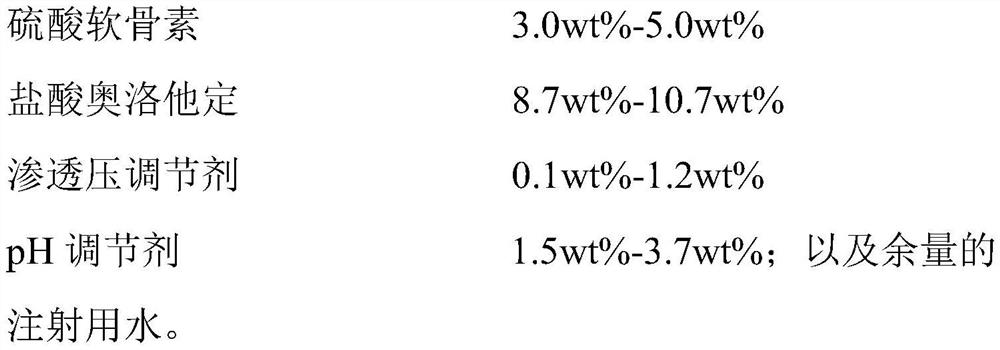Patents
Literature
35 results about "Corneal disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
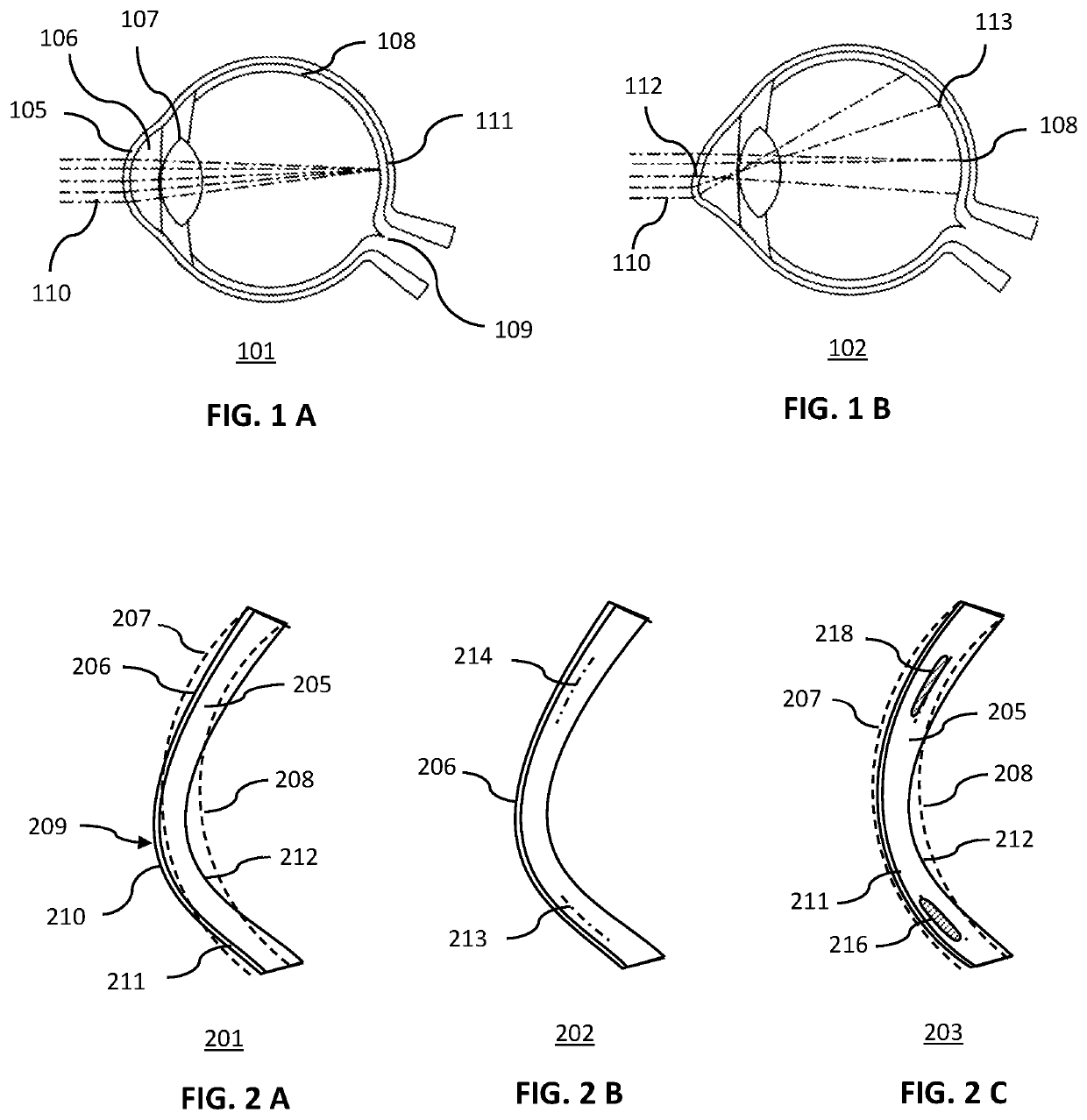
Corneal Disease. The term "corneal disease" refers to a variety of conditions that affect mainly the cornea. These include infections, degenerations, and many other disorders that may arise mostly as a result of heredity.
Stem cell regenerating surface cornea and its application in corneal transplantation
InactiveCN1398644AGuaranteed long-term effectivenessRich sourcesEye implantsArtificial cell constructsDiseaseCorneal disease
In the present invention an embryo or adult cornea epithelium is cut, digested with digesting liquid and centrifugated to prepare single cell suspension, and the cell suspension is cultured in culture dish or bottle with proper amount of culture medium and CO2 in 5% at 37 deg.c. The cultured cell is passed after cell converges to 80-90% and the second and sixth generation of cell is passed directly to amnion for culture for another 8-20 days to obtain the stem cell regenerating surface cornea of the present invention. The stem cell regenerating surface cornea may be used as material for treating corneal disease. The present invention provides a new material for treating corneal disease with rich material source, no danger of mouse-originated pollution, no or slight immunological rejection.
Owner:北京科宇联合干细胞生物技术有限公司
Therapeutic Agent for Corneal Diseases
InactiveUS20090163432A1Maintain transparencyLittle abilityOrganic active ingredientsSenses disorderCorneal diseasePharmacology
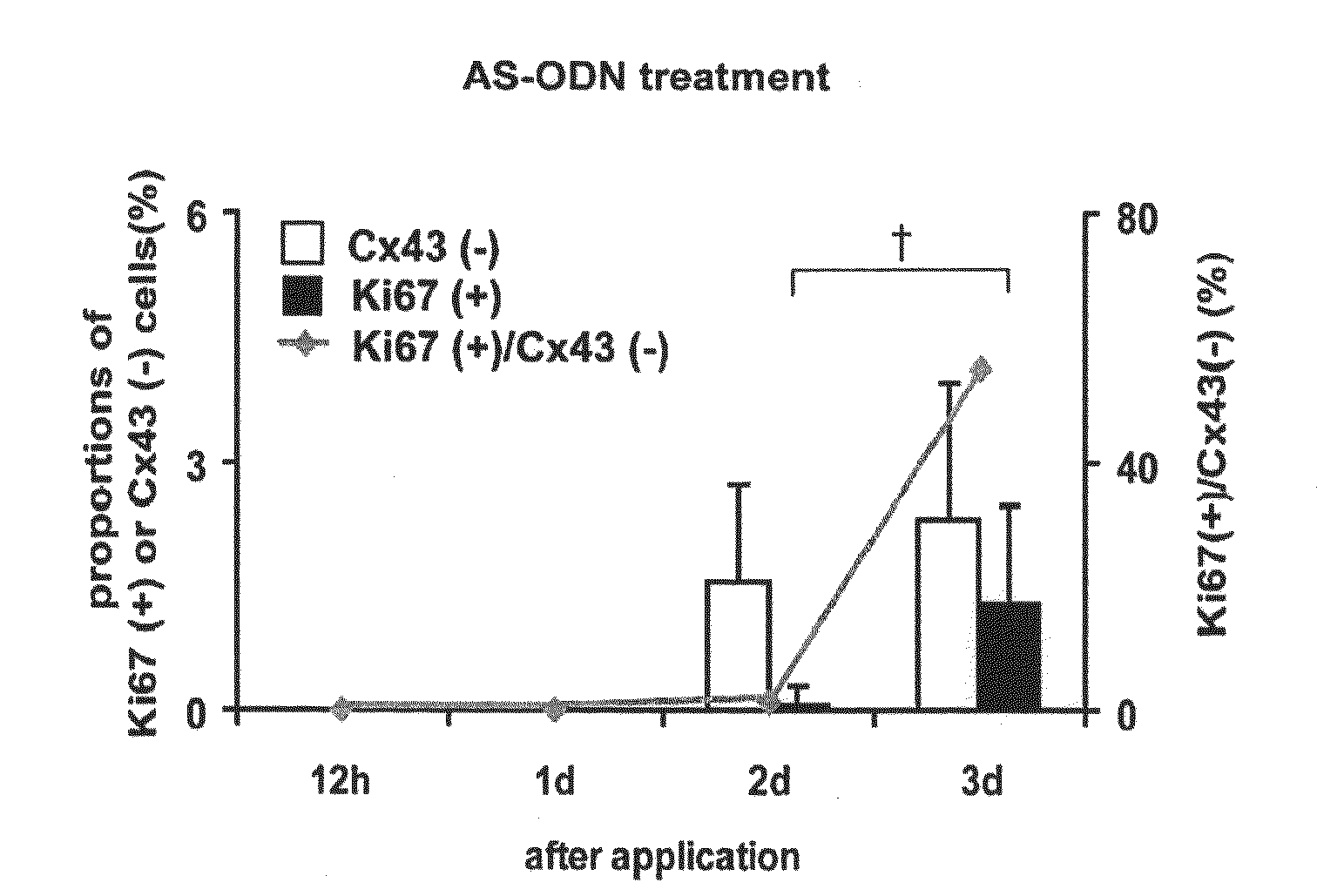
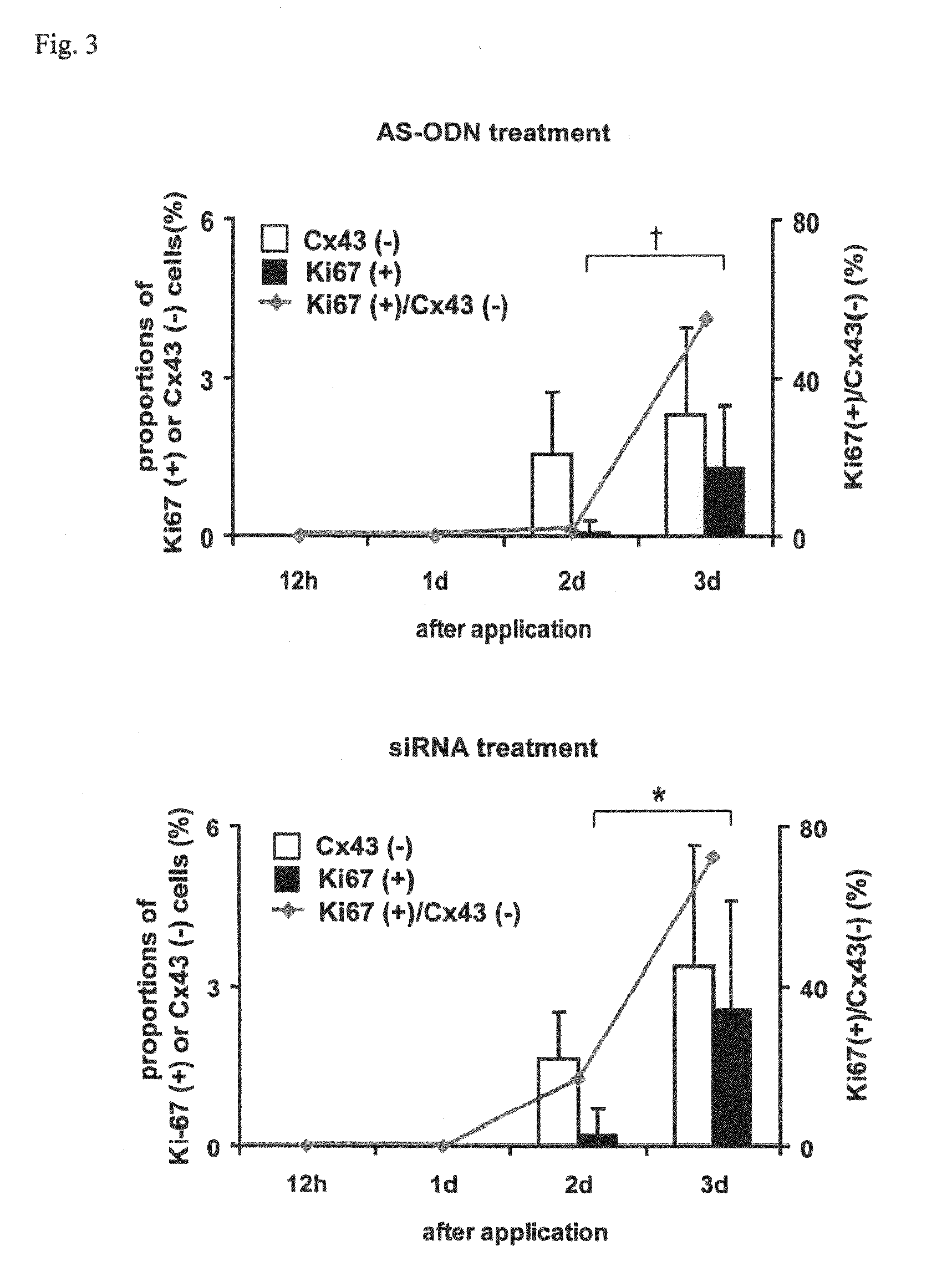
The present invention relates to a treatment agent for a disease or a disorder caused by a reduction in corneal endothelial cells, comprising as an active component at least one nucleic acid molecule inhibiting the expression of a connexin 43 gene.
