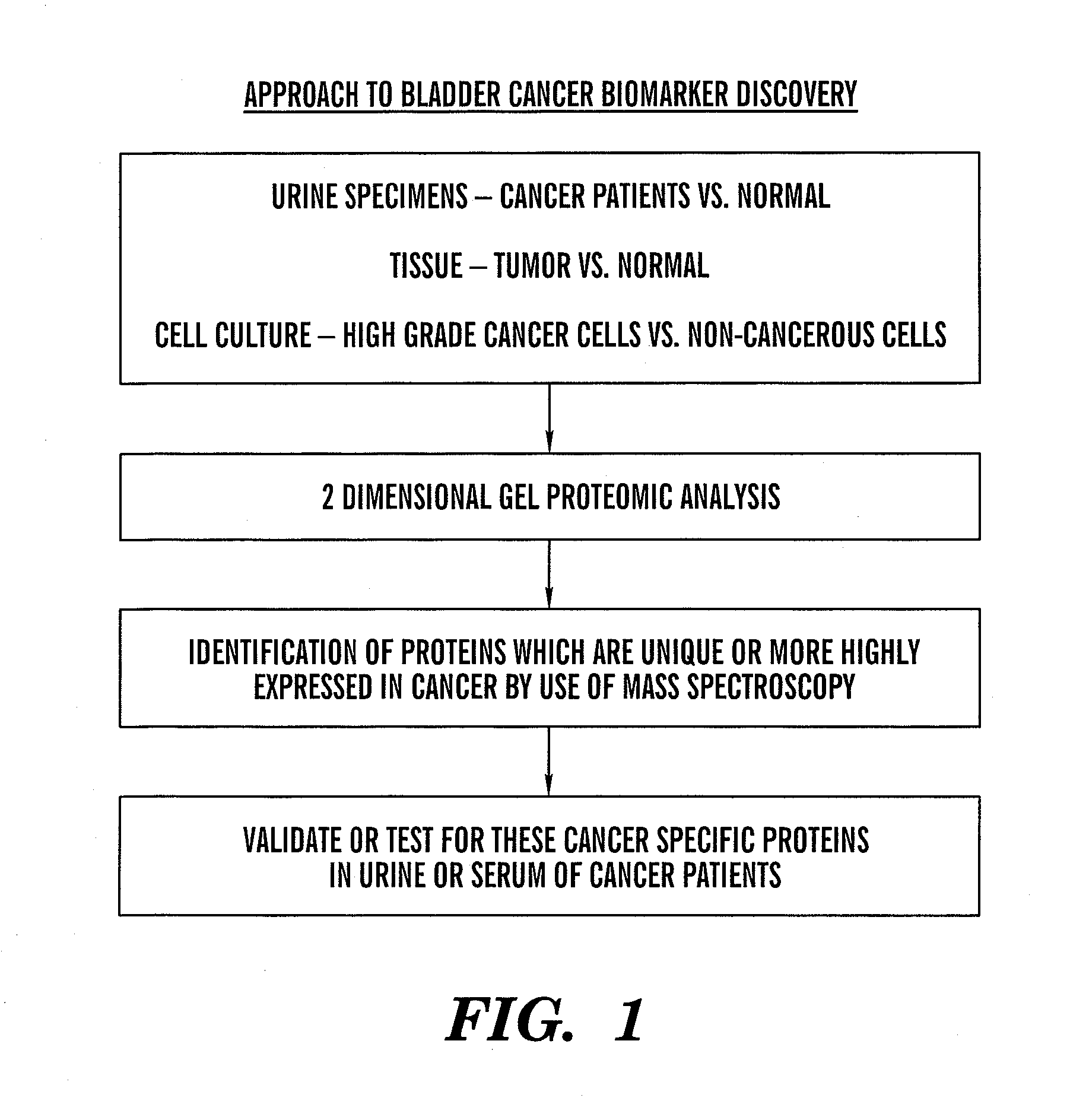
Patents
Literature
85 results about "Bladder cancer patient" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
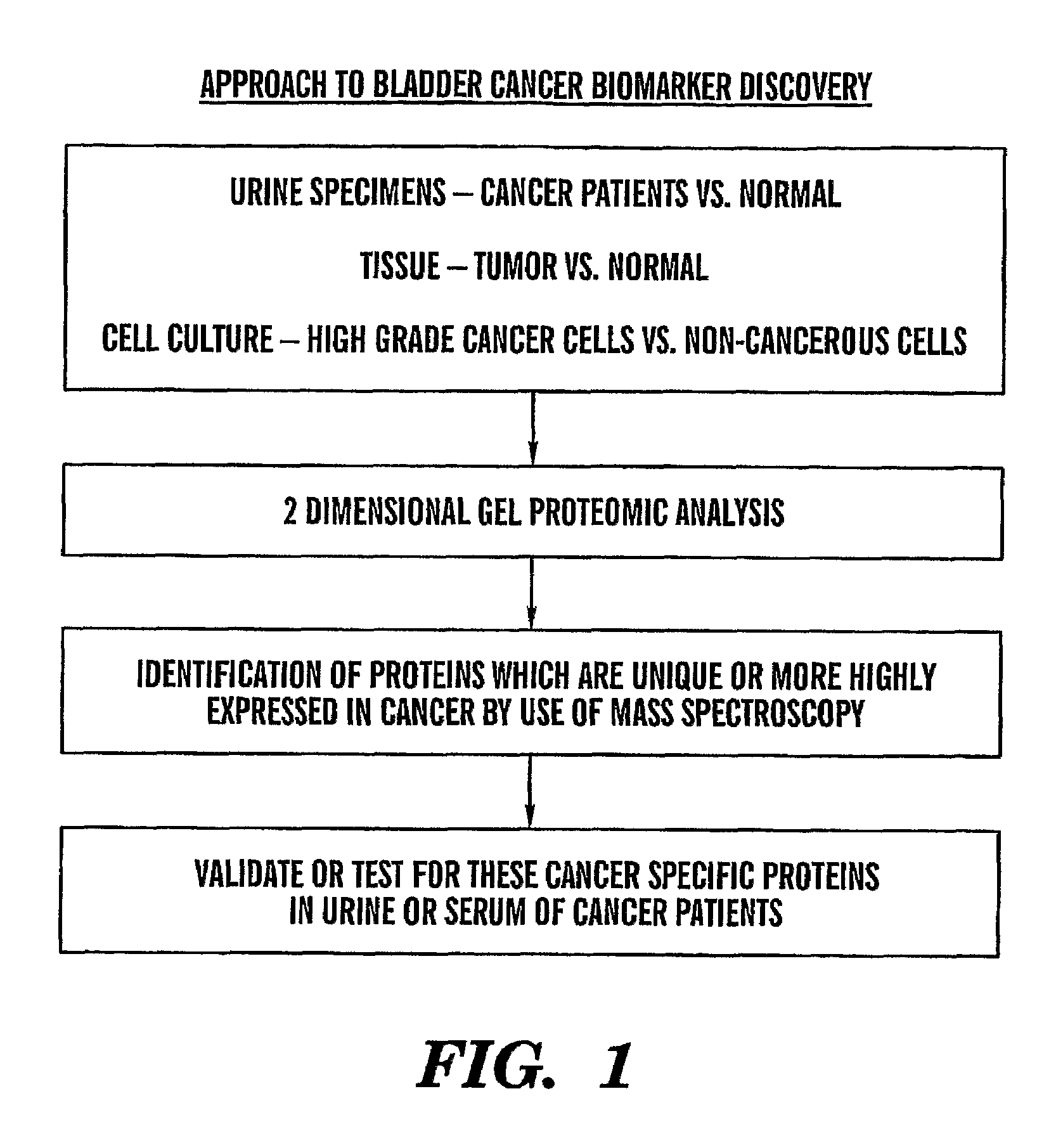
Bladder Cancer Treatment. Treatment for bladder cancer depends on the stage of the disease, the type of cancer, and the patient's age and overall health. Options include surgery, chemotherapy, radiation, and immunotherapy. In some cases, treatments are combined (e.g., surgery or radiation and chemotherapy, preoperative radiation).
Adam12 as a biomarker for bladder cancer
InactiveUS20090029372A1Improve the level ofLower Level RequirementsMicrobiological testing/measurementMaterial analysisTissue ArraysAffymetrix genechip
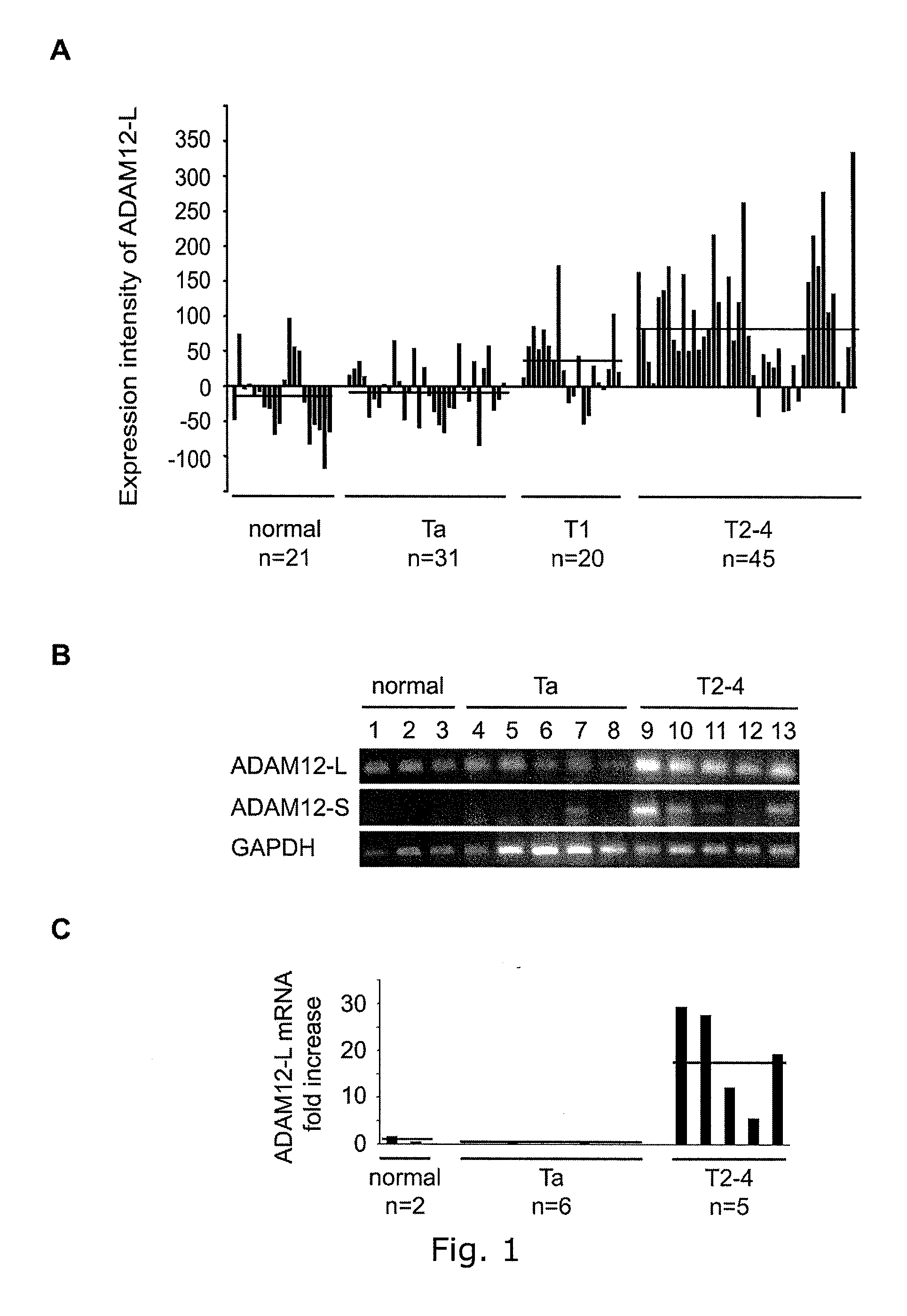
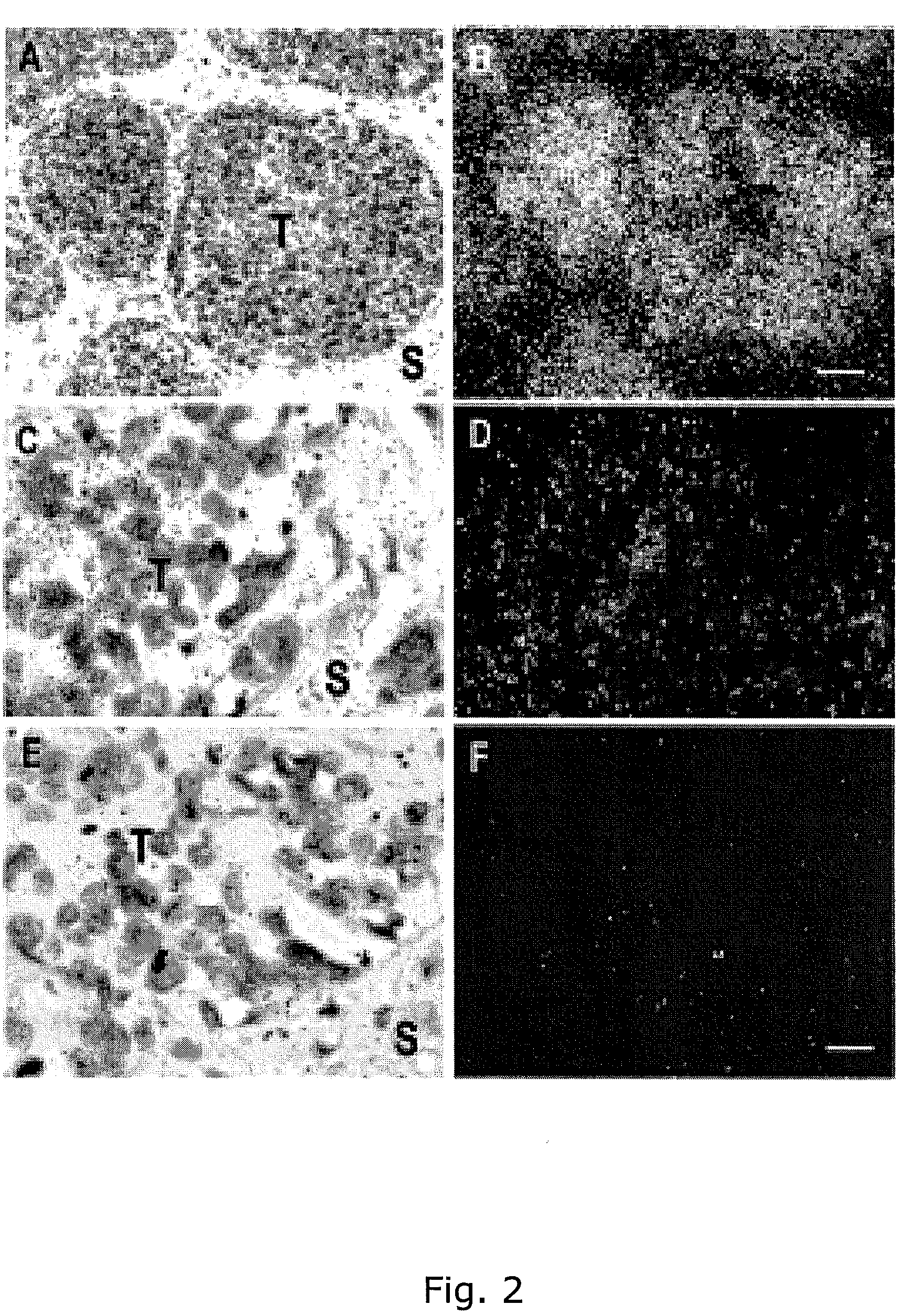
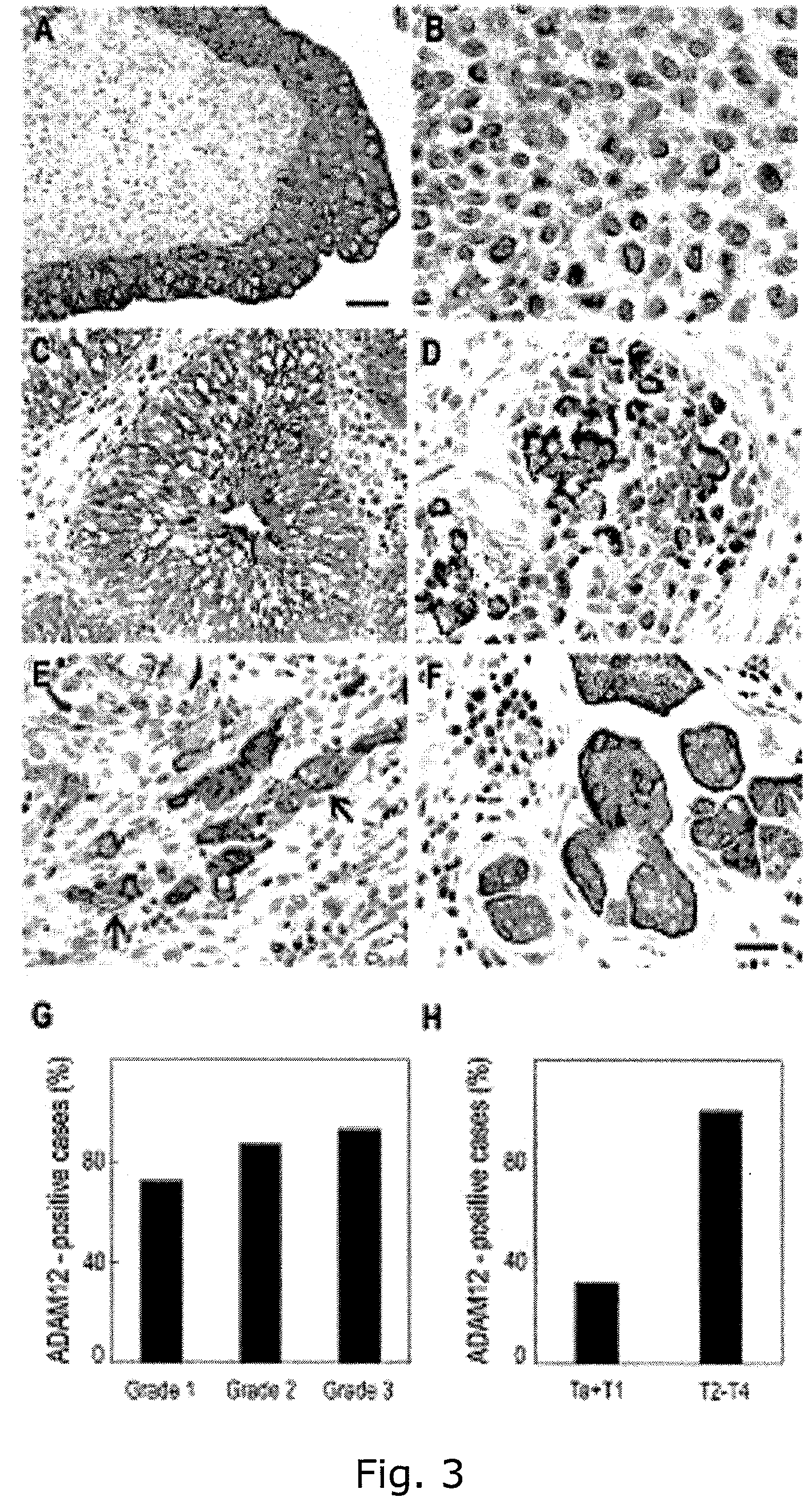
The present inventors have shown that the gene and protein expression profiles of ADAM8, ADAM10 and ADAM12 in different grades and stages of bladder cancer.ADAM12 gene expression was evaluated in tumors from 96 patients with bladder cancer using a customized Affymetrix GeneChip. Gene expression in bladder cancer was validated using reverse transcription-polymerase chain reaction (RT-PCR), quantitative PCR, and in situ hybridization. Protein expression was evaluated by immunohistochemical staining on tissue arrays of bladder cancers.The presence and relative amount of ADAM12 in the urine of cancer patients were determined by Western blotting and densitometric measurements, respectively.Particularly ADAM12 mRNA expression was significantly upregulated in bladder cancer, as determined by microarray analysis, and the level of ADAM12 mRNA correlated with disease stage. ADAM12 protein expression correlated with tumor stage and grade. ADAM12 was present in higher levels in the urine from bladder cancer patients than in urine from healthy individuals. Significantly, following removal of tumor by surgery, in most bladder cancer cases examined the level of ADAM12 in the urine decreased and, upon recurrence of tumor, increased.
