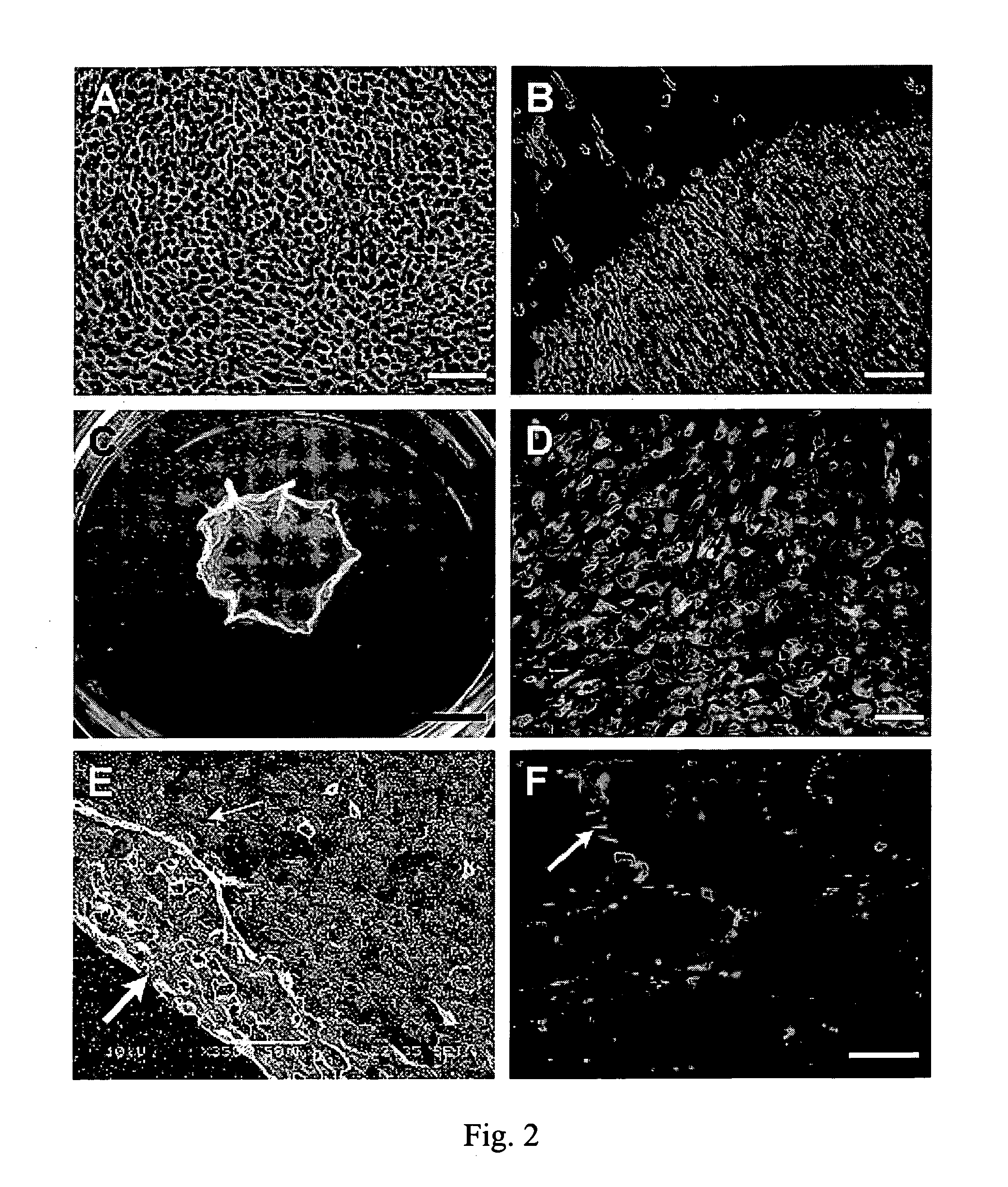
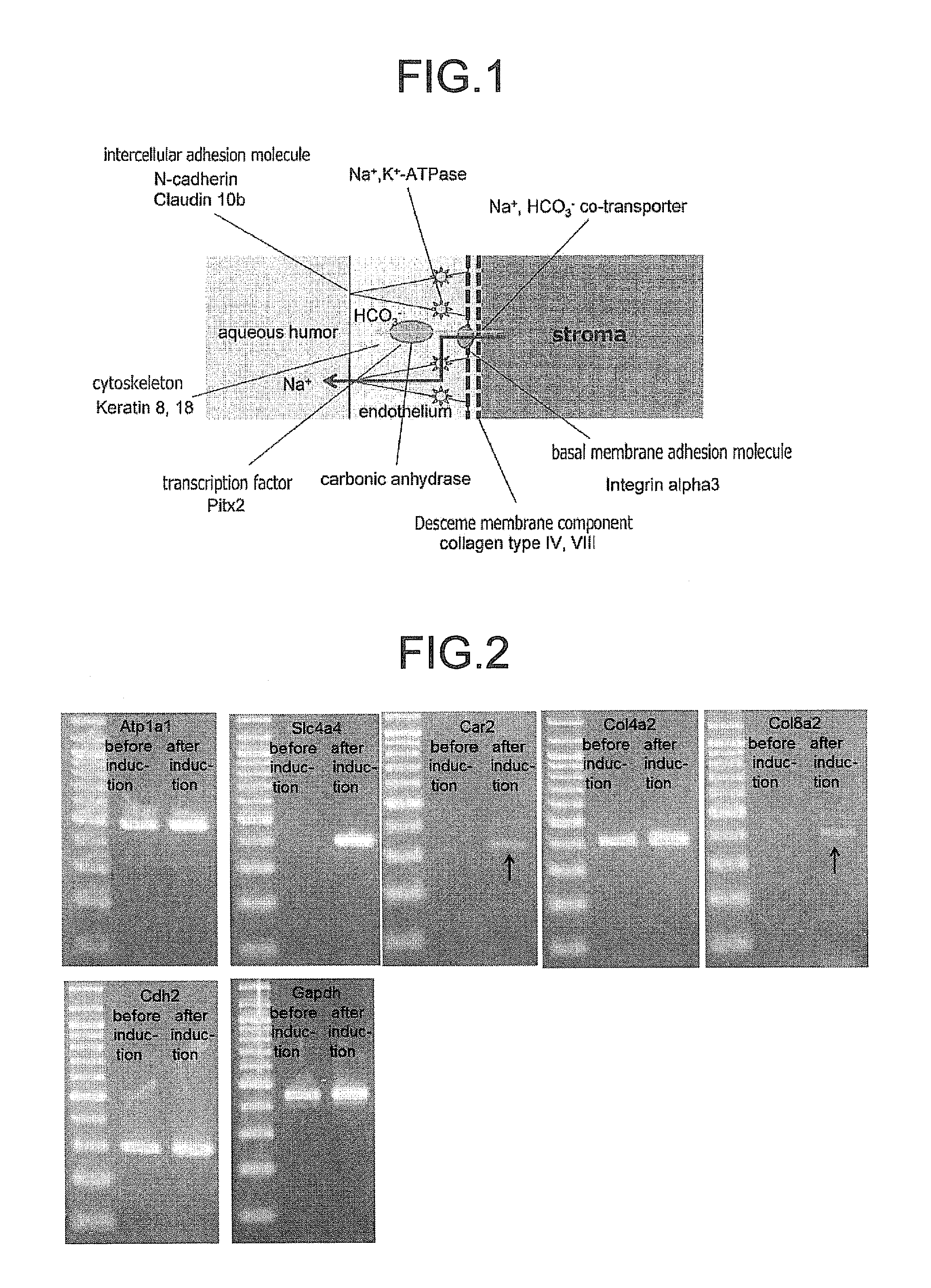
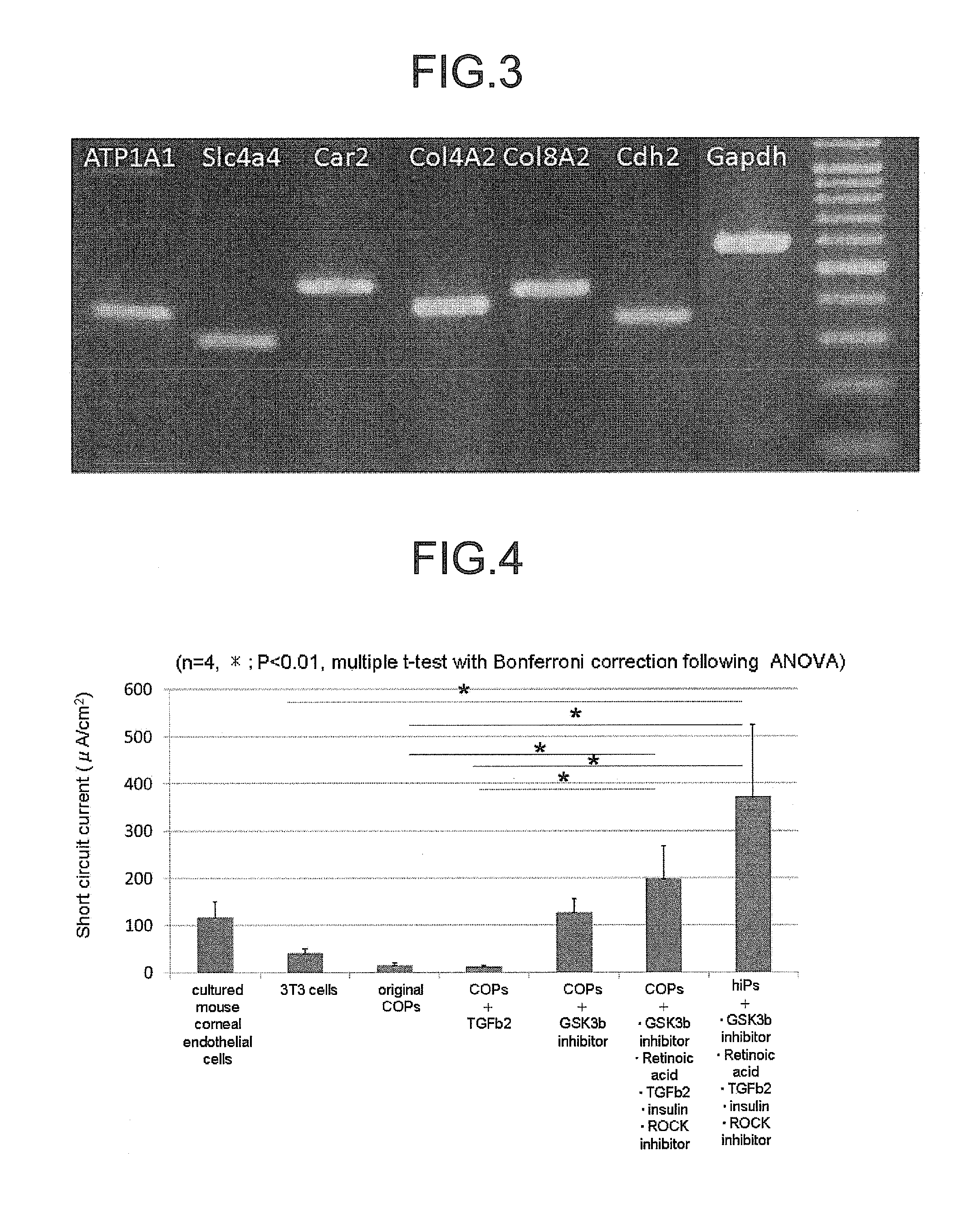
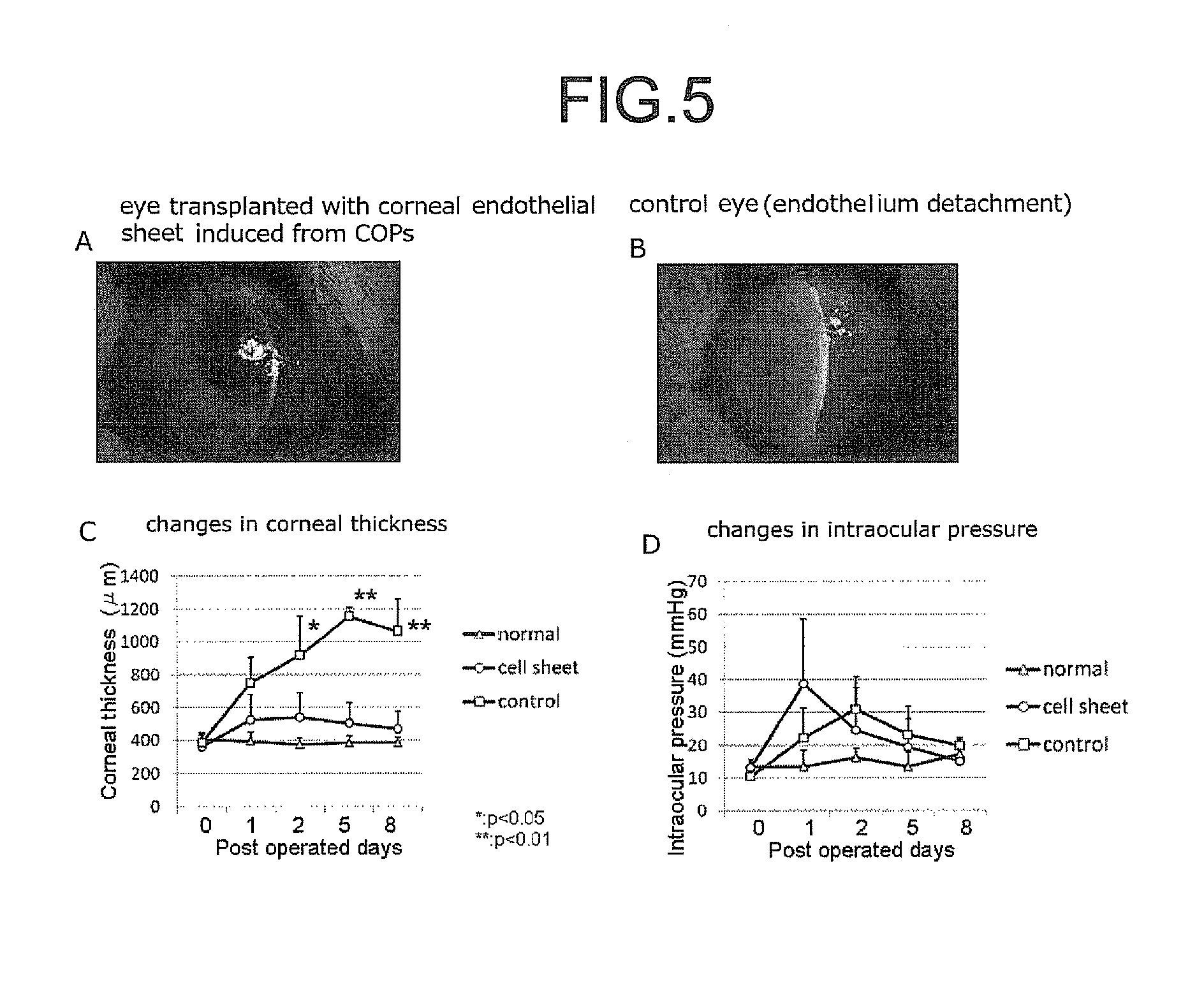
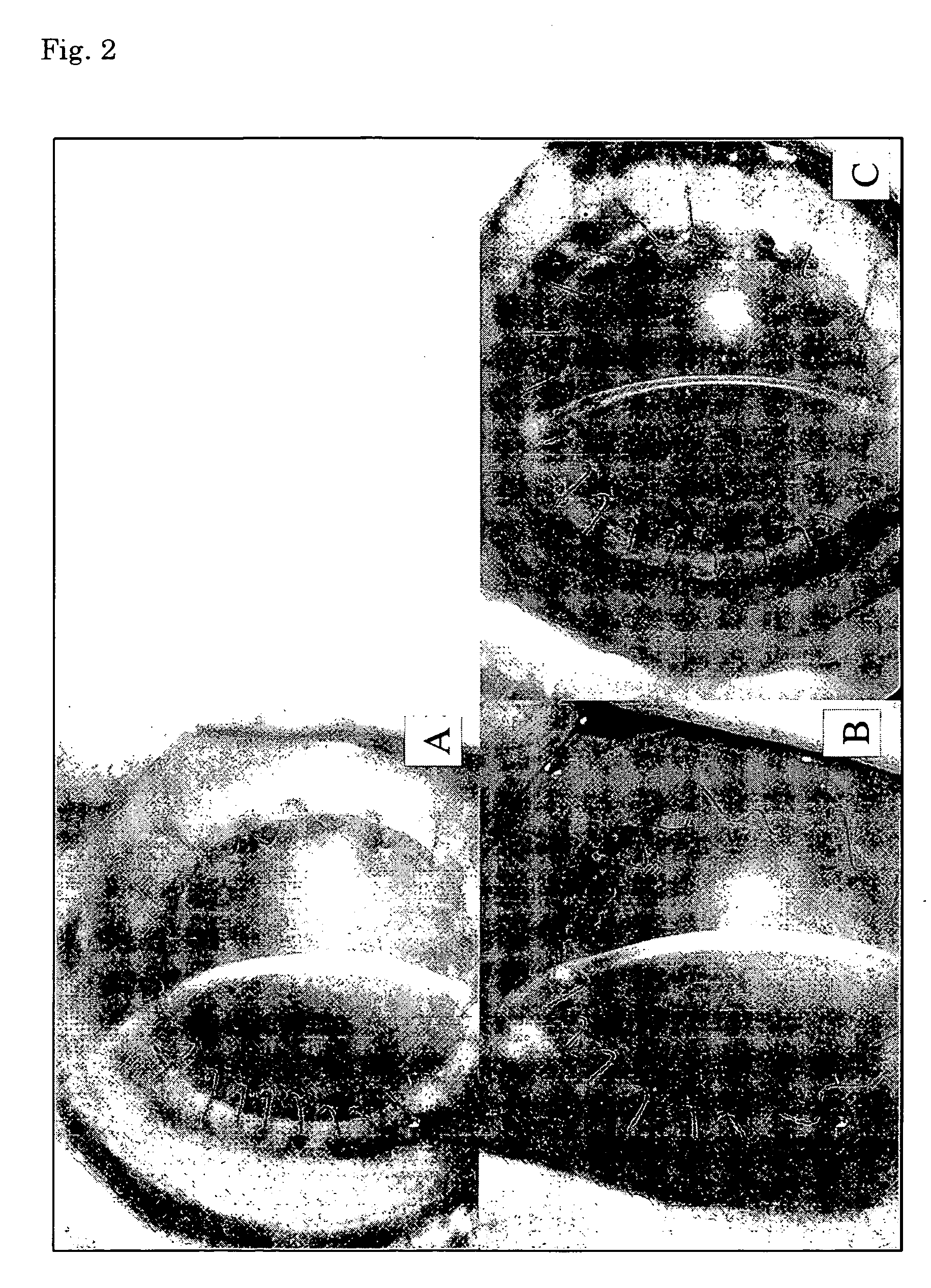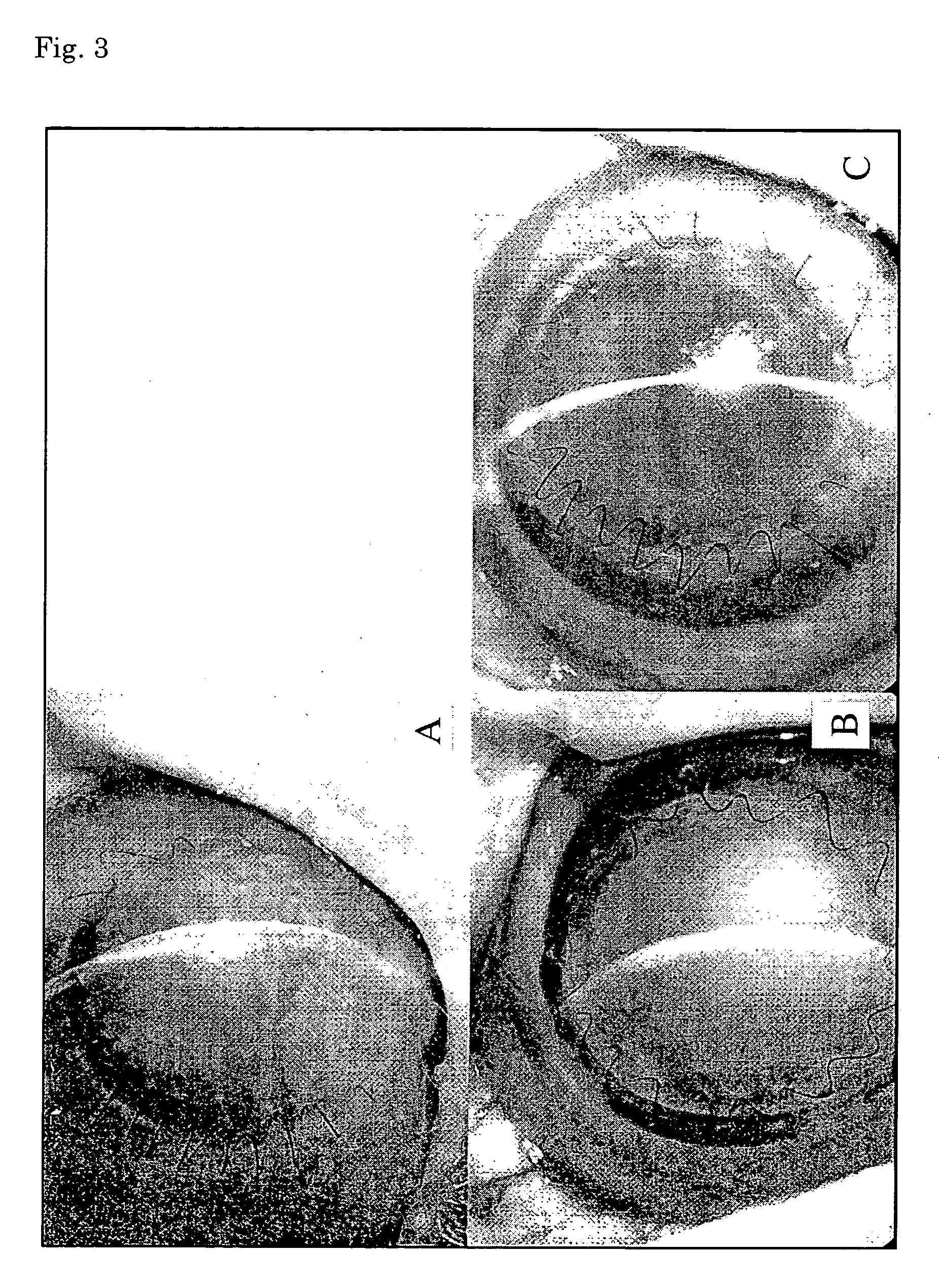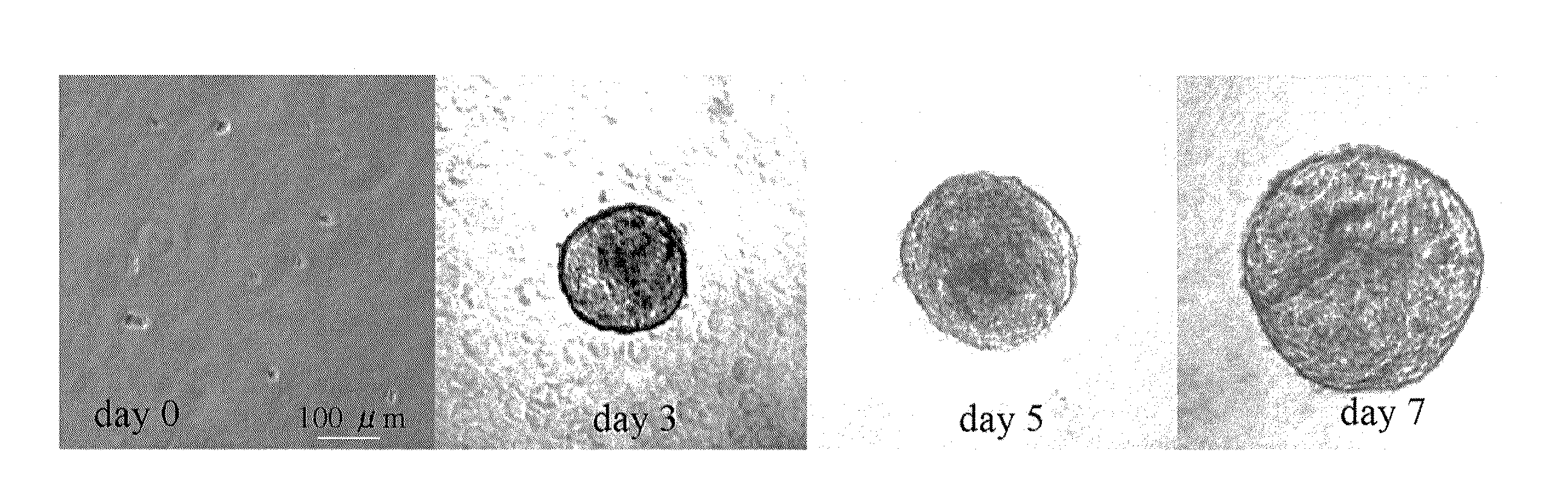Patents
Literature
177 results about "Corneal endothelial cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
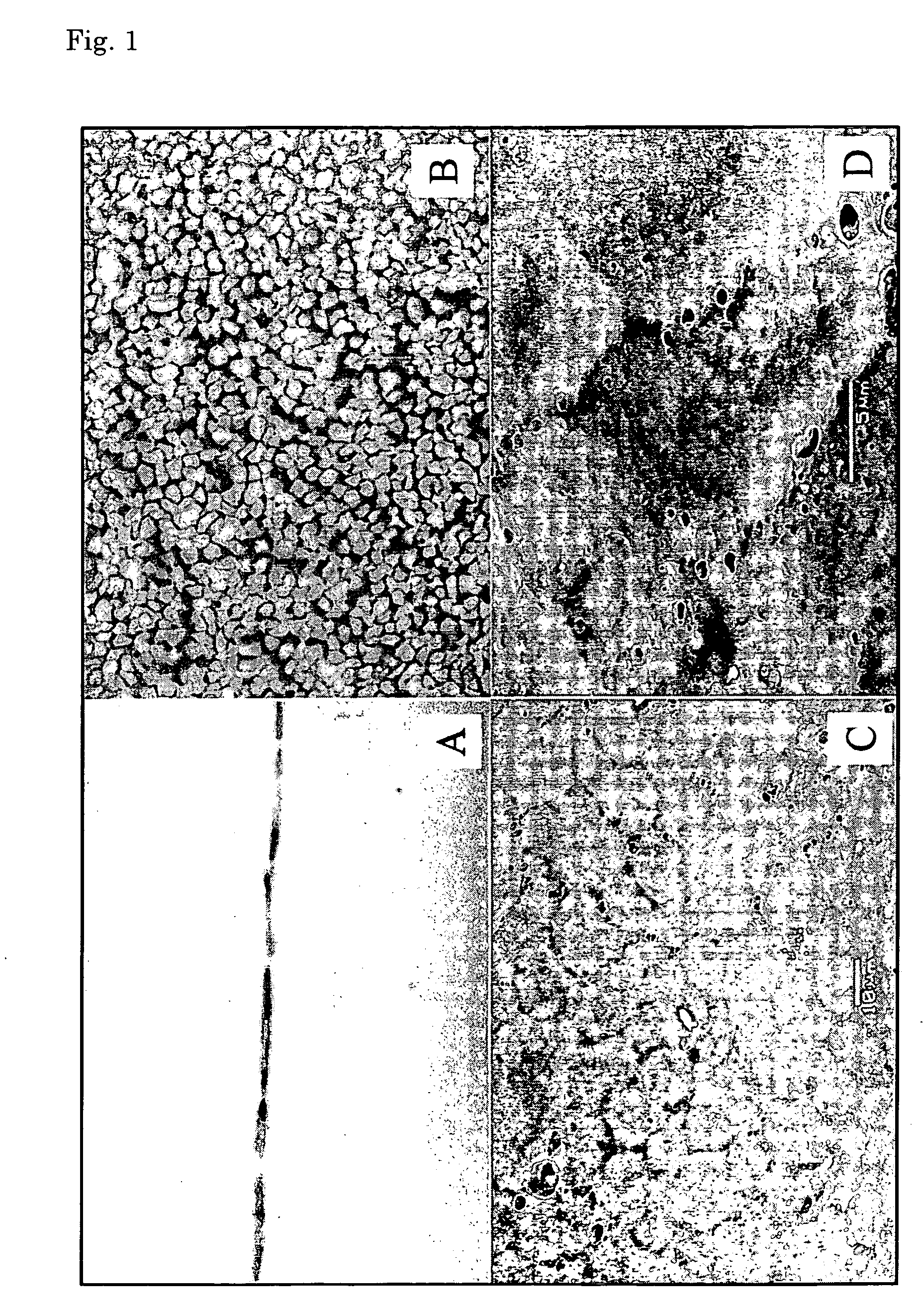
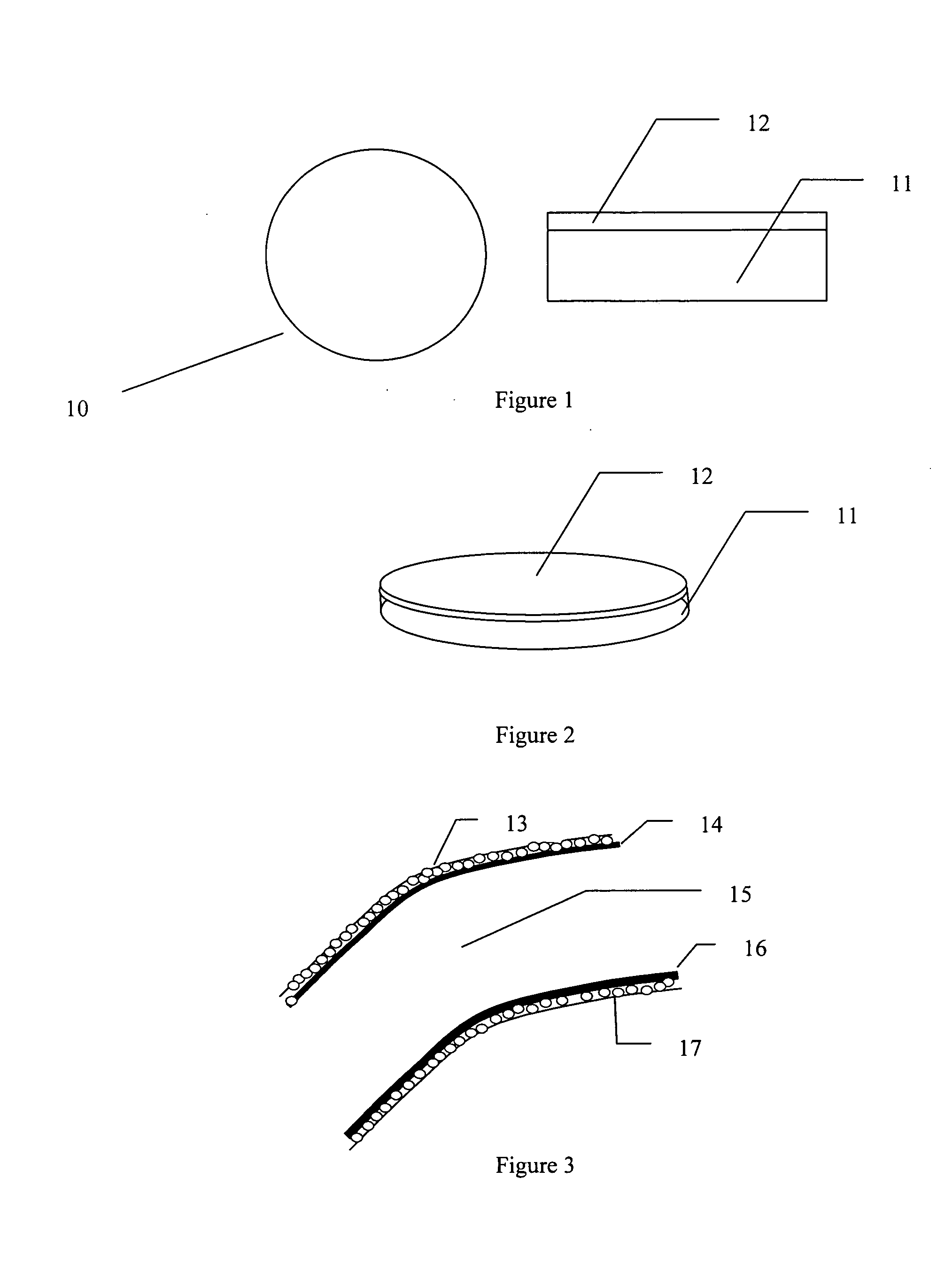
Corneal endothelium-like sheet and method of constructing the same
It is our intention to provide a transplantation material applicable to the treatment of various diseases that require corneal endothelium transplantation. Corneal endothelial cells are collected, proliferated and then sown on amnion and cultured. Thus, a corneal endothelium-like sheet, which has a cell layer made up of the corneal endothelium-origin cells formed on the amnion, can be obtained.
Owner:ARBLAST
Resorbable Cornea Button
InactiveUS20090222086A1Improve tolerancePromote cell growthBiocideSenses disorderCorneal endothelial cellBiodegradable polymer
The present invention provides a resorbable corneal button comprised of a biodegradable polymer which is capable of supporting the growth and expansion of endothelial cells on it surface for use in transplantation healthy corneal endothelial cells to cornea tissue in need of a transplant and a method of using same.
Owner:CELLULAR BIOENG
