Blood type method system and device
A technology for blood and red blood cells, applied in measurement devices, biochemical cleaning devices, biochemical equipment and methods, etc., can solve the problems of not allowing cross-matching, large test tubes, false negative results, etc., and achieve fast and accurate results. The effect of obtaining results, low error tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
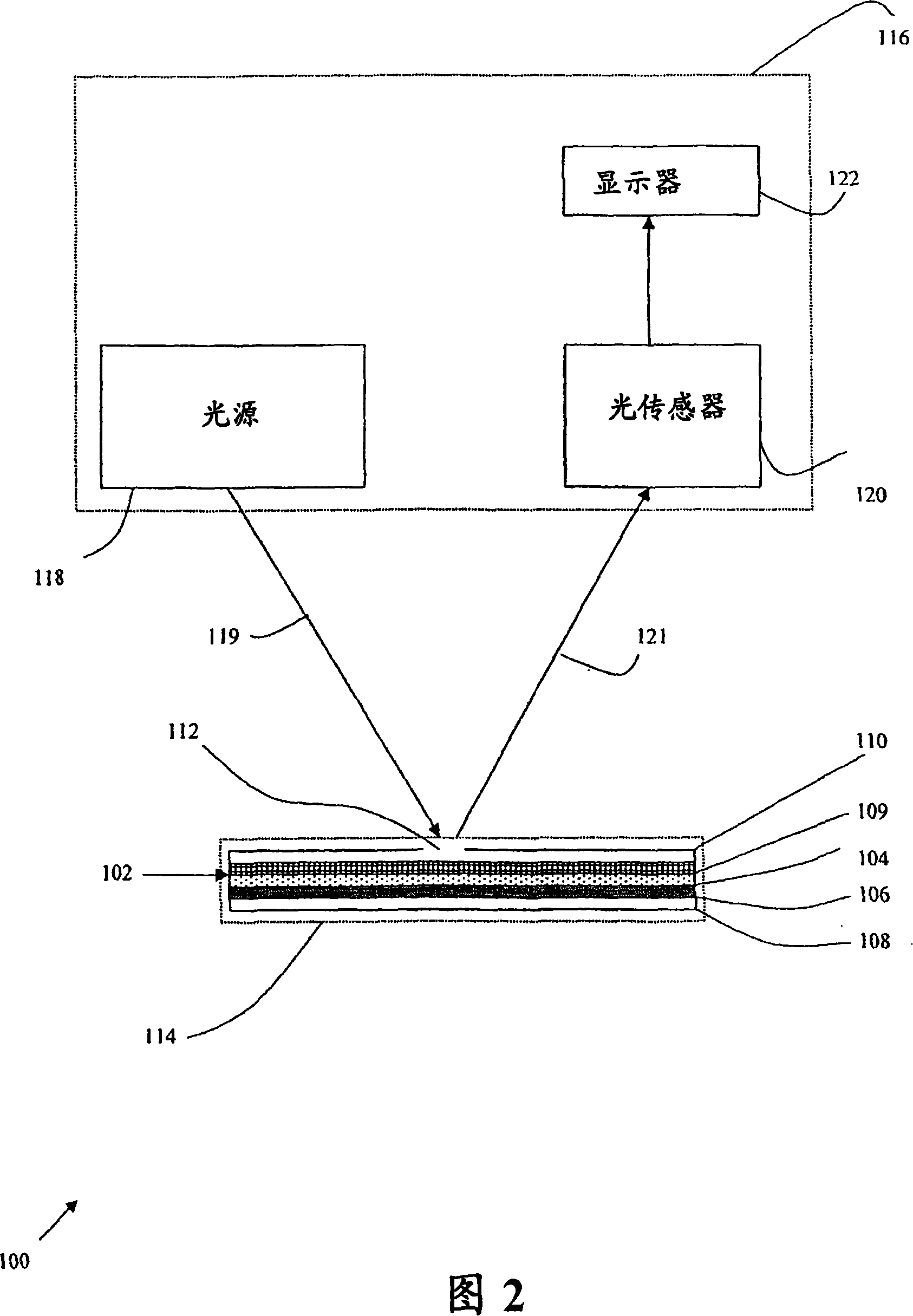
Image
Examples
example
[0150] The examples that follow are exemplary implementations of methods and systems according to the invention. These examples are not intended to be limiting in any way.
[0151] Example 1 - Lotion
[0152] The washes used to generate the instances are:
[0153] A. Dulbecco's Phosphate Buffered Saline (PBS) obtained from Biological Industries, Beit Ha'emek, Israel.
[0154] B. A solution prepared from PBS diluted 1:1 in water with 4% w / v polyethylene glycol (PEG) 15000-20000 MW (Fluka) and 0.3% w / v dextran sulfate sodium salt (Amersham Biosciences).
