Patents
Literature
67 results about "Corneal Transplant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The corneal tissue from a donor.
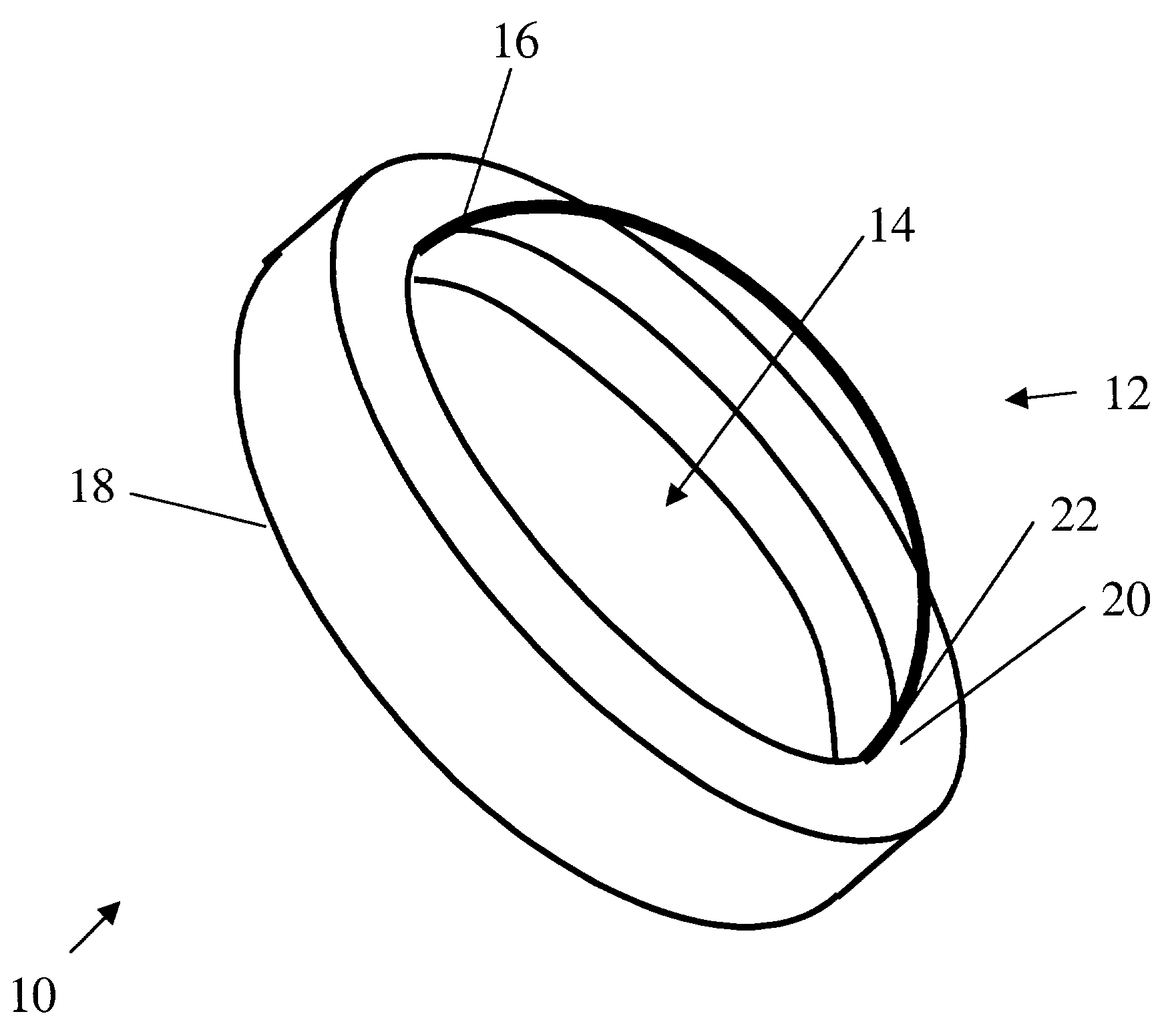
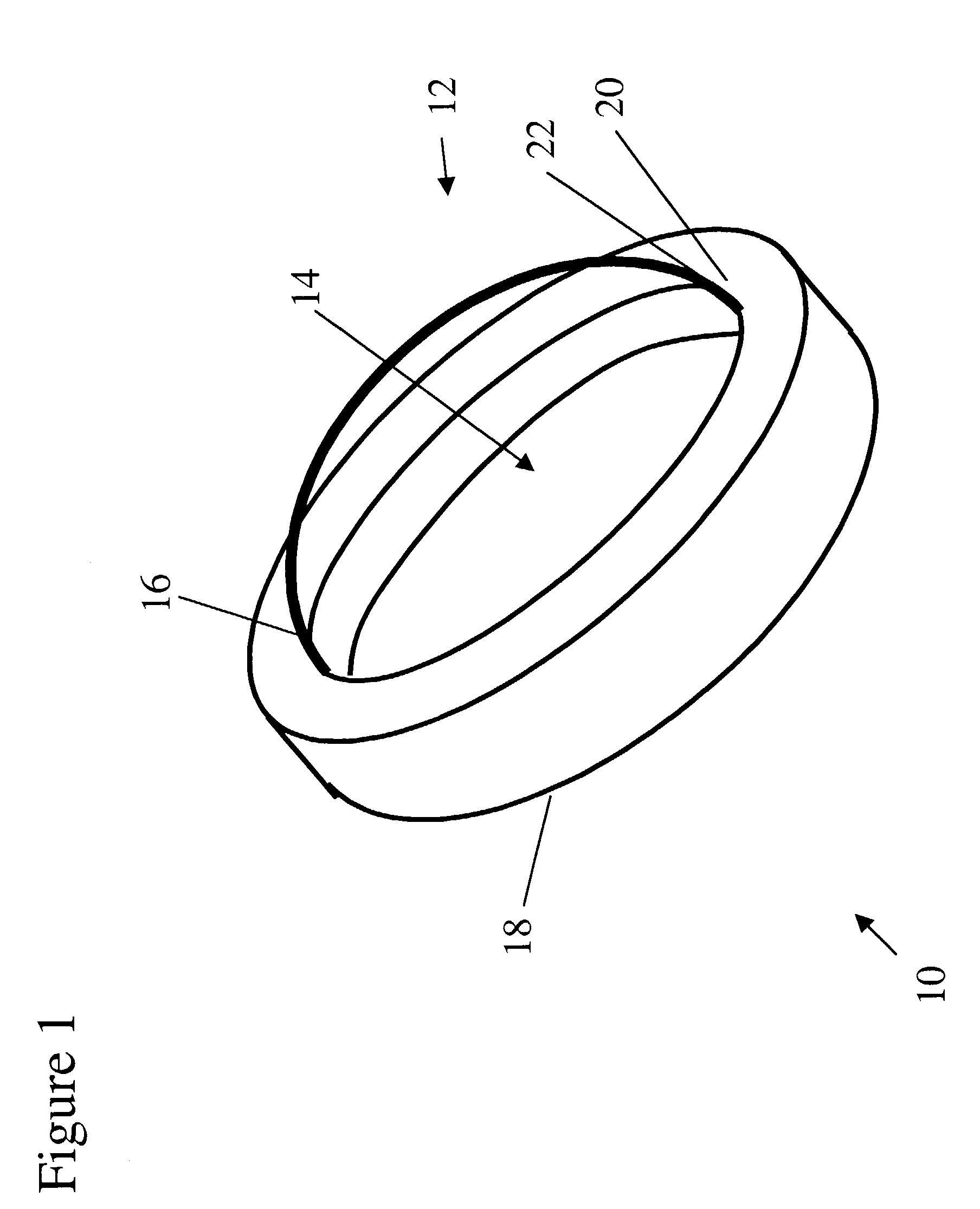
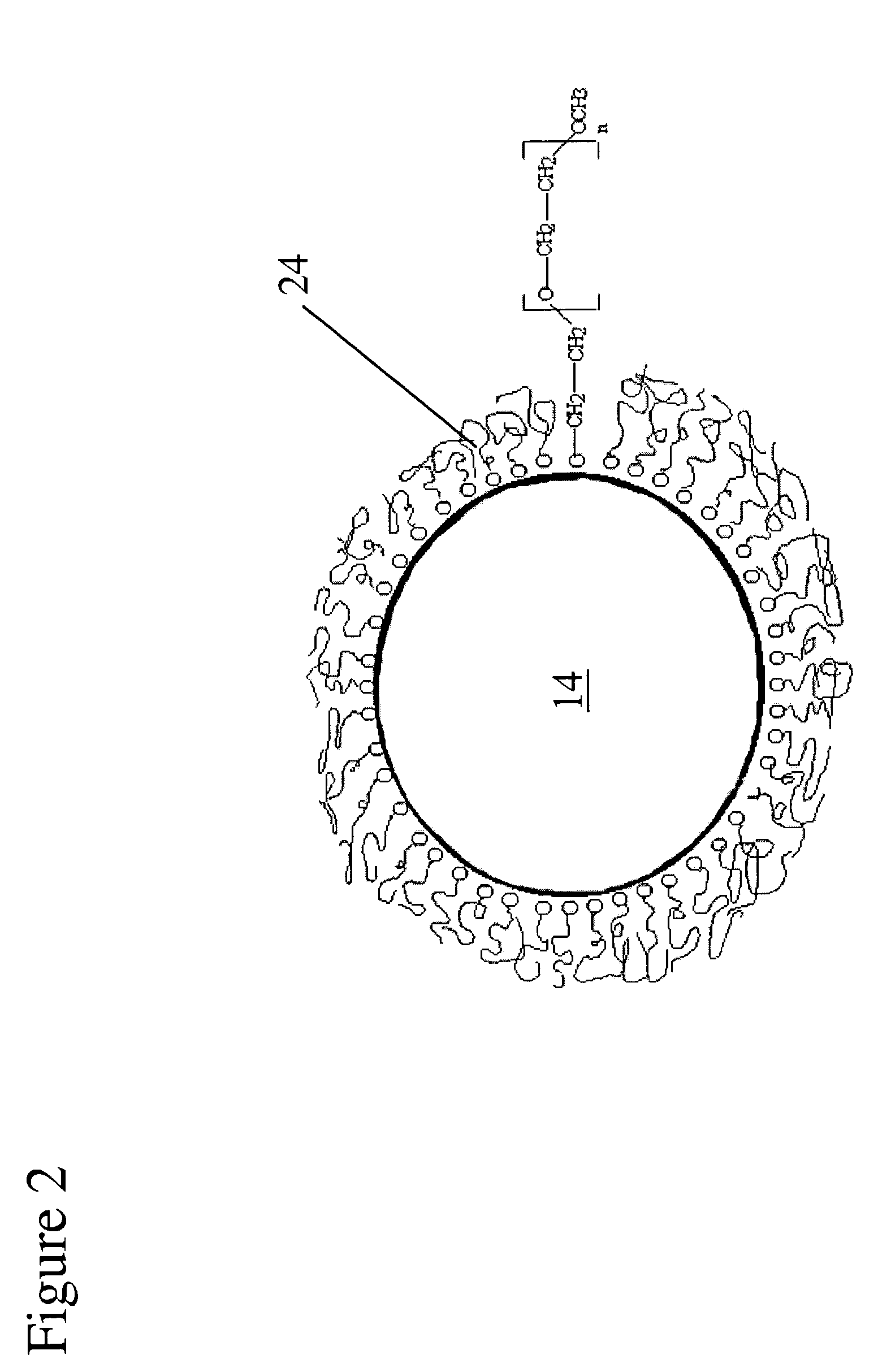
Artificial cornea
InactiveUS6976997B2Improve mechanical propertiesEasy to suture onto recipient bedMaterial nanotechnologyCoatingsDiseasePostoperative inflammation
The invention provides implants suitable for use as an artificial cornea, and methods for making and using such implants. Artificial corneas having features of the invention may be two-phase artificial corneas, or may be three phase artificial corneas. These artificial corneas have a flexible, optically clear central core and a hydrophilic, porous skirt, both of which are biocompatible and allow for tissue integration. A three-phase artificial cornea will further have an interface region between the core and skirt. The artificial corneas have a high degree of ocular tolerance, and allow for tissue integration into the skirt and for epithelial cell growth over the surface of the prosthesis. The use of biocompatible material avoids the risk of disease transmission inherent with corneal transplants, and acts to minimize post-operative inflammation and so to reduce the chance or severity of tissue necrosis following implantation of the synthetic cornea onto a host eye.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
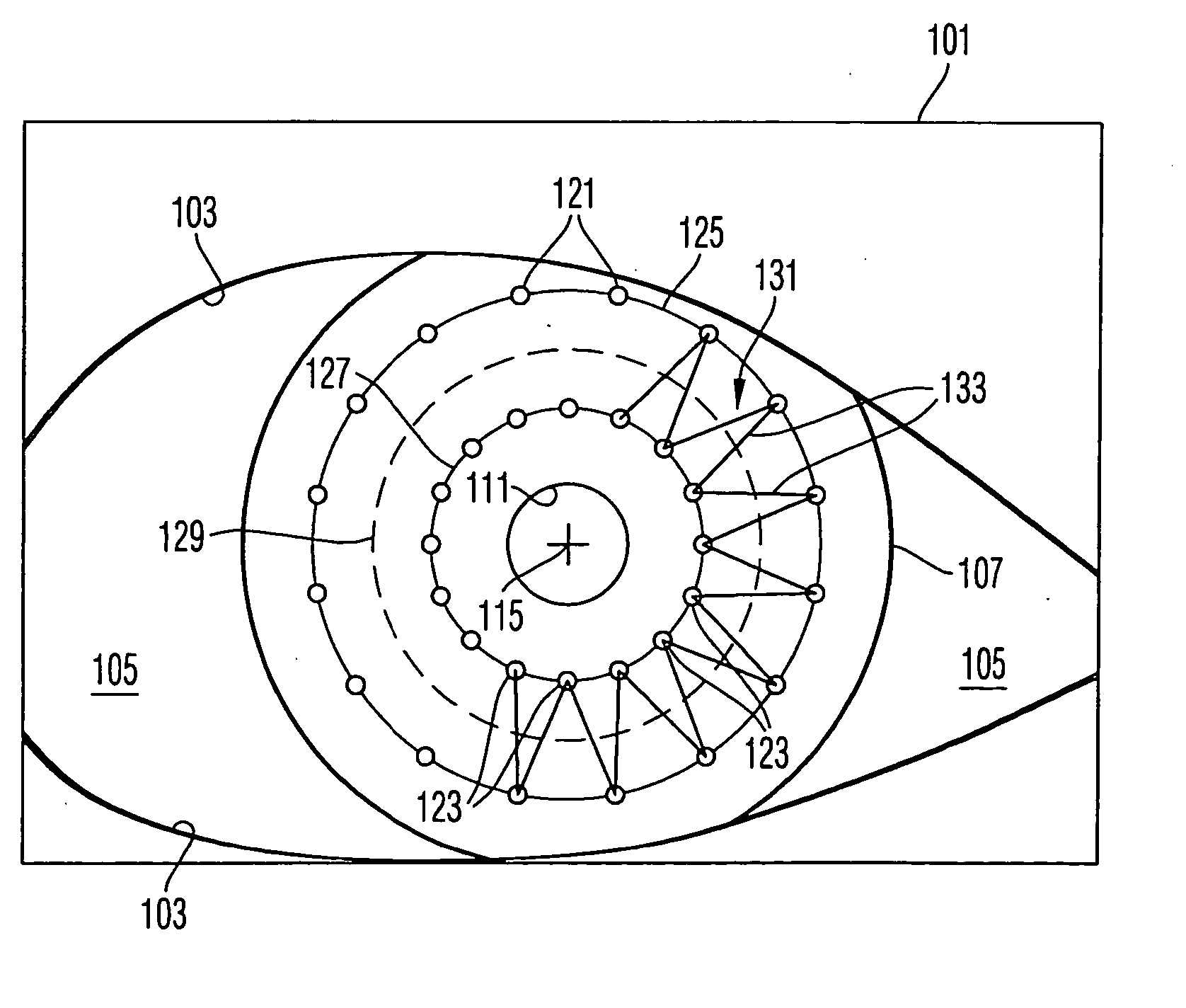
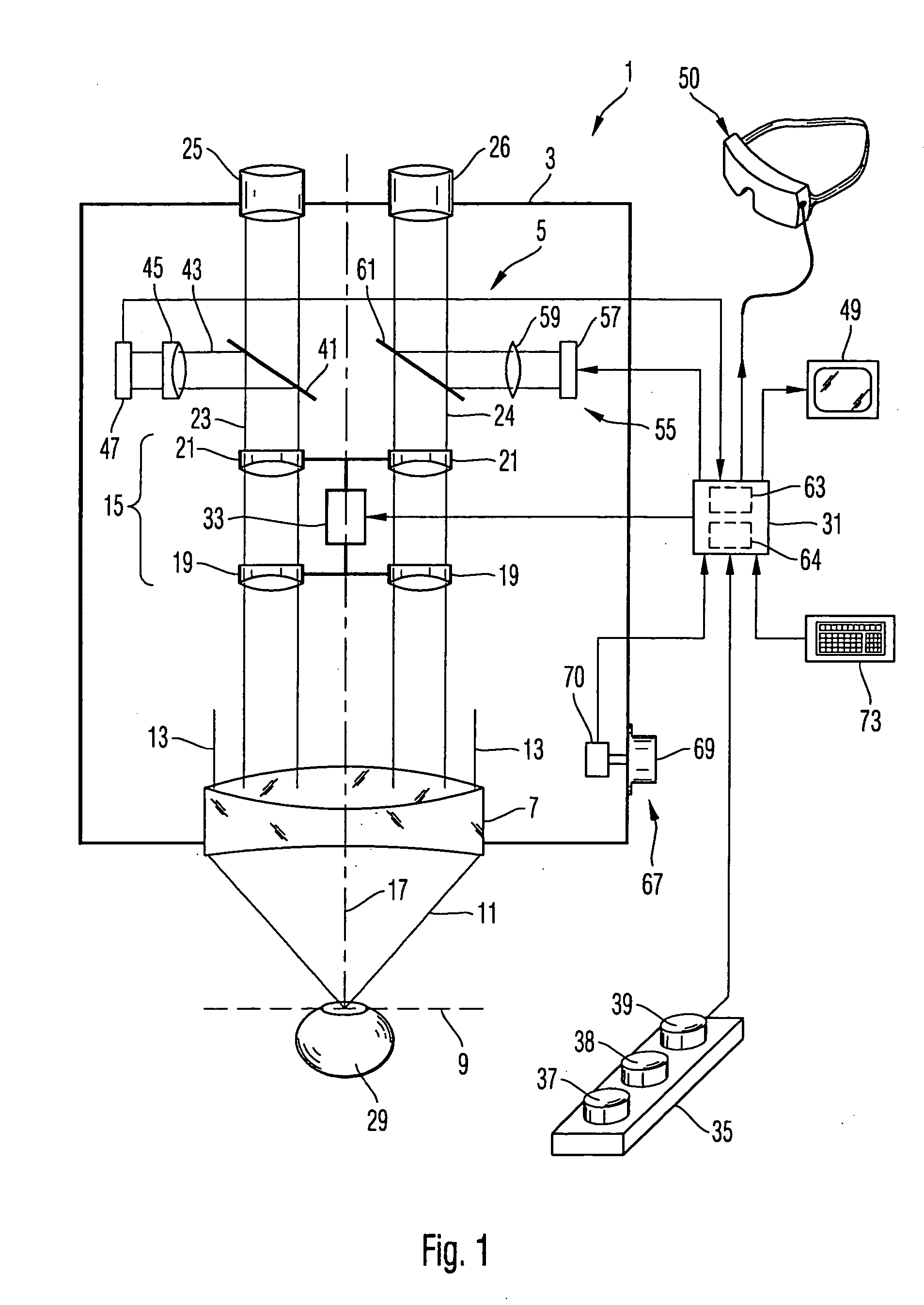
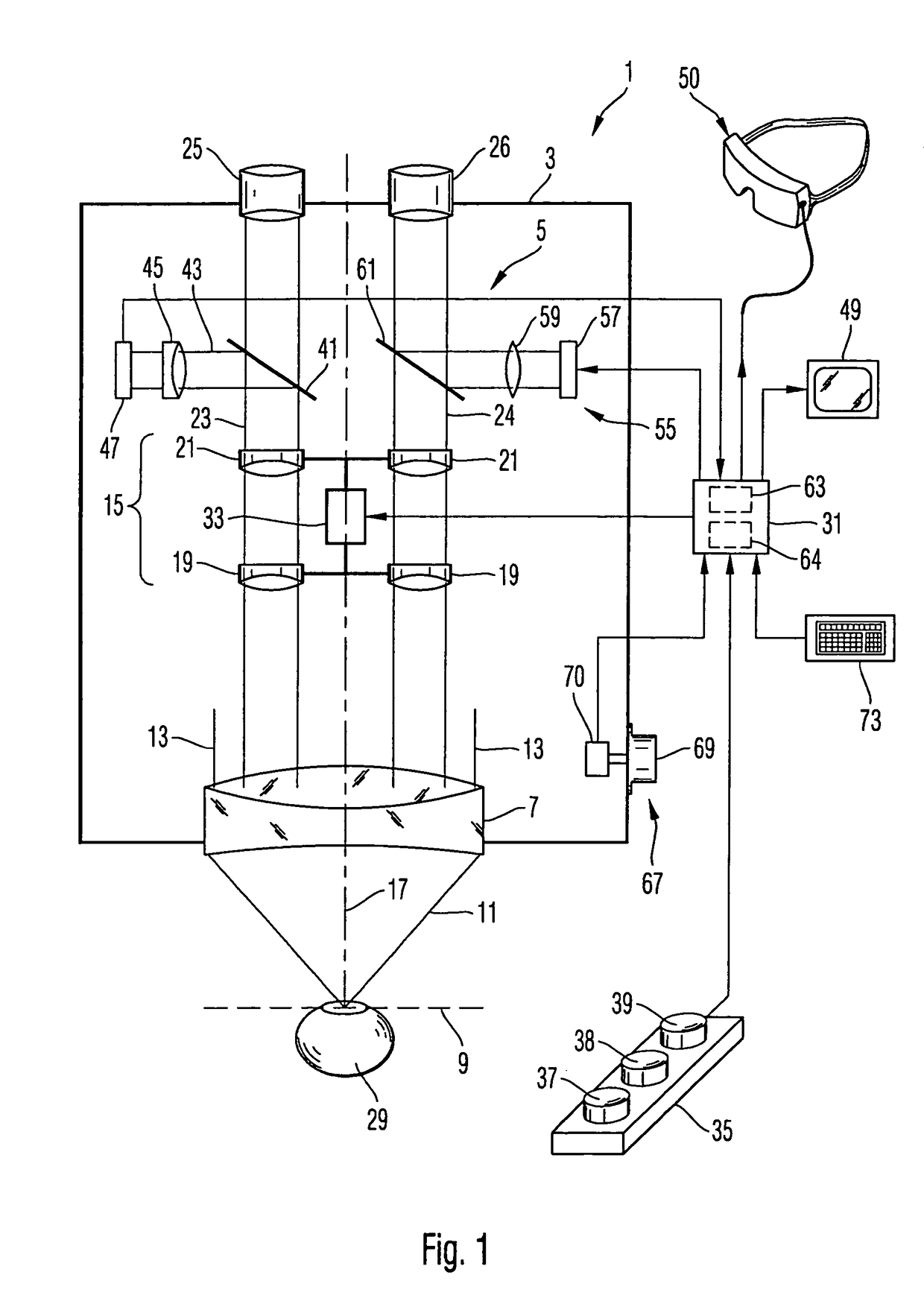
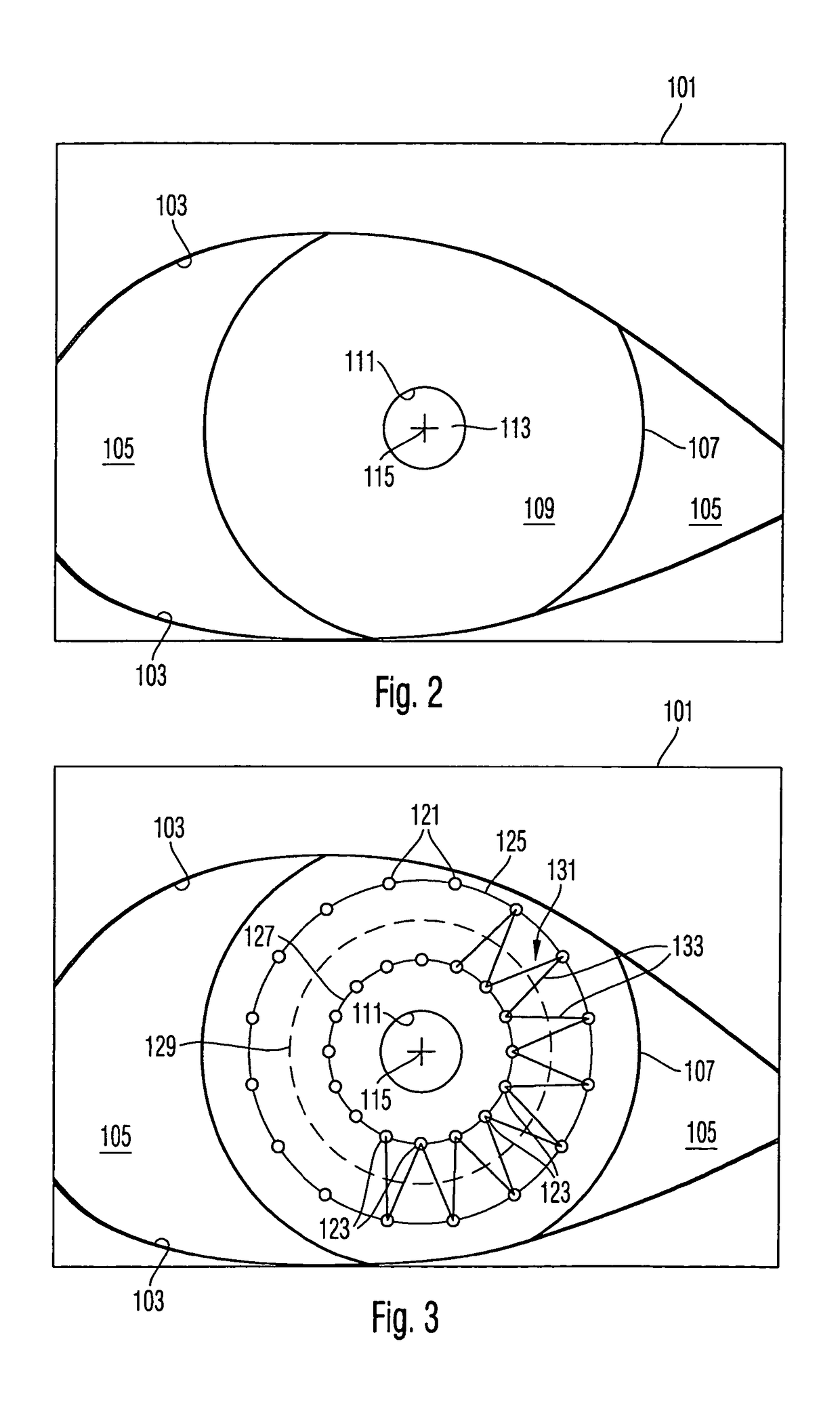
Surgical microscopy system and method for performing eye surgery
ActiveUS20060247659A1For precise cuttingPrecise other manipulationEye surgeryMicroscopesCorneal TransplantSurgery procedure
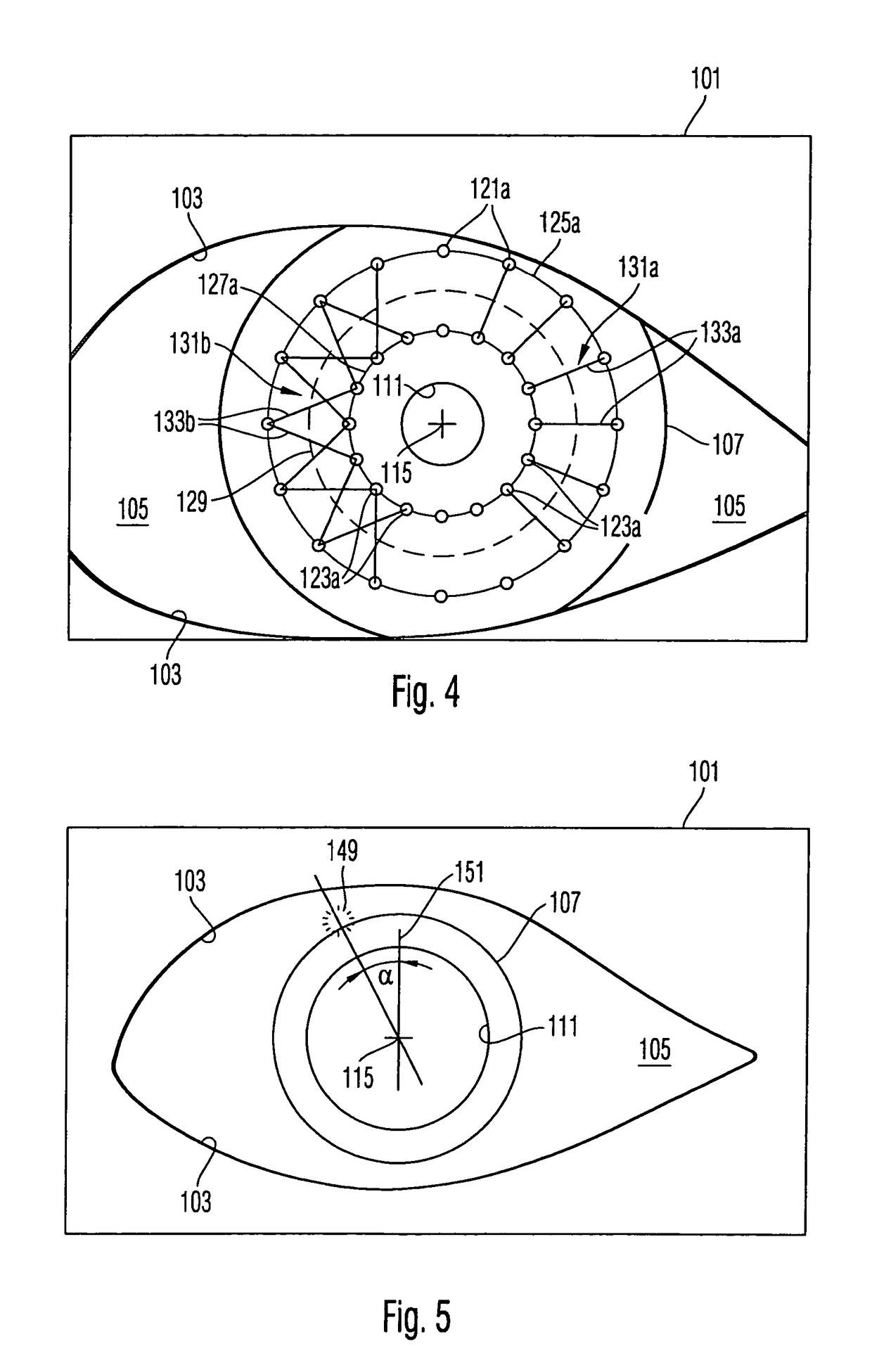
A surgical microscopy system comprises microscopy optics for generating an image of an eye under surgery. A pattern generator generates a pattern to be superimposed with the image. An eye-tracker is provided for tracking a position of the superimposed pattern with respect to the image in case of a movement of the eye. The superimposed pattern comprises pattern elements that are equally distributed on first and second circles of different sizes, in order to give assistance when placing a suture during a corneal transplant. The superimposed pattern may also provide an assistance for orientating a toric intra-ocular lens.
