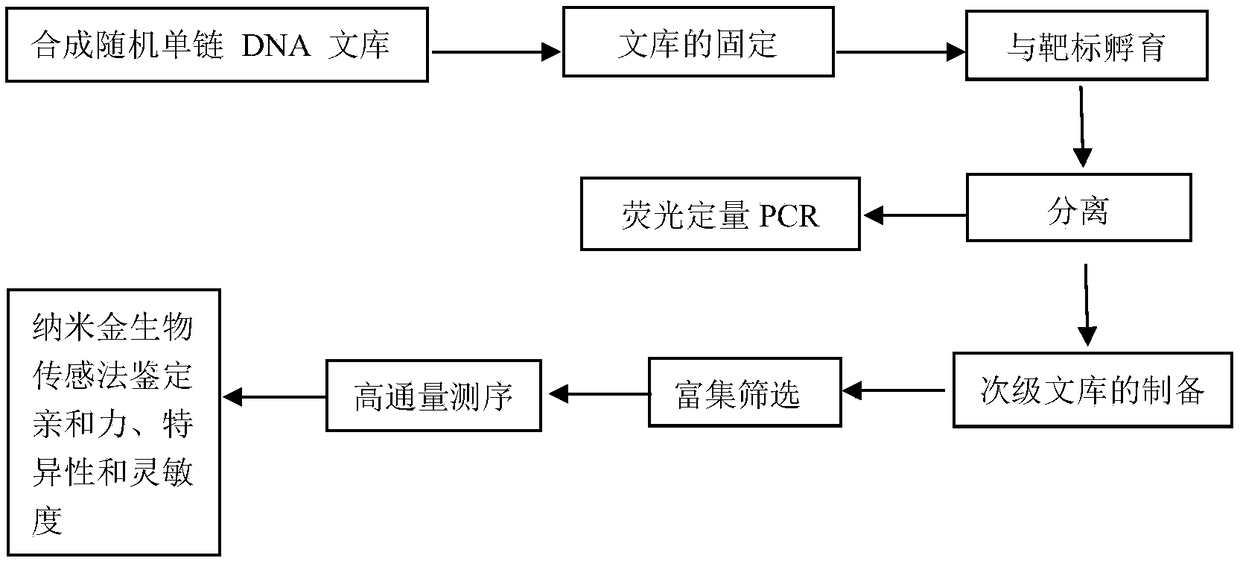
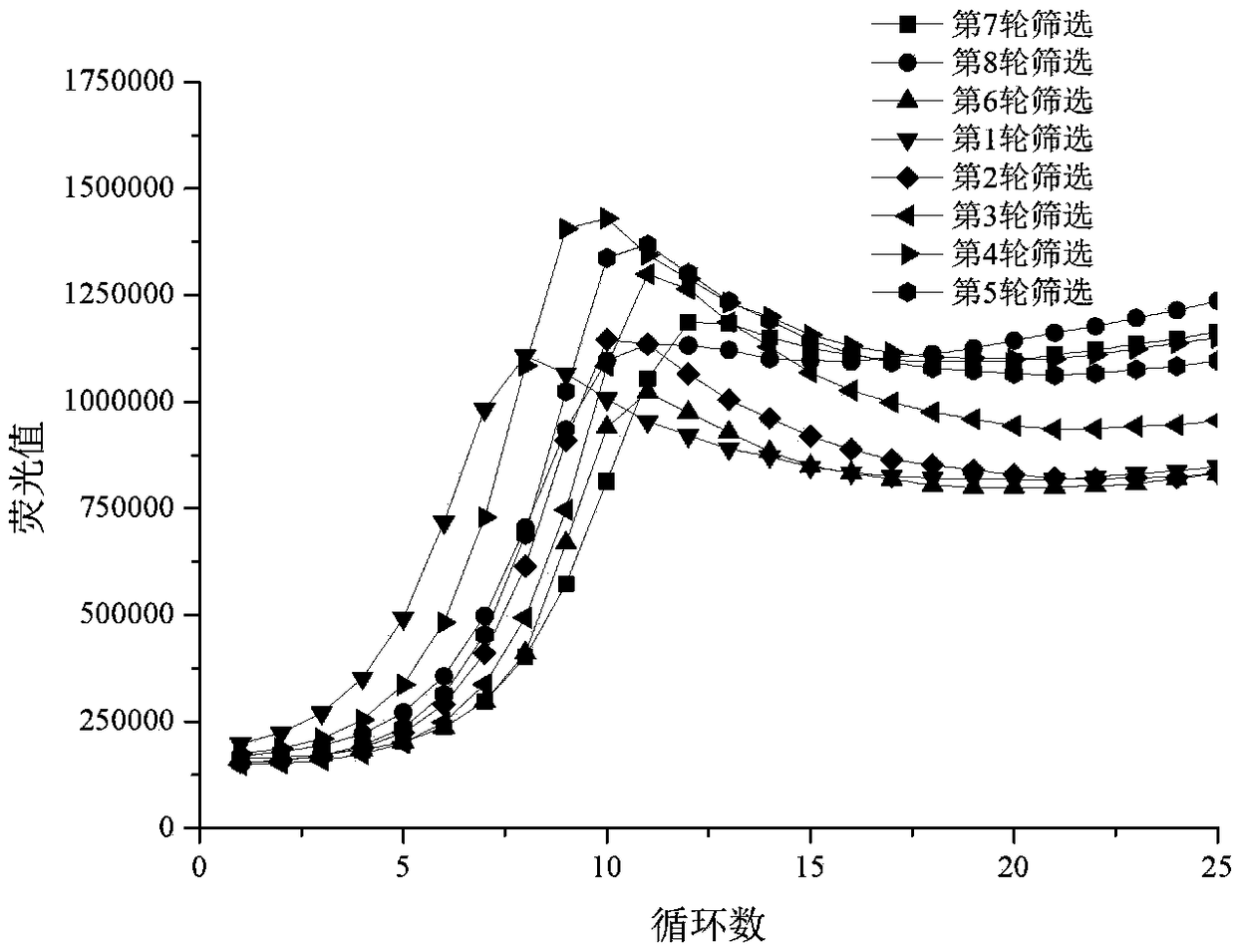
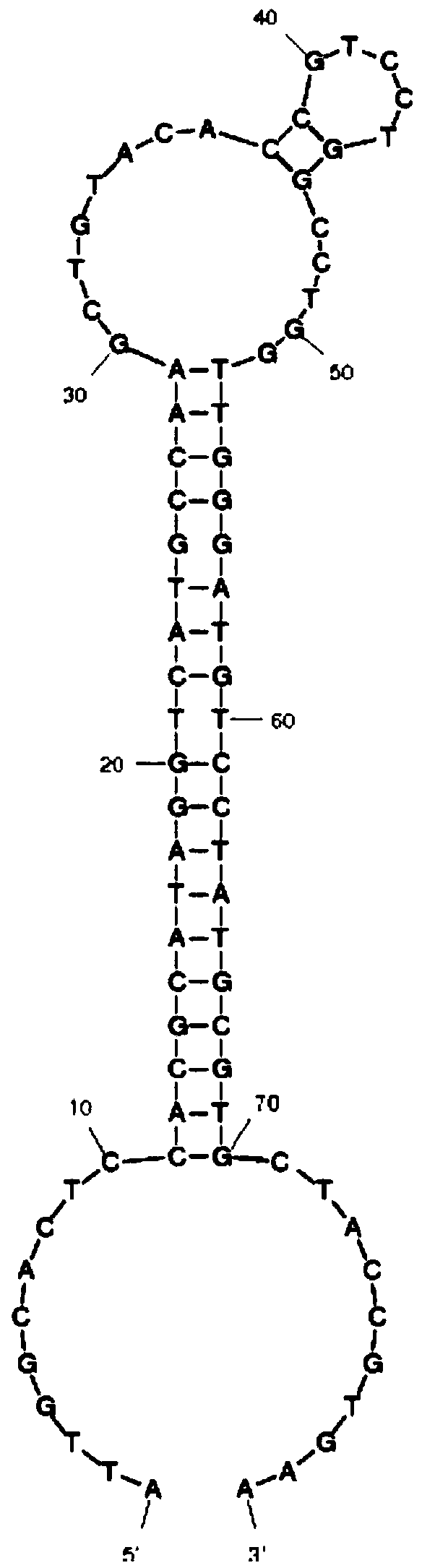
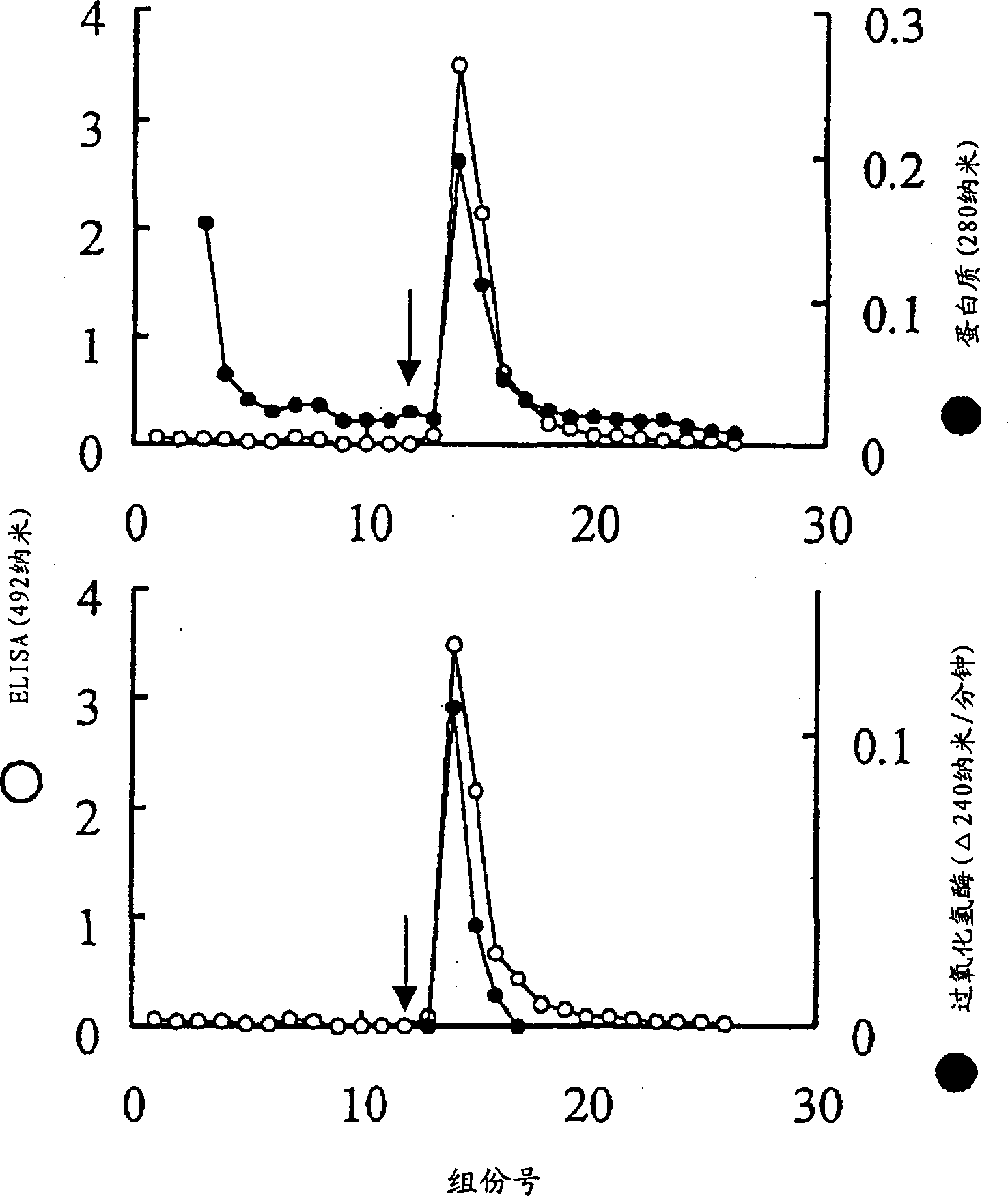
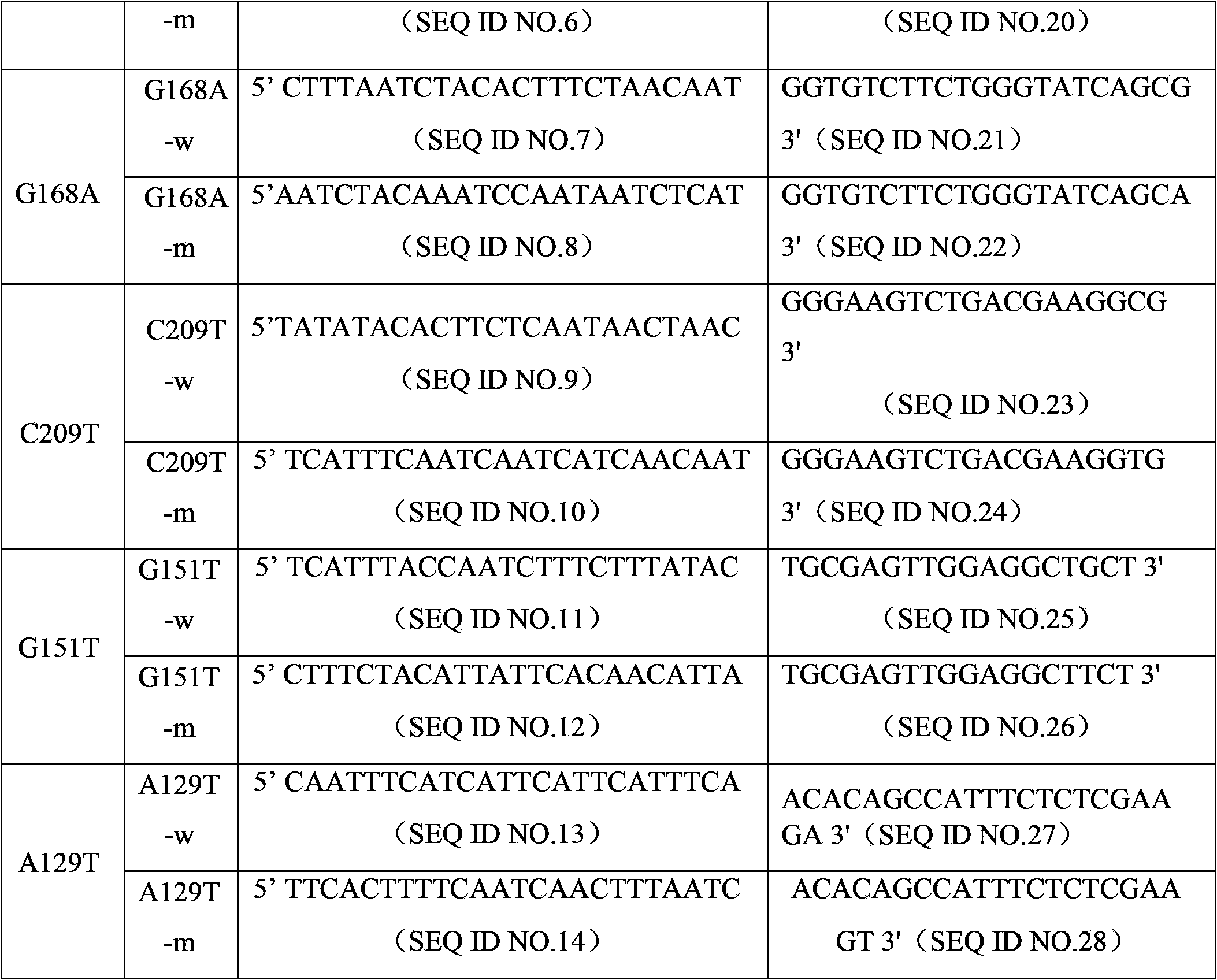
Patents
Literature
172results about How to "No cross-reactivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
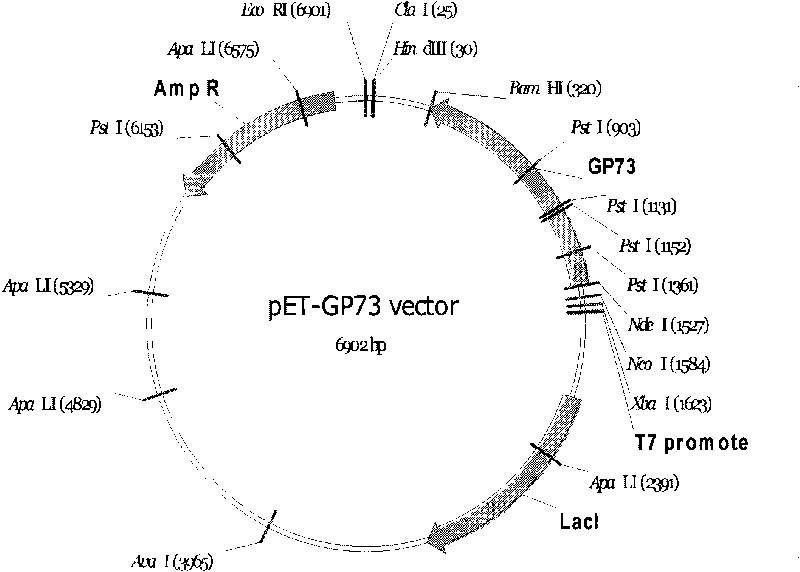
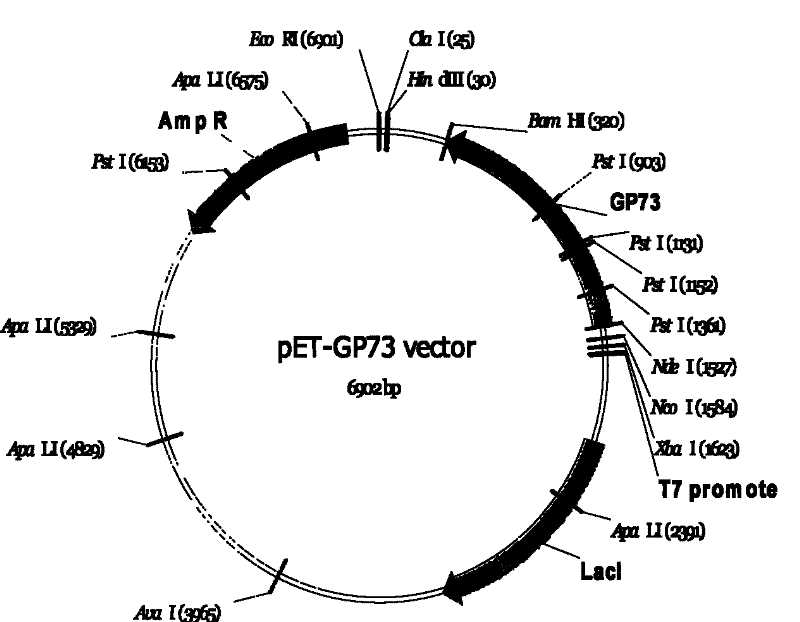
Monoclonal antibody against GP73 protein, preparation method and application thereof
ActiveCN101735319ANo cross reactivityStrong specificityChemiluminescene/bioluminescenceImmunoglobulins against animals/humansMonoclonal antibodyChronic hepatitis
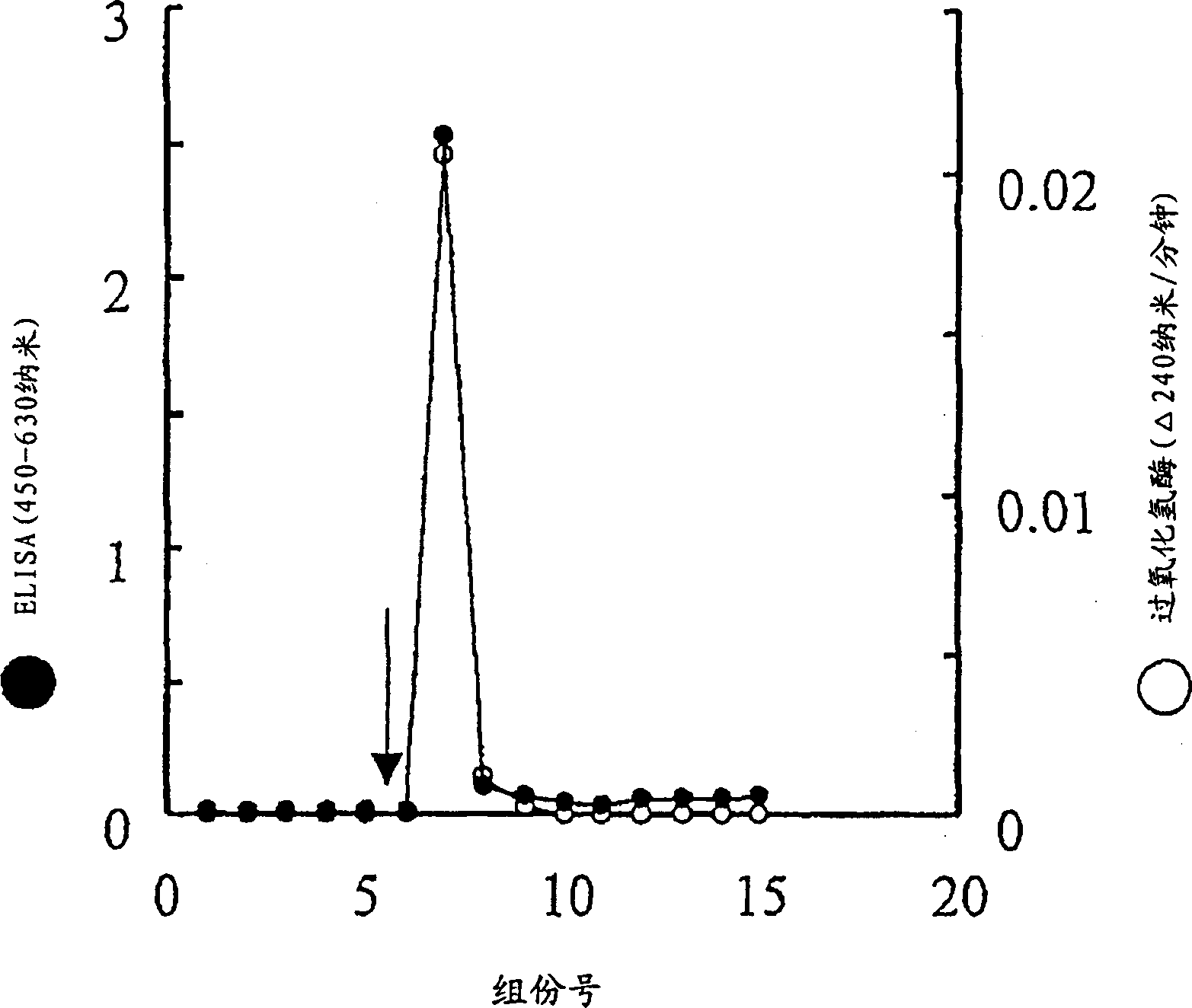
The invention provides a monoclonal antibody against GP73 protein, a preparation method and an application thereof. The invention also provides an immunological quantitative detection kit for detecting GP73 in a PBLs sample and a preparation method thereof. The kit in the invention can be used for monitoring the development level of chronic hepatitis and prognosis, and can be used for early diagnosis of liver cancer. By using the kit in the invention to monitor the expression level of the GP73, the prognosis level and probability to cause cirrhosis and liver cancer can be determined for patients, and the most direct support is provided for preventing, diagnosing and curing liver cancer and hepatitis.