Regenerated corneal endothelial cell sheets, processes for producing the same, and methods of using the same
ActiveUS20070148137A1Prevent shrinkageImprove adhesionBiocideNervous system cellsCorneal endothelial cellBiology
An improved process for producing a regenerated corneal endothelial cell sheet, comprising the steps of allowing corneal endothelial cells collected from a tissue to be cultivated on a cell culture support having its surface covered with a polymer of which the hydrating force varies in a temperature range of 0-80° C., and after the culture, (1) adjusting the temperature of the culture solution to the temperature at which the polymer on the substrate surface is hydrated, (2) bringing the cultured corneal endothelial cell sheet into close contact with a carrier, and (3) detaching the sheet together with the carrier. The regenerated corneal endothelial cell sheet obtained by the process will adhere very well to living tissues.
Owner:CELLSEED +2
Therapeutic Agent for Corneal Diseases
InactiveUS20090163432A1Maintain transparencyLittle abilityOrganic active ingredientsSenses disorderCorneal diseasePharmacology
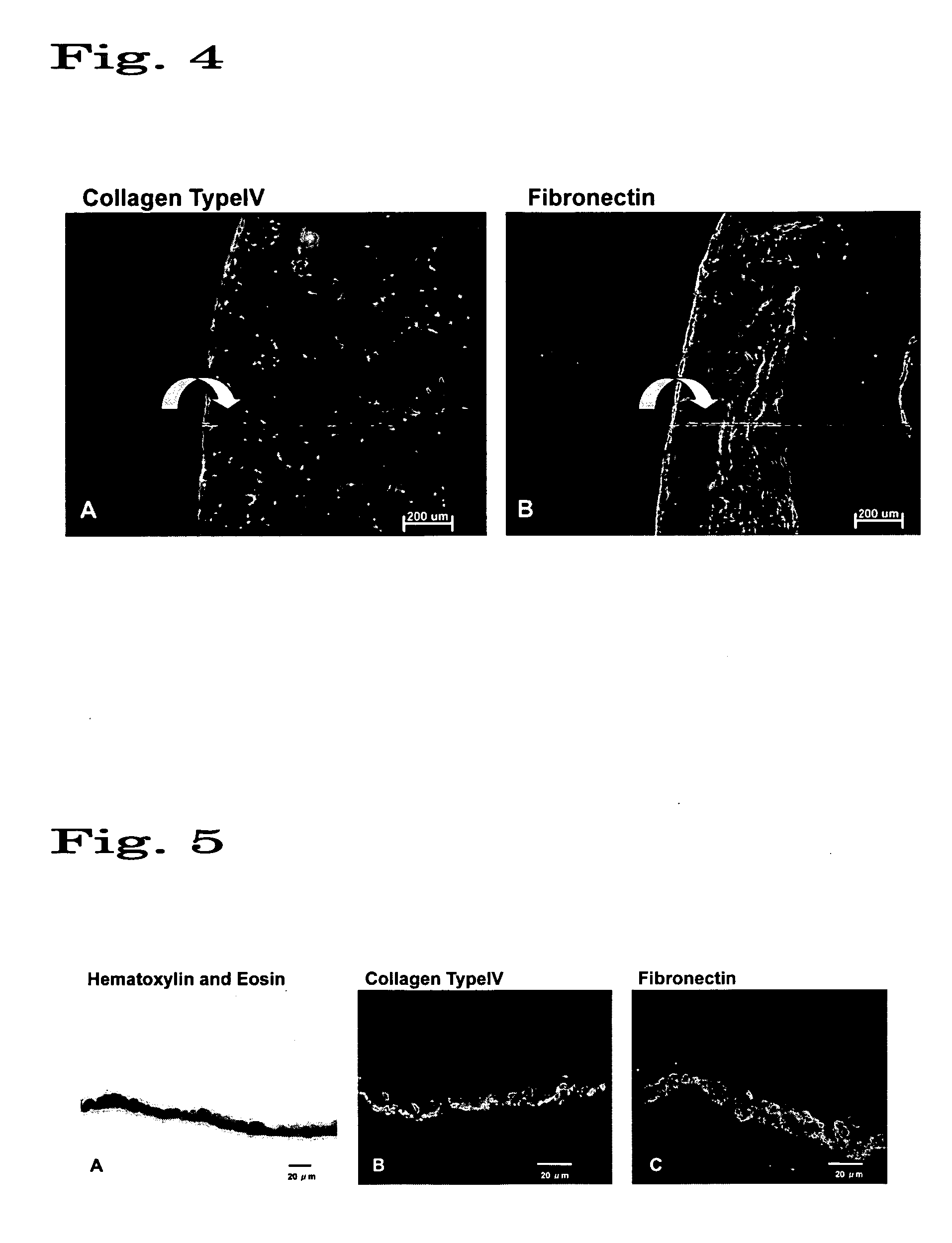
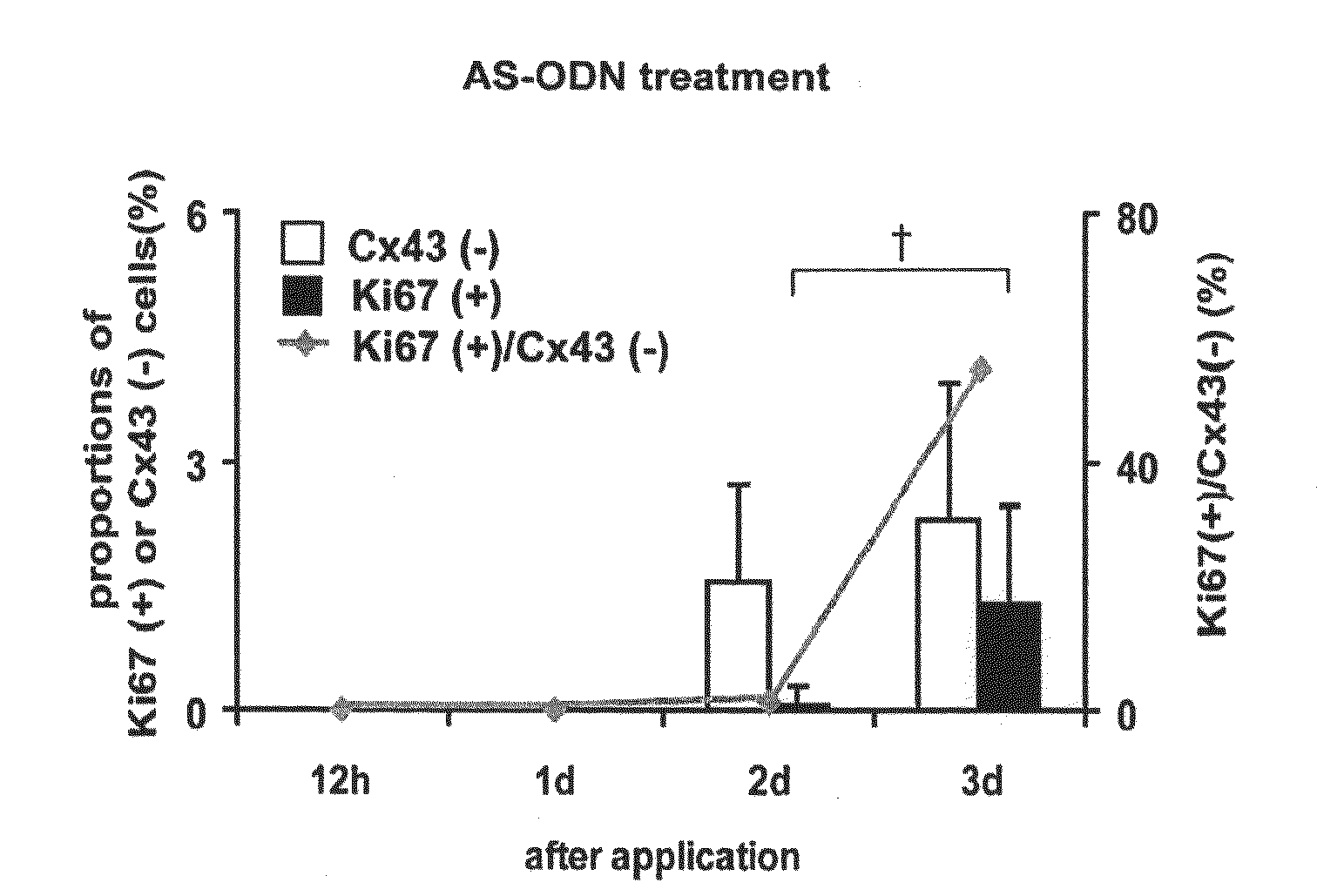
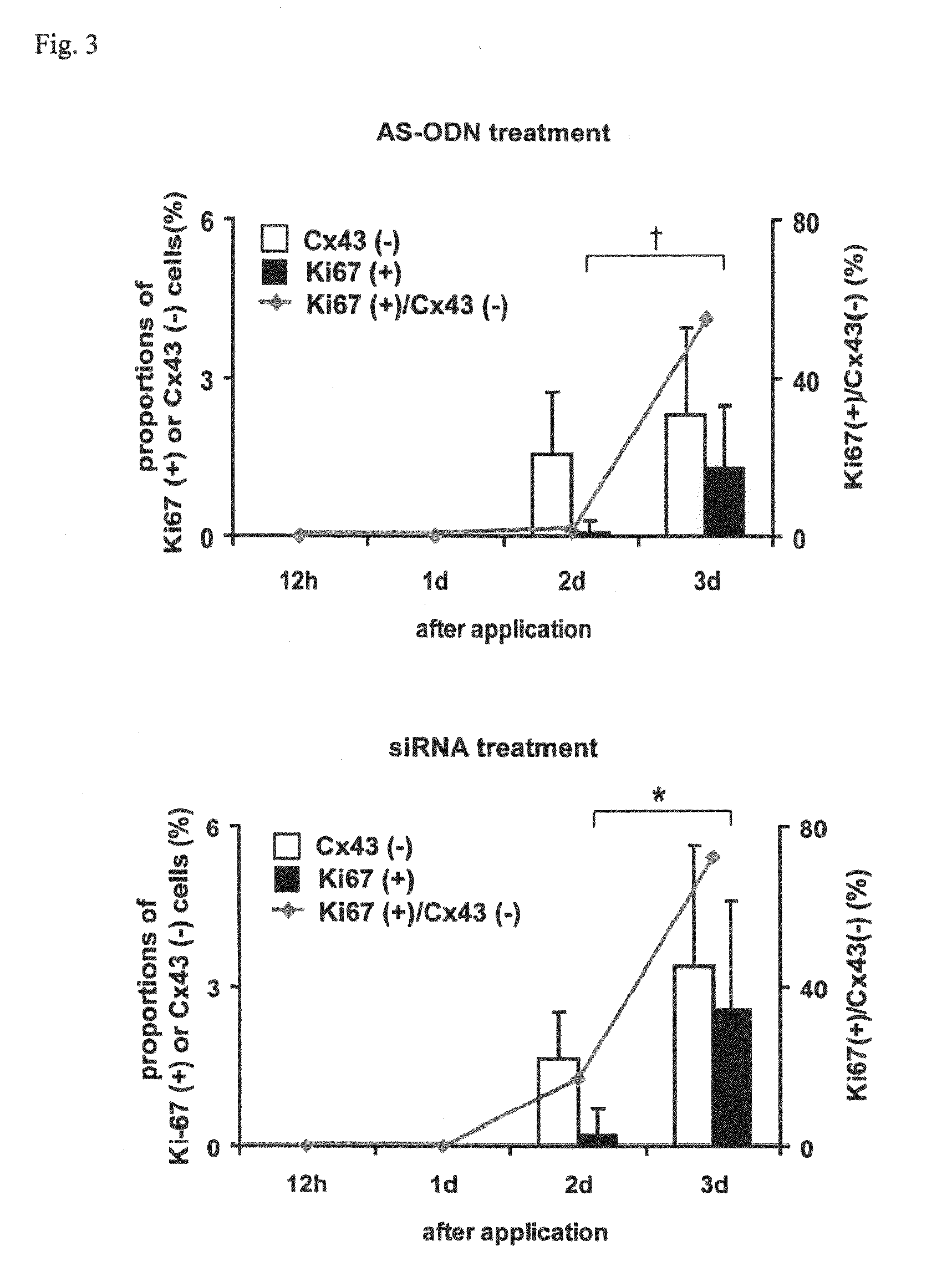
The present invention relates to a treatment agent for a disease or a disorder caused by a reduction in corneal endothelial cells, comprising as an active component at least one nucleic acid molecule inhibiting the expression of a connexin 43 gene.
