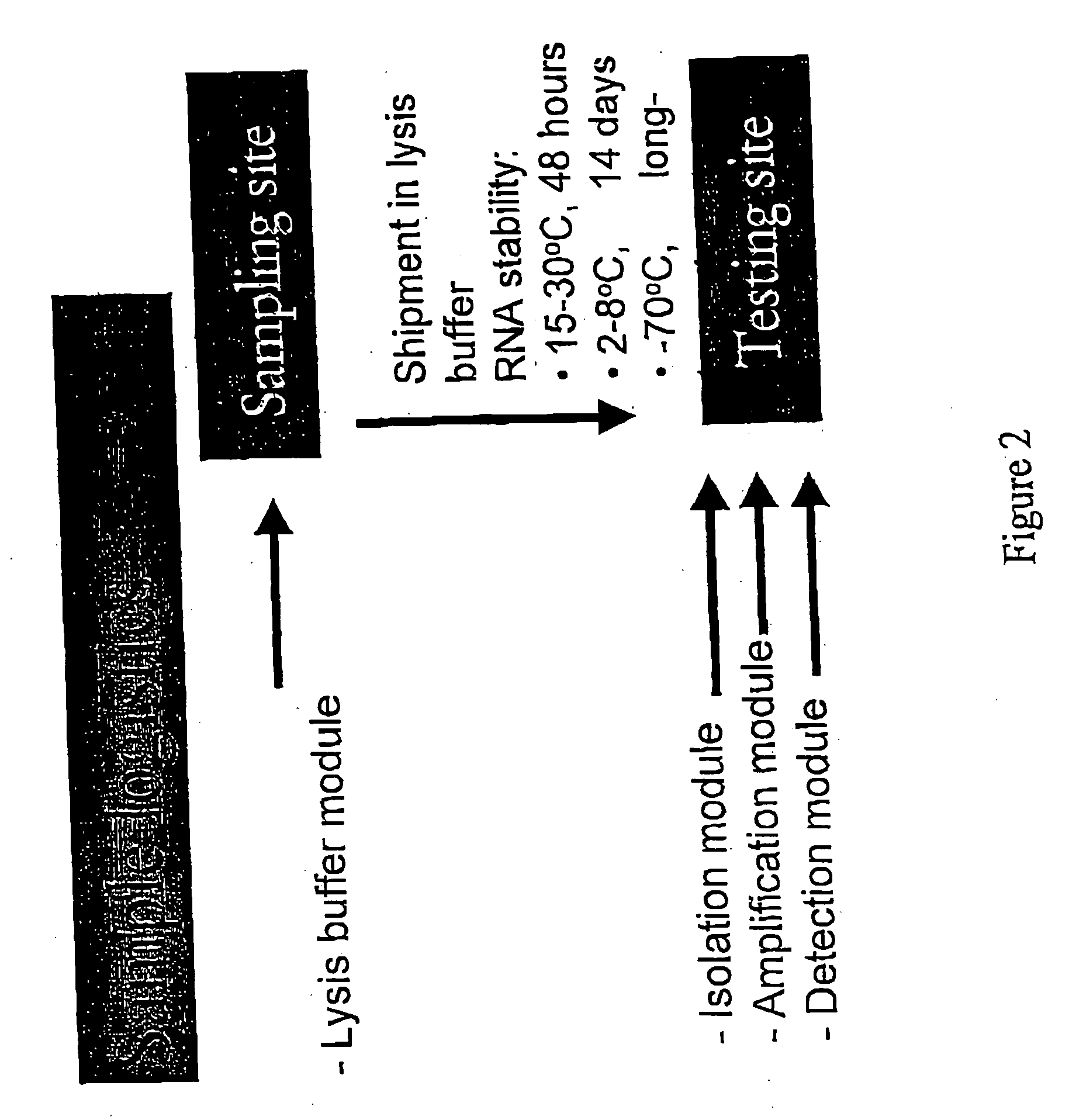
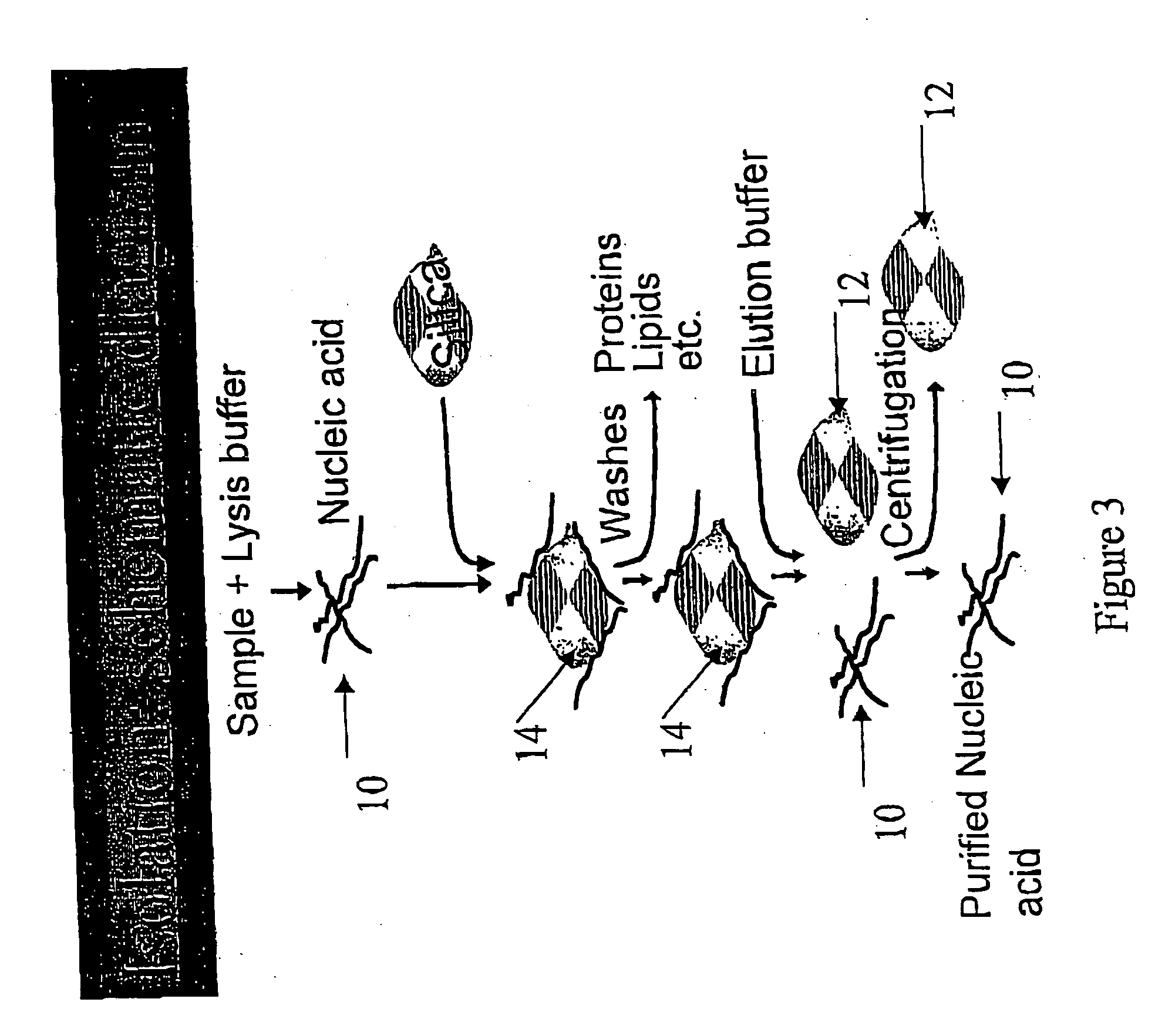
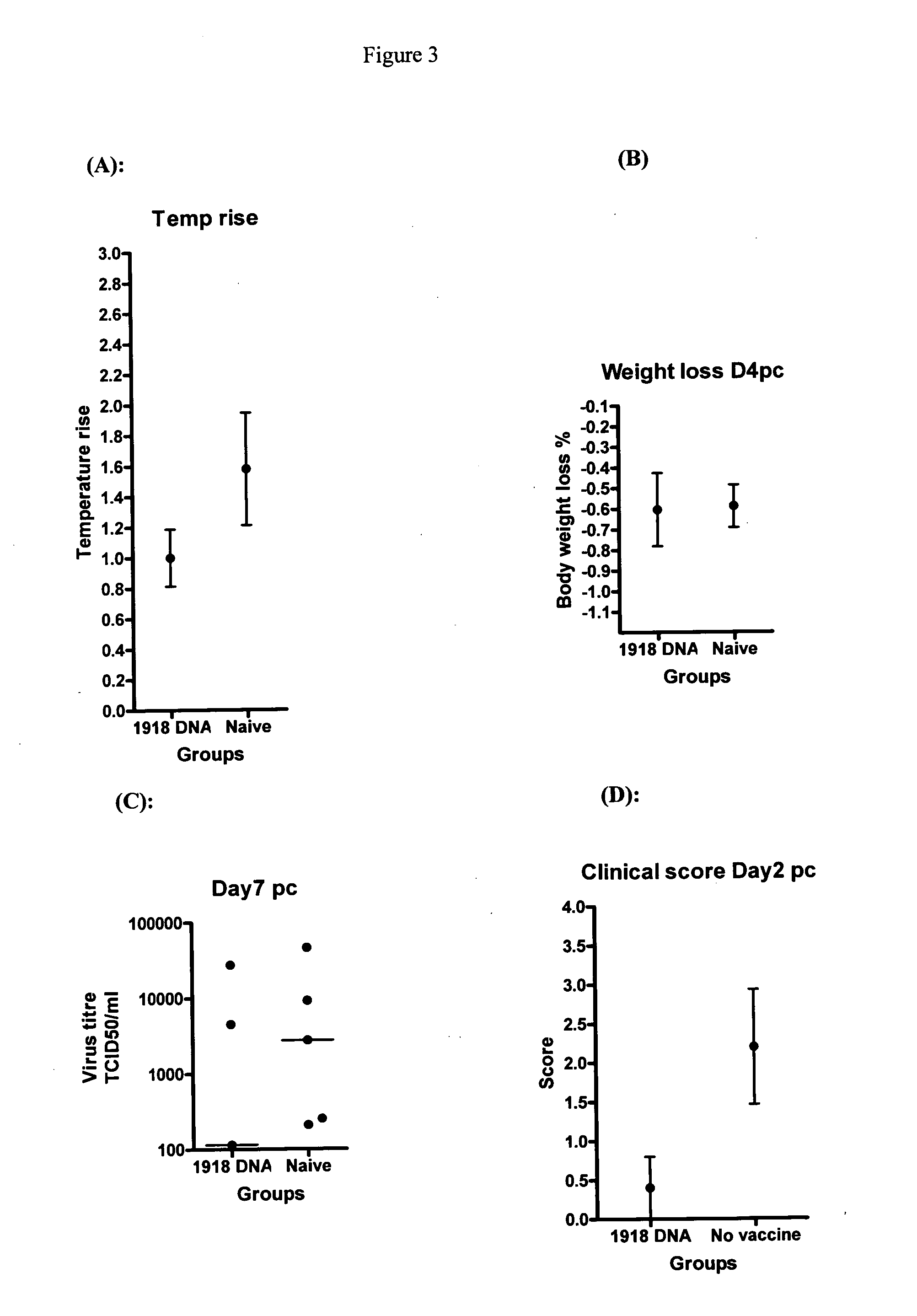
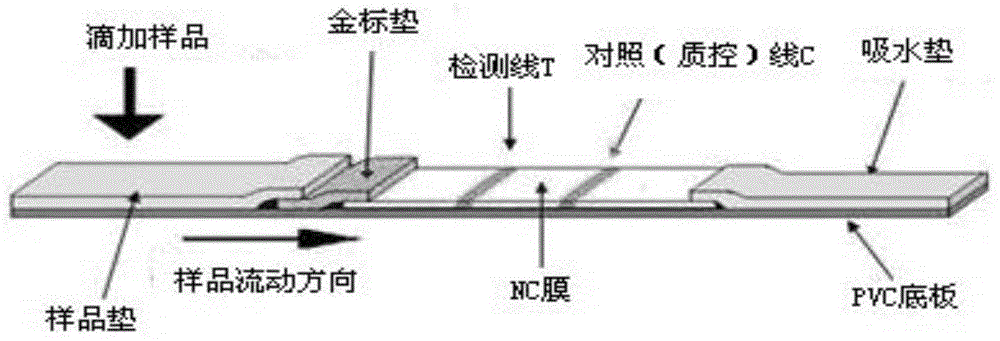
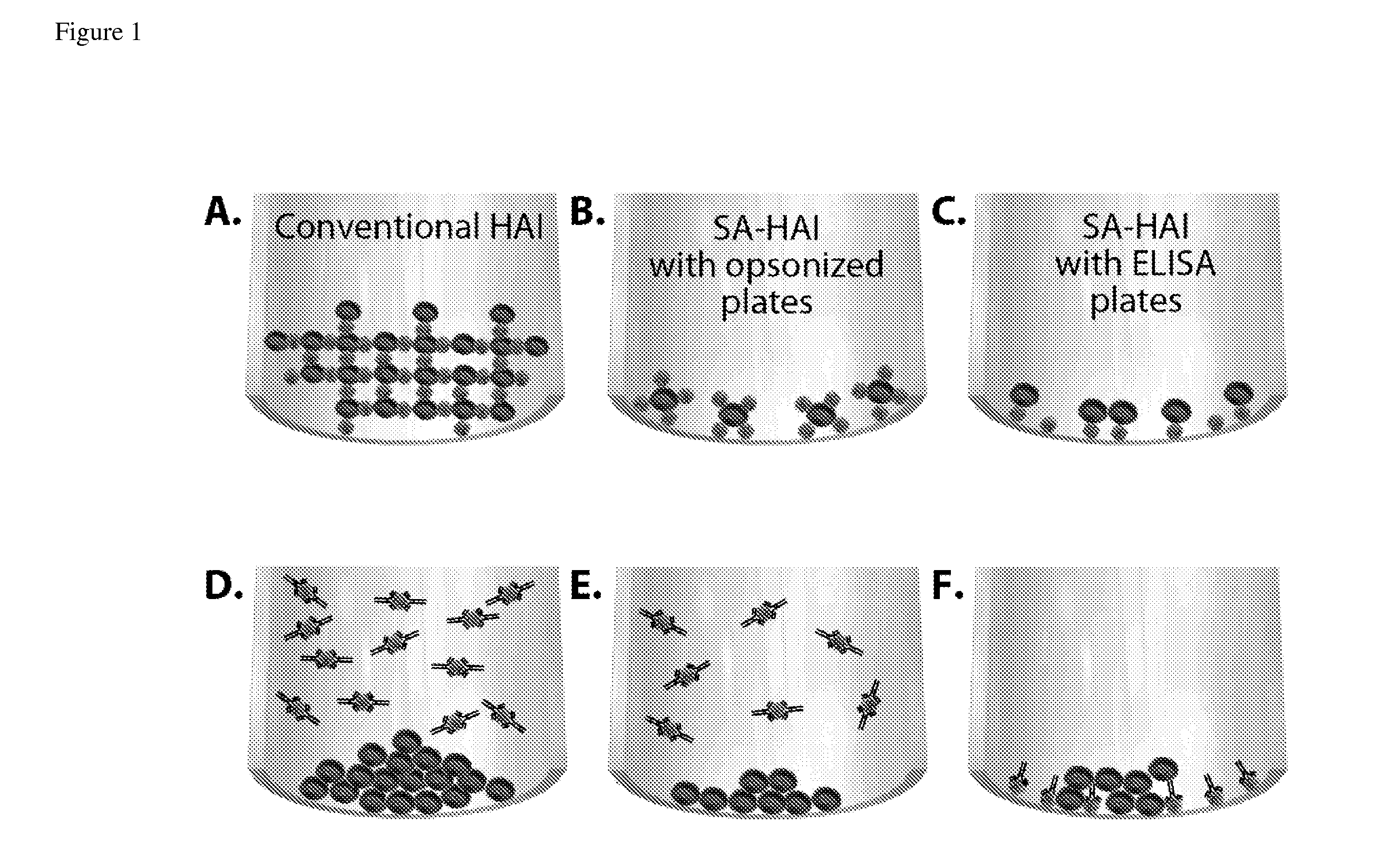Patents
Literature
221 results about "Hemagglutination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hemagglutination, or haemagglutination, is a specific form of agglutination that involves red blood cells (RBCs). It has two common uses in the laboratory: blood typing and the quantification of virus dilutions in a haemagglutination assay.
