Patents
Literature
48 results about "Donor tissue" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Donated tissue includes tendons, ligaments, bone, and cartilage. Surgical procedures that may be done using donor tissues include: ACL reconstruction. cartilage transplants. meniscus transplant. spinal fusion. fracture repair.
High-throughput tissue microarray technology and applications
InactiveUS20030215936A1Easy to analyzeGood for comparisonBioreactor/fermenter combinationsBiological substance pretreatmentsClinical informationTissue microarray
A method and apparatus are disclosed for a high-throughput, large-scale molecular profiling of tissue specimens by retrieving a donor tissue specimen from an array of donor specimens, placing a sample of the donor specimen in an assigned location in a recipient array, providing substantial copies of the array, performing a different biological analysis of each copy, and storing the results of the analysis. The results may be compared to determine if there are correlations or discrepancies between the results of different biological analyses at each assigned location, and also compared to clinical information about the human patient from which the tissue was obtained. The results of similar analyses on corresponding sections of the array can be used as quality control devices, for example by subjecting the arrays to a single simultaneous investigative procedure. Uniform interpretation of the arrays can be obtained, and compared to interpretations of different observers.
Owner:UNITED STATES OF AMERICA
Tissue transplantation compositions and methods
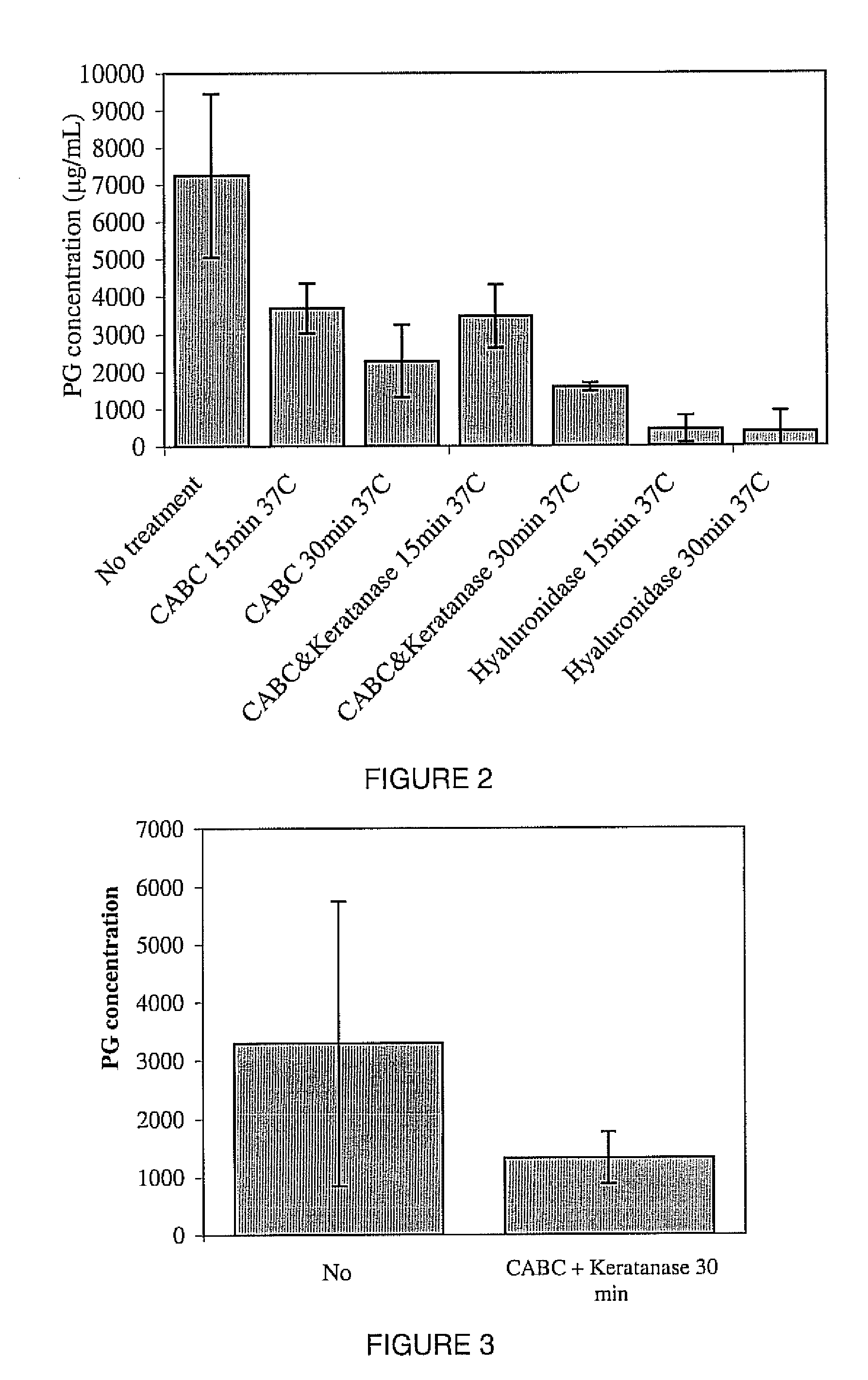
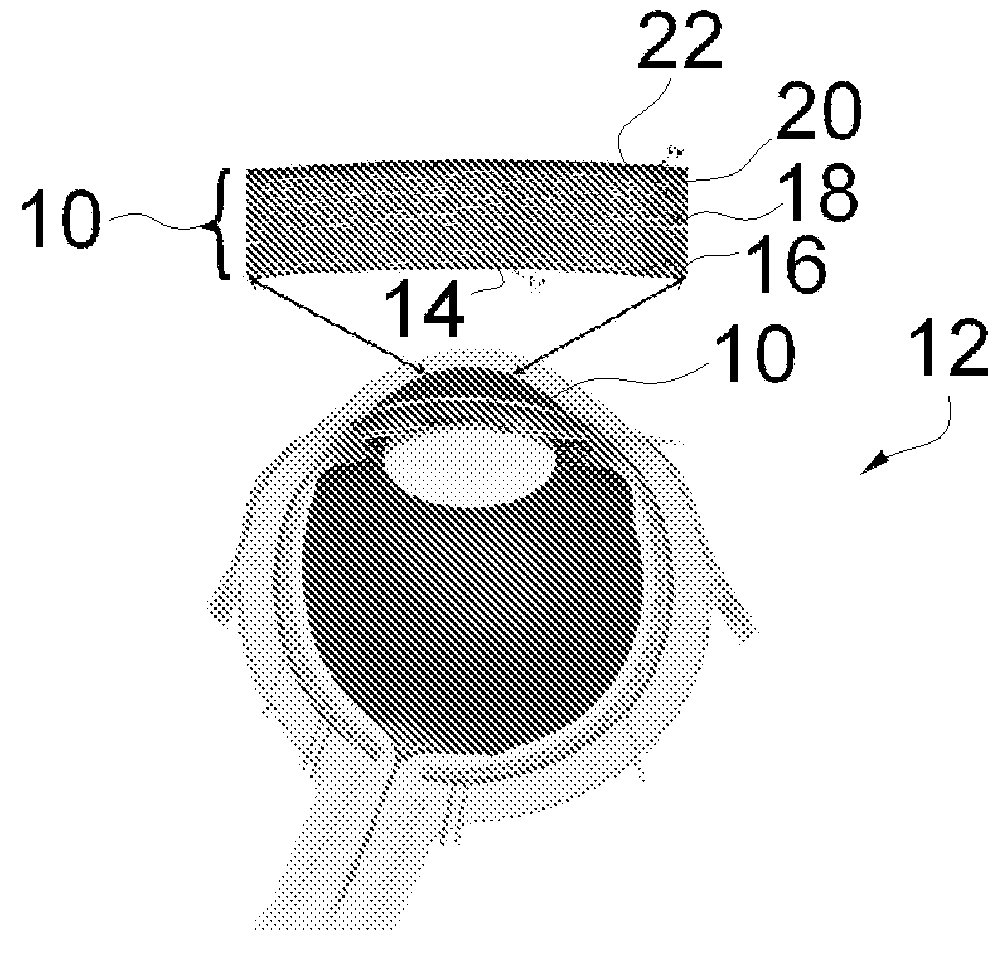
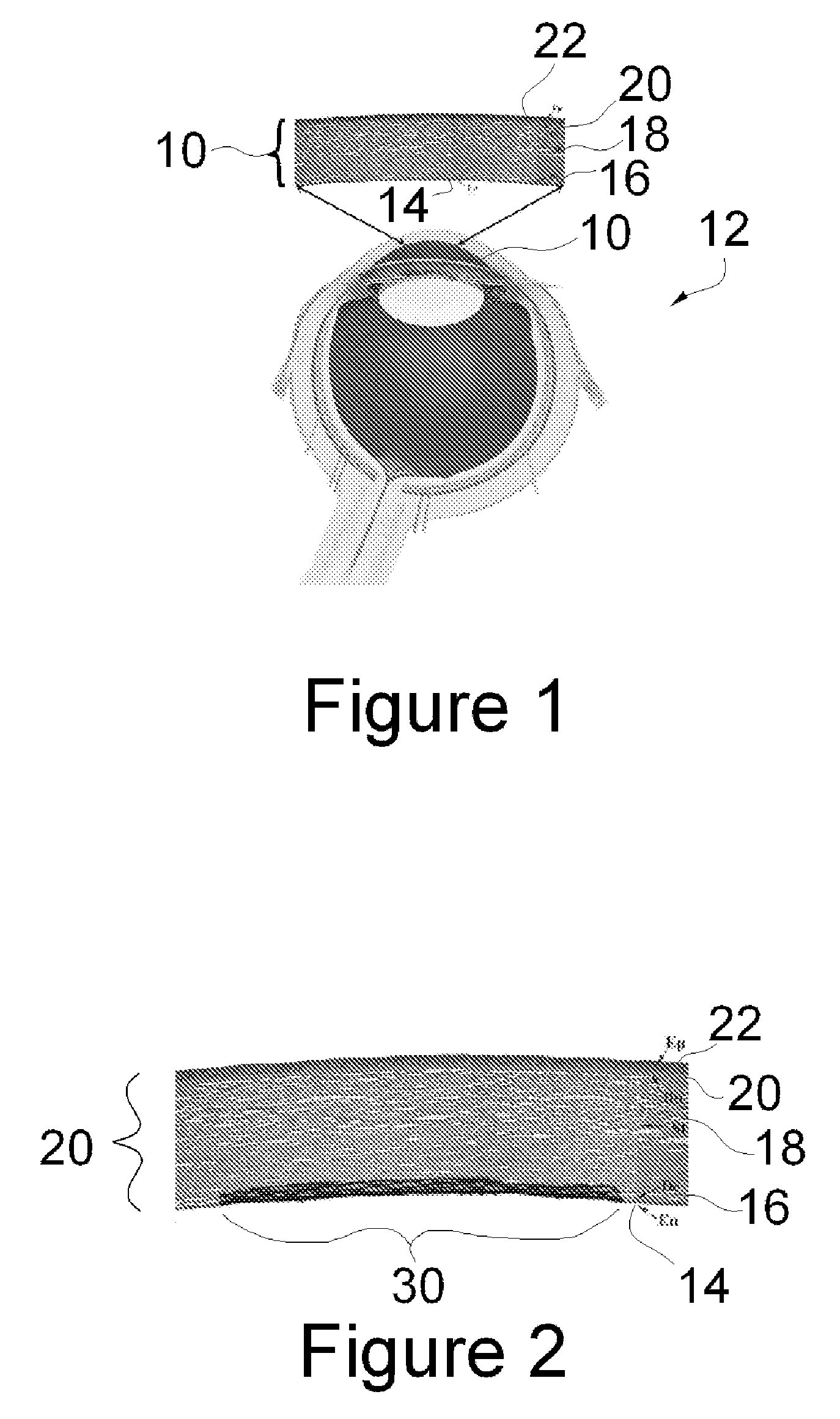
A biomedical material for transplant to a subject is provided according to embodiments of the present invention which includes an isolated donor tissue enzyme-treated to reduce the amount of proteoglycans in the donor tissue compared to untreated tissue. Isolated cells are optionally added to the enzyme-treated donor tissue, including leukocytes, particularly monocytes; macrophages; platelets; cells derived from an intervertebral disc such as chondrocyte-like nucleus pulposus cells; fibrocytes; fibroblasts; mesenchymal