Patents
Literature
205 results about "Enzymatic digestion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Enzymatic digestion occurs primarily in the mouth, stomach and small intestine. Both physical and chemical digestion begin in the mouth: the process of chewing grinds up food and mixes it with saliva, which contains an enzyme called salivary amylase.
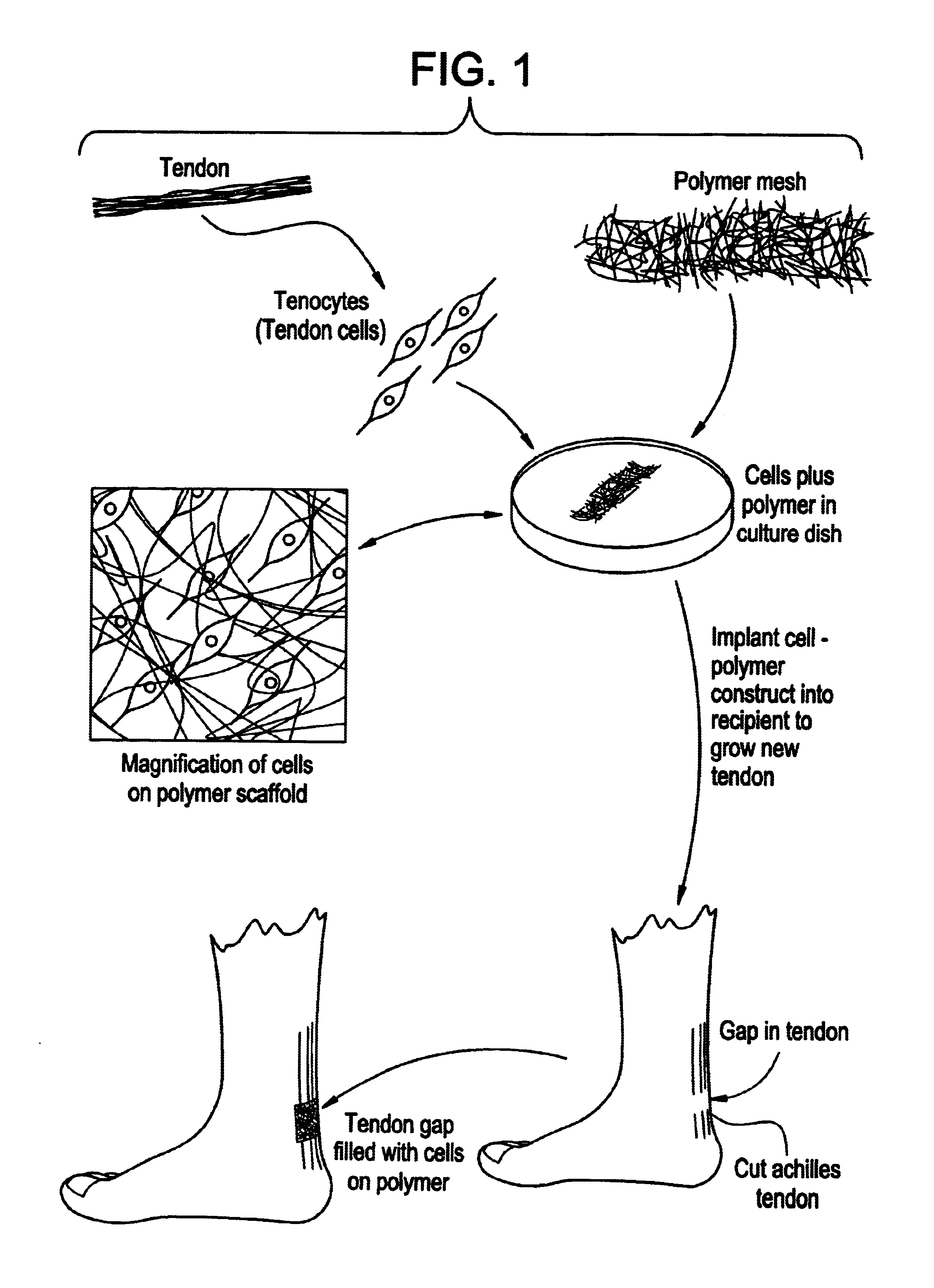
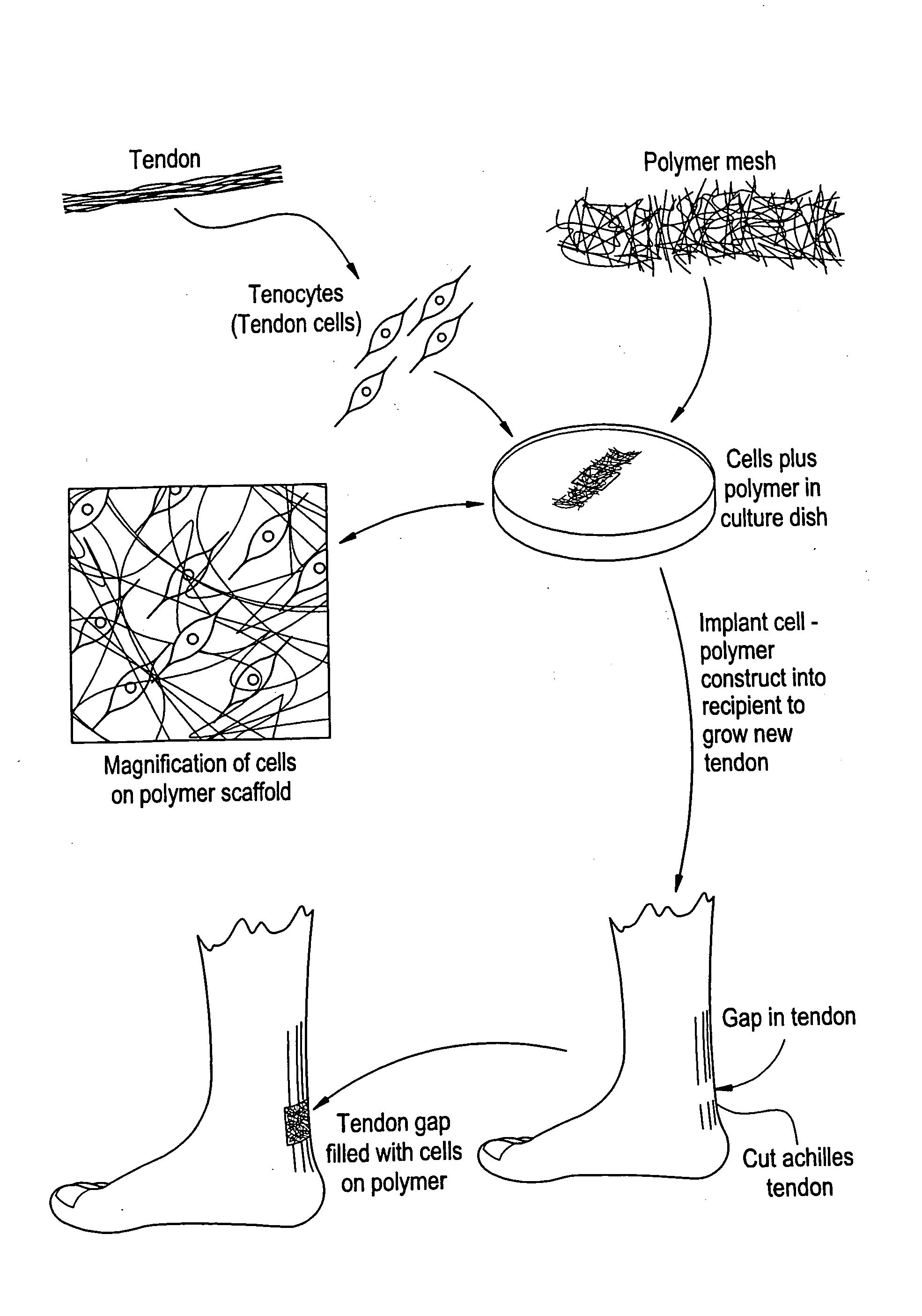
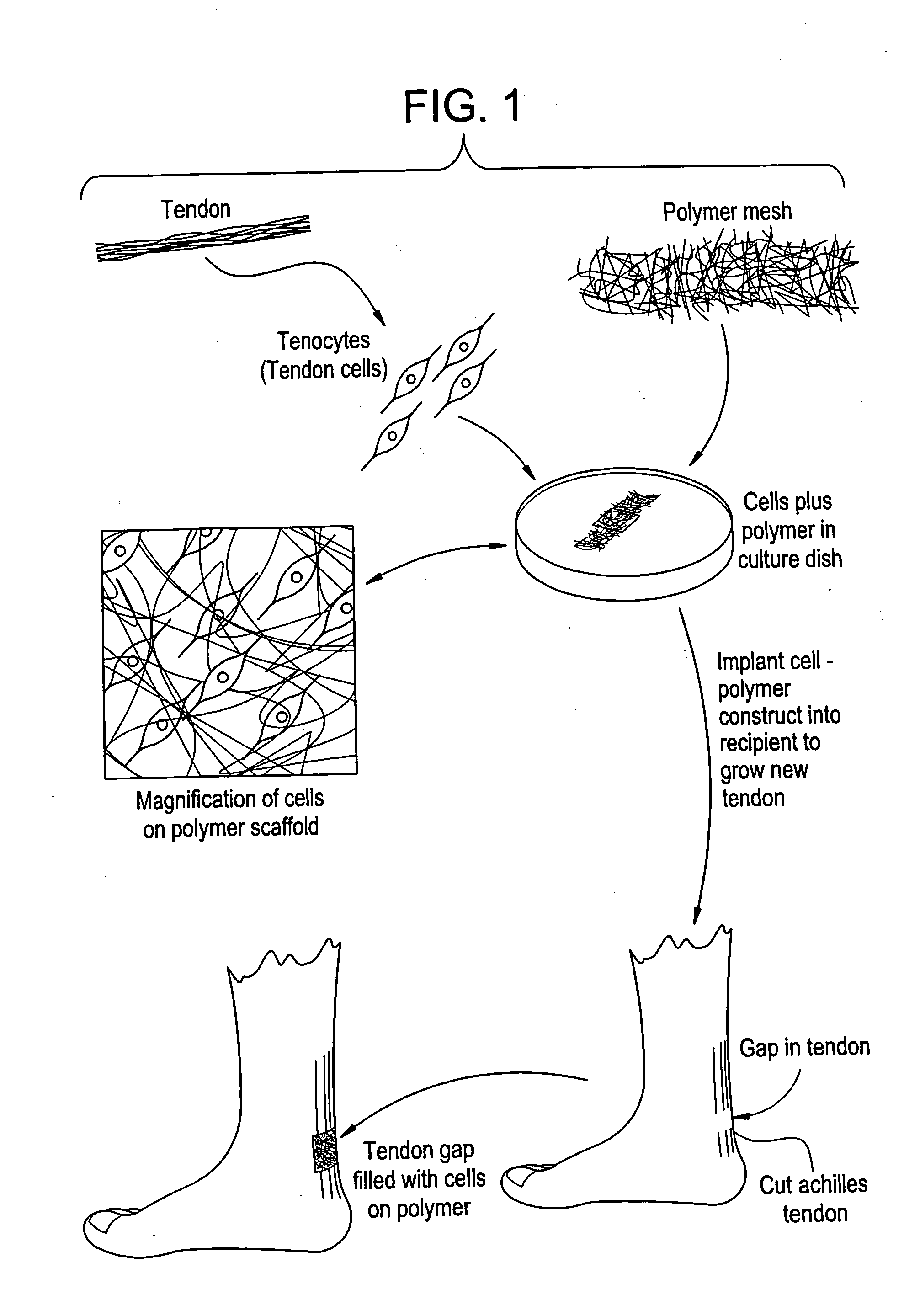
Tissue engineered tendons and ligaments
InactiveUS6840962B1Moderate strengthReduce inflammationLigamentsMusclesEnzymatic digestionLigament structure
Connective tissue, including neo-tendons and ligaments, has been constructed using biodegradable synthetic scaffolds seeded with tenocytes. The scaffolds are preferably formed from biodegradable fibers formed of a polymer such as polyglycolic acid-polylactic acid copolymers, and seeded with cells isolated from autologous tendon or ligament by means of enzymatic digestion or direct seeding into tissue culture dishes from explants. The cell polymer constructs are then surgically transplanted to replace missing segments of functioning tendon or ligament.
Owner:MASSACHUSETTS INST OF TECH +1
Tissue engineered tendons and ligaments
Connective tissue, including neo-tendons and ligaments, has been constructed using biodegradable synthetic scaffolds seeded with tenocytes. The scaffolds are preferably formed from biodegradable fibers formed of a polymer such as polyglycolic acid-polylactic acid copolymers, and seeded with cells isolated from autologous tendon or ligament by means of enzymatic digestion or direct seeding into tissue culture dishes from explants. The cell polymer constructs are then surgically transplanted to replace missing segments of functioning tendon or ligament.
Owner:VACANTI CHARLES A +5
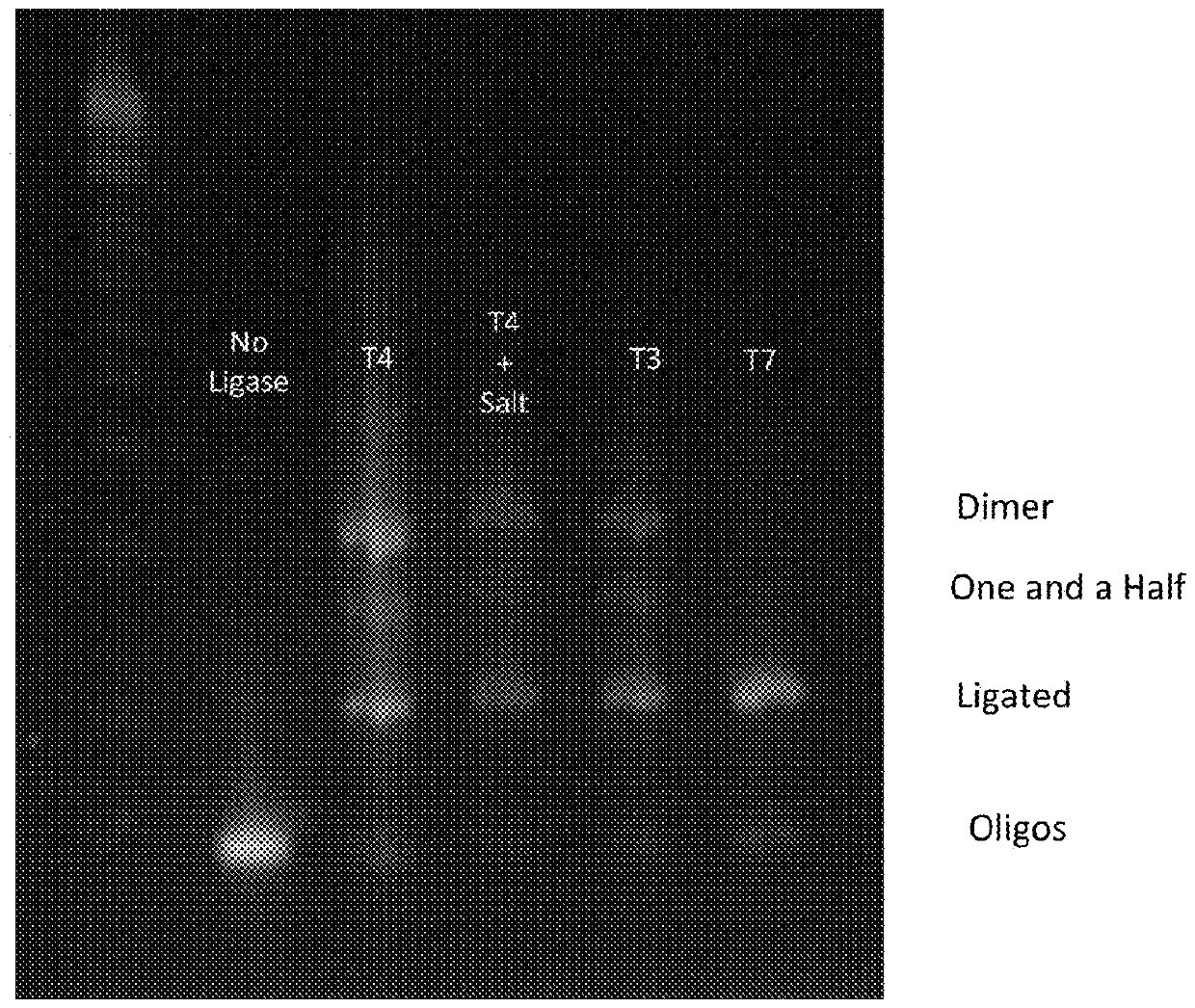
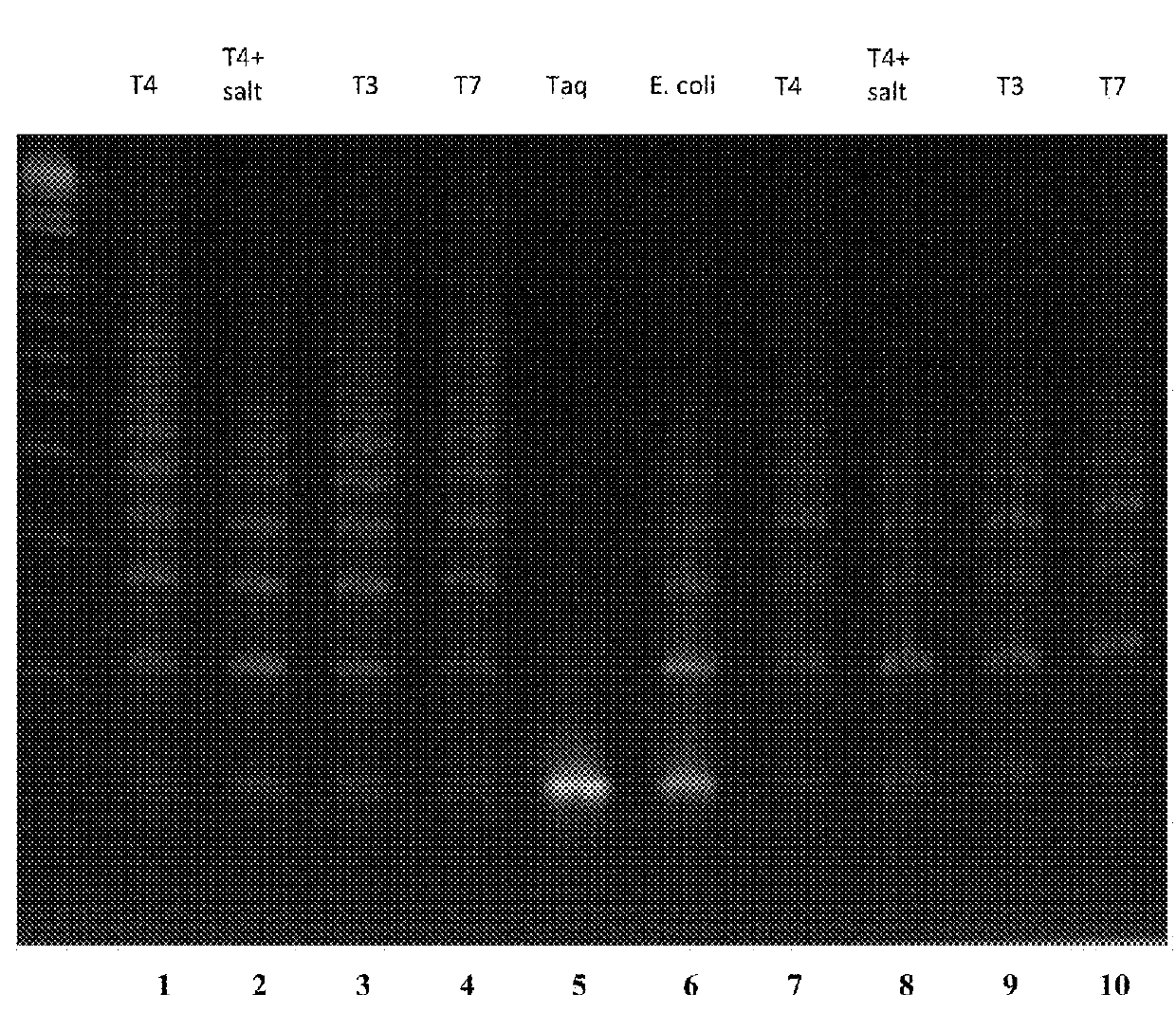
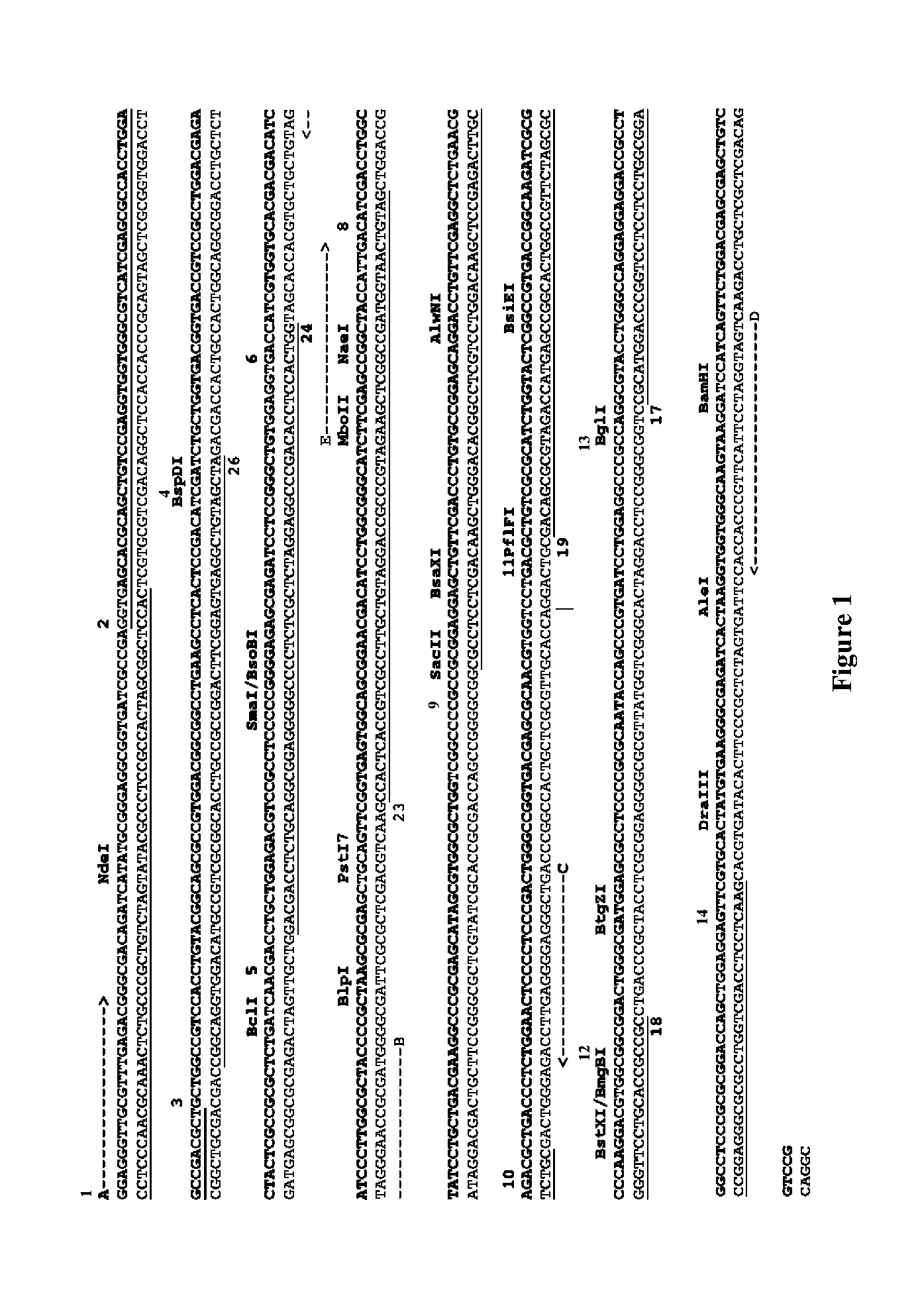
Compositions and Methods For High Fidelity Assembly of Nucleic Acids
InactiveUS20130059296A1Nucleotide librariesMicrobiological testing/measurementEnzymatic digestionPolymerase L
Aspects of the invention relate to methods, compositions and algorithms for designing and producing a target nucleic acid. The method can include: (1) providing a plurality of blunt-end double-stranded nucleic acid fragments having a restriction enzyme recognition sequence at both ends thereof; (2) producing via enzymatic digestion a plurality of cohesive-end double-stranded nucleic acid fragments each having two different and non-complementary overhangs; (3) ligating the plurality of cohesive-end double-stranded nucleic acid fragments with a ligase; and (4) forming a linear arrangement of the plurality of cohesive-end double-stranded nucleic acid fragments, wherein the unique arrangement comprises the target nucleic acid. In certain embodiments, the plurality of blunt-end double-stranded nucleic acid fragments can be provided by: releasing a plurality of oligonucleotides synthesized on a solid support; and synthesizing complementary strands of the plurality of oligonucleotides using a polymerase based reaction.