Owner:BEIJING HOTGEN BIOTECH CO LTD
Papillomavirus detection and parting method as well as liquid phase chip thereof
InactiveCN101250593AImprove detection efficiencyOvercoming missed detection of latent infectionMicrobiological testing/measurementFluorescenceSignal-to-noise ratio (imaging)
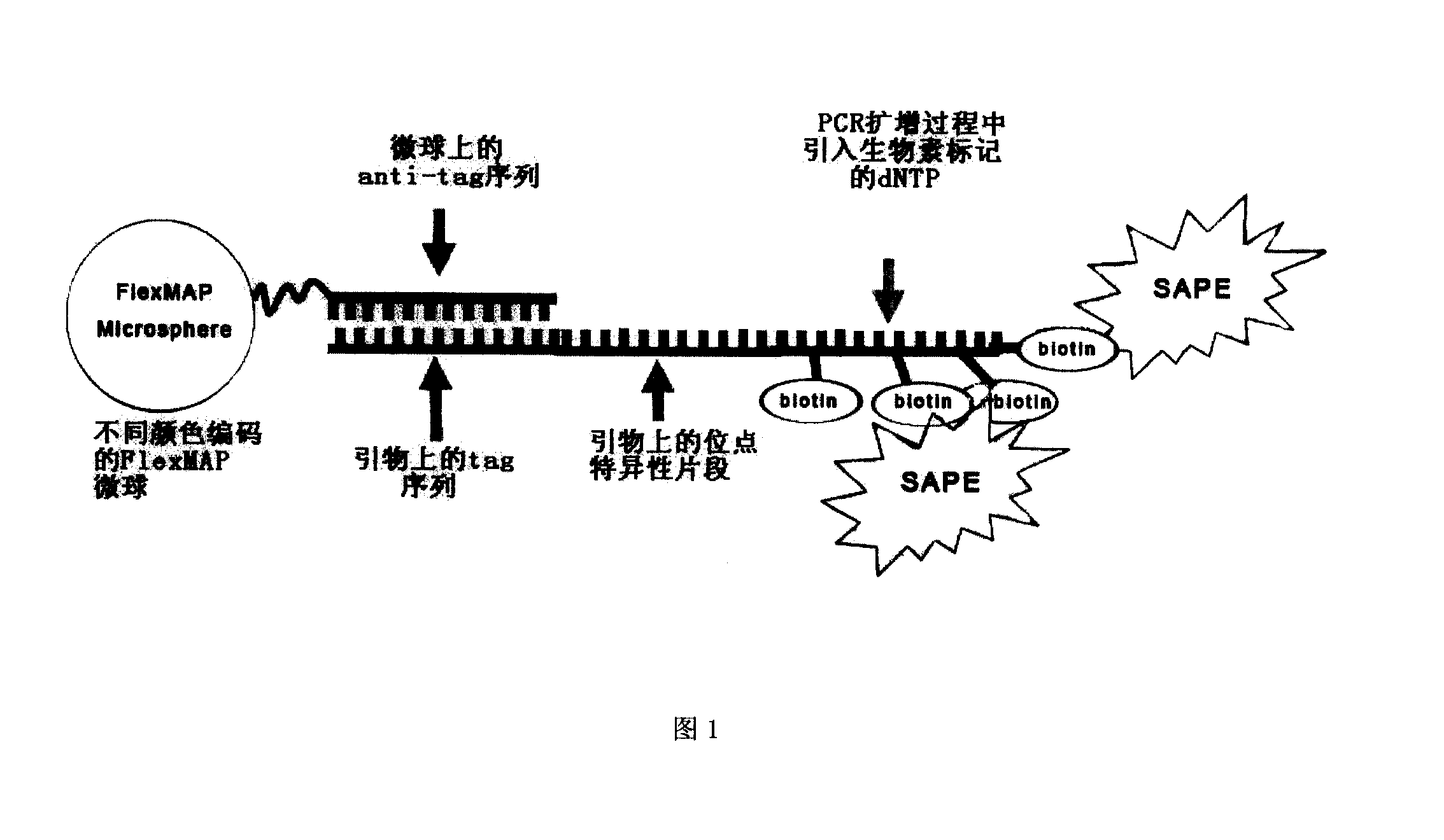
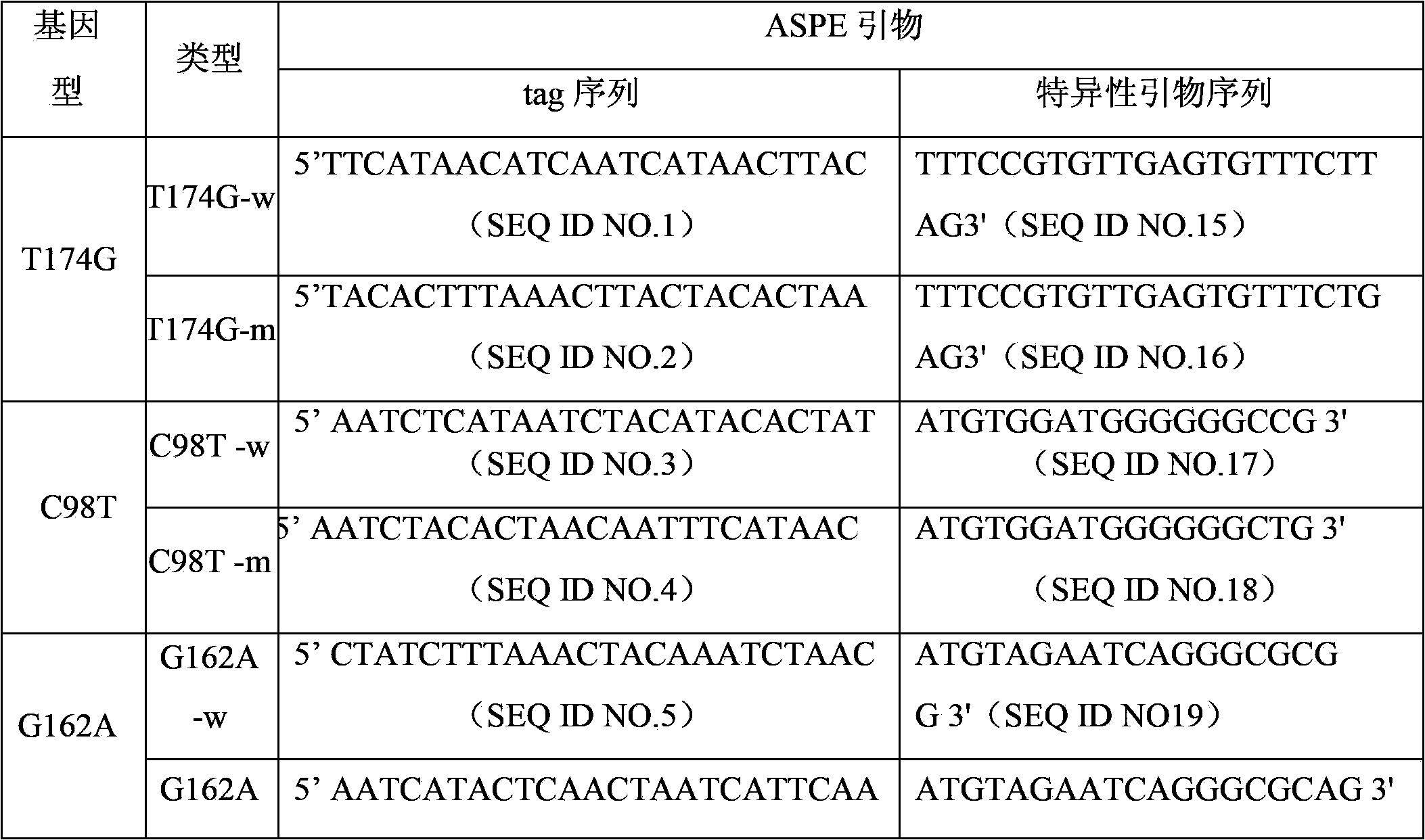
The invention discloses papillomavirus detection and a typing liquid chip, and a process which is used to the detection and typing papillomavirus. The liquid chip mainly comprises microsphere which is respectively enveloped with specific anti-tag label sequence and 7-10 T spacer arm sequence which is arranged between anti-tag label sequence and microsphere, wherein anti-tag label sequence is chosen from two or more than two sequences in SEQ ID NO. 25- SEQ ID NO.47, specific ASPE primer is respectively designed aiming at each typing HPV, ASPE primer is chosen from two or more than two sequence from SEQ ID NO.1-SEQ ID NO.23, and primer of target sequence which has mutant sites is amplified. The liquid chip improves the current liquid chip technology, which makes ribonucleotide probe microspheres which is prepared be suitable to different detection projects, largely increases fluorescent signal value which is detected, thereby further increasing sensitivity of the detection, strengthening signal-to-noise ratio, and making detection results more accurate and reliable.
Owner:SUREXAM BIO TECH
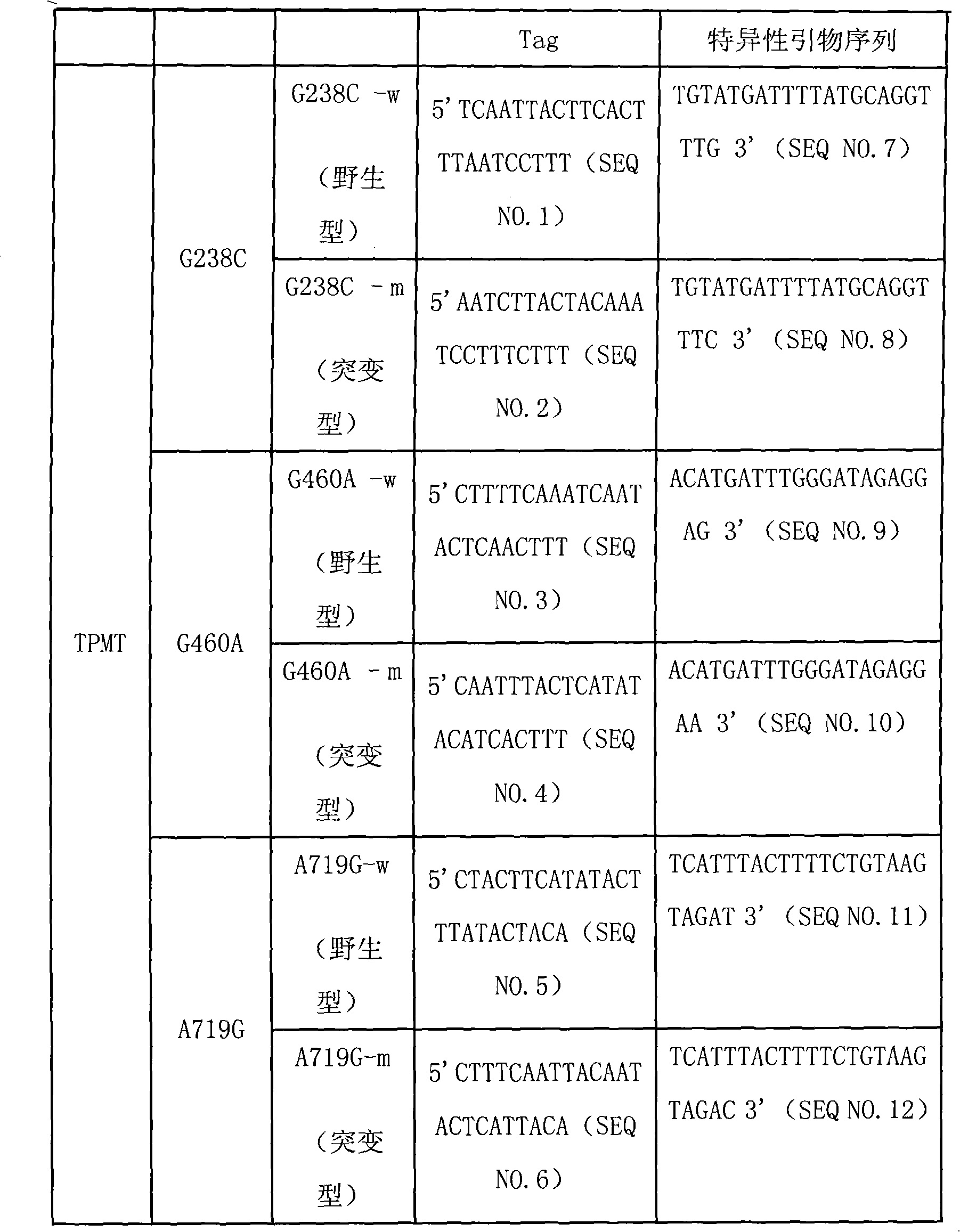
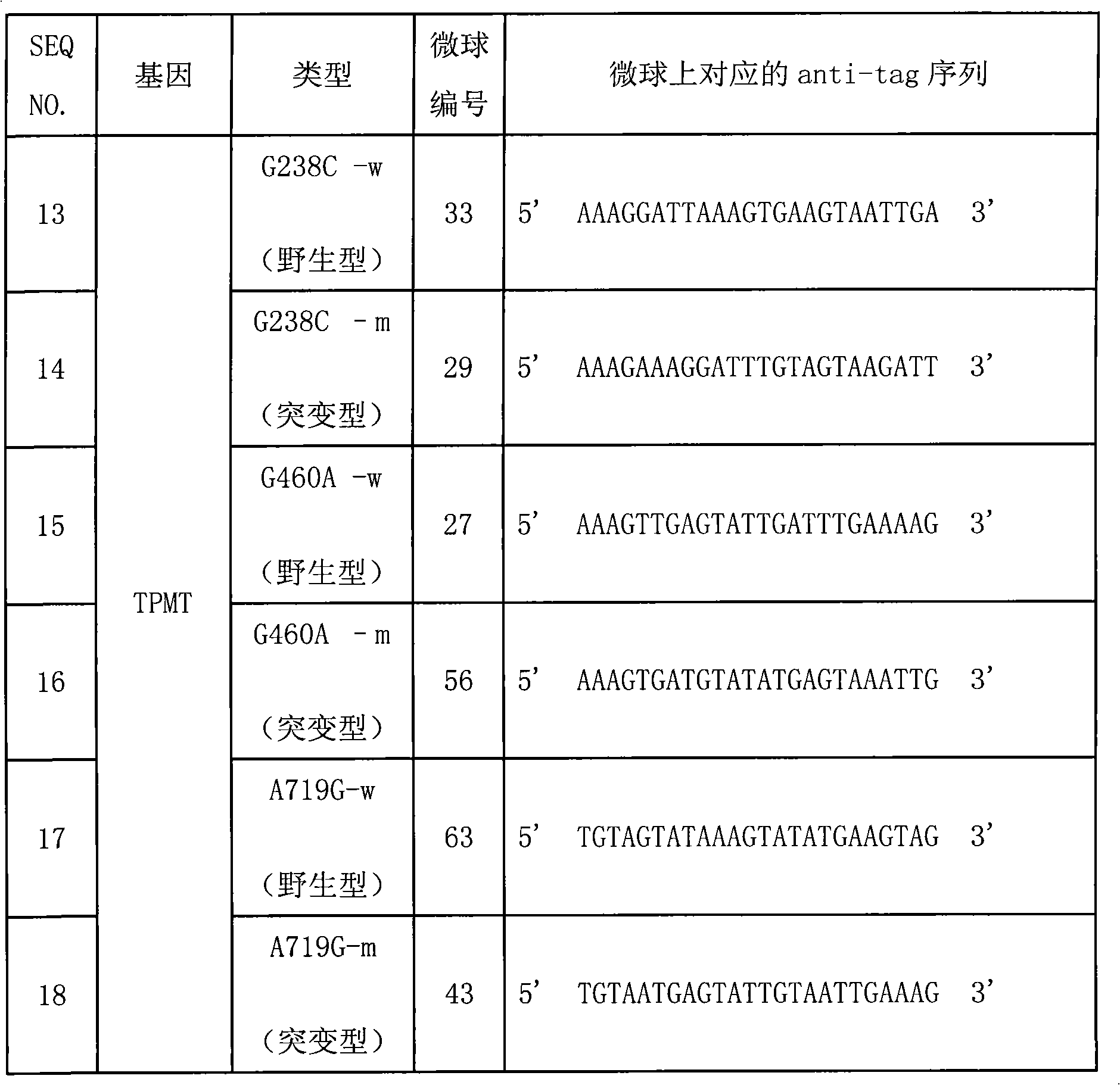
Specific sequence, liquid phase chip and method for SNP detection of TPMT gene
ActiveCN101671739AImprove signal-to-noise ratioNo cross-reactivityMicrobiological testing/measurementDNA/RNA fragmentationSignal-to-noise ratio (imaging)Type specific
The invention discloses a liquid phase chip, a specific prime and a method for the SNP detection of TPMT gene, the liquid phase chip comprises wild-type and mutant-type specific ASPE primer pairs designed respectively in accordance with mutant sites of each type, microspheres respectively enveloped with specific anti-tag sequences, and primers for amplifying target sequences having SNP sites of TMPT gene G238C, G460A and / or A719G. The prepared liquid phase chip for the SNP detection of TPMT gene has quite excellent signal-to-noise ratio and, basically, no cross reaction is present between thedesigned probe and the anti-tag sequence. The ASPE primers designed according to the invention has quite excellent specificity and can accurate distinguish the mutant sites of each type. The detectionmethod has simple steps and convenient operation, can complete the detection of 3 types of mutant sites in one step, and can avoid plenty of uncertain factors existing in the process of repeated operation, therefore, the accuracy of the detection can be enhanced enormously.
