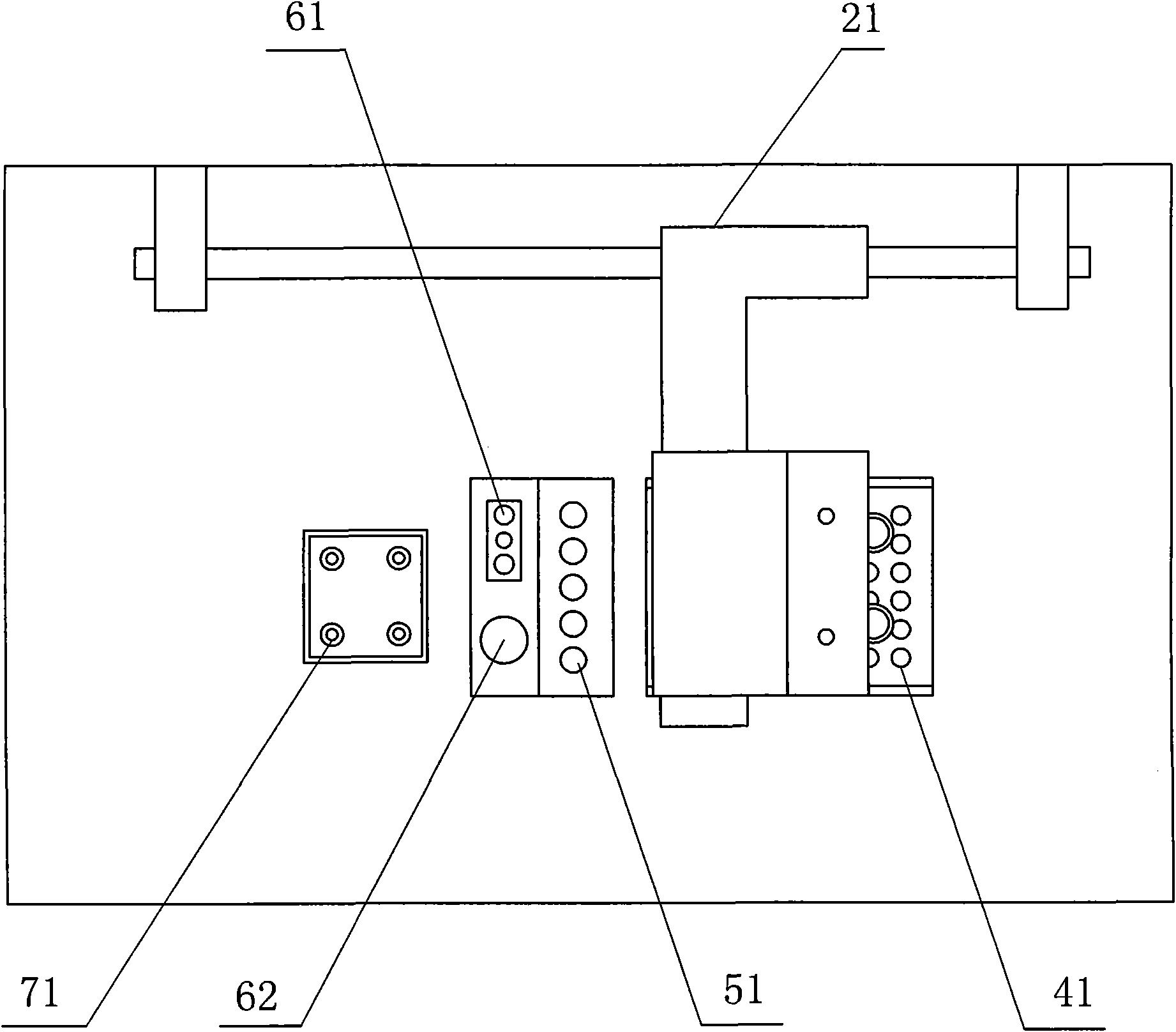
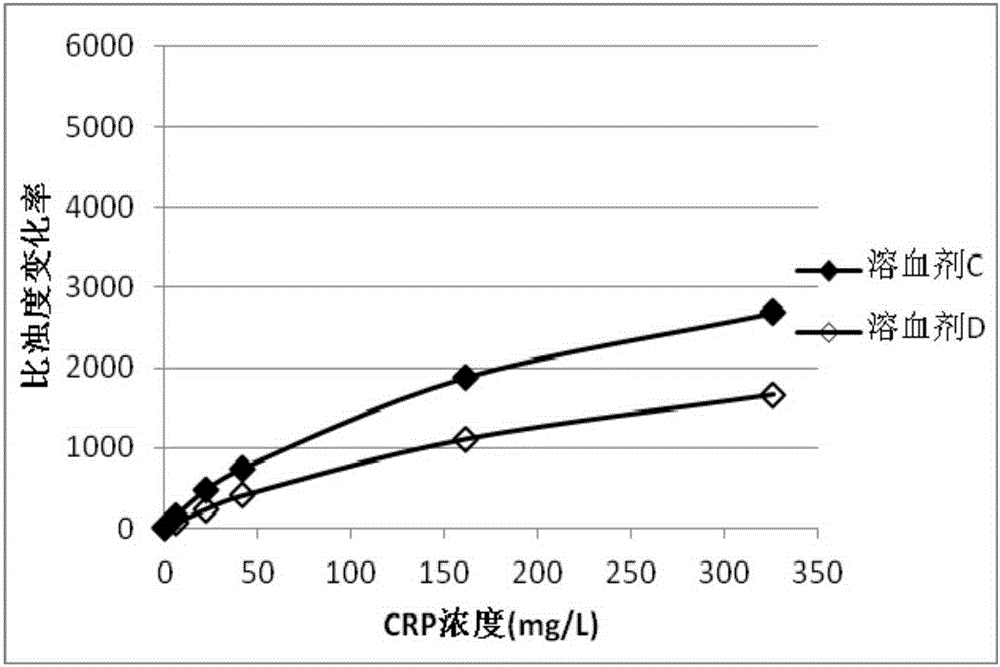
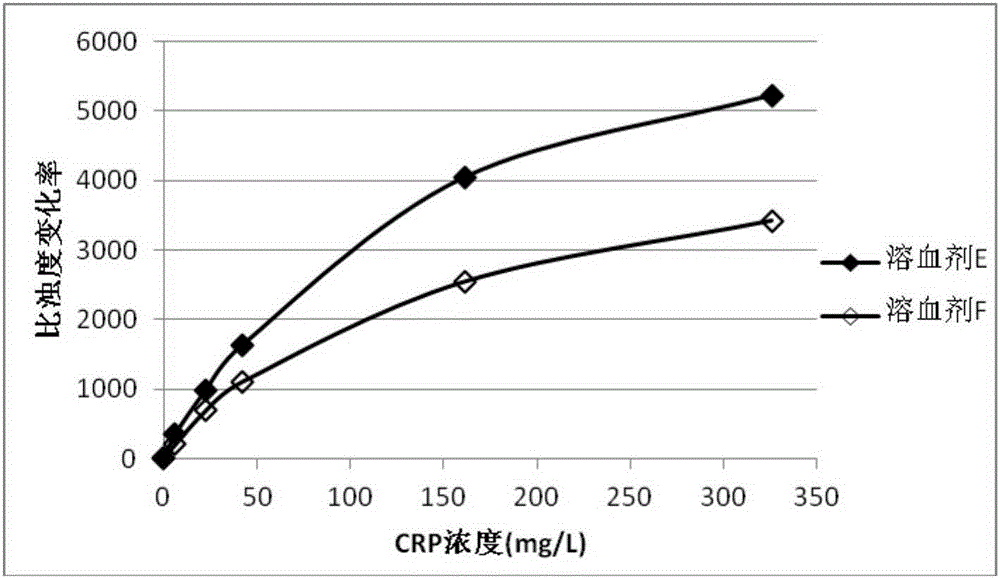
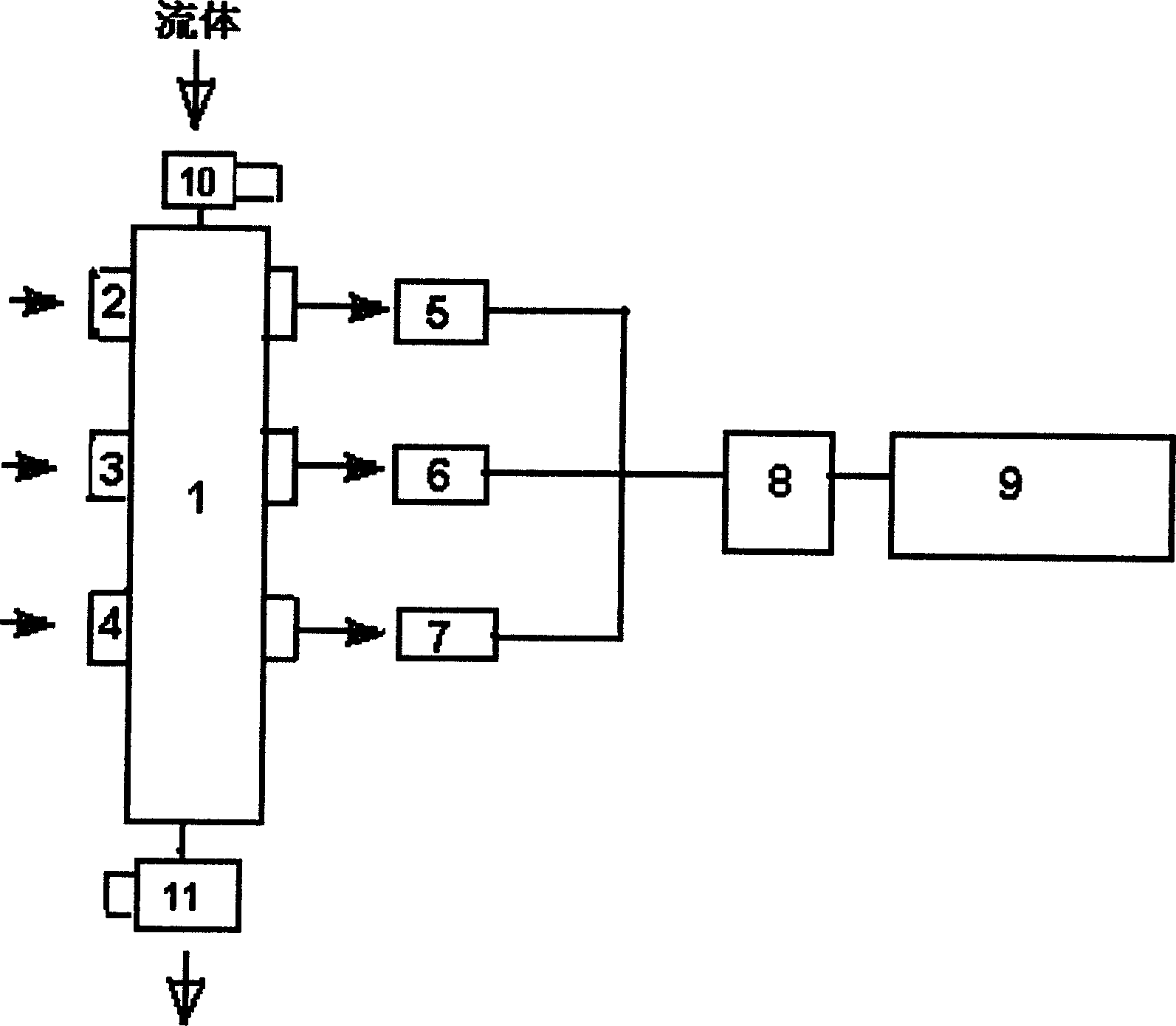
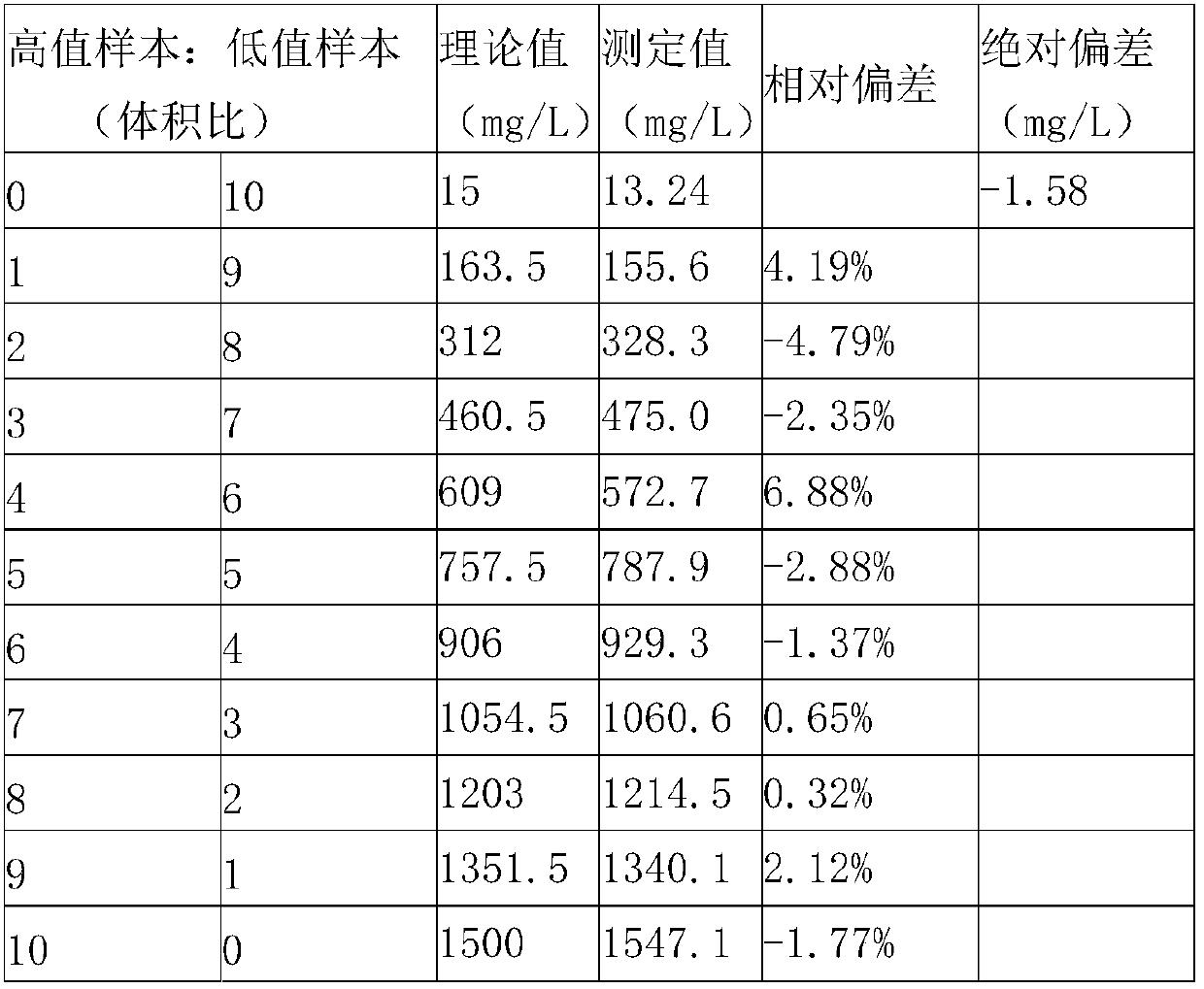
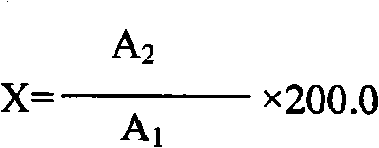Patents
Literature
137 results about "Turbidimetry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Turbidimetry (the name being derived from turbidity) is the process of measuring the loss of intensity of transmitted light due to the scattering effect of particles suspended in it. Light is passed through a filter creating a light of known wavelength which is then passed through a cuvette containing a solution. A photoelectric cell collects the light which passes through the cuvette. A measurement is then given for the amount of absorbed light.
