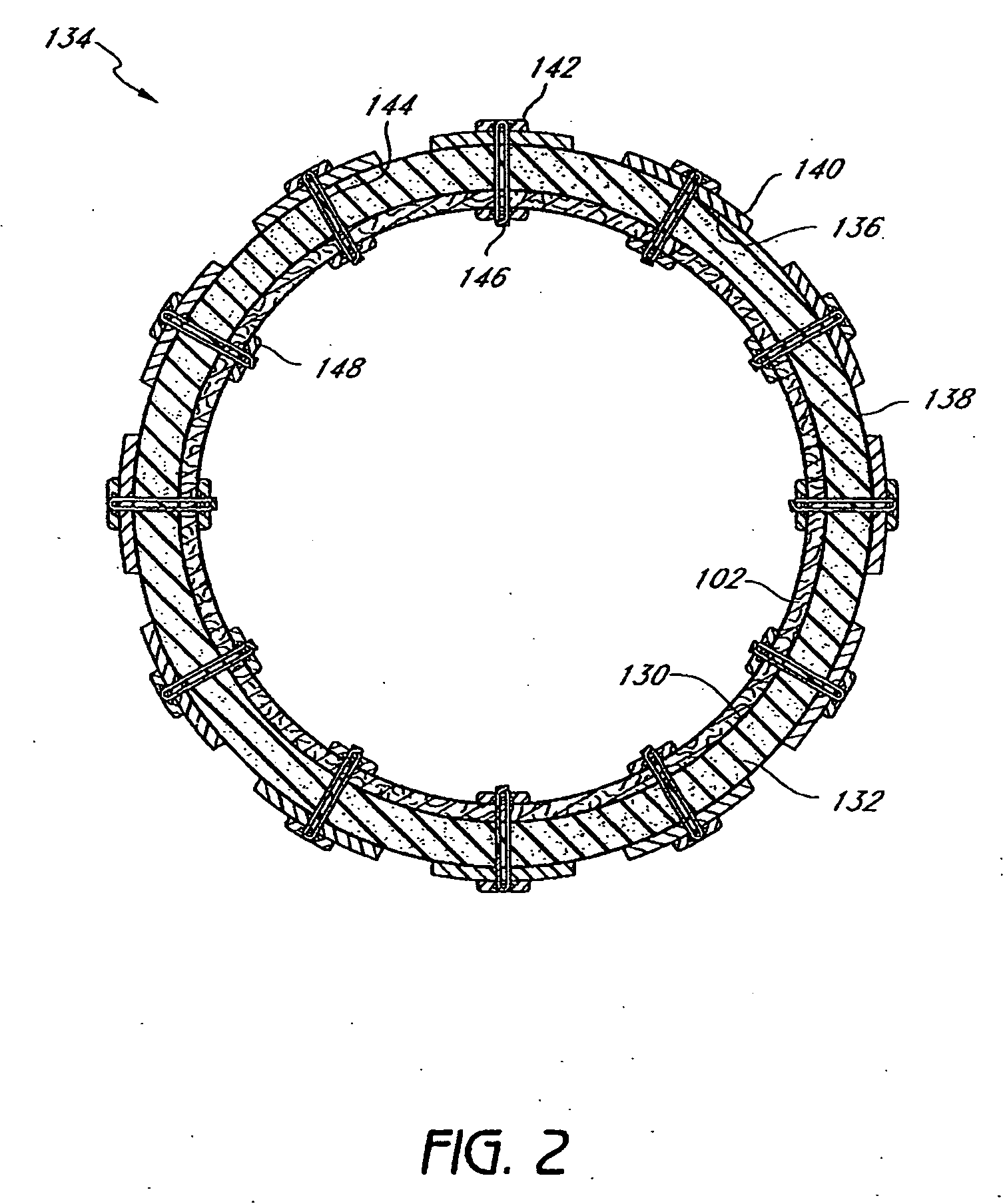
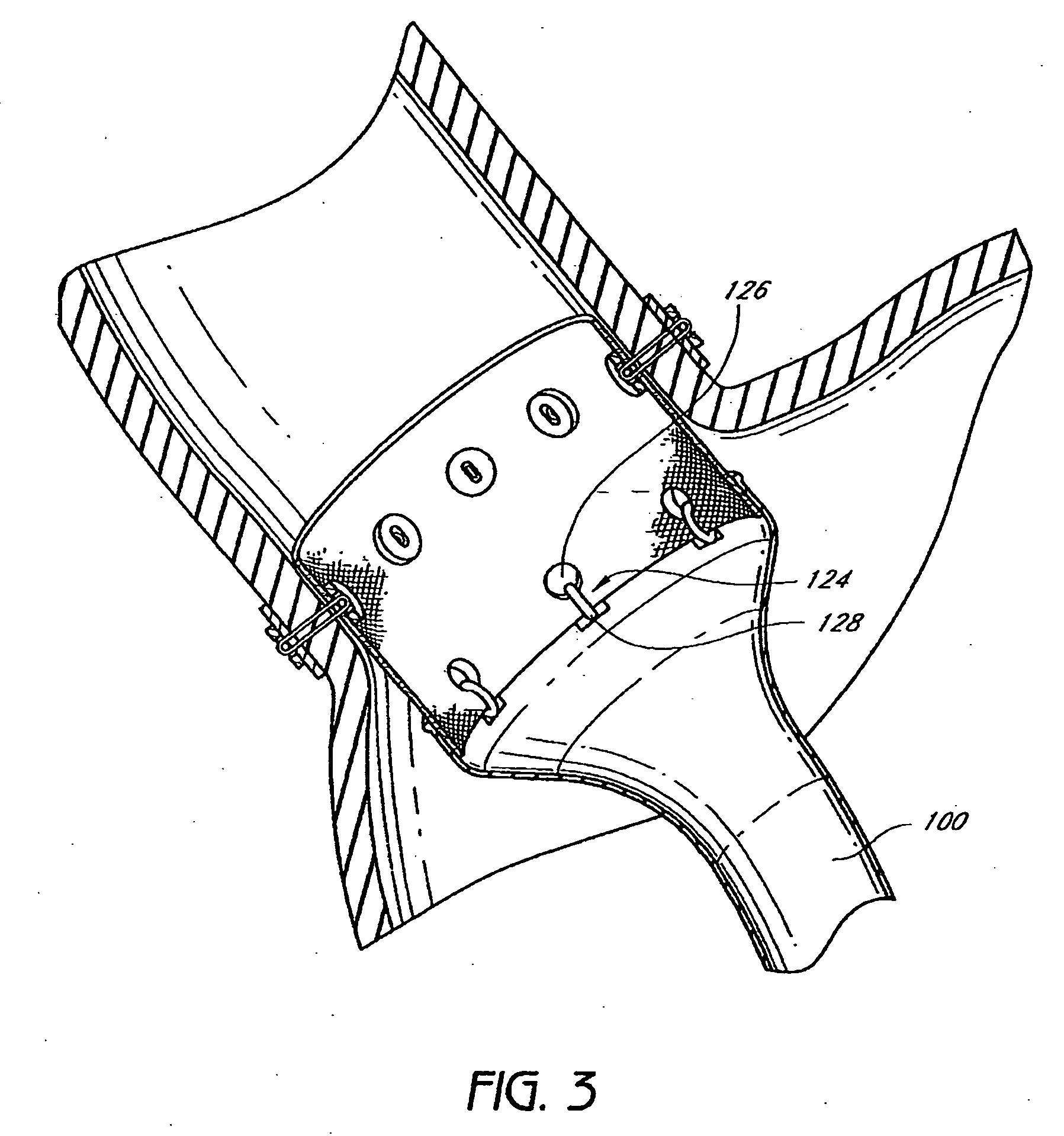
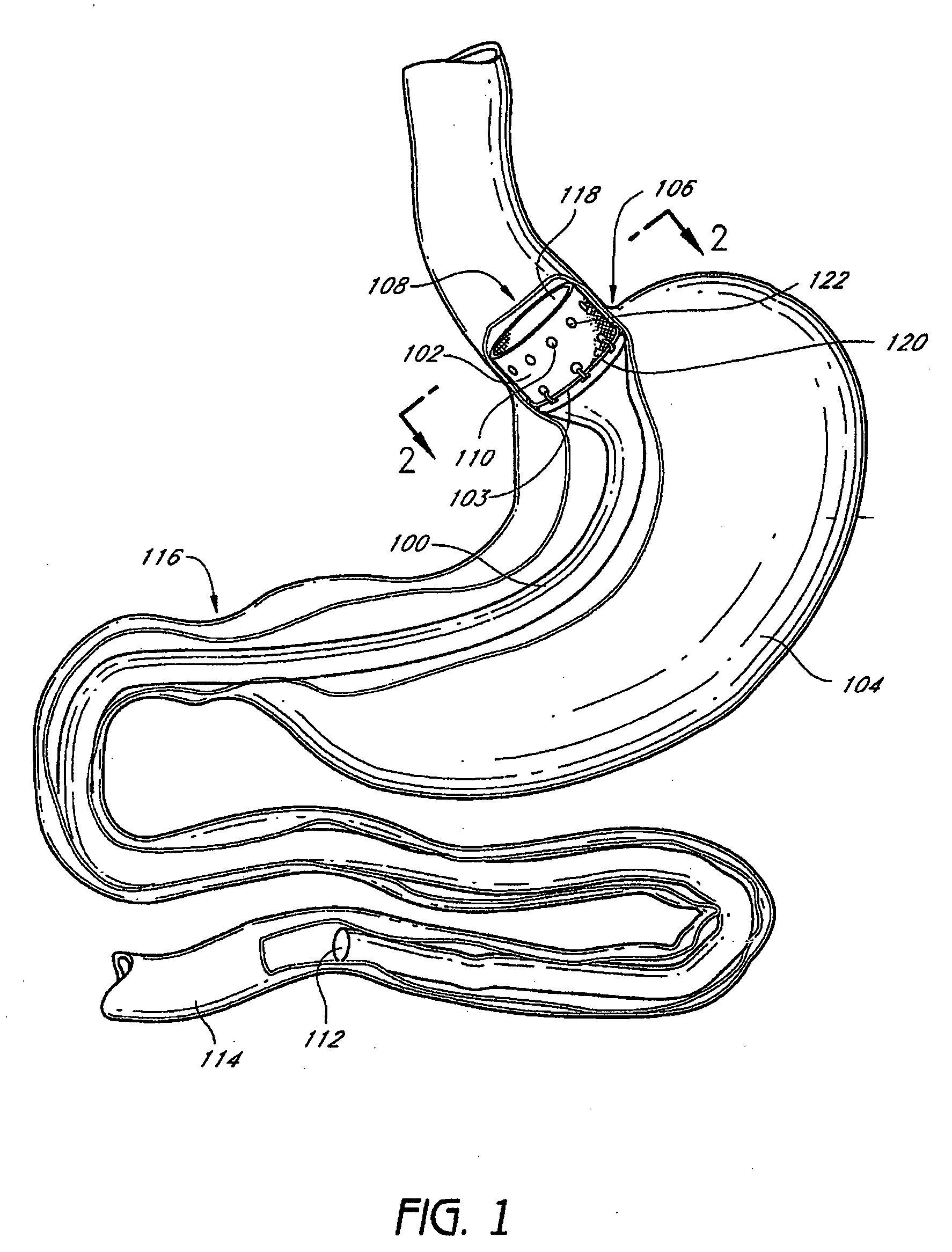
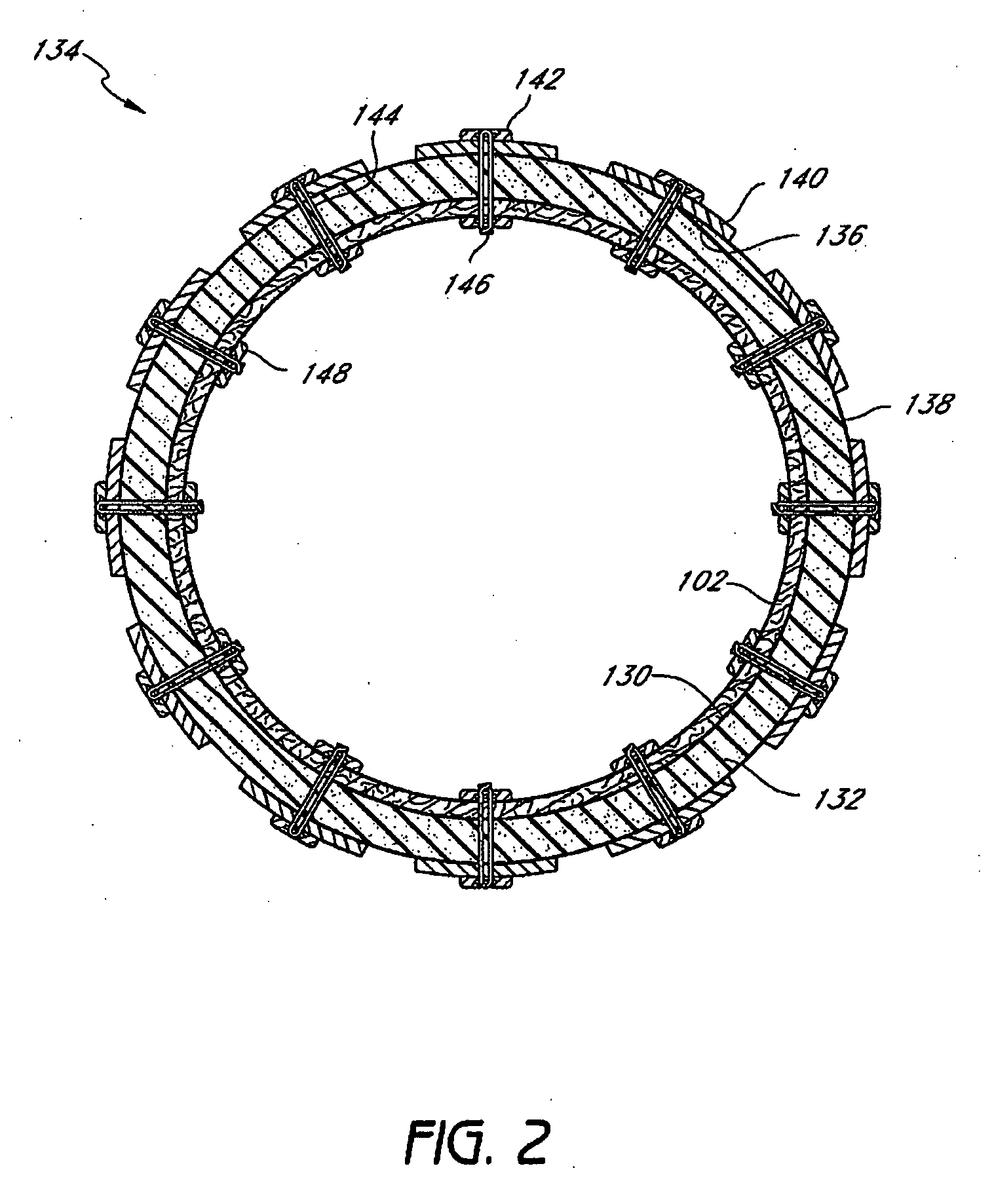
Patents
Literature
48 results about "Gastrointestinal device" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
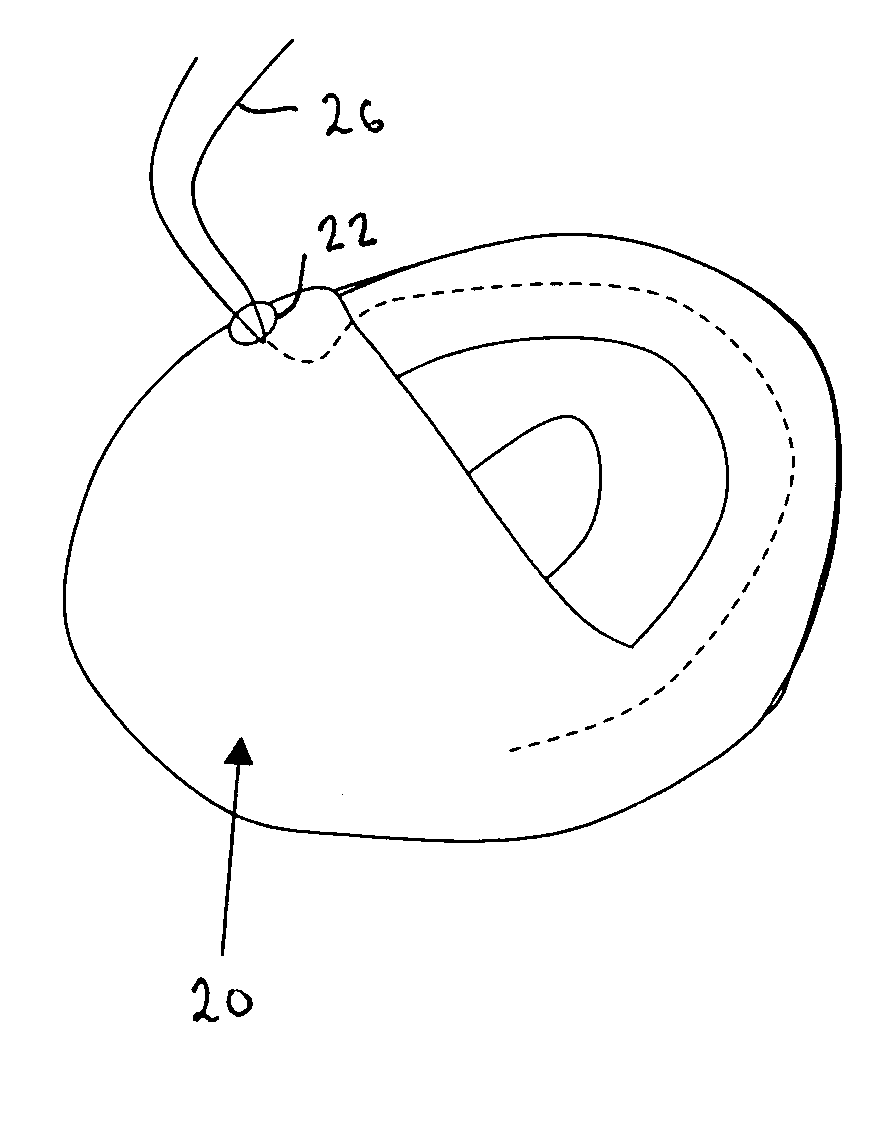
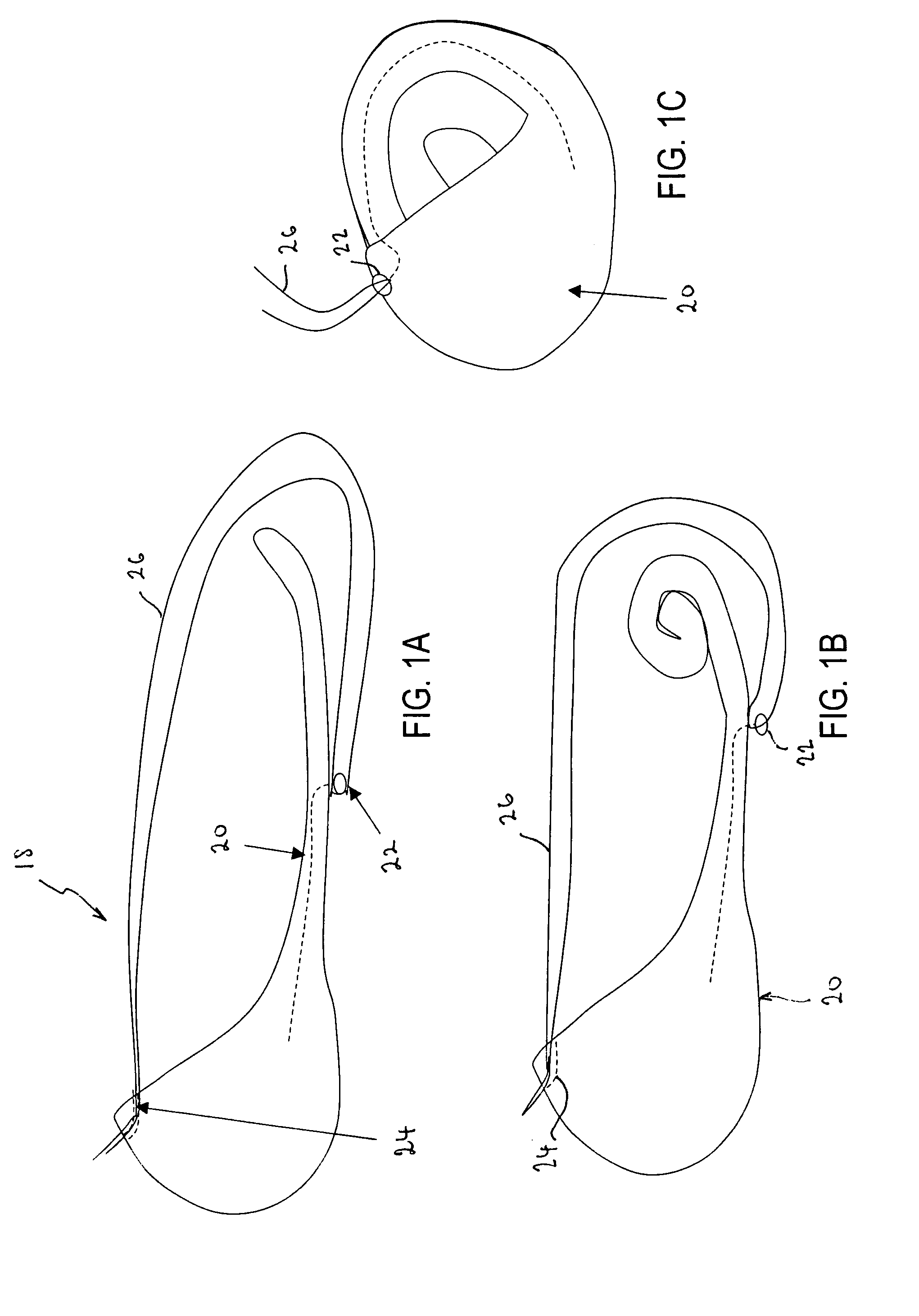
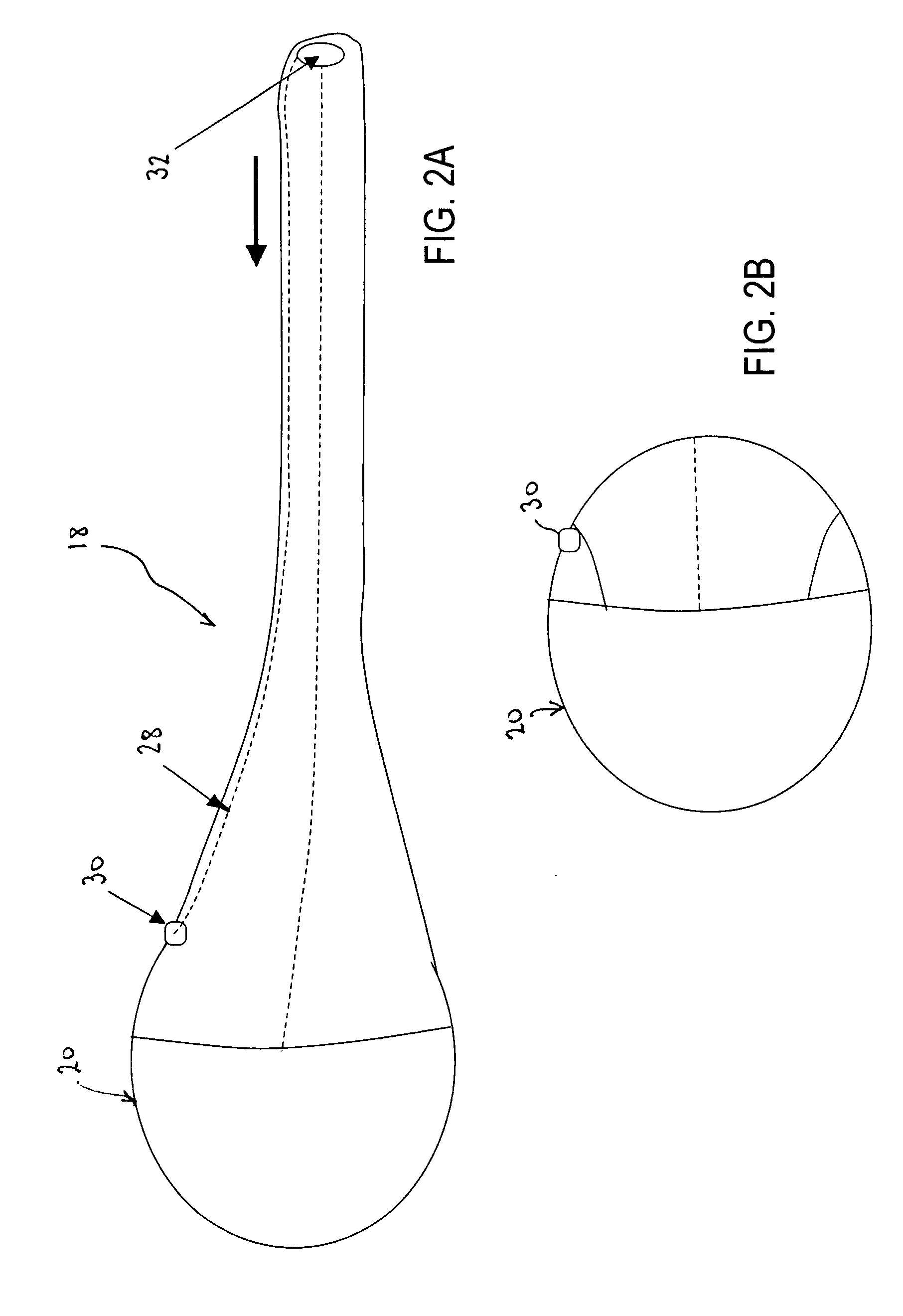
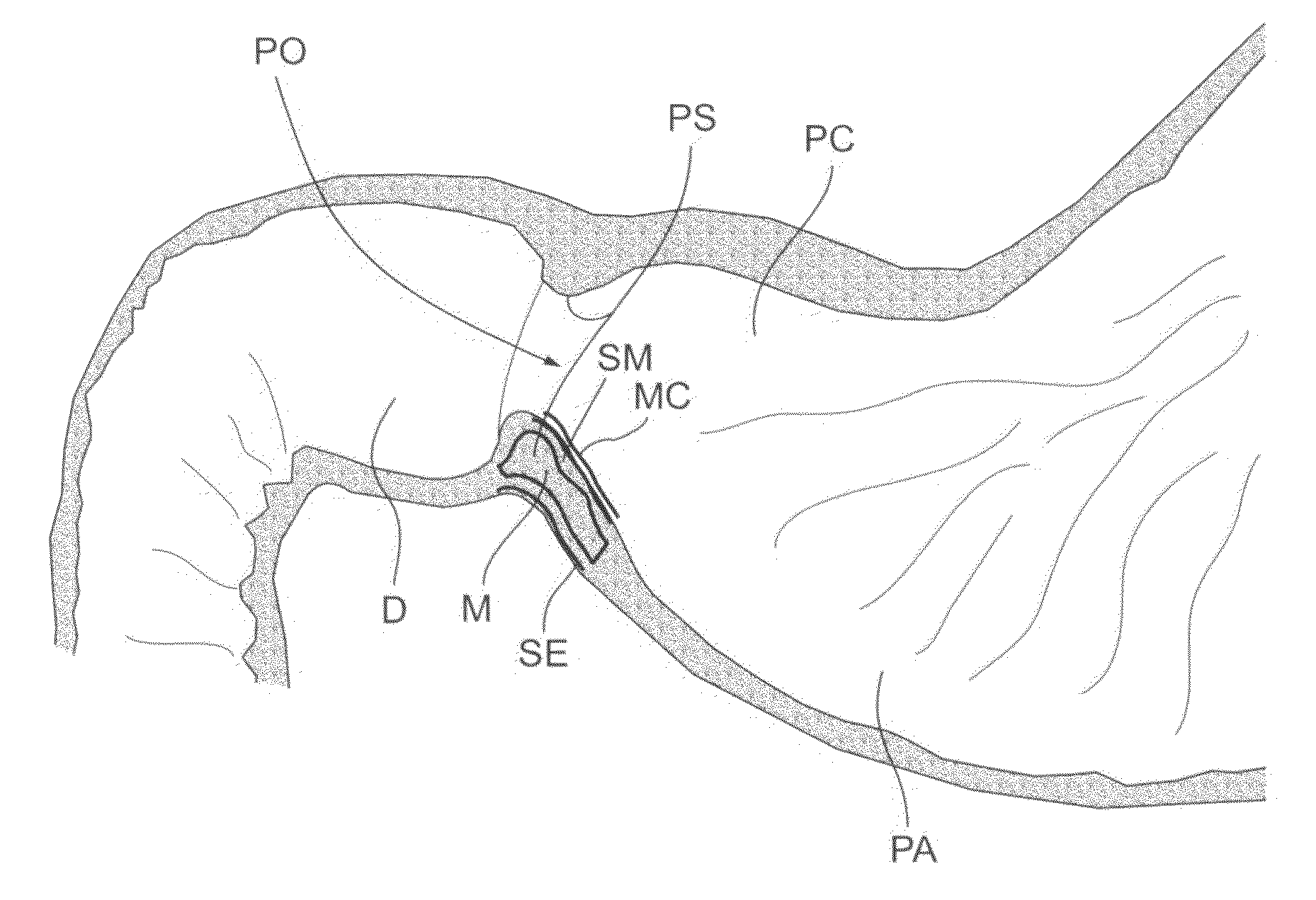
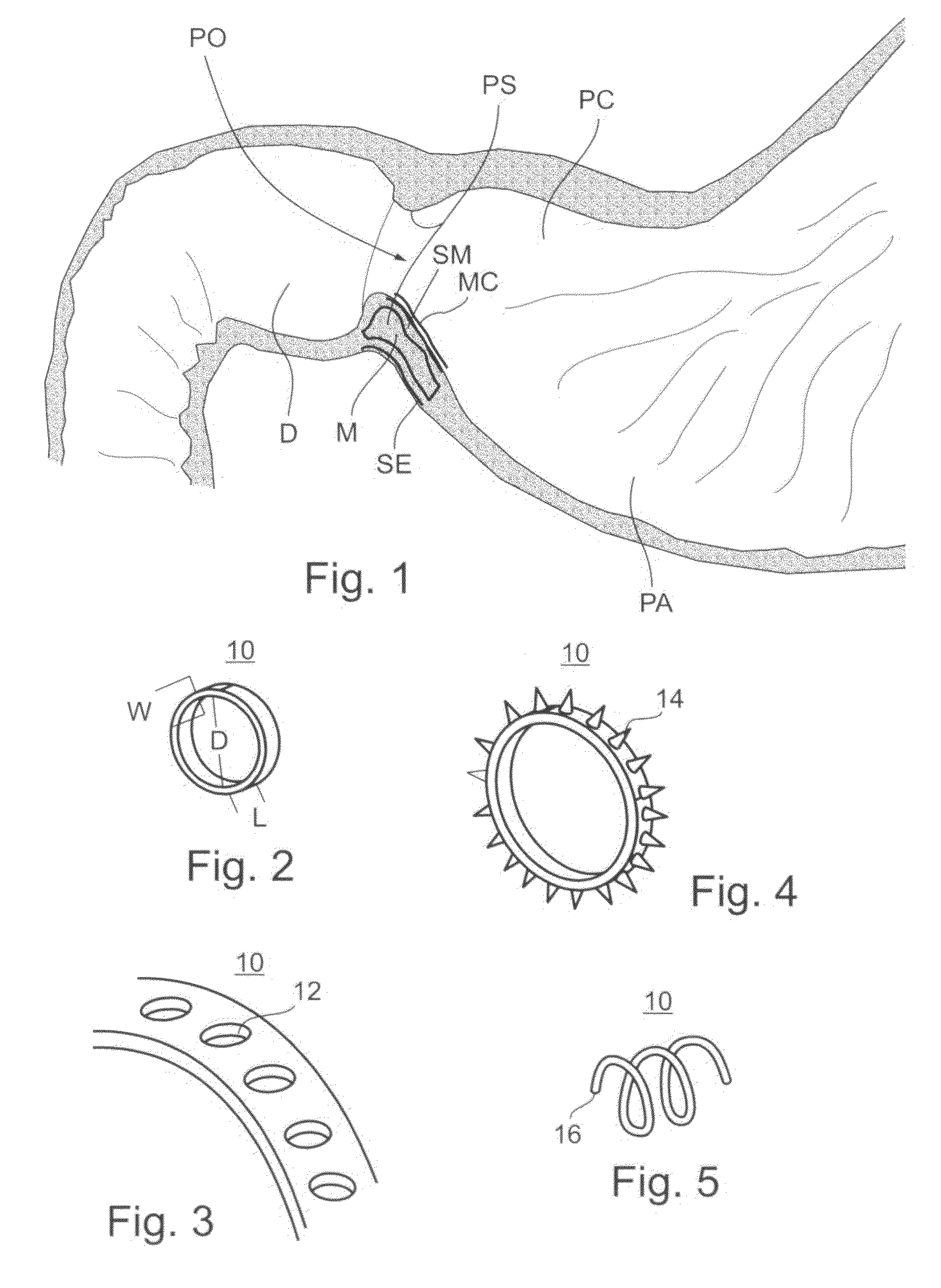
Bariatric sleeve
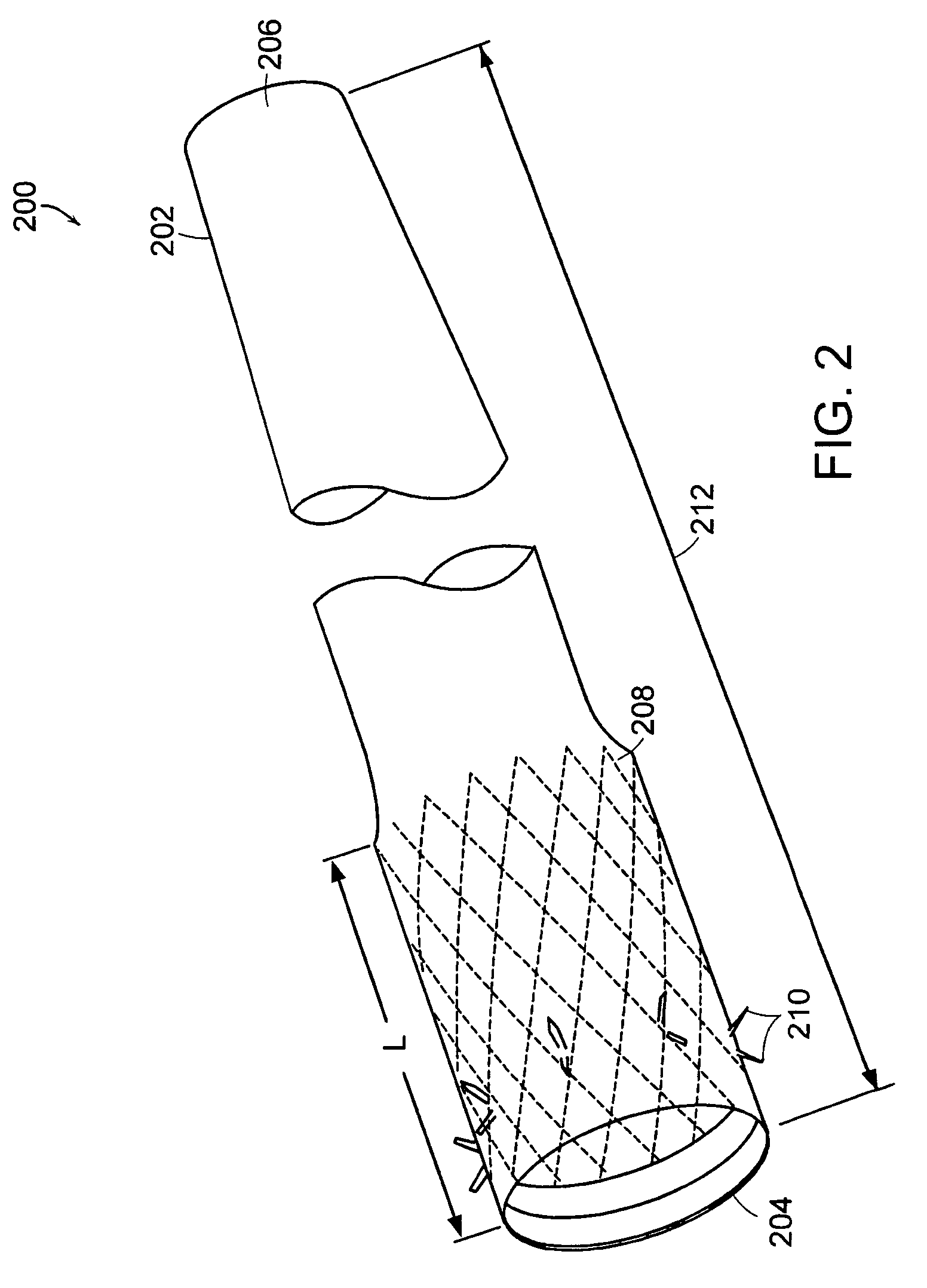
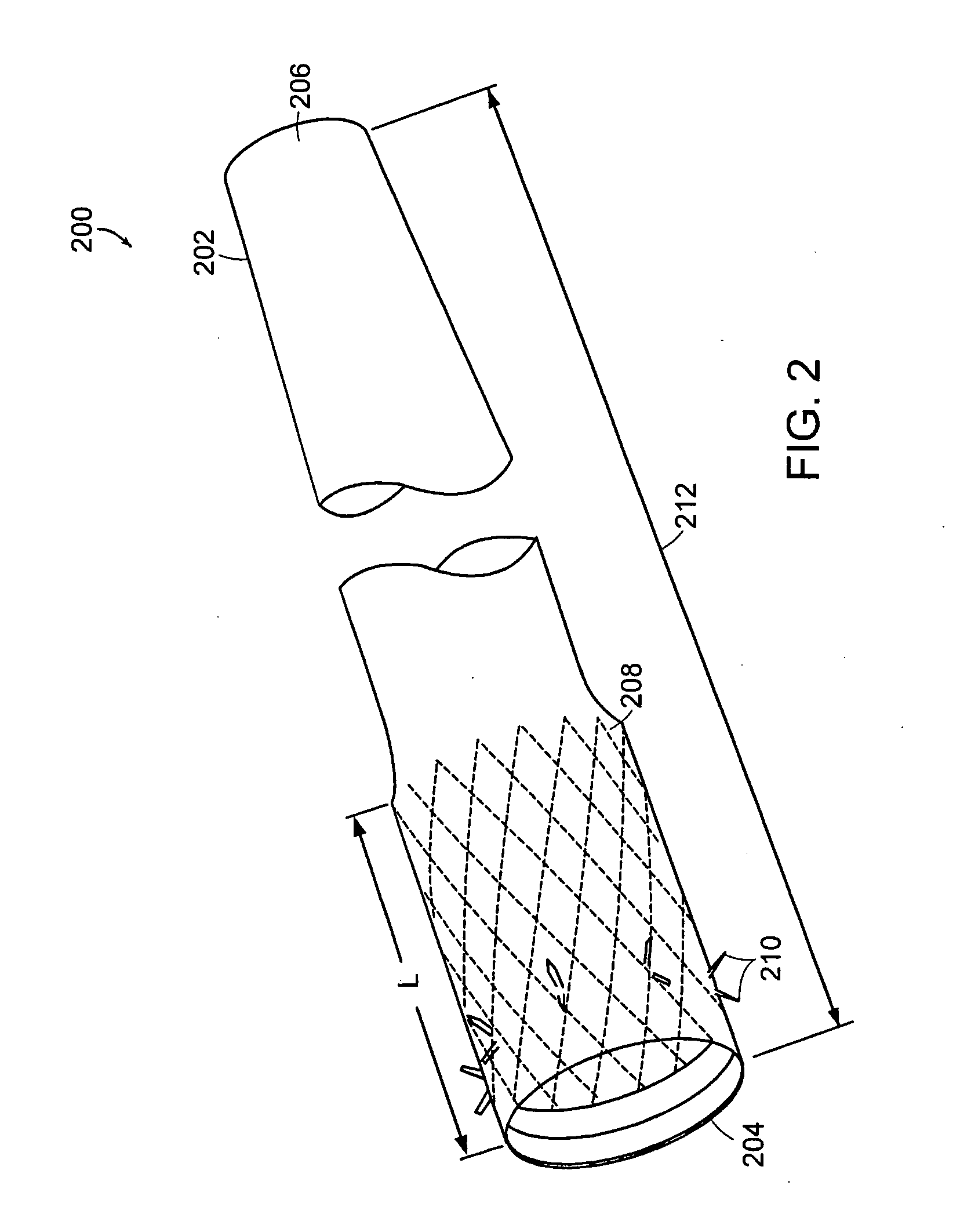
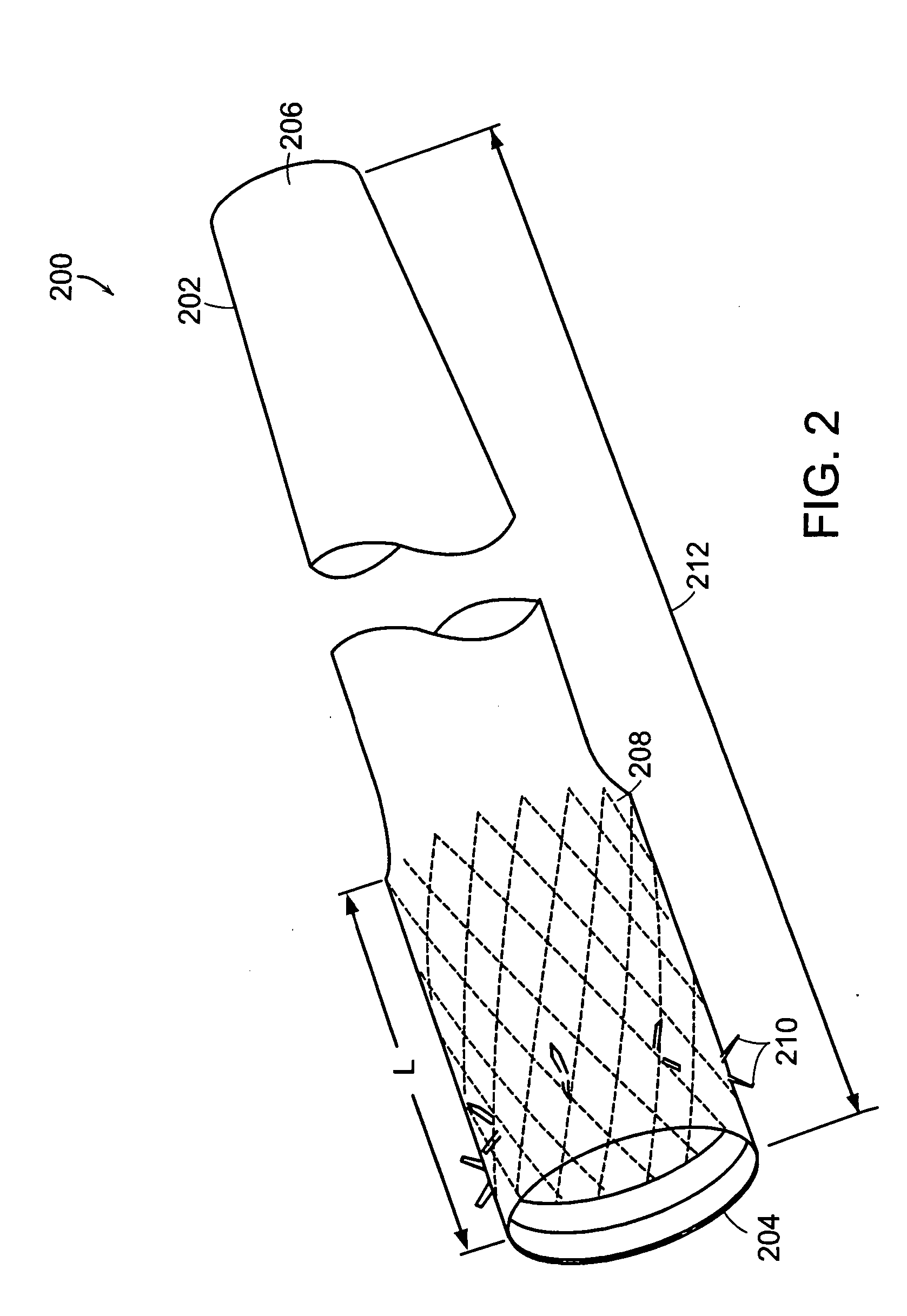
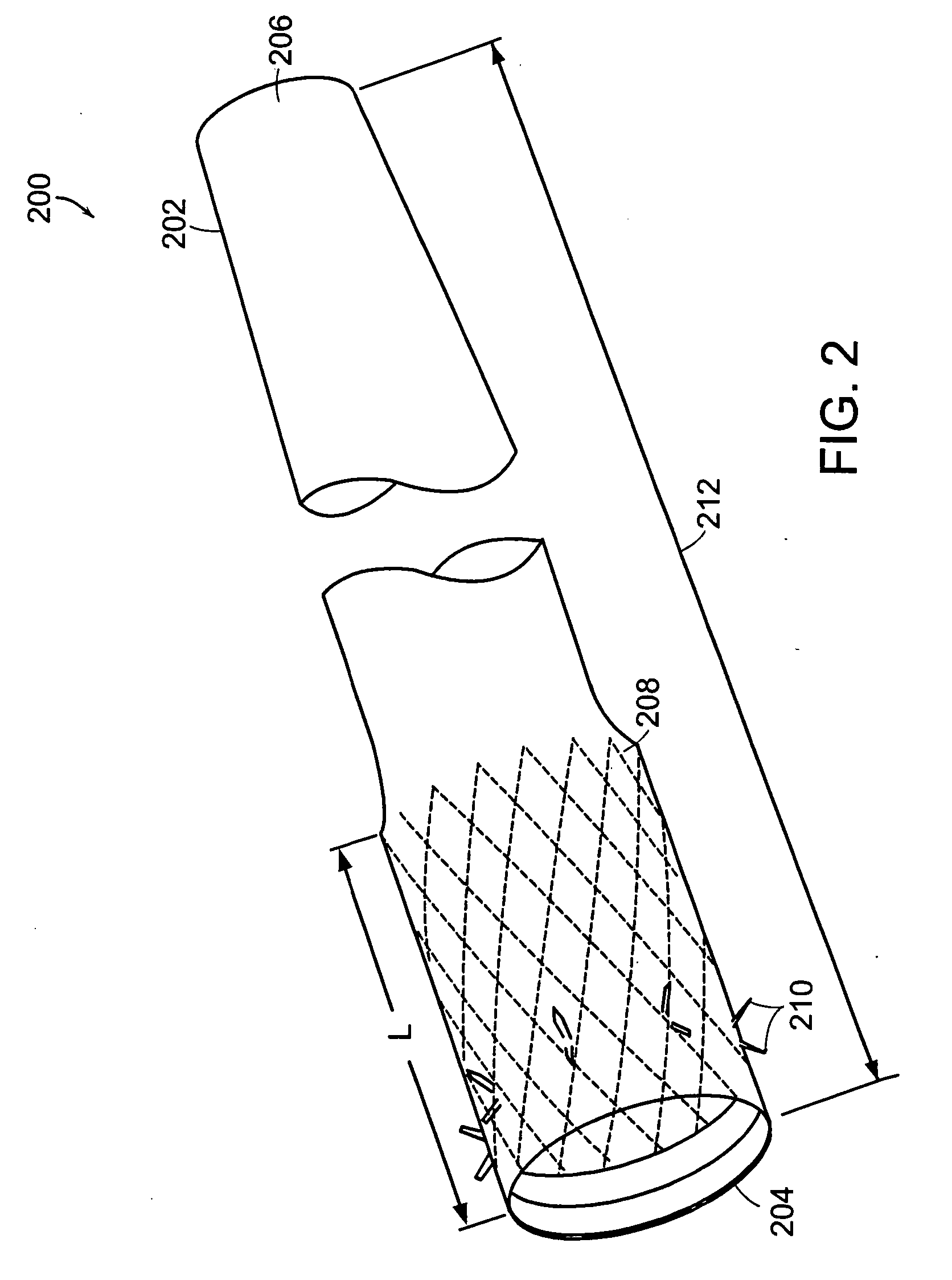
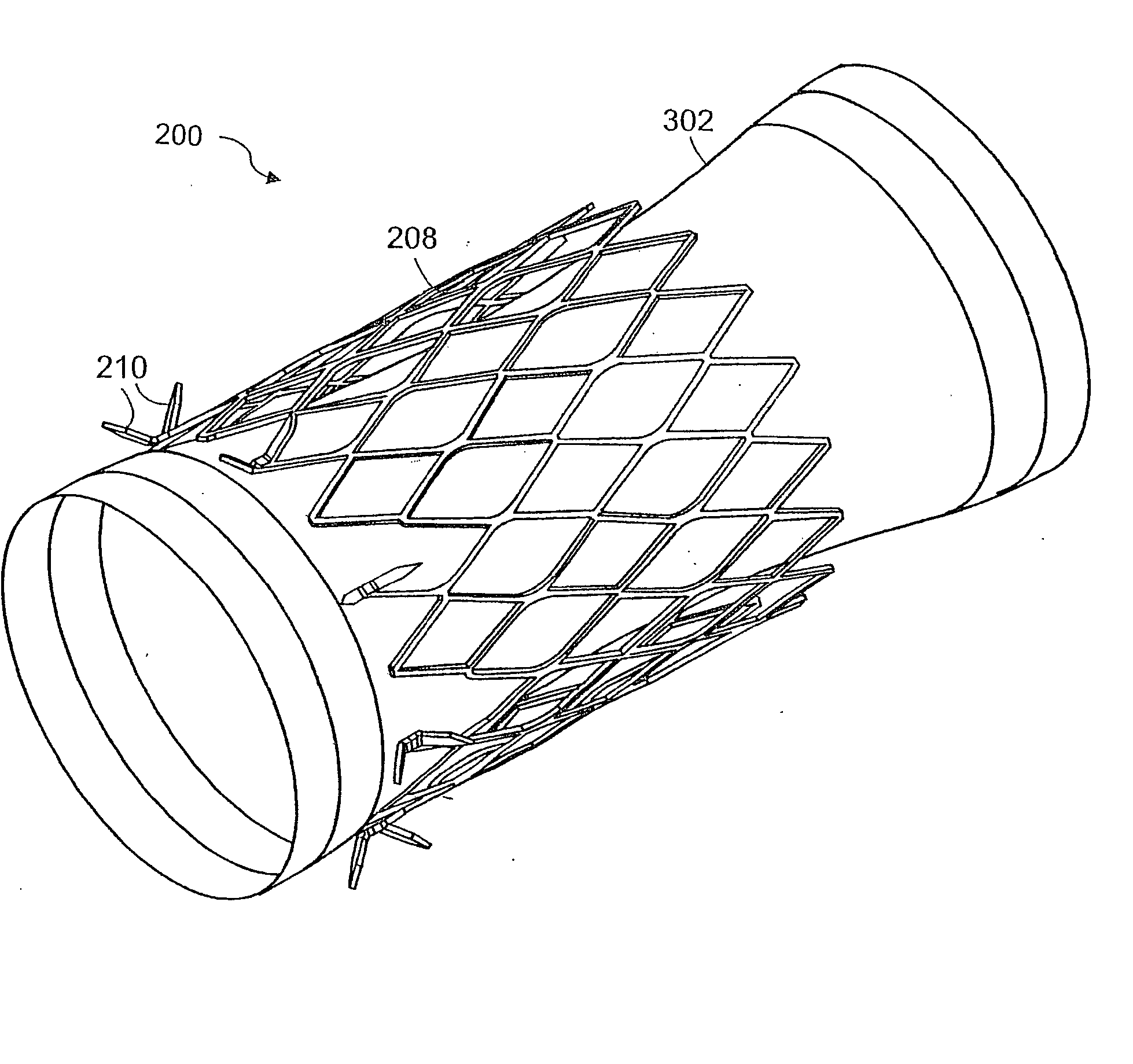
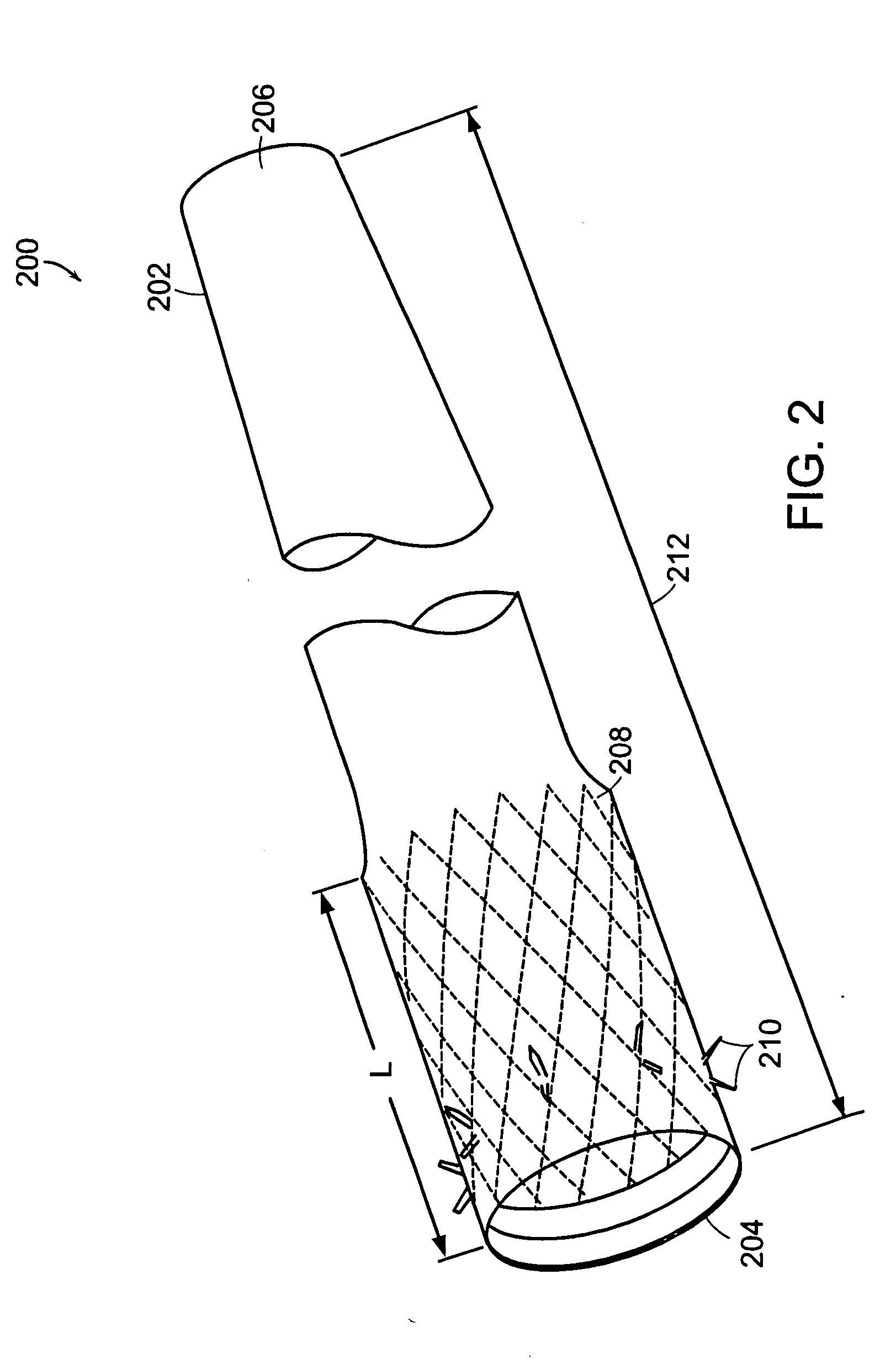
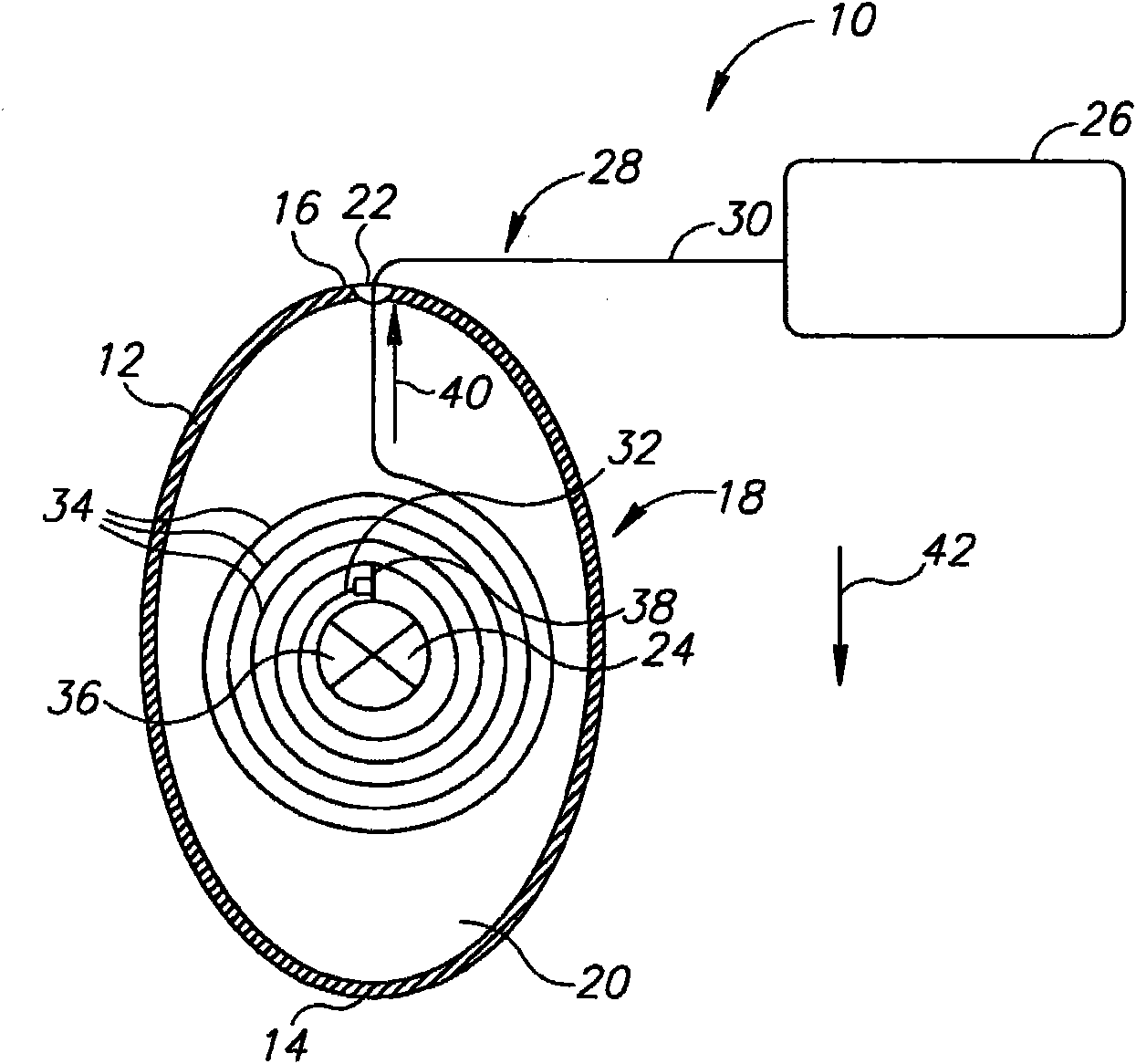
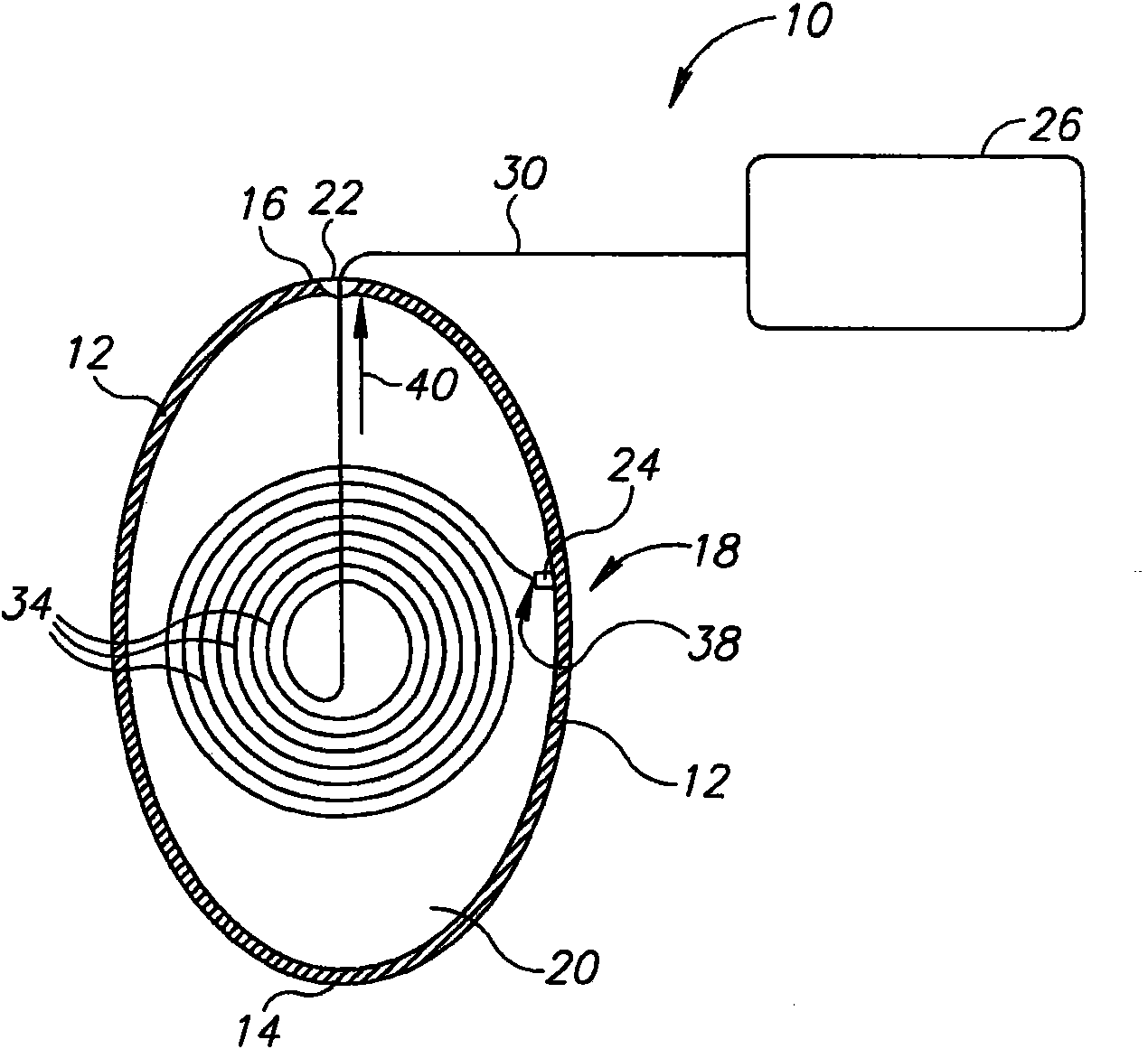
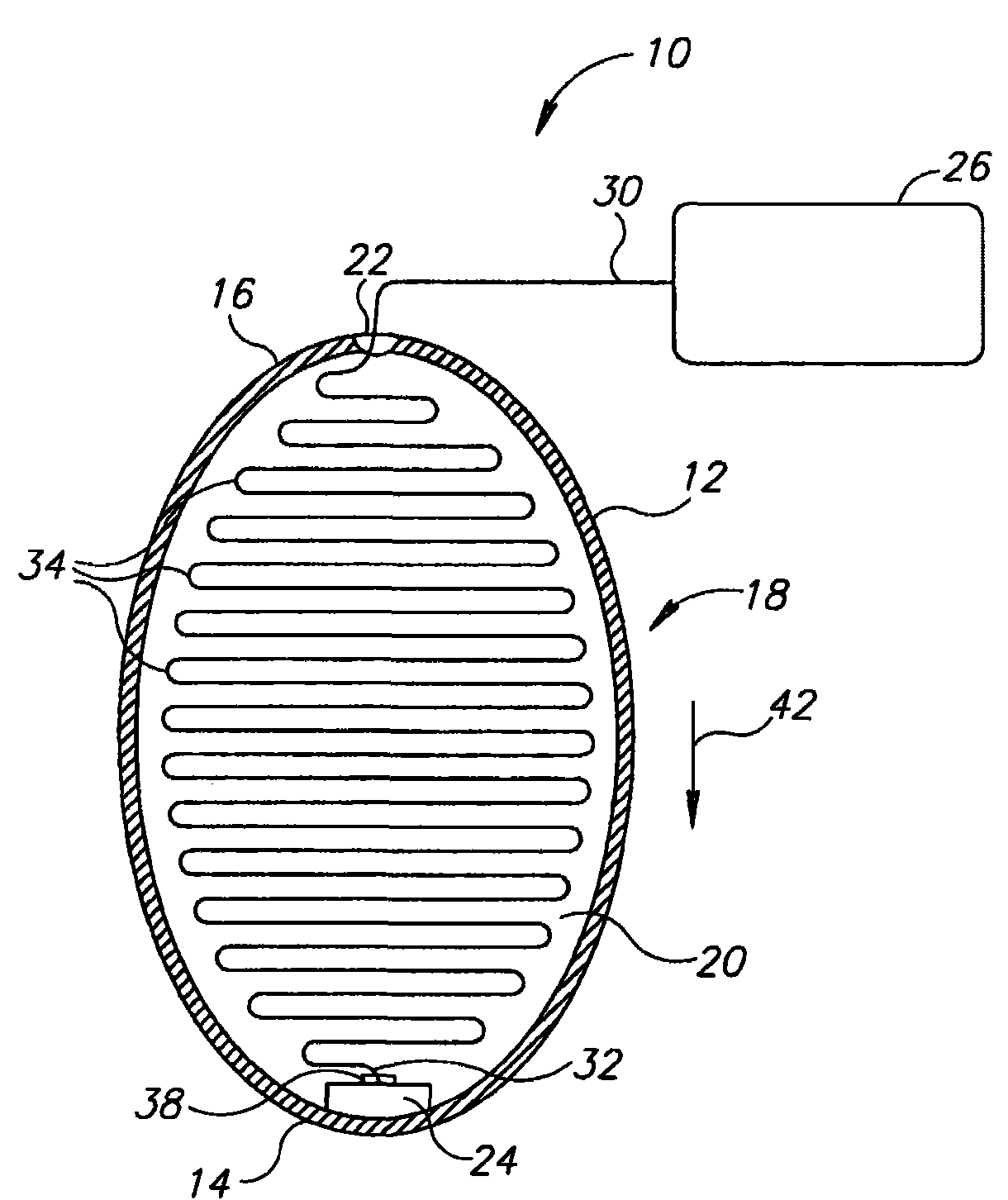