Owner:广州益善医学检验所有限公司
Method for detecting diarrhea shellfish toxin
InactiveCN101975768AReduce usageEasy extractionPreparing sample for investigationFluorescence/phosphorescenceActin depolymerizationDepolymerization
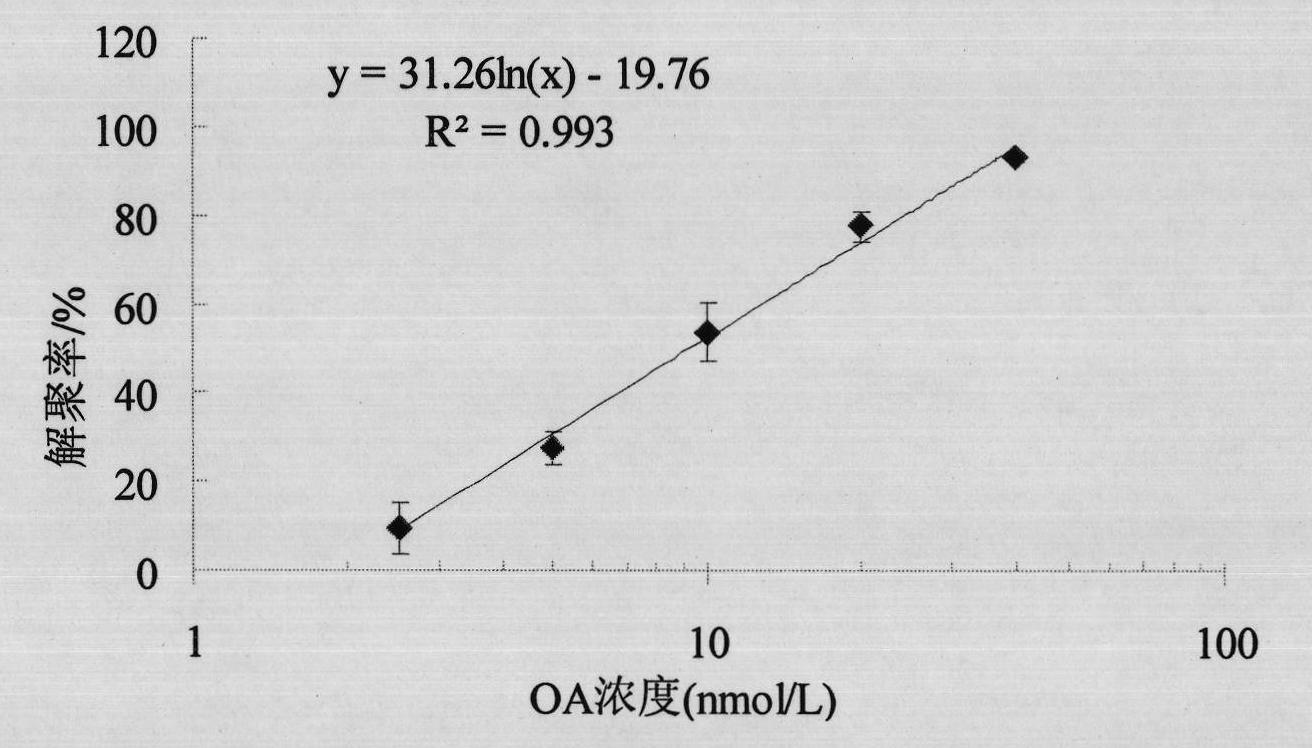
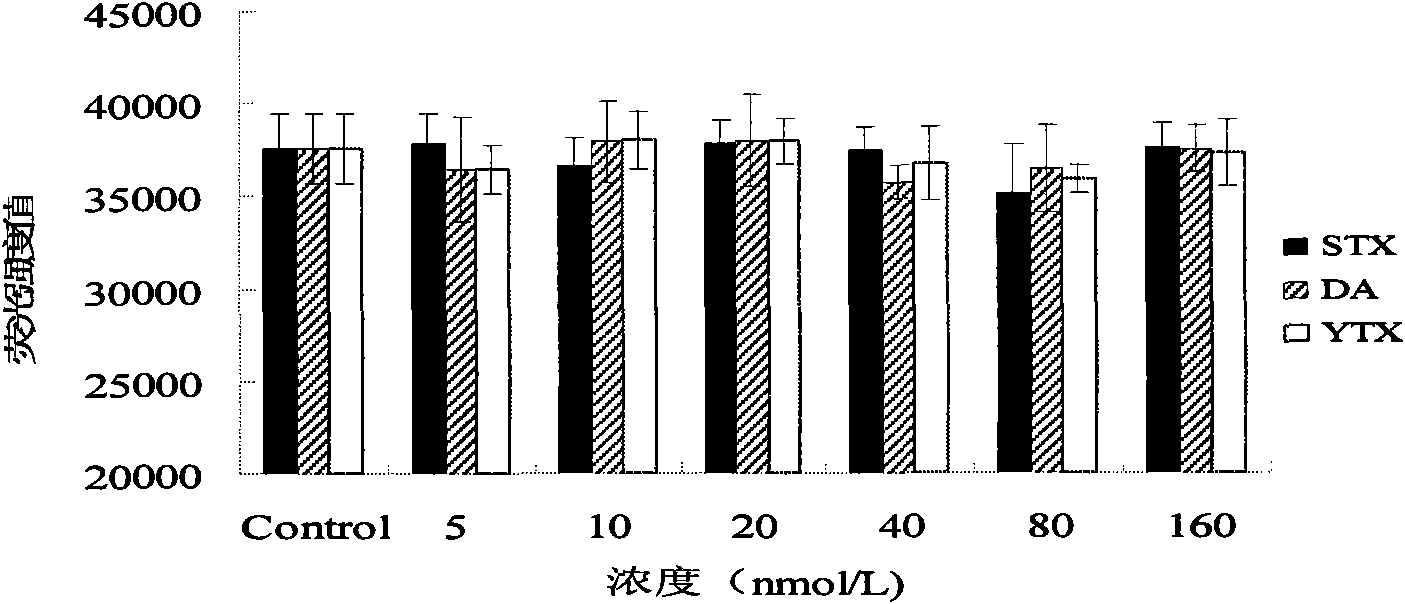
The invention discloses a method for detecting diarrhea shellfish toxin, which comprises the following steps: A) establishing a standard curve of depolymerization of okadaic acid (OA) induced HL-7702 hepatocyte F-actin; B) carrying out the calculation on the concentration of the OA according to the standard curve of the F-actin depolymerization of the HL-7702 hepatocyte, establishing a detection method of the hepatocyte F-actin of the diarrhea shellfish toxin to determine the possible detection limit range; C) selecting one or more of components (STX, DA and YTX) which are possibly coexist with the diarrhea shellfish to act on the HL-7702 hepatocyte, determining the specificity of the method for detecting the OA content by detecting the degree of damaging the polymerizing power of the HL-7702 hepatocyte F-actin by the above components. The detection method of the invention has the advantages: (1) taking cells as experimental subjects to avoid the using of experimental animals and conforming to the rule of 3R; (2) having high sensitivity; (3) having good specificity; (4) having good repeatability; and (5) having simple and convenient sample extraction and low matrix effects.
Owner:SHENZHEN CENT FOR DISEASE CONTROL & PREVENTION
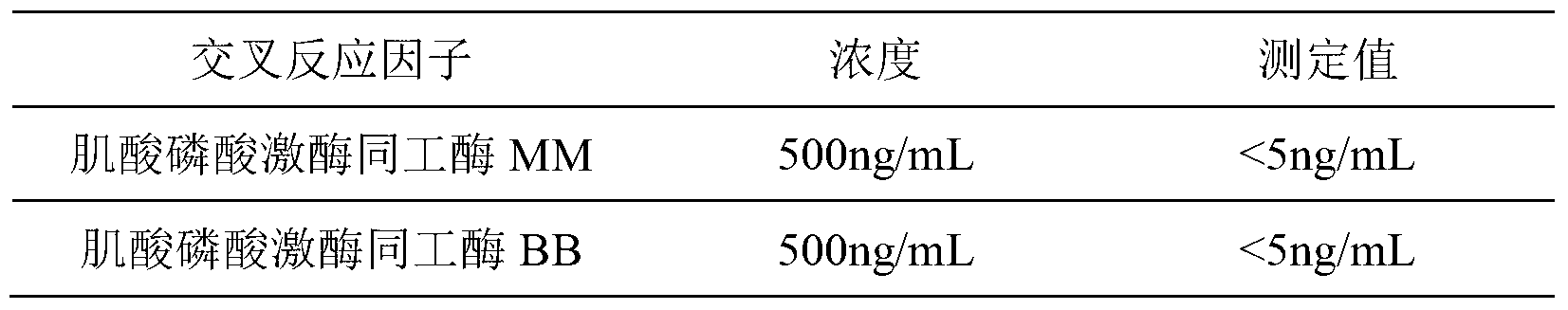
Kit for chemiluminescence immunity quantitative detection of CK-MB (creatine kinase- isoenzyme) nano magnetic particle and preparation method of kit
ActiveCN103278623ANo cross-reactivityStrong specificityBiological testingCreatine kinaseHorse radish peroxidase
The invention discloses a kit for chemiluminescence immunity quantitative detection of a CK-MB nano magnetic particle. The kit comprises a CK-MB calibrator; a nano magnetic particle suspension liquid coupled with streptavidin, bioti-labeled CK-MB antibodies, CK-MB abzyme conjugate, a CK-MB quality control product, a chemiluminescence liquid A and a chemiluminescence liquid B, a 20-time concentrated washing liquor and a reaction tube, wherein for the CK-MB abzyme conjugate, used enzyme adopts horse radish peroxidase with purity RZ larger than or equal to 3.0 and activity larger than or equal to 250 U / mL. Besides, the invention further discloses a preparation method of the kit. Compared with the conventional kit, the kit provided by the invention has the advantages of high sensibility and test automation, wide measurable concentration range, long validity of a reagent, simple operation and the like.
Owner:BIOSCIENCE (TIANJIN) DIAGNOSTIC TECH CO LTD
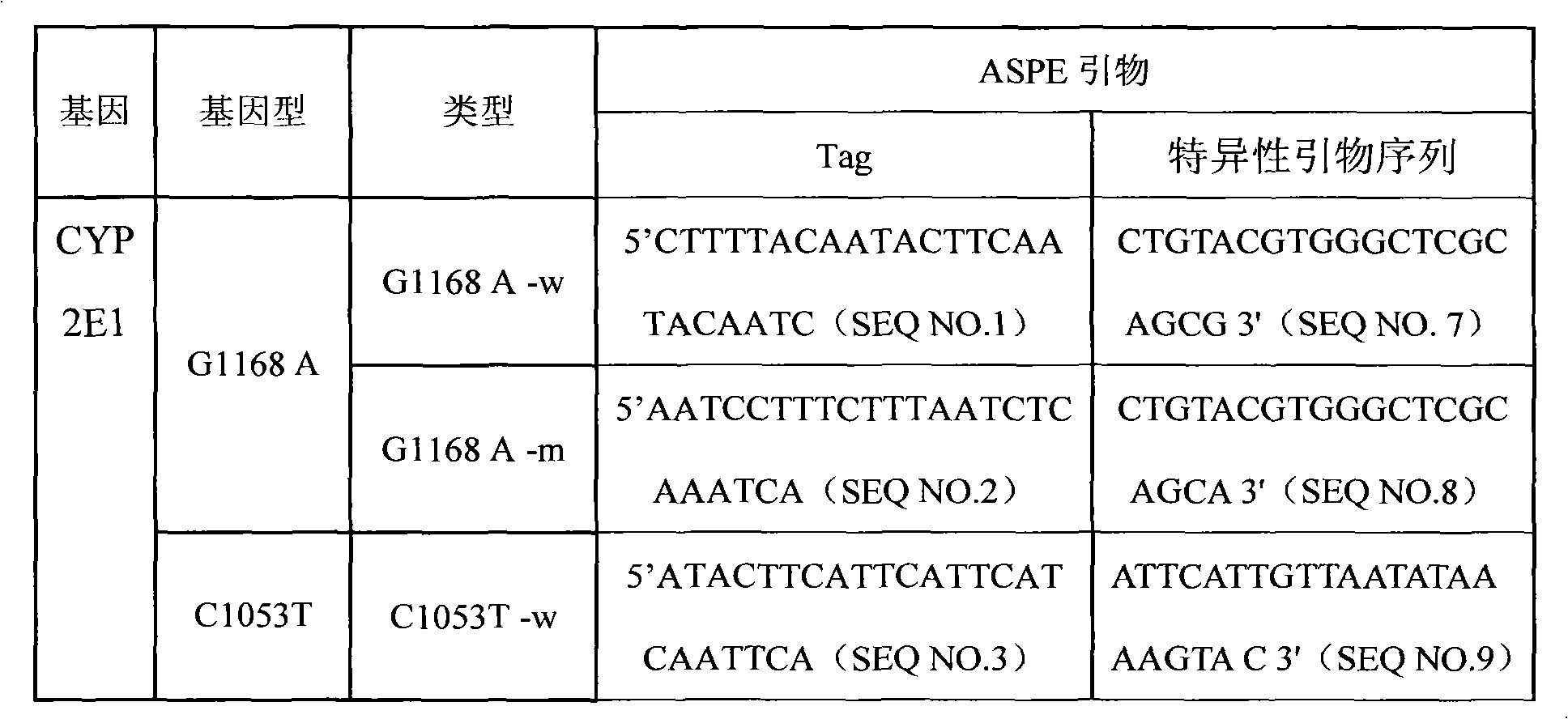
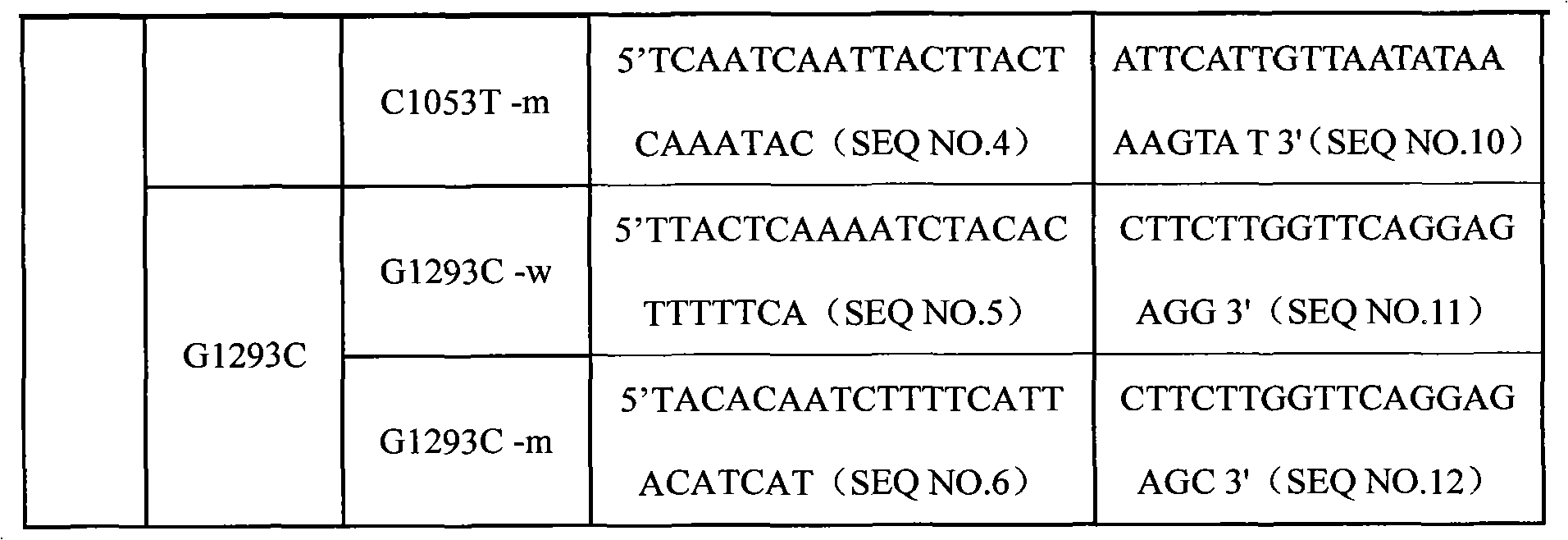
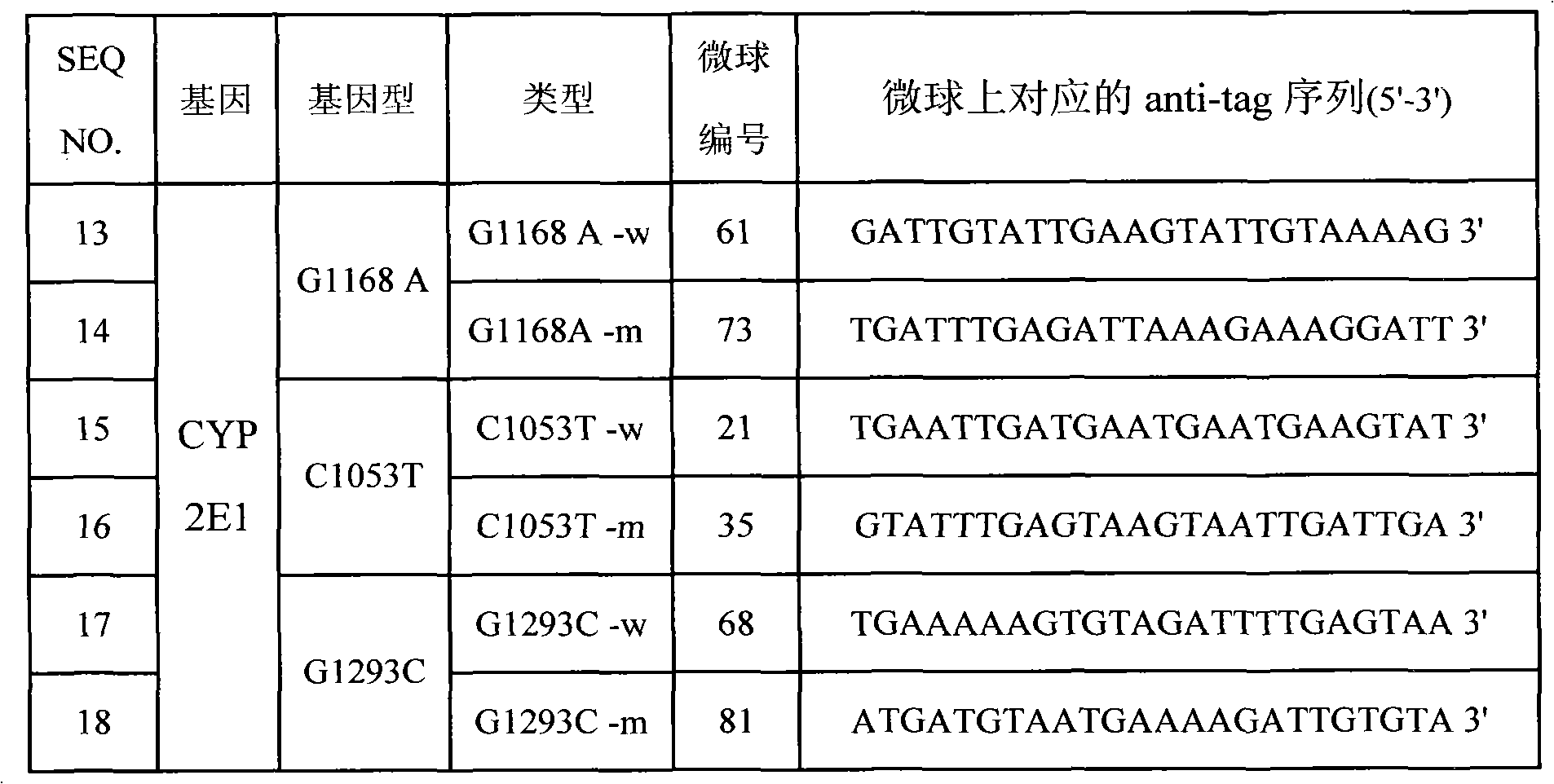
Specific primmer, liquid phase chip and detection method for CYP2E1 (Cytochrome P450 2E1) gene SNP (Single Nucleotide Polymorphism) detection
InactiveCN101805798AImprove signal-to-noise ratioImplement parallel detectionMicrobiological testing/measurementDNA/RNA fragmentationMicrosphereWild type
The invention discloses a specific primmer, a liquid phase chip and a detection method for CYP2E1 (Cytochrome P450 2E1) gene SNP (Single Nucleotide Polymorphism) detection. The liquid phase chip comprises wild-type and mutant ASPE (Allele-Specific Primer Extension) primer pairs respectively designed to each type of mutant site, microspheres respectively encapsulated with specific anti-tag sequences, and primers for respectively amplifying a CYP2E1 gene target sequence with G1168A, C1053T and / or G1293C SNP site. The prepared liquid phase chip for CYP2E1 gene SNP detection has excellent signal-to-noise ratio, and the designed probe and the anti-tap sequence do not exit the cross reaction basically. The ASPE primers have excellent specificity and can exactly distinguish all kinds of mutant sites. The detection method has simple steps, the detection of three kinds of SNP bits can be finished once with simple operation, and a plurality of undetermined factors existing in multiple operation processes are avoided, thus the precision rate of the detection can be greatly improved.