Owner:KANSAI TLO KK
Inducing method for directionally differentiating human embryonic stem cells to corneal endothelial cells
The invention discloses an inducing method for directionally differentiating human embryonic stem cells to corneal endothelial cells. The method comprises the steps of: cultivating the human embryonic stem cells on a mouse embryonic fibroblast feed layer; sorting human embryonic stem cell clone groups in good state; grafting the groups on a human corneal stromal fibroblast layer processed by mitomycin C and cultivating for 7 days, wherein the human embryonic stem cells are differentiated to rosettes; separating and transferring the rosettes from the human corneal stromal fibroblast layer to a culture bottle; cultivating continuously for 7 days by using a neural crest stem cell culture medium; sorting the neural crest stem cells by a flow cytometry; adding the neural crest stem cells into the culture bottle; placing in a 5% CO2 incubator for incubating and cultivating at 37 DEG C by using a human corneal endothelial cell culture medium; changing the liquid every other day; and cultivating for about 10 days to obtain the corneal endothelial cells. The multiplication capacity of the corneal endothelial cells are similar to that of human corneal endothelial cells and the corneal endothelial cells can be transferred to 1-2 generations in vitro maximally. The corneal endothelial cells can be used as seed cells for cornea construction and transplant in tissue engineering.
Owner:SHANDONG UNIV
Laminate of Cultured Human Corneal Endothelial Cells Layer and Method for Manufacturing Same
InactiveUS20070238173A1Avoiding immunologic rejectionMaintain transparencyBiocideEye implantsCorneal endothelial cellCollagen i
A laminate comprising a transparent collagen I type sheet and a human corneal endothelial cell culture layer provided on the sheet. An endothelial cell culture layer laminate usable in transplantation is provided.
Owner:YAMAGAMI +1
Human corneal endothelial cell-derived precursor cells, cellular aggregates, methods for manufacturing the same, and methods for transplanting precursor cells and cellular aggregates
InactiveUS20090232772A1Easy to stickImprove abilitiesBiocideSenses disorderDescemets MembraneCorneal endothelial cell
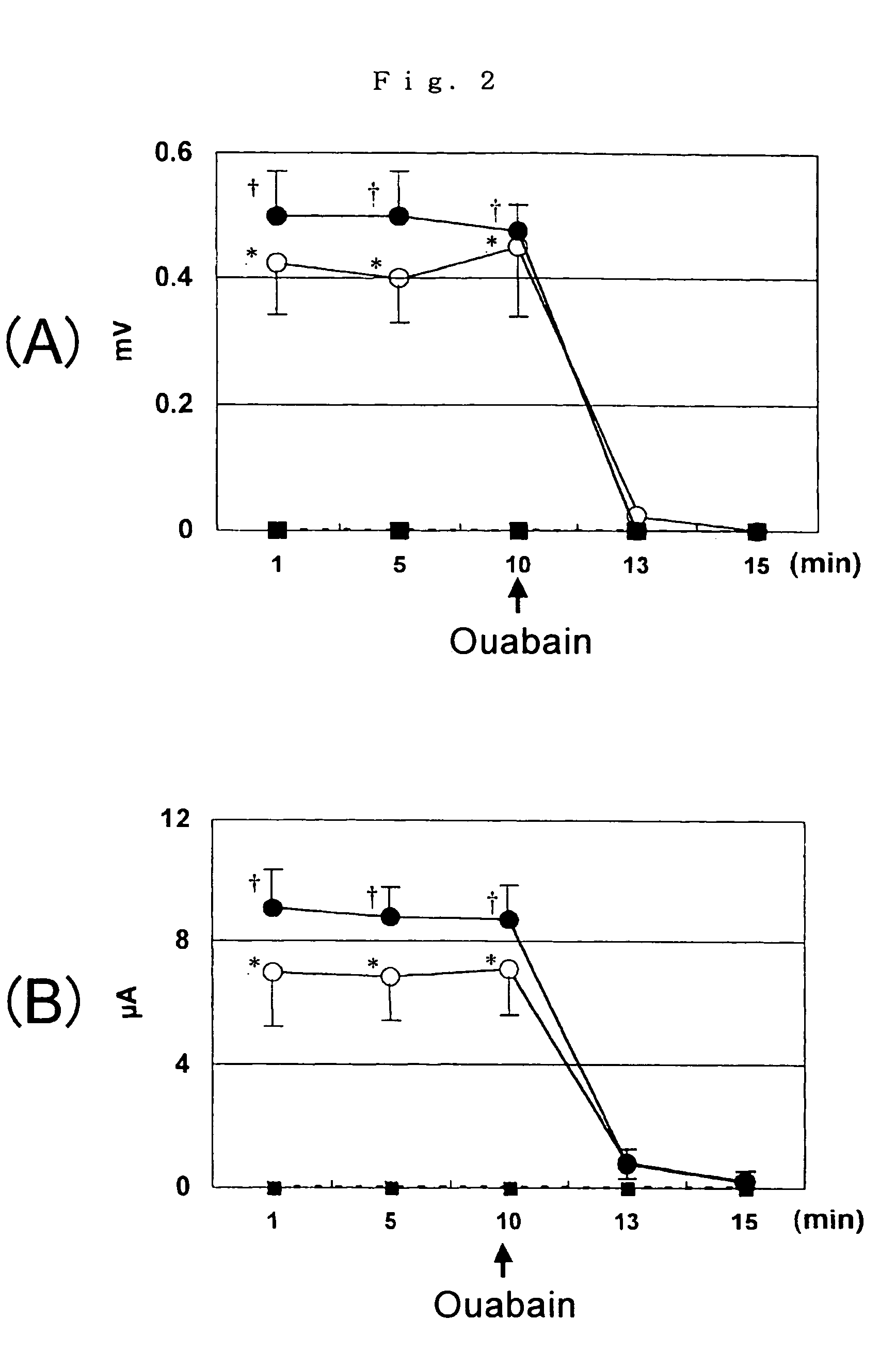
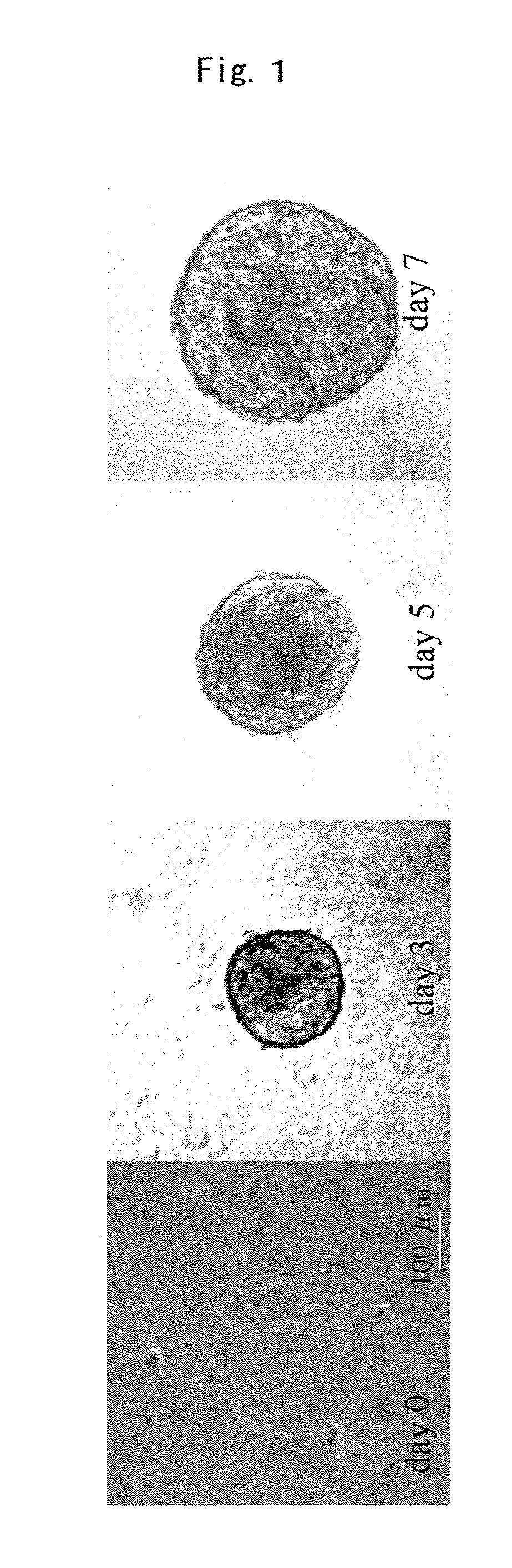
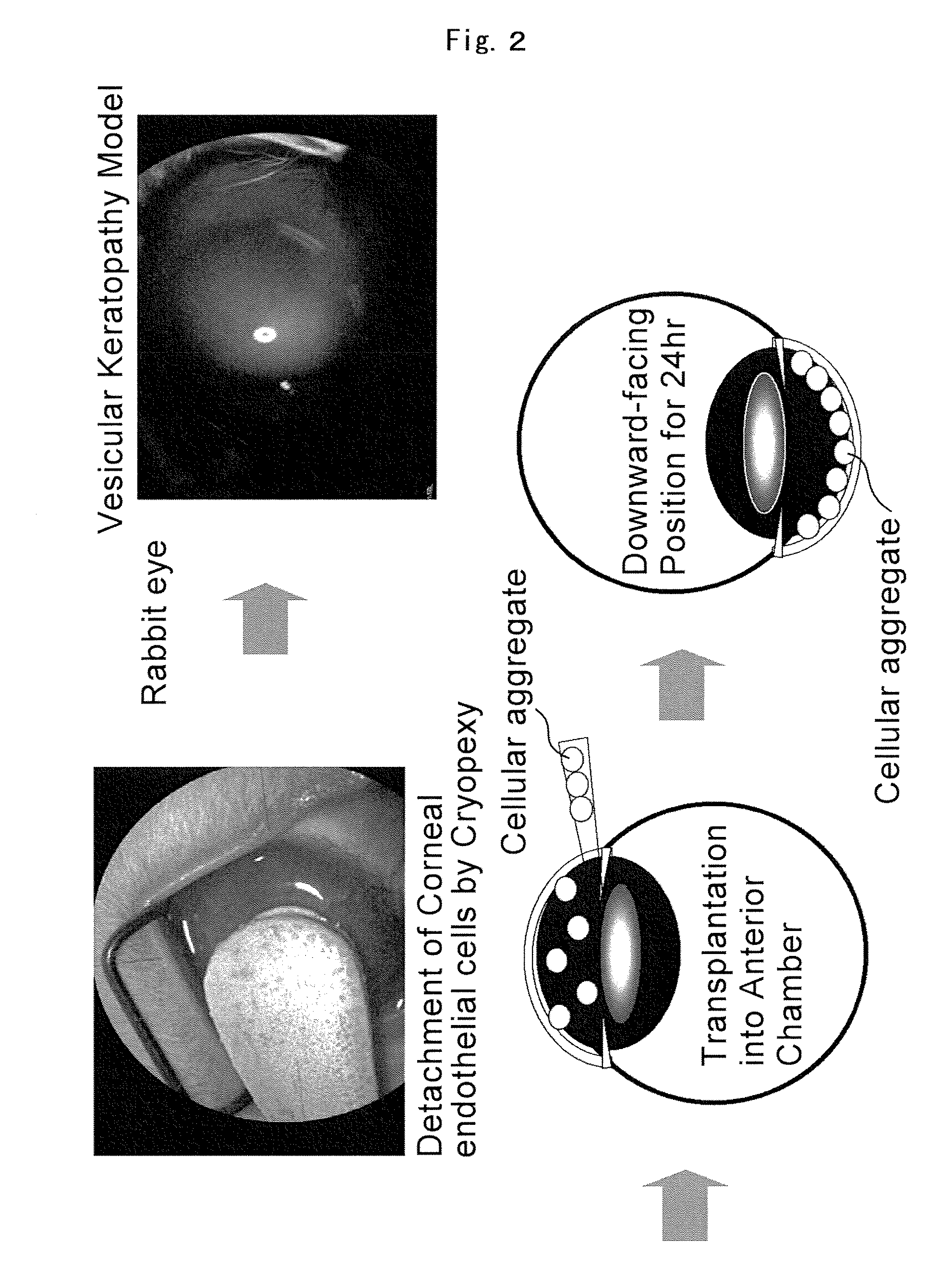
Providing is cellular aggregates derived from corneal endothelial cells that, when transplanted, readily adhere to the parenchyma of cornea and function in a manner equivalent to corneal endothelial cells, and a method of transplantation of the cellular aggregates. Cellular aggregates derived from corneal endothelial cells. The cellular aggregates derived from corneal endothelial cells is prepared by culturing human corneal endothelial cells in a medium containing fetal bovine serum, growth factor and glucose; and then float culturing the cells obtained in a medium containing growth factor. A method of transplantation into the anterior chamber the cellular aggregate or the cellular aggregate prepared by the above method, comprising inserting a tube into the parenchyma of cornea, introducing the cellular aggregate into the anterior chamber through the inserted tube, and causing the cellular aggregate that has been introduced to adhere to Descemet's membrane by assuming in a downward-facing position.
Owner:AMANO SHIRO +2
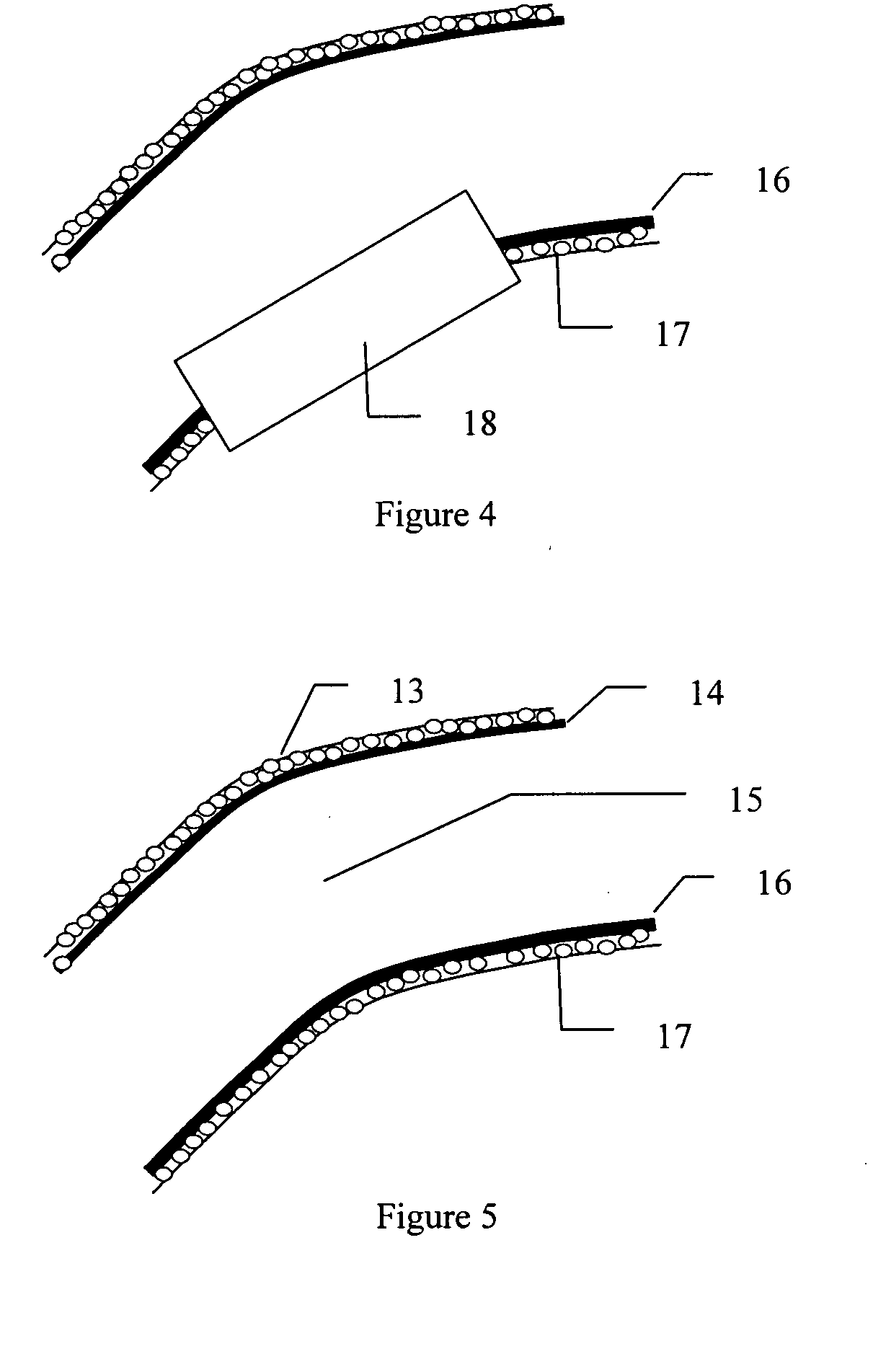