Owner:PHYSICIANS CHOICE LAB SERVICES +1
Methods for diagnosis and prognosis of epithelial cancers
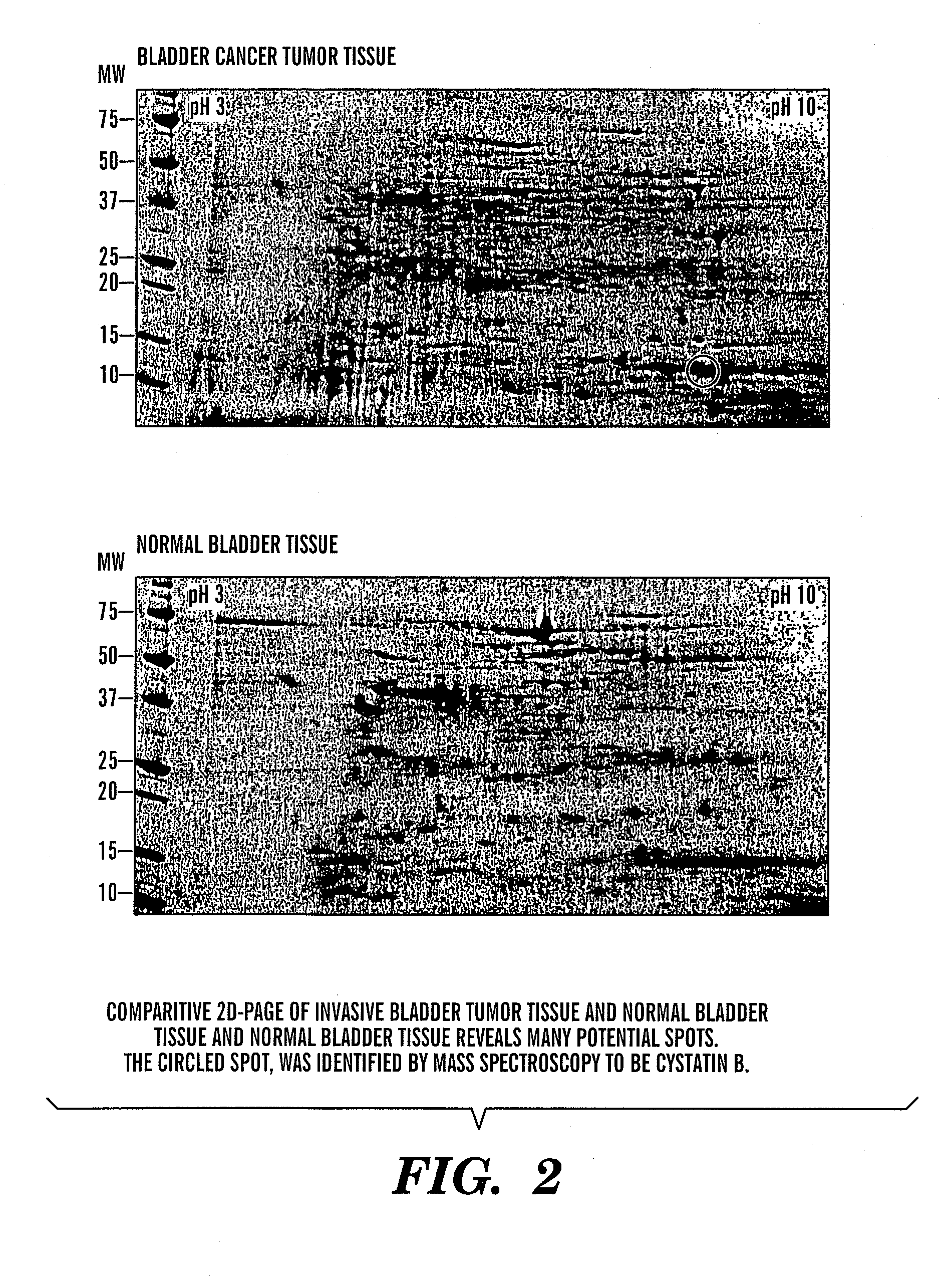
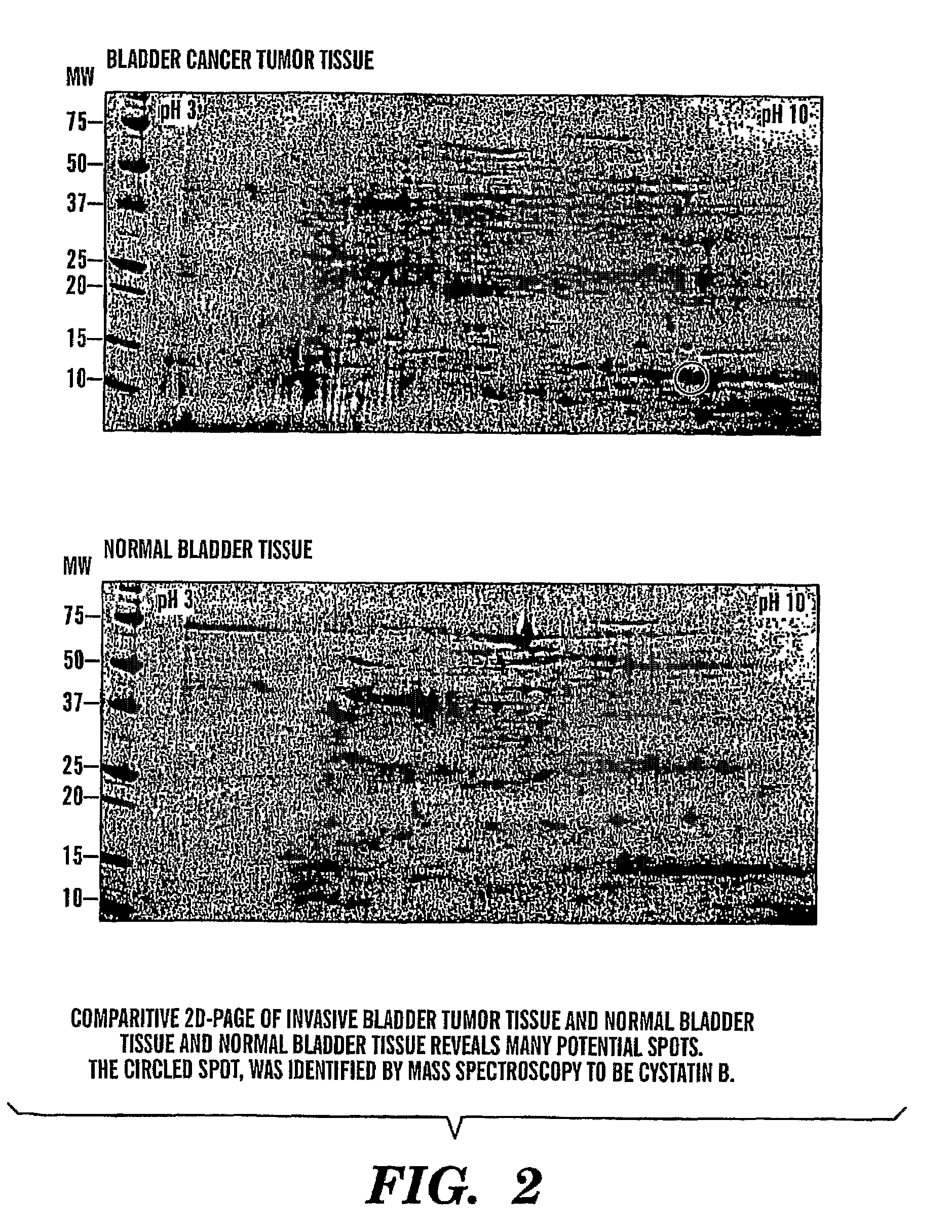
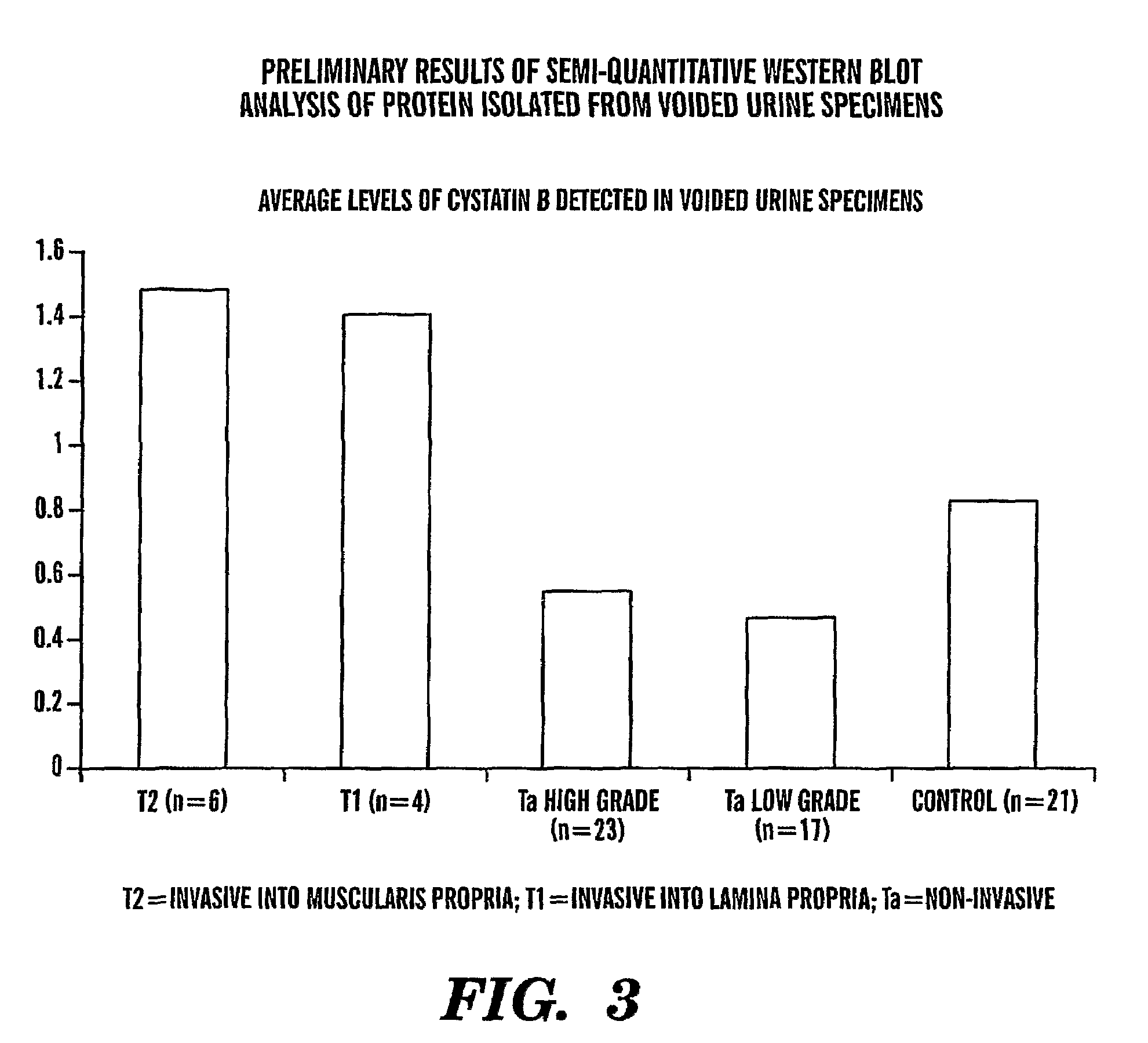
InactiveUS20080064047A1Easy diagnosisQuick and easy and safeBiological material analysisBiological testingBladder cancer patientCystatin B
The present invention is based on the discovery that three proteins, Cystatin B, Chaperonin 10, and Profilin are present in the urine of patients with bladder cancer, a cancer of epithelial origin. Accordingly, the present invention is directed to methods for prognostic evaluation of cancers of epithelial origin and to methods for facilitating diagnosis of cancers of epithelial origin by monitoring the presence of these markers in biological samples. The invention is also directed to markers for therapeutic efficacy.
Owner:THE GENERAL HOSPITAL CORP +1
Bladder cancer patient urine specific metabolite spectrum, establishing method and application
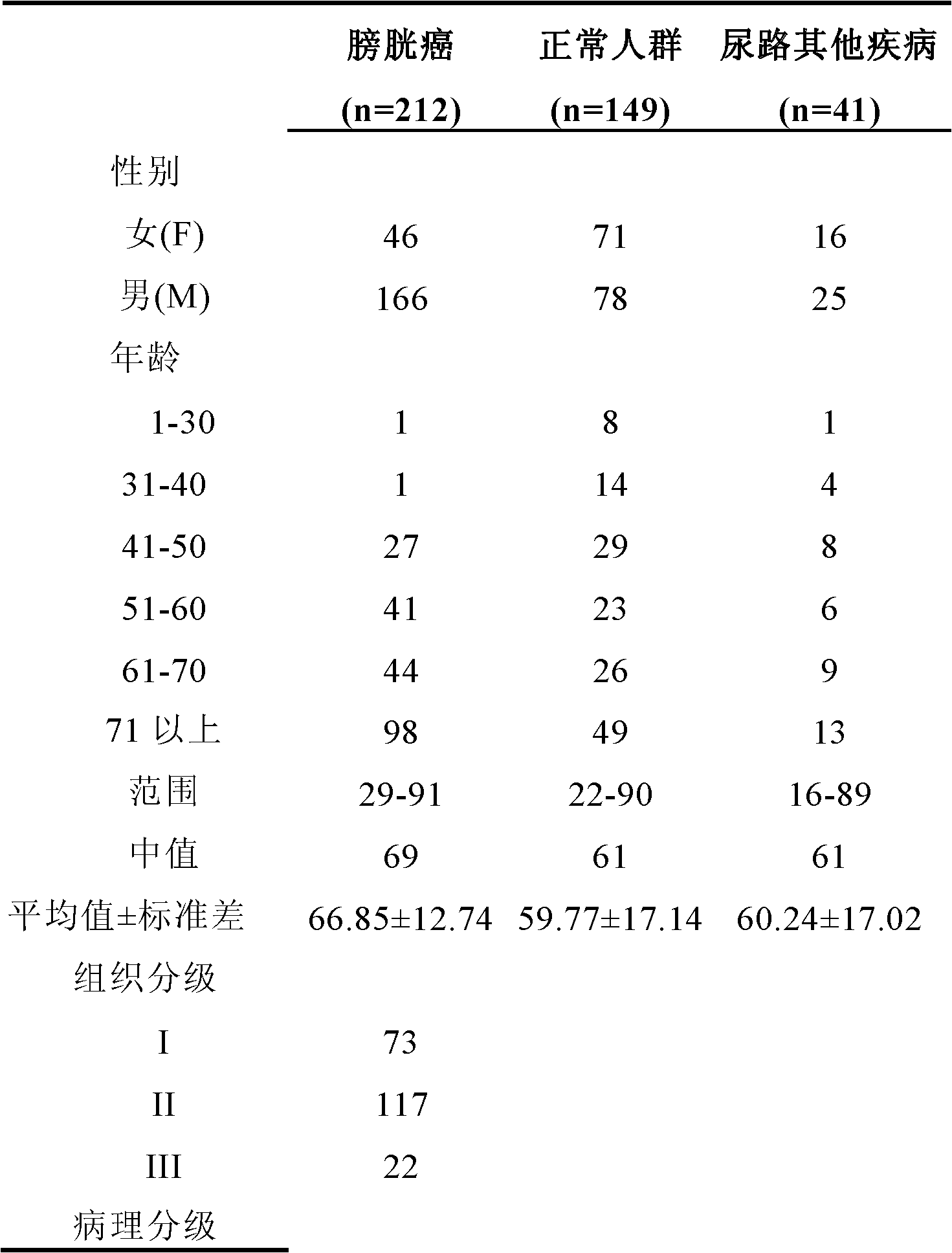
The invention provides a bladder cancer patient urine specific metabolite spectrum, an establishing method and an application, and especially provides a method for establishing a spectrum of specific metabolites in urine of bladder cancer patients, and a method for screening a related specific biomarker. Through metabonomics, especially metabonomics based on liquid chromatography-mass spectrometry combined technology, the invention establishes a bladder cancer patient urine specific metabolite spectrum. The invention provides a basis for early diagnosis of bladder cancer and bladder cancer diseases with different pathological stages, and also provides a spectrum of specific metabolites in urine of bladder cancer patients and a related specific biomarker. The method provided by the invention has the characteristics of noninvasiveness, convenience, and rapidness, can accurately reflect the difference of metabolite spectra of bladder cancer patients and normal people, and has high specificity.
Owner:BGI GENOMICS CO LTD
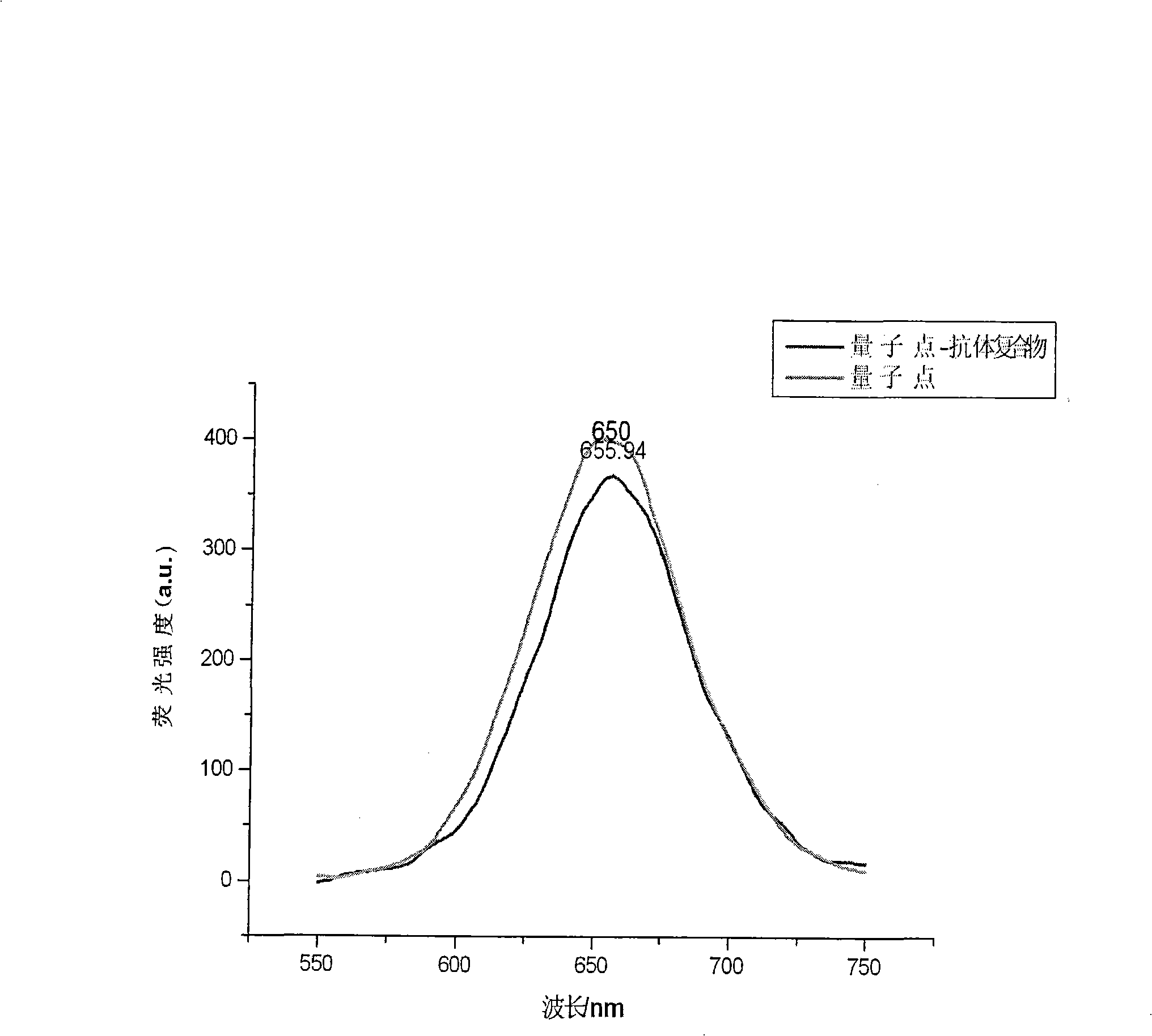
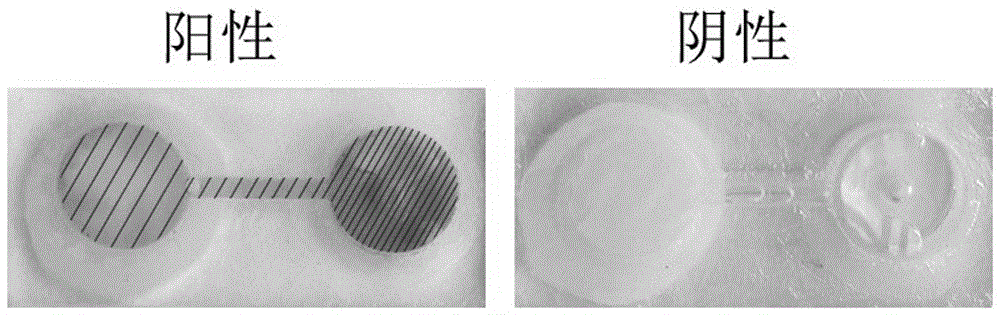
Rapid checking method for bladder cancer by quantum dot mark immunity-chromatography test paper
The invention discloses a method for rapidly detecting the bladder cancer with quantum dot labeling immune chromatographic test paper. The existing method for bladder cancer detection comprises the cystoscope, ultrasonic waves, CT, and the like, but the methods are trivial in detection procedures and patients are subjected to pains. By making use of the quantum dot labeling immune chromatographic test paper, the method rapidly detects the amount of fibronectin protein contained in urine of bladder cancer patients and diagnoses and judges the bladder cancer. The detection method which is low in cost and simple in method is suitable for samples of blood, urine, saliva, and the like, can be applied to the detection of hormones, proteins, viruses, drugs, bacteria, environmental pollution, and the like, and can be used in places such as hospitals, customs, , airports and families.