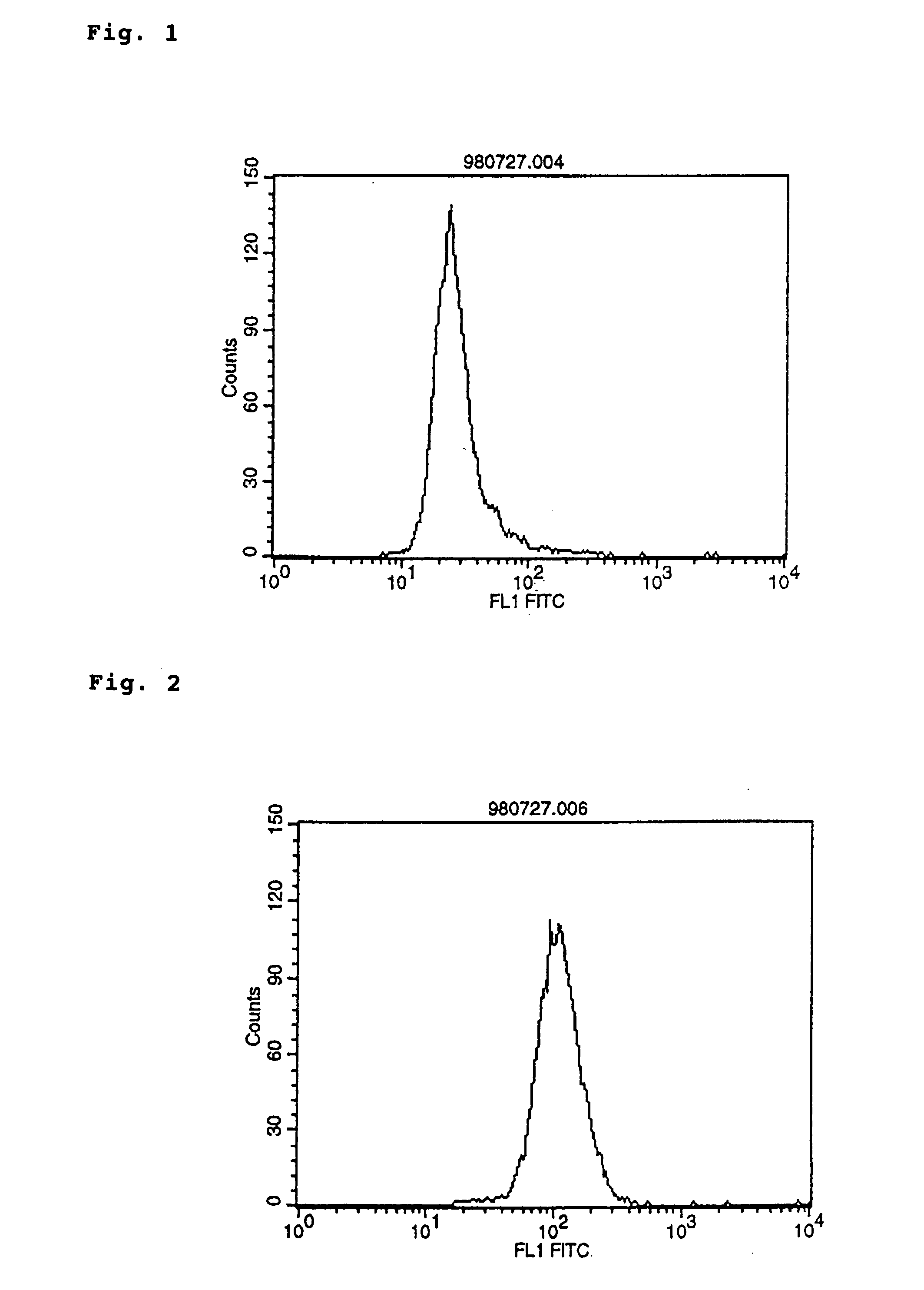
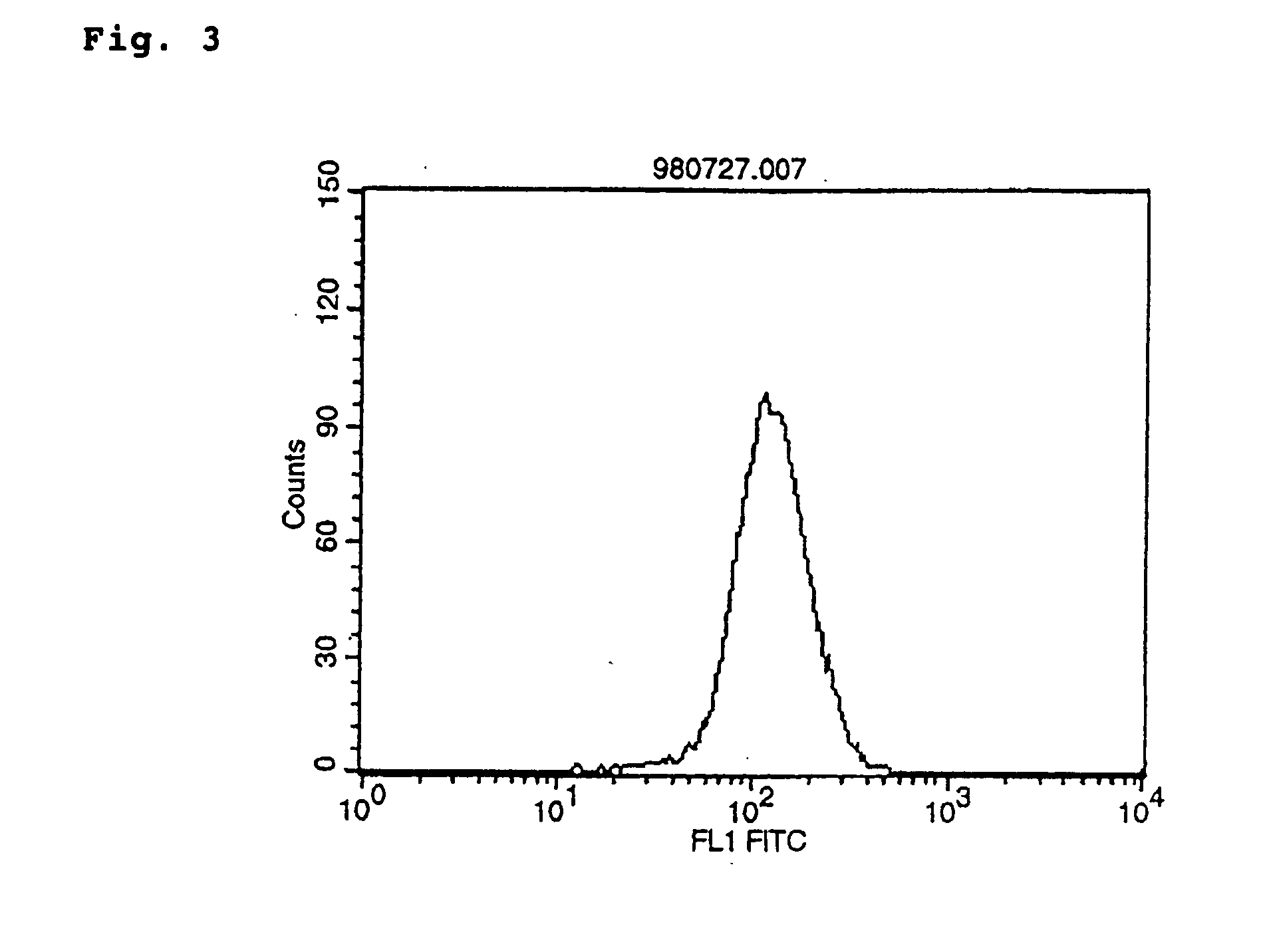
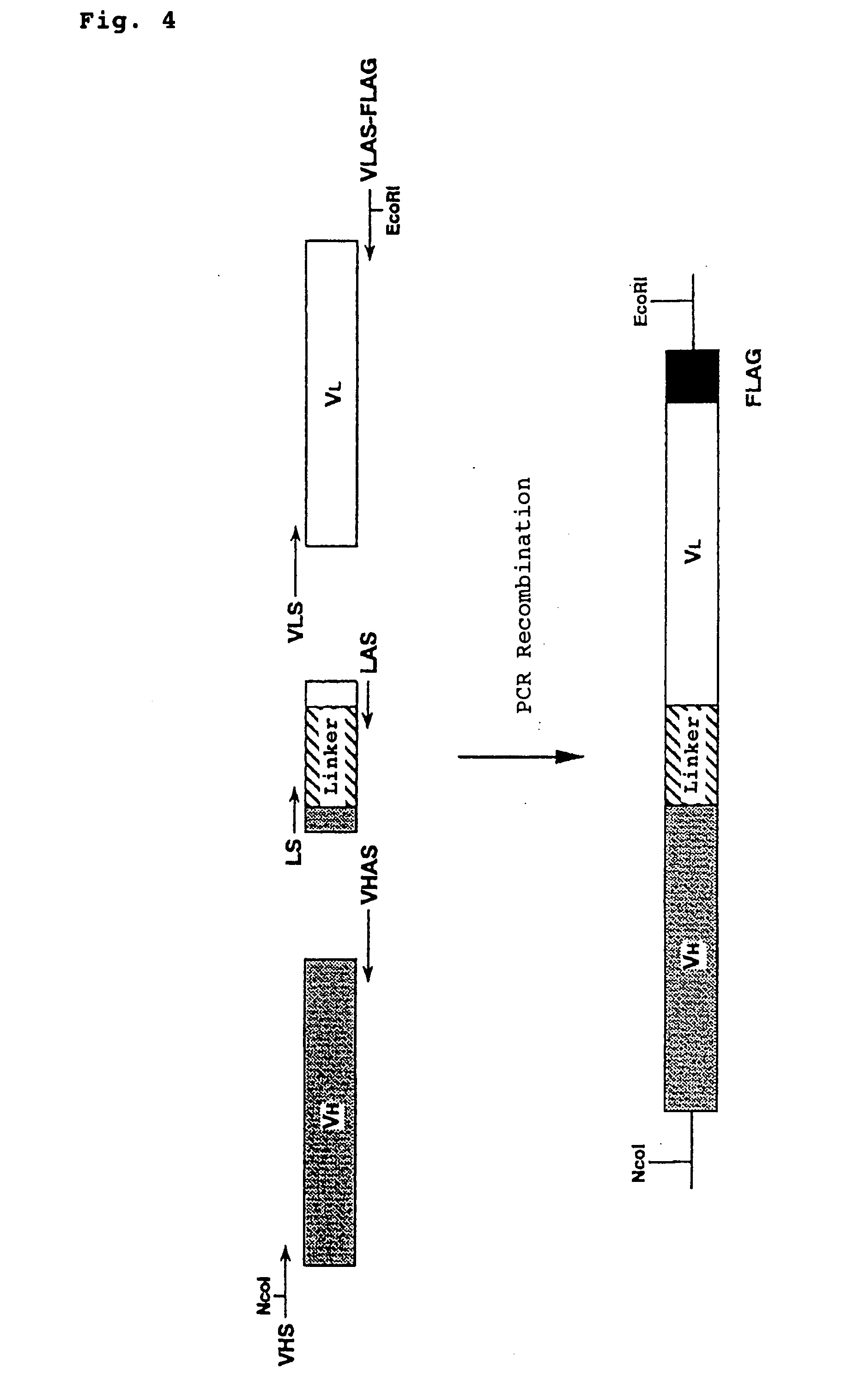
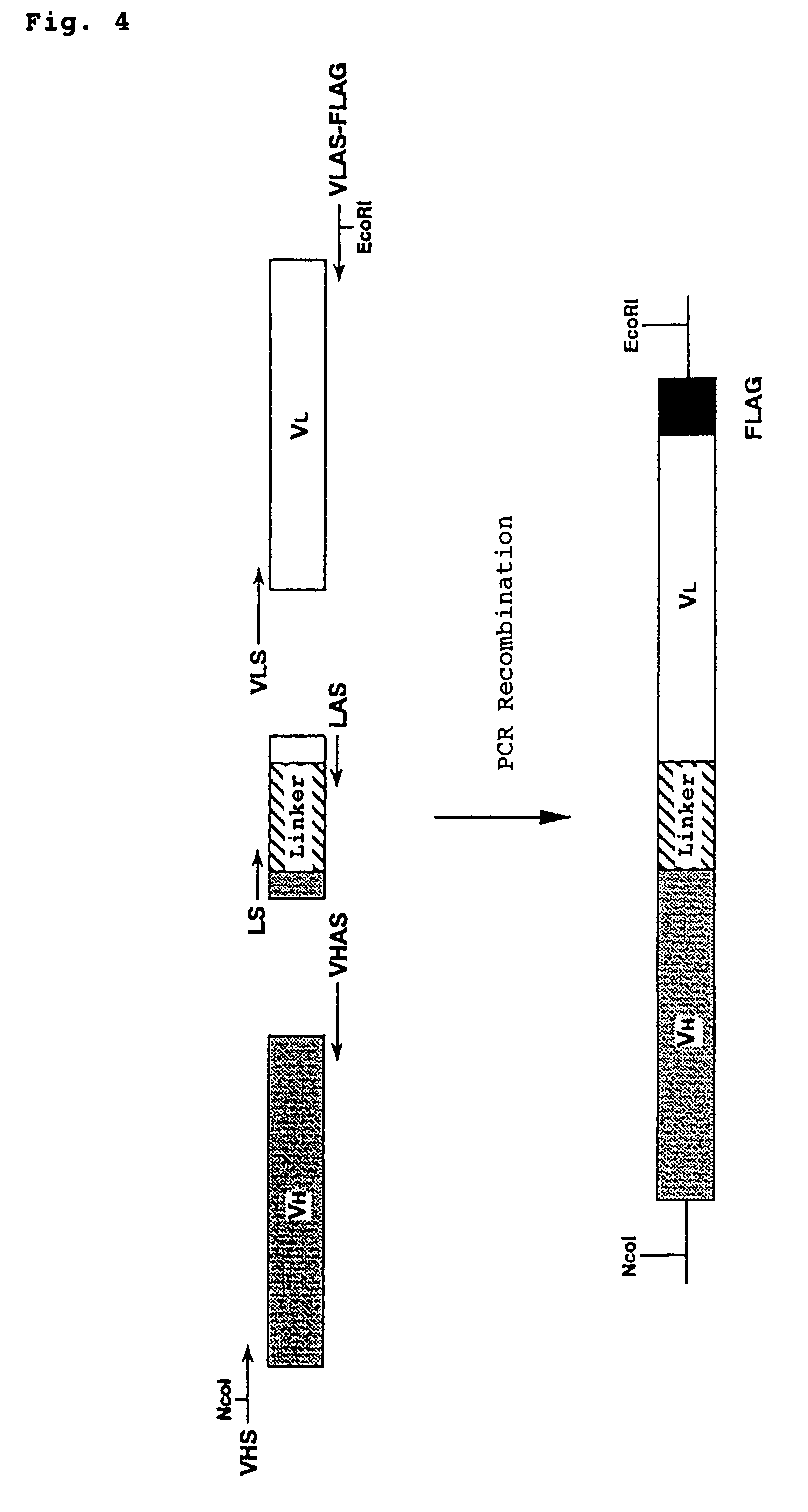
Polypeptide inducing apoptosis
InactiveUS20040073013A1Efficient purificationEasy to produceAnimal cellsSugar derivativesMonoclonal antibodyApoptosis
This invention relates to reconstructed polypeptides which have properties of inducing apoptosis of nucleated blood cells having Integrin Associated Protein (IAP) and causing no hemagglutination. The reconstructed polypeptides comprise two or more H chain V regions and two or more L chain V regions of a monoclonal antibody which induces apoptosis of nucleated blood cells having IAP. The reconstructed polypeptides are useful as a therapeutic agent for blood dyscrasia such as leukemia.
