Patents
Literature
123 results about "Corneal endothelium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The corneal endothelium is a single layer of cells on the inner surface of the cornea. It faces the chamber formed between the cornea and the iris. The corneal endothelium are specialized, flattened, mitochondria-rich cells that line the posterior surface of the cornea and face the anterior chamber of the eye. The corneal endothelium governs fluid and solute transport across the posterior surface of the cornea and maintains the cornea in the slightly dehydrated state that is required for optical transparency.
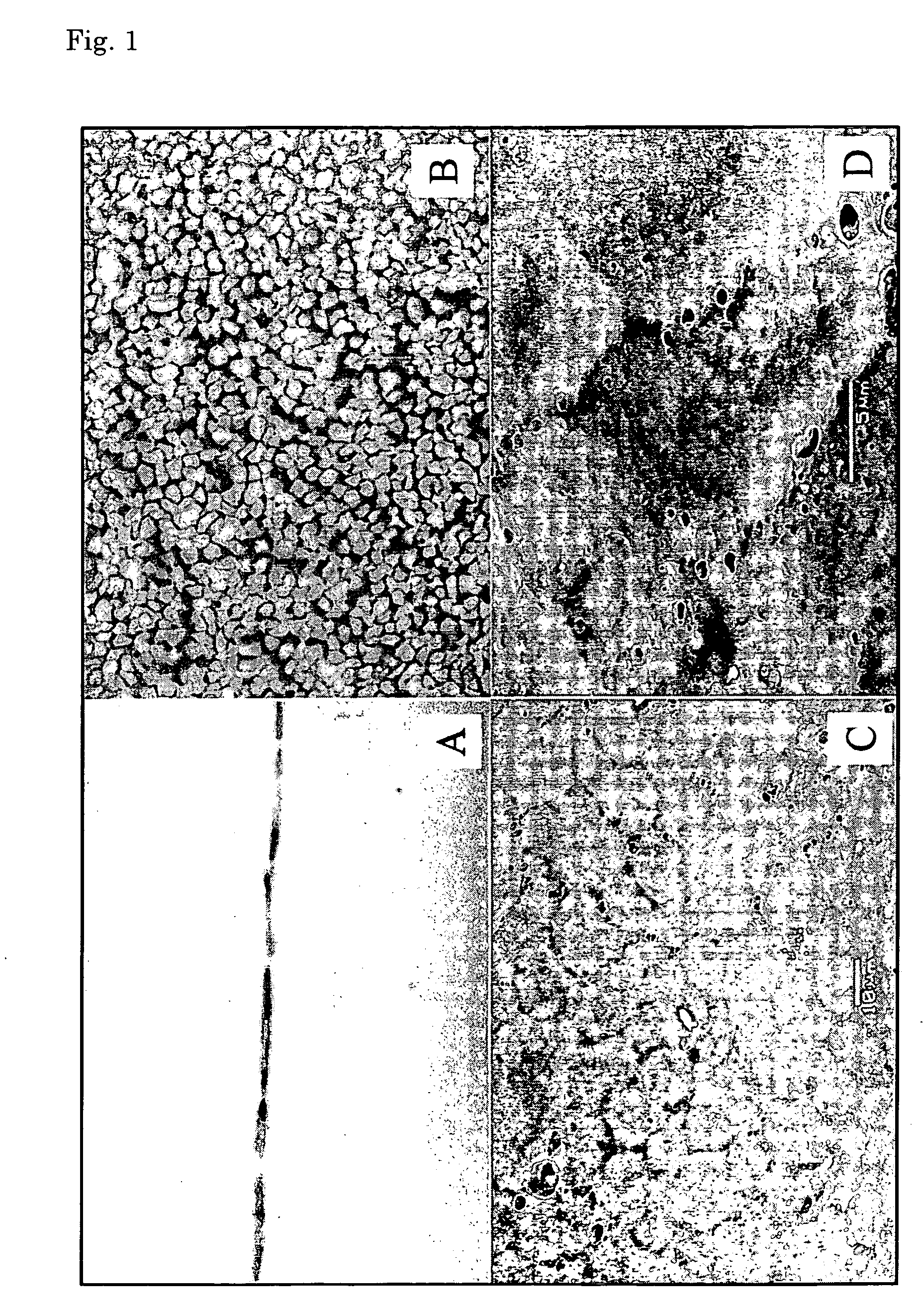
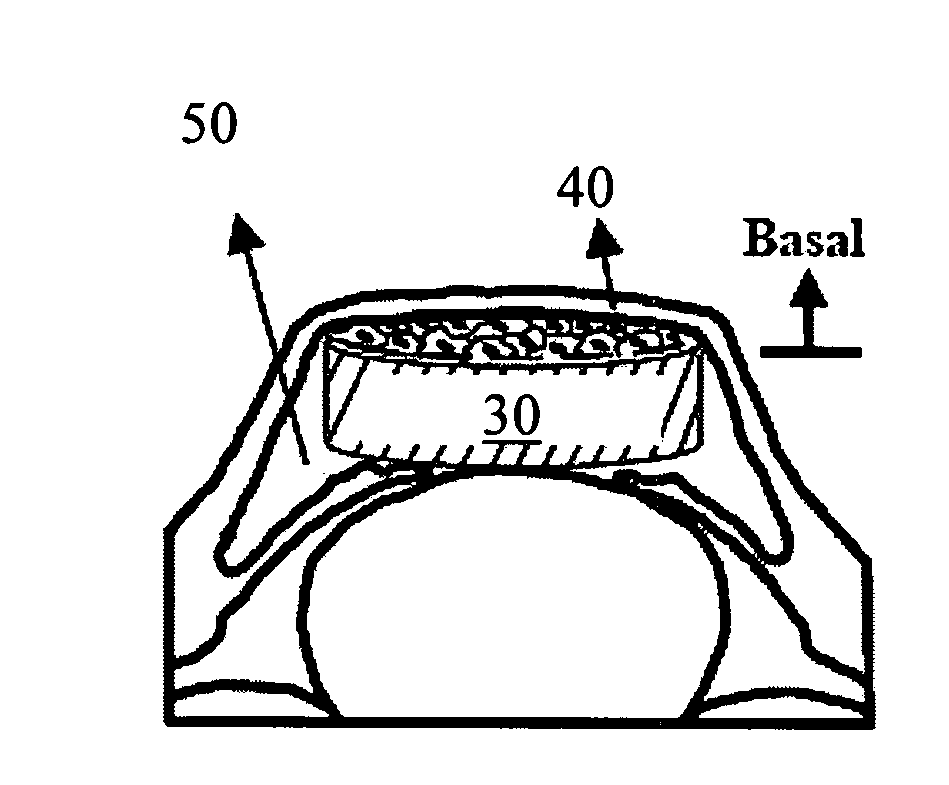
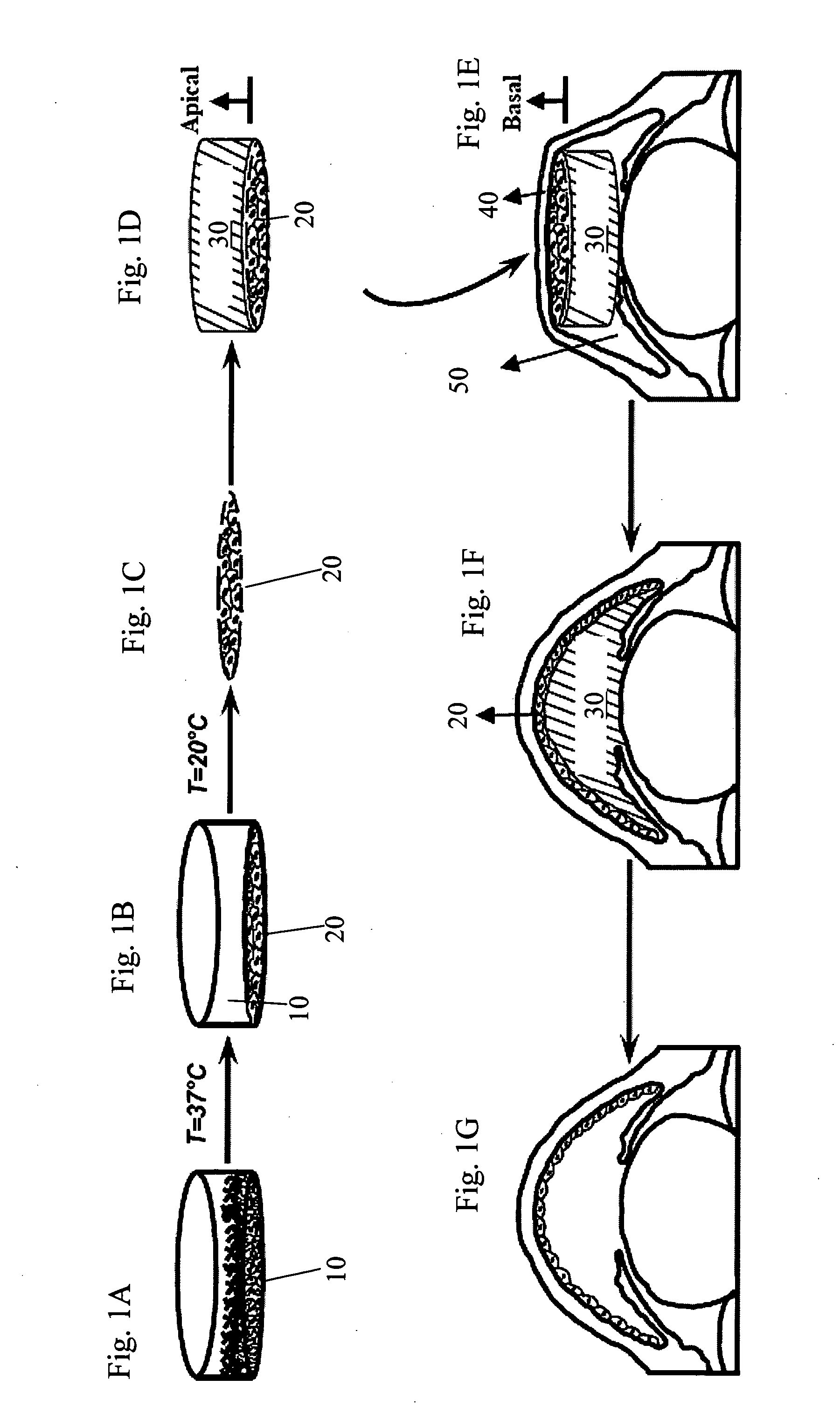
Corneal endothelium-like sheet and method of constructing the same
It is our intention to provide a transplantation material applicable to the treatment of various diseases that require corneal endothelium transplantation. Corneal endothelial cells are collected, proliferated and then sown on amnion and cultured. Thus, a corneal endothelium-like sheet, which has a cell layer made up of the corneal endothelium-origin cells formed on the amnion, can be obtained.
Owner:ARBLAST
Acellular cornea or acellular corneal stroma, preparation method and application thereof
InactiveCN101985051ARetain toughnessLow immunogenicityProsthesisFreeze thawingVaccine Immunogenicity
The invention discloses acellular cornea or acellular corneal stroma, a preparation method and application thereof. The method comprises the following steps of: (1) obtaining fresh animal full-thickness cornea or corneal stroma; (2) removing corneal epithelium, corneal endothelium and stroma cells, namely 1, soaking the full-thickness cornea or the corneal stroma in pure water at room temperature; 2, placing the soaked full-thickness cornea or corneal stroma into enzyme solution, digesting with oscillating, and washing with balanced salt solution with oscillating; and 3, repeating freeze-thaw processes of the full-thickness cornea or the corneal stroma for 4 to 8 times and washing with balanced salt solution with oscillating to obtain the acellular cornea or the acellular corneal stroma; (3) dehydrating; and (4) sterilizing and storing. In the method, the decellularization processing time of the cornea is short; the influence on the structure and the physiological property of the cornea is small; and the processed cornea has very low immunogenicity which is similar to the property of natural cornea. The acellular cornea or the acellular corneal stroma can be applied to artificial cornea construction of tissue engineering and also can serve as a medical material applied to corneal transplantation and refraction surgery.
