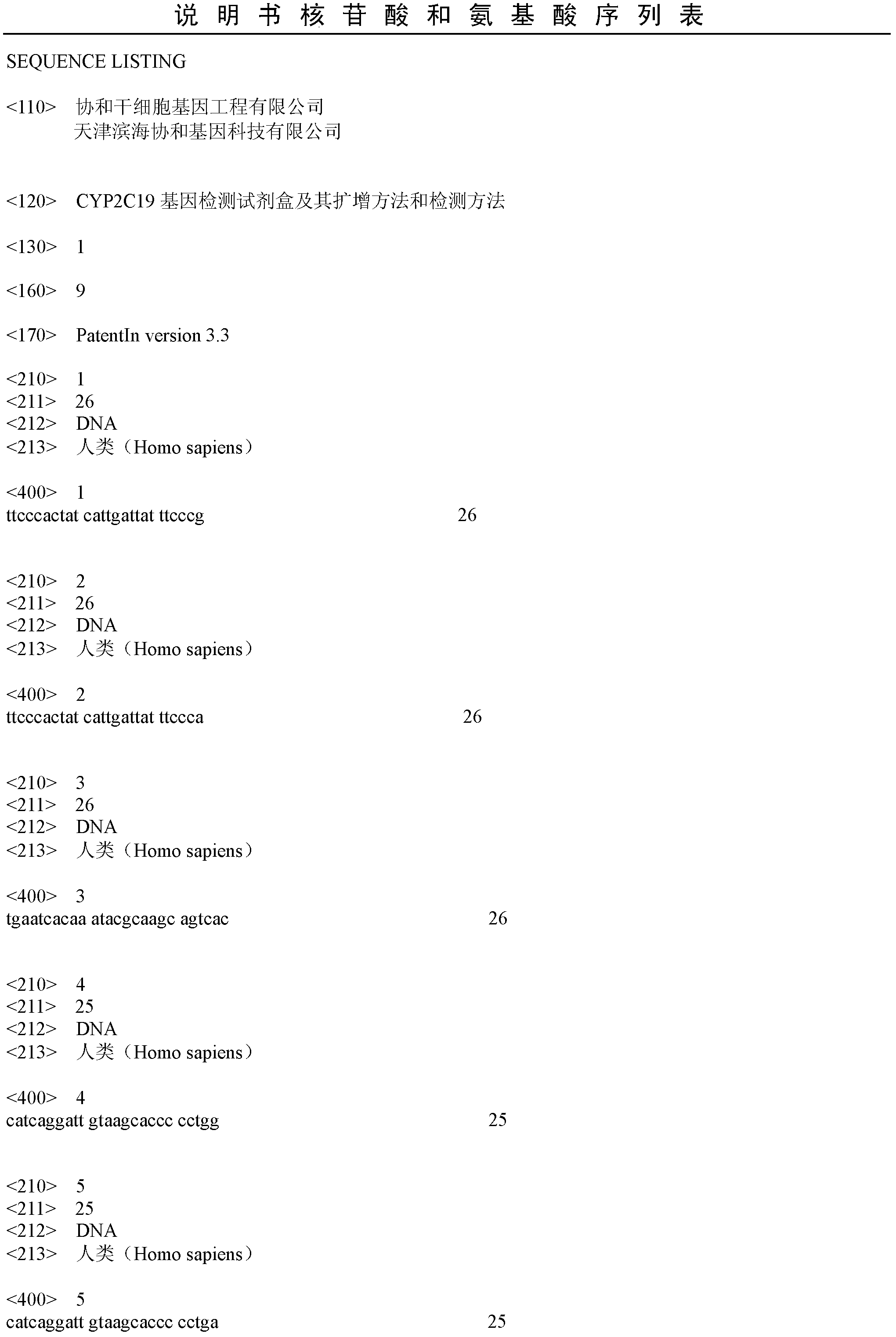Patents
Literature
327results about How to "Avoid False Positive Results" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
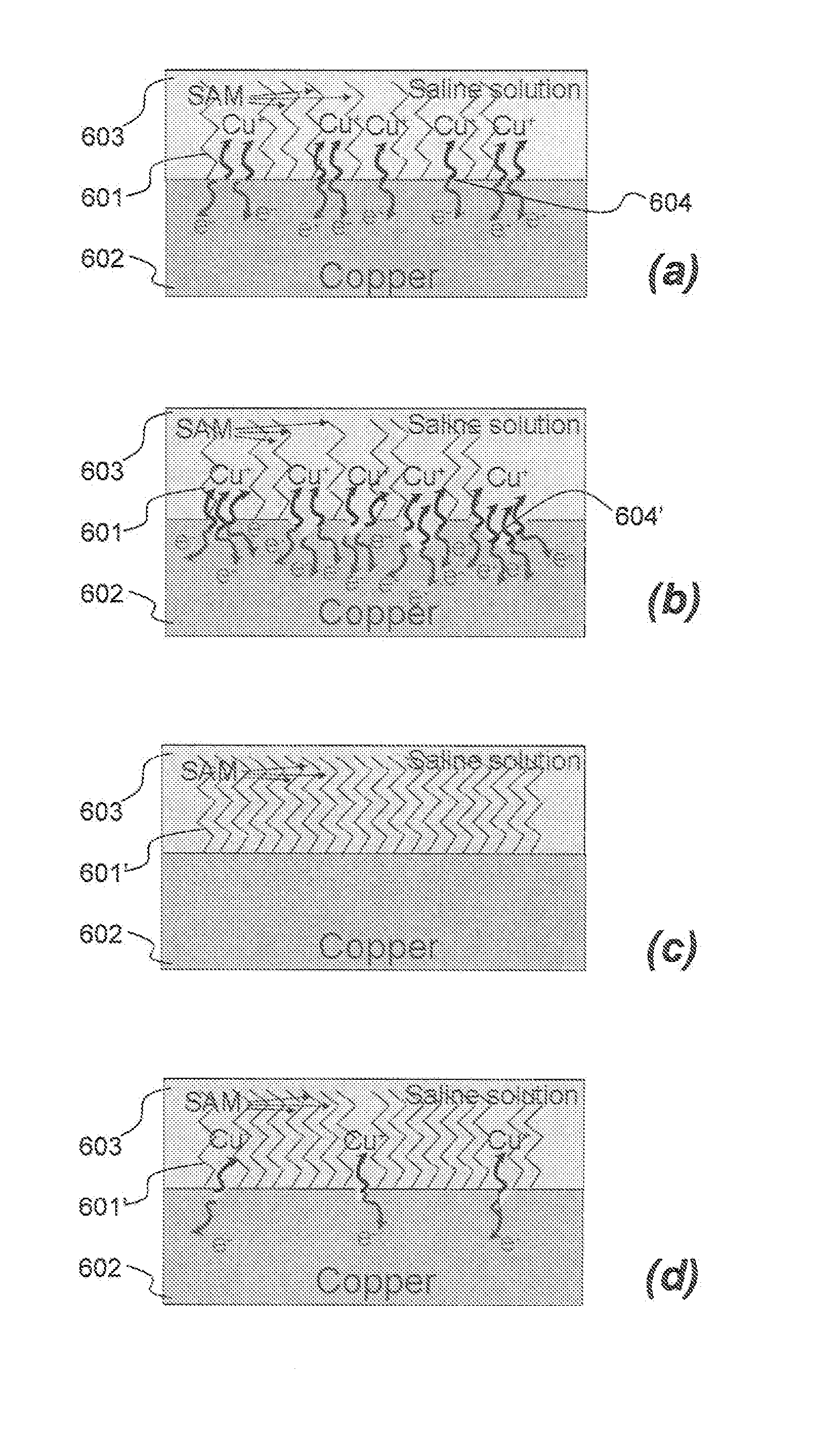
Device having self-assembled-monolayer
InactiveUS20120088315A1Avoid inhibitionEffective corrosion inhibitionComponent separationBiological material analysisSelf-assembled monolayerSensing applications
A device for bio-sensing applications is disclosed, comprising a substrate such as a semiconductor chip having Cu electrodes thereon, and a self assembled monolayer bonded to at least one of the Cu electrodes, wherein molecules of the self-assembled monolayer comprise a head group which bonds to Cu, a carbon-comprising chain comprising a chain of at least 12 C atoms, and a terminal group which is hydrophilic and for binding a bio-receptor. The terminal group is hydrophilic to allow binding to the bio-receptor, and inclusion of the carbon-comprising chain, limits or avoids corrosion of the copper. Also disclosed is a method of providing such a device, activating the terminal group and coupling a bio-receptor to the activated terminal group. Disclosure further extends to use of such a device for bio-sensing applications.
Owner:NXP BV
Polymorphism detection system and kit for gene relevant to personalized medication for cardiovascular disease
ActiveCN107641645AReduce operating intensityReduce testing costsMicrobiological testing/measurementDNA/RNA fragmentationDeoxyuridine TriphosphatePersonalization
The invention discloses a polymorphism detection system and a kit for a gene relevant to personalized medication for a cardiovascular disease. The system relates to a quantitive fluorescent PCR (Polymerase Chain Reaction) amplification (Quantitive Fluorescent PCR, QF-PCR) technique and a capillary electrophoresis detection technique. The system has the characteristics that 1, through the multiplePCR, the amplification of 27 polymorphic sites of 17 genes is realized in a one-tube manner; 2, the reference can be provided for the use of common medicines of an antihypertensive drug, an antiplatelet drug, oral anticoagulant drug, a blood lipid regulation drug and the like by a detection result; 3, the system can be used for realizing the direct amplification of blood and blood card, and is used for avoiding the step of extracting DNA (Deoxyribonucleic Acid); 4,the system can be used for integrating a UDG-dUTP (Uracil DNA Glycosylase-Deoxyuridine Triphosphate) pollution prevention measure,and can be used for effectively preventing a product from being polluted. The polymorphism detection system and the kit have the advantages of being complete in detection site coverage, high in specificity, high in sensitivity, high in reliability, simple and convenient to operate, high in practicability and low in cost, and has large-batch detection ability.
Owner:BEIJING MICROREAD GENE TECH
Kit for detecting SNP (Single Nucleotide Polymorphism) sites related to Warfarin individualized application, and multiplex PCR (Polymerase Chain Reaction) amplification method and detection method using same
InactiveCN102251043AImprove detection efficiencyReduce testing costsMicrobiological testing/measurementCYP4F2 geneGlycerol
The invention discloses a kit for detecting SNP (Single Nucleotide Polymorphism) sites related to Warfarin individualized application by combining multiplex PCR (Polymerase Chain Reaction) technology and SNP sensitive molecule switch technology and a detection method using the same. The kit types four SNP sites related to Warfarin application, including rs9934438 SNP site on VKORC1 gene, rs1799853 and rs1057910 SNP sites on CYP2C9 gene, and rs2108622 SNP site on CYP4F2 gene. The kit comprises a wild type 2*amplification buffer, a mutant type 2*amplification buffer, polymerase, a cell lysis solution and glycerol, wherein the two buffers respectively contain corresponding SNP wild type and mutant phenotype sequence specific primers and beta-actin primers; and the four SNP sites can be typed in the two multiplex PCR reactions, thereby providing molecular biology references for reasonable application of Warfarin.
Owner:UNION STEMCELL & GENE ENG +1
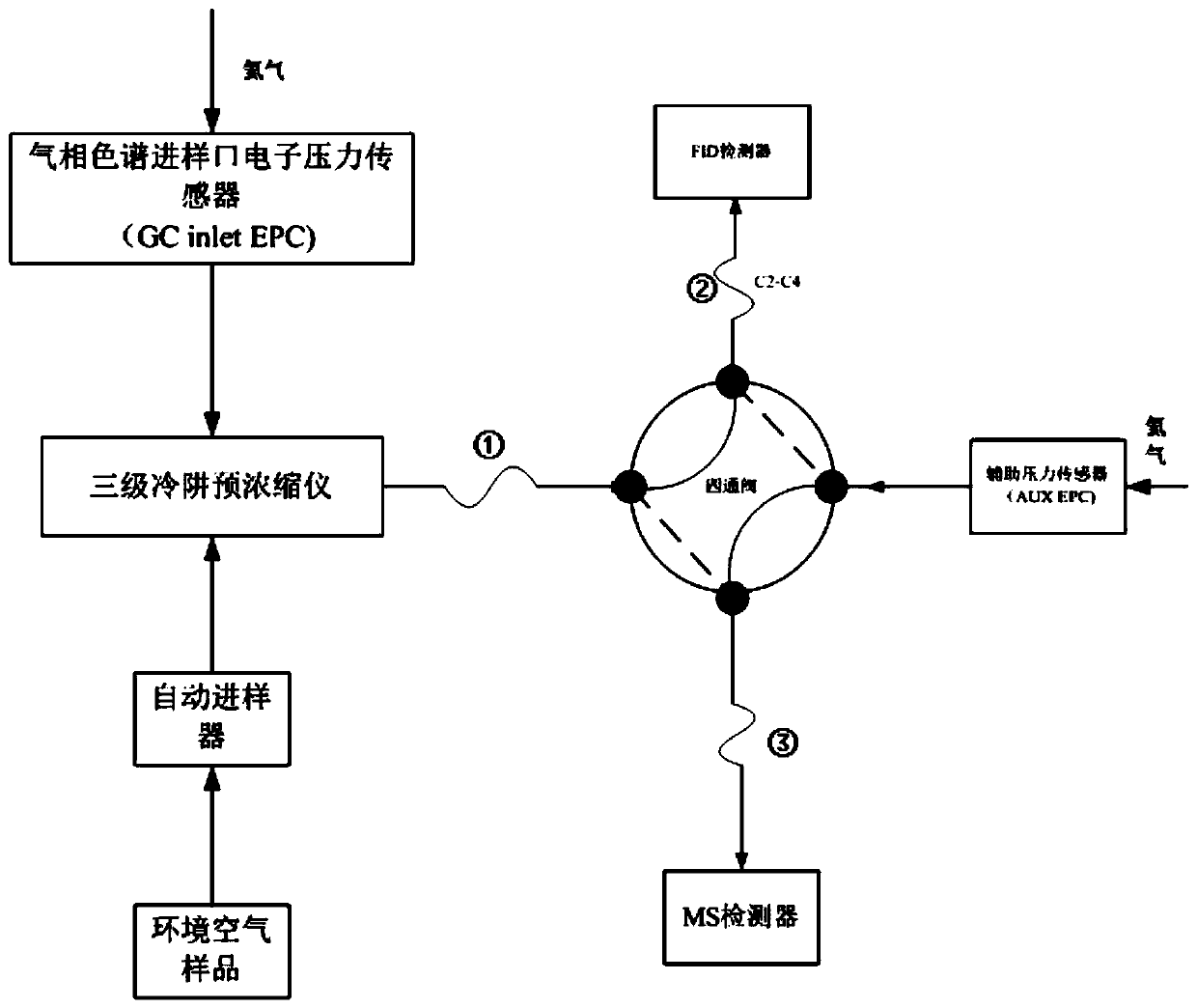
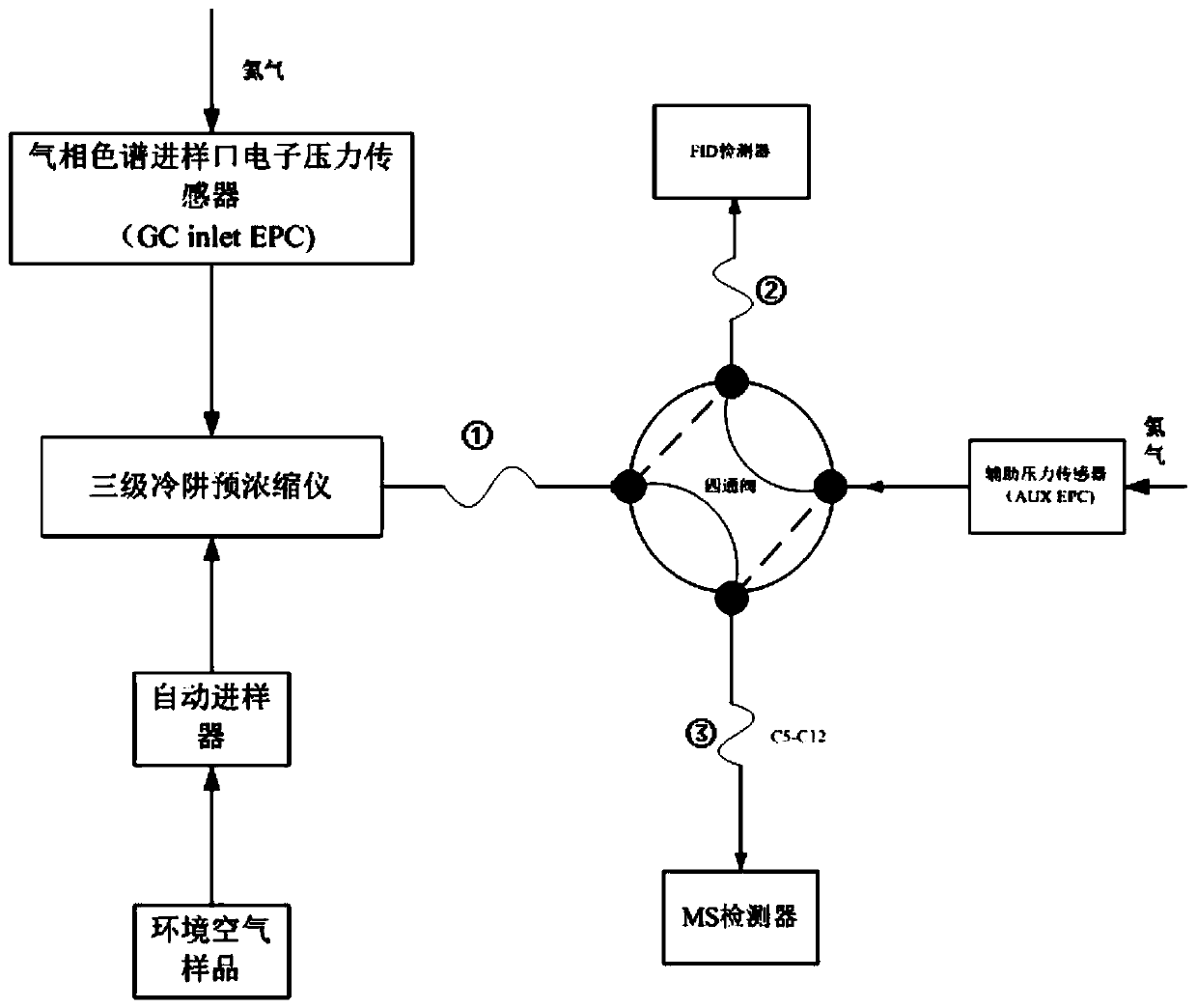
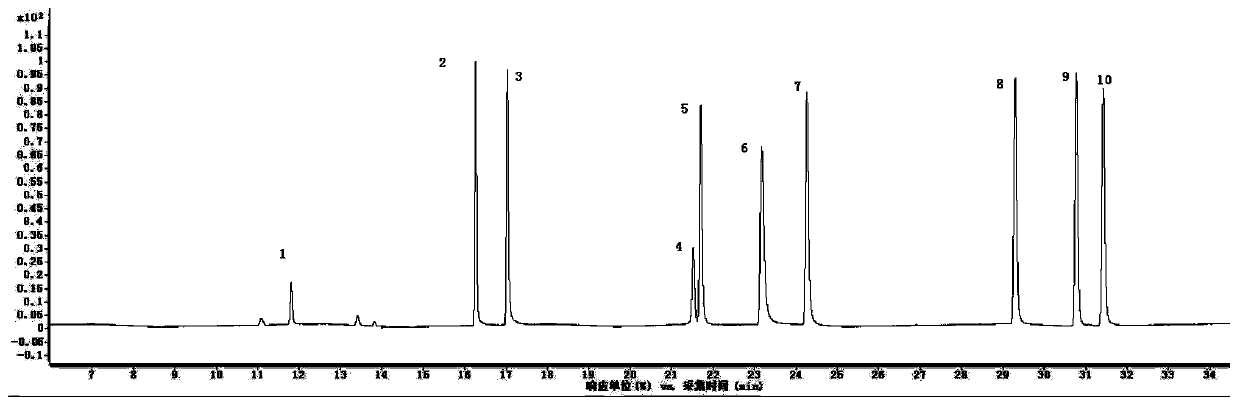
System and method for measuring content of 57 volatile organic compounds in ambient air
ActiveCN110187037AEfficient enrichmentHigh analytical sensitivityComponent separationInjection volumeFour-way valve
The invention provides a system and a method for measuring the content of 57 volatile organic compounds in ambient air. The system comprises a four-way valve, a gas chromatograph and a three-stage cold trap preconcentrator; the four ports of the four-way valve are respectively connected with a pre-separation column, a first secondary separation column, a second secondary separation column and a helium gas source; and the gas chromatograph is provided with a flame ionization detector and a mass spectrometry detector. The method comprises the following steps: (1) preparing a mixed standard use gas; (2) preparing an internal standard use gas; (3) collecting an ambient air sample; (4) establishing a standard curve of a target component; and (5) taking the ambient air sample to be tested, removing interfering substances through the three-stage cold trap preconcentrator, performing gas chromatography-mass spectrometry analysis in the mass spectrometry detector after focusing is performed, obtaining the content of each component according to a peak area and the standard curve established in the step (4), and calculating the concentration of the sample to be tested according to a sample injection volume of the sample to be tested. By adopting the method provided by the invention, the simultaneous analysis of two detectors is achieved by once sample injection, thereby avoiding the influence of the interfering substances on the measurement result simply and effectively.