Owner:CHUGAI PHARMA CO LTD
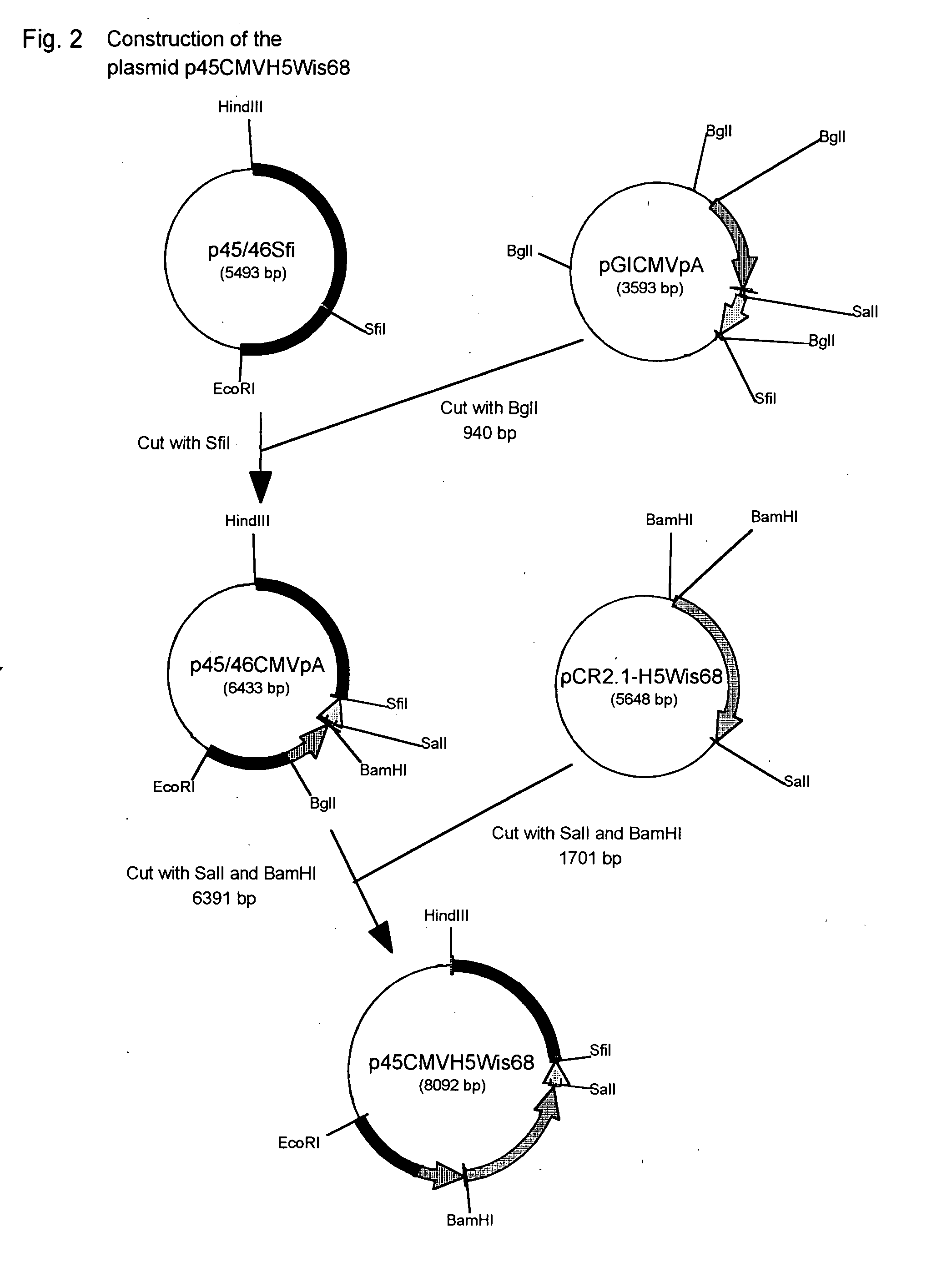
Kit for detecting non-pathogenic or pathogenic influenza a subtype h5 virus
InactiveUS20040142319A1High sensitivityStrong specificitySugar derivativesMicrobiological testing/measurementReverse transcriptaseHaemagglutination inhibition
Current methods for detecting influenza A subtype H5 virus, for example cell culture, haemagglutination-inhibition, fluorescent antibody and enzyme immunoassay, and reverse transcriptase polymerase chain reaction (RT-PCR) may have the disadvantages of low sensitivity and low specificity. Furthermore, such methods are relatively difficult to use, and may not be suitable for routine detection on a daily basis. The kit for detecting H5 virus of this invention may provide a user-friendly alternative that is relatively more sensitive and specific to H5 virus. The detection kit utilizes two specially designed primers A and B for the replication of H5 virus, and a specific capture probe for immobilizing the amplified viral RNA. An additional primer C is also designed for the detection of pathogenic H5 virus. The detection of H5 virus by the detection kit may be accomplished within one day if desired.
Owner:HAI KANG LIFE
Influenza vaccine
InactiveUS20090304730A1Overcomes drawbackSsRNA viruses negative-senseViral antigen ingredientsEpitopeInfluenza vaccine
The present invention relates to influenza vaccines for human and veterinary use. In particular, the present invention provides a vaccine able to effect long term and cross-strain protection by including at least two influenza virus epitopes expressed as a chimeric polypeptide wherein at least one epitope is influenza A virus matrix protein epitope and the second epitope is a haemagglutinin peptide epitope.
Owner:YEDA RES & DEV CO LTD
GeXP rapid detection kit capable of simultaneously identifying nine pathogens of chicken respiratory tract diseases
ActiveCN102899424AStrong specificityImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationInfectious laryngotracheitisRespiratory tract disease
The invention discloses a GeXP rapid detection kit capable of simultaneously identifying nine pathogens of chicken respiratory tract diseases. The kit is used based on a GeXP system and comprises ten polymerase chain reaction (PCR) primer pairs; the kit is used for identifying and detecting avian influenza virus, H5, H7 and H9 subtype avian influenza virus, newcastle disease virus, infectious bronchitis, infectious laryngotracheitis, mycoplasma gallisepticum, bursa synovialis mycoplasma and haemophilus paragallinarum; and the kit is good in specificity, high in sensitivity and can detect 100 copy / mu l. Compared with an identifying result of the conventional experiment method of a pathogen separation and hemagglutination inhibition experiment or a serology experiment and the like, the GeXP rapid detection kit has the advantage that the coincidence rate reaches 100 percent. The kit is generally used for detecting the main chicken respiratory tract diseases and the pathogens thereof, so that a simple and high-flux detection kit and a detection system are provided, an actual requirement is met, and the application prospect is wide.
Owner:GUANGXI VETERINARY RES INST
Polypeptide inducing apoptosis
InactiveUS7696325B2Efficient purificationEasy to produceAnimal cellsSugar derivativesApoptosisMonoclonal antibody
This invention relates to reconstructed polypeptides which have properties of inducing apoptosis of nucleated blood cells having Integrin Associated Protein (IAP) and causing no hemagglutination. The reconstructed polypeptides comprise two or more H chain V regions and two or more L chain V regions of a monoclonal antibody which induces apoptosis of nucleated blood cells having IAP. The reconstructed polypeptides are useful as a therapeutic agent for blood dyscrasia such as leukemia.
Owner:CHUGAI PHARMA CO LTD
GeXP (Gene Expression) rapid detection kit capable of simultaneously identifying six virus of chicken respiratory disease
ActiveCN102899423AStrong specificityImprove throughputMicrobiological testing/measurementMicroorganism based processesBovine respiratory diseaseInfectious bronchitis virus
The invention discloses a GeXP (Gene Expression) rapid detection kit capable of simultaneously identifying six virus of chicken respiratory disease. The GeXP rapid detection kit is used basing on a CeXP system and contains seven PCR (Polymerase Chain Reaction) primer pairs, the specificity for simultaneously detecting avian influence virus, H5, H7 and H9 sub type of the avian influence virus, Newcastle disease virus, infectious bronchitis virus and infectious laryngotracheitis virus is strong, the sensitivity can be up to 100 copy / mu l, and compared with an identifying result of regular test methods such as virus isolation and hemagglutination inhibition, the coincidence rate is up to 100%. According to the GeXP rapid detection kit disclosed by the invention, a simple and high-throughput detection kit and a detection system are provided for the detection of common main chicken viral respiratory disease, the actual needs are accordant, and the application prospect is wide.
Owner:GUANGXI VETERINARY RES INST
Turkey herpesvirus vectored recombinant containing avian influenza genes
InactiveUS20080241188A1Easy to distinguishEasy to detectSsRNA viruses negative-senseVectorsVaccinationElisa kit
The present invention provides a recombinant turkey herpesvirus modified by the presence of the cDNA encoding the hemagglutinin protein of avian influenza virus under a promoter. A poultry vaccine comprising the recombinant turkey herpesvirus described in the present invention can induce serological responses that may be easily detected by the hemagglutination inhibition assay but not by commercially available diagnostic ELISA kits; thus enabling easy differentiation between vaccination and field infection.
Owner:ZEON CORP +1
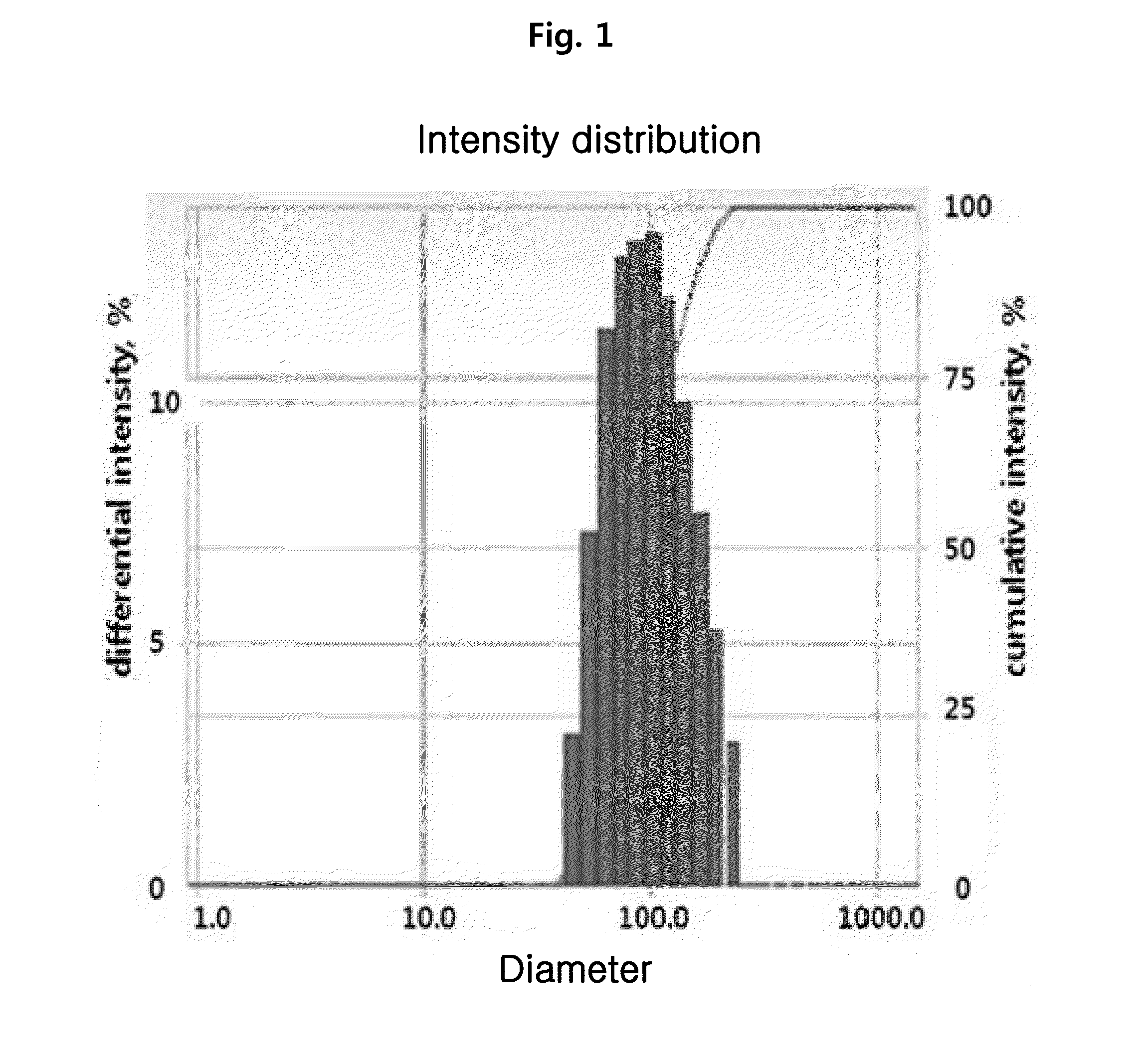
Lipid nanomaterial containing lysophosphatidylcholine or derivative thereof and method for preparing same
ActiveUS20160022711A1Easy to usePromote aggregationAntibacterial agentsOrganic active ingredientsDiseasePhospholipid
The present invention relates to a lipid nanomaterial containing lysophosphatidylcholine or an ether derivative thereof, a method for preparing the same, a method for reducing erythrocyteolysis and hemagglutination using the compound, and a pharmaceutical agent containing the lipid nanomaterial. The present invention contains lysophosphatidylcholine or an ether derivative thereof as an active ingredient, and can be useful as a treatment agent for septicemia, bacterial infection diseases, and the like.
