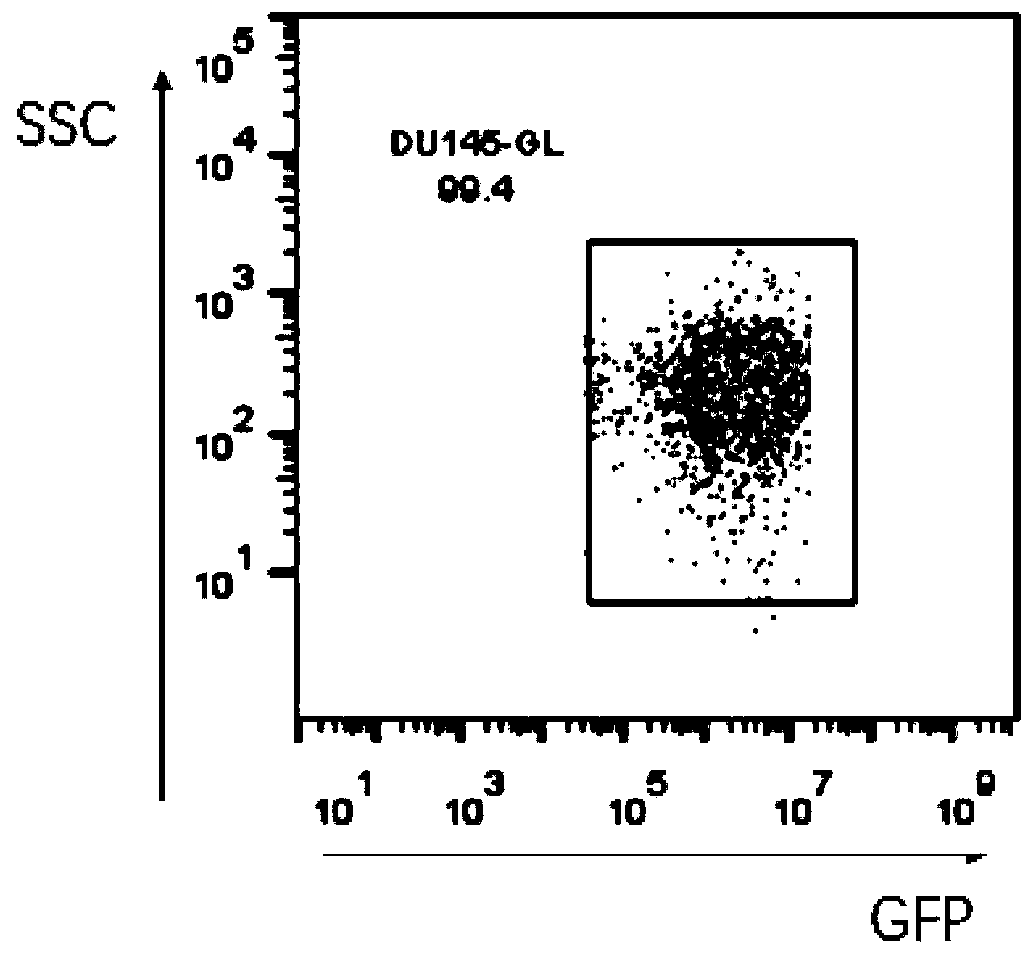
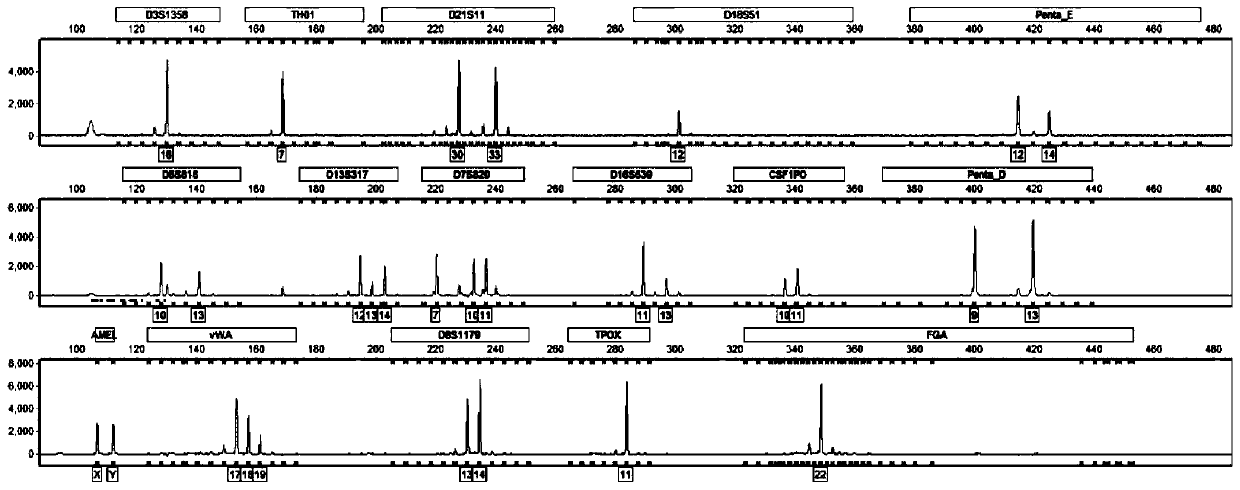
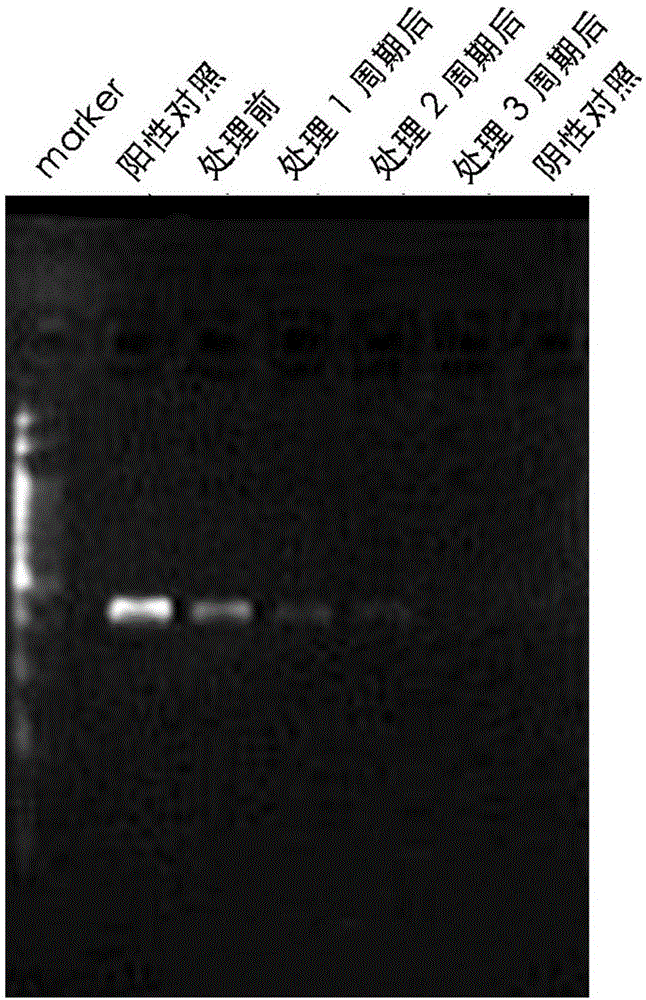
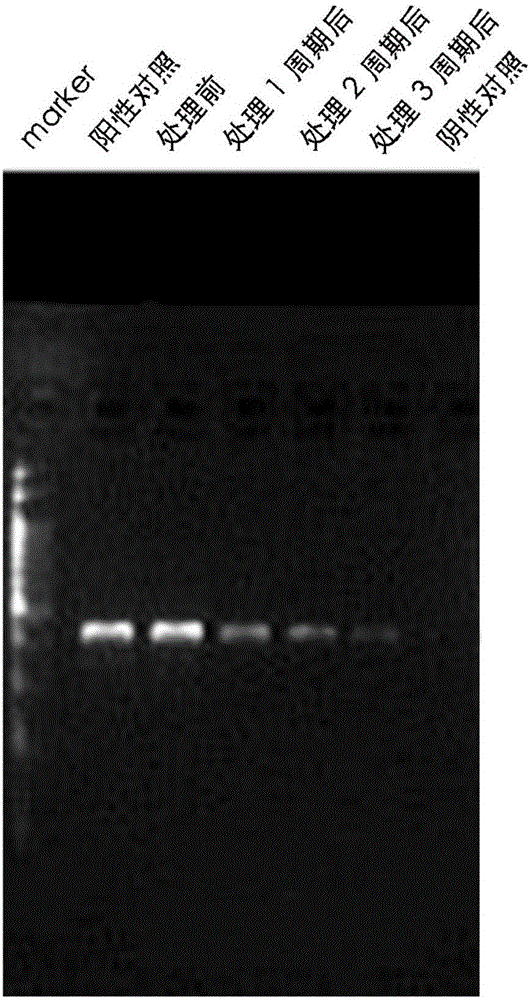
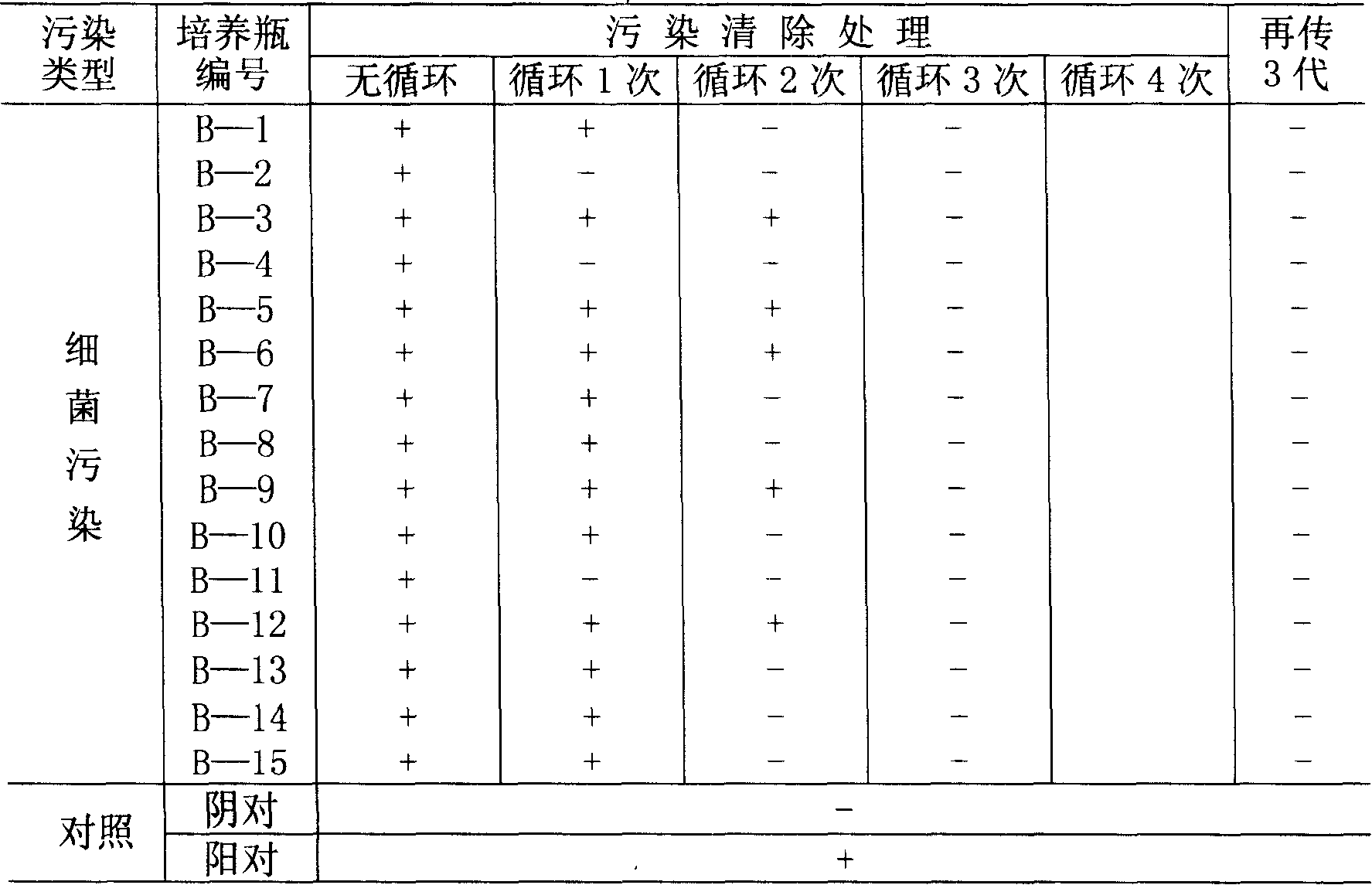
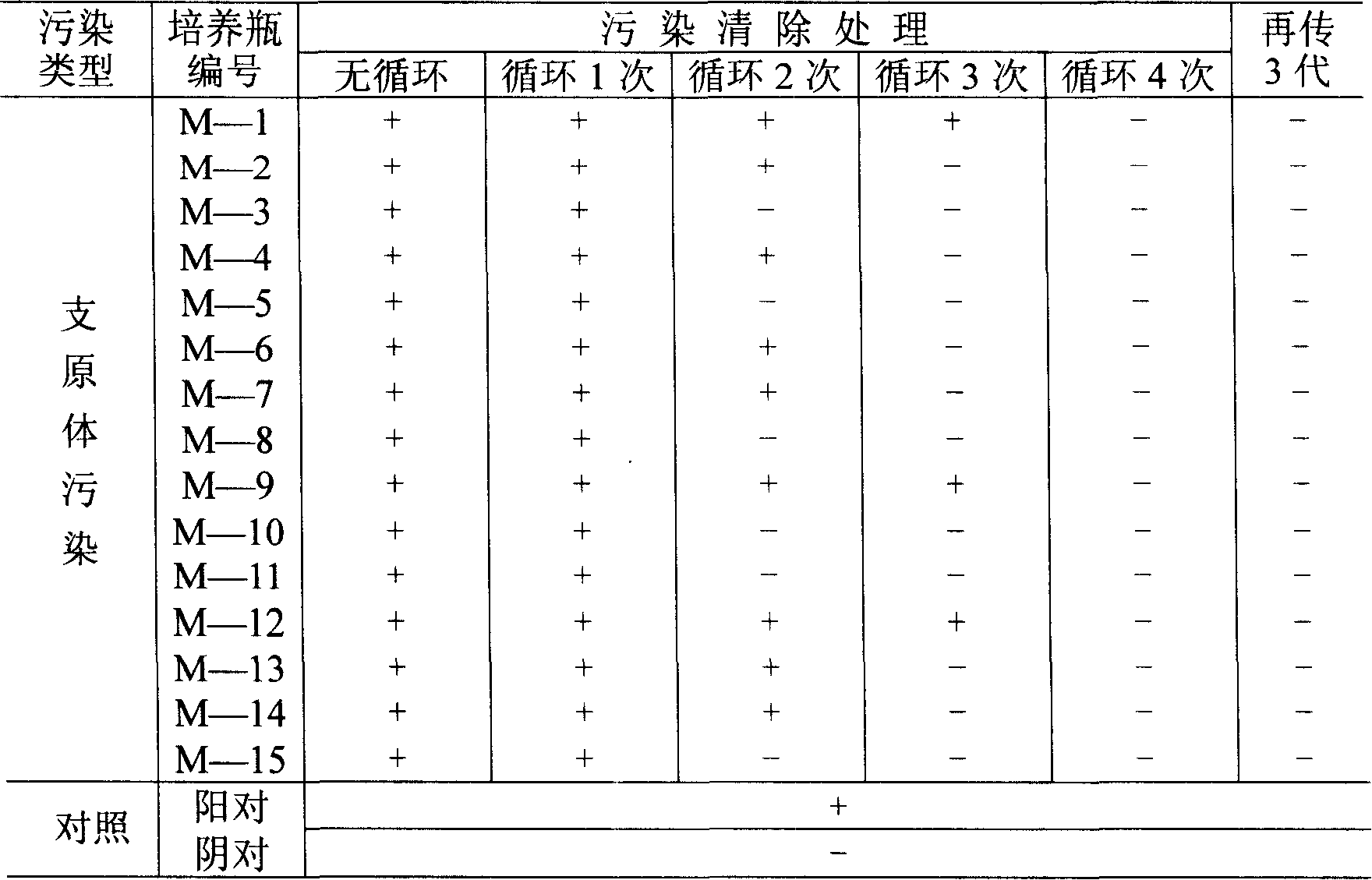
Patents
Literature
39 results about "Mycoplasma contamination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing human umbilical cord mesenchymal stem cell exosomes
PendingCN109880797AHigh purityClear sourceSkeletal/connective tissue cellsMesenchymal stem cellMycoplasma contamination
The invention discloses a method for preparing human umbilical cord mesenchymal stem cell exosomes. The method uses a newborn umbilical cord as a source of umbilical cord mesenchymal stem cells, usesa medium prepared by a fetal calf serum or a serum substitute in which exosome carried by itself is removedfor culturing, collects a supernatant, and further separates and extracts the exosomesby differential centrifugation, the purity is high, and the source of the umbilical cord mesenchymal stem cell exosomes is ensured; the exosomes is prepared by the differential centrifugation, the operationis simple, and the method is suitable for large-scale production; during the whole process, sterility is guaranteed, the contamination ofmycoplasma is prevented, and the product safety is higher.