Owner:CARL ZEISS MEDITEC AG
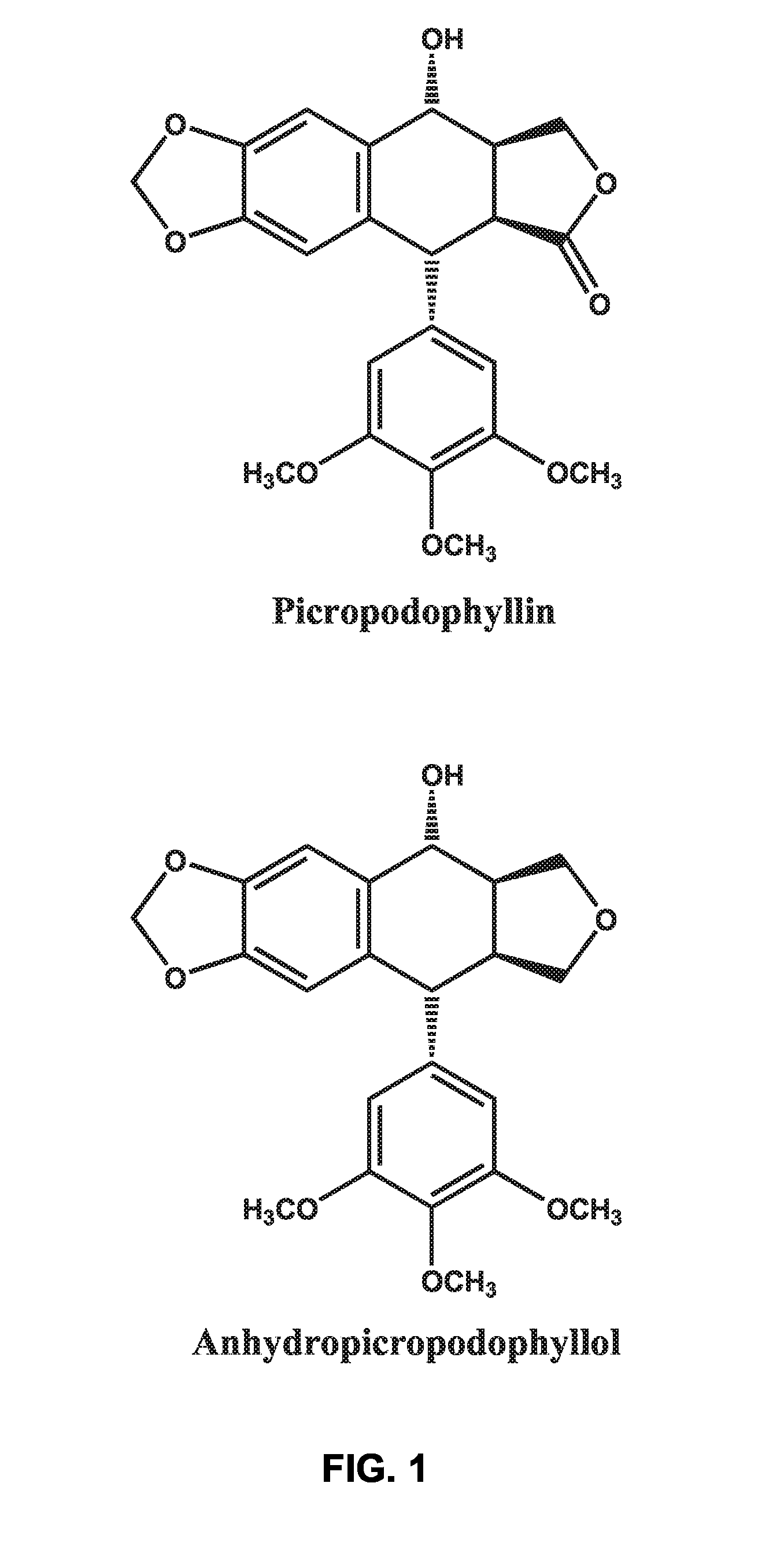
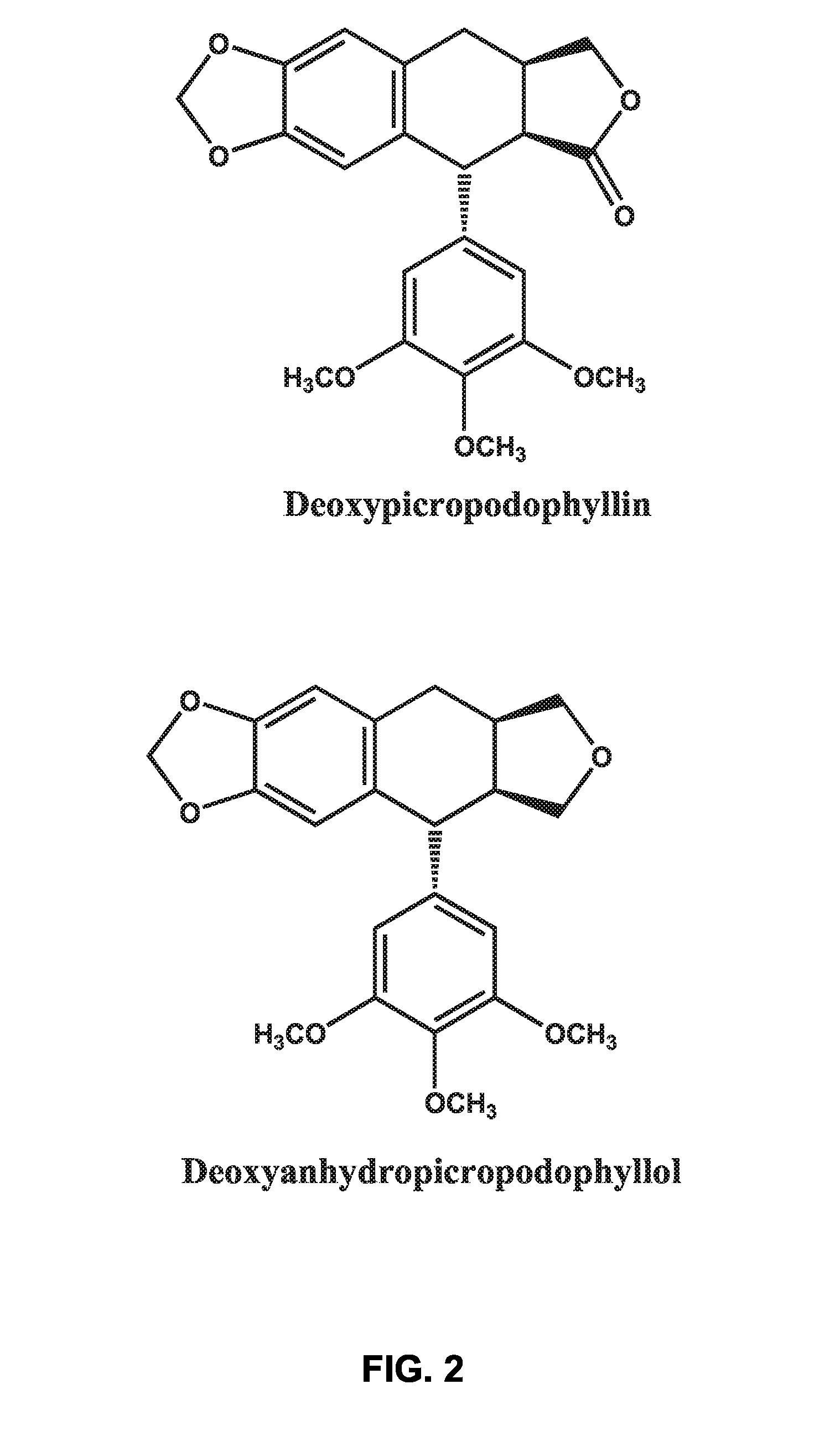
Use of cyclolignans for the treatment of type 2 diabetes and as contraceptives
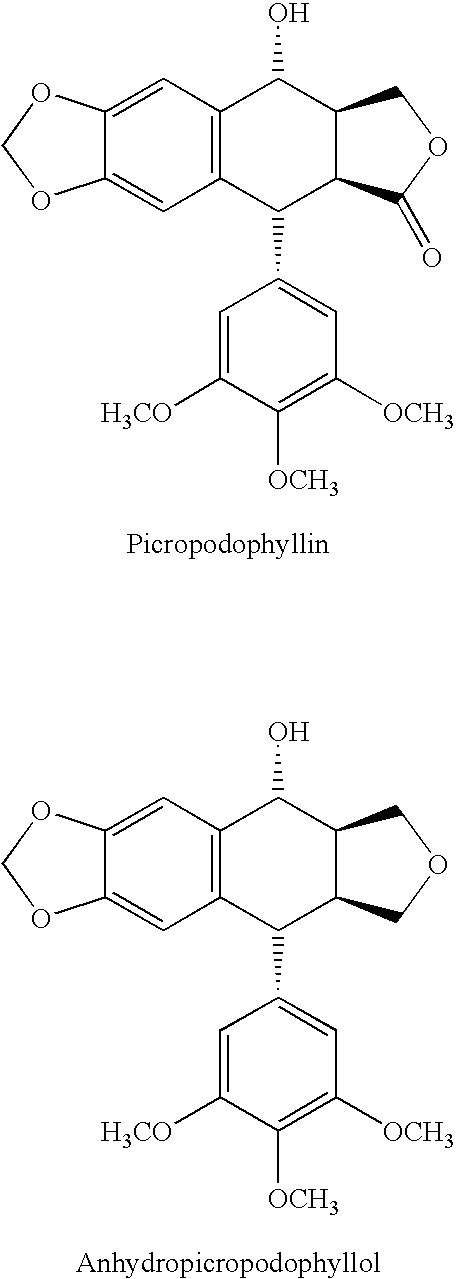
There is disclosed use of certain cyclolignans for prophylaxis or treatment of diabetes mellitus type 2, nephropathy, retinopathy, macular degeneration, retinopathy of prematurity, central retinal vein occlusion, branch retinal vein occlusion, rubeotic glaucoma, thyroid eye disease, corneal graft rejection and corneal chemical burns; and for contraception. Preferred compounds are picropodophyllin, deoxypicropodophyllin and anhydropicropodophyllol. There is also described a method of treatment of an eye disease.
Owner:AXELAR
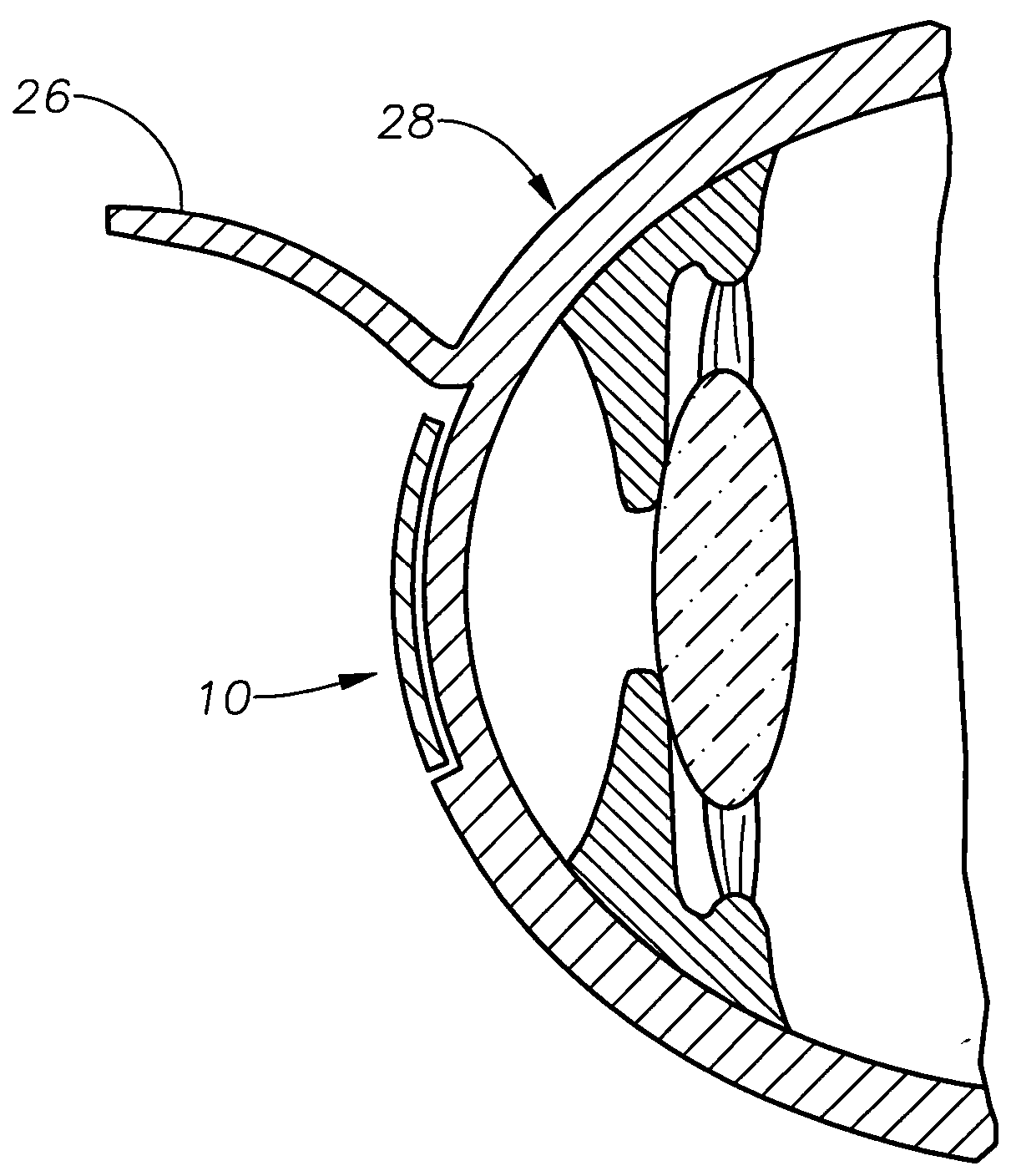
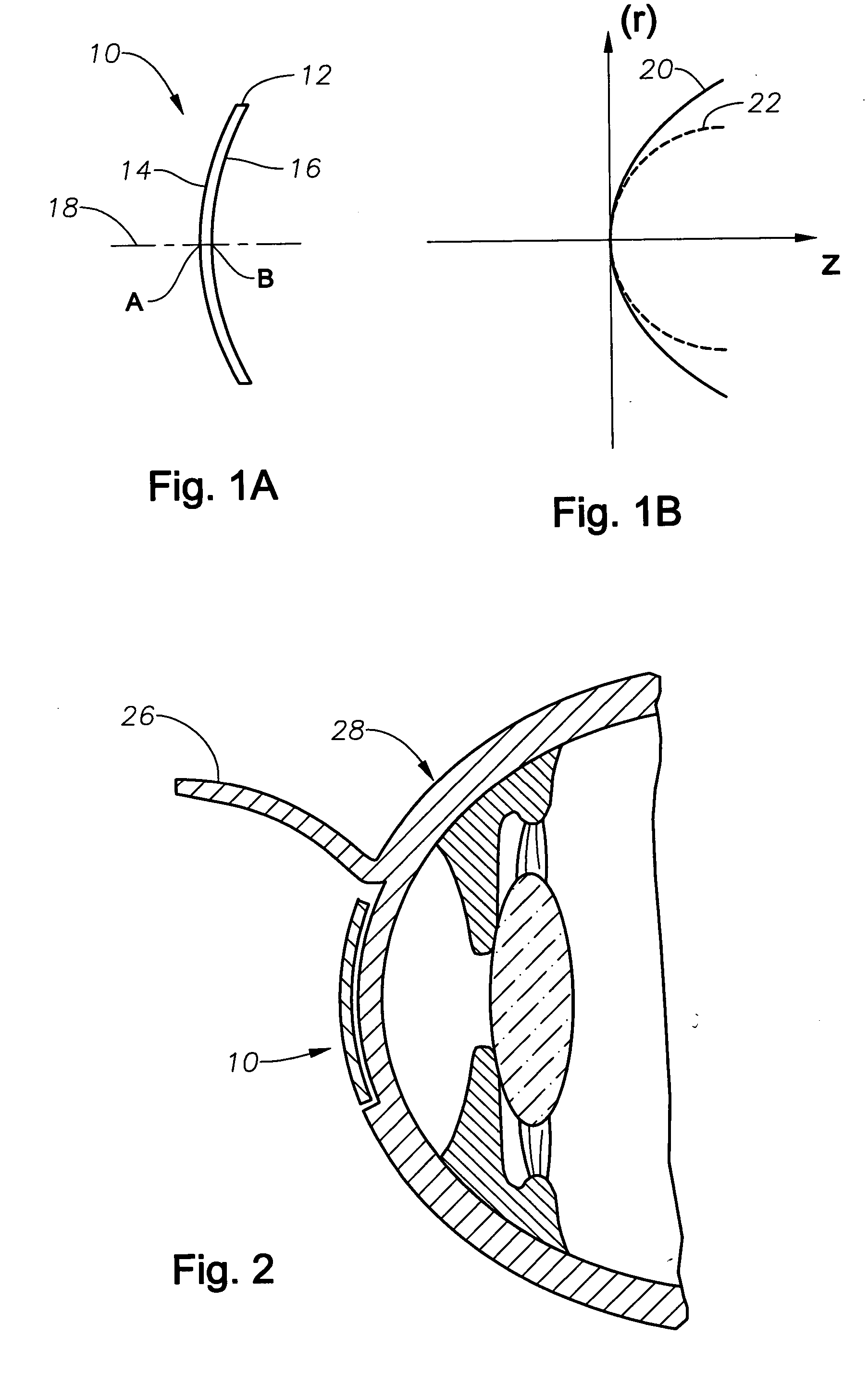
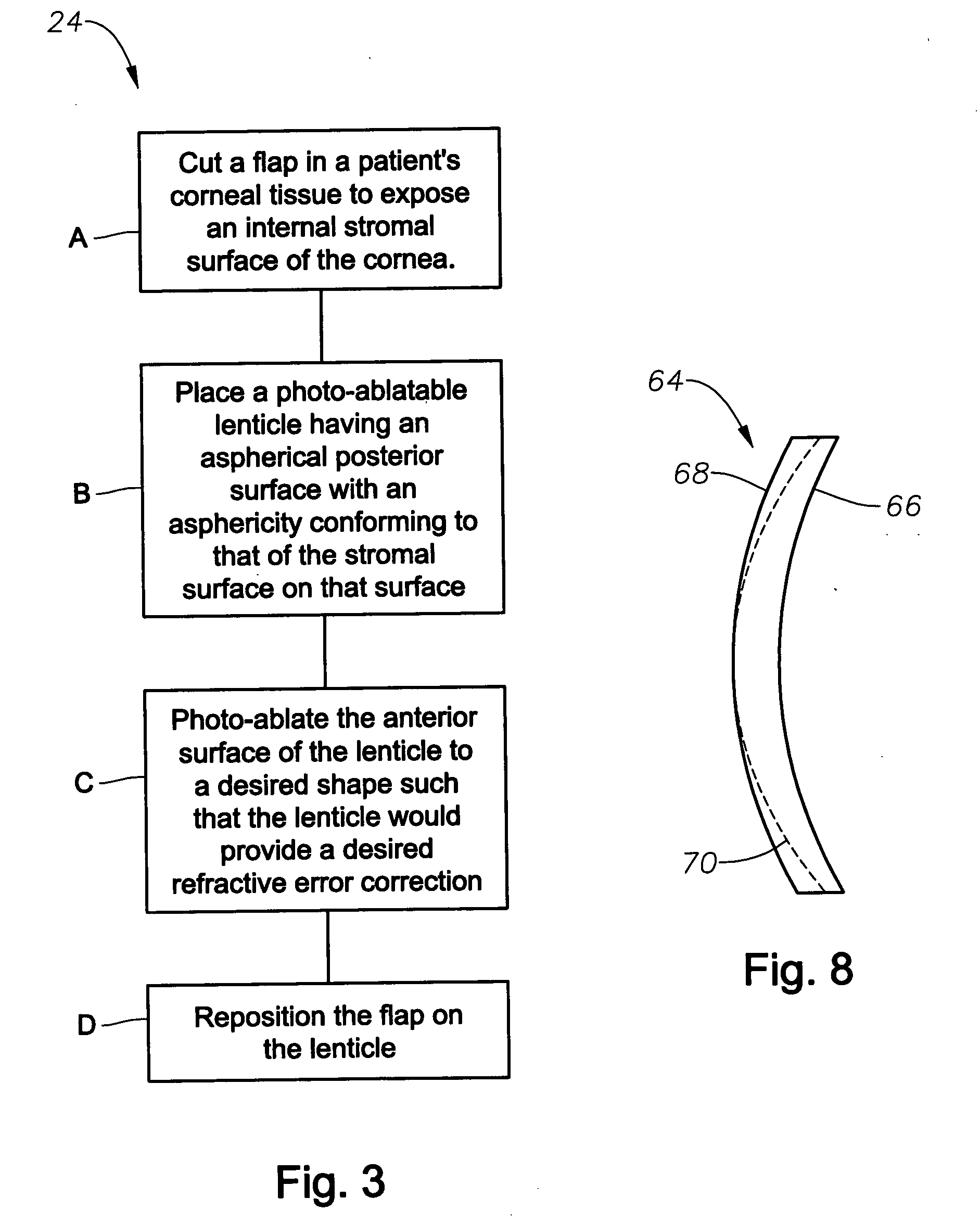
Aspheric lenticule for keratophakia
InactiveUS20060116762A1Correction errorImprove image contrastLaser surgeryEye implantsContour matchingRefractive error
Contour-matching, aspheric lenticules are disclosed for implantation in a subject's cornea to correcting refractive errors. The lenticules include a photoablatable anterior surface and a posterior surface having an aspheric profile that can substantially match the asphericity exhibited by the corneal stromal surface, on which the lenticule is placed. The posterior surface can have a generally concave shape while the anterior surface can have a generally convex shape, though other shapes can also be utilized in some embodiments. In some embodiments, the asphericity of the lenticule's posterior surface can differ from an asphericity exhibited by the corneal stromal surface by less than about 50%, or preferably by less than about 20%.