Owner:SHANGHAI NORMAL UNIVERSITY
Method and kit for diagnosing bladder cancer with urine
InactiveCN102311953AMicrobiological testing/measurementDNA/RNA fragmentationCommunity health centerBiomarker (petroleum)
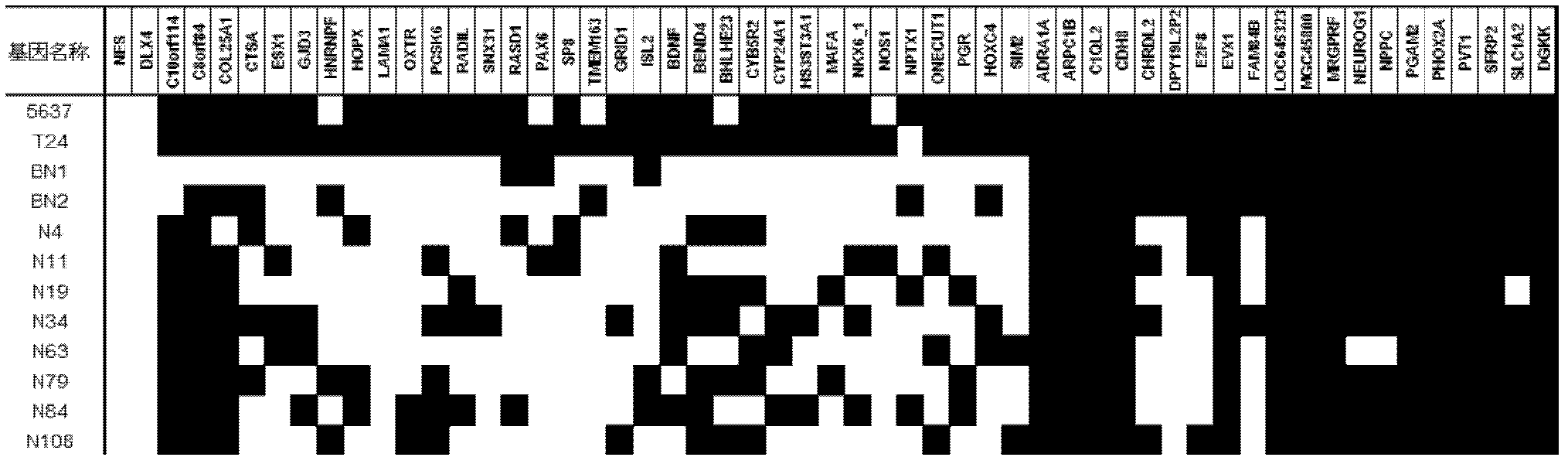
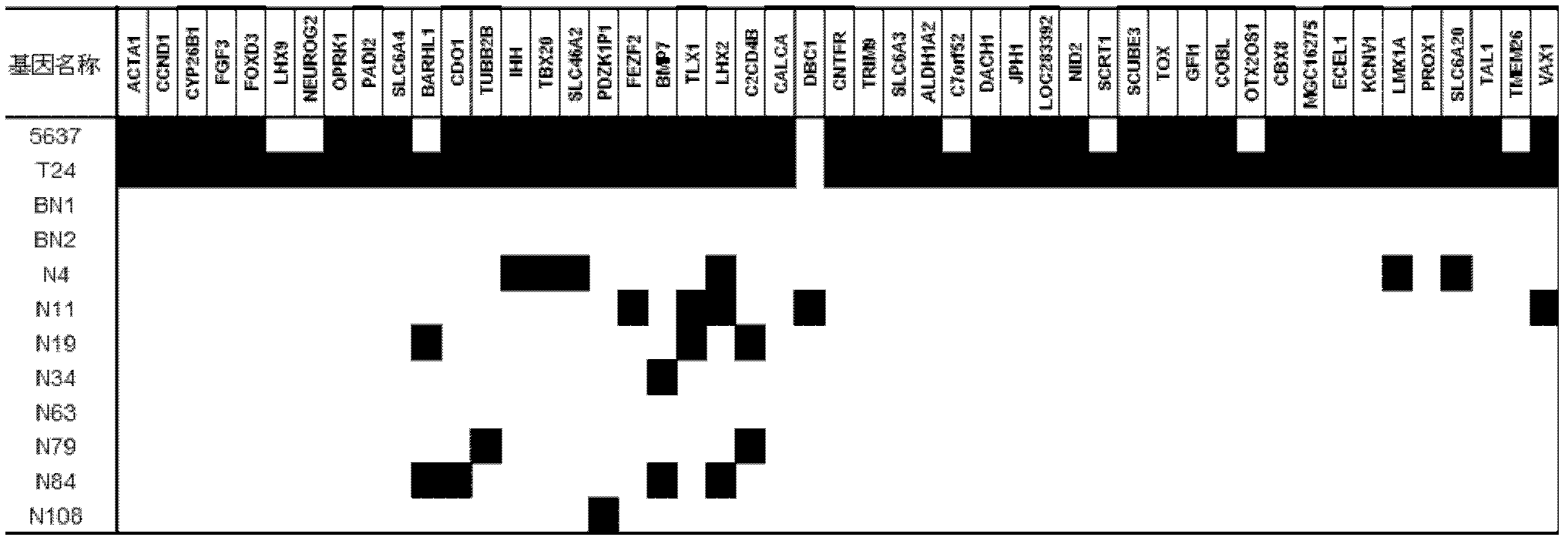
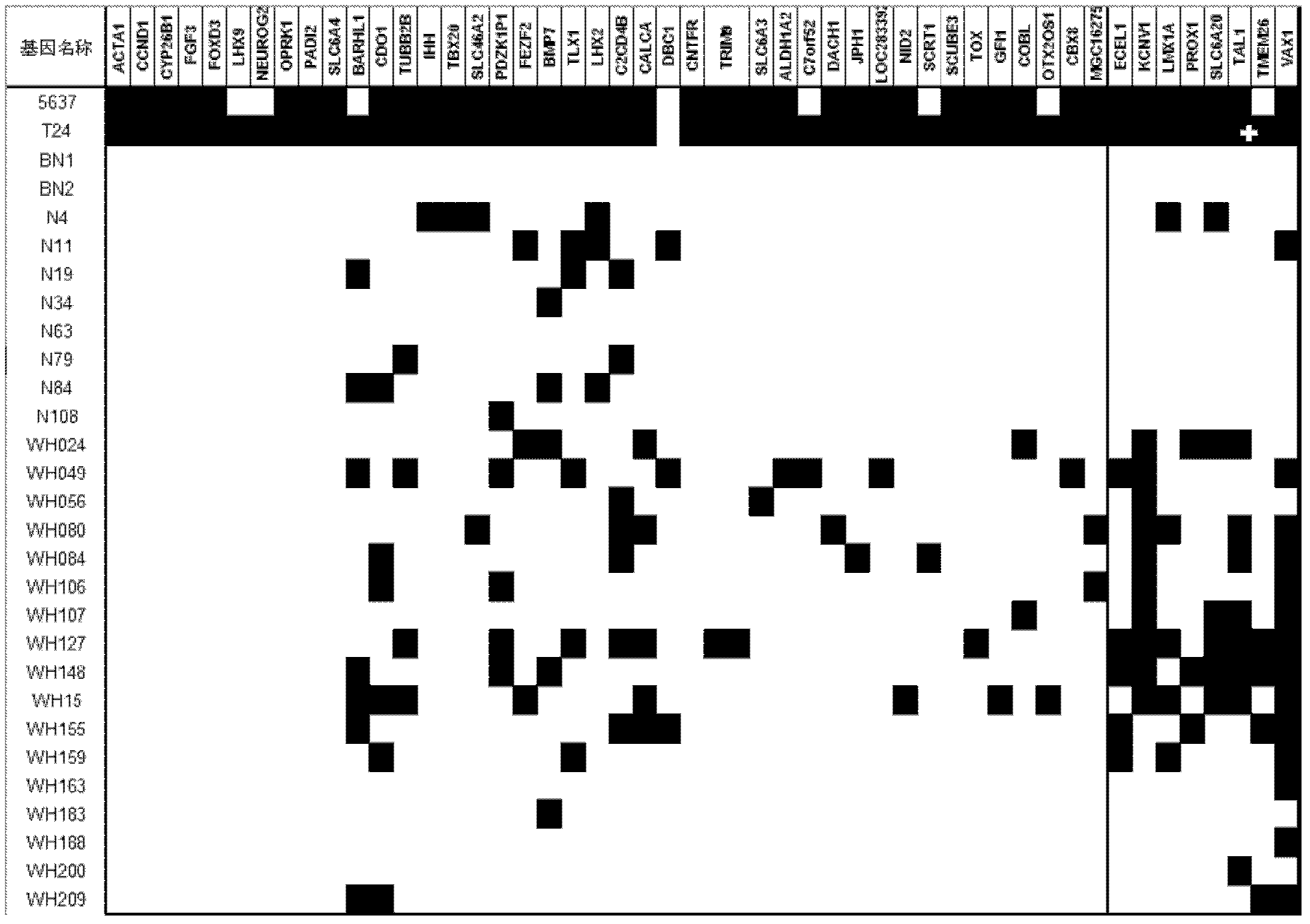
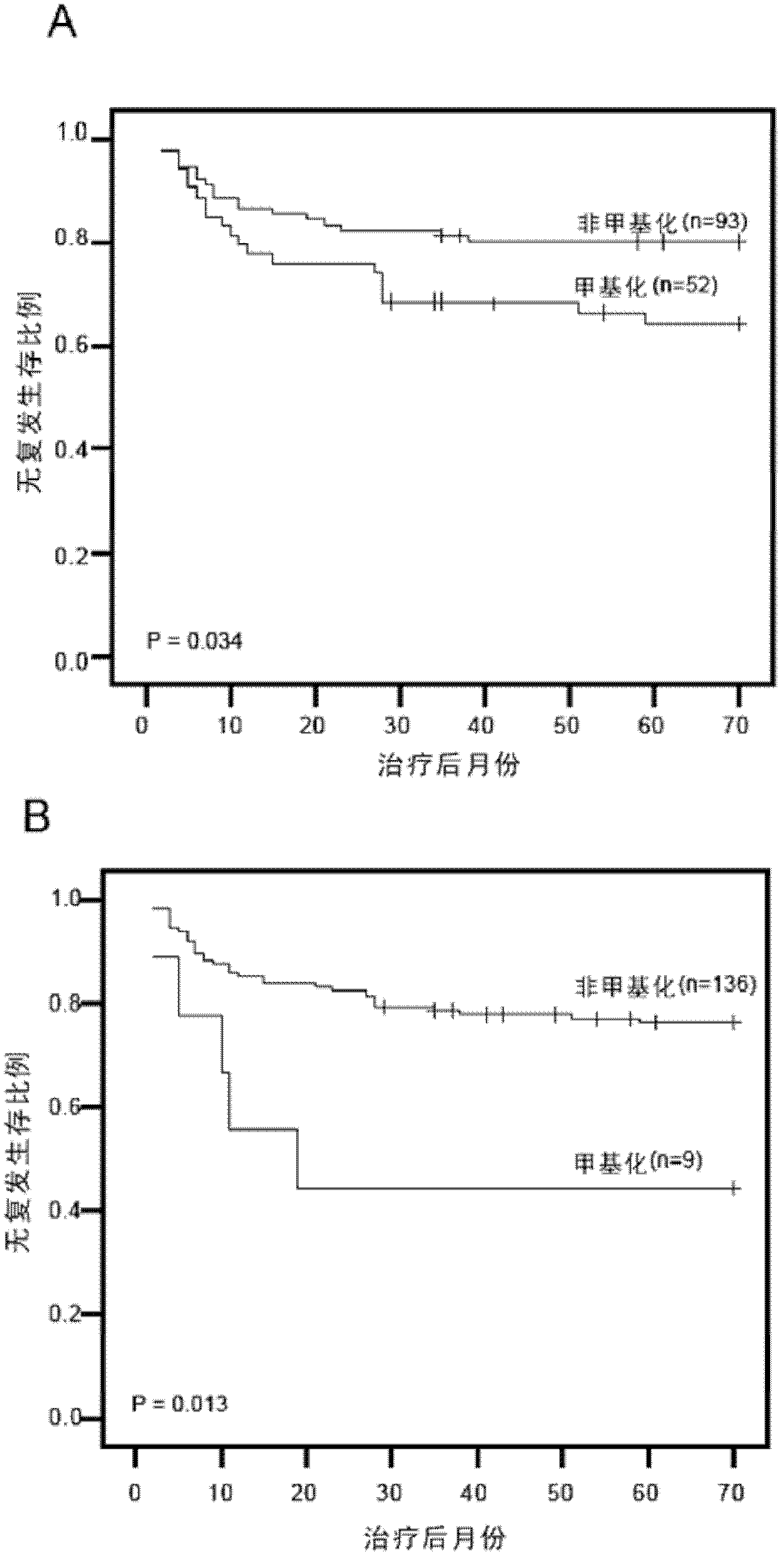
The invention relates to a method and a kit for diagnosing bladder cancer with urine. The invention discloses a group of methylated sensitive genes, comprising ECEL1, KCNV1, LMX1A, PROX1, SLC6A20, TAL1, TMEM 26, and VAXI gene. In urine samples of bladder cancer patients, the specific CpG sites of the genes are showed the highest methylation levels. Accordingly, the genes are the biological marker of bladder cancer. The method and the kit can be used as the basic of designing diagnostic reagents of bladder cancer, and are suitable for auxiliary detection of cancer in hospitals, postoperative followup, screening of high risk group of bladder cancer in community health centers, and screening of common people and high risk practitioners of bladder cancer in physical examination center.
Owner:SHANGHAI INST OF ONCOLOGY
A kind of bladder cancer quantitative detection test paper
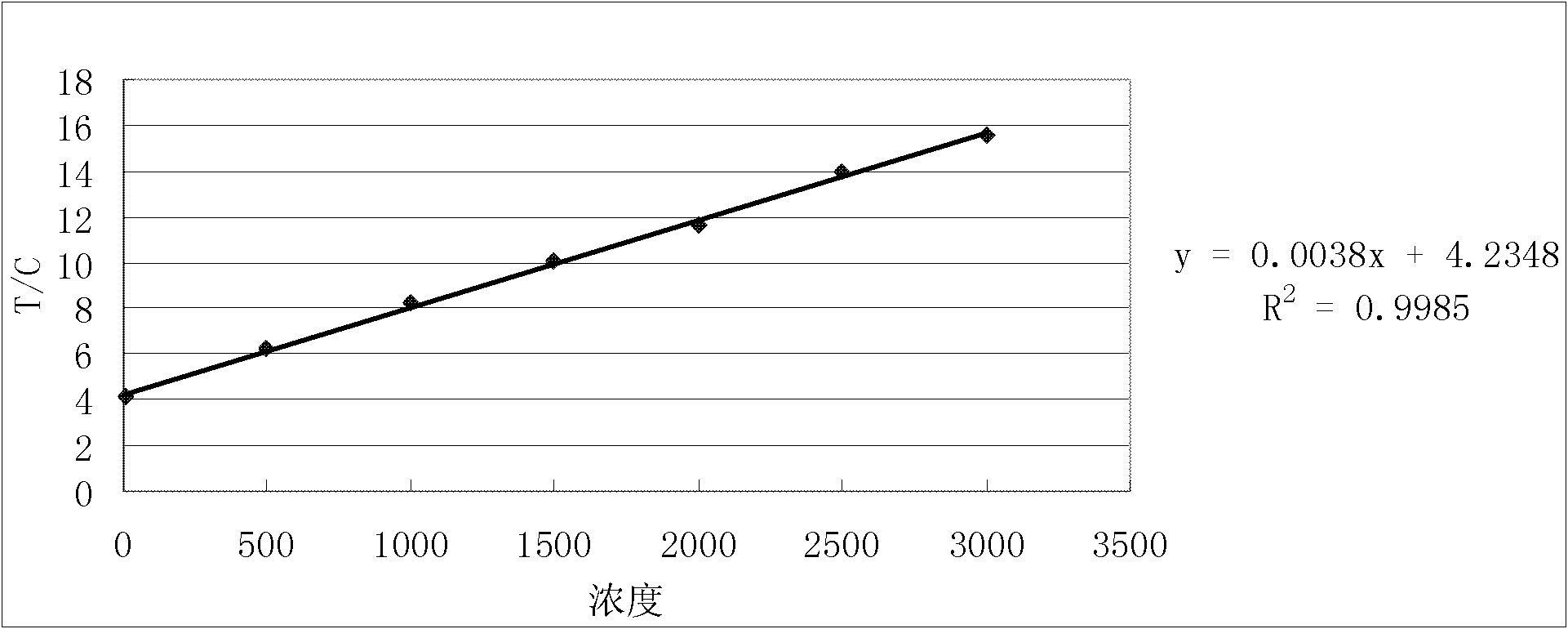
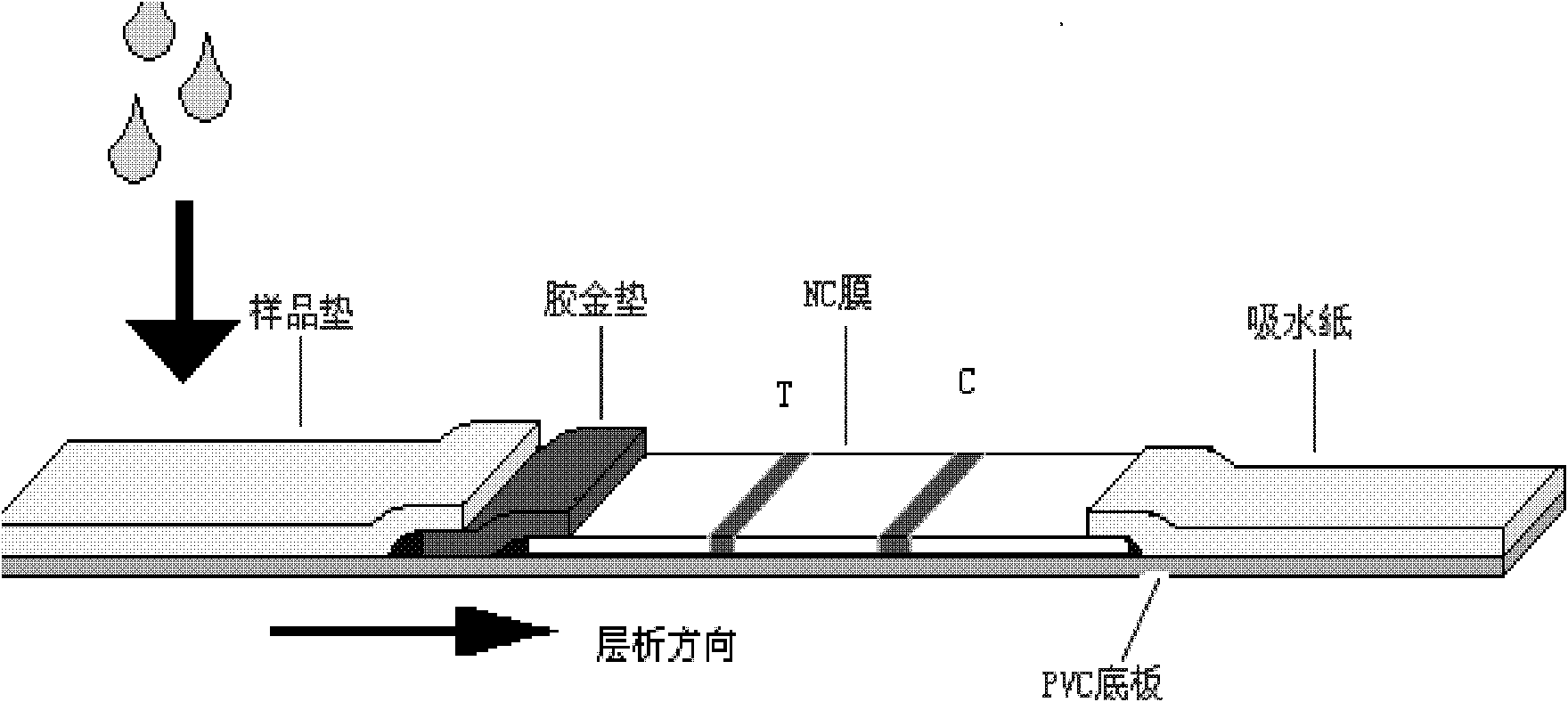
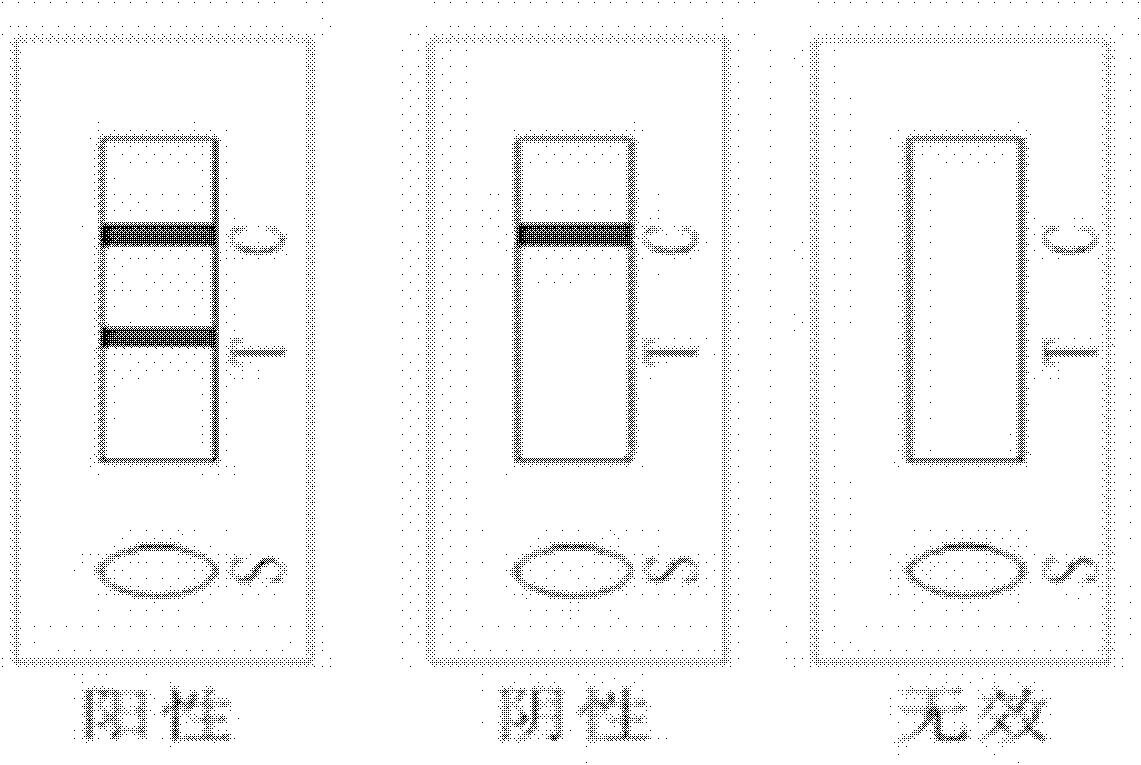
The invention relates to a test paper for quantitative detection of bladder cancer, which is composed of a sample pad, a colloidal gold pad, a nitrocellulose membrane, an absorbent paper and a bottom plate. On the nitrocellulose membrane, there is a detection area fixed with urinary fibronectin and sheep antibody. In the control area of the mouse secondary antibody, there is a gold-labeled anti-urinary fibronectin antibody on the colloidal gold pad, wherein the gold-labeled antibody is dispersed on the colloidal gold pad after being dispersed in a pH 5-10 dilution. The sensitivity of quantitative detection is up to 10ng / mL, the linear range is from 10-3000ng / mL, and it has the advantages of strong specificity, high precision, good stability, easy operation, low cost, etc. It is suitable for primary screening and surgical treatment of bladder cancer. After tracking.