Owner:SUREXAM BIO TECH
Chemiluminescent ligand analysis method for quantitative detection of human auto-antibody
InactiveCN101470117ASolve the problem of inaccurate quantitative detectionNo cross-reactivityBiological testingAutoantibodyBiomedical technology
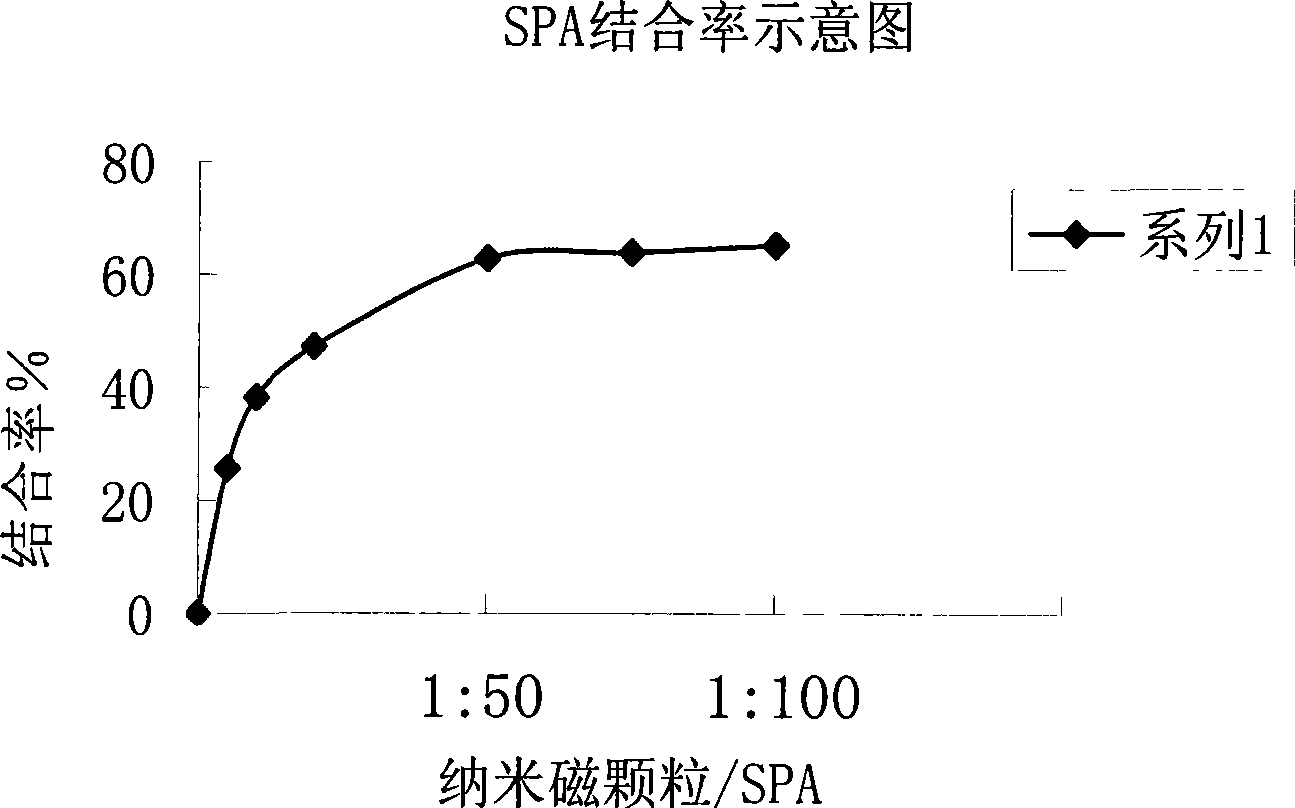
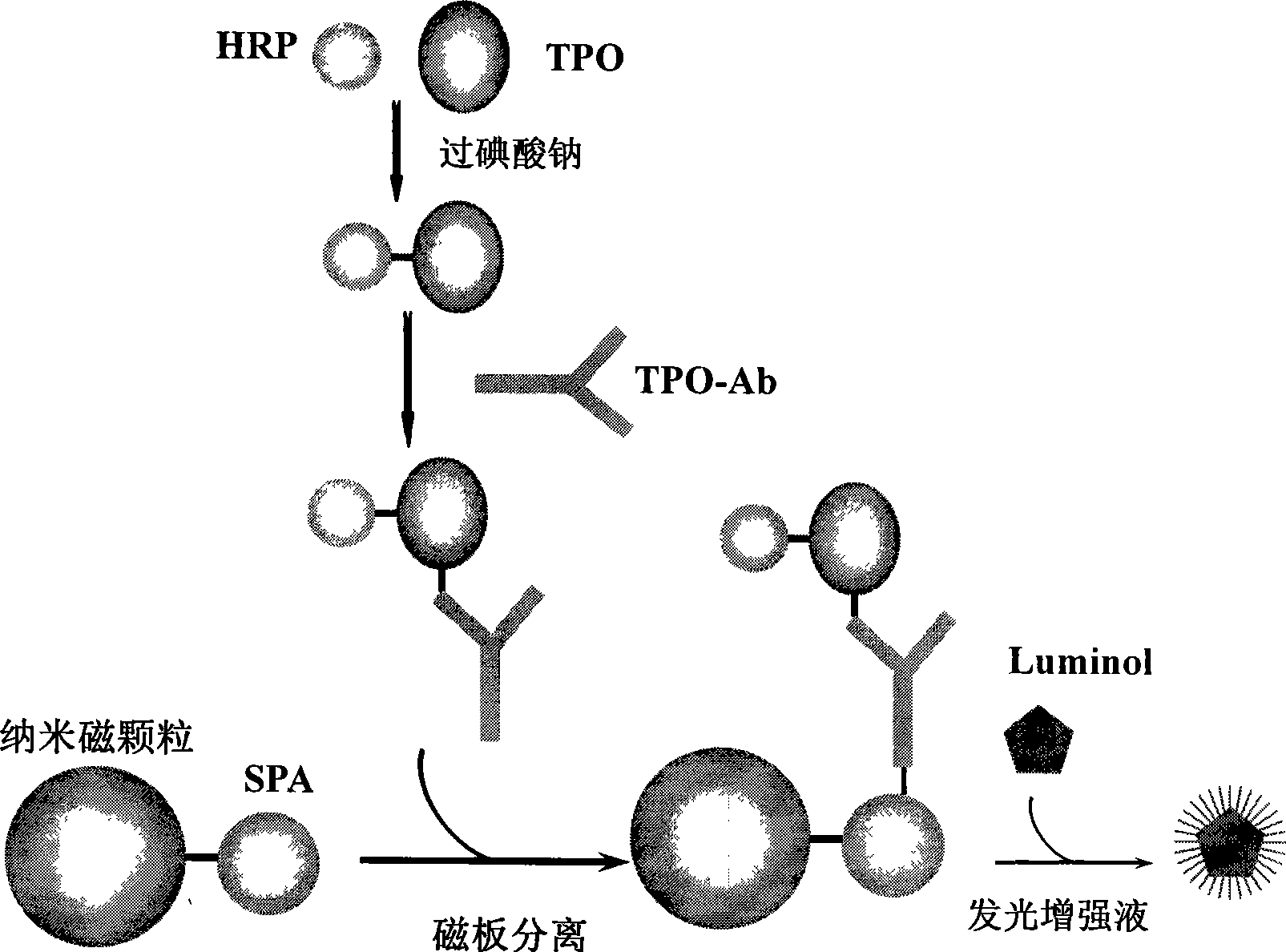
A chemical luminous ligand analysis method for quantitatively checking human antibodies belongs to the biomedical technical field, which comprises: using the generality that staphylococcal protein A (SPA) can react with Fc point of IgG in mice; using the monoclonal antibody of high affinity and specificity to prepare a standard product to establish a standard curve; respectively reacting the monoclonal antibody and the antibody in the sample with enzyme-labeled antigens; using the nanometer magnetic particles coated by SPA as ligand to separate solid and liquid; and using enzymatic chemical illumination reaction catalyzed by horseradish peroxidase (HRP) to process illumination check; thereby establishing a chemical luminous ligand quantitative analysis on human antibodies. The chemical luminous ligand analysis method is suitable for quantitatively checking all human antibodies, for resolving the problem of prior art while most human antibodies can not be accurately and quantitatively checked, avoiding cross reaction and avoiding false negative result or false positive result.
Owner:天津市协和医药科技集团有限公司
PCR (Polymerase Chain Reaction) primer, kit and liquid chip for detecting RET fusion gene
ActiveCN103805688AHigh coincidence rateSimplify the stepsNucleotide librariesMicrobiological testing/measurementMicrosphereMicrobiology
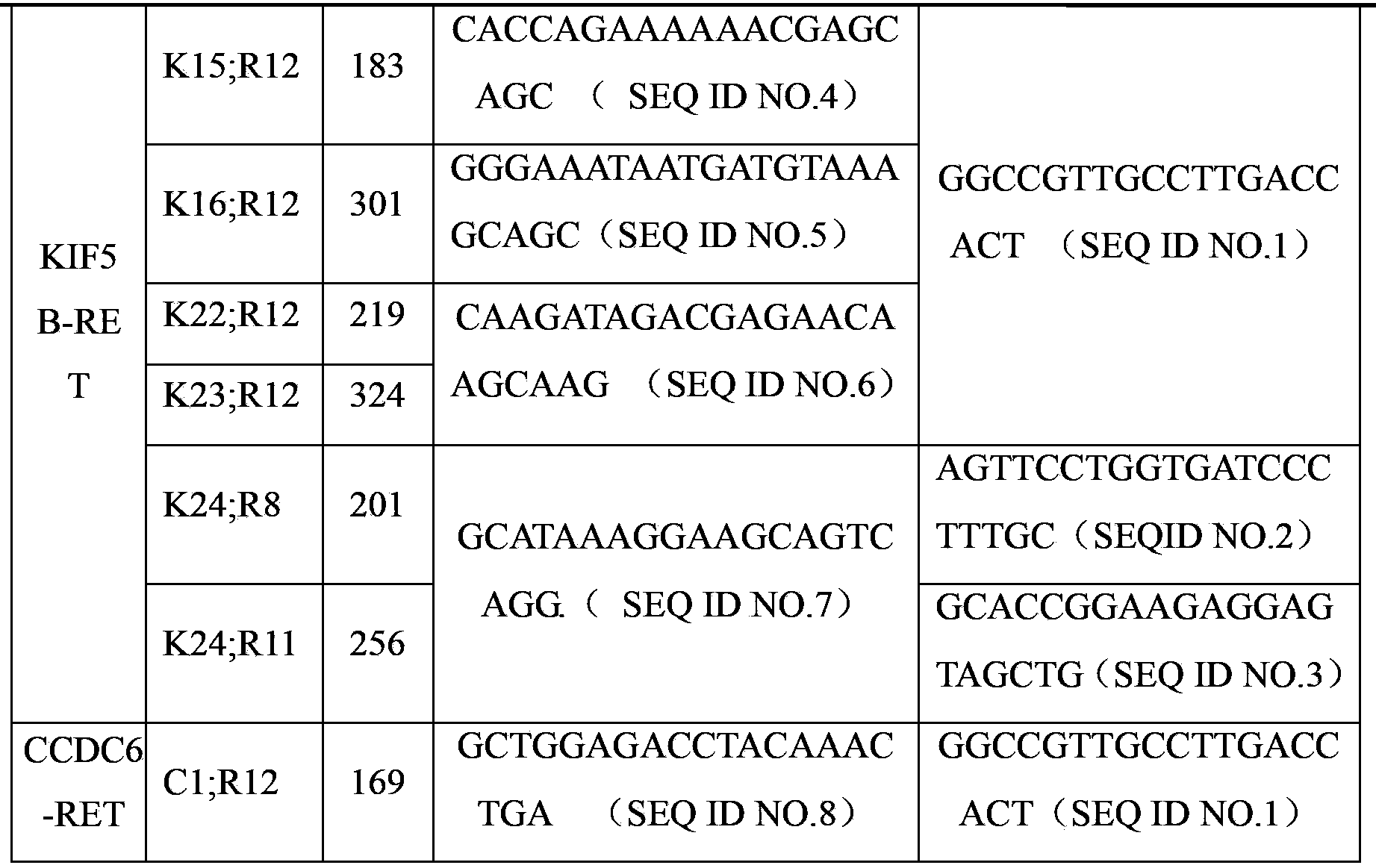
The invention discloses a PCR (Polymerase Chain Reaction) primer, a kit and a liquid chip for detecting an RET fusion gene. The liquid chip comprises a PCR amplification primer, an ASPE (Allele Specific Primer Extension) primer which consists of tag sequences and specific primers, and microspheres, wherein the specific primers comprise the following sequences: SEQ ID NO.23 for K15;R12, SEQ ID NO.24 for K16;R12, SEQ ID NO.25 for K22;R12, SEQ ID NO.26 for K23;R12, SEQ ID NO.27 for K24;R8, SEQ ID NO.28 for K24;R11 and / or SEQ ID NO.29 for C1;R12. The liquid chip disclosed by the invention has a quite good signal to noise ratio and can be used for implementing amplification of seven fusion subtypes through one step, and the specific primers have quite good specificity.
Owner:SUREXAM BIO TECH
Indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) diagnostic kit of mycoplasma gallisepticum
InactiveCN102221616AIndirect ELISA method optimizationAntigenic stabilityBiological testingSorbentElution
The invention discloses an indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) diagnostic kit of mycoplasma gallisepticum, which comprises an ELISA plate coated by species specificity proteins PvpA, an elution buffer solution, an antibody diluting solution, an ELISA secondary antibody, a substrate color developing solution and a stop solution. The kit disclosed by the invention selects the species specificity proteins PvpA, covers the high-frequency variation regions: DR-1 and DR-2 regions of the PvpA proteins, has high specificity and immunogenicity, improves the specificity and sensitivity of the detection result, lowers the production cost and is suitable for popularization and use in grassroots veterinarian mechanisms.
Owner:SOUTH CHINA AGRI UNIV
Injectable hyaluronic acid/polyethylene glycol hydrogel as well as preparation method and application thereof
ActiveCN106519072AEasy to prepareHigh yieldPharmaceutical delivery mechanismProsthesisCross-linkChemical reaction
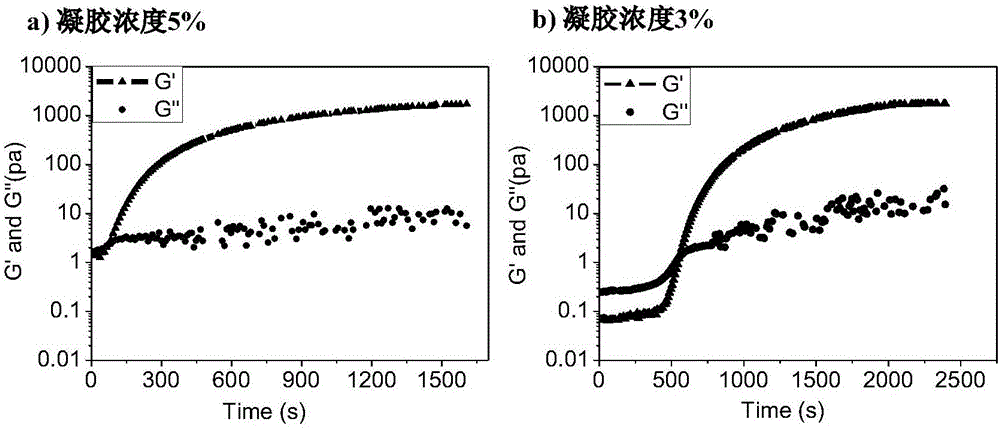
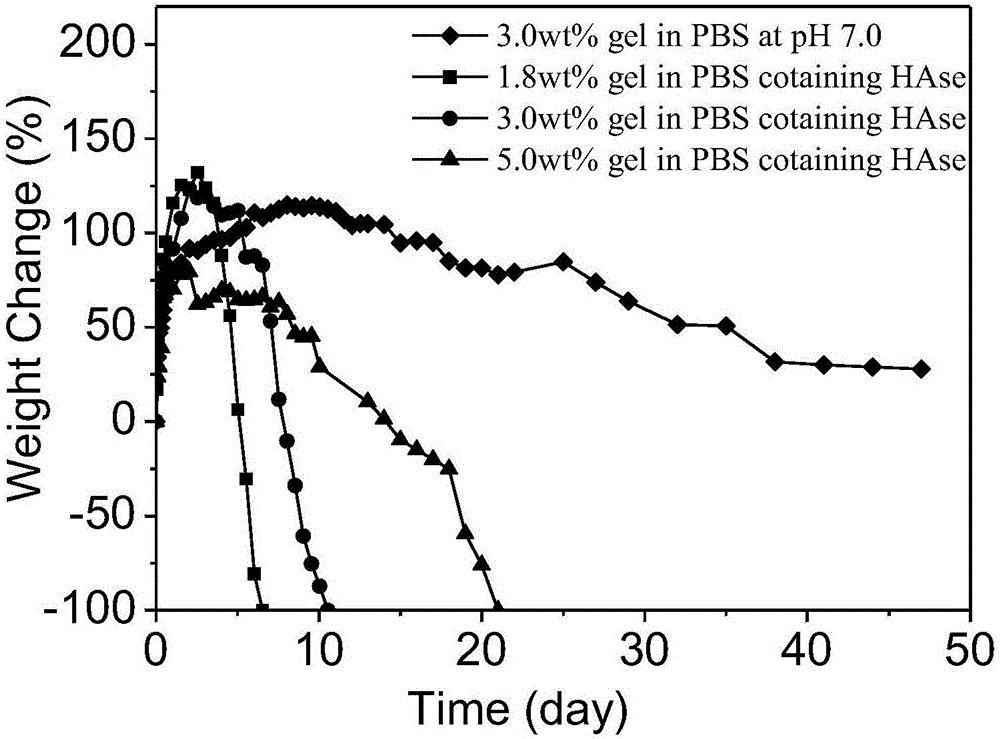
The invention discloses injectable hyaluronic acid / polyethylene glycol hydrogel as well as a preparation method and application thereof, and belongs to the fields of plastic and cosmetic biological materials. The method is characterized in that a cyclooctyne-modified hyaluronic acid (HA) solution is mixed with a nitrine-modified polyethylene glycol (PEG) solution, a hydrogel precursor solution with favorable flowability is formed, and the hydrogel precursor solution can be quickly cross-linked through a ring tension promoted click chemical reaction to form the hydrogel after being injected into a body, so as to play roles in water retention, plastic and the like. The preparation process of the material is simple; the additional addition of a cross-linking agent is not needed; the heating is not needed; the ultraviolet treatment or the radiation and the like are not needed; one-step cross-linking is adopted; the injectable hyaluronic acid / polyethylene glycol hydrogel is non-toxic and has no stimulation; therefore, the made hydrogel is good in biocompatibility, and is insignificant in inflammatory reaction. Meanwhile, the gel is slower in degradation speed, and is excellent in mechanical performance; therefore, the material is good in durability and molding performance, is quite applicably used as a face-lifting filling material in the fields of wrinkle resisting, breast enlargement, nose augmentation, filling-padding and the like.