stem cells; mesenchymal precursor cells; chondrocytes; or a combination of any of these. The isolated donor tissue is articular cartilage or an intervertebral disc tissue such as nucleus pulposus tissue and / or annulus fibrosis tissue enzyme-treated to remove proteoglycans normally present in these tissues. A biomedical material of the present invention is administered to a subject to treat a disorder or injury, such as a disorder or injury to connective tissue.
Owner:FERREE BRET
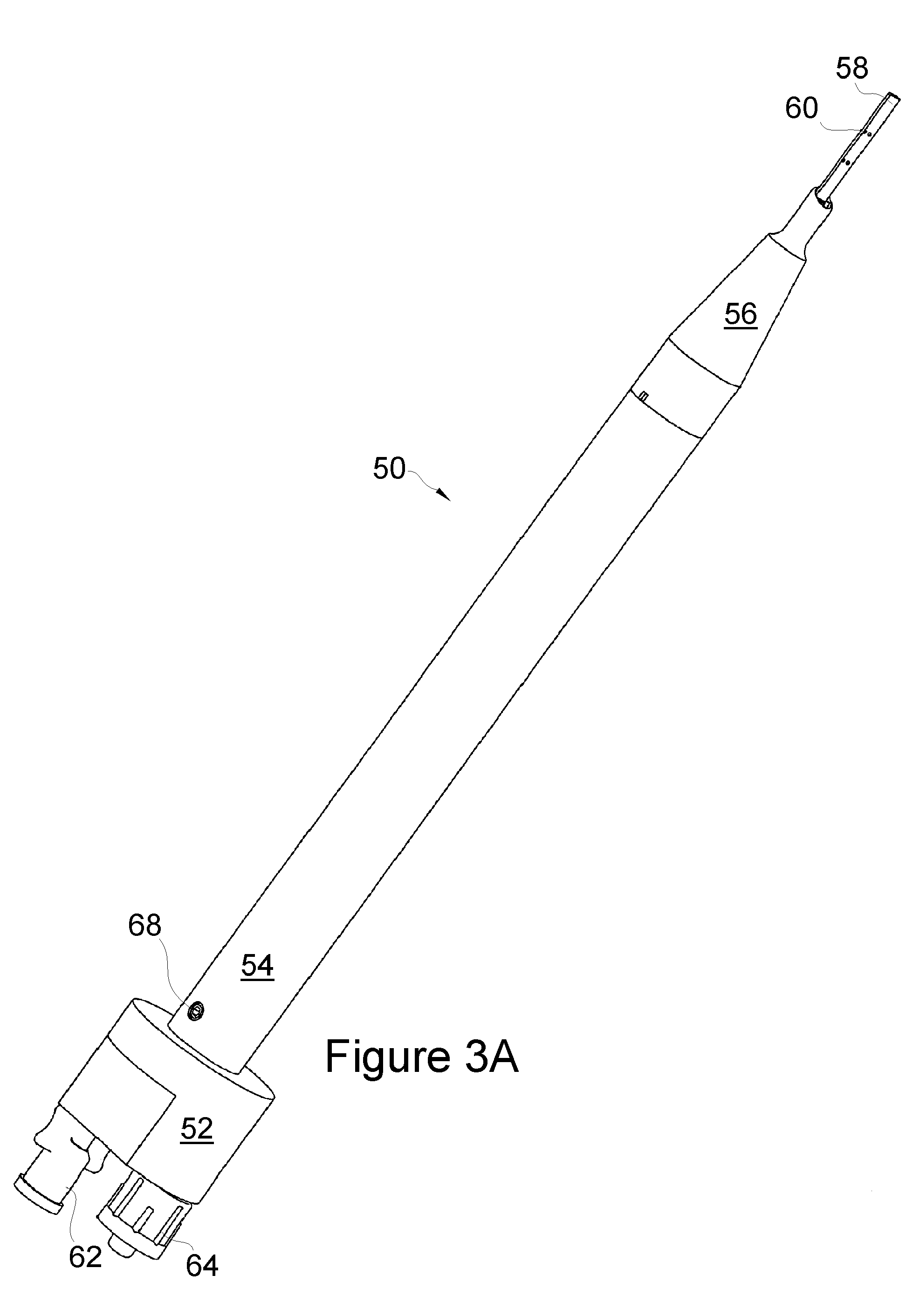
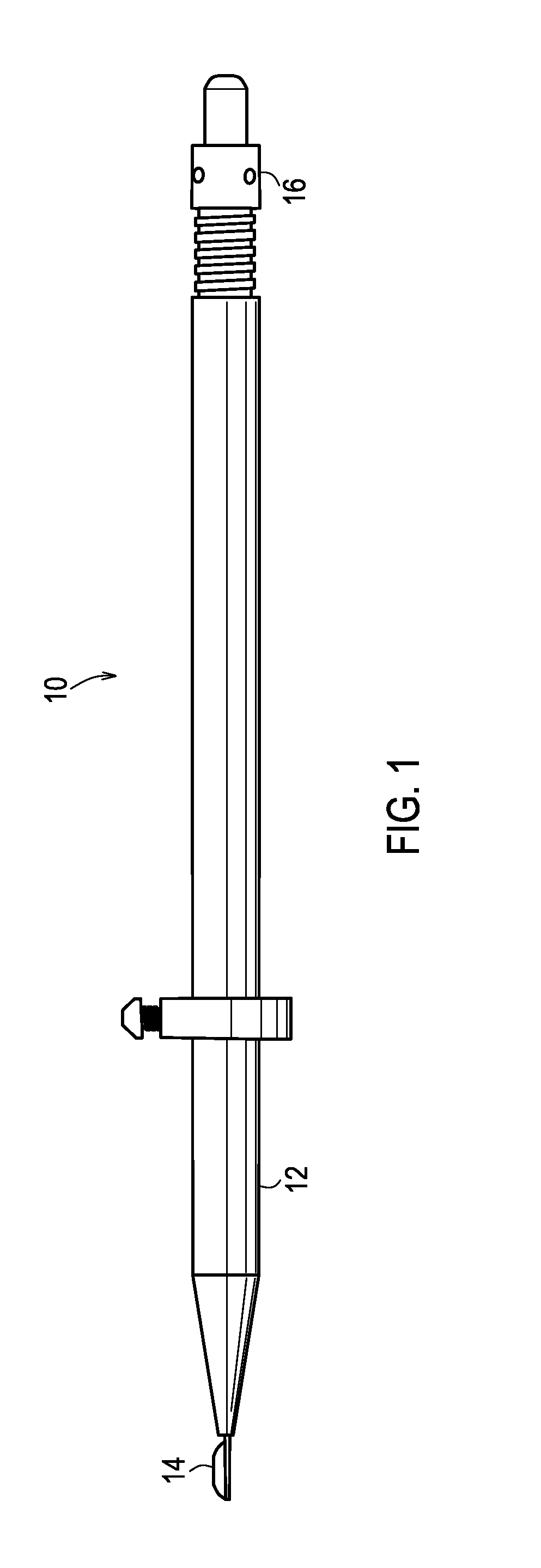
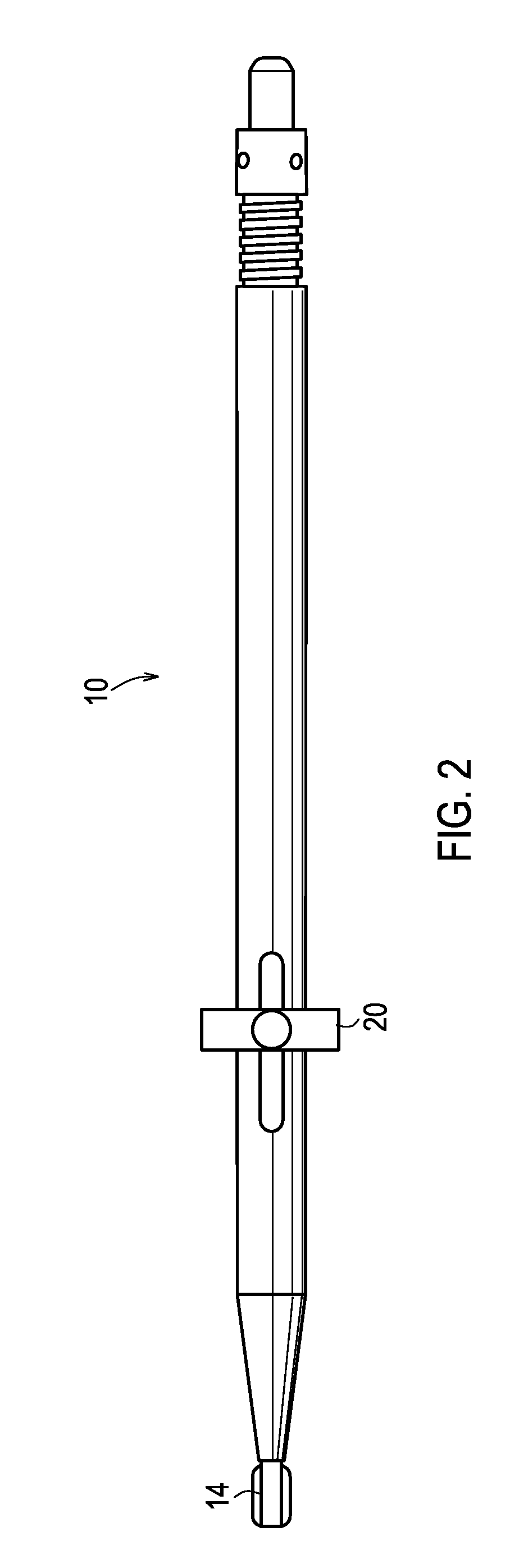
Apparatus and method for endothelial keratoplasty donor tissue transport and delivery
InactiveUS20080281341A1Minimizes endothelial layer damageEasy to operateEye implantsEye surgeryDonor tissueBiomedical engineering
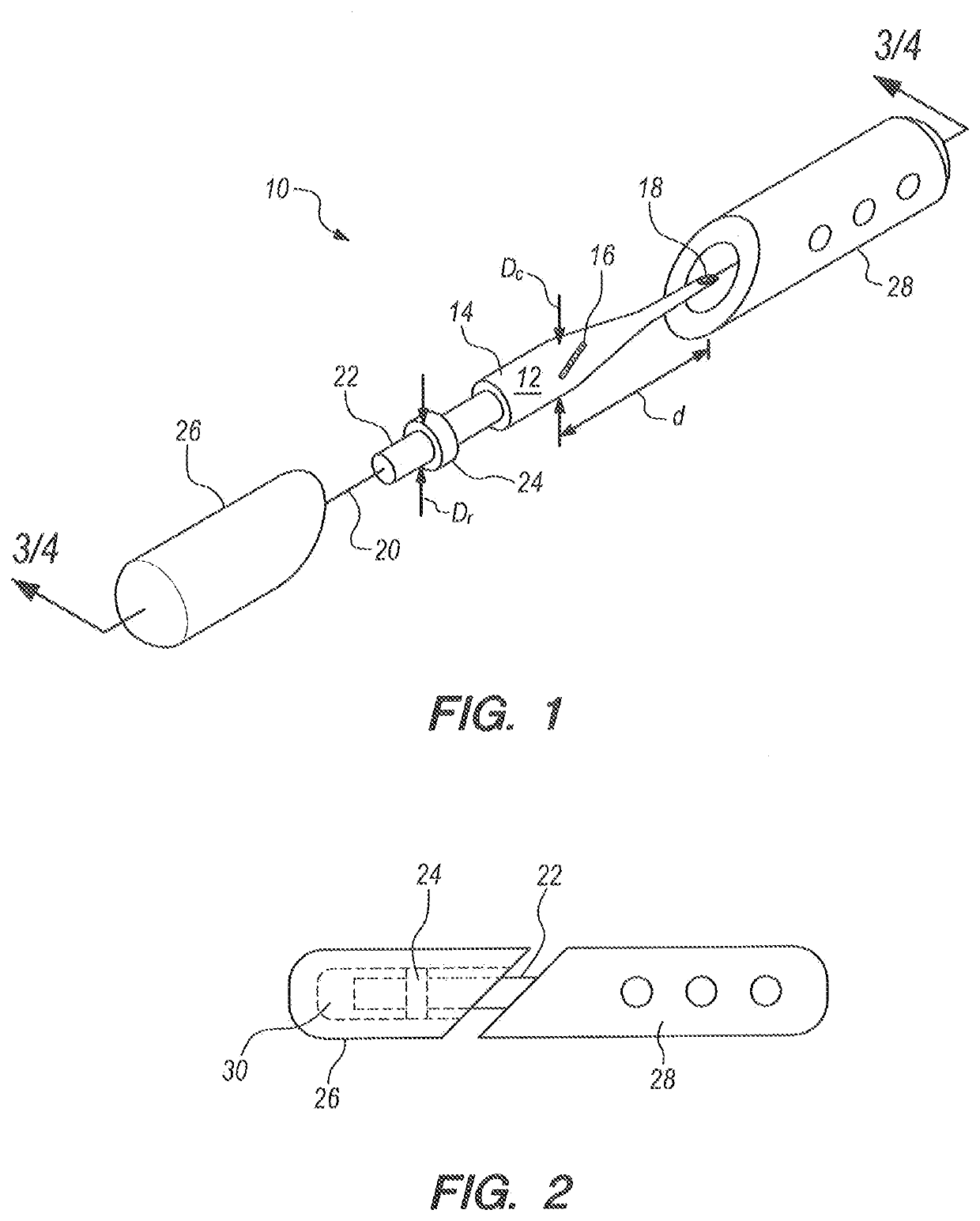
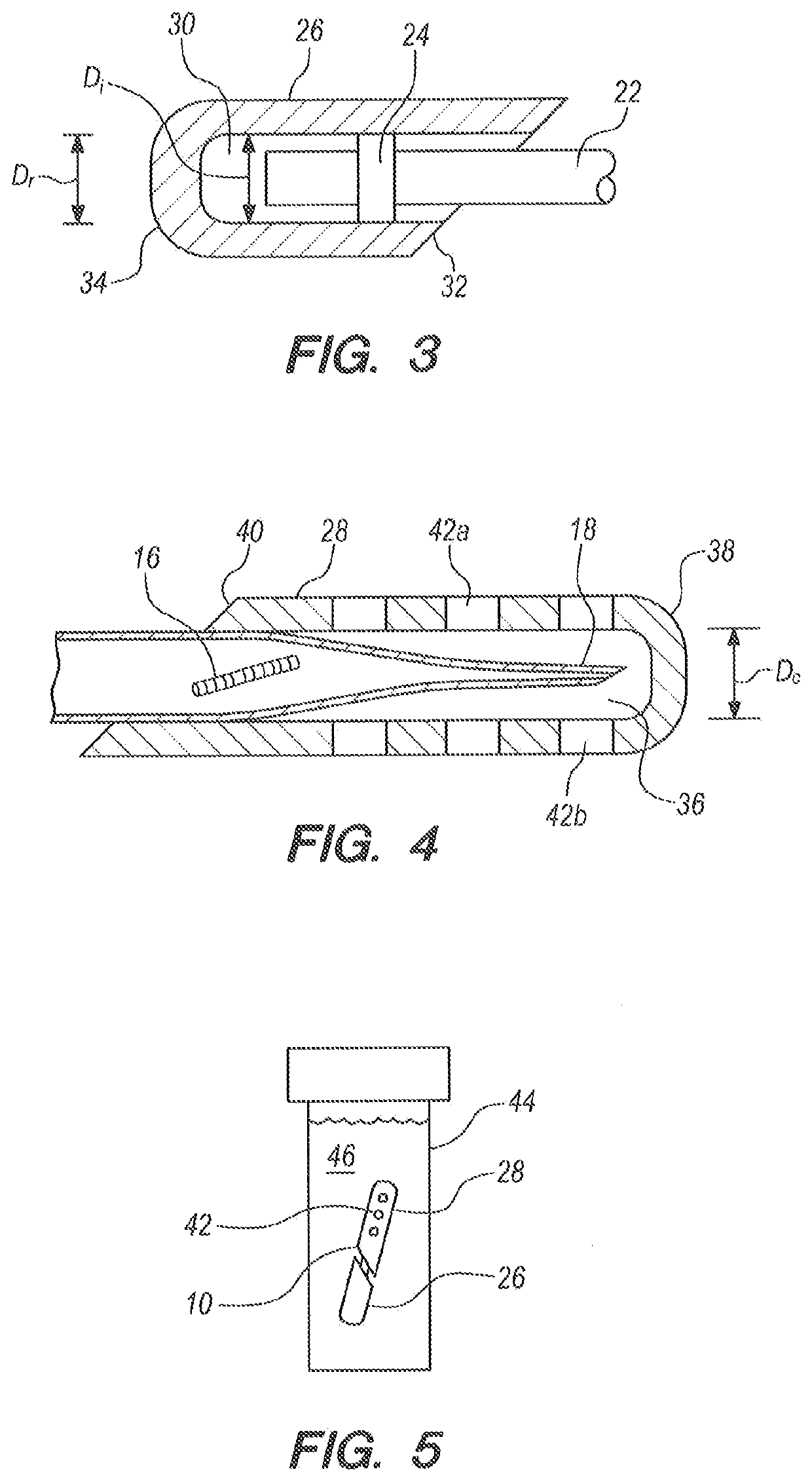
A method for endothelial keratoplasty donor tissue transport and delivery to a recipient's eye, such as in DLEK, DSEK and DSAEK procedures, comprises the steps of: attaching endothelial keratoplasty donor tissue to a tissue holder, wherein the attachment between the tissue holder and the donor tissue is not on the endothelial layer of the endothelial keratoplasty donor tissue; placing the tissue holder and the attached donor tissue within a surrounding insertion tip; transporting the insertion tip to a position adjacent an access opening in the recipient's eye; inserting the tissue holder with the attached donor tissue through the opening and extending the tissue holder from within the insertion tip to position the donor tissue within the recipient's eye; and separating the attached donor tissue from the tissue holder with the tissue holder within the recipient's eye. An associated apparatus is disclosed.
Owner:ENSION +1
Tissue processing system
ActiveUS7651507B2Small sizeEasy to disassembleDead animal preservationSurgical sawsTissue ProcessorDonor tissue
