Patents
Literature
33 results about "Particle agglutination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Agglutination is the clumping together of particles which forms non-dissolvable or what is called as insoluble aggregates. The common substances used in the PAT are latex particles, preserved blood cells from mammals or birds, colloidal particles, and gelatine beads.
Particle use for image display media, image display panel using the particles, and image display device
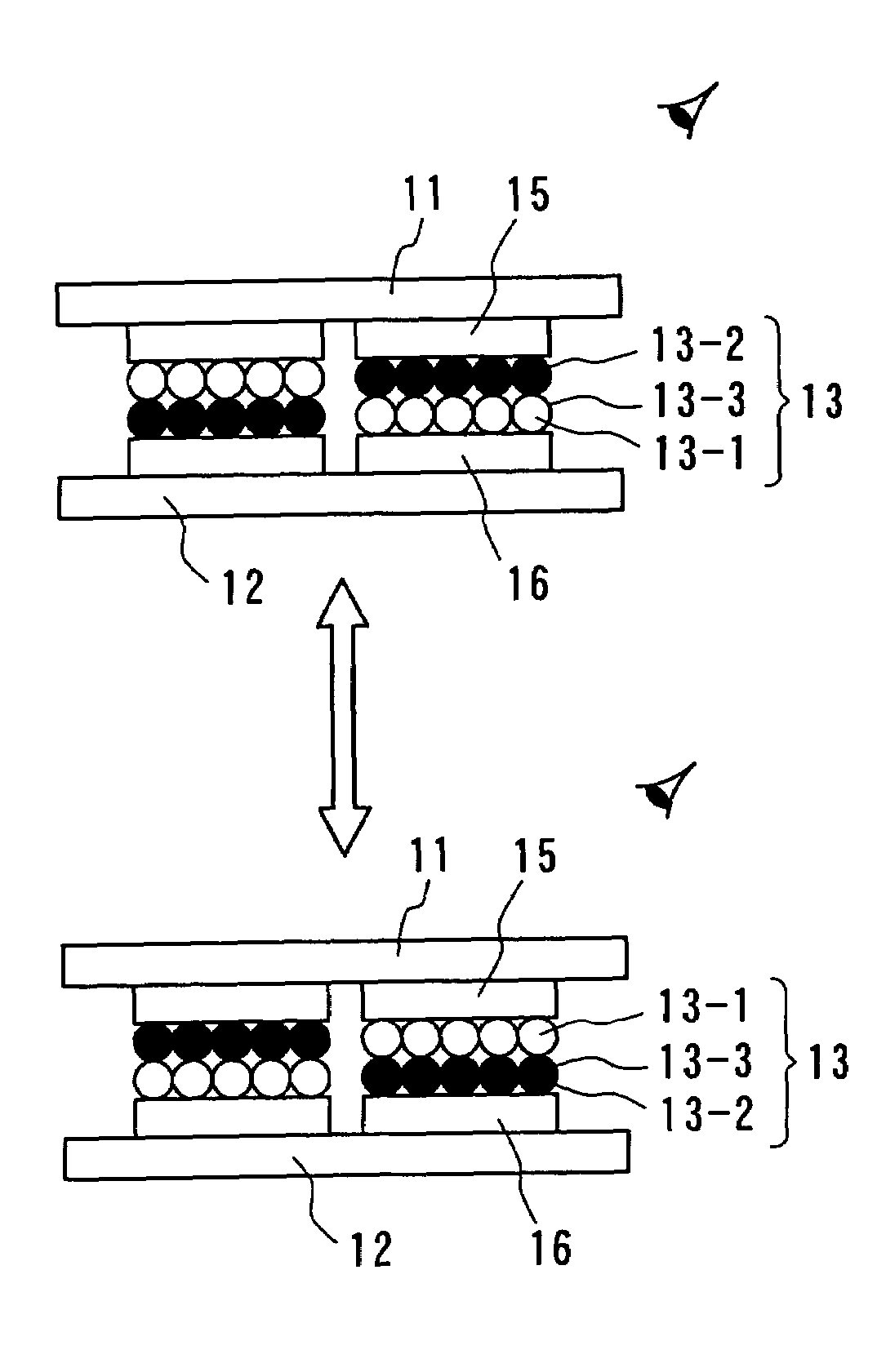
InactiveUS7236291B2Increase brightness factorImprove reflectivityStatic indicating devicesNon-linear opticsComputer graphics (images)Display device
An image display device, in which image display media are sealed between opposed substrates, at least one of two substrates being transparent, and in which the image display media, to which an electrostatic field is applied, are made to move so as to display an image. A construction of particles used as the image display media is improved (the first, second, fourth, fifth, sixth, eighth and ninth aspects of the invention), and a material of the particles used as the image display media is improved (the third, seventh and tenth aspects of the invention). Whiteness of the particles and a liquid powder using the particles is improved, particle agglutination is prevented, and the charge property is controlled. Moreover, durability is improved such that a contrast of the image display during a repetition display is not decreased. As a result, an excellent image display can be achieved.
Owner:BRIDGESTONE CORP
Method and device for ultrasound assisted particle agglutination assay
InactiveUS20090053688A1Accurate assessmentSensitive detectionBioreactor/fermenter combinationsBiological substance pretreatmentsActive matterAgglutination assay
Ultrasound-assisted particle agglutination assay methods and apparatuses are described based on first providing a standing wave ultrasound field at a resonance frequency of a test liquid in a resonator cell containing microparticles covered with a binding agent with high affinity to an analyte sought to be detected by the assay test. Formation of the specifically-bound and nonspecifically-bound aggregates of these microparticles is then followed by effective stirring of the liquid with swept-frequency sonication causing disintegration of nonspecifically-bound aggregates and leaving specifically-bound aggregates in place for further detection and measurement. The methods and devices of the invention allow significant improvement in the sensitivity and specificity of agglutination tests and are advantageously applicable to detecting various proteins, DNA, RNA and other biologically active substances. Specific examples are provided.
Owner:ALLIED INNOVATIVE SYST
Homogeneous double receptor agglutination assay for immunosuppressant drugs
A homogeneous, non-competitive, double receptor agglutination assay for measuring immunosuppressant drugs is described. The assay employs at least two receptors wherein each receptor is specific for a separate binding site on the drug and wherein each receptor is bound to a detection particle. The immunosuppressant drug binds to the receptors and causes particle agglutination, which can be measured and correlated with the presence or amount of immunosuppressant drug in a sample.
Owner:SIGLER GERALD F +5
Particles for immunoassays and methods for treating the same
InactiveUS6890765B2Preparing sample for investigationPretreated surfacesAntigen bindingOrganic chemistry
A method of treating particles to be used in immunoassays reduces interference in particle agglutination assays. For particles having covalently bound antibodies and residual NHS-ester or sNHS-ester groups on the surface, the reactive esters are treated with an aqueous mixture containing an amine compound of formula (I):H2N—R—X (I). The moiety —X is —NH2, —OH, or —CO2CH2CH3; and R is an alkyl group or an alkyl ether group. When —X is —NH2 or —CO2CH2CH3, R contains from 1 to 20 carbon atoms; and when —X is —OH, R contains from 4 to 20 carbon atoms.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Particle agglutination detection method and device
InactiveCN1849514AEasy to useAvoid False Positive ResultsMicrobiological testing/measurementBiological testingCrossmatching bloodBlood typing
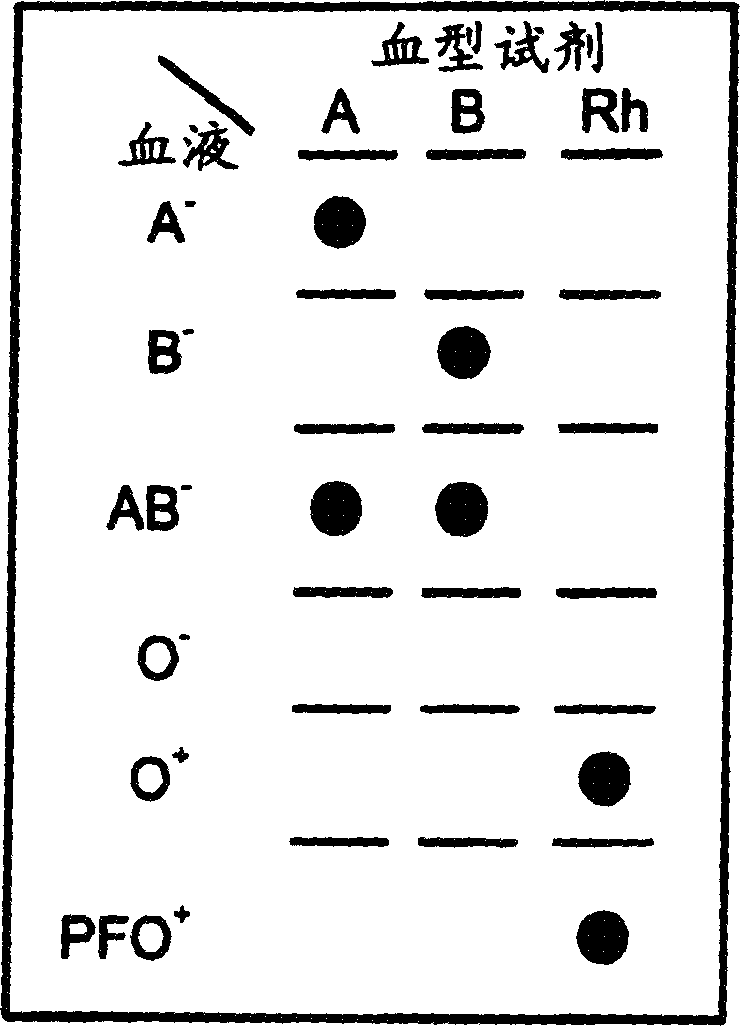
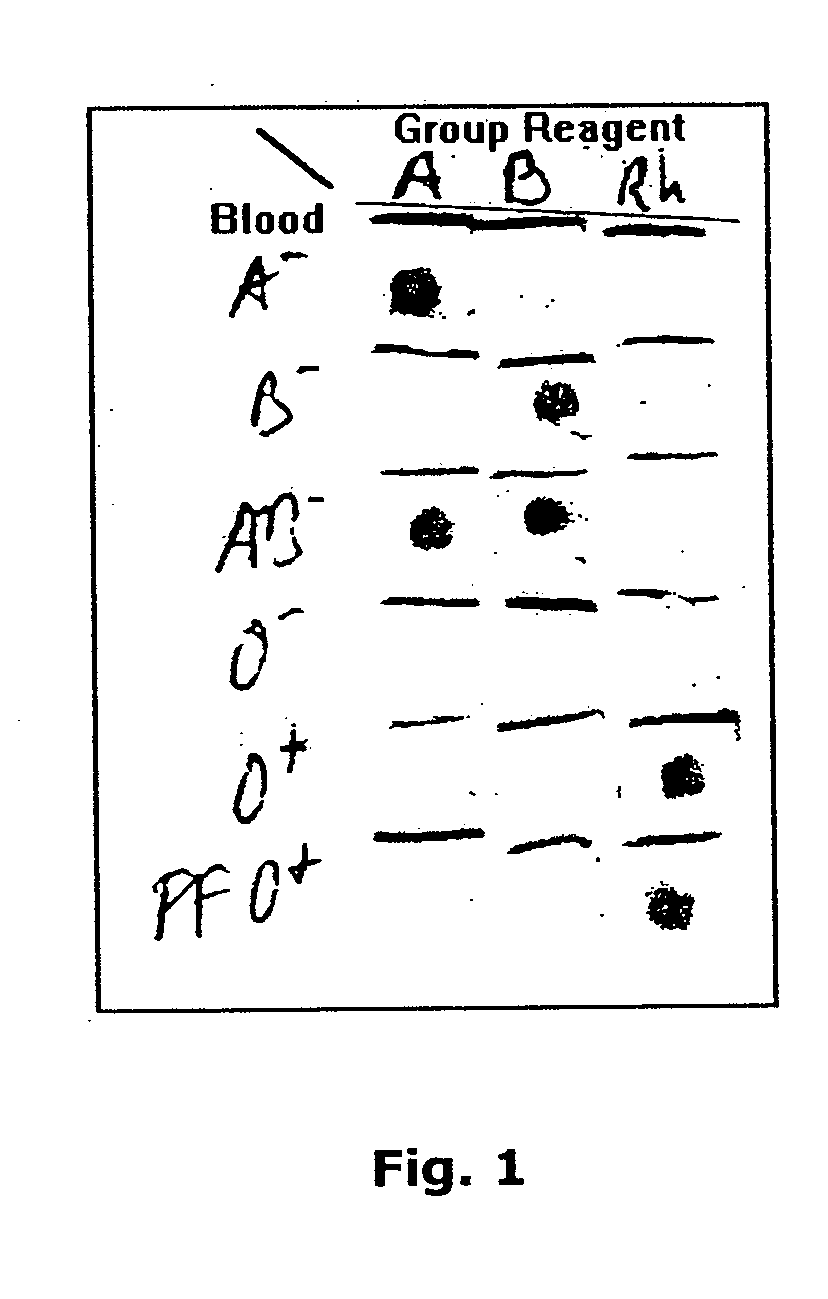
A method for detecting and / or observing particle agglutination comprising: placing a volume of particle suspension on a filter constructed to allow passage of individual particles; placing a volume of solution or suspension containing an agglutination reagent on the particle The location of the suspension; optionally placing the washing solution at the location of the agglutinating reagent; and observing the surface of the location for the presence of particles, which presence indicates that particle agglutination has occurred. Also provided are methods for detecting agglutination in general and hemagglutination, eg, for blood typing and cross-matching in particular. The method consists of continuous vertical addition of whole blood, blood typing reagents, and detergent to the filter. In the case of hemagglutination, colored, preferably red or reddish spots appear after washing. Devices and kits based on the present invention are also claimed which facilitate blood typing and cross matching in a non-laboratory environment without the need for laboratory instruments.
Owner:INVERNESS SWITZERLAND GMBH
Latex reagent for adiponectin analysis and method of adiponectin analysis
A latex reagent for analyzing adiponectin, comprising a suspension of latex particles carrying a substance which specifically binds to adiponectin, is disclosed. Further, a method for analyzing adiponectin, comprising (1) obtaining a biological liquid possibly containing adiponectin, and (2) bringing the biological liquid, while maintaining the state in which the biological liquid is obtained, into contact with a suspension of latex particles carrying a substance which specifically binds to adiponectin, and optically analyzing a degree of latex-particles-agglutination, is disclosed. According to the latex reagent and the method for analyzing adiponectin, a predilution or pretreatment of the biological liquid to be analyzed is not necessary. Further, the analysis can be performed rapidly and conveniently, and facilities therefor are not limited.
Owner:MITSUBISHI CHEM MEDIENCE +1
Particle agglutination detection method and device
InactiveUS20050084879A1Easy to useAvoid False Positive ResultsMicrobiological testing/measurementBiological testingAgglutinationHemagglutination
A method for the detection and / or visualization of particle agglutination, comprising: placing a volume of a suspension of the particles on a filter, the filter being constructed so as to permit passage of individual particles; placing a volume of a solution or suspension containing an agglutinating agent at the location of the particle suspension; optionally placing a wash solution at the location of the agglutinating agent; and observing the surface at the location for the presence of particles, such presence indicating that agglutination of the particles occurred. There is also provided a method for detection of agglutination reactions in general and hemagglutination reactions, such as used in blood grouping and cross-matching, in particular. The method is comprised of successive vertical additions of whole blood, blood grouping reagent and wash to a filter. In case of hemagglutination a colored, preferably red or reddish dot becomes visible after washing. A device and a kit based on the invention is also claimed and facilitates blood grouping and matching in non-laboratory environment without the need for laboratory instruments.