Owner:深圳浦瑞健康科技有限公司
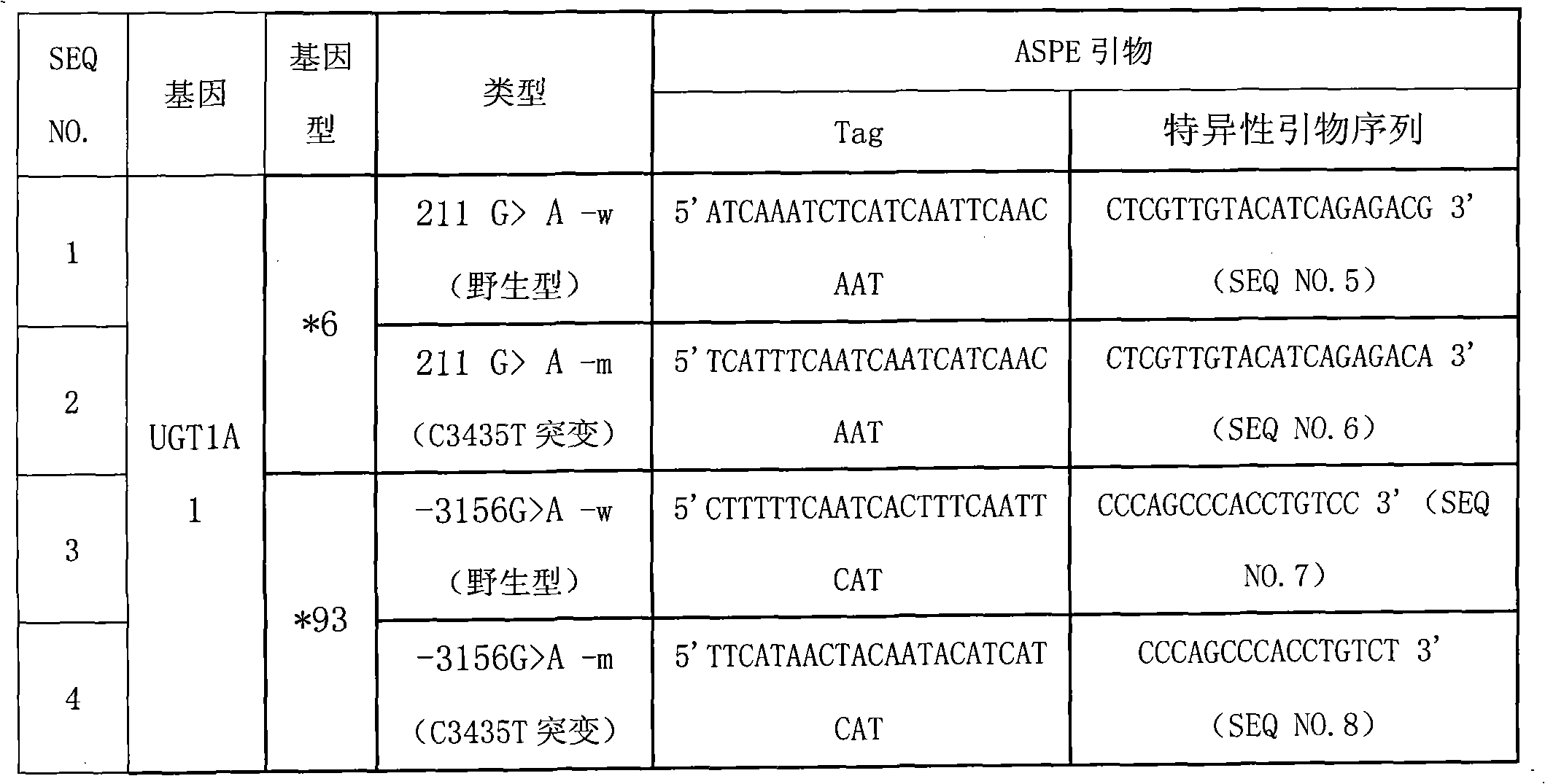
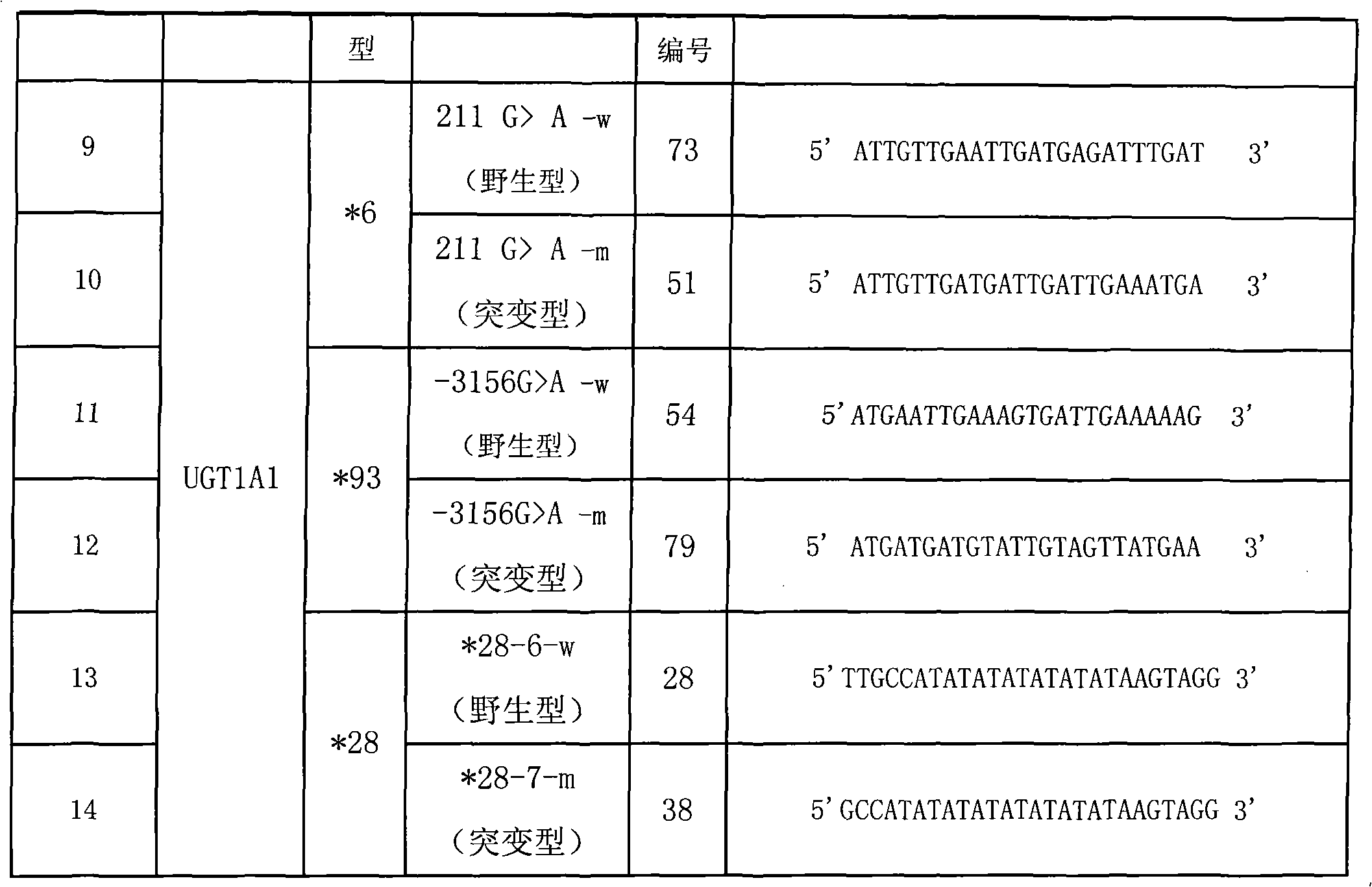
Method for detecting gene polymorphism of UGT1A1 and liquid phase chip
ActiveCN101671740AImprove signal-to-noise ratioNo cross-reactivityMicrobiological testing/measurementSignal-to-noise ratio (imaging)Color-coding
The invention discloses a liquid phase chip for detecting gene polymorphism of UGT1A1 and a detection method using the liquid phase chip, the liquid phase chip comprises microspheres respectively enveloped with specific corresponding wide-type and variant-type anti-tag sequences, wherein the anti-tag sequences are selected from the group consisting of SEQ ID NO.13 and SEQ ID NO.14 directed to UGT1A1*28 gene type, SEQ ID NO.9 and SEQ ID NO. 10 directed to UGT1A1*6 gene type, and / or SEQ ID NO.11 and SEQ ID NO.12 directed to UGT1A1*93 gene type; each of the above microspheres includes different color codings, primers including variant target sequences of the UGT1A1*28gene type, the UGT1A1*6 gene type and / or the UGT1A1*93 gene type are amplified, and the primers are modified by biotin so thatthe corresponding PCR reaction product contains a biotin labeling and has a sequence capable of complementary pairing with anti-tag. The matching rate of the detection method provided by the inventionand sequencing method reaches as high as 100%. The prepared liquid phase chip for detecting gene polymorphism of UGT1A1 has quite excellent signal-to-noise ratio and, basically, no cross reaction ispresent between the designed probe and the anti-tag sequence.
Owner:SUREXAM BIO TECH
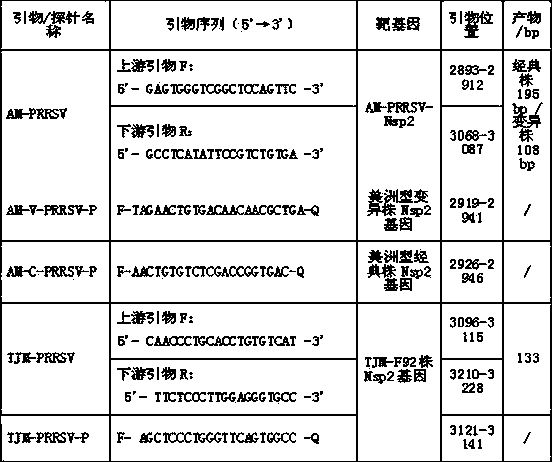
Multiple fluorescent quantitative RT-PCR (reverse transcriptase-polymerase chain reaction) method for detecting PRRSV (porcine reproductive and respiratory syndrome virus) and application thereof
InactiveCN103725793ARapid amplification reactionEfficient amplification reactionMicrobiological testing/measurementFluorescence/phosphorescenceHighly pathogenicMedicine
The invention discloses a multiple fluorescent quantitative RT-PCR (reverse transcriptase-polymerase chain reaction) method for detecting PRRSV (porcine reproductive and respiratory syndrome virus) and an application thereof. The method is implemented by fluorescent quantitative RT-PCR primers and probes for detecting the PRRSV, wherein the primers comprise an AM-PRRSV primer pair and a TJM-PRRSV primer pair, and the probes comprise an AM-V-PRRSV-P probe, an AM-C-PRRSV-P probe and a TJM-PRRSV-P probe; and the multiple fluorescent quantitative RT-PCR method for detecting the PRRSV comprises the steps as follows: the primers and the probes are utilized to perform fluorescent quantitative RT-PCR amplification, fluorescence signals are collected after the amplification, the fluorescence signals which have amplification curves are positive, and the method is characterized in that an RT-PCR amplification system comprises the primers and the probes. The multiple fluorescent quantitative RT-PCR method can detect and distinguish PRRSV American type classical strains, HP-PRRSV and highly-pathogenic PRRS living-vaccine TJM-F92 strains simultaneously, and has the advantages of high specificity, high sensibility and high degree of automation.
Owner:广西壮族自治区动物疫病预防控制中心
Liquid phase chip for detecting EGFR (epidermal growth factor receptor) gene mutation
ActiveCN102234683AImprove signal-to-noise ratioNo cross-reactivityMicrobiological testing/measurementMicrosphereEGFR Gene Mutation
The invention discloses a liquid phase chip for detecting EGFR (epidermal growth factor receptor) gene mutation. The liquid phase chip mainly comprises a wild-type and mutant-type allele specific primer extension (ASPE) primer pair designed for mutational sites of an EGFR gene respectively, microspheres which are respectively coated with specific anti-tag sequences and have different colors of codes and amplification primers for respectively amplifying the target sequences with the corresponding mutational sites of the Exon 19, Exon 20 and / or Exon 21, wherein each ASPE primer comprises the tag sequences at the 5' terminal and the specific primers aiming at the mutational sites of the target gene at the 3' terminal; and the anti-tag sequences can correspondingly complement and form pair with the tag sequences selected in (A). The liquid phase chip has the following advantages: the coincidence rate of the detection method provided by the invention and a sequencing method is as high as 100%; the prepared liquid phase chip has very good signal to noise ratio; and cross reactions do not exist between the designed probes and the anti-tag sequences.
Owner:SUREXAM BIO TECH
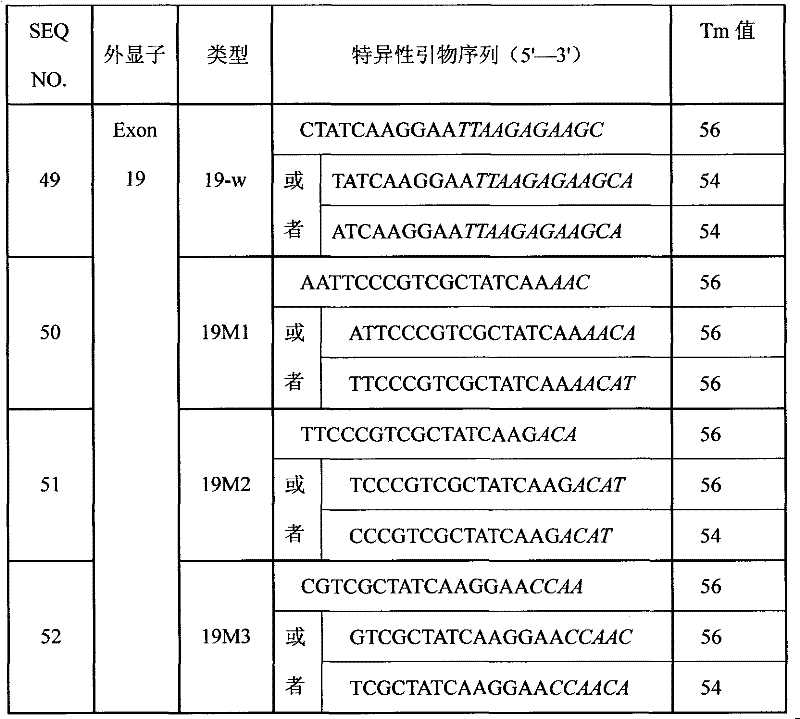
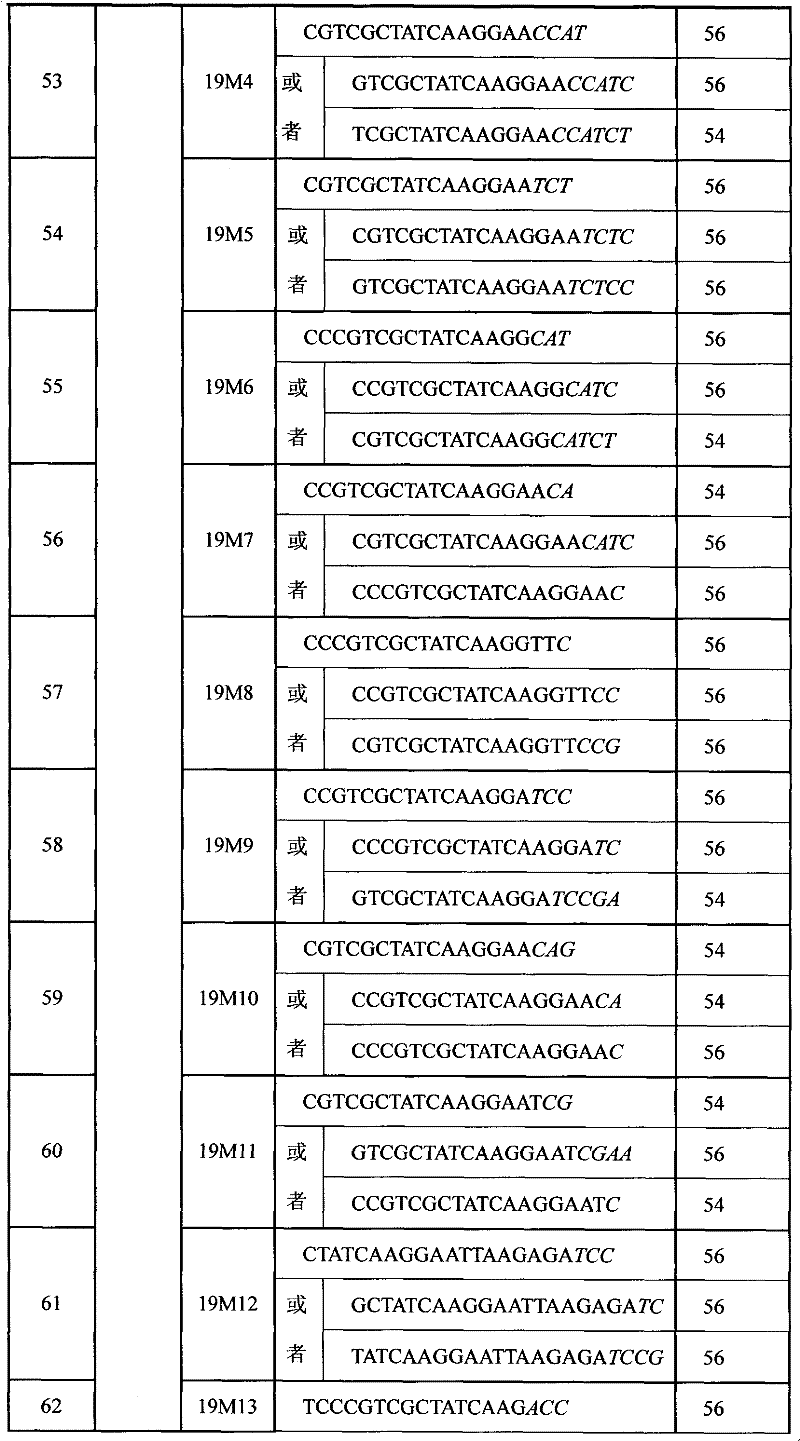