Owner:SHANDONG UNIV
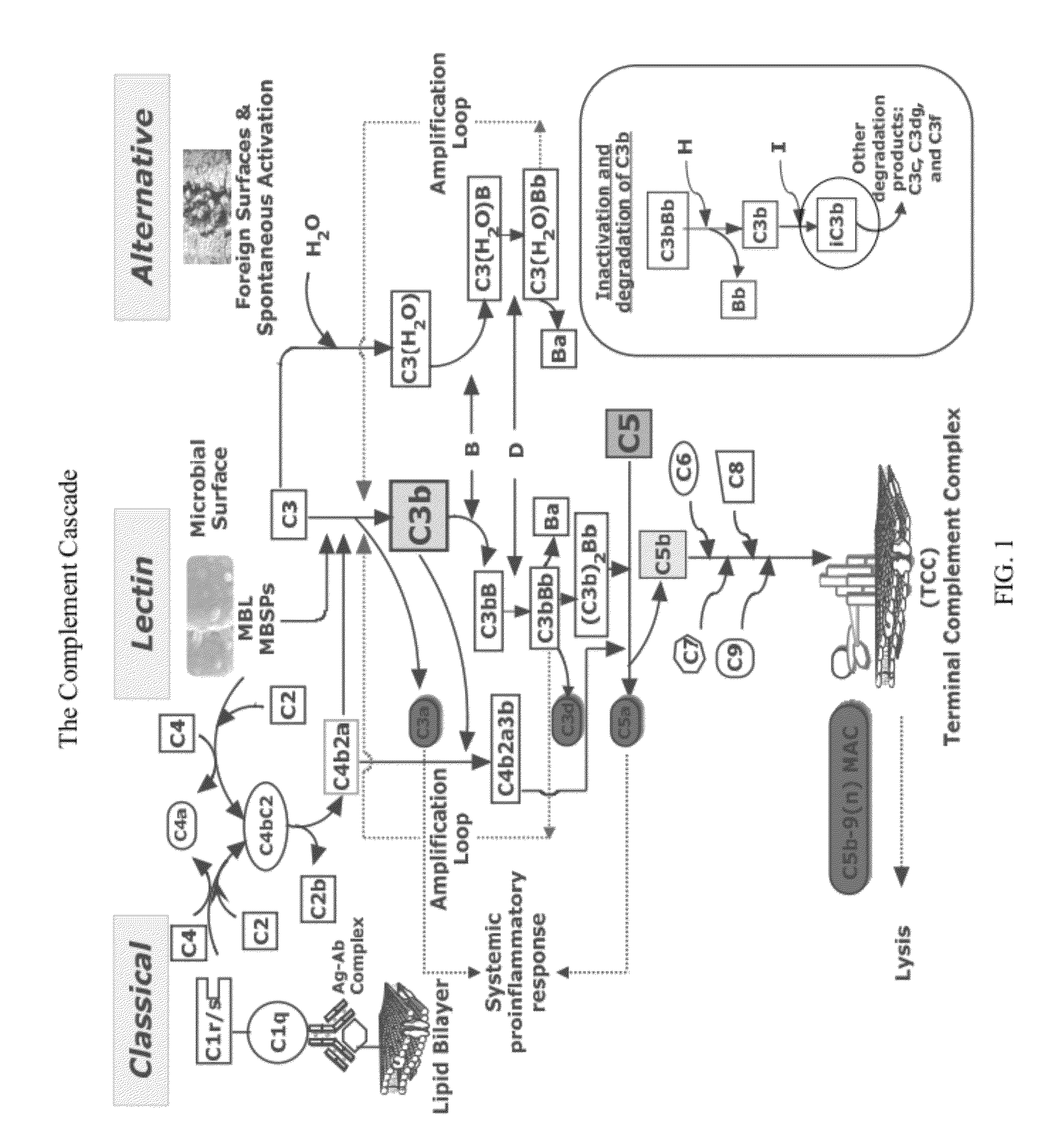
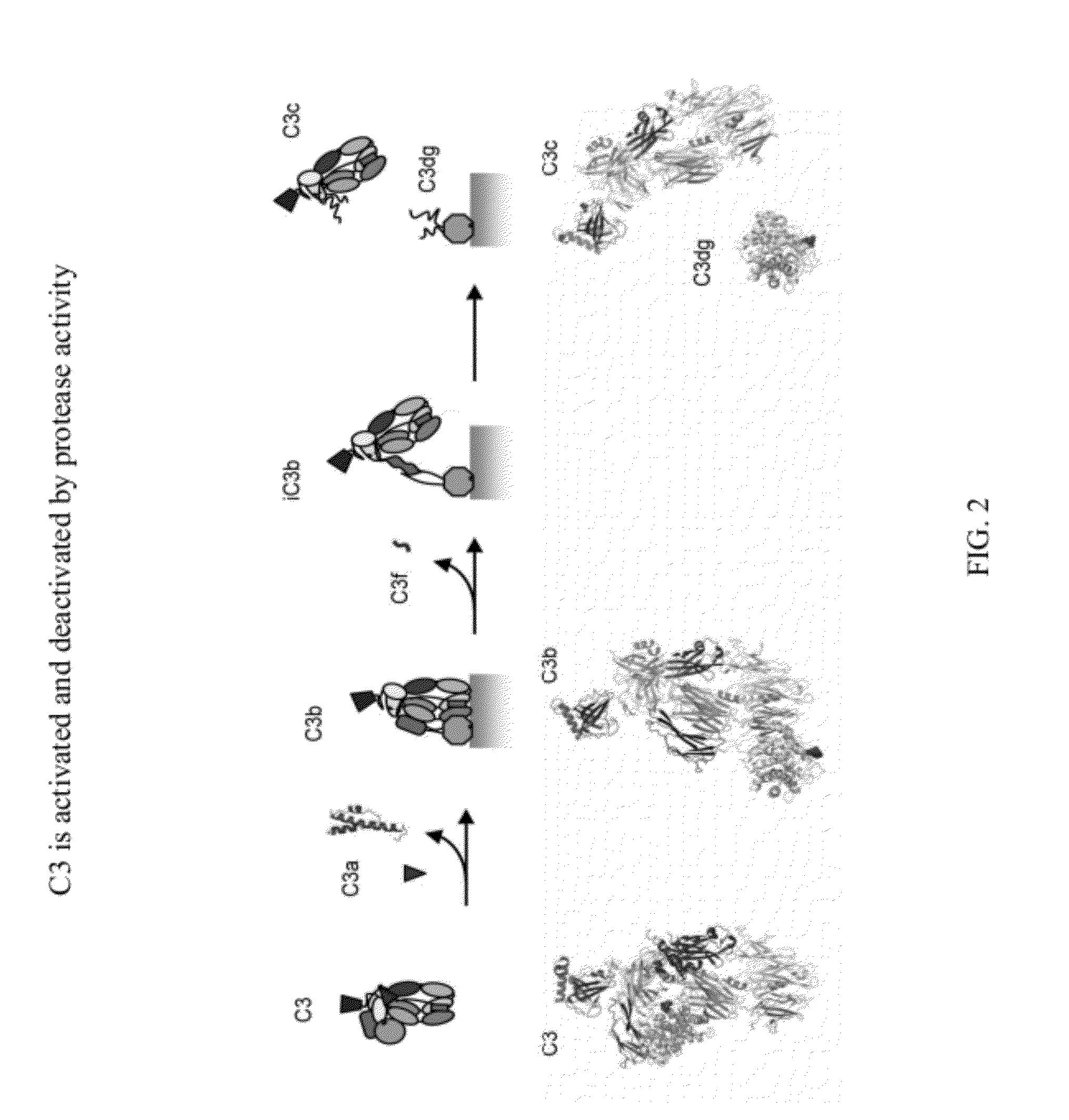
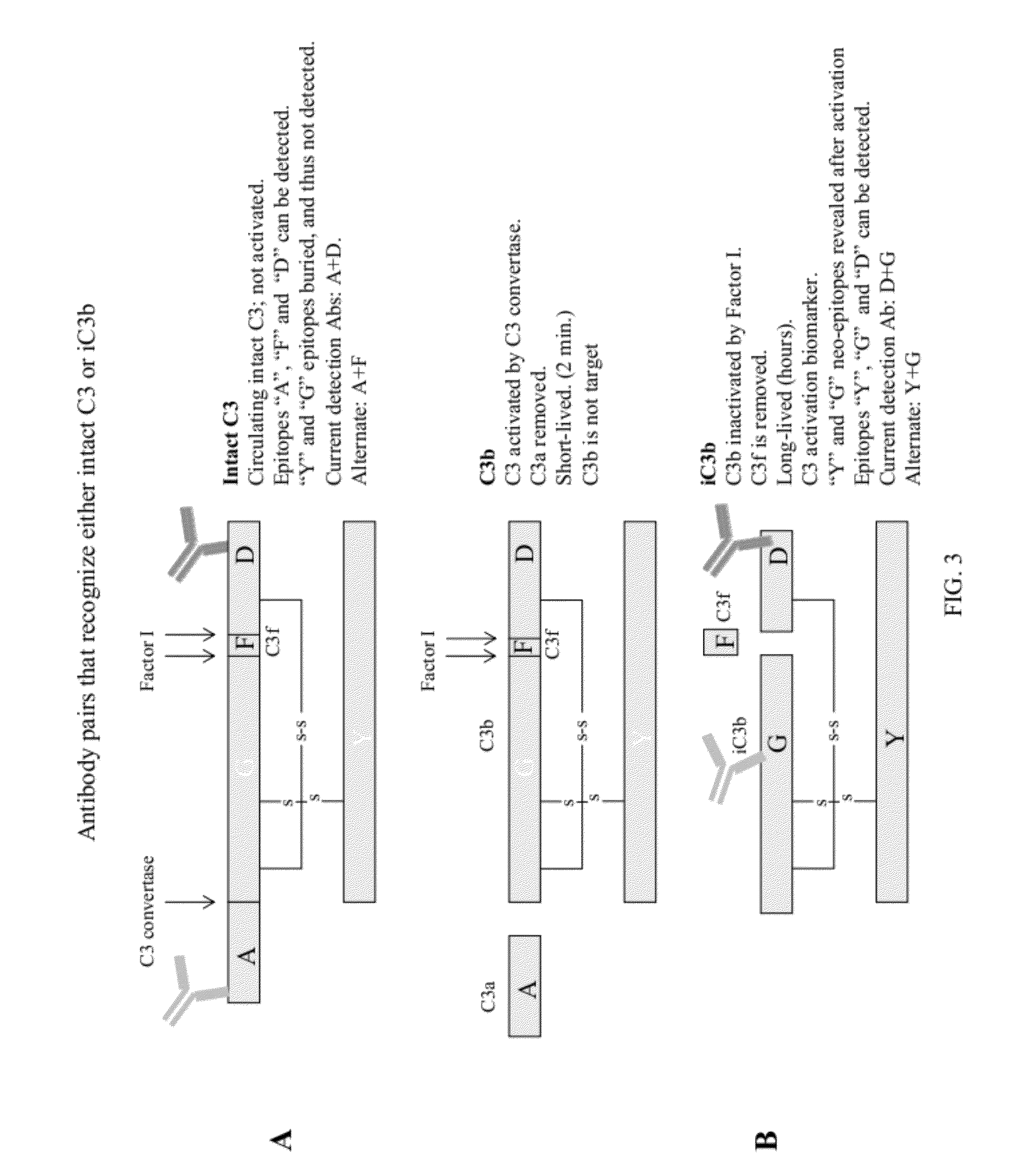
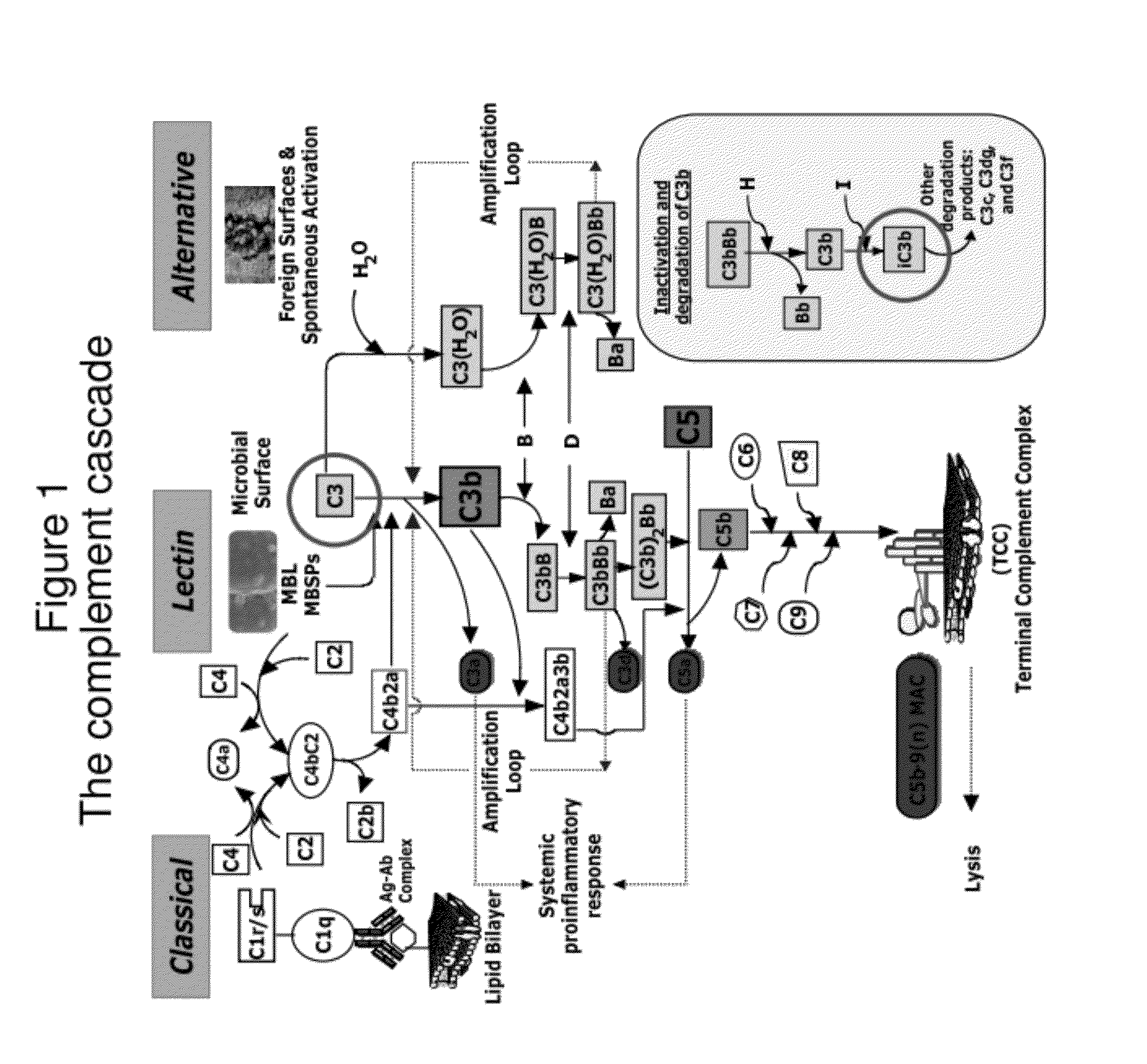
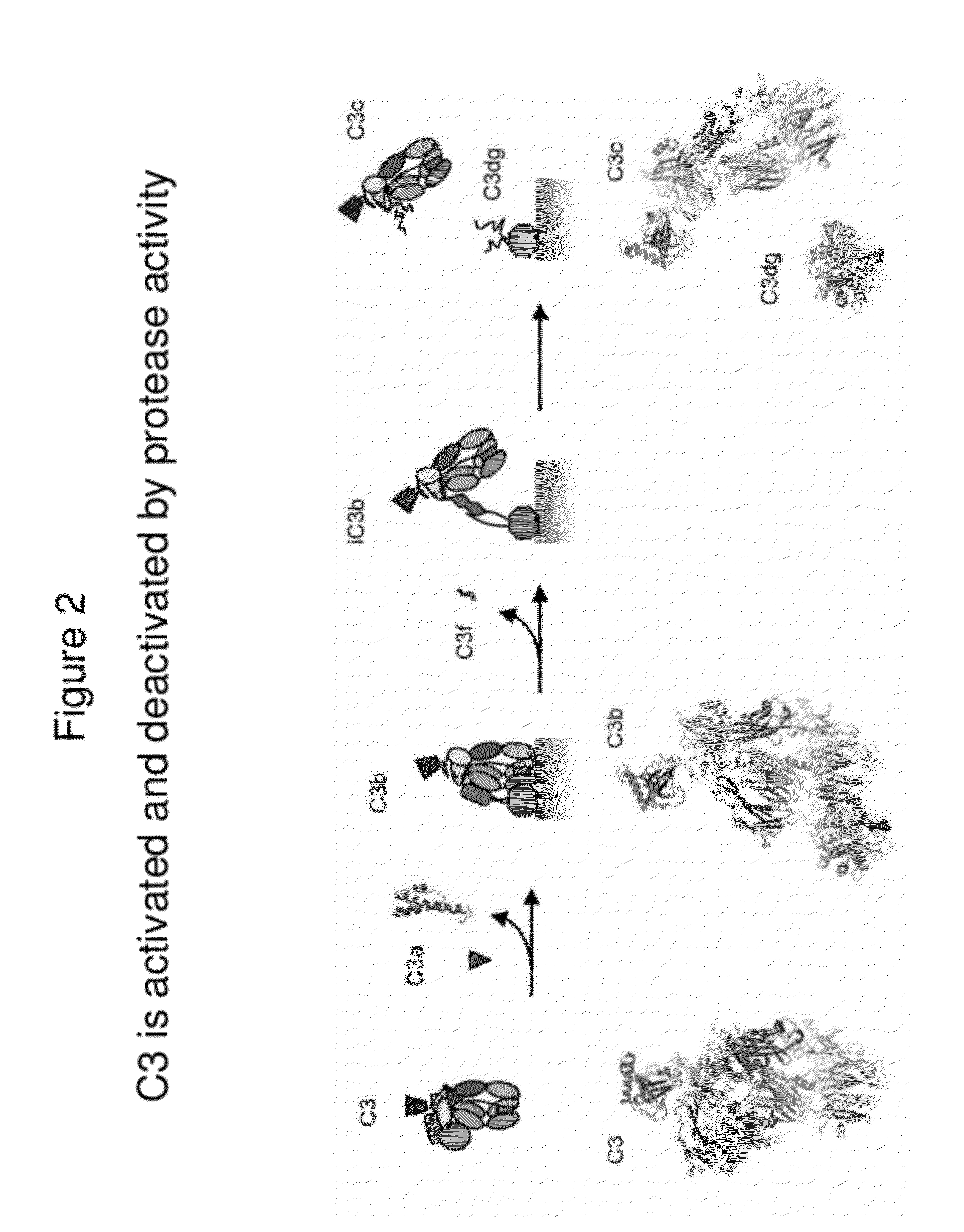
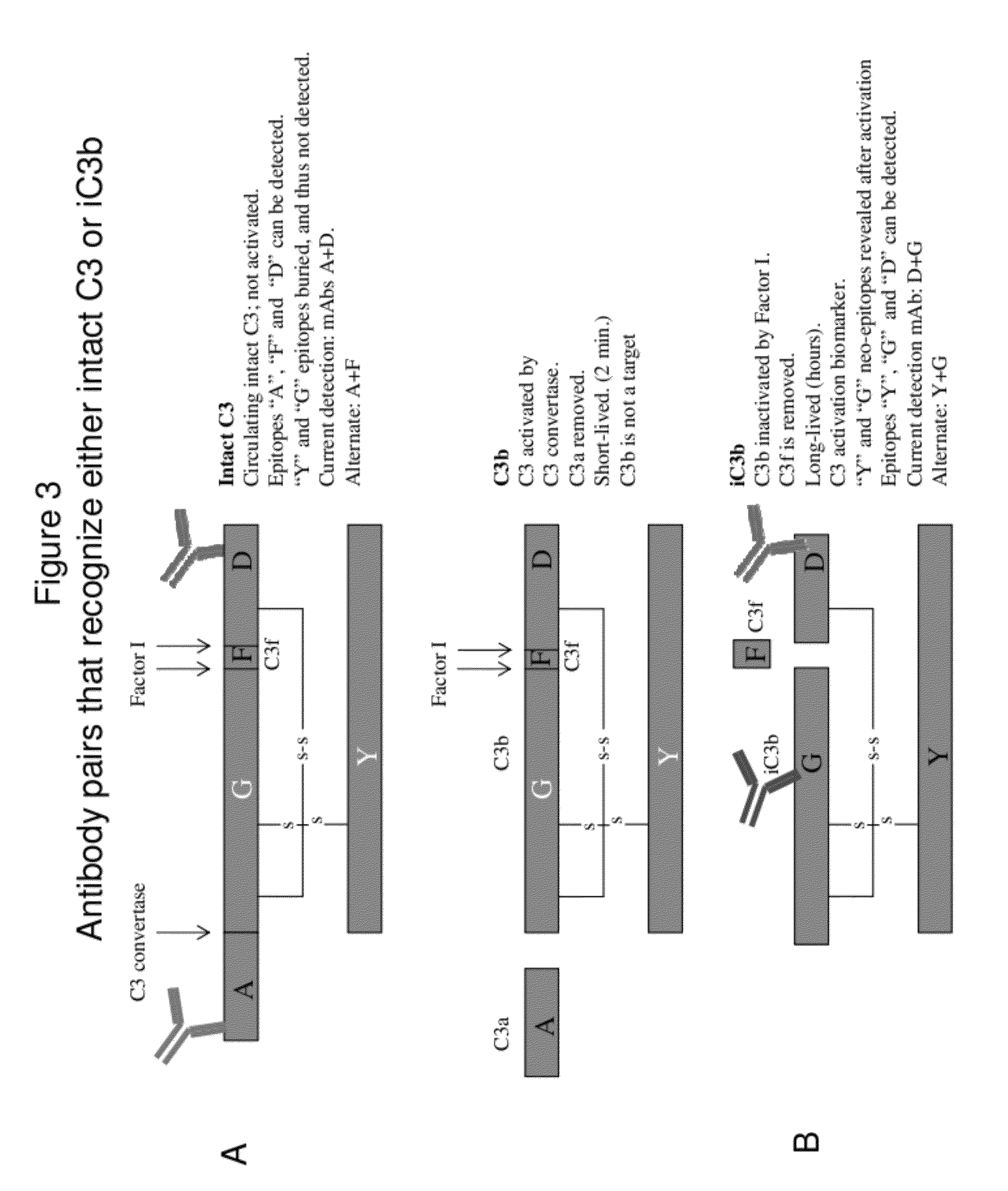
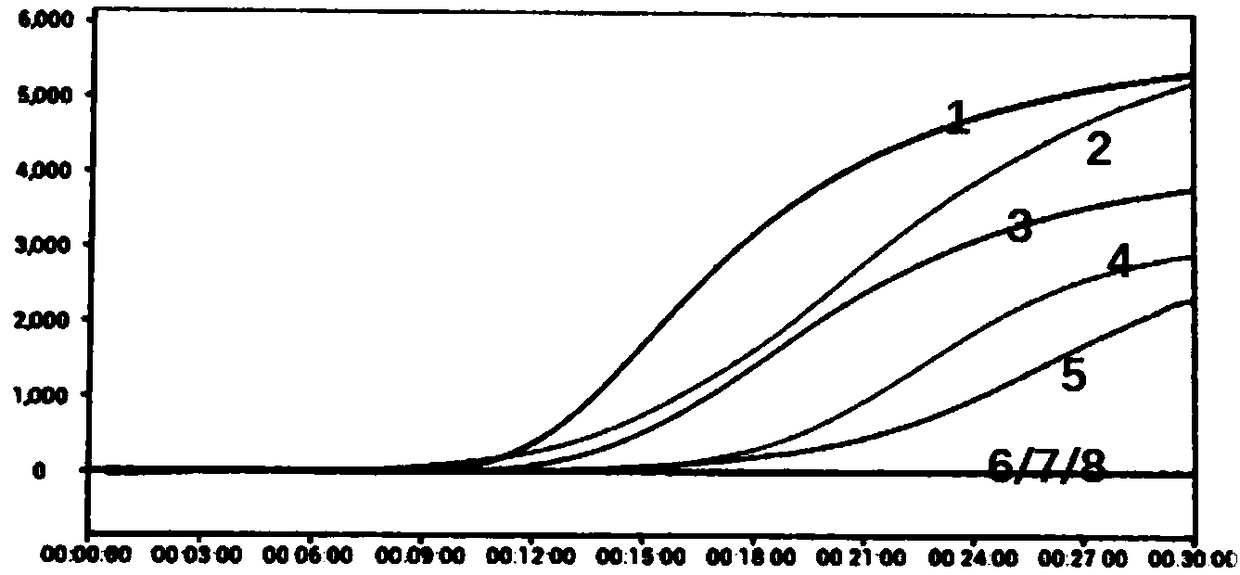
Detecting complement activation
ActiveUS20120315266A1Minimizing spontaneous complement activationReduce materialSenses disorderNervous disorderActivation methodCross reactive antibodies
Methods of detecting complement activation including steps of detecting in a sample from a subject a level of iC3b wherein the detecting involves specific interaction between the iC3b and a non-cross-reactive antibody thereto, comparing the detected level with a reference level, which reference level is within a range of about 10 ng / ml to about 5,000 ng / ml, wherein determination that the detected level is above the reference level indicates that the subject is suffering from or susceptible to undesirable and / or pathologic complement activation, and administering treatment to treat undesired complement activation if the detected level is above the reference level. Other methods of detecting complement activation with or without measuring iC3b are also provided.
Owner:KYPHA
Kit for detecting aldehyde dehydrogenase 2 gene polymorphism and amplification method and detection method thereof
InactiveCN103184268AImprove detection efficiencyReduce testing costsMicrobiological testing/measurementGlycerolPolymerase L
The present invention discloses a kit for detecting aldehyde dehydrogenase 2(ALDH2) gene polymorphism by using the single-tube bidirectional allele-specific amplification technology combined with the SNP sensitivity molecular switch technology, and an amplification method and a detection method thereof. The kit genotypes the Glu487Lys(rs671) site on the aldehyde dehydrogenase 2. The kit includes amplification buffer, polymerase, cell lysis buffer and glycerol, and can complete the genotyping for the SNP site in one PCR reaction so as to understand the genotype of the aldehyde dehydrogenase 2 subject and the product activity thereof.