Owner:ALCON INC
Whole layer biological cornea as well as construction method and use thereof
The invention aims at providing a novel full-thickness biological cornea used for transplantation. The full-thickness biological cornea takes an animal cornea acellular matrix as a carrier, and the acellular matrix comprises animal cornea matrix cells, epithelial cells and endothelial cells which are cultured and augmented in vitro. The cornea is characterized in that the xenogenic corneal acellular matrix prepared by a biochemical method can be used as a good carrier for in vitro constructing the biological cornea in the aspects of shape, structure and biological compatibility, the matrix cells, epithelial cells and endothelial cells of the cornea are planted respectively, and dynamically cultured in the simulated in vivo environment of a biological reactor, so that the full-thickness biological cornea with nearly normal tissue structure and characteristics can be constructed in vitro, and the biological cornea can be used to simulate the physiological cornea for fundamental research on physiology, pathology and pharmacology; moreover, the biological cornea can also be directly used as the donator for corneal transplantation.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Ply tissue engineering corneal frame and manufacturing method and application thereof
ActiveCN101947144AThe production process is simple and reliableShorten the timeEye implantsDiseaseFiber
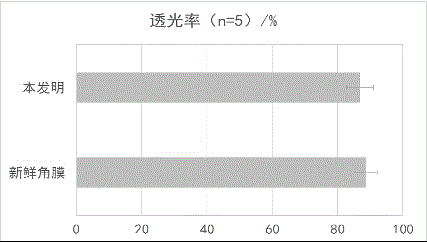
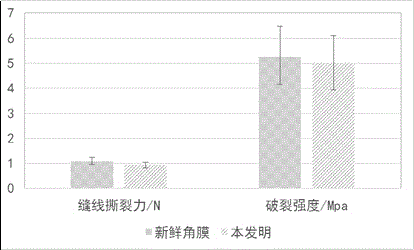
The invention discloses a ply tissue engineering corneal frame and a manufacturing method and an application thereof, relating to a ply tissue engineering corneal frame of the biomedicine. Compared with the existing materials, the invention provides a ply tissue engineering corneal frame and a manufacturing method and an application thereof, which has abundant resources, high transparency, good biocompatibility, thorough decellularization and strong safety, and the performance approaches to that of a fresh cornea, so that the ply tissue engineering corneal frame can be accepted by majority of patients and applied clinically for a long term. The ply tissue engineering corneal frame is an animal derived decellularized ply corneal stroma sheet and does not contain cellular constituents; collagenous fibers are tidily arranged, and gaps are regular; corneal light transmittance is 80-95%, and tensile strength is 2-5N / mm<2>. The ply tissue engineering corneal frame can be served as the substitute for various donor materials for corneal transplantation and can be used for treating a series of diseases of corneal trauma and chemical burn, corneal tumor and a series of hyperplastic diseases, a series of diseases of corneal neovascularisation and scar, corneal immune diseases, a series of diseases caused by corneal transplantation exclusive reaction and other keratopathy.
Owner:XIAMEN DAKAI BIOTECH CO LTD
Surgical microscopy system and method for performing eye surgery
ActiveUS7905887B2Less manipulationGood successEye treatmentMicroscopesCorneal TransplantSurgical department
A surgical microscopy system comprises microscopy optics for generating an image of an eye under surgery. A pattern generator generates a pattern to be superimposed with the image. An eye-tracker is provided for tracking a position of the superimposed pattern with respect to the image in case of a movement of the eye. The superimposed pattern comprises pattern elements that are equally distributed on first and second circles of different sizes, in order to give assistance when placing a suture during a corneal transplant. The superimposed pattern may also provide an assistance for orientating a toric intra-ocular lens.
