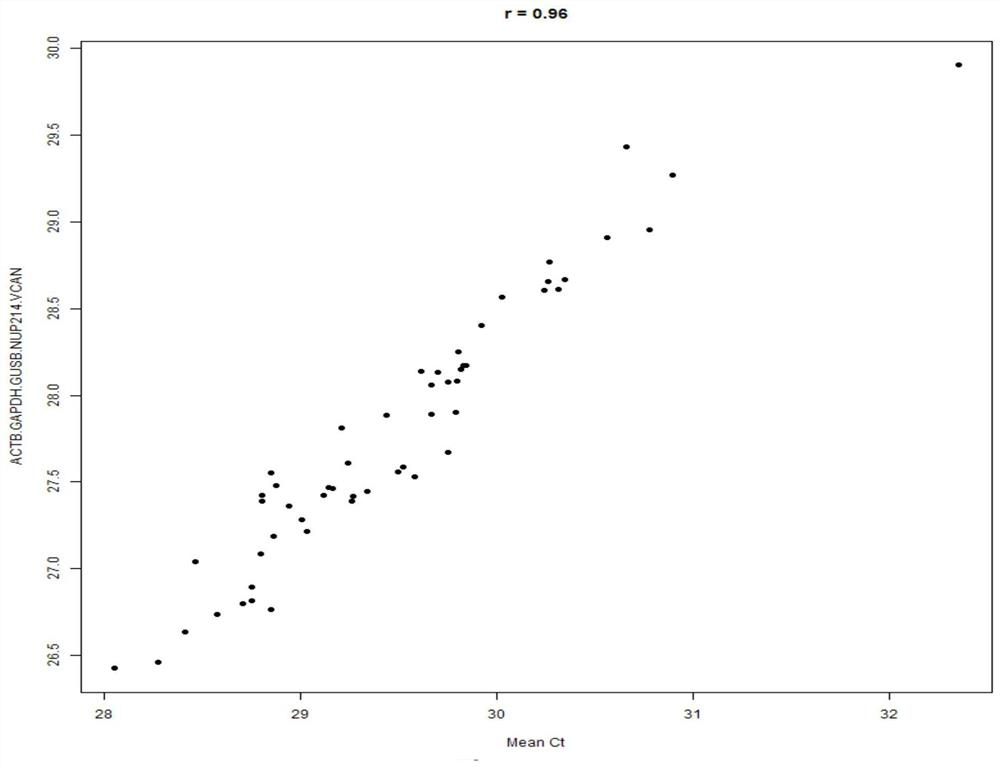
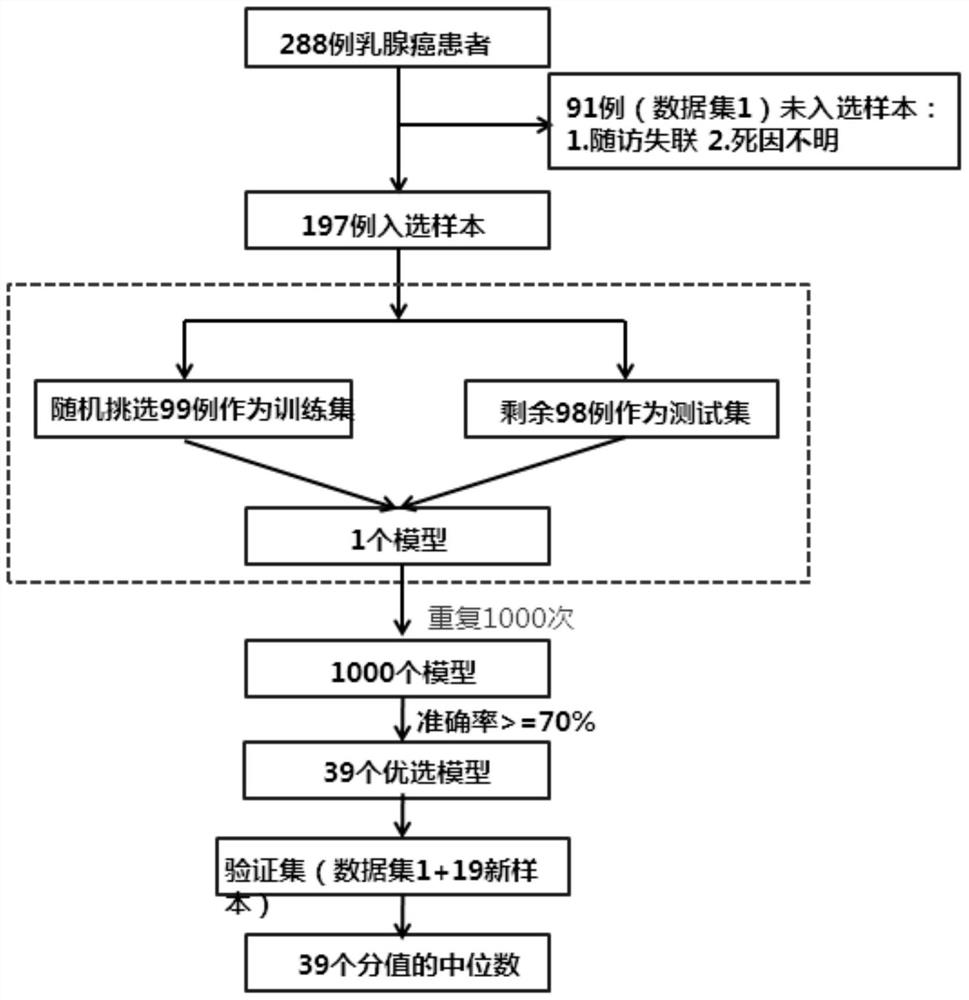
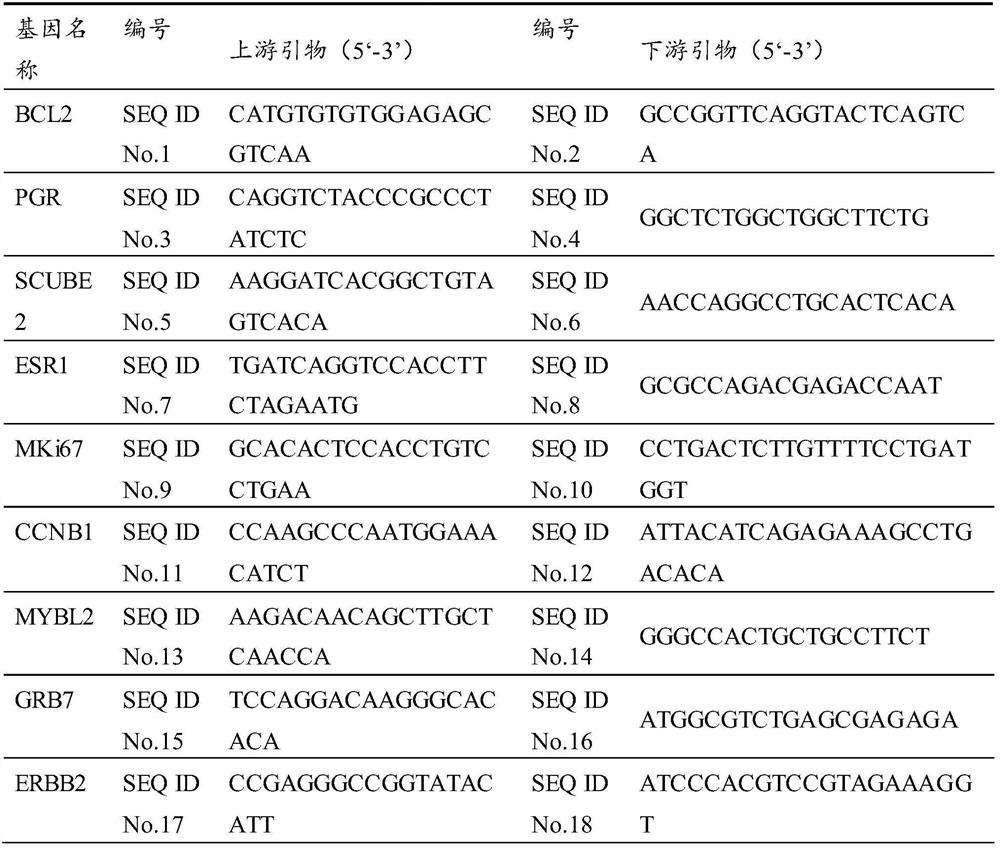
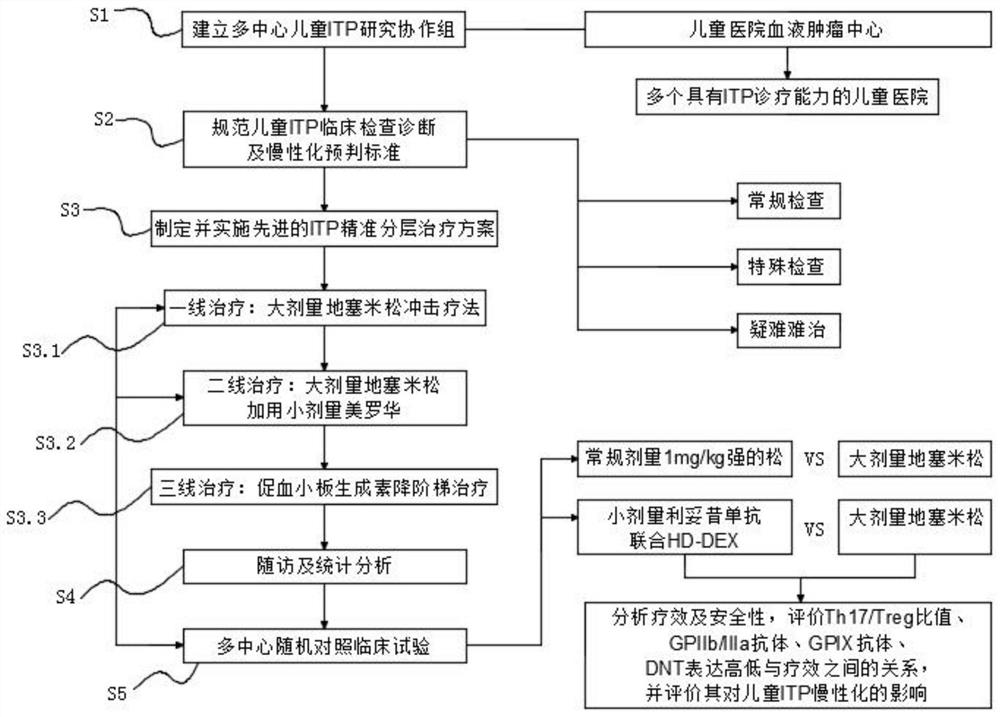
Patents
Literature
37 results about "Newly diagnosed" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Molecular determinants of myeloma bone disease and uses thereof
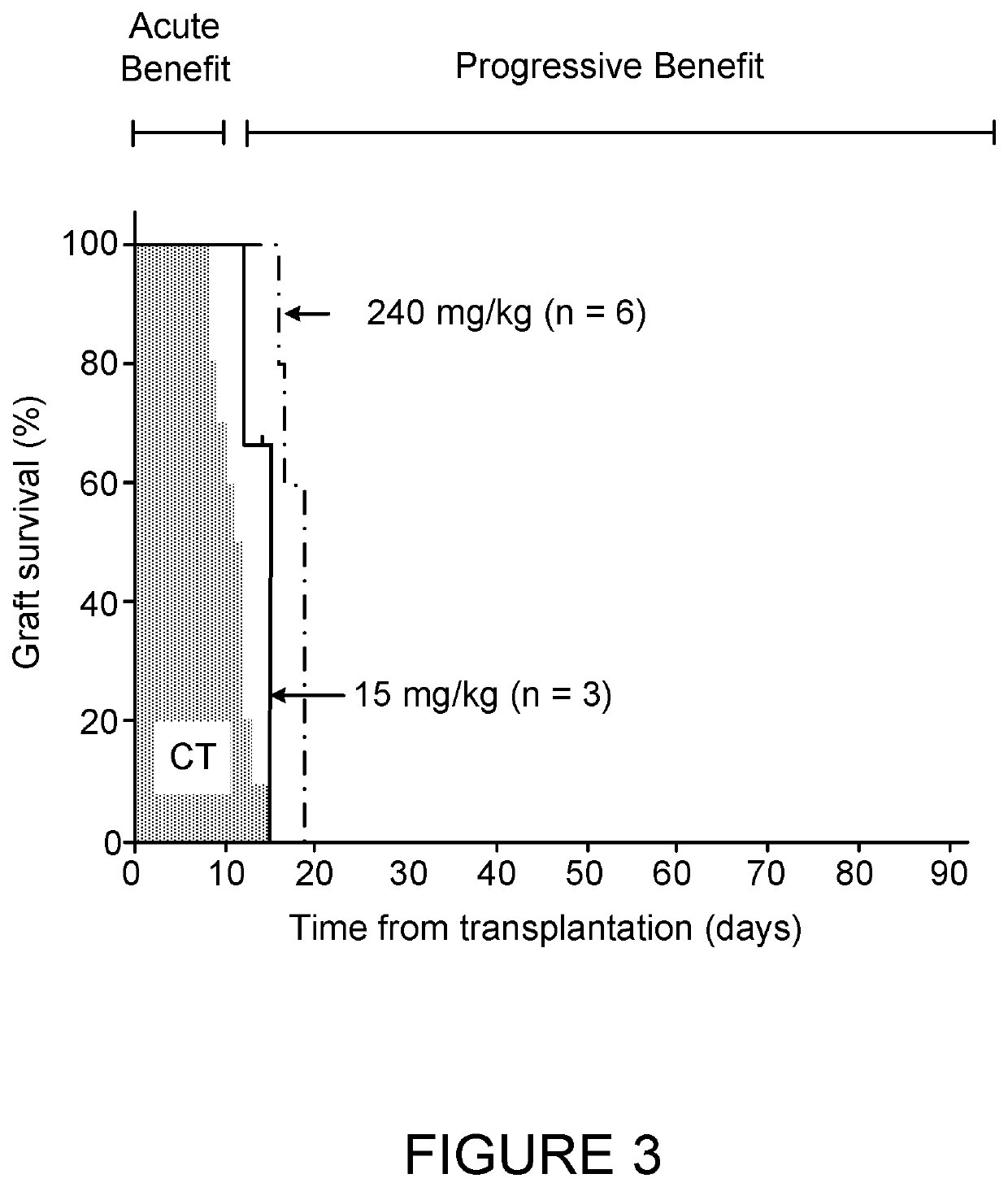
InactiveUS7642238B2Prevent and reverse bone lossPrevent bone lossPeptide/protein ingredientsMicrobiological testing/measurementNormal boneNewly diagnosed
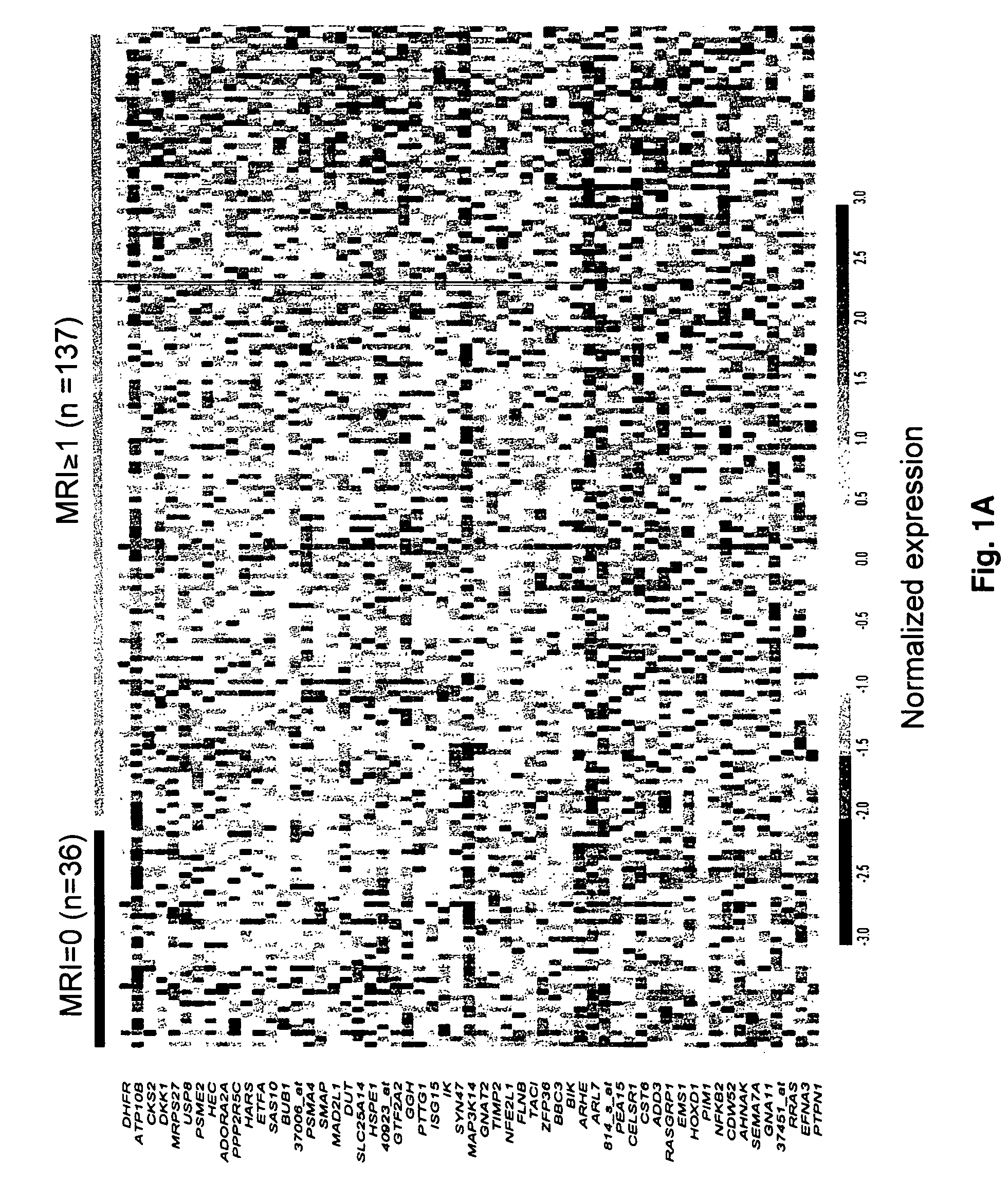
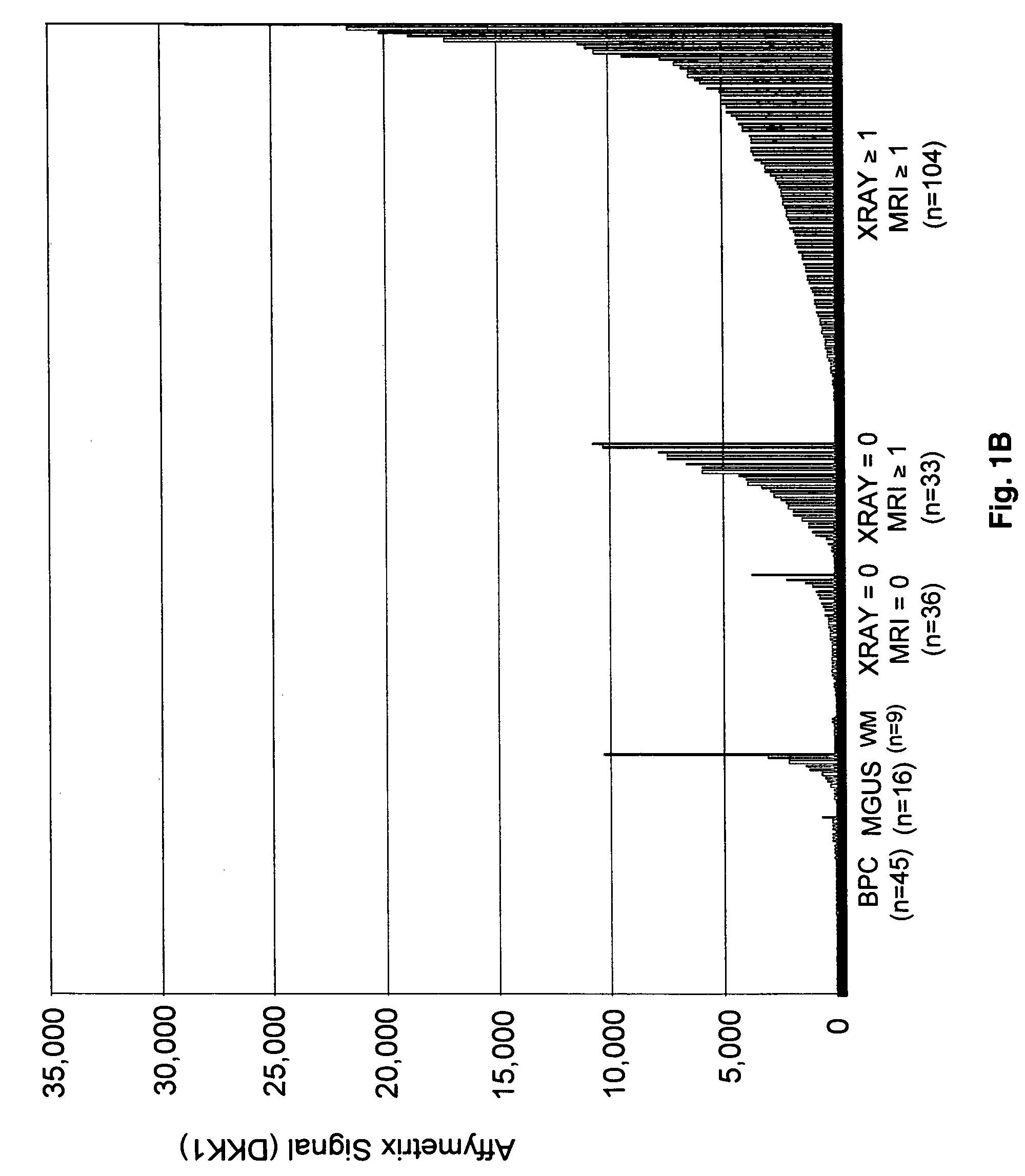
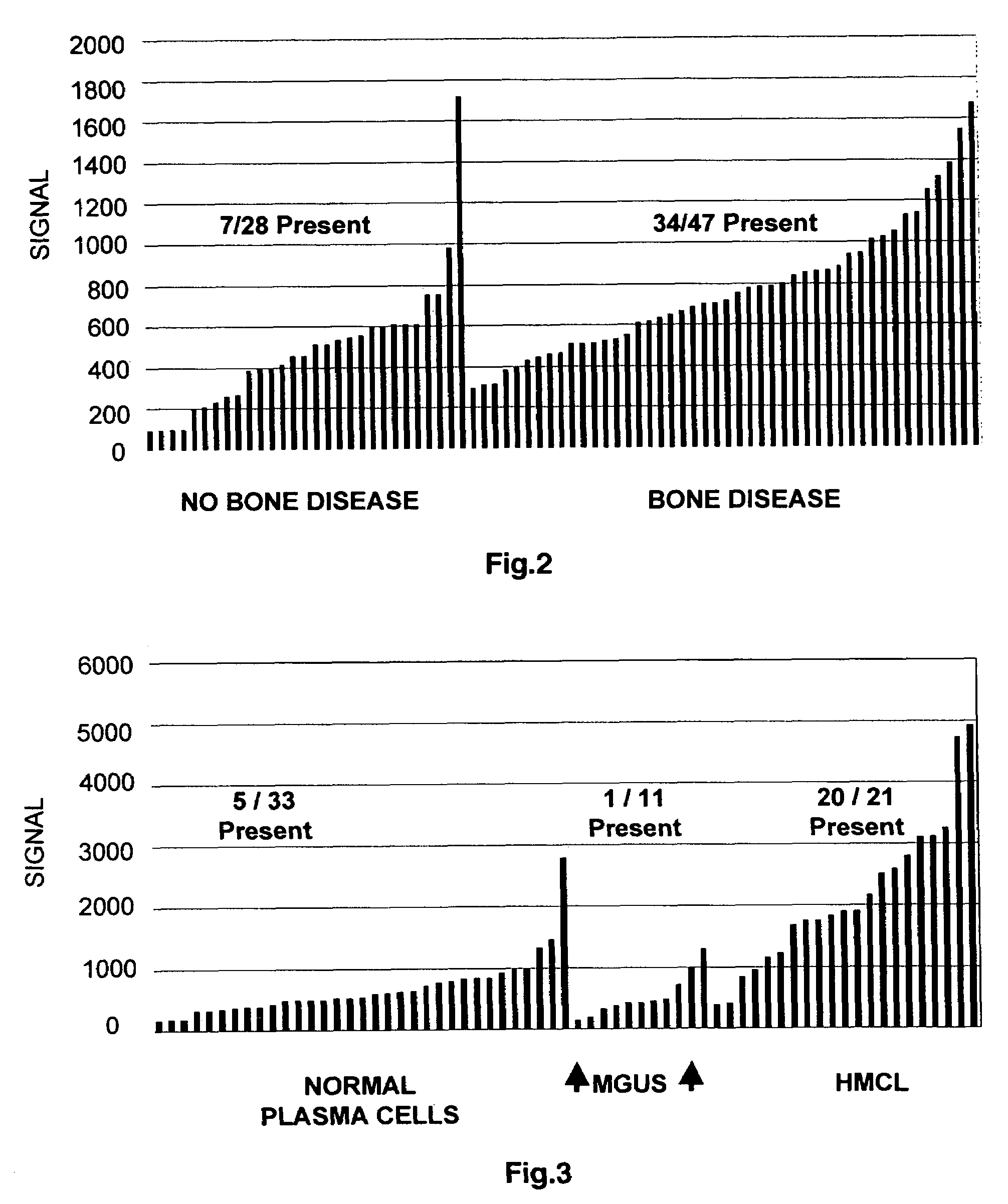
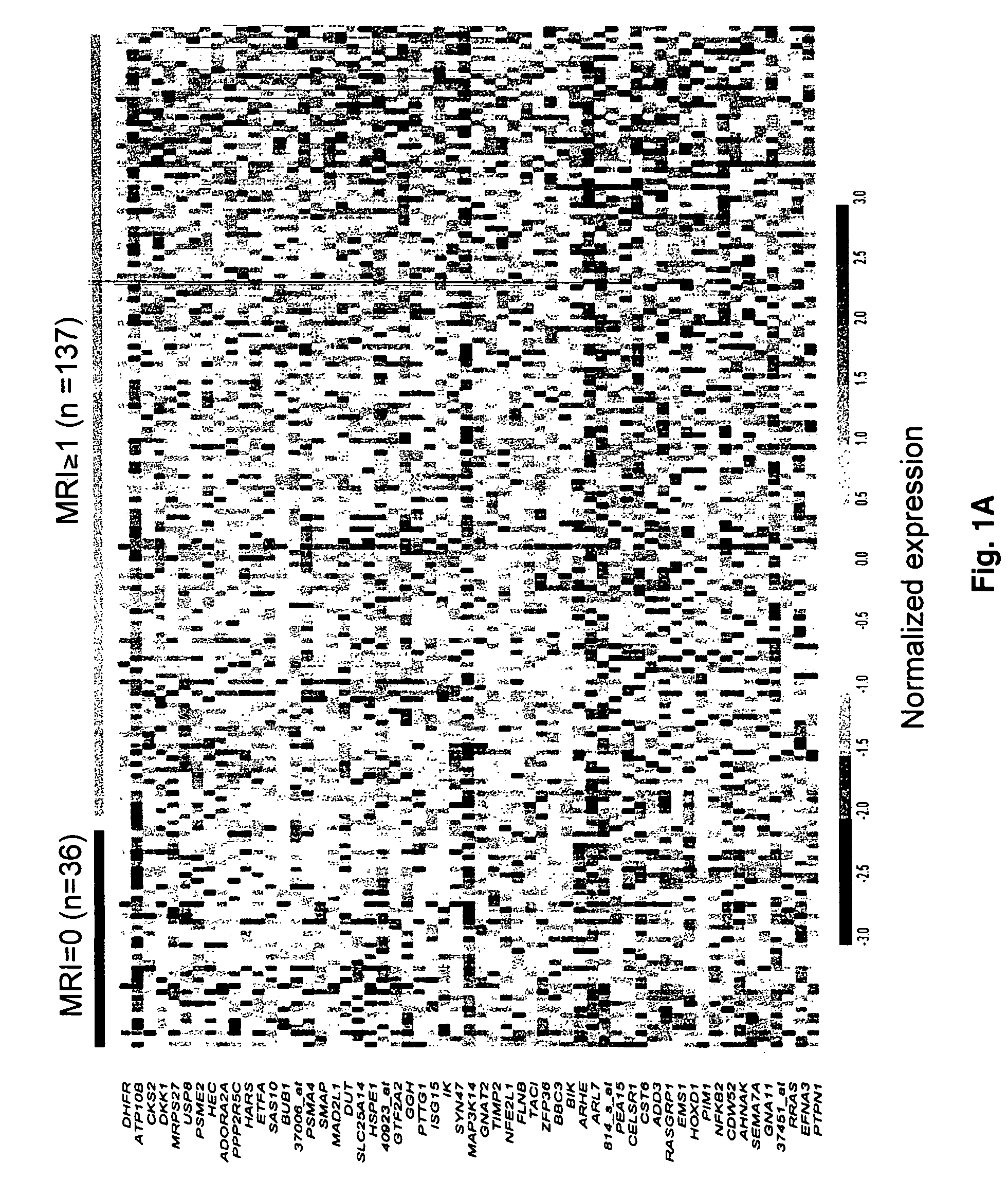
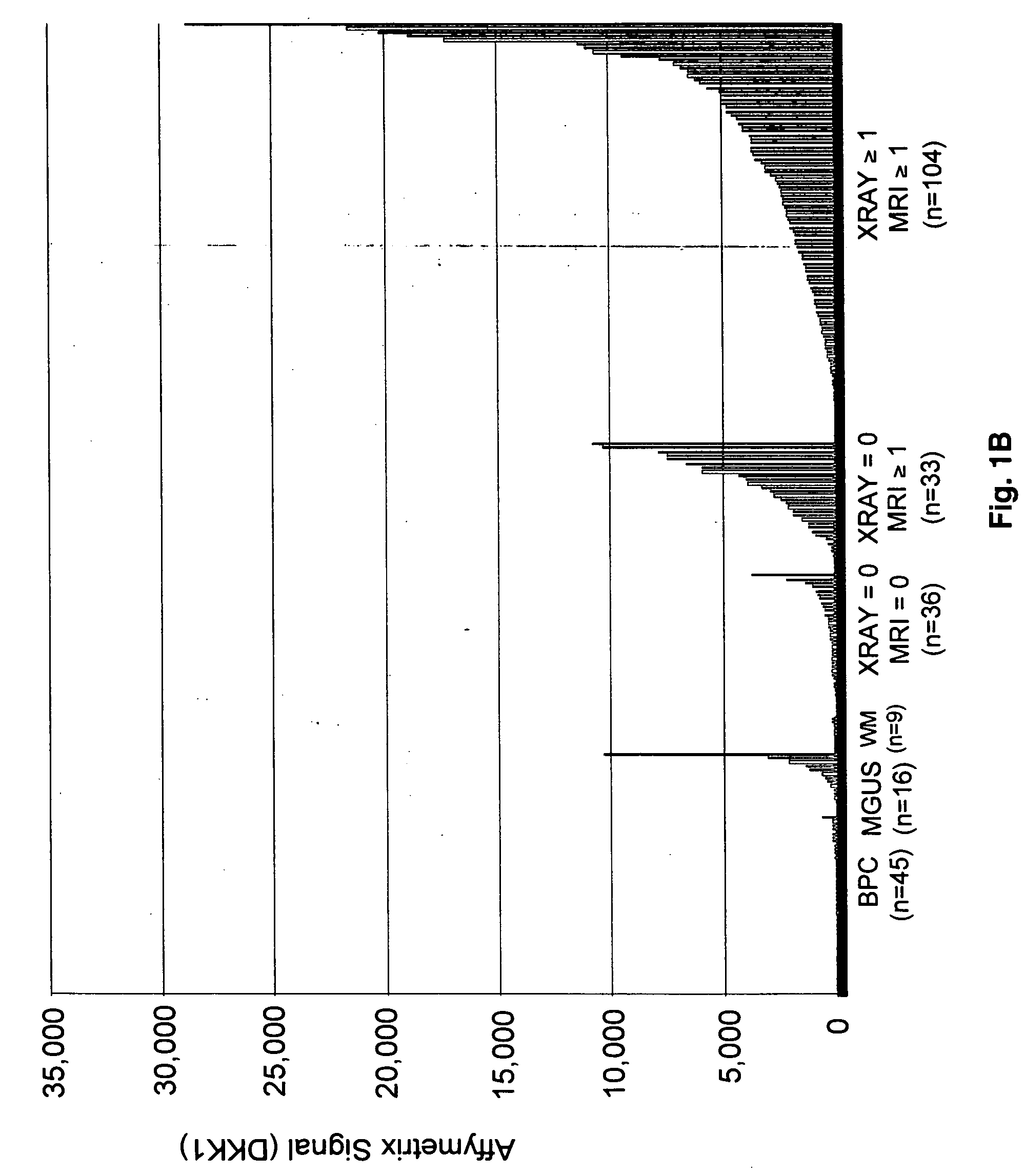
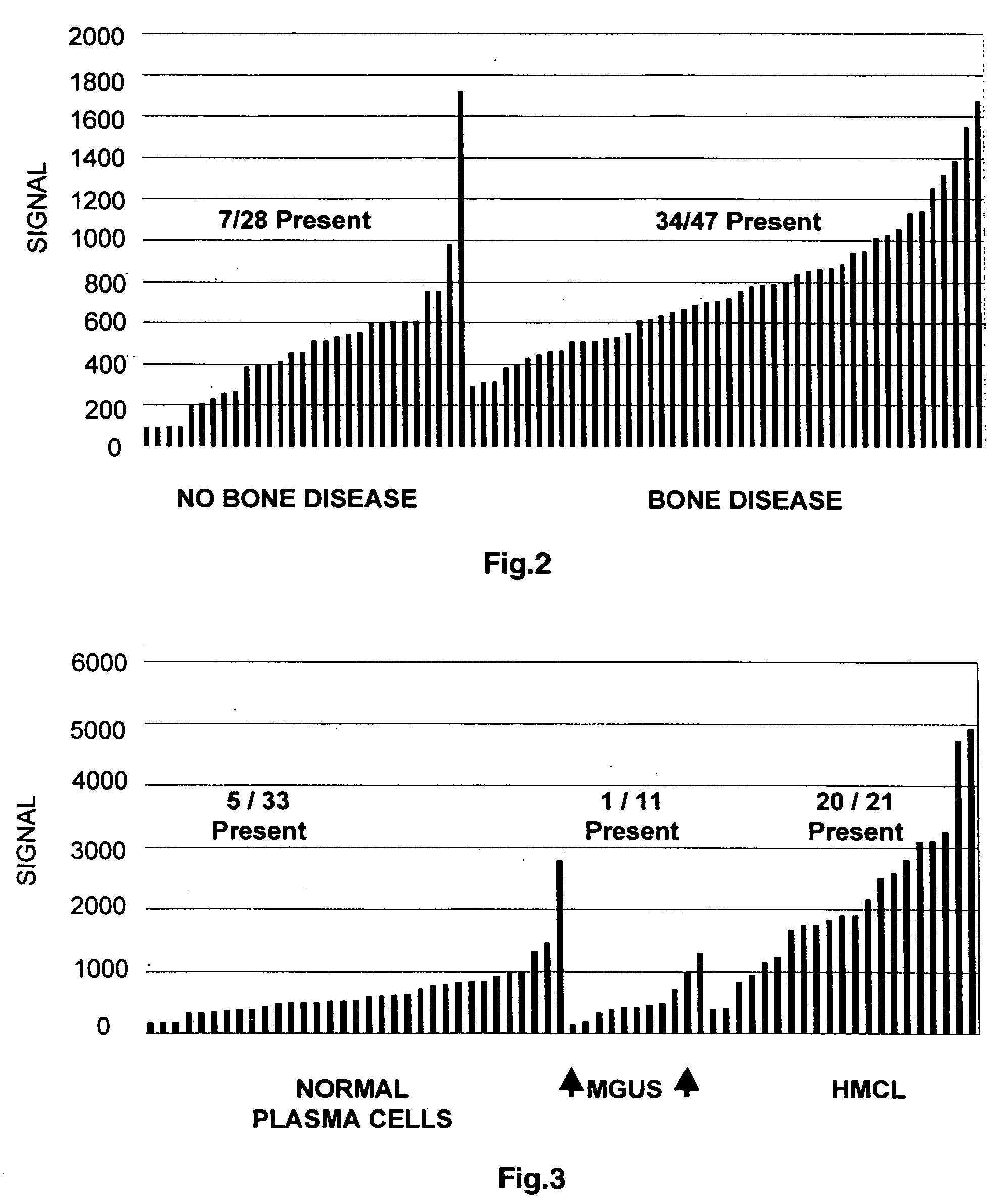
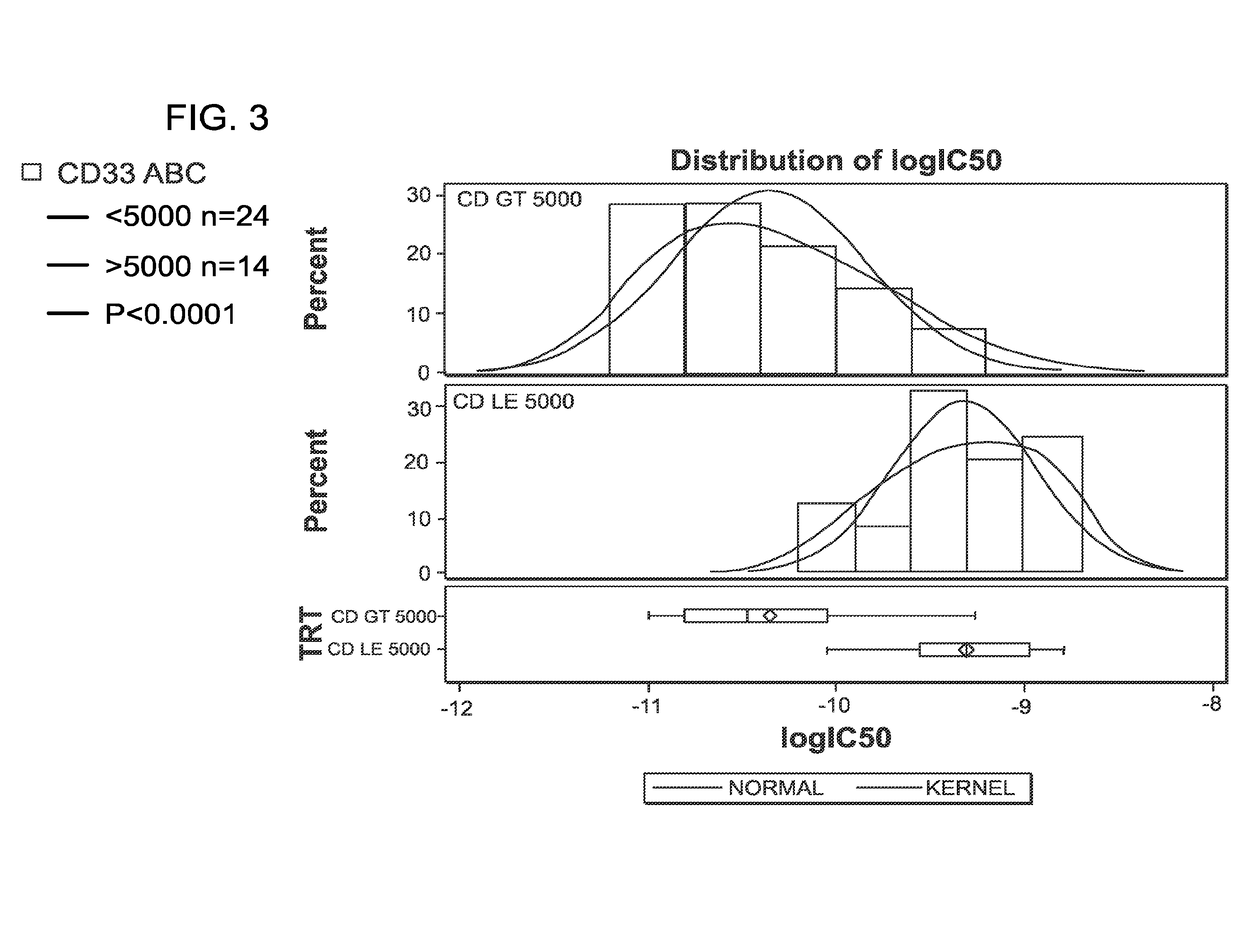
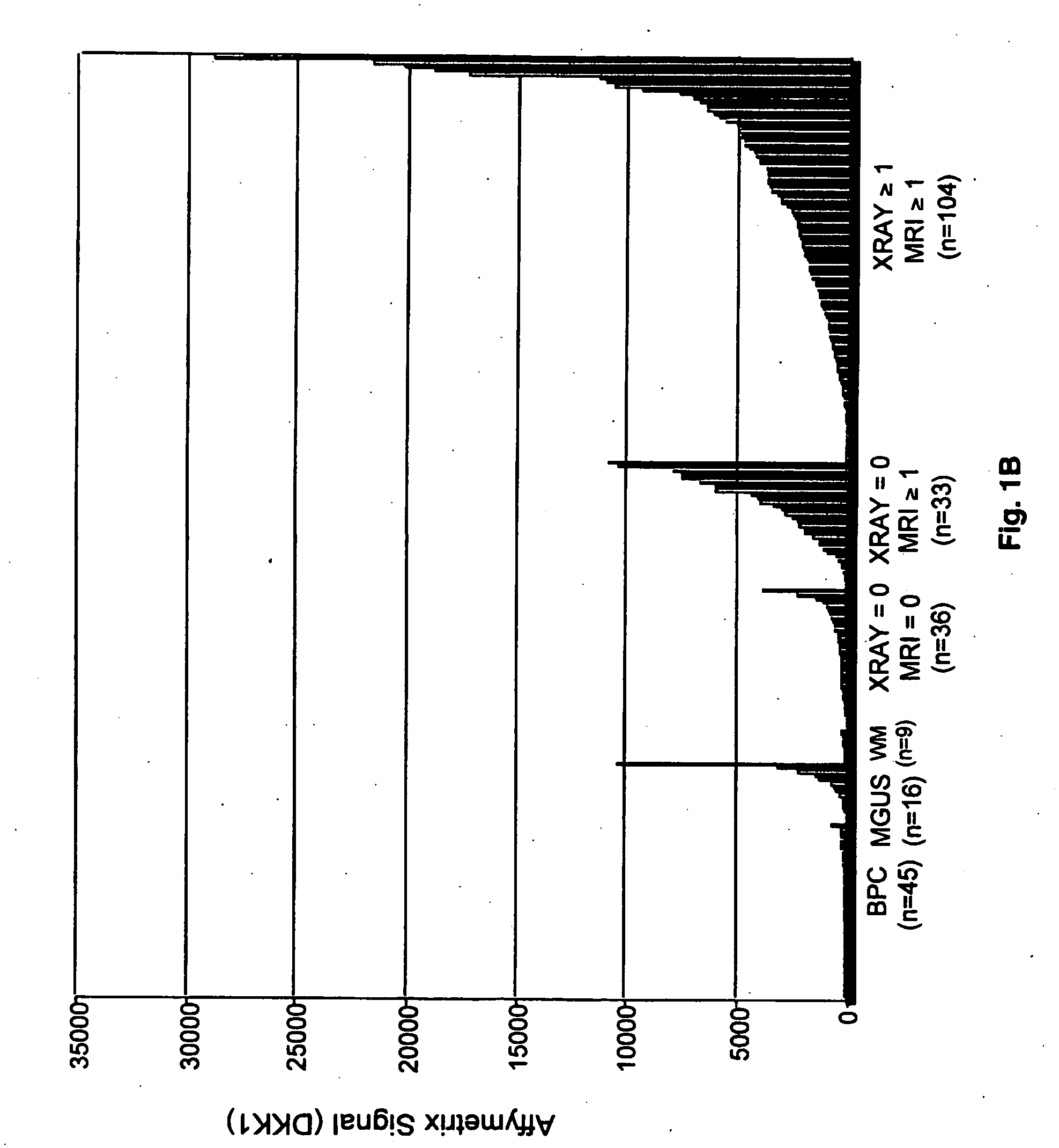
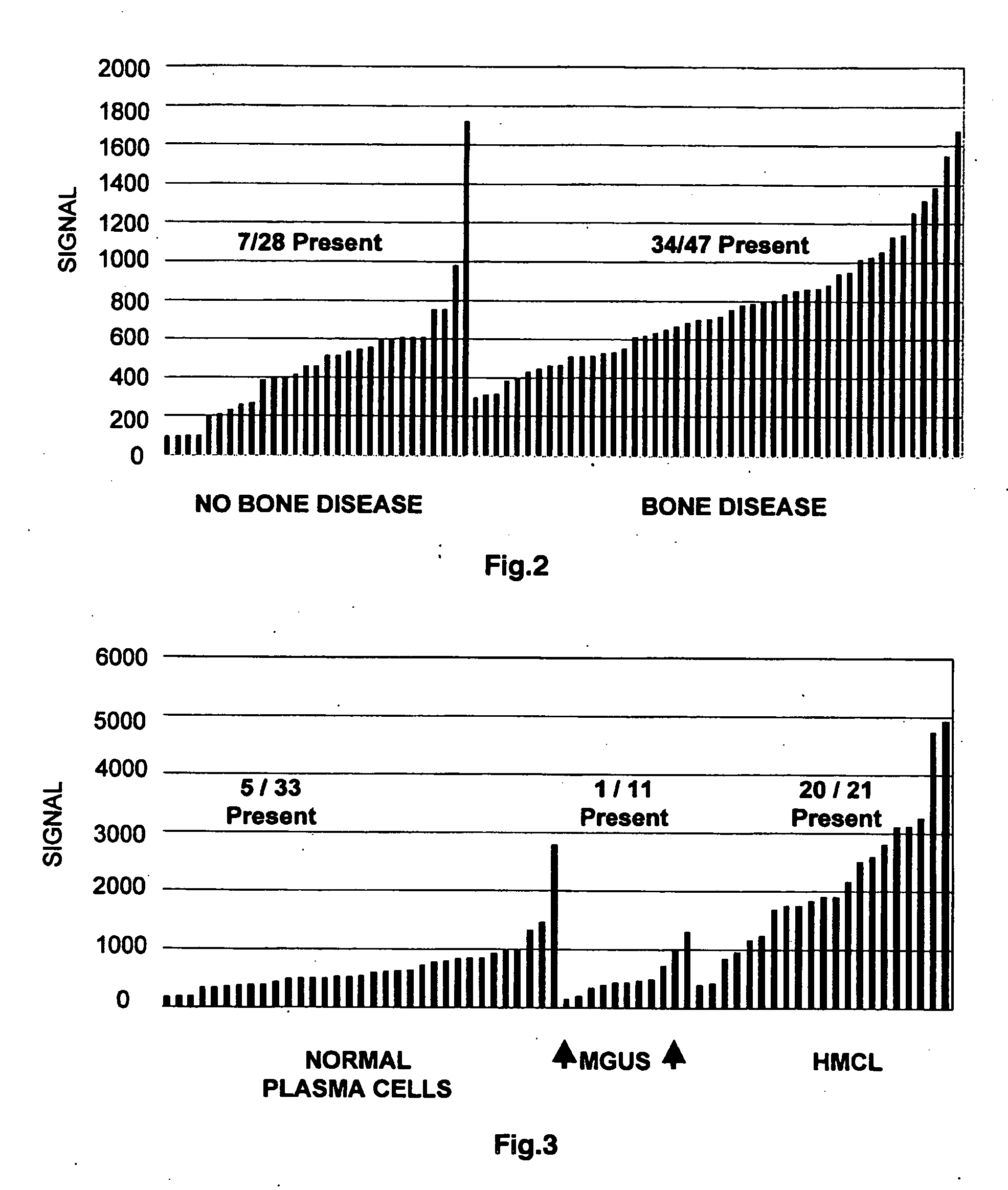
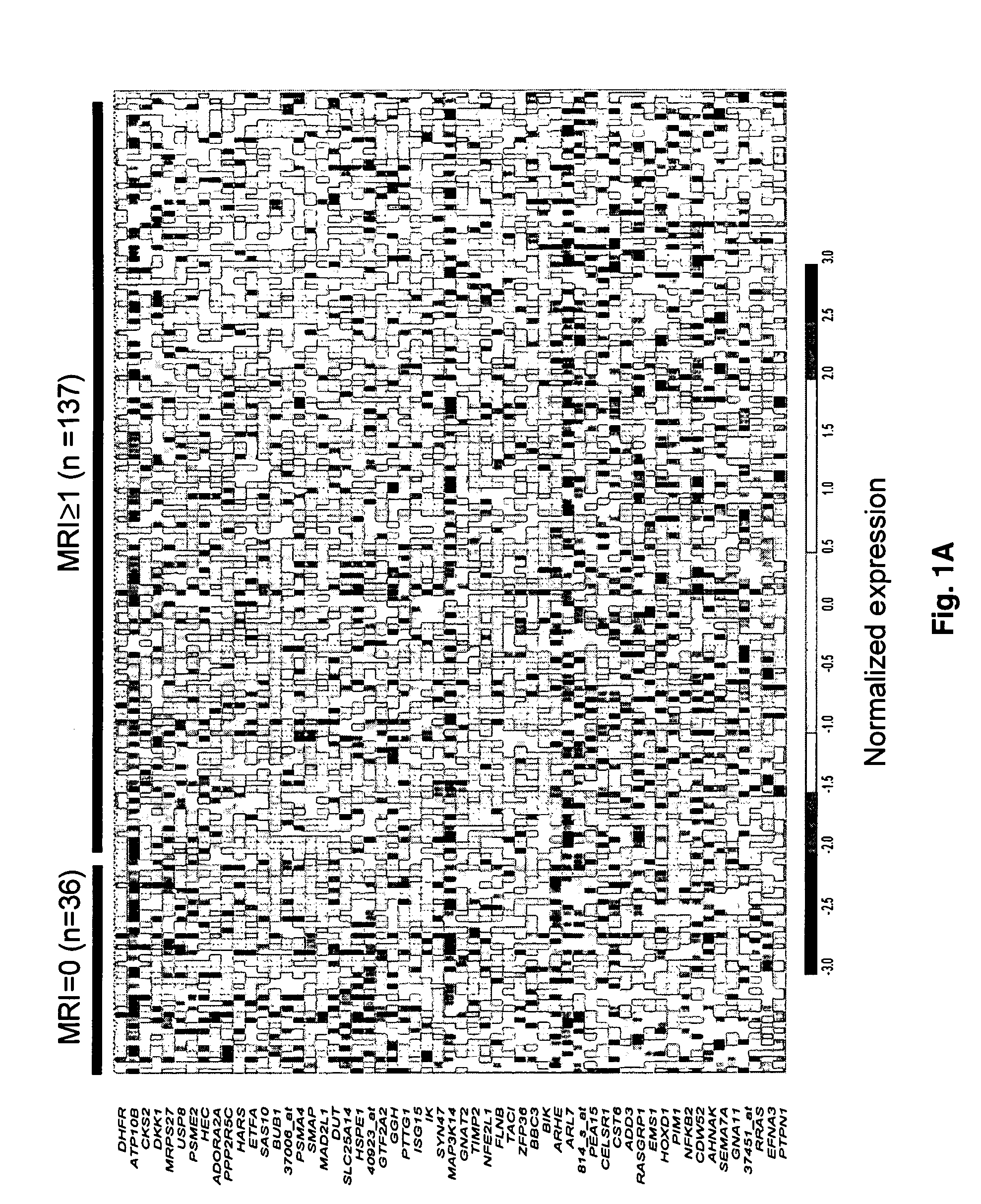
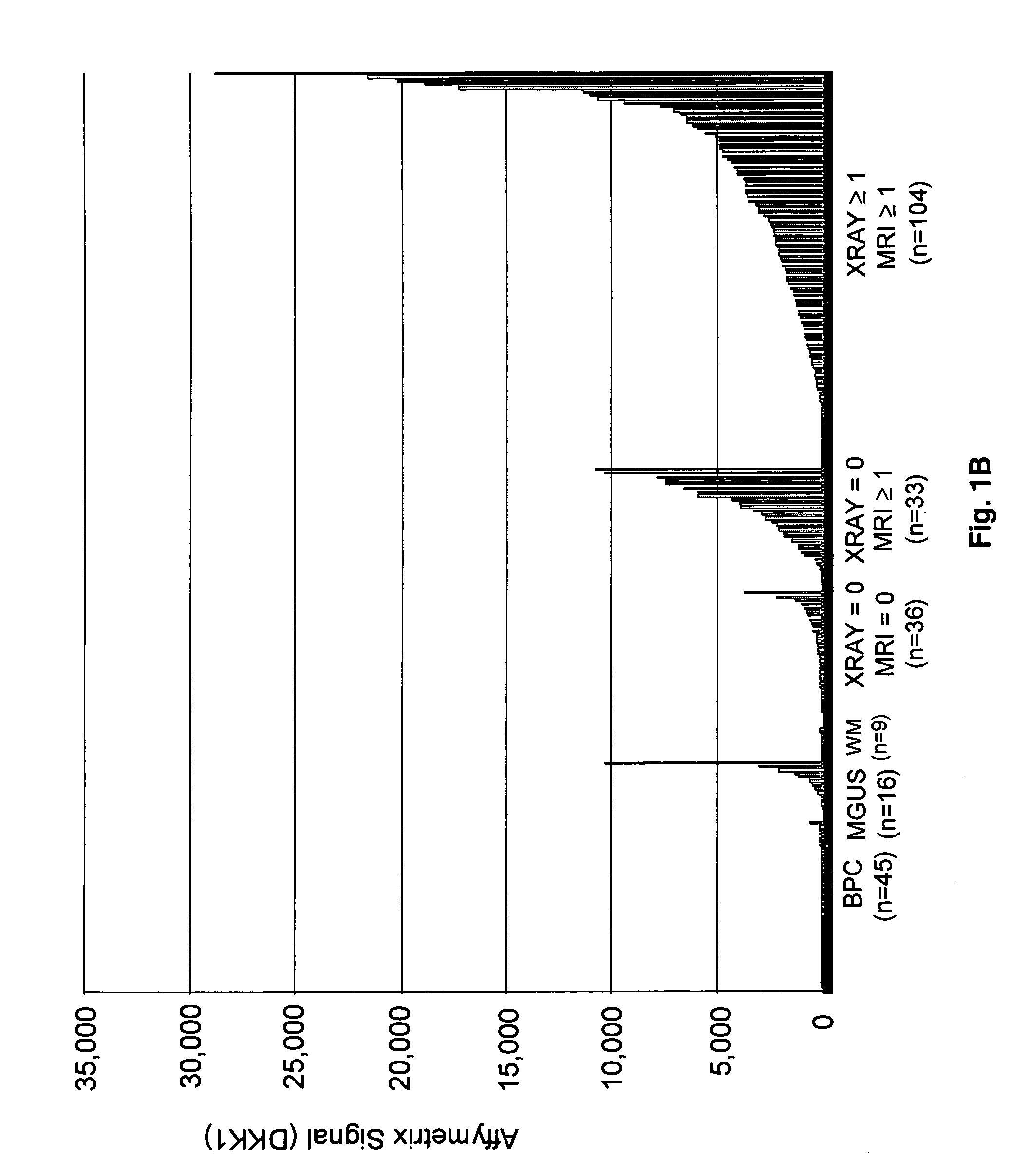
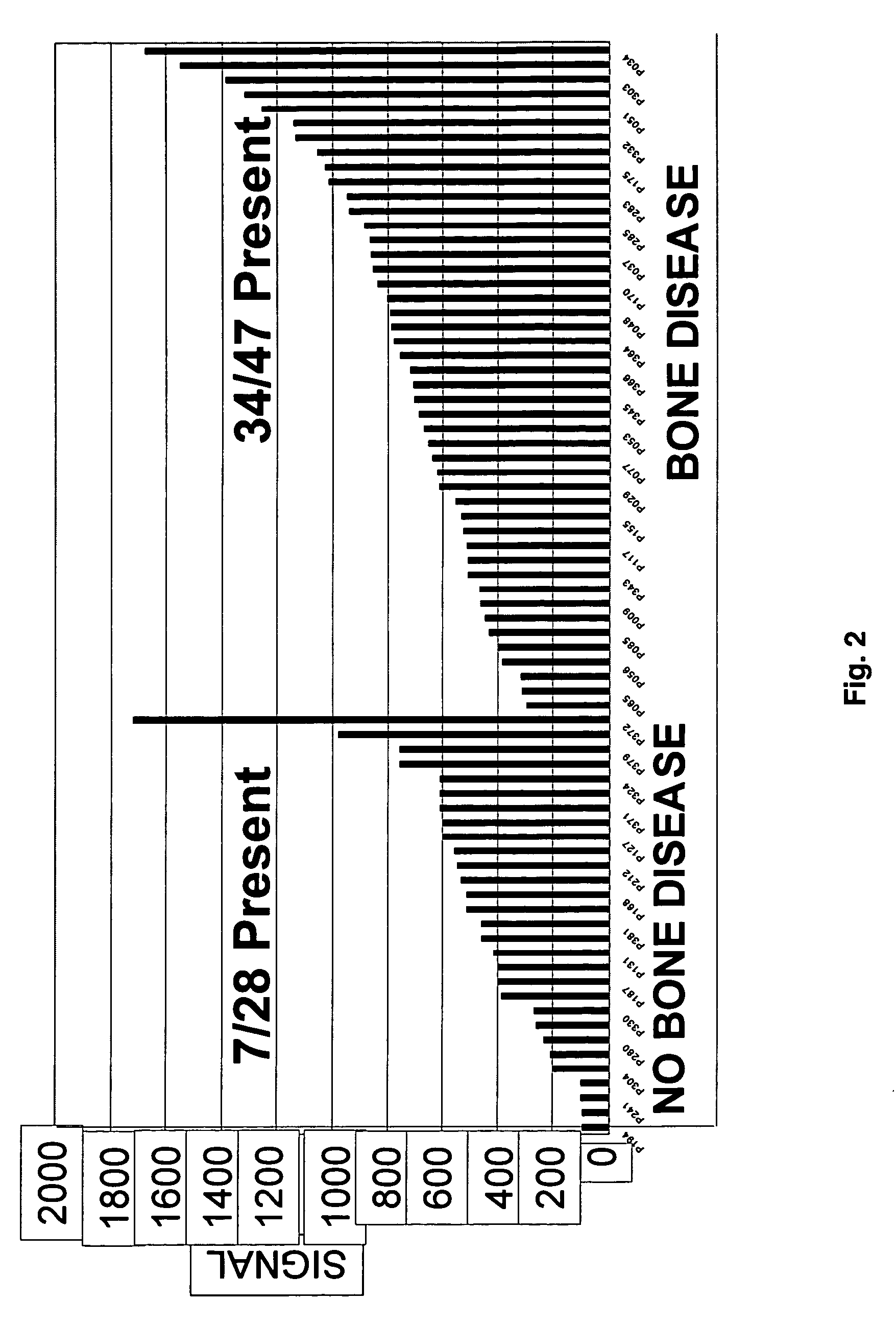
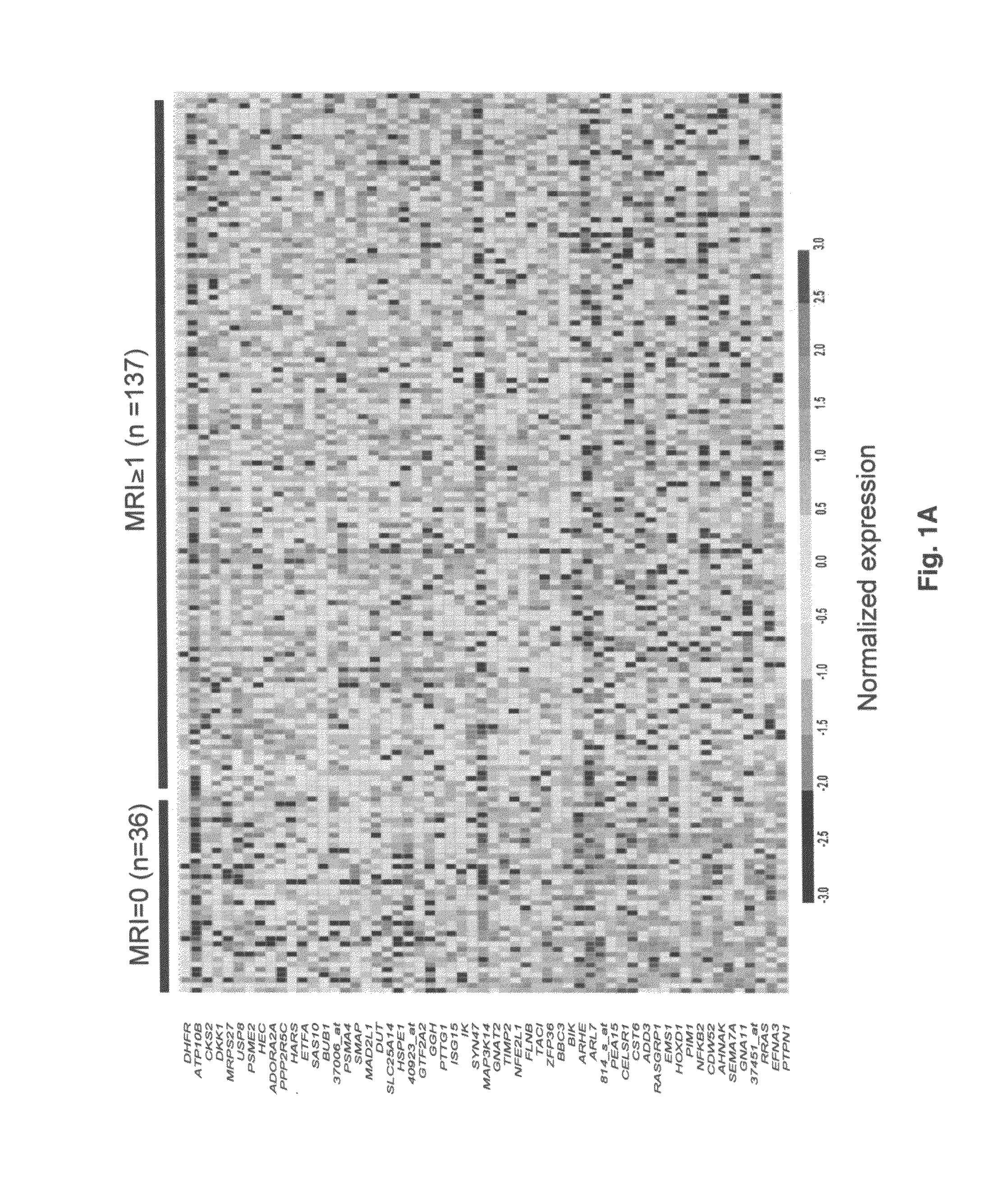
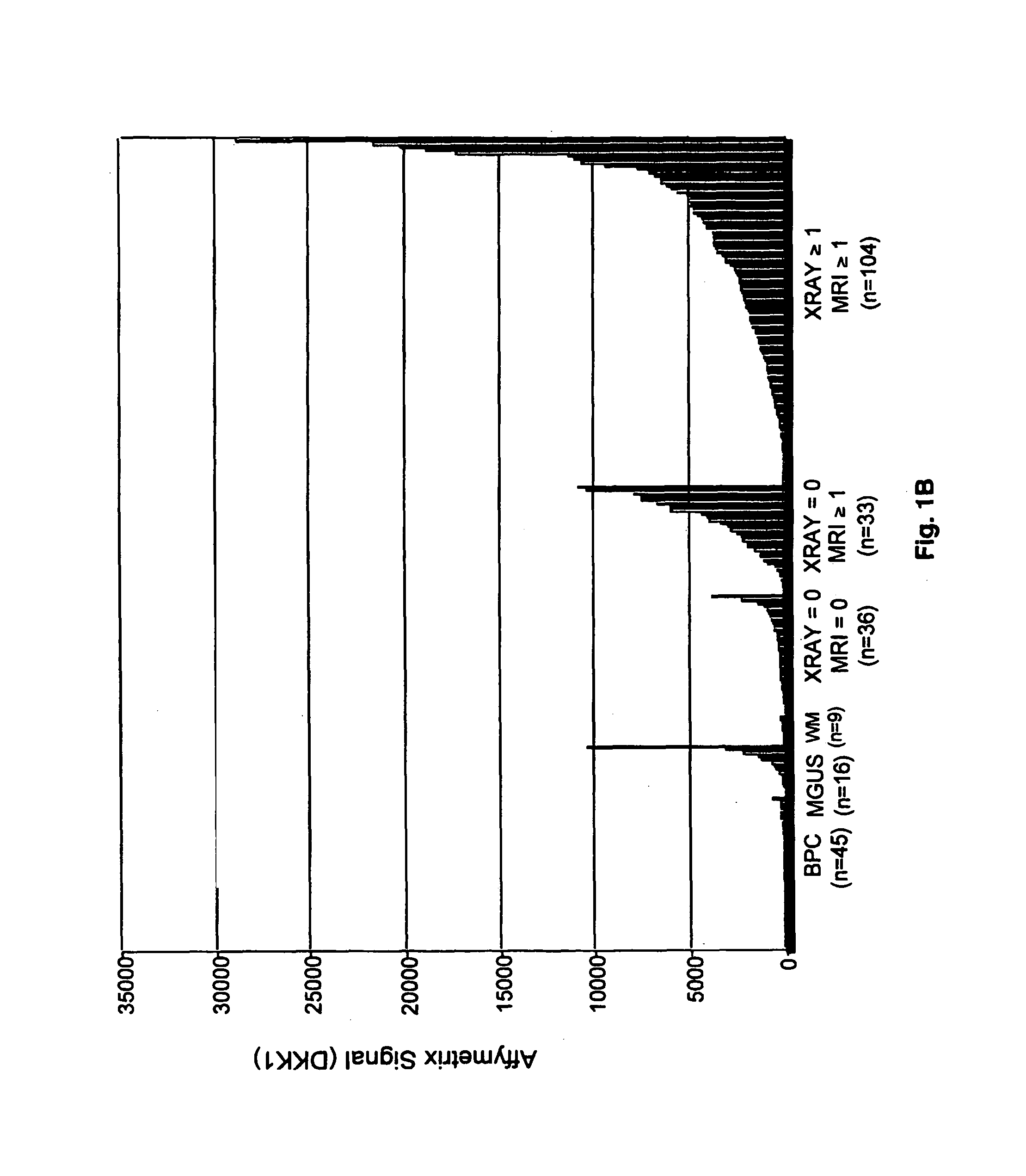
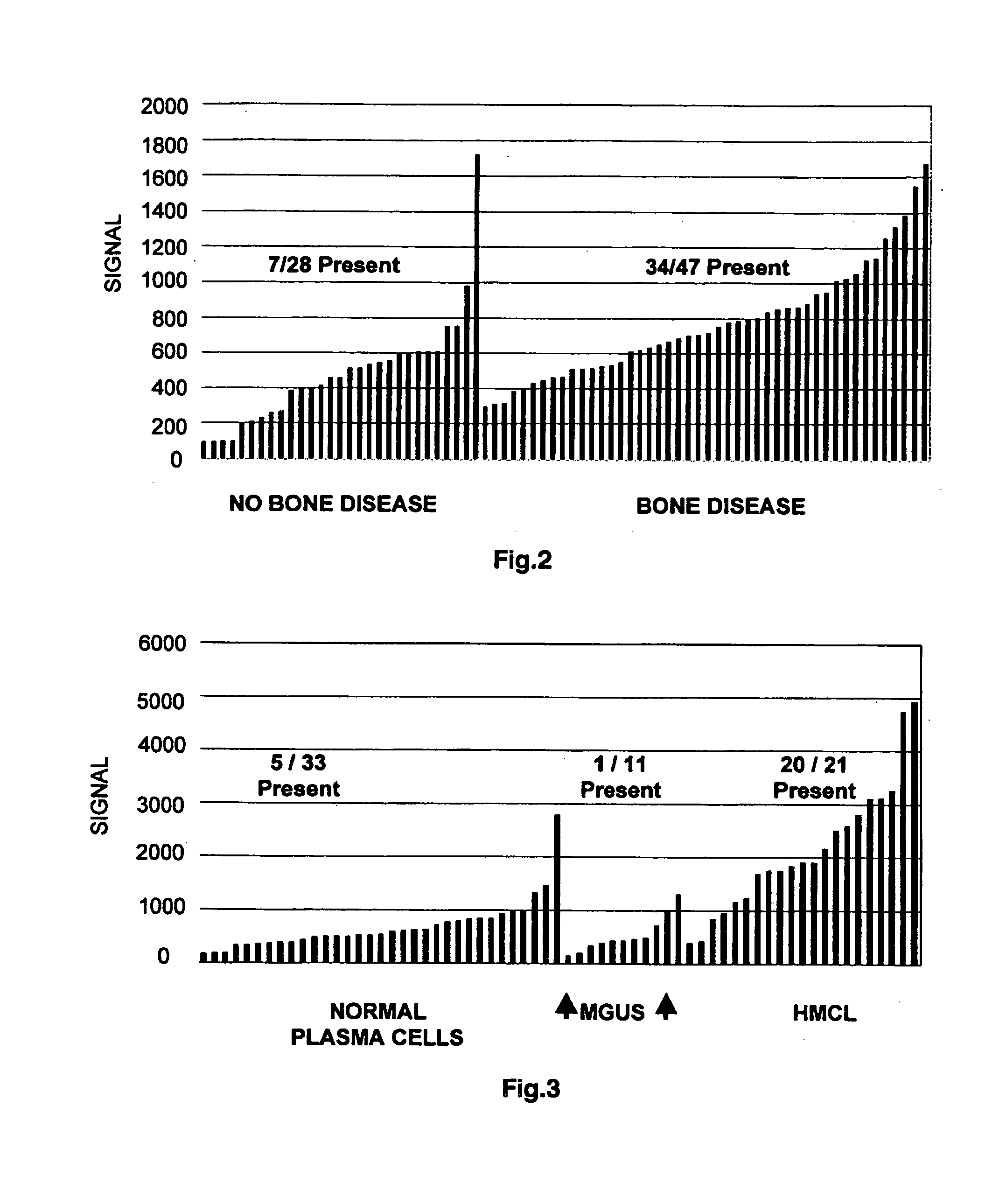