Integrated detection method for separating, enriching and detecting urine exosome as well as detection chip
The invention provides an integrated detection method for separating, enriching and detecting an exosome. The method comprises the following steps: designing and assembling a dual-membrane filtration chip, constructing an ELISA detection standard curve, collecting a sample, injecting and washing, performing chip ELISA detection, and performing data processing analysis. The invention also provides an integrated detection chip for separating, enriching and detecting a urine exosome. A reaction system and the detection method of the present invention can be used for detecting the content of a urine sample of a bladder cancer patient and the exosome in a supernatant of a bladder cancer cell line nutrient solution. The detection chip has characteristic of high specificity, generates no influence or wound on human body, and the detection method does not require the expensive precision experiment apparatus (such as an ultra centrifuge and a fluorescence microscope), and has large application prospect.
Owner:ZHEJIANG UNIV
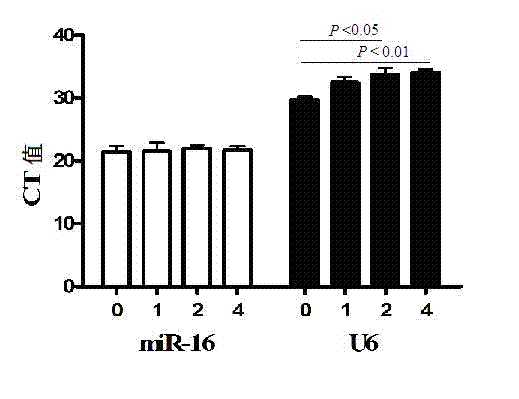
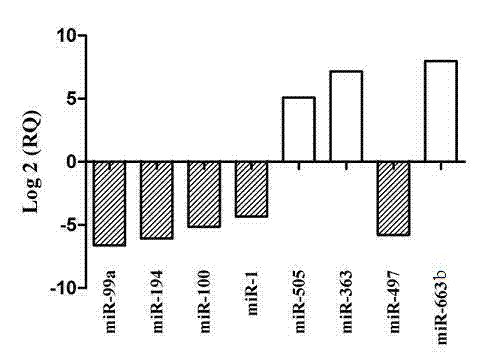
Serum micro ribonucleic acid (miRNA) biomarker of bladder cancer and detection method of expression quantity thereof
ActiveCN102925444AMicrobiological testing/measurementFluorescence/phosphorescenceSerum igeBladder cancer patient
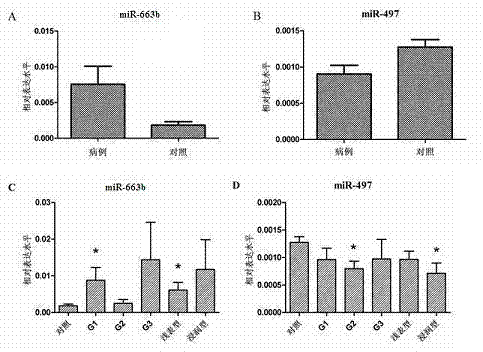
The invention relates to a serum micro ribonucleic acid (miRNA) biomarker used for detection of bladder cancer, overcomes the defects in the prior art, provides a miRNA marker circulating in serum of a patient having the bladder cancer, establishes a blood circulation miRNA expression profile of the patient having the bladder cancer, discloses the value of serum miRNAs in diagnosis of the bladder cancer, and provides support for early detection and early treatment of the patient having the bladder cancer clinically. According to the serum miRNA biomarker of the bladder cancer and a detection method of expression quantity of the serum miRNA biomarker, for the first time, the serum miRNAs, especially miR-663b and miR-497 can be used as an important indicator of biological detection and plays roles in clinical early diagnosis of the bladder cancer, differential diagnosis and effective observation, a theoretical basis is provided for research on the serum miRNAs of urinary system tumor in the future, novel ideas are provided for the diagnosis of the bladder cancer at the molecular level, and the serum miRNA biomarker of the bladder cancer and the detection method of expression quantity of the serum miRNA biomarker have great theoretical significance and potential practical value.
Owner:NANJING MEDICAL UNIV
Urine-based method and kit for diagnosing relapse risk of bladder cancer patient
The invention relates to a urine-based method and kit for diagnosing the relapse risk of a bladder cancer patient. The invention discloses two methylation-sensitive genes, namely an LMX1A gene and a VAX1 gene. A urine sample is kept before the operation of a detected bladder cancer patient, and the specific CpG sites of the genes are subjected to high methylation among people who are determined to suffer from a relapse in the follow-up visit process. Thus, the two genes can be used as biological markers for the relapse of bladder cancer. The invention can be used as the basis for designing a kit for prognosing and diagnosing the relapse of bladder cancer, and is applicable to the prognosis detection of bladder cancer in hospital, the postoperative follow-up visit and the postoperative monitoring of the community health center on people subjected to bladder cancer operation.
Owner:SHANGHAI INST OF ONCOLOGY
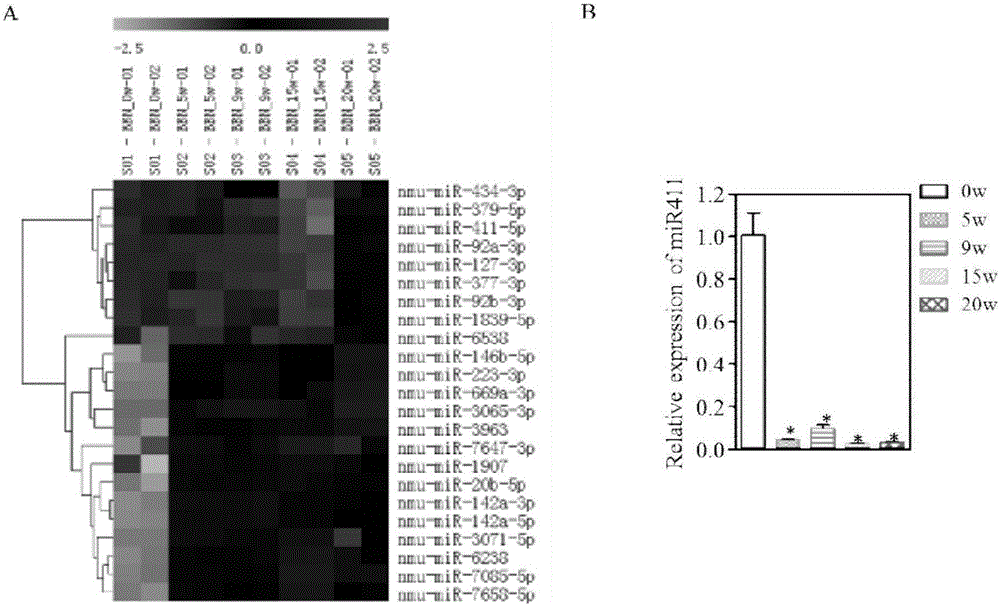
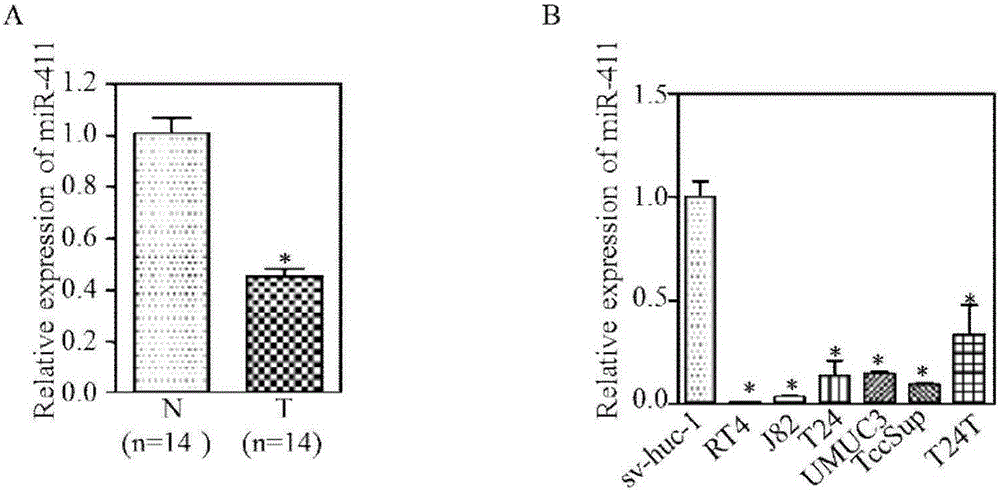
MiR-411 serving as target of bladder cancer and application of miR-411
InactiveCN105999269AIncreased sensitivityAuxiliary diagnosisOrganic active ingredientsMicrobiological testing/measurementMirna microarrayIn vivo
The invention belongs to the field of biotechnology, and specifically relates to miR-411 as a bladder cancer target and its application. The invention uses bladder cancer T24 and UMUC3 cells as models, and overexpressing miR-411 can inhibit the proliferation of bladder cancer cells in vivo and in vitro, and induce bladder cancer Cancer cell cycle G2 / M phase arrest, while enhancing the sensitivity of chemotherapy drugs (mitomycin and gemcitabine) to bladder cancer cells. It can be seen that miR-411 expression promoter can prevent or / and treat bladder cancer. Using miRNA chip technology, real-time fluorescent quantitative PCR technology, bioinformatics analysis of TCGA database and other methods to detect the expression of miR‑411 in bladder cancer primary animal models and clinical bladder cancer tissues showed a significant down-regulation trend, and the expression of miR‑411 was correlated with bladder cancer There is a certain positive correlation with the survival time of patients. It can be seen that by detecting the expression of miR‑411, bladder cancer can be assisted in the diagnosis and / or prognosis.
Owner:WENZHOU MEDICAL UNIV
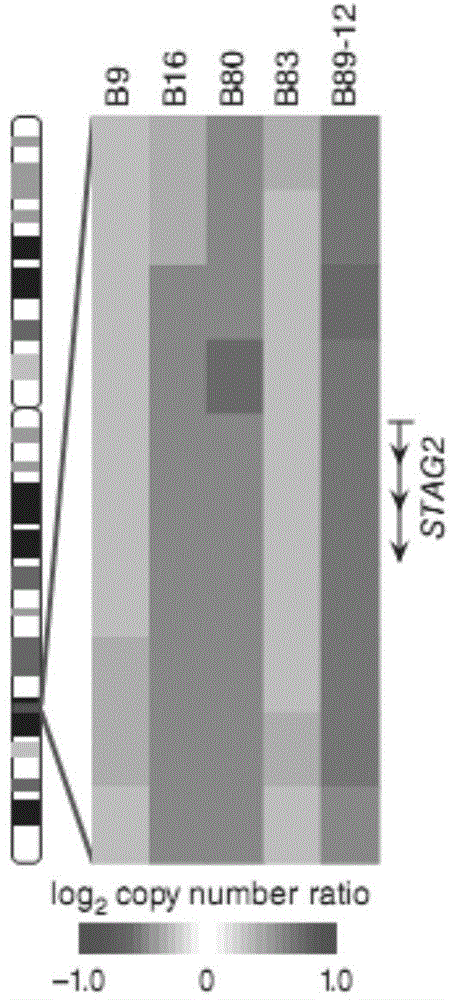
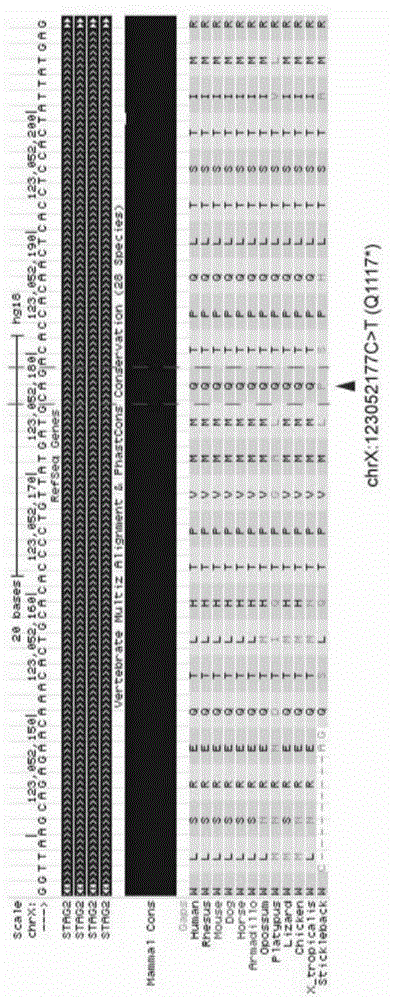
Application of long-chain non-coding RNA DUXAP8 as biomarker for prognosis of bladder cancer
The invention provides application of long-chain non-coding RNA DUXAP8 as a biomarker for prognosis of bladder cancer. The application is characterized in that the long-chain non-coding RNA DUXAP8 isused for predicting the overall survival time or recurrence-free survival time of bladder cancer patients. The application successfully provides a high-efficiency biomarker resource for the prognosisof the disease by detecting the change in the frequency of the genomic site copy number of the long-chain non-coding RNA DUXAP8.
Owner:JIANGYIN PEOPLES HOSPITAL
Bladder drug flushing and perfusion device after urinary system surgery
InactiveCN110237338AEasy to useImprove user experienceEnemata/irrigatorsMedical devicesCold weatherPerfusion
The invention discloses a bladder drug flushing and perfusion device after a urinary system surgery. By means of a drug adding mechanism, drug components can be added to perfusate, an affected part can be treated by diminishing inflammation and arresting bleeding in a perfusion process, and even bladder chemotherapy drugs can be perfused for a bladder cancer patient after a surgery; by means of a heating mechanism, the perfusate can be heated, and proper heating can reduce the stress response, improve the use experience of the patient in colder weather, and improve the drug effect; by means of a temperature sensor, the internal temperature of the bladder can be detected to realize temperature control, and when the internal temperature is lower than the lower limit of the preset temperature, liquid circulation is accelerated; and by means of a pressure sensor, the liquid pressure inside the bladder can be detected, so that on one hand, proper filling inside the bladder is ensured, and on the other hand, when the urinary tract is blocked by blood clots or other reasons, early warning can be given to avoid discomfort caused by overfilling of the bladder and improve the use experience of the patient. Therefore, the device disclosed by the invention has certain application prospects.
