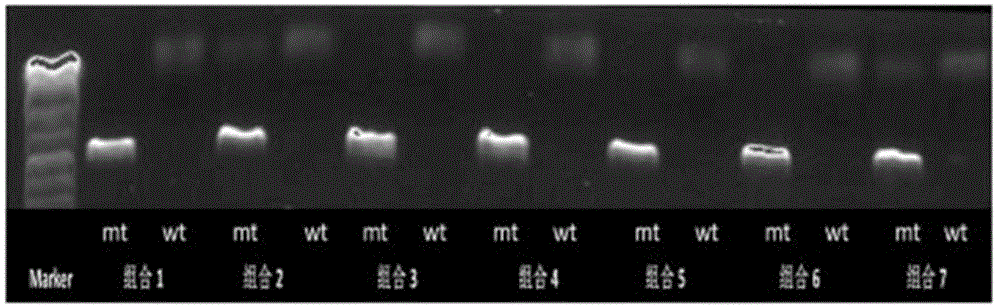
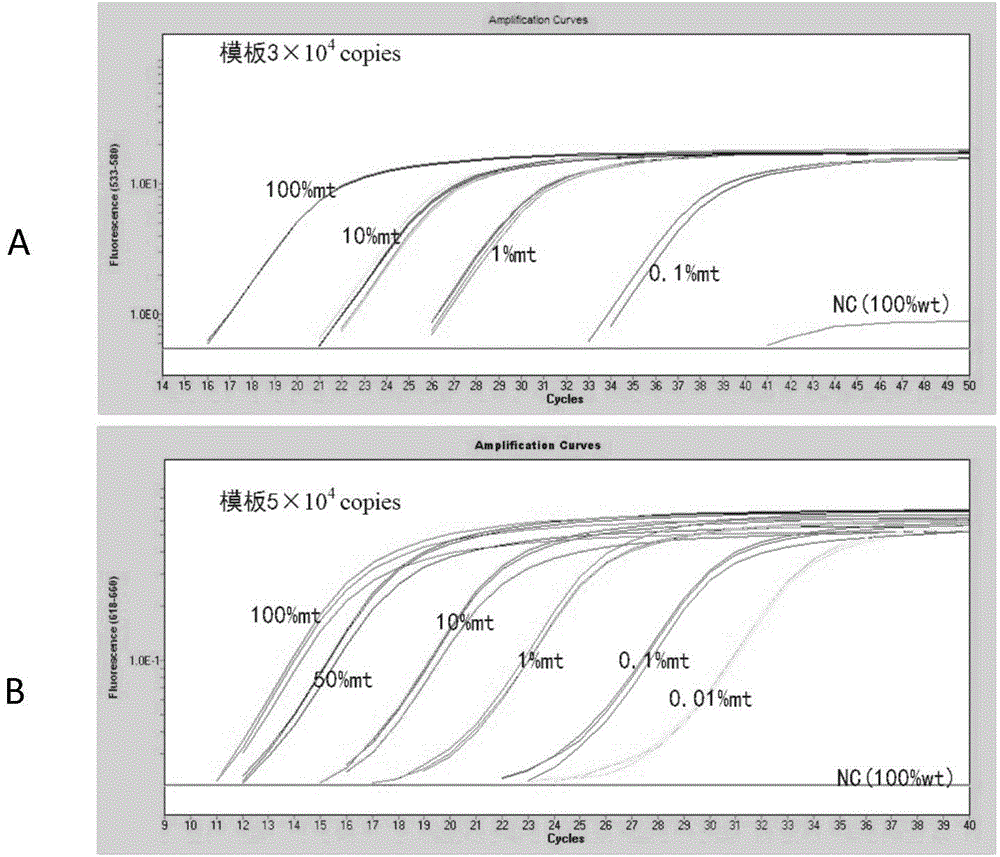
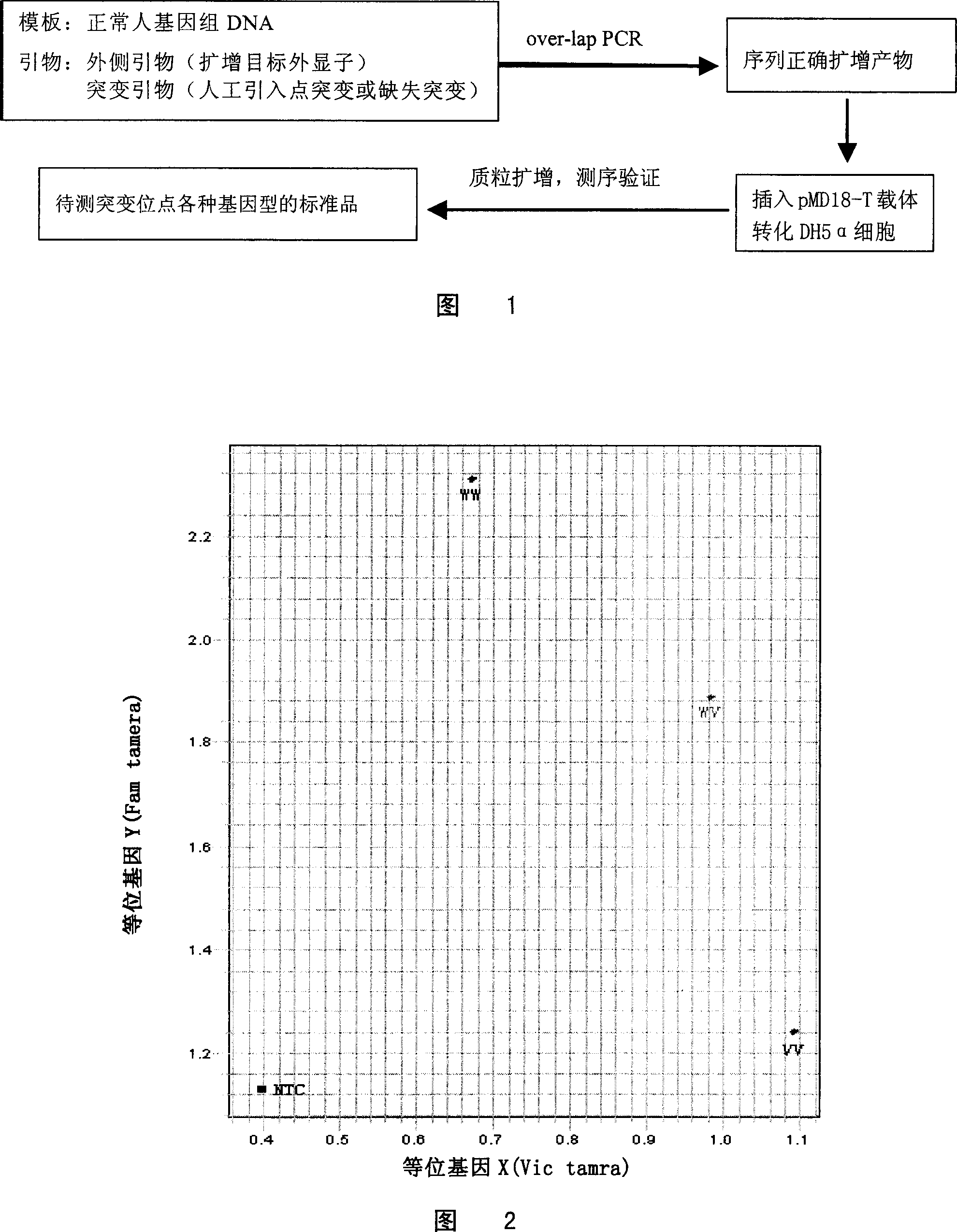
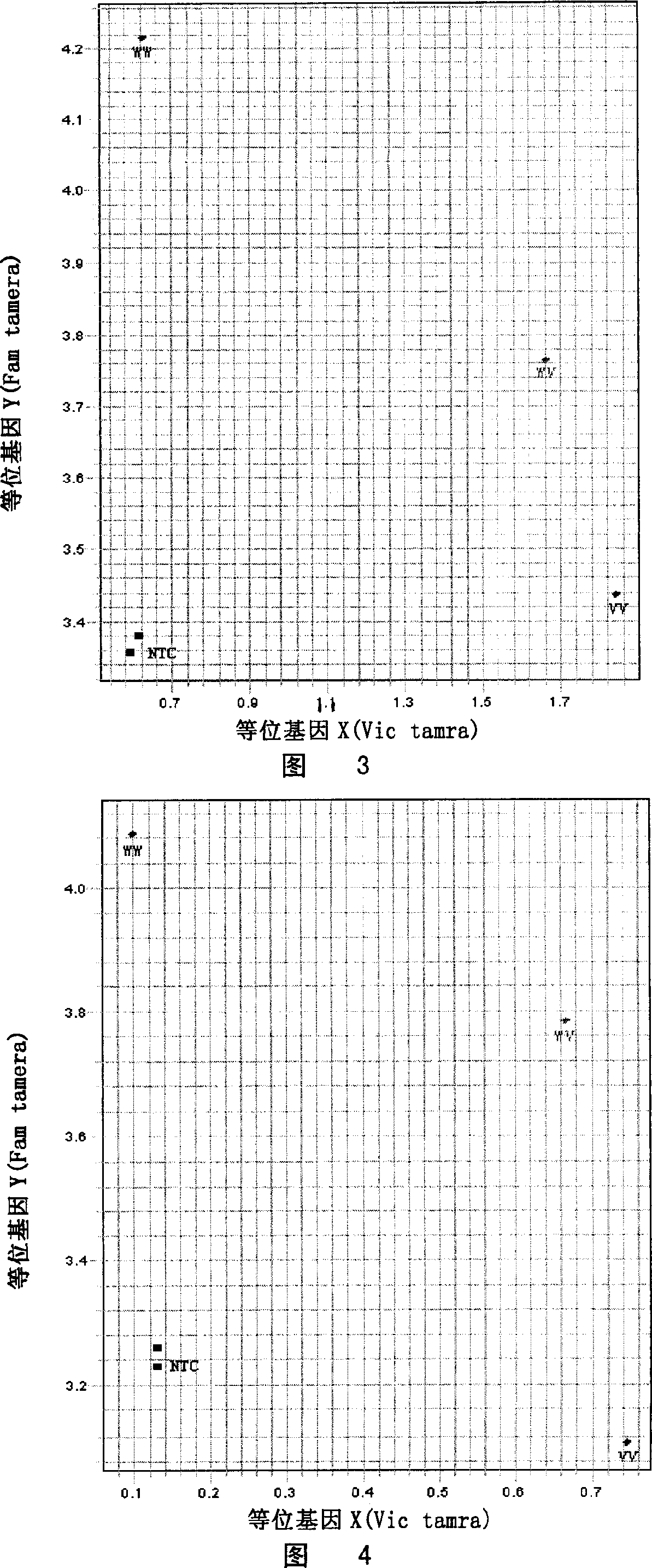
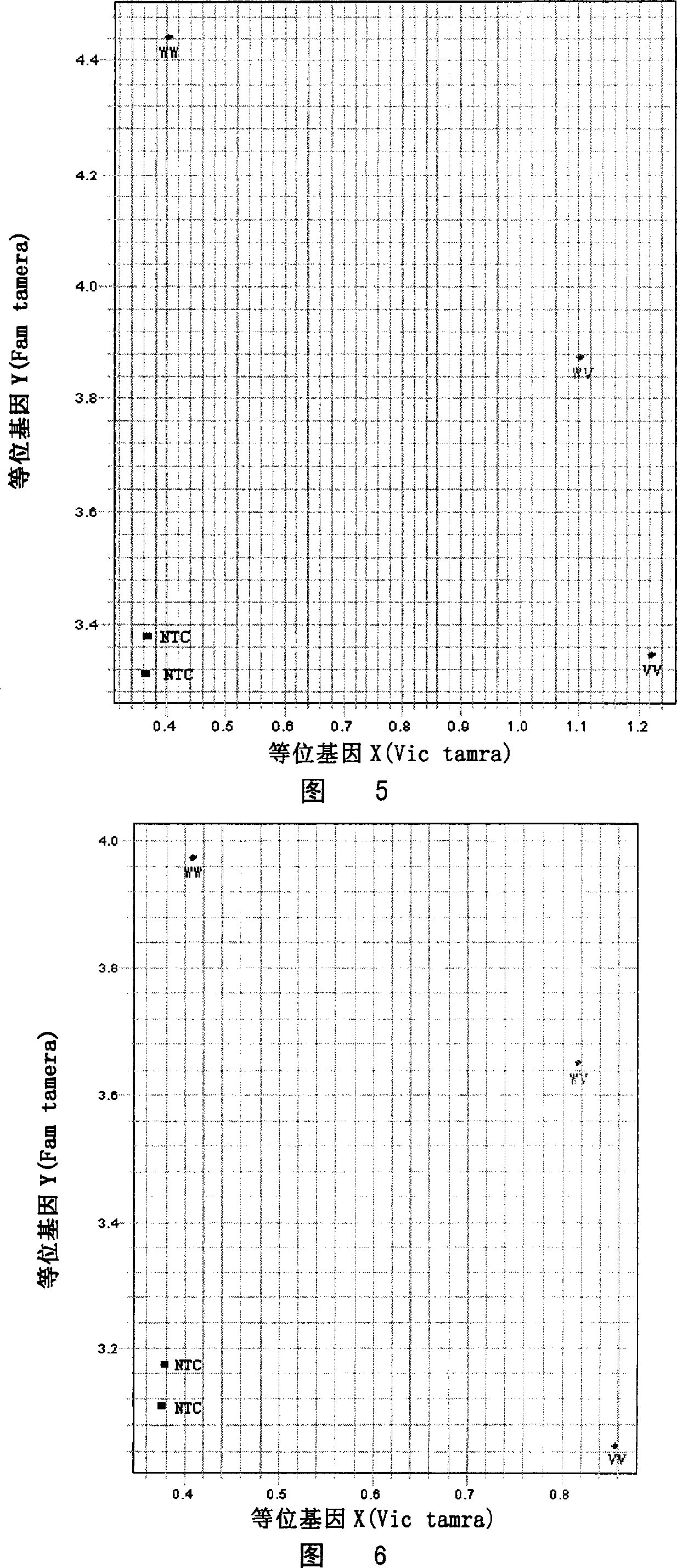
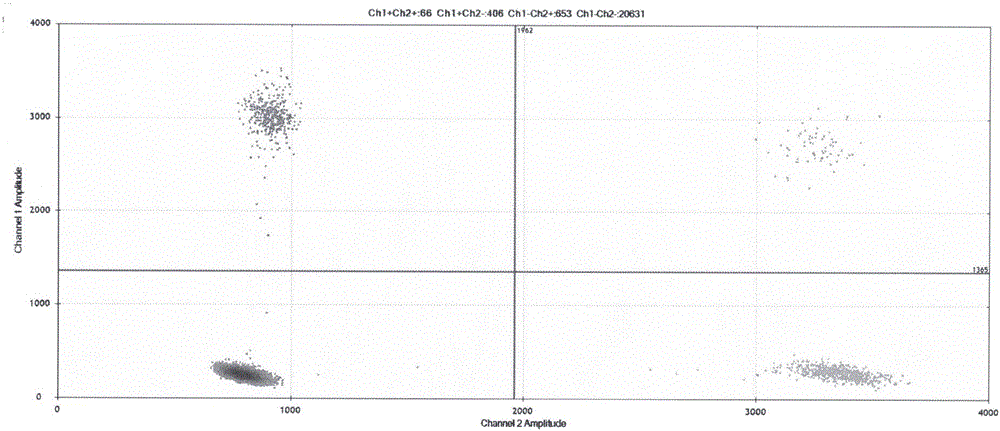
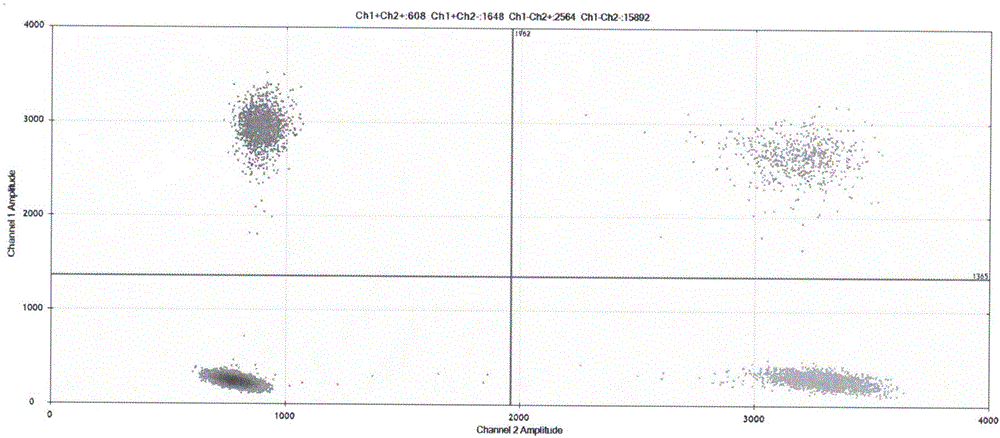
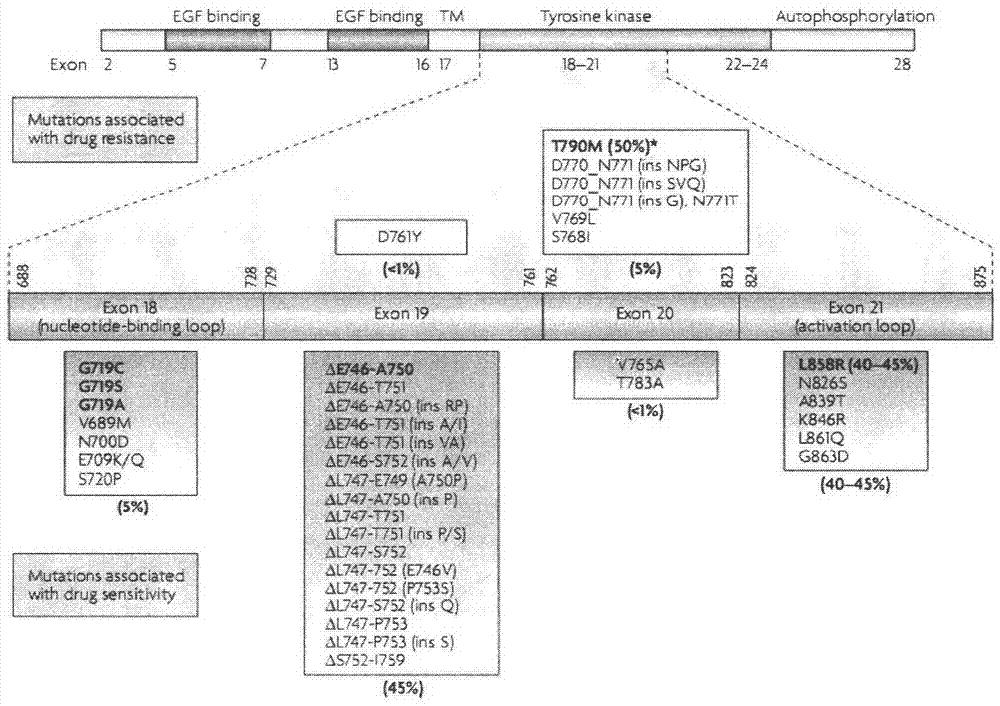
