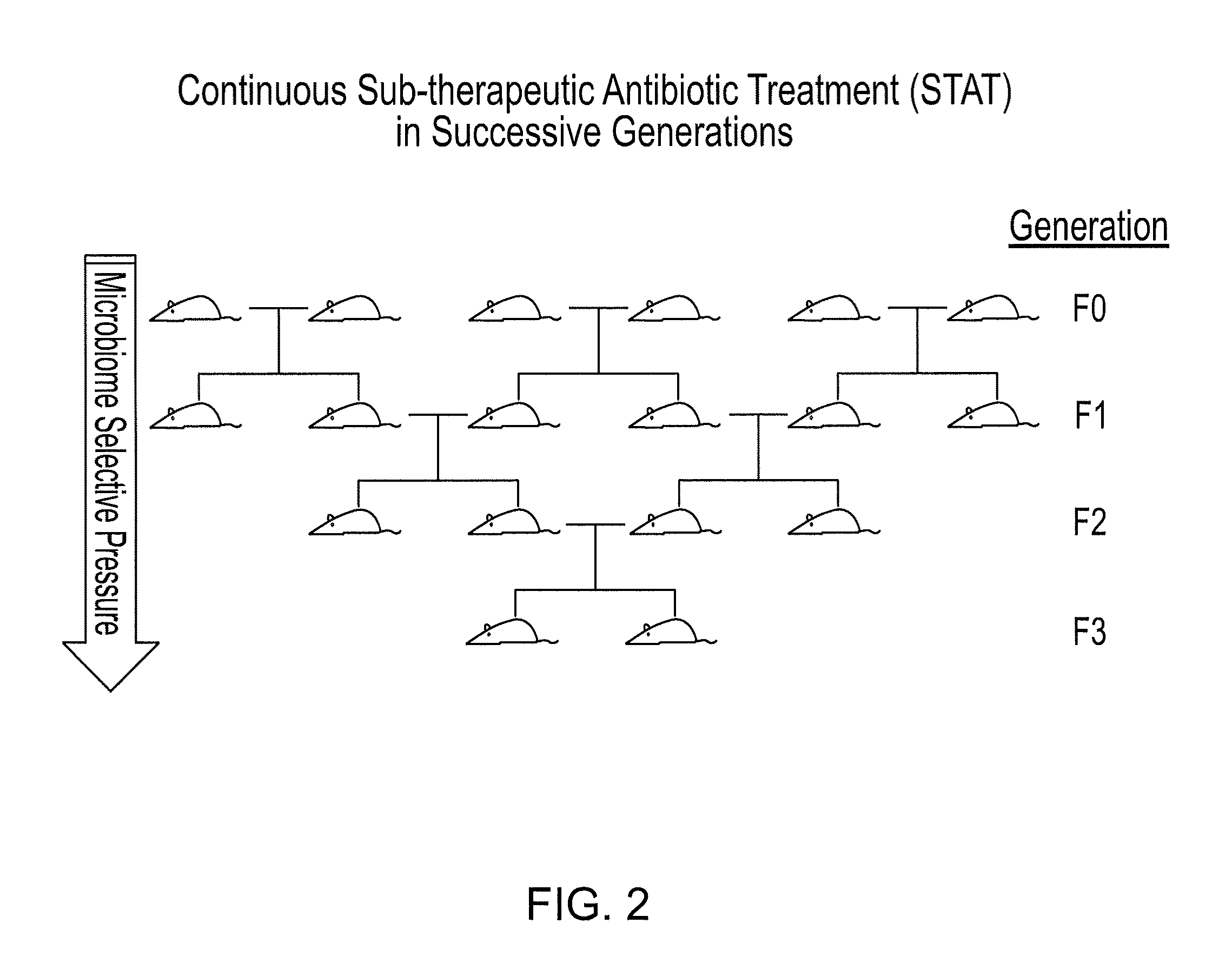
Compositions and methods for treating obesity and related disorders by characterizing and restoring mammalian bacterial microbiota
ActiveUS20110280840A1Increased use of antibioticIncreasing adult height and muscle massBiocideMetabolism disorderIntestinal microorganismsBone formation
The present invention relates to characterizing changes in mammalian gastrointestinal microbiota associated with antibiotic treatment and various disease conditions (such as obesity, metabolic syndrome, insulin-deficiency or insulin-resistance related disorders, glucose intolerance, diabetes, non-alcoholic fatty liver, abnormal lipid metabolism, short stature, osteoporosis, and other disorders of bone formation and mineralization, etc.) and related diagnostic and therapeutic methods. Therapeutic methods of the invention involve the use of probiotics, prebiotics, or narrow spectrum antibiotics / anti-bacterial agents that are capable of restoring healthy mammalian bacterial gastrointestinal microbiota.
Owner:NEW YORK UNIV
Stable probiotic microsphere compositions and their methods of preparation
The invention relates to viable and stable probiotic formulations for intestinal targeting made of microspheres comprising each a core of one or more probiotic bacteria, microcrystallline cellulose with a degree of polymerization from 165-365 and mean diameter from 45 to 180 μm, a disintegrant and a stabilizer, the core being coated with a non-enteric coating and further coated with an enteric coating. Each probiotic microsphere has a residual moisture level of less than 5% and a water activity (aw) between 0.1 and 0.5. Such a probiotic microsphere shows no reduction in viable bacteria after one hour in simulated gastric fluid. The present invention also relates to the process of preparing such formulation.