Owner:KANSAI TLO KK
Therapeutic agent for corneal disease
A therapeutic agent for a corneal disease comprising irsogladine or a salt thereof as an active ingredient. The purpose is to find a substance capable of effectively treating / ameliorating a corneal disease which has been increased in the number of cases thereof in recent years and to provide a therapeutic agent for a corneal disease comprising the substance as an active ingredient.
Owner:TEIKA PHARMA CO LTD
Ophthalmic elastography
InactiveUS20150313573A1Accurate measurementInfrasonic diagnosticsSonic diagnosticsDiseaseCorneal disease
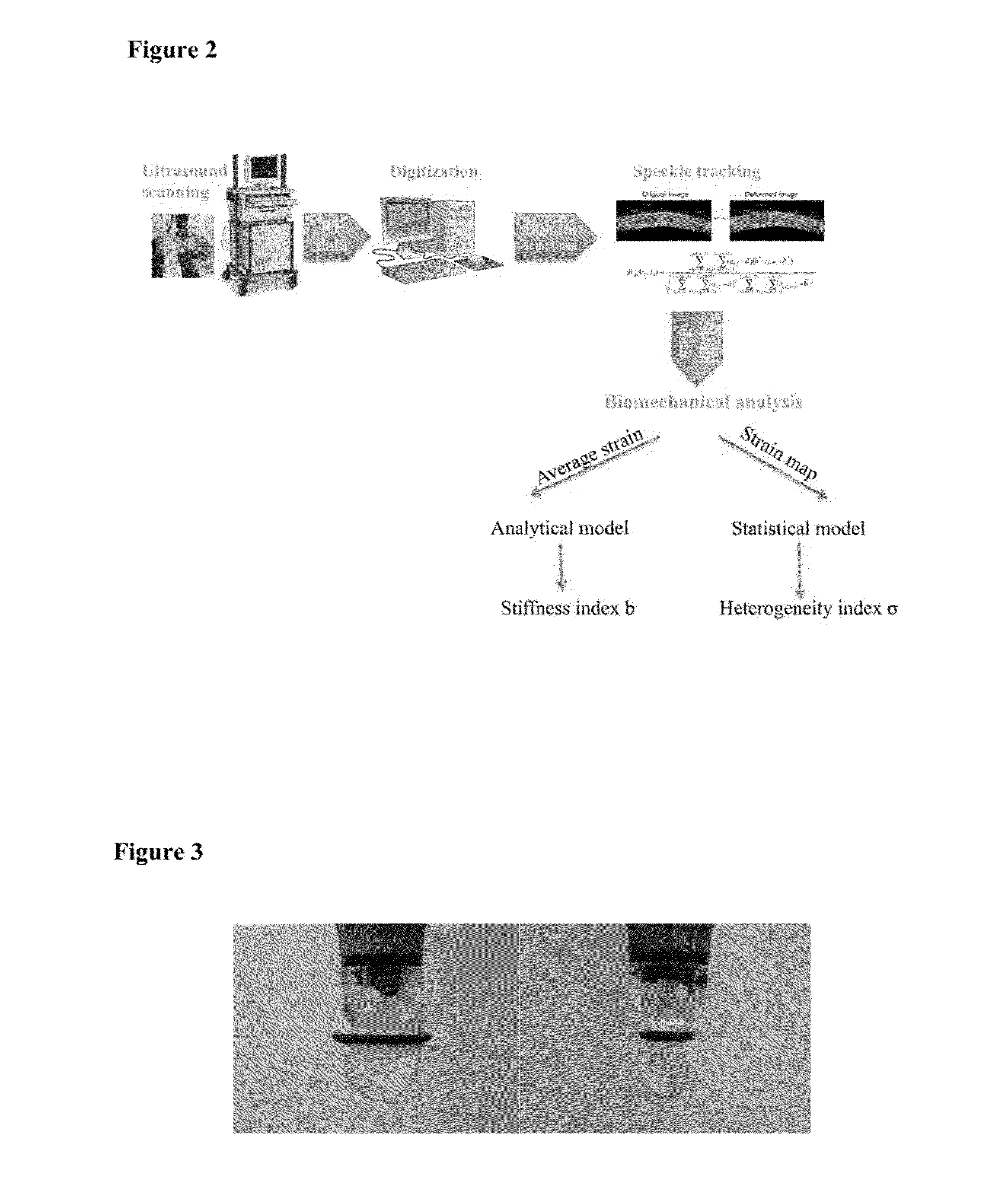
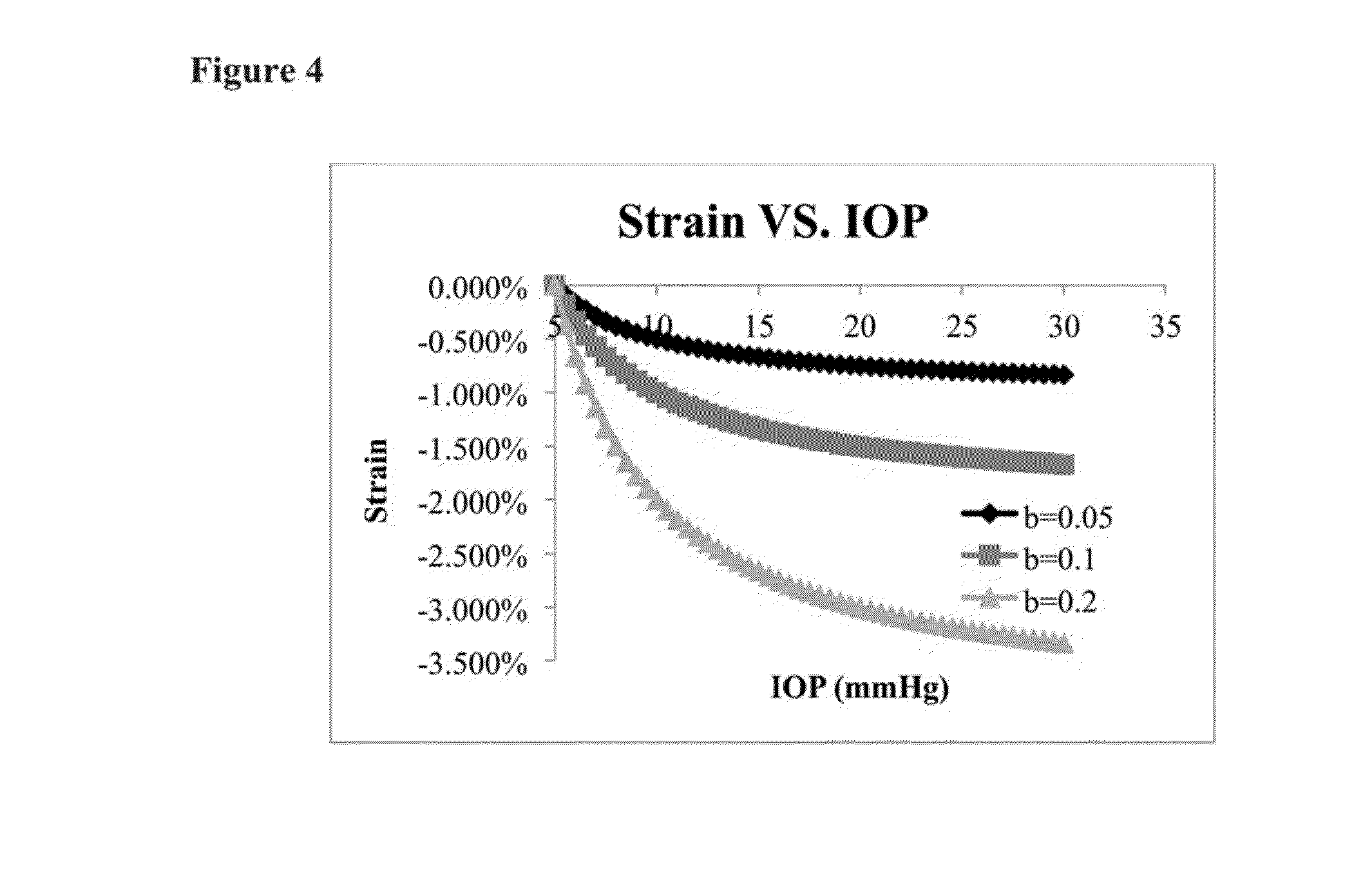
This invention describes an ultrasound technique that maps out the mechanical properties of the cornea and the sclera to the intrinsic mechanical loadings in the eye. It helps identify the abnormally weaker or stiffer regions in the eye, to add functional information for early and definitive diagnosis of corneal diseases, surgical planning, prevention of surgical complications, as well as better interpretation of tonometric readings. This technique will allow a spatial mapping of the mechanical strains developed in the cornea or the sclera during ocular pulse or other intraocular pressure fluctuations. The envisioned use of this technique resembles the current clinical ophthalmic ultrasound in terms of the patient experience, but provides functional information about the eye tissue that is not available from current clinical ultrasound.
Owner:OHIO STATE INNOVATION FOUND
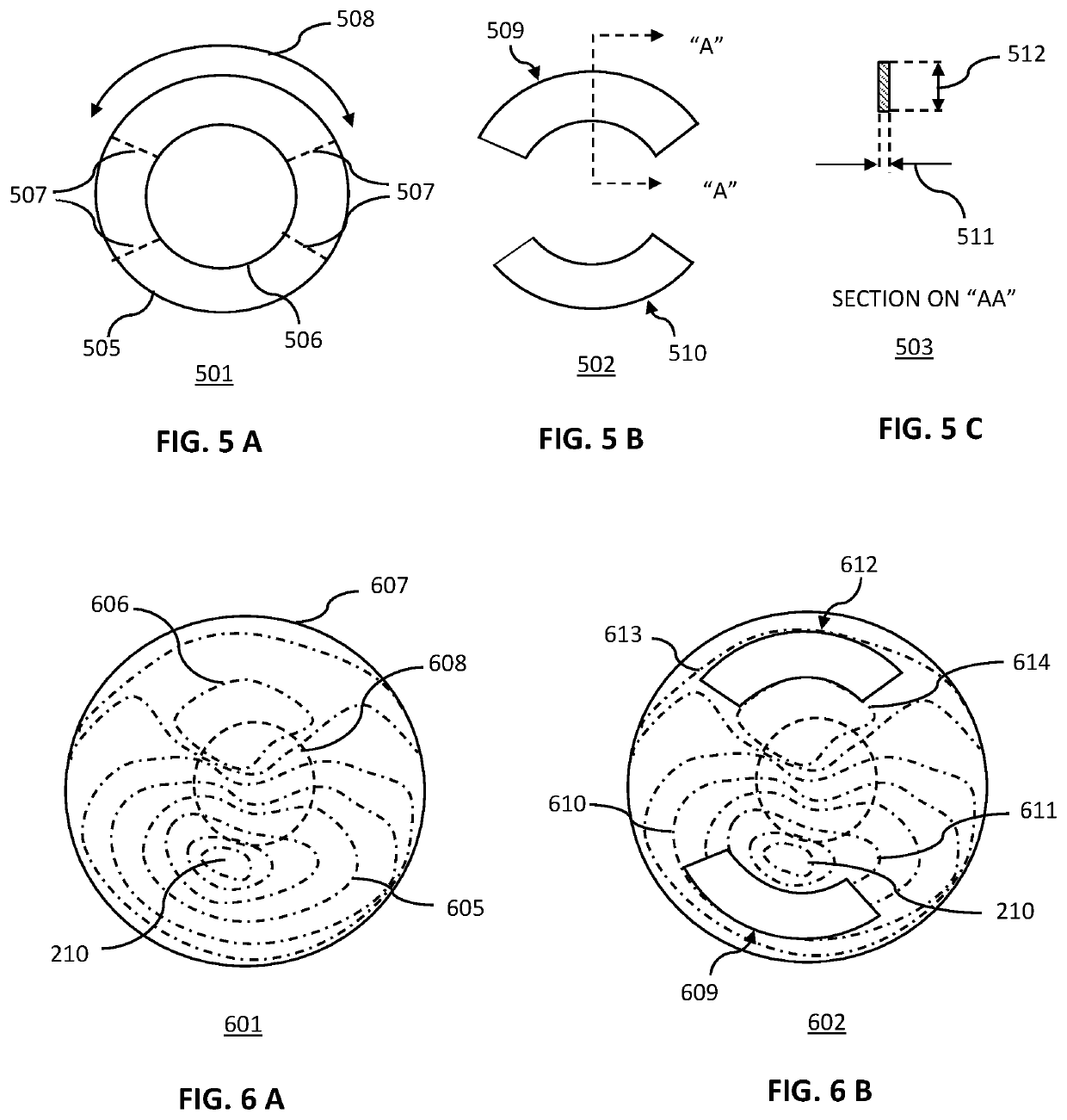
Method for expressing corneal irregularity structure changes based on change consistency parameters of anterior segment tomography technology
ActiveCN110717884AImprove diagnostic capabilitiesReduce complexityImage enhancementReconstruction from projectionDiseaseCorneal disease
The invention discloses a method for expressing corneal irregularity structure changes based on change consistency parameters of an anterior segment tomography technology. According to the method, a method for establishing objective indexes of parameter change relative positions is provided, the positions, where morphological changes may occur, of the irregular corneas are comprehensively expressed, the early diagnosis performance of irregular corneal diseases such as the conic corneas can be theoretically improved, and meanwhile the complexity of combined interpretation of a large number of parameters in clinical actual work is reduced.