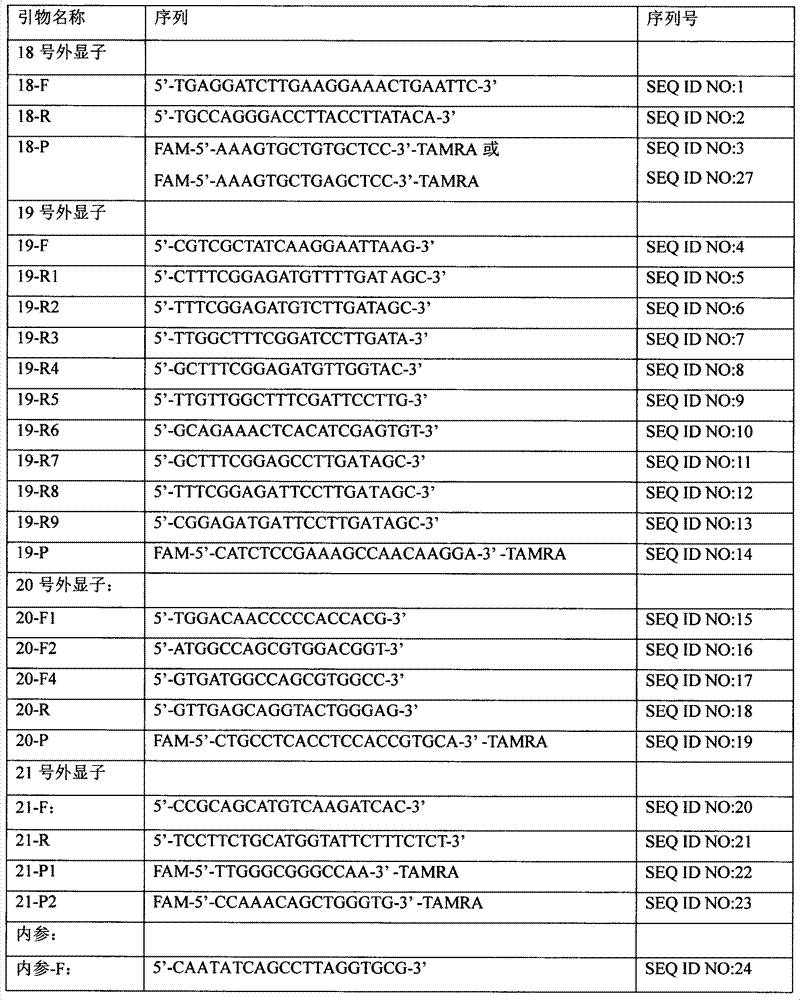
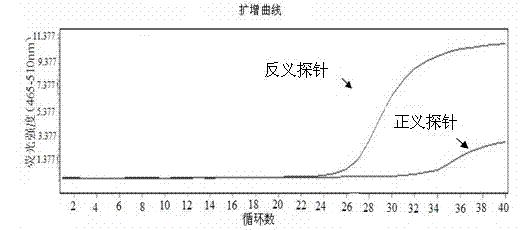
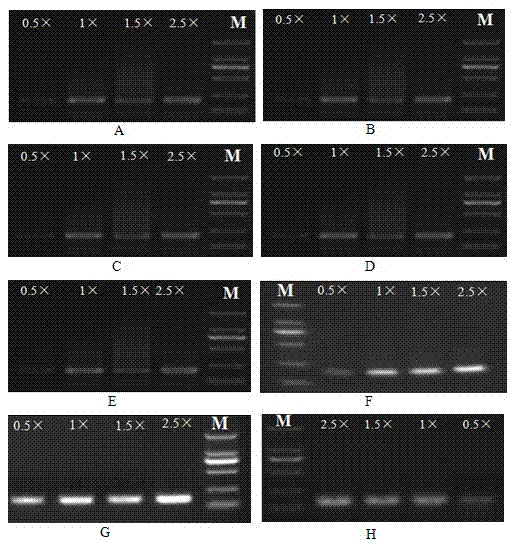
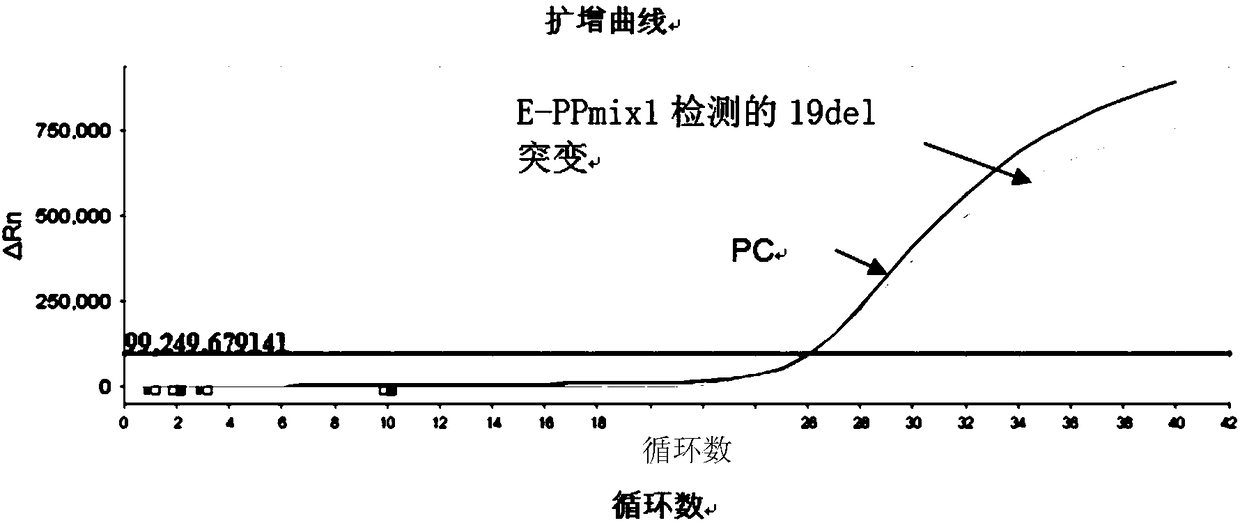
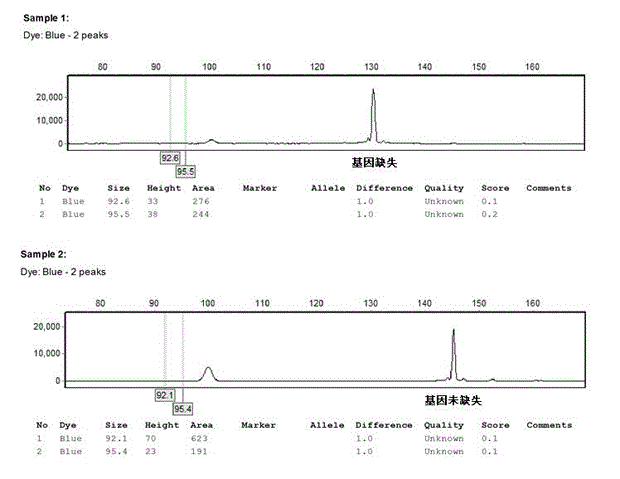
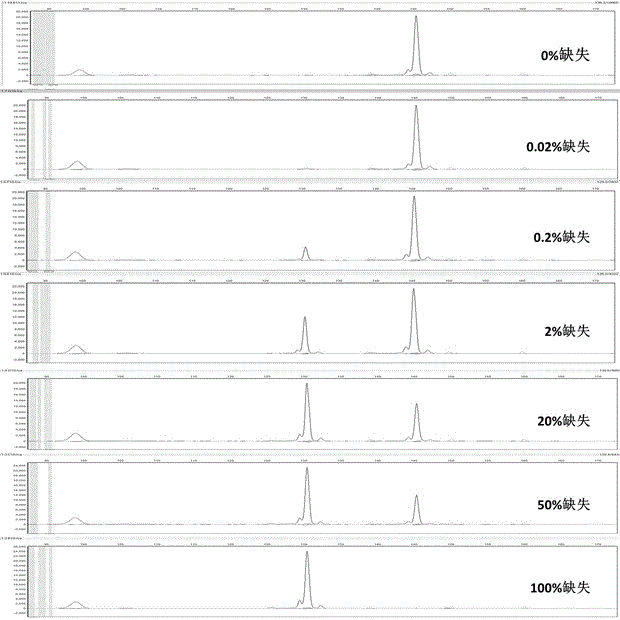
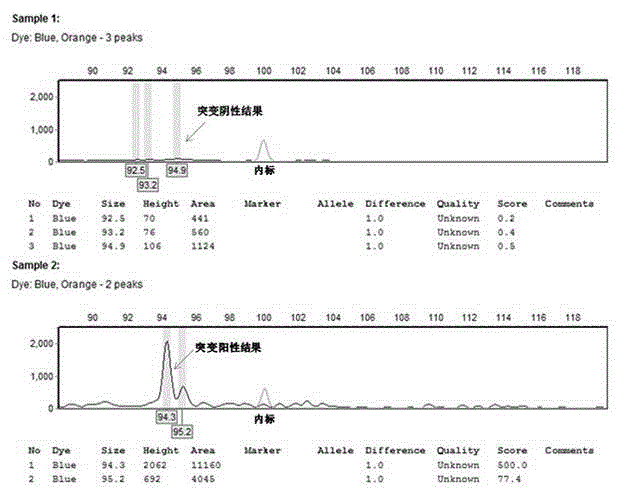
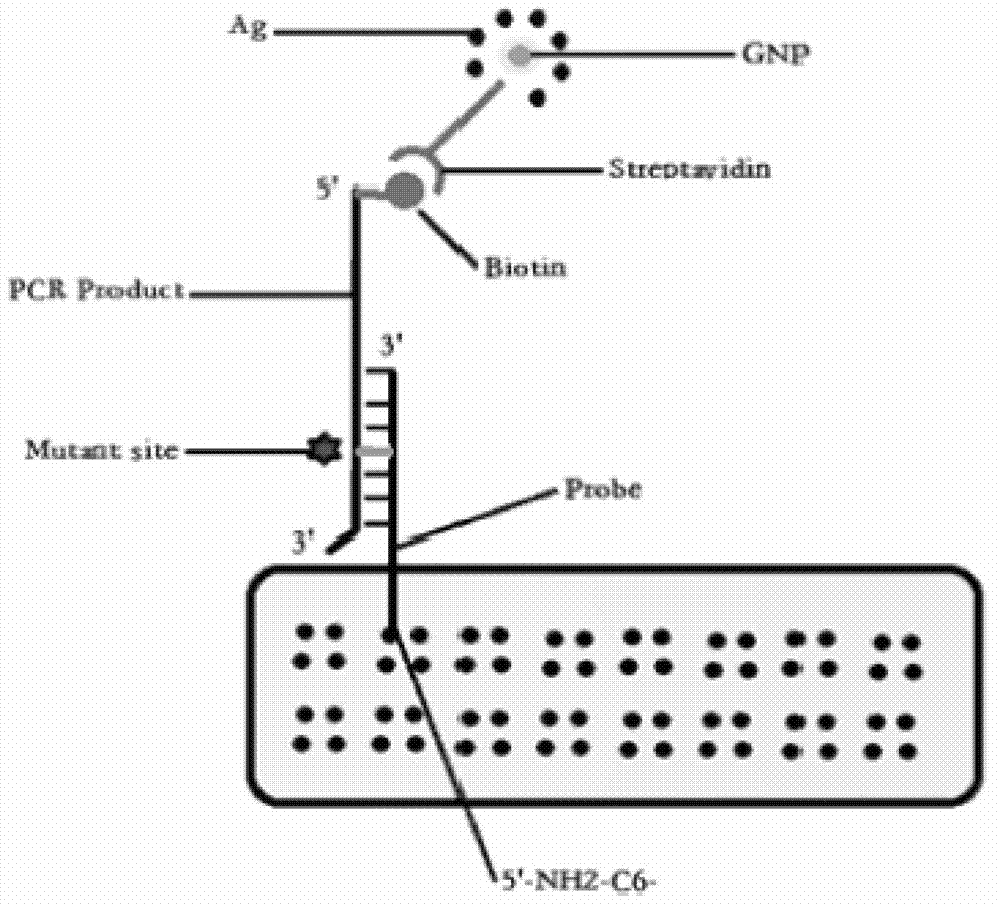
