Patents
Literature
233 results about "False Negative Result" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A false negative is a test result that indicates a person does not have a disease or condition when the person actually does have it, according to the National Institute of Health (NIH).
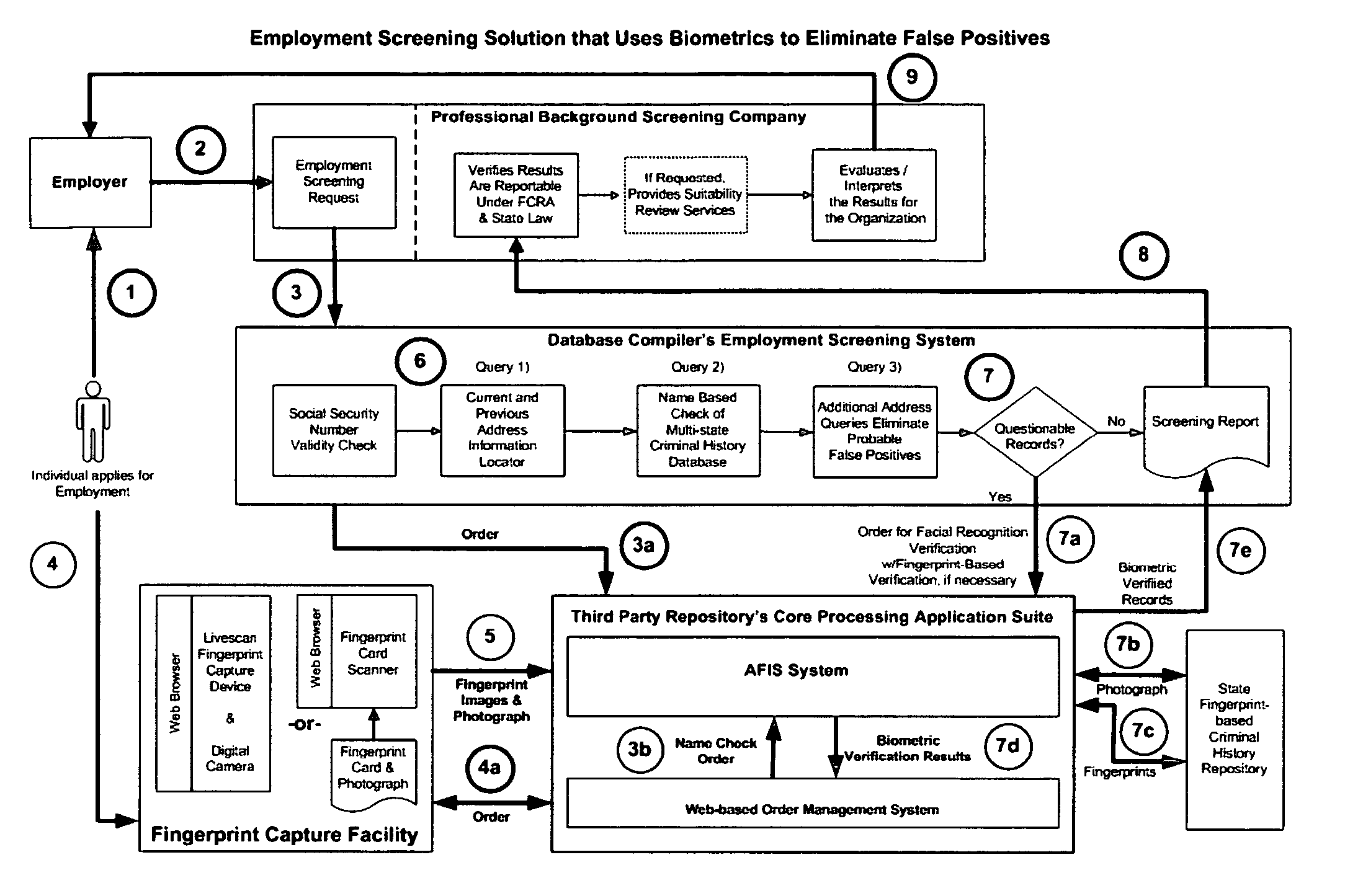
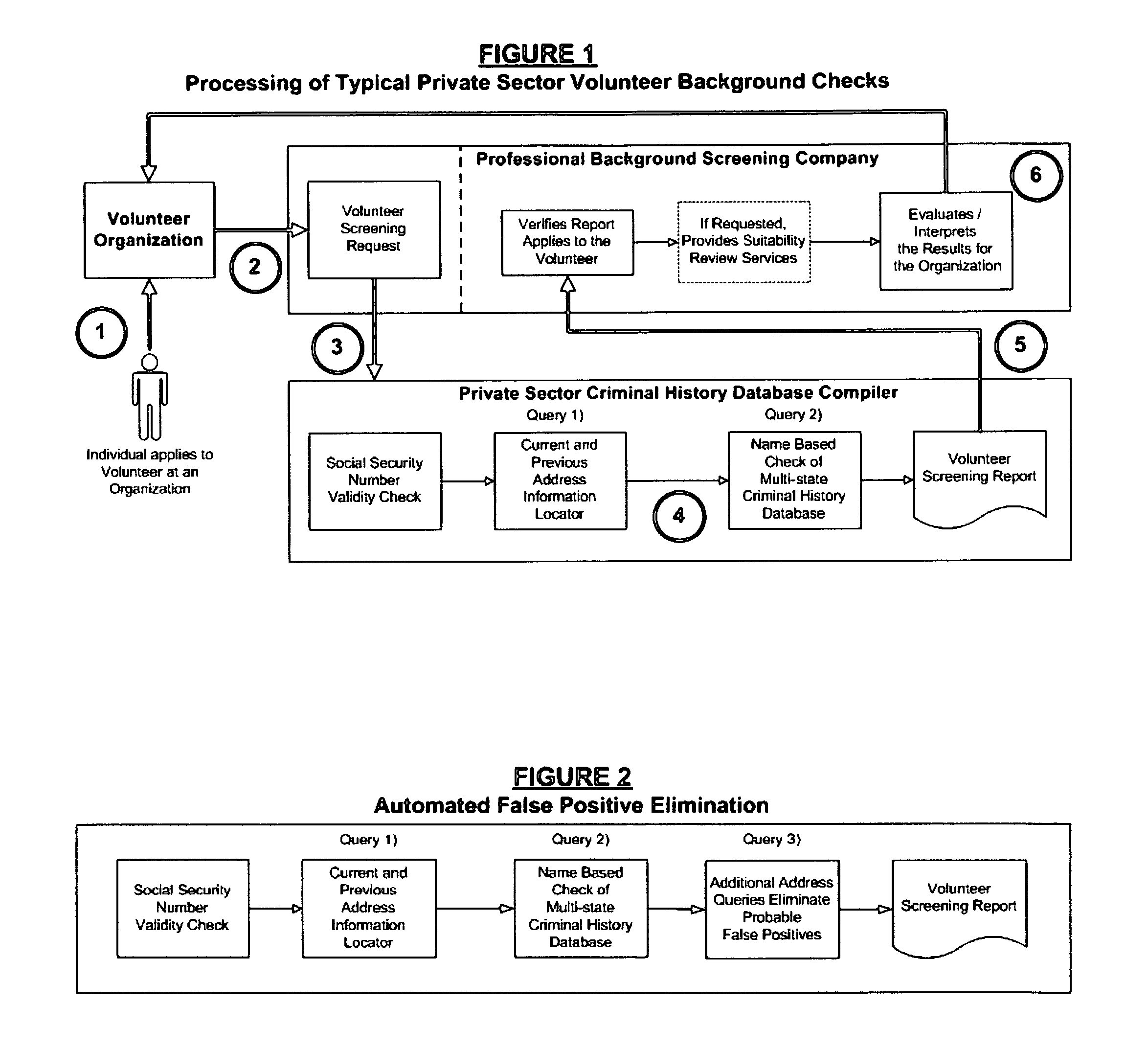
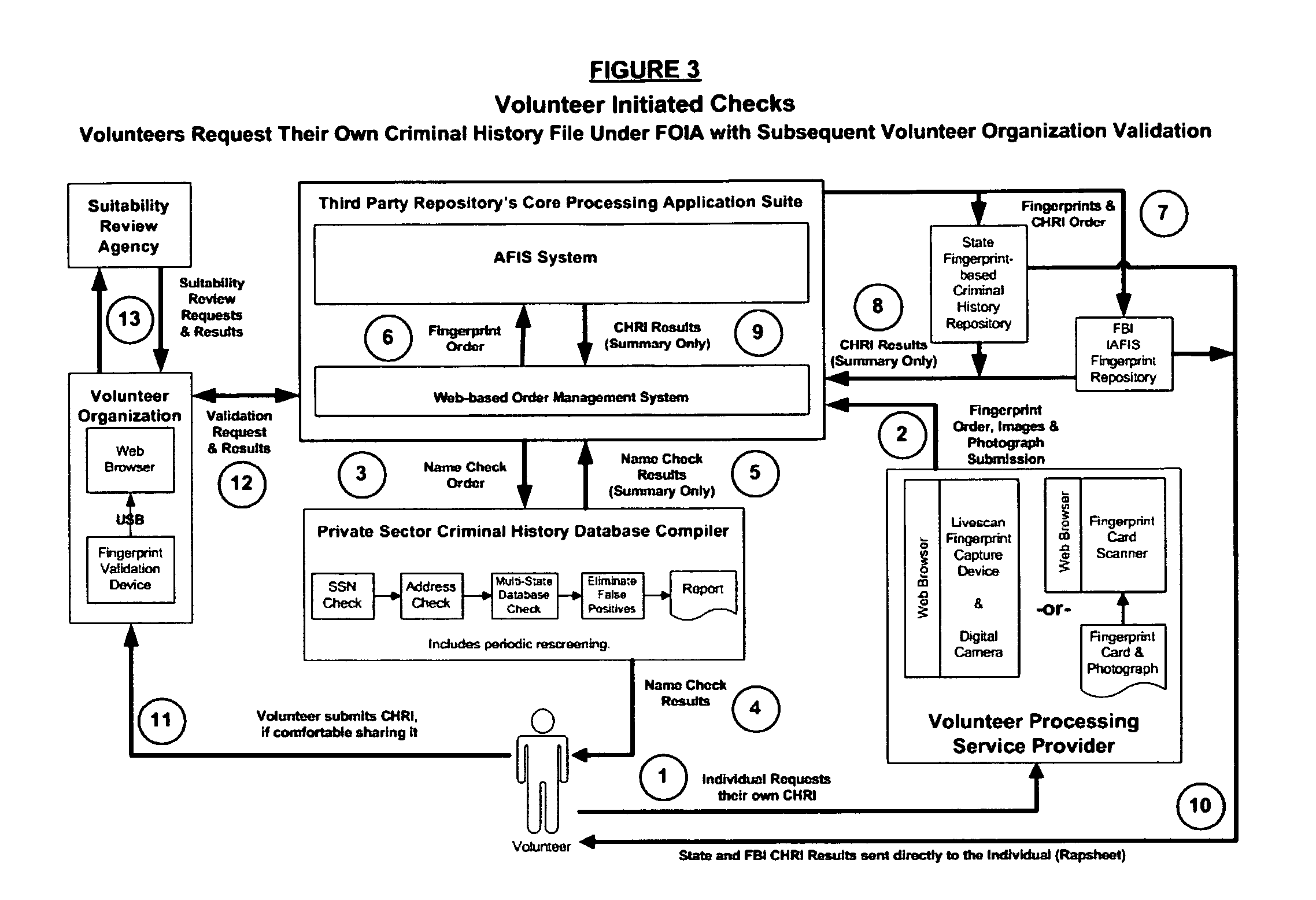
Biometric-supported name-based criminal history background checks
InactiveUS20060018520A1Improve recognition accuracyEasy to useCharacter and pattern recognitionOffice automationThird partyBiometric data
A method and apparatus for conducting biometric-supported name-based criminal history background investigations is disclosed as a means of eliminating both false-positive and false-negative results. Each of the embodiments disclosed employ the cooperative efforts of a professional background screening company, a database compiler, a trusted independent third-party evaluator of biometric data, and at least one government criminal history repository for providing more accurate screening reports for employers.
Owner:NAT BACKGROUND DATA
High-throughput diagnostic assay for the human virus causing severe acute respiratory syndrome (SARS)
InactiveUS20050181357A1Rapid and reliable diagnostic assayHigh detection sensitivitySugar derivativesMicrobiological testing/measurementDiagnostic testPresent method
The present invention relates to a high-throughput diagnostic assay for the virus causing Severe Acute Respiratory Syndrome (SARS) in humans (“hSARS virus”). In particular, the invention relates to a high-throughput reverse transcription-PCR diagnostic test for SARS associated coronavirus (SARS-CoV). The present assay is a rapid, reliable assay which can be used for diagnosis and monitoring the spread of SARS and is based on the nucleotide sequences of the N (nucleocapsid)-gene of the hSARS virus. The present method eliminates false negative results and provides increased sensitivity for the assay. The invention also discloses the S (spike)-gene of the hSARS virus. The invention further relates to the deduced amino acid sequences of the N-gene and S-gene products of the hSARS virus and to the use of the N-gene and S-gene products in diagnostic methods. The invention further encompasses diagnostic assays and kits comprising antibodies generated against the N-gene or S-gene product.