Owner:CANACURE CORP
Freeze dried fecal microbiota for use in fecal microbial transplantation
The present invention provides freeze-dried compositions that include an extract of human feces and a cryoprotectant, and methods for making and using such compositions, including methods for replacing or supplementing or modifying a subject's colon microbiota, and methods for treating a disease, pathological condition, and / or iatrogenic condition of the colon.
Owner:RGT UNIV OF MINNESOTA
Diagnosis and treatment of autism spectrum disorder
ActiveUS20140065132A1Improve behaviorImprove performanceBiocideNervous disorderClinical psychologyAutism spectrum disorder
Disclosed herein are compositions, systems, and methods for diagnosing and treatment of subjects suffering from anxiety, autism spectrum disorder (ASD), or a pathological condition with one or more of the symptoms of ASD.
Owner:CALIFORNIA INST OF TECH
Alteration of microbial populations in the gastrointestinal tract
InactiveUS6348452B1Increase the number ofHigh activityBiocideBacteria material medical ingredientsMicroorganismResistant starch
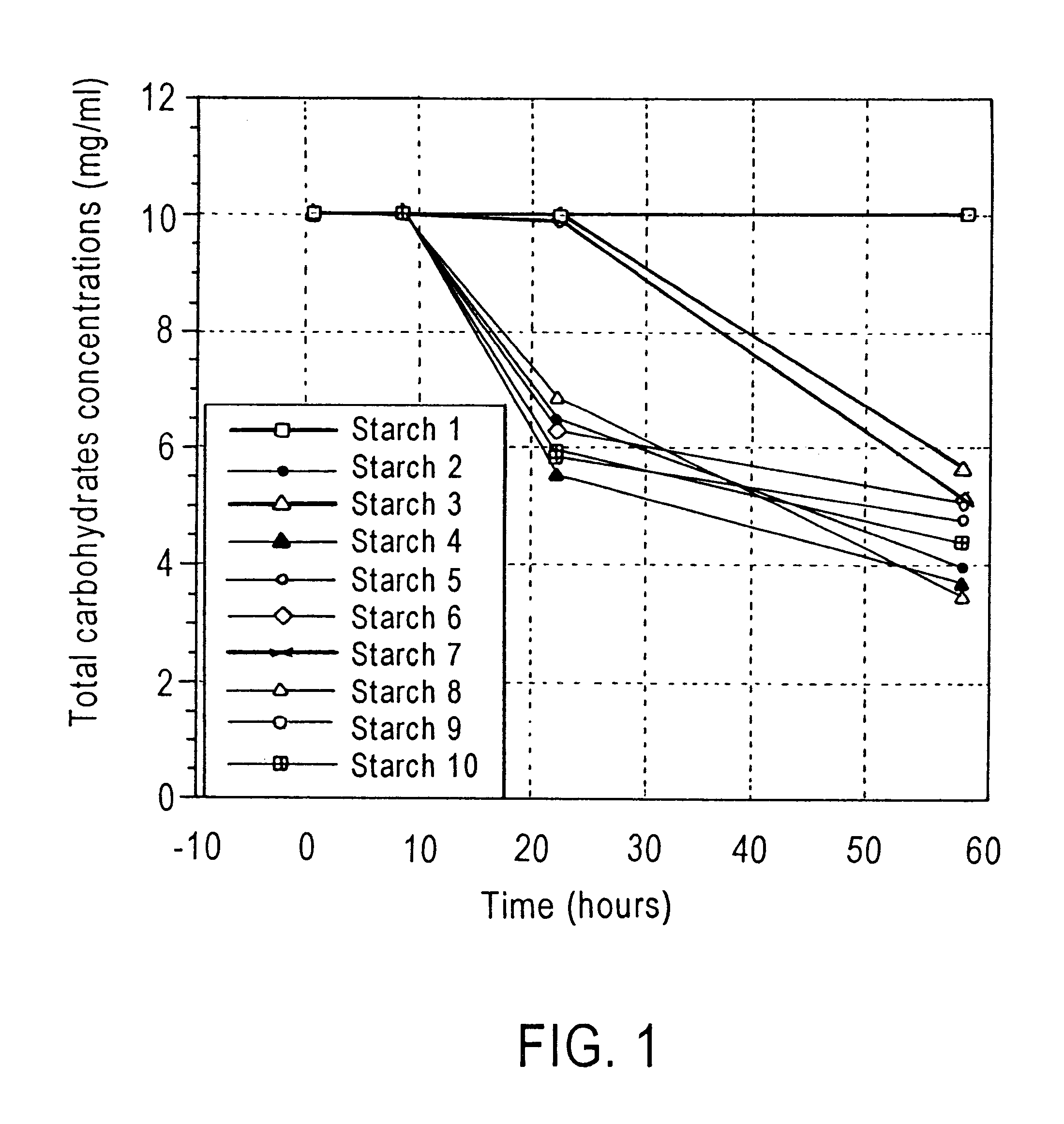
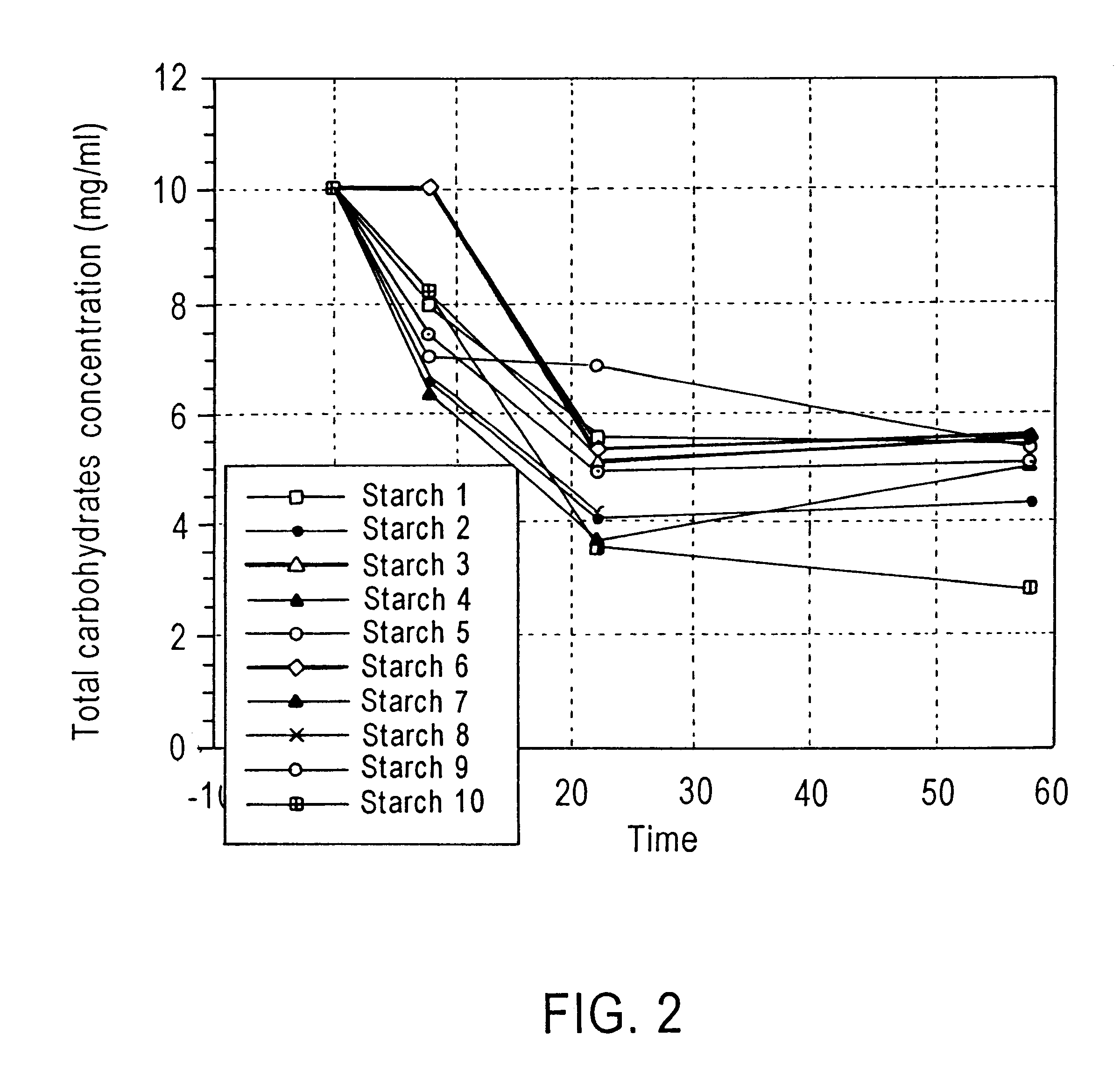
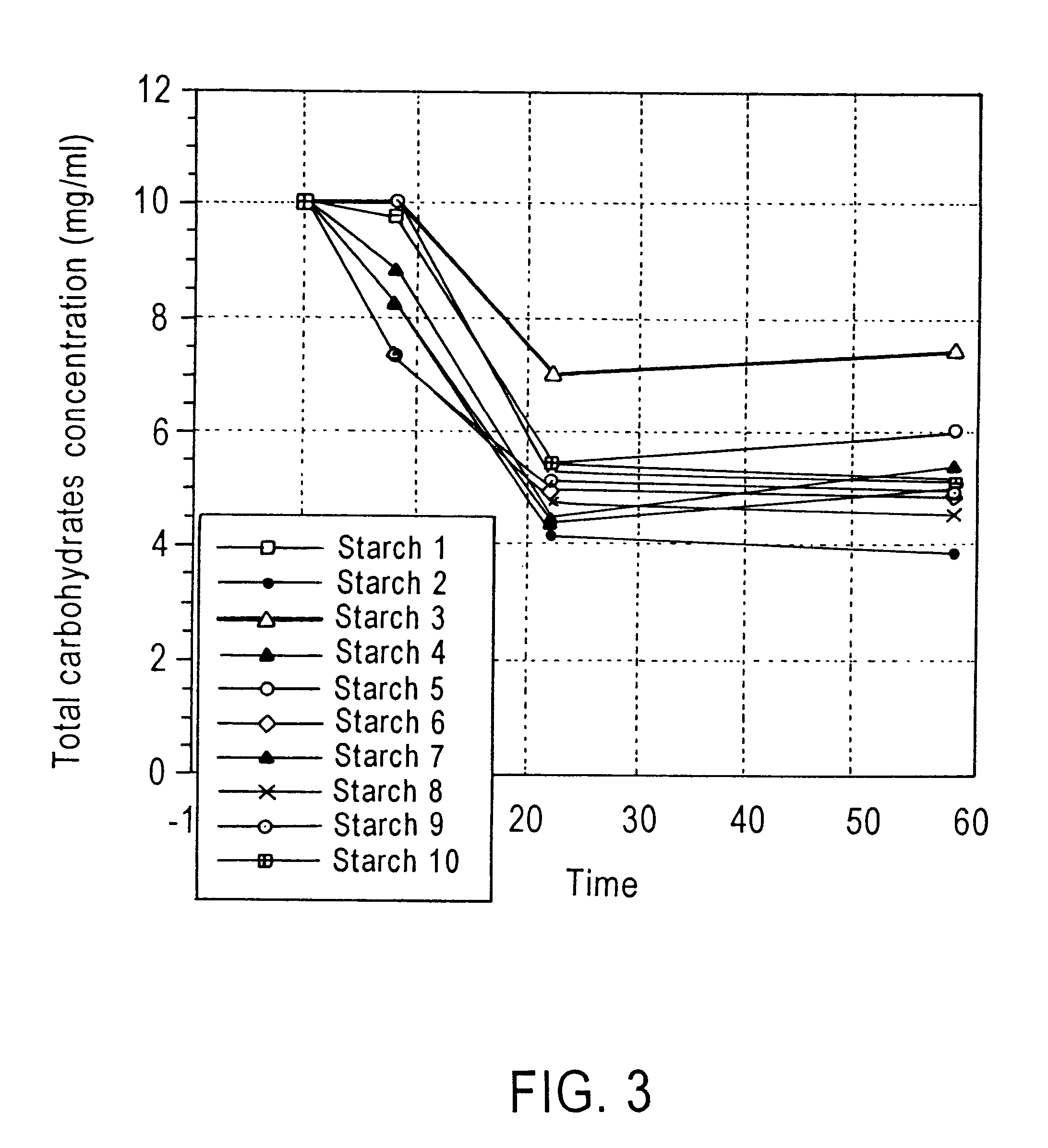
Method of enhancing a resident population of microorganism in a selected site of the gastrointestinal tract of an animal, the method comprising providing to the animal a selected modified or unmodified resistant starch or mixtures thereof in combination with one or more probiotic microorganisms such that upon ingestion the starch passes through the gastrointestinal tract substantially unutilized until it reaches the selected site where it is utilized by the resident and / or the probiotic microorganisms thereof causing an increase in number and / or activity of the microorganisms.
Owner:CORN PROD DEV INC