Owner:JINAN UNIVERSITY
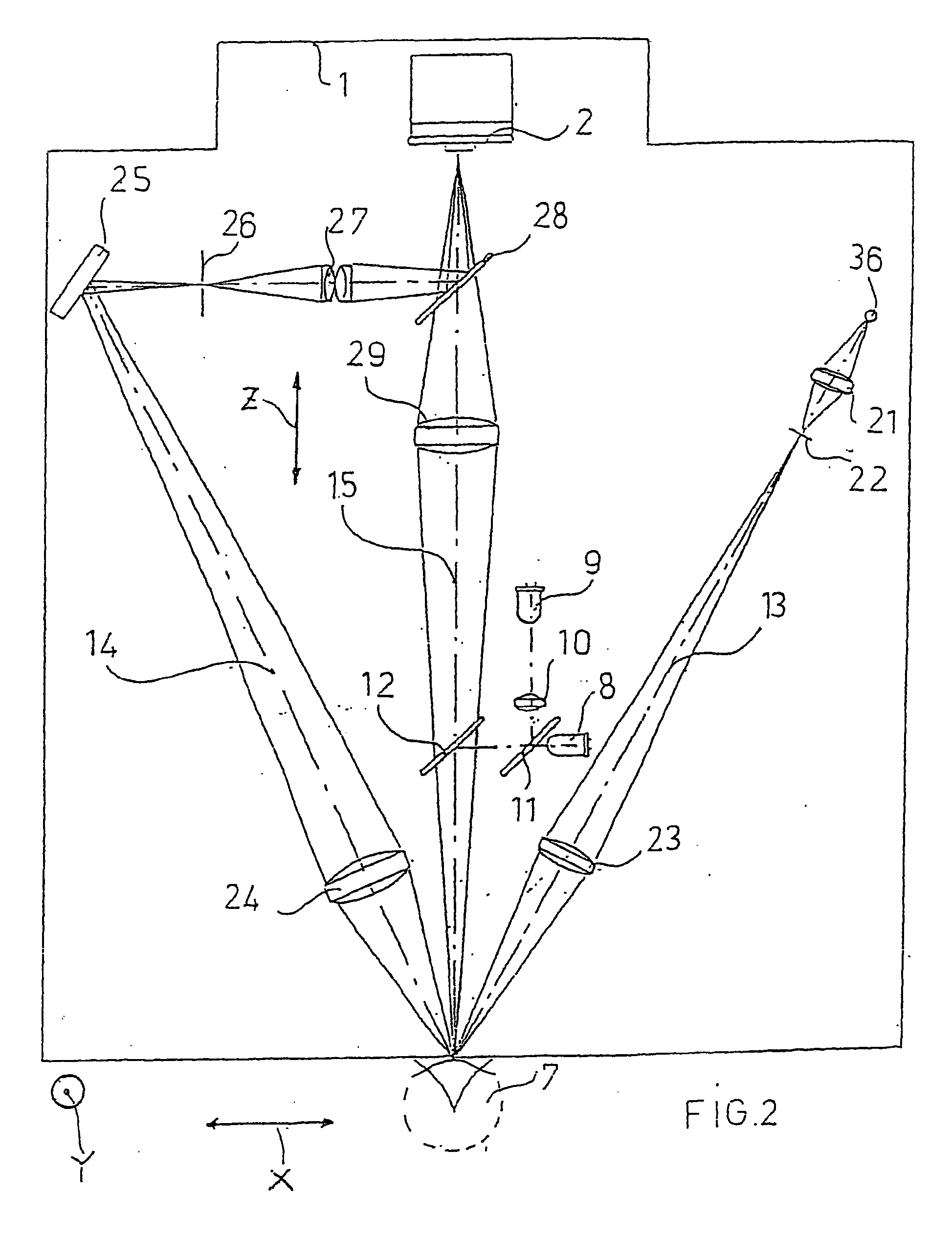
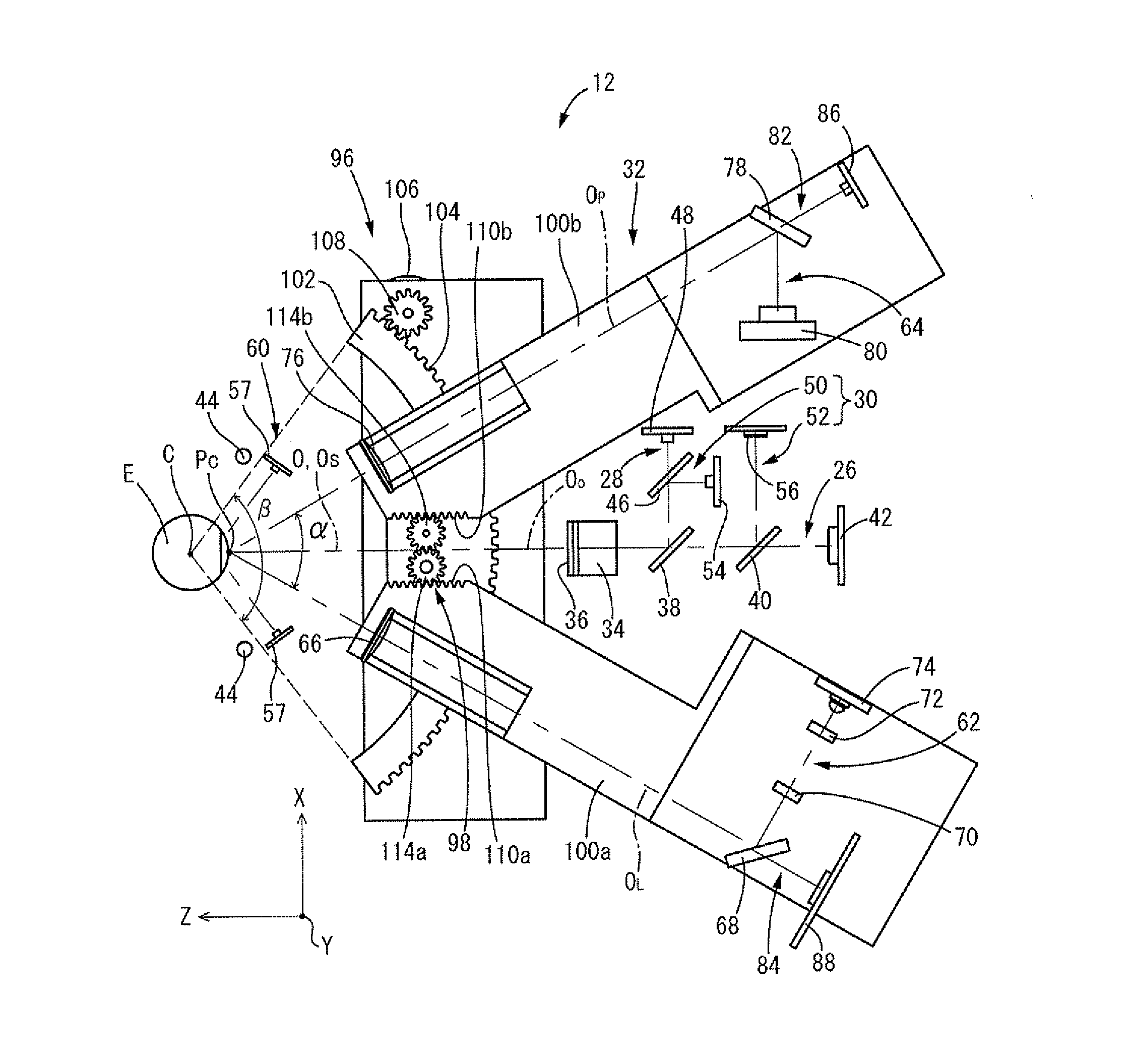
Reflection Microscope for Examination of the Corneal Endothelium and Method of Operating Same
ActiveUS20070263172A1Quality improvementReduce usageMicroscopesOthalmoscopesAutomatic controlLight spot
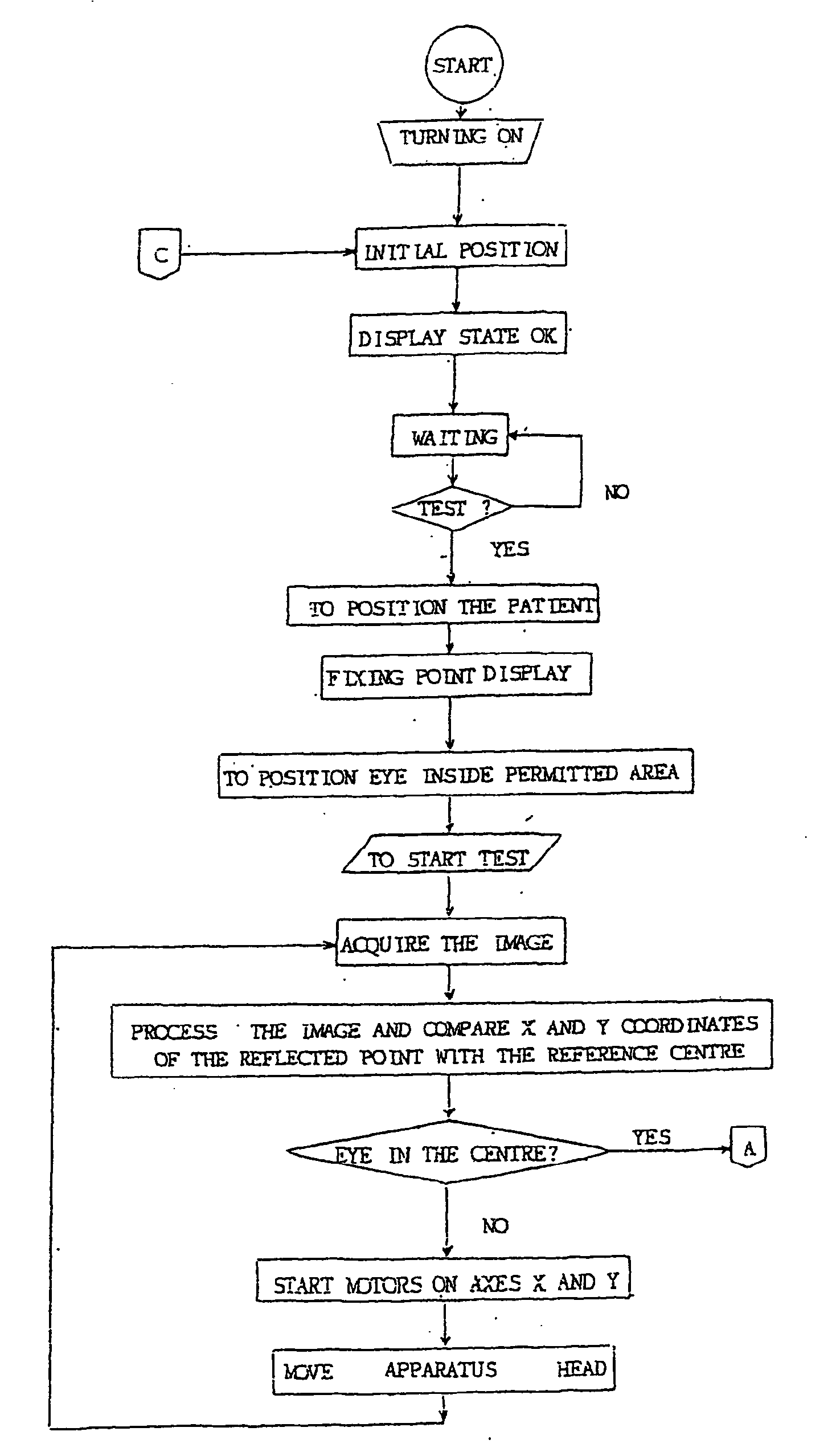
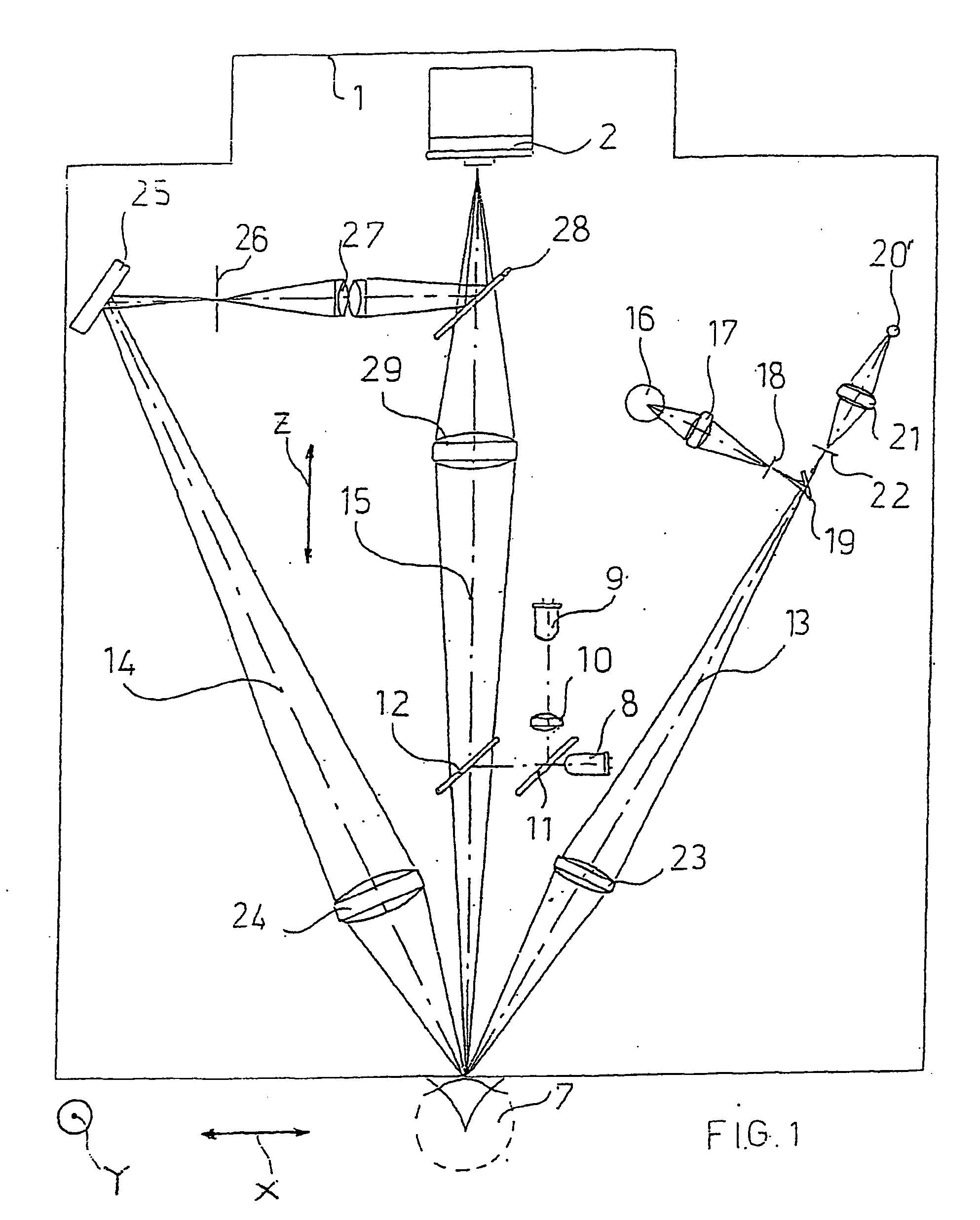
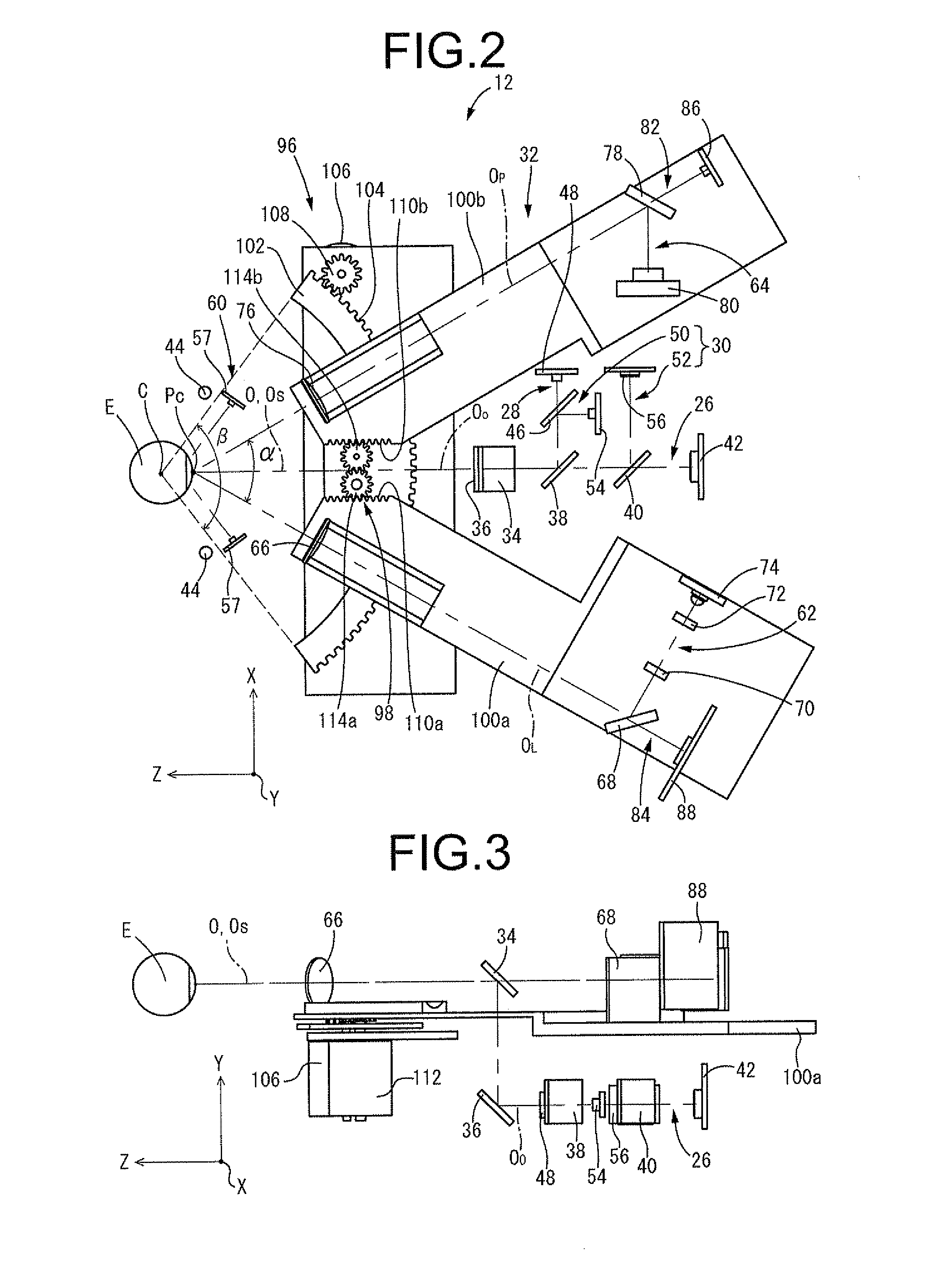
An apparatus and a method for operating an endothelium reflection microscope. The apparatus comprises an optical head including: an illuminating system, an eye-front observation optical system along a central channel in which an alignment-use light spot is received and imaged by a camera comprising a digital optical sensor, and an enlarged-imaging optical system for enlarged observation or photographing of the subject part of the digital camera. The apparatus further comprises a drive for moving the optical head and a CPU controller for automatically controlling the drive, the illuminating system and the eyefront optical system. The method comprises an endothelium image acquisition procedure in which the grey level inside a check area of the camera sensor is constantly checked during the advancement along an advancement direction (Z-); when the grey level reaches a predetermined threshold value, a delay time (Δt) is triggered; and when the delay time (Δt) lapses, the acquisition of one or more images of the endothelium by the digital camera is enabled.
Owner:C S O SRL
Fabrication of gelatin hydrogel sheet for the transplantation of corneal endothelium
The invention provides a corneal endothelial composition comprising a transparent hydrogel scaffold and a single layer of cultured corneal endothelial cells on the surface of the scaffold. The hydrogel scaffold I comprised of at least one biopolymer, preferably gelatin. Also provided are methods of making a corneal endothelial scaffold
Owner:WAKE FOREST UNIV HEALTH SCI INC
Biopolymer-bioengineered cell sheet construct
The present invention discloses a biopolymer-bioengineered human corneal endothelial cell (HCEC) sheet construct for reconstructing corneal endothelium in a patient. The construct includes a biopolymer carrier which is bioresorable and deformable; and a bioengineered cell sheet comprising a monolayer of interconnected HCECs with substantially uniform orientation, wherein the bioengineered cell sheet is attached to a surface of the carrier with apical surfaces of the HCECs facing said carrier.