Owner:WENZHOU MEDICAL UNIV
Amelioration of cataracts, macular degeneration and other ophthalmic diseases
InactiveUS7825134B2Halts development of cataractsInhibit progressBiocidePharmaceutical delivery mechanismUveitisDisease
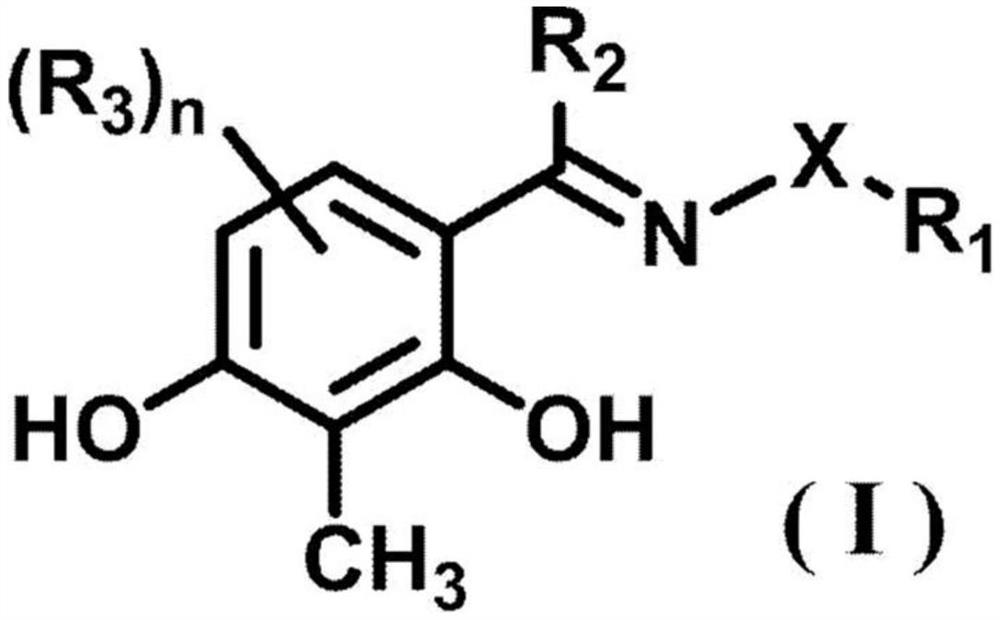
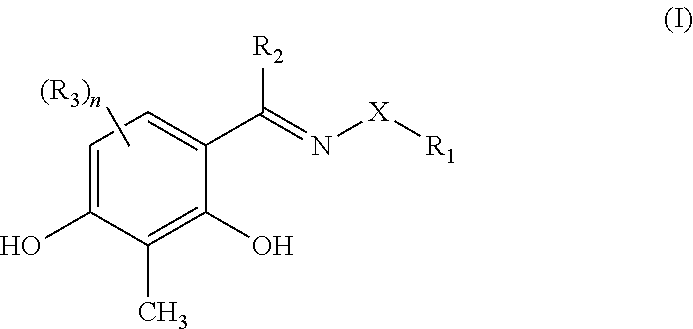
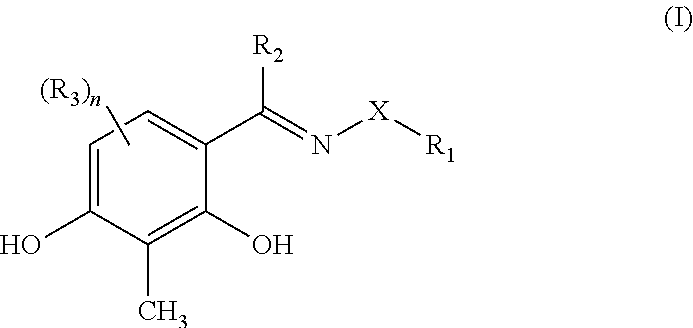
Ophthalmically acceptable compositions used in arresting the development of macular degeneration, retinopathy, glaucoma, eyelid disorders, corneal disease, or uveitis are disclosed. The compositions comprise a pharmaceutically acceptable carrier or diluent and at least one compound having the formula:wherein R1, R2, R3, R4, R5, R6, and R7 are as defined herein.
Owner:COLBY PHARMA CO
Novel peptides and medicinal uses thereof
InactiveUS20050009752A1Easy curingUseful in therapyBiocideSenses disorderDiseaseInsulin-like growth factor
It is an object of the invention to examine the minimum unit of the exhibition of the activity of insulin-like growth factor-I and find a pharmaceutical use thereof in the fields of ophthalmology and dermatology. A joint administration of a peptide containing the amino acid sequence represented by Ser-Ser-Ser-Arg as the minimum unit of the exhibition of the activity of insulin-like growth factor-I and a peptide containing the amino acid sequence represented by Phe-Gly-Leu-Met-NH2 is effective for curing corneal disorders and can significantly promote the healing of skin wounds.
Owner:NISHIDA TERUO +1
Medical cornea paster and its preparation method
InactiveCN1634610AHas the function of treating corneal diseasesSenses disorderEye implantsCornea diseasesDisease
A medical cornea patch and its preparing method are disclosed, which implants autogenous mesenchyma stem cell to amnion for being carrier to treat cornea disease. The process includes: obtaining bone marrow 10ml from healthy person in clinical practice, diluting by PBS in ratio of 1:1, adding 5ml of Ficoll, centrifuging for 20 minutes, sucking out the nebulous single nuclei cell suspension, washing three times by PBS, implanting to 10% FBS containing culture medium of a-MEM, fetching 2í‡105 cell of the fourth generation to treated amnion with size of 1.5cmí‡1.5cm after 10 to 14 days, adding to culture medium to continue culturing, till the cell grow fully of the whole amnion.
Owner:北京科宇联合干细胞生物技术有限公司
Therapeutic agent for corneal sensory nerve damage containing semaphorin inhibitor as active ingredient
ActiveCN103379906APrevention of sensory neuropathySenses disorderNervous disorderCorneal diseaseHydrogen atom
A compound represented by formula (1) (wherein R1 represents a hydrogen atom or a carboxyl group, R2 represents a hydrogen atom or a hydroxy group, R3 represents a hydrogen atom or a carboxyl group, and R4 represents a hydrogen atom or a hydroxy group) or a pharmaceutically acceptable salt thereof is effective as a therapeutic agent or a prophylactic agent for a corneal disease or sensory nerve damage due to corneal surgery, and as a regeneration accelerator for corneal sensory nerves.
Owner:KEIO UNIV +1
Substituted tricyclic pyrazole derivatives with protein kinase activity
Chemical compounds that are derivatives of 2H[1]-benzothiepino[5,4-c]pyrazole, 2H[1]-benzoxepino[5,4-c]pyrazole, 1-benzothiopyrano[4,3-c]pyrazole, 1-benzopyrano[4,3-c]pyrazole, 4,5-dihydro-2h-furano[2,3-g]indazole and 4,5-dihydro-2h-thieno[2,3-g]indazole derivatives 2H[1]-benzothiepino[5,4-c]pyrazole, [1]-benzothiopyrano[4,3-c]pyrazole, 4,5-dihydro-2H-furano[2,3-g]indazole and 4,5-dihydro-2H-thieno[2,3-g]indazole derivatives are inhibitors of protein kinase activity. Several of the protein kinases, whose activity is inhibited by these chemical compounds, are involved in angiogenic and / or edematous processes. Thus, these chemical compounds can ameliorate disease states where angiogenesis, edema or endothelial cell hyperproliferation or hyperpermeability is a factor. Like cancer, artritis, atherosclerosis, psoriasis, hemangioma, myocardial angiogenesis, coronary and cerebral collaterals, ischemic limb angiogenesis, corneal disease, rubeosis, neovascular glaucoma, macular degeneration, wound healing, peptic ulcer Helicobacter related diseases, fractures, diabetic retinopathy, and cat scratch fever.
Owner:ABBOTTGMBH & CO
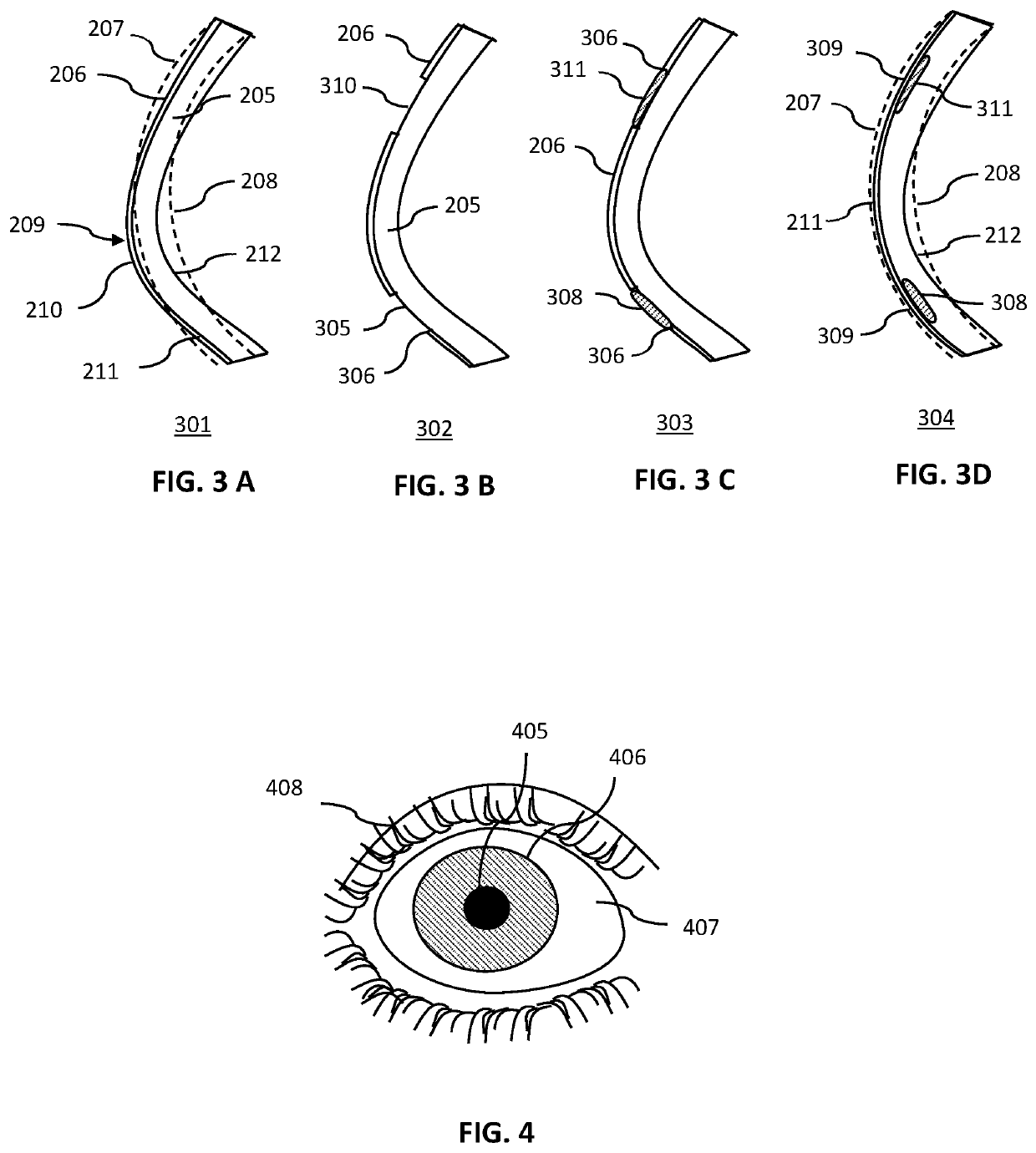
Method for the amelioration of ectatic and irregular corneal disorders
Methods for the amelioration of ectatic corneal disorders using corneal augmentations are disclosed. The shape of the augmentation is determined using data obtained from mapping of a patient's cornea based on computerized corneal topography and tomography. Factors considered include the maximum keratometry and specific iso-deviation contours. In one embodiment, an augmentation is inlayed into a femtosecond created, intrastromal pocket. In a further embodiment, an onlay augmentation is positioned over a region of the cornea from which the epithelial layer has been removed. The onlay is held in place by glue, sutures, tucking under a perimeter chamfer, or some combination thereof, until the epithelial layer regrows over the augmentation. In a further embodiment, the inlay or only augmentation is followed by a post-augmentation, further reshaping of the corneal augmentation. In one embodiment, this further reshaping is photorefractive keratectomy (PRK) surgery. In another and a phototherapeutic keratectomy (PTK) surgery.