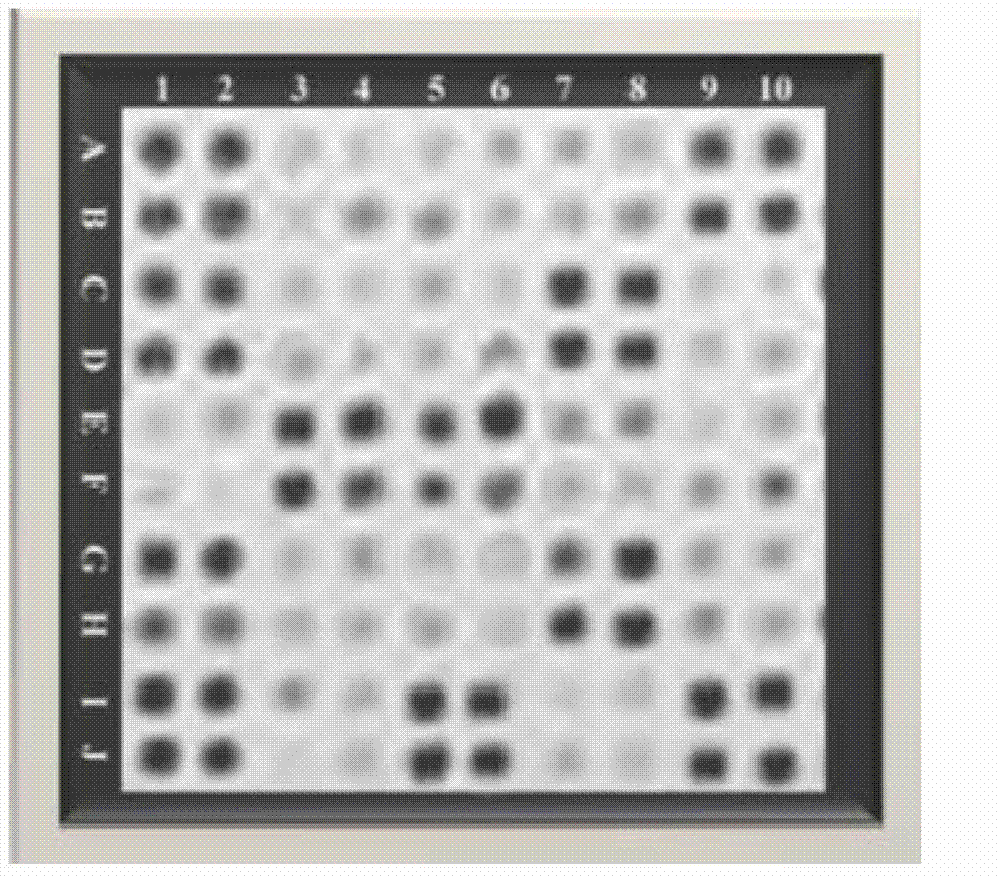
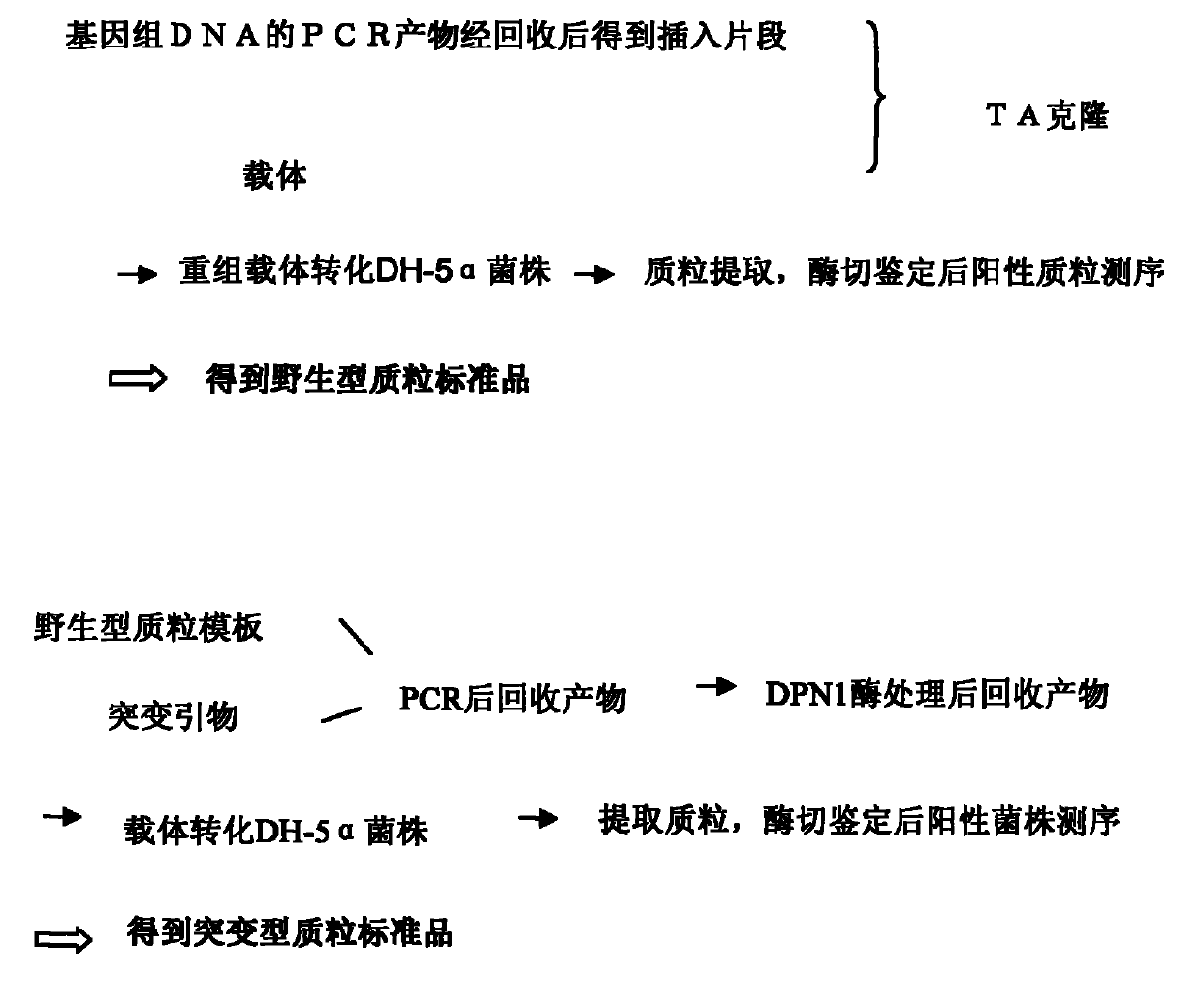
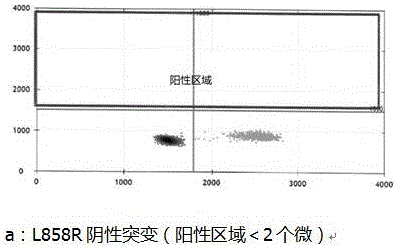
Patents
Literature
126 results about "EGFR Gene Mutation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
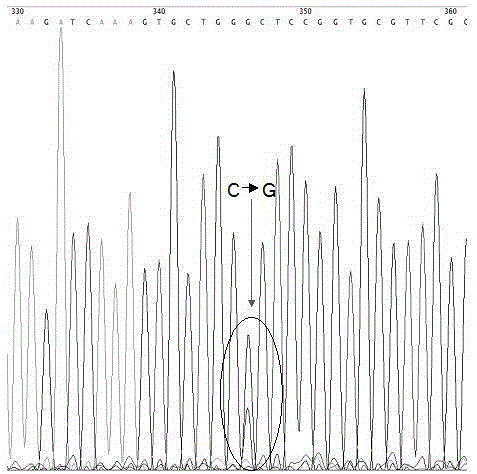
A change in the nucleotide sequence of the EGFR gene.