Spectrophotometric system and method for the identification and characterization of a particle in a bodily fluid
InactiveUS7027134B1Rapid and inexpensive and convenient for diagnosisRapidly and inexpensively disease diagnosisScattering properties measurementsDiagnostic recording/measuringTurbidimetryWavelength
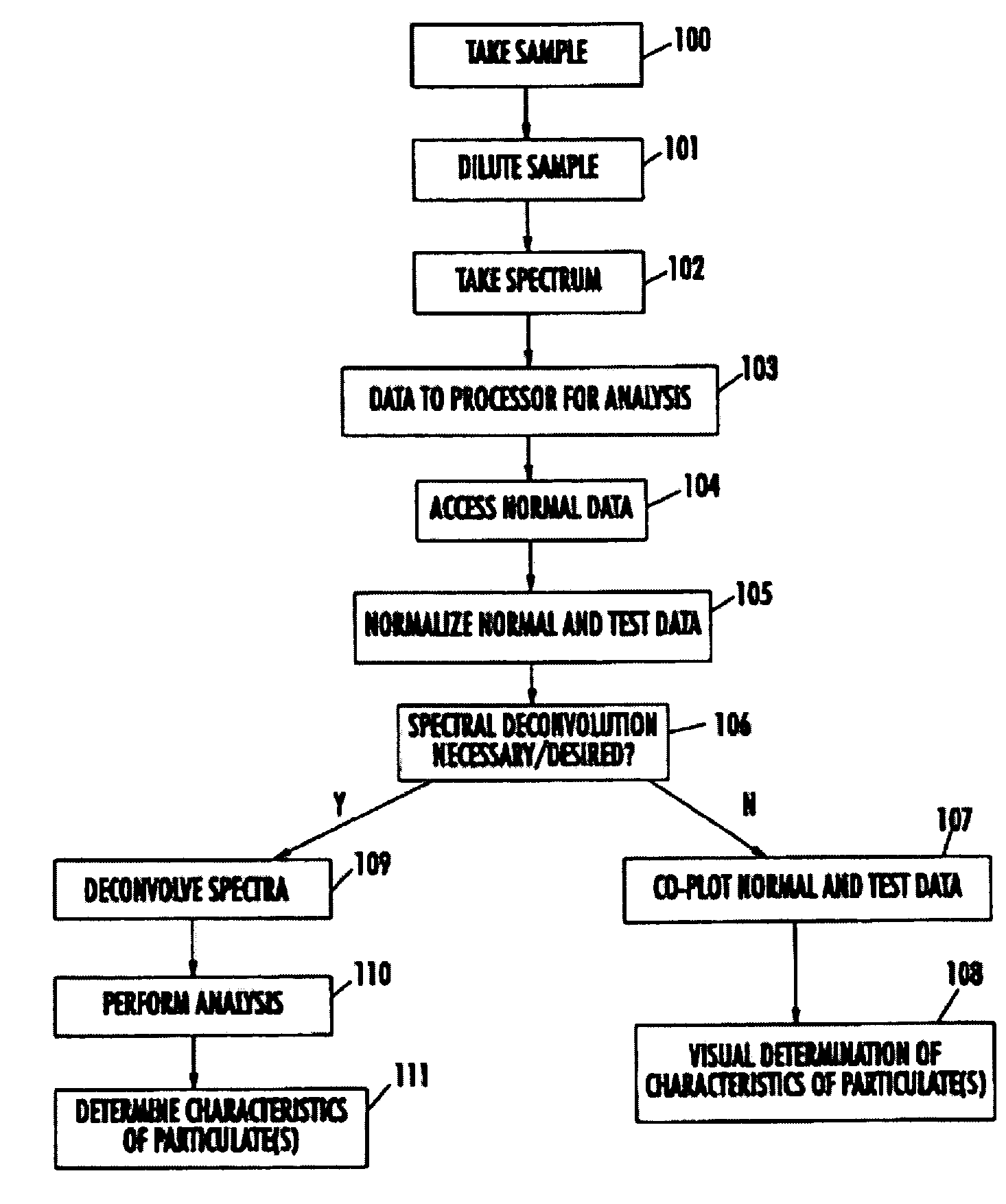
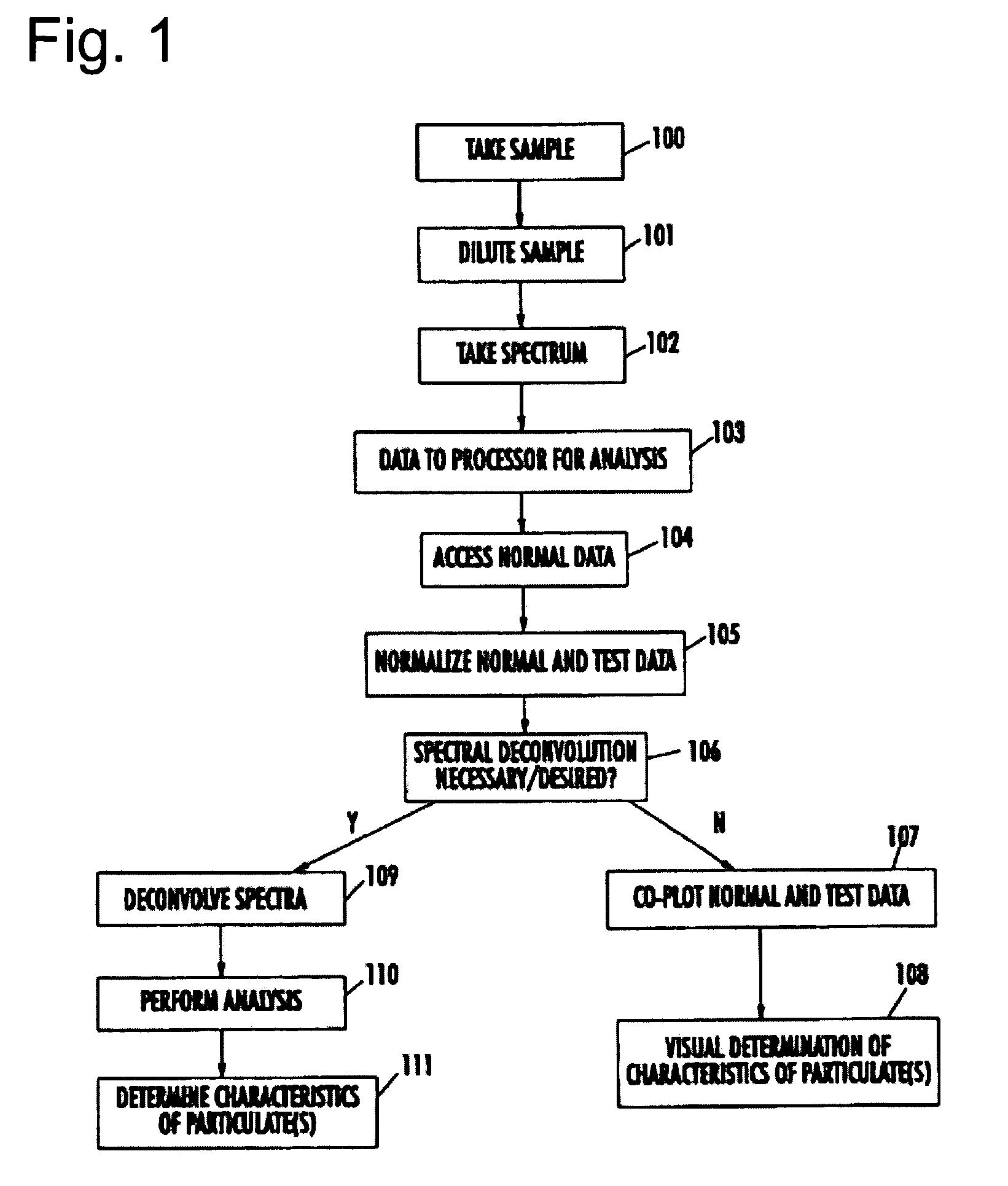
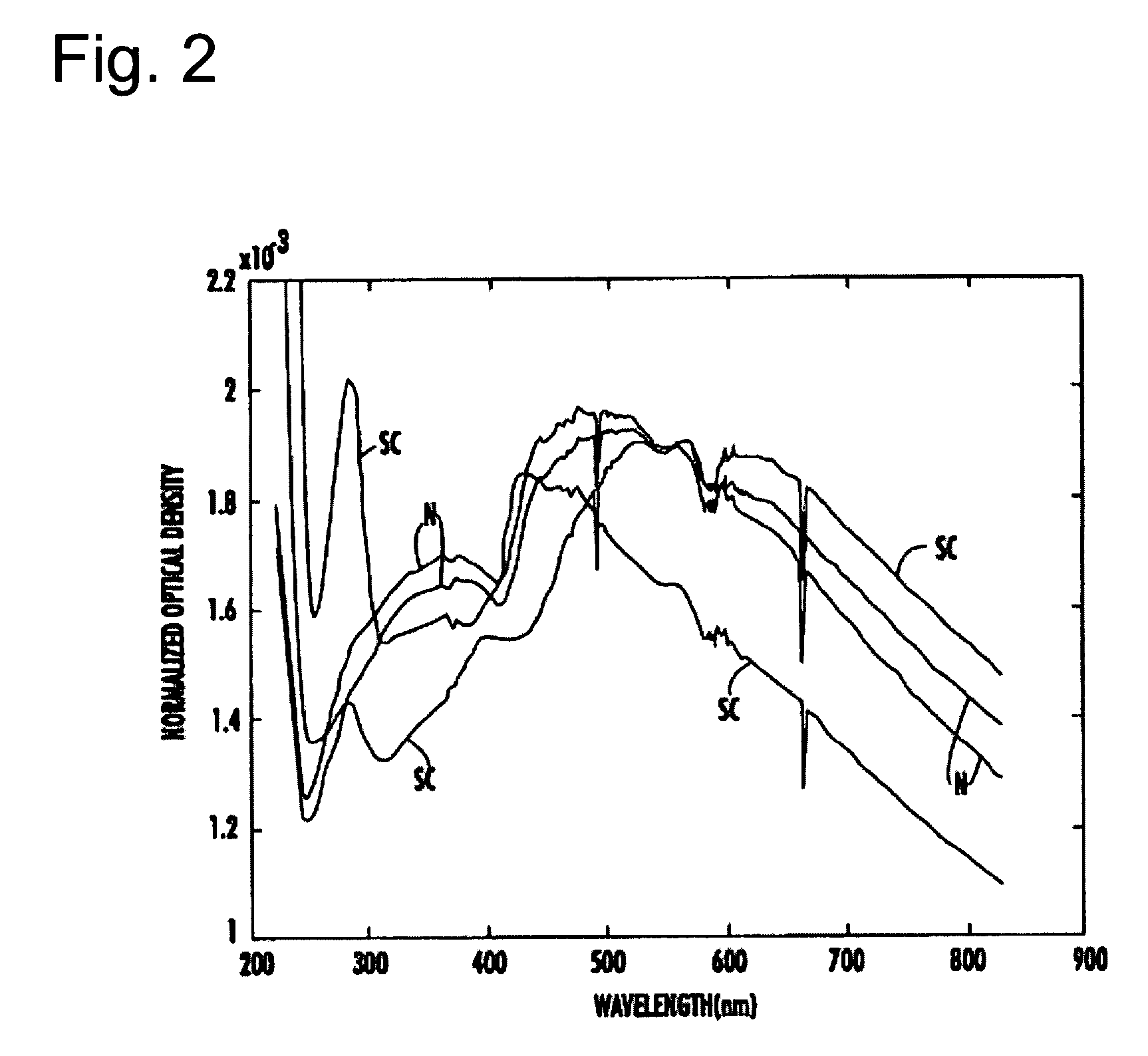
The present invention provides a method and apparatus for the detection of an infectious disease or disorder in a fluid, such as a mammalian blood sample, the detection of a specific protein in a urine sample, or the detection of a particle in a plasma. The identification of the particles of interest is enable by taking a transmission spectrum of a test sample in at least a portion of the ultraviolet, visible, near-infrared portion of the spectrum and comparing the spectrum with a standard sample spectrum. From the comparison it is then determined whether the fluid from the test sample contains an particle of interest, and an identity of the particle of interest is determined. Spectroscopic and multiwavelength turbidimetry techniques provide a rapid, inexpensive, and convenient means for diagnosis. The comparison and determination steps may be performed visually or by spectral deconvolution.
Owner:UNIV OF SOUTH FLORIDA
Method and reagent for latex sensitization
ActiveCN102161716AHigh clinical application valueShorten the timeBiological testingAntigenAntistreptolysin O
The invention relates to a method and a reagent for latex sensitization. Specifically, the method for latex sensitization comprises the following steps: combining latex with an unrelated protein by a chemical bond; and then, crosslinking an antigen or an antibody on the unrelated protein by glutaraldehyde so as to cause latex sensitization. The antistreptolysin O latex prepared with the method enhances an immune turbidimetry reagent. With the method disclosed by the invention, time for preparing the reagent can be shortened, and the sensitivity of the reagent is improved.