Owner:INVERNESS MEDICAL SWITZERLAND GMBH
Inert carrier indirect agglutination test detection system and application thereof
ActiveCN111537712ANo self-condensationSelf-condensation does not occurBiological material analysisDepsipeptidesSerum/Whole bloodAntigen
The invention discloses an inert carrier indirect agglutination test detection system and application thereof. The system comprises inert carrier bacteria S9 and a complex S9-P which is shown and expressed on the surface of the inert carrier bacteria S9 and carries a P-antibody factor. The system only carries the P-antibody factor, is single in component and specific in targeting, generates macroscopic positive particle agglutination reaction with whole blood or serum of chicken infected by pullorum disease and salmonella typhimurium under a certain concentration condition, and does not generate non-specific cross agglutination reaction with chicken-derived serum and whole blood of different backgrounds infected by non-pullorum disease and salmonella typhimurium. The S9-P is based on a glass plate agglutination reaction operation platform. The S9-P is simple and convenient to operate, sensitive and rapid in reaction, macroscopic in agglutination reaction particles and clear and easy inresult judgment, tests and result judgment are completed within two minutes, and the S9-P is suitable for targeted specific detection of pullorum disease and salmonella gallinarum infection in chicken flocks and has a good application prospect in on-site monitoring and diagnosis of the chicken flocks.
Owner:YANGZHOU UNIV
Latex particles for measuring particle agglutination
Latex particles for a particle agglutination assay, with which it is possible to carry out high-sensitivity assay while highly suppressing a non-specific reaction are disclosed. The latex particles being obtained by emulsion polymerization with a polymerizable monomer having a phenyl group, and a polymerizable monomer having a phenyl group and a sulfonate in an aqueous medium including a nonionic surfactant at a concentration of 0.005 to 0.02 wt % based on the aqueous medium, the latex particles having an average particle size of 0.005 to 1.0 μm.
Owner:SEKISUI MEDICAL CO LTD
Blood serum or blood plasma diluting solution applied to detection method of trace gelatin particle agglutinated HIV-1 (Human Immunodeficiency Virus-1) recent infection
InactiveCN102608312ARealize independent productionRespond effectivelyMaterial analysisMedicineBovine serum albumin
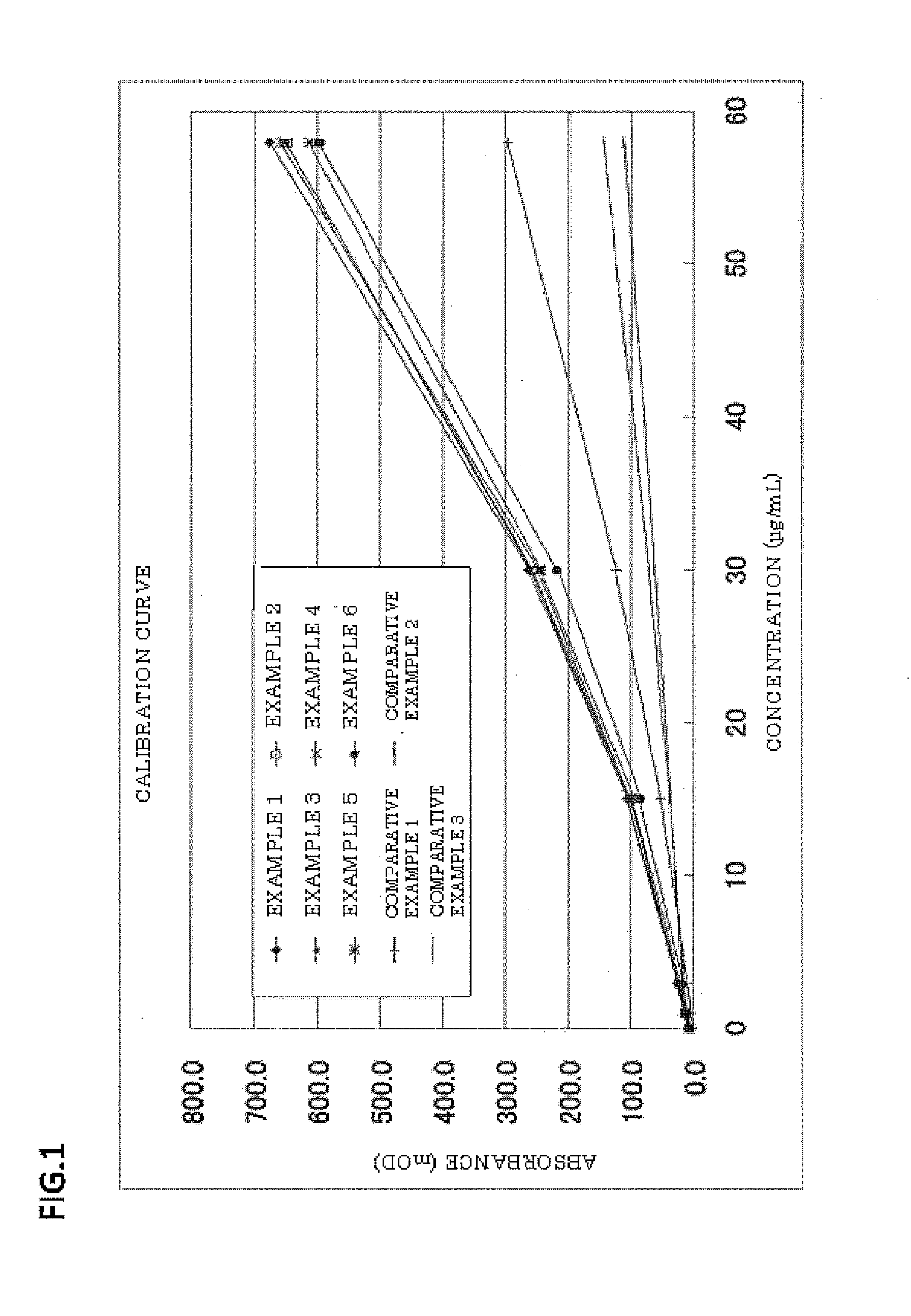
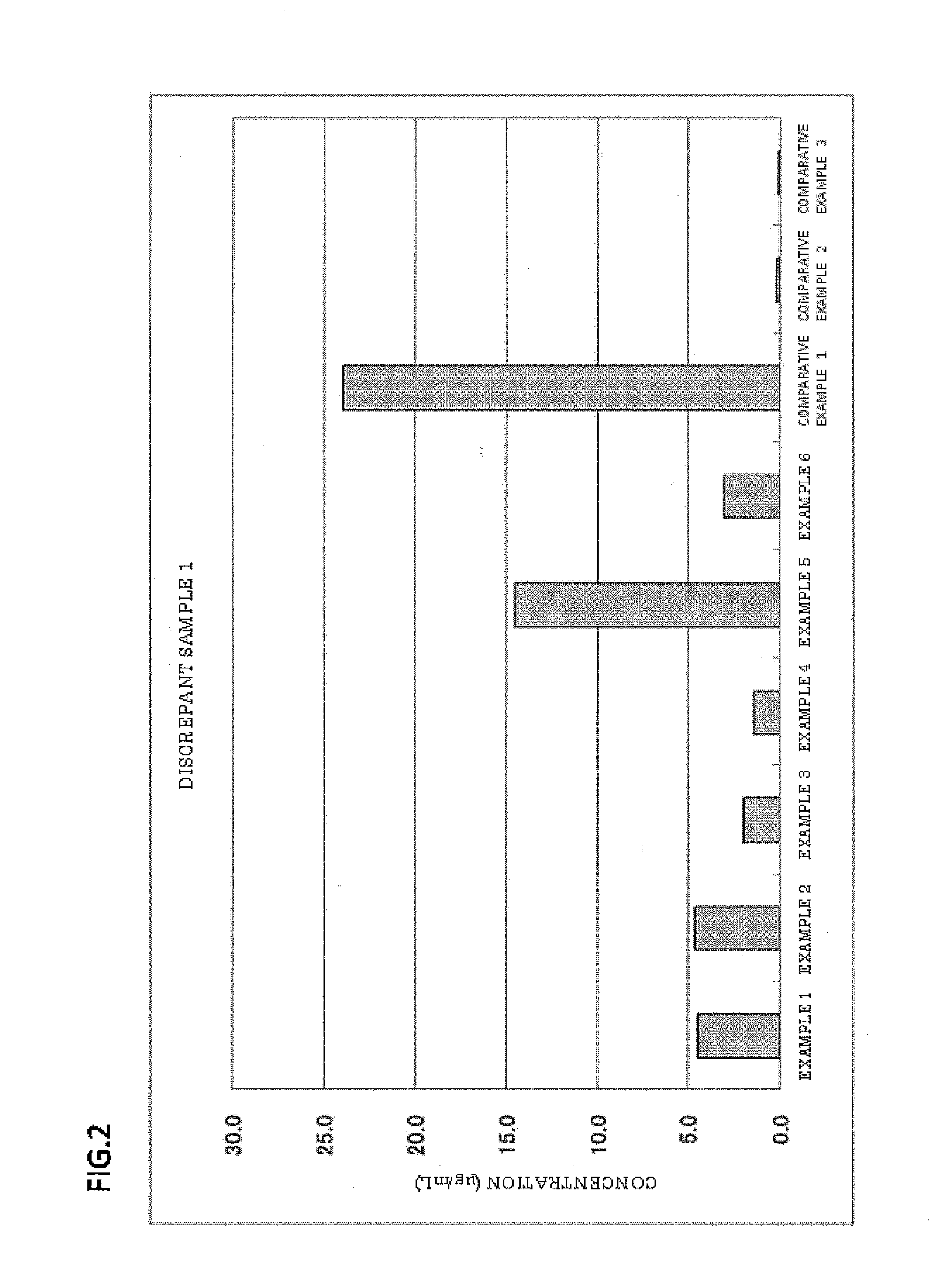
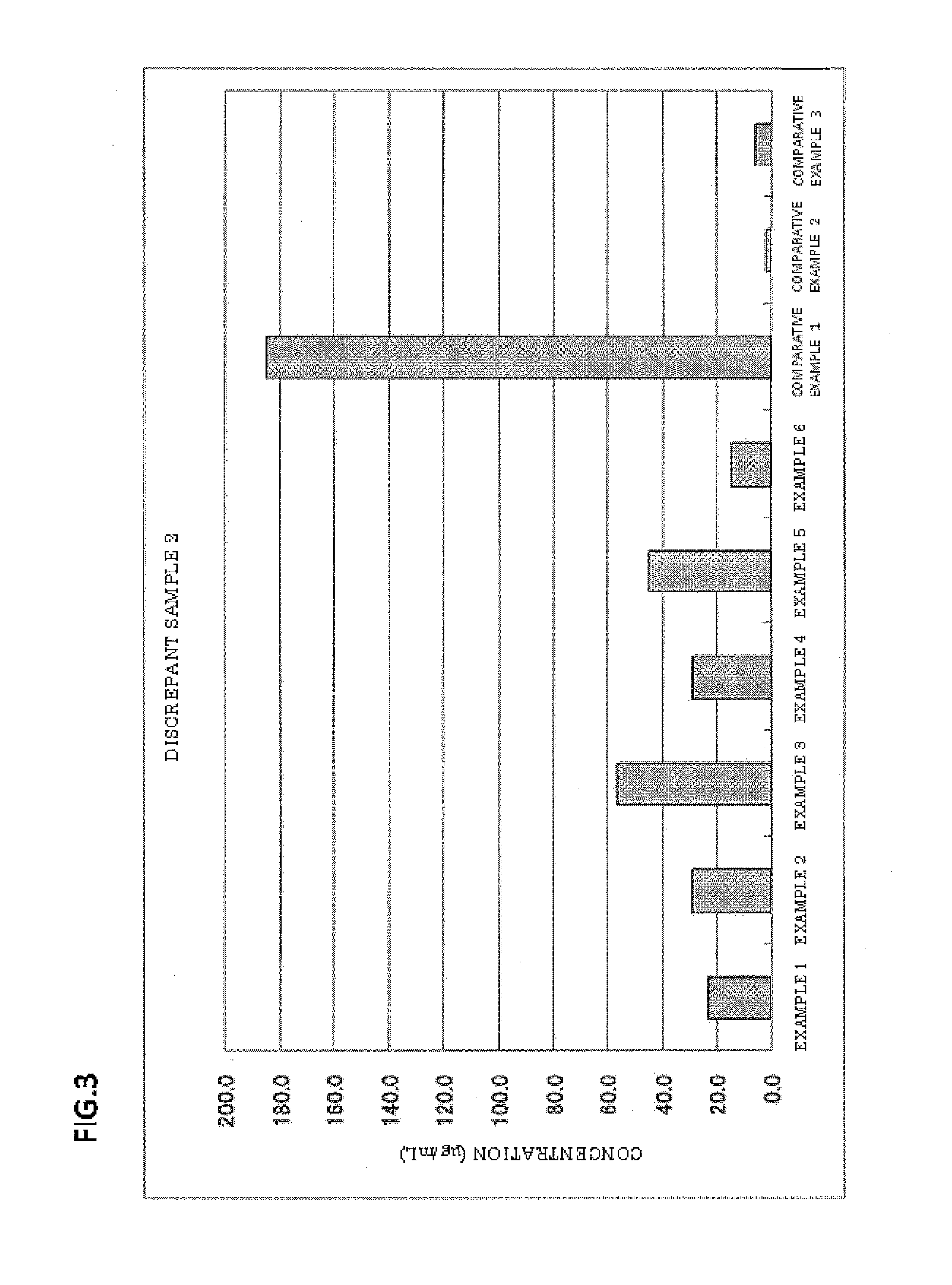
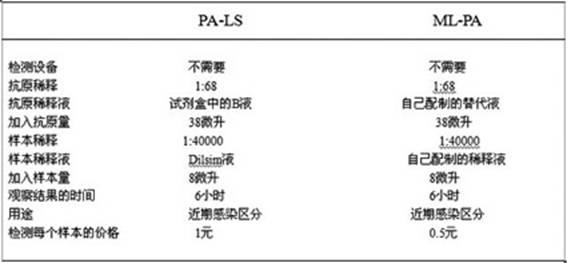
The invention provides a blood serum or blood plasma sample diluting solution applied to a method for detecting trace gelatin particle agglutinated HIV-1 (Human Immunodeficiency Virus-1) recent infection, belonging to the field of a biological technology. The invention particularly provides a reagent applied to detecting the HIV-1 recent infection. The diluting solution is prepared from a buffering solution, TritonX100 with the concentration of 30%, NaN3 and bovine serum albumin by mixing according to a ratio. The diluting solution disclosed by the invention can completely replace a DilsimTM11 diluting solution and can be used for a detection method of the trace gelatin particle agglutinated HIV-1 recent infection.
