Methods for diagnosis and prognosis of epithelial cancers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Proteomic Analysis of Voided Urine, Bladder Cancer Tissue and Cell Lines for Biomarker Discovery in Transitional Cell Carcinoma
Introduction
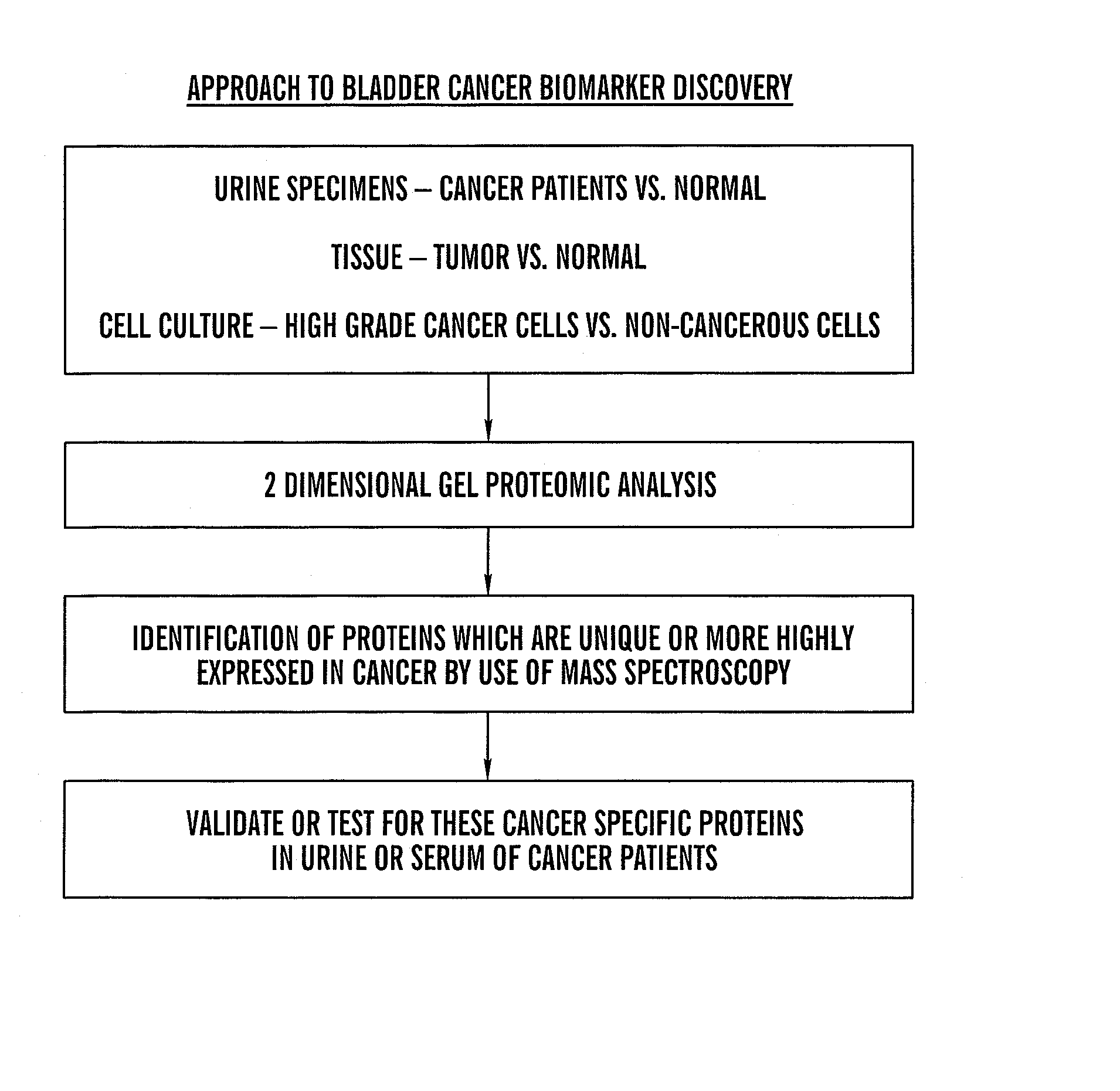
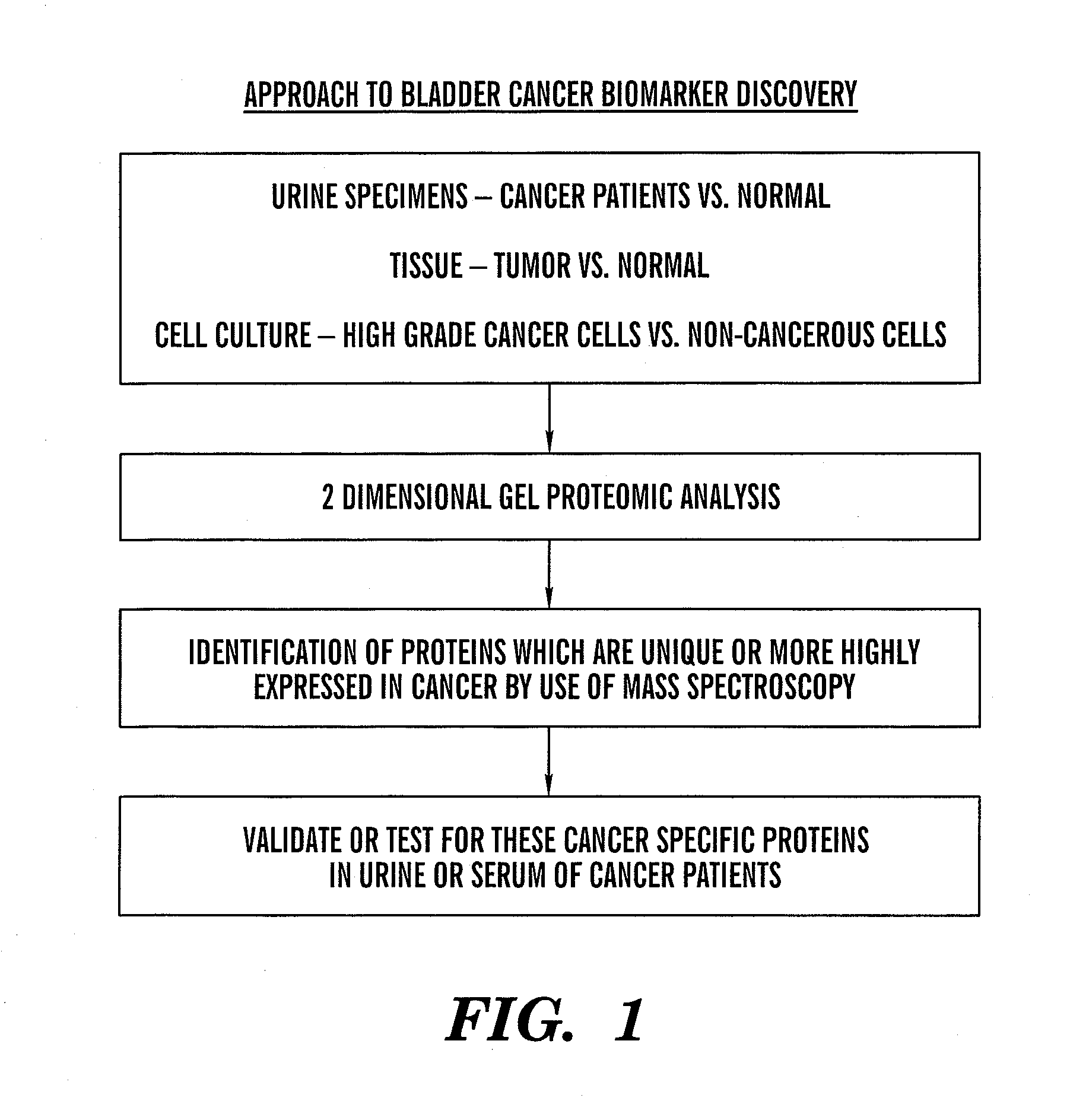
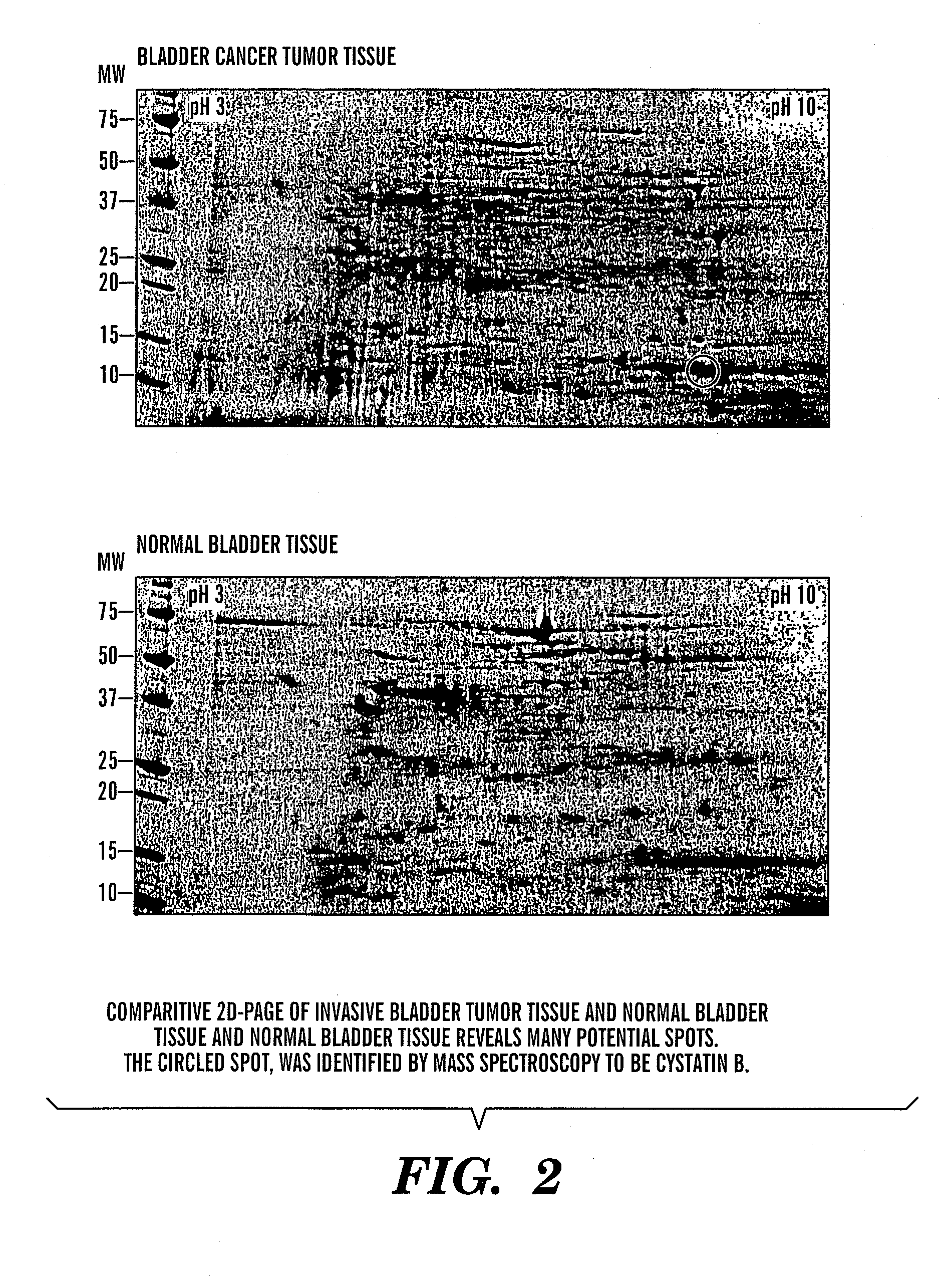
[0102] There is a need for new biomarkers to aid in the diagnosis and management of cancers of epithelial origin. Urine can serve as an excellent medium for epithelial cancer biomarker discovery and analysis. Proteomic analysis by two-dimensional polyacrylamide gel electrophoresis (2D PAGE) is one effective tool to analyze the proteome of human specimens. 2D PAGE was used to analyze voided urine, human bladder tumor and normal tissue, and human derived bladder cancer cell lines as a method for biomarker discovery.
Methods
[0103] Under IRB approved protocol, voided urine specimens were collected from sixty-three patients prior to diagnostic cystoscopy with biopsy and twenty-two age-matched control patients with no clinical evidence of bladder cancer and no history of malignancy. Total urinary protein was isolated and quantified. Equival...