Owner:BEIJING MDC NEW SPRING MEDICAL DEVICES
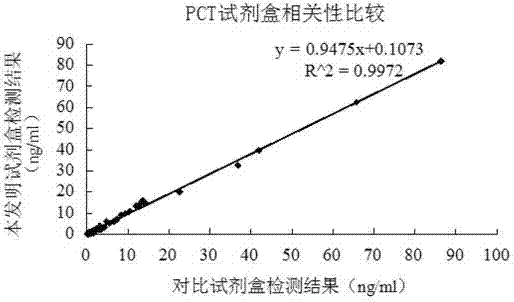
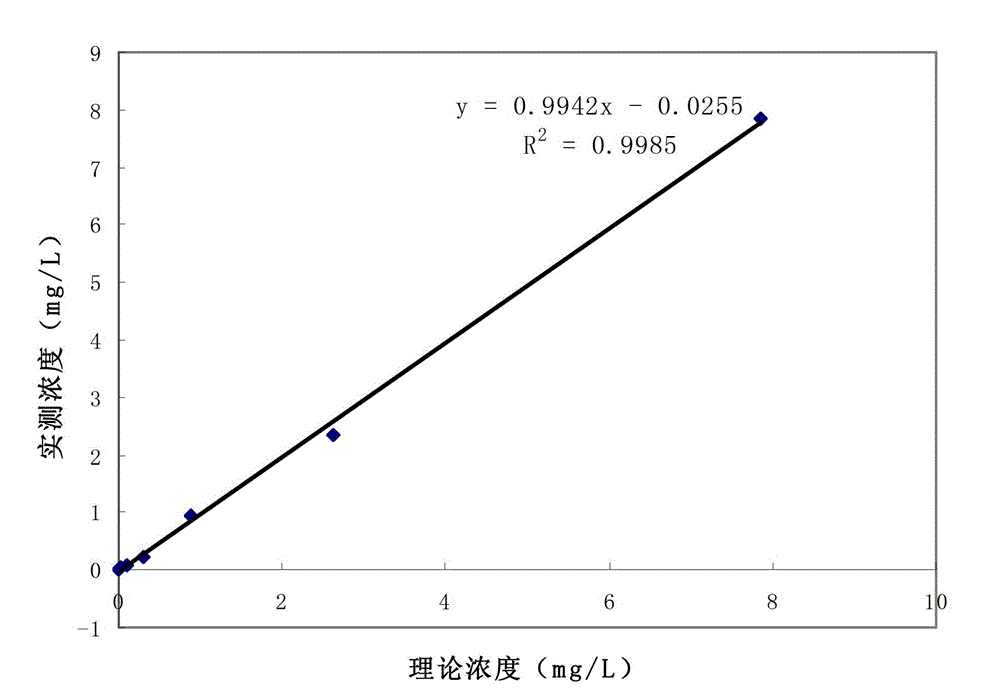
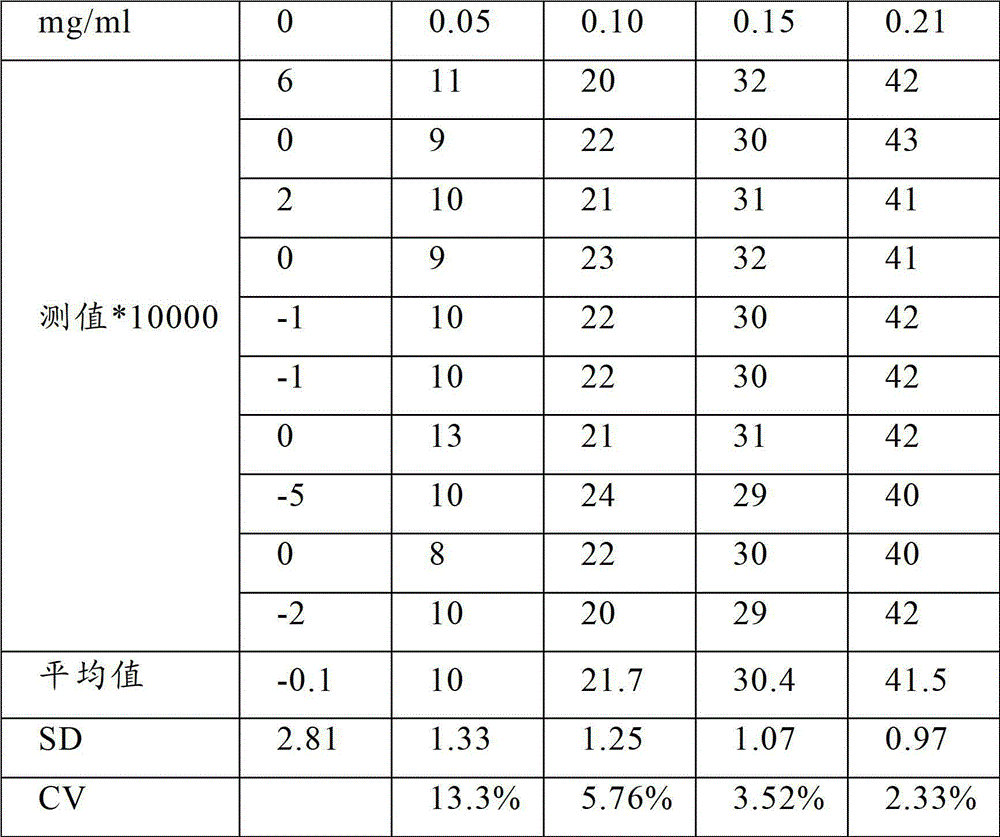
Latex enhanced turbidimetric immunoassay kit of quantitatively detecting procalcitonin PCT
ActiveCN102759631AHigh detection sensitivityMeet the needs of clinical applicationsMaterial analysis by observing effect on chemical indicatorBiotin-streptavidin complexTurbidimetry
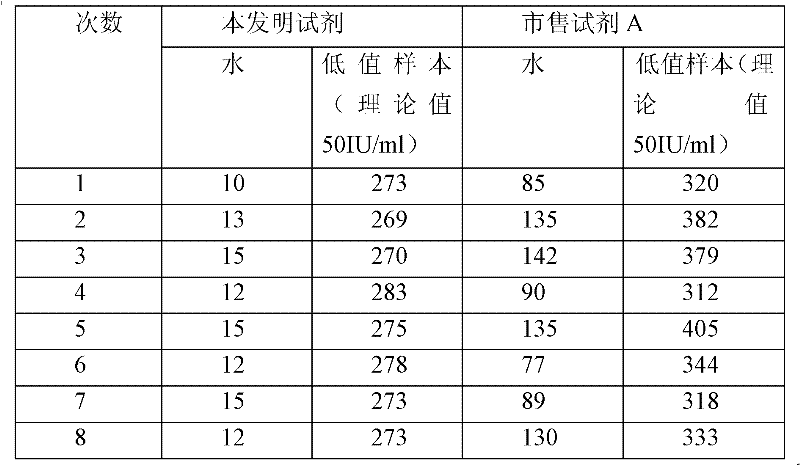
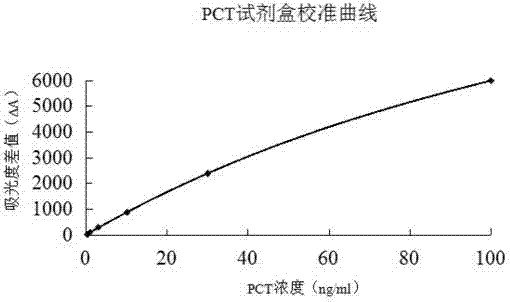
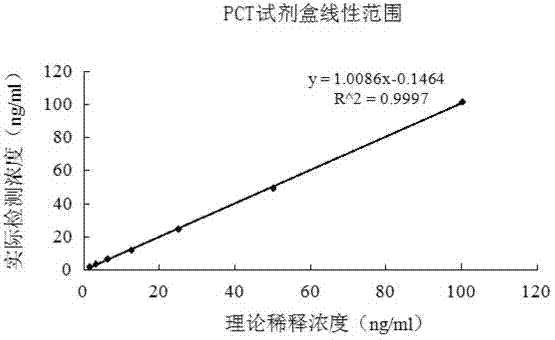
The invention relates to a latex enhanced turbidimetric immunoassay kit of quantitatively detecting procalcitonin PCT. The kit comprises an R1 reagent, an R2 reagent and a calibrator, wherein the R1 reagent comprises a protecting agent, a reaction enhancing agent, a preservative and buffer solution; the R2 reagent comprises a protecting agent, a preservative, buffer solution and anti-human PCT antibody coated sensitization polystyrene latex particles; the calibrator comprises a protecting agent, a preservative, buffer solution and PCT recombinant protein; the human PCT antibody in the R2 reagent is linked with polystyrene latex particles through streptavidin-biotin; and the particle diameter of the latex particles in the R2 reagent is 40-500 nm. The kit can be used on a biochemical analyzer and a scatter turbidimetry analyzer for quantitatively detecting the PCT content in human blood. The invention provides the PCT detection kit which has the advantages of convenience, quickness, high sensitivity, strong specificity and accurate quantification; and the kit has high instrument compatibility, is low in detection cost and meets the requirements on PCT turbidimetric products in clinical use.
Owner:NANJING NORMAN BIOLOGICAL TECH
Kit for performing retinol binding protein detection by using latex turbidimetry
InactiveCN102944679AHigh detection sensitivityGuaranteed SensitivityColor/spectral properties measurementsHydrogenTurbidimetry
The invention relates to the technical field of biotechnology, and particularly discloses a kit for detecting retinol binding protein content by using latex immunoturbidimetry. The kit comprises a reagent R1, a reagent R2 and a standard product, wherein the reagent R1 is buffer solution with pH (Potential of Hydrogen) value of 6-9; the reagent R2 is latex reagent coated by anti-retinol binding protein double antibodies; and the standard product is retinol binding protein solution with pH value of 5-8. According to the kit, the retinol binding protein content in a sample can be detected by using the latex immunoturbidimetry; the sensitivity is high and can reach 0.042 mg / L; and the kit has the advantages of high stability, easiness and quickness in operation, high specificity, low probability of interference, accurate quantification and broad application prospect.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
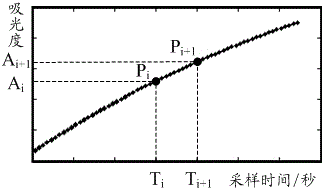
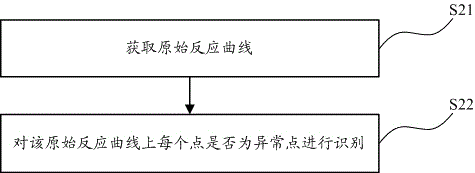
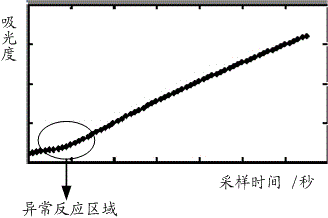
Identification method, correction method and alarm method for turbidimetry abnormal reaction curve
ActiveCN105466927AImprove accuracyMaterial analysis by observing effect on chemical indicatorBiological testingTurbidimetryReaction curve
The present invention provides an identification method, a correction method and an alarm method for a turbidimetry abnormal reaction curve. The identification method comprises: acquiring an original reaction curve; and identifying whether the abnormal point exists on the original reaction curve. With the embodiments of the present invention, the abnormal point identifying and alarming is innovatively performed on the turbidimetry reaction curve, the condition that the data possibly being subjected to the abnormal reaction is adopted as the detection result without the treatment is avoided, and the detection accuracy can be easily improved; and the identified abnormal point is corrected, such that the significantly-abnormal reaction curve can be well made up, and the detection accuracy is substantially improved.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD
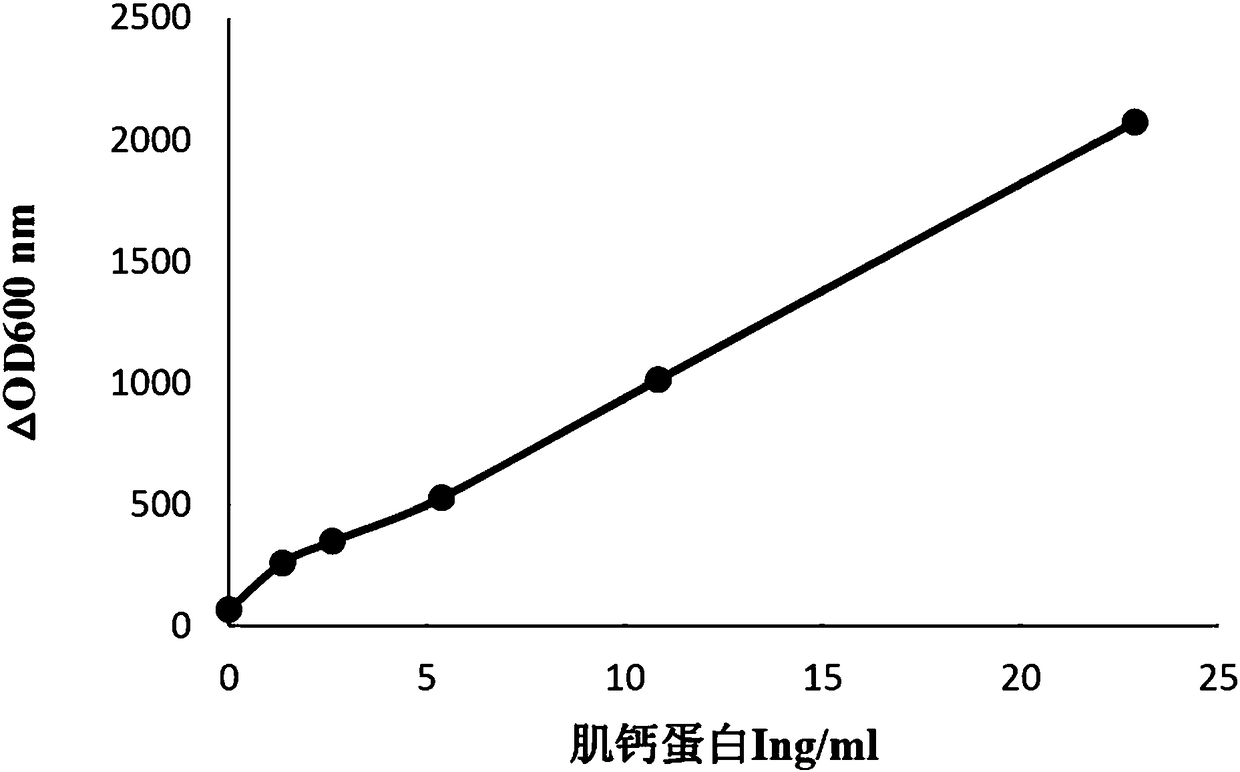
Troponin I latex-enhanced immunological turbidimetry detection kit
The invention relates to a troponin I latex-enhanced immunological turbidimetry detection kit which particularly comprises a first reagent and a second reagent. The first reagent comprises buffer solution, blocking agents, surface active agents, electrolyte, stabilizers, polyethylene glycol, chaotropic agents and preservatives; the second reagent comprises latex particles coated with human troponin I monoclonal antibodies, buffer solution, surface active agents, stabilizers and preservatives. The kit displays better interference resistance.
Owner:BEIJING STRONG BIOTECH INC
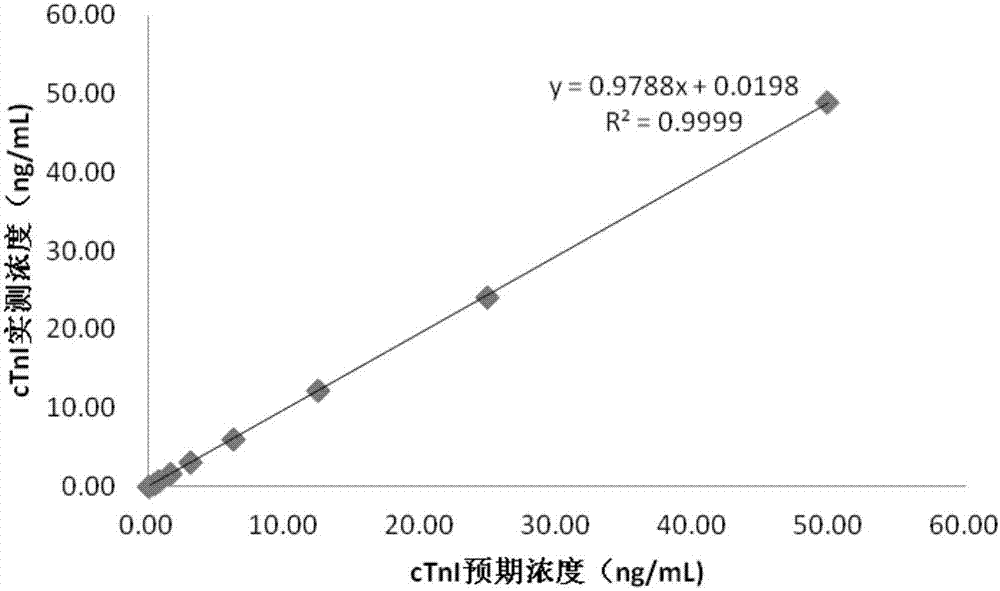
Method for detecting immunological turbidimetry of hypersensitive cardiac troponin I magnetic microspheres and detection kit
InactiveCN107422129ARapid responsePromote formationDisease diagnosisBiological testingTurbidimetryAntibody
The invention relates to the technical field of clinic kits and particularly relates to a method for detecting immunological turbidimetry of hypersensitive cardiac troponin I magnetic microspheres and a detection kit. The kit comprises a reaction diluent reagent, a cTnI antibody-coupled magnetic microsphere reagent and a cTnI correction product. Compared with a detection result of an existing cTnI chemiluminiscence detection kit, the method and the detection kit have the advantages that by carrying out statistic analysis, the relativity is good, no remarkable difference exists, the kit can be clinically popularized and used, and a relatively good choice is provided for the detection of cardiac troponin I.
