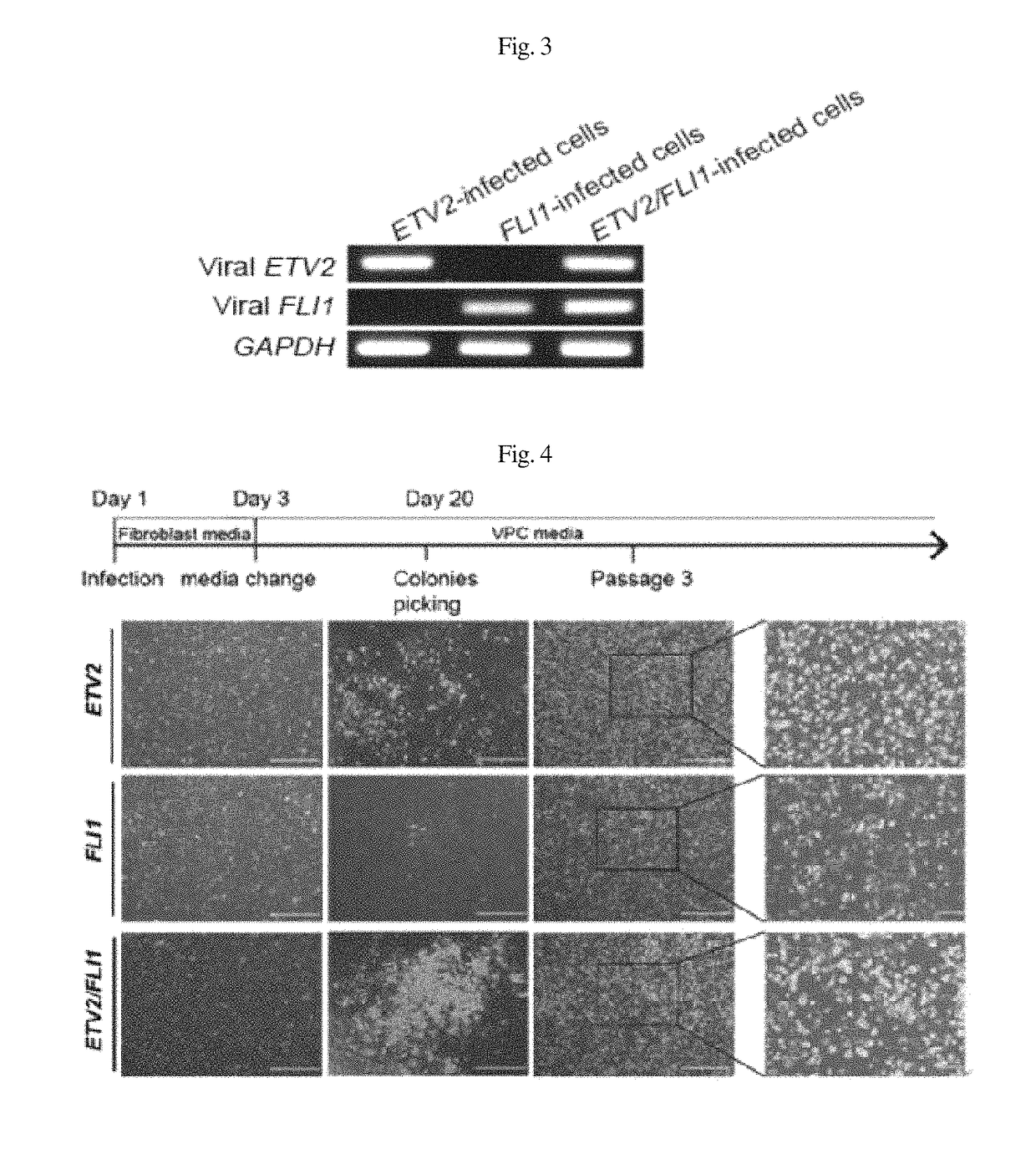
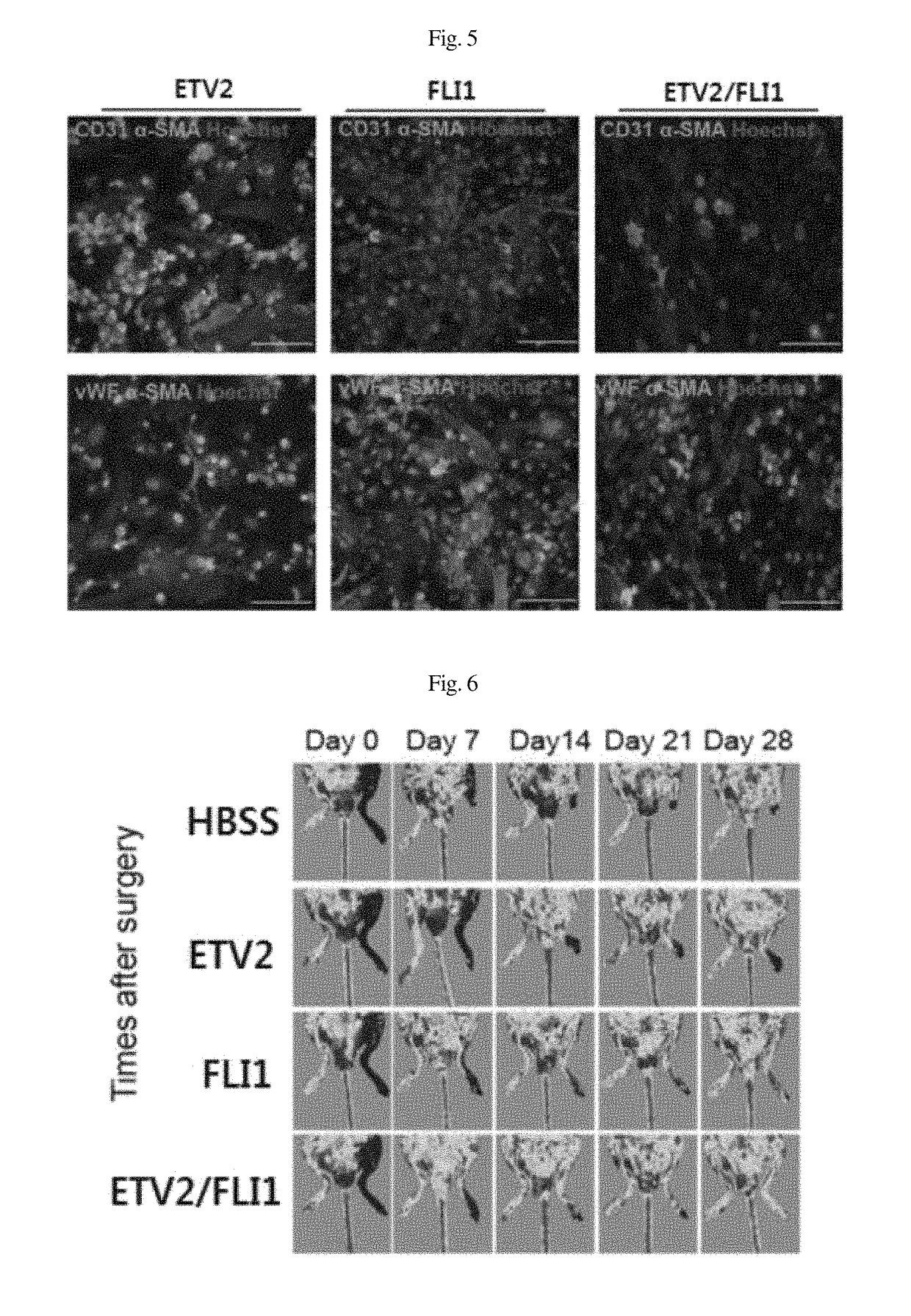
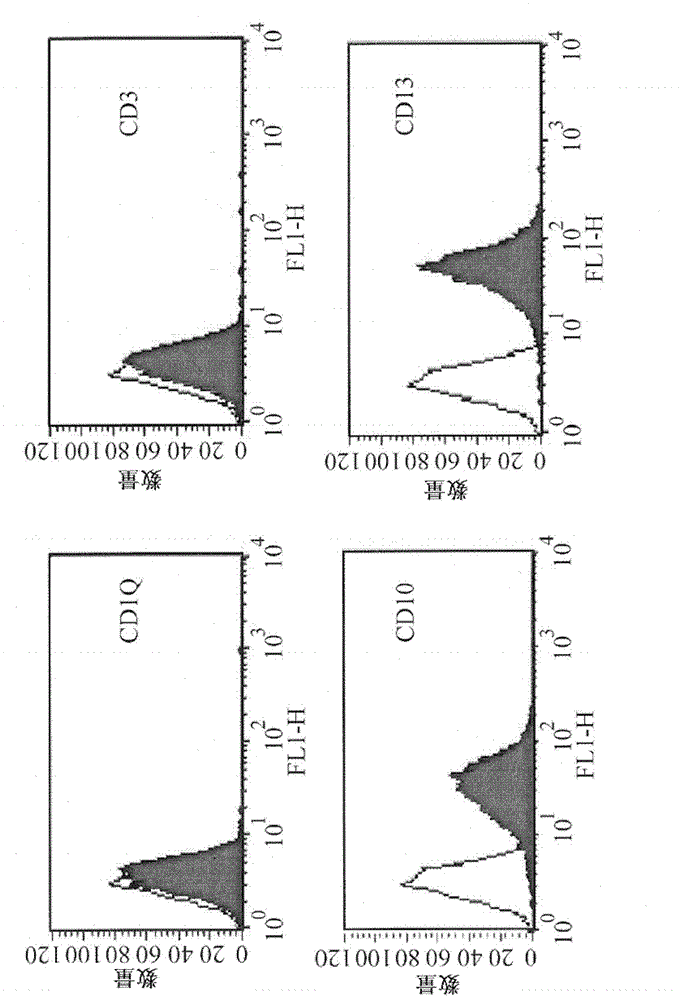