Owner:UNION STEMCELL & GENE ENG +1
Detection method of chromosome copy number variation
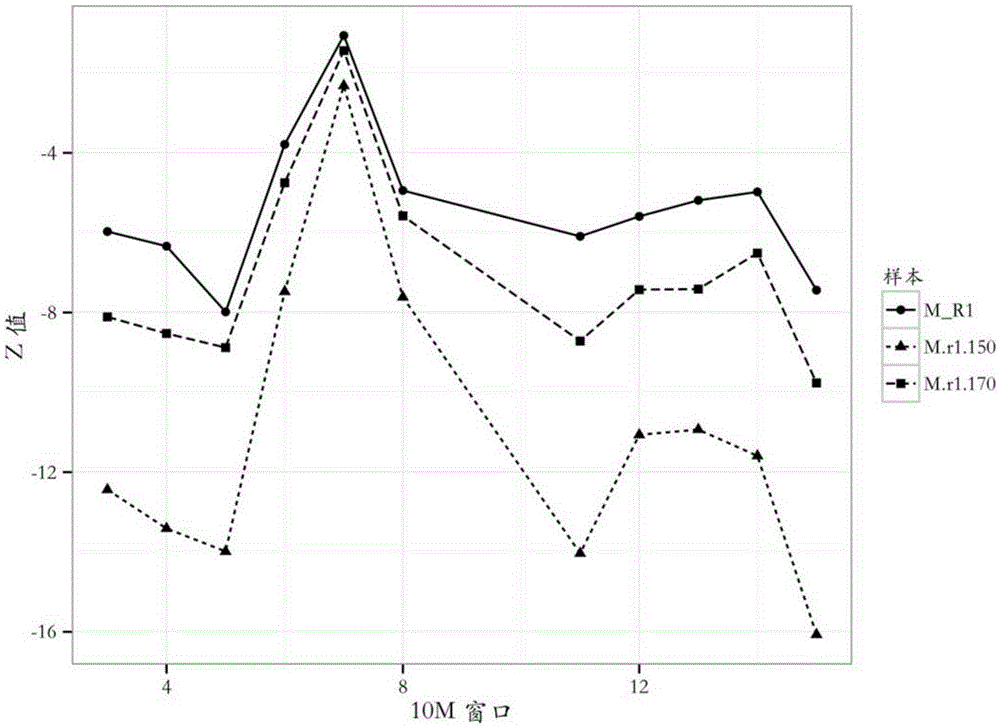
InactiveCN105349678AOvercoming the pitfalls of noninvasive testingThe result is accurateMicrobiological testing/measurementReference genome sequenceAbsolute volume
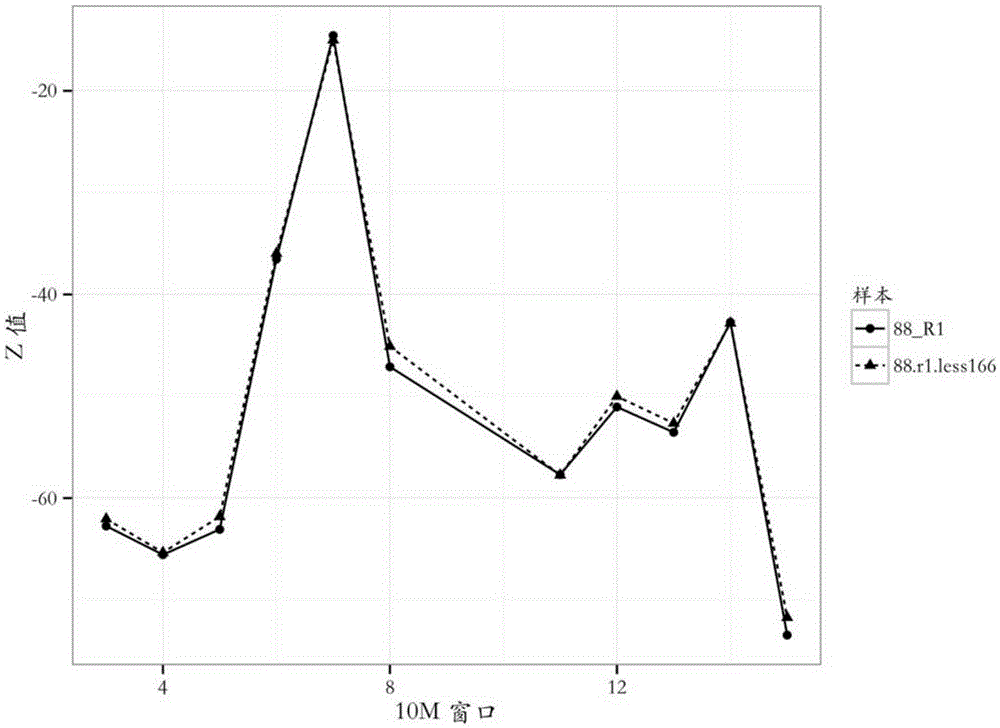
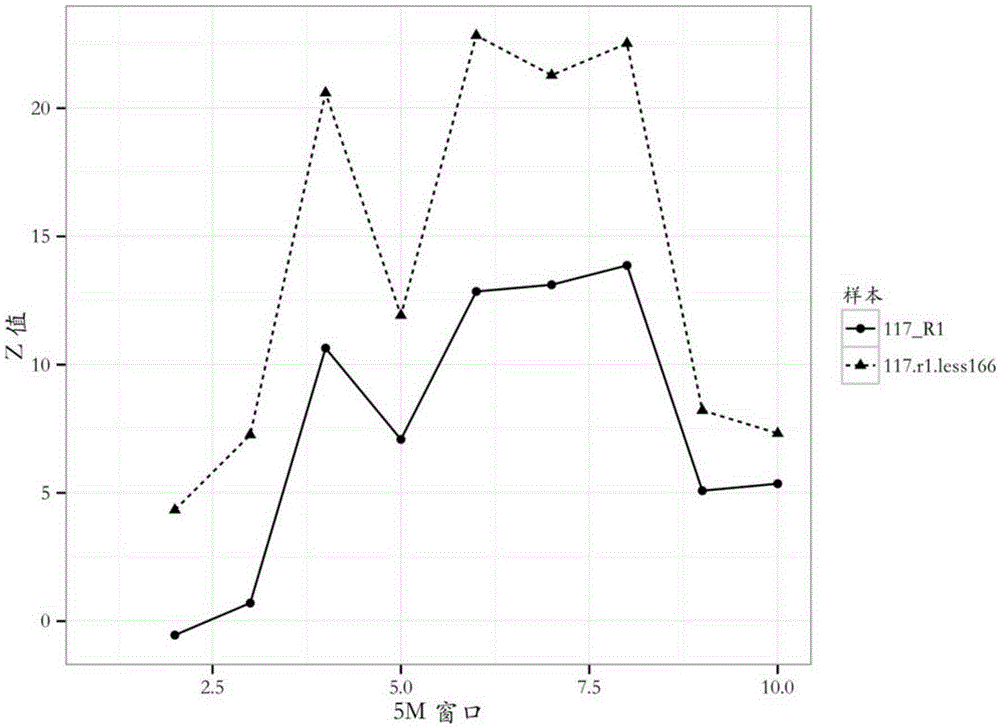
The invention provides a detection method of chromosome copy number variation. The detection method comprises the following steps: preparing cell-free DNA from body fluid of a parent body with an appendage and taking the cell-free DNA as a sample to be detected; preparing cell-free DNA from body fluid of a normal parent body without appendages and taking the cell-free DNA as a check sample; comparing to a reference genome sequence after all sequencing is finished; counting matching number and calculating z value; determining that cnv originates from the appendage if the length of the sample to be detected is smaller than the absolute value of the z value of a DNA sequence of N bp and greater than the absolute value of the z value of all DNA sequences of the sample to be detected in the same window; and determining that the cnv originates from the parent body if the length of the sample to be detected is not smaller than the absolute value of the z value of the DNA sequence of N bp and not greater than the absolute value of the z value of all the DNA sequences of the sample to be detected in the same window. By the detection method, whether the cnv originates from the parent body or the appendage can be judged effectively, accuracy of the result of existing non-invasive detection can be the same with that of the result of the traditional intrusion detection, loss caused by false positive is avoided effectively, and meanwhile, the detection method is easy to operate and has application value.