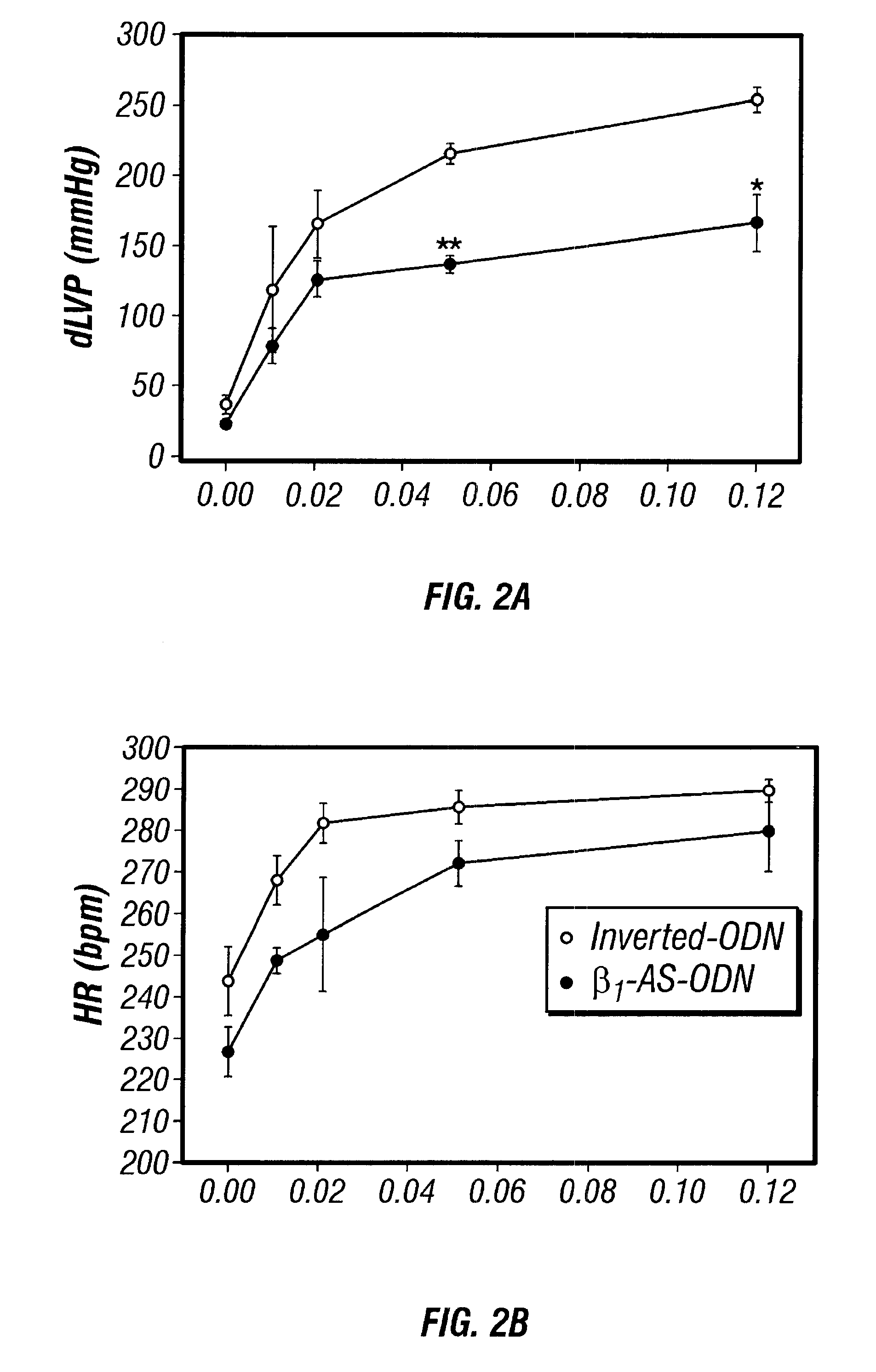
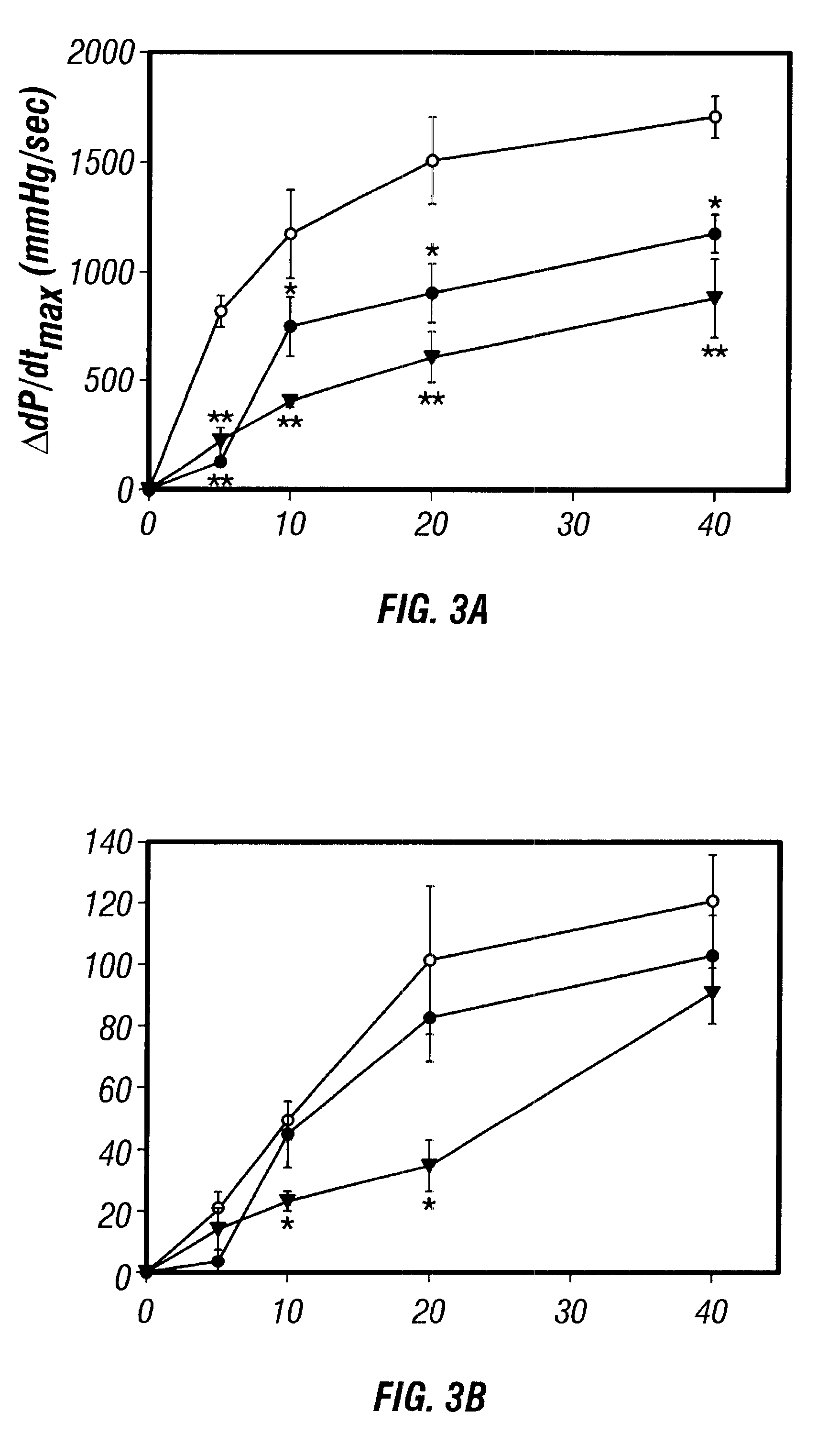
Antisense compositions targeted to beta1-adrenoceptor-specific mRNA and methods of use
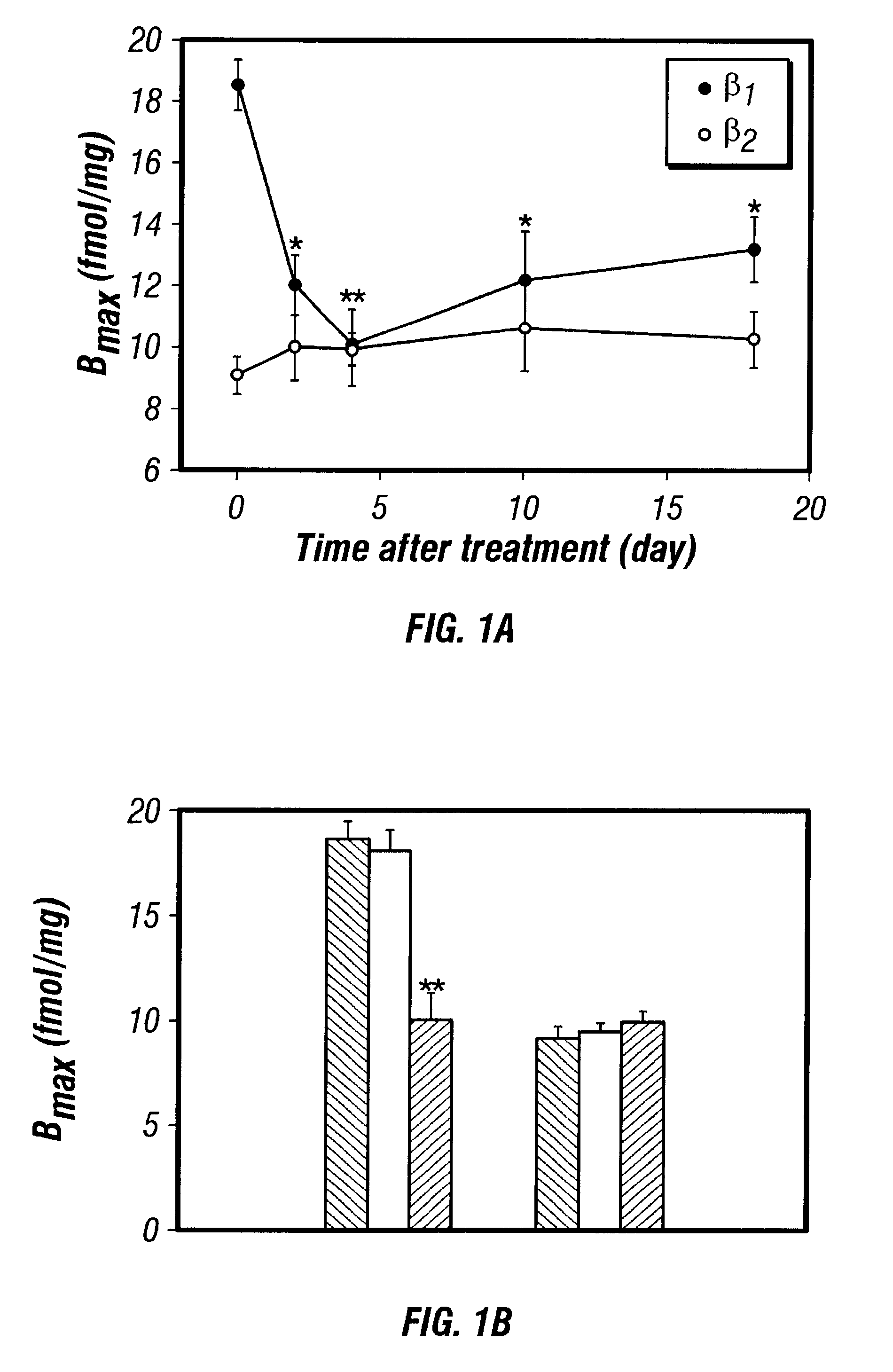
InactiveUS6489307B1Reduce inhibitionInhibit and reduce expressionBiocideOrganic active ingredientsEccentric hypertrophyMammal
Disclosed are antisense oligonucleotide, polynucleotide, and peptide nucleic acid compounds that specifically bind to mammalian mRNA encoding a beta1-adrenoceptor polypeptide and that are useful in the control and / or treatment of cardiac dysfunction, hypertension, hypertrophy, myocardial ischemia, and other cardiovascular diseases in an affected mammal, and preferably, in a human subject. The antisense compounds disclosed herein, and pharmaceutical formulations thereof, provide sustained control of beta1-adrenoceptor expression over prolonged periods, and achieve therapeutic