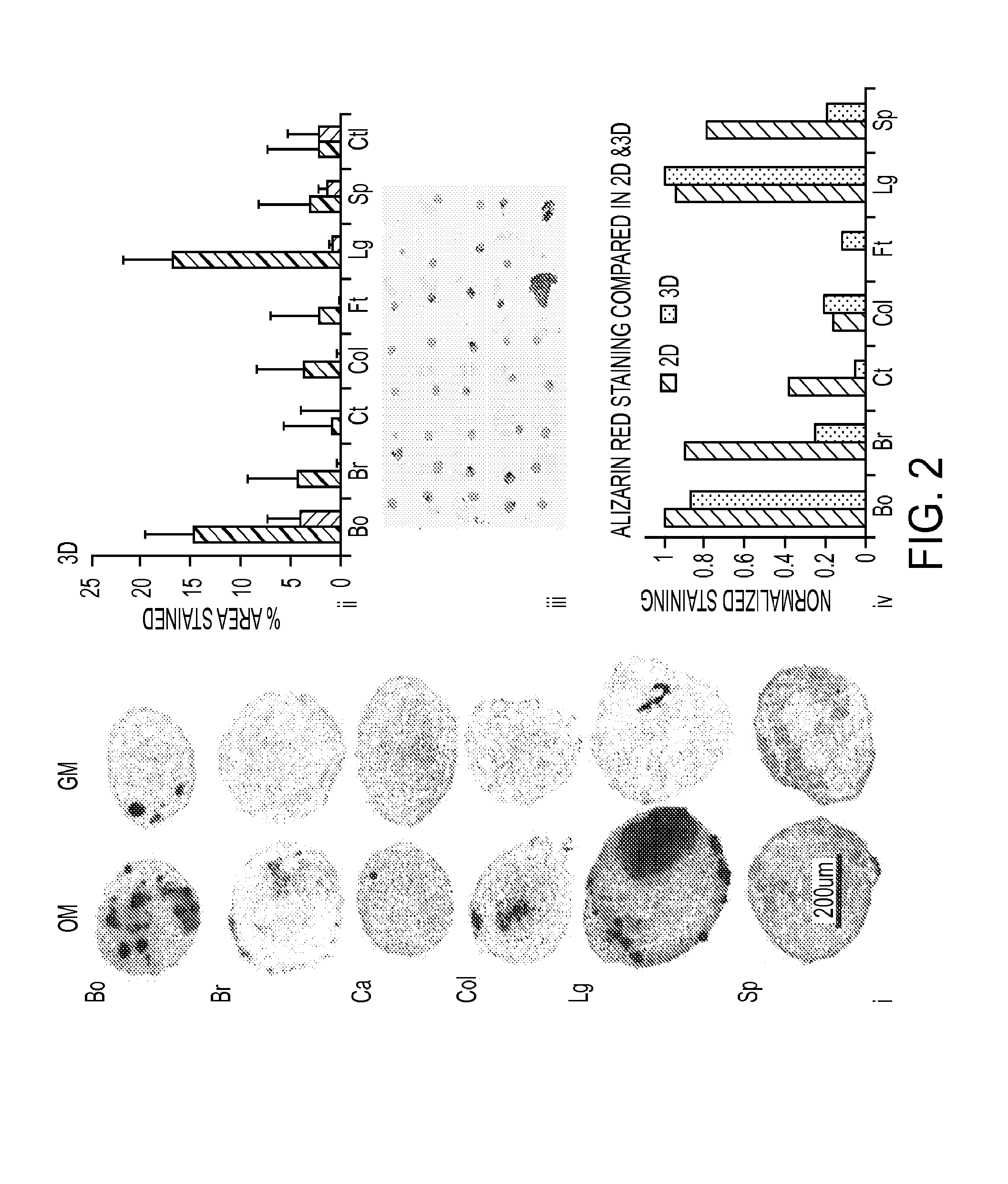
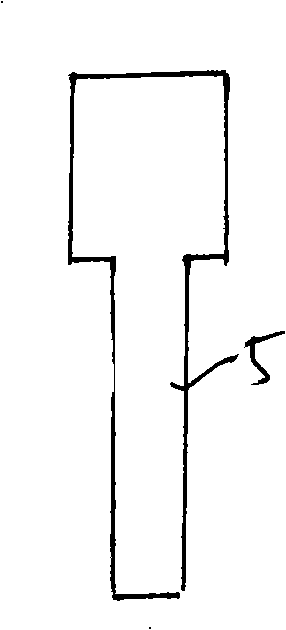Patents
Literature
35 results about "Tissue Arrays" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
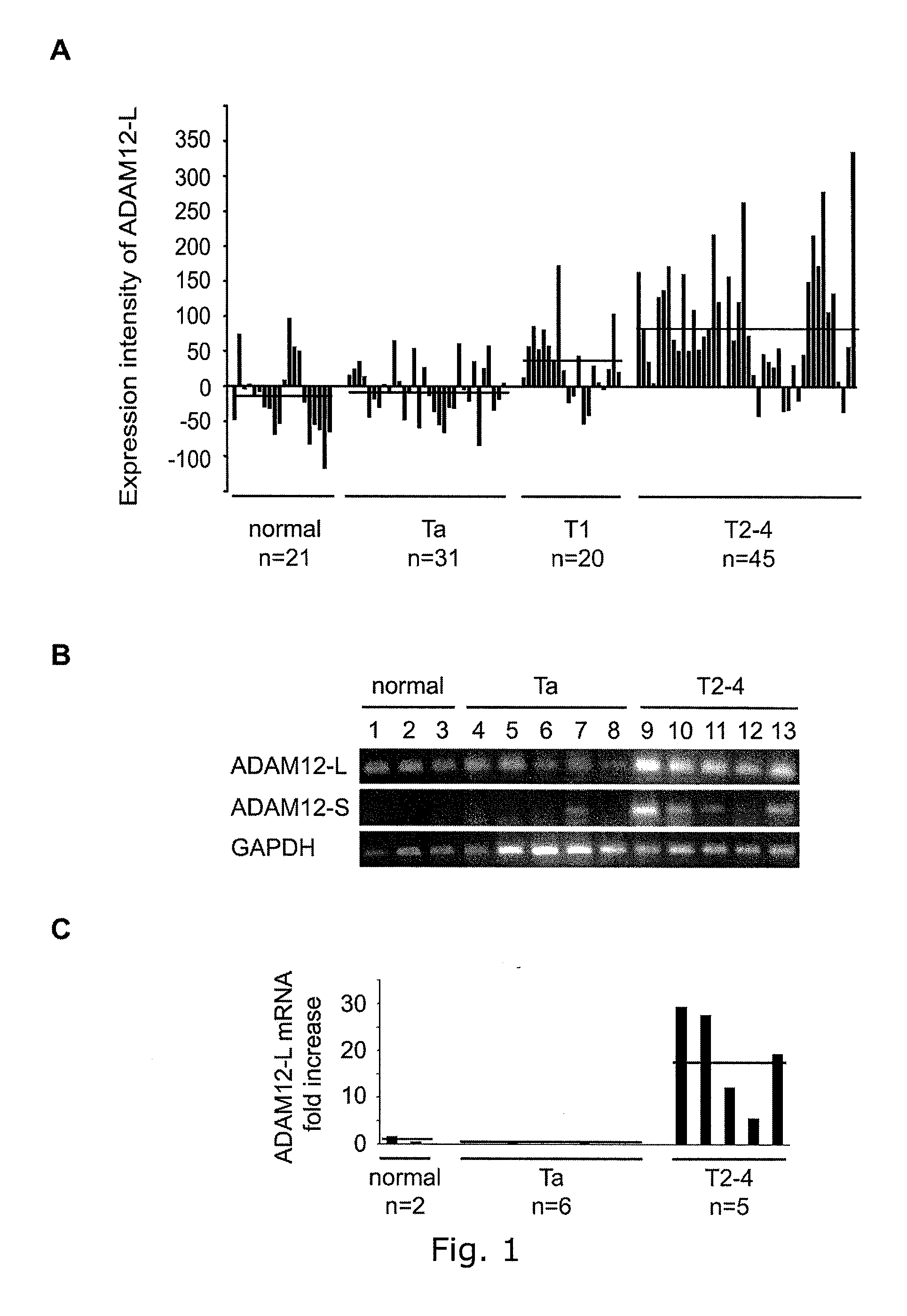
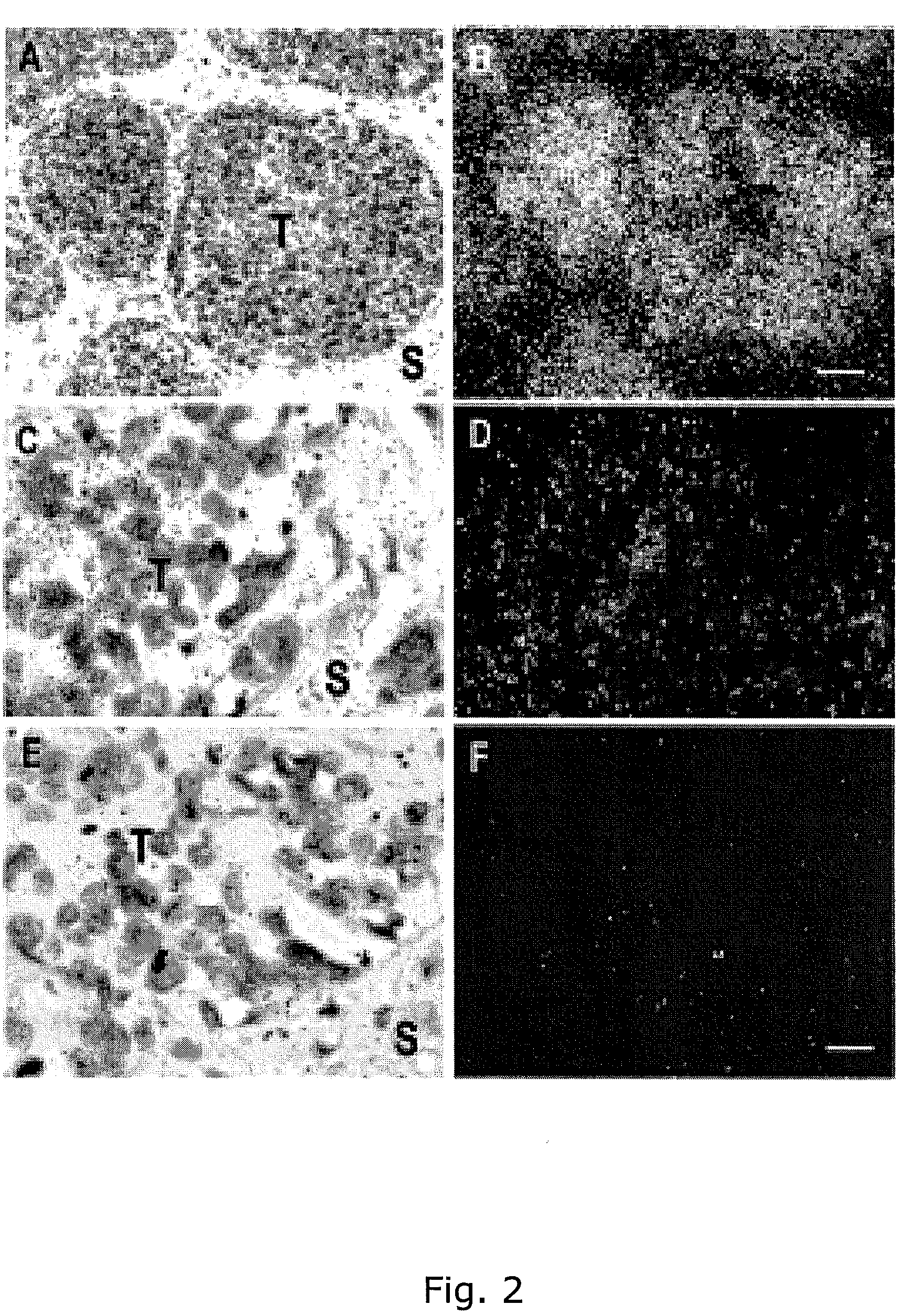
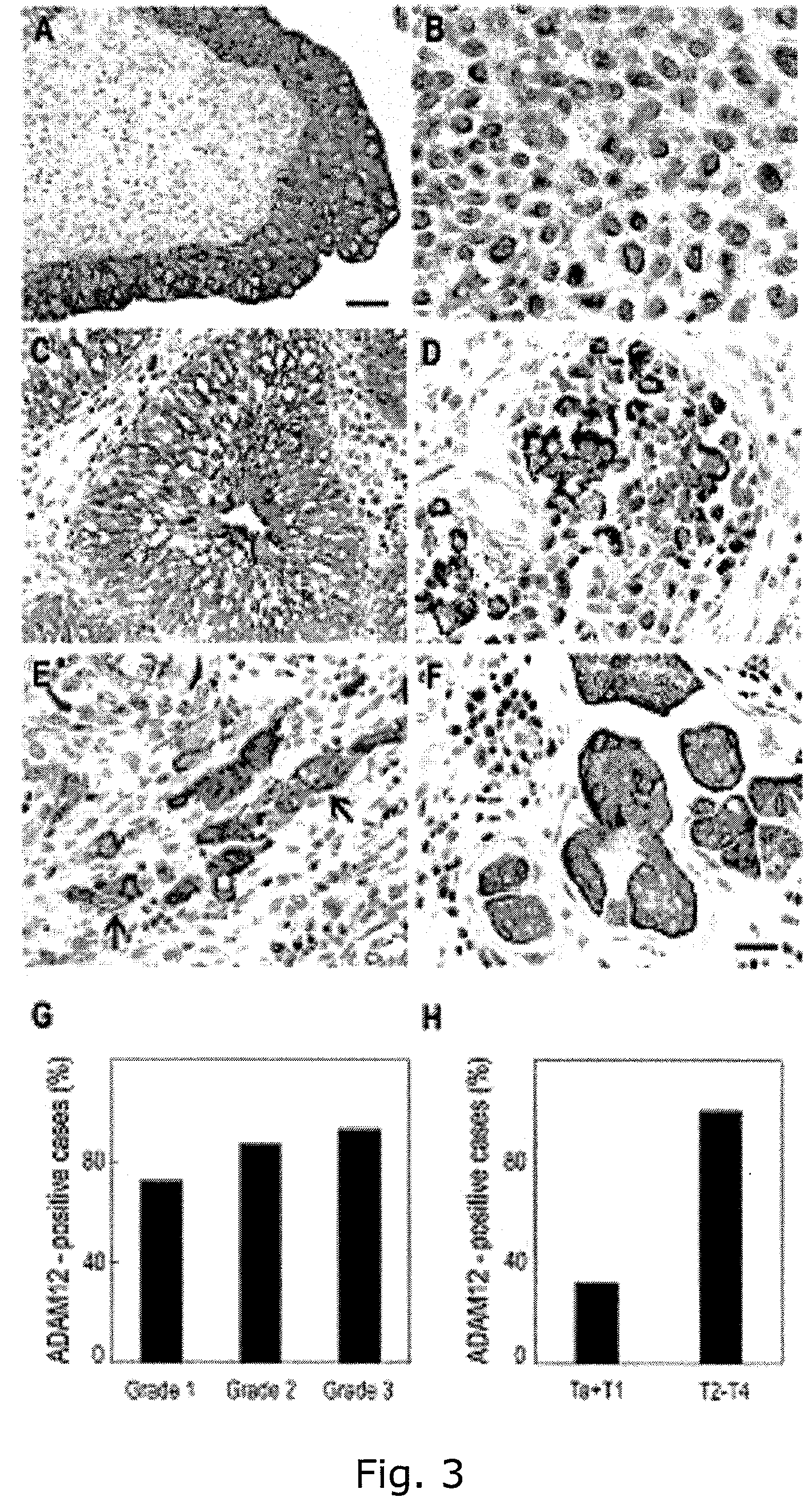
Adam12 as a biomarker for bladder cancer
InactiveUS20090029372A1Improve the level ofLower Level RequirementsMicrobiological testing/measurementMaterial analysisTissue ArraysAffymetrix genechip
The present inventors have shown that the gene and protein expression profiles of ADAM8, ADAM10 and ADAM12 in different grades and stages of bladder cancer.ADAM12 gene expression was evaluated in tumors from 96 patients with bladder cancer using a customized Affymetrix GeneChip. Gene expression in bladder cancer was validated using reverse transcription-polymerase chain reaction (RT-PCR), quantitative PCR, and in situ hybridization. Protein expression was evaluated by immunohistochemical staining on tissue arrays of bladder cancers.The presence and relative amount of ADAM12 in the urine of cancer patients were determined by Western blotting and densitometric measurements, respectively.Particularly ADAM12 mRNA expression was significantly upregulated in bladder cancer, as determined by microarray analysis, and the level of ADAM12 mRNA correlated with disease stage. ADAM12 protein expression correlated with tumor stage and grade. ADAM12 was present in higher levels in the urine from bladder cancer patients than in urine from healthy individuals. Significantly, following removal of tumor by surgery, in most bladder cancer cases examined the level of ADAM12 in the urine decreased and, upon recurrence of tumor, increased.
Owner:PHYSICIANS CHOICE LAB SERVICES +1
Frozen tissue microarray technology for analysis RNA, DNA, and proteins
InactiveUS6893837B2Withdrawing sample devicesPreparing sample for investigationAnalysis dnaTissue Arrays
The invention disclosed herein improves upon existing tissue microarray technology by using frozen tissues embedded in tissue embedding compound as donor samples and arraying the specimens into a recipient block comprising tissue embedding compound. Tissue is not fixed prior to embedding, and sections from the array are evaluated without fixation or post-fixed according to the appropriate methodology used to analyze a specific gene at the DNA, RNA, and / or protein levels. Unlike paraffin tissue arrays which can be problematic for immunohistochemistry and for RNA in situ hybridization analyses, the disclosed methods allow optimal evaluation by each technique and uniform fixation across the array panel. The disclosed arrays work well for DNA, RNA, and protein analyses, and have significant qualitative and quantitative advantages over existing methods.
Owner:RGT UNIV OF CALIFORNIA
Tissue punch and tissue sample labeling methods and devices for microarray preparation, archiving and documentation
InactiveUS20060199169A1Avoid mistakesWaste of tissueBioreactor/fermenter combinationsBiological substance pretreatmentsVertical tubeTissue Arrays
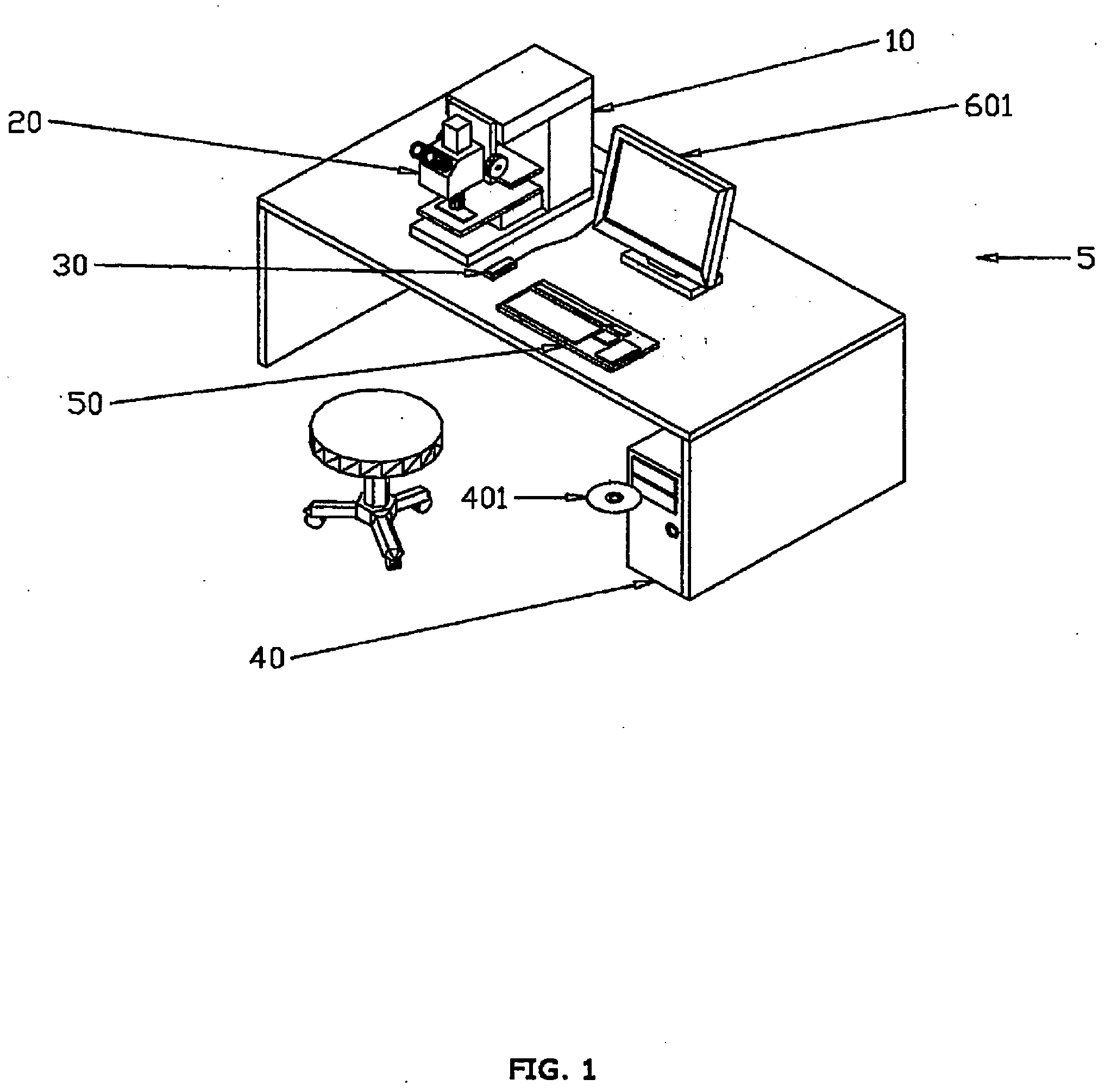
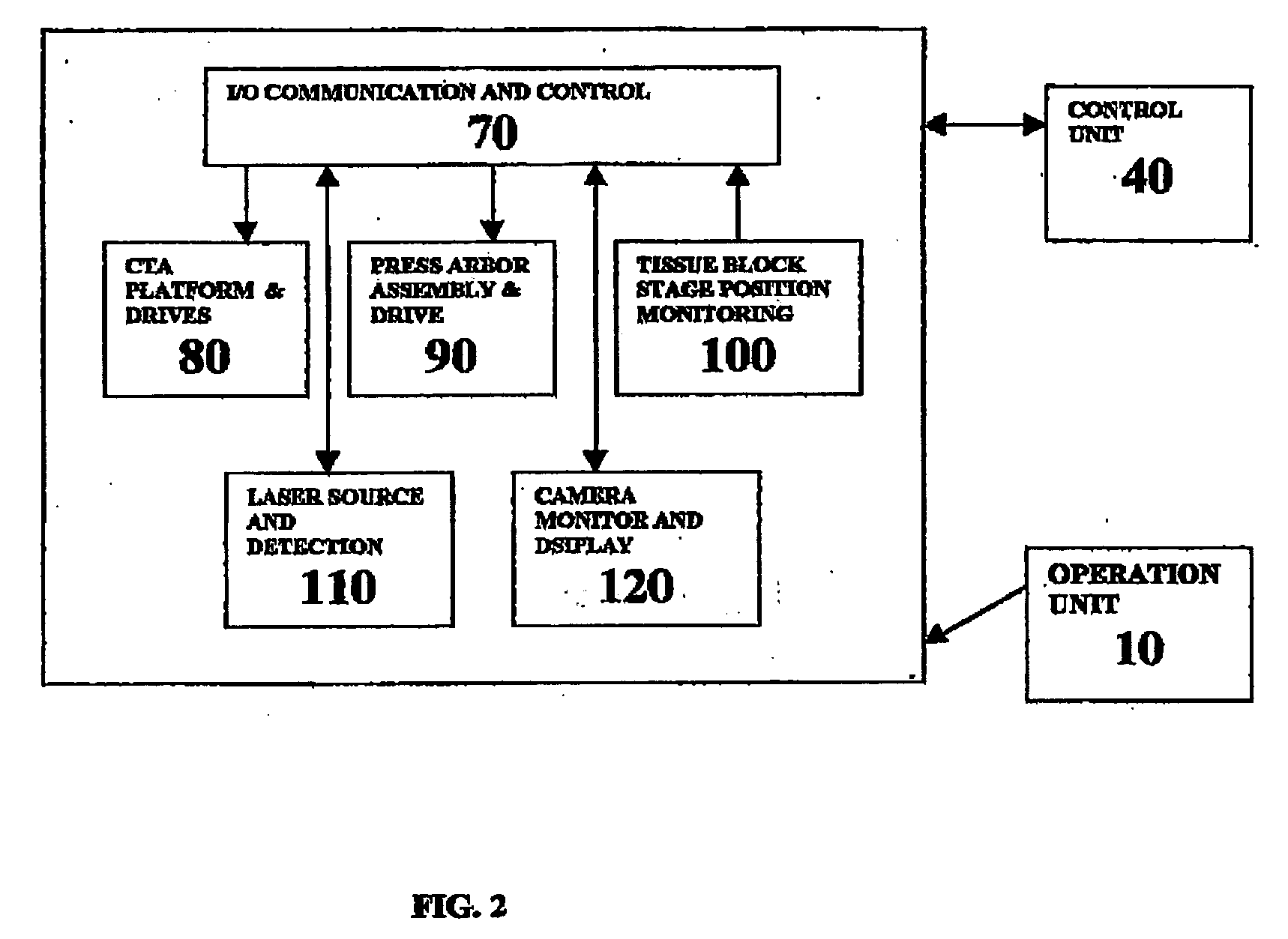
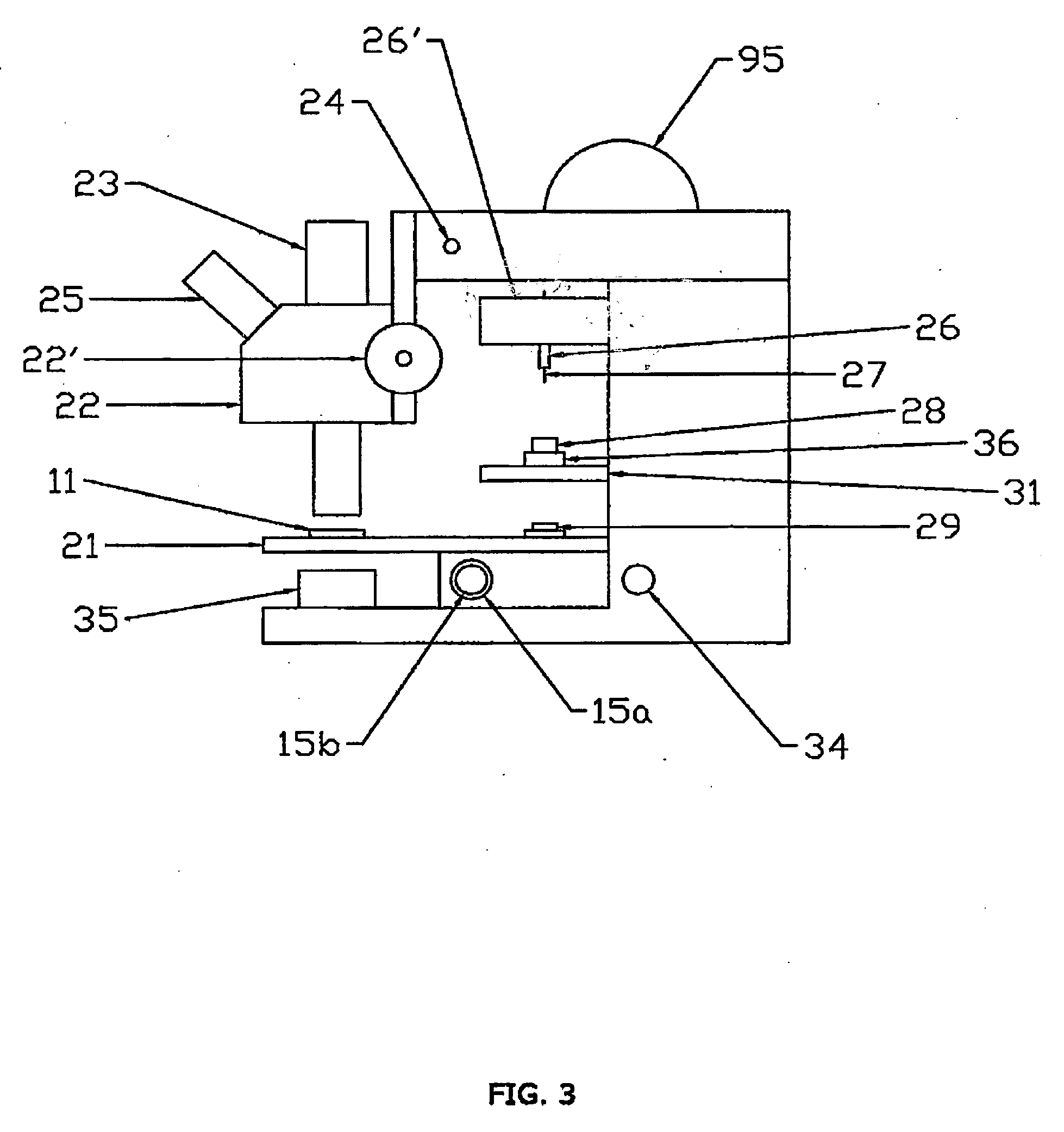
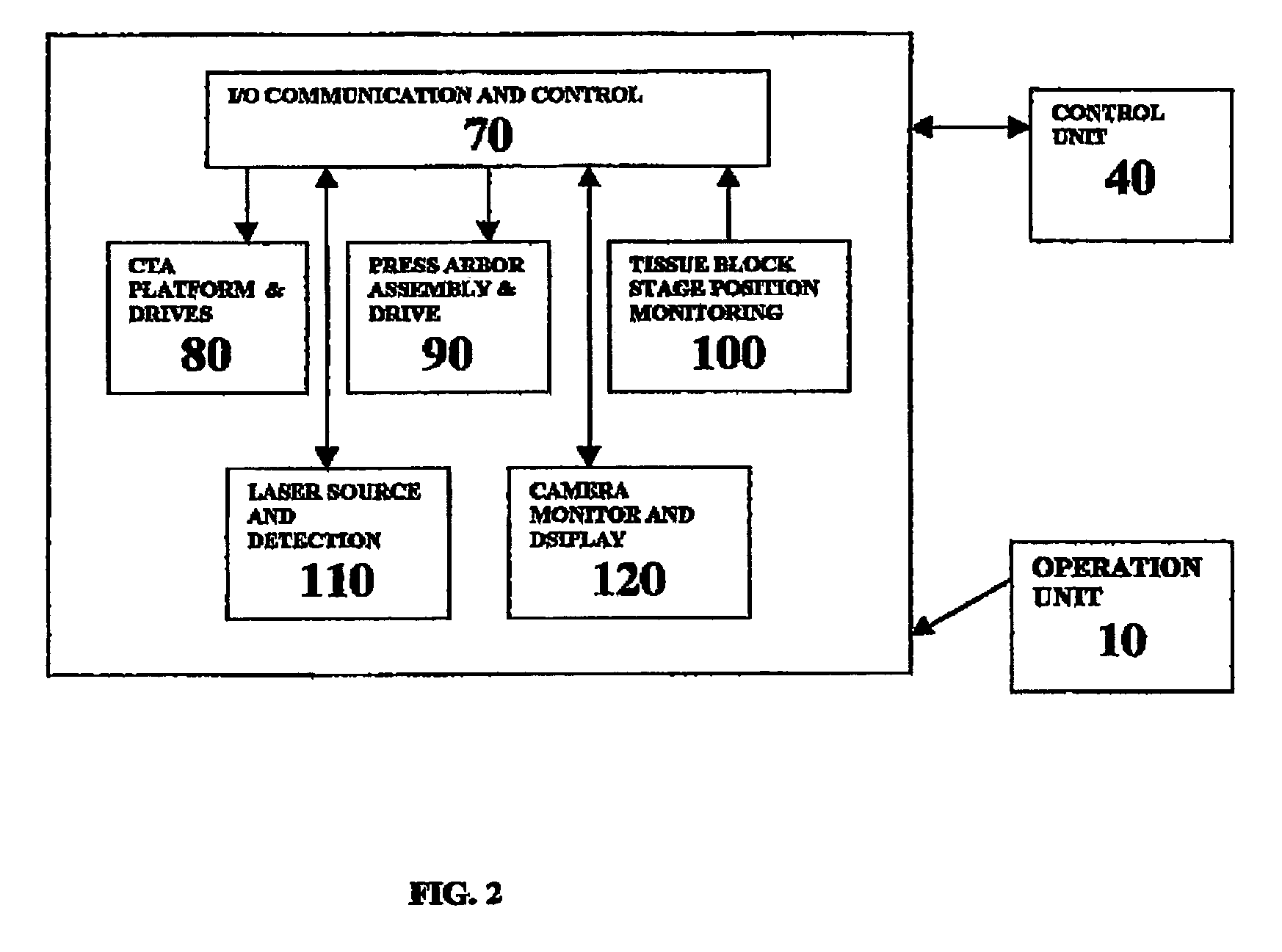
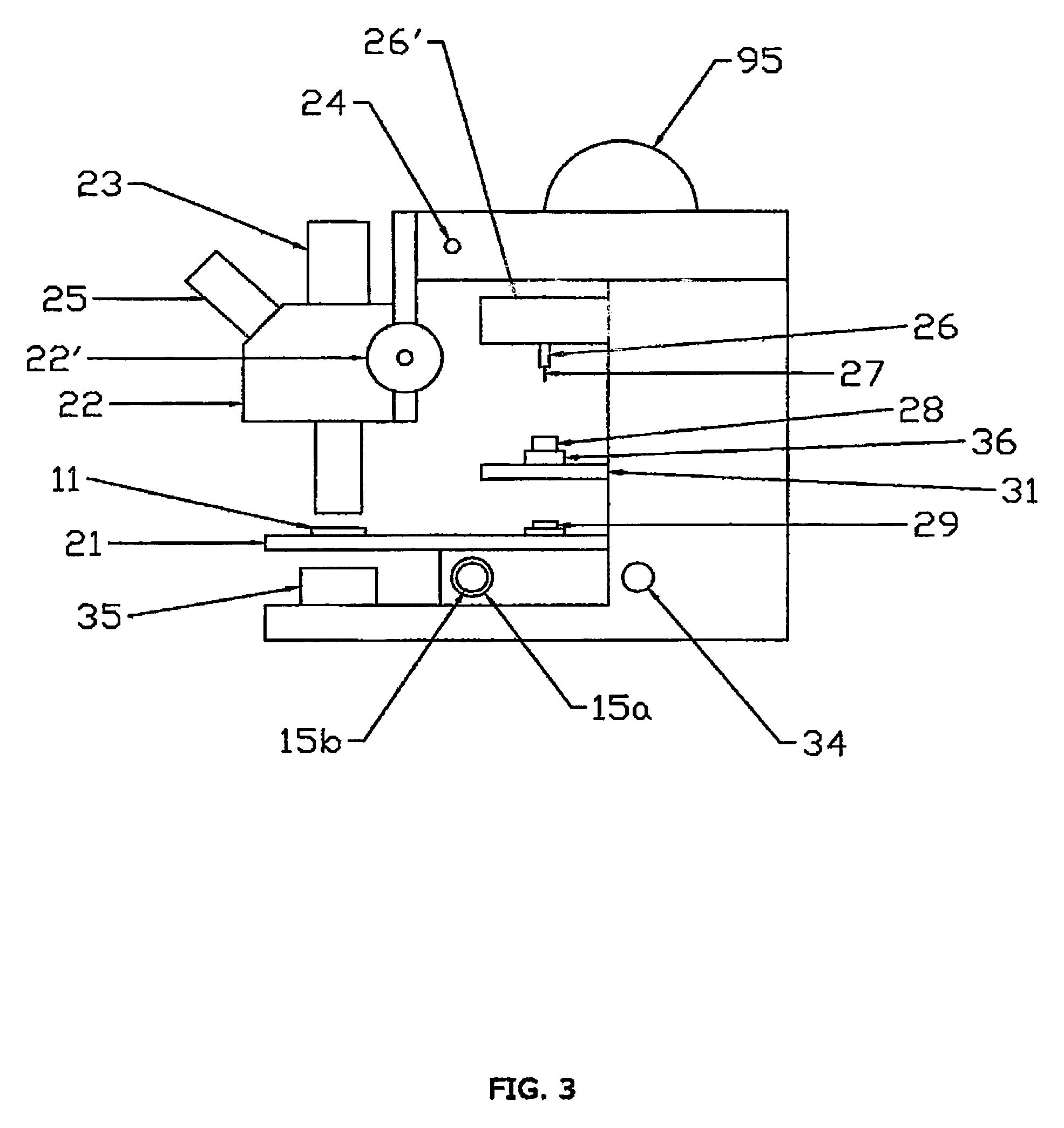
A workstation that provides an efficient method to collect biological tissues in a column tissue array format from blocks of embedded, frozen tissues, or fresh tissues. The workstation has a control unit for directing operations of the workstation and the operation unit for performing the production of the tissue column array. The operation unit comprises an array of vertical tubes in a platform, an arbor which engages and presses down the designated tube in the array, the embedded tissue block which is mounted directly below the designated tube, assemblies of motors responsive to the control unit for driving the platform and the tissue block, a light source block for generating an alignment signal, and a light detector block which measures the signal from the light source to determine the degree of alignment between the arbor, punch tubes, and the specimen block.