Owner:VERSITECH LTD
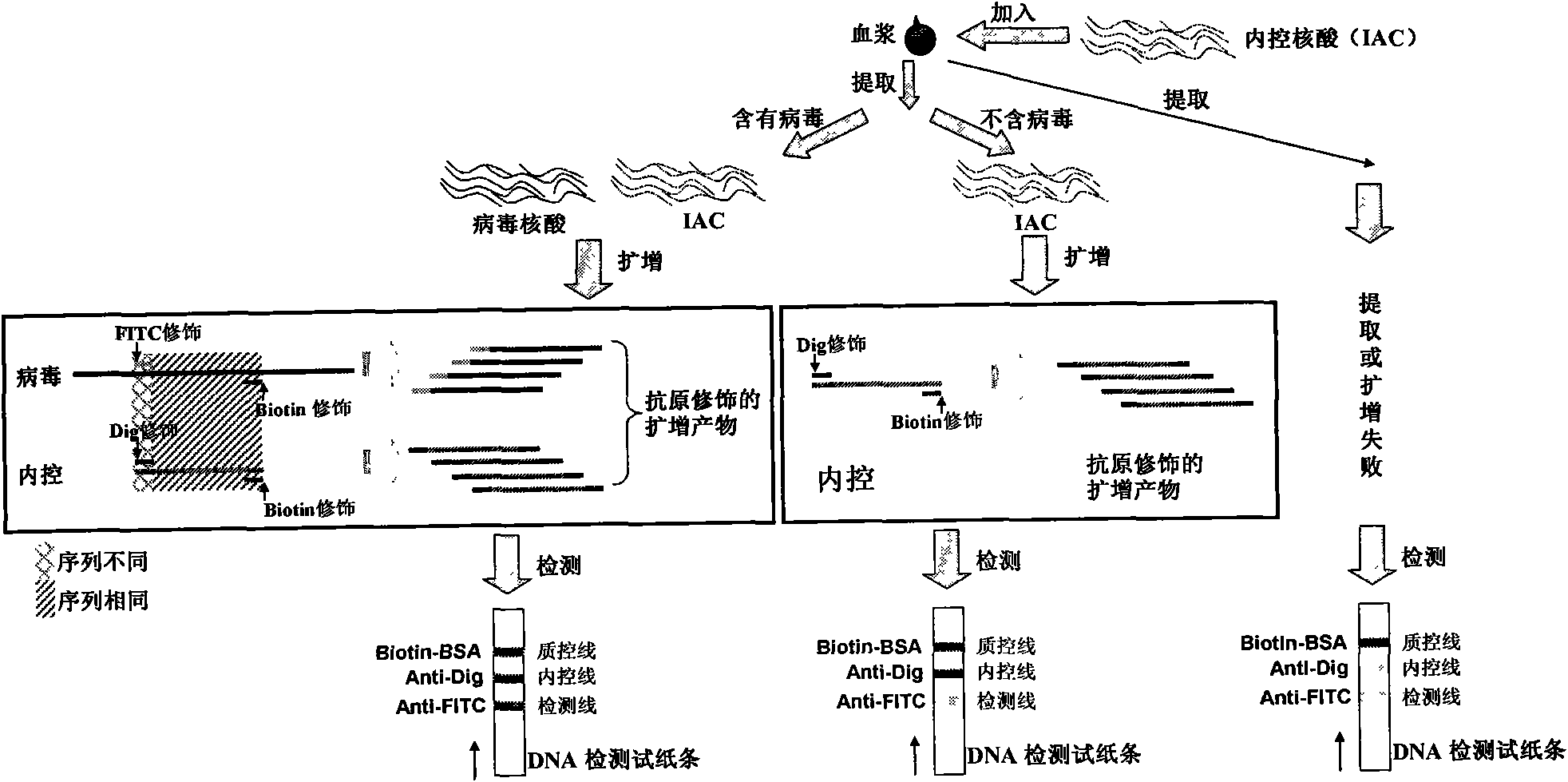
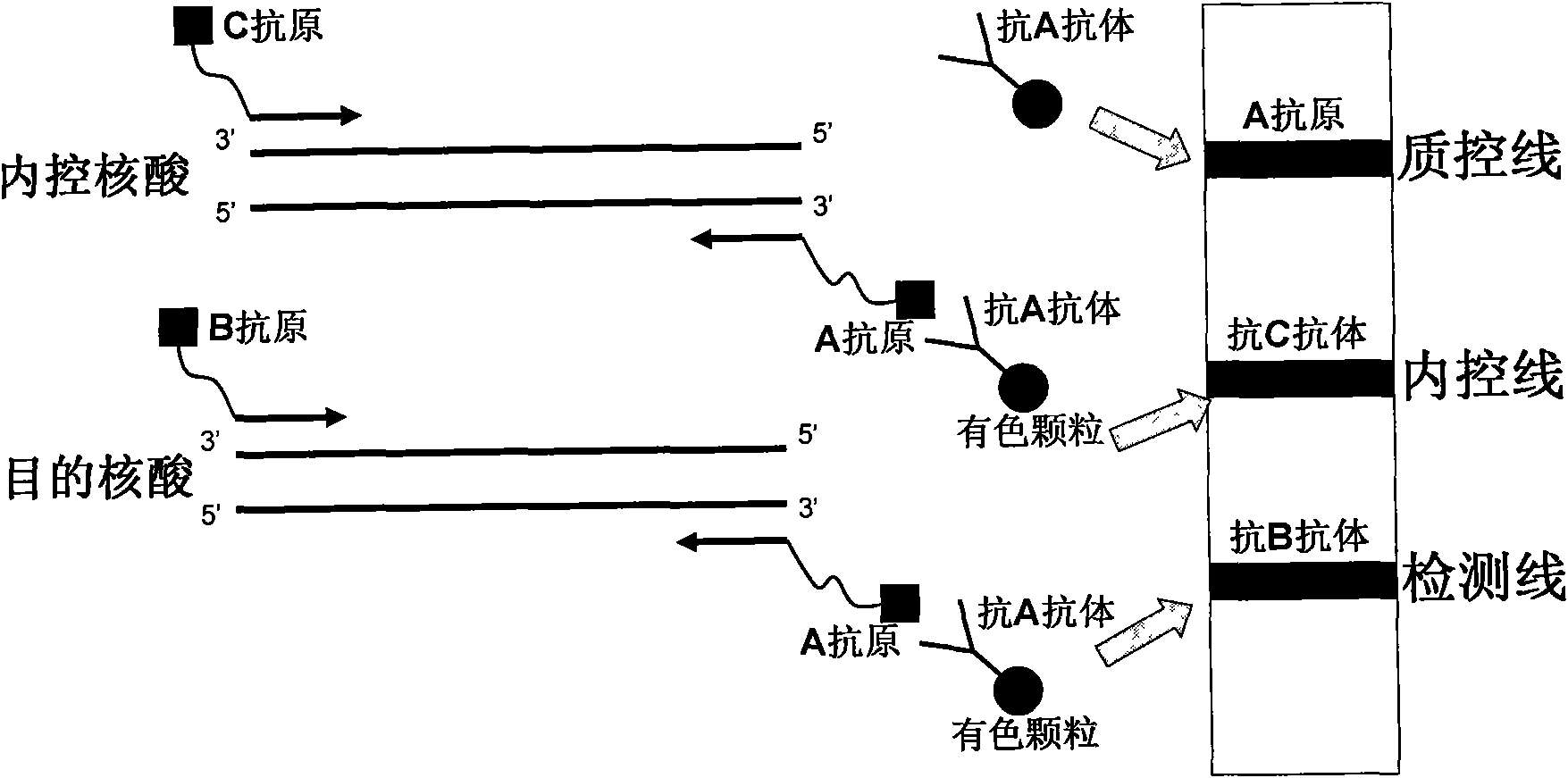
Method for semi-quantitatively detecting pathogenic nucleic acid by adding internal control nucleic acid
InactiveCN101957373AAvoid diagnostic problems that are prone to false negativesAvoid problems prone to false negativesMicrobiological testing/measurementMaterial analysisTest sampleQuality control
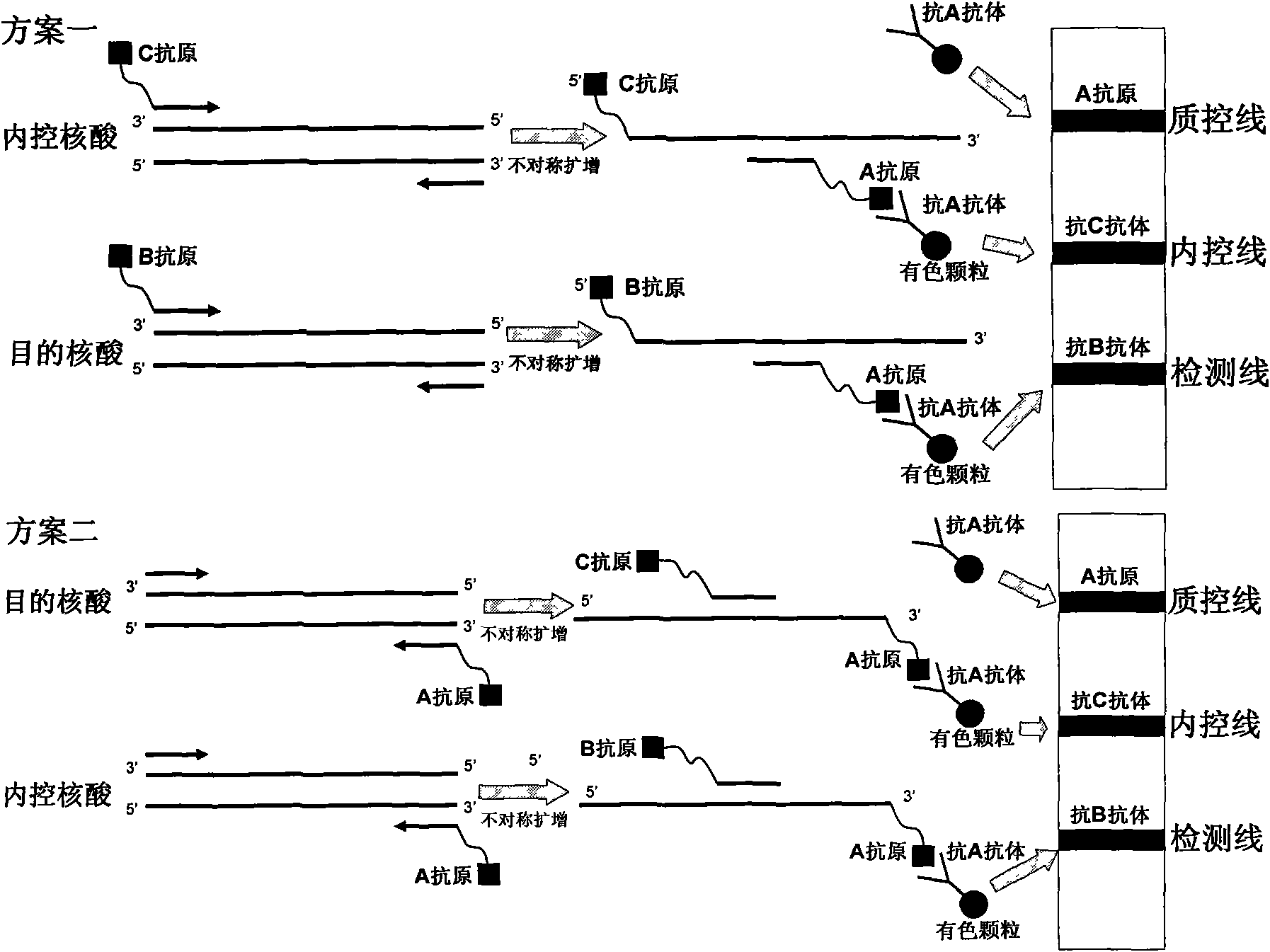
The invention belongs to the field of nucleic acid detection and discloses a method for semi-quantitatively detecting pathogenic nucleic acid by adding internal control nucleic acid. Corresponding internal control is added in the whole process of extracting and amplifying target nucleic acid and testing by using a test paper, so that the internal control and a target segment are parallelly operated, and the semi-quantitative detection is performed finally through color development and intensity contrast of three strips, namely a detection line, an internal control line and a quality control line on the test paper. In the method, in the whole process of processing the target nucleic acid, the corresponding internal control is taken as a positive contrast, and false negative results due to links such as extraction, amplification or sample application errors are avoided in the processing of detecting by using the test paper. Meanwhile, by comparing color development intensity of the internal control line and a sample line and introducing the semi-quantitative function on the basis of the qualitative function of the immunochromatographic test paper to estimate the copy number of tested samples, the detection results are more detailed, accurate and reliable. The method has the advantages of convenient and quick operation and capacity of meeting the actual clinical requirement.
Owner:HUADONG RES INST FOR MEDICINE & BIOTECHNICS
Method for improving the accuracy of the semi-quantitative determination of analyte in fluid samples
InactiveUS6306660B1Microbiological testing/measurementBiological testingAnalyteQuantitative determination
Disclosed is an improved method for determining the concentration of a first analyte in a fluid test sample as a function of a second analyte also present in the sample whose concentration in the fluid sample is clinically related to that of the first analyte. The method involves determining the concentration of the first analyte, and, if this concentration is outside of its useful analytical range, dividing this concentration by the normal concentration of the second analyte. This method of ratioing the concentrations of the first and second analyte is advantageous because accuracy is increased with fewer false positive and false negative results being reported.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
High-throughput diagnostic assay for the human virus causing severe acute respiratory syndrome (SARS)
InactiveUS7547512B2Rapid and reliable diagnostic assayHigh detection sensitivitySugar derivativesMicrobiological testing/measurementPresent methodSevere acute respiratory syndrome