Owner:李洪
Use of superhydrophobic surfaces for liquid agglutination assays
ActiveUS20120264113A1Wide detection rangeFast aggregationBioreactor/fermenter combinationsBiological substance pretreatmentsThermal energyAnalyte
This invention relates to the use of thermodynamically incompatible surfaces in agglutination assays for the express purpose of using the sample as a key component of the detection instrument. Specifically, the invention relates to formation of a lense and a virtual container for rapid mixing via thermal energy by a sample liquid disposed on a superhydrophobic surfaces, and a subsequent specific analyte or overall protein concentration assay using particles agglutination for use in the industrial, environmental, and clinical laboratory test fields.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
Insoluble carrier particle nephelometric immunoassay reagent
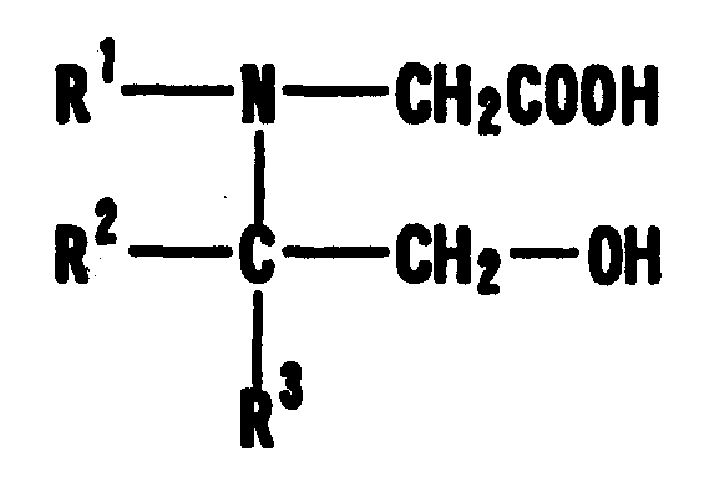
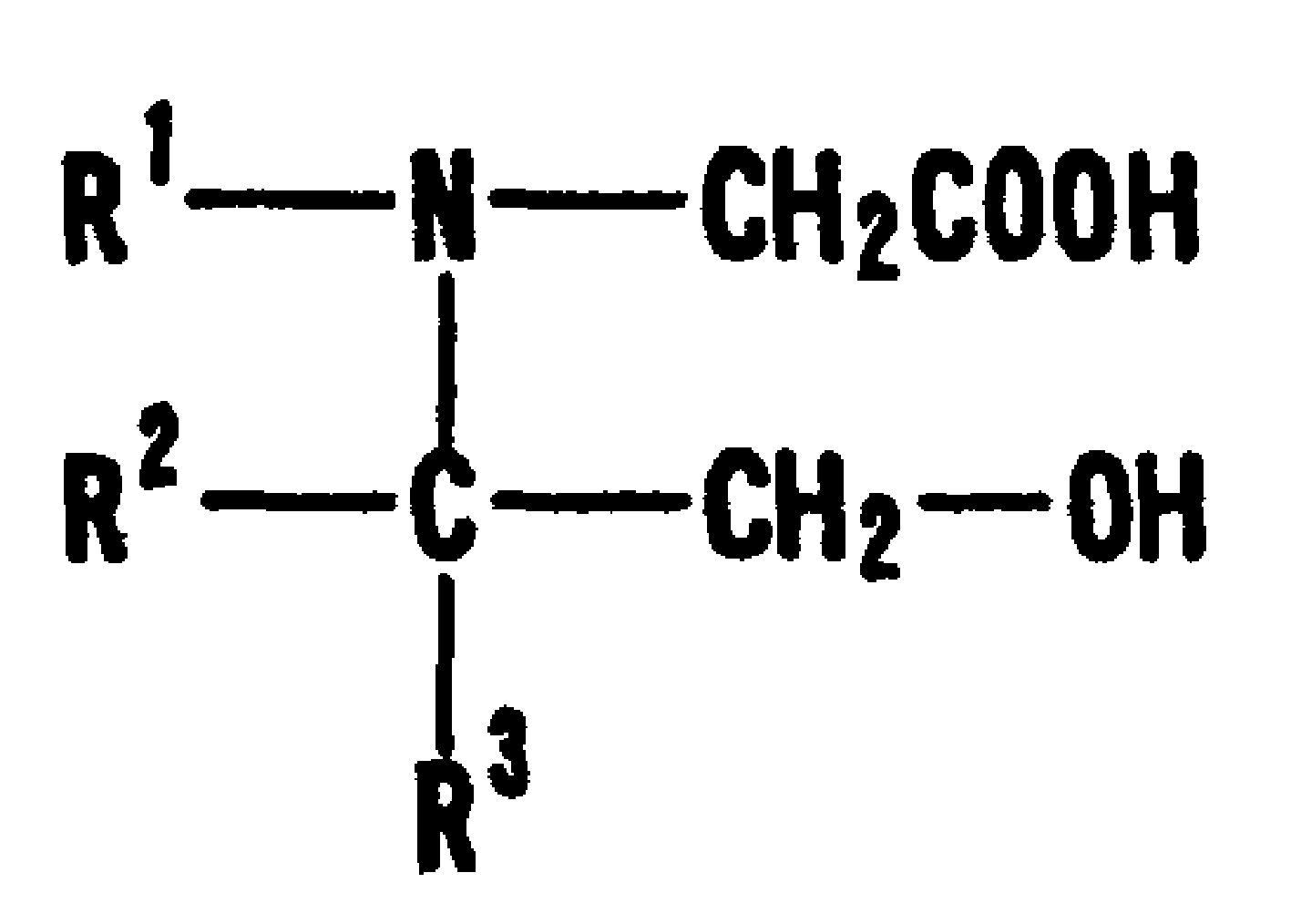
Turbidimetric immunoassay of insoluble carrier particles that can inhibit the agglutination reaction of insoluble carrier particles such as latex by inhibiting the effect of plasma components that affect the measured value, stabilize the agglutination reaction, stabilize the absorbance of the reaction solution, and obtain accurate measurement results A reagent for measurement, a kit for insoluble carrier particle turbidimetric immunoassay, and a method for insoluble carrier particle turbidimetric immunoassay using the reagent or kit. In the presence or absence of a buffer containing a compound containing the following groups in the molecule, such as broad bean pyrimidine glucoside, tricoflavone, etc., the insoluble carrier particles are loaded with antibodies or antigens, and then, in the presence of the above buffer , the insoluble carrier particle suspension sensitive to the antibody or antigen is in contact with the test body, and an immunoagglutination reaction occurs, and the concentration produced is measured through the agglutination reaction of the insoluble carrier particles, and the antigen or antibody in the test body is quantified. (In the formula, R1, R2, and R3 can be the same or different, and represent a hydrogen atom, a hydroxyalkyl group, etc.)
Owner:KYOWA MEDEX CO LTD +1
Method and device for ultrasound assisted particle agglutination assay
InactiveUS7989177B2Accurate assessmentSensitive detectionBioreactor/fermenter combinationsBiological substance pretreatmentsAgglutinationMicroparticle
Ultrasound-assisted particle agglutination assay methods and apparatuses are described based on first providing a standing wave ultrasound field at a resonance frequency of a test liquid in a resonator cell containing microparticles covered with a binding agent with high affinity to an analyte sought to be detected by the assay test. Formation of the specifically-bound and nonspecifically-bound aggregates of these microparticles is then followed by effective stirring of the liquid with swept-frequency sonication causing disintegration of nonspecifically-bound aggregates and leaving specifically-bound aggregates in place for further detection and measurement. The methods and devices of the invention allow significant improvement in the sensitivity and specificity of agglutination tests and are advantageously applicable to detecting various proteins, DNA, RNA and other biologically active substances. Specific examples are provided.
Owner:ALLIED INNOVATIVE SYST
Use of superhydrophobic surfaces for liquid agglutination assays
ActiveUS9995688B2Wide detection rangeFast aggregationBioreactor/fermenter combinationsBiological substance pretreatmentsThermal energyAnalyte
This invention relates to the use of thermodynamically incompatible surfaces in agglutination assays for the express purpose of using the sample as a key component of the detection instrument. Specifically, the invention relates to formation of a lense and a virtual container for rapid mixing via thermal energy by a sample liquid disposed on a superhydrophobic surfaces, and a subsequent specific analyte or overall protein concentration assay using particles agglutination for use in the industrial, environmental, and clinical laboratory test fields.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
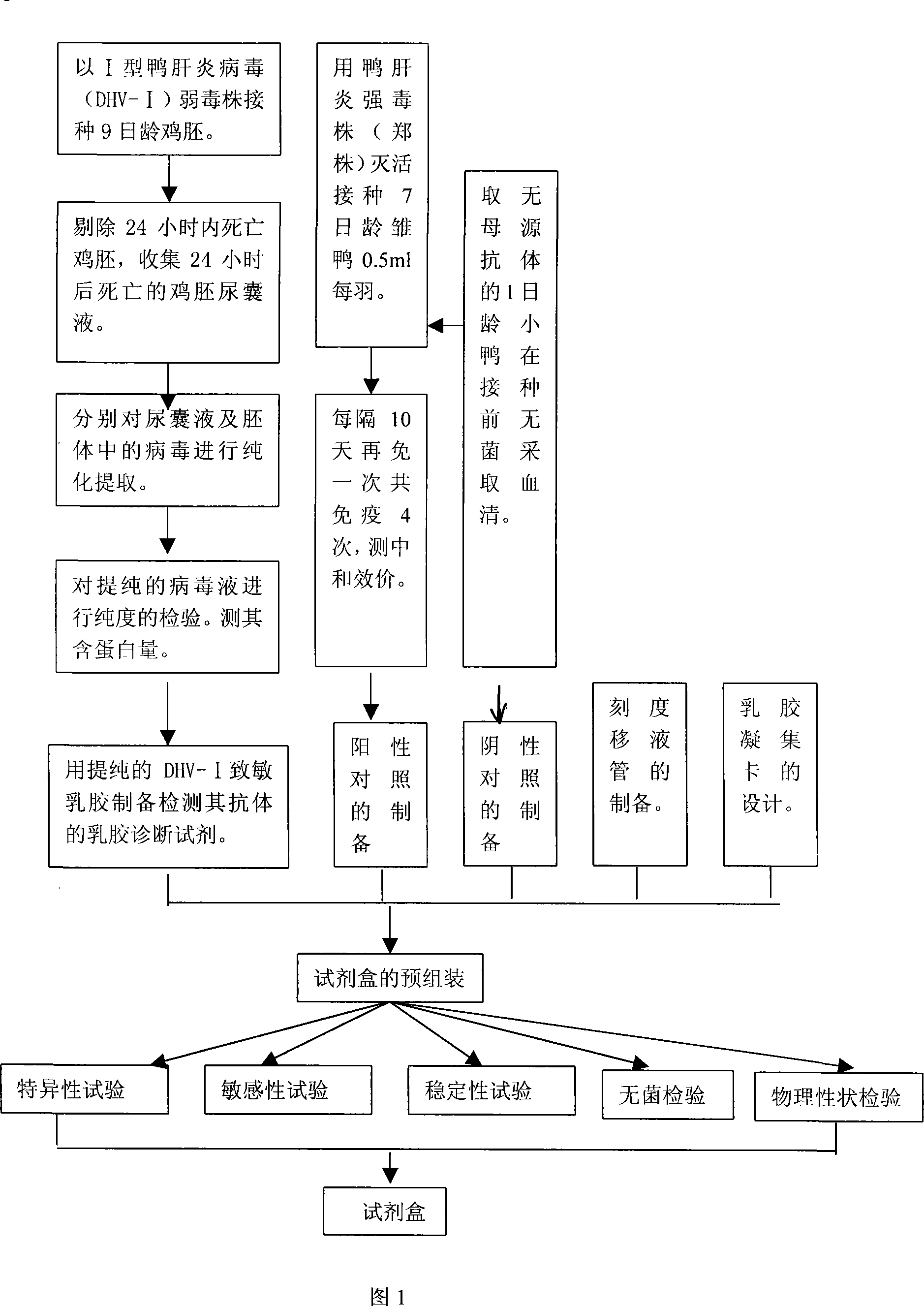
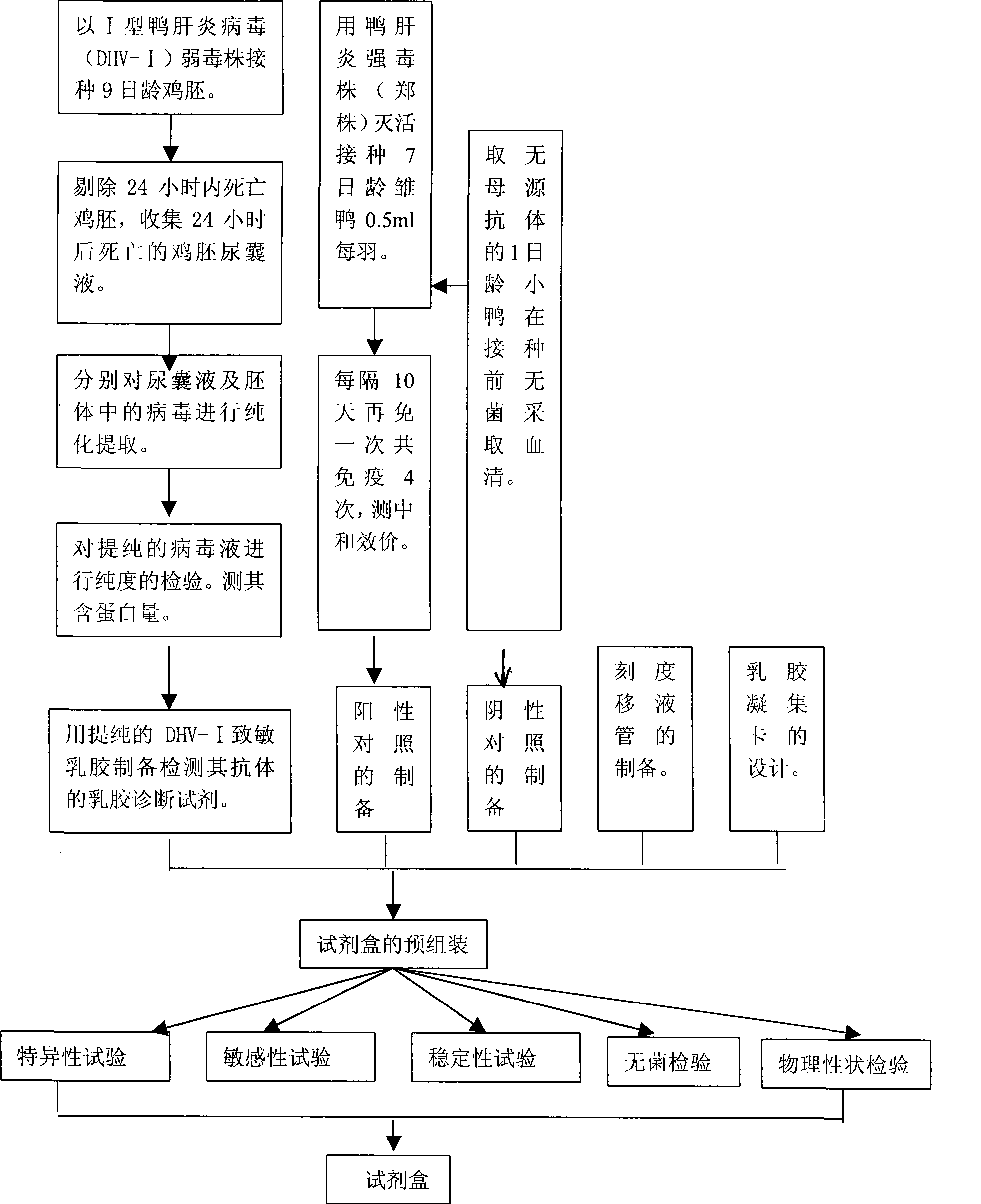
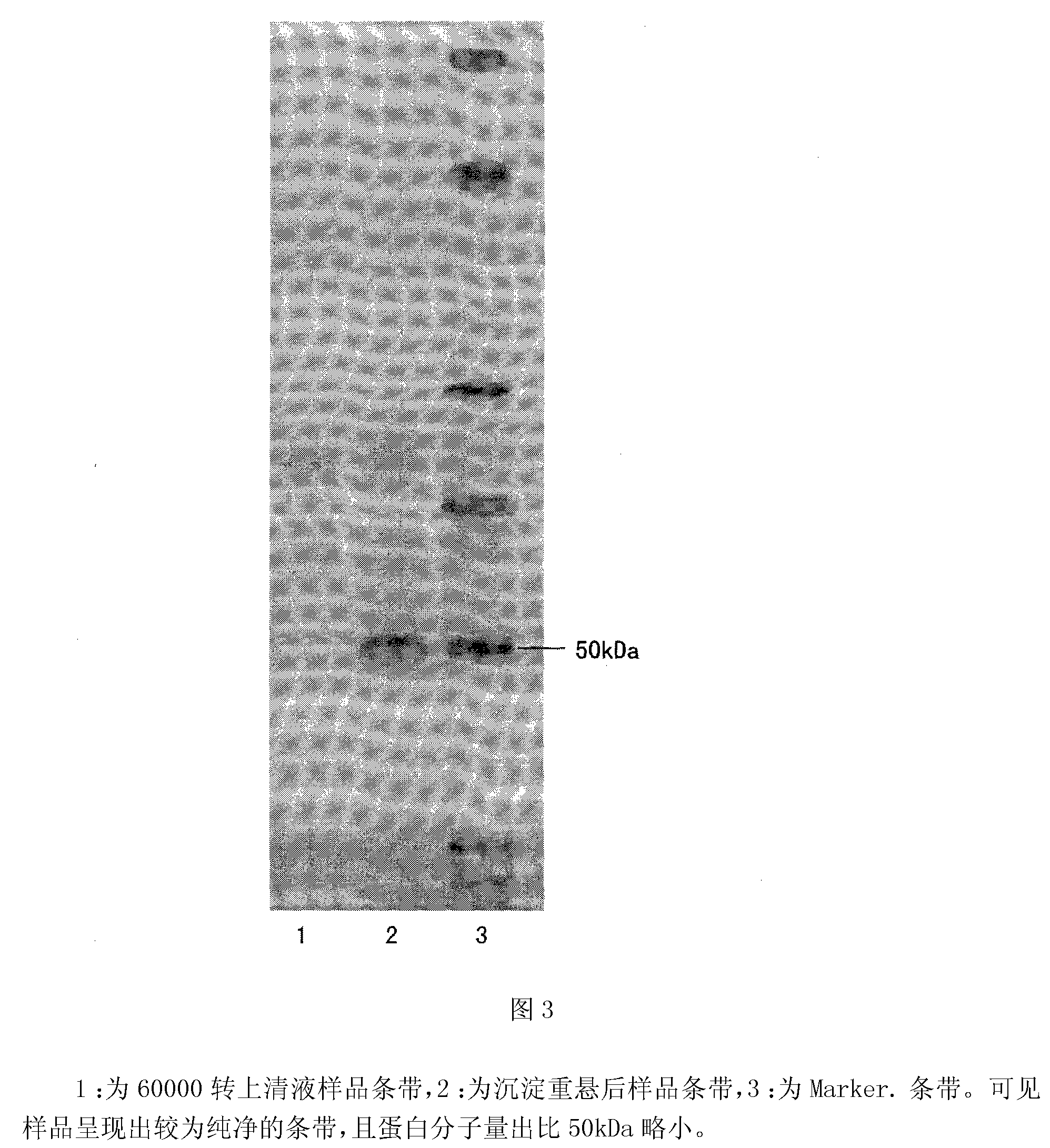
Gelatin particle-agglutination detection reagent kit suitable for 1type duck hepatitis virus antibody and its uses
InactiveCN101101297AStrong specificityHigh sensitivityMaterial analysisDuck hepatitis A virusAgglutination
The invention belongs to the bird immunology technology area, it concretely relates to a kit of acceptable for the one type duck hepatitis virus antibody emulsion agglutination and the application of testing one type duck hepatitis virus antibody emulsion agglutination. The kit of this invention contains box body and core reagent in the box body, positive check sample, negative check sample, sample cell, scale sample sucker and the carrier of observed result. The characteristic is that core reagent is latex antigen from sublimating e duck hepatitis virus AV2111 and inactivating one type duck hepatitis virus sensitization polystyrene latex, the positive check sample is one type duck hepatitis virus antiserum, the negative check sample is the green duck of a day old of no one type duck hepatitis mother source antibody. The carrier is the paper characteristic latex agglutination card of having hollowness. The invention also discloses the preparation method and application of this kit. The kit has the significance merit of high specificity, high sensitivity, handy operation, and quickly diagnose.
