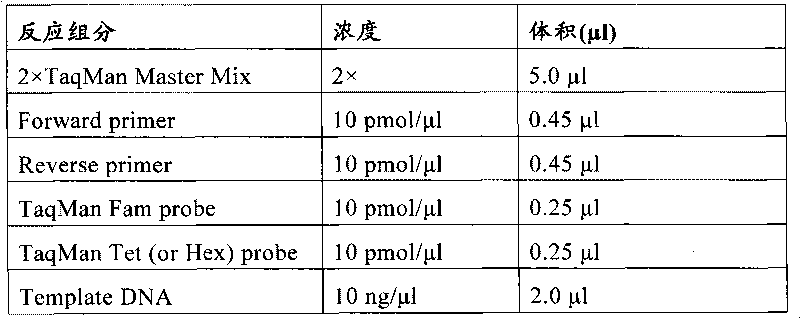
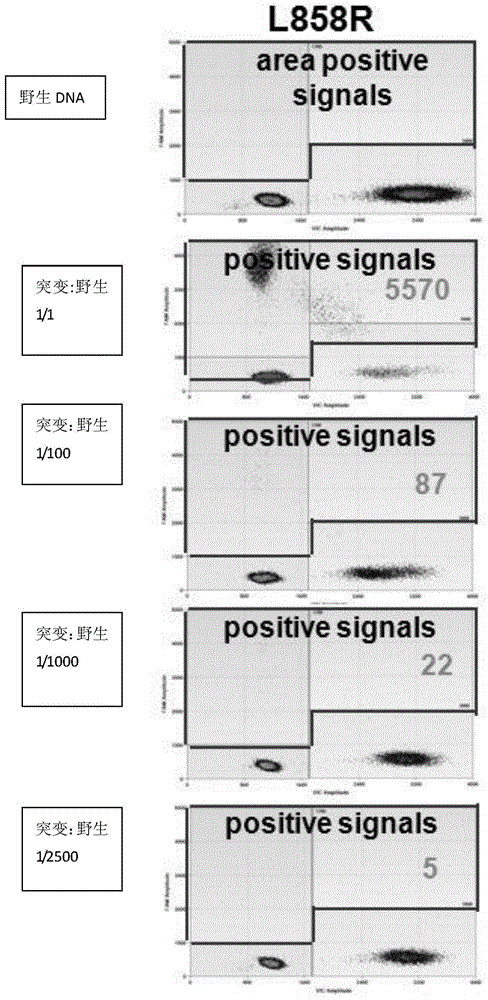
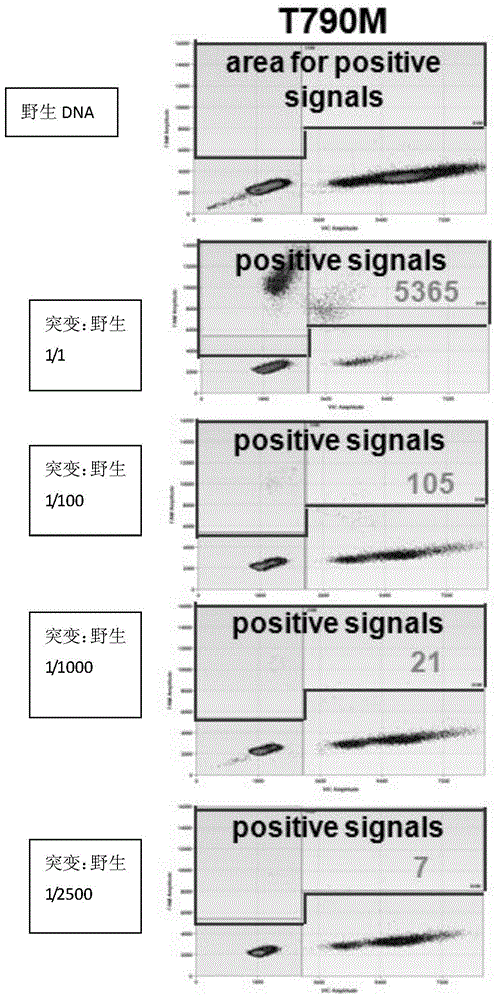
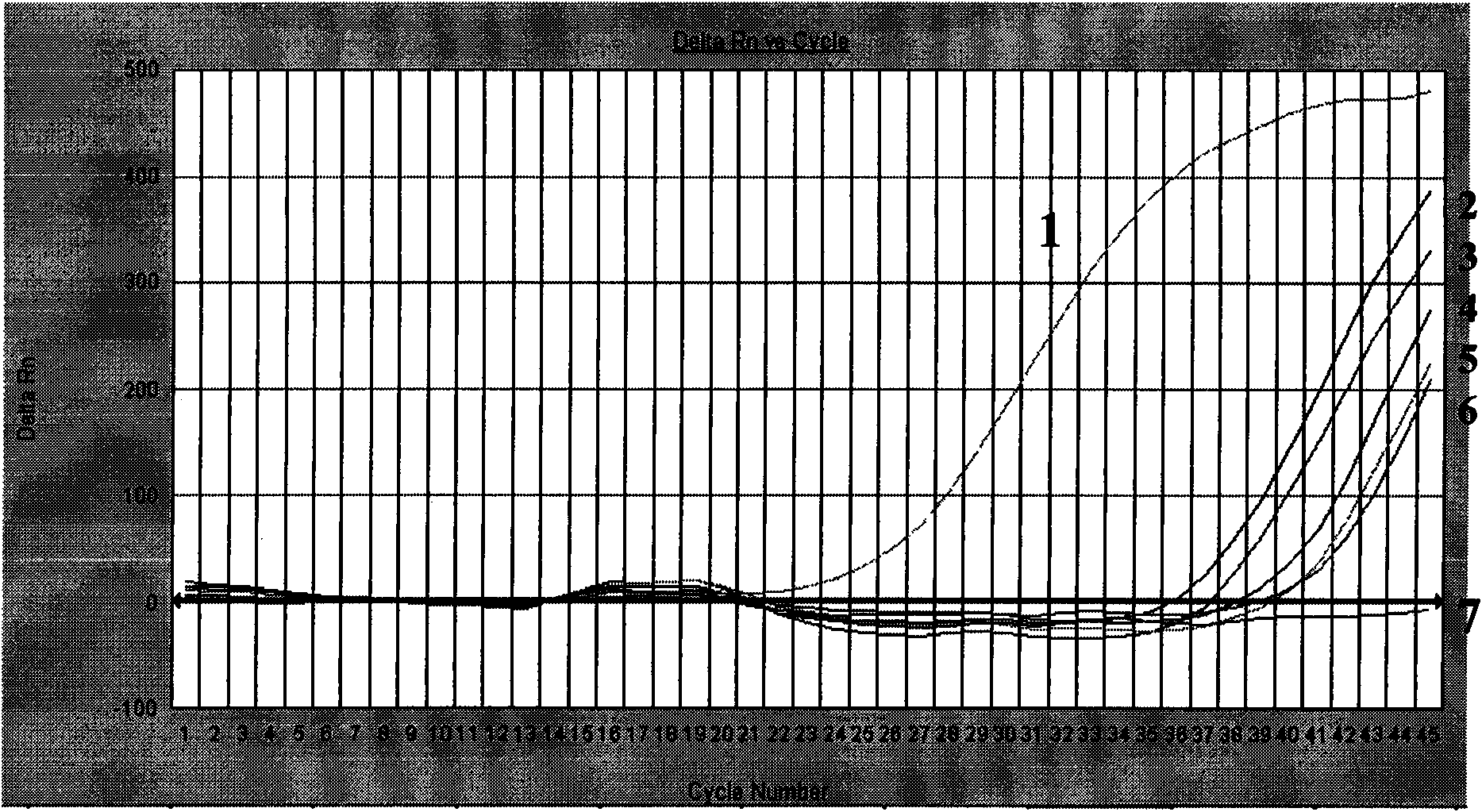
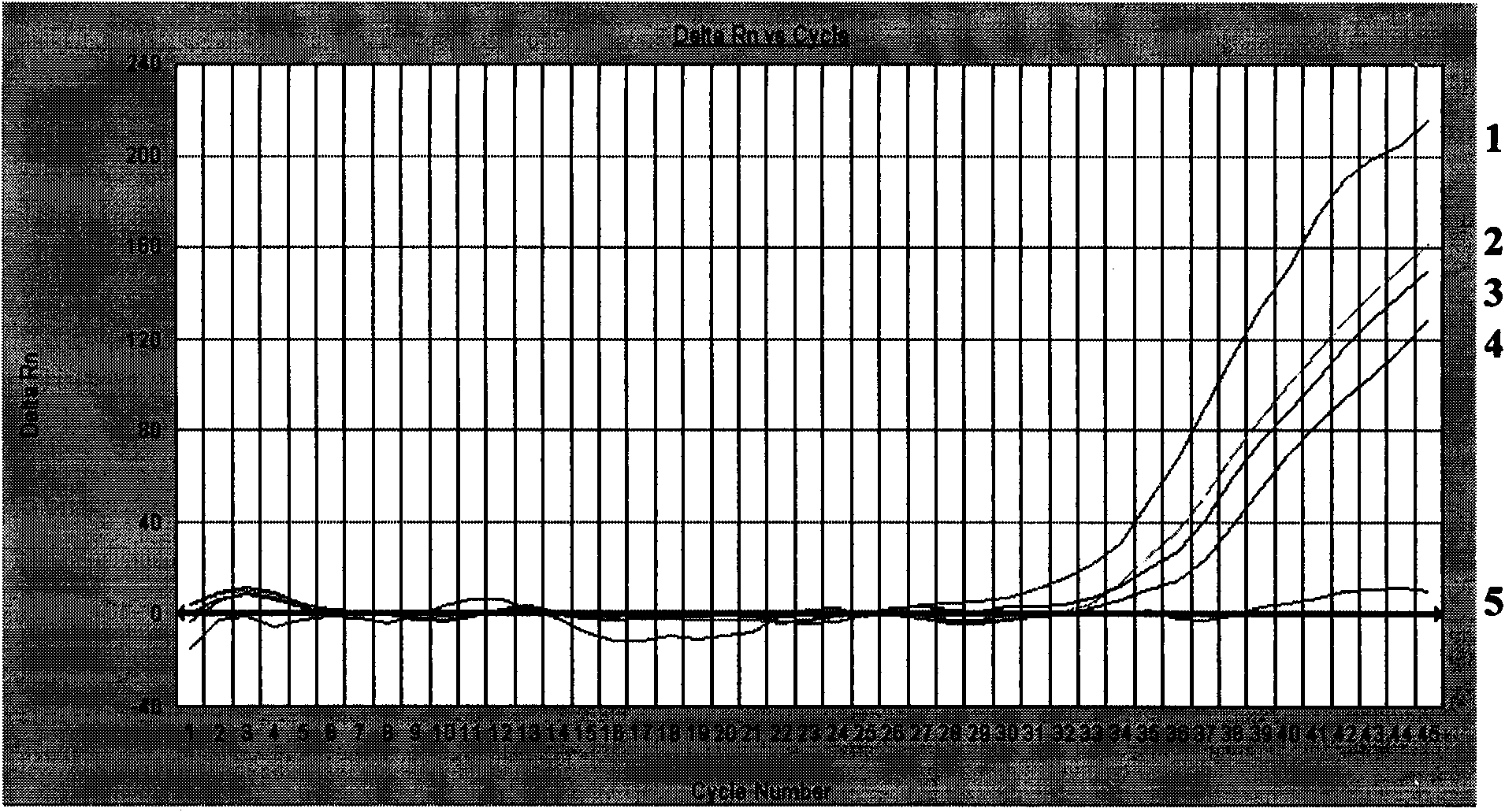
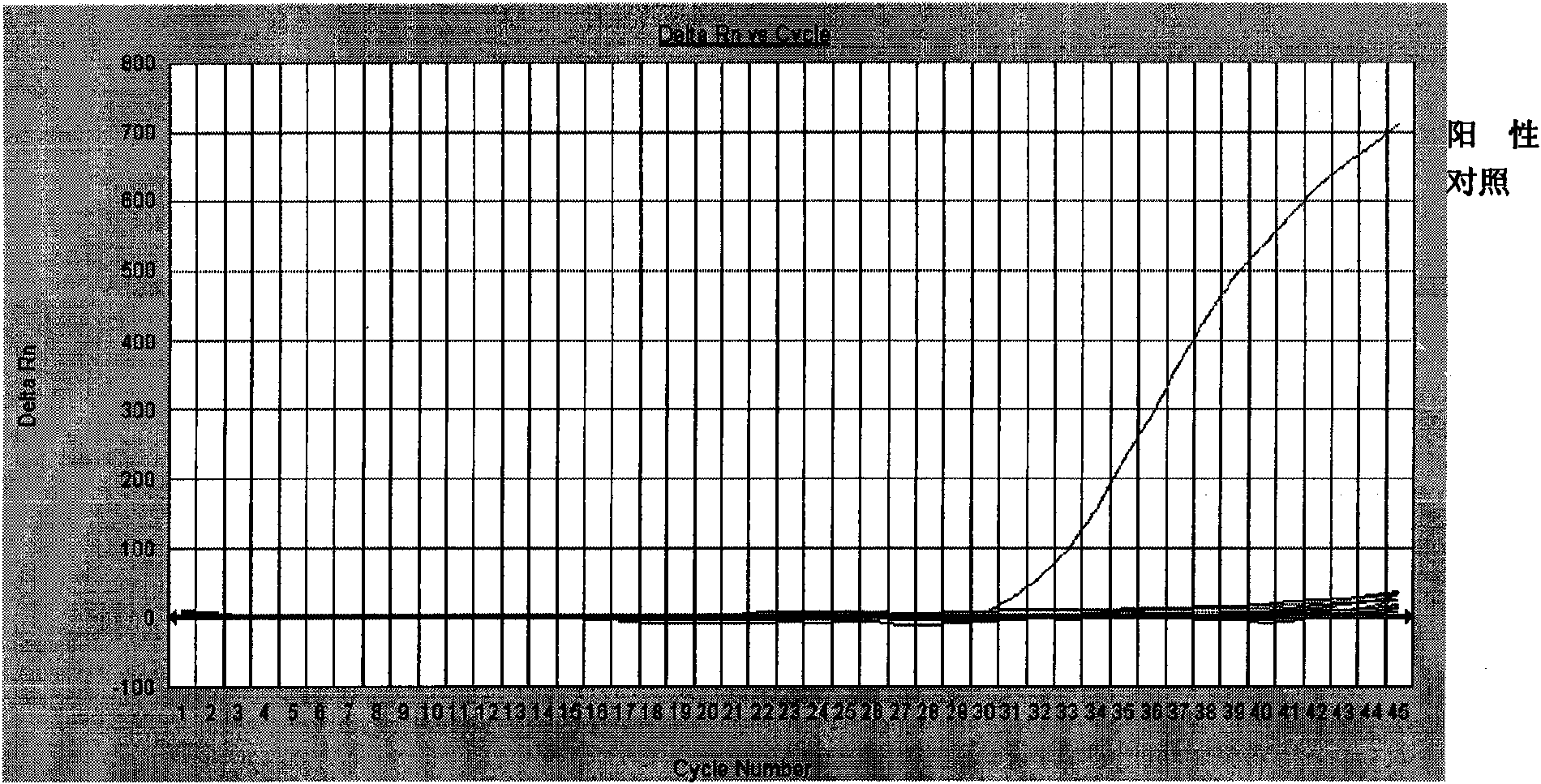
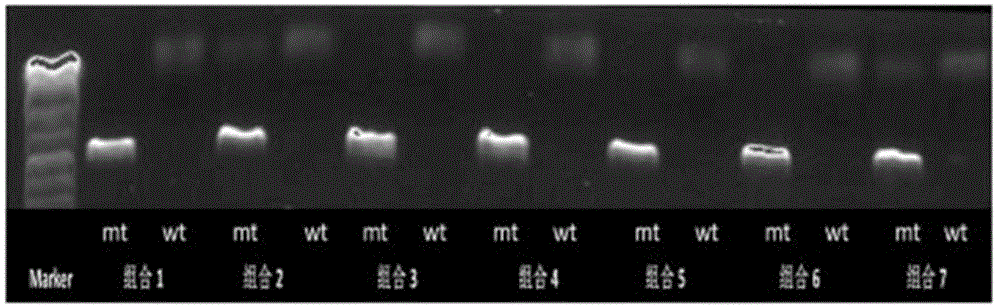
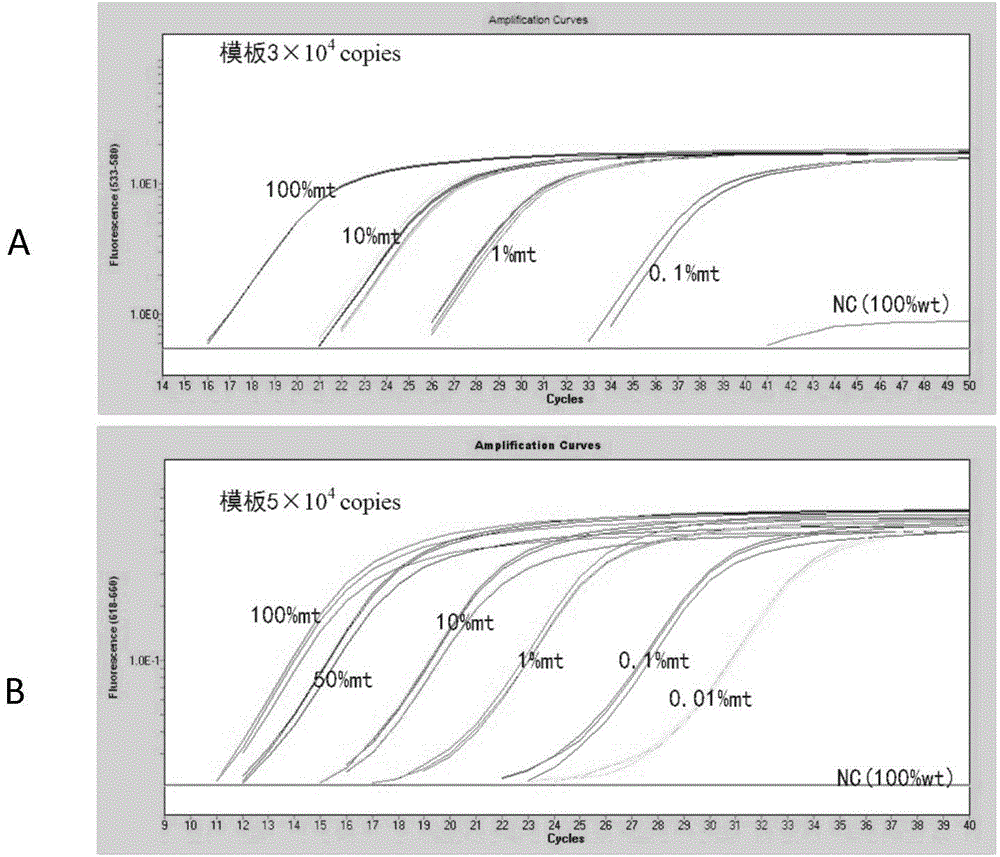
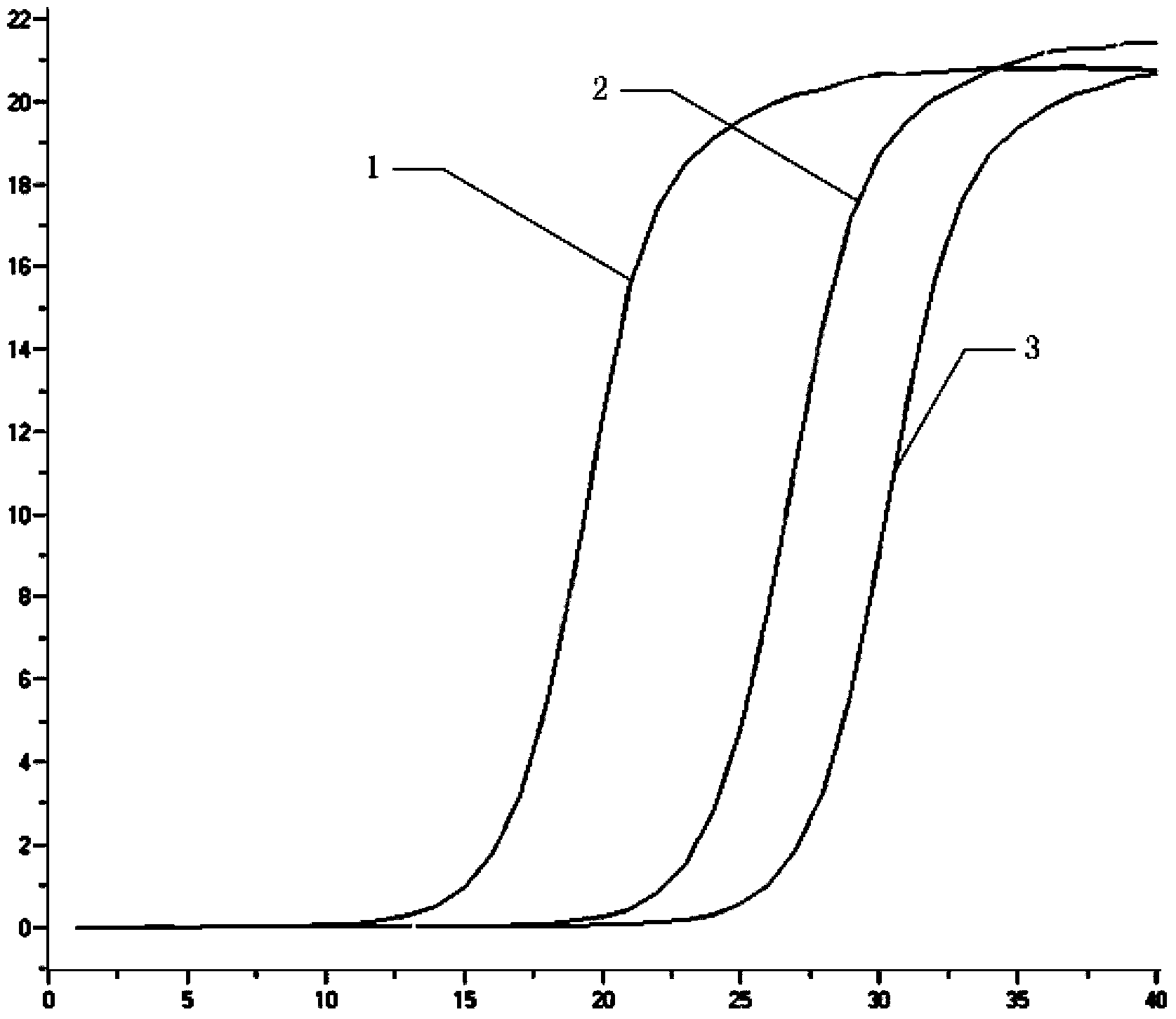
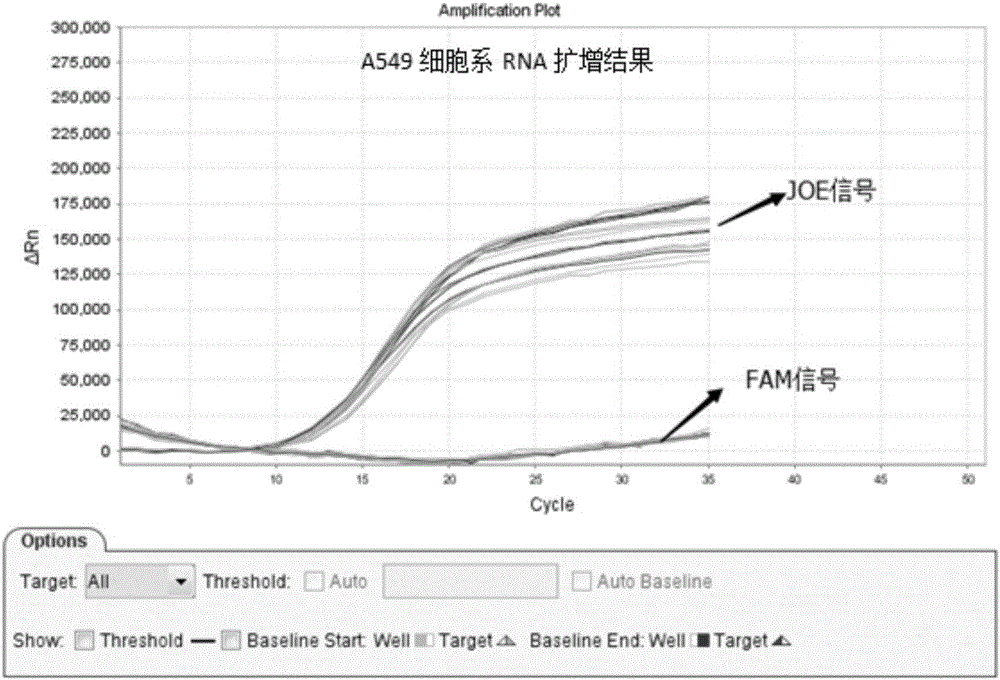
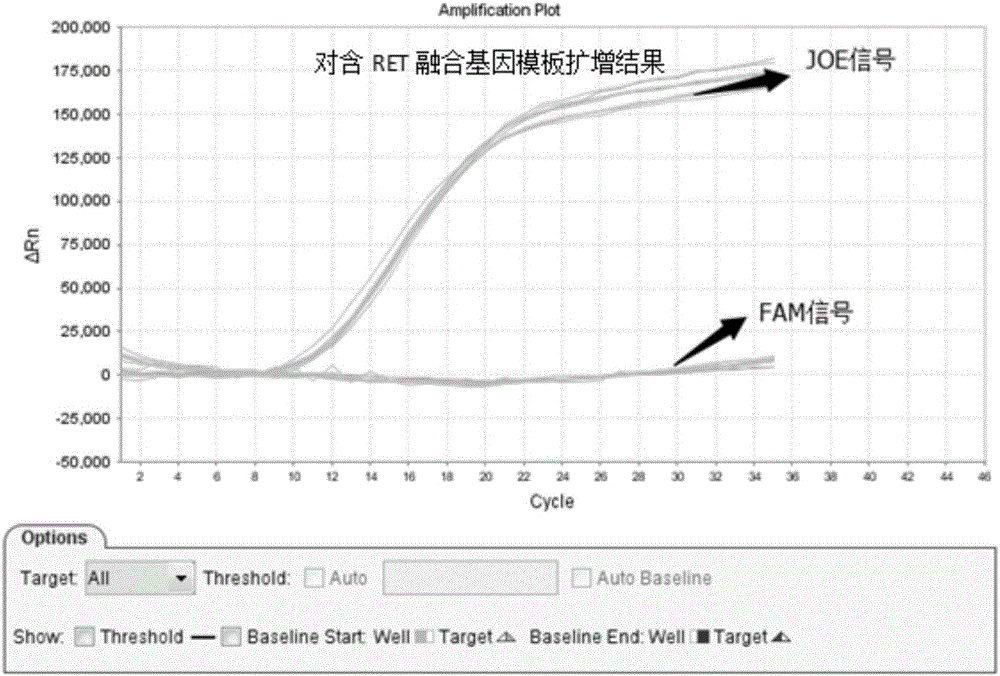
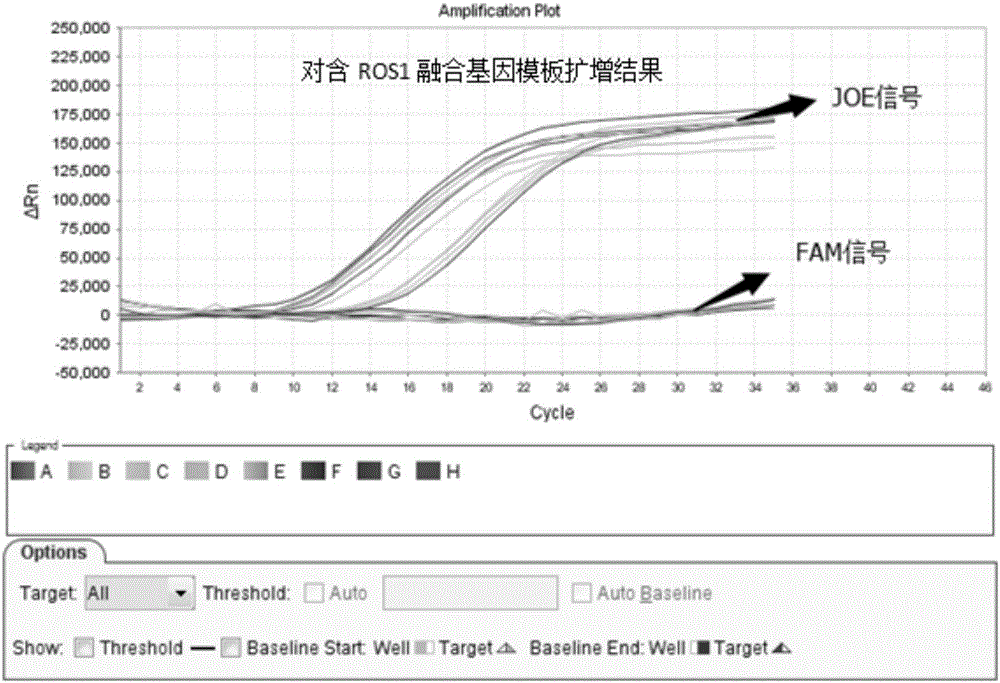
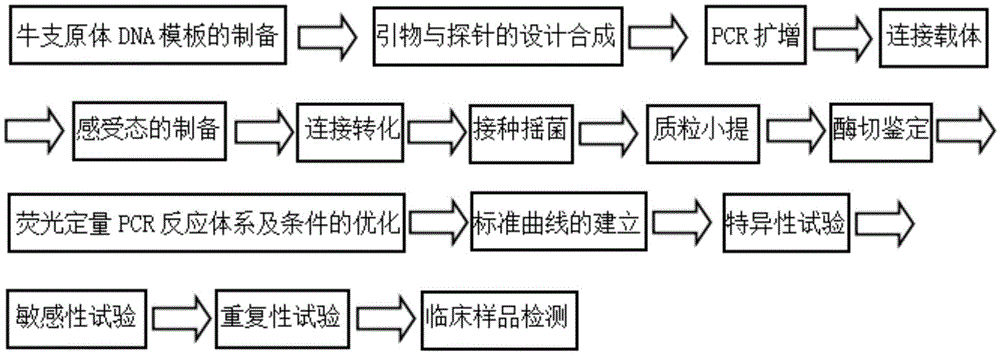
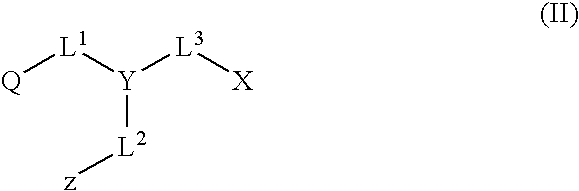Patents