Owner:THE PEOPLES HOSPITAL SHAANXI PROV
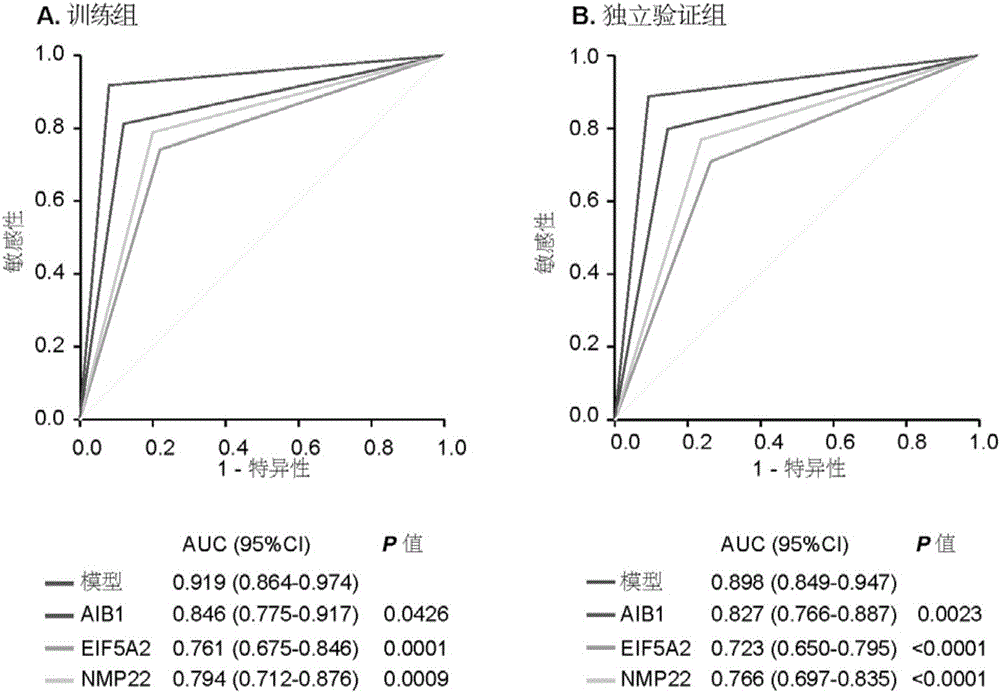
Molecular markers for non-invasive diagnosis of bladder cancer
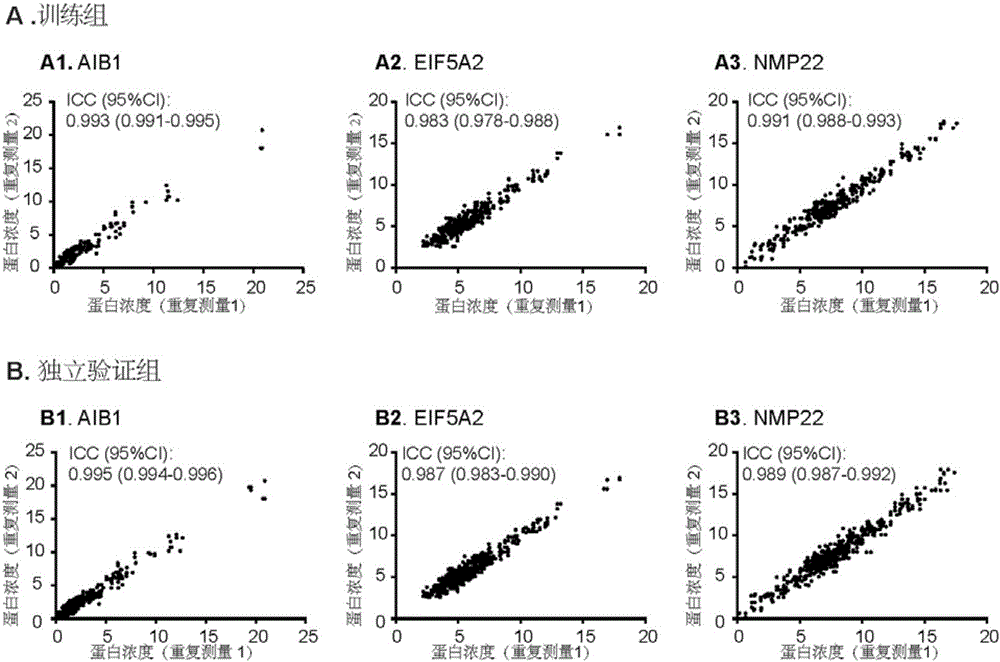
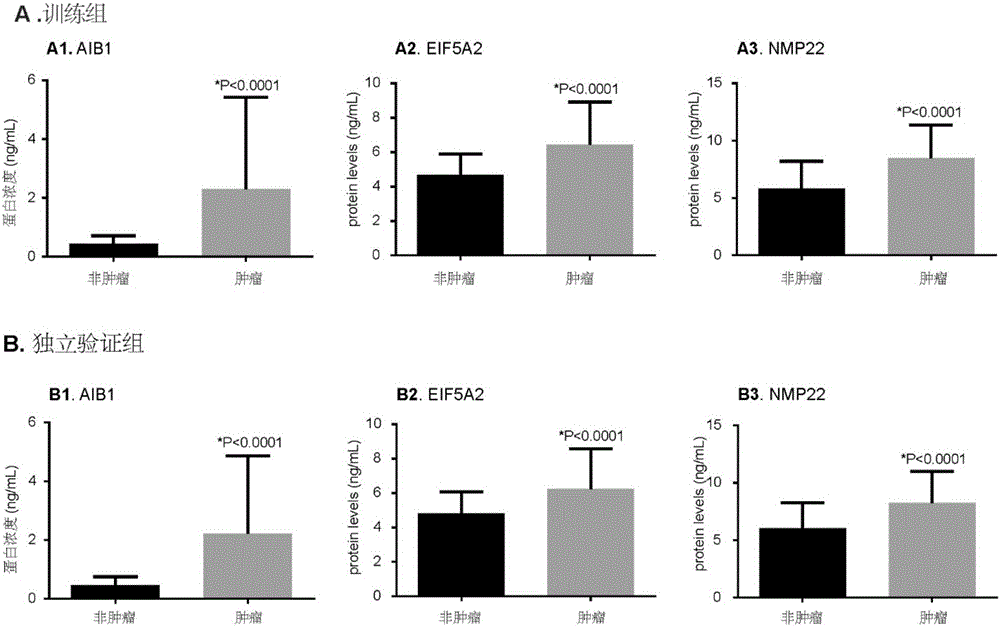
The invention discloses molecular markers for non-invasive diagnosis of bladder cancer. Based on previous research basis, the inventor detects the protein expression level of AIB1 and EIF5A2 in urine of bladder cancer patients and non-bladder cancer population, and finds that AIB1 and EIF5A2 can serve as new tumor markers for non-invasive diagnosis of bladder cancer, and through combination of AIB1, EIF5A2 and NMP22, the diagnosis accuracy of bladder cancer can further be improved.
Owner:陈卫 +1
Drug for treating bladder cancer and preparation method thereof
InactiveCN103908581AInhibited DiffusionRelieve painAntineoplastic agentsPlant ingredientsArundinaSide effect
The invention discloses a drug for treating bladder cancer and a preparation method thereof and belongs to the field of traditional Chinese medicine. The effective components of the drug disclosed by the invention are prepared from the following raw materials: chufa, duckweed, Chinese arundina herb, large-leaved gentian, sow thistle, caulis lonicerae, talc, dayflower, dianthus superbus, herba pyrrosiae, plantain herb, mangnolia officinalis, large angiopteris rhizome, fructus viticis, akebiaquinata and honewort; the matching of the selected medicinal raw materials is proper and meets the theory of traditional Chinese medicine and modern medicine; the drug has the functions of clearing away heat and toxic materials, relieving swelling and pain, promoting diuresis for stranguria and treating stranguria, has a unique curative effect on bladder cancer, and can inhibit cancer cell diffusion to helt bladder cancer patients to get rid of pain; the drug is remarkable in curative effect, safe, free of side effect, efficient and practical, low in cost and worthy of wide promotion and application; and the bladder cancer patients can normally work and live during a medicine taking period.
Owner:朱华亭
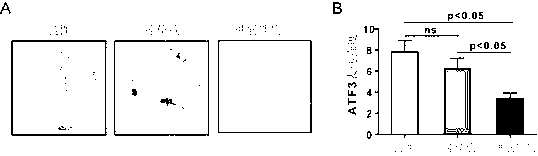
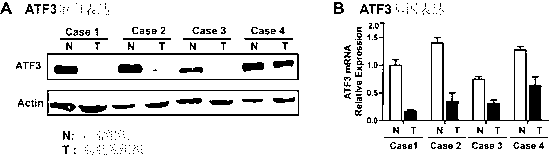
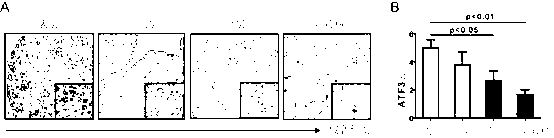
Application of ATF (activating transcription factor) 3 to predicting bladder cancer metastasis and judging prognosis
InactiveCN103235141AInhibit transferImprove prognosis survivalBiological testingBladder cancer patientCancer metastasis
The invention relates to application of an ATF (activating transcription factor) 3 protein to preparation of a kit for detecting or judging bladder cancer metastasis and judging bladder cancer prognosis. The invention also provides the kit for detecting or judging bladder cancer metastasis and judging bladder cancer prognosis. The invention has the following advantages: the invention provides a new protein marker for predicting bladder cancer metastasis of patients with bladder cancers and judging prognosis and the protein marker can be used for detecting or judging bladder cancer invasion and metastasis degrees and judging prognosis by an immunohistochemical detection method; the invention provides the new kit for predicting bladder cancer metastasis of the patients with bladder cancers and judging prognosis; and the invention provides the new application of the ATF 3 protein to suppressing bladder cancer metastasis and lengthening the prognostic lifetime of the patients with bladder cancers.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Marker for predicting bladder cancer chemosensitivity and application
InactiveCN110423820ASensitivity quickly and accurately masteredImprove clinical treatment effectMicrobiological testing/measurementBladder cancer patientTreatment effect
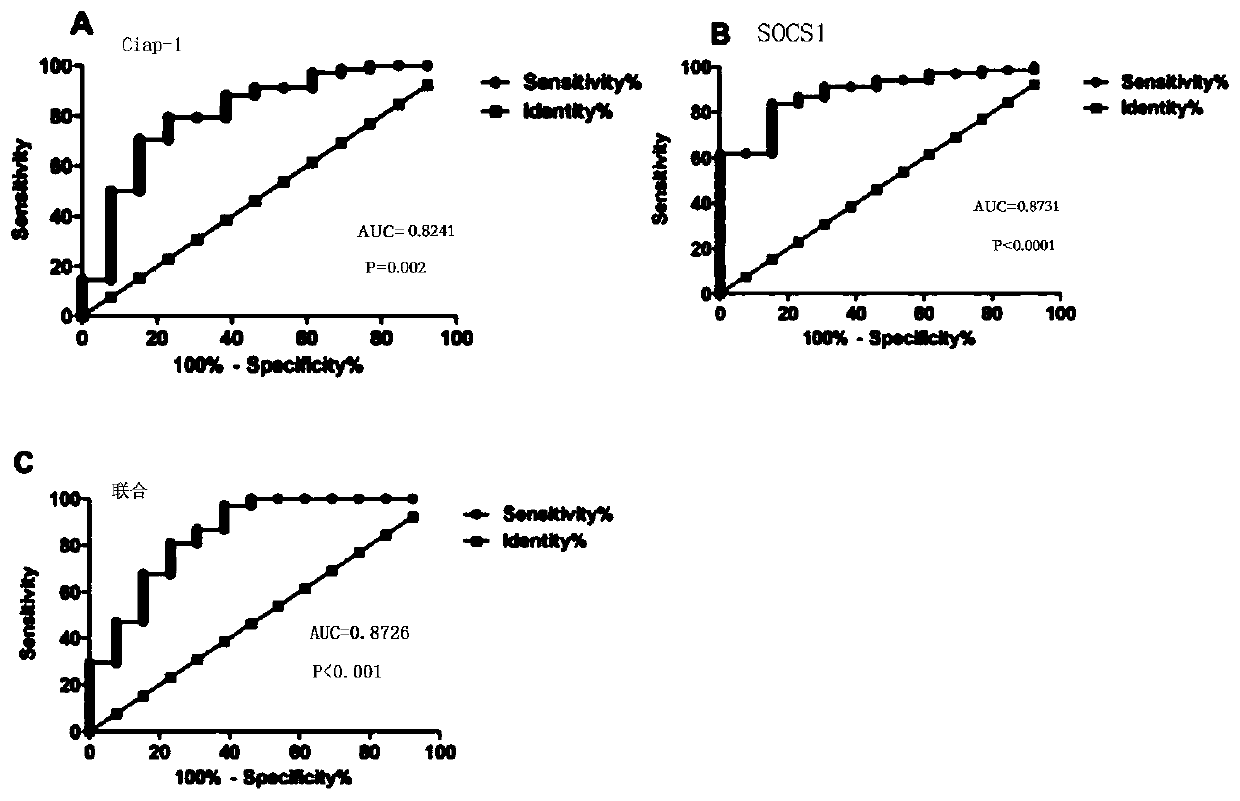
The invention provides a maker for predicting bladder cancer chemosensitivity. The marker is a combinationof SOCS1 and CYLD genes. According to the marker for predicting the bladder cancer chemosensitivity and the marker being the combination of the SOCS1 and the CYLD genes, the jointly-predicted area under a ROC curve by the combination of the SOCS1 and the CYLD genes is 0.9641, sensitiveness reaches 92.51%, and specificity reaches 91.31%. According to the marker for predicting the bladder cancer chemosensitivity and application, sensibility of a bladder cancer patient to chemotherapy drug gemcitabine can be quickly and accurately mastered by a clinician, the foundation is laid for the clinic treatment effect, help is provided for discoveringnovel drug targets which have potential treatment values and enhance the sensibility of the chemotherapy drug gemcitabine, and good application values and prospects are achieved.
Owner:昆明医科大学第二附属医院
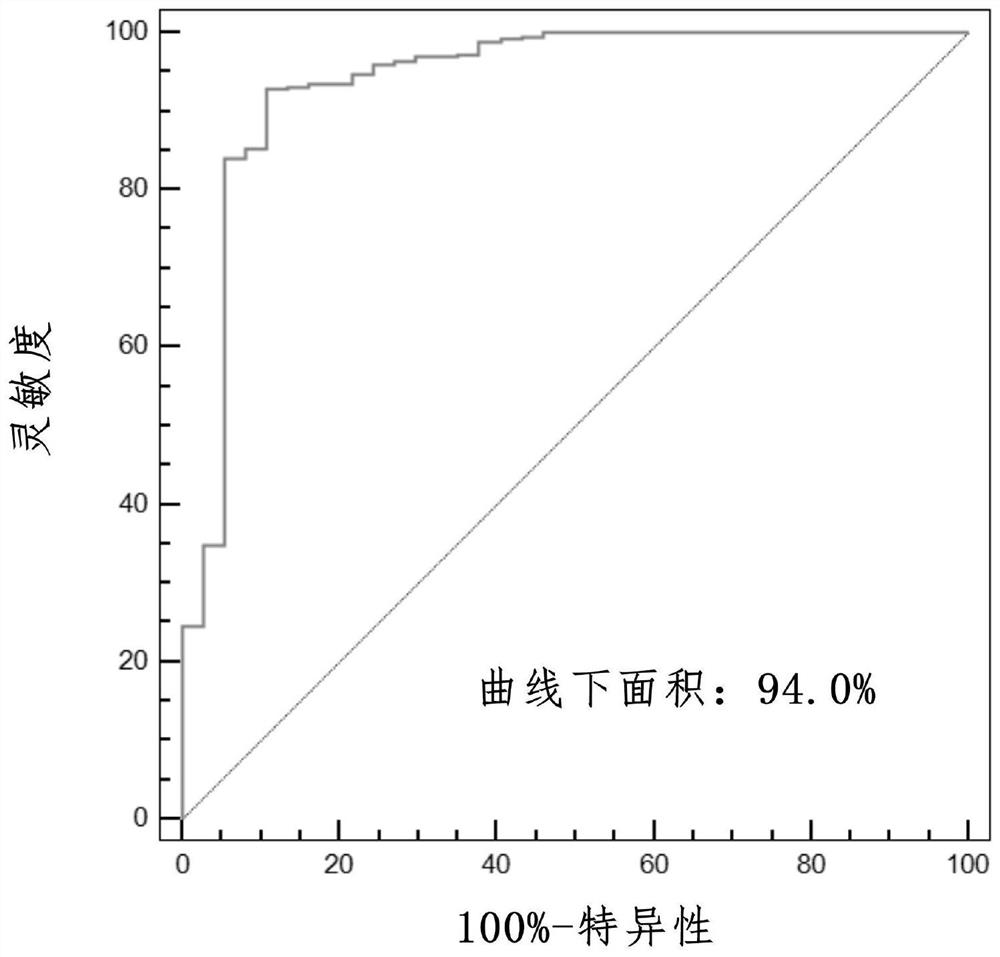
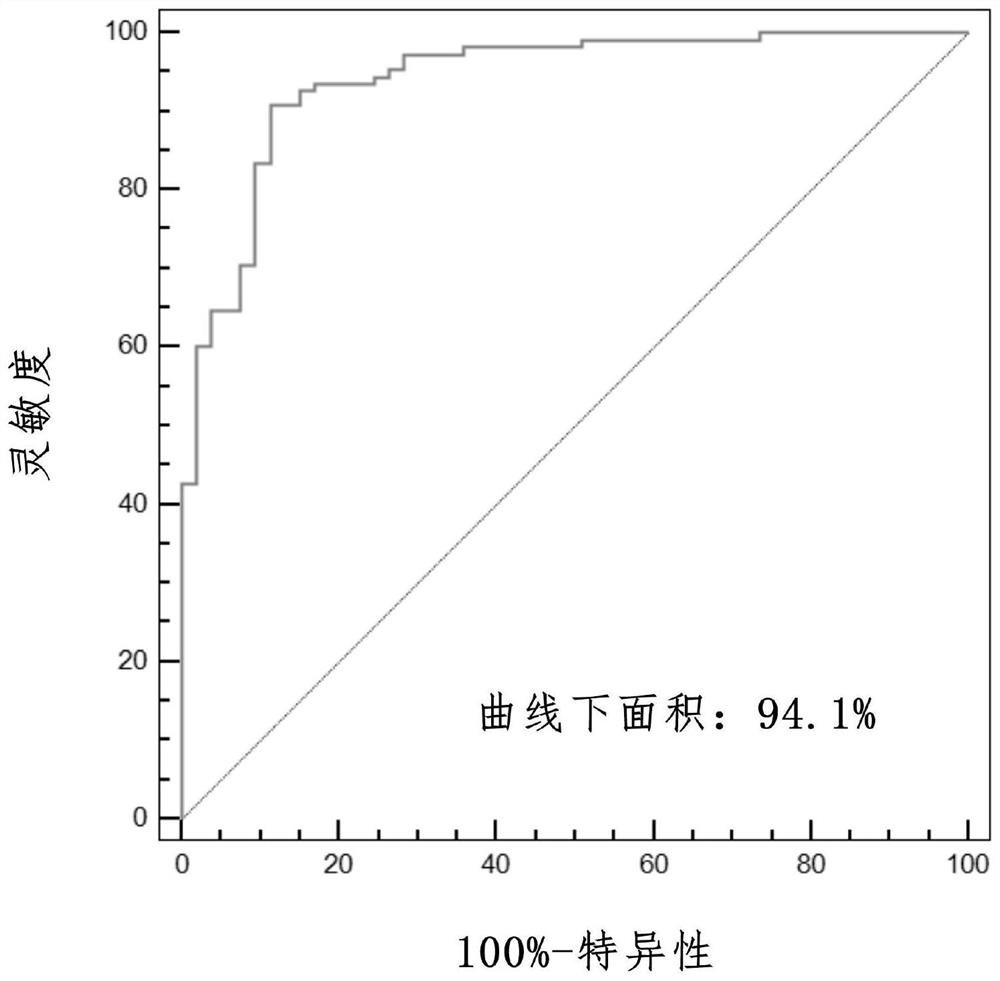
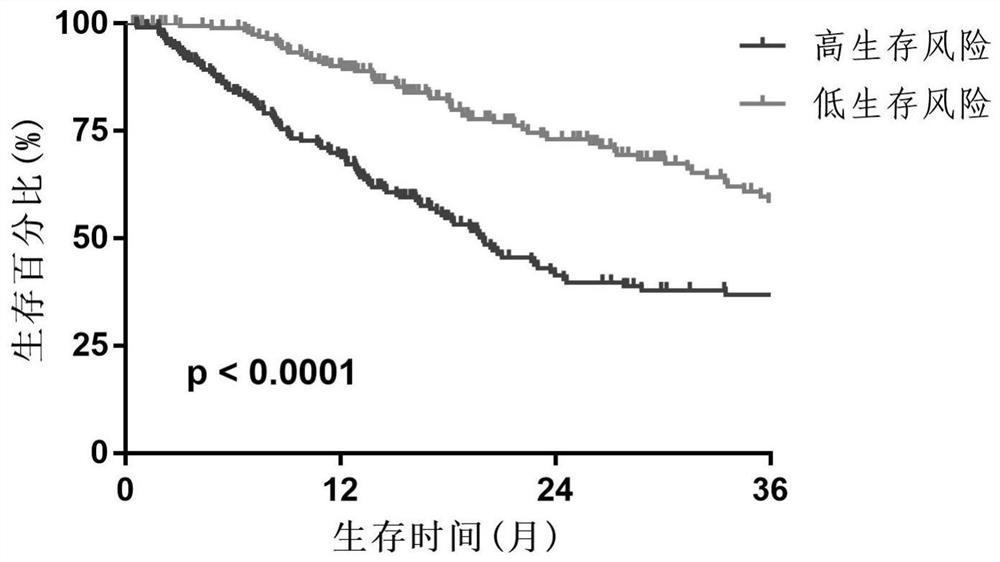
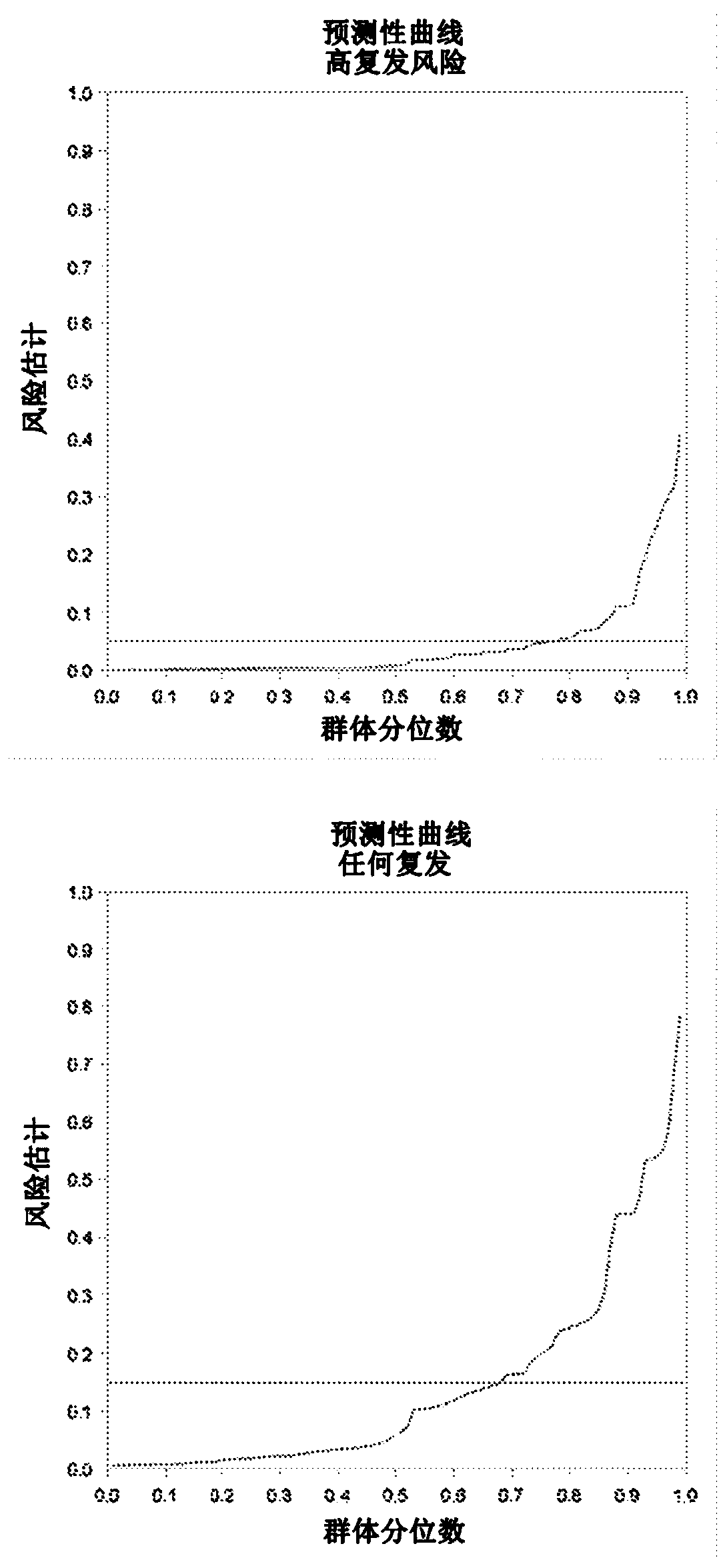
Prognosis model for total survival rate of bladder cancer patient
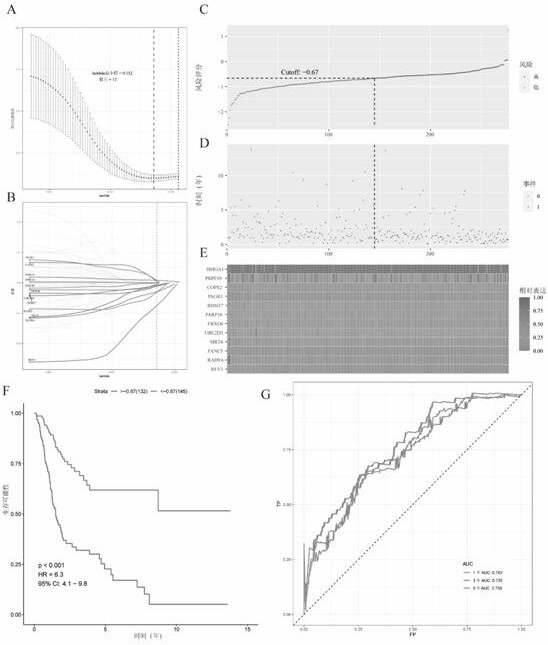
ActiveCN112725454APredictive validationMicrobiological testing/measurementProteomicsBladder cancer patientRisk groups
The invention belongs to the technical field of bioengineering and tumor markers, and relates to a prognosis model for the total survival rate of a bladder cancer patient, in particular to a prognosis model capable of predicting the total survival rate of the bladder cancer patient based on 12 DNA repair related genes. According to the invention, a prognosis model with 12 DRRGs is established, and bladder cancer patients are divided into high-risk and low-risk groups. The risk score of the bladder cancer patient in the training queue is significantly related to the OS (Plt, 0.001, HR = 6.3 [4.1, 9.8]). ROC curve analysis shows that AUC is 0.763 and 0.735 and 0.735 and 0.735 respectively in follow-up visits in one year, three years and five years. The prediction performance has been verified in the test set.