Owner:GEN9
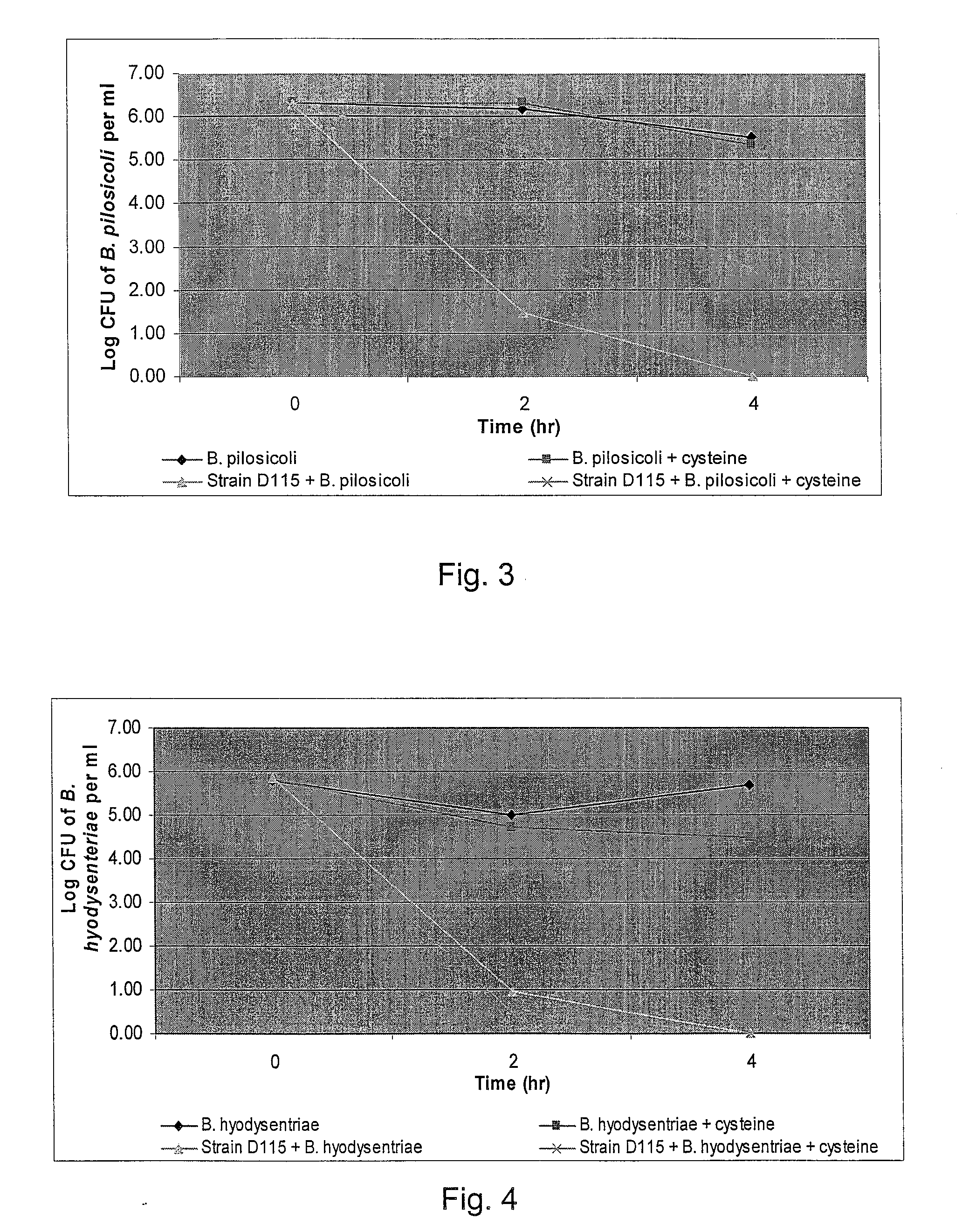
Broad-Spectrum Antibacterial and Antifungal Activity of Lactobacillus Johnsonii D115
The present invention demonstrated the potential use of Lactobacillus johnsonii D115 as a probiotic, as a prophylactic agent or as a surface treatment of materials against human and animal pathogens such as Brachyspira pilosicoli, Brachyspira hyodysenteriae, Shigella sonnei, Vibrio cholera, Vibrio parahaemolyticus, Campylobacter jejuni, Streptococcus pneumoniae, Enterococcus faecalis, Enterococcus faecium, Clostridium perfringens, Yersinia enterocolitica, Escherichia coli, Klebbsiella pneumoniae, Staphylococcus aureus, Salmonella spp., Bacillus cereus, Aspergillus niger and Fusarium chlamydosporum. The proteineous antimicrobial compound was partially characterized and found to be heat tolerant up to 121° C. for 15 min, and acid tolerant up to pH1 for 30 min at 40° C. The compound is also stable to enzymatic digestion, being able to retain more than 60% antimicrobial activity when treated with pepsin and trypsin.
Owner:KEMIN IND INC
Compositions and Methods for High Fidelity Assembly of Nucleic Acids
InactiveUS20150203839A1Microbiological testing/measurementLibrary member identificationEnzymatic digestionPolymerase L
Aspects of the invention relate to methods, compositions and algorithms for designing and producing a target nucleic acid. The method can include: (1) providing a plurality of blunt-end double-stranded nucleic acid fragments having a restriction enzyme recognition sequence at both ends thereof; (2) producing via enzymatic digestion a plurality of cohesive-end double-stranded nucleic acid fragments each having two different and non-complementary overhangs; (3) ligating the plurality of cohesive-end double-stranded nucleic acid fragments with a ligase; and (4) forming a linear arrangement of the plurality of cohesive-end double-stranded nucleic acid fragments, wherein the unique arrangement comprises the target nucleic acid. In certain embodiments, the plurality of blunt-end double-stranded nucleic acid fragments can be provided by: releasing a plurality of oligonucleotides synthesized on a solid support; and synthesizing complementary strands of the plurality of oligonucleotides using a polymerase based reaction.
Owner:GEN9
Enzymatic digestion of tissue
InactiveUS20060188892A1Promotes fast digestionMicrobiological testing/measurementNucleic acid reductionEnzymatic digestionNuclease inhibitor
The present invention concerns a compositions and method for isolating a nucleic acid from a cell-containing sample. There is disclosed a method comprising obtaining at least one cell-containing sample, which comprises a cell containing nucleic acid, obtaining at least one catabolic enzyme, obtaining at least one nuclease inhibitor, preparing an admixture of the sample, the catabolic enzyme, and the nuclease inhibitor, maintaining the admixture under conditions where the catabolic enzyme is active, and agitating the admixture, where the sample is digested to produce a nucleic acid-containing lysate of the sample.
Owner:APPL BIOSYSTEMS INC
Methods and Systems for Pretreatment and Processing of Biomass
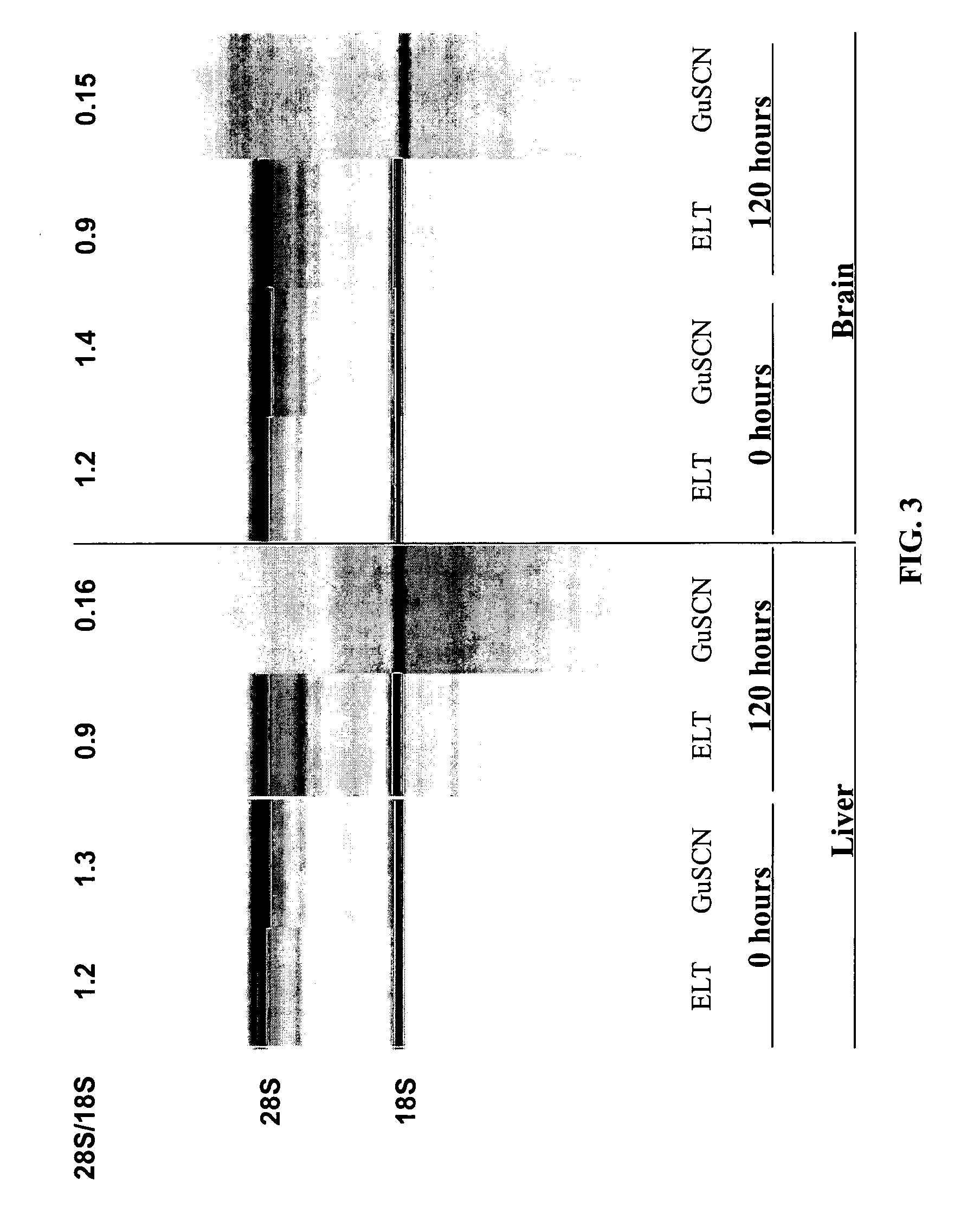
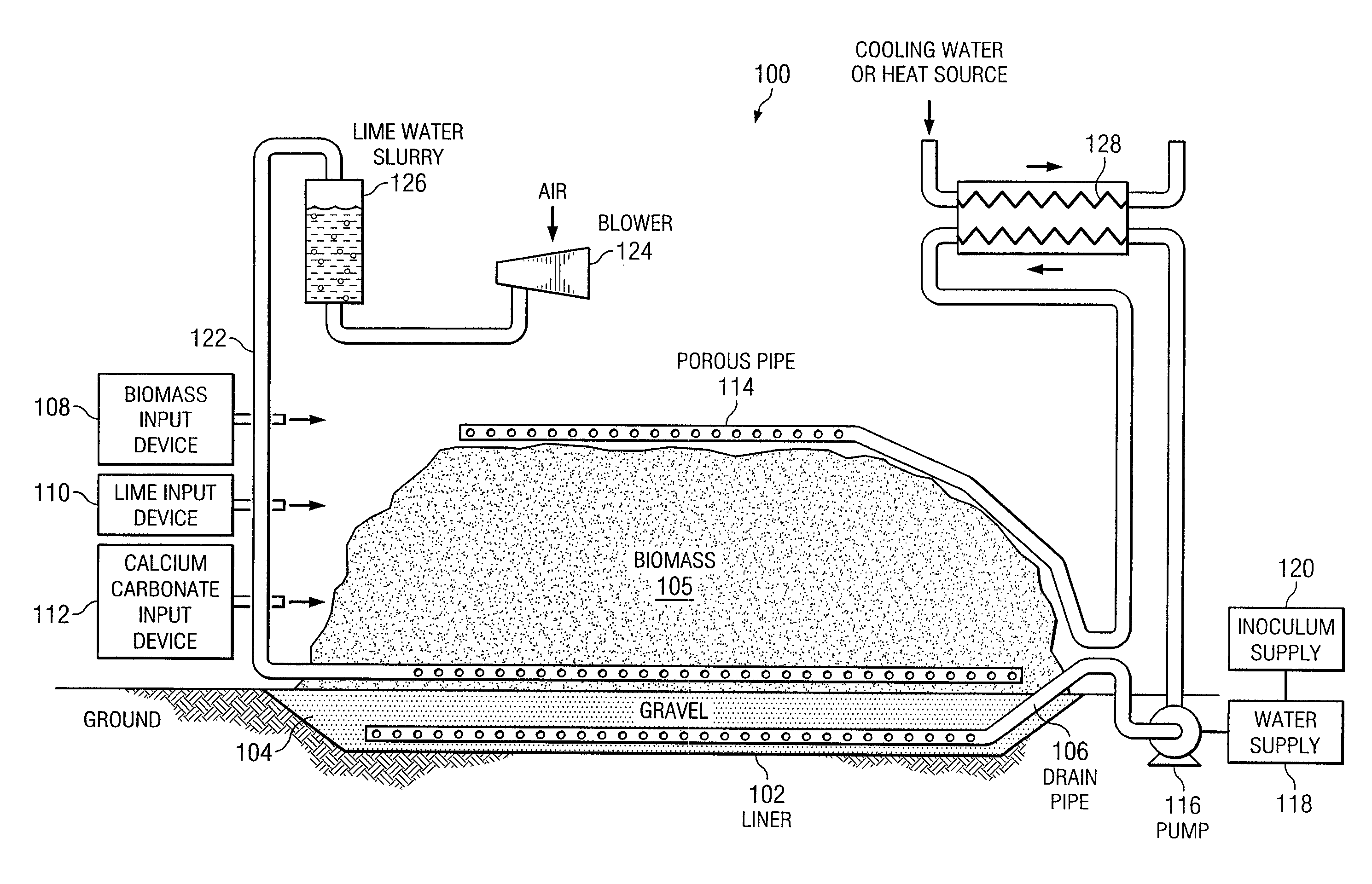
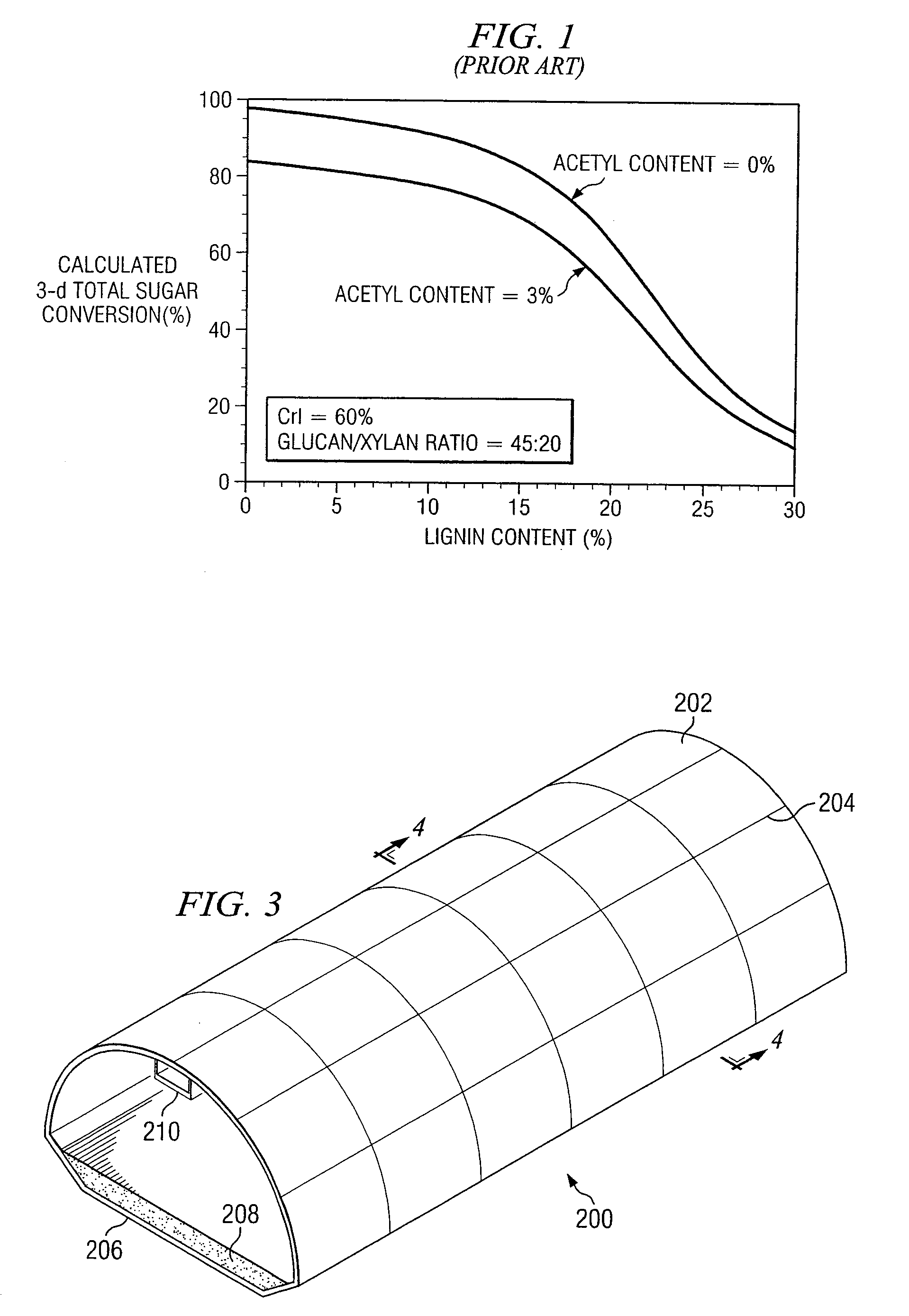
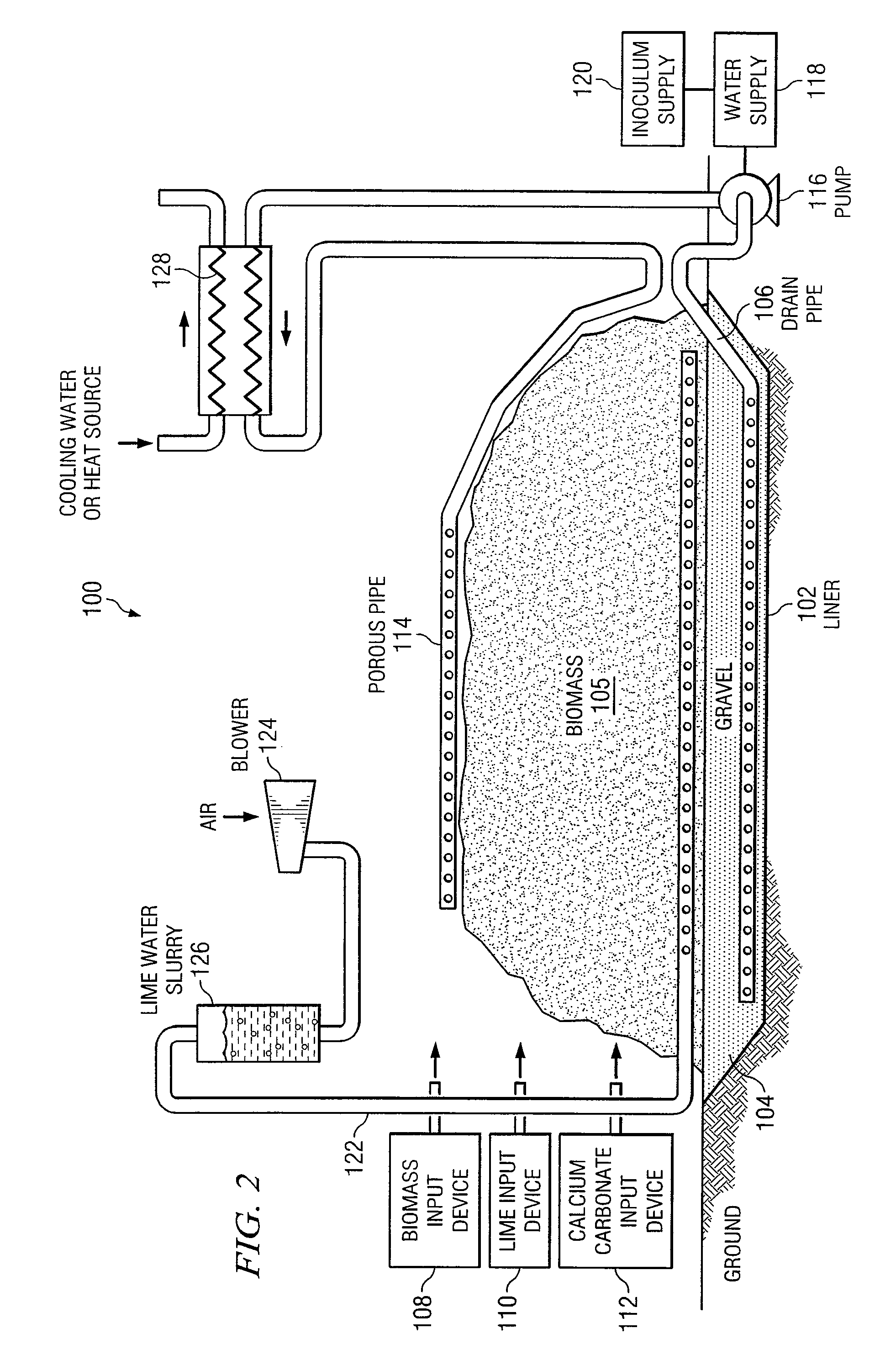
InactiveUS20080121359A1Increasing enzyme digestibilityLow lignin contentBio-organic fraction processingProductsEnzymatic digestionCellulose
According to one embodiment of the invention, a system for processing biomass includes a water-impermeable bottom liner, a gravel layer supported by the bottom liner, a drain pipe disposed within the gravel layer, a biomass input device operable to deliver biomass over the gravel layer to form a biomass pile, a lime input device operable to deliver lime to the biomass for pretreating the biomass, a distribution pipe elevated above the gravel layer, and a pump operable to circulate water through the biomass pile by delivering water to the distribution pipe and receiving water from the drain pipe after it has traveled through the biomass pile.According to another embodiment, a method for biomass pretreatment with alkali, conducted at ambient pressure for approximately 4-16 weeks at temperatures ranging from approximately 25° C. to 95° C. Biomass may be lignocellulosic biomass and may be rendered suitable for enzymatic digestion or pulp production.