InactiveUS20060161265A1Increased axial stabilityLess movementSuture equipmentsStentsIntestinal structureGastrointestinal device
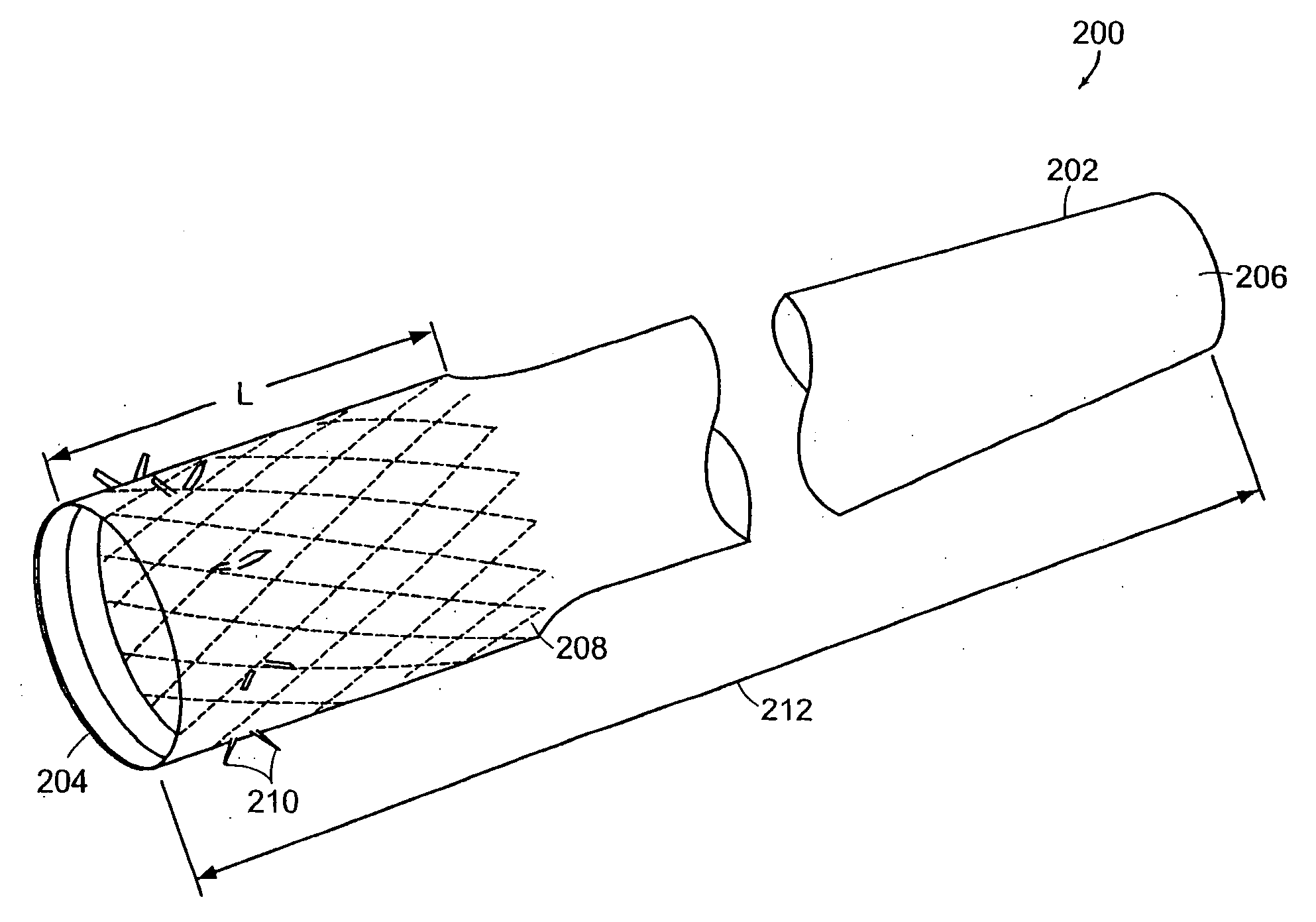
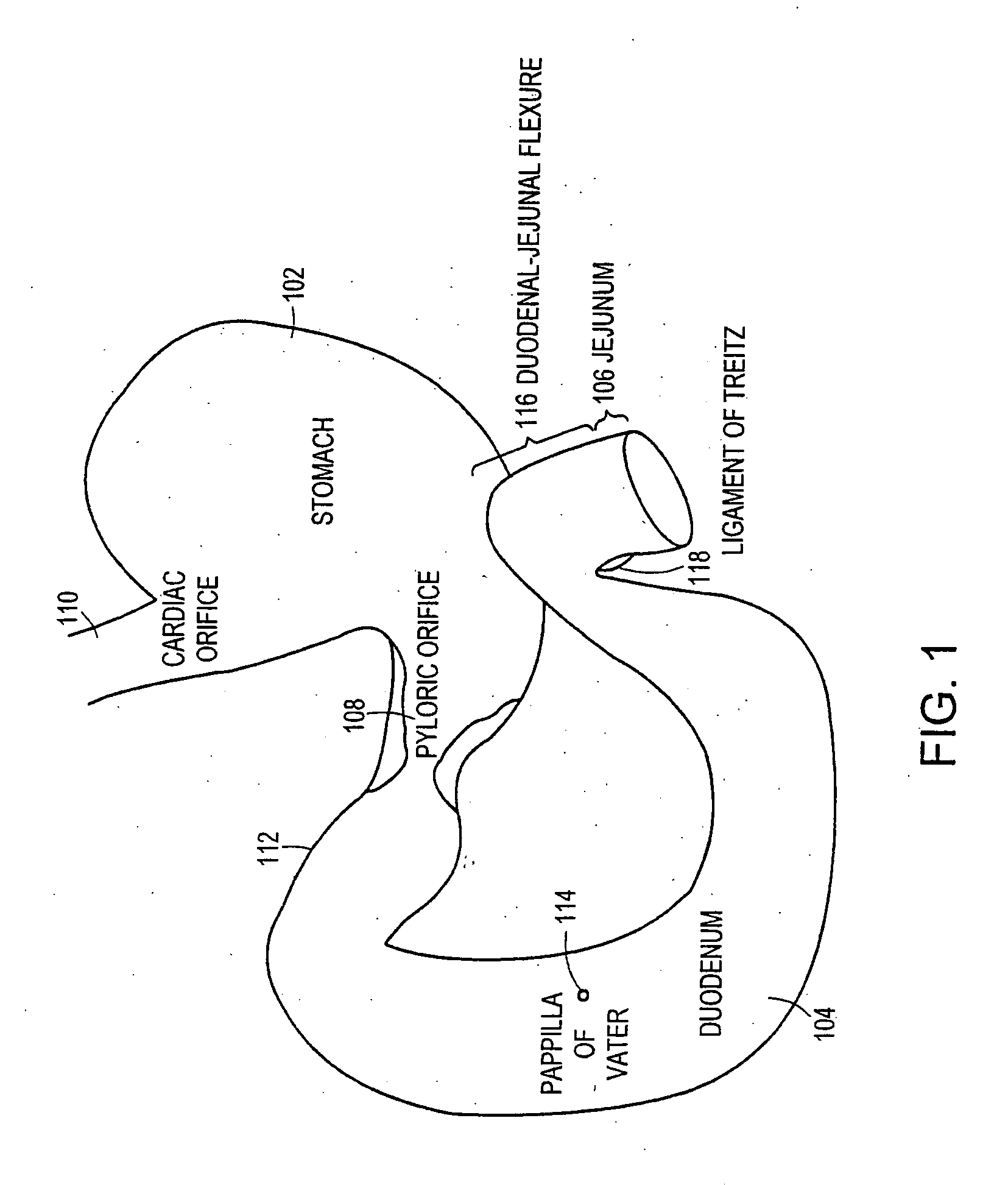
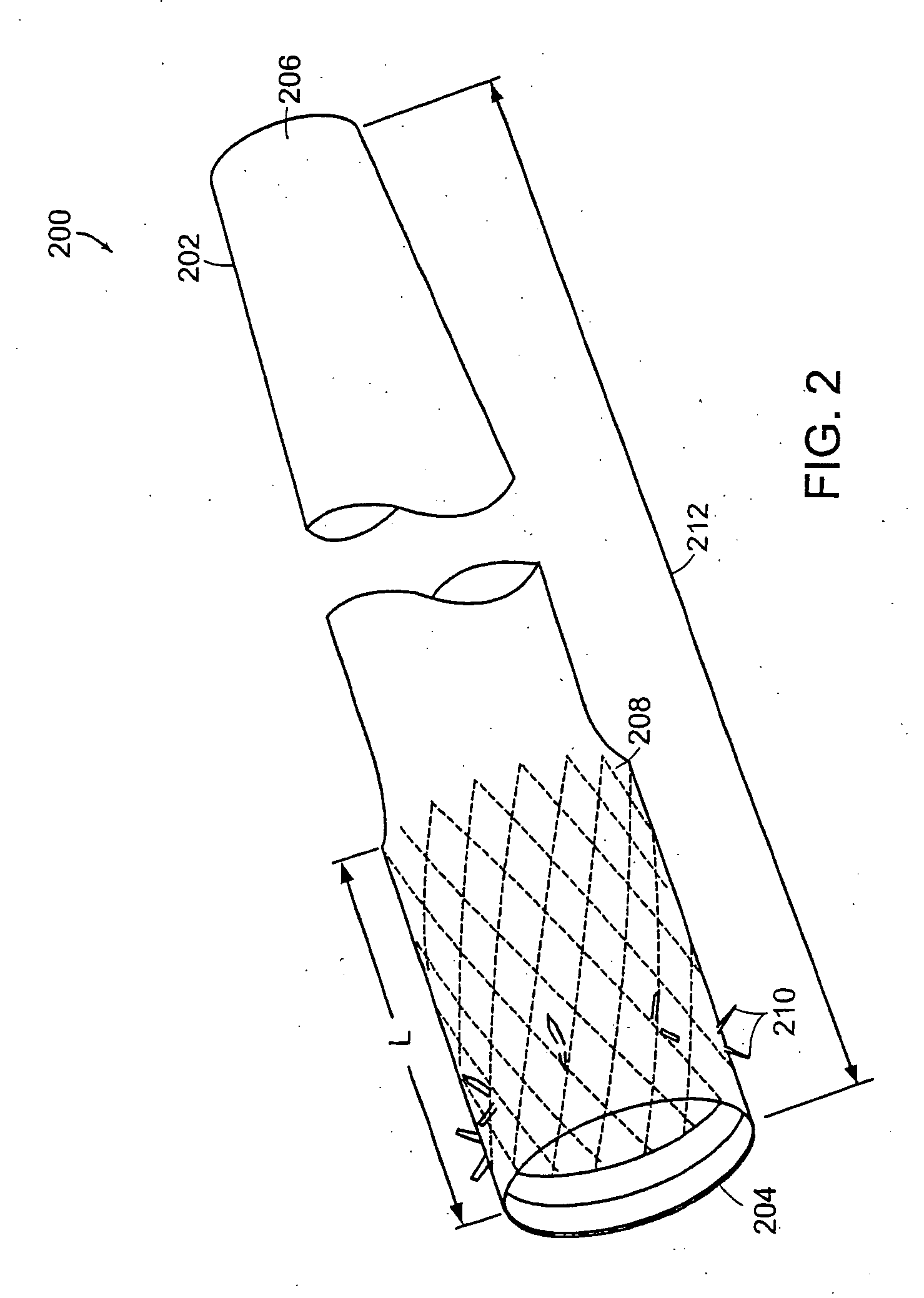
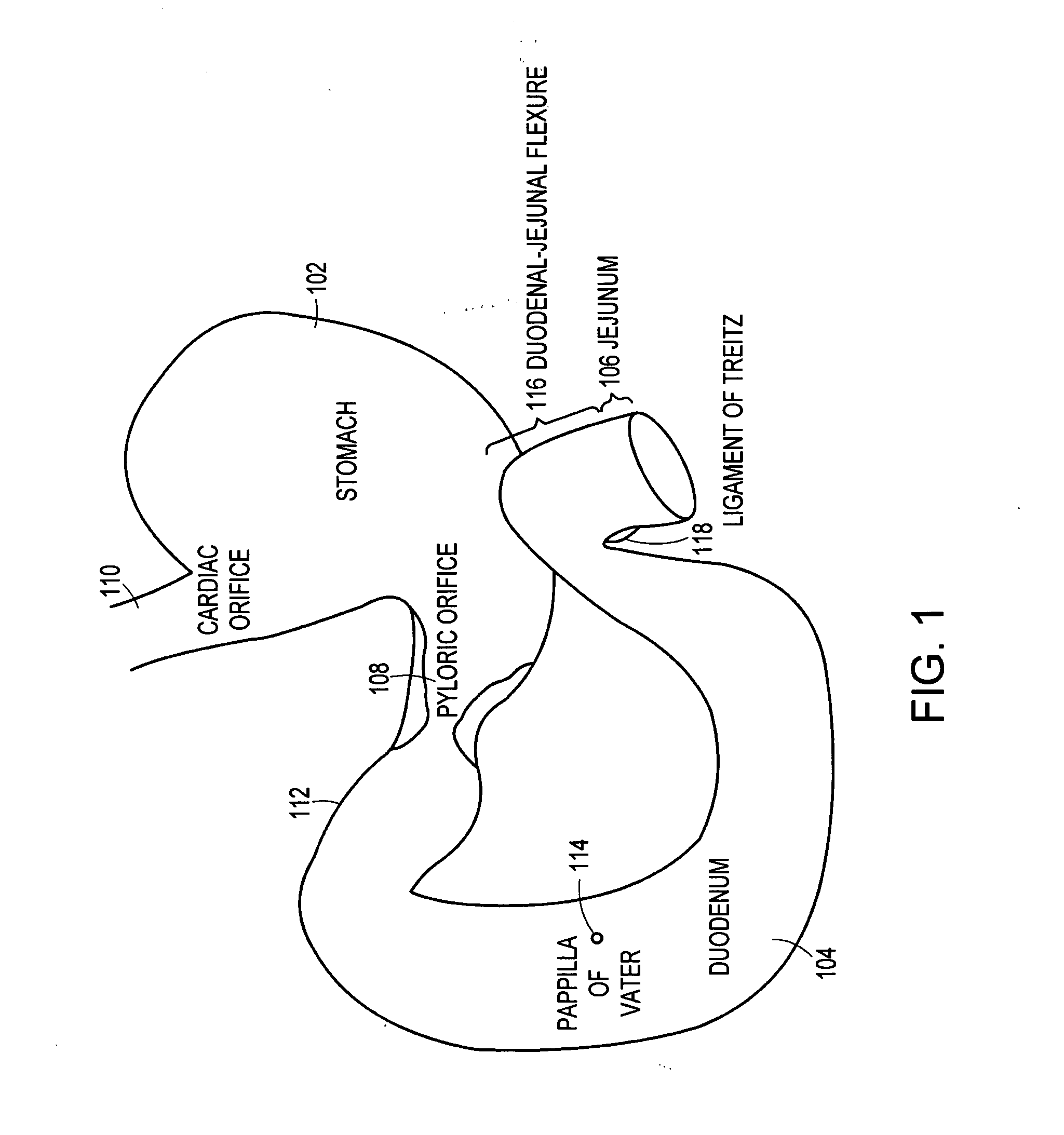
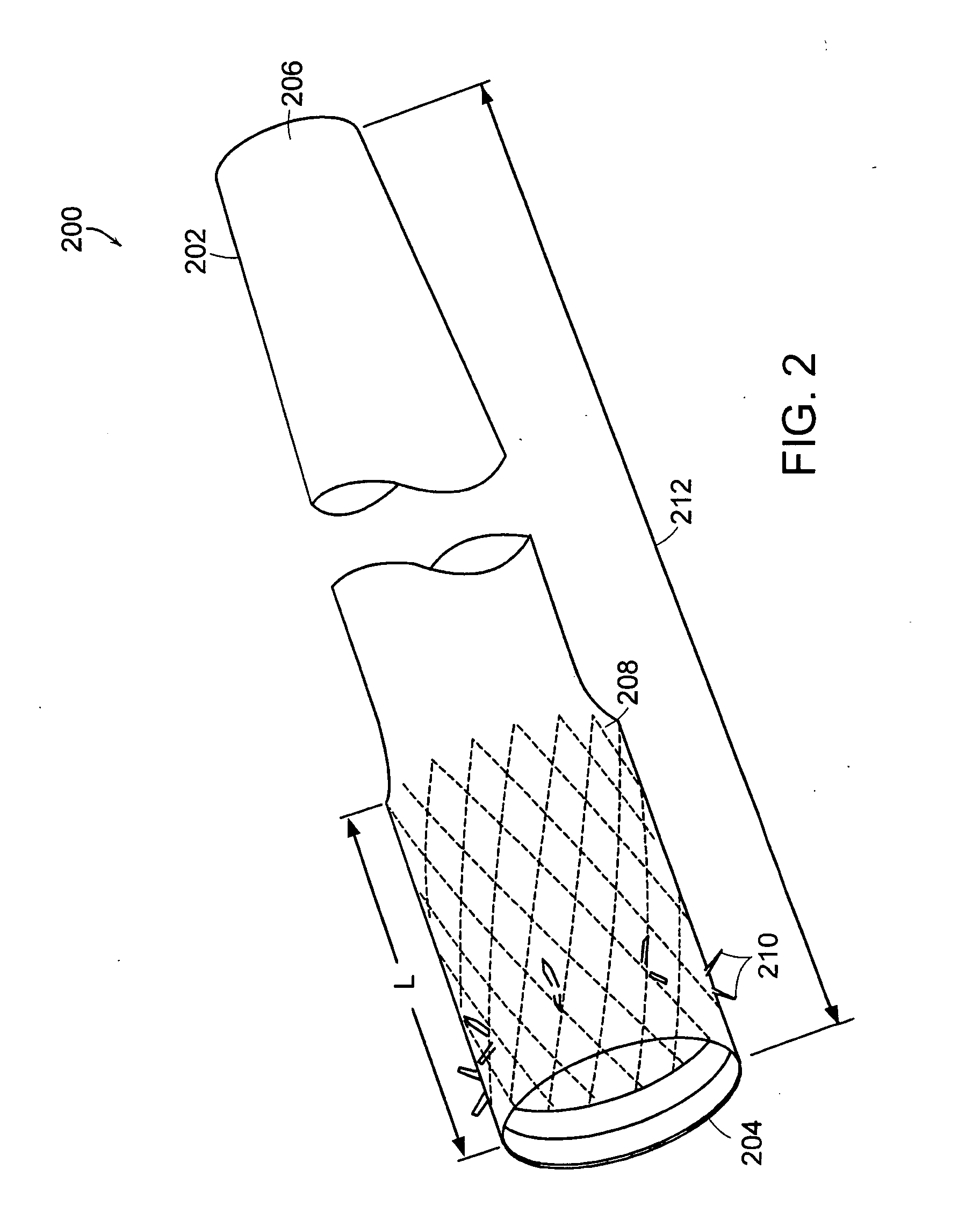
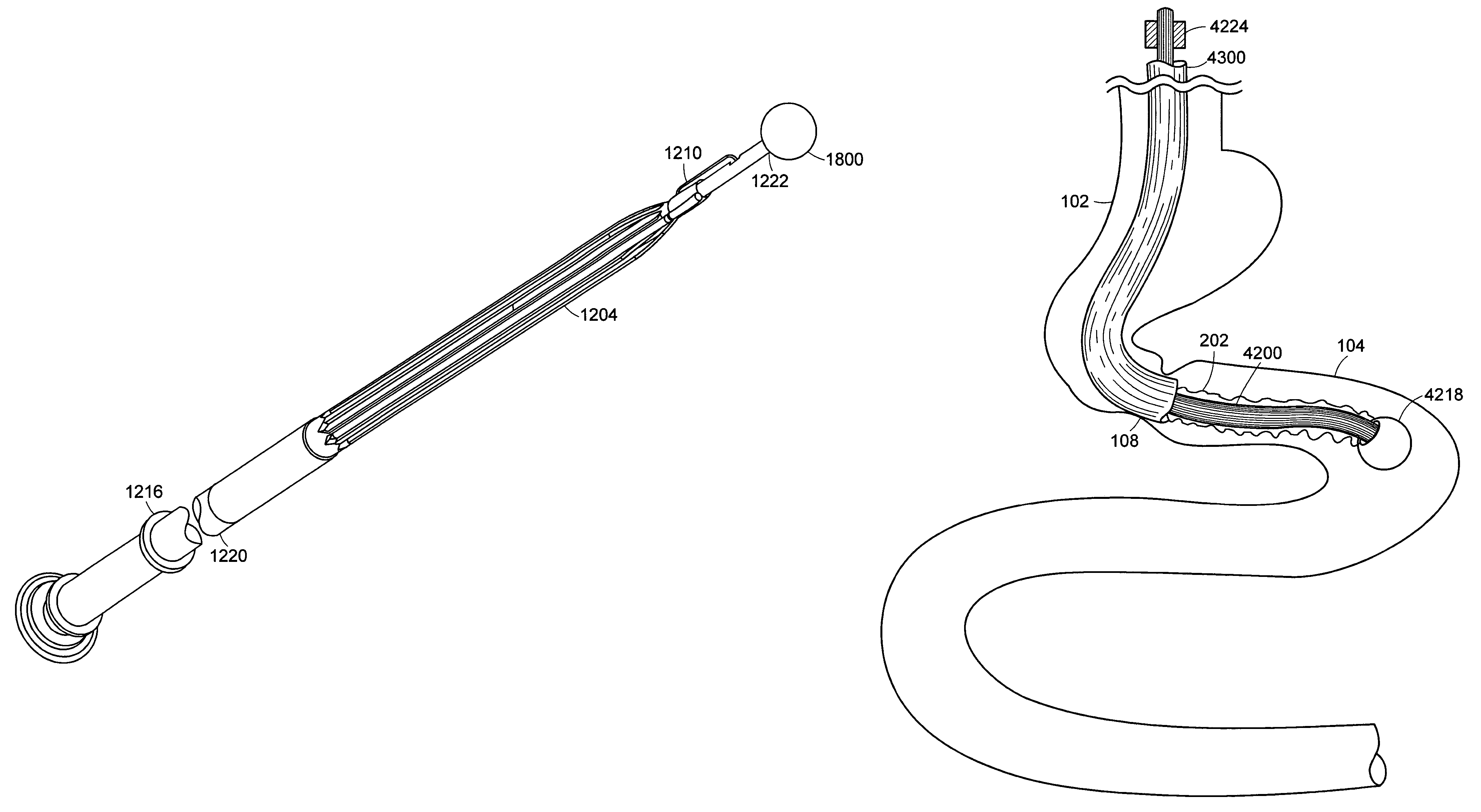
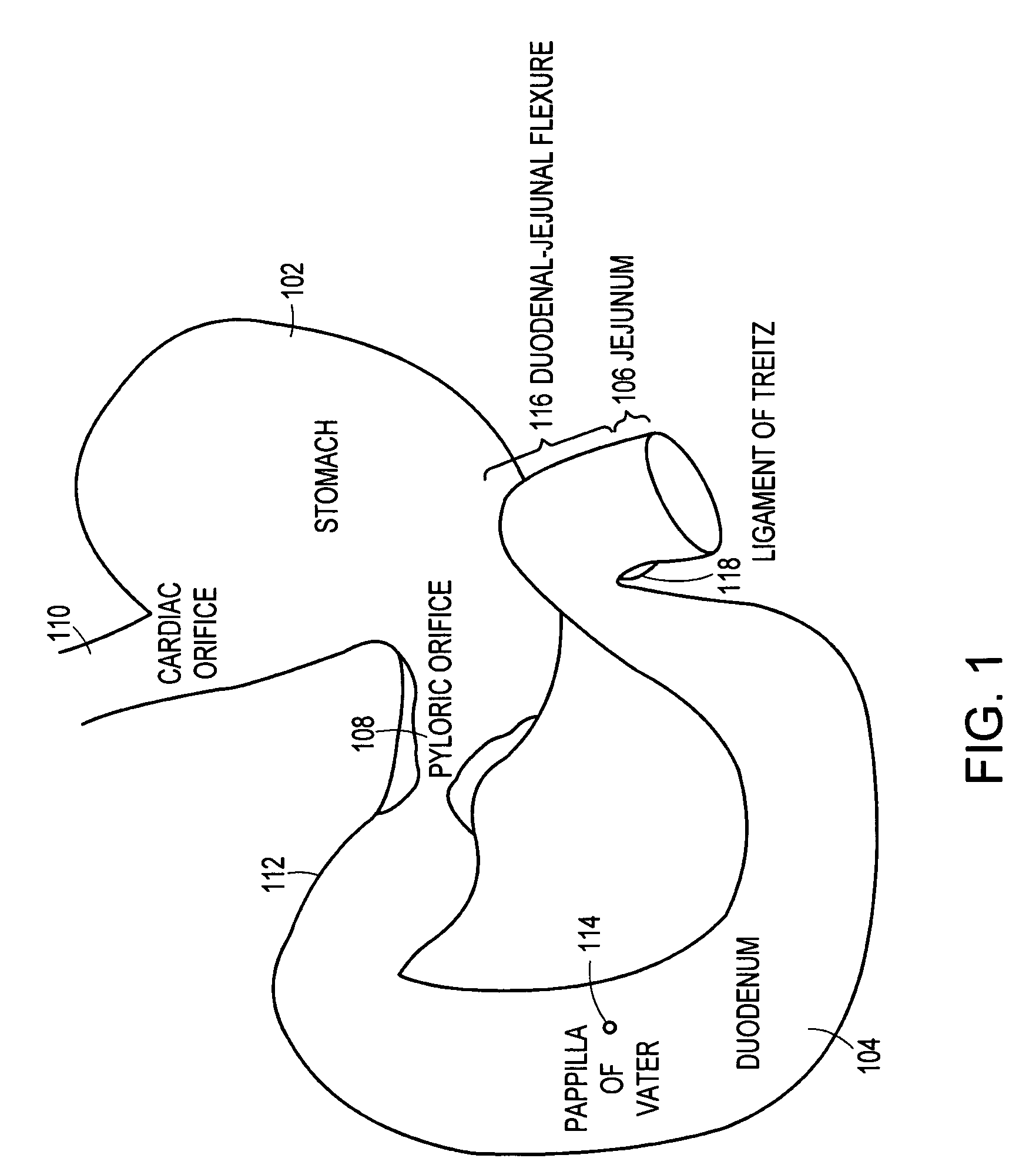
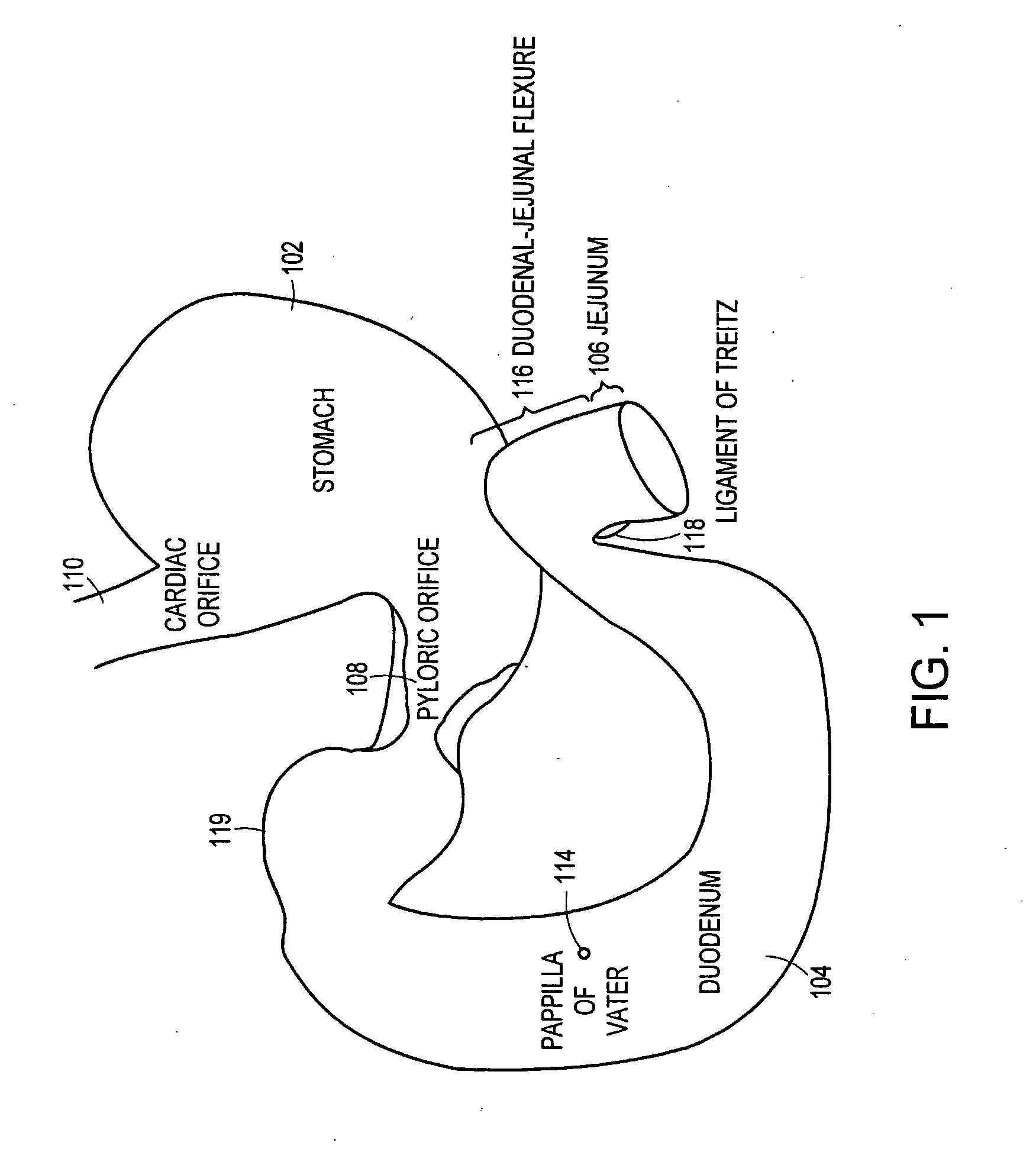
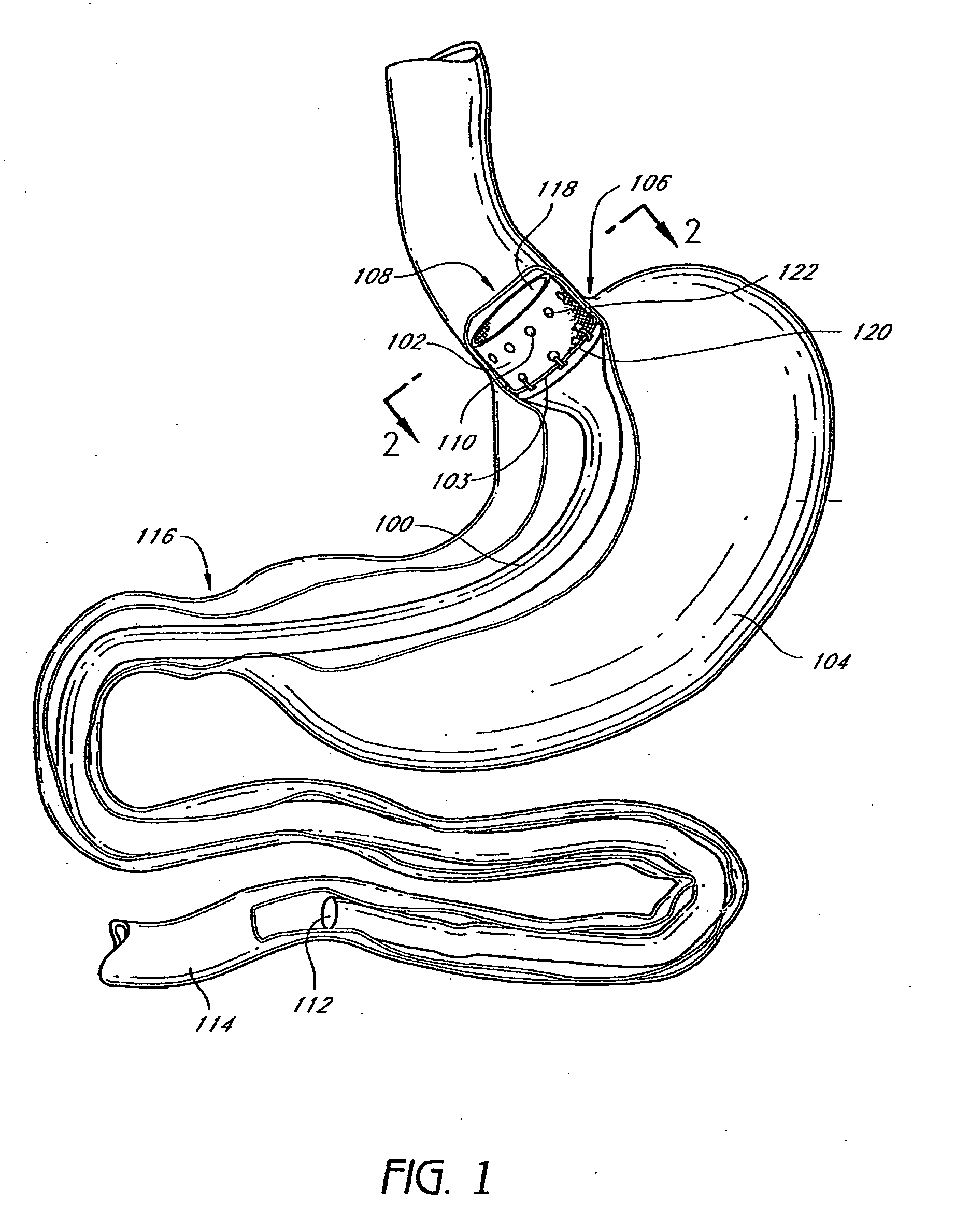
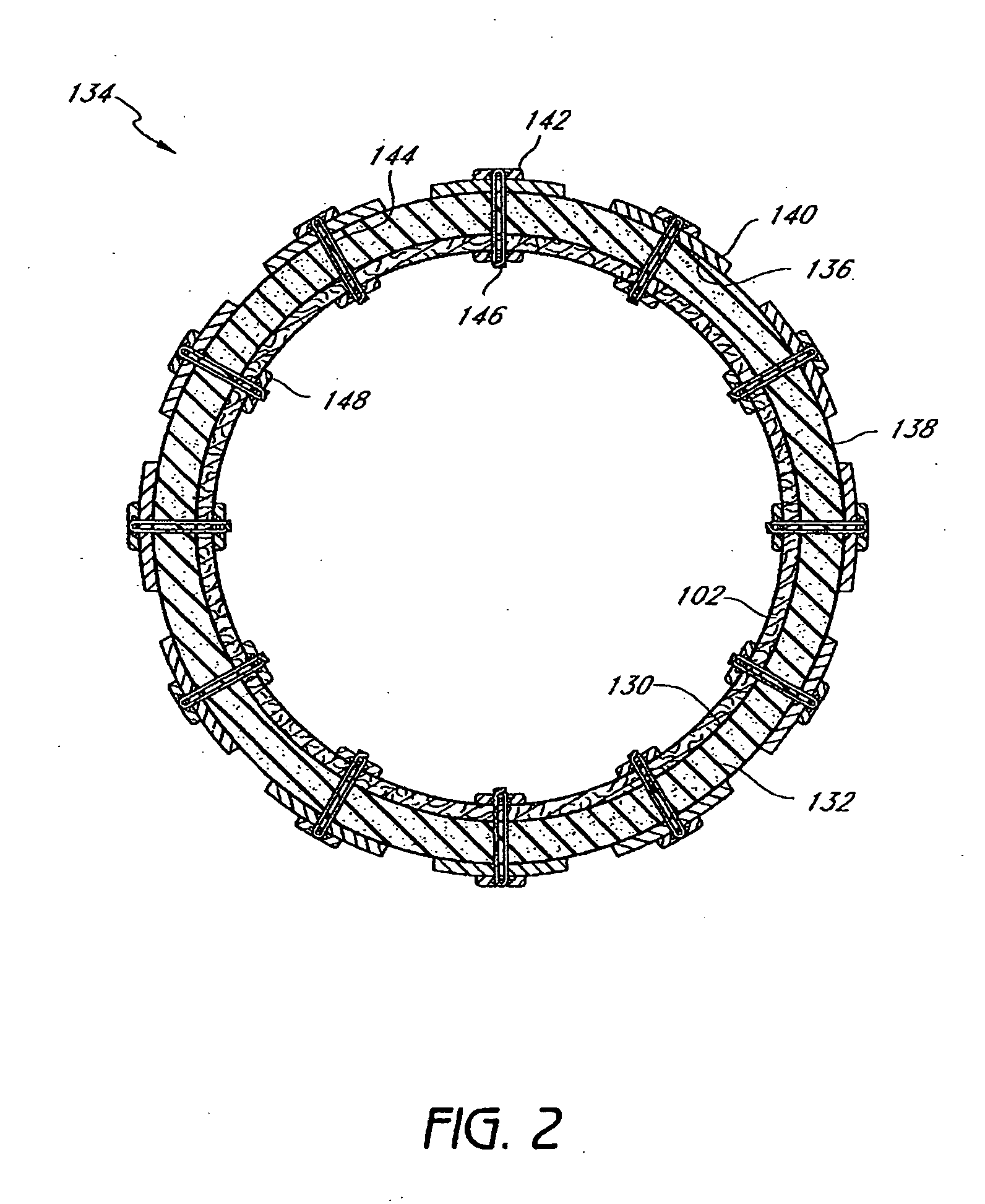
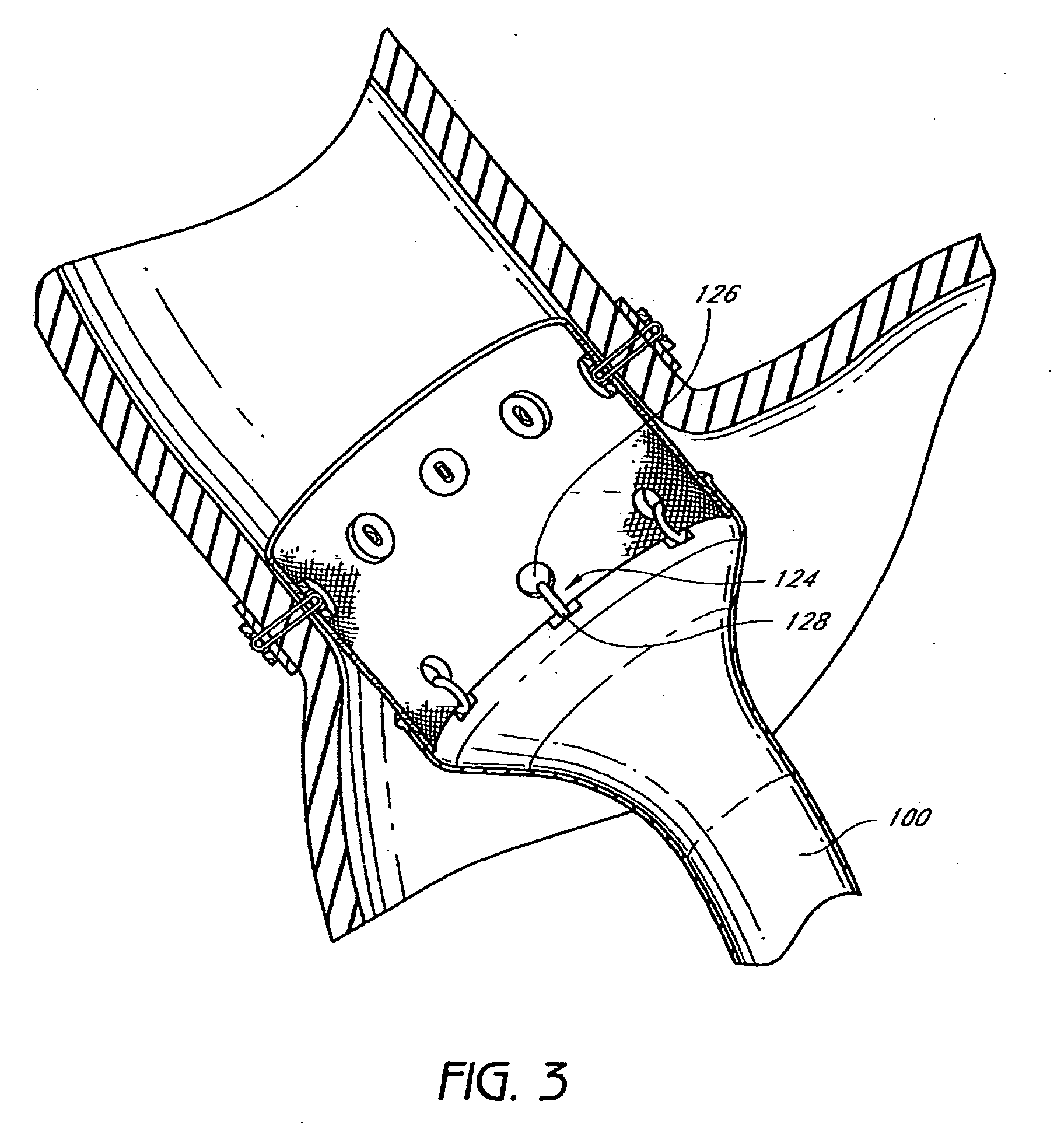
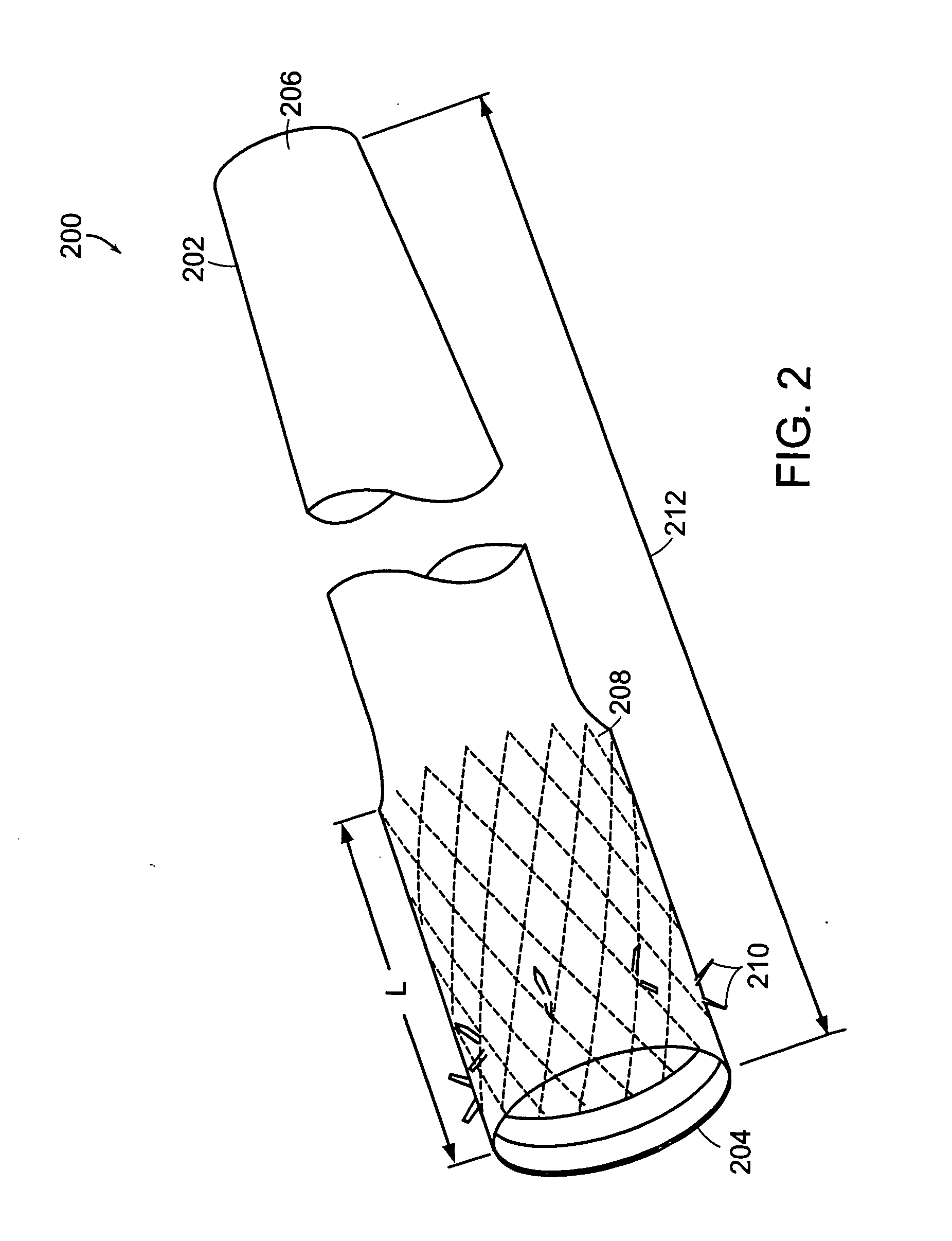
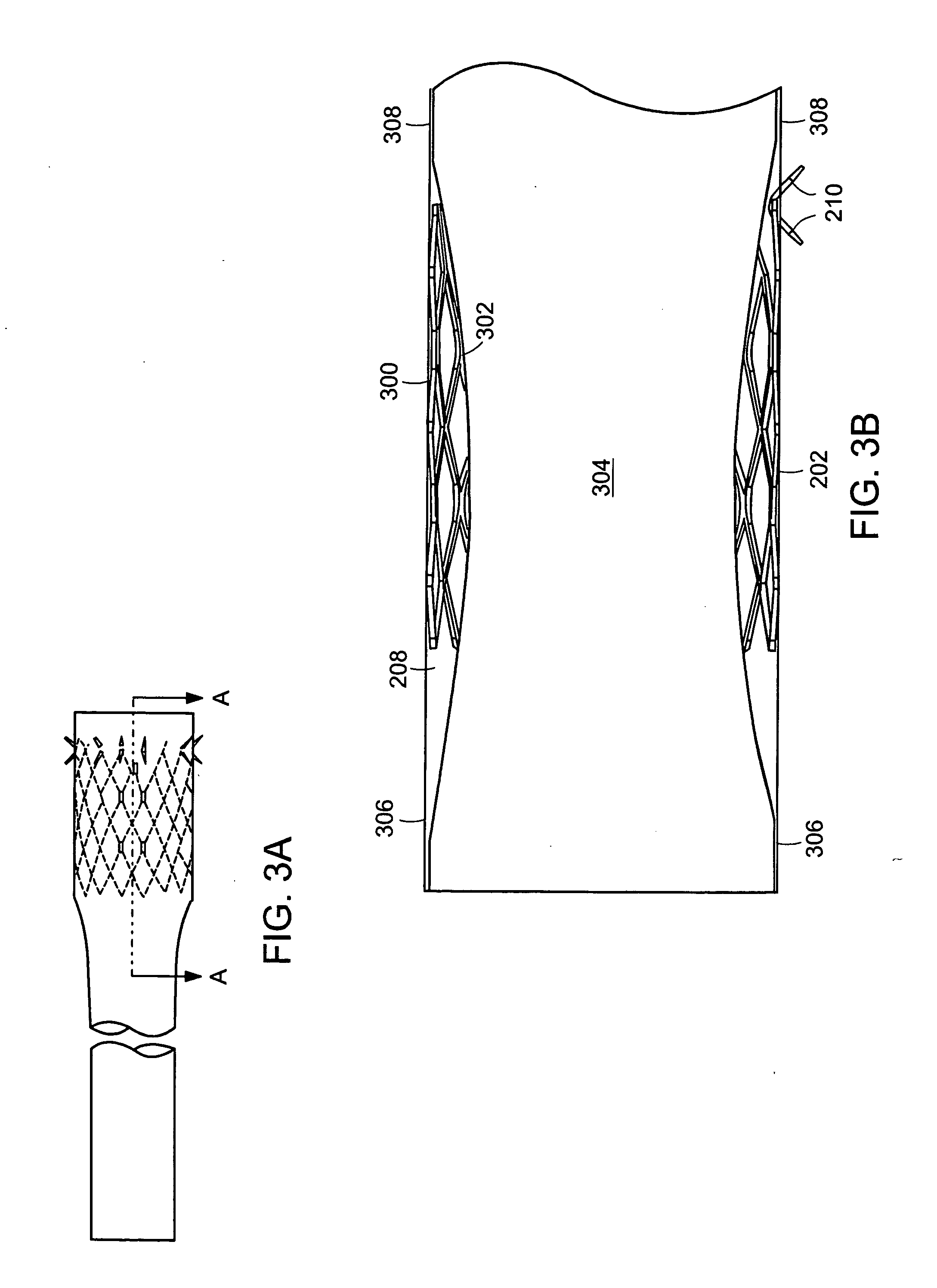
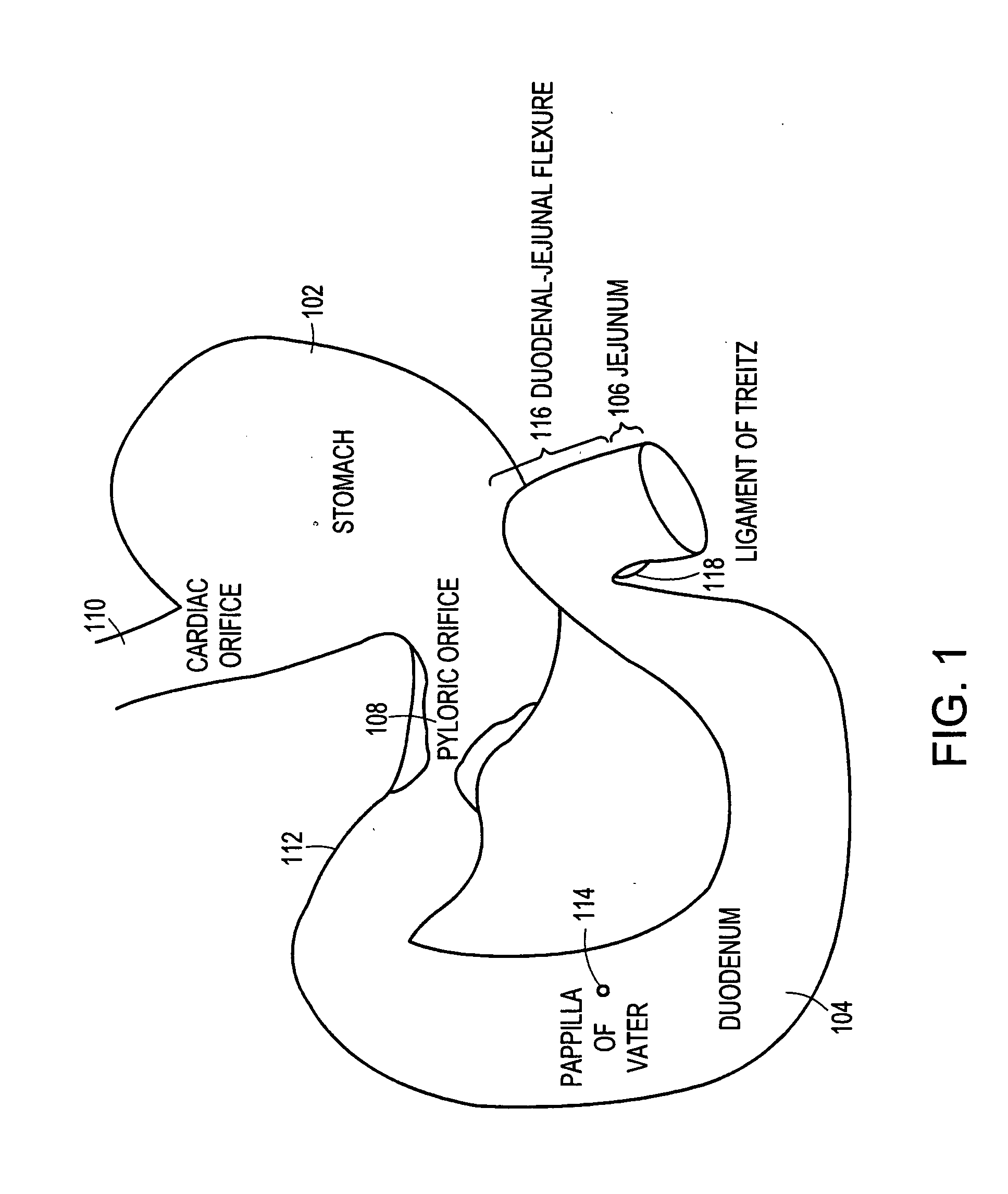
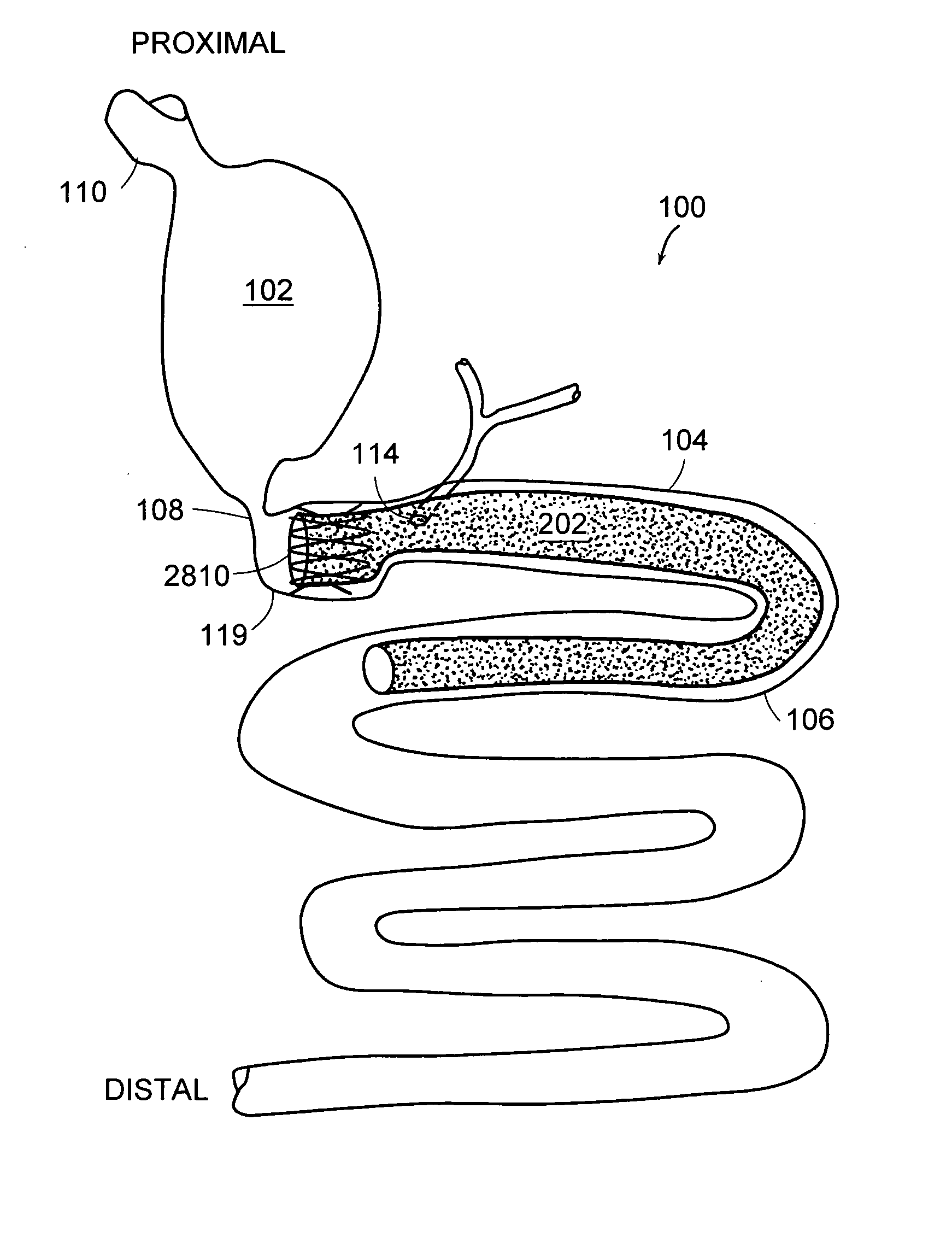
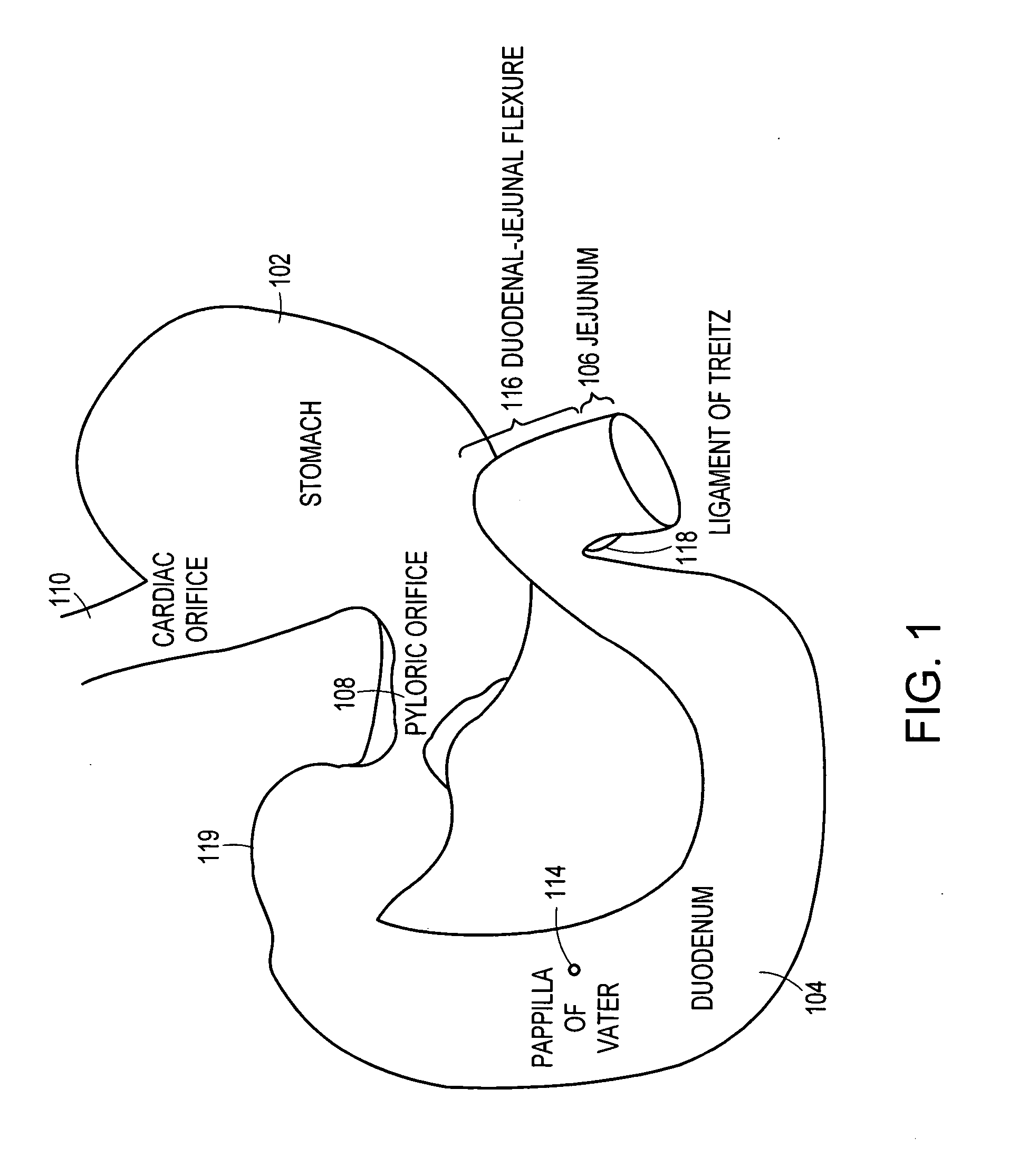
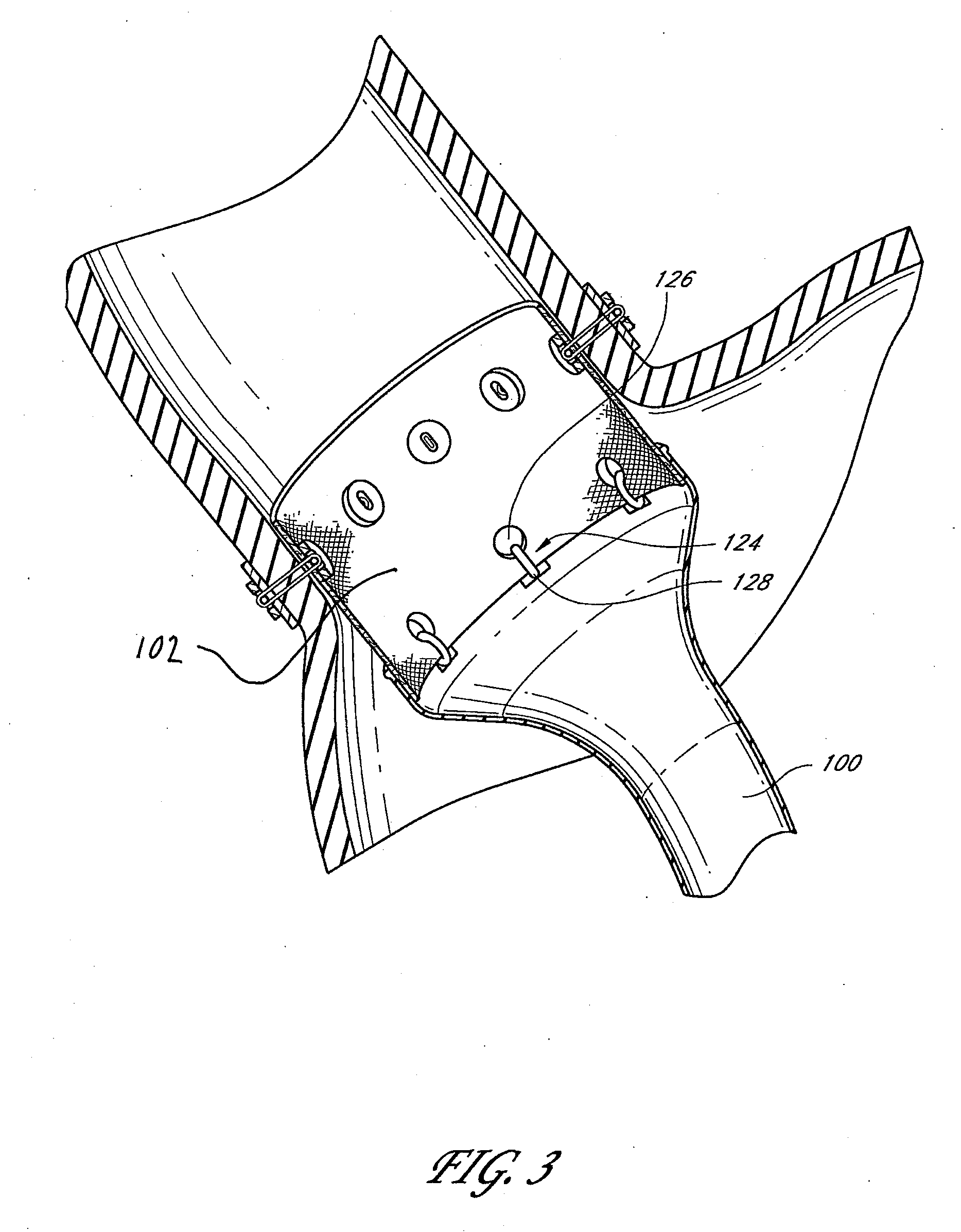
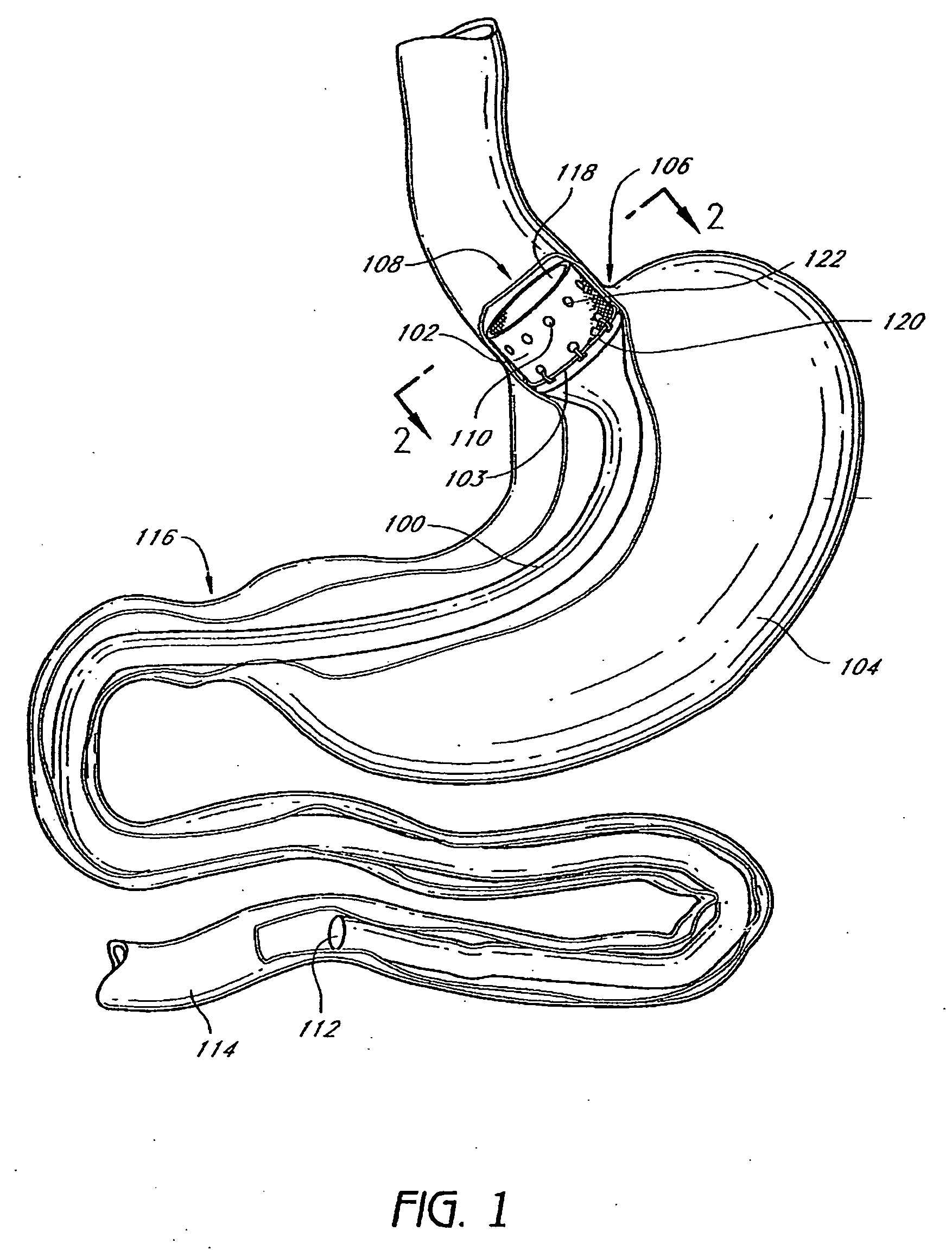
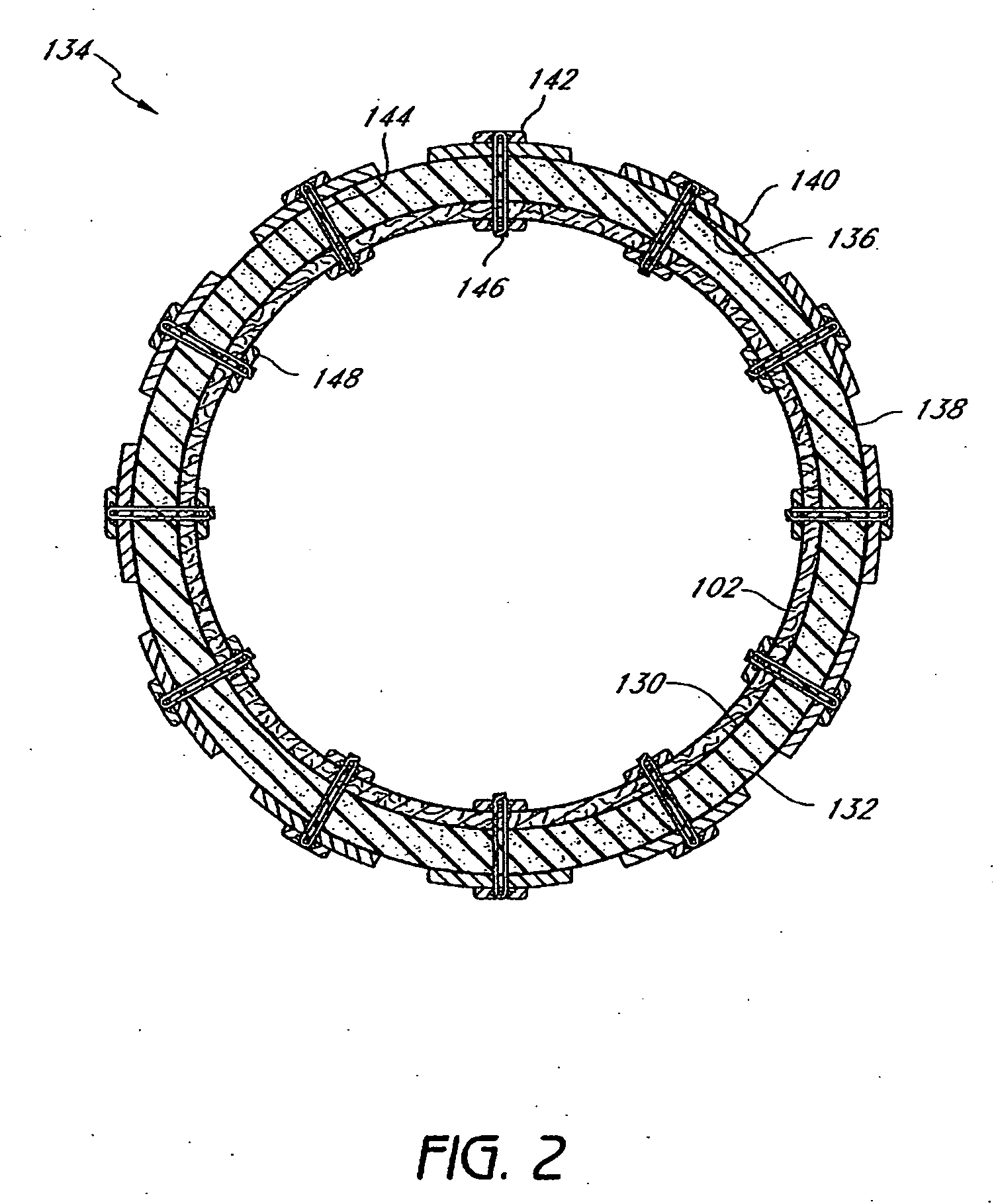
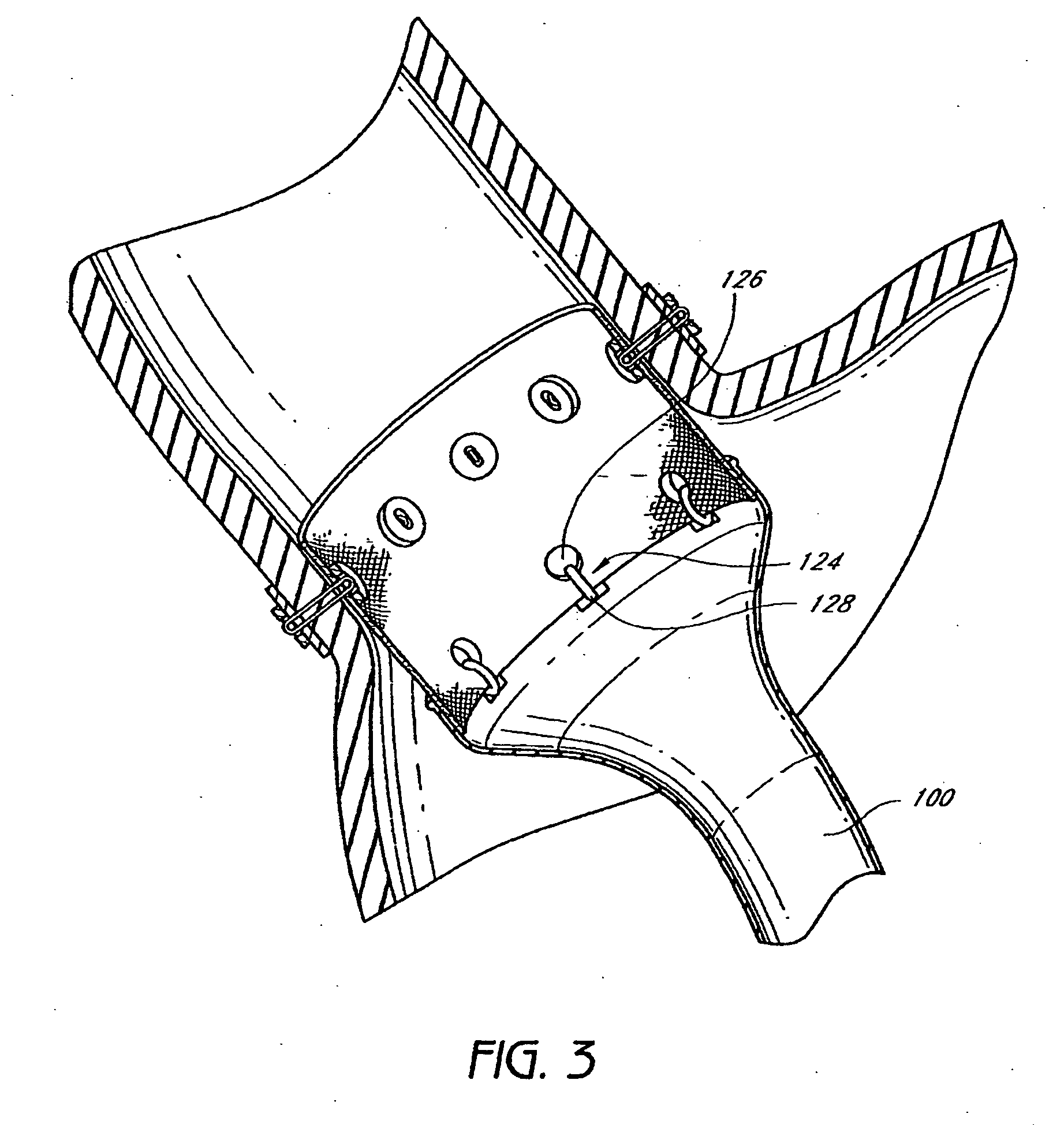
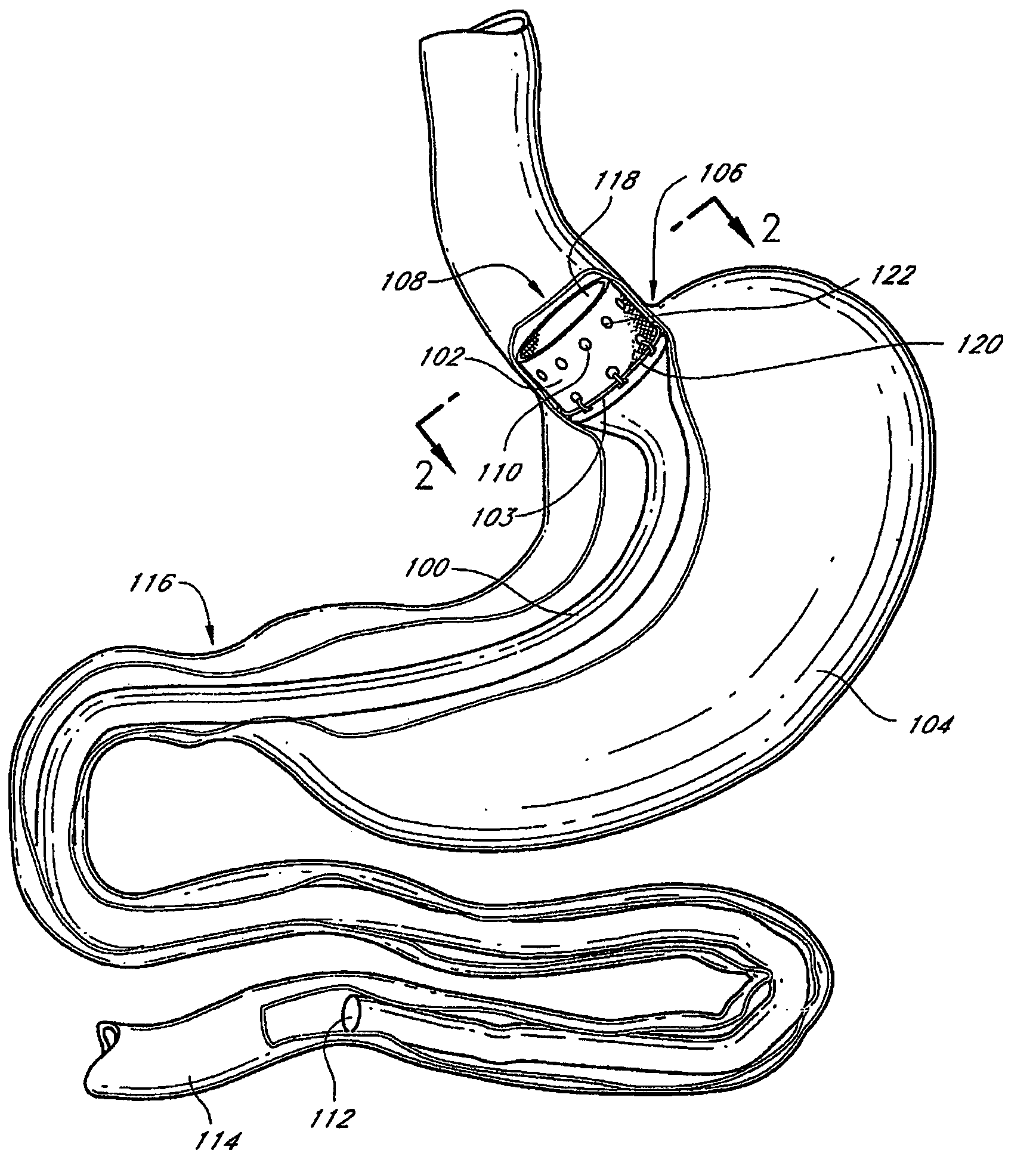