Owner:SHANDONG PROVINCIAL HOSPITAL AFFILIATED TO SHANDONG FIRST MEDICAL UNIVERSITY
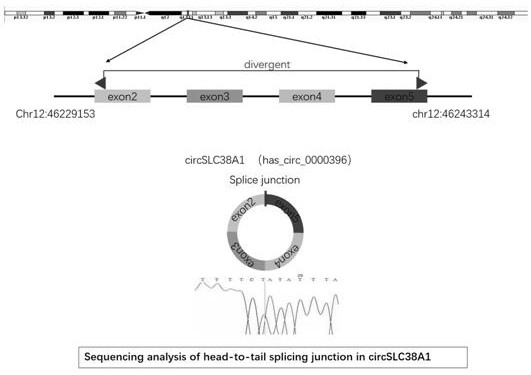
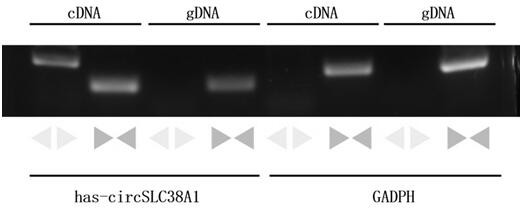
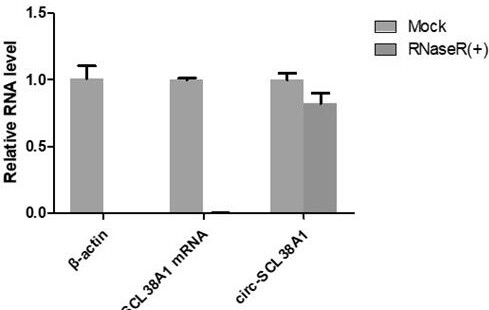
Application of circ-SLC38A1 as target point in medicine for inhibiting bladder cancer cells
ActiveCN112011620AInhibit migrationAbility to inhibit invasionOrganic active ingredientsMicrobiological testing/measurementCancer cellBladder cancer patient
The invention belongs to the technical field of tumor biological gene therapy, and relates to a bladder cancer diagnosis and treatment biomarker and a target spot, in particular to application of circ-SLC38A1 as a target spot in a bladder cancer cell inhibition medicine. The invention discloses an expression condition of circ-SLC38A1 in tumor tissues of a bladder cancer patient and a function of circ-SLC38A1 in bladder cancer cells. It is found for the first time that circ-SLC38A1 can be used as a bladder cancer diagnosis biomarker and a drug therapy target. Compared with normal control, circ-SLC38A1 is significantly up-regulated in tumor tissues of bladder cancer patients. The result of the invention shows that interference on circ-SLC38A1 can inhibit the migration and invasion ability ofbladder cancer cells, and it is shown that the circ-SLC38A1 plays a role of a cancer-promoting gene in the occurrence and development of bladder cancer, and provides a new target for clinical treatment of bladder cancer.
Owner:THE SECOND HOSPITAL OF SHANDONG UNIV
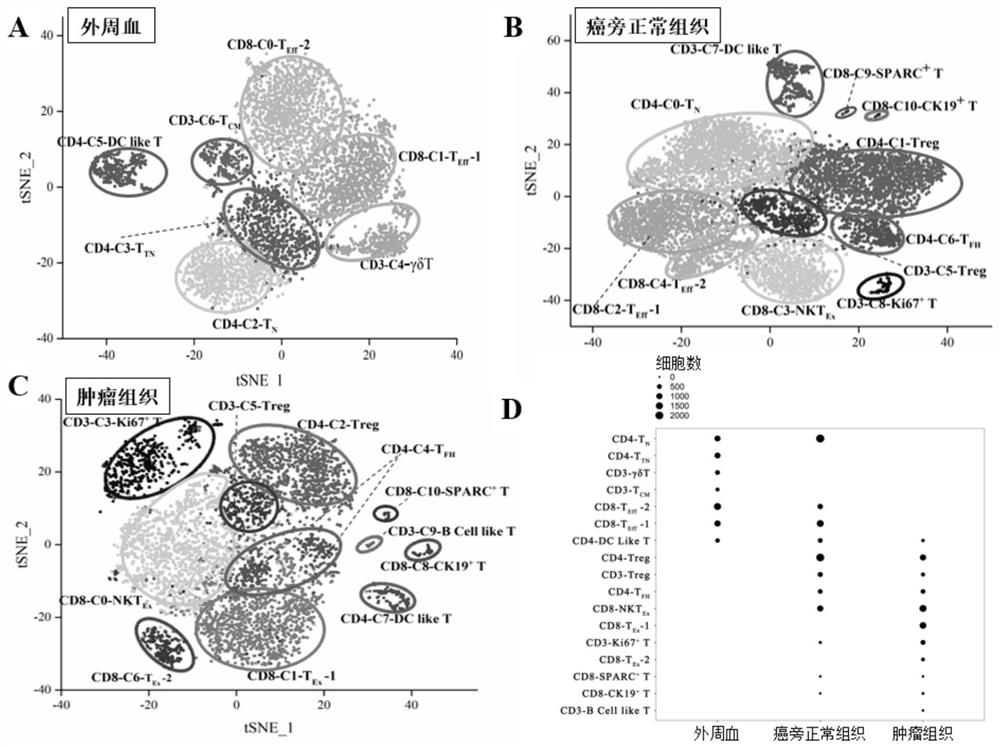
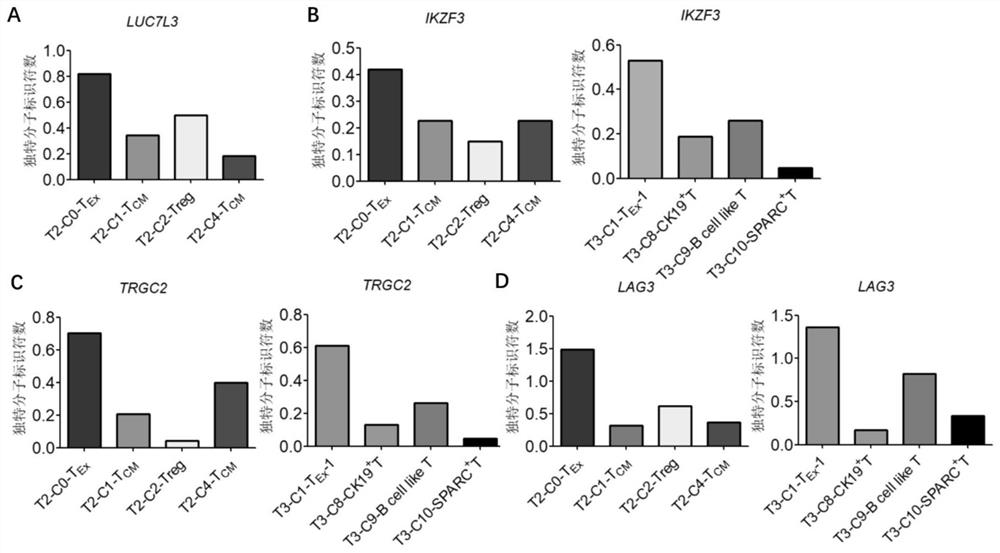
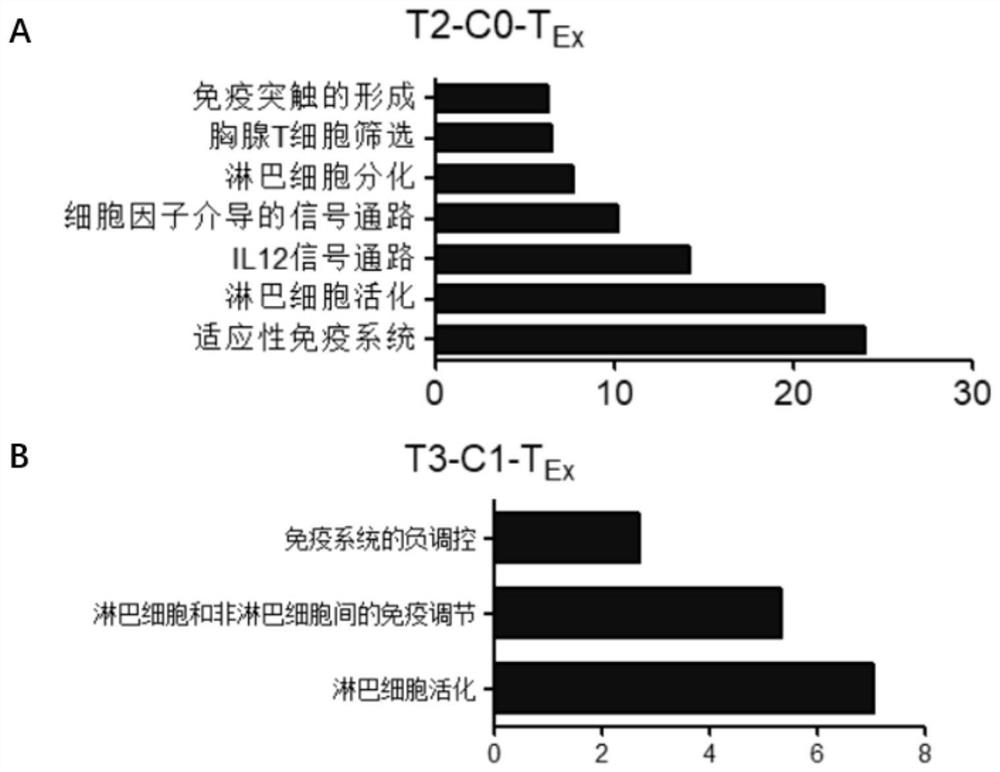
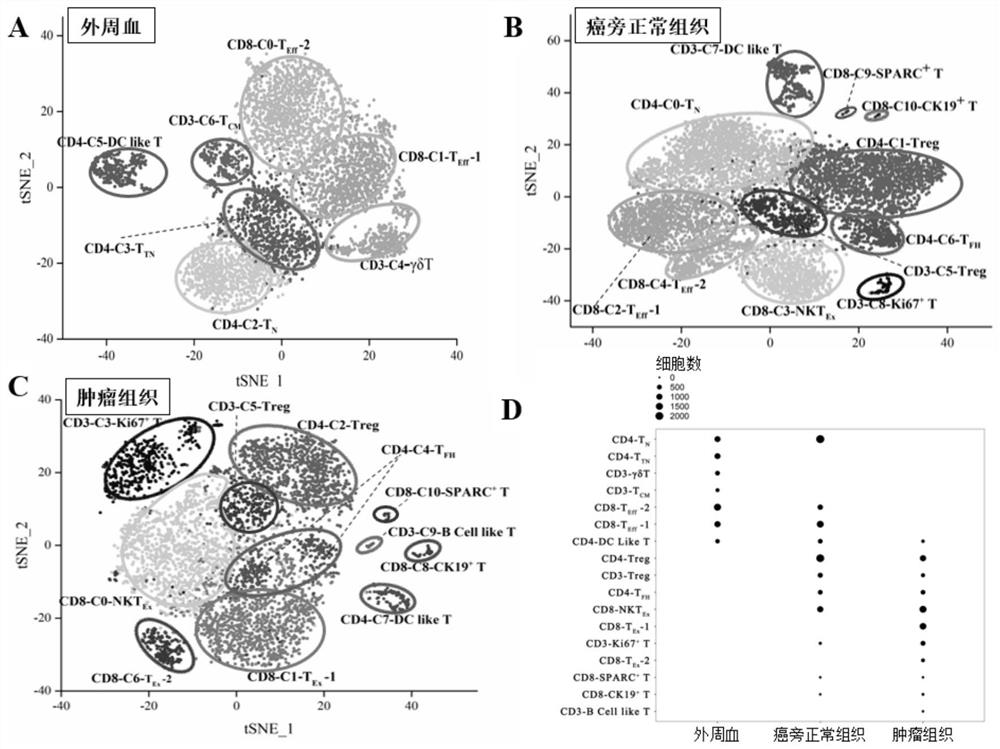
Bladder cancer exhausted T cell subset as well as characteristic genes and application thereof
InactiveCN111748627AMicrobiological testing/measurementBlood/immune system cellsSingle cell transcriptomeCD8
According to the invention, single-cell transcriptome sequencing is carried out on peripheral blood, normal bladder tissues and bladder cancer tissue-derived T cells of an infiltrated bladder cancer patient by utilizing a single-cell transcriptome sequencing analysis technology, a bladder cancer specific T cell subset, namely CD8<+> exhausted T cells, is identified, and characteristic genes, namely genes LUC7L3, IKZF3 and TRGC2, expressed by the CD8<+> exhausted T cells are determined. The characteristic genes can be used for identifying or authenticating CD8<+> exhausted T cells from bladdercancer, can be further used for auxiliary diagnosis, prognosis and immunotherapy of bladder cancer, and provide related detection reagents or kits.
Owner:BEIJING UNIV OF CHEM TECH
Bladder cancer pathomics intelligent diagnosis method based on machine learning and prognosis model thereof
PendingCN112435743AAlleviate shortagesEffective automatic pathological diagnosisMedical simulationMedical data miningMicroscopic imageBladder cancer patient
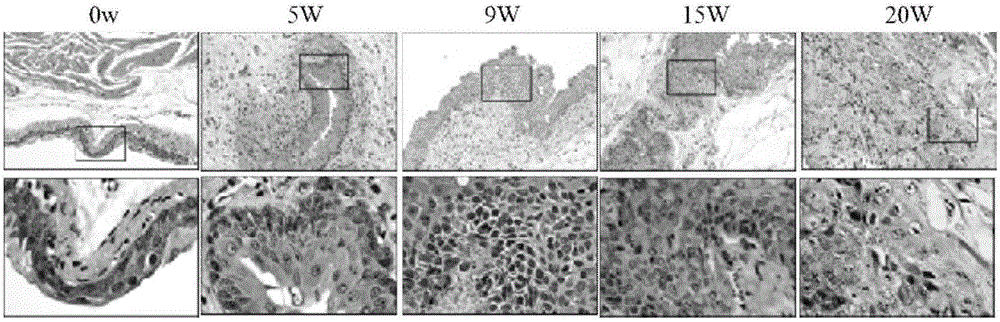
The invention relates to a bladder cancer pathomics intelligent diagnosis method based on machine learning and a prognosis model thereof, and the method is characterized in that the method comprises the following steps: S1, preforming data acquisition; S2, processing a microscopic pathology image; S3, performing bladder cancer pathological image feature extraction; S4, constructing and inspectinga bladder cancer automatic pathological diagnosis model based on machine learning; and S5, constructing and checking a bladder cancer survival prognosis prediction model based on machine learning. Thebladder cancer pathomics intelligent diagnosis method has the advantages that the bladder cancer pathomics intelligent diagnosis method is constructed based on pathological section microscopic imagemachine learning, effective automatic pathological diagnosis can be achieved, pathological diagnosis is expected to be further promoted to be developed to the efficient and accurate field, and the current situation that domestic pathology physicians are in shortage is relieved; the postoperative survival condition of the bladder cancer patient can be efficiently and accurately predicted, and important guidance opinions are provided for clinical decisions of clinicians.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Method for diagnosis and prognosis of epithelial cancers
InactiveUS8685659B2Easy diagnosisQuick and easy and safeBiological material analysisBacteriuriaBladder cancer patient
The present invention is based on the discovery that three proteins, Cystatin B, Chaperonin 10, and Profilin are present in the urine of patients with bladder cancer, a cancer of epithelial origin. Accordingly, the present invention is directed to methods for prognostic evaluation of cancers of epithelial origin and to methods for facilitating diagnosis of cancers of epithelial origin by monitoring the presence of these markers in biological samples. The invention is also directed to markers for therapeutic efficacy.