The present invention relates to a high-throughput diagnostic assay for the virus causing Severe Acute Respiratory Syndrome (SARS) in humans (“hSARS virus”). In particular, the invention relates to a high-throughput reverse transcription-PCR diagnostic test for SARS associated coronavirus (SARS-CoV). The present assay is a rapid, reliable assay which can be used for diagnosis and monitoring the spread of SARS and is based on the nucleotide sequences of the N (nucleocapsid)-gene of the hSARS virus. The present method eliminates false negative results and provides increased sensitivity for the assay. The invention also discloses the S (spike)-gene of the hSARS virus. The invention further relates to the deduced amino acid sequences of the N-gene and S-gene products of the hSARS virus and to the use of the N-gene and S-gene products in diagnostic methods. The invention further encompasses diagnostic assays and kits comprising antibodies generated against the N-gene or S-gene product.
Owner:VERSITECH LTD
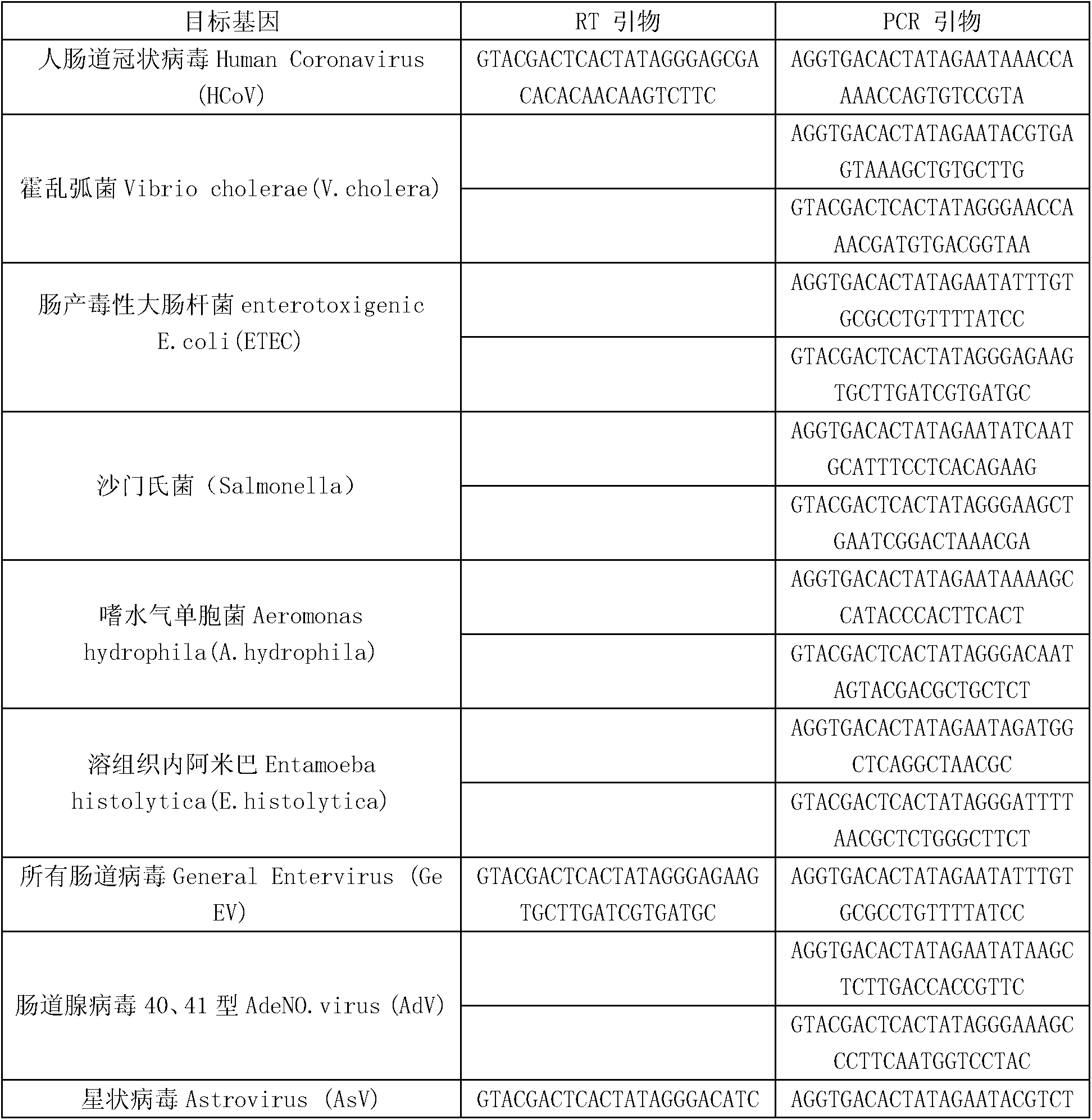
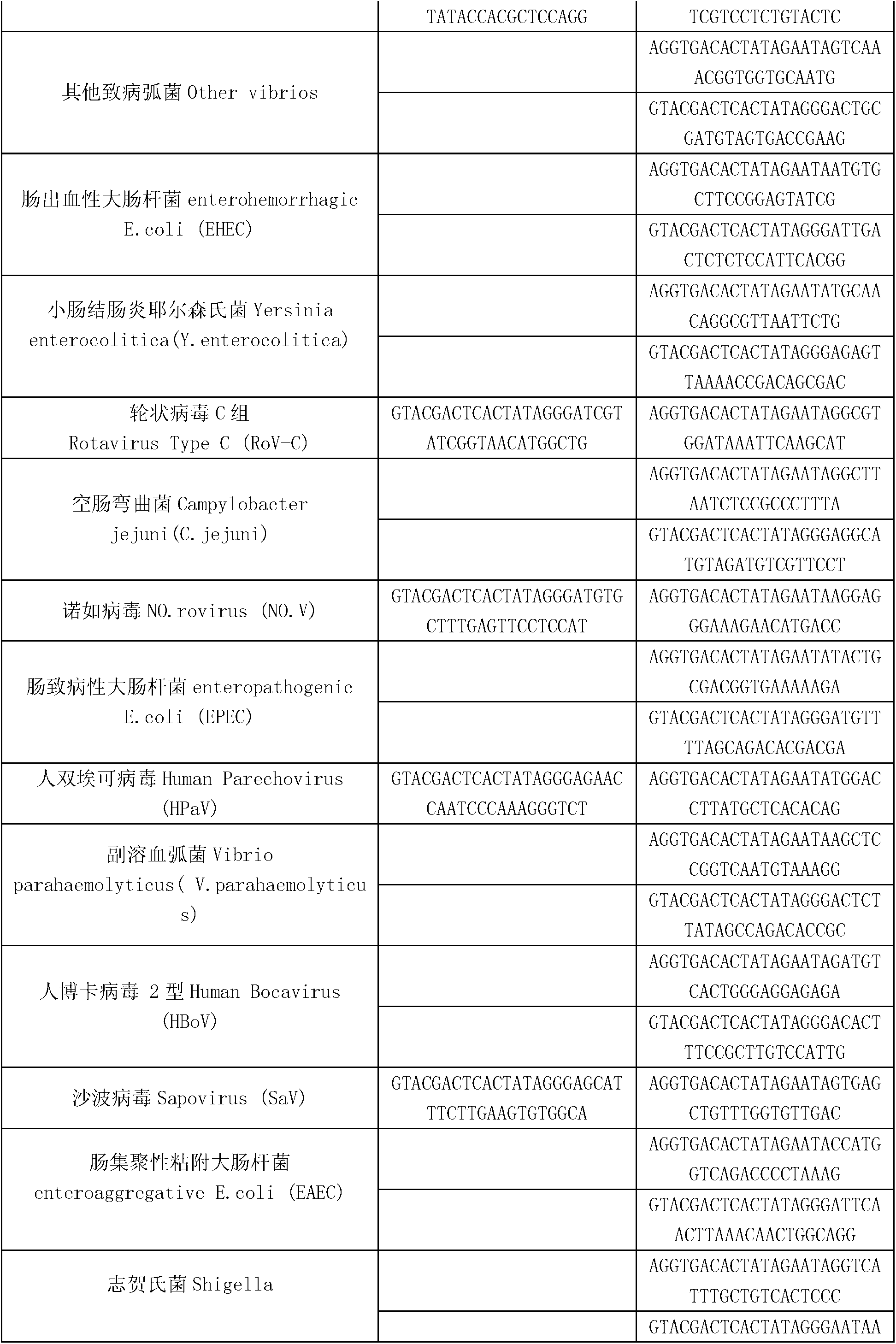
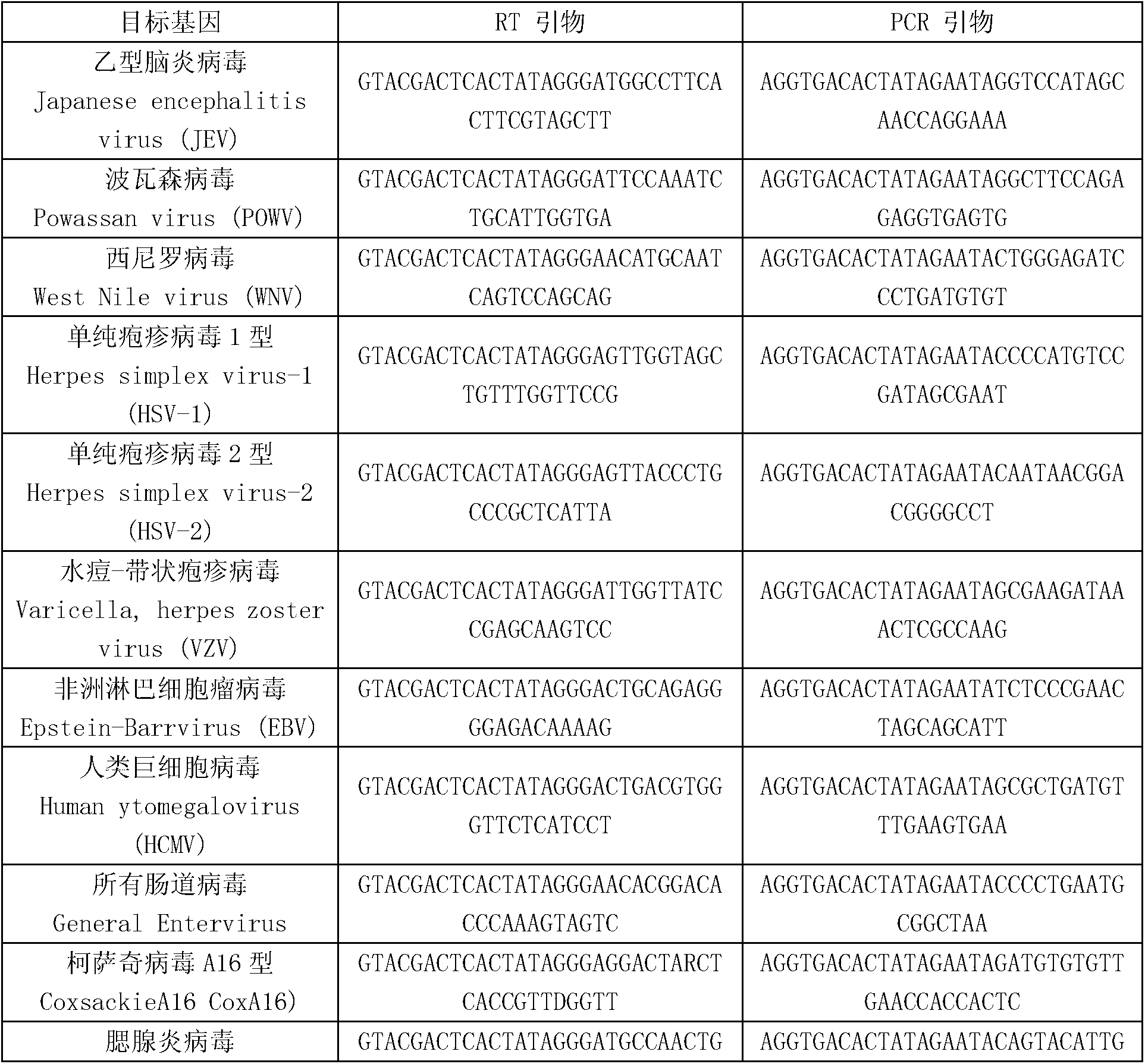
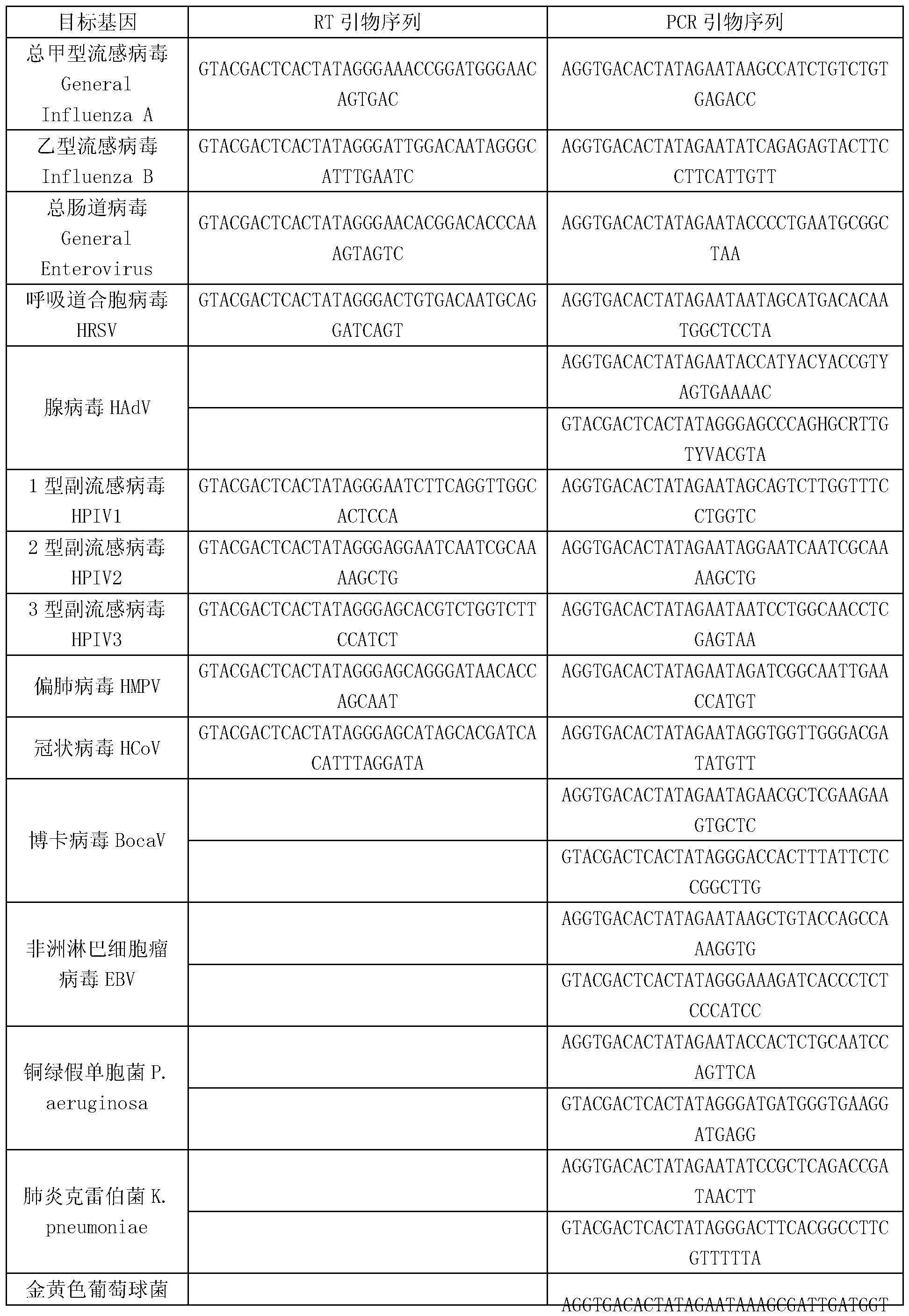
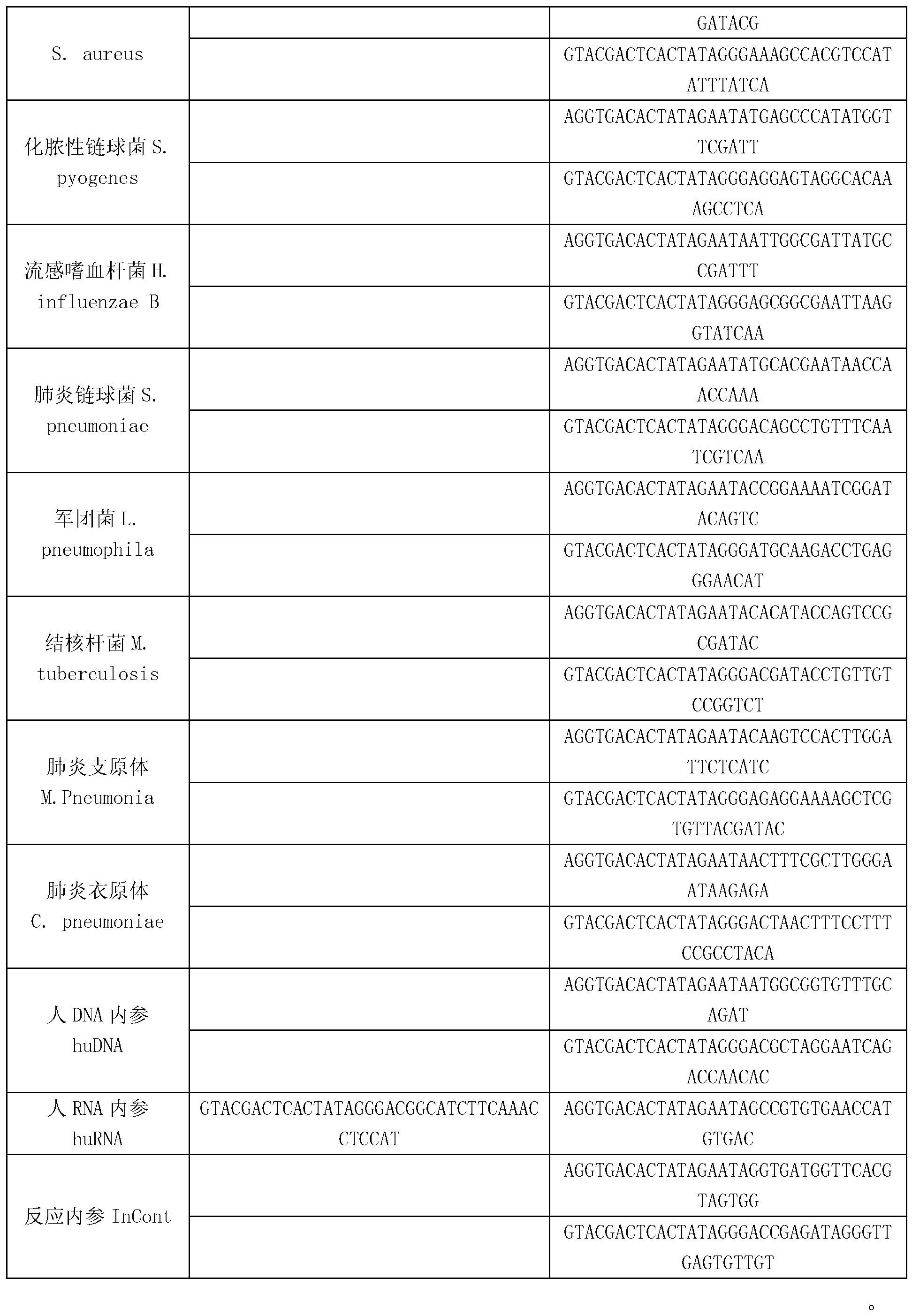
Kit for synchronously detecting thirty diarrhea pathogens and detection method of kit
ActiveCN103074450AStrong specificityImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
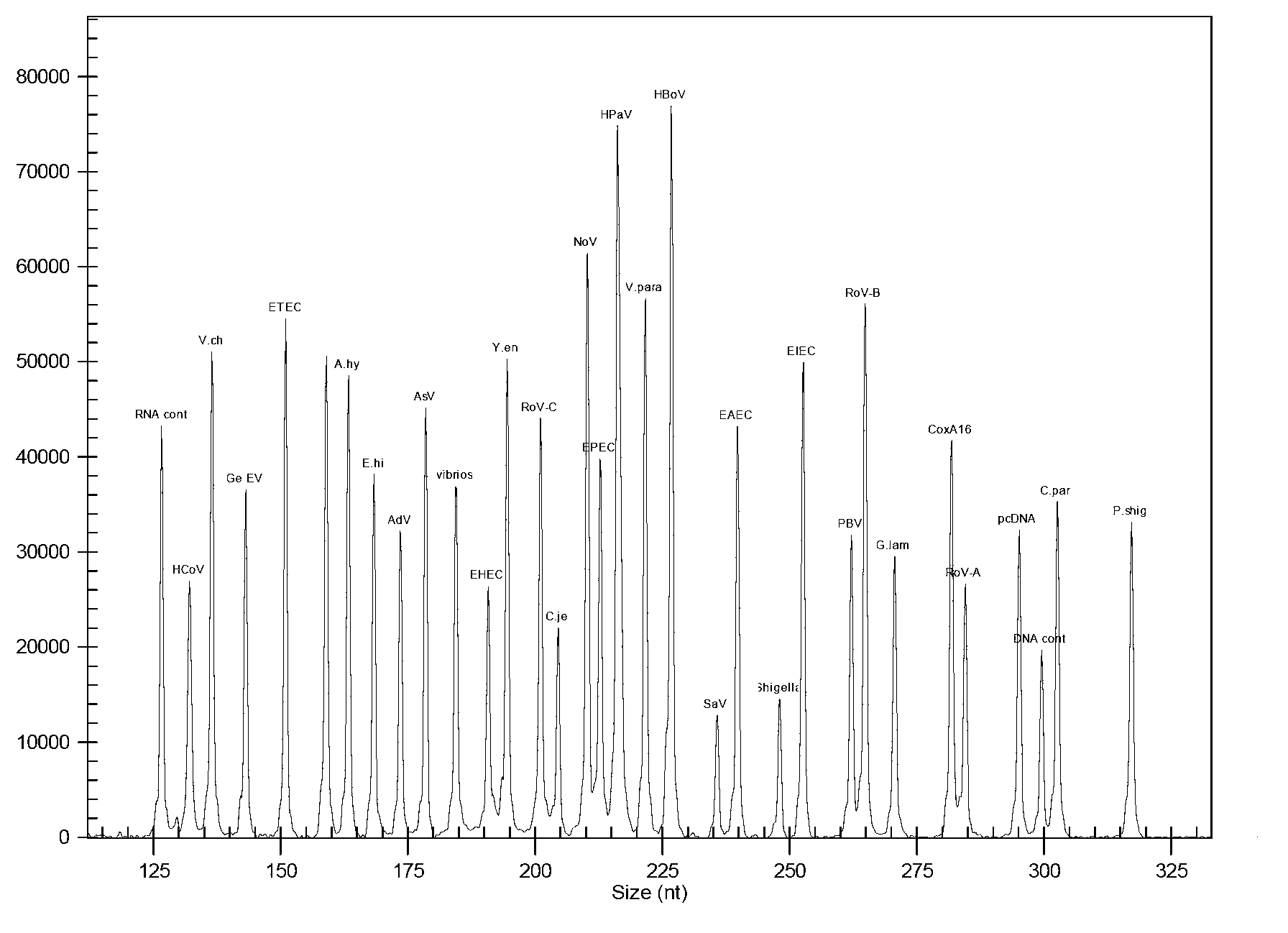
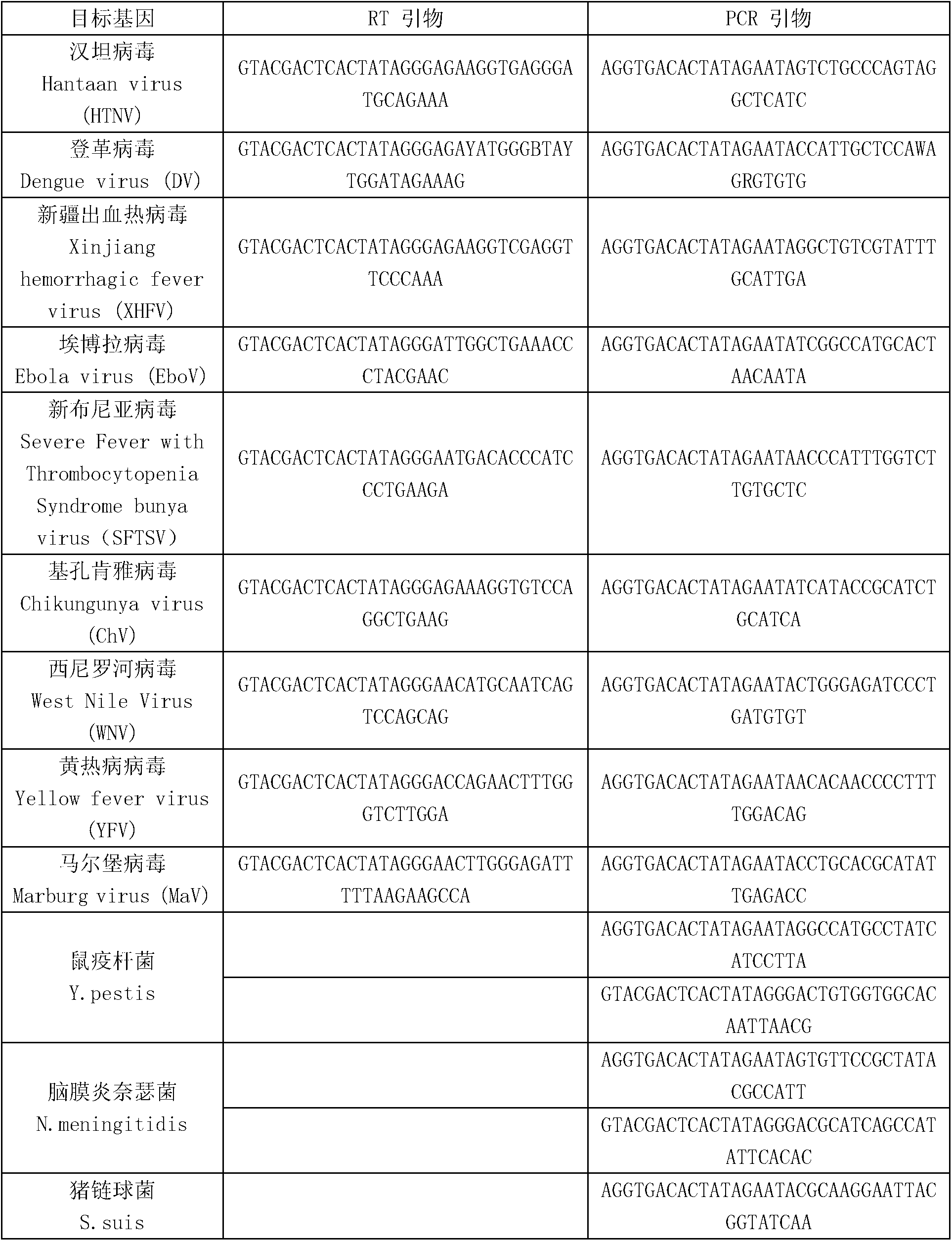
The invention discloses a kit for synchronously detecting thirty diarrhea pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of eleven diarrhea RNA viruses and a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-12 (sequence identifier number 1-12), and the PCR primer comprises forward and reverse PCR amplification primers of the rest nineteen diarrhea pathogens, a human DNA internal reference and a reaction internal reference, and PCR amplification primers of eleven diarrhea RNA viruses and the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 13-66. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Quantitative detection kit of hepatitis B virus (HBV) nucleic acid
ActiveCN103642941AAvoid PCR false negativesHighly conservativeMicrobiological testing/measurementMagnetic beadReference product
The invention discloses a quantitative detection kit of a hepatitis B virus (HBV) nucleic acid applied to the field of biomedical clinic diagnosis. The kit comprises a paramagnetic particle method extraction kit and an HBV nucleic acid amplification kit, wherein the paramagnetic particle method extraction kit comprises a pyrolysis binding solution, a rinsing solution, an eluant and magnetic bead liquid; the HBV nucleic acid amplification kit comprises an HBV-PCR (Polymerase Chain Reaction) reaction solution, an enzyme mixed solution, an HBV-interior label, HBV quantitative reference products 1-4, a negative quality product, a clinical positive quality product and a strong positive quality product. The quantitative detection kit is simple, convenient and fast in operation, low in cost, high in detection sensitivity, good in repeatability, high in conservative property of primer and probe, and strong in specificity, and covers different subtypes or variants of the hepatitis B virus, improvement of the accuracy and the specificity of the hepatitis B detection is facilitated, an efficient interior label system is led in, the problems such as reciprocal inhibition, interference and the like caused by simultaneous amplification of a target gene and the interior label are solved, the overall PCR amplification process can be effectively monitored, and a false negative result is avoided.