Owner:JINAN PANSHENG BIOTECH
Kit and method for detecting mycoplasma pollution in CHO cultured cells
InactiveCN103627781AMicrobiological testing/measurementMicroorganism based processesMicrobiologyMycoplasma contamination
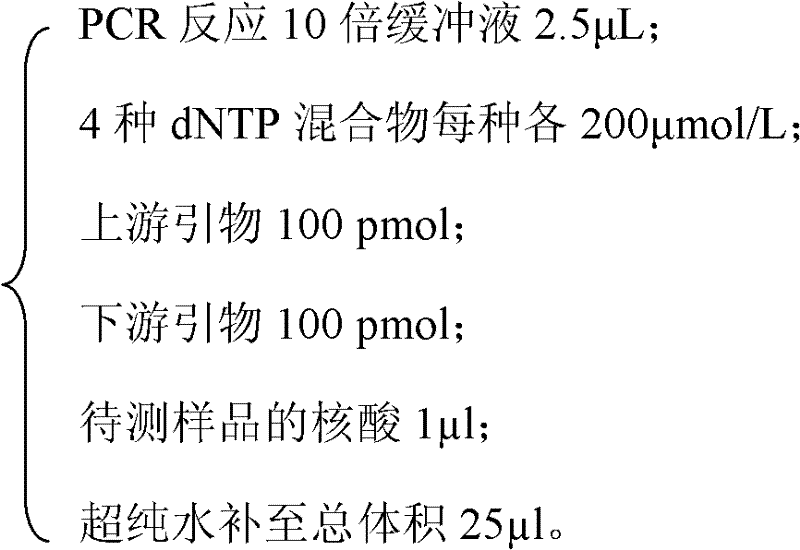
The invention relates to a kit and a method for detecting mycoplasma pollution in CHO cultured cells, and belongs to the field of biological technology detection. The kit comprises a primer pair of the DNA sequence of a specific amplified CHO cell glyceraldehyde-3-phosphatedehydrogenase and a primer pair of the DNA sequence of a specific amplified mycoplasma 16srRNA conserved domain, and the two above primer pairs are placed in a same PCR system. According to the kit, when mycoplasma pollution is detected by employing the PCR technology, the determination of reliability of a detection result can be finished at the same time. The kit and the method help to substantially improve the reliability of the detection result on mycoplasma pollution in CHO cultured cells, the operation is simple, the detection period is short, and the sample detection operationality is good.