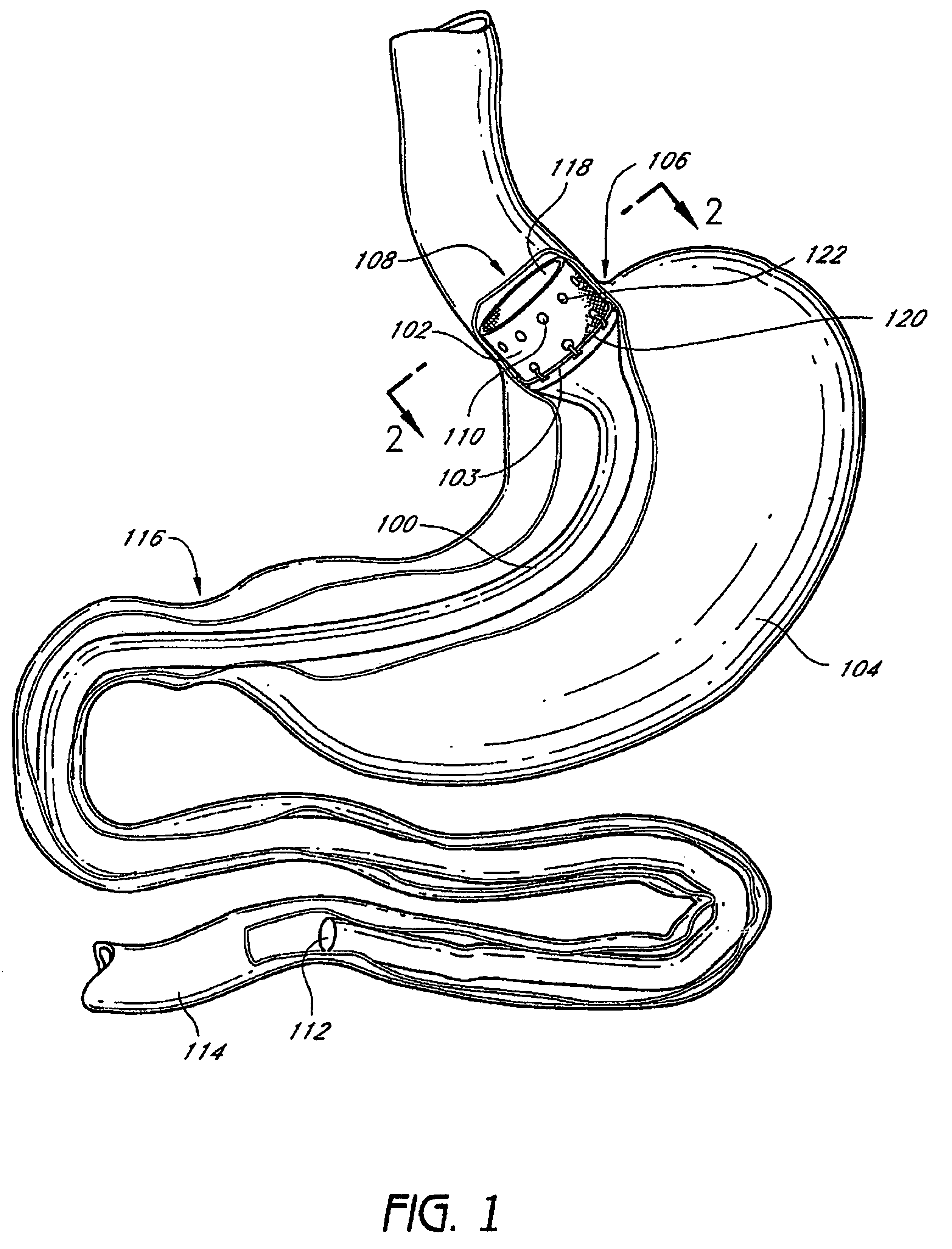
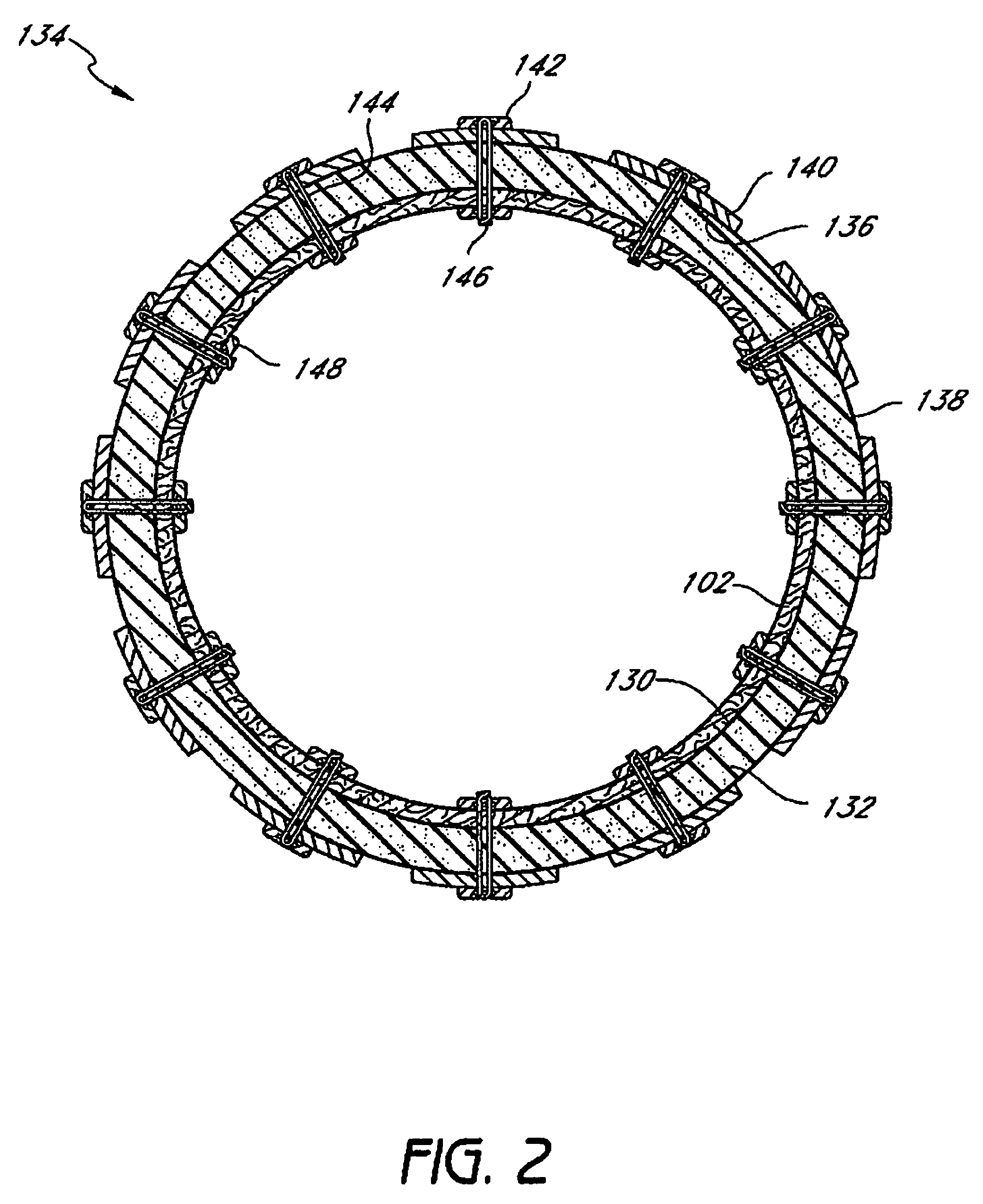
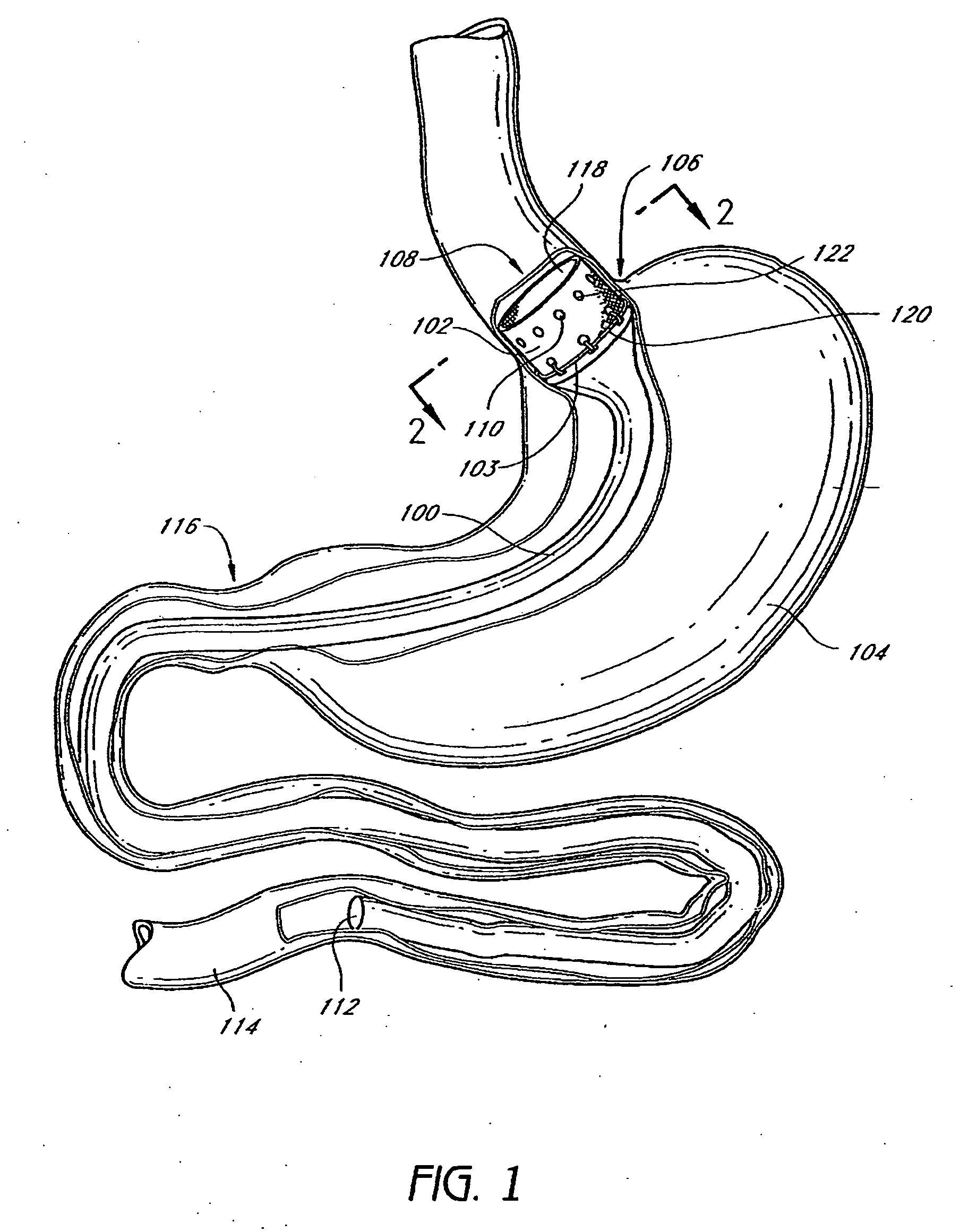
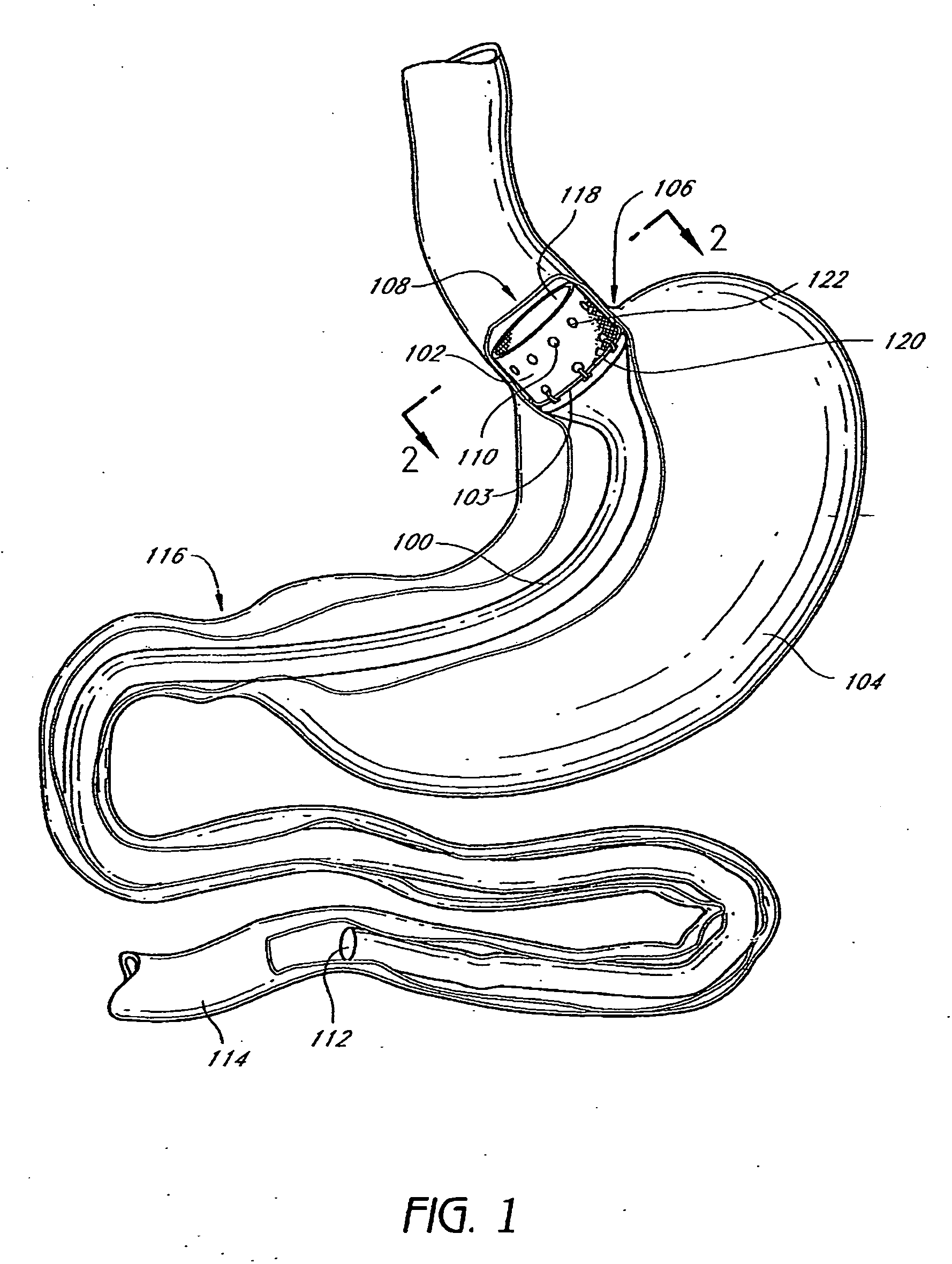
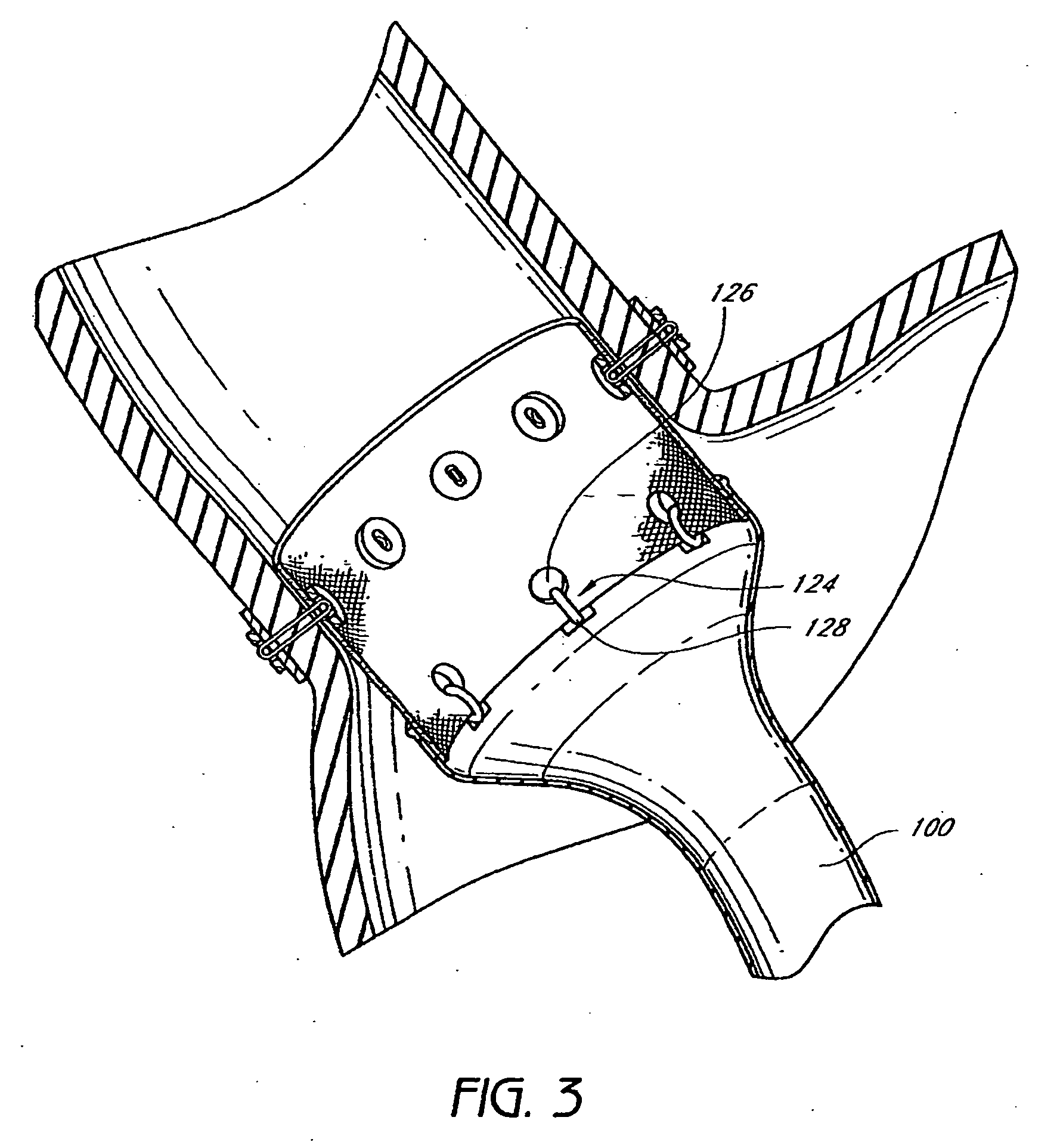
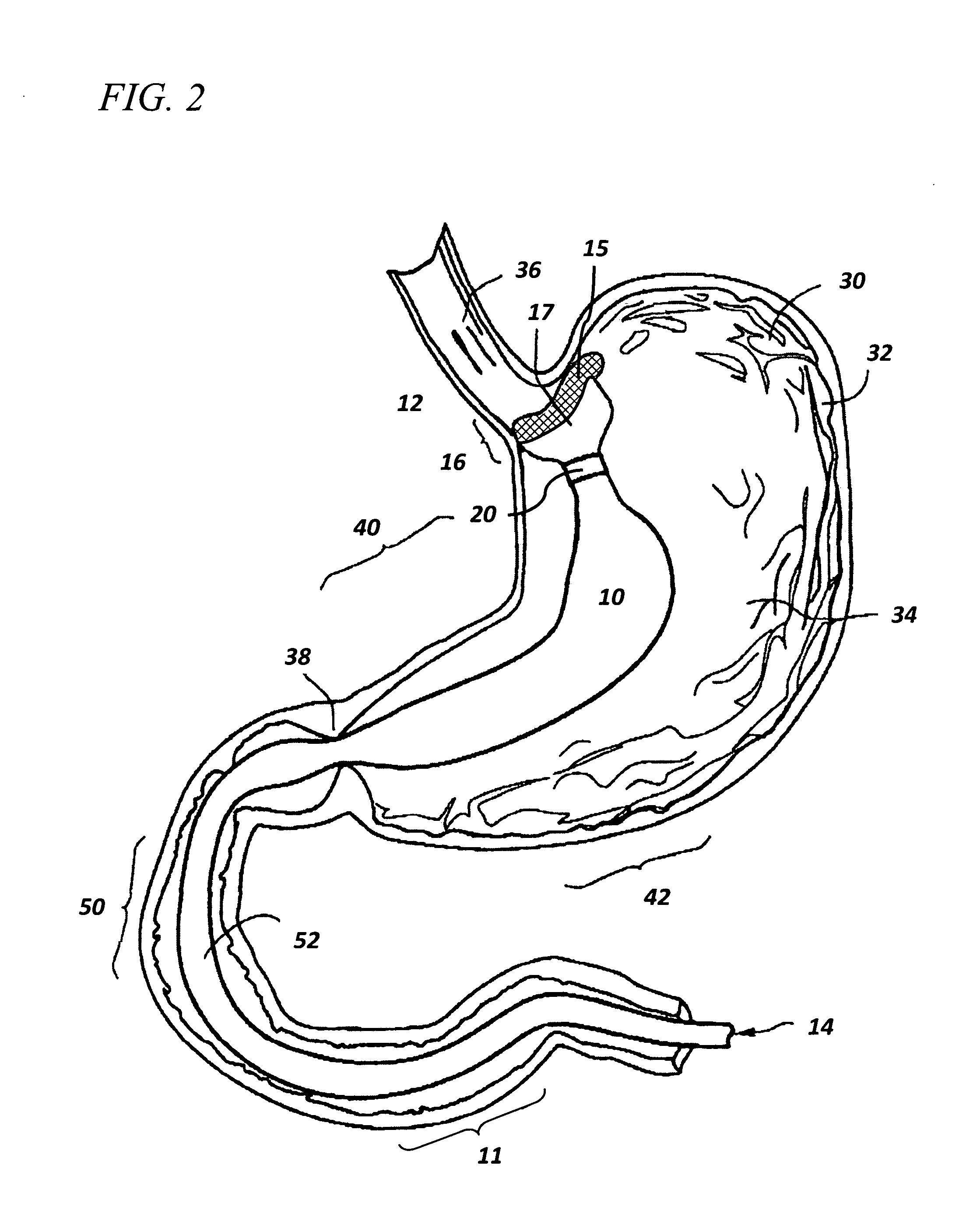
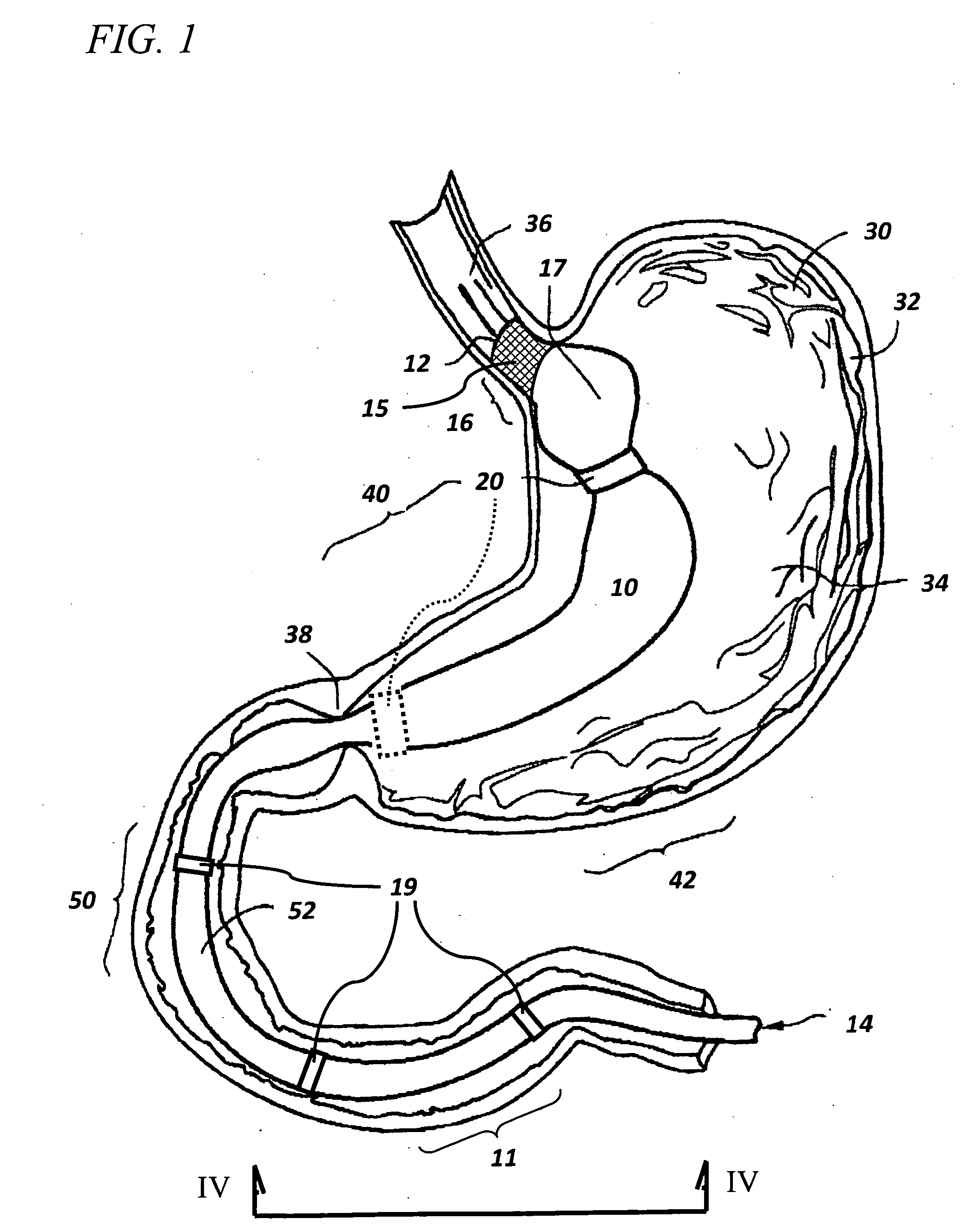
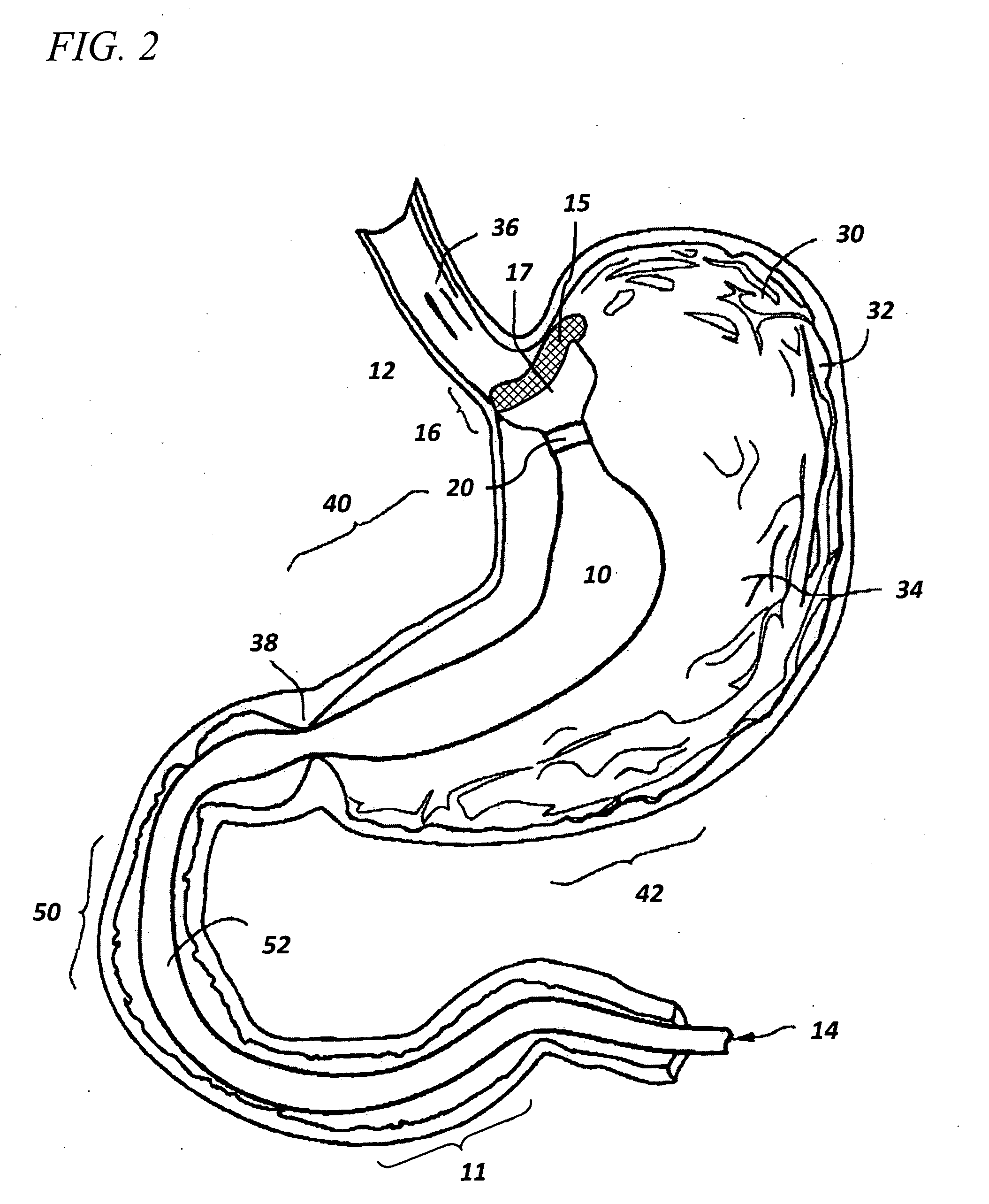
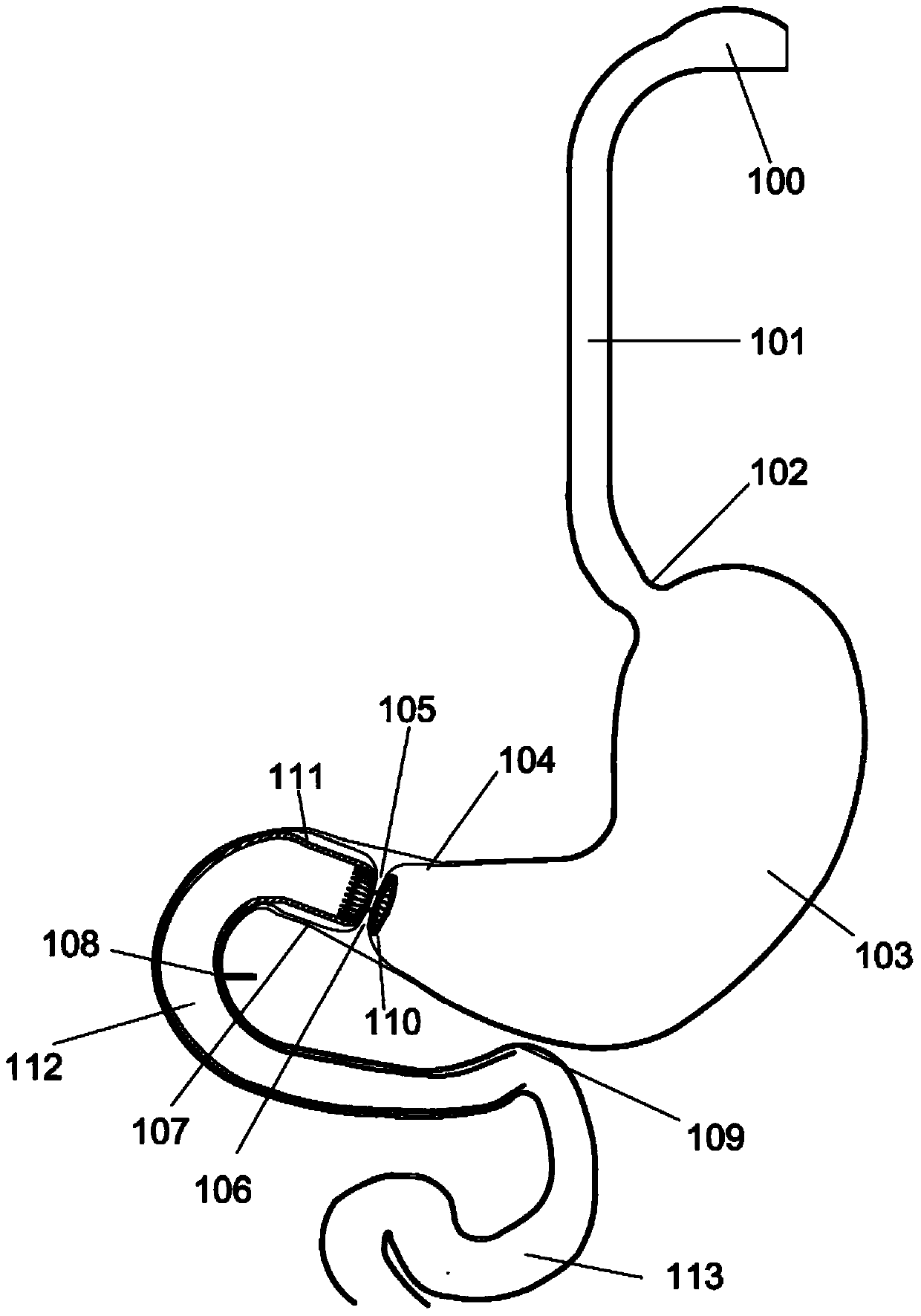
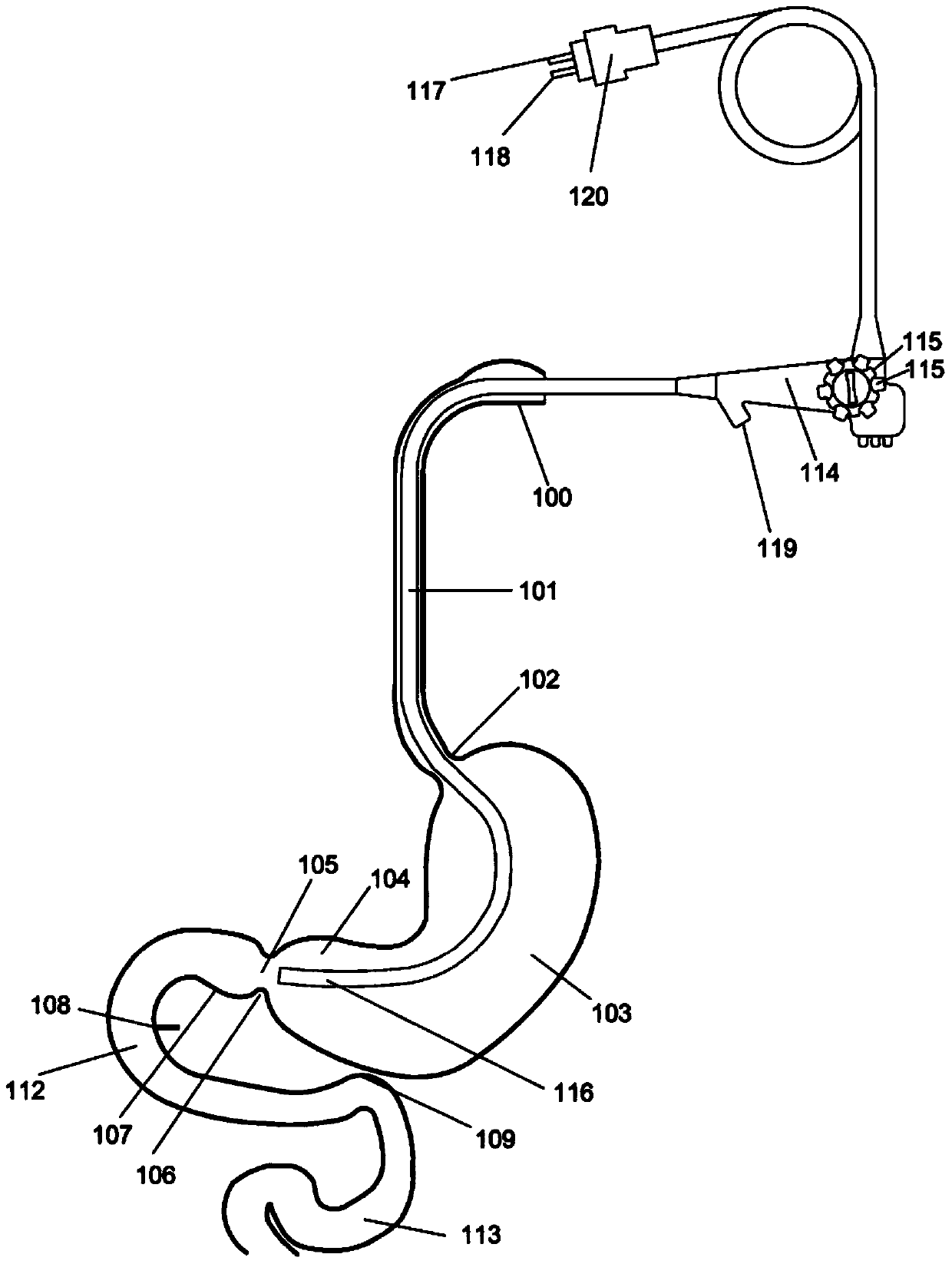
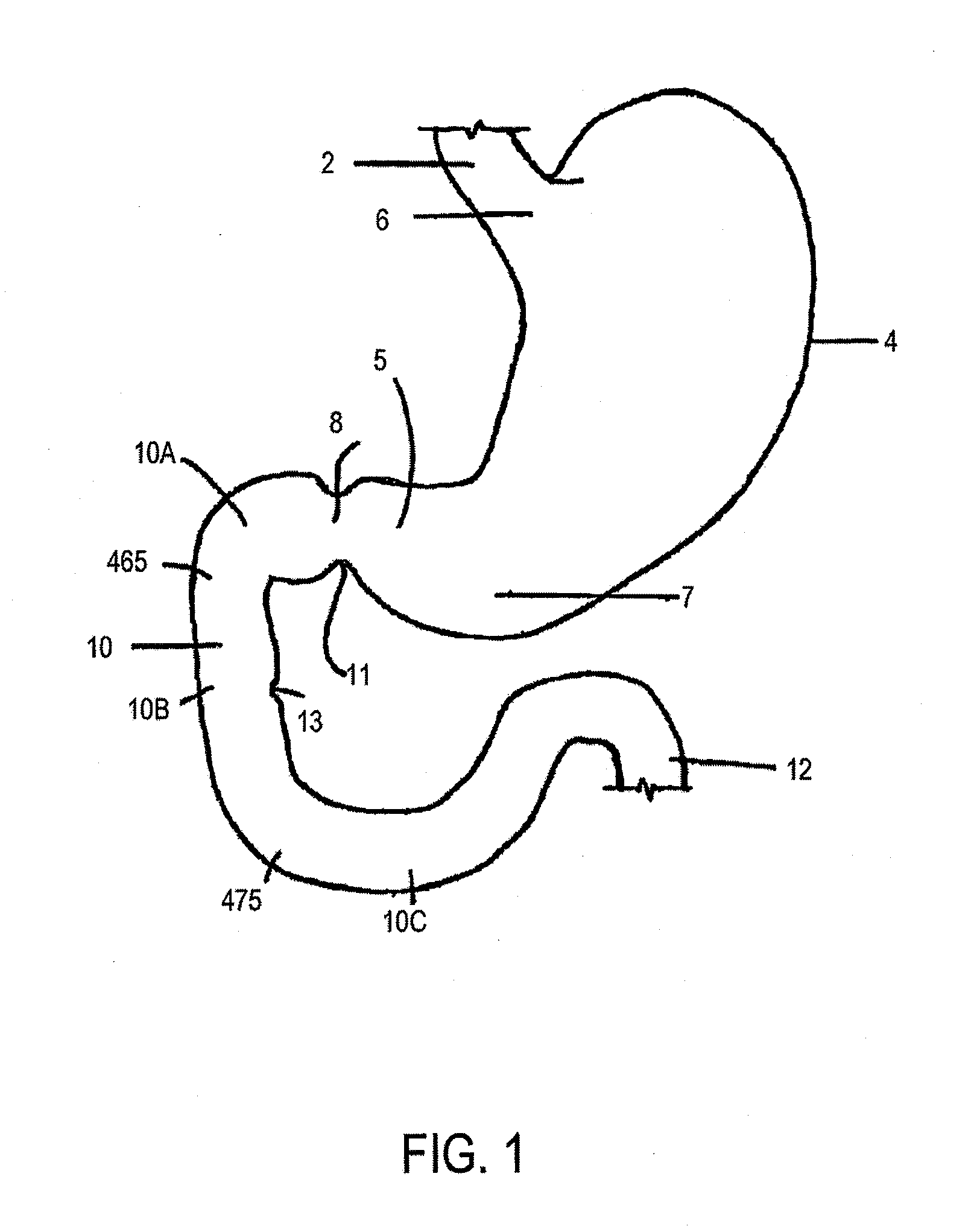
Method and apparatus for limiting absorption of food products in specific parts of the digestive system is presented. A gastrointestinal implant device is anchored in the stomach and extends beyond the ligament of Treitz. All food exiting the stomach is funneled through the device. The gastrointestinal device includes an anchor for anchoring the device to the stomach and a flexible sleeve. When implanted within the intestine, the sleeve can limit the absorption