Owner:SHANGHAI MAJORBIO BIO PHARM TECH
Quantitative detection method of gene rare mutation
InactiveCN101381766ARealize real-time detectionAvoid pollutionMicrobiological testing/measurementFluorescence/phosphorescenceNucleotidePyrophosphate
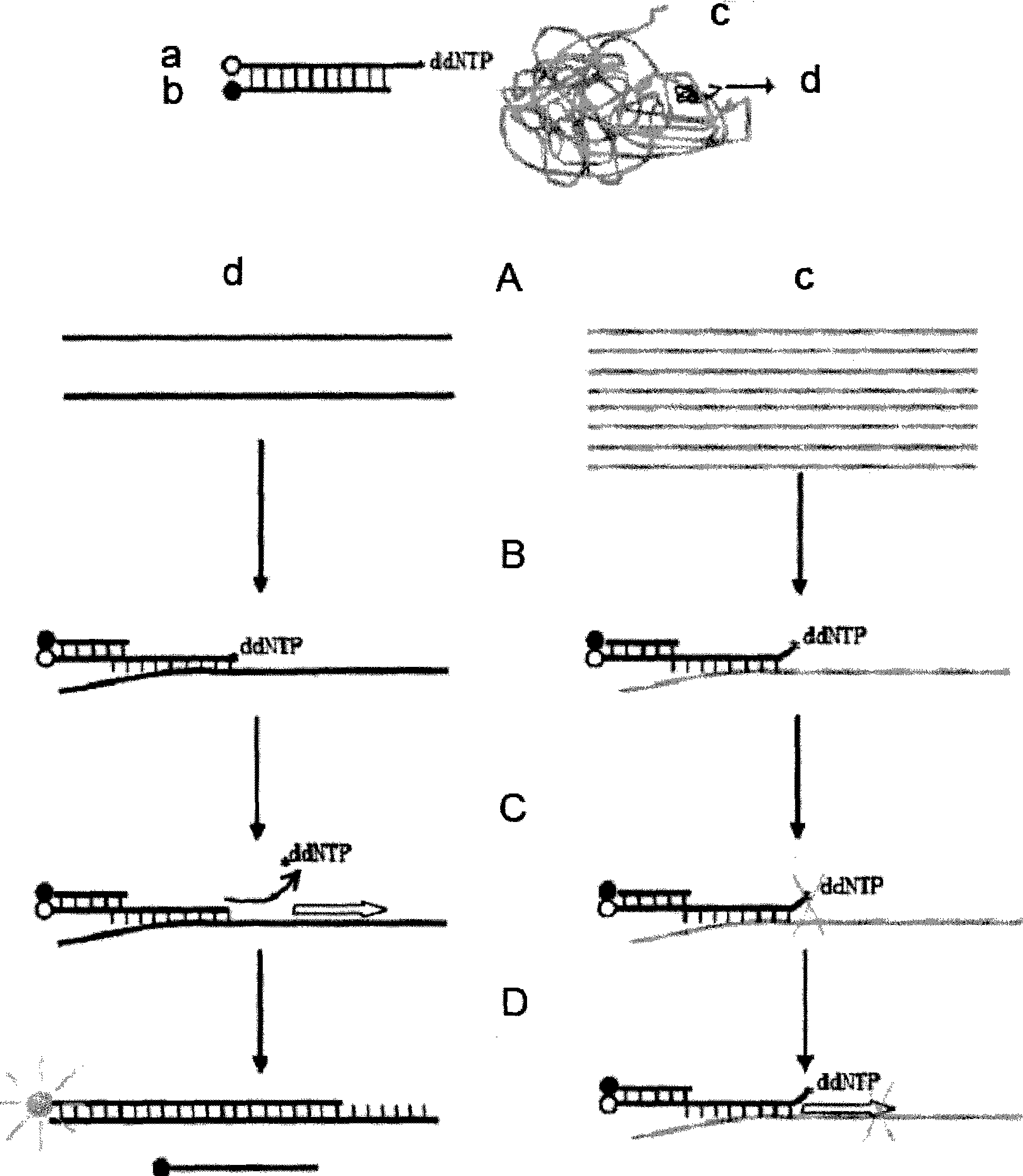
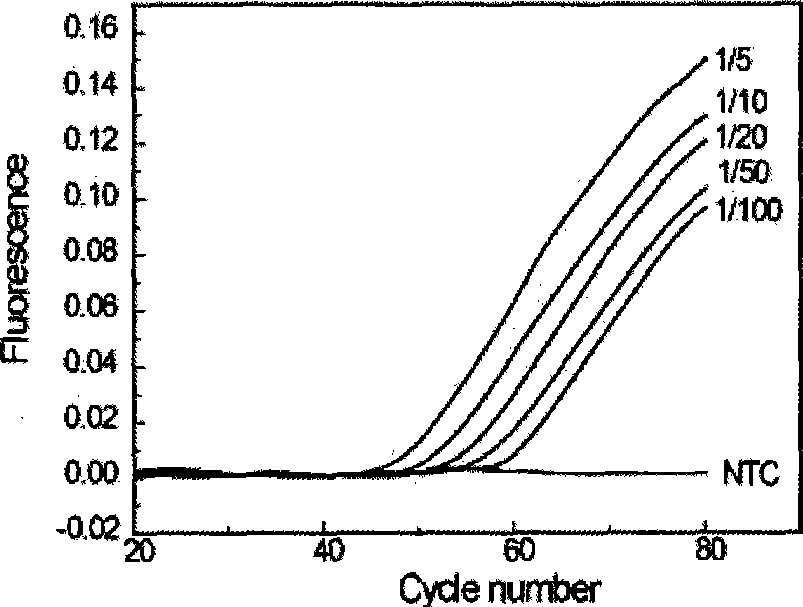
The invention relates to a genetic detection method, in particular to a quantitative detection method for unusual genetic mutation. The quantitative detection method for unusual genetic mutation comprises the following steps: firstly, corresponding primer sequences are designed and prepared according to the unusual genetic mutation required for quantitative detection, wherein the 3' end of a primer is provided with a mutant site - special nucleotide; except for the final site of the nucleotide, all the other sequences can be synthesized by commercial means; and the final site of the nucleotide is modified nucleotide, so as to make the primer incapable of extending in the general PCR reaction; and the modified nucleotide is added to the tail of the primer; and secondly, in a reaction system which contains special DNA polymerase and pyrophosphate, the primer designed and prepared in the first step is adopted for amplifying the unusual genetic mutation for quantitative detection, and a real-time fluorescent PCR instrument is utilized for quantitative detection; and the quantitative detection information of the unusual genetic mutation can be acquired through analysis of an amplifying curve recorded by the real-time fluorescent PCR instrument.
Owner:XIAMEN UNIV
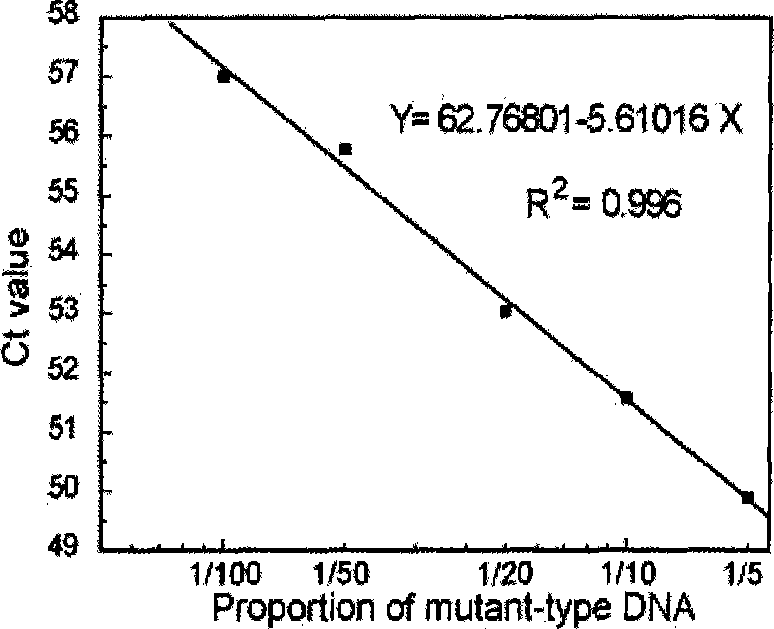
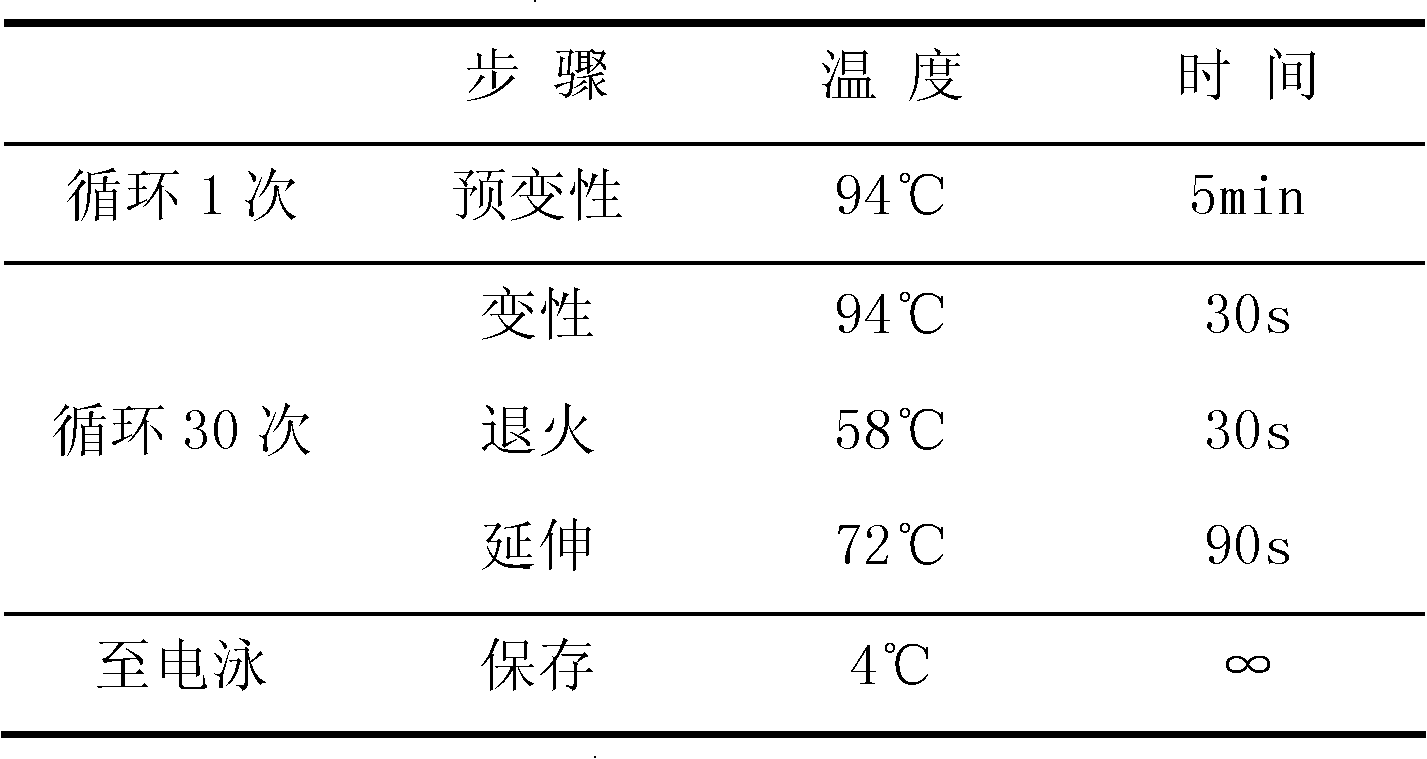
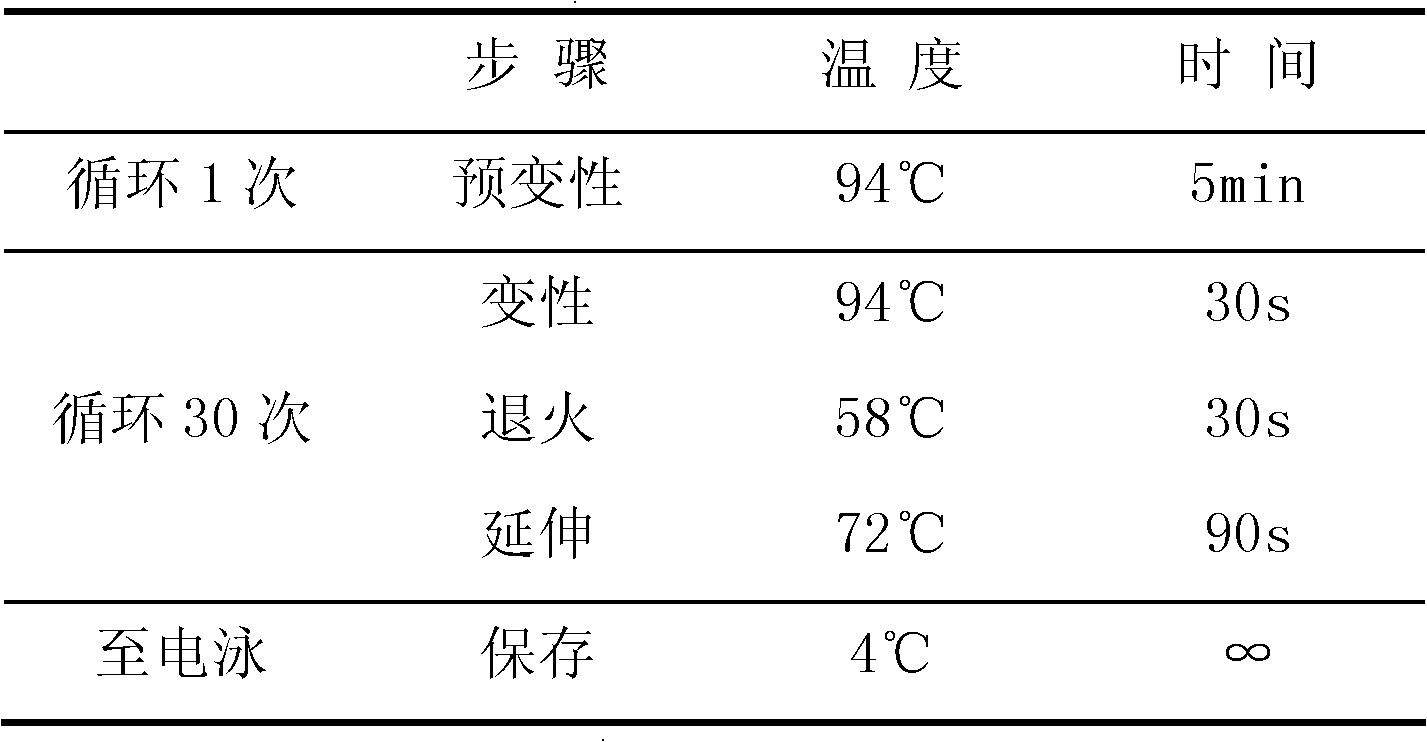
CYP2C19 gene detection kit, amplification method and detection method
InactiveCN103184265AImprove detection efficiencyReduce testing costsMicrobiological testing/measurementWild typePolymerase L
The present invention discloses a gene detection kit using the multiplex PCR technology combined with the SNP sensitive molecular switch technology for genotyping of cytochrome 4502C19 gene, an amplification method and a detection method. Three common polymorphic sites on the CYP2C19 gene significantly changing the product activity are genotyped by the kit, wherein the three common polymorphic sites include: rs4244285SNP site, rs4986893 site and rs12248560 site. The kit includes a wild-type 2*PCR buffer, a 2*mutant amplification buffer, and a polymerase. The two buffers respectively includes a sequence-specific primer and an internal reference primer corresponding to SNP wild and mutant phenotype, and can complete genotyping for the three SNP sites in two multiplex PCR reactions, and thus the CYP2C19 gene is genotyped, and molecular biological evidence is provided for the gene-expressed enzyme activity prediction.
Owner:UNION STEMCELL & GENE ENG +1
Septin 9 gene methylation detection method and kit
PendingCN110305940AHigh detection sensitivityGood specificityMicrobiological testing/measurementFluorescenceEnzyme
The invention provides a Septin 9 gene methylation detection kit. The kit comprises at least one of the following three groups of specific primers and probes aiming at the detection of Septin 9-v2 exon methylation, SEQ ID NO.5-SEQ ID NO.7, SEQ ID NO.8-SEQ ID NO.10, and SEQ ID NO.11-SEQ ID.13. The invention further provides a Septin 9 gene methylation detection method and a sample pretreatment method. The Septin 9 gene methylation detection method comprises the following steps of TET enzyme oxidation; pyridine borane reaction; and multiple fluorescence PCR reaction. The kit and the detection method have the advantages of high detection sensitivity, good specificity and high detection flux.