Owner:NCPC NEW DRUG RES & DEV
Reagent for eliminating mycoplasma contamination in cell culture and use method thereof
ActiveCN102409019AMetabolic effectsEfficient removalMicrobiological testing/measurementTissue cultureMycoplasma contaminationPleuromutilin
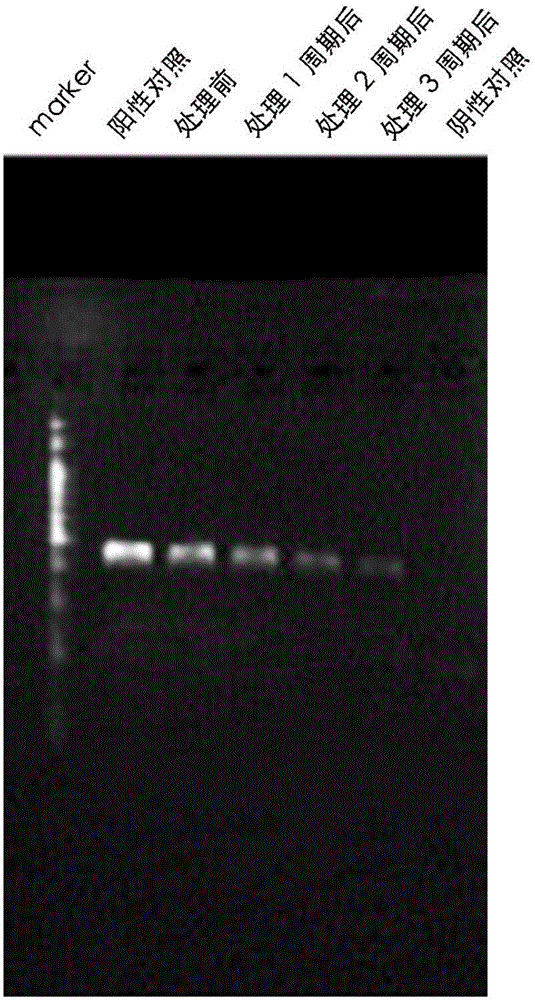
The invention provides a reagent for eliminating mycoplasma contamination in cell culture and a use method thereof. The reagent is prepared by mixing liquid A and liquid B isometrically or mixing liquid A, liquid B and liquid C isometrically, wherein the liquid A is prepared by mixing semisynthetic pleuromutilin derivatives and 0.01MPBS, and the mass-volume percentage concentration of the solute after mixing is 0.5-1.5%; the liquid B is prepared by mixing quinolone derivatives and 0.01MPBS, and the mass-volume percentage concentration of the solute after mixing is 0.5-1.5%; and the liquid C is prepared by mixing tetracycline derivatives and 0.01MPBS, and the mass-volume percentage concentration of the solute after mixing is 0.25-0.75%. The reagent and method provided by the invention havethe following beneficial effects: if contamination is not severe, the effect can be displayed within a week; and after the treatment cycle is finished, black spots do not exist among the treated contaminated cells, the growth cycles of the cells return to normal, the cell morphology is normal, and aggregated growth and netting phenomena are avoided.
Owner:胡晓鹏
Swine testicular cell strain ST-S suitable for suspension culture as well as acquisition method and application of swine testicular cell strain ST-S
Owner:JINYUBAOLING BIO PHARMA CO LTD +1
Mycoplasma contamination detection method and application
InactiveCN108359737AImprove convenienceImprove featuresMicrobiological testing/measurementMicroorganism based processesPolymerase LMycoplasma contamination
The invention provides a new detection method for detecting mycoplasma contamination in a cell culture process and a detection kit. According to the new detection method and the detection kit, relatively conservative rDNA in mycoplasma is taken as a detection target segment, the detection is performed with a recombinase and polymerase based isothermal amplification technology by combination of specific primers and probes, so that operation is simple and convenient, detection time is shortened, and specificity and accuracy of mycoplasma contamination detection are improved.
Owner:SUZHOU GENDX BIOTECH CO LTD
Product for prevention and control of pollution of cell culture
The invention provides a kind of product that can prevent the contamination in cell cultivating. The characteristic of the product is that every liter product contains 1-50g ethyl ciprofloxacin. The invention adds drug of certain density which can prevent and clear the contamination to the cell culture fluid to prevent the common contamination of bacteria and mycoplasma. The invention has overcome many defects of common drugs such as short drug action period, short shelf life, high price and unable to preserve in ambient temperature. The invention can not only be used in cell cultivating, it can also be used to clarify other organic medium materials, wash the commonly used apparatus or clarify the separating and purifying materials in emergency.
Owner:YUNNAN ANIMAL SCI & VETERINARY INST
Reporting gene amplification kit for detecting mycoplasma
InactiveCN101580867AAvoid cross contaminationHigh sensitivityMicrobiological testing/measurementInfection diagnosisHybridization probe
The difficult problem of the contamination of cell cultured mycoplasma and the infection diagnosis of various mycoplasma urgently need a reliable and effective kit. Mycoplasma culture by a fluorescent staining method is reliable but takes time and trouble, and culture pollution is intensified. A direct PRC method for amplifying a mycoplasma gene, which is used for the routine detection of the mycoplasma is easy for cross contamination, and high false masculinity is only used as an auxiliary reference. The invention relates to a mycoplasma conservative gene which across links a plant arabidopsis LEY sequence into a reporting gene DNA by a hybridization probe and amplifies the indirect detection of a reporting gene. The mycoplasma conservative gene is characterized in that mycoplasma 16sRNA is hybridized with the head part and the tail part of the LEY reporting gene DNA whose head part and tail part are provided with mycoplasma conservative sequence probes; the reporting gene DNA is catalyzed into a ring by heat-proof ligase, and the annular reporting gene is reversely amplified by PCR so as to indirectly reflect the detection of the mycoplasma; by adjusting ligase reaction, the reporting gene is difficultly across linked by the cross contamination, thereby reducing the false masculinity; and if a system is contaminated, the reporting gene can be changed.
Owner:BEIJING TAG ARRAY MOLECULAR TEST +2
Kit for obtaining lung stem cells through separation and method
ActiveCN107267437AIncrease costLow costCell dissociation methodsCulture processStem Cell IsolationFibroblast
The invention discloses a kit for obtaining lung stem cells through separation and a method. The lung stem cell separation kit comprises tissue digestive juice, embryo fibroblasts subjected to radiation treatment and a lung stem cell separation culture solution. With the adoption of the kit, a large quantity of amplified lung stem cells can be obtained effectively, and the obtained lung stem cells are good in activity, high in purity and free of bacterium and mycoplasma contamination.
Owner:REGEND THERAPEUTICS CO LTD
PCR (Polymerase Chain Reaction) detection method of mycoplasma
InactiveCN102242213AStrong specificityShort detection cycleMicrobiological testing/measurementPcr methodMycoplasma contamination
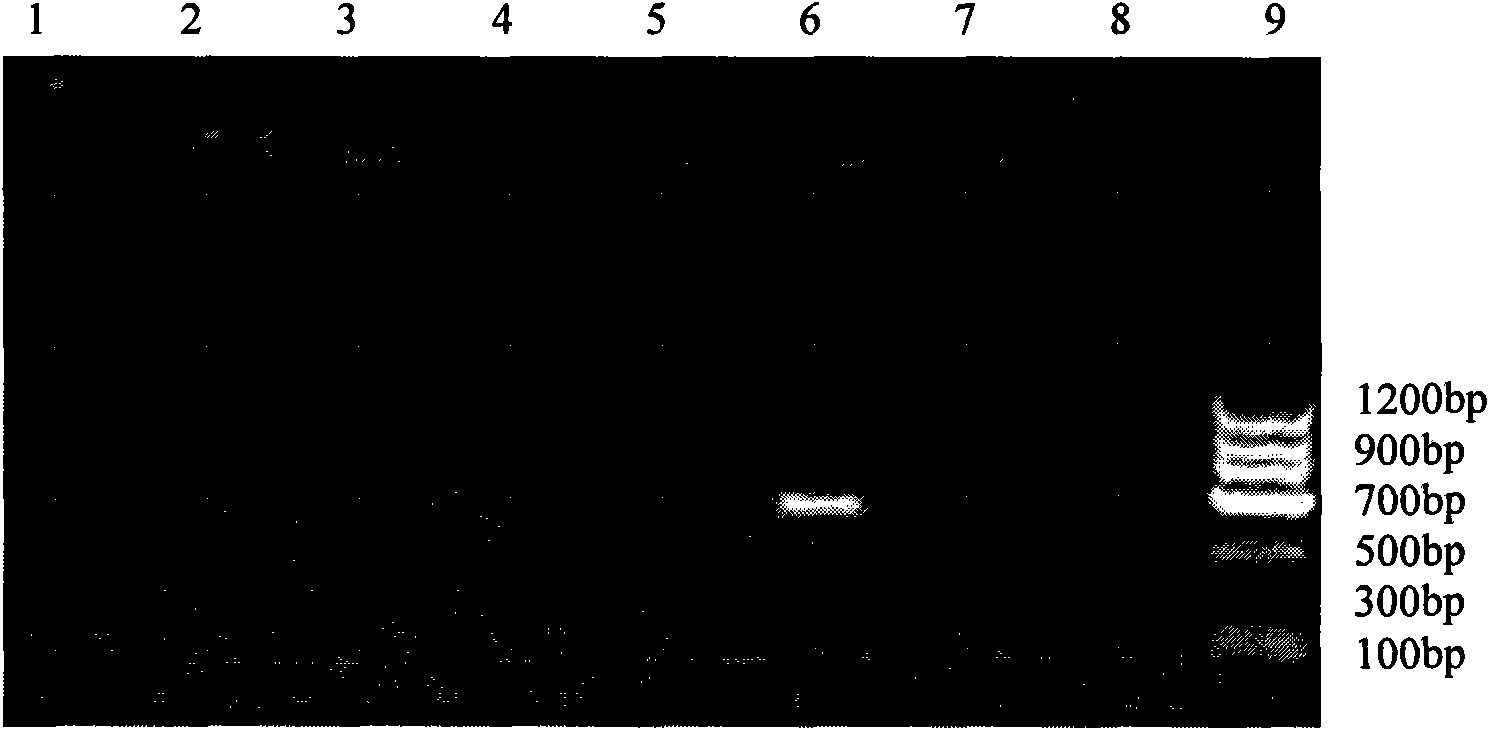
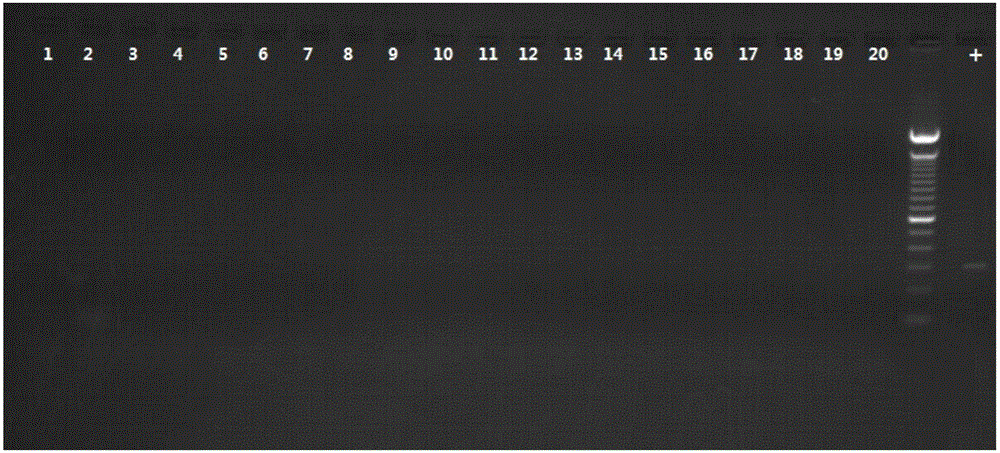
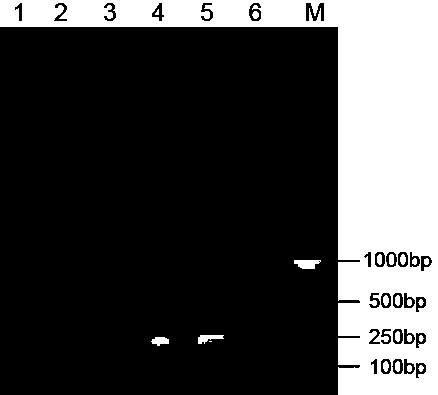
The invention relates to a PCR (Polymerase Chain Reaction) method for detecting mycoplasma, belonging to the technical field of biological detection. In the invention, PCR amplification and electrophoresis are carried out by extracting mycoplasma nucleic acids and applying mycoplasma specific primers to judge whether mycoplasma contamination exists in a detection sample or not, wherein if 435bp specific strips appear in an electrophoretogram, the result shows that the mycoplasma exists in the sample, and if 435bp specific strips do not appear, the result shows that the mycoplasma does not exist in the sample. By the detection and verification, the detection system provided by the invention has the characteristics of high specificity, good stability, short checking period and the like.