Owner:北京科跃中楷生物技术有限公司
Method and device for testing platelet aggregation
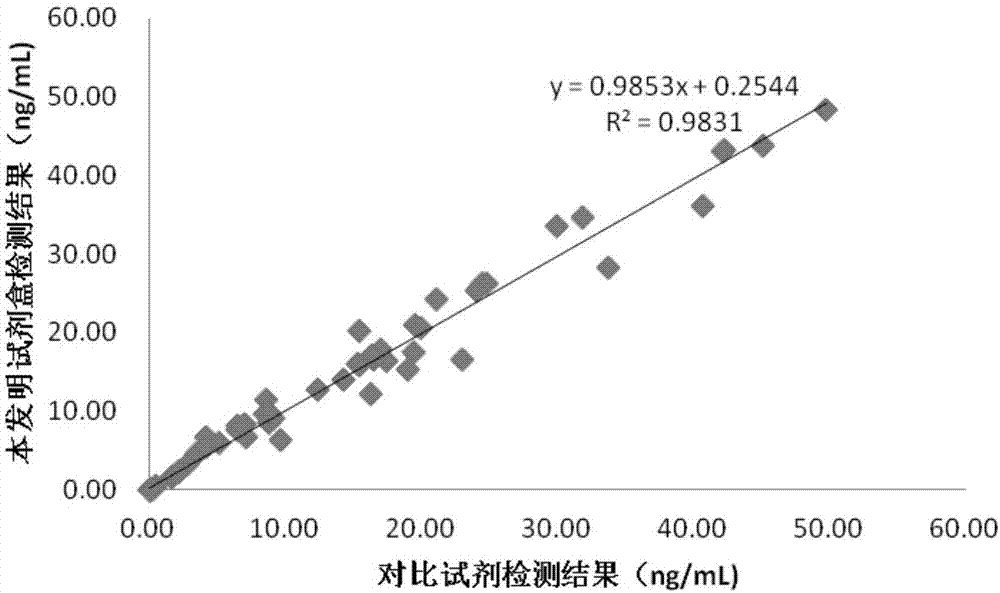
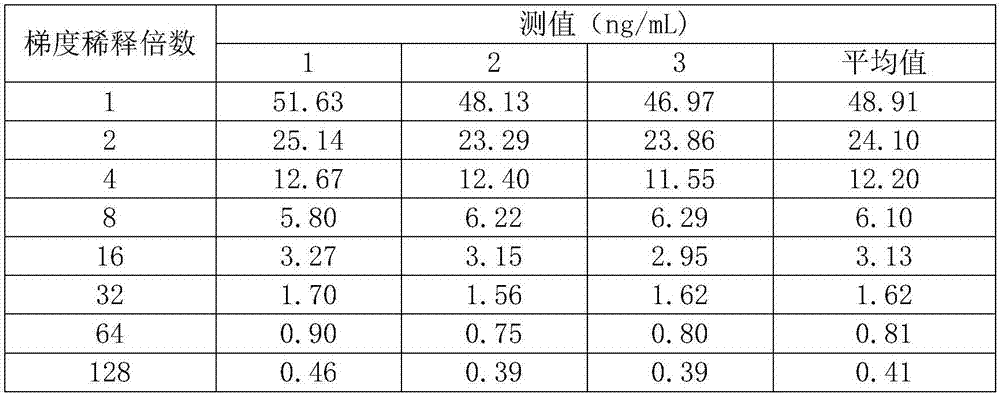
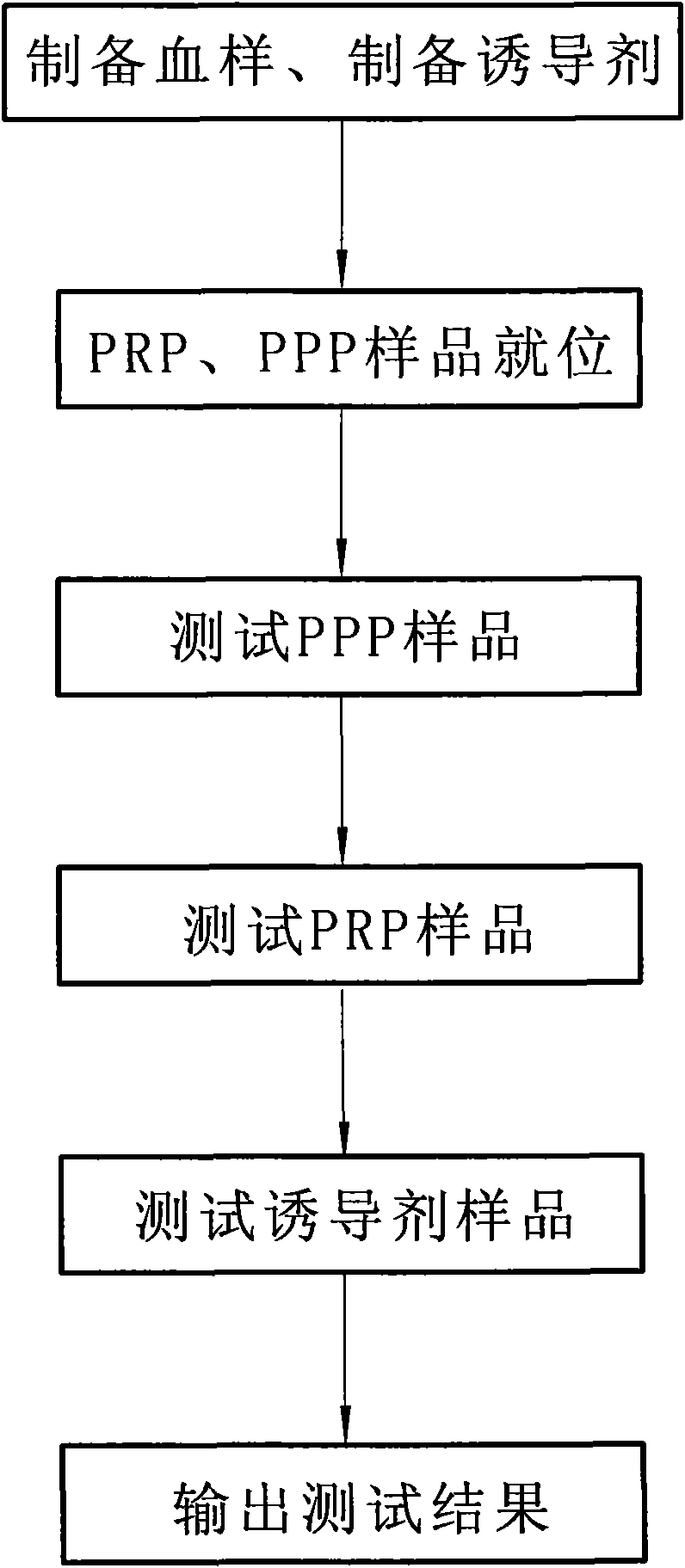
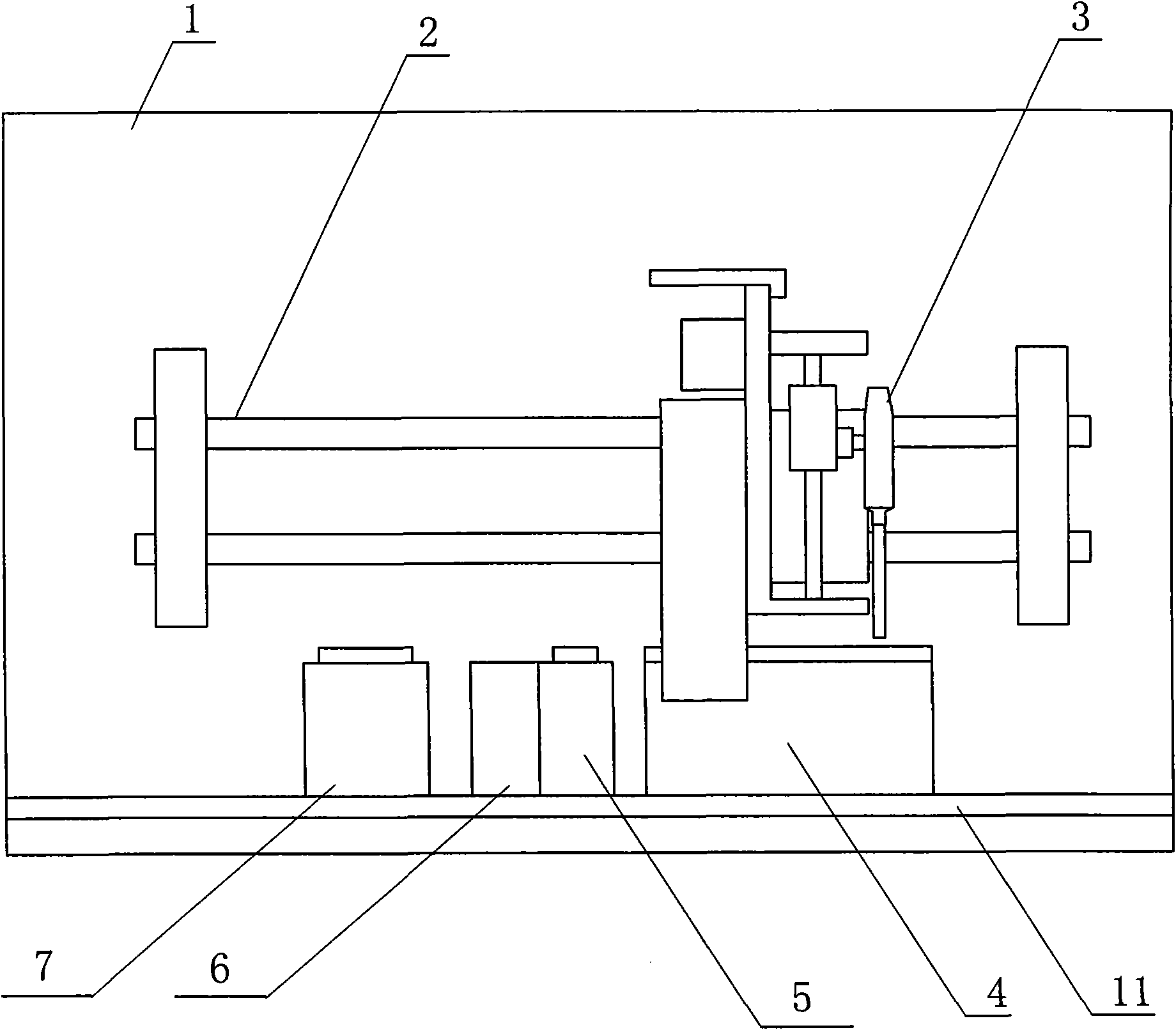
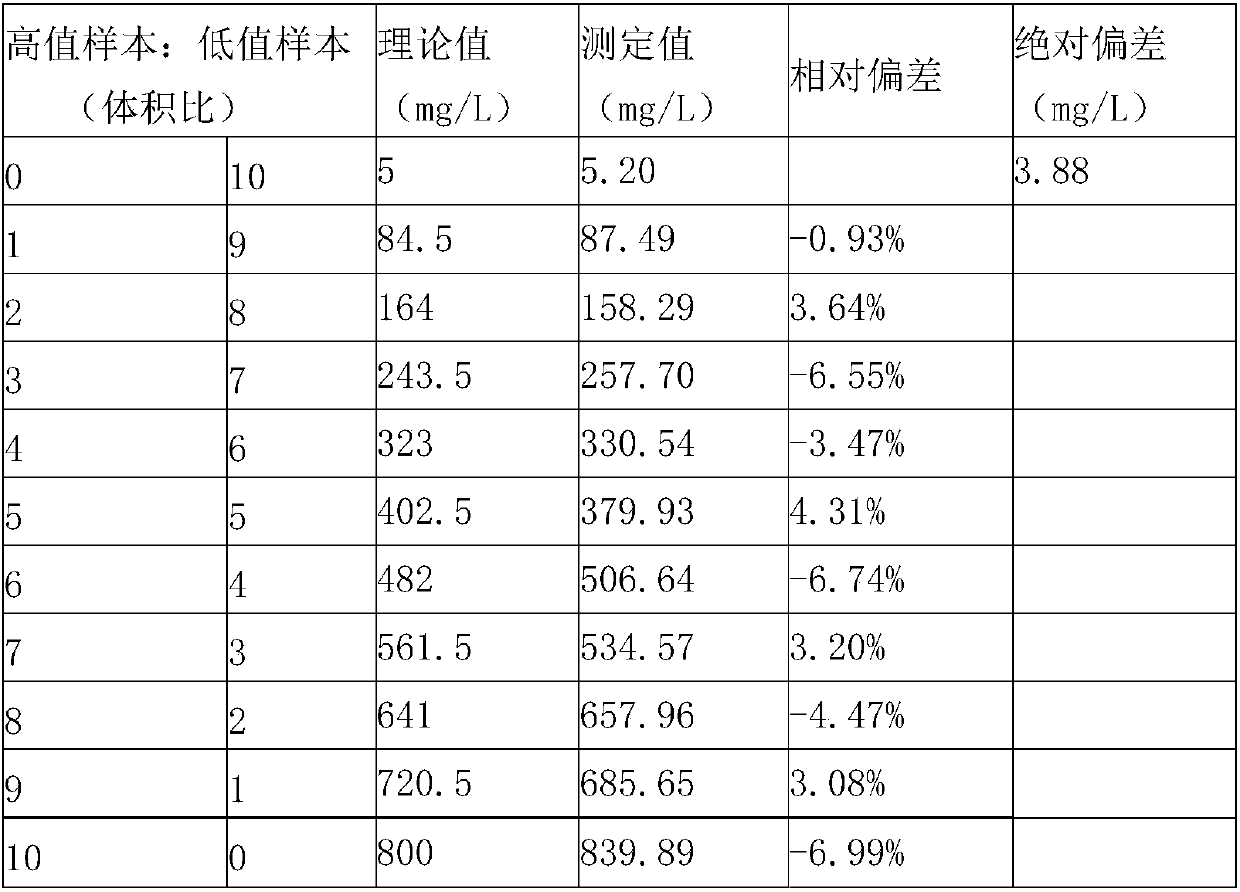
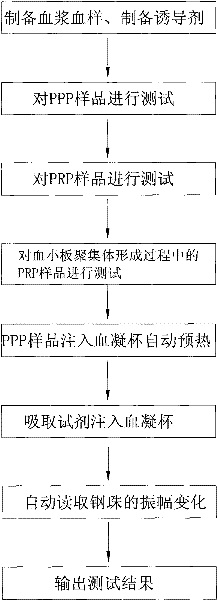
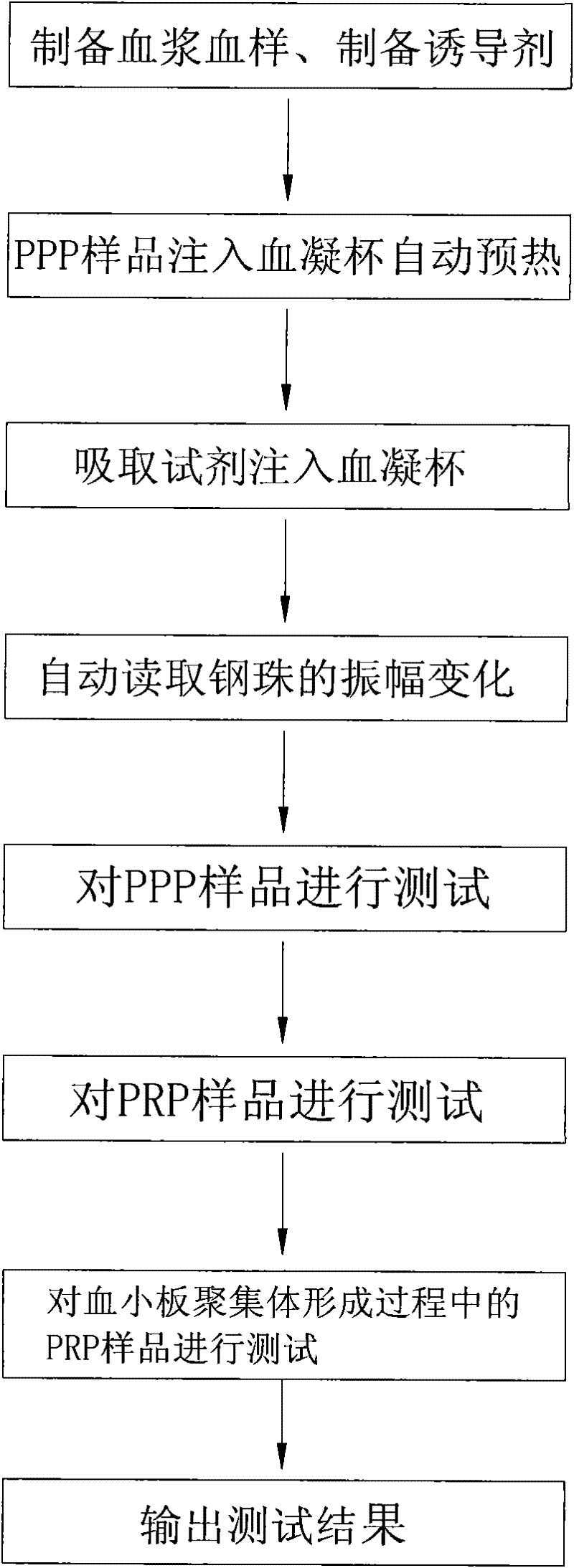
ActiveCN101881737AFast testFully automatedInvestigating moving fluids/granular solidsTransmissivity measurementsTurbidimetryBlood plasma
The invention discloses a method for testing platelet aggregation. A fully automatic platelet aggregometer is used for testing according to photoelectric turbidimetry. The method comprises the following test steps of: preparing a platelet aggregation inducer, putting prepared platelet rich plasma and platelet poor plasma into a sample disk device, instructing a sample adding needle to absorb the platelet poor plasma, injecting the platelet poor plasma into a test cup of a test core device and reading the transmittance data of a of a platelet poor plasma sample; instructing the sample adding needle to absorb the platelet rich plasma, injecting the platelet rich plasma into the test cup of the test core device and reading the transmittance data of a platelet poor plasma sample; instructing the sample adding needle to absorb the platelet aggregation inducer, injecting the platelet aggregation inducer into the test cup of the test core device and reading the transmittance data of an inductor sample; and calculating the maximum platelet aggregation rate of a tested blood sample and generating a test report according to input patient information.