A tissue processing system includes a series of blades arranged in parallel to form a tissue processor. The blades may be adjusted to create a spaced between each blade in the range of 250-1000 microns. A slicer is included to remove a donor tissue to be processed by the processor. The processor is rotatable 90 degrees so as to create uniform micrografts of tissue for transplanting to a recipient site. An extractor is included to remove tissue particles from the processor after they have been cut into an appropriate size. A cutting block may be provided to ensure uniform thickness of cuts of the donor tissue.
Owner:KCI LICENSING INC
Tissue harvesting device and method
A tissue harvesting method and device for obtaining micrograft tissue particles within the size range of 50-1500 microns, and more preferably 500-1000 microns, and most preferably 600 microns. The particles may be processed after a piece of donor tissue has been excised from the donor site, or processed into the desired size directly at the donor site, and thereafter excised. Cutters having blades or cutting edges spaced in the range of 50-1500 microns are utilized to obtain particles within the desired size range. An elastomer is positioned between the cutting edges to push the excised particles out of the blades for ease of use.
Owner:KCI LICENSING INC
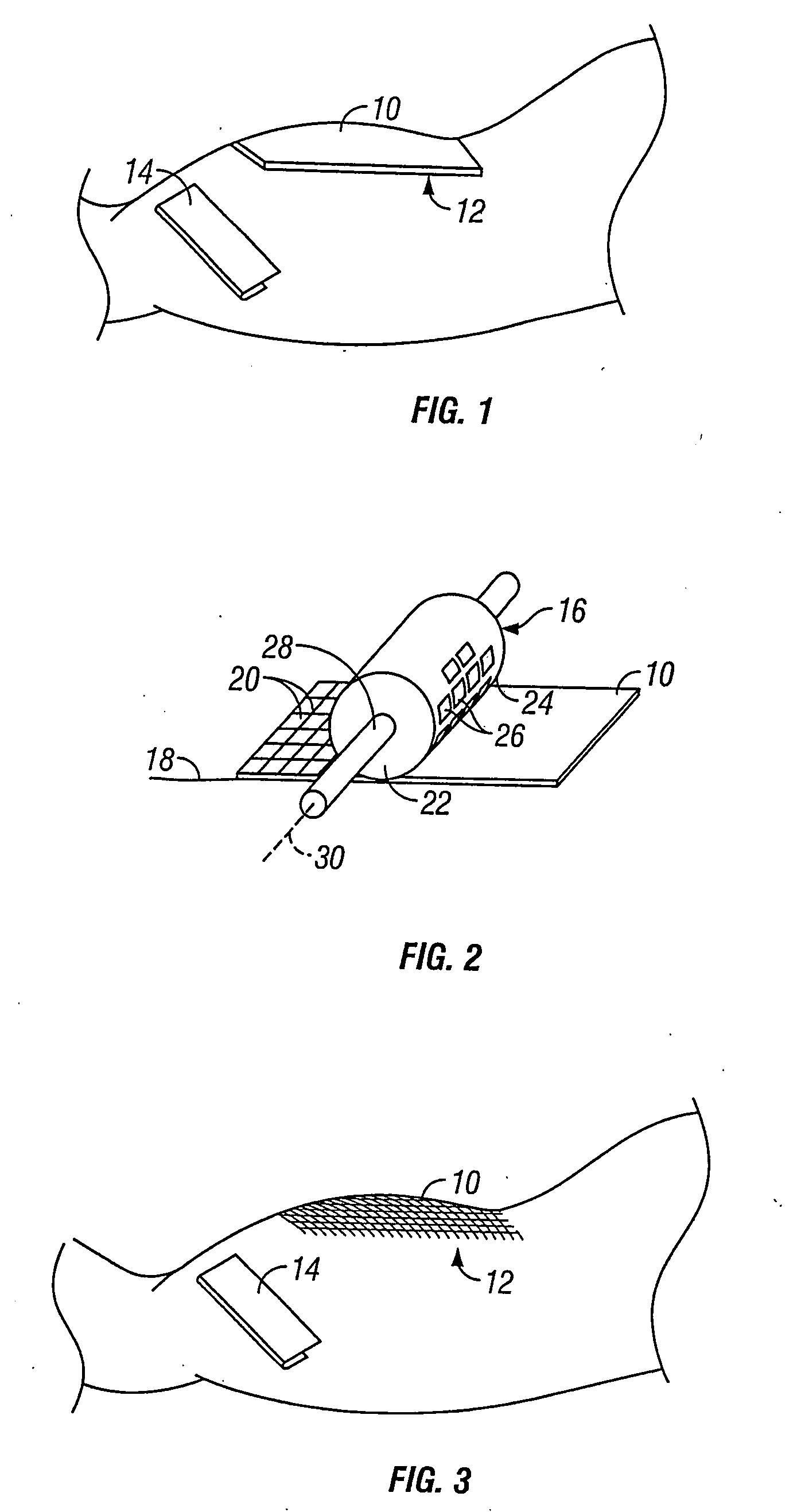
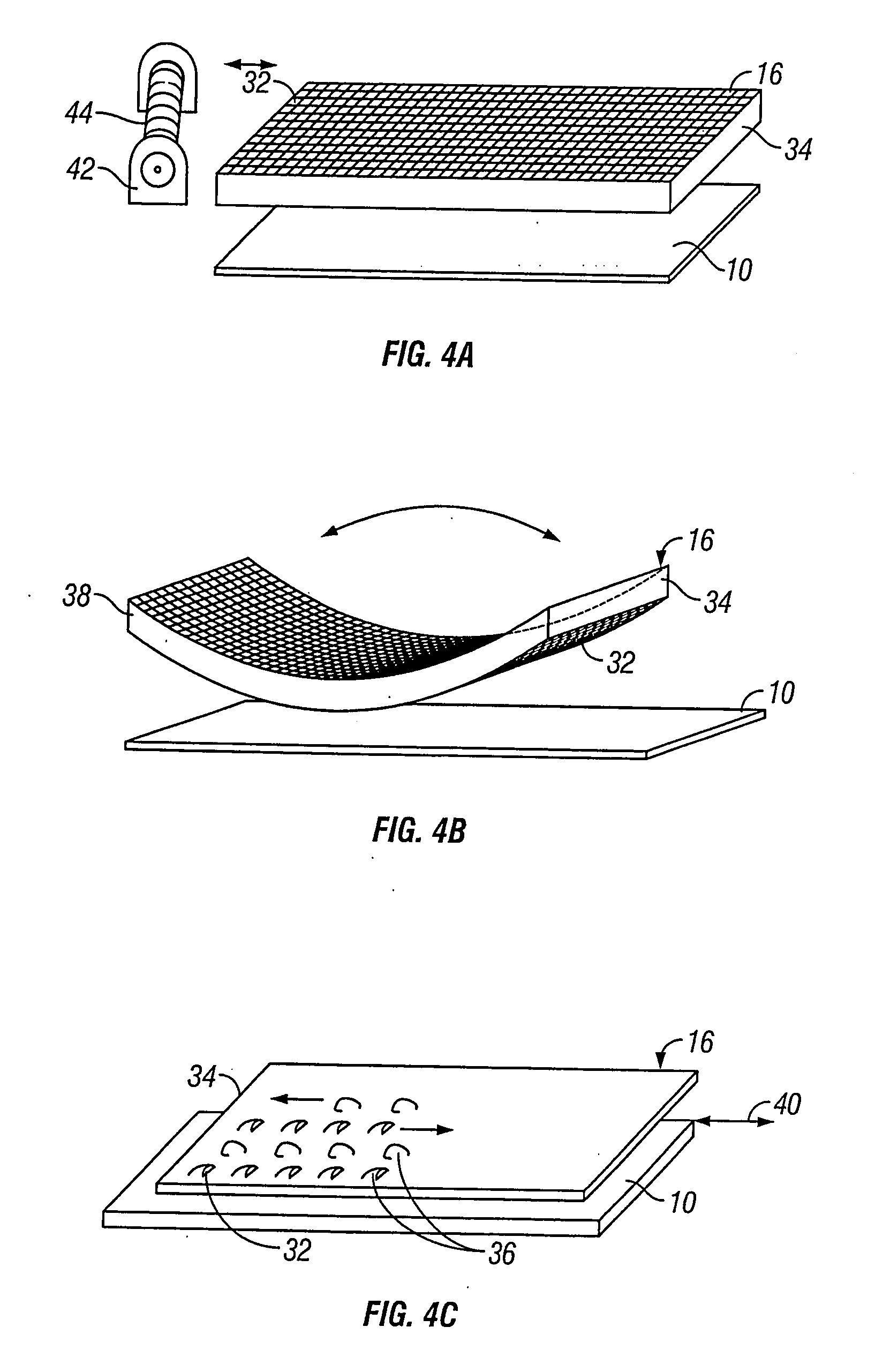
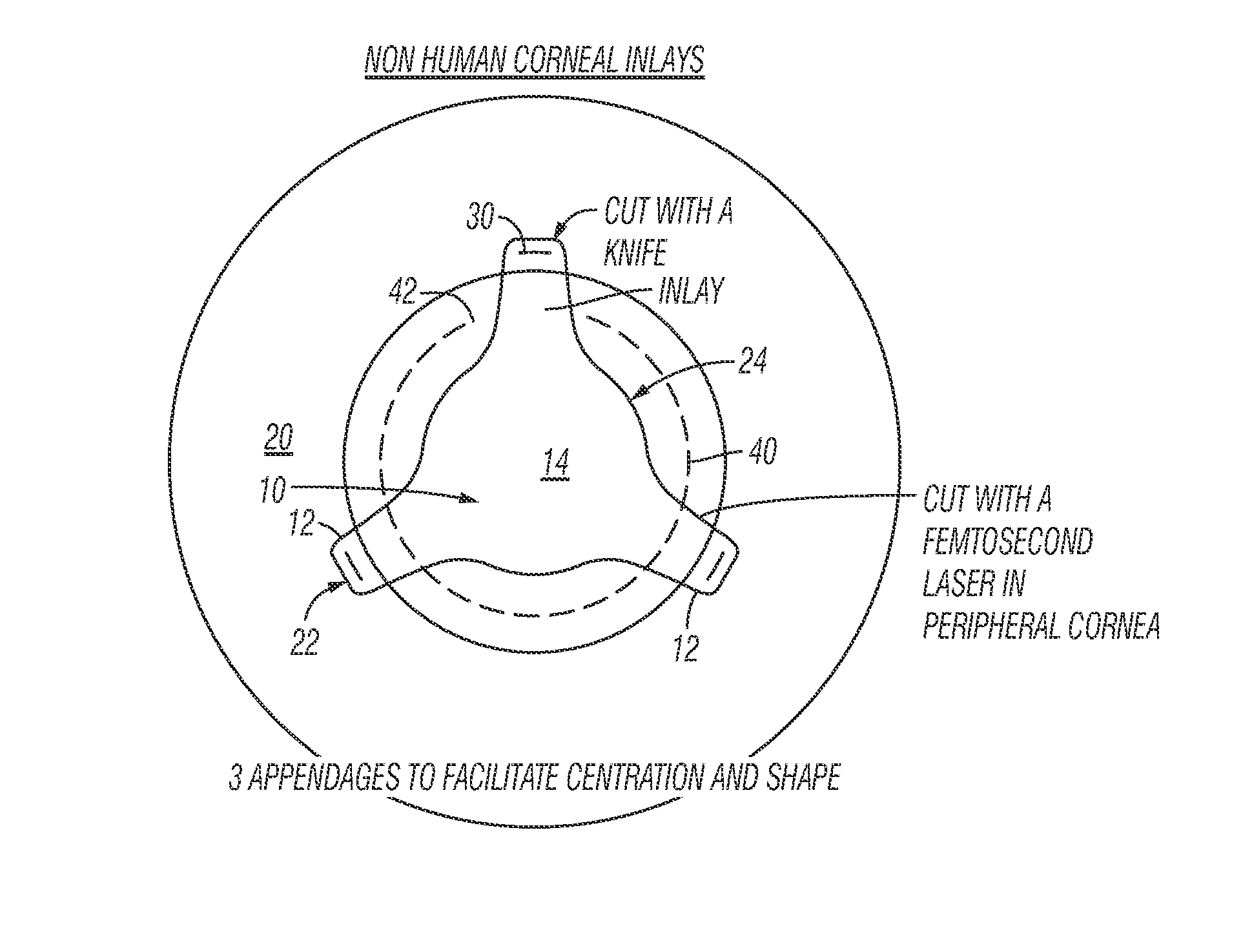
Method for preparing corneal donor tissue for refractive eye surgery utilizing the femtosecond laser
InactiveUS20140155871A1High precisionKeep clearLaser surgerySurgical instrument detailsFiberCross-link
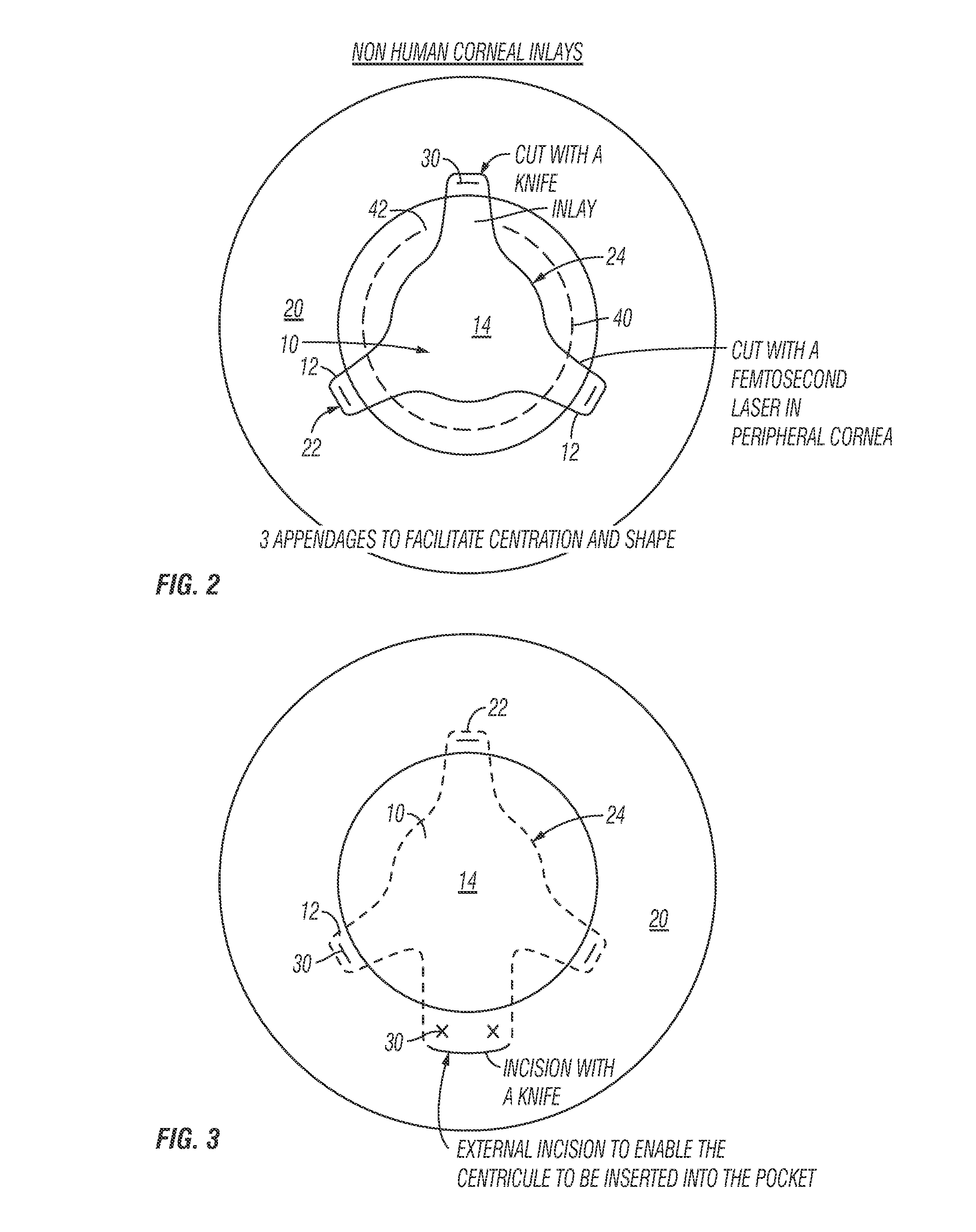
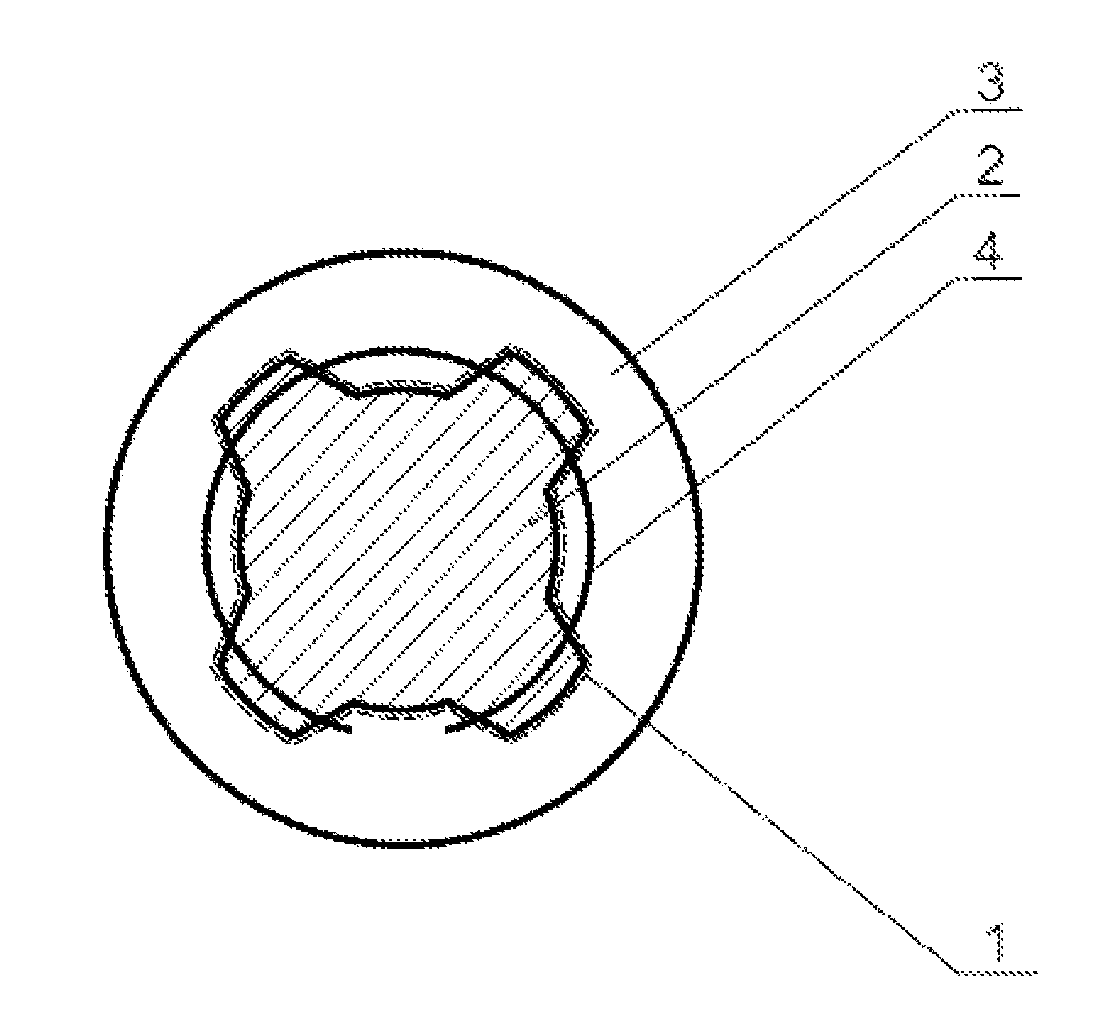
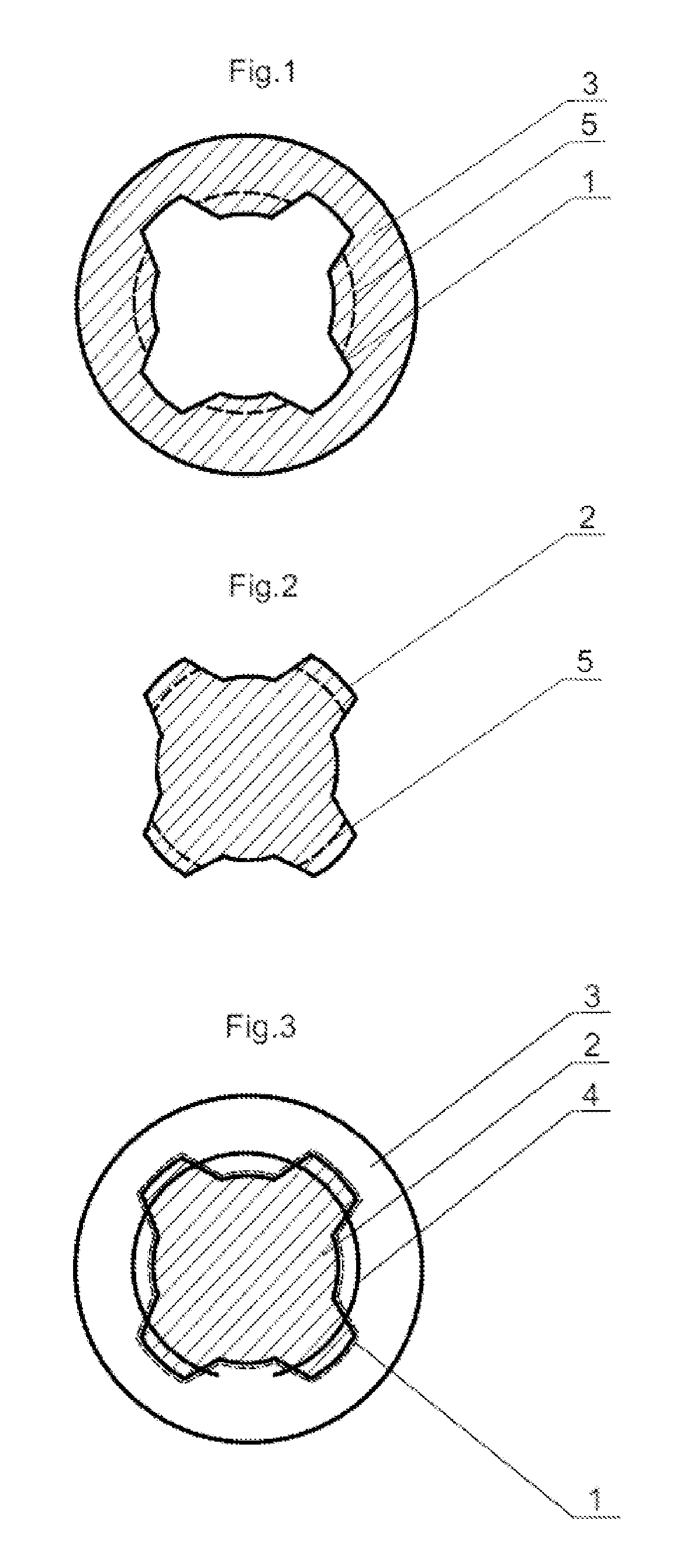
A method of accurately fabricating a non-human donor corneal tissue for implantation into a recipient human cornea, the method comprising the steps of: removing corneal tissue from a donor with a femtosecond laser; placing the corneal tissue in a fixative solution for a selected time interval to cross-link the collagen fibrils in the tissue and prevent swelling of the corneal tissue; and shaping the tissue to provide a conical inlay of a selected shape and thickness having one or more radial extensions. The method is such that the corneal inlay may be attached to the peripheral corneal and / or the sclera. The method is such that the corneal inlay may be stored for a period of up to two years prior to attachment.