Owner:HUAZHONG AGRI UNIV
Aqueous pharmaceutical suspensions containing rebamipide and manufacturing process thereof
InactiveUS20100029714A1Promote recoverySimple processBiocideSenses disorderPolyvinyl alcoholSodium salt
The invention provides a rebamipide-containing aqueous pharmaceutical suspension which can be prepared by a simple process and keep the dispersed fine-particle state of rebamipide stable without having the fine particle agglutinated. The rebamipide-containing aqueous pharmaceutical suspension of the invention is prepared by mixing polyvinyl alcohol and additionally a sodium salt compound with rebamipide.
Owner:OTSUKA PHARM CO LTD
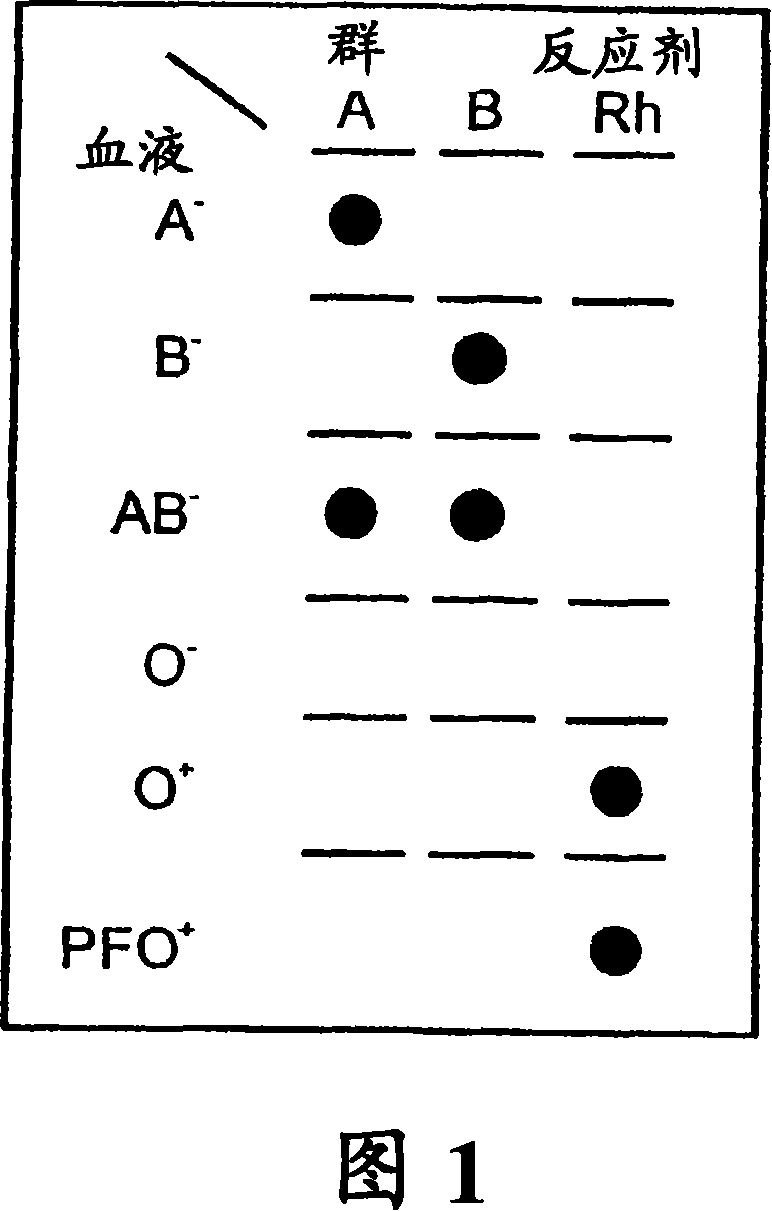
Blood type method system and device
InactiveCN101120249ANo centrifugation stepEasy to useBioreactor/fermenter combinationsBiological substance pretreatmentsMedicineGroup A - blood
A method for the detection and / or visualization of particle agglutination in a particle suspension is provided, comprising placing a volume of the particle suspension and a volume of a solution or suspension containing an agglutinating agent at substantially the same selected location on a surface of a filter constructed so as to permit passage of individual unagglutinated particles in a direction perpendicular to the surface. A wash solution at optionally placed at substantially the same location as that of said agglutinating agent, and the surface is observed for the presence of