Inert carrier indirect agglutination test detection system and its application
A technology of agglutination test and detection system, which is applied in the fields of biomedicine and immunodiagnosis, can solve the problems of low antibody detection rate and possible positive reaction of Enterobacteriaceae, and achieve clear and easy-to-judgment results and rapid positive reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The Salmonella S9 used in the present invention has been preserved in the China General Microorganism Culture Collection Management Center (CGMCC), the preservation address is Beijing, China, the preservation number is CGMCC No.17340, the preservation date is March 18, 2019, and the classification is named Salmonella (Salmonella sp.), strain code S9. Example 1 Identification and verification of Salmonella pullorum P antigenic factor
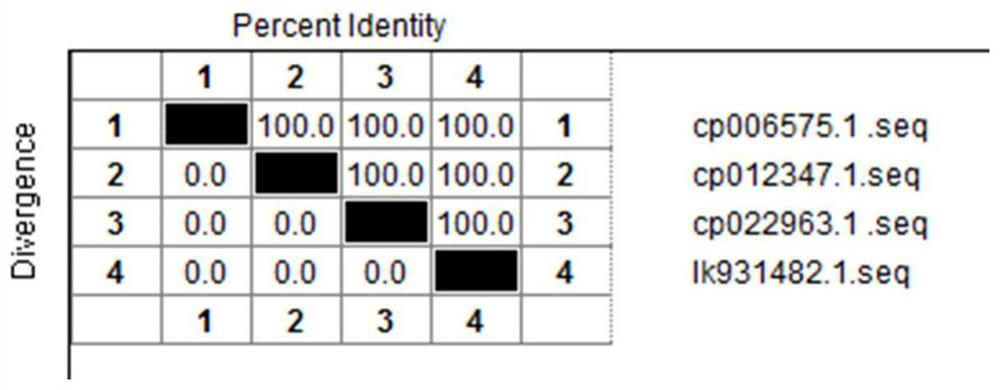
[0049] Search the uploaded P antigen factor coding gene P in Salmonella pullorum pullorum (Table 1) through the GenBank database on NCBI, and compare the downloaded p gene sequences with DNAMAN Windows version software, and the results show that p in different Salmonella pullorum pullorum strains The gene sequence is highly conserved ( figure 1 ). In fact, it is also highly conserved with the p gene sequences of multiple strains of Salmonella pullorum isolates isolated and identified by our laboratory and determined by DNA sequence.
[...
Embodiment 2
[0054] Example 2 Construction of inert carrier indirect agglutination test detection system S9-P
[0055] According to the full-length fragment of the p gene of Salmonella pullorum in NCBI, the amplification primers were designed with Olige7 software, and NheI and BamHI restriction sites and protective bases were added to the 5' ends of the upstream and downstream primers respectively. The upstream and downstream primers were respectively:
[0056] p-UP: 5′-ATG AAA CGT TCA CTT ATT GCT GCT-3′
[0057] p-LO: 5'-TTAA TCA GTT AAT ACC GTC ATC GTC AG-3';
[0058] Prepare CVCC526 Salmonella pullorum template by boiling method, p-PCR system: 5×pfu DNA polymerase buffer 10 μL, dNTP 5 μL, upstream primer 2 μL, downstream primer 2 μL, template 2 μL, pfu high-fidelity enzyme (2.5 units / uL) 2 μL , 27 μL of deionized water. PCR reaction conditions: 94°C for 5min, 94°C for 1min, 52°C for 1min, 72°C for 5min, 30 cycles, 72°C for 10min. After the above PCR reaction was completed, 2.4 μL of ...
Embodiment 3
[0069] Verification of Example 3 Inert carrier detection system bacterial strain expressing P factor
[0070] Single colonies of strain S9 and recombinant strain S9-P were inoculated on LB and ampicillin-resistant LB agar medium respectively, cultured at 37°C for 24 hours, and single colonies were picked and inoculated in LB and ampicillin-resistant LB liquid medium respectively, blindly After two passages, draw a small amount of bacterial liquid and inoculate them in LB and ampicillin-resistant LB liquid medium respectively. After static culture at 37°C for 48 hours, centrifuge at 10,000 rpm for 2 minutes, resuspend the pellet with sterilized PBS, absorb a small amount of supernatant, and suspend On the copper grid, and negatively stained with phosphotungstic acid for 5 minutes, PhilipsTecnai 12 transmission electron microscope TEM observation and shooting results showed that the surface of S9 seems to have no P antigen factor components, while the surface of the inert carrier...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com