Owner:山东泰利信医疗科技有限公司
Detection kit for fibrous protein or fibrinogen degradation products
ActiveCN104198724AHigh refractive indexHigh chemical inertnessBiological testingFiberFibrinogen degradation product
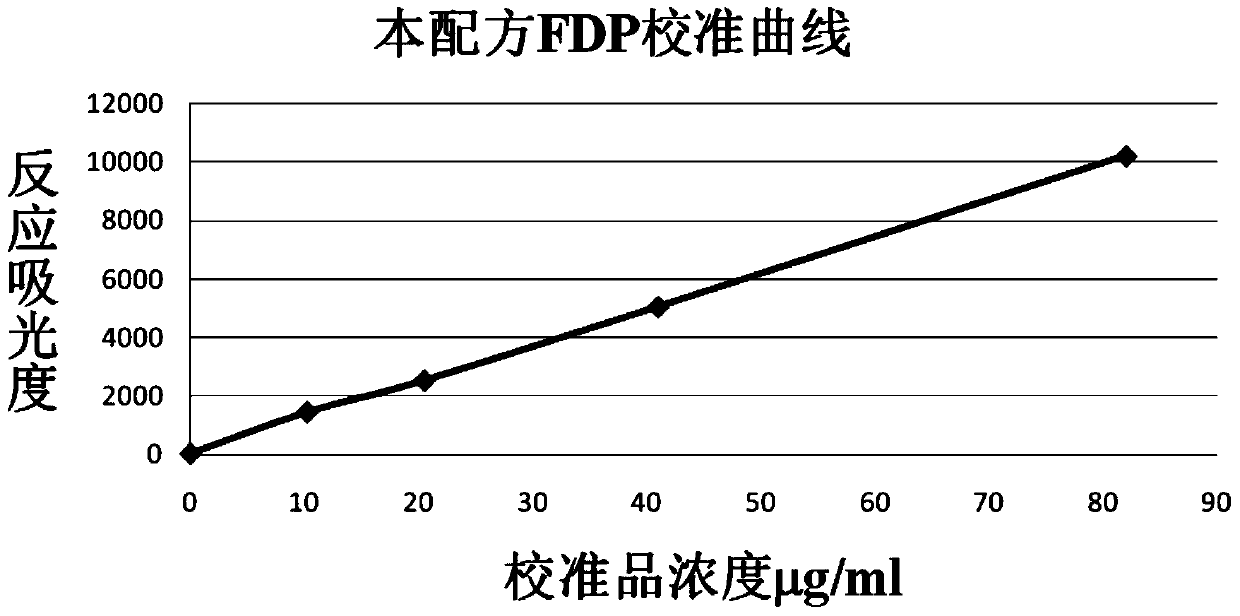
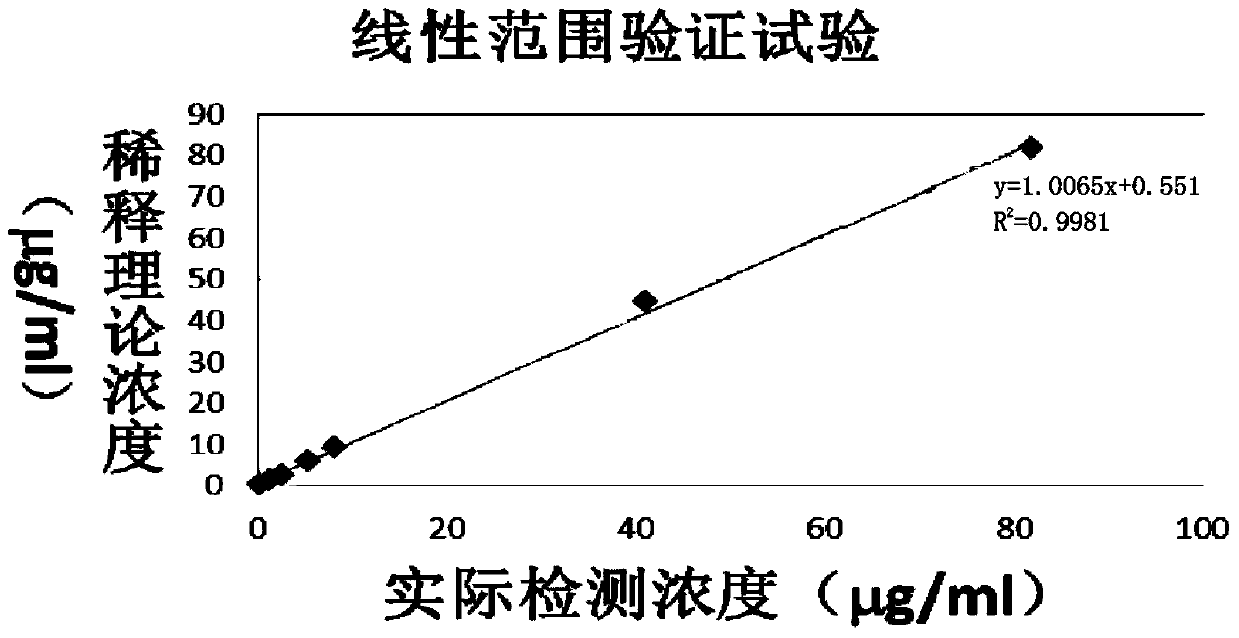
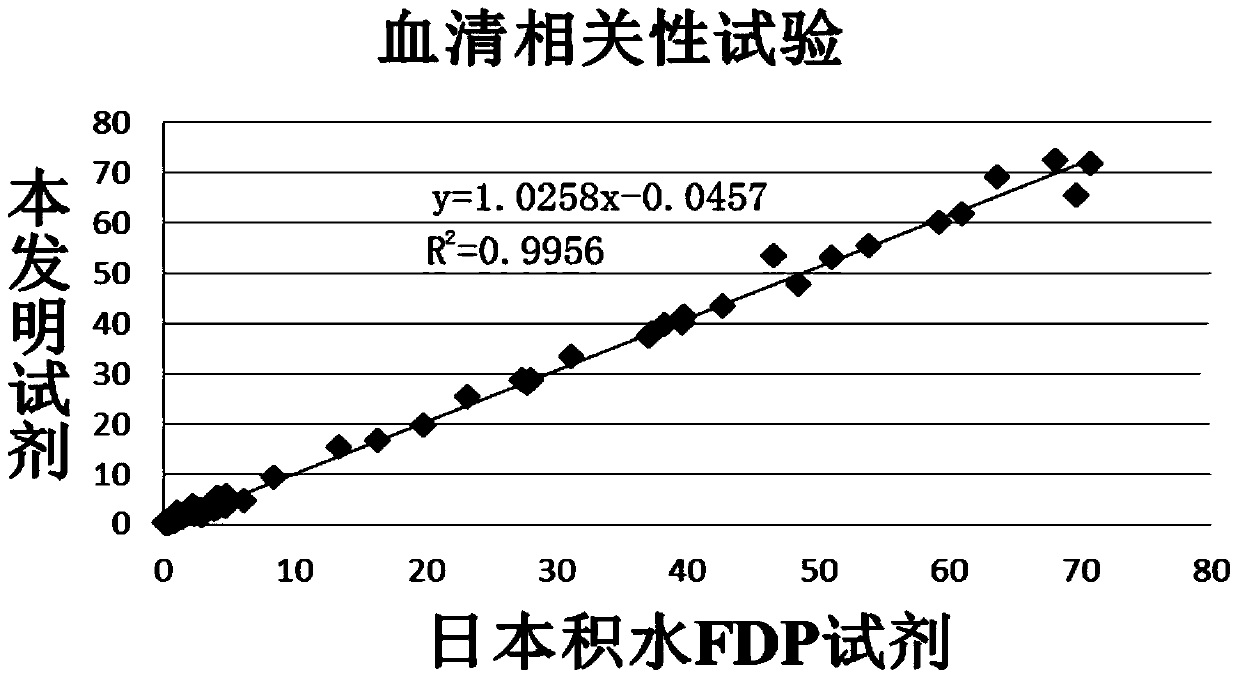
The invention relates to a detection kit for of fibrous protein or fibrinogen degradation products. The detection kit consists of an FDP R1 reagent, an FDP R2 reagent and an FDP standard product which are independent respectively. The invention establishes a method for determining the content of FDP in serum or blood plasma of a human body by coupling FDP antibodies and latex particles and adopting a latex-enhanced turbidimetry. Compared with the prior art, the kit can be used for detecting the FDP with the concentration range being 0.25-80microgram / ml in the serum or the blood plasma, and the problem of narrow detection linearity of the existing kit is solved; simultaneously, when being applied to carry out FDP test, the kit has the advantages of simplicity in operation, high accuracy, good repeatability and high sensitivity, and can be used on a full-automatic biochemical analyzer, a special protein instrument and a spectrophotometer.
Owner:上海睿康生物科技有限公司
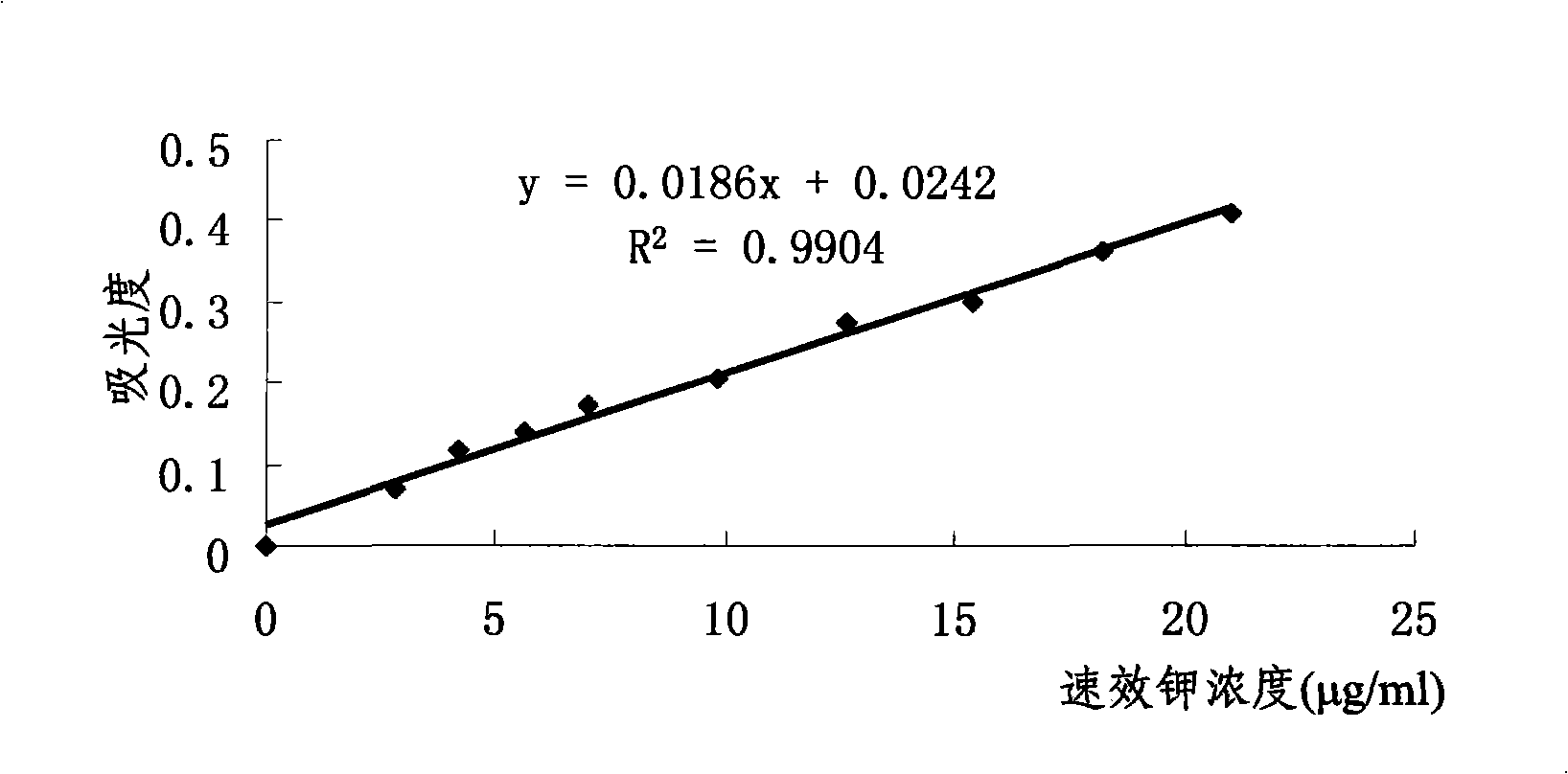
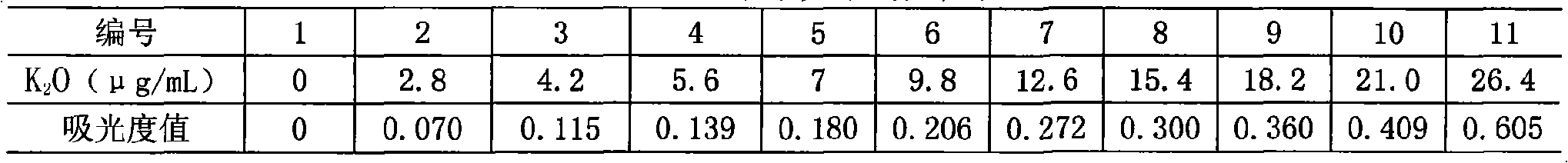
Method for measuring kalium in soil by tetraphenylboron sodium nephelometery and its screening agent
InactiveCN101271072AEliminate distractionsDoes not affect linearityMaterial analysis by observing effect on chemical indicatorPotassiumTurbidimetry
The invention discloses a method for measuring potassium by the sodium tetraphenylborate turbidimetry and a masking agent which is used by the method. The masking agent is a water solution containing 0.01 to 0.04mol / l of Cu<2+>, 0.04 to 0.10mol / l of tartaric acid and 5 to 10mol / l of H<+>; the method for measuring the potassium by the sodium tetraphenylborate turbidimetry is that: the masking agent is used for masking ammonium ion and other interfering ions in liquid under test, basic EDTA disodium salt solution is further added to carry out the auxiliary masking, basic sodium tetraphenylborate water solution is finally added, and the turbidity measurement is immediately carried out after shaking the reaction solution well. The measurement method has the advantages of simple operation, rapid and accurate measurement and low cost; the linear range thereof meets the requirements of the rapid measurement, which is suitable for the application of grass roots and agriculture popularization departments; and the masking agent used does extremely small harm to the human body and the environment.
Owner:河南农大迅捷测试技术有限公司
Hemolytic agent, method for pretreatment of biological sample, method for determining content of target substance, and kit
ActiveCN106546455AReduce the binding forceOptimize the reaction systemMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationHemolytic AgentsTurbidimetry
The invention discloses a hemolytic agent, a method for pretreatment of a biological sample, a method for determining the content of a target substance, and a kit. The invention provides a use of a boric acid buffer system in preparation of the hemolytic agent, the hemolytic agent for immunological turbidimetry determination, the method for pretreatment of the biological sample, the method for determining the content of the target substance in the biological sample, and the kit for immunological turbidimetry determination. The hemolytic agent is used for immunological turbidimetry determination. When the hemolytic agent prepared by the boric acid buffer system is used for immunological turbidimetry determination, the hemolytic agent has at least one of the following advantages of high sensitivity, fast and simple detection process and strong specificity.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD
Online method for measuring oil density in oil containing sewage and device thereof
InactiveCN1687746AAvoid inhomogeneityEnsure stabilityPreparing sample for investigationColor/spectral properties measurementsUltrasonic emulsificationDisplay device
The invention discloses the online measuring the oil concentration of the sewage and its equipment. The method adopts the ultrasonic emulsification oil-water mixture and uses the optical nephelo method to test the oil concentration. The equipment comprises the measuring section vertically put on the sewage pipeline, the ultrasonic oscillator set at the input and the output ends of the measuring section; three pairs of white gem windows transmitting beams set at the outside pipe wall, and the windows arrange in 120 Deg angle mutually; three groups of photoelectric converters are set corresponding to the windows; connect the industrial controller with a displayer through the signal receiver and the amplifier. When the sewage flows through the test section, the ultrasonic oscillator emulsificates the sewage and mixes it evenly; the testing beam is the violet beam with fixed wavelength; the photoelectric converter converses the optical signal into the electric signal and the optical nephelometer calculates the oil concentration of the sewage.