of nutrients, delay the mixing of chyme with digestive enzymes, altering hormonal triggers, providing negative feedback, and combinations thereof. The anchor is collapsible for endoscopic delivery and removal.
Owner:GI DYNAMICS
Anti-obesity devices
InactiveUS20050085923A1Controlled absorptionChange habitsSuture equipmentsStentsGastrointestinal deviceLigament structure
Method and apparatus for limiting absorption of food products in specific parts of the digestive system is presented. A gastrointestinal implant device is anchored in the stomach and extends beyond the ligament of Treitz. All food exiting the stomach is funneled through the device. The gastrointestinal device includes an anchor for anchoring the device to the stomach and a flexible sleeve to limit absorption of nutrients in the duodenum. The anchor is collapsible for endoscopic delivery and removal.
Owner:GI DYNAMICS
Anti-obesity devices
InactiveUS7122058B2Limit absorptionModify their heating habitsSuture equipmentsStentsGastrointestinal deviceImplanted device
Owner:GI DYNAMICS
Intestinal sleeve
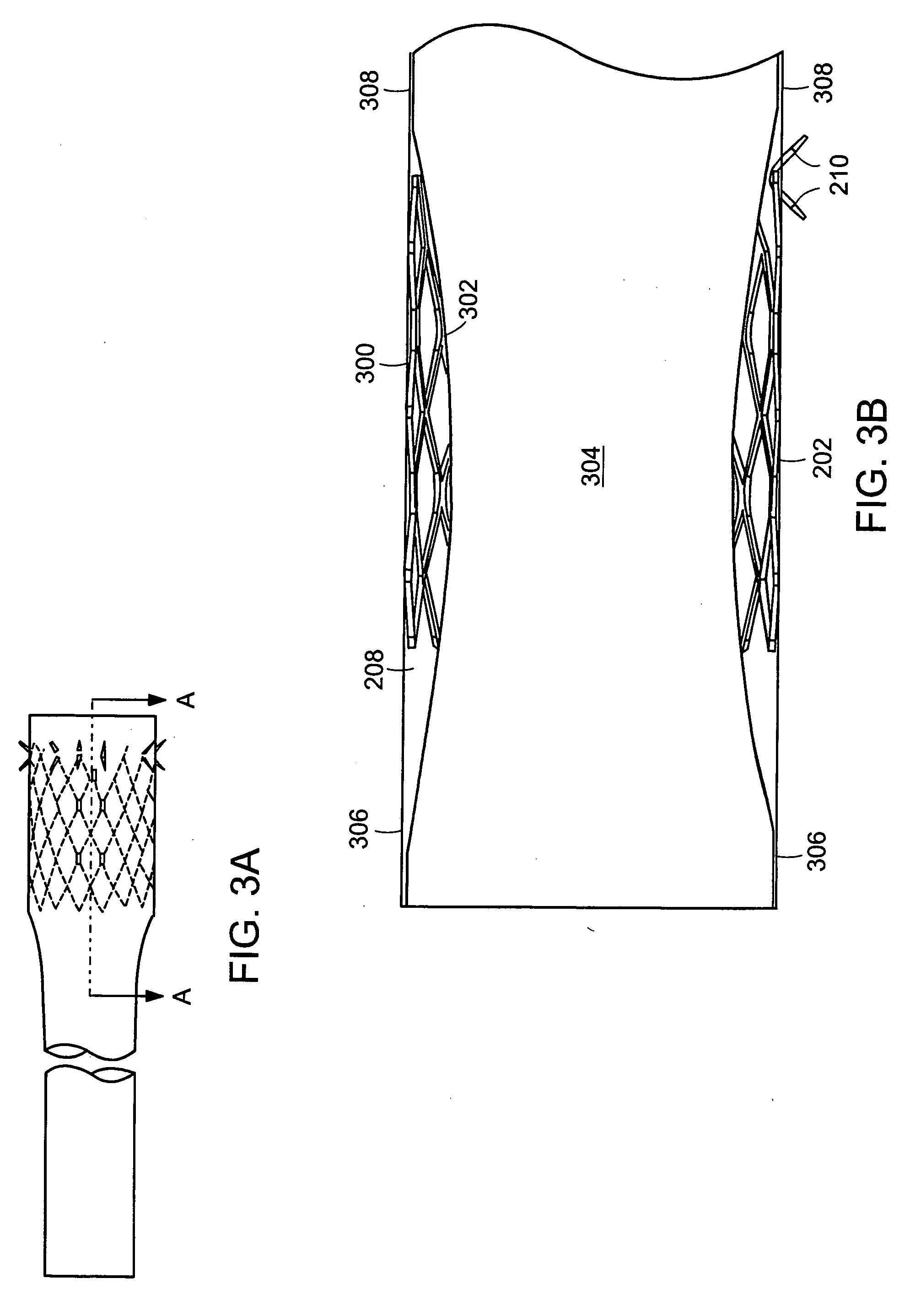
ActiveUS20050125075A1Controlled absorptionChange habitsSuture equipmentsStentsGastrointestinal deviceInsertion stent
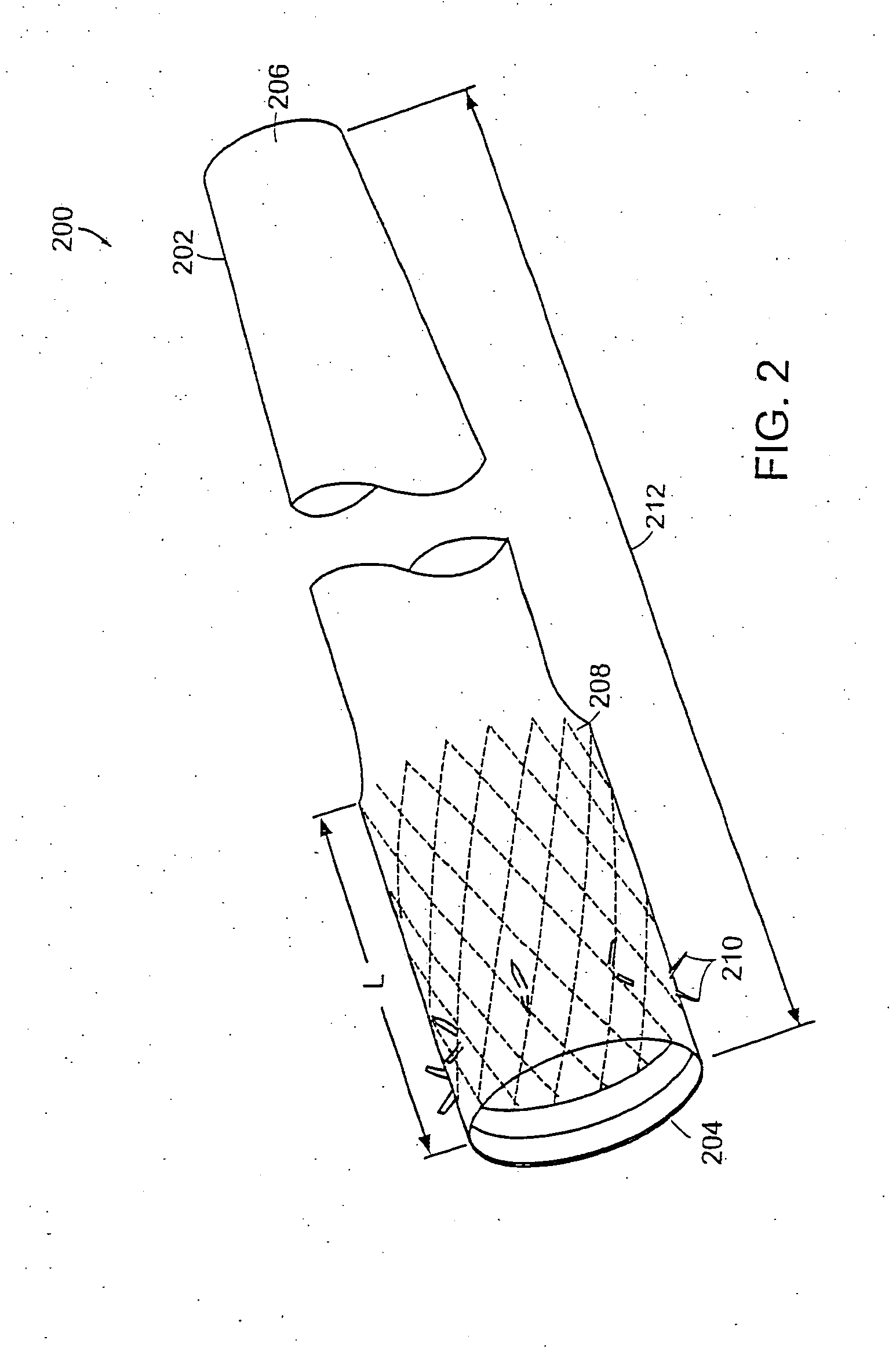
Method and apparatus for limiting absorption of food products in specific parts of the digestive system is presented. A gastrointestinal implant device is anchored in the duodenum and extends beyond the ligament of Treitz. All food exiting the stomach is funneled through the device. The gastrointestinal device includes an anchor for attaching the device to the duodenum and an unsupported flexible sleeve to limit absorption of nutrients in the duodenum. The anchor can include a stent and / or a wave anchor and is collapsible for catheter-based delivery and removal.