Owner:NATIONAL TSING HUA UNIVERSITY
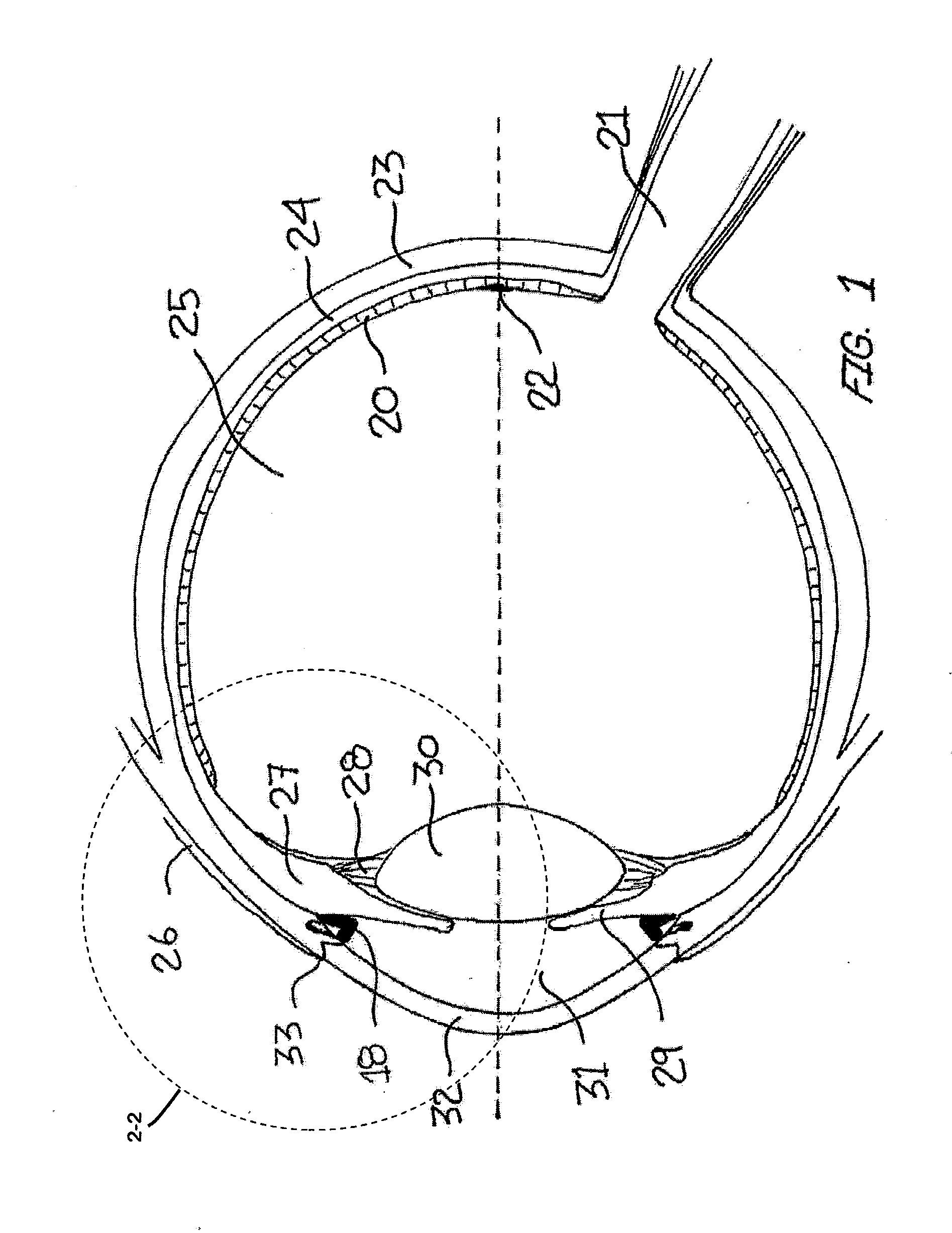
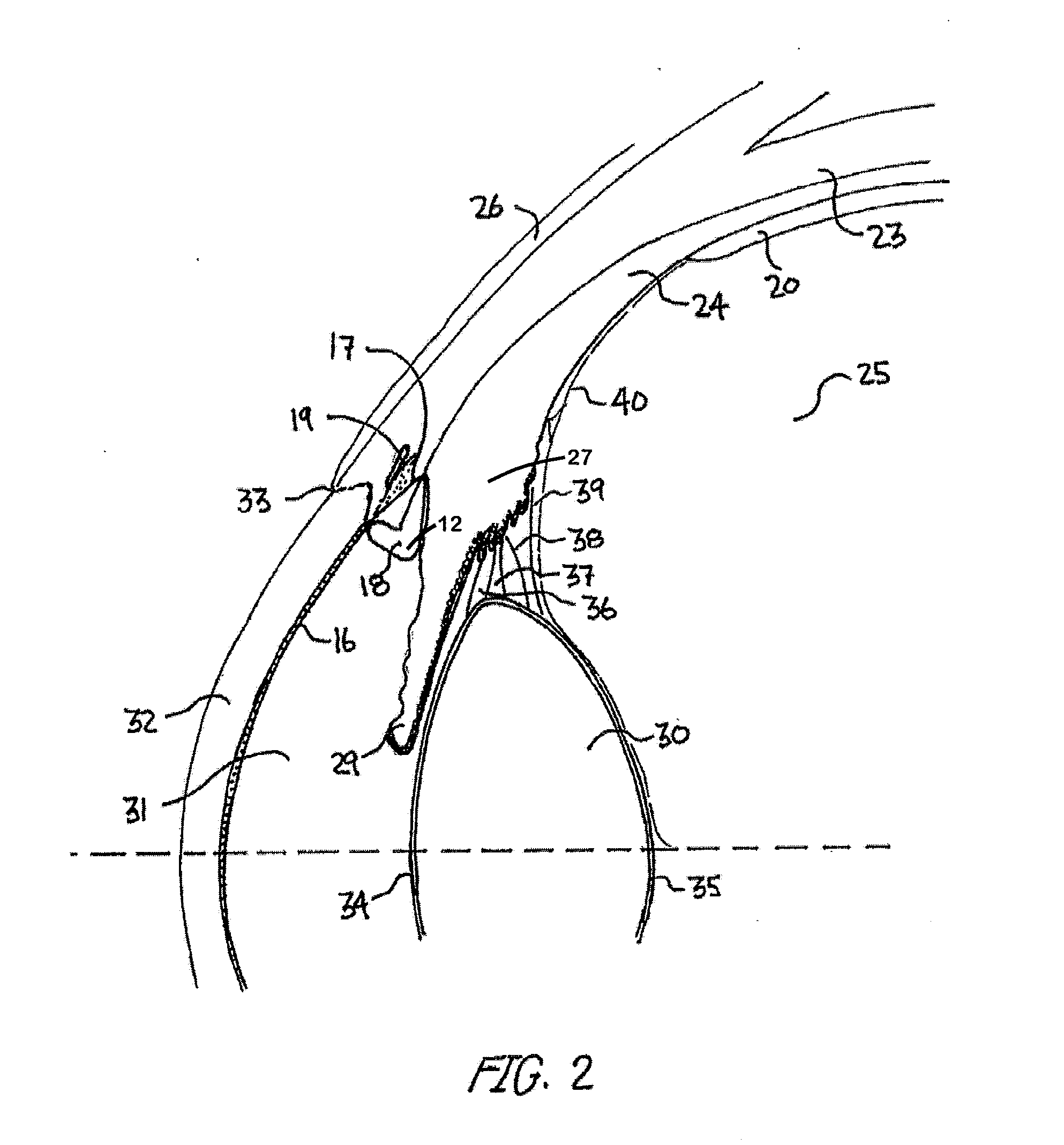
Ocular Collar Stent for Treating Narrowing of the Irideocorneal Angle
InactiveUS20140046437A1Restore some accommodative abilityRestore levelStentsEye implantsCorneal endotheliumInsertion stent
An ocular stent for insertion in an anterior chamber of an eye is provided. The stent facilitates the restoration of the structure of an irideocorneal angle of the anterior chamber for treating structural changes from ocular aging. The stent includes an annular body sized to fit in the irideocorneal angle of the eye. The body includes: an anterior portion configured to contact a surface of a transition zone between a trabecular meshwork and a corneal endothelium of the eye; a posterior portion configured to contact a peripheral iris of the eye; and a central portion connecting the anterior portion and the posterior portion of the body. A method for stabilizing the iredeocorneal angle of the anterior chamber using a stent is also disclosed herein.
Owner:REGENEYE L L C
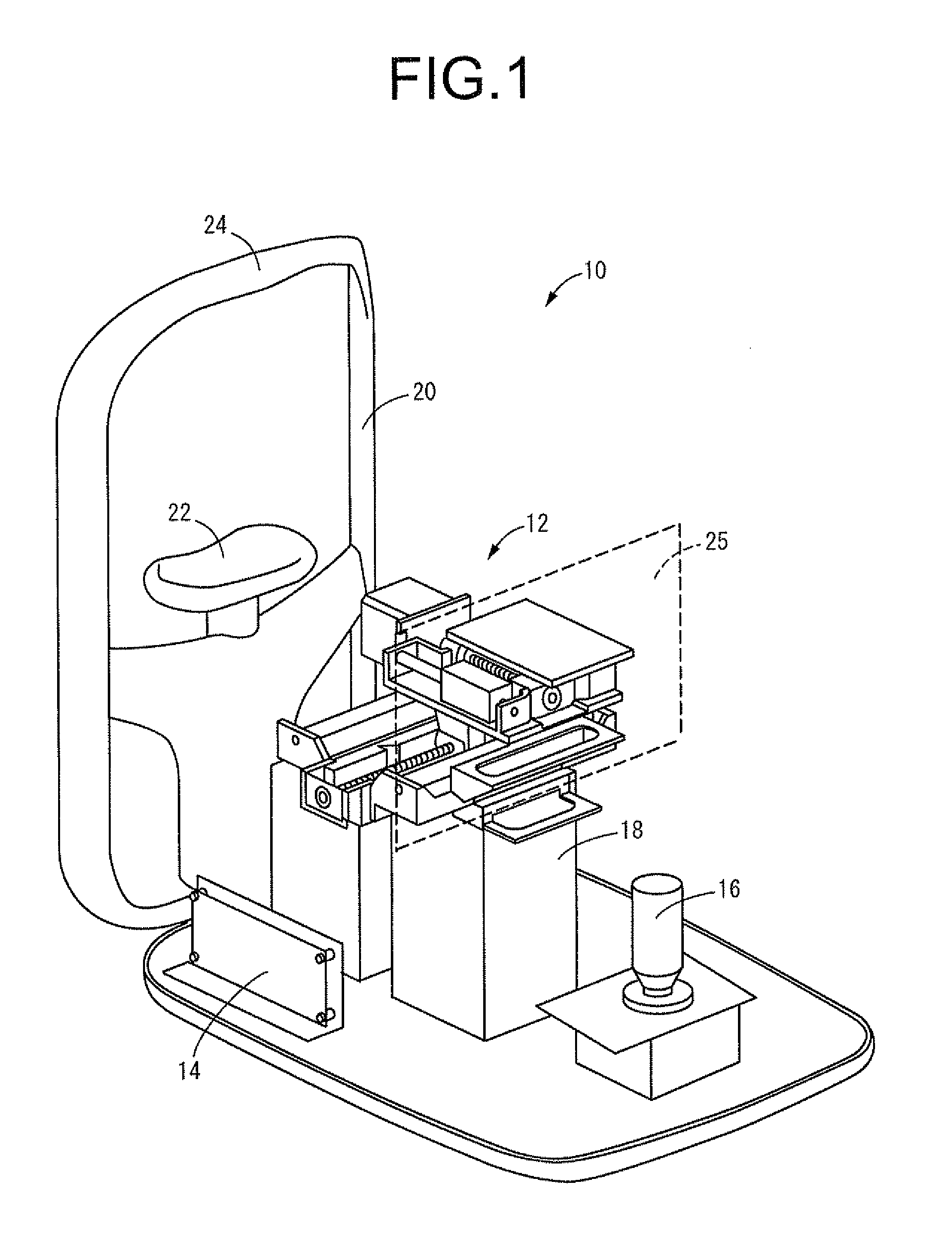
Cornea imaging apparatus and cornea imaging method
A cornea imaging apparatus including: a collimation axis holding mechanism; an imaging mechanism; a Z-direction actuating means; an X-direction actuating means and a Y-direction actuating means; an inclination angle changing means that changes an inclination angle of an imaging center axis of the imaging mechanism against a collimation axis of an eye under examination; an endothelial configuration computing means that determines a corneal endothelial configuration; and a normal vector computing means that determines a normal vector direction at a given imaging position of the corneal endothelial configuration, wherein at the imaging position, the inclination angle and positions in the Z and X directions are set adjusted so as to align the imaging center axis of the imaging mechanism with the normal vector direction determined by the normal vector computing means.
Owner:TOMEY CORP
Positive power anterior chamber ocular implant
A positive power anterior chamber ocular implant for placement in a phakic eye is disclosed to correct refractive errors caused by hyperopia which includes at least one convex surface and haptics for positioning the lens in the anterior chamber of the eye. Contact between the lens and other anatomic bodies, such as the anatomical lens, the corneal endothelium and iris is avoided, and the haptics avoid contact with the iris.
Owner:NOVARTIS AG
Method for preparing corneal endothelial cell
ActiveUS20140170751A1Improve proliferative abilityIncrease the number ofNervous system cellsUnknown materialsDiseaseProgenitor
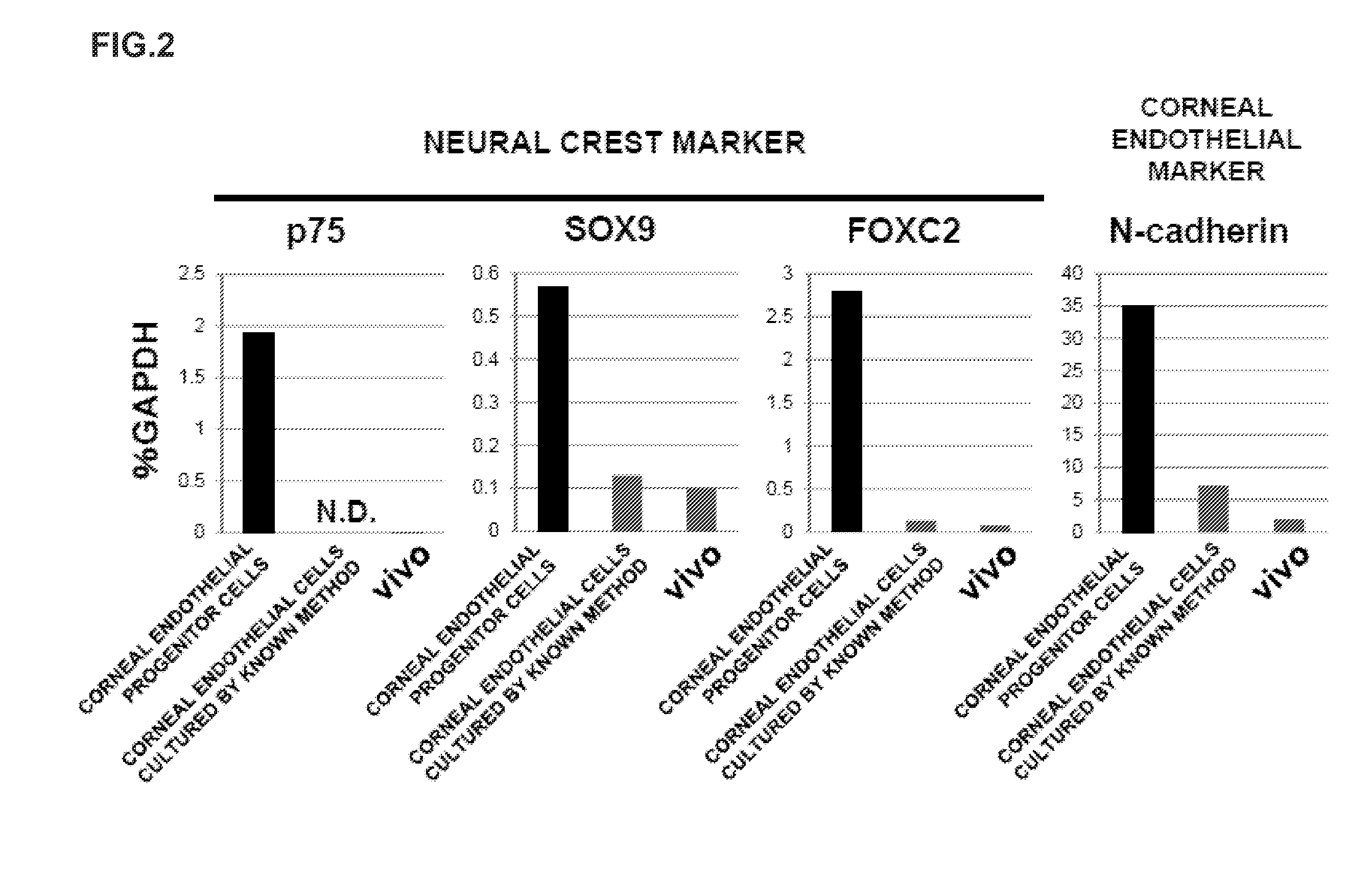
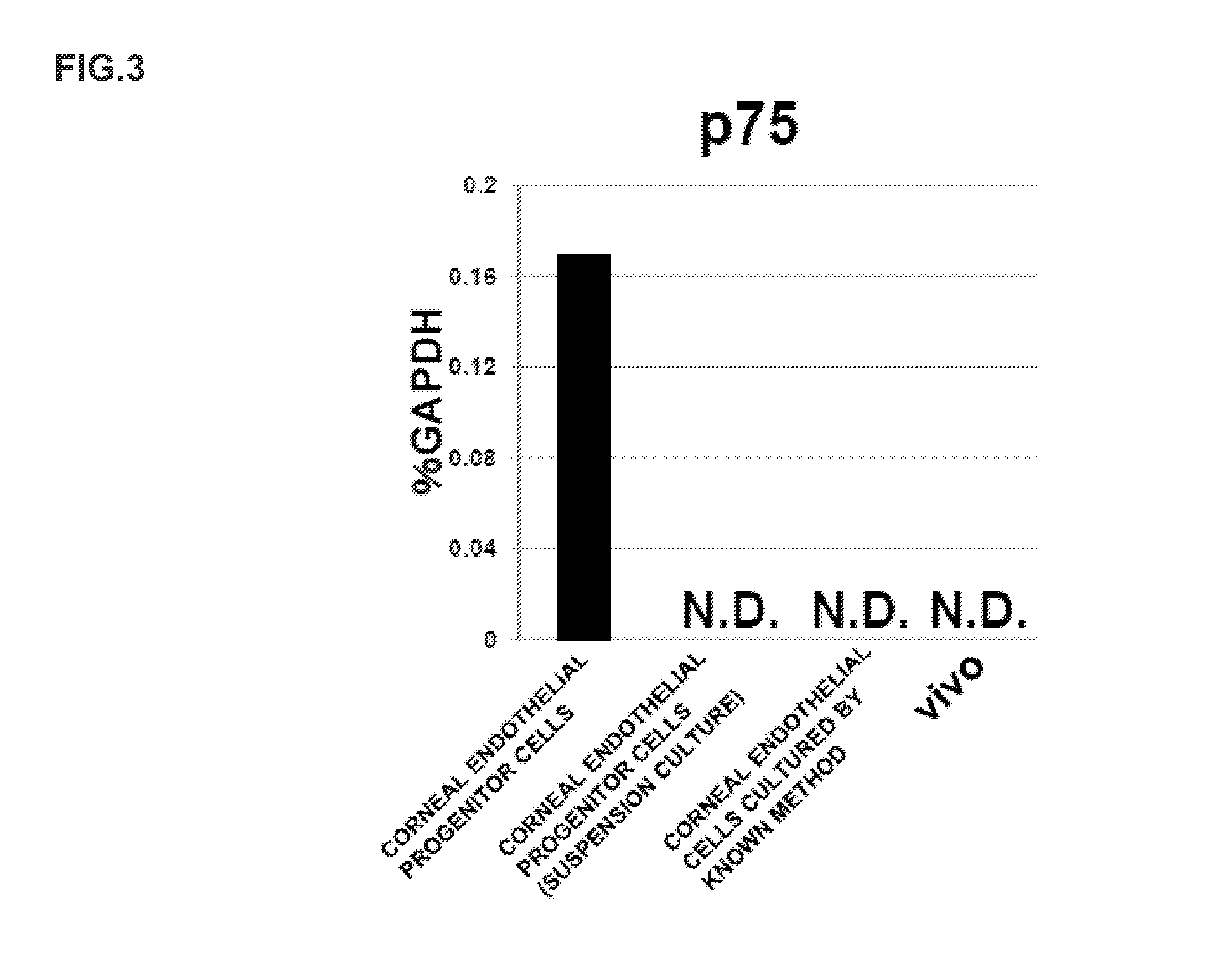
The present invention relates to a method for preparing corneal endothelial progenitor cells by adherent culturing a cell population isolated from corneal endothelial cell tissue at a low density by using a serum-free medium. The present invention also relates to a method for preparing corneal endothelial cells by differentiation-inducing the corneal endothelial progenitor cells obtained by the aforementioned method. According to the present invention, corneal endothelial progenitor cells can be selectively grown from a corneal tissue-derived cell population, and corneal endothelial cells obtained by inducing the corneal endothelial progenitor cells can be applied to treatment of corneal endothelial diseases. As a result, problems of corneal transplantation such as shortage of donors and occurrence of rejection can be solved.