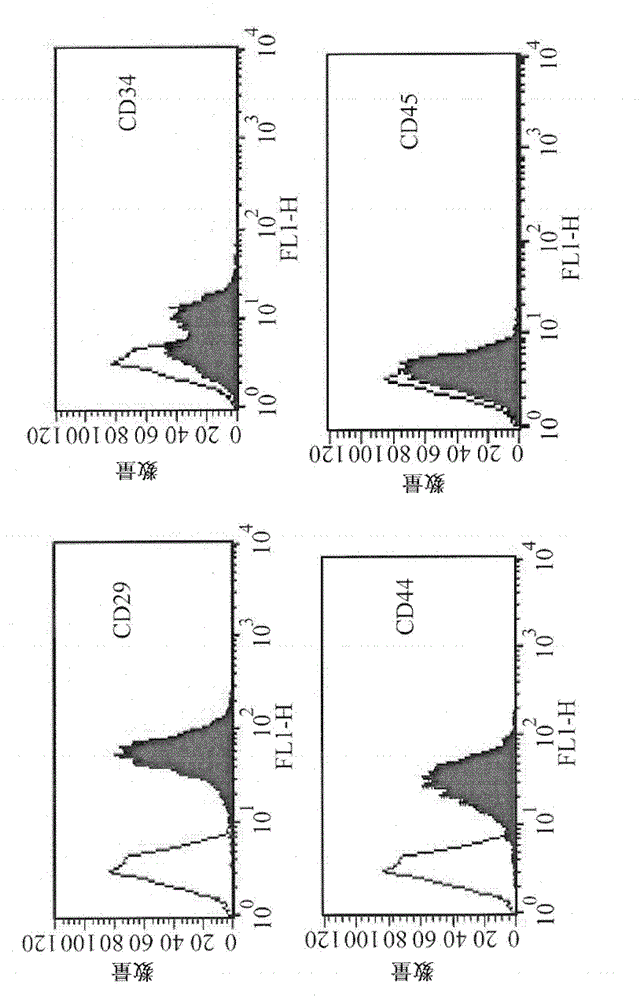
Patents
Literature
49 results about "Teratoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A type of tumor made of different tissues such as hair, muscle or bone.