Owner:EXB TECH
Tissue punch and tissue sample labeling methods and devices for microarray preparation, archiving and documentation
InactiveUS7405056B2Waste of tissueWaste of timeBioreactor/fermenter combinationsBiological substance pretreatmentsVertical tubeTissue Arrays
A workstation that provides an efficient method to collect biological tissues in a column tissue array format from blocks of embedded, frozen tissues, or fresh tissues. The workstation has a control unit for directing operations of the workstation and the operation unit for performing the production of the tissue column array. The operation unit comprises an array of vertical tubes in a platform, an arbor which engages and presses down the designated tube in the array, the embedded tissue block which is mounted directly below the designated tube, assemblies of motors responsive to the control unit for driving the platform and the tissue block, a light source block for generating an alignment signal, and a light detector block which measures the signal from the light source to determine the degree of alignment between the arbor, punch tubes, and the specimen block.
Owner:EXB TECH
Core sampling device intended to assemble tissue arrays
ActiveUS20060121596A1Simple designEasy to implementBioreactor/fermenter combinationsBiological substance pretreatmentsTissue ArraysWaste management
A core sampling device intended to assemble tissue arrays of the type incorporating a core sampling punch, a core excision punch intended to make recesses in one or several so-called receiver blocks, and means to expel the sample cores into one or several paraffin receiver blocks or into any frozen milieu or not, wherein the core excision punch is mounted substantially coaxially in the core sampling punch, the sampling punch being in the external position, both punches being able to move in translation and / or rotation with respect to one another, and the ejection means being arranged to as to expel the cores from each punch.
Owner:MITOGEN
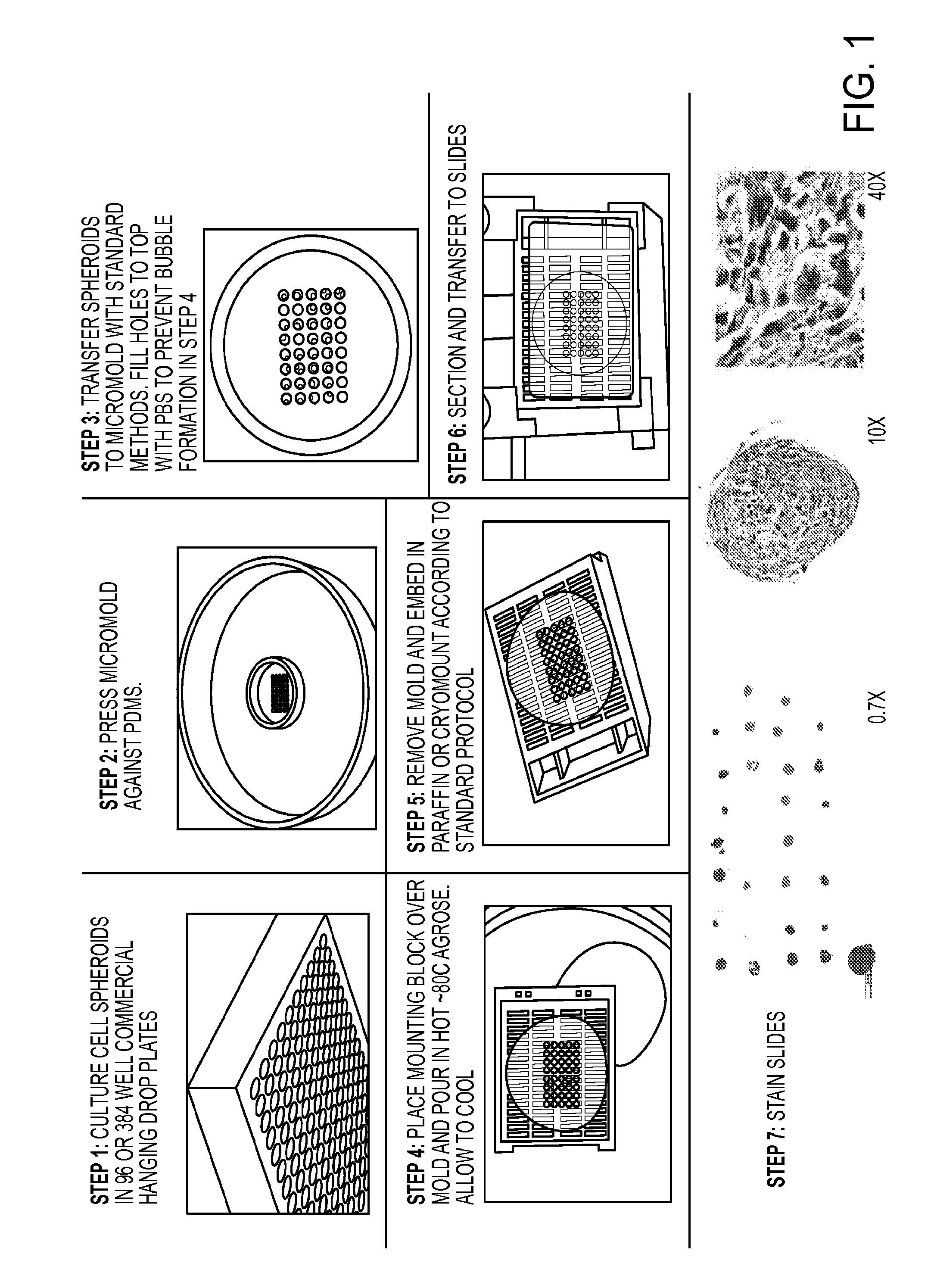
Tissue array for cell spheroids and methods of use
InactiveUS20160281061A1Avoid small quantitiesDiagnosticsPreparing sample for investigationTissue ArraysHistology
The present invention generally features methods of preparing a microarray of cell spheroids, methods of preparing a micromold for embedding spheroids for histology, and methods of screening a library of agents.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Transcriptome microarray technology and methods of using the same
InactiveCN101115848AFacilitate cross-validation researchRaise the potentialTissue ArraysTissue sample
Provided herein are arrays comprising transcriptomes of diseased tissues and methods of using the arrays for diagnosis, prognosis, screening, and identification of diseases. The genetic profile of a tissue sample for a disease state is analyzed using diseased tissue transcriptome arrays for disease diagnosis. The genetic profile is then correlated with the effectiveness of specific therapeutic agents. Correlation of therapeutic agent effects with expression profiles provides for further screening and selection of patients predicted to respond to these therapeutic agents, thus minimizing unnecessary exposure to ineffective treatments.