Owner:OSAKA UNIV
RNAi Methods and Compositions for Stimulating Proliferation of Cells with Adherent Functions
ActiveUS20100003299A1Promotes HCEC adhesionReduce thicknessBiocideOrganic active ingredientsSurgical GraftIn vivo
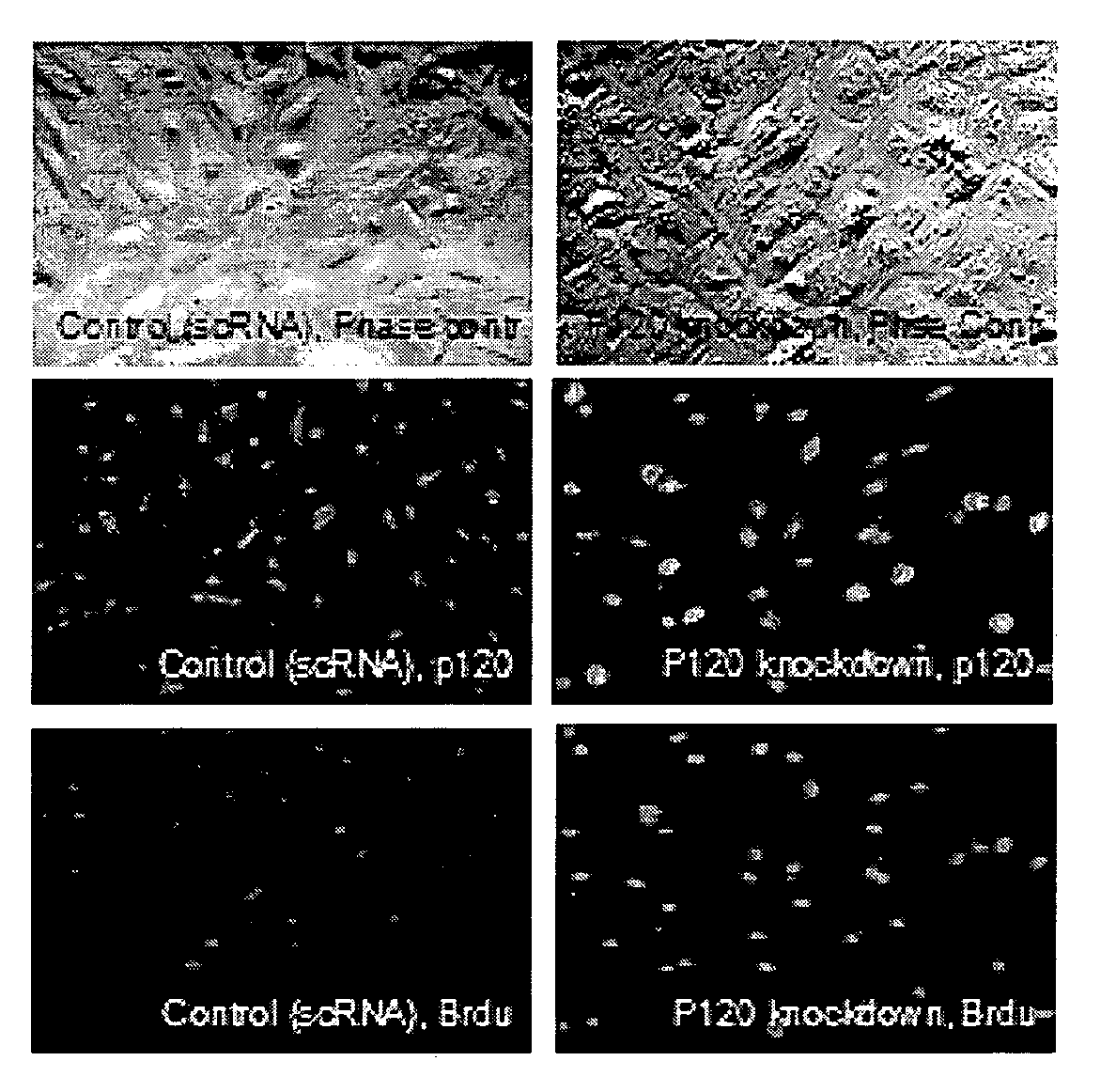
Described herein are methods and compositions for stimulating proliferation of cells that express adherent junctions and cease proliferation, for example, human corneal endothelial cells, by downregulation of certain cell-cell junctions. In one embodiment, downregulation is achieved using RNA interference, and contacting the cells with mitogenic growth factors and an agent that elevates intracytoplasmic cAMP. Furthermore, described herein are methods of isolating human corneal endothelial cells from keratocytes, and methods of preserving and maintaining viability of human corneal endothelial cell aggregates. Also described are surgical grafts comprising human corneal endothelial cells that have been isolated, optionally stored, and transiently contacted with an agent that downregulates expression of p 120, and a biocompatible support. The methods and compositions described herein can be used in novel therapies to help expand human corneal endothelial cells during in vitro tissue engineering and for in vivo treatment of corneal endothelial dysfunction.
Owner:TISSUETECH INC
Method for expansion of human corneal endothelial cells
A method for expanding human corneal endothelial cells includes: (a) providing an amniotic membrane with or without amniotic cells, wherein the amniotic membrane has an extracellular matrix; (b) placing onto the amniotic membrane, a sheet of endothelial layer, or a cell suspension including human corneal endothelial stem cells; and (c) culturing the corneal endothelial cells on the amniotic membrane for a duration sufficient for the corneal endothelial stem cells to expand to an appropriate area. The invention also relates to a method for creating a surgical graft for a recipient site of a patient using the method for expanding human corneal endothelial cells, and the surgical graft prepared therefrom.
Owner:TSAI RAY JUI FANG +1
Femtosecond laser cataract surgery treatment device
ActiveCN104042403AAvoid postoperative astigmatismAvoid EndophthalmitisLaser surgeryUltrasonic emulsificationFemto second laser
The invention discloses a femtosecond laser cataract surgery treatment device which comprises an optical coherence tomography scanning imaging system, a data processing system, a laser light source system, a laser energy detection system, a laser scanning system and a press mirror. The laser light source system is provided with a femtosecond laser. The laser light source system and the laser energy detection system, the laser energy detection system and the laser scanning system, the laser scanning system and the optical coherence tomography scanning imaging system are respectively connected through a light beam transmission system. The femtosecond laser cataract surgery treatment device has the advantages that the device is precise, efficient and safe, and astigmatism and endophthalmitis after operation are avoided; envelope tearing and suspensory ligament injury are reduced, surrounding tissue injuries are small; complications produced during crystal nucleus emulsification and artificial lens implantation are decreased as far as possible; the operating difficulty is reduced; the required energy for ultrasonic emulsification is greatly decreased, and the corneal endothelium is effectively protected.
Owner:广东麦特维逊医学研究发展有限公司
Membrane Associated Tumor Endothelium Markers
InactiveUS20110104059A1Reduced activityHigh activitySenses disorderPeptide/protein ingredientsMolecular levelFhit gene
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Compositions and Methods of Treatment of Corneal Endothelium Disorders
InactiveUS20160175380A1Enhance cell viabilityGood effectSenses disorderTetrapeptide ingredientsDiseaseCorneal endothelial cell
Owner:THE SCHEPENS EYE RES INST
Blade for preparing an endothelial graft, and associated preparation method
InactiveUS20140012295A1Reduce riskEasy to prepareEye implantsEye surgeryCorneal endotheliumOphthalmology
The invention relates to an instrument for preparing an corneal endothelial graft, the tool being a trephine blade (1) having a cross section in the form of an arc of a circle of between 270° and 355°. The invention also relates to a method of preparing a graft using such an instrument.
Owner:CENT HOSPITALER UNIV DE ROUEN
Constructing method for human corneal endothelium cell system
The construction of human corneal endothelium cell line with corneal endothelium as material includes the steps of: the first adherent culture of human corneal endothelium cell inside DMEM / F12 culture liquid with ox embryo serum in 20 wt%, chondroitin sulfate oxidizing and degrading matter, epidermal cell growth factor, basic fibroblast growth factor and ox eye auxin to obtain pure corneal endothelium cell; and the subsequent secondary culture in trypsinization process. The technological process is scientific and reasonable, and up to now, passage cell of 106-th generation has been obtained. The human corneal endothelium cell line of the present invention has no any virus or cancer genetic transfection, has no any tumorigenicity, and is expected to be used in direct artificial cornea production and clinical application.
Owner:青岛彩晖生物科技有限公司
Corneal endothelial tissue inserter
ActiveUS20100274257A1Avoids inadvertent adherenceLow costSurgeryEye treatmentCorneal endotheliumNose
A device transfers donor endothelial cells to the cornea of a recipient without injuring the cells or the cornea during the transfer process. The device transfers living endothelial cells from a donor to a recipient with minimal or no trauma. The device includes a handle, an irrigation tube lengthwise within the handle, a button upon the handle concentric with the irrigation tube, a tapered nose opposite the button, a forward tube extending outwardly from the nose, and a platform joined to the irrigation tube. The forward tube has an elliptical cross section and two beveled features. The platform has two wings that curl gently around donor tissue upon retracting the platform into the forward tube. The handle has a groove for a stem of a knob that surgeon presses and pulls to move the platform.
Owner:FISCHER SURGICAL
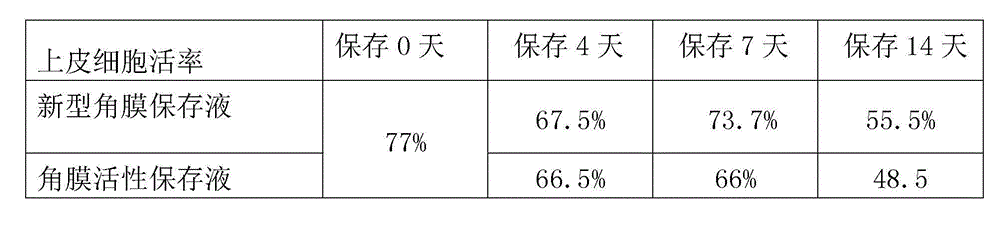
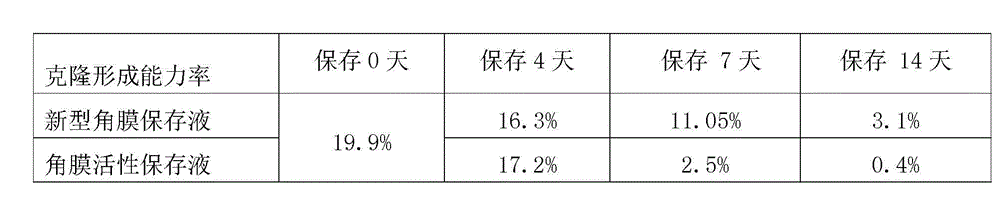
Cornea metaphase preservation solution, and preparing and using methods thereof
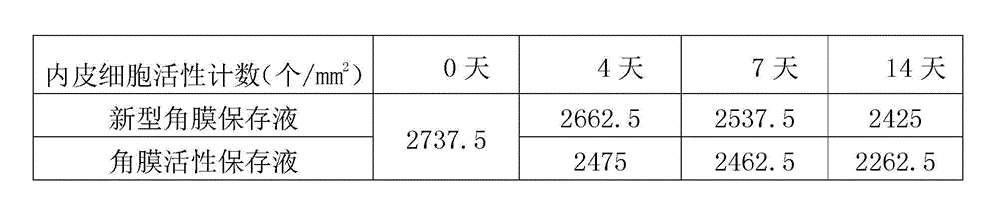
The invention provides a cornea metaphase preservation solution, and preparing and using methods thereof. The cornea metaphase preservation solution is a cell culture minimum essential medium (MDM) with chondroitin sulfate, low molecular dextran, L-glutamine, dexamethasone, tobramycin, 2-hydroxyethyl and Y-27632 added. The preservation solution not only can keep activity and normal morphology of cornea endothelial cells, but also can enhance viability of corneal limbus epithelial cells and improve clone ability of corneal limbus stem cells. And especially, a medium to long term preservation effect is obvious, phenomena of deformation, conjugation and the like of endothelial cells of a control group cornea do not appear in long term preservation, endothelial morphology of the control group cornea is consistent with endothelial morphology of a cornea preserved for 4 days, and the endothelial cells of the control group cornea are still regular and few in cell conjugation phenomena. The preservation solution can effectively prevent cell apoptosis phenomena during the process of preserving isolated cornea materials, increases activity and clone forming ability of the corneal limbus stem cells, and enables the cornea to maintain a transparent feature in a long preservation time.