Literature
1038 results about "TaqMan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
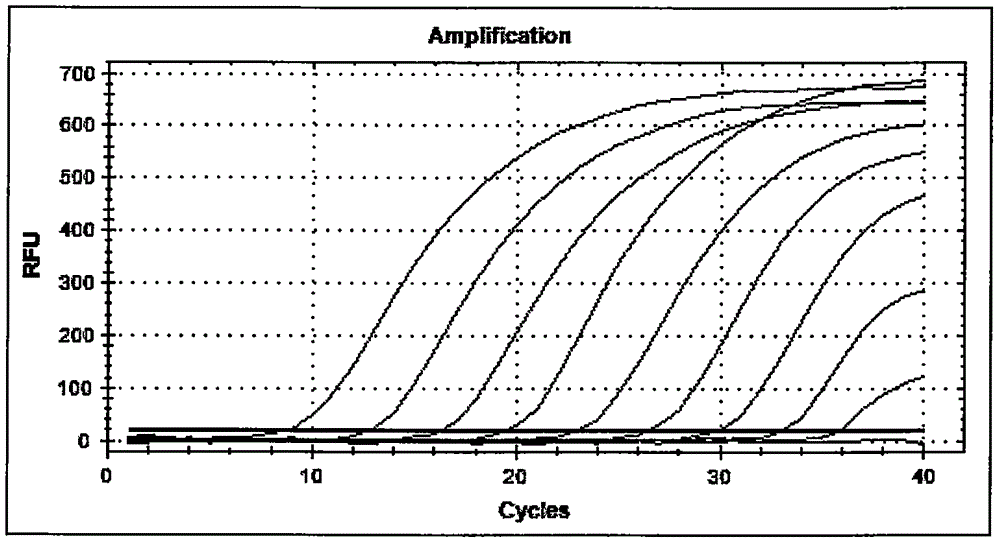
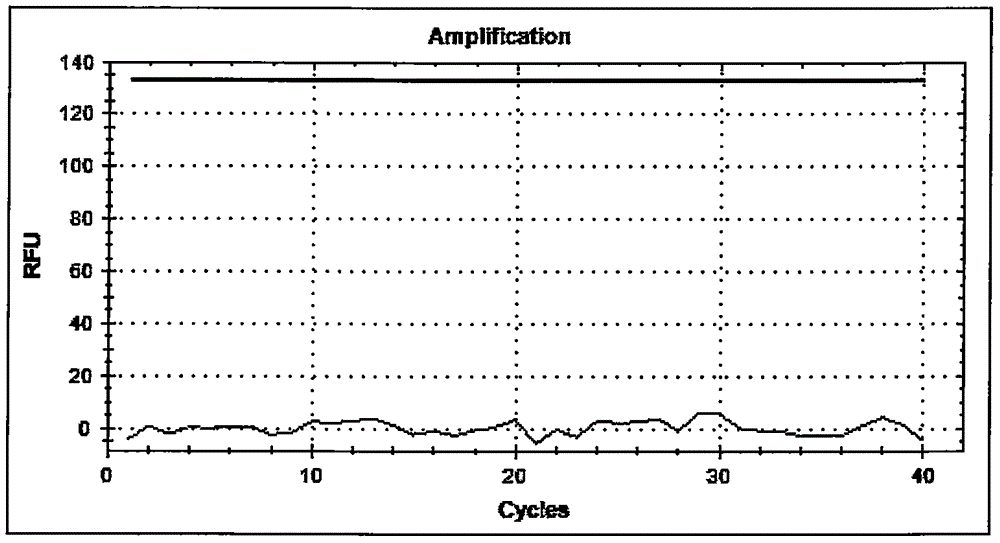
TaqMan probes are hydrolysis probes that are designed to increase the specificity of quantitative PCR. The method was first reported in 1991 by researcher Kary Mullis at Cetus Corporation, and the technology was subsequently developed by Roche Molecular Diagnostics for diagnostic assays and by Applied Biosystems (now part of Thermo Fisher Scientific) for research applications.