Owner:ALMAC DIAGNOSTICS LIMITED
Preparation method of paraffin organization chip
InactiveCN101319971AEasy to learnEasy to masterPreparing sample for investigationBiological testingTissue ArraysParaffin oils
The invention discloses a preparing method for an olefin tissue core which includes the steps of customizing an olefin model, customizing a tissue array hole plate, customizing a mandrel, customizing a sampling needle, customizing the olefin block of the tissue chip array of a receptor, sampling the olefin block of a supplier, preparing the olefin block of the tissue chip of the supplier, etc. The preparing method is simple and easy with good experimental result.
Owner:NANTONG UNIVERSITY
Needle biopsy tissue chip
The invention provides a technology and method for preparing high throughput needle biopsy tissue chip. The method comprises the following steps: collecting 42 residual paraffin-embedded tissue samples of needle biopsy of patients who have been diagnosed as the breast cancer patients through pathological examination on mammary gland needle biopsy tissues; taking the corresponding normal pathological HE stained sections as the guide, cutting the paraffin-embedded tissue sections (1mm to 4mm) corresponding to the cancer parts of the HE-stained sections, preparing paraffin-embedded tissue cores, preparing breast cancer needle biopsy tissue arrays by utilizing a tissue chip instrument, cutting paraffin into slices, and preparing paraffin-embedded tissue chip of breast cancer needle biopsy tissue. The prepared needle biopsy tissue chip can be applied to molecular medical research of related diseases such as immunocytochemistry, in-situ hybridization, and the like.
Owner:泰州医药城博奥邦科生物科技有限公司
Tissue array production method
ActiveUS8835130B2Promote safe productionWithdrawing sample devicesPreparing sample for investigationTissue ArraysBiomedical engineering
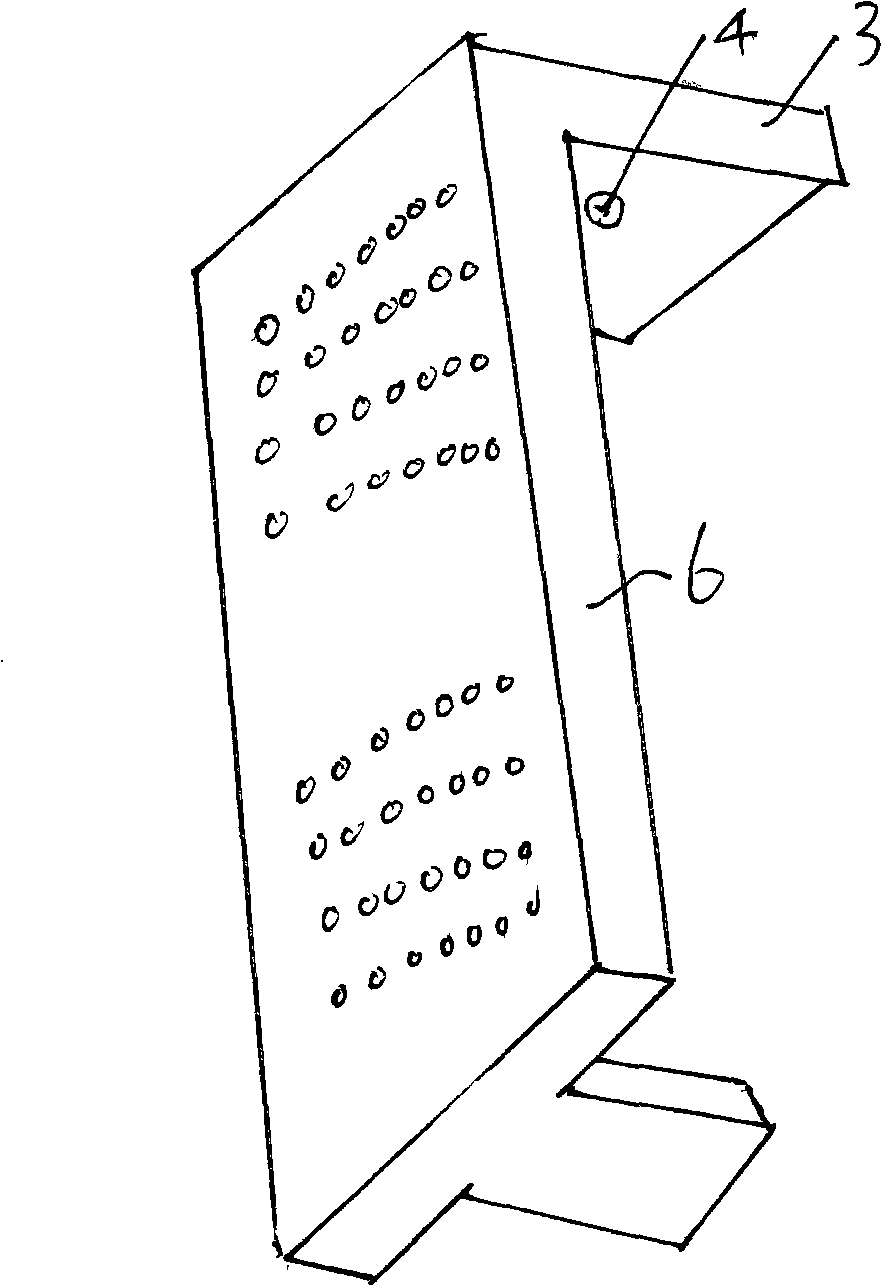
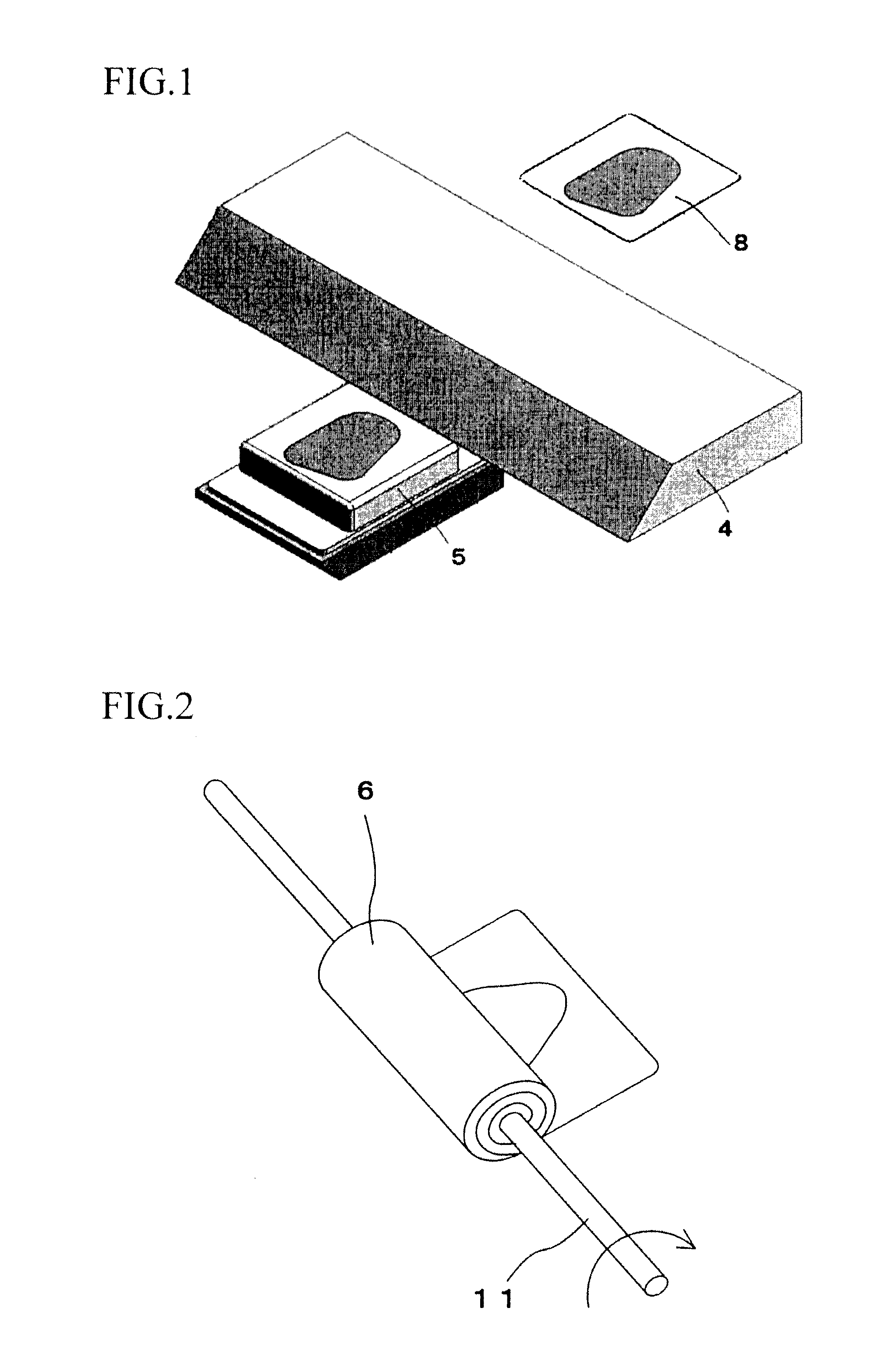
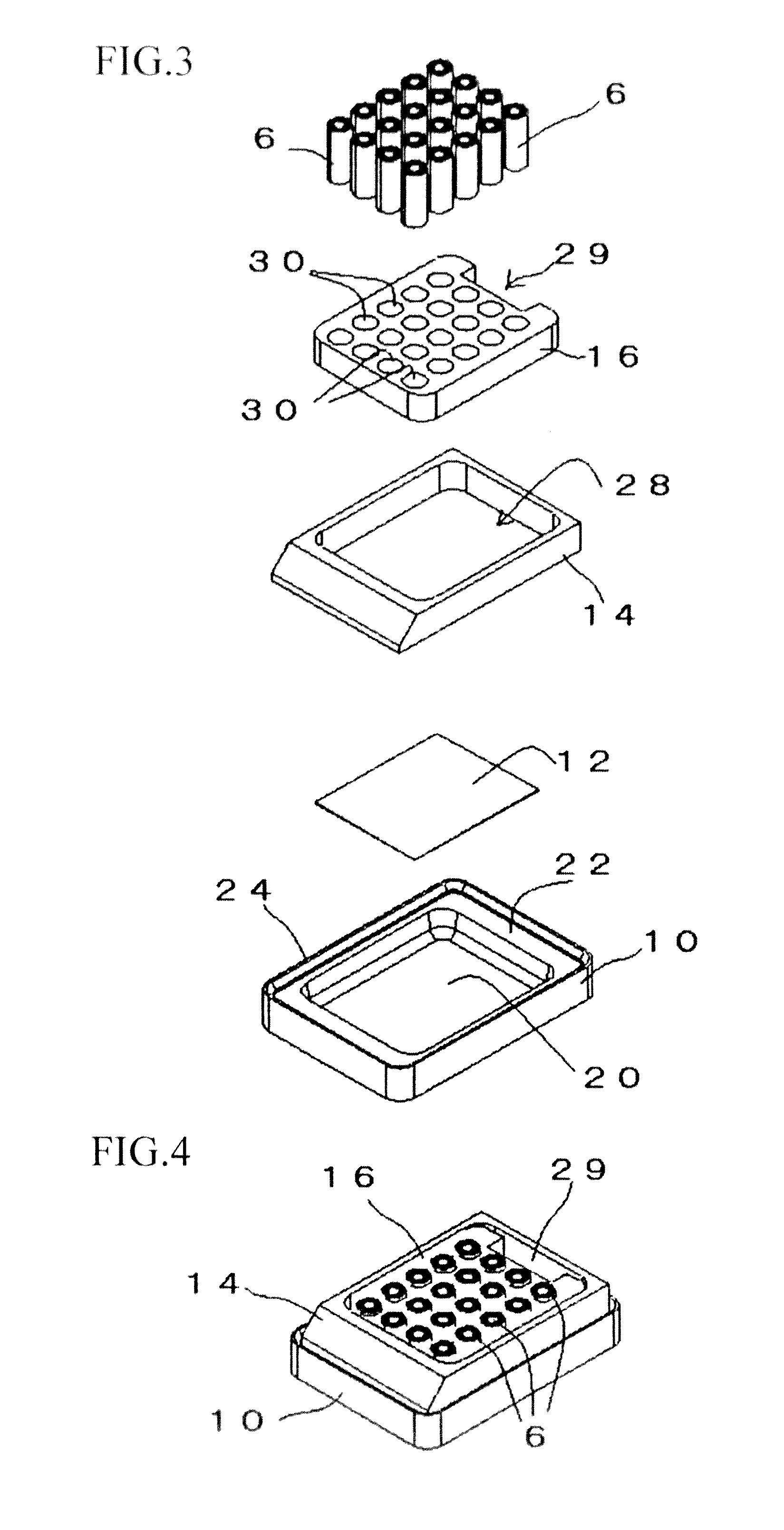
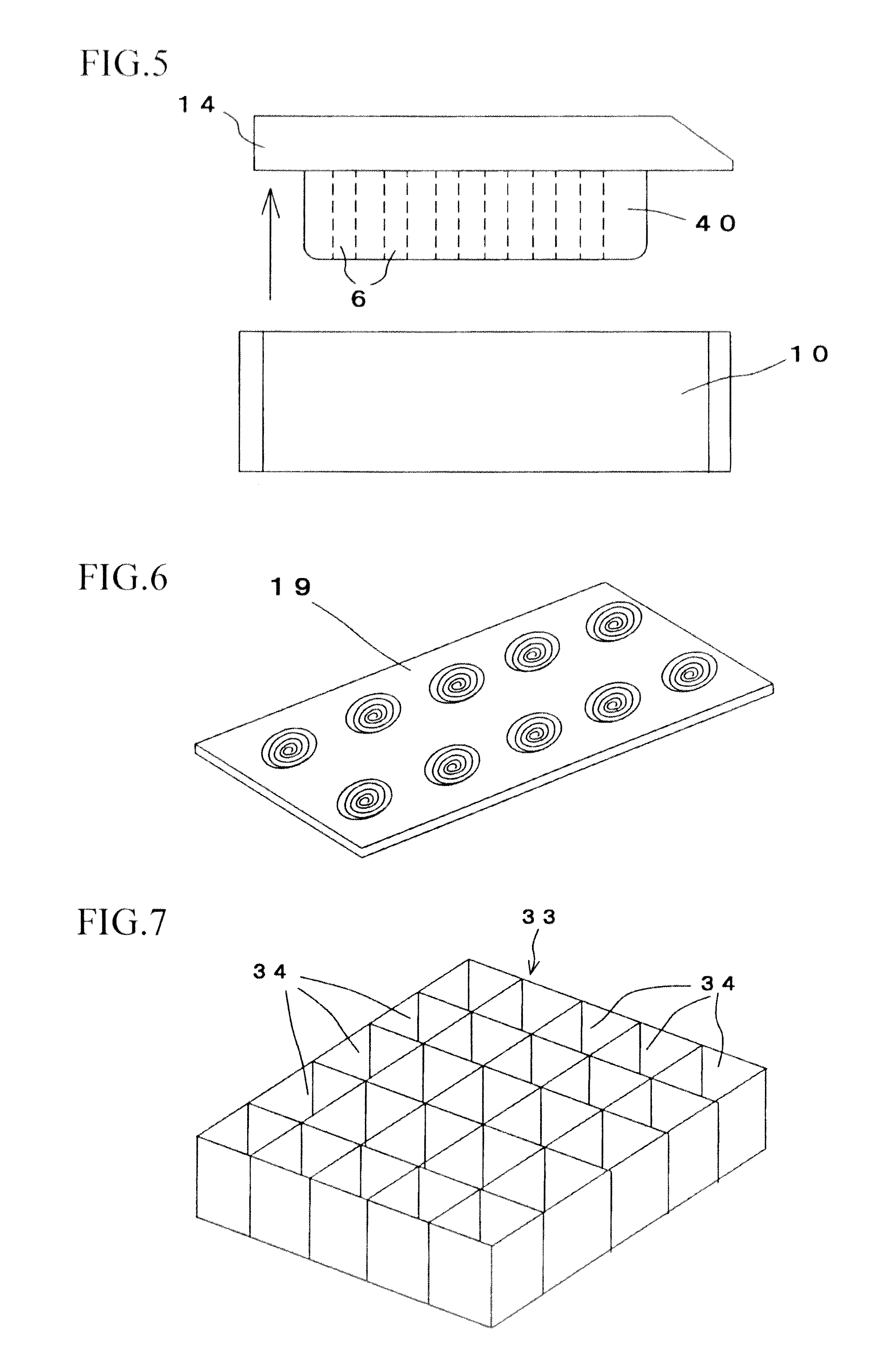
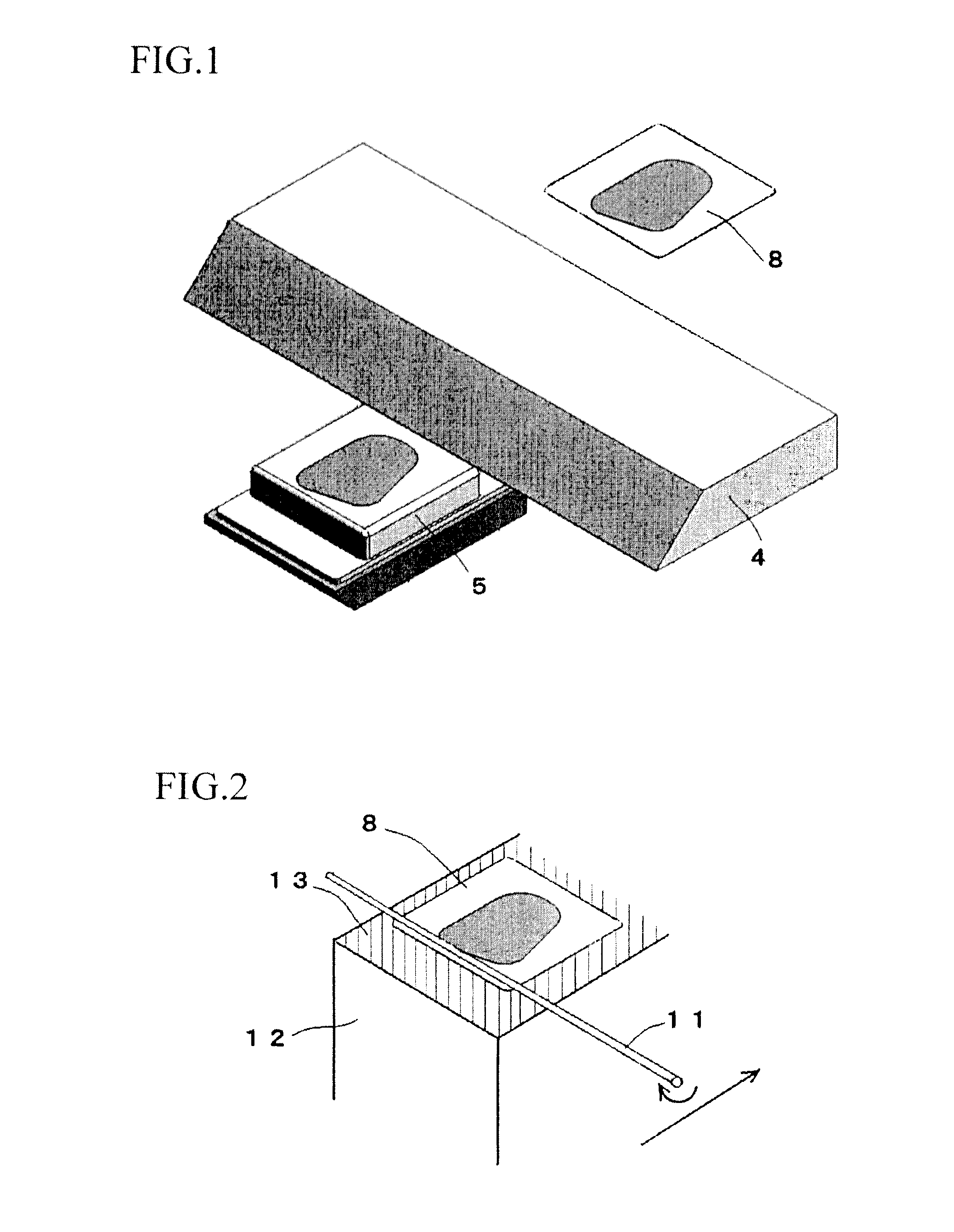
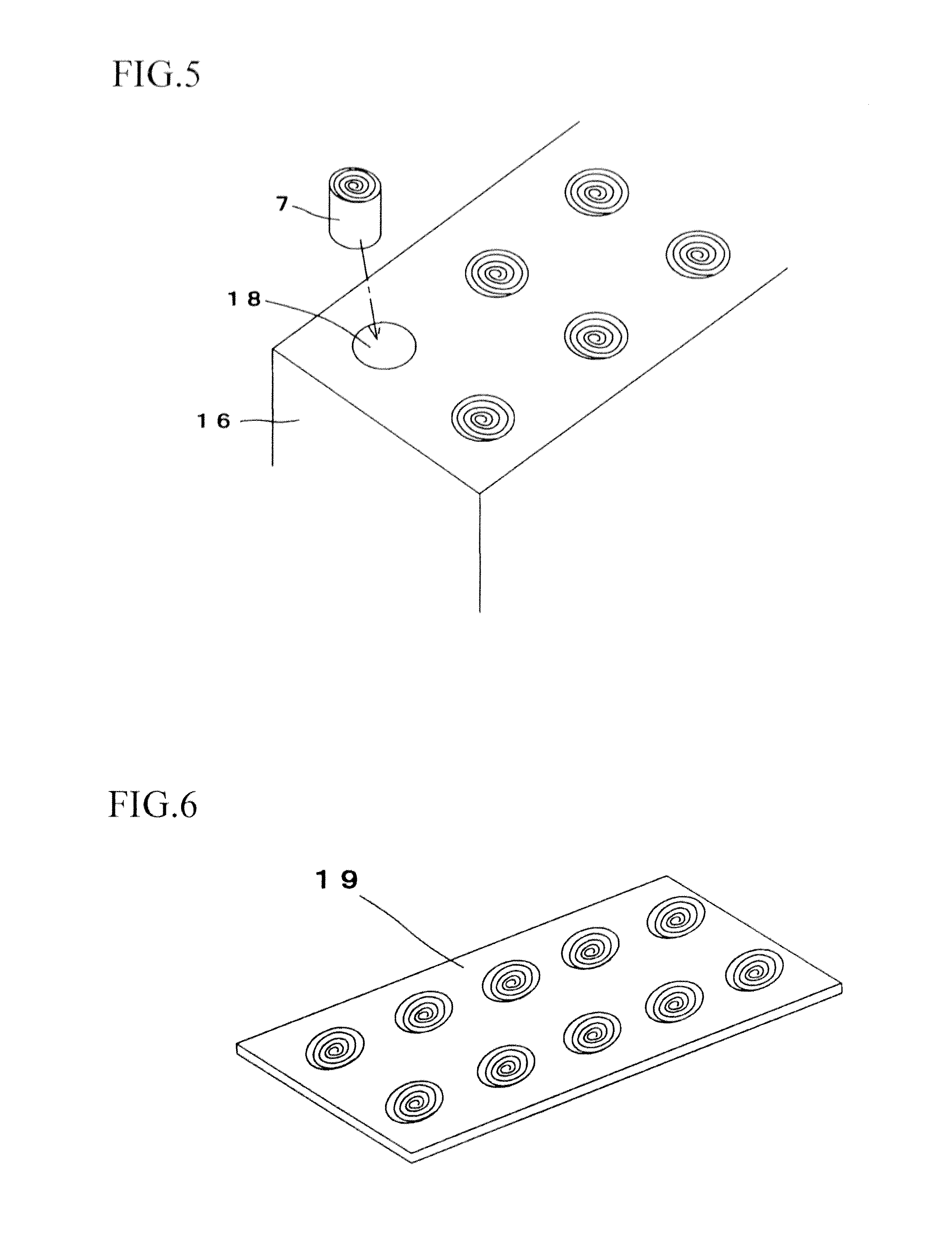
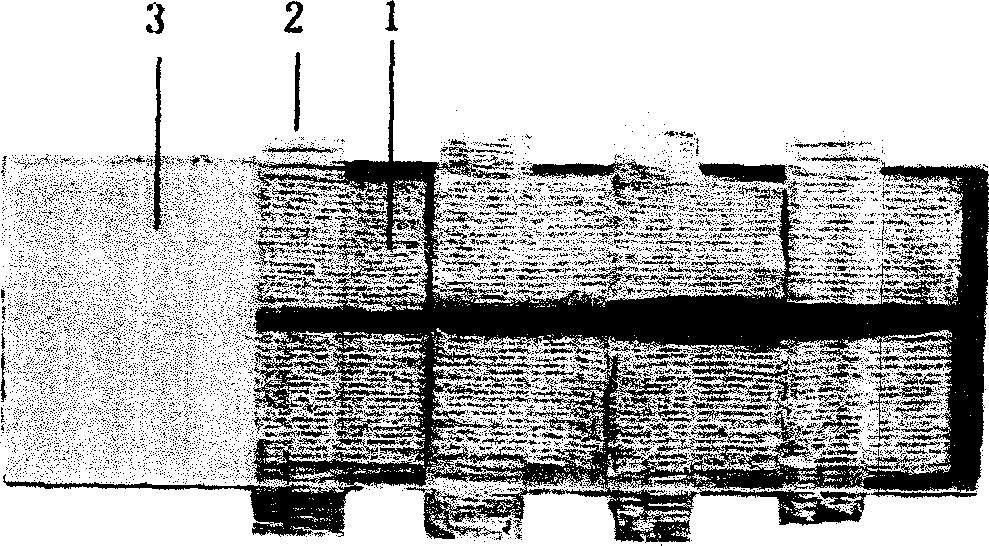
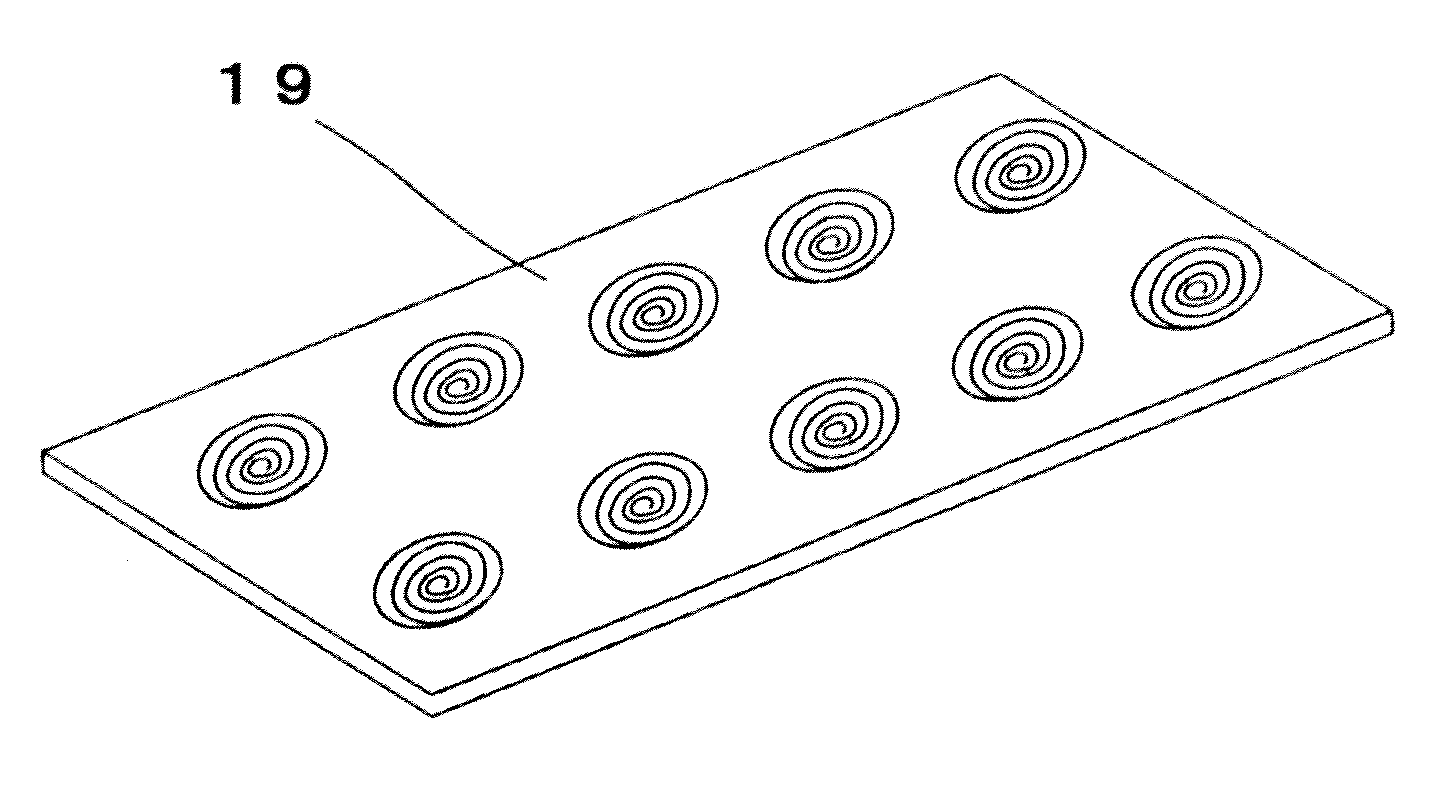
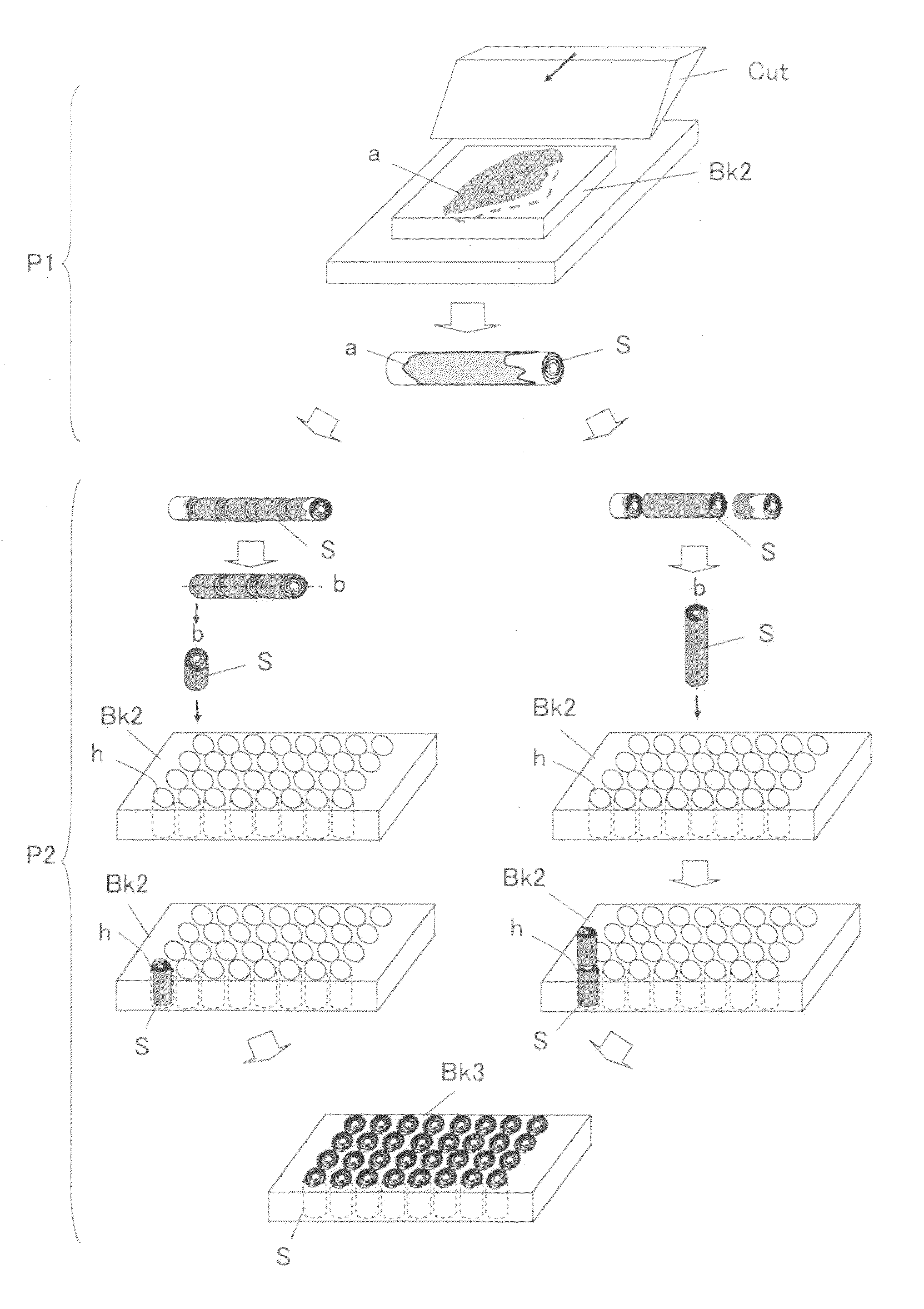
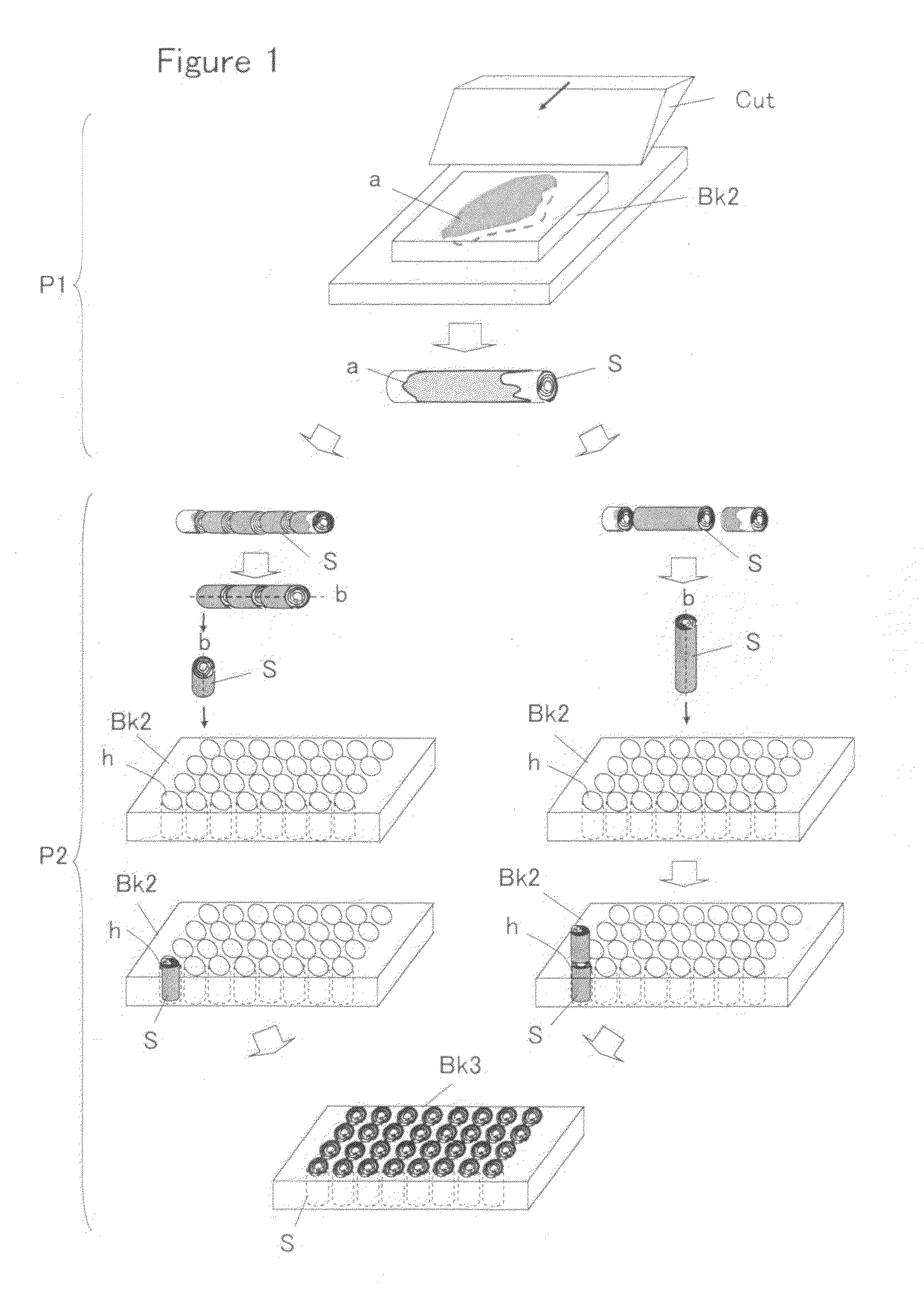
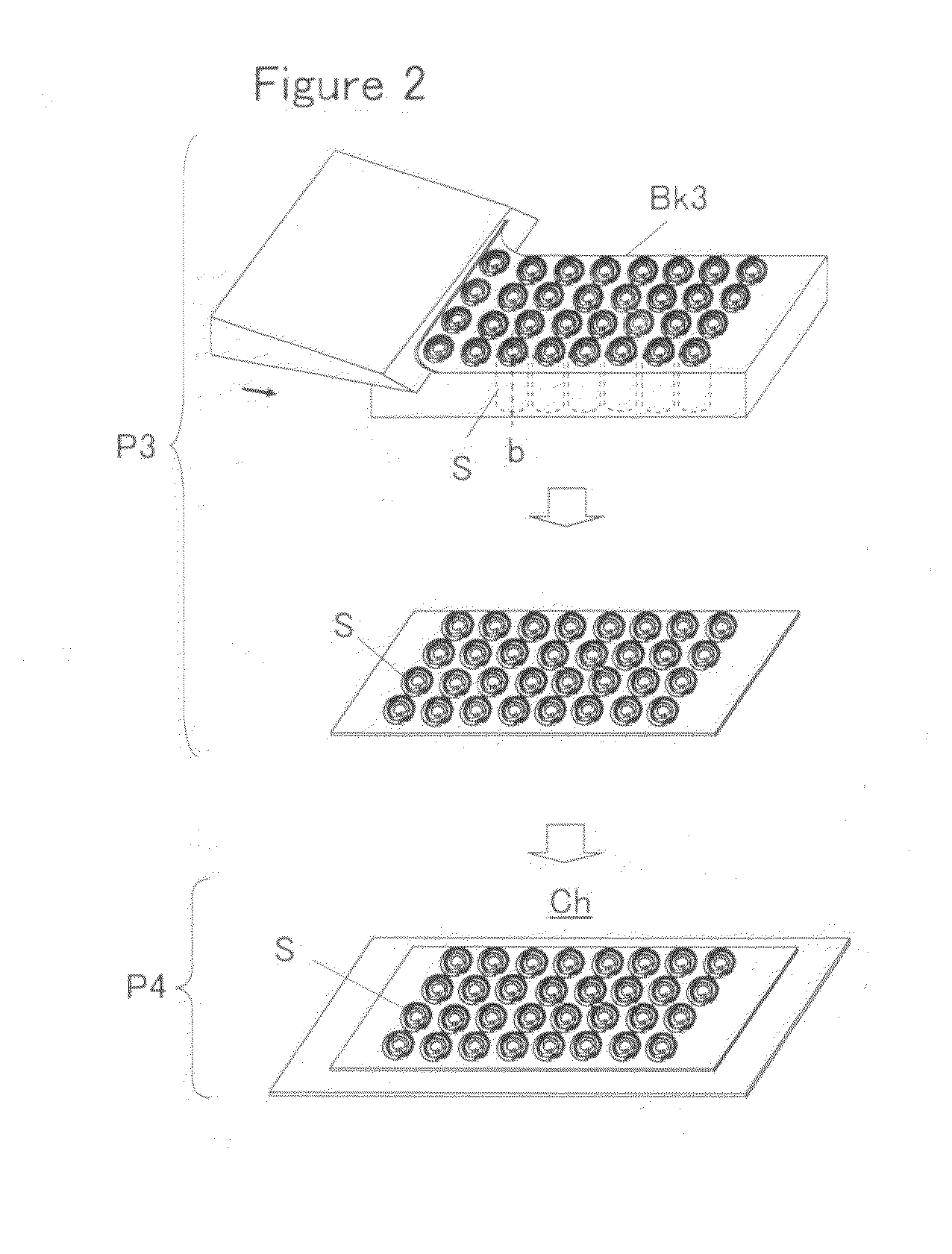
Tissue array production method enables even roll-shaped tissue pieces having various diameters to be steadily fixed to a substrate block is provided. In a method in which roll-shaped tissue pieces formed by rolling sheet-like tissue pieces in the shape of a roll are arranged on a substrate in an array form, a tissue array is produced by placing a holding member which holds a plurality of roll-shaped tissue pieces so that their axis directions are vertically directed in a container into which a melted embedding medium is poured for its accumulation, to hold the respective roll-shaped tissue pieces in the holding member; pouring the embedding medium into the container, to form a substrate block constituted so that the plurality of roll-shaped tissue pieces is arranged in the array form; and slicing the substrate block so that the roll-shaped tissue pieces are ring-shaped.