Owner:TEXAS A&M UNIVERSITY
Preparation method of acellular matrix gels and acellular matrix gels
ActiveCN105169483AIncrease biological functionSuitable for in vivo applicationProsthesisEnzymatic digestionFiber
The invention discloses a preparation method of acellular matrix gels, which is implemented through that natural tissues or organs are treated by using a decellation technology, the obtained object is subjected to subsequent chalking and enzymatic digestion treatment so as to obtain an acellular matrix solution, and the acellular matrix solution can be prepared into acellular matrix gels with various shapes and properties under mild conditions. Acellular matrix gels prepared by using the preparation method disclosed by the invention can be used in injectable gels and can be processed and formed into gels, the formed gel has a nano fiber microstructure, and the microstructure has a positive effect on regulating the behaviors of cells.
Owner:SUN YAT SEN UNIV
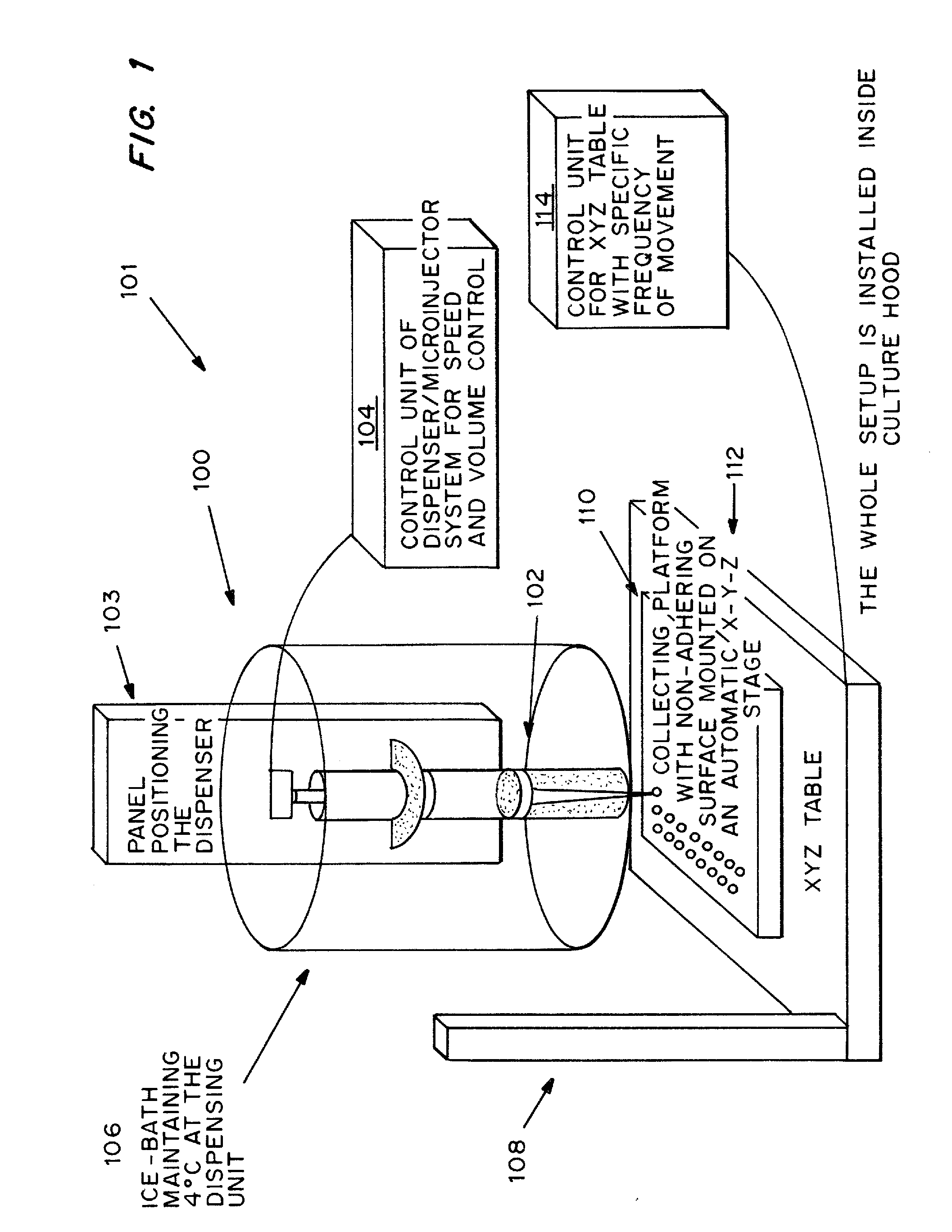
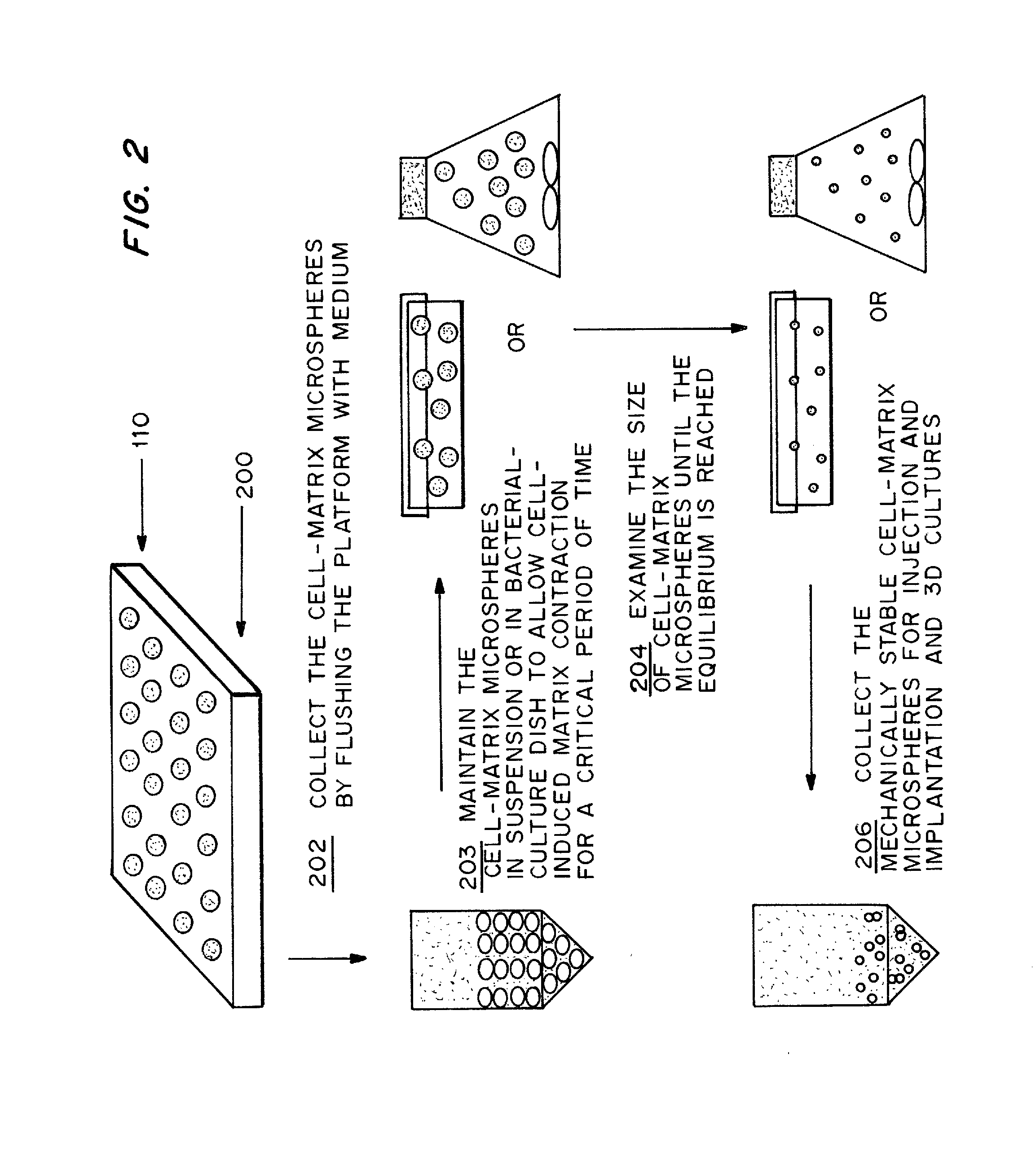
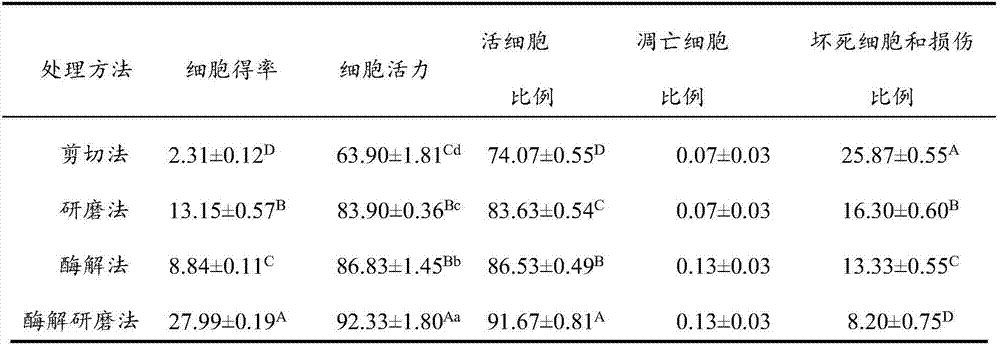
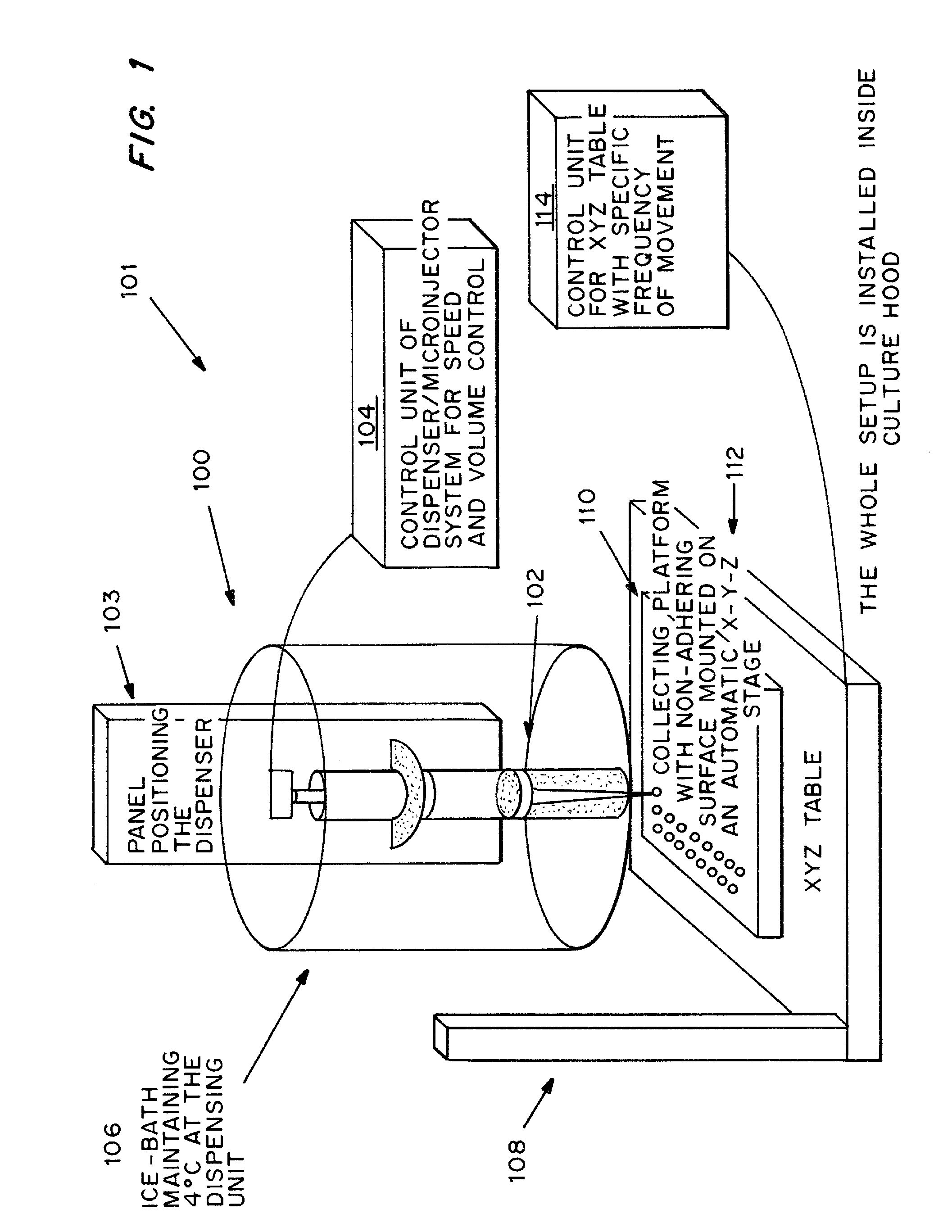
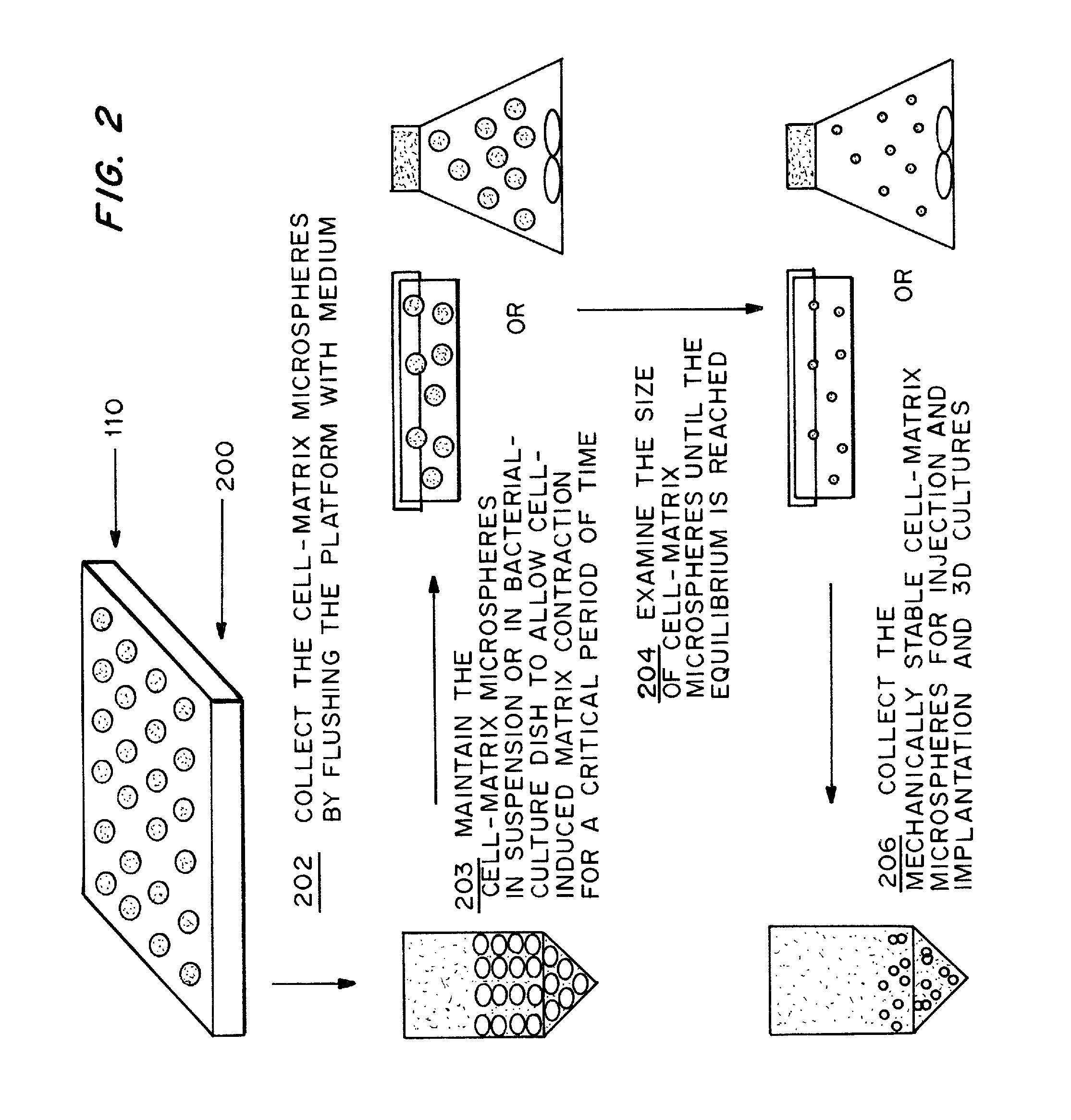
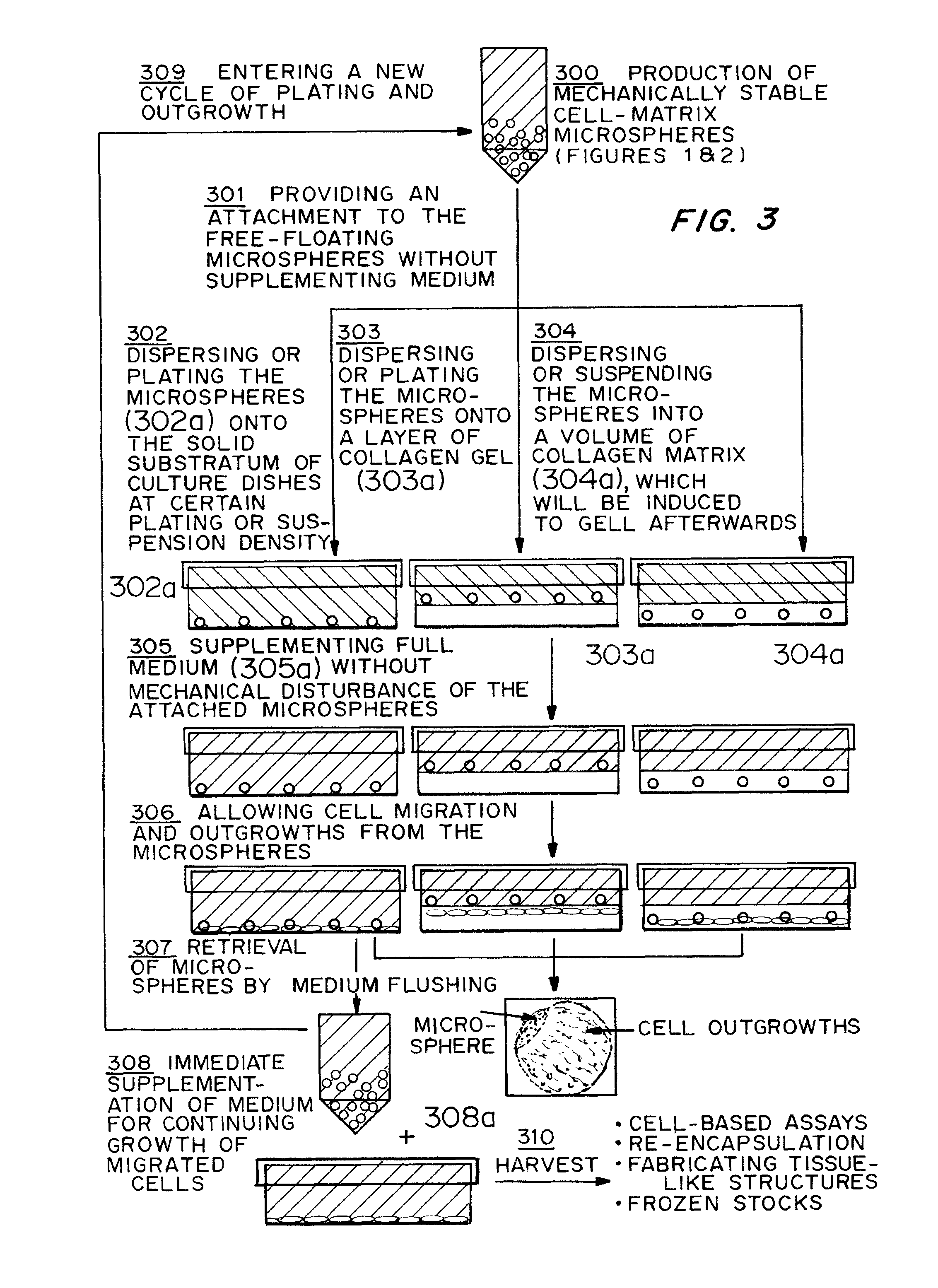
Cell-matrix microspheres, methods for preparation and applications
ActiveUS20080031858A1Increased protein productivityStable cell-matrix microspheresBiocideBioreactor/fermenter combinationsEnzymatic digestionHigh cell
A method has been developed to produce stable cell-matrix microspheres with up to 100% encapsulation efficiency and high cell viability, using matrix or biomaterial systems with poor shape and mechanical stability for applications including cell therapeutics via microinjection or surgical implantation, 3D culture for in vitro expansion without repeated cell splitting using enzymatic digestion or mechanical dissociation and for enhanced production of therapeutic biomolecules, and in vitro modeling for morphogenesis studies. The modified droplet generation method is simple and scalable and enables the production of cell-matrix microspheres when the matrix or biomaterial system used has low concentration, with slow phase transition, with poor shape and mechanical stability.
Owner:VERSITECH LTD
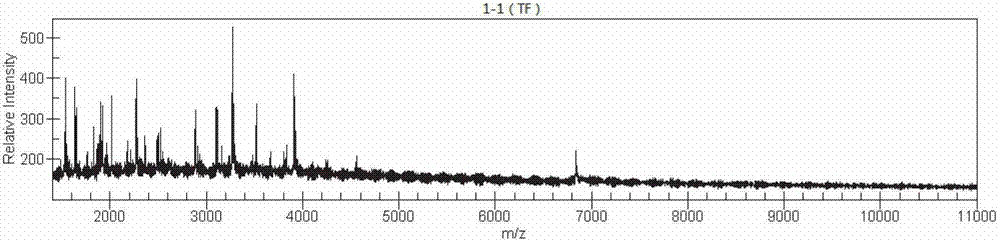
Method for building helicobacter pylori nucleic acid fingerprint spectrum and product thereof
ActiveCN102827930AAccurate identificationMicrobiological testing/measurementMicroorganism based processesEnzymatic digestionMass spectrometry
The invention discloses a method for building a helicobacter pylori nucleic acid fingerprint spectrum, which comprises PCR (polymerase chain reaction) amplification, SAP (severe acute pancreatitis) enzymatic digestion, transcription and nuclease digestion, purification, mass spectrometer detection and the like. A helicobacter pylori nucleic acid fingerprint spectrum database is set up on the basis of the method. According to the generated mass peak spectrum of the experiment, the helicobacter pylori of a sample to be detected can be quickly identified, so the method can be widely applied in the fields of helicobacter pylori types and classification, environmental sanitation, public safety qunarantine and the like.