Methods of Reducing Teratoma Formation During Allogeneic Stem Cell Therapy
ActiveUS20120315252A1Reduce infarct sizeImprovement in ventricular functionBiocideMammal material medical ingredientsCell therapyCell harvest
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Rat embryonic stem cell
ActiveUS20120142092A1Microbiological testing/measurementHybrid cell preparationEmbryoNa k atpase activity
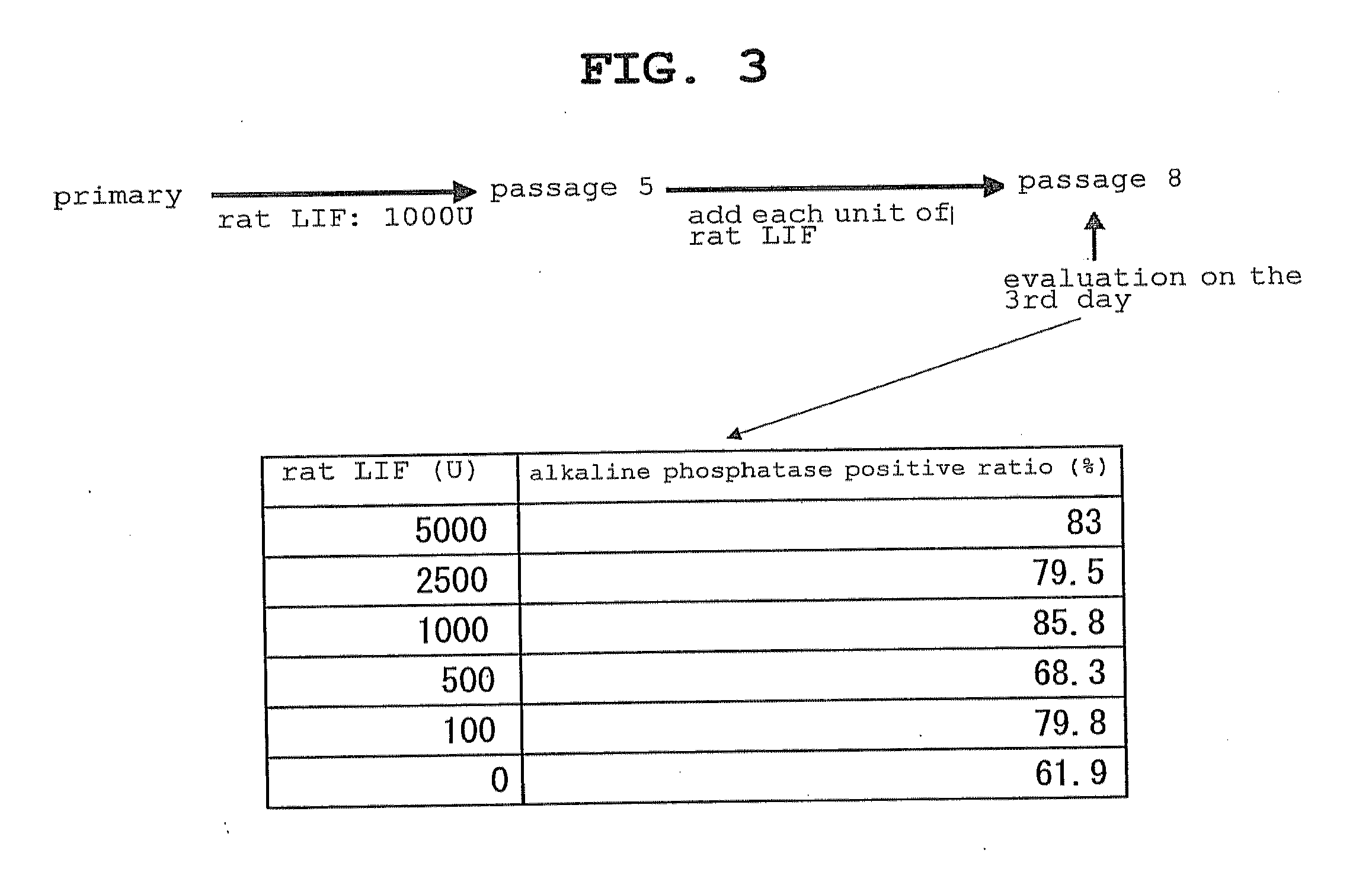
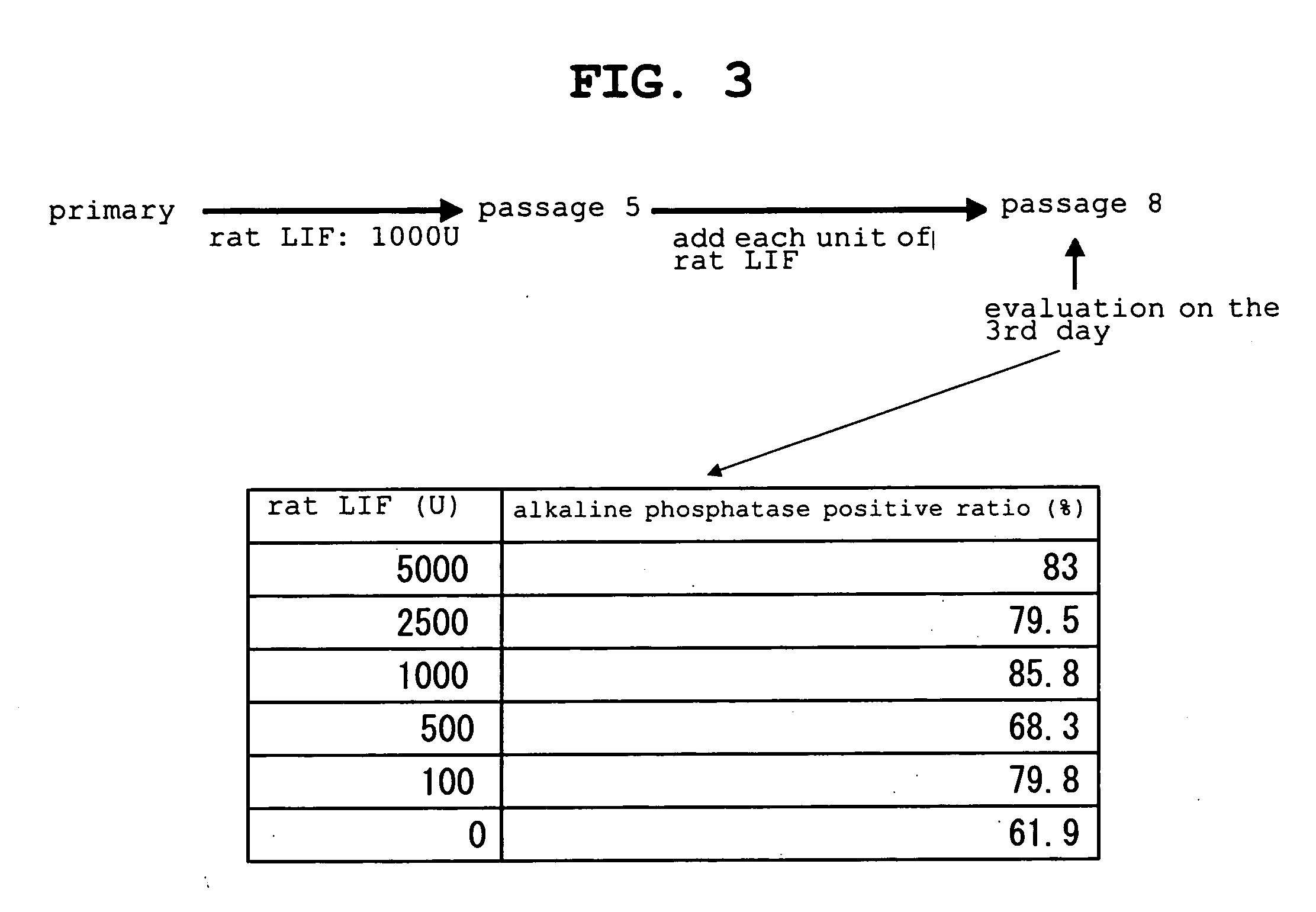