Owner:XI AN JIAOTONG UNIV
Kit for measuring microalbuminuria by adopting immune competition turbidimetry
InactiveCN107741493AMeet the screeningTreatment Level MonitoringMaterial analysisNormal peopleMicrosphere
The invention provides a kit for measuring microalbuminuria by adopting immune competition turbidimetry. The kit comprises an anti-human albumin antibody, human albumin marked leatexbeads and a buffersolution. The anti-human albumin antibody and the human albumin marked leatexbeads are separately placed. The particle size of the leatexbeads is 80-267 nm. When the kit is used for measuring microalbuminuria in human urine, the maximum linear range is 5 mg / L-1500 mg / L. The kit can meet screening of clinical normal people, and can also avoid result underestimation or even false negative result arising from a hook effect caused by antigen excess.
Owner:HUNAN HY BIOPOCT TECH CO LTD
A kind of assay method and equipment for platelet aggregation and coagulation factor
ActiveCN102262090AFast testProtect your healthMaterial analysis by optical meansPlatelet aggregation ratioTurbidimetry
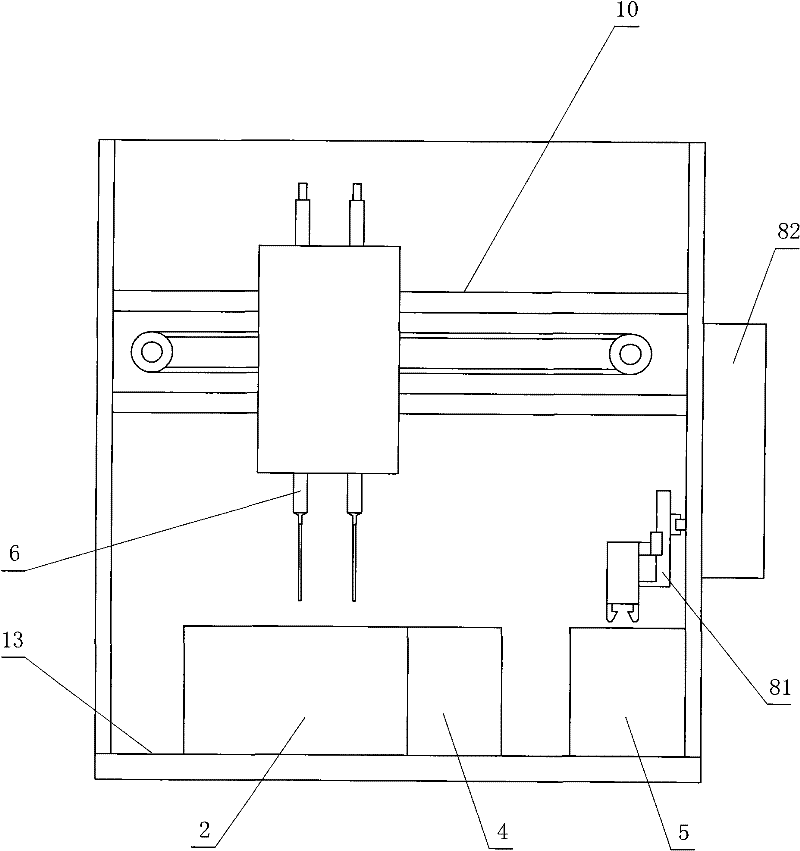
The invention relates to a method for measuring the platelet aggregation and a blood coagulation factor. In the method, a full-automatic coagulate blood analytical instrument is used, the platelet aggregation is measured by using photoelectric turbidimetry and the blood coagulation factor is measured by using a double magnetic circuit and bead method. The method comprises the following measuring steps of: preparing a platelet aggregation inductive agent, platelet rich plasma and platelet poor plasma; respectively reading light transmittance data of a platelet poor plasma sample and light transmittance data of a platelet rich plasma sample as well as light transmittance data of the platelet rich plasma during the forming process of the platelet aggregation within the specified time; calculating the platelet aggregation rate of the measured blood sample; absorbing the platelet poor plasma and injecting the platelet poor plasma in a hemagglutination cup; absorbing the selected reagent according to a test program and injecting the selected reagent into the hemagglutination cup; automatically monitoring the amplitude of a steel bead; and generating a test report by using measuring results of the platelet aggregation and the blood coagulation factor according to input information of patients.
Owner:BEIJING PRECIL INSTR CO LTD +1
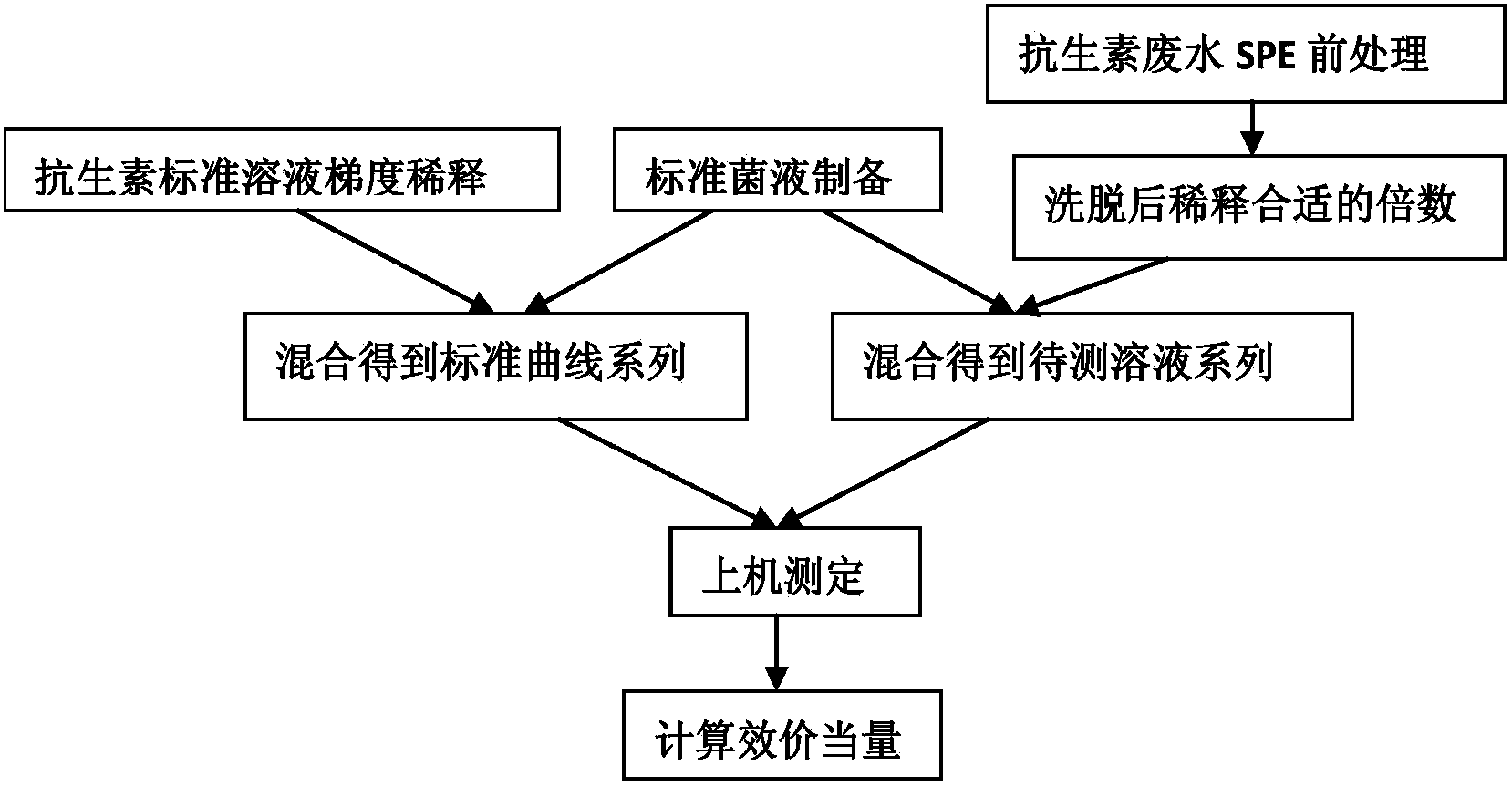
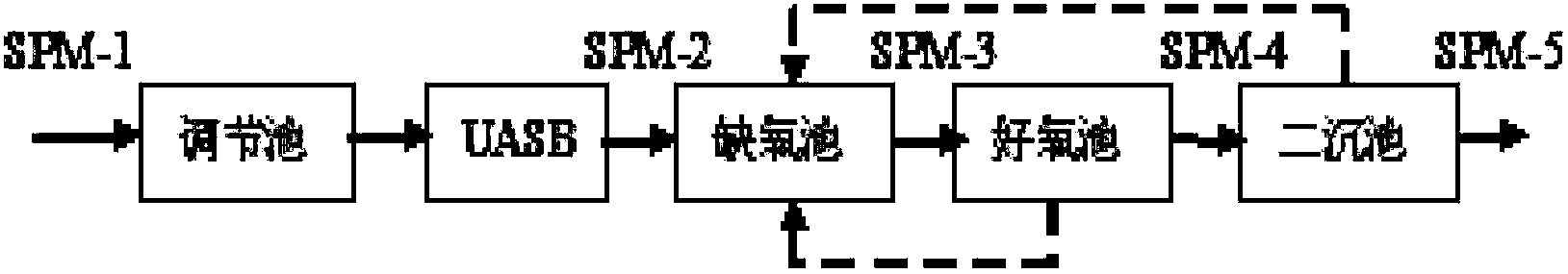
Method for evaluation of residual biopotency of antibiotics in waste water
ActiveCN103913426AUniversalLess resistant bacteriaMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationMetaboliteWastewater
The invention discloses a method for evaluation of comprehensive biopotency (potency equivalent) of antibiotics in waste water, and the method comprises determination of absorbancy of the waste water by use of turbidimetry and calculation of the residual biopotency of the antibiotics in the waste water. According to the method, different antibiotics in the wastewater and antibiotic metabolites with antimicrobial activity groups can be s measured accurately, and the operation is simple, so that the method can be used for comparison of waste water with different types of antibiotics or comprehensive evaluation of mixed waste water with various antibiotics, and has universality on evaluation of the residual biopotency of the waste water with different types of antibiotics.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Full-automatic determining instrument of red blood cell osmotic fragility
ActiveCN103033482AFully automatedThe detection process is fastColor/spectral properties measurementsData displayPeristaltic pump
The invention discloses a full-automatic determining instrument of red blood cell osmotic fragility. The full-automatic determining instrument is based on the principle of light-proof turbidimetry and consists of a sample absorbing device, an automatic electromechanical device, a light detector and a circuit system, wherein the sample absorbing device is used for absorbing a blood sample through the instrument; the automatic electromechanical device is used for accomplishing various control actions in the automatic measurement of the instrument; the light detector is used for radiating reaction liquid to generate a permeable light signal; the circuit system is used for controlling various stepper motors, electromagnetic valves and peristaltic pumps, converting the light signal into an electric signal, amplifying the electric signal and converting the electric signal into a digital signal so as to carry out data processing analysis, data display and communication between the instrument and a PC (Personal Computer). The full-automatic determining instrument is convenient to operate, rapid in measuring speed, high in precision and good in repeatability, and the information of the red blood cell osmotic fragility of the blood sample can be obtained through full-automatic detection.
Owner:广东华赢医疗科技有限公司
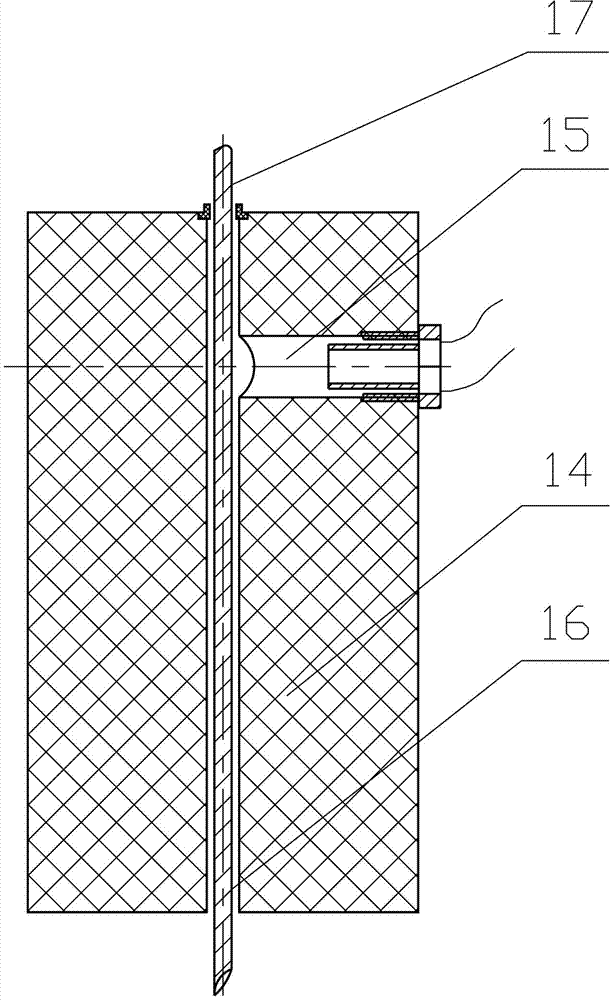