Owner:JINYUBAOLING BIO PHARM CO LTD
Primary isolation method of skin mesenchymal stem cells
ActiveCN109234230ACultivate stableGood energyCell dissociation methodsSkeletal/connective tissue cellsCell membraneMesenchymal stem cell
The invention belongs to the field of stem cells, and discloses a primary isolation method of skin mesenchymal stem cells. The method comprises: after skin tissue is pretreated, digested with DispaseII disperse enzyme, washed, repeatedly blown to separate cells, filtered and centrifuged to obtain cell precipitates which are resuspended in Lonza complete medium and then inoculated into a culture dish for 60-90min; The unattached cell suspension was inoculated into fibronectin-coated culture plates, and the cells were passaged when the cell confluence reached 80% - 90%. A large numb of skin mesenchymal stem cells can be isolate by using that primary isolation method of the invention, and the cell membrane of the skin mesenchymal stem cells isolated and cultured is not damage, the cell culture is stable, and mycoplasma pollution is not introduced, the cell vigor is good, and the skin mesenchymal stem cells can be stably cultured in the follow-up.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Treatment method of mycoplasma contamination
InactiveCN106635950AImprove survivabilityConvenient researchTissue cultureMycoplasma contaminationCulture mediums
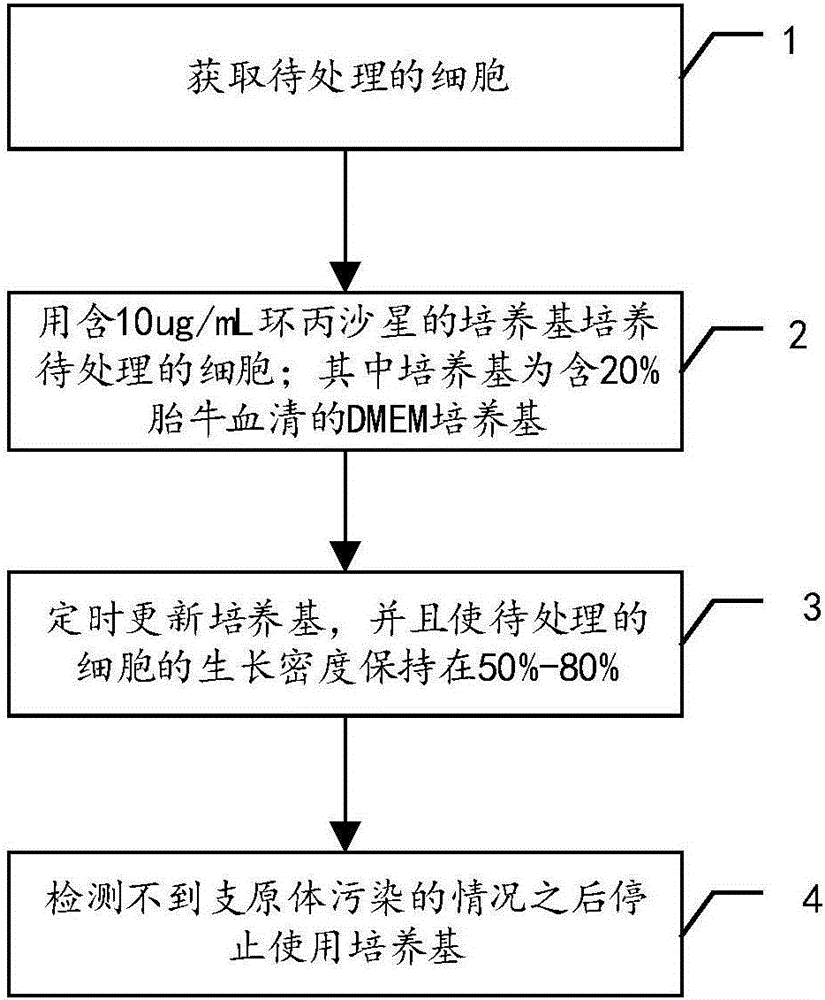
The invention discloses a treatment method of mycoplasma contamination. The treatment method comprises the following steps: obtaining cells to be treated; culturing the cells to be treated by utilizing a culture medium containing 10mu.g / mL of ciprofloxacin; updating the culture medium at fixed time, and enabling the growth density of the cells to be treated to be kept at 50 percent to 80 percent; stopping utilizing the culture medium until no mycoplasma contamination condition is detected. According to the treatment method of the mycoplasma contamination, disclosed by the invention, mycoplasmas are removed by adopting a method of adding the ciprofloxacin into the culture medium and the aim that the mycoplasma contamination is completely removed under the condition that influences on the cells are small is realized, without the need of adopting a manner of inactivating firstly and discarding secondly; meanwhile, the treatment method also has the advantage that the mycoplasma contamination does not reoccur after the ciprofloxacin is not used.
Owner:ZHUJIANG HOSPITAL SOUTHERN MEDICAL UNIV
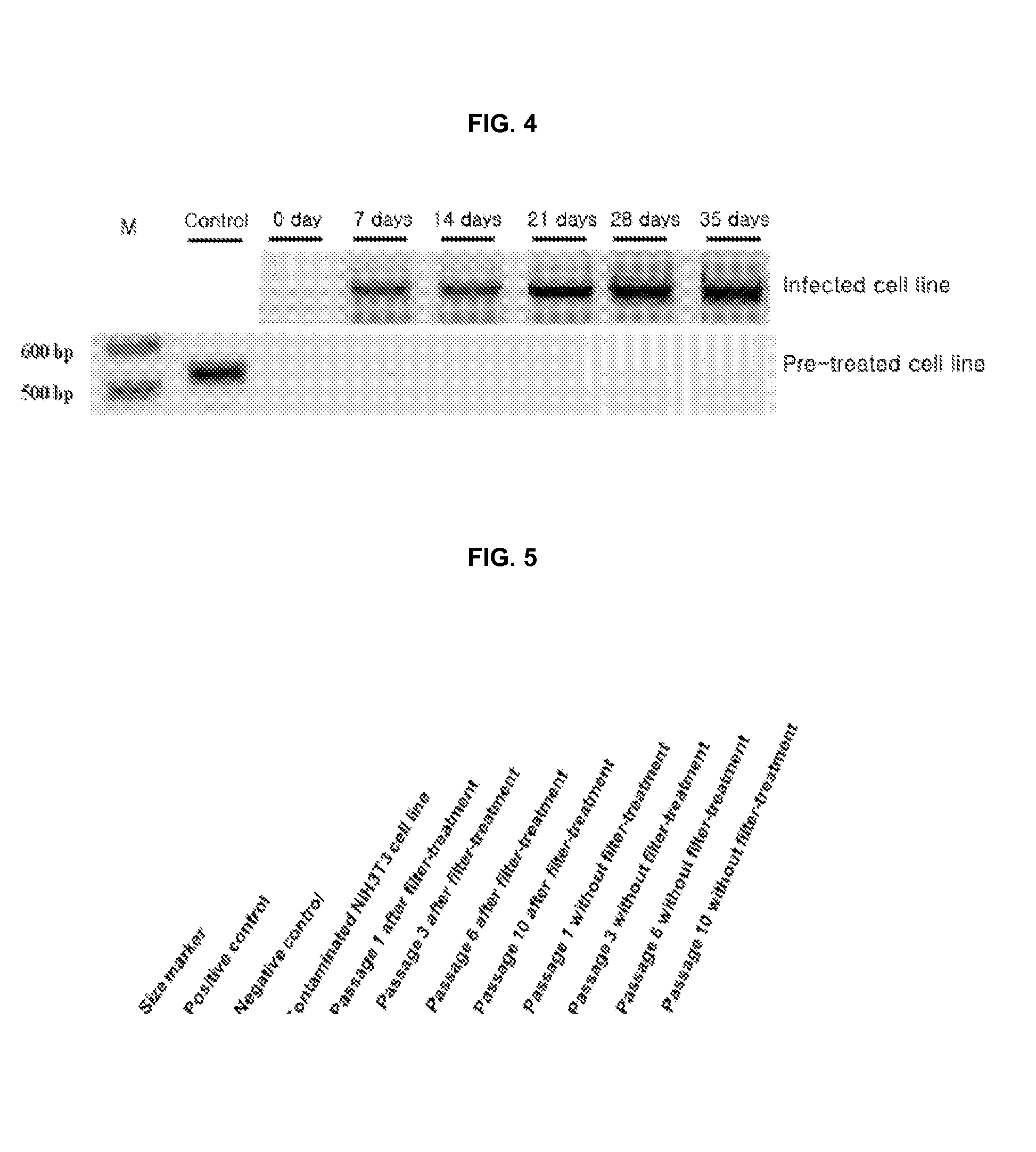
Method for culturing mycoplasma contamination-free cells and method for removing mycoplasma contamination of cells
ActiveUS20120244614A1Prevent mycoplasma contaminationRemove mycoplasma contaminationFungiOrganic chemistryBiotechnologyMycoplasma contamination
Owner:CELLSAFE
Human umbilical cord and placenta protection solution mycoplasma detection primer pair, kit and detection method thereof
InactiveCN106967826AEasy to carry outEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationPositive controlMycoplasma contamination
The invention relates to a human umbilical cord and placenta protection solution mycoplasma detection primer pair, a kit and a detection method thereof, and belongs to the technical field of in-vitro diagnosis reagents. The kit comprises a primer pair, a PCR (Polymerase Chain Reaction) reagent, a positive control, a negative control and a PCR reaction tube, wherein the primer pair comprises an upstream primer Myco-F: 5'-ggcgaatgggtgagtaacacg-3', and a downstream primer Myco-R: 5'-cggataacgcttgcgacctatg-3'. The detection kit provided by the invention is simple and convenient to operate, short in time and very high in sensitivity, whether cells are contaminated by mycoplasma or not can be detected before culture, the economic loss caused by aimless cell culture can be avoided, the mycoplasma can be fundamentally prevented from entering a cell culture chamber, and possibility of mycoplasma cross contamination of cells can be reduced.