Owner:SHANDONG EYE INST
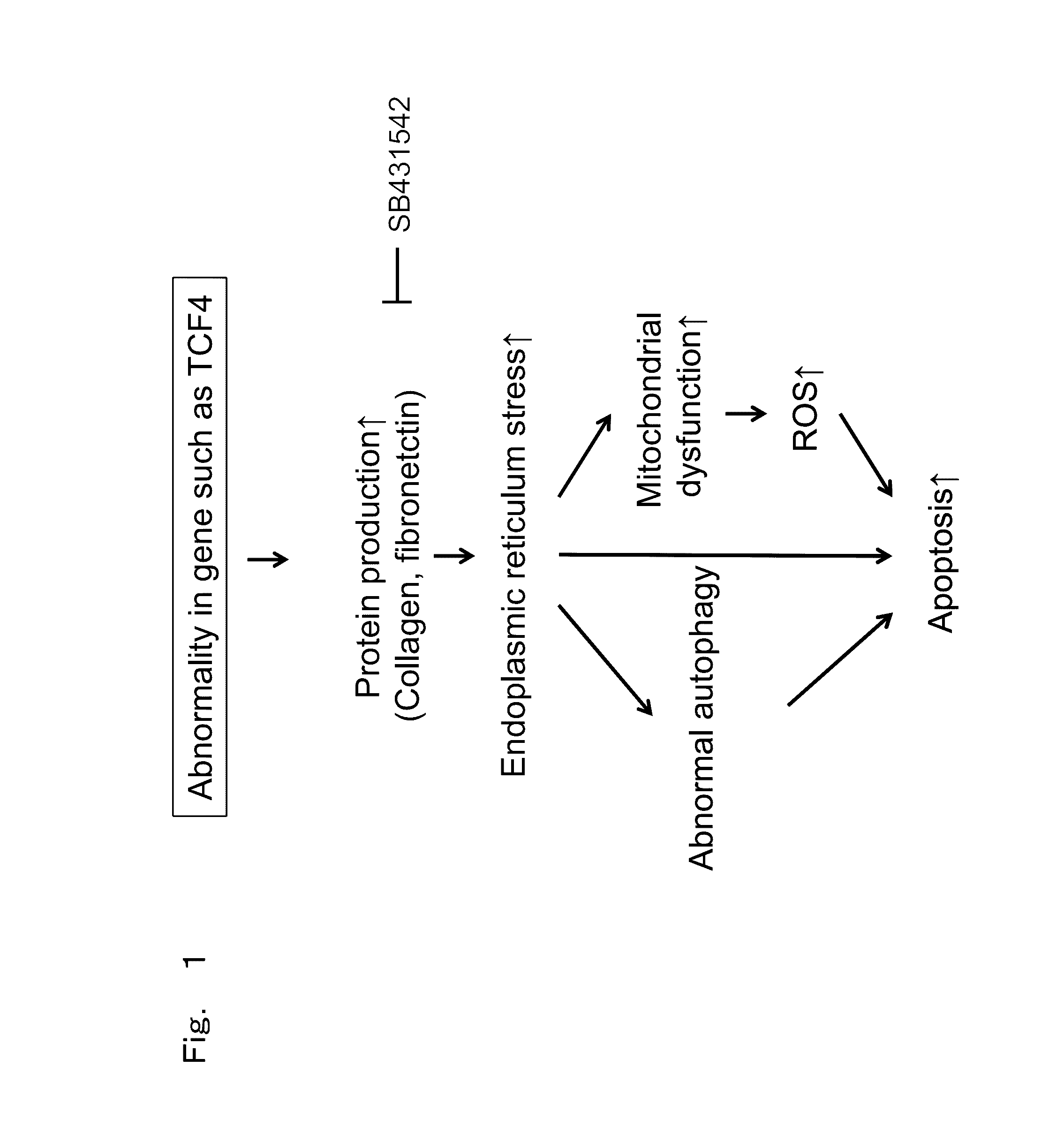
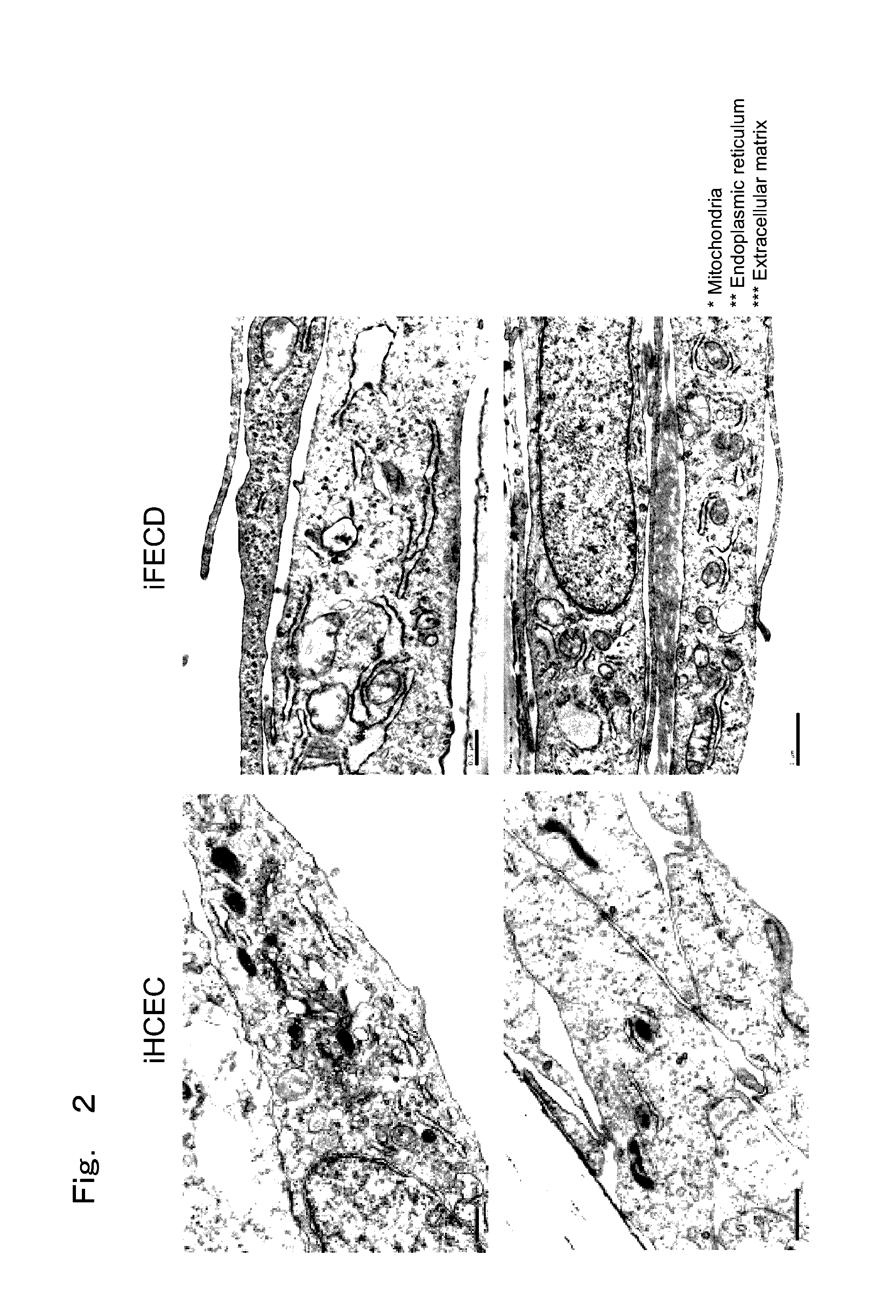
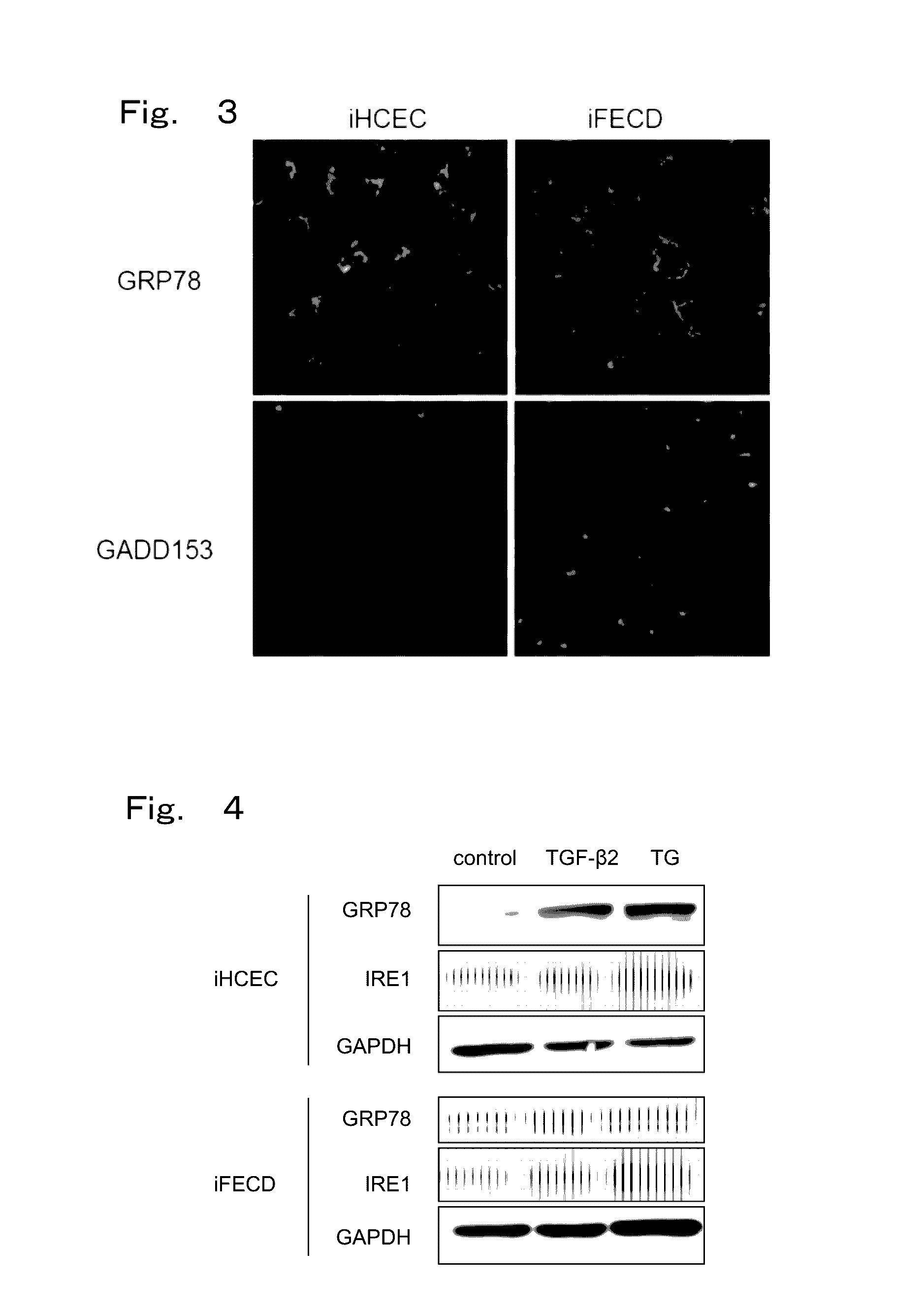
Therapeutic drug for diseases related to endoplasmic reticulum cell death in corneal endothelium
The present invention provides a treatment drug or prophylactic drug for diseases, disorders, or conditions related to endoplasmic reticulum (ER) stress. Specifically, the present invention provides a treatment drug or prophylactic drug for diseases, disorders, or conditions related to endoplasmic reticulum (ER) stress in the corneal epithelium, the drug containing a TGFβ-signal inhibitor. As a preferred TGFβ-signal inhibitor, the drug contains 4-[4-(1,3-benzodioxole-5-yl)-5-(2-pyridinyl)-1H-imidazole-2-yl]benzamide.
Owner:KYOTO PREFECTURAL PUBLIC UNIV CORP +2
Corneal posterior lamella and use thereof
InactiveCN101745152AImprove proliferative abilityStrong self-renewal abilityEye implantsCorneal endothelial cellCorneal endothelium
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Human corneal endothelial cell sheet
InactiveUS20150374881A1Simple materialBiocideSenses disorderCorneal endothelial cellCorneal endothelium
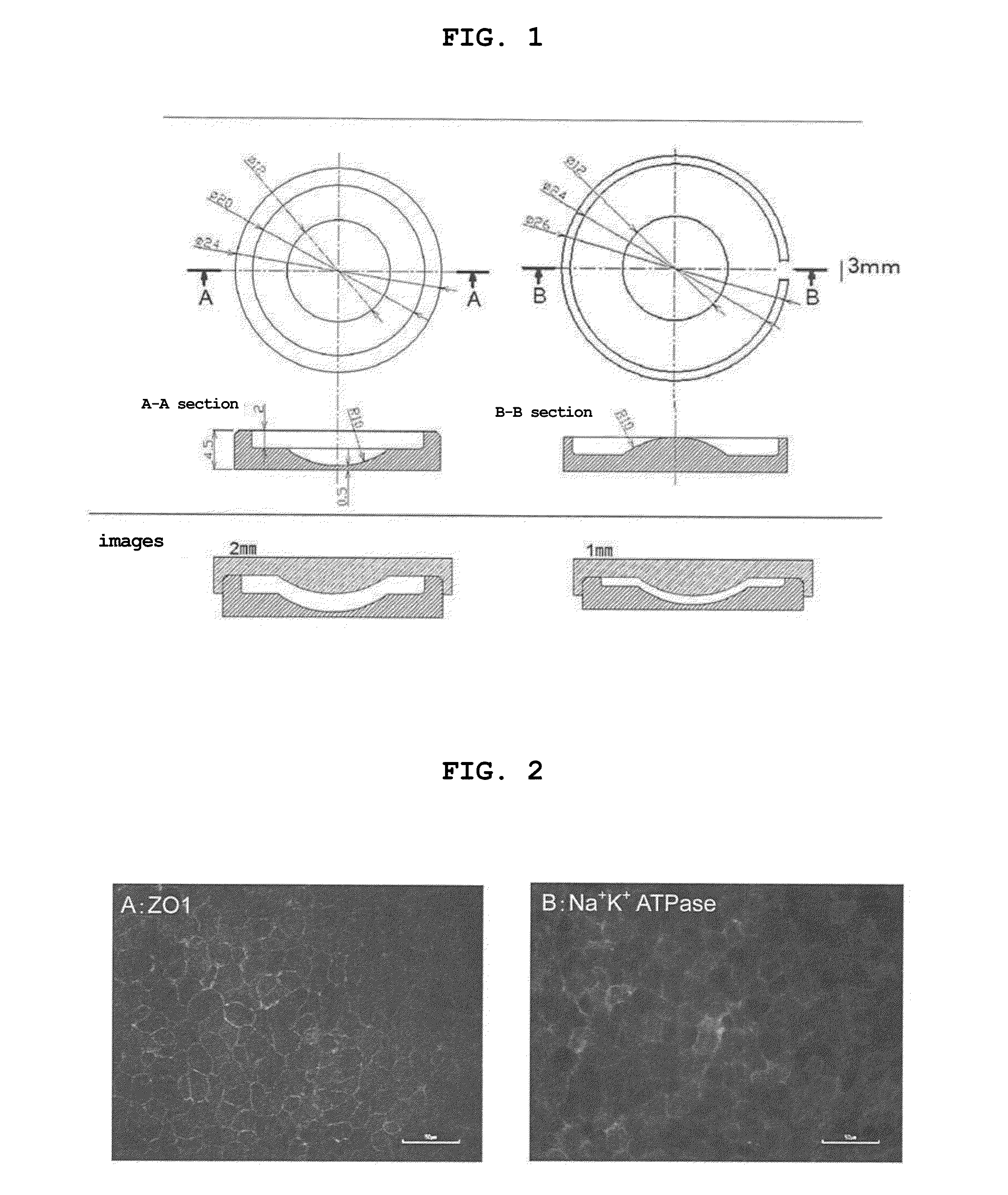
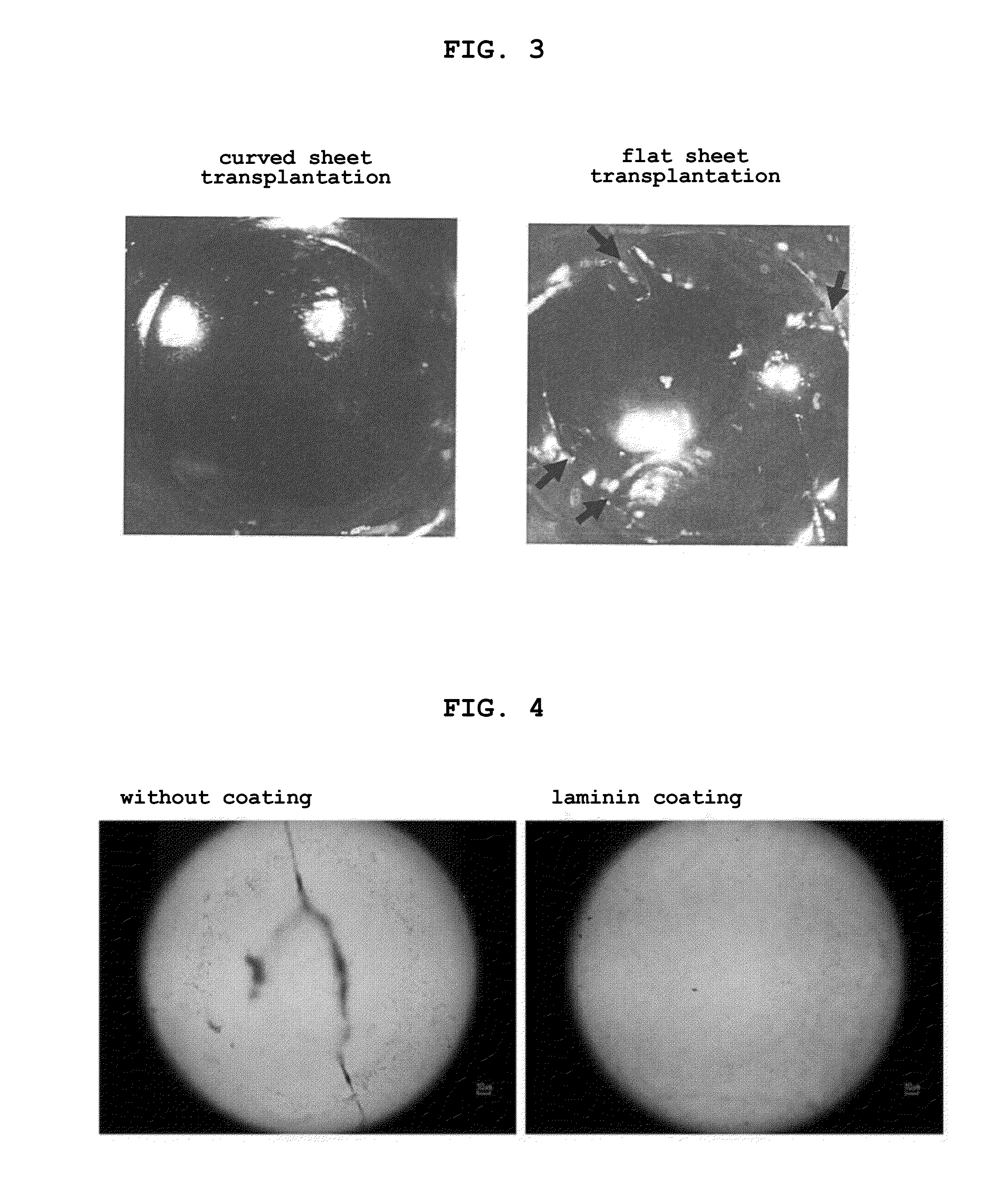
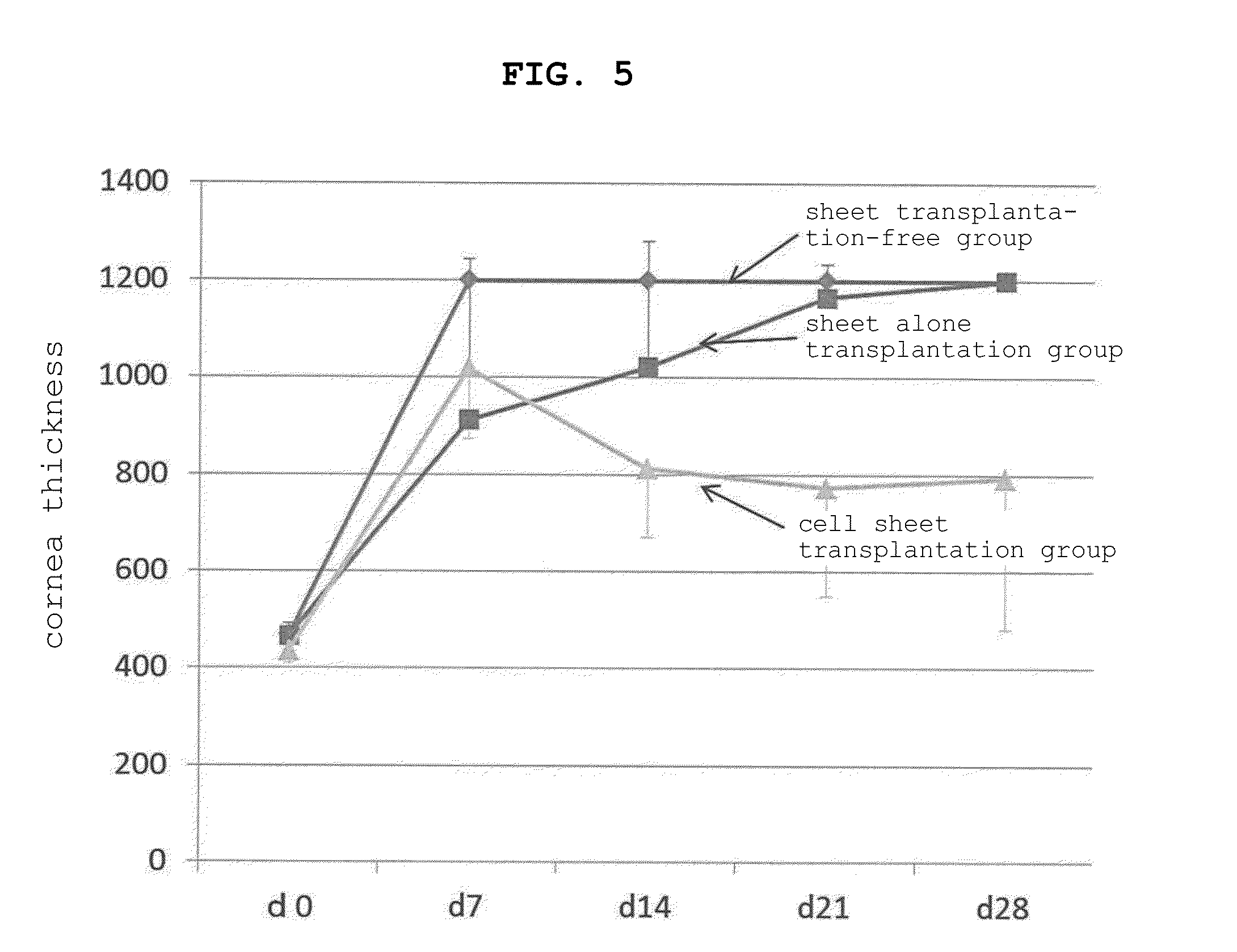
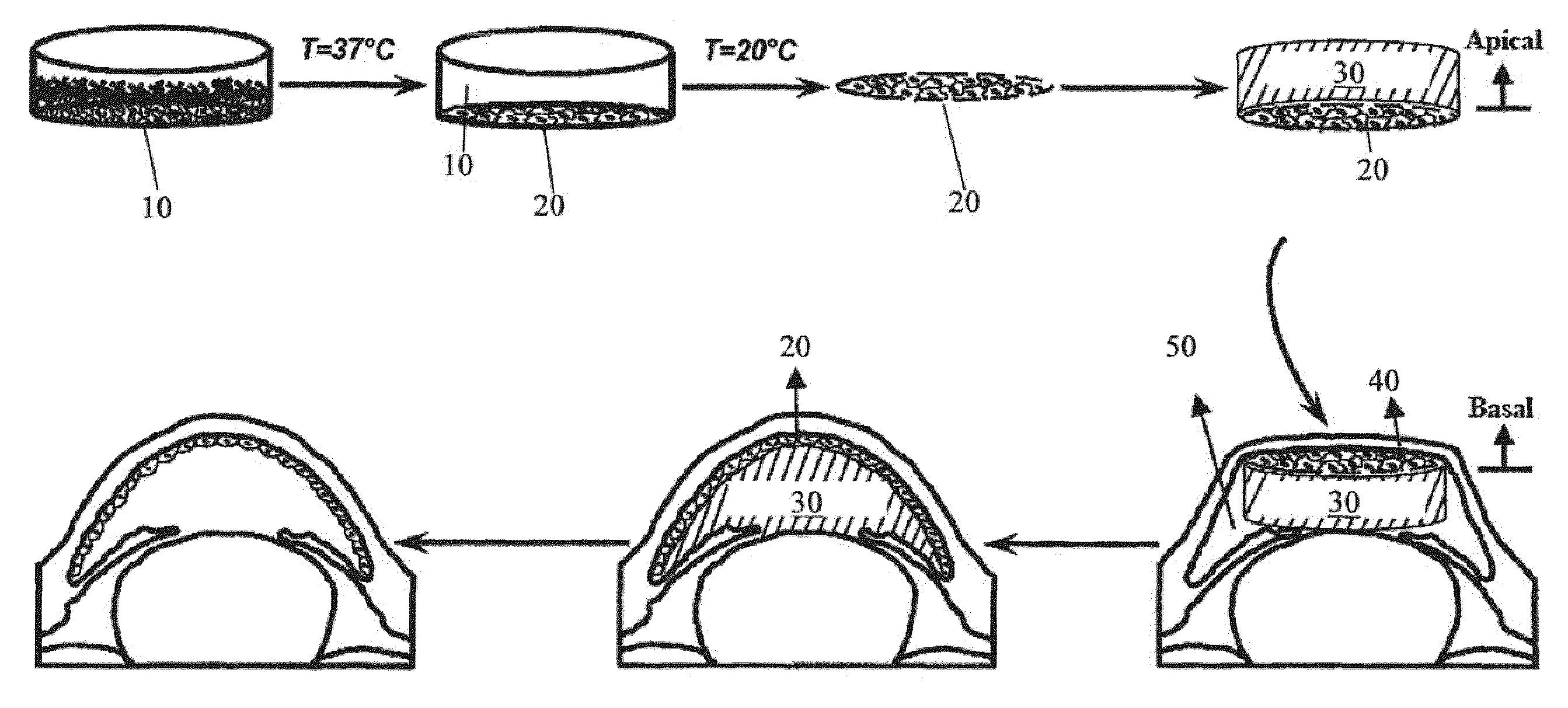
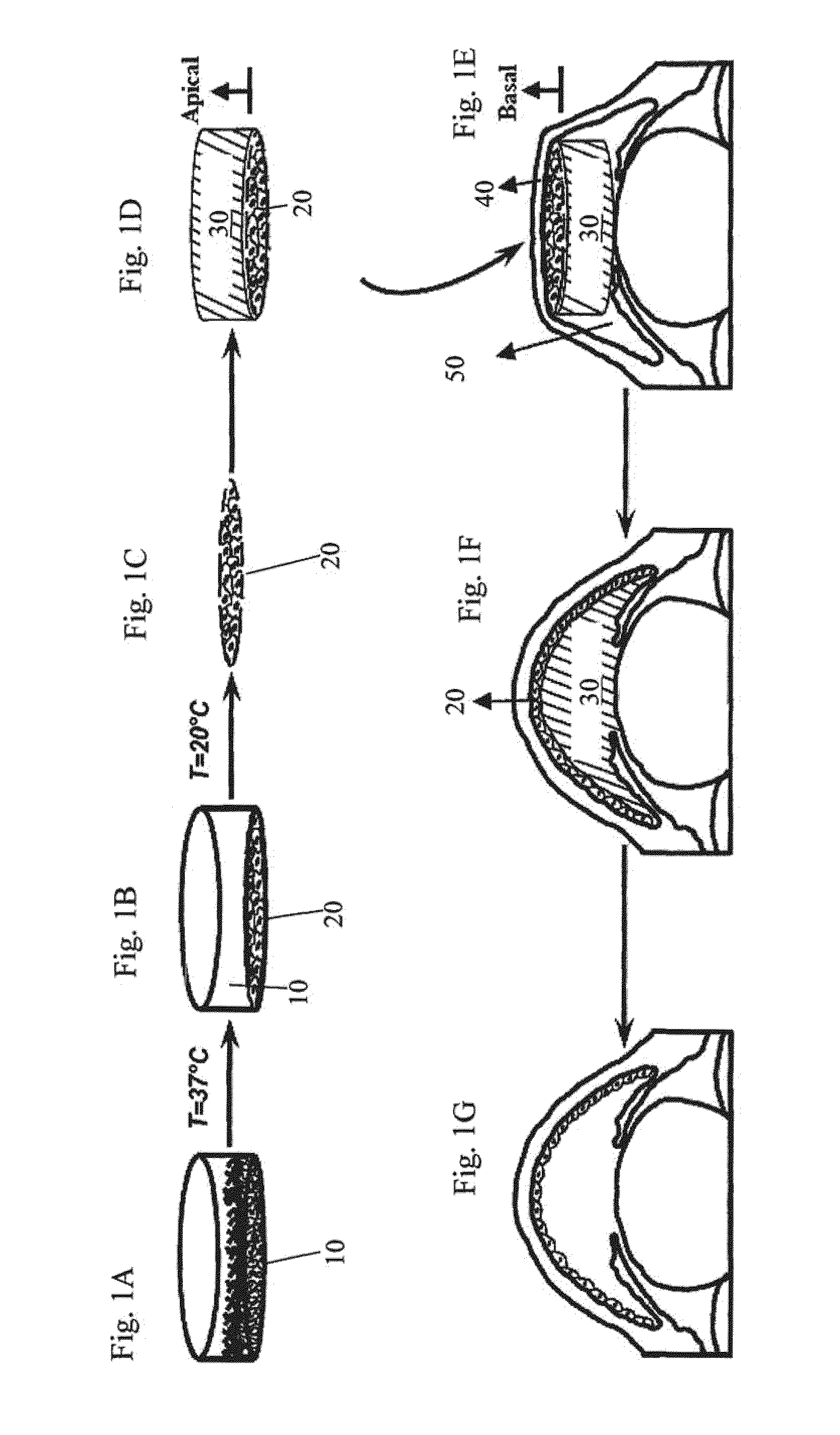
The present invention provides a human corneal endothelial cell sheet having a curvature fitting a human corneal endothelial surface, particularly, the human corneal endothelial cell sheet supported by a cell support having a curvature fitting a human corneal endothelial surface, and a production method of a human corneal endothelial cell sheet having a curvature fitting a human corneal endothelial surface, which includes the following steps:(1) a step of gelling a gelatin aqueous solution poured into a template having a curvature fitting a human corneal endothelial surface;(2) a step of forming a sheet-like gelatin by drying the gelled gelatin obtained in (1);(3) a step of obtaining a gelatin sheet by thermally crosslinking the sheet-like gelatin obtained in (2);(4) a step of seeding human corneal endothelial cells on the gelatin sheet obtained in (3) to form a human corneal endothelial cell layer.
Owner:NITTA GELATIN INC +1
Biopolymer-Bioengineered Cell Sheet Construct
InactiveUS20090263465A1BiocideMammal material medical ingredientsCorneal endothelial cellCorneal endothelium
A biopolymer-bioengineered human corneal endothelial cell (HCEC) sheet construct for reconstructing corneal endothelium in a patient is recited. The construct includes a biopolymer carrier which is bioresorable and deformable; and a bioengineered cell sheet containing a monolayer of interconnected HCECs with substantially uniform orientation. The bioengineered cell sheet is attached to a surface of the biopolymer carrier with apical surfaces of the HCECs facing said carrier.