Owner:ARIBIO CO LTD
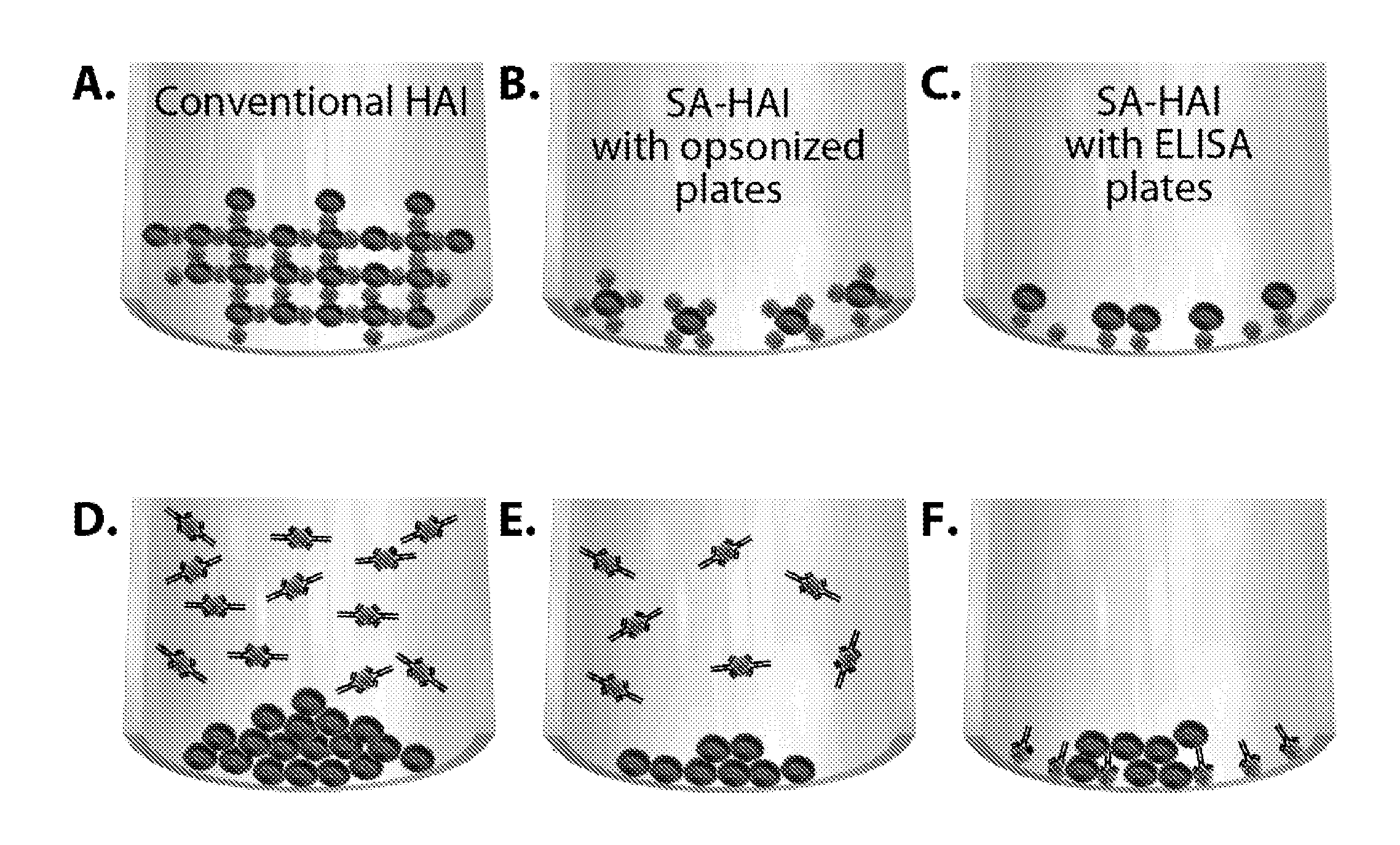
Surface-assisted hemagglutination and hemagglutination inhibition assays
ActiveUS20110097705A1Low variabilityHigh sensitivityMicrobiological testing/measurementBiological testingSerum igeRed blood cell
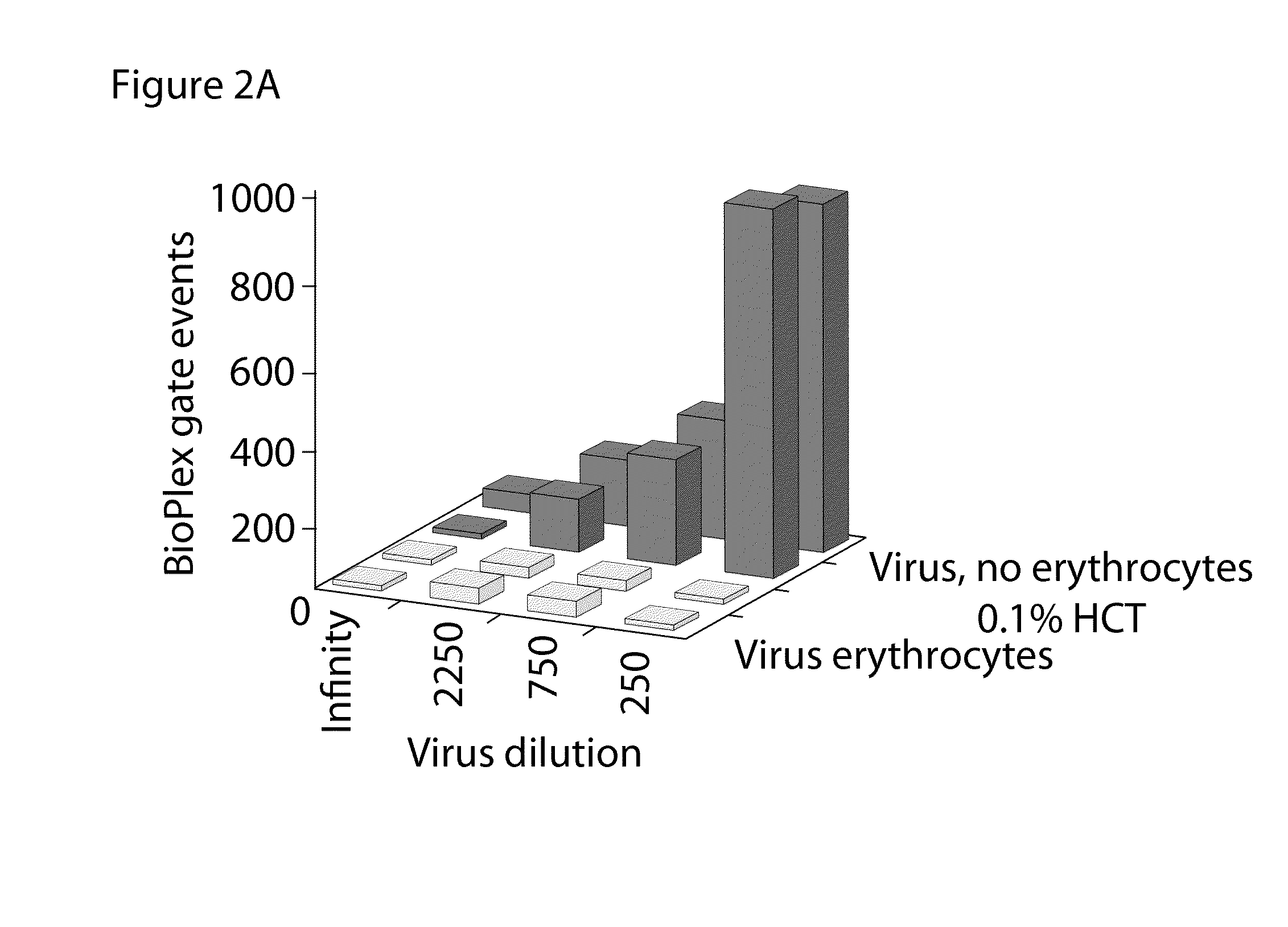
Hemagglutination (HA) and hemagglutination inhibition (HAI) functional assays remain important instruments of analysis of virus-cell interaction and protecting efficacy of virus-specific antibodies and sera. However, they demonstrate limited sensitivity towards many viruses, and require significant volumes of viruses, erythrocytes, sera, and antibodies. The present invention comprises new and significantly more sensitive versions of the HA and HAI assays based on observing agglutination on activated surfaces of specifically opsonized plates and ELISA plates rather than in solution. A version of the new assay that uses ELISA plates additionally allows characterizing the affinity of functional antibodies in the tested sera and fluids, which is not possible in the classical HAI assay. The methods of the present invention can also be used to improve the sensitivity of agglutination methods based on latex beads and to develop agglutination methods using target cells other than erythrocytes.
Owner:SANOFI PASTEUR VAX DESIGN
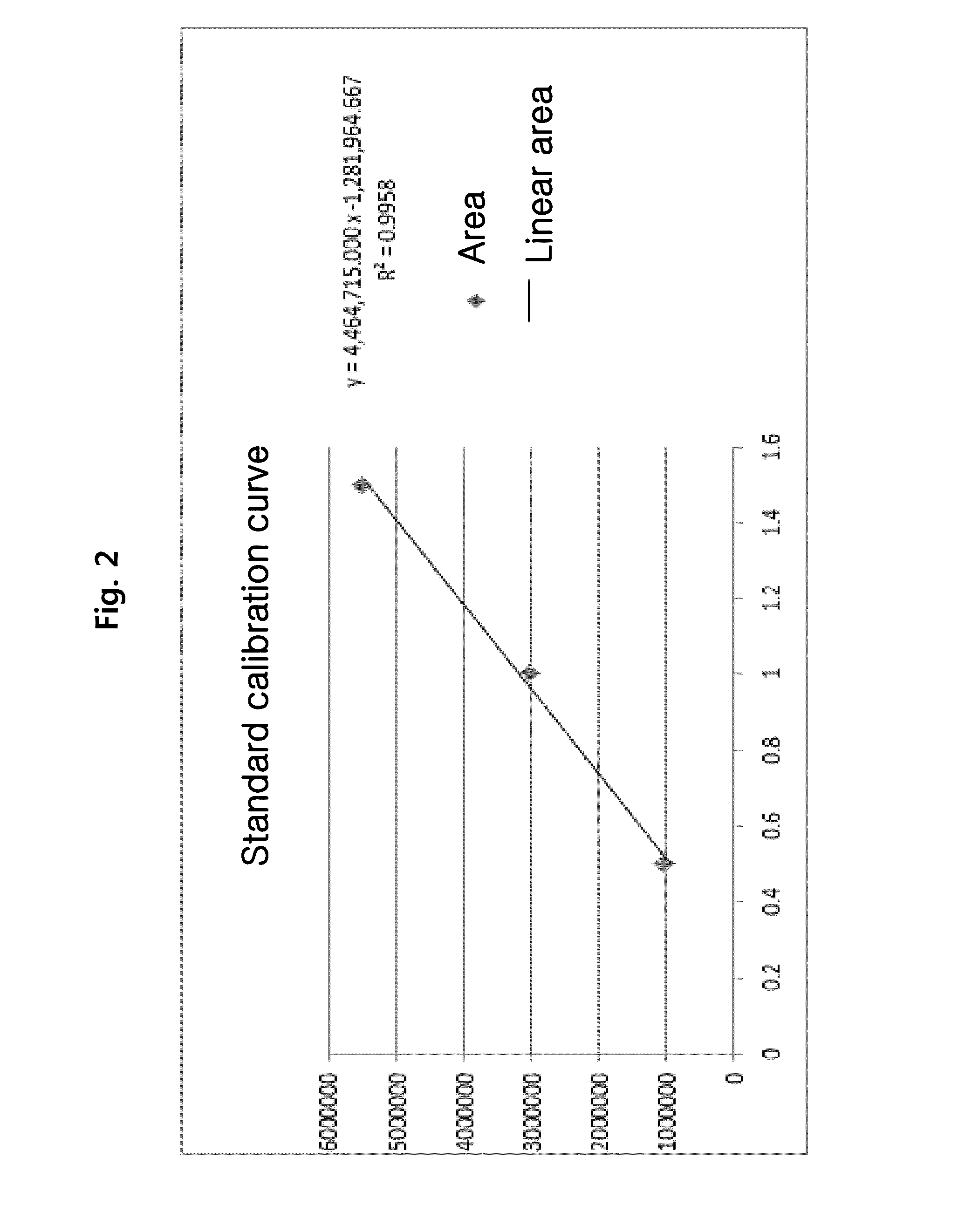
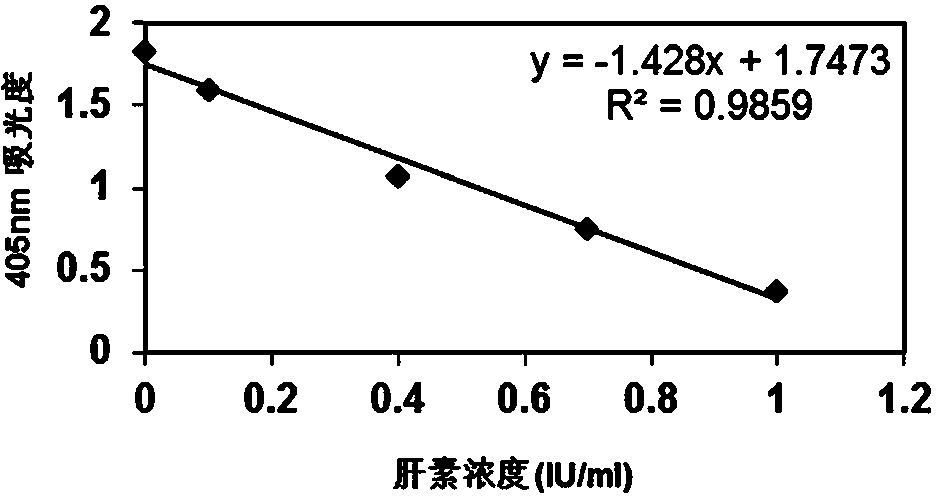
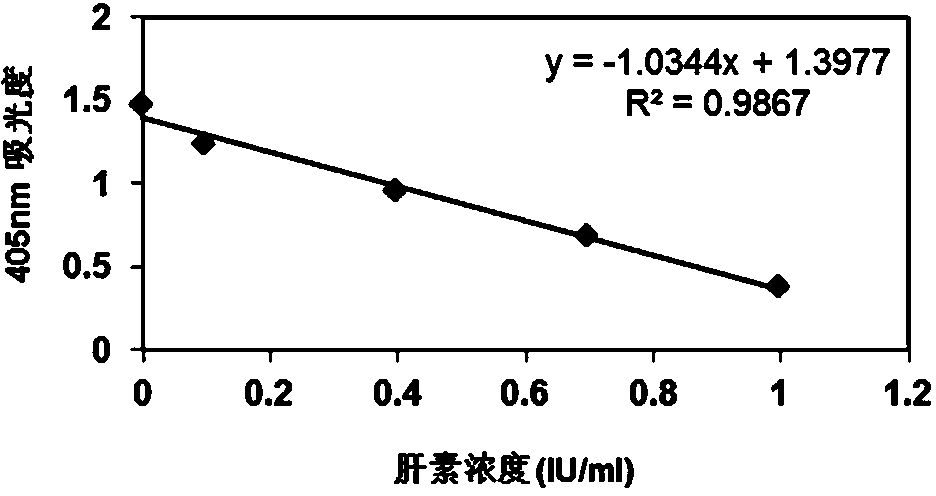
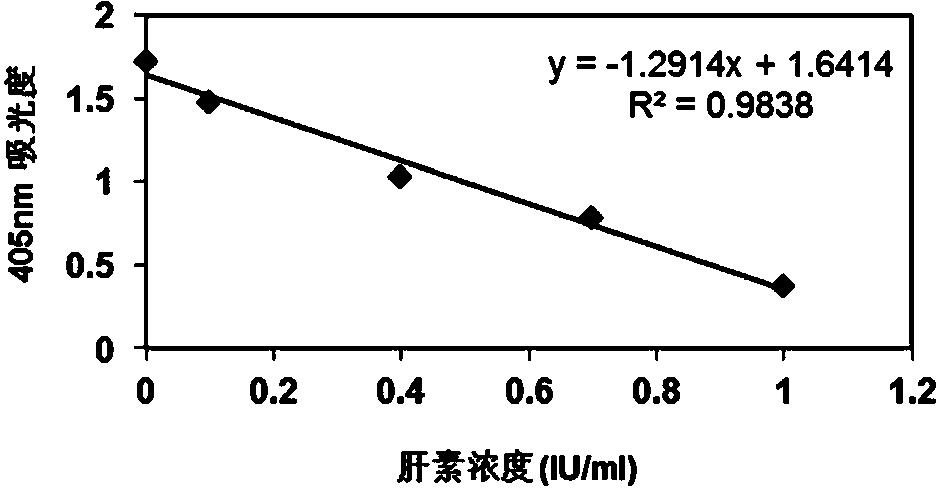
Heparin content detection method
InactiveCN104048931AEasy to measureHigh sensitivityMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsPharmacologyHeparin-DHE
The invention discloses a heparin content detection method. The heparin content detection method comprises the following steps of by a developing substrate method, mixing a sample to be detected and a FXa activity detection reagent, carrying out incubation, detecting signal intensity of the developing substrate, calculating signal intensity and comparing the signal intensity and a standard curve to obtain heparin content of the sample to be detected, wherein the developing substrate is a developing substrate of an activation factor X(FXa) and is selected from Benzoyl-Ile-Glu-Gly-Arg-p-nitroanilide.HCl, CH3O-CO-D-CHA-Gly-Arg-p-nitroanilide.AcOH, Acetyl-D-Arg-Gly-Arg-p-nitroanilide.2HCl, and 4-Nz-D-Arg-Gly-Arg-p-nitroanilide.2HCl. The heparin content detection method can be used for detecting common unclassified heparin, low-molecular weight heparin and fondaparinux, has good detection stability and good repeatability, can accurately show heparin content, has high sensitivity and accuracy, can fast find the optimal dosage scope of heparin and is free of multitime detection on patients. The heparin content detection method can be used for automation apparatuses such as a hemagglutination analyzer or a biochemical analyzer, realizes automation, is conducive to clinical popularization use and has the characteristics of simple operation, high sensitivity and good repeatability.