Owner:YUNNAN SHUNXI REGENERATION MEDICAL ENG CO LTD
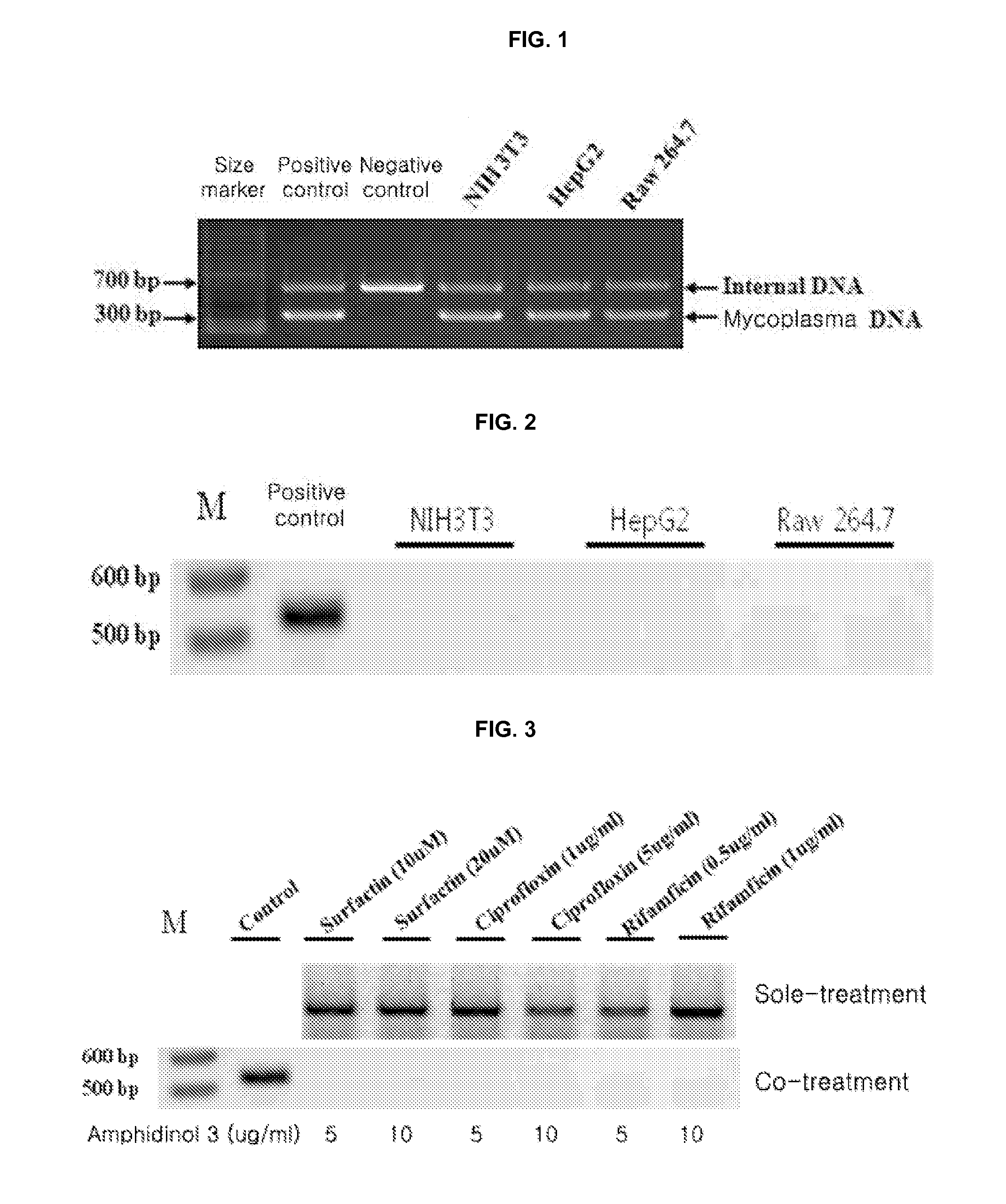
Method for culturing mycoplasma contamination-free cells and method for removing mycoplasma contamination of cells
ActiveUS20140093955A1Stable executionAvoid contaminationFungiOrganic chemistrySolution treatmentCulture cell
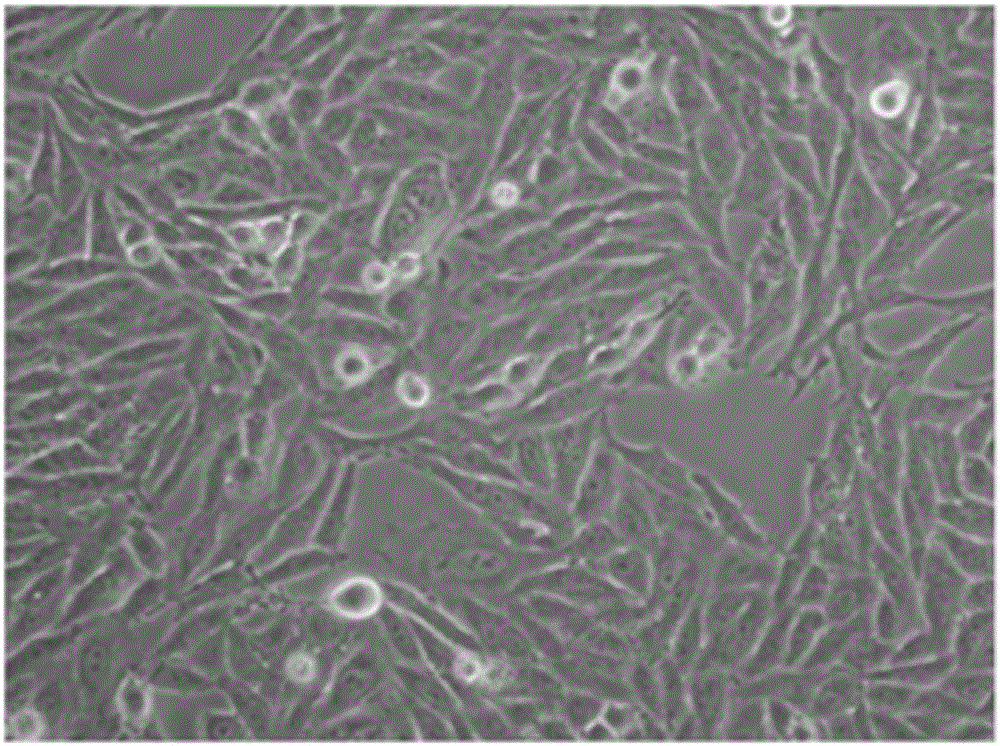
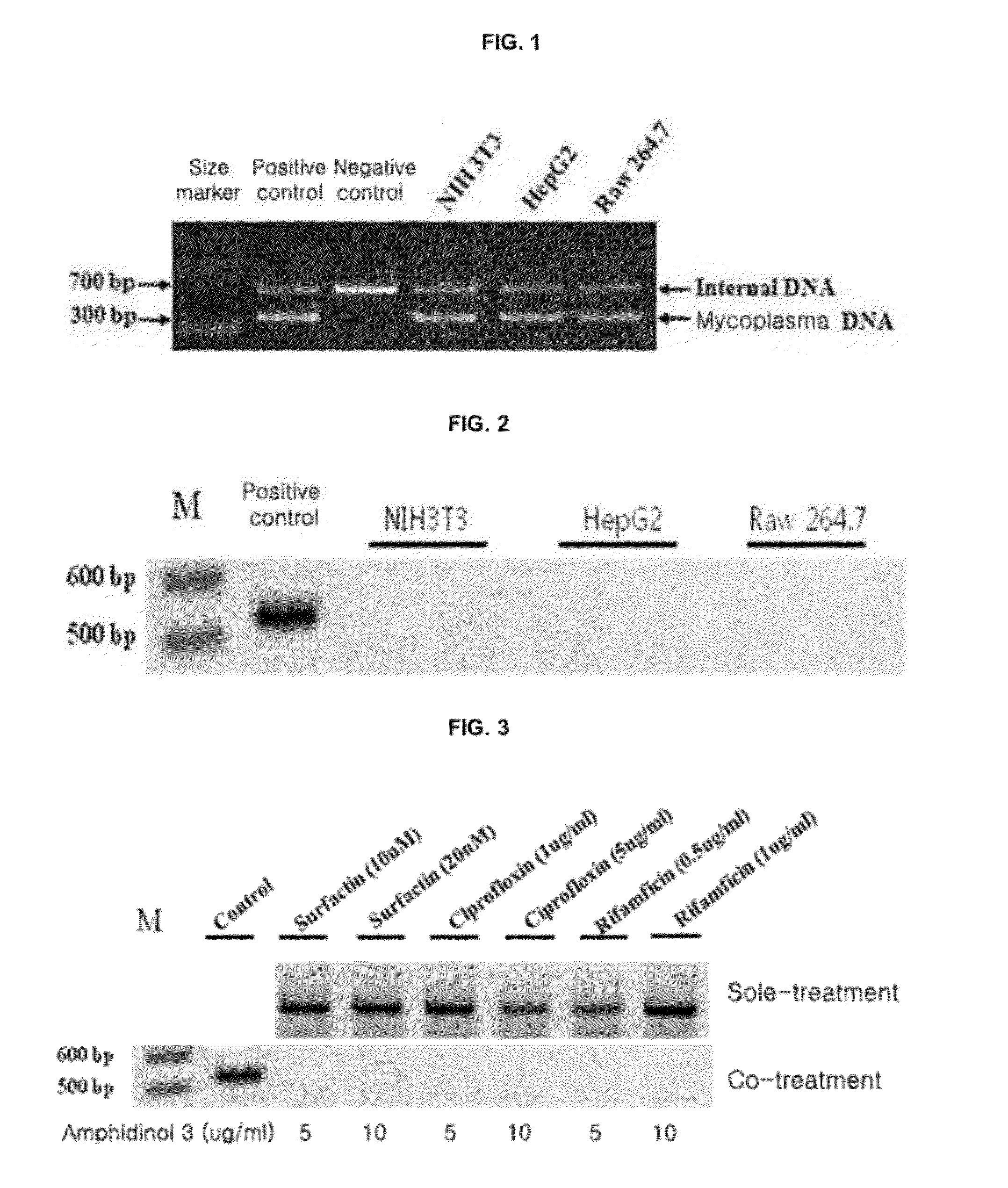
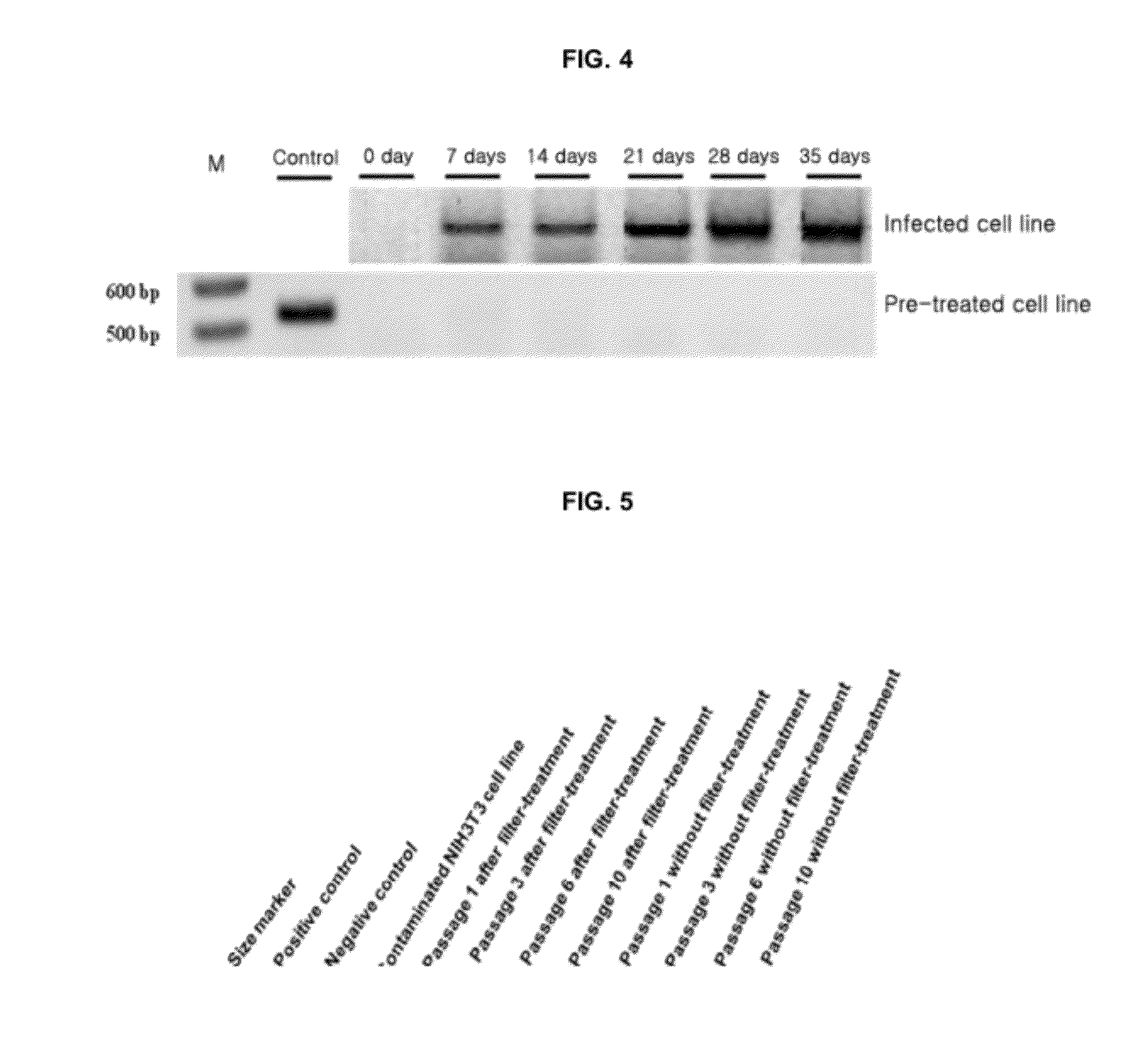
Provided is a method for culturing cells, which prevents the possibility of mycoplasma contamination by culturing the cells using biomass extract obtained by culturing a strain with amphidinol productivity, preferably a strain such as Amphidinium klebsii, Amphididinium carterae, and the like belonging to Dinoflagellates, or using a fraction obtained from the extract; and a method for removing mycoplasma contamination of the cells by culturing the cells contaminated by mycoplasma using the amphidinol derivatives. In addition, provided is a method for removing mycoplasma contamination of the cells, including a combination of single cell-isolating enzyme solution treatment and cell aggregate removal.
Owner:CELLSAFE
Detection primers, kit and detection method for cell mcoplasma
InactiveCN108753996ASimple amplification procedureEasy to operateMicrobiological testing/measurementMicroorganism based processesMycoplasma contaminationBuffer solution
The invention belongs to the technical field of biology and particularly relates to detection primers, a kit and a detection method for cell mcoplasma. The kit comprises a combination of two pairs ofprimers required for PCR (Polymerase Chain Reaction) amplification, wherein the primer combination can specifically amplify approximate 250bp sequences between 16S genes and 23S genes in various mycoplasmas; meanwhile, the amplification of two pairs of primers ensures