Patents
Literature
311 results about "Jejunum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
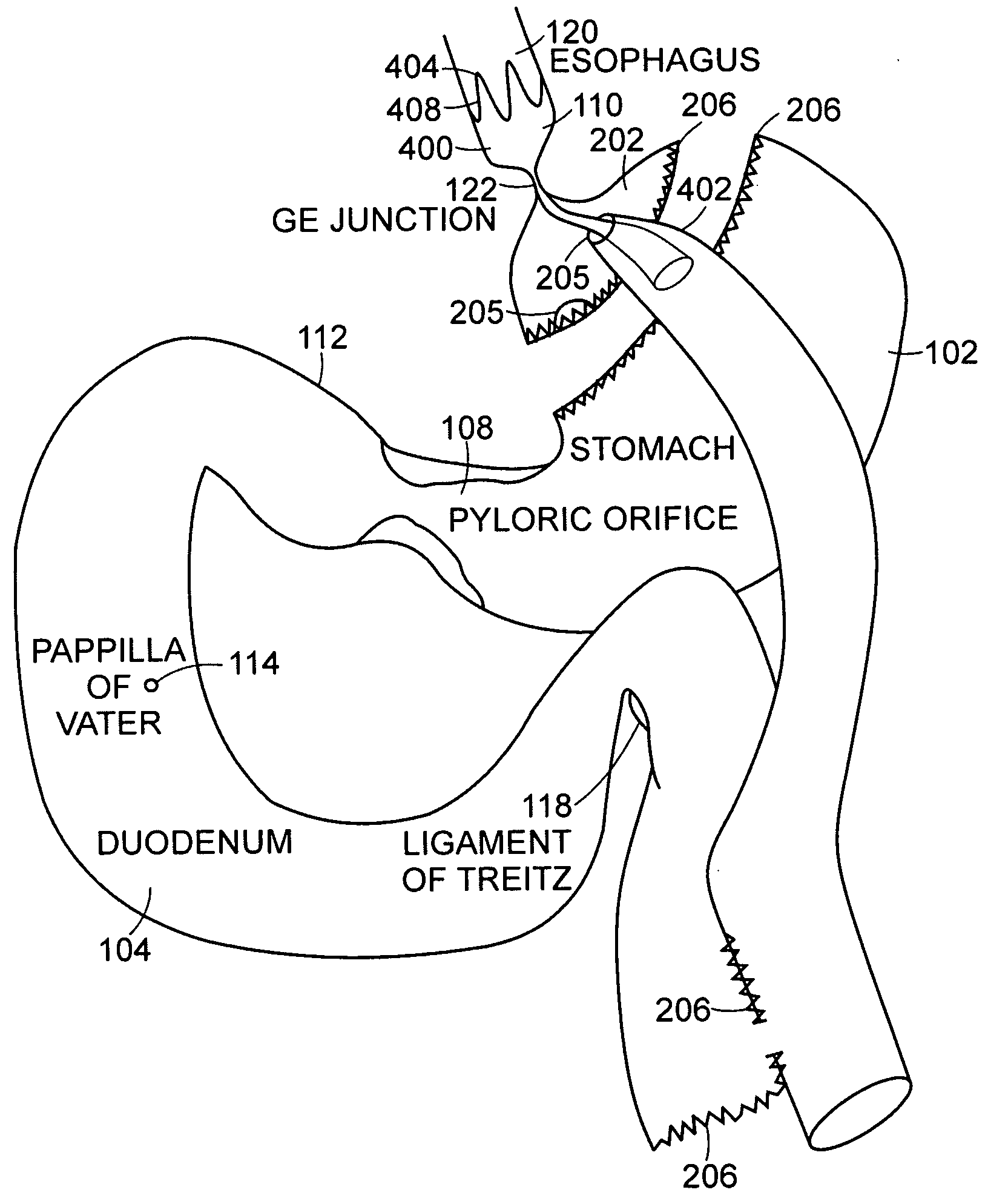
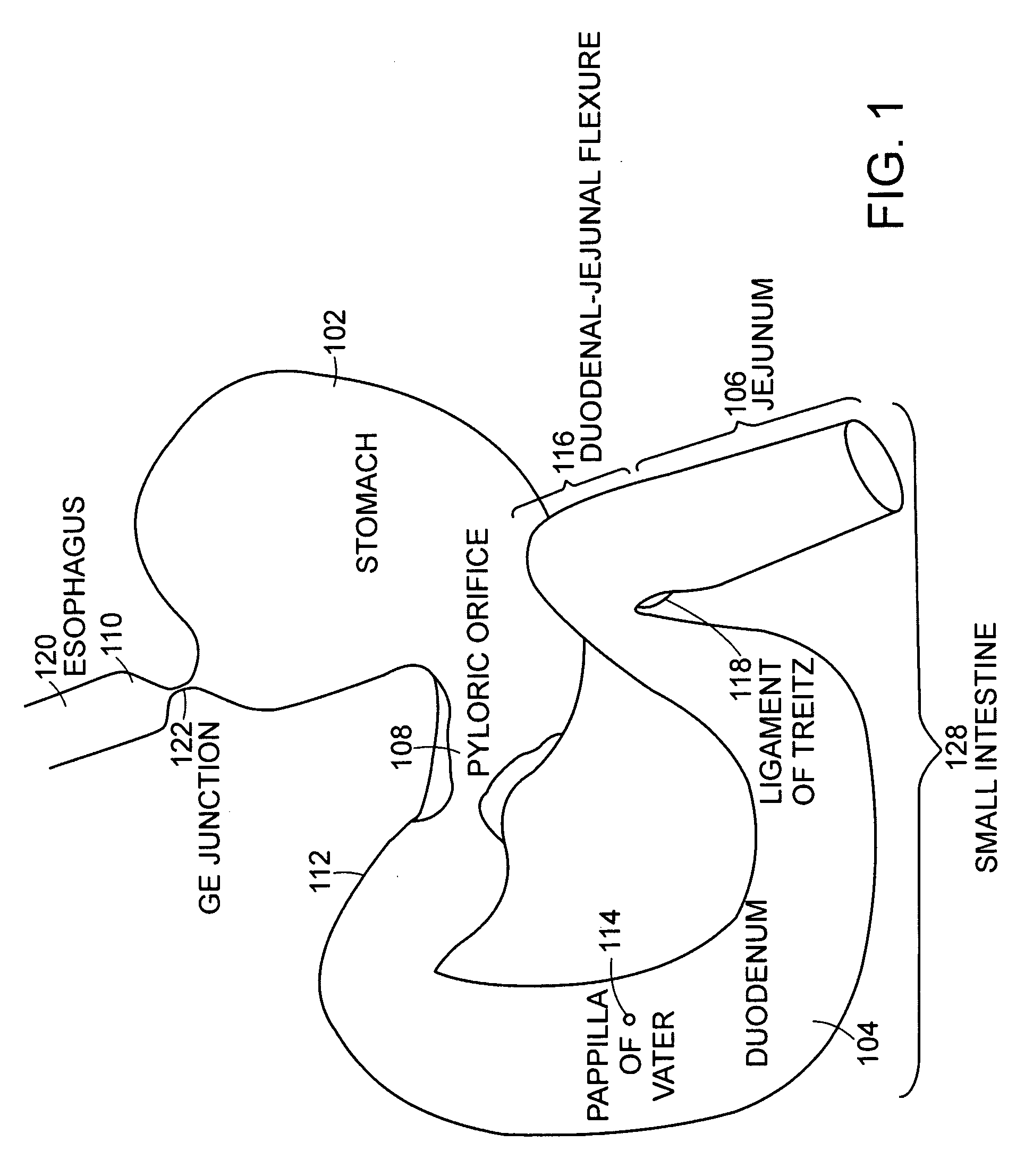
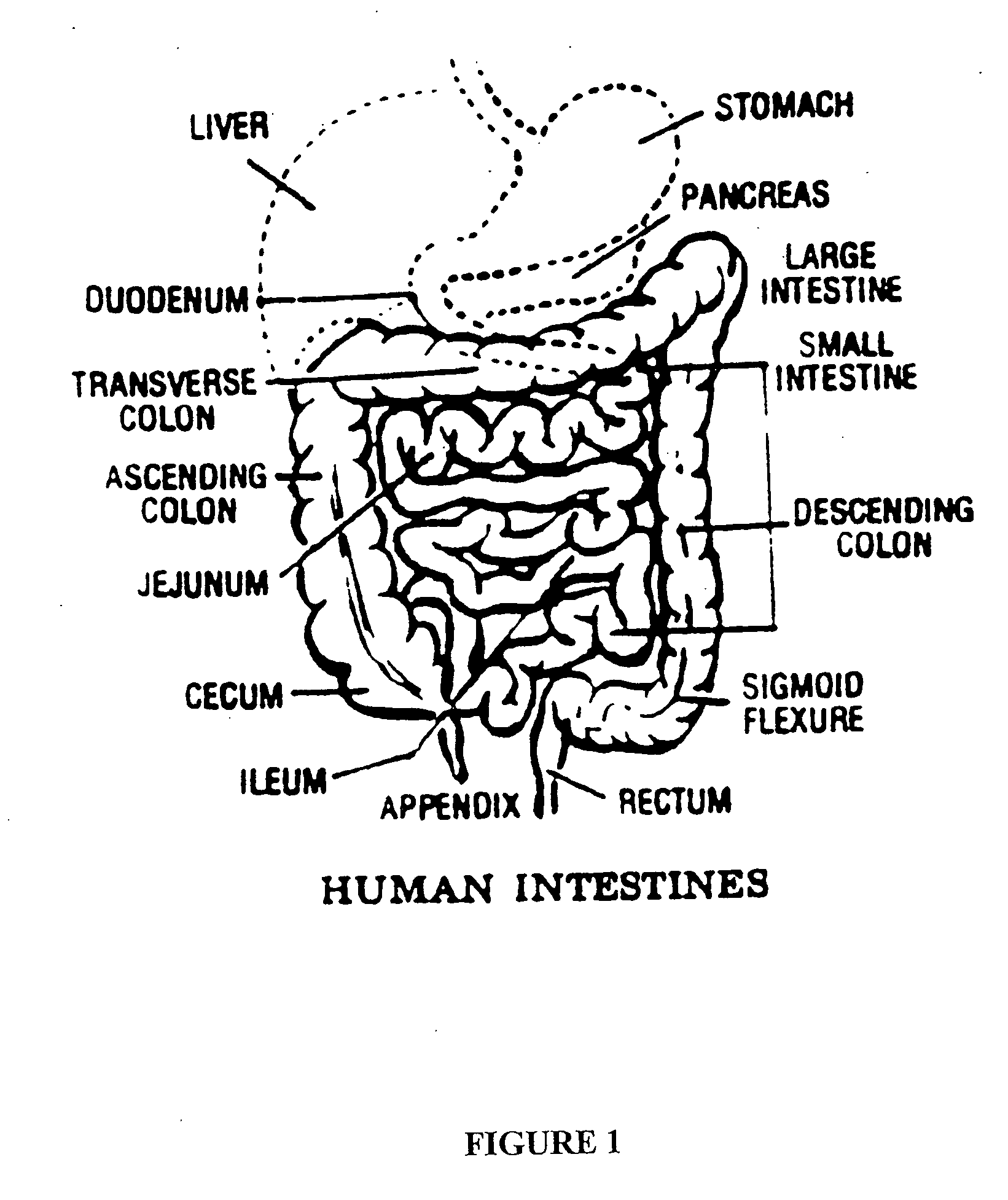
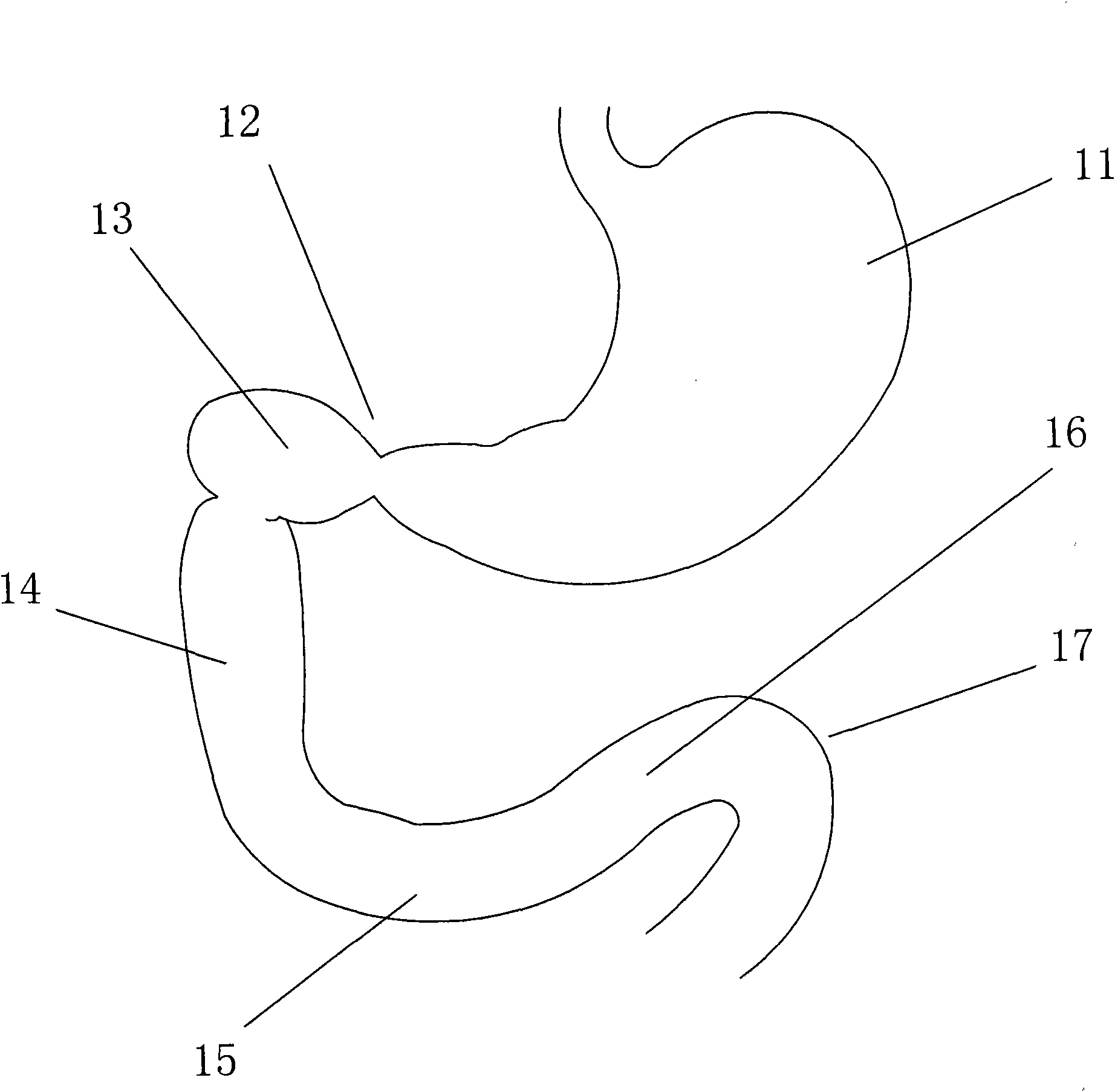
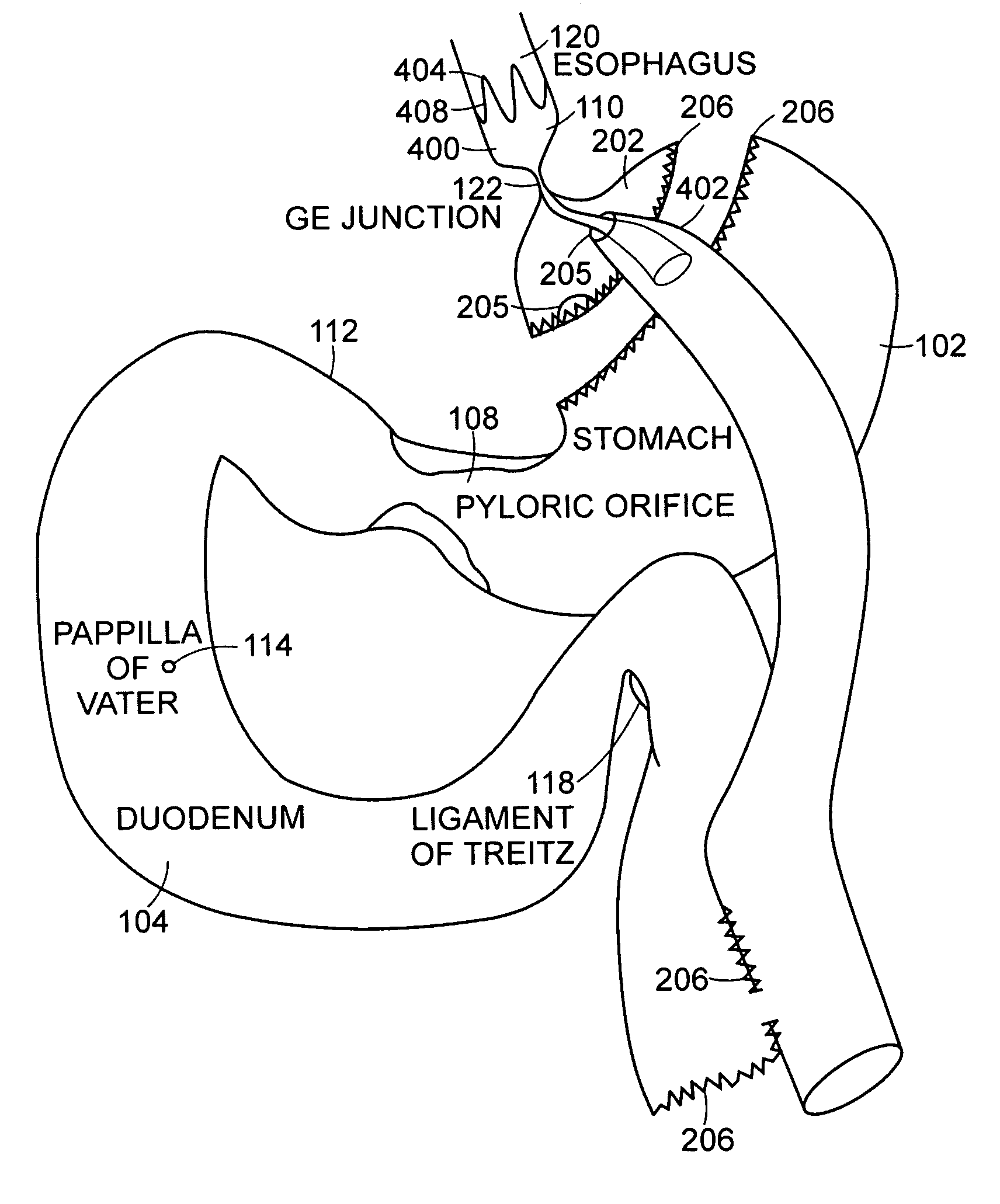
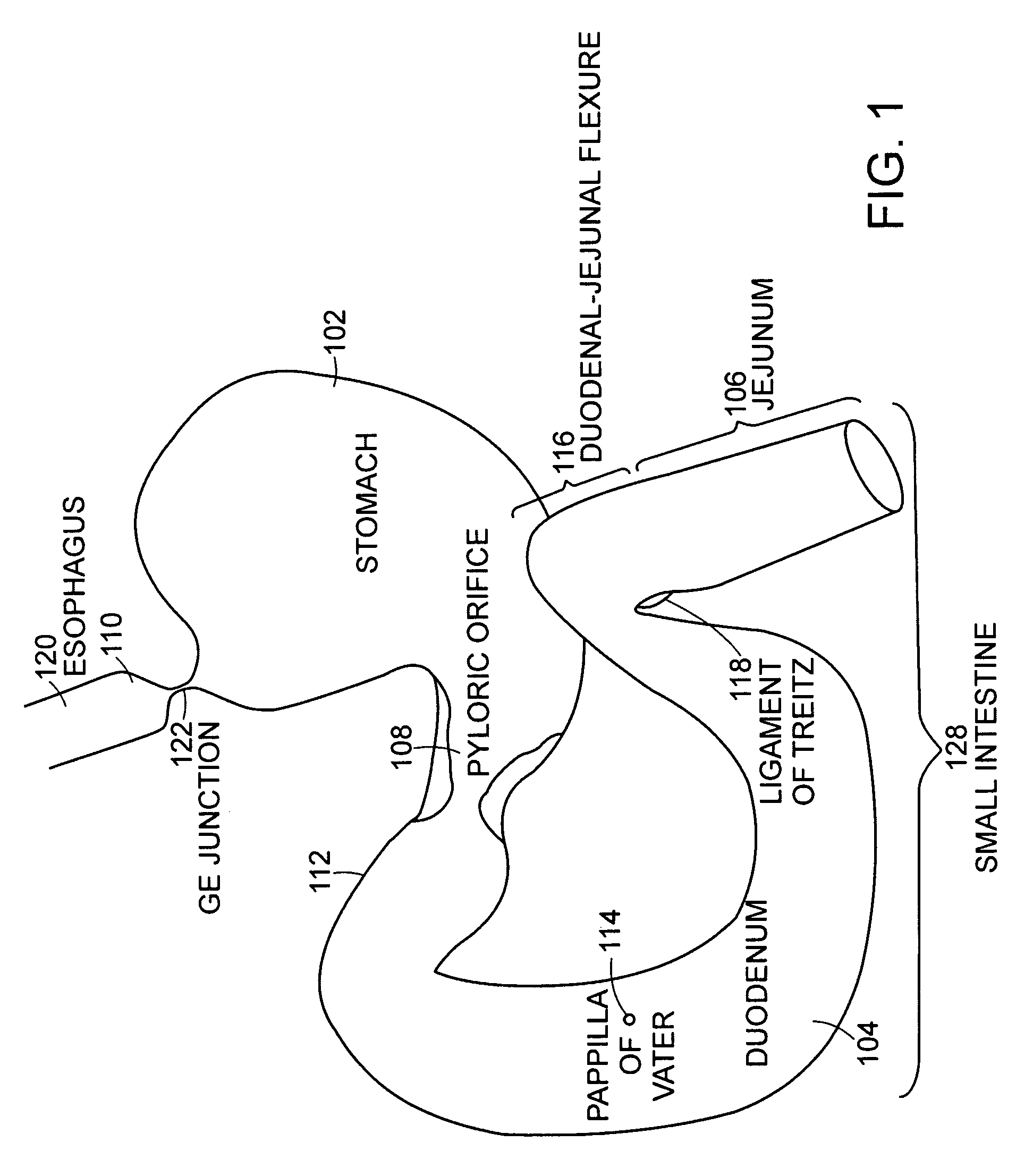
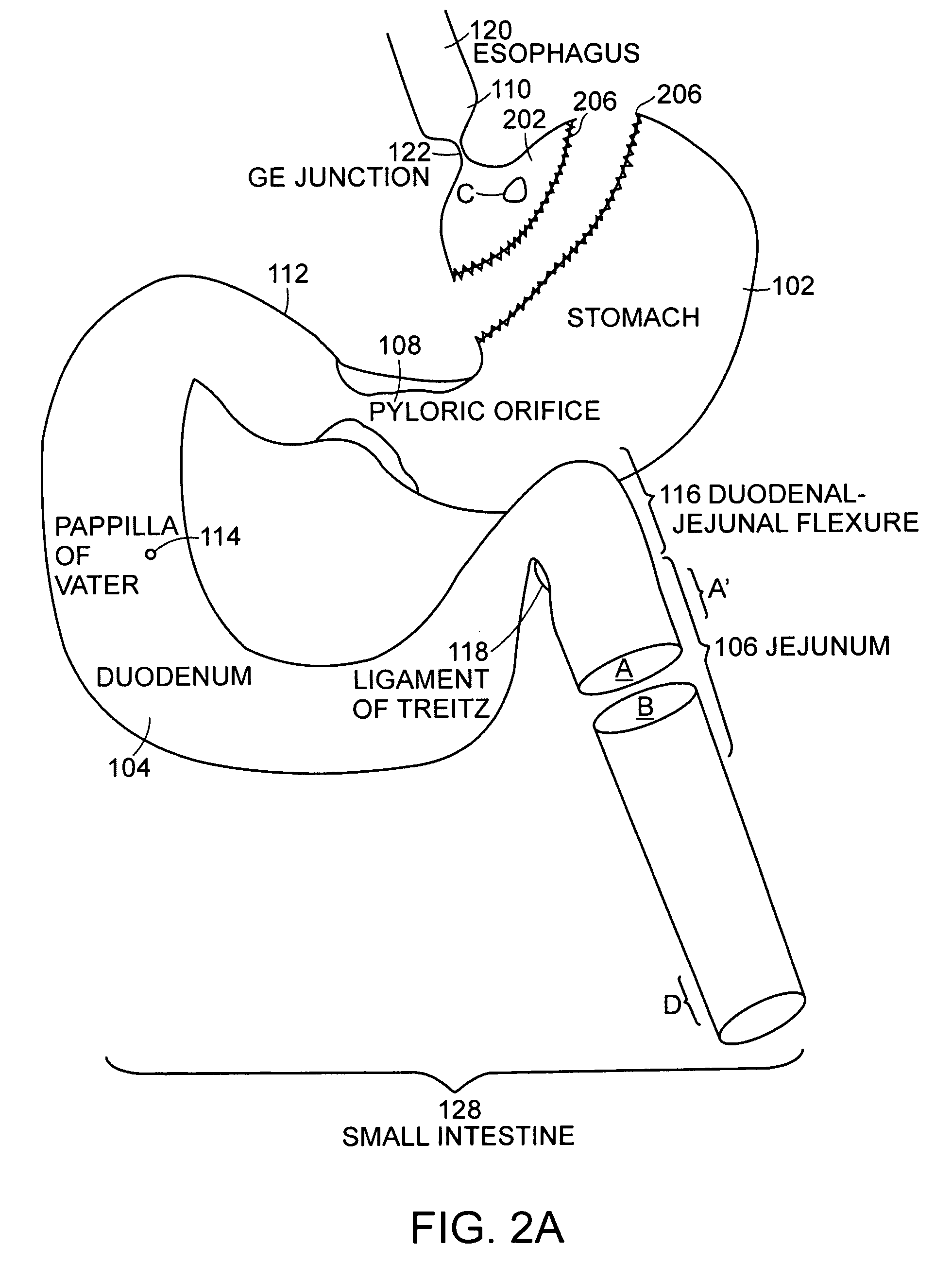
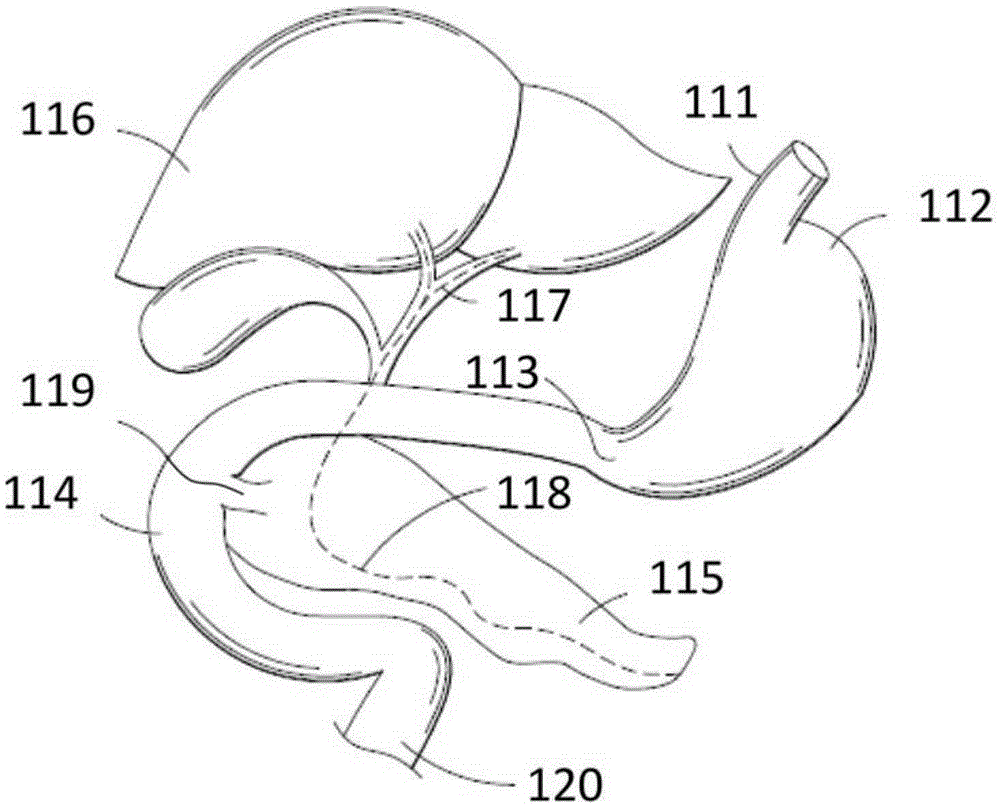
The jejunum (/dʒɪˈdʒuːnəm/) is the second part of the small intestine in humans and most higher vertebrates, including mammals, reptiles, and birds. Its lining is specialized for the absorption by enterocytes of small nutrient molecules which have been previously digested by enzymes in the duodenum.