Owner:THE GENERAL HOSPITAL CORP +1
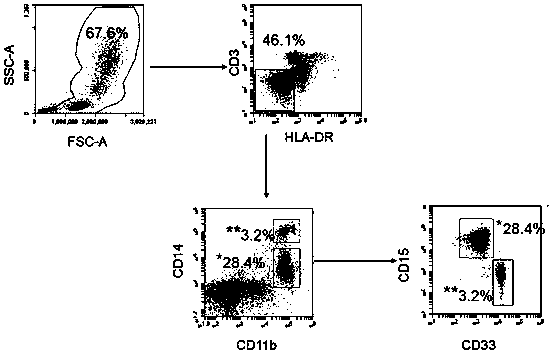
Use of granulocyte type myeloid derived suppressor cell as diagnostic biomarker
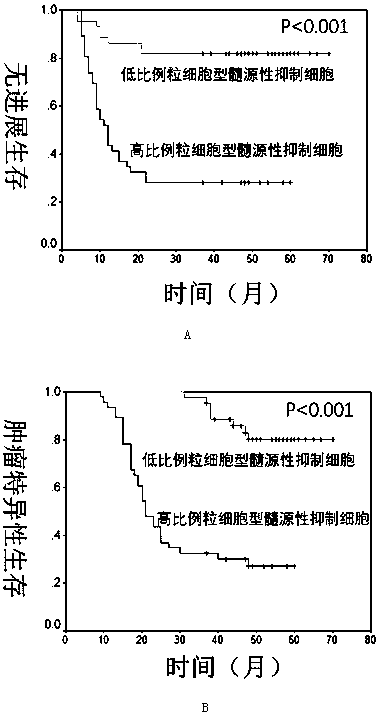
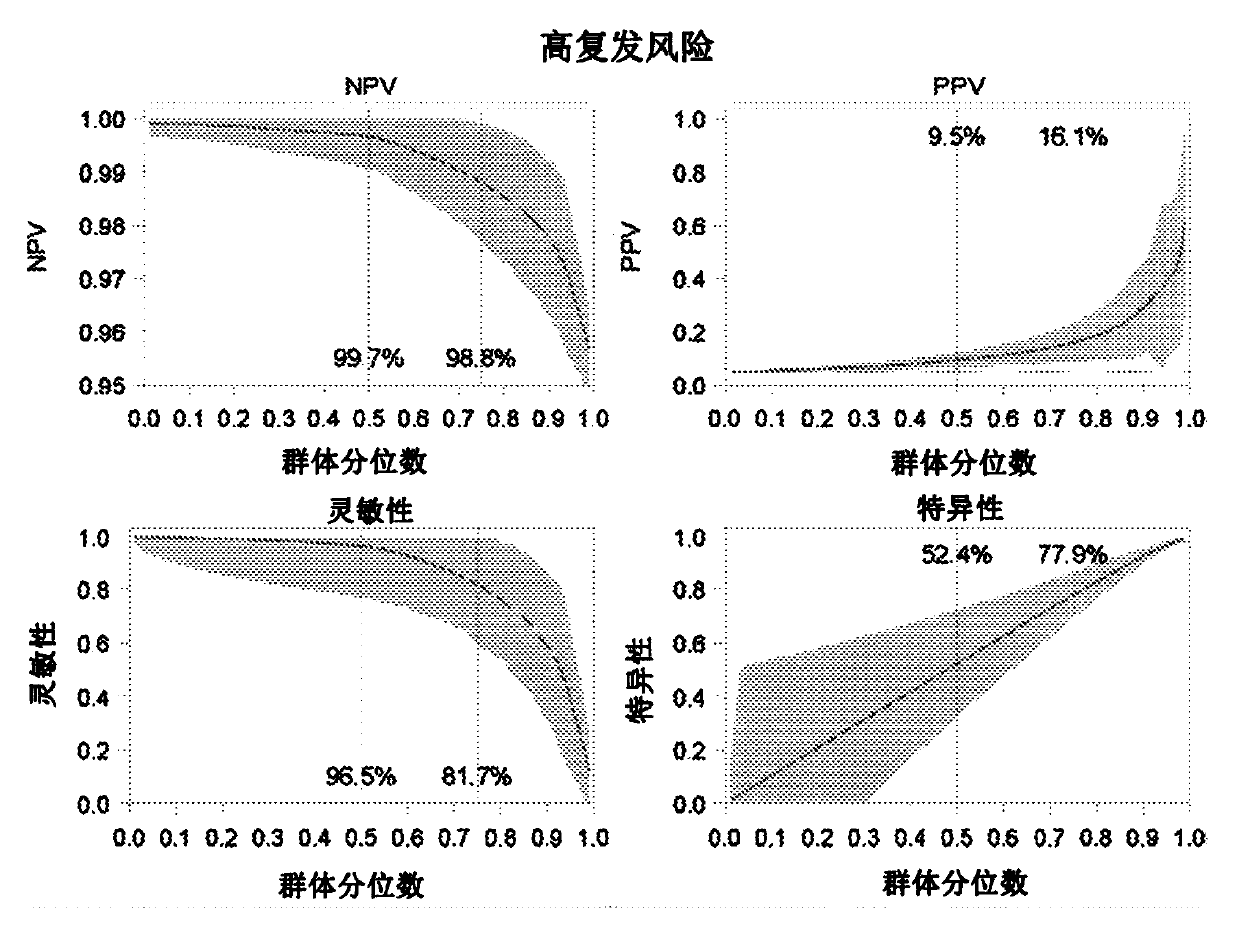
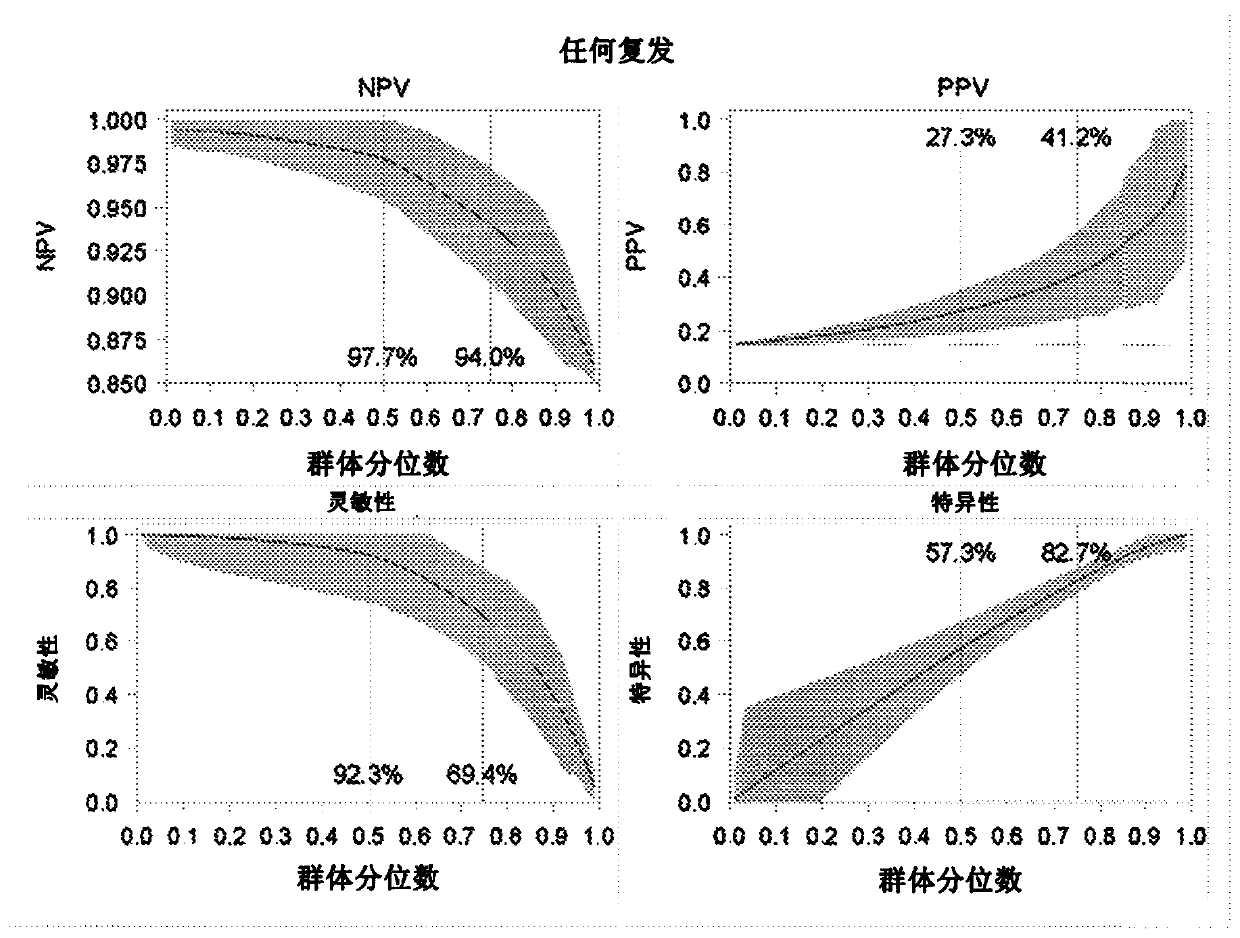
InactiveCN108254301ASignificant technological progressImprove survival rateIndividual particle analysisBladder cancer patientImproved survival
The invention provides use of a granulocyte type myeloid derived suppressor cell in the preparation of a diagnostic biomarker for diagnosing and predicting a neoadjuvant chemosensitivity effect of muscle-invasive bladder cancer. The invention further provides use of the granulocyte type myeloid derived suppressor cell in the preparation of a kit for diagnosing and predicting the neoadjuvant chemosensitivity effect of the muscle-invasive bladder cancer. The invention further provides a kit for diagnosing and predicting the neoadjuvant chemosensitivity effect of the muscle-invasive bladder cancer. The kit contains a reagent for detecting the granulocyte type myeloid derived suppressor cell. The invention discovers the granulocyte type myeloid derived suppressor cell as a novel biomarker fordiagnosing and predicting the neoadjuvant chemosensitivity effect of the muscle-invasive bladder cancer. A muscle-invasive bladder cancer patient with low-content granulocyte type myeloid derived suppressor cells can be sequentially subjected to neoadjuvant chemosensitivity and total cystectomy operation, so that the survival rate is increased, the excessive treatment is avoided, and the operationopportunity is not delayed.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
DNA methylation and mutational analysis methods for bladder cancer surveillance
PendingCN110621788AMicrobiological testing/measurementDisease diagnosisDNA methylationGenes mutation
The present disclosure relates to methods of monitoring bladder cancer patients and analyzing patient samples for presence of methylated DNA and optionally particular gene mutations. In some embodiments, analysis results are correlated with clinical outcome measures such as risk of bladder cancer recurrence.
Owner:基美健有限公司
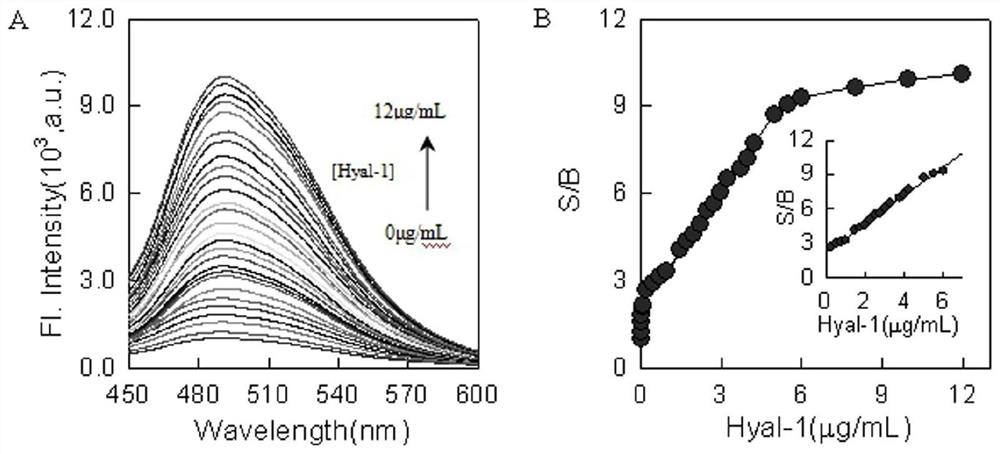
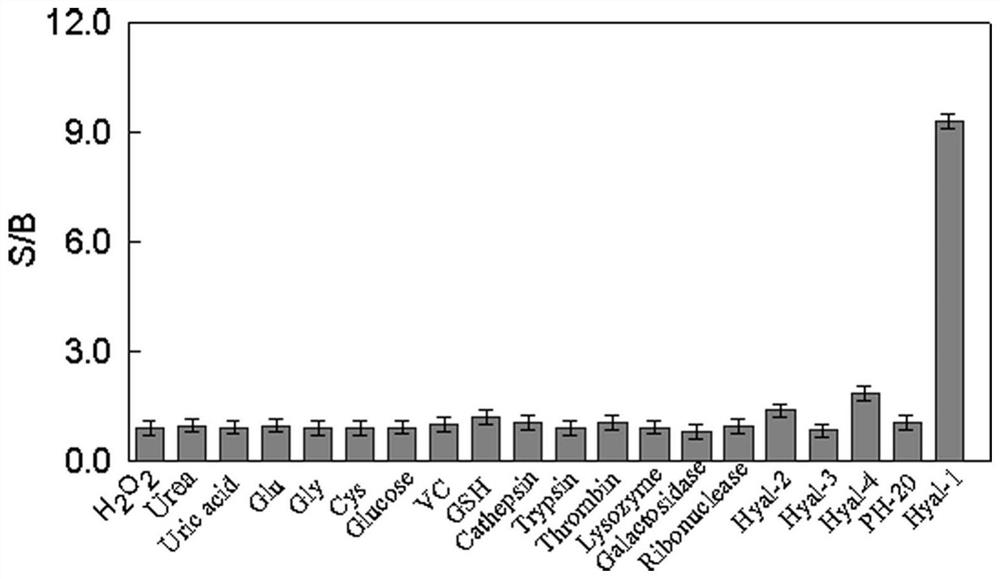
Preparation and application of type I hyaluronidase fluorescent nano sensor
ActiveCN112179875ARealize determinationOriginal and innovativeNanosensorsFluorescence/phosphorescenceBladder cancer patientHyaluronidase
The invention discloses a polymer nano sensor and application of the polymer nano sensor in detection of hyaluronidase type 1 (Hyal-1). Based on the principle that different hyaluronidase differentially degrades hyaluronic polymer nano-micelles, hydrogen bond induced enhanced dye molecules are wrapped in nano-micelle particles, and a high-selectivity and high-sensitivity Hyal-1 fluorescent nano-sensor is designed by utilizing stimulus-responsive nano-materials to adjust the interaction between dye and water molecules. A type I hyaluronidase detection kit comprises a type I hyaluronidase fluorescent nano sensor and a solvent. Results show that the kit has the advantages of high specificity, good sensitivity, simplicity and convenience in operation, economy, practicability and the like; andthe detection of Hyal-1 in an actual urine sample is realized. Therefore, the method provided by the invention has original innovativeness, good social values and application prospects, and is expected to be applied to detection and analysis of Hyal-1 in urine of bladder cancer patients.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method for Diagnosis of Bladder Cancer and Related Kits
InactiveUS20130203059A1Strong PTPD immunoreactive signalMicrobiological testing/measurementBiological material analysisBacteriuriaBladder cancer patient
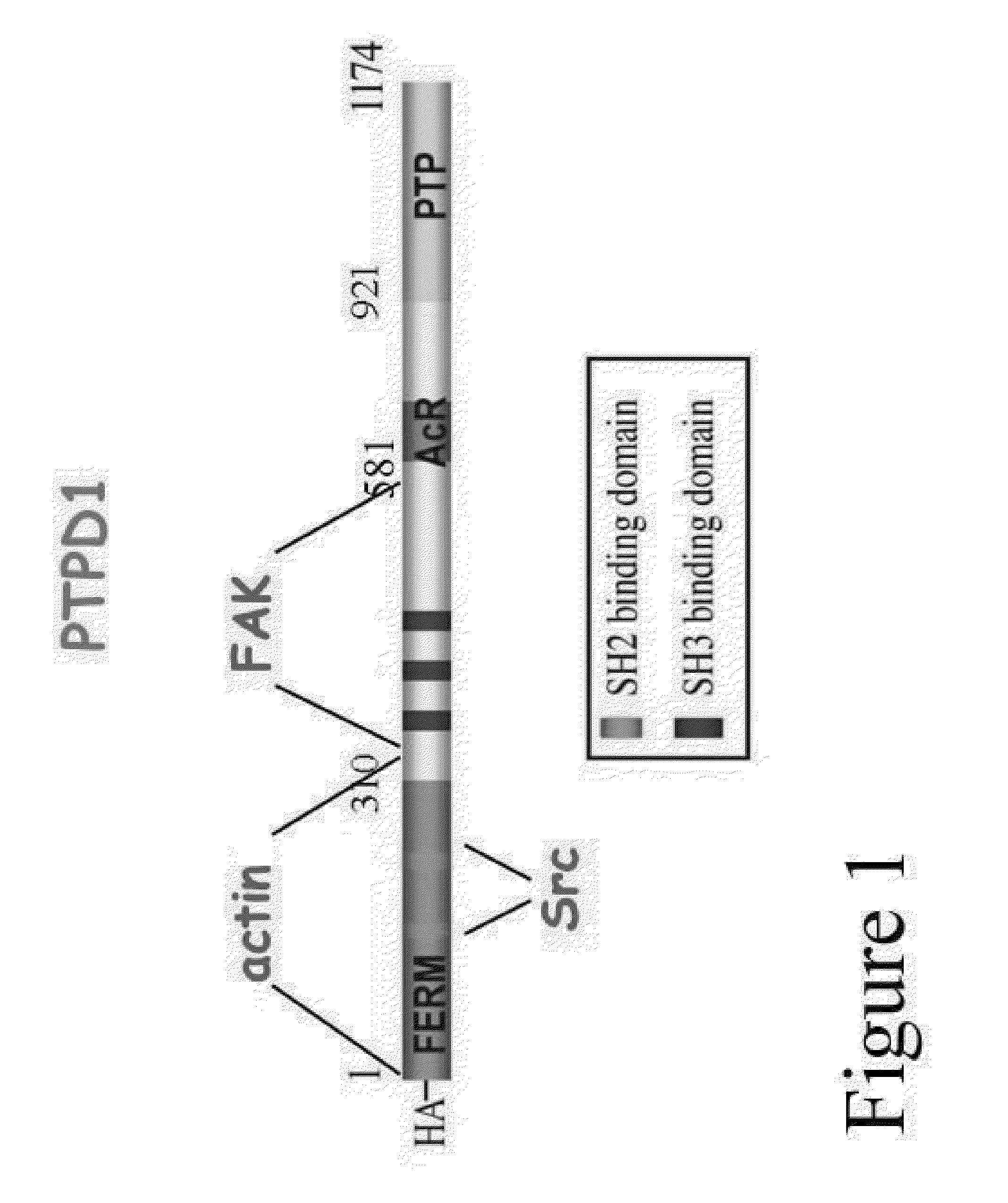
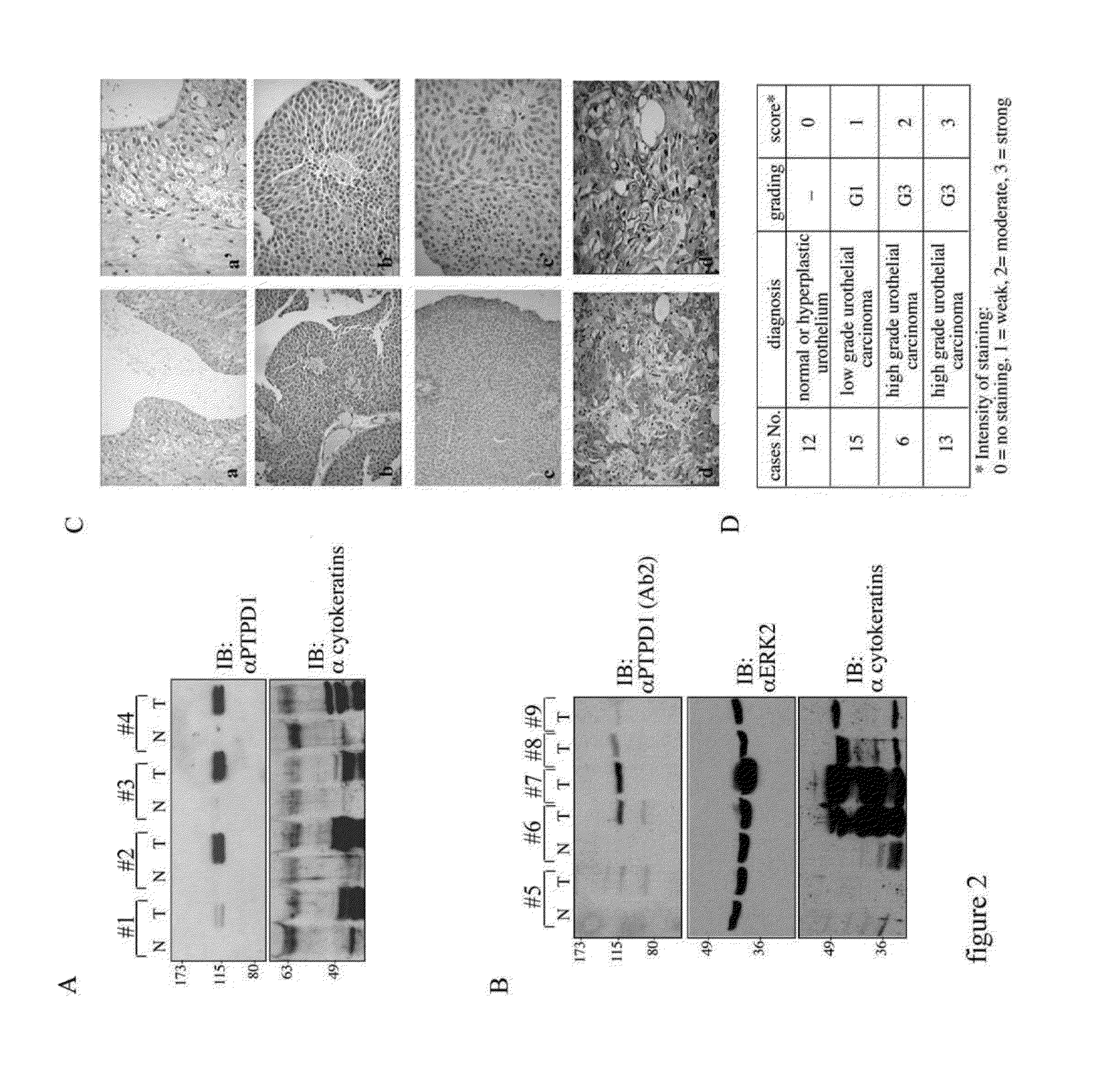
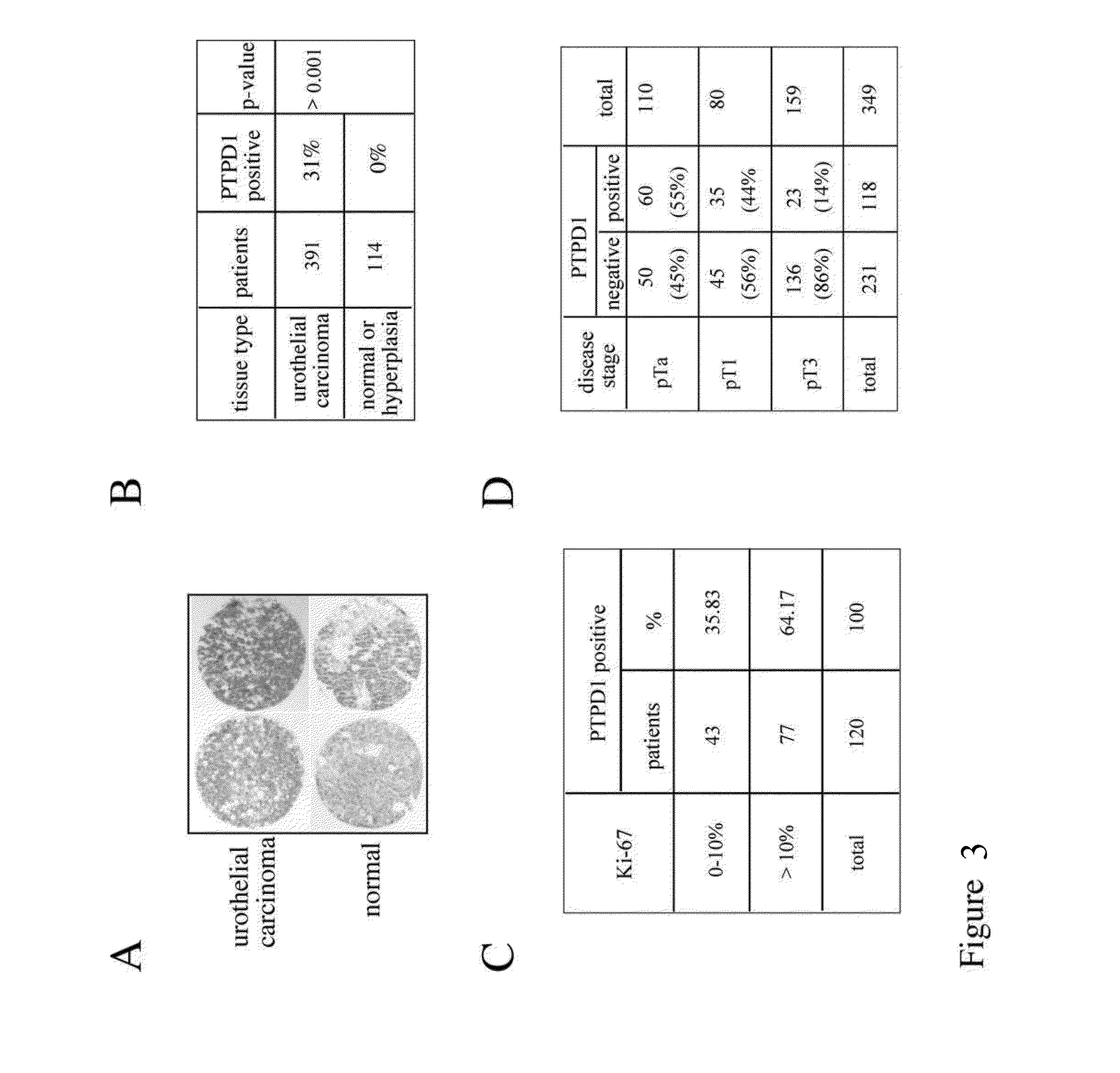
The invention refers to a novel molecular biomarker, namely PTPD1, that is markedly increased in human bladder cancers. PTPD1 expression positively correlated with the grading and invasiveness potential of these tumors. PTPD1 can be detected at high levels in exfoliated bladder cells isolated from urine of bladder cancer patients, while no PTPD1 signal was evident in normal exfoliated bladder cells. Thus, PTPD1 detection in urine samples may represent a novel and reliable marker for non-invasive diagnosis of aggressive bladder cancer.