To identify molecular determinants of lytic bone disease in multiple myeloma, the expression profiles of ˜12,000 genes in CD138-enriched plasma cells from newly diagnosed multiple myeloma patients exhibiting no radiological evidence of lytic lesions (n=28) were compared to those with ≧3 lytic lesions (n=47). Two secreted WNT signaling antagonists, soluble frizzled related protein 3 (SFRP-3 / FRZB) and the human homologue of Dickkopf-1 (DKK1), were expressed in 40 of 47 with lytic bone lesions, but only 16 of 28 lacking bone lesions (P<0.05). DKK1 and FRZB were not expressed in plasma cells from 45 normal bone marrow donors or 10 Waldenstrom's macroglobulinemia, a related plasma cells malignancy that lacks bone disease. These data indicate that these factors are important mediators of multiple myeloma bone disease, and inhibitors of these proteins may be used to block bone disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Methods of determining acute myeloid leukemia response to treatment with farnesyltransferase
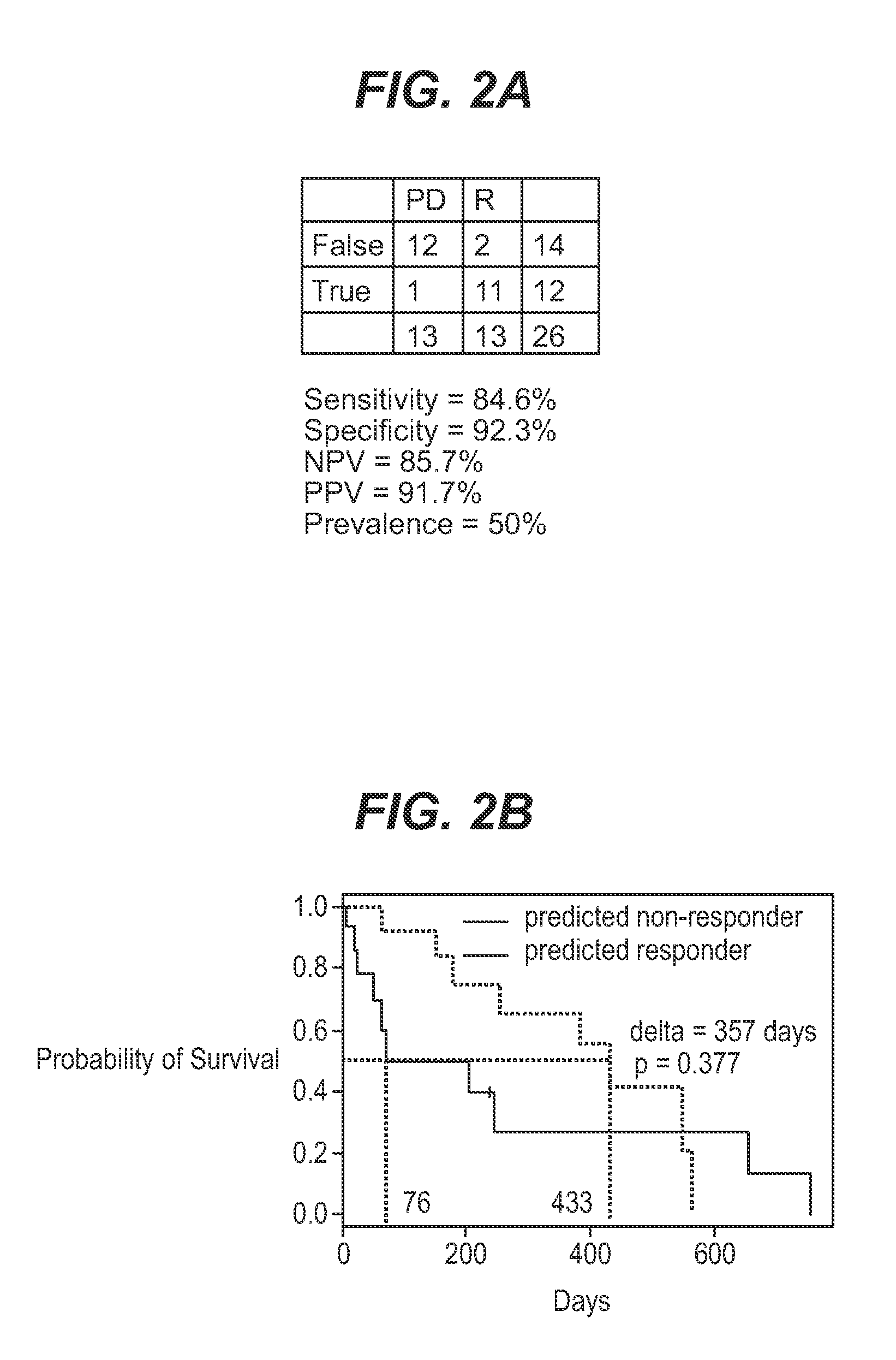
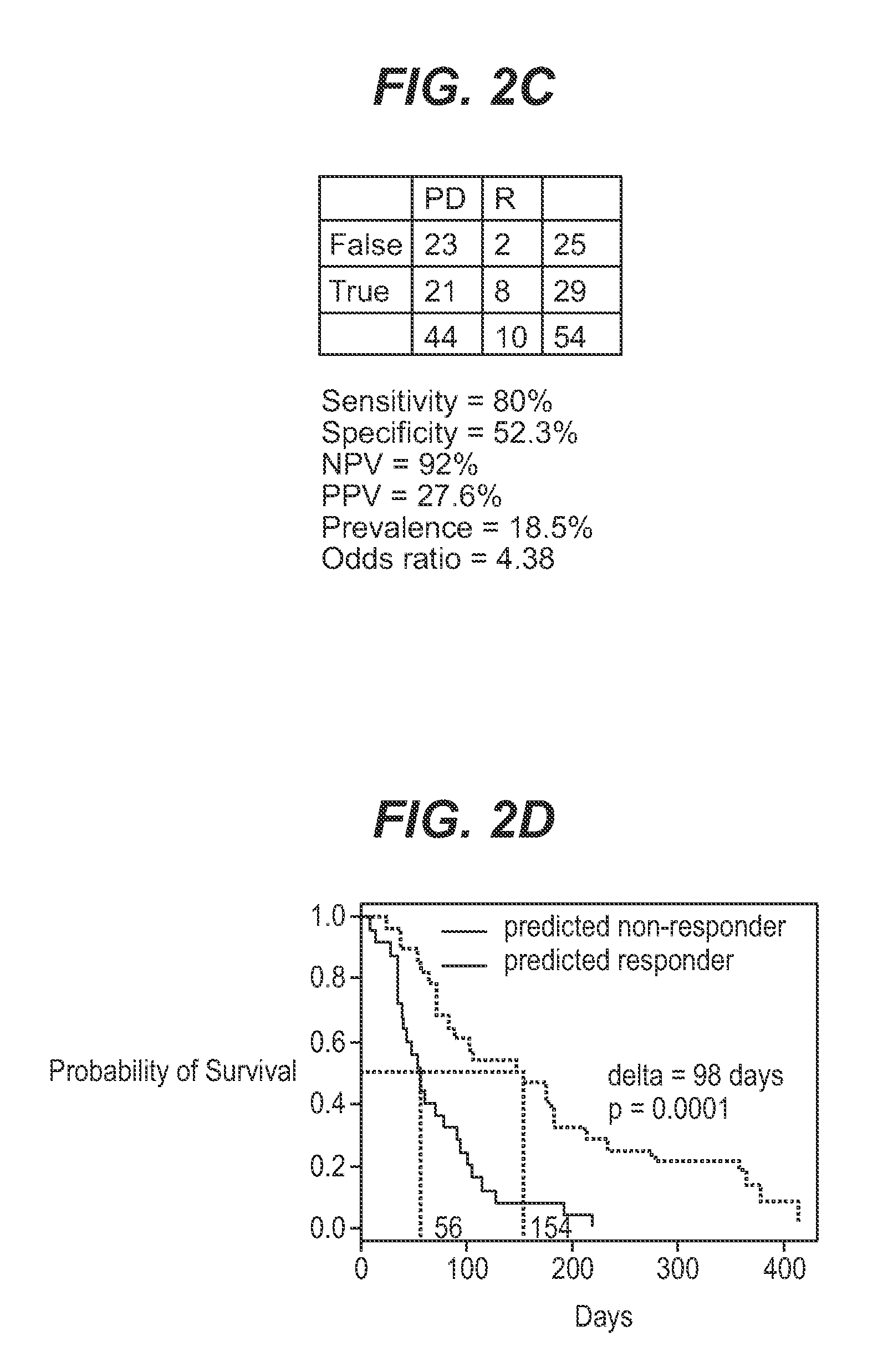
InactiveUS7932036B1Improve accuracyHigh response rateSugar derivativesMicrobiological testing/measurementNewly diagnosedClinical study
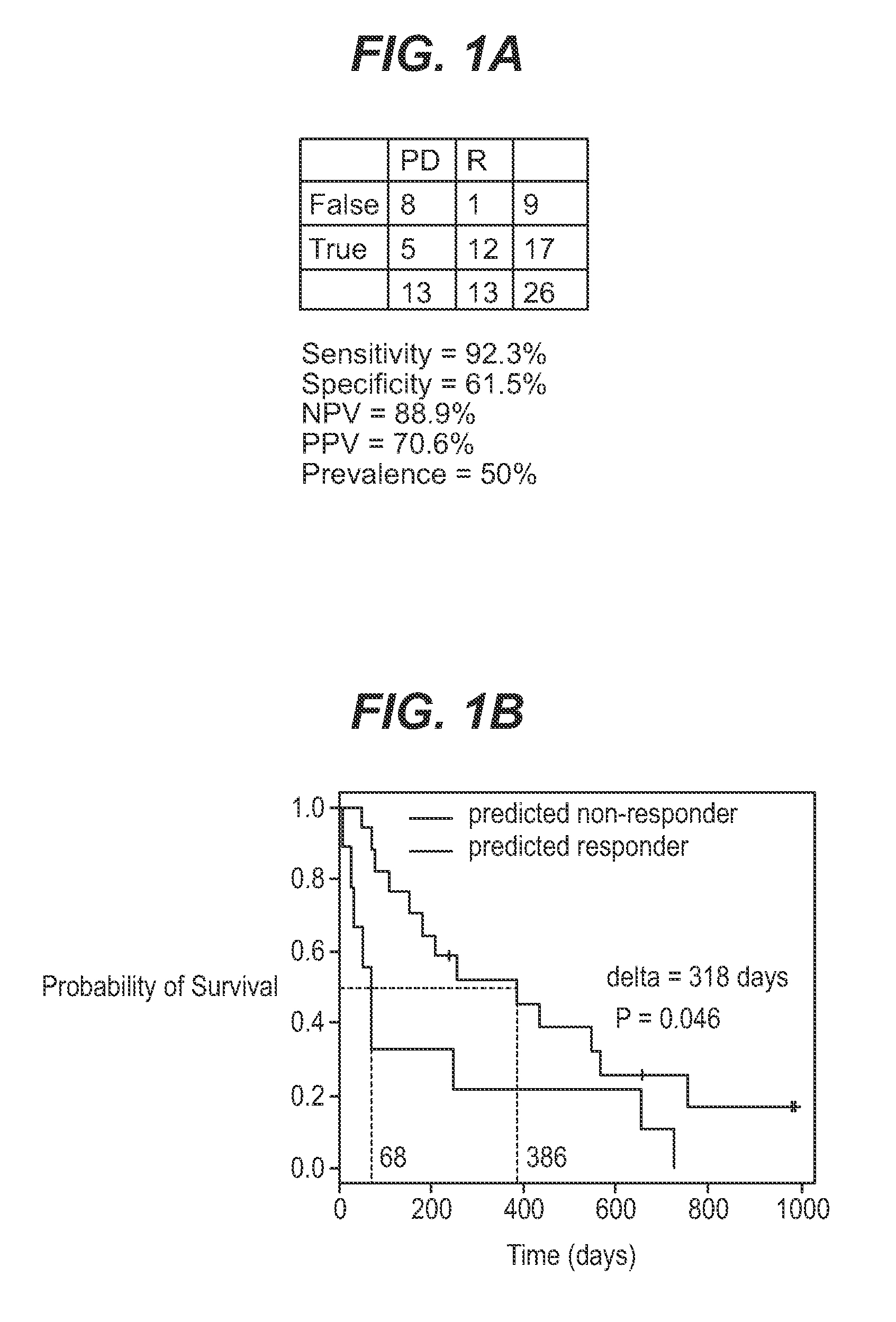
We analyzed bone marrow from 67 patients from a phase 2 study of farnesyltransferase inhibition with tipifarnib (R115777, ZARNESTRA®), in older adults with previously untreated, poor-risk acute myeloid leukemia (AML) for N-Ras mutations, global gene expression, and / or quantitative PCR (qPCR) of specific genes. Microarray profiling identified a two-gene expression ratio (RASGRP1:APTX) which provided the greatest accuracy for predicting response to tipifarnib. We demonstrated that this classifier could predict response to tipifarnib in an independent set of 54 samples from relapsed or refractory AML, with a NPV and PPV of 92% and 28%, respectively (odds ratio of 4.4). Therefore, in both newly diagnosed and relapsed or refractory AML, this classifier improves the overall response rate by approximately 50% while maintaining a high NPV, and significantly improves patient overall survival. The two-gene classifier was also validated by qPCR in thirty AML samples from the same clinical study demonstrating a negative predictive value (NPV) and positive predictive value (PPV) of 81% and 50%, respectively (odds ratio of 4.3). These data indicate that a simple two-gene expression assay may have utility in diagnosing a population of AML patients who are more likely to respond to tipifarnib.