Owner:SHANGHAI VASCUTECH DIAGNOSIS CO LTD
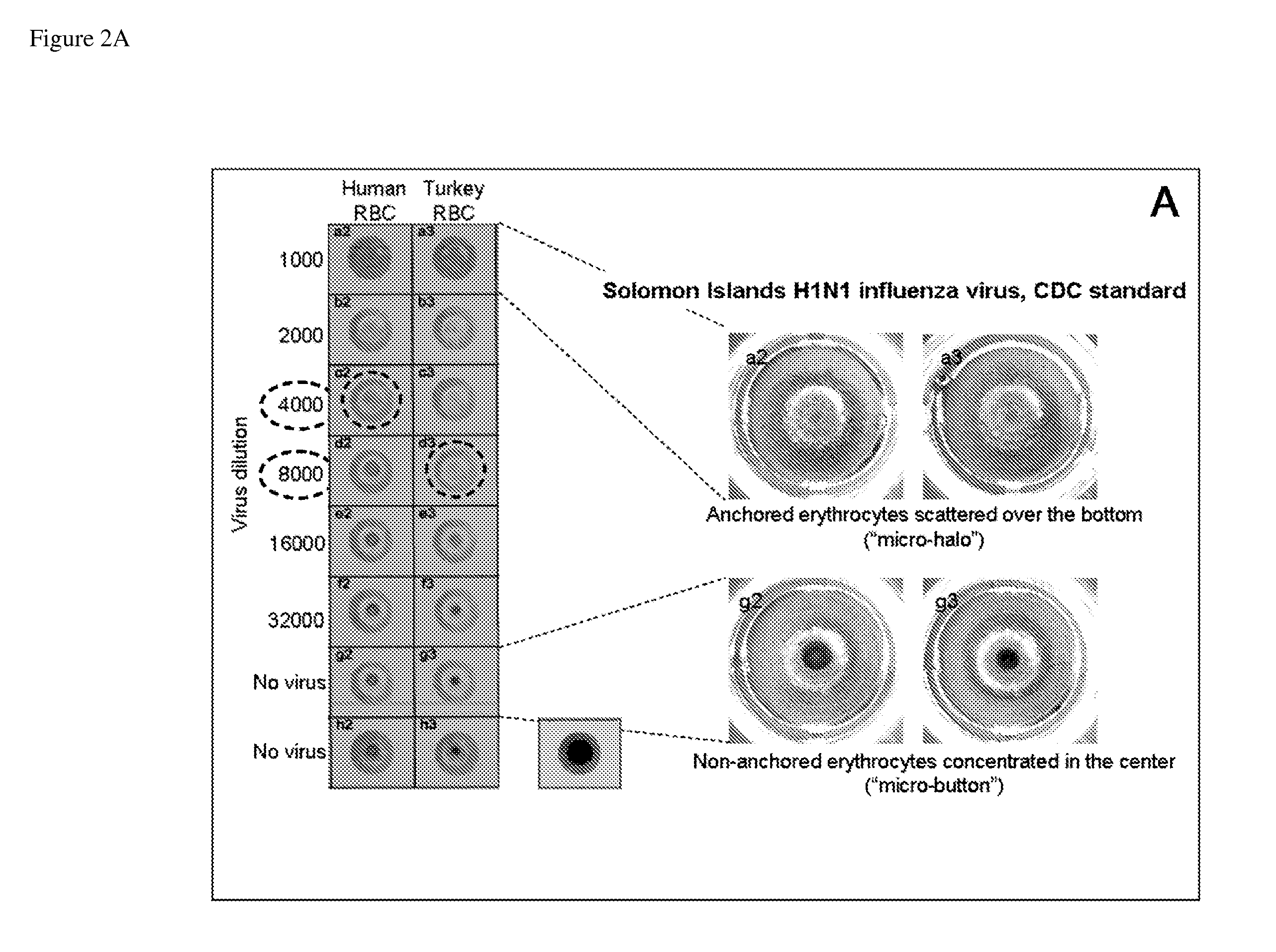
Bead Array Reader Based-Hemagglutination and Hemagglutination Inhibition Assay
InactiveUS20090325148A1High sensitivityMicrobiological testing/measurementBiological material analysisViral VaccineAnti viral immunity
Hemagglutination assays and hemagglutination inhibition assays were introduced in medical and virology practice more than 60 years ago. Since then, these assays have become important tools for measuring concentrations and strengths of viral cultures, the efficacy of the anti-viral immunization, and for studying the neutralizing capacity of virus-specific antibodies. The present invention comprises an improved hemagglutination inhibition assay (HAI), with at least about a 10-fold increase in sensitivity versus the traditional the HAI, to provide more accurate measurements of components in, for example, fluids from the in vitro MIMIC® system when assessing the effects of anti-viral vaccines (e.g., for seasonal influenza).
Owner:SANOFI PASTEUR VAX DESIGN
Particle agglutination detection method and device
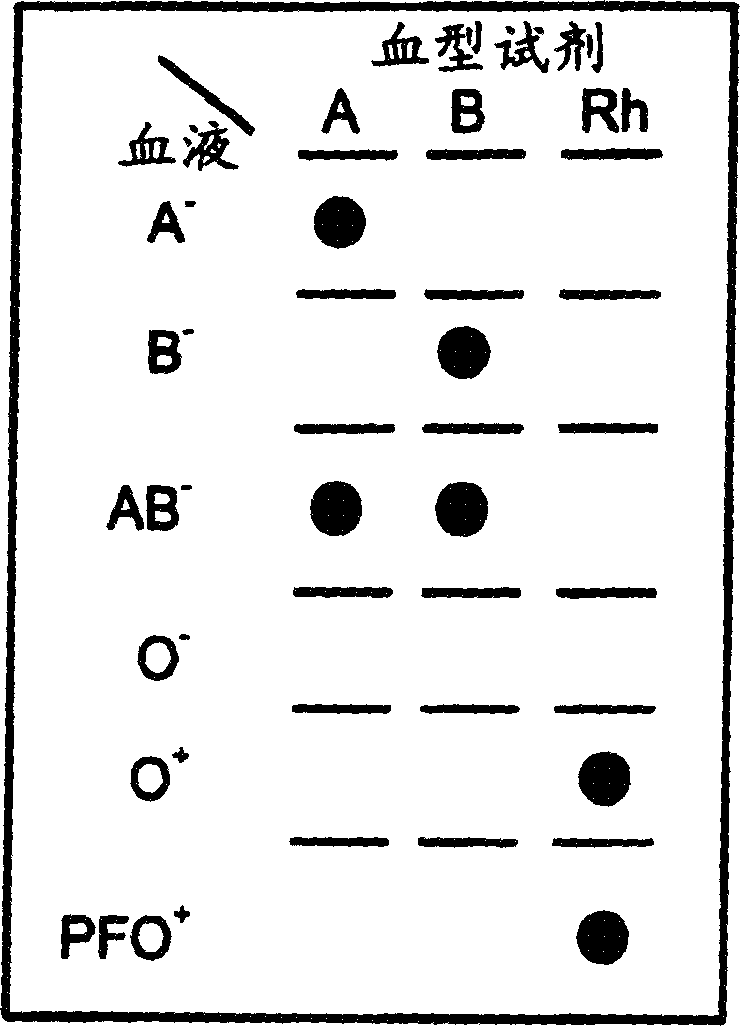
InactiveCN1849514AEasy to useAvoid False Positive ResultsMicrobiological testing/measurementBiological testingCrossmatching bloodBlood typing
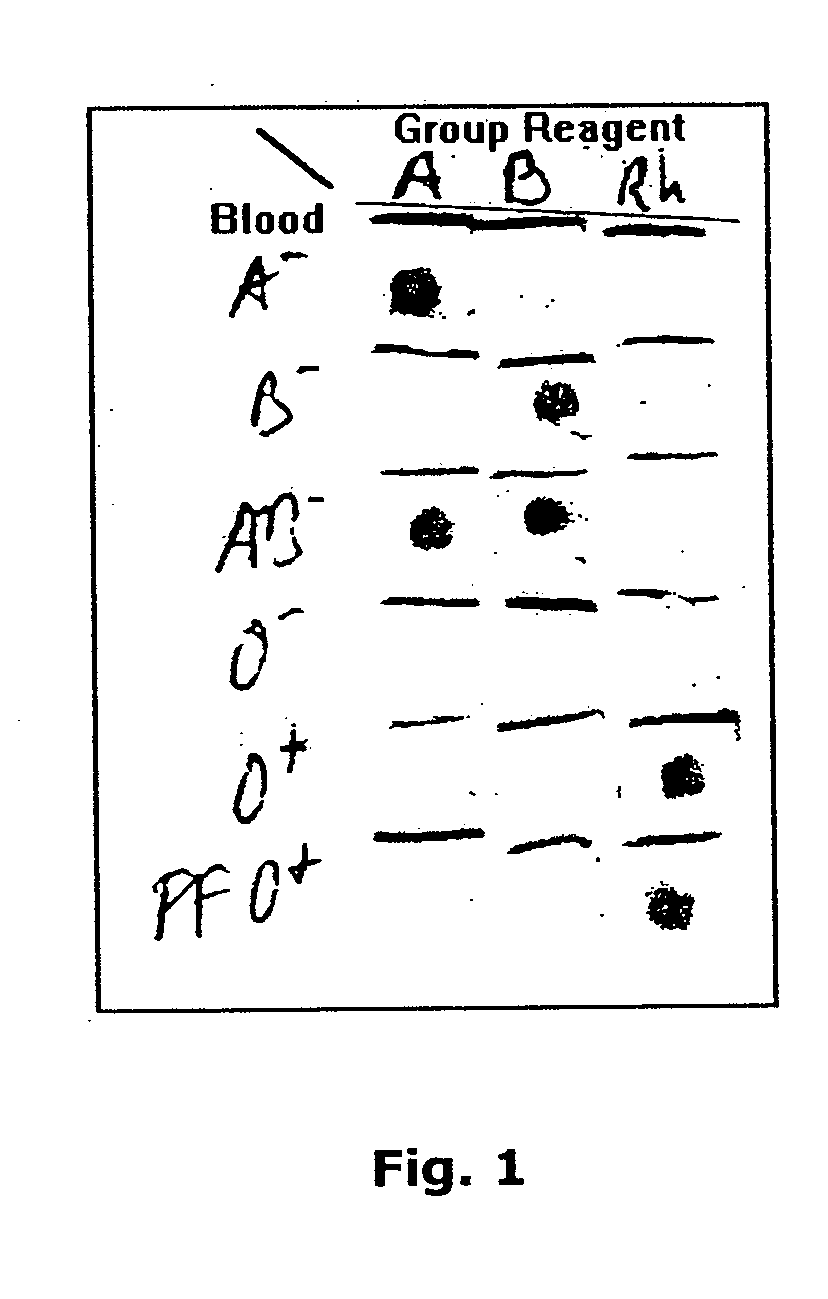
A method for detecting and / or observing particle agglutination comprising: placing a volume of particle suspension on a filter constructed to allow passage of individual particles; placing a volume of solution or suspension containing an agglutination reagent on the particle The location of the suspension; optionally placing the washing solution at the location of the agglutinating reagent; and observing the surface of the location for the presence of particles, which presence indicates that particle agglutination has occurred. Also provided are methods for detecting agglutination in general and hemagglutination, eg, for blood typing and cross-matching in particular. The method consists of continuous vertical addition of whole blood, blood typing reagents, and detergent to the filter. In the case of hemagglutination, colored, preferably red or reddish spots appear after washing. Devices and kits based on the present invention are also claimed which facilitate blood typing and cross matching in a non-laboratory environment without the need for laboratory instruments.
