GPD1L-deleted human embryonic stem cell strain and construction method and application thereof
A technology of human embryonic stem cells and construction methods, applied in the field of GPD1L-deficient human embryonic stem cell lines and their construction, can solve the problems of large species and physiological differences, physiological activity cannot be maintained, and the pathological process of heart disease cannot be effectively simulated. Achieve good stability and stable cell karyotype
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A method for constructing a GPD1L-deleted human embryonic stem cell line, comprising the following:
[0037] 1. Human embryonic stem cell maintenance culture and passage,
[0038] (1). In the biological safety cabinet, discard the old culture medium, wash once with PBS buffer solution, and replace with new E8 culture medium.
[0039] (2). Cells can be subcultured when the growth confluence reaches 70% to 80%. Wash once with PBS buffer, add 0.05mM EDTA, digest at 37°C for 5 minutes, gently absorb the digestion solution, add new E8 culture medium, blow and beat to make the cells fall off, the passage ratio can be determined according to the experimental situation, the general ratio is 1:6.
[0040] 2. GPD1L gene knockout sgRNA design,
[0041] (1) Download and save the CDS sequence of GPD1L: run Snapgene software, create a new DNA file, paste the CDS region sequence of GPD1L into New DNAFile, name and save for later use;
[0042] (2) Download and save the GPD1L gene sequ...
Embodiment 2
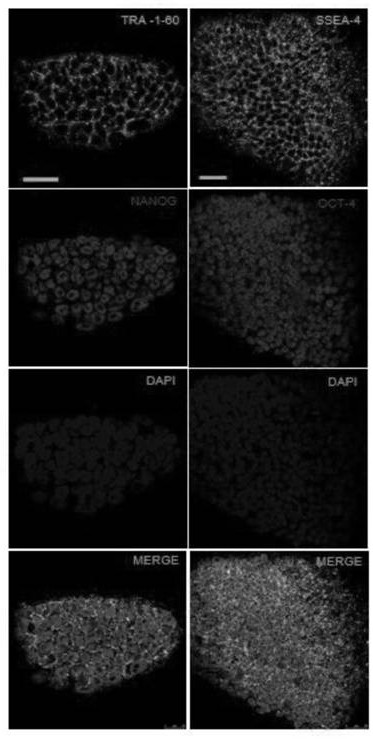
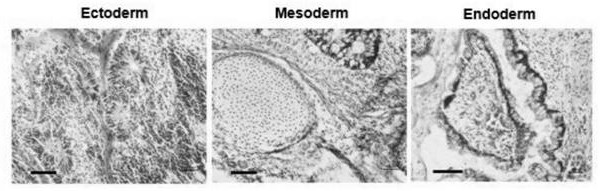
[0082] GPD1L missing human embryonic stem cell line GPD1L protein loss identification method, the specific method is
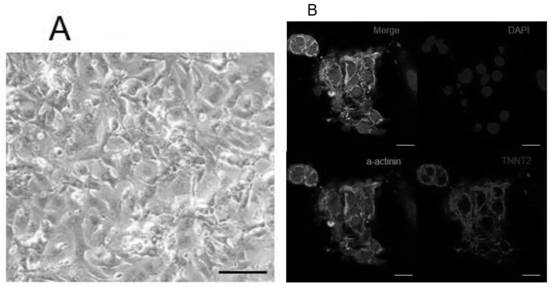
[0083] 1. Differentiation of GPD1L-deficient embryonic stem cells into cardiomyocytes
[0084] (1). Add 4ml of myocardial differentiation medium 1 to each well of GPD1L-deficient embryonic stem cells in a 6-well plate, and then add 4ml of myocardial differentiation medium 2 after culturing for 48 hours. After culturing for 48 hours, add 4ml of myocardial differentiation medium 3, and thereafter Change the myocardial differentiation medium 3 every 2 days;
[0085] (2). Taking the first day of myocardial differentiation culture medium 1 as the calculation, generally on the 8th to 10th day of myocardial differentiation, beating cardiomyocyte clusters can be seen under the microscope, and GPD1L-deficient embryonic stem cells can differentiate into cardiomyocytes.
[0086] 2. Extraction of total protein from cardiomyocytes derived from GPD1L-deficient human embryo...
Embodiment 3
[0107] GPD1L deletion human embryonic stem cell karyotype identification test, the specific method is as follows:
[0108] (1). GPD1L-deficient human embryonic stem cells are passaged into 6-well plates, and the confluence reaches about 50% for karyotype analysis;
[0109] (2). Replace the E8 culture medium containing 100ng / ml Colcemid (Gibco#15210040) for 2h;
[0110] (3). Wash once with PBS, add 0.05mM EDTA, digest for 5min, and centrifuge the cell suspension at 1000rpm for 3min to retain the cell pellet;
[0111] (4). Add 3ml of 0.075mol / L potassium chloride at 37°C to the cell pellet, mix well, and treat with hypotonicity at 37°C for 20 minutes;
[0112] (5). Add 1ml of newly prepared fixative solution (methanol:glacial acetic acid=3:1, volume ratio) for pre-fixation, mix the cell suspension, centrifuge at 1000rpm for 3min, and discard the supernatant;
[0113] (6). Add 3ml of fixative in step (5), mix gently, and let stand at room temperature for 20 minutes;
[0114] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com