Methods and compositions for growing corneal endothelial and related cells on biopolymers and creation of artifical corneal transplants
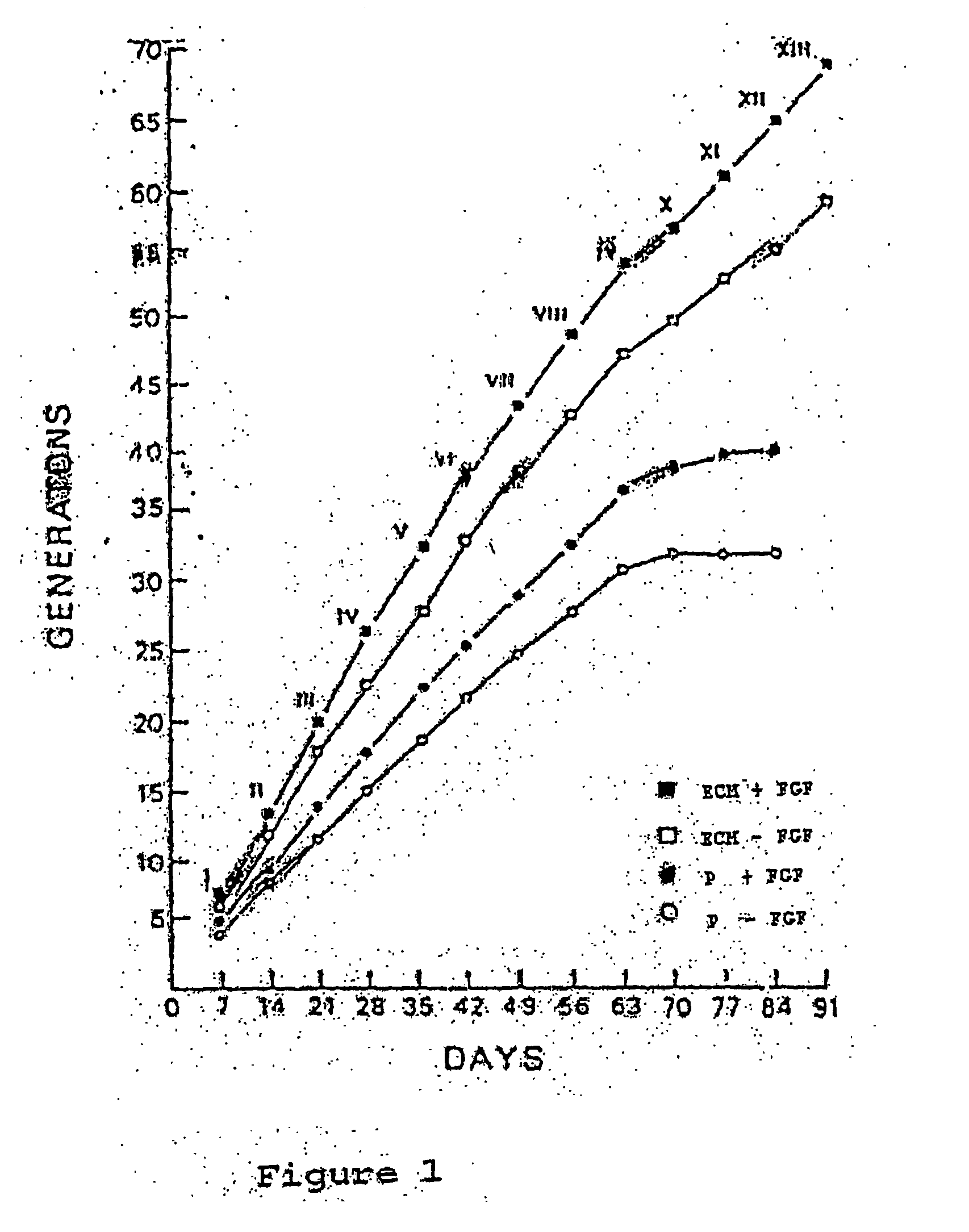
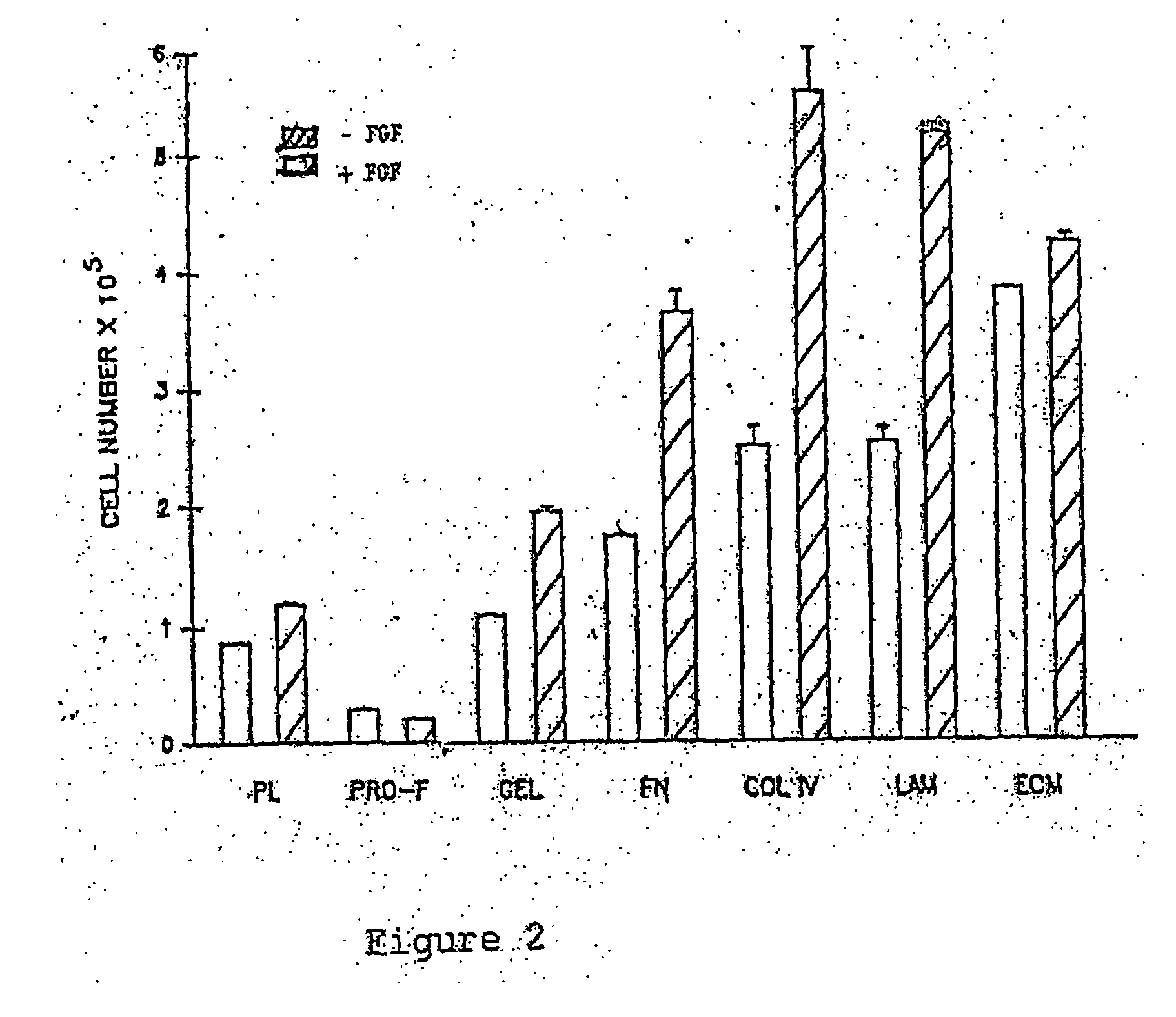
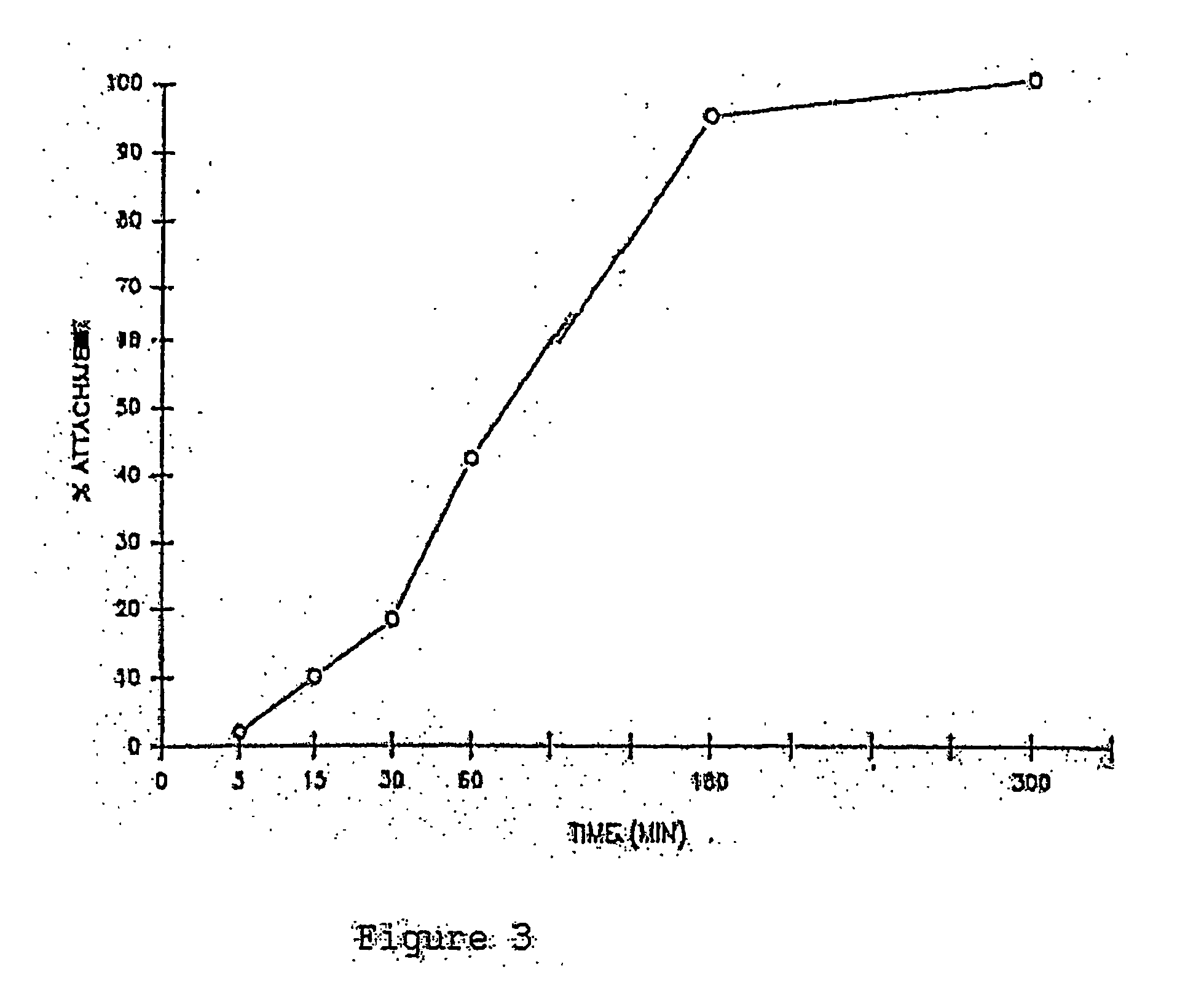
This invention discloses methods to attach and grow a monolayer of cultured human corneal endothelial cells onto the endothelial side of the stroma synthesized from biopolymer to generate a more bio-equivalent artificial cornea. The approaches will include the use of attachment and growth promoting agents such as fibronectin, laminin, RGDS, collagen type IV, bFGF conjugated with polycarbophil, and EGF conjugated with polycarbophil. The patent also describes a method to create a self-sustaining polymer containing adhesive molecules and growth factors to support the attachment and proliferation of cultured human corneal endothelial cells for corneal transplantation either as a half-thickness device or full-thickness button replacement. An approach for the implantation of cultured retinal pigment epithelial (RPE) cells into the sub-retinal space for treatment of age-related macular degeneration (ARMD) is disclosed in this invention. This method will enable the delivery of the transplanted RPE in a sheet of monolayer cells and will be better suited to perform their physiological function.
Owner:CELLULAR BIOENG
Fabrication of gelatin hydrogel sheet for the transplantation of corneal endothelium
The invention provides a corneal endothelial composition comprising a transparent hydrogel scaffold and a single layer of cultured corneal endothelial cells on the surface of the scaffold. The hydrogel scaffold I comprised of at least one biopolymer, preferably gelatin. Also provided are methods of making a corneal endothelial scaffold
Owner:WAKE FOREST UNIV HEALTH SCI INC
Biopolymer-bioengineered cell sheet construct
The present invention discloses a biopolymer-bioengineered human corneal endothelial cell (HCEC) sheet construct for reconstructing corneal endothelium in a patient. The construct includes a biopolymer carrier which is bioresorable and deformable; and a bioengineered cell sheet comprising a monolayer of interconnected HCECs with substantially uniform orientation, wherein the bioengineered cell sheet is attached to a surface of the carrier with apical surfaces of the HCECs facing said carrier.
Owner:NATIONAL TSING HUA UNIVERSITY
Method for producing corneal endothelial cell
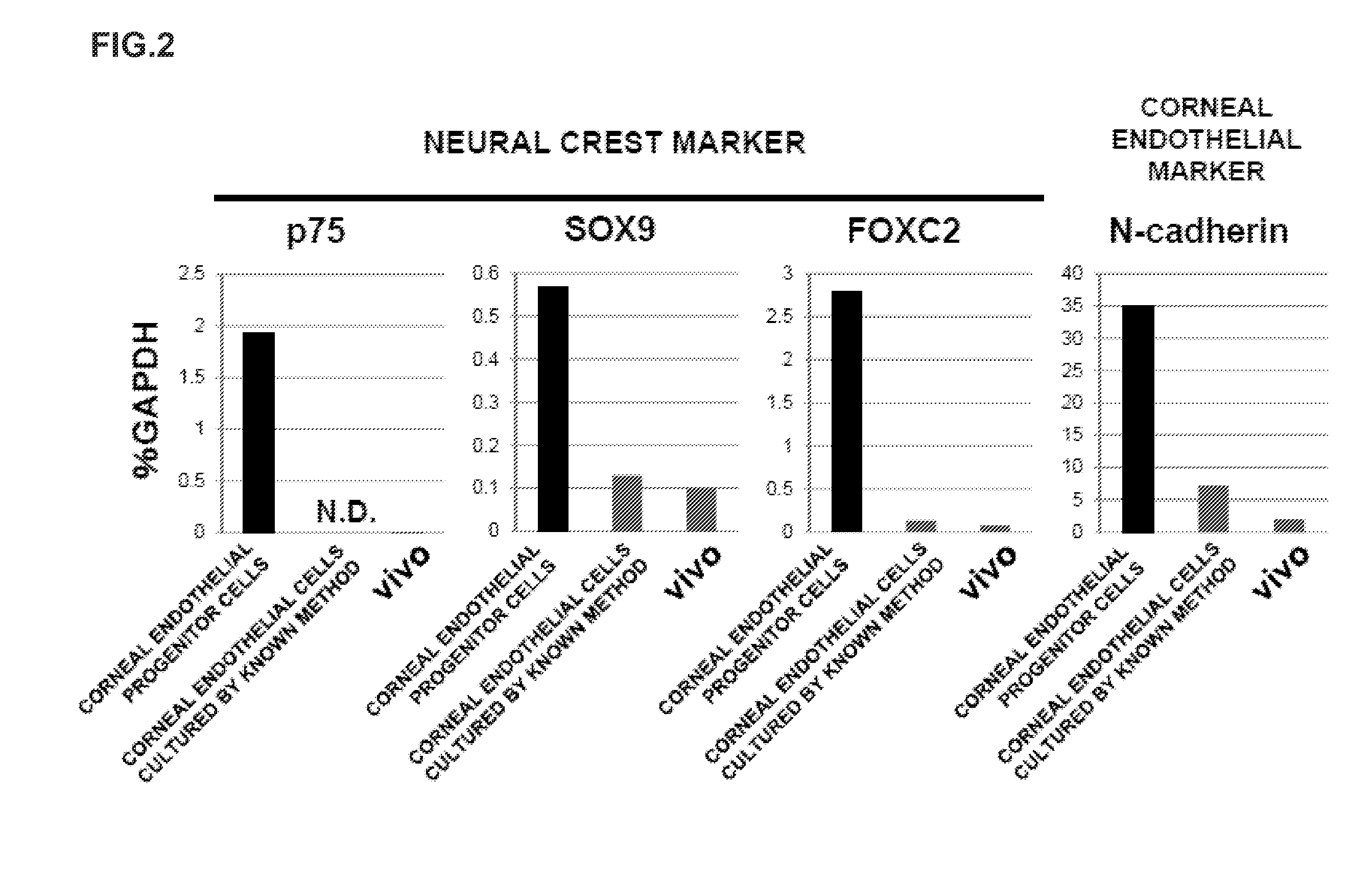
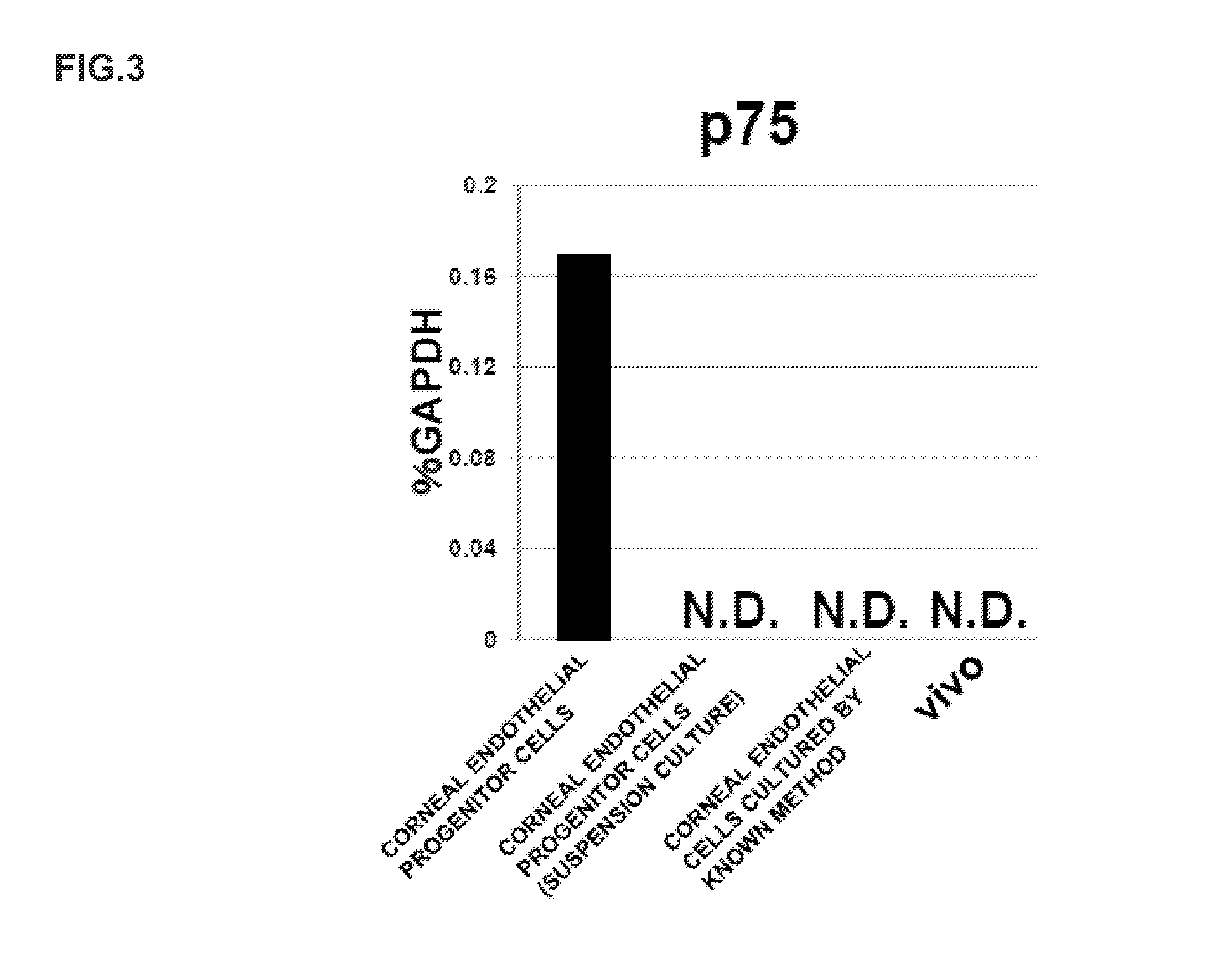
ActiveUS9347042B2Efficient productionSenses disorderEpidermal cells/skin cellsCorneal endothelial cellNeural crest
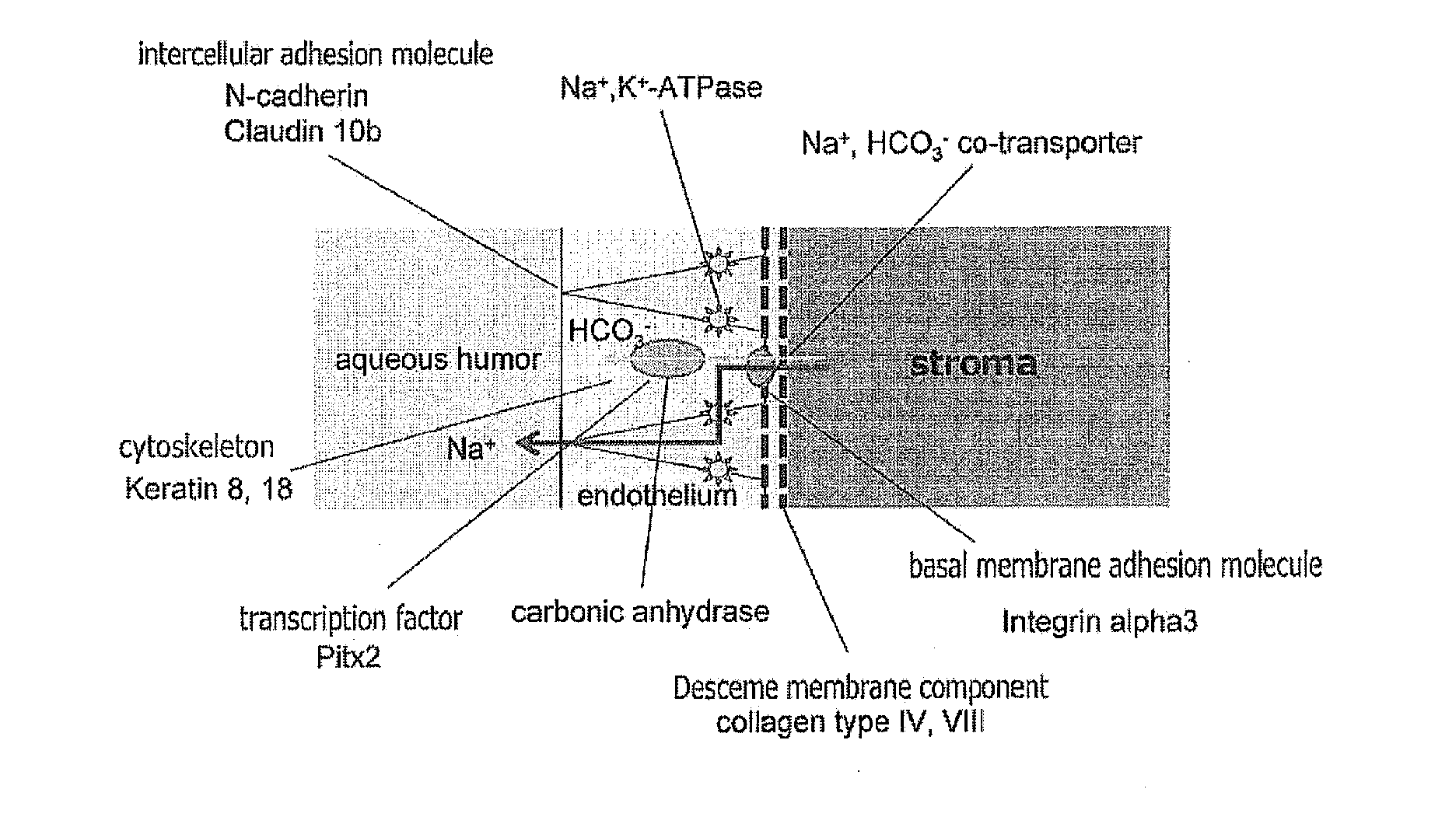
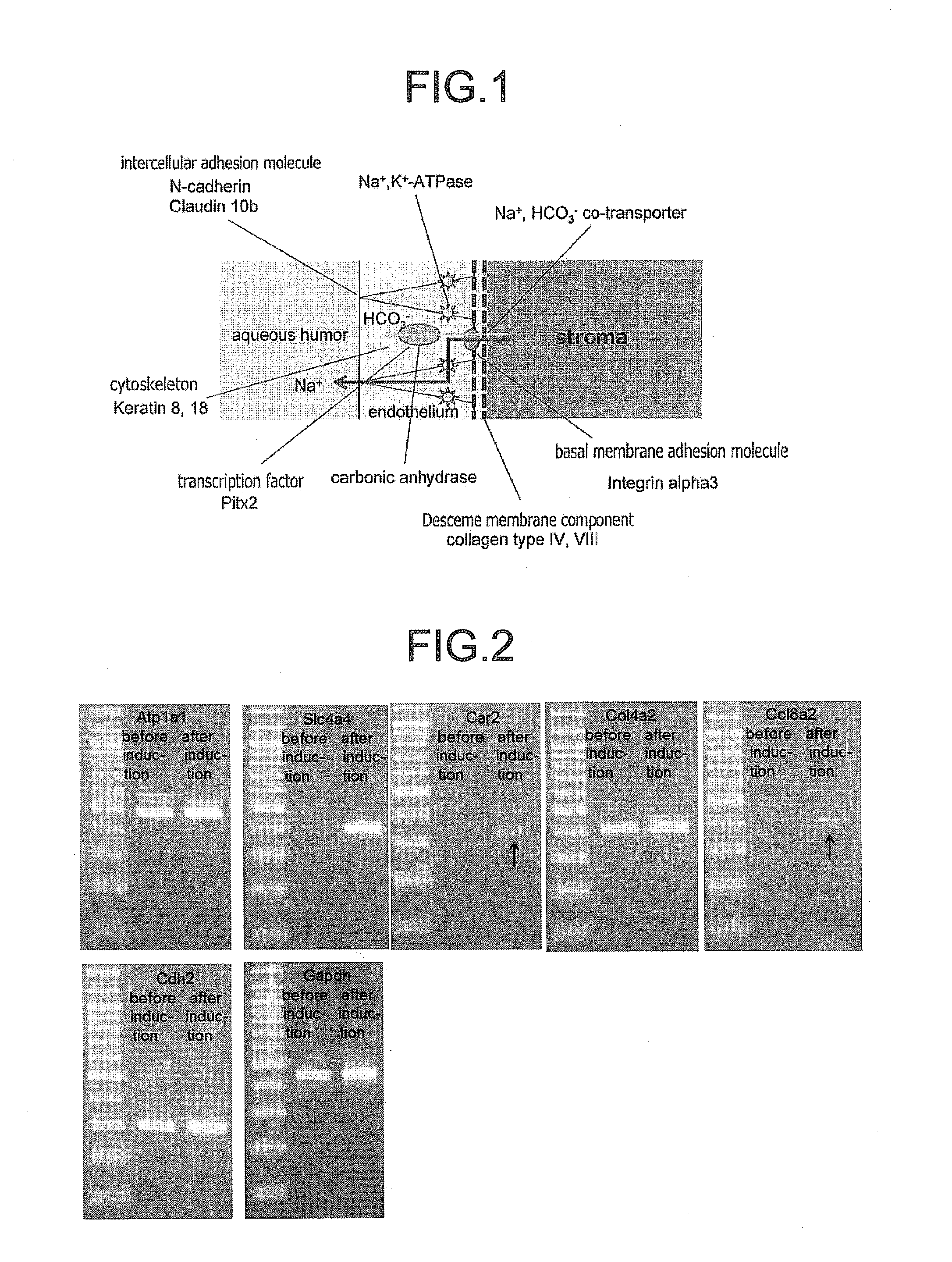
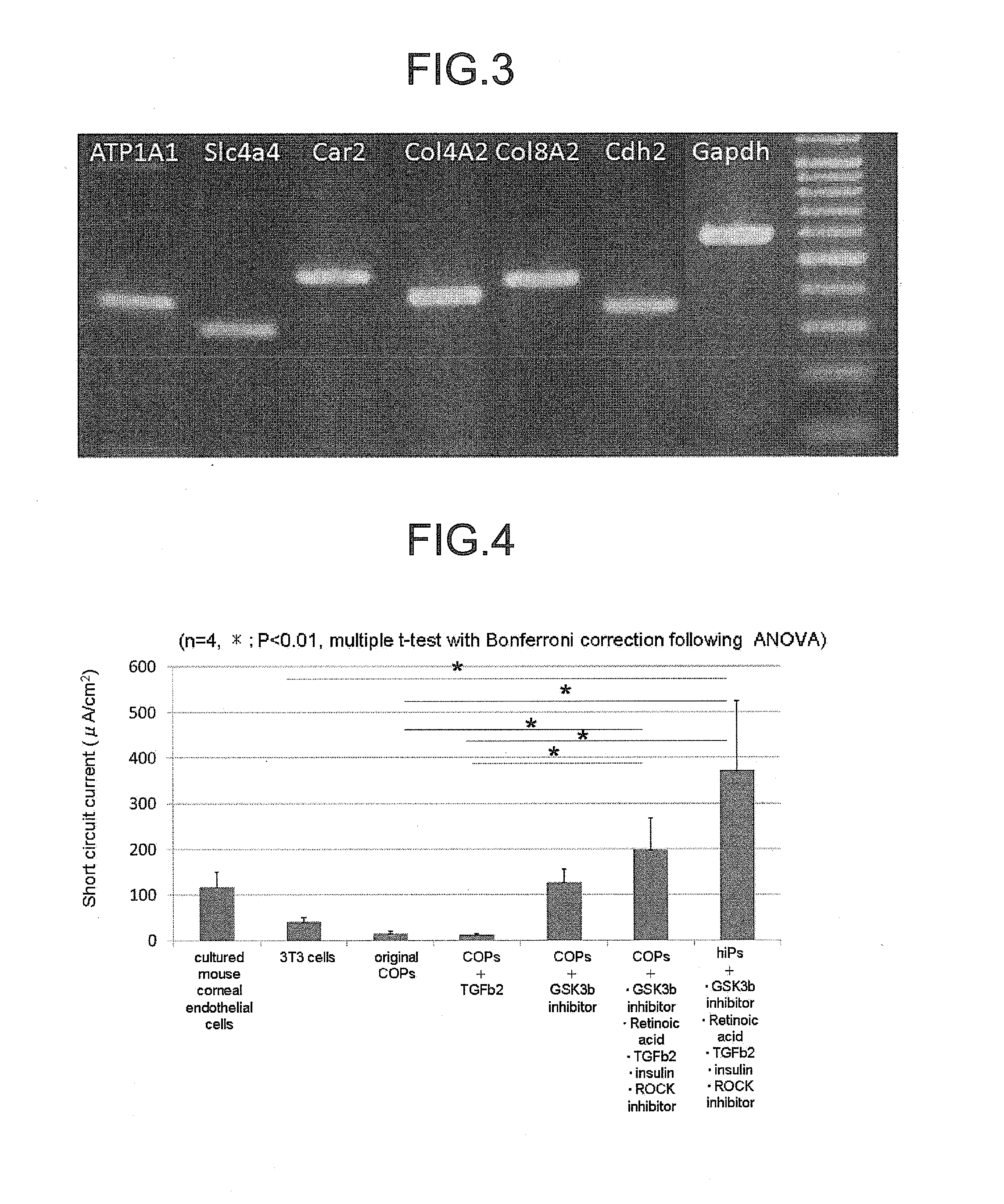
The invention provides a method of efficiently producing corneal endothelial cells, particularly from corneal stroma or iPS cell-derived neural crest stem cells, a method of producing corneal endothelial cells stably in a large amount by inducing more efficient differentiation of stem cells into corneal endothelial cells, and a medicament containing corneal endothelial cells. The method of inducing differentiation of stem cells into corneal endothelial cells includes a step of culturing the stem cells in a differentiation induction medium containing a GSK3 inhibitor (preferably a GSK3β inhibitor) and retinoic acid, with the differentiation induction medium preferably further containing one or more of TGFb2, insulin, a ROCK inhibitor, and the like.