Owner:CTAK LLC
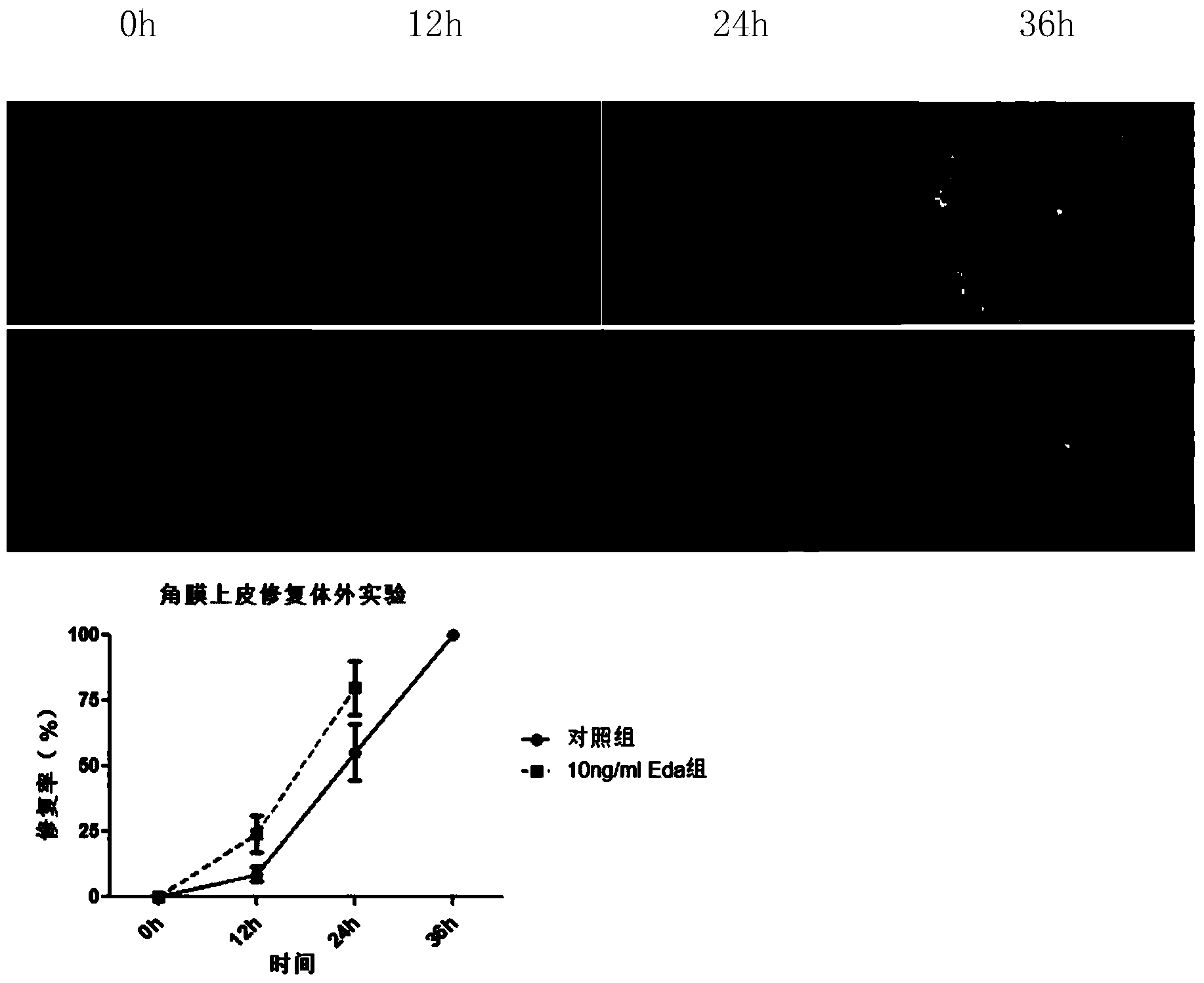
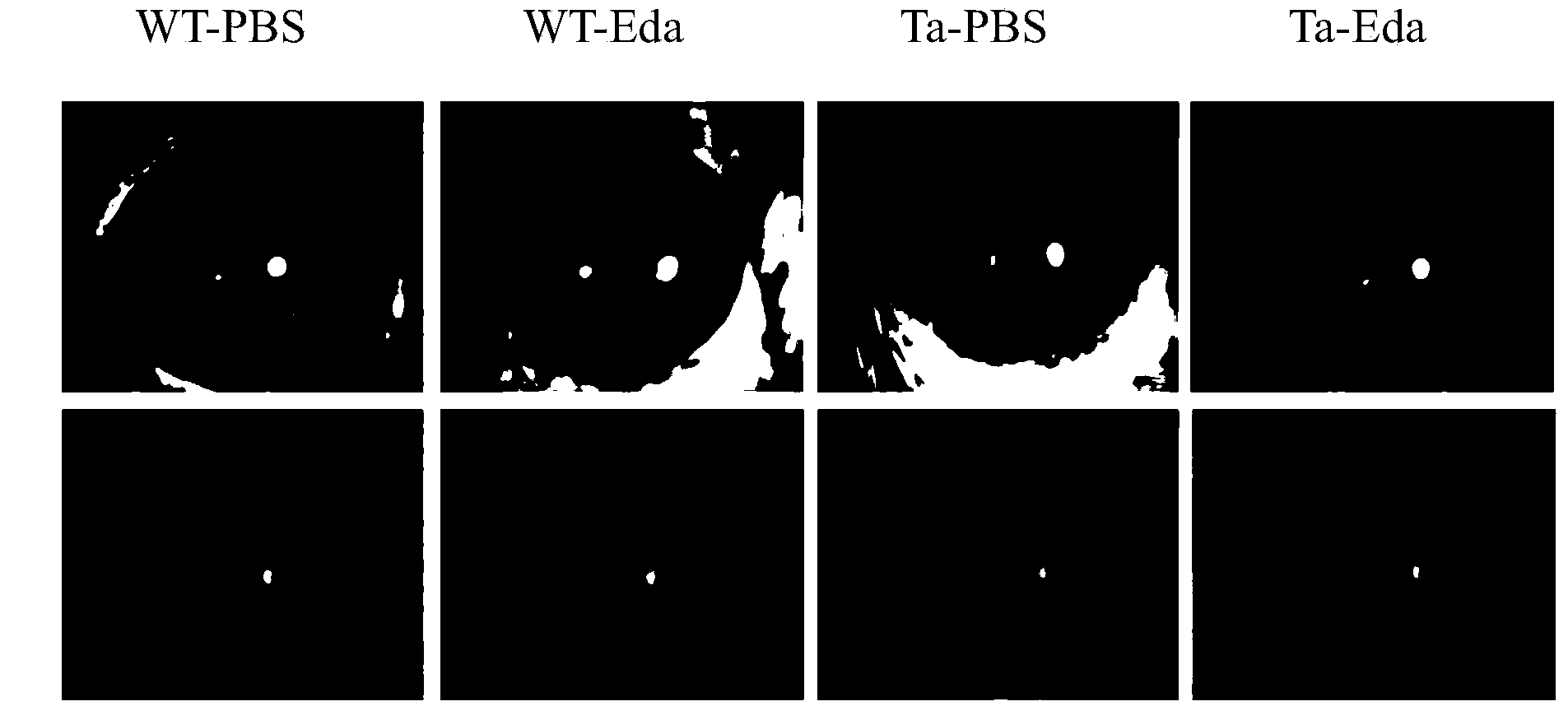
Ectodysplasin (Eda)-containing medicine for treating ocular surface diseases and corneal diseases
InactiveCN103961689ANo toxicityPromote epithelial repairSenses disorderPeptide/protein ingredientsDiseaseCorneal disease
The invention discloses an ectodysplasin (Eda)-containing medicine for treating ocular surface diseases and corneal diseases. An effective component of the ectodysplasin-containing medicine is ectodysplasin with the concentration of 1ng / mL-1mg / mL, and the ectodysplasin is natural ectodysplasin and / or recombinant ectodysplasin. The ectodysplasin (Eda)-containing medicine has the advantages that the ectodysplasin is used as the effective component, and therefore, the ectodysplasin-containing medicine has no toxicity on corneas and has the effects of promoting corneal epithelium restoration and treating the ocular surface diseases caused by Eda gene mutation.
Owner:XIAMEN UNIV
Stem cell regenerating surface cornea and its application in corneal transplantation
InactiveCN1218755CGuaranteed long-term effectivenessRich sourcesEye implantsArtificial cell constructsDiseaseCorneal disease
Owner:北京科宇联合干细胞生物技术有限公司
Medical cornea paster and its preparation method
InactiveCN100336567CHas the function of treating corneal diseasesSenses disorderEye implantsFicollCorneal disease
A medical cornea patch and its preparing method are disclosed, which implants autogenous mesenchyma stem cell to amnion for being carrier to treat cornea disease. The process includes: obtaining bone marrow 10ml from healthy person in clinical practice, diluting by PBS in ratio of 1:1, adding 5ml of Ficoll, centrifuging for 20 minutes, sucking out the nebulous single nuclei cell suspension, washing three times by PBS, implanting to 10% FBS containing culture medium of a-MEM, fetching 2X105 cell of the fourth generation to treated amnion with size of 1.5cmX1.5cm after 10 to 14 days, adding to culture medium to continue culturing, till the cell grow fully of the whole amnion.
Owner:北京科宇联合干细胞生物技术有限公司
Therapeutic agent for corneal disease
A therapeutic agent for a corneal disease comprising irsogladine or a salt thereof as an active ingredient. The purpose is to find a substance capable of effectively treating / ameliorating a corneal disease which has been increased in the number of cases thereof in recent years and to provide a therapeutic agent for a corneal disease comprising the substance as an active ingredient.
Owner:TEIKA PHARMA CO LTD
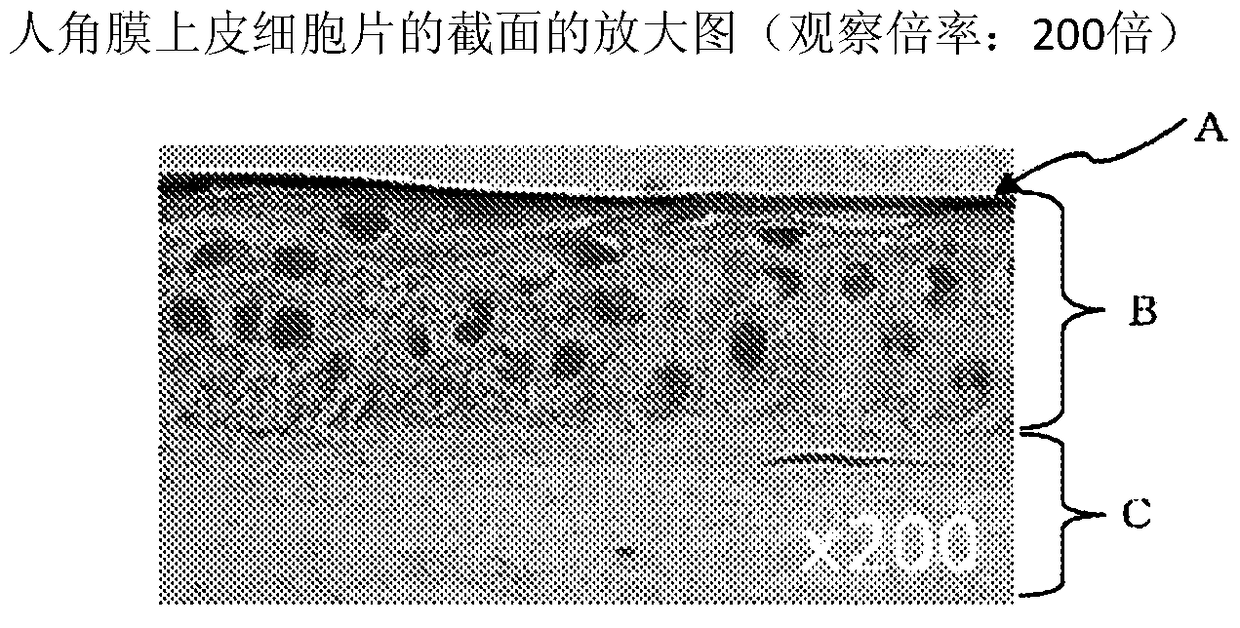
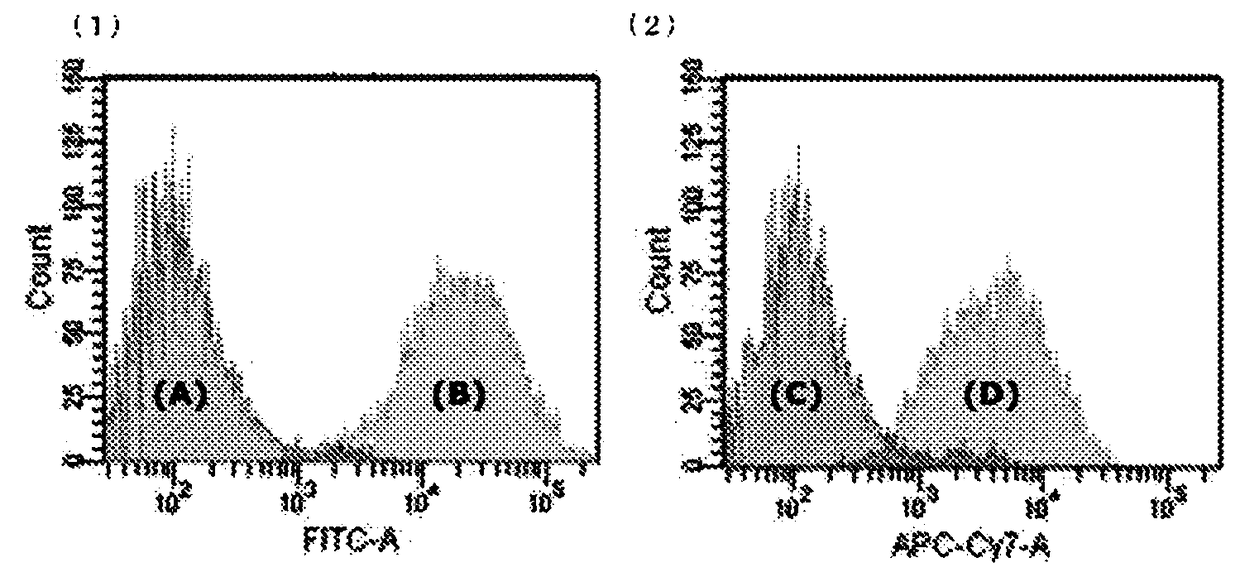
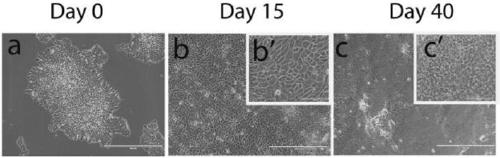
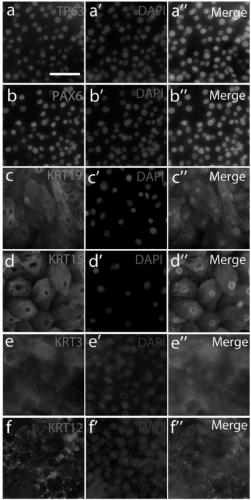
Production method of human corneal epithelial sheet
ActiveCN105308175BImprove qualityOvercome deficienciesSenses disorderNervous system cellsPhosphodiesteraseCorneal disease
Subject: To provide a method for producing human corneal epithelial sheets for transplantation to patients with corneal diseases, and for culturing cells derived from human corneal epithelium obtained by culturing human corneal epithelial cells on stromal amniotic membrane. Solution: culture method of human corneal epithelial cells using human mesenchymal stem cells as supporting cells, and using various combinations of ROCK inhibitors, phosphodiesterase inhibitors, MAP kinase inhibitors, and TGF‑β receptor inhibitors culture medium of human corneal epithelial cells.