Owner:JANSSEN DIAGNOSTICS LLC
Evaluation system and method for efficacy of synchro-neoadjuvant chemoradiotherapy before rectal cancer surgery
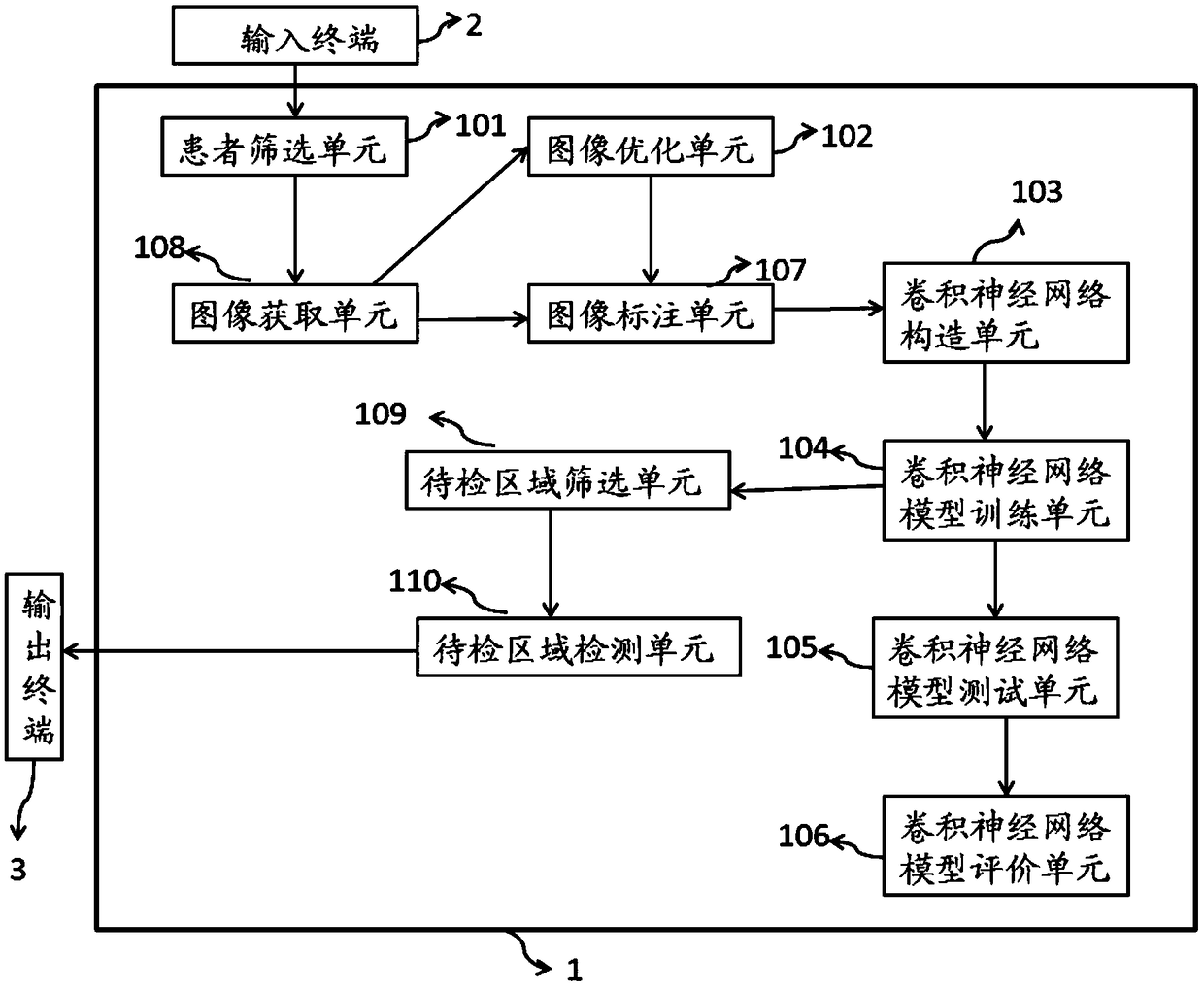
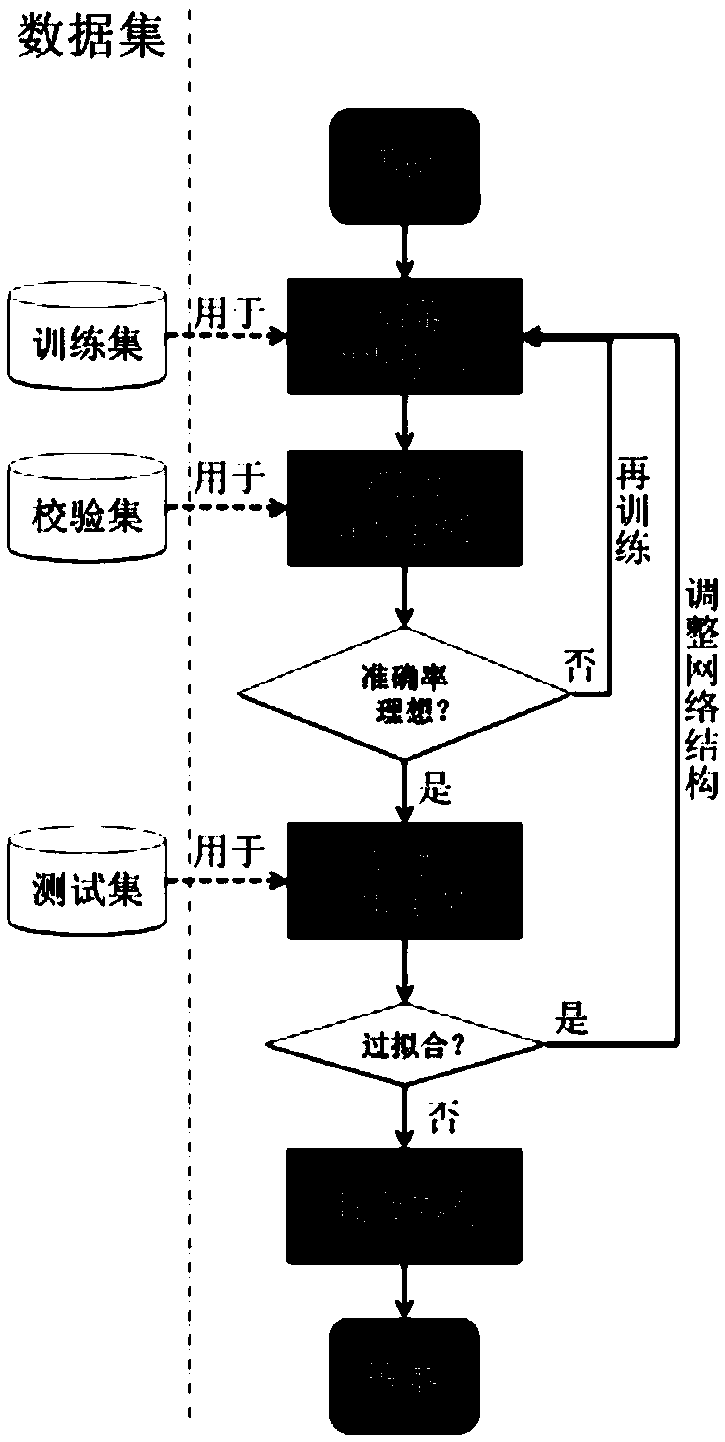
InactiveCN108694718AReduce the need for consultationsFast evaluationImage enhancementImage analysisNewly diagnosedSynchro
The invention relates to an evaluation system and method for the efficacy of synchro-neoadjuvant chemoradiotherapy before the rectal cancer surgery. The evaluation system comprises an image acquisition unit used for acquiring pathological biopsy slice scan images and neoadjuvant chemoradiotherapy treatment pre-MRI images of newly diagnosed locally advanced rectal cancer patients, and classifying the rectal cancer patients into a training set, a check set and a test set, to serve as input image data, an image labeling unit used for respectively labeling the pathological biopsy slice scan imagesand the MRI images of the training set, the check set and the test set, a convolutional neural network constructing unit used for constructing a first convolutional neural network model, and a convolutional neural network model training unit used for obtaining a second convolutional neural network model for evaluating the efficacy of synchro-neoadjuvant chemoradiotherapy before the rectal cancersurgery. The evaluation system for the efficacy of synchro-neoadjuvant chemoradiotherapy before the rectal cancer surgery has multiple advantages such as being high in accuracy, short in time consumption, long in working duration, objective and three-dimensional.
Owner:THE SIXTH AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
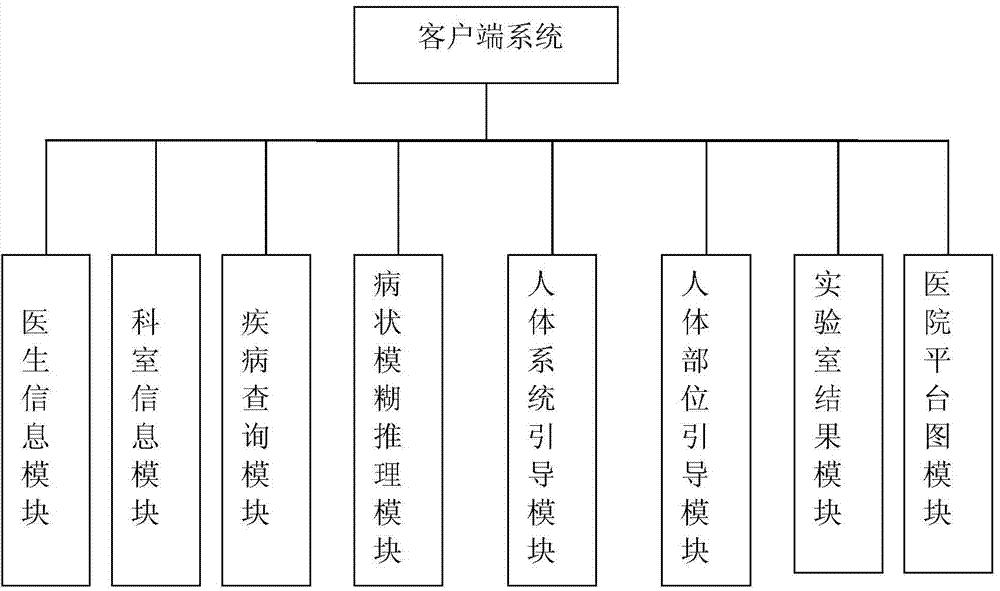
Medical guiding system based on body disease of patient
ActiveCN104537275AImprove service levelImprove service capabilitiesSpecial data processing applicationsNewly diagnosedDisease
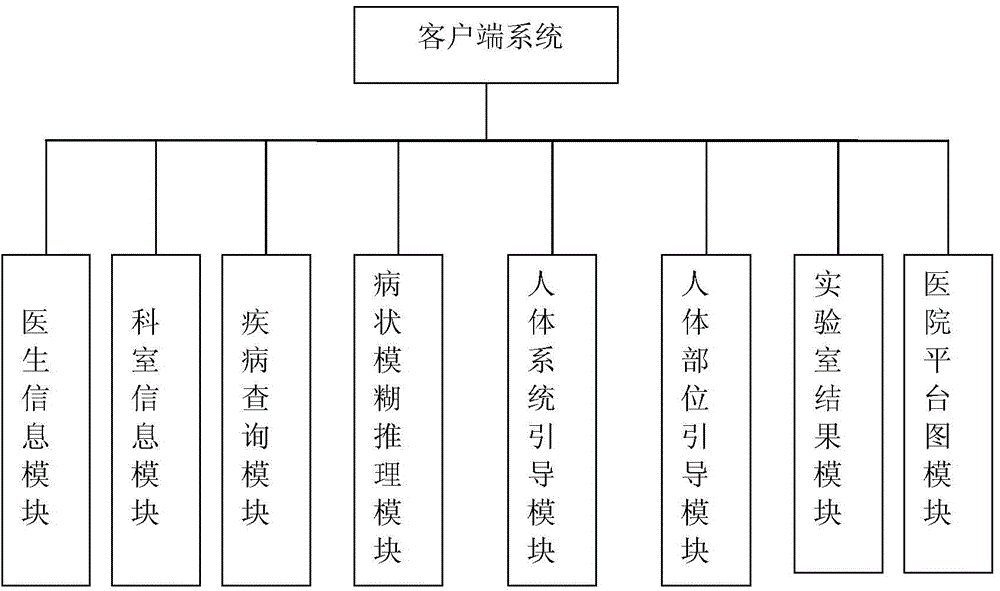
The invention discloses a medical service guiding system based on patient physical symptoms. The medical service guiding system comprises a client side system and a server system, wherein the client side system and the server system are connected with each other through a network. The client side system comprises a doctor information module, a department information module, a disease inquiry module, a symptom fuzzy reasoning module, a human body system guiding module, a human body part guiding module, a laboratory result module and a hospital planar graph module. Users can log into the medical service guiding system through a hospital terminal device or a personal handheld mobile device and select the corresponding modules to conduct inquiry; meanwhile, by setting up a symptom-department-doctor fuzzy reasoning model, newly-diagnosed patients can be helped to rapidly know prevalence of themselves according to the symptoms of themselves and find appropriate doctors in the unfamiliar environment, and therefore flexibility, accuracy and diagnostic efficiency are improved compared with an existing system. By means of the medical service guiding system, the current requirement of people for medical treatment and health is met, and the hospital service level and the service capacity are improved.
Owner:CHENGDU RUIGAN TECH
Molecular determinants of myeloma bone disease and uses thereof
InactiveUS20060019895A1Increase bone massBone lossPeptide/protein ingredientsMicrobiological testing/measurementFrzbNewly diagnosed
To identify molecular determinants of lytic bone disease in multiple myeloma, the expression profiles of ˜12,000 genes in CD138-enriched plasma cells from newly diagnosed multiple myeloma patients exhibiting no radiological evidence of lytic lesions (n=28) were compared to those with ≧3 lytic lesions (n=47). Two secreted WNT signaling antagonists, soluble frizzled related protein 3 (SFRP-3 / FRZB) and the human homologue of Dickkopf-1 (DKK1), were expressed in 40 of 47 with lytic bone lesions, but only 16 of 28 lacking bone lesions (P<0.05). DKK1 and FRZB were not expressed in plasma cells from 45 normal bone marrow donors or 10 Waldenstrom's macroglobulinemia, a related plasma cells malignancy that lacks bone disease. These data indicate that these factors are important mediators of multiple myeloma bone disease, and inhibitors of these proteins may be used to block bone disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Medical guide system based on patient physical symptoms
InactiveCN103942463AImprove service levelImprove service capabilitiesSpecial data processing applicationsNewly diagnosedHuman body
The invention discloses a medical service guiding system based on patient physical symptoms. The medical service guiding system comprises a client side system and a server system, wherein the client side system and the server system are connected with each other through a network. The client side system comprises a doctor information module, a department information module, a disease inquiry module, a symptom fuzzy reasoning module, a human body system guiding module, a human body part guiding module, a laboratory result module and a hospital planar graph module. Users can log into the medical service guiding system through a hospital terminal device or a personal handheld mobile device and select the corresponding modules to conduct inquiry; meanwhile, by setting up a symptom-department-doctor fuzzy reasoning model, newly-diagnosed patients can be helped to rapidly know prevalence of themselves according to the symptoms of themselves and find appropriate doctors in the unfamiliar environment, and therefore flexibility, accuracy and diagnostic efficiency are improved compared with an existing system. By means of the medical service guiding system, the current requirement of people for medical treatment and health is met, and the hospital service level and the service capacity are improved.
Owner:CHENGDU RUIGAN TECH
Gene expression profiling based identification of genomic signature of high-risk multiple myeloma and uses thereof
InactiveUS20080274911A1High-risk indexIncreased risk of deathMicrobiological testing/measurementLibrary screeningNewly diagnosedComparative genomic hybridization
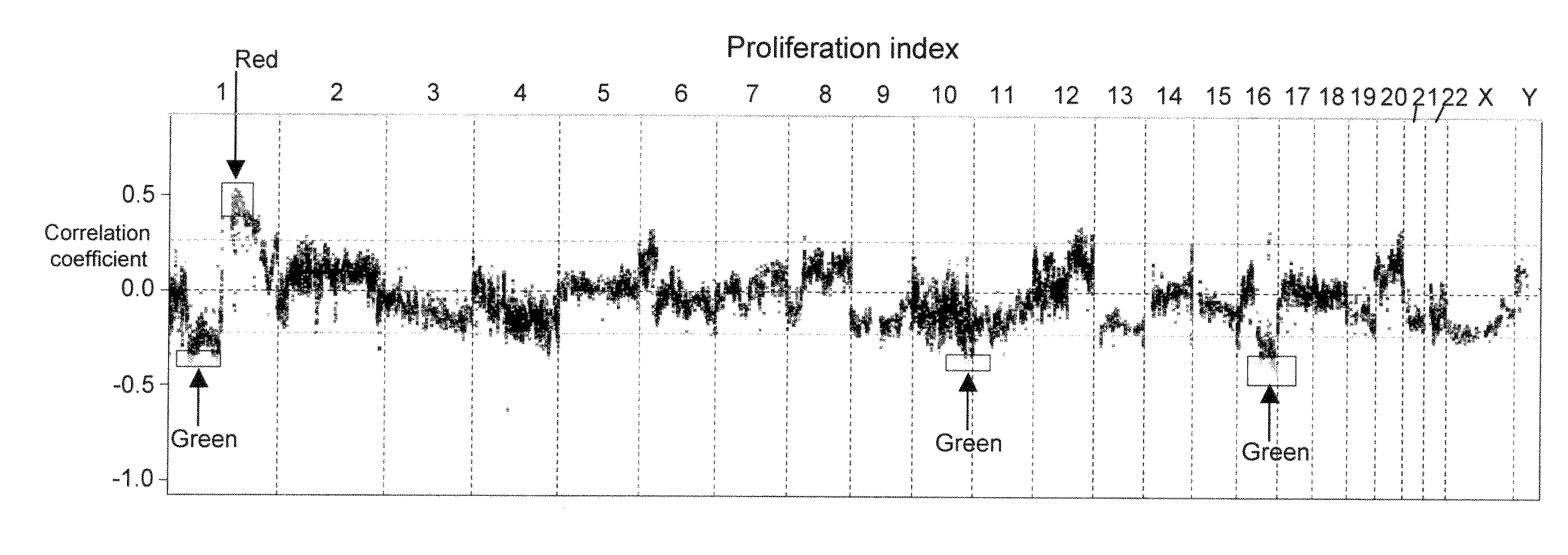
The present invention discloses a method of applying novel bioinformatics and computational methodologies to data generated by high-resolution genome-wide comparative genomic hybridization and gene expression profiling on CD138-sorted plasma cells from a cohort of 92 newly diagnosed multiple myeloma patients treated with high dose chemotherapy and stem cell rescue. The results revealed that gains the q arm and loss of the p arm of chromosome 1 were highly correlated with altered expression of resident genes in this chromosome, with these changes strongly correlated with 1) risk of death from disease progression, 2) a gene expression based proliferation index, and 3) a recently described gene expression-based high-risk index. Importantly, we also found a strong correlation between copy number gains of 8q24, and increased expression of Argonate 2 (AGO2) a gene coding for a master regulator of microRNA expression and maturation, also being significantly correlated with outcome. Our novel findings significantly improve our understanding of the genomic structure of multiple myeloma and its relationship to clinical outcome.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Methods of treating juvenile type 1 diabetes mellitus
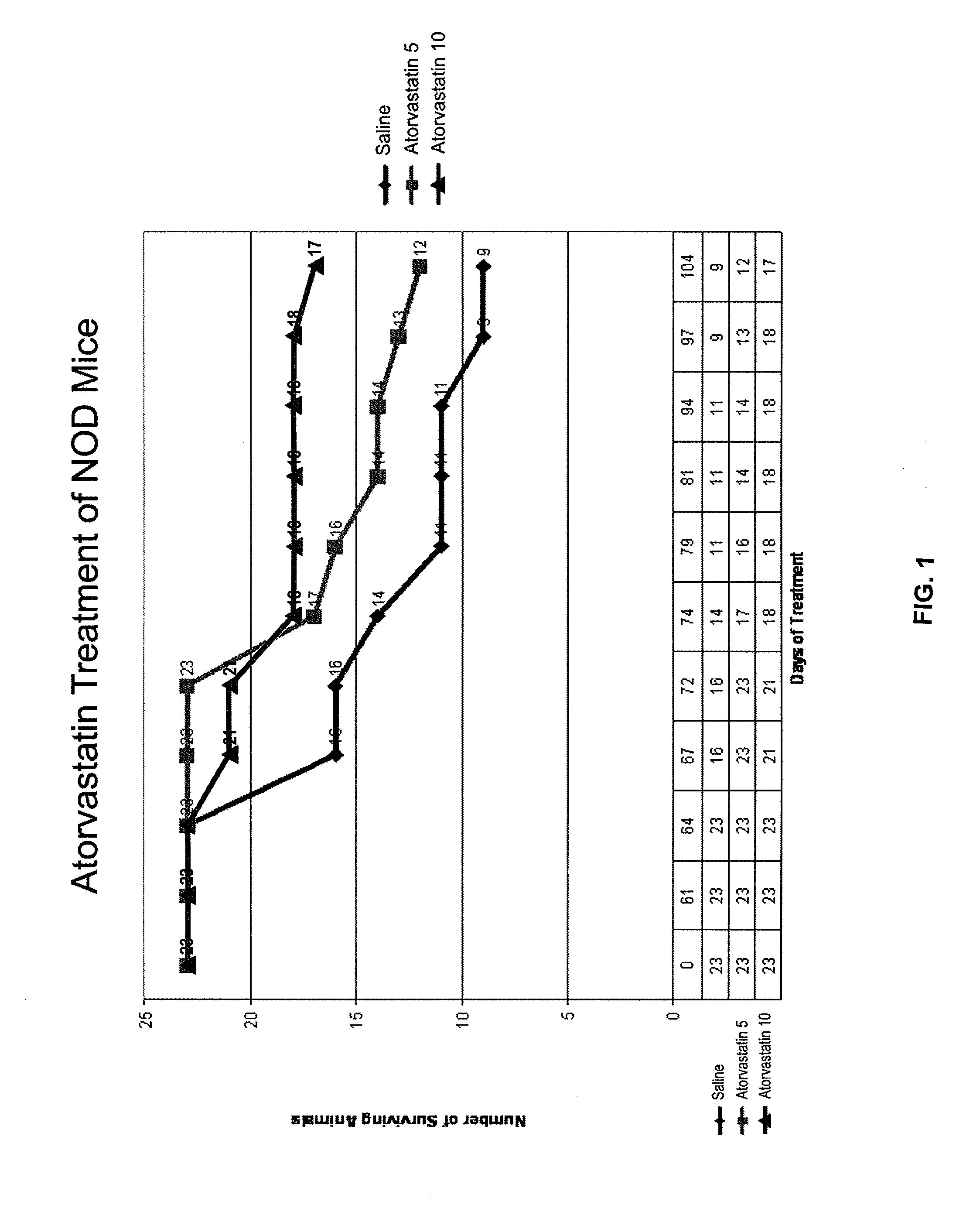
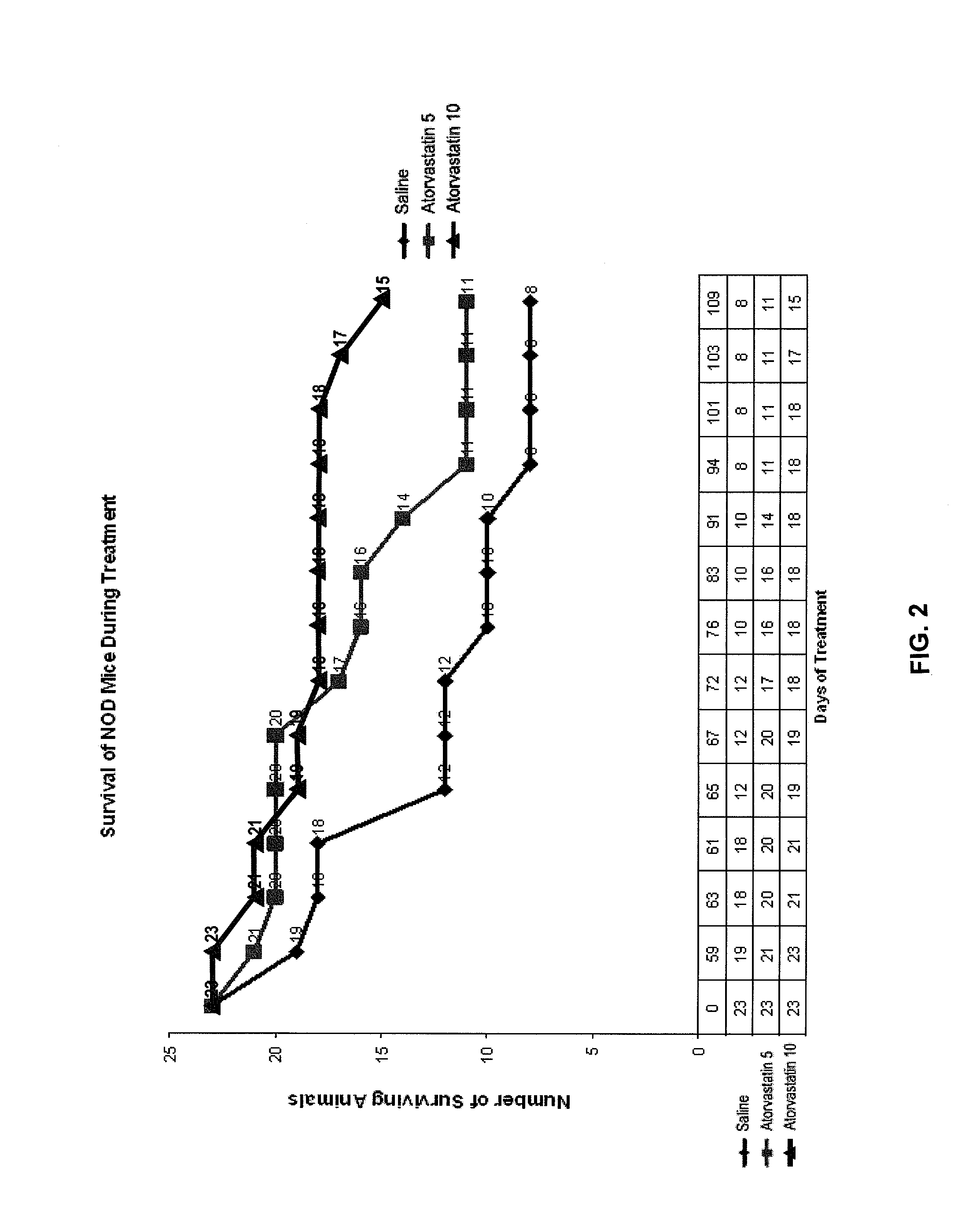
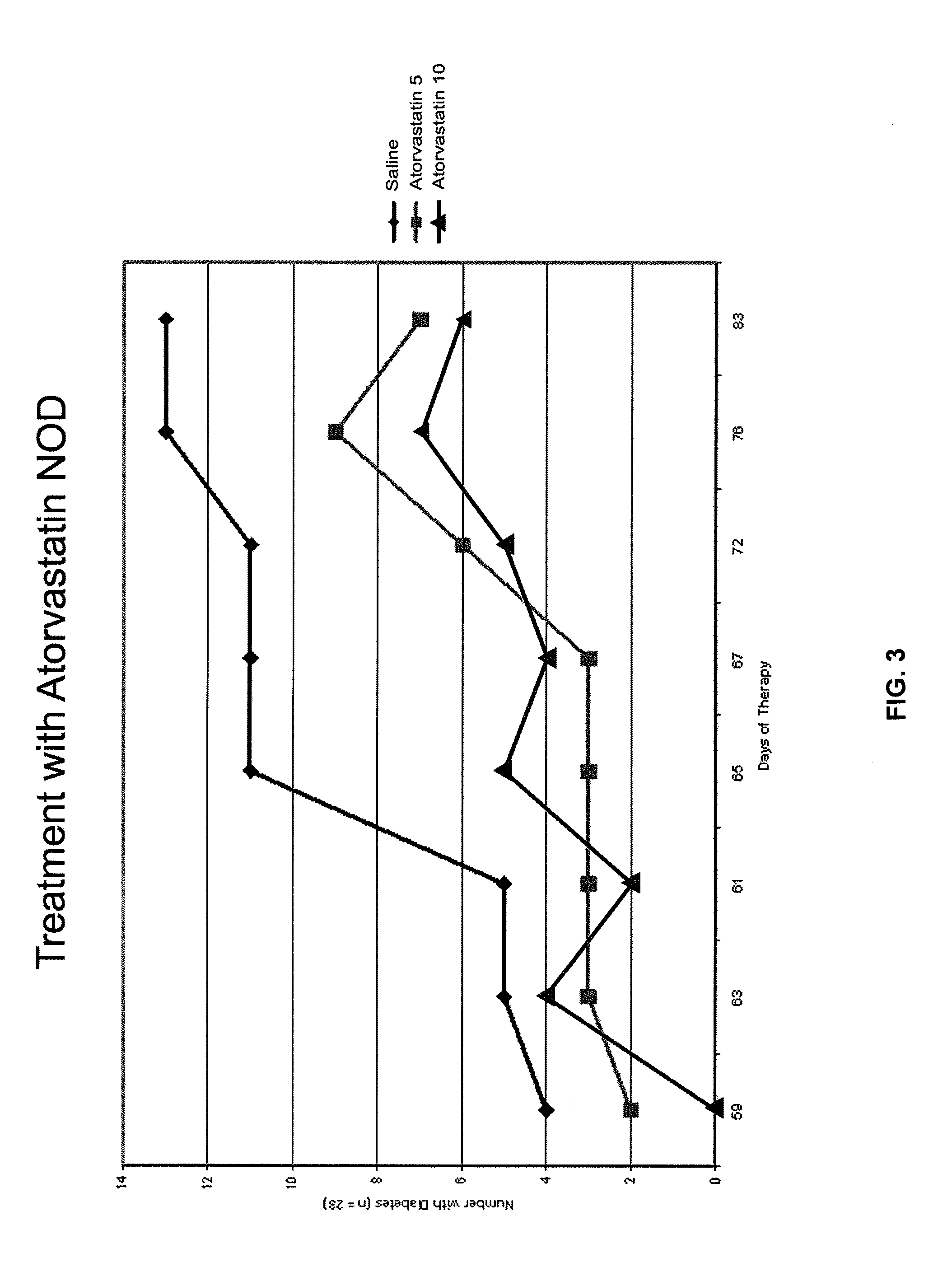
InactiveUS20080227846A1Reducing chronic complicationReducing premature deathBiocideMetabolism disorderNewly diagnosedIslet cells
The present disclosure describes methods for treating or preventing Type 1 diabetes mellitus in juveniles, particularly in juveniles newly diagnosed with Type 1 diabetes. This prevention or treatment of Type 1 diabetes is achieved by administering one or more therapeutic agents to a juvenile in need, wherein the therapeutic agent is, for example, a competitive inhibitor of mevalonate synthesis, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, or an inducer of AMP protein kinase (AMPK) activity. In certain embodiments, juveniles with Type 1 diabetes are treated with an HMG-CoA reductase inhibitor such as a statin, thereby decreasing the destruction of islet cells, or maintaining endogenous insulin production, in the juvenile.