Owner:KEIO UNIV
Preparation method and application thereof of acellular conjunctiva matrix
InactiveCN101590292AEasy to prepareGood biocompatibilityEye implantsDead animal preservationTreatment effectBiocompatibility Testing
The invention relates to a preparation method and application thereof of an acellular conjunctiva matrix used as a tissue engineering corneal scaffold material. The method comprises the following steps: removing cell components in a bulbar conjunctiva first; preparing the acellular conjunctiva matrix; and taking the acellular conjunctiva matrix as a tissue engineering corneal scaffold. A rabbit corneal epithelium or an endothelial cell can form a good cell single layer on the scaffold, can construct a tissue engineering corneal epithelium or endothelium, and can successfully transplant the tissue engineering corneal epithelium or endothelium onto rabbit animal model eyes. The degradation time of the built tissue engineering cornea is longer, rabbit eyes have no obvious immune reject reaction, and the therapeutic effects on the lack of corneal limbus stem cells or the decompensation of the corneal endothelial cell function are obvious. The method and the application thereof have the advantages of simple preparation method, good biocompatibility, low antigenicity, easy growth and proliferation of seed cells, slow degradation, extensive bulbar conjunctiva sources and wide application and development prospects; and the transparency can be maintained all the time in the training process and after transplantation.
Owner:SHANDONG EYE INST
Method for preparing corneal endothelial cell
ActiveUS20140170751A1Improve proliferative abilityIncrease the number ofNervous system cellsUnknown materialsDiseaseProgenitor
The present invention relates to a method for preparing corneal endothelial progenitor cells by adherent culturing a cell population isolated from corneal endothelial cell tissue at a low density by using a serum-free medium. The present invention also relates to a method for preparing corneal endothelial cells by differentiation-inducing the corneal endothelial progenitor cells obtained by the aforementioned method. According to the present invention, corneal endothelial progenitor cells can be selectively grown from a corneal tissue-derived cell population, and corneal endothelial cells obtained by inducing the corneal endothelial progenitor cells can be applied to treatment of corneal endothelial diseases. As a result, problems of corneal transplantation such as shortage of donors and occurrence of rejection can be solved.
Owner:OSAKA UNIV
Preparation method for artificial corneas
The invention discloses a preparation method for artificial corneas. The preparation method comprises the following steps of (1) collecting age-appropriate pig eyeballs; (2) scraping corneal epithelial cells and corneal endothelial cells of the pig eyeballs, reserving bowman layers, preparing lamellar corneas by using a lamellar blade, and drilling size-specific corneal slices; (3) putting the corneal slices obtained in step (2) in liquid nitrogen for 8-35 minutes, slowly rewarming the corneal slices to room temperature under normal temperature and normal pressure after the corneal slices are taken out, repeating the operation for 4-12 times; (4) putting the corneal slices which are subjected to freezing-thawing treatment in step (3) in normal saline for carrying out ultrasonic cleaning, wherein the ultrasonic working frequency is 20-250 kHz, and the ultrasonic time is 15-65 minutes; (5) putting the corneal slices which are subjected to ultrasonic treatment in a buffer system, carrying out enzymolysis on DNA (Deoxyribose Nucleic Acid) of corneal stromal cells by using endonuclease, and obtaining the artificial corneas. According to the artificial corneas prepared by the preparation method disclosed by the invention, gaps between natural collagen fibers of the artificial corneas are beneficial for inducing the growth of the corneal cells and the growth of nerve fibers of an acceptor and promoting the repairing and the reconstructing of corneas of a donor.
Owner:XIAMEN DAKAI BIOTECH CO LTD
A directional differentiation induction method of human embryonic stem cells to obtain corneal endothelial cells
ActiveCN106167790AConsistent proliferative abilityCulture processNervous system cellsCorneal endothelial cellNeural crest
A directional differentiation induction method of human embryonic stem cells to obtain corneal endothelial cells is provided. The method includes inducing the human embryonic stem cells to differentiate into human neural crest stem cells, inducing the human neural crest stem cells to differentiate into the human corneal endothelial cells, and other steps. The method adopts a primary human corneal stroma cell culture supernatant fluid, a human corneal endothelial cell culture supernatant fluid and a human lens cell culture supernatant fluid as a conditioned medium. Through adding retinoic acid and a plurality of recombinant proteins into the medium, and combining three-dimensional and two-dimensional culture methods, a corneal endothelial cell development process is simulated to induce the human embryonic stem cells to directionally differentiate into the human corneal endothelial cells, the corneal endothelial cells morphological structures of which are similar to those of normal human corneal endothelial cells can be obtained, and in-vitro subculture results show that proliferation of the obtained human corneal endothelial cells is consistent with proliferation of the normal human corneal endothelial cells.
Owner:GENERAL HOSPITAL OF PLA
RNAi Methods and Compositions for Stimulating Proliferation of Cells with Adherent Functions
ActiveUS20100003299A1Promotes HCEC adhesionReduce thicknessBiocideOrganic active ingredientsSurgical GraftIn vivo
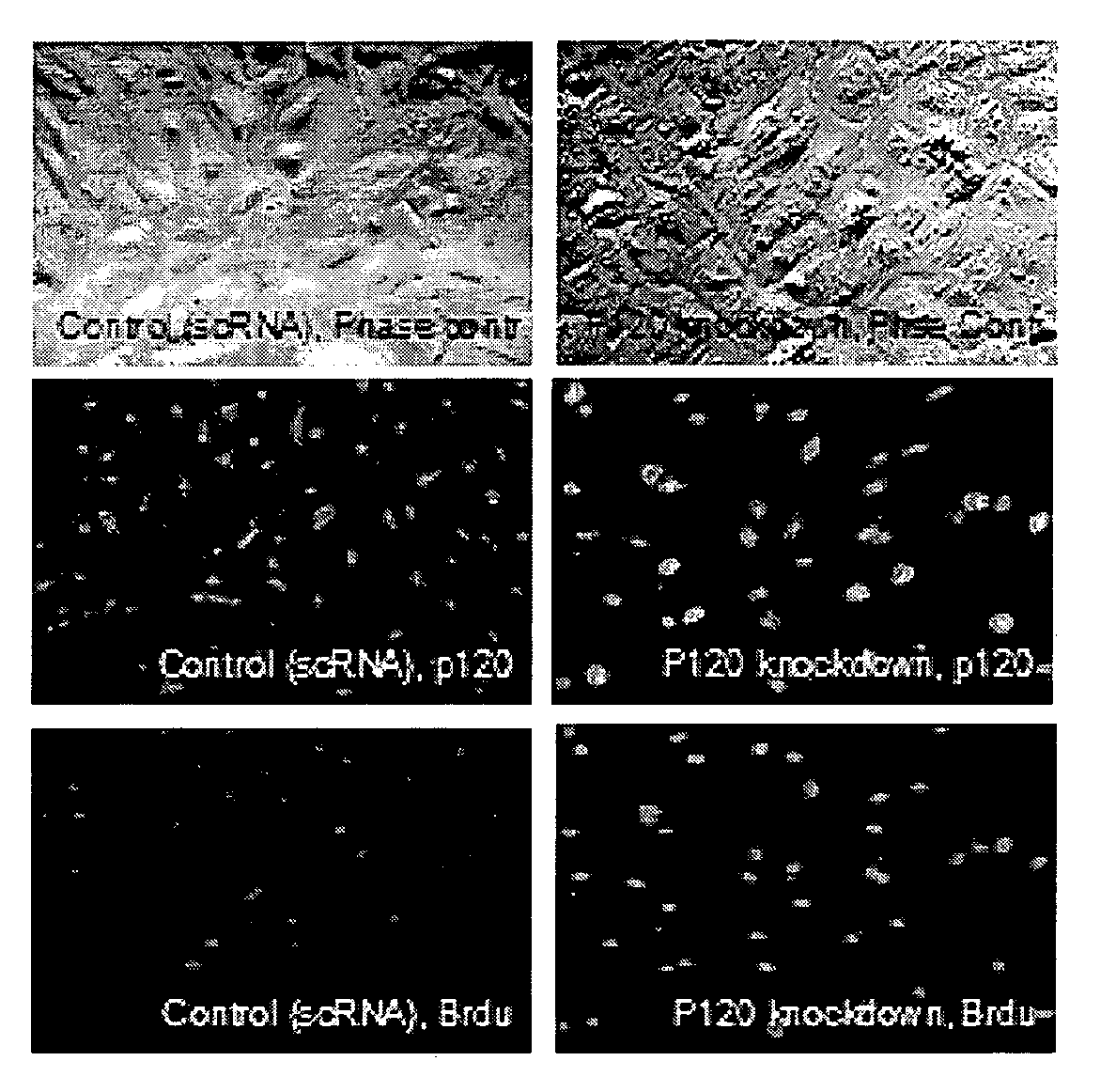
Described herein are methods and compositions for stimulating proliferation of cells that express adherent junctions and cease proliferation, for example, human corneal endothelial cells, by downregulation of certain cell-cell junctions. In one embodiment, downregulation is achieved using RNA interference, and contacting the cells with mitogenic growth factors and an agent that elevates intracytoplasmic cAMP. Furthermore, described herein are methods of isolating human corneal endothelial cells from keratocytes, and methods of preserving and maintaining viability of human corneal endothelial cell aggregates. Also described are surgical grafts comprising human corneal endothelial cells that have been isolated, optionally stored, and transiently contacted with an agent that downregulates expression of p 120, and a biocompatible support. The methods and compositions described herein can be used in novel therapies to help expand human corneal endothelial cells during in vitro tissue engineering and for in vivo treatment of corneal endothelial dysfunction.
Owner:TISSUETECH INC
Method for expansion of human corneal endothelial cells
A method for expanding human corneal endothelial cells includes: (a) providing an amniotic membrane with or without amniotic cells, wherein the amniotic membrane has an extracellular matrix; (b) placing onto the amniotic membrane, a sheet of endothelial layer, or a cell suspension including human corneal endothelial stem cells; and (c) culturing the corneal endothelial cells on the amniotic membrane for a duration sufficient for the corneal endothelial stem cells to expand to an appropriate area. The invention also relates to a method for creating a surgical graft for a recipient site of a patient using the method for expanding human corneal endothelial cells, and the surgical graft prepared therefrom.
Owner:TSAI RAY JUI FANG +1
Method for producing corneal endothelial cell
ActiveUS20140315305A1Efficient productionProduce efficientlySenses disorderEpidermal cells/skin cellsCorneal endothelial cellNeural crest
The invention provides a method of efficiently producing corneal endothelial cells, particularly from corneal stroma or iPS cell-derived neural crest stem cells, a method of producing corneal endothelial cells stably in a large amount by inducing more efficient differentiation of stem cells into corneal endothelial cells, and a medicament containing corneal endothelial cells. The method of inducing differentiation of stem cells into corneal endothelial cells includes a step of culturing the stem cells in a differentiation induction medium containing a GSK3 inhibitor (preferably a GSK3β inhibitor) and retinoic acid, with the differentiation induction medium preferably further containing one or more of TGFb2, insulin, a ROCK inhibitor, and the like.
Owner:KEIO UNIV
Compositions and Methods of Treatment of Corneal Endothelium Disorders
InactiveUS20160175380A1Enhance cell viabilityGood effectSenses disorderTetrapeptide ingredientsDiseaseCorneal endothelial cell
Owner:THE SCHEPENS EYE RES INST
Laminate of cultured human corneal endothelial cells layer and method for manufacturing same
InactiveUS7959939B2Maintain transparencyImprove adhesionBiocideEye implantsCorneal endothelial cellCollagen i
A laminate comprising a transparent collagen I type sheet and a human corneal endothelial cell culture layer provided on the sheet. An endothelial cell culture layer laminate usable in transplantation is provided.
Owner:YAMAGAMI SATORU +1
Constructing method for human corneal endothelium cell system
The construction of human corneal endothelium cell line with corneal endothelium as material includes the steps of: the first adherent culture of human corneal endothelium cell inside DMEM / F12 culture liquid with ox embryo serum in 20 wt%, chondroitin sulfate oxidizing and degrading matter, epidermal cell growth factor, basic fibroblast growth factor and ox eye auxin to obtain pure corneal endothelium cell; and the subsequent secondary culture in trypsinization process. The technological process is scientific and reasonable, and up to now, passage cell of 106-th generation has been obtained. The human corneal endothelium cell line of the present invention has no any virus or cancer genetic transfection, has no any tumorigenicity, and is expected to be used in direct artificial cornea production and clinical application.
Owner:青岛彩晖生物科技有限公司
Endothelial cell growth factor, methods of isolation and expression
A novel growth factor specific for vascular endothelial cells has been identified in conditioned medium of bovine pituitary derived folliculo stellate cells. This factor, named folliculo stellate derived growth facto (FSdGF) or vascular endothelial growth factor (VEGF), was purified to homogeneity by a combination of heparin sepharose affinity chromatography, Bio Gel P-60 exclusion chromatography, Mono S ion exchange chromatography and hydrophobic chromatography on a C4 reverse phase HPLC column. The factor is also found in the murine AtT-20 cell line. Alternatively, the growth factor is purified by a first reverse phase HPLC using acetonitrile gradient followed by a second reverse phase HPLC using an isopropanol gradient. FSdGF, having a molecular weight of about 43,000 da, was characterized as a glycoprotein composed of two homologous sub units with MW of about 23 kDa. FSdGF was a potent mitogen for vascular endothelial cells with activity detectable at 10 pg / ml and saturation at 500 pg / ml. It did not stimulate the proliferation of other cell types such as bovine corneal endothelial cells, adrenal cortex cells, granulosa cells, BALB / MK cells or BHK-21 cells. Microsequencing revealed an amino terminal sequence containing no significant homology to any known protein. The release of FSdGF by pituitary cells and its unique target cell specificity indicate that FSdGF is useful in angiogenesis.