Owner:INVERNESS SWITZERLAND GMBH
Canine influenza virus monoclonal antibody hybridoma cell strain F112 and application thereof
ActiveCN107034197AStrong antiviral activityRapid test diagnosisImmunoglobulins against virusesAntiviralsLymphocyteTiter
The invention relates to canine influenza virus monoclonal antibody hybridoma cell strain F112 and belongs to the technical field of biology. Immune antigens are prepared by using canine influenza virus epidemic strain A / Canine / Nanjing / 11 / 2012 (H3N2) through lymphocyte hybridoma technique, the efficient hybridoma cell strain F112 is screened out from the antigens, and the canine influenza virus monoclonal antibody hybridoma cell strain F112 has hemagglutination inhibition titer of 212 and neutralizing titer of 105 for canine influenza virus. A canine influenza preventive therapeutic agent prepared by using F112 monoclonal antibody is used for preventing and treating canine influenza, under the effective rate of 100%. The efficient F112 monoclonal antibody is applicable to the detection and diagnosis of canine influenza and the preventive therapy, and has a promising application prospect.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
A kind of assay method and equipment for platelet aggregation and coagulation factor
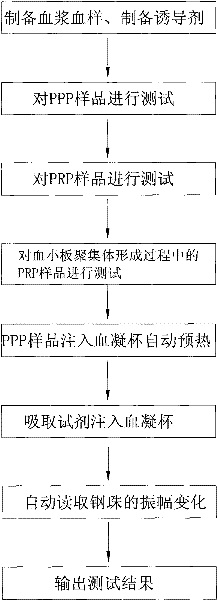
ActiveCN102262090AFast testProtect your healthMaterial analysis by optical meansPlatelet aggregation ratioTurbidimetry
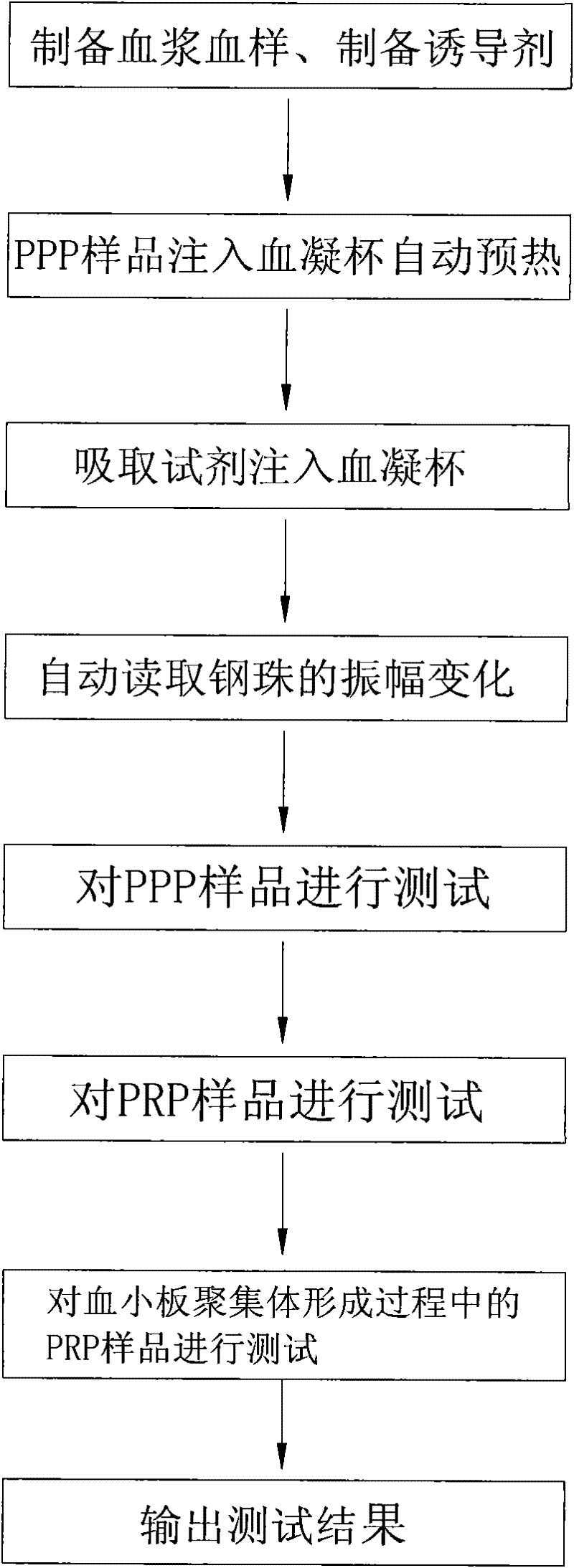
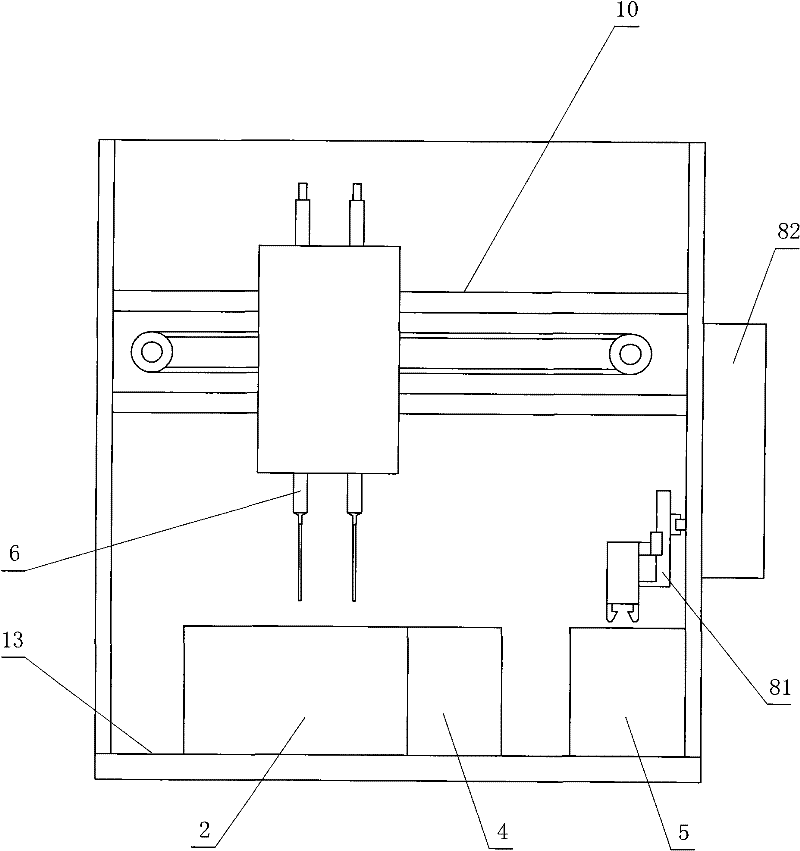
The invention relates to a method for measuring the platelet aggregation and a blood coagulation factor. In the method, a full-automatic coagulate blood analytical instrument is used, the platelet aggregation is measured by using photoelectric turbidimetry and the blood coagulation factor is measured by using a double magnetic circuit and bead method. The method comprises the following measuring steps of: preparing a platelet aggregation inductive agent, platelet rich plasma and platelet poor plasma; respectively reading light transmittance data of a platelet poor plasma sample and light transmittance data of a platelet rich plasma sample as well as light transmittance data of the platelet rich plasma during the forming process of the platelet aggregation within the specified time; calculating the platelet aggregation rate of the measured blood sample; absorbing the platelet poor plasma and injecting the platelet poor plasma in a hemagglutination cup; absorbing the selected reagent according to a test program and injecting the selected reagent into the hemagglutination cup; automatically monitoring the amplitude of a steel bead; and generating a test report by using measuring results of the platelet aggregation and the blood coagulation factor according to input information of patients.
Owner:BEIJING PRECIL INSTR CO LTD +1
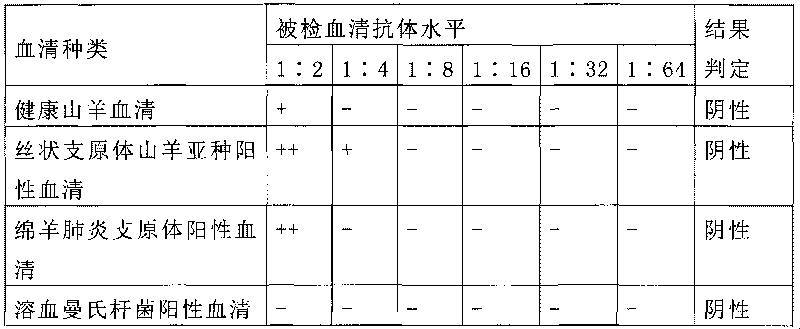
Mycoplasma capricolum subsp. pneumonia antigen of goats and preparation method thereof
ActiveCN101712971AAvoid timeAvoid the disadvantage of low sensitization potencyBiological testingFermentationRed blood cellSubspecies
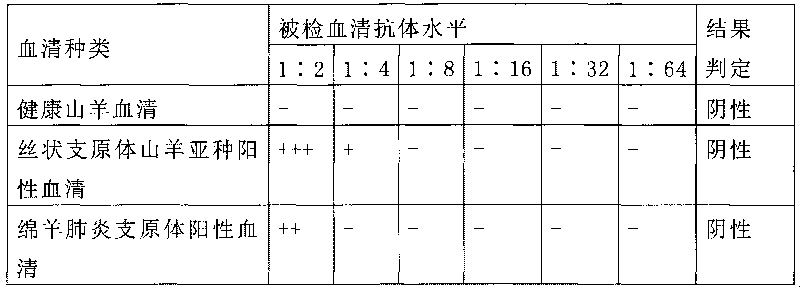
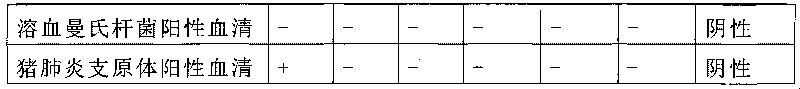
A mycoplasma capricolum subsp. pneumonia polysaccharide antigen of goats is characterized by extracting polysaccharides secreted by thalli from the supernatant of a mycoplasma capricolum subsp. pneumonia culture of goats. The agent is prepared by the following processing steps: a. culturing mycoplasma and b. extracting mycoplasma polysaccharide antigen. The invention has the following advantages:(1) the antigen in the invention is the polysaccharide antigen extracted from the supernatant of the mycoplasma capricolum subsp. pneumonia culture of goats and is polysaccharide secreted by thalli, has strong specificity and can truly detect the mycoplasma capricolum subsp. pneumonia antibody of goats; (2) the aldehyde-tanned sheep red cells sensitized with polysaccharide are used, thus avoidingthe defects of short preservation time and low sensitization titer of the fresh sheep red cells, being easily preserved for long time and having high hemagglutination titer;(3) the method is simple, convenient and fast in operation and dispenses with special equipment and apparatuses, thus being suitable for popularization and application at the grassroots level; (4) the invention is the unique diagnostic reagent for detecting the mycoplasma capricolum pneumonia antibodies of goats at home at present.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
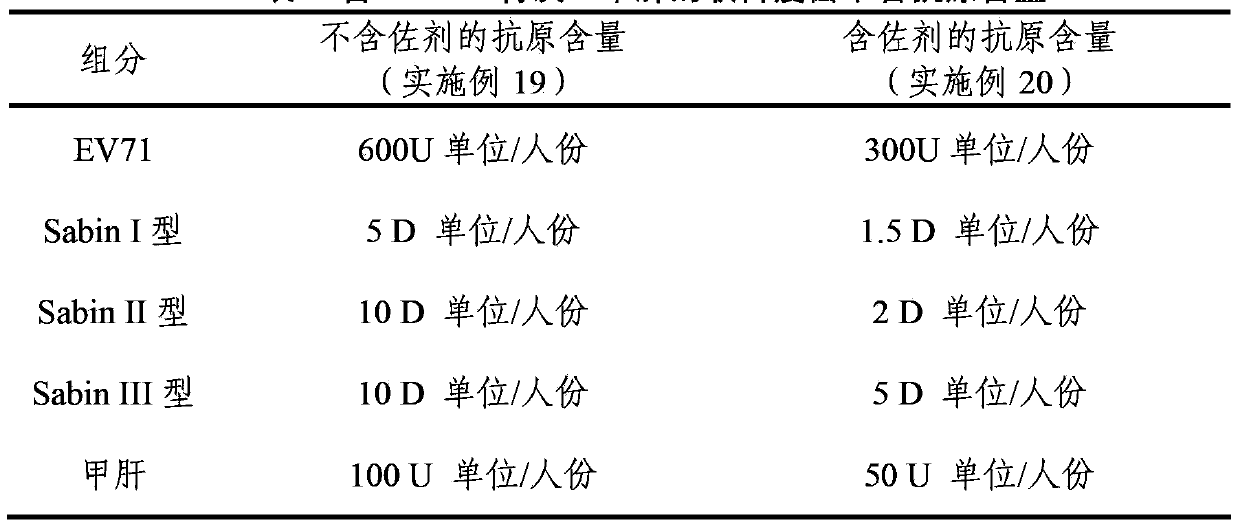
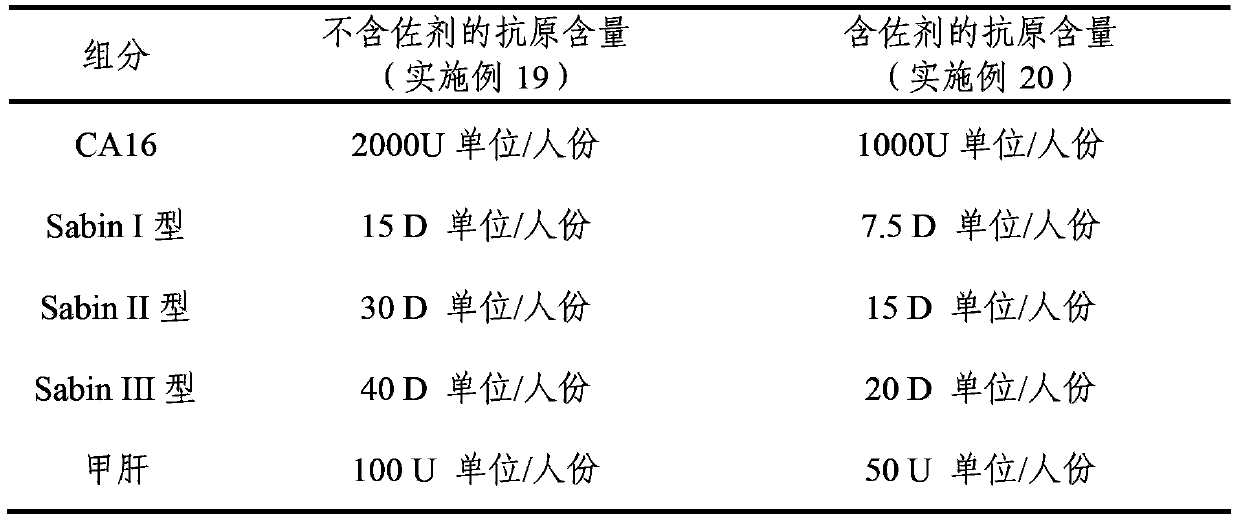
Multivalent immunogenic composition
ActiveCN103394082AImprove securitySave the number of seedsBacterial antigen ingredientsViral antigen ingredientsHemagglutininTetanus toxoids
The invention provides a multivalent immunogenic composition, which includes an inactivated hepatitis A antigen and an inactivated poliovirus. The composition also can further include over one or two of a purified pertussis antigen, diphtheria toxoid, tetanus toxoid, filamentous hemagglutinin, Haemophilus influenzae type b polysaccharide, Neisseria meningitidis capsular polysaccharide, a hepatitis B virus antigen, enterovirus 71 and a coxsackievirus A16 antigen, and a physiologically acceptable carrier. The composition involved in the invention is employed to immunize the inoculated population in the form of a bivalent vaccine or more combined vaccines. Without reducing the immune effects of each immunizing antigen, the inoculation number of times can be reduced at the same time, and the time and human resources can also be saved.
Owner:SINOVAC BIOTECH
Duplex bacteria-free medicine mixer
InactiveCN1486755AGood hemostatic effectSpeed up healingInfusion syringesSurgeryAir filterEngineering
The duplex bacteria-free medicine mixer includes injectors, duplex frame on the injectors, push rods stretching into the duplex frame and injector seat as well as characterized nozzles communicated via locking parts with the injector seat, which has inner cavity separated into two sealed hollow areas. The structure with nozzles makes the fibrinogen and the catalyst from the injectors well mixed to raise the hemorrhage arresting effect of the mixing formed gel matter, short the hemagglutination period and speed wound heal. The improved injector seat eliminates the need of power unit and air filter unit resulting in saving in cost and simple operation. In addition, the humanized design of the locking unit, pull rod and support rod also simplifies the operation of the device.
Owner:上海米沙瓦医科工业有限公司
Group I type 4 aviadenovirus strain WZ and application of strain WZ
ActiveCN106754754AImprove featuresImproving immunogenicitySsRNA viruses negative-senseSsRNA viruses positive-senseViral VaccineAvian adenovirus
The invention discloses a group I type 4 aviadenovirus strain WZ. The strain has a preservation number of China Center for Type Culture Collection (CCTCC) No: V201662, is named as group I type 4 aviadenovirus strain WZ in a classified way, and is preserved in December 14, 2016; a group I type 4 viral vaccine of the aviadenovirus is prepared from the inactivated group I type 4 aviadenovirus strain which is taken as an active ingredient; the invention also provides application of the group I type 4 aviadenovirus strain WZ in preparation of an agar gel precipitating antigen, a hemagglutination inhibition (HI) antigen and positive serum antigen reagent which are used for diagnosing the group I type 4 virus of the aviadenovirus, and preparation of egg yolk antibody and antiserum which are used for treating the group I type 4 virus of the aviadenovirus. After the vaccine prepared by using the group I type 4 aviadenovirus strain is used for immunizing, the toxin attacking protection rate for the group I type 4 aviadenovirus strain WZ reaches 90-100%. As a vaccine strain with good manufacturing effect, the group I type 4 aviadenovirus strain WZ can be used for preventing avian inclusion body hepatitis and pericardial effusion syndrome, and also can be used for virus identification and epidemiological investigation.