Owner:SUREXAM BIO TECH
Novel coronavirus detection method and kit
PendingCN111334615AEasy to prepareAvoid pollutionMicrobiological testing/measurementMicroorganism based processesNucleic acid sequencingFluorescent pcr
The invention provides a novel coronavirus detection method and kit. The method adopts a fluorescent nucleic acid amplification method with multiple pairs of primers and multiple probes, and can realize detection of multiple target nucleic acid sequences in a single tube. The novel coronavirus ORF1ab gene, N gene and E gene are used as target sequences to design specific primers and TaqMan probes,and the novel coronavirus is detected through multi-channel fluorescent PCR amplification. The design of the primers and probes, a reaction system and an amplification program are optimized, the fluorescence detection can be completed in 70 minutes, and the sensitivity of the kit reaches 200 copies / mL.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
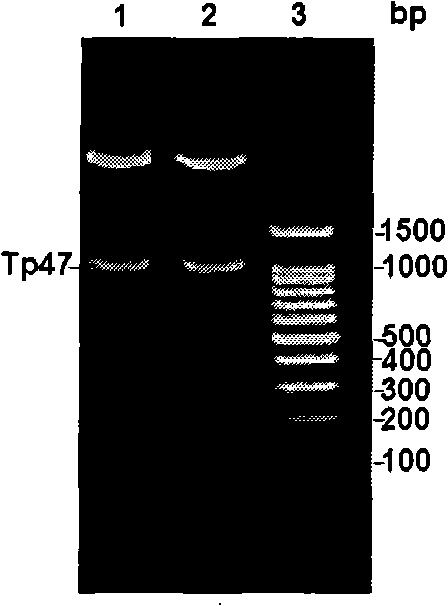
Syphilis spirochete membrane antigen with shorten expression and uses thereof
InactiveCN101293919ASignificant antigen reactivityAntigen reactivity verificationBacteriaDepsipeptidesAntigenSpiroplasma
The invention discloses a DNA sequence expressing a truncated treponema pallidum membrane antigen and an amino acid sequence The membrane antigen is removed of the part having high homology with human fibronectin, so as to avoid false positive and improve the specificity of the serological test for diagnosis of treponema pallidum infection. The invention also discloses the application of the membrane antigen in preparing diagnostic reagents for detecting treponema pallidum infection.
Owner:ARMY MEDICAL UNIV
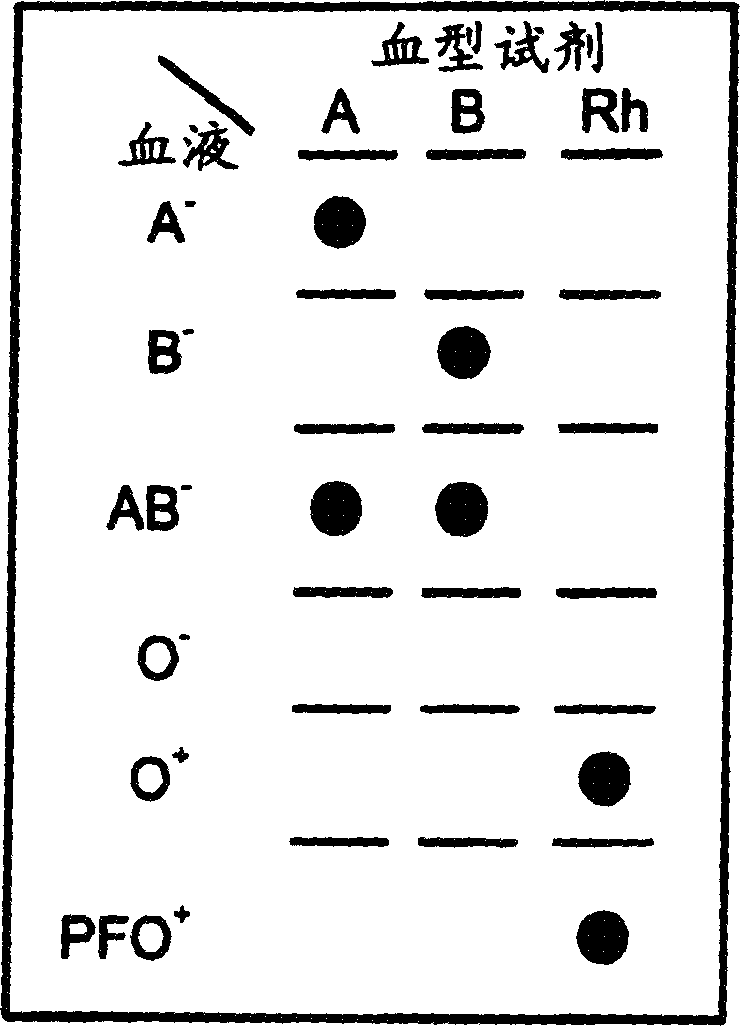
Particle agglutination detection method and device
InactiveCN1849514AEasy to useAvoid False Positive ResultsMicrobiological testing/measurementBiological testingCrossmatching bloodBlood typing
A method for detecting and / or observing particle agglutination comprising: placing a volume of particle suspension on a filter constructed to allow passage of individual particles; placing a volume of solution or suspension containing an agglutination reagent on the particle The location of the suspension; optionally placing the washing solution at the location of the agglutinating reagent; and observing the surface of the location for the presence of particles, which presence indicates that particle agglutination has occurred. Also provided are methods for detecting agglutination in general and hemagglutination, eg, for blood typing and cross-matching in particular. The method consists of continuous vertical addition of whole blood, blood typing reagents, and detergent to the filter. In the case of hemagglutination, colored, preferably red or reddish spots appear after washing. Devices and kits based on the present invention are also claimed which facilitate blood typing and cross matching in a non-laboratory environment without the need for laboratory instruments.
Owner:INVERNESS SWITZERLAND GMBH
Tumor cell microsatellite instable state detection system
ActiveCN108374046AReduce the number of amplification cyclesReduce dosageMicrobiological testing/measurementDNA/RNA fragmentationWilms' tumorQuantitative fluorescence
The invention discloses a tumor cell microsatellite instable state detection system, and relates to quantitative fluorescence PCR (polymerase chain reaction) amplification technologies and capillary electrophoresis detection technologies. The tumor cell microsatellite instable state detection system has the advantages that six mononucleotide repeat sites NR-21, NR-24, NR-27, MONO-27, BAT-25 and BAT-26 can be amplified by the aid of the tumor cell microsatellite instable state detection system, and sites BrafV600E, UGT1A1*6 and UGT1A1*28 can be typed by the aid of the tumor cell microsatelliteinstable state detection system; reference can be provided to checking Lynch syndromes and using irinotecan medicines by detection results on the basis that MSI (microsatellite instable) states are determined. The invention further provides a detection reagent kit designed according to the tumor cell microsatellite instable state detection system.
Owner:BEIJING MICROREAD GENE TECH
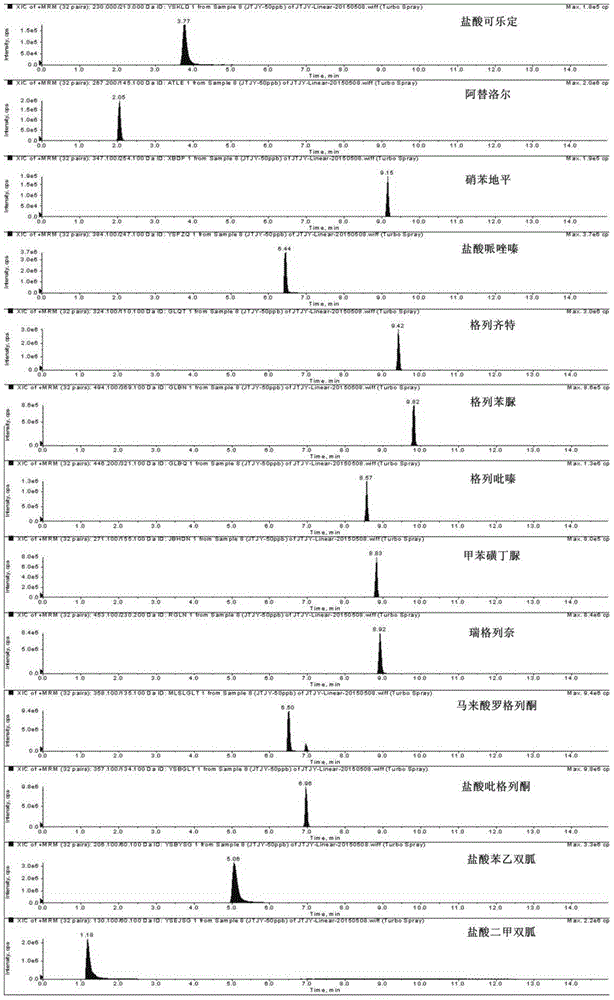
Method for determination of illegally added substances in traditional Chinese medicines and health-care products
The invention discloses a method for determination of illegally added substances in traditional Chinese medicines and health-care products; with use of high performance liquid chromatography-tandem quadrupole linear ion trap mass spectrometry, the method which is capable of simultaneously qualitative and quantitative detection (multiple reaction monitoring sMRM-information dependent acquisition IDA-enhanced product ion scanning EPI detection) of 13 kinds of illegally added hypoglycemic hypotensive chemical drugs such as clonidine hydrochloride and gliclazide in the traditional Chinese medicines and the health-care products is developed. And through one-time sampling detection, not only can a quantitative result obtained, but also occurrence of a false positive result is effectively avoided through qualitative database screening. The establishment of the method provides a technical support for enacting detection standards of the illegally added hypoglycemic hypotensive chemical drugs in the traditional Chinese medicine and the health-care products.
Owner:SHANDONG ANALYSIS & TEST CENT
Kit for detecting polymorphism of interleukin 28B gene by utilizing fluorescence PCR (Polymerase Chain Reaction) technology
ActiveCN103667514AStrong specificityImprove accuracyMicrobiological testing/measurementAntiviral drugInterleukin 28B
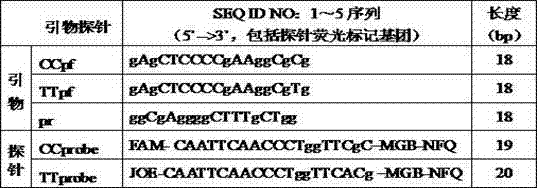
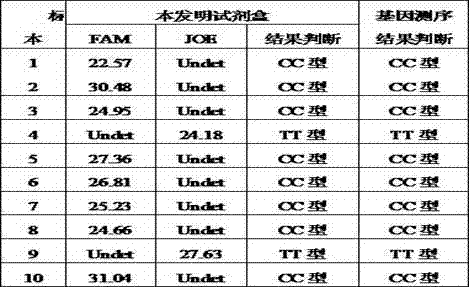
The invention relates to a kit for detecting the polymorphism of an interleukin 28B (IL28B) gene by utilizing a fluorescence PCR (Polymerase Chain Reaction) technology. The kit is characterized in that three primers are adopted in a single reaction tube to augment sequences of CC type and TT type of an rs12979860 gene, and the CC type and the TT type of the gene are detected by two MGB fluorescence probes with different wavelengths. The kit is simple in operation and accurate and reliable in detection result, can be applied to detection of the polymorphism of the IL28B rs12979860 gene of a patient with clinical hepatitis C and further predicts the treatment effect of antiviral drugs.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
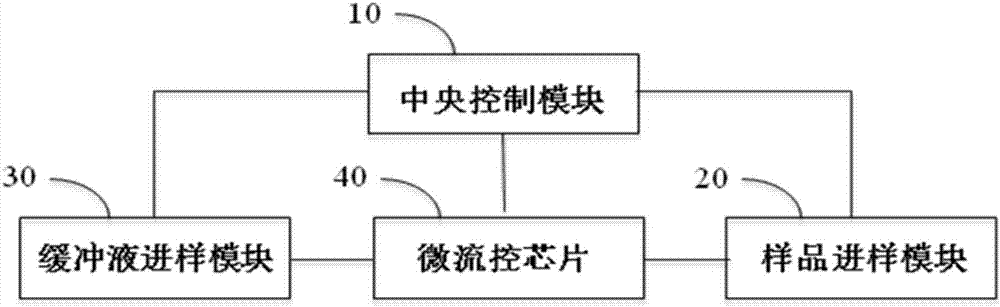
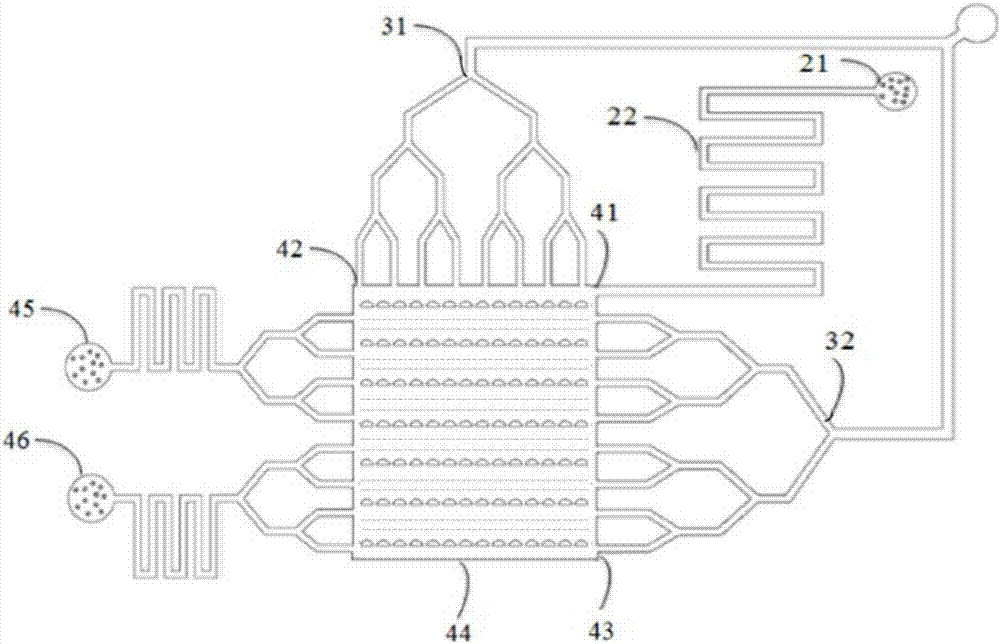
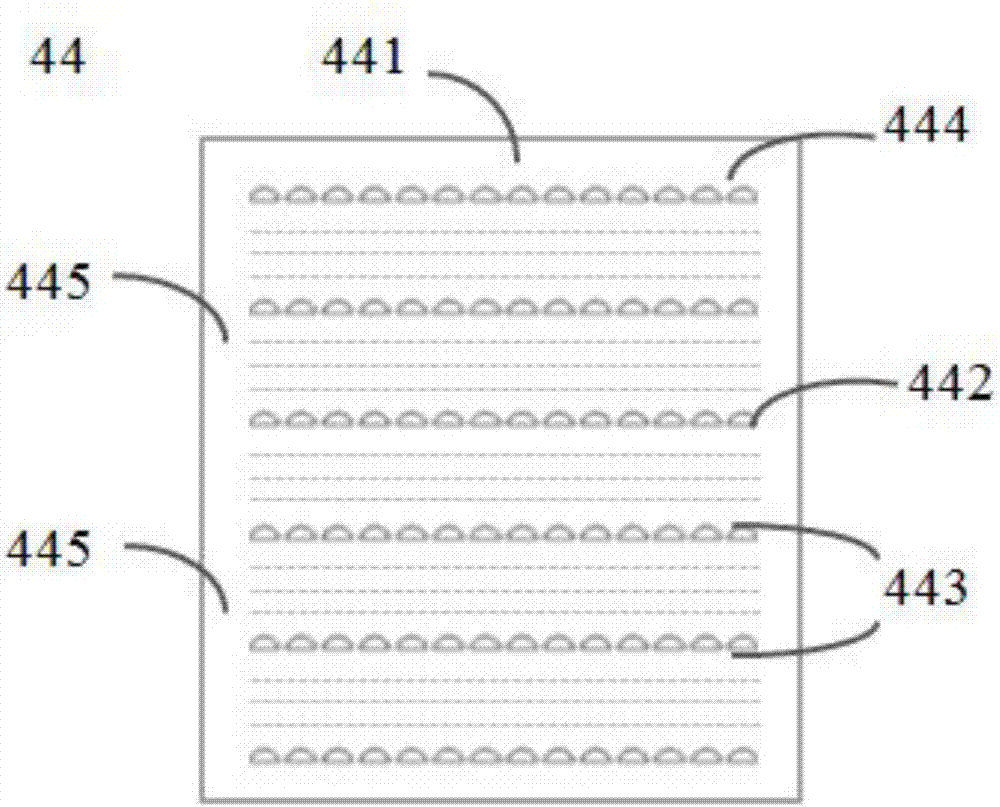
Circulation tumor cell automatic capturing micro-fluidic chip and automatic capturing method thereof
ActiveCN107400623AReduce human interventionAchieve continuous captureBioreactor/fermenter combinationsBiological substance pretreatmentsContinuous flowNon targeted
The invention discloses a circulation tumor cell automatic capturing micro-fluidic chip and an automatic capturing method thereof. The circulation tumor cell automatic capturing micro-fluidic chip comprises a sample feeding module, a sample separation module and a buffer liquid sample feeding module, wherein the sample feeding unit is connected with the sample separation module; the sample feeding unit is used for feeding a sample to be detected into the micro-fluidic chip; the buffer liquid sample feeding module comprises an oscillation flow unit and a continuous flow unit; the oscillation flow unit and the continuous flow unit are respectively connected with the micro-fluidic chip; the sample separation unit is used for separating cells of the sample to be detected; a target cell collection unit is used for collecting target cells obtained through separation; the micro-fluidic chip comprises a sample separation unit, a target cell collection unit and a non-target cell collection unit; and the non-target cell collection unit is used for collecting non-target cells and generated waste liquids obtained after separation. The circulation tumor cell automatic capturing micro-fluidic chip is capable of achieving automatic separation of cells and is low in false positive rate.