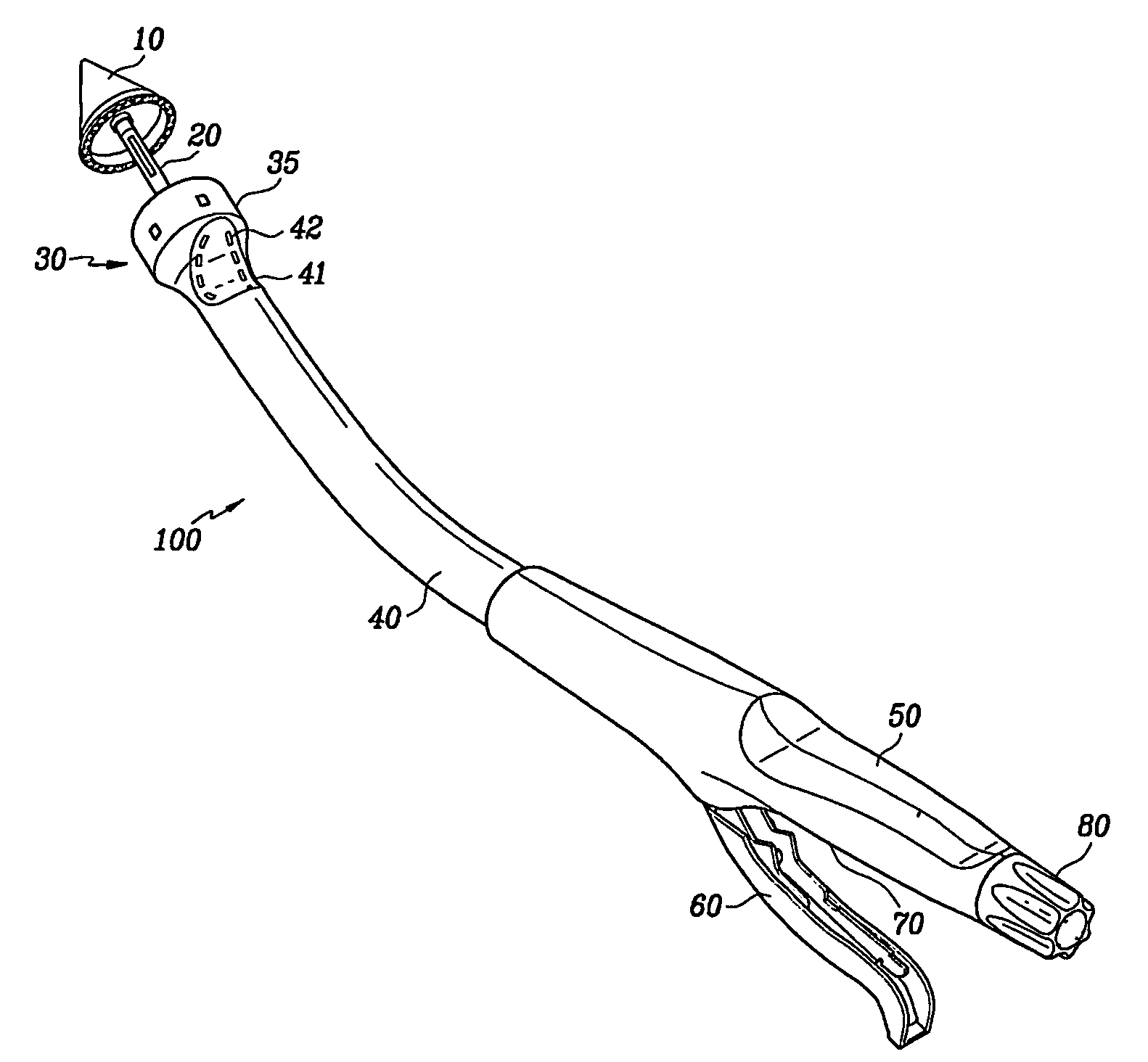
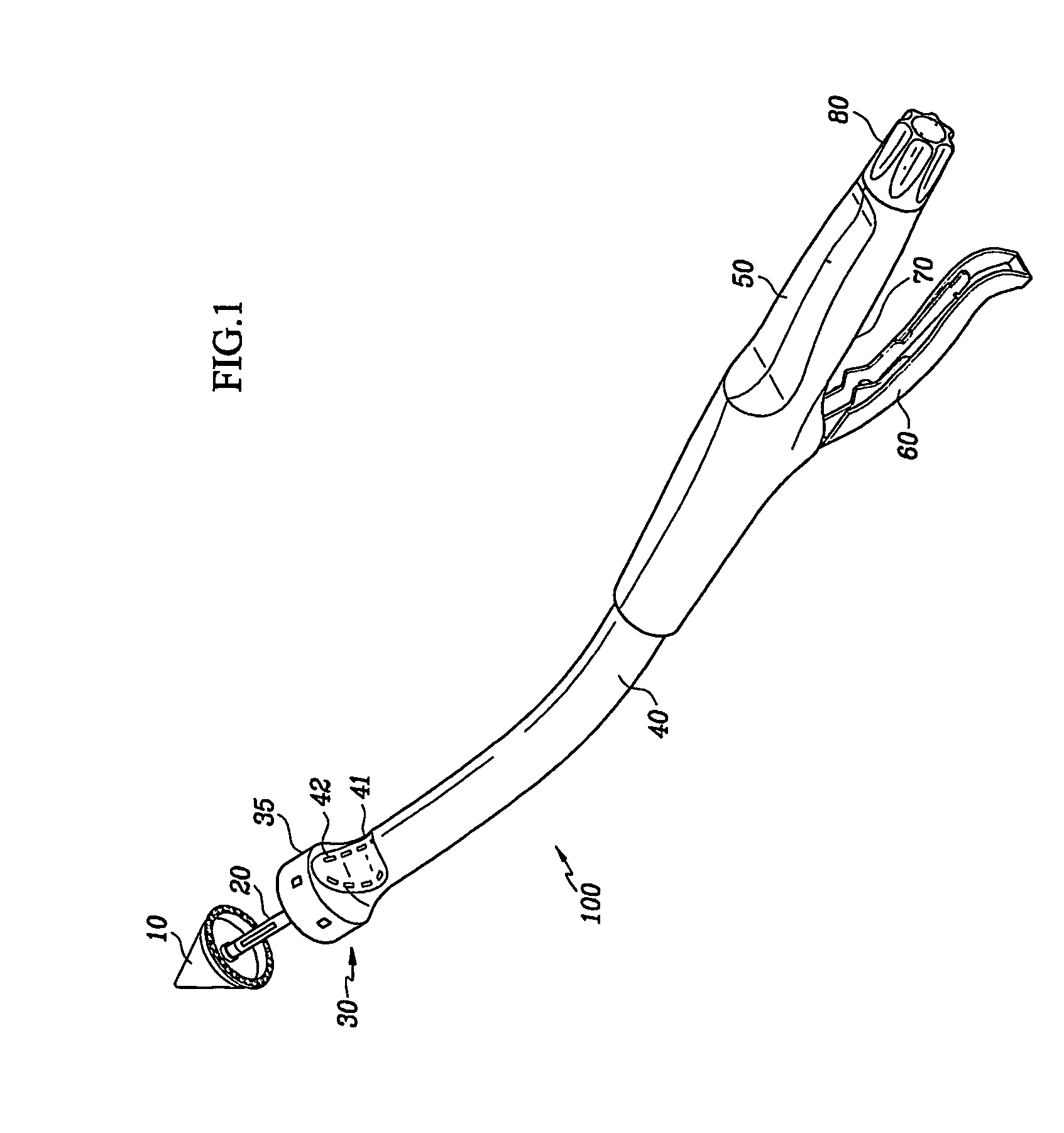
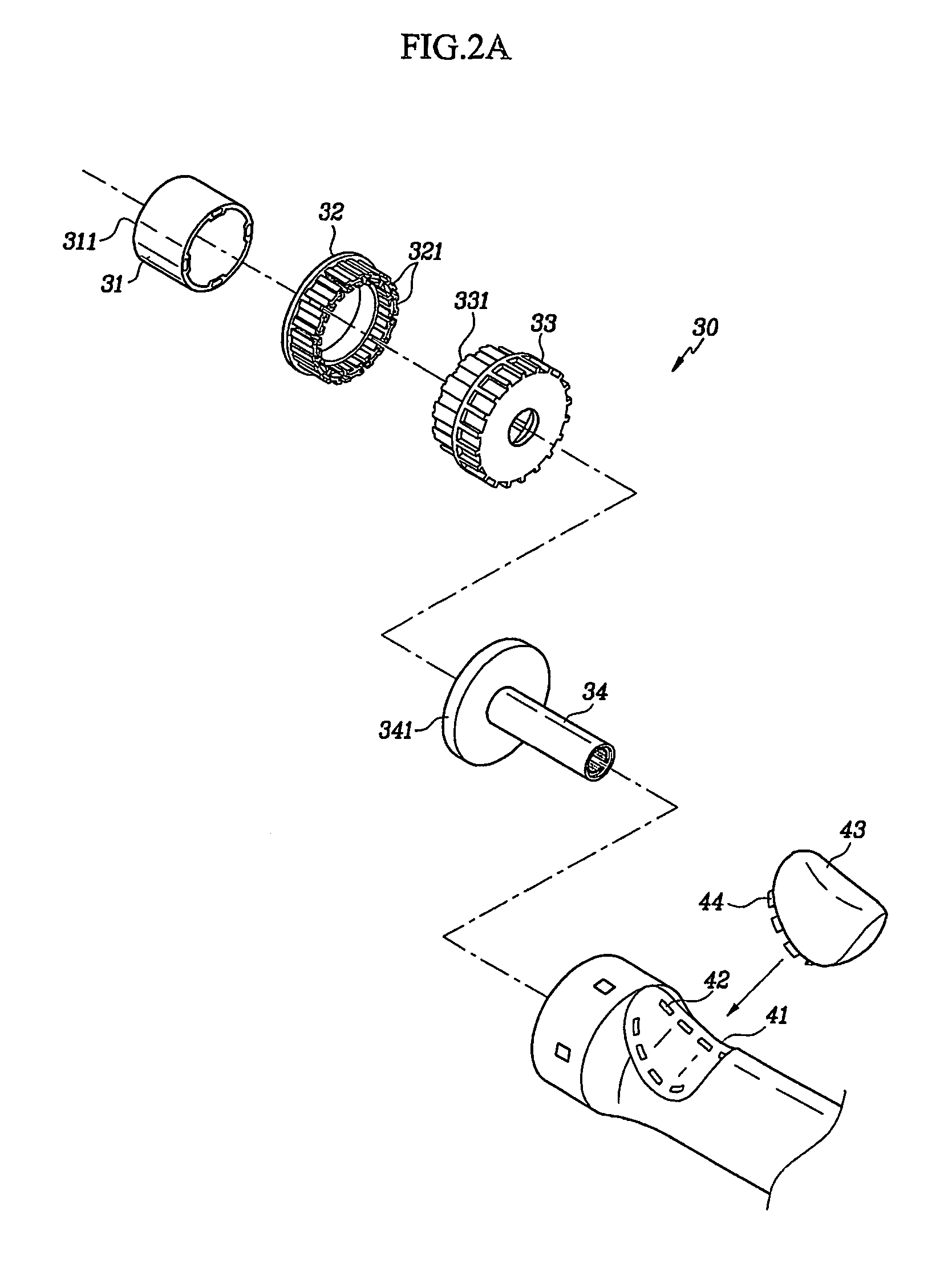
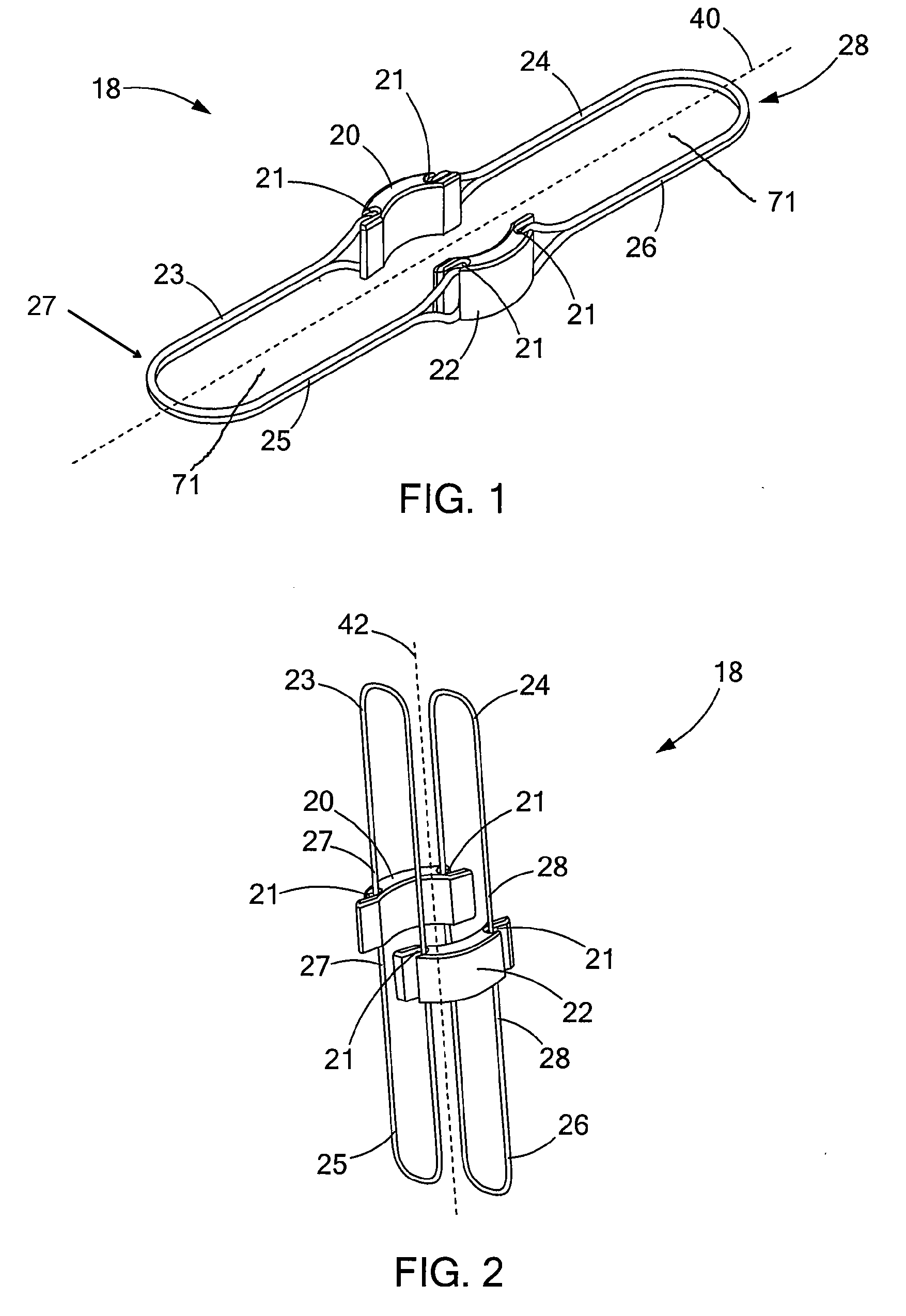
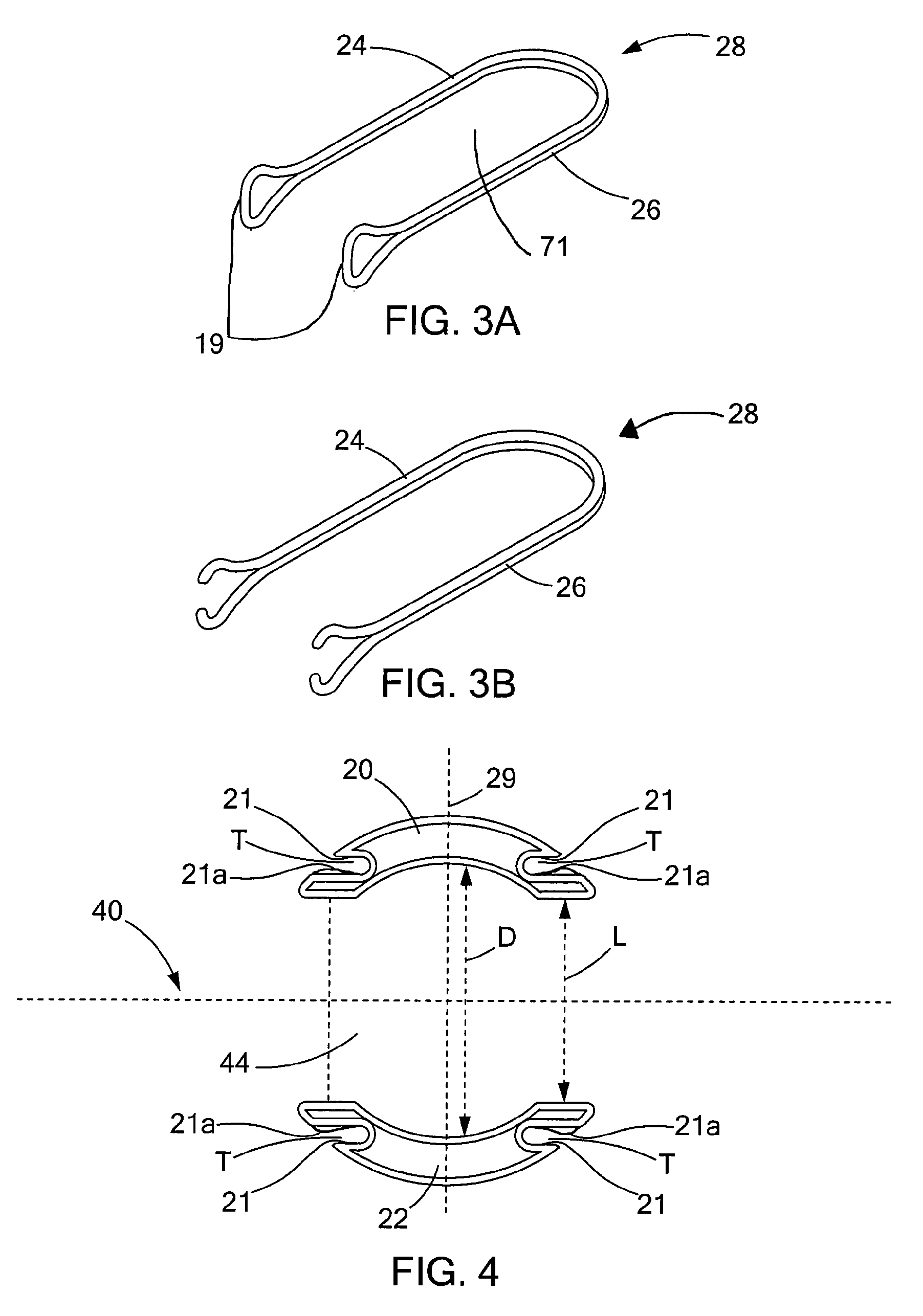
Circular surgical stapler with a detachable anvil
Owner:INHA UNIV RES & BUSINESS FOUNDATION
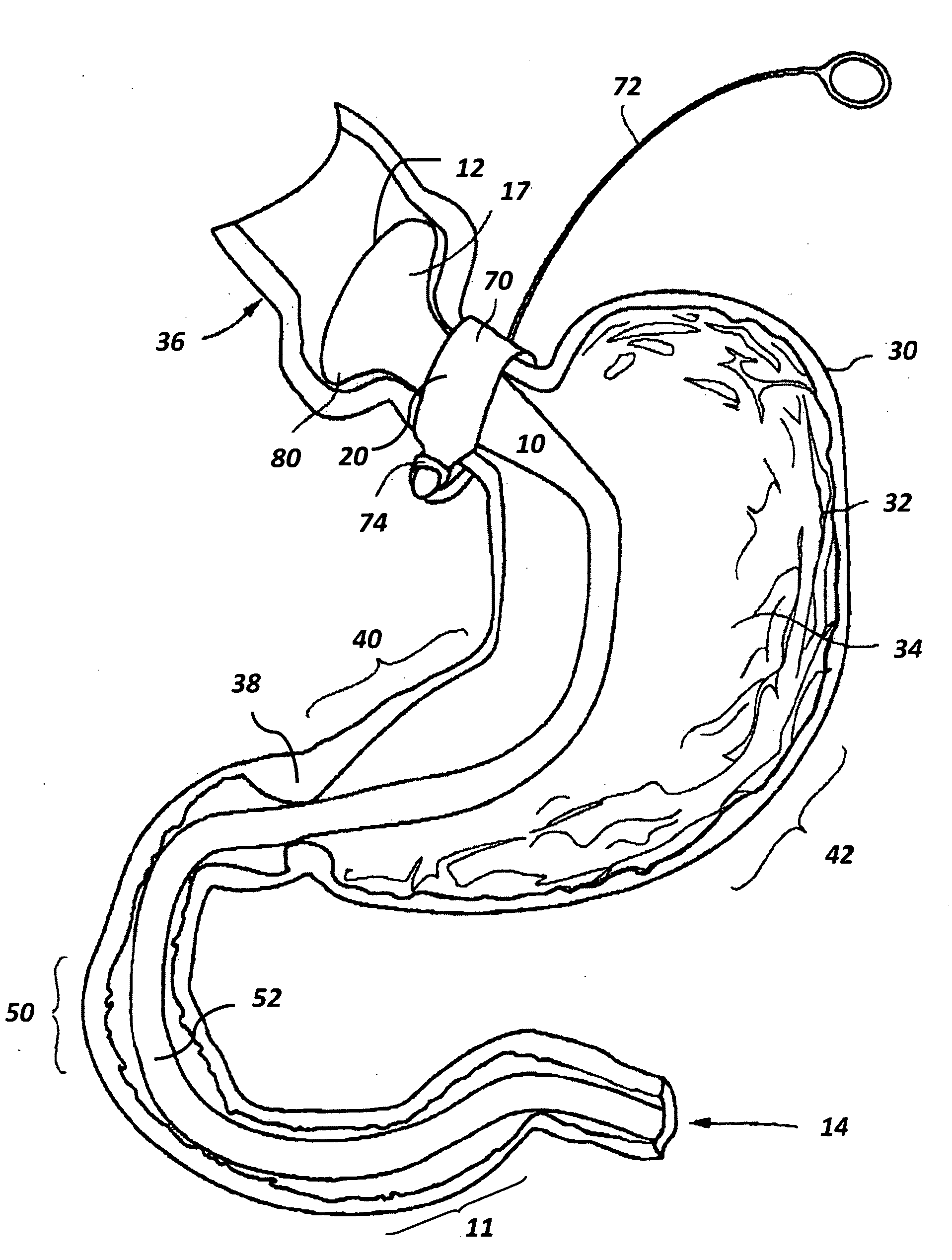
Use of a gastrointestinal sleeve to treat bariatric surgery fistulas and leaks
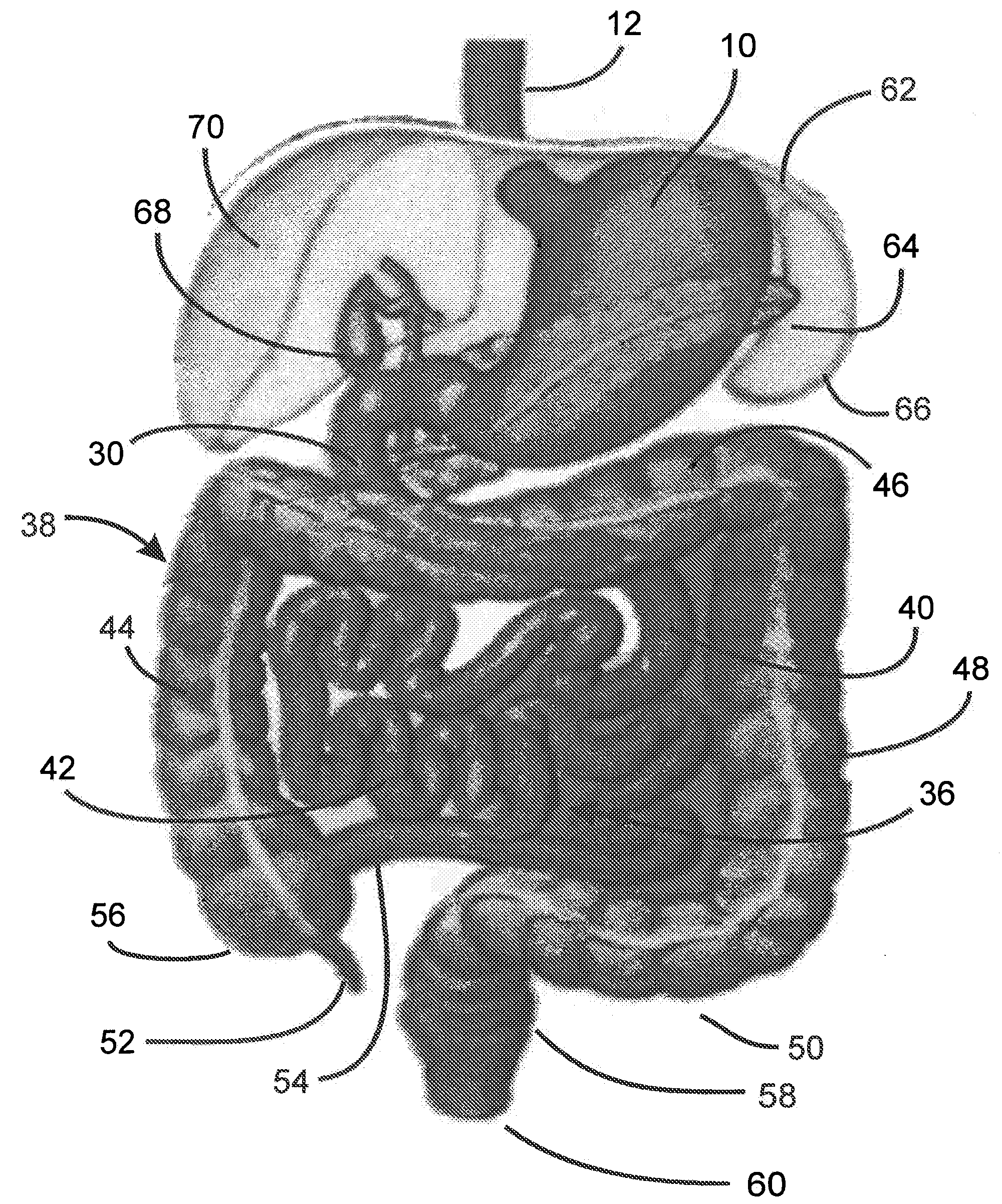
ActiveUS20080208357A1Minimize traumaCurved bigOesophagiObesity treatmentIntestinal structureDamages tissue
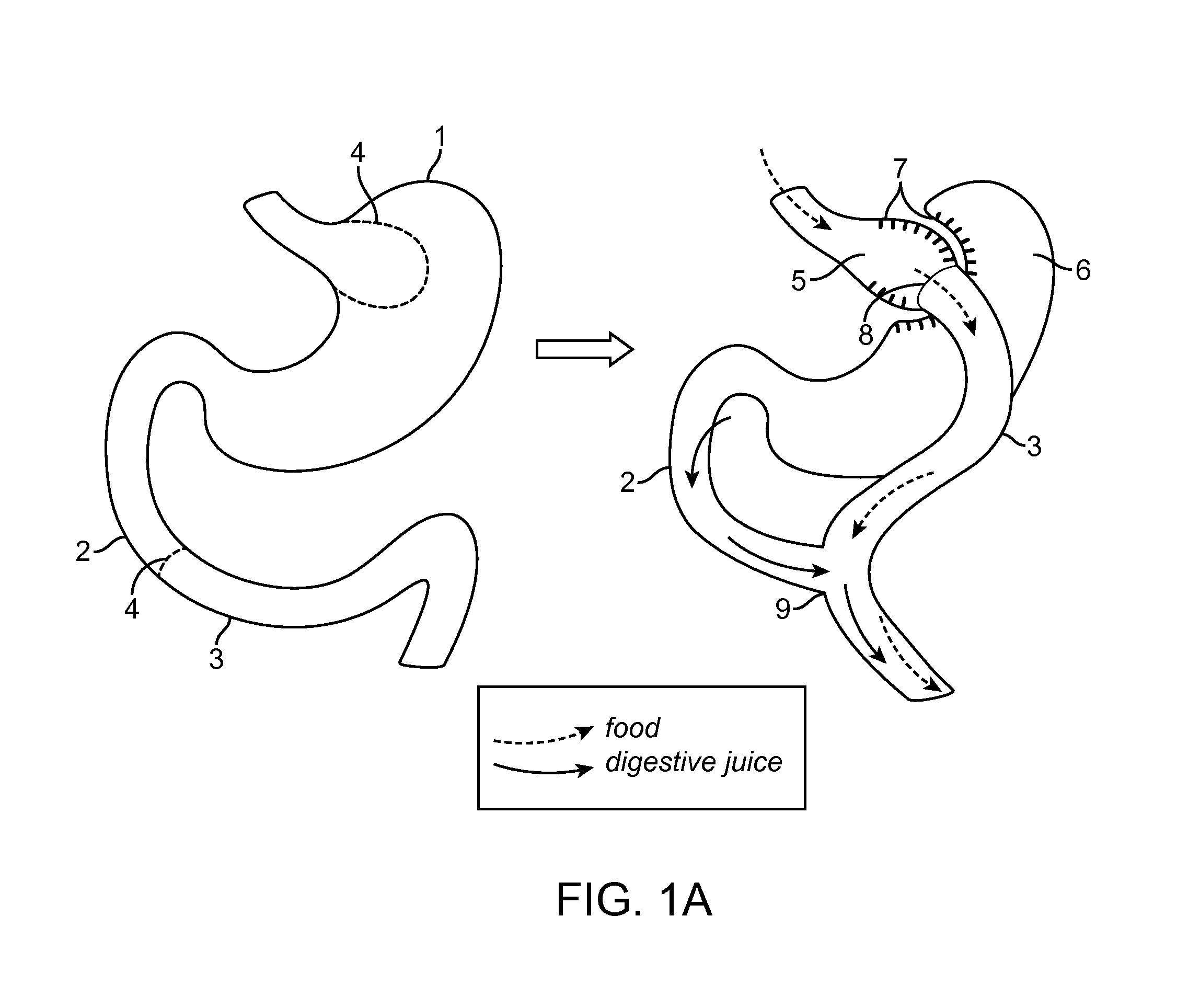
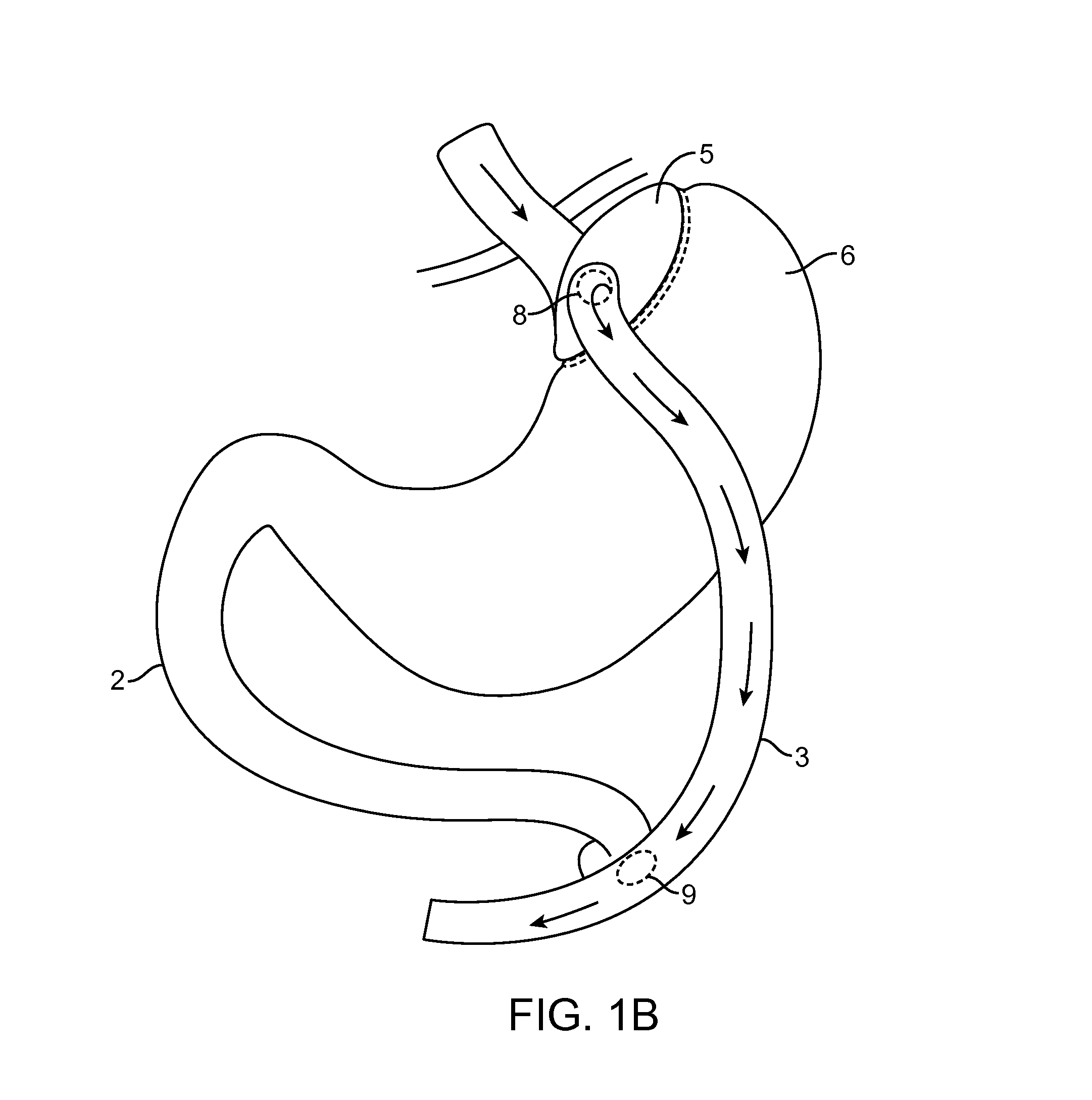
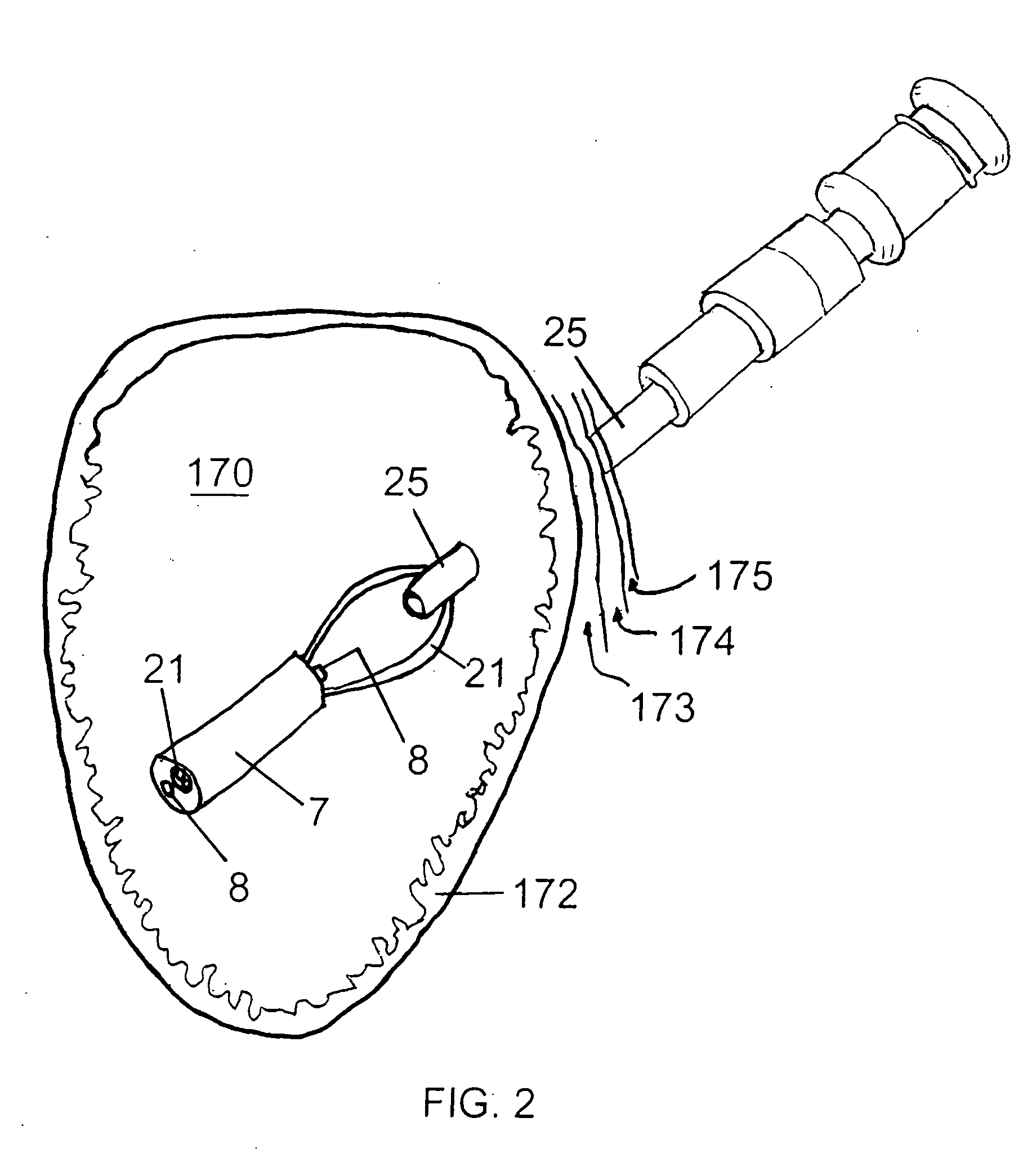
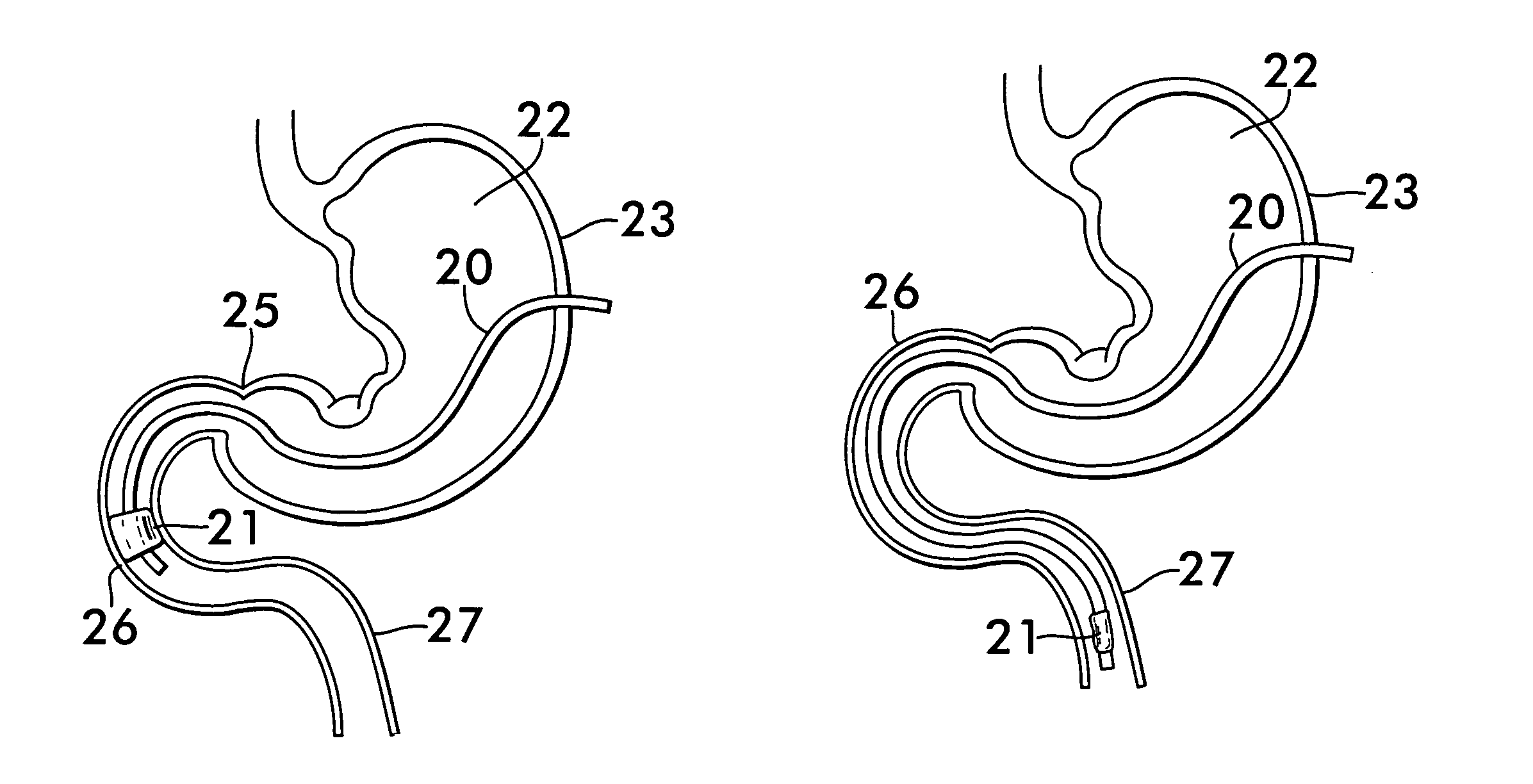
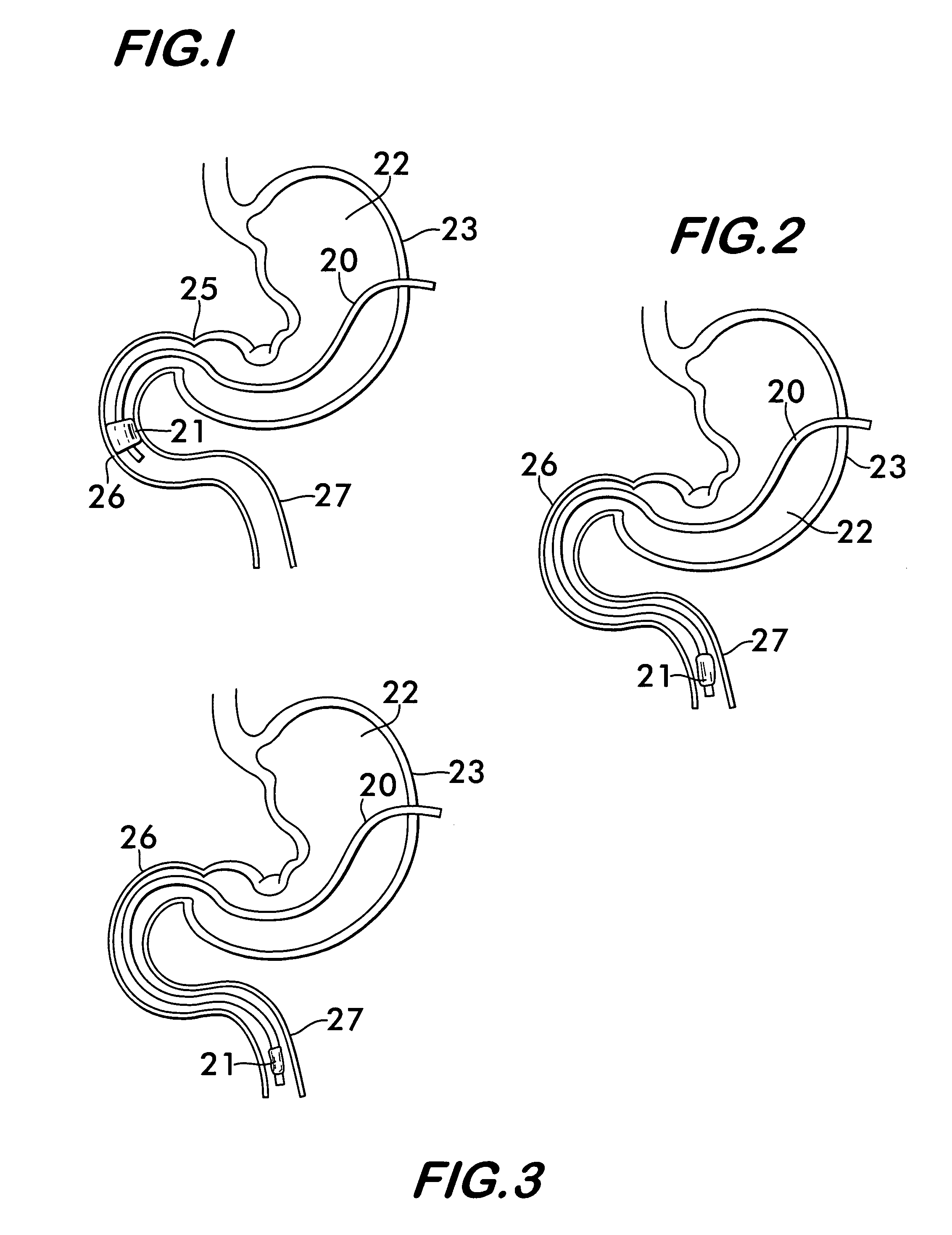
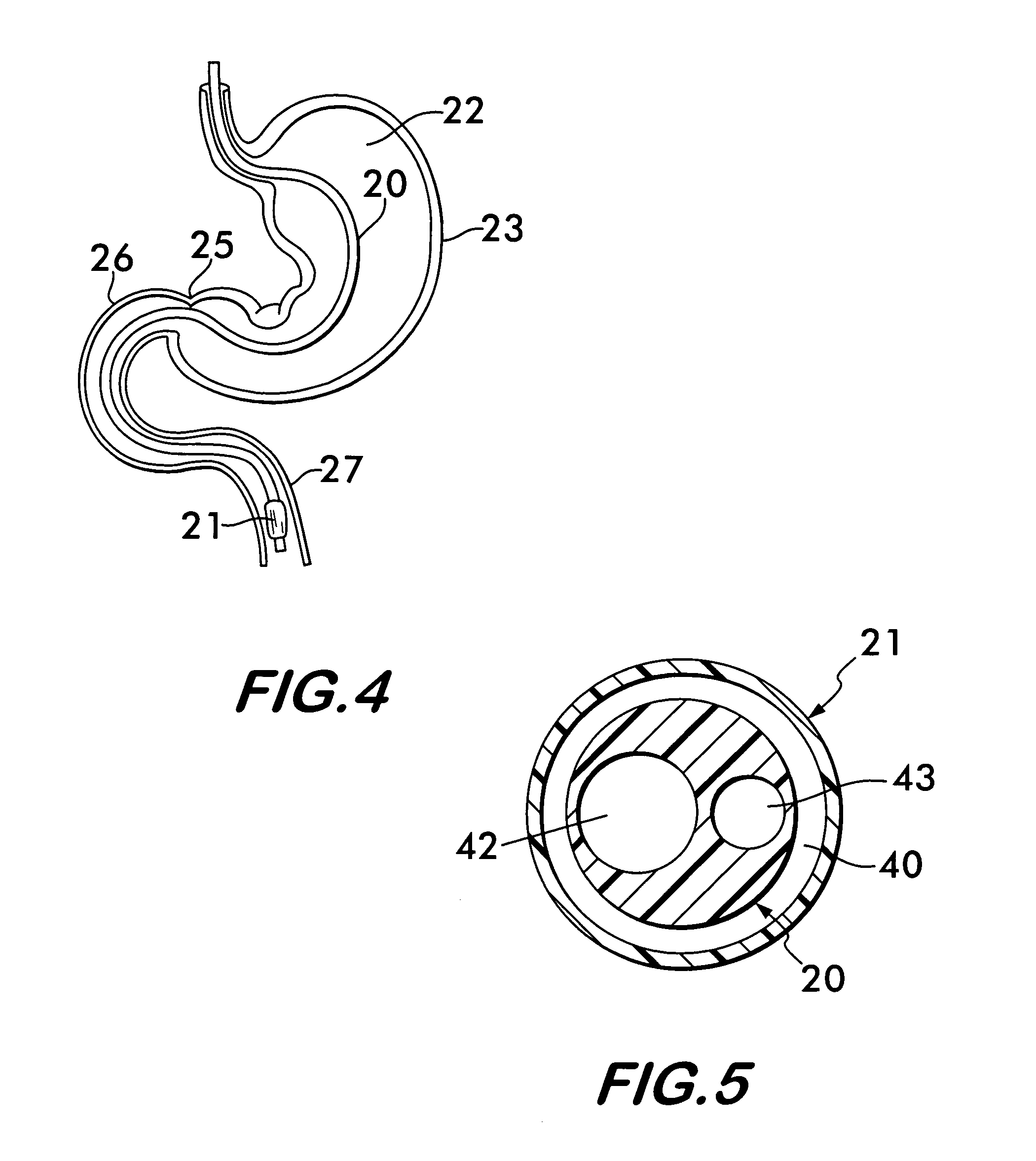
Method for treating a Roux-en-Y patient having fistulas and leaks as a result of bariatric surgery. A gastrointestinal implant device is anchored in the esophagus and extends through a stomach pouch into an intestine anastomosed to the stomach pouch to prevent fistulas and other damaged tissue from making contact with food and fluids entering the esophagus. The gastrointestinal implant device includes an unsupported flexible sleeve and an anchor coupled to a proximal portion of the sleeve. The flexible sleeve is open at both ends, and adapted to extend below a jejunum. The anchor is adapted to be retained within the esophagus, preferably just above the gastroesophageal (GE) Junction. The anchor can include a stent such as a wave anchor and is collapsible for catheter-based delivery and removal.