Owner:HENAN AGRICULTURAL UNIVERSITY
Compound interferon inducing agent lozenge
InactiveCN1261161CLong duration of actionAvoid smallViral antigen ingredientsAntiviralsTreatment effectSide effect
The present invention is compound interferon inducing agent lozenge, relates to biomedicine engineering, and aims at providing lozenge capable of inducing body to generate endogenous interferon and with high curative effect, less side effect, small dosage and wide application. Each of the compound interferon inducing agent lozenge contains attenuated Newcastle disease virus vaccine or deactivated Newcastle disease virus vaccine 10-120 hemagglutination units, astragalus root 1-2000 mg, liquiritigenin 1-1000 mg, supplementary material and stabilizer. The compound interferon inducing agent lozenge is suitable for preventing SARS and other viral infectious respiratory tract diseases, and has also auxiliary treatment effect on various kinds of tumor, hepatitis B and hepatitis C.
Owner:钱汶光
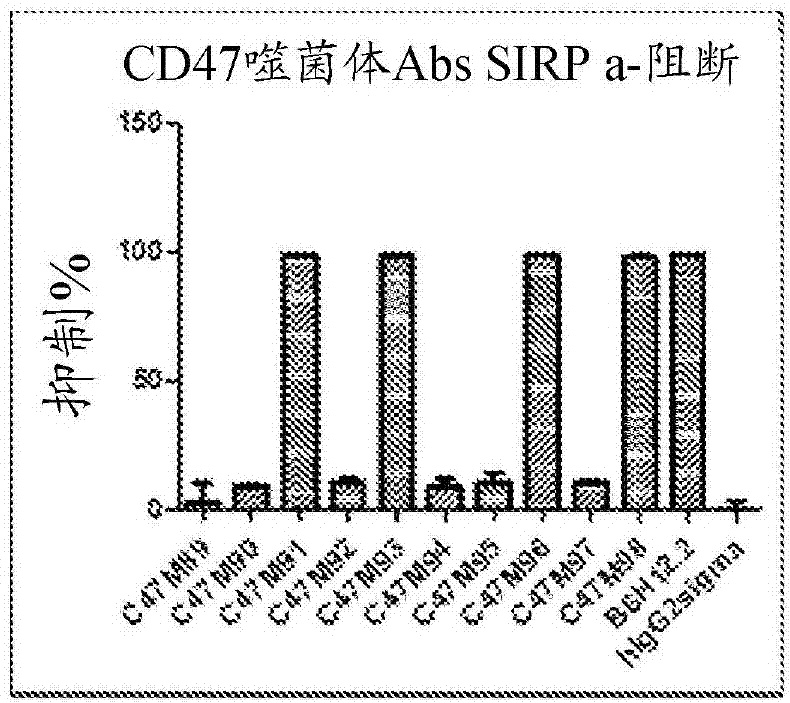
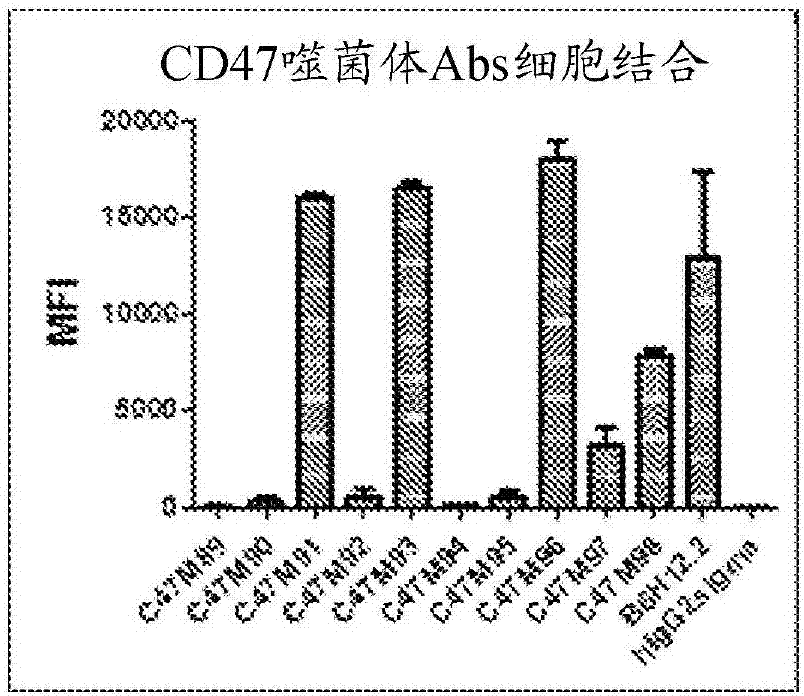
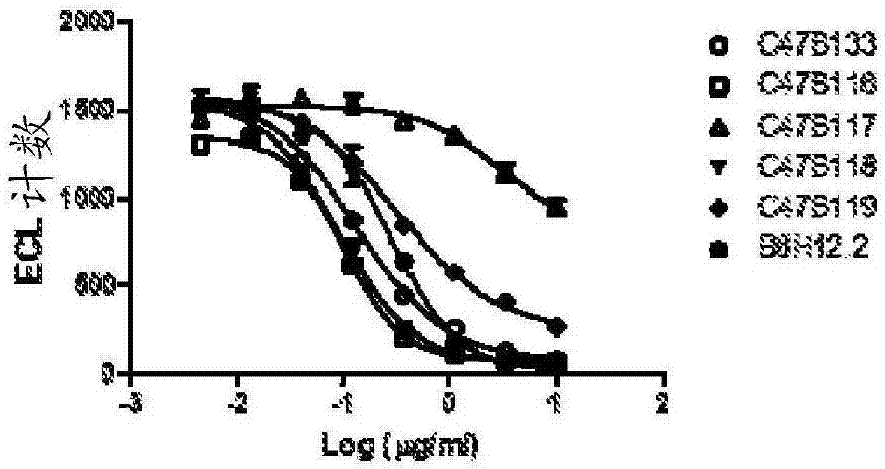
Cd47 antibodies, methods, and uses
ActiveCN107406503AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesPlatelet aggregation
The present disclosure relates generally to monoclonal antibodies that specifically bind to CD47, more specifically to CD47 antibodies that do not have significant platelet aggregation activity and do not have significant hemagglutination activity. Methods of generating these antibodies and methods of using these monoclonal antibodies as therapeutics are also provided.
Owner:JANSSEN PHARMA NV
Diagnostic kit for Newcastle disease virus antibody
The invention relates to a diagnostic kit for a Newcastle disease virus antibody, which comprises 10-time concentrated washing liquid, serum diluent, standard serum, an anti-chicken immunoglobulin G (IgG) enzyme-labeled antibody, enzyme-labeled antibody diluent, a color development agent A, a color development agent B and stop solution. In an enzyme-linked immunosorbent assay (ELISA) diagnostic kit for detecting the Newcastle disease virus antibody, the established method has specificity, sensitivity and operability. In the aspect of sensitivity tests, the coincidence rate of the established method and a method of immunofluorescence technology is 86 percent, and compared with the method of the immunofluorescence technology, the established method is more sensitive and has the zero relevance ratio in other causes of disease pathogeny; and compared with hemagglutination inhibition (HI), the method has the advantages of quickness and low cost, and can be used for diagnosing and monitoring Newcastle disease virus, and the result is easy to determine.
Owner:河南后羿生物工程股份有限公司
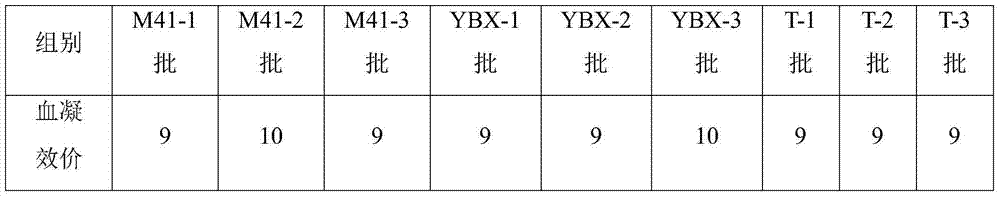
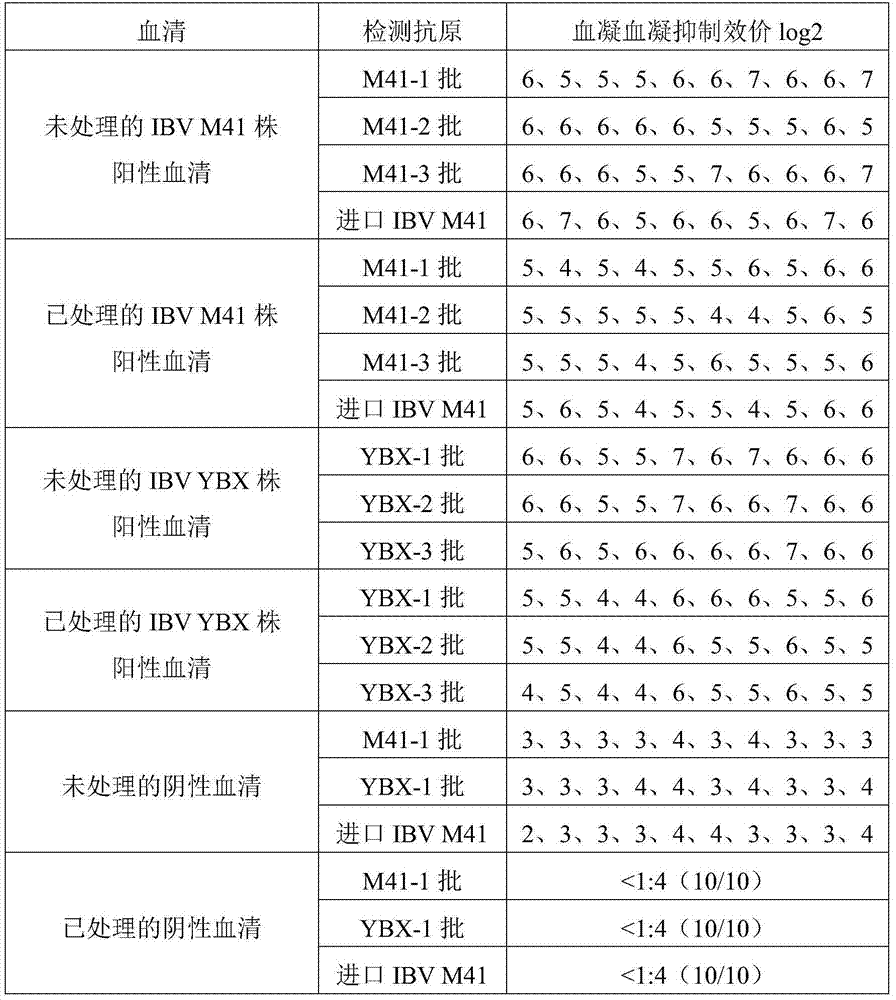
Method for detecting hemagglutination inhibition antibody of chicken infectious bronchitis
The invention relates to the technical field of livestock vaccine, and in particular relates to a method for detecting a hemagglutination inhibition antibody of chicken infectious bronchitis. The method comprises the following steps: treating serum to be detected, namely, by taking kaoline suspension as a serum treatment liquid for treating serum to be detected, adding the supernate into chicken erythrocyte blood corpuscle mud so as to obtain serum which is diluted in a ratio of 1:4 and is to be detected; performing hemagglutination inhibition test, namely, adding 25mu l of the serum which is diluted in a ratio of 1:4 and is to be detected into 25mu l of antigen with 4HA units, and performing hemagglutination judgment. By adopting the negative serum treated by using a method for removing non-specific reaction of the serum, the infectious bronchitis hemagglutination inhibition evaluation titer is less than 1:4, and by adopting the infectious bronchitis positive serum treated by using the method, the infectious bronchitis hemagglutination inhibition evaluation titer is reduced by 0.5-1 titer, the non-specificity reaction is removed, and the accuracy of the infectious bronchitis hemagglutination inhibition experiment result is improved.