Owner:FERRARA NAPOLEONE +2
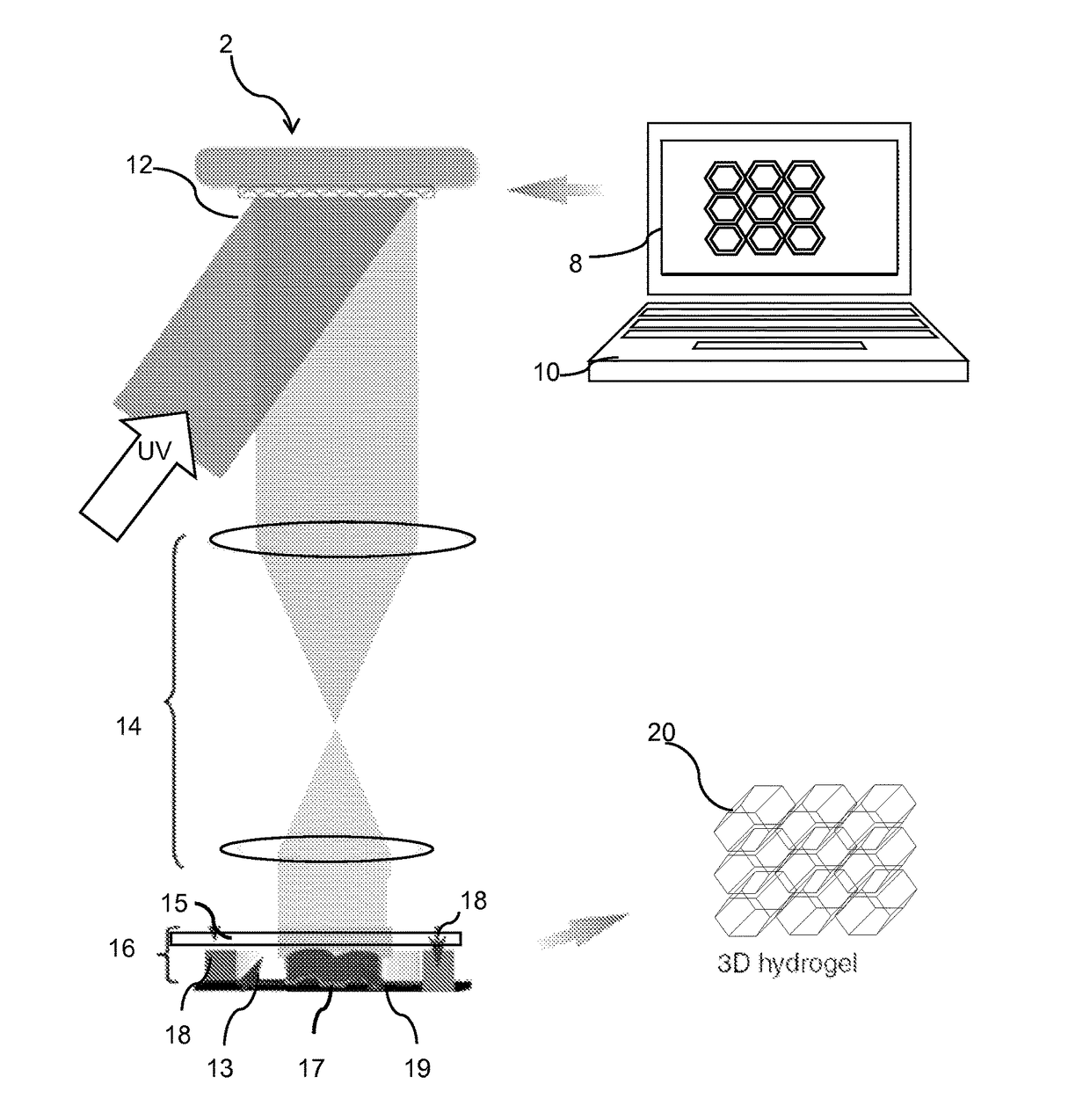
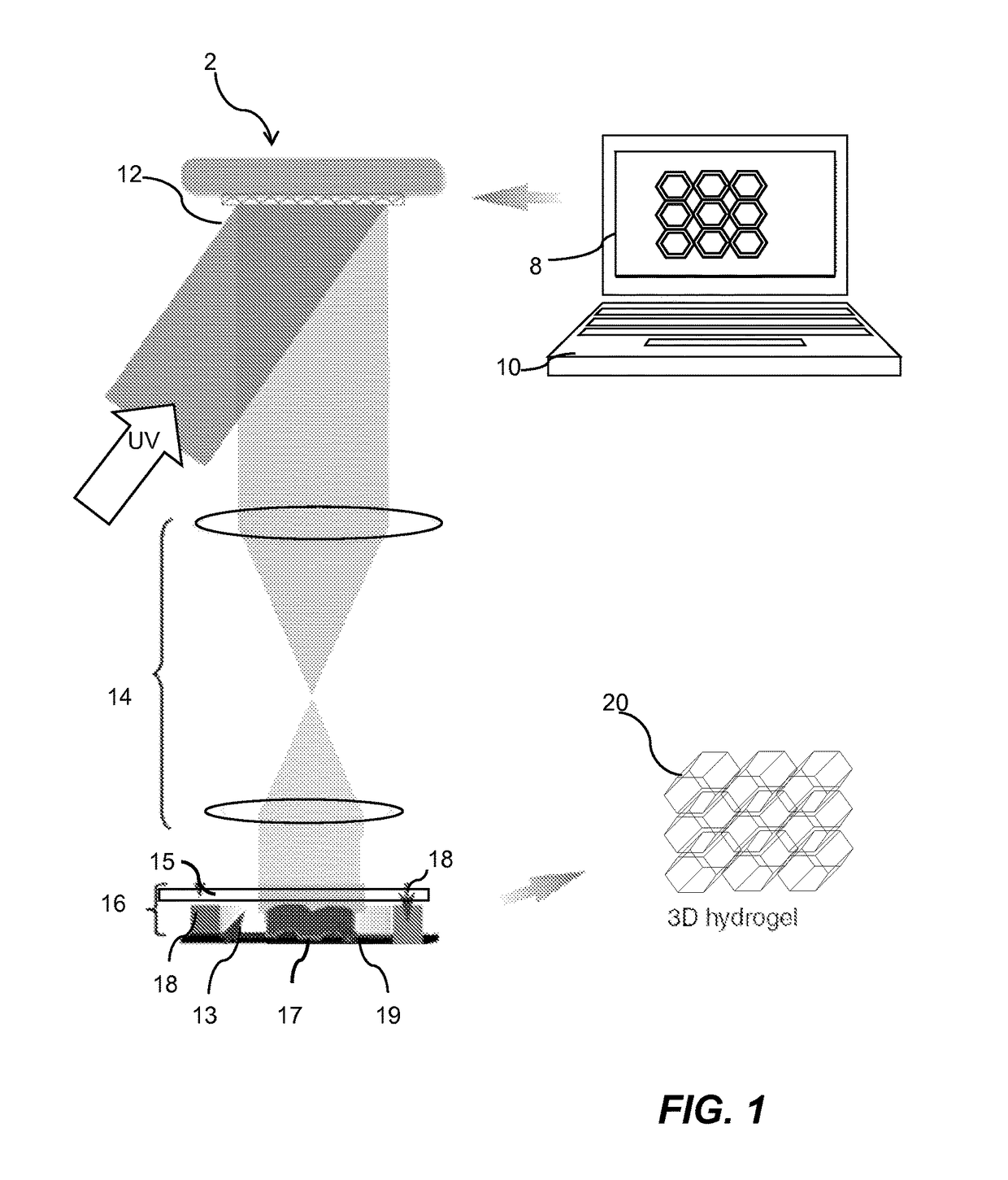
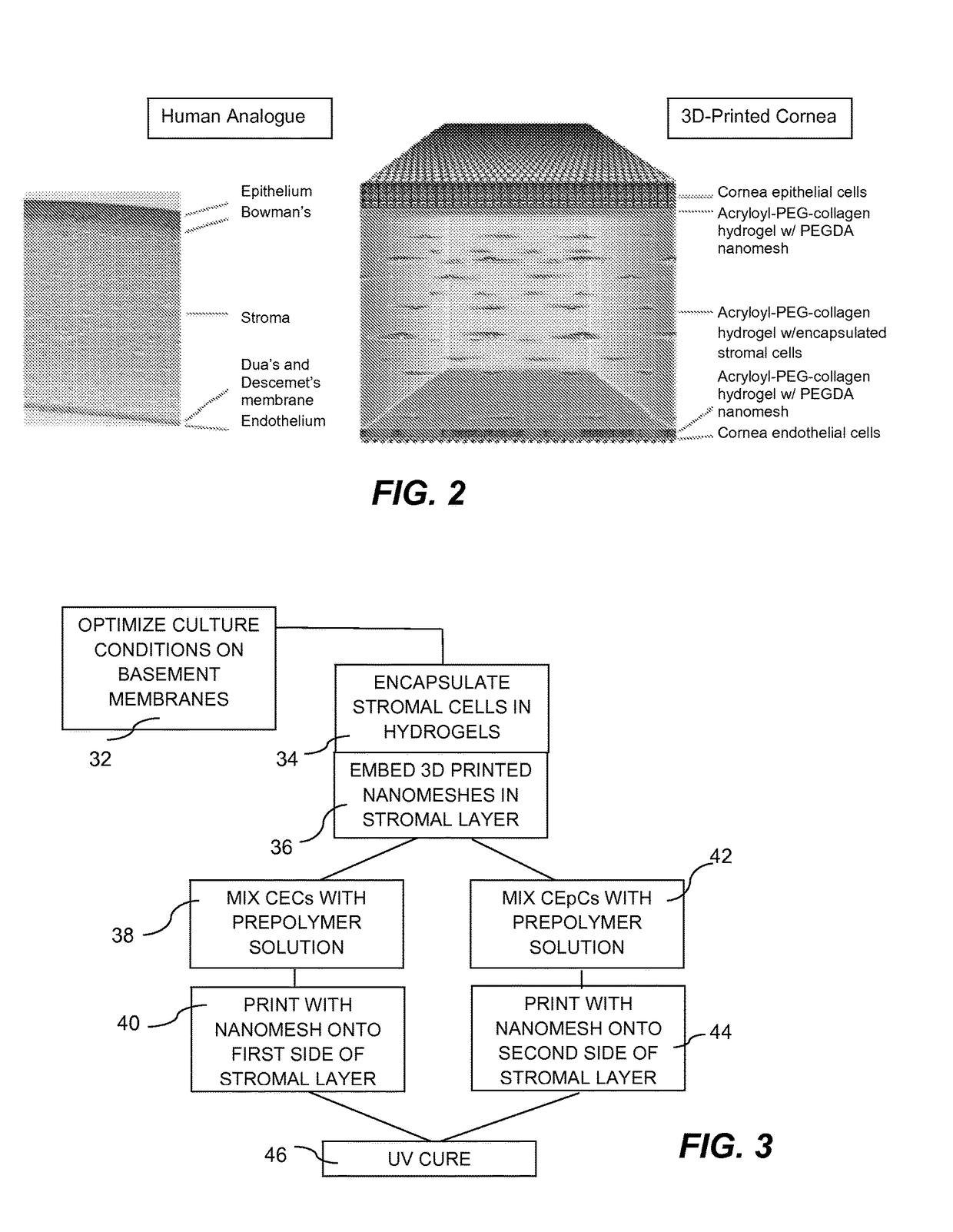
Three-dimensional bioprinted artificial cornea
InactiveUS20170281828A1Restore visionEliminate dependenciesAdditive manufacturing apparatusPharmaceutical delivery mechanismCorneal endothelial cellStromal cell
An artificial cornea is fabricated by separately culturing live stromal cells, live corneal endothelial cells (CECs) and live corneal epithelial cells (CEpCs), and 3D bioprinting separate stromal, CEC and CEpC layers to encapsulate the cells into separate hydrogel nanomeshes. The CEC layer is attached to a first side of the stromal layer and the CEpC layer to a second side of the stromal layer to define the artificial cornea.
Owner:RGT UNIV OF CALIFORNIA
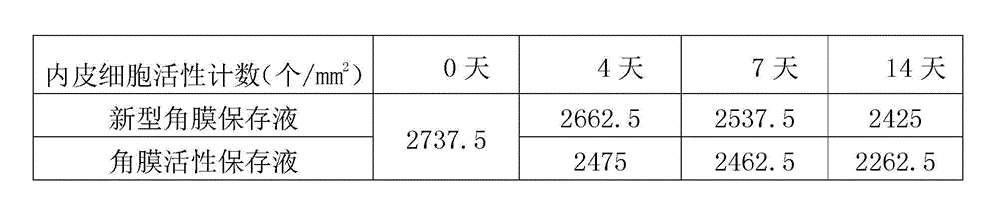
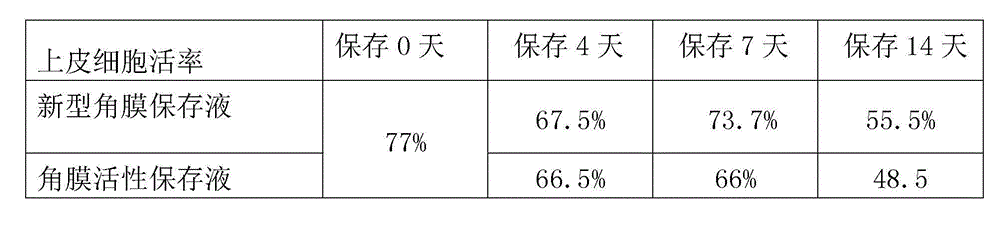
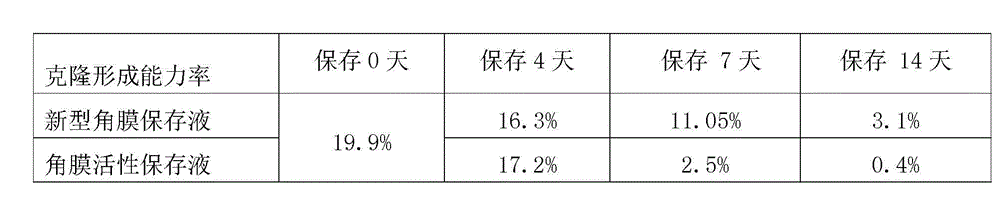
Cornea metaphase preservation solution, and preparing and using methods thereof
The invention provides a cornea metaphase preservation solution, and preparing and using methods thereof. The cornea metaphase preservation solution is a cell culture minimum essential medium (MDM) with chondroitin sulfate, low molecular dextran, L-glutamine, dexamethasone, tobramycin, 2-hydroxyethyl and Y-27632 added. The preservation solution not only can keep activity and normal morphology of cornea endothelial cells, but also can enhance viability of corneal limbus epithelial cells and improve clone ability of corneal limbus stem cells. And especially, a medium to long term preservation effect is obvious, phenomena of deformation, conjugation and the like of endothelial cells of a control group cornea do not appear in long term preservation, endothelial morphology of the control group cornea is consistent with endothelial morphology of a cornea preserved for 4 days, and the endothelial cells of the control group cornea are still regular and few in cell conjugation phenomena. The preservation solution can effectively prevent cell apoptosis phenomena during the process of preserving isolated cornea materials, increases activity and clone forming ability of the corneal limbus stem cells, and enables the cornea to maintain a transparent feature in a long preservation time.
Owner:SHANDONG EYE INST
Human corneal endothelial cell culture solution as well as preparation method and application thereof
The invention relates to a human corneal endothelial cell culture solution as well as a preparation method and an application thereof. The solution comprises bovine corneal endothelial cell lysis solution with 10-200 mug / ml of protein as the main active component, fetal calf serum with the content being 10%, 100U / ml of penicillin, 100U / ml of streptomycin and the remainder being supplemented by D / F12 basal culture solution. The method comprises of: firstly culturing bovine corneal endothelial cell in vitro; preparing bovine corneal endothelial cell lysis solution and formulating in a disinfection chamber to obtain the product. The invention, for the first time, prepares bovine corneal endothelial cell lysis solution into cell culture solution and applies to in vitro culture of human corneal endothelial cells, can promote great augmentation of human corneal endothelial cells cultured in vitro, maintain form feature of corneal endothelial cells, inhibit cells from ageing and apoptosis, and facilitate the subculture of endothelial cells; wherein, the raw material bovine corneal endothelial cell has abundant source and is easy for in vitro culture, the preparation method of the culture solution is simple, easy to implement, conducive for industrialization, and has wide application and development prospect.
Owner:SHANDONG EYE INST
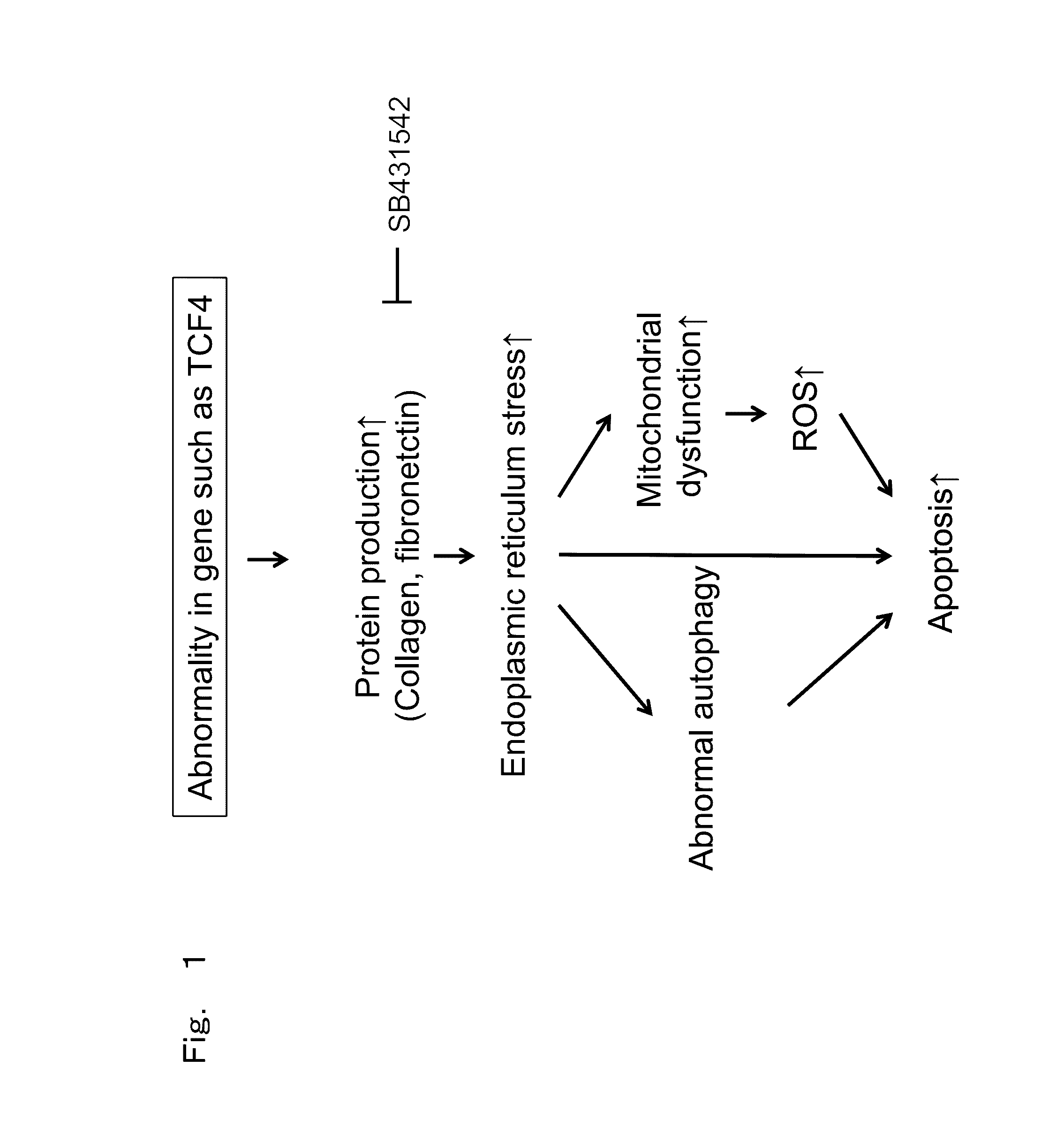
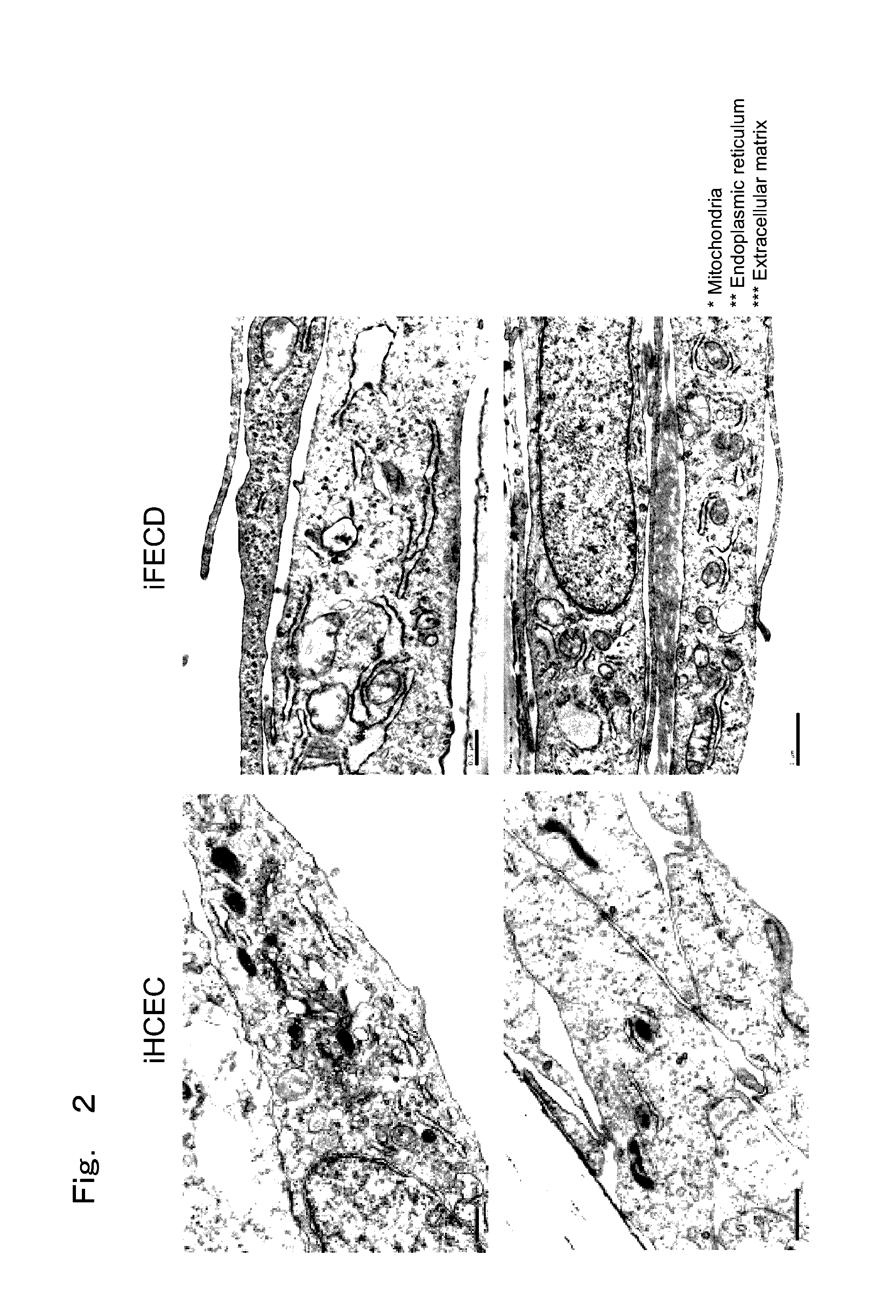
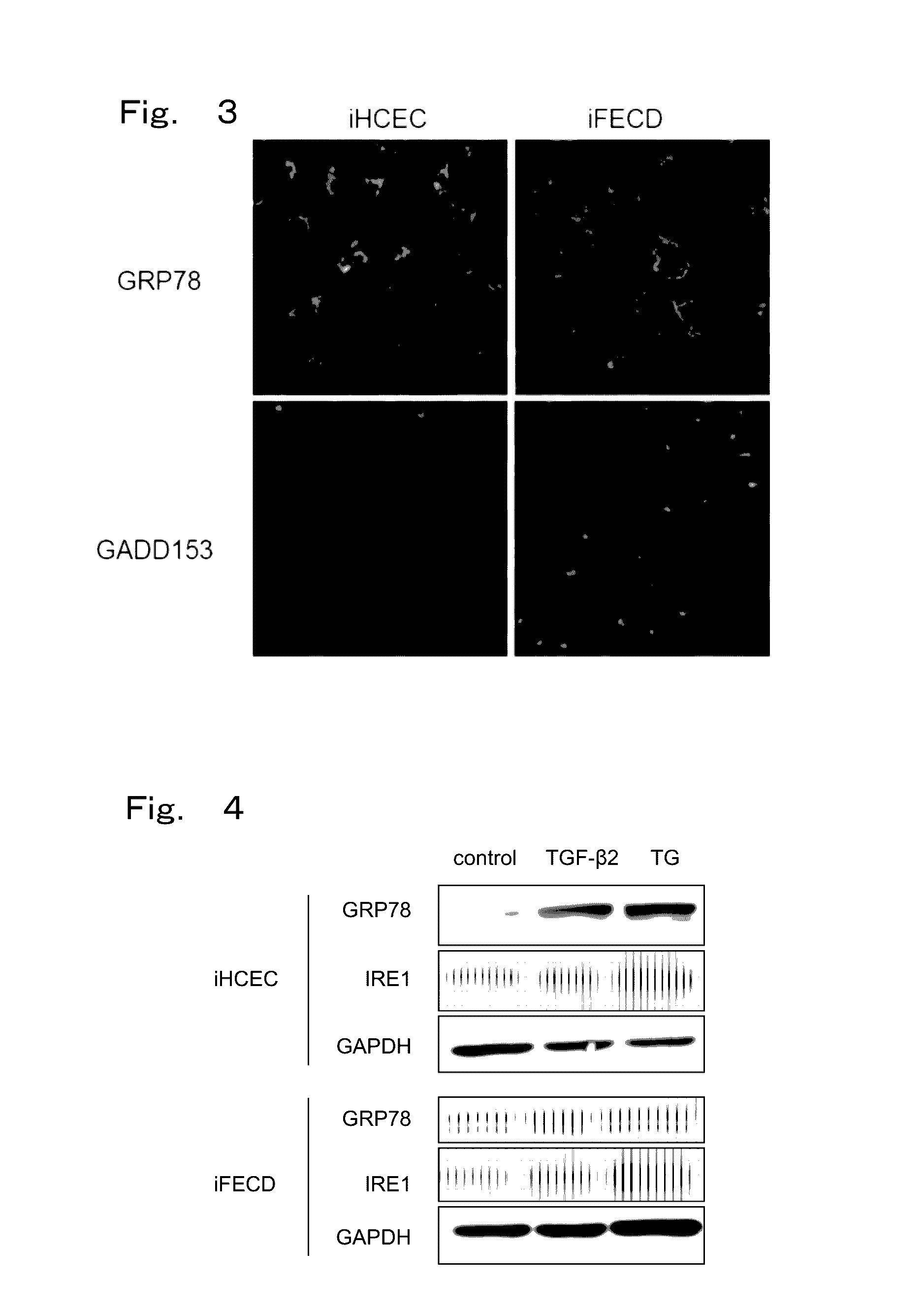
Therapeutic drug for diseases related to endoplasmic reticulum cell death in corneal endothelium
The present invention provides a treatment drug or prophylactic drug for diseases, disorders, or conditions related to endoplasmic reticulum (ER) stress. Specifically, the present invention provides a treatment drug or prophylactic drug for diseases, disorders, or conditions related to endoplasmic reticulum (ER) stress in the corneal epithelium, the drug containing a TGFβ-signal inhibitor. As a preferred TGFβ-signal inhibitor, the drug contains 4-[4-(1,3-benzodioxole-5-yl)-5-(2-pyridinyl)-1H-imidazole-2-yl]benzamide.