Owner:东北制药集团辽宁生物医药有限公司
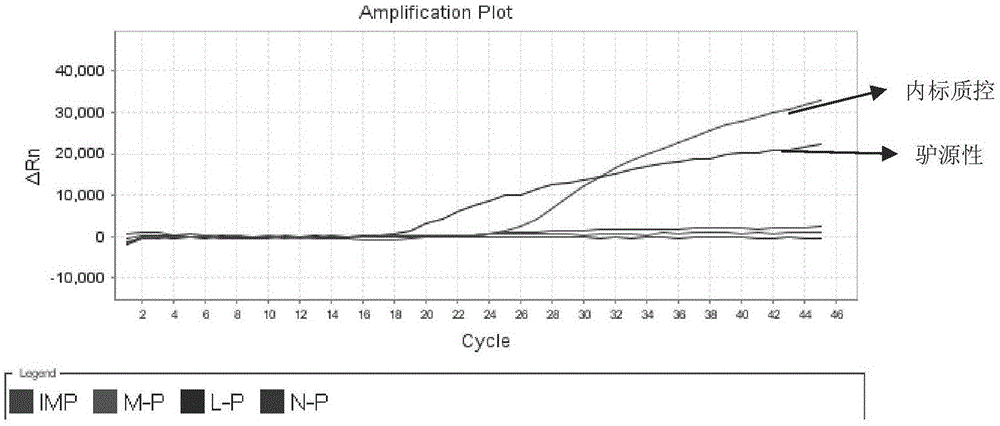
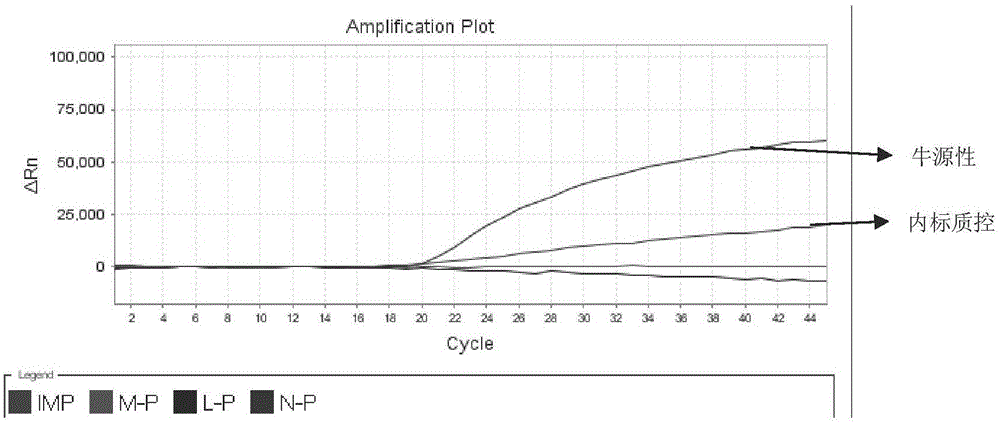
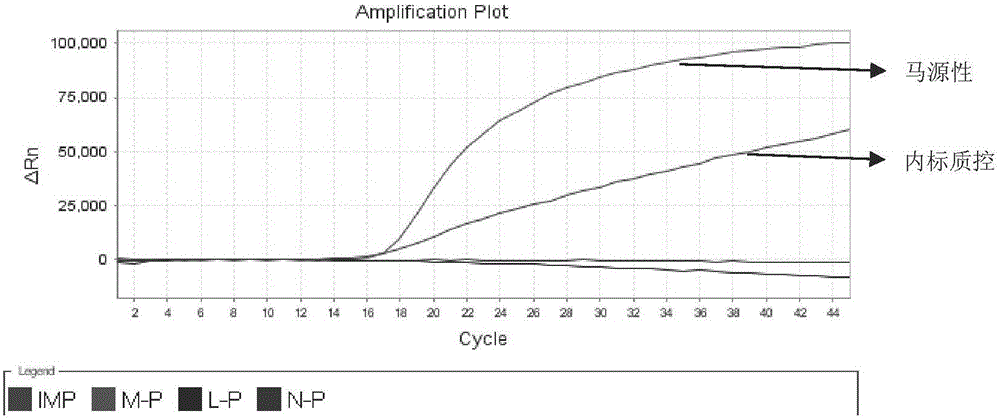
Detection kit for quickly identifying donkey skin, horse skin and mule skin
ActiveCN104046700AIncrease productionHigh purityMicrobiological testing/measurementCreatine kinaseFluorescence
The invention discloses a detection kit for quickly identifying donkey skin, horse skin and mule skin, which is used for accurately identifying donkeys, horses, mules and jennets by creatively applying 16SrRNA genes of CKM (creatine kinase) karyogene (nDNA) and mitochondrial genome DNA (mtDNA) at the same time from genetic background of the horses, the donkeys and the mules. The kit adopts an molecular beacon probe (MB) method to carry out a quintuple multicolor fluorescence quantitative PCR (polymerase chain reaction) detection technology, and also can detect donkey, horse, mule and jennet components; and moreover, the kit is added with exogenous internal reference as an interior label, can detect and avoid false negative results produced by PCR inhibitor contained in a sample. The quintuple multicolor fluorescence quantitative PCR is used for detecting in the same tube without opening a cover, so that the detection kit is not easy to pollute, and is accurate and stable, simple to operate, extremely high in sensitivity, strong in specificity, and the like, and therefore, a novel way is explored for identifying the donkey skin, horse skin and mule skin.
Owner:VEGETABLE RES INST OF SHANDONG ACADEMY OF AGRI SCI
Method of polymerase chain reaction with ultra-low denaturing temperatures and applications thereof
The invention relates to a method polymerase chain reaction (PCR) and the application thereof A method of PCR performed at ultra-low denaturing temperatures is provided. The denaturing temperatures of the templates adopted are 93-98° C. in the primary 2-3 cycles, and 60-87° C. in the follow-up cycles, those are much lower than 94-96° C., the conventional denaturing temperatures. It is found in the experiment that this method could not only become a universally applied PCR, but also control the reaction specificity by the template selection at ultra-low temperatures. The method possesses unique functions in excluding non-specific amplified products and false-negative results, excluding false-positivity brought about by the contaminants in products and discriminating genomic DNA from cDNA.
Owner:XU DINGBANG +3
African swine fever virus nucleic acid amplification primer, detection method and kit
InactiveCN101921878AQuick checkEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationGold particlesHybrid compound
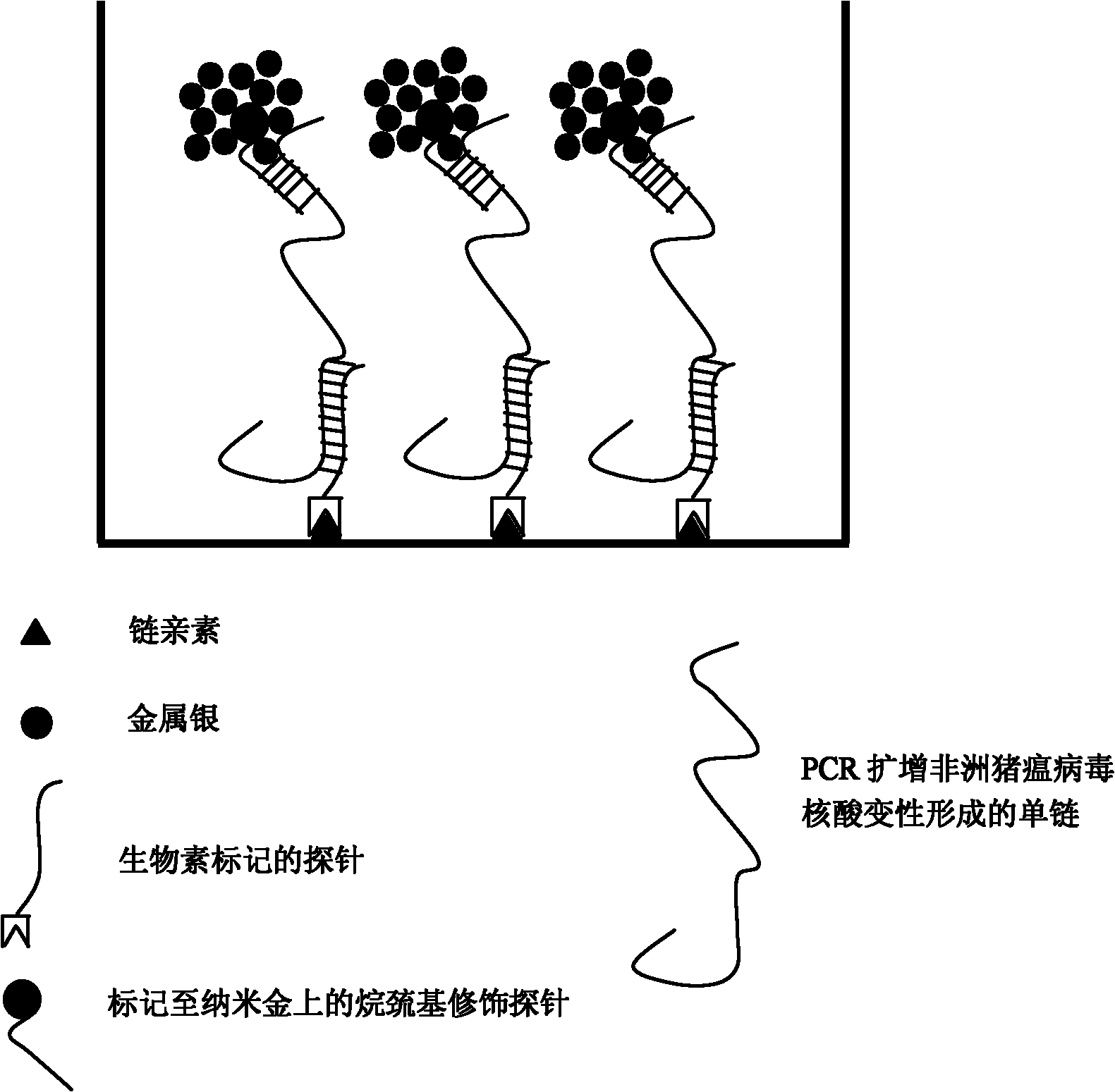
The invention relates to an African swine fever virus nucleic acid amplification primer, a detection method and a kit. The primer pair and the specific nucleic acid probe are selected from SEQ ID NO: 1-12. The detection method comprises the following steps: (a) amplifying nucleic acid in the sample to be detected by using a PCR primer; (b) labeling the detection probe labeled by alkyl sulfydryl group on nano gold particles; (c) hybridizing the capture probe labeled by biotin and the detection probe labeled by nano gold in step (b) with a metamorphic PCR product; (d) adding the hybrid system in step (c) to a Streptavidin-coated ELISA plate to capture the hybrid compound; (e) carrying out silver enhancement to capture nano gold; (f) and stoping the silver enhancement reaction, and visually inspecting the grey scale judgment result. The invention has the characteristics of high detection sensitivity, strong detection specificity and low detection cost, can effectively eliminate false positive or false negative result when the PCR method is used for detecting African swine fever virus, and quickly detect the African swine fever virus nucleic acid.
Owner:YANGZHOU UNIV
Primers and probes for detecting mutation of cancer gene BRAFV600E
InactiveCN102242207AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationCancer genesForward primer
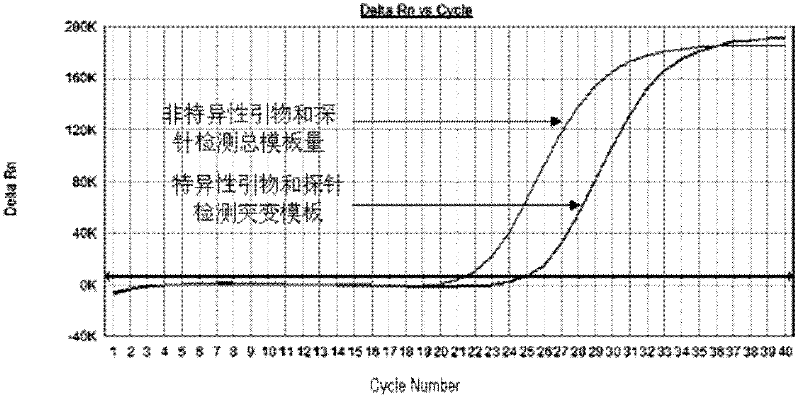
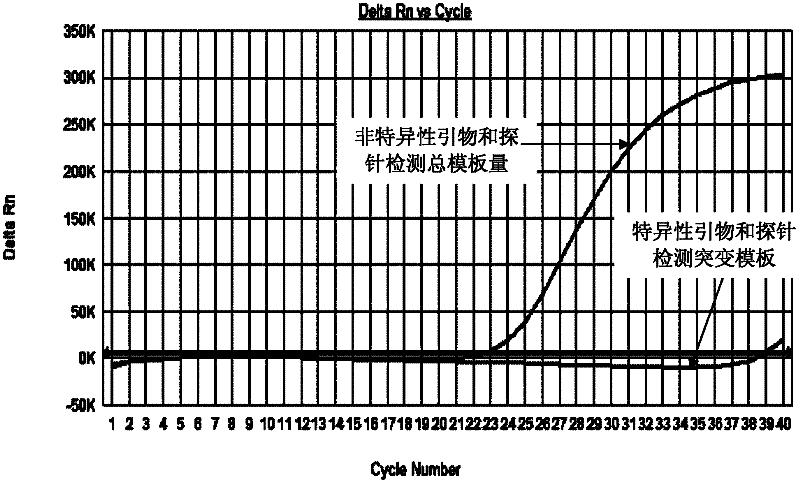
The invention relates to the field of biotechnology and aims to provide multiple primers and probes for detecting mutation of a cancer gene BRAFV600E. The primers and probes provided by the invention respectively comprise a mutation specific forward primer, a mutation non-specific forward primer, universal reverse primers and universal probes shown in SEQ NO.1-11. The detection sensitivity of themutation specific primer and probe provided by the invention can reach 500 copies / ml, thus the sensitivity is good. If a sample does not contain the cancer gene BRAFV600E, the ct value of the amplification curve of the sample is greater than or equal to 36, and the specificity is strong. Because the mutation non-specific primer is simultaneously designed in the invention to be used for detecting the total template quantity of samples, the false negative result is avoided and the quality control is convenient.
Owner:ZHEJIANG UNIV
Method for screening xanthine oxidase inhibitor by ultra performance liquid chromatography and mass spectrometry
ActiveCN102095825AQuick checkAccurate detectionComponent separationMass spectrometry imagingGenistein
The invention provides a method for screening a xanthine oxidase inhibitor by ultra performance liquid chromatography and mass spectrometry. The method is used for analyzing the in-vitro inhibition rate of the extract or monomer of a natural product on the xanthine oxidase and the catalytic activity of the xanthine oxidase. By using the ultra performance liquid chromatography-mass spectrometry in the xanthine oxidase inhibitor screening, the method is rapid and accurate in sample detection, and the correlation coefficients of the linear equation reaches 0.998. The mass spectrometry has high accuracy and good specificity in detecting the mass electron ratio of a compound, and can be used for screening the xanthine oxidase inhibitor and for the kinetic study of the xanthine oxidase inhibitor without false positive and false negative results which occur in the spectrometry. The results showed that the inhibition rate I of 20 mumol / L allopurinol is 80%; the inhibition rate of 20 mumol / L isorhamnetin is 73%; the inhibition rate of 20 mumol / L genistein is 50%; and the inhibition rate of 0.1 mg / mL aqueous extract of Ginkgo biloba is 27%.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Design method for realtime fluorescent quantitative PCR experiment interior label
InactiveCN101475988AEffective monitoring errorAvoid false negative resultsMicrobiological testing/measurementInternal standardFluorescence
A design method of real-time fluorescence quantitative PCR internal standard includes the following steps: (1) designing a primer sequence according to the activating genes to be tested, select the corresponding internal standard genes, whose primer sequence is same to that of the activating genes; (2) designing the probe of the activating genes, determining the concentration, and labeling a fluorescent reporting group at the 5' end of the probe; (3) designing two probes of the internal standard genes, one probe corresponding to the internal standard genes, the other probe corresponding to another sequence of the activating genes, and respectively labeling a same fluorescent reporting group at the 5' end of the two probes; (4) extracting and purifying the activating nucleic acid, and performing real-time fluorescence quantitative PCR amplification. The present invention can effectively monitor errors occurring in the nucleic acid extraction, amplification and product analysis processes, thus avoiding false-negative results.