the accuracy of test results. The kit also comprises a pre-reactive mixture required for the PCR amplification; the mixture comprises Taq enzymes, dNTP, a buffer solution and the like; the kit disclosed by the invention can quickly and efficiently distinguish whether mycoplasma contamination in the cell occurs or not and can obtain accurate detection result; meanwhile, batch detection can be realized.
Owner:JIANGSU UNIV
Method for removing mycoplasma contamination in tumor cells and application of method
PendingCN110317789AReduce harmEasy to operateCell dissociation methodsTumor/cancer cellsMycoplasma contaminationTreatment period
The invention relates to a method for removing mycoplasma contamination in tumor cells and application of the method. The method comprises the steps that a tumor cell line with the positive mycoplasmas is transplanted into a mouse body with the NSI immune deficiency, the mouse is bred, then the tumor cell line is separated from the inside of the mouse body, and a tumor cell line with the negativemycoplasmas is obtained. Compared with a frequently-used method for removing mycoplasma contamination in cells at present, the provided method is simpler in operation process and safer, the factors ofantibiotics and high temperature are avoided, the damage to the cells can be reduced to the minimum, the treatment period is short, mycoplasma contamination in the tumor cells can also be thoroughlyremoved, and the relapse phenomenon cannot be easily caused.
Owner:CANCER CENT OF GUANGZHOU MEDICAL UNIV
Reagent, kit and treatment for treating mycoplasma contamination
InactiveCN106047797AImprove the effect of pollution treatmentReduce harmCulture processVertebrate cellsTreatment effectMedicine