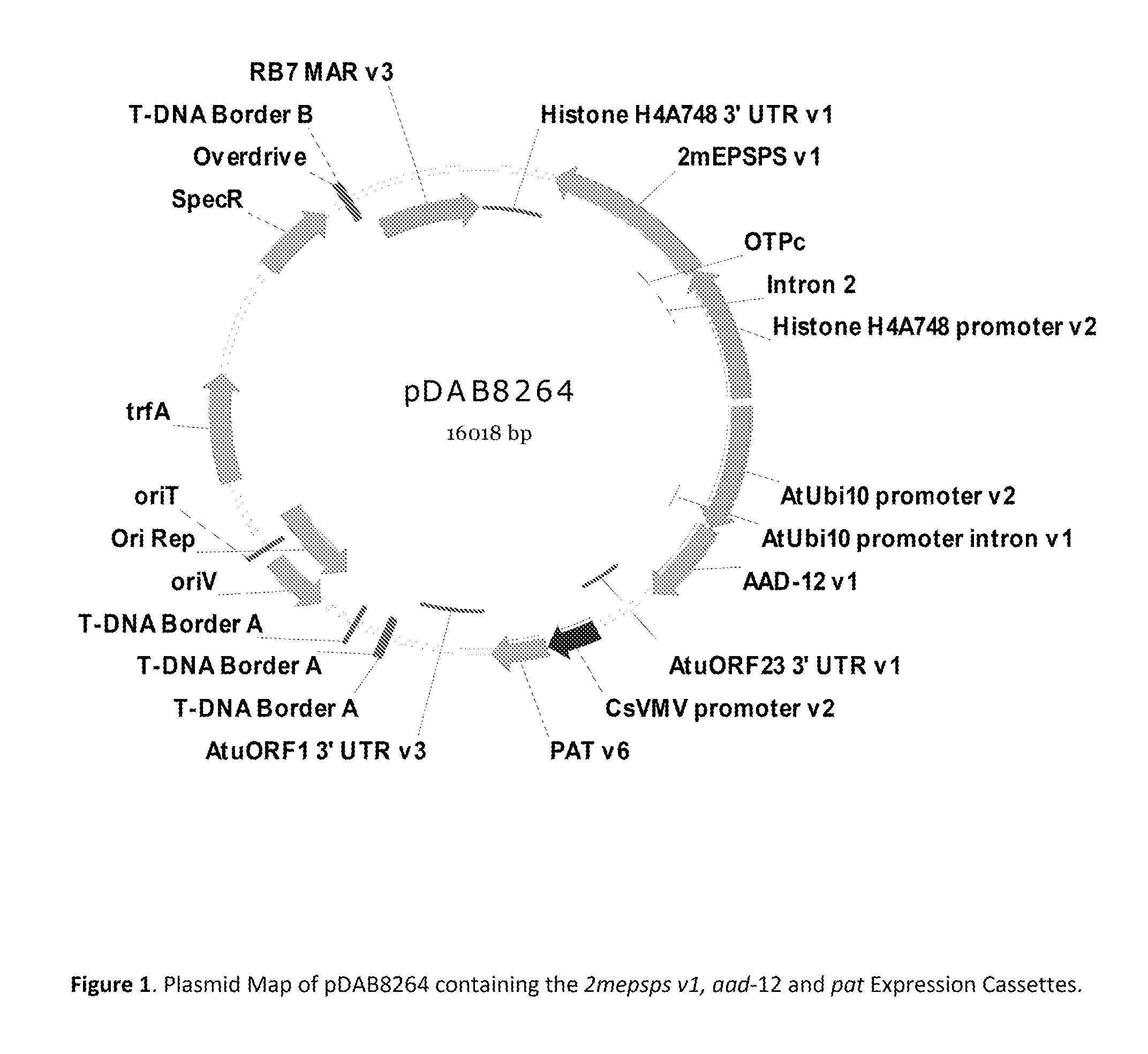
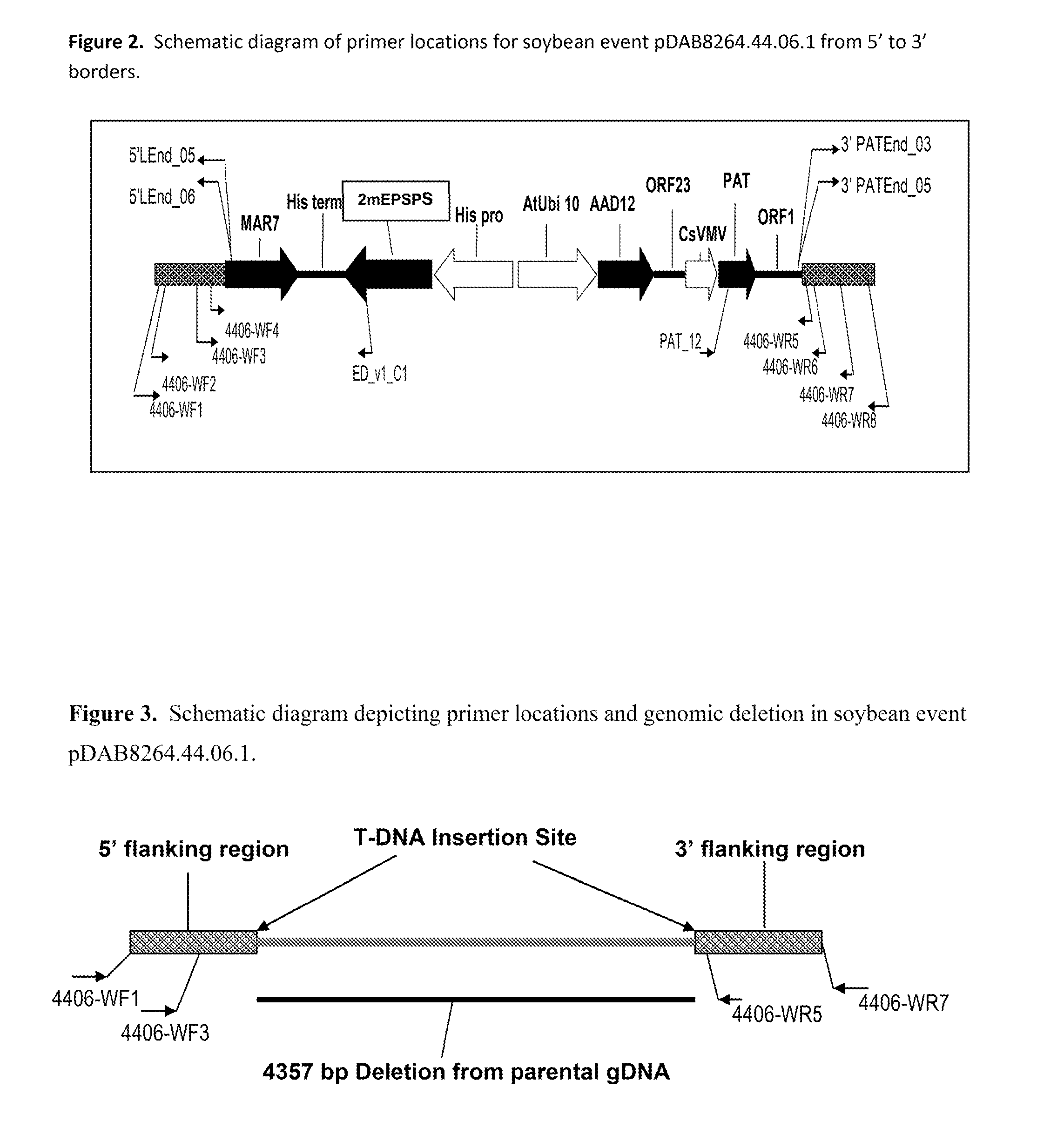
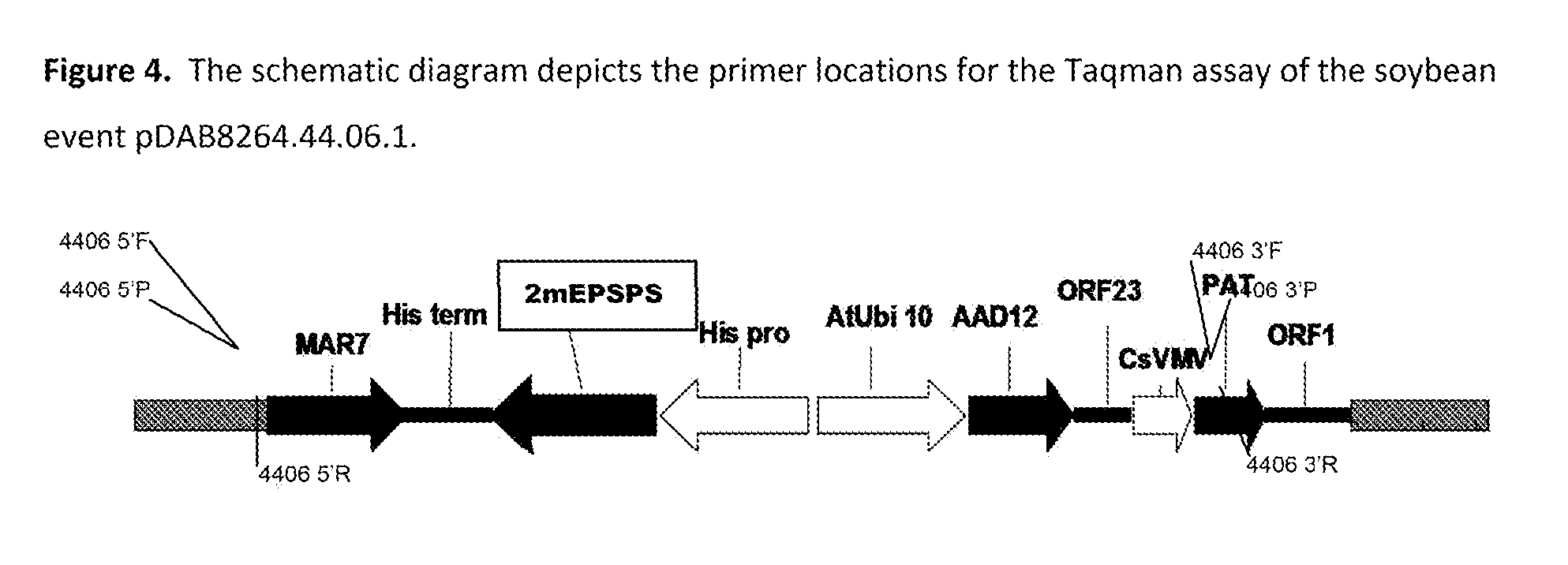
Stacked herbicide tolerance event 8264.44.06.1, related transgenic soybean lines, and detection thereof
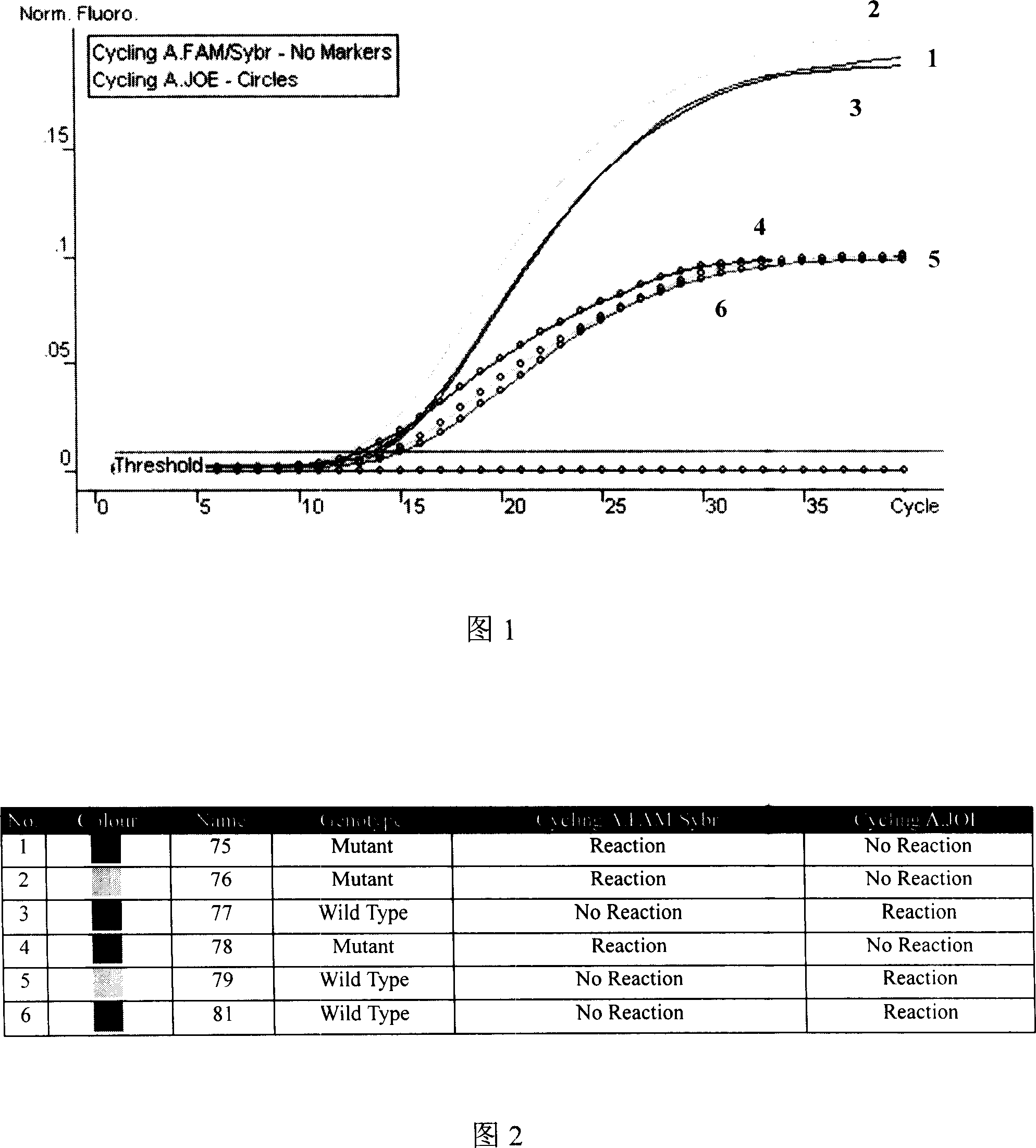
ActiveUS9540655B2Preserve usefulnessIncrease flexibilityBiocideMicrobiological testing/measurementPcr assayMultiple traits
Owner:M S TECH +1
Methods and compositions for identifying a fetal cell
InactiveUS20100304978A1High expressionMicrobiological testing/measurementLibrary screeningCandidate Gene Association StudyTrophoblast
The present invention provides methods and compositions for specifically identifying a fetal cell. An initial screening of approximately 400 candidate genes by digital PCR in different fetal and adult tissues identified a subset of 24 gene markers specific for fetal nucleated RBC and trophoblasts. The specific expression of those genes was further evaluated and verified in more defined tissues and isolated cells through quantitative RT-PCR using custom Taqman probes specific for each gene. A subset of fetal cell specific markers (FCM) was tested and validated by RNA fluorescent in situ hybridization (FISH) in blood samples from non-pregnant women, and pre-termination and post-termination pregnant women. Applications of these gene markers include, but are not limited to, distinguishing a fetal cell from a maternal cell for fetal cell identification and genetic diagnosis, identifying circulating fetal cell types in maternal blood, purifying or enriching one or more fetal cells, and enumerating one or more fetal cells during fetal cell enrichment.