Owner:戴立忠
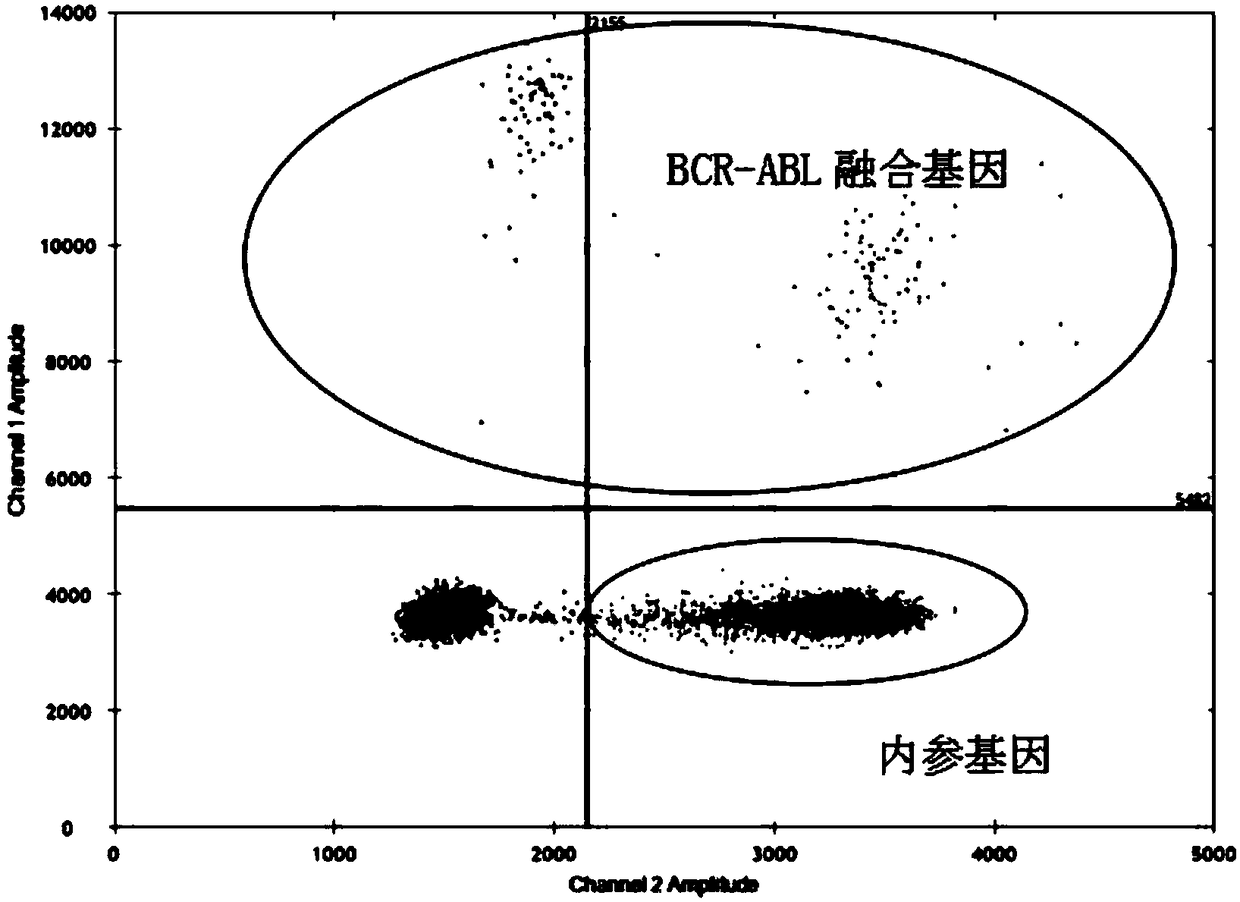
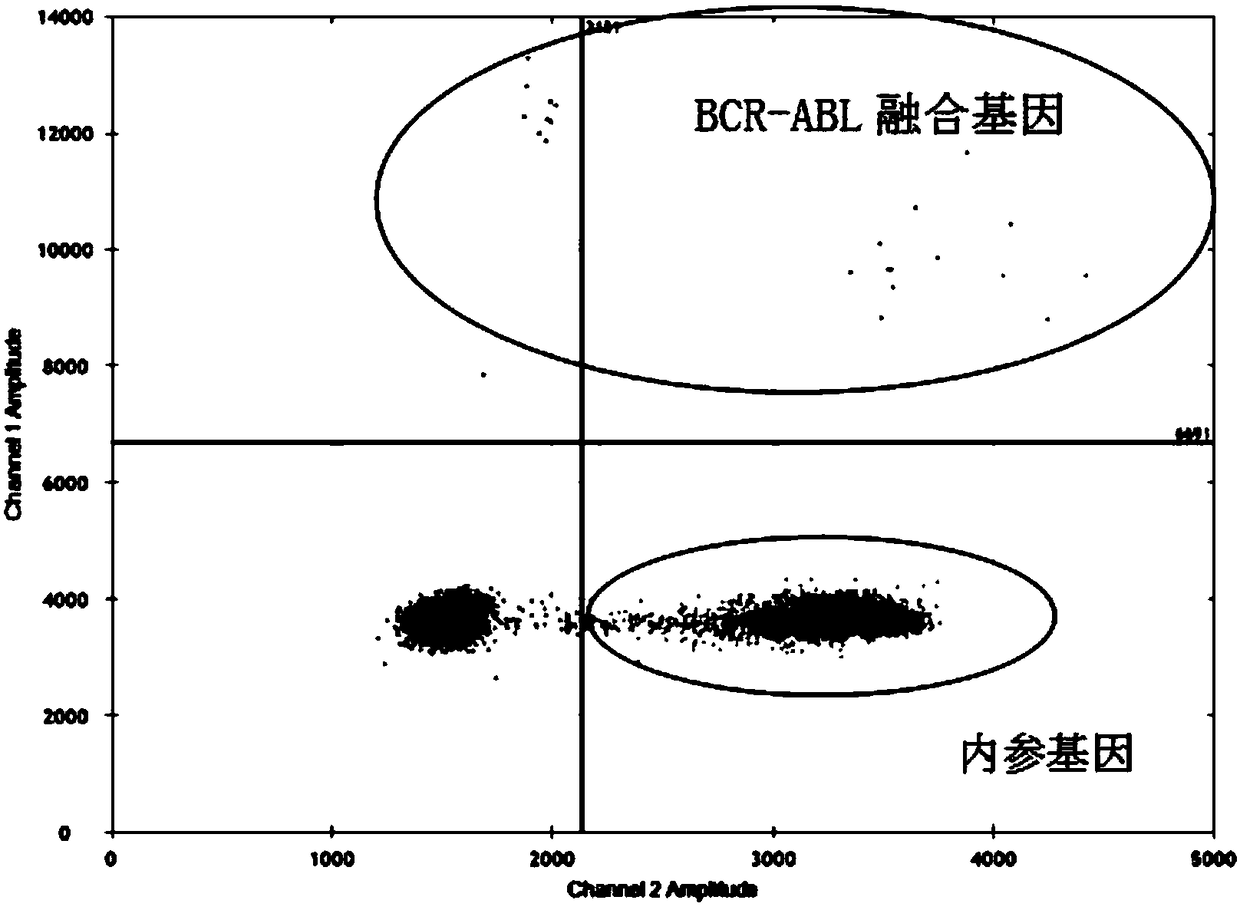
Primers for detecting BCR/ABL fusion genes by ddPCR technology and detection method thereof
InactiveCN108103155AStrong design specificityHigh detection sensitivityMicrobiological testing/measurementDNA/RNA fragmentationFluorescencePcr ctpp
The invention discloses primers for detecting BCR / ABL fusion genes by a ddPCR technology and a detection method thereof. With digital PCR as a detection platform, in allusion to three types of BCR / ABLfusion genes, upstream and downstream primers for detecting the BCR / ABL fusion genes, a BCR / ABL fusion gene detection probe, upstream and downstream primers for detecting an internal reference gene and an internal reference gene detection probe are designed and synthesized, cDNA templates of a sample to be detected, the upstream and downstream primers for detecting the BCR / ABL fusion genes, the BCR / ABL fusion gene detection probe, the upstream and downstream primers for detecting an internal reference gene, the internal reference gene detection probe and a PCR premixed solution are mixed forpreparing ddPCR microreaction liquid drops for a PCR amplification reaction, and whether fusion gene templates are contained in the sample to be detected and the number and the content thereof are judged according to the types of fluorescence signals. The design specificity of the primers and the probes are strong, the detection sensitivity is higher through combination with the digital PCR technology, and a false negative result is avoided by automatically reading the result by software, so that the primers and the probes can be applied to detection of a small number of leukemia cells and thepossibility of relapse of a patient is reduced.
Owner:PRIMBIO GENES BIOTECH WUHAN CO LTD
Chemiluminescent ligand analysis method for quantitative detection of human auto-antibody
InactiveCN101470117ASolve the problem of inaccurate quantitative detectionNo cross-reactivityBiological testingAutoantibodyBiomedical technology
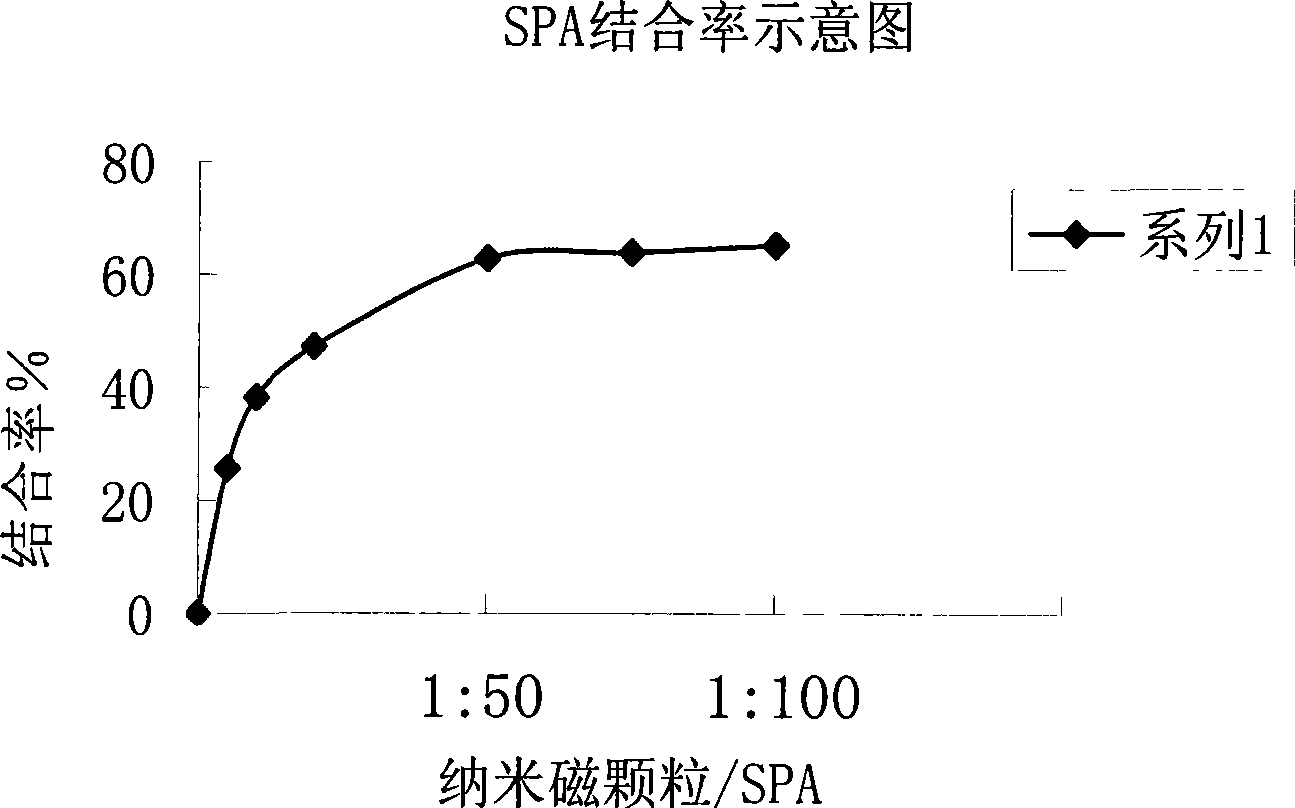
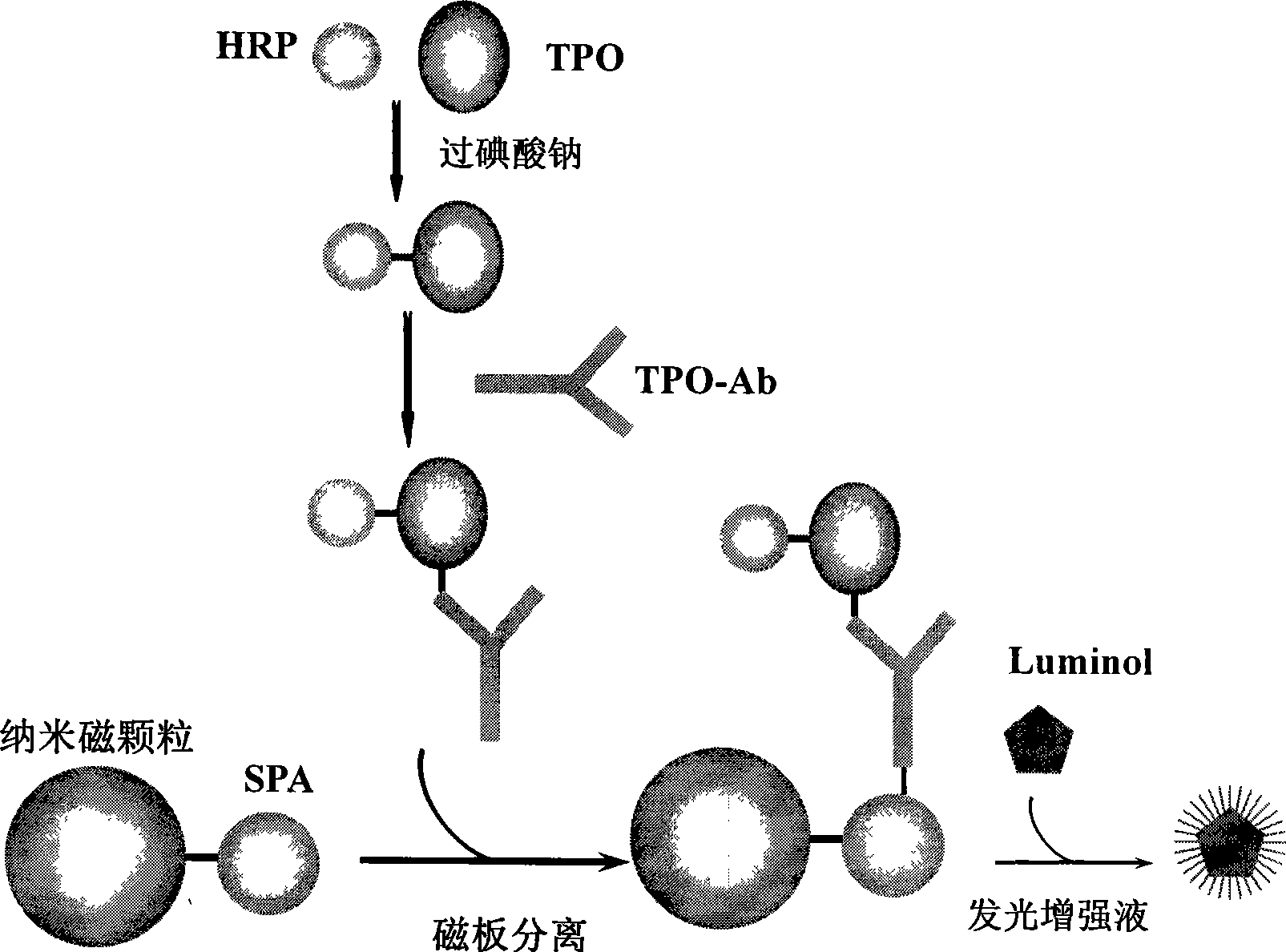
A chemical luminous ligand analysis method for quantitatively checking human antibodies belongs to the biomedical technical field, which comprises: using the generality that staphylococcal protein A (SPA) can react with Fc point of IgG in mice; using the monoclonal antibody of high affinity and specificity to prepare a standard product to establish a standard curve; respectively reacting the monoclonal antibody and the antibody in the sample with enzyme-labeled antigens; using the nanometer magnetic particles coated by SPA as ligand to separate solid and liquid; and using enzymatic chemical illumination reaction catalyzed by horseradish peroxidase (HRP) to process illumination check; thereby establishing a chemical luminous ligand quantitative analysis on human antibodies. The chemical luminous ligand analysis method is suitable for quantitatively checking all human antibodies, for resolving the problem of prior art while most human antibodies can not be accurately and quantitatively checked, avoiding cross reaction and avoiding false negative result or false positive result.
Owner:天津市协和医药科技集团有限公司
3'-Based sequencing approach for microarray manufacture
InactiveUS20090082218A1Easy to detectReduce false positiveNucleotide librariesMicrobiological testing/measurementPoly-A RNAPolyadenylation
Methods are described to derive design sequences for the production of nucleic acid microarrays. The present methods use high throughput 3′ sequencing of transcripts in a tissue sample or diseased state to design probes for nucleic acid microarrays. Also described are nucleic acid microarrays that possess probes directed to the extreme 3′ end of transcripts in a tissue. These microarrays preferably represent alternate polyadenylation sequences that are specific to the tissue from which the transcripts are derived. Also described are methods of using the microarrays directed to the extreme 3′ end of the transcript for evaluating gene expression in a tissue where there are reduced false positive and false negative results.
Owner:ALMAC DIAGNOSTICS LIMITED
Primers, probe composition, kit and multiplex fluorescence PCR detection method for detecting ass-derived component, horse-derived component and bovine-derived component in cosmetics
InactiveCN105132543AImprove throughputStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationFluorescent pcrBiology
The invention discloses primers and a probe composition for detecting an ass-derived component, a horse-derived component and a bovine-derived component in cosmetics, as well as a kit comprising the primers and the probe composition, wherein the primers comprise a pair of universal primers, the probe composition comprises four Taqman probes, and the nucleotide sequences of the primers and the probe composition are shown as SEQ ID NO.1-7. The invention further discloses a multiplex fluorescence PCR detection method for detecting the ass-derived component, the horse-derived component and the bovine-derived component in the cosmetics. The multiplex fluorescence PCR detection method comprises the following steps: taking a to-be-tested sample, extracting the DNA, carrying out PCR amplification on the DNA by utilizing the primers, the probe composition or the kit, collecting fluorescence signals, and determining that whether the to-be-tested sample contains the ass-derived component, the horse-derived component and the bovine-derived component or not according to the fluorescence signals. With the adoption of the detection technology, the false negative result during the PCR reaction can be effectively avoided, and the detection technology has the advantages of being accurate, stable, easy to operate, extremely high in sensitivity, strong in specificity, and the like.