Owner:GI DYNAMICS
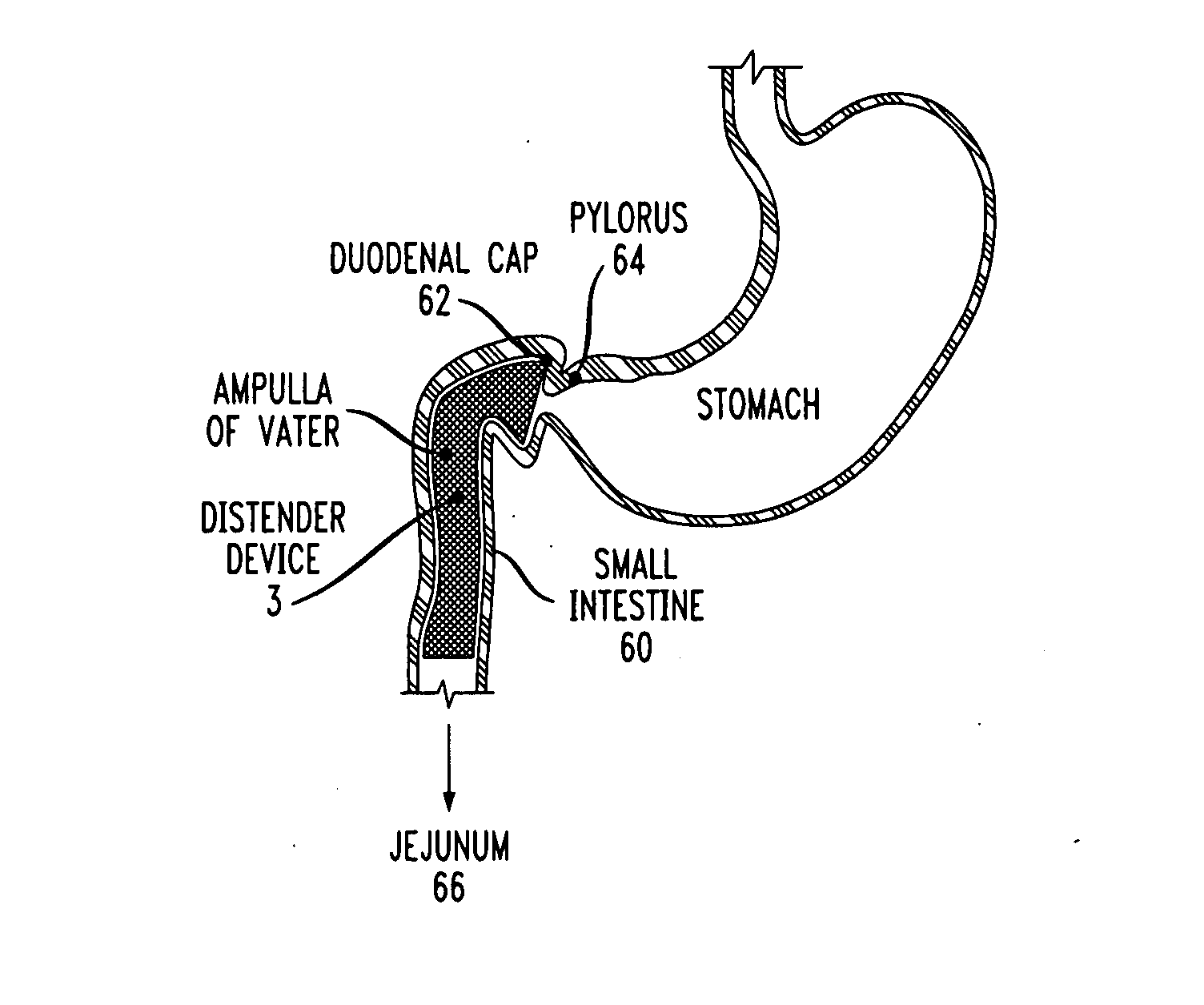
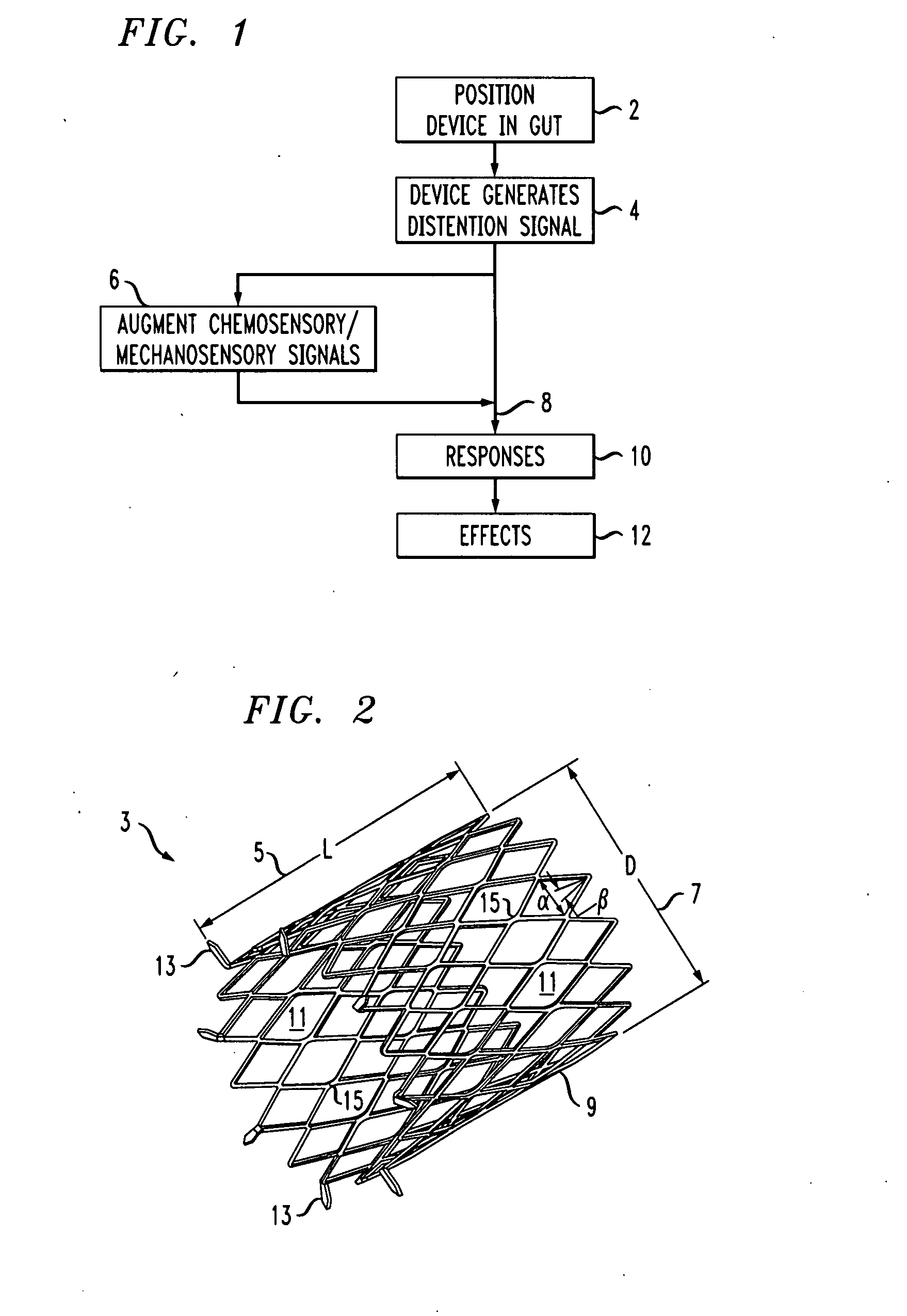
Distender device and method for treatment of obesity and metabolic and other diseases
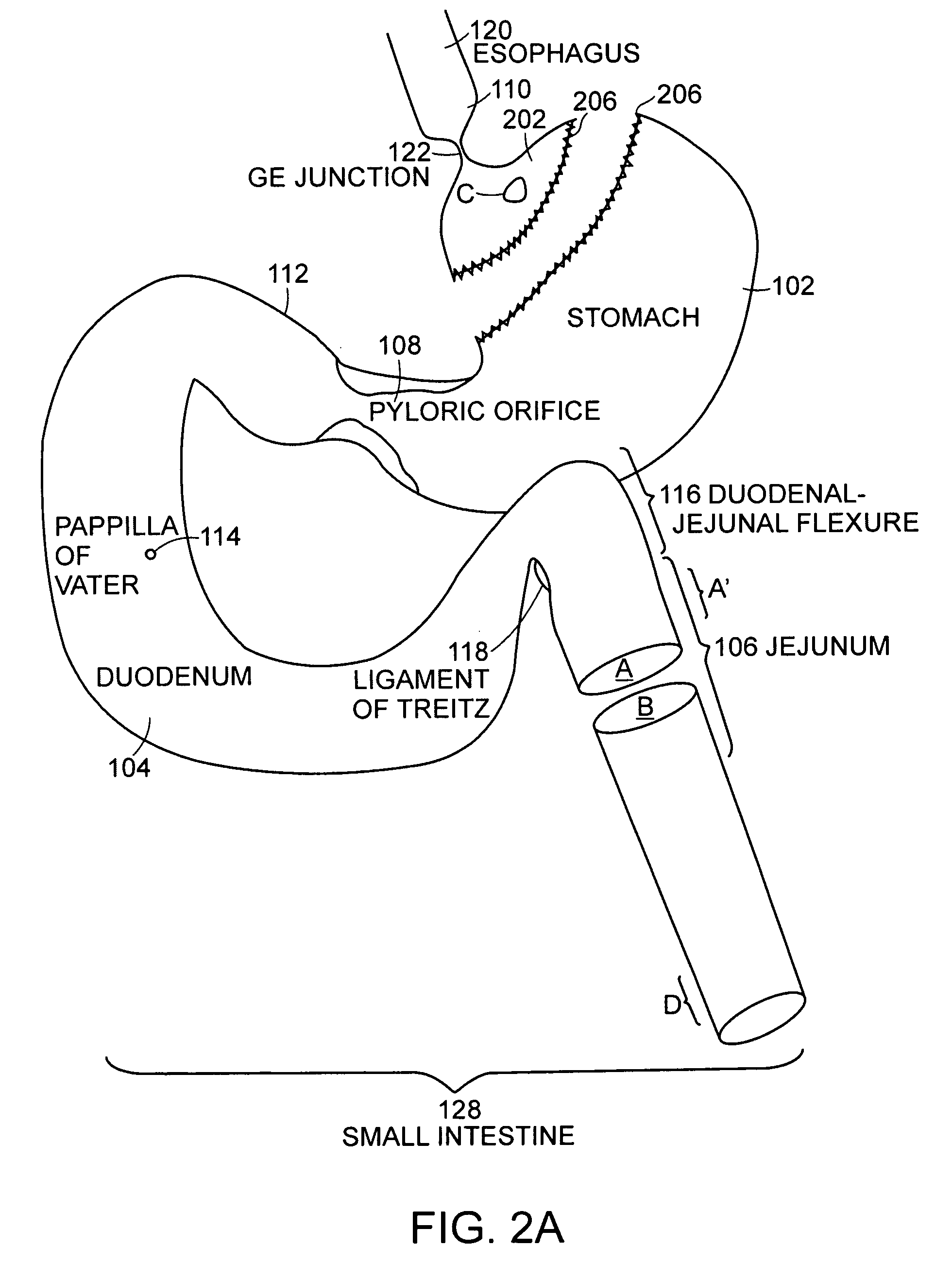
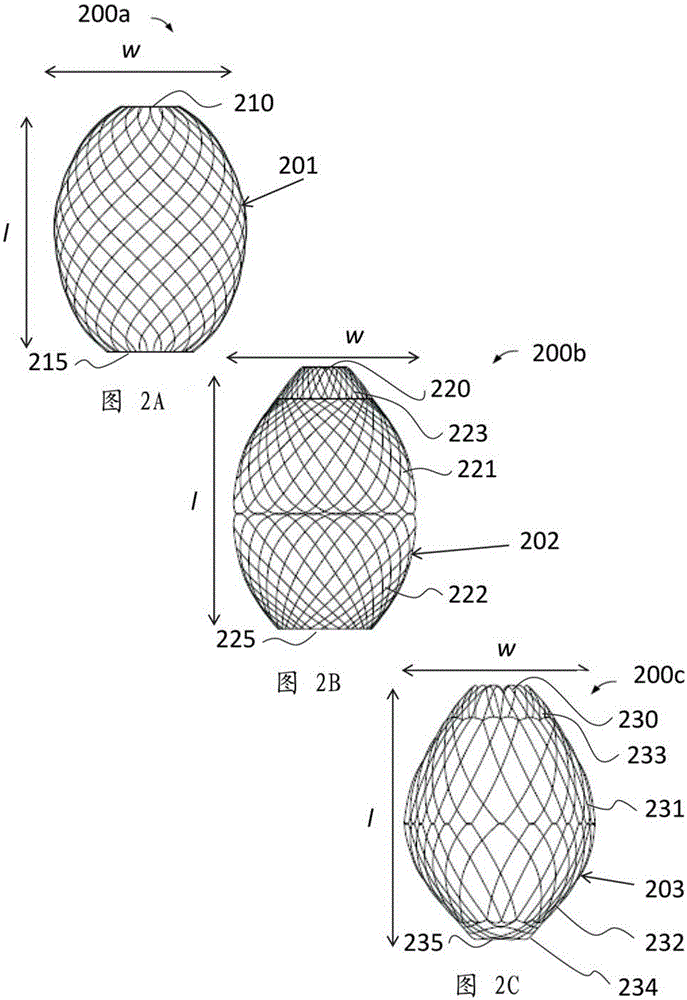
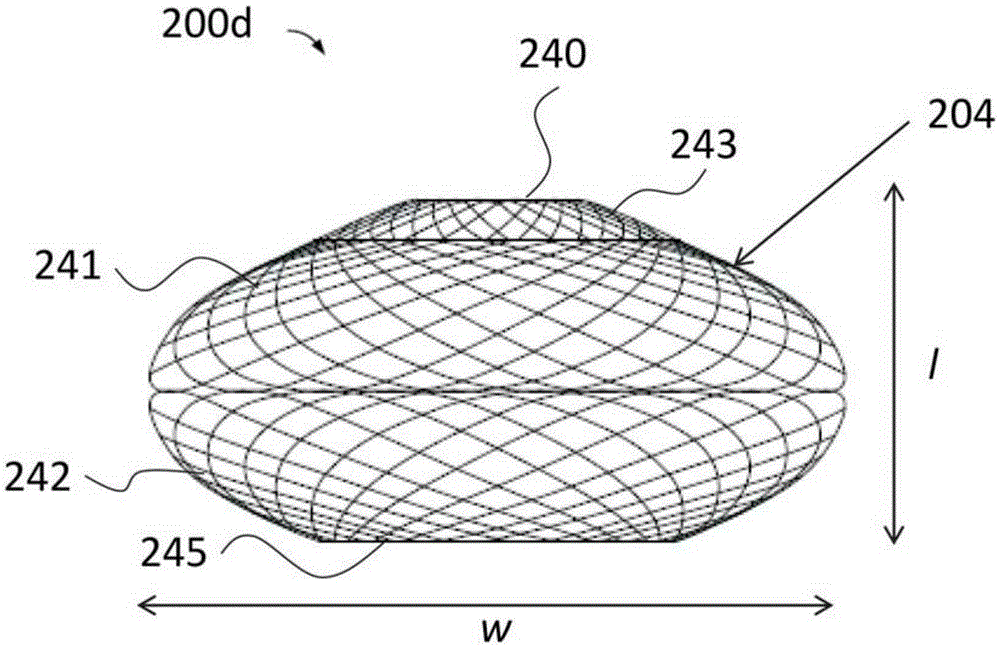
A gastrointestinal implant device is positioned in a patient's small intestine or rectum and produces an outward force that itself produces a distension signal which is a therapeutically useful neural or humoral signal that evokes satiogenic or weight loss effects by itself. The device may advantageously be placed in the duodenum adjacent the pylorus or in the jejunum, ileum or rectum. The distension signals may amplify chemosensory or mechanosensory signals such as enteroendocrine secretions within the patient. The device may be a mesh and include a low material density that allows for unrestricted chyme absorption within the small intestine and unrestricted chyme flow through the gastrointestinal system. A method includes inserting the device into the patient then either retrieving the device after treatment is complete or allowing a device formed of a biodegradable material to degrade in time after treatment is complete.