Owner:CUMMING JAMES STUART
Method and implant for attachment of the transplanted cornea
Owner:INDYWIDUALNA SPECJALISTYCZNA PRAKTYKA LEKARSKA DR MED BARTLOMIEJ KALUZNY
In vivo, animal model for expression of hepatitis C virus
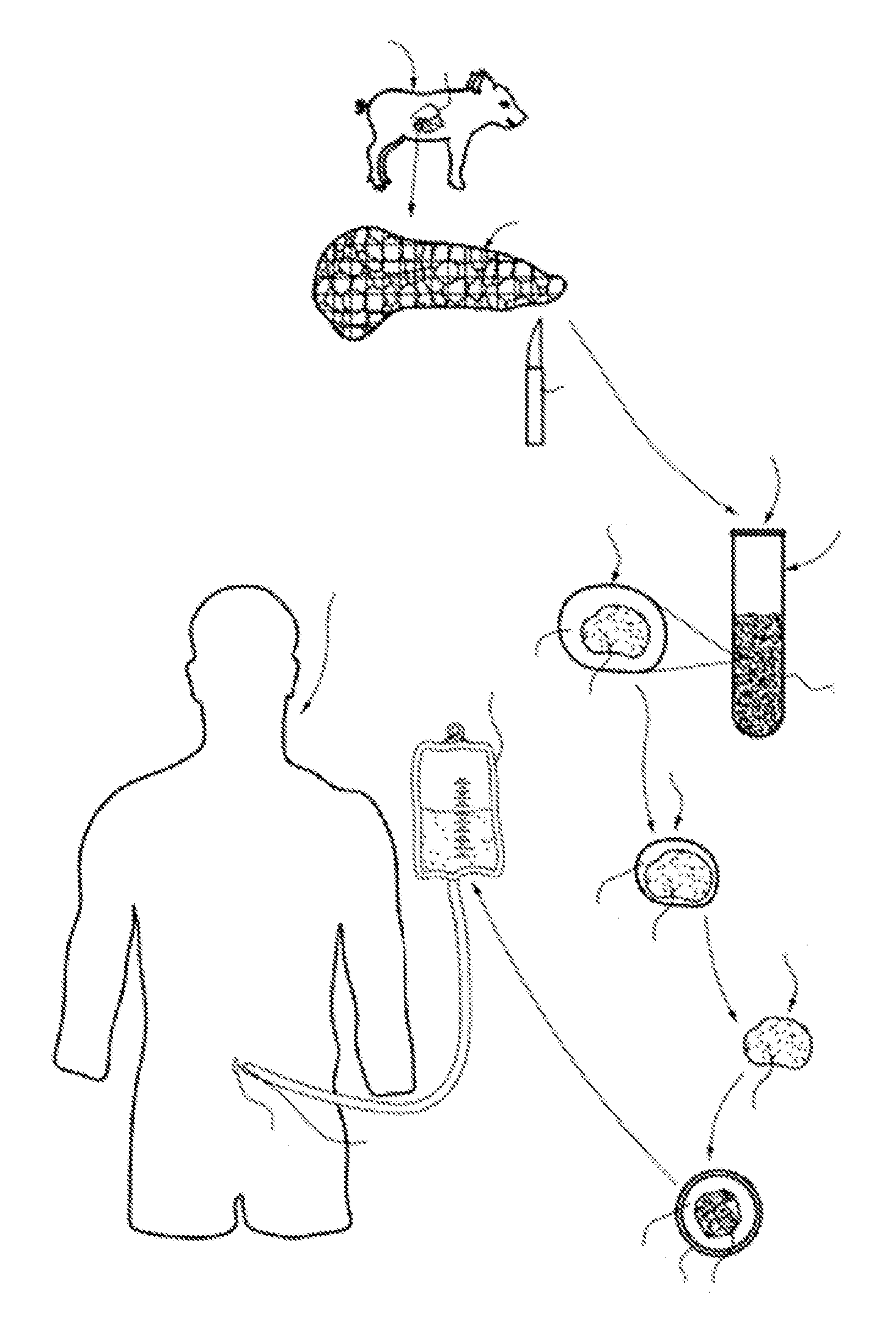
Disclosed is a method for expressing hepatitis C virus in an in vivo, animal model. Viable, hepatitis C virus-infected, human hepatocytes are transplanted into a liver parenchyma of a scid / scid mouse host. The scid / scid mouse host is then maintained in a viable state, for up to five days or greater, whereby viable, morphologically intact human hepatocytes persist in the donor tissue and hepatitis C virus is replicated in the persisting human hepatocytes.
Owner:CEDARS SINAI MEDICAL CENT
Antigen-presenting cell populations and their use as reagents for enhancing or reducing immune tolerance
InactiveUS20060292618A1Low levelImprove toleranceMicrobiological testing/measurementBlood/immune system cellsImmunologic disordersDisease
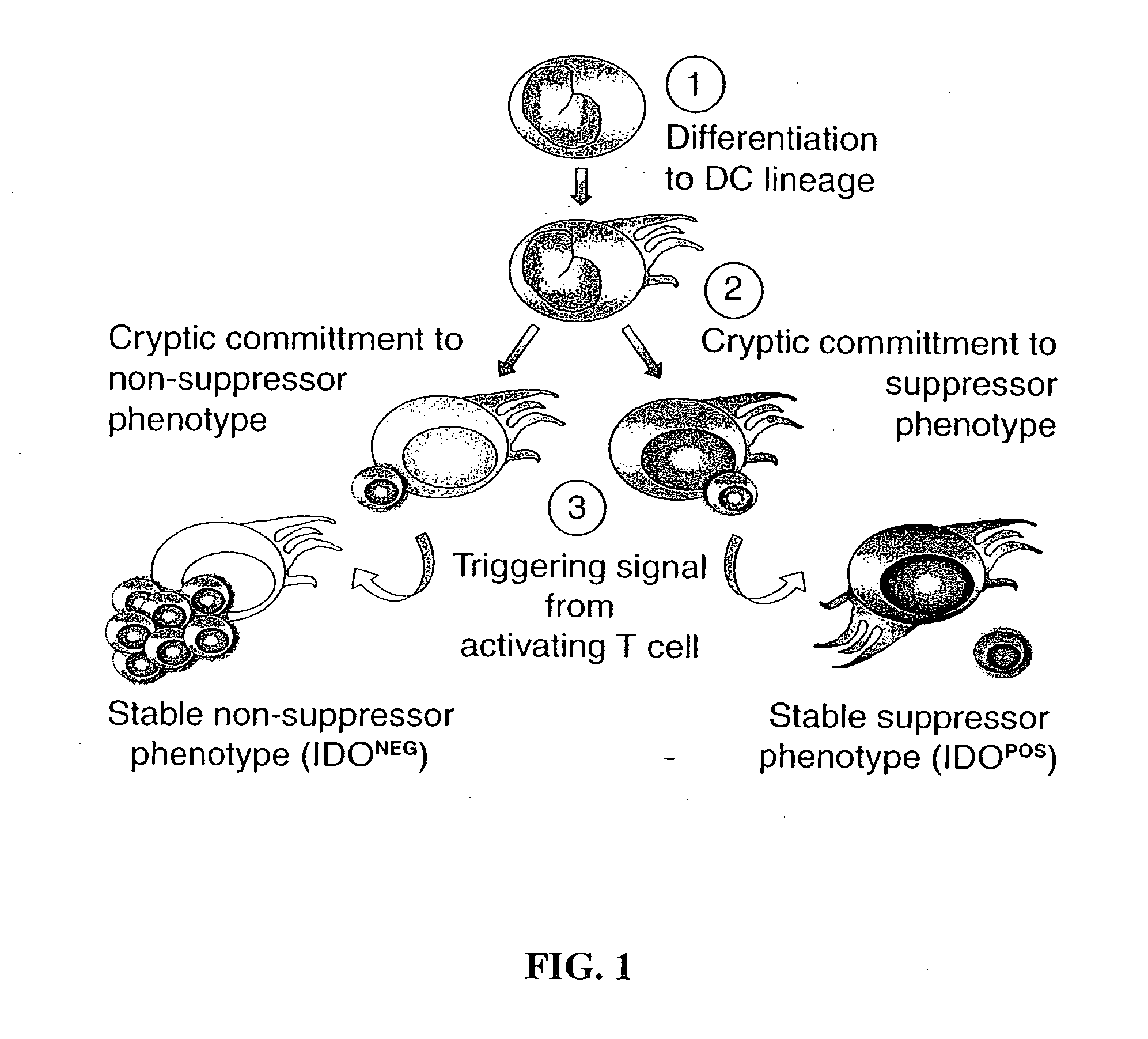
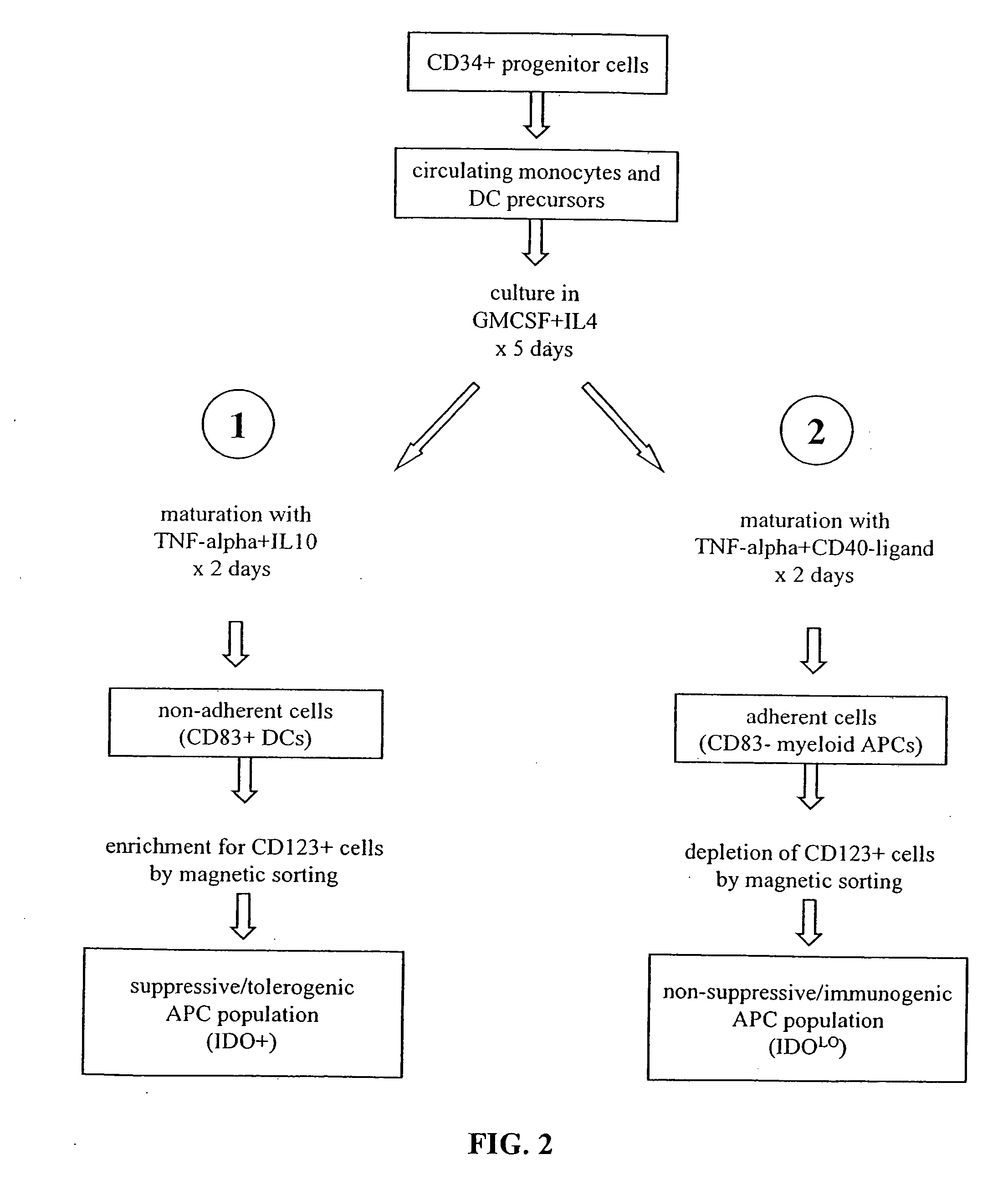
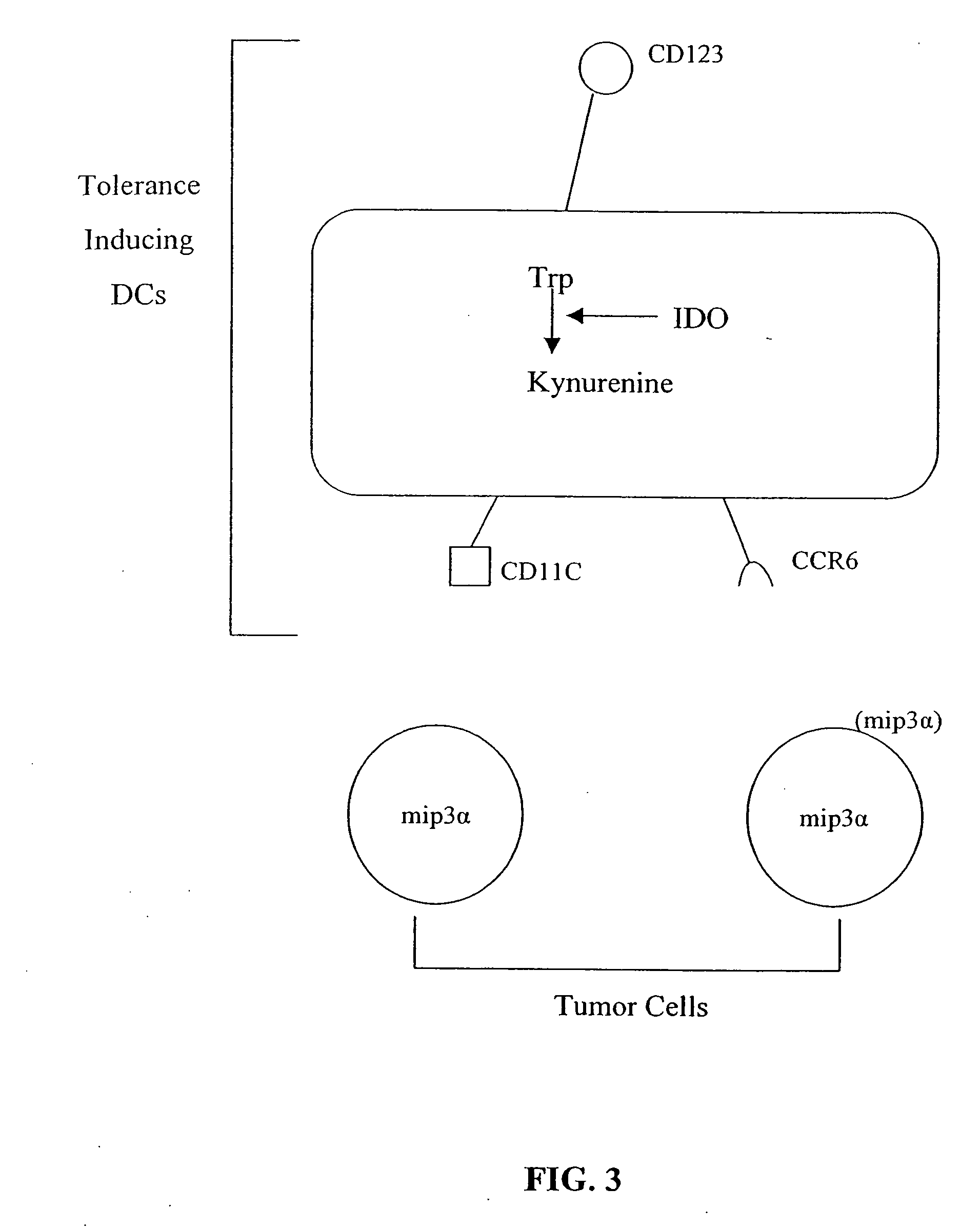
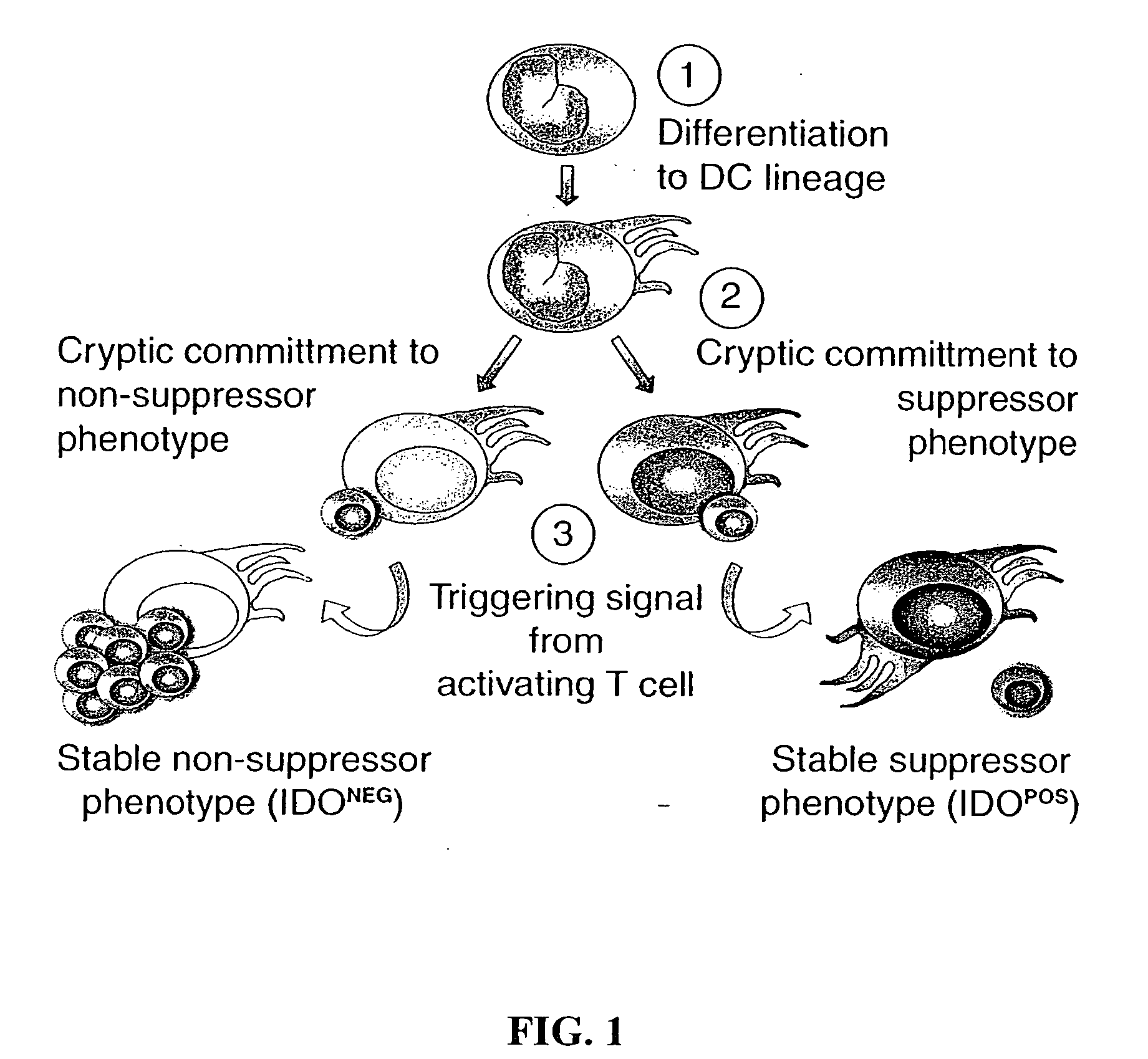
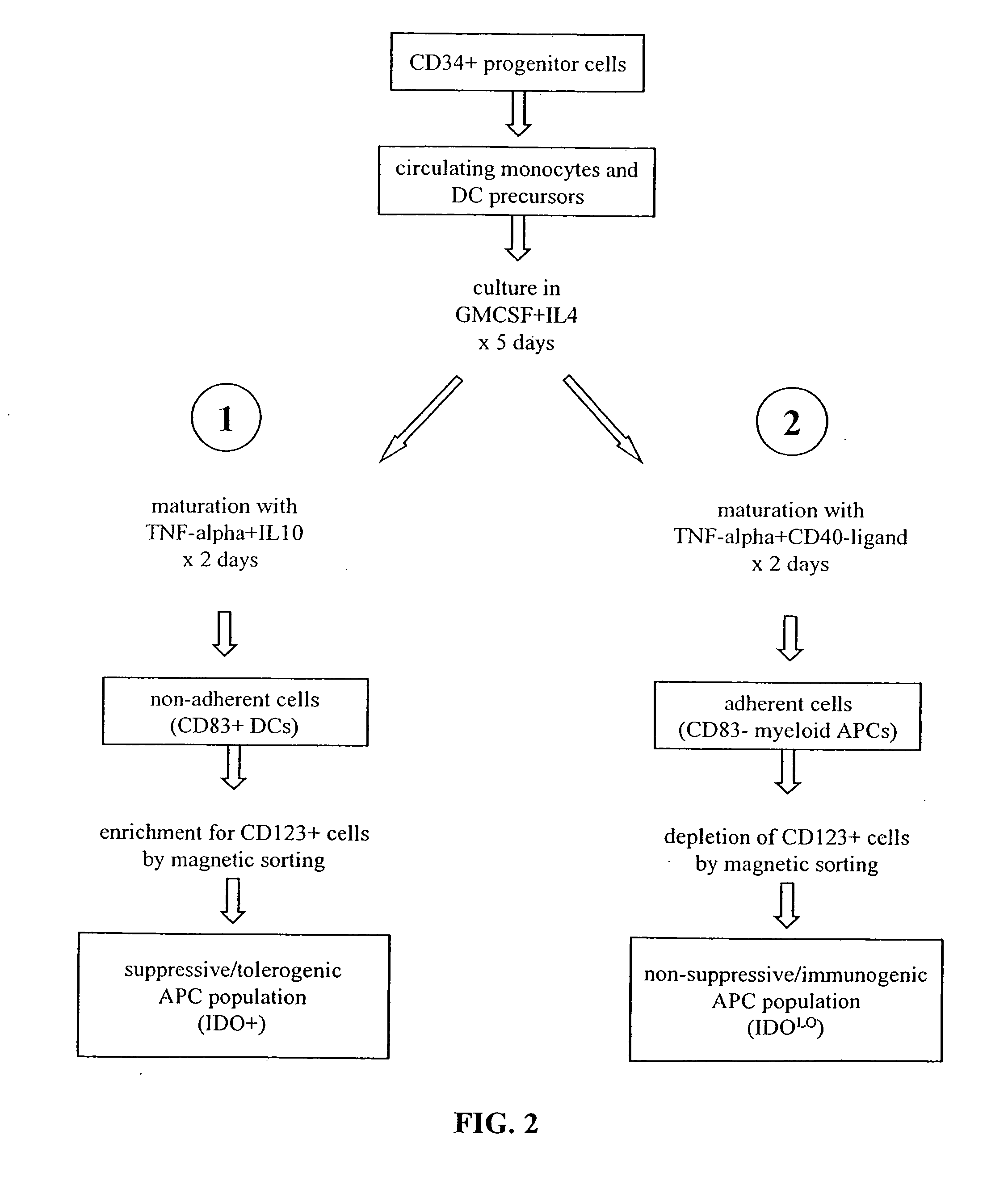
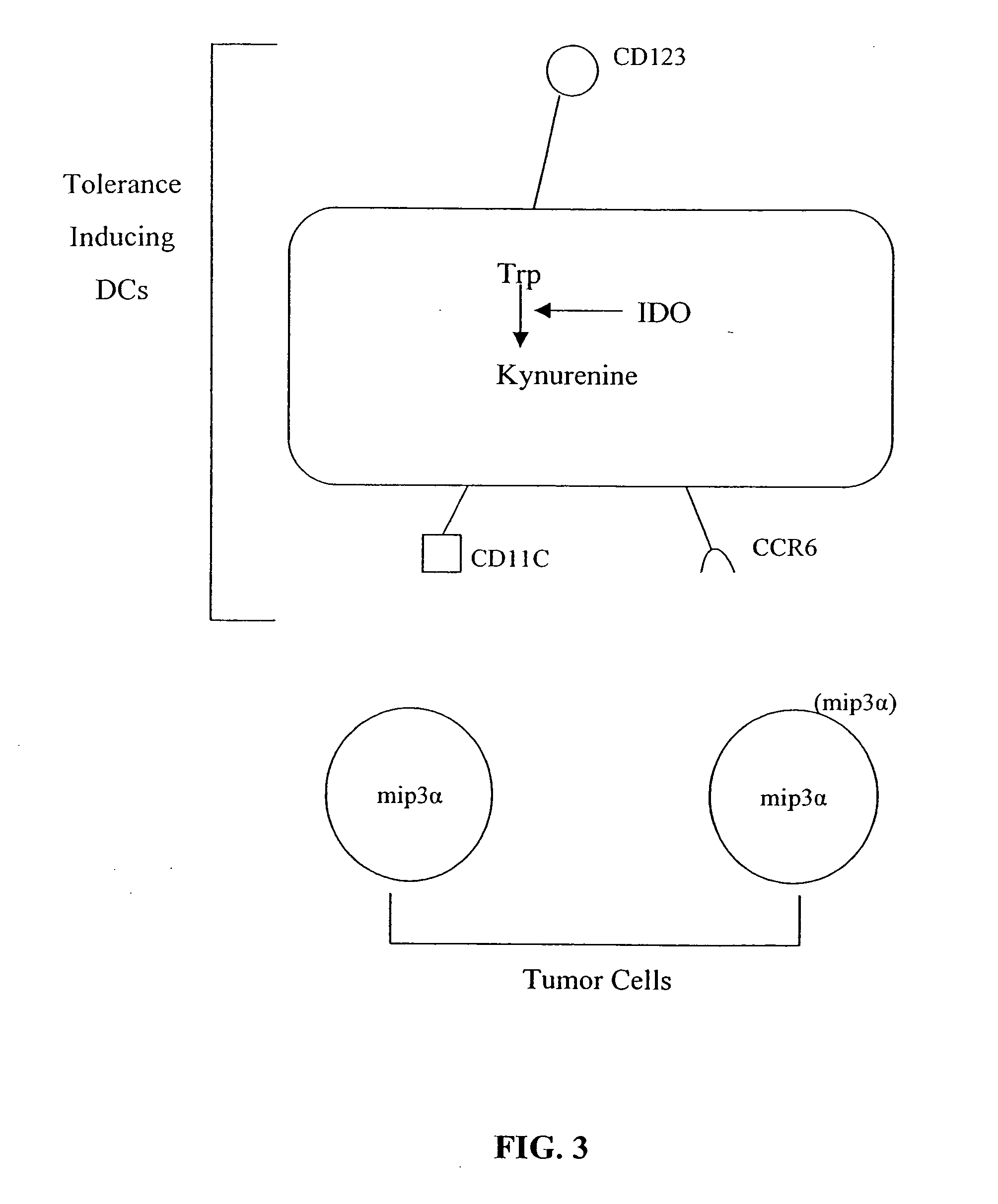
The present invention is based on the discovery antigen-presenting cells (APCs) may be generated to have predetermined levels of expression of the intracellular enzyme, indoleamine 2,3-dioxygenase (IDO). Because expression of high levels of IDO is correlated with a reduced ability to stimulate T cell responses and an enhanced ability to induce immunologic tolerance, APCs having high levels of IDO may be used to increase tolerance in the immune system, as for example in transplant therapy or treatment of autoimmune disorders. For example, APCs having high levels of IDO, and expressing or loaded with at least one antigen from a donor tissue may be used to increase tolerance of the recipient to the donor's tissue. Alternatively, APCs having reduced levels of IDO expression and expressing or loaded with at least one antigen from a cancer or infectious pathogen may be used as vaccines to promote T cell responses and increase immunity.
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
Tissue engineered fine particle tissue and method for preparing the same
A tissue engineering particle tissue and preparing process is characterized in that the tissue engineering particle tissue which is formed by cells to be compounded on micro-carriers comprises larger particle tissues which are formed by mutual connection of a plurality of particle tissues. The invention uses the tissue engineering technology to culture highly active tissue engineering particle tissues to implant in the body for repairing defected tissues and organs. The invention has a good cell growth state and higher cell activity, and can clinically solve the difficulties of less donor tissues and large repairing area, and the micro-carriers can finally be degraded, after the body is cured, no influences can be caused to the body. In the clinical treatment, the invention can be used as a transplant for repairing large area tissue defection and as filling material for repairing body depressed deformation, and can be transplanted to wound surfaces. The invention can also be used by injection, and is extensive in application range and convenient in utilization.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Antigen-presenting cell populations and their use as reagents for enhancing or reducing immune tolerance
InactiveUS20070048769A1Low levelImprove toleranceMicrobiological testing/measurementAntibody ingredientsAntigenDisease
The present invention is based on the discovery antigen-presenting cells (APCs) may be generated to have predetermined levels of expression of the intracellular enzyme, indoleamine 2,3-dioxygenase (IDO). Because expression of high levels of IDO is correlated with a reduced ability to stimulate T cell responses and an enhanced ability to induce immunologic tolerance, APCs having high levels of IDO may be used to increase tolerance in the immune system, as for example in transplant therapy or treatment of autoimmune disorders. For example, APCs having high levels of IDO, and expressing or loaded with at least one antigen from a donor tissue may be used to increase tolerance of the recipient to the donor's tissue. Alternatively, APCs having reduced levels of IDO expression and expressing or loaded with at least one antigen from a cancer or infectious pathogen may be used as vaccines to promote T cell responses and increase immunity.
Owner:GEORGIA HEALTH SCI UNIV RES INST
Cell Suspension Preparation Technique and Device