The present invention discloses a reagent, a kit and a treatment method for treating mycoplasma contamination. The reagent is a solution containing antibiotic tiamulin, minocycline and antibiotic ciprofloxacin, wherein a concentration ratio of the antibiotic tiamulin to the minocycline to the antibiotic ciprofloxacin is 4:1:3. According to the present invention, with the reagent, the kit and the treatment method, the combination comprising the antibiotic tiamulin, the minocycline and the antibiotic ciprofloxacin according to the specific ratio is used, such that the mycoplasma contamination treatment effect can be significantly increased; and the mycoplasma contamination problem can be simply, safely, rapidly and effectively treated.
Owner:SHANGHAI XP BIOMED
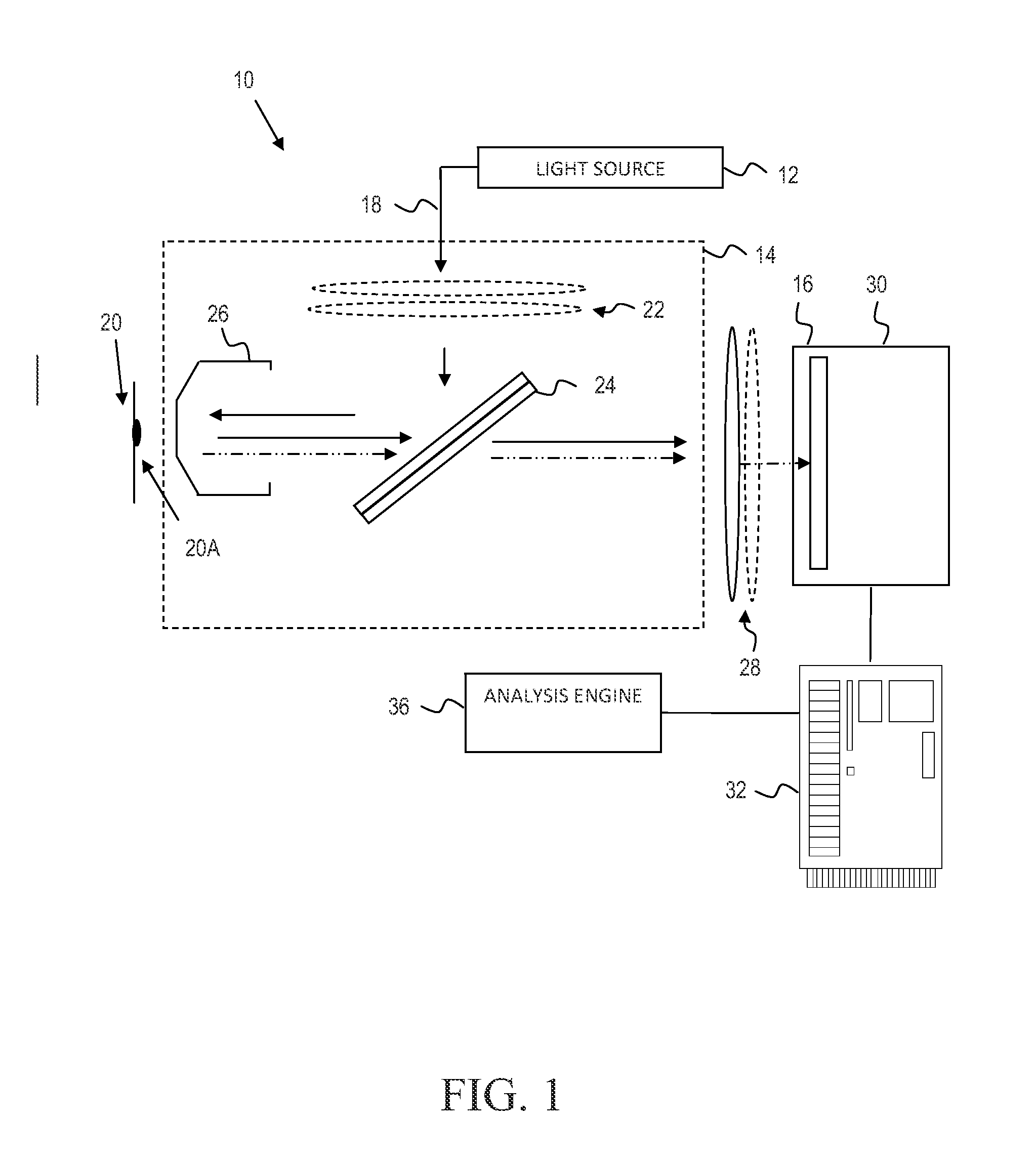
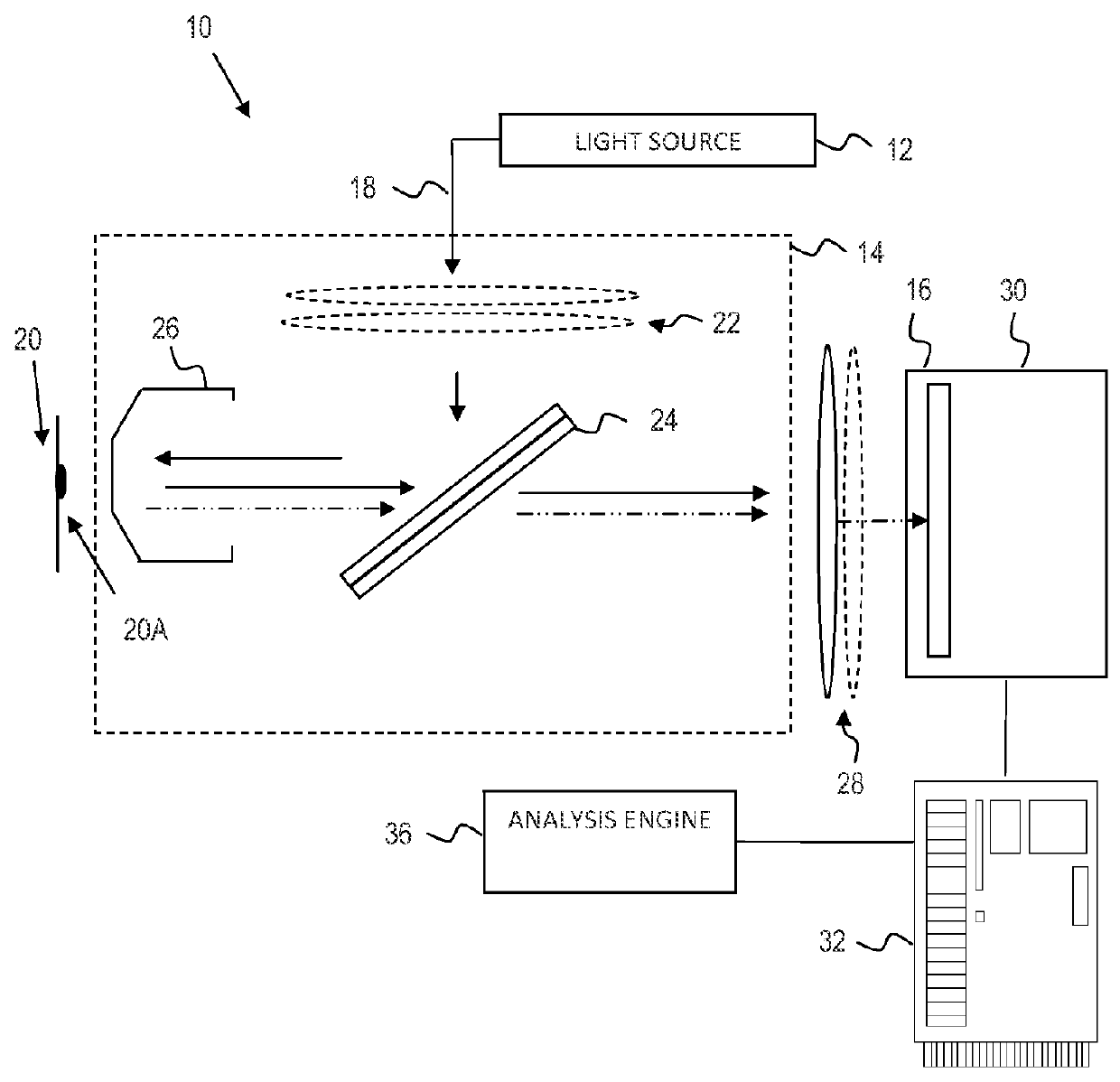
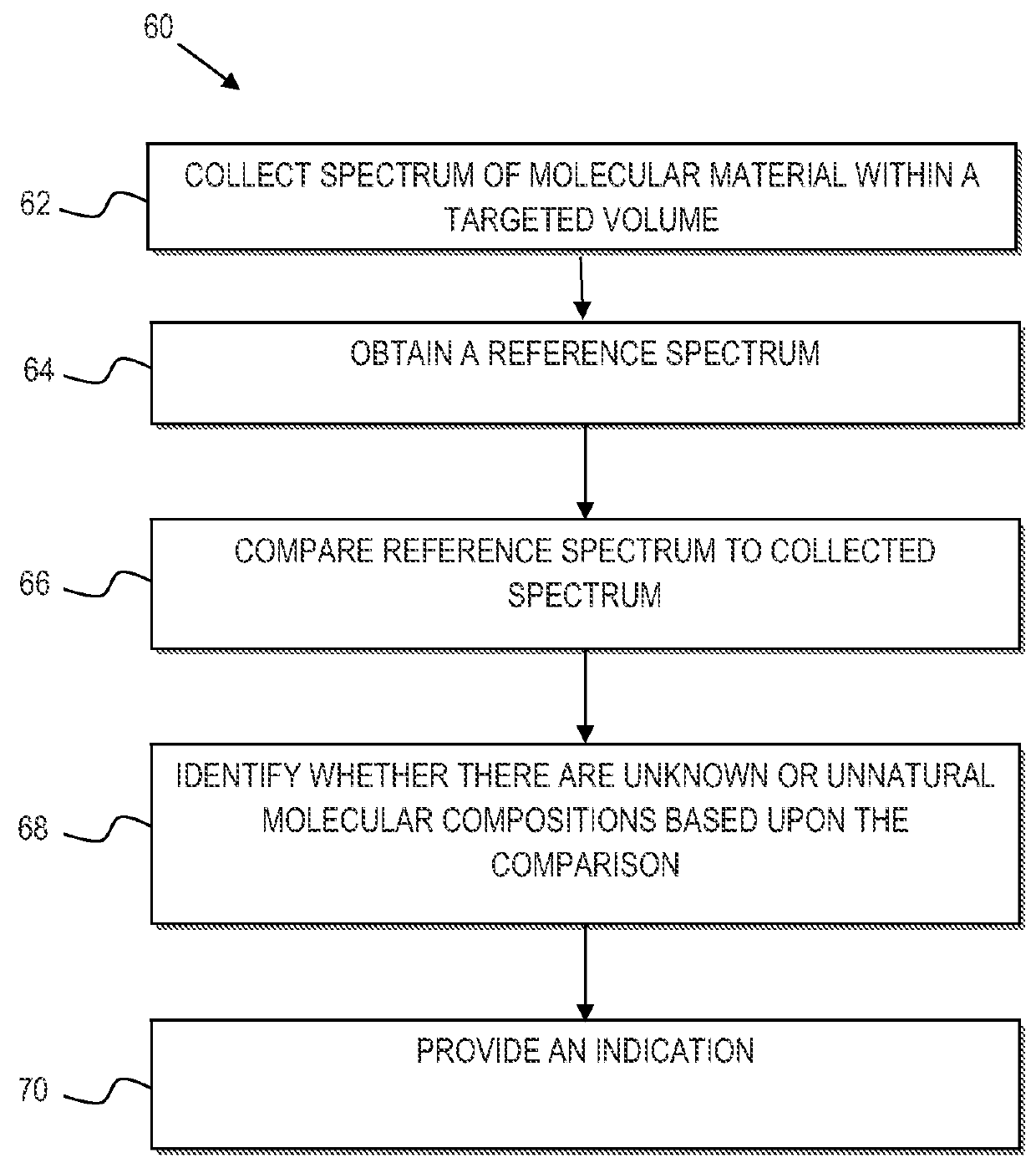
Identification of mycoplasm contamination in biotechnology production using raman spectroscopy
ActiveUS20140178924A1Bioreactor/fermenter combinationsBiological substance pretreatmentsMolecular compositionFrequency spectrum
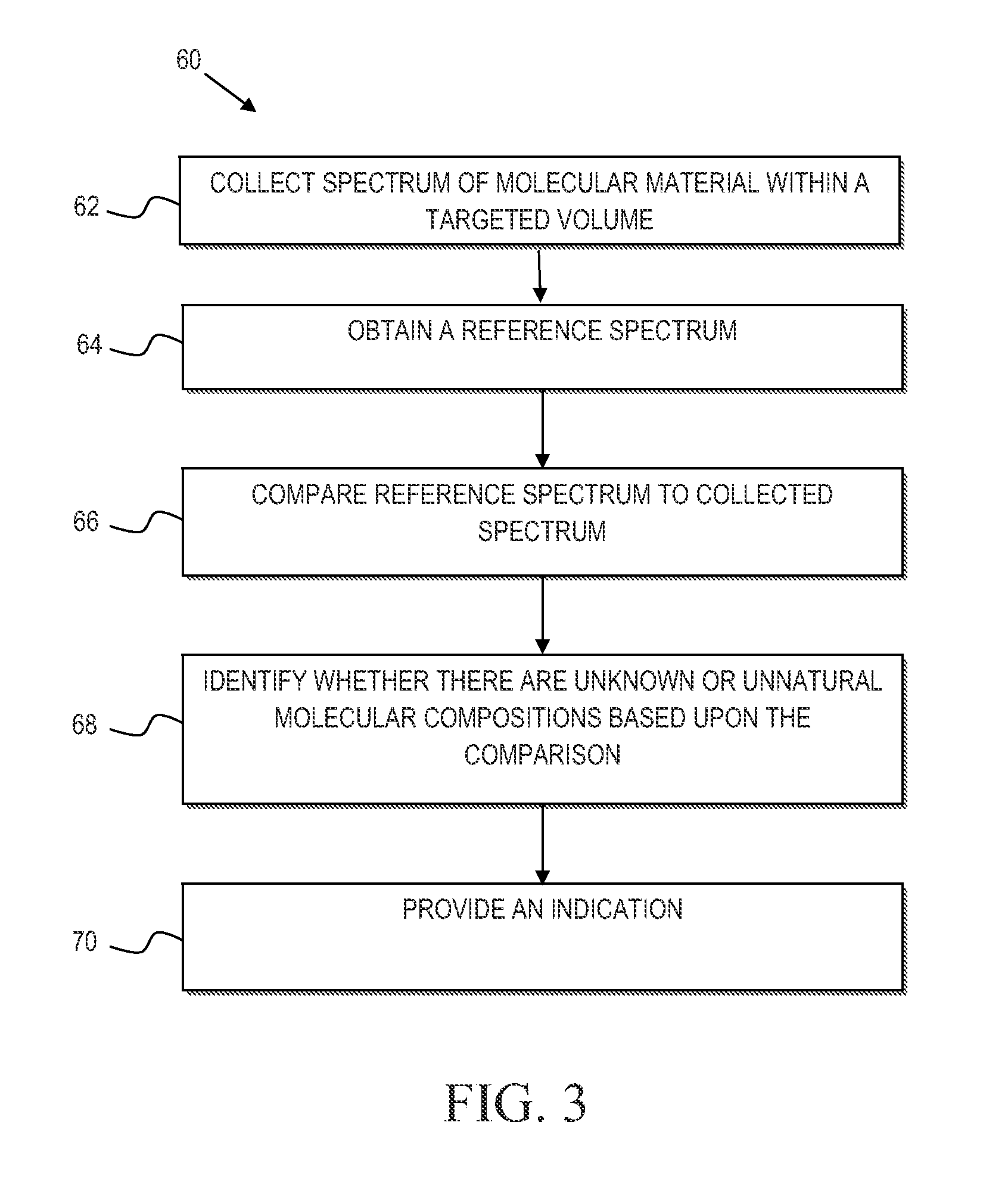
Mycoplasma contamination of a known cell line is detected by collecting a Raman spectrum of a targeted volume within a sample, the targeted volume containing a known cell line of interest, obtaining a reference spectrum uniquely associated with the known cell line where the obtained reference spectrum is known to be free of mycoplasma and comparing, using a processing device, the reference spectrum to the collected spectrum. Mycoplasma is further detected by identifying whether there are unnatural molecular compositions within the collected spectrum based upon the comparison of the reference spectrum to the collected spectrum and providing an indication as to whether mycoplasma is detected in the collected Raman spectrum based upon whether unnatural molecular compositions are identified within the collected spectrum.