Owner:ADVANCED NEUROMODULATION SYST INC
Probiotic/prebiotic composition and delivery method
A prebiotic, composition comprising a probiotic and prebiotic, and method of delivering a probiotic, prebiotic or composition directly into the intestinal tract of a mammal are disclosed. The probiotic is any beneficial bacteria and the prebiotic is a substance beneficial to a probiotic. Most preferably, the prebiotic includes a mucopolysaccharide. The method preferably involves delivering the prebiotic, probiotic or composition via a delivery tube, such as an enteral feeding tube, directly to a position downstream of the stomach, most preferably to the jejunum.
Owner:SOC DES PROD NESTLE SA
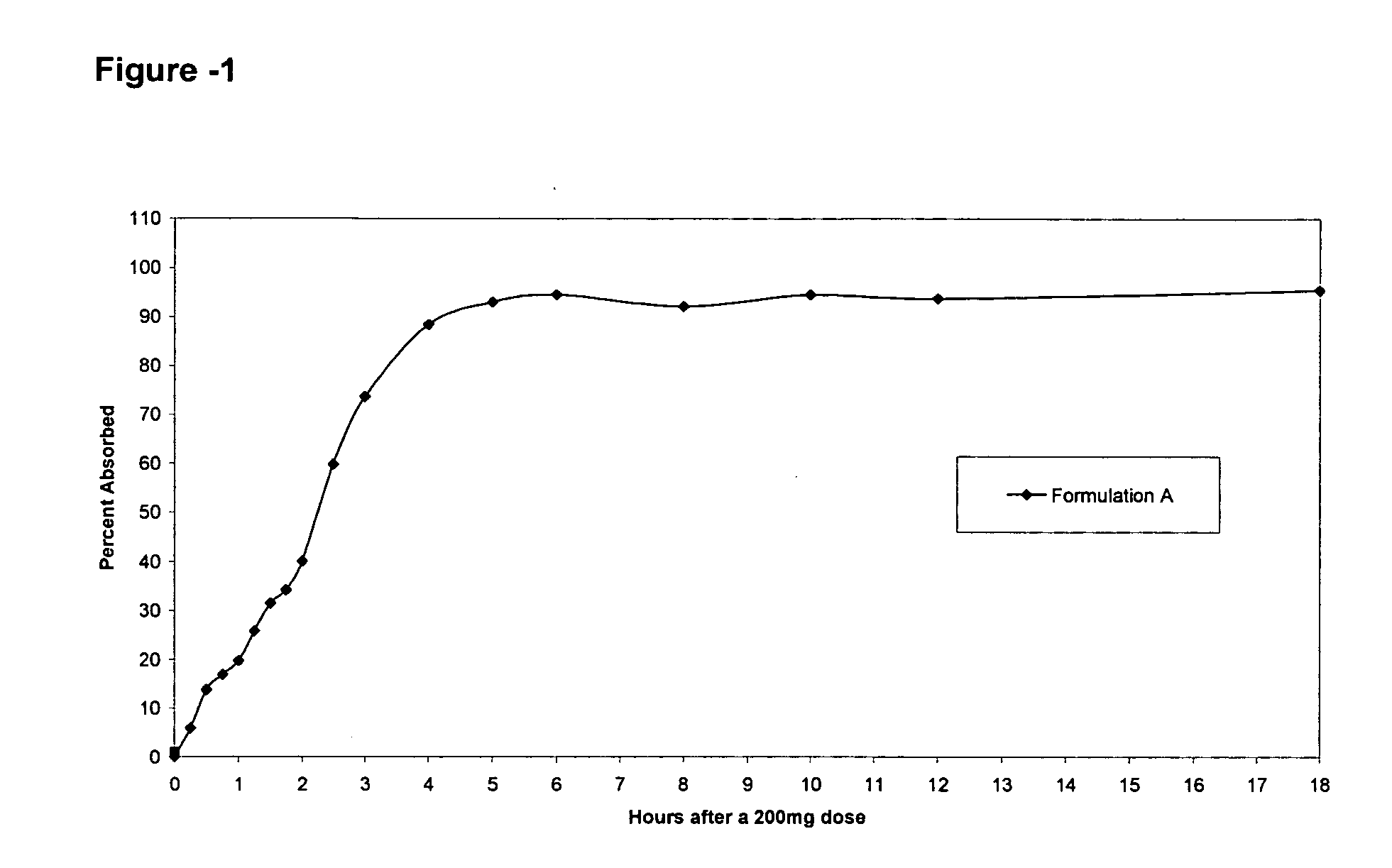
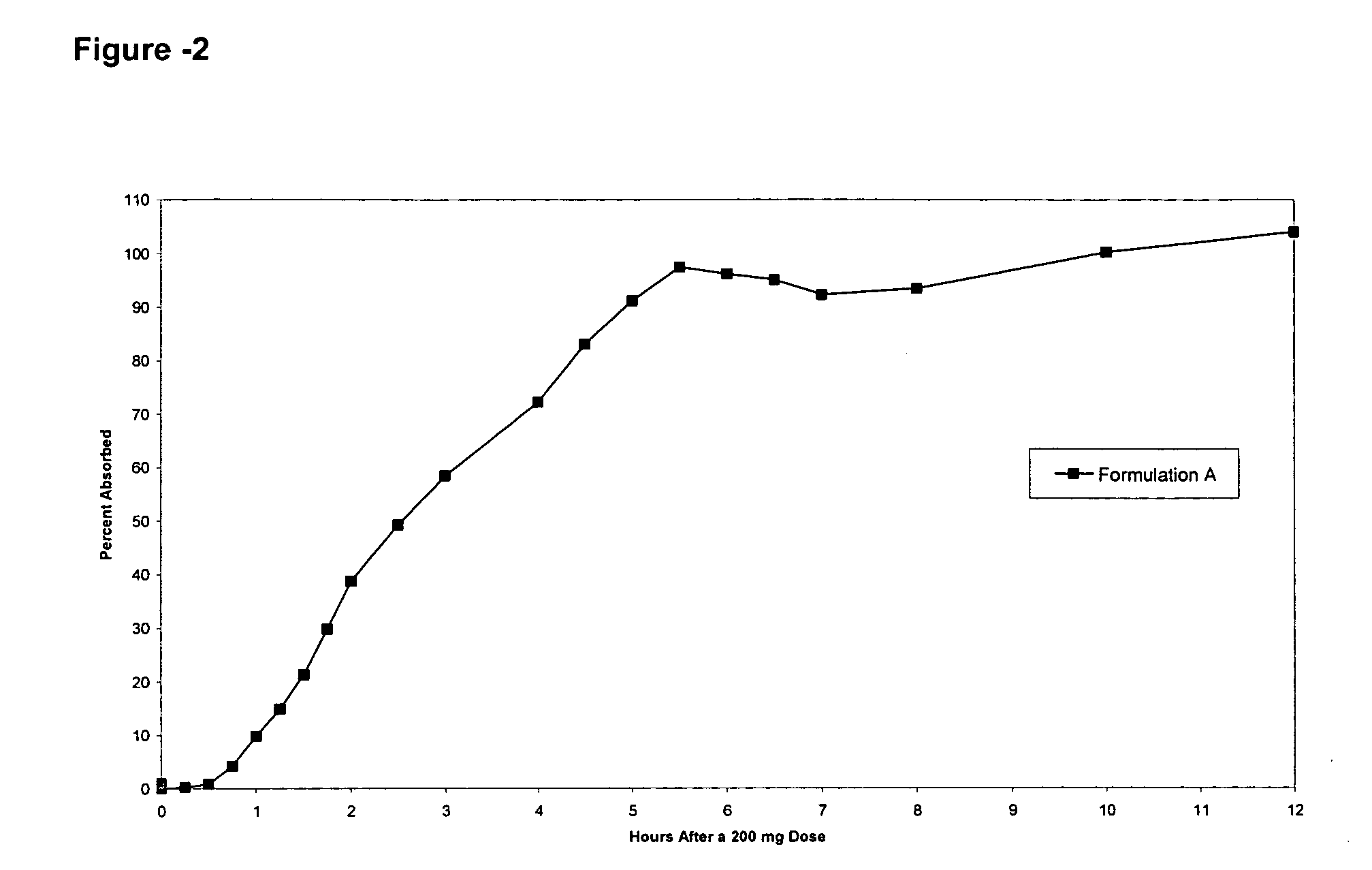
Pharmaceutical formulations targeting specific regions of the gastrointesinal tract
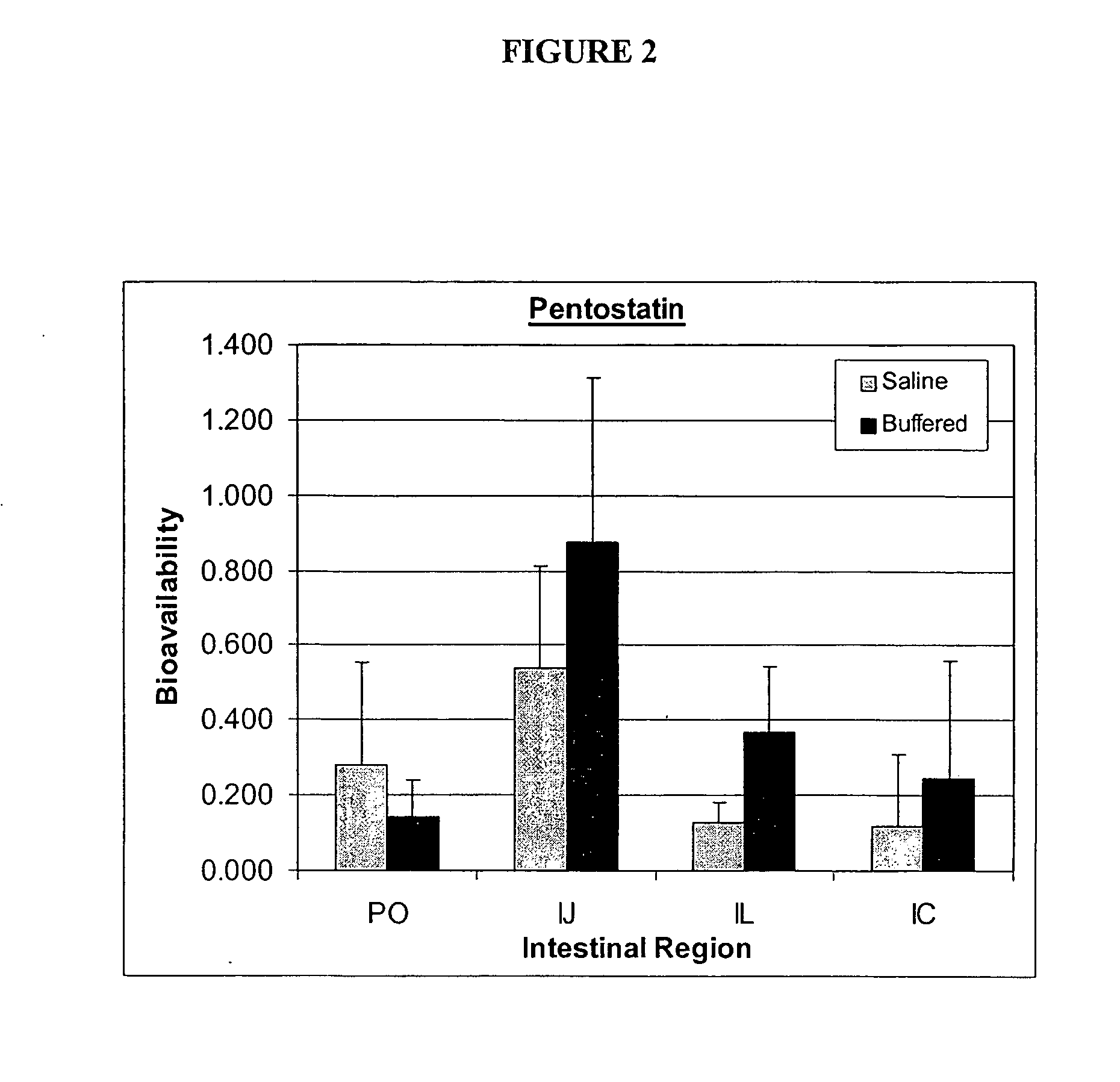
Oral formulations of pharmaceuticals are provided with enhanced bioavailability by targeting specific regions of the gastrointestinal tract. Particularly, water soluble and acid-labile drugs such as cytidine analogs (e.g., decitabine) and 2'-deoxyadenosine analogs (e.g., pentostatin) are formulated with pH-sensitive polymers so that these drugs are preferably absorbed in the upper regions of the small intestine, such as the jejunum. In addition, drugs with poor oral bioavailability such as camptothecin compounds (e.g., 9-nitro-camptothecin) can also be formulated using similar strategies in order to significantly improve their oral bioavailability. These formulations can be used to treat a wide variety of diseases or conditions, such hematological disorders, benign tumors, cancer, restenosis, inflammatory diseases, and autoimmune diseases.
Owner:MAYNE PHARMA USA
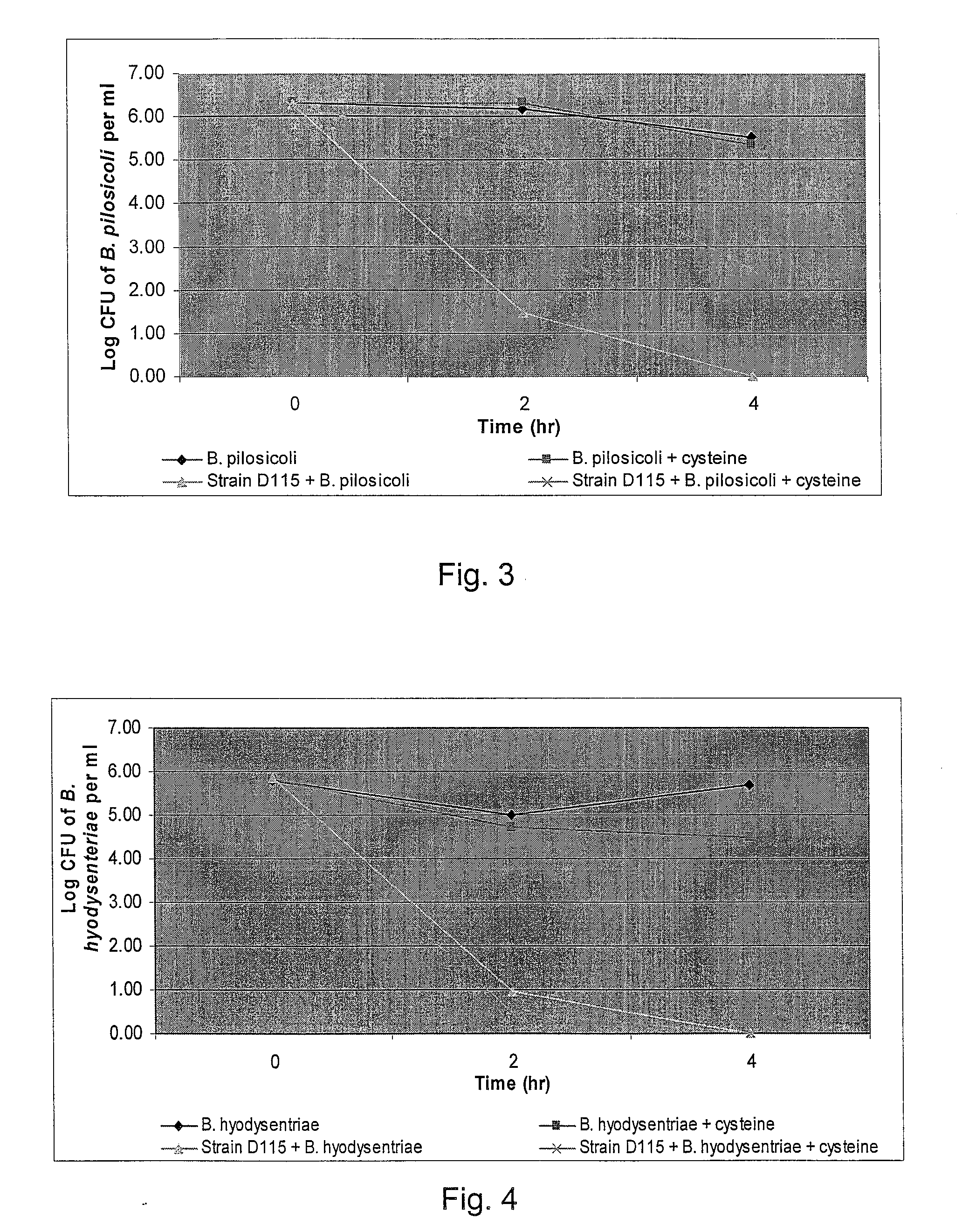
Broad-Spectrum Antibacterial and Antifungal Activity of Lactobacillus Johnsonii D115
The present invention demonstrated the potential use of Lactobacillus johnsonii D115 as a probiotic, as a prophylactic agent or as a surface treatment of materials against human and animal pathogens such as Brachyspira pilosicoli, Brachyspira hyodysenteriae, Shigella sonnei, Vibrio cholera, Vibrio parahaemolyticus, Campylobacter jejuni, Streptococcus pneumoniae, Enterococcus faecalis, Enterococcus faecium, Clostridium perfringens, Yersinia enterocolitica, Escherichia coli, Klebbsiella pneumoniae, Staphylococcus aureus, Salmonella spp., Bacillus cereus, Aspergillus niger and Fusarium chlamydosporum. The proteineous antimicrobial compound was partially characterized and found to be heat tolerant up to 121° C. for 15 min, and acid tolerant up to pH1 for 30 min at 40° C. The compound is also stable to enzymatic digestion, being able to retain more than 60% antimicrobial activity when treated with pepsin and trypsin.
Owner:KEMIN IND INC
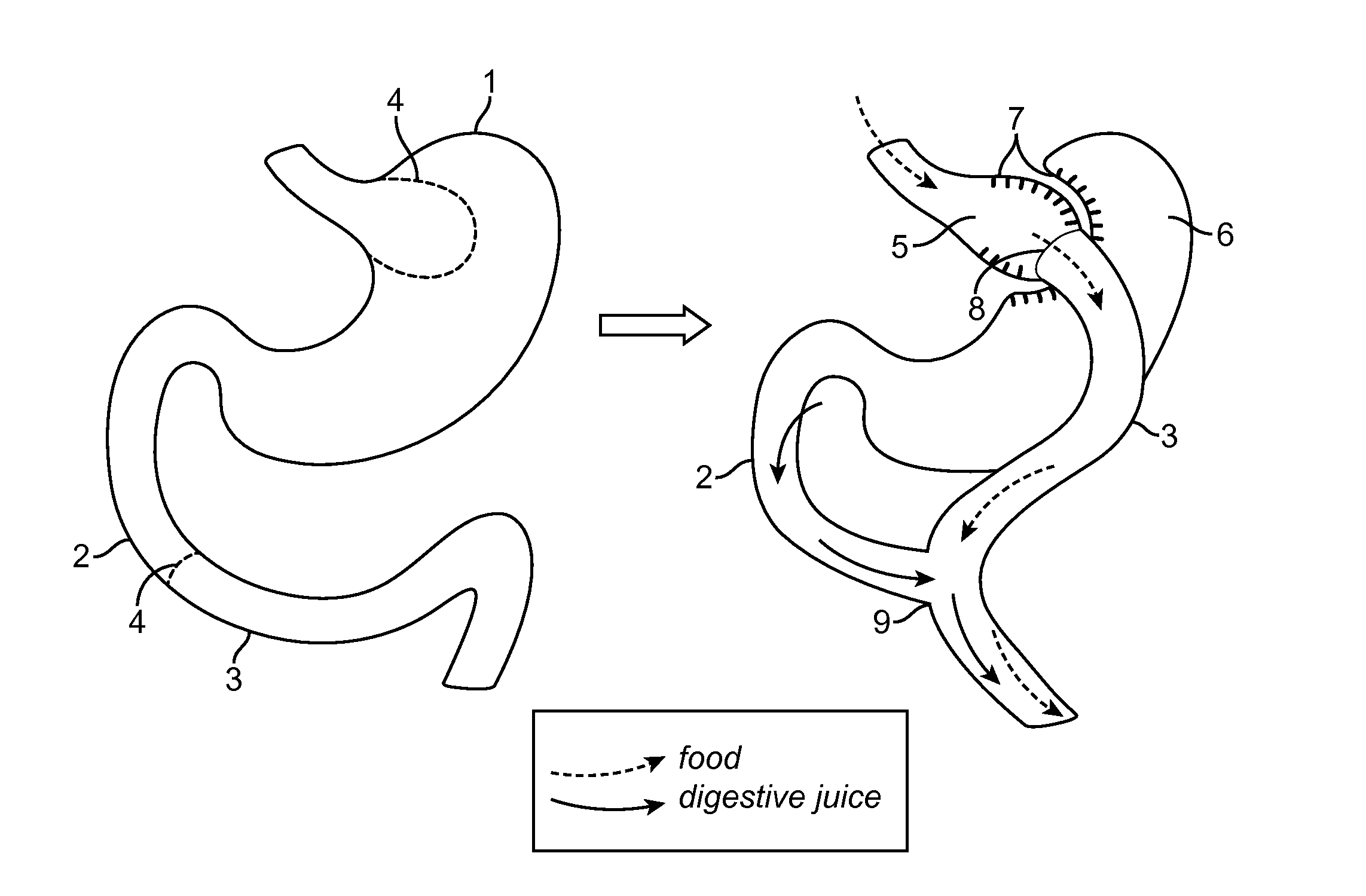
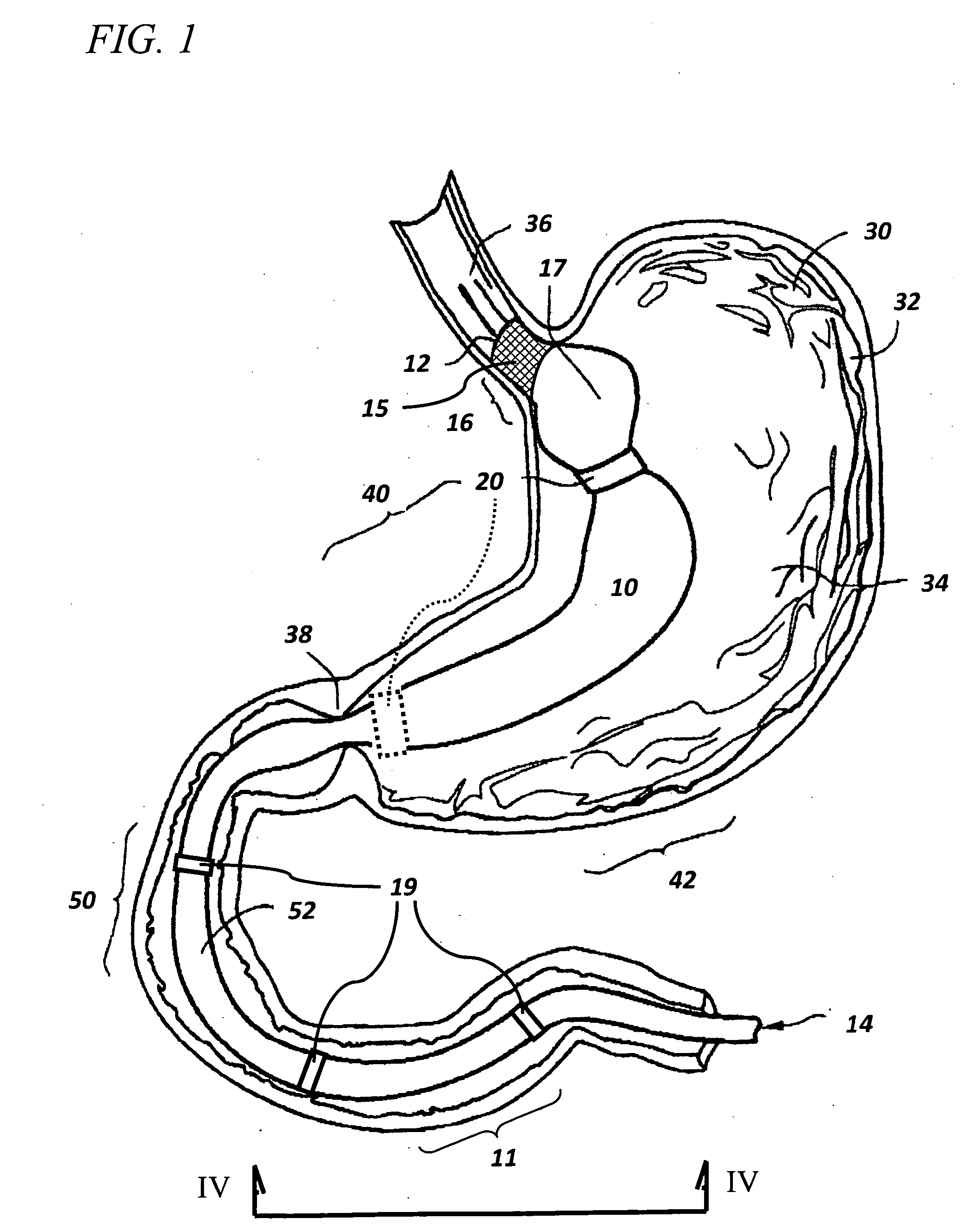
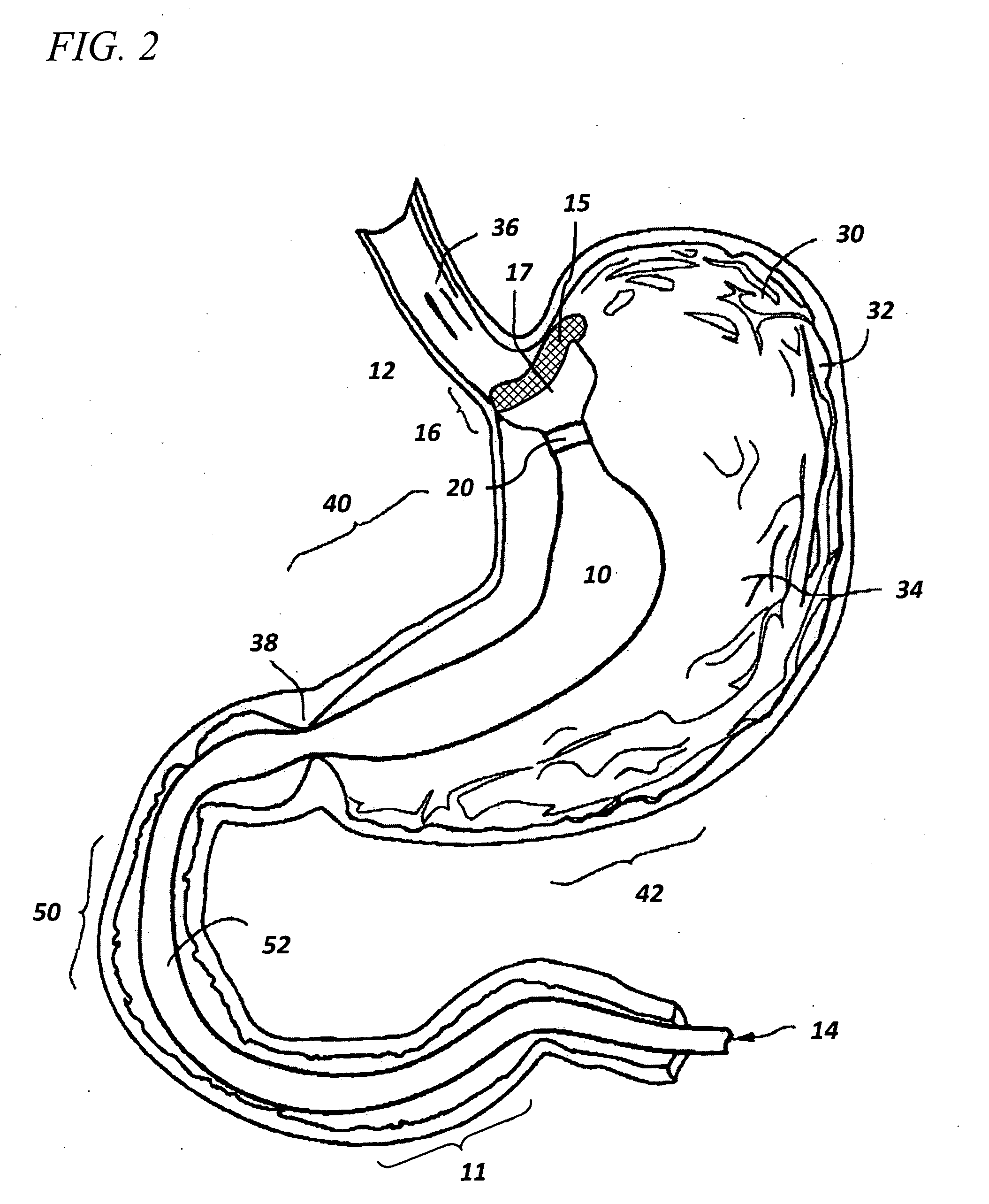
Devices and methods for forming an anastomosis
Devices and methods for deploying an anastomotic stent between portions of the gastro-intestinal (GI) tract are disclosed. The anastomotic stents are configured to atraumatically engage the tissue walls and to permit the flow of fluid, partially digested food, and food. The stents can be deployed using endoscopic catheter devices, laparoscopic tools, and combinations of both endoscopic tools and laparoscopic tools. Examples of anastomoses include anastomoses between the stomach and a portion of the intestines such as the jejunum. Anastomoses can also be formed between two closed ends of the intestines, such as two closed ends of the colon formed during a colon resection procedure. Anastomoses can also be formed between a fundal pouch formed during a gastric bypass procedure and the jejunum. Laparoscopic tools are disclosed to deploy a stent by selectively removing a radial restraint on a self expanding stent with the restraint removed through the laparoscopic access points.