Owner:JCR PHARMA +1
Composition for Treating Corneal Diseases or Conjunctival Diseases
ActiveUS20180264064A1Effective preventionEffective treatmentCosmetic preparationsSenses disorderDiseaseCorneal disease
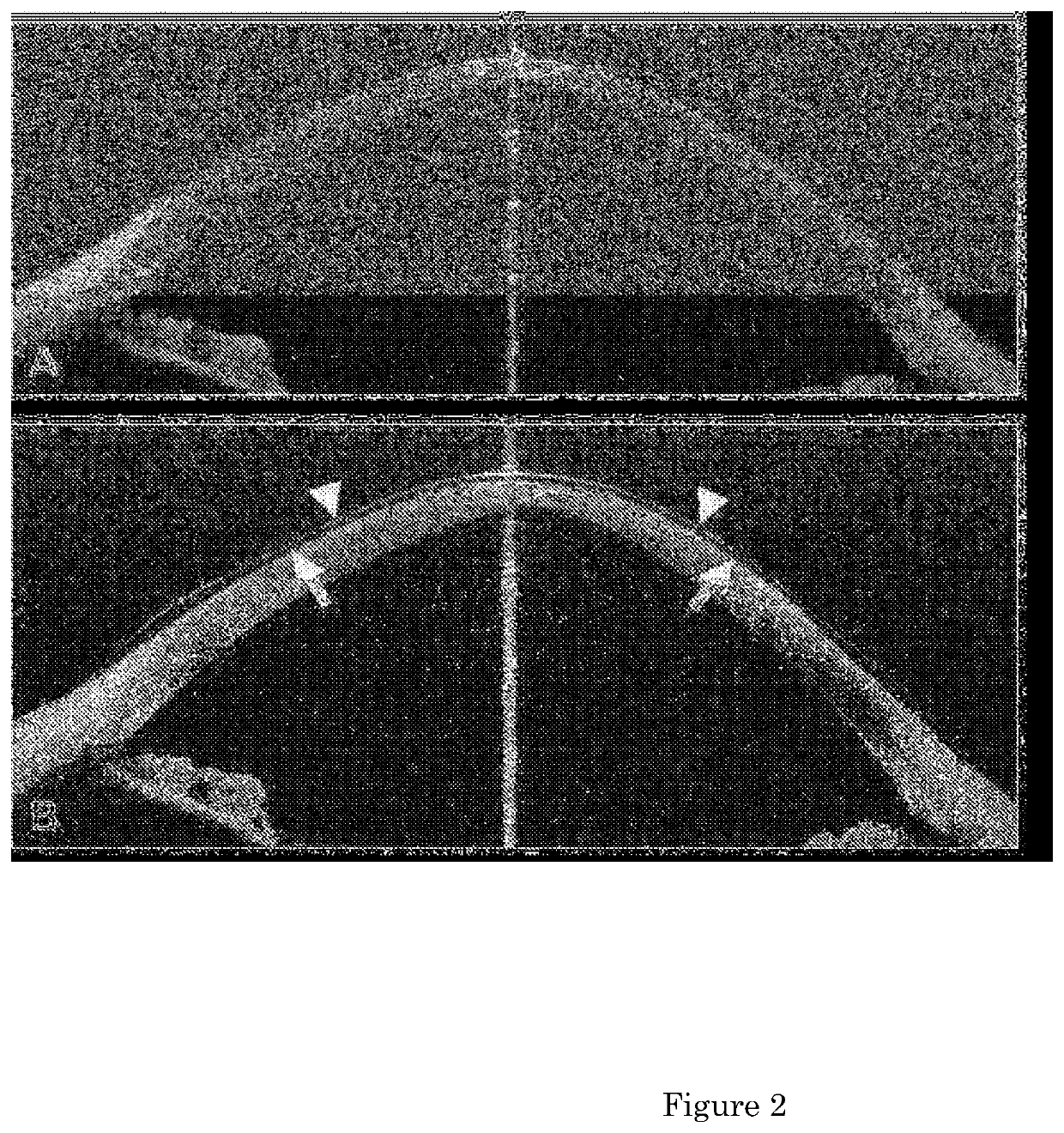
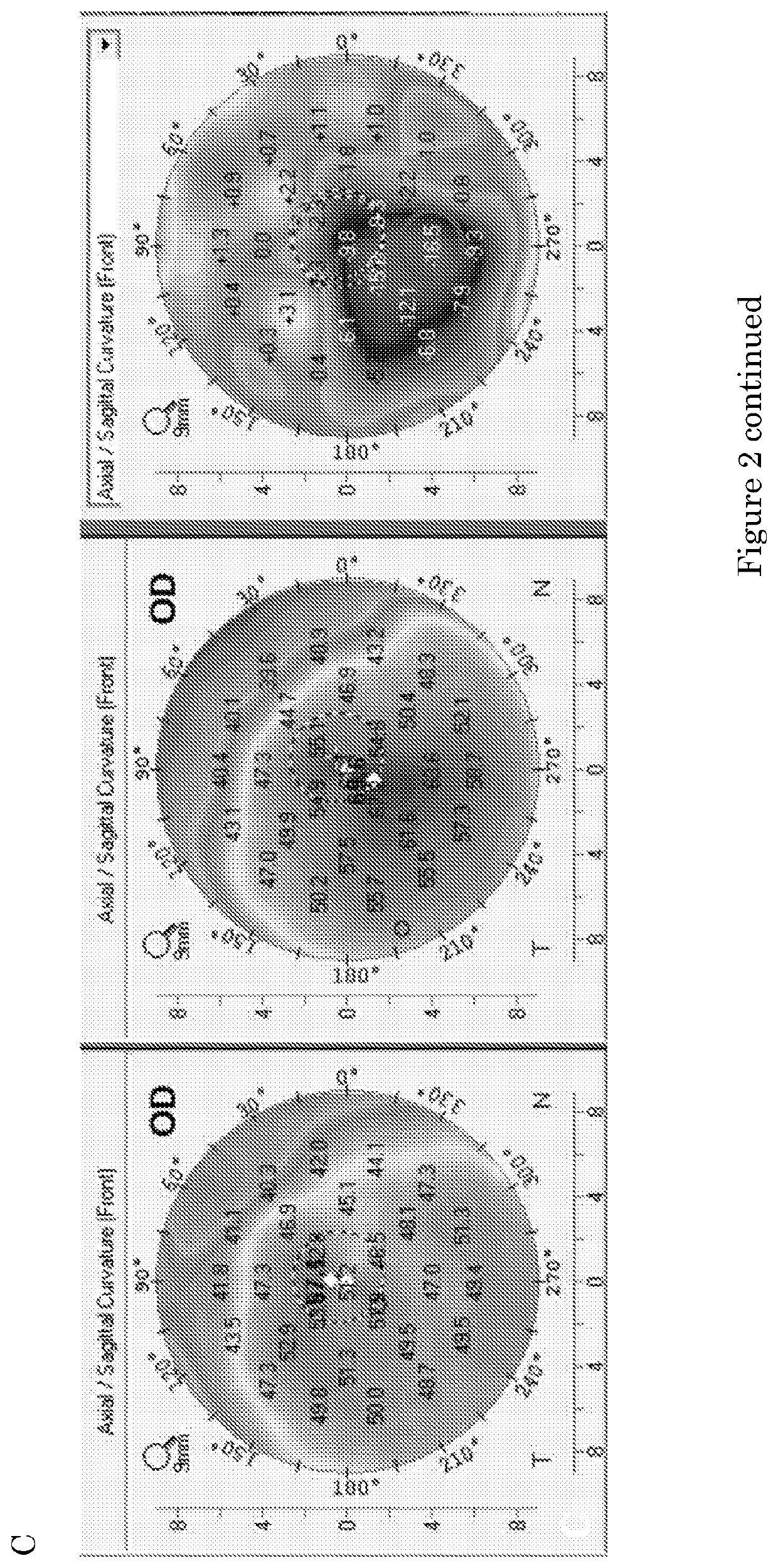
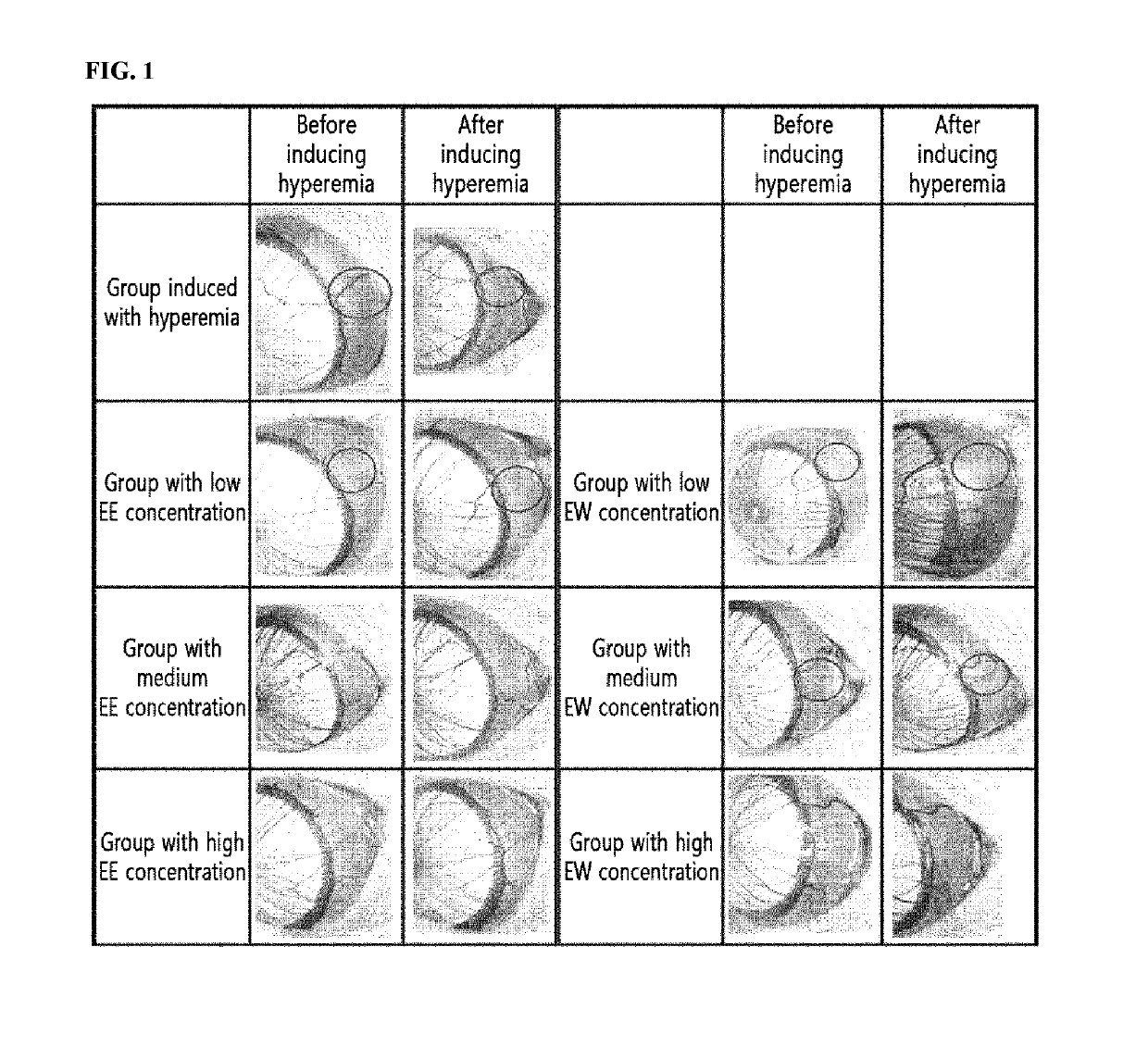
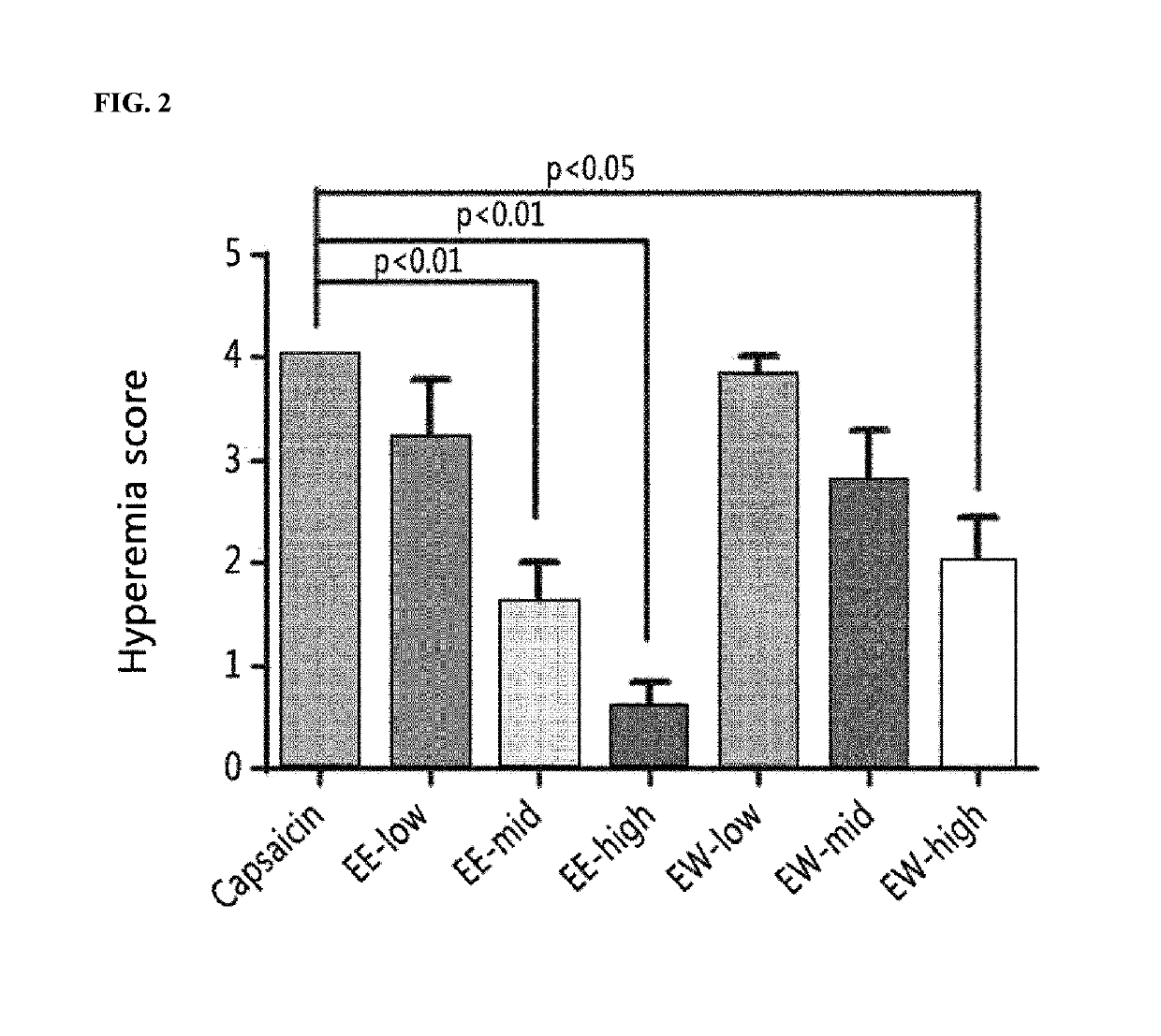
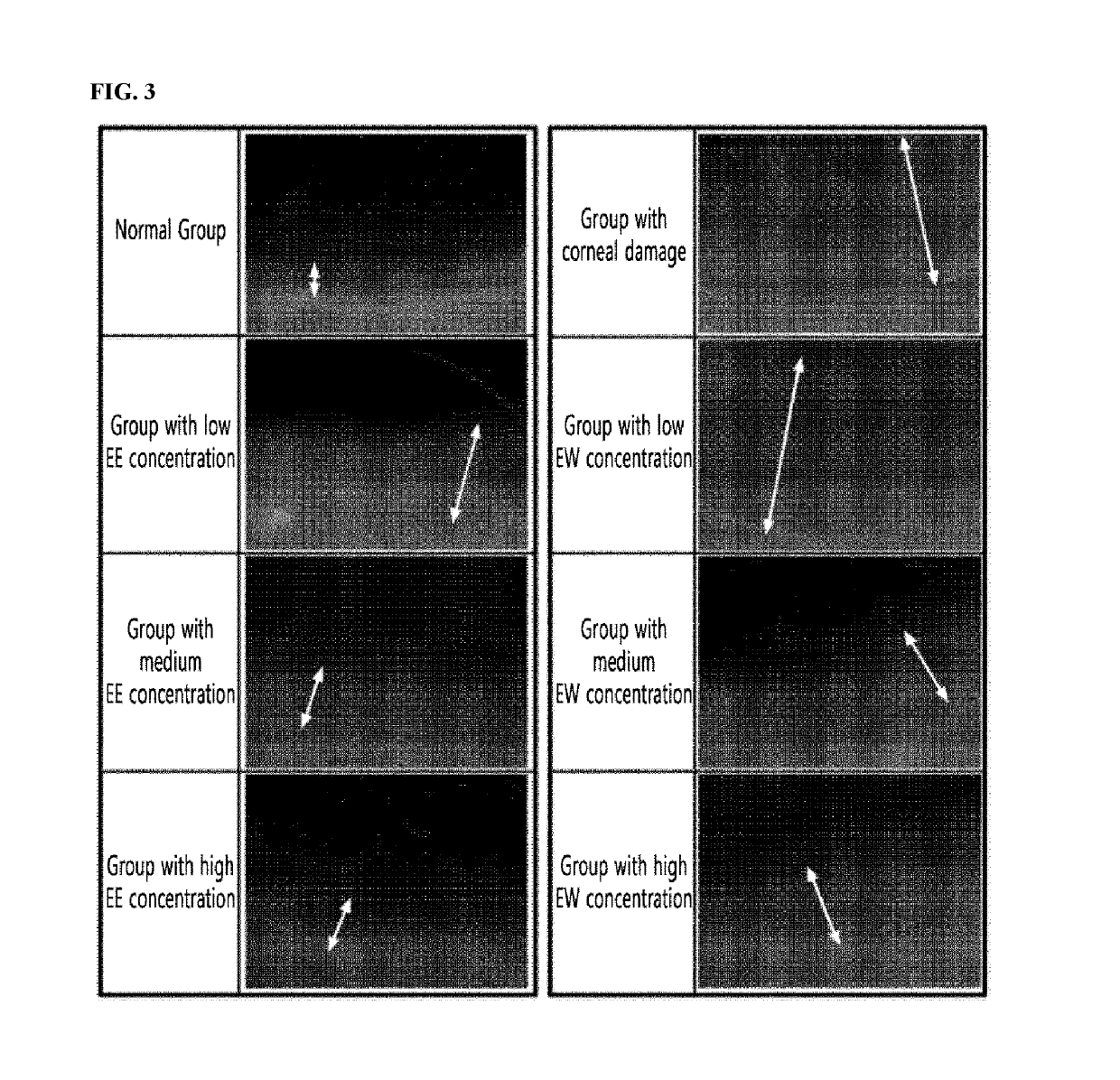
The present invention relates to a pharmaceutical composition for preventing and treating corneal diseases or conjunctival diseases, containing a maple leaf extract as an active ingredient. The maple leaf extract exhibits an effect of inhibiting hyperemia in the eyeball in which hyperemia has been induced and an effect of inhibiting angiogenesis in the eyeball in which corneal damage has been induced, thus being effectively used in a pharmaceutical composition for preventing and treating corneal diseases or conjunctival diseases.
Owner:KOREA INST OF ORIENTAL MEDICINE
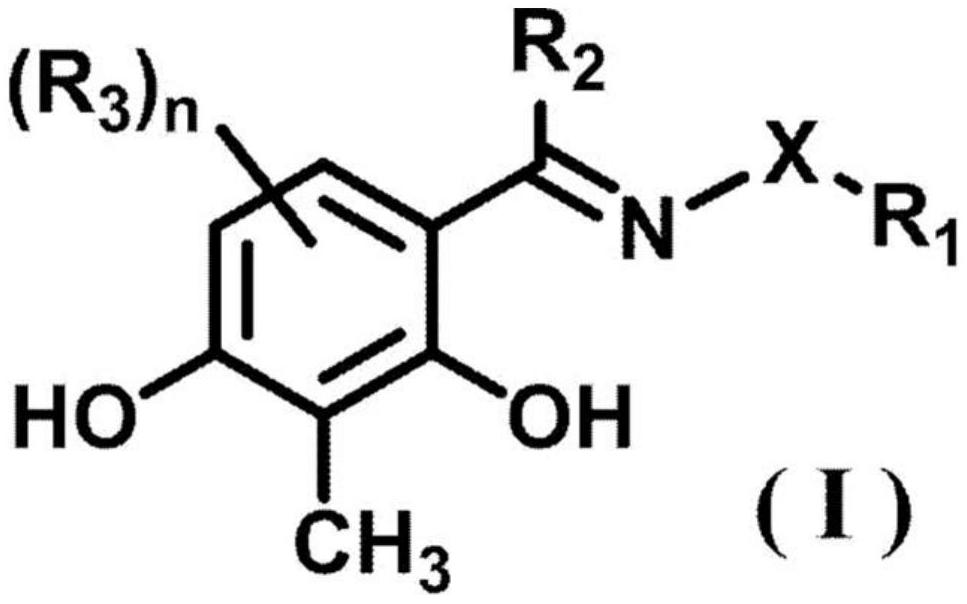
Therapeutic agent for corneal diseases
PendingCN113557059APromote proliferationEfficient preparationOrganic active ingredientsSenses disorderDiseaseCorneal disease
Owner:NISSAN CHEM IND LTD
Remedy for corneal diseases
Owner:TEIKA PHARMA CO LTD
Screening method for transparency of membranous heterogeneous biomaterials and acellular dermal matrix
ActiveCN109324001BHigh transparencySolve the contradiction of shortageColor/spectral properties measurementsTissue regenerationDiseaseCorneal disease