Owner:SUREXAM BIO TECH
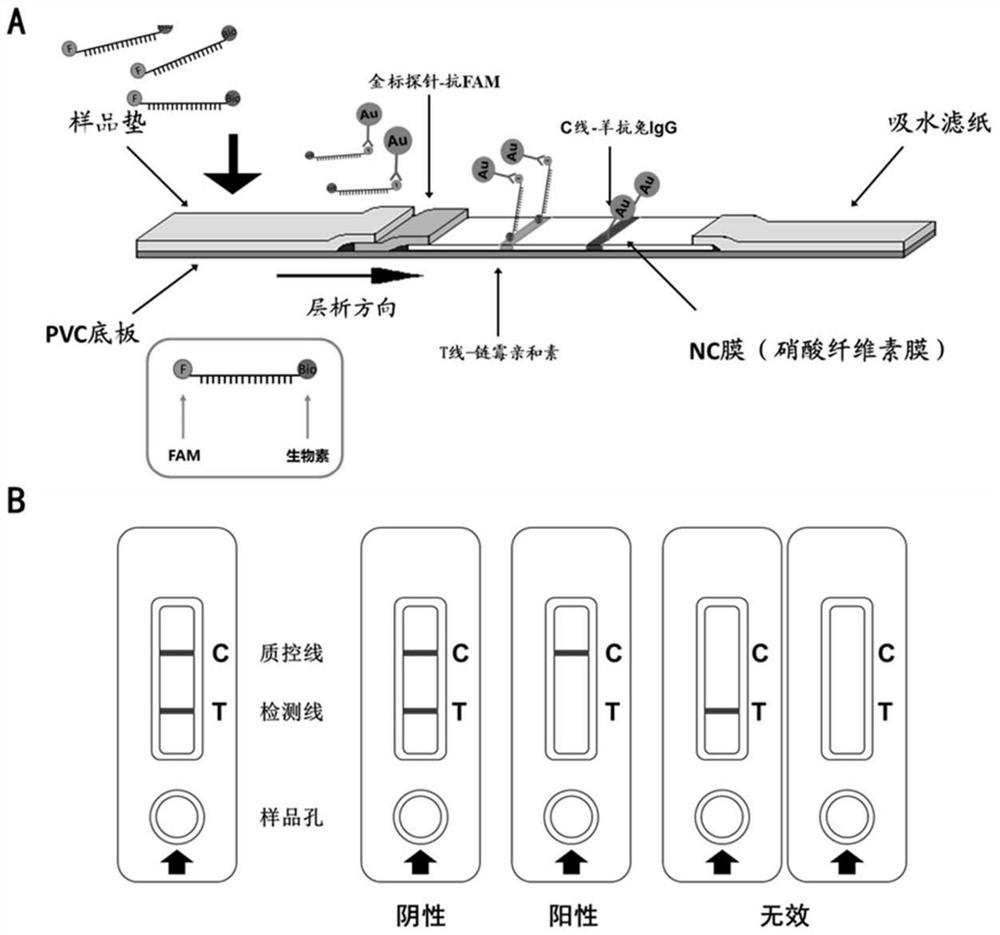
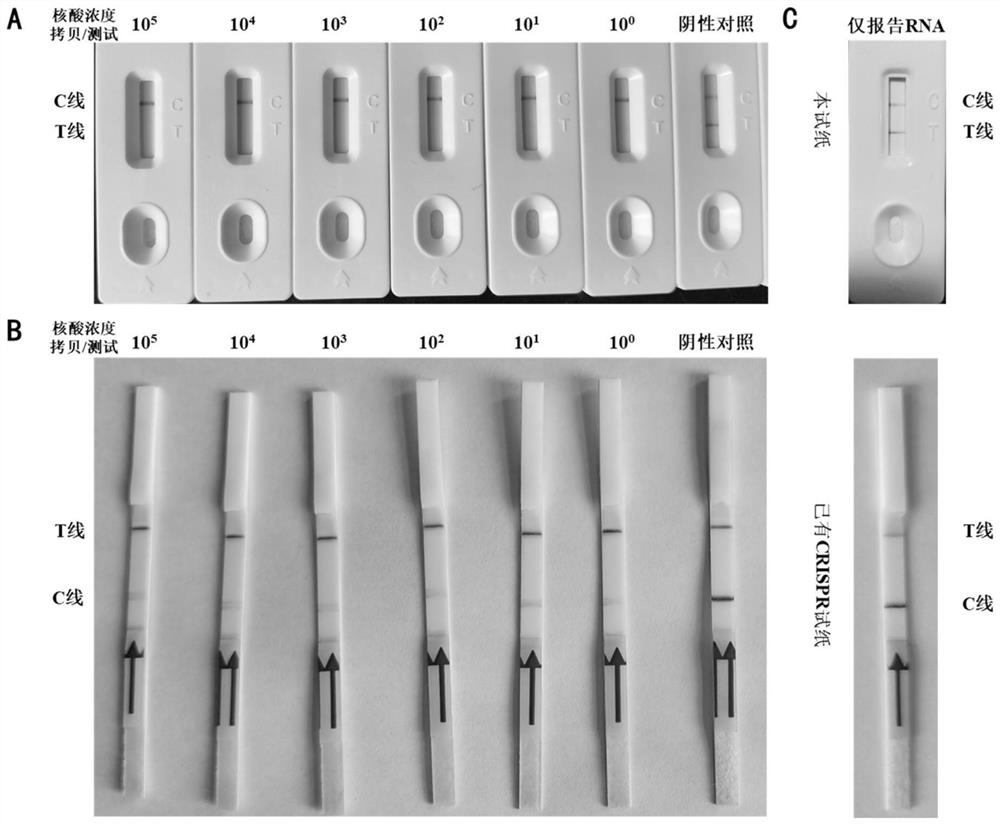
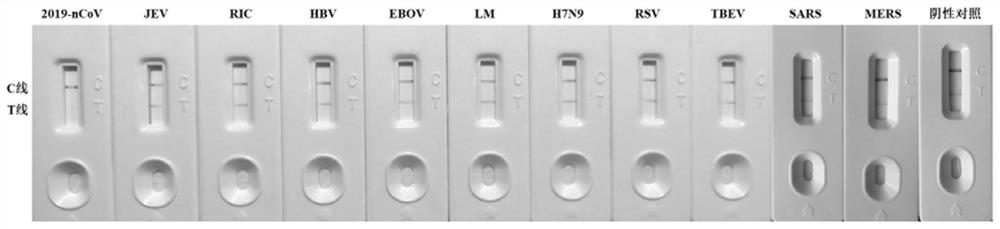
Line eliminating method immunochromatography test paper and application thereof in CRISPR nucleic acid test
ActiveCN111621598AGuaranteed detection sensitivityImprove accuracyHydrolasesMicrobiological testing/measurementNucleic acid detectionNucleic acid test
The invention discloses line eliminating method immunochromatography test paper and application thereof in a CRISPR nucleic acid test. A kit provided by the invention comprises: 1) immunochromatography test paper; and 2) a CRISPR reaction system, wherein in a sample flow direction of the immunochromatography test paper sequentially comprises a sample pad containing a colloidal gold labelled antibody, an NC membrane contain a T line and a C line and water-absorbent filter paper; the T line is formed by streptavidin; the C line is formed by a secondary antibody of the colloidal gold labelled antibody; the CRISPR reaction system comprises report RNA, crRNA and Cas protein; and the Cas protein is in a second-class type V or type VI CRISPR system. Experiments of the invention prove that the test paper in the kit can realize high-sensitivity, high-specificity and convenient detection of multiple specific nucleic acids (pathogens, gene mutation and drug-resistant mutation) by binding a CRISPRnucleic acid detection system.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Reagent kit for detecting high-risk human mammilla papillomavirus, as well as preparation and application thereof
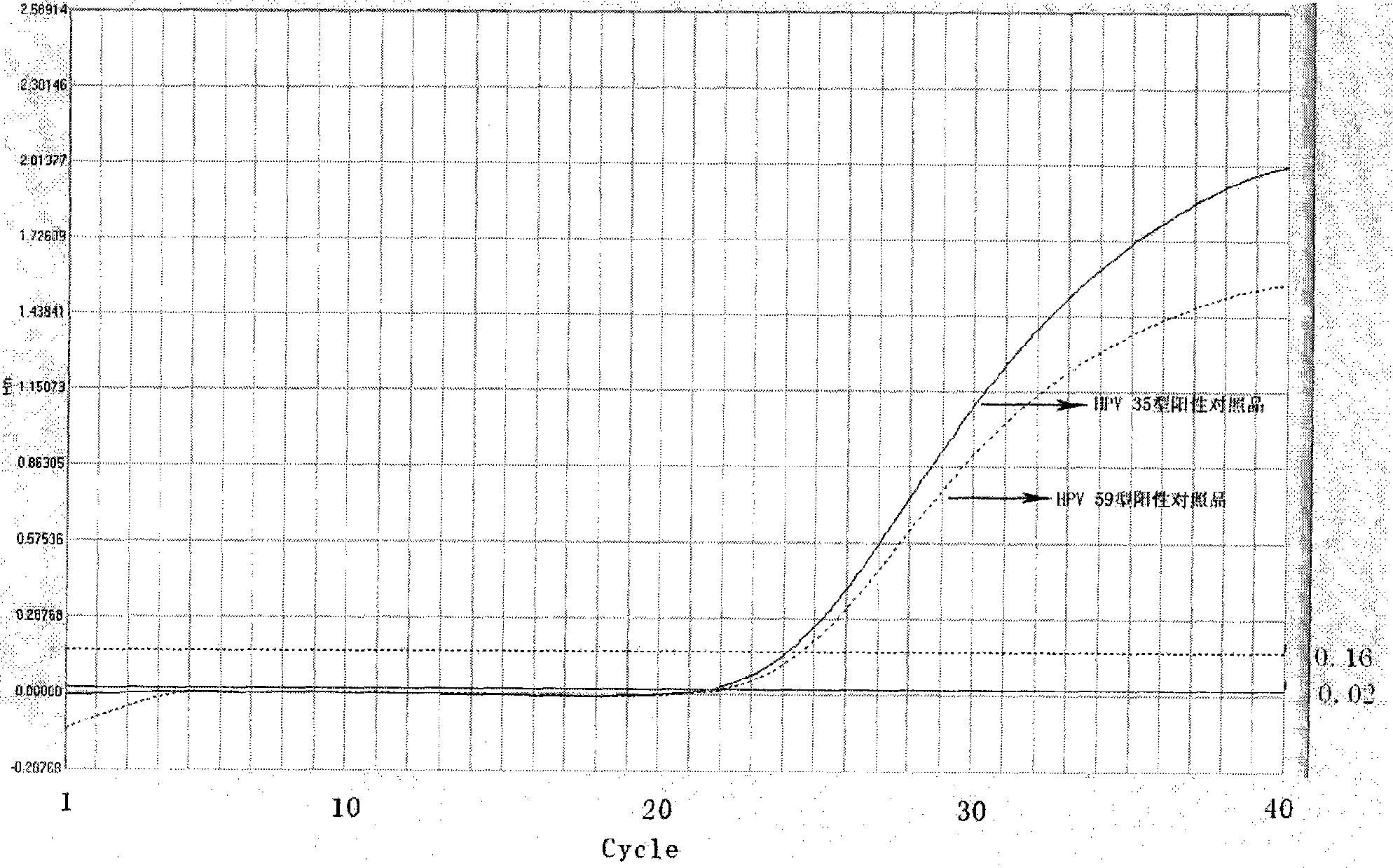
ActiveCN101503741AAvoid False Positive ResultsIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesFluorescent pcrBiology
The invention relates to a kit for auxiliary diagnosis of cervical carcinoma and high-grade cervical intraepithelial lesion, and discloses a kit for detecting high-risk HPV. The kit for detecting the high-risk HPV comprises a high-risk HPV nucleic acid fluorescent PCR detection mixture and Taq enzyme. The invention also discloses a method for preparing the kit for detecting the high-risk HPV and a method for using the same. The kit can be used for detecting the high-risk HPV and overcomes the defects of high false positive rate, low specificity, high cost and the like of typing detection of the high-risk HPV in the prior art.
Owner:SHANGHAI ZJ BIO TECH
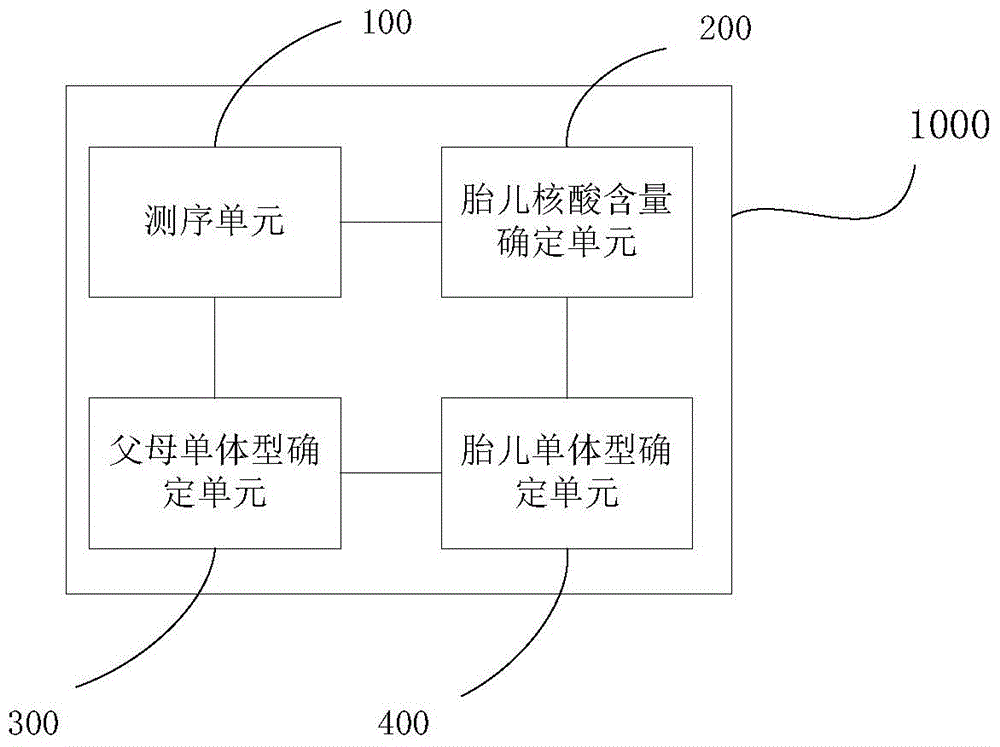
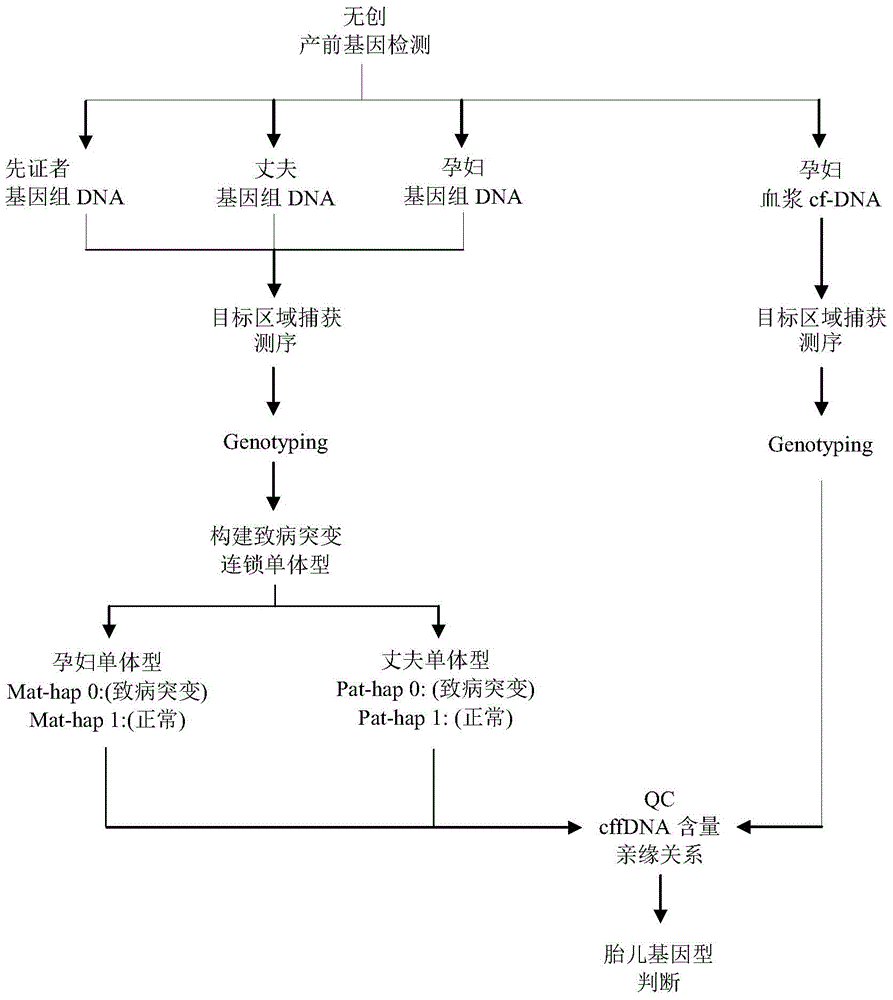
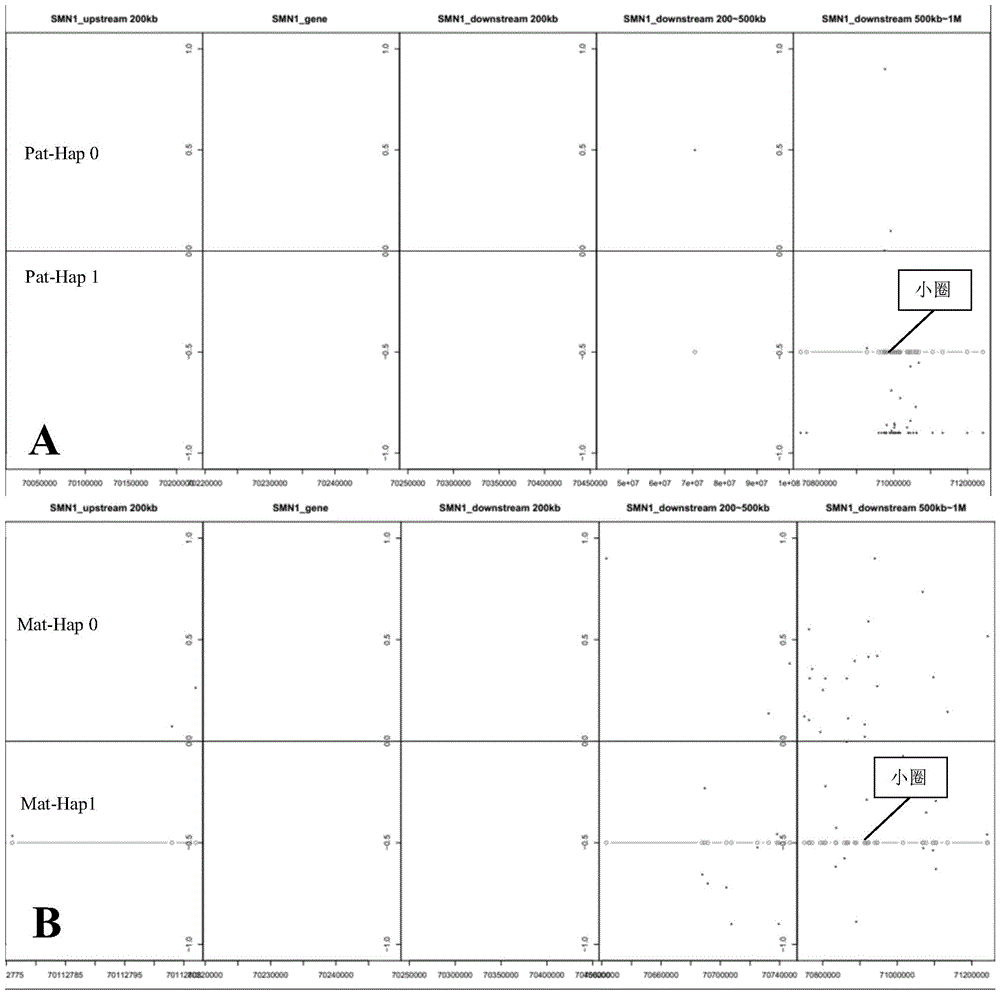
Method and apparatus for determining fetus target area haplotype
ActiveCN105648045AEfficient detectionAvoid False Positive ResultsBioreactor/fermenter combinationsBiological substance pretreatmentsHaplotypeBody fluid
The present invention provides a method and an apparatus for determining the fetus target area haplotype. The method comprises: carrying out sequence sequencing on the target area of free nucleic acids in a pregnant woman body fluid so as to obtain first sequencing data; carrying out sequence sequencing on the same target areas of fetus family members so as to obtain second sequencing data, third sequencing data and fourth sequencing data, wherein the second sequencing data is the sequencing data of the fetus mother, the third sequencing data is the sequencing data of the fetus father, and the fourth sequencing data is the sequencing data of a proband; based on the first sequencing data, the second sequencing data and the optionally selected sequencing data, determining the fetus nucleic acid content in the pregnant woman body fluid; based on the second sequencing data, the third sequencing data and the fourth sequencing data, respectively constructing the target area haplotype of the fetus mother and the target area haplotype of the fetus father; and based on the target area haplotype of the fetus mother, the target area haplotype of the fetus father, and the fetus nucleic acid content, constructing the fetus target area haplotype.