Owner:GI DYNAMICS
Gastrointestinal implant system
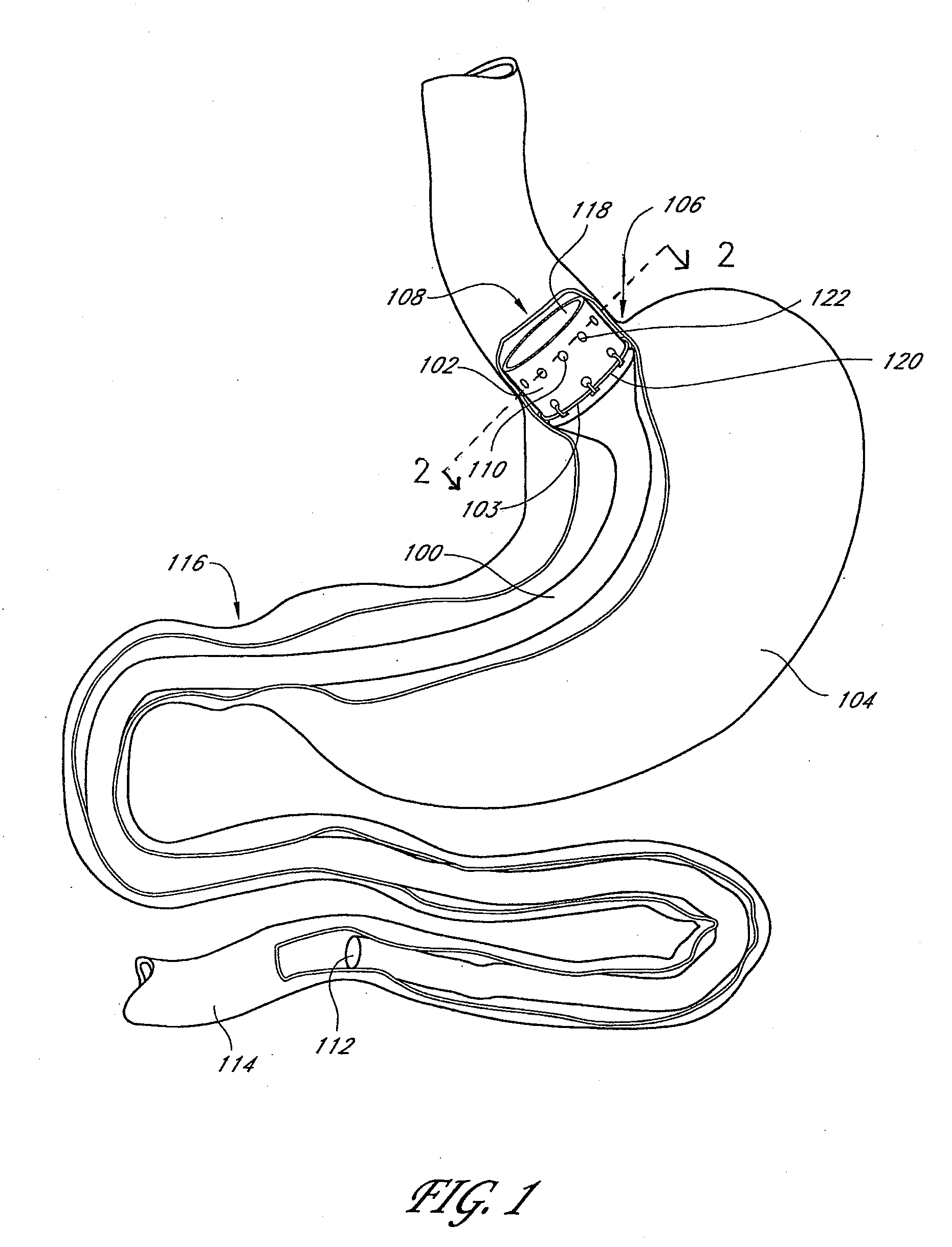
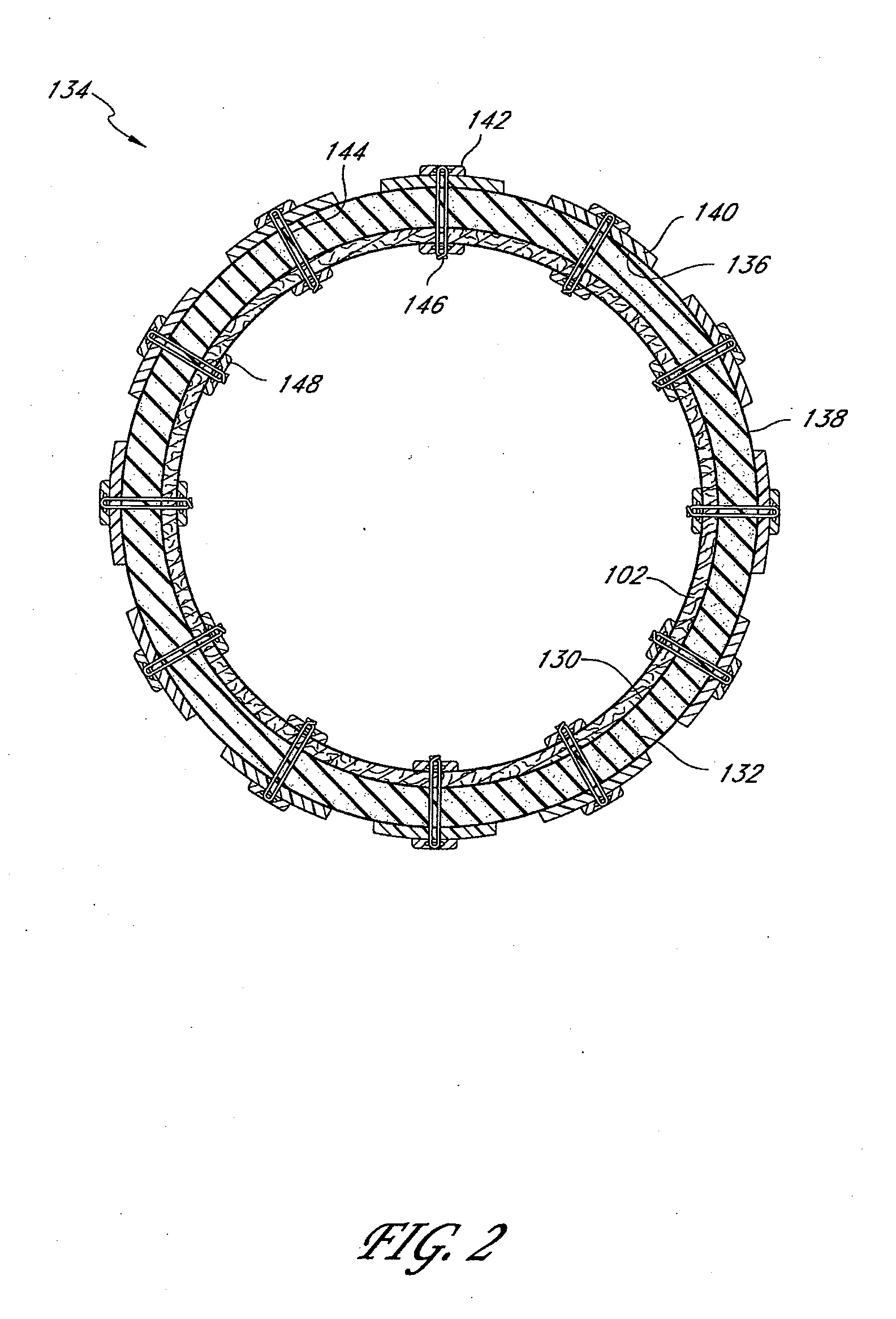
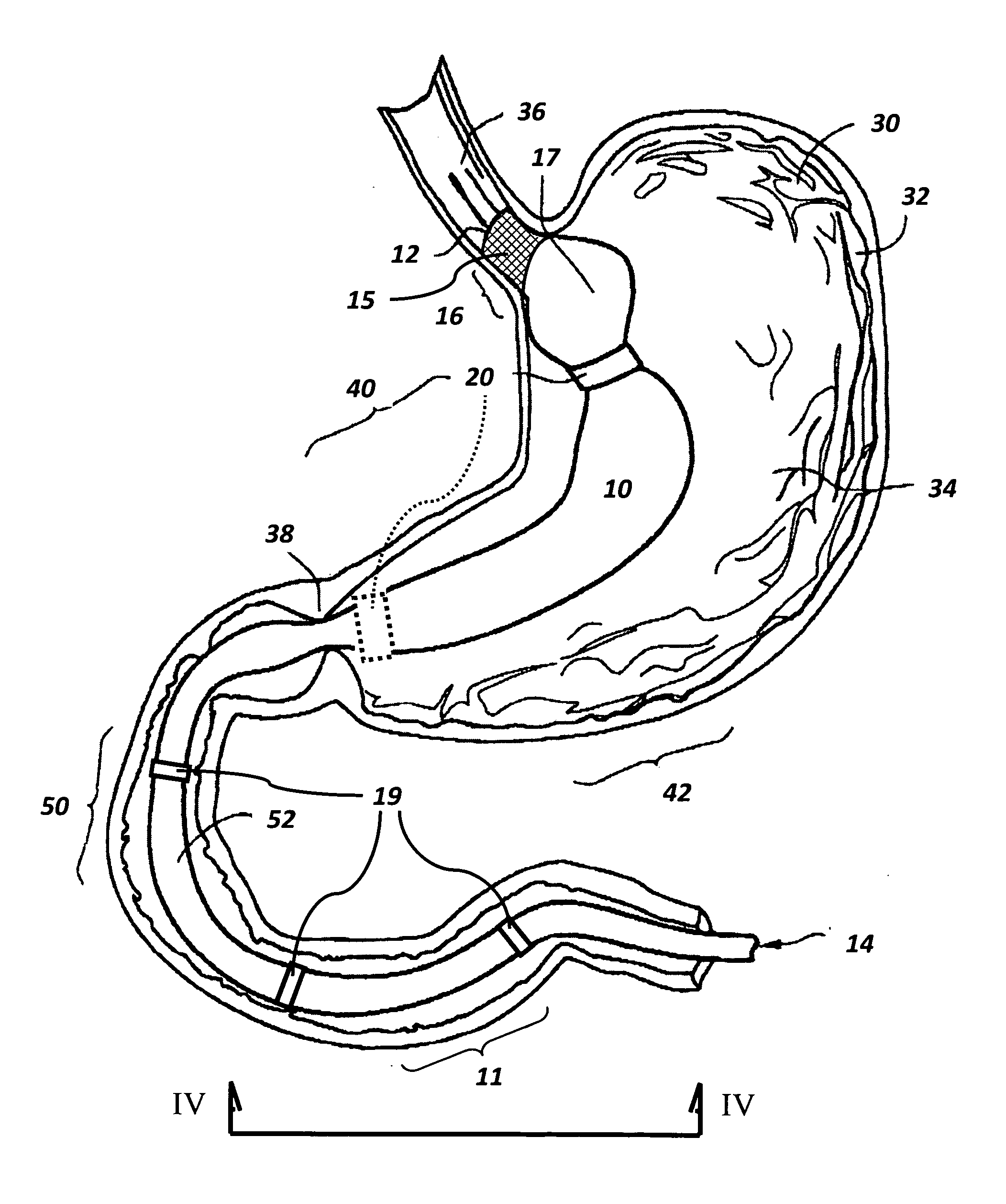
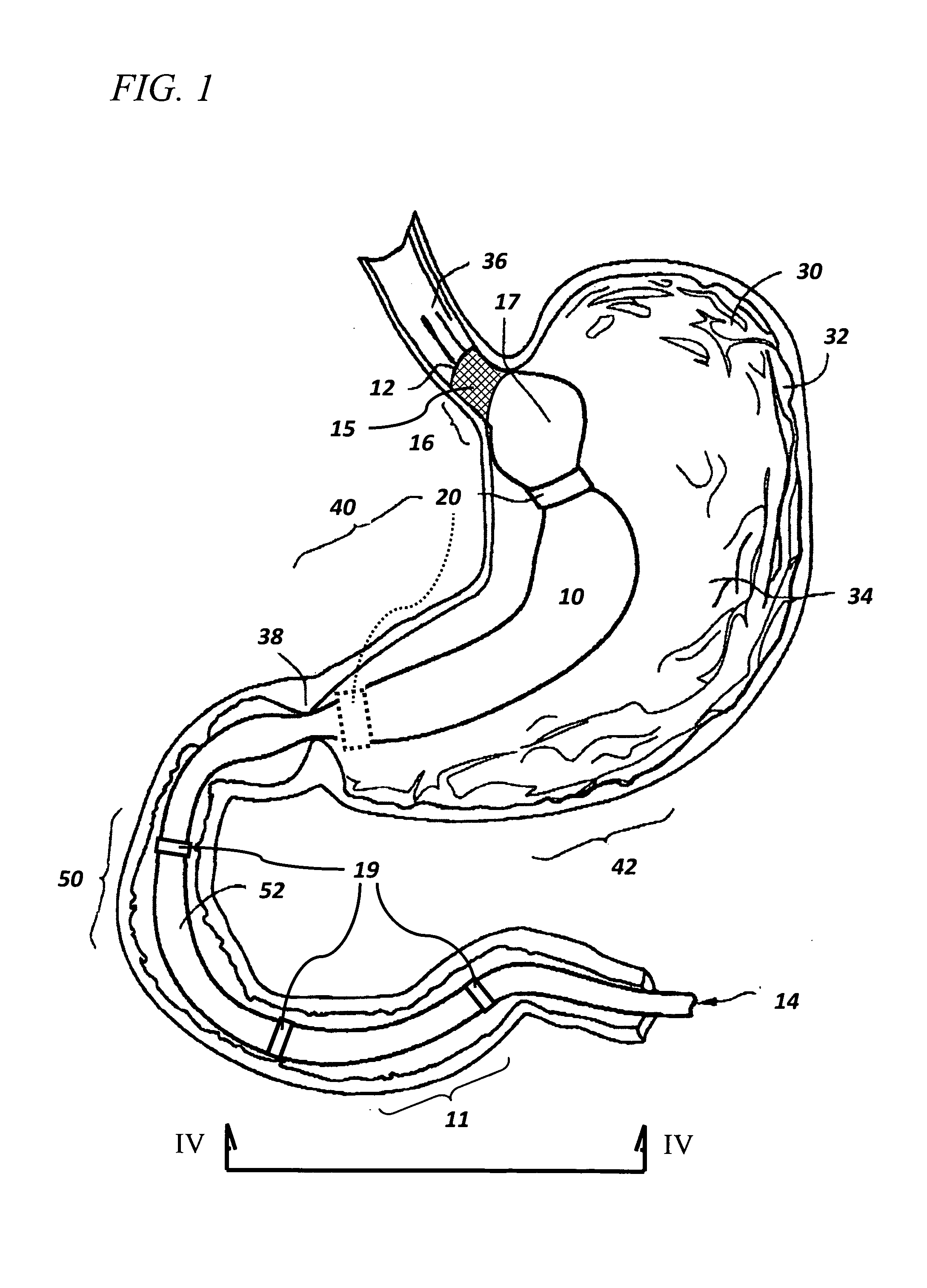
The present invention provides devices and methods for attachment of an endolumenal gastrointestinal device, such as an artificial stoma device, a gastrointestinal bypass sleeve or other therapeutic or diagnostic device, within a patient's digestive tract. In one application of the invention, an endolumenal bypass sleeve is removeably attached in the vicinity of the gastroesophageal junction to treat obesity and / or its comorbidities, such as diabetes. The bypass sleeve may be at least partially deployed by eversion.
Owner:DANN MITCHELL +4
Bariatric sleeve
InactiveUS20050075622A1Reduce twistDelayed bucklingSuture equipmentsStentsGastrointestinal deviceLigament structure
Owner:GI DYNAMICS
Bariatric sleeve removal devices
Method and apparatus for limiting absorption of food products in specific parts of the digestive system is presented. A gastrointestinal implant device is anchored in the stomach and extends beyond the ligament of Treitz. All food exiting the stomach is funneled through the device. The gastrointestinal device includes an anchor for anchoring the device to the stomach and a flexible sleeve to limit absorption of nutrients in the duodenum. The anchor is collapsible for endoscopic delivery and removal.
Owner:GI DYNAMICS
Methods of treatment using a bariatric sleeve
InactiveUS20050080395A1Reduce twisting and bucklingReduce penetrationSuture equipmentsStentsGastrointestinal deviceLigament structure
Method and apparatus for limiting absorption of food products in specific parts of the digestive system is presented. A gastrointestinal implant device is anchored in the stomach and extends beyond the ligament of Treitz. All food exiting the stomach is funneled through the device. The gastrointestinal device includes an anchor for anchoring the device to the stomach and a flexible sleeve to limit absorption of nutrients in the duodenum. The anchor is collapsible for endoscopic delivery and removal.
Owner:GI DYNAMICS
Bariatric sleeve delivery devices
Method and apparatus for limiting absorption of food products in specific parts of the digestive system is presented. A gastrointestinal implant device is anchored in the stomach and extends beyond the ligament of Treitz. All food exiting the stomach is funneled through the device. The gastrointestinal device includes an anchor for anchoring the device to the stomach and a flexible sleeve to limit absorption of nutrients in the duodenum. The anchor is collapsible for endoscopic delivery and removal.
Owner:GI DYNAMICS
Intestinal sleeve
InactiveUS20060265082A1Reduce twisting and bucklingMinimize traumaSuture equipmentsStentsGastrointestinal deviceInsertion stent
A gastrointestinal implant device is anchored in the duodenum and extends beyond the ligament of Treitz. All food exiting the stomach is funneled through the device. The gastrointestinal device includes an anchor for attaching the device to the duodenum and an unsupported flexible sleeve. The anchor can include a stent and / or a wave anchor and is collapsible for catheter-based delivery and removal.
Owner:GI DYNAMICS
Everting gastrointestinal sleeve
The present invention provides devices and methods for attachment of an endolumenal gastrointestinal device, such as an artificial stoma device, a gastrointestinal bypass sleeve or other therapeutic or diagnostic device, within a patient's digestive tract. In one application of the invention, an endolumenal bypass sleeve is removeably attached in the vicinity of the gastroesophageal junction to treat obesity and / or its comorbidities, such as diabetes. The bypass sleeve may be at least partially deployed by eversion.
Owner:DANN MITCHELL +4
Attachment cuff for gastrointestinal implant
The present invention provides devices and methods for attachment of an endolumenal gastrointestinal device, such as an artificial stoma device, a gastrointestinal bypass sleeve or other therapeutic or diagnostic device, within a patient's digestive tract. In one application of the invention, an endolumenal bypass sleeve is removeably attached in the vicinity of the gastroesophageal junction to treat obesity and / or its comorbidities, such as diabetes. The bypass sleeve may be at least partially deployed by eversion.
Owner:VALENTX
Devices and methods for endolumenal gastrointestinal bypass
The present invention provides devices and methods for attachment of an endolumenal gastrointestinal device, such as an artificial stoma device, a gastrointestinal bypass sleeve or other therapeutic or diagnostic device, within a patient's digestive tract. In one application of the invention, an endolumenal bypass sleeve is removeably attached in the vicinity of the gastroesophageal junction to treat obesity and / or its comorbidities, such as diabetes. The bypass sleeve may be at least partially deployed by eversion.
Owner:VALENTX
Gastro-intestinal device and method for treating addiction
ActiveUS20070178160A1Prevents patient tamperingReduce appetitePowder deliveryElectrotherapyGastrointestinal deviceHazardous substance
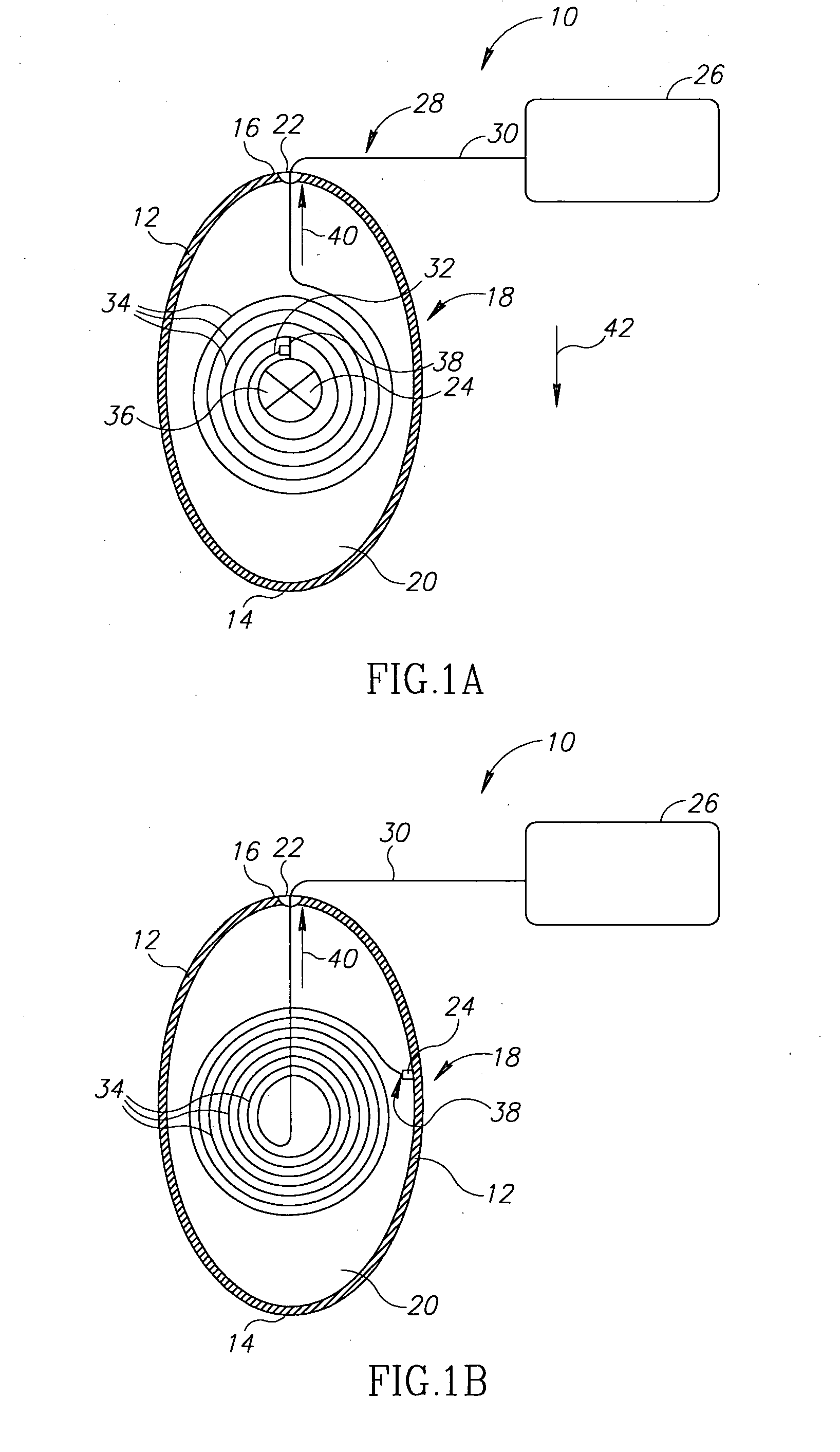
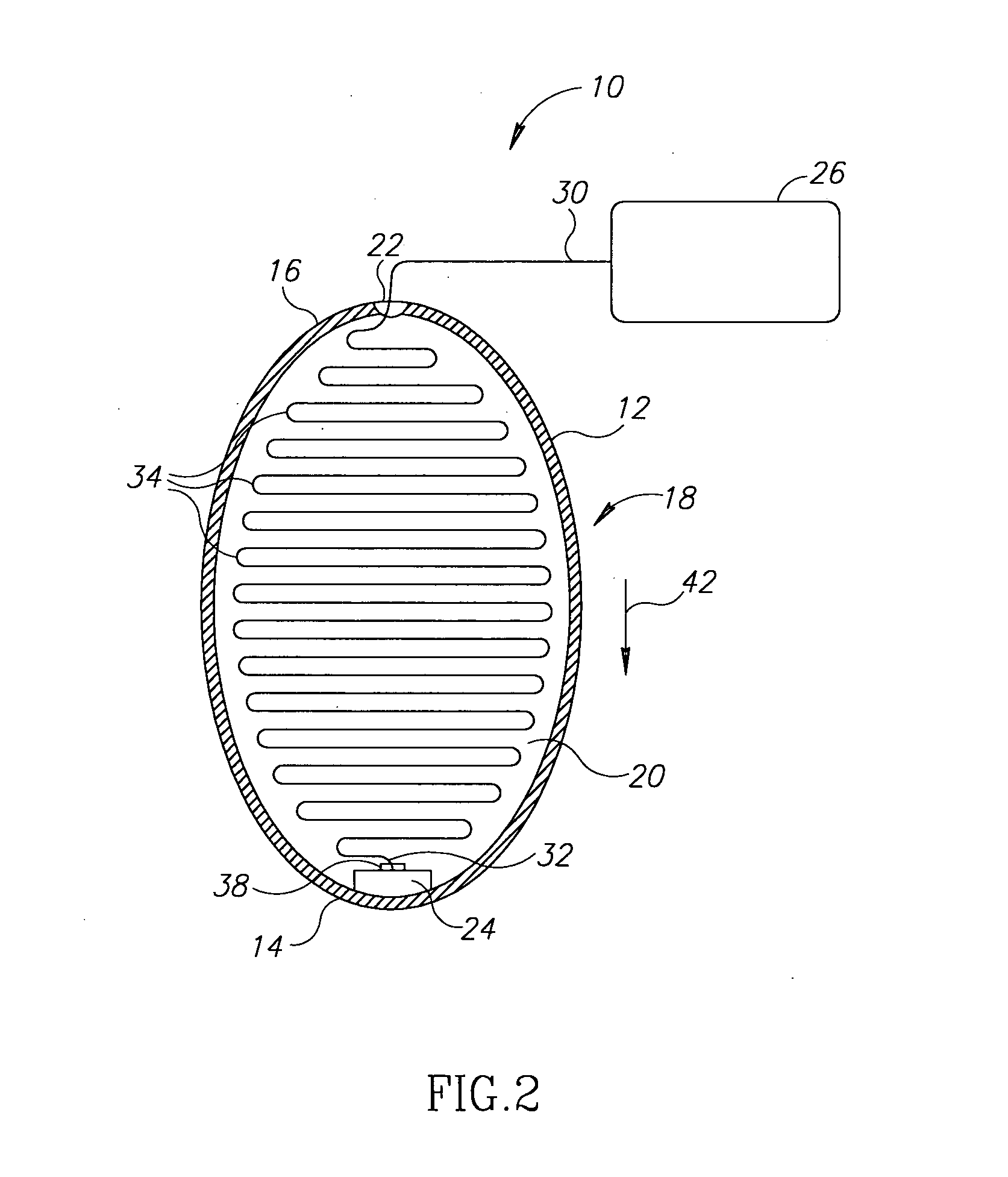
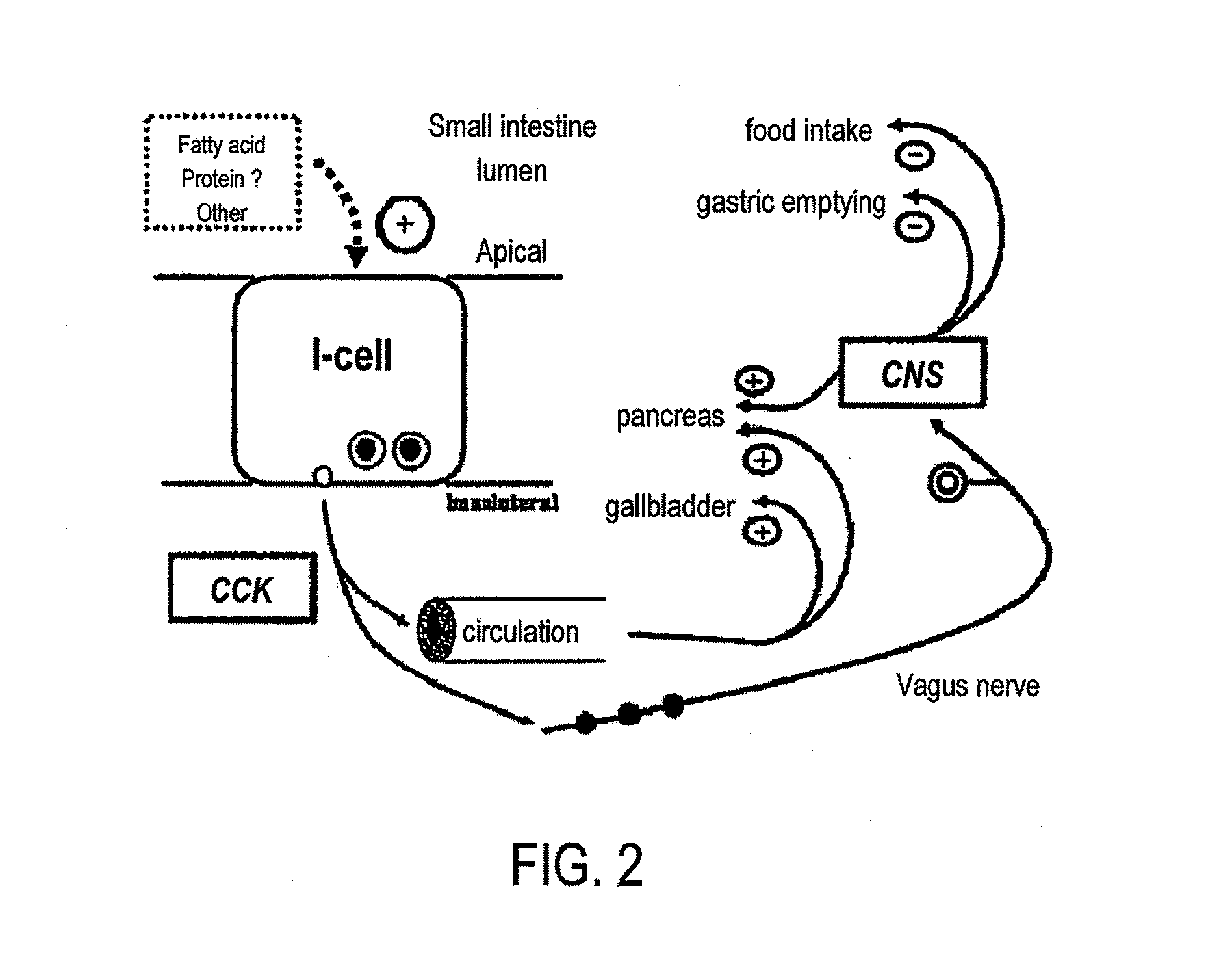
A device and a method for treating a medical condition include a reversible member disposed in a patient's gastro-intestinal tract, and a dispensing member coupled to the reversible member that delivers a drug and / or a noxious when a predetermined substance is detected. In a different embodiment, the device and method of the present invention include a polymer infused with a drug and disposed into a preformed shell inside the gastric space, where it expands and hardens, releasing the drug over time. Both the casing and the polymer may be biocompatible. The present invention enables the slow-release of anti-addictive agents without patient tampering and with the appropriate dosage. Ancillary systems such as sensors, actuators, refill and recharge ports, and communication and data processing units may also be included.