Owner:BATTELLE MEMORIAL INST
Method for the specific detection of low abundance RNA species in a biological sample
ActiveUS20100124748A1Low abundanceMicrobiological testing/measurementSpecific detectionMycoplasma contamination
The present invention relates to a method and a kit for the detection of low abundance RNA species in a biological sample and to a method and a kit for the detection of a mycoplasma contamination in a biological sample.
Owner:TAKEDA PHARMA CO LTD
GPD1L-deleted human embryonic stem cell strain and construction method and application thereof
PendingCN113801852ANo pollution in the processStable karyotypeGenetically modified cellsMicrobiological testing/measurementGerm layerEmbryo
The invention provides a GPD1L-deleted human embryonic stem cell strain and a construction method and application thereof, and relates to the technical field of genetic engineering. According to a gene editing principle, sgRNA of a targeted GPD1L gene exon 4 is synthesized, a gene knockout plasmid is constructed, screening is carried out after human embryonic stem cells are transfected, a basic group A is inserted into the obtained GPD1L diallele exon 4, a termination codon TGA appears at the 157 amino acid position of protein after GPD1L expression, and protein translation is terminated in advance. The GPD1L-deleted human embryonic stem cell line has the advantages of GPD1L protein deletion, normal karyotype, normal stem cell pluripotent marker and good continuous passage stability, trigerm teratoma can be formed in an animal body, and mycoplasma pollution is avoided. The GPD1L-deleted human embryonic stem cell H9 line can be used for researching the influence of GPD1L deletion on the function of differentiated myocardial cells and the screening of therapeutic drugs.
Owner:QIQIHAR MEDICAL UNIVERSITY
Composition for efficiently removing mycoplasma contamination in cell culture process
Owner:南京千济诺生物科技有限公司
A processing method for contaminated suspension cultured cell
InactiveCN107779425AEasy to handleBiological preparation purgeAfter treatmentMycoplasma contamination
The invention discloses a method for treating contamination of suspension culture cells. The specific steps of the treatment method for suspension culture cells after contamination are as follows: S1, identification of pollution types, S2, treatment of fungal pollution, S3 treatment of mycoplasma pollution, S4 Bacterial contamination treatment. The method first distinguishes different pollution categories through different identification methods, and then treats different pollution sources separately. Compared with the traditional treatment method after cell pollution occurs, the present invention is simple and easy to implement, and has better treatment effect.
Owner:金时代进出口贸易重庆有限公司
LAMP primer group for detecting broad-spectrum mycoplasma, kit and application of LAMP primer group
InactiveCN105755161AAvoid pollutionExcellent broad spectrumMicrobiological testing/measurementMicroorganism based processesMycoplasma contaminationBioinformatics
The invention discloses an LAMP primer group for detecting broad-spectrum mycoplasmas. The LAMP primer group consists of an outer primer F3, an outer primer B3, an inner primer FIP, an inner primer BIP, a loop primer LF and a loop primer LB, wherein the primers are respectively shown as SEQ ID NO.1-6. The invention further discloses a mycoplasma visible detection kit with the primer group, and application of the mycoplasma visible detection kit. The kit disclosed by the invention has advantages of good mycoplasma specificity, sensitivity, rapid and efficient amplification, visible identification and the like. By adoption of the detection system disclosed by the invention, normal mycoplasma contamination can be rapidly and sensitively detected in the cell culture process under an isothermal condition of 60-62 DEG C, no complex instrument is needed, and mycoplasma contamination detection in a cell laboratory at regular time can be relatively well achieved.
Owner:YEASEN BIOTECHNOLOGY (SHANGHAI) CO LTD
Method capable of improving cell state and application thereof
PendingCN110272866AReduce harmEasy to operateCell dissociation methodsBlood/immune system cellsImmunodeficient MouseMycoplasma contamination
The invention relates to a method capable of improving the cell state and application thereof. The method comprises the steps of transplanting a mycoplasma positive cell line into the body of an immunodeficient mouse, raising the mouse, and separating a cell line from the body of the mouse to obtain a mycoplasma negative cell line. Compared with current common methods for removing mycoplasma contamination in cells, the method for removing mycoplasma contamination in cells has a simpler and safer operation flow; meanwhile, the method avoids antibiotic and high-temperature factors, can minimize the damage to cells, has a shorter treatment cycle and can also completely remove mycoplasma contamination without a relapse.
Owner:CANCER CENT OF GUANGZHOU MEDICAL UNIV
Method for eliminating mycoplasma contamination of PCV2 (Porcine Circovirus Virus 2)
The invention relates to the technical field of biology, and aims to provide a method for eliminating mycoplasma contamination of PCV2 (Porcine Circovirus Virus 2). The method for eliminating the mycoplasma contamination of the PCV2 comprises the following steps: filtering the PCV2 through a 0.1-micrometer bacteria filter, and breeding for 4 generations; treating two generations of the PCV2 by using Plasmocin with the final concentration of 2.5 micrograms / milliliter, and conventionally breeding the PCV2 for two generations. The method provided by the invention saves the experiment cost and more importantly, has the advantages of few antibiotic usage times, low concentration and short mycoplasma removing time by adopting a method of firstly filtering and then using antibiotics and can complete the integral treatment process for only 8 generations and 16-20 days without lowering the titer of the PCV2 viruses, provide the mycoplasma-free and high-titer PCV2 viruses for vaccine production and provide the guarantee for the increase of the virus content of a vaccine semi-finished product within unit volume.
Owner:HANGZHOU JIANLIANG VETERINARY BIOLOGICAL PREPARATIONS CO LTD
PCR (Polymerase Chain Reaction) kit for detecting mycoplasma pollution in cell culture and application of PCR kit
PendingCN114350825AHigh sensitivityHighly spectralMicrobiological testing/measurementMicroorganism based processesMycoplasma contaminationNucleic acid sequencing
The invention provides a PCR (Polymerase Chain Reaction) kit for detecting mycoplasma pollution in cell culture and application of the PCR kit. The composition disclosed by the invention comprises the following sequences: (1) an upstream primer Primer-Myc-F: TGAGTAGTATGCTCGCAAGAGTG, (2) an upstream primer, (3) a downstream primer, (4) an upstream primer and (5) a downstream primer, wherein the upstream primer is used for PCR (Polymerase Chain Reaction) amplification of a mycoplasma nucleic acid sequence; and (2) a downstream primer Primer-Myc-R: CGACACGAGCTGACGACAAC, which is used for carrying out PCR (Polymerase Chain Reaction) amplification on the nucleic acid sequence of the mycoplasma. The composition can be used for preparing a PCR kit. The composition or the kit can be used for mycoplasma detection. Compared with the existing PCR (Polymerase Chain Reaction) primer amplification method, the primer pair disclosed by the invention is optimized, so that Primer-BLAST shows that 528 products can be amplified, and the primer pair has high spectral property.
Owner:苏州鉴达生物科技有限公司
Identification of mycoplasm contamination in biotechnology production using Raman spectroscopy
ActiveUS9334520B2Radiation pyrometryMicrobiological testing/measurementMolecular compositionMycoplasma contamination
Owner:BATTELLE MEMORIAL INST
Porcine testicular cell line st-s that can be cultured in suspension and its obtaining method and application
The invention discloses a swine testicular cell strain ST-S suitable for suspension culture as well as an acquisition method and an application of the swine testicular cell strain ST-S. ST cells, which are subjected to adherent culture, are domesticated into ST cells which are suitable for suspension culture and can be passaged stably, and the ST cells are named as ST-S; and the ST-S cells keep sensitivity to classical swine fever virus (CSFV) and are capable of avoiding mycoplasma contamination. The invention provides a large-scale industrial suspension culture mode on the ST-S cells, and the suspension culture mode is applicable to culture by virtue of a shake flask and a bioreactor. By virtue of the ST-S cells obtained from the suspension culture, vaccines or other bio-products can be produced, so that a virus production process is simplified, product stability is enhanced, serum dosage and production cost are reduced and production efficiency is improved.
Owner:JINYUBAOLING BIO PHARMA CO LTD +1
Product for prevention and control of pollution of cell culture
Owner:YUNNAN ANIMAL SCI & VETERINARY INST
Reagent for eliminating mycoplasma contamination in cell culture and use method thereof
The invention provides a reagent for eliminating mycoplasma contamination in cell culture and a use method thereof. The reagent is prepared by mixing liquid A and liquid B isometrically or mixing liquid A, liquid B and liquid C isometrically, wherein the liquid A is prepared by mixing semisynthetic pleuromutilin derivatives and 0.01MPBS, and the mass-volume percentage concentration of the solute after mixing is 0.5-1.5%; the liquid B is prepared by mixing quinolone derivatives and 0.01MPBS, and the mass-volume percentage concentration of the solute after mixing is 0.5-1.5%; and the liquid C is prepared by mixing tetracycline derivatives and 0.01MPBS, and the mass-volume percentage concentration of the solute after mixing is 0.25-0.75%. The reagent and method provided by the invention have the following beneficial effects: if contamination is not severe, the effect can be displayed within a week; and after the treatment cycle is finished, black spots do not exist among the treated contaminated cells, the growth cycles of the cells return to normal, the cell morphology is normal, and aggregated growth and netting phenomena are avoided.
Owner:胡晓鹏
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com