particles. There is also provided a method for detection of agglutination reactions, such as used in blood grouping, reverse grouping and cross-matching. A device and a kit based on the invention is also claimed, which facilitates blood grouping, reverse grouping and matching in non-laboratory environment without the need for laboratory instruments.
Owner:INVERNESS SWITZERLAND GMBH
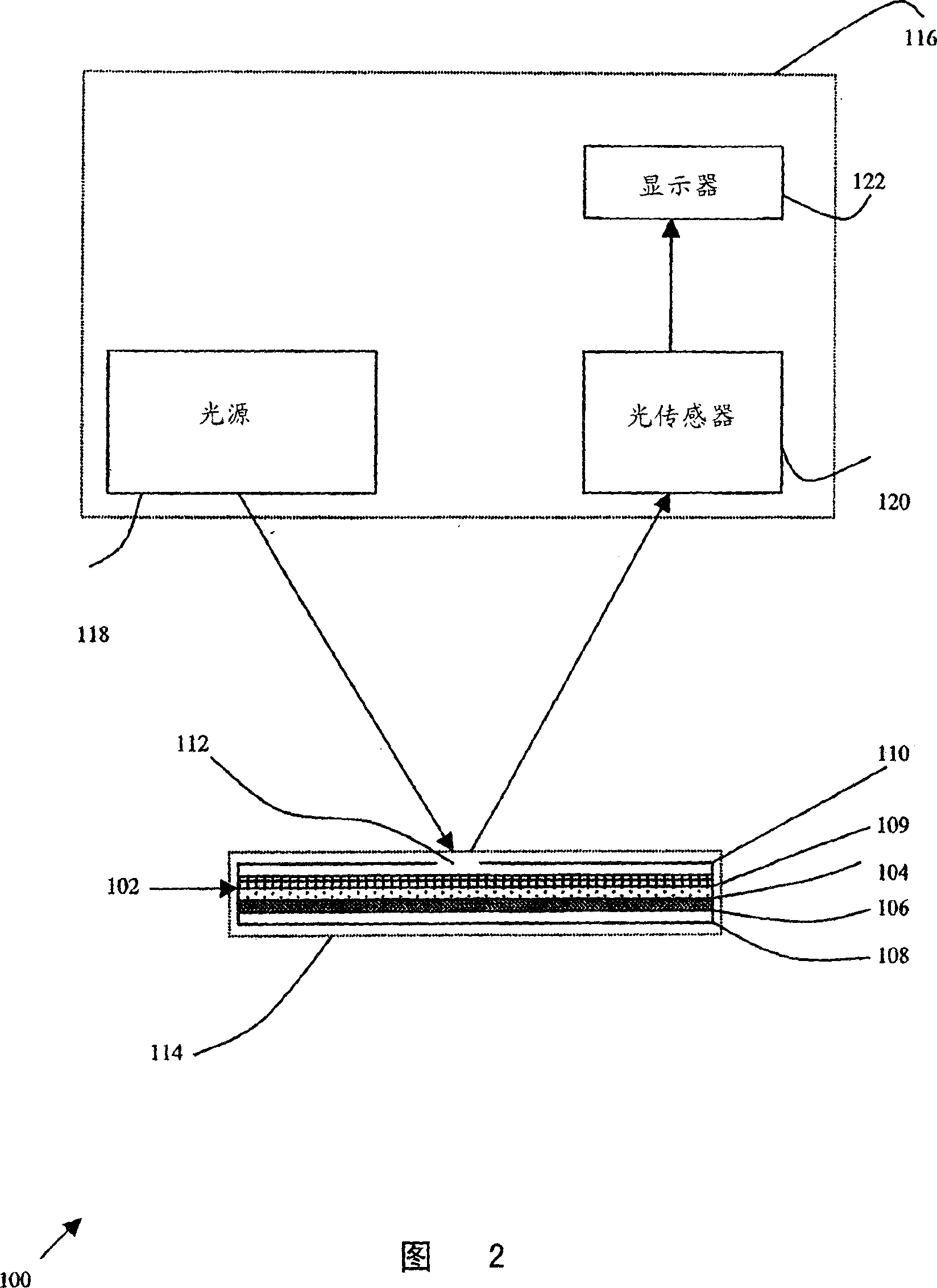
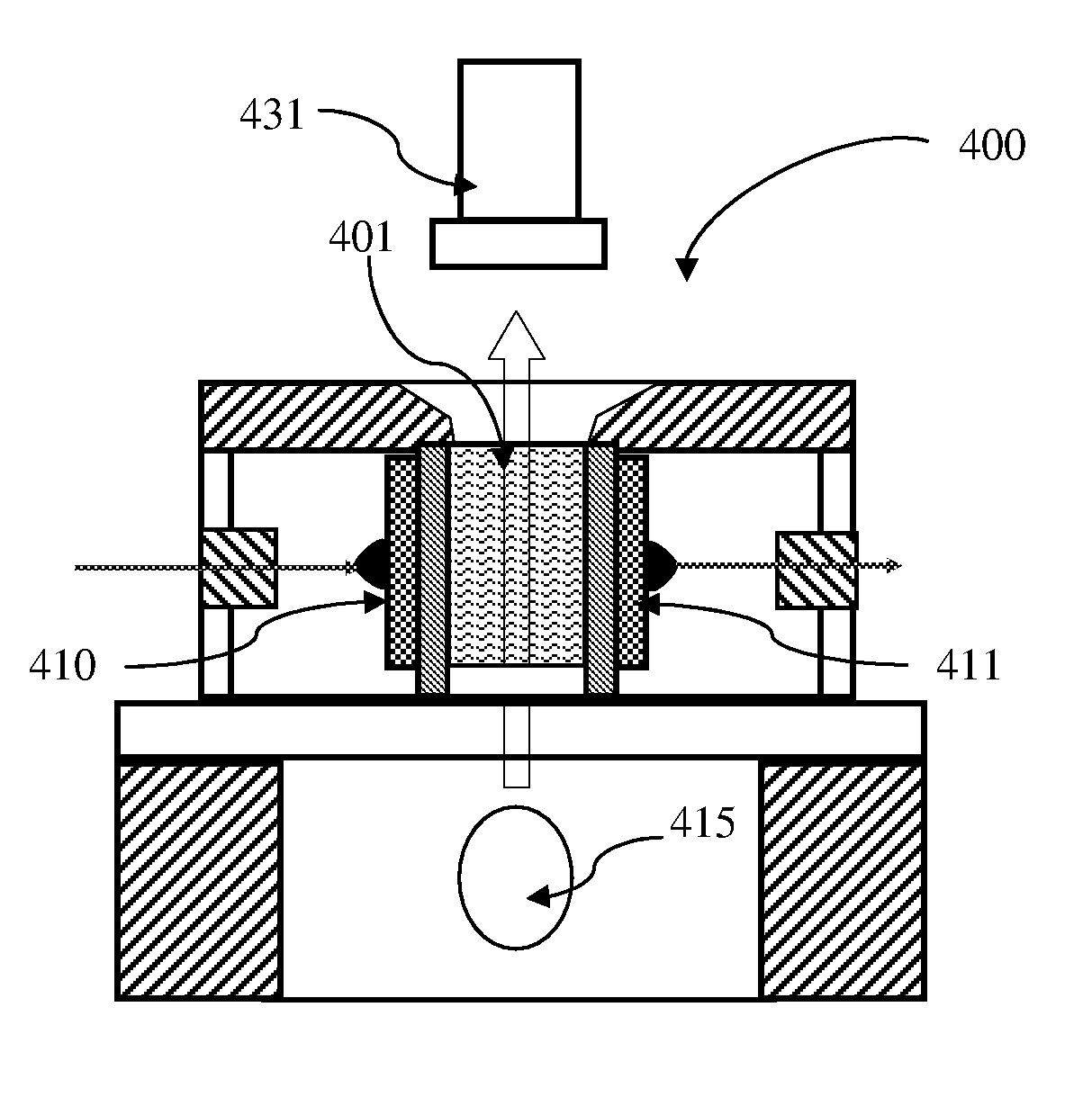
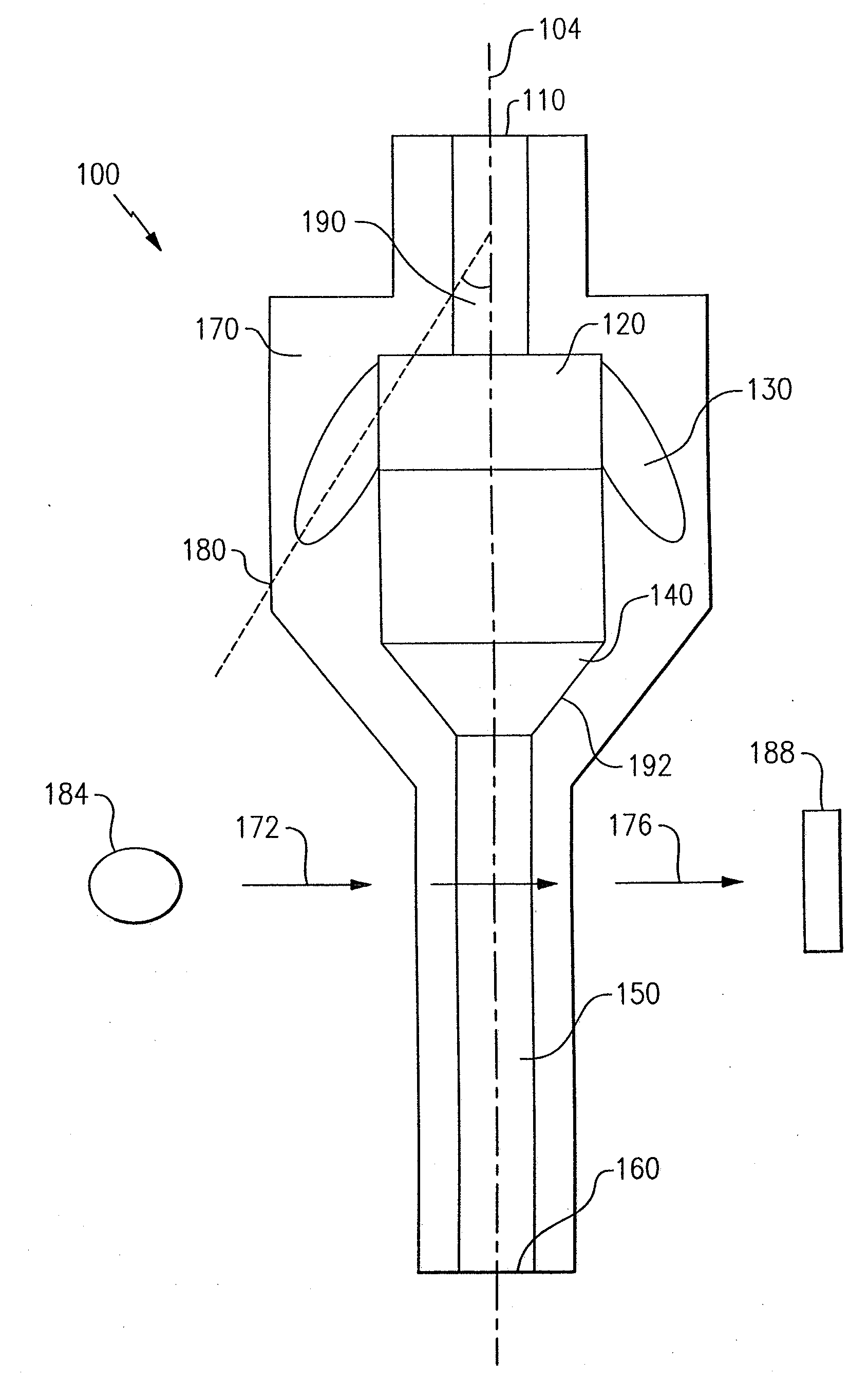
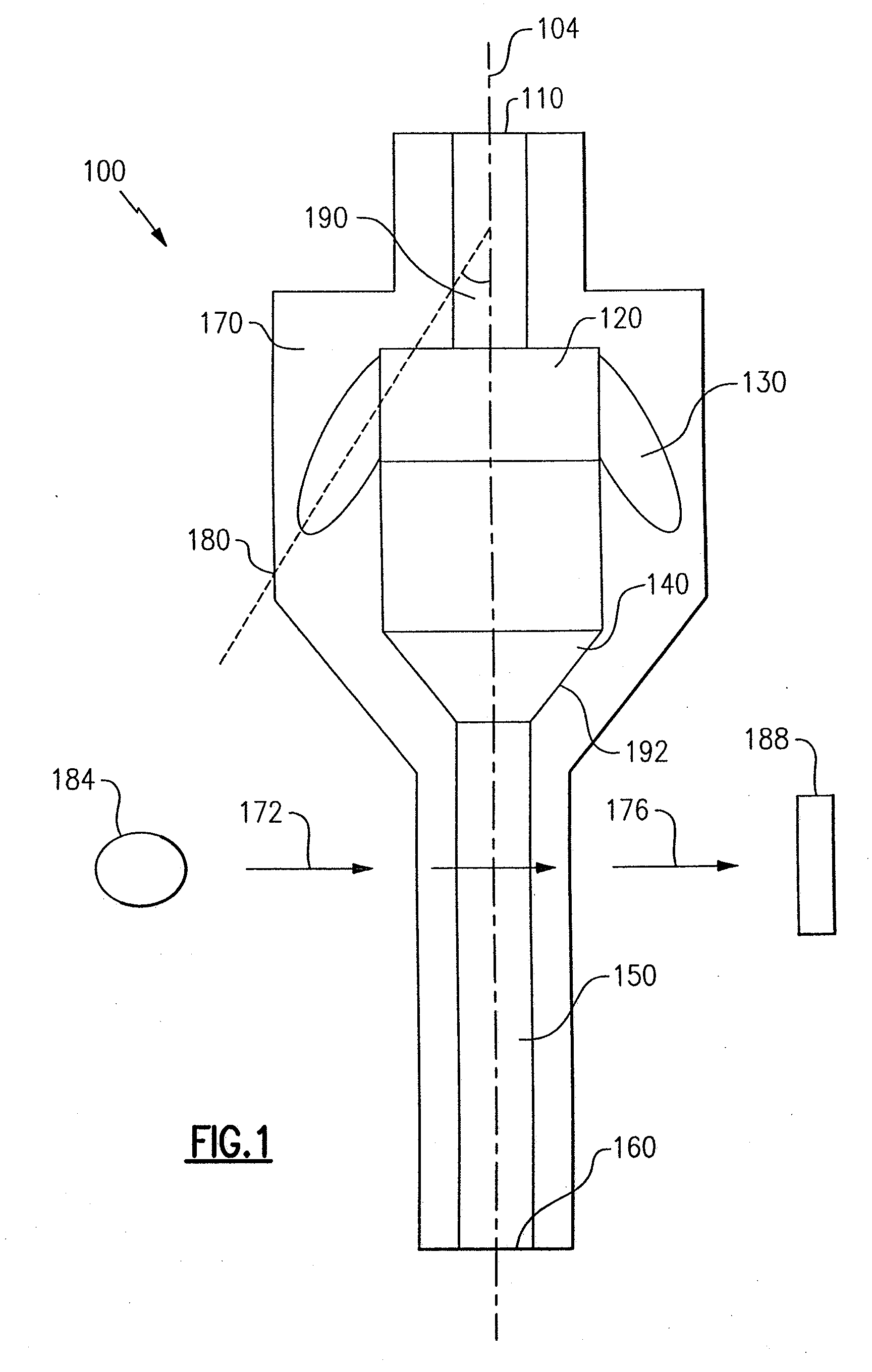
Particle agglutination in a tip
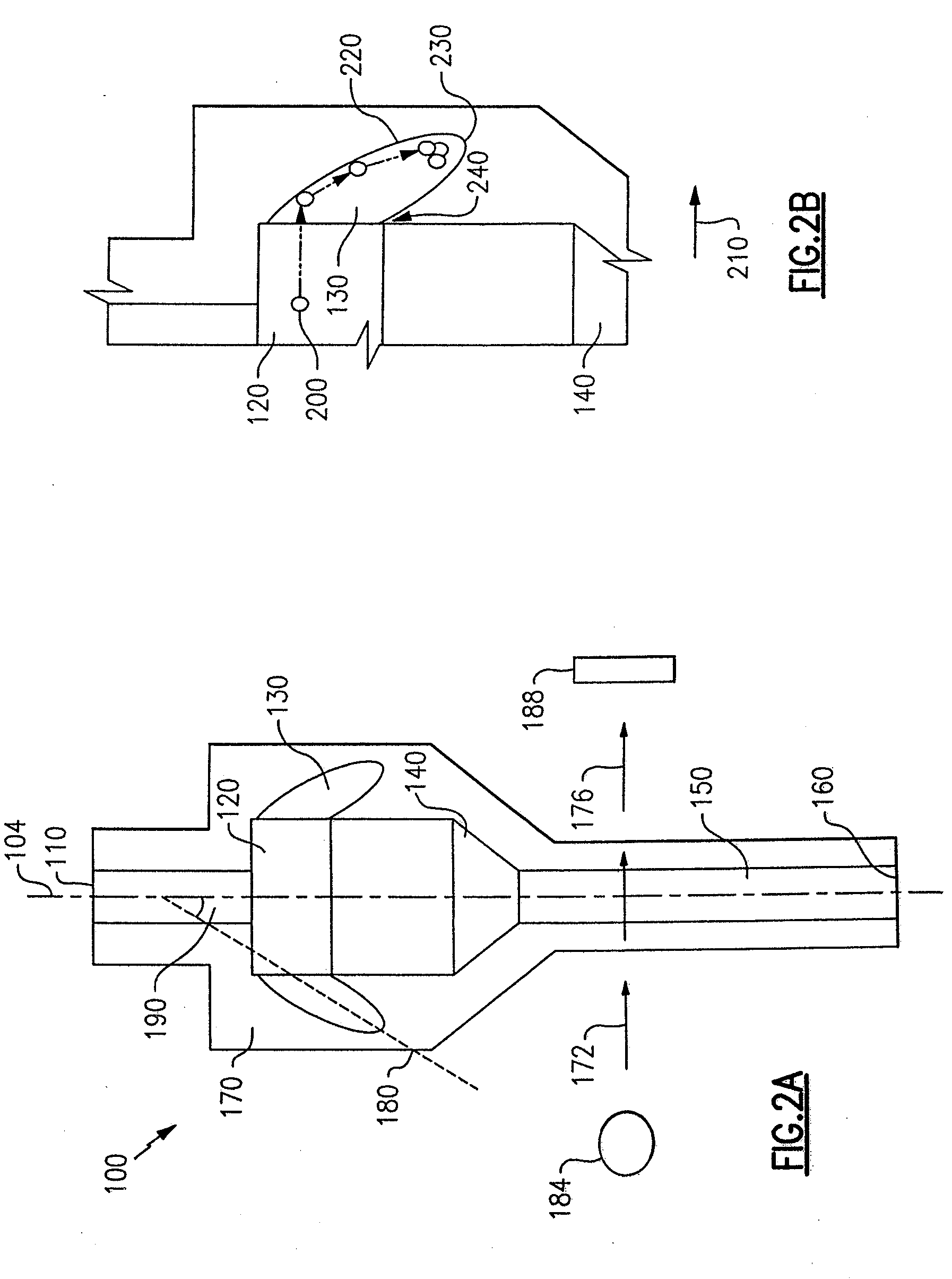
ActiveUS7850917B2Cost-effectiveMinimal interventionBioreactor/fermenter combinationsBiological substance pretreatmentsCentrifugationAgglutination
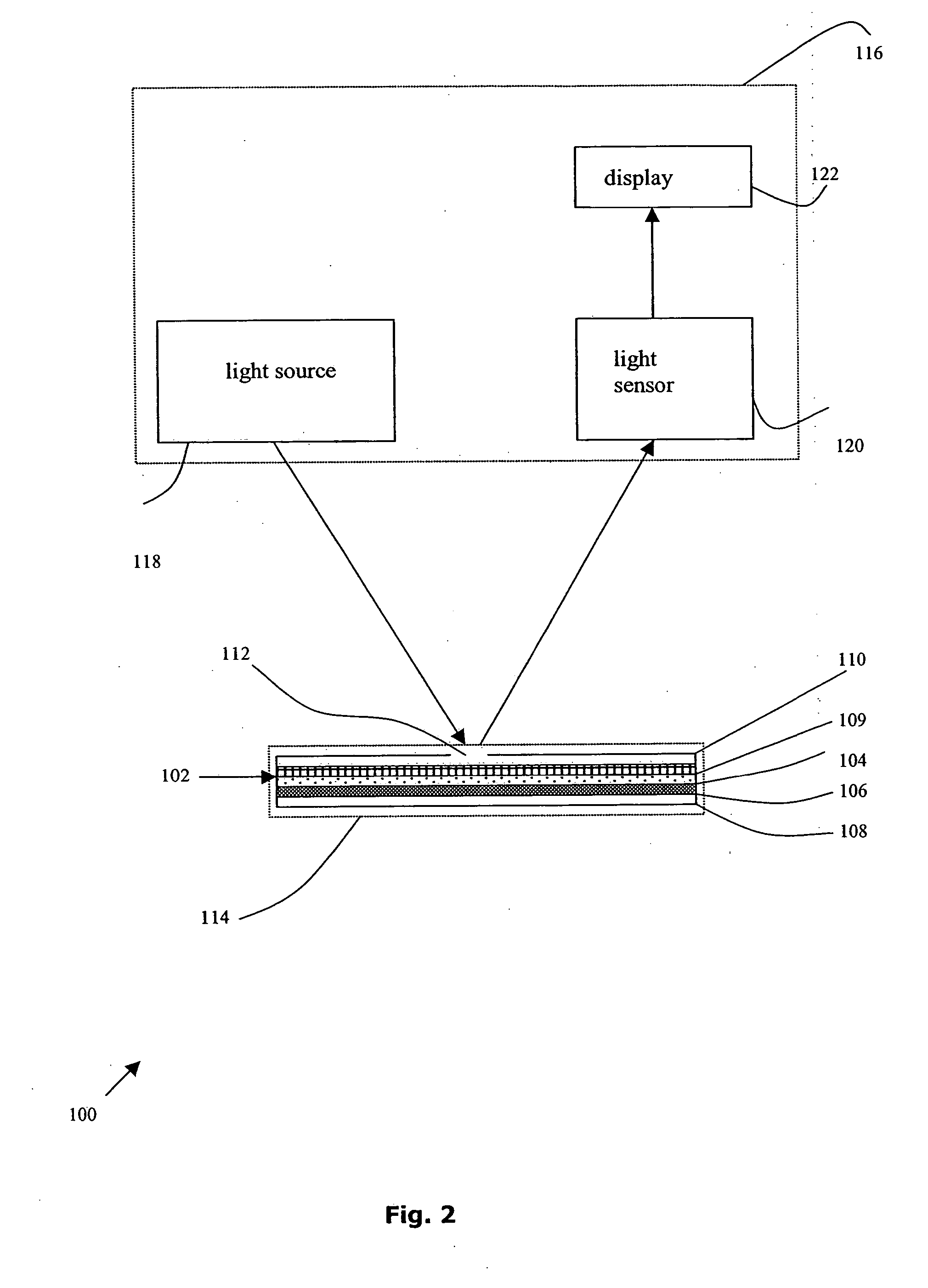
An apparatus and a related method for performing particle agglutination reactions in a single, disposable probe tip are disclosed. The probe tip includes a sample cavity for sample acquisition, at least one flanking cavity for the capture of particles by centrifugation or other means, a transition zone for the mixing of the sample with reagents for agglutination and a detection zone for the optical detection of particle agglutination. A mechanism may be attached to the probe tip for the controlled movement of fluids through the internal volume of the probe tip. The probe tip is particularly useful for the automation of high-throughput agglutination-type assays.