Owner:SAKURA SEIKI +1
Method for forming tissue pieces for tissue array and device for forming tissue pieces for tissue array
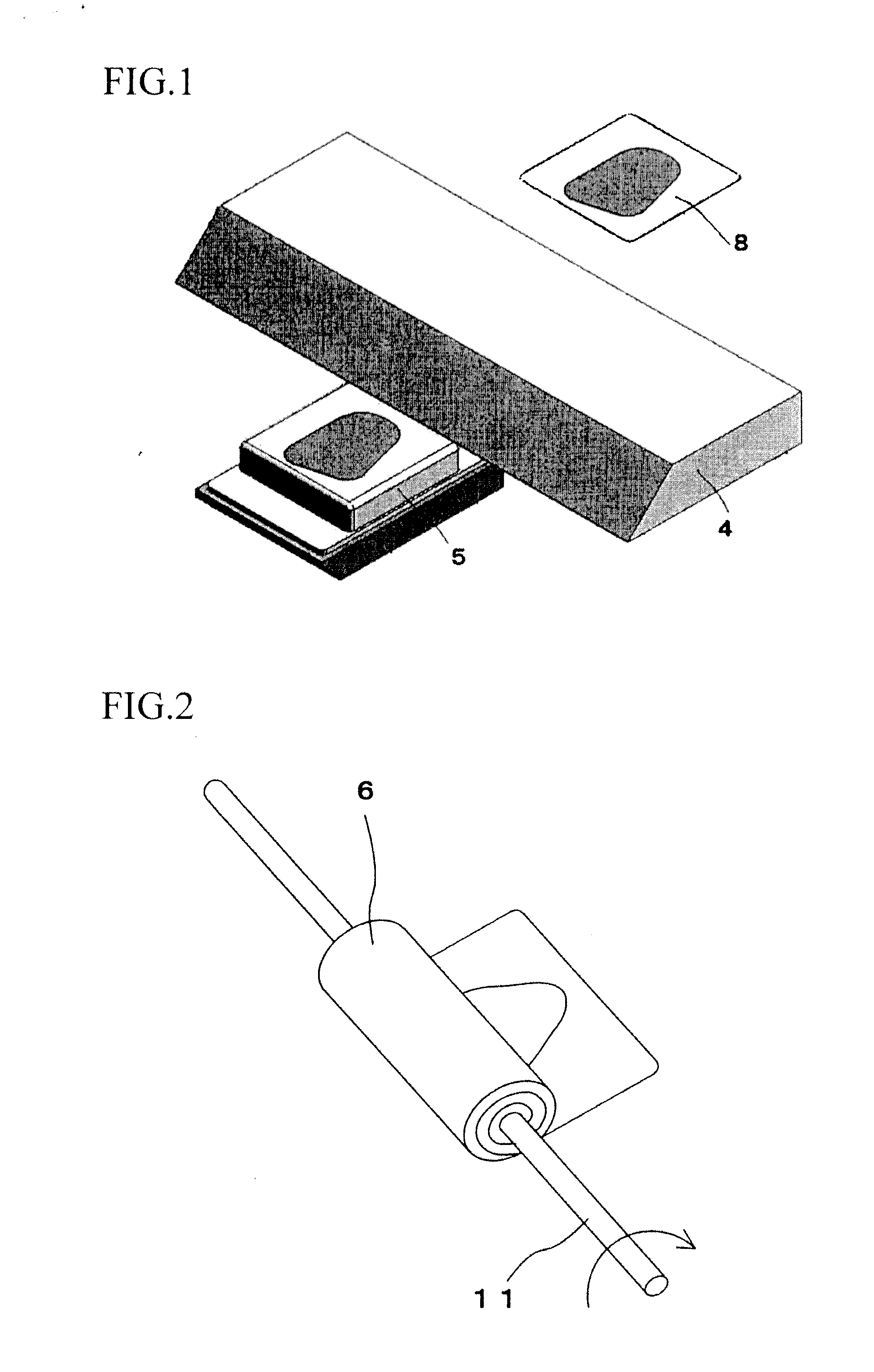
ActiveUS20130122540A1Improve performanceBiological substance pretreatmentsPreparing sample for investigationTissue ArraysBiomedical engineering
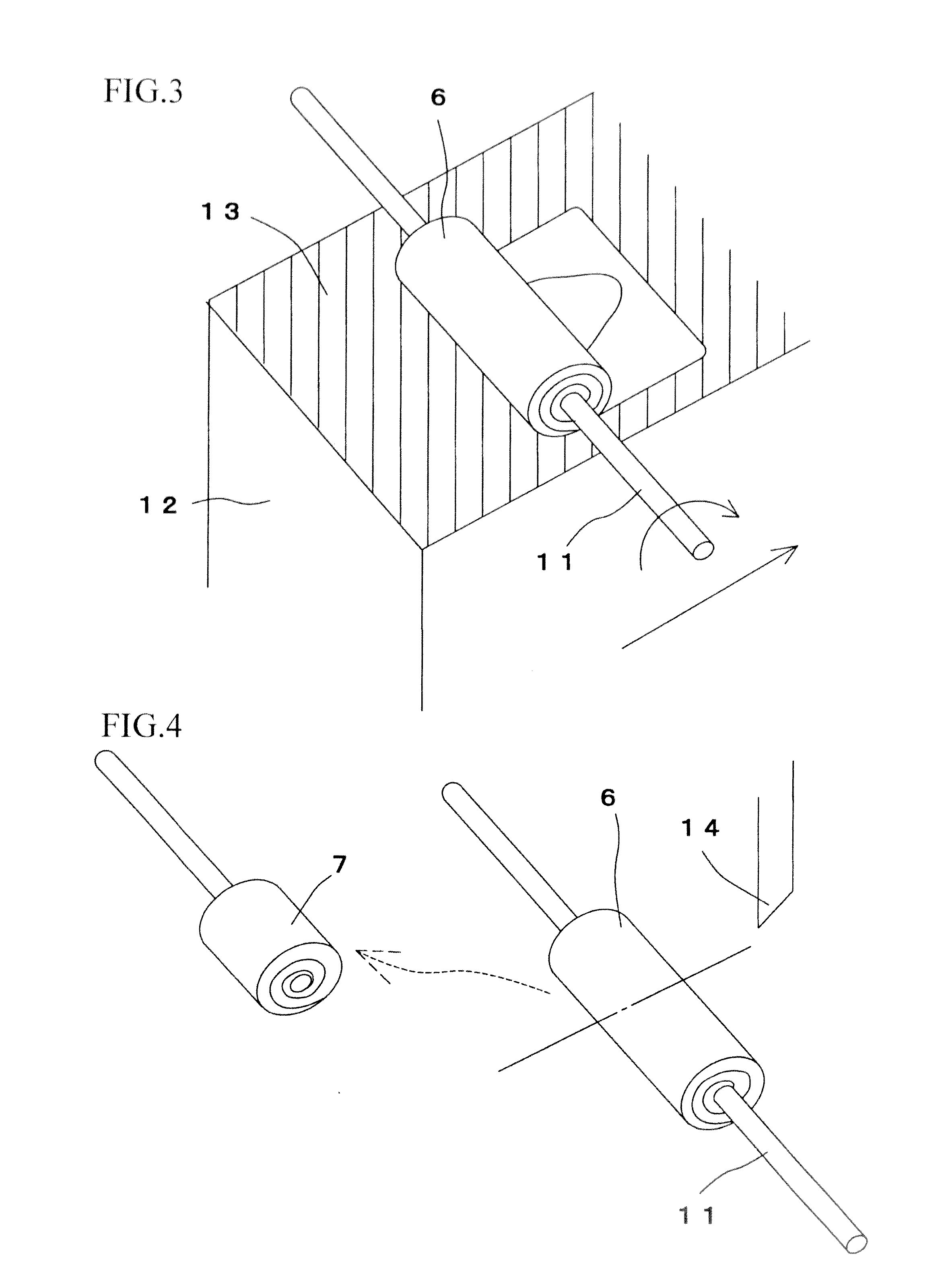
The invention provides a method for forming tissue pieces which allows stable and accurate winding around a core rod in rolling a sheet-like tissue piece, and a device therefor.In the tissue pieces used as each tissue piece in a tissue array obtained by arranging the tissue pieces on a substrate in an array form, the method for forming a roll-shaped tissue piece 6 obtained by rolling a sheet-like tissue piece 8 is characterized by the facts that the sheet-like tissue piece 8 is produced by slicing a tissue block 5 obtained by embedding the tissues with an embedding medium, the sheet-like tissue piece 8 is placed on a mounting block 12, peeling means 13 which easily enables peeling of the tissue piece 8 from the mounting block 12 is applied between the mounting block 12 and the tissue piece 8, and the tissue piece 8 is wound around a core rod 11 while heating the tissue piece 8 on the mounting block 12.
Owner:SAKURA SEIKI +1
Core sampling device intended to assemble tissue arrays
ActiveUS7572410B2Simple designEasy to implementBioreactor/fermenter combinationsBiological substance pretreatmentsTissue ArraysNuclear engineering
A core sampling device includes a core sampling punch, a core excision punch that makes a recess in a receiver body, and an ejector that expels a sample core into the receiver body. The core excision punch is mounted inside the core sampling punch and is substantially coaxial with the core sampling punch. Both punches are able to move in translation and / or rotation with respect to one another, and the ejector is arranged so as to expel cores from each punch.
Owner:MITOGEN
Large capacity tissue array making method
InactiveCN1763228ALarge capacitySaving and timeMicrobiological testing/measurementBiological testingTissue ArraysTissue microarray
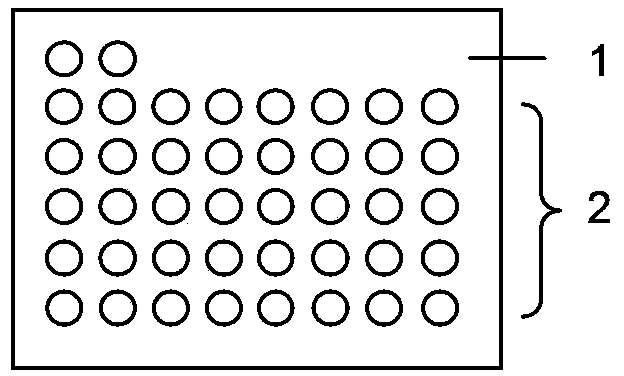
The process of preparing great capacity tissue array includes the following steps: preparing several tissue array slices and arranging the tissue array chips on the identical glass slide; dropping water in 43-49 deg.c onto the glass slide to develop the slices; exhausting water on the glass slide to make the slices close to the glass slide; and final heating at 60 deg.c for 1-2 hr and dewaxing with xylene. The tissue array thus prepared has great capacity, and the preparation process is simple, convenient and easy to control. The micro array has great sample number and this can avoid pseudo negative in detection.
Owner:SOUTHERN MEDICAL UNIVERSITY
Method for manufacturing tissue array paraffin block
ActiveCN101738338BEasy to sliceHigh yieldPreparing sample for investigationBiological testingTissue ArraysAdhesion strength
The invention discloses a method for manufacturing a tissue array paraffin block, which comprises the following steps: attaching the tissue array to the same plane of an embedding bottom mold by using tissue array adhesive, embedding paraffin, cooling, and reducing the adhesion strength of the tissue array by using a releasing agent, thereby obtaining the tissue array paraffin block. The method comprises the steps of sampling, arranging, array planting, paraffin filling and demolding. The invention is applied to the techniques for manufacturing paraffin tissue arrays in the field of biochips.The method can be used for manufacturing the tissue array paraffin blocks with ease and high efficiency.
Owner:苏州新芯生物技术有限公司
Tissue array production method
ActiveUS20130115652A1Promote safe productionWithdrawing sample devicesPreparing sample for investigationTissue ArraysBiomedical engineering
A tissue array production method which enables even roll-shaped tissue pieces having various diameters to be steadily fixed to a substrate block is provided.In the tissue array production method in which roll-shaped tissue pieces 6 formed by rolling sheet-like tissue pieces 8 in the shape of a roll are arranged on a substrate in an array form, the tissue array is produced by placing a holding member 16 which holds a plurality of roll-shaped tissue pieces 6 so that their axis directions are vertically directed in a container 10 into which a melted embedding medium is poured for its accumulation, to hold the respective roll-shaped tissue pieces 6 in the holding member 16; by pouring the embedding medium into the container 10, to form a substrate block 40 constituted so that the plurality of roll-shaped tissue pieces 6 is arranged in the array form; and by slicing the substrate block 40 so that the roll-shaped tissue pieces 6 are ring-shaped, to place the sliced pieces on the substrate.
Owner:SAKURA SEIKI +1
Tissue piece forming device and tissue piece forming method
InactiveUS20110218558A1Easy to integrateHighly-integratedPreparing sample for investigationWound clampsTissue ArraysBiomedical engineering
A tissue piece forming device suitable for forming a tissue piece required in the process of fabricating a novel tissue array chip having many benefits such as the one that the tissue array chip can be fabricated even if the tissue included in a tissue block has a small thickness. The tissue piece forming device (100) for forming a sheet-like tissue piece (S) into a roll shape includes a rotary shaft (1) to which one end of the sliced tissue piece (S) is secured and a drive means for rotating the rotary shaft (1). The tissue piece (S) can be formed into a tight roll shape while rotating the rotary shaft (1).
Owner:UNIVERSITY OF TOYAMA
Microscopic precision construction of tissue array block related application data
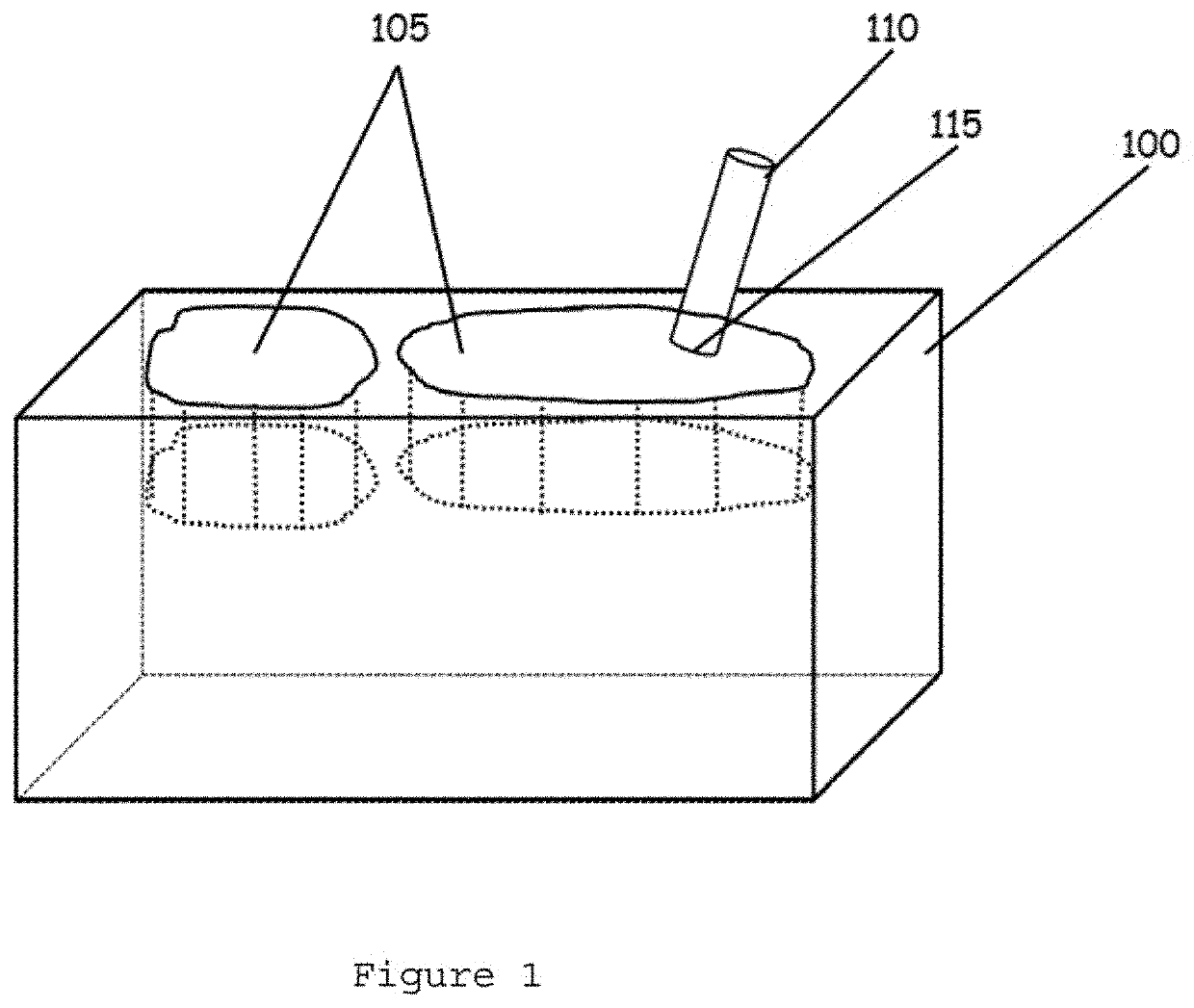
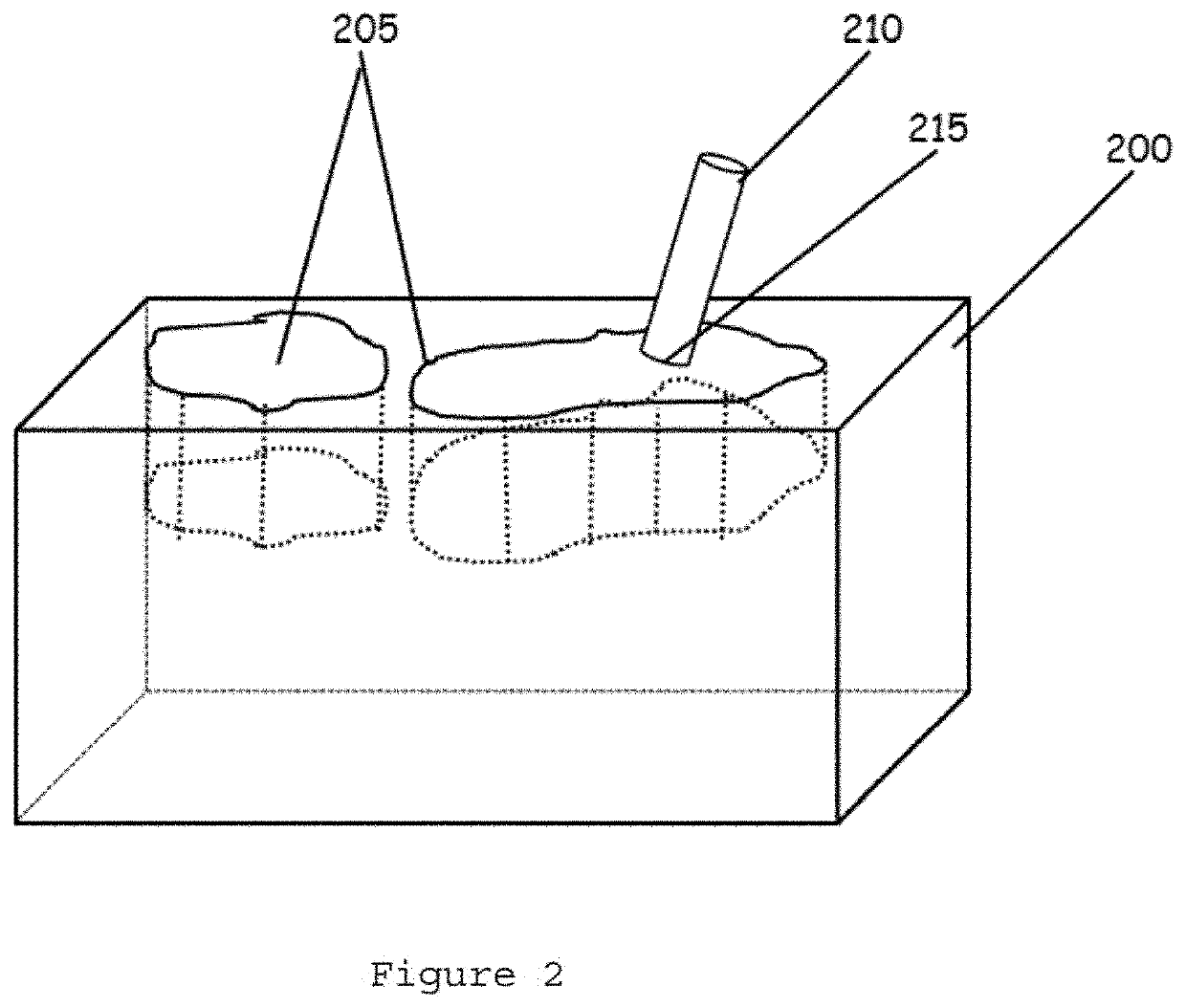
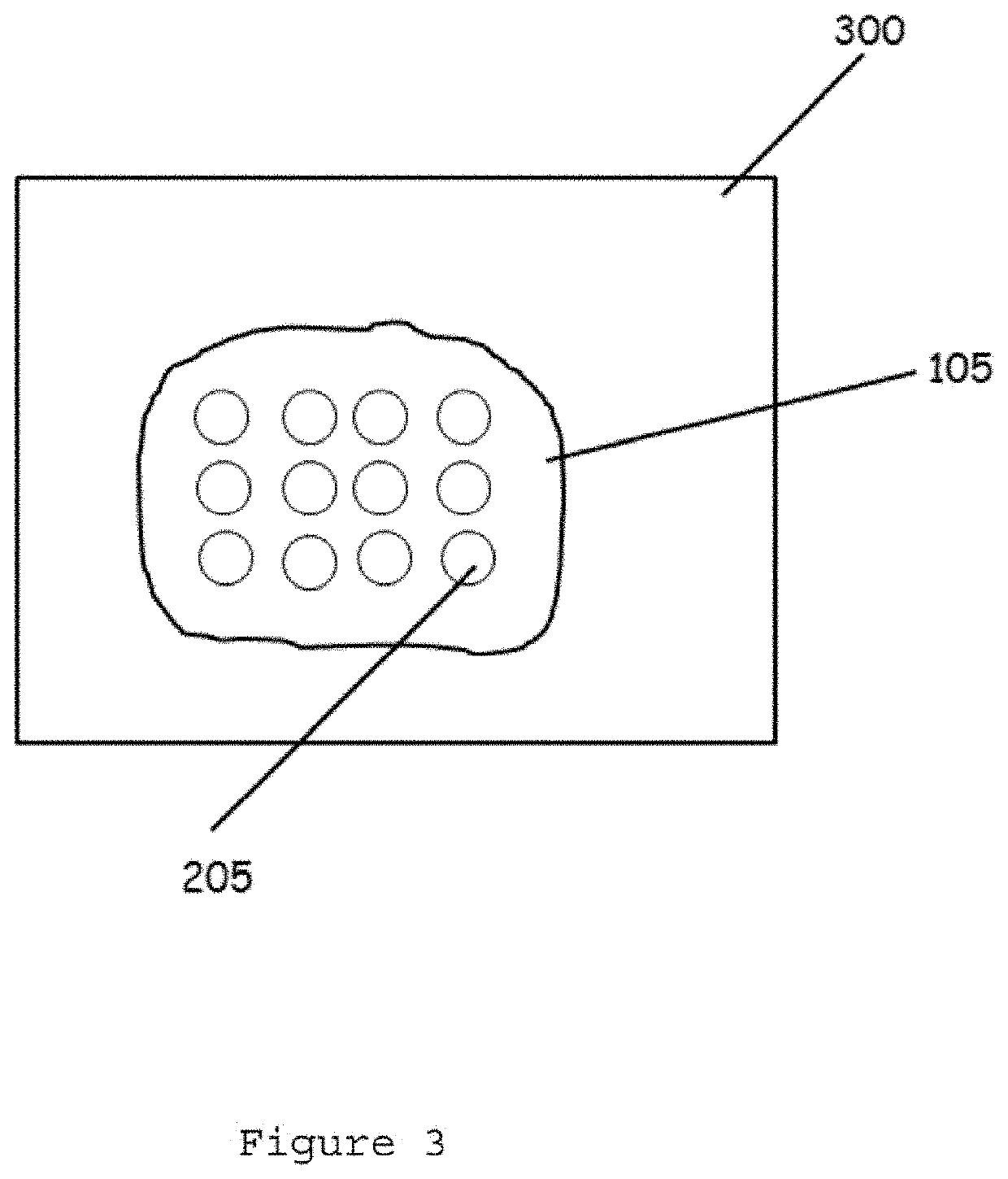
InactiveUS20060099653A1Choose accuratelyEasy to assemblePreparing sample for investigationBiological testingTissue ArraysTissue sample
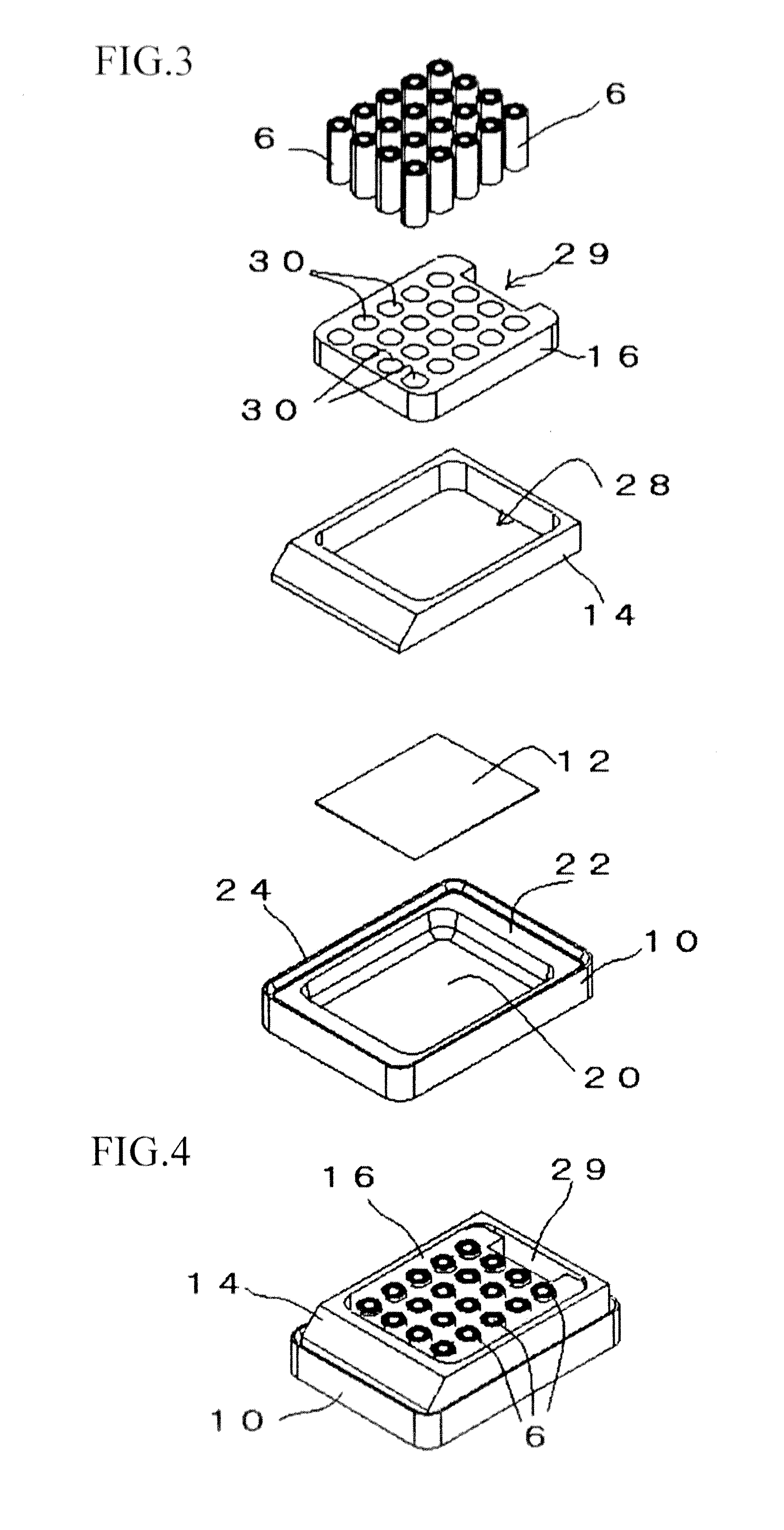
This present invention covers novel means, devices and instruments for production of a tissue array block that is further sectioned into duplicates of tissue arrays. An integral microscope is incorporated into the instrument for viewing and examining a stained reference slide and selecting donor tissue core region(s) from the reference slide. The reference slide is held in a reference slide station that is operatively linked and indexed with a station or platform holding a source donor tissue block, which is further indexed and precisely positioned with reference to the donor needle punch for punching the donor tissue core(s). A recipient block indexed to the donor block punch is placed under the donor punch station and donor tissue cores are delivered into pre-existing hole(s) by a stylet to construct the tissue array block. The instrument includes a donor punch station, optionally a second recipient punch station, with each operable independently or removable. The present invention also provides pre-loading needles with donor tissue cores for constructing tissue array blocks in pre-gridded and pre-punched recipient block. The tissue arrays produced from the tissue array blocks made are useful for testing such freshly-made and / or archival tissue specimens in both scientific and clinical research and applications.