Owner:BOSTON SCI SCIMED INC
Devices and methods for endolumenal therapy
InactiveUS20090093767A1Promoting tissue in-growthAltering abilityDiagnosticsSurgeryGastrointestinal deviceLower esophagus
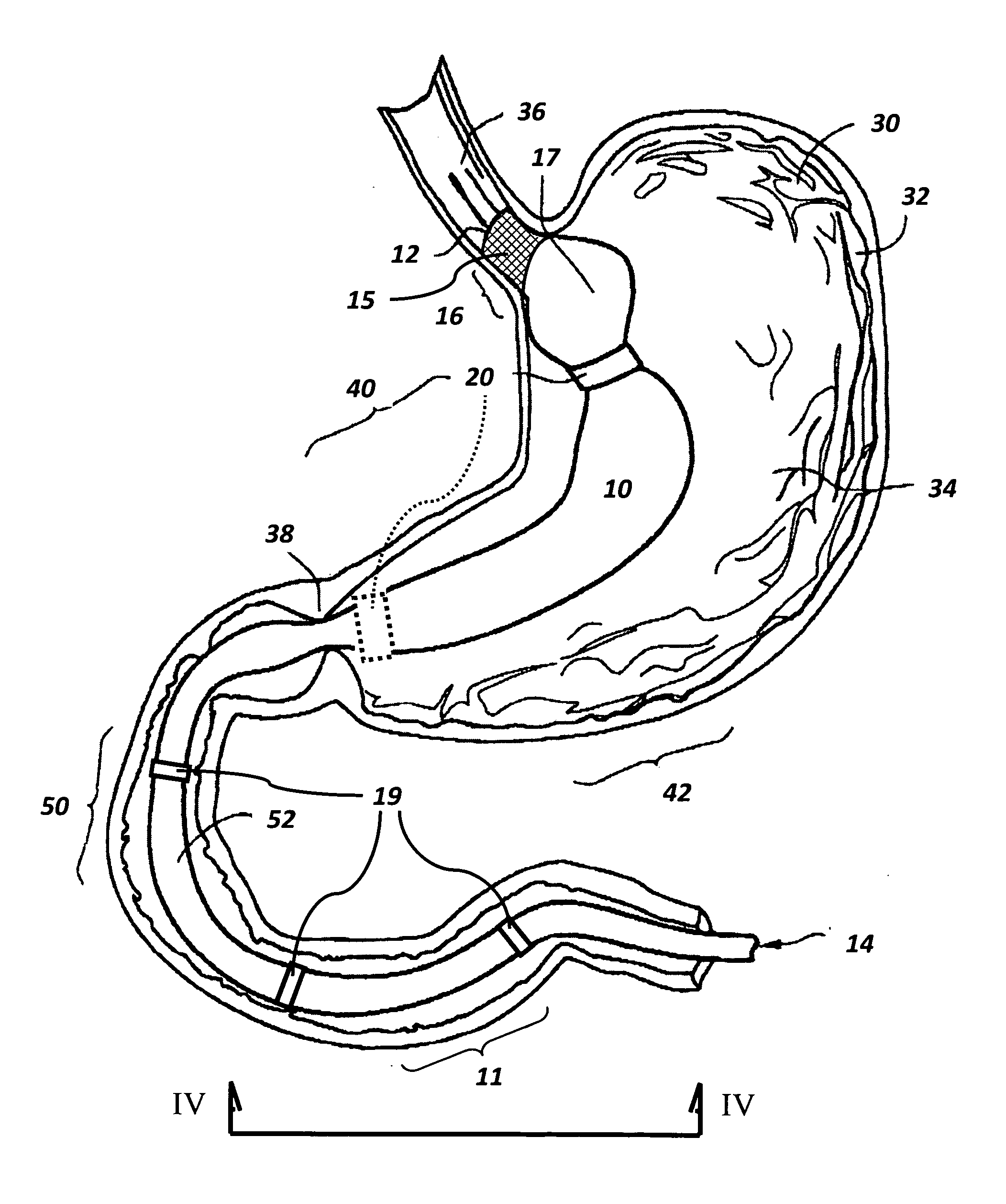
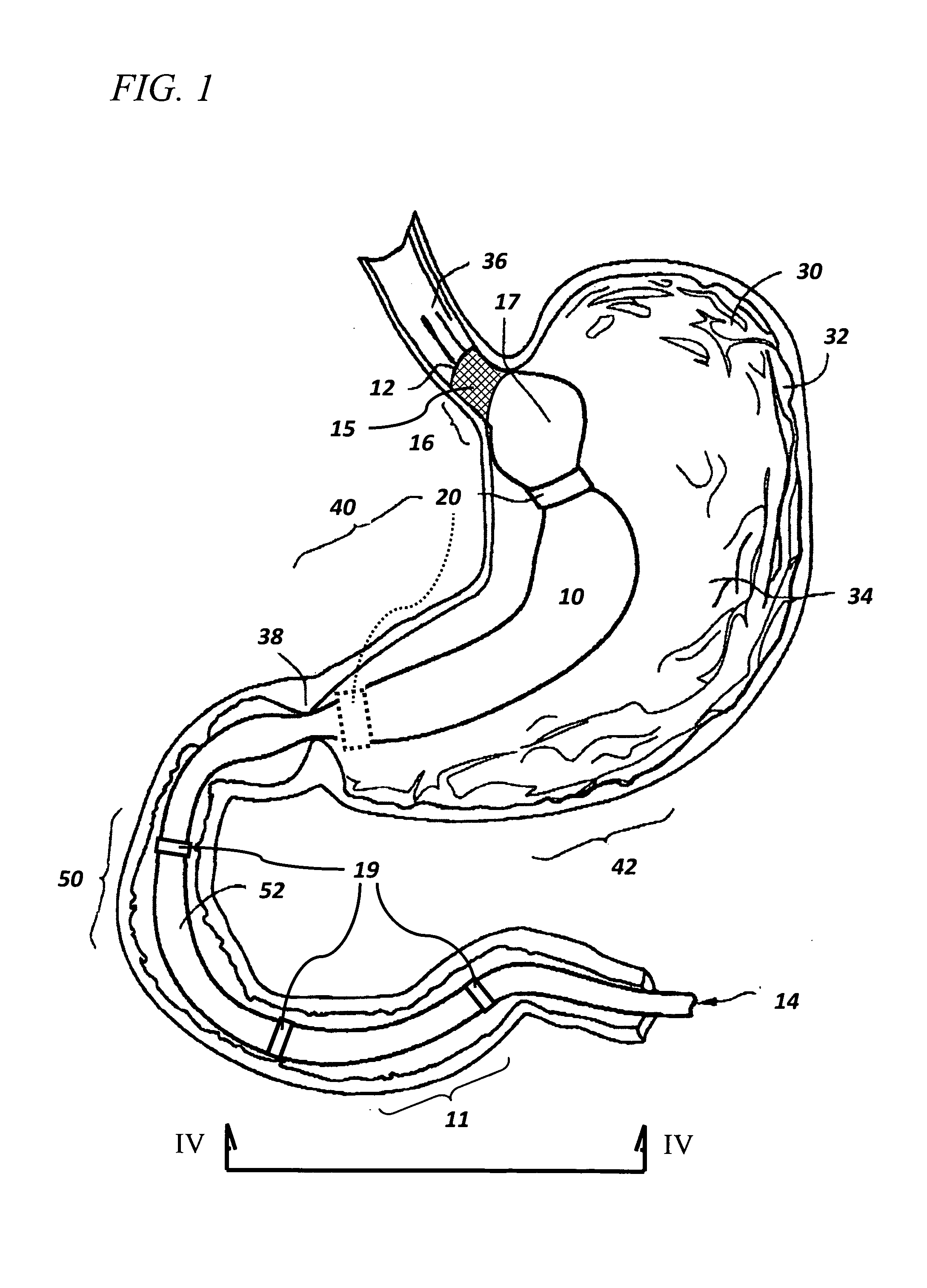
The present invention is directed generically to a means for altering the ability of the mammalian body to absorb nutritive content from ingested foodstuffs, and more specifically to an apparatus and method of use for an endolumenal sleeve (referred to also as an “intragastrointestinal device” or “gastrointestinal device”) positioned in the mammalian gastrointestinal (GI) tract. A suitable endolumenal sleeve is comprised of an anchor element and an opening at a proximal end, an elongate lumen or hollow open-ended tube having a transverse dimension, and a distal orifice. Optionally, an exterior aspect of the elongate lumen may include additional modes of attachment to the tissues walls of the GI tract through the use of one or more means for promoting tissue in-growth. The endolumenal sleeve is retained in the GI tract such that a substantial fraction of the food and liquids passing through the GI tract is channeled into the proximal opening and through an interlumenal space defined within the interior space of the endolumenal sleeve. Within the endolumenal sleeve there may be one or more restrictive means to constrain, impede or otherwise control the operative flow of material through the device. An individual restrictive means can either be of a fixed geometry or such means may include one or more elements which are adjustable in nature or function. The elongate lumen of the endolumenal sleeve is formed of a polymer composition suitable for controlled ingress of biological secretions, egress of certain selected nutritional elements, and may comprise either a single tubular structure or a multi-section (i.e. articulated and / or multiple lumen) assembly. When the endolumenal sleeve is in situ within the mammalian gastro-intestinal system, ingested foodstuffs are conveyed from the proximal end to said distal orifice. In typical applications, the proximal end of the endolumenal sleeve is positioned within the physiological region extending from the lower esophagus to the duodenum and the distal orifice is positioned within the physiological region extending from the upper duodenum to the lower jejunum, though further extension into the lower intestine is possible. Through proper selection of position for the endolumenal sleeve proximal and distal ends, combined by selection of the composition used in the fabrication of the elongate lumen, it is possible to finitely control the degree of nutritive absorption performed by the gastrointestinal tract.
Owner:KELLEHER BRIAN
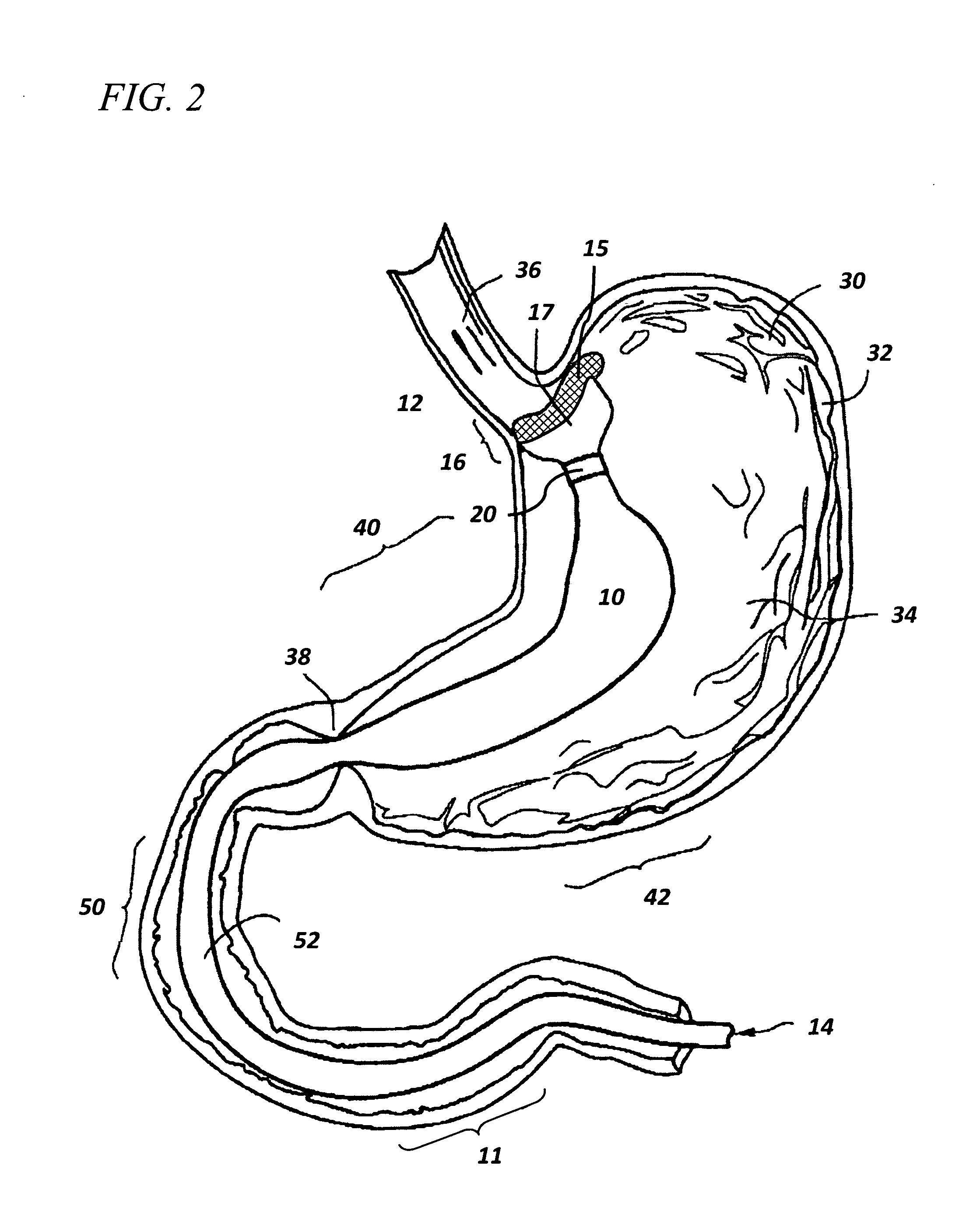
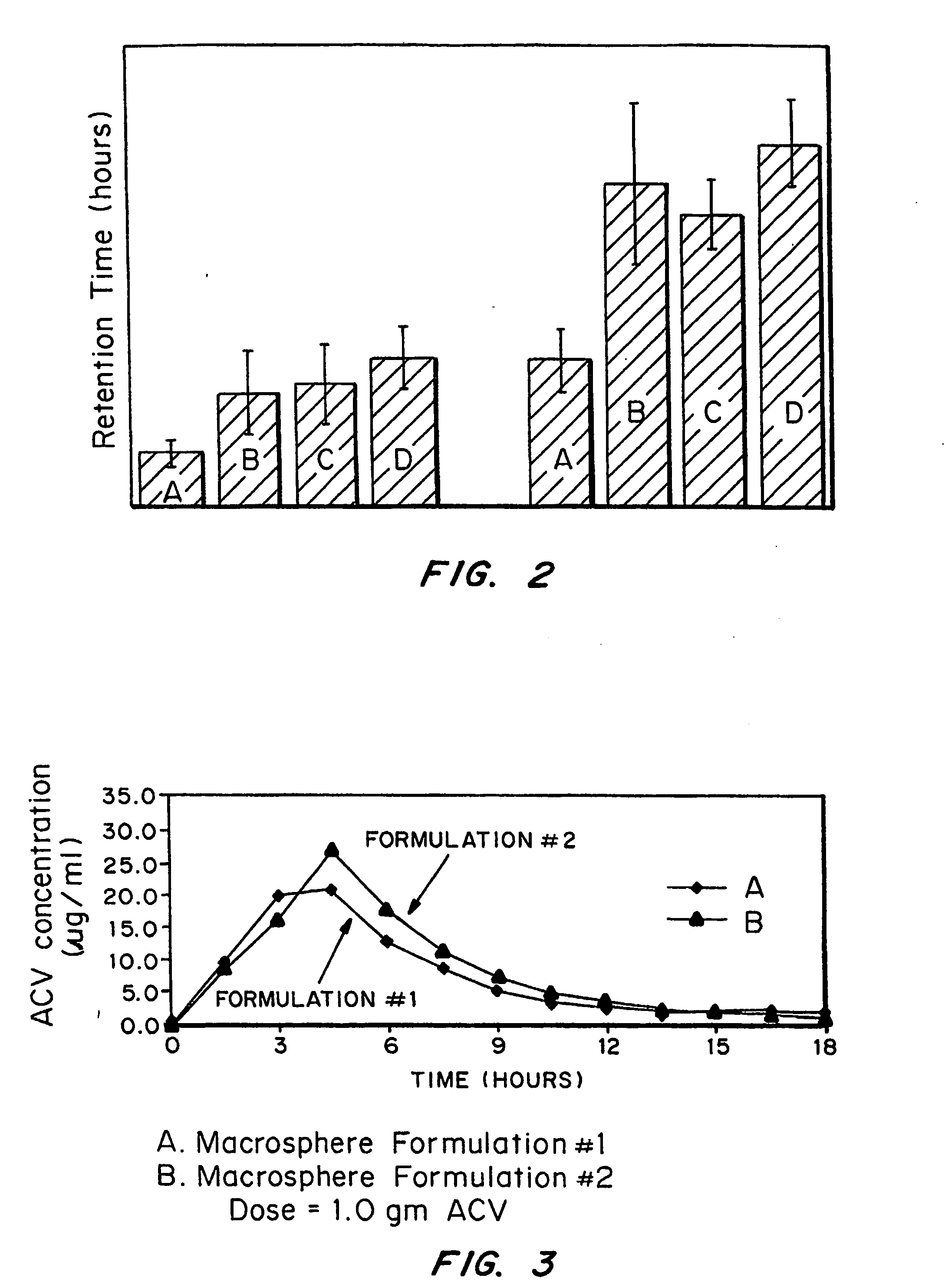
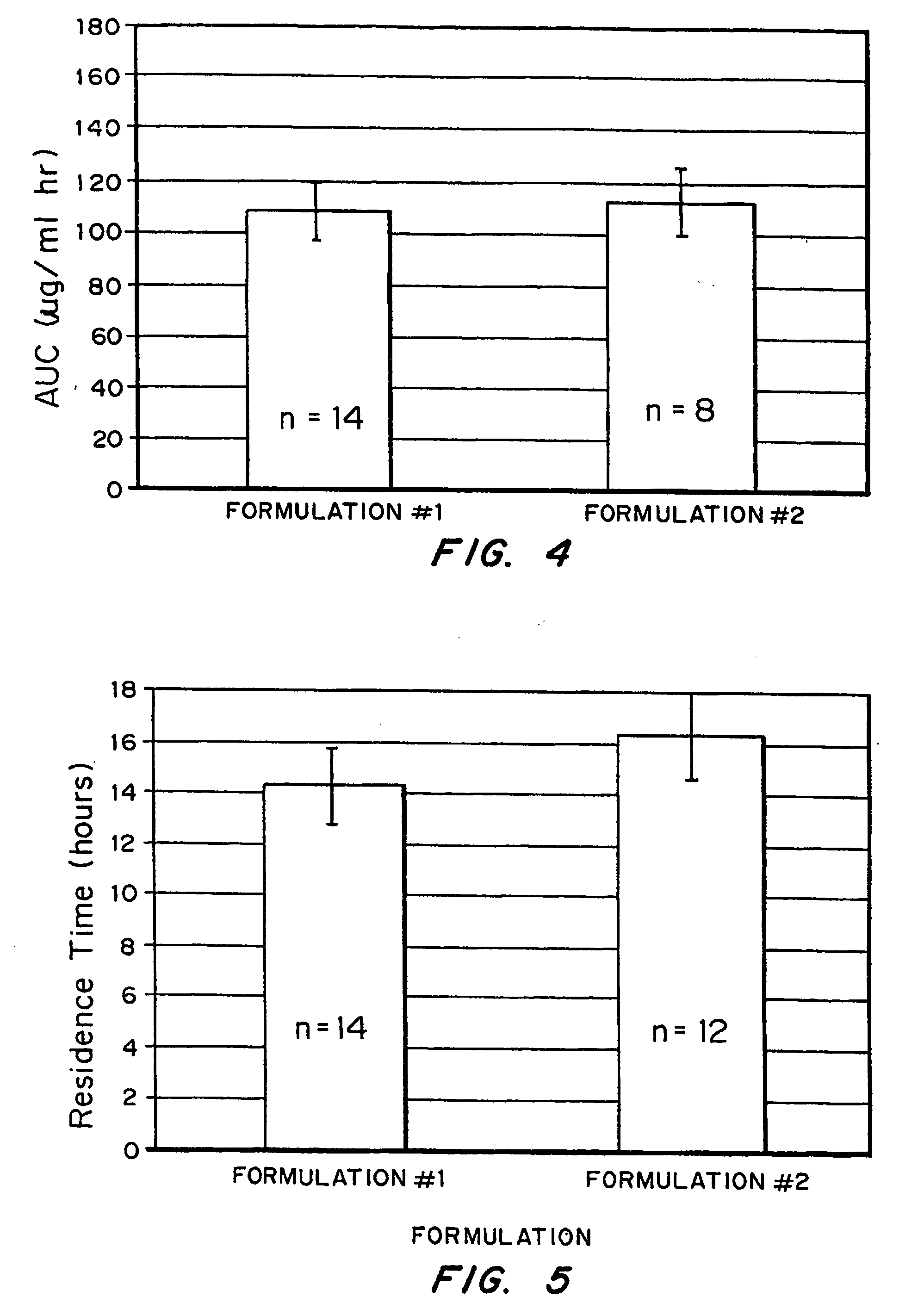
Bioadhesive drug delivery system with enhanced gastric retention
InactiveUS20050064027A1Prolonged gastric retention timeHigh retention rateBiocideCosmetic preparationsWhole bodyRetention time
Bioadhesive macrosphere delivery systems (“BDDS”) having prolonged gastric retention time due to bioadhesion rather than physical density or size are described. In general, the macrospheres have diameters that are greater than 200 microns, more preferably greater than 500 microns. The bioadhesive macrospheres are released in the stomach where they reside in close proximity to the gastric mucosa for a prolonged period of time. Increased residence of BDDS in the upper GI can lead to increased systemic absorption of drug in the preferred site of systemic absorption, namely the upper GI tract (upper to mid-jejunum). The BDDS may be engineered either as a capsule with drug delivery controlled by a diffusion-limited membrane or degradable shell, or as a solid matrix system with drug delivery controlled by a combination of diffusion and polymer degradation kinetics.
Owner:SPHERICS
Devices and methods for augmenting extragastric banding
InactiveUS20090093839A1Promoting tissue in-growthAltering abilitySurgeryDilatorsLower esophagusGastrointestinal device
The present invention is directed generically to a means for altering the ability of the mammalian body to absorb nutritive content from ingested foodstuffs, and more specifically to an apparatus and method of use for an endolumenal sleeve (referred to also as an “intragastrointestinal device” or “gastrointestinal device”) positioned in the mammalian gastrointestinal (GI) tract. A suitable endolumenal sleeve is comprised of an anchor element and an opening at a proximal end, an elongate lumen or hollow open-ended tube having a transverse dimension, and a distal orifice. Optionally, an exterior aspect of the elongate lumen may include additional modes of attachment to the tissues walls of the GI tract through the use of one or more means for promoting tissue in-growth. The endolumenal sleeve is retained in the GI tract such that a substantial fraction of the food and liquids passing through the GI tract is channeled into the proximal opening and through an interlumenal space defined within the interior space of the endolumenal sleeve. Within the endolumenal sleeve there may be one or more restrictive means to constrain, impede or otherwise control the operative flow of material through the device. An individual restrictive means can either be of a fixed geometry or such means may include one or more elements which are adjustable in nature or function. The elongate lumen of the endolumenal sleeve is formed of a polymer composition suitable for controlled ingress of biological secretions, egress of certain selected nutritional elements, and may comprise either a single tubular structure or a multi-section (i.e. articulated and / or multiple lumen) assembly. When the endolumenal sleeve is in situ within the mammalian gastro-intestinal system, ingested foodstuffs are conveyed from the proximal end to said distal orifice. In typical applications, the proximal end of the endolumenal sleeve is positioned within the physiological region extending from the lower esophagus to the duodenum and the distal orifice is positioned within the physiological region extending from the upper duodenum to the lower jejunum, though further extension into the lower intestine is possible. Through proper selection of position for the endolumenal sleeve proximal and distal ends, combined by selection of the composition used in the fabrication of the elongate lumen, it is possible to finitely control the degree of nutritive absorption performed by the gastrointestinal tract.
Owner:KELLEHER BRIAN
Lactobacillus plantarum CCFM8724 and application thereof
ActiveCN102533618AWith acidityWith fragranceAntibacterial agentsMilk preparationBiotechnologySoybean product
The invention relates to lactobacillus plantarum CCFM8724 and application thereof. The lactobacillus plantarum CCFM8724 has ability of suppressing growth of campylobacter jejuni in vitro, good resistance to acid and cholate and good adhesive ability to intestinal epithelial cells, and can control the campylobacter jejuni adhering to the intestinal epithelial cells so as to suppress growth of the campylobacter jejuni in chicken. The lactobacillus plantarum CCFM8724 can be used for preparing a medicinal composition for suppressing campylobacter jejuni; a fermentation agent containing the strain of lactobacillus plantarum CCFM8724 can be used for producing fermented dairy products, fermented bean products, fermented fruit / vegetable products and silage; the fermentation agent enables the products to obtain certain acidity and particular flavor; and meanwhile, the product preserving time is prolonged, and the nutritional value, digestibility and safety of the products are improved.