Primers, probes, kit and method for detecting human EGFR (epidermal growth factor receptor) gene mutations
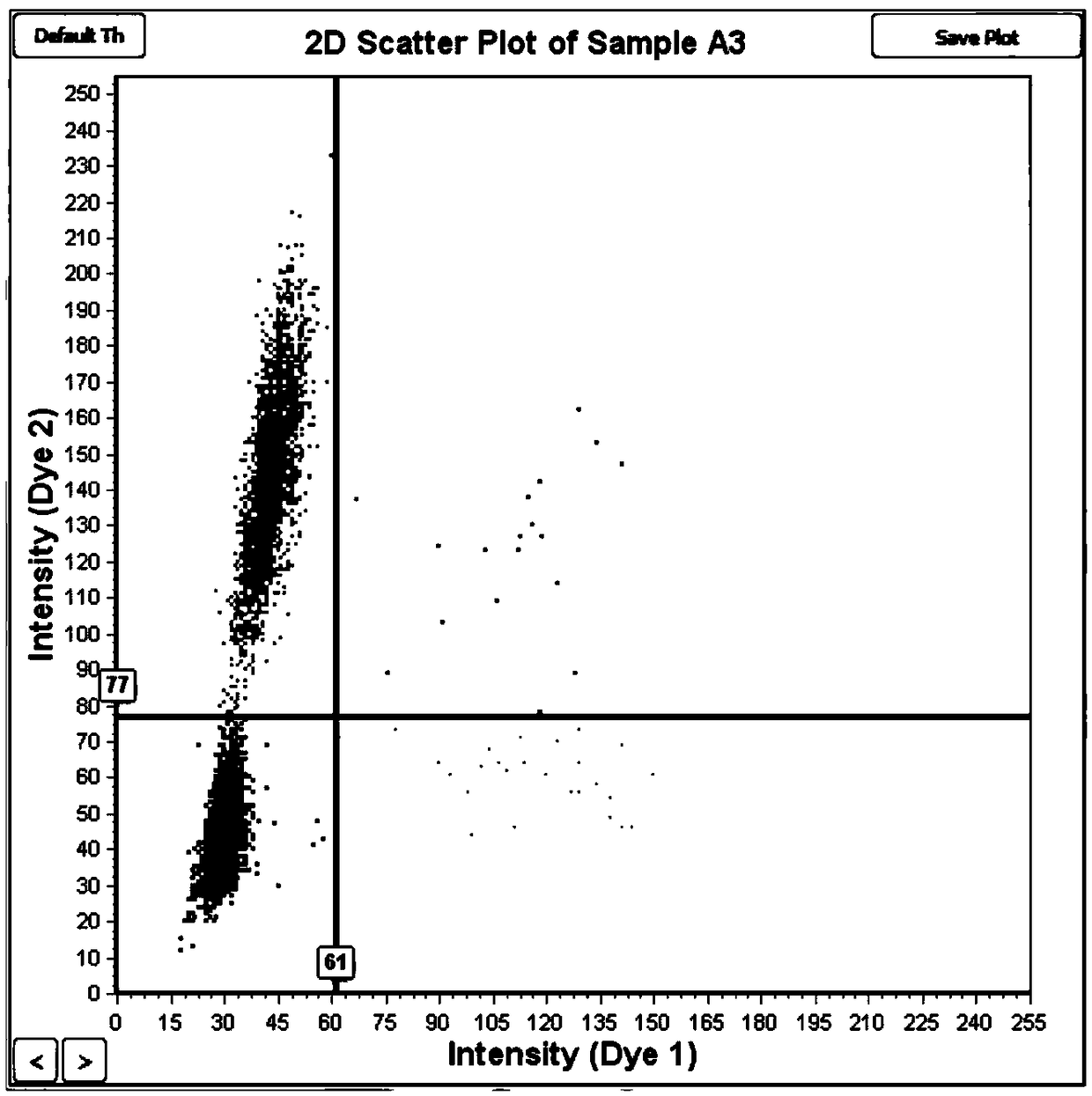
ActiveCN102747157AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationEGFR Gene MutationWild type
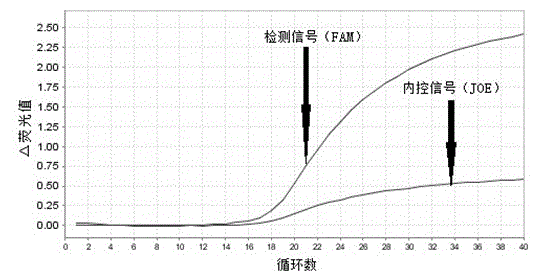
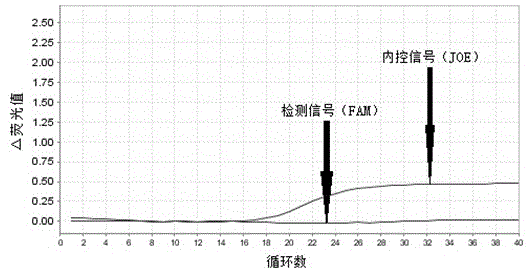
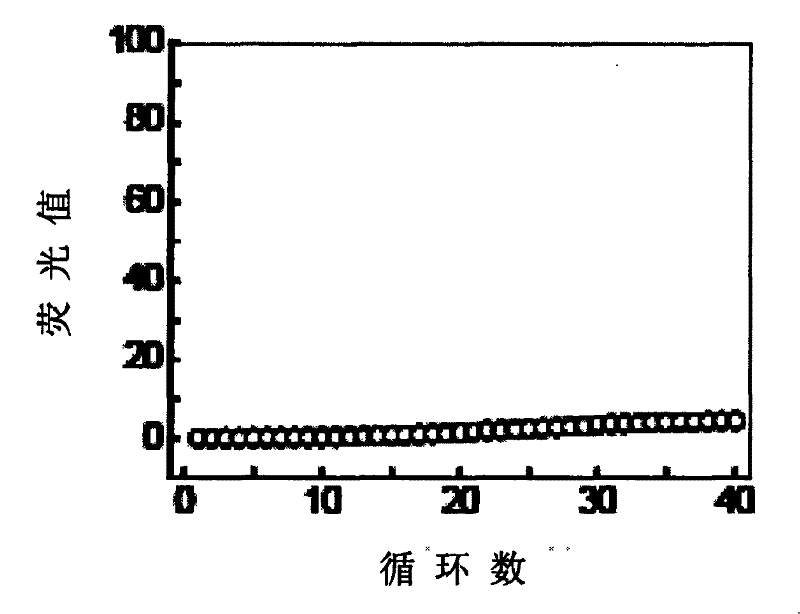
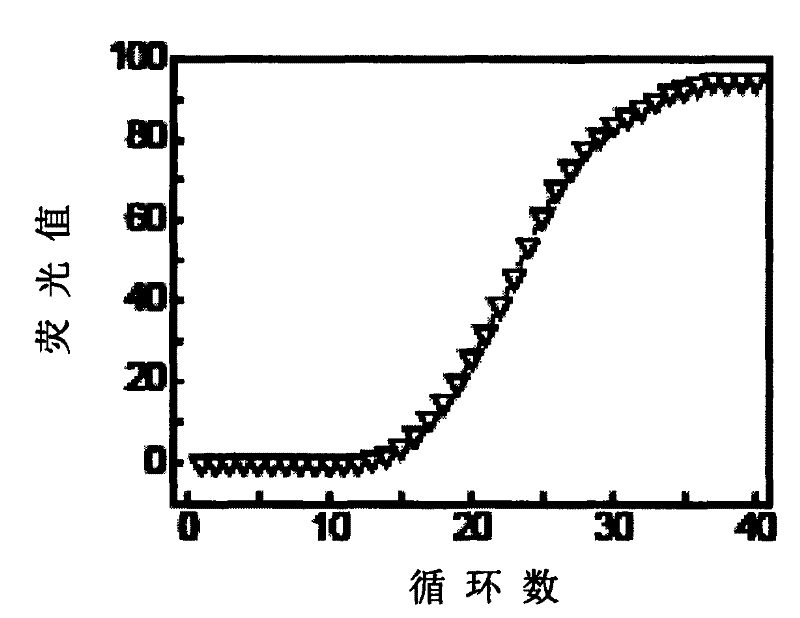
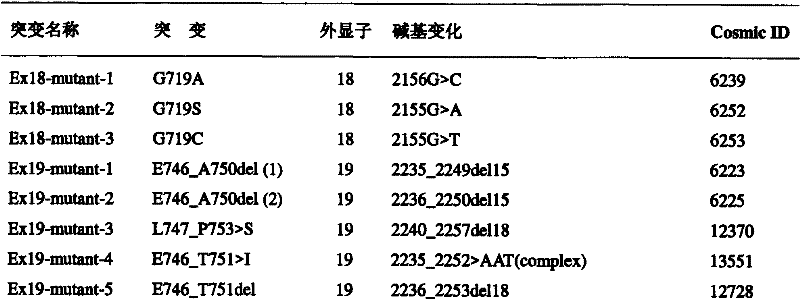
The invention provides primers, probes, kit and method for detecting human EGFR (epidermal growth factor receptor) gene mutations. The method comprises the following steps of: (1) providing the primers and the probes; (2) processing detected samples and extracting DNA (deoxyribonucleic acid); (3) carrying out fluorescent quantitation on components of a PCR (polymerase chain reaction) system; (4) amplifying target sequences of an EGFR gene mutation to be detected; and (5) distinguishing wild type genes and mutant type genes by utilizing ARMS (amplification refractory mutation system) primers and judging results via the fluorescence intensity of an FAM and a JOE. The primers, probes and method provided by the invention have the beneficial effects that 29 kinds of mutations on the EGFR genes can be simultaneously detected, so that the sensitivity is high, the specificity is strong and the detection speed is high; and the whole detection process only takes 60 minutes.
Owner:武汉海吉力生物科技有限公司
Primers, probes, detection system and kit for one time detection of lung cancer multiple genes
ActiveCN104818320AWill not interfere with each otherLow costMicrobiological testing/measurementDNA/RNA fragmentationTumor targetBRAF Gene Mutation
The present invention discloses primers, probes, a detection system and a kit for one time detection of lung cancer multiple genes, wherein the primers, the probes, and the distribution way for detecting 25 EGFR gene mutations, 7 KRAS gene mutations, 6 BRAF gene mutations, 9 NRAS gene mutations, 5 HER2 gene mutations, 2 PIK3CA gene mutations, 5 fusion genes of ALK5, 13 fusion genes of ROS1, and 6 fusion genes of RET are provided. According to the present invention, the detection kit adopts the 12 linking PCR reaction strip design, each 12 linking PCR strip detects multiple genes of a sample, and the corresponding fusion detection reagents and the internal control reagents are filled in the pipes 1-4 of the 12 linking PCR strip; and with the primers, the probes, the detection system and the kit, the one-time detection of the 24 fusions and the 54 mutations of the lung cancer can be achieved, such that the detection time is substantially shortened, the sensitivity is high, the specificity is strong, the operation is simple and rapid, and the reference for selection of tumor targeting drug therapy on lung cancer patients can be provided for clinician.
Owner:AMOY DIAGNOSTICS CO LTD
EGFR gene mutation detection primer probe and kit thereof
ActiveCN106520931ASolving insufficient sample sizeNon-invasive detection is goodMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceEGFR Gene Mutation
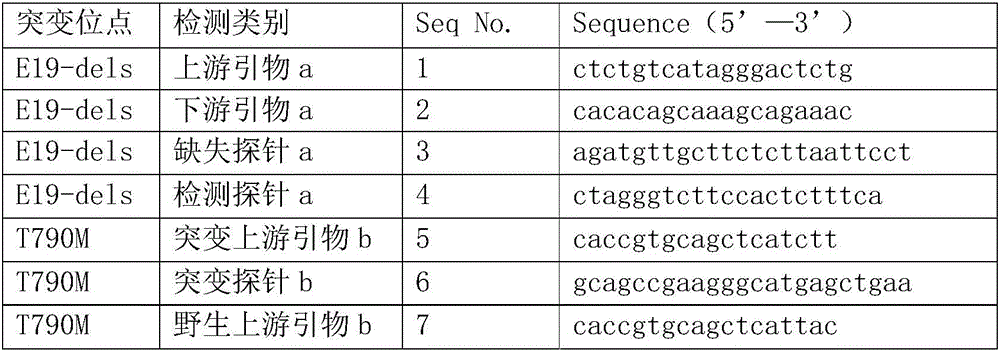
The invention relates to an EGFR gene mutation detection primer probe and a kit thereof. The kit comprises a digital PCR master mix used for preparing digital PCR reaction liquor, a liquid droplet stabilizer and primer probe mixed liquor. The primer probe mixed liquor comprises an upstream primer a, a downstream primer a, a deficiency probe a and a detection probe a, wherein the upstream primer a, the downstream primer a, the deficiency probe a and the detection probe a are used for detecting 19 exon E19-Dels locus; the primer probe mixed liquor further comprises a mutation upstream primer b, a mutation probe b, a wild upstream primer b, a wild probe b, and an universal downstream primer b, wherein the mutation upstream primer b, the mutation probe b, the wild upstream primer b, the wild probe b and the universal downstream primer b are used for detecting a 20 exon T790M locus; the primer probe mixed liquor further comprises a mutation upstream primer c, a mutation probe c, a wild upstream primer c, a wild probe c and an universal downstream primer c, wherein the mutation upstream primer c, the mutation probe c, the wild upstream primer c, the wild probe c and the universal downstream primer c are used for detecting 21 exon L858R locus. The EGFR gene mutation detection kit is capable of quickly, conveniently and accurately detecting situations of EGFR gene mutation in a patient with non-small cell lung cancer.