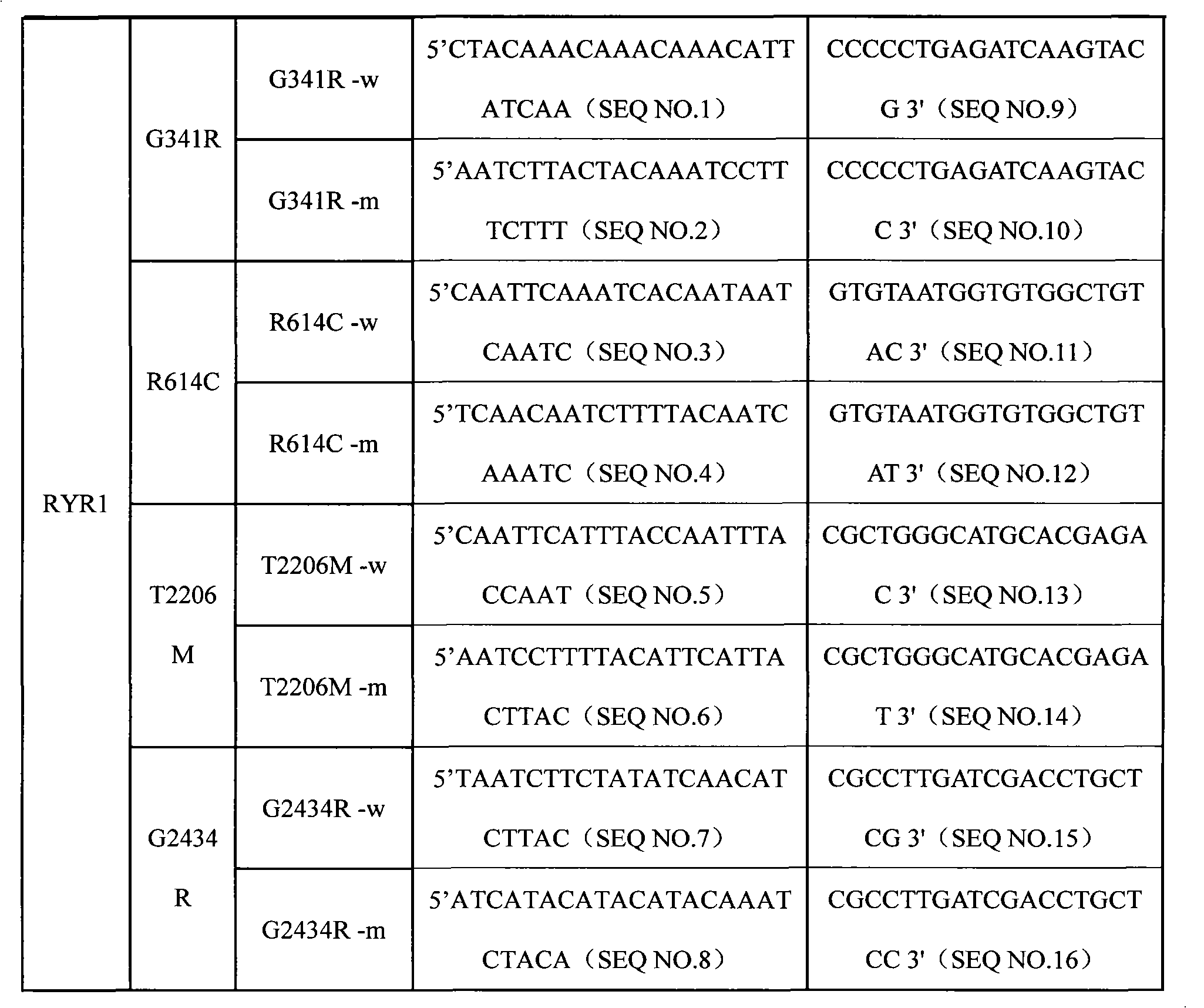
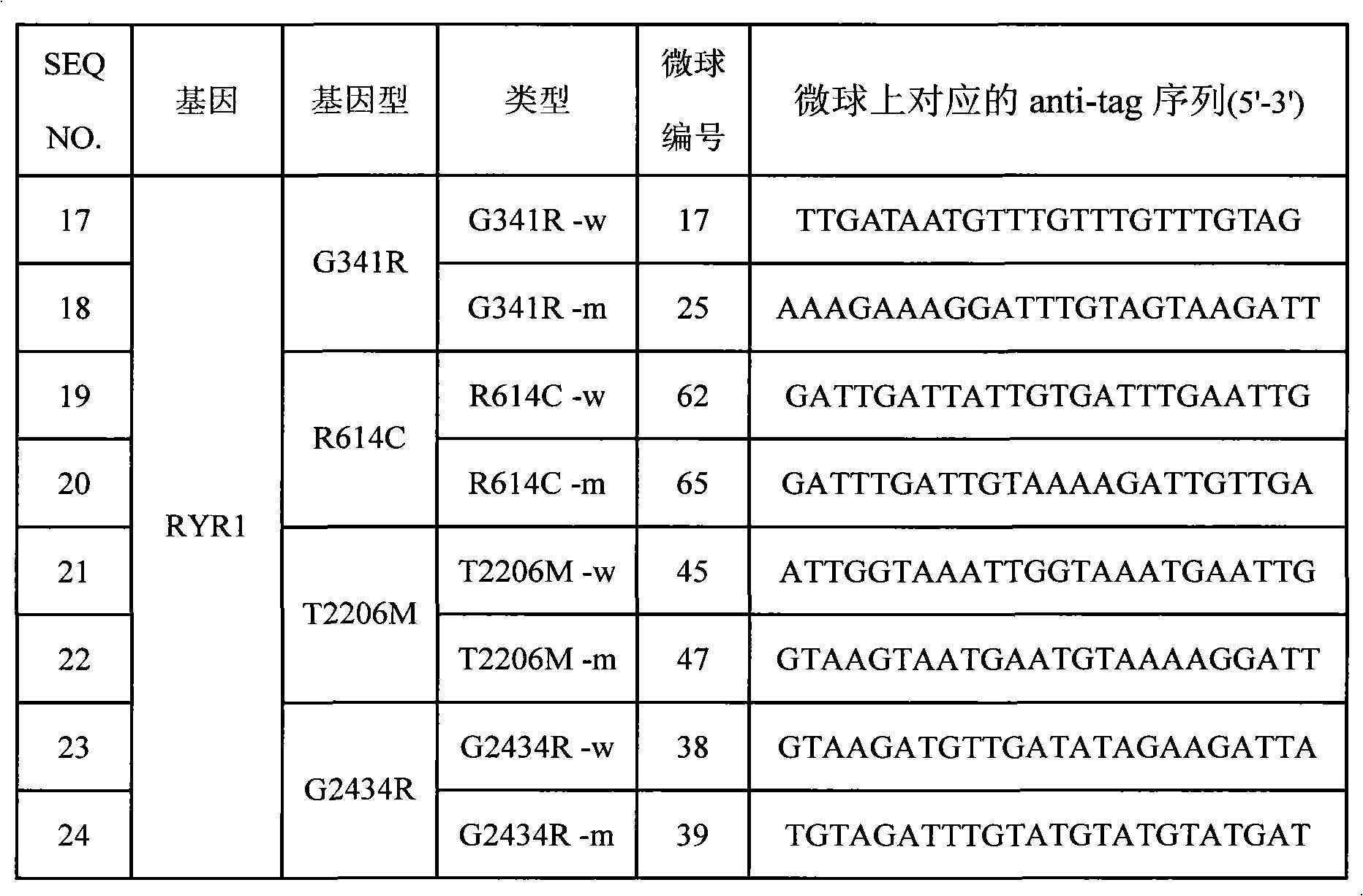
SNP (Single Nucleotide 0olymorphism) detection specific primer, liquid-phase chip and detection method of RYR1 (Ryanodine Receptors 1) gene
InactiveCN101812523AImprove signal-to-noise ratioNo cross-reactivityMicrobiological testing/measurementDNA/RNA fragmentationNucleotideWild type
The invention discloses an SNP (Single Nucleotide Polymorphism) detection specific primer, a liquid-phase chip and a detection method of an RYR1 (Ryanodine Receptors 1) gene. The liquid-phase chip comprises wild type and mutant type ASPE (Allele Specific Primer Extension) primer pairs respectively designed aiming at the mutational sites of each type, microballoons respectively coated with a specific anti-tag sequence and primers used for respectively amplifying target RYR1 gene sequences with G341R, R614C, T2206M and / or G2434R sites. The SNP detection liquid-phase chip of the RYR1 gene has very good signal-to-noise ratio, and a designed probe and the anti-tag sequences have no cross reactions basically. The designed ASPE primers have very good specificity and can accurately distinguish various types of mutational sites. The detection method has simple steps, and four SNP sites can finish being detected in one step, thus the operation is convenient; and moreover, various uncertain factors existing in the processes of multiple operations are avoided, thus the detection precision is greatly improved.
Owner:SUREXAM BIO TECH
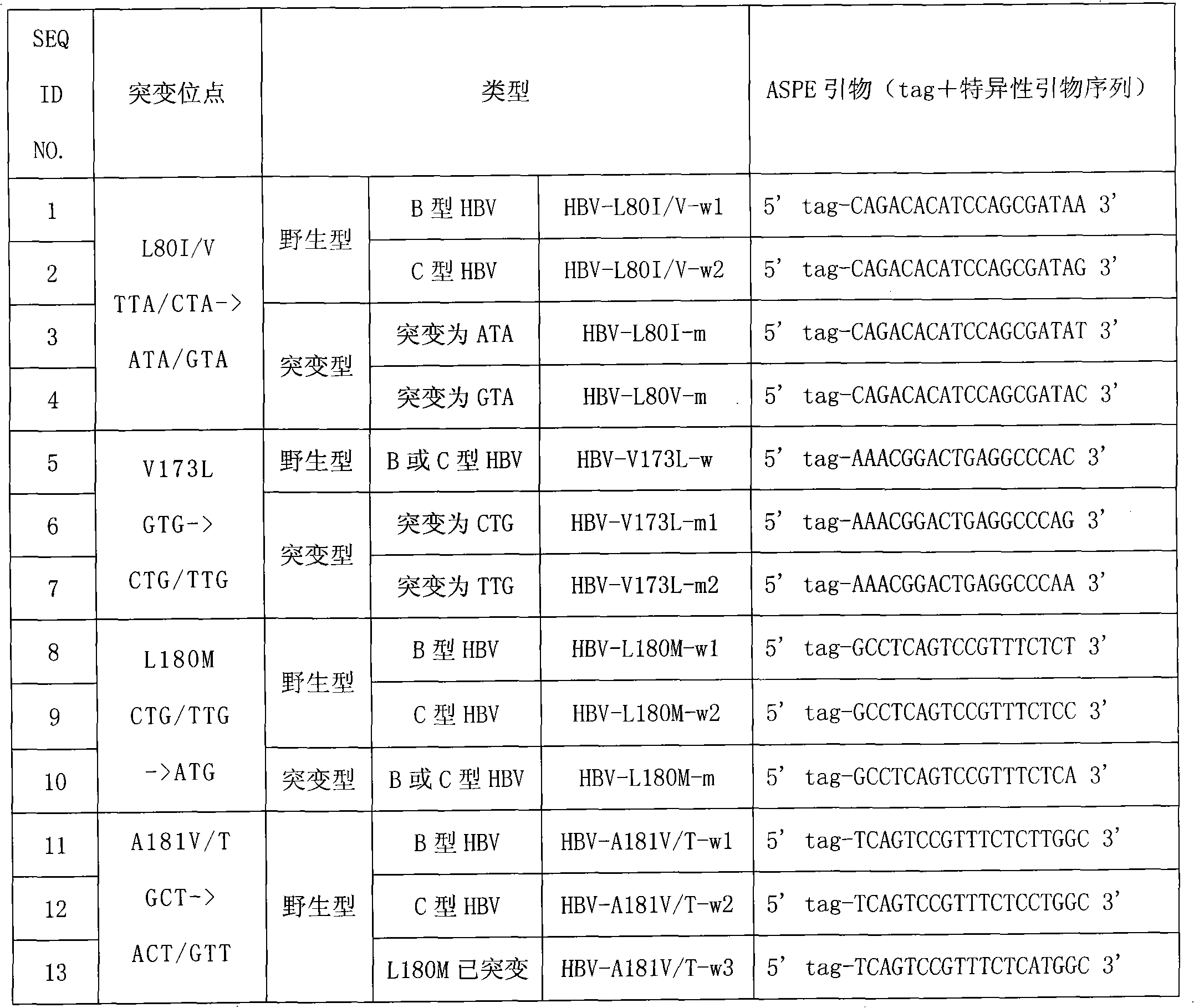
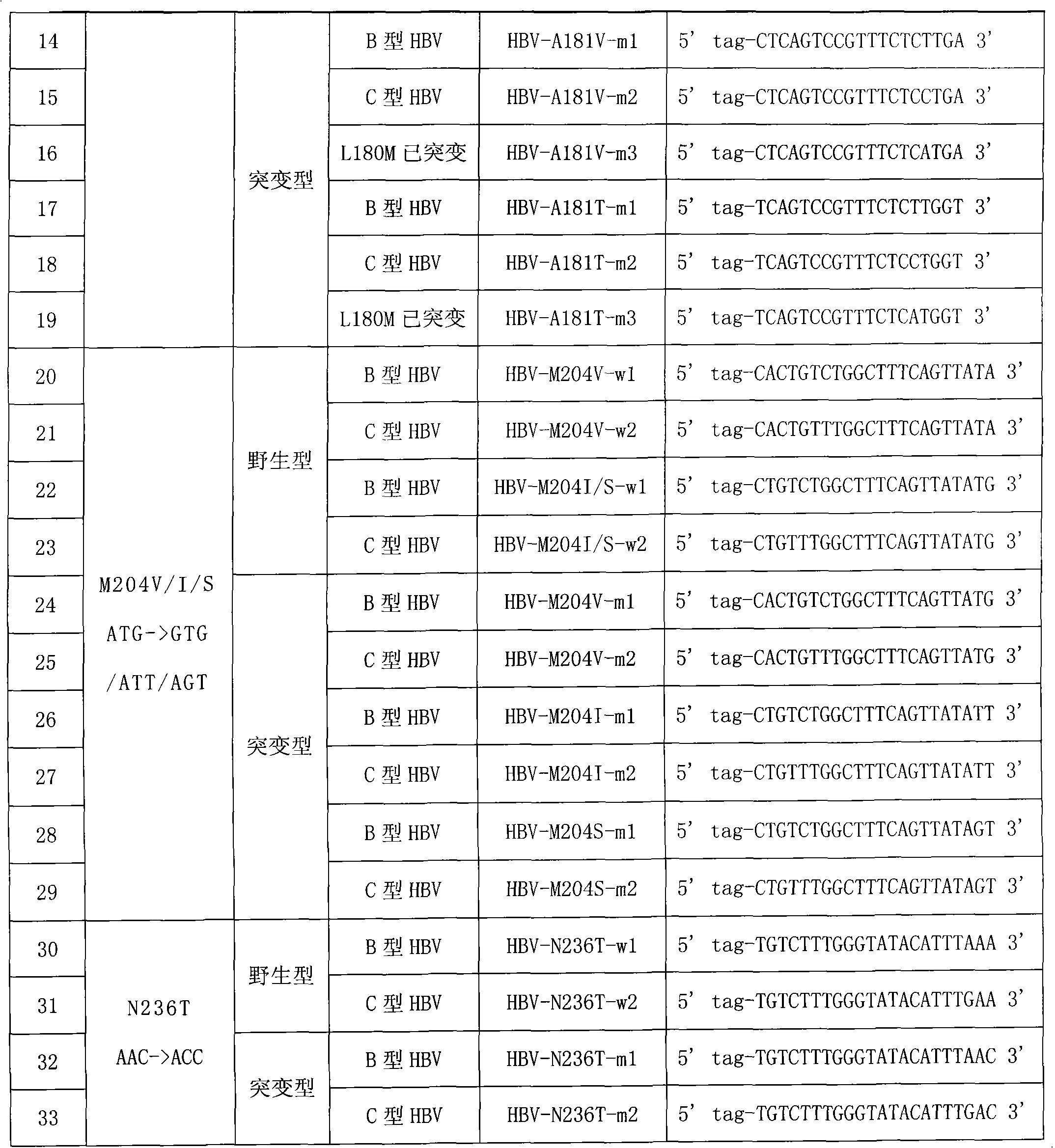
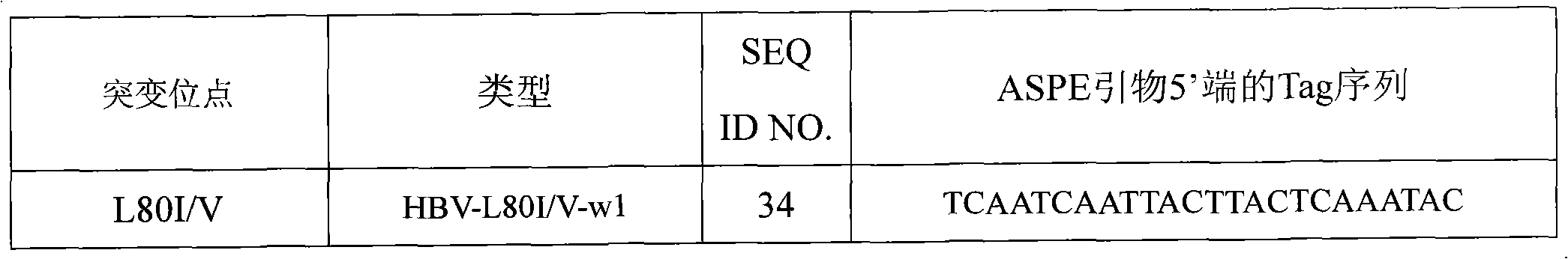
Hepatitis B virus YMDD motif mutation detection specific primer and liquid phase chip and method thereof
ActiveCN101633963AImprove signal-to-noise ratioNo cross-reactivityMicrobiological testing/measurementMicroorganism based processesSignal-to-noise ratio (imaging)Mutation detection
The invention discloses a hepatitis B virus YMDD motif mutation detection specific primer and liquid phase chip and a method thereof. The liquid phase chip mainly comprises an ASPE primer, a microsphere coated with an anti-tag sequence and a primer used for amplifying target sequences having mutation sites of rtL801 / V, rtV173L, rtL180M, rtA181V / T, rtM204V / I / S and / or rtN 236T. The detection liquid phase chip and the detection method have high detection sensitivity, high signal-to-noise ratio and more accurate and reliable detection results. Moreover, detecting 6 mutation sites can be completed in the same reaction, i.e., the operation is convenient, and many uncertain factors existing in many times of operational processes are avoided, thereby greatly improving the detection accuracy.
Owner:SUREXAM BIO TECH
Nucleic acid aptamer for detecting clenbuterol hydrochloride and screening method and application thereof
ActiveCN108977448AQuick checkEasy to detectMicrobiological testing/measurementBiological testingAptamerNucleotide
The invention provides a nucleic acid aptamer for detecting clenbuterol hydrochloride. The nucleic acid aptamer is selected from one of following nucleotide sequences shown in SEQ ID NO.1; the nucleicacid aptamers with same lengths are cut from both ends of the nucleotide sequence shown in SEQ ID NO.1, and the cutting length is 1 to 11bp; the nucleic acid aptamers with same cutting lengths of 1 to 11bp at the two ends respectively have the corresponding nucleotide sequences shown in SEQ ID NO.12 to SEQ ID NO.2. The invention also provides a screening method and application of the nucleic acidaptamer. The nucleic acid aptamer has the advantages that the specificity is strong, and the nucleic acid aptamer does not generate crossing reaction with clenbuterol hydrochloride analogues; the affinity is high, and the affinity constant is (42.17+ / -8.97) nM; the sensitivity is high; the minimum detection limit of clenbuterol hydrochloride is 1.2ng / mL.
Owner:HUBEI NORMAL UNIV
Monoclonal antibody, hybridoma, immunoassay method and diagnosis kit
InactiveCN1384886ANo cross reactivityStrong specificityBiological material analysisImmunoglobulins against bacteriaImmuno assayQuality control
The present invention provides a diagnostic method by which Helicobacter pylori infection can be diagnosed economically without causing any pain to the patient and without resorting to any special devices. Due to the use of one antibody in this method, a high degree of specificity can be achieved without showing any cross-reactivity resulting in batch-to-batch variation, thereby facilitating quality control. Furthermore, high sensitivity can be achieved even when using simple monoclonal antibodies. The invention also provides a monoclonal antibody with Helicobacter pylori catalase as antigen.