Owner:ORTHO-CLINICAL DIAGNOSTICS
Particle agglutination-evaluating container
ActiveUS20080213131A1Easily and accuratelyEasy and accurate evaluationBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenEngineering
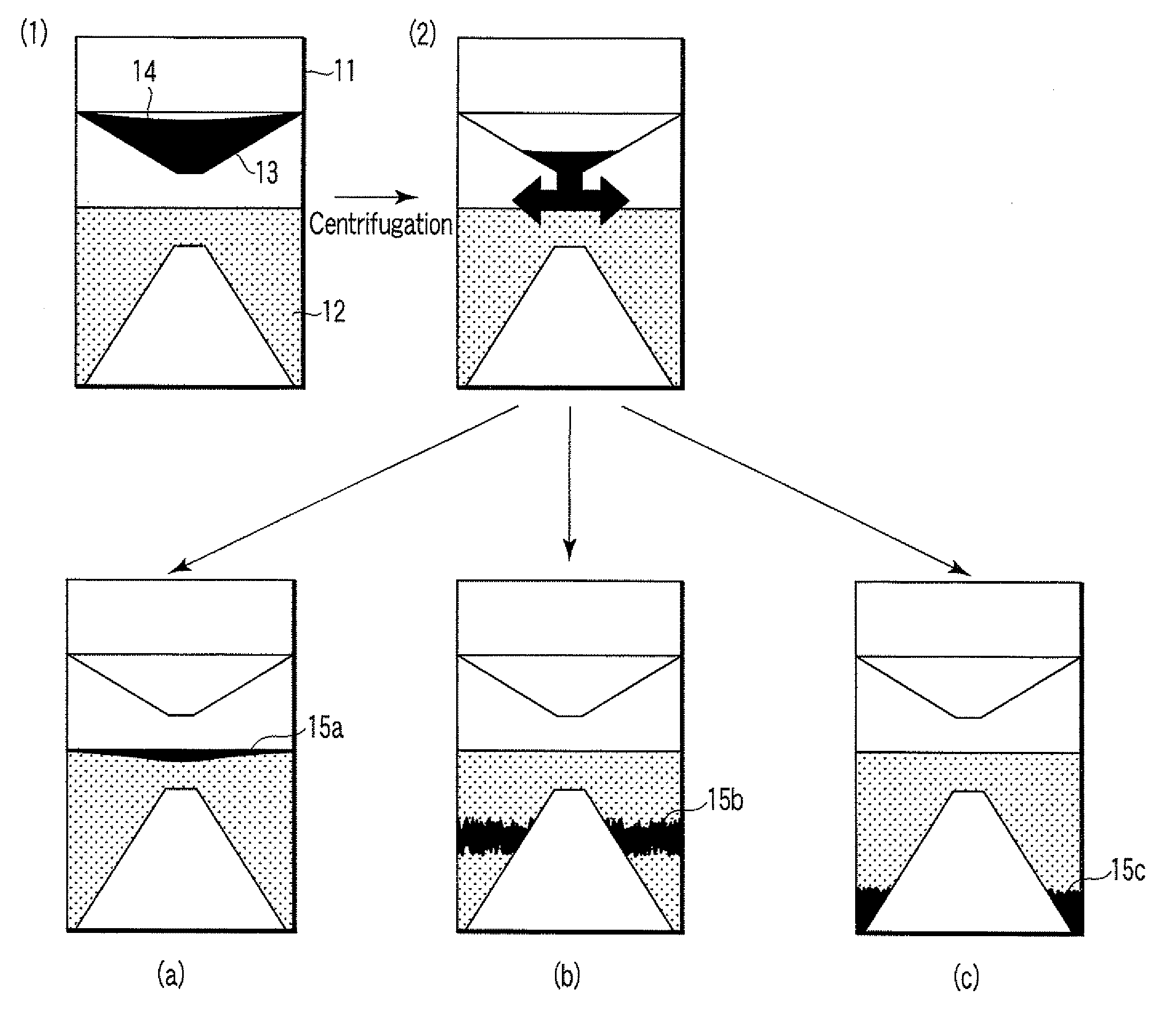
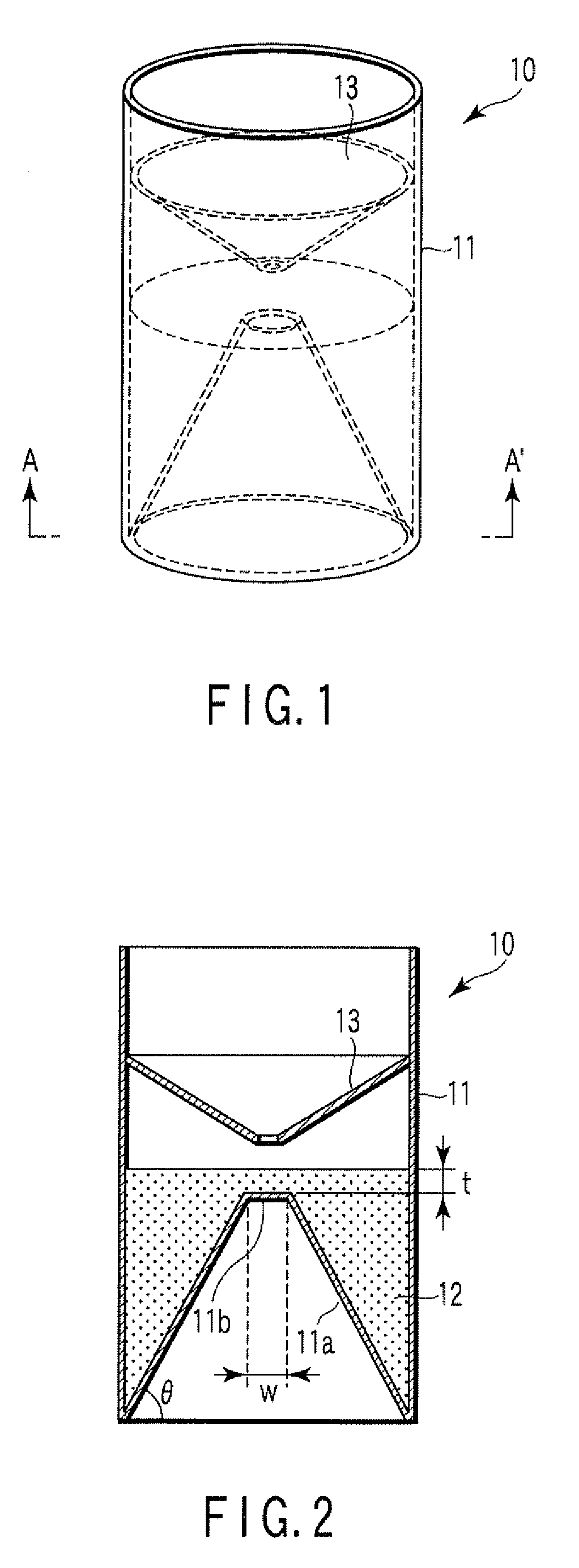
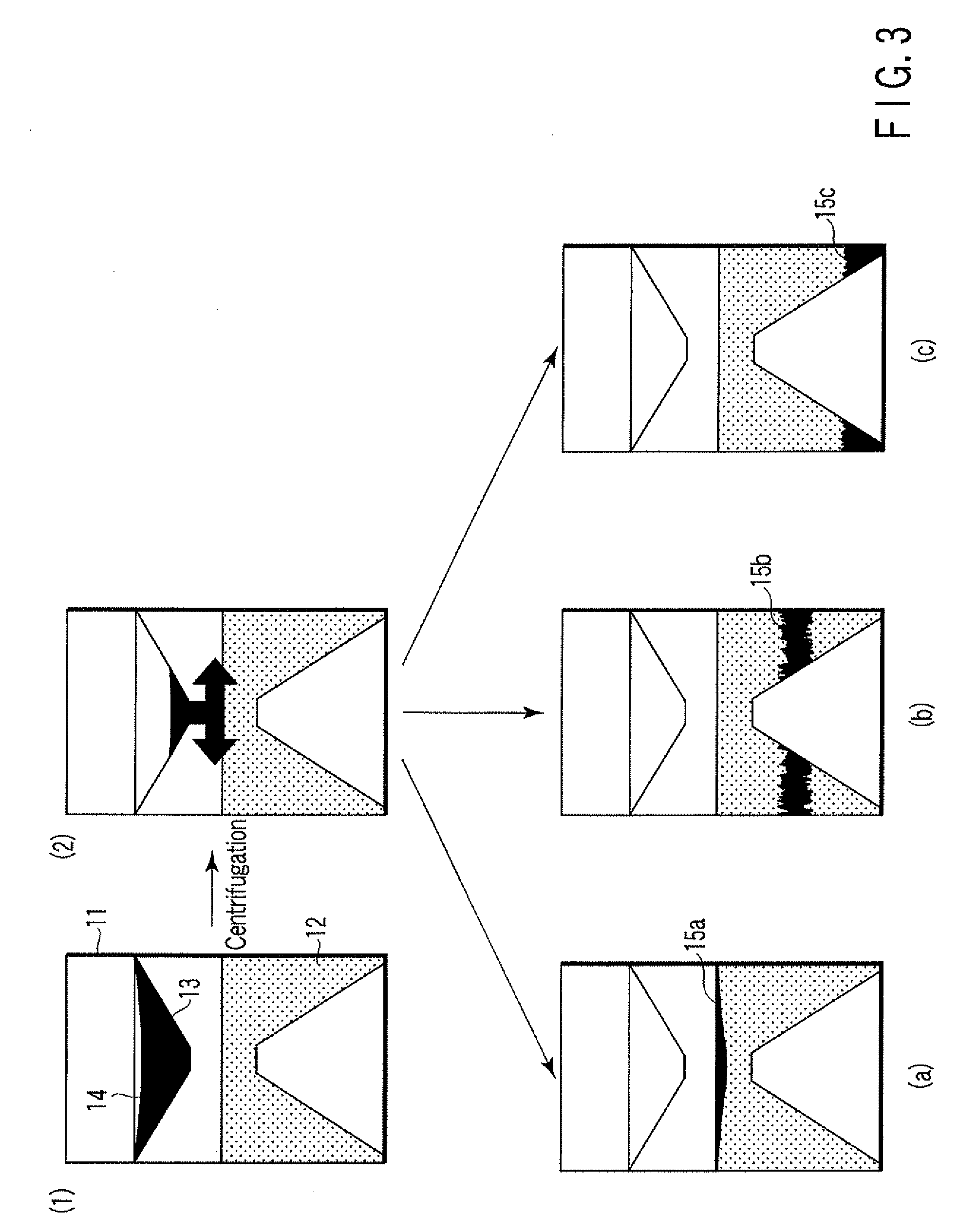
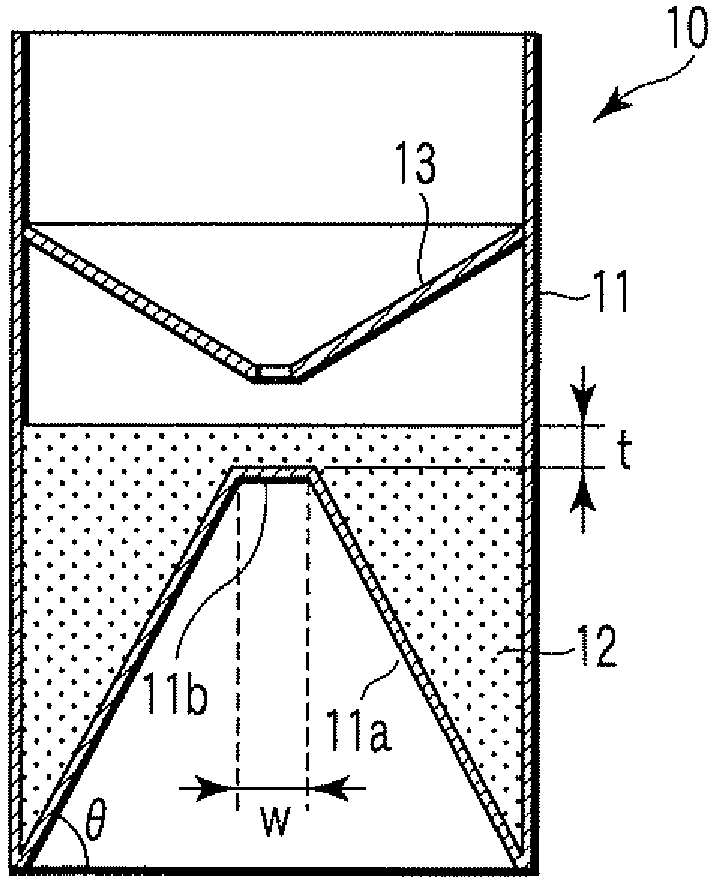
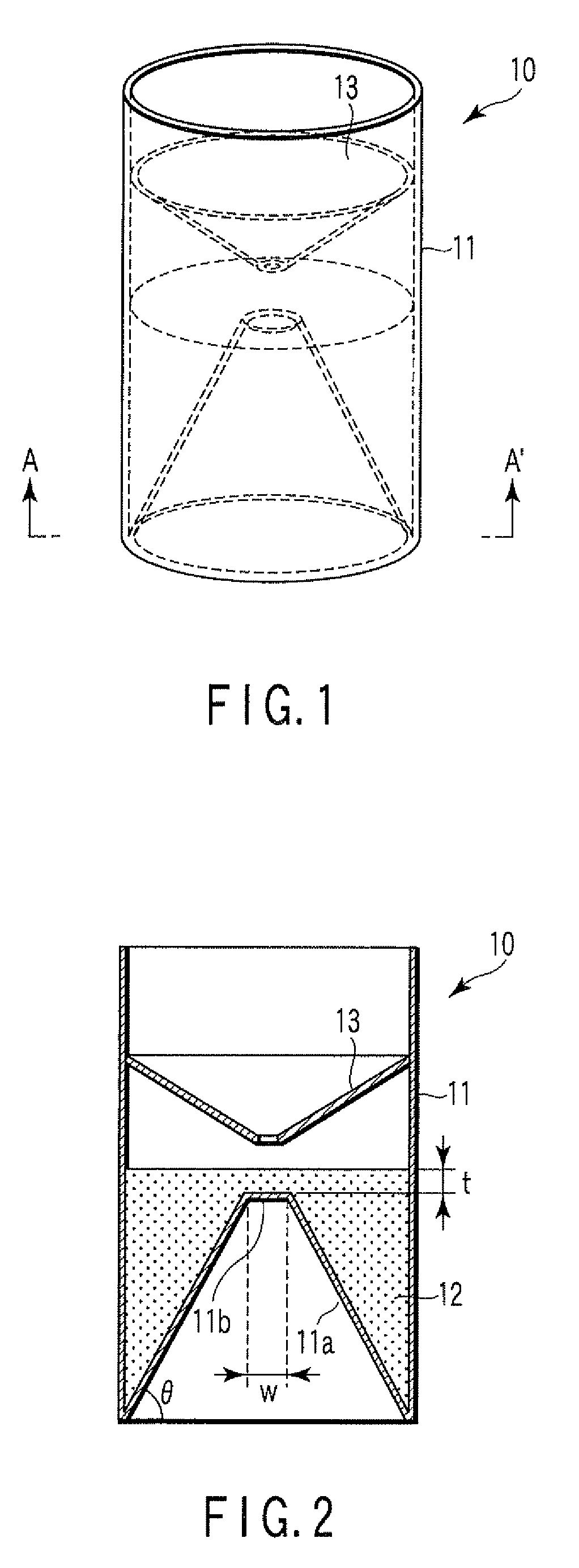
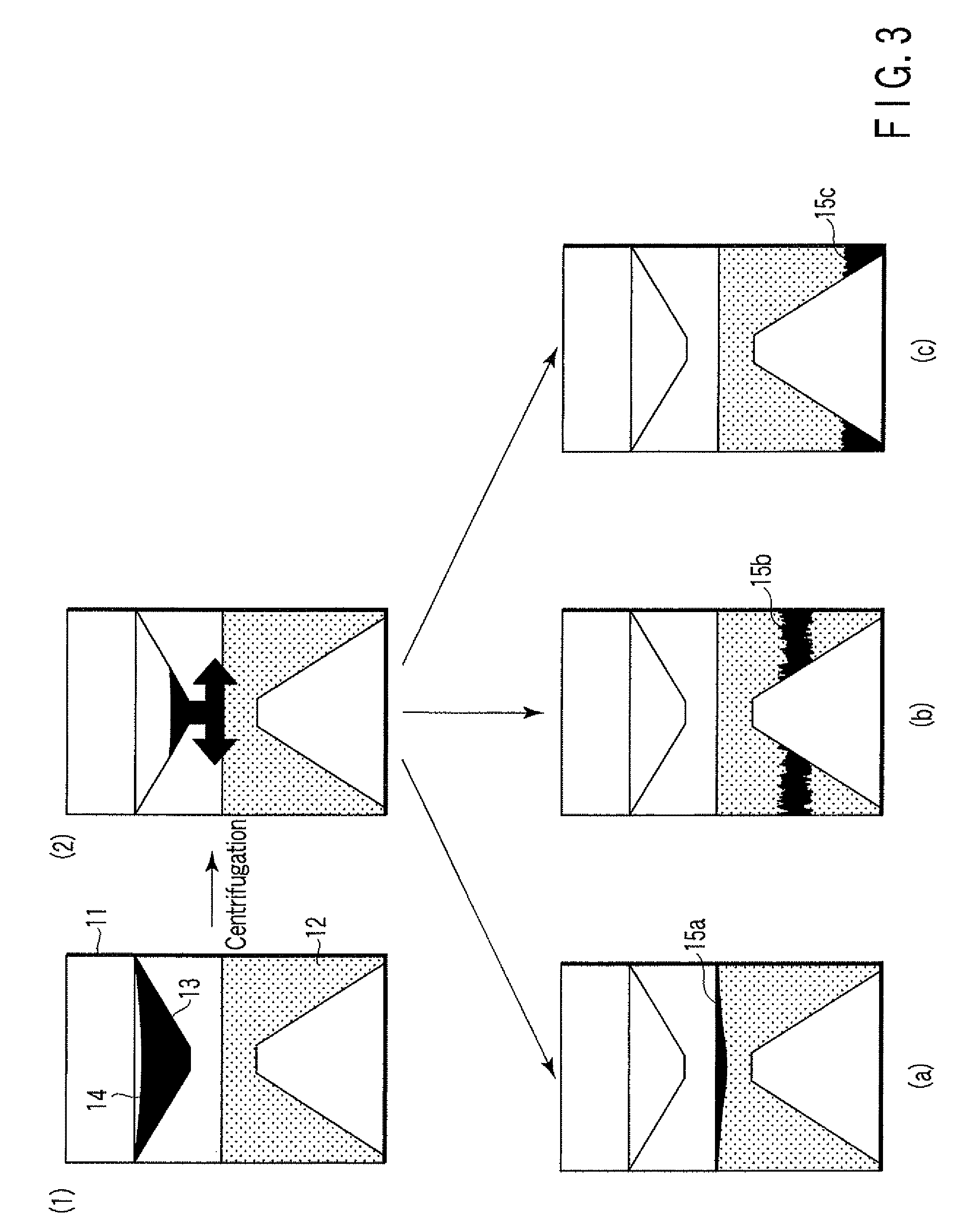
A particle agglutination-evaluating container for immunological analysis, based on an agglutinate formed in an agglutination reaction between an antibody- or antigen-containing sample and agglutination particles, includes a transparent container body having a bottom face including a sloping bottom face and a raised bottom face extended in a horizontal direction at the top of the sloping bottom face to form an obtuse angle with the sloping bottom face, and a fluidal separation layer containing insoluble particles which are filled in the container body and where the agglutination particles are separated according to the agglutination degree, wherein agglutination is evaluated by observation from the bottom of the container body, where the agglutination reaction is judged positive when the agglutination particles are observed through the raised bottom face, negative when observed at the bottom end of the sloping bottom face, and weakly positive when observed in the middle of the sloping bottom face.