Owner:VERINATA HEALTH INC
Use Of Genes As Molecular Markers In Diagnosis Of Schizophrenia And Diagnostic Kit For The Same
InactiveUS20080274455A1Sugar derivativesMicrobiological testing/measurementQuantitative Real Time PCRScreening method
Drug-naive and drug-free schizophrenic PBL were screened to identify additional markers that are differentially expressed compared to healthy individuals using microarray and quantitative real-time PCR (QRT-PCR) techniques. Genes for dopamine D2 receptor (DRD2) and inwardly rectifying potassium channel (Kir2.3) were found to be overexpressed in microarray analysis. Increased mRNA levels were confirmed by QRT-PCR using SybrGreen method and dual labeled TaqMan probes.The invention relates to a method for diagnosing schizophrenia in a subject comprising assessing the level or the expression level of at least one of the following genes or proteins: Kir2.3 or DRD2 or a gene encoding Kir2.3 or DRD2. The invention further relates to agents and uses thereof, said agents specifically binding to said proteins or nucleic acids encoding them, diagnostic kits and screening methods.Use of both molecular markers allow prediction of schizophrenia and help to follow efficiency of drugs in therapy in order to provide a more tailored medication for schizophrenic patients.
Owner:THE BIOLOGICAL RES CENT OF THE HUNGARIAN ACAD OF SCI
ARMS-qPCR (Allele Refractory Mutation System-quantitative Polymerase Chain Reaction) detection kit for KRAS (Kirsten Rat Sarcoma Viral Oncogene Homolog) gene mutation subtype and detection method
InactiveCN102367478AIncreased sensitivityQuick checkMicrobiological testing/measurementViral OncogenePositive control
The invention relates to the field of molecular biology and aims to provide an ARMS-qPCR (Allele Refractory Mutation System-quantitative Polymerase Chain Reaction) detection kit for KRAS (Kirsten Rat Sarcoma Viral Oncogene Homolog) gene mutation subtype and a detection method. The kit comprises a qPCR hybrid reaction solution, a locked nucleic acid retardant probe, a reference primer, an ARMS primer and a positive control sample, wherein the qPCR hybrid reaction solution comprises a PCR buffer solution, dNTPs (Deoxynucleotide Triphosphates), MgCl2, GoldStarbest Taq enzyme, a universal PCR reverse primer and a universal TaqMan probe. The kit provided by the invention can be used for rapidly and accurately detecting specific locus mutation of KRAS genes in various cancer tissues with high sensitivity, has high sensitivity, and can be used for detecting genome DNA with various tissue origins, specially free DNA segments adopting cell-free systems, such as blood serum and blood plasma, orother body fluid origins, wherein the genome DNA is derived from cell systems. Compared with direct sequencing and other mutation detection technologies, the kit and the detection method thereof havethe advantages of strong specificity, high sensitivity, simplicity and rapidness in operation, high throughput, safety, definiteness and objectivity in result identification and the like for detecting the KRAS gene mutation.
Owner:ZHEJIANG UNIV
Diagnostic assay for the human virus causing severe acute respiratory syndrome (SARS)
ActiveUS7267942B2Sugar derivativesMicrobiological testing/measurementPcr assaySevere acute respiratory syndrome
The present invention relates to a diagnostic assay for the virus causing Severe Acute Respiratory Syndrome (SARS) in humans (“hSARS virus”). In particular, the invention relates to a real-time quantitative PCR assay for the detection of hSARS virus using reverse transcription and polymerase chain reaction. Specifically, the quantitative assay is a TaqMan® assay using the primers and probes constructed based on the genome of the hSARS virus. The invention further relates to a diagnostic kit that comprises nucleic acid molecules for the detection of the hSARS virus.
Owner:HONG KONG THE UNIV OF +1
Probe for detecting matrilinear inheritance chondriosome deafness gene A1555G and its use
ActiveCN1987462ASimplified electrophoresis detectionReduce the risk of contaminationMicrobiological testing/measurementBiological testingFluorescenceGenotype Analysis
The invention designs two pieces of Taqman mutant type and wild type probe and a pair of primer. Using Taqman probe method of real time fluorescence quantitation carries out genotype analysis for A1555G mutation of deaf gene of maternal inheritance mitochondria so as to diagnose genetic deaf disease of maternal mitochondria. The method is suitable to large-scale screening or preventative inspecting A1555G mutation of deaf mitochondria gene of maternal inheritance. Features are: simple, time saving, high specificity, high sensitivity, intuitive tested result, accurate and reliable.
Owner:金政策
Medicine metabolic relevant loci detection method
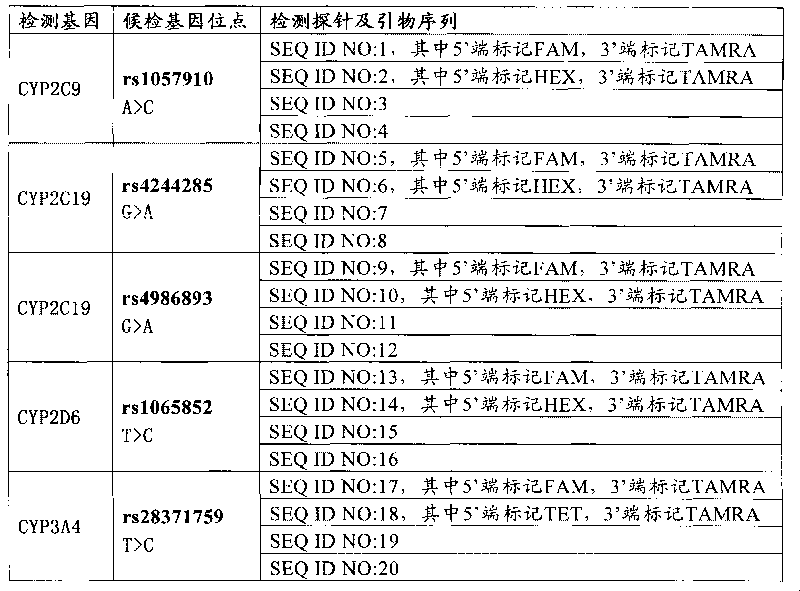
InactiveCN101760528AAccurate and reliable metabolic strengthAvoid adverse reactionsMicrobiological testing/measurementDrug metabolismFluorescence
The invention relates to a medicine metabolic relevant loci detection method, which comprises the following steps: extracting genome DNA from human samples; respectively designing a Taqman probe pair and a primer pair according to at least two medicine metabolic relevant genes; respectively marking the 5' end and the 3'end of the Taqman probe pair with fluorescence reporting genes and fluorescence quenching genes; carrying out fluorescence quantitative PCR augmentation on the genome DNA; and judging whether the medicine metabolic relevant genes have the mutation according to the fluorescence quantitative PCR augmentation results. Preferably, the number of the medicine metabolic relevant genes is four, the Taqman probe pair and the primer pair are used for detecting a loci rs1057910 of a gene CYP2C9, a loci rs4244285 of a gene CYP2C19, a loci rs4986893of a gene CYP2C19, a loci rs1065852 of a gene CYP2D6 and a loci rs28371759 of a gene CYP3A4. The invention has the advantages of ingenious design, simple operation and accurate and reliable detection results, and provides the reference frame for determining whether professional doctors are needed to be consulted so as to make sure the medicine can be taken or not or the proper dosage and the like when a certain medicine is taken.
Owner:SHANGHAI CHROMYSKY MEDICAL RES
Methods, compositions, and kits for detecting allelic variants
ActiveCN102428190AMicrobiological testing/measurementDNA/RNA fragmentationNucleotideMutation detection
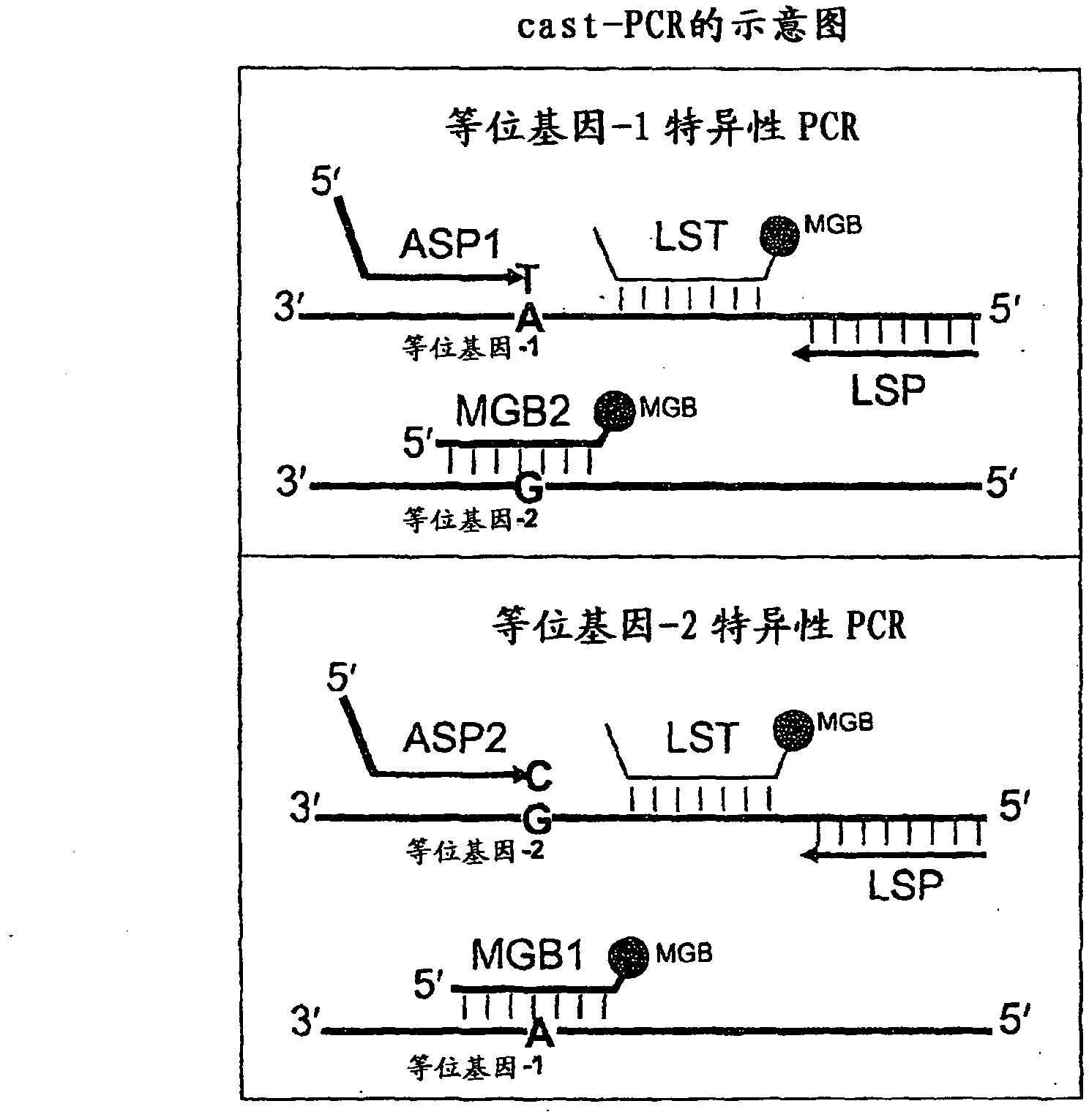
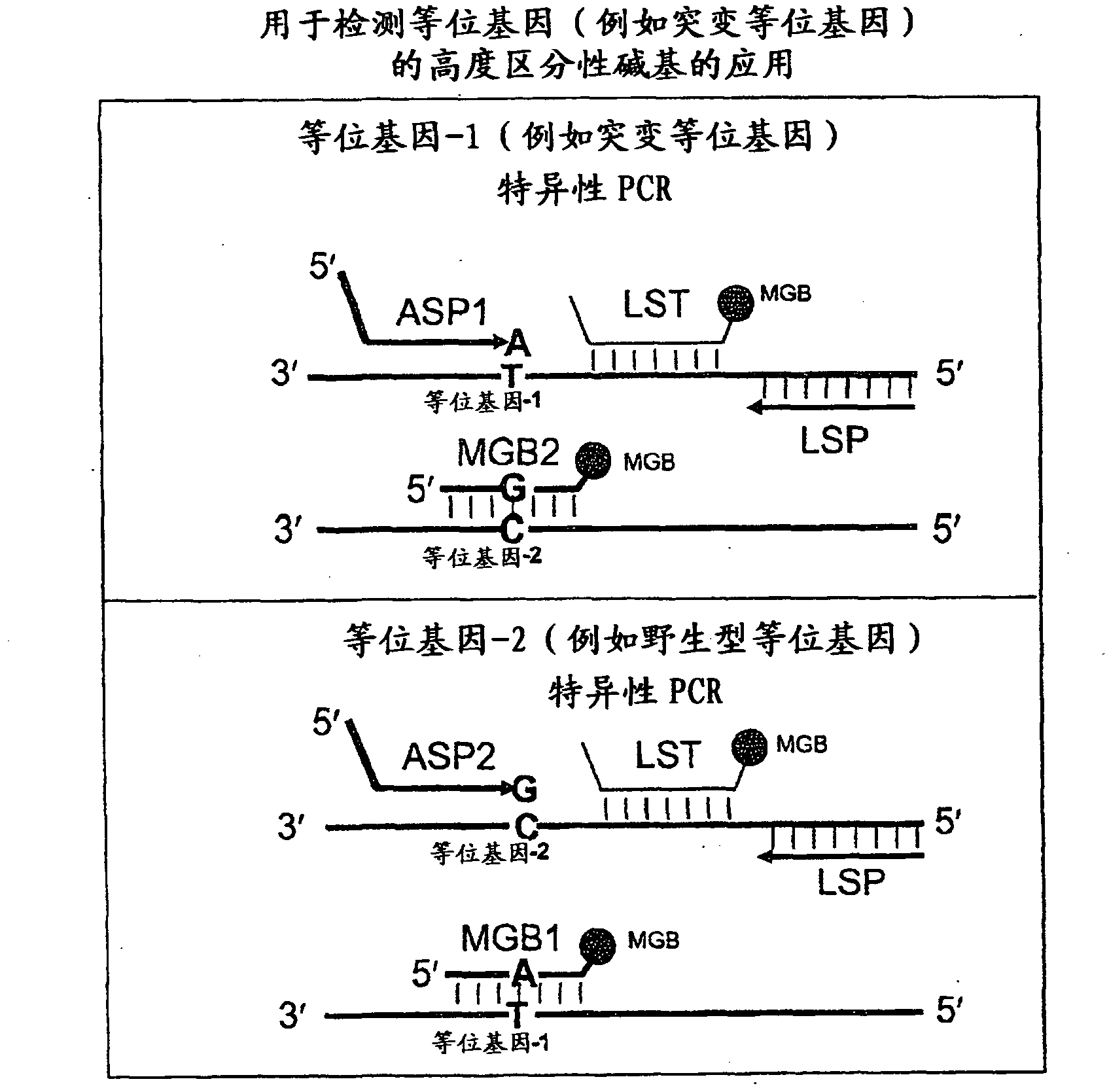
In some embodiments, the present inventions relates generally to compositions, methods and kits for use in discriminating sequence variation between different alleles. More specifically, in some embodiments, the present invention provides for compositions, methods and kits for quantitating rare (e.g., mutant) allelic variants, such as SNPs, or nucleotide (NT) insertions or deletions, in samples comprising abundant (e.g., wild type) allelic variants with high specificity and selectivity. In particular, in some embodiments, the invention relates to a highly selective method for mutation detection referred to as competitive allele-specific TaqMan PCR ('cast-PCR').