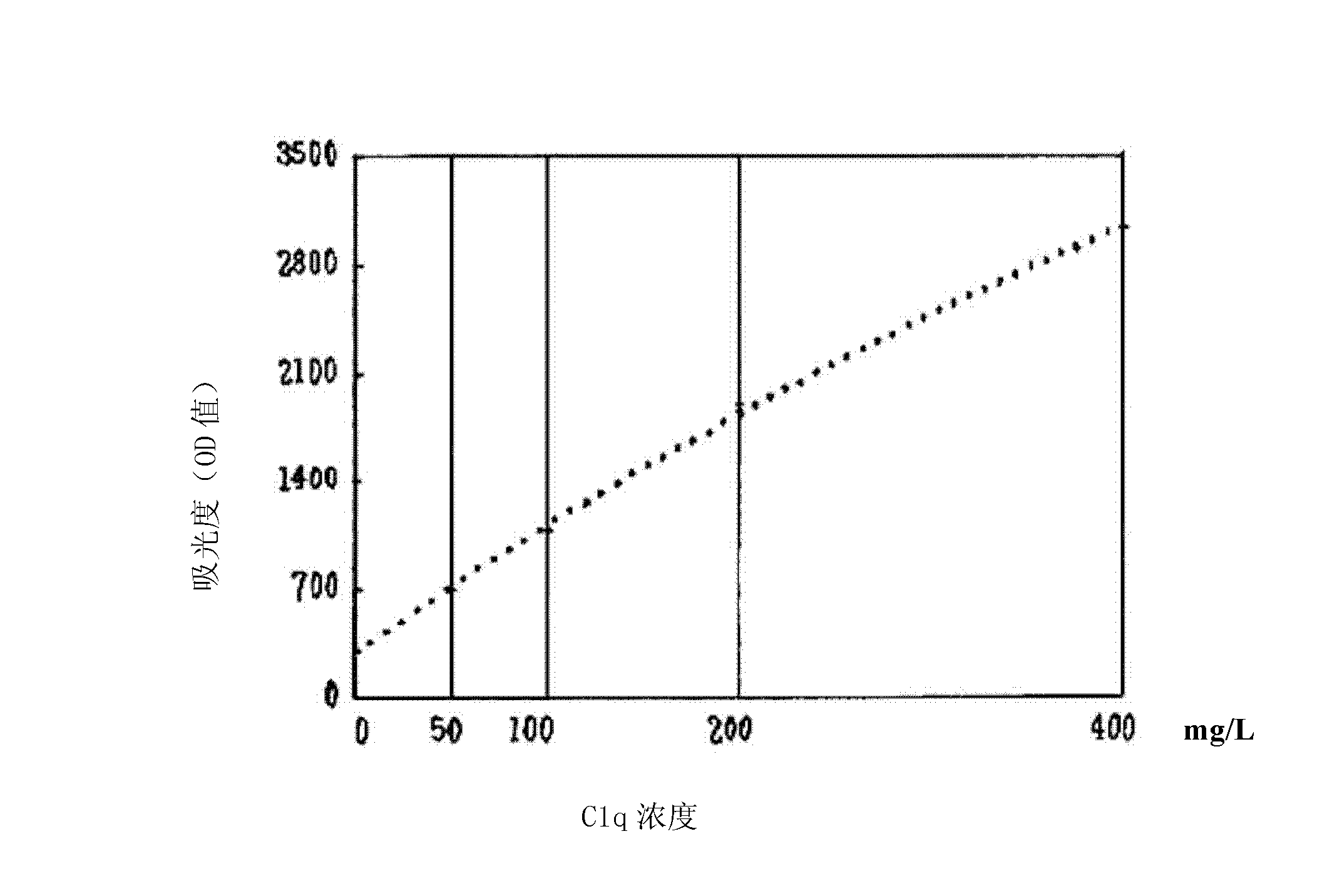
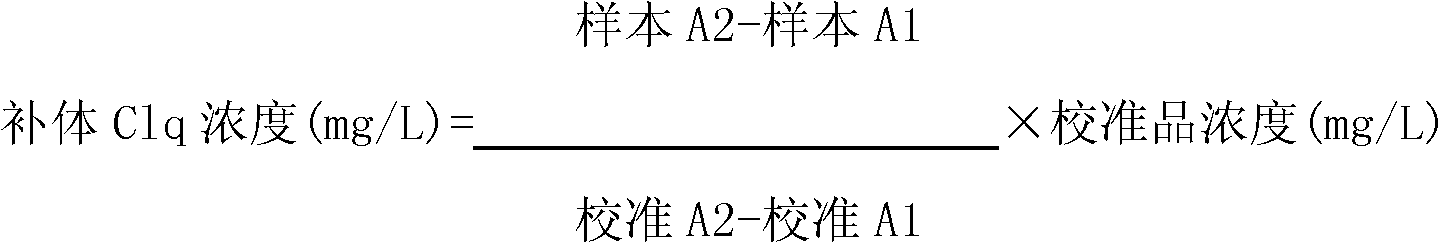
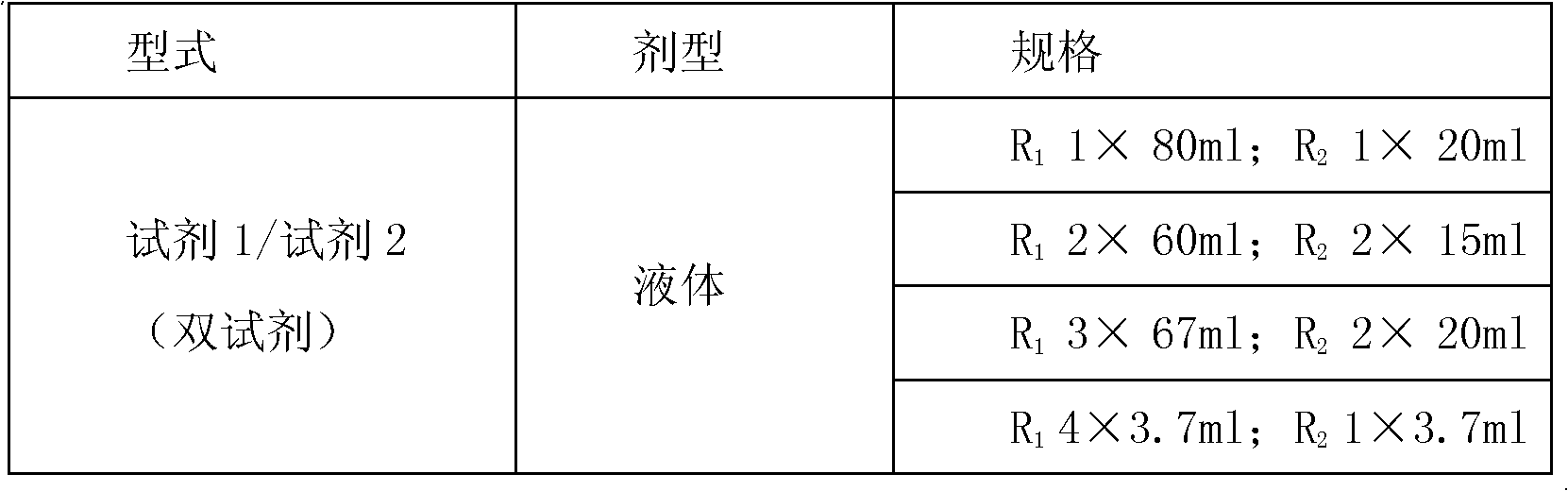
Kit and method for detecting concentration of complement Clq in human serum
ActiveCN102323427ASignificant technological progressEasy to measureMaterial analysis by observing effect on chemical indicatorBiological testingSorbentTurbidimetry
The invention belongs to the field of biological engineering, and provides a kit for detecting the concentration of a complement Clq in human serum by an immune transmission turbidimetry and an immune scattering turbidimetry. The kit solves the technical problems that an immune diffusion method and an enzyme-linked immune sorbent assay (ELISA) double antibody sandwich technology for measuring theconcentration of the complement Clq have complicated steps and are low in accuracy and repeatability in the prior art. The kit comprises two reagents, wherein a first reagent consists of disodium hydrogen phosphate, monopotassium phosphate, polyethylene glycol 6000 (PEG6000), ethylene diamine tetraacetic acid (EDTA)-NA2 and TX-100; and a second reagent consists of the disodium hydrogen phosphate,the monopotassium phosphate, the PEG 6000, the EDTA-NA2, the TX-100 and rabbit antihuman complement Clq antiserum. The invention also provides a method for detecting the concentration of the complement Clq in the human serum by using the kit. The kit and the method for measuring the concentration of the complement Clq have simple and convenient steps, are high in accuracy and repeatability and are used for automated analysis meters.
Owner:上海北加生化试剂有限公司
Cystatin C latex-particle-enhanced turbidimetry detection reagent kit and application thereof
ActiveCN105738617AEnhanced detection signalHigh detection sensitivityMaterial analysisLatex particleTurbidimetry
The invention provides a cystatin C latex-particle-enhanced turbidimetry detection reagent kit and application thereof.The reagent kit comprises a reagent R1 and a reagent R2.The reagent R1 is prepared from a buffer solution, inorganic salt, a surfactant, a preservative, a stabilizer and interference elimination protein.The reagent R2 is prepared from a buffer solution, inorganic salt, a surfactant, a preservative, a stabilizer and a polystyrene latex particle mixture, and the polystyrene latex particle mixture is interlinked with a cystatin C antibody.The reagent kit based on latex-particle-enhanced turbidimetric immunoassay (PETIA) can be generally applied to analysis of various full-automatic biochemical analyzers and is short in assay time, good in specificity, high in precision and good in accuracy when used.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
Pullorum staining agglutination antigen as well as preparation method and application thereof
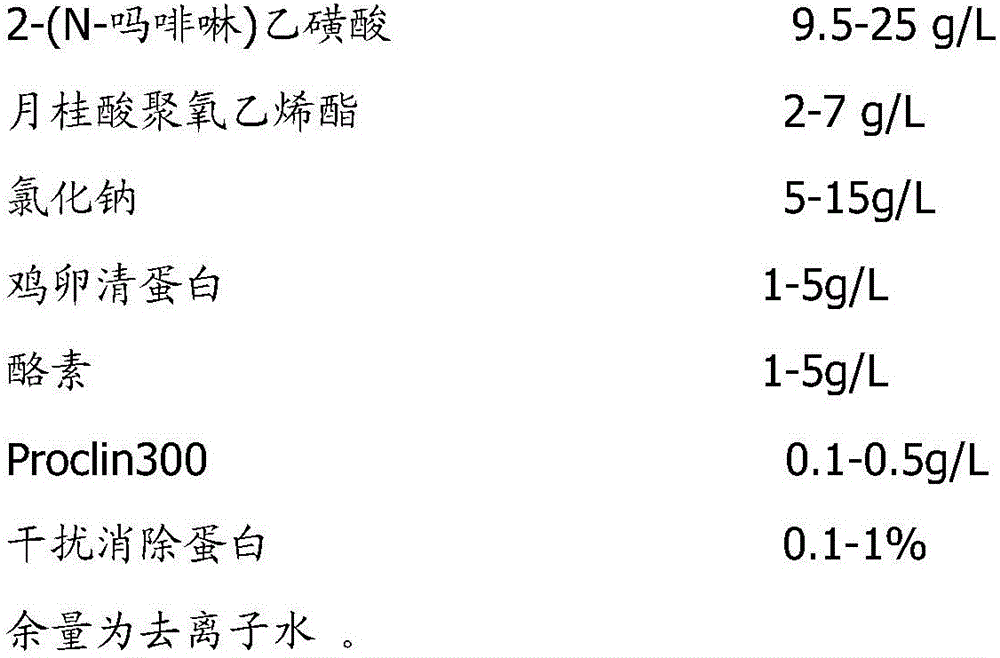
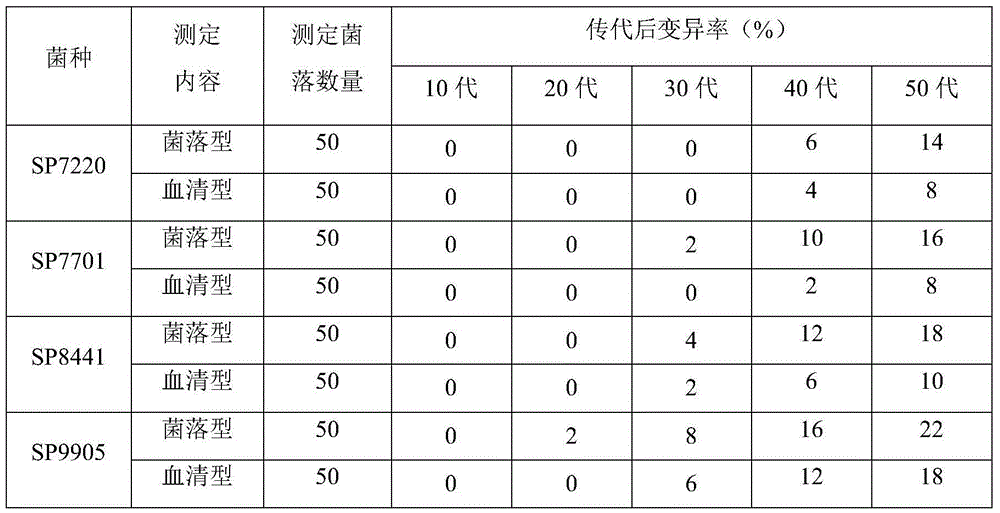
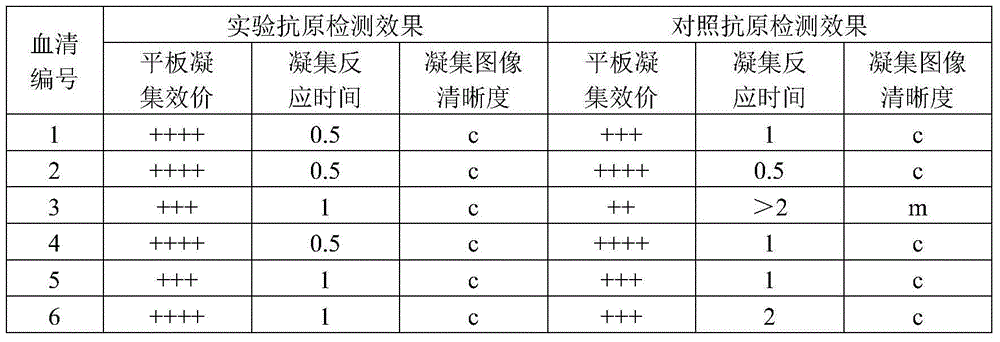
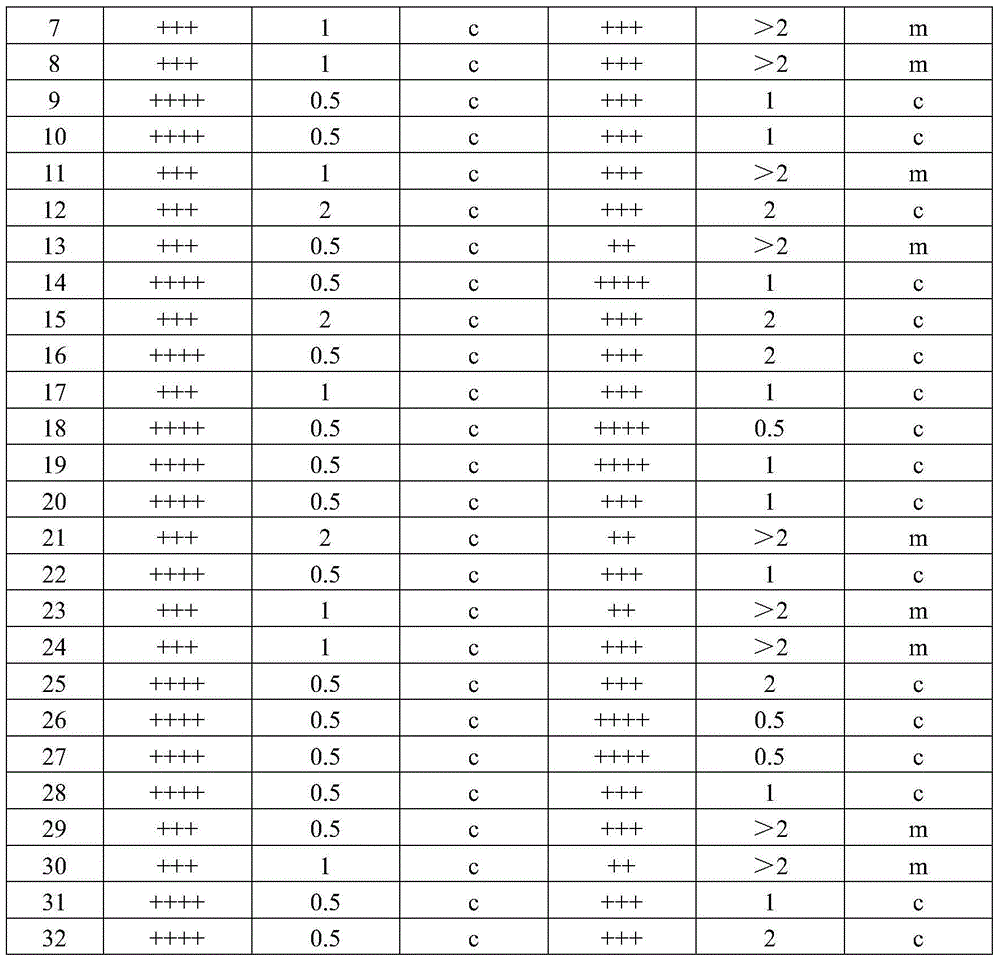
ActiveCN104789500ALow variabilityGood antigenicityBacteriaMicroorganism based processesTurbidimetryAgglutination
The invention discloses a pullorum staining agglutination antigen as well as a preparation method and an application thereof, and belongs to the technical field of veterinarian diagnosis. Salmonella pullorum SP7220, SP7701, SP8441 and SP9905 are subjected to recovery and passage respectively to form a seed bacterium liquid, then the seed bacterium liquid is inoculated with a solid culture medium for proliferation, after inactivation with formalin, the concentration of the bacterium liquid is adjusted with turbidimetry, finally, the bacterium is fully and evenly mixed and packaged after a crystal violet solution is added for staining, and the pullorum staining agglutination antigen is prepared. The technological method is simple, reasonable, scientific, stable in production and low in cost, the selected production stains of the pullorum staining agglutination antigen are good in antigenicity and low in mutation rate, the prepared pullorum staining agglutination antigen product has the advantages of high sensitivity, high specificity, quickness in diagnosis and clearness in agglutination image, the staining agglutination antigen is relatively ideal for pullorum detection.
Owner:JIANGSU INST OF POULTRY SCI
Immune turbidimetry for detection of apoliprotein M
In the method, reaction between polyclonal antibody of anti apolipoprotein M and apolipoprotein M of sample to be tested forms compound, which causes change of cloudiness. The change is expressed by intensity of light transmission or intensity of light scattering. Content of apolipoprotein M of sample to be tested is looked out from curve between concentrations of standard apolipoprotein M and relevant intensity of light transmission or intensity of light. Titer of polyclonal antibody is at least 1:128 containing multi antigenic determinant. Sample to be tested is human blood plasma or serum in empty stomach diluted. Polyclonal antibody of anti apolipoprotein M is mixed with diluted sample. Features of thee method are: convenience, good repeatability, and high adaptability.