Owner:BIOTECH RES CENT SHANDONG ACADEMY OF AGRI SCI
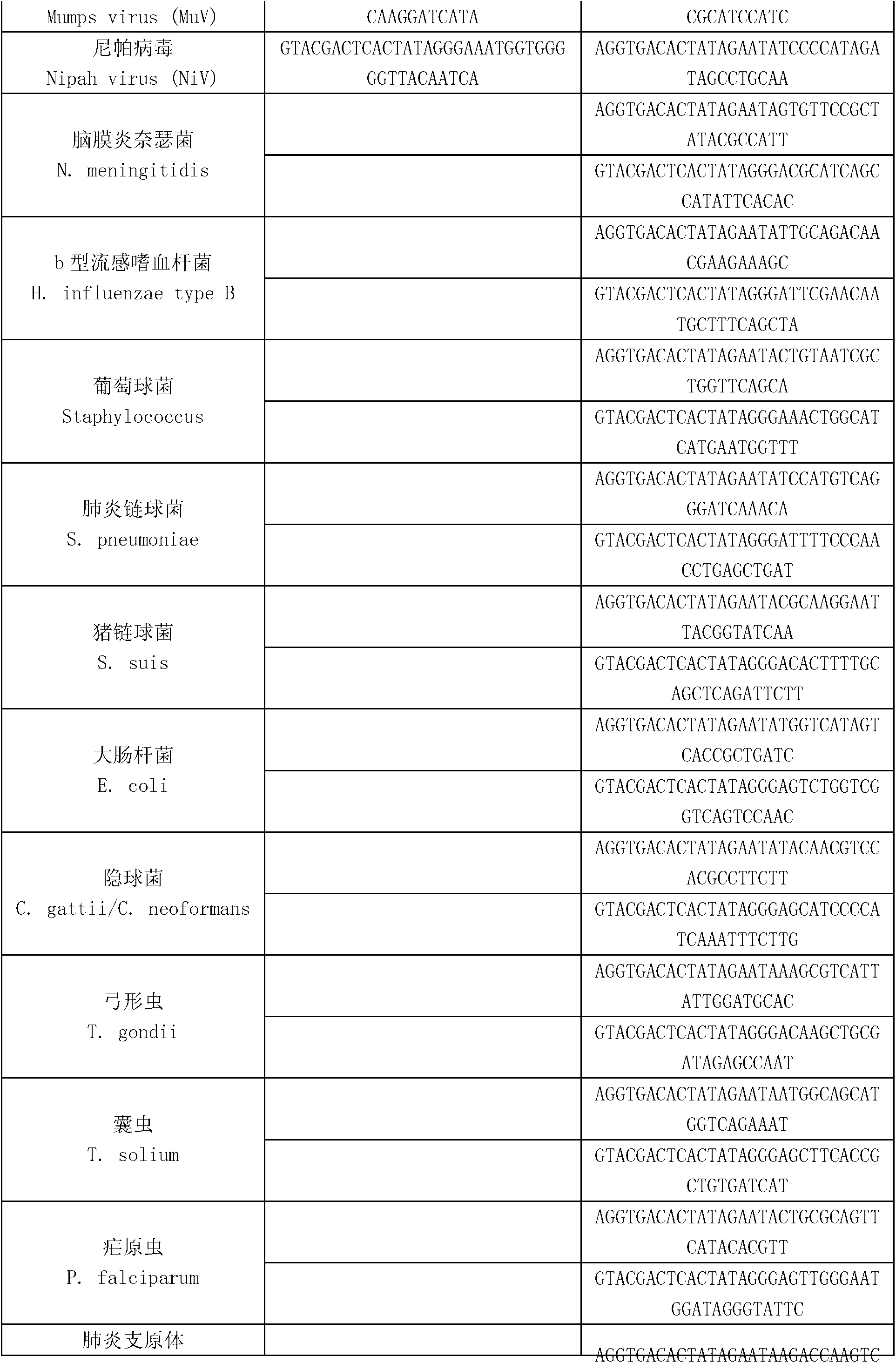
Kit for synchronously detecting twenty-three meningitis pathogens and detection method of kit
ActiveCN103074448AEnsuring Quality JudgmentsStrong specificityMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting twenty-three meningitis pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the twelve meningitis pathogens and a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-13 (sequence identifier number 1-13), and the PCR primer comprises forward and reverse PCR amplification primers of the rest eleven meningitis pathogens, a human DNA internal reference and a reaction internal reference, and PCR amplification primers of the twelve meningitis pathogens and the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 14-52. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
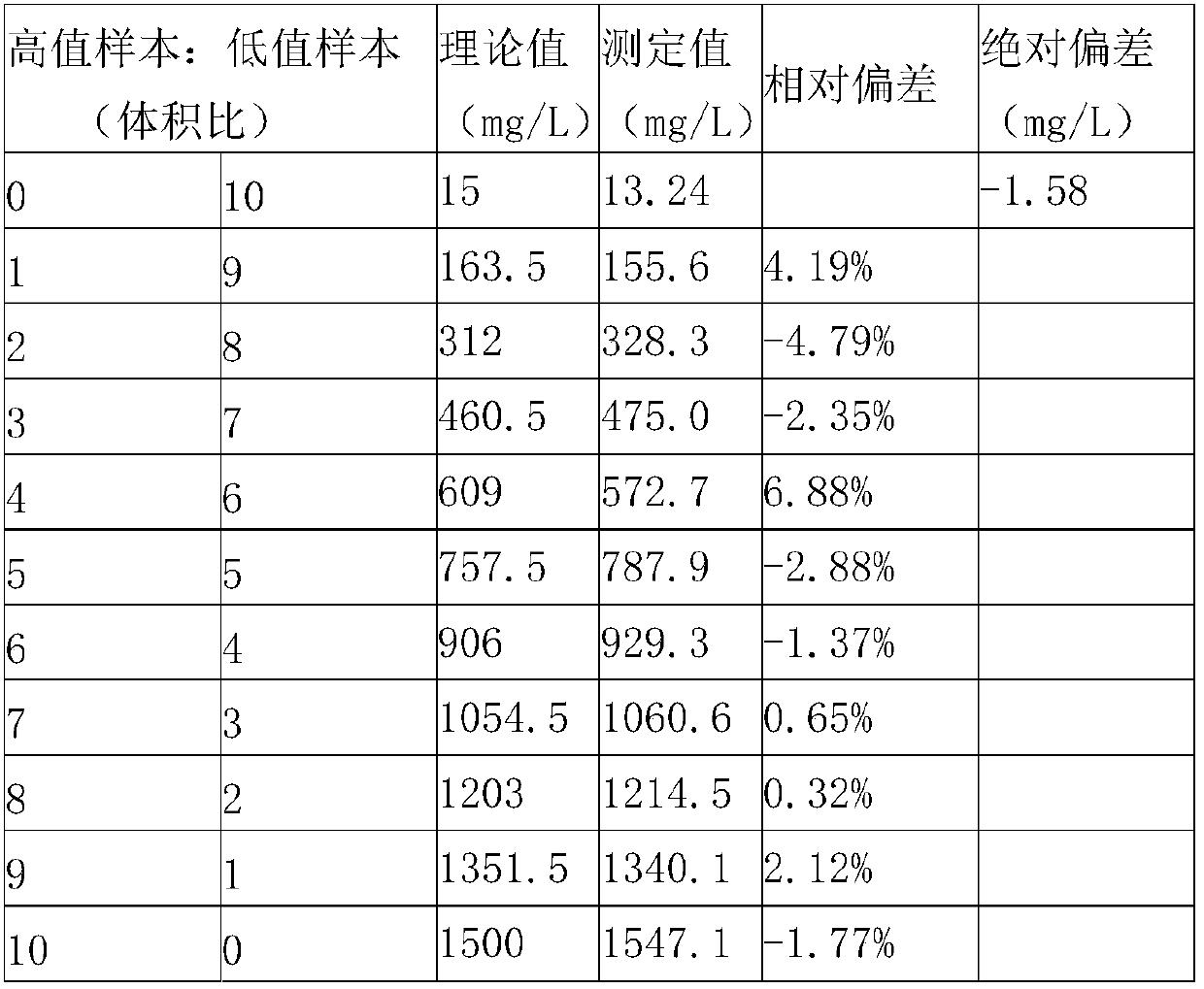
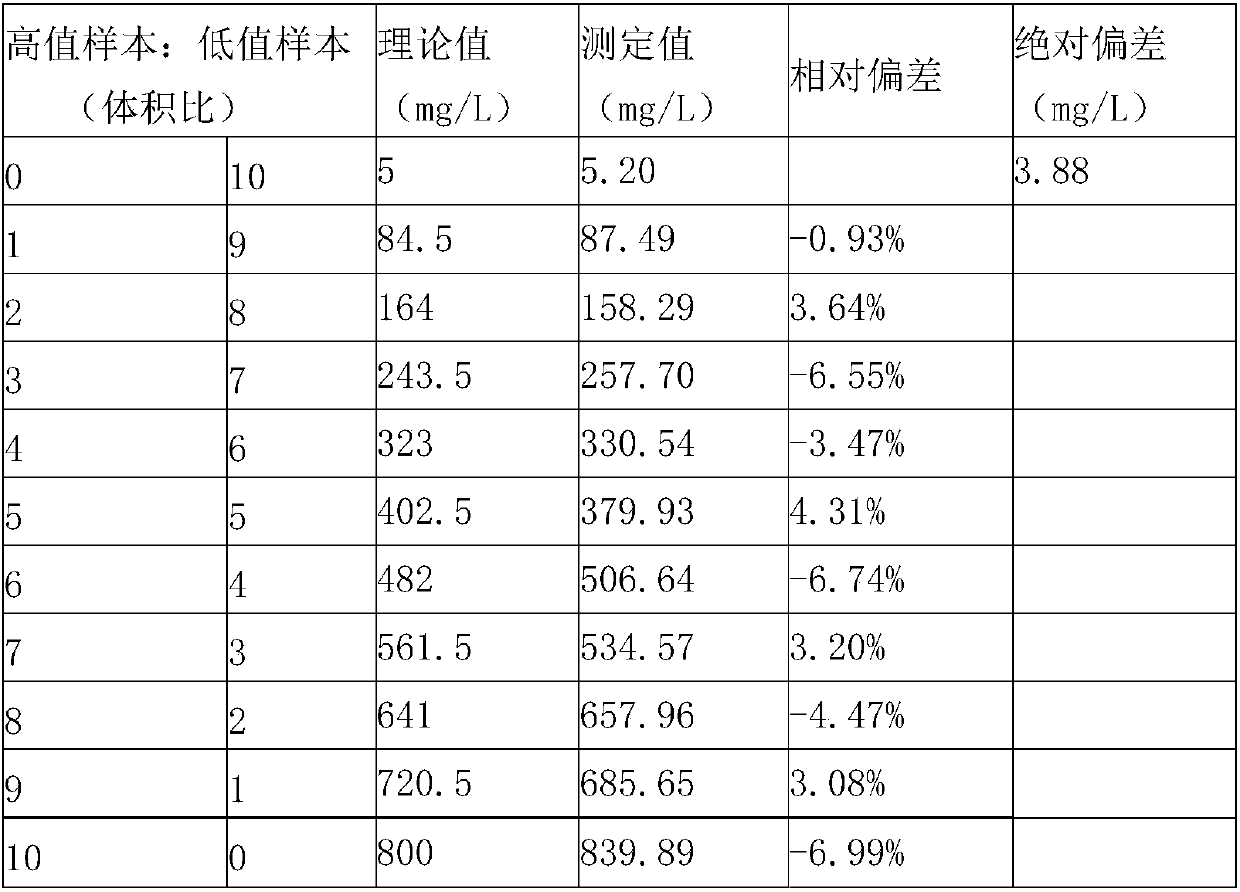
Kit for measuring microalbuminuria by adopting immune competition turbidimetry
InactiveCN107741493AMeet the screeningTreatment Level MonitoringMaterial analysisNormal peopleMicrosphere
The invention provides a kit for measuring microalbuminuria by adopting immune competition turbidimetry. The kit comprises an anti-human albumin antibody, human albumin marked leatexbeads and a buffersolution. The anti-human albumin antibody and the human albumin marked leatexbeads are separately placed. The particle size of the leatexbeads is 80-267 nm. When the kit is used for measuring microalbuminuria in human urine, the maximum linear range is 5 mg / L-1500 mg / L. The kit can meet screening of clinical normal people, and can also avoid result underestimation or even false negative result arising from a hook effect caused by antigen excess.
Owner:HUNAN HY BIOPOCT TECH CO LTD
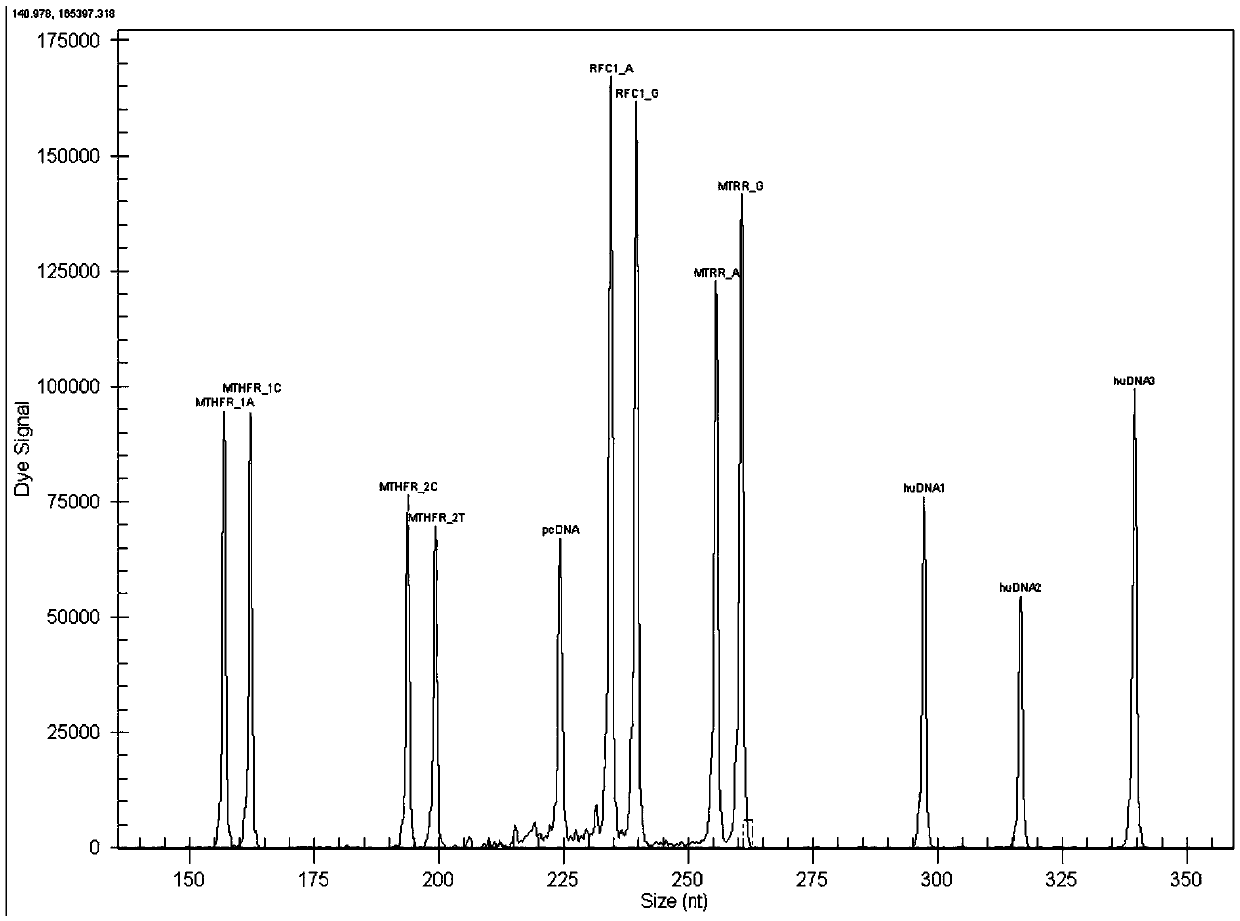
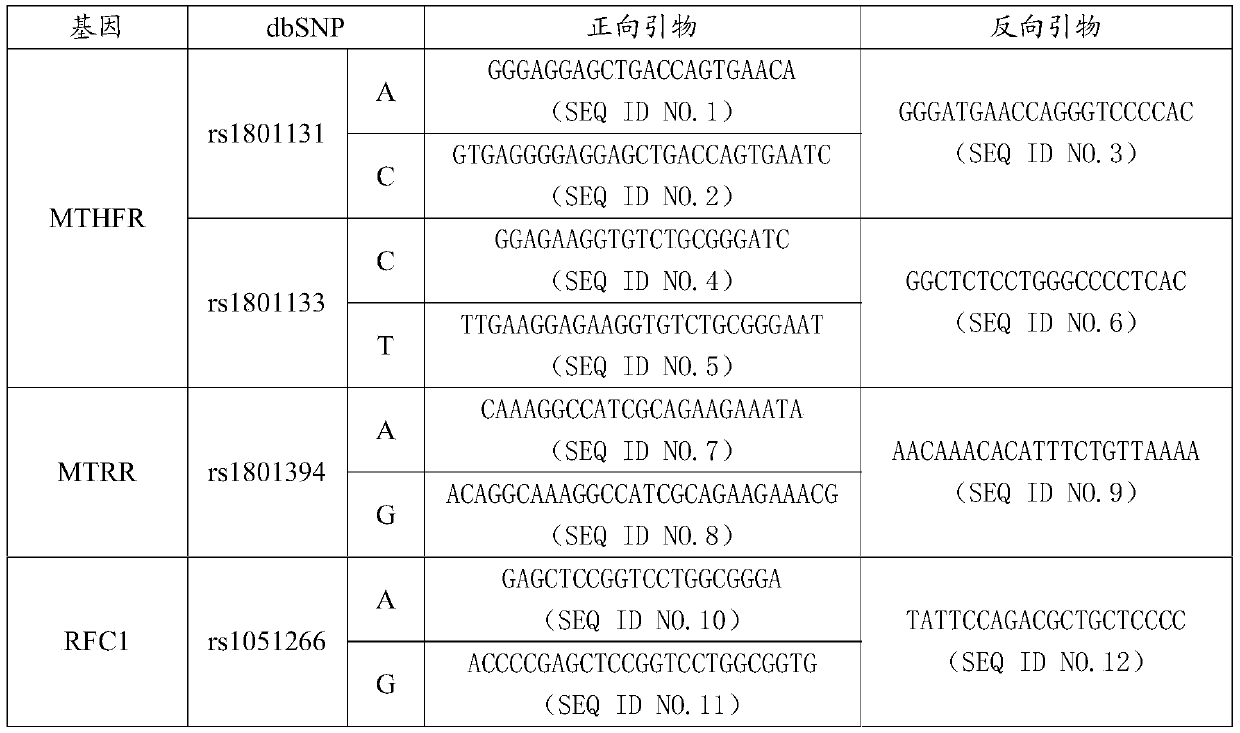
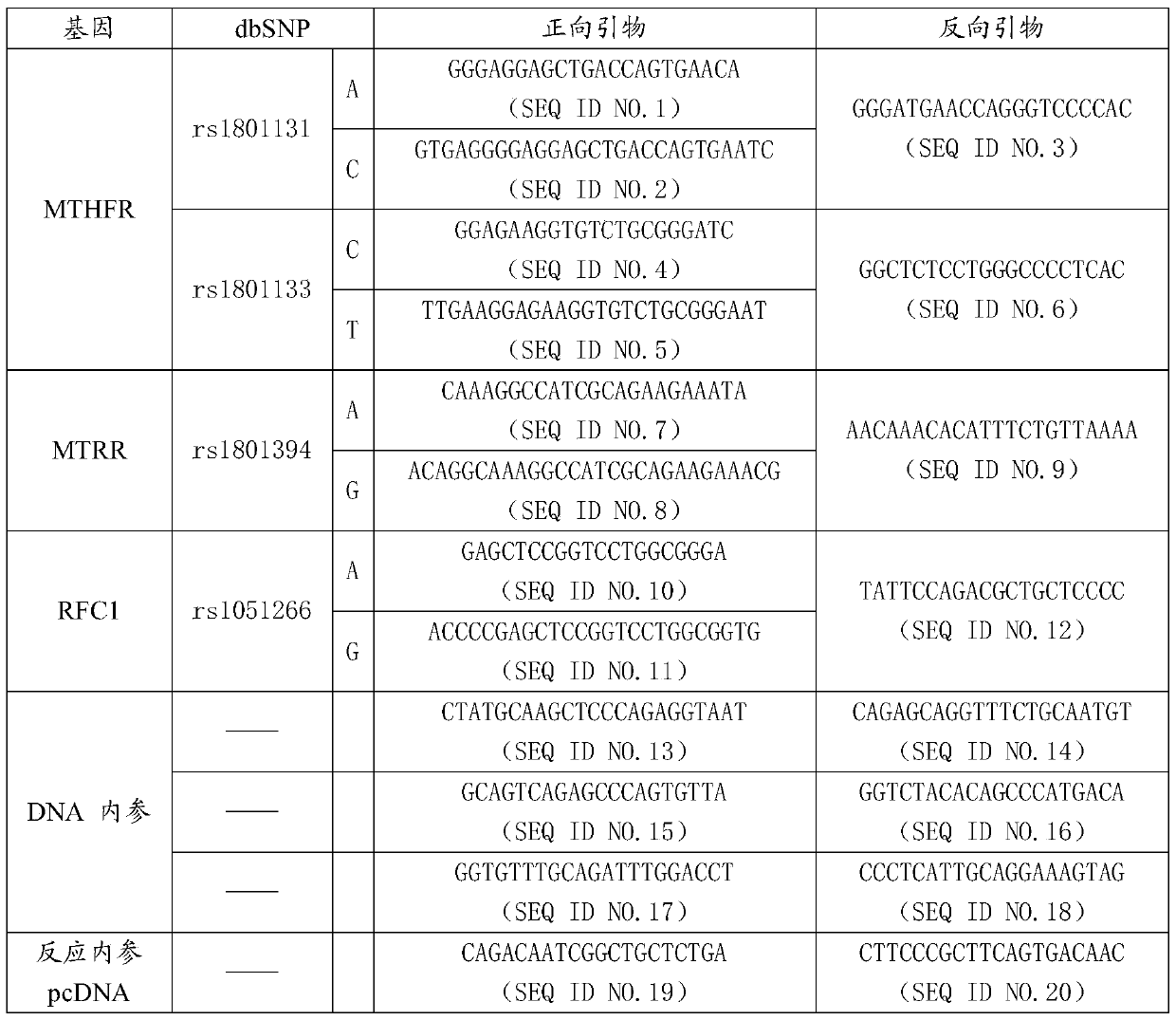
MTHFR, MTRR and RFC1 gene polymorphism detection primer combination and kit and application of MTHFR, MTRR and RFC1 gene polymorphism detection kit
ActiveCN105463122ASave production costSave testing costMicrobiological testing/measurementDNA/RNA fragmentationPositive controlRFC1
The invention discloses an MTHFR, MTRR and RFC1 gene polymorphism detection primer combination and kit and application of the MTHFR, MTRR and RFC1 gene polymorphism detection kit. The detection kit comprises ultrapure water, an X solution, a 10*PCR buffering solution, a PCR primer, a 25 mM magnesium chloride solution, DNA polymerase and a positive control product, wherein the PCR primer comprises a forward and reverse amplification primer, a DNA internal reference forward and reverse amplification primer and a reaction internal reference forward and reverse amplification primer of different gene types, the three primers are located at four SNP sites on a gene related to folic acid metabolism, and the gene sequence of the PCR primer is shown in SEQ ID NO.1-NO.20. The detection kit has the advantages of being high in specificity, accuracy, flux and reliability, low in cost and free of a false-negative result.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
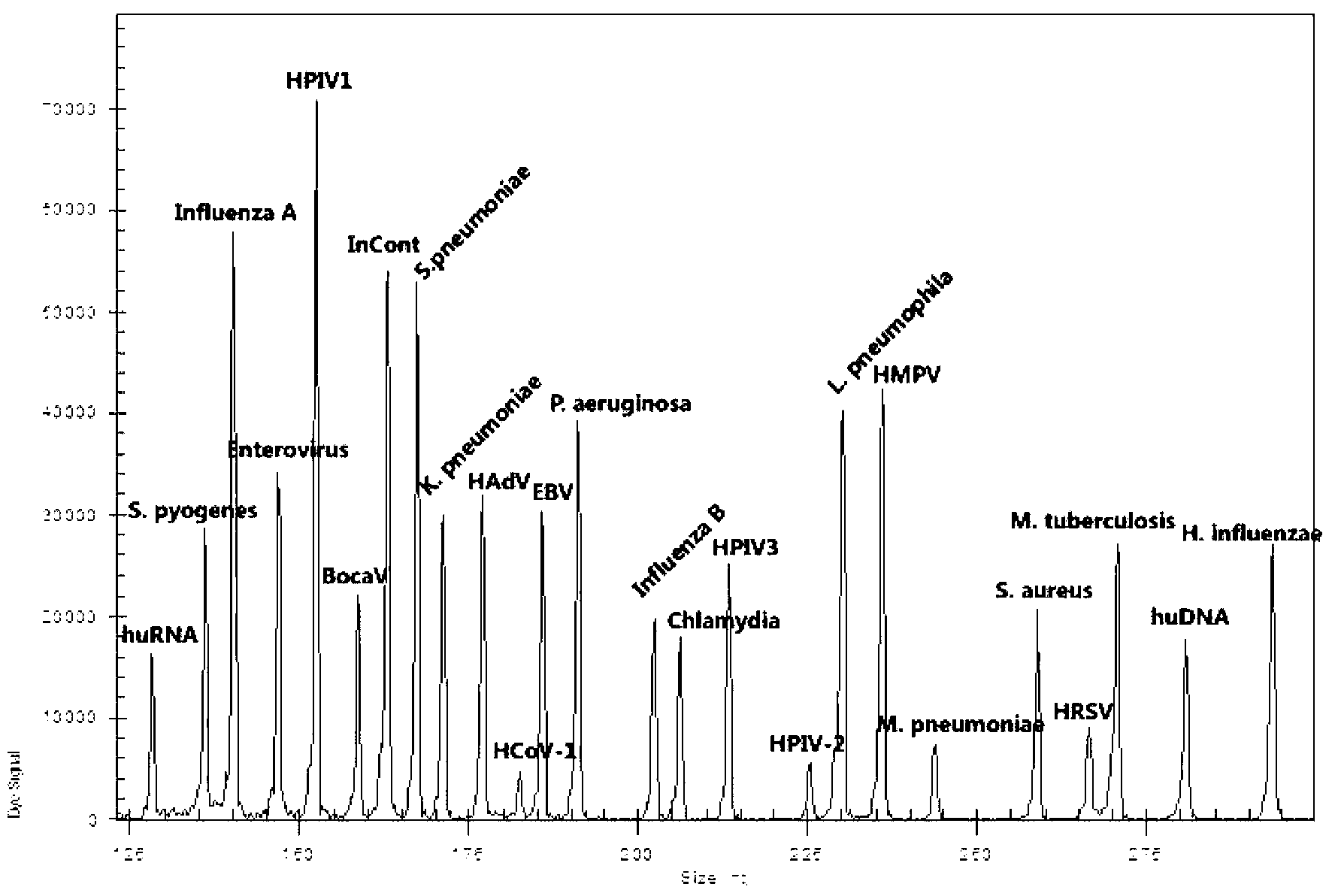
Kit for synchronously detecting twenty-two respiratory tract pathogens and detection method of kit
ActiveCN103074451AEnsuring Quality JudgmentsImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting twenty-two respiratory tract pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the nine respiratory tract pathogens, and a reverse primer of a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-6 (sequence identifier number 1-6), and the PCR primer comprises forward and reverse PCR amplification primers of the rest thirteen respiratory tract pathogens, a forward and reverse amplification primer of a human DNA internal reference, a forward and reverse PCR amplification primer of a reaction internal reference, PCR amplification primers of the nine respiratory tract pathogens, and a PCR amplification primer of the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 7-44. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
KIR and ligand genetic typing experimental method
InactiveCN108624665ASmall sample sizePromote amplificationMicrobiological testing/measurementDiseaseNatural Killer Cell Inhibitory Receptors
The invention discloses a KIR and ligand genetic typing experimental method. By the technology, the time and labor are saved, and meanwhile, DNA sample capacity is further saved. A multi-PCR technology is adopted, meanwhile, 36 pairs of primers are further combined according to the sizes of PCR products, and finally, amplified reaction is finished in 12 reaction holes. The KIR and ligand genetic typing experimental method is conveniently applied to amplification of 96 pore plates, conventional Taq enzyme is used, typing of all KIR genes and ligands thereof can be finished at a time under the same reaction conditions, and the practicality of the KIR and ligand genetic typing experimental method is greatly improved. Two pairs of primers are used for a KIR gene, the accuracy is improved, anda false negative result is avoided. The KIR gene can be combined to MHC-I type ligand molecules on the surfaces of targeting cells, inhibiting or activating signals are transmitted to regulate the activity of NK cells and T cells, and the KIR and ligand genetic typing experimental method plays an important regulation role in hematopoietic stem cell transplantation, feto-matemal tolerance, anti-infectious immunity, tumor immunity and autoimmune diseases. Therefore, KIR genetic typing facilitates understanding of influences of KIR to tumor immunity, hematopoietic stem cell transplantation and autoimmune diseases.
Owner:韩瑜
Full-automatic microorganism detecting enrichment system and enrichment method thereof