Owner:BARONOVA
Cuff and sleeve system for gastrointestinal bypass
Owner:VALENTX
Devices and methods for endolumenal therapy
InactiveUS20090093767A1Promoting tissue in-growthAltering abilityDiagnosticsSurgeryGastrointestinal deviceLower esophagus
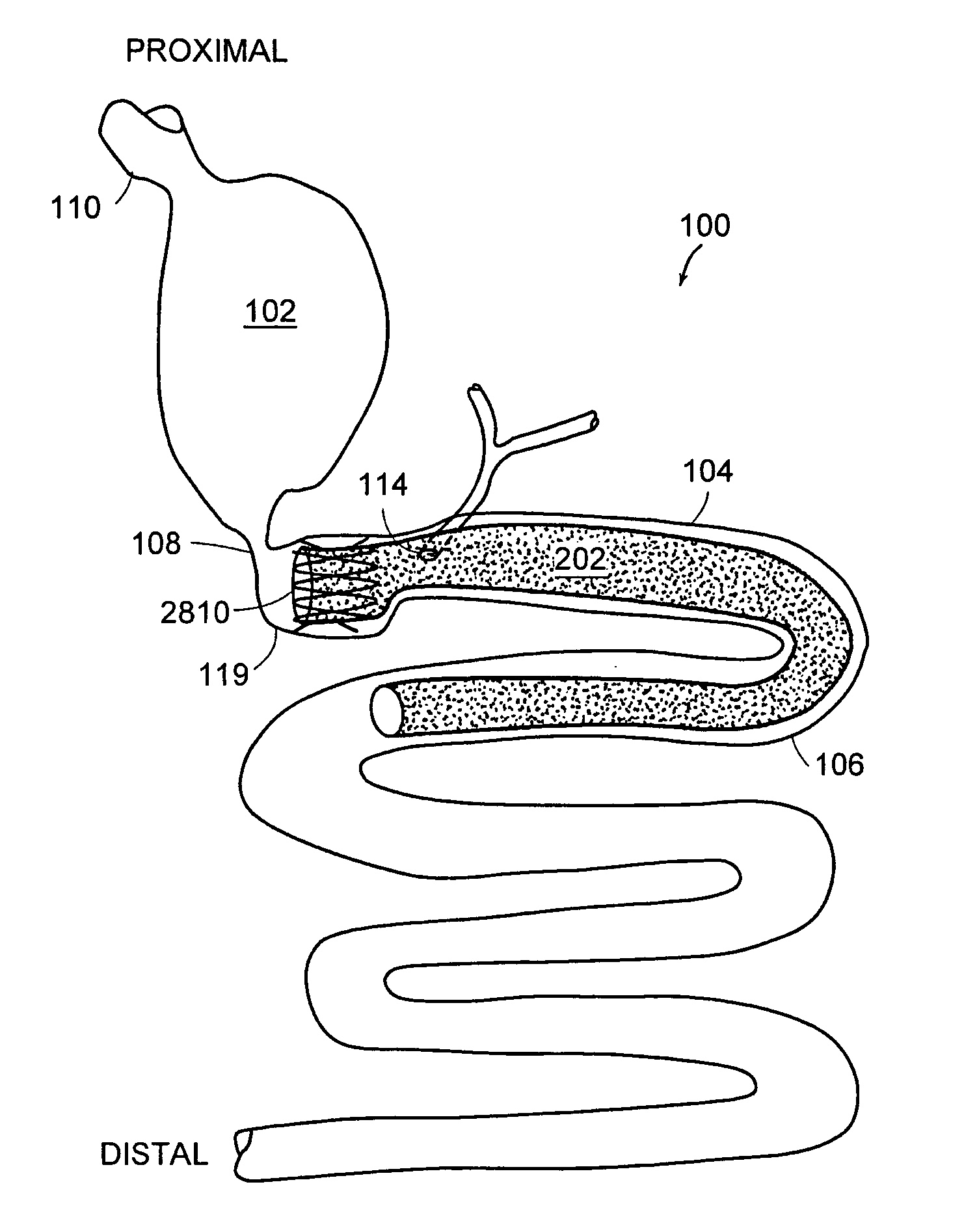
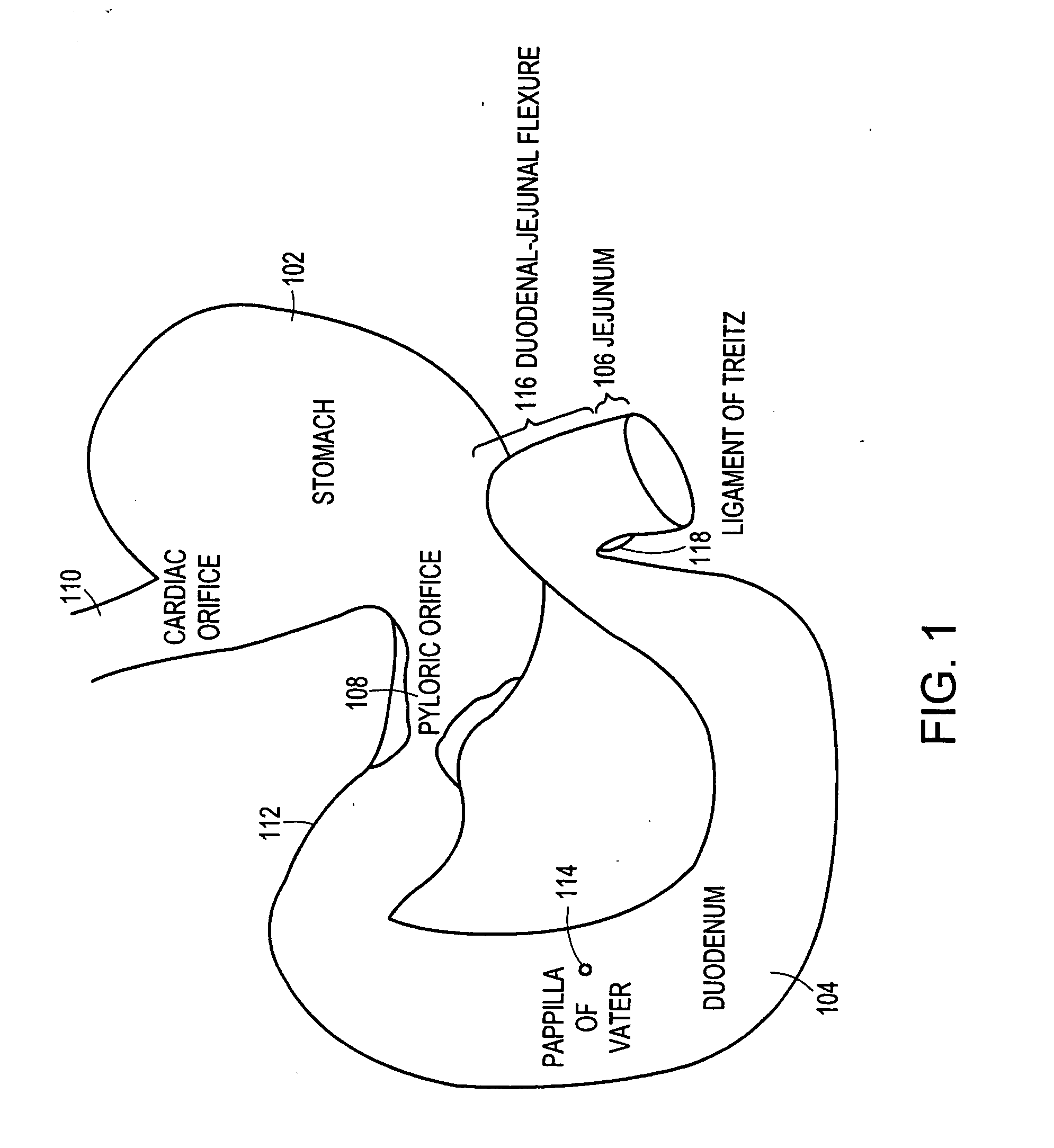
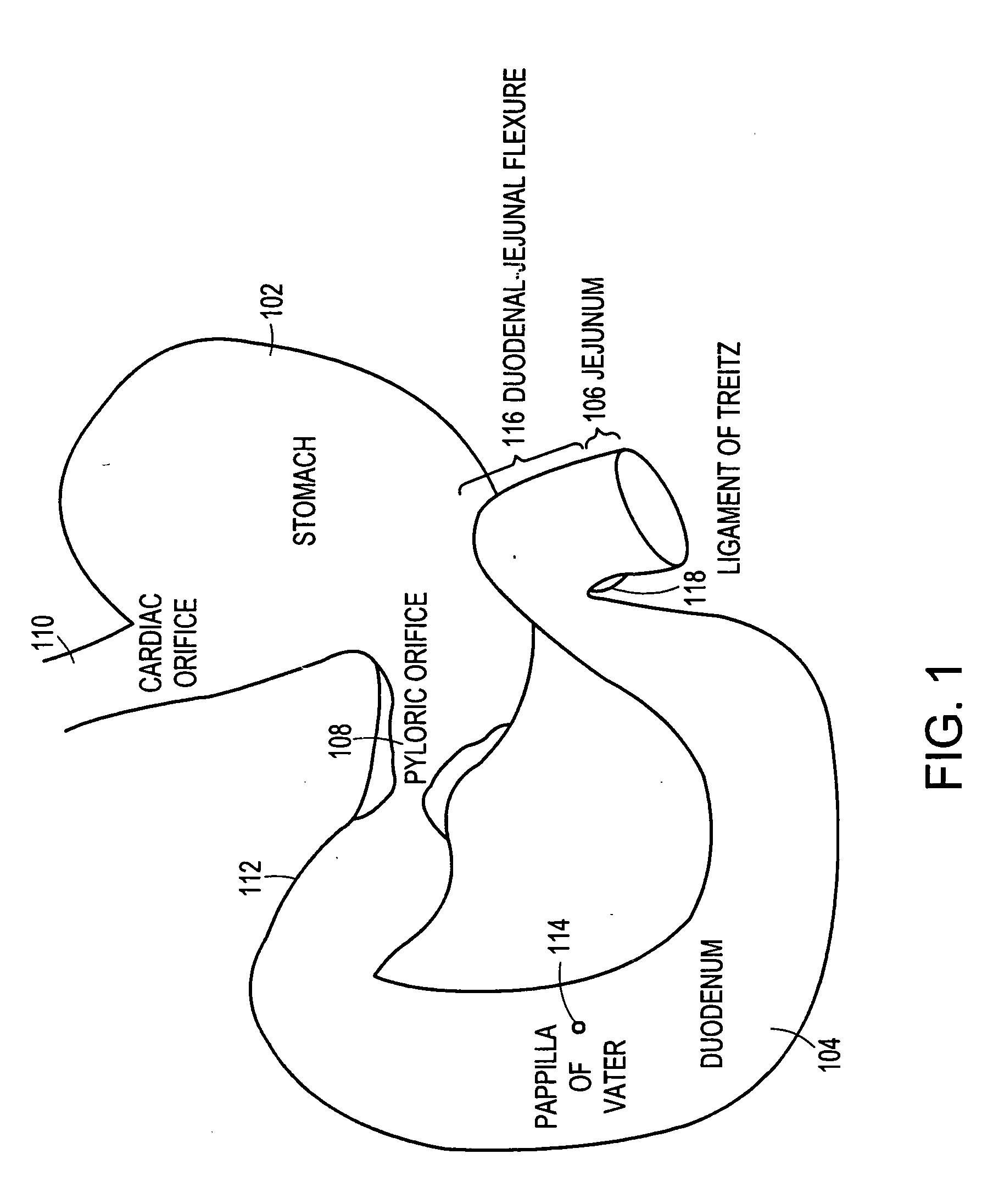
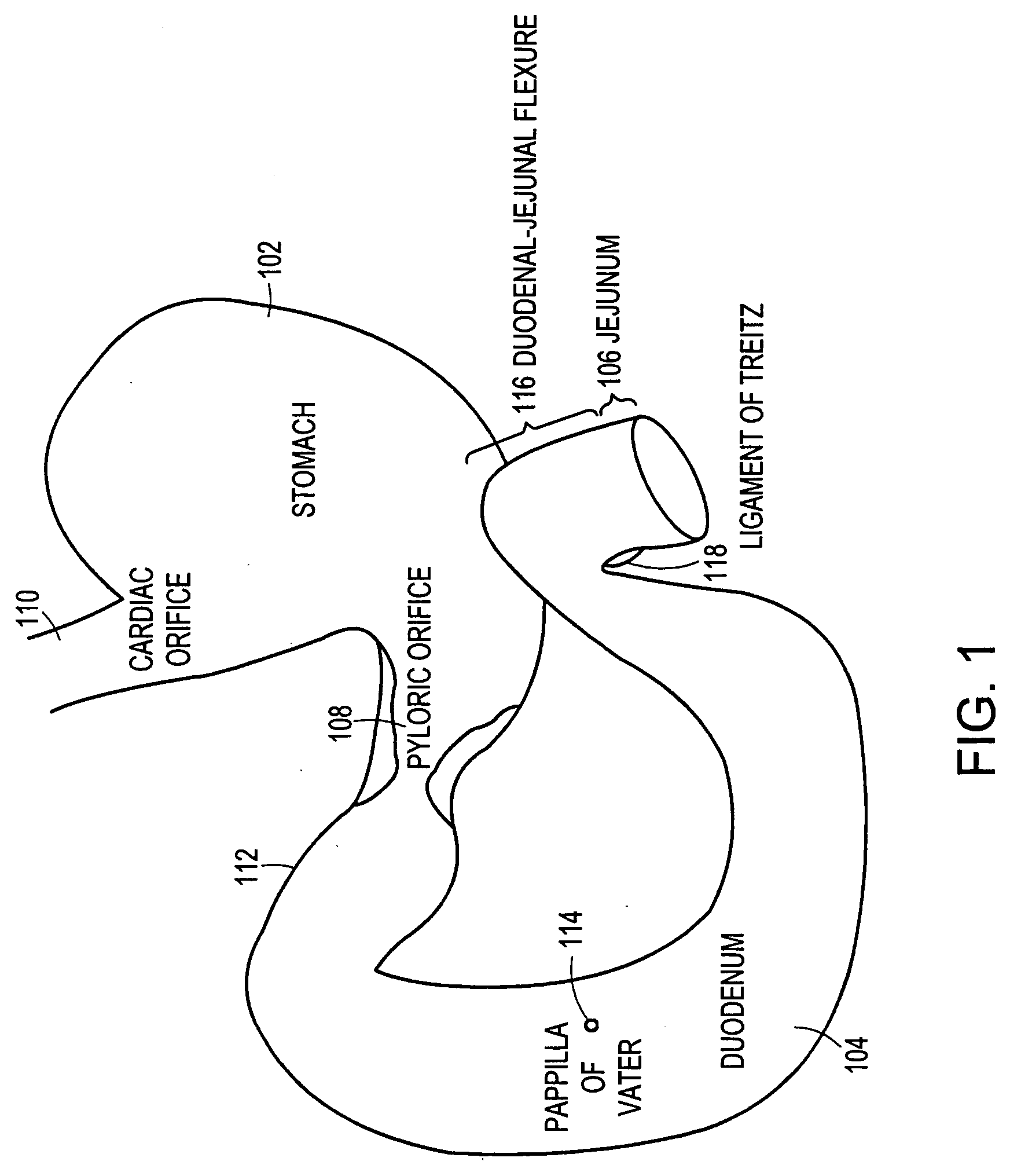
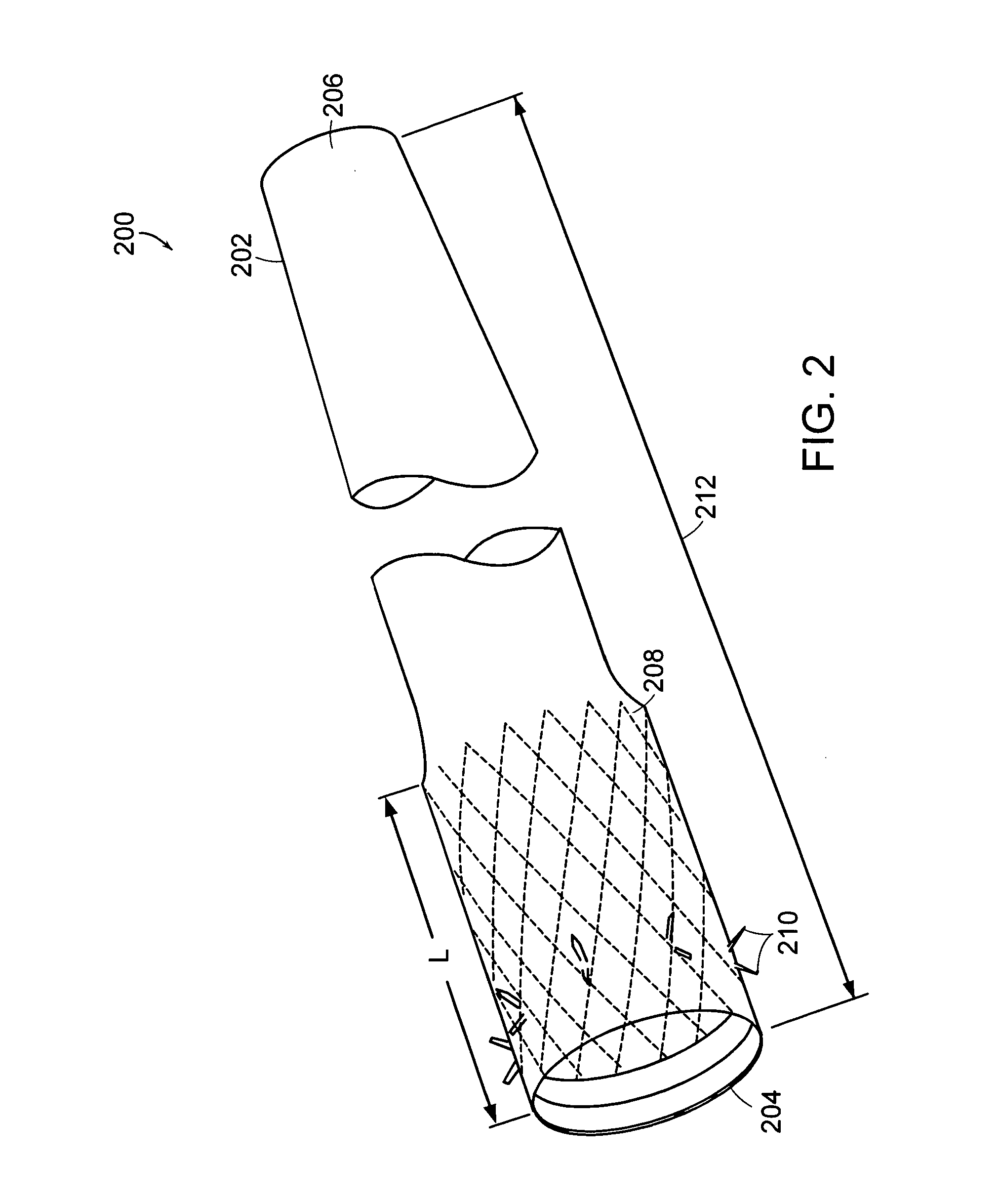
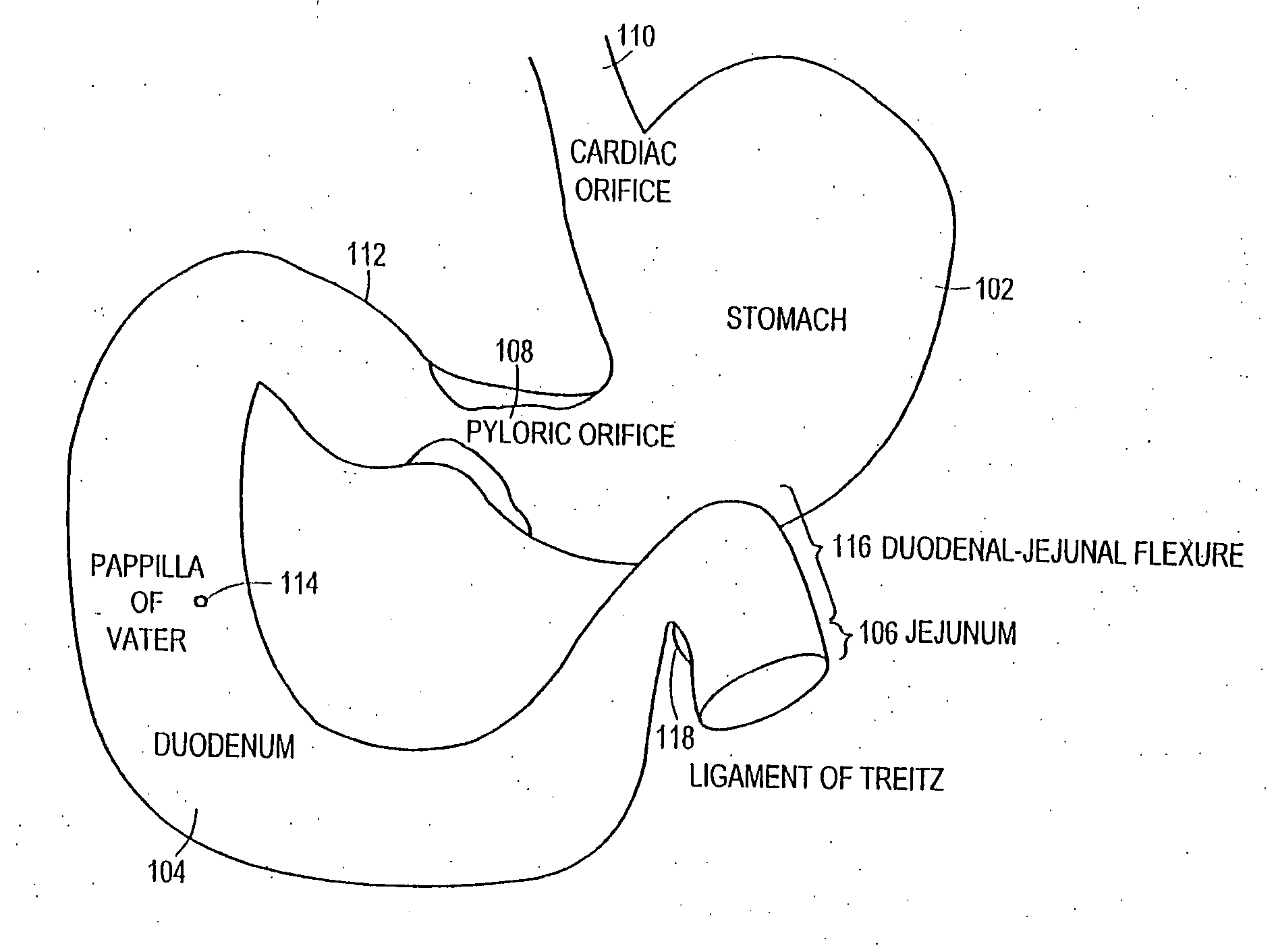
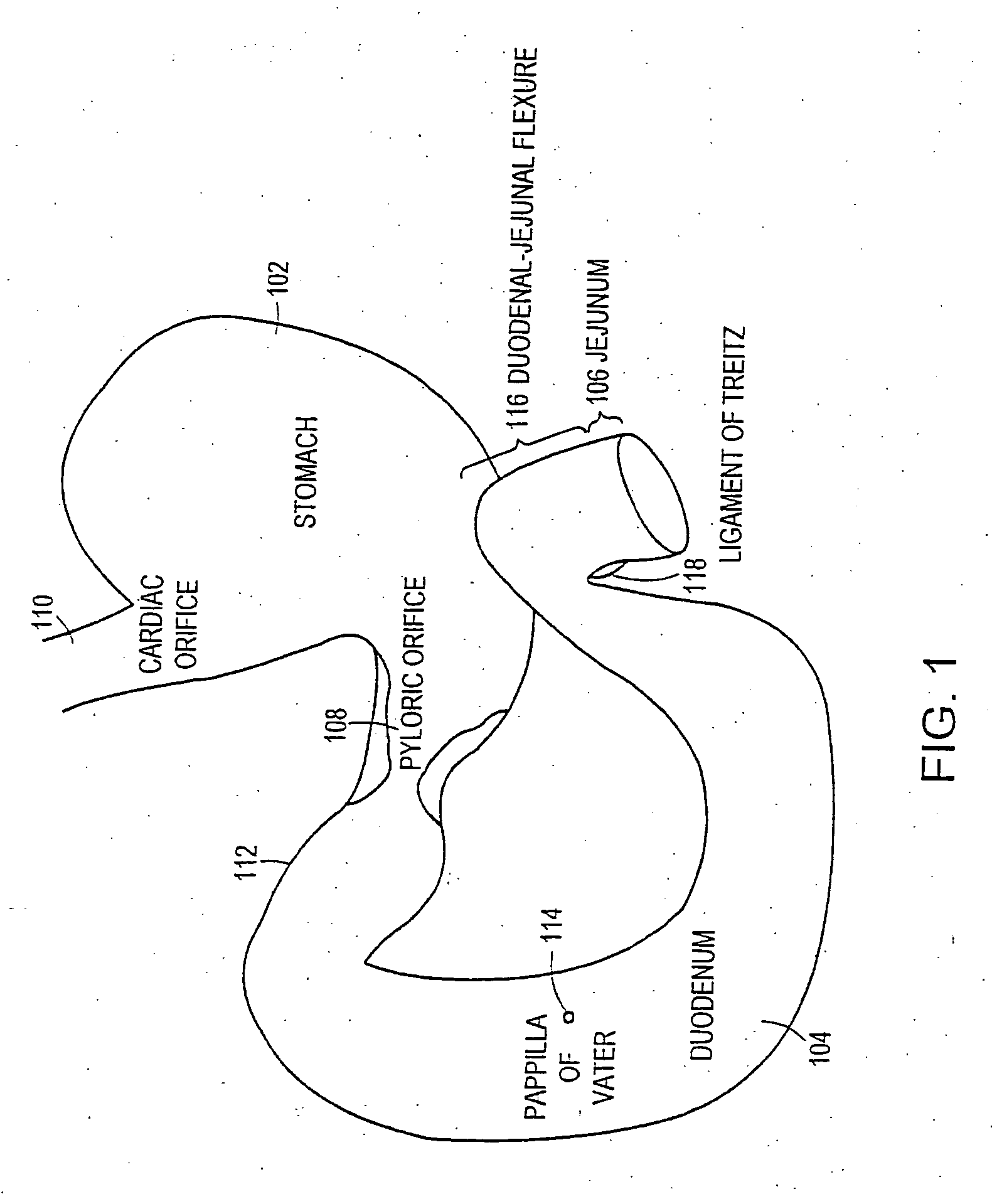
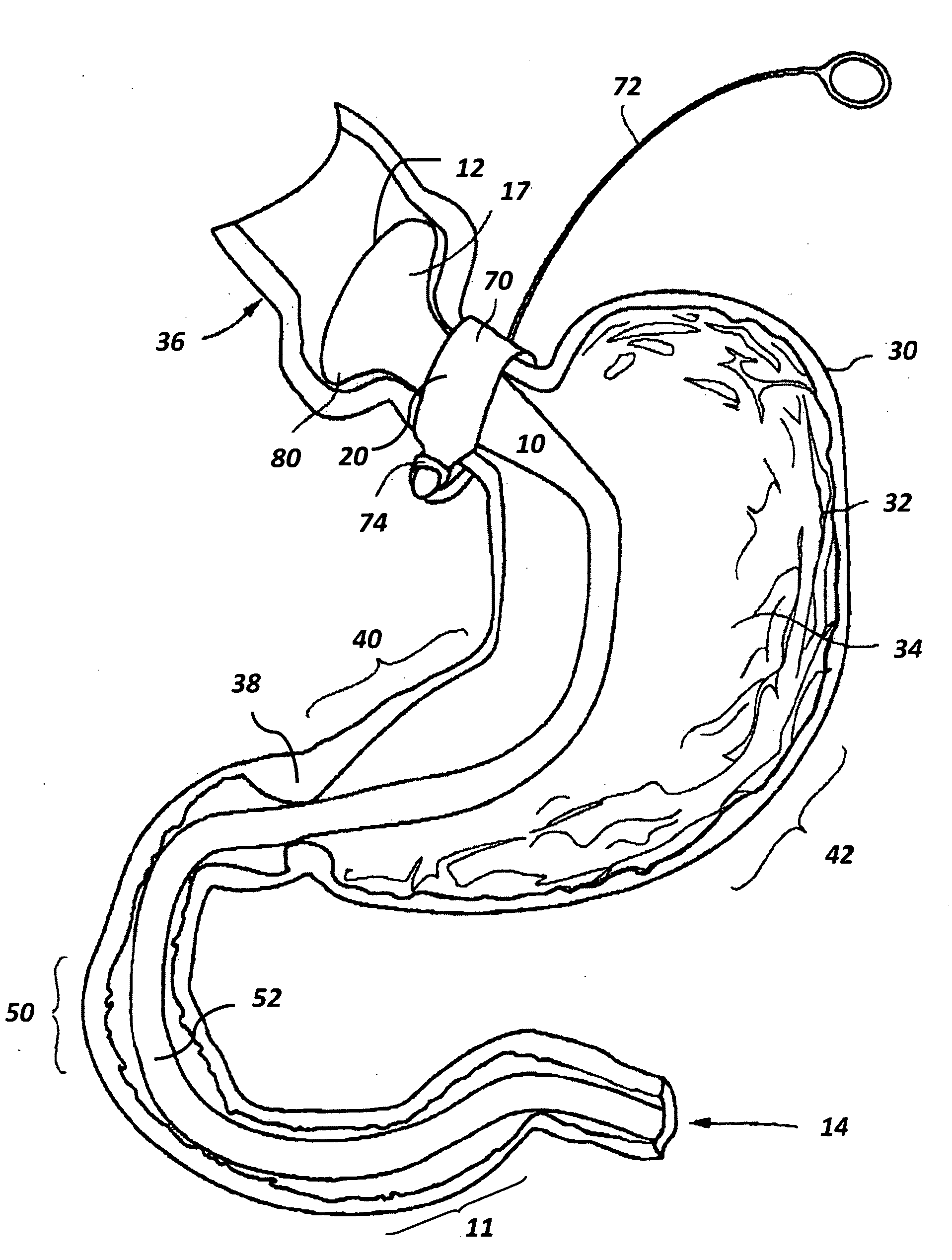
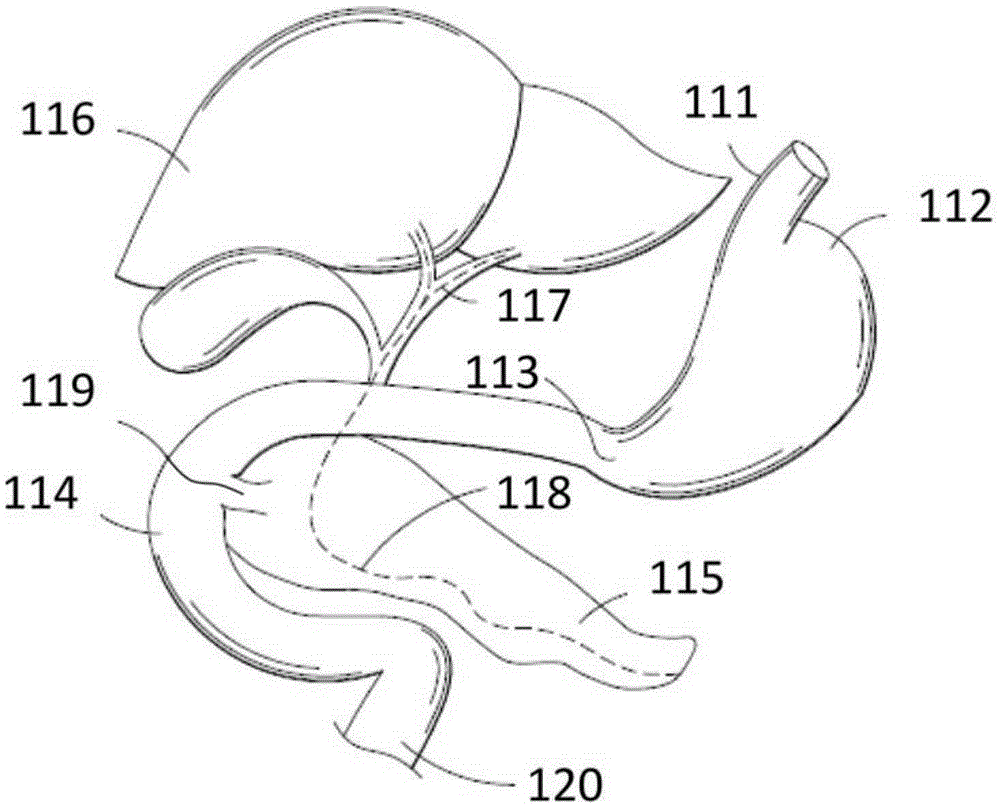
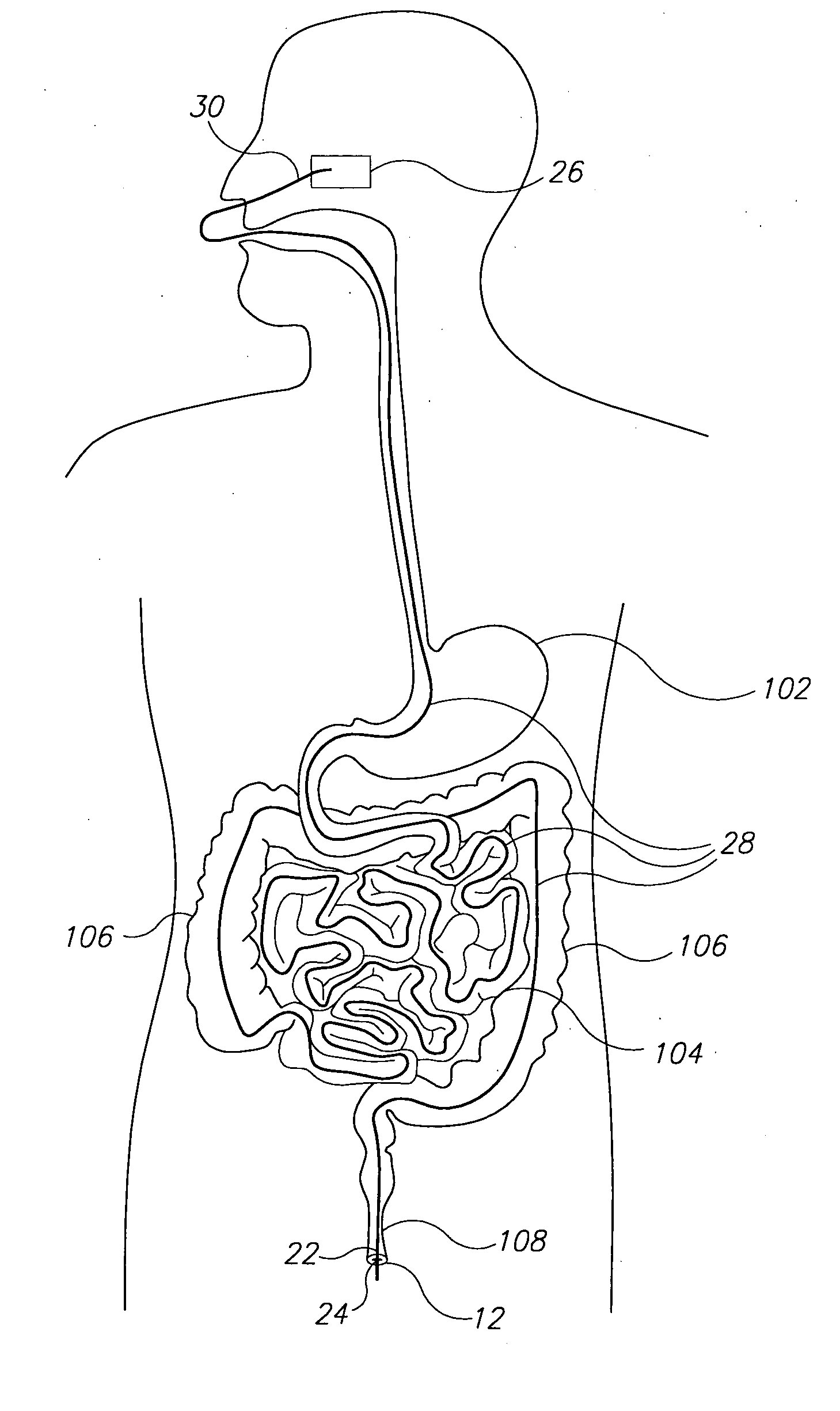
The present invention is directed generically to a means for altering the ability of the mammalian body to absorb nutritive content from ingested foodstuffs, and more specifically to an apparatus and method of use for an endolumenal sleeve (referred to also as an “intragastrointestinal device” or “gastrointestinal device”) positioned in the mammalian gastrointestinal (GI) tract. A suitable endolumenal sleeve is comprised of an anchor element and an opening at a proximal end, an elongate lumen or hollow open-ended tube having a transverse dimension, and a distal orifice. Optionally, an exterior aspect of the elongate lumen may include additional modes of attachment to the tissues walls of the GI tract through the use of one or more means for promoting tissue in-growth. The endolumenal sleeve is retained in the GI tract such that a substantial fraction of the food and liquids passing through the GI tract is channeled into the proximal opening and through an interlumenal space defined within the interior space of the endolumenal sleeve. Within the endolumenal sleeve there may be one or more restrictive means to constrain, impede or otherwise control the operative flow of material through the device. An individual restrictive means can either be of a fixed geometry or such means may include one or more elements which are adjustable in nature or function. The elongate lumen of the endolumenal sleeve is formed of a polymer composition suitable for controlled ingress of biological secretions, egress of certain selected nutritional elements, and may comprise either a single tubular structure or a multi-section (i.e. articulated and / or multiple lumen) assembly. When the endolumenal sleeve is in situ within the mammalian gastro-intestinal system, ingested foodstuffs are conveyed from the proximal end to said distal orifice. In typical applications, the proximal end of the endolumenal sleeve is positioned within the physiological region extending from the lower esophagus to the duodenum and the distal orifice is positioned within the physiological region extending from the upper duodenum to the lower jejunum, though further extension into the lower intestine is possible. Through proper selection of position for the endolumenal sleeve proximal and distal ends, combined by selection of the composition used in the fabrication of the elongate lumen, it is possible to finitely control the degree of nutritive absorption performed by the gastrointestinal tract.
Owner:KELLEHER BRIAN
Bariatric sleeve
InactiveUS20090240340A1Limit absorptionReducing hormone triggersSuture equipmentsStentsGastrointestinal deviceIntestinal structure
Method and apparatus for limiting absorption of food products in specific parts of the digestive system is presented. A gastrointestinal implant device is anchored in the stomach and extends beyond the ligament of Treitz. All food exiting the stomach is funneled through the device. The gastrointestinal device includes an anchor for anchoring the device to the stomach and a flexible sleeve. When implanted within the intestine, the sleeve can limit the absorption of nutrients, delay the mixing of chyme with digestive enzymes, altering hormonal triggers, providing negative feedback, and combinations thereof. The anchor is collapsible for endoscopic delivery and removal.
Owner:GI DYNAMICS
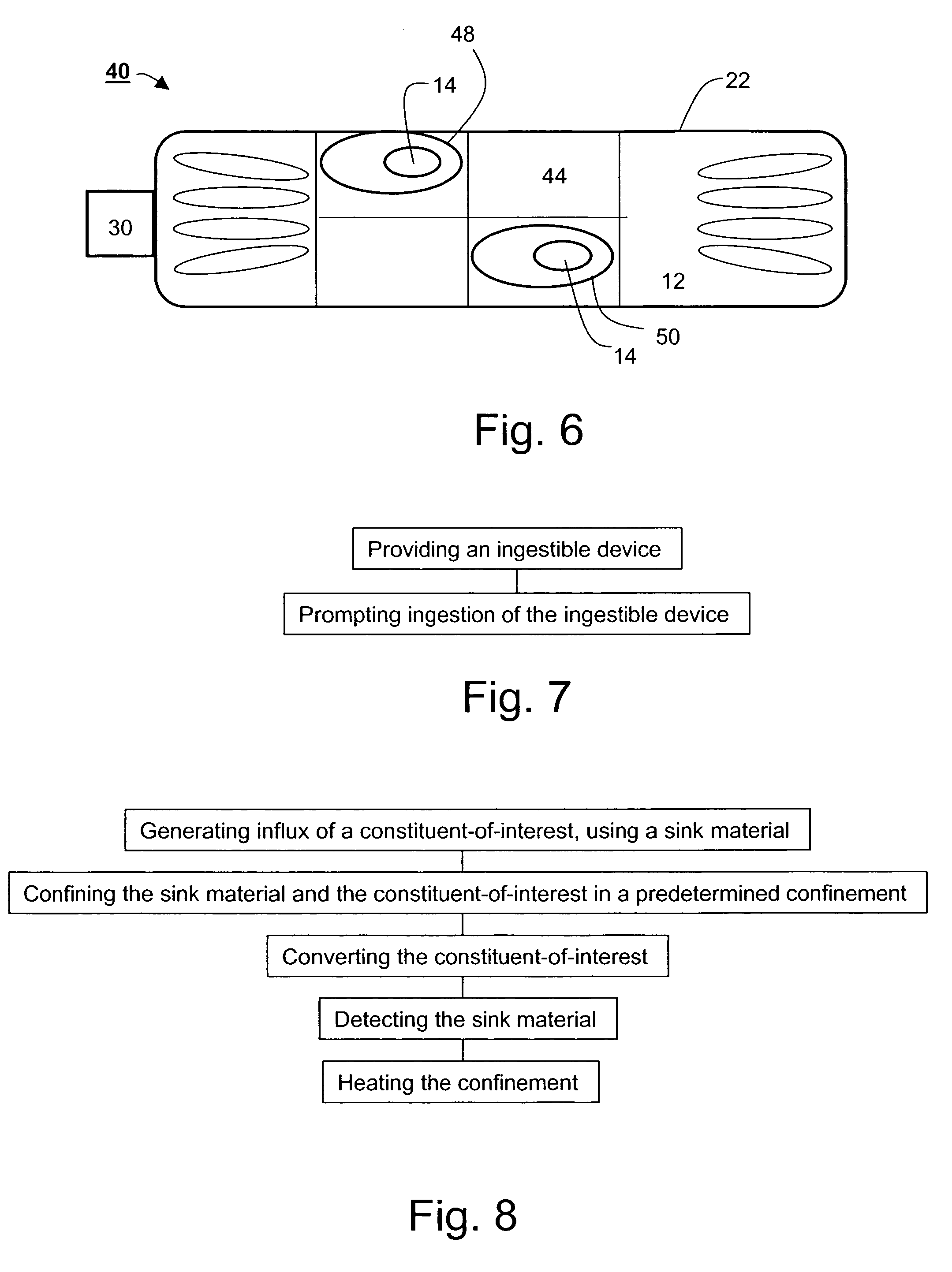
Ingestible gastrointestinal device
InactiveUS20040214311A1Bioreactor/fermenter combinationsBiological substance pretreatmentsGastrointestinal deviceGastroenterology
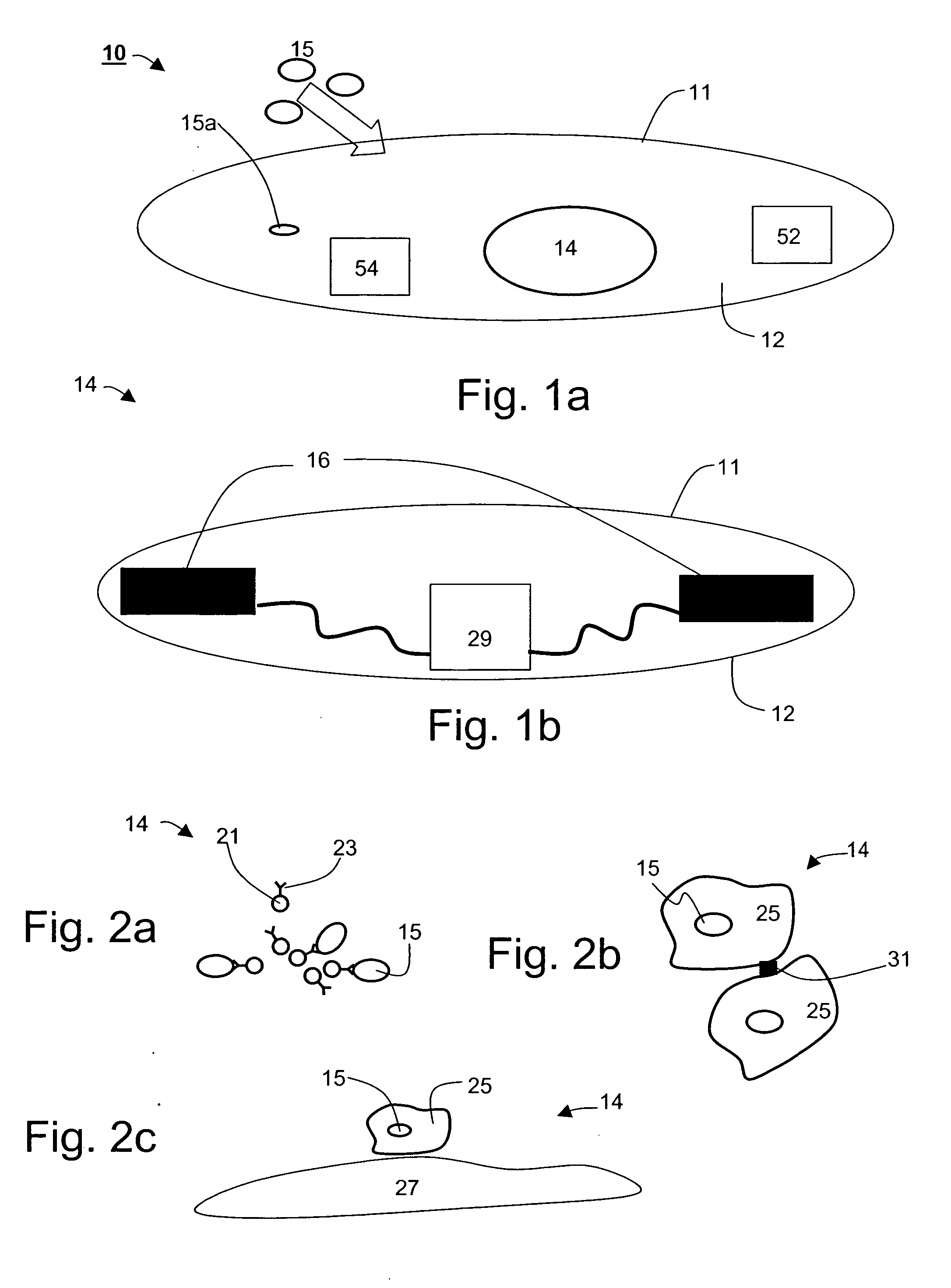
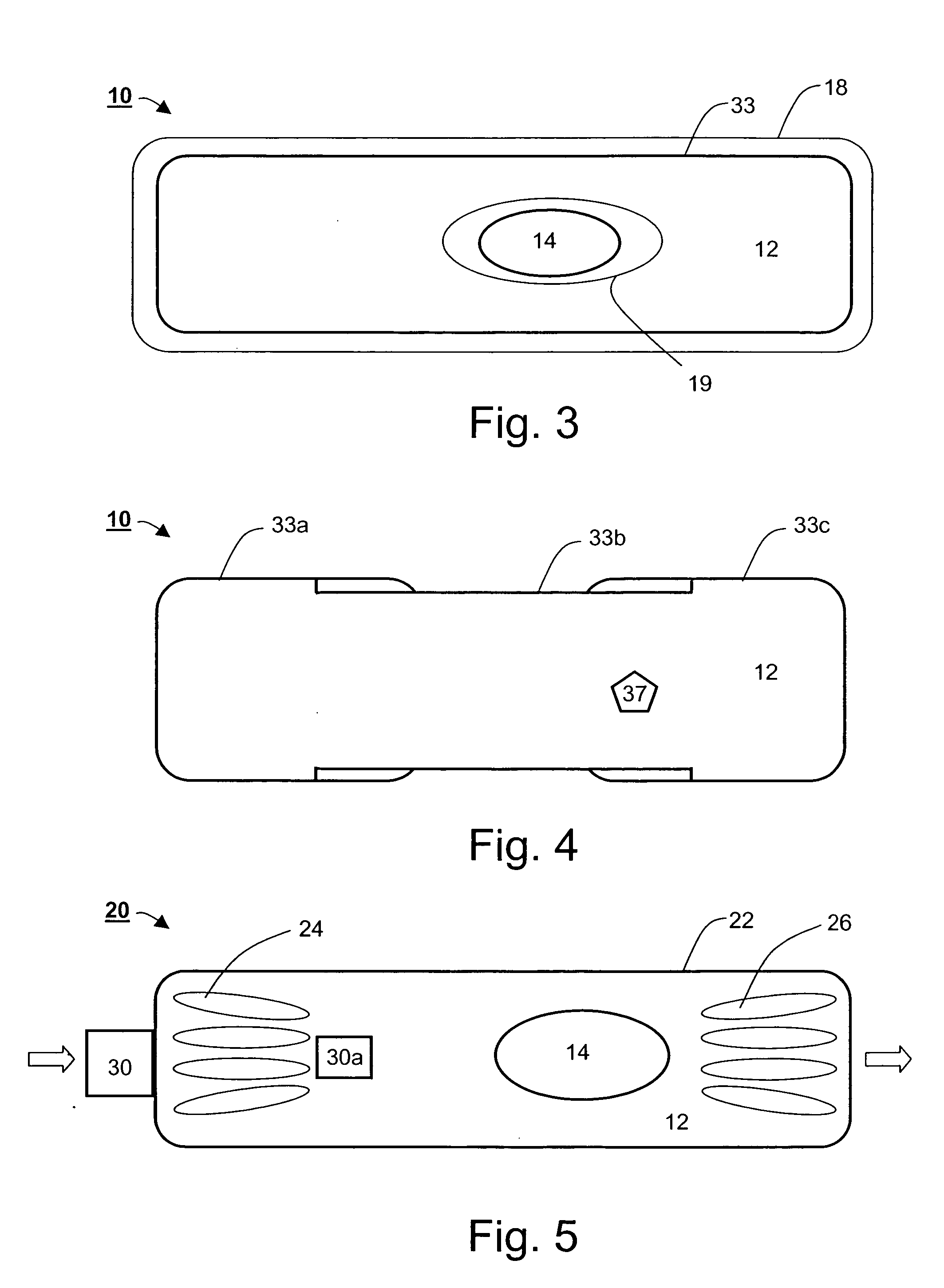
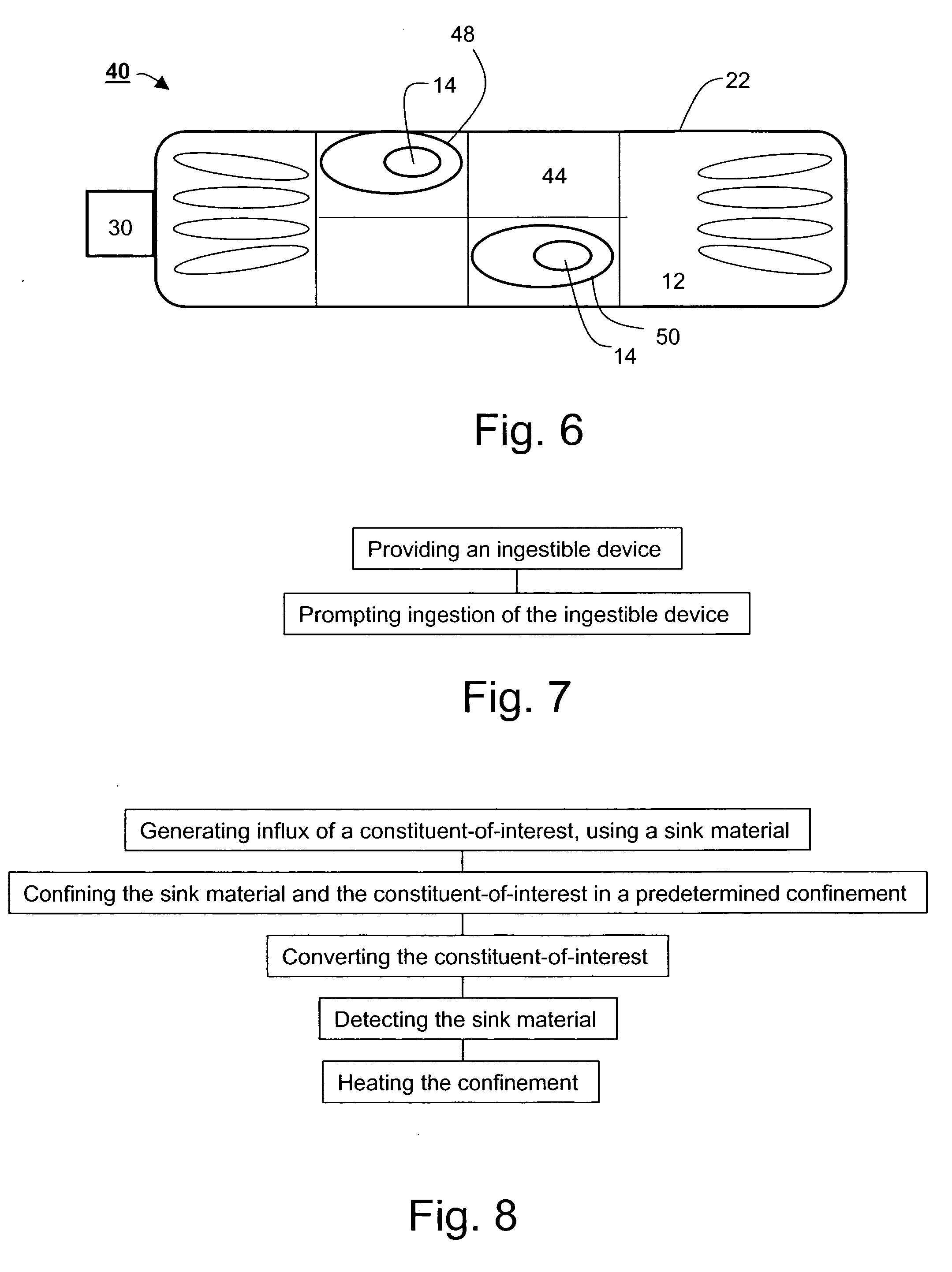
An ingestible device is provided. The ingestible device comprises a sink mechanism, for generating net influx of at least one constituent-of-interest present in a gastrointestinal tract of an individual, and a confining mechanism for confining the sink mechanism in a predetermined confinement.