Owner:ADVANCED EDM AUTOMATION
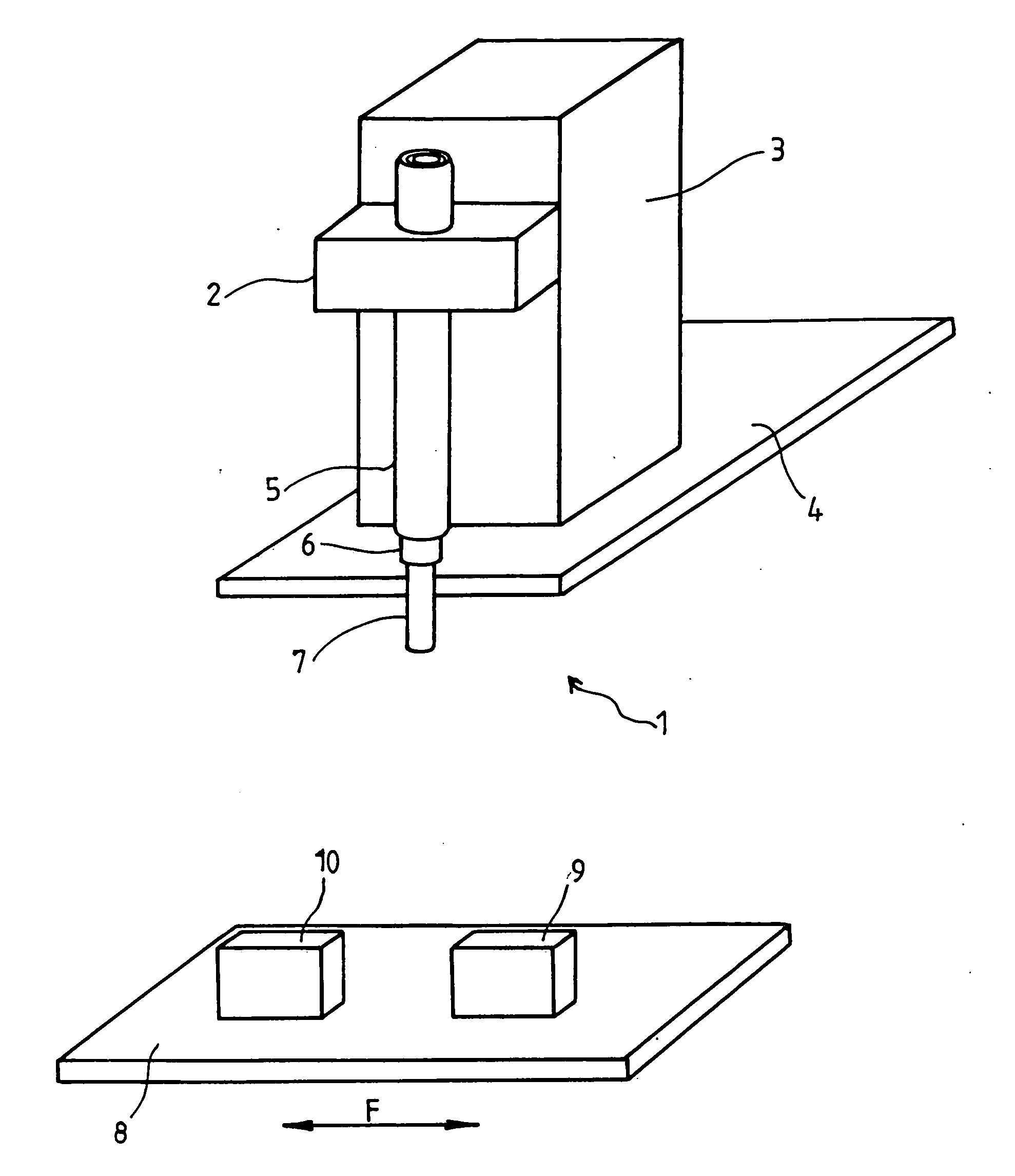
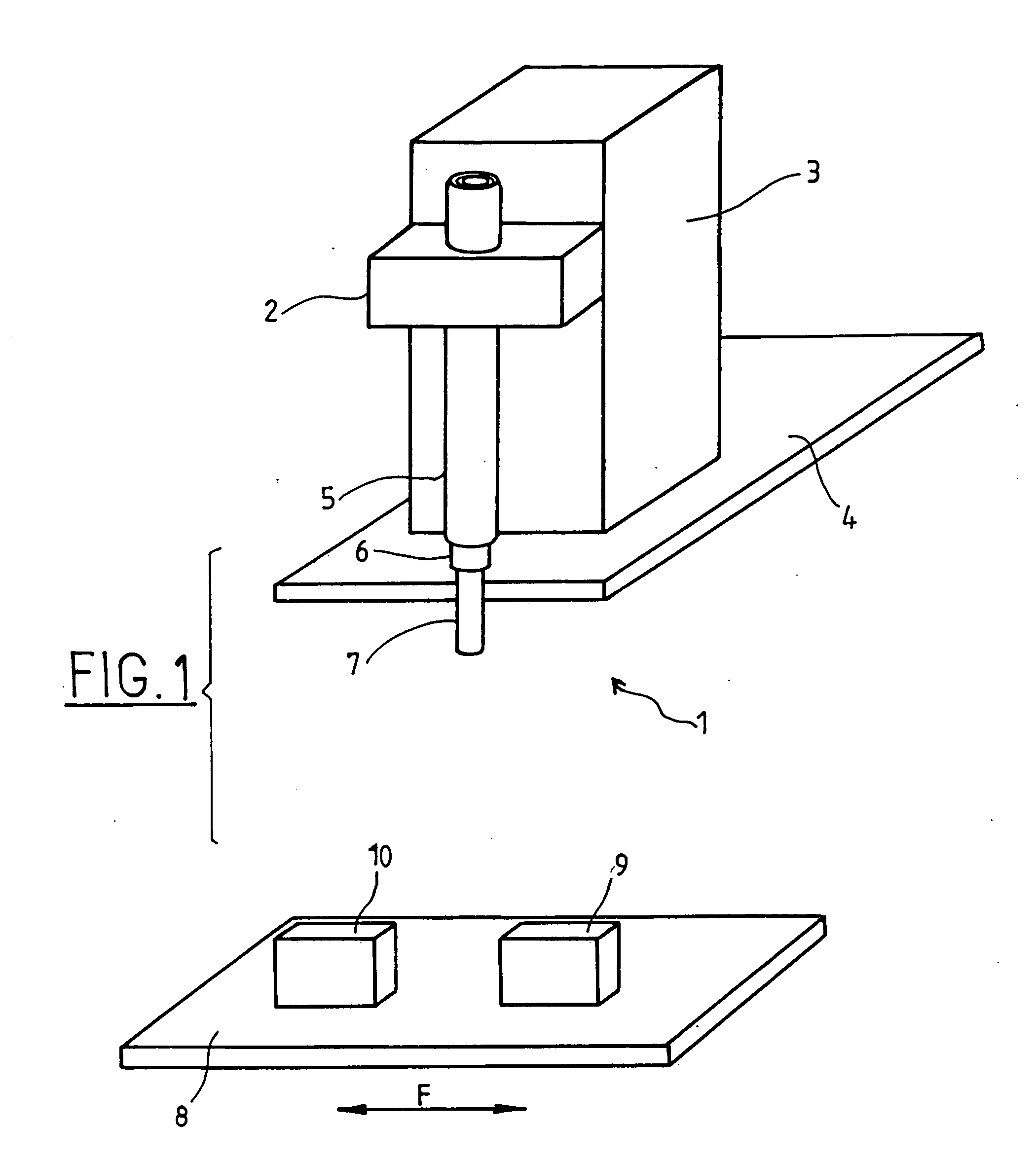
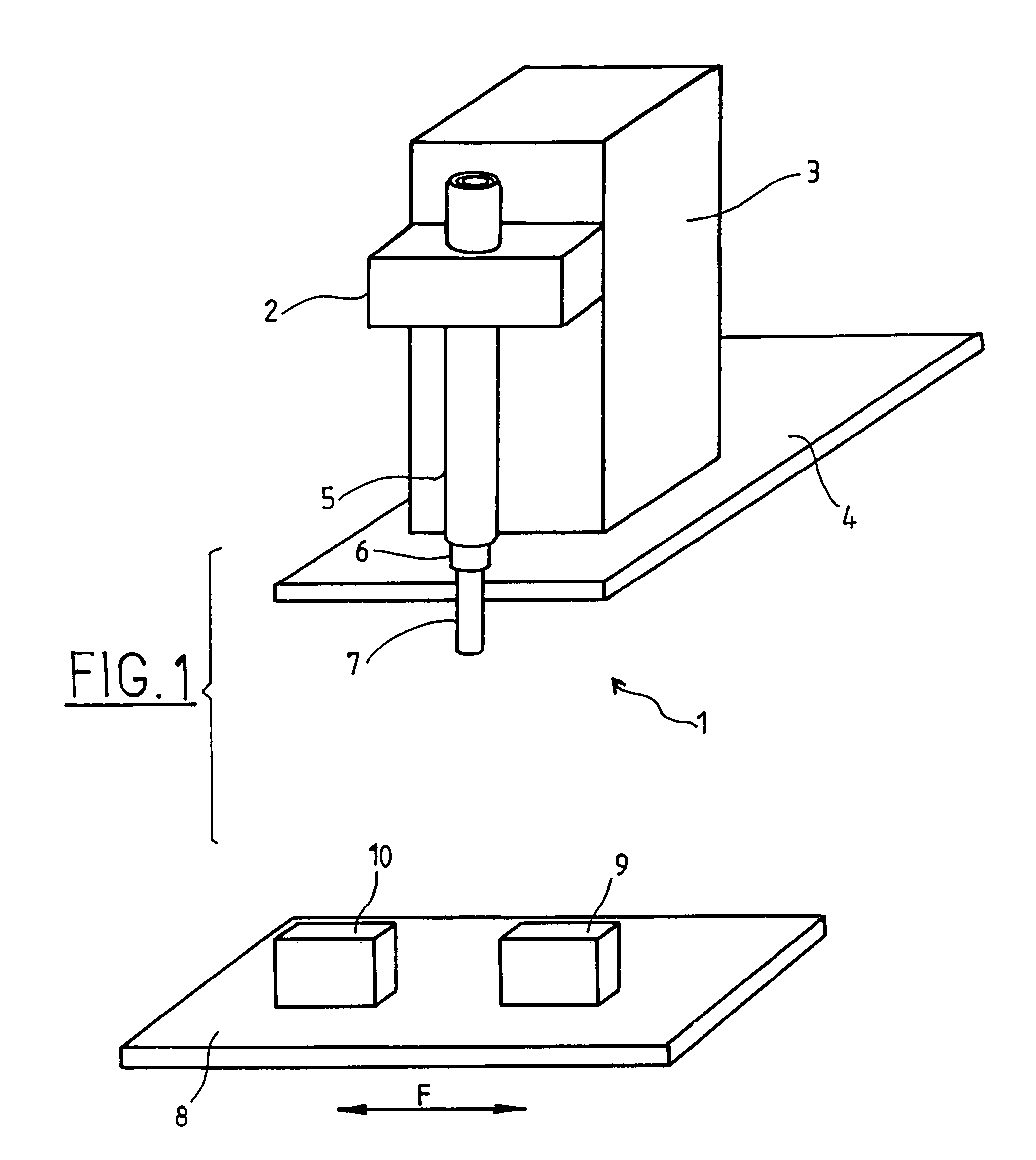
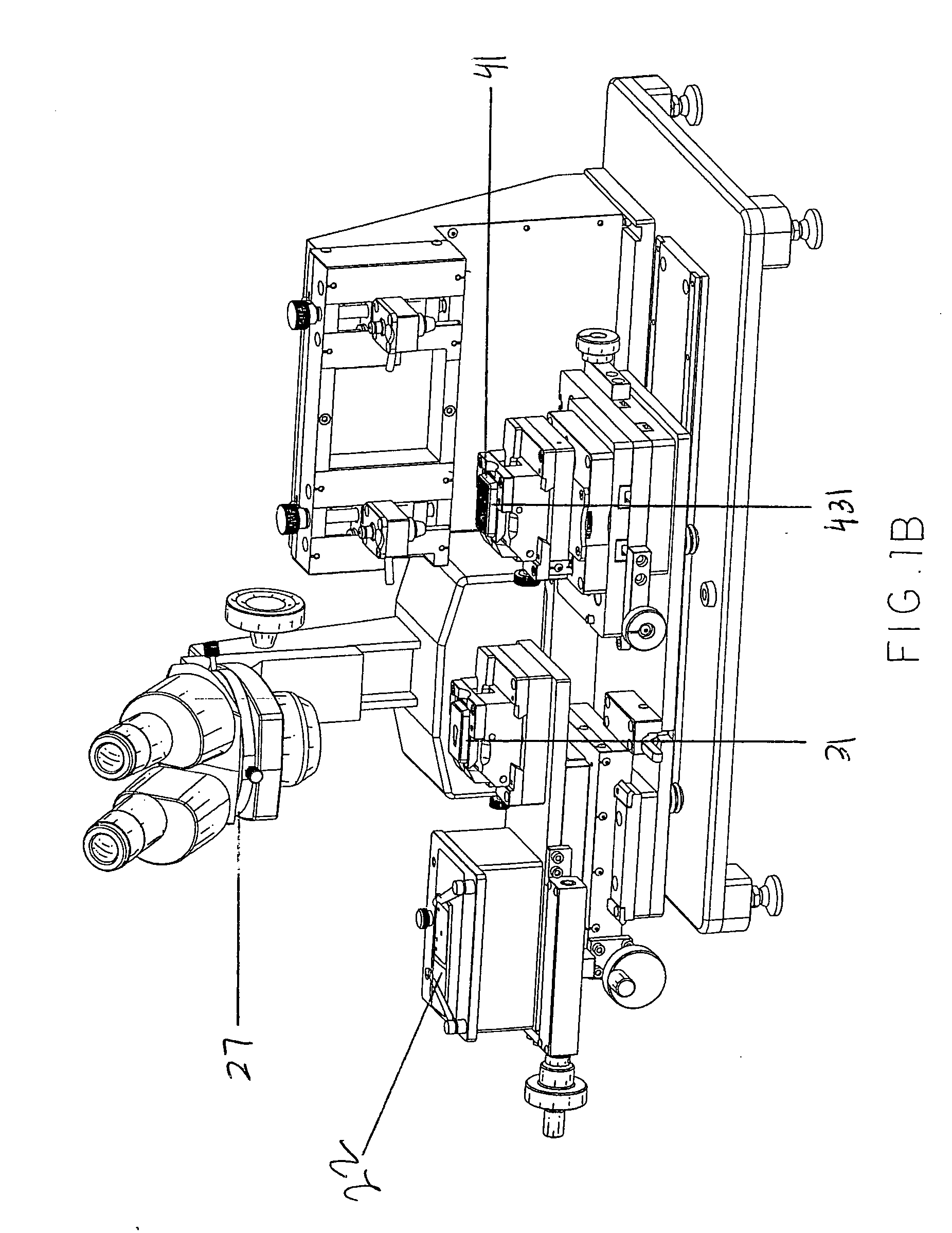
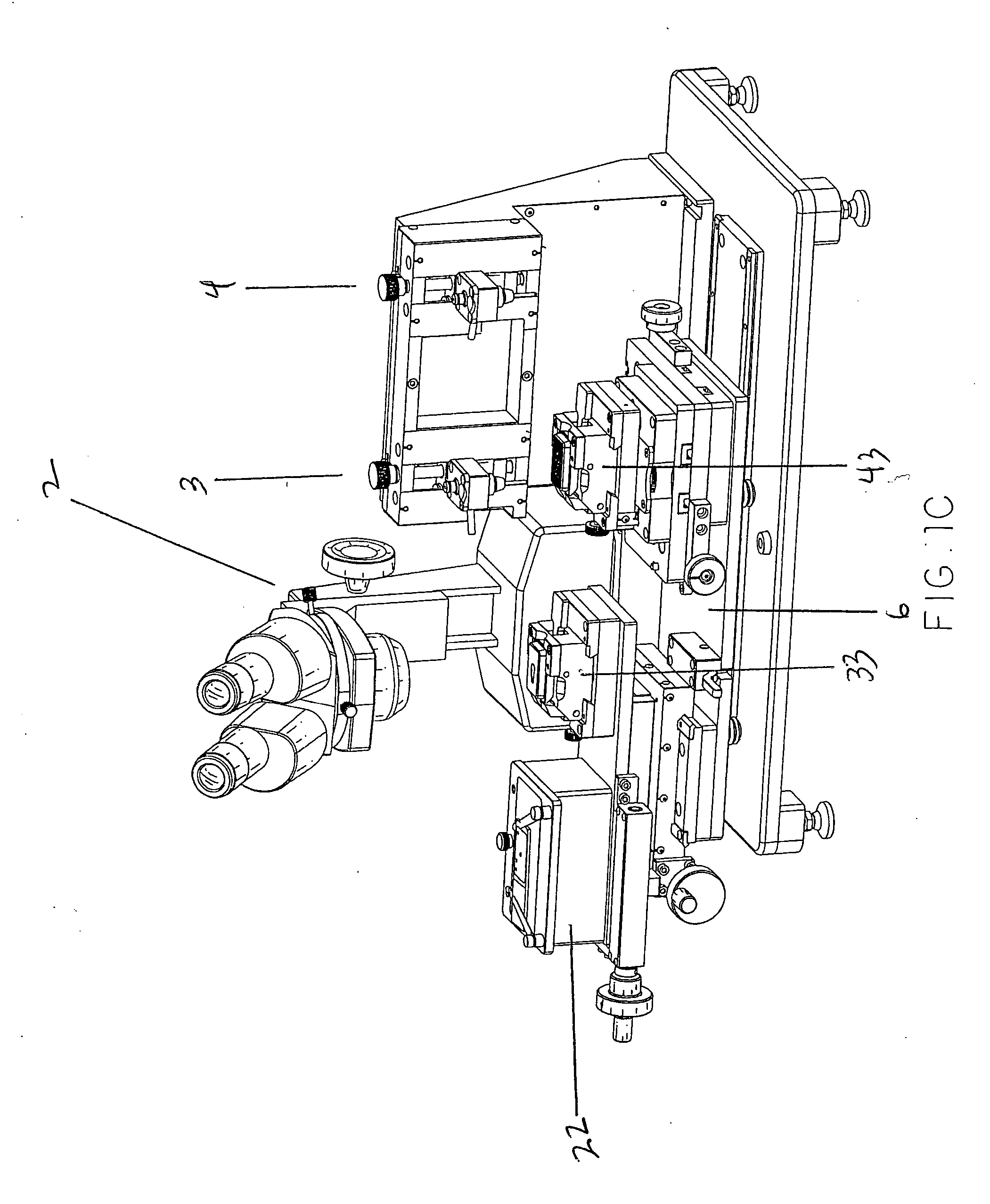
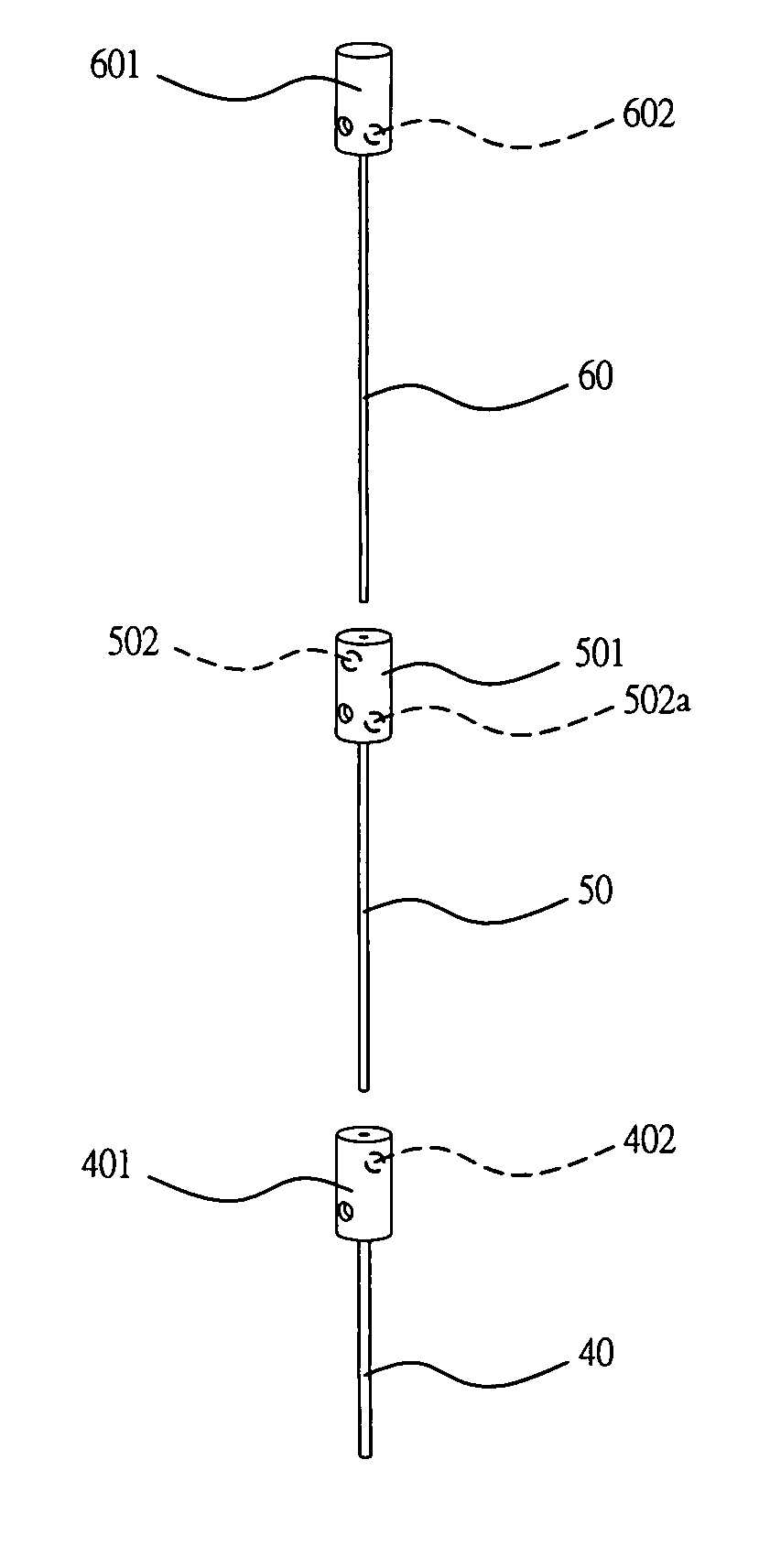
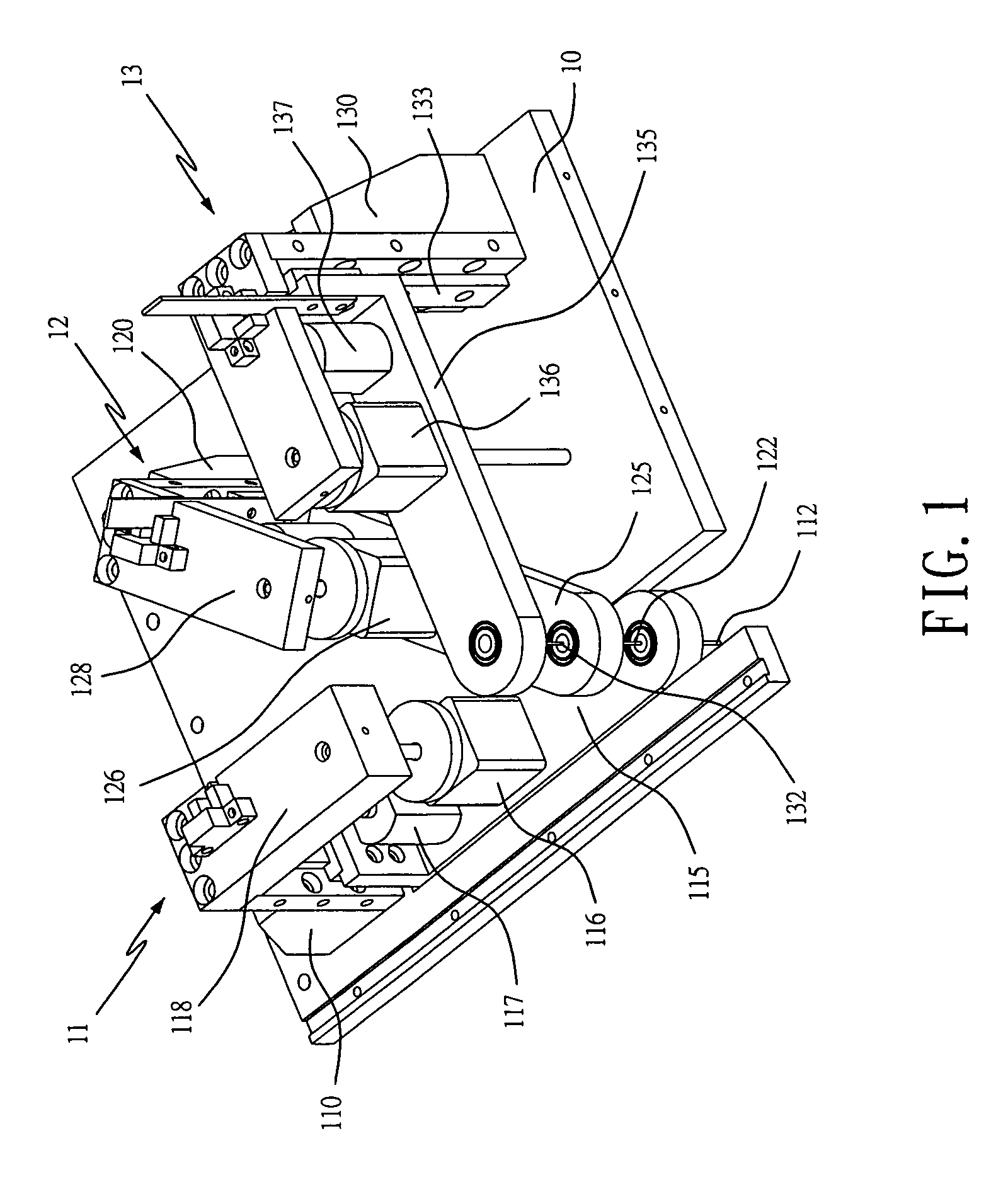
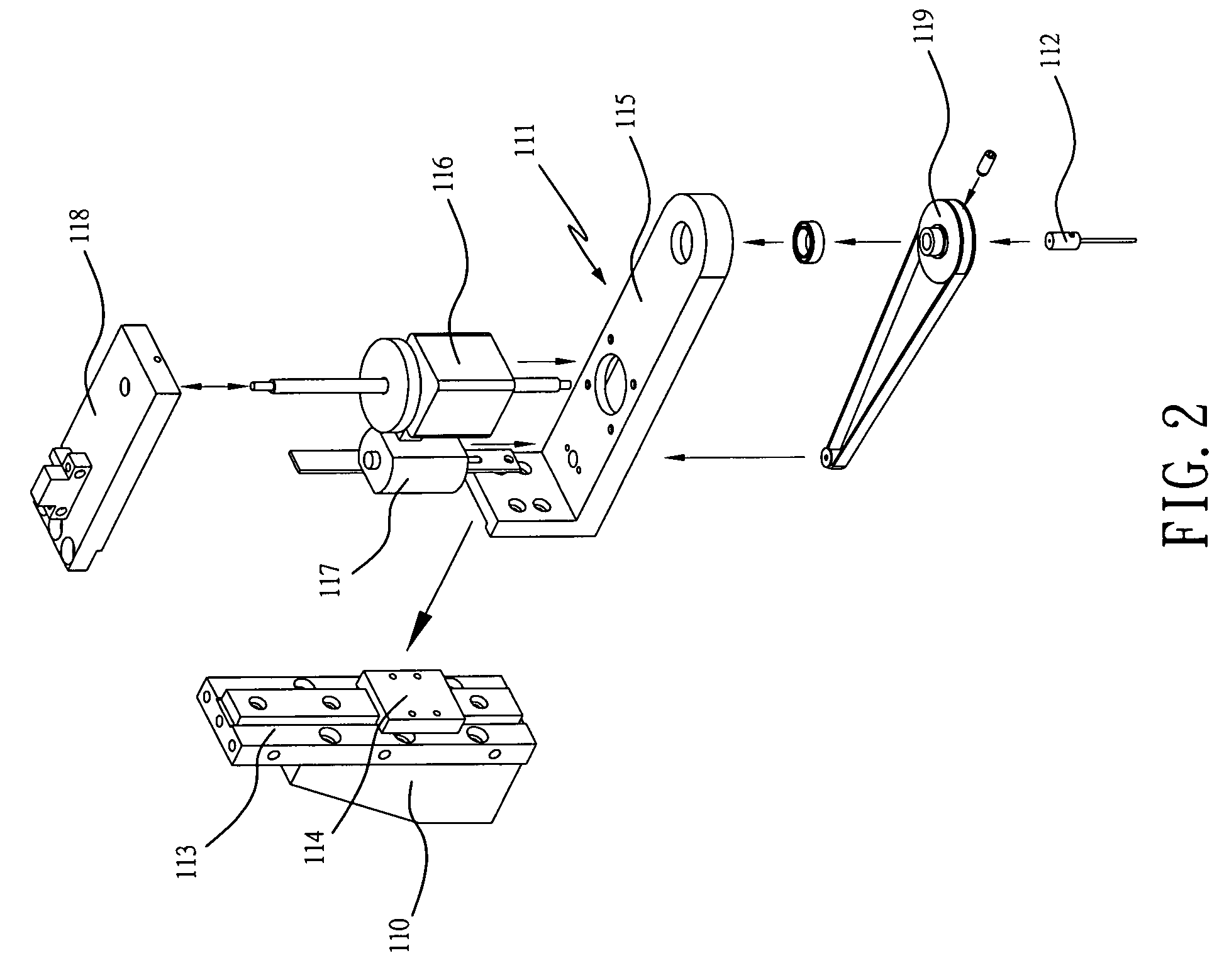
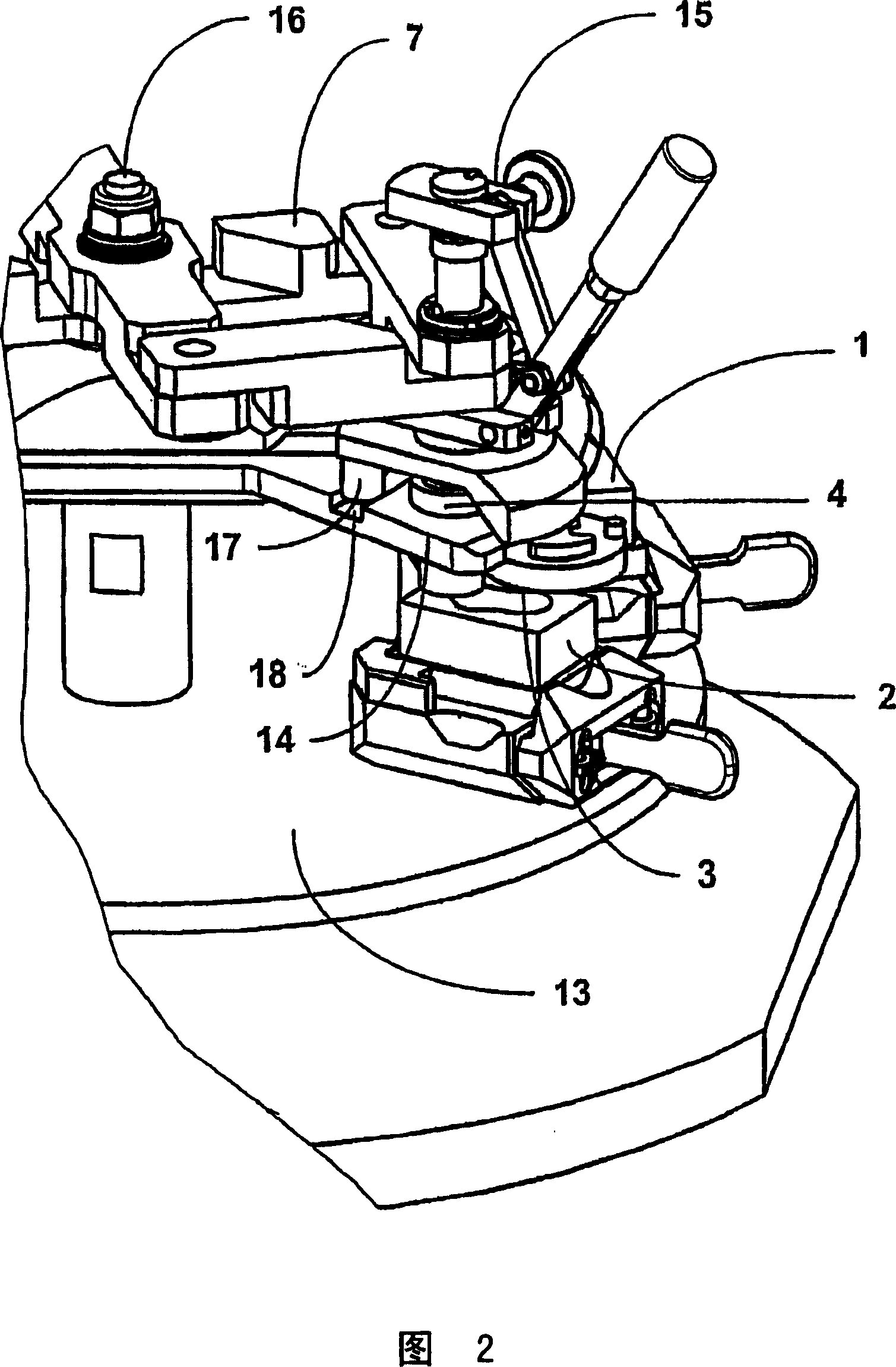
Apparatus For Producing Tissue Arrays
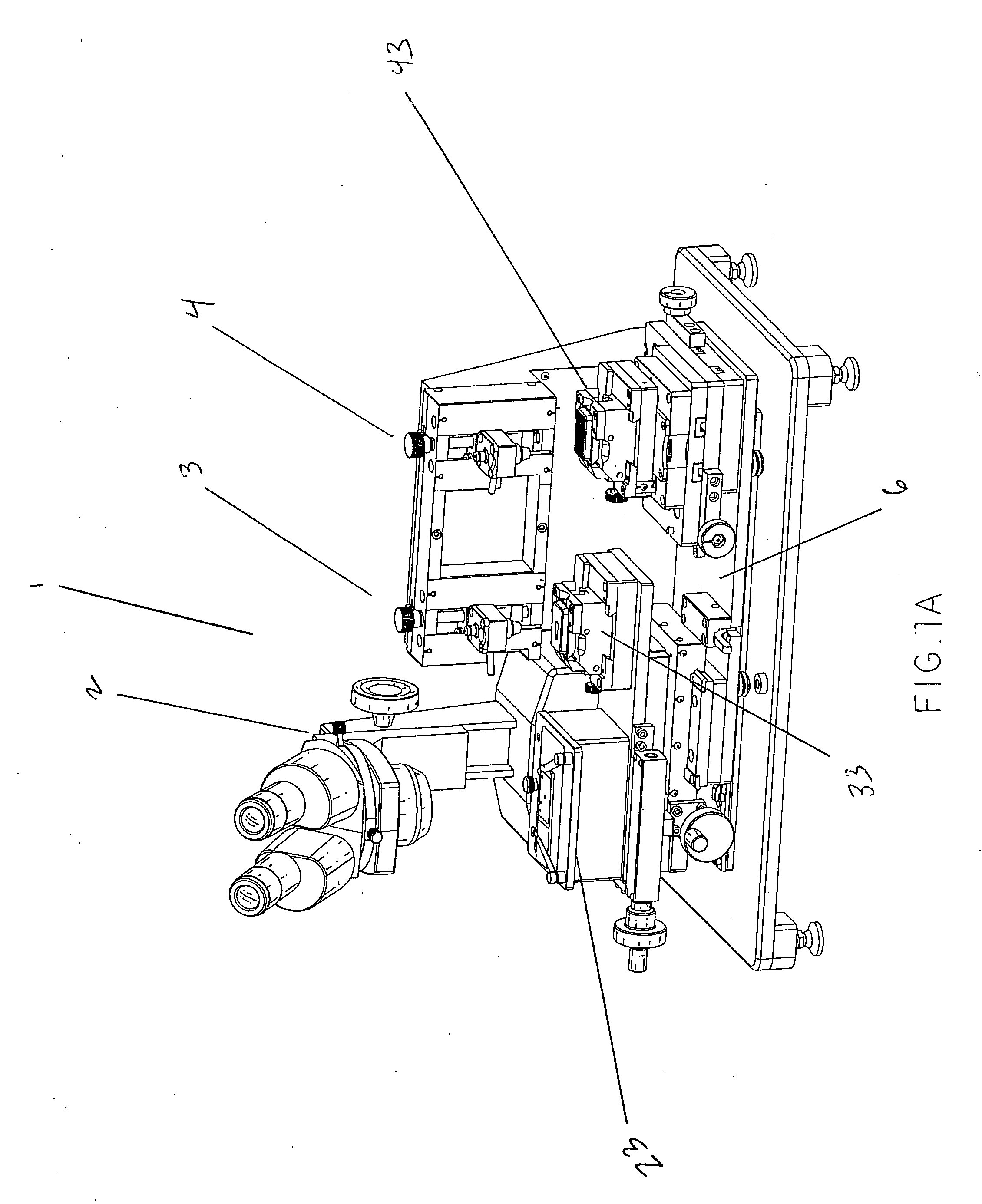
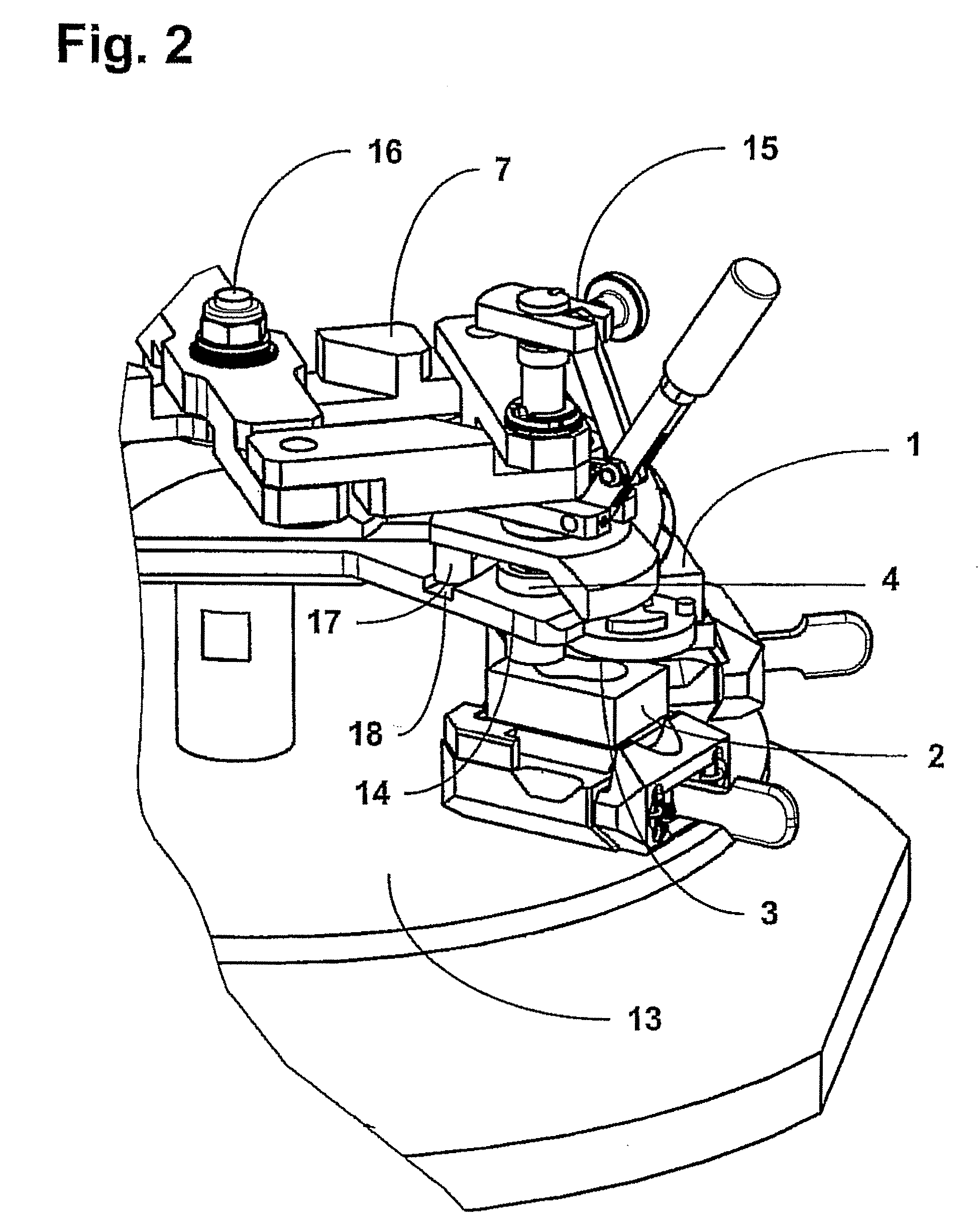
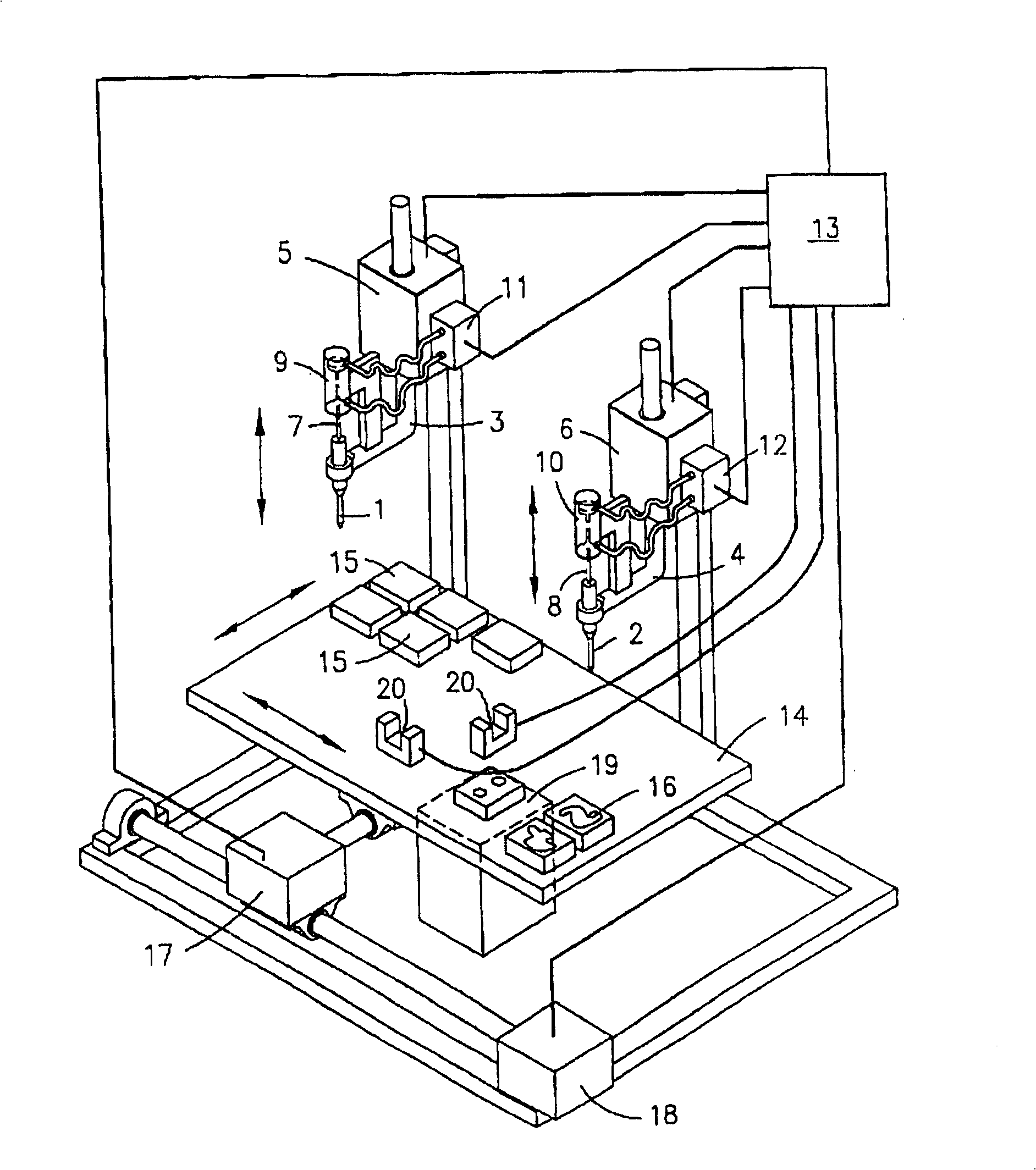
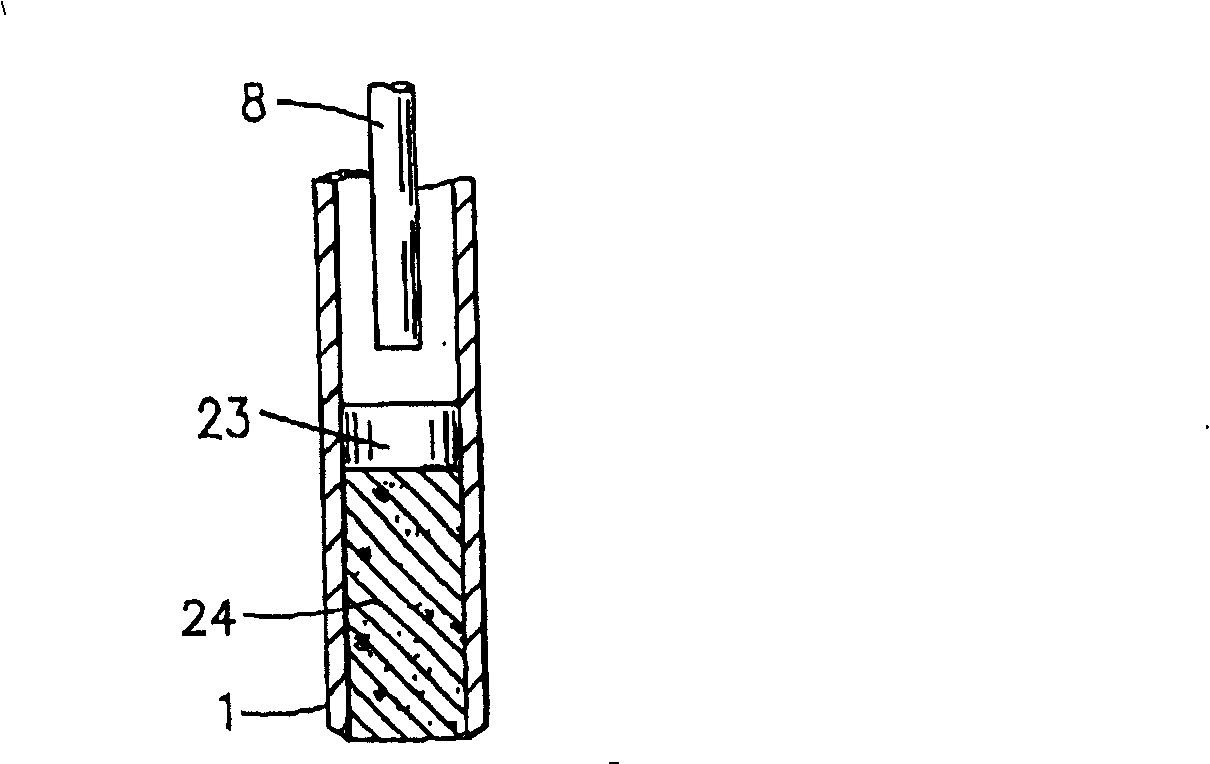
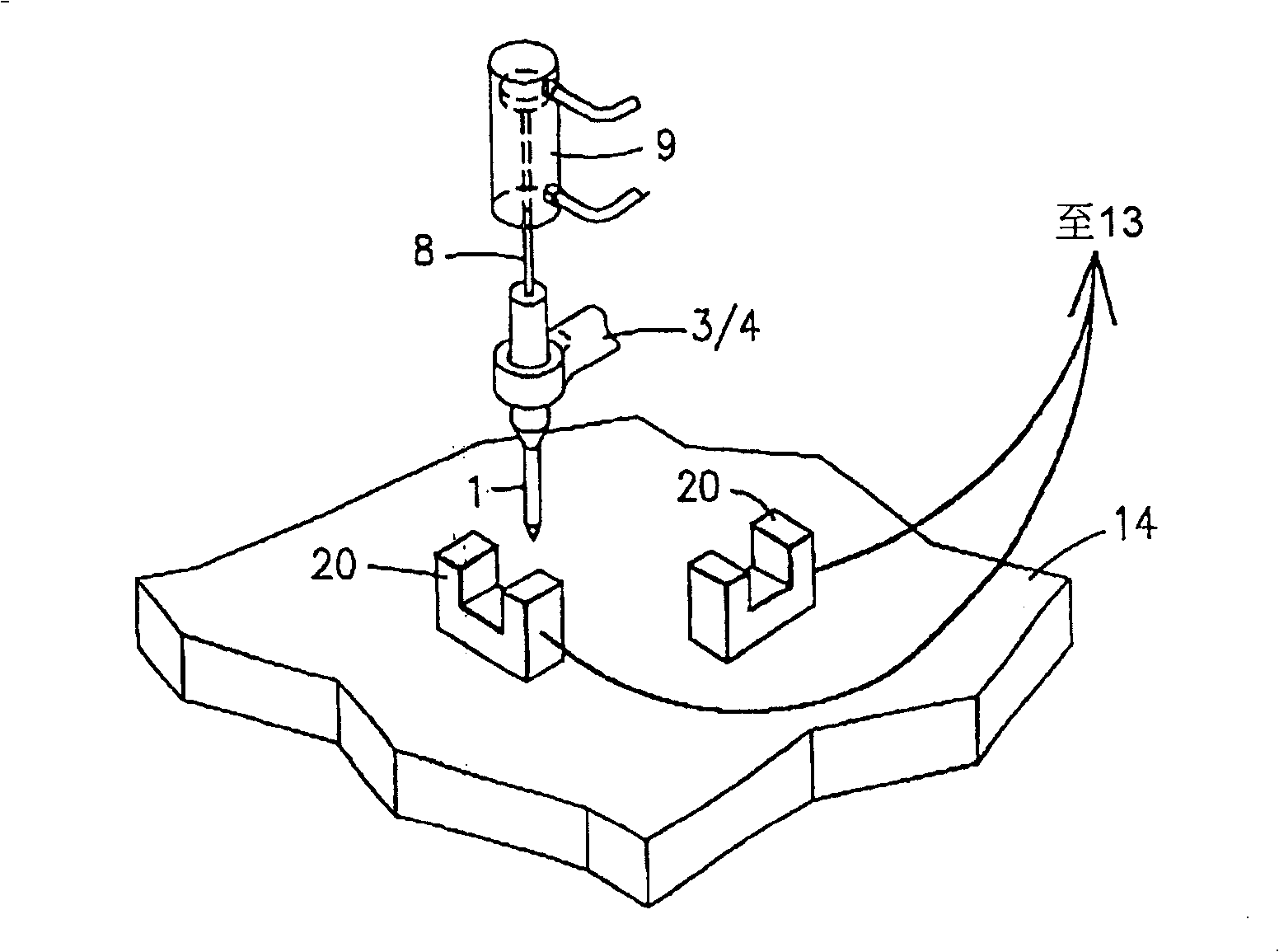
InactiveUS20070059816A1Simple and reliable positioningRemoved positioningBioreactor/fermenter combinationsBiological substance pretreatmentsTissue ArraysBiomedical engineering
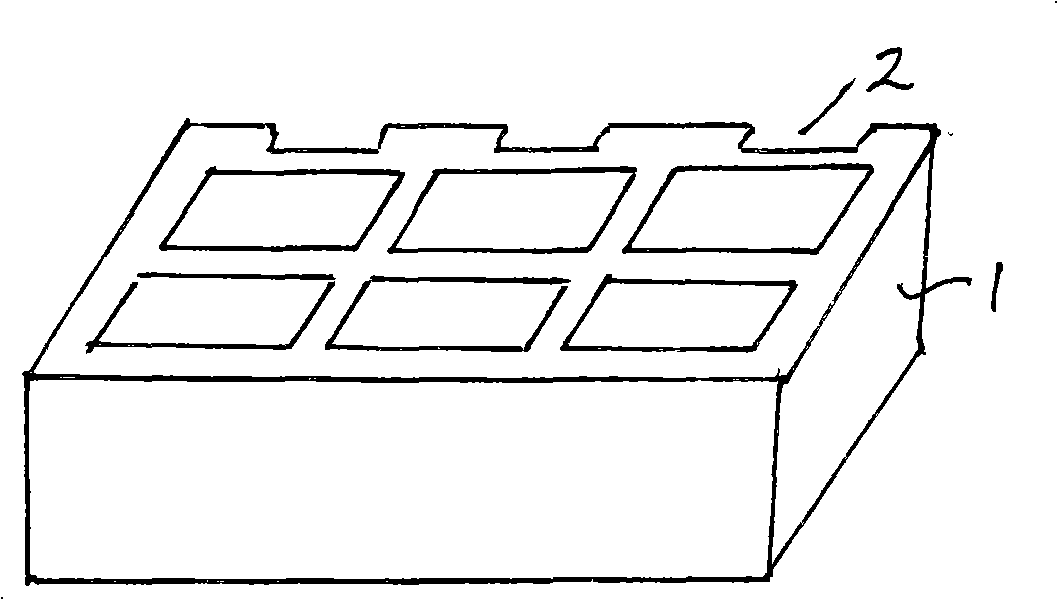
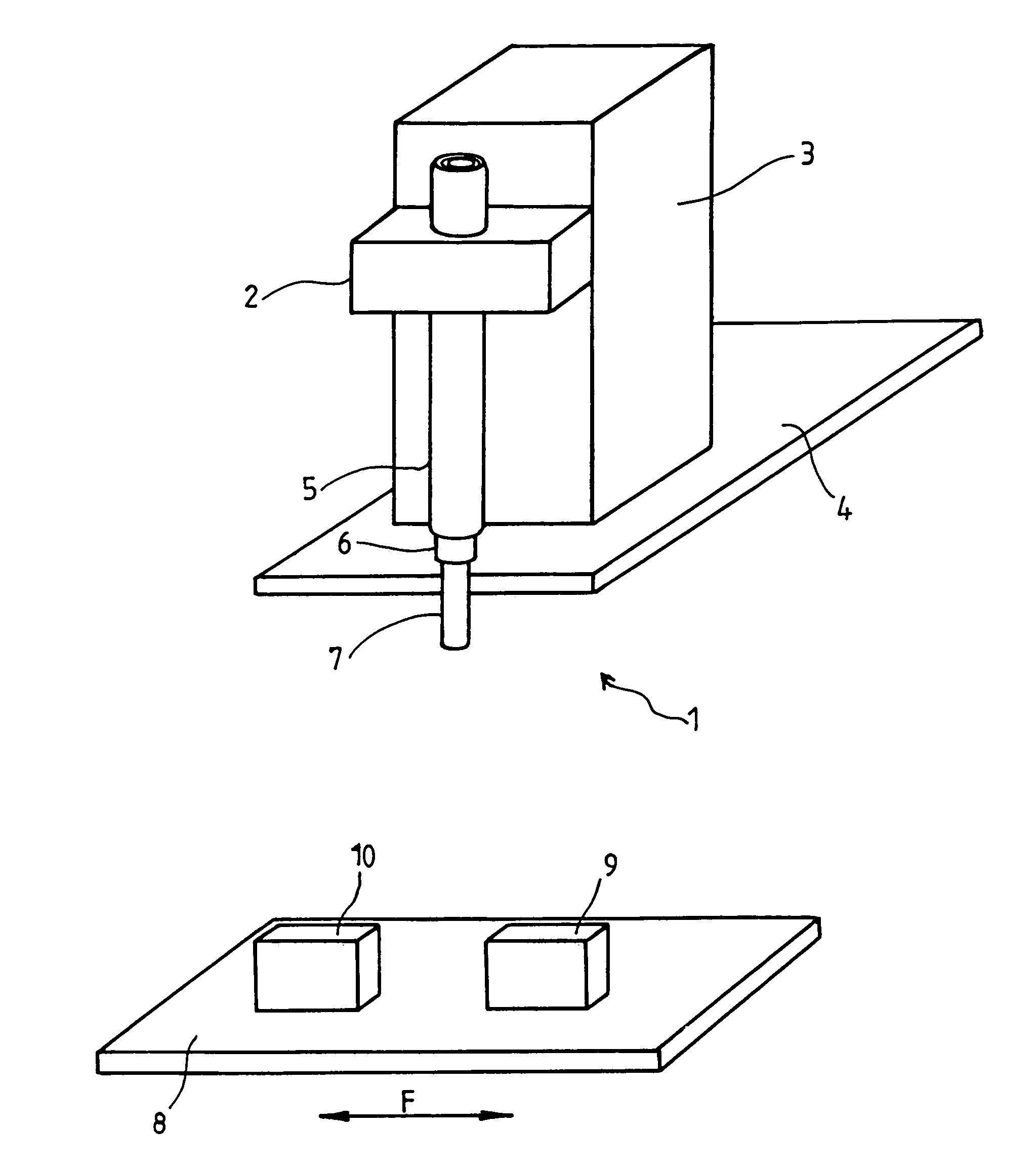
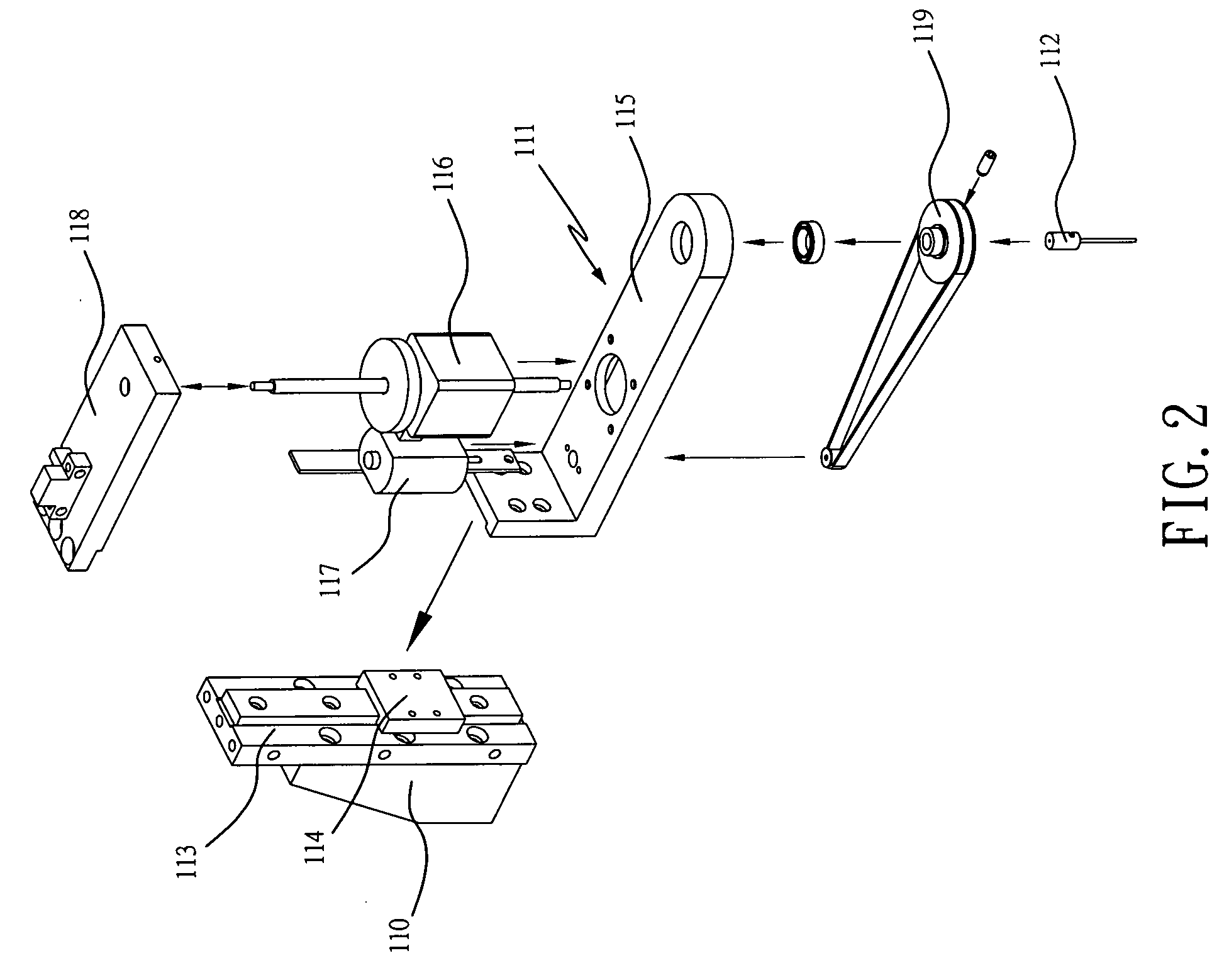
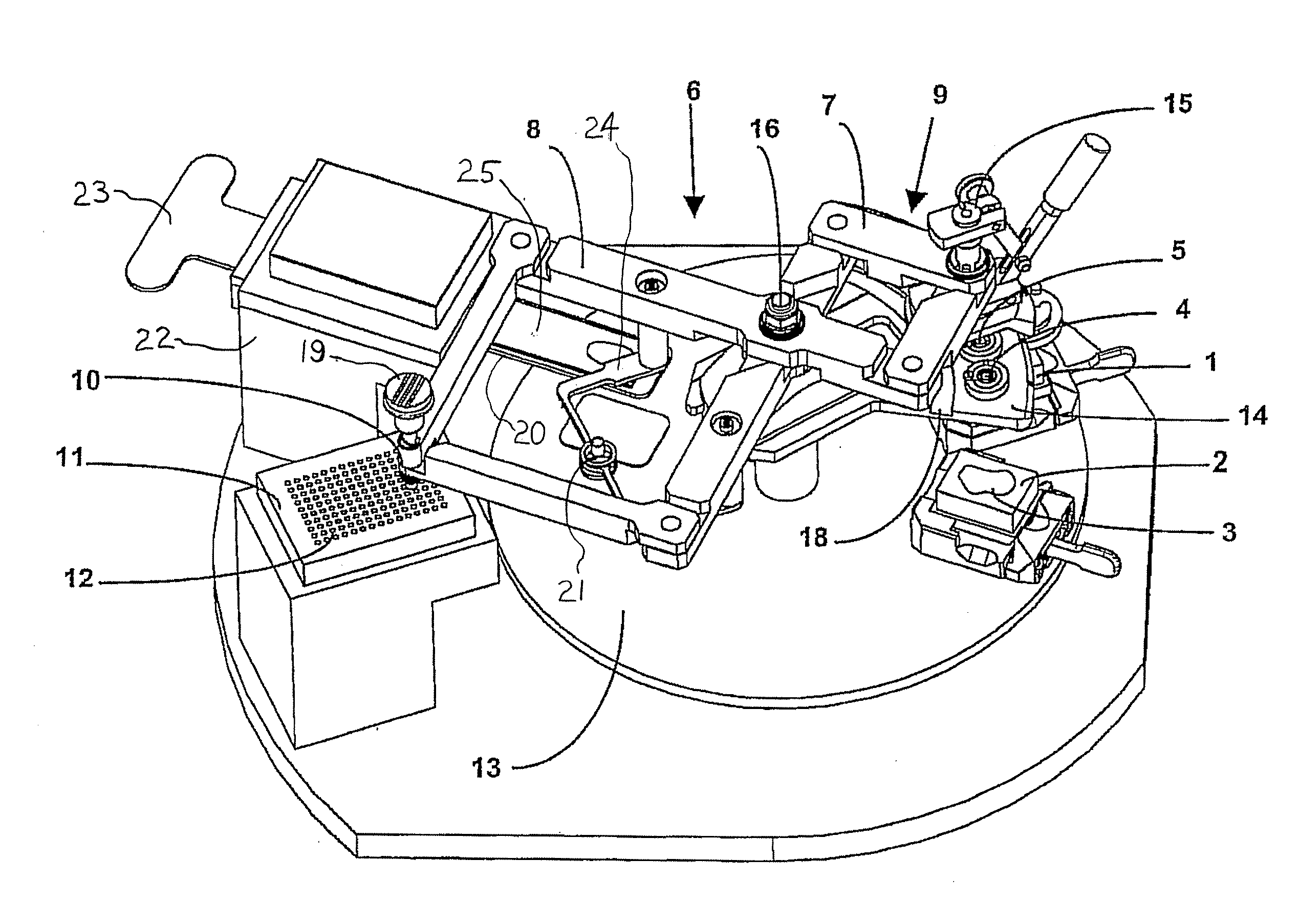
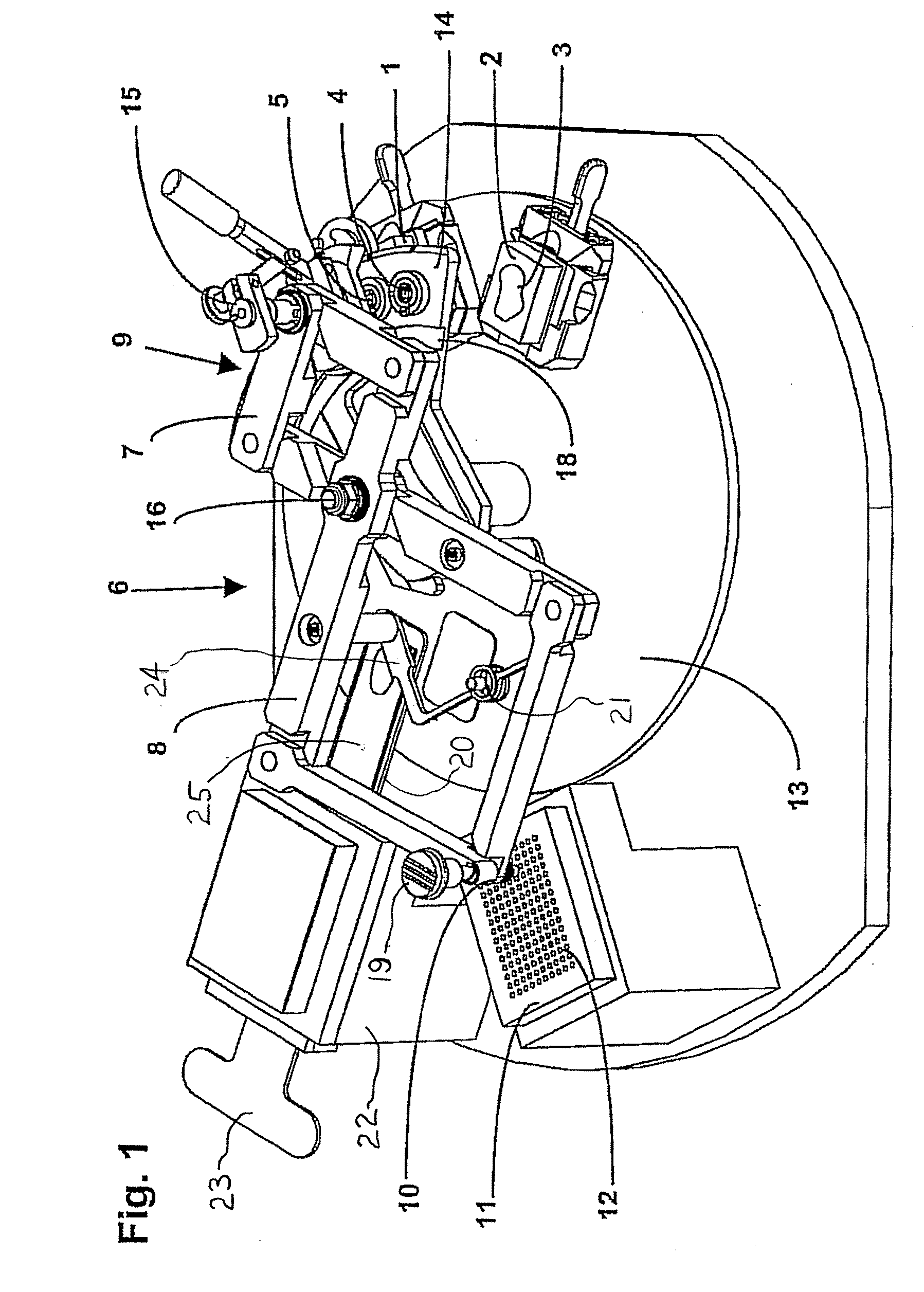
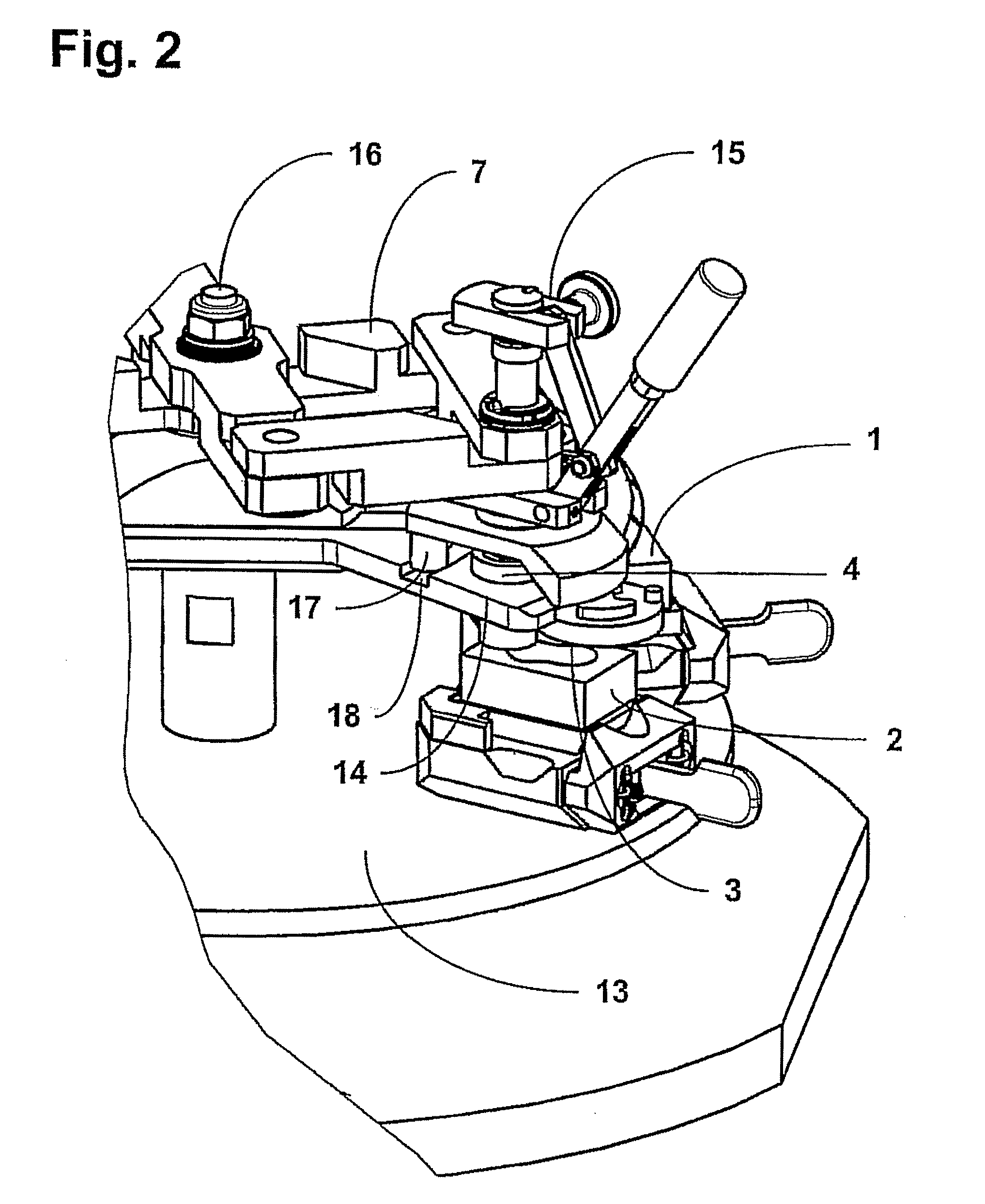
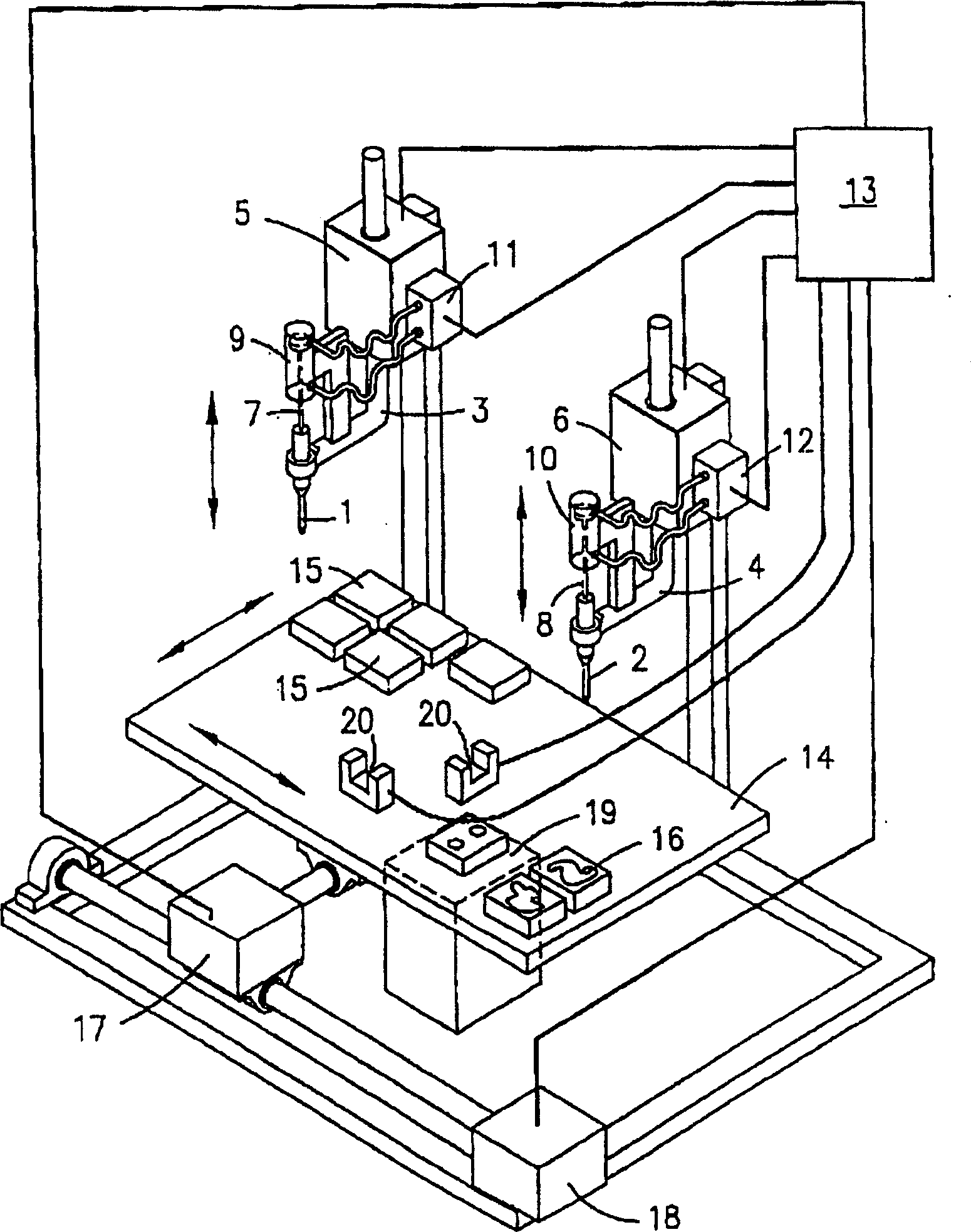
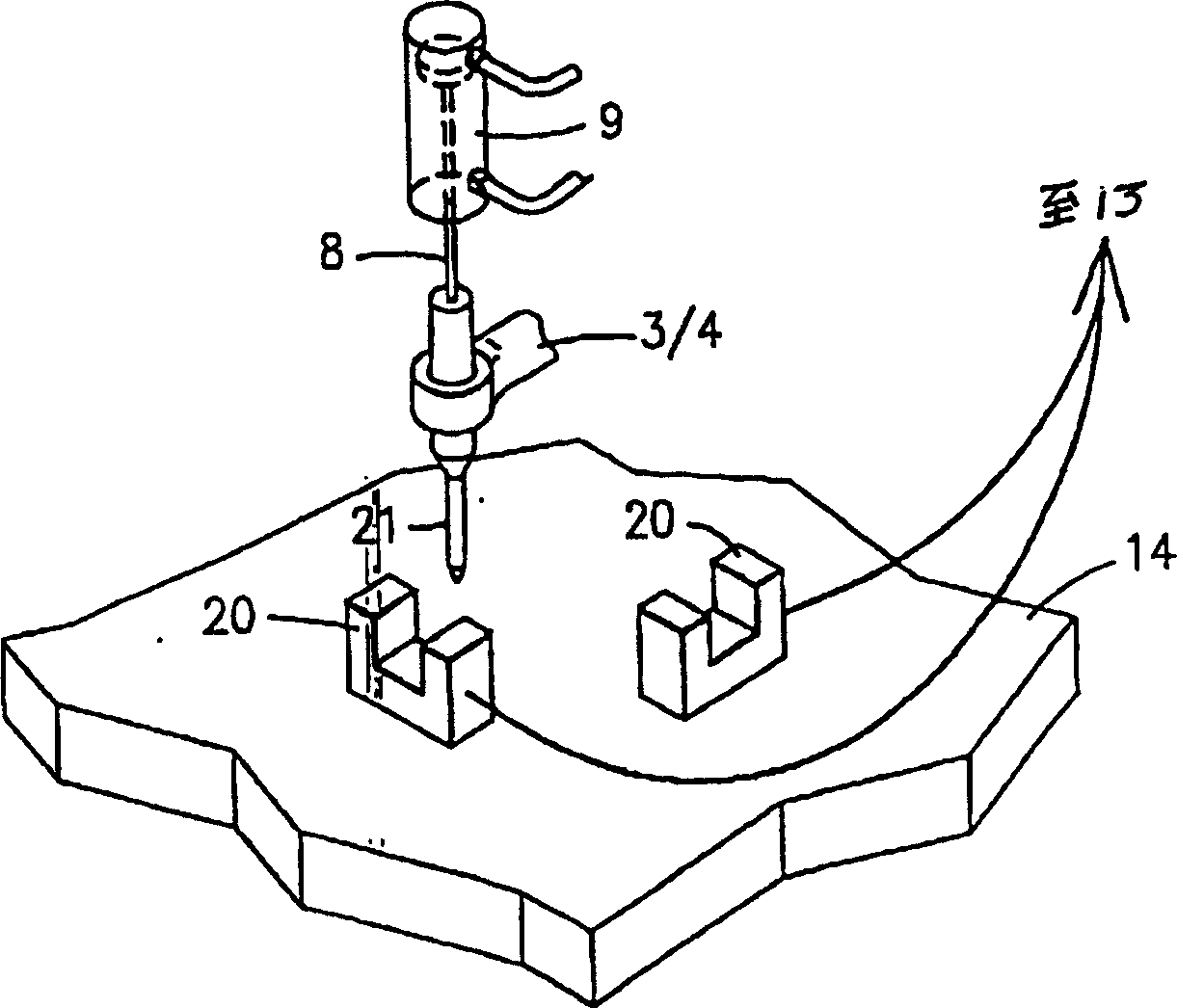
An apparatus for producing a tissue array, having at least one receiver block (1) and at least one donor block (2) that comprises tissue (3) to be investigated, is described. The donor block (2) comprises tissue (3) to be investigated, a first hollow needle (4) for creating a cavity in the receiver block (1), and a second hollow needle (5) for removing a sample from the tissue (3) and introducing the sample (3) into the cavity of the receiver block (1), being provided. For positioning of the first and / or the second hollow needle (4; 5) above the receiver block (1) and / or the donor block (2), a positioning array (11) having predefined markings, as well as a movably mounted lever, are provided for transferring the position of the markings onto a corresponding position on the donor block (2) and / or onto a corresponding position on the receiver block (1).