example ii
Immunostaining for Cystatin B in Bladder Cancer Tissue
Methods
[0109] Normal bladder and bladder cancer tissue were immunostained using mouse monoclonal anti-cystatin B antibody and counterstained with Haematoxylin. Immunostaining was performed using the bladder cancer tissue microarray BL801 (US Biomax Inc, Rockville, Md.). The tissues were deparaffinized, endogenous peroxide blocked in 3% hydrogen peroxide in methanol, and microwave antigen retrieval performed using Antigen Unmasking Solution. Blocking was performed using 5% normal horse serum and endogenous biotin blocked using Avidin / Biotin kit. Tissue was incubated with mouse monoclonal anti-cystatin B / Stefin B antibody, clone A6 / 2 (GeneTex, Inc, San Antonio, Tex.), followed by anti-mouse biotinylated secondary antibody, amplified using ABC kit, and developed using DAB. Tissue was counterstained using Gill's Hematoxylin #3 (Sigma-Aldrich, St. Louis, Mo.), and blued using Tacha's Bluing Solution (Biocare, Concord, Calif.). All ...
example iii
Cystatin B as a Tissue and Urinary Biomarker of Bladder Cancer Recurrence and Disease Progression
Introduction
[0111] Bladder cancer is the second most common genitourinary malignancy in the United States. In 2006, there were an estimated 61,420 newly diagnosed cases of bladder cancer and an estimated 13,060 deaths due to cancer of the bladder (Jemal, A. et al. CA Cancer J Clin 2006;56: 106-30). Among all newly diagnosed cases, approximately 70% present as superficial tumors (stages Ta, T1, or Tis); however, up to 50-70% of those cases will recur after resection and approximately 10-20% will progress to invasive disease (T2 or greater) (Rubben, H. et al. J Urol 1988;139: 283-5). Pathologic data, including grade, stage, and associated carcinoma in-situ at initial presentation have provided some insight into the risk of disease progression to muscularis propria invasion (Althausen, A. F. et al. J Urol 1976;116: 575-80; Herr, H. W. J Urol 2000;163: 60-1). Nevertheless, the ability to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com