Owner:CARL ZEISS MEDITEC AG
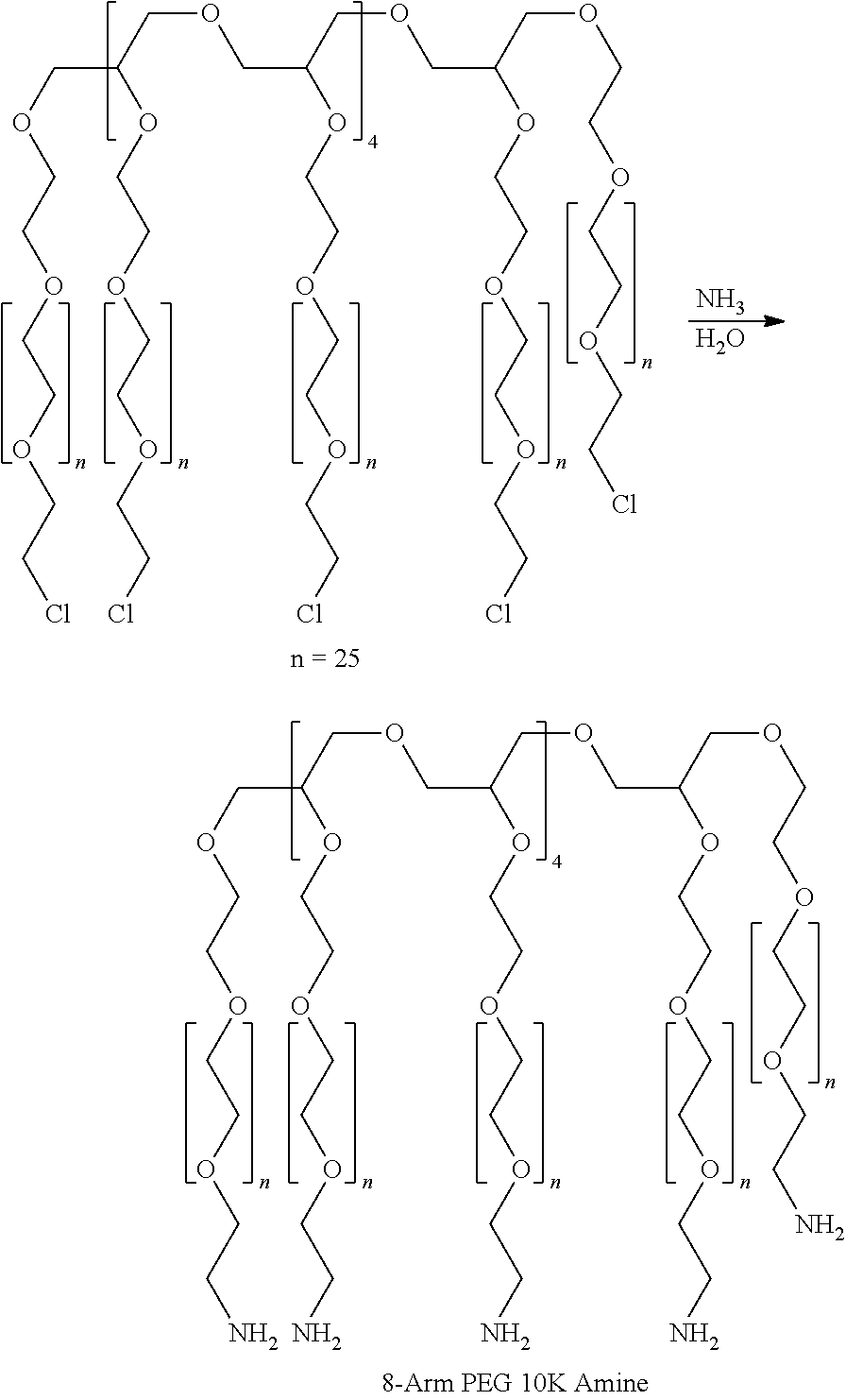
Hydrogel tissue adhesive having increased degradation time
InactiveUS20100160960A1Long degradation timeProlong degradation timeCosmetic preparationsSurgical adhesivesSurgical operationFistula
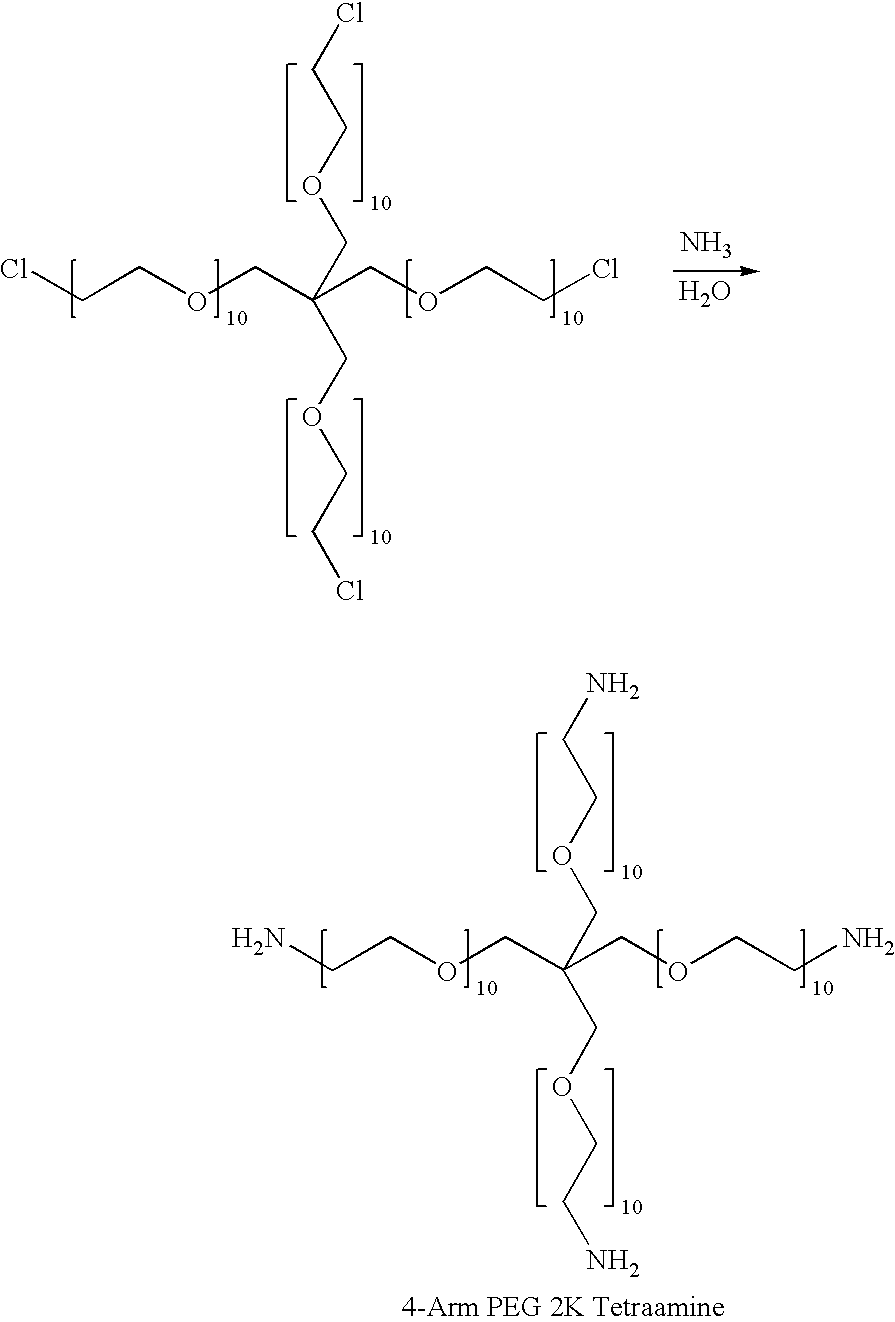
A hydrogel tissue adhesive having increased degradation time is described. The hydrogel tissue adhesive is formed by reacting an oxidized polysaccharide with a water-dispersible, multi-arm amine in the presence of a polyol additive, which retards the degradation of the hydrogel. The hydrogel may be useful as a tissue adhesive or sealant for medical applications, including but not limited to, ophthalmic applications such as sealing wounds resulting from trauma such as corneal lacerations, or from surgical procedures such as vitrectomy procedures, cataract surgery, LASIK surgery, glaucoma surgery, and corneal transplants; neurosurgery applications, such as sealing the dura; as a plug to seal a fistula or the punctum; adhesion prevention to prevent undesired tissue to tissue adhesions resulting from trauma or surgery; and as a hemostat sealant.
Owner:ACTAMAX SURGICAL MATERIALS
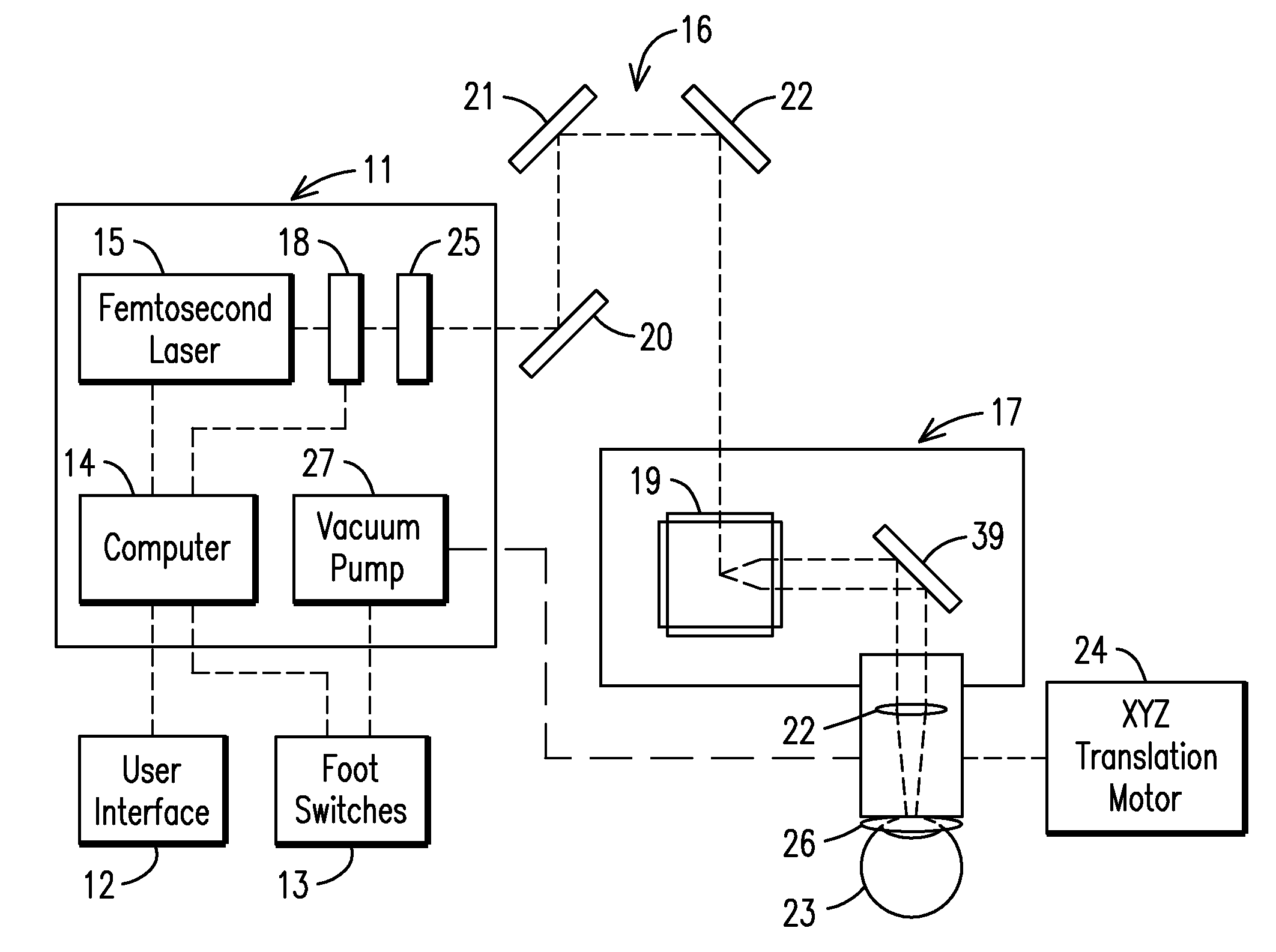
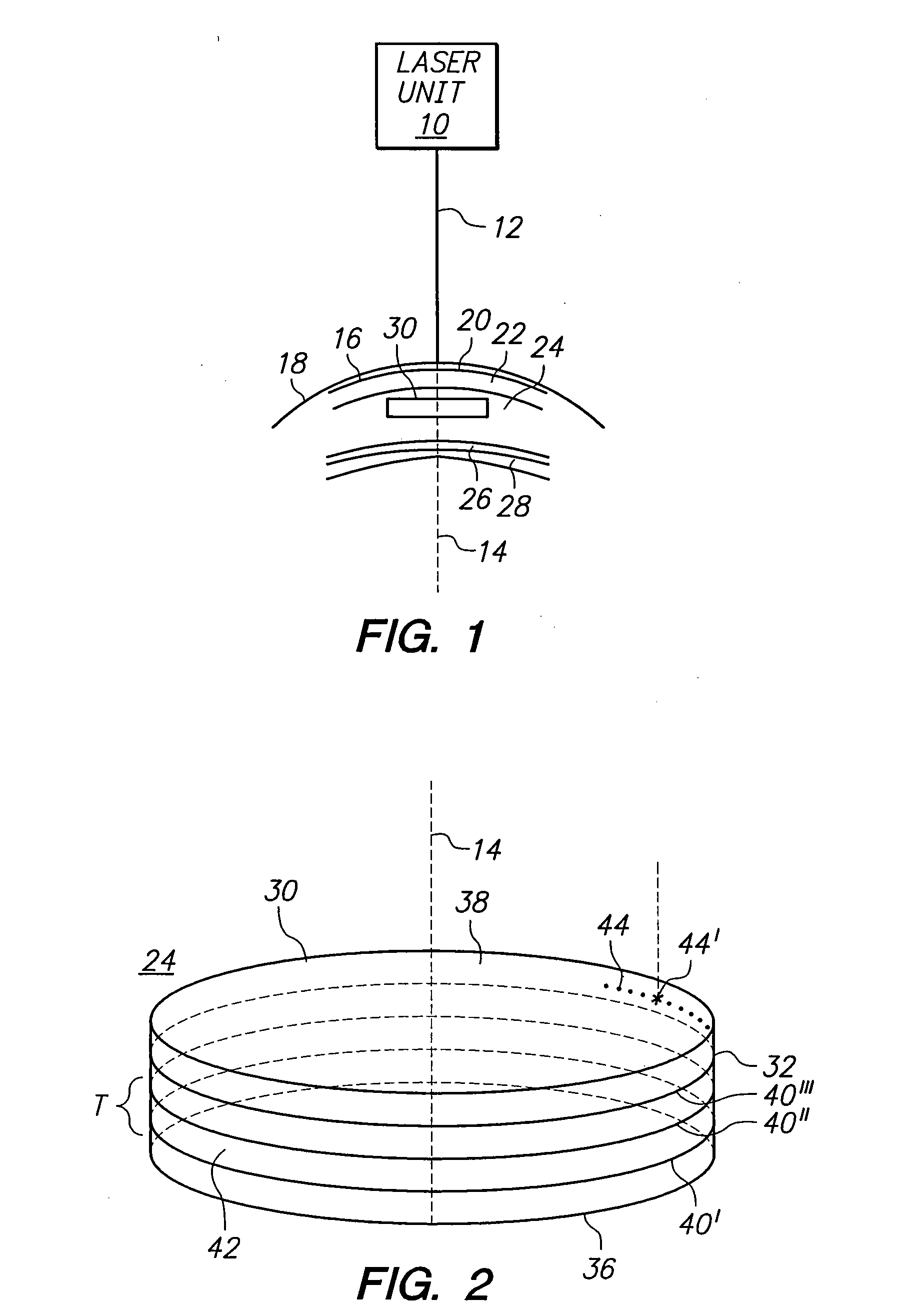
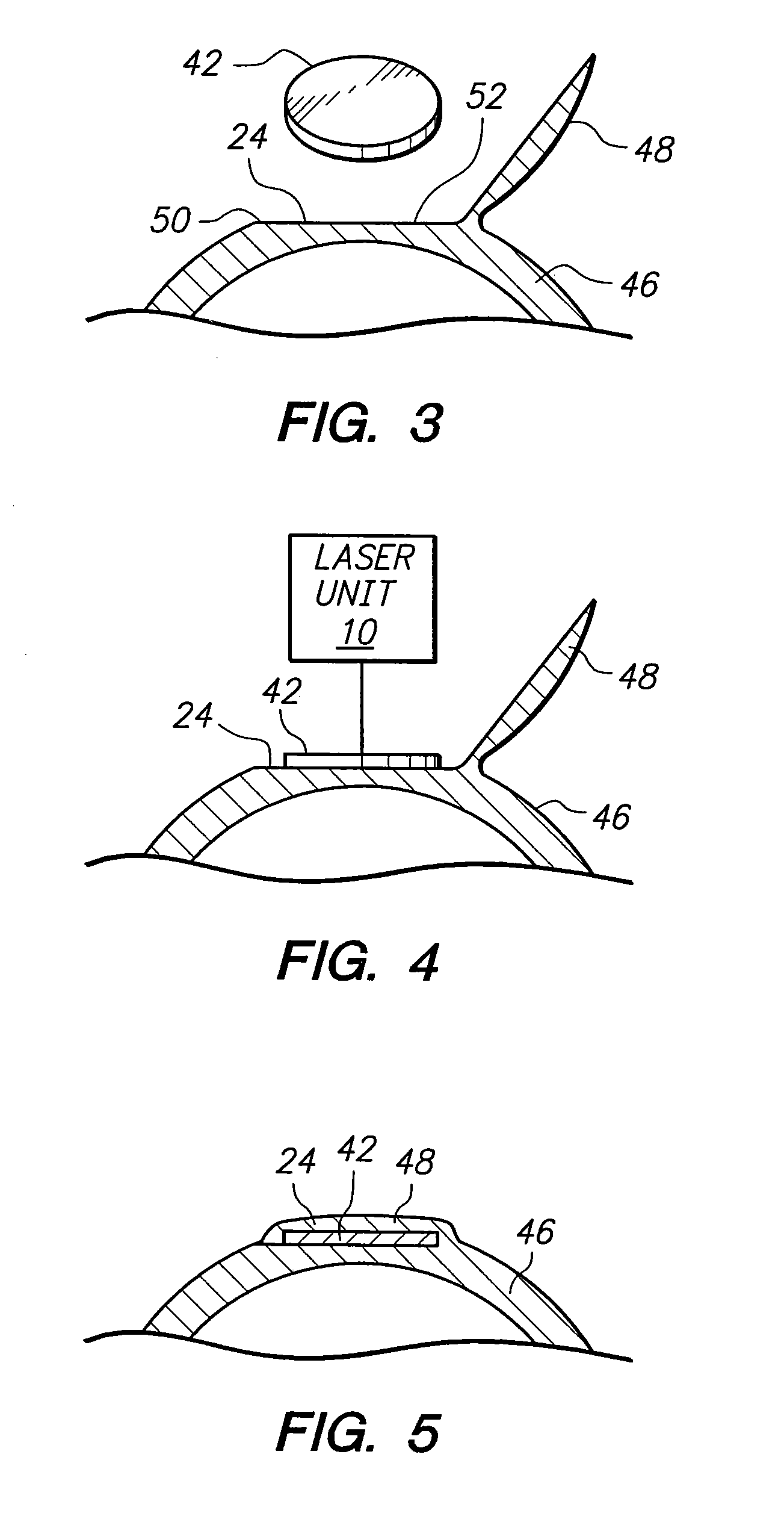
Ophthalmological laser method and apparatus
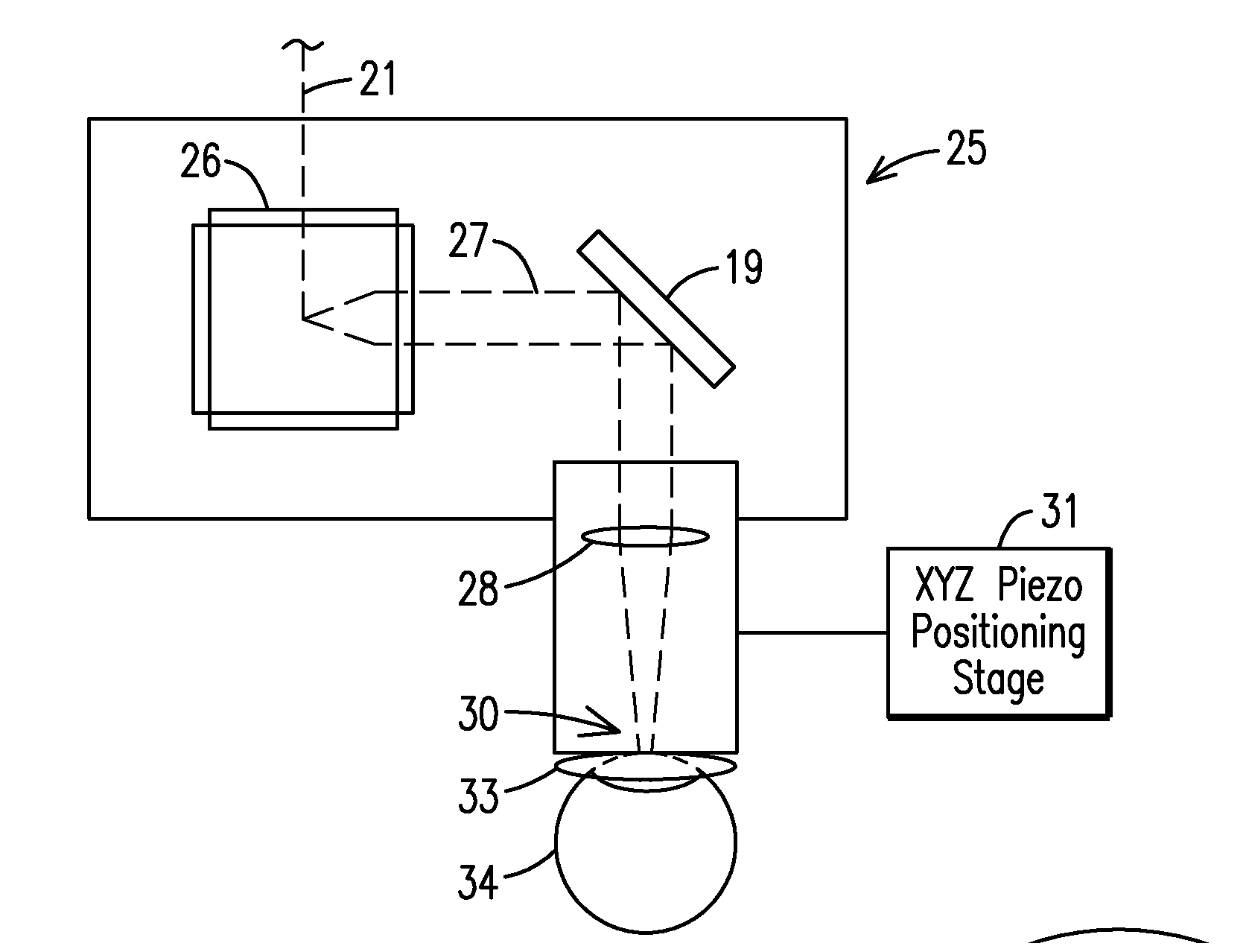
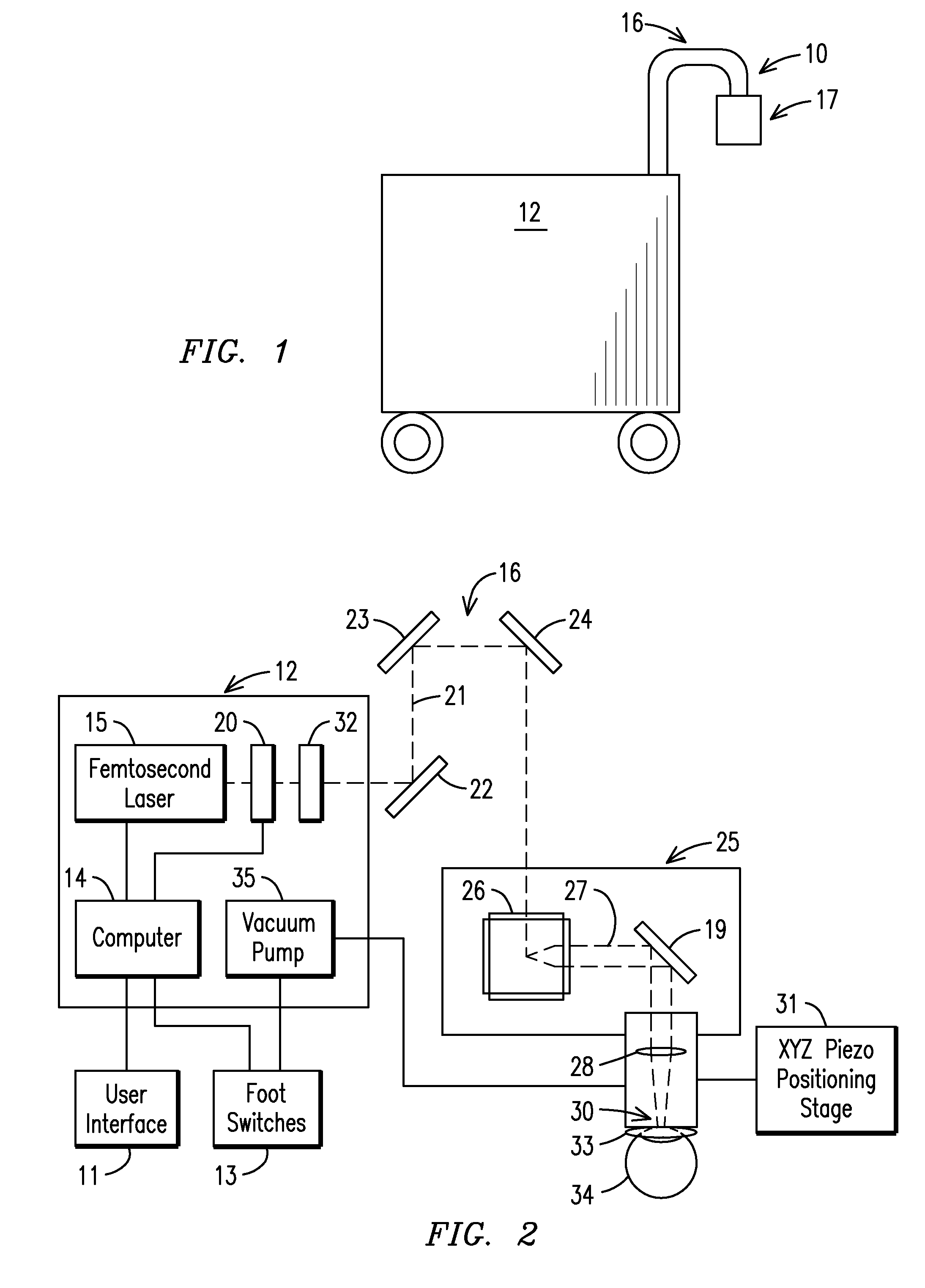
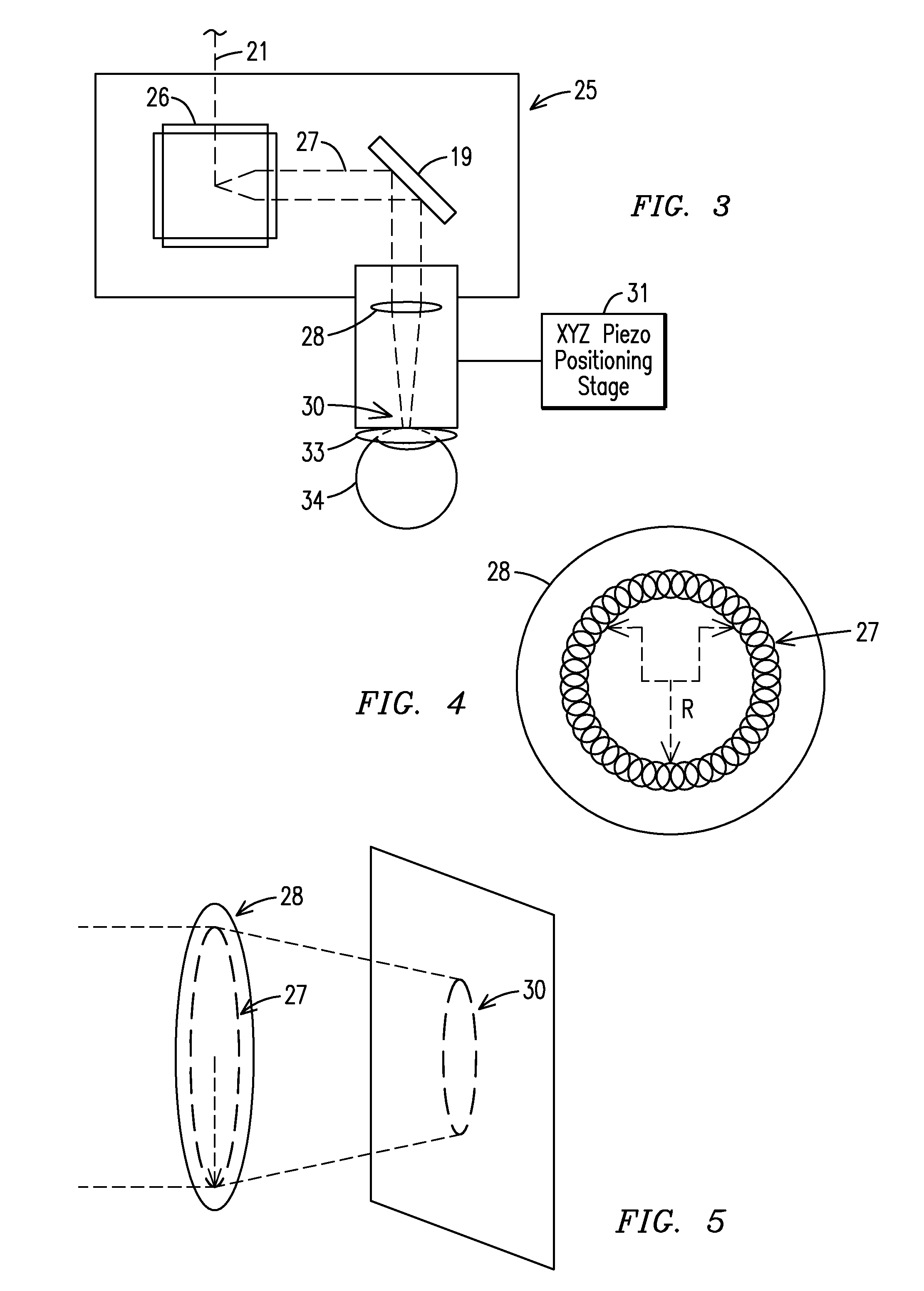
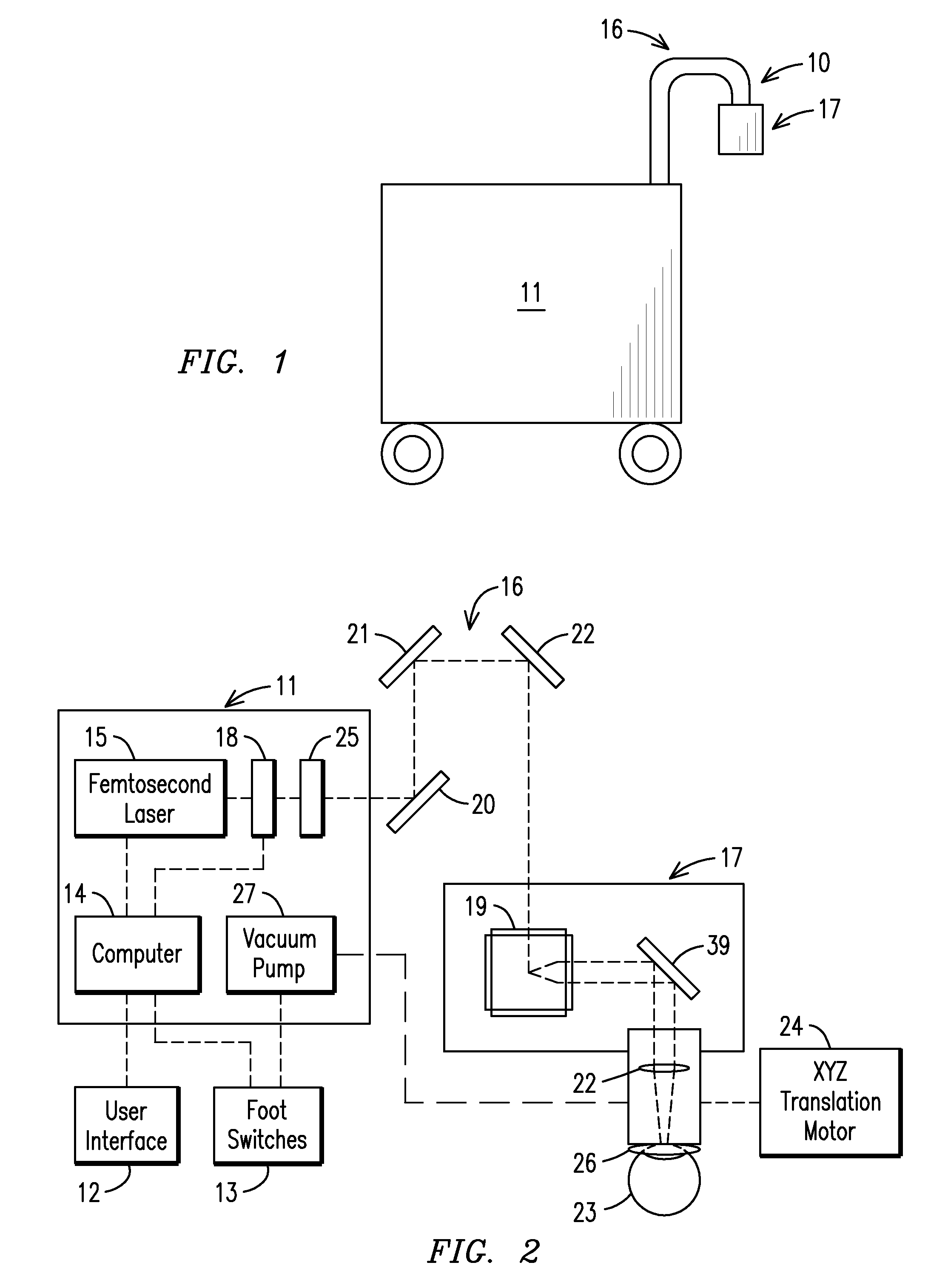
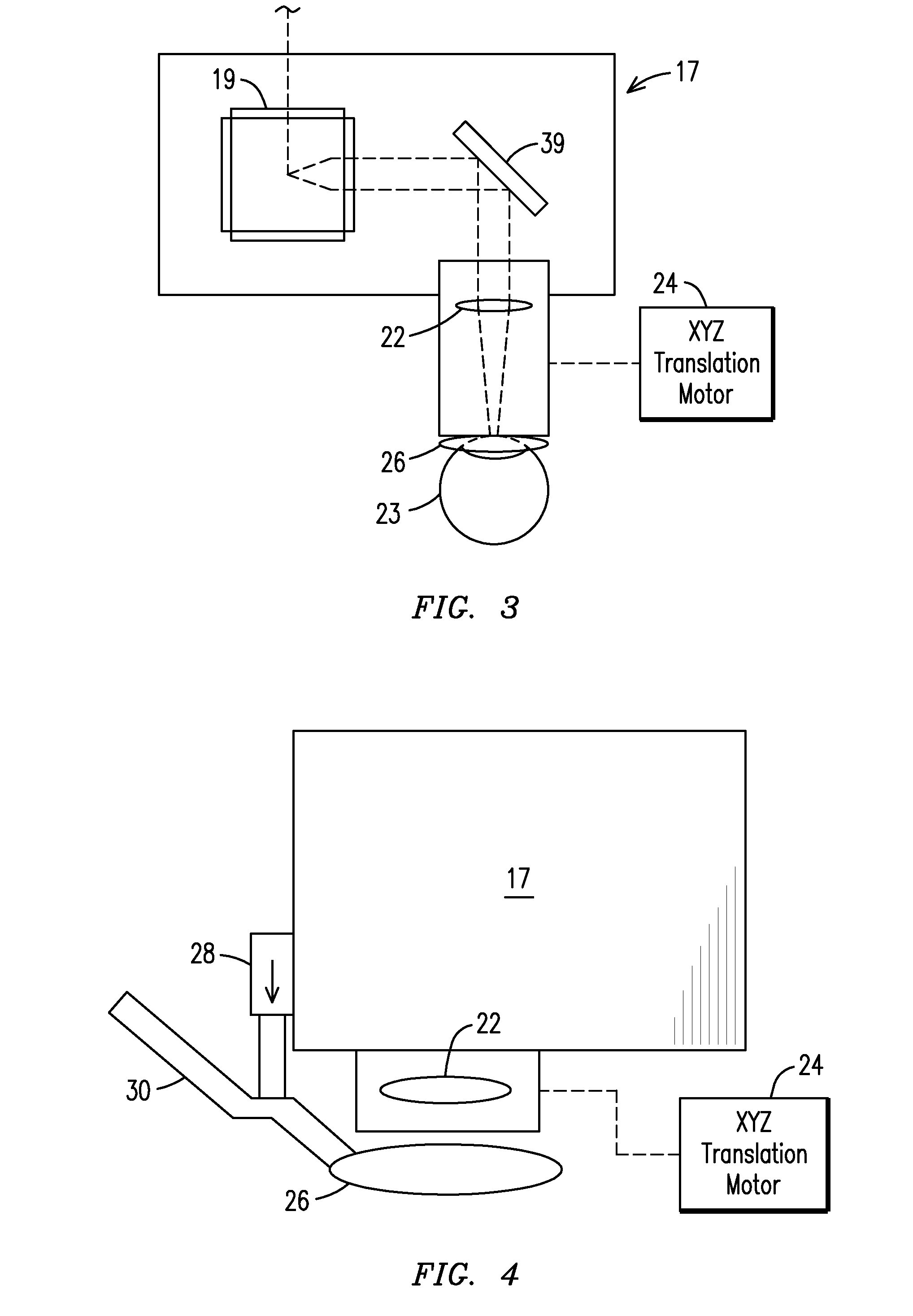
The present invention relates to a femtosecond laser ophthalmological apparatus and method that creates a flap on the cornea for LASIK refractive surgery or for other applications that require removal of corneal and lens tissue at specific locations such as in corneal transplants, stromal tunnels, corneal lenticular extraction and cataract surgery. The femtosecond laser is transferred to a hand piece module via a rotating mirror arm module. In the hand piece, the femtosecond laser beam is scanned into overlapping circles of laser pulses which are then moved in an overlapping trajectory on a patient's eye to ablate the eye tissue in a predetermined pattern.
Owner:HUANG CHENG HAO
Decellularized heterogeneous corneal stroma carrier and its preparation method and application
The invention discloses a decellularized heterogeneous corneal stromal carrier and its preparation method and application. The carrier is an animal lamellar cornea from which epithelial cells and stromal cells have been removed through hypertonic solution combined with enzyme digestion. The preparation method of the carrier is as follows: firstly, take Fresh animal eyeballs are aseptically operated under an operating microscope, and the lamellar cornea with a thickness of 150 μm to 400 μm is drilled with a graduated trephine drill with a diameter of 5 mm to 12 mm, and then removed under the combined action of hypertonic solution and trypsin / pancreatin substitute The cells are finally dehydrated and dried to obtain the decellularized heterogeneous corneal stroma carrier, which is stored for future use. The decellularized heterogeneous corneal stroma carrier can be used as a corneal transplant donor to directly perform therapeutic corneal transplantation, and can also be used as an artificial biological corneal scaffold to construct a full-layer or lamellar artificial biological cornea. The decellularized heterogeneous corneal stroma carrier prepared by the invention has the following characteristics: the collagen is neatly arranged, similar to normal corneal tissue, and has good transparency after rehydration.