Owner:KYOTO PREFECTURAL PUBLIC UNIV CORP +2
Corneal posterior lamella and use thereof
InactiveCN101745152AImprove proliferative abilityStrong self-renewal abilityEye implantsCorneal endothelial cellCorneal endothelium
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Human corneal endothelial cell sheet
InactiveUS20150374881A1Simple materialBiocideSenses disorderCorneal endothelial cellCorneal endothelium
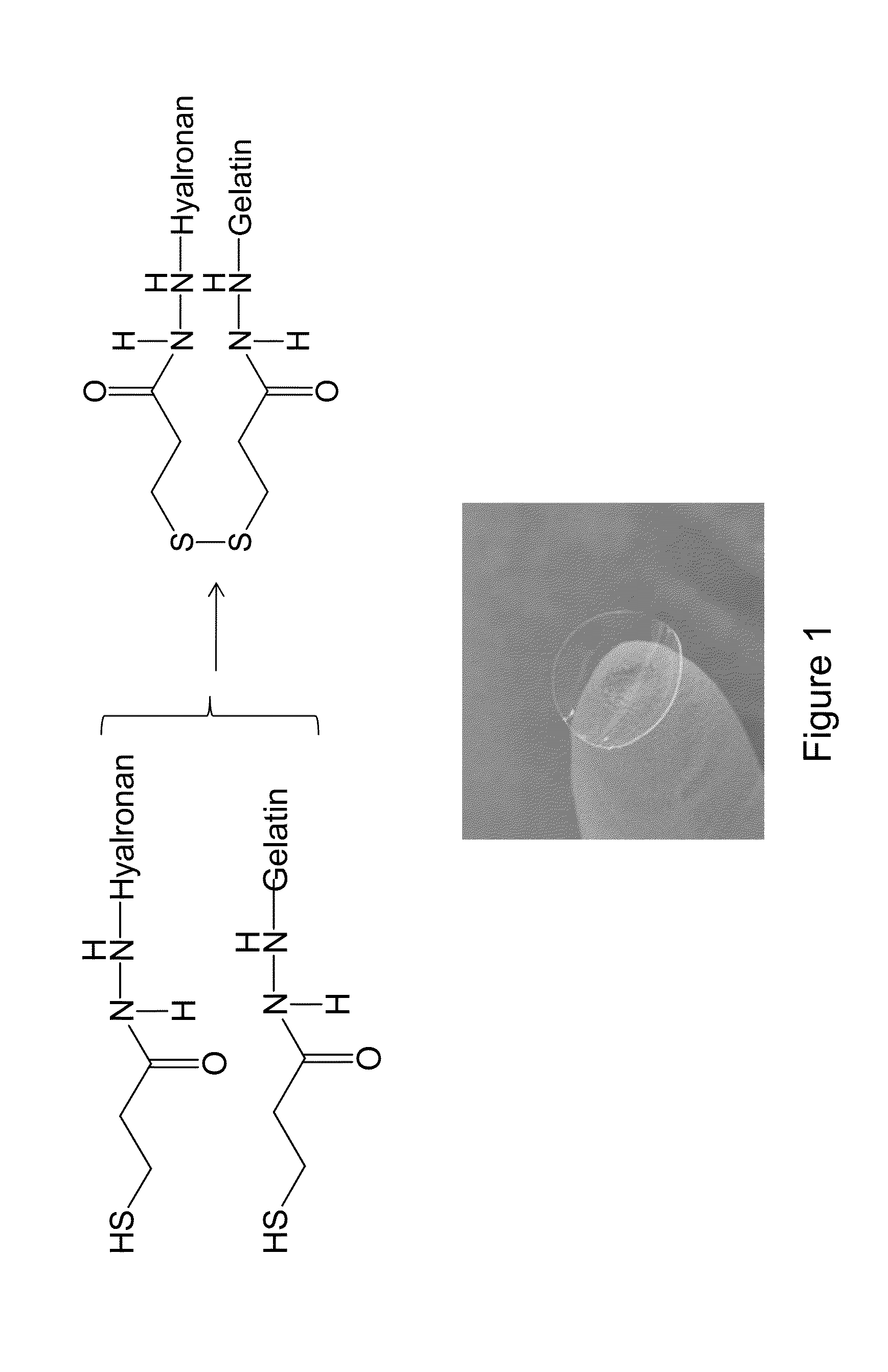
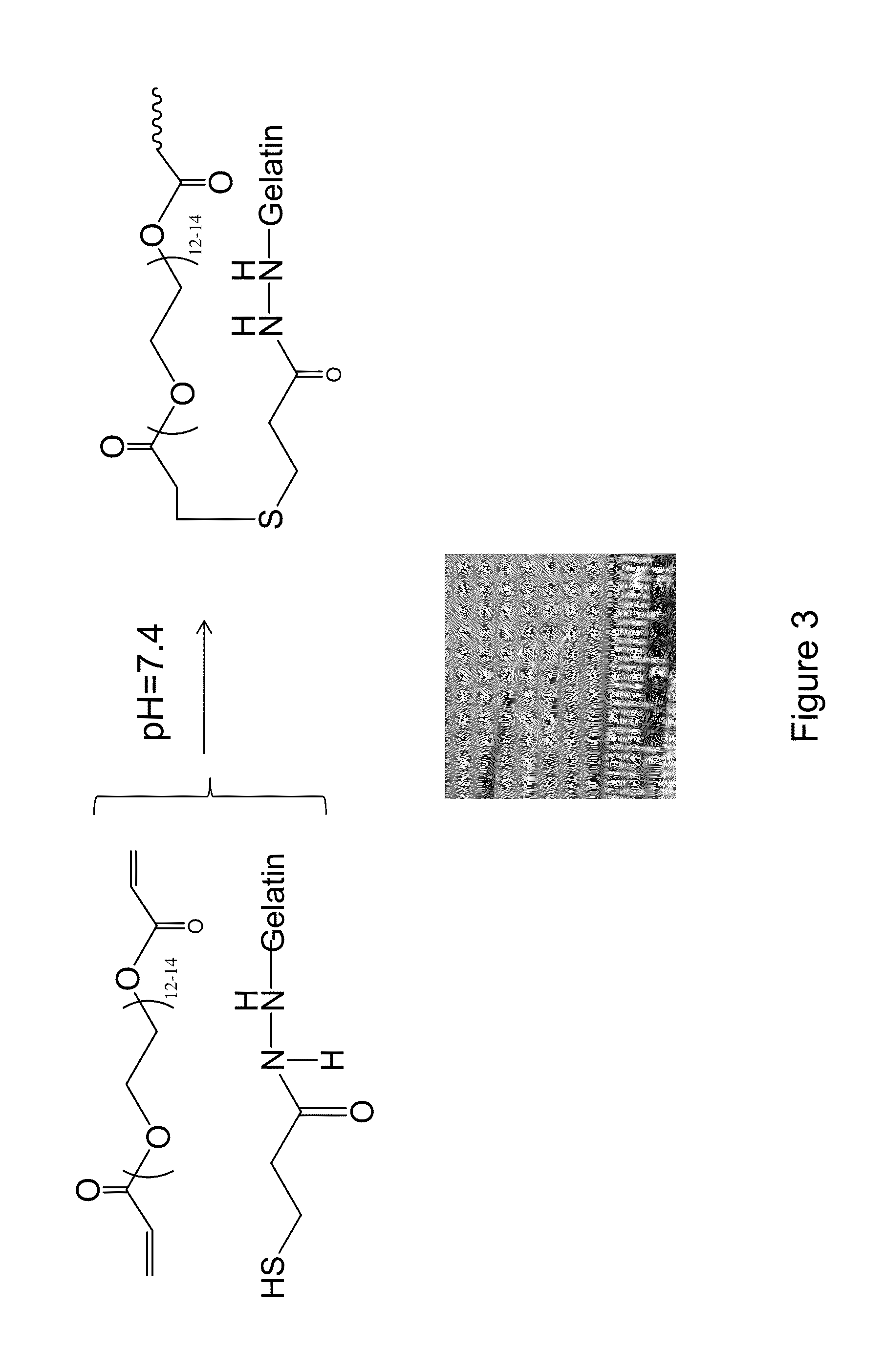
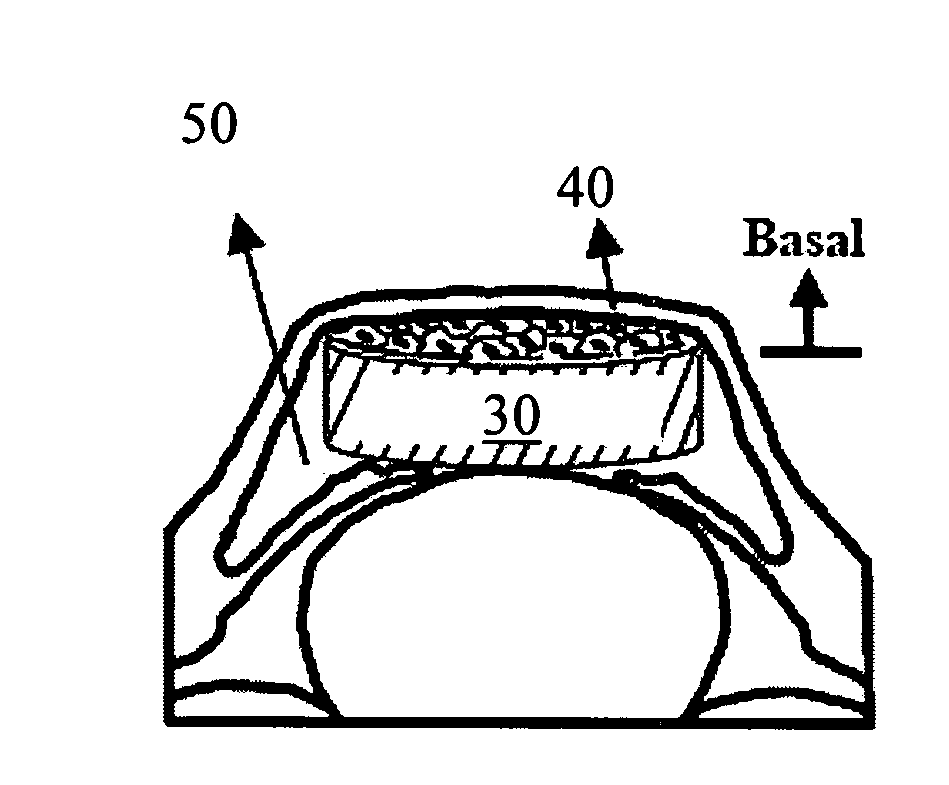
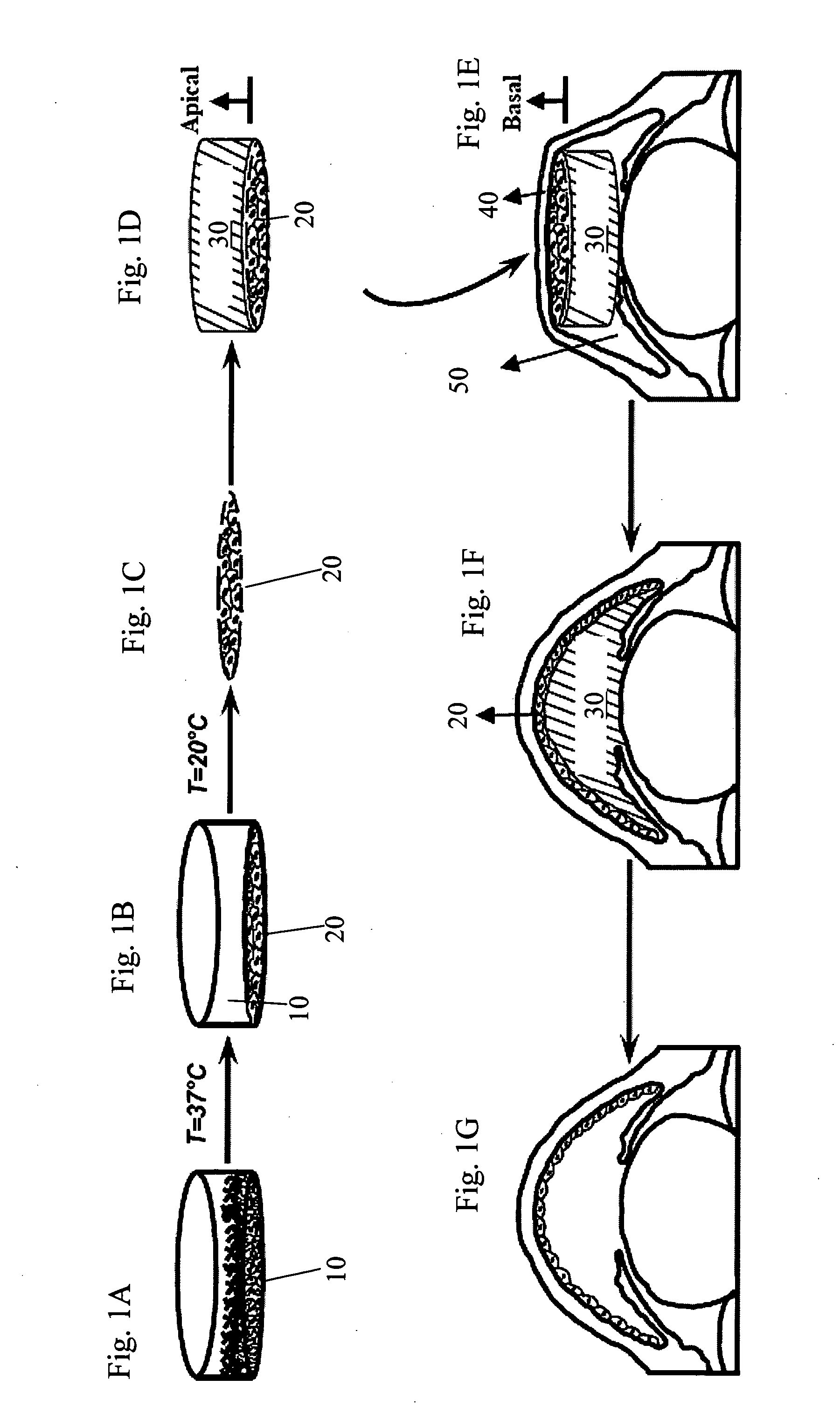
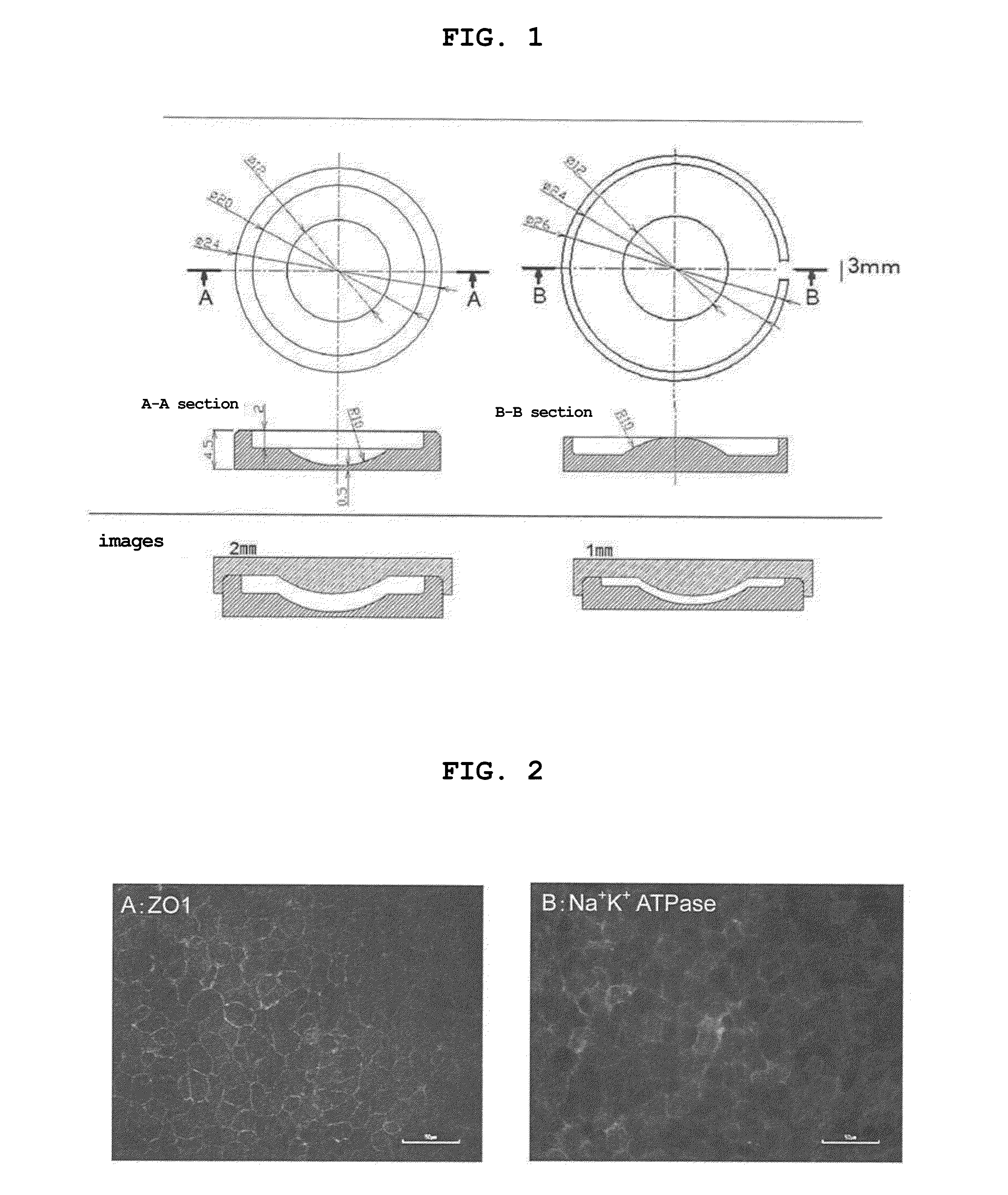
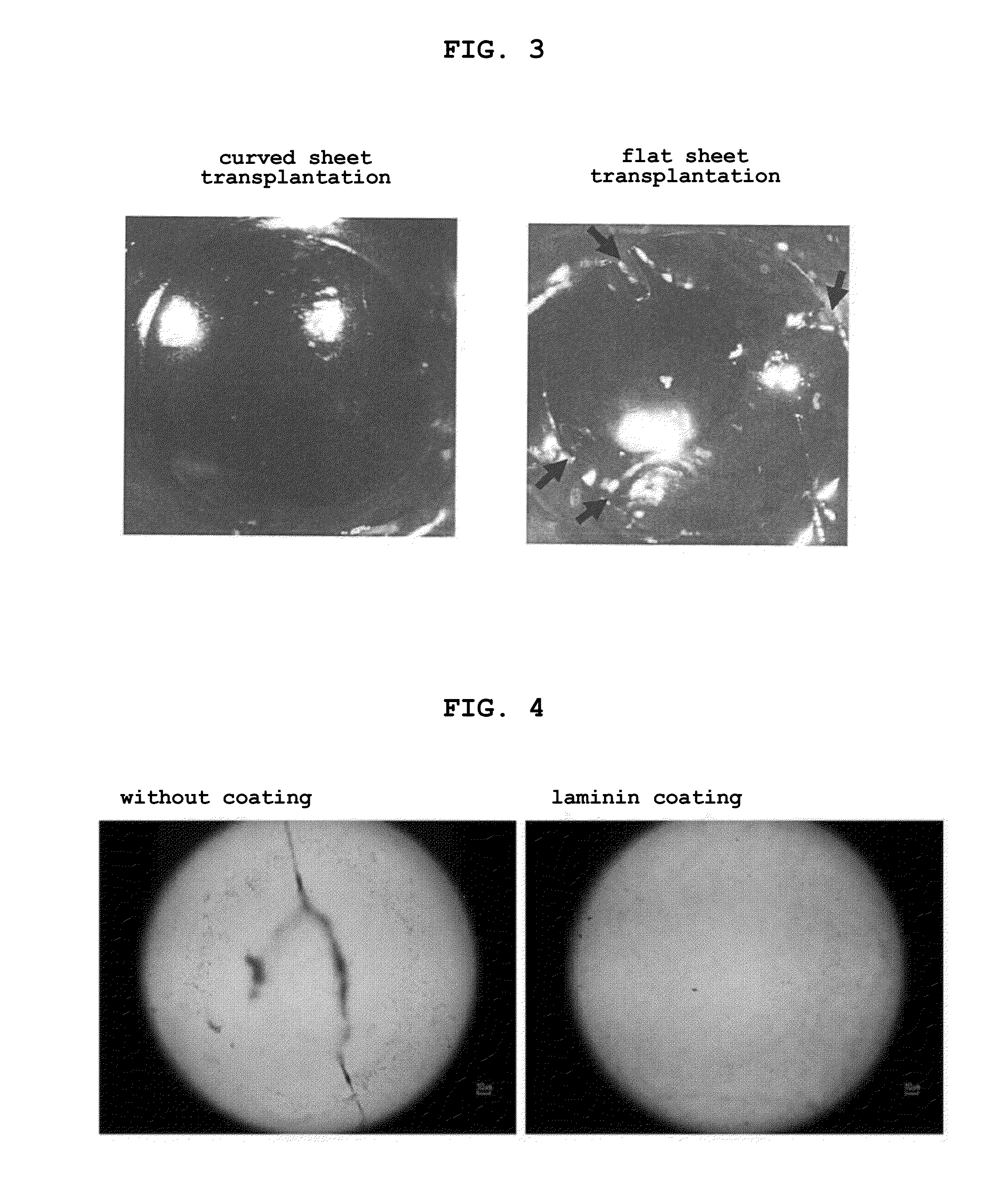
The present invention provides a human corneal endothelial cell sheet having a curvature fitting a human corneal endothelial surface, particularly, the human corneal endothelial cell sheet supported by a cell support having a curvature fitting a human corneal endothelial surface, and a production method of a human corneal endothelial cell sheet having a curvature fitting a human corneal endothelial surface, which includes the following steps:(1) a step of gelling a gelatin aqueous solution poured into a template having a curvature fitting a human corneal endothelial surface;(2) a step of forming a sheet-like gelatin by drying the gelled gelatin obtained in (1);(3) a step of obtaining a gelatin sheet by thermally crosslinking the sheet-like gelatin obtained in (2);(4) a step of seeding human corneal endothelial cells on the gelatin sheet obtained in (3) to form a human corneal endothelial cell layer.