Owner:北京金匙基因科技有限公司
Kit for detecting EGFR gene mutation and application of kit
ActiveCN104946739ADoes not generate non-specific signalStrong specificityMicrobiological testing/measurementSerum igeFluorescence
The invention discloses a kit for detecting EGFR gene mutation and an application of the kit. The kit is characterized by containing 20 specific amplification primers for an EGFR gene mutation site, 5 efficient blocking probes for wild sequences, and 4 EGFR gene specific TaqMan fluorescent probes. The kit can be used for detecting samples of which the mutation copy number is as low as 5-10 copies and the mutation content is as low as 0.1%. The kit disclosed by the invention can be used for simultaneously detecting 5 types of gene mutations of an EGFR gene, is high in sensitivity, simple to operate, low-cost in detection and wide in clinical application range, the samples can be fresh pathological tissues, paraffin embedded tissues, pleural fluid, serum or plasma, the detection speed is high, and the detection process can be finished in only 90 minutes.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI +1
Quick detecting gene mutation correlative to curative effect of non small-cell carcinoma of the lung
ActiveCN101092644AUniform reaction conditionsMicrobiological testing/measurementSmall-cell carcinomaEGFR Gene Mutation
This invention discloses a test kit for detecting EGFR gene mutation associated with curative effects of non-small cell lung cancer. This invention also discloses a method for rapidly detecting EGFR gene mutation associated with curative effects of non-small cell lung cancer. The method is rapid and precise, and also has such advantages as easy operation, low requirement for apparatus, and high detection rate (higher than 90%).
Owner:庶安永康(厦门)健康产业有限公司
PCR micro droplet kit for examining human EGFR gene mutation
InactiveCN106319042AWith absolute quantificationStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationEGFR Gene MutationMutant
The application relates to the examining field of gene mutation and particularly relates to a PCR micro droplet kit for examining human EGFR gene mutation. The kit includes a nucleic acid amplification reagent; a reference substance and micro droplet PCR generated oil. The nucleic acid amplification reagent includes a primer and a probe for examining the No. 18 extron mutant, a primer and a probe for examining the No. 19 extron mutant, a primer and a probe for examining the No. 20 extron mutant, and a primer and a probe for examining the No. 21 extron mutant. The present application can be used for examining the seven species of the No. 18, 19, 20, and 21 extron mutants for the EGFR genes in the DNAs extracted from the paraffin-embedded pathology tissue slices and the peripheral blood dissociate DNAs. The present application provides a reference for the patients to choose the tumor molecularly targeted drug remedy and monitor. The present invention has absolute ration, high specificity, accuracy and high sensitivity.
Owner:上海睿昂基因科技股份有限公司
Kit, reaction system and method for detecting human EGFR gene mutation
InactiveCN106434941AGood choiceHigh sensitivityMicrobiological testing/measurementFluorescenceEGFR Gene Mutation
The invention discloses a kit, reaction system and method for detecting human EGFR gene mutation. The kit comprises an upstream primer, a downstream primer, a fluorescent probe for detecting an L858R mutation and a fluorescent probe for detecting wild types. The primers with specific sequences and the probes with the specific sequences are designed in the kit according to the L858R mutation of an EGFR gene. In an optimized reaction system, high-sensitivity detection of the L858R mutation of the EGFR gene is achieved through a digital PCR platform. Besides, negativity and positivity of the L858R mutation are detected, meanwhile the proportion of the mutation in a sample can be obtained, sources of samples capable of being detected in the kit are further diversified, the samples can be tumor tissue and ctDNAs, and the application range of the kit, the reaction system and the method is widened.
Owner:GENETRON HEALTH (BEIJING) CO LTD
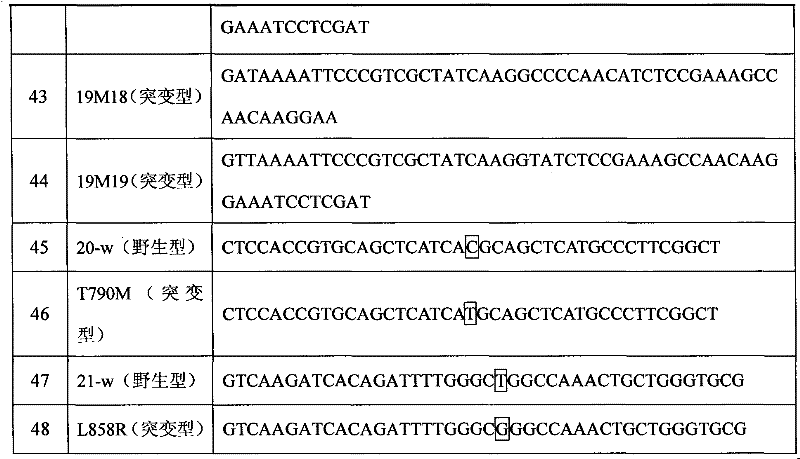
Primers and probe for detecting EGFR gene mutation, kit thereof, and application method of kit
ActiveCN102851375AAccurate typingStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationEGFR Gene MutationGenotyping
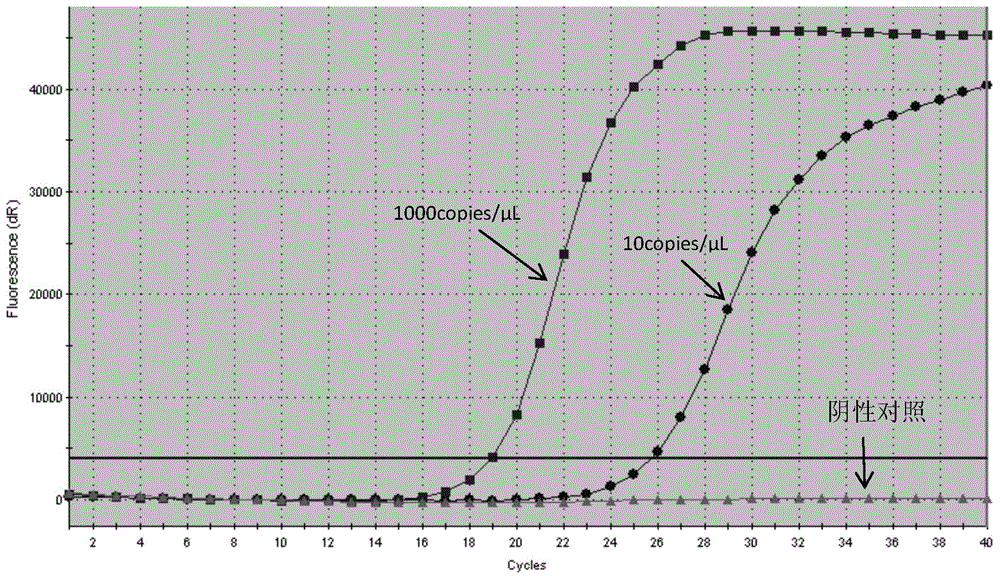
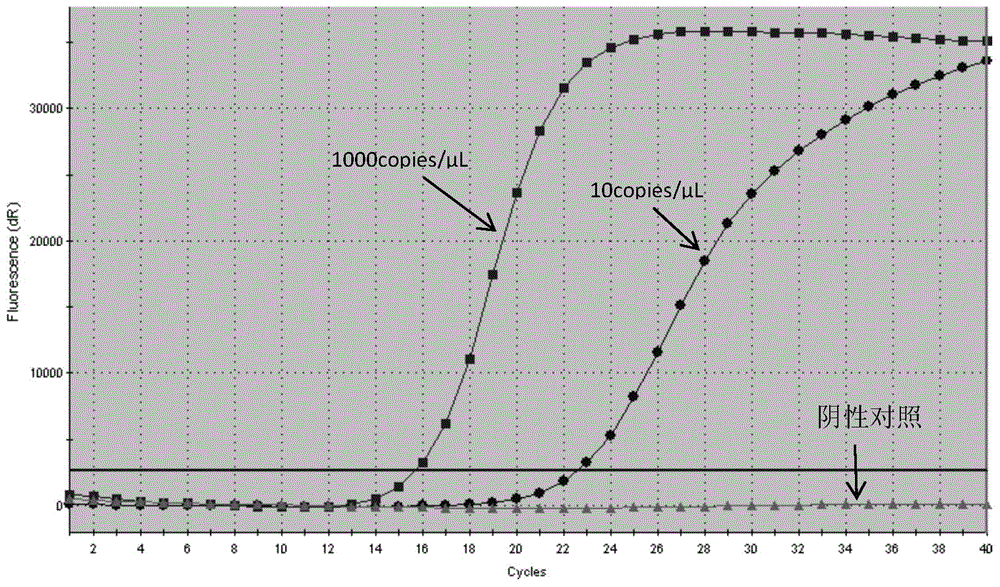
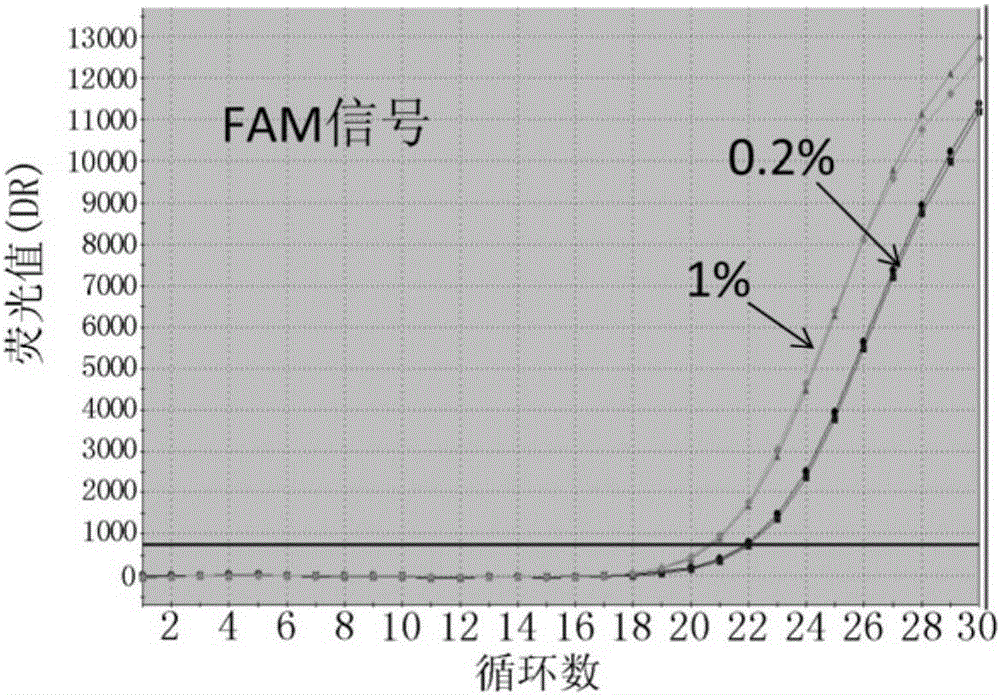
The invention relates to the field of gene mutation detection, and specifically relates to primers and a probe used for detecting EGFR gene mutation, a kit thereof, and an application method of the kit. With the kit provided by the invention, a sample containing 0.1-1% of EGFR gene mutation DNA can be detected; and accurate genotyping can be carried out upon a sample containing 10% of EGFR No. 19 exon gene mutation DNA through sequencing. The sensitivity of the kit provided by the invention is high, wherein a minimum detection limit is 5-copy gene. With the kit, qualitative detection can be carried out upon EGFR gene mutation. Different sensitivity reference ranges can be provided for assisting clinical determinations of mutation amount volumes, such that final treatment programs can be provided according to different mutations.
Owner:上海源奇生物医药科技有限公司
EGFR (epidermal growth factor receptor) gene mutation site detection kit
ActiveCN102808027AHigh sensitivityStrong specificityMicrobiological testing/measurementPharmacySpecific detection
The invention discloses an EGFR (epidermal growth factor receptor) gene mutation site detection kit which detects EGFR gene mutation sites by combining the ARMS (amplification refractory mutation system) specific mutant enrichment technology with the Taqman-MGB (minor groove binder) probe specific detection technology. The kit comprises ARMS primer pairs and corresponding Taqman-MGB probes, and the ARMS primer pairs and the corresponding Taqman-MGB probes are used for detecting the EGFR gene mutation sites. The EGFR gene mutation site detection kit is high in sensitivity, capable of detecting multiple samples simultaneously and low in cost, provides guidance for pharmacy of clinicians, realizes individualized treatment of patients suffering from tumors and is capable of reducing treatment risk and burden of the patients and wide in application prospect.
Owner:JIANGSU MICRODIAG BIOMEDICINE TECH CO LTD
Primer probe combination for detection of EGFR gene mutation and application of same
ActiveCN108611412AGrowth inhibitionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationMutation detectionEGFR Gene Mutation
The invention relates to a detection product for gene mutation and particularly relates to a primer probe combination for detection of EGFR gene mutation and an application of the same. The primer probe combination includes a detection primer probe, which includes an EGFR gene mutation detection specific primer pair, an EGFR gene specific probe, an amplification blocking probe, and an internal reference system, wherein the loci of mutation detection of the EGFR gene are the mutation loci of 18-21 exons. The primer probe combination has high specificity and sensitivity on the EGFR gene mutationand simple and quick operation. The detection result has great accuracy and repeatability. The primer probe combination can detect a tumor tissue sample and can help clinical treatment, so that the primer probe combination has important value.