Owner:陕西省眼科研究所
Aseptic processing preparation method for allogeneic corneal grafts
The invention discloses an aseptic processing preparation method for allogeneic corneal grafts. By virtue of whole treatment on eyeballs, the method comprises the following steps: two-step virus inactivation, namely ultraviolet irradiation and sodium hypochlorite solution soaking, epithelial layer removal, two-step decellularization, namely serum soaking and an osmotic pressure method, cutting and dewatering, and glycerine solution storage. According to the preparation method disclosed by the invention, drying and terminal sterilizing steps are not needed, so that the preparation method is simple in process, short in production cycle and low in production cost; through soft decellularization treatment, the structure and components of the natural corneal stroma are kept to the maximal extent; no significant difference is formed among the transparency, the mechanical strength and the natural corneal stroma; the degradation speed is matched with the regeneration speed of newborn cornea tissues; antigen removal and virus inactivation are thorough; the biocompatibility and the biosecurity are high; the structure and performance of glycerine storage can be kept stable for a long period of time; clinical application is convenient; and corneal transplantation can be carried out instead of the allogeneic cornea.
Owner:SHAANXI BOYU REGENERATIVE MEDICINE CO LTD
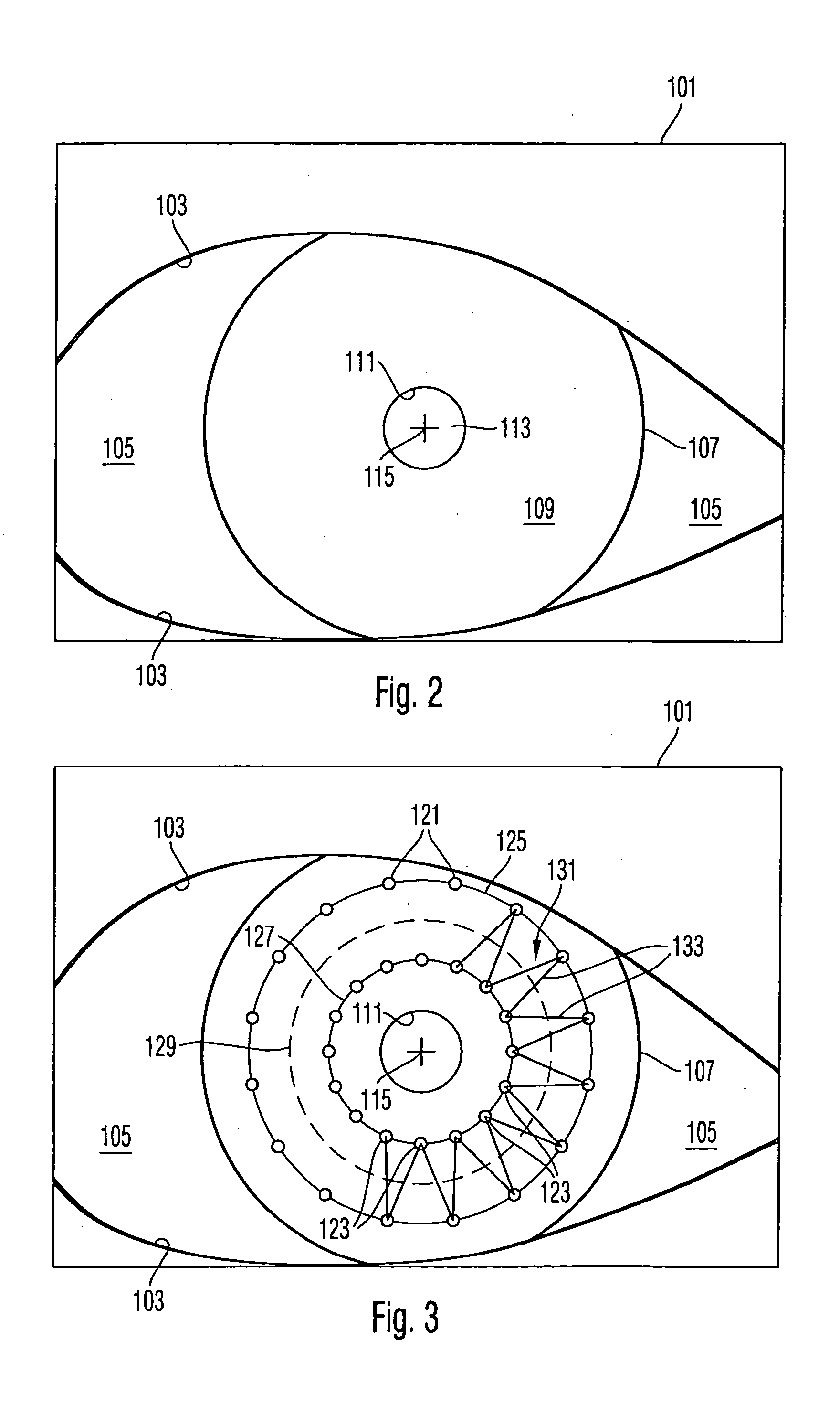
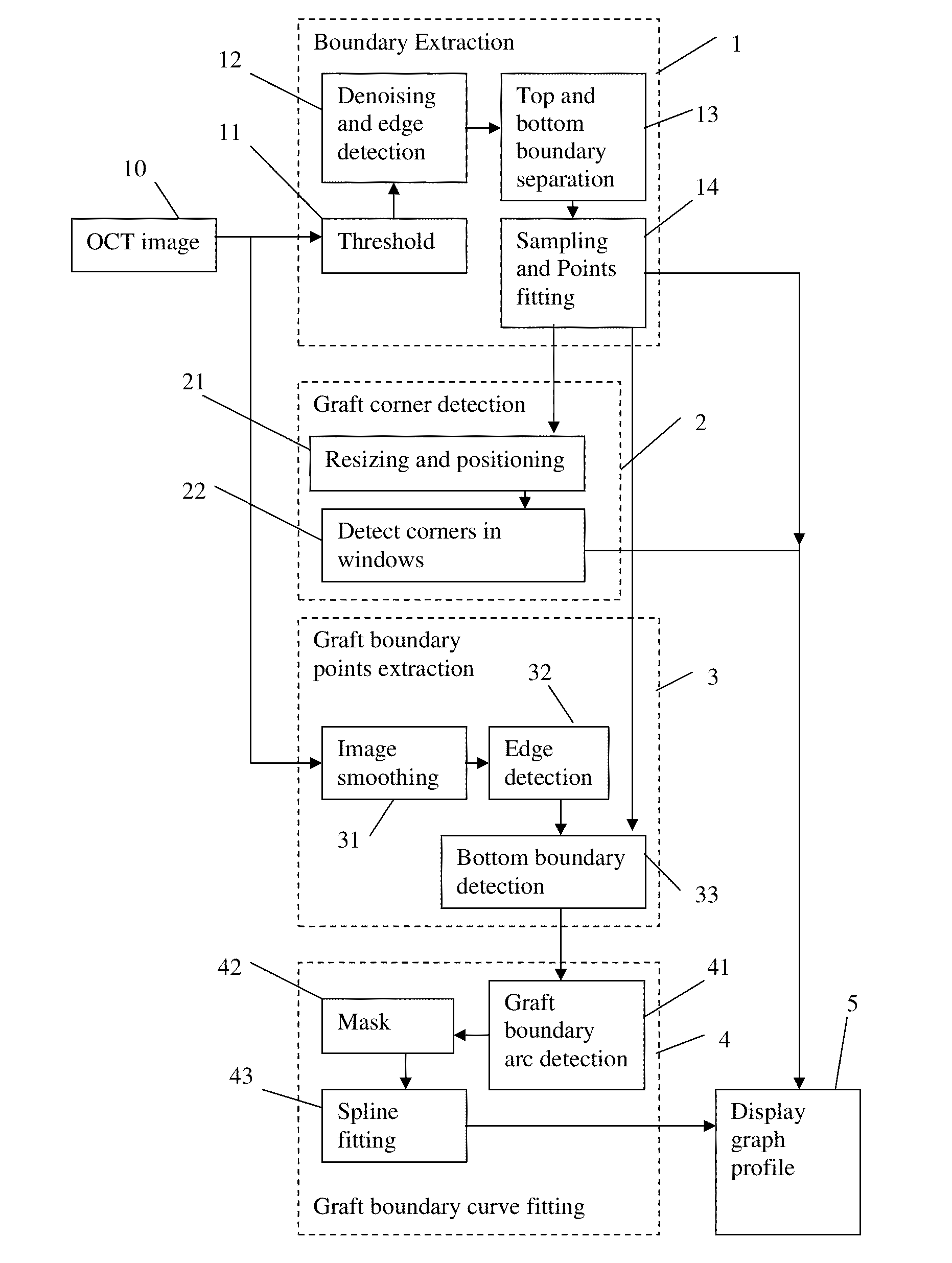
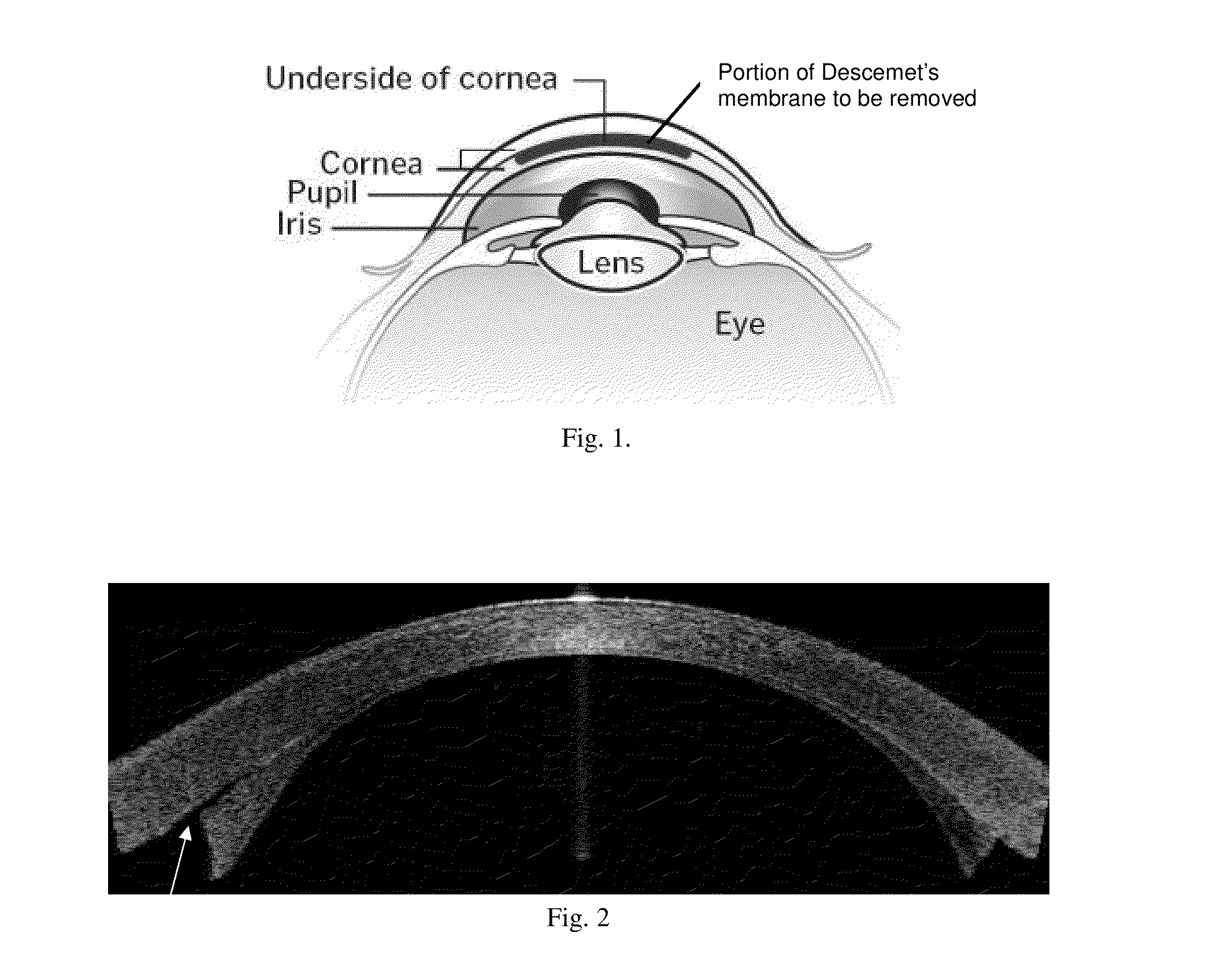
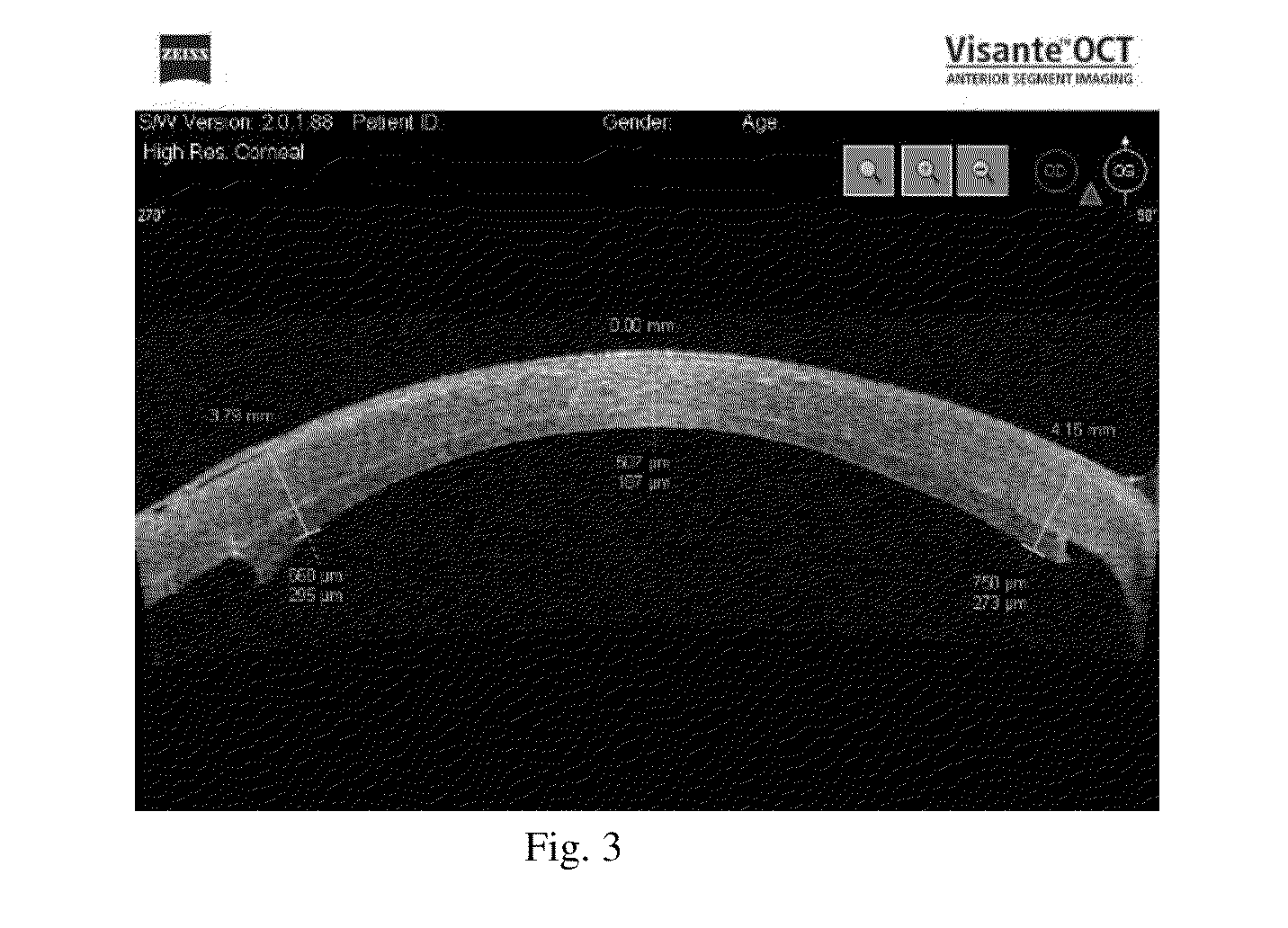
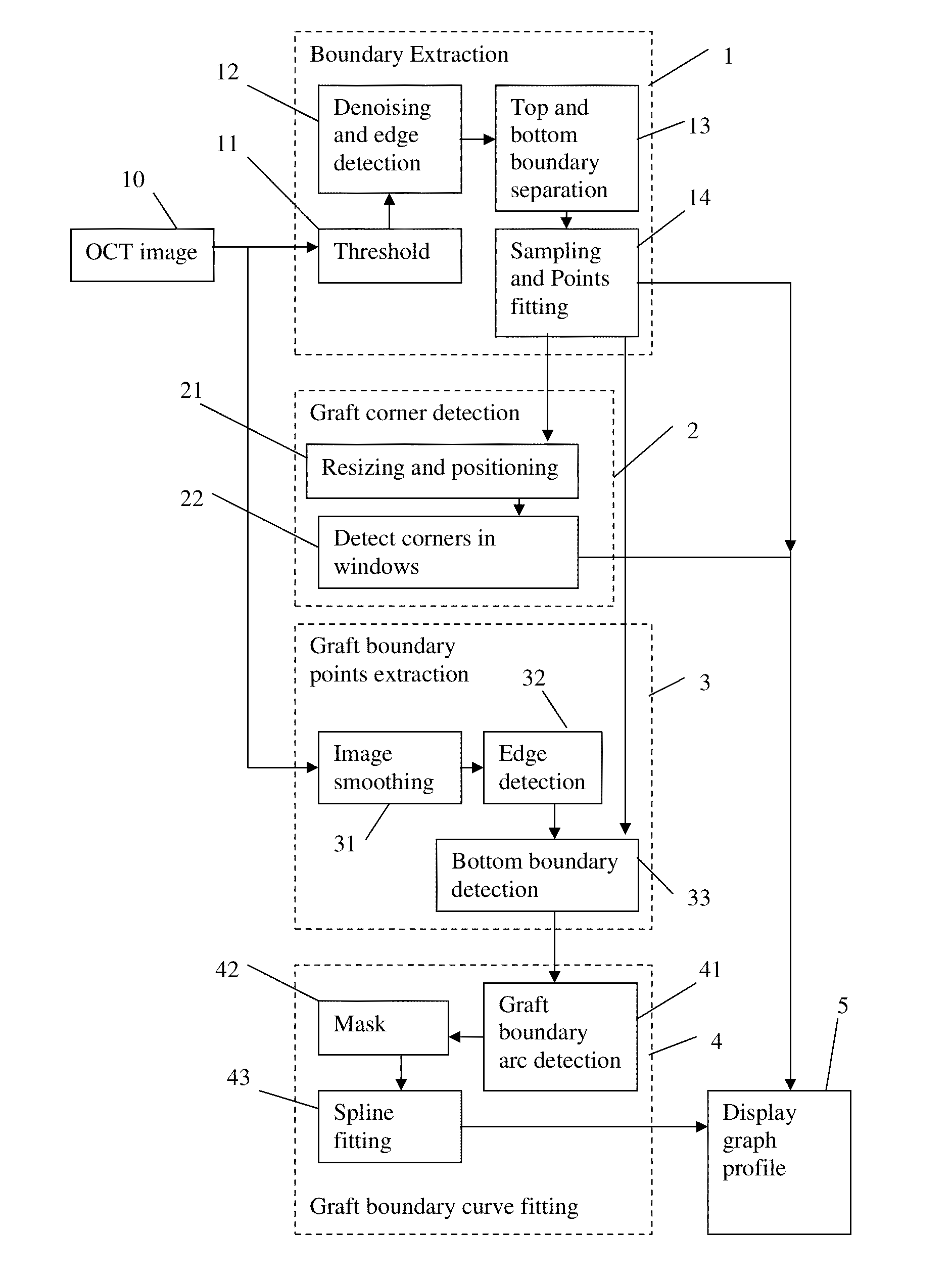
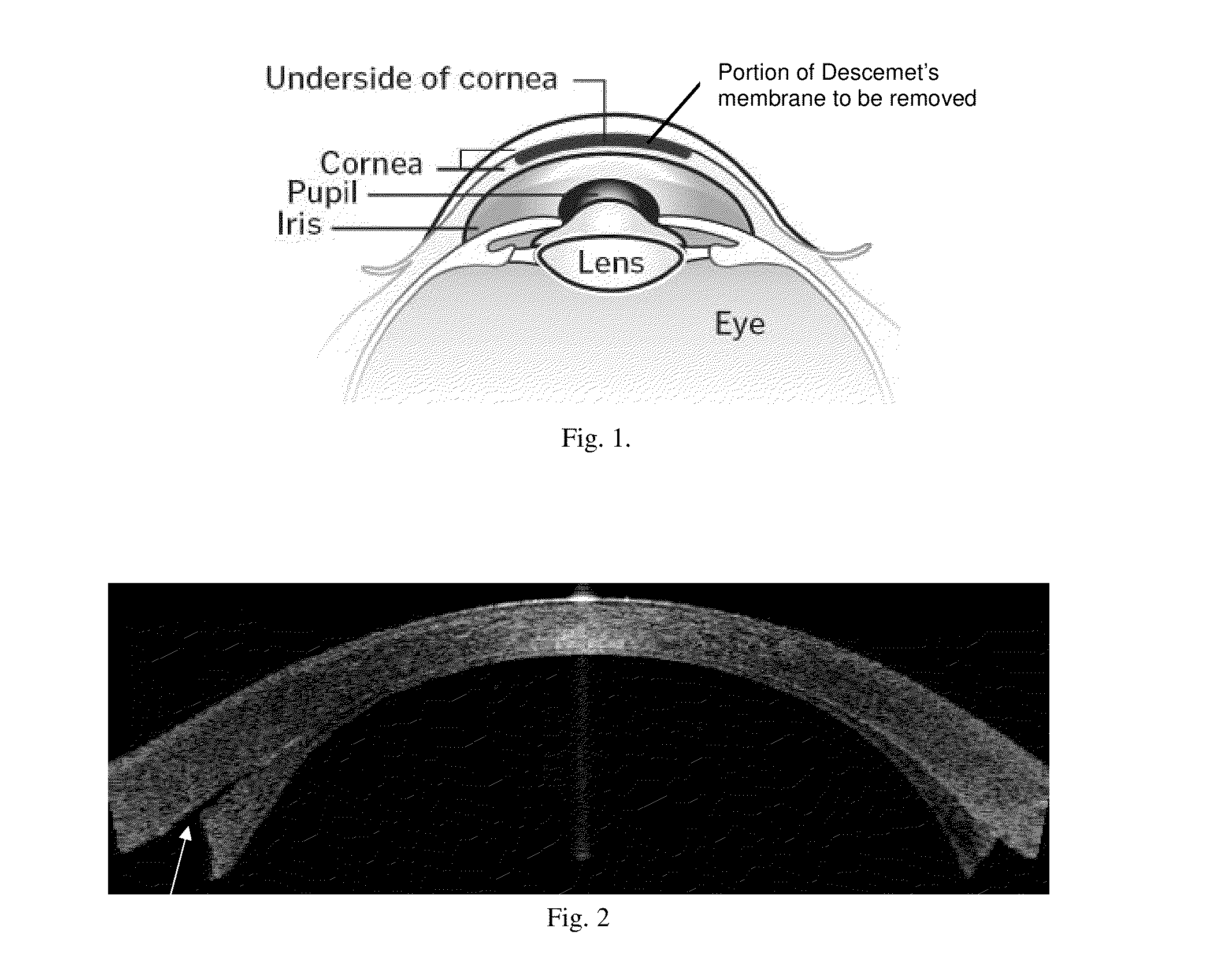
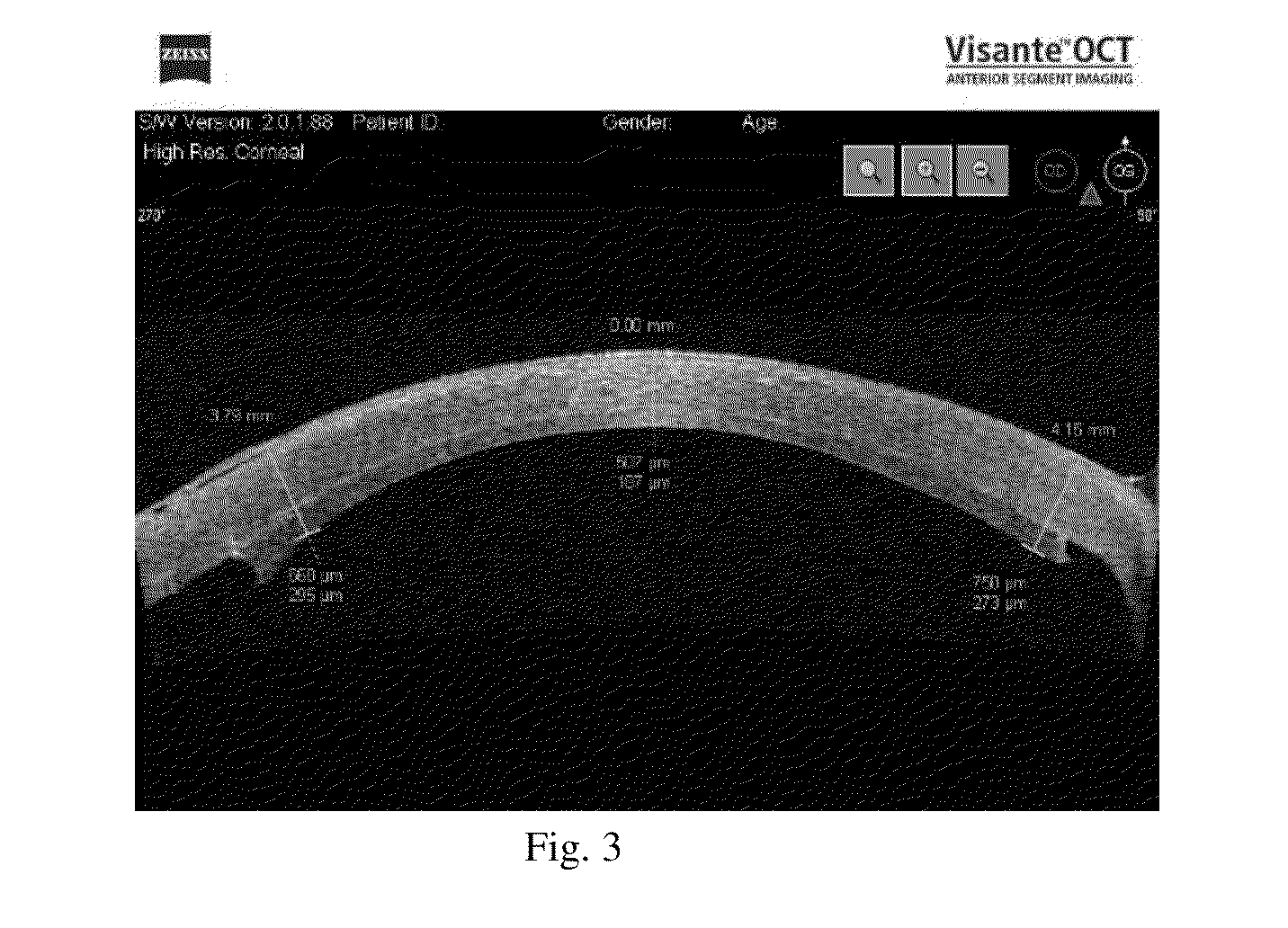
Corneal graft evaluation based on optical coherence tomography image
An OCT image of an eye which has been subject to a DSAEK corneal transplant, in which a Descement's membrane in the cornea has been replaced by a graft, is processed to identify the outline of the graft. The process includes the steps of: computationally extracting the boundary of the cornea including the graft; computationally detecting the corners of the graft; computationally extracting points on the boundary between the graft and the original cornea; and computationally fitting the points on the boundary between the graft and the original cornea smoothly into a curve. The outline of the graft is then displayed. A graft profile may be generated, indicating the thickness of the graft at each point along its length.