Owner:天津华大基因科技有限公司 +1
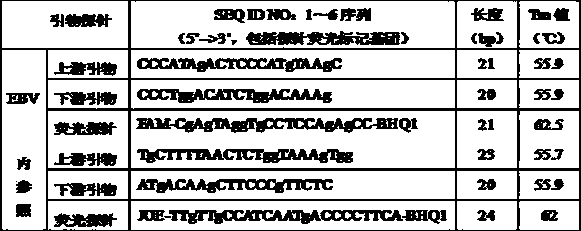
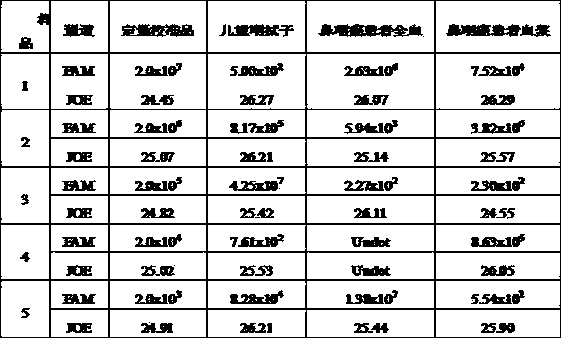
Reference-containing high-sensitivity fluorescent quantitative polymerase chain reaction (PCR) kit for Epstein-Barr virus
ActiveCN103642945AHigh detection sensitivityAvoid misdiagnosisMicrobiological testing/measurementFluoProbesViral nucleic acid
The invention relates to a kit for quantitative detection of an Epstein-Barr virus (EBV) nucleic acid by using a fluorescent polymerase chain reaction (PCR) technology. A pair of primers is used for amplifying an EBV specific nucleic acid sequence and EBVDNA is quantitatively detected through a fluorescence probe and meanwhile, an internal reference DNA is detected by using a human endogenous gene specificity primer and the fluorescence probe. By adopting the kit, existence of the EBV and internal reference nucleic acid is simultaneously detected by a single-tube double-wavelength fluorescent PCR technology, the EBVDNA in whole blood, plasma and a nasopharyngeal secretion sample can be quantitatively detected, and the whether a PCR inhibitor or template loss caused by a misoperation in detection exists in the sample is judged by the detection result of the internal reference nucleic acid. Thus, the kit is simple, convenient and fast in operation, can provide sensitive and accurate quantitative result, and can be widely applied to quantitative detection of clinical EBV virus infection.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
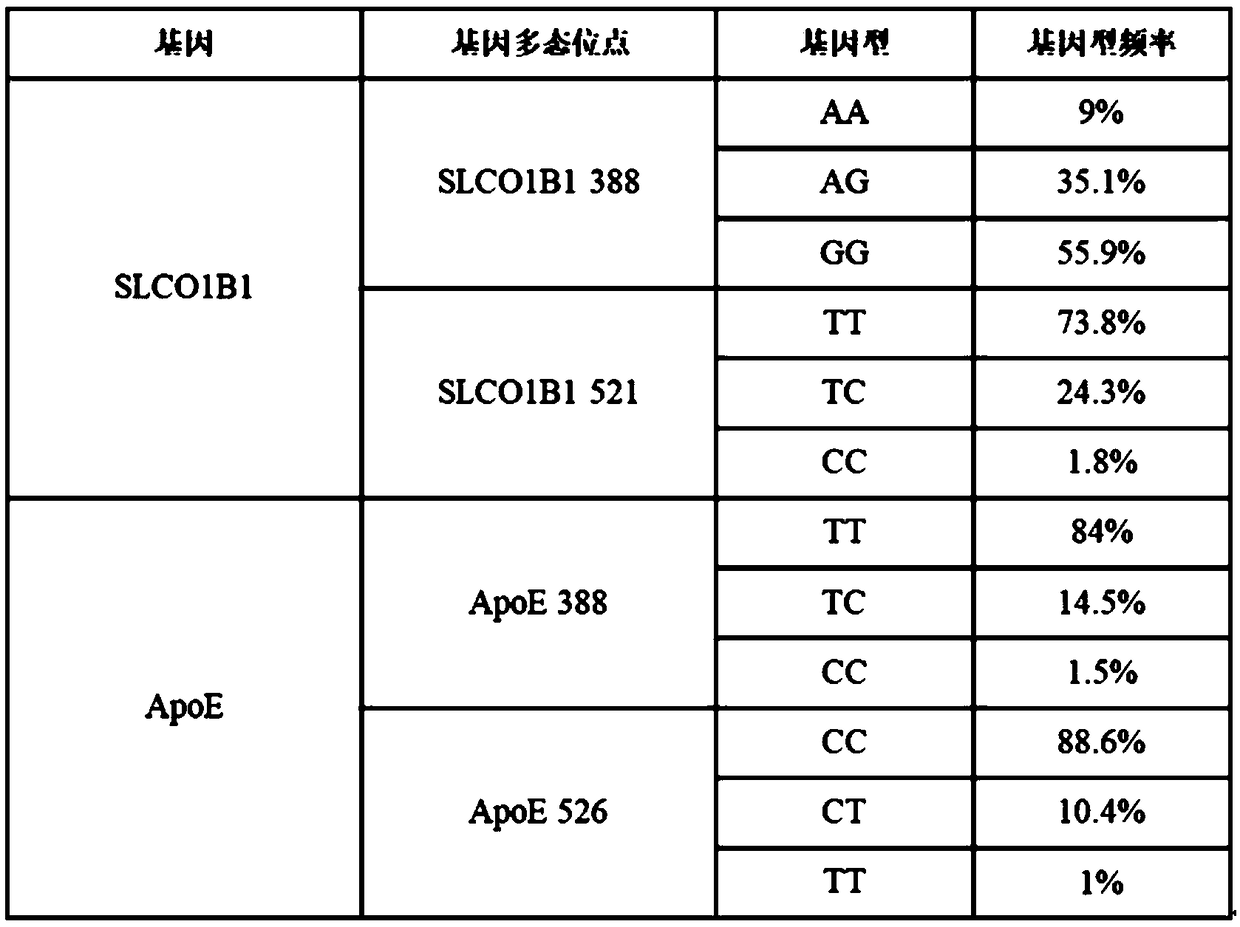
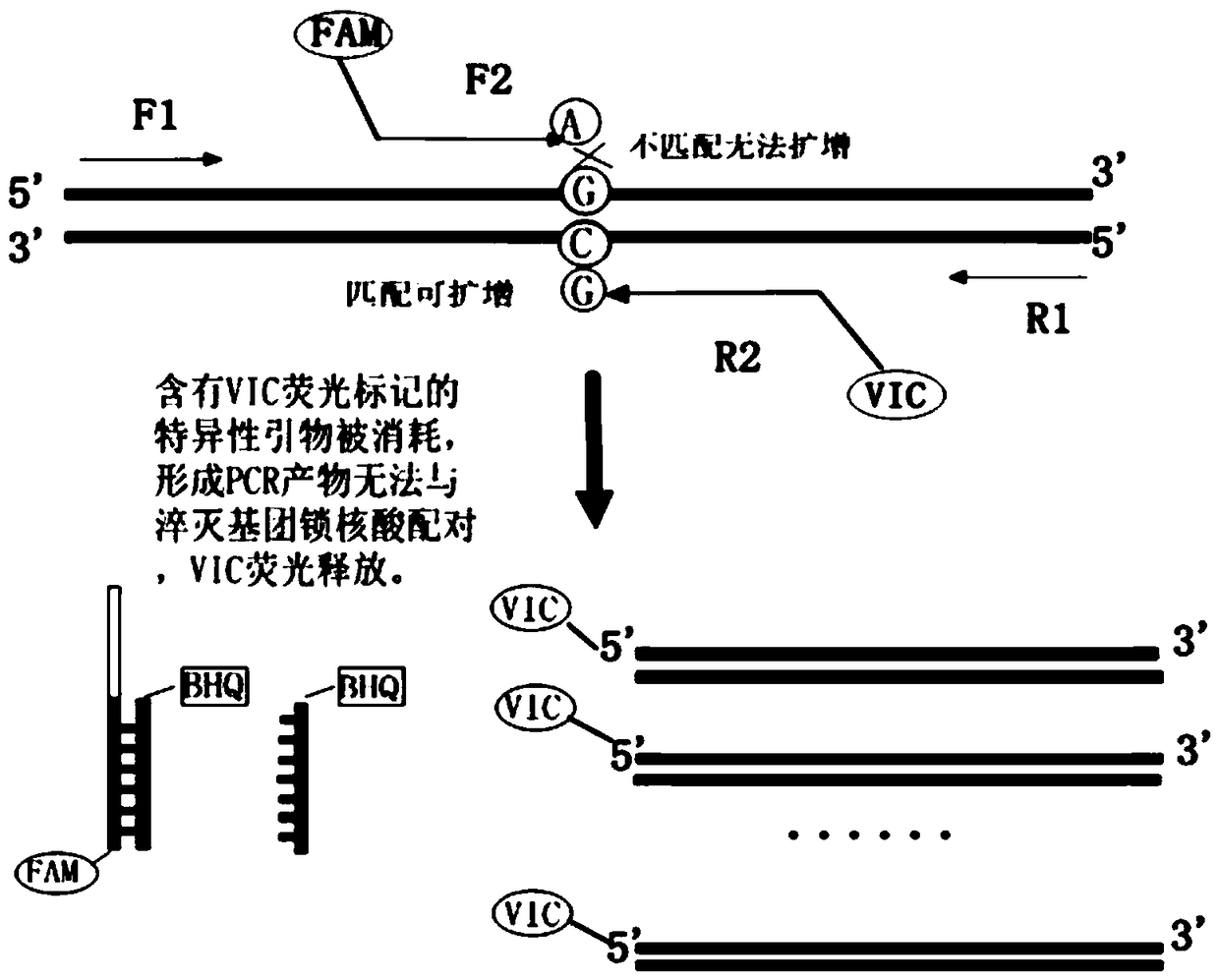
Human SLCO1B1 and ApoE gene polymorphism detection kit, and preparation method and application of same
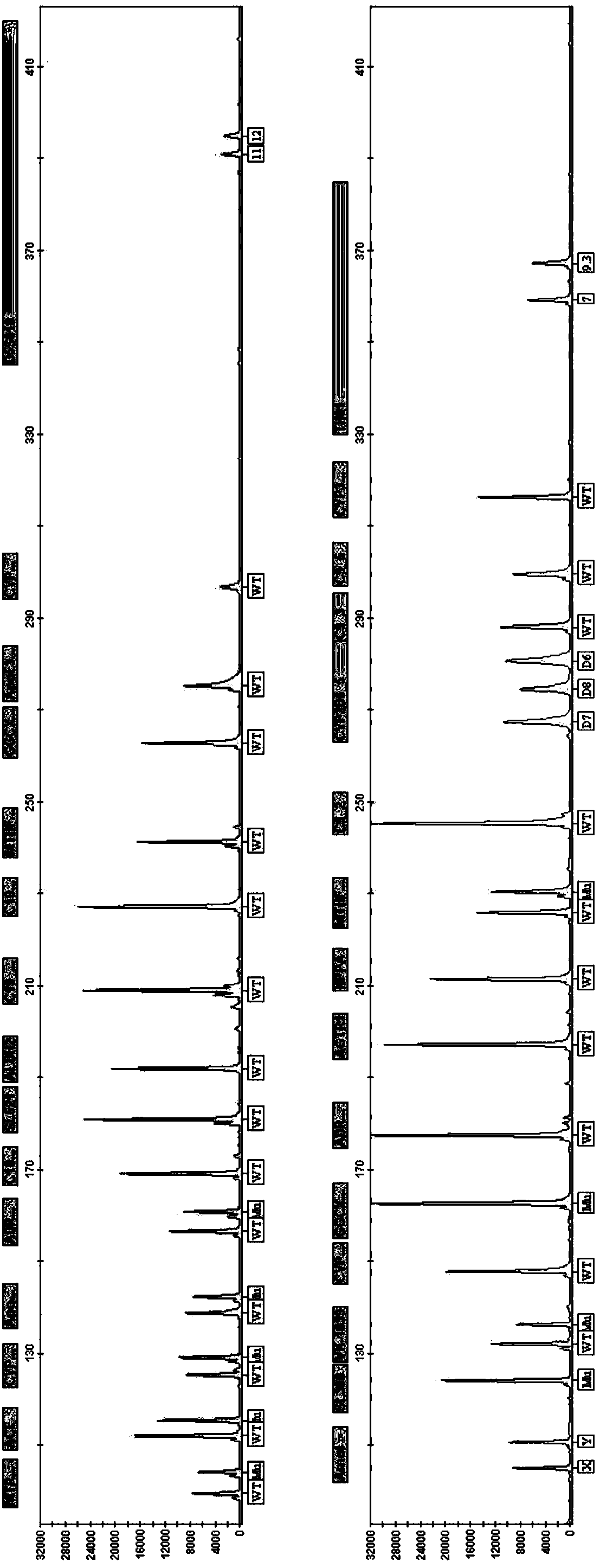
ActiveCN108998517AGuaranteed accuracyReduce concentrationMicrobiological testing/measurementDNA/RNA fragmentationSLCO1B1True positive rate
The invention belongs to the field of biotechnologies, and particularly relates to a human SLCO1B1 and ApoE gene polymorphism detection kit, and a preparation method and an application of same. The kit is composed of: a PCR premix reaction solution respectively used for detecting the rs2306283 loca of the SLCO1B1 gene, the rs4149056 loca of the SLCO1B1 gene, the rs429358 loca of the ApoE gene andthe rs7412 loca of the of the ApoE gene, and a positive reference substance and a negative reference substance. The PCR premix reaction solution includes specific primer sequence groups, probe groupsand a PCR reaction solution, which are used for amplifying the mentioned loci. The specific primer sequence groups are composed of a regular outer primer and specific ARMs primers having fluorescencetags. The kit is used for detecting the polymorphism of human SLCO1B1 and ApoE gene, is high in sensitivity and specificity, is easy to use and has reliable results, can complete the detection withinone hour, and is simple and objective in result interpretation.
Owner:WUHAN HEALTHCHART BIOLOGICAL TECH
Maleimide propionyl piperazine heptamethine cyanine salt fluorescence carrier and preparation method and application thereof
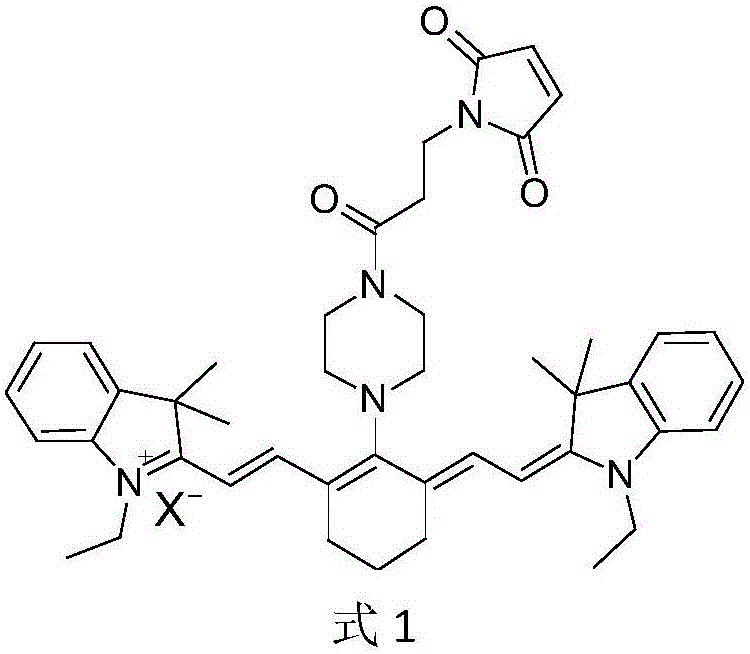
ActiveCN105884748AEasy to detectIn vivo imaging and tracingOrganic chemistryFluorescence/phosphorescenceIn vivoMaleimide
The invention belongs to the technical field of drug carriers, and discloses a maleimide propionyl piperazine heptamethine cyanine salt fluorescence carrier and a preparation method and application thereof. On the basis of near-infrared fluorescent cyanine dye, a maleimide structure is introduced, the carrier is obtained, the carrier can be effectively subjected to a Michael addition reaction with micromolecules, polypeptide, protein and the like which contain nucleophilic groups such as mercapto groups, fluorescence labeling is conducted on corresponding molecules, and in vitro detection and in vivo developing tracking on the micromolecules, polypeptide and the protein are achieved. The fluorescence carrier has good light stability, and compared with indocyanine green (ICG), the fluorescence carrier has higher quantum efficiency and larger Stokes shift and can be used for preparing a detection reagent or kit of the micromolecules, polypeptide and the protein which contain the nucleophilic groups.