InactiveUS20170029760A1The test result is accurateLabor savingBioreactor/fermenter combinationsBiological substance pretreatmentsTime efficientEnrichment methods
A full-automatic microorganism detecting enrichment system and the enrichment method thereof are provided. A transmission apparatus is controlled by a industrial control computer to transmit a to-be-detected sample placed on a preset operating position, a culture medium comprising culture, a pipeline filter specially for collecting bacteria and a filter head sequentially into a sterilization cabin, an middle package removing cabin, an inner package removing cabin, an enrichment operating cabin, a buffer cabin and a positive bacteria filling cabin to perform corresponding operations and enrich the microorganism in the to-be-detected samples. Said system can achieve a fully automated enrichment of the microorganism in the to-be-detected samples, needs no manual operation, and saves time and labor force. Further, it can avoid false positive or false negative result caused by an effect of human factor, and achieve sterile and automatic operation with an accurate detection result.
Owner:GEM TEMP +2
Method for quantitative detection of microorganisms in mixed microbial fermentation process
ActiveCN108588188AShorten detection timeImprove efficiencyMicrobiological testing/measurementMicroorganism based processesMicroorganismCell membrane
The invention relates to a method for quantitative detection of viable count of specific microorganisms in a vinegar solid fermentation process, and belongs to the field of fermented food. The methodhas the characteristics of accurate results, fast and efficient properties and high sensitivity, overcomes the shortcomings that consuming time is long, operating intensity is high and VBNC bacteria cannot be counted of a traditional viable counting method, and is optimized on the basis of existing PMA-qPCR. In particular, a repair solution is developed for repairing the membranes of sub-lethal bacteria cells and is suitable for repairing the cell membranes of bacteria with damaged cell membranes, false negative results caused by sub-lethal damage of a bacterium body is reduced, the accuracy of quantitative results is improved, and the purpose of quickly and accurately quantifying the number of live bacteria in the sample is achieved.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Biochip utilizing hook effect to enlarge detection range and detection method thereof
ActiveCN105823880AWide detection rangeHigh detection sensitivityMaterial analysisTime efficientAnalyte
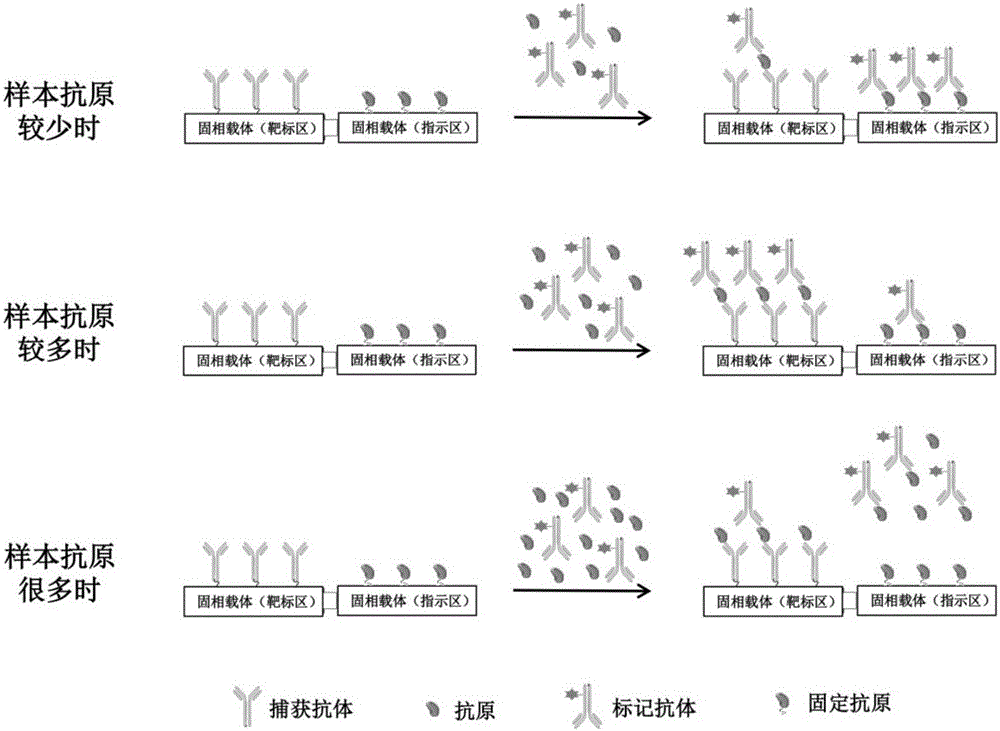
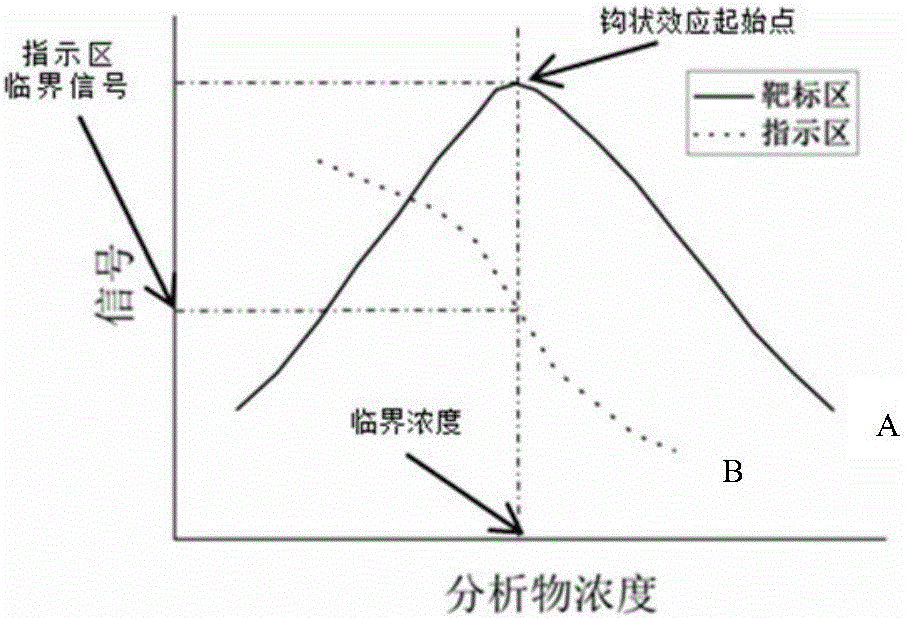
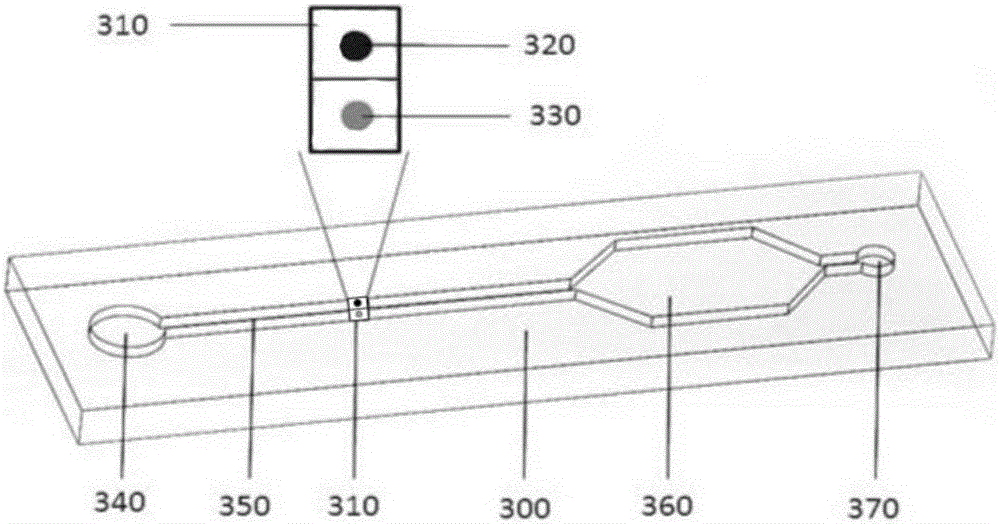
The invention discloses a biochip utilizing the hook effect to enlarge the detection range and a detection method thereof. The biochip comprises at least one target area, a capturing antibody is fixed in the target area, the capturing antibody is combined with an analyte, through the labeling antibody combined with the analyte, detectable signals can be generated and can be used to quantitatively analyze the analyte in a sample; the biochip also comprises at least one indicating area, an antigen is fixed in the indicating area; and the fixed antigen is not combined with the analyte and is combined with labeling antibody that is not combined with the analyte to generate a detectable signal, which can be used to judge whether a hook effect is generated or not. The biochip has the advantages of flexible and convenient operation, time saving, and high sensitivity, largely enlarges the detection range of biological molecules, and avoids the false negative results.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI +1
Cloned enzyme-donor immunoassay (CEDIA) ImmunoChip drug detecting kit
Rapid drug detection is a regular detection item for public security departments, drug control organizations, sports events, enlistment and entry departments. At present, colloidal gold test paper is a basic choice for rapid drug detection, but the sensitivity of the colloidal gold test paper is low, the possibility of fake positive and fake negative results is high, the repeatability is low and the quantification is impossible. The invention discloses a cloned enzyme-donor immunoassay (CEDIA) ImmunoChip drug detecting kit. The kit comprises an immuno chip on which the combination of an enzyme donor and an enzyme receptor is immobilized, wherein the enzyme donor can be coupled with a drug micromolecule by a chemical bond to form an enzyme-donor-labeled exogenous antigen so as to be used for detecting whether a specific anti-drug antibody which can be competitively combined with the enzyme-donor-labeled exogenous antigen exists in a sample to be detected by a CEDIA ImmunoChip drug detecting method and calibration is carried out by using a novel fluorogenic substrate. The kit has the advantages of sensitive reaction, high accuracy, no cross reaction and high repeatability, and is simple and convenient to operate.
Owner:GUANGZHOU YIHANG BIOTECH
Multi-allelic molecular detection of SARS-associated coronavirus
InactiveCN1906306AMicrobiological testing/measurementAgainst vector-borne diseasesQuantitative determinationSARS-associated coronavirus
The subject invention relates to a multiple-allelic RT-real-time polymerase chain reaction (PCR) assay for coronaviruses including the SARS virus. Multiple target sequences within the SARS-CoV, S, E, M and N genes are identified. The use of the four different targets enhances the likelihood that the fundamental genetic drift of the virus will not lead to a false negative result. Multiplex assays format for the assay are envisioned. Thus, the present invention allows for early diagnosis of a SARS infection. The assay would be useful in the context of monitoring treatment regimens, screening potential anti SARS agents, and similar applications requiring qualitative and quantitative determinations.