[0155] C. Dulbecco's Phosphate Buffered Saline (PBS) with 0.001-0.01% w / v polyoxyethylene-10-tridecyl ether (Sigma).
example 2
[0156] Example 2 - Blood Grouping
[0157] Ahlstrom #142 filter paper of size 0.5 x 0.5 cm was placed on the absorbent pad. Two μL of whole blood was pipetted into the center of the filter followed by 2 μL of anti-blood typing reagent (Gamma Biologicals Inc., Houston, Texas, USA). The filter was then rinsed with a few drops (from the dropper bottle) of wash solution and dried. Alternatively, use Ahlstrom #142 filter paper with a 4mm circle radius, 10 μL of blood, and 10 μL of antiblood group reagent, followed by a few drops of the wash solution of Example 1.
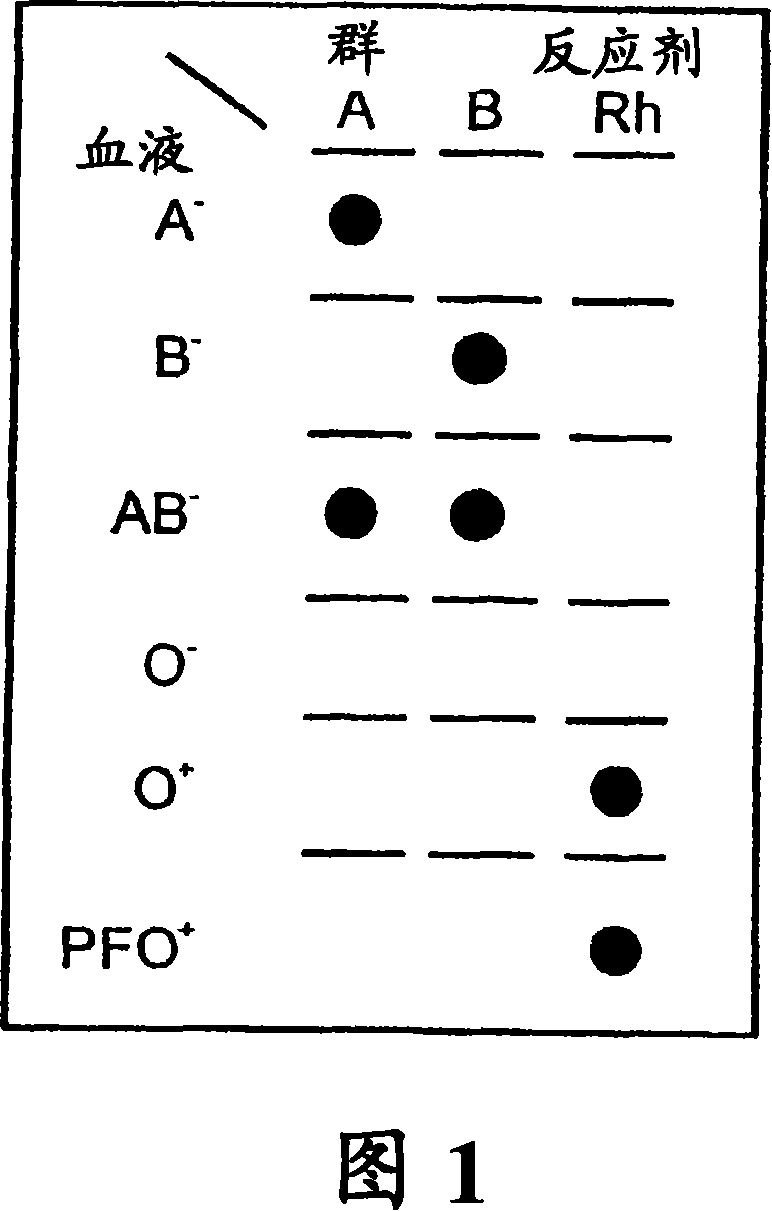
[0158] This test was repeated with various blood samples of different blood types (received from and pre-tested by the Blood Service Center of Israel RedMagen David, Tel Hashomer, Israel) and tested with anti-A, anti-B, anti-AB and anti-D (=Rh ) blood group reagent for testing. A - Blood only produces red spots with anti-A and anti-AB reagents. B - Blood was spotted with anti-B and anti-AB reagents. AB - Blood ...
example 3
[0159] Example 3 - Grouping of Blood Using Dried Reagents
[0160] Ahlstrom #142 filter discs of size 0.5 x 0.5 cm were placed on a non-absorbent surface (hollow bottom, disposable Petri dish). 50 [mu]L of anti-blood typing reagent (Gamma Biologicals, Inc., Houston, TX, USA) was pipetted onto each piece of filter paper. Petri dishes were incubated overnight at 37°C with filter paper containing anti-blood typing reagents. At this point the filter is dry.
[0161] To test the ability of the dried antibody-dipped discs to correctly identify blood groupings of whole blood samples, they were placed on absorbent pads and 1 μL of test blood was placed approximately in the center of each filter pad of the filter pad series. The series comprising pads are each impregnated with one of anti-A, anti-B or anti-D (=Rh) reagents.
[0162] Rinse remaining blood from the filter pad by placing 5-10 drops of wash solution A or B. The filters are then air dried for record keeping. The resu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com