Owner:重庆高晋生物科技有限公司
Method and kit for detecting streptomycin medicine resistant mutation of Mycobacterium tuberculosis
InactiveCN102229991AAvoid False Positive ResultsSimple and fast operationMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceNucleotide
The invention discloses a method and a kit for detecting the streptomycin medicine resistant mutation of Mycobacterium tuberculosis. The invention relates to medicine resistant mutation detection technique and provides a method for detecting streptomycin medicine resistant mutation of Mycobacterium tuberculosis, which effectively improves sensitivity and specificity, and is simple and convenient in operation and short in period. The method comprises: designing primers and probes according to the complete sequence of the Mycobacterium tuberculosis and the gene sequences of the genomes rpsL and rrs of the Mycobacterium tuberculosis; extracting the DNA of a sample of the Mycobacterium tuberculosis; constructing a polymerase chain reaction (PCR) reaction system; and performing PCR amplification and analysis on a fusion curve. In the method, experiments are performed in two tubes respectively by using the specific primers and probes, and the amplification of the nucleic acid fragment of a target nucleotide sequence and subsequent analysis on the fusion curve are realized by using heat-resistance DNA polymerase, four kinds of nucleotide monomers and other components and by using real-time PCR technique. A fluorescent PCR fusion curve method with high specificity can quickly and accurately detects common medicine-resistance mutation of Mycobacterium tuberculosis and is expected to be directly used for medicine-resistance detection of a clinic Mycobacterium tuberculosis sample.
Owner:XIAMEN UNIV +1
Micro-fluidic chip, preparation method thereof, micro-fluidic device and pathogenic bacterium detection method
ActiveCN111804356AHighly species-specificEfficient ConcentrationMicrobiological testing/measurementChemiluminescene/bioluminescenceAptamerEngineering
The invention discloses a micro-fluidic chip, a preparation method thereof, a micro-fluidic device and a pathogenic bacteria detection method. The micro-fluidic chip comprises a chip layer, a channelstructure is arranged in the chip layer, and an array structure of which the surface is modified with an aptamer is arranged in the channel structure. The micro-fluidic device prepared by adopting themicro-fluidic chip can be used for rapid and high-sensitivity detection of pathogenic bacteria in food, and the detection effect is high in stability.
Owner:TSINGHUA UNIV
Conditionallly replication-competent adenovirus
InactiveUS20140199688A1Simple and highly sensitive detectionAvoid False Positive ResultsVectorsMicrobiological testing/measurementCancer cellCancers diagnosis
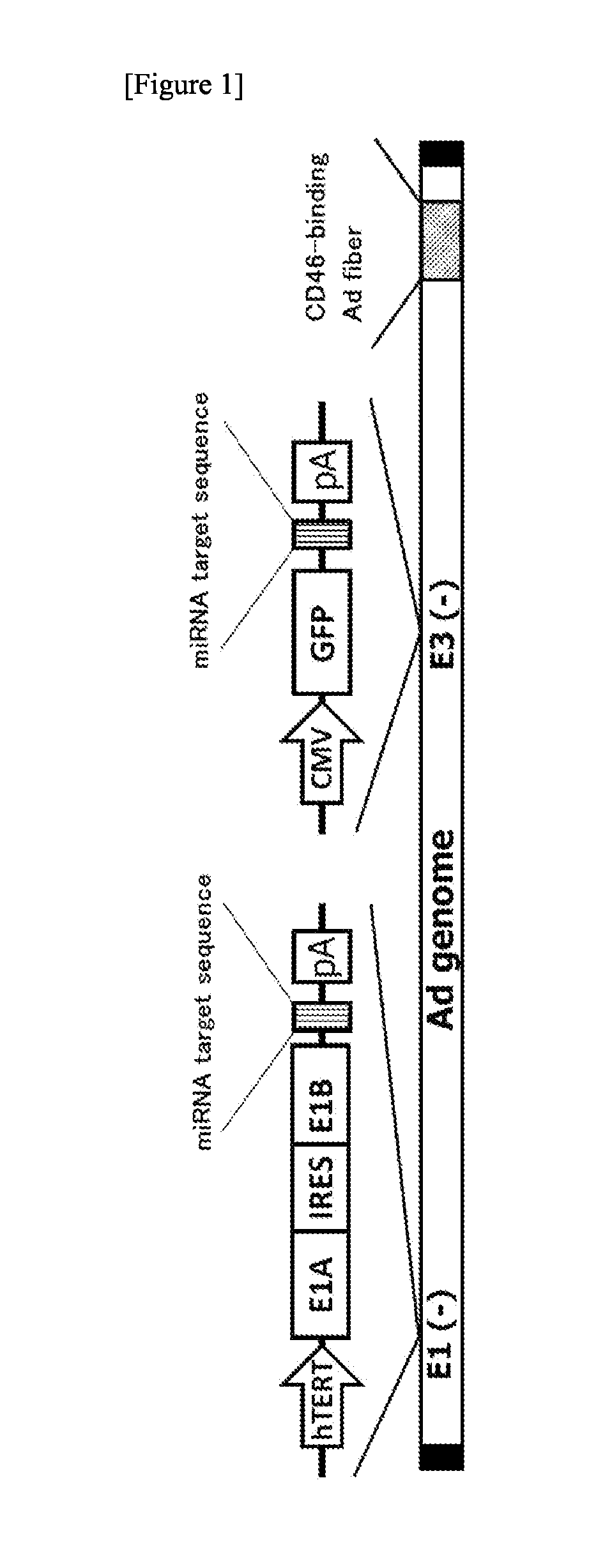
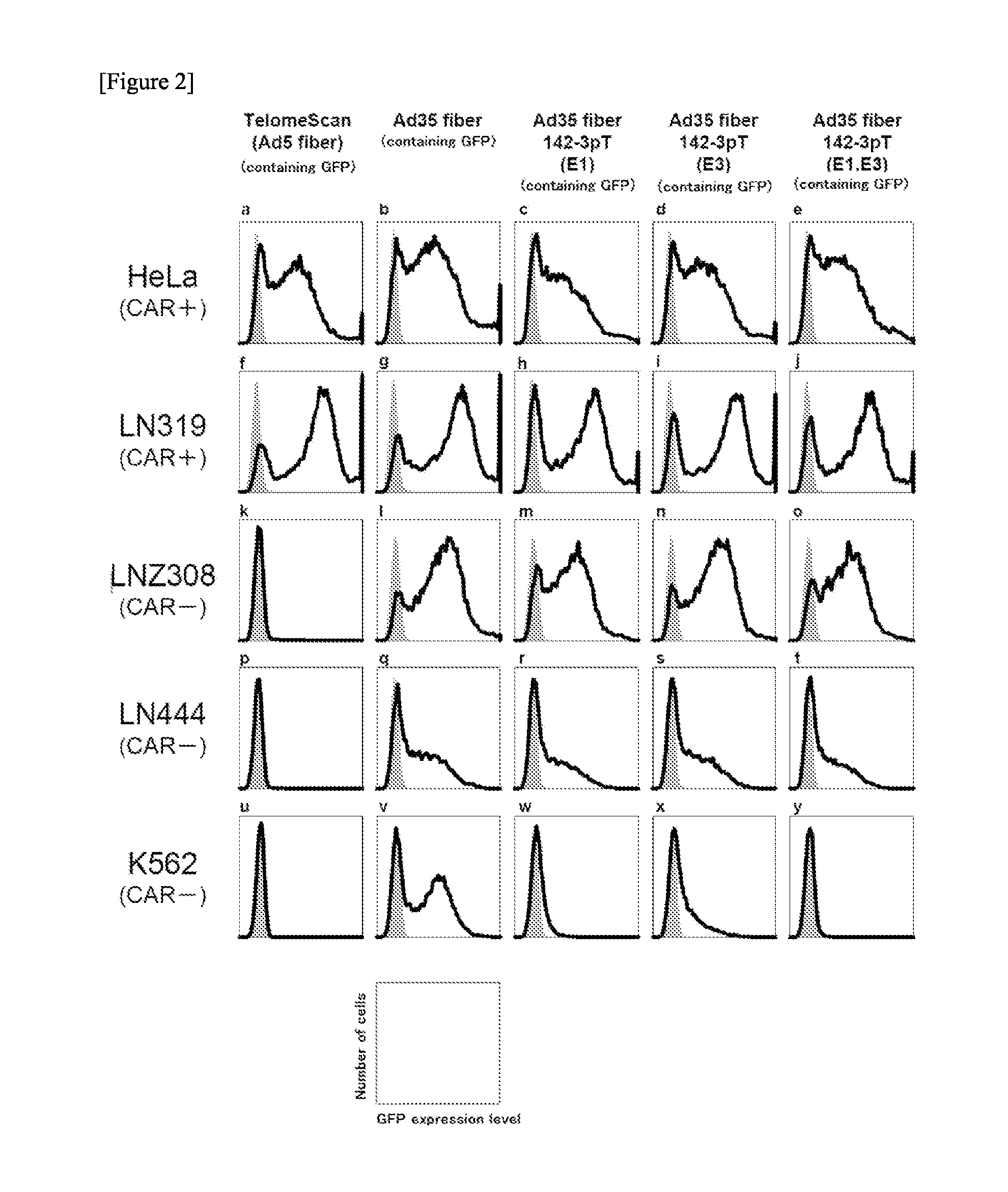
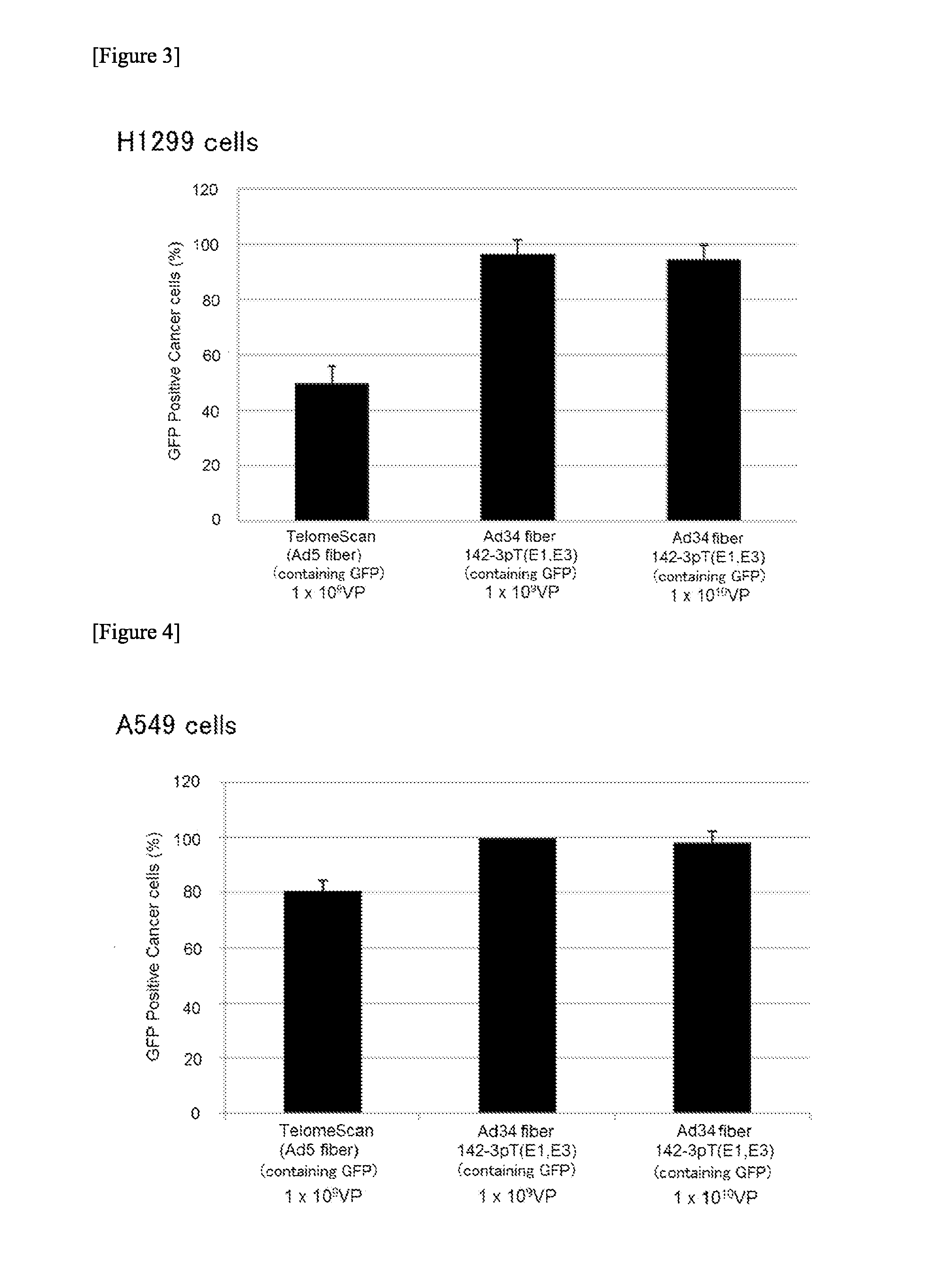
The object of the present invention is to provide a novel conditionally replicating adenovirus and a reagent comprising the same for cancer cell detection or for cancer diagnosis.The present invention provides a polynucleotide, which comprises human telomerase reverse transcriptase (hTERT) promoter, E1A gene, IRES sequence and E1B gene in this order and which comprises a target sequence of a first miRNA. The present invention also provides a recombinant adenovirus, which comprises a replication cassette comprising the above polynucleotide, wherein the replication cassette is integrated into the E1 region of the adenovirus genome.
Owner:NAT INST OF BIOMEDICAL INNOVATION HEALTH & NUTRITION
Lateral Flow Immunoassay for Complement Activation and Methods of Use for Point-of-Care Assessment of Complement-Associated Disorders
ActiveUS20120141457A1Avoid false positive resultAvoid False Positive ResultsAntibacterial agentsSenses disorderPoint of careLateral flow immunoassay
A method for treating an individual at risk for a complement-associated disorder is provided, including: (a) obtaining a sample of a body fluid from the individual; (b) measuring a complement activation level in the sample via a point-of-care lateral flow immunoassay; (c) correlating the complement activation level in the sample to a risk of a complement-associated disorder by comparing the complement activation level in the sample to a reference level in a control, wherein a deviation in complement activation level in the sample compared to the reference level in the control indicates the individual is at risk for a complement-associated disorder; (d) selecting a treatment for the individual, based on the correlating of step (c); and (e) treating the individual with the treatment selected in accordance with step (d). Lateral flow immunoassays and a method of monitoring an individual suffering from a complement-associated disorder are also provided herein.
Owner:KYPHA
Mycoplasma contamination detection method and application
InactiveCN108359737AImprove convenienceImprove featuresMicrobiological testing/measurementMicroorganism based processesPolymerase LMycoplasma contamination
The invention provides a new detection method for detecting mycoplasma contamination in a cell culture process and a detection kit. According to the new detection method and the detection kit, relatively conservative rDNA in mycoplasma is taken as a detection target segment, the detection is performed with a recombinase and polymerase based isothermal amplification technology by combination of specific primers and probes, so that operation is simple and convenient, detection time is shortened, and specificity and accuracy of mycoplasma contamination detection are improved.
Owner:SUZHOU GENDX BIOTECH CO LTD
Klebsiella pneumoniae nucleic acid detection kit (PCR-fluorescent probe method)
InactiveCN103160574AAvoid pollutionImprove reliabilityMicrobiological testing/measurementFluorescence/phosphorescenceK pneumoniaeFluoProbes
The invention relates to a real-time fluorescent PCR kit, and especially relates to a kit used for detecting klebsiella pneumonia (KP) by using a real-time fluorescent PCR technology. With the kit, KP in samples can be detected. The kit can be widely applied in auxiliary diagnosis of KP infection.
Owner:DAAN GENE CO LTD
Primer, fluorescence probe and kit for quantitative detection of streptococcus pneumonia nucleic acid and detection method of streptococcus pneumonia nucleic acid
ActiveCN102876774AHigh sensitivityPrevent false negative and false positive resultsMicrobiological testing/measurementMicroorganism based processesFluorescenceSample collection
The invention provides a primer, a fluorescence probe and a kit for the quantitative detection of streptococcus pneumonia nucleic acid and a detection method of streptococcus pneumonia nucleic acid, wherein the primer comprises a forward primer and a backward primer; and the kit is used for the quantitative detection of streptococcus pneumonia nucleic acid and comprises the primer and the fluorescence probe, and PCR reaction liquid, a DNA extraction solution, a negative quality control material, a positive quality control material, a critical positive quality control material and a working standard. The method for the quantitative detection of streptococcus pneumonia nucleic acid through using the primer, the fluorescence probe and the kid comprises the following steps: step 1, sample collection; step 2, sample processing; step 3, sample application; step 4, PCR amplification; and step 5, analysis and judgment. According to the real-time TaqMan fluorescence quantitative PCR provided by the embodiment of the invention, the primer and the fluorescence probe have high specificity and high sensitivity, the kit has precise quantification, and the detection method can rapidly detect the streptococcus pneumonia.
Owner:WUHAN BIOTECH GENE ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com