Owner:MUSC FOUND FOR RES DEV
Device for predicting bone metastasis risk of newly-diagnosed prostate cancer
InactiveCN102968558AAvoid overuseClinically convenientSpecial data processing applicationsNewly diagnosedMedicine
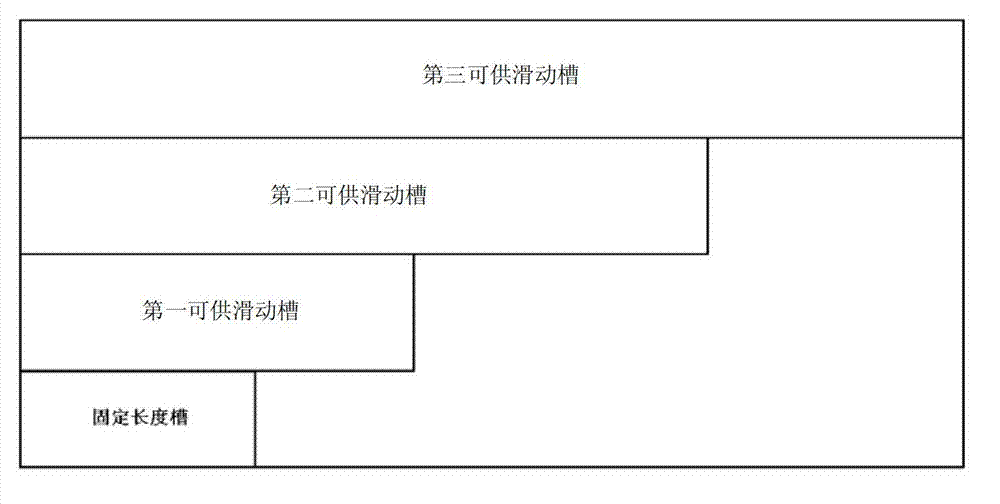
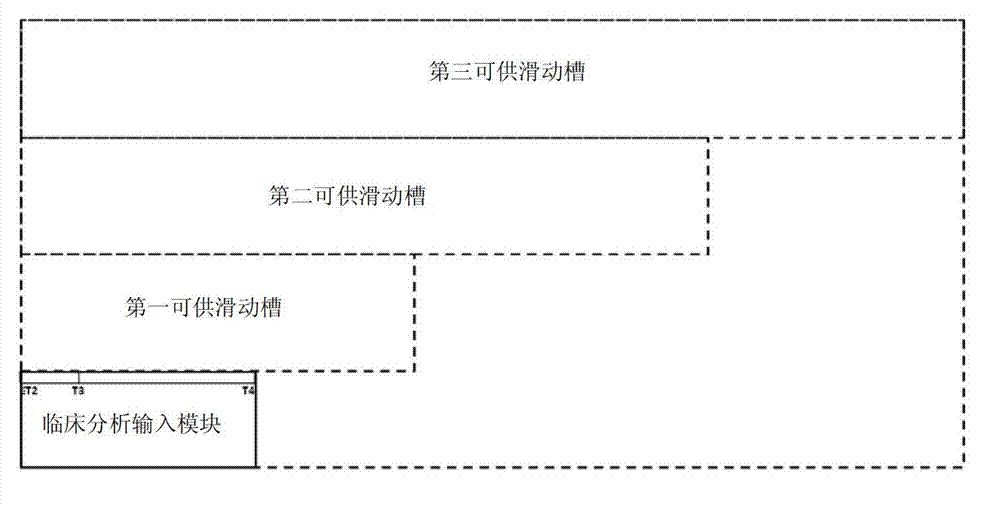
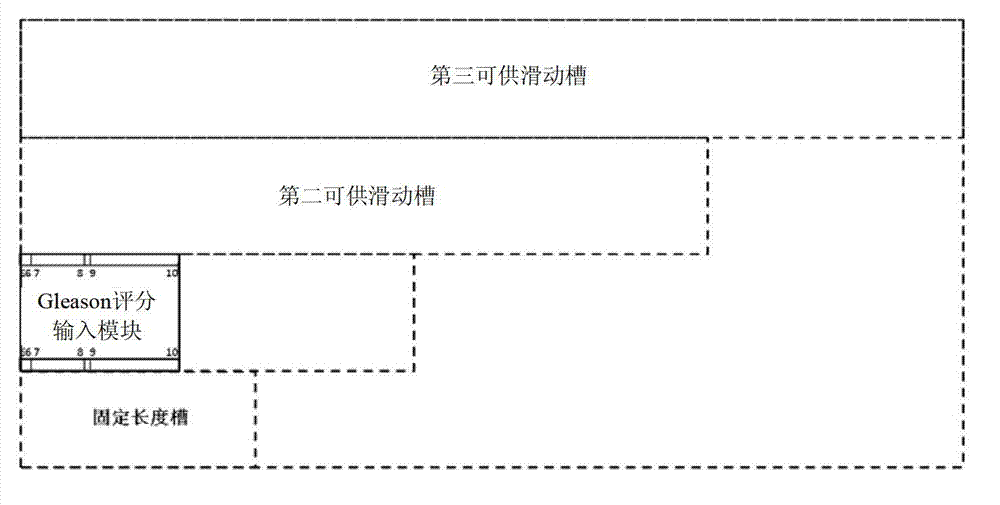
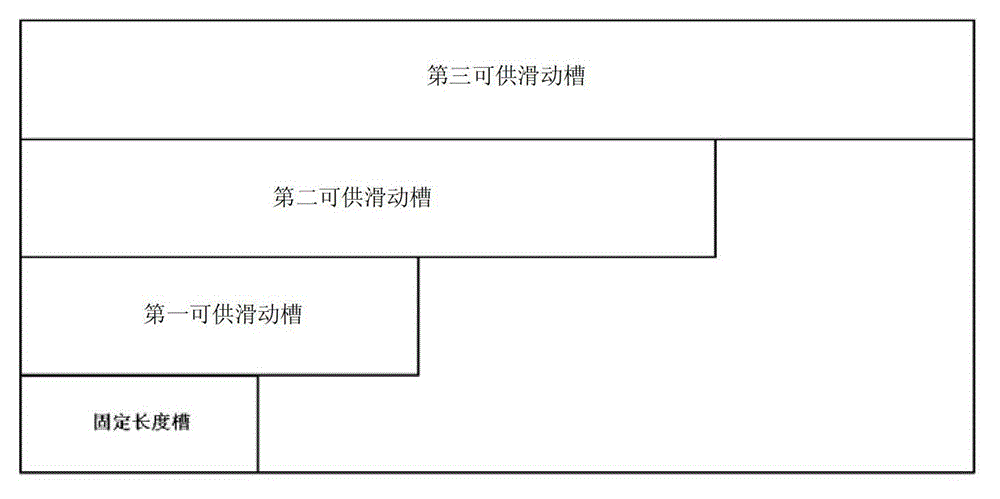
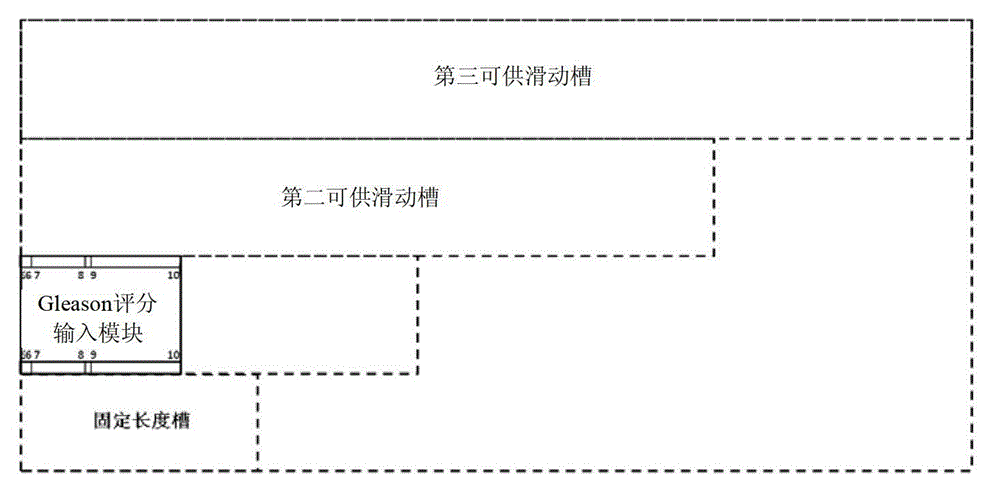
The invention discloses a device for predicting the bone metastasis risk of newly-diagnosed prostate cancer. According to the device, the risk of bone metastasis is predicted, bone scanning is carried out on patients with high bone metastasis risk and the overuse of the bone scanning is avoided under the condition of not increasing missed diagnosis. The technical scheme provided by the invention is that the device comprises a storage module, four input modules, a processing module and an output module, wherein four input slots are formed in the storage module and are a slot with fixed length, a first slot for sliding, a second slot for sliding and a third slot for sliding respectively; the four input modules are a clinical stage input module placed in the slot with the fixed length, a Gleason scoring input module placed in the first slot for sliding, a PSA (Prostate-Specific Antigen) value input module placed in the second slot for sliding and an age input module placed in the third slot for sliding; the processing module is used for adjusting the positions of the four input modules in the input slots of the storage module to obtain an output locus; and the output module is provided with a staff gauge for predicting a risk value of the bone metastasis, and a corresponding locus of the output locus on the staff gauge is a predicted risk value.
Owner:叶定伟
Methods for characterizing and treating acute myeloid leukemia
InactiveUS20170080102A1Reduce probabilityTreating or preventing acute myeloid leukemia relapseOrganic active ingredientsMicrobiological testing/measurementBenzodiazepineNewly diagnosed
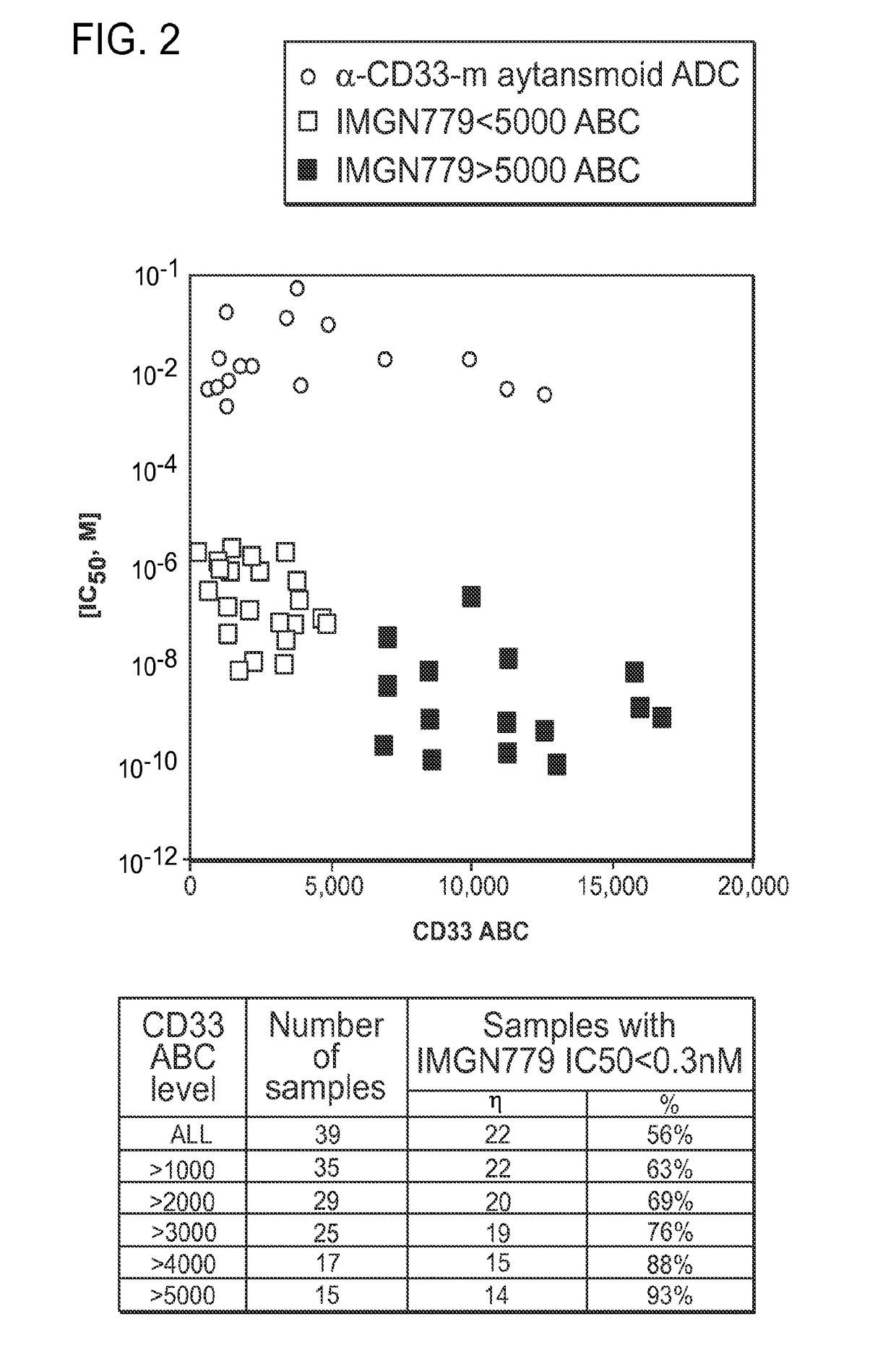
The invention features methods for characterizing and treating acute myeloid leukemia (AML) (e.g., newly diagnosed, relapsed, and refractory AML) in a subject using immunoconjugates of the invention. In one aspect, the invention generally features a method of treating acute myeloid leukemia in a subject (e.g., a human), the method involving administering an effective amount of an immunoconjugate to a pre-selected subject, where the immunoconjugate contains a humanized or chimeric antibody or fragment conjugated to a cytotoxic benzodiazepine dimer compound via a cleavable disulfide linker.
Owner:IMMUNOGEN INC
Molecular determinants of myeloma bone disease and use thereof
InactiveUS20070066558A1Increase bone massBone lossGenetic material ingredientsMicrobiological testing/measurementNewly diagnosedNormal bone
To identify molecular determinants of lytic bone disease in multiple myeloma, the expression profiles of ˜12,000 genes in CD138-enriched plasma cells from newly diagnosed multiple myeloma patients exhibiting no radiological evidence of lytic lesions (n=28) were compared to those with ≧3 lytic lesions (n=47). Two secreted WNT signaling antagonists, soluble frizzled related protein 3 (SFRP-3 / FRZB) and the human homologue of Dickkopf-1 (DKK1), were expressed in 40 of 47 with lytic bone lesions, but only 16 of 28 lacking bone lesions (P<0.05). DKK1 and FRZB were not expressed in plasma cells from 45 normal bone marrow donors or 10 Waldenstrom's macroglobulinemia, a related plasma cells malignancy that lacks bone disease. These data indicate that these factors are important mediators of multiple myeloma bone disease, and inhibitors of these proteins may be used to block bone disease reduce tumor burden in multiple myeloma and to block bone disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Molecular determinants of myeloma bone disease and uses thereof
InactiveUS7459437B2Block alkaline phosphatase productionReduce decreasePeptide/protein ingredientsMicrobiological testing/measurementWaldenstrom macroglobulinemiaNormal bone
To identify molecular determinants of lytic bone disease in multiple myeloma, the expression profiles of ˜12,000 genes in CD138-enriched plasma cells from newly diagnosed multiple myeloma patients exhibiting no radiological evidence of lytic lesions (n=28) were compared to those with ≧3 lytic lesions (n=47). Two secreted WNT signaling antagonists, soluble frizzled related protein 3 (SFRP-3 / FRZB) and the human homologue of Dickkopf-1 (DKK1), were expressed in 40 of 47 with lytic bone lesions, but only 16 of 28 lacking bone lesions (P<0.05). DKK1 and FRZB were not expressed in plasma cells from 45 normal bone marrow donors or 10 Waldenstrom's macroglobulinemia, a related plasma cells malignancy that lacks bone disease. These data indicate that these factors are important mediators of multiple myeloma bone disease, and inhibitors of these proteins may be used to block bone disease.
Owner:UNIV OF ARKANSAS FOR MEDICAL SCI THE
Kit for detecting expression index of mRNA (messager Ribose Nucleic Acid) of WT1 (Wilms Tumor 1) gene
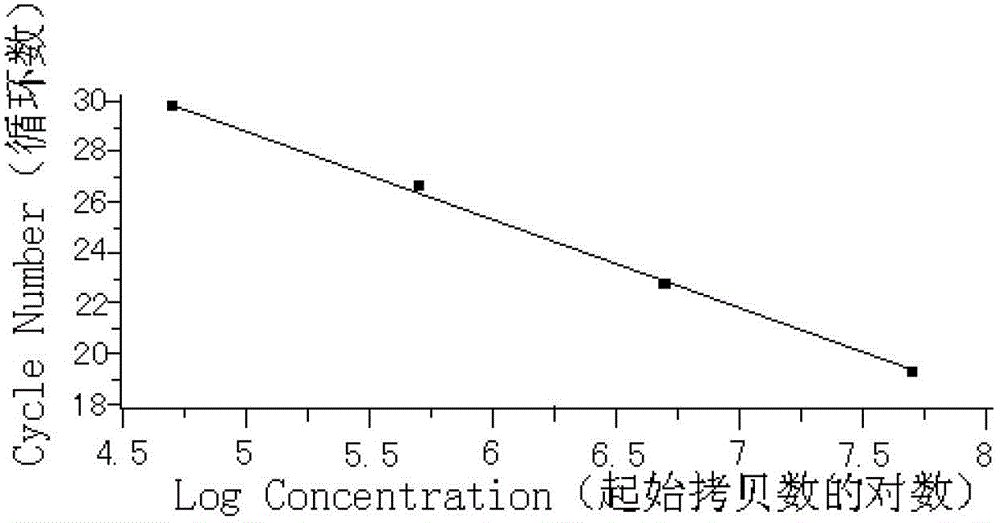
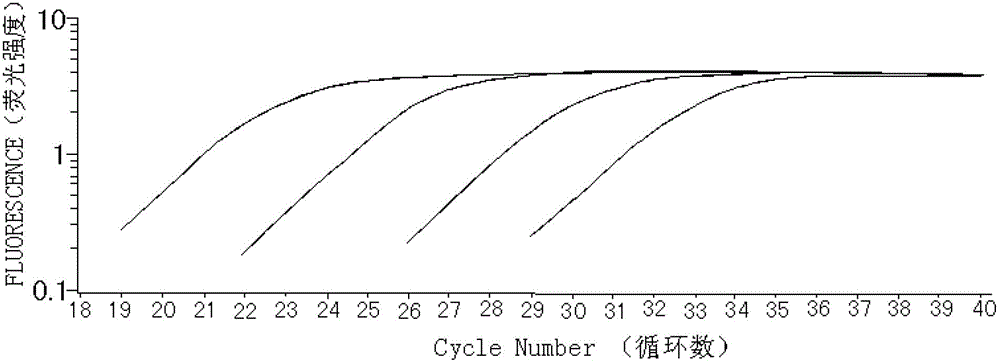
ActiveCN102912018AStrong specificityImprove accuracyMicrobiological testing/measurementFluorescence/phosphorescenceNewly diagnosedDisease monitoring
The invention relates to a kit for detecting an expression index of mRNA (messager Ribose Nucleic Acid) of a WT1 (Wilms Tumor 1) gene, and belongs to the field of biotechnology. The kit comprises detection primers, a fluorescent probe, a cDNA (complementary Deoxyribose Nucleic Acid) first strand synthesis reagent, a fluorescent quantitative PCR (Polymerase Chain Reaction) mixed solution, negative reference and positive reference, wherein the detection primers and the fluorescent probe comprise a WT1 gene primer, an internal reference gene ABL primer and a Taqman fluorescent probe. The WT1 gene is related with hematopoietic tumor incidence, is of over-expression in about 80% of patients with newly diagnosed acute myelocytic leukemia and acute lymphocytic leukemia, is recognized as a leukemia marker gene, and can serve as an independent minimal residue disease monitoring and prognosis prompting index. The level of the mRNA of the WT1 gene is detected by adopting a fluorescent quantitative PCR technology with higher sensitivity and specificity, and both the specificity and the sensitivity of a detection result are remarkably improved. The kit provides a brand-new quick, simple and convenient gene diagnosis technology for prognosing the acute myelocytic leukemia and the acute lymphocytic leukemia and confirming chemotherapy regimens.
Owner:童永清 +1
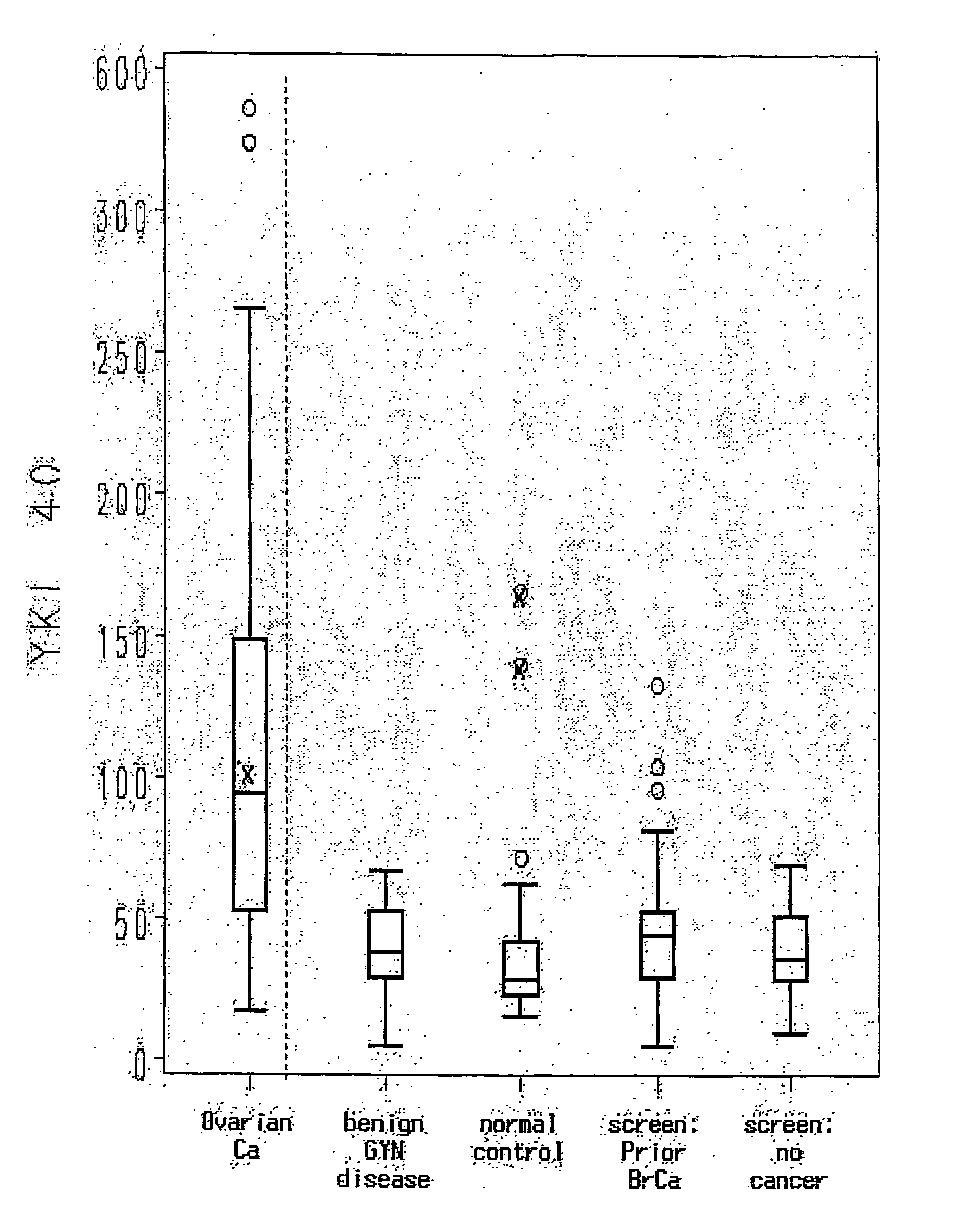
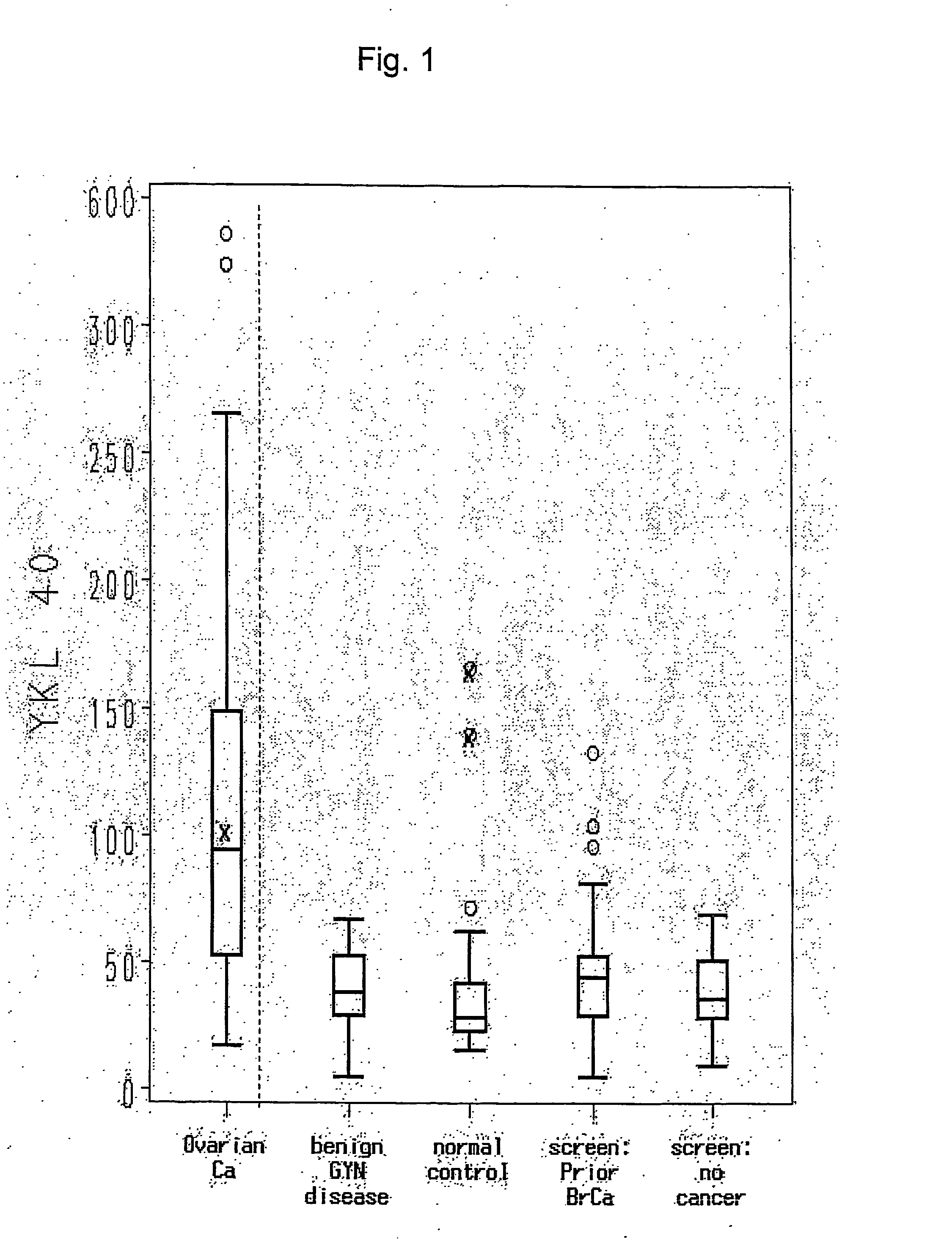
Method for Early Detection of Ovarian Cancer
InactiveUS20070269831A1Facilitating early treatmentIncrease risk factorMedical preparationsBiological testingNewly diagnosedTreatment choices
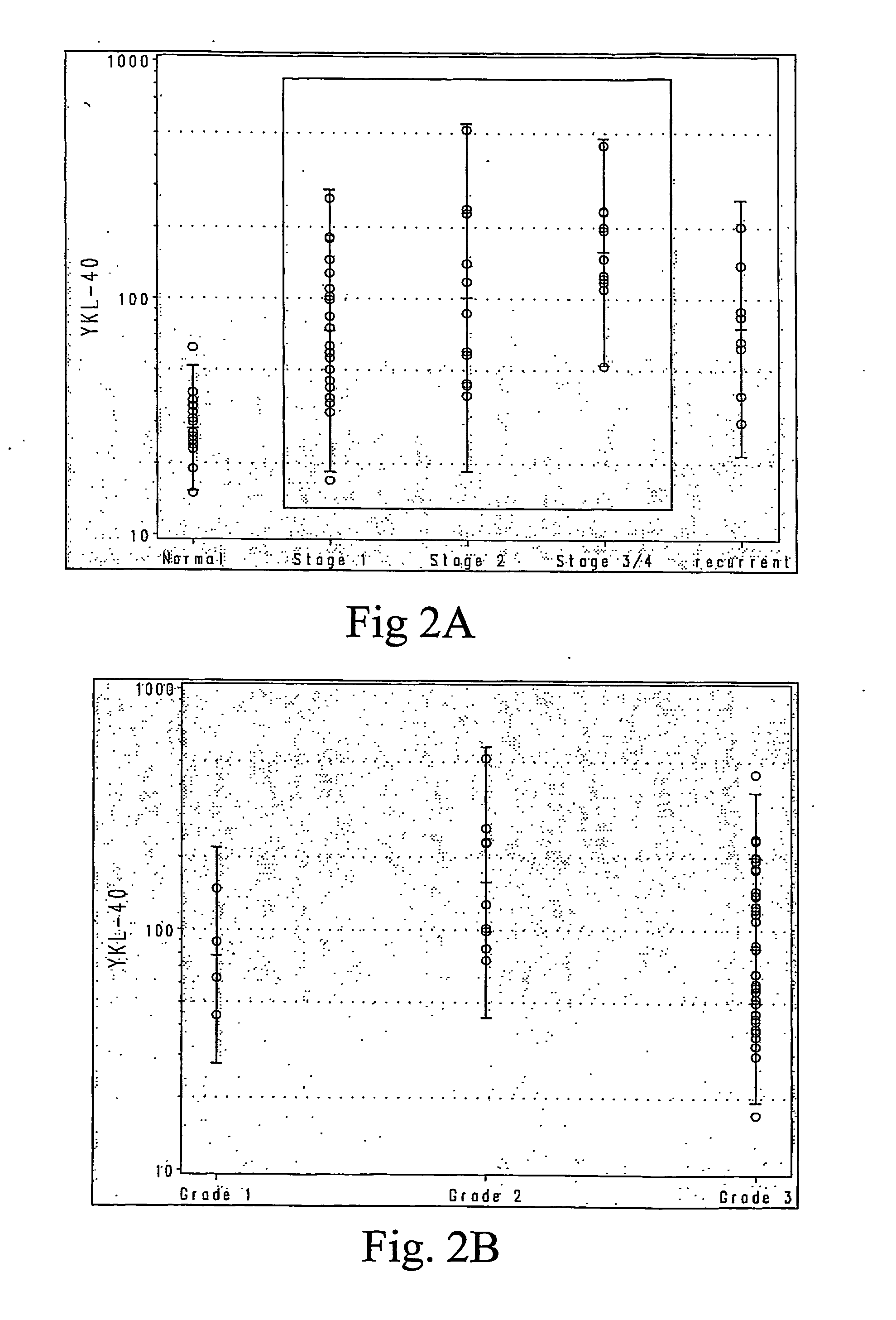
YKL-40 levels in serum and plasma samples provide as an indicator of both the presence of early stage ovarian cancer and of its aggressiveness. Thus testing for YKL-40 levels in patients at risk but not yet displaying symptoms associated with ovarian cancer, or in patients newly diagnosed with early stage ovarian cancer (Stage I or II) enhance both the detection of early stage ovarian cancer and selection of treatment protocols used for early stage ovarian cancer patients.