InactiveUS20100196334A1Rapid and efficient and simple to prepare and applyReduce complexityBiocideBioreactor/fermenter combinationsDonor tissueBiomedical engineering
The present invention provides for methods and devices suitable for producing a transplantable cellular suspension of living tissue suitable for grafting to a patient. In applying the method and / or in using the device, donor tissue is harvested, subjected to a cell dissociation treatment, cells suitable for grafting back to a patient are collected and dispersed in a solution that is suitable for immediate dispersion over the recipient graft site.
Owner:AVITA MEDICAL LTD
Kits for treatment of hematologic disorders
InactiveUS7408039B2Reduce GVHDSignificant graft-versus-leukemia (GVL) effectBiocideGenetic material ingredientsGraft versus leukaemiaHematological Diseases
The inventors have discovered that hematologic disorders, e.g., both neoplastic (hematologic cancers) and non-neoplastic conditions, can be treated by the induction of mixed chimerism using myeloreductive, but not myeloablative, conditioning. Methods of the invention reduce GVHD, especially GVHD associated with mismatched allogeneic or xenogeneic donor tissue, yet provide, for example, significant graft-versus-leukemia (GVL) effect and the like.
Owner:THE GENERAL HOSPITAL CORP
Corneal endothelial tissue inserter
ActiveUS20100274257A1Avoids inadvertent adherenceLow costSurgeryEye treatmentCorneal endotheliumNose
A device transfers donor endothelial cells to the cornea of a recipient without injuring the cells or the cornea during the transfer process. The device transfers living endothelial cells from a donor to a recipient with minimal or no trauma. The device includes a handle, an irrigation tube lengthwise within the handle, a button upon the handle concentric with the irrigation tube, a tapered nose opposite the button, a forward tube extending outwardly from the nose, and a platform joined to the irrigation tube. The forward tube has an elliptical cross section and two beveled features. The platform has two wings that curl gently around donor tissue upon retracting the platform into the forward tube. The handle has a groove for a stem of a knob that surgeon presses and pulls to move the platform.
Owner:FISCHER SURGICAL
Treatment of hematologic disorders
InactiveUS7892578B2Reduce GVHDRemarkable effectBiocideOrganic active ingredientsGraft versus leukaemiaHematological Diseases
The inventors have discovered that hematologic disorders, e.g., both neoplastic (hematologic cancers) and non-neoplastic conditions, can be treated by the induction of mixed chimerism using myeloreductive, but not myeloablative, conditioning. Methods of the invention reduce GVHD, especially GVHD associated with mismatched allogeneic or xenogeneic donor tissue, yet provide, for example, significant graft-versus-leukemia (GVL) effect and the like.
Owner:THE GENERAL HOSPITAL CORP
System for detecting bacteria in blood, blood products, and fluids of tissues
InactiveUS20100129836A1Bioreactor/fermenter combinationsBiological substance pretreatmentsWhole blood productMammal
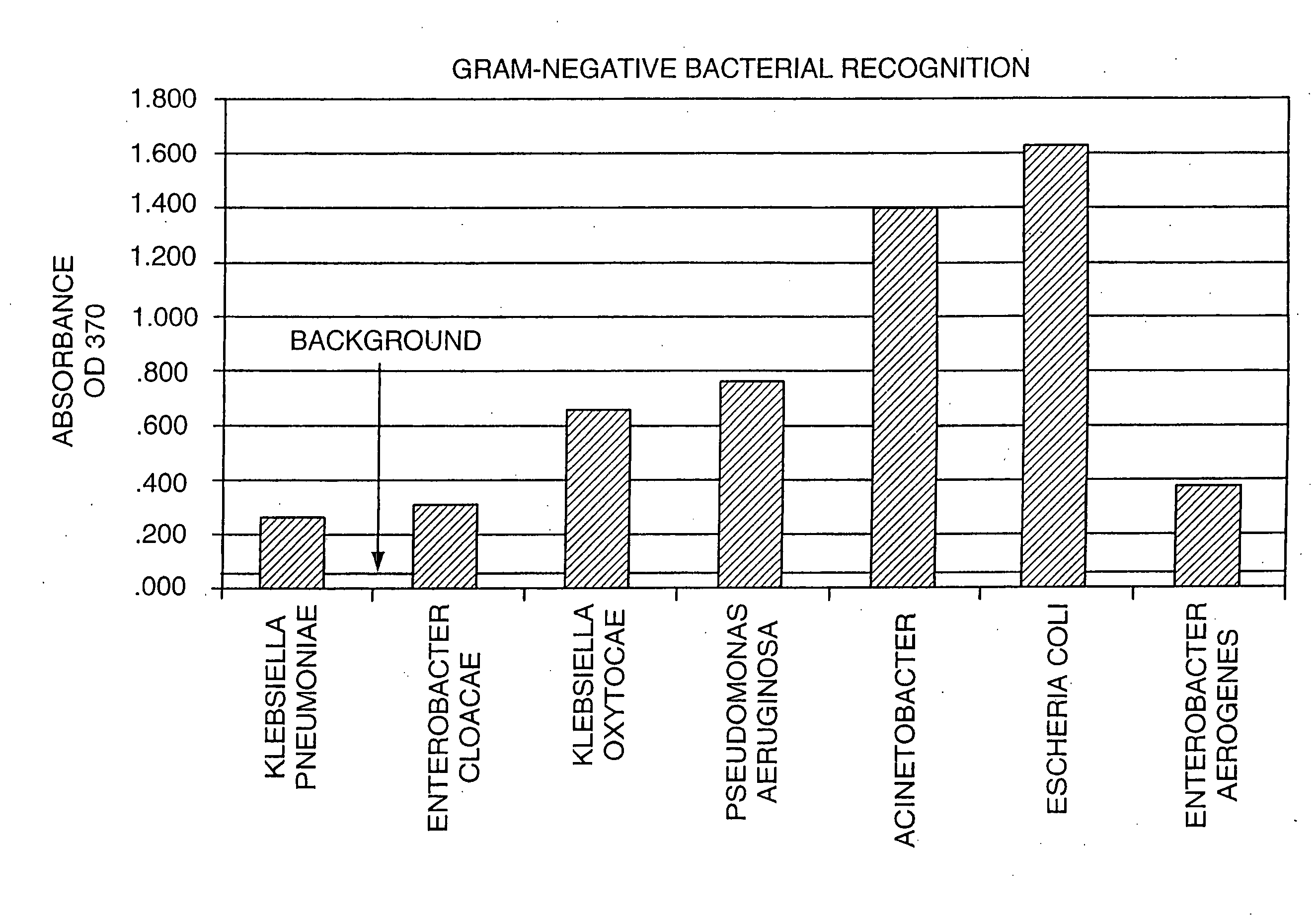
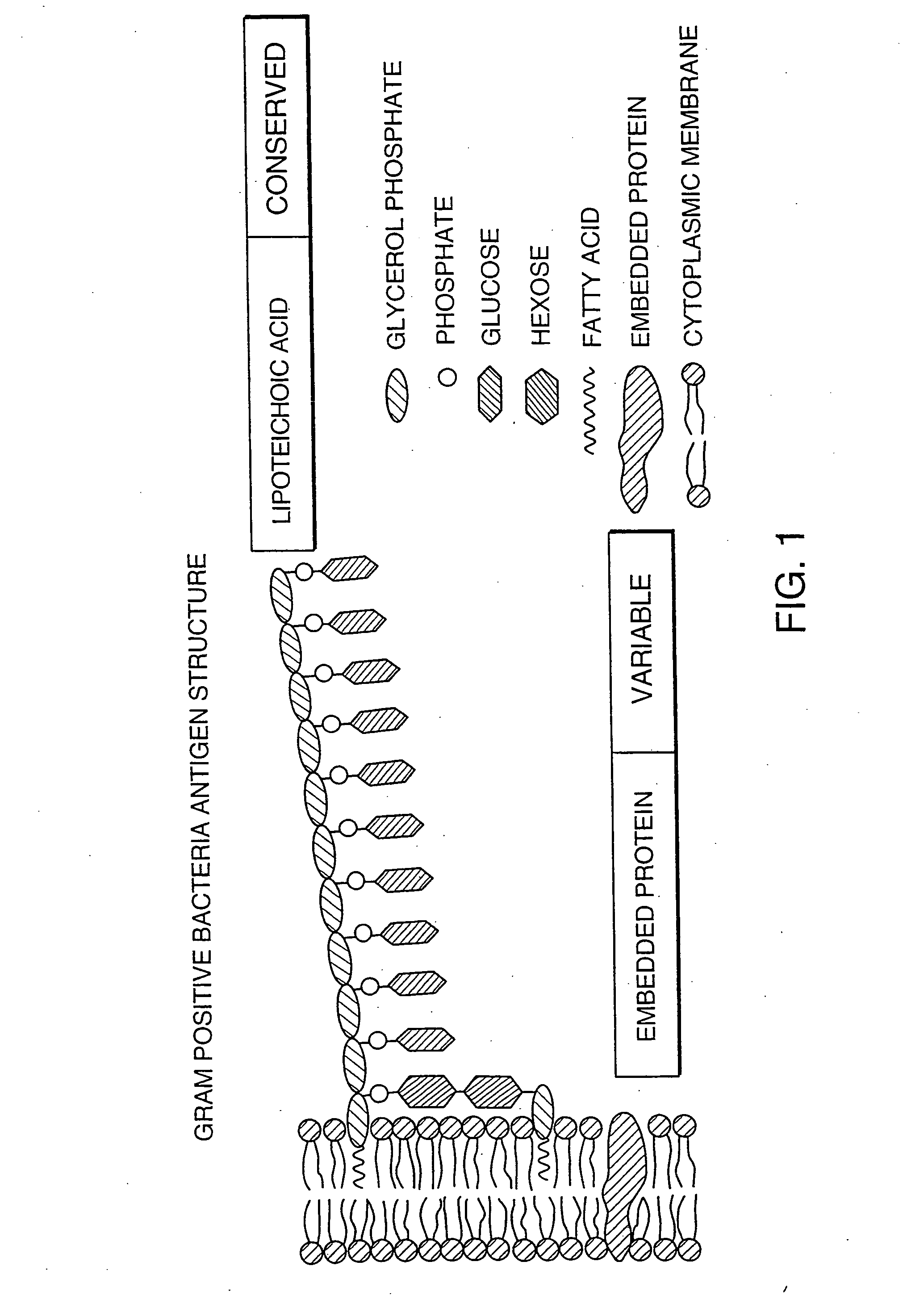
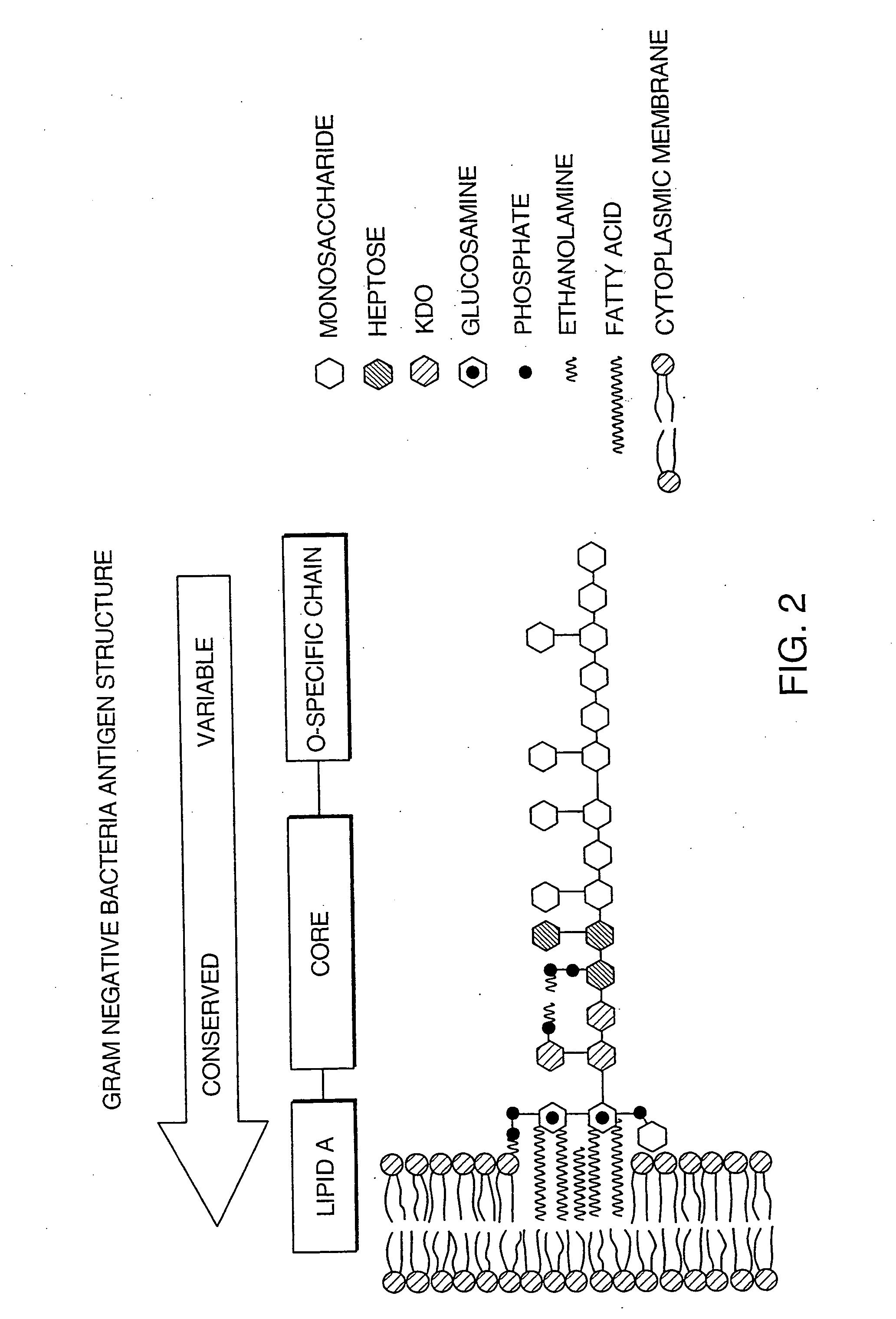
The invention provides methods for screening for the presence of a clinically relevant amount of bacteria in donor blood or a blood product from a donor mammal, particularly blood or a blood product that will be transferred from the donor mammal to a recipient mammal. The method comprises contacting a sample of the donor blood or a blood product with a set of binding agents that comprises binding agents that specifically bind to Gram-negative bacterial antigen and / or binding agents that specifically bind to Gram-positive bacterial antigen, and determining binding of the set of binding agents to the sample, wherein binding indicates the presence of a clinically relevant amount of Gram-positive bacteria and / or Gram-negative bacteria in the donor blood or blood product and no binding indicates the absence of a clinically relevant amount of Gram-positive bacteria and / or Gram-negative bacteria in the donor blood or blood product.The invention further provides methods and kits for screening for the presence of a clinically relevant amount of Gram-positive bacteria, Gram-negative bacteria, or both Gram-positive and Gram-negative bacteria in a donor tissue by screening the fluid in which the donor tissue is stored.
Owner:VERAX BIOMEDICAL
Method of Screening for Binding Interaction Using Sets of Microparticles and Unique Probes