Owner:LIFE TECH CORP
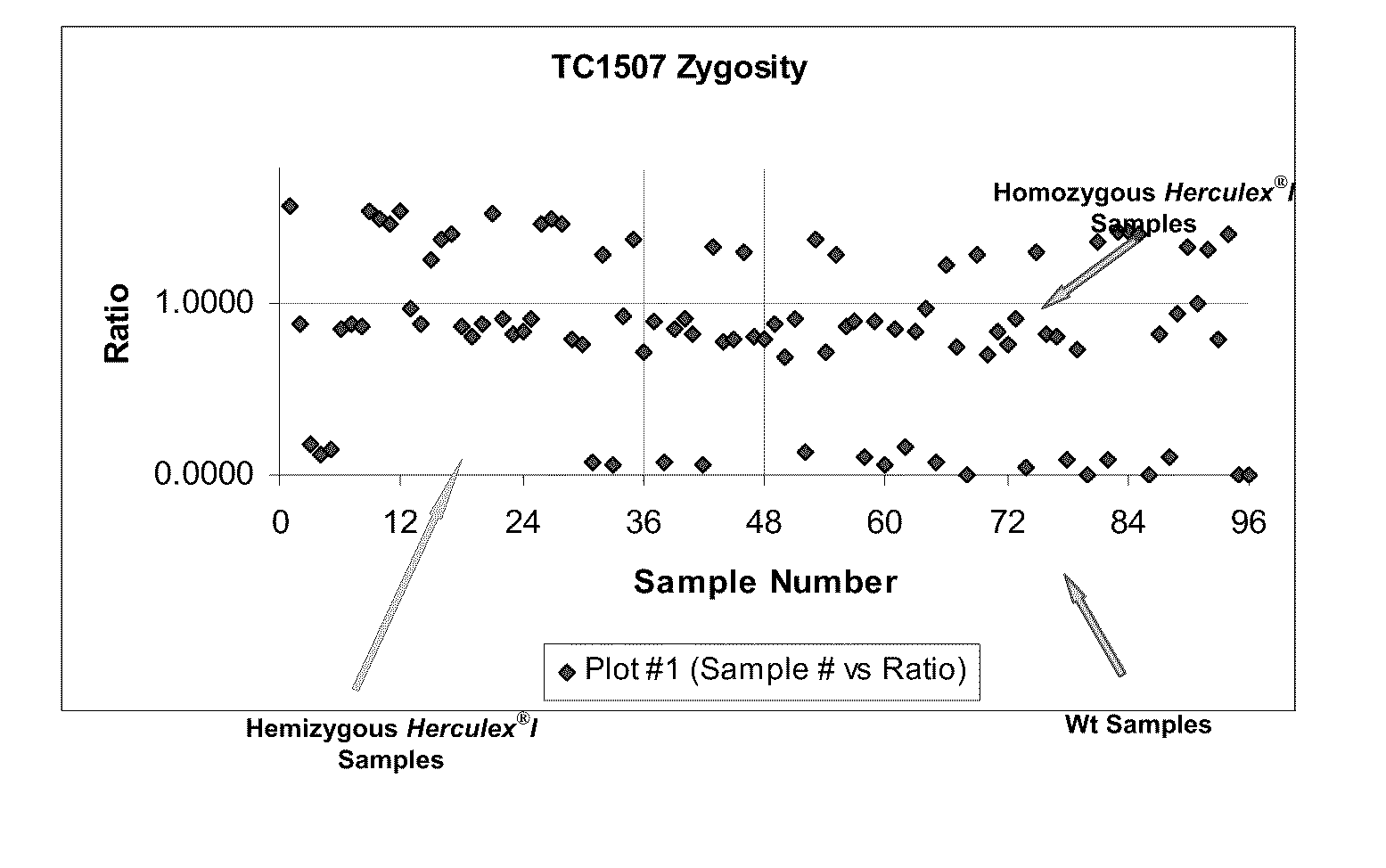
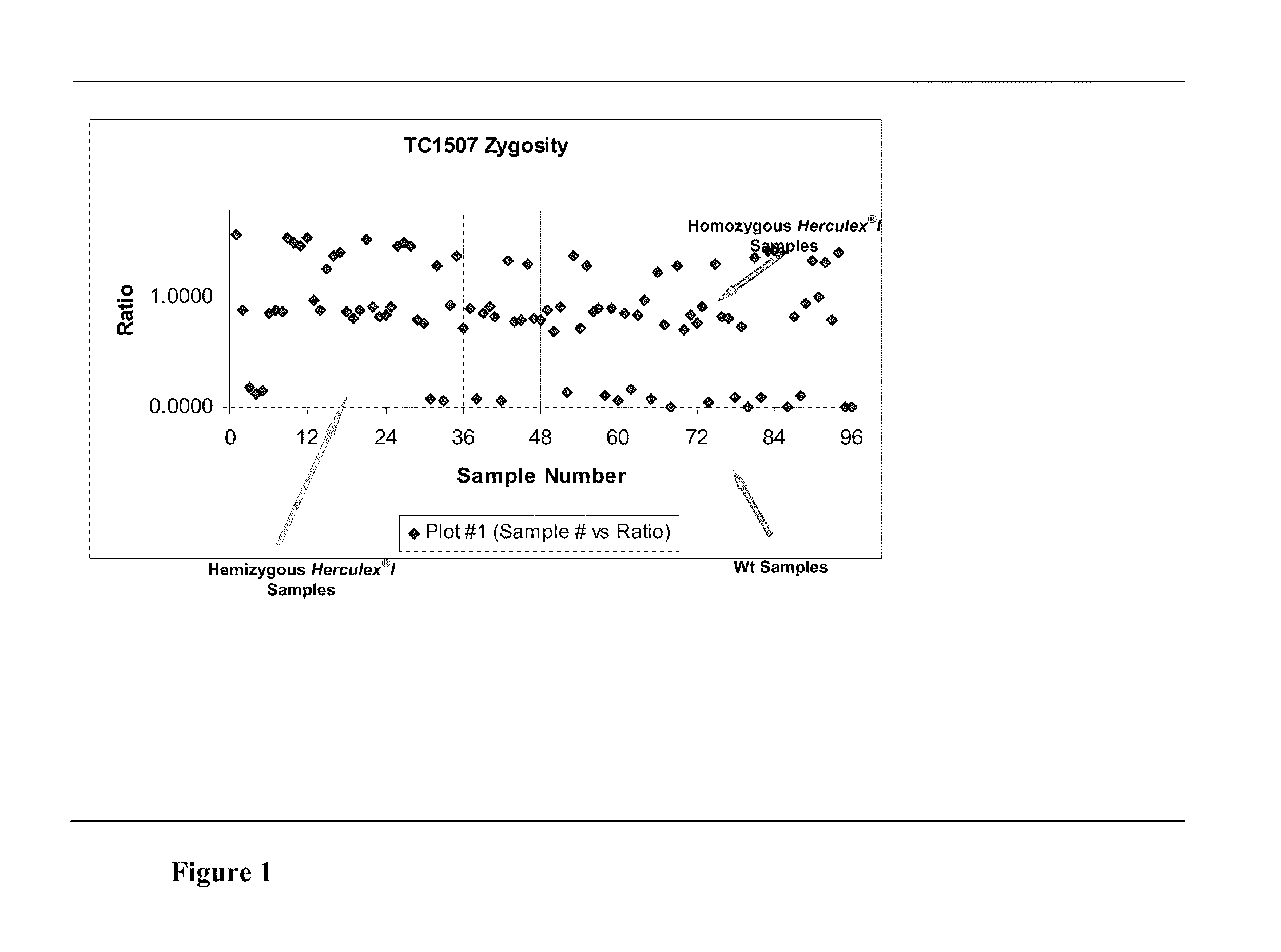
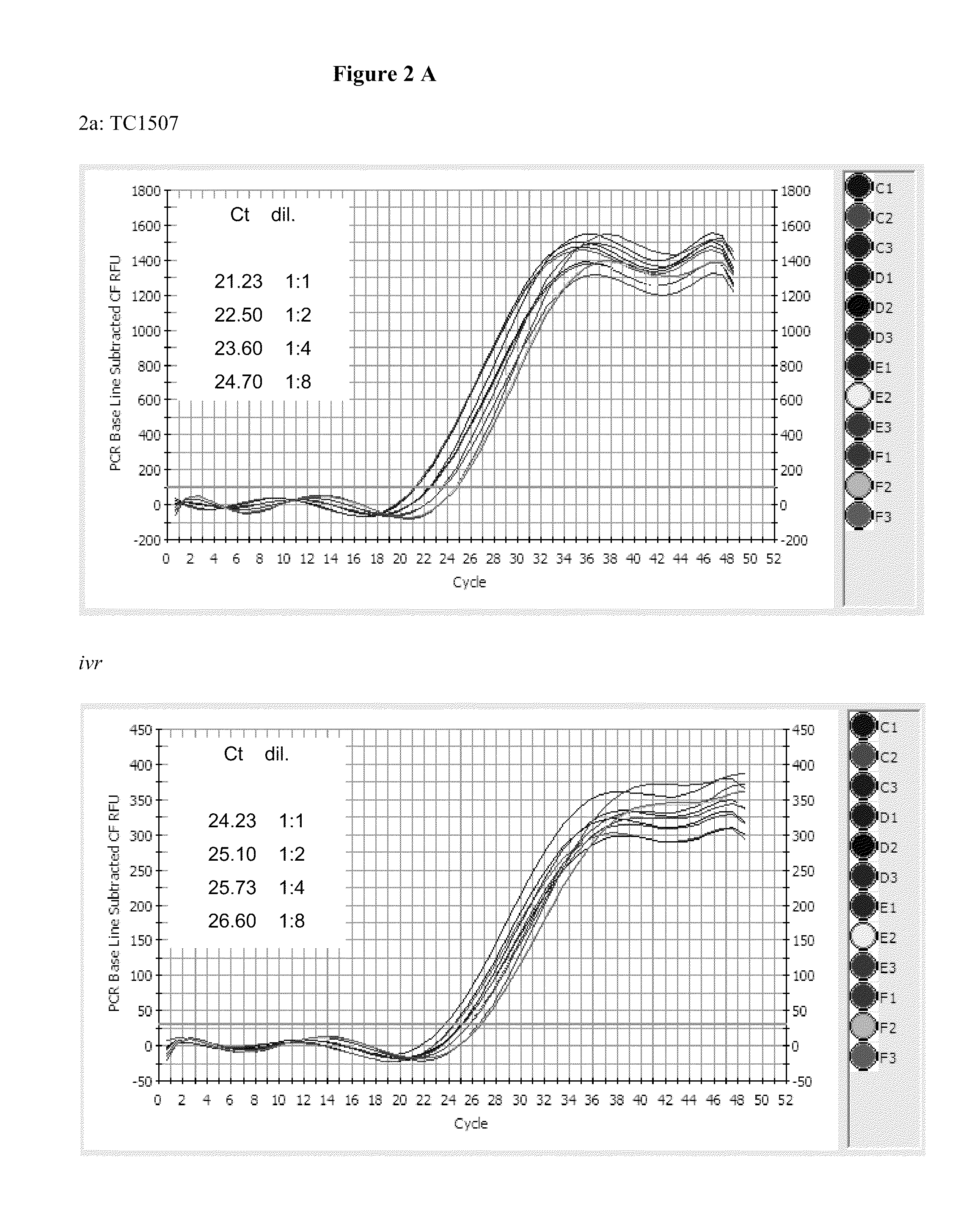
Endpoint taqman methods for determining zygosity of corn comprising tc1507 events
InactiveUS20110151441A1High throughput zygosity analysisSugar derivativesMicrobiological testing/measurementReference genesPcr assay
A method for zygosity analysis of the maize Cry1F event TC1507 is provided. The method provides TC1507 event-specific and maize endogenous reference gene-specific primers and TaqMan probe combinations for use in an endpoint biplex TaqMan PCR assay capable of producing robust genotype calls for assisting in molecular breeding of TC1507.
Owner:DOW AGROSCIENCES LLC
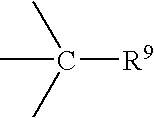
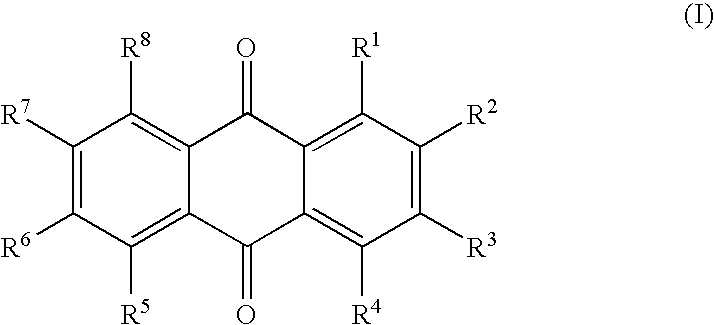
Quencher compositions comprising anthraquinone moieties
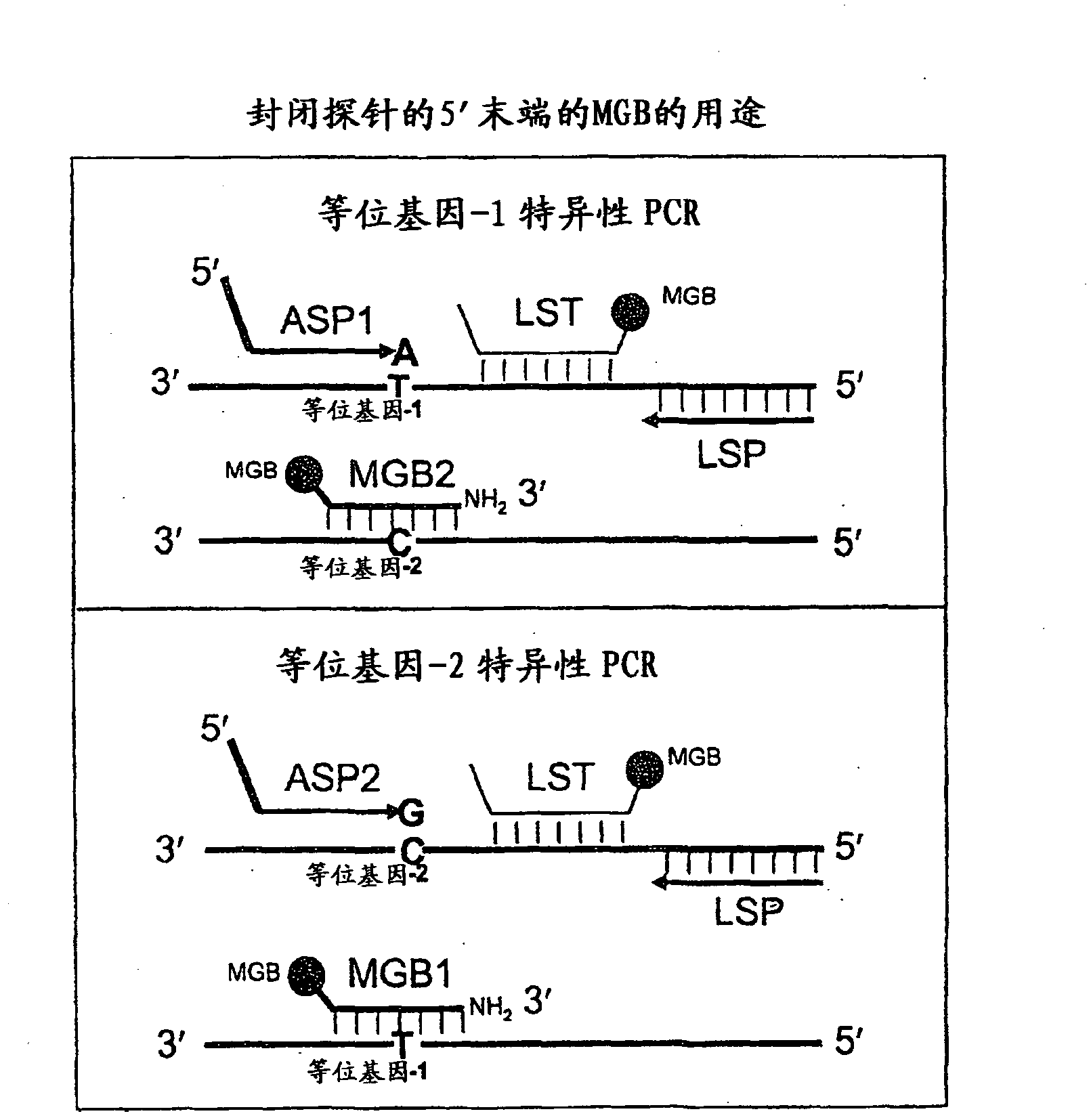
The present invention provides novel quencher composition comprising anthraquinone quencher moieties. The anthraquinone quencher moieties are useful as quencher labels when attached to biomolecules such as natural or modified polynucleotides, oligonucleotides, nucleosides, nucleotides, carbohydrates and peptides. For example, polynucleotides can be labeled at the 3′ terminus with fluorescence quencher solid support compositions, and polynucleotides can be labeled at internally or at the 5′ terminus. The detectable probes may have a format like molecular beacons, scorpion probes, sunrise probes, conformationally assisted probes and TaqMan probes.