Owner:SINGAPORE HEALTH SERVICES PTE +1
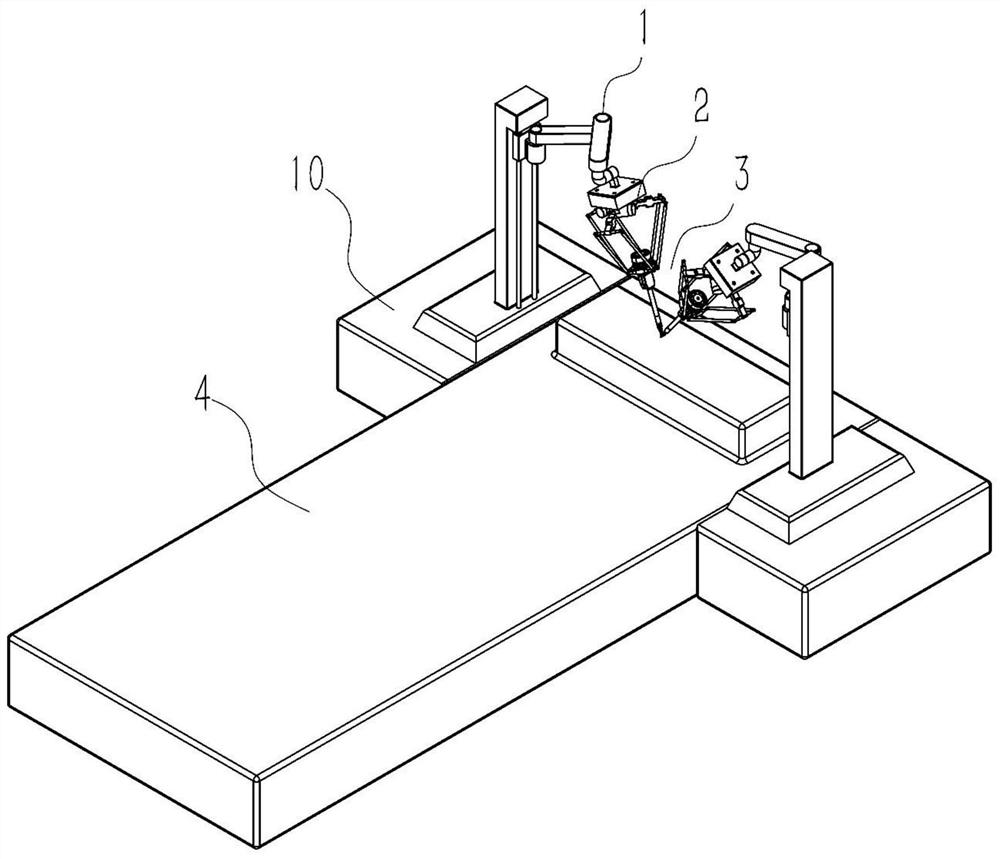
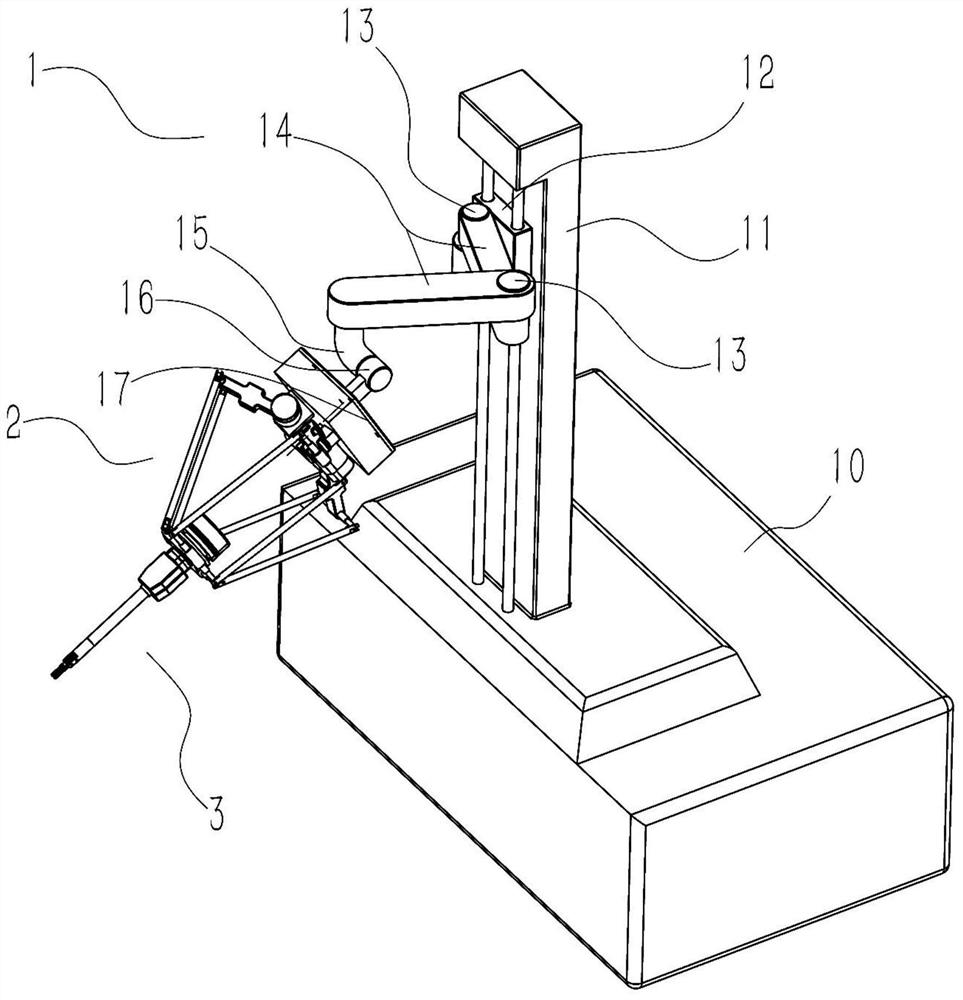
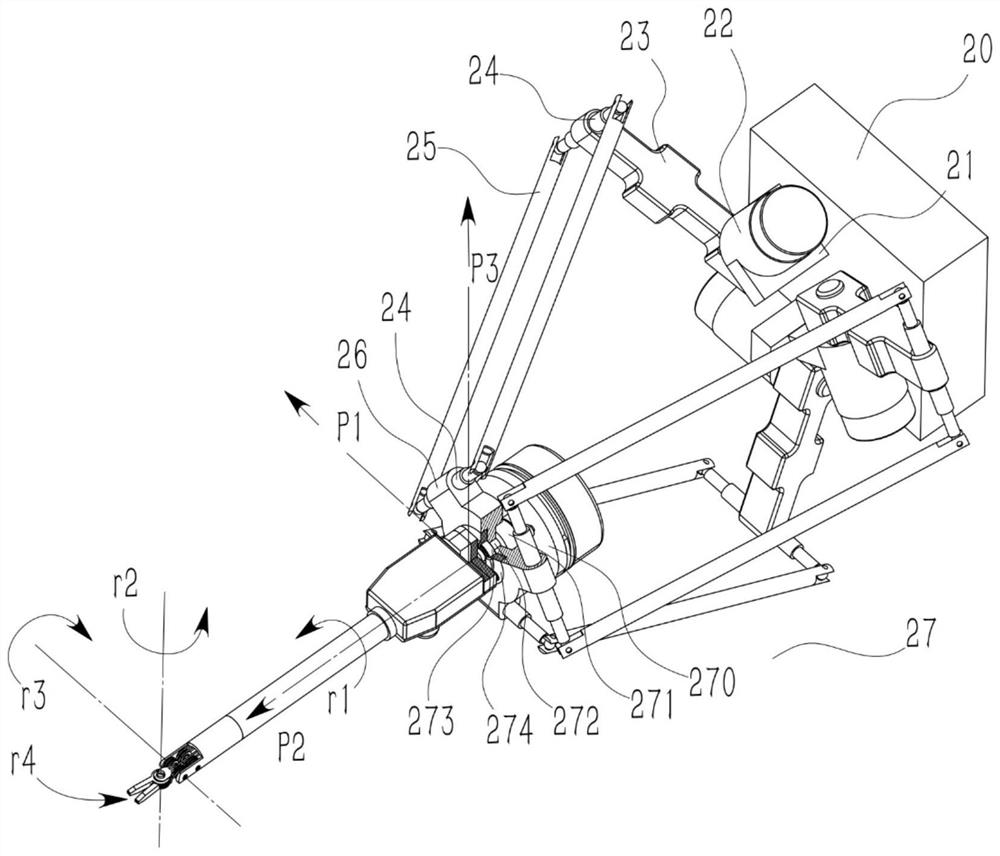
Operation mechanism for ophthalmic corneal transplantation surgical robot
PendingCN112168482ASmall sizeAvoid interferenceEye surgerySurgical robotsSurgical ManipulationOphthalmology department
The invention discloses an operation mechanism for an ophthalmic corneal transplantation surgical robot. The operation mechanism includes two bases arranged beside an operating table, two robotic armsrespectively fixed on vertical lifting platforms of the bases, at least two instrument-holding arms to simulate the hands of a surgeon and a mirror-holding arm to place imaging equipment; each instrument-holding arm includes a preoperative positioning mechanism and an intraoperative instrument-manipulating mechanism; the preoperative positioning mechanism is used for positioning the intraoperative instrument-manipulating mechanism; the intraoperative instrument-manipulating mechanism includes a position adjusting mechanism and a pose adjusting mechanism; the position adjusting mechanism can realize the position adjusting of the pose adjusting mechanism in space; and the motion and force transmission of the pose adjusting mechanism can be achieved through a rope-pulley mechanism so as to control tools to perform operations. Through the operation mechanism, the movement track of operation tools at any position and pose in a working space can be realized, and corneal drilling and suturing during corneal transplantation can be accomplished; and the operation mechanism is good in flexibility, high in precision, high in motion stiffness and large in motion space range.
Owner:XI AN JIAOTONG UNIV
Ectocornea-like sheet and method of constructing the same
InactiveUS20050003532A1Reduce differentiationHigh divisional potentialEye implantsArtificial cell constructsDiseaseOral mucosal epithelial cell
It is intended to provide a transplantation material applicable to ocular surface diseases with a need for ectocornea transplantation (i.e., an ectocornea-like sheet). Oral mucosal epithelial cells are inoculated onto an amnion and then cultured in the coexistence of supporter cells. When a layered structure of the oral mucosal epithelial cells is formed, the outermost layer is brought into contact with air, thereby inducing differentiation. Thus, an ectocornea-like sheet having an oral mucosal epithelial cell layer on the amnion is obtained.
Owner:AMNIOTEC +1
Low swell, long-lived hydrogel sealant
A low swell, long-lived hydrogel sealant formed by reacting a highly oxidized polysaccharide containing aldehyde groups with a multi-arm amine is described. The hydrogel sealant may be particularly suitable for applications requiring low swell and slow degradation, for example, ophthalmic applications such as sealing wounds resulting from trauma such as corneal lacerations, or from surgical procedures such as vitrectomy procedures, cataract surgery, LASIK surgery, glaucoma surgery, and corneal transplants; neurosurgery applications, such as sealing the dura; and as a plug to seal a fistula or the punctum. The low swell, long-lived hydrogel sealant may also be useful as a tissue sealant and adhesive, and as an anti-adhesion barrier.
Owner:ACTAMAX SURGICAL MATERIALS
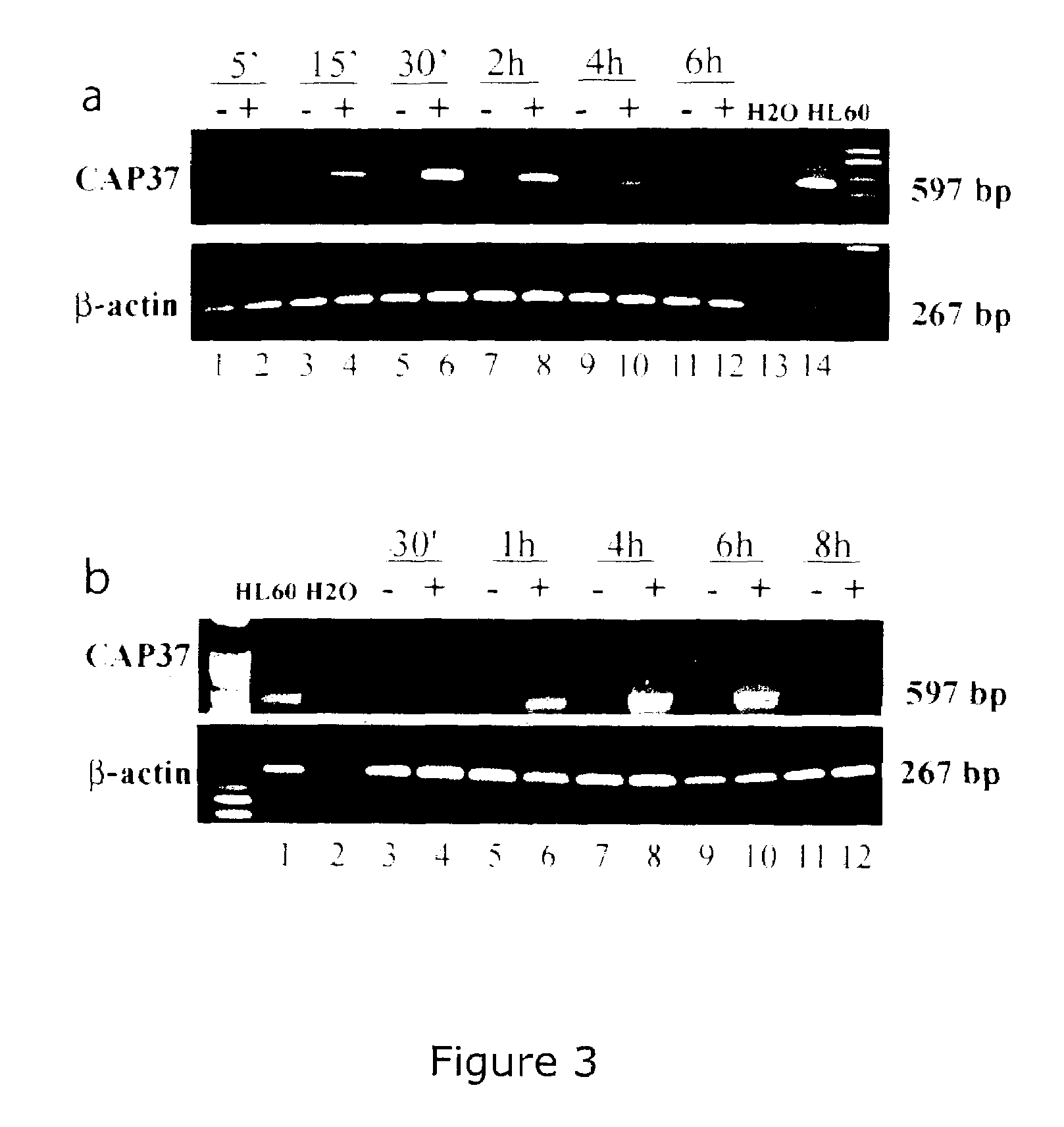
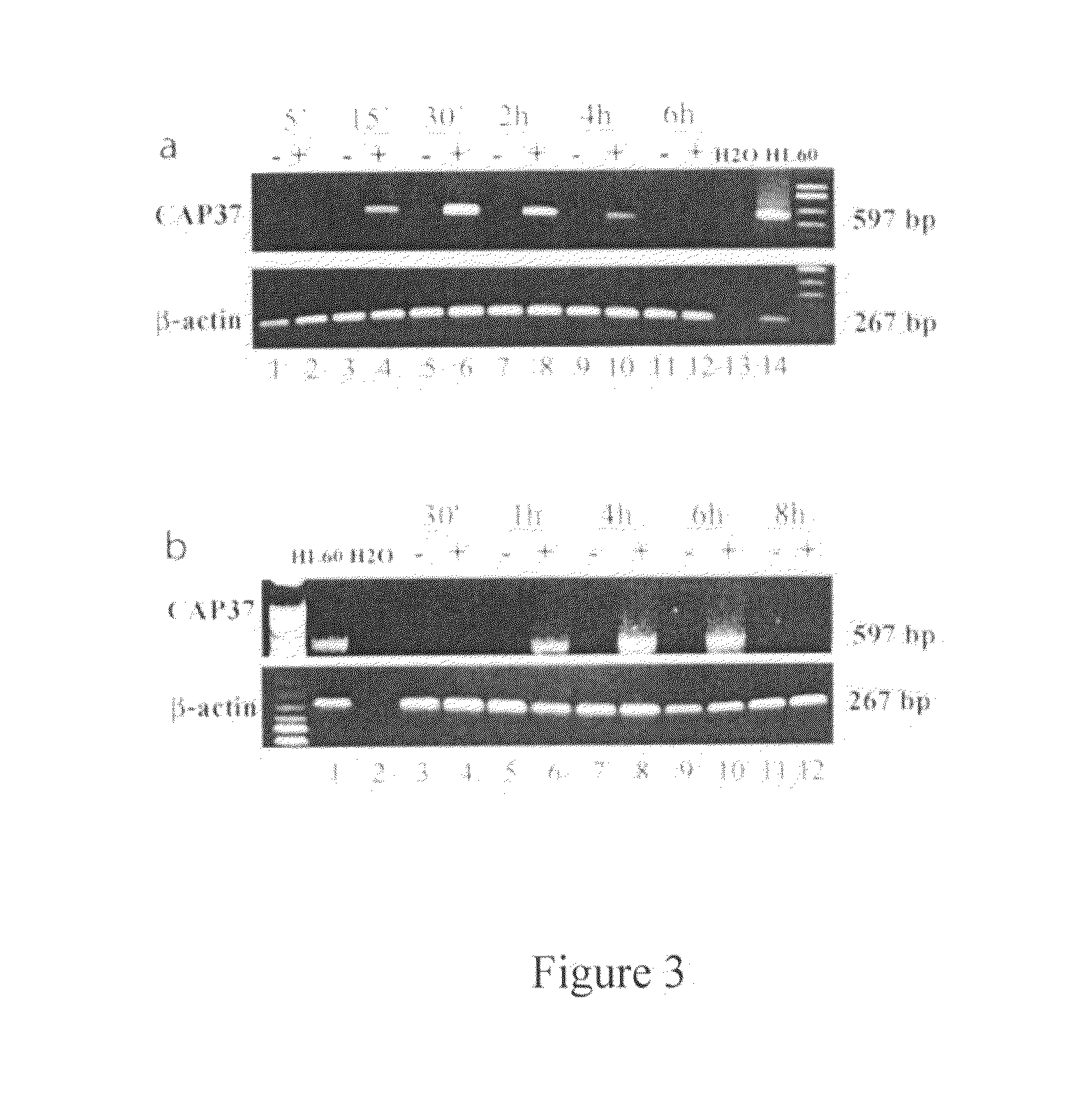
Treatment and inhibition of ocular infections and wounds by CAP37 and CAP37 peptides
ActiveUS7354900B2Promote healingAvoid infectionAntibacterial agentsBiocideBacterial ConjunctivitisMammal
A method for treating ocular conditions such as bacterial keratitis, bacterial conjunctivitis, corneal ulcers and wounds, endophthalmitis, and blebitis in mammals, by using a native, synthetic, or recombinant CAP37, or effective peptide portions thereof including CAP37 peptides 20-44, 23-42, 102-122, and 120-146 and monocysteine derivatives of peptides 20-44 and 23-42. The CAP37, peptides, and peptide derivatives can also be used to store, clean, sterilize, or coat contact lenses, and may be used in media for storing mammalian corneal transplants.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Ophthalmological laser method and apparatus
The present invention relates to a femtosecond laser ophthalmological apparatus and method that creates a flap on the cornea for LASIK refractive surgery or for other applications that require removal of corneal and lens tissue at specific locations, such as in corneal transplants, stromal tunnels, corneal lenticular extraction and cataract surgery. The femtosecond laser is transferred from the main cabinet to a hand piece module via a rotating mirror set module. In the hand piece, the femtosecond laser beam is scanned and guided to the patient's eye. The ablation pattern is based on dividing the area of the ablation area into a matrix grid made up of cells. Predetermined ablation pattern is completed in an individual cell before moving on to the next cell until ablation is complete in the entire matrix grid mapped on the ablation area.
Owner:EXCELSIUS MEDICAL
Ophthalmic surgical irrigating solutions containing hyaluronidase and method for preventing post-operative intraocular pressure increases
InactiveUS20070137657A1Protection is in progressIncrease pressureSenses disorderPeptide/protein ingredientsIntraocular Pressure RiseAntioxidant
An ophthalmic irrigating solution containing hyaluronidase and an antioxidant is provided. The solution prevents a post-operative intraocular pressure rise when used during an ophthalmic surgical procedure, and also protects corneal endothelial cells. A method for preventing post-operative intraocular pressure increases in an eye during ophthalmic surgery involves irrigating an anterior chamber of the eye with an ophthalmic-solution containing hyaluronidase and an antioxidant. Kits containing hyaluronidase and base medium solutions are also provided. The kits are designed such that the hyaluronidase and base medium solution are combined and administered together to an eye of a patient during or following an ophthalmic surgical procedure, such as cataract surgery, intraocular lens surgery, corneal transplant surgery, or glaucoma surgery. Administration of the components of the kit prevents an increase in post-operative intraocular pressure and also protects corneal endothelial cells.