Owner:WAKAMOTO PHARMA
Chemiluminiscence kit for detecting human epididymis secretory protein 4
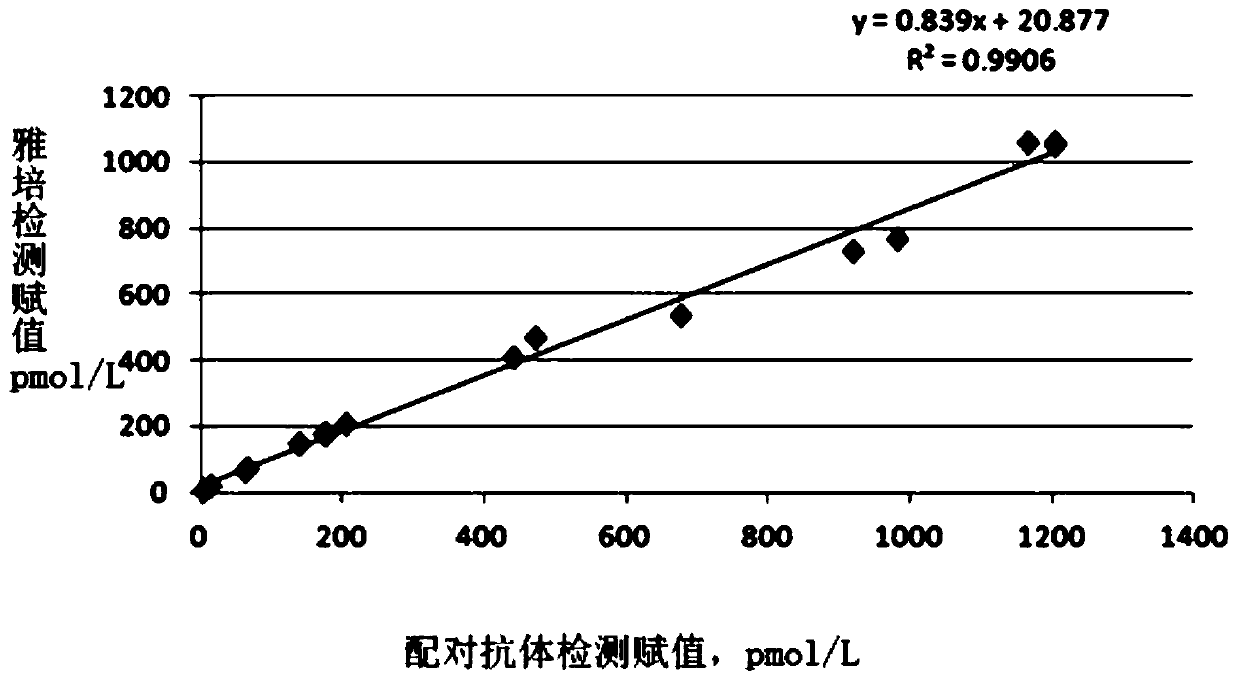
ActiveCN110895279AGood response specificityHigh sensitivityChemiluminescene/bioluminescenceImmunoglobulins against protease inhibitorsCapture antibodySubcloning
The invention discloses a chemiluminiscence kit for detecting human epididymis secretory protein 4. The kit comprises the human epididymis secretory protein 4, an acridinium ester labeled 69-G10-B8-G8detection antibody and a biotin labeled 9-C3-D12-B1 capture antibody, wherein the acridinium ester labeled 69-G10-B8-G8 detection antibody and the biotin labeled 9-C3-D12-B1 capture antibody are respectively obtained by secreting respective corresponding hybridoma cell strains 69-G10-B8-G8 and 9-C3-D12-B1. The two hybridoma cell strains are obtained by performing recombinant plasmid, protein expression, animal immunization and screening and finally subcloning on an artificially synthesized HE4 gene. The double-antibody sandwich compound disclosed by the invention is good in detection sensitivity and precision, wide in linear range, good in antigen reaction specificity and free of cross reaction.
Owner:普健生物(武汉)科技有限公司
Primer and method for detecting CYP2D6 gene polymorphism
InactiveCN105506095AGood specificityGood accuracyMicrobiological testing/measurementDNA/RNA fragmentationTreatment effectGene
The invention belongs to the technical field of biological detection, and in particular relates to a primer and a method for detecting CYP2D6 gene polymorphism. The primer for detecting the CYP2D6 gene polymorphism comprises a nested PCR amplification primer and an SNaPshot PCR primer; the nested PCR amplification primer comprises a nested A-turn amplification primer and a nested B-turn amplification primer. The primer for detecting the CYP2D6 gene polymorphism has the advantages of good specificity, no cross reaction and high accuracy, detection of the CYP2D6 gene polymorphism is realized, and the primer has very important significance for realizing gene oriented individualized treatment, continuously improving treatment effects and reducing untoward effects.
Owner:GUANGZHOU KINGMED DIAGNOSTICS GRP CO LTD
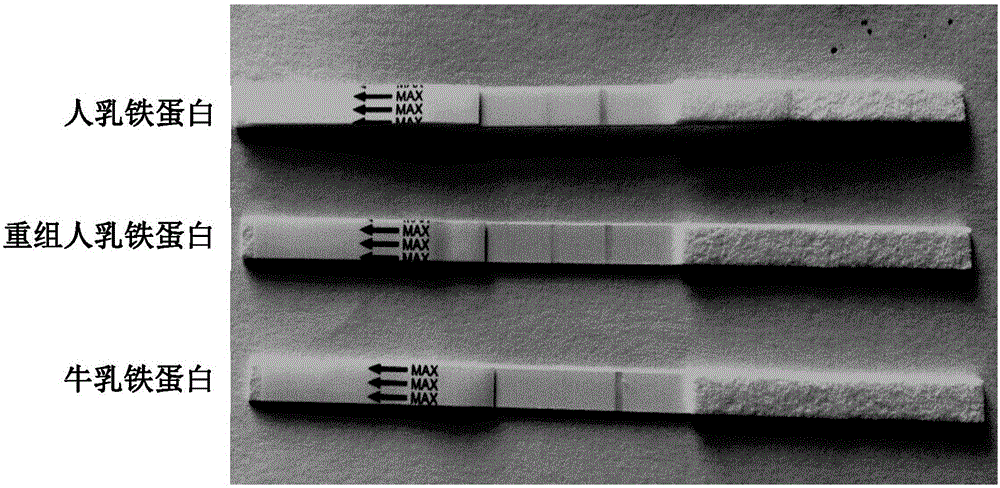
Human lactoferrins double-antibody sandwich ELISA kit
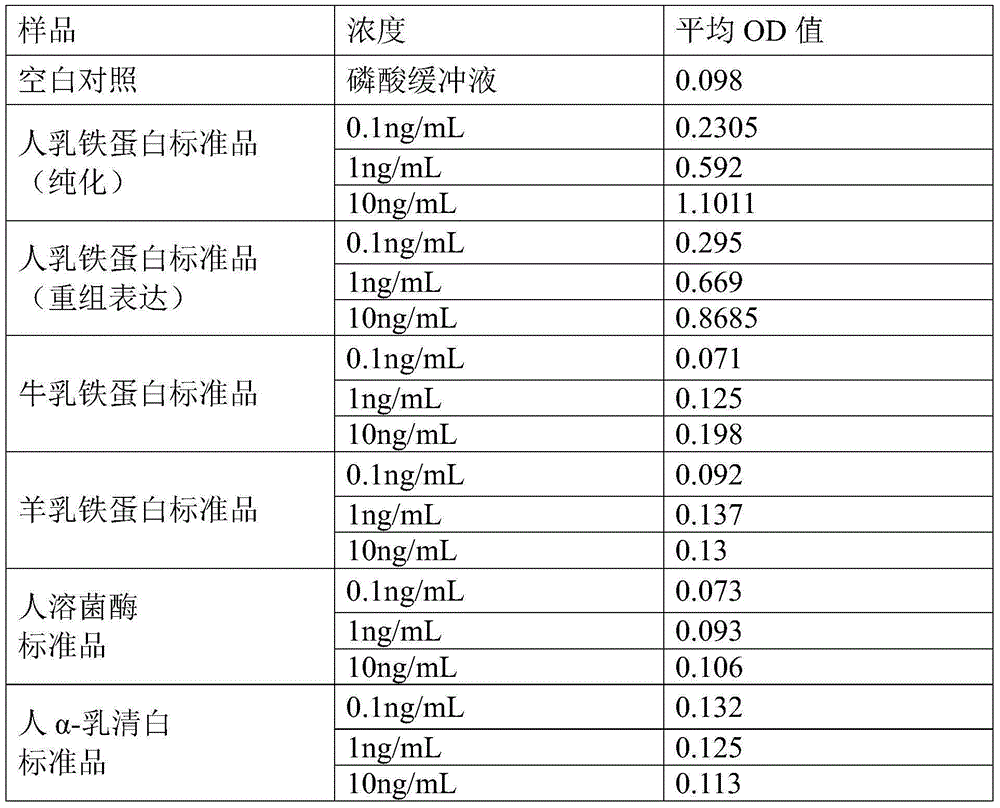
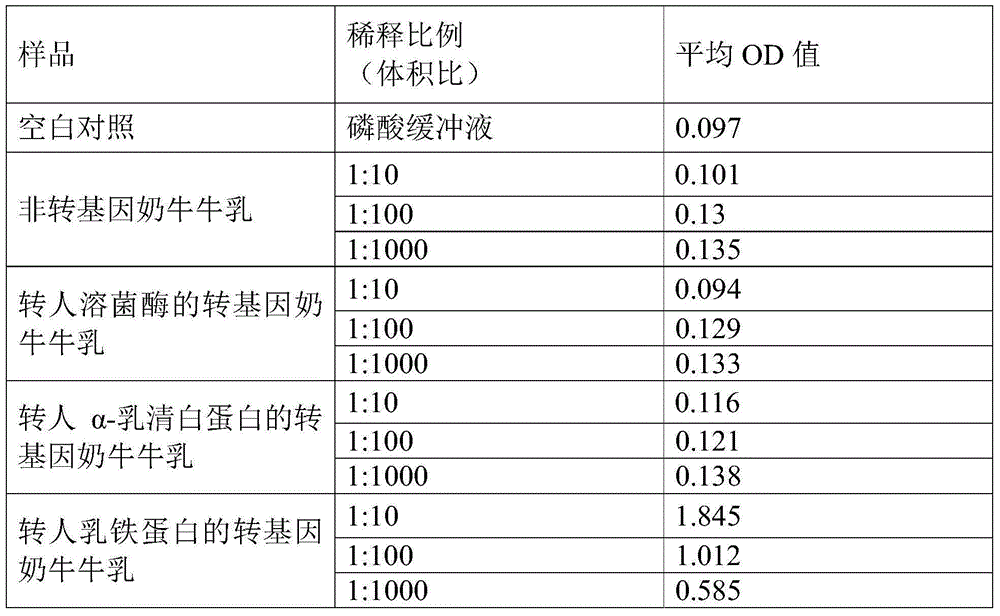
ActiveCN105158478ANo cross-reactivityIncreased sensitivityBiological testingElisa kitChromogenic Substrates
The invention discloses a human lactoferrins double-antibody sandwich ELISA kit. The kit comprises an enzyme-linked plate, a coating buffer, a first monoclonal antibody, a blocking solution, anenzyme-labelled second monoclonal antibody, a chromogenic substrate and a stopping solution; the first monoclonal antibody is secreted by a hybridoma cell strain with the preservation number of CGMCC NO. 10594; and the second monoclonal antibody is secreted by a hybridoma cell strain with the preservation number of CGMCC NO. 10595. According to the above technical scheme, detection on human lactoferrins can reach the sensitivity of 1 ng / mL by employing the double-antibody sandwich ELISA kit, cross reactions do not happen aiming at cattle lactoferrin and sheep lactoferrin, also cross reactions do not happen aiming at human lysozyme transgenic cattle and human alpha-whey alhumin transgenic cattle, and the double-antibody sandwich ELISA kit possesses relatively high sensitivity and specificity.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
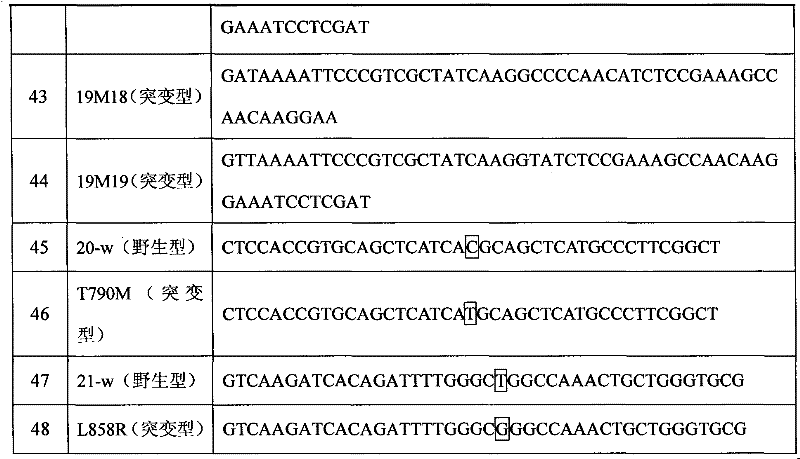
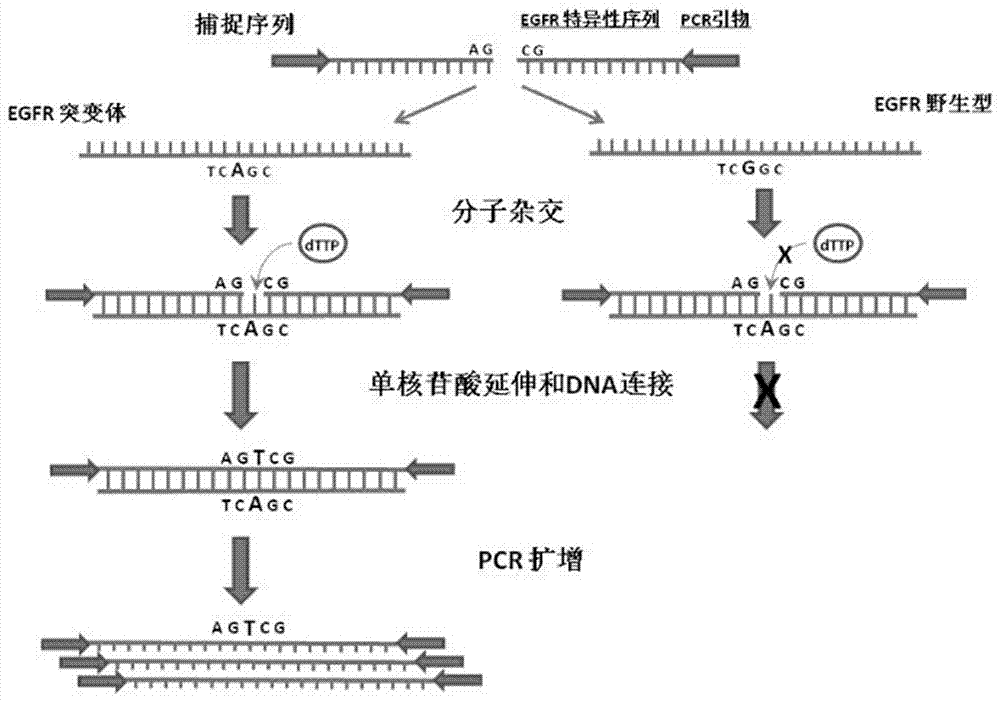
Method for efficiently detecting EGFR T790M mutant, probe and kit for detection
InactiveCN107513577AImprove stabilityAccurate distinctionMicrobiological testing/measurementDNA/RNA fragmentationEGFR T790MCancer therapy
The invention discloses a method for efficiently detecting EGFR T790M mutant, a probe and a kit for detection. According to the invention, the method for EGFR mutant capture primer hybridization, mononucleotide extension and DNA linkage is adopted for recognizing the EGFR T790M mutant in a sample, converting the EGFR T790M mutant into a capture sequence containing a mutation site and adopting the specific probe and PCR amplification specific to the mutation site for detecting the capture sequence with the mutation site, thereby detecting the existence of the EGFR T790M mutant in the sample. The invention also provides the probe and the kit for detecting according to the method; the detection according to the invention has the characteristics of high sensitivity, high specificity and simple operation; the method is suitable for the clinical examination of the trace EGFR T790M mutant in a blood sample of a tumor patient; new method and thought are supplied for personalized cancer therapy.
Owner:GNOMEGEN
TERT gene mutation detection specific primers and liquid chip
ActiveCN103451267AImprove signal-to-noise ratioImplement parallel detectionNucleotide librariesMicrobiological testing/measurementMutation detectionGenetics
Owner:SUREXAM BIO TECH
Primer for detecting alcohol metabolizing genes by aid of pyrosequencing joint sequencing methods and application of primer
ActiveCN106987623AGood specificityGood accuracyMicrobiological testing/measurementDNA/RNA fragmentationAlcohol dehydrogenasePyrosequencing
The invention discloses a primer for detecting alcohol metabolizing genes by the aid of pyrosequencing joint sequencing methods, and belongs to the technical field of biological detection. The primer comprises amplification primers shown as SEQ ID NO.1-4 and sequencing primers shown as SEQ ID NO.5-6. Biotin labeling is carried out by 5' ends of the primers shown as SEQ ID NO.1 and SEQ ID NO.3. The invention further discloses application of the primer to simultaneously detecting the polymorphism of SNP (single nucleotide polymorphism) loci rs671 of ALDH2 (acetaldehyde dehydrogenase 2) genes and SNP loci rs1229984 of ADH1B (alcohol dehydrogenase 1B) genes. The primer is high in detection throughput as compared with the traditional ordinary pyrosequencing with only single-SNP polymorphism detection capacity during sequencing reaction in each procedure, the detection time can be effectively shortened, and labor and the cost can be effectively reduced. Besides, the primer and the application have the advantages of accurate detection results, good specificity, high sensitivity, short detection cycle, simplicity in operation and capability of meeting clinical examination requirements.