effects from as little as a single dose. Administration of these antisense compositions to approved animal models resulted in a decrease in blood pressure, but no significant change in heart rate. Use of such antisense compositions in the reduction of beta1-adrenoceptor polypeptides in a host cell expressing beta1-adrenoceptor-specific mRNA, and in the preparation of medicaments for treating human and animal diseases, and in particular, hypertension and other cardiac dysfunction is also disclosed.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Compositions and methods for treating pancreatic insufficiency
InactiveUS20060121017A1Stable enzyme componentEffective low dose treatment regimenPeptide/protein ingredientsHydrolasesProteinase activityPancreas
The present invention relates to compositions for the treatment of conditions, including pancreatic insufficiency. The compositions of the present invention comprise lipase, protease and amylase in a particular ratio that provides beneficial results in patients, such as those afflicted with pancreatic insufficiency. This invention also relates to methods using such compositions for the treatment of pancreatic insufficiency.
Owner:ELI LILLY & CO
Probiotic/prebiotic composition and delivery method
A prebiotic, composition comprising a probiotic and prebiotic, and method of delivering a probiotic, prebiotic or composition directly into the intestinal tract of a mammal are disclosed. The probiotic is any beneficial bacteria and the prebiotic is a substance beneficial to a probiotic. Most preferably, the prebiotic includes a mucopolysaccharide. The method preferably involves delivering the prebiotic, probiotic or composition via a delivery tube, such as an enteral feeding tube, directly to a position downstream of the stomach, most preferably to the jejunum.