Owner:BIOYONG TECH
Method and kit for detecting non-small cell lung cancer drive gene mutation spectrum, and application
ActiveCN103305625AImprove throughputSmall fluxMicrobiological testing/measurementEnzymatic digestionCapillary electrophoresis
The invention discloses a method and a kit for detecting a non-small cell lung cancer drive gene mutation spectrum, and an application. The method comprises the following steps of: designing 15 pairs of amplification primers for amplifying the exon segments of the seven related genes of non-small cell lung cancer, dividing the amplification primers into 6 groups, and preparing an amplification primer mixed solution, performing multiple PCR (polymerase chain reaction) amplification on the to-be-detected samples by the amplification primer mixed solution respectively, and then performing enzymatic digestion; and designing 39 extension primers used for detecting hotspot mutation sites, dividing the extension primers into 6 groups corresponding to the amplification primer mixed solution, and preparing an extension primer mixed solution, performing extension reaction on the digested PCR product, then performing enzymatic digestion, performing capillary electrophoresis on the obtained product, and making a result judgment via software analysis. The kit provided by the invention comprises the amplification primers for amplifying the exon segments of the seven related genes of non-small cell lung cancer, and the extension primers used for detecting hotspot mutation sites. The method and the kit provided by the invention are simple, high in flux, and short in time consumption.
Owner:GUANGDONG GENERAL HOSPITAL
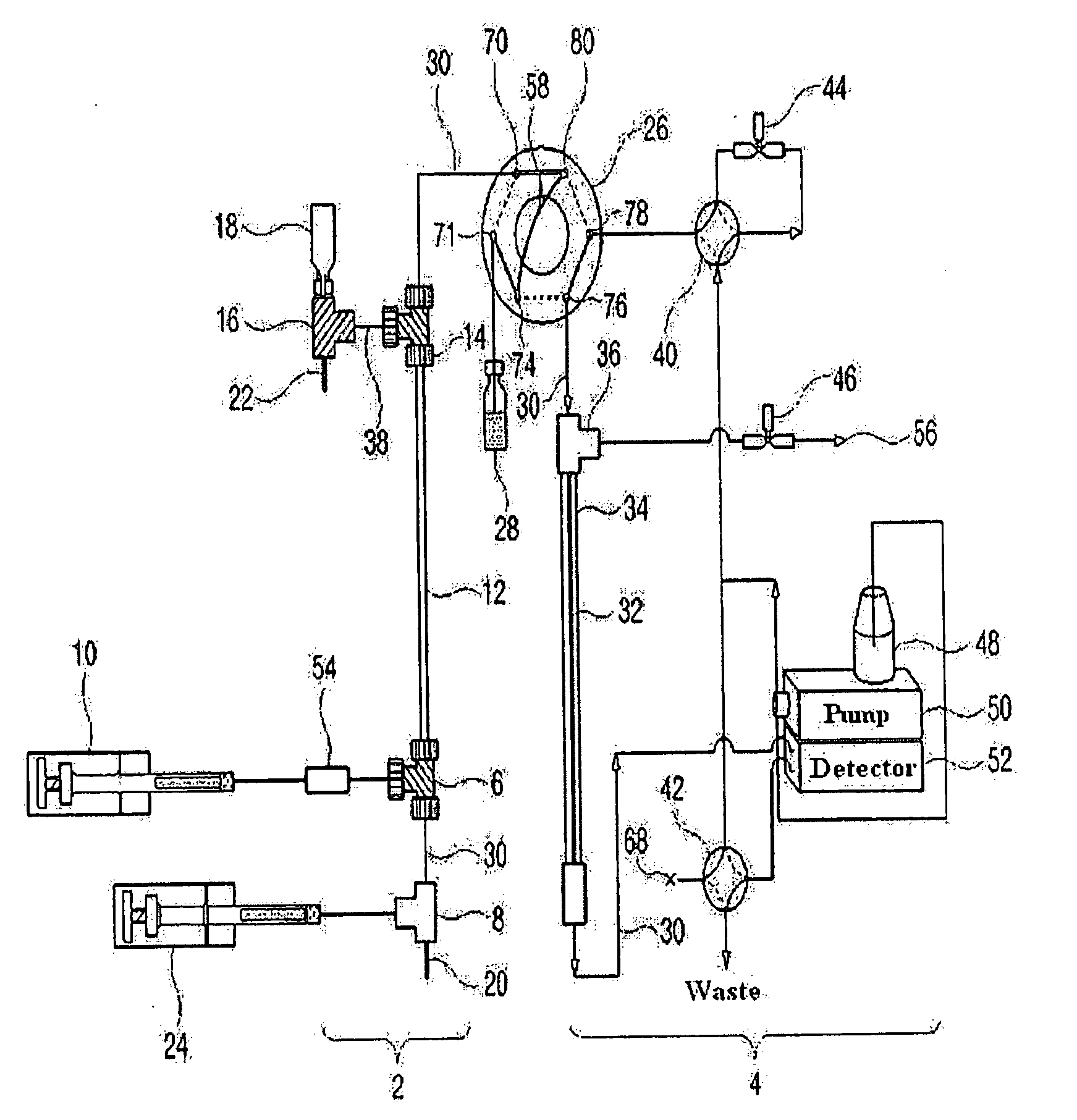
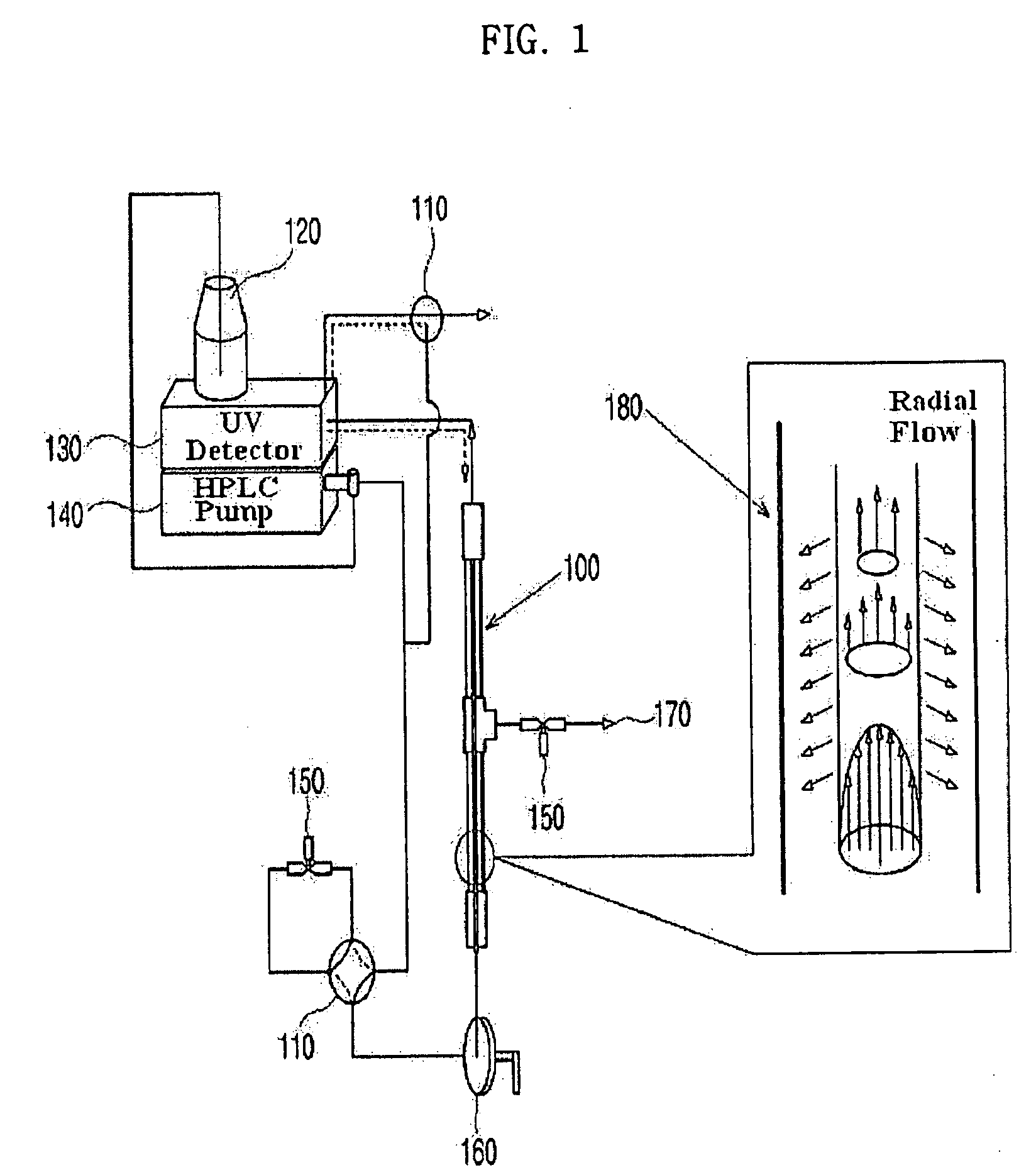
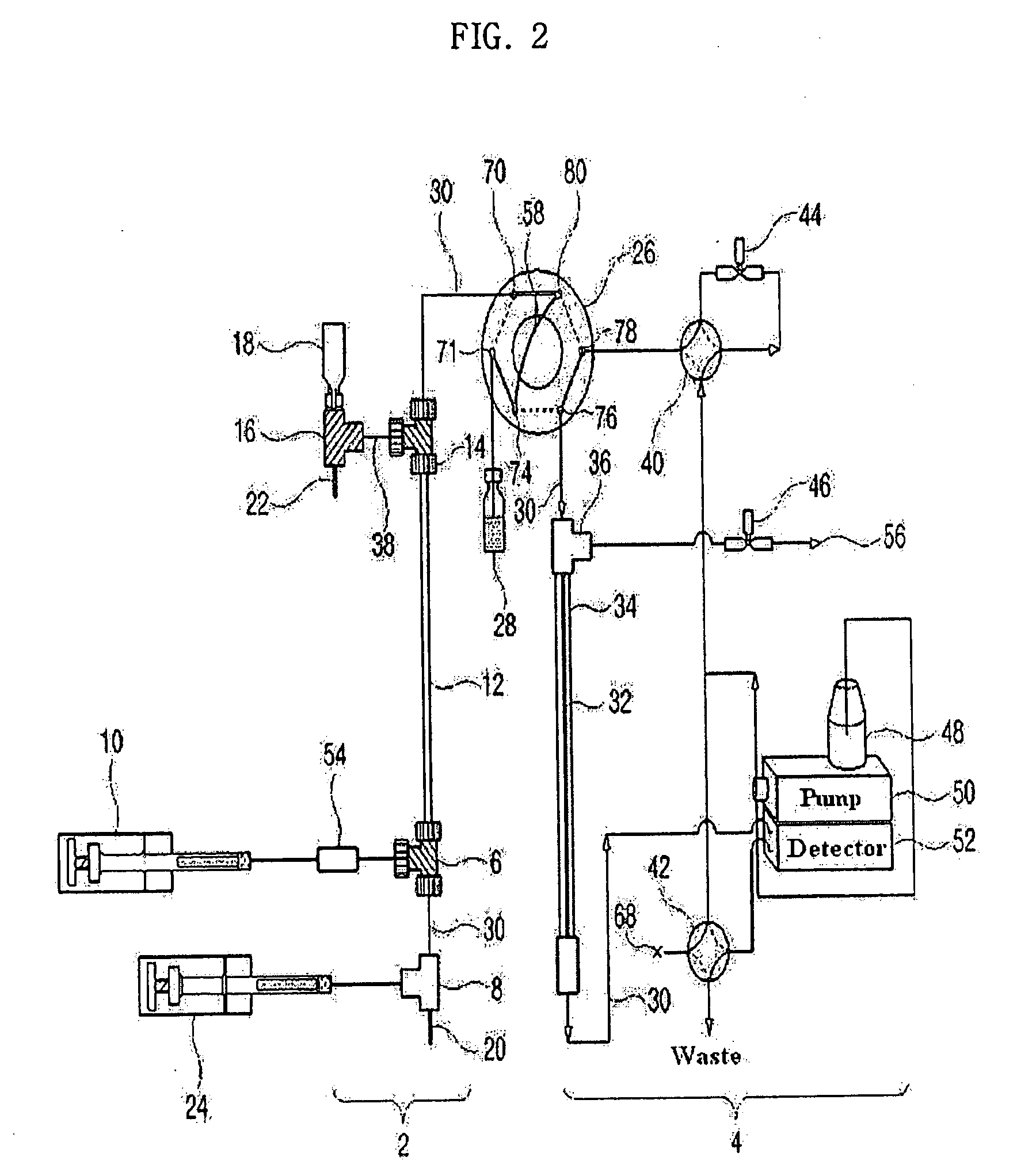
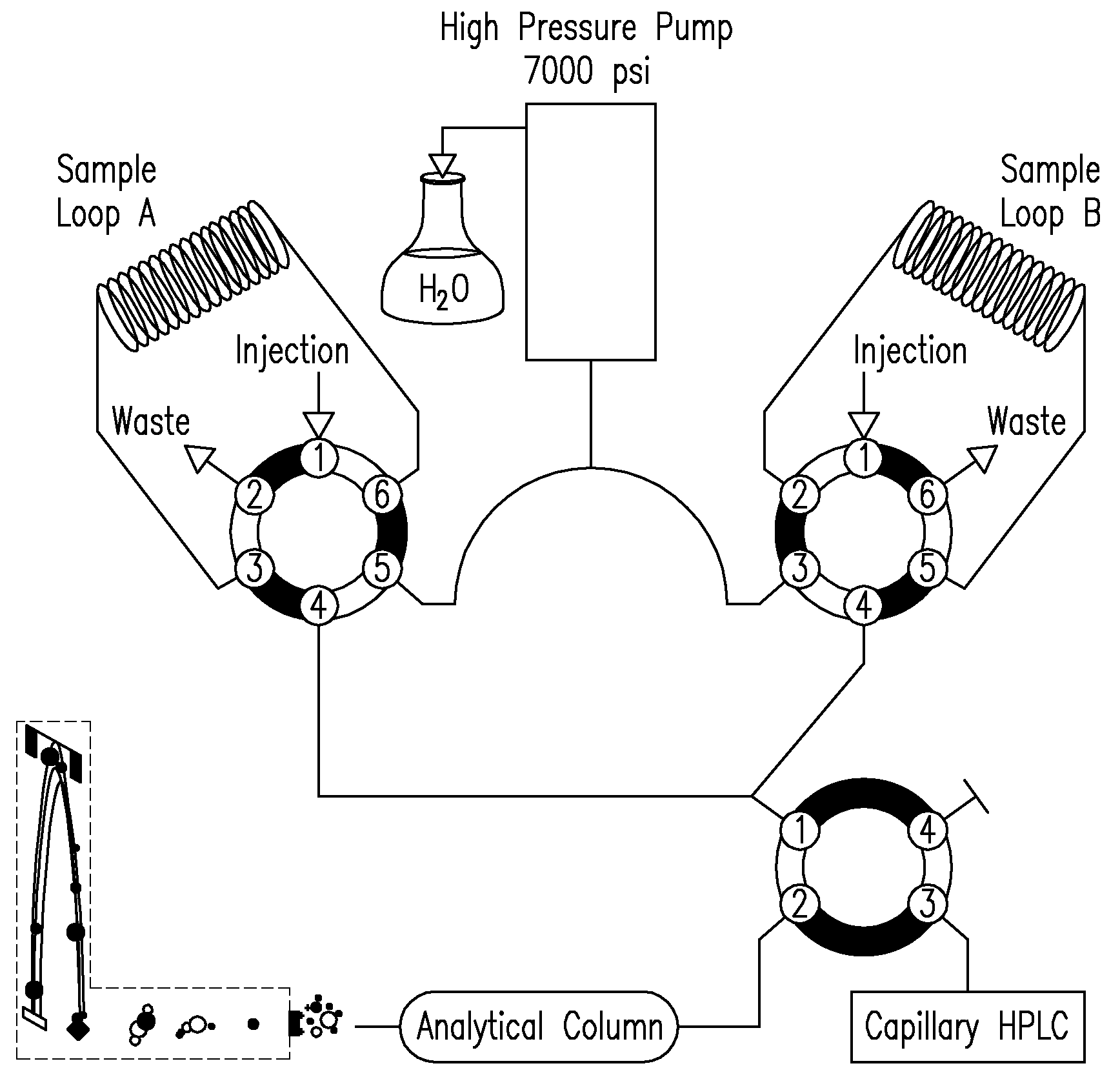
Apparatus for Protein Separation Using Capillary Isoelectric Focusing-Hollow Fiber Flow Field Flow Fractionation and Method Thereof
Disclosed herein is an apparatus for the separation of proteins, comprising a capillary isoelectric focusing unit (2) for primarily separating protein samples on the basis of pi; and a hollow fiber flow field flow fractionation unit (4), connected to one side of the capillary isoelectric focusing unit (2), for secondarily separating the protein samples. The apparatus allows proteins to be separated on the basis of pi and molecular weight without denaturation and can further be applied for the identification of proteins in conjunction with nanoflow liquid chromatography-electrospray ionization-tandem mass spectrometry after enzymatic digestion of the protein fractions.