Owner:YEBIO BIOENG OF QINGDAO
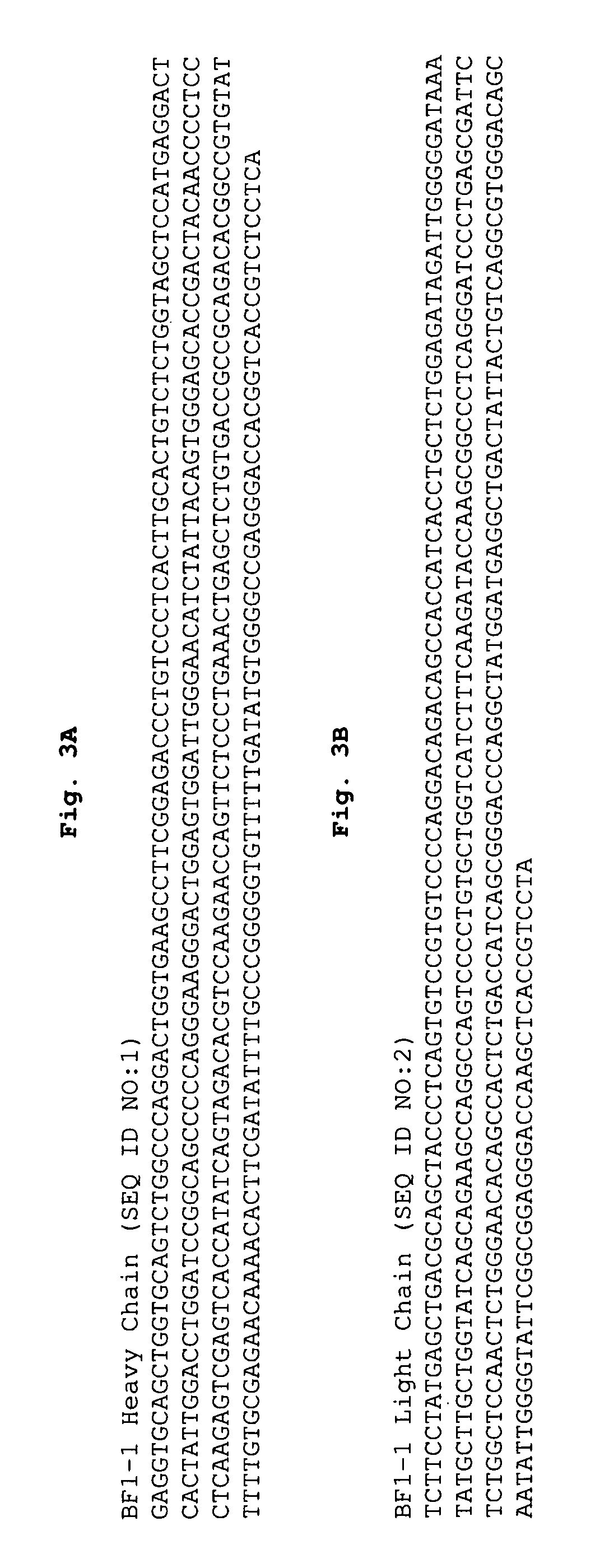
Human neutralizing monoclonal antibodies to H5N1 influenza A virus
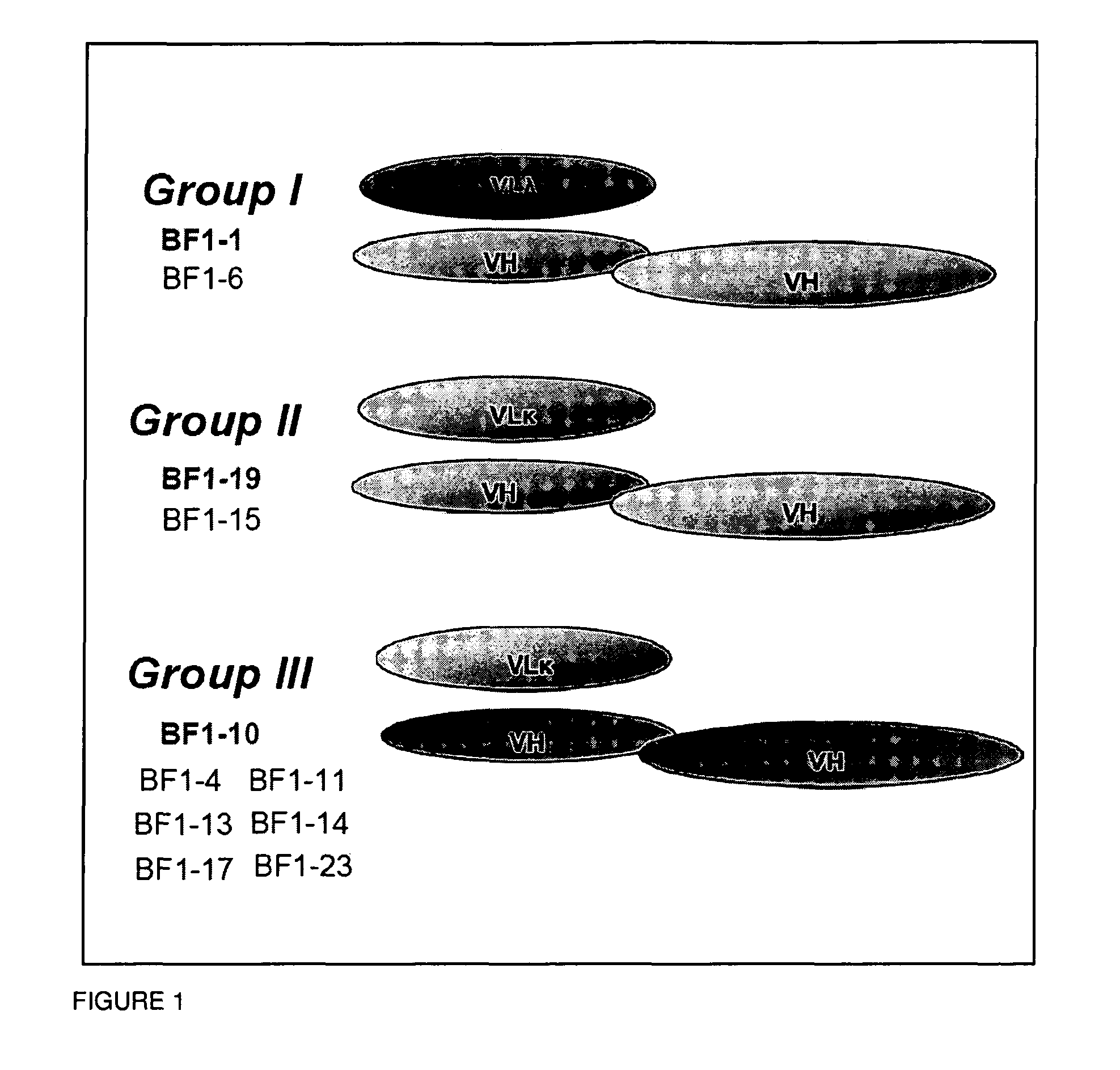
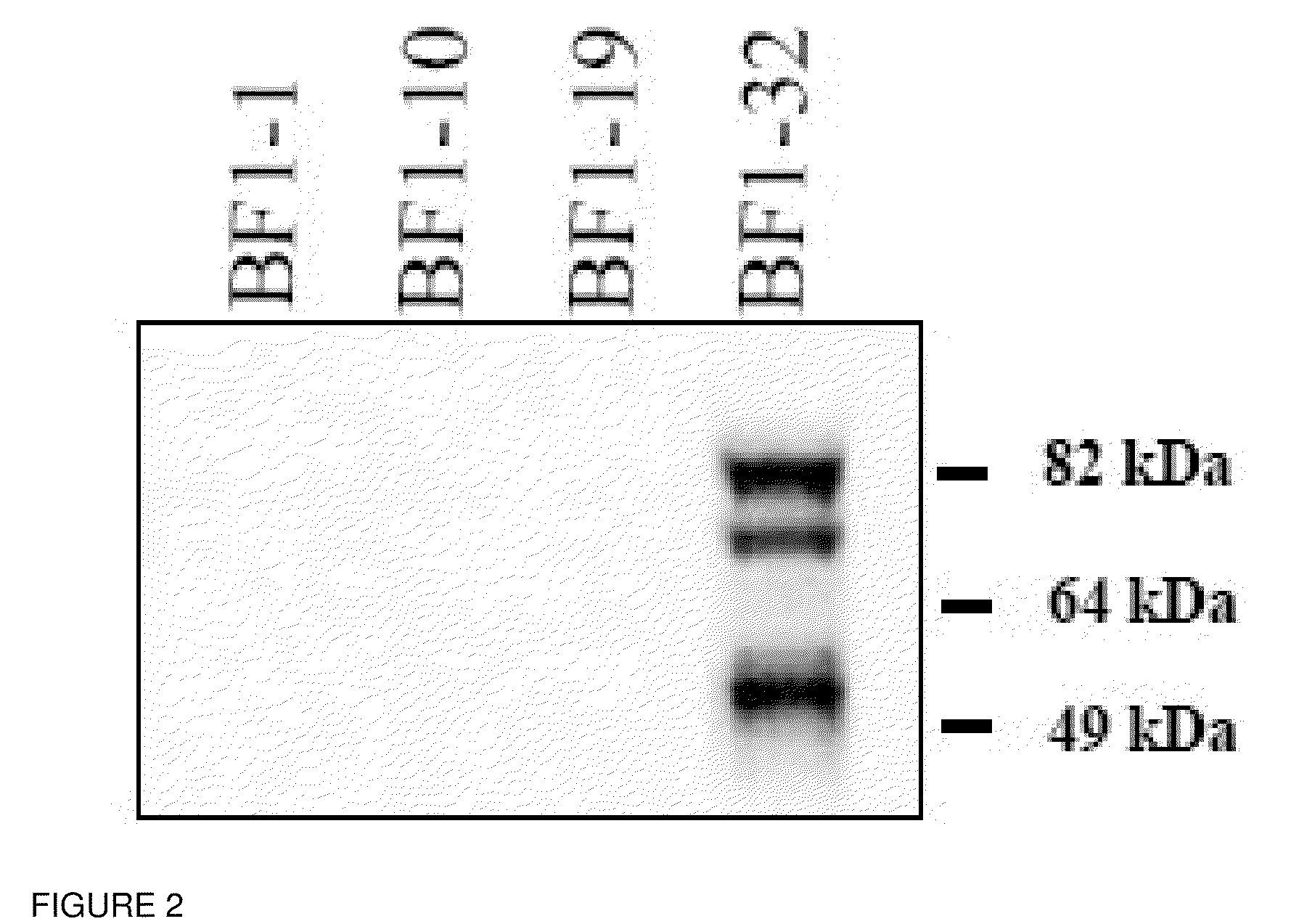
A panel of IgG1 human monoclonal antibodies (HMAbs) identified by hemagglutination inhibition (HI) assay has been produced from peripheral B cells of an individual immunized with prototype H5N1 vaccine. Sequence analysis of antibody clones showed three clusters of different HMAbs as represented by HMAbs designated as BF1-1, BF1-19 and BF1-10. BF1-1 and BF1-10 have distinct CDR 1, 2 and 3 regions of both heavy and light chains. BF1-19 has the same heavy chain as BF1-1 but the light chain of BF1-10. Antibody binding affinity, KD, studies showed all three HMAbs ranging from at least about 10−8 to at least about 10−9. In vivo protection studies showed that these antibodies afforded significant protection against infection. These findings demonstrate that the antibodies of the invention are cross-neutralizing and therapeutic.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Particle agglutination detection method and device
InactiveUS20050084879A1Easy to useAvoid False Positive ResultsMicrobiological testing/measurementBiological testingAgglutinationHemagglutination
A method for the detection and / or visualization of particle agglutination, comprising: placing a volume of a suspension of the particles on a filter, the filter being constructed so as to permit passage of individual particles; placing a volume of a solution or suspension containing an agglutinating agent at the location of the particle suspension; optionally placing a wash solution at the location of the agglutinating agent; and observing the surface at the location for the presence of particles, such presence indicating that agglutination of the particles occurred. There is also provided a method for detection of agglutination reactions in general and hemagglutination reactions, such as used in blood grouping and cross-matching, in particular. The method is comprised of successive vertical additions of whole blood, blood grouping reagent and wash to a filter. In case of hemagglutination a colored, preferably red or reddish dot becomes visible after washing. A device and a kit based on the invention is also claimed and facilitates blood grouping and matching in non-laboratory environment without the need for laboratory instruments.
Owner:INVERNESS MEDICAL SWITZERLAND GMBH
Avian influenza H5N1 subtype Re-5 strain hemagglutination inhibition antigen standard substance and preparation method
InactiveCN102012430AImprove the level of prevention and controlMaterial analysisImmune effectsImmune monitoring
The invention relates to an avian influenza H5N1 subtype Re-5 strain hemagglutination inhibition antigen standard substance and a preparation method thereof. The standard substance is prepared by performing the following series of steps of: preparing and inspecting liquid of avian influenza H5N1 subtype Re-5 strain viruses; inactivating the liquid of the viruses and inspecting a semi-finished product; freeze-drying, inspecting a finished product, homogeneity and stability, demarcating the standard substance, valuing and the like. The standard substance is fundamental guarantee of accurately diagnosing avian influenza H5N1 subtype, performing immune monitoring of an H5N1 subtype Re-5 strain hemagglutination inhibition antibody and accurately evaluating the immune effect of vaccine and improves the prevention and control level of the avian influenza.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Optimized influenza vaccines
ActiveUS20110229518A1Broad cross-reactivityPrevent/limit infection and spread of infectionSsRNA viruses negative-senseSugar derivativesInfluenza aHemagglutination
The invention concerns nucleotides vaccines encoding influenza proteins with few or no glycosylation sites. Since these first introductions of pandemic influenzas the viruses have drifted, accumulating mutations at antigenic sites, but also the N-glycosylation pattern has changed during the drifted years, accumulating N-linked glycosylation sequons that help mask the antigenic sites for recognition by the host immune system. These “naked” initial haemagglutinins induce a broad cross reactivity against widely drifted influenza subtypes. The origin of the DNA or RNA can be both pandemic influenza strains, which codes for proteins which have a naturally low content of glycosylation sites and / or DNA or RNA from non-pandemic influenza strains where the nucleotides have been mutated or changed so it encodes for proteins with less or no glycosylation sites. The invention also discloses DNA or RNA encoding the haemagglutinin (HA) from pandemic influenza A, e.g. the 1918 H1N1 and / or the 1957 H2N2 and / or the 1968 H3N2 influenza A virus, optionally with the Neuraminidase (NA) and / or matrix protein (M) and / or the nucleoprotein (NP) from these pandemic influenza virus included, mixed together with DNA or RNA from non-pandemic influenza A as a vaccine against present day and future influenza A viruses.
Owner:STATENS SERUM INST
Anti-H7 subtype avian influenza virus monoclonal antibody and application thereof
ActiveCN104312979AHemagglutination inhibition HI titer is highHigh potencyImmunoglobulins against virusesMicroorganism based processesAvian virusHemagglutination
The invention relates to an anti-H7 subtype avian influenza virus monoclonal antibody and application thereof, belonging to the technical field of biology. A hybridoma cell strain 5D2 is screened; the hemagglutination inhibition (HI) titer of the rat ascites antibody prepared from the hybridoma cell strain 5D2 for H7-AIV is 11log2, and the ELISA titer is 2*10<5>. The anti-H7 monoclonal antibody secreted by the hybridoma cell strain has high HI and ELISA titer; the colloidal gold immunochromatography detection test strip assembled by the anti-H7 monoclonal antibody has high specificity, except that the result for H7-AIV is positive, the results for the other avian viruses are negative; the anti-H7 monoclonal antibody can be used for on-site diagnosis on fowl, mammals or the like infected by H7-AIV; and the result can come out within 10 minutes, is visual, and can be easily judged with naked eyes. The sensitivity of the diagnosis kit assembled by the hybridoma cell strain secretory antibody is not lowered after the diagnosis kit is stored for one year, so the diagnosis kit has favorable stability.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES +1
Method of preparing porcine parvovirus virus-like particle subunit vaccine by using Escherichia coli expression system and application of method
InactiveCN106148358AImprove solubilityGood specific immune responseBacteriaViral antigen ingredientsEscherichia coliEukaryotic plasmids
The invention discloses an encoding gene of porcine parvovirus VP2 protein, a method of prokaryotically expressing VP2 protein virus-like particles, and application of the method in vaccine preparation. Sequences are optimized, VP2 gene is artificially synthesized, the synthesized gene is inserted into pET28a vector, the gene and chaperone protein plasmids are co-transferred to BL21(DE3) host bacteria, the VP2 protein and chaperone protein are co-expressed to promote correct folding of the VP2 protein. Experiments prove that recombinant bacteria expressed VP2 protein can be self-assembled in vitro and has good immunogenicity; by immunizing mice and guinea pigs with the virus-like particle subunit vaccine prepared with the VP2 protein expressed herein, it is possible to induce the production of a high level of hemagglutination inhibition antibodies and neutralizing antibodies, and the vaccine can prevent guinea pigs from being affected by strong porcine parvovirus. The recombinant bacteria according to the invention can be utilized to efficiently prepare porcine parvovirus virus-like particles, the production cost is low, operation is simple, and biosafety is better.
Owner:HENAN ACAD OF AGRI SCI +1
A rapid antigen detection method for inactivated oil emulsion vaccine against avian influenza finish products
ActiveCN103235139ADoes not destroy hemagglutination titerDoes not destroy antigenicityBiological testingOil emulsionHaemagglutination inhibition
The present invention discloses a rapid antigen detection method for inactivated oil emulsion vaccine against avian influenza finish products, and the method comprises the following steps of: 1) mixing uniformly isopropyl myristate and the inactivated oil emulsion vaccine against avian influenza by thoroughly shaking, centrifuging, and separating a water phase layer; 2) performing HA titer detection to the water phase of the vaccine obtained in the step 1); 3) according to the detection result of the HA titer detection of the water phase of the vaccine in step 2), taking the water phase of the inactivated oil emulsion vaccine against avian influenza of step 1) to prepare a 4HAU vaccine antigen diluent; and 4) performing hemagglutination inhibition tests by using the 4HAU vaccine antigen diluent prepared in step 3), wherein a HI titer is expressed as the highest dilution serum that completely inhibits the 4HAU antigen. The method of the invention does not destroy the hemagglutination titer and antigenicity of vaccine antigens, can quickly and accurately determine the HI titer of the water phase of the inactivated oil emulsion vaccine against avian influenza finish products and analyze differences in antigenicity, and reagents in use are safety and non-toxic for human and environment, and are cheap and readily available.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA +2
Compound interferon inducing agent lozenge
InactiveCN1616097AConvenient sourceMature technologyViral antigen ingredientsAntiviralsTreatment effectSide effect
The present invention is compound interferon inducing agent lozenge, relates to biomedicine engineering, and aims at providing lozenge capable of inducing body to generate endogenous interferon and with high curative effect, less side effect, small dosage and wide application. Each of the compound interferon inducing agent lozenge contains attenuated Newcastle disease virus vaccine or deactivated Newcastle disease virus vaccine 10-120 hemagglutination units, astragalus root 1-2000 mg, liquiritigenin 1-1000 mg, supplementary material and stabilizer. The compound interferon inducing agent lozenge is suitable for preventing SARS and other viral infectious respiratory tract diseases, and has also auxiliary treatment effect on various kinds of tumor, hepatitis B and hepatitis C.
Owner:钱汶光
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com