The invention discloses a method for screening the transparency of a film-shaped heterogeneous biological material, which comprises the steps of dehydration of the biological material sucrose, OCT embedding, layer-by-layer frozen sectioning, and light transmittance measurement with a spectrophotometer. Also provided is a highly transparent decellularized dermal matrix obtained by the above method. Through the present invention, the acellular dermal matrix with the best transparency can be effectively screened out, which can be used for lamellar keratoplasty, and the surgeon can use the above-mentioned matrix to replace the existing lamellar corneal stroma during the operation. At the same time, it can also effectively solve the contradiction of the current shortage of donor corneas.
Owner:PEKING UNIV THIRD HOSPITAL
Corneal epithelial cells and corneal stent, and preparation methods and applications thereof
ActiveCN110468094ARapid ProliferationLow immunogenicitySenses disorderEpidermal cells/skin cellsDiseaseCorneal disease
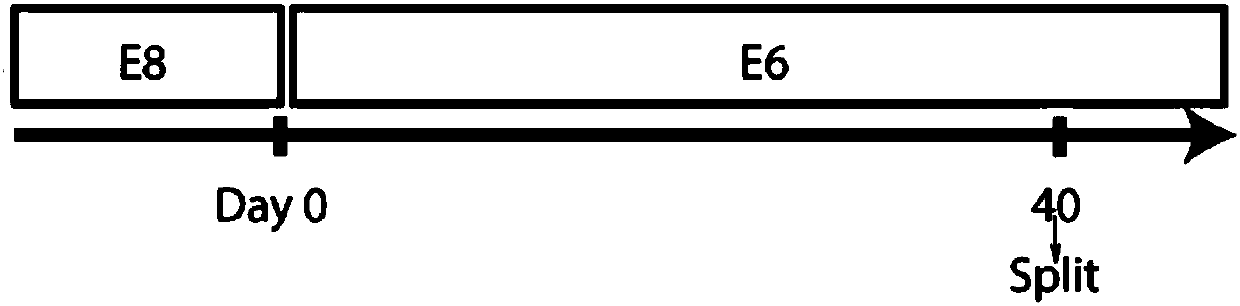
The invention discloses corneal epithelial cells and a corneal stent, and preparation methods and applications thereof, and relates to the field of medicines for corneal diseases. According to a preparation method of the corneal epithelial cells provided by the present invention, human embryonic stem cells are cultured in an E6 medium by inoculation, so that the human embryonic stem cells are differentiated into corneal epithelial cells. By the method, the corneal epithelial cells with rapid proliferation ability can be continuously provided, and can be used for preparing the corneal stent orfor preparing medicines for treating corneal diseases.