ActiveUS20090142762A1Increase the number ofFew characteristicMicrobiological testing/measurementMaterial analysisTissue typingMicroparticle
The present invention relates to methods for screening for binding interactions using multiple sets of microparticles, wherein said set has the same identifiable characteristic and wherein one of more sets comprise subsets of microparticles and said subset presents at least one unique probe that acts as a binding partner for a target molecule in a biological sample. In particular, the invention provides for methods of detecting tissue-typing antigens in donor tissue or recipient tissue using these multiple sets of microparticles.
Owner:ONE LAMBDA INC
Tissue harvesting device and method
A tissue harvesting method and device for obtaining micrograft tissue particles within the size range of 50-1500 microns, and more preferably 500-1000 microns, and most preferably 600 microns. The particles may be processed after a piece of donor tissue has been excised from the donor site, or processed into the desired size directly at the donor site, and thereafter excised. Cutters having blades or cutting edges spaced in the range of 50-1500 microns are utilized to obtain particles within the desired size range. An elastomer is positioned between the cutting edges to push the excised particles out of the blades for ease of use.
Owner:KCI LICENSING INC
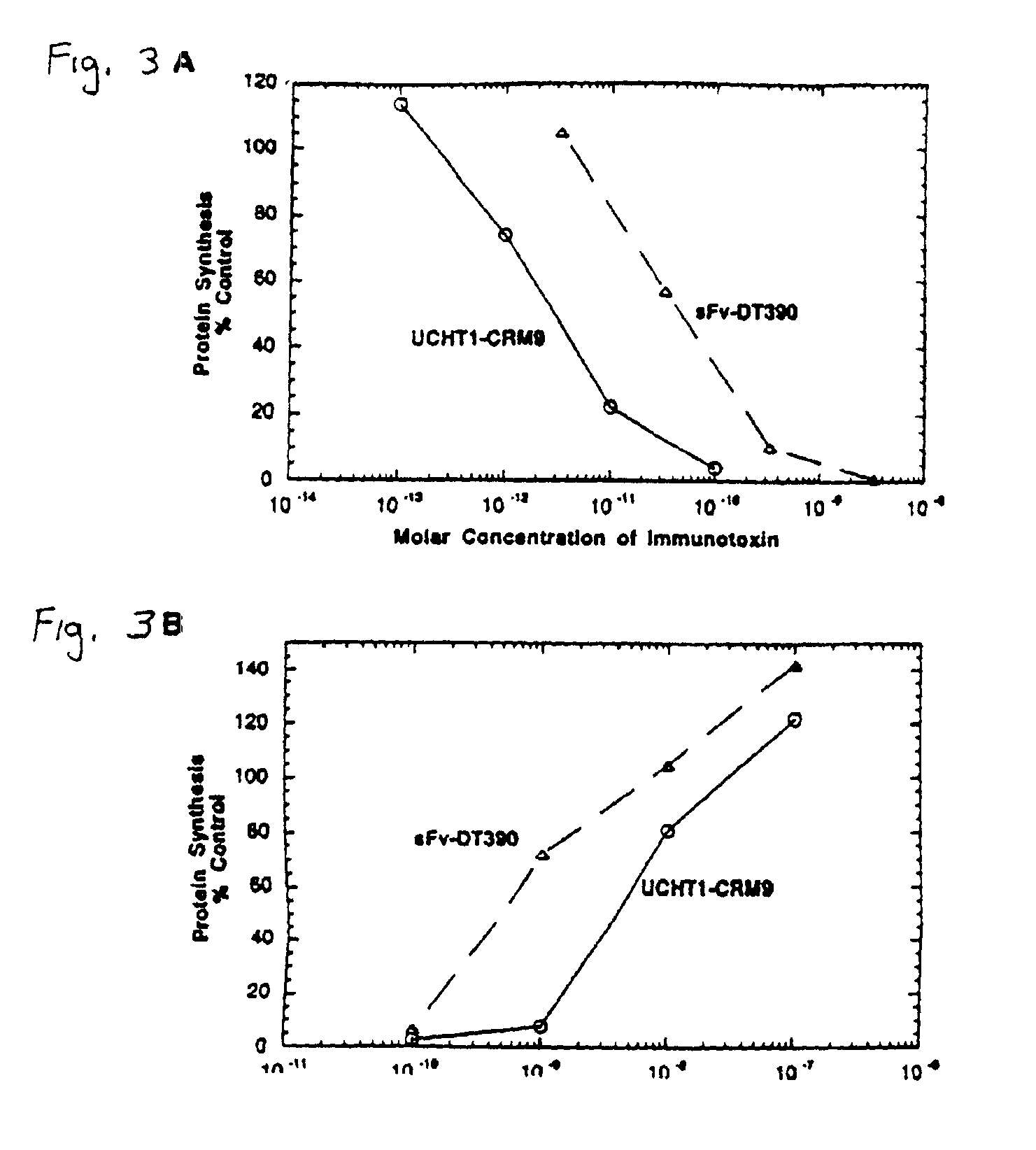
Methods of inducing immune tolerance using immunotoxins
InactiveUS7125553B1Induce immune toleranceSnake antigen ingredientsAntibody ingredientsPresent methodBinding site
Provided is a method of treating an immune system disorder not involving T cell proliferation, comprising administering to the animal an immunotoxin comprising a mutant diphtheria toxin moiety linked to an antibody moiety which routes by the anti-CD3 pathway, or derivatives thereof under conditions such that the disorder is treated. Thus, the present method can treat graft-versus-host disease. Also provided is a method of inhibiting a rejection response by inducing immune tolerance in a recipient to a foreign mammalian donor tissue or cells, comprising the steps of: a) exposing the recipient to an immunotoxin so as to reduce the recipients's peripheral blood T-cell lymphocyte population by at least 80%, wherein the immunotoxin is anti-CD3 antibody linked to a diphtheria protein toxin, wherein the protein has a binding site mutation; and b) transplanting the donor cells into the recipient, whereby a rejection response by the recipient to the donor organ cell is inhibited, and the host is tolerized to the donor cell.
Owner:UNITED STATES OF AMERICA +1
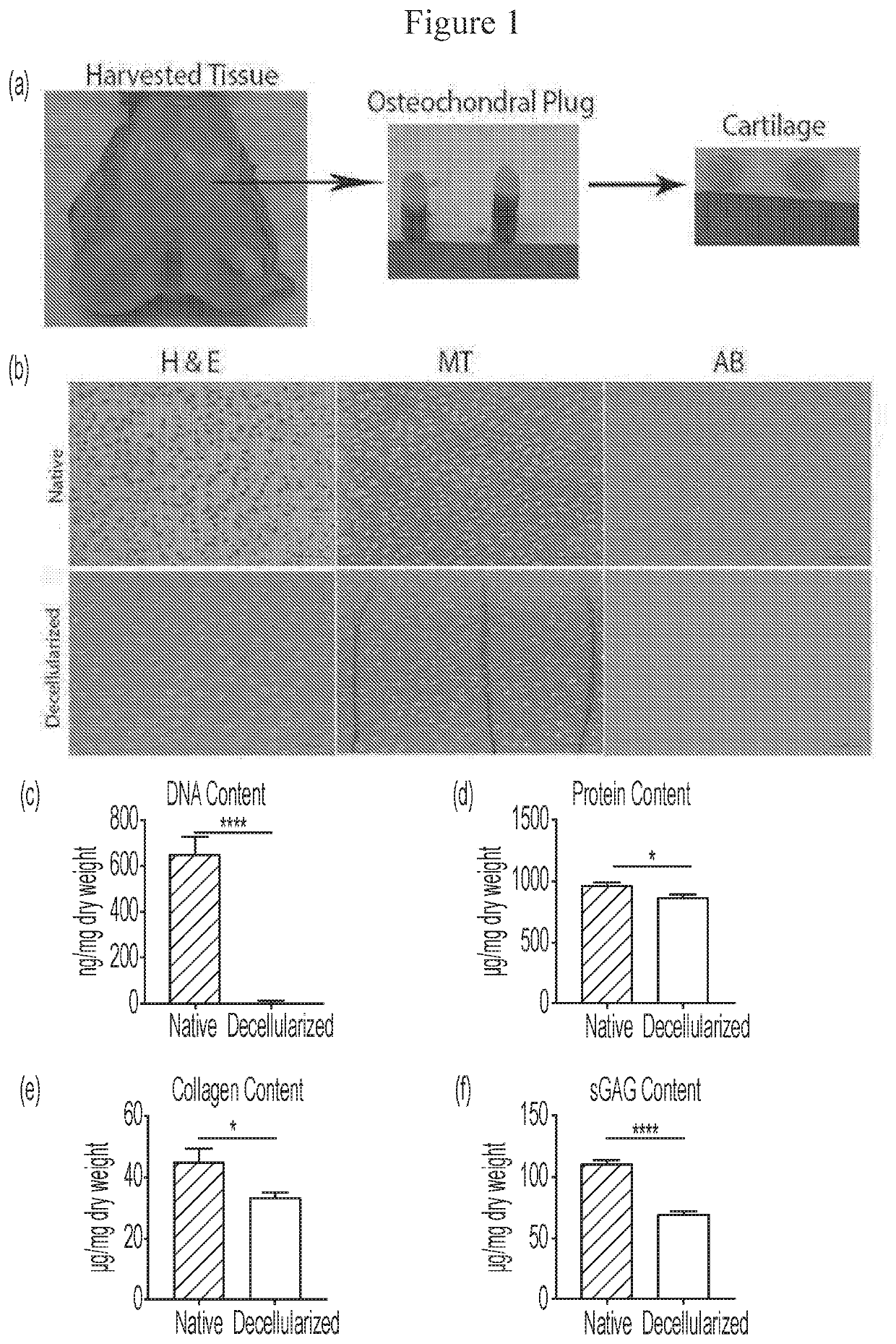
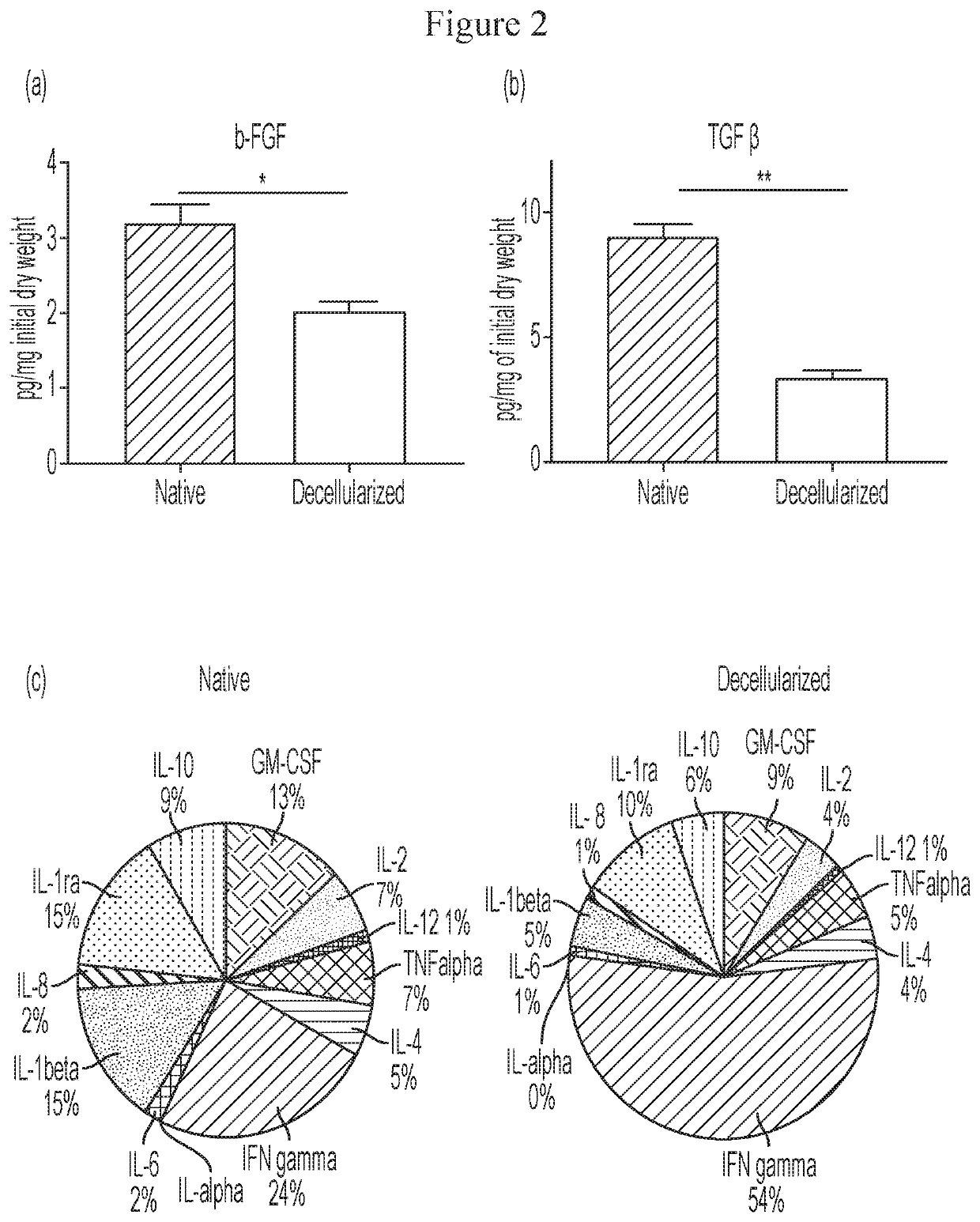
Microspheres Containing Decellularized Donor Tissue and Their Use in Fabricating Polymeric Structures
PendingUS20200289702A1Promote migrationIncreased proliferationAdditive manufacturing apparatusPeptide/protein ingredientsMicrosphereDonor tissue
Decellularized matrix microspheres comprising a polymeric material and a donor tissue are provided. Also disclosed are structures containing a plurality of decellularized matrix microspheres incorporating a first polymer and a donor tissue; and a second polymer, wherein the decellularized matrix microspheres and the second polymer are in the form of a filament. Methods of treating a tissue injury employing the matrix microspheres and structures described as well as their methods of manufacture are also provided.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
Composition and method for improving function of embryonic kidney transplants
Owner:WASHINGTON UNIV IN SAINT LOUIS
Treatment of Hematologic Disorders
InactiveUS20090028870A1Reduce GVHDRemarkable effectBiocideOrganic active ingredientsGraft versus leukaemiaHematological Diseases
Owner:THE GENERAL HOSPITAL CORP
Tissue Processing System
Owner:KCI LICENSING INC
Methods for increasing isolation yields of cellular products
Methods of isolating cellular products, such as pancreatic islets, may be used in diabetes research and therapeutic transplantation. The methods may involve providing a donor tissue having desired cells and undesired cells, perfusing the donor tissue with a perfusion solution, developing edema during perfusion of the donor tissue to form a swelled tissue, and separating the desired cells from undesired cellular material to obtain a cellular product. The methods may also include disrupting the tissue, and separating the desired cells from undesired cellular material to obtain the cellular product. The methods may result in an increased yield of cellular product that retains sufficient functional integrity to be useful as a transplantation resource.
