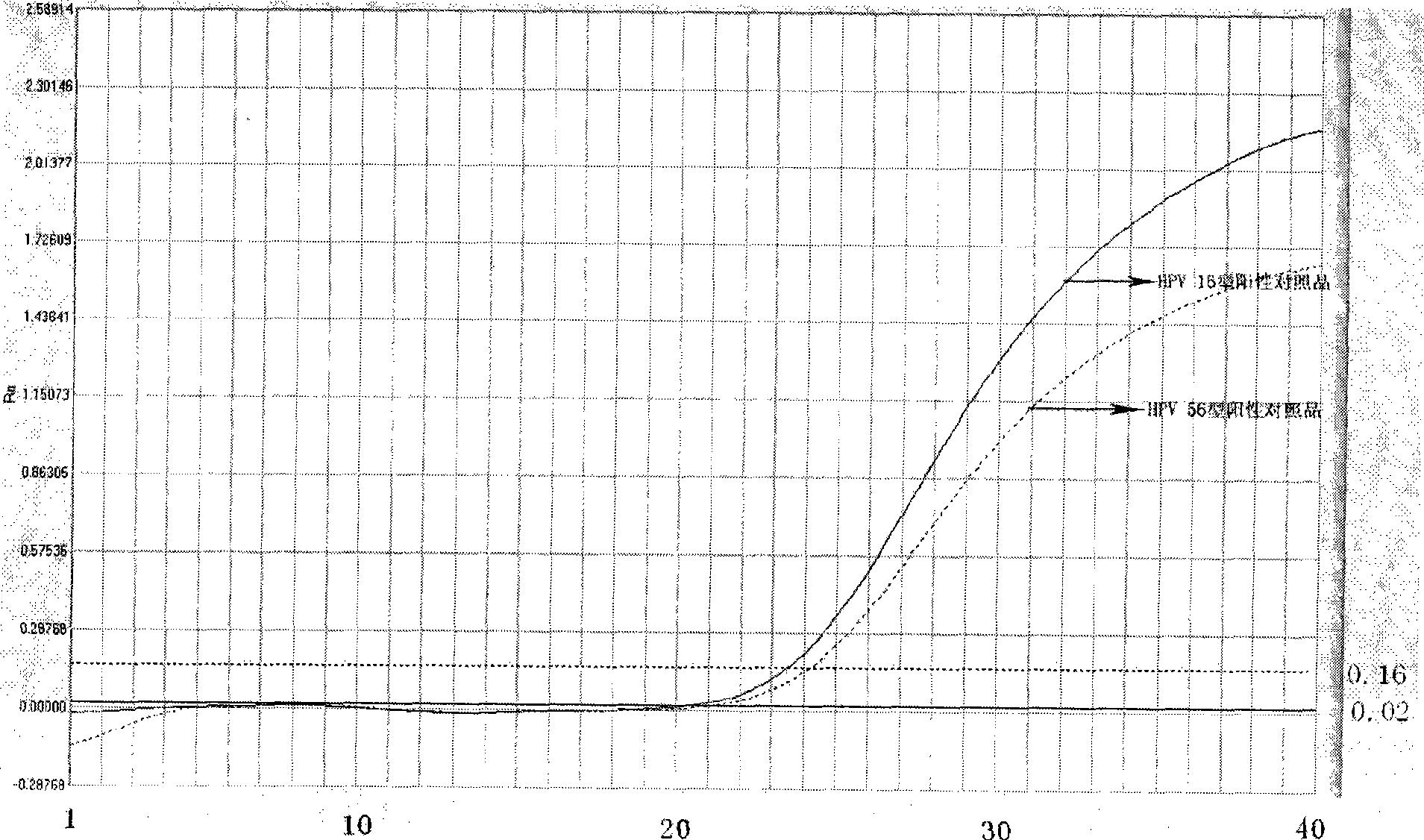
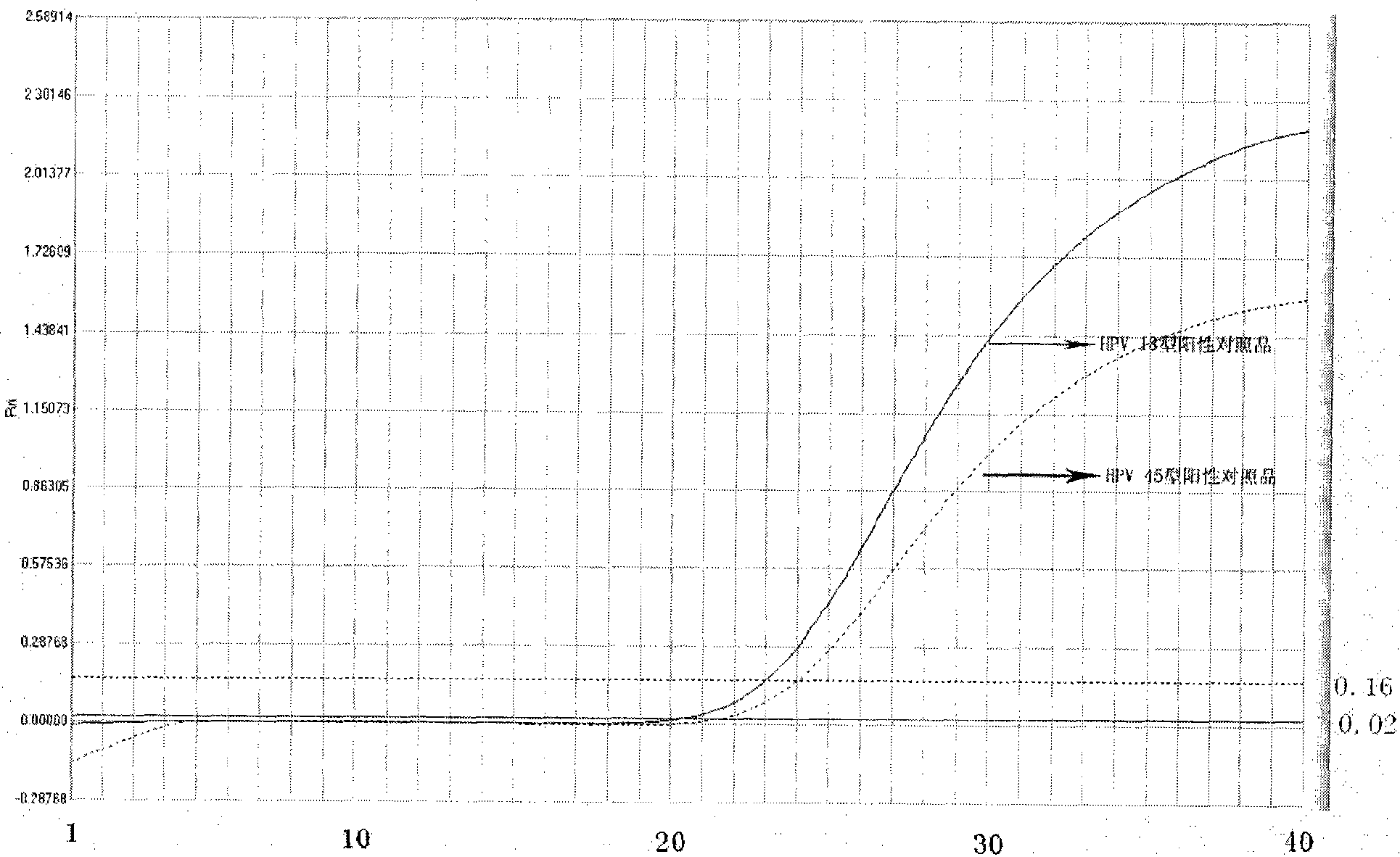
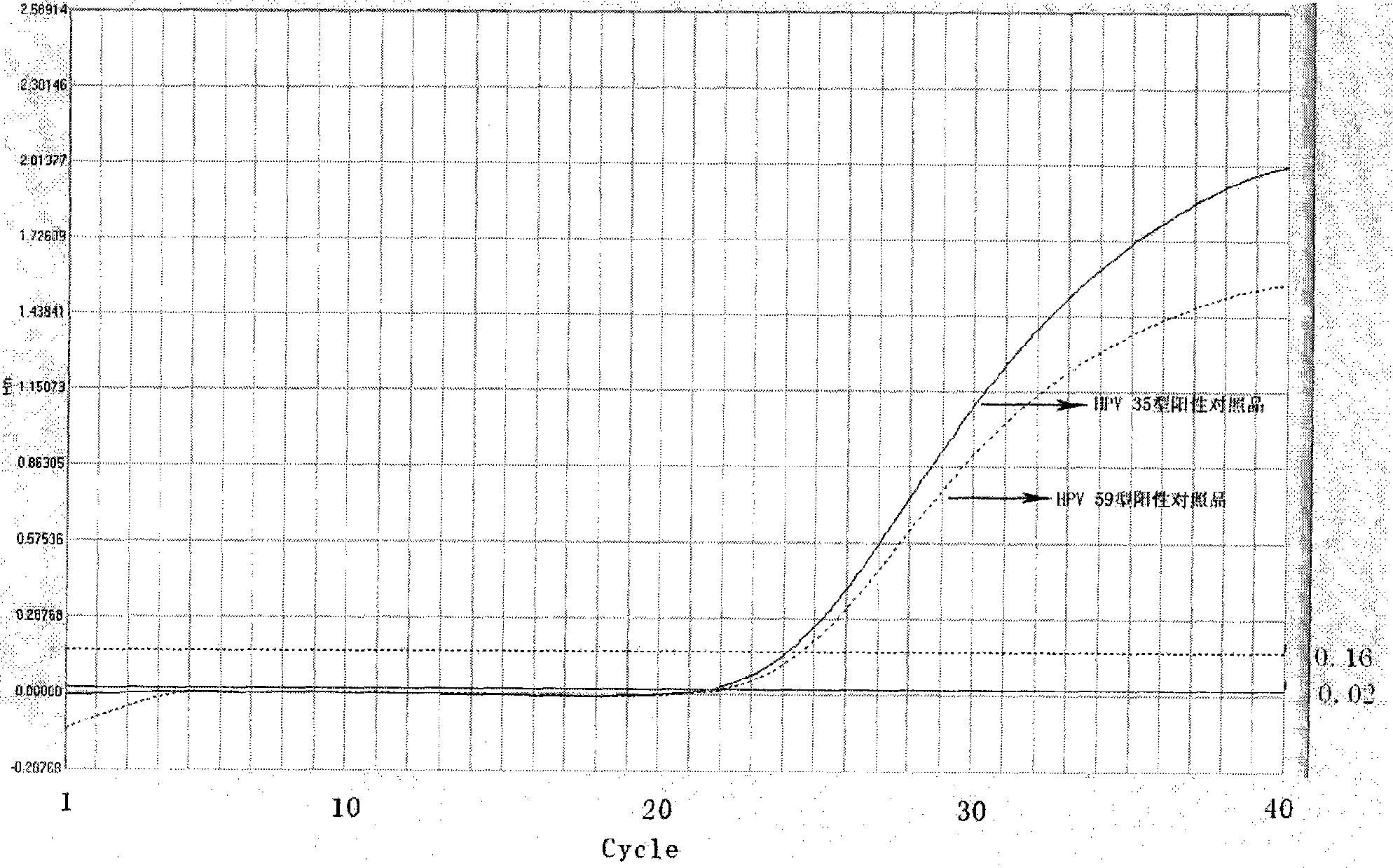
Reagent kit for detecting high-risk human mammilla papillomavirus, as well as preparation and application thereof
A human papillomavirus and kit technology, applied in the field of human papillomavirus diagnostic reagents, can solve the problems of high false positive rate, easy to have alternate contamination and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1 The preparation method of the human papillomavirus (HPV) high-risk type typing detection kit detection mixed solution: 1. Synthesize the oligonucleotide probe according to the following sequence:
[0105]
[0106]
[0107] 2. Preparation of nucleic acid fluorescent PCR detection mixture:
[0108] (1), preparation of HPV16 type and HPV 56 type nucleic acid fluorescent PCR detection mixture:
[0109] Human papillomavirus (HPV) type 16 upstream primer 1.2 μl / test; downstream primer 1.2 μl / test; probe 0.2 μl / test; human papillomavirus (HPV) type 56 upstream primer 1.2 μl / test; downstream primer 1.2 μl / test; probe 0.2μl / test; PCRMIX-20μl / test; deionized water 10.4μl / test and mix well.