Owner:BECKMAN COULTER INC
Particle agglutination-evaluating container
ActiveUS7807107B2Easy and accurate evaluationHigh-throughput analysisBioreactor/fermenter combinationsBiological substance pretreatmentsAgglutinationEngineering
Owner:BECKMAN COULTER INC
Quinidine conjugates and their use in immunoassays
InactiveUS6140530ACarbamic acid derivatives preparationOrganic compound preparationQuinidineImmunogenicity
This invention relates to quinidine derivatives and immunogenic conjugates and reporter conjugates prepared therefrom. The immunogenic conjugates and reporter conjugates are useful for eliciting antibodies and for performing immunoassays for quinidine. A particle agglutination immunoassay for quinidine is also provided.
Owner:DADE BEHRING
Regenerated particle aggregate, process for producing regenerated particle aggregate, regenerated-particle-aggregate-containing paper containing regenerated particle aggregate as internal additive, an
InactiveCN101321913AImprove effective utilizationExcellent bulkinessSludge treatmentSilicaMaterials scienceCoated paper
The present invention relates to a regeneration particle aggregate, a method thereof for making the regeneration particle aggregate, a regeneration particle aggregate integrated paper which is integrated with the regeneration particle aggregate at the inner side, and a printing used coated paper which is coated with the regeneration particle aggregate. The aim of the invention is to provide various papers which use the recyclable regeneration particle aggregate to make a contribution to environment protection. In the making process of various papers, the regeneration particle aggregate is at least contained to be used as an added filling agent, coating used paint, etc. The regeneration particle aggregate uses the voided naps discharged from the wastepaper treating process as a principal raw material. The regeneration particle aggregate is obtained mainly through the processes of dewatering process, drying process, burning process and crushing process.
Owner:DAIO PAPER CORP
Gelatin particle-agglutination detection reagent kit suitable for 1type duck hepatitis virus antibody and its uses
InactiveCN101101297BStrong specificityHigh sensitivityMaterial analysisDuck hepatitis A virusAgglutination
The invention belongs to the bird immunology technology area, it concretely relates to a kit of acceptable for the one type duck hepatitis virus antibody emulsion agglutination and the application of testing one type duck hepatitis virus antibody emulsion agglutination. The kit of this invention contains box body and core reagent in the box body, positive check sample, negative check sample, sample cell, scale sample sucker and the carrier of observed result. The characteristic is that core reagent is latex antigen from sublimating e duck hepatitis virus AV2111 and inactivating one type duck hepatitis virus sensitization polystyrene latex, the positive check sample is one type duck hepatitis virus antiserum, the negative check sample is the green duck of a day old of no one type duck hepatitis mother source antibody. The carrier is the paper characteristic latex agglutination card of having hollowness. The invention also discloses the preparation method and application of this kit. The kit has the significance merit of high specificity, high sensitivity, handy operation, and quickly diagnose.
Owner:HUAZHONG AGRI UNIV
Particle agglutination in a tip
ActiveUS20110070600A1Cost-effectiveMinimal interventionBioreactor/fermenter combinationsBiological substance pretreatmentsCentrifugationAgglutination
An apparatus and a related method for performing particle agglutination reactions in at least one disposable probe tip are disclosed. The at least one probe tip includes a sample cavity for sample acquisition, at least one flanking cavity for the capture of particles by centrifugation or other means, a transition zone for the mixing of the sample with reagents for agglutination and a detection zone for the optical detection of particle agglutination. A mechanism may be attached to the probe tip for the controlled movement of fluids through the internal volume of the probe tip. The probe tip is particularly useful for the automation of high-throughput agglutination-type assays.
Owner:ORTHO-CLINICAL DIAGNOSTICS
Mixing method and mixing apparatus for particle agglutination
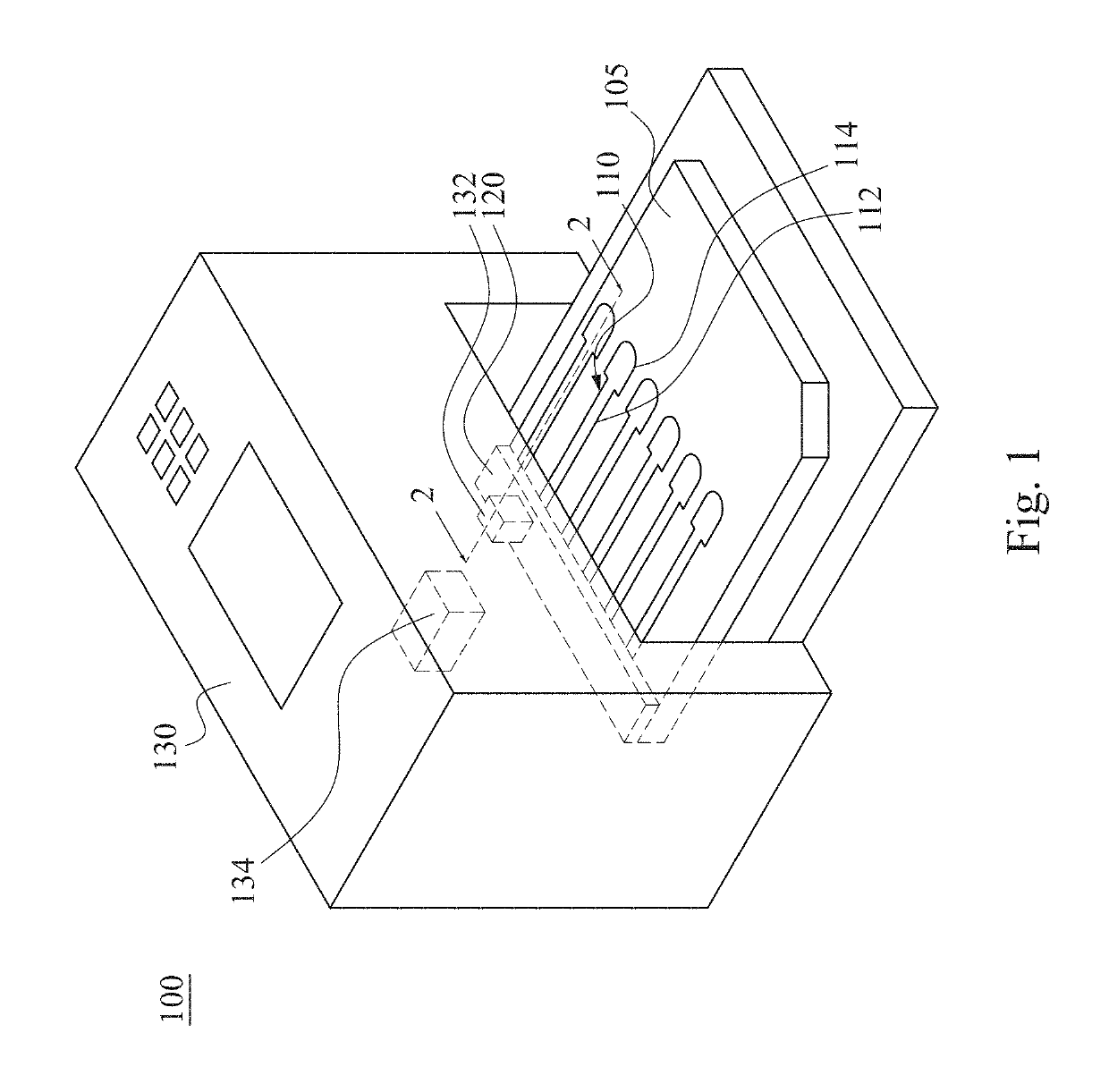
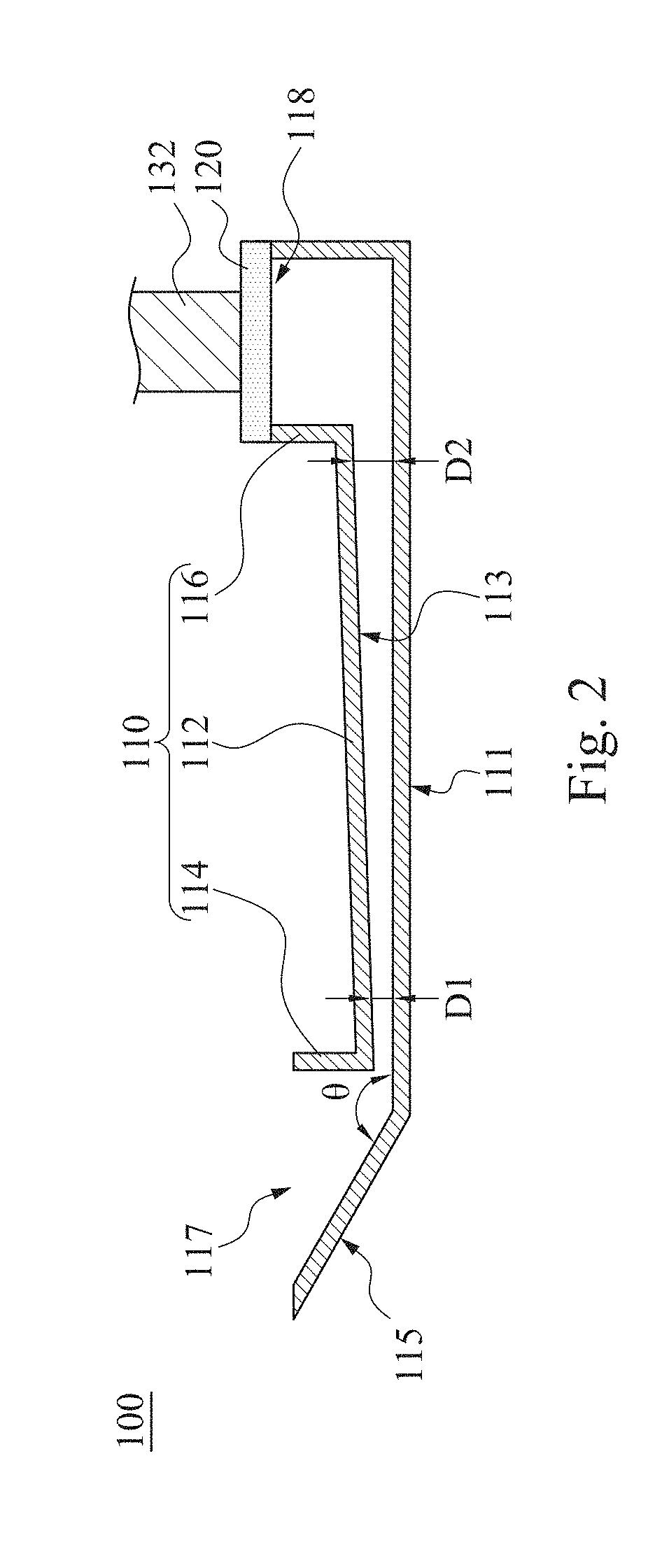
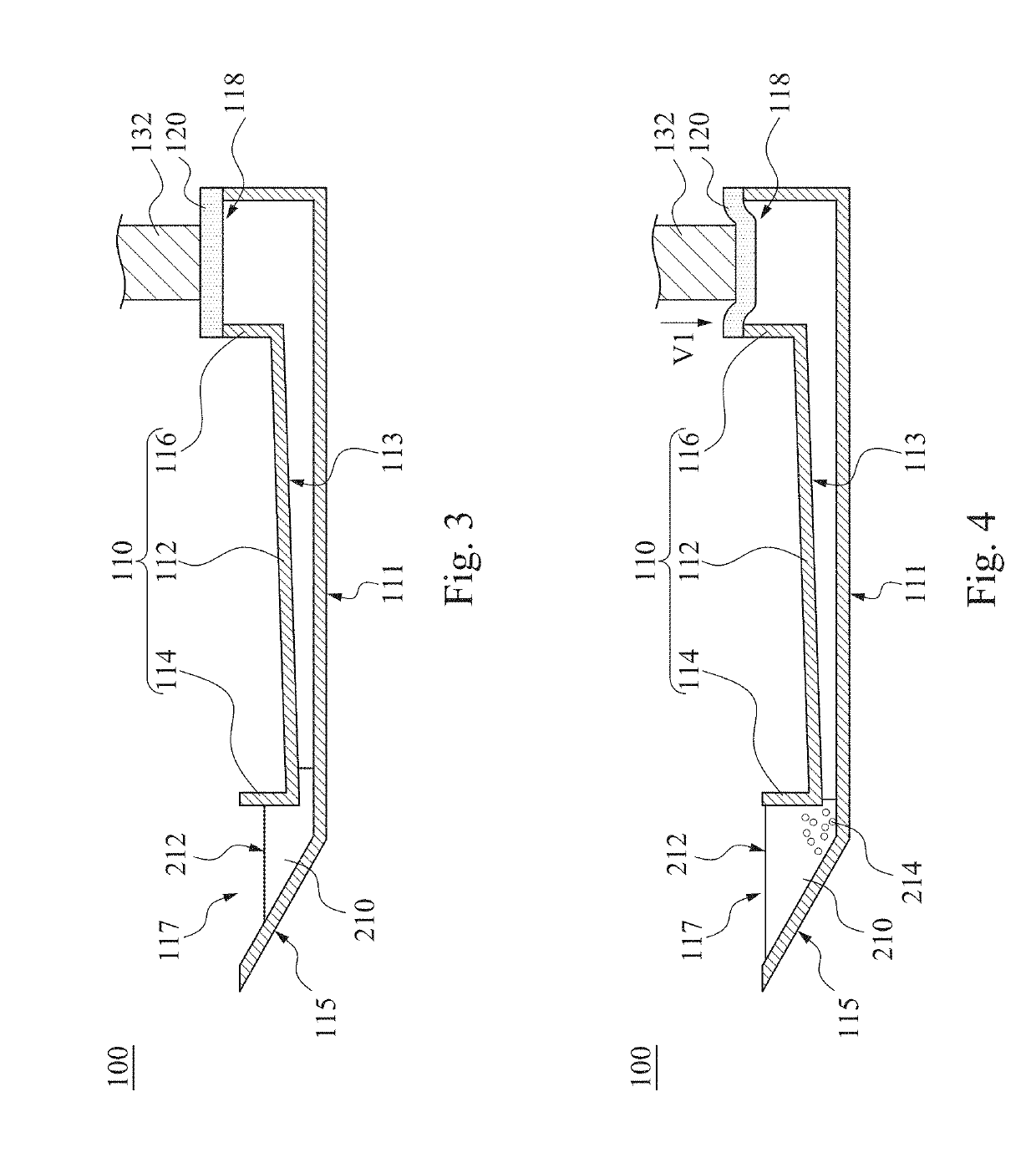
InactiveUS20190120829A1Reduce test stepsShorten test timeShaking/oscillating/vibrating mixersTransportation and packagingEngineeringTest material
A mixing method for particle agglutination includes the following steps of: dropping testing materials into an accommodating recess at one end of a channel structure; pressing a flexible layer on the other end of the channel structure; and releasing the flexible layer to an initial position after pressing the flexible layer such that a negative pressure is generated by an air chamber that is covered by the flexible layer and draws the testing materials that are in the accommodating recess to move toward the air chamber along a diverging channel of the channel structure. The testing materials are mixed with each other in the diverging channel, in which the depth of the diverging channel is gradually increased from the accommodating recess to the air chamber.