Owner:UNIVERSITY OF MACAU
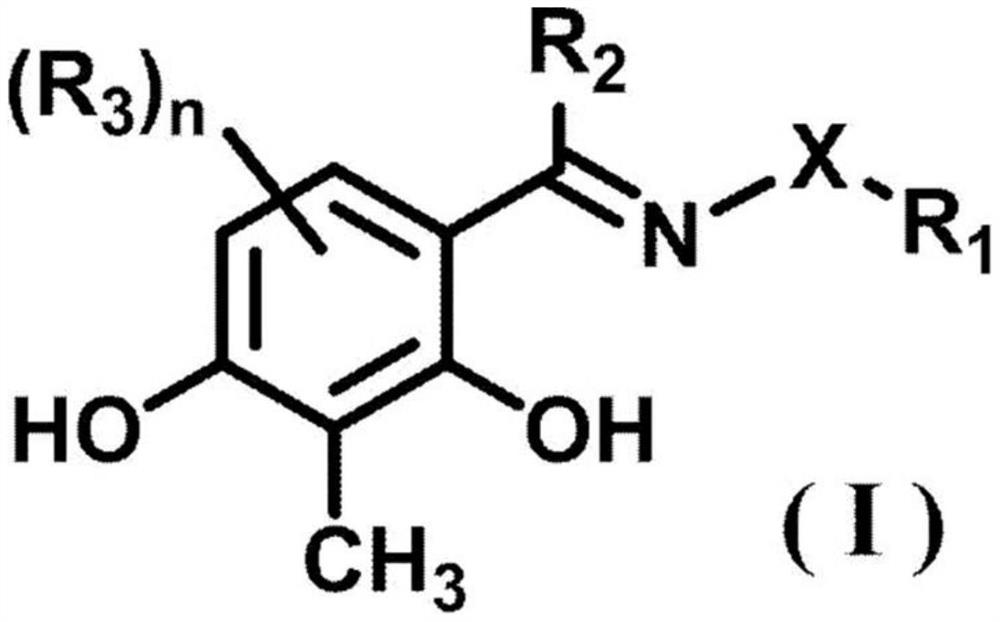
Therapeutic agent for corneal diseases
PendingUS20220133659A1Facilitated DiffusionEfficient productionOrganic active ingredientsSenses disorderDiseaseCorneal disease
Owner:NISSAN CHEM IND LTD
Cell Composition, Method of Production and its Use in Corneal Diseases
PendingUS20210093674A1Enhanced corneal clarityEnhanced visual recoverySenses disorderPeptide/protein ingredientsDiseaseCorneal disease
The present invention provides cell compositions, methods of production and its uses in corneal diseases. The invention discloses cell compositions and multilayer cell compositions comprising limbal epithelial cells and limbal stromal cells. The inventions are highly efficacious and represents an advancement over the existing therapeutic approaches in treatment or prevention of corneal diseases. The invention also discloses methods for preparing the compositions, methods of treatment and the uses of the composition in preventing and treating corneal diseases.
Owner:HYDERABAD EYE RES FOUND
Remedy for corneal diseases
Owner:TEIKA PHARMA CO LTD
Multifunctional corneal graft piece
The invention discloses a multifunctional corneal graft piece, which contains releasable drug factors or biological factors with the same or different functions in the corneal graft piece body in a manner of blocking, layering, dividing regions or dividing different chambers, different regions / positions of the corneal graft piece can carry different kinds of drugs to prevent and treat multiple corneal diseases, and different or synergistic prevention and treatment effects can be realized in different regions / positions of the same corneal graft piece. The corneal graft piece achieves versatility by carrying multiple factors or proteins in different regions / positions of the corneal graft piece. By the arrangement of corresponding parts of the corneal graft piece, fixed-point quantitative drug factor release can be realized to prevent and treat inflammation. The multifunctional corneal graft piece has multiple structures, is easy to process, has good biocompatibility, and can well play arole in preventing and treating diseases.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
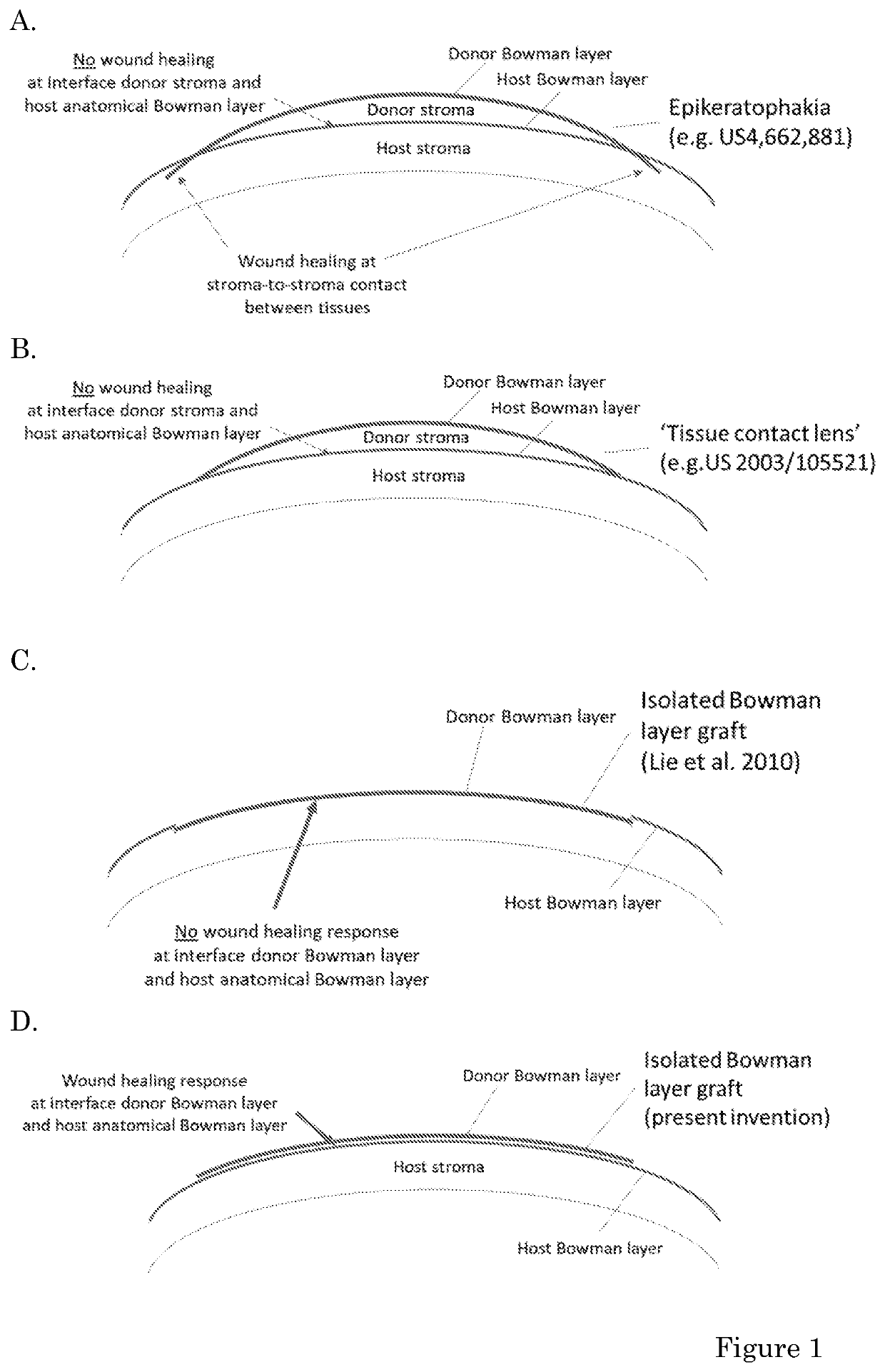
Donor overlay for treatment or alleviation of anterior corneal disorders
The invention relates to methods for the treatment or alleviation of an anterior corneal disorder in a subject in need thereof comprising removing corneal epithelial cells from an eye of said subject without removing any corneal tissue or other ocular tissue located posterior to the corneal epithelial cell layer; and positioning an overlay comprising a Bowman layer (BL), Descemet membrane (DM) and / or crystalline lens capsule on the anterior surface of said corneal tissue located posterior to the corneal epithelial cell layer. The invention further relates to freeze-dried and / or gamma irradiated Bowman layer, Descemet membrane and / or crystalline lens capsule and compositions comprising the same that are useful in such methods.
Owner:NIIOS USA INC
Composition for treating corneal diseases or conjunctival diseases
ActiveUS10357528B2Effective preventionEffective treatmentCosmetic preparationsSenses disorderDiseaseCorneal disease
The present invention relates to a pharmaceutical composition for preventing and treating corneal diseases or conjunctival diseases, containing a maple leaf extract as an active ingredient. The maple leaf extract exhibits an effect of inhibiting hyperemia in the eyeball in which hyperemia has been induced and an effect of inhibiting angiogenesis in the eyeball in which corneal damage has been induced, thus being effectively used in a pharmaceutical composition for preventing and treating corneal diseases or conjunctival diseases.
Owner:KOREA INST OF ORIENTAL MEDICINE
Ophthalmic composition as well as preparation method and application thereof
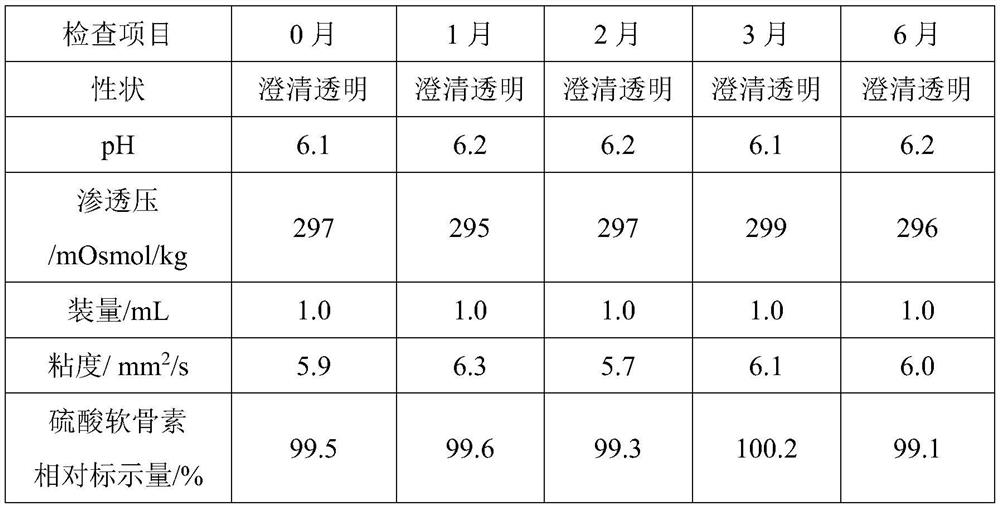
ActiveCN112263545ALittle side effectsAlleviate irritation symptomsOrganic active ingredientsSenses disorderCorneal diseaseEye irritation
The invention relates to an ophthalmic composition which comprises chondroitin sulfate and olopatadine hydrochloride. The invention further relates to a preparation method of the ophthalmic composition. The preparation method comprises the following steps: S1, mixing a pH regulator, an osmotic pressure regulator and injection water to obtain a mixed solution I; S2, homogeneously stirring and mixing chondroitin sulfate, olopatadine hydrochloride and injection water for 10-15 minutes at 3000-4500 r / min to obtain a mixed solution II; and S3, mixing the mixed solution I and the mixed solution II with the injection water, and filtering the mixture to obtain the ophthalmic composition. Furthermore, the invention relates to application of the ophthalmic composition in preparation of medicines forimproving eye stimulation symptoms caused by treatment of corneal diseases. The ophthalmic composition serving as eye drops can be used for effectively relieving eye irritation caused by chondroitinsulfate at conventional dosage for corneal disease treatment, so that the medication compliance of a patient can be improved, and the treatment time of the patient can be effectively shortened.