Owner:CLEO COSMETIC & PHARMA
Treatment of ocular wounds and ulcers
InactiveUS20090233867A1Promote healingAvoid infectionAntibacterial agentsBiocideDiseaseBacterial Conjunctivitis
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Corneal graft evaluation based on optical coherence tomography image
An OCT image of an eye which has been subject to a DSAEK corneal transplant, in which a Descement's membrane in the cornea has been replaced by a graft, is processed to identify the outline of the graft. The process includes the steps of: computationally extracting the boundary of the cornea including the graft; computationally detecting the corners of the graft; computationally extracting points on the boundary between the graft and the original cornea; and computationally fitting the points on the boundary between the graft and the original cornea smoothly into a curve. The outline of the graft is then displayed. A graft profile may be generated, indicating the thickness of the graft at each point along its length.
Owner:SINGAPORE HEALTH SERVICES PTE +1
Ophthalmic surgical irrigating solutions containing hyaluronidase and method for preventing post-operative intraocular pressure increases
InactiveUS7976833B2Increase pressureProtects corneal endothelial cellsSenses disorderPeptide/protein ingredientsIntraocular Pressure RiseAntioxidant
An ophthalmic irrigating solution containing hyaluronidase and an antioxidant is provided. The solution prevents a post-operative intraocular pressure rise when used during an ophthalmic surgical procedure, and also protects corneal endothelial cells. A method for preventing post-operative intraocular pressure increases in an eye during ophthalmic surgery involves irrigating an anterior chamber of the eye with an ophthalmic solution containing hyaluronidase and an antioxidant. Kits containing hyaluronidase and base medium solutions are also provided. The kits are designed such that the hyaluronidase and base medium solution are combined and administered together to an eye of a patient during or following an ophthalmic surgical procedure, such as cataract surgery, intraocular lens surgery, corneal transplant surgery, or glaucoma surgery. Administration of the components of the kit prevents an increase in post-operative intraocular pressure and also protects corneal endothelial cells.
Owner:CLEO COSMETIC & PHARMA
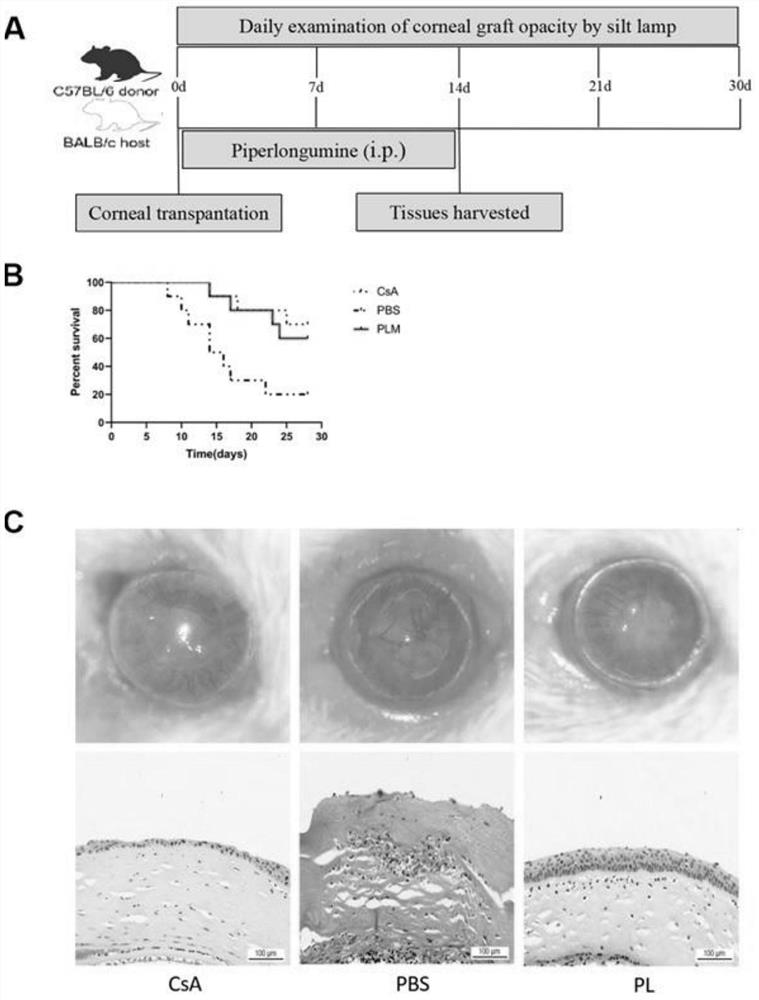
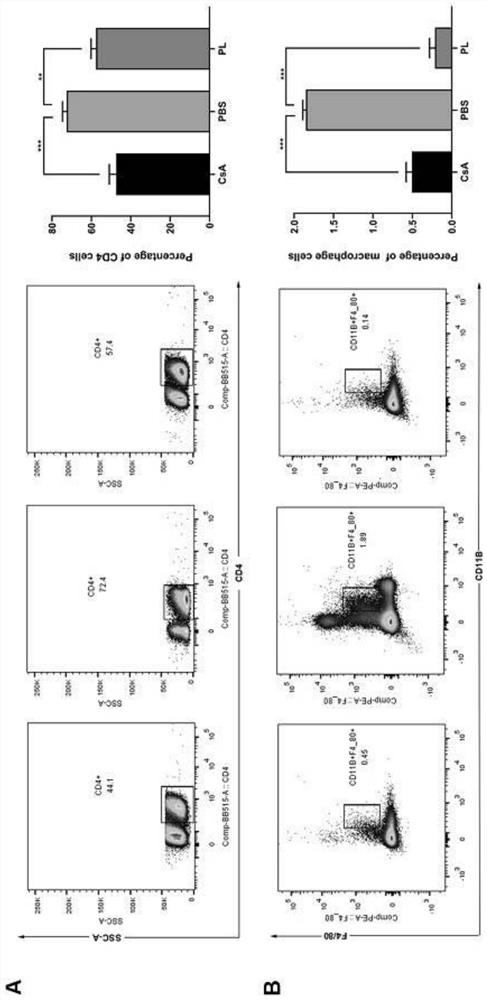
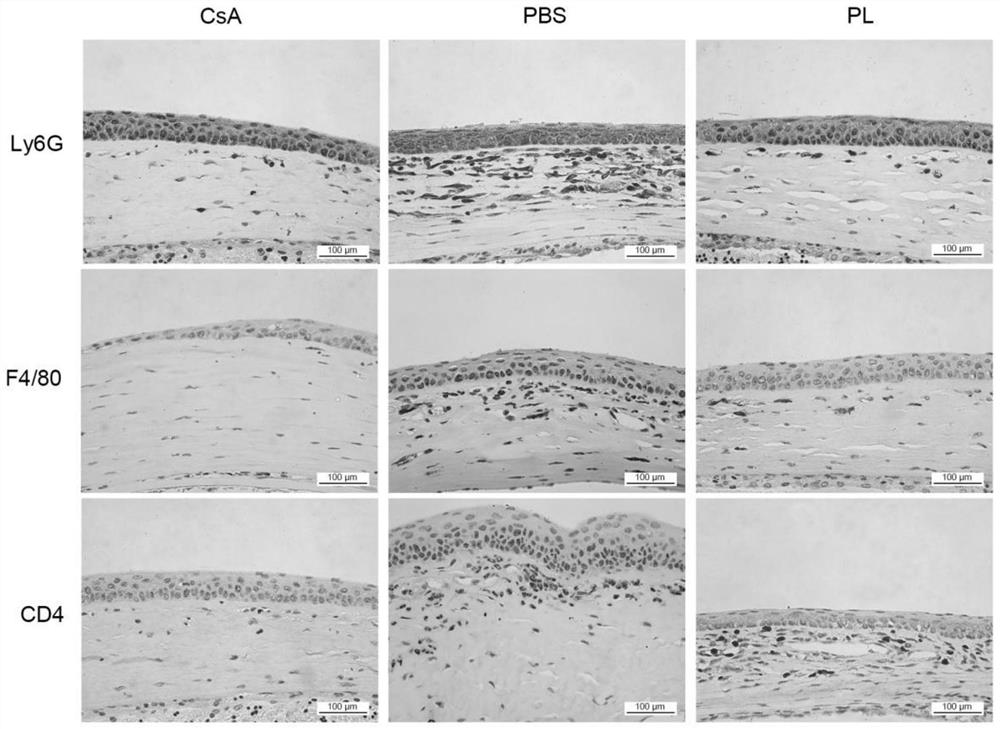
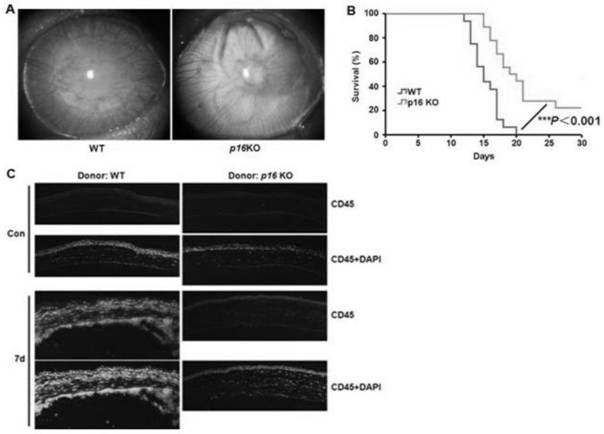
Application of piperlongumine in preparation of medicine for preventing and treating immunological rejection in corneal transplantation
InactiveCN113244233AAlleviate immune rejection of transplantProlong survival timeOrganic active ingredientsSenses disorderOphthalmologyCytokine
The invention relates to application of piperlongumine in preparation of a medicine for preventing and treating immunological rejection in corneal transplantation. Researches prove that the natural compound piperlongumine can effectively relieve immunological rejection in corneal transplantation and prolong the survival time of the corneal graft in a transplant rejection animal model, and possible mechanisms comprise inhibition of generation of immune cells and local infiltration and reduction of secretion of related inflammatory cytokines. The invention proves the effectiveness of the natural compound piperlongumine in treating the immunological rejection after the corneal transplantation, and shows that the piperlongumine has great development potential and research value when being used as a new anti-rejection medicine after the corneal transplantation.
Owner:EYE & ENT HOSPITAL SHANGHAI MEDICAL SCHOOL FUDAN UNIV
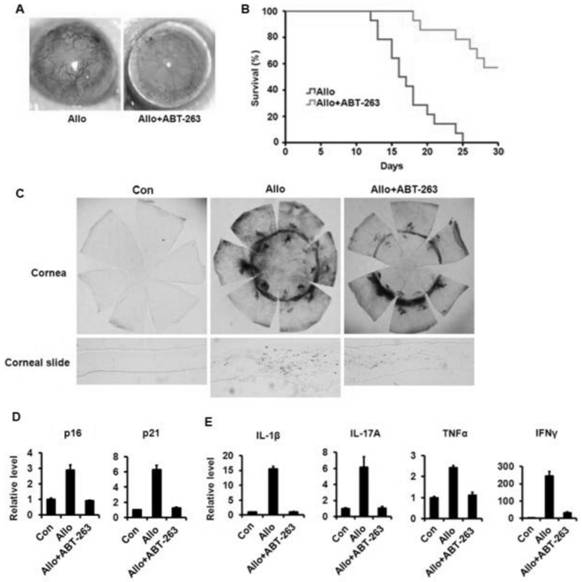
Application of ABT-263 in preparation of medicine for inhibiting corneal transplantation immunological rejection
PendingCN113633642AProlong survival timeReduce inflammationOrganic active ingredientsPharmaceutical delivery mechanismDiseaseOphthalmology
The invention provides application of ABT-263 in preparation of a medicine for inhibiting corneal transplantation immunological rejection, and belongs to the technical field of chemical medicines. The invention provides an application of a micromolecular Bcl-2 inhibitor Navitclax(ABT-263) capable of inducing apoptosis in preparation of a product for inhibiting immunological rejection after corneal transplantation. Researches show that the Bcl-2 inhibitor ABT-263 can prolong the survival time of a corneal graft after corneal transplantation, reduce immune inflammatory response in the corneal graft, maintain transparency of the corneal graft and reduce immunological rejection, and can be used for preventing and treating diseases such as immunological rejection after corneal transplantation, corneal endothelial cell decompensation and the like.
Owner:山东第一医科大学附属青岛眼科医院
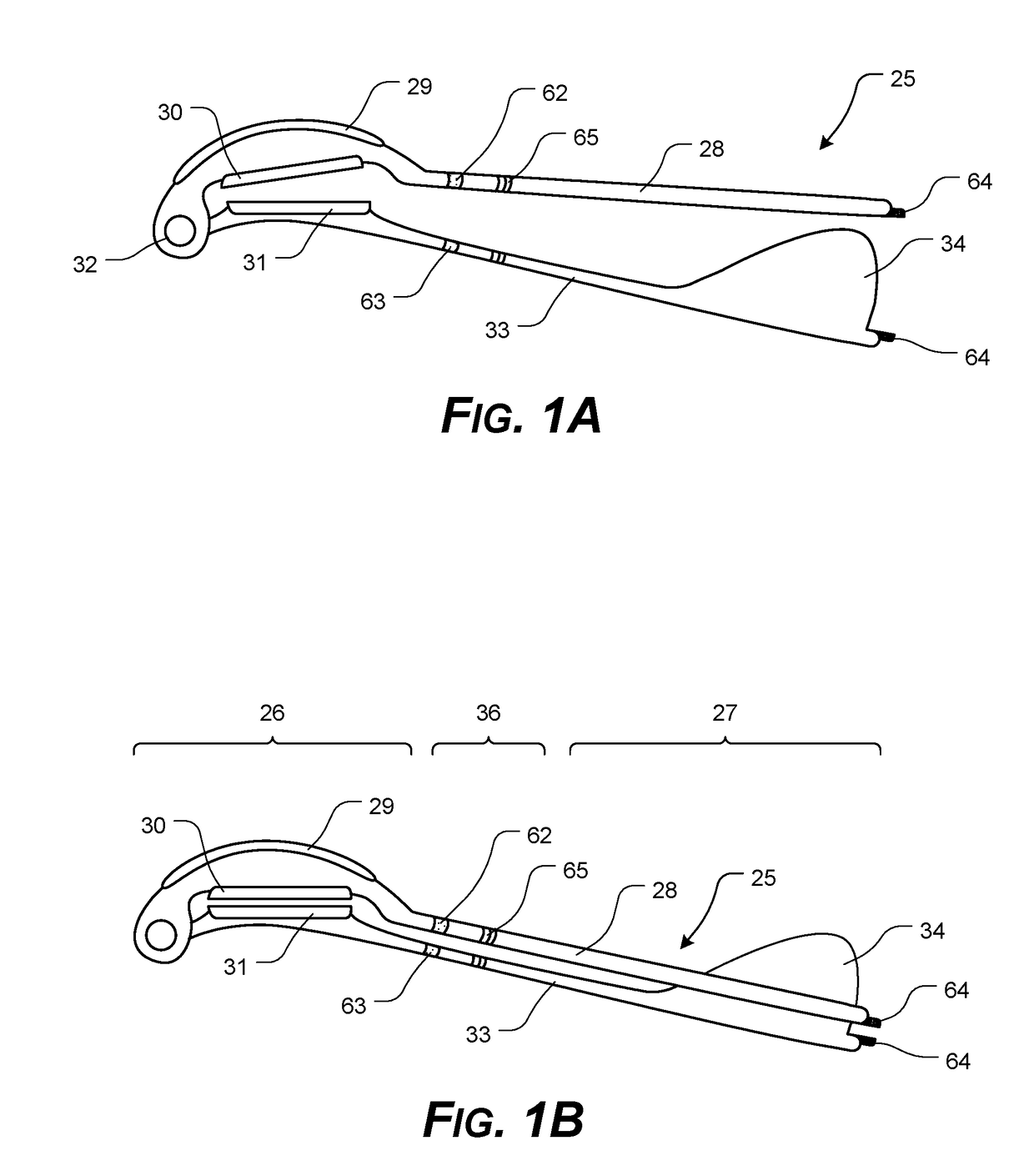
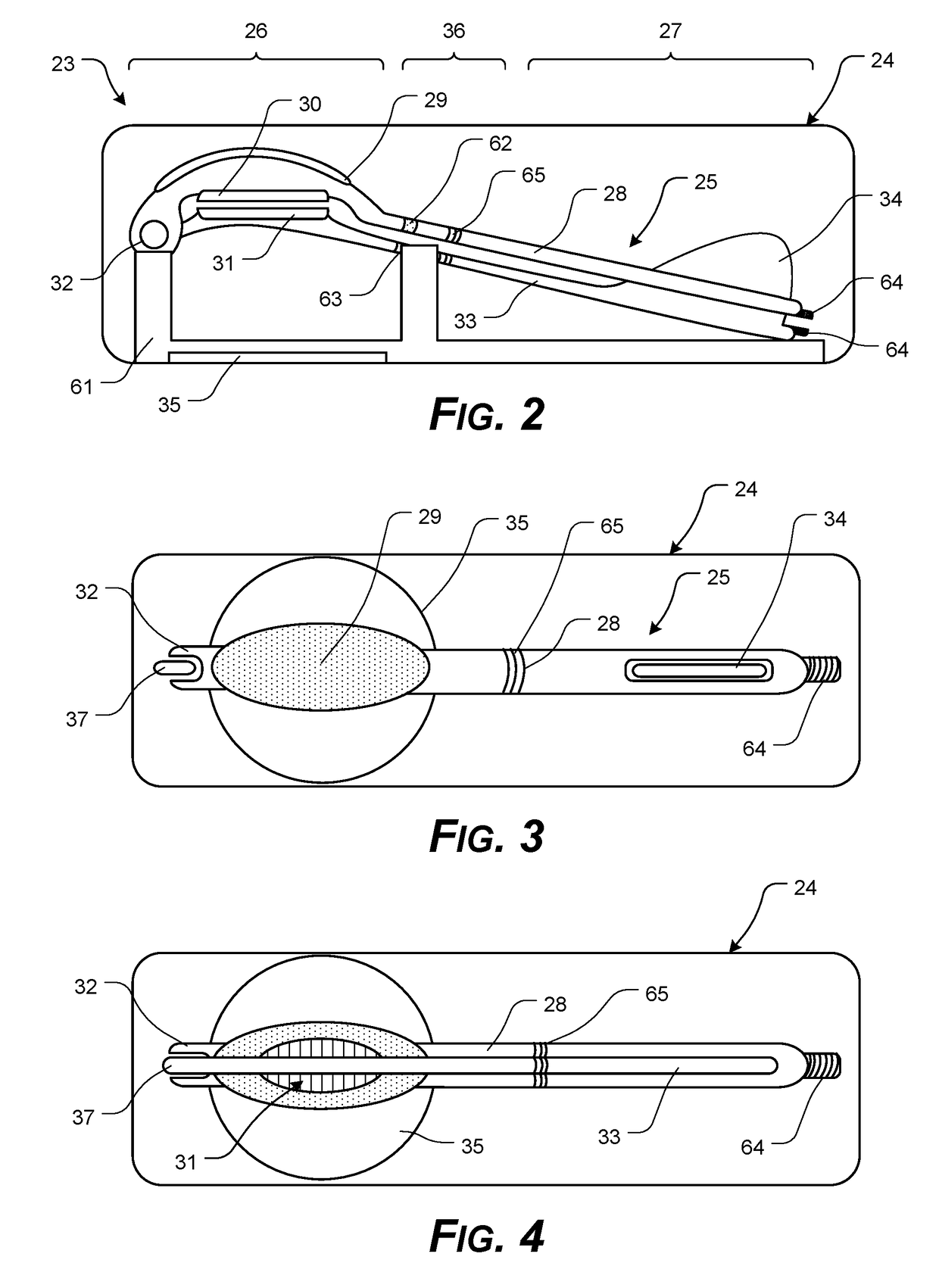
Corneal Transplant Systems, Methods, and Apparatuses
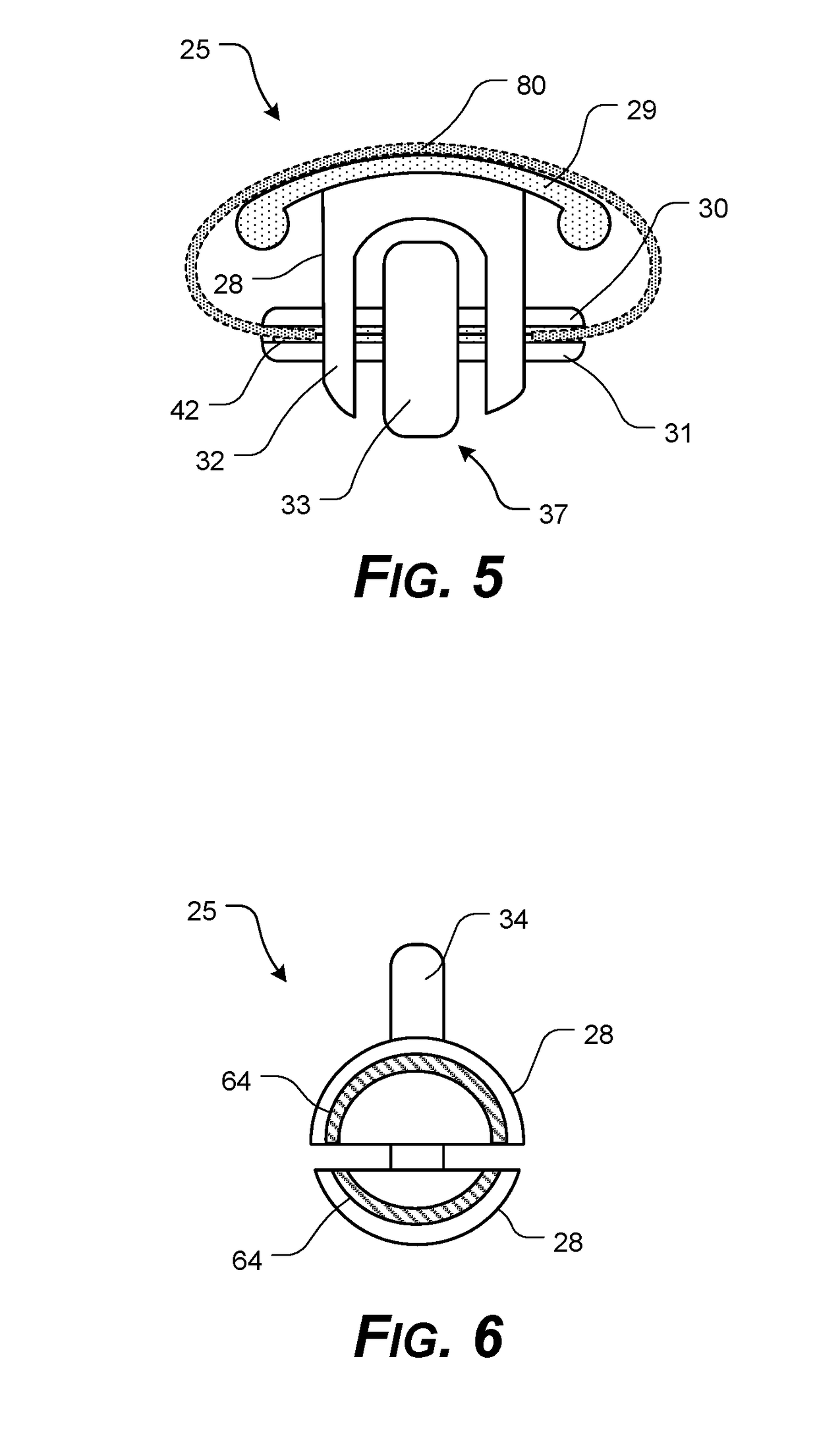
InactiveUS20190038400A1Easy to operateTransportationEye surgeryIntraocular lensDistal portionCorneal Transplant
A transplant tissue storage, transport, and / or placement apparatus, including at least some of a transplant device extending from a distal portion to a proximal portion; a tissue support element formed in the distal portion, the tissue support element having an upper surface having a tissue support element configured to support a central portion of transplant tissue, and a transplant tissue securing mechanism with opposing first securing pad and the second securing pad configured for clasping opposing peripheral portions of transplant tissue in a draped configuration; and a lockable arrangement formed in the proximal portion, the lockable arrangement configured to release a secured transplant tissue from the mounting surface, upon actuation.