Owner:QIAGEN GMBH
TaqMan(TM)-PCR for the detection of pathogenic E. coli strains
InactiveUS6664080B1Addressing slow performanceIncrease valueSugar derivativesMicrobiological testing/measurementPathogenicityOligonucleotide Primer
The present invention relates to a method for the detection of pathogenic E. coli in a sample comprising PCR amplification of DNA isolated from said sample using oligonucleotide primers specific for pathogenic E. coli.
Owner:BAVARIAN NORDIC RES INST AS
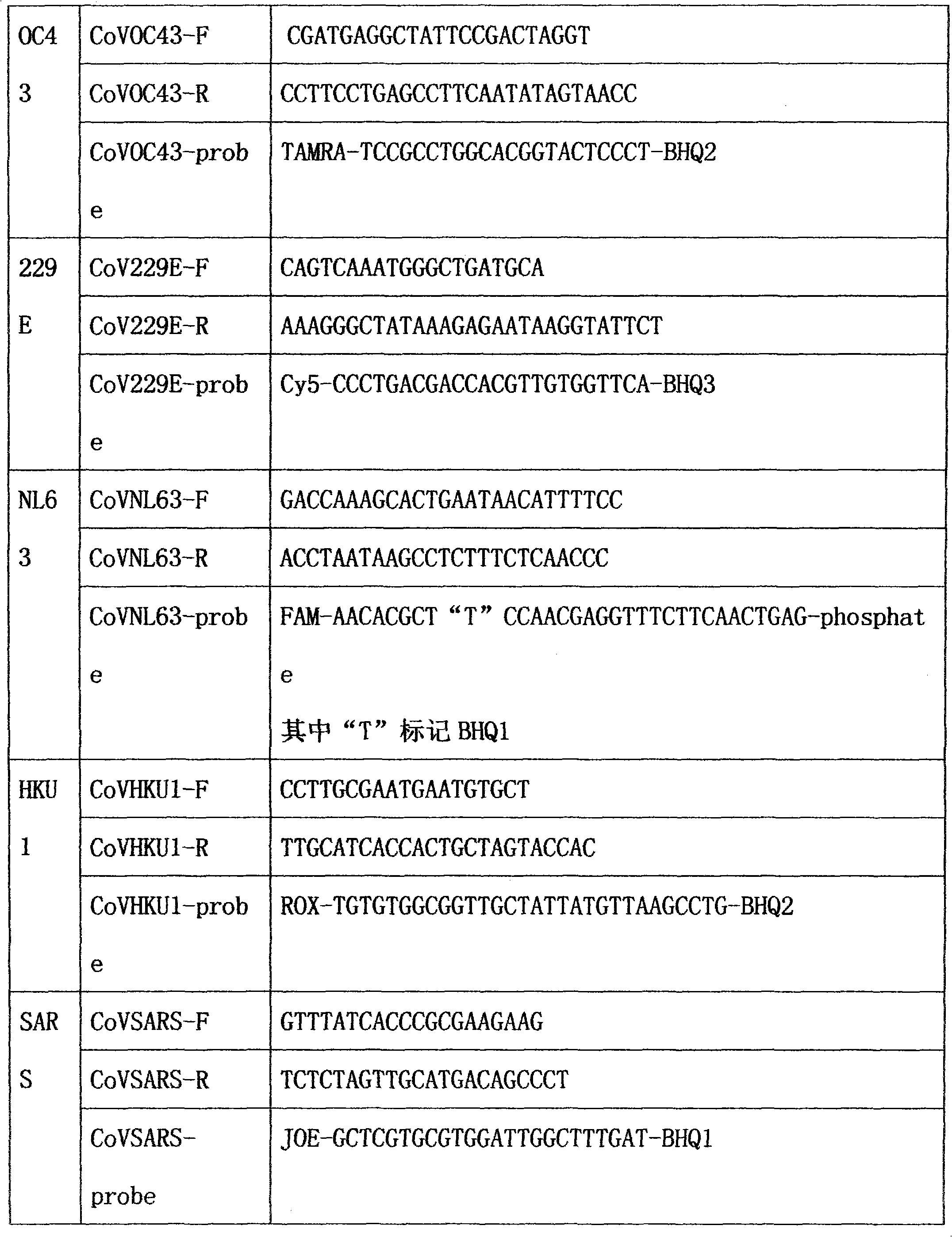
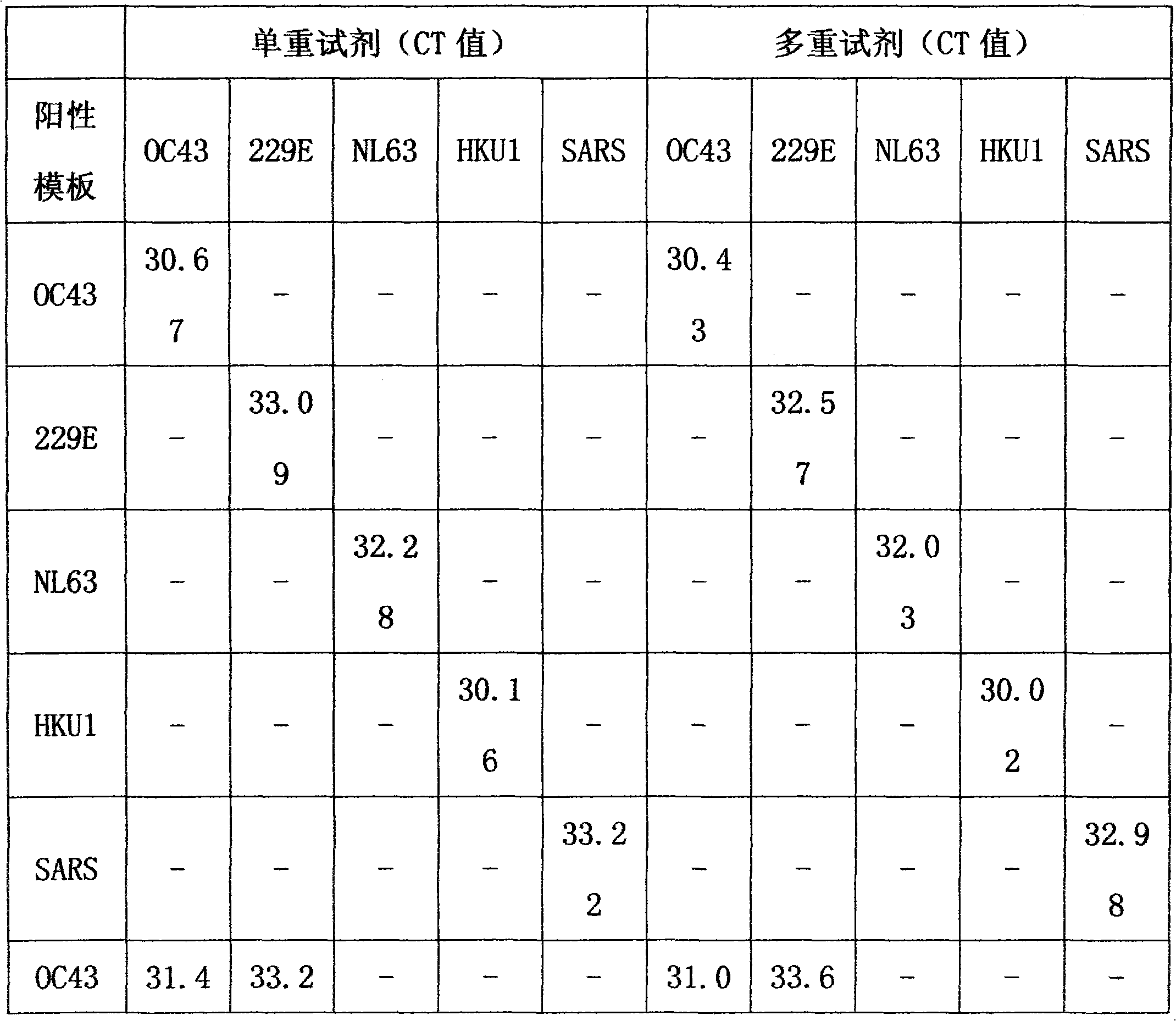
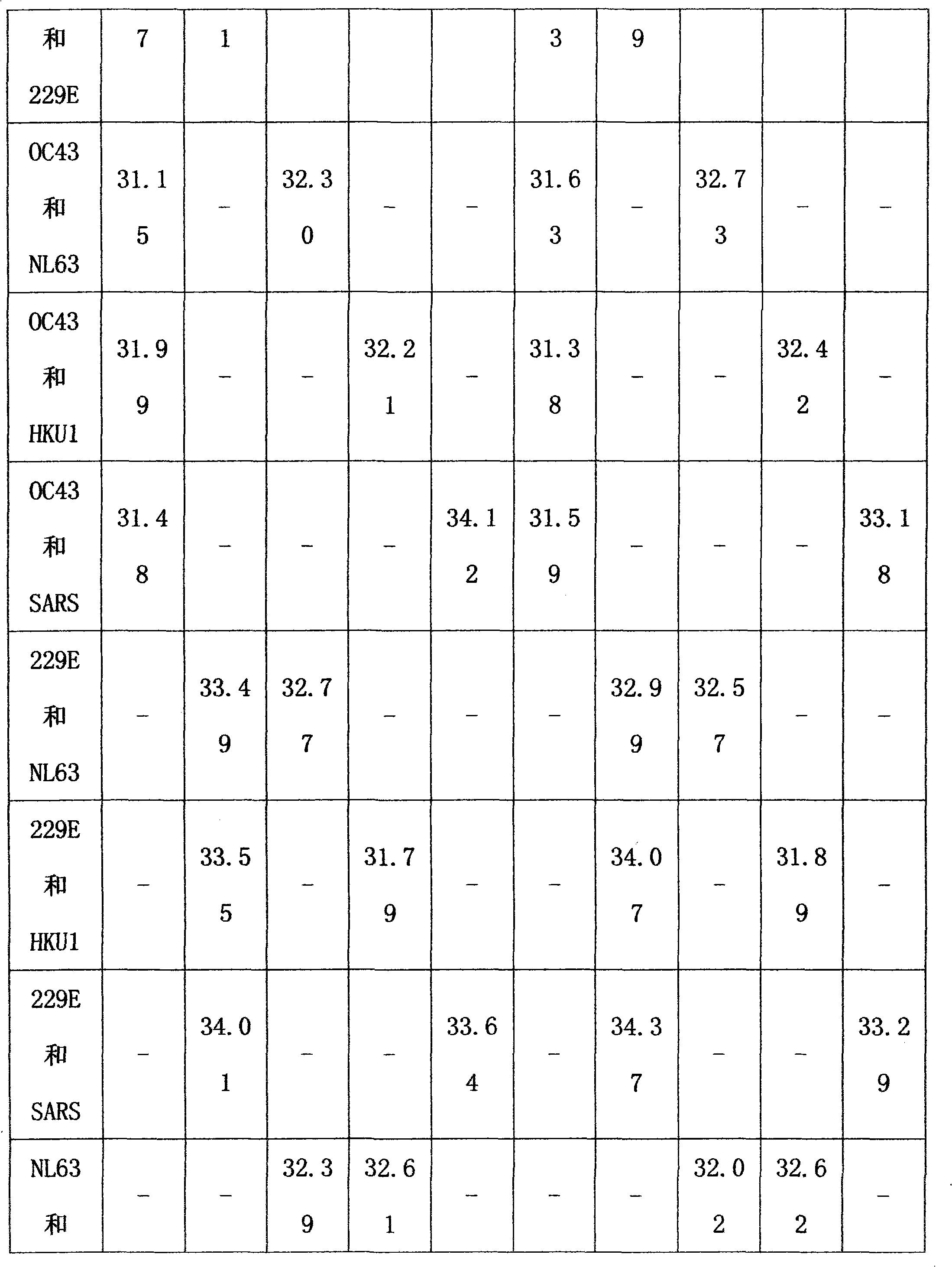
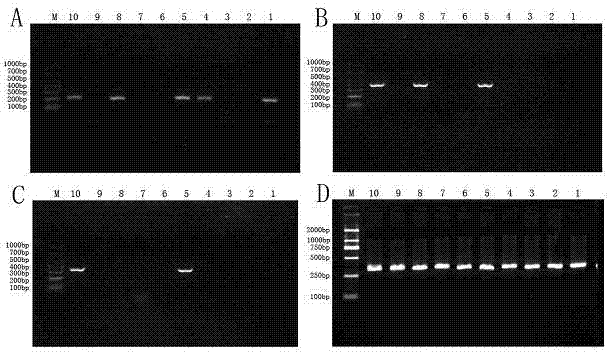
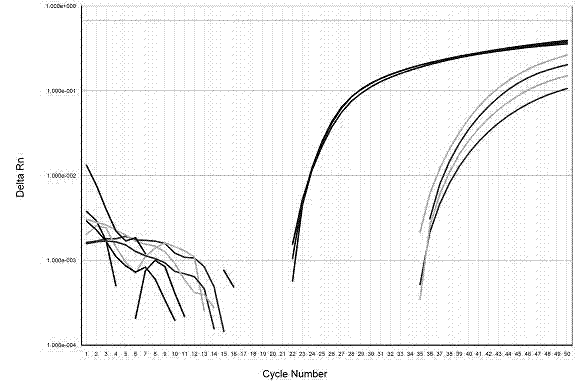
Method for single-tube multiplex fluorescent polymerase chain reaction (PCR) detection of human coronavirus OC43, 229E, NL63, HKU1 and SARS, and primers, probes and kit adopted by the method
InactiveCN102732638AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationHuman coronavirusFluorescein
The invention provides a method for single-tube multiplex fluorescent polymerase chain reaction (PCR) detection of human coronavirus OC43, 229E, NL63, HKU1 and SARS. The method adopts primers having sequences of SEQ ID NO: 1-10, and probes having sequences of SEQ ID NO: 11-15. The invention also provides a kit for single-tube multiplex fluorescent PCR detection of human coronavirus OC43, 229E, NL63, HKU1 and SARS. The kit contains the primers and the probes. The method provided by the invention adopts the primers which are specific primers of OC43, 229E, NL63, HKU1 and SARS, and the probes which are Taqman probes, utilizes TAMRA / CY5 / FAM / ROX / JOX multiple fluorescein labeling, realizes single-tube multiplex detection of human coronavirus OC43, 229E, NL63, HKU1 and SARS, and has the advantages of strong specificity, high sensitivity, fast detection speed, simple and convenient operation and low cost. The primers and the probes can be used as detection reagents for a scientific research and clinical application.
Owner:SUN YAT SEN UNIV
Method used for detecting HLA-B*5801 alleles
ActiveCN103484533AReliable resultsFlexible detection methodMicrobiological testing/measurementDNA/RNA fragmentationGenomicsA-DNA
The invention belongs to the field of pharmacogenomics and genetic diagnosis, and relates to a method used for detecting HLA-B*5801 alleles. The method comprises following steps: a DNA sample to be detected is taken, three pairs of specific primers and a pair of internal primers are taken, amplification of DNA segments is realized by using sequence specific primer method, and then the results of the amplification are analyzed by agarose gel electrophoresis; or sample DNA is extracted, a pair of specific primers, a pair of internal primers and three fluorescence probes are taken, amplification of DNA segments is realized by Taqman probe method using a fluorescence ration PCR instrument, and then the amplification curve is analyzed so as to obtain results. Results analysis methods such as agarose gel electrophoresis, high resolution melting curve and SYBRGreen fluorogenic quantitative PCR are employed in the method. The method has advantages of speediness, convenience, flexibility, high resolution and no contamination; is suitable for detection of HLA-B*5801 alleles in samples such as peripheral blood and hair; and can be used for determining the probability of severe skin adverse reaction of patients with gout or hyperuricemia caused by taking of allopurinol.
Owner:安徽同科生物科技有限公司
Method and kit for detecting gene point mutation based on digital PCR platform
InactiveCN103911427AHigh speedImprove accuracyMicrobiological testing/measurementFluorescenceBiology
The invention relates to the field of molecular biology and especially relates to a method and a kit for detecting gene point mutation based on a digital PCR platform. Digital PCR is used as a platform, PCR primers and TaqMan probes are added into a reaction system, the TaqMan probes labeled with different types of fluorescent light groups are used for detecting wild and mutational DNA templates, and according to fluorescent light types, types and an amount ratio of the DNA templates in a sample are determined.