Owner:NATIONAL TSING HUA UNIVERSITY
Method for preparing carrier bracket of tissue engineering artificial corneal endothelium by using fresh amniotic membrane
The invention relates to a method for preparing a carrier bracket of tissue engineering artificial corneal endothelium by using a fresh amniotic membrane. The method comprises the following steps: soaking and disinfecting the fresh amniotic membrane by tobramycin sulfate injection according to the mass ratio of 1:1000; after reverse digestion using trypsin-EDTA digestive juice, lightly scraping the epithelial surface of the amniotic membrane by a cell scraper to completely remove residual epithelial cells to obtain the denuded amniotic membrane which is tiled in culture plate holes for fixingand dry-posting; coating by special coating liquid for the denuded amniotic membrane; and sucking and then drying the coating liquid to obtain the carrier bracket of the tissue engineering artificialcorneal endothelium. The method has scientific and reasonable process, the prepared carrier bracket can be mass produced to meet the heavy demand of scale reconstruction of the tissue engineering artificial corneal endothelium and create conditions for sight rehabilitation of corneal endothelium blindness through clinical corneal transplantation, and the preparation method of the carrier bracket has low cost in in-vitro reconstruction and clinical treatment of the tissue engineering artificial corneal endothelium.
Owner:青岛宇明生物技术有限公司
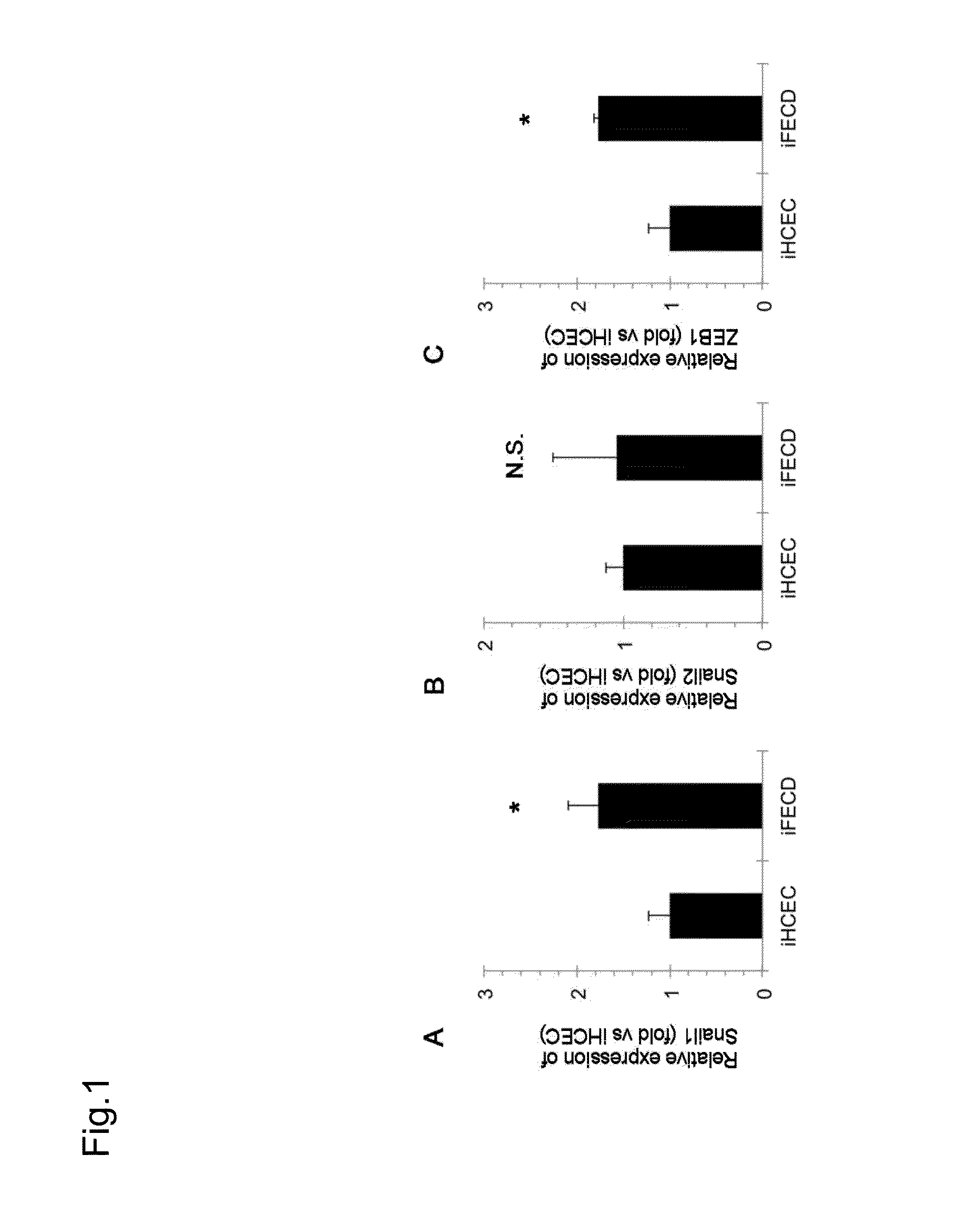
Corneal endothelial cell marker
ActiveUS20160266114A1FunctionalTreat or prevent corneal endothelial diseaseSenses disorderBiological material analysisCorneal endothelial cellSurface marker
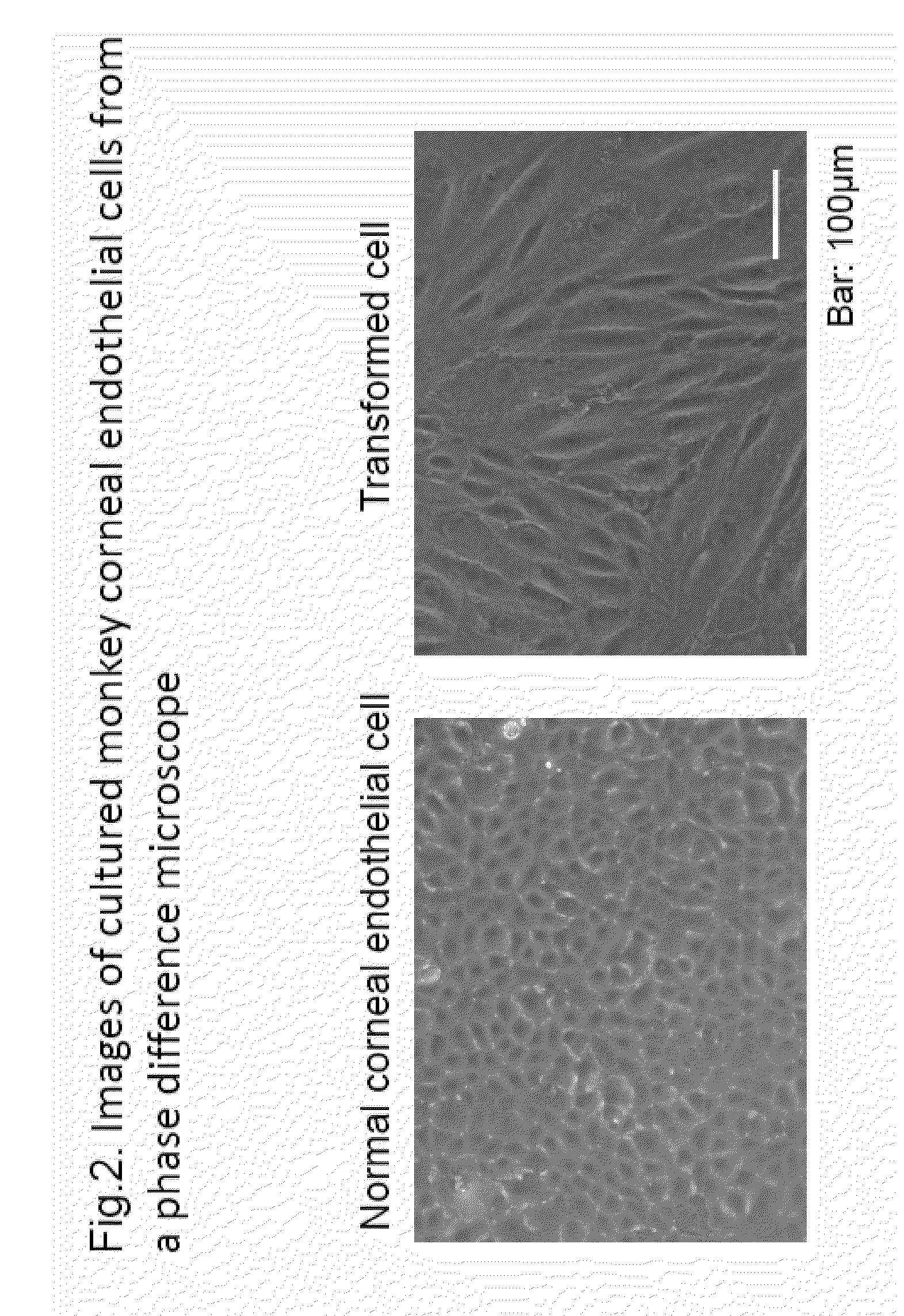
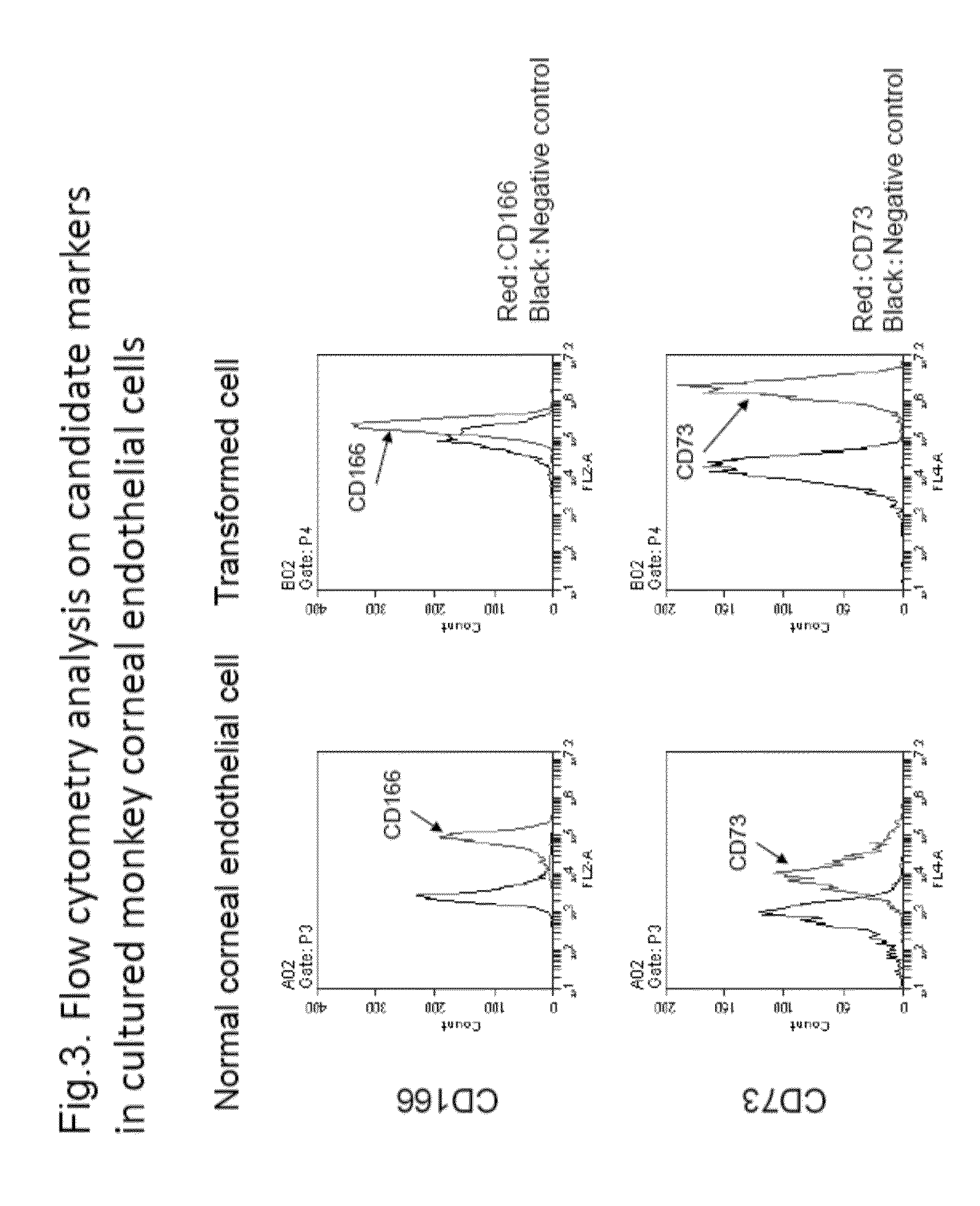
The purpose of the present invention is to provide a method of purification and preparation of cultured corneal endothelial cells, and in particular, to provide cell surface markers for use in corneal endothelial cells not including transformed cells. Provided are cell markers for distinguishing normal cells and transformed cells, in particular normal and transformed corneal endothelium cells. These cell markers relate to specific cell surface markers, for example, to a normal corneal endothelial surface marker such as CD166, and a transformed cell surface marker such as CD73. By using the transformed cell surface marker such as CD73 to remove transformed cells by sorting, it becomes possible to improve purity of a normal cultured corneal endothelium. By using normal corneal endothelial surface marker such as CD166, or by combined use with the transformed cell surface marker, it becomes possible to provide a means for verifying the purity of a prepared corneal endothelium.