Owner:WUXI SHENRUI BIO PHARMA
Kit for quantitatively detecting EGFR (Epidermal Growth Factor Receptor) gene mutation and application thereof
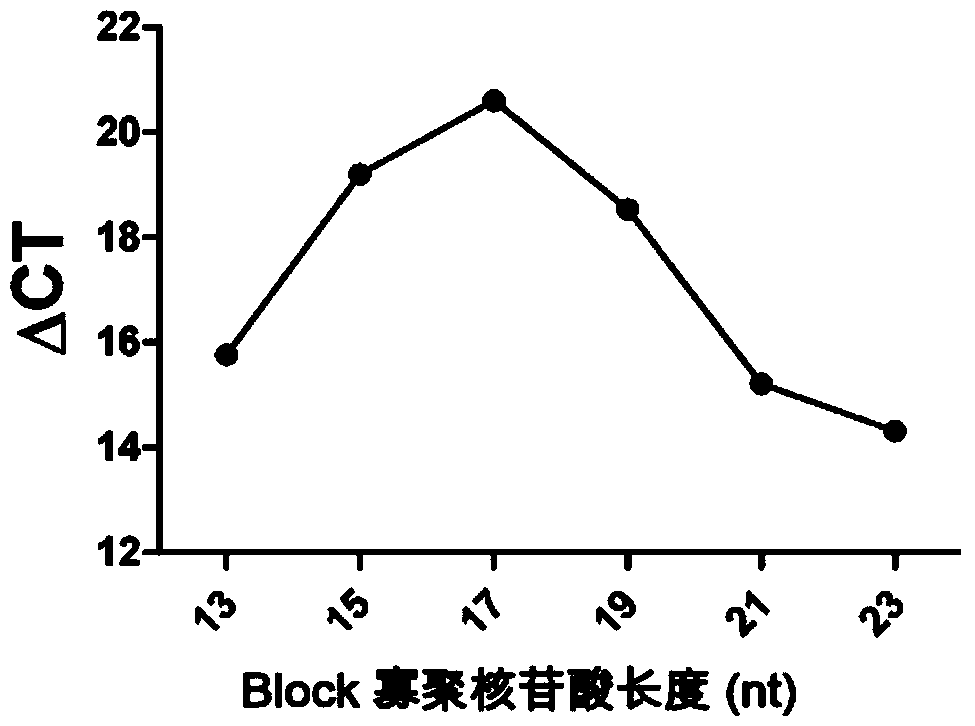
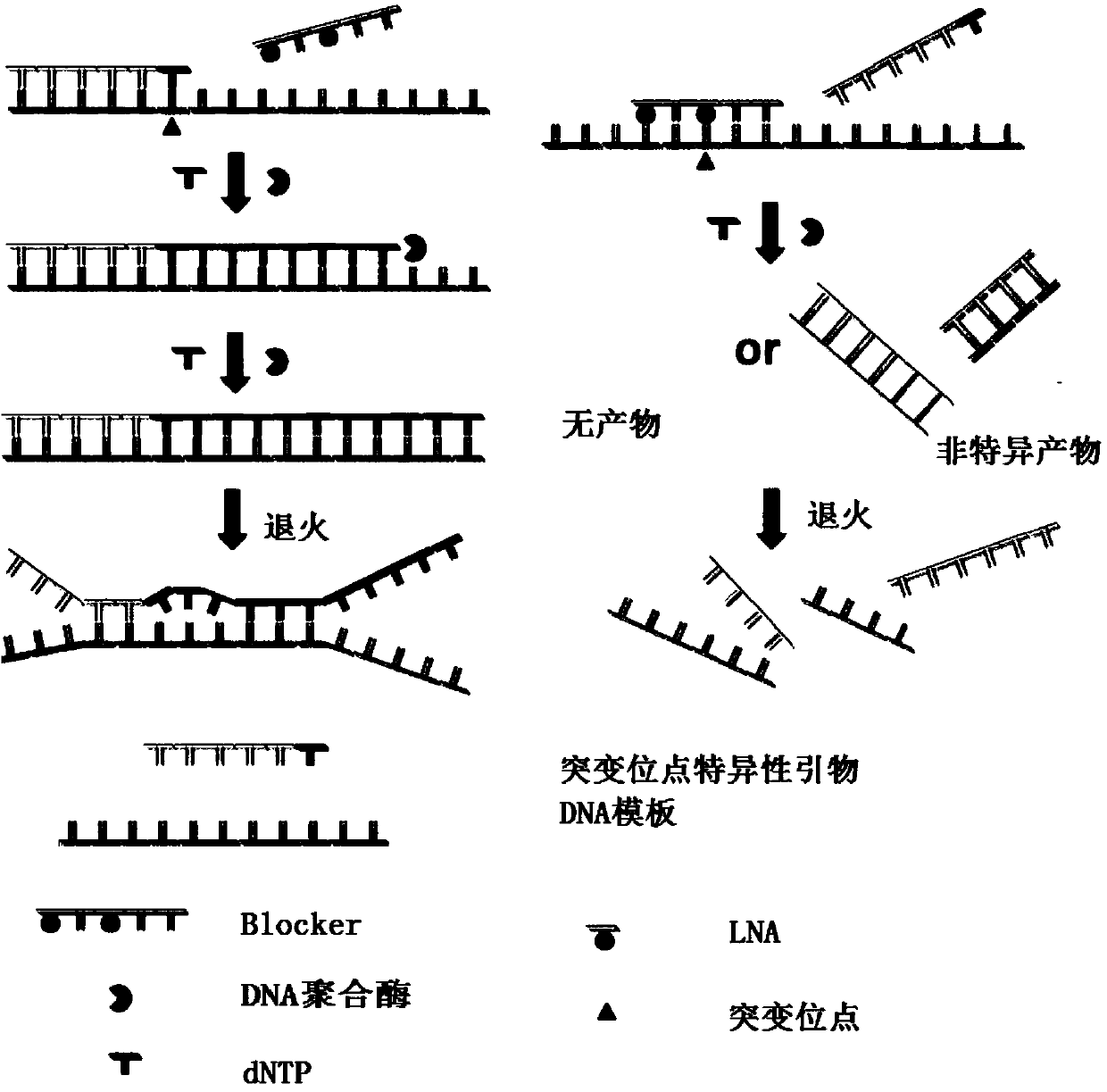
The invention relates to a high-sensitivity kit for quantitatively detecting EGFR (Epidermal Growth Factor Receptor) gene mutation and application thereof. The application comprises the following steps: respectively designing a forward primer and a backward primer in a sequence with the length of 50-100bp in a specific amplification gene mutation site area aiming at a to-be-detected gene template, designing the mutation sites at corresponding positions of the forward primer, and designing competitive Block oligonucleotides for inhibiting non-mutant amplification nearby the mutation sites. The detection is performed by utilizing the specificity aiming at a probe of the amplification product, and the gene mutation conditions are obtained by detecting the detectable markers on the probe. The invention provides a gene mutation analysis method which is stable in result, high in sensitivity and high in repeatability.
Owner:GENOSABER BIOTECH CO LTD +1
Kit for detecting EGFR gene mutation and detection method thereof
ActiveCN104762408AStrong specificityHigh sensitivityMicrobiological testing/measurementUse medicationWild type
The invention discloses a kit for detecting EGFR gene mutation and a detection method thereof. The kit comprises a specific probe modified with LNA locked nucleic acid. The specific probe aims at an EGFR gene mutation site. The specific probe can be combined with a wild type DNA and can detect a DNA sample contained with 0.01 percent of the EGFR gene mutation. The detecting method has the advantages that the specificity is strong, the sensitivity is high, pollution is small, the operation is simple and quick, security is high and the like. The detection method is especially suitable for detecting the gene mutation from body fluid contained with low-content mutation like blood plasma, urine and saliva and is suitable for conducting early screening and diagnosis on lung cancer, and guidance is provided for individualized medication.
Owner:JIANGSU MICRODIAG BIOMEDICINE TECH CO LTD
Probes, primers, detection system and kit for detecting mutations of EGFR gene
ActiveCN105349654AHigh sensitivityMeet the actual needs of rapid detectionMicrobiological testing/measurementDNA/RNA fragmentationEGFR Gene MutationBlood plasma
The invention discloses probes, primers, a detection system and a kit for detecting mutations of an EGFR gene, and the probes and the primers have the sequences of SEQ ID NO.1 to SEQ ID NO.24. The invention is characterized in that (1), the amplification efficiency is greatly improved to a largest extent; (2) the sensitivity is high, and the detection sensitivity can reach 0.2%; (3) compared with a digital PCR method, operations are simple, the cost is saved, and the clinical application scope is wide; (4) plasma samples with large reaction volume are detected, the DNA sampling quantity of the plasma samples becomes larger, the system is more stable, and the detection rate of the plasma samples is increased; (5) 25 mutations of the EGFR gene can be detected simultaneously in three reaction tubes, and results are intuitive and clear; (6) the detection speed is fast, and consumed time is only 1 / 2 that of the digital PCR; and (7) a detection method can detect peripheral blood samples, has convenient sampling, and can dynamically monitor curative effect of EGFR-TKI drugs on patients.
Owner:AMOY DIAGNOSTICS CO LTD
Kit and method for detecting mutation of EGFR gene
ActiveCN108949990AEfficient amplificationHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationEGFR Gene MutationAbsolute quantification
The invention provides a kit and method for detecting the mutation of an EGFR gene and particularly discloses a method, a primer and a probe for detecting the mutation of the EGFR gene, a kit including prime probe mixing liquid, a primer and a probe for detecting the mutation of a C797S locus, a primer and a probe for detecting the mutation of a T790M locus, a primer and a probe for detecting themutation of a 19del locus and a primer and a probe for detecting the mutation of a L858 locus. According to the method provided by the invention, 0.1% mutation rate can be detected based on a digitalPCR platform; and the method has the advantages that the optimization process is simple, much detected mutation types can be detected, the absolute quantification is realized, the sensitivity is high,samples are easily acquired, and the like.
Owner:DAAN GENE CO LTD
Primers and probes for detecting human EGFR gene mutations as well as use method thereof
ActiveCN101608240BHigh sensitivityStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceGene targetsEGFR Gene Mutation
The invention discloses primers and probes for detecting human EGFR gene mutations as well as a use method, relating to the detection of gene mutation. The method of the invention comprises the following steps: (1) providing the primers and the probes; (2) processing the sample to be detected and extracting a template; (3) preparing a reaction system of fluorescent PCR amplification mutation genesequence; (4) amplifying the mutable gene target sequence by the primers and the probes in the step (1); (5) detecting the fluorescence intensity of FAM and HEX or ROX of the reaction system as the judgment standard of results by adopting hybridization of a dual ring probes and an amplification product. The primers, the probes and the method of the invention can simultaneously detect 29 deletion mutations of EGFR gene, and features high sensitivity, strong specificity, strong selective capacity and fast detection speed demonstrated by completing the whole detection process in only 90 minutes.
Owner:AMOY DIAGNOSTICS CO LTD
Primer and probe assembly and kit for detecting EGFR mutation sites as well as application of primer and probe assembly or kit
InactiveCN108642154AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceMutation detection
The invention provides a primer and probe assembly for detecting specific mutation on 18, 19, 20 and 21 exons of EGFR genes, a kit which comprises the primer and probe assembly and is used for detecting EGFR mutation sites through multiple droplet digital PCR as well as an application and a use method of the primer and probe assembly or the kit. The primer and probe assembly or the kit can performhigh-throughput detection on 29 mutation sites of the EGFR genes, has high specificity, high sensitivity and good stability, has great significance in mutation detection of EGFR genes of NSCLC (non-small cell lung cancer) and can dynamically guide medication for patients.
Owner:PILOT GENE TECH HANGZHOU CO LTD
Primer and method for detecting human EGFR gene mutation
ActiveCN104131091AMicrobiological testing/measurementDNA/RNA fragmentationGrowth Factor GeneEGFR Gene Mutation
The invention belongs to the field of biotechnology, especially the detection of gene mutation. More specifically, the invention discloses 9 groups of primers for detection of 41 mutation of a human epidermal growth factor EGFR gene and a detection method by using the primers.
Owner:艾吉泰康(嘉兴)生物科技有限公司
Method and chip for detecting tumor cell EGFR (epidermal growth factor receptor) gene mutation
InactiveCN102732636AImprove accuracyAccurate readingNucleotide librariesMicrobiological testing/measurementBiotin-streptavidin complexErlotinib
The invention relates to the technical field of biology, and discloses a method for detecting tumor cell EGFR (epidermal growth factor receptor) gene mutation. The method comprises the following steps: taking sequences shown in SEQ ID NO:1-8 as primers and DNA (deoxyribonucleic acid) of a sample to be detected as a template to carry out PCR (polymerase chain reaction) amplification; and respectively hybridizing probes having sequences shown in SEQ ID NO:9-24 with the obtained amplification products, then washing to remove the uncombined amplification products, finally incubating hybrid products with streptavidin coupled with gold nanoparticles, and judging the mutation result according to color variations of the gold nanoparticles. Based on the chip technology, by utilizing the characteristic of specific binding of biotins and streptavidin, the streptavidin coupled with gold nanoparticles is specifically bound with the biotins labelled on the amplification products after the probes aiming at EGFR gene mutation related to sensitivity or drug resistance to gefinitib, erlotinib and the like compose a chip array and are hybridized with the biotin labelled amplification products, thus accurately interpreting the mutation result.