[0110] (2), preparation of HPV18 type and HPV 45 type nucleic acid fluorescent PCR detection mixed solution:
[0111] Human papillomavirus (HPV) type 18 upstream primer 1.2 μl / test; downstream primer 1.2 μl / test; probe 0.2 μl / test; human papillomavirus (HPV) type 45 upstream...
Embodiment 2
[0124] Example 2 Use of the Human Papillomavirus (HPV) High-Risk Typing Kit:
[0125] Source of specimens: The specimens used come from clinical medical units.
[0126] 2.1 Processing of test specimens:
[0127] Add 1ml of sterile saline to the specimen, shake well, absorb the liquid and transfer it to a 1.5ml centrifuge tube, and centrifuge at 13,000rpm for 5 minutes. Add 1 ml of sterile saline to the precipitate, mix well, centrifuge at 13,000 rpm for 5 minutes, and repeat the washing once. The precipitate was directly added to 50 μl of nucleic acid extraction solution and mixed thoroughly, bathed in boiling water for 10 minutes, then centrifuged at 13,000 rpm for 5 minutes, and 4 μl of the supernatant was taken as a PCR reaction template.
[0128] 2.2 Reagent preparation:
[0129] (1) Take 36 μl HPV16 and HPV56 nucleic acid fluorescent PCR detection mixture and 0.4 μl Taq enzyme, shake and mix for a few seconds, and centrifuge at 3000 rpm for a few seconds.
[0130] (2)...
Embodiment 3
[0154] Example 3 Human papillomavirus (HPV) high-risk type typing detection kit clinical sensitivity and specificity test:
[0155] 3.1 Specimen: 50 specimens that have been clinically classified into HPV high-risk types
[0156] 3.2 Sample processing, reagent configuration, sample loading and PCR amplification are the same as Example 1
[0157] 3.3 Test results:
[0158] Among the 50 specimens, the test results of the high-risk HPV typing test kit and the clinical unit were both positive in 42 cases, and the sensitivity of the high-risk HPV typing test kit was 97.67% ; Human papillomavirus (HPV) high-risk type detection kit detection results and clinical unit detection results were negative in 7 cases, and the specificity of human papillomavirus (HPV) high-risk type detection kit was 100%; The accuracy of the HPV high-risk typing detection kit is 98%. The specific test results are shown in the table below.
[0159] Test results
[0160] Specimen ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com