Owner:ACTUALEYES INC +2
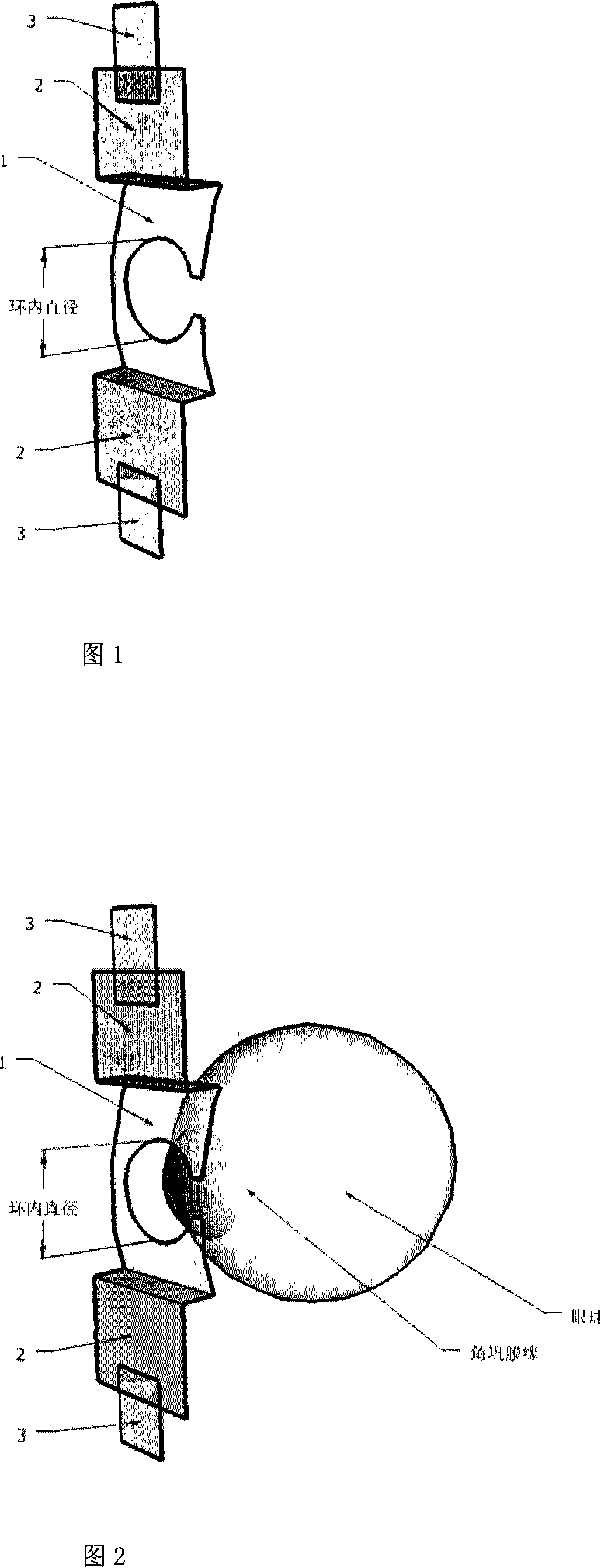
Eyelid retractor capable of fixing eyeball and application thereof
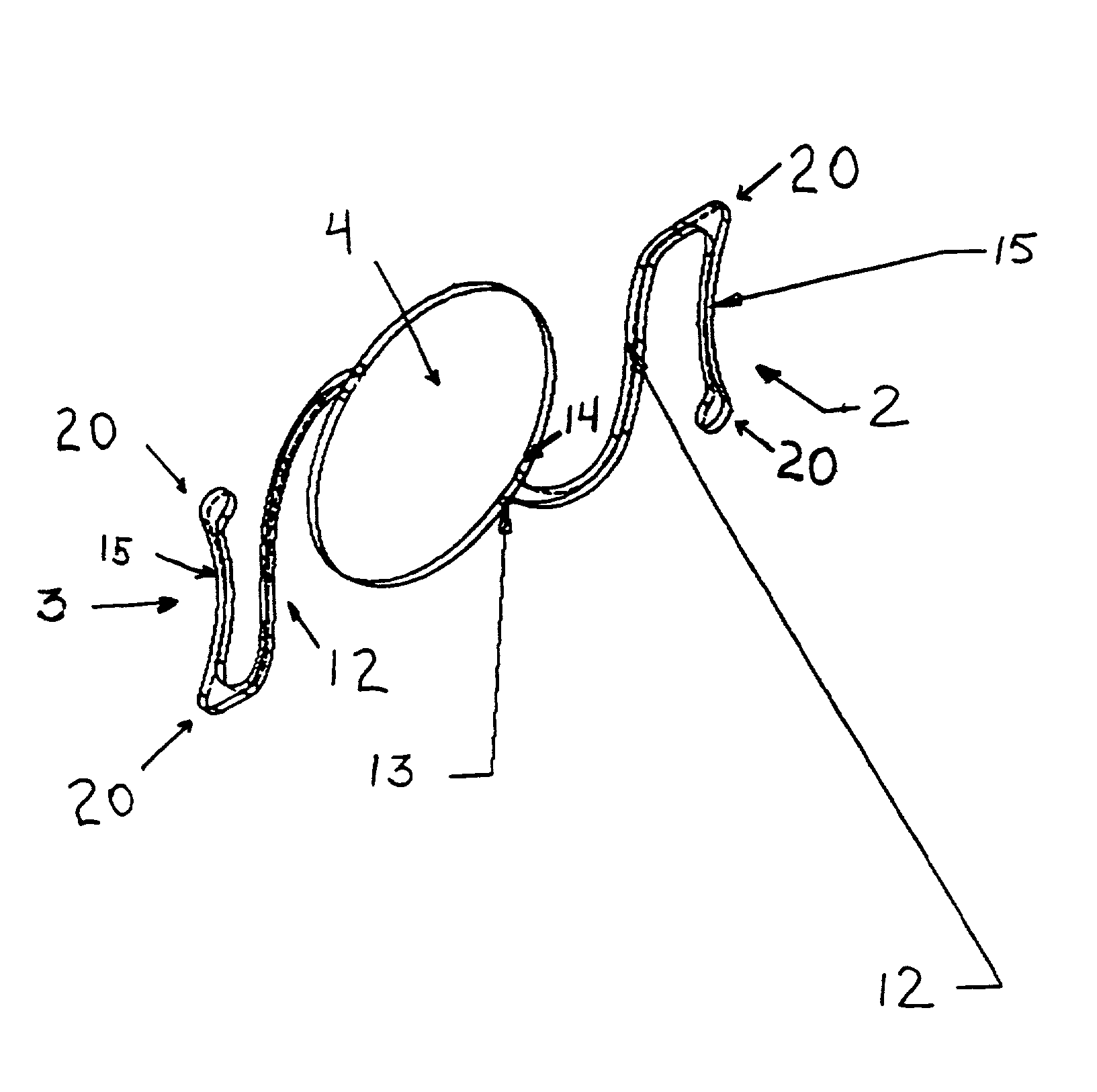
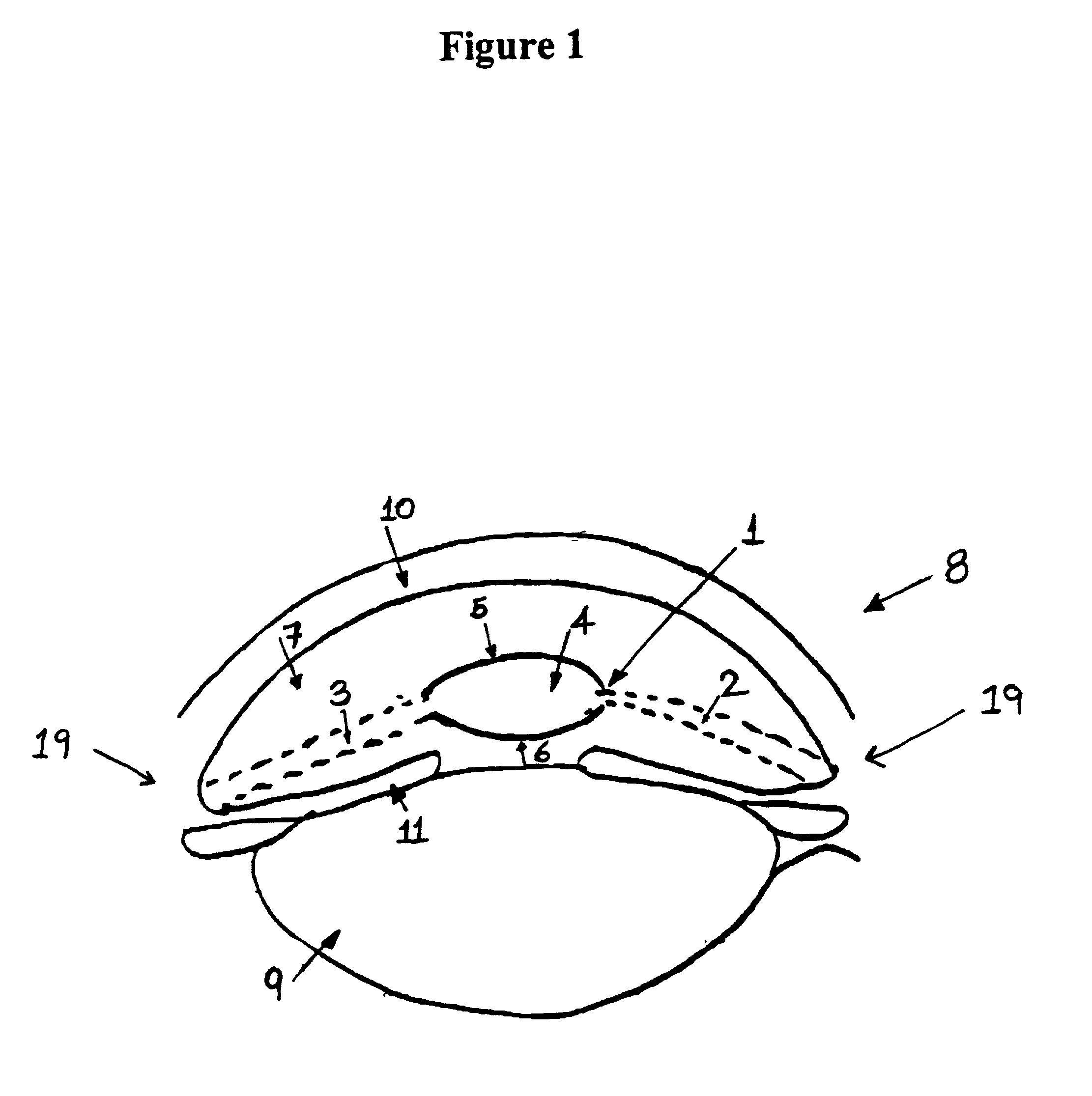
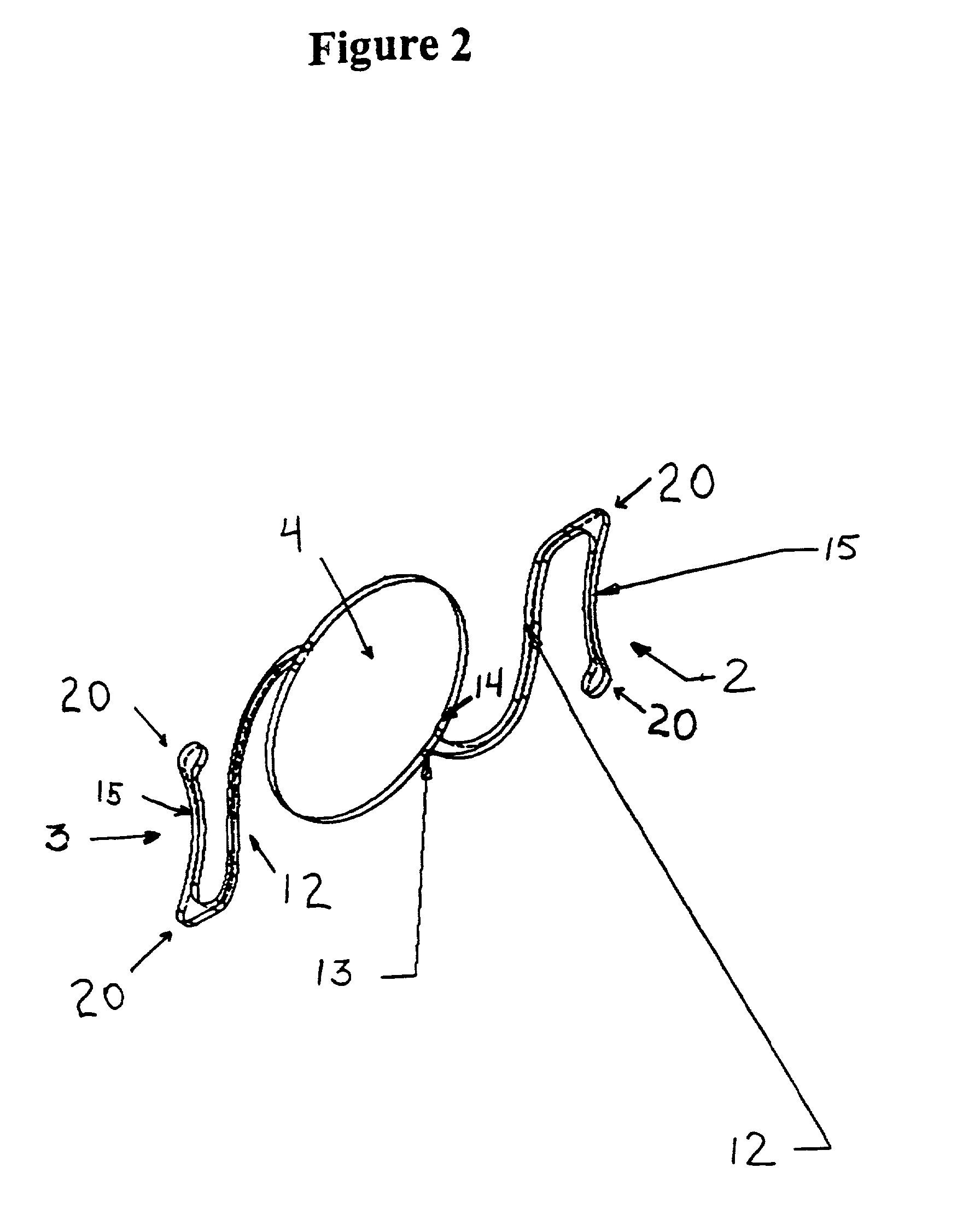
The invention provides an eyelid retractor which can fix eyeballs, and comprises a C type body (1), two arms (2), and components (3) which are adhered on the ends of an upper and a lower two arms and can be fixed on the skin, wherein the intra-annular diameter of the C type body (1) is the same as the diameter of an adult cornea, a base face of the C type body (1) is arranged at a radian at which the C type body (1) is in the anastomosis with a conjunctiva sac, and the C type body (1) of the eyelid retractor can be blocked at the edge of a corneosclera, thereby achieving the purpose of fixing eyeballs. The eyelid retractor can be used in the ophthalmic surgeries and / or ophthalmic examinations in which patients are asked to widely open eyes and keep fixed eyesight, such as the forward centesis, the anterior segment laser surgery, the optical coherence tomography technique (OTC) examination, the heidelberg retina tomograph (HRT) examination, the optic nerve fiber thickness analyzing (GDx) or the corneal endothelium counting.