Owner:上海涌泰生物医药科技有限公司
Method for detecting allergen almond component in foods by fluorescent PCR technology
InactiveCN101643786AAvoid complex processingReduce distractionsMicrobiological testing/measurementFluorescence/phosphorescenceFluorescent pcrBiology
The invention discloses a method for detecting an allergen almond component in foods by a fluorescent PCR technology, belonging to allergen detecting technologies, in particular a method for detectingan allergen almond component in foods by an exonuclease probe fluorescent PCR technology (TaqMan). Aiming at an allergen almond component Pru du 1.06B DNA sequence a primer and a TaqMan probe are designed, the fluorescent PCR detecting method is established. The method includes the designed primer and a probe of the almond component specificity, and the fluorescent PCR reaction condition matchedwith the primer and the probe. The method has no cross reaction with peanuts, hazelnuts, chestnuts, walnuts, pine nuts, macadamia nuts, and the like and has specificity; and the detecting sensitivitycan reach 5mg / kg. The method can be used for detecting the allergen in the foods and preventing anaphylactic reaction caused by the foods and has practical meanings.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Kit for detecting EGFR gene mutation and application of kit
ActiveCN104946739ADoes not generate non-specific signalStrong specificityMicrobiological testing/measurementSerum igeFluorescence
The invention discloses a kit for detecting EGFR gene mutation and an application of the kit. The kit is characterized by containing 20 specific amplification primers for an EGFR gene mutation site, 5 efficient blocking probes for wild sequences, and 4 EGFR gene specific TaqMan fluorescent probes. The kit can be used for detecting samples of which the mutation copy number is as low as 5-10 copies and the mutation content is as low as 0.1%. The kit disclosed by the invention can be used for simultaneously detecting 5 types of gene mutations of an EGFR gene, is high in sensitivity, simple to operate, low-cost in detection and wide in clinical application range, the samples can be fresh pathological tissues, paraffin embedded tissues, pleural fluid, serum or plasma, the detection speed is high, and the detection process can be finished in only 90 minutes.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI +1
Kit and method for detecting polymorphism of CYP2C19 gene
ActiveCN103468818AStrong specificityImprove accuracyMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceTrue positive rate
The invention relates to a kit and a method for detecting polymorphism of CYP2C19 gene, and belongs to the field of fluorogenic quantitive PCR (Polymerase Chain Reaction). The kit comprises a detection primer and a fluorescent probe, which comprise at least one group of specific primer and specific Taqman fluorescent probe of CYP2C19 gene CYP2C19*2 polymorphism, specific primer and specific Taqman fluorescent probe of CYP2C19 gene CYP2C19*3 polymorphism, and specific primer and specific Taqman fluorescent probe of CYP2C19 gene CYP2C19*17 polymorphism. The kit is adopted for detecting the CYP2C19 gene, the sensitivity and specificity are both obviously improved, the detection time is short and the predication of medicament dosage is facilitated.
Owner:刘辉
Kit for simultaneously detecting SLCO1B1, APOE and LDLR gene multisite mutation
The invention belongs to the technical field of gene mutation detection, and concretely discloses a kit for simultaneously detecting SLCO1B1, APOE and LDLR gene multisite mutation. Through meticulous design, multi-time verification, screening and optimization, specific primers and probes based on a Taqman allelic gene resolution analysis method are obtained; eight functional variation of the SLCO1B1, APOE and LDLR genes can be detected; the time from DNA (Deoxyribonucleic Acid) extraction to fluorescent PCR (Polymerase Chain Reaction) to result obtaining is less than four hours; and the manual operation time is less than two hours. The kit comprising the primer pairs and the probe pairs has the advantages that the time is saved; convenience is realized; the sensitivity is high; and both the positive conformity rate and the negative conformity rate of a sample are higher than 99 percent, and the like. A detection method provided by the invention is mainly used for the personalized medication auxiliary diagnosis of statins such as simvastatin, atorvastatin, fluvastatin and rosuvastatin.
Owner:钟诗龙
Variant porcine reproductive and respiratory syndrome virus (PRRSV) TaqMan fluorescence quantitative RT-PCR detecting kit and application thereof
InactiveCN101736094AGuaranteed specificityStrong specificityMicrobiological testing/measurementMicroorganism based processesHighly pathogenicFluorescence
The invention discloses variant porcine reproductive and respiratory syndrome virus (PRRSV) TaqMan fluorescence quantitative RT-PCR detecting kit and application thereof. A primer and a TaqMan probe are designed and synthesized by referring to an NSP2 fragment gene sequence of the variant PRRSV and common PRRSV of a GenBank. By optimizing the reaction condition and constructing a standard plasmid product, a method for diagnosing the variant PRRSV by TaqMan fluorescence quantitative RT-PCR is established. A result indicates that the method has the advantages of strong specificity, high sensitivity, and the like and can detect the standard plasmid product with 264 copy numbers, and the virus quantity of 0.5623TICD50 is 10 times more sensitive than RT-PCR. By detecting 22 disease samples, 8 disease samples are positive, and the positive rate is 36.4 percent. Because the method has the advantages of quantification, high speed, accuracy, sensitivity, and the like, the invention is suitable for the diagnosis on the swinery infected variant PRRSV in the early stage, the medium stage and the later stage and plays an important role in effectively diagnosing, preventing and treating the highly pathogenic PRRSV.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI +6
Micro-fluidic chip reagent kit for detecting ten respiratory tract infection pathogens and use method of reagent kit
ActiveCN107603866AStrong specificityBioreactor/fermenter combinationsBiological substance pretreatmentsFluorescent pcrBiology
The invention provides a micro-fluidic chip reagent kit for detecting ten respiratory tract infection pathogens and a use method of the reagent kit. The reagent kit adopts a combination of a Taqman probe fluorescent PCR (polymerase chain reaction) technology and a micro-fluidic chip, detects the ten common respiratory tract infection pathogens, can obtain a detection result within 2h, and is highin specificity, and the sensitivity can reach 100 copies / microliter. The kit comprises a sample introduction chamber, at least ten reaction chambers and a micro-fluidic flow channel, wherein the reaction chambers are mutually independent; each reaction chamber is provided with a reagent dry powder for amplifying one respiratory tract pathogen in advance; each reagent dry powder comprises a primerfor amplifying the corresponding respiratory tract pathogen and a TaqMan probe; the reagent dry powder arranged in each reaction chamber in advance can amplify any one of the ten respiratory tract pathogens; the respiratory tract pathogens amplified by the reagent dry powder in all the reaction chambers can include the ten respiratory tract pathogens.
Owner:NANJING LANSION BIOTECH CO LTD
Zika virus fluorescent PCR detecting kit
InactiveCN105734171AAccurate detectionEasy to operateMicrobiological testing/measurementMicroorganism based processesFluorescenceEnzyme system
The invention provides a Zika virus fluorescent PCR detecting kit, and relates to a kit for detecting Zika virus, in particular to simultaneous detection of Zika virus RNA by using a real-time fluorescent polymerase chain type reaction technology.The kit mainly comprises RT-PCR reaction liquid, primer probe mixed liquid, an RT-PCR reaction enzyme system, DEPC H2O and a packaging box for separating and packaging reagent bottles or tubes in a centralized manner.The kit adopts a Taqman probe detection mode and a one-step real-time fluorescent PCR reaction mode, can accurately and quickly detect the Zika virus RNA, and can be widely applied to various fields such as Zika virus disease clinic early diagnosis, disease prevention and scientific research.
Owner:DAAN GENE CO LTD
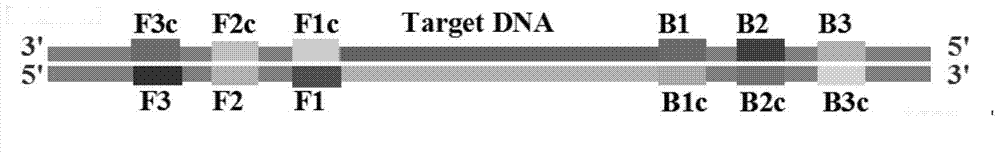
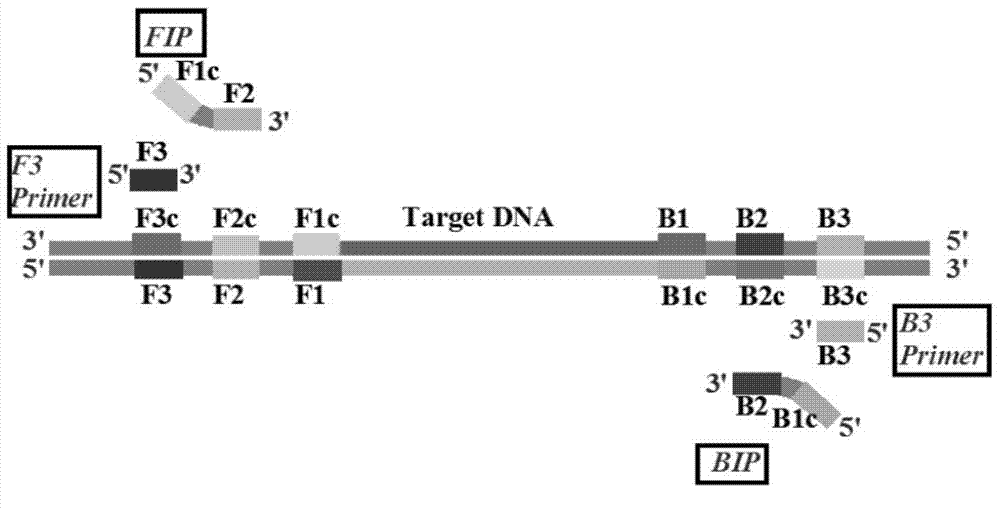
Loop-mediated isothermal amplification (LAMP) method based on TaqMan probe, and LAMP primer and kit special for same
ActiveCN103540660AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseSocial benefits
The invention provides a loop-mediated isothermal amplification (LAMP) method based on a TaqMan probe, and an LAMP primer and a kit special for the same. The loop-mediated isothermal amplification method based on the TaqMan probe, and the LAMP primer and the kit special for the same are used for detecting a target gene. According to the invention, the TaqMan probe is combined with the LAMP technology to solve the problem of nonspecific amplification fundamentally; the loop-mediated isothermal amplification method is capable of detecting the target gene quickly, conveniently and efficiently at high specificity and high sensitivity under the isothermal condition, thus providing a new technology platform for nucleic acid detection; therefore, the loop-mediated isothermal amplification method can be applied to screening and detecting pathogenic bacteria (such as superbacteria) for grass-roots medical treatment and public health units and various disease preventing and control centers, and has wide market prospect and high economic and social benefits; consequently, the loop-mediated isothermal amplification method is suitable for large-range popularization and application.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
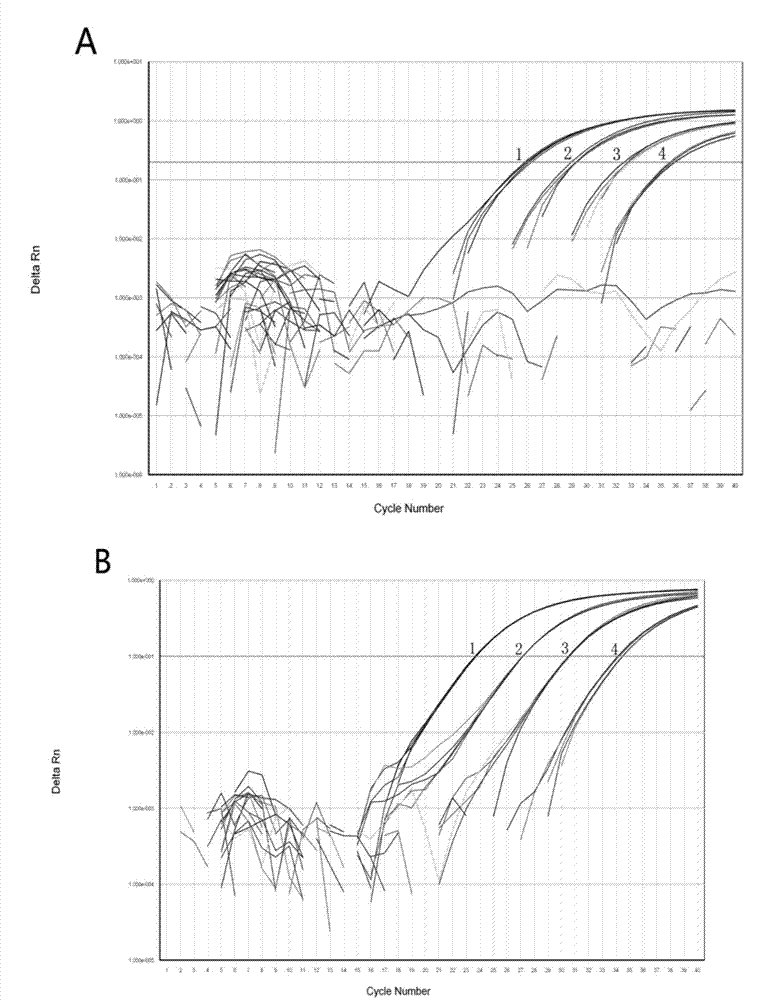
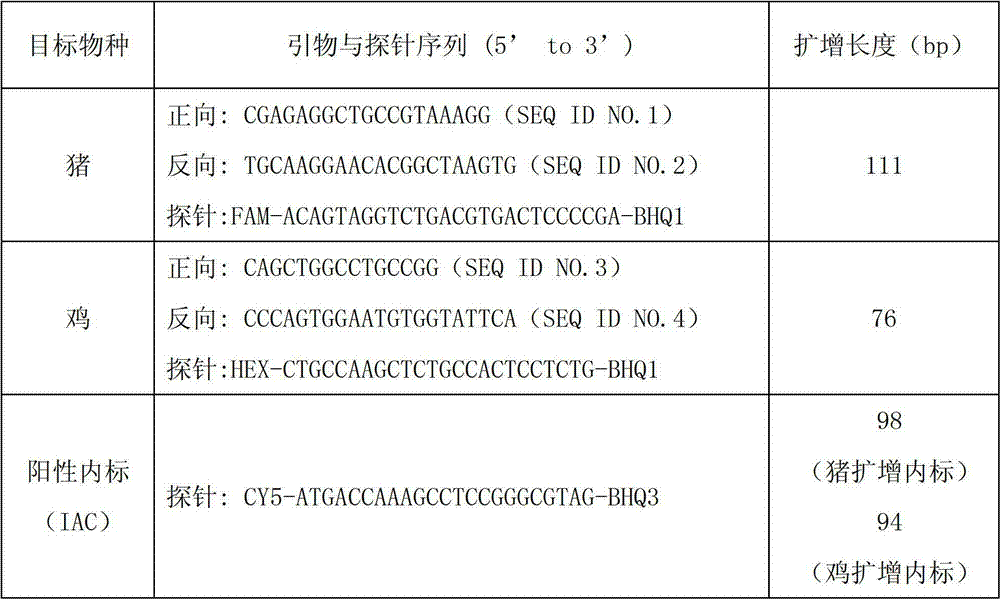
Taqman probe fluorescent quantitation polymerase chain reaction (PCR) method for rapidly detecting pork or chicken compositions in food added with internal amplification control
ActiveCN102864243AReduce the risk of interferenceDoes not affect detection sensitivityMicrobiological testing/measurementAdditive ingredientInternal standard
The invention discloses a probe fluorescent quantitation polymerase chain reaction (PCR) method for rapidly detecting pork or chicken compositions in food added with internal amplification control. The method includes designing a primer and a probe respectively based on an animal nuclear gene; and artificially synthesizing one section of competitive internal amplification control and a corresponding probe,and establishing an internal standard fluorescent quantitation PCR system respectively, using ABI 7500 Software SDS 1.4 to analyze experiment results and taking amplification with a Ct value smaller than 36 as a detection positive result. According to the method, the method is provided with good specificity aimed at target species, false negative test results are avoided by monitoring PCR reaction in real time, a novel way is explored for identification of animal origin ingredients in food, and the method has the advantages of being accurate and stable, convenient to operate and the like.