Owner:JIANGNAN UNIV
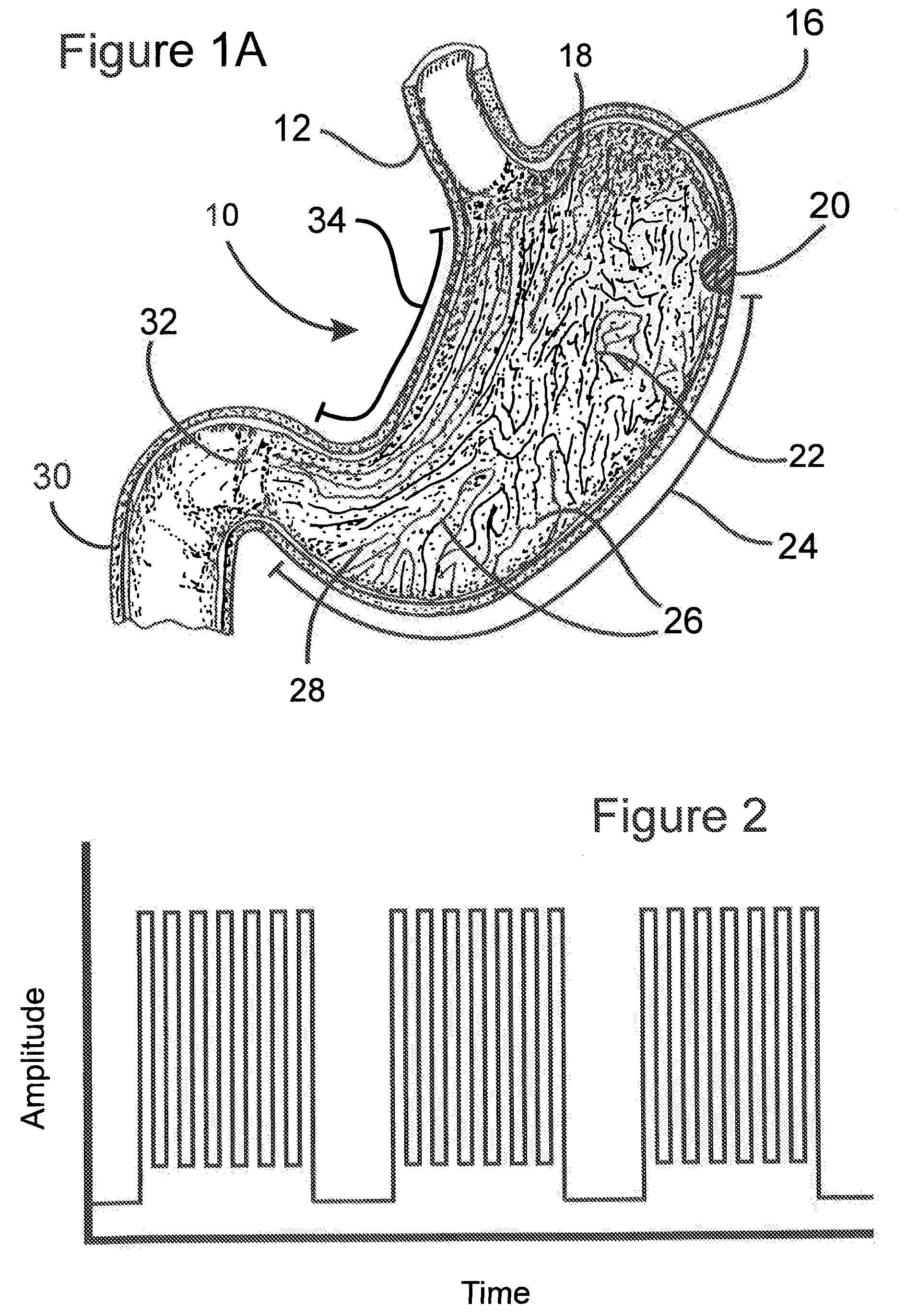
Process for electrostimulation treatment of morbid obesity
InactiveUS20080183238A1Prevent and slow down stomach emptyingTransit delayInternal electrodesExternal electrodesMorbid obesityPulse rate
An improved process using electrostimulation for treating obesity, especially morbid obesity, is provided. The improved method of this invention provides electrostimulation on or along the small intestines, preferably on or along the duodenum and / or jejunum, which provides improved control of obesity. In one embodiment, the process employs stimulation of the lesser curvature at a rate of about 2 to about 30 pulses / minute with each pulse lasting about 0.1 to about 4 seconds such that there is a pause of about 3 to about 30 seconds between the pulses. More preferably, the pulse rate is about 12 to about 14 pulses / minute with each pulse lasting about 0.1 to about 0.5 seconds with a pause of about 4.5 to about 5 seconds between pulses. Preferably, the pulse amplitude is about 0.5 to about 15 milliamps. More preferable, each pulse consists of a train of micro-bursts with a frequency of about 5 to about 100 Hz.
Owner:MEDTRONIC INC
Formulations for a tight junction effector
ActiveUS20070196501A1Stable in gastric fluidAntibacterial agentsBiocideIntestinal fluidSmall intestine
Enteric compositions comprising one or more tight junction agonists and / or one or more tight junction antagonists are provided. Compositions of the invention may comprise a delayed-release coating disposed over a tight junction agonist and / or tight junction antagonist layer which may be disposed over an inert core. Delayed-release coatings may be substantially stable in gastric fluid and substantially unstable in intestinal fluid, thus providing for substantial release of the tight junction agonist and / or antagonist from the composition in the duodenum or jejunum of the small intestine.
Owner:ALBA THERAPEUTICS CORP
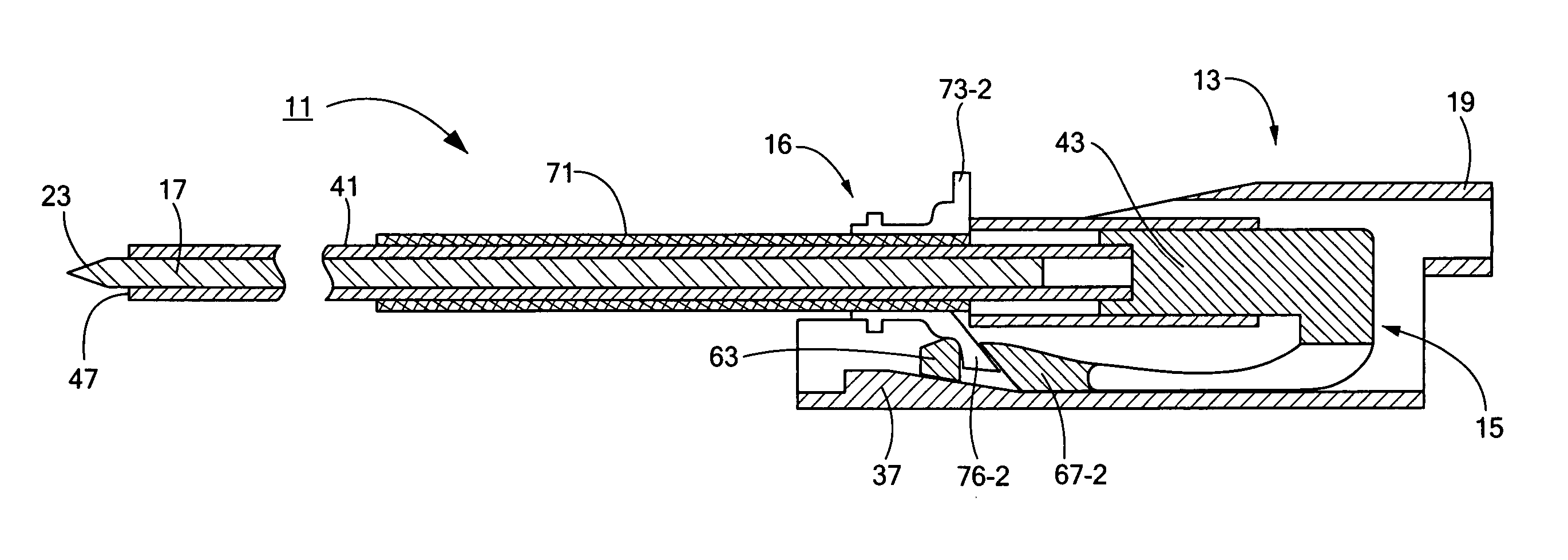
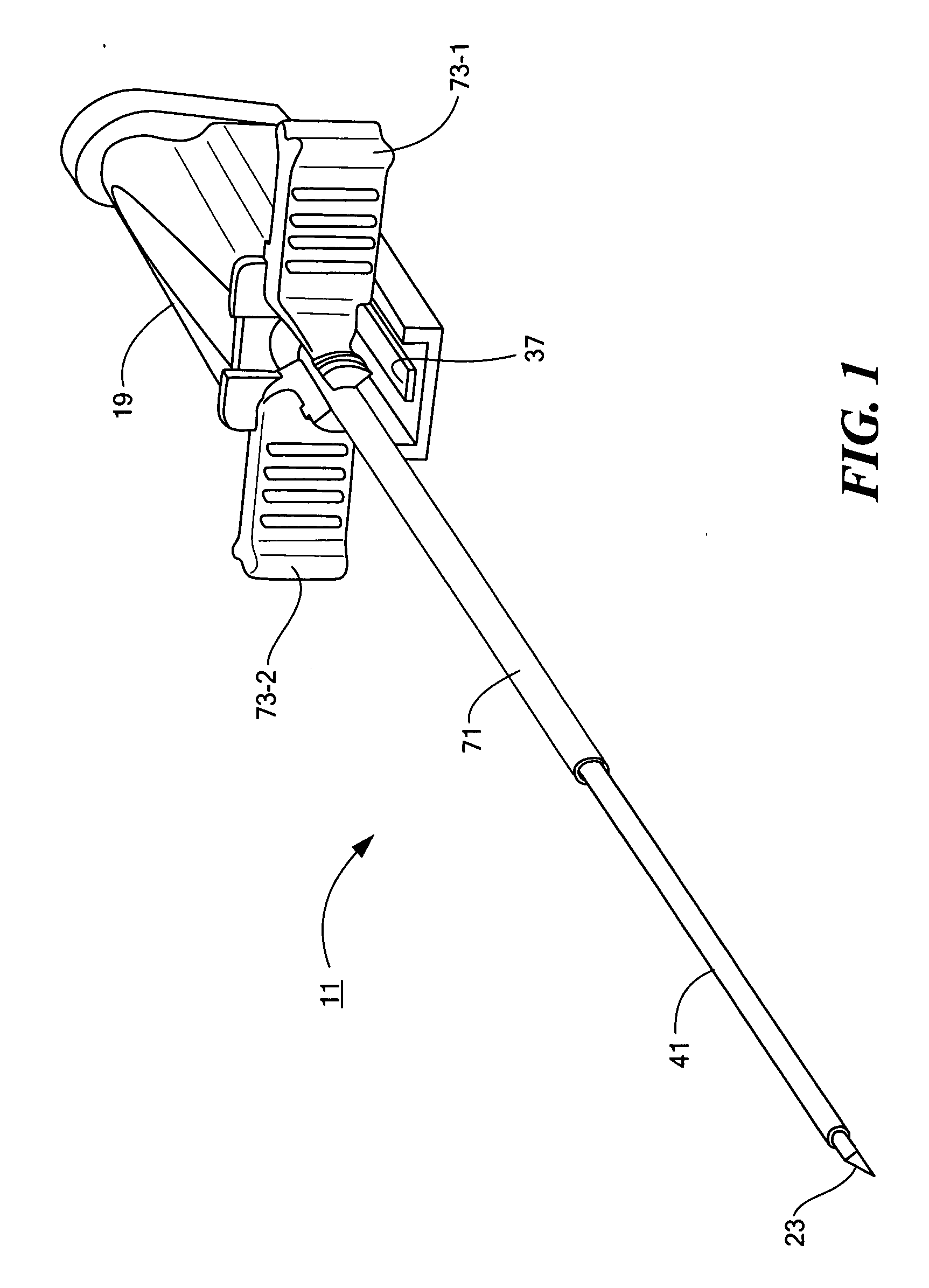
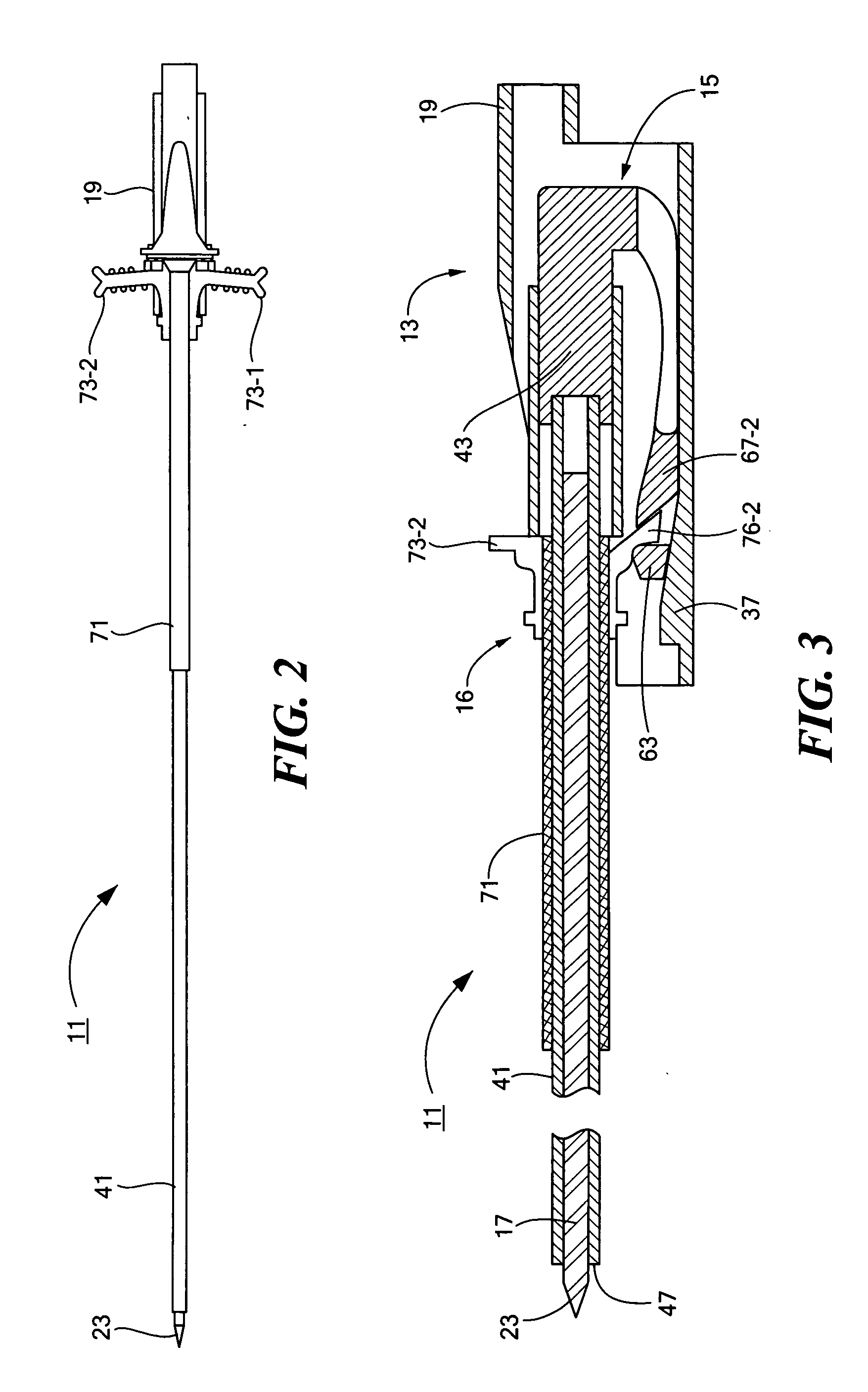
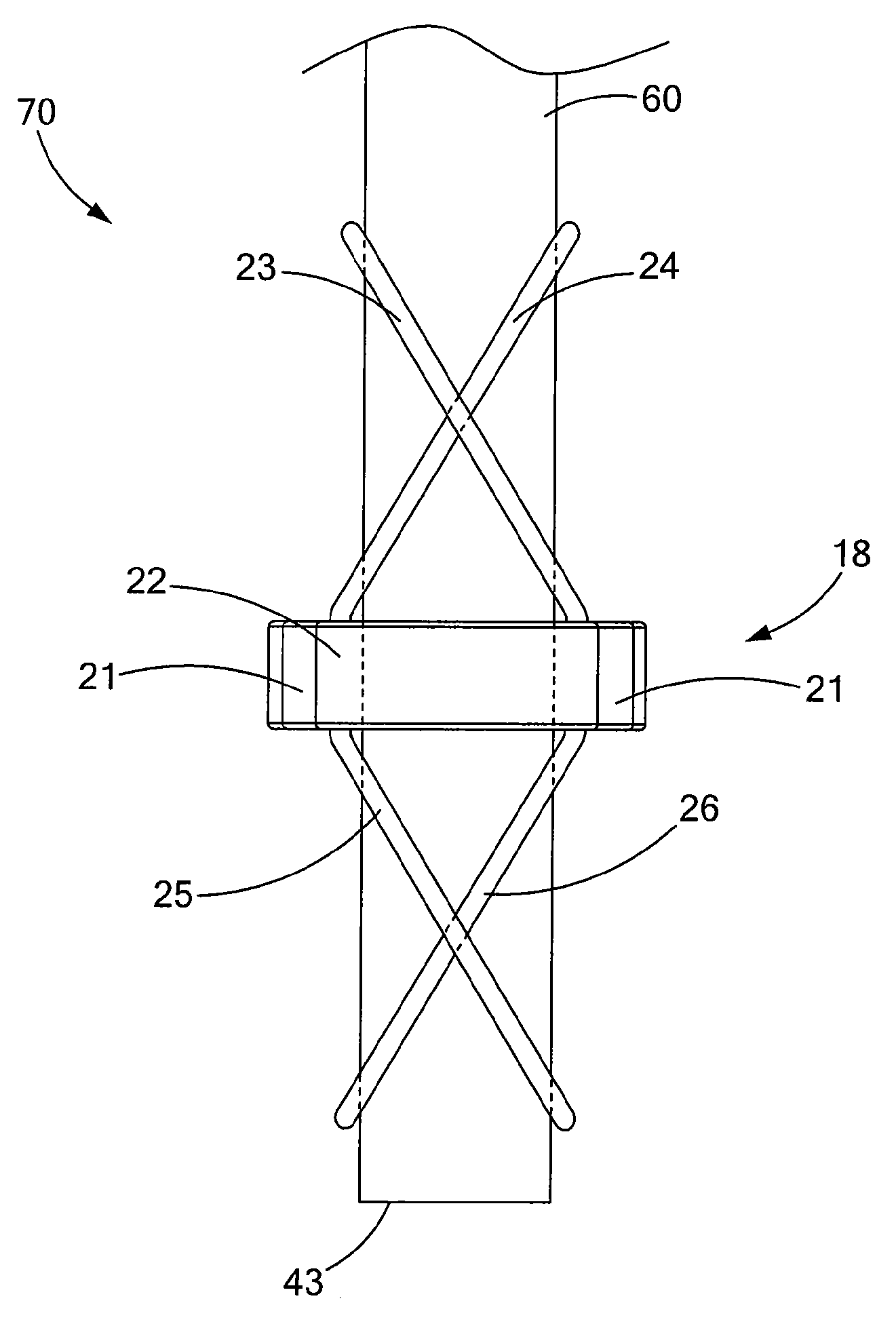
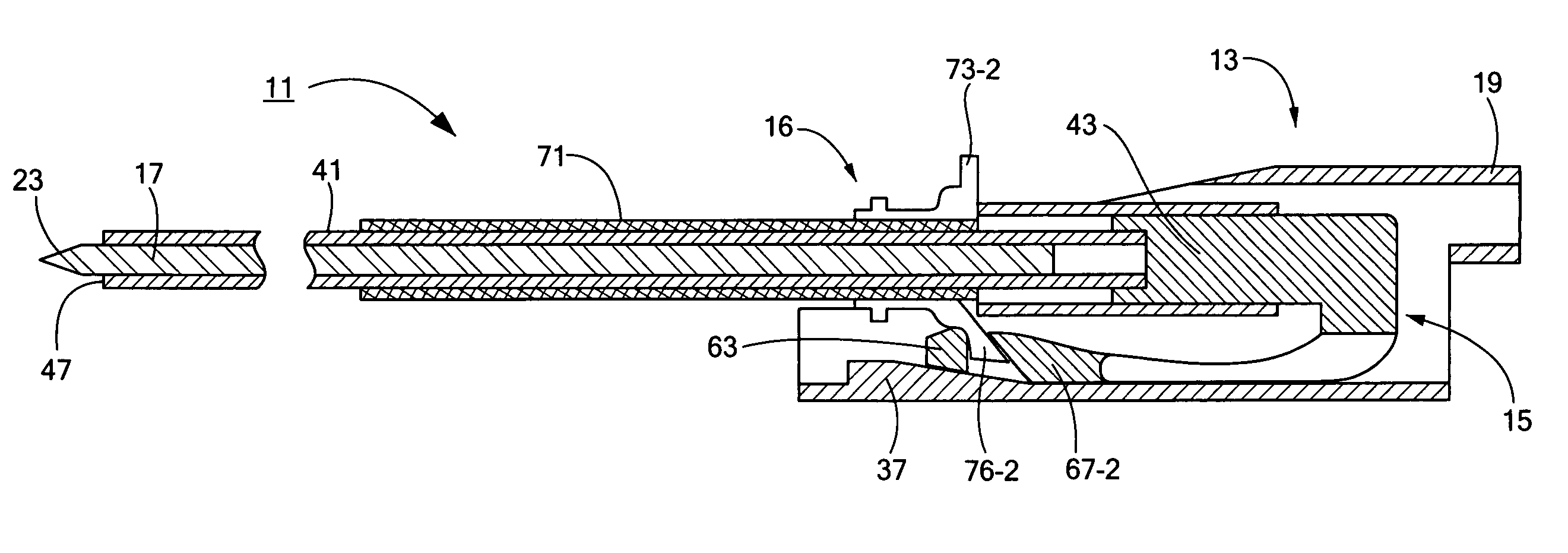
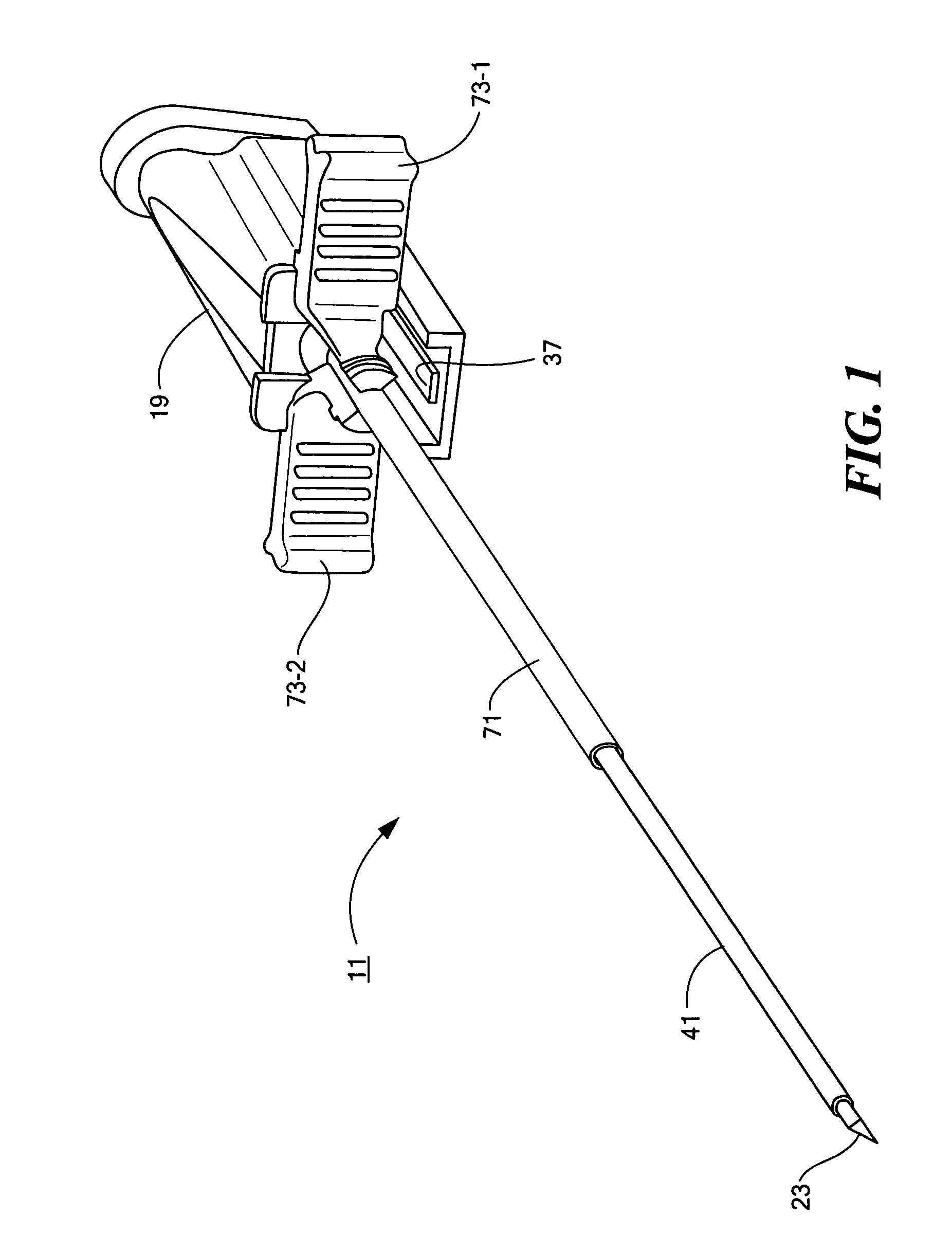
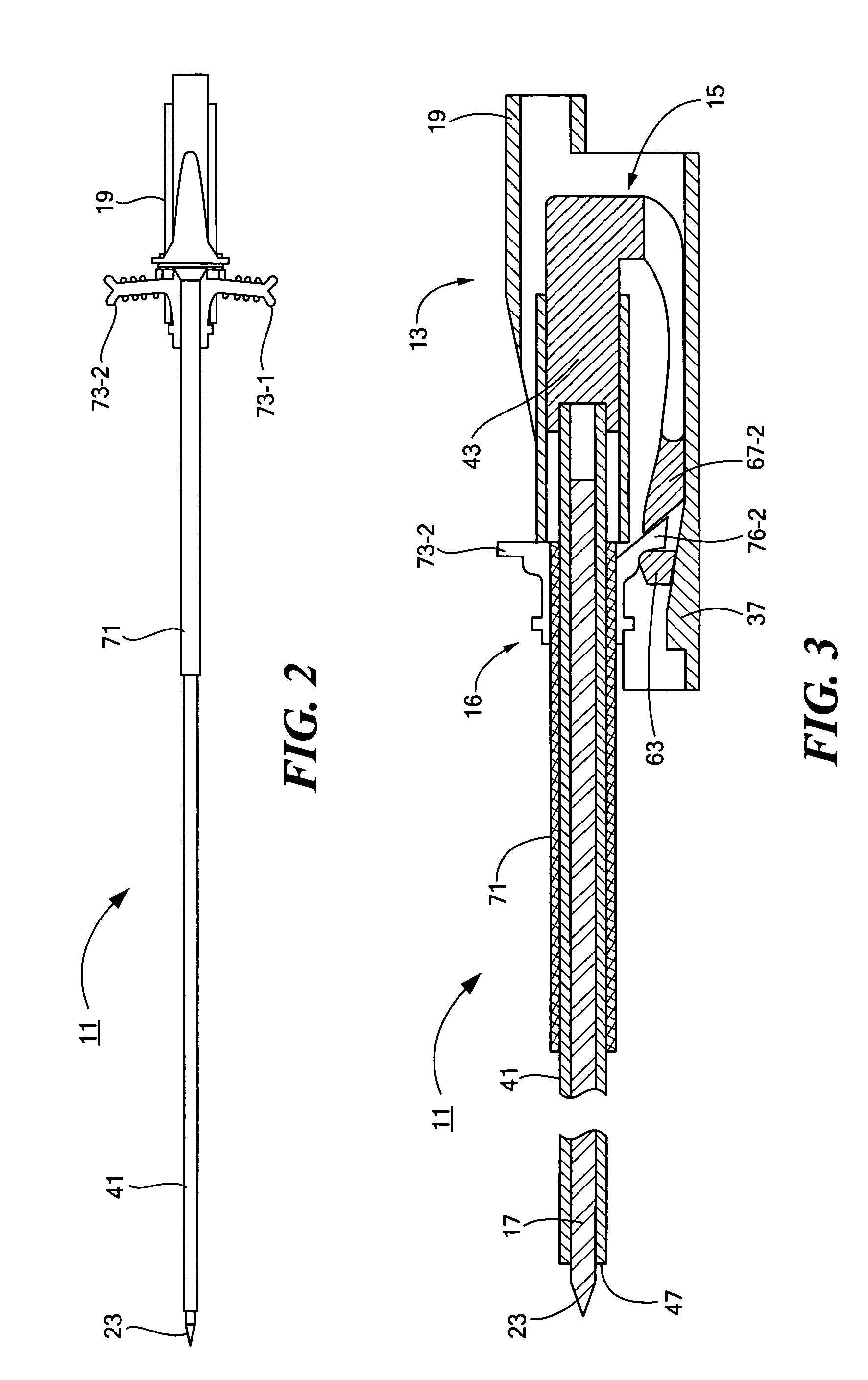
Percutaneous endoscopic jejunostomy access needle
An access needle suitable for use in percutaneous endoscopic jejunostomy procedures. In one embodiment, the access needle includes a stylet assembly, a safety sheath assembly and a cannula assembly. The stylet assembly includes a stylet and a stylet hub. The stylet has a proximal end and a sharpened distal end, the proximal end of the stylet being mounted within the stylet hub. The safety shield assembly includes a sheath and a shuttle. The sheath, which has a blunt distal end, coaxially surrounds the stylet. The proximal end of the sheath is fixed to the shuttle, the shuttle being movable between a first position in which the sharpened distal end of the stylet is exposed and a second position in which the sharpened distal end of the stylet is shielded by the blunt distal end of the sheath. The cannula assembly includes a cannula and a cannula hub, the cannula hub being fixed to the proximal end of the cannula. The cannula hub is detachably engageable with the shuttle to move the shuttle between its first and second positions.