Owner:肖真
In-vitro separation and purification method for tree-shrew corneal endothelial cells
ActiveCN106244549AEnhance cell viabilityTypical endothelial cell morphologyCell dissociation methodsMicrobiological testing/measurementHigh cellPurification methods
The invention discloses an in-vitro separation and purification method for tree-shrew corneal endothelial cells. The in-vitro separation and purification method includes the steps that tree-shrew corneal endothelial cells are separated, purified and subjected to passage cultivation through CECs, and corneal endothelial cells are obtained. The separated corneal endothelial cells have high cell activities, purification of the corneal endothelial cells can be achieved in the passed P3 generation is passed, the purified cells have typical endothelial cell morphology, and are identified through NSE immunofluorescence, and the dyeing rate is high. The purification efficiency of the method is greatly improved, the cost is low, using is convenient, and the large quantity of tree-shrew corneal endothelial cells can be obtained. In-vitro cultivation of the tree-shrew corneal endothelial cells is achieved, and the void that a specific corneal-endothelial-primary-cell in-vitro cultivation technology for a tree-shrew species does not exist ate present is filled; the material taking and purification methods of primary cells are easy to operate, the cost is low, and in-vitro cultivation tree-shrew corneal endothelial cells can be kept the good cell states in the long-term passage cultivation process.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Method of constructing corneal endothelium of tissue engineering
ActiveCN103725643AGuaranteed functionImprove edemaVertebrate cellsArtificial cell constructsSkin graftingCorneal endothelium
The invention discloses a method of constructing a corneal endothelium of tissue engineering by utilizing a live amnion upper skin graft. The method comprises the steps: acquiring the live amnion upper skin graft; and constructing the corneal endothelium of the tissue engineering by virtue of the live amnion upper skin graft. With the adoption of the method, as shapes, the arrangement, tissue structures, biological functions and the like of cells of the adopted live amnion upper skin graft are similar to the corneal endothelium, the live amnion upper skin graft can be adopted to replace the corneal endothelium with dysfunction, the normal functions of cornea is maintained, and the cornea transparency is improved.
Owner:XIAMEN UNIV
Method for inducing embryonic stem cell to be differentiated into corneal endothelial cells and inducing culture medium
InactiveCN106754717AAvoid Source PollutionHigh biosecurityCulture processNervous system cellsFeeder LayerL-glutamine
The invention provides an inducing culture medium for inducing to produce corneal endothelial cells. The inducing culture medium is characterized by taking a DMEM (Dulbecco Modified Eagle Medium) / F12 culture medium as a basic culture medium, and is prepared from the following components: beta-mercaptoethanol of which the molar concentration is 0.05 to 0.15 mmol / L, glutamine of which the molar concentration is 0.05 to 0.15 mmol / L, bFGF (Basic Fibroblast Growth Factors) of which the mass concentration is 4 to 8 ng / ml, NEAA (Non Essential Amino Acid) of which the molar concentration is 0.05 to 0.15 mmol / L, KSR (Knockout Serum Replacement) of which the volume concentration is 18 to 25 percent and RA of which the molar concentration is 0.5 to 2 mu mol / L. The invention also provides a method for inducing an embryonic stem cell to be differentiated into the corneal endothelial cells, wherein the corneal endothelial cells which are obtained through inducing are obvious in morphology rule feathers. The method does not involve a feeder layer, so that other animal origin pollution is avoided, and the biological safety is high; the corneal endothelial cells are used as corneal endothelium seed cells in tissue engineering, so that a qualified material source is provided for clinical corneal endothelium transplantation.
Owner:SHANDONG EYE INST
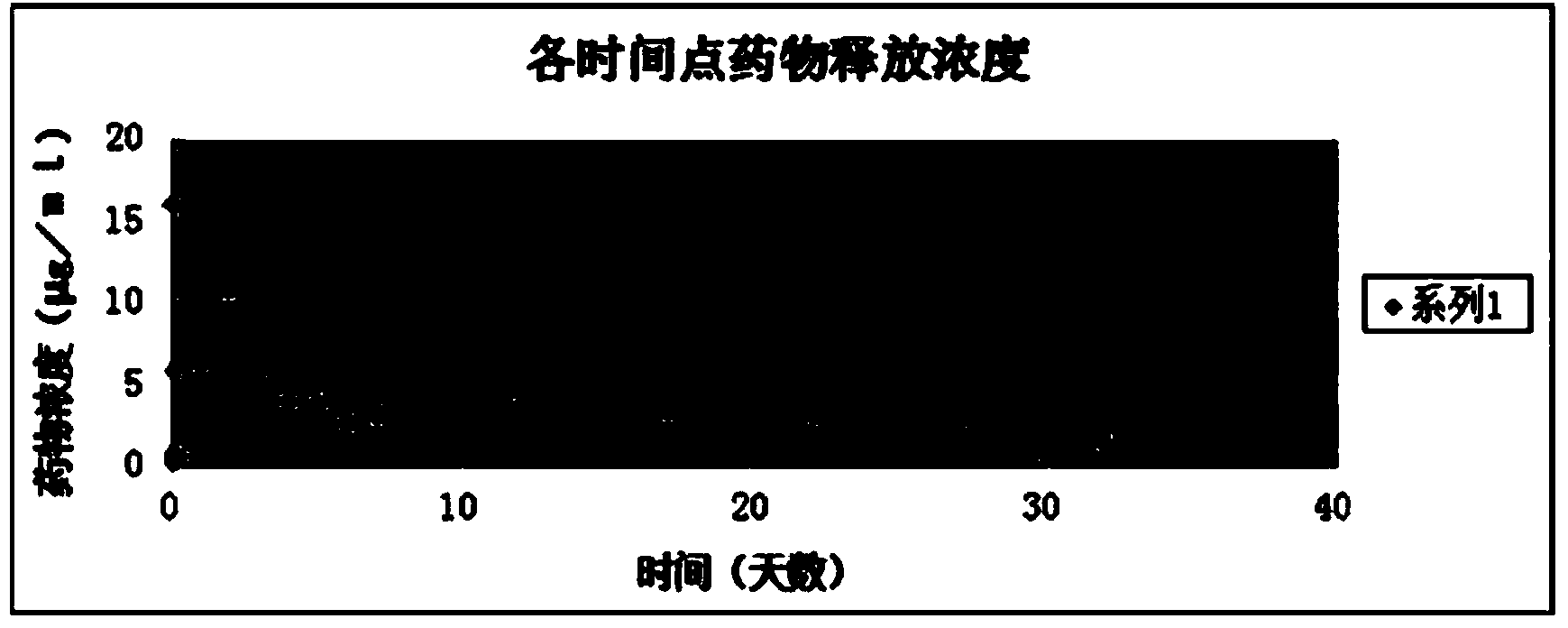
Intraocular lens loaded with drug slow-releasing thin layers on loop surfaces
InactiveCN104352289ADoes not affect mechanicsDoes not affect optical propertiesIntraocular lensSide effectIntraocular inflammation
The invention relates to an intraocular lens loaded with drug slow-releasing thin layers on loop surfaces. The purpose of the invention is that the provided intraocular lens can effectively prevent and control microbial infection and antagonism inflammatory response after a cataract surgery, reduce the occurrence rate of intraocular inflammation after the cataract surgery and prevent and inhibit after-cataract, therefore improving the postoperative visual quality; at the same time, the stability on aspects such as optics and mechanics of the intraocular lens is not influenced; the intraocular lens does not have a toxic or side effect on intraocular tissues such as the corneal endothelium of a body, epithelial cells of a ciliary body, irises, choroids, retina and the like; the provided production method is simple, mature and practical, the cost of raw materials is low, and industrialized mass production can be realized. According to the technical scheme, the intraocular lens loaded with the drug slow-releasing thin layers on the loop surfaces comprises an optical part and at least two loops symmetrically arranged on the side edge of the optical part. The intraocular lens is characterized in that the surfaces of the loops are coated with the drug slow-releasing thin layers, and the thin layers contain antibacterial or / and anti-inflammatory or / and after-cataract cataract resisting drugs.
Owner:ZHEJIANG UNIV
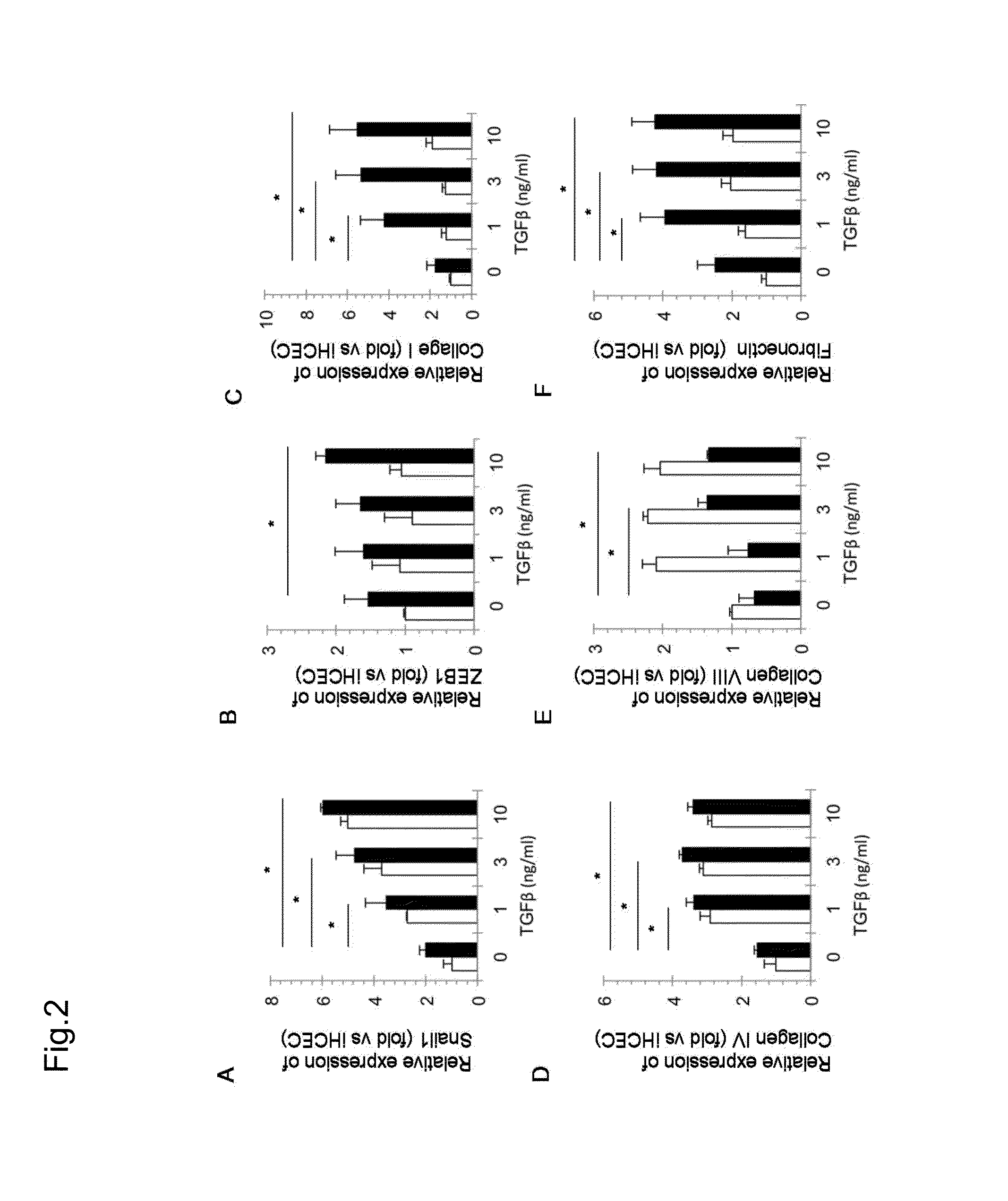
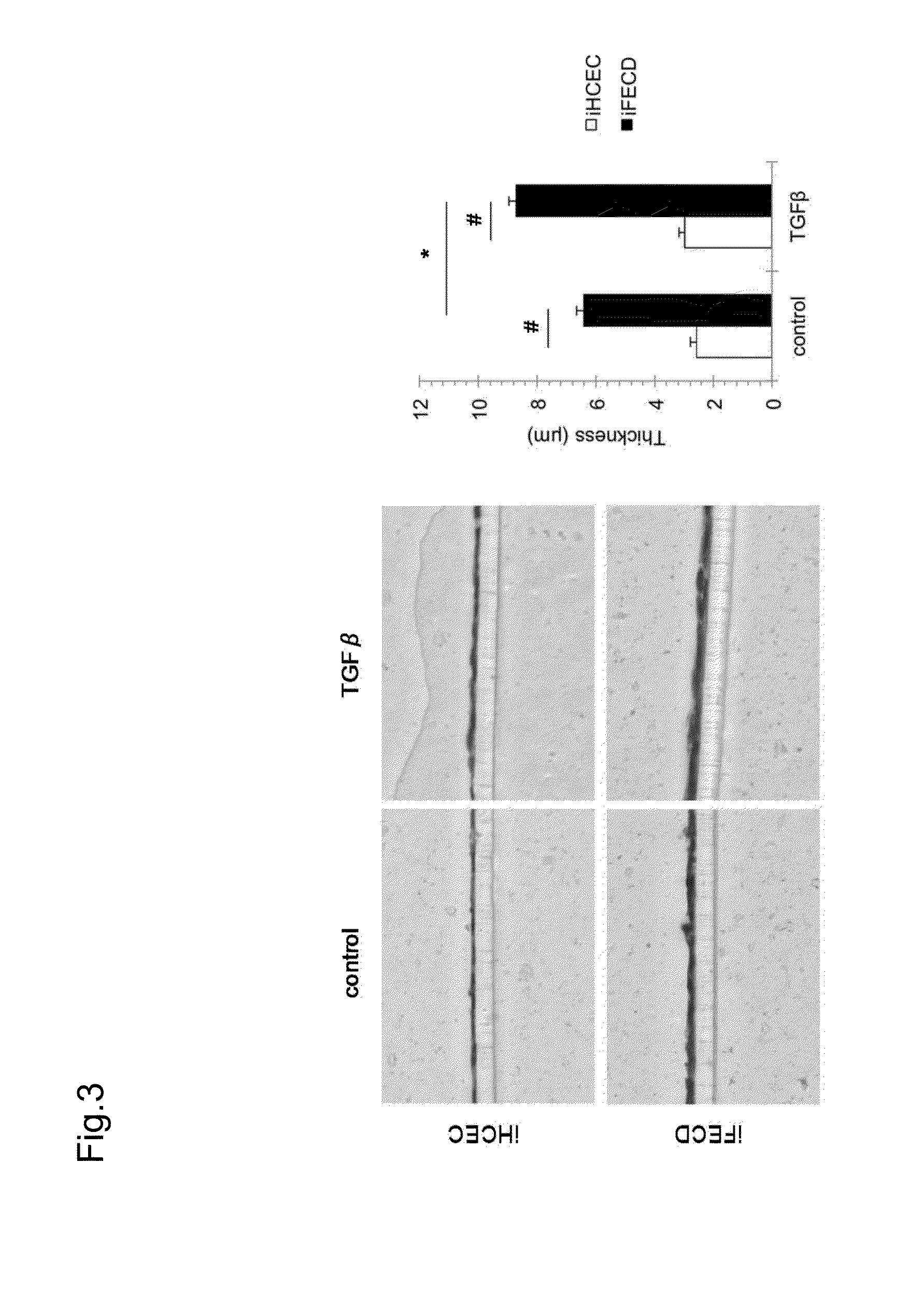
Corneal endothelium ecm therapeutic medicaments
The present invention provides medicaments for treating or preventing a disease, disorder, or condition associated with extracellular matrix (ECM) abnormality in a corneal endothelium, wherein the medicaments comprise a TGF-beta signal inhibiting agent. More specifically, this disease, disorder, or condition is a disorder associated with Fuchs' endothelial corneal dystrophy. Such a disorder includes photophobia, blurred vision, vision disorder, eye pain, lacrimation, hyperemia, pain, bullous keratopathy, ophthalmic unpleasantness, a decrease in contrast, glare, edema in corneal stroma, bullous keratopathy, corneal opacity, and the like. A preferable TGF-beta signal inhibiting agent includes 4-[4-(1,3-benzodioxol-5-yl)-5-(2-pyridinyl)-1H-imidazol-2-yl]benzamide.
Owner:KYOTO PREFECTURAL PUBLIC UNIV CORP +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com