Owner:张晓膺
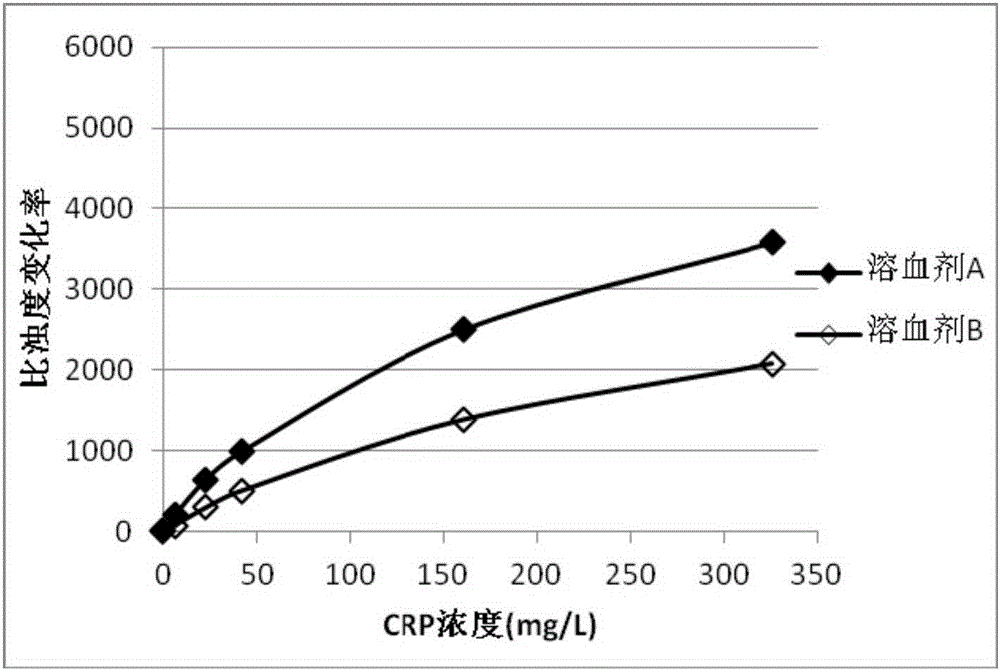
Detection kit and detection method for detecting animal C-reactive protein based on amide group nanometer latex enhanced turbidimetry
ActiveCN105067615AEasy to operateRapid responseMaterial analysis by observing effect on chemical indicatorBiological testingPreservativeTurbidimetry
The invention provides a detection kit and detection method for detecting animal C-reactive protein based on amide group nanometer latex enhanced turbidimetry. The detection kit comprises R1 reagent and R2 reagent. The R1 reagent comprises 10 to 50 mmol / L of first buffer solution, 0.2% to 2.5% w / v of coagulant, 0.1 to 2% w / v of surfactant and 0.01% to 0.1% w / v of first preservative. The R2 reagent comprises 0.5% to 2% w / v of C-labbeled reactive protein antibody crosslinking amide group nanometer latex, 0.2 to 2% w / v of stabilizer, 10 to 50 mmol / L of second buffer solution and 0.01 to 0.1 % w / v of second preservative. The detection kit and the detection method have the advantages of being simple to operate, quick to react, high in sensitivity and good in specificity.
Owner:SHENZHEN HUISONG TECH DEV
Method for detecting chlorine content in zirconium oxide
InactiveCN104677843AReduce the impactQuality assuranceColor/spectral properties measurementsTest samplePhysical chemistry
The invention relates to the technical field of zirconium oxide, and in particular relates to a method for detecting chlorine content in zirconium oxide. The method comprises the following steps: enabling a test sample to react with silver nitrate in a slightly acidic solution to form curdy silver chloride suspension liquid, and performing turbidimetry. By adopting the method disclosed by the invention, the content of chlorine in zirconium oxide powder can be accurately detected, the influences of chlorine impurities to zirconium oxide ceramic products can be reduced, and the quality of the zirconium oxide ceramic products can be ensured.
Owner:DONGGUAN XINBO STRUCTURAL CERAMICS CO LTD
Application of enzyme labeling meter in latex enhancing immune transmittance turbidimetry
InactiveCN1786713AThe detection process is fastShort timeColor/spectral properties measurementsAntigenExtinction
The invention offers immunity transmission turbidimetry used enzyme mark instrument to detect insolubility immunity grain compound enhanced turbidity by latex extinction value. The method includes the following steps: combining antibody with polystyrene latex to form antibody latex; combining the antibody latex with albumen antigen of blood serum sample; when immune response happens they will do chain reaction that a lot of latex is gathered to form insolubility immunity grain compound; the grain size of the latex changes and its transmission capacity and absorbance value also changes. The invention adopts enzyme mark instrument with certain transmitting wavelength to measure the change of the latex grain absorbance value, and draws reaction standard curve of the antigen and corresponding antibody latex grain to calculate identity antigen density in sample.
Owner:陈金华
Blood coagulation factor and fibrinolysis measuring method
ActiveCN102565317ASimple and fast operationLow costColor/spectral properties measurementsBiological testingMagnetic beadSemi automatic
The invention relates to a blood coagulation factor and fibrinolysis measuring method, which is used for testing by using a semi-automatic blood coagulation analyzer. The blood coagulation factor and fibrinolysis measuring method comprises the following testing steps of: collecting a patient blood sample and separating blood plasma out; injecting the blood plasma into a testing cup by using a sample feeding gun; putting the testing cup in a blood coagulation factor measuring device, adding a reagent, starting a timing button of the blood coagulation factor testing device, and testing the blood plasma in the testing cup by the blood coagulation factor testing device according to a double magnetic circuit magnetic bead method; and injecting the blood plasma into a fibrinolysis testing cup by the sample feeding gun, putting the fibrinolysis testing cup in a fibrinolysis testing device, adding a reagent, starting a timing button of the fibrinolysis testing device, testing the blood plasmain the fibrinolysis testing cup by the fibrinolysis testing device according to a photoelectric turbidimetry, and printing and outputting fibrinolysis data after the testing by the fibrinolysis testing device is ended. The blood coagulation factor and fibrinolysis measuring method disclosed by the invention is simple and convenient and greatly reduced in cost.
Owner:BEIJING PRECIL INSTR CO LTD
Reagent kit for detecting blood serum folic acid concentration
InactiveCN101430330ALow costAvoid cumbersomeMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementPenicillinNutrition
The invention belongs to the technical field of reagent equipment, and more particularly relates to a kit for detecting folacin concentration of blood serum (plasma), which is designed according to a detecting method based on a principle that the growth of lactobacillus casei penicillin drag-resistant strain (ATCC7469) requires folacin as essential nutrientsubstance, and prepared by the following steps that necessary reagents required by testing are matched reasonably and then assembled into the kit that is convenient for use; the solution concentration of folacin standard series is 0, 4, 8, 12, 16, 20ug / l respectively; a test sample is unnecessary to be diluted in advance, and the concentration just can be measured by sampling 5 microlitre of test sample directly; a culture medium bottle, a folacin standard liquid bottle, a lactobacillus casei liquid bottle and an antiseptic liquid bottle are arranged in the kit. The reagent is a folacin detecting method which is based on a 96-porous micro-reaction board and carries out quantitative determination by applying the turbidimetry determination of an immunization enzyme-linked determinator; and the method is low in folacin detection cost, convenient for operation and is suitable for developing folacin nutrition screening in large scale in women and children institutions.
Owner:天津市妇女儿童保健中心
Fast drug sensitivity detection kit for aquatic product vibrio
ActiveCN103013822APromote growthGrowth does not affectBioreactor/fermenter combinationsBiological substance pretreatmentsBuretteTurbidimetry
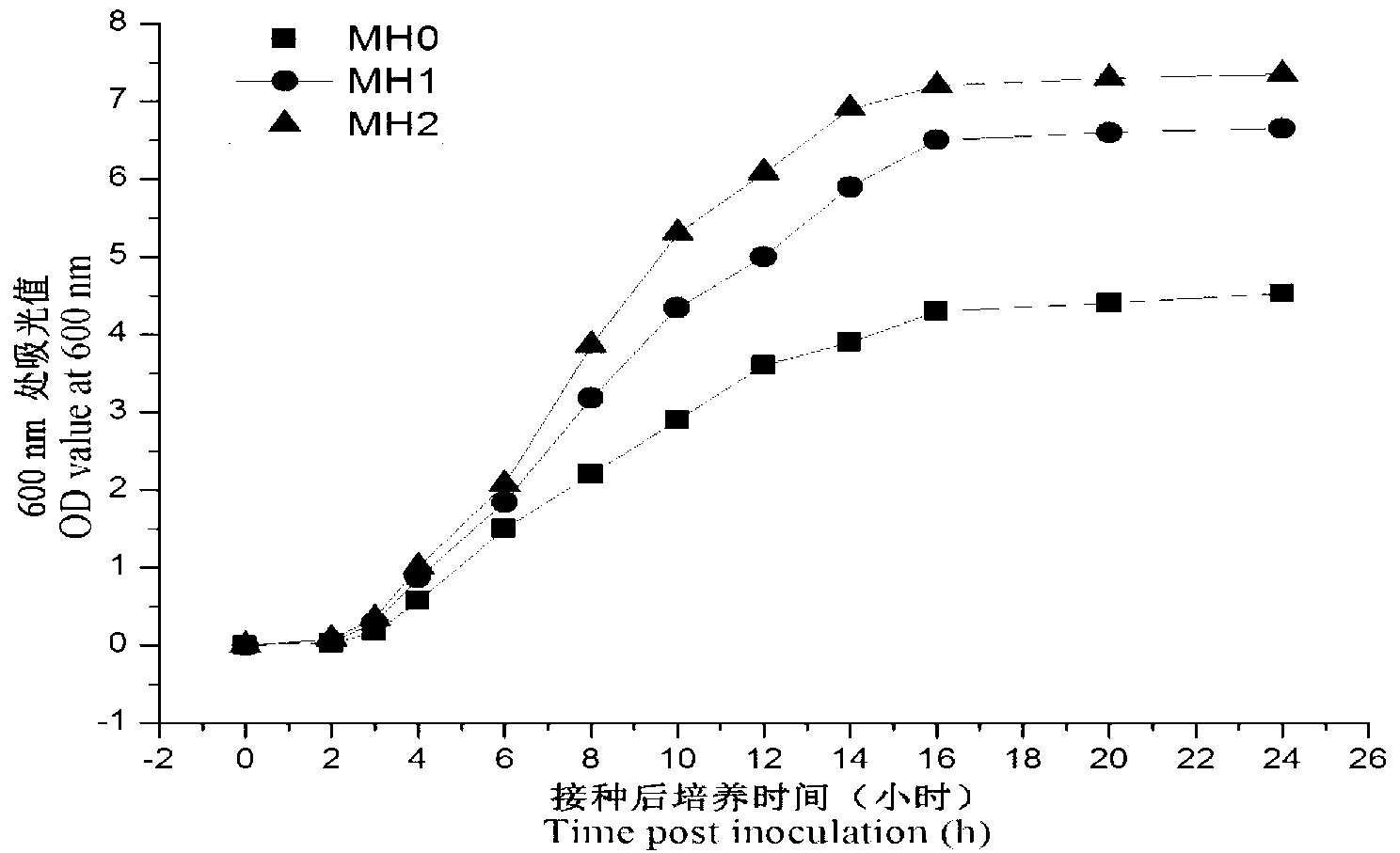
The invention relates to a fast drug sensitivity detection kit for vibrio, which can be applied to an aquiculture site. According to the invention, the detection kit comprises a detection plate, a thallus collection tool, bacterium enrichment liquid, a drug sensitivity detection culture medium, a turbidimetry tube, an inoculation burette and a colorimetric card, wherein the detection plate comprises inspection holes enveloping drugs and color indicators for drug sensitivity detection, and the color indicators are Alamar Blue. According to the invention, fish peptone, Ca2+ and Mg2+ are added based on the MH culture medium and the concentration of NaCl is adjusted, and the culture medium is used as the bacterium enrichment liquid of vibrio, which can effectively promote the growth of the vibrio and enables the bacterium enrichment liquid to reach the required concentration in a short time, so as to shorten the time of drug sensitivity detection. At the same time, a blue dye, namely the Alamar Blue, which is safe and nontoxic to the cells is used; the Alamar Blue has no toxic effect on cells, and can be enveloped in the micropores of the drug sensitivity detection plate directly without influencing the growth of the bacteria, so that the detection operation is simpler and more convenient and quick; and the proliferation dynamic of the bacteria can be continuously monitored.
Owner:MARINE BIOLOGY INST OF SHANDONG PROVINCE
Immune scatter turbidimetry based full-automatic detection device and method thereof
InactiveCN104535776ARealize fully automatic detectionImprove efficiencyMaterial analysisMiniaturizationTurbidimetry
The invention discloses an immune scatter turbidimetry based full-automatic detection device and a method thereof. By means of a sampling needle and two reagent needles, individual loading of a sample and double reagent needles can be realized, cross contamination between liquid reagents can be avoided, and the detection accuracy can be improved. The sample adding and cleaning between channels are independent to each other, thus greatly improving the efficiency of the detection device. The reaction cup can be used repeatedly, not only saves cost, but also realizes miniaturization and intelligentization of immune scatter turbidimetry full-automatic detection equipment, thus being better suitable for the detection needs of hospitals and small clinics. At the same time, a magnetic field transformation bottom spiral eddy current mixing and cleaning technology is adopted to achieve good stirring and cleaning effects of the reaction cup.
Owner:SHENZHEN LIFOTRONIC TECH
Retinol conjugated protein detection kit
The invention discloses a retinol conjugated protein detection kit. The kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 is a buffer solution, the reagent R2 is the mixture of retinol conjugated protein antibody sensitized polystyrene latex particles and the buffer solution. The retinol conjugated protein detection kit has the advantages that the kit is simple and quick to detect, high in sensitivity, good in accuracy, good in anti-interference capacity and low in production cost; a lipoprotein a (retinol conjugated protein) detection method adopted by the kit is a latex enhanced immuno-turbidimetry and enables the lipoprotein a (retinol conjugated protein) detection to be more economical, convenient and quicker, and the retinol conjugated protein detection kit is applicable to automatic biochemical analyzers of most of hospitals and especially can achieve quick quantitative detection for emergency treatment.
Owner:宁波天康生物科技有限公司
Micro-fluidic chip and preparation method thereof
ActiveCN107213928ARapid Quantitative DetectionEasy to operateLaboratory glasswaresMaterial analysisTurbidimetryPre treatment
The invention discloses a micro-fluidic chip which comprises a substrate, a sampling part and a bottom cover, wherein a sample feeding cavity, a pre-treating region, a detection cavity and a reaction region are formed in the substrate; the sampling part comprises a sample feed inlet, a sample accommodating cavity and a sample outlet; the pre-treating region comprises a pre-treating reagent cavity, a pre-treating cavity and a gas cavity; the reaction region comprises a reaction reagent cavity and a reaction cavity; elastic cavity walls are formed on the pre-treating reagent cavity, the gas cavity, the reaction reagent cavity and the reaction cavity; a transparent panel is arranged on the upper surface of the detection cavity and a transparent bottom plate is arranged on the lower surface of the detection cavity; and the transparent panel and the transparent bottom plate correspond to each other. Based on a transmitting turbidimetry, according to the micro-fluidic chip, the content of a to-be-detected component in a sample is quickly and quantitatively detected by means of a micro-fluidic chip technology, so that the micro-fluidic chip has the characteristics of being easy to operate, high in sensitivity and accurate in result. A preparation method of the micro-fluidic chip comprises the steps of preparing a reagent; and assembling the chip.
Owner:深圳市海拓华擎生物科技有限公司
Urinary transferrin immuno-turbidimetry reagent and preparation method thereof
InactiveCN105044352ASolve shipping problemsActivity is not affectedDisease diagnosisBiological testingFreeze-dryingTurbidimetry
The invention discloses a urinary tranferrin immuno-turbidimetry kit. The kit comprises a freeze-dried R1 reagent and a freeze-dried R2 reagent, which are fixed in a same container. The freeze-dried R1 reagent and freeze-dried R2 reagent are prepared by adding an excipient and a protective agent into a liquid R1 reagent and a liquid R2 reagent, and then freeze-drying the reagents. The liquid R1 reagent is a buffer solution containing PEG. The liquid R2 reagent is anti-human transferrin polyclonal or matched monoclonal antibody couple latex, or anti-human transferrin polyclonal antibody or matched monoclonal antibody coupled colloidal gold. The detection operation is convenient, the time is saved, at the same time, the transportation and preservation are convenient, the reagent can be transported in cold areas, and the reagent stability is improved.
Owner:NANJING PERLONG MEDICAL EQUIP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com