Owner:IND ACADEMIC CORP FOUND YONSEI UNIV
Scaffolds of umbilical cord decellularized Wharton jelly for tissue engineering and preparation method thereof
InactiveCN102198292AControllable fine structureModerate degradation rateProsthesisFine structureEnzymatic digestion
The invention discloses scaffolds of umbilical cord decellularized Wharton jelly for tissue engineering and a preparation method thereof. Umbilical cords are employed as the raw material and their outer membranes and vascular tissues are peeled off. And the rest part of the umbilical cords is subjected to hypotonic freeze-thaw, mechanical pulverization, differential centrifugation, enzymatic digestion for decellularization. Then the umbilical cord Wharton jelly is collected and injected into a mold. After freeze drying and crosslinking, multiple three dimensional porous sponge scaffolds and composite scaffolds can be obtained. The method of the invention has the advantages of wide material source, low cost, simple technology. And the prepared scaffolds are characterized by controllable fine structure, appropriate degradation rate, good biocompatibility, and biomechanical strength, which are in favor of cell adhesion and the uniform distribution of seed cells within the scaffolds, as well as seed cell multiplication, migration and growth. Thus, the scaffolds of umbilical cord decellularized Wharton jelly in the invention can be widely applied in the tissue engineering field such ascartilage, bone, skin and nerve, with a favorable clinical application prospect.
Owner:卢世璧
Method for extracting type I collagen
InactiveCN106701879AImprove extraction yieldGood biocompatibilityConnective tissue peptidesPeptide preparation methodsEnzymatic digestionActive enzyme
The invention relates to a method for extracting type I collagen. The method comprises the following steps: cleaning bovine tendon, refrigerating and slicing; disinfecting and degreasing the sliced bovine tendon with 75-percent alcohol and normal hexane; adding a proper amount of active enzyme into homogenate for performing enzymatic digestion after removing impure proteins and smashing the homogenate; performing salting-out and dialysis treatment on supernatant; performing freeze drying and vacuum packaging on a collagen stock solution obtained after the dialysis to obtain a type I collagen product. The method is simple in preparation process, can be used for realizing large-scale production, and is an economical and effective extraction method. The type I collagen extracted by the method has superior biocompatibility and bioactivity, can be applied to biomedicine, and has an important application value in the field of makeups; meanwhile, epithelial cells can be activated, the hyperplasia of the epithelial cells and the generation of collagenase are facilitated, and the skin becomes tight and elastic.
Owner:WUHAN YIJIABAO BIOMATERIAL CO LTD
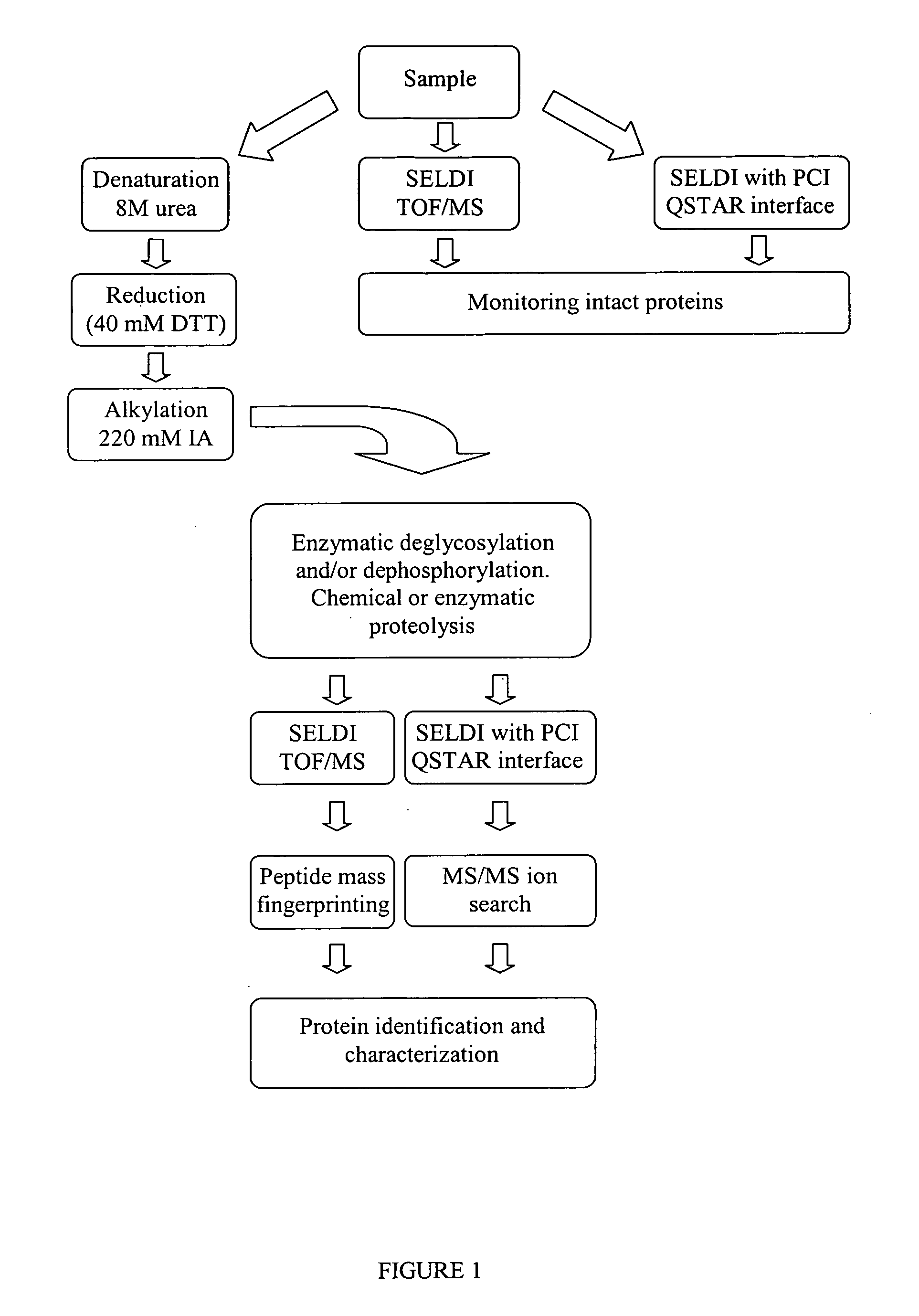
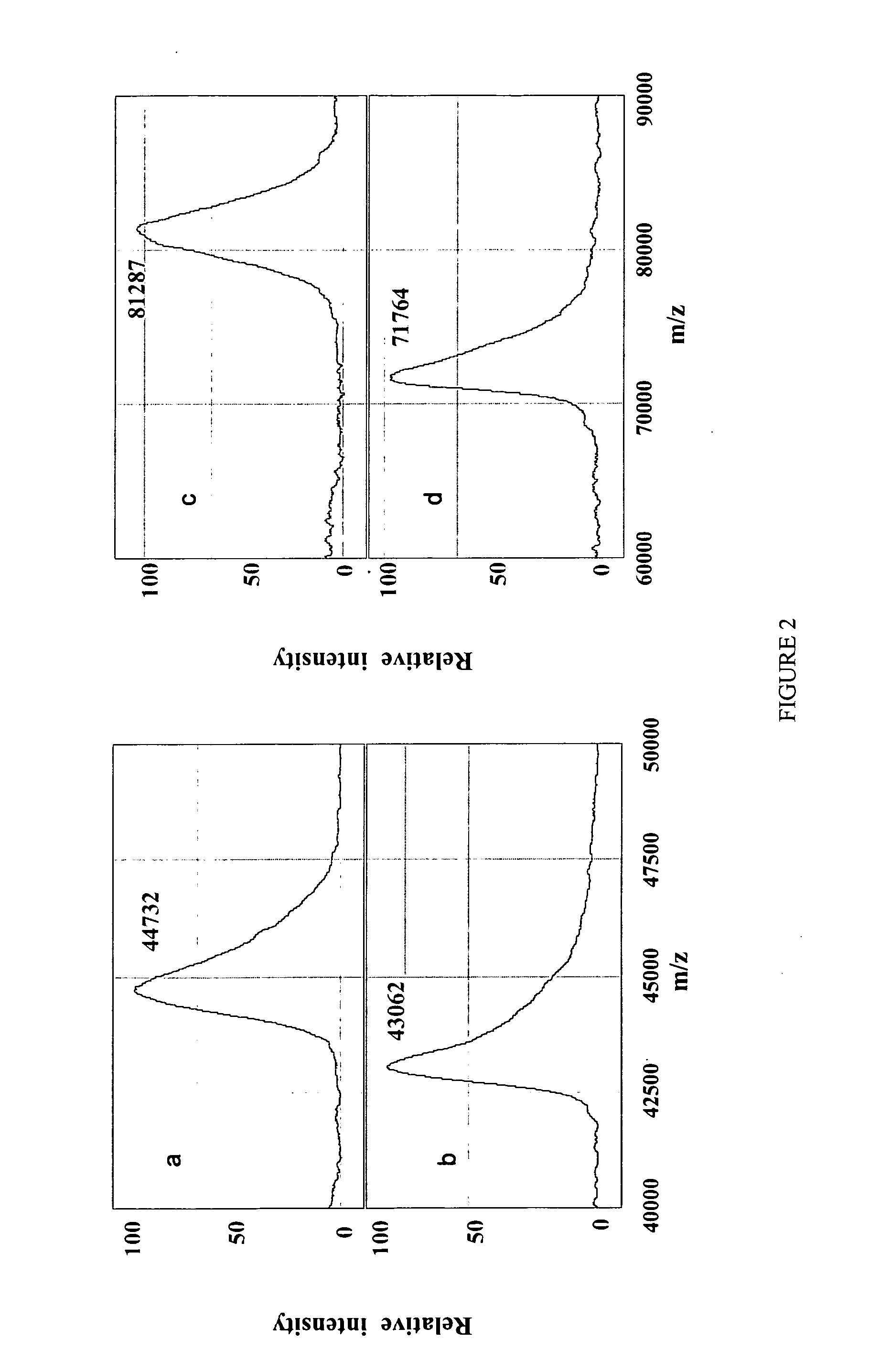
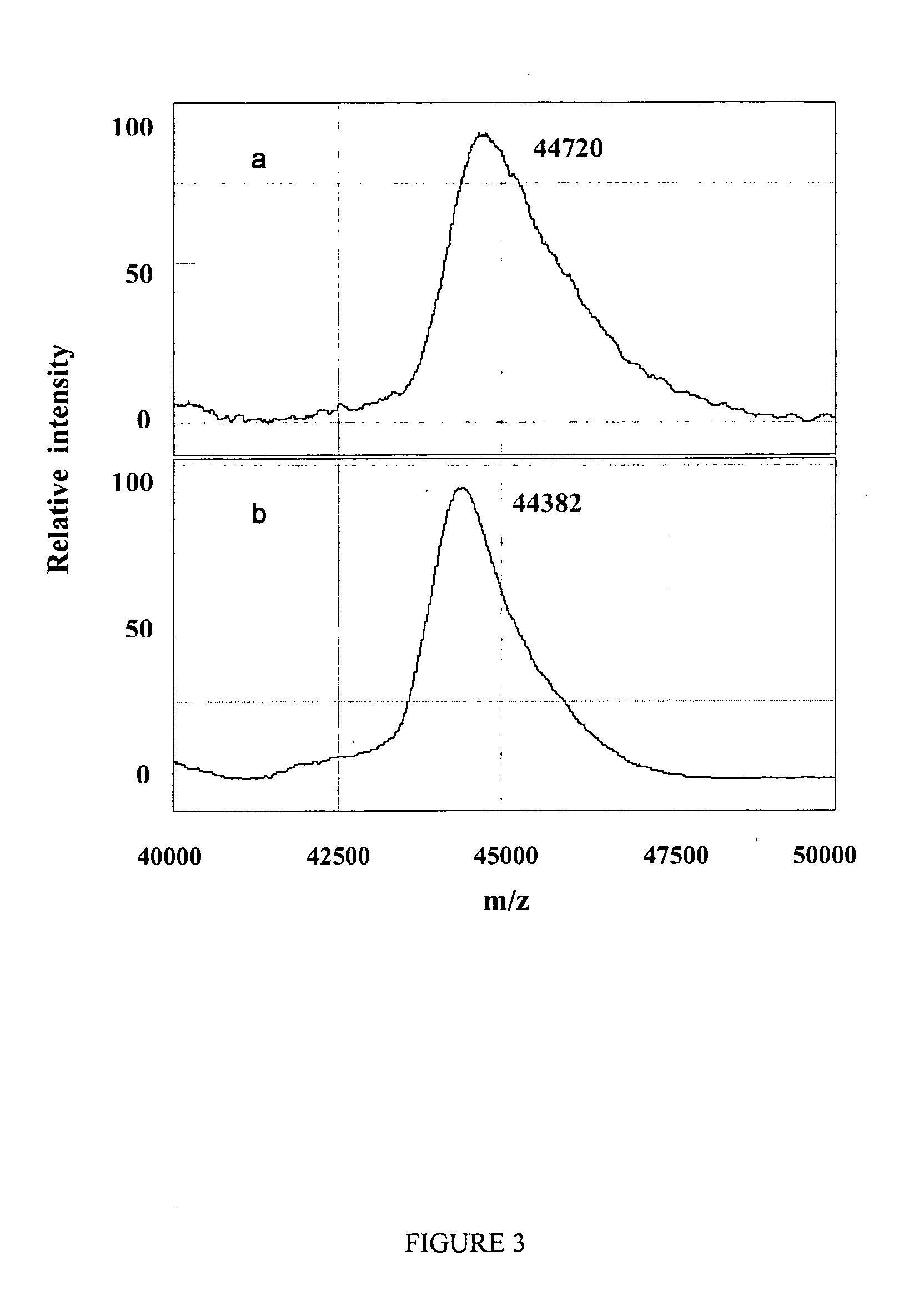
Complete chemical and enzymatic treatment of phosphorylated and glycosylated proteins on protein chip arrays
InactiveUS20060269980A1Quick upgradeSimpler, more sensitive methodologiesMicrobiological testing/measurementBiological material analysisEnzymatic digestionChemical treatment
A simple and quick protocol for chemical treatment, enzymatic or chemical digestion, and subsequent identification of proteins on protein chip arrays is disclosed, together with kits therefor. The chemical treatment comprises denaturation, reduction and alkylation while enzymatic digestion encompasses deglycosylation, dephosphorylation, and digestion by various proteases. Digestion is also accomplished by various chemicals that are known to induce proteolysis. All reactions are carried out sequentially on chip. Subsequent peptide mass fingerprinting or product ion searches allow the identification of specific peptides which can be correlated to proteins. The method of the present invention can be applied to the analysis of biological samples such as urine and plasma to identify biomarkers in diseased states. The methods of the present invention allow complete on chip treatment, which can be used for rapid protein identification and structural characterization of heavily posttranslationally modified proteins.
Owner:GIBBS BERNARD F
Protein mixture analysis by mass spectrometry
ActiveUS7070949B2Overcomes time pressureReduce measurementElectrolysis componentsPeptide/protein ingredientsEnzymatic digestionChromatographic separation
The invention relates to the analysis of complex protein mixtures such as entire proteomes, and in particular the rapid detection of previously unknown or unusually expressed proteins by common enzymatic digestion, subsequent chromatographic separation and analysis of the digestion peptides by mass spectrometry. The invention consists in subjecting fractions of the digestion peptides separated by liquid chromatography to analysis by mass spectrometry, at a time other than that of the chromatography, in a tandem time-of-flight mass spectrometer with ionization by matrix assisted laser desorption (MALDI). This method finds many times more proteins than are found through the procedures predominantly used until now of two-dimensional gel electrophoresis with subsequent time-of-flight mass spectrometry. It also removes the time pressure that dominates the real-time analysis of coupled LC-MS processes, and it allows measurements to be reduced to the interesting proteins by intermediate analysis.