Owner:BOSTON SCI SCIMED INC
Linear clamps for anastomosis
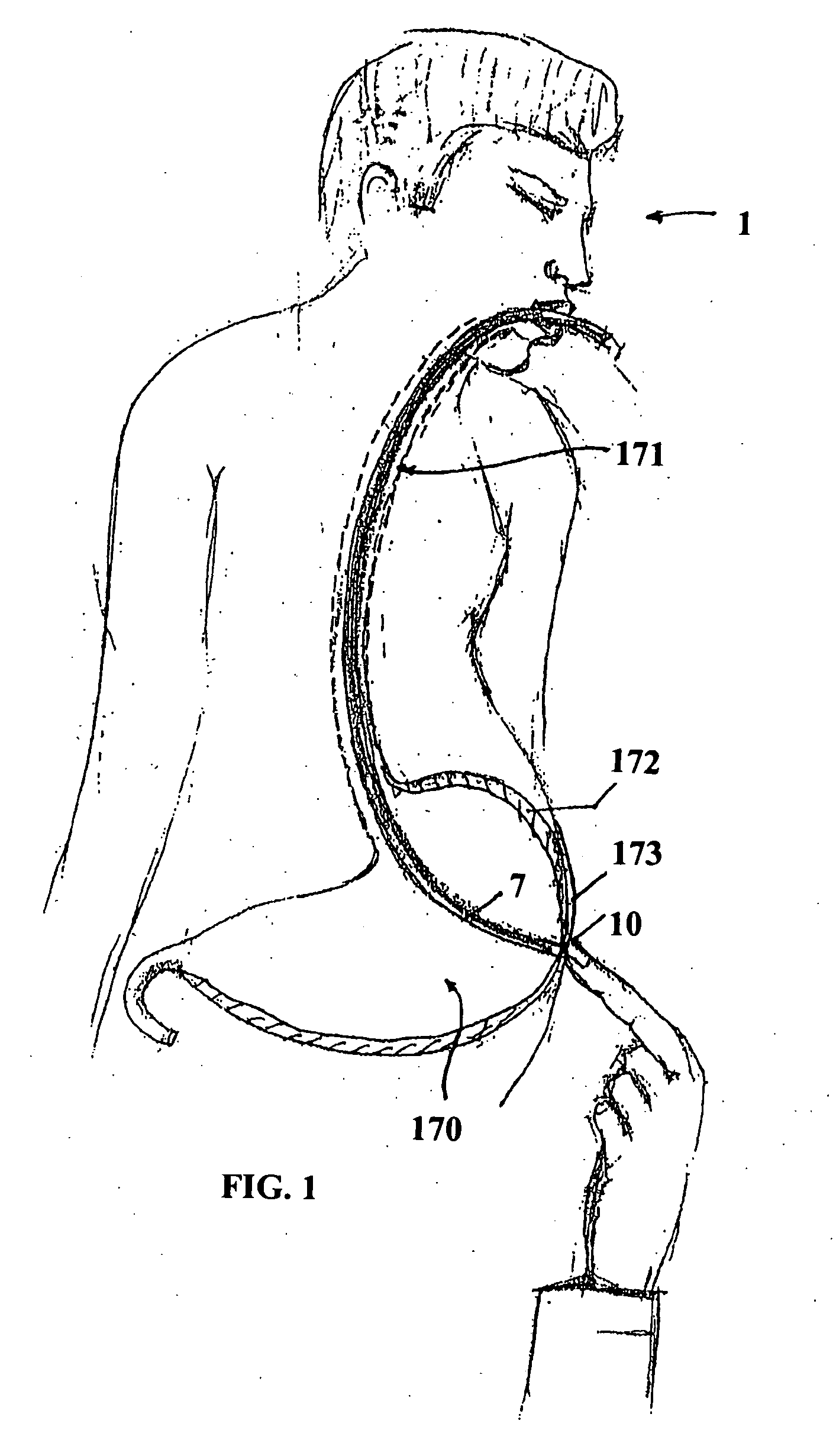
ActiveUS20100331866A1Reducing technical challengeMinimizing potential riskWound clampsStomaEngineering
The present embodiments provide medical apparatuses, systems, and methods for rapidly forming an anastomosis between two viscera. The medical apparatus generally comprises two bases with two clamp members rotatably attached to the bases, the clamp members being capable of compressing tissue. The system generally comprises positioning and then deploying the medical apparatus between and within two stomas via an elongate member. The method generally comprises using the medical system to create an anastomosis between the stomach and the jejunum.
Owner:COOK MEDICAL TECH LLC
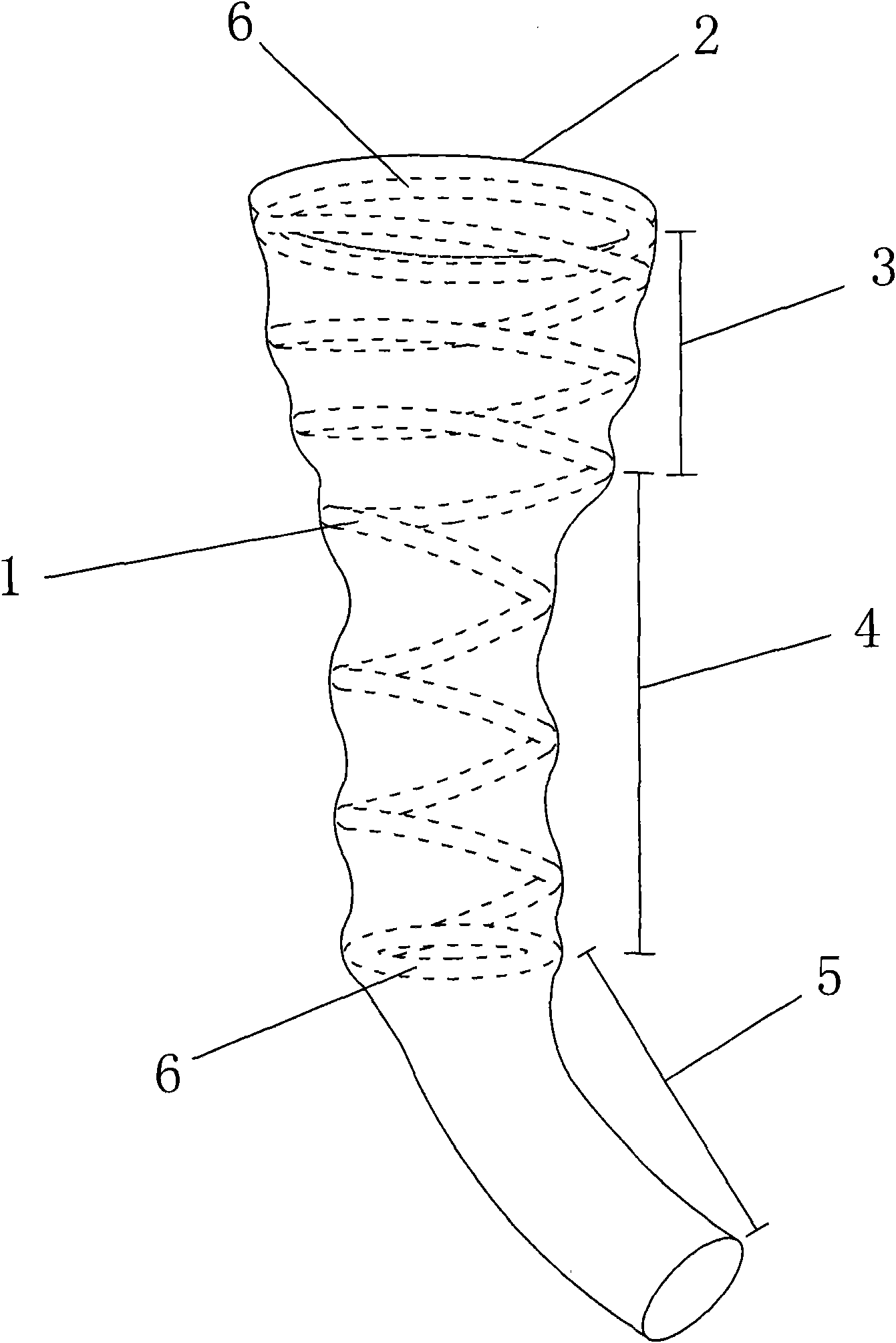
Duodenal sleeve and conveyor thereof
InactiveCN101843536AAvoid absorptionInhibit metabolismNon-surgical orthopedic devicesPylorusMetal framework
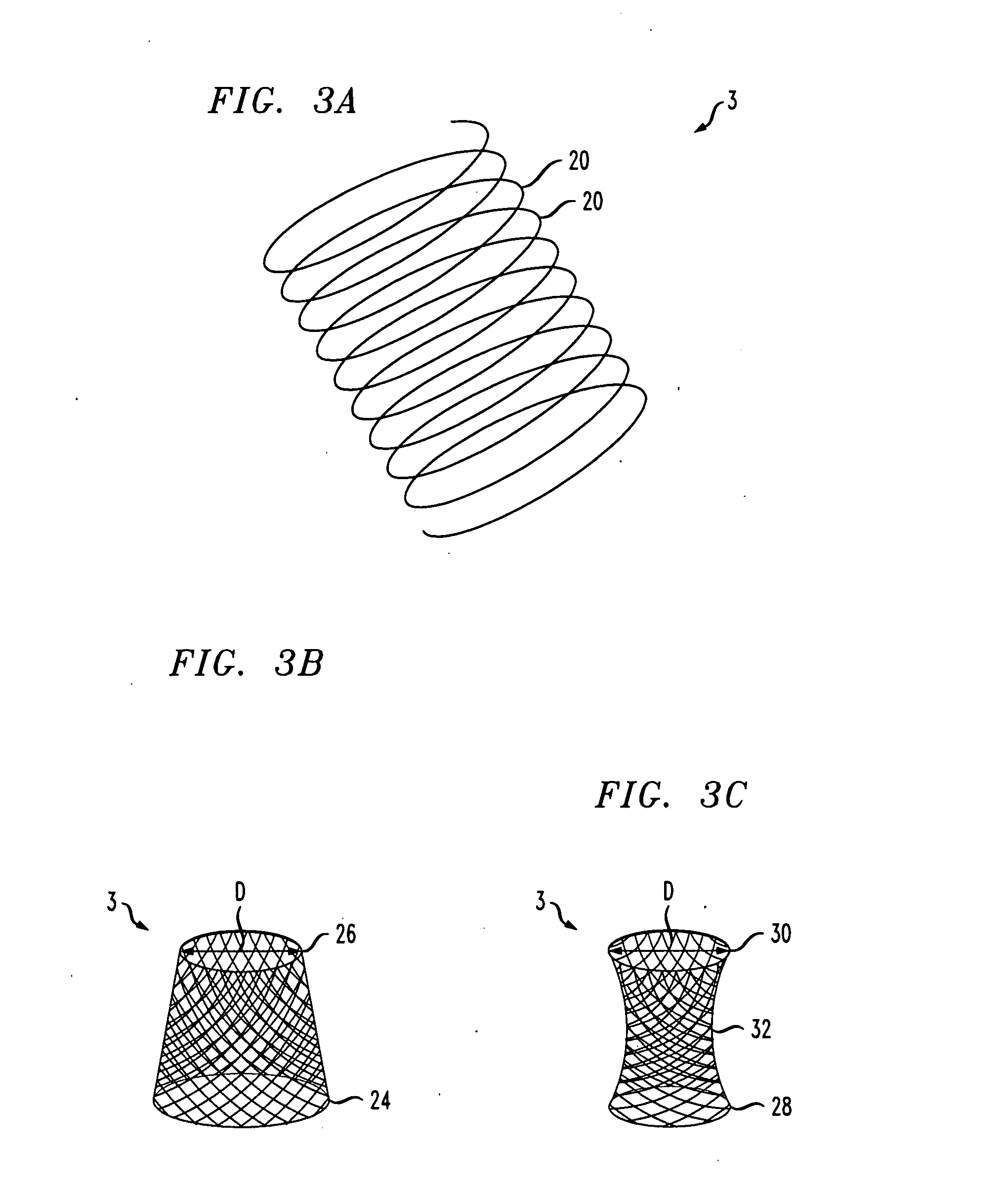
The invention provides a duodenal sleeve which is composed of a metal framework and an outer sleeve coated outside the metal framework. The metal framework is of a spiral ring structure and divided into a duodenal spherical cavity section for the metal framework and a duodenal spherical rear section for the metal framework; the spiral ring of the duodenal spherical cavity section for the metal framework is bigger and denser than that of the duodenal spherical rear section for the metal framework; the spiral ring structure of the duodenal spherical cavity section for the metal framework is in a bowl or funnel shape; the top and the bottom of the metal framework are equipped with circular or oval annular closed opening; and the outer sleeve is coated outside the metal framework and the lower end thereof is longer than the bottom end of the metal framework. The invention further provides a conveyor for the duodenal sleeve, comprising a plastic hose, wherein, longitudinal cracks are arranged on the wall of the plastic hose from the head end to the tail end thereof. The duodenal sleeve is used for transporting stomach effluents from a stomach pylorus, replaces gastric bypass operation, avoids that the stomach effluents are directly digested, absorbed and metabolized in duodenum, and treats adiposis and diabetes.
Owner:南京速能医疗器械设计有限公司
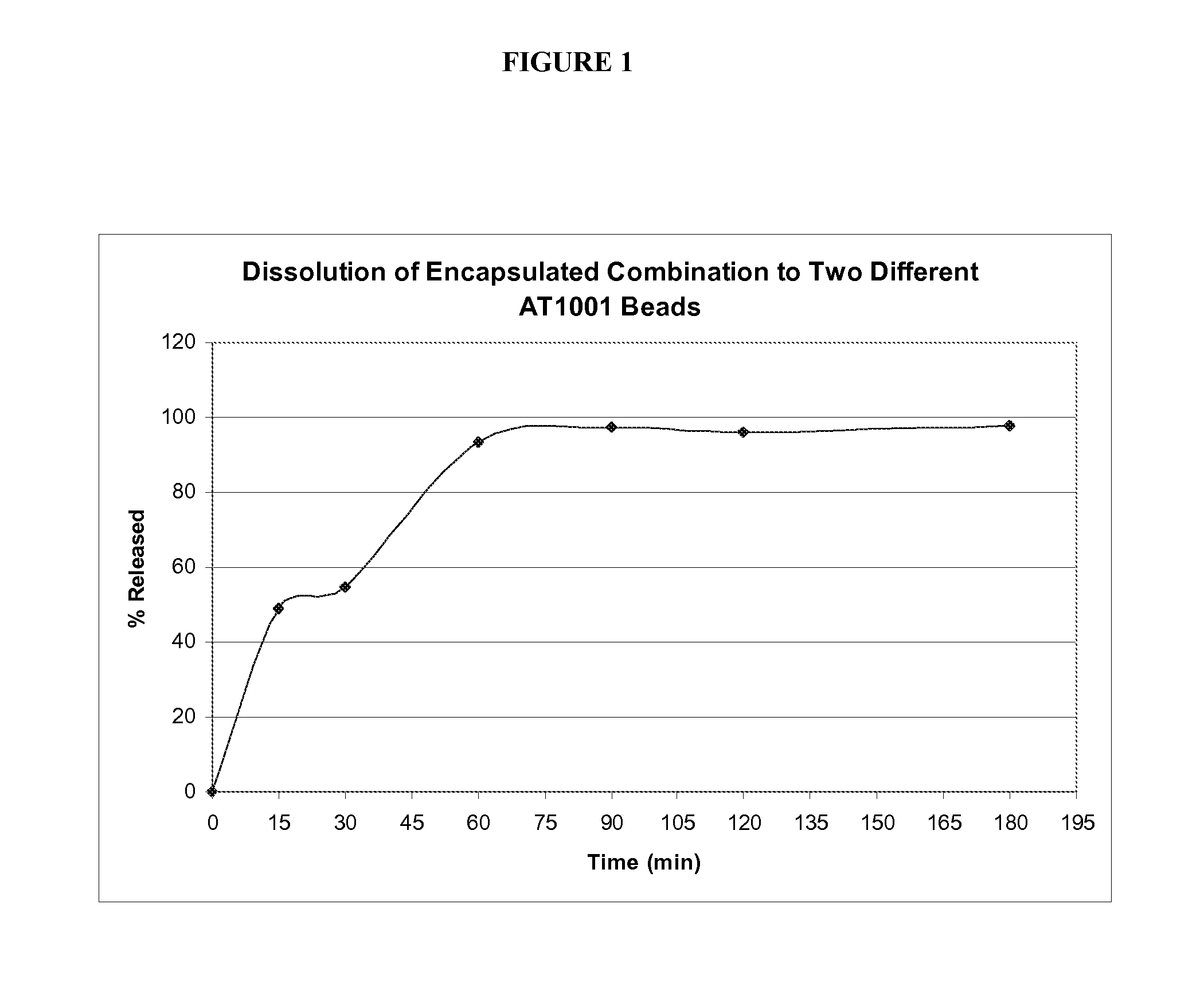
Controlled release compositions comprising nimesulide
InactiveUS20070128276A1Extended stayDecreased gastrointestinal motilityBiocideNervous disorderActive agentArthritis
A controlled release composition comprising nimesulide as an active agent formulated as a gastroretentive system, preferably as a solid oral dosage form is provided, wherein the residence time of the active agent is increased in the stomach, duodenum, jejunum or ileum. The present invention also provides process of preparing such dosage form and methods of using such dosage form compositions. The dosage form compositions are preferably administered once-a-day or twice-a-day and are particularly very useful in the prophylaxis or treatment of NSAID indicated disorder(s) such as acute painful conditions like post-operative trauma, pain associated with cancer, sports injuries, migraine headache and the like, or chronic diseases such as arthritis and the like.
Owner:PANACEA BIOTEC
Inner coverage membrane for duodenum
The invention provides an inner coverage membrane for duodenum. The inner coverage membrane is obtained from biocompatibility materials, and is formed by an elastic kettle belly part and a tubular part, wherein the kettle belly part is arranged in a sphere part of the duodenum, the tubular part can be extended to jejunum, the kettle belly part comprises a wave-shaped, a V-shaped, a ladder-shaped or a wall-shaped elastic circle which is continuously wound, the elastic circle is made of memorized or non-memorized biocompatibility materials, a peak, a valley and a curved angle of the elastic circle are single-circled bending springs and are with an outward anchor and hook, a retrieve thread is wound on the single-circled bending spring on the lower edge in a penetrating manner, the upper edge of the kettle belly part is a wave-shaped, a V-shaped, ladder-shaped or a wall-shaped elastic membrane, the elastic circle and the overall kettle belly part move along with the duodenum and the sphere part, and the kettle belly part and the tubular part can be closed or folded into a sphere shape, a cylindrical shape, a capsule shape or a spindle shape outside a human body. The single-circled bending springs have the advantages of strong elasticity, weak sharp injury, excellent fixing performance for the retrieve thread attached on the single-circled bending spring, and benefit for an anatomized structure and basic physiologic functions of the duodenum. The inner coverage membrane for duodenum can be prepared into a medical appliance for preventing and curing obesity and diabetes.
Owner:万平
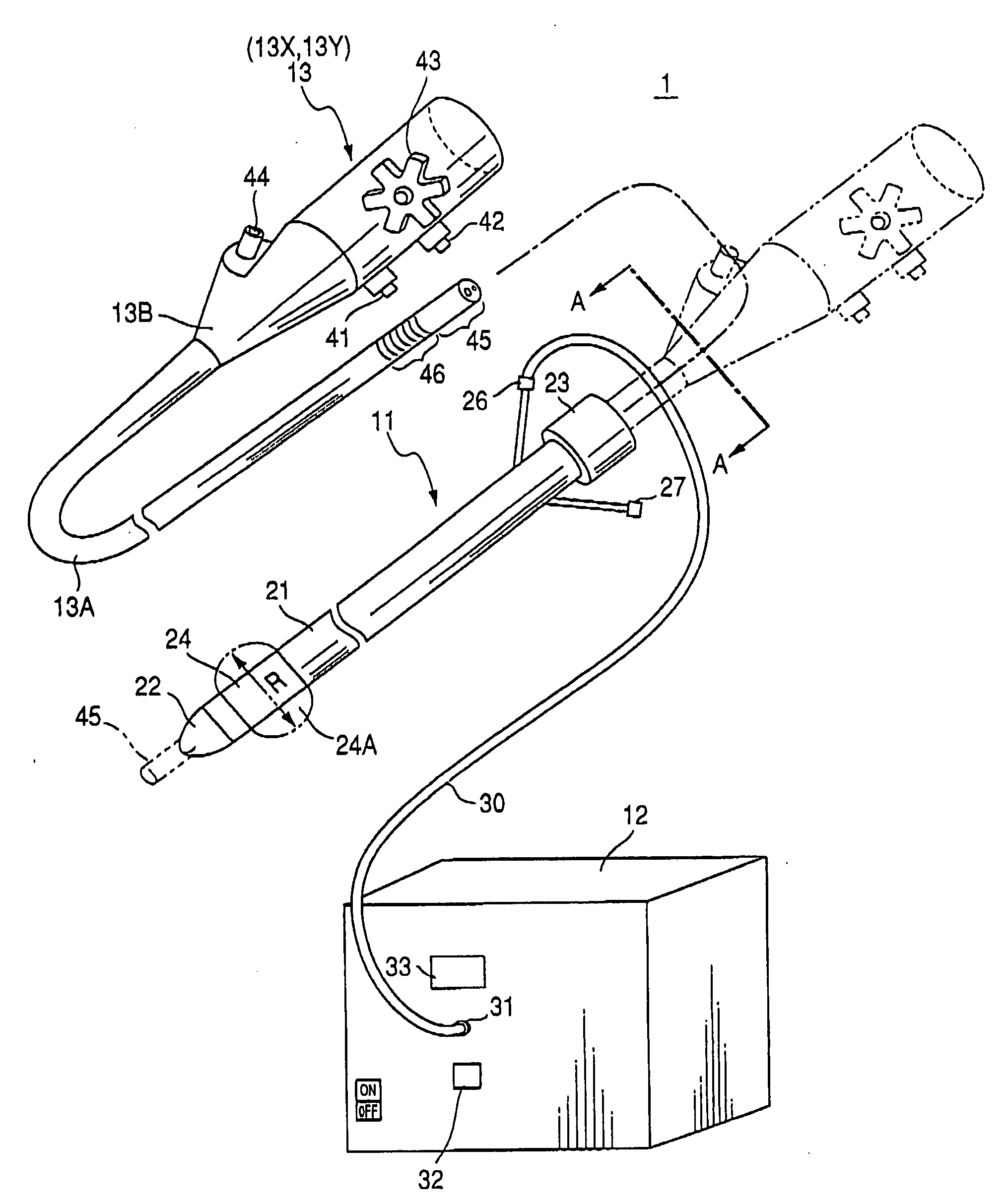
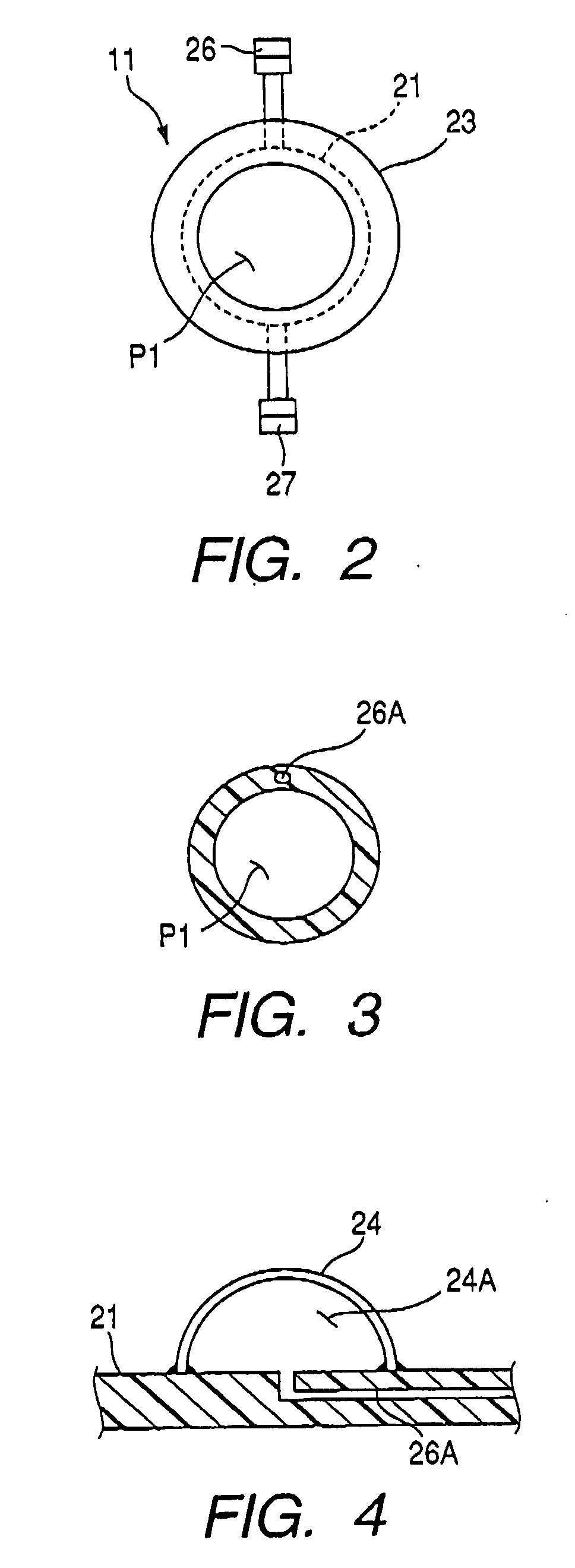
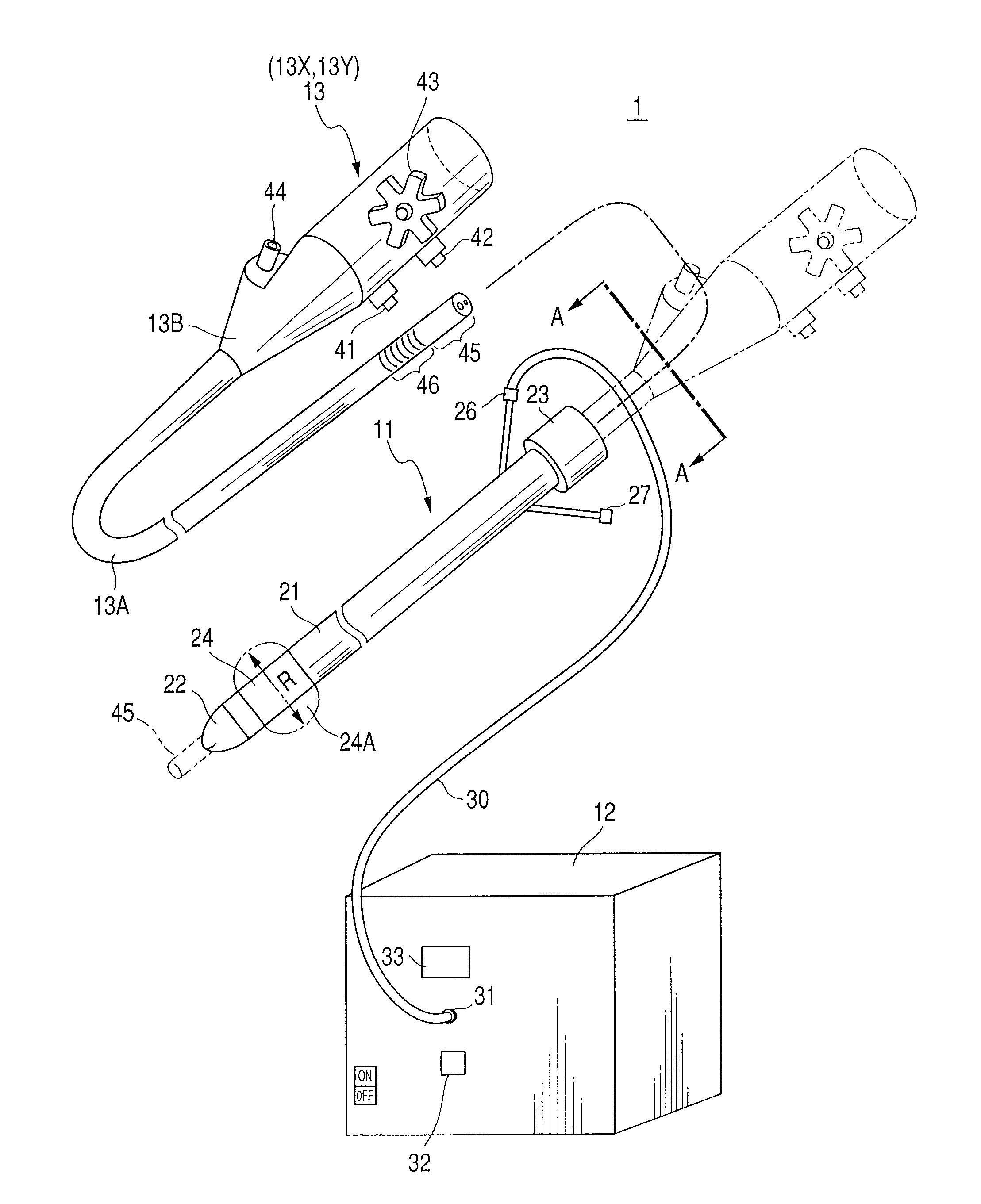
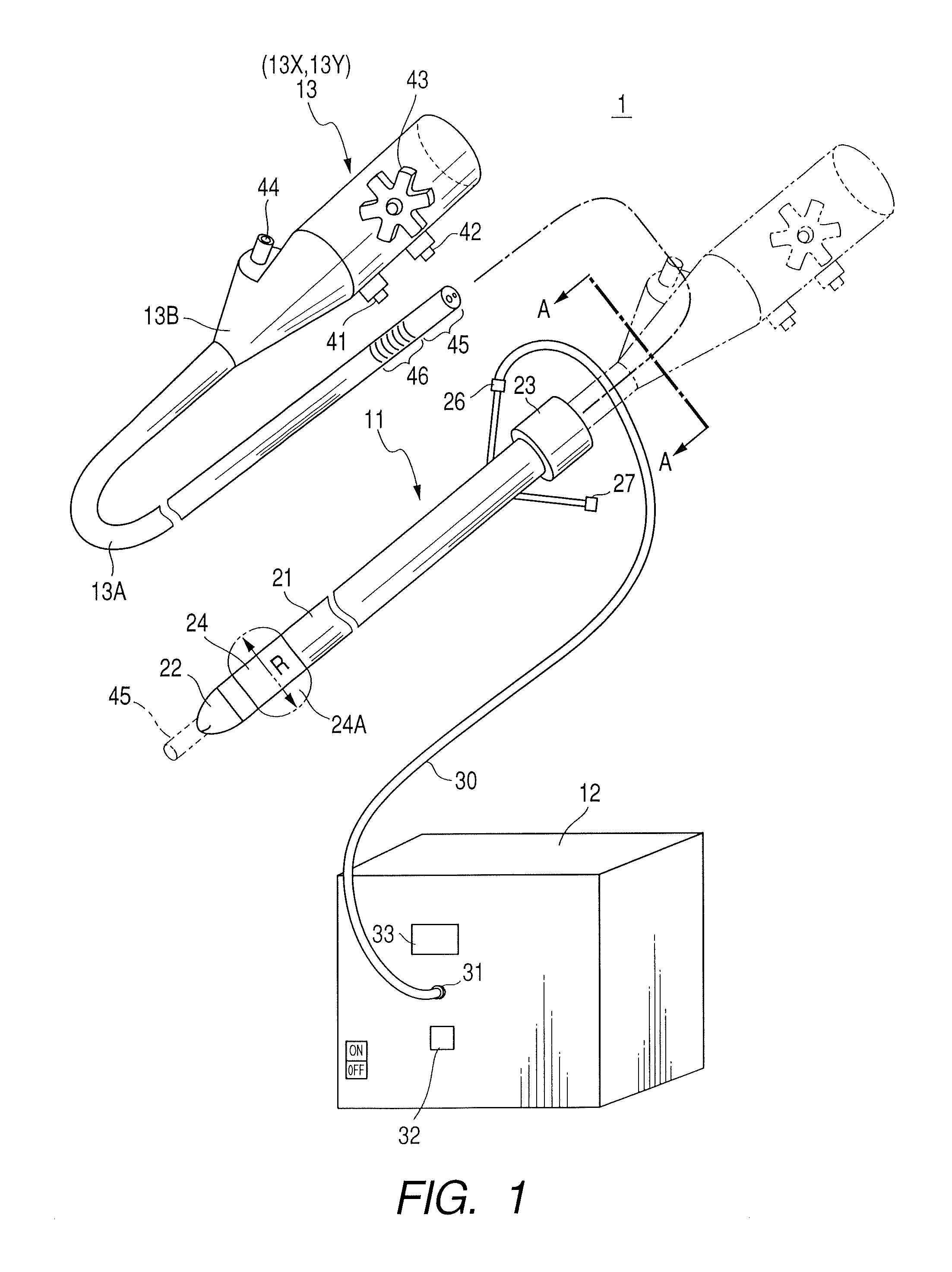
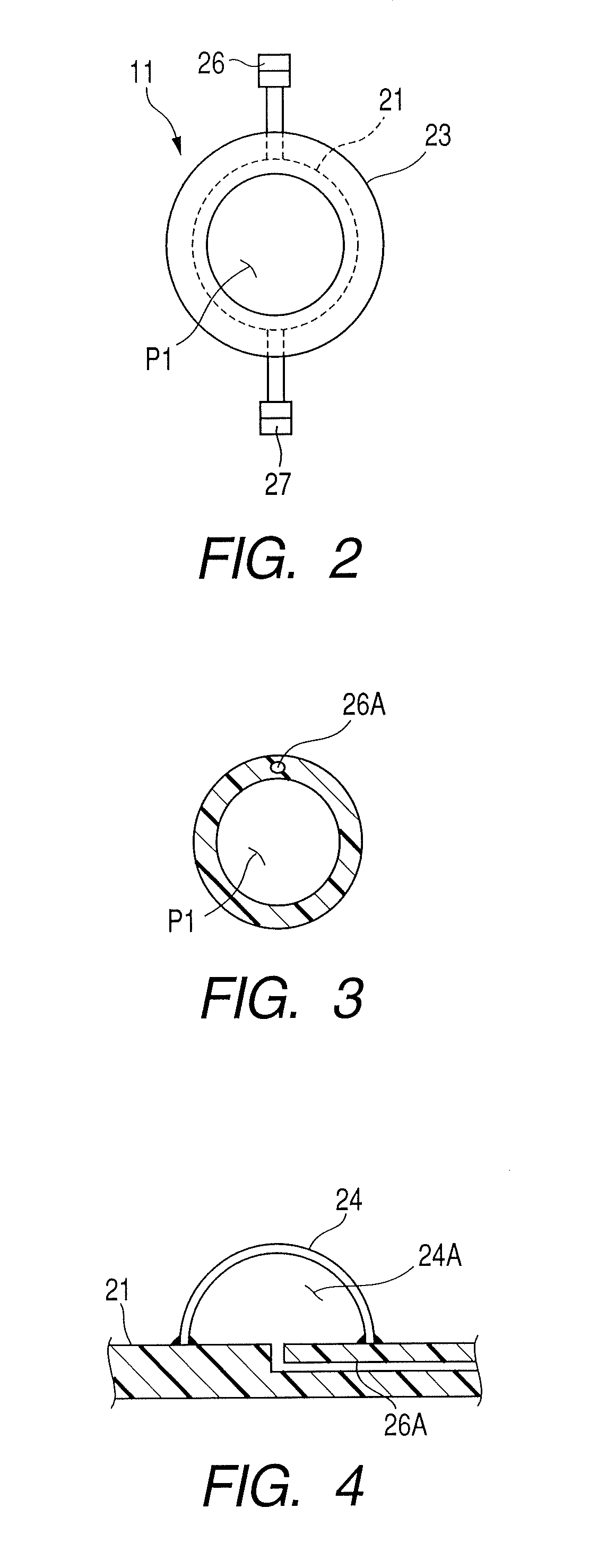
Therapeutic method and endoscopic system that use overtube
With a first endoscope inserted through an overtube, the overtube is orally inserted into an object being examined, and a distal end portion of an insertion tube of the first endoscope is made to advance, within the object, from a jejunum via a jejunum-to-jejunum anastomosed portion and a jejunum-anastomosed bent portion so that the distal end portion is located adjacently to a Vater's papilla of a duodenum. The overtube is then sent to a position which is adjacent to the Vater's papilla along the insertion tube of the first endoscope. After this, the first endoscope is pulled from the overtube. A second endoscope is then inserted into the overtube to make a distal end portion of its insertion tube protrude from the distal end of the overtube and is located adjacently to the Vater's papilla. The second endoscope thus-approached is used to treat the Vater's papilla and the tissue therearound.