Owner:LEICA BIOSYST NUSSLOCH
Tissue array using a carrier medium and method for providing the same
An exemplary tissue array, and a method for producing the same, can be provided which can include providing an accepter biological structure(s), providing a donor tissue(s), removing a portion(s) of the donor tissue(s), and removing a portion(s) of the accepter biological structure(s). The removed portion(s) of the accepter biological structure(s) can have a size that is substantially similar to a size of the removed portion(s) of the donor tissue(s). The removed portion(s) of the donor tissue(s) can be inserted into the accepter biological structure(s) at a location substantially corresponding to the removed portion(s) of the accepter biological structure(s).
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Tissue array instrument
InactiveCN100413955CRaise the possibilityBioreactor/fermenter combinationsBiological substance pretreatmentsTissue ArraysEngineering
Owner:BEECHER INSTR
Preparation method of paraffin organization chip
InactiveCN101319971BSimple methodImprove the experimental effectPreparing sample for investigationBiological testingTissue ArraysParaffin oils
The invention discloses a preparing method for an olefin tissue core which includes the steps of customizing an olefin model, customizing a tissue array hole plate, customizing a mandrel, customizing a sampling needle, customizing the olefin block of the tissue chip array of a receptor, sampling the olefin block of a supplier, preparing the olefin block of the tissue chip of the supplier, etc. The preparing method is simple and easy with good experimental result.
Owner:NANTONG UNIVERSITY
Coaxial Tissue Block Puncher Set
InactiveUS20090239294A1Effectiveness of easyEasy to operateBioreactor/fermenter combinationsBiological substance pretreatmentsTissue ArraysParaffin oils