Owner:BRUKER DALTONIK GMBH & CO KG
DNA profiling and SNP detection utilizing microarrays
InactiveUS20060008823A1High sensitivityImprove accuracyMicrobiological testing/measurementDNA microarrayEnzymatic digestion
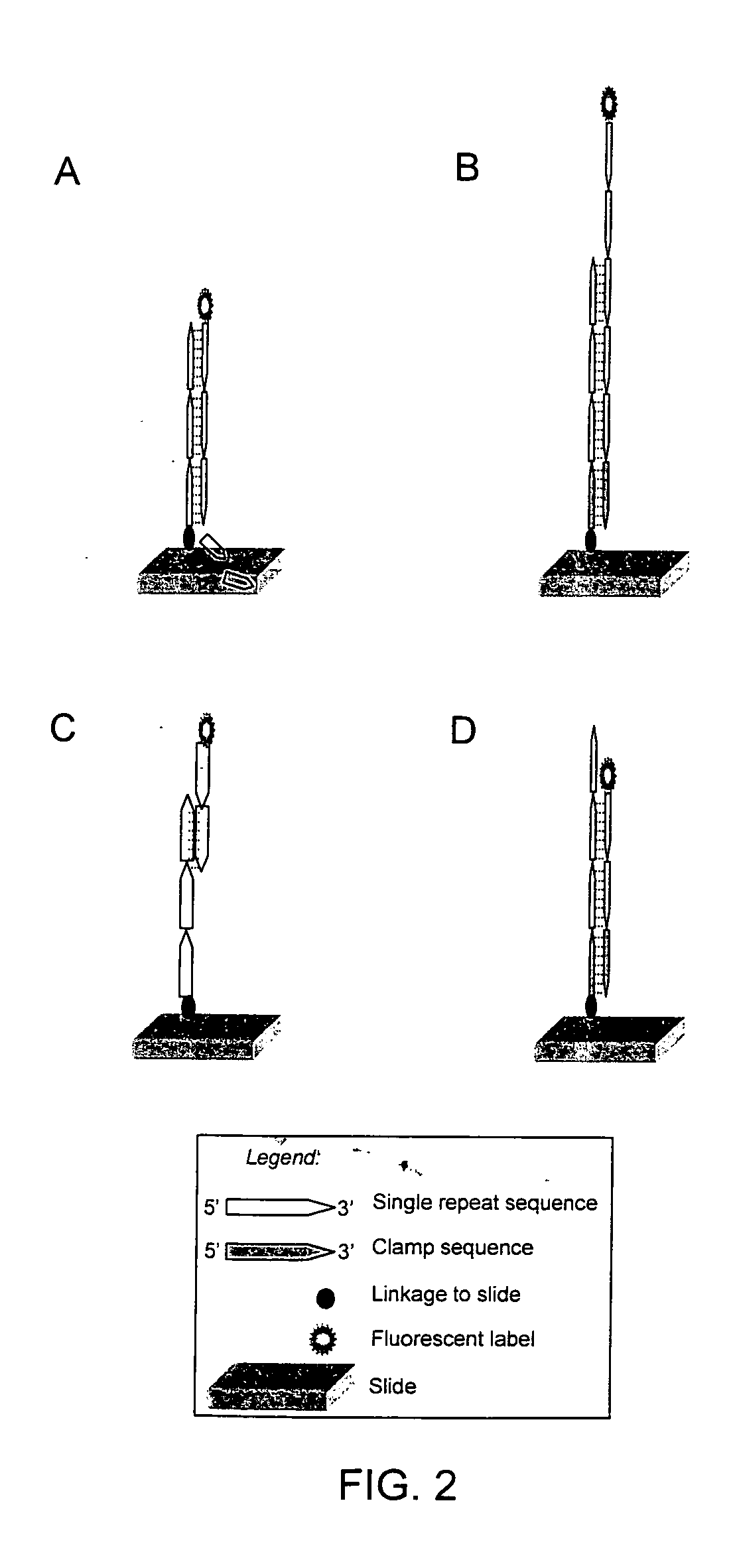
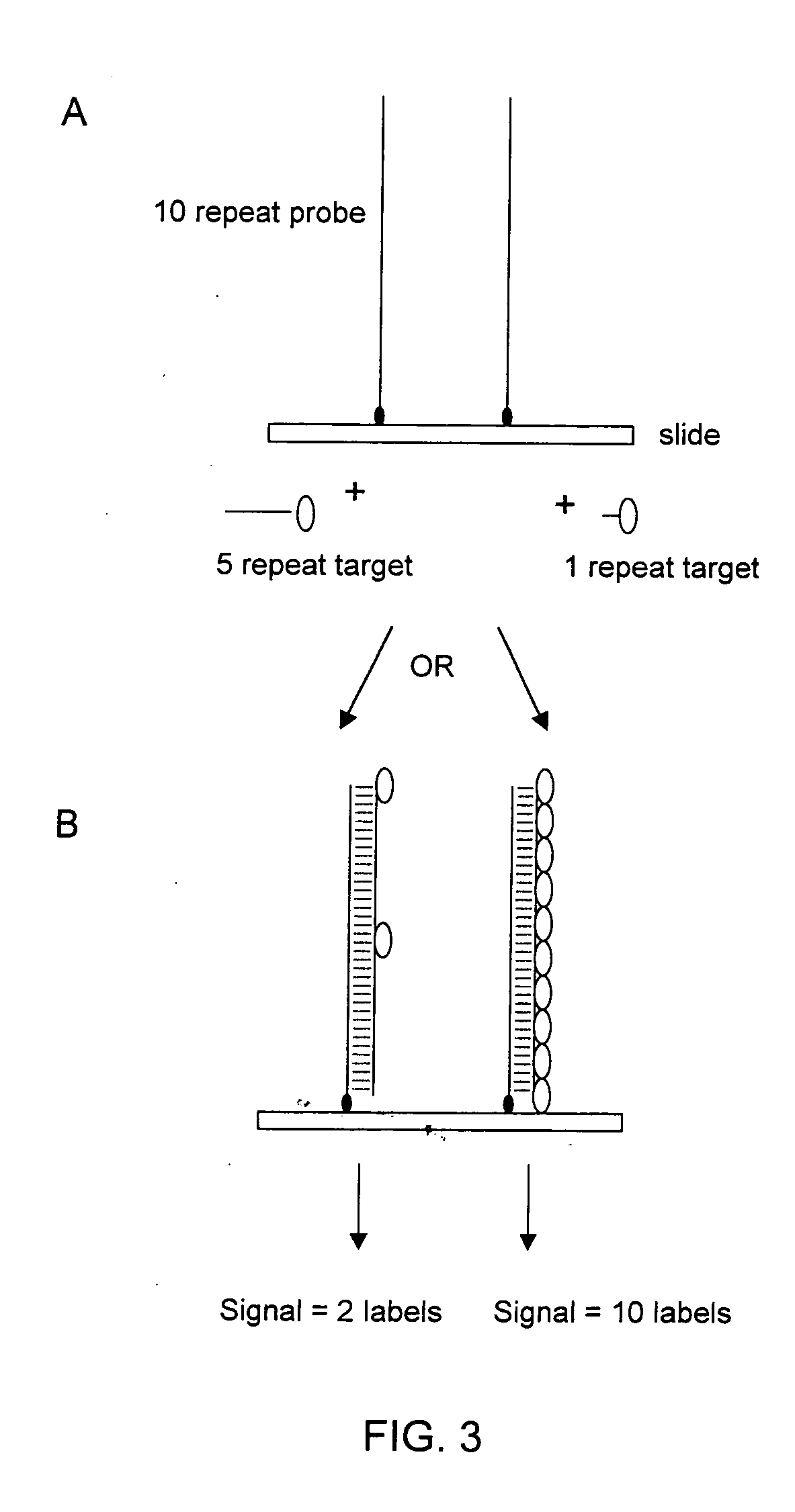
The present invention provides methods for rapidly identifying and distinguishing between different DNA sequences utilizing short tandem repeat (STR) analysis and DNA microarrays. Specifically, these methods facilitate the deduction of a target molecule's identity, length, and number of STRs. In an embodiment, a labeled STR target sequence is hybridized to a DNA microarray carrying complementary probes. These probes vary in length to cover the range of possible STRs. The labeled single-stranded regions of the DNA hybrids are selectively removed from the microarray surface utilizing a post-hybridization enzymatic digestion. The number of repeats in the unknown target is deduced based on the pattern of target DNA that remains hybridized to the microarray. The DNA profiling techniques described herein are useful for performing forensic analysis to uniquely identify individual humans or other species.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Decellularized heterogeneous corneal stroma carrier and its preparation method and application
The invention discloses a decellularized heterogeneous corneal stromal carrier and its preparation method and application. The carrier is an animal lamellar cornea from which epithelial cells and stromal cells have been removed through hypertonic solution combined with enzyme digestion. The preparation method of the carrier is as follows: firstly, take Fresh animal eyeballs are aseptically operated under an operating microscope, and the lamellar cornea with a thickness of 150 μm to 400 μm is drilled with a graduated trephine drill with a diameter of 5 mm to 12 mm, and then removed under the combined action of hypertonic solution and trypsin / pancreatin substitute The cells are finally dehydrated and dried to obtain the decellularized heterogeneous corneal stroma carrier, which is stored for future use. The decellularized heterogeneous corneal stroma carrier can be used as a corneal transplant donor to directly perform therapeutic corneal transplantation, and can also be used as an artificial biological corneal scaffold to construct a full-layer or lamellar artificial biological cornea. The decellularized heterogeneous corneal stroma carrier prepared by the invention has the following characteristics: the collagen is neatly arranged, similar to normal corneal tissue, and has good transparency after rehydration.
Owner:陕西省眼科研究所
Method for sequential culture of human umbilical cord blood mesenchymal stem cells by using two culture media
ActiveCN102559590APromote growthExcellent adhesionSkeletal/connective tissue cellsEnzymatic digestionStem cell culture
The invention discloses a method for sequential culture of human umbilical cord blood mesenchymal stem cells by using two culture media. A lymphocyte separation medium density gradient centrifugation method is used for separating umbilical cord blood mononuclear cells, a dulbecco modified eagle medium (DMEM) / F12 is used for primary culture of the mesenchymal stem cells of the human umbilical cord blood, and the method increases adherence of the cells, reduces growth of osteoclast like cells remarkably, facilitates formation of human umbilical cord blood-mesenchymal stem cells (hUCB-MSCs) colonies, and greatly improves successful rate of culture of the hUCB-MSCs. The DMEM / F12 is used continuously for subculturing to a P2 generation, the method is combined by a using enzymatic digestion and differential velocity adherent method simultaneously, and the method facilitates purification of P1-P2 generation cells remarkably. A P3 generation and the following generations are cultured by using an Oricell human umbilical cord mesenchymal stem cell culture medium, marker proteins and good morphological characteristics and growth characteristics of the hUCB-MSCs are maintained, and multilineage differentiation potential of the hUCB-MSCs is maintained. In addition, costs of the culture media adopted by the method are reduced remarkably.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
Enzymatic digestion of tissue
InactiveUS20090286304A1Promotes fast digestionMicrobiological testing/measurementNucleic acid reductionEnzymatic digestionNuclease inhibitor
The present invention concerns a compositions and method for isolating a nucleic acid from a cell-containing sample. There is disclosed a method comprising obtaining at least one cell-containing sample, which comprises a cell containing nucleic acid, obtaining at least one catabolic enzyme, obtaining at least one nuclease inhibitor, preparing an admixture of the sample, the catabolic enzyme, and the nuclease inhibitor, maintaining the admixture under conditions where the catabolic enzyme is active, and agitating the admixture, where the sample is digested to produce a nucleic acid-containing lysate of the sample.
Owner:LIFE TECH CORP
Universal peptide-binding scaffolds and protein chips
InactiveUS20050191706A1Bioreactor/fermenter combinationsPeptide librariesEnzymatic digestionProtein chip