Owner:OLYMPUS CORP
Endoscopic system that uses overtube
With a first endoscope inserted through an overtube, the overtube is orally inserted into an object being examined, and a distal end portion of an insertion tube of the first endoscope is made to advance, within the object, from a jejunum via a jejunum-to-jejunum anastomosed portion and a jejunum-anastomosed bent portion so that the distal end portion is located adjacently to a Vater's papilla of a duodenum. The overtube is then sent to a position which is adjacent to the Vater's papilla along the insertion tube of the first endoscope. After this, the first endoscope is pulled from the overtube. A second endoscope is then inserted into the overtube to make a distal end portion of its insertion tube protrude from the distal end of the overtube and is located adjacently to the Vater's papilla. The second endoscope thus-approached is used to treat the Vater's papilla and the tissue therearound.
Owner:OLYMPUS MEDICAL SYST CORP
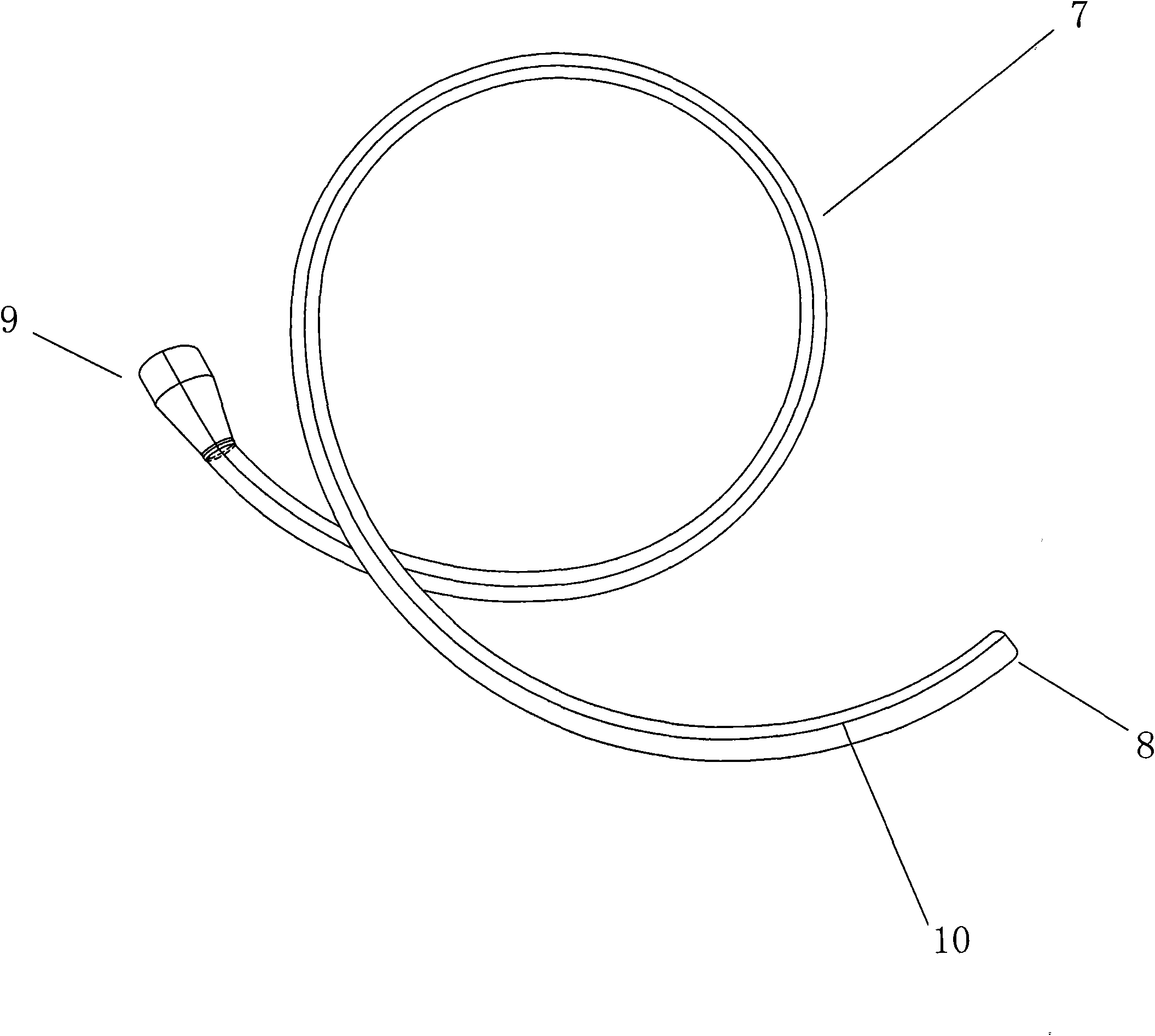
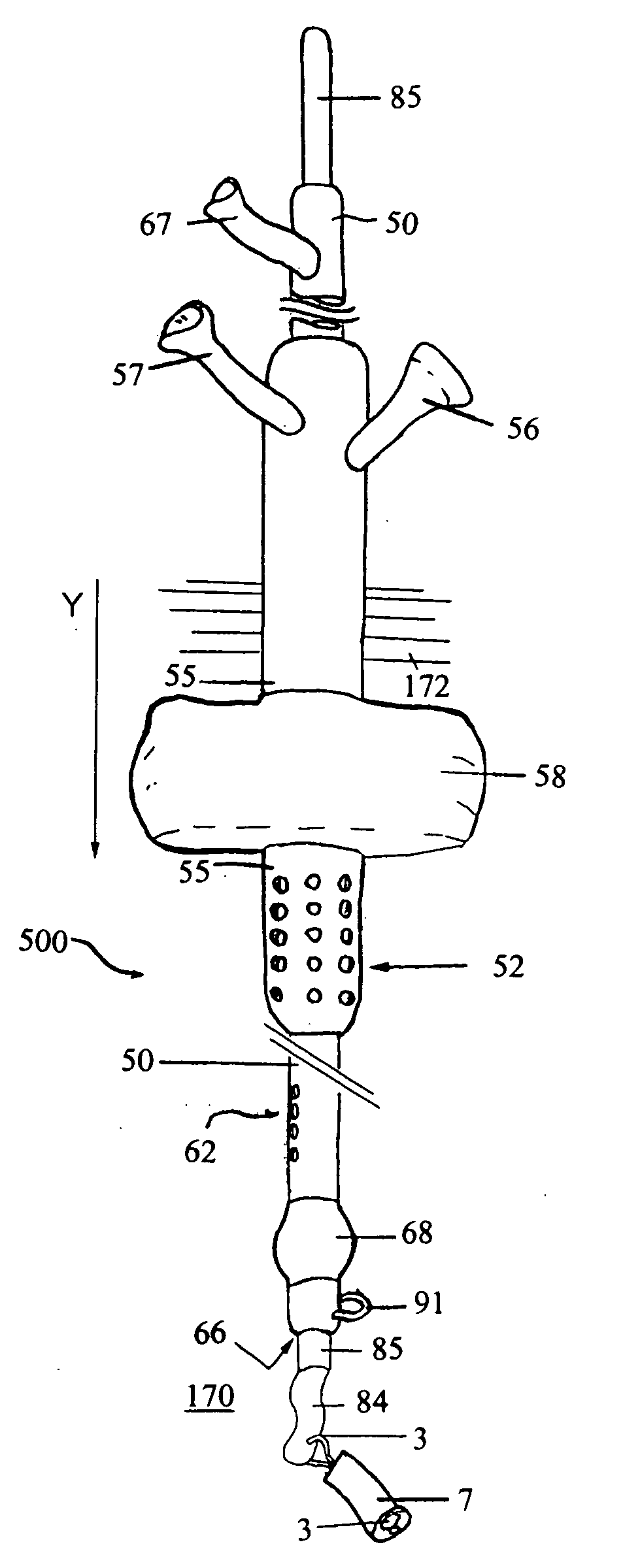
Feeding tube
ActiveUS20090216186A1Prevents large pointReduce displacementStentsBalloon catheterOesophageal tubeDilator
An feeding tube and a kit for installation of a feeding tube includes a balloon anchored gastric tube. A protective sleeve on a dilator protects the toroidal balloon from damage during surgical insertion of the feeding tube. In one example, a jejunal tube is integrated with the kit and the gastric tube serves as a gastric sleeve forming an annular region or channels for fluid flow to and / or from the stomach and to / from the jejunum. An integrated gastro jejunal feeding tube unit may include a jejunal tube outlet port at one end, a jejunal balloon port, a gastric sleeve outlet port, one or more gastric balloon ports, one or more gastric balloons, gastric drainage holes, a jejunal balloon positioned at end of the jejunal tube and sleeve. A black silk loop may affix an end of the jejunal tube at a position in the jejunum, and insertion of the jejunal tube may be monitored continuously using an endoscope. Drainage holes may be provided in the jejunal tube and / or the gastric sleeve. Depending on the clinical state, feeding may be initiated through the gastric sleeve outlet port or via the jejunal tube outlet port and may be altered without surgical intervention, as required. Drainage holes in the gastric sleeve may be used to deflate the stomach, while nutrition is provided by a jejunal tube inserted in the jejunum, simultaneously.
Owner:SAINATH INTPROP
Oligonucleotide primer for detecting common pathogenic bacteria by adopting fluorescent quantitation PCR (Rich Client Platform) technology, method thereof for detecting common pathogenic bacteria and application thereof
InactiveCN101928773AEfficient and wideWide range of applicationsMicrobiological testing/measurementAgainst vector-borne diseasesBiotechnologyFood poisoning
The invention discloses an oligonucleotide primer for detecting common pathogenic bacteria by adopting a fluorescent quantitation PCR (Rich Client Platform) technology, a method thereof for detecting common pathogenic bacteria and the application thereof. The method comprises the following steps of: providing 10 pairs of specific oligonucleotide primer sequences at annealing temperature of 50-60 DEG C without differing 5 DEG C; and simultaneously, quickly, accurately and effectively identifying and quantificationally detecting various pathogenic bacteria at the same time. A detection range comprises bacillus cereus, enterobacter sakazakii, vibrio parahaemolyticus, enterohemorrhagic escherichia coli O157, salmonella, Listeria monocytogenes, Shigella, campylobacter jejuni, pseudomonas aeruginosa, klebsiella pneumoniae, and the like. The invention also can be used for the fields of disease diagnosis, environmental monitoring, water-quality and food supervision and detection, food poisoning pathogenicbacteria detection, bacteriological classification, epidemiological investigation, biological agent detection, and the like, is convenient, quick, accurate and effective and has wide application range.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
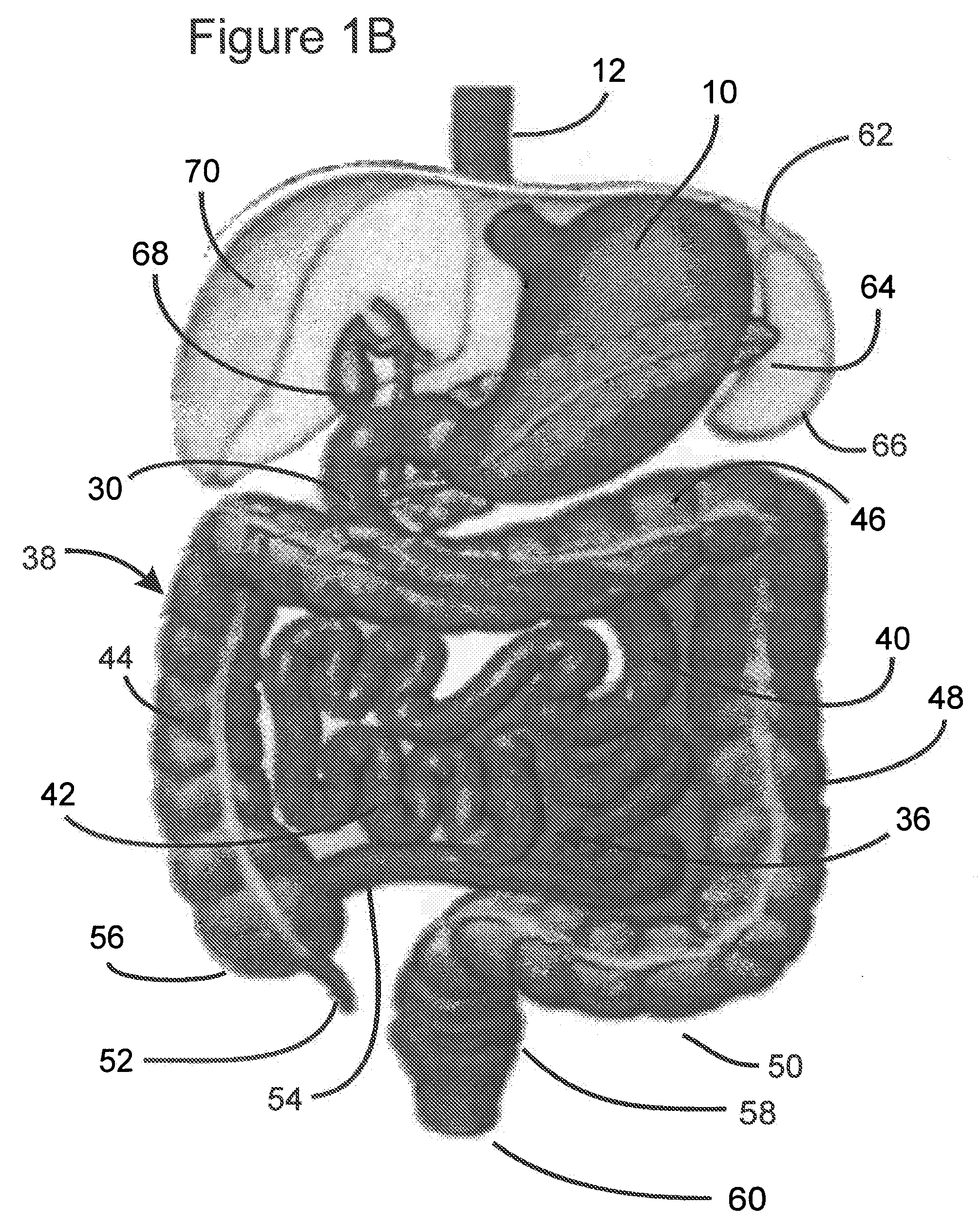
Use of a gastrointestinal sleeve to treat bariatric surgery fistulas and leaks
ActiveUS8801647B2Minimize traumaCurved bigOesophagiIntravenous devicesIntestinal structureDamages tissue
Method for treating a Roux-en-Y patient having fistulas and leaks as a result of bariatric surgery. A gastrointestinal implant device is anchored in the esophagus and extends through a stomach pouch into an intestine anastomosed to the stomach pouch to prevent fistulas and other damaged tissue from making contact with food and fluids entering the esophagus. The gastrointestinal implant device includes an unsupported flexible sleeve and an anchor coupled to a proximal portion of the sleeve. The flexible sleeve is open at both ends, and adapted to extend below a jejunum. The anchor is adapted to be retained within the esophagus, preferably just above the gastroesophageal (GE) Junction. The anchor can include a stent such as a wave anchor and is collapsible for catheter-based delivery and removal.
Owner:GI DYNAMICS
Food-originated pathogenic bactenium quick detection gene chip and its application
InactiveCN1536090AMicrobiological testing/measurementAgainst vector-borne diseasesFood poisoningBeta-hemolytic streptococcus
The present invention provides a gene chip for quickly detecting pathogens from food source, discloses the preparation method of said gene chip and provides 26 oligonucleotide probe sequences for detection. Said gene chip can quickly, accurately and high-effectively detect and identify the class of the pathogens in food, and its detection range includes staphylococcus aureus, Shiga's bacillus, salmonella, colibacillus 0157, bacillus proteus, mononuclear hyperplastic listerella, enterocolitis yersinia, aeruginous pseudomonads, vibrio parahaemolyticus, vibrio cholerae, bacillus cereus, beta hemolytic streptococcus, coconut fermentation pseudomonads, boticin, vibrio jejuni and bacillus perfringens, etc.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
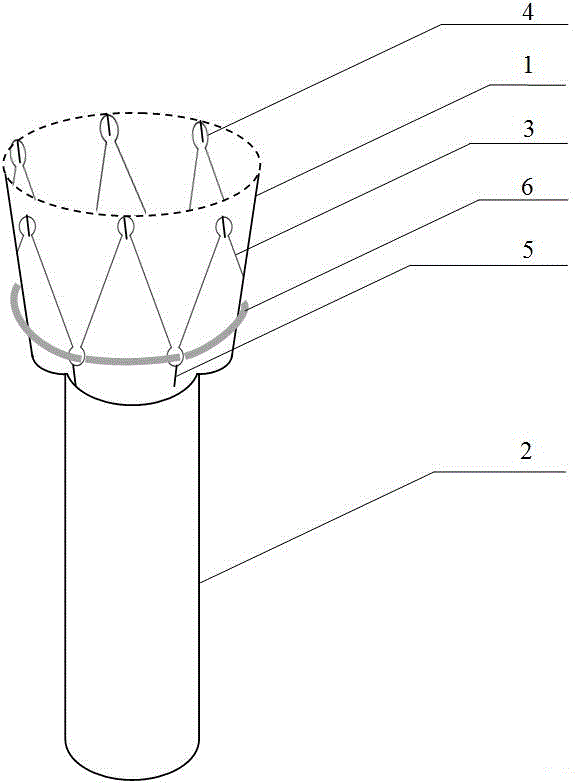
Intragastric device for treating obesity
A gastrointestinal device for treating obesity includes a three-dimensional porous structure configurable between a compressed pre-deployment configuration to facilitate delivery and an expanded post-deployment configuration. The porous structure includes a first opening at its proximal end and a larger second opening at its distal end. The porous structure also includes a sleeve coupled to its distal end. Optionally, the device further includes a suture at the proximal end of the wire mesh structure to facilitate retrieval and an anti-migration component positioned at the junction of the porous structure with the sleeve. The porous structure is deployed in a patient's stomach such that the anti-migration component sits proximal to the patient's pylorus and prevents migration of the entirety of the device into and through the pylorus. The sleeve extends through the pylorus, into the duodenum and ends in the duodenum or jejunum. Food enters the device from the first opening at the proximal end of the porous structure, passes through the porous structure and sleeve, and exits at the distal end of the sleeve. The device treats obesity by providing a relatively immovable volume occupying structure in the stomach and a bypass for food past the pylorus and proximal portion of the small intestine. Optionally, the device further acts to slow the passage of food through the digestive tract. Patients with the device experience satiety more quickly and have a prolonged sensation of satiety.
Owner:SYNERZ MEDICAL
Percutaneous endoscopic jejunostomy access needle
An access needle suitable for use in percutaneous endoscopic jejunostomy procedures. In one embodiment, the access needle includes a stylet assembly, a safety sheath assembly and a cannula assembly. The stylet assembly includes a stylet and a stylet hub. The stylet has a proximal end and a sharpened distal end, the proximal end of the stylet being mounted within the stylet hub. The safety shield assembly includes a sheath and a shuttle. The sheath, which has a blunt distal end, coaxially surrounds the stylet. The proximal end of the sheath is fixed to the shuttle, the shuttle being movable between a first position in which the sharpened distal end of the stylet is exposed and a second position in which the sharpened distal end of the stylet is shielded by the blunt distal end of the sheath. The cannula assembly includes a cannula and a cannula hub, the cannula hub being fixed to the proximal end of the cannula. The cannula hub is detachably engageable with the shuttle to move the shuttle between its first and second positions.
Owner:BOSTON SCI SCIMED INC
Methods for producing enhanced antigenic Helicobacter sp.
InactiveUS6051416AEnhanced antigenic propertyIncrease proteinAntibacterial agentsBiocideBacteroidesVirulent characteristics
Methods using in vitro processes are disclosed for inducing or enhancing expression of enteric bacterial antigens or virulence factors. The methods, therefore, produce antigenically enhanced enteric bacteria. Also methods for using the antigenically enhanced bacteria are also disclosed, as well as vaccines containing the enteric bacteria. Specifically a whole enteric bacterium or components thereof are provided by Helicobacter species. Also there are other enteric bacteria which are useful for the disclosed invention; such as Campylobacter jejuni.
Owner:BIOPORT R&D
Probiotic/Prebiotic Composition and Delivery Method
InactiveUS20040086491A2Increase the number ofBiocideOrganic active ingredientsEnteral feedingsProbiotic bacteria
Abstract of the Disclosure A prebiotic, composition comprising a probiotic and prebiotic, and method of delivering a probiotic, prebiotic or composition directly into the intestinal tract of a mammal are disclosed. The probiotic is any beneficial bacteria and the prebiotic is a substance beneficial to a probiotic. Most preferably, the prebiotic includes a mucopolysaccharide. The method preferably involves delivering the probiotic, prebiotic or composition via a delivery tube, such as an enteral feeding tube, directly to a position downstream of the stomach, most preferably to the jejunum.
Owner:SOC DES PROD NESTLE SA
Administration of serine protease inhibitors to the stomach
ActiveUS9504736B2The way is simple and fastNervous disorderPeptide/protein ingredientsMulti organHigh doses
The inventors have unexpectedly discovered that shock and / or potential multi-organ failure due to shock can be effectively treated by administration of liquid high-dose protease inhibitor formulations to a location upstream of where pancreatic proteases are introduced into the gastrointestinal tract. Most preferably, administration is directly to the stomach, for example, via nasogastric tube under a protocol effective to treat shock by such administration without the need of providing significant quantities of the protease inhibitor to the jejunum and / or ileum.
Owner:LEADING BIOSCI +1
Gastrojejunal feeding tube
InactiveUS7220253B2Avoid pullingAvoid unnecessary influenceBalloon catheterSurgerySmall intestineEndoscope
An adjustable size balloon at the distal end of a feeding tube is used to aid in positioning, along with an endoscope, the tube in the jejunum or small bowel of a patient, without creating an obstruction in the intestines.The balloon is fully inflated when the endoscope that is used to place the tube in the duodenum is withdrawn, and then partially deflated to a size that allows peristaltic action on the balloon to move the tube into the jejunum, after which the balloon is further deflated to avoid creating an obstruction in the intestine.
Owner:GI SUPPLY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com