Owner:SLOAN KETTERING INST FOR CANCER RES
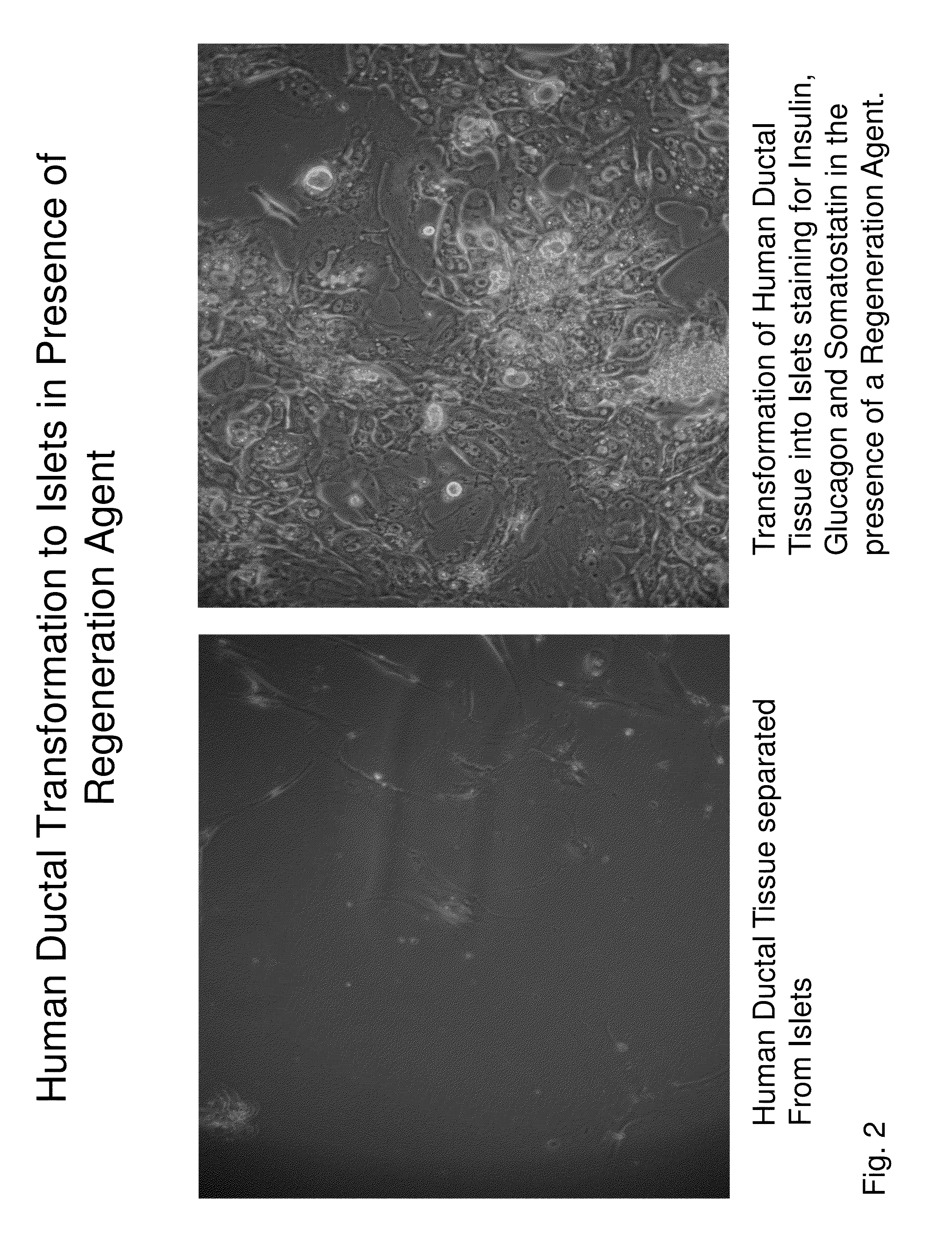
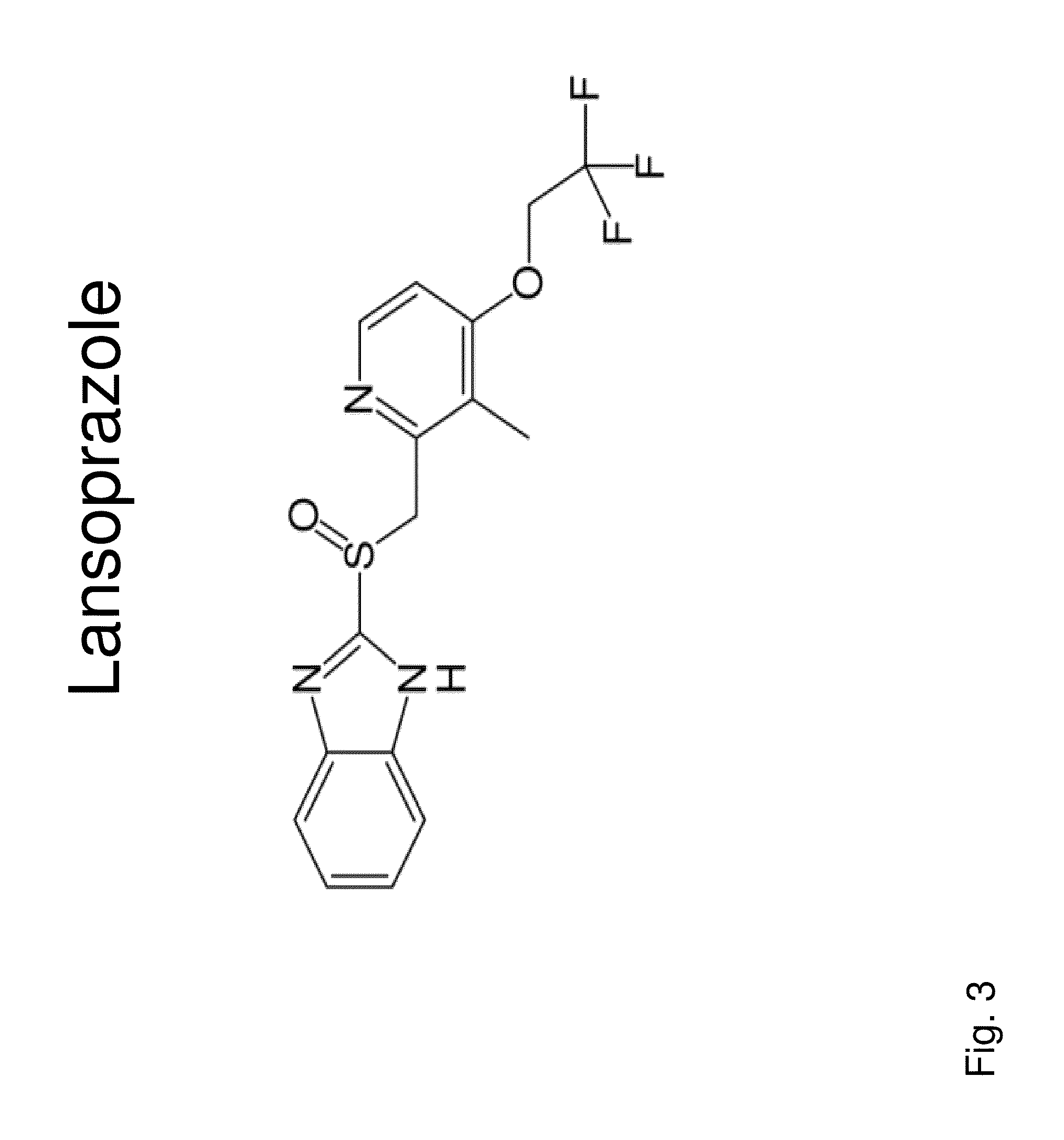
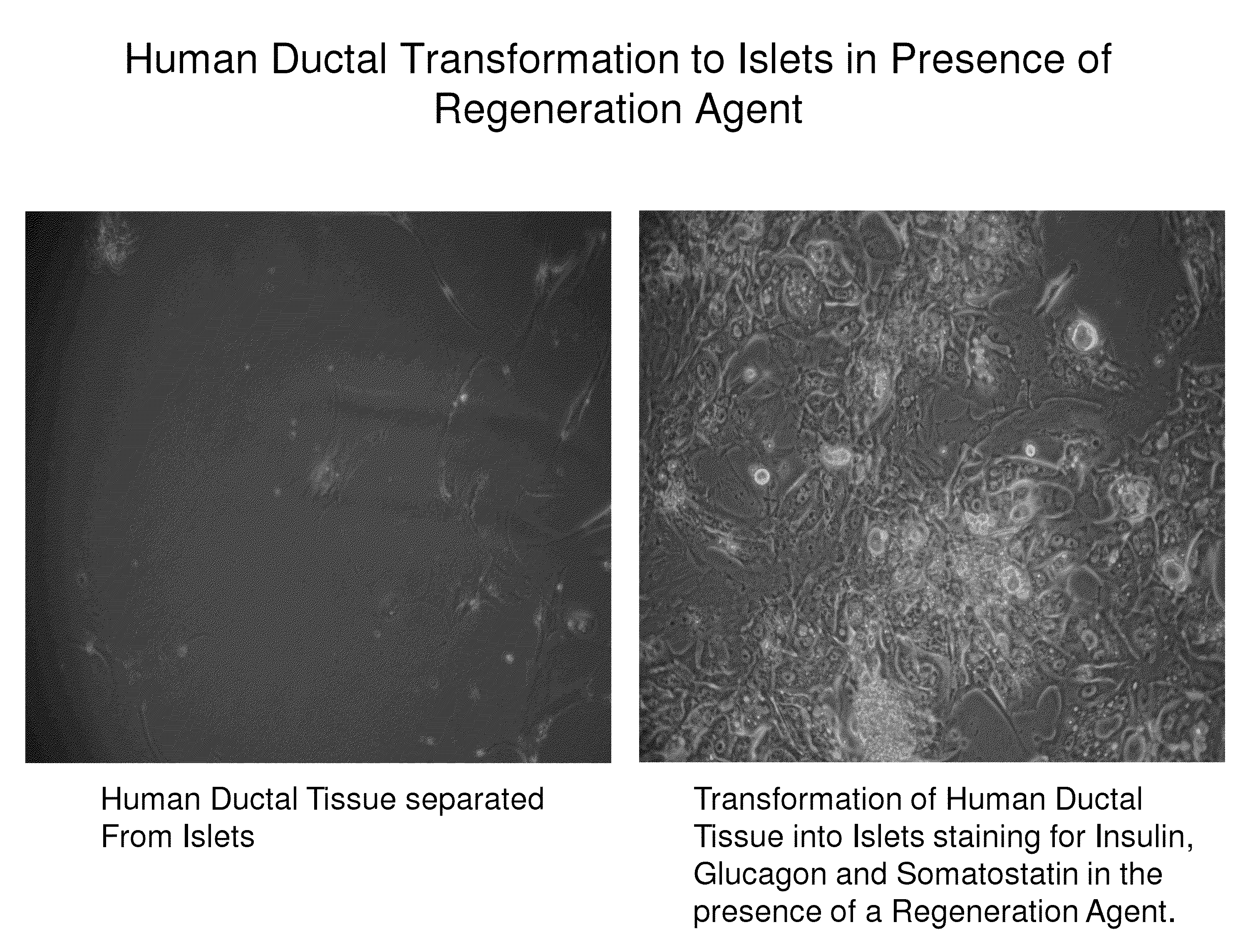
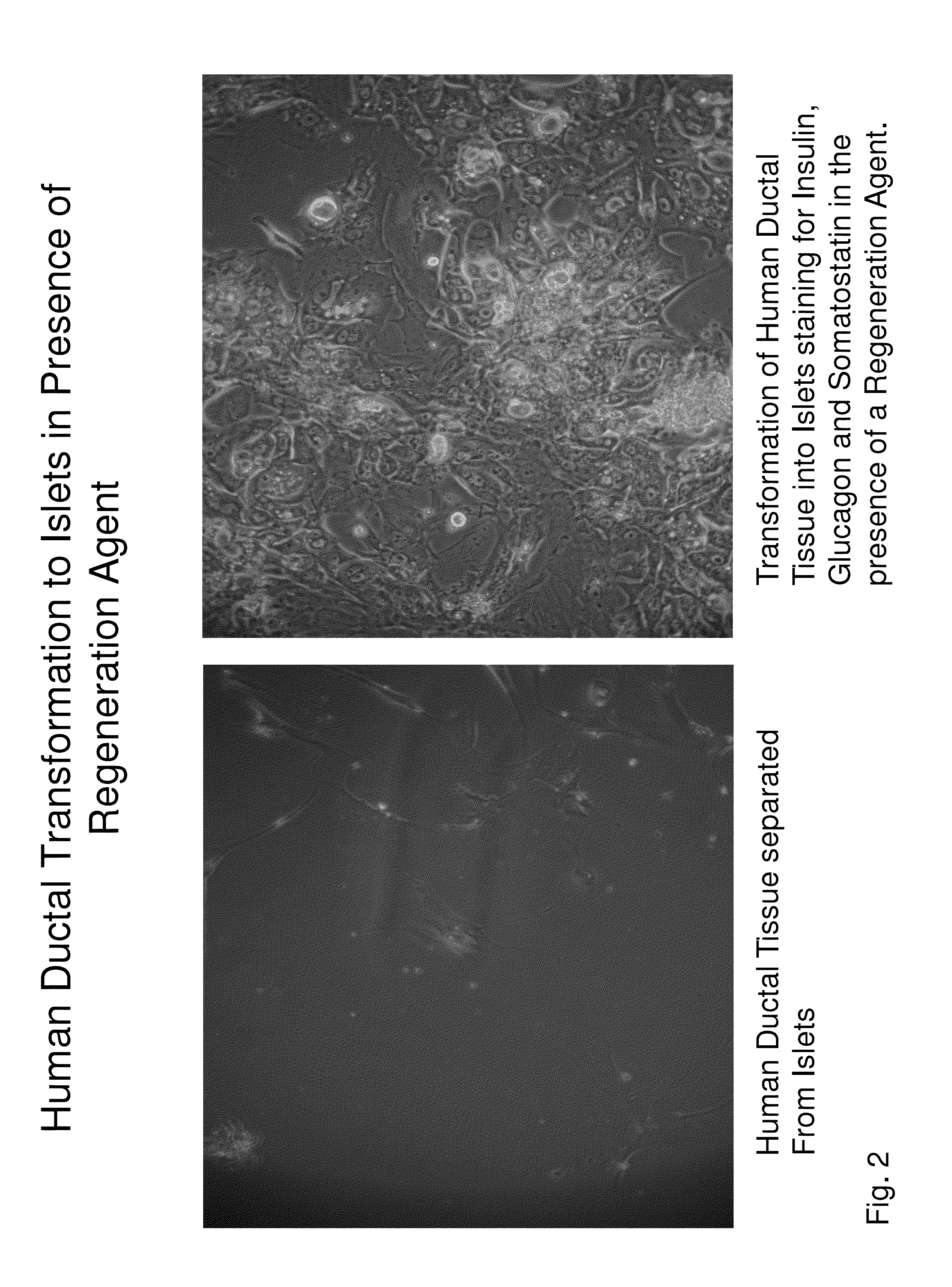
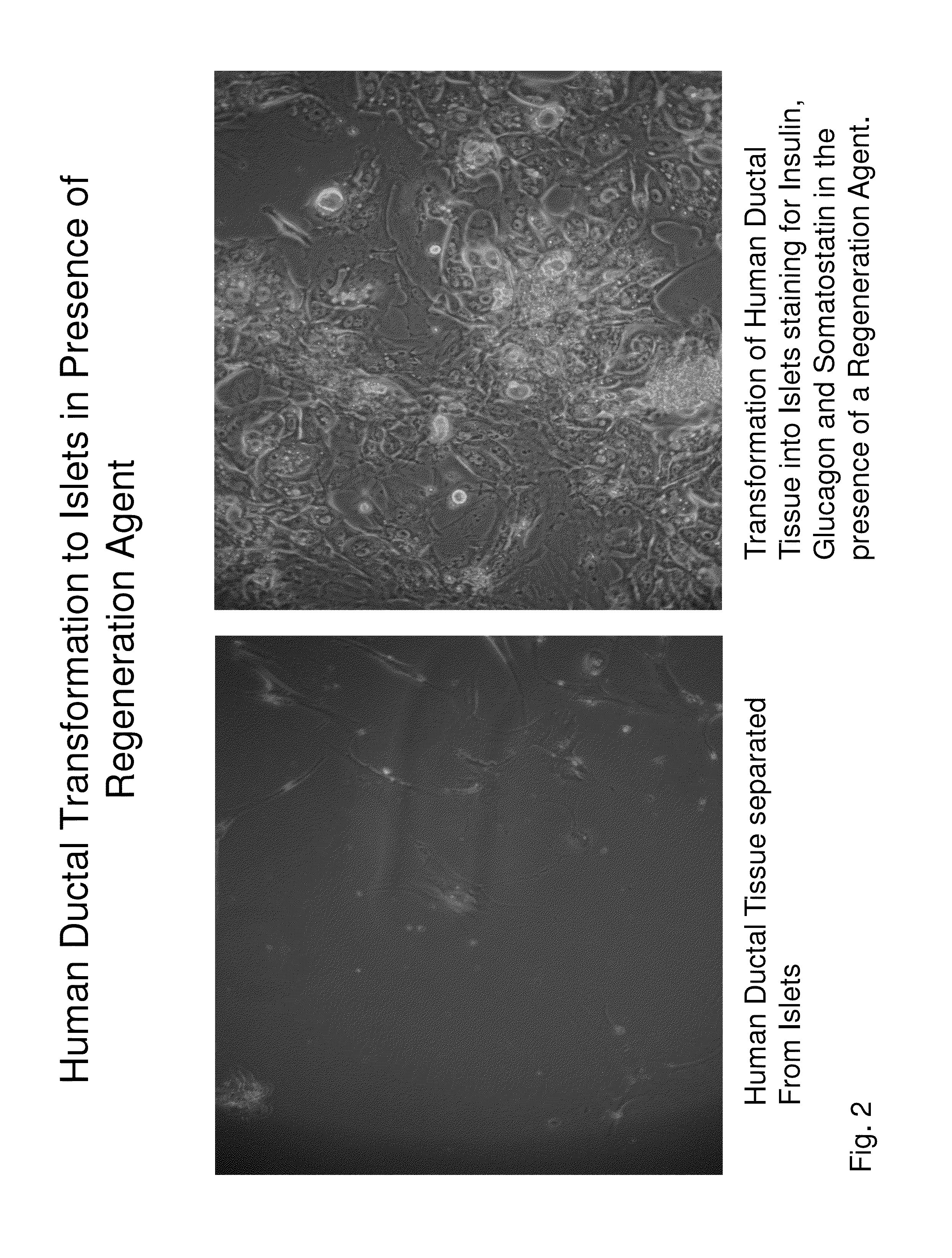
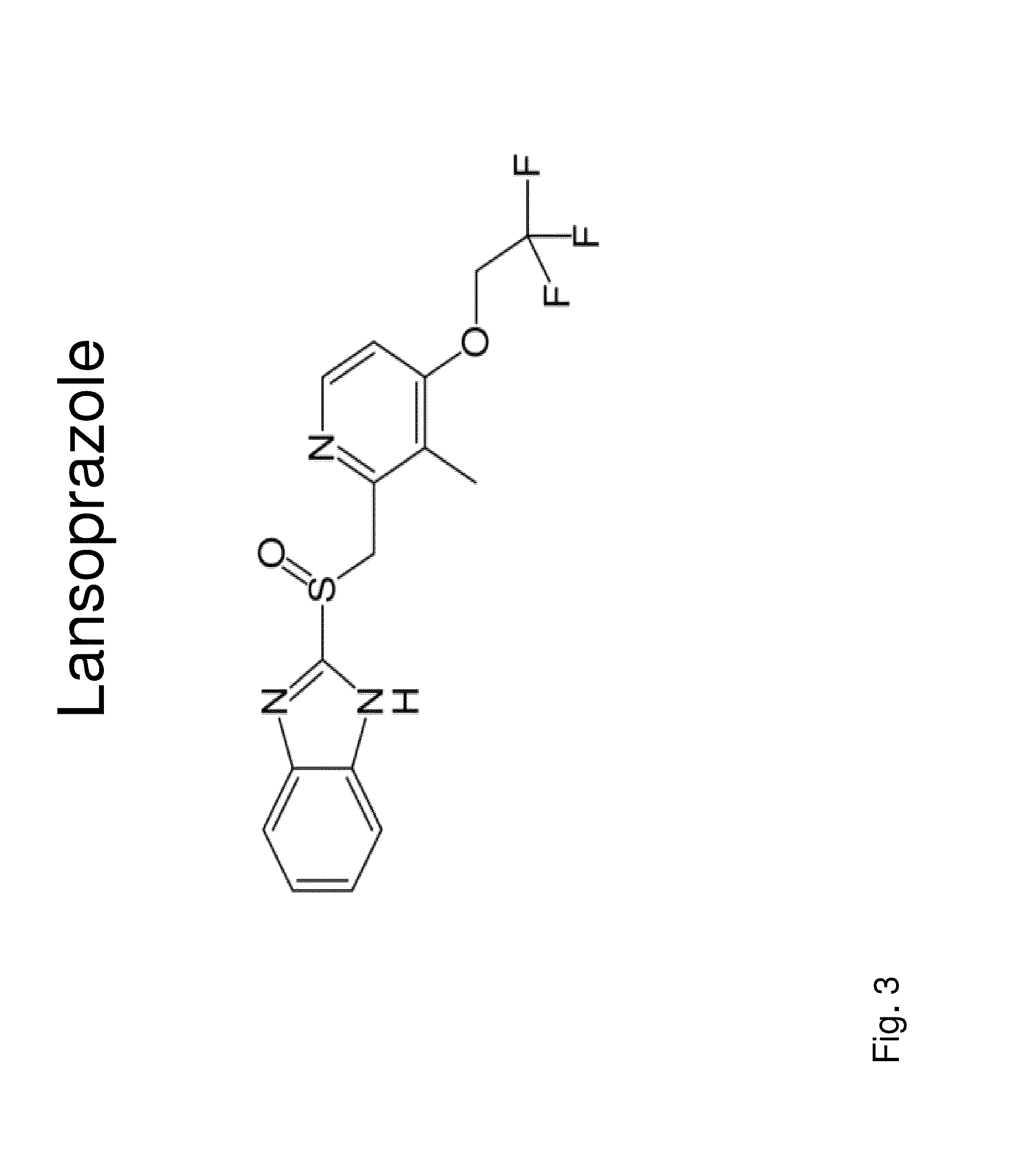
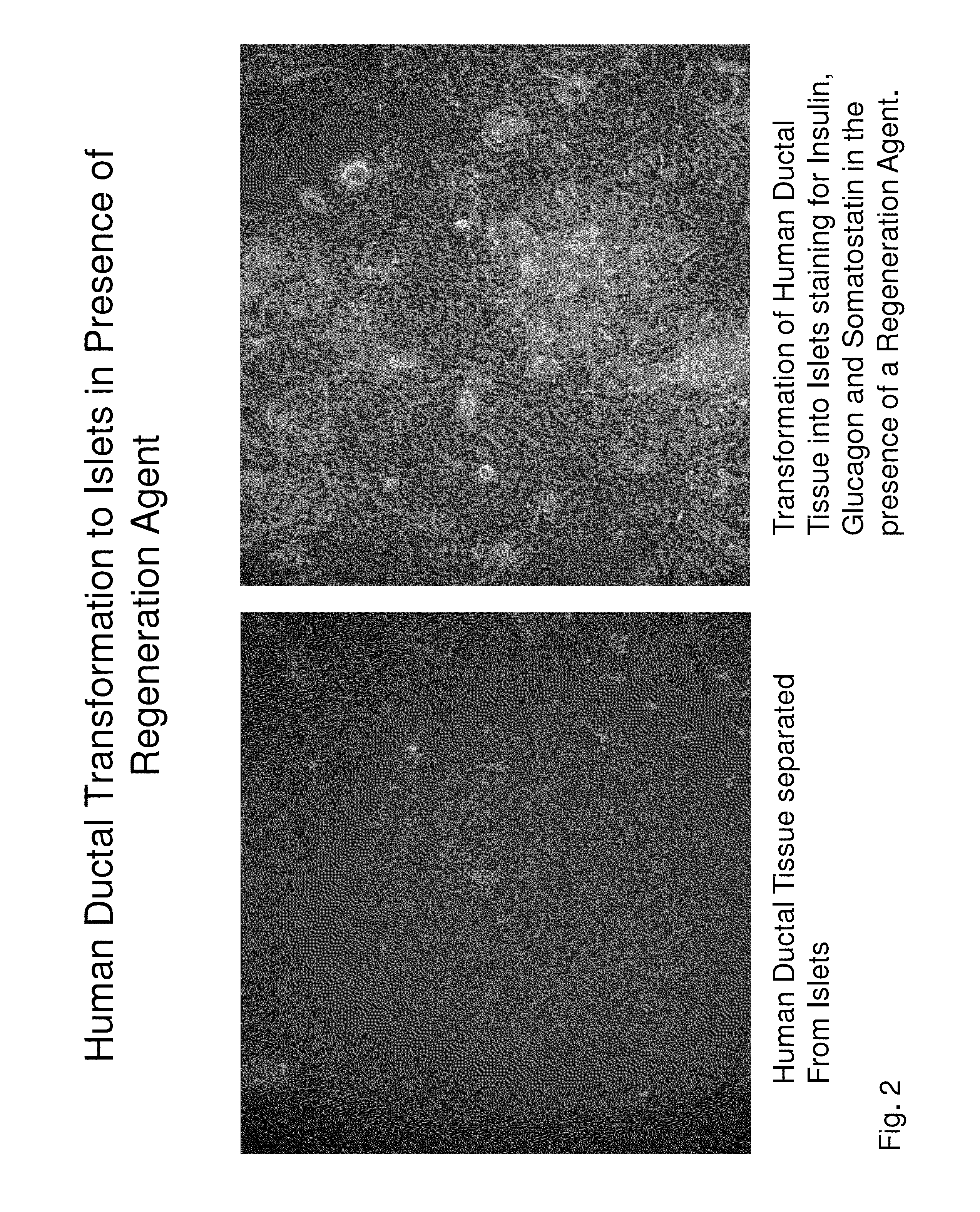
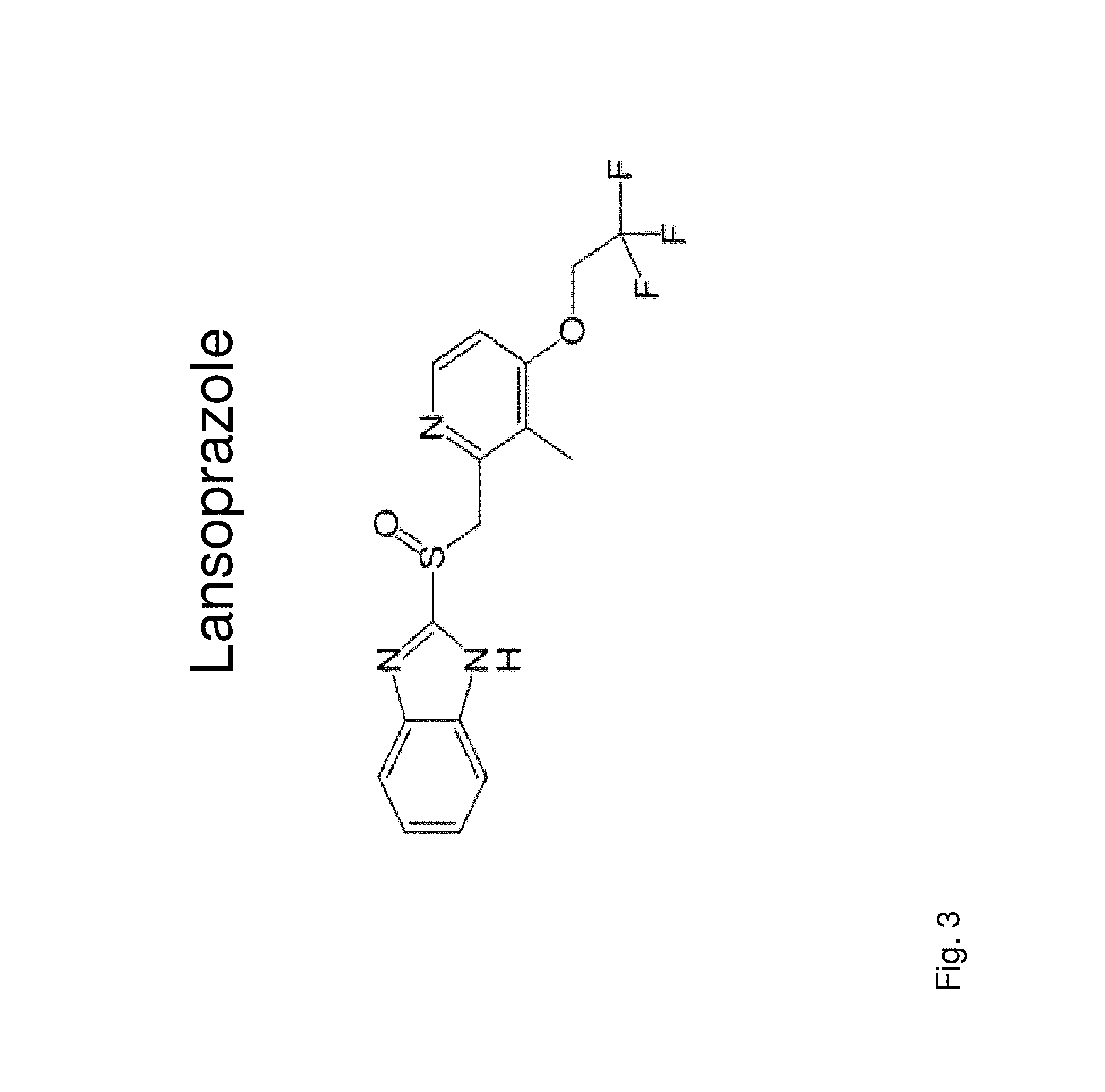
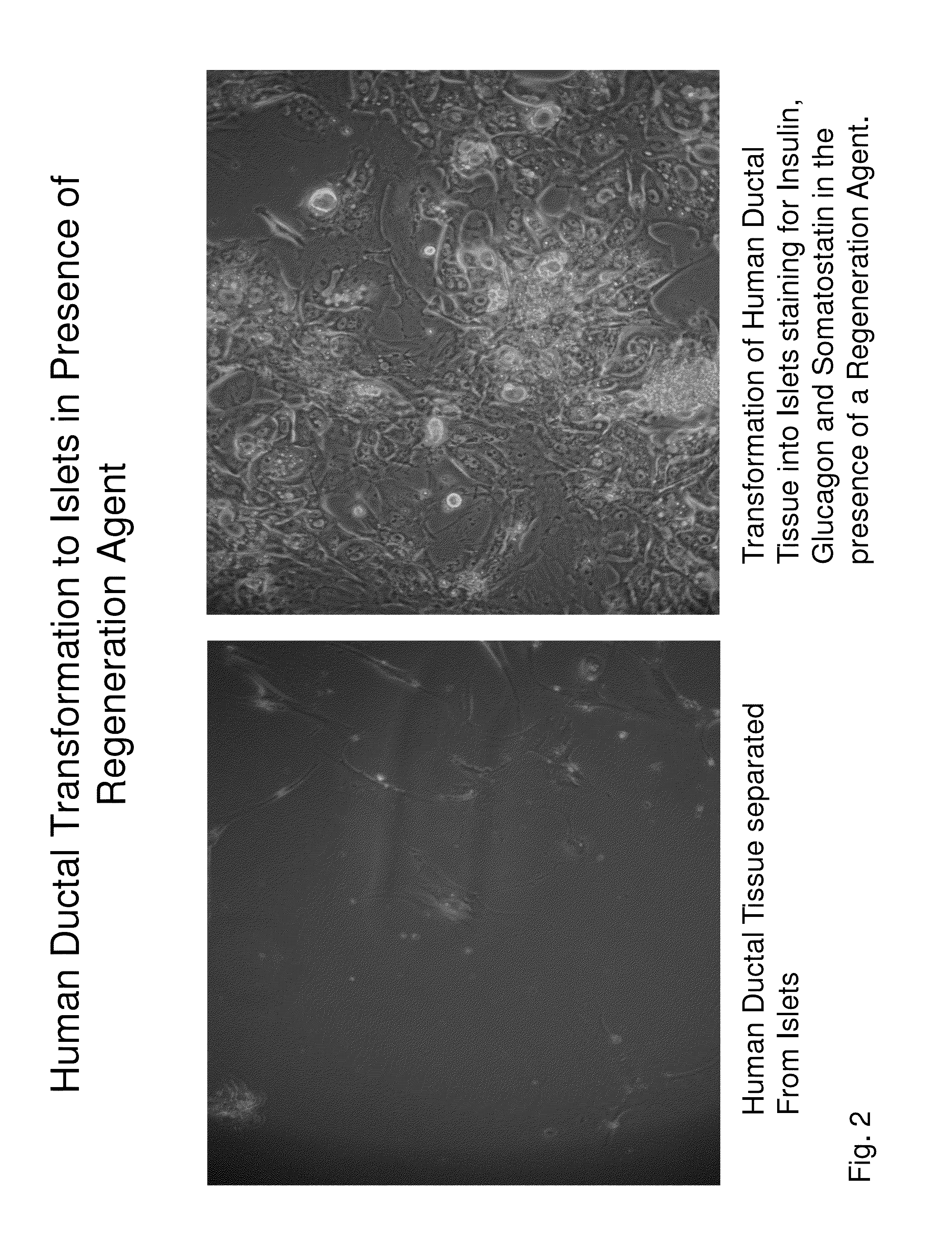
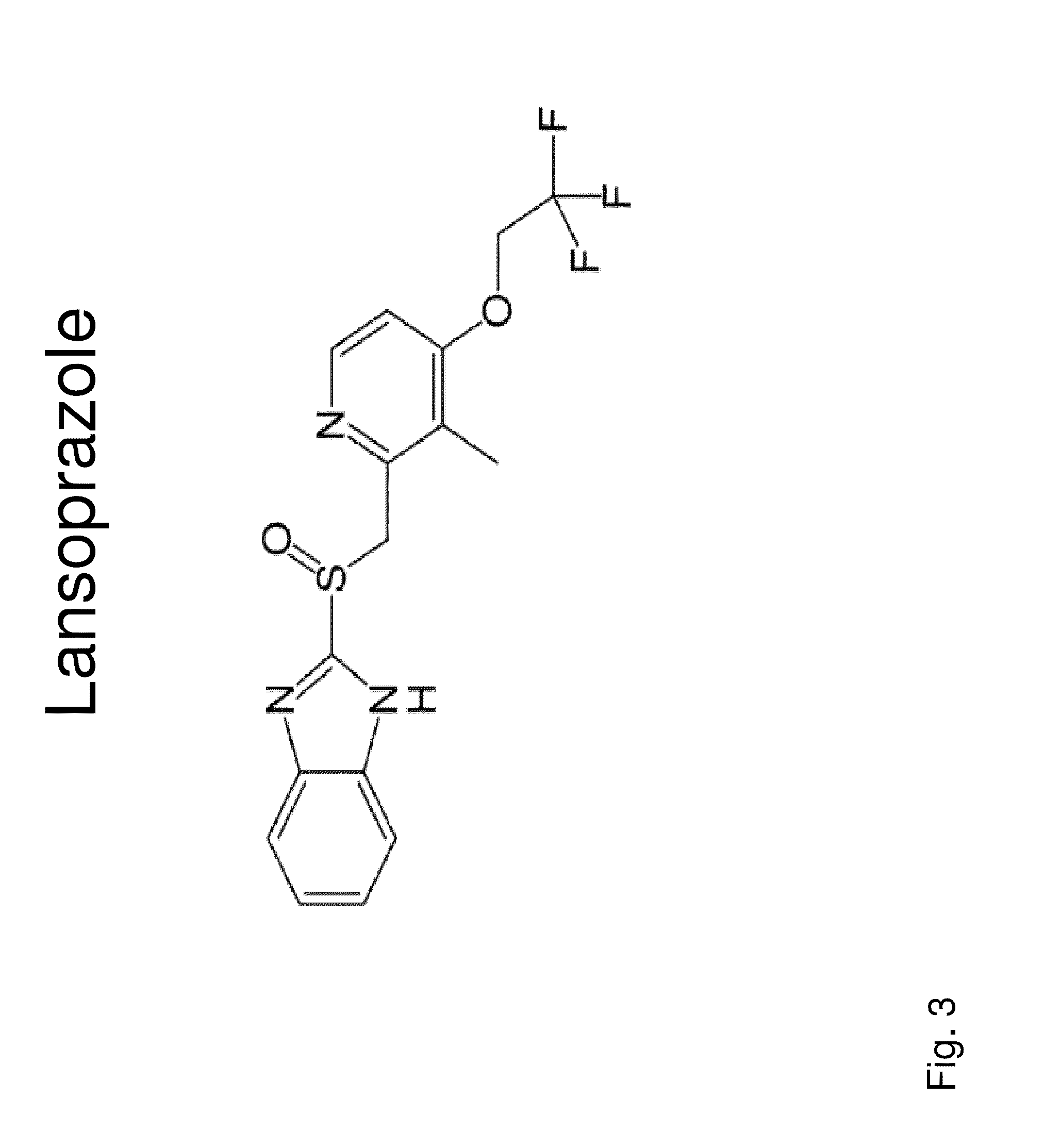
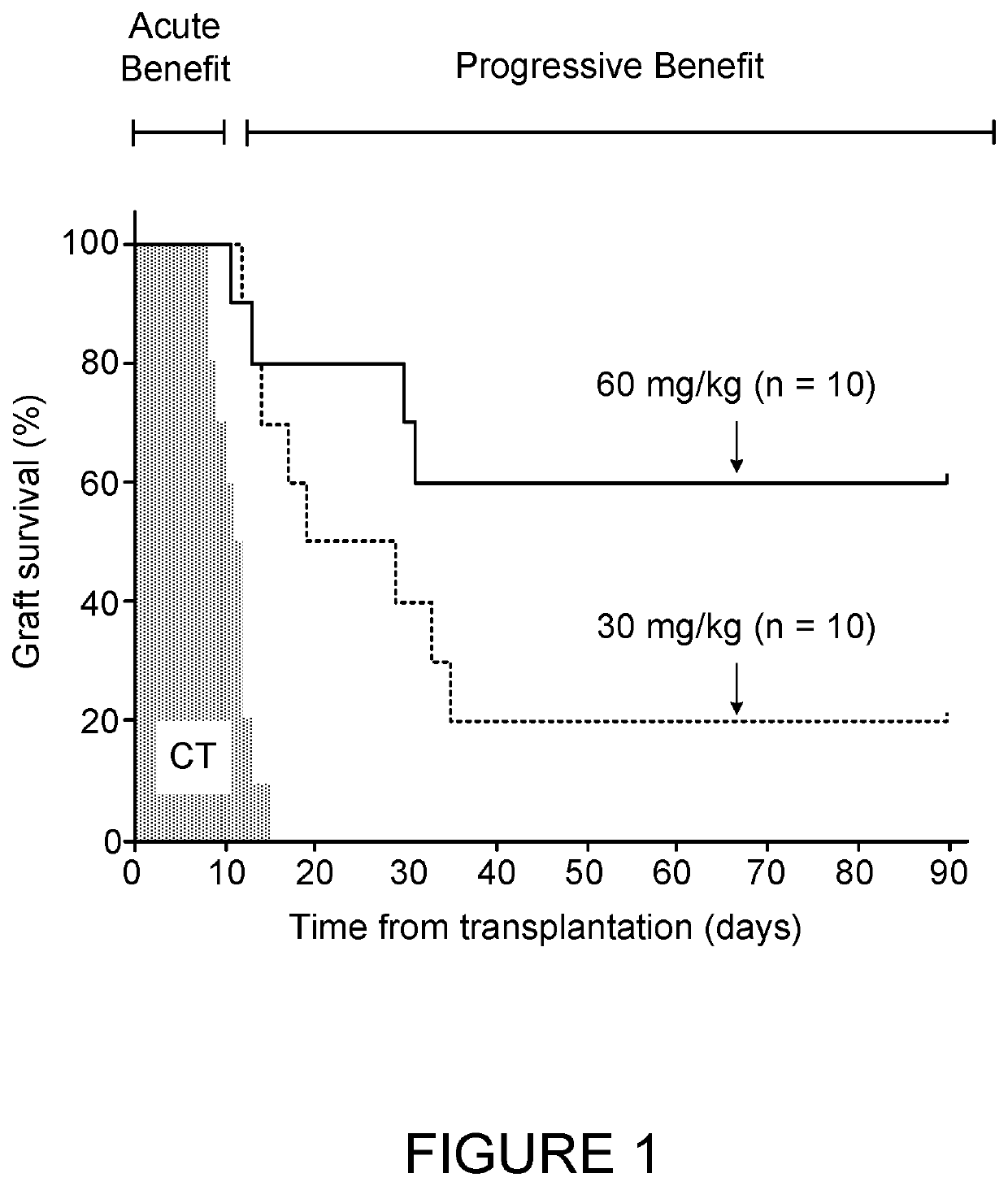
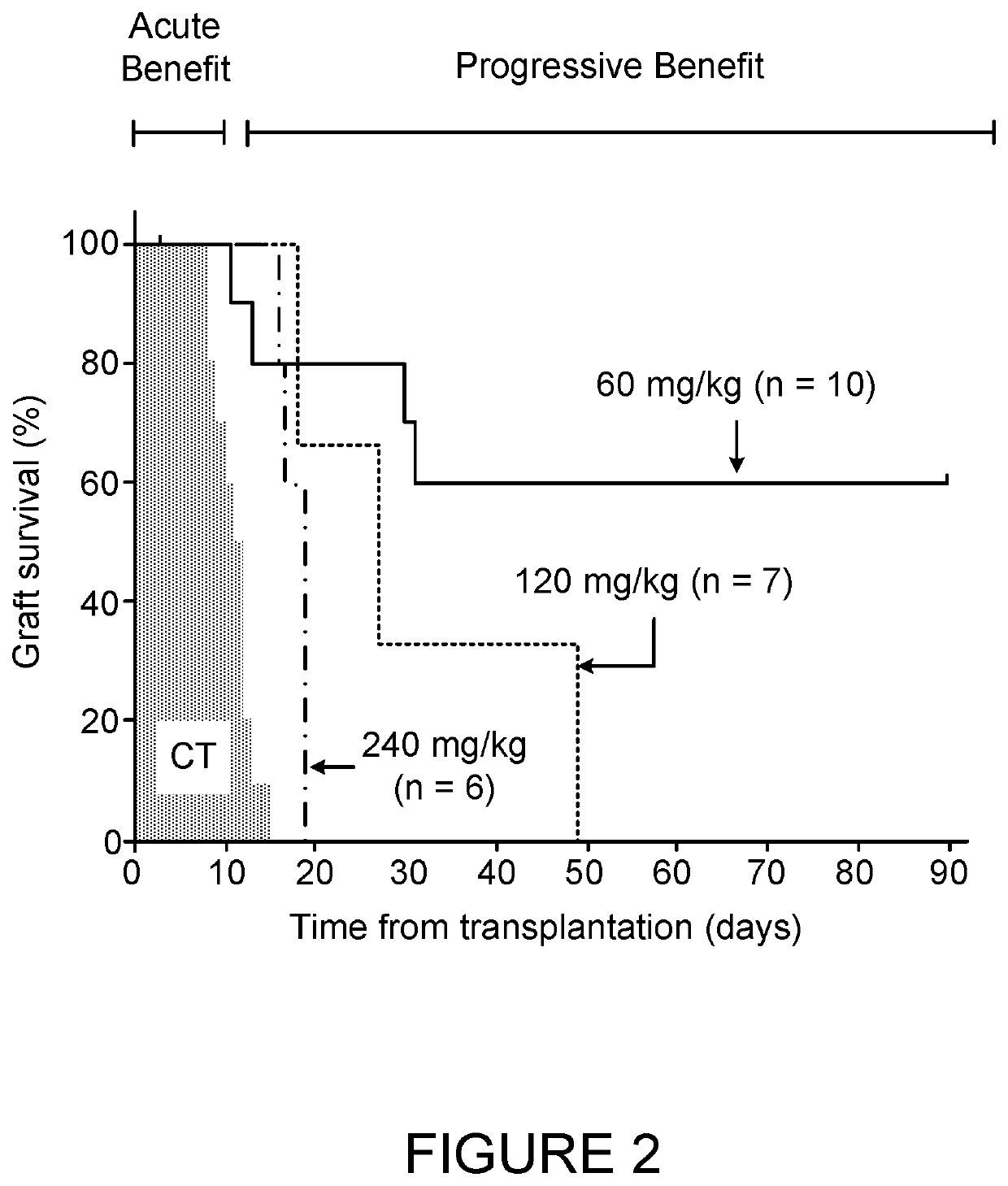
Insulin independence among patients with diabetes utilizing a ppi in combination with an immune tolerance agent
InactiveUS20150174111A1Improve the level ofProtected from vandalismBiocideBacterial antigen ingredientsNewly diagnosedImmune tolerance
To date, no immune tolerance agent or combination of immune tolerance agents has been able to sustain insulin-independence among type 1 diabetes patients. This patent provides methods and pharmaceutical compositions for providing insulin independence among newly diagnosed and existing type 1 diabetes. Methods include utilization of PPIs, which increase gastrin resulting in the transformation of human ductal tissue into insulin-secreting new beta cells, used in combination with an immune tolerance agent to protect the new insulin-producing beta cells generated by the PPI from immune destruction. Compositions and methods are provided for beta cell generation therapy comprising at least one member from a group of PPIs with formulations selected from immune tolerance agents, when used in combination result in insulin-independence among new and existing type 1 patients whom currently require insulin to sustain life. Compositions and methods are provided for insulin-independence among type 2 patients using PPIs when combined with therapeutic agents utilized for the treatment of type 2 diabetes.
Owner:LEVETAN CLARESA
Insulin independence among patients with diabetes utilizing a PPI in combination with an immune tolerance agent
InactiveUS20140234405A1Improve the level ofIncrease in plasma levelBiocideMetabolism disorderNewly diagnosedImmune tolerance
To date, no immune tolerance agent or combination of immune tolerance agents has been able to sustain insulin-independence among type 1 diabetes patients. This patent provides methods and pharmaceutical compositions for providing insulin independence among newly diagnosed and existing type 1 diabetes. Methods include utilization of PPIs, which increase gastrin resulting in the transformation of human ductal tissue into insulin-secreting new beta cells, used in combination with an immune tolerance agent to protect the new insulin-producing beta cells generated by the PPI from immune destruction. Compositions and methods are provided for beta cell generation therapy comprising at least one member from a group of PPIs with formulations selected from immune tolerance agents, when used in combination result in insulin-independence among new and existing type 1 patients whom currently require insulin to sustain life. Compositions and methods are provided for insulin-independence among type 2 patients using PPIs when combined with therapeutic agents utilized for the treatment of type 2 diabetes.
Owner:PERLE BIOSCI
Orthopedic persistent ailment treating medicament and preparation method thereof
InactiveCN101518610AIncrease weightPrevent diarrheaAntibacterial agentsHeavy metal active ingredientsNewly diagnosedSalvia miltiorrhiza