Owner:TOPOGEN
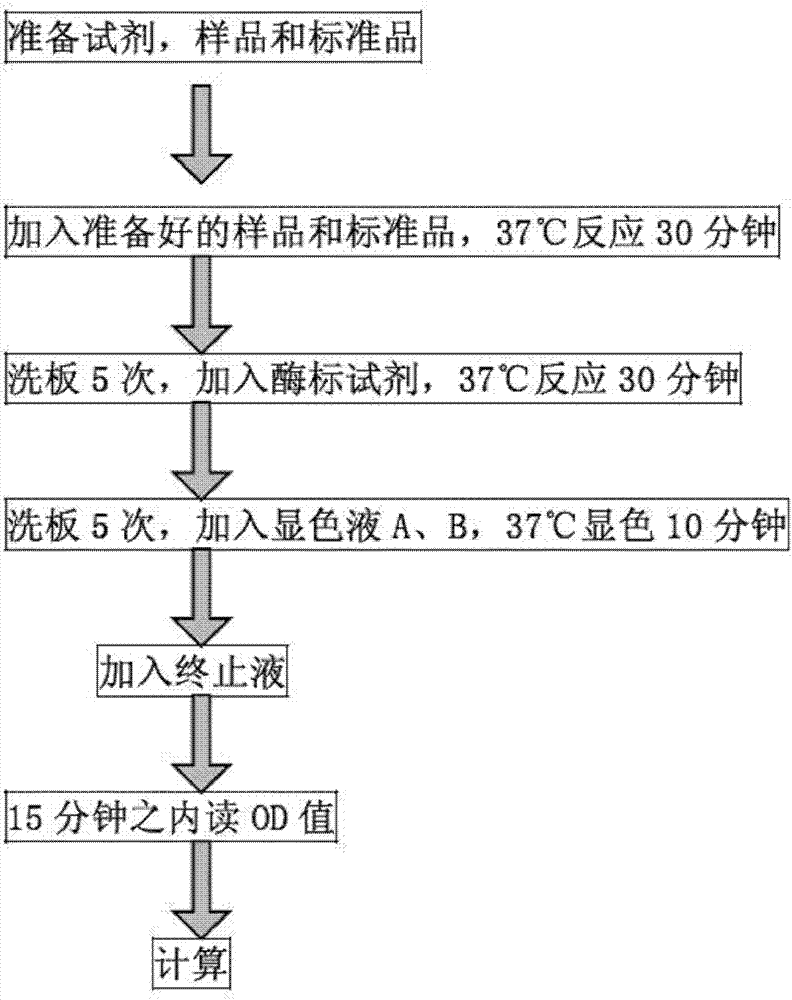
Method for detecting urine fibronectin, and kit thereof
InactiveCN102901824ARelieve painReduce financial burdenBiological testingBladder cancer patientPhases of clinical research
The invention relates to a method for detecting the urine fibronectin, and a kit thereof. The method comprises the steps of specimen acquisition, experiment preparation, sample addition, incubation, plate washing, enzyme addition, incubation, washing, color development and termination. A bladder cancer diagnosis technology having the advantages of simplicity, rapidness, non-invasion, low price, high sensitivity and strong specificity is established in the invention to determine the concentrations of the urine fibronectin of a bladder cancer patient in different pathological phases, and the index of the bladder cancer infiltration degree can be discriminated through the difference of the urine fibronectin.
Owner:沈周俊 +2
Application of bladder cancer prognosis lifetime marker, evaluation device and computer readable medium
ActiveCN114807377AGood forecastMicrobiological testing/measurementProteomicsGenomic sequencingBladder cancer patient
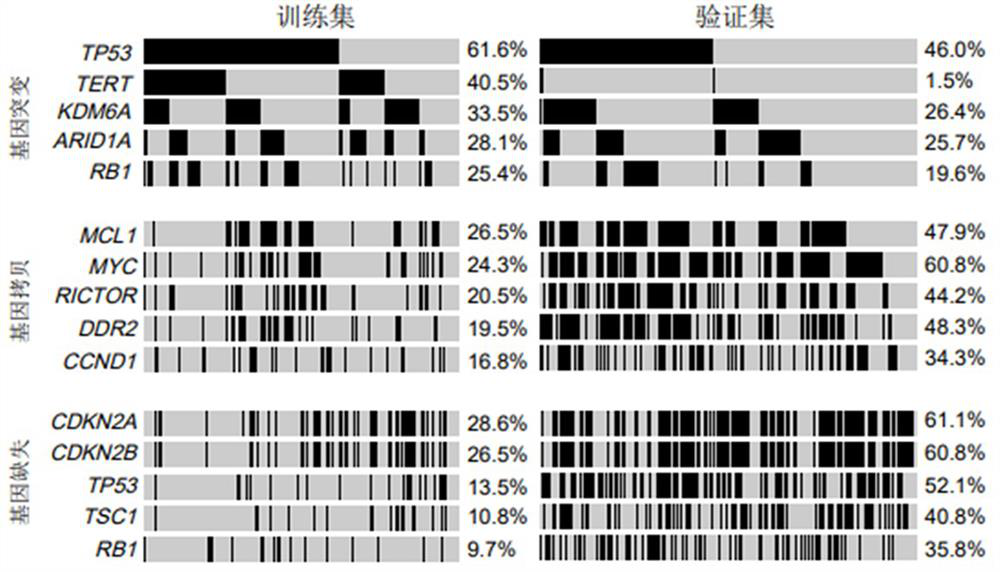
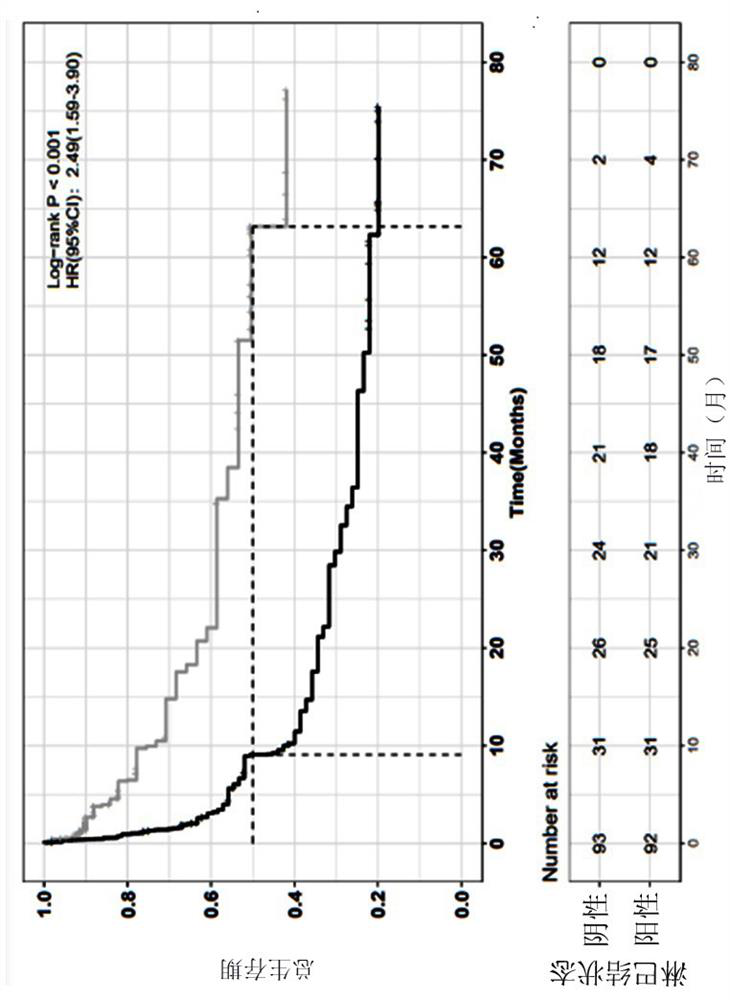
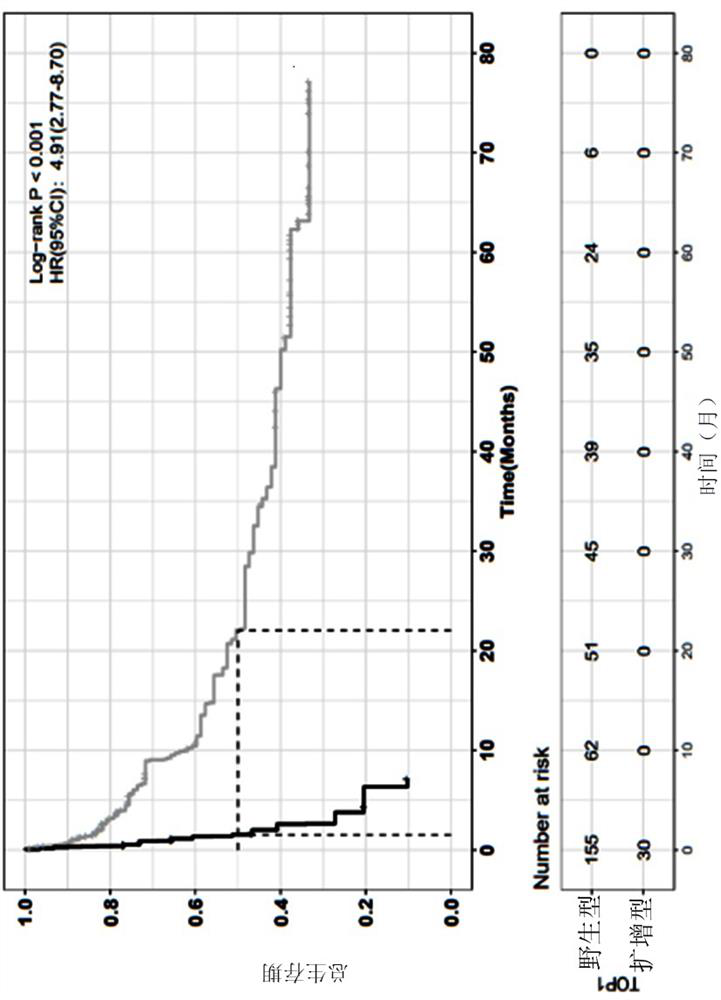
The invention relates to application of a bladder cancer prognosis lifetime marker, an evaluation device and a computer readable medium, and belongs to the technical field of tumor medicine. Single-factor and multi-factor analysis is carried out on genome sequencing data of tumor samples of a large number of bladder cancer patients, the correlation between each factor and the total lifetime is evaluated, a five-feature prognosis model including a lymph node state, TOP1 amplification, MYC amplification, CREBBP mutation and a CDKN2A deletion state is established, the model can effectively carry out prognosis stratification of the bladder cancer patients, and the prognosis stratification of the bladder cancer patients is realized. A reference basis is provided for clinical diagnosis and treatment.
Owner:GENESEEQ TECH INC +1
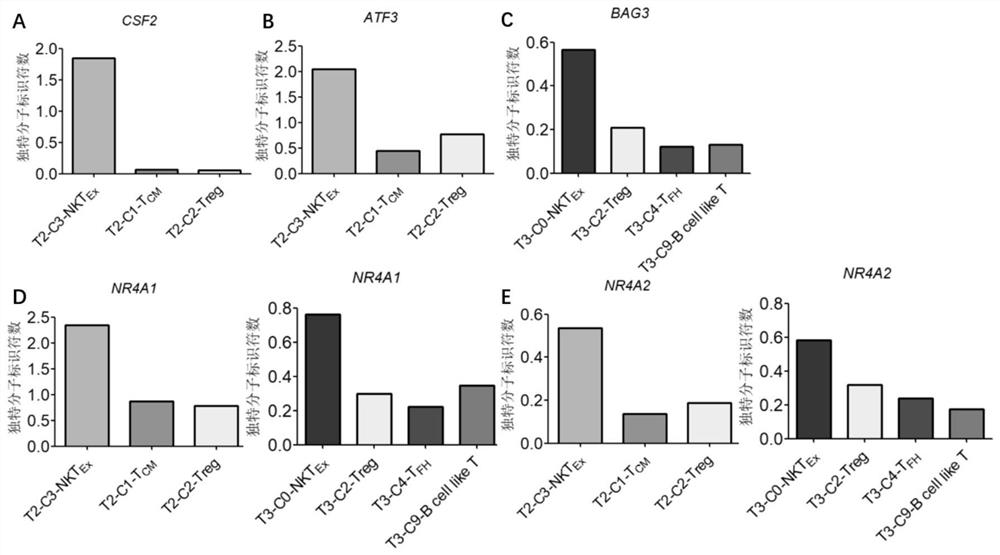
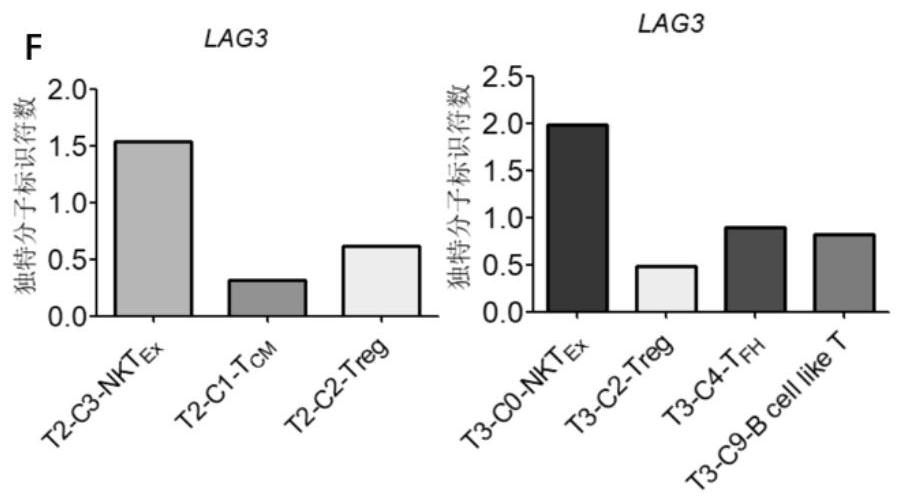
Bladder cancer depleted NKT cell subset, characteristic gene and application thereof
PendingCN111733244AMicrobiological testing/measurementDisease diagnosisSequence analysisSingle cell transcriptome
The invention discloses a bladder cancer depleted NKT cell subset, a characteristic gene and application thereof. The bladder cancer depleted NKT cell subset is characterized in that a single cell transcriptome sequencing analysis technology is utilized to perform single cell transcriptome sequencing on NKT cells from peripheral blood, normal bladder tissues and bladder cancer tissues of an infiltrative bladder cancer patient, a bladder cancer specific NKT cell subset, namely CD8<+> depleted NKT cells, is identified, and characteristic genes expressed by the CD8<+> depleted NKT cells, namely genes CSF2, ATF3, BAG3, NR4A1 and NR4A2, are determined. Moreover, the characteristic genes can be used for identifying or identifying the CD8<+> depleted NKT cells from the bladder cancer, and furtherused for auxiliary diagnosis, prognosis and immunotherapy of the bladder cancer, and provide a related detection reagent or kit.
Owner:BEIJING UNIV OF CHEM TECH
Rapid detection kit for nuclear matrix protein 22 and application thereof
The invention belongs to the technical field of biological detection, and specifically relates to a rapid detection kit for nuclear matrix protein 22 and application thereof. The rapid detection kit for the nuclear matrix protein 22 disclosed by the invention mainly consists of a solid-phase carrier enveloped by a monoclonal antibody to the nuclear matrix protein 22, the monoclonal antibody to the nuclear matrix protein 22 labelled by horseradish peroxidase, acridinium ester, washing liquid and nuclear matrix protein 22 calibrator. The rapid detection kit for the nuclear matrix protein 22 disclosed by the invention can detect an expression level of the nuclear matrix protein 22 in body fluid of a patient after surgical treatment or recurrent and metastatic tumor patient, and has an important meaning for clinical diagnosis of bladder cancer; and the rapid detection kit for the nuclear matrix protein 22 disclosed by the invention has the advantages of high accuracy and stability in a detection result and good sensitivity, and is favorable for early diagnosis and prognosis treatment of the bladder cancer.
Owner:ANHUI IPROCOM BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com