Owner:湖北远大天天明制药有限公司
Opthalmic compositions comprising viscosifying polymers and nucleic acids
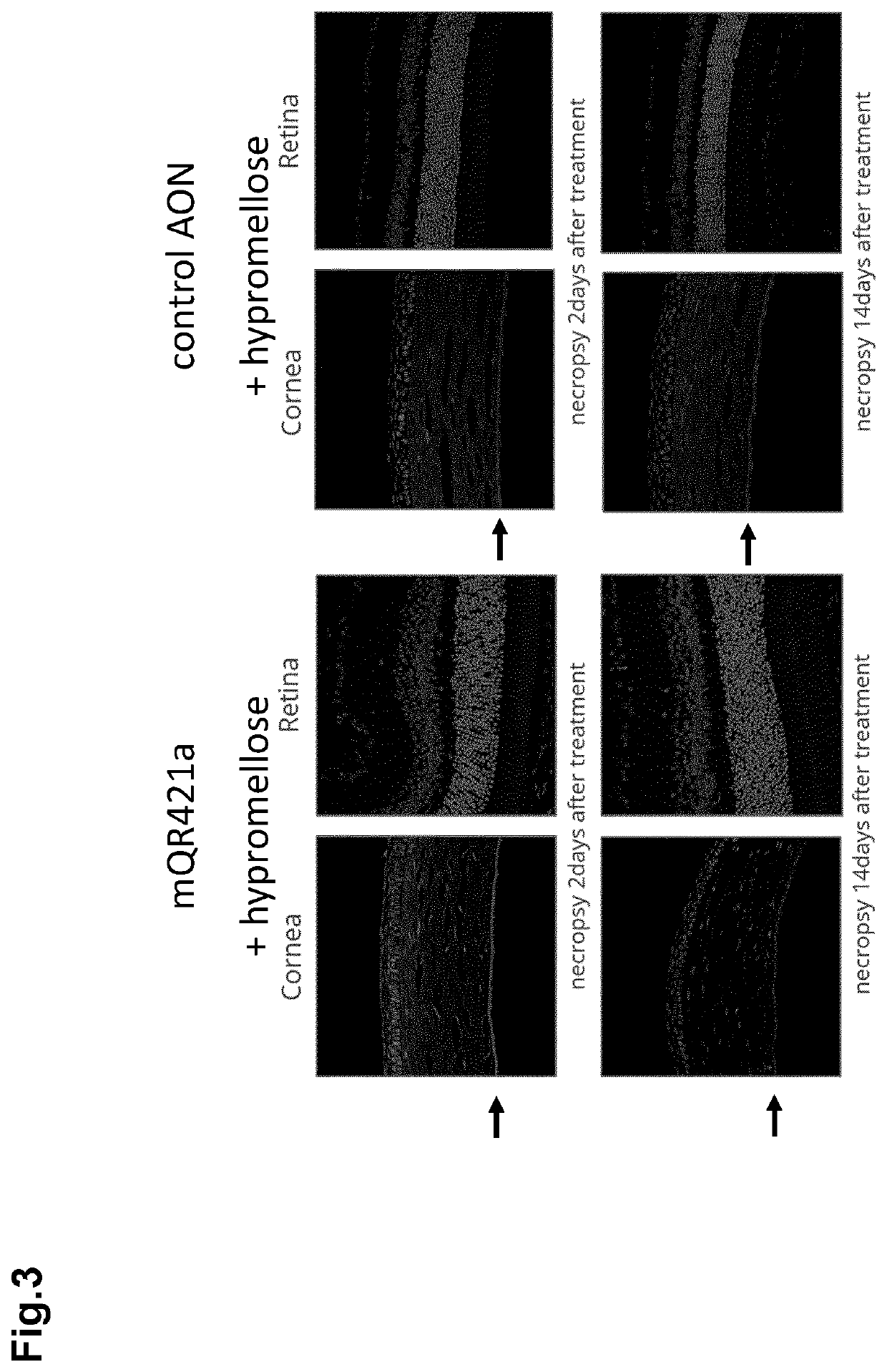
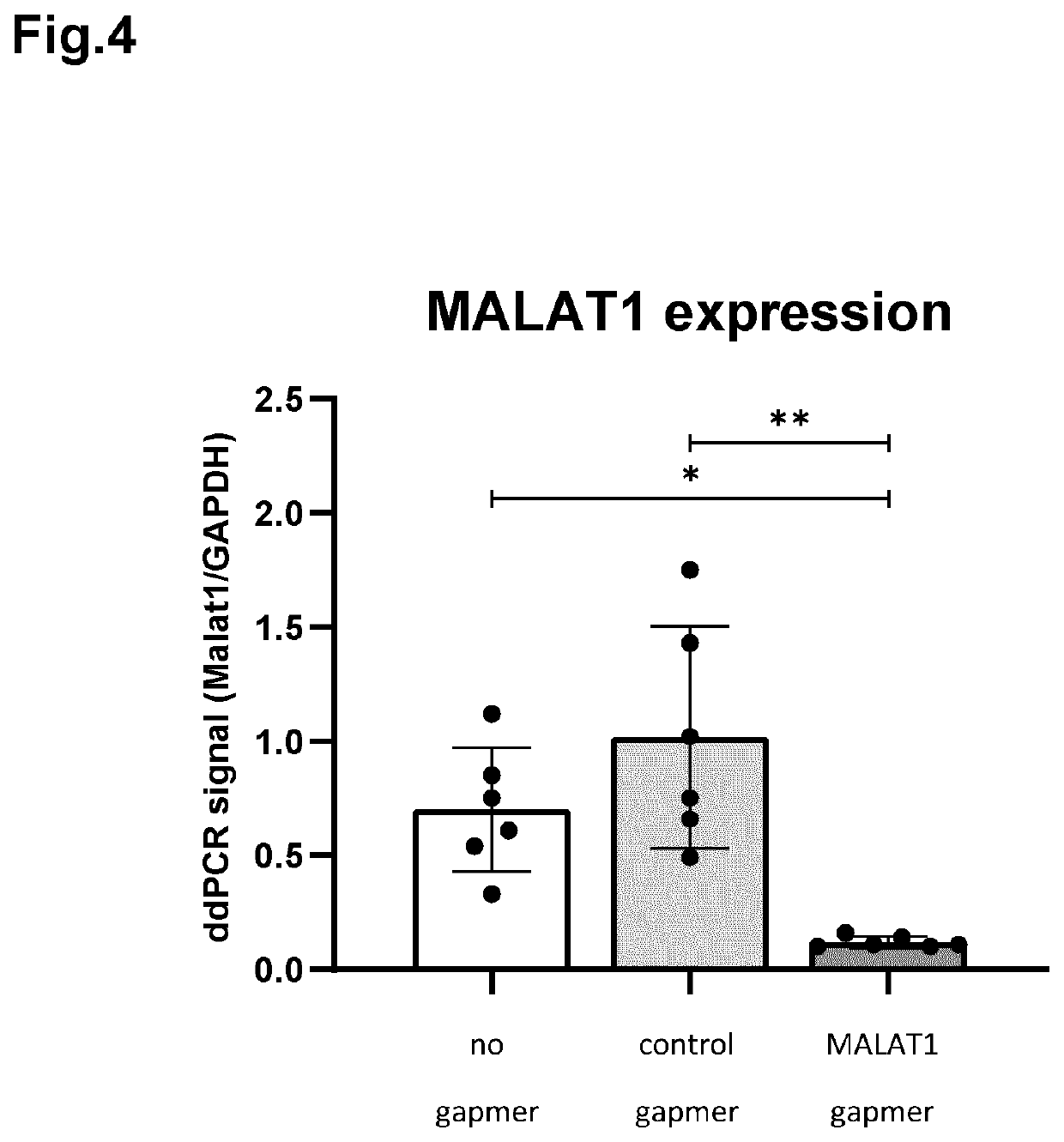
PendingUS20220265695A1Prevents and reduces RNA toxicityPrevents and diminishes formationOrganic active ingredientsOintment deliveryDescemets MembraneCorneal disease
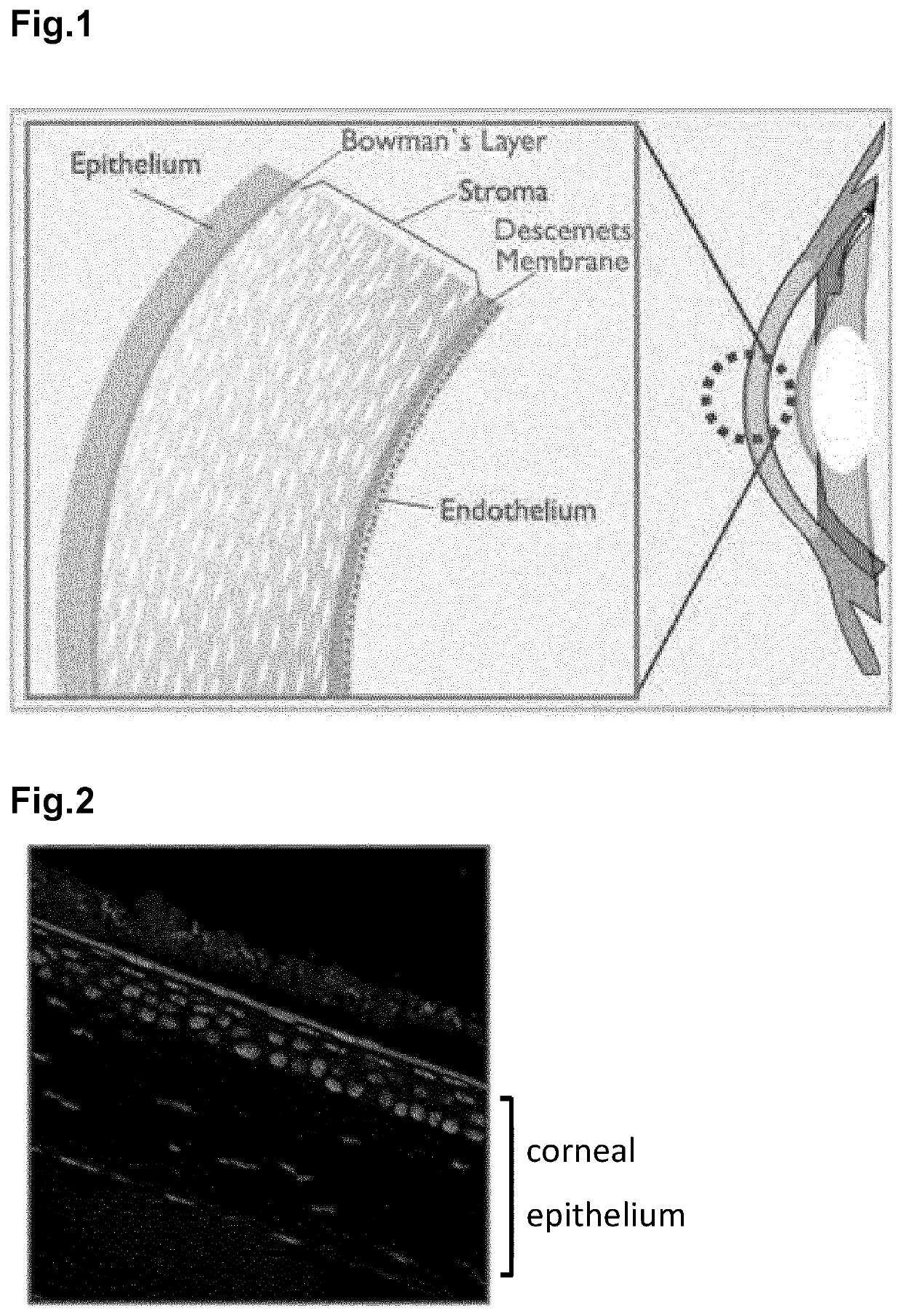
The invention relates to ophthalmic compositions comprising: i) a nucleic acid molecule, preferably an antisense oligonucleotide, such as an single-stranded antisense oligonucleotide that modulates splice modulation or prevention of RNA toxicity due to trinucleotide repeats in a target RNA molecule, or a gapmer that induces breakdown of a target RNA molecule after formation of a double-stranded RNA / gapmer complex; and ii) a viscosifying polymer. The ophthalmic compositions are for topical administration in the eye of a mammalian subject suffering from a corneal disease, such as a hereditary corneal dystrophy. The viscosifying polymer in the compositions of the invention allows the entry of the nucleic acid molecule to the different layers of the cornea: the corneal epithelium, Bowman's membrane, stroma, Dua's layer, the Descemet's membrane and / or the corneal endothelium.
Owner:PROQR THERAPEUTICS II BV
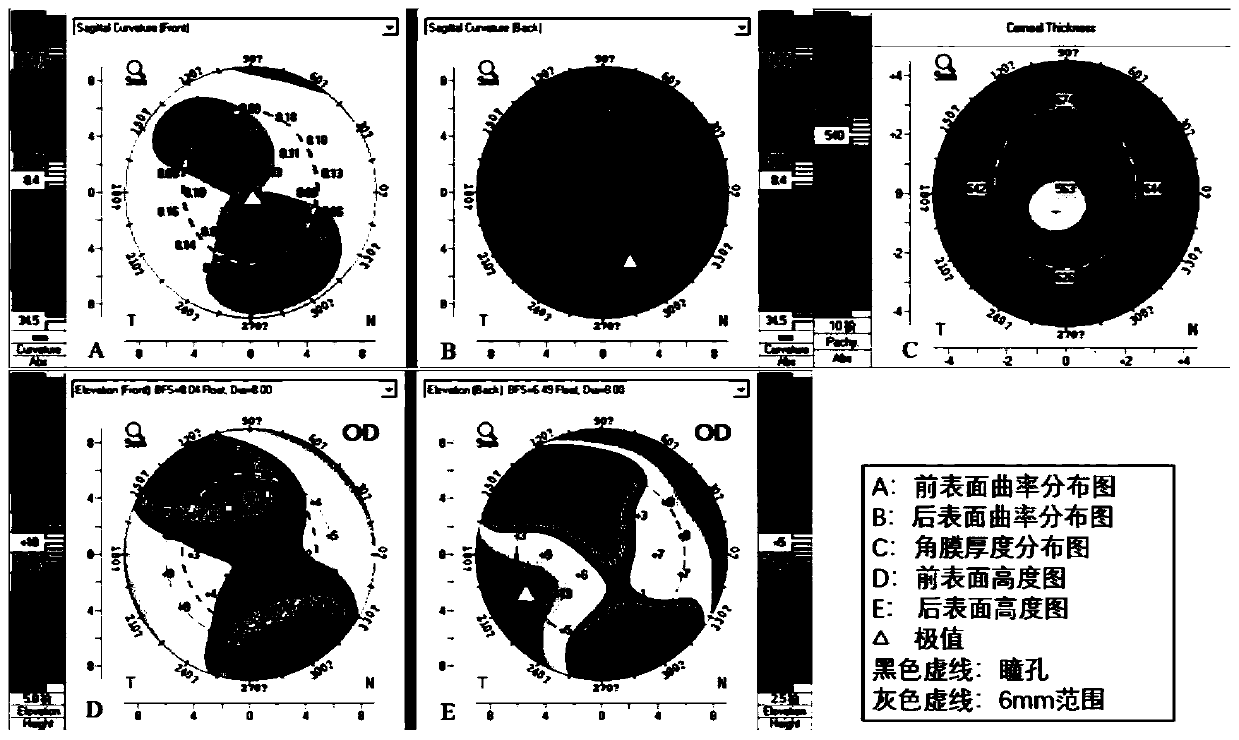
A device for measuring the working distance of a corneal topograph
ActiveCN112932404BHigh restoration accuracyAccurate diagnosisEye diagnosticsTesting optical propertiesCorneal curvatureCorneal disease
Owner:NINGBO MING SING OPTICAL R & D
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com