Owner:BIRCH BIOMEDICAL RES
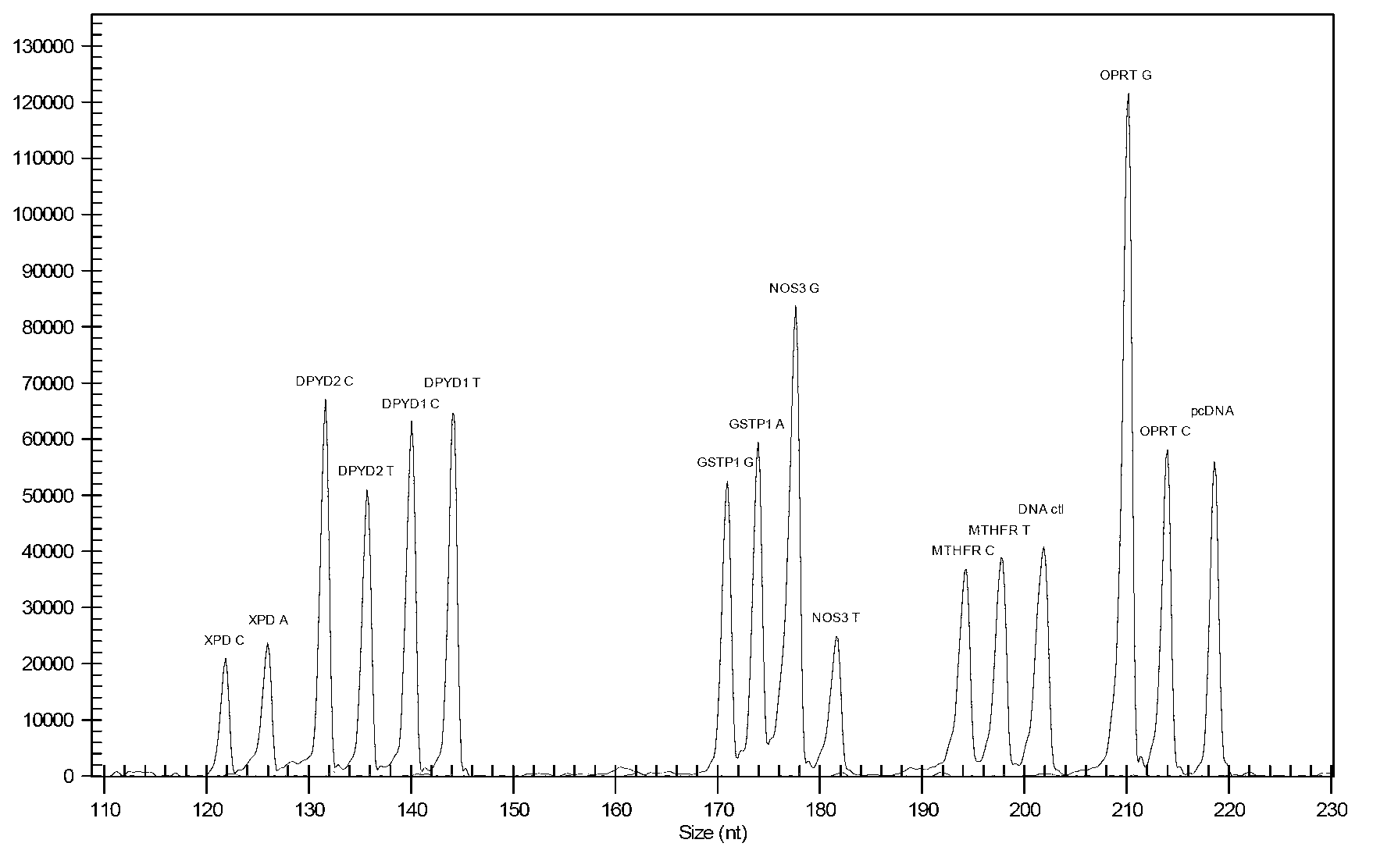
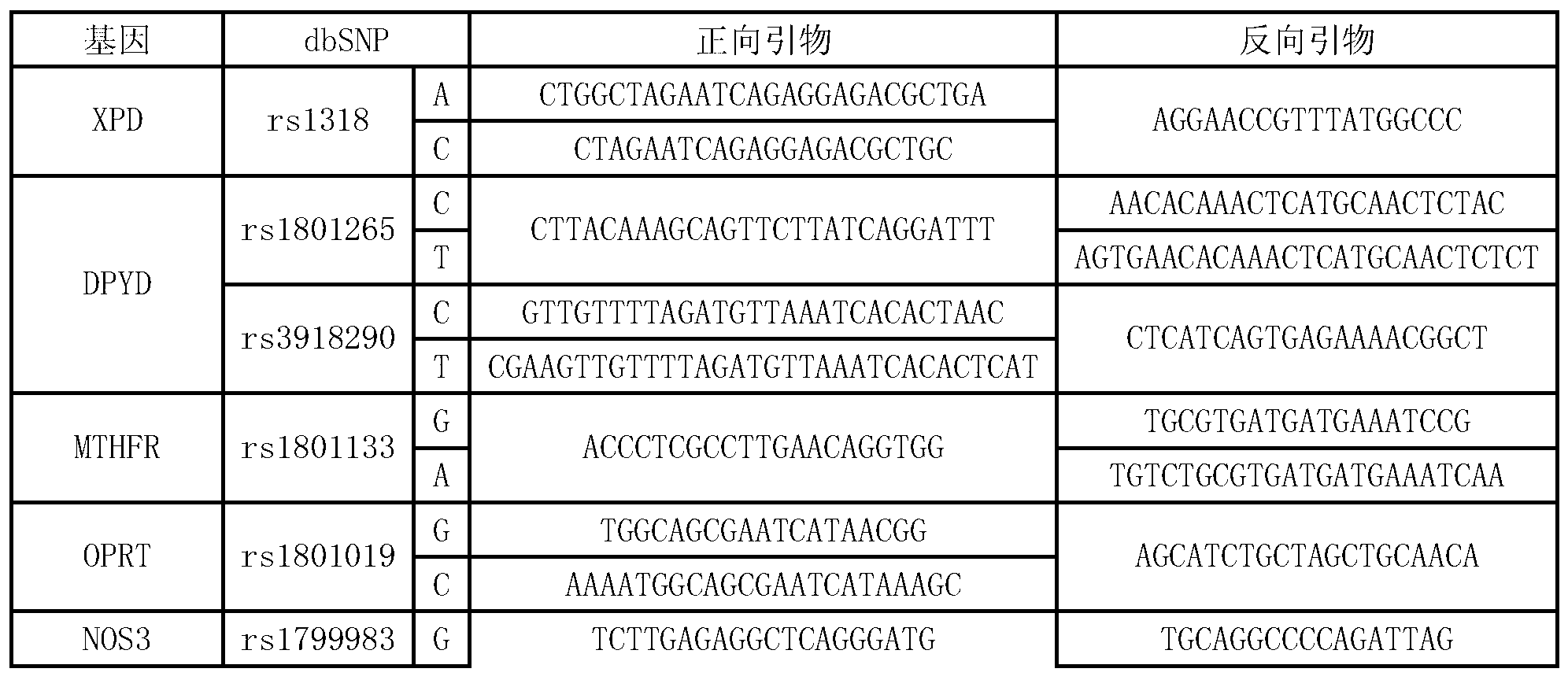
Multi-gene detection kit for guiding administration of 5-fluorouracil and detection method of multi-gene detection kit
ActiveCN103074436AImprove detection efficiencyShorten the timeMicrobiological testing/measurementPositive controlPolymerase L
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
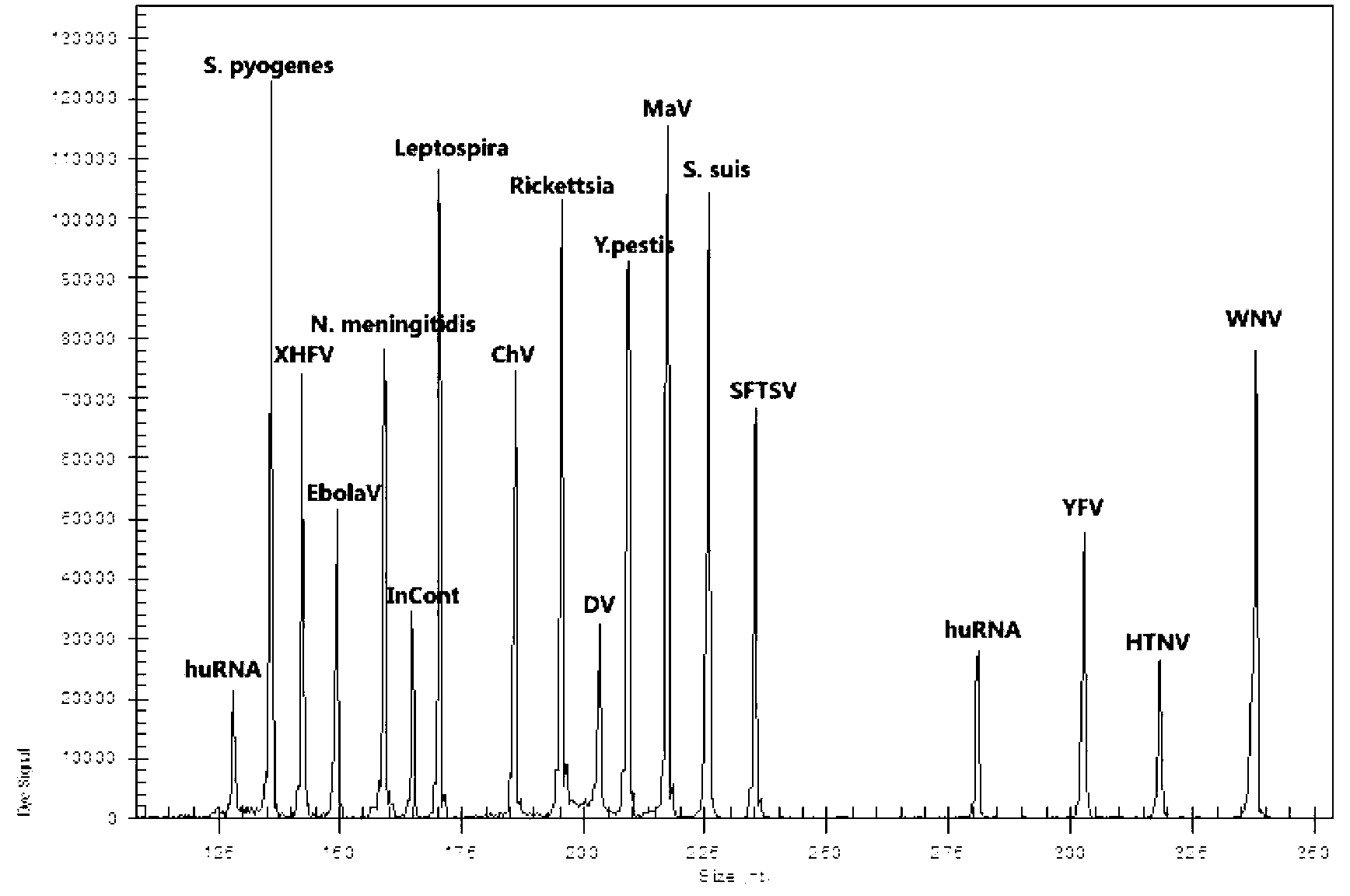
Kit for synchronously detecting fifteen hemorrhagic fever pathogens and detection method of kit
ActiveCN103074452AMonitor reaction efficiencyEnsuring Quality JudgmentsMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting fifteen hemorrhagic fever pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the nine hemorrhagic fever pathogens and a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-10 (sequence identifier number 1-10), and the PCR primer comprises forward and reverse PCR amplification primers of the rest six hemorrhagic fever pathogens, a human DNA internal reference and a reaction internal reference, and PCR amplification primers of the nine hemorrhagic fever pathogens and the human RNA internal reference, and has a gene sequence show as SEQ ID NO. 10-36. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Stomach cancer serological detection and identification kit and method
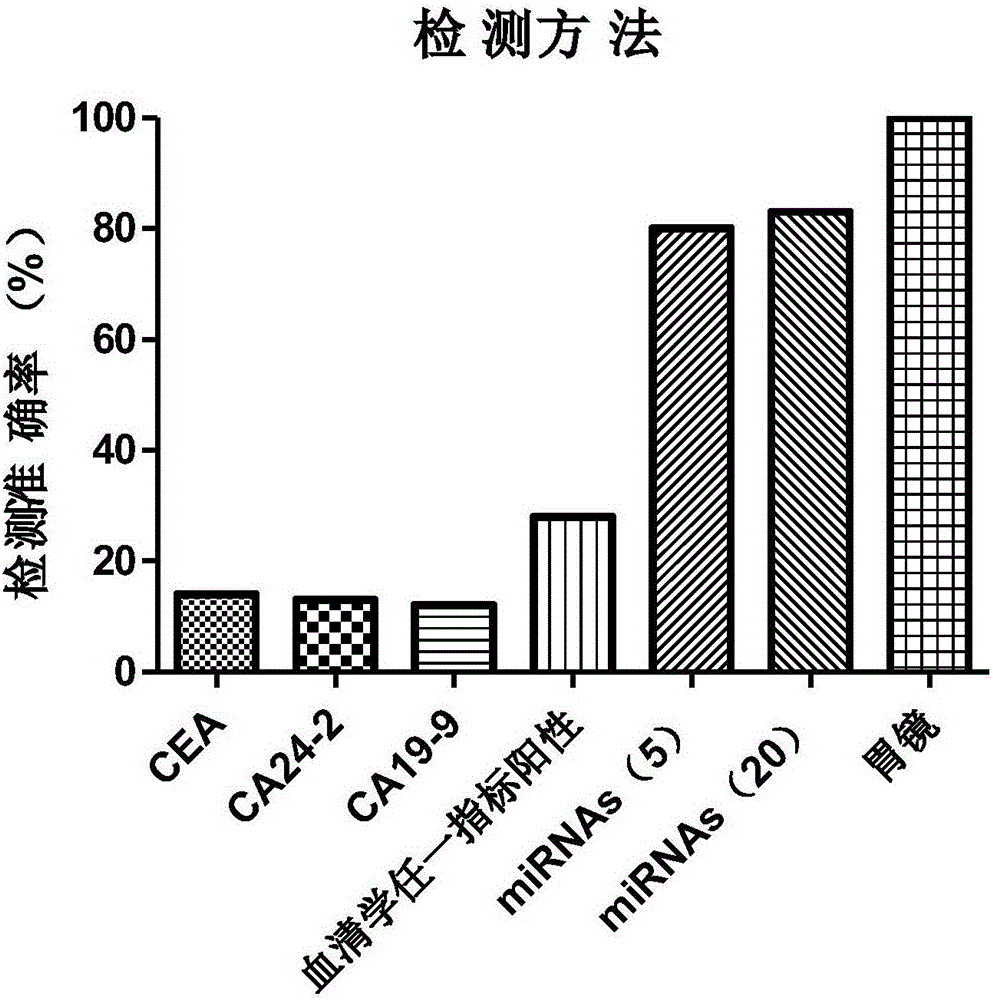
ActiveCN106755377AExclude false positive resultsAvoid pressure lossMicrobiological testing/measurementAntigenCarbohydrate antigen
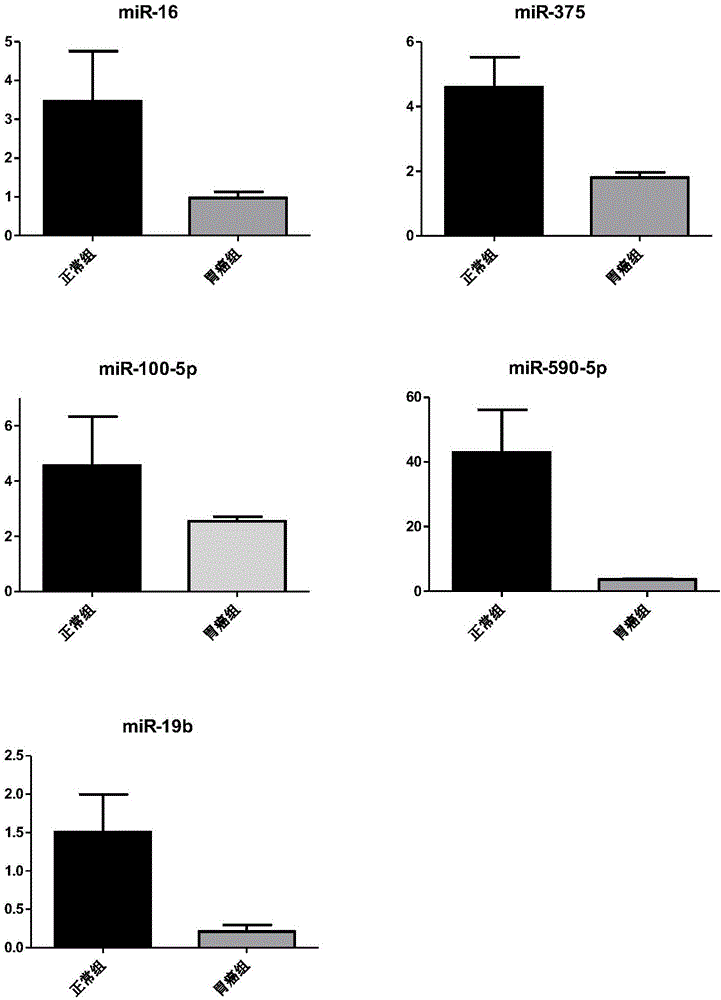
The invention discloses a stomach cancer serological detection and identification kit and a method. The stomach cancer serological detection and identification kit is prepared from a cel-miR-39-5p fragment, a primer, an exosome isolating reagent, an exosome miRNA (Micro Ribonucleic Acid) extracting reagent, a reverse transcription reagent and a real-time fluorescent quantitative PCR (Polymerase Chain Reaction) reagent, wherein the primer comprises a cel-miR-39-5p internal reference primer, an hsa-miR-375 primer, an hsa-miR-590-5p primer, an hsa-miR-19b-3p primer, an hsa-miR-100-5p primer and an hsa-miR-16 primer. When any results of detecting stomach cancer CA (Carbohydrate Antigen)19-9, CA24-2 and CEA (Carcino Embryonie Antigen) of a patient by using serum are positive, and the stomach cancer serological detection and identification kit provided by the invention is then used for carrying out aided detection, false positive results of serological detection can be effectively removed, so that huge mental stress and property loss brought to the patient can be avoided; meanwhile, aiming at the situation that when the results of detecting the stomach cancer CA19-9, CA24-2 and CEA of the patient by using the sera are negative, and the stomach cancer serological detection and identification kit provided by the invention is then used for carrying out the aided detection,, the false negative results of the serological detection can be effectively found, so that the life of the patient can be rescued in time.
Owner:ZHEJIANG PROVINCIAL HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com