The invention relates to an orthopedic persistent ailment treating medicament and a preparation method thereof. The medicament takes danshen root, red paeony root, cortex phellodendri, Thunberg Fritillary bulb, native copper, Niaotong leaf, lytta, Digen, Huechys sanguine and pubescent holly root as raw materials. The preparation method comprises that: selected medication parts are washed, soaked and sliced, dried by Qi phase heat technology and ground into powder; the medicinal powder is filled into a small cloth bag of which the opening is then fastened; and Chinese medicament drinking tablets are directly filled into a medicinal pot, the drinking tablets are put on the bottom of the medicinal pot, the medicinal powder is put on the drinking tablets, and the drinking tablets and the medicinal powder are slowly decocted by slow fire, and added with a small amount of edible salt for decoction together. The orthopedic persistent ailment treating medicament, which is synthesized by Chinese medicaments and herbal medicaments, clears Qi phase heat and assists removing gore, treats various bone diseases such as osteomyelitis, bone tuberculosis, newly diagnosed myeloma, arthritis, hyperosteogeny and the like, and the cure rate is 90 percent.
Owner:吴翠青
Device for predicting prostate cancer risk in prostate aspiration biopsy
InactiveCN105653886AAvoid overuseReach painHealth-index calculationSpecial data processing applicationsNewly diagnosedHealth index
Owner:FUDAN UNIV SHANGHAI CANCER CENT
Molecular determinants of myeloma bone disease and use thereof
InactiveUS7811750B2Prevent and reverse bone lossPrevent bone lossMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsWaldenstrom macroglobulinemiaNewly diagnosed
To identify molecular determinants of lytic bone disease in multiple myeloma, the expression profiles of ˜12,000 genes in CD138-enriched plasma cells from newly diagnosed multiple myeloma patients exhibiting no radiological evidence of lytic lesions (n=28) were compared to those with ≧3 lytic lesions (n=47). Two secreted WNT signaling antagonists, soluble frizzled related protein 3 (SFRP-3 / FRZB) and the human homologue of Dickkopf-1 (DKK1), were expressed in 40 of 47 with lytic bone lesions, but only 16 of 28 lacking bone lesions (P<0.05). DKK1 and FRZB were not expressed in plasma cells from 45 normal bone marrow donors or 10 Waldenstrom's macroglobulinemia, a related plasma cells malignancy that lacks bone disease. These data indicate that these factors are important mediators of multiple myeloma bone disease, and inhibitors of these proteins may be used to reduce tumor burden in multiple myeloma and to block bone disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Application of isoalantolactone in preparation of medicines for resisting chronic myeloid leukemia
InactiveCN104739825ASmall molecular weightLow toxicityOrganic active ingredientsAntineoplastic agentsNewly diagnosedMyeloid leukemia
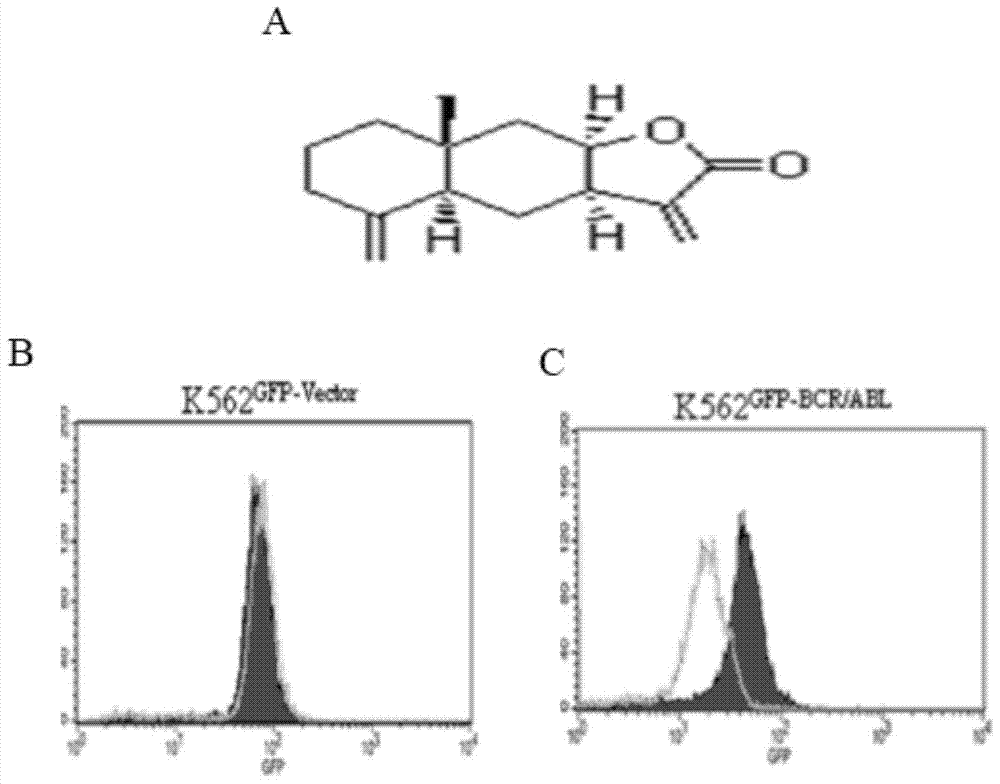
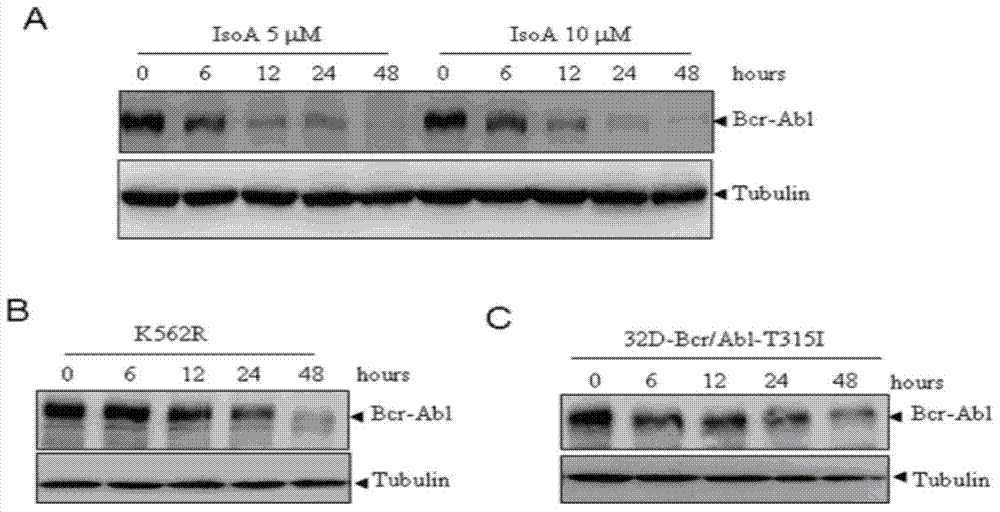
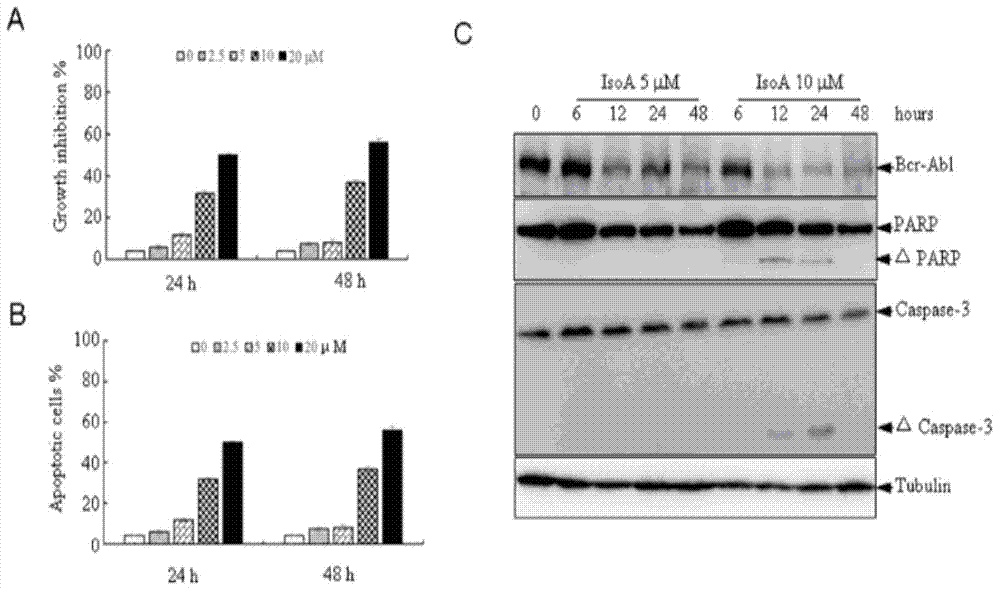
The invention provides application of isoalantolactone in preparation of medicines for resisting chronic myeloid leukemia by screening cell lines with built GFP-Bcr-Abl. The inventor finds out that the anti-CML activity of the isoalantolactone is realized by specifically inducing autolysosome in a K562 cell to actively degrade Bcr-Abl fusion protein, and simultaneously finds out that the isoalantolactone is capable of significantly inhibiting formation of Bcr-Abl positive stem cell cloning in newly diagnosed chronic myeloid patients and medicine-resistant patients. The possibility is provided for treatment medicine-resistant or recurrent CML patients by inducing degradation of the Bcr-Abl fusion protein through a natural small molecular compound of the isoalantolactone.