Owner:石家庄迪安医学检验实验室有限公司
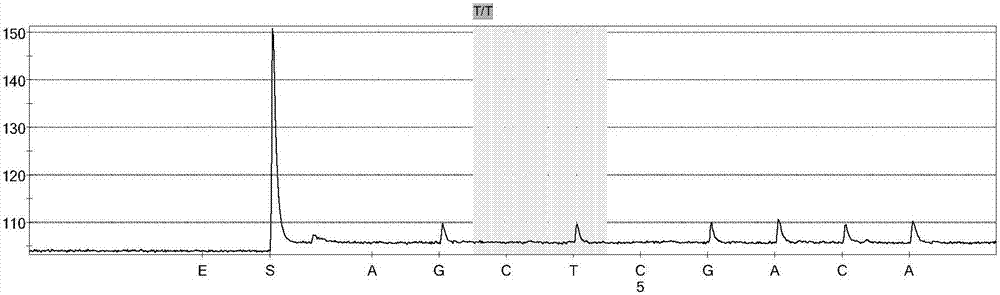
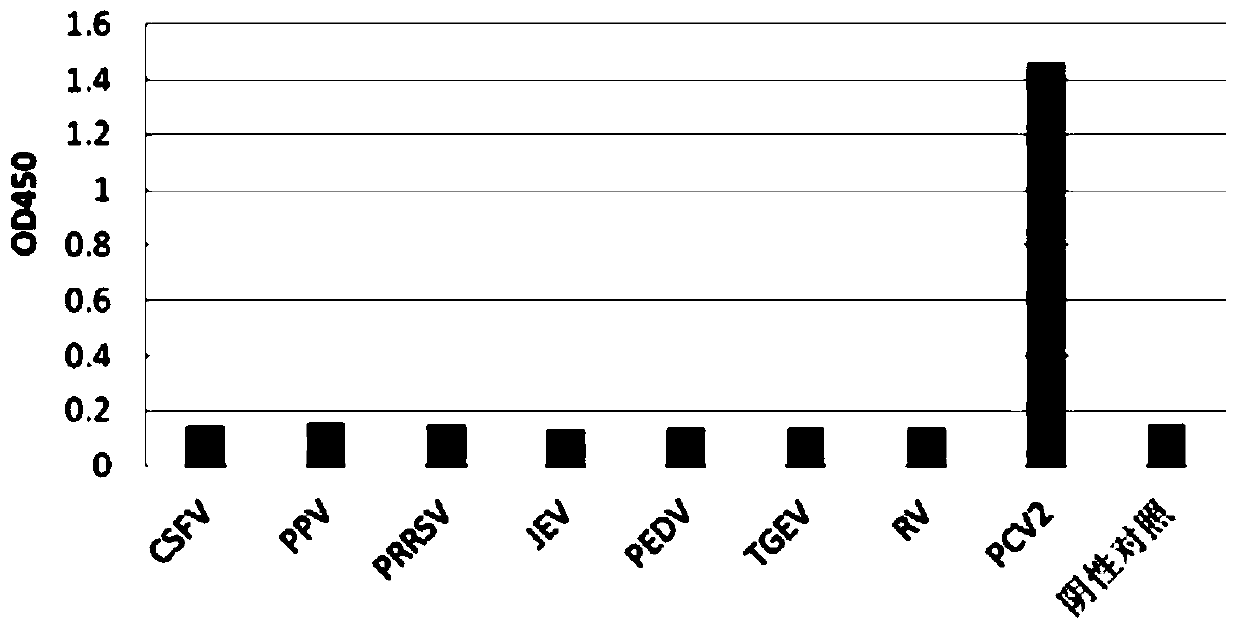
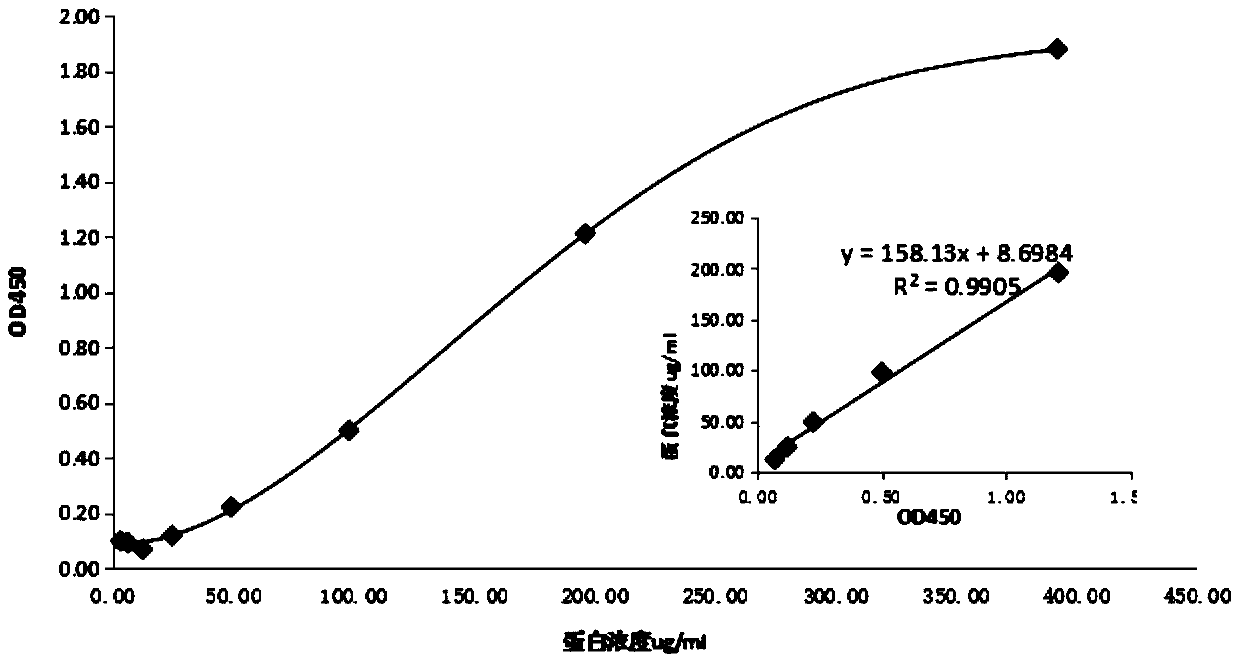
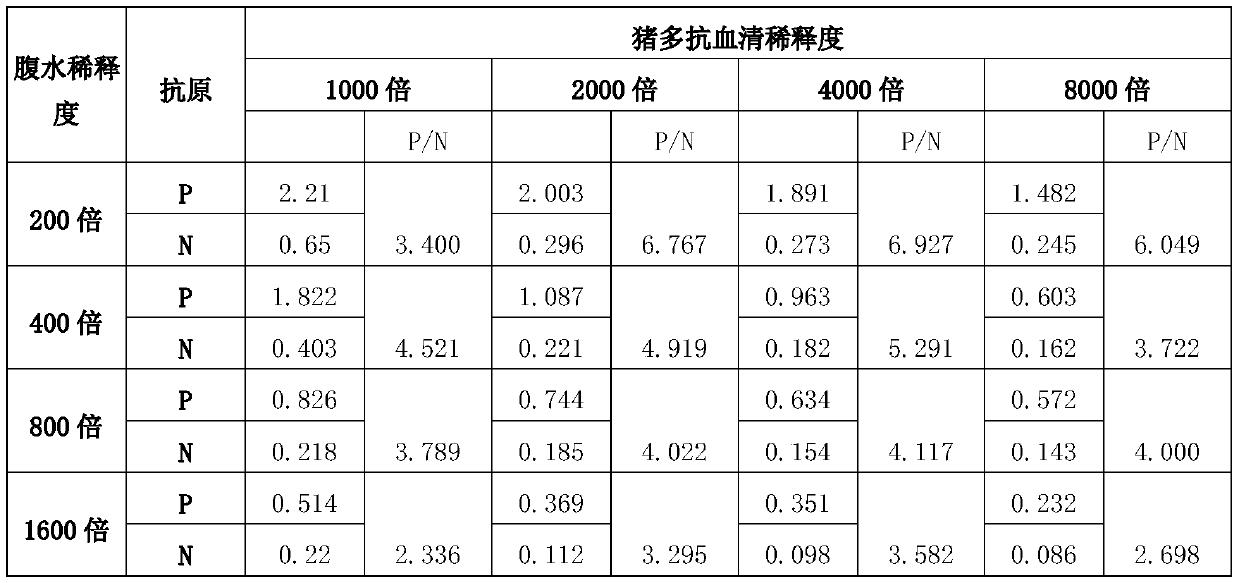
Porcine circovirus type 2 double antibody sandwich ELISA reagent kit and application thereof
InactiveCN109799351ANo cross reactivityGood reactogenicityBiological testingAntigenPorcine circovirus
The invention relates to a porcine circovirus type 2 double antibody sandwich ELISA reagent kit and a detection method. The reagent kit includes an enzyme label plate coated with a monoclonal antibodyagainst a porcine circovirus Cap protein, a blocking buffer, a primary antibody, an enzyme-labeled secondary antibody, concentrated washing liquid, a colored solution, a stopping solution, a standard, a positive contrast, and a negative contrast. The porcine circovirus type 2 double antibody sandwich ELISA has the advantages of short detection cycle, simple operation, high sensitivity, strong specificity and simple equipment requirements and can perform qualitative, semi qualitative and quantitative analysis. Moreover, the reagent kit can achieve rapid detection of large quantities of samples. The invention also provides an application of the reagent kit in detecting an antigen of a porcine circovirus type 2 inactivated vaccine.
Owner:TIANJIN RINGPU BIO TECH
Monoclonal antibody against GP73 protein, preparation method and application thereof
ActiveCN101735319BNo cross reactivityStrong specificityChemiluminescene/bioluminescenceImmunoglobulins against animals/humansMonoclonal antibodyChronic hepatitis
Owner:BEIJING HOTGEN BIOTECH CO LTD
Recombined expression plamid vector, expression antige of N gene of bubble cell stomatitis virus and preparation method
This invention relates to a diagnostic reagent used in preparing VSV N protein recombination antigen and its preparation method. The reagent includes its N gene recombination expression plasmid vector and its antigen. The preparation method includes 1) cloning the VSV coding group specific antigen N gene fragment to pMD18-T plasmid vectors to make up of N gene clone recombination plasmid, 2) sub-cloning plugging into the pBAD / Thio TOPO expression vector, 3, converting TOP10 cells, 4) screening positive clones getting SVS N reading code frame.
Owner:CHECKOUT & QUARANTINE TECH CENT YUNNAN ENTRY &EXIT CHECKOUT & QUARANTINE BUR
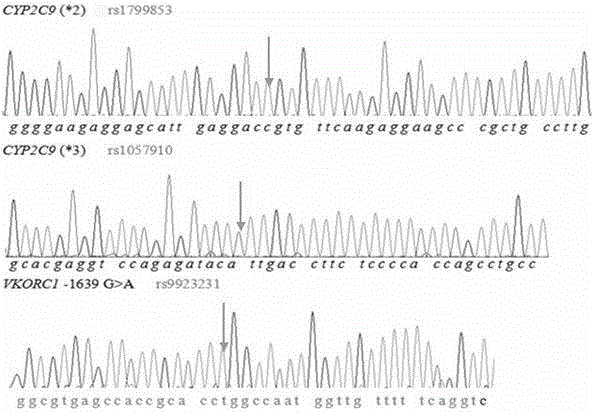
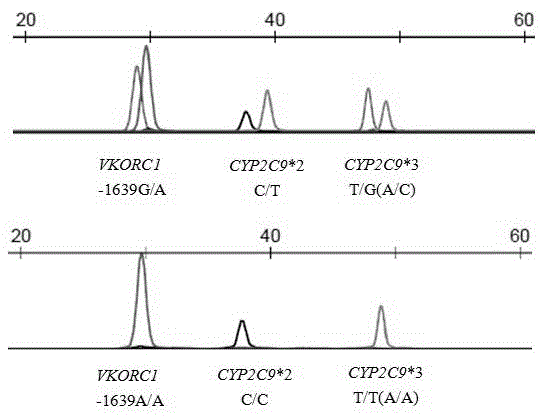
Primer and method for simultaneously detecting VKORC1 and CYP2C9 gene polymorphisms
InactiveCN104988223AStrong specificityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationVKORC1Specific detection
The invention belongs to the technical field of biological detection, and provides a primer for simultaneously detecting VKORC1 and CYP2C9 gene polymorphisms. The primer comprises a PCR amplification primer and a SNaPshot PCR primer. The primer can achieve specific detection on VKORC1 and CYP2C9 gene polymorphisms, causes no cross reaction and is good in accuracy.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT +1
Human lactoferrin monoclonal antibody pair
ActiveCN105131111ANo cross-reactivityIncreased sensitivityImmunoglobulins against animals/humansBiological testingSerum protein albuminBacteriolysin
The invention discloses a human lactoferrin monoclonal antibody pair, which is composed of a first monoclonal antibody and a second monoclonal antibody; wherein the first monoclonal antibody is secreted by a hybridoma cell strain with a preservation number of CGMCC No.10595, and the second monoclonal antibody is secreted by a hybridoma cell strain with a preservation number of CGMCC No.10594. The invention also provides an application method of the provided human lactoferrin monoclonal antibody pair in the preparation of a kit for detecting human lactoferrin. Through the technical scheme mentioned above, a double-antibody sandwich immunity test paper detection method is adopted to detect human lactoferrin, the sensitivity can reach 10 ng / mL; moreover, cross reaction does not happen between bovine lactoferricin and goat lactoferricin; cross section does not happen in human bacteriolysin transgene cattle and human alpha-serum albumin transgene cattle; and the sensitivity and specificity are high.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
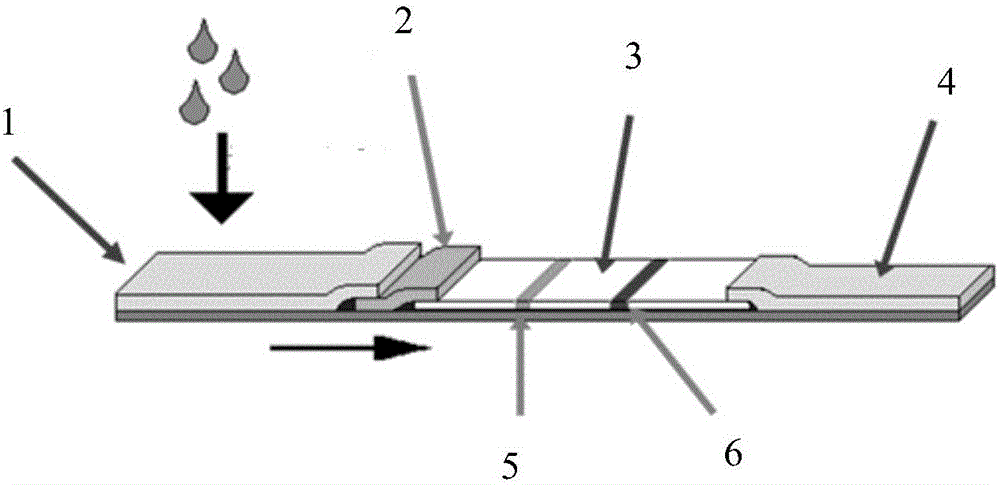
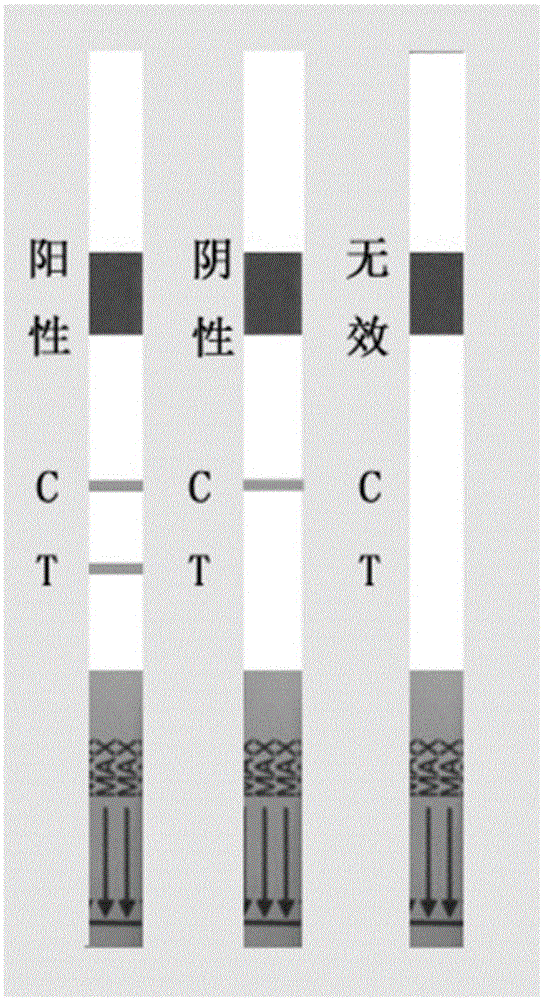
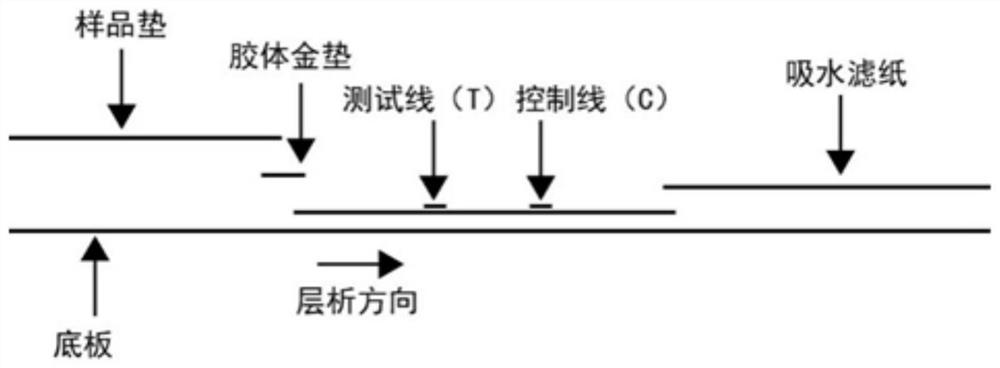
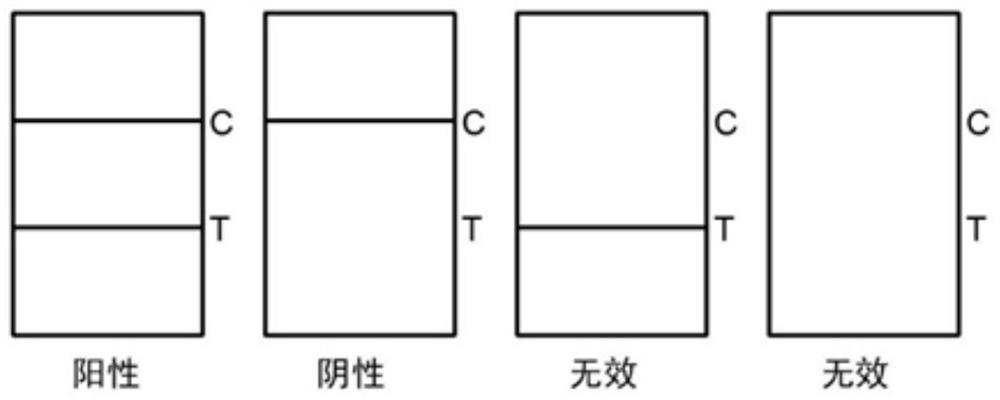
Colloidal gold immune test strip for detecting tomato brown rugose fruit virus and preparation method thereof
ActiveCN113564128ANo cross-reactivityAccurate detectionImmunoglobulins against virusesMicroorganism based processesCelluloseNitrocellulose
The invention discloses a colloidal gold immune test strip for detecting tomato brown fruit rugose fruit virus and a preparation method thereof. The test strip is formed by sequentially overlapping and sticking a sample pad, an immune colloidal gold pad, a nitrocellulose membrane and water-absorbing filter paper on a bottom plate. The colloidal gold marked by the anti-ToBRFV monoclonal antibody is coated on the colloidal gold pad, and the other anti-ToBRFV monoclonal antibody and the anti-mouse IgG secondary antibody are respectively coated on a detection (T) line and a control (C) line of the nitrocellulose membrane. The ToBRFV colloidal gold immune test strip disclosed by the invention can be used for accurately, specifically and sensitively detecting the ToBRFV in field tomatoes. According to the test strip, the detection result can be observed by naked eyes within 5 minutes, and the test strip is very suitable for large-batch sample detection in the field, has a very good application value in actual production, and provides material support for diagnosis, monitoring and early warning of ToBRFV.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
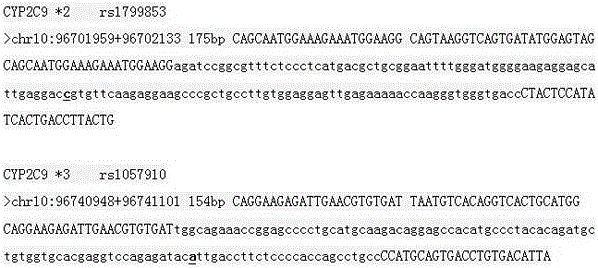
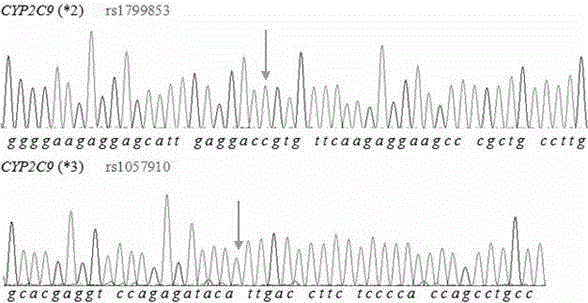
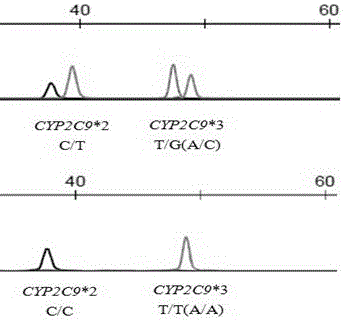
Primer and method for simultaneously detecting polymorphism of CYP2C*2 and CYP2C*3 genes
InactiveCN104988222AGood specificityGood accuracyMicrobiological testing/measurementDNA/RNA fragmentationMolecular biologyBioinformatics
The invention provides a primer for simultaneously detecting polymorphism of CYP2C*2 and CYP2C*3 genes and belongs to the technical field of biological detection. The primer comprises a PCR amplification primer and a SNaPshot PCR primer. By the adoption of the primer, specific detection of polymorphism of the CYP2C*2 and CYP2C*3 genes can be achieved, cross reaction does not occur, and accuracy is good.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com