Owner:LEVY MARK M
Devices and methods for augmenting extragastric banding
InactiveUS20090093839A1Promoting tissue in-growthAltering abilitySurgeryDilatorsLower esophagusGastrointestinal device
The present invention is directed generically to a means for altering the ability of the mammalian body to absorb nutritive content from ingested foodstuffs, and more specifically to an apparatus and method of use for an endolumenal sleeve (referred to also as an “intragastrointestinal device” or “gastrointestinal device”) positioned in the mammalian gastrointestinal (GI) tract. A suitable endolumenal sleeve is comprised of an anchor element and an opening at a proximal end, an elongate lumen or hollow open-ended tube having a transverse dimension, and a distal orifice. Optionally, an exterior aspect of the elongate lumen may include additional modes of attachment to the tissues walls of the GI tract through the use of one or more means for promoting tissue in-growth. The endolumenal sleeve is retained in the GI tract such that a substantial fraction of the food and liquids passing through the GI tract is channeled into the proximal opening and through an interlumenal space defined within the interior space of the endolumenal sleeve. Within the endolumenal sleeve there may be one or more restrictive means to constrain, impede or otherwise control the operative flow of material through the device. An individual restrictive means can either be of a fixed geometry or such means may include one or more elements which are adjustable in nature or function. The elongate lumen of the endolumenal sleeve is formed of a polymer composition suitable for controlled ingress of biological secretions, egress of certain selected nutritional elements, and may comprise either a single tubular structure or a multi-section (i.e. articulated and / or multiple lumen) assembly. When the endolumenal sleeve is in situ within the mammalian gastro-intestinal system, ingested foodstuffs are conveyed from the proximal end to said distal orifice. In typical applications, the proximal end of the endolumenal sleeve is positioned within the physiological region extending from the lower esophagus to the duodenum and the distal orifice is positioned within the physiological region extending from the upper duodenum to the lower jejunum, though further extension into the lower intestine is possible. Through proper selection of position for the endolumenal sleeve proximal and distal ends, combined by selection of the composition used in the fabrication of the elongate lumen, it is possible to finitely control the degree of nutritive absorption performed by the gastrointestinal tract.
Owner:KELLEHER BRIAN
Ingestible gastrointestinal device
InactiveUS7459305B2Avoid damageBioreactor/fermenter combinationsBiological substance pretreatmentsGastrointestinal deviceBiology
An ingestible device is provided. The ingestible device comprises a sink mechanism, for generating net influx of at least one constituent-of-interest present in a gastrointestinal tract of an individual, and a confining mechanism for confining the sink mechanism in a predetermined confinement.
Owner:LEVY MARK M
Pyloric Devices and Methods
InactiveUS20090118749A1Shorten the lag periodEffectively alter satietyObesity treatmentWound clampsGastrointestinal devicePyloric sphincter
A gastrointestinal device is provided. The device includes a band sized and configured for residing in or around a pyloric sphincter region of the subject. The band is functional in maintaining the pyloric sphincter at a fixed opening size.
Owner:SVIP 2
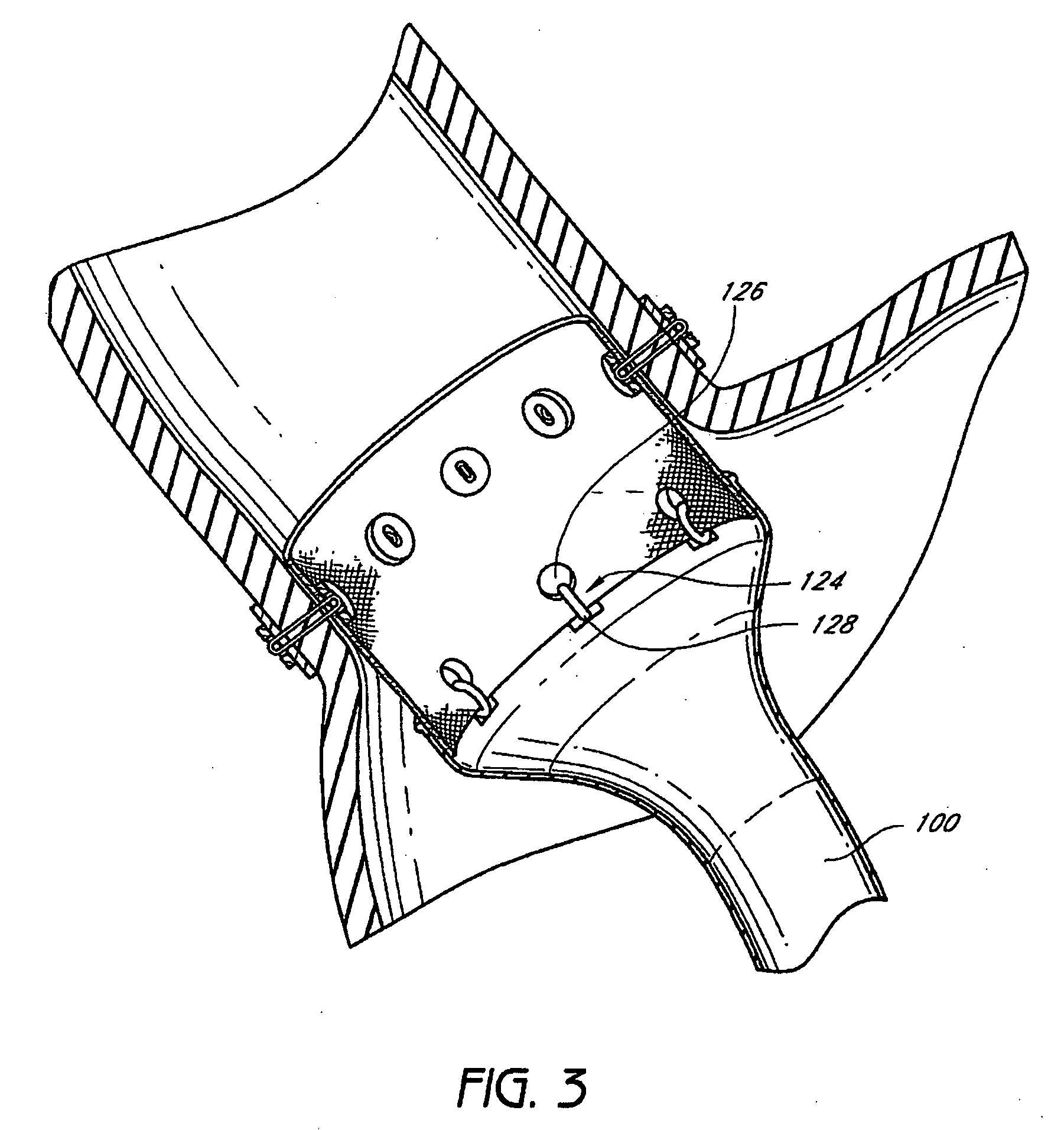
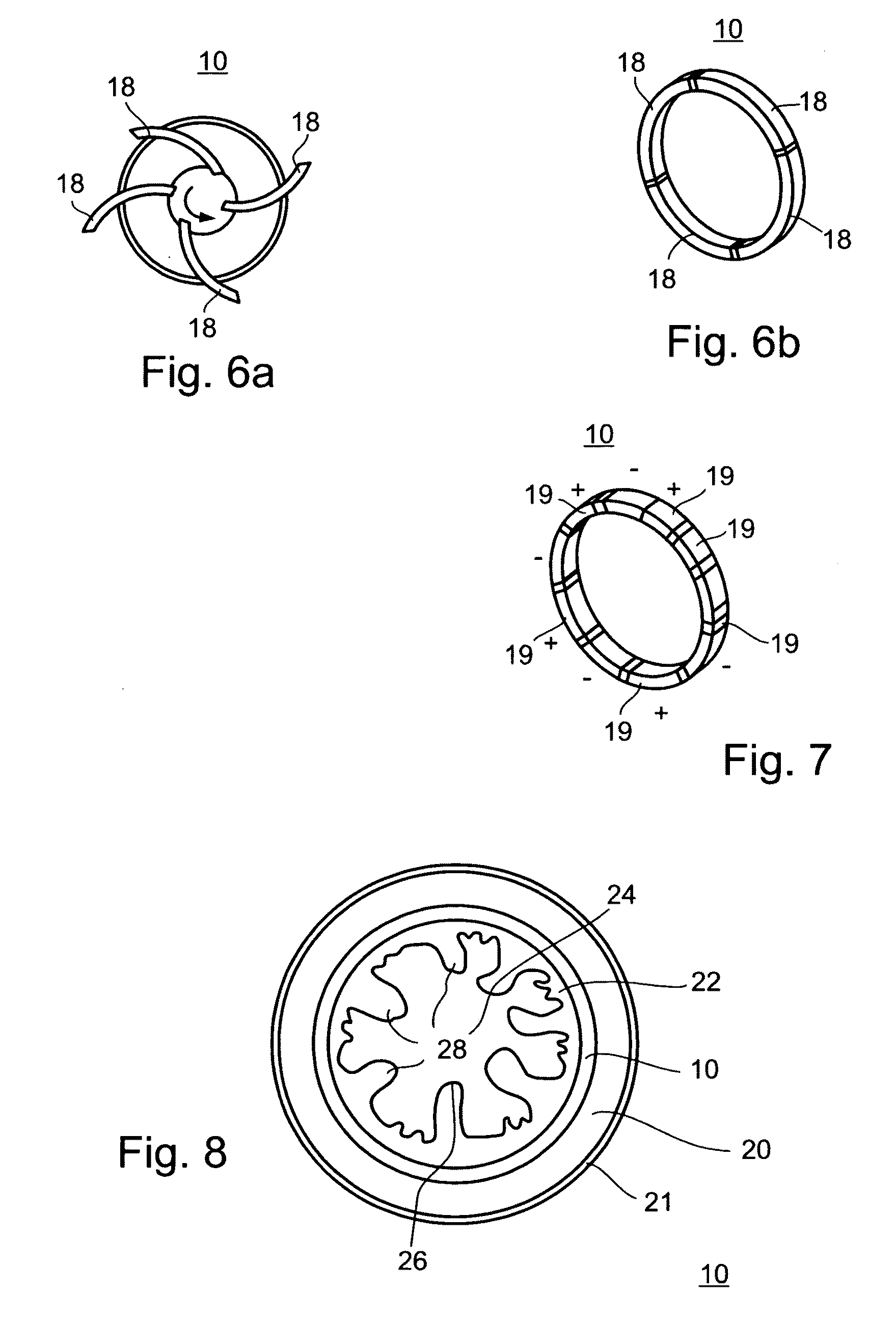
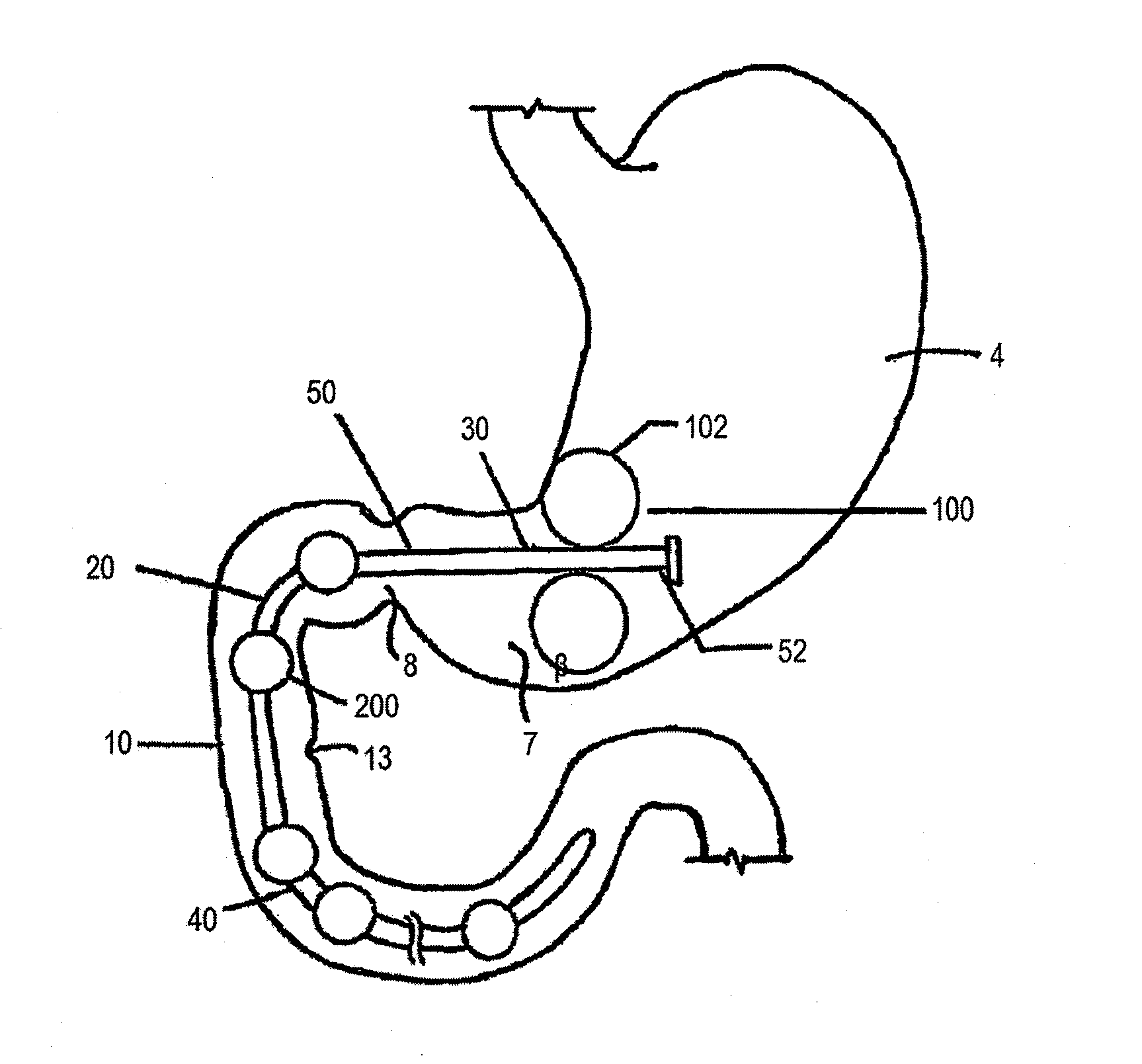
Anchors and methods for intestinal bypass sleeves
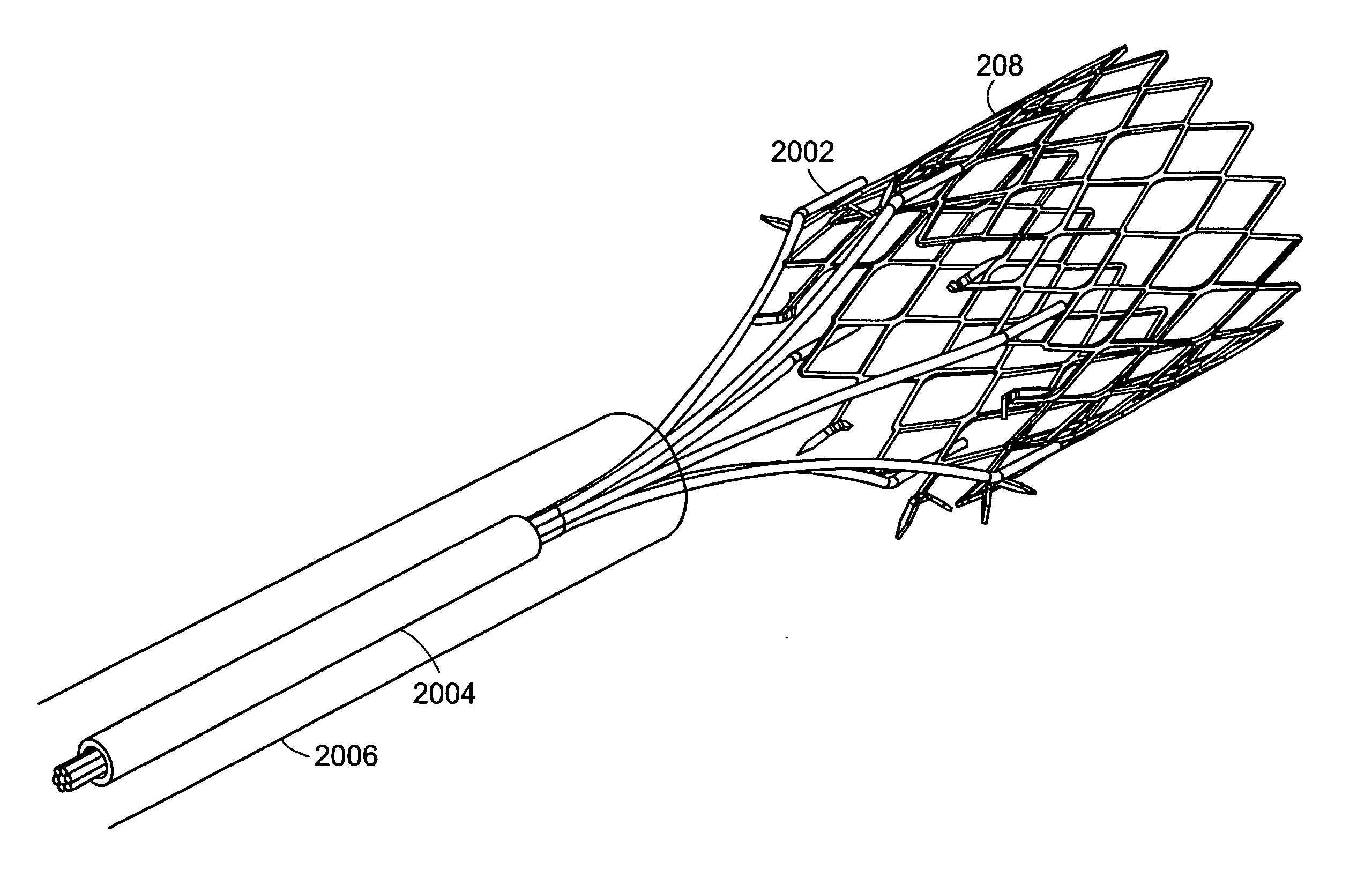
A gastrointestinal device for implanting within a pylorus, a duodenal bulb, and a duodenum of a patient's gastrointestinal tract includes an expandable structure includes a proximal portion having a plurality of spring arms and a distal portion having a plurality of spring arms, the proximal and distal portions coupled by a rigid central cylinder having a diameter capable of fitting within the pylorus and having a length greater than a width of the pylorus. A membrane is coupled to and covering at least a portion of one of the proximal portion and the distal portion of the expandable structure. An intestinal bypass sleeve is coupled to at least one of the proximal and distal portions of the expandable structure and having a length sufficient to extend at least partially into the duodenum. In the expanded configuration, the proximal portion has a diameter larger than a maximum opening diameter of the pylorus and further wherein, in the expanded configuration, the distal portion has a diameter larger than a maximum opening diameter of the pylorus.
Owner:METAMODIX
Thermogelling polymer blends for biomaterial applications
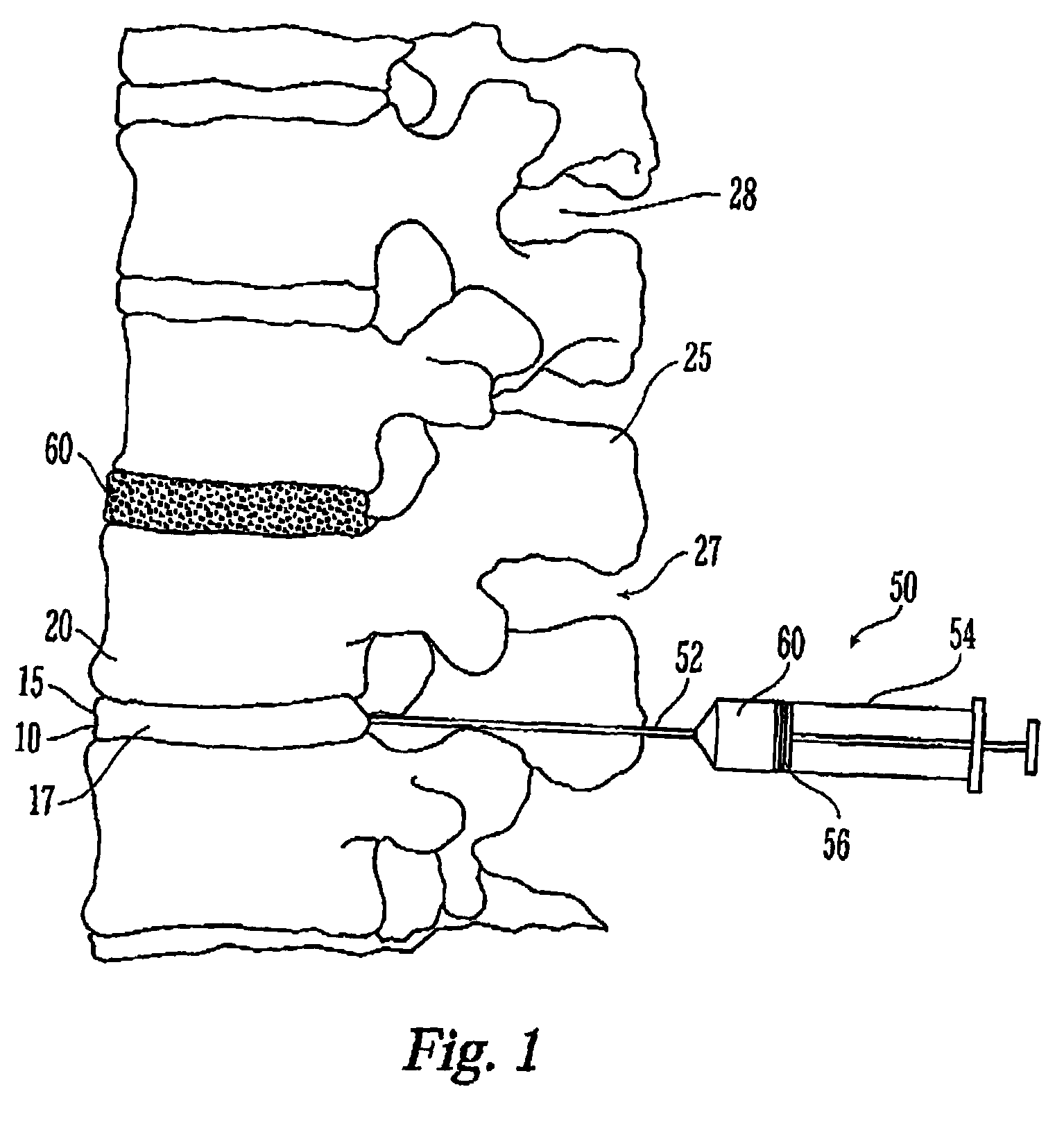
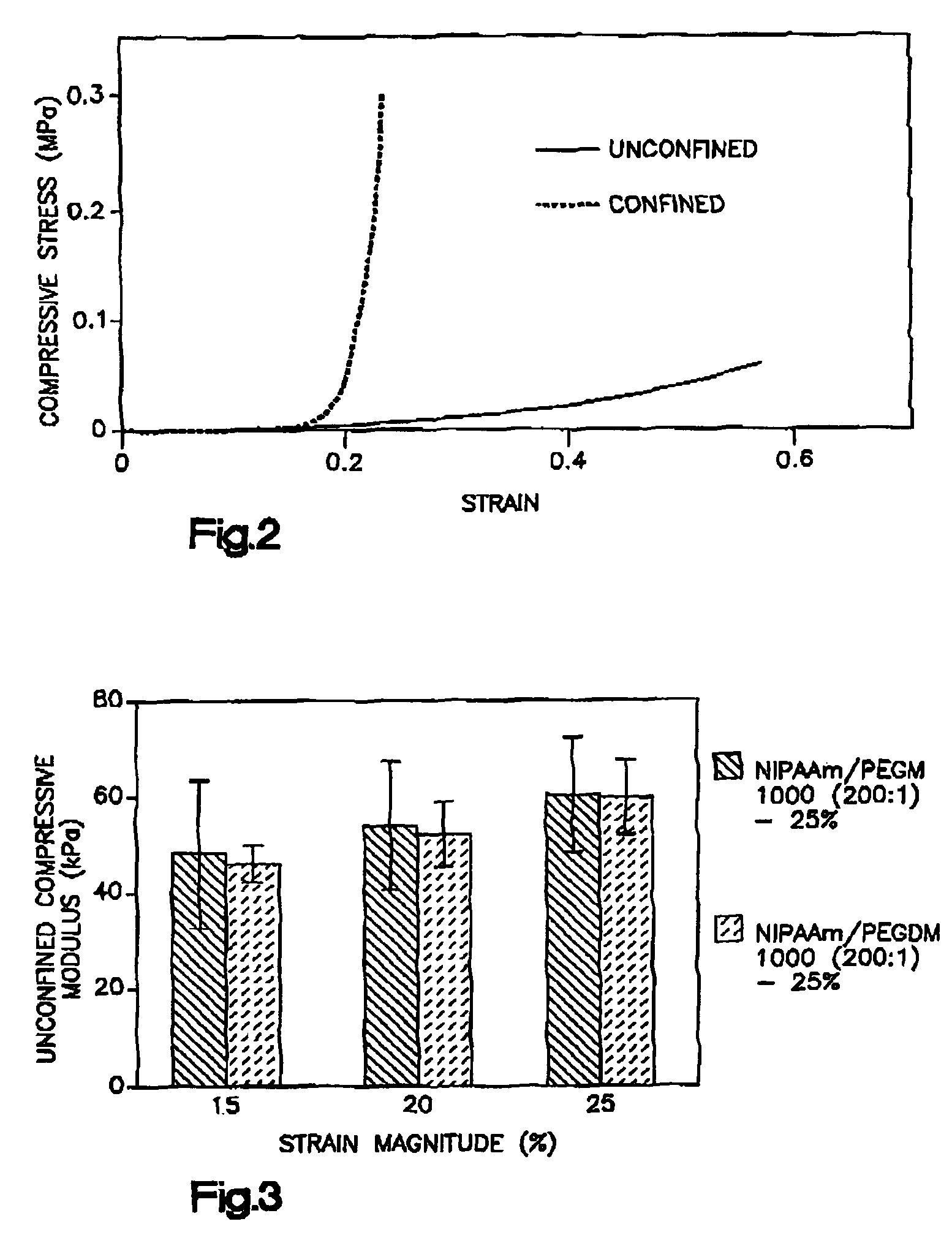
Thermogelling polymers are described containing poly (n-isopropyl acrylamide). Solutions of this polymer, copolymers or mixtures of the polymer with a second polymer such as poly(ethylene glycol), poly (vinyl pyrrolidone) or poly(vinyl alcohol) are liquids at room temperature and solids at body temperature. Thus, also provided are methods of implanting a hydrogel into a mammal by injecting the solution as a liquid at a temperature below body temperature into a selected site in the mammal at a temperature below body temperature, which then undergoes thermal phase transition to form a solid hydrogel in situ in the body as the implant warms to body temperature. Methods for using these thermal gelling materials in various applications including nucleus pulposus replacement / augmentation, wound care, disk replacement, cartilage replacement, joint replacement, surgical barriers, gastrointestinal devices, cosmetic and reconstructive surgery, and breast enlargement are also provided.
Owner:SYNTHES USA +1
Intragastric device for treating obesity
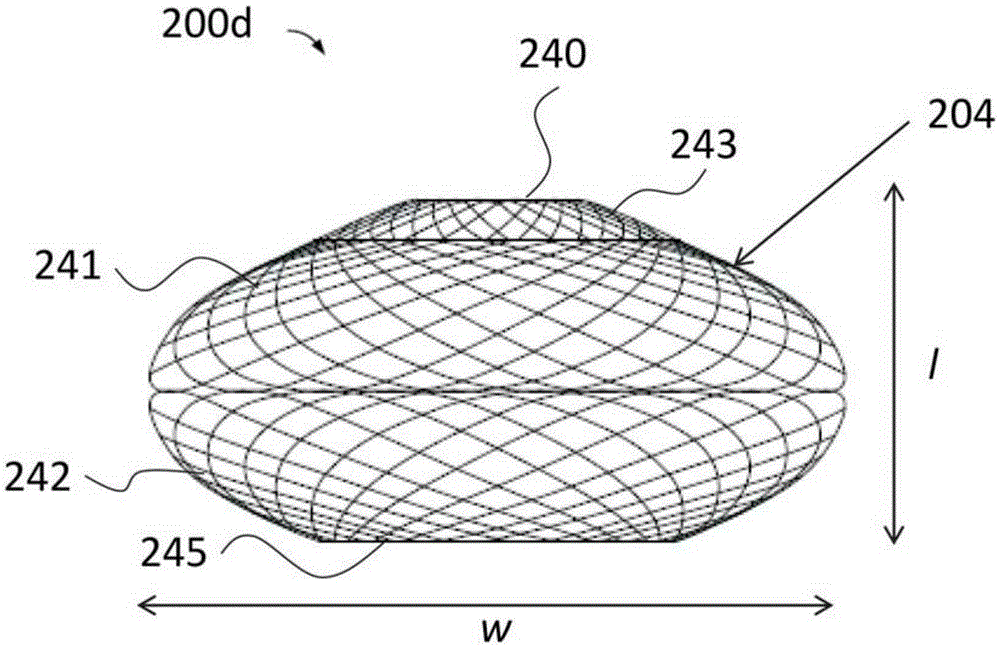
A gastrointestinal device for treating obesity includes a three-dimensional porous structure configurable between a compressed pre-deployment configuration to facilitate delivery and an expanded post-deployment configuration. The porous structure includes a first opening at its proximal end and a larger second opening at its distal end. The porous structure also includes a sleeve coupled to its distal end. Optionally, the device further includes a suture at the proximal end of the wire mesh structure to facilitate retrieval and an anti-migration component positioned at the junction of the porous structure with the sleeve. The porous structure is deployed in a patient's stomach such that the anti-migration component sits proximal to the patient's pylorus and prevents migration of the entirety of the device into and through the pylorus. The sleeve extends through the pylorus, into the duodenum and ends in the duodenum or jejunum. Food enters the device from the first opening at the proximal end of the porous structure, passes through the porous structure and sleeve, and exits at the distal end of the sleeve. The device treats obesity by providing a relatively immovable volume occupying structure in the stomach and a bypass for food past the pylorus and proximal portion of the small intestine. Optionally, the device further acts to slow the passage of food through the digestive tract. Patients with the device experience satiety more quickly and have a prolonged sensation of satiety.
Owner:SYNERZ MEDICAL
System and method for guiding of gastrointestinal device through the gastrointestinal tract
Systems and methods for guidance of a gastrointestinal device through a gastrointestinal tract are provided. A gastrointestinal guidewire is positioned through the gastrointestinal tract, by introduction of an introducing element into the gastrointestinal tract. One end of the gastrointestinal guidewire is attached to the introducing element, and the other end of the gastrointestinal guidewire is attached to an anchoring element, anchored to a location outside of the gastrointestinal tract. Movement of the introducing element through the gastrointestinal tract results in positioning of the guidewire through the gastrointestinal tract. The guidewire is then used as a scaffold to guide the gastrointestinal device, wherein the gastrointestinal device may be externally controlled. This allows for speeding up, slowing down, reversal and stopping of the gastrointestinal device during its descent through the gastrointestinal tract.
Owner:BEN HORIN SHOMRON SILAN
System and method for guiding of gastrointestinal device through the gastrointestinal tract
Owner:TEL HASHOMER MEDICAL RES INFRASTRUCTURE & SERVICES
Duodenal gastrointestinal devices and related treatment methods
Owner:ENDOSPHERE
Medical swallowing image collection device and image processing method thereof
InactiveCN103202703AAccurate image dataAccurate Diagnosis ReferenceImage analysisRadiation diagnosticsGastrointestinal deviceImaging processing
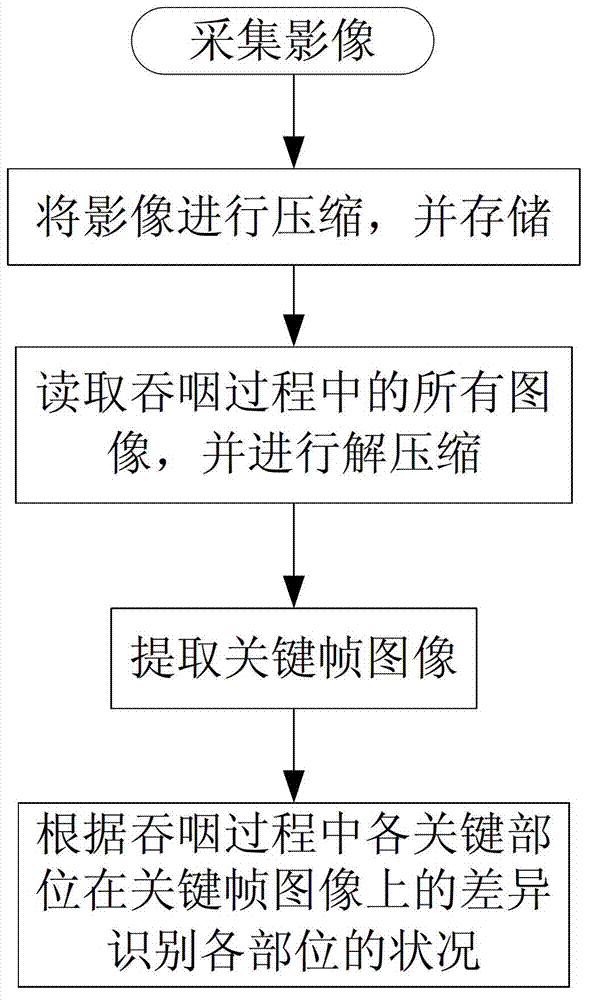
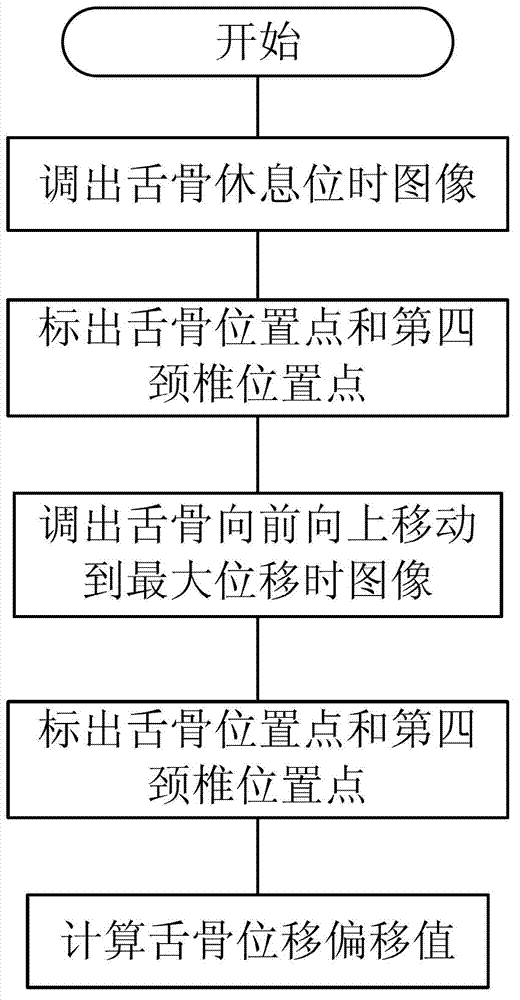
The invention discloses a medical swallowing image collection device and an image processing method thereof. The device comprises a digital collection card, a computer and a display. The digital collection card is inserted into a slot of a peripheral component interconnect-express (PCI-E) of the computer, is connected with a digital gastrointestinal device through a data line and sends collected image data of the digital gastrointestinal device to the computer in real time. The computer further comprises a memory for storing the image data and a processing module for processing images. The method comprises the steps of reading images in the swallowing process; extracting key frame images in the swallowing process; and identifying conditions of every part according to difference of every key part on the key frame images in the swallowing process. The medical swallowing image collection device is high in collecting precision; by image data processing, detailed quantitative analysis can be performed on the dynamic swallowing process, and every key parameter is measured and calculated; and image data can be called at any time so as to be used for reassessment and therapeutic effect comparison.
Owner:GUANGZHOU LONGEST SCI & TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com