The present invention provides a universal peptide-binding scaffold. This scaffold is used to bind a target. The target can be a peptide or peptides of interest (for example, peptides associated with a disease state) or can represent the entire proteome. The target can be either protein fragments prepared by enzymatic digestion of the entire proteome or N- or C-terminal short sequences exposed by chemical denaturation of the entire proteome (unfolded proteins). The universal peptide-binding scaffold can be tailored to specifically bind a target using the methods described herein.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Peptide for inhibiting dipeptidyl-peptidase iv
InactiveUS20140193463A1Metabolism disorderTetrapeptide ingredientsGelatin hydrolysateEnzymatic digestion
Owner:CHINA MEDICAL UNIVERSITY(TW)
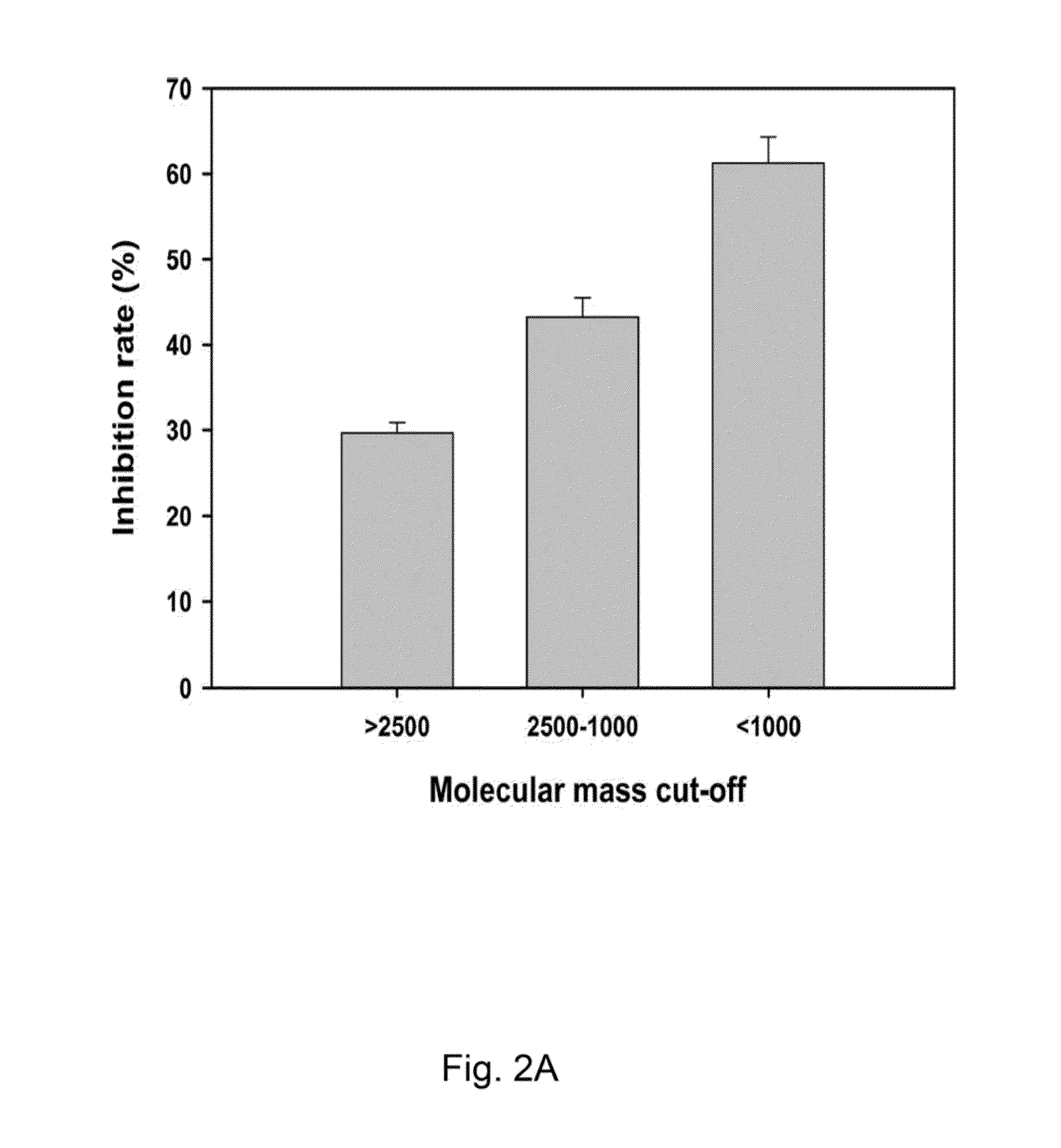
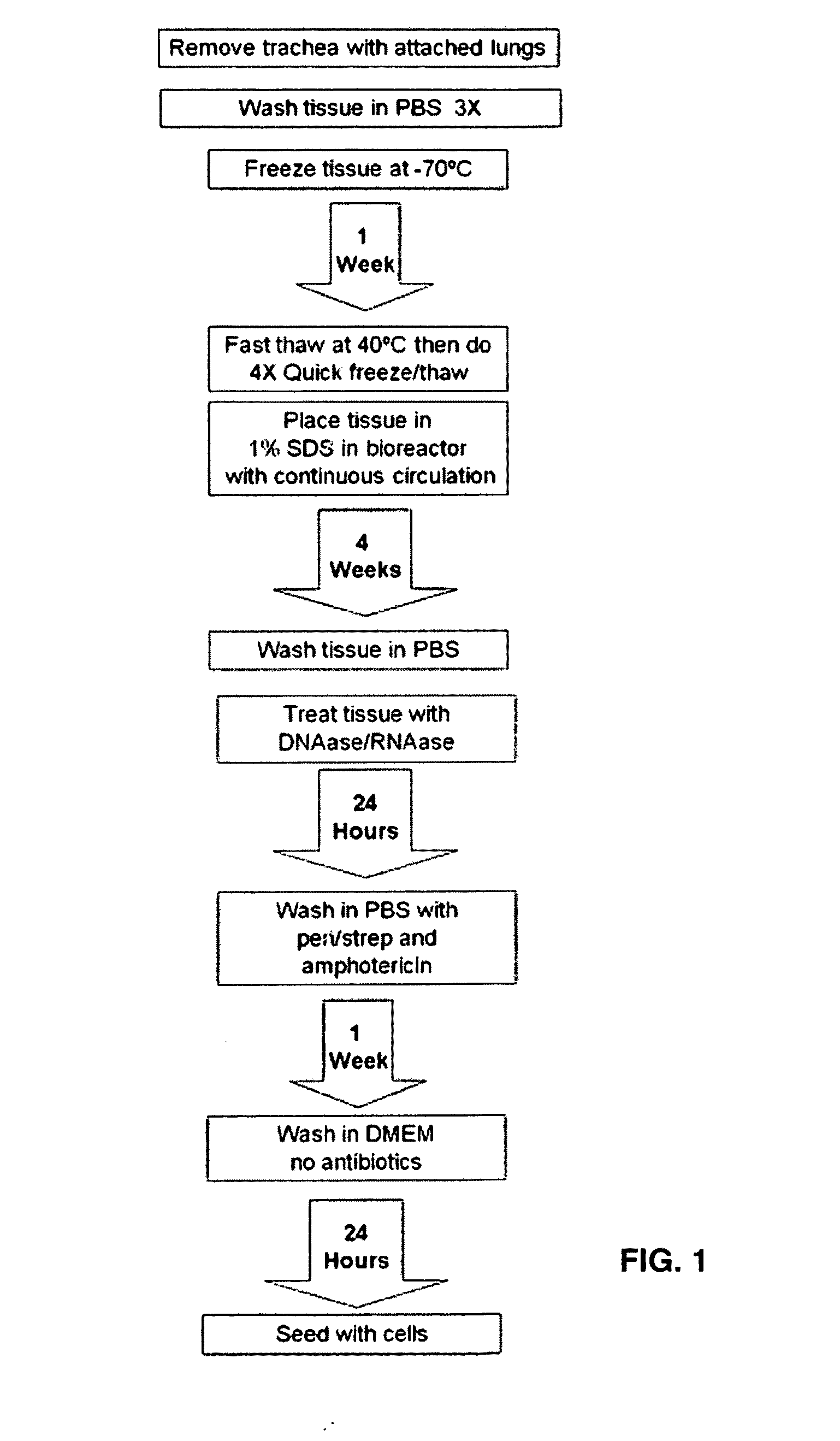
Production of and uses for decellularized lung tissue
Owner:BOARD OF RGT UNIV OF TEXAS SYST THE
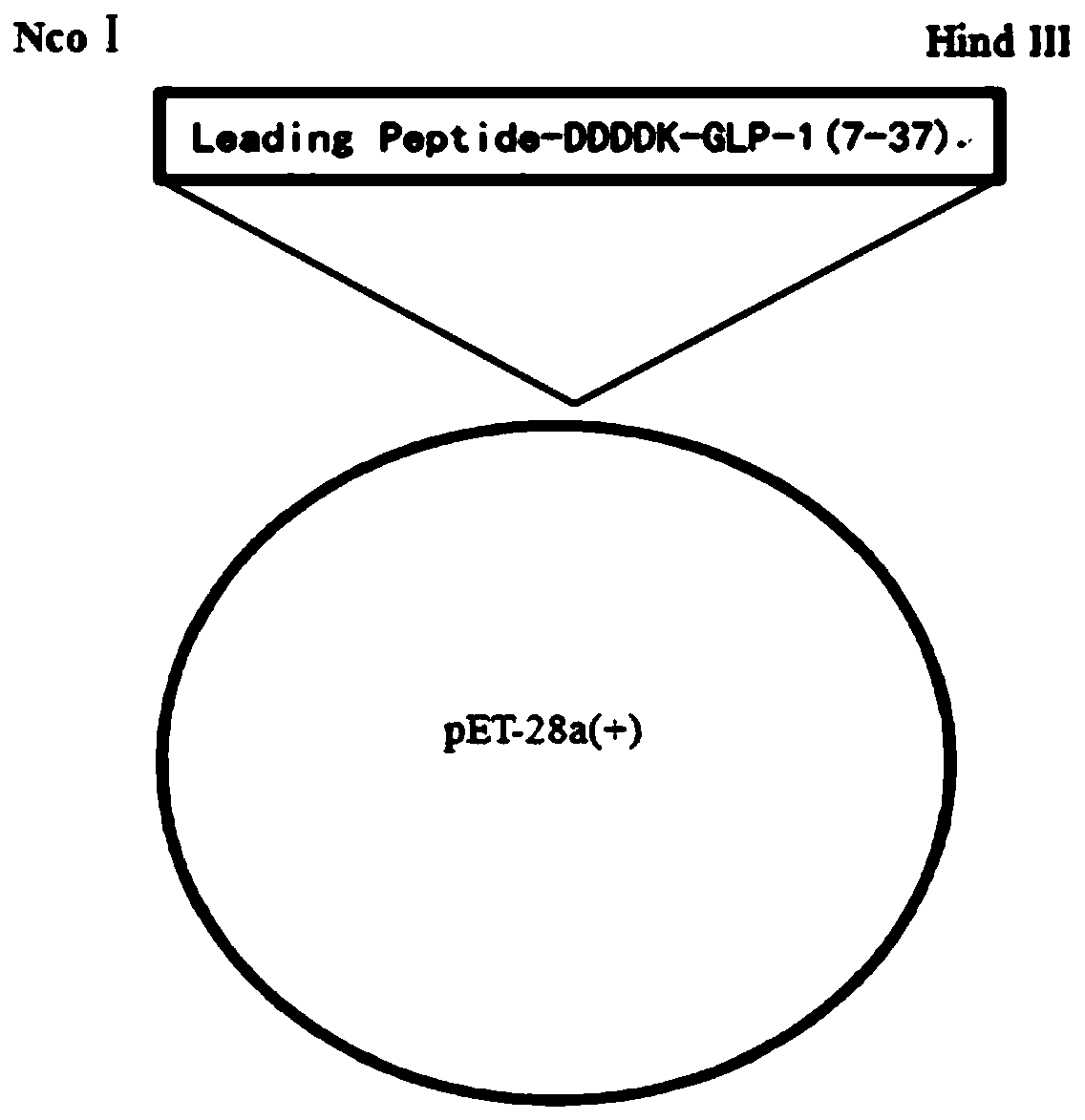
Fusion protein and method for preparing liraglutide intermediate polypeptide
ActiveCN110128552AShorten the enzymatic digestion timeLow costBacteriaAntibody mimetics/scaffoldsEnzymatic digestionEscherichia coli
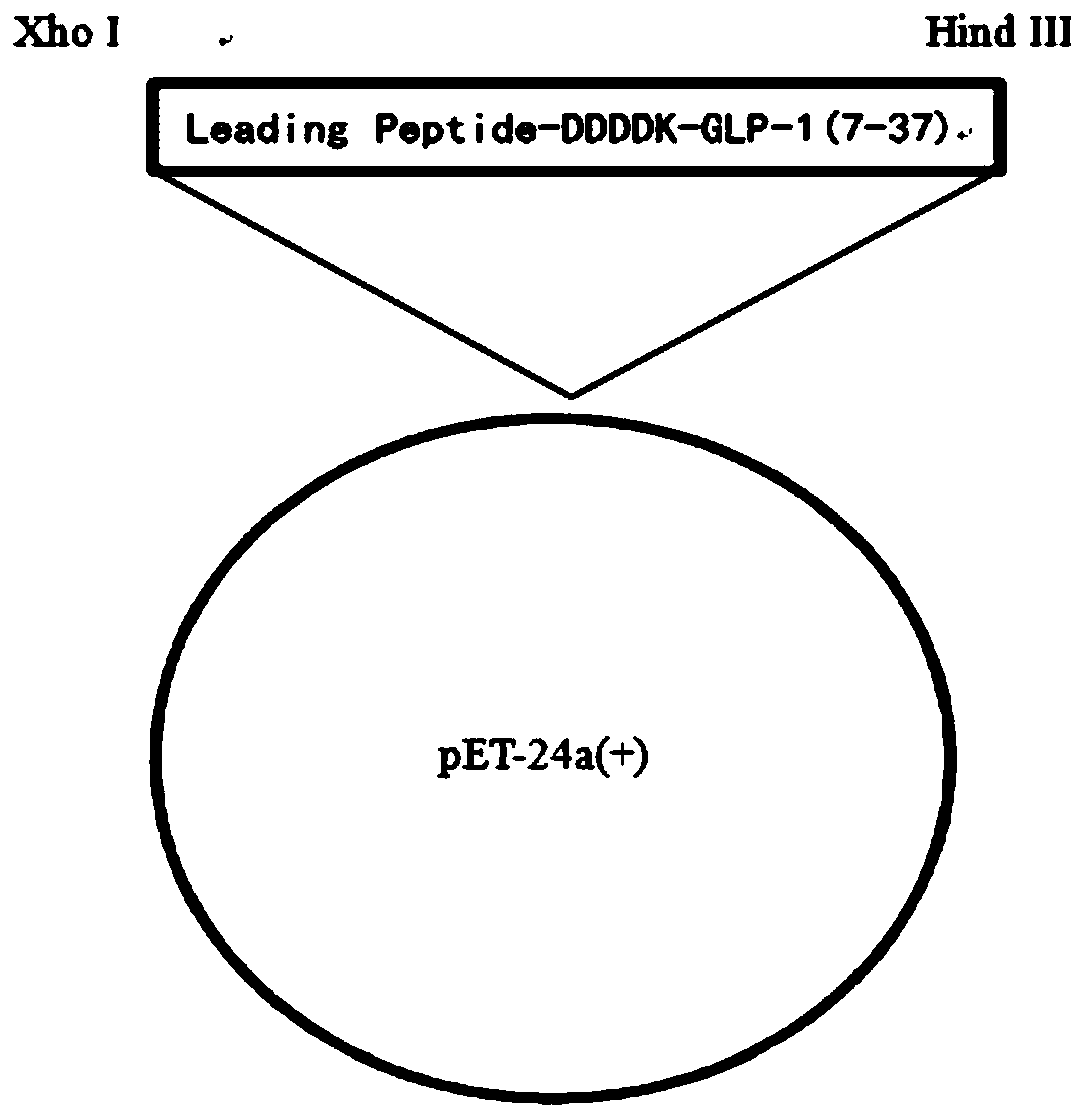
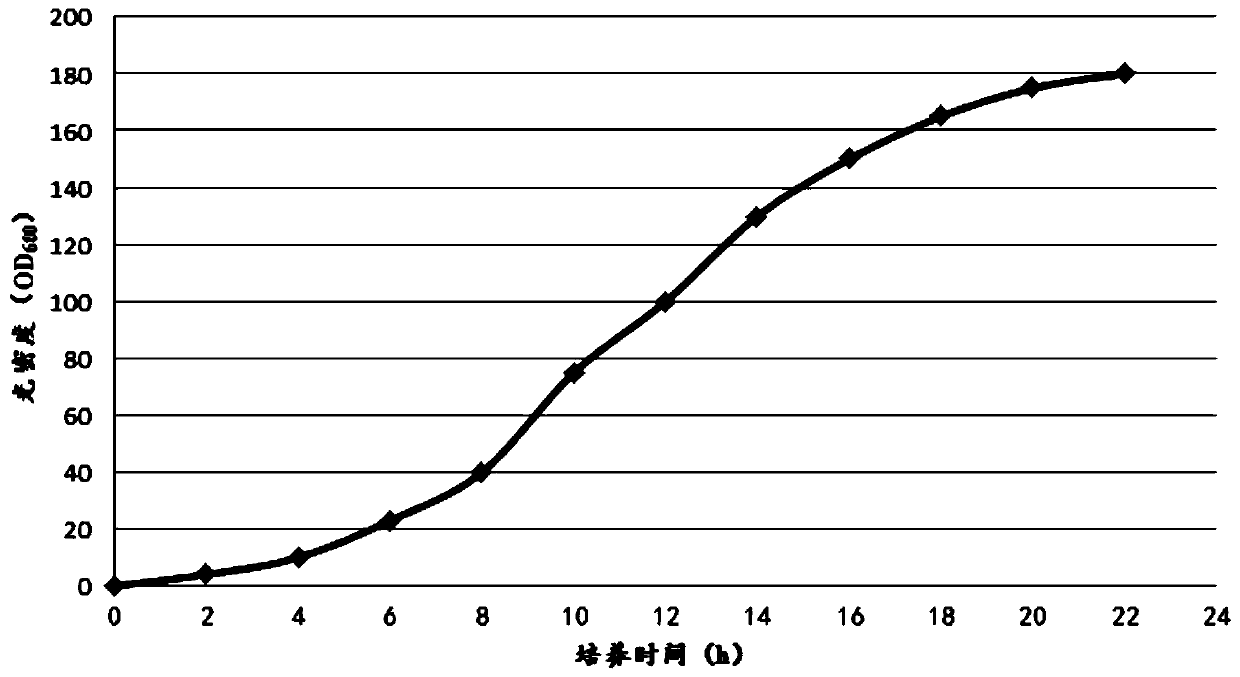
The invention belongs to the technical field of polypeptide preparation, and in particular, relates to a fusion protein and a method for preparing a liraglutide intermediate polypeptide GLP-1 (7-37).The method includes the main steps: constructing recombinant liraglutide intermediate engineering bacteria, expressing a GLP-1 (7-37) fusion protein by culture and induction of escherichia coli, and carrying out denaturation, renaturation, enzyme digestion and separated purification to obtain the intermediate polypeptide GLP-1 (7-37). Through changing a sequence of leading peptide, the expressionmode becomes an intracellular insoluble inclusion body expression, and the expression quantity is remarkably increased; inclusion bodies after washing are dissolved at high pH, and a large amount of denaturants are not needed to use, the inclusion bodies after washing are added to a inclusion body dissolved buffer solution at a high protein concentration of 5-40 g / L, the renaturation time does notexceed 1 h, and enterokinase digestion is carried out immediately after dissolution; the renaturation process can be reduced, the enzymatic digestion system is reduced, the cost of chemical reagentscan be reduced, and industrial amplification is facilitated; ion exchange separation and purification can be used, and the separation degree is high. The prepared liraglutide intermediate polypeptideGLP-1 (7-37) reaches 92% or more and the yield is more than 87%.
Owner:AMPHASTAR NANJING PHARMA +1
Method for enzymaticly digesting porcine fat tissue and applications thereof
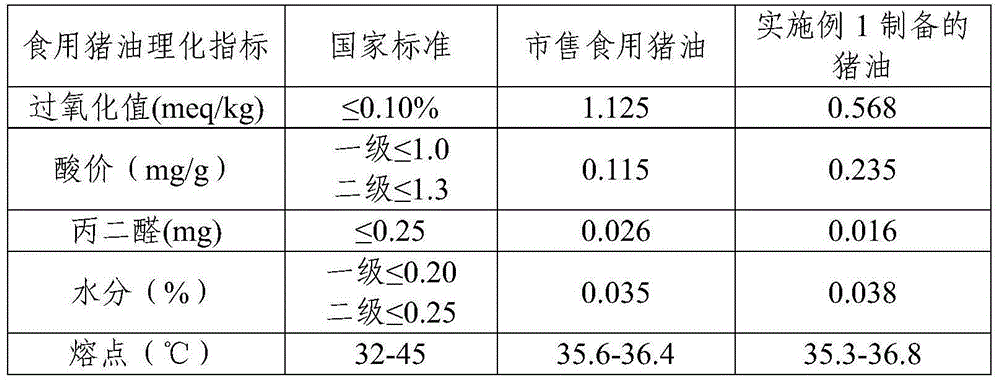
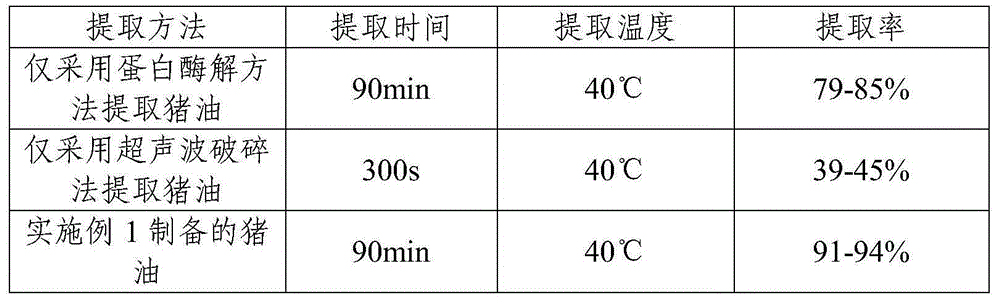
InactiveCN105018210ANatural dissolutionReduce moisture contentFatty-oils/fats refiningFatty-oils/fats productionTissue proteinEnzymatic digestion
The present invention relates to a method for enzymaticly digesting porcine fat, wherein fat cells are fragmented by using an ultrasonic wave-assisted enzymatic digestion method. The present invention also provides a method that is based on the foregoing method and that is for further refinement of advanced edible lard and fat tissue protein hydrolysate. The method comprises: extracting lard in in a cryogenic condition; obtaining the advanced edible lard after refining the lard; and obtaining a sauce by performing homogenizing, flavouring, thermal reacting, filling, and sterilizing on the protein hydrolysate obtained during enzymatic extraction.
Owner:CHINA MEAT RES CENT
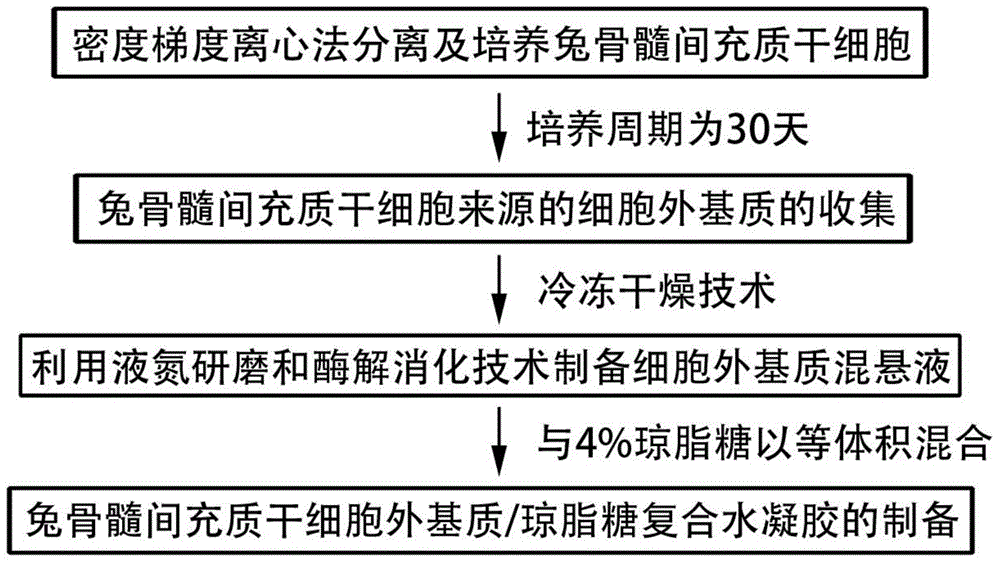
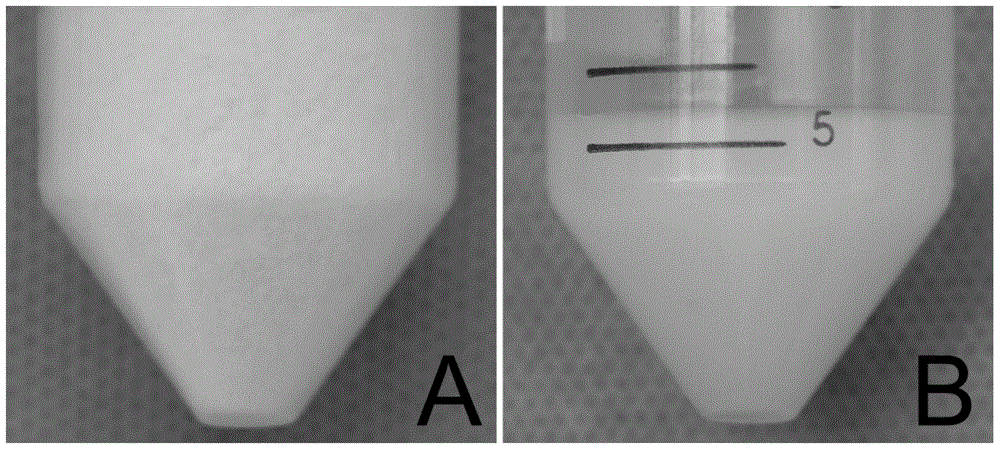
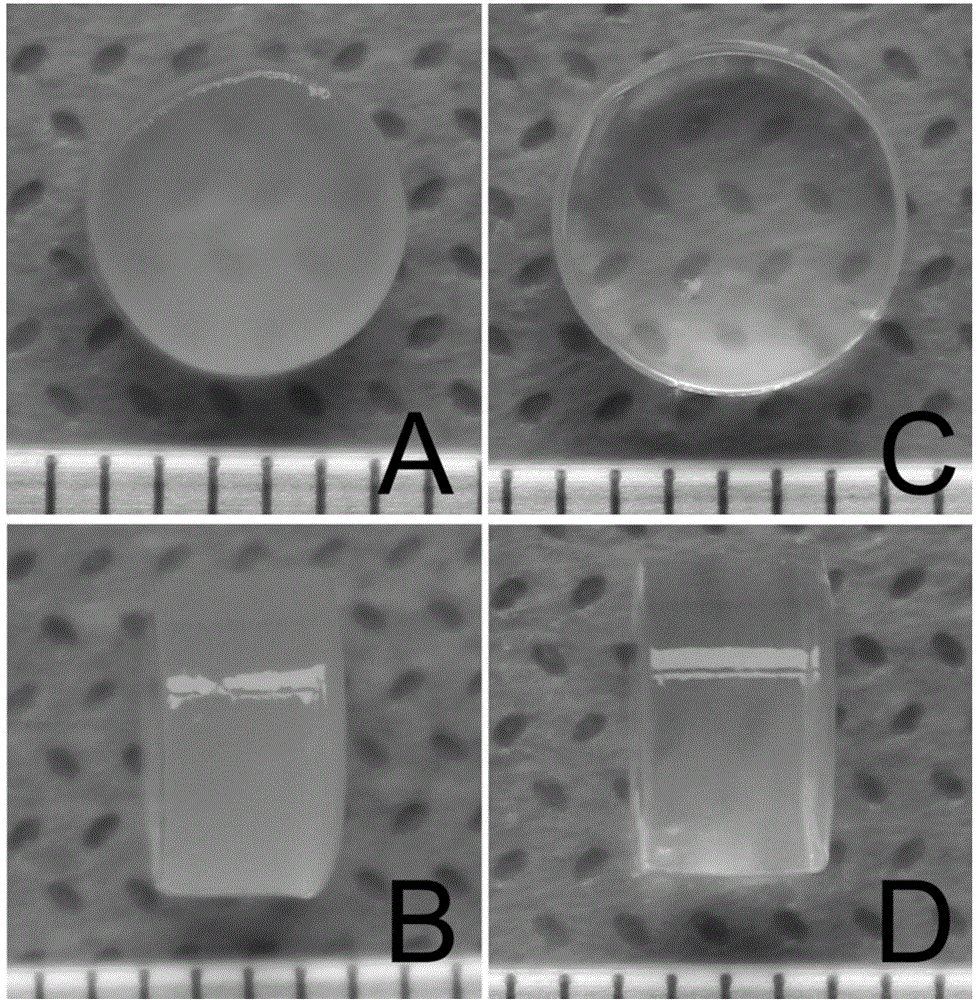
Injectable mesenchymal stem cell extracellular matrix/agarose composite hydrogel as well as preparation method and application thereof
InactiveCN104307046AImprove adhesionImprove plasticitySkeletal/connective tissue cellsProsthesisEnzymatic digestionCell-Extracellular Matrix
The invention discloses injectable mesenchymal stem cell extracellular matrix / agarose composite hydrogel as well as a preparation method and an application thereof. The preparation method comprises the following steps: culturing mesenchymal stem cells of a rabbit in vitro with monolayer high density, collecting a secreted extracellular matrix of the mesenchymal stem cells, and acquiring an extracellular matrix suspension by utilizing freeze drying, grinding under liquid nitrogen and an enzymatic digestion technology; and then fully and uniformly mixing the extracellular matrix suspension and agarose with same volume to prepare the mesenchymal stem cell extracellular matrix / agarose composite hydrogel material. The injectable mesenchymal stem cell extracellular matrix / agarose composite hydrogel can be injected into cartilage defect parts in arbitrary shapes by virtue of a simple injection mode and has good adhesion integration capability with peripheral host tissues,is sufficient in cell source, avoids potential risks caused by xenogenic resource materials, and is convenient to repair cartilage defects in arbitrary shapes to reach purpose of good integration with the peripheral host tissues.