Owner:HAINAN MEDICAL COLLEGE
Liquid phase chip for detecting EGFR (epidermal growth factor receptor) gene mutation
ActiveCN102234683AImprove signal-to-noise ratioNo cross-reactivityMicrobiological testing/measurementMicrosphereEGFR Gene Mutation
The invention discloses a liquid phase chip for detecting EGFR (epidermal growth factor receptor) gene mutation. The liquid phase chip mainly comprises a wild-type and mutant-type allele specific primer extension (ASPE) primer pair designed for mutational sites of an EGFR gene respectively, microspheres which are respectively coated with specific anti-tag sequences and have different colors of codes and amplification primers for respectively amplifying the target sequences with the corresponding mutational sites of the Exon 19, Exon 20 and / or Exon 21, wherein each ASPE primer comprises the tag sequences at the 5' terminal and the specific primers aiming at the mutational sites of the target gene at the 3' terminal; and the anti-tag sequences can correspondingly complement and form pair with the tag sequences selected in (A). The liquid phase chip has the following advantages: the coincidence rate of the detection method provided by the invention and a sequencing method is as high as 100%; the prepared liquid phase chip has very good signal to noise ratio; and cross reactions do not exist between the designed probes and the anti-tag sequences.
Owner:SUREXAM BIO TECH
Kit for quantitatively detecting EGFR mutation
InactiveCN102021232AAccurate determination of contentEasy to operateMicrobiological testing/measurementVector-based foreign material introductionEGFR Tyrosine Kinase InhibitorsEGFR Gene Mutation
Owner:BEIJING ACCB BIOTECH
Method of detecting EGFR (epidermal growth factor receptor) gene mutation in urine of colorectal cancer patient by using digital PCR (polymerase chain reaction)
InactiveCN106755450ASolve the cumbersomeTime consuming to solveMicrobiological testing/measurementDifferential displayNon traumatic
The invention discloses a method of detecting EGFR (epidermal growth factor receptor) gene mutation in urine of a colorectal cancer patient by using digital PCR (polymerase chain reaction). The method comprises the particular following steps: extracting a DNA (deoxyribonucleic acid) template from the urine, preparing ddPCR (differential display polymerase chain reaction) liquid, preparing a droplet, performing PCR amplification, detecting the droplet, analyzing data, and displaying a result, wherein a lysate comprises 10mM of Tris-HCl, 1mM of EDTA (ethylene diamine tetraacetic acid) and 0.5% of SDS (sodium dodecyl sulfonate); a thermal circulation mode of detection comprises the steps of incubating at 95 DEG C for 10min, at 95 DEG C for 15s, at 63 DEG C for 1min (45 cycles in total), and finally putting at 4 DEG C. According to the method, ARMS is combined with the digital PCR; the method solves the problems of complication, time consuming, low sensitivity and the like during EGFR gene mutation detection of the colorectal cancer patient in the prior art by detecting the EGFR mutation in the urine of the patient; the non-traumatic detection in addition to pathologic histology is provided particularly.
Owner:安徽安龙基因科技有限公司
Method for detecting EGFR (epidermal growth factor receptor) mutant gene based on multiple-fluorescence PCR (polymerase chain reaction)
PendingCN109457018AEasy to judgeImprove accuracyMicrobiological testing/measurementFluorescenceWild type
The invention discloses a method for detecting EGFR (epidermal growth factor receptor) mutant gene based on multiple fluorescence, relates to the field of detection of the EGFR mutant gene, and provides a method for detecting the EGFR mutant gene based on the multiple-fluorescence PCR (polymerase chain reaction). The method comprises the following steps of preparing multiple specific ARMS (amplification refractory mutation system)-amplification primers for different EGFR mutant genes and blockers for inhibiting amplification of wild genes, amplifying a fragment containing the mutant gene in ato-be-detected sample, and judging whether the different samples have the corresponding EGFR gene mutation or not according to the fluorescence value. The invention also provides a kit for detecting the EGFR mutant gene through the method. The method has the advantages that the sensitivity is high, the accuracy is high, and the specificity is strong; the multiple EGFR mutant genes can be simultaneously detected.
Owner:SHANGHAI MAG GENE NANOTECH CO LTD
Sequence composition and kit for detecting mutation of human EGFR gene
ActiveCN108949927AStrong specificityNo non-specific amplification signal occursMicrobiological testing/measurementDNA/RNA fragmentationEGFR Gene MutationFhit gene
The invention discloses a group of primer sequences and blocker sequences for detecting the mutation of a human EGFR gene and relates to detection of the mutation of the human EGFR gene. The inventionfurther discloses a kit including the primer sequences and blocker sequences and a use method of the kit. The primer sequences and blocker sequences provided by the invention can be used for detecting multiple different types of mutant genes in a single reaction and are high in sensitivity, strong in specificity and high in single detection flux.
Owner:SHANGHAI MAG GENE NANOTECH CO LTD
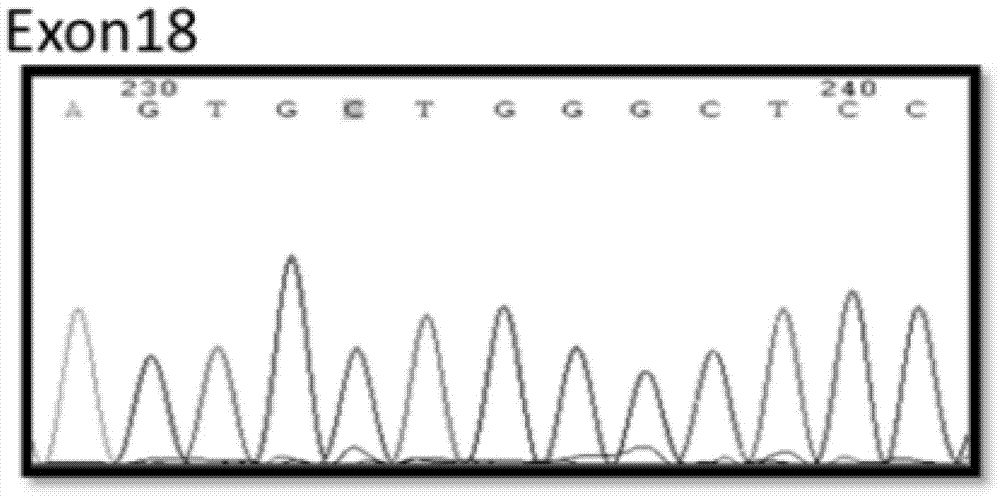
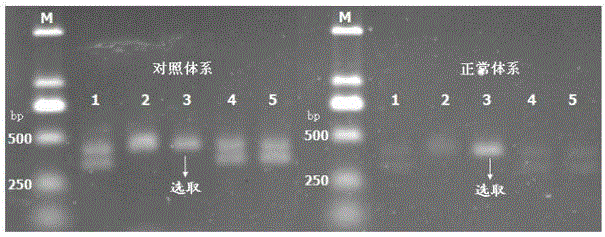
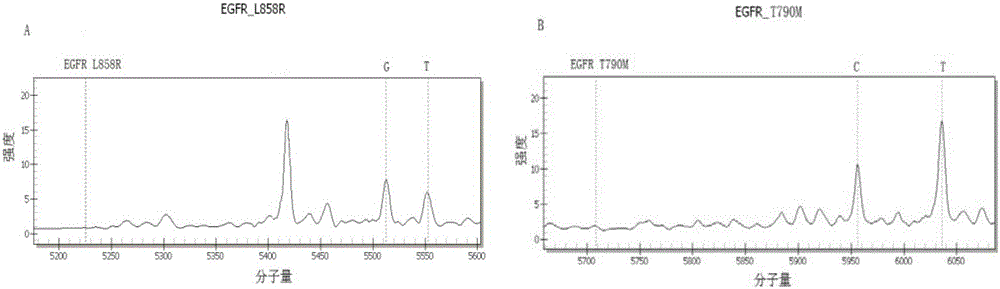
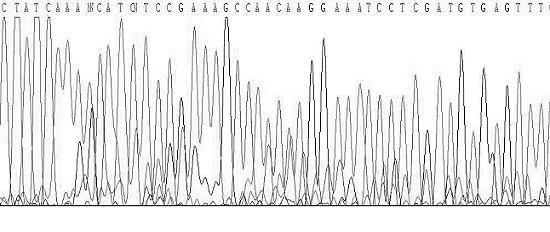
Method and kit for sensitively detecting human EGFR (epidermal growth factor receptor) gene mutation on basis of Sanger sequencing
ActiveCN105256021AGrowth inhibitionImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationMedicineEGFR Gene Mutation
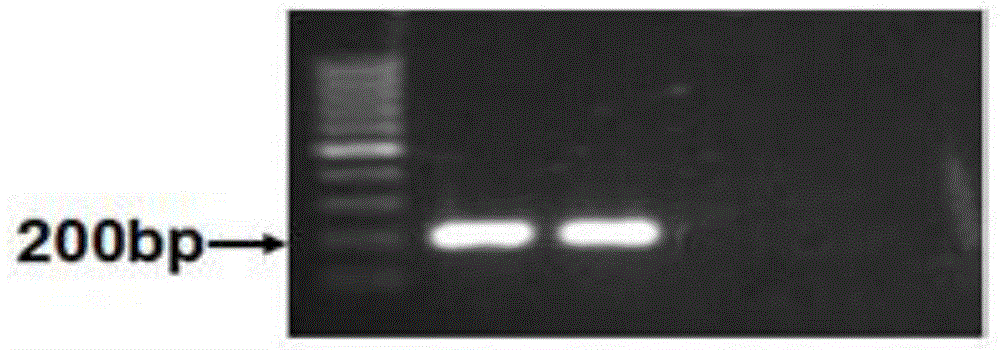
The invention belongs to the field of gene mutation detection and particularly relates to a method and a kit for sensitively detecting human EGFR (epidermal growth factor receptor) gene mutation on the basis of Sanger sequencing. In order to overcome the defects of a conventional human EGFR gene mutation detection method and kit, the method and the kit for flexibly detecting the human EGFR gene mutation on the basis of the Sanger sequencing are provided; heatproof DNA ligase and a specific connecting primer system aiming at a mutation site are introduced into an EGFR gene segment amplification system, so that amplification of wild type EGFR gene segments in a sample is greatly inhibited, and a wild type sample can be directly determined on the basis of amplified segment bands; or the proportion of mutation segments in a final PCR (polymerase chain reaction) product of a sample with low mutation content is enabled to be higher than 50%, accordingly, the accurate identification of the Sanger sequencing on a heterozygous gene segment system is guaranteed; the method and the kit can detect the EGFR gene mutation with the content as low as 1% in the sample on the basis of the Sanger sequencing, the accuracy rate is very high, the detection cost is low, and new unknown mutation can be possibly detected.
Owner:FUJIAN MEDICAL UNIV
Human multi-gene mutation detection kit based on high-throughput sequencing method
InactiveCN107164513AHigh sensitivityStrong specificityMicrobiological testing/measurementLibrary creationMutation detectionEGFR Gene Mutation
The invention belongs to the technical field of biology and particularly relates to a human multi-gene mutation detection kit based on a high-throughput sequencing method and an application thereof. The kit includes a KRAS gene mutation detection primer pair, an EGFR gene mutation detection primer pair and an ALK gene fusion detection primer pair. The kit can simultaneously detect DNA mutation and RNA fusion, is high in sensitivity and low in detection limit, is high in specificity, has good repeatability, and can reach 100% in both accuracy and positive coincident rate.
Owner:SINGLERA GENOMICS (SHANGHAI) LTD
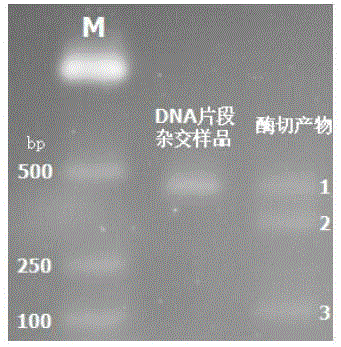
Method for sensitively detecting human EGFR gene mutation based on enzyme digestion and reagent kit
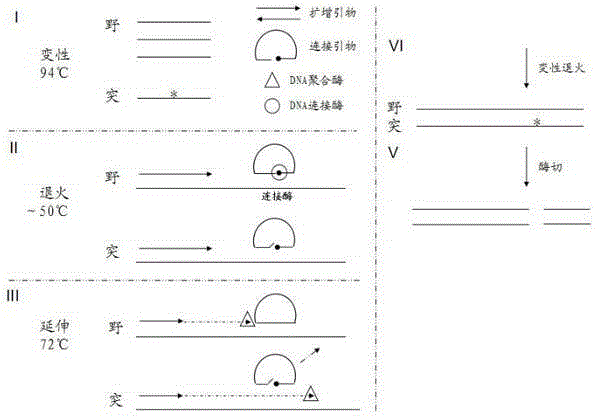
ActiveCN105420361AGrowth inhibitionEfficient detectionMicrobiological testing/measurementEnzyme digestionMutation detection
In order to overcome defects of existing methods and reagent kits, the invention provides a method for sensitively detecting human EGFR gene mutation based on enzyme digestion and a reagent kit thereof, and belongs to the field of gene mutation detection. The method comprises the steps that heat-resisting DNA ligase and a specific connection primer set for a mutation site are introduced into an EGFR gene segment amplification system, a wild type sample can be directly determined on the basis of an amplified segment strip, or the proportion of a final PCR product of a low-mutation-content sample is made to be higher than 50%; then, a DNA segment hybridization sample containing a mismatched structure at the mutation site is prepared through denaturation and annealing, and therefore a heterozygosis gene segment sample can be effectively detected by means of a mismatched digestion enzyme. The method and the reagent kit can detect EGFR gene mutation with the content as small as 1% in the sample through enzyme digestion, and are high in accuracy rate, short in detection time, low in cost and suitable for clinical detection.