A coaxial tissue block puncher set comprises a carrier mechanism, the first, second and third operating mechanisms being respectively installed at proper positions thereon, while each of the operating mechanisms respectively has a base and a lifting unit being movingly installed on the base, wherein lifting unit of the first operating mechanism is movingly installed with a first punch needle tube, lifting unit of the second operating mechanism being movingly installed with a second punch needle tube is pierced through the first punch needle tube, and lifting unit of the third operating mechanism being movingly installed with a thimble is pierced through the second punch needle tube. Therefore, user is able to punch-extract relevant pathological paraffin and put to relevant position in the empty block thereby forming a tissue array without the need for tedious manual methods thus achieving the effectiveness of fastness, precision and easy operation, etc.
Owner:LOR KUO LUNG
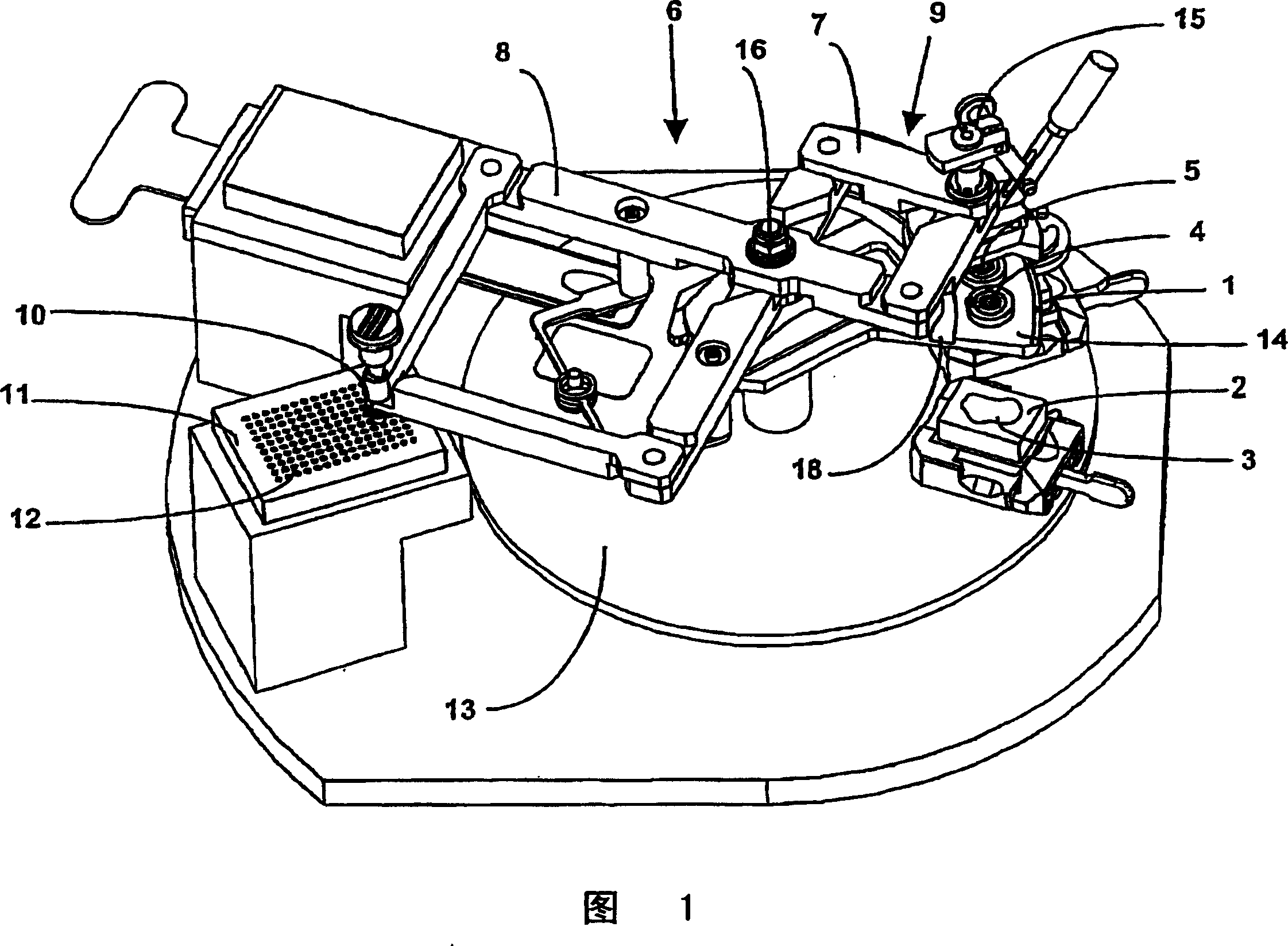
Instrument for constructing tissue arrays
InactiveCN1255527CShorten the timeHigh positioning accuracyBioreactor/fermenter combinationsBiological substance pretreatmentsTissue ArraysEngineering
A simple, robust and precise instrument for constructing tissue arrays. The instrument includes multiple punches mounted on a punch platform, the punch platform displaceable between precisely defined positions. Mechanical d+E,acu e+EE tentes or stops are provided which mechanically arrest the movement of the punch platform in the precisely defined positions. This arrangement greatly saves time and improves accuracy over use of conventional precision linear positioning means. By the simple step of moving the punch platform from a first position to a second position, either punch can be quickly brought into operating position (and the other moved into a non-interfering position) by either manually or by automatic means, making it possible to quickly alternate from one punch to the other.