Owner:南京医科大学附属南京医院
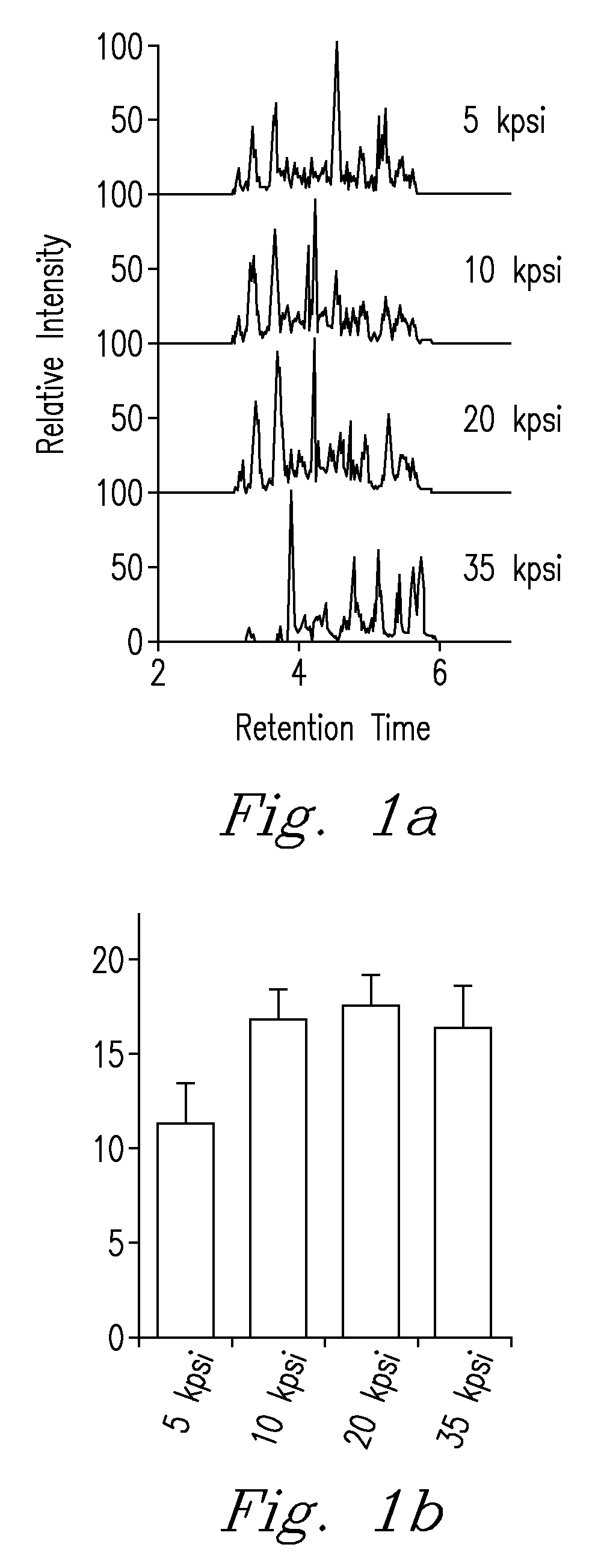
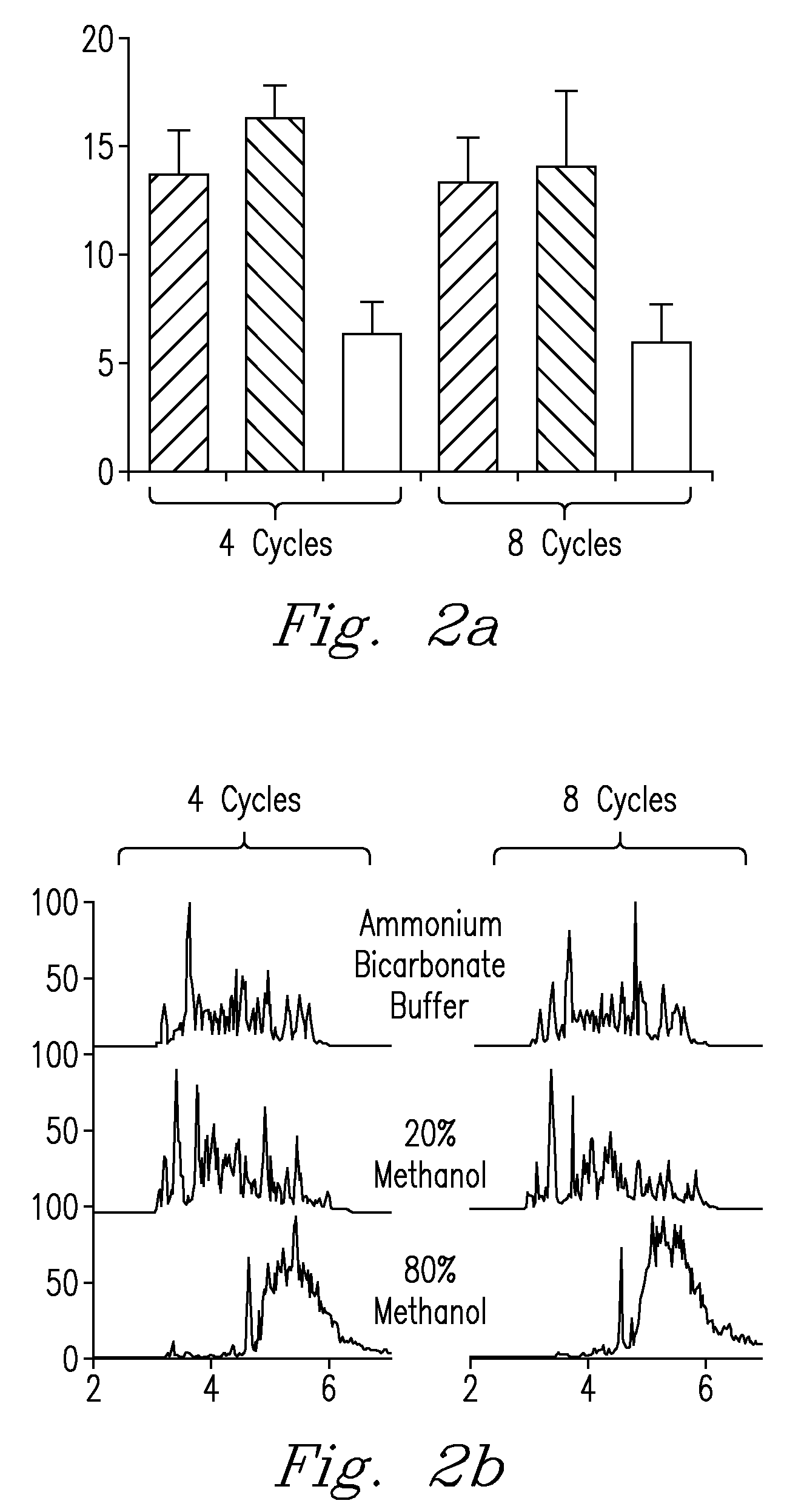
High Pressure Enzymatic Digestion System For Protein Characterization
InactiveUS20090203068A1Quick fixBioreactor/fermenter combinationsBiological substance pretreatmentsEnzymatic digestionPressure cycle
A method and system for obtaining samples for proteomic analysis that utilizes pressure and a preselected agent to obtain a processing sample in a significantly shorter period of time than prior art methods and which maintains the integrity of the processing sample through the preparatory process. In one embodiment of the invention, a sample and an enzyme are combined and subjected to a pressure, preferably a pressure cycle range that varies between 0 to 35 kpsi, for a period of time of preferably less than 60 seconds. This process results in producing a sample suitable for analysis, which is preferably introduced to another analytical instrument such as a mass spectrometry instrument, or other device.
Owner:BATTELLE MEMORIAL INST
Preparation method of fish single-cell suspension
InactiveCN106929468ABest preparation methodGood dispersionCell dissociation methodsArtificial cell constructsEnzymatic digestionFiltration
The application discloses a preparation method of a fish single-cell suspension. The preparation method comprises the following steps: preparing a PBS reagent, a D-Han liquid and an IV type collagenase liquid, preparing a kidney tissue block of a fish, and washing the kidney tissue for more than three times with PBS; putting the kidney tissue block in a flat vessel of an ice bath, adding PBS, shearing the kidney tissue block into small pieces, and putting the tissue blocks in a high throughput tissue centrifuge tube of a burnisher; adding the IV type collagenase liquid into the centrifuge tube and meanwhile, adding PBS to fix the volume to 4ml, putting a zirconium oxide wall to grind, and performing enzymatic digestion on a tissue homogenate; and performing filtration, centrifugalization, washing with PBS, performing centrifugalization to abandon supernate and removing cell debris, filtering the cell suspension and fixing the volume to 3ml with PBS, and storing the cell suspension for later use at 4 DEG C. By adopting an enzymatic hydrolysis grinding method, advantages of a griding method and an enzymatic hydrolysis method are combined. The tissue is more fully treated by enzymatic hydrolysis grinding, so that the problem that the cell yield is low and the cell viability is low for preparing the fish single-cell suspension can be solved.
Owner:SOUTHWEST UNIV
Cell-matrix microspheres, methods for preparation and applications
ActiveUS8679809B2Poor shapeReduced stabilityBioreactor/fermenter combinationsBiocideHigh cellEnzymatic digestion
A method has been developed to produce stable cell-matrix microspheres with up to 100% encapsulation efficiency and high cell viability, using matrix or biomaterial systems with poor shape and mechanical stability for applications including cell therapeutics via microinjection or surgical implantation, 3D culture for in vitro expansion without repeated cell splitting using enzymatic digestion or mechanical dissociation and for enhanced production of therapeutic biomolecules, and in vitro modeling for morphogenesis studies. The modified droplet generation method is simple and scalable and enables the production of cell-matrix microspheres when the matrix or biomaterial system used has low concentration, with slow phase transition, with poor shape and mechanical stability.
Owner:VERSITECH LTD
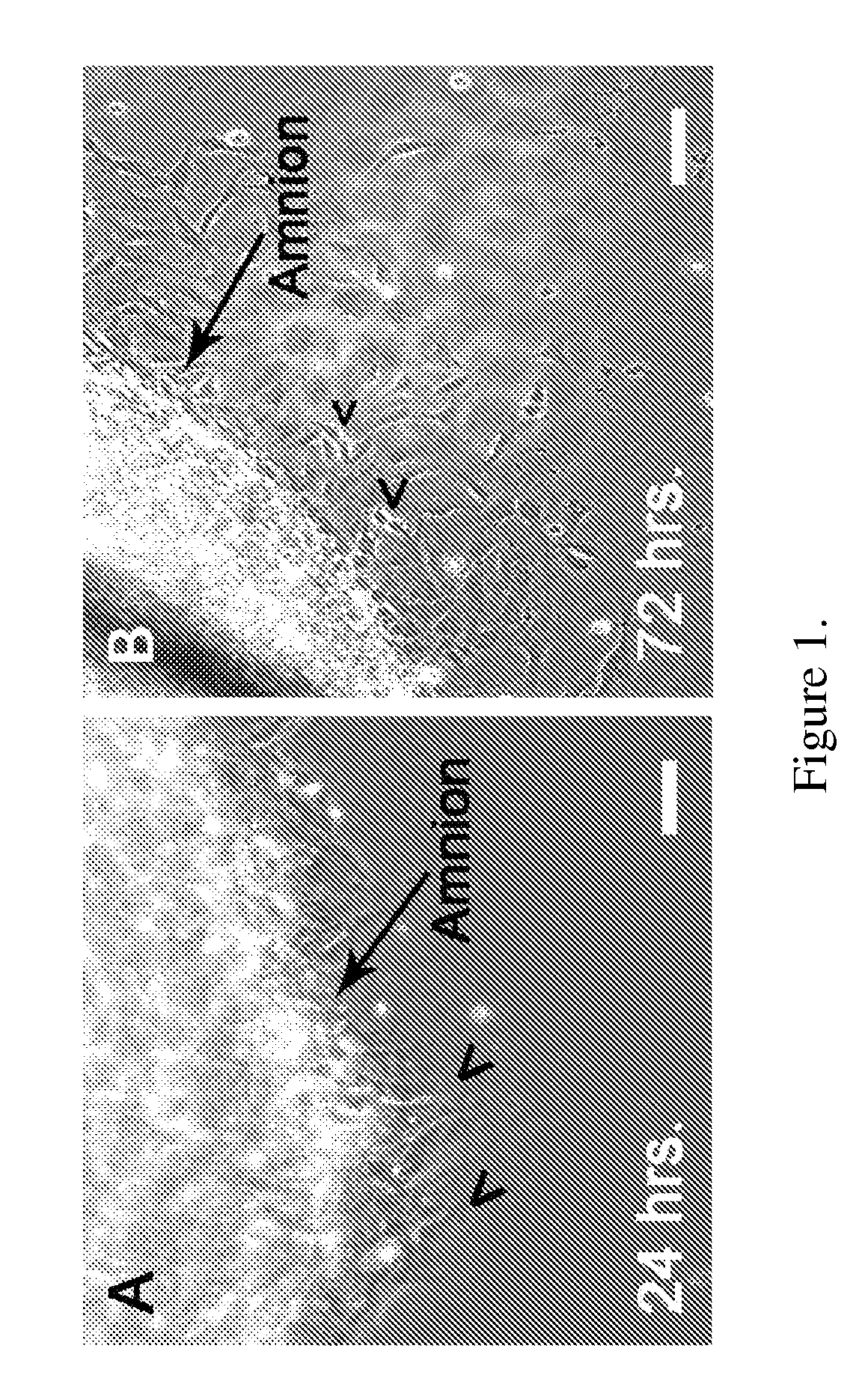
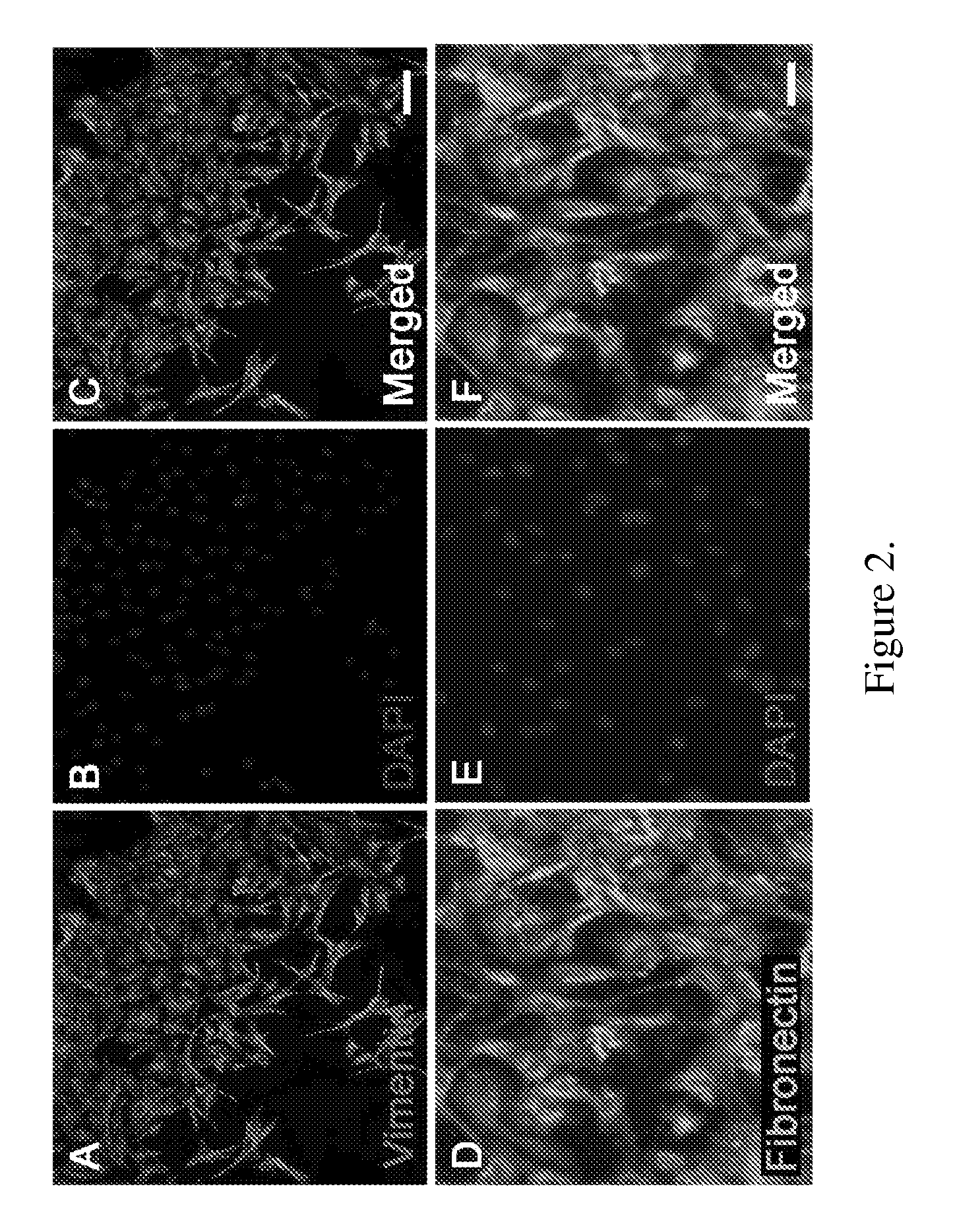
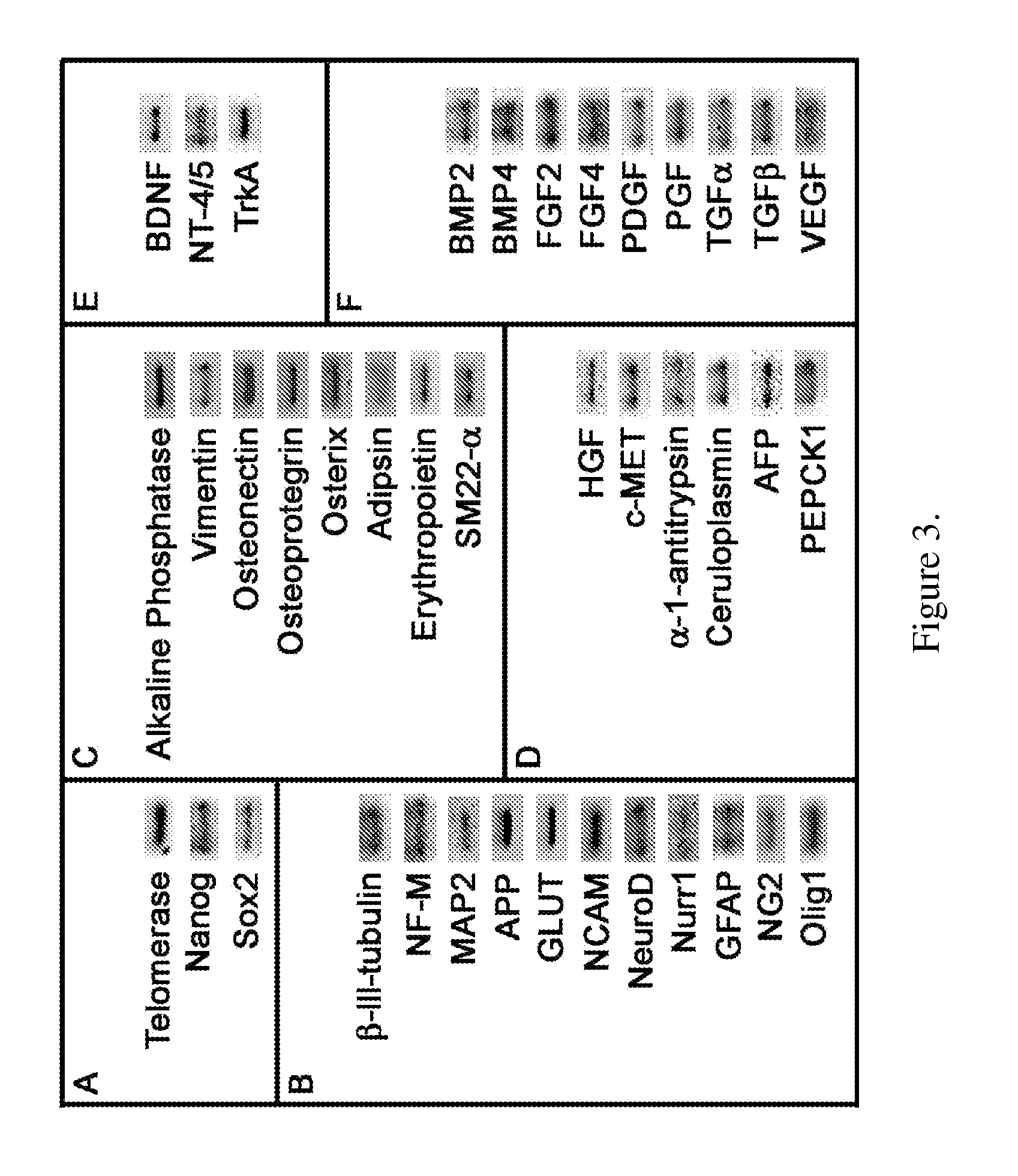
Obtaining multipotent amnion-derived stem cell (ADSC) from amniotic membrane tissue without enzymatic digestion
Owner:RUTGERS THE STATE UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com