Owner:NANJING AGRICULTURAL UNIVERSITY
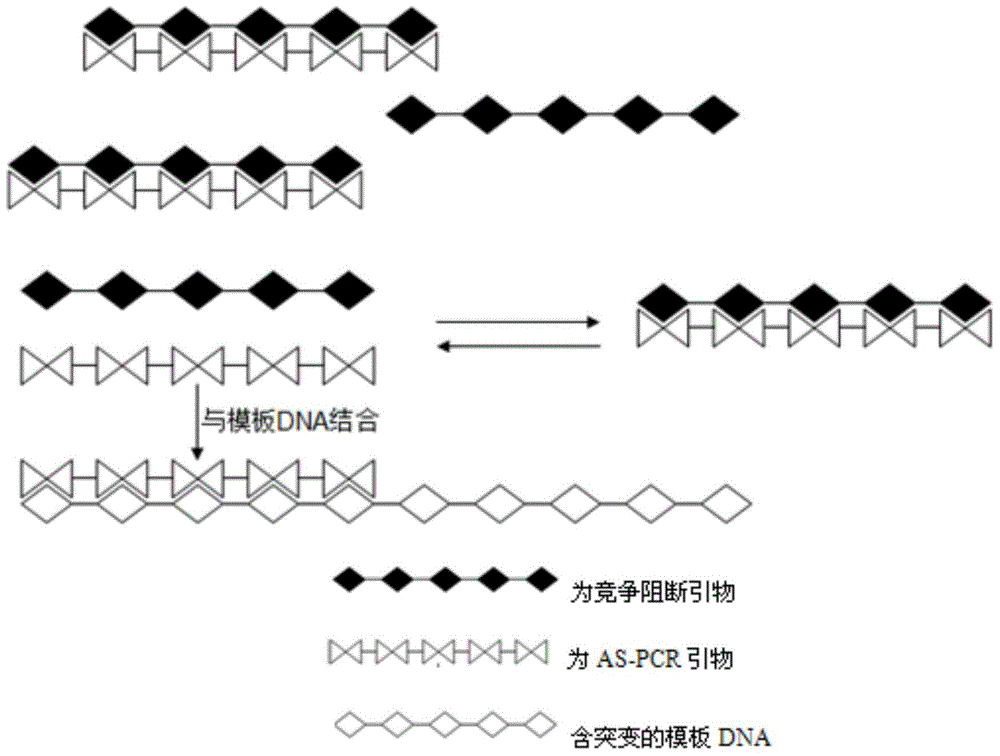
AS-PCR (allele-specific polymerase chain reaction) primer design method, gene mutation detection method and kit
InactiveCN104611427AStrong specificityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationPolymerase LOligonucleotide
The invention relates to the field of molecular biology, in particular to an AS-PCR (allele-specific polymerase chain reaction) primer design method, a gene mutation detection method and a kit. The AS-PCR primer design method comprises the following steps: (1), an AS-PCR primer is designed for a target sequence containing a to-be-detected allele mutation region; (2), a competition blocking primer is designed for the AS-PCR primer and adopts oligonucleotide in reverse complement with the AS-PCR primer. According to the gene mutation detection method, the AS-PCR primer and the competition blocking primer have a PCR amplification reaction. The kit comprises the AS-PCR primer, the competition blocking primer, a TaqMan probe, an internal control primer, an internal control probe, polymerase, dNTP (deoxynucleotide) and a buffer solution. The AS-PCR primer design method, the gene mutation detection method and the kit have the advantage that the difference of specificity of the AS-PCR primers on mutation sites due to strong and weak mismatch of the mutation sites is reduced effectively.
Owner:江苏宏泰格尔生物医学工程有限公司
African hog cholera virus fluorescent quantitative PCR detecting reagent and preparation and use thereof
InactiveCN101463396AFast detection methodSensitive highMicrobiological testing/measurementLower limitFluorescence
The invention discloses a fluorescence quantitative PCR detection reagent for African swine fever virus, and a preparation method and the application thereof. A set of specific primers and Taqman probes are designed and synthesized to be used for detecting ASFV P54 in relevant porcine products. A standard curve drawn in the invention provides a standard for the quantitative detection of ASFV P54. The invention establishes a fast and simple real-time fluorescence quantitative PCR detection system with strong specificity and high flexibility. The detection time is only several hours, and the detection lower limit can be 15 copies. The invention can be applied to the diagnosis and quarantine technology towards the imported relevant porcine products at port, and the invention provides reliable and effective technical condition for the import quarantine work of the country without ASF.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
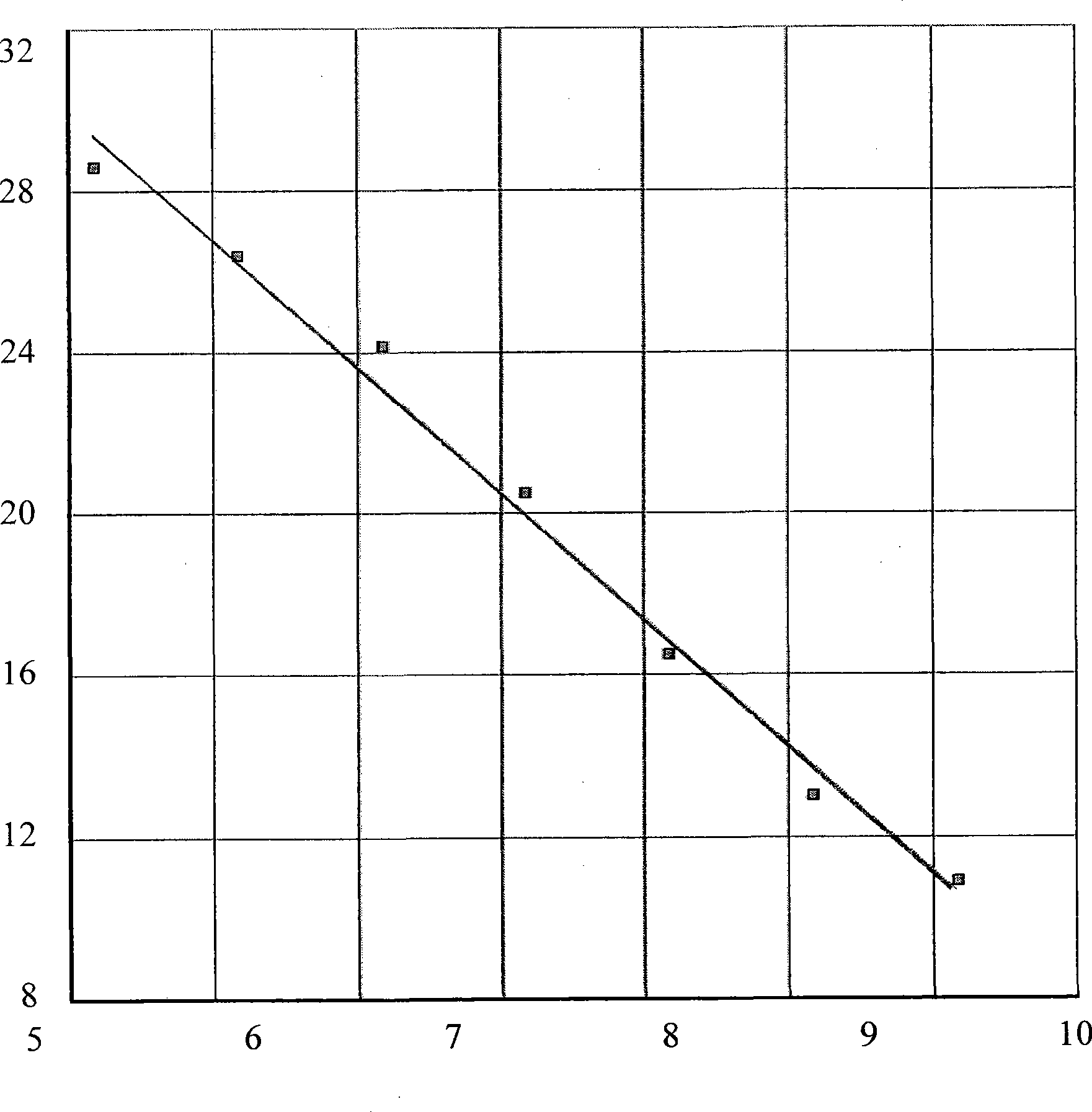
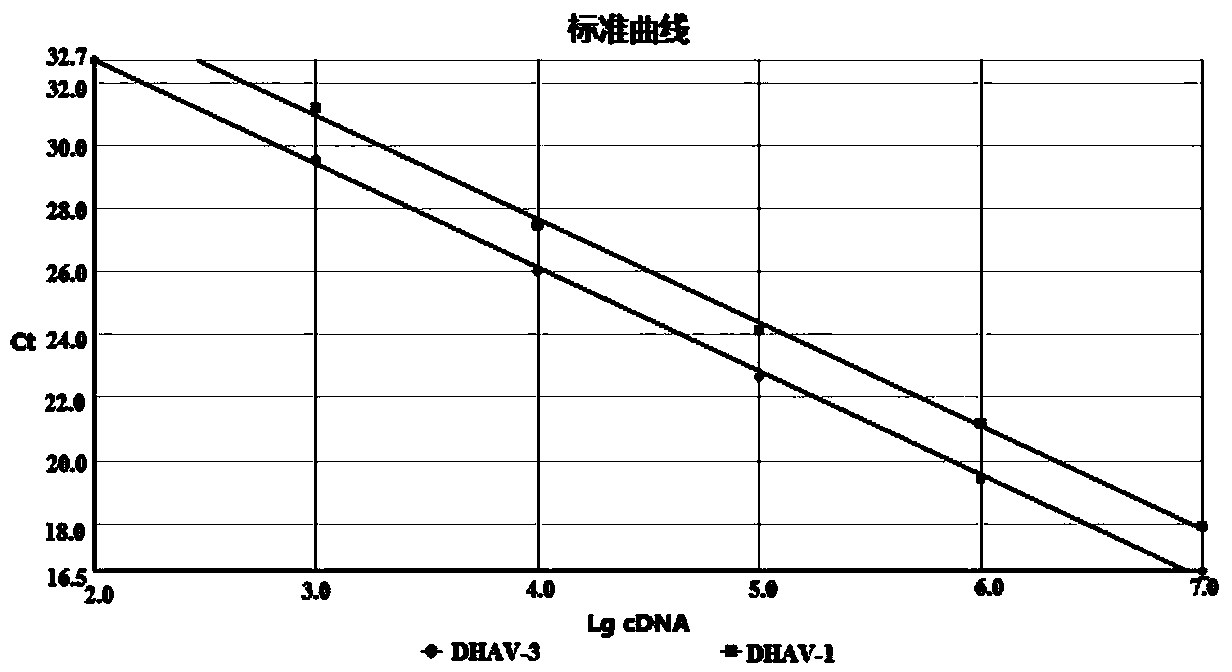
Dual fluorescence quantitative method for quickly identifying type 1 and type 3 duck hepatitis A viruses
InactiveCN104046704ARapid identificationOptimization parametersMicrobiological testing/measurementAgainst vector-borne diseasesDuck hepatitis A virusSpecific detection
The invention provides a dual fluorescence quantitative RT-PCR (reverse transcription-polymerase chain reaction) specific primer for detecting type 1 and type 3 duck hepatitis A viruses. A dual fluorescence quantitative method comprises the following steps: comparing sequences of DHAV-1 and DHAV-3 published on GenBank, and designing a pair of specific detection primers SEQ1 and SEQ2 aiming at DHAV-1 and a Taqman probe (probe1) as well as a pair of specific detection primers SEQ3 and SEQ4 aiming at DHAV-3 and a Taqman probe (probe2) respectively in a region with conservation and relatively large difference between gene sequences of the two viruses; confirming the concentration of the dual fluorescence quantitative RT-PCR specific primer and the Taqman probe aiming at DHAV-1 and DHAV-3. The dual fluorescence quantitative method built by virtue of the group of primers is good in specificity and high in sensitivity, and can be used for quick serum type identification and real-time quantitative analysis of the type 1 and type 3 duck hepatitis A viruses.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Reagent kit for quantitatively assessing long-term recurrence risks of breast cancer
InactiveCN102586410AEasy to operateTest results are stableMicrobiological testing/measurementFluorescence/phosphorescenceGenomicsBreast cancer metastasis
The invention relates to the functional genomic and gene expression detection technology and the analysis technology, and discloses a reagent kit for quantitatively assessing long-term recurrence risks of breast cancer. Particularly, a type of genes capable of being used for breast cancer metastasis and prognostic molecular classification is screened within a human functional genome expression profile range, the detection technology is created, and the reagent kit is prepared and applied to breast cancer metastasis and prognostic assessment for a patient. The reagent kit for quantitatively assessing long-term recurrence risks of breast cancer comprises 21 pairs of primers, 21 specific taqman fluorescent probes, 10XRT-PCR (reverse transcription-polymerase chain reaction) buffer solution, 2.5mM of dNTP (diethyl-nitrophenyl thiophosphate) mixed liquor, reverse transcriptase, DNA (deoxyribose nucleic acid) polymerase, 10XPCR buffer solution and RNA (ribonucleic acid) enzyme inhibitor. The reverse transcription PCR technology is combined with the taqman fluorescent quantitative PCR technology, reverse transcription primers, real-time PCR primers and the taqman fluorescent probes are self-designed and optimized, reverse transcription PCR reagent and taqman fluorescent quantitative PCR reagent are integrated to prepare the detection reagent kit, operation is simple and fast, detection results are more stable, and detection cost is lower.
Owner:苏州科贝生物技术有限公司
Human ALK fusion gene detection primer set and detection kit
InactiveCN105039580AMultiple detection fusion typesSpecify the fusion typeMicrobiological testing/measurementDNA/RNA fragmentationGene typeReaction system
The invention discloses a human ALK fusion gene detection primer set and a detection kit. The primer set comprises one or more detection primers in sixteen ALK fusion gene types A01 to A16 and Taqman probes corresponding to the gene types. As many as sixteen fusion gene types except for EML4 can be detected through the detection kit formed by the primer set and the probes, the requirement for the sample RNA obtaining quality is low, and the ideal detection effect can be achieved through samples in different existence forms; by means of the kit, an operator only needs to directly feed extracted sample RNA once, inverse transcription and PCR amplification are carried out in one closed tube reaction system, and the pollution probability and the result error probability are reduced. One-time detection only takes eighty minutes. The human ALK fusion gene detection primer set and the detection kit have the remarkable advantages that the number of the detection types is large, sensitivity is high, experiment operation is simple, the period is short, the human ALK fusion gene detection primer set and the detection kit are safe and nontoxic, and cost is low.
Owner:武汉海吉力生物科技有限公司
Real-time fluorescence quantification PCR detecting kit for cow mycoplasma and special primers and TaqMan probe thereof
InactiveCN105420379ANo follow-up work requiredReduce workloadMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceMycoplasma bovis
The invention discloses a real-time fluorescence quantification PCR detecting kit for cow mycoplasma, special primers and a TaqMan probe thereof and application of the detecting kit in detection of cow mycoplasma. The primers and the TaqMan probe for real-time fluorescence quantification PCR detecting of the cow mycoplasma are designed according to a specific conserved sequence of an OPPD / F gene of the cow mycoplasma and are used for detecting the cow mycoplasma of a sample to be detected qualitatively and quantitatively. The nucleotide sequence of the upstream primer (OF-A) is shown as SED ID NO:1 in a sequence table, the nucleotide sequence of the downstream primer (OF-B) is shown as SED ID NO:2 in the sequence table, and the nucleotide sequence of the TaqMan probe (OF-P) is shown as SEQ IDNO:3 in the sequence table. By means of the detecting kit, the special primers and the TaqMan probe thereof, the cow mycoplasma can be detected quickly, conveniently, efficiently, highly specifically and highly sensitively, and a novel technical platform is provided for detection of the cow mycoplasma.
Owner:JINYUBAOLING BIO PHARM CO LTD +1
Fluorescent PCR (Polymerase Chain Reaction) detection kit for IDH1/IDH2 (isocitrate dehydrogenase 1/isocitrate dehydrogenase 2) gene mutation and application thereof
InactiveCN103436613AStrong specificityEnrichmentMicrobiological testing/measurementDNA/RNA fragmentationNucleotideBlood plasma
The invention relates to a fluorescent PCR detection kit for the IDH1 / IDH2 (isocitrate dehydrogenase 1 / isocitrate dehydrogenase 2) gene mutation and application thereof, and particularly provides a nucleotide sequence used for detecting the IDH1 / IDH2 gene mutation, a kit with the nucleotide sequence and the application of the kit to detection of the IDH1 / IDH2 gene mutation. The nucleotide sequence comprises a specific ARMS (Amplification Refractory Mutation System) primer, a general mutation detection TaqMan probe and a nucleic acid amplification retardation primer. The kit can be used for quickly detecting the IDH1 / IDH2 gene mutation with high throughput and at a low cost, is high in sensitivity, good in specificity, low in pollution and quick and safe to operate, can be suitable for high-sensitivity detection of trace mutation in general clinic samples such as fresh frozen tissues and paraffin tissues, especially non-traumatic serums or plasma samples in addition to pathological tissues.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com