Owner:NITTA GELATIN INC +1
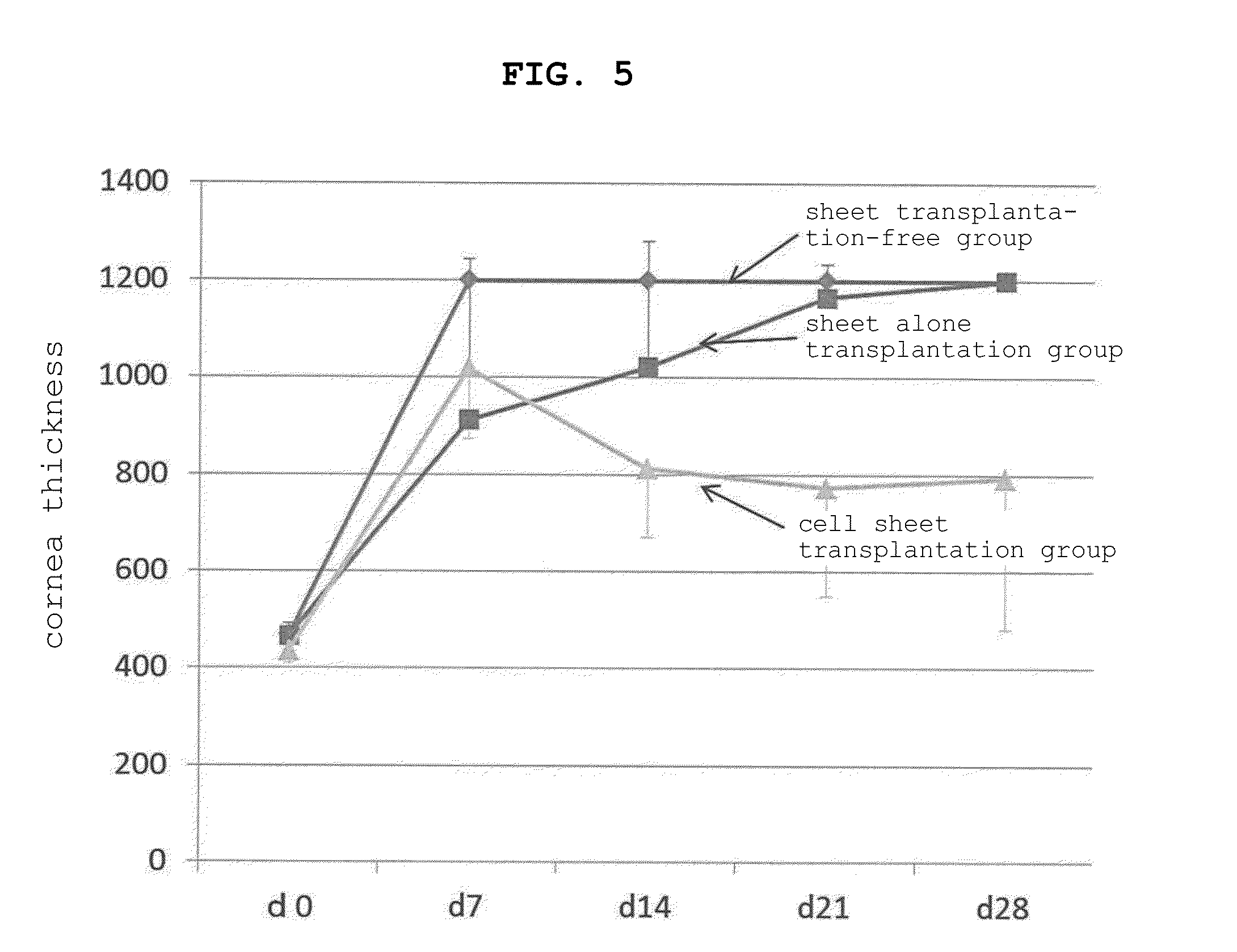
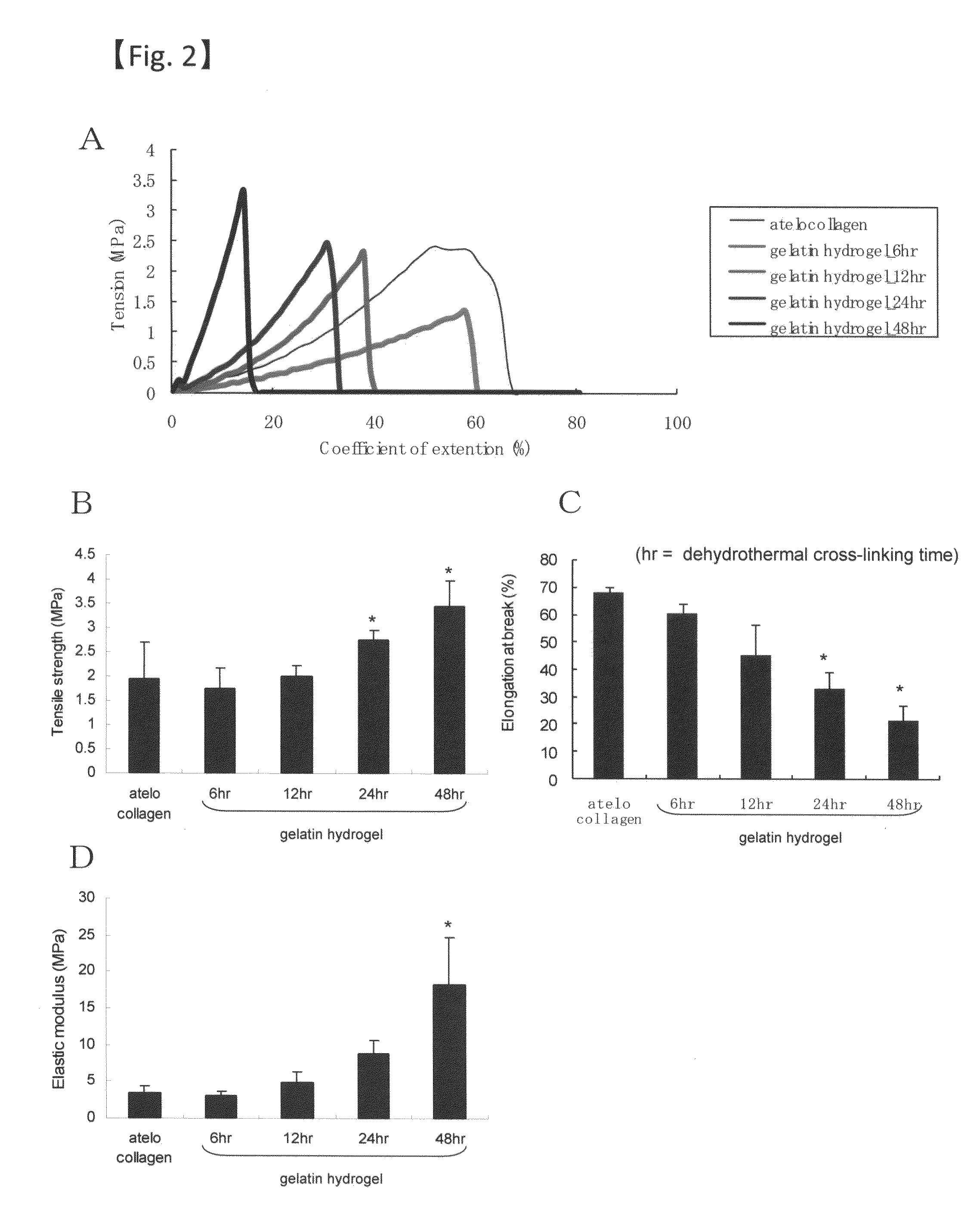
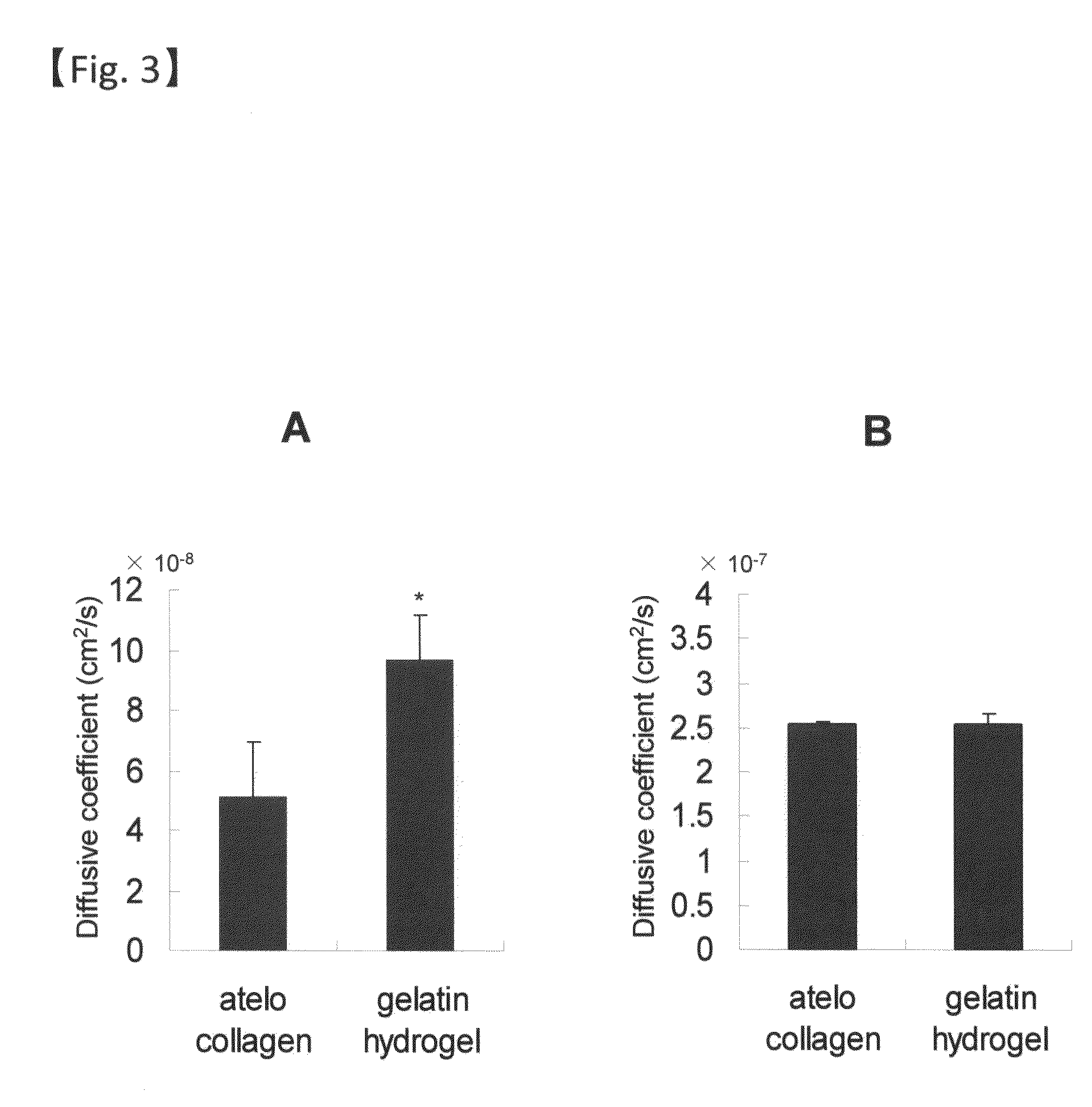
Sheet for corneal transplants
InactiveUS20120282318A1Stable transplantation resultAppropriate strengthBiocideSenses disorderCorneal endothelial cellBiocompatibility Testing
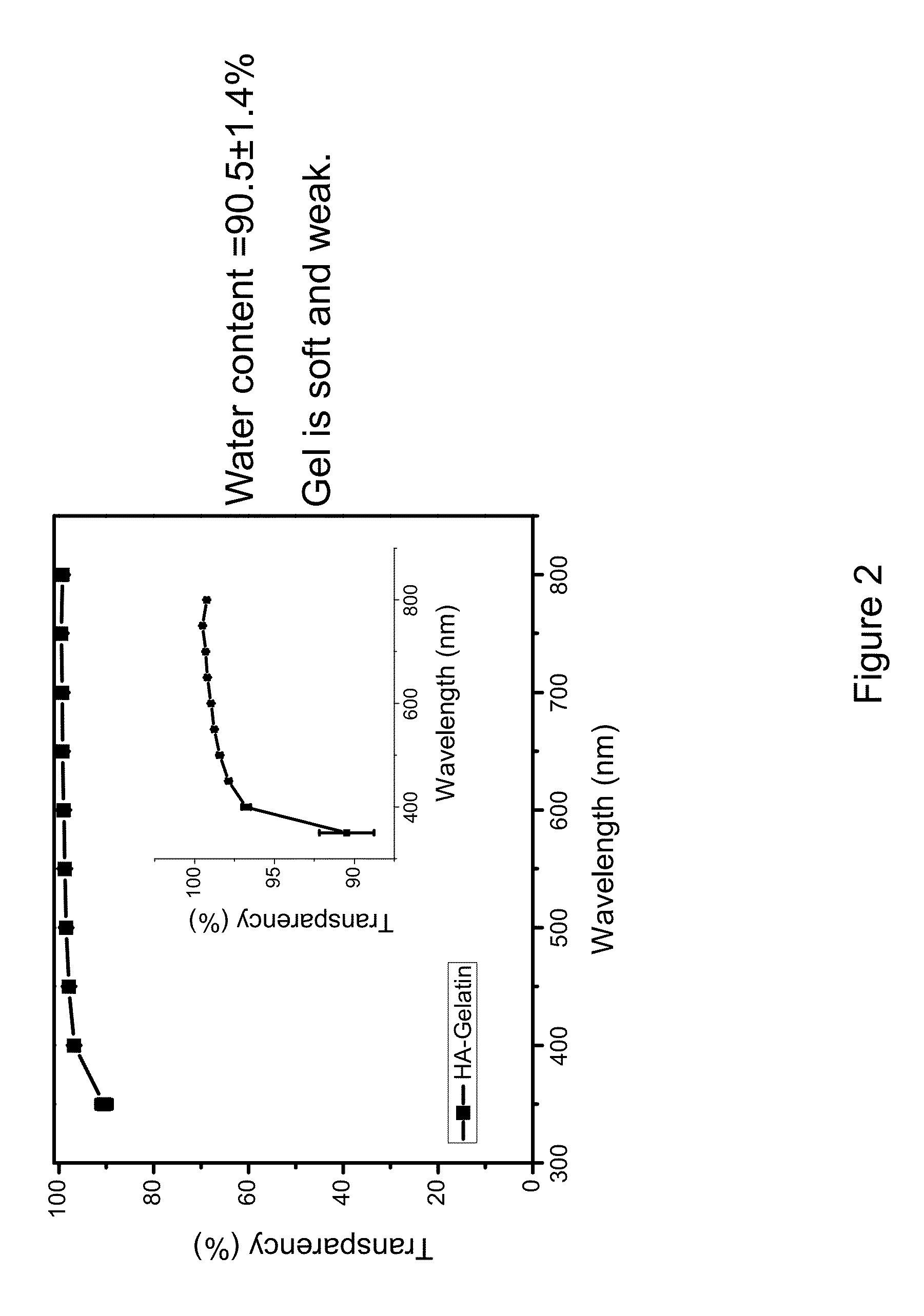
The present invention relates to a sheet for corneal transplants comprising corneal endothelial cells on a gelatin hydrogel, which is obtainable by seeding and culturing corneal endothelial cells on a gelatin hydrogel coated with collagen. The sheet of the present invention is extremely useful as a sheet for corneal transplants not only for its biocompatibility and biodegradability, but also for its high transparency.
Owner:OSAKA UNIV
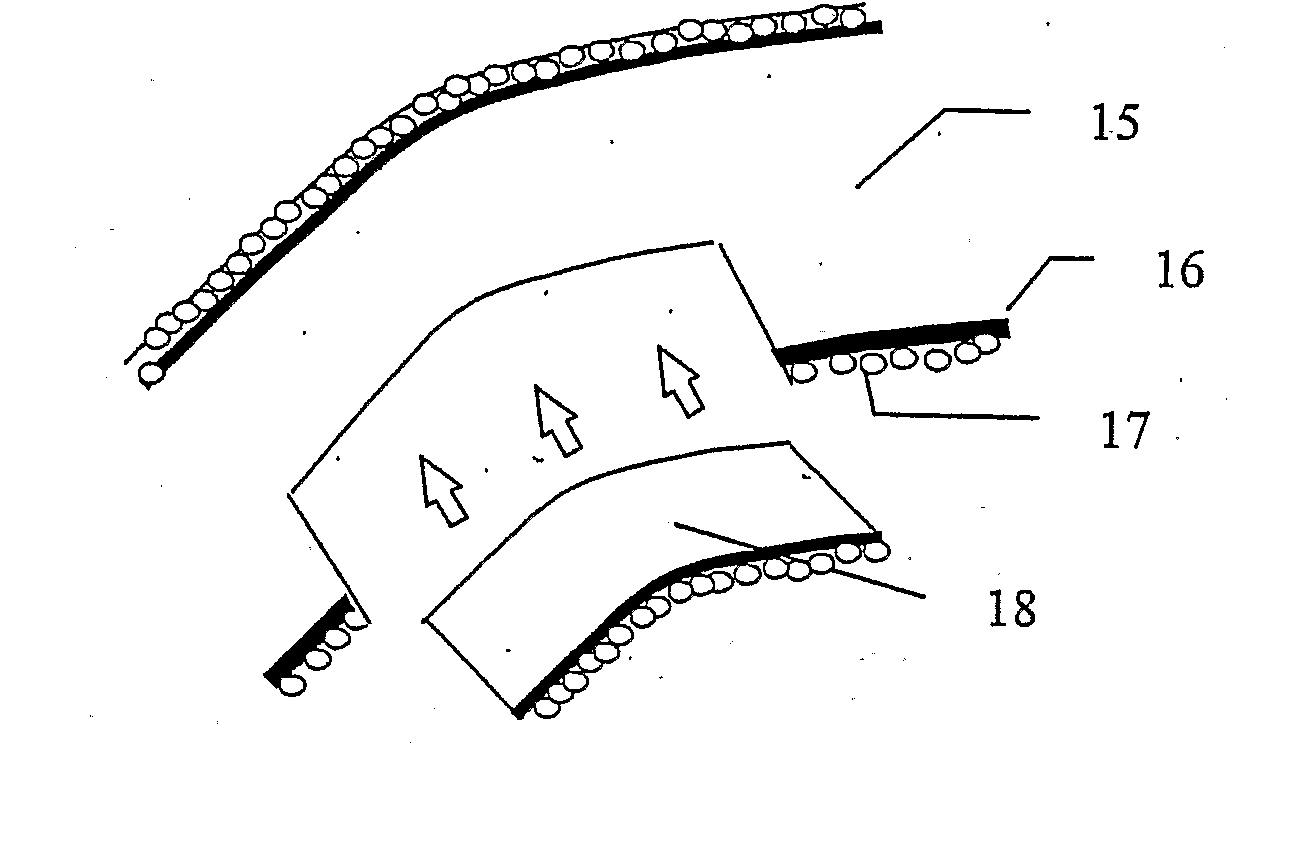
Tissue-engineered cornea and preparation method thereof
ActiveCN110613862AIncreasing the thicknessIncreased mechanical toughnessEye implantsTissue regenerationFiberCell-Extracellular Matrix
The invention discloses a tissue-engineered cornea and a preparation method thereof. The tissue-engineered cornea comprises three or more layers of laminated structures, each layer of the laminated structure is a composite layer formed in the mode that a fiber scaffold prepared by electrospinning or electrostatic direct writing or light curing 3D printing is cured and composited with one or more of collagen, an acellular matrix and silk fibroin, the space between every two adjacent layers is inoculated with corneal stromal cells and neurons, the corneal stromal cells and the neurons can grow in the direction of fibers of the scaffold, the corneal stromal cells between every two adjacent layers secrete extracellular matrixes to form a tissue structure similar to the human corneal stroma, and the upper surfaces and the lower surface of the laminated structures are inoculated with corneal epithelial cells and corneal endothelial cells. The tissue-engineered cornea can simulate the corneastructure well, is easy to prepare and good in biocompatibility and is easily fused with tissue.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com