Owner:BEECHER INSTR
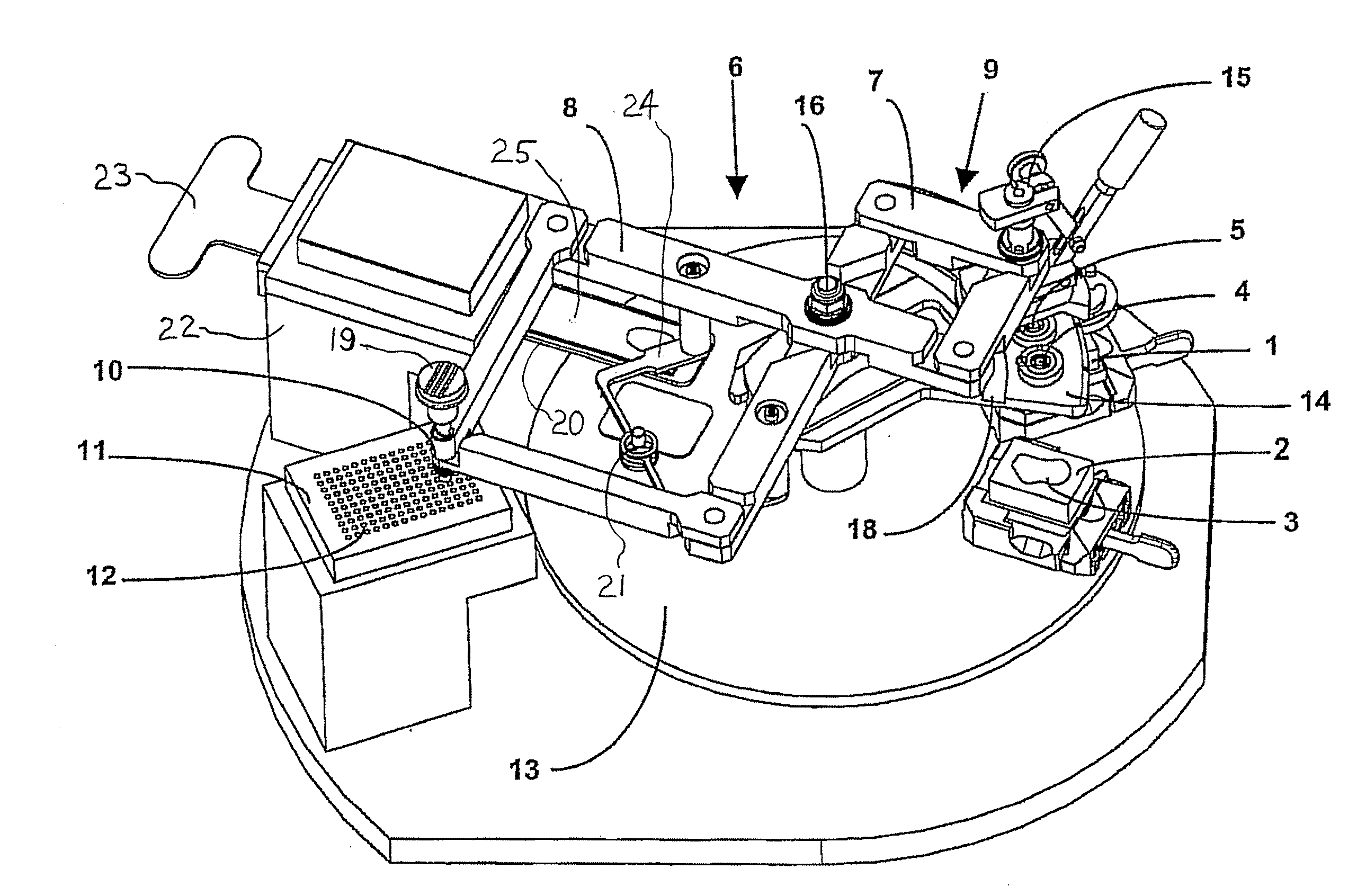
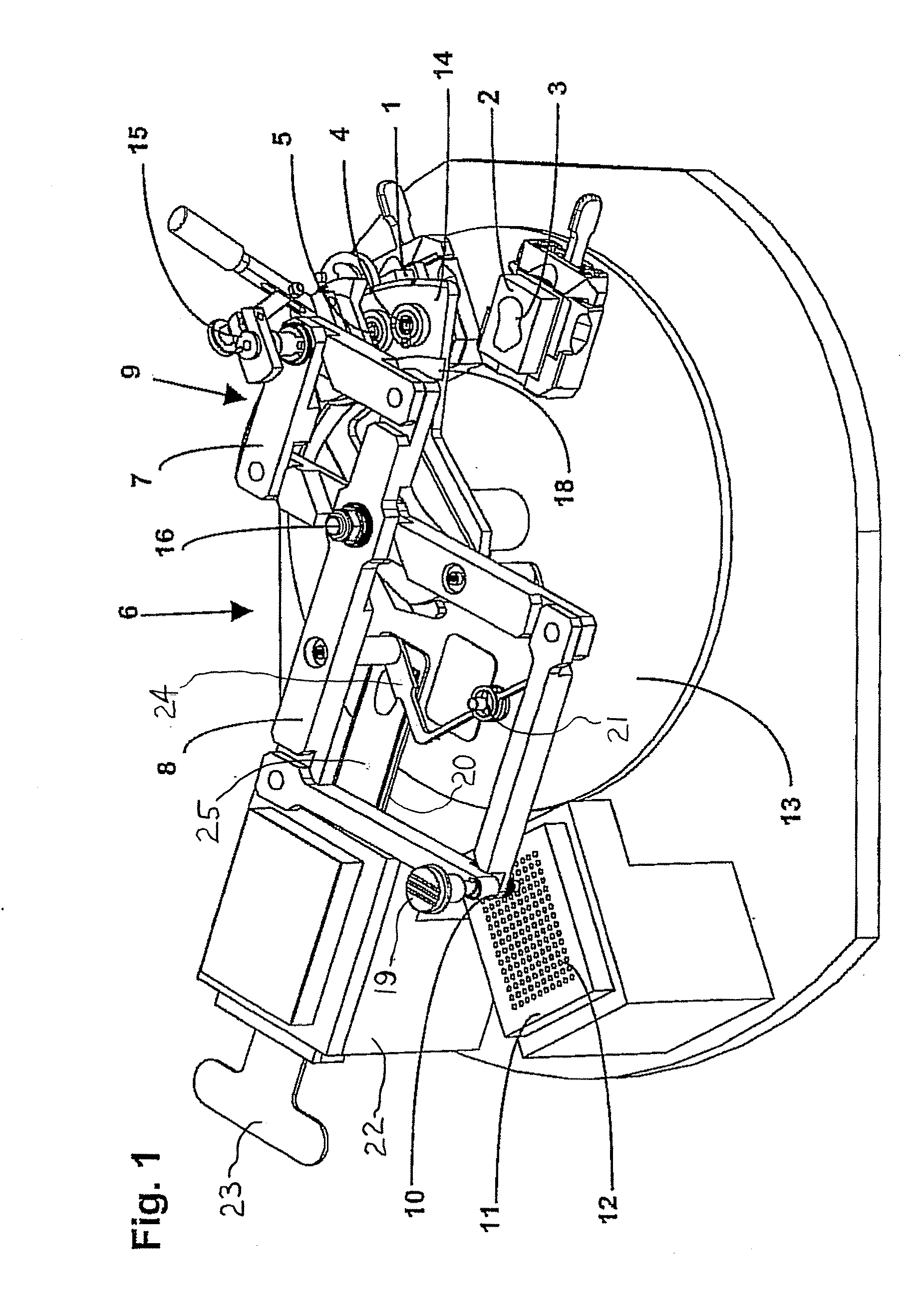
Apparatus For Producing Tissue Arrays
ActiveUS20070087428A1Simple and reliable introductionPrecise positioningBioreactor/fermenter combinationsBiological substance pretreatmentsTissue ArraysBiomedical engineering
An apparatus for producing a tissue array is described. At least one receiver block (1), and at least one donor block (2) that comprises tissue (3) to be investigated, are provided, a first hollow needle (4) for creating a cavity in the receiver block (1), and a second hollow needle (5) for removing a sample from the tissue (3) and introducing the sample (3) into the cavity of the receiver block (1), being present. A pantograph (6) is provided for positioning the first and / or the second hollow needle (4; 5) above the receiver block (1).
Owner:LEICA BIOSYST NUSSLOCH
Preventing hyaluronan-mediated tumorigenetic mechanisms using intronic RNAs
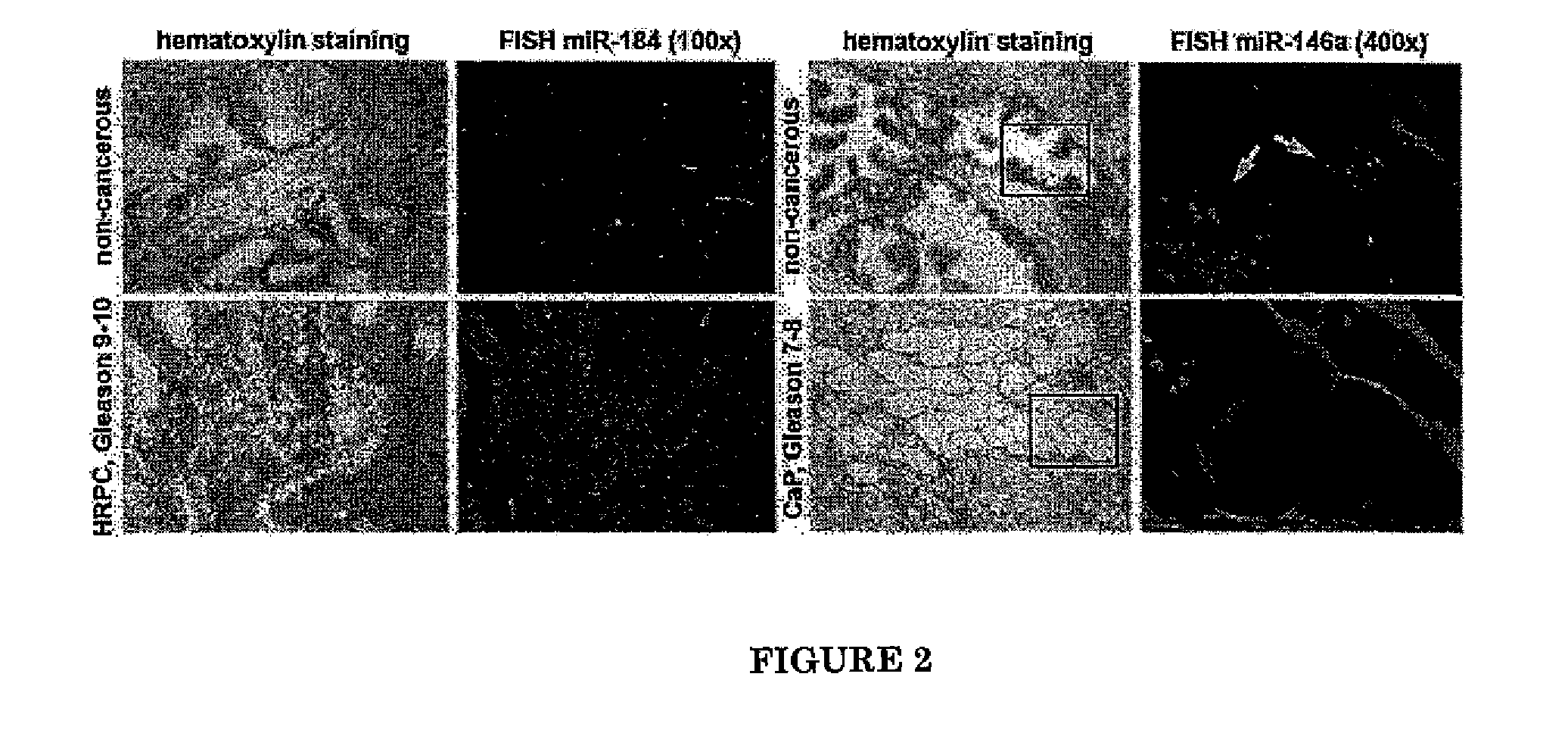
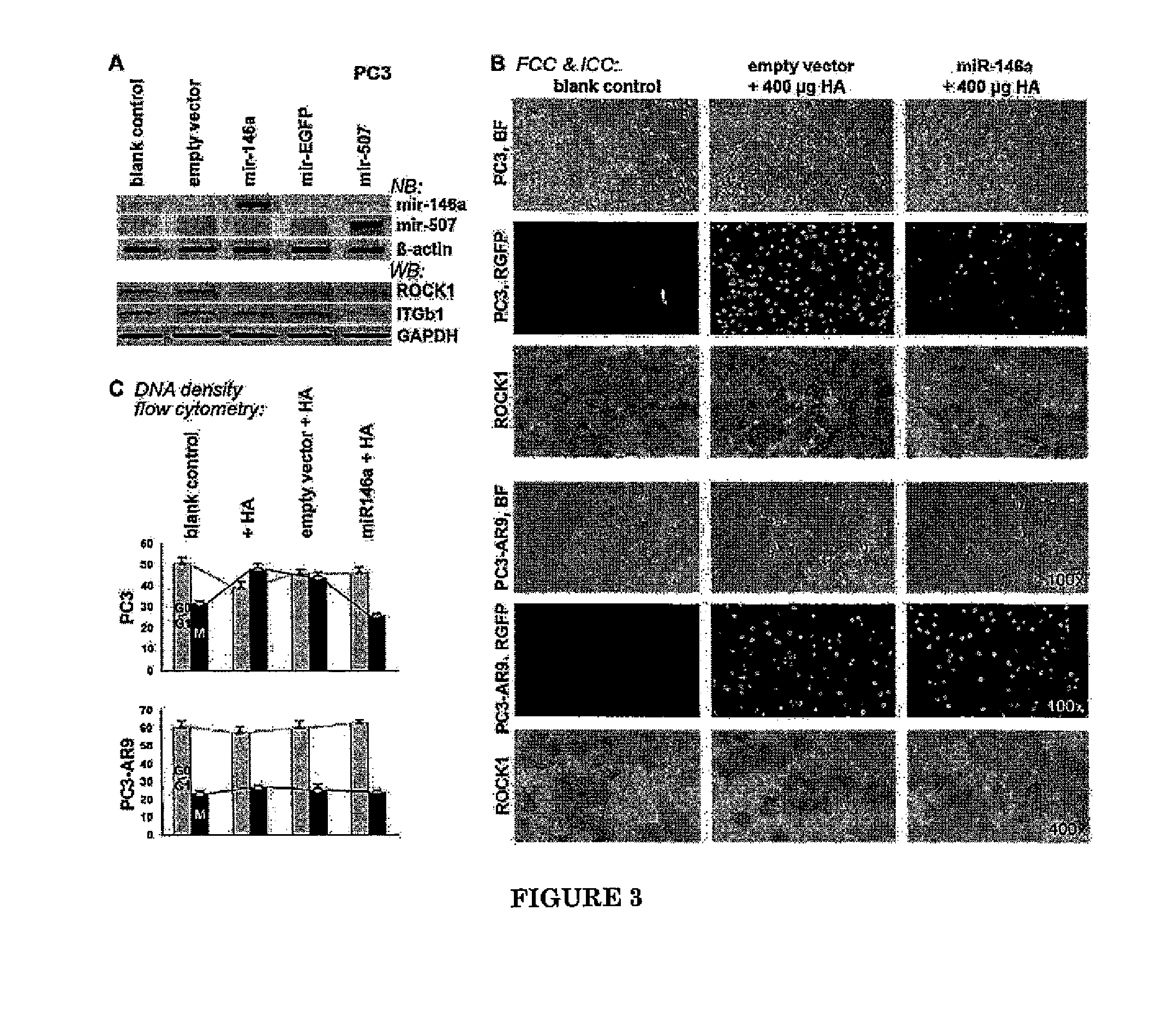
InactiveUS8895525B2High similarityReduced its tumorigenecitySugar derivativesMicrobiological testing/measurementLymphatic SpreadTissue Arrays
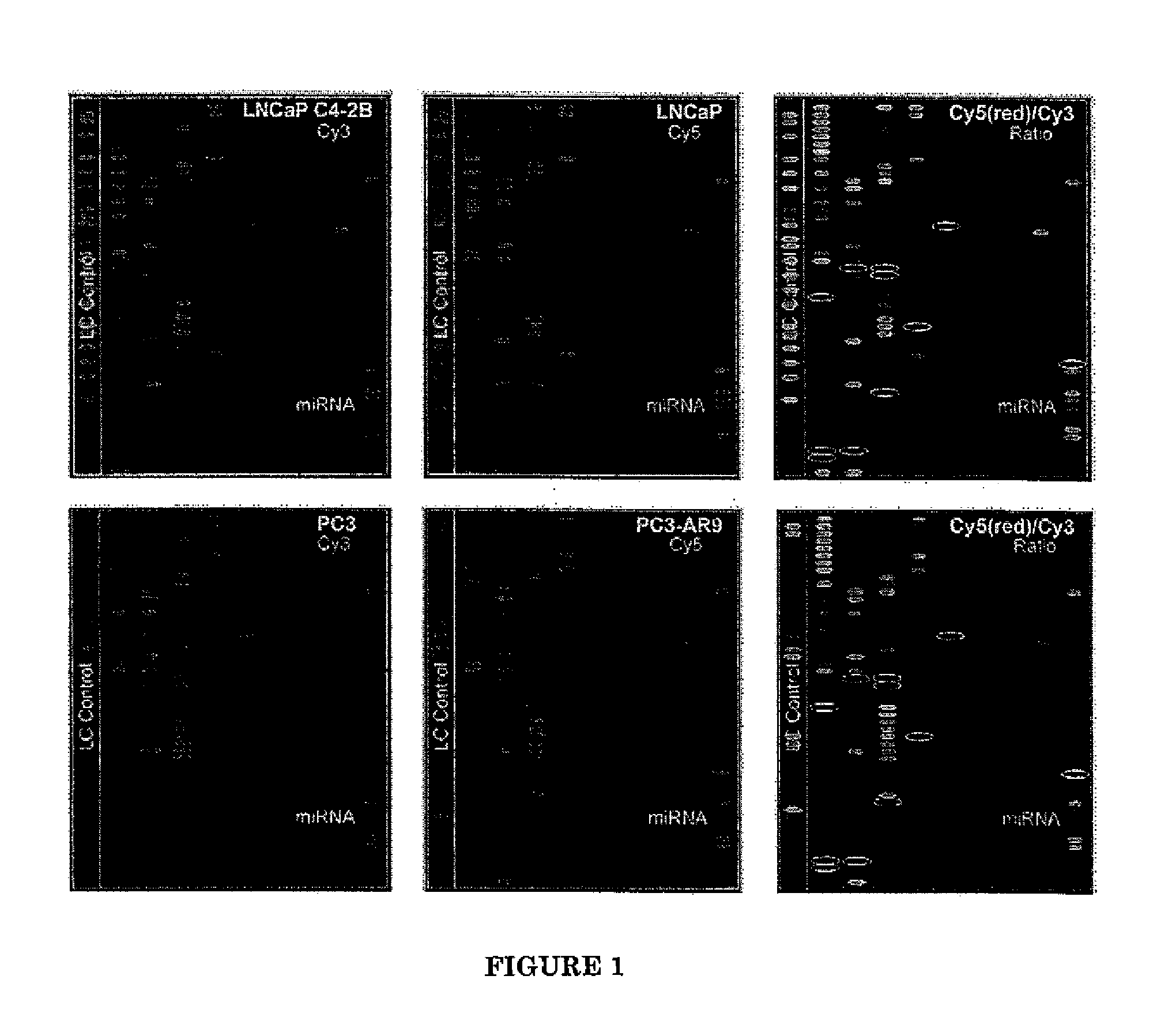
Patterns of microRNA (miRNA) expression are correlated to the degrees of tumor cell differentiation in human prostate cancer. MiRNAs can complementarily bind to either oncogenes or tumor suppressor genes, resulting in targeted gene silencing and thus changes of cellular tumorigenecity. Using miRNA microarray analysis, 8 down-regulated and 3 up-regulated known miRNAs in androgen-independent human prostate cancer cell lines, such as LNCaP C4-2B and PC3, compared to those androgen-dependent cell lines, such as LNCaP and PC3-AR9 were consistently detected. Fluorescent in-situ hybridization assays in human prostate cancer tissue arrays containing sixty patients at different stages also showed the same miRNA expression patterns in hormone-refractory prostate carcinomas (HRPC) compared to androgen-sensitive non-cancerous prostate epithelium. In-vitro tumorigenecity assays using one of the identified miRNAs, mir-146a, were performed to provide validation of its function in prostate cancer. Gain-of-function transfection of mir-146a markedly suppressed its targeted ROCK1 gene expression in androgen-independent PC3 cells, consequently resulting in reduced cancer cell proliferation, invasion and metastasis to human bone marrow endothelial cell monolayers. Since ROCK1 is the key kinase for activating hyaluronan-mediated HRPC transformation in vivo and in PC3 cells, mir-146a should function as a tumor-suppressor gene in modulating the ROCK1-associated tumorigenecity.
Owner:UNIV OF SOUTHERN CALIFORNIA
Tissue array instrument
InactiveCN1294251CRaise the possibilityBioreactor/fermenter combinationsBiological substance pretreatmentsTissue ArraysEngineering
Arrays of biological tissue can be created by removing cores from regions of interest in a series of donor blocks (16) of embedded tissues. The cores removed are placed in a regular array in a recipient block (15). This is typically done with two different punches, one for obtaining the cores of interest and the other for creating the receiving holes in the recipient block (15). The present invention comprises such a system including two separate z axes, one for each punch. Each punch has its own stylet and the axis of each punch is parallel to the axis of its drive. Alternatively, the invention is made by providing a single z axis, with a mechanism for automatically changing two or more punches in and out of a holder on the z axis.
Owner:BEECHER INSTR
Tissue array using a carrier medium and method for providing the same
An exemplary tissue array, and a method for producing the same, can be provided which can include providing an accepter biological structure(s), providing a donor tissue(s), removing a portion(s) of the donor tissue(s), and removing a portion(s) of the accepter biological structure(s). The removed portion(s) of the accepter biological structure(s) can have a size that is substantially similar to a size of the removed portion(s) of the donor tissue(s). The removed portion(s) of the donor tissue(s) can be inserted into the accepter biological structure(s) at a location substantially corresponding to the removed portion(s) of the accepter biological structure(s).
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Coaxial tissue block puncher set
InactiveUS7862777B2Effectiveness of easyEasy to operateBioreactor/fermenter combinationsBiological substance pretreatmentsTissue ArraysParaffin oils
A coaxial tissue block puncher set comprises a carrier mechanism, the first, second and third operating mechanisms being respectively installed at proper positions thereon, while each of the operating mechanisms respectively has a base and a lifting unit being movingly installed on the base, wherein lifting unit of the first operating mechanism is movingly installed with a first punch needle tube, lifting unit of the second operating mechanism being movingly installed with a second punch needle tube is pierced through the first punch needle tube, and lifting unit of the third operating mechanism being movingly installed with a thimble is pierced through the second punch needle tube. Therefore, user is able to punch-extract relevant pathological paraffin and put to relevant position in the empty block thereby forming a tissue array without the need for tedious manual methods thus achieving the effectiveness of fastness, precision and easy operation, etc.
Owner:LOR KUO LUNG
Apparatus for producing tissue arrays
InactiveCN1924540AReduce positioningEliminate complex positioningPreparing sample for investigationBiological testingTissue ArraysBiomedical engineering
An apparatus for producing a tissue array, having at least one receiver block ( 1 ) and at least one donor block ( 2 ) that comprises tissue ( 3 ) to be investigated, is described. The donor block ( 2 ) comprises tissue ( 3 ) to be investigated, a first hollow needle ( 4 ) for creating a cavity in the receiver block ( 1 ), and a second hollow needle ( 5 ) for removing a sample from the tissue ( 3 ) and introducing the sample ( 3 ) into the cavity of the receiver block ( 1 ), being provided. For positioning of the first and / or the second hollow needle ( 4; 5 ) above the receiver block ( 1 ) and / or the donor block ( 2 ), a positioning array ( 11 ) having predefined markings, as well as a movably mounted lever, are provided for transferring the position of the markings onto a corresponding position on the donor block ( 2 ) and / or onto a corresponding position on the receiver block ( 1 ).
Owner:LEICA MICROSYST NUSSLOCH
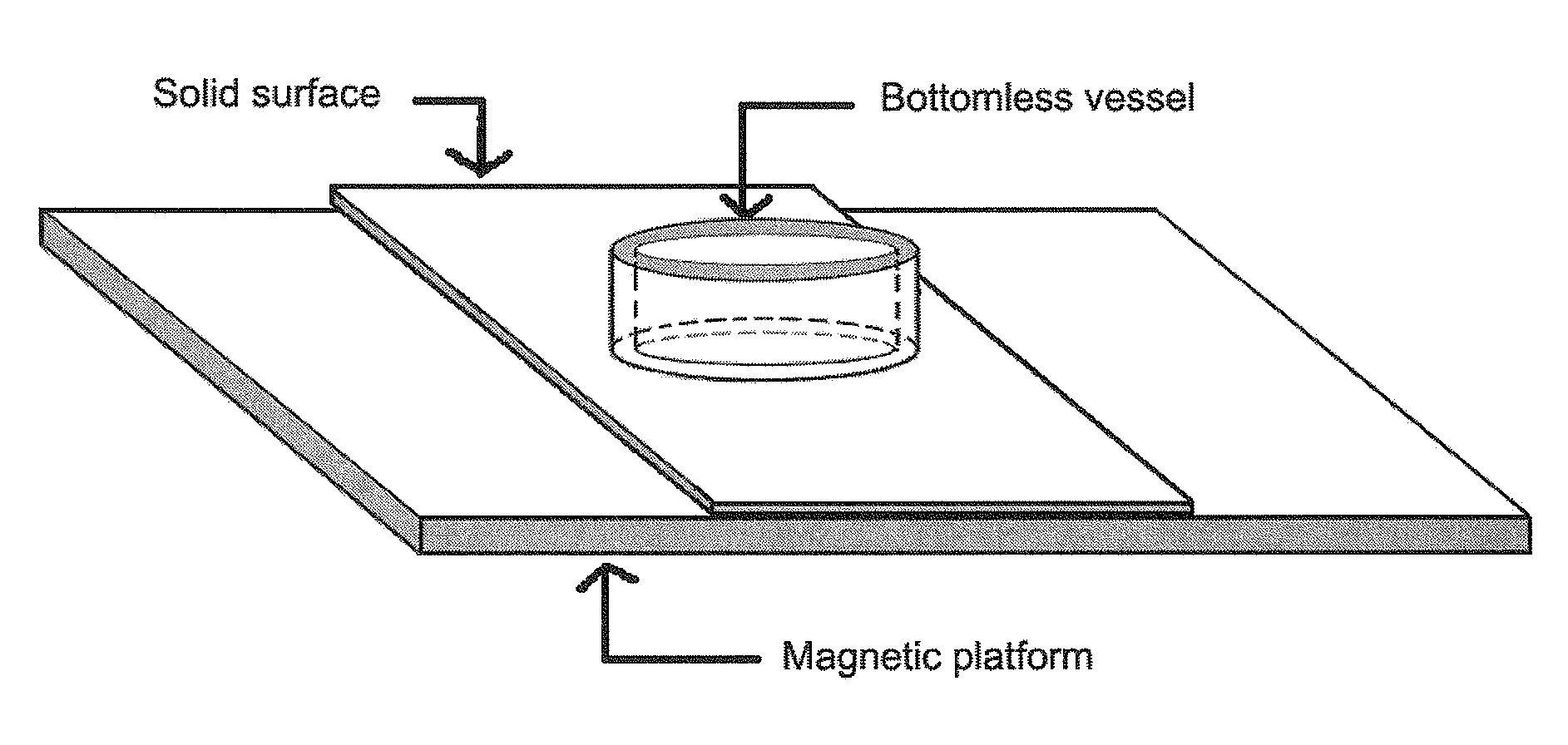
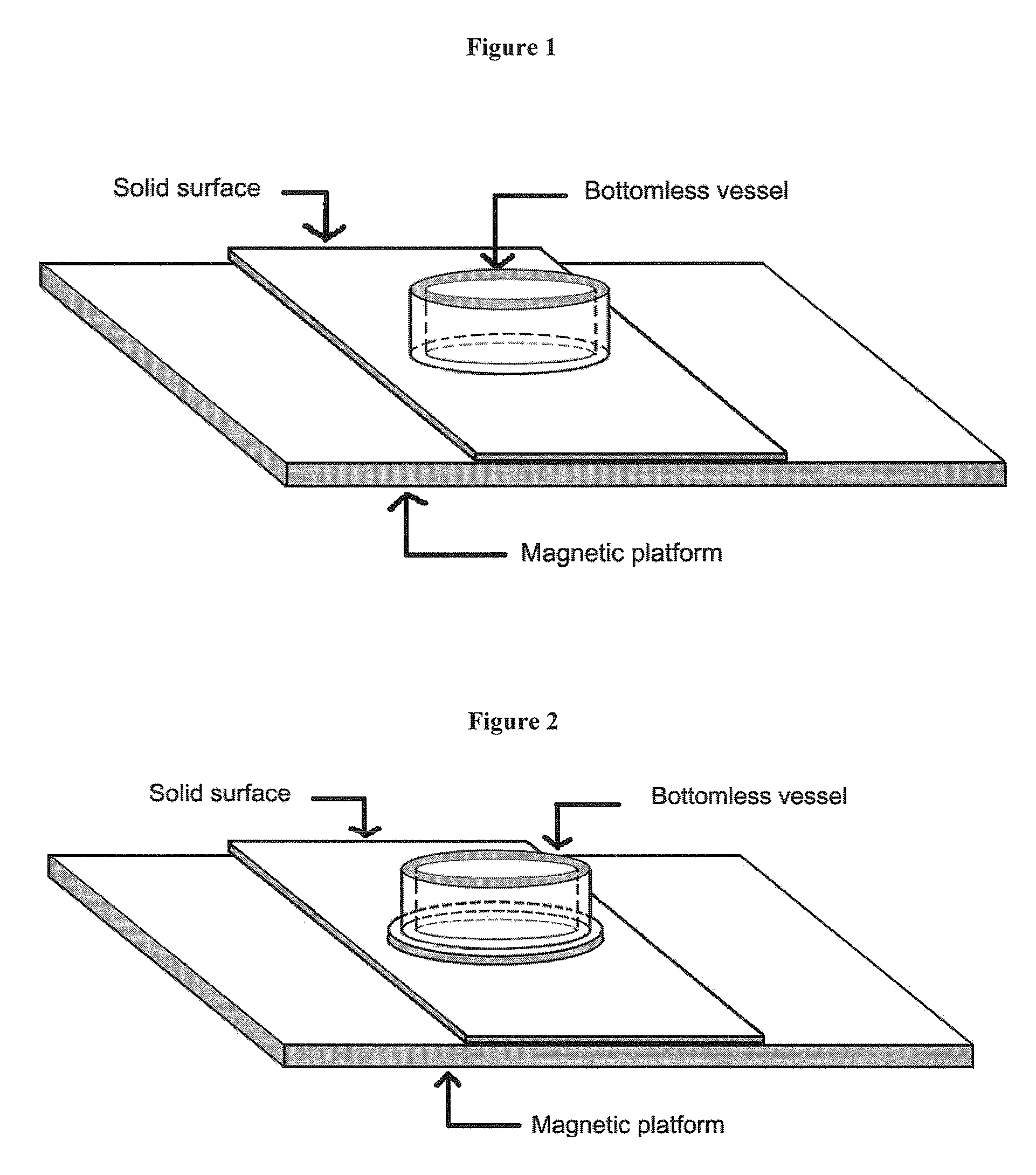
Solid surface reservoirs
An apparatus for forming a surface reservoir to hold a sample for a desirable period of time is described. The apparatus contains a platform, a solid surface disposed onto the platform, and an assembly of a bottomless vessel mounted on the solid surface. Also described is an apparatus that forms an array of surface reservoirs on a solid surface when multiple bottomless vessels are used, which can be used for high throughput applications. The apparatus can be used in applications on a solid surface, such as immunohistochemistry (IHC), oligo synthesis, peptide synthesis, ELISA, DNA array, peptide array, protein array, antibody array, tissue array, cell culturing, etc.
Owner:NANJING GENSCRIPT BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com