Owner:SOC DES PROD NESTLE SA
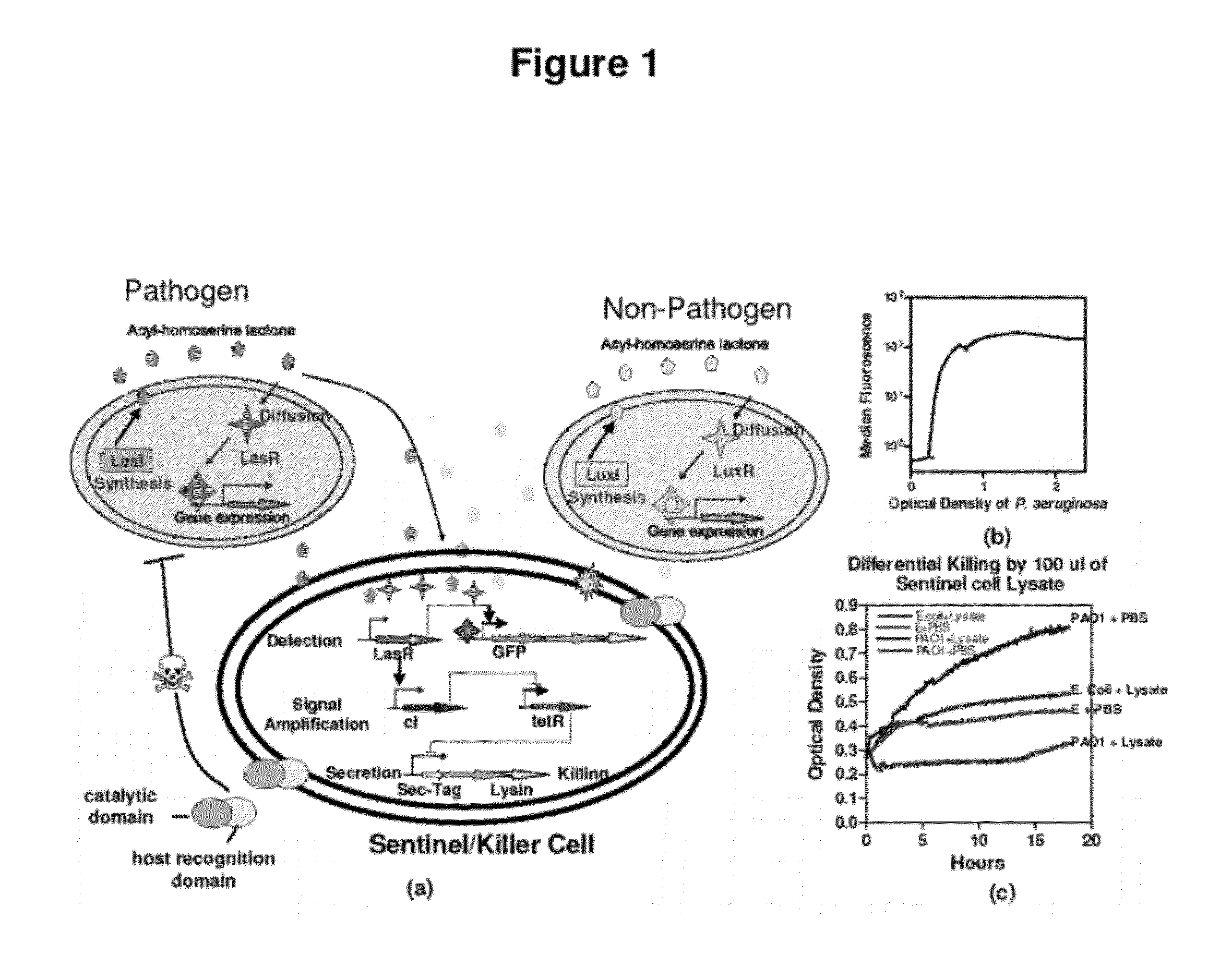
Genetically programmable pathogen sense and destroy
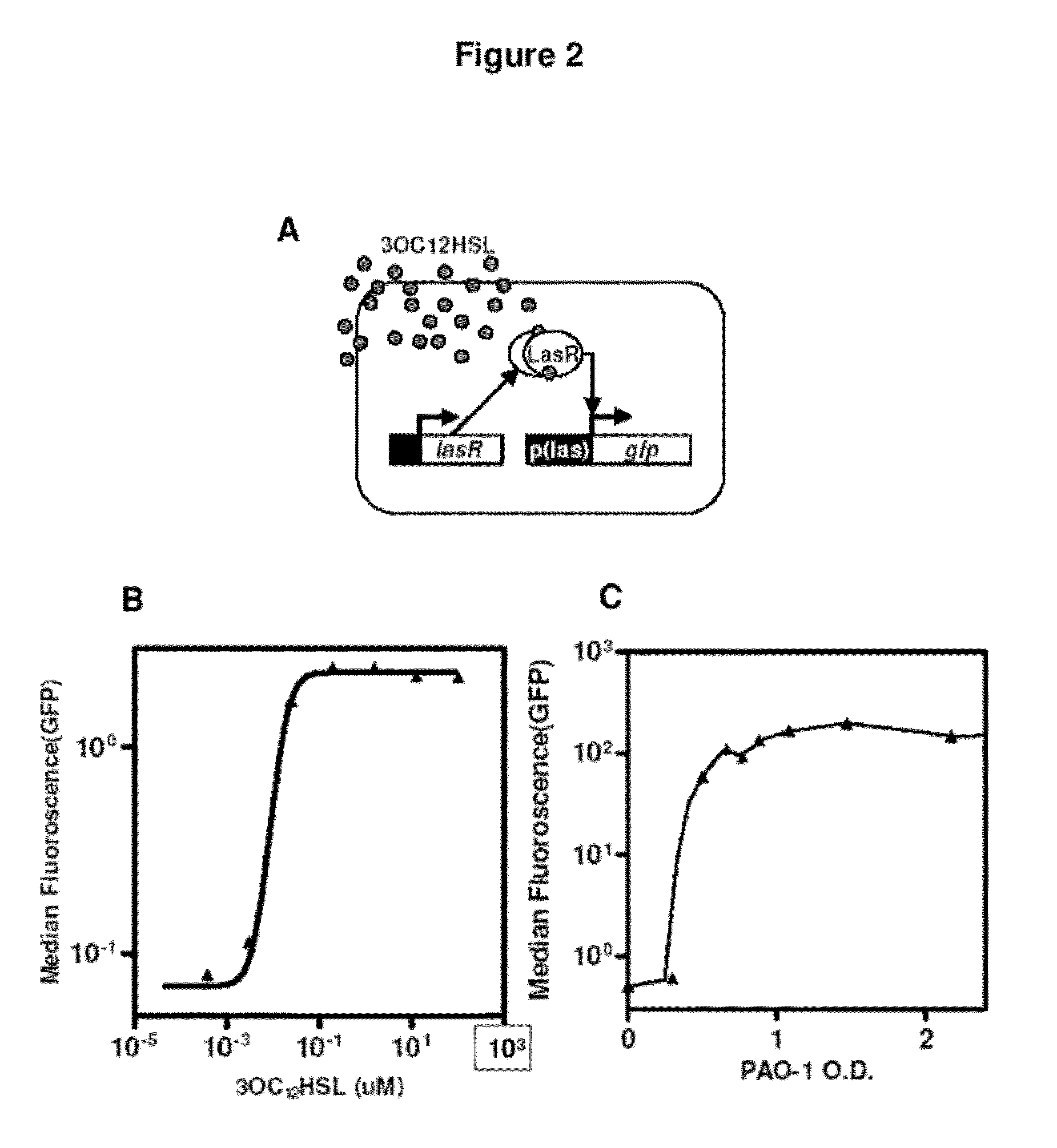
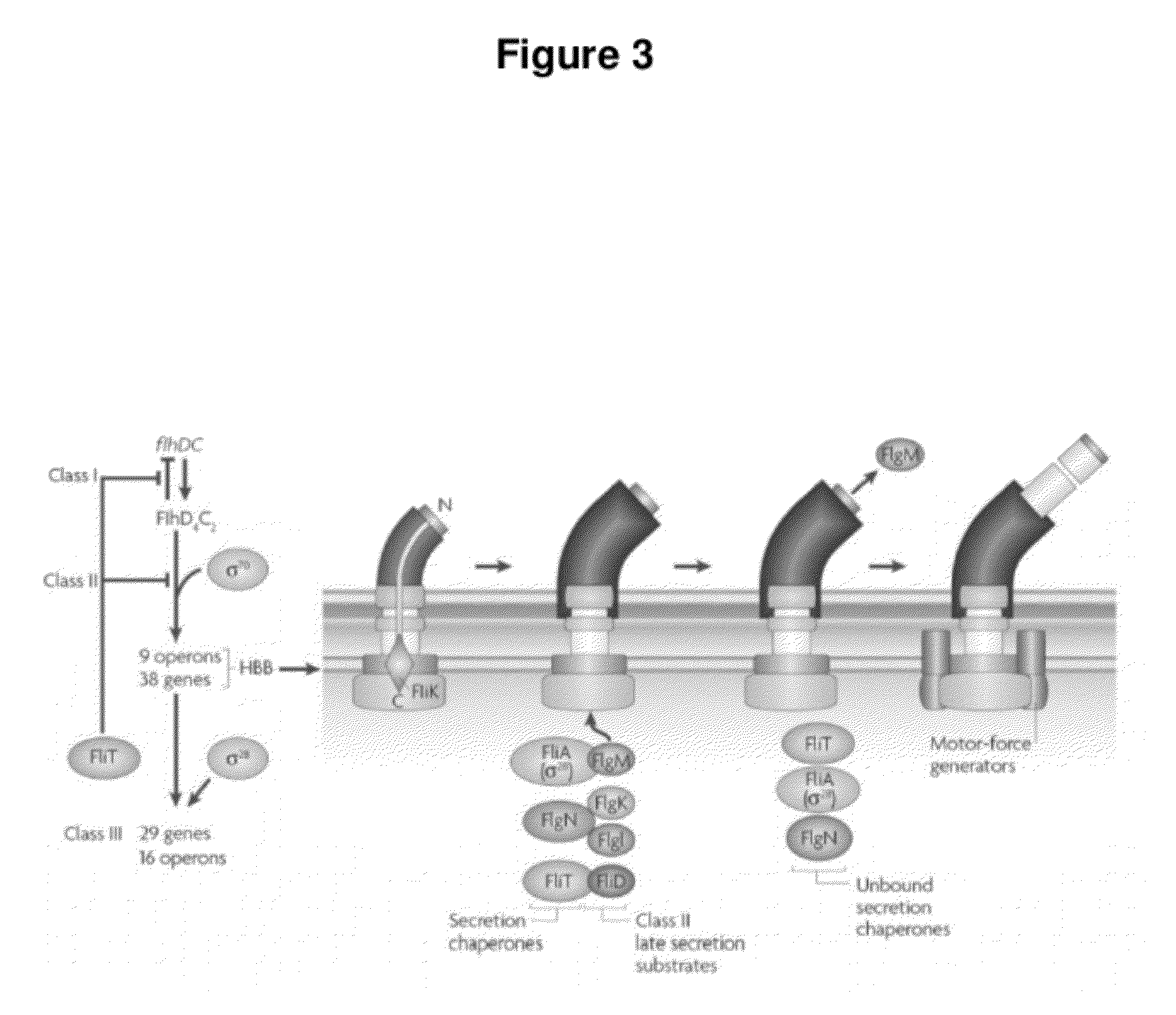
InactiveUS20120027786A1Easy to deployWide range of applicationsAntibacterial agentsBiocideSomatosensory systemPathogen
Owner:MASSACHUSETTS INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com