Owner:SAMUDRE SANDEEP
Formula of medicament for treating non-infectious ocular inflammations, and inhibiting corneal neovascularization and anti-rejection reaction generated after corneal grafting
InactiveCN102210864AHigh activityEasy to storeSenses disorderPeptide/protein ingredientsMicrosphereRetention time
The invention discloses a formula of a medicament for treating non-infectious ocular inflammations, and inhibiting the corneal neovascularization and the anti-rejection reaction generated after corneal grafting. Through the selection of an isotonizing agent and a buffering agent and the addition of an antiseptic agent, a release microsphere preparation, a recombinant human interleukin-8 receptor antagonist and a chemokine-like factors 1 (CKLF1), the activity of a recombinant human interleukin-1 receptor antagonist is enhanced, and the retention time thereof is extended. In the invention, the problem that in the prior art, because of having a high environmental requirement on the outside, the existing recombinant human interleukin-1 receptor antagonist is easy to inactivate is solved; and through adding the recombinant human interleukin-8 receptor antagonist and the chemokine-like factors 1 (CKLF1) into the medicament, a synergistic effect is generated among the recombinant human interleukin-8 receptor antagonist, the chemokine-like factors 1 (CKLF1) and the recombinant human interleukin-1 receptor antagonist, thereby reducing the dosage of the medicament.
Owner:BEIJING DAZHOU HEKANG BIO TECH
Method for harvesting corneal donor plugs for use in keratophakia procedures
A method for harvesting a plurality of corneal donor plugs for use in keratophakia procedures requires the determination of a perimeter for the plurality of plugs. Further, the method involves the selection of posterior and anterior boundaries for the plurality of donor plugs. In the method, interfaces between adjacent donor plugs are identified. Thereafter, a pulse laser beam is directed along the perimeter, the boundaries and the interfaces to establish the plurality of plugs. After the plugs are removed from the donor cornea, individual plugs are mechanically separated from one another.
Owner:TECHNOLAS PERFECT VISION
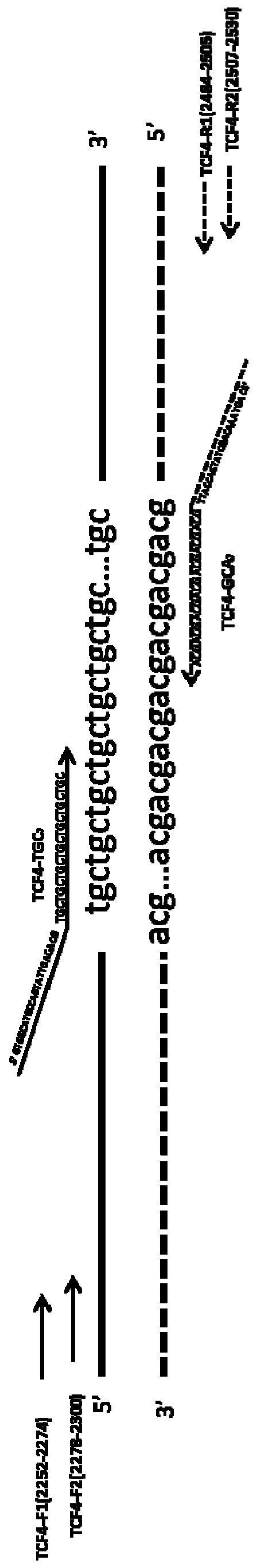
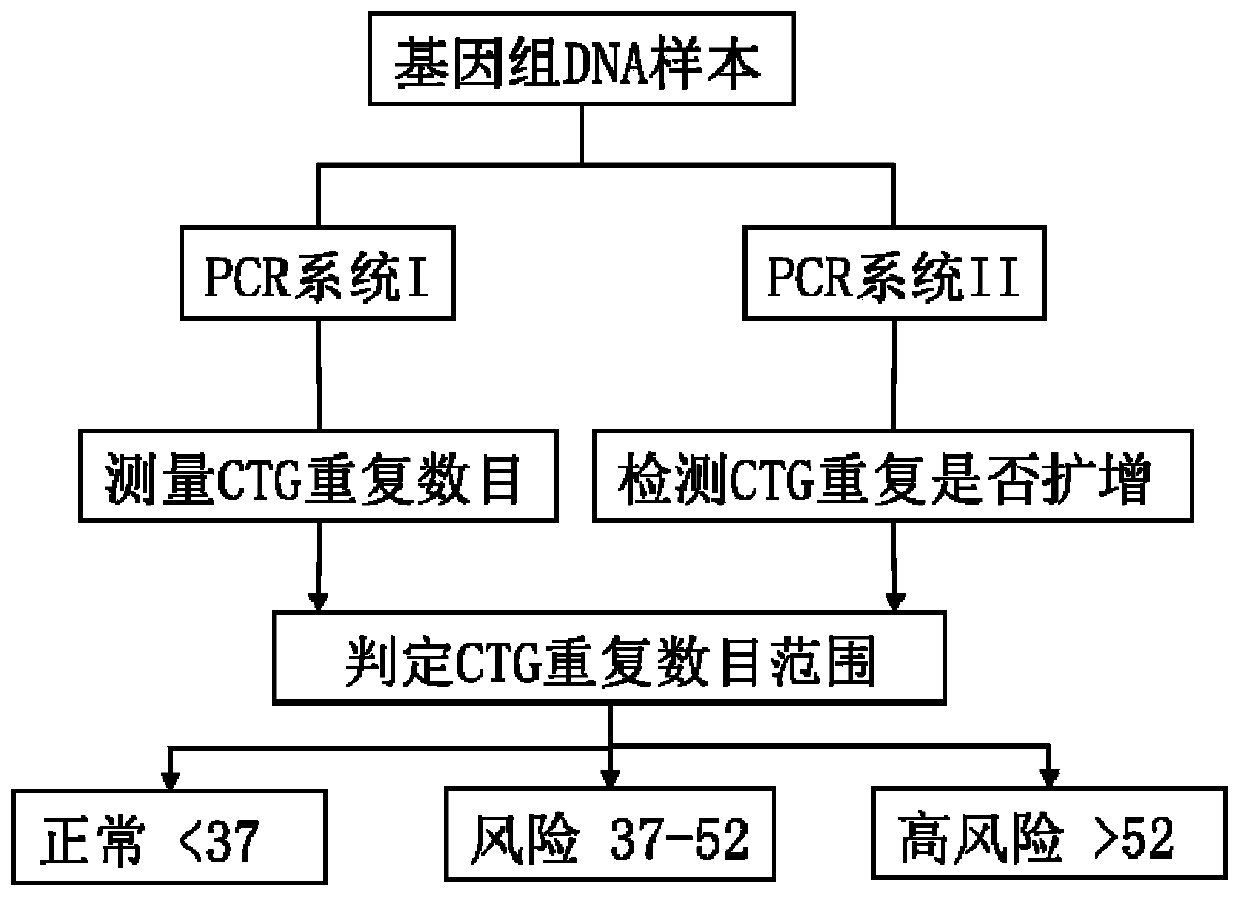
Kit for rapid screening CTG repetition of high-risk gene TCF4 for Fuchs endothelial corneal dystrophy
InactiveCN110964722AImprove throughputSmall sample sizeMicrobiological testing/measurementDNA/RNA fragmentationBiologyLarge sample
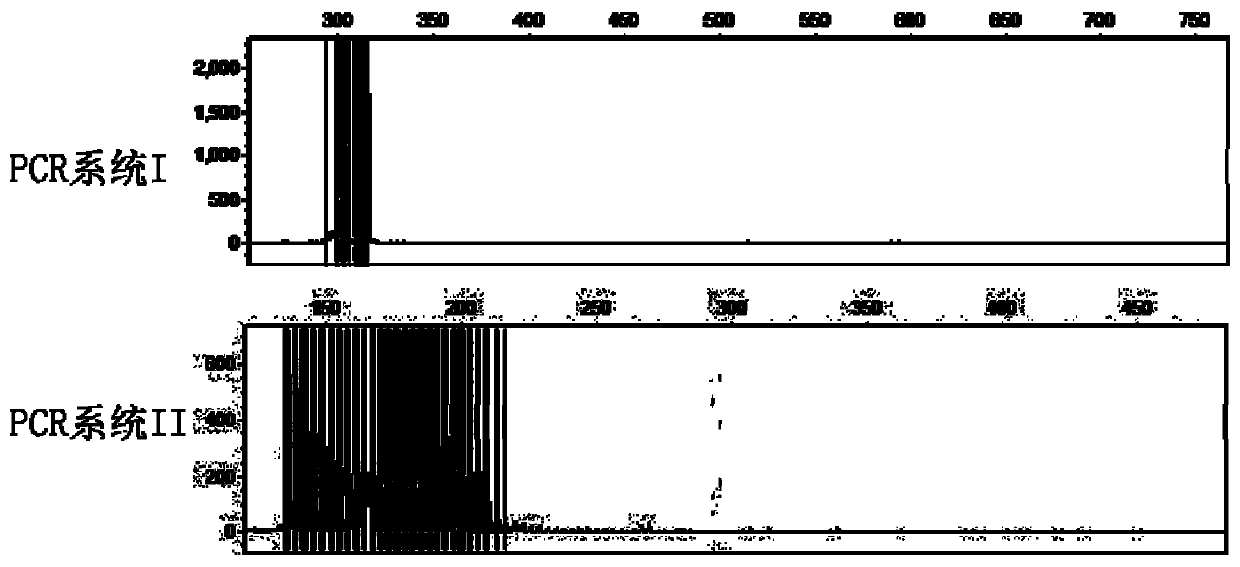
The invention provides a detection method and a kit for rapid screening CTG repeated amplification of a high-risk gene TCF4 for Fuchs endothelial corneal dystrophy. The incidence rate of the Fuchs endothelial corneal dystrophy in people over 40 years of age in Europe and America is 5 percent, with about 70 percent of patients presenting CTG repeated amplification of the TCF4. Components in the kitinclude DNA polymerases, a reaction solution, an enhancer, dNTPs, a specific primer and a standard control. Through a CG-content PCR system, after fluorescence scanning of high-thermal-stability DNApolymerase amplification CTG repetition, an interval, in which CTG of a sample to be tested can be accurately distinguished from a standard substance. The kit provided by the invention is suitable forrapid screening whether the number of CTG repetitions of Fuchs endothelial corneal dystrophy sites TCF4 is normal or amplified individuals of all ages or with a large sample quantity, so that effective treatment strategies for various stages are proposed and made for high-risk people, and screening is performed on a corneal transplantation donor. The kit has the characteristics of accuracy, rapidness, high flux, small sample quantity and low cost.
Owner:杭州方夏生物科技有限公司
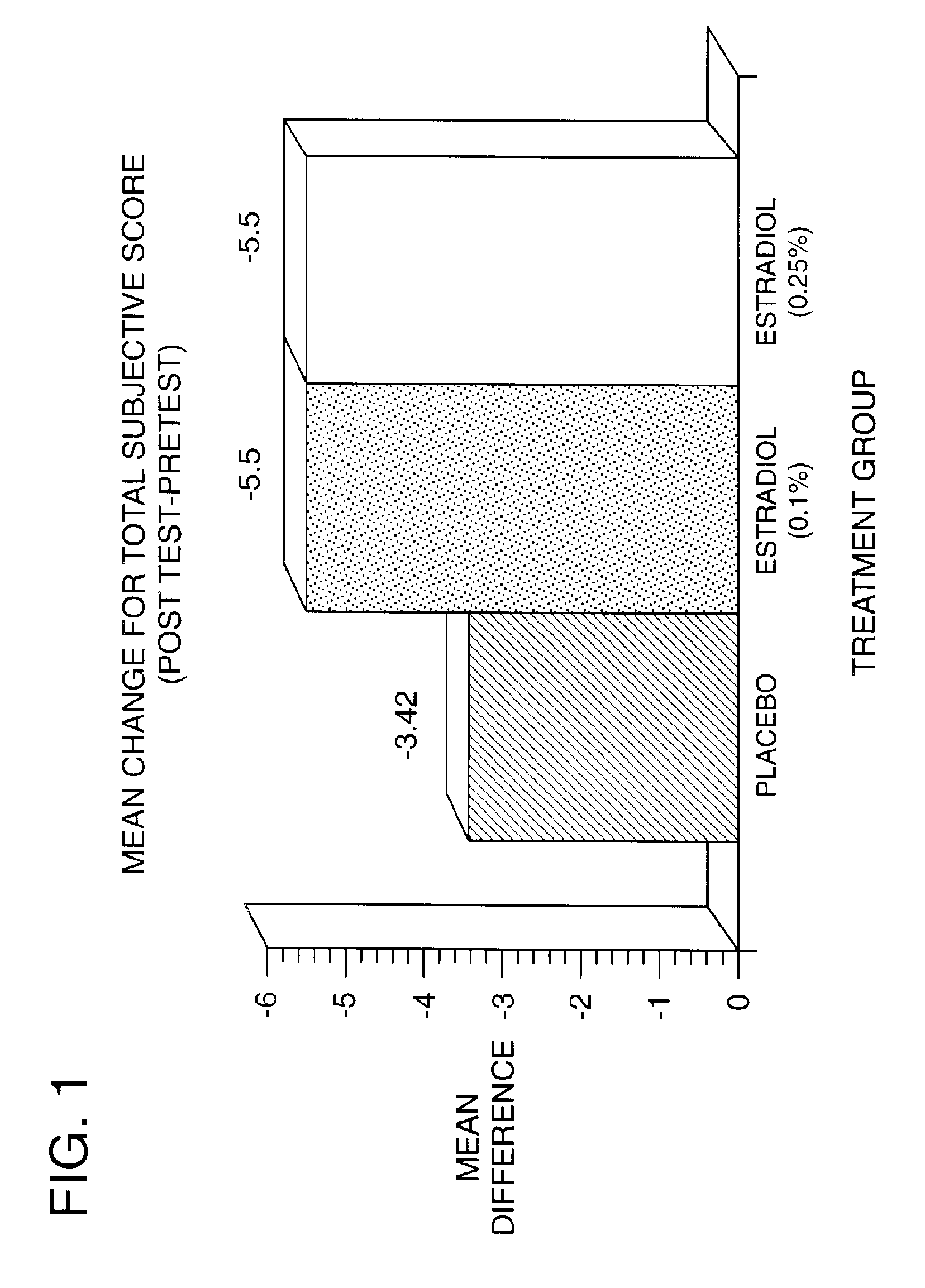
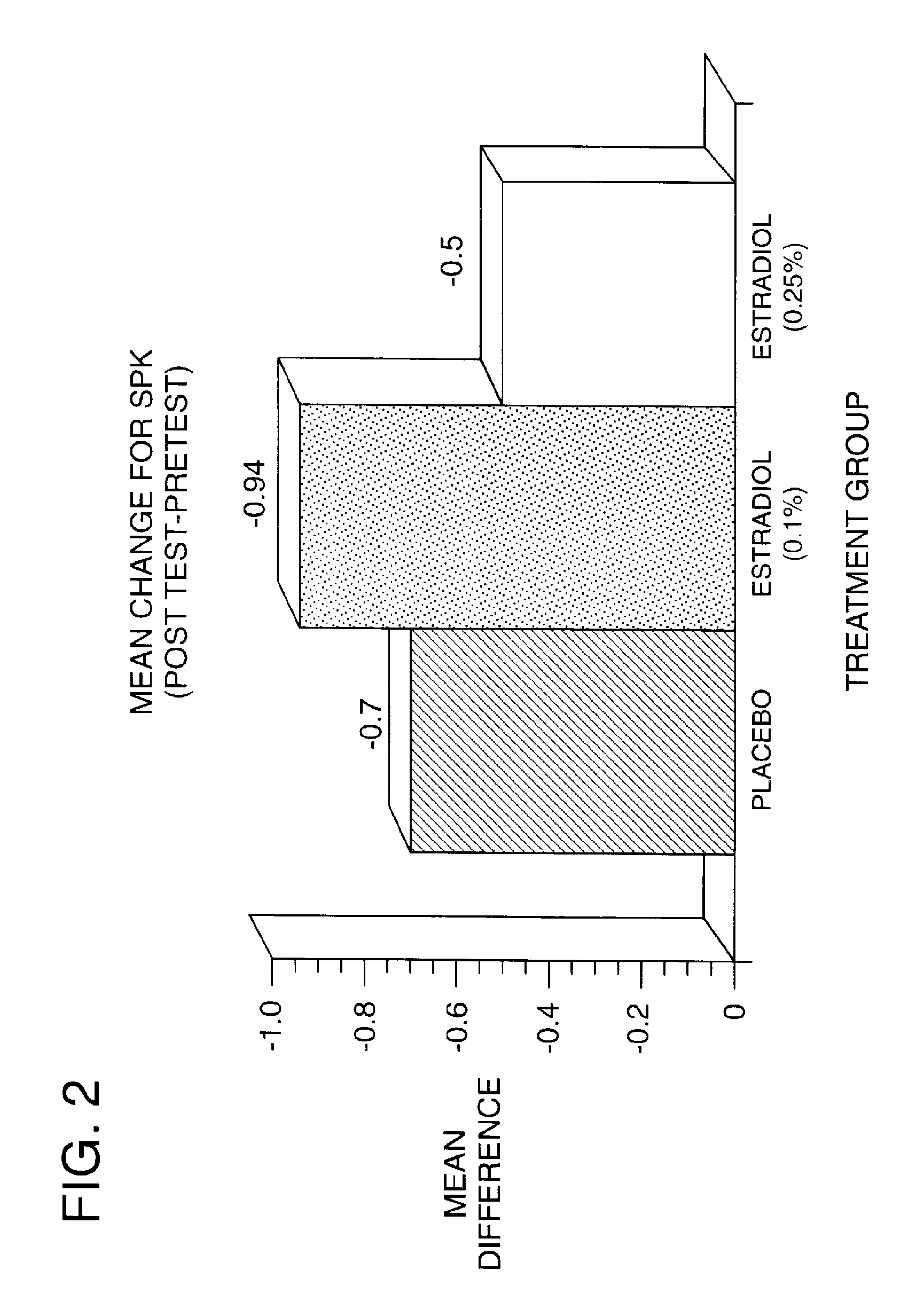
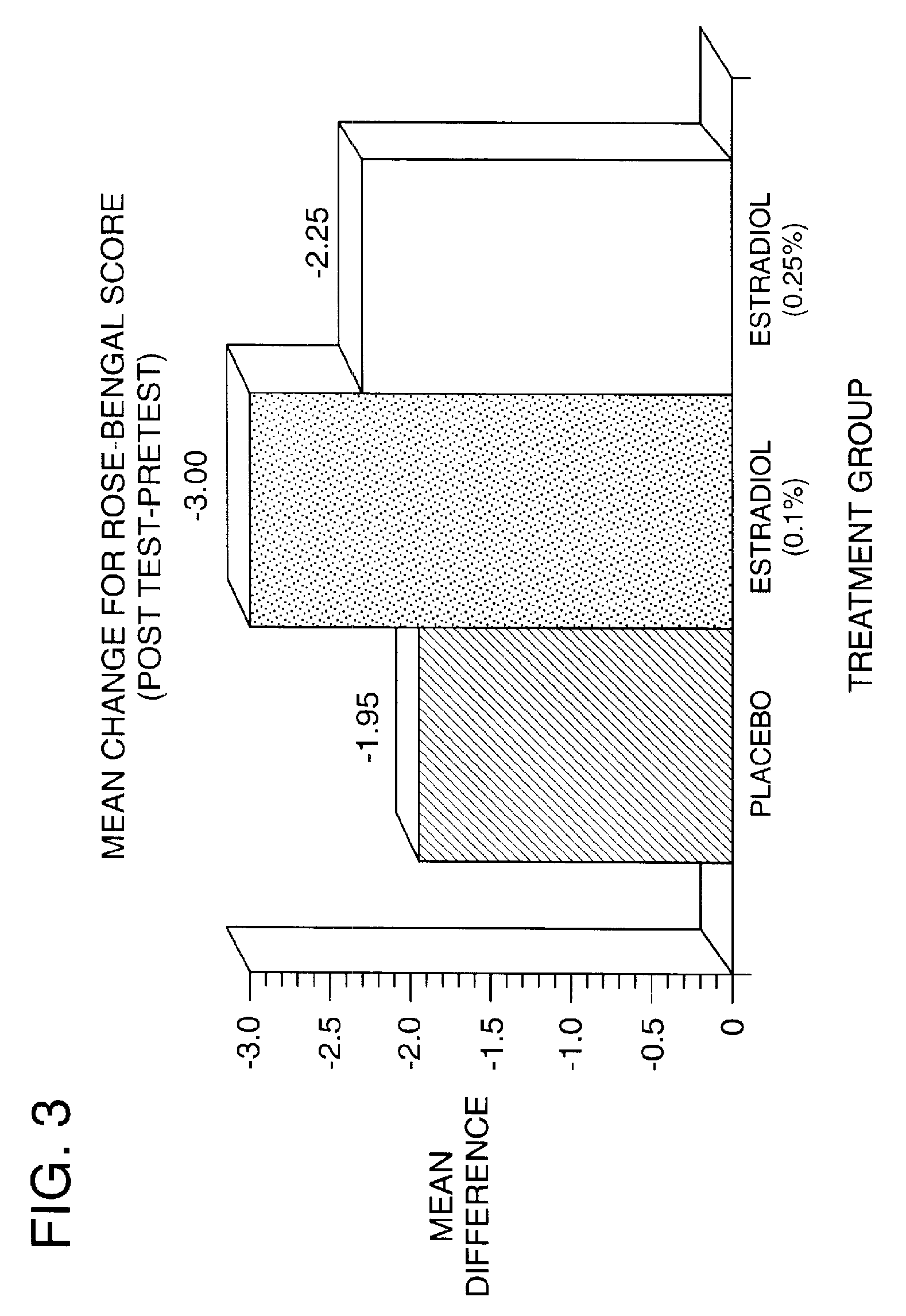
Time-release and micro-dose formulations for topical application of estrogen and estrogen analogs or other estrogen receptor modulators in the treatment of dry eye syndrome, and methods of preparation and application
ActiveUS8987241B2Alleviate dry-eye syndromeFull efficacyOrganic active ingredientsBiocide17 β estradiolGynecology
A topical application formulation of estrogen and estrogen analogs or other estrogen receptor modulators is disclosed for the treatment of primary or secondary dry eye syndrome (also known as keratoconjunctivitis sicca (KCS)). Preferred formulations include 17-β-estradiol and its derivatives in lipid, liposomes, polymers, or aqueous or non-aqueous vehicles for the topical treatment of the ocular surface tissues particularly as time-release or micro-dose formulations. These formulations may also be useful in treating other conditions where KCS may occur, such as post-operative refractive surgery and corneal transplant patients.
Owner:REDWOOD PHARMA AB
Membrane non-uniformity biological material transparency screening method and decellularized dermal matrix
ActiveCN109324001AHigh transparencySolve the contradiction of shortageColor/spectral properties measurementsTissue regenerationLamellar keratoplastyScreening method
The invention discloses a membrane non-uniformity biological material transparency screening method. The membrane non-uniformity biological material transparency screening method comprises the steps of biological material saccharose dehydration, OCT embedding, layer-by-layer frozen section, transparency measurement by using a spectrophotometer and the like. The invention also provides a high-transparency decellularized dermal matrix obtained by using the method. Through the invention, the decellularized dermal matrix with optimal transparency can be screened effectively, the decellularized dermal matrix can be used for lamellar keratoplasty, the matrix can be used for replacing an existing lamellar cornea matrix in the operation by an operating doctor, and the contradiction of donor corneashortage at present can be further solved effectively while the cornea diseases of patients are cured.
Owner:PEKING UNIV THIRD HOSPITAL
Medium, corneal stromal slice prepared by medium and preparation method
ActiveCN111235109AGood biocompatibilityGood cell affinityEye implantsCulture processFibrosisBiocompatibility
The invention relates to the technical field of medical biomaterials, in particular to a culture medium, a corneal stromal slice prepared by the culture medium and a preparation method. The medium provided by the invention is a Dulbecco's modified eagle medium / F12 (DMEM / F12) medium containing a porcine corneal extract, Y-27632, insulin-transferrin-selenium (ITS), fibroblast growth factors (FGF), ascorbic acid and fetal bovine serum. According to the medium, by selecting suitable active components and proportion, a corneal stromal cell and corneal stromal slice prepared by employing the mediumhave the functions of maintaining the corneal stromal cell phenotype, promoting proliferation, improving vitality, resisting fibrosis and resisting apoptosis, and the like in comparison with a cornealstromal cell and corneal stromal slice prepared by a traditional medium. The corneal stromal slice disclosed by the invention is prepared by taking a corneal lens as a raw material and culturing thecorneal lens by employing the medium; and compared with animal-derived corneal repair material, the corneal lens has good biocompatibility and no antigenicity, can be used as substitutes of various donor materials for corneal transplantation, and can be accepted by the majority of patients and is clinically applied for long.
Owner:AIER EYE HOSPITAL GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com