Owner:DELTA ELECTRONICS INC
Insoluble carrier particle nephelometric immunoassay reagent
The present invention provides: a reagent and a kit for an insoluble carrier particle nephelometric immunoassay which stabilize the agglutination reaction by suppressing the action of blood plasma components that are involved in the agglutination reaction of insoluble carrier particles such as latex and affect the values to be determined, to provide the stable absorbances of the reaction solutions and the accurate determination results; and a method of an insoluble carrier particle nephelometric immunoassay utilizing said reagent or kit. An antigen or an antibody is carried on an insoluble carrier particle in the presence or absence of a buffer solution comprising a compound having within its molecule a group shown below such as bicine, tricine, then an antibody- or antigen-sensitized insoluble carrier particle suspension is allowed to contact a sample in the presence of the above mentioned buffer solution to cause the immune agglutination reaction, and the turbidity generated by the insoluble carrier particle agglutination reaction is measured to determine the antigen or antibody in the sample (wherein R<1>, R<2> and R<3> may be the same or different, and independently represent a hydrogen atom, a hydroxyalkyl group or the like).
Owner:KYOWA MEDEX CO LTD +1
Latex particles for measuring particle agglutination
Latex particles for a particle agglutination assay, with which it is possible to carry out high-sensitivity assay while highly suppressing a non-specific reaction are disclosed. The latex particles being obtained by emulsion polymerization with a polymerizable monomer having a phenyl group, and a polymerizable monomer having a phenyl group and a sulfonate in an aqueous medium including a nonionic surfactant at a concentration of 0.005 to 0.02 wt % based on the aqueous medium, the latex particles having an average particle size of 0.005 to 1.0 μm.
Owner:SEKISUI MEDICAL CO LTD
Mixing method and mixing apparatus for particle agglutination
InactiveCN109701408AGuaranteed mixEasy to observeShaking/oscillating/vibrating mixersFlow mixersEngineeringHybrid device
The invention provides a mixing method and a mixing apparatus for particle agglutination. A mixing method for particle agglutination includes the following steps of: dropping testing materials into anaccommodating recess at one end of a channel structure; pressing a flexible layer on the other end of the channel structure; and releasing the flexible layer to an initial position after pressing theflexible layer such that a negative pressure is generated by an air chamber that is covered by the flexible layer and draws the testing materials that are in the accommodating recess to move toward the air chamber along a diverging channel of the channel structure. The testing materials are mixed with each other in the diverging channel, in which the depth of the diverging channel is gradually increased from the accommodating recess to the air chamber.
Owner:DELTA ELECTRONICS INC
Magnesium oxide particle aggregate
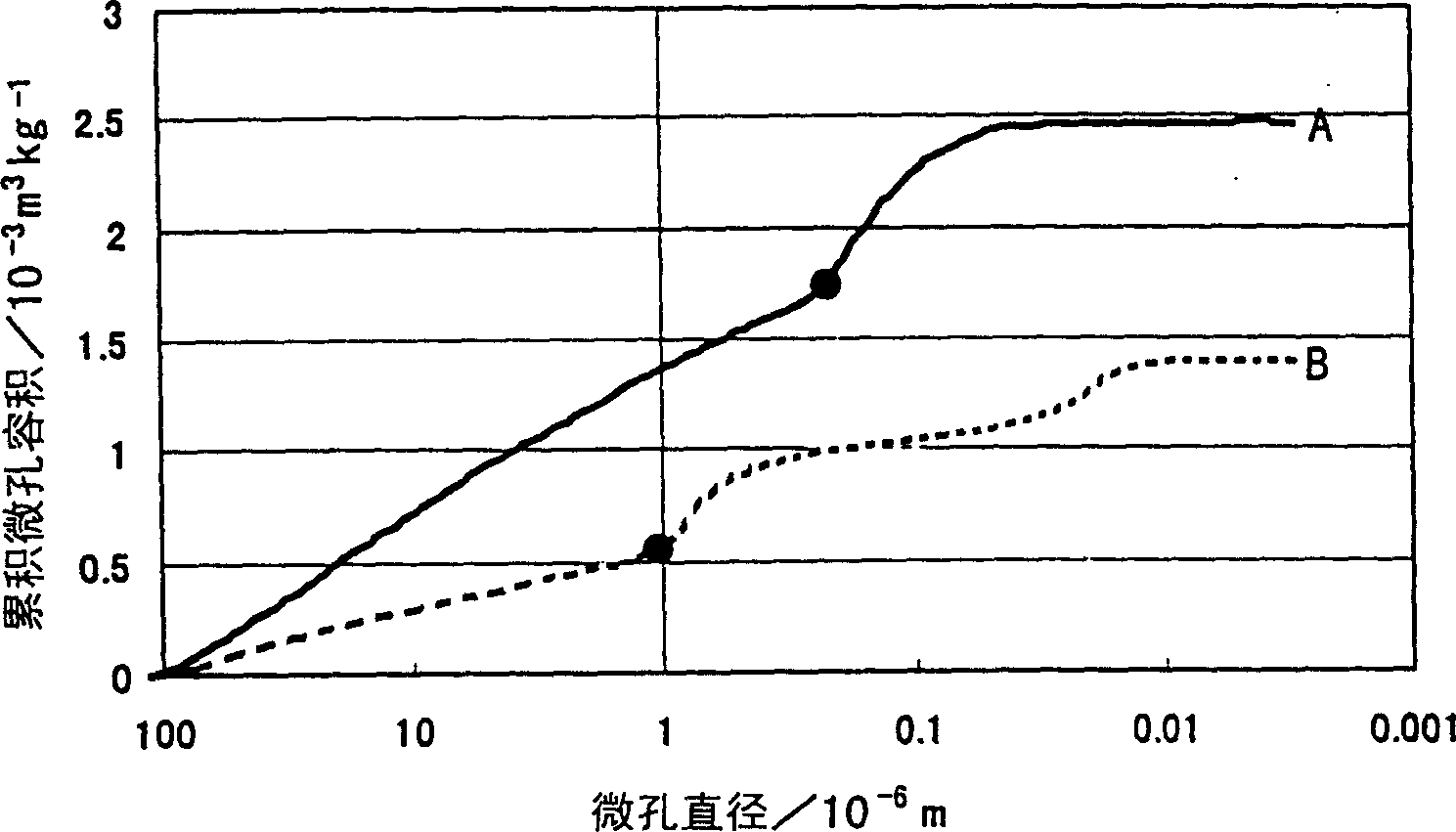
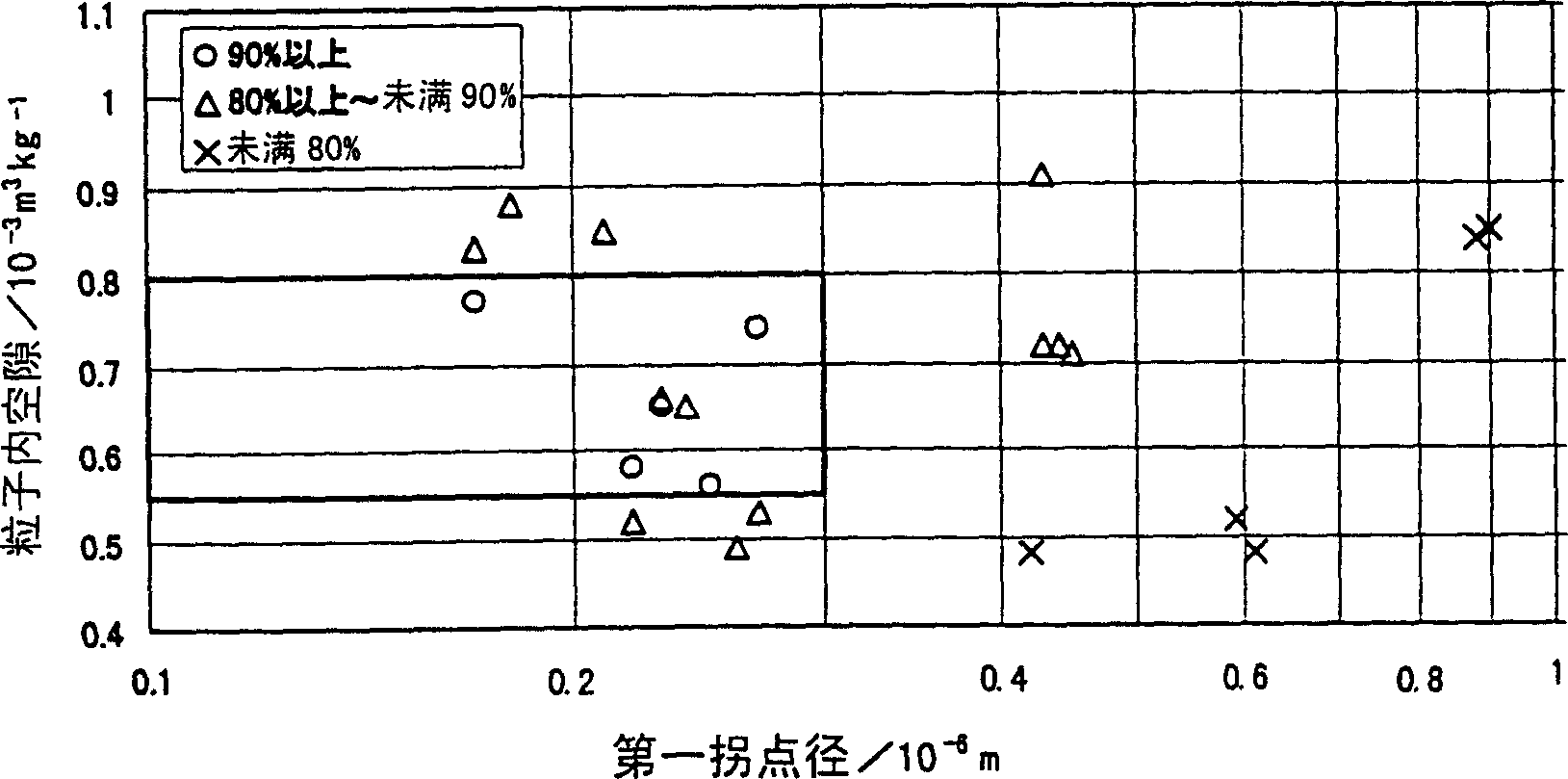
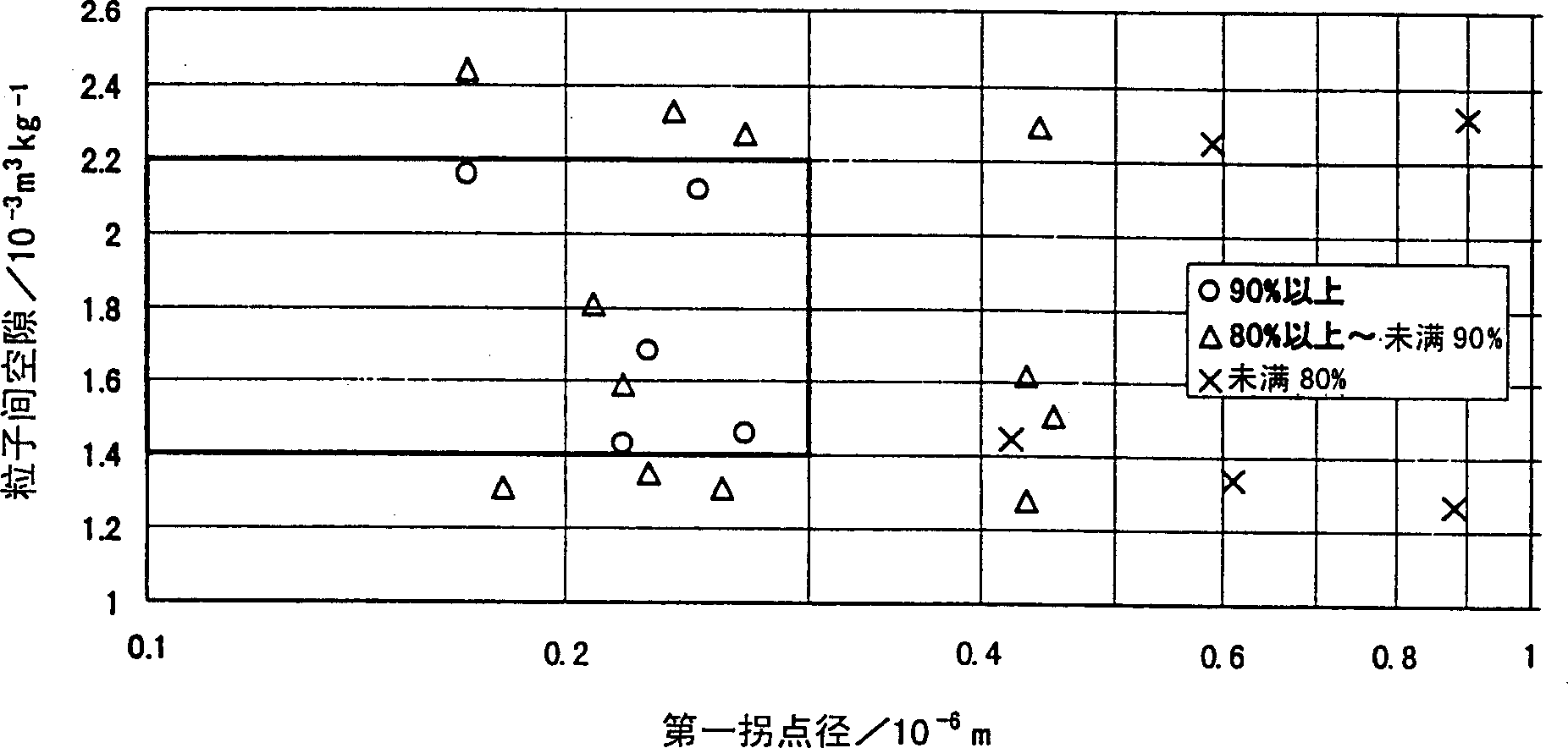
The present invention is to provide appropriate control of magnesium oxide and surface SiO by controlling particle agglomeration structure 2 The solid-phase-solid-phase reaction of the thin film is an agglomeration of magnesium oxide particles. This subject is based on the cumulative micropore volume curve of the particles, the first inflection point diameter is 0.30 × 10 -6 m or less, the amount of voids between particles is 1.40×10 -3 ~2.20×10 -3 m 3 / kg, the amount of voids in the particles is 0.55×10 -3 ~0.80×10 -3 m 3 / kg of magnesium oxide particle aggregates.
Owner:TATEHO CHEM IND CO
Inert carrier indirect agglutination test detection system and its application
ActiveCN111537712BAvoid non-specific cross-agglutination reactionsPotential application value is goodBiological material analysisDepsipeptidesSerum/Whole bloodAntigen
Owner:YANGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com