Owner:LIFELINE SCI
System and method for resecting corneal tissue
A system and method of resecting corneal tissue for transplantation is disclosed. In each of the recipient cornea and the donor cornea, an annular incision is made at a predetermined incision depth. A first sidecut incision is made in each cornea, running from the outer periphery of the annular incision to one of the anterior corneal surface or the posterior corneal surface. The first sidecut incision forms an acute angle with the annular incision. A second sidecut incision is also made in each cornea, running from the inner periphery of the annular incision to the other of the anterior corneal surface or the posterior corneal surface. The second sidecut incision forms an acute angle with the annular incision. The combination of the incisions in each cornea resects corneal tissue from the recipient cornea and donor tissue from the donor cornea. The donor tissue is grafted into the recipient cornea.
Owner:AMO DEVMENT
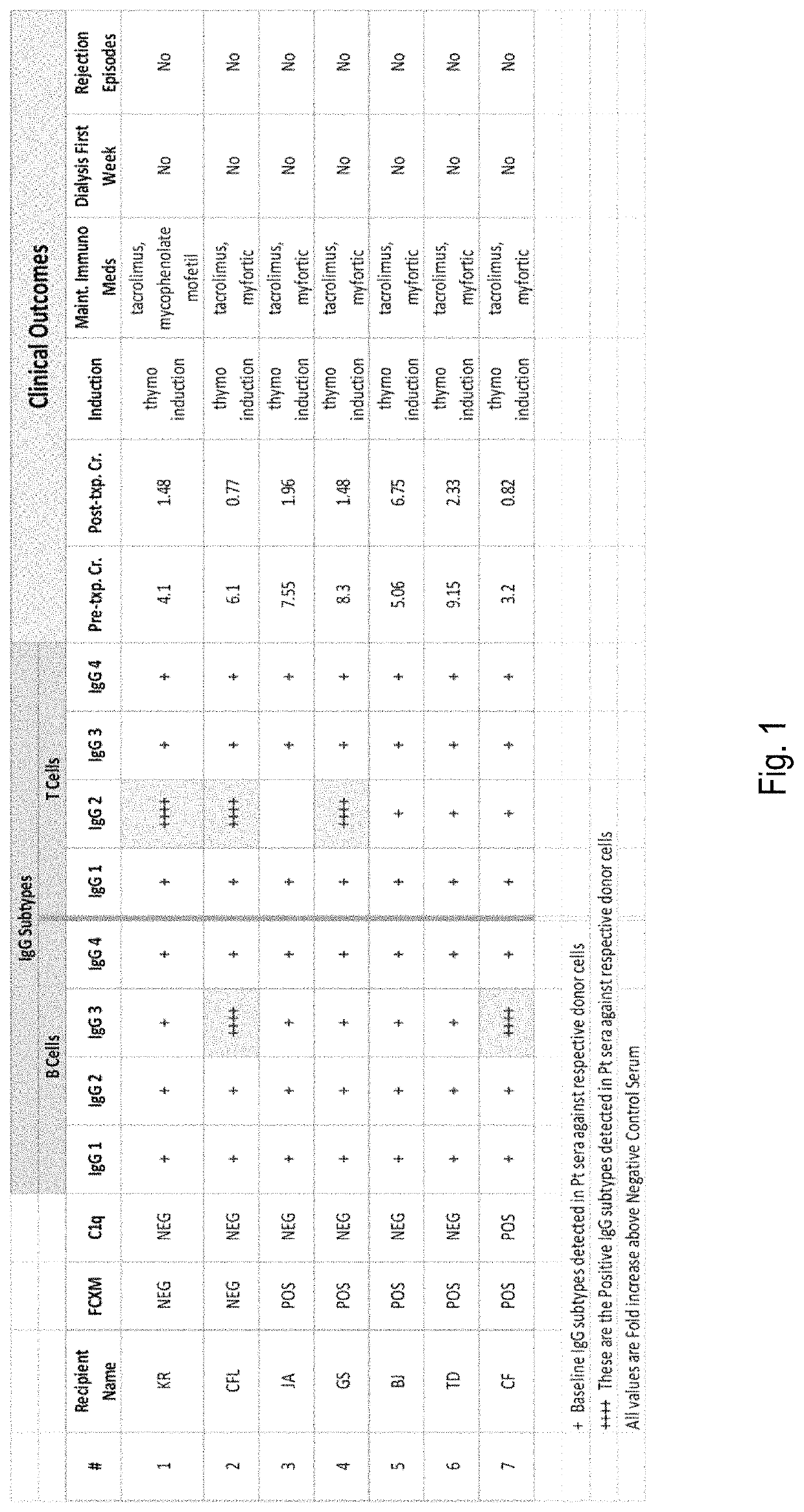
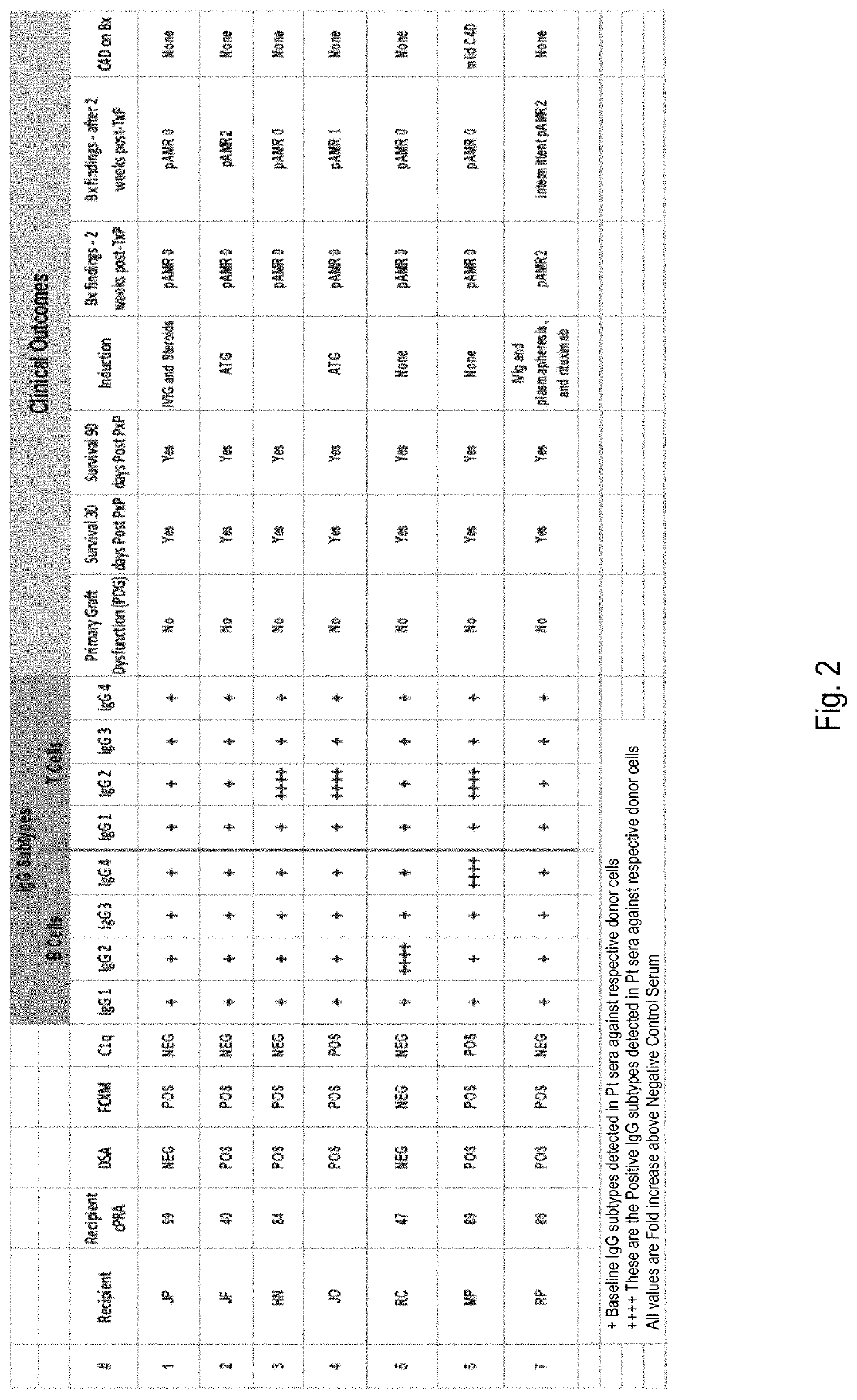
IgG subtyping assay for identifying transplantable tissue samples
ActiveUS10648980B2Accurate predictionIncrease the number ofUnknown materialsBiological testingAntiendomysial antibodiesAssay
The disclosure relates to methods for assessing the suitability of a tissue obtained from a vertebrate (e.g., human) donor for grafting to a recipient, such as another vertebrate of the same species. The method involves contacting leukocytes obtained from the donor with serum from the recipient. Binding between recipient antibodies and the leukocytes is assessed, specifically focusing on the subtype(s) of IgG antibodies which bind with donor leukocytes. Detected binding between recipient antibodies of subtypes IgG1 and IgG3 indicates that the donor tissue is not suitable for grafting to the recipient. Detected binding between recipient antibodies of subtypes IgG2 and / or IgG4 indicates that the donor tissue can suitably be grafted to the recipient.
Owner:NJ SHARING NETWORK
Microscopic precision construction of tissue array block related application data
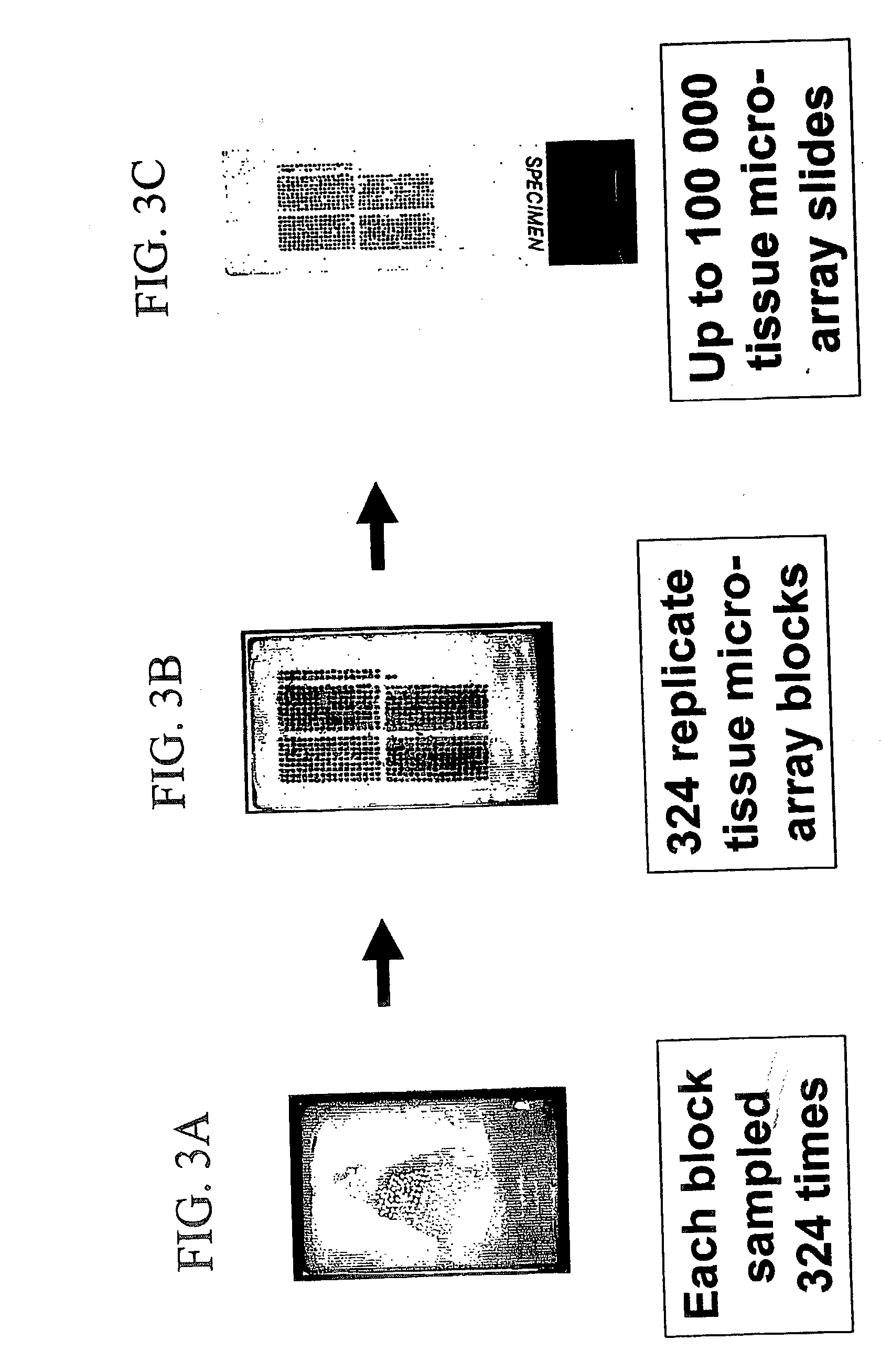
InactiveUS20060099653A1Choose accuratelyEasy to assemblePreparing sample for investigationBiological testingTissue ArraysTissue sample
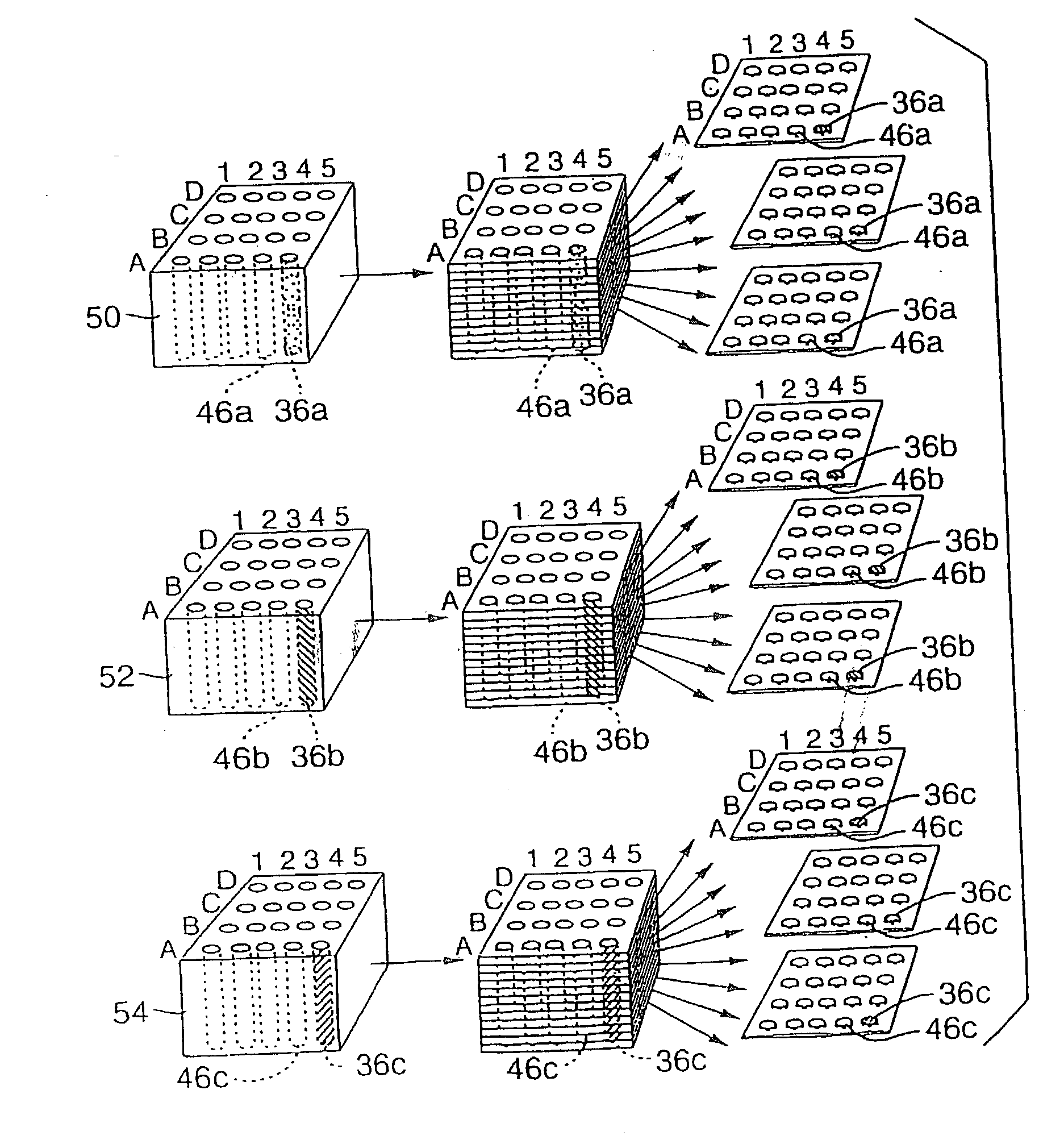
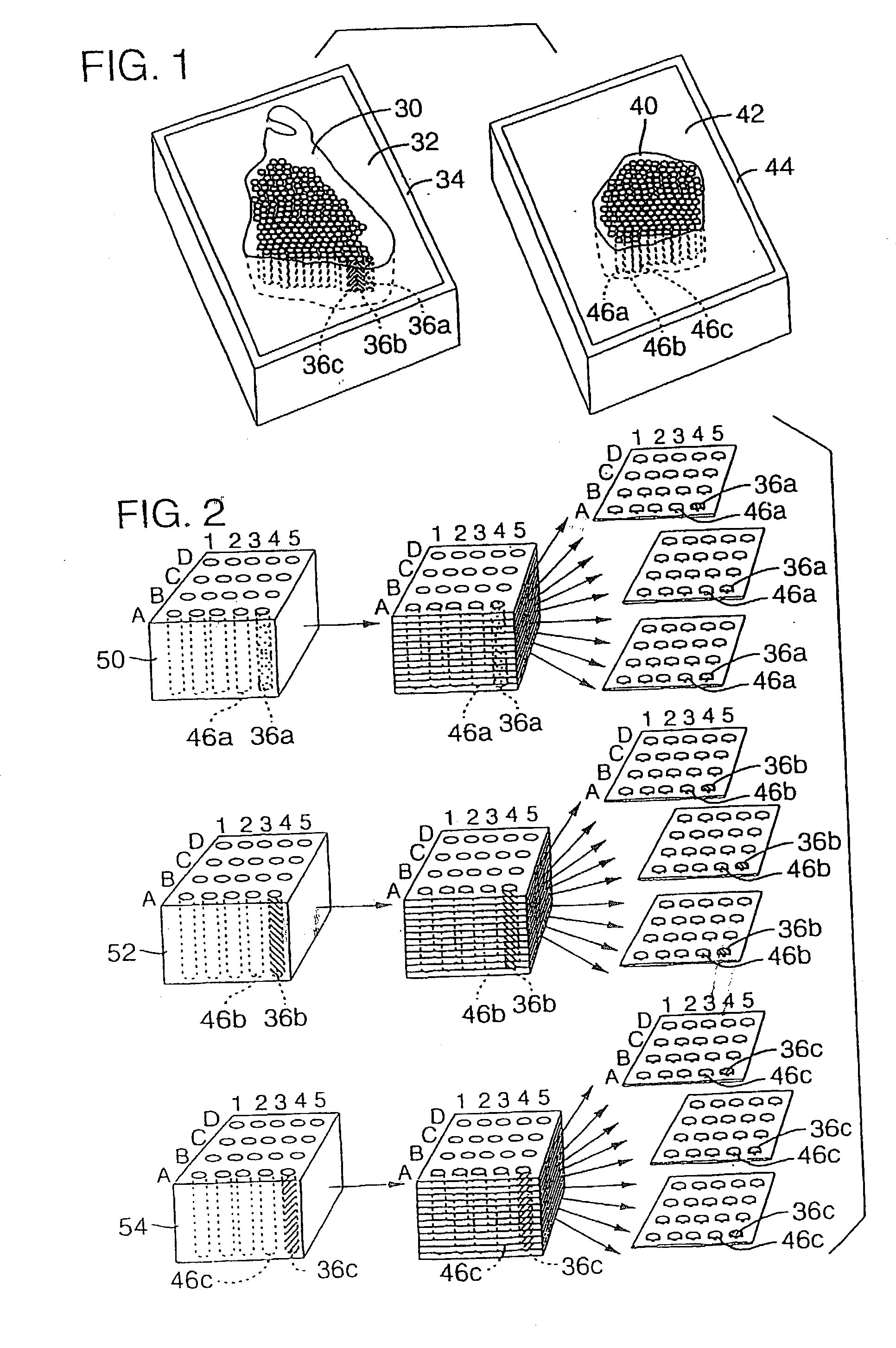
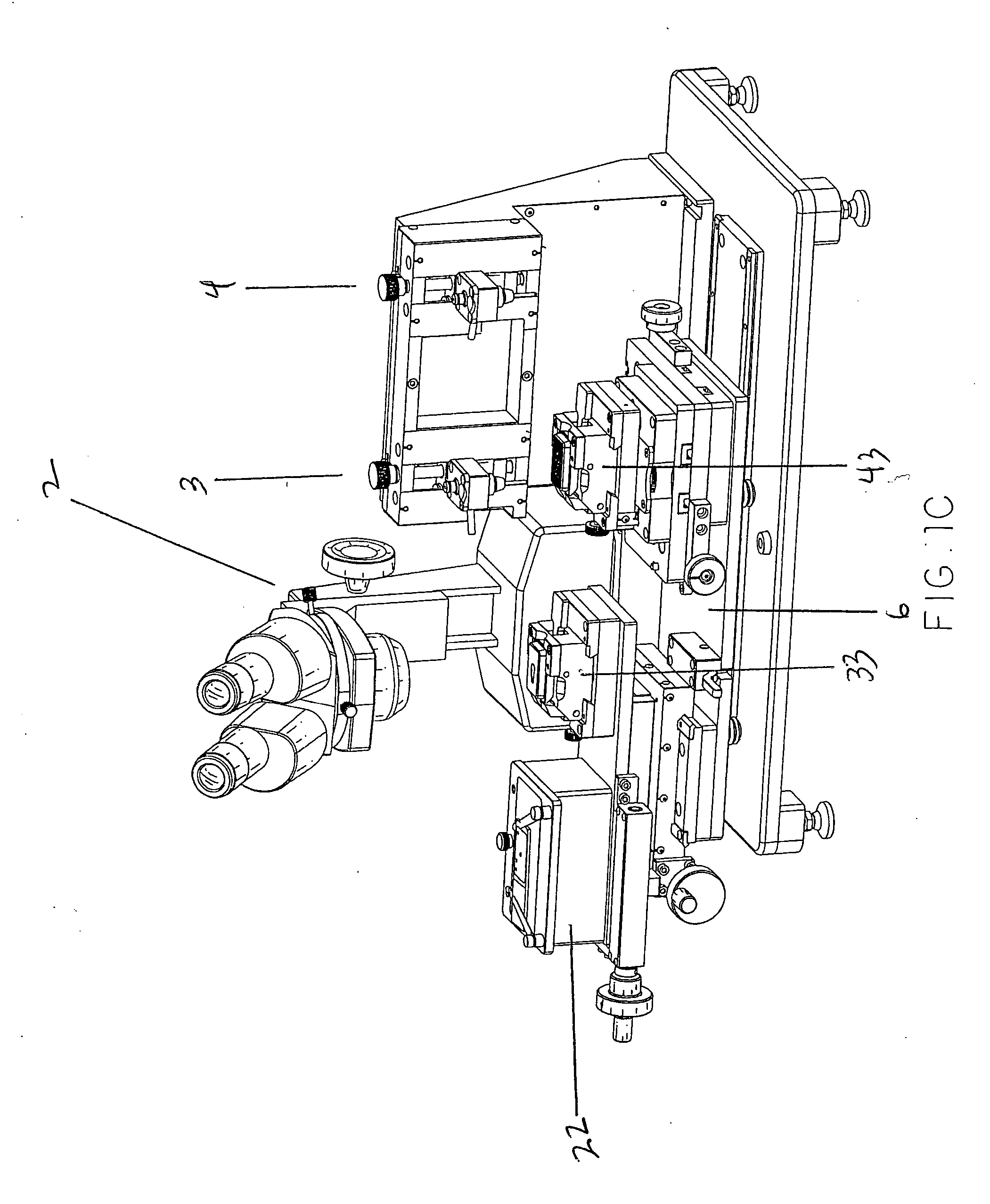
This present invention covers novel means, devices and instruments for production of a tissue array block that is further sectioned into duplicates of tissue arrays. An integral microscope is incorporated into the instrument for viewing and examining a stained reference slide and selecting donor tissue core region(s) from the reference slide. The reference slide is held in a reference slide station that is operatively linked and indexed with a station or platform holding a source donor tissue block, which is further indexed and precisely positioned with reference to the donor needle punch for punching the donor tissue core(s). A recipient block indexed to the donor block punch is placed under the donor punch station and donor tissue cores are delivered into pre-existing hole(s) by a stylet to construct the tissue array block. The instrument includes a donor punch station, optionally a second recipient punch station, with each operable independently or removable. The present invention also provides pre-loading needles with donor tissue cores for constructing tissue array blocks in pre-gridded and pre-punched recipient block. The tissue arrays produced from the tissue array blocks made are useful for testing such freshly-made and / or archival tissue specimens in both scientific and clinical research and applications.