Owner:上海交通大学医学院附属第三人民医院
Gelatin-coated pill for preventing and treating calculi in vivo
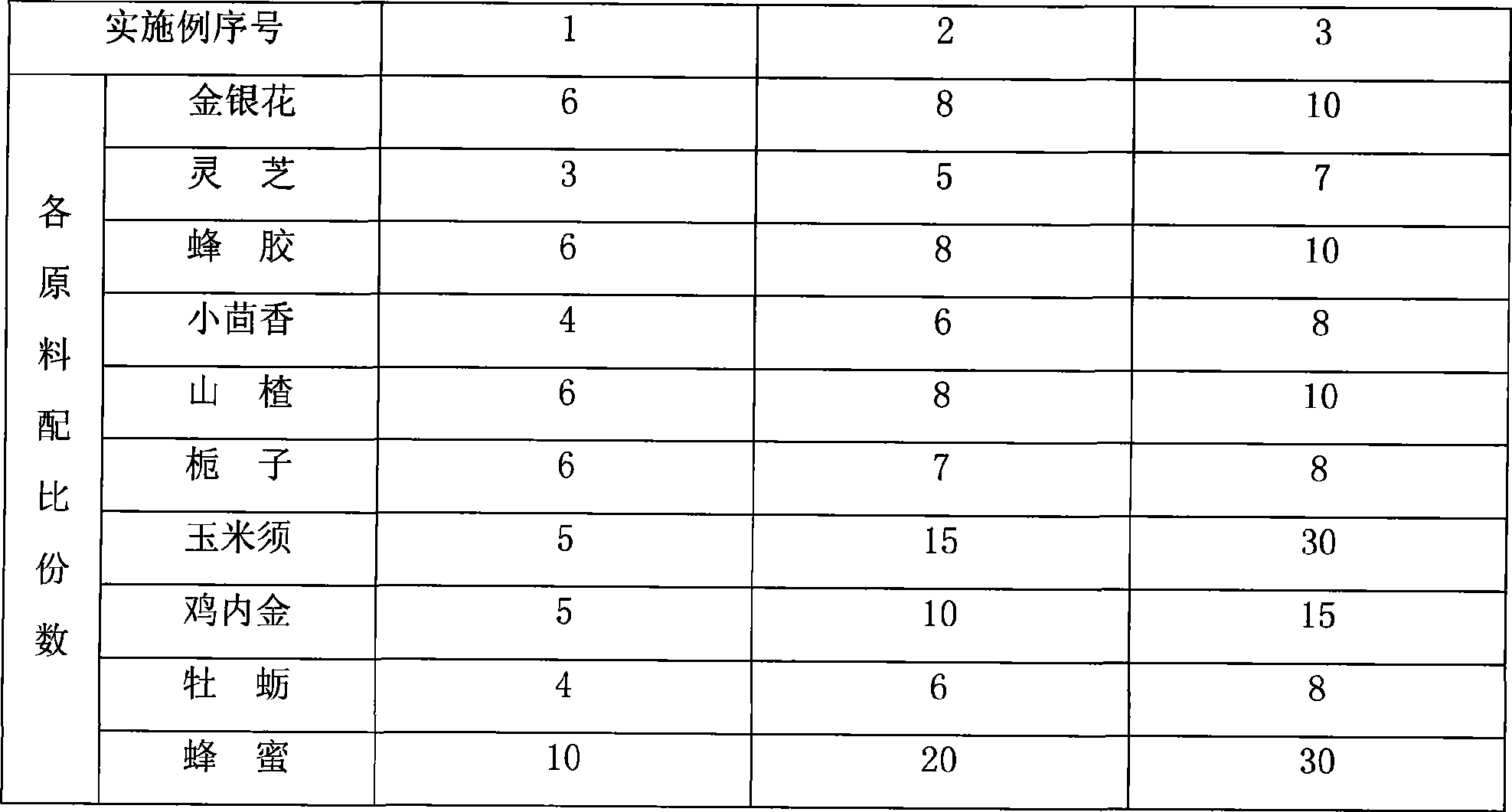
InactiveCN101371908APlay a therapeutic roleInhibition formationAnthropod material medical ingredientsDigestive systemGizzardNewly diagnosed
The invention discloses a soft capsule for treating intracorporeal stones and relates to the field of health care products, the stone symptoms have various types, the causes are different, the traditional Chinese medicine uses different formulas to treat different stone symptoms, the prescribe is difficult to suit the symptoms of newly diagnosed patients, an appropriate formula is more difficult to be selected for the prevention, so a broad-spectrum formula is needed to avoid the difficulty of selection, the soft capsule is proposed for solving the problem, the soft capsule adopts ten materials including chicken's gizzard-membrane, honeysuckle flower, hawthorn fruit, lucid ganoderma, fennel fruit, corn stigma, and the like, the soft capsule is prepared by extracting, concentrating, drying and smashing into powder, the soft capsule has no toxicity or side effects, and the soft capsule further has two efficacies of treatment and prevention and simultaneously has the function of being used with operation therapy, thereby having the beneficial effects of promoting the discharge of residual stones, alleviating the symptoms, and the like.
Owner:吴云
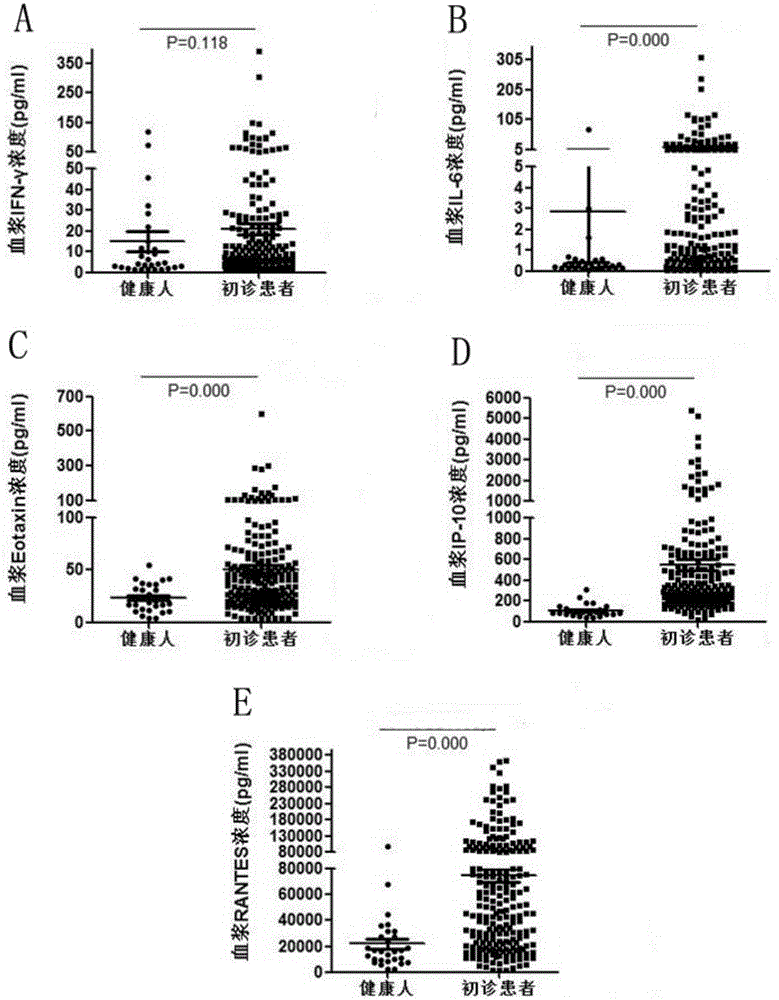
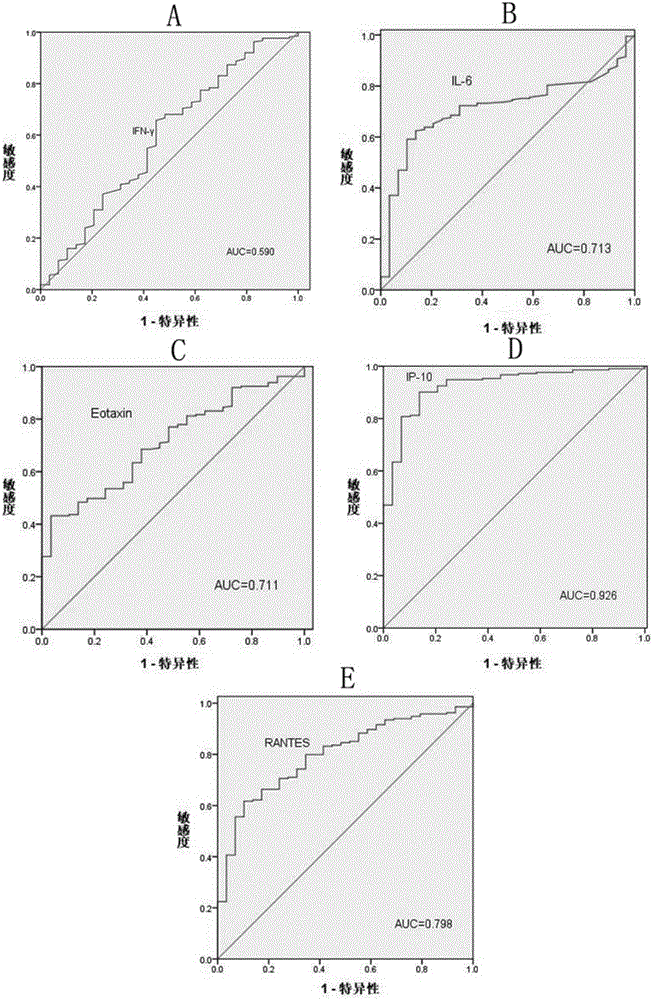
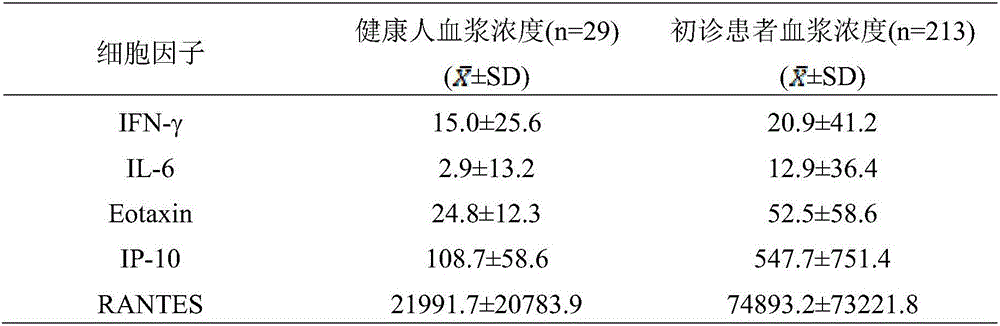
IP10 and interleukin combined cytokine for diagnosis of tuberculosis and kit thereof
InactiveCN106568970AHigh sensitivityImprove featuresDisease diagnosisBiological testingDiseaseNewly diagnosed
The invention relates to application of cytokines IP-10 and IL-6 as a combined marker in preparation of a kit for diagnosis or monitoring of tuberculosis. The cytokines and a combination thereof provided by the invention can realize detection of cytokines in the plasma of newly diagnosed tuberculosis patients, can significantly improve the diagnosibility of pulmonary tuberculosis, and are conducive to prognosis of diseases and treatment situations thereof.
Owner:SHANGHAI RONGSHENG BIOLOGICAL PHARM CO LTD +1
Insulin independence among patients with diabetes utilizing a ppi in combination with an immune tolerance agent
InactiveUS20140235552A1Improve the level ofIncrease in plasma levelBiocideBacterial antigen ingredientsNewly diagnosedImmune tolerance
To date, no immune tolerance agent or combination of immune tolerance agents has been able to sustain insulin-independence among type 1 diabetes patients. This patent provides methods and pharmaceutical compositions for providing insulin independence among newly diagnosed and existing type 1 diabetes. Methods include utilization of PPIs, which increase gastrin resulting in the transformation of human ductal tissue into insulin-secreting new beta cells, used in combination with an immune tolerance agent to protect the new insulin-producing beta cells generated by the PPI from immune destruction. Compositions and methods are provided for beta cell generation therapy comprising at least one member from a group of PPIs with formulations selected from immune tolerance agents, when used in combination result in insulin-independence among new and existing type 1 patients whom currently require insulin to sustain life. Compositions and methods are provided for insulin-independence among type 2 patients using PPIs when combined with therapeutic agents utilized for the treatment of type 2 diabetes.
Owner:PERLE BIOSCI
Insulin independence among patients with diabetes utilizing a PPI in combination with an immune tolerance agent
ActiveUS8808689B1Improve the level ofIncrease in plasma levelBiocidePancreatic cellsNewly diagnosedImmune tolerance
To date, no immune tolerance agent or combination of immune tolerance agents has been able to sustain insulin-independence among type 1 diabetes patients. This patent provides methods and pharmaceutical compositions for providing insulin independence among newly diagnosed and existing type 1 diabetes. Methods include utilization of PPIs, which increase gastrin resulting in the transformation of human ductal tissue into insulin-secreting new beta cells, used in combination with an immune tolerance agent to protect the new insulin-producing beta cells generated by the PPI from immune destruction. Compositions and methods are provided for beta cell generation therapy comprising at least one member from a group of PPIs with formulations selected from immune tolerance agents, when used in combination result in insulin-independence among new and existing type 1 patients whom currently require insulin to sustain life. Compositions and methods are provided for insulin-independence among type 2 patients using PPIs when combined with therapeutic agents utilized for the treatment of type 2 diabetes.
Owner:LEVETAN CLARESA
A device for predicting the risk of bone metastases in newly diagnosed prostate cancer
InactiveCN102968558BAvoid overuseClinically convenientSpecial data processing applicationsNewly diagnosedMedicine
The invention discloses a device for predicting the bone metastasis risk of newly-diagnosed prostate cancer. According to the device, the risk of bone metastasis is predicted, bone scanning is carried out on patients with high bone metastasis risk and the overuse of the bone scanning is avoided under the condition of not increasing missed diagnosis. The technical scheme provided by the invention is that the device comprises a storage module, four input modules, a processing module and an output module, wherein four input slots are formed in the storage module and are a slot with fixed length, a first slot for sliding, a second slot for sliding and a third slot for sliding respectively; the four input modules are a clinical stage input module placed in the slot with the fixed length, a Gleason scoring input module placed in the first slot for sliding, a PSA (Prostate-Specific Antigen) value input module placed in the second slot for sliding and an age input module placed in the third slot for sliding; the processing module is used for adjusting the positions of the four input modules in the input slots of the storage module to obtain an output locus; and the output module is provided with a staff gauge for predicting a risk value of the bone metastasis, and a corresponding locus of the output locus on the staff gauge is a predicted risk value.
Owner:叶定伟
Insulin independence among patients with diabetes utilizing a ppi in combination with an immune tolerance agent
ActiveUS20140234264A1Improve the level ofProtected from vandalismBiocidePancreatic cellsNewly diagnosedImmune tolerance
Owner:LEVETAN CLARESA
Multiple-variable dose regimen for treating diabetes
InactiveUS20200046815A1FunctionalReduce inflammationPowder deliverySpray deliveryDosing regimenNewly diagnosed
The present invention relates to the field of preservation of functional pancreatic islet (beta-cells) and treatment of diabetes, providing improved dosage regimen of AAT administration to Type 1 Diabetes Mellitus (T1DM) patients, particularly to newly diagnosed T1DM patients. The improved dose regiment is a multiple variable dosage regimen, comprising and induction phase and a treatment phase.
Owner:KAMADA
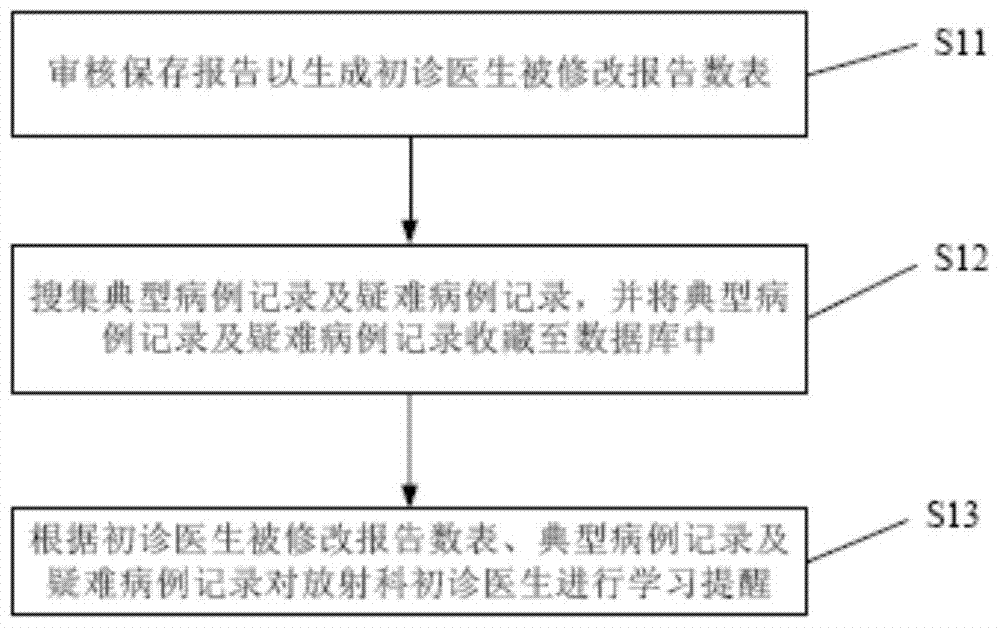
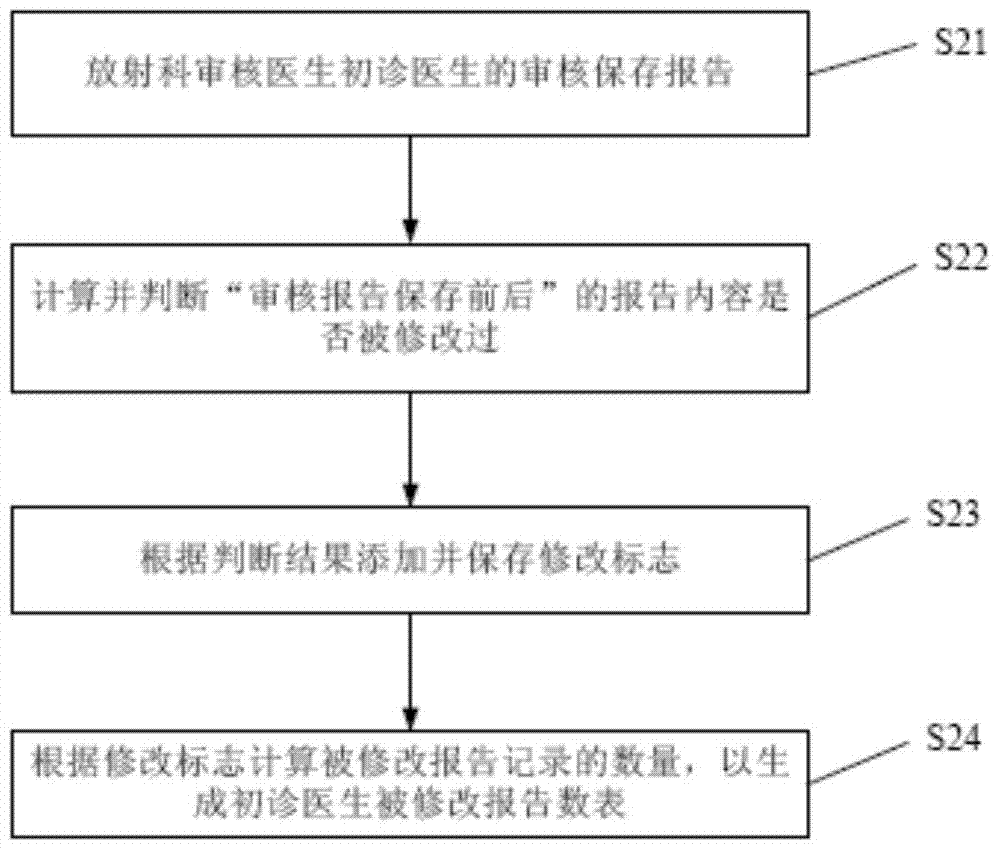
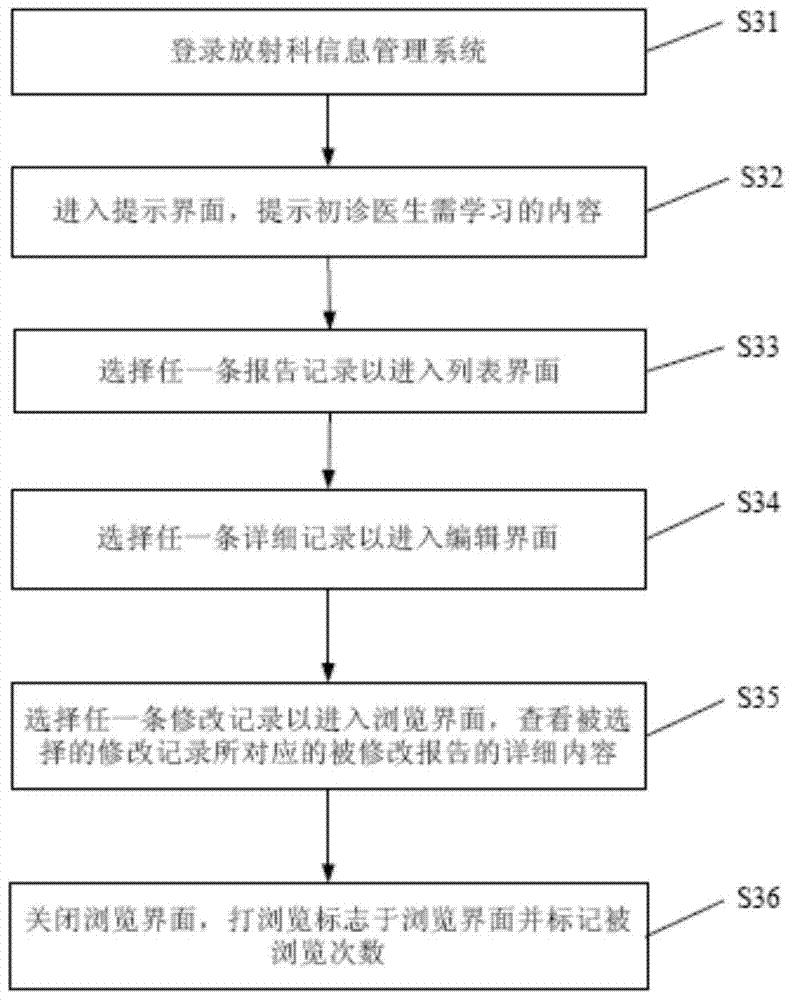
A method to realize the learning function of first-time doctors in radiology department
The invention discloses a method for realizing the learning function of first-time doctors in the department of radiology, which includes: reviewing and saving reports to generate a list of modified reports for first-time doctors; collecting typical case records and difficult case records, and storing the typical case records and difficult case records To the database; according to the revised report number table of first-time doctors, typical case records and difficult case records, learning reminders are given to new-time doctors in the radiology department. Compared with the prior art, the method of the present invention can automatically remind the first-time radiologists to learn in a timely and targeted manner, which promotes the continuous improvement of the medical level of the first-time doctors of the radiology department, and helps improve the overall medical level of the radiology department of the hospital. And then reached the purpose of improving the overall medical level of the hospital.
Owner:LANWON TECH
Molecular markers, internal reference genes and their applications, detection kits and methods for constructing detection models
ActiveCN107574243BImproving technical methods in prognostic predictionMeet the needs of individualized precision treatmentMicrobiological testing/measurementDNA/RNA fragmentationReference genesNewly diagnosed
Owner:CAPITALBIO CORP +1
Detection method for children immune thrombocytopenia
PendingCN111613323AImprove the level of diagnosis and treatmentAchieve interactionMedical data miningHealth-index calculationNewly diagnosedClinical exam
The invention discloses a detection method for children immune thrombocytopenia, and particularly relates to the field of thrombocytopenia detection methods. The method comprises the following specific detection steps: S1, establishing a multi-center child ITP research cooperation group; S2, standardizing ITP clinical examination diagnosis and chronic prejudgment standards of children; S3, formulating and implementing an advanced ITP precise layering treatment scheme: evaluating a plurality of newly diagnosed ITP child patients entering the group according to the scheme; S4, performing follow-up visit and statistical analysis; and S5, performing multi-center random control clinical test. According to the invention, a child ITP diagnosis and treatment research cooperation group is established, a core expert group is established to manage the cooperation group, a unified and standard diagnosis and treatment scheme is implemented, a child ITP diagnosis and treatment research network withcooperative access and rapid response is formed, and the child ITP diagnosis and treatment research network is radiated downwards to primary hospitals by taking member hospitals as backbones; throughscientific research and innovation, key clinical problems existing in current ITP treatment are promoted to be solved, and the overall diagnosis and treatment level of children ITP in China is greatlyimproved.
Owner:BEIJING CHILDRENS HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com