Owner:FUJIAN MEDICAL UNIV
Kit for detecting lung cancer EGFR gene mutation targeted therapy related drug-resistant variation sites
InactiveCN106544427AReduce clinical testing costsOptimizing clinical resource allocationMicrobiological testing/measurementAnalysis dataKRAS
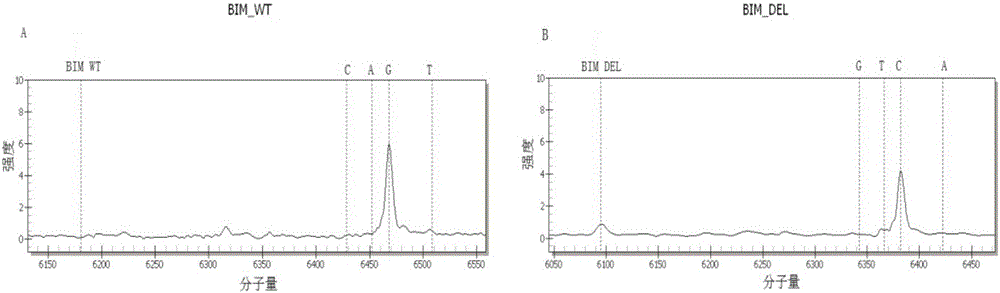
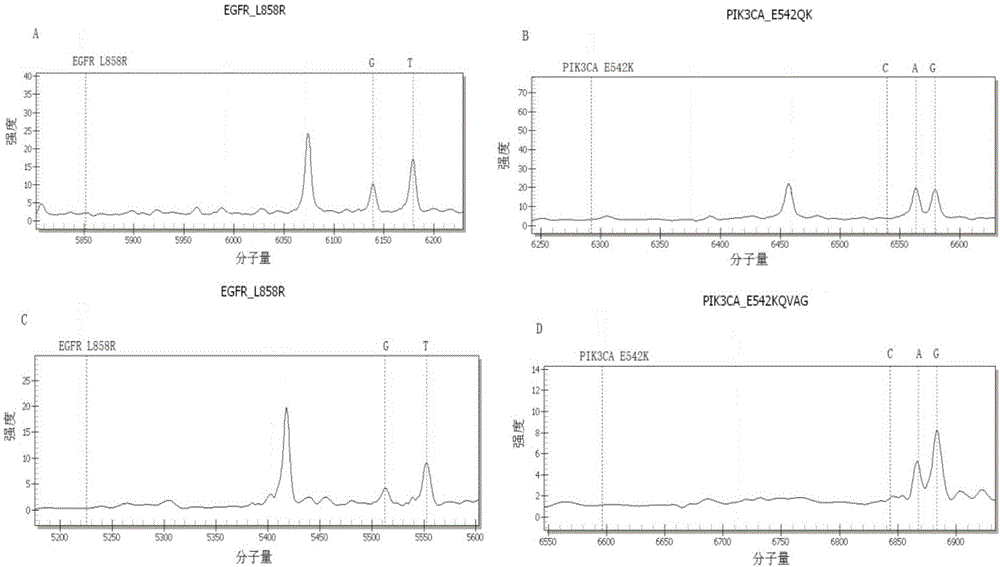
The invention discloses a kit for detecting lung cancer EGFR gene mutation targeted therapy related drug-resistant variation sites. The kit comprises amplimers represented by SEQ ID NO.1 to SEQ ID NO.122 and extension primers represented by SEQ ID NO.123 to SEQ ID NO.183. The kit can detect 60 nucleic acid sites of the following seven relevant genes: EGFR, KRAS, PIK3CA, BRAF, HER2, BIM and NRAS, provides gene analysis data for successful or failed EGFR targeted therapy, and provides basis for subsequent therapy and researches.
Owner:GUANGDONG GENERAL HOSPITAL
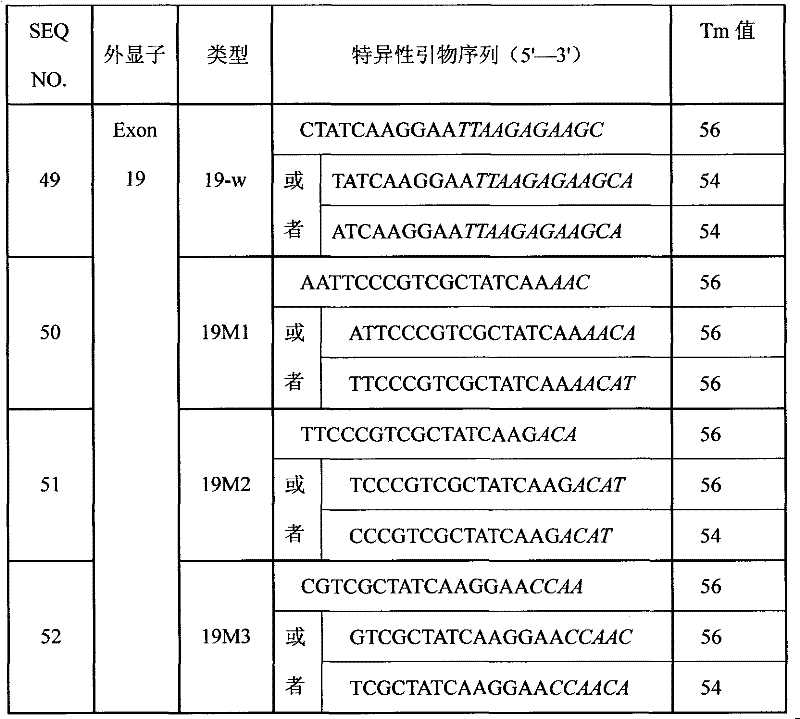
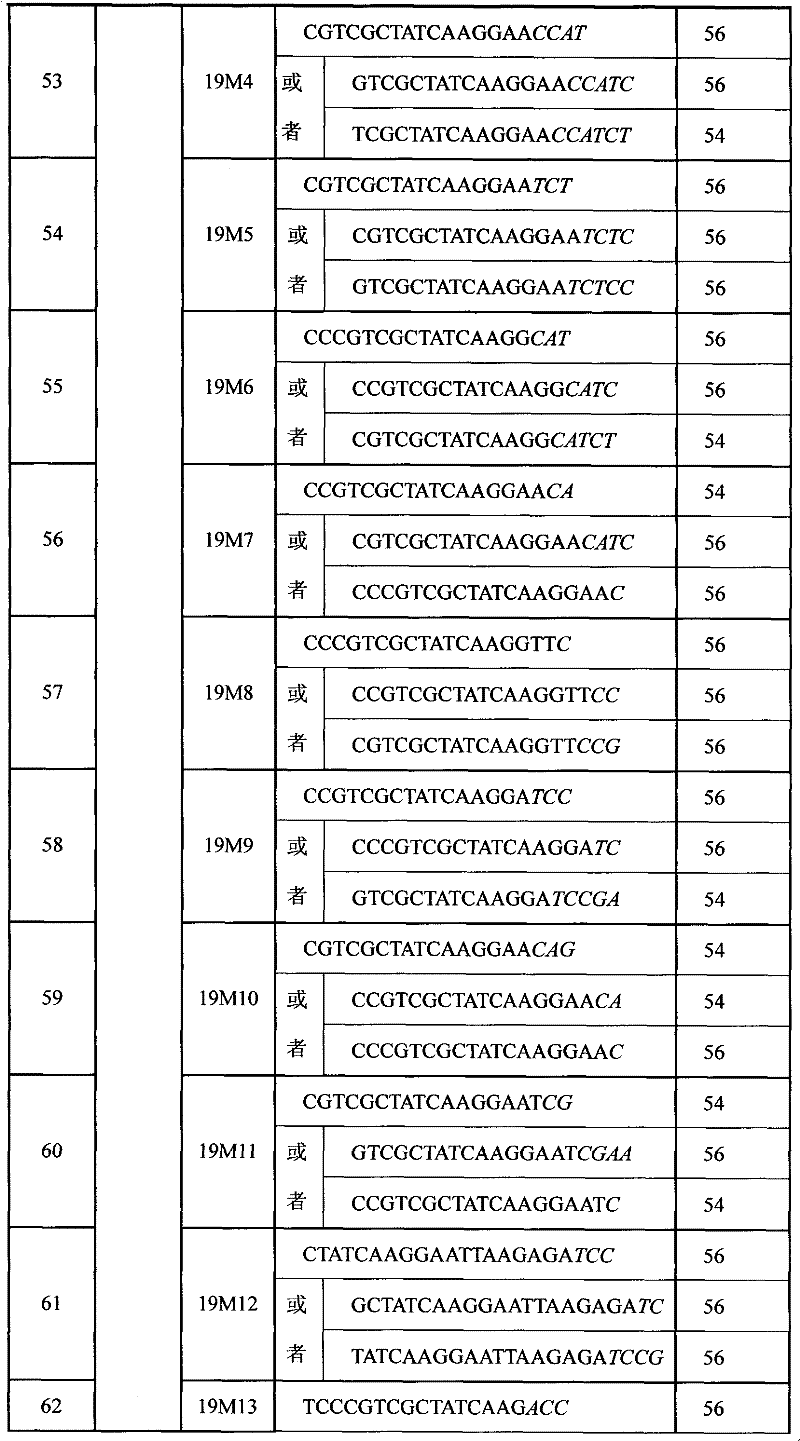
Primer composition, kit and method for detecting mutation of exon 19 of human EGFR gene
InactiveCN102181536AHigh sensitivityHigh speedMicrobiological testing/measurementDNA/RNA fragmentationNucleotideEGFR Gene Mutation
Owner:宁波基内生物技术有限公司
Kit for detecting EGFR gene hot spot mutation and detection method
ActiveCN103484551AHigh sensitivityImprove the detection rateMicrobiological testing/measurementEGFR Gene MutationMicrobiology
Belonging to the field of biotechnology, the invention discloses a kit for detecting EGFR gene hot spot mutation and a detection method. The kit provided by the invention comprises a PNA reagent, and clamp technology is utilized for enclosing a wild type EGFR gene sequence, thereby raising sensitivity of a sanger sequencing method and detection rate of EGFR gene mutation. The detection method provided by the invention is simple and safe, does not require many expensive reagents, and is economical and efficient; meanwhile, the detection method has the advantages of short operation time and can be completed within 4-6 h.
Owner:武汉思泰得医学检验实验室有限公司
Kit for detecting gene mutation of EGFR and application of kit
InactiveCN104531893AMicrobiological testing/measurementDNA/RNA fragmentationSolubilityEGFR Gene Mutation
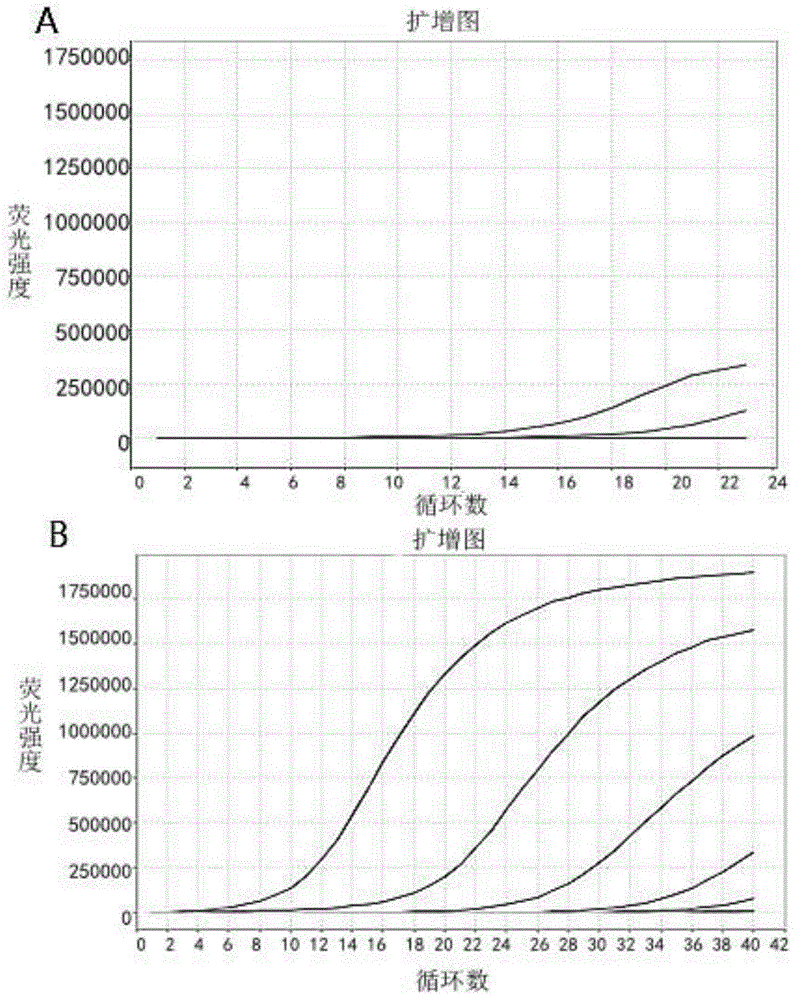
The invention provides a kit for detecting the gene mutation of EGFR and application of the kit. Specific primers are designed according to different gene mutations of the EGFR and are modified relatively, and whether related mutations of the EGFR exists is judged through an amplification curve and a solubility curve by adopting sample DNA as a template combined with real-time quantitative PCR (polymerase chain reaction). The kit provided by the invention and a method for detecting a mutant site have high sensitivity and specificity, can be used for detecting trace mutation and have good clinical application value.
Owner:舒雄 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com