Owner:ADVANCED EDM AUTOMATION
Protector assembly for handling and transporting corneal tissue
A protector assembly for handling, storing and transporting donor tissue in a transfer pipette includes a distal cap and a proximal plug that are respectively engageable with opposite ends of the pipette. Structurally, the pipette includes a distally tapered fluid chamber which is in fluid communication with an extension tube. The distal cap is dimensioned for friction engagement with the fluid chamber of the pipette and it is formed with a plurality of vents to establish fluid communication through the vents with the fluid chamber. On the other hand, the proximal plug is dimensioned for a friction engagement with the extension tube to prevent leakage of solution from the fluid chamber of the pipette when the proximal plug is engaged with the pipette. In combination, an engagement of the protector assembly with a pipette holding a donor tissue will protect and preserve the donor tissue during handling and transport.
Owner:SAN DIEGO EYE BANK
Modulating Ischemic Injury
InactiveUS20160000874A1Preventing modulating reducing treating ameliorating ischemic injuryExtension of timePeptide/protein ingredientsAntipyreticIntact tissueActive agent
The invention is directed to methods of modulating ischemic injury in tissues and organs, including donor tissue and organs and intact tissue and organs. The invention is further directed to methods of increasing time to ischemic injury in such tissues and organs. The invention is further directed to storing and preserving donor tissues and organs. Such methods utilize compositions comprising Amnion-derived Cellular Cytokine Solution (herein referred to as ACCS). The ACCS compositions may be formulated for sustained-release, targeted-release, timed-release, extended-release, etc. and may be used alone or in combination with various suitable active agents.
Owner:STEMNION
Ex vivo maturation of islet cells
InactiveUS20150132849A1Enhances glucose responsivenessEnhances glucose-dependent insulin secretionMetabolism disorderPancreatic cellsIslet cellsPancreas
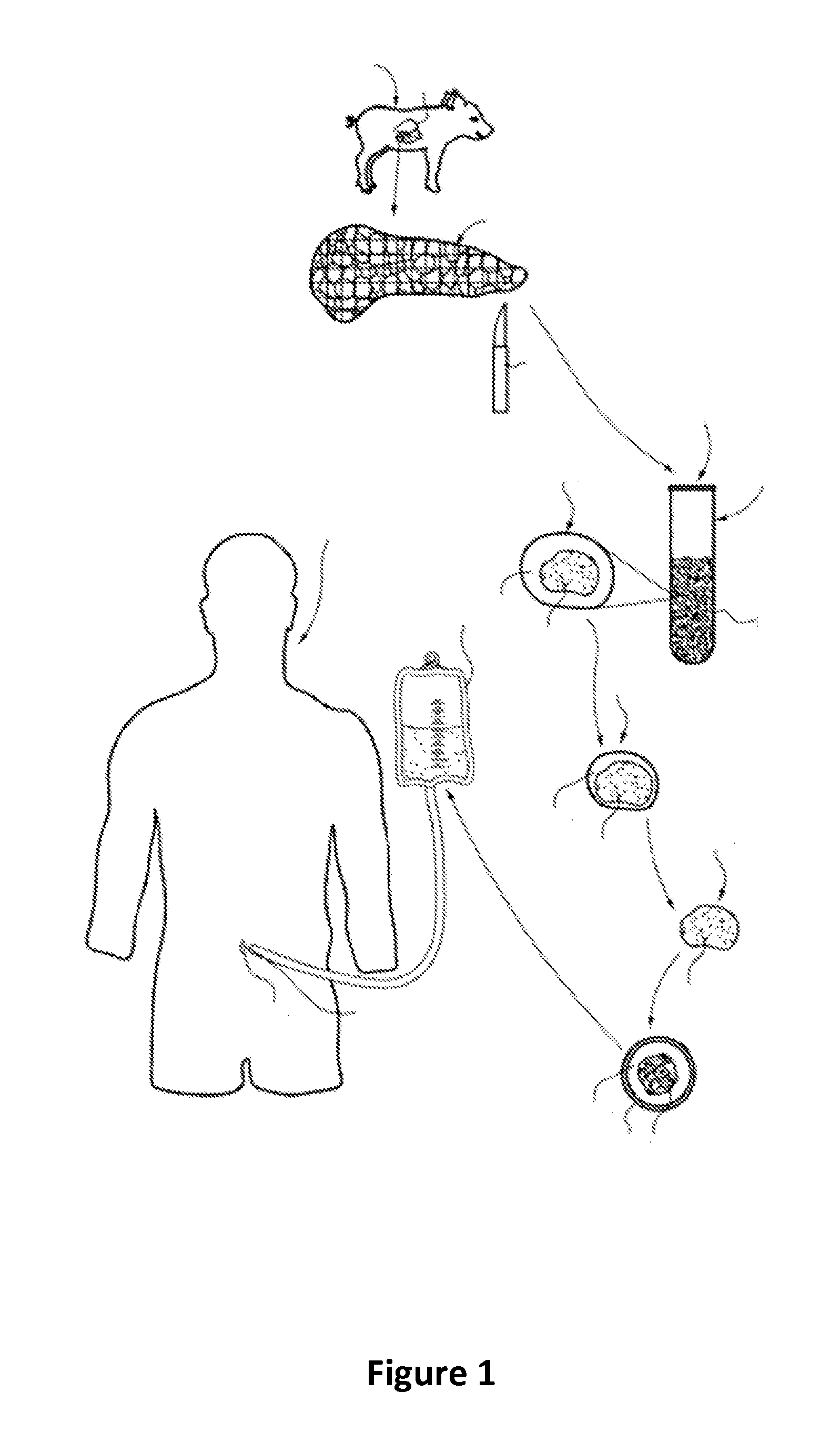
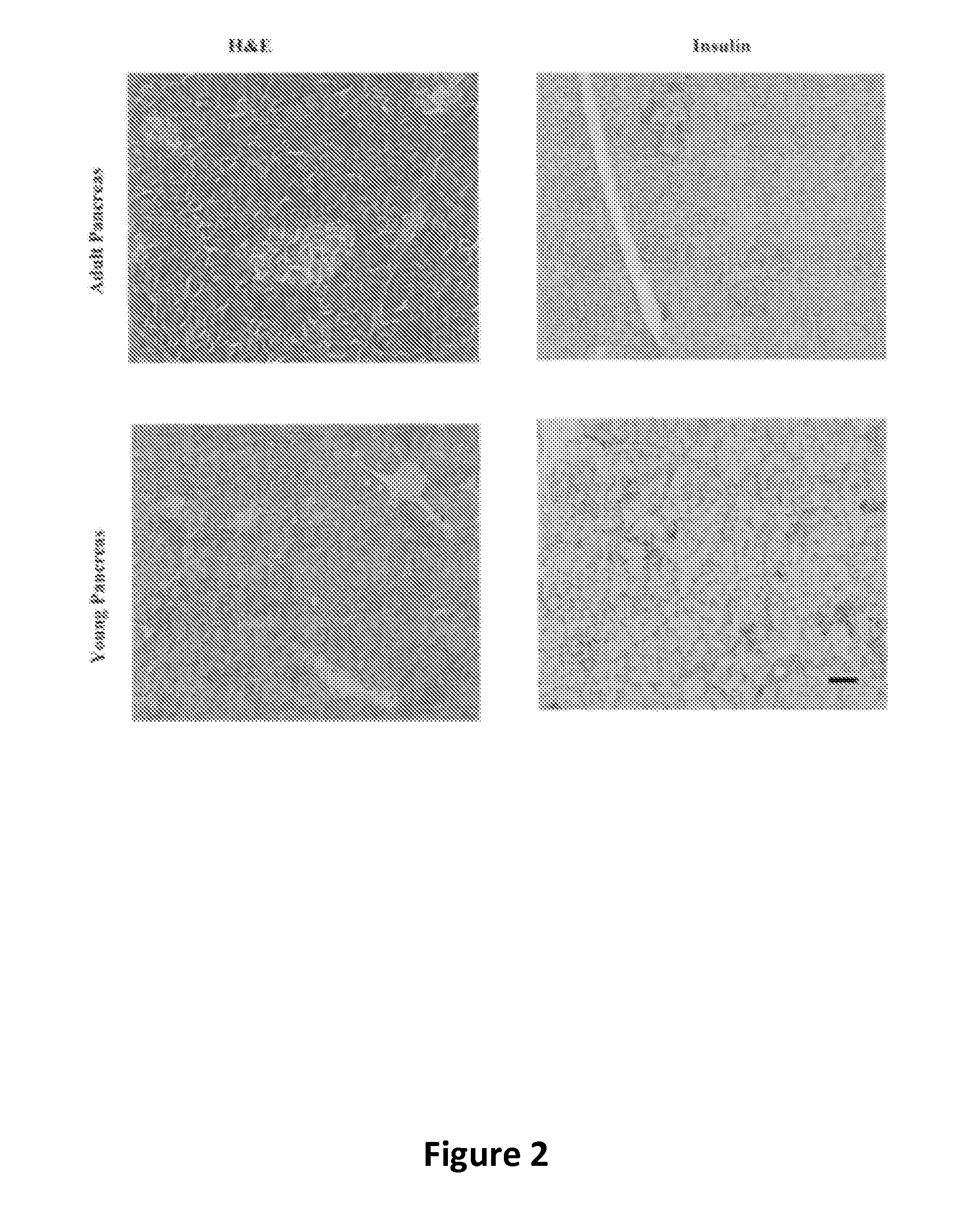
The invention relates to methods for promoting maturation of islet cells from pre-weaned mammals for the purpose of optimizing the islets for their use as donor tissue for xenotransplantation. The method of the invention removes the pancreas from donor animals and reduces the pancreas tissue to fragments that are greater than the size of an intact islet while retaining islets in their whole, insulin-producing condition. The method of the invention also serially cultures the digested tissue in novel maturation media that enhance the glucose responsiveness of the cultured islets, and selects islets that are sufficiently glucose-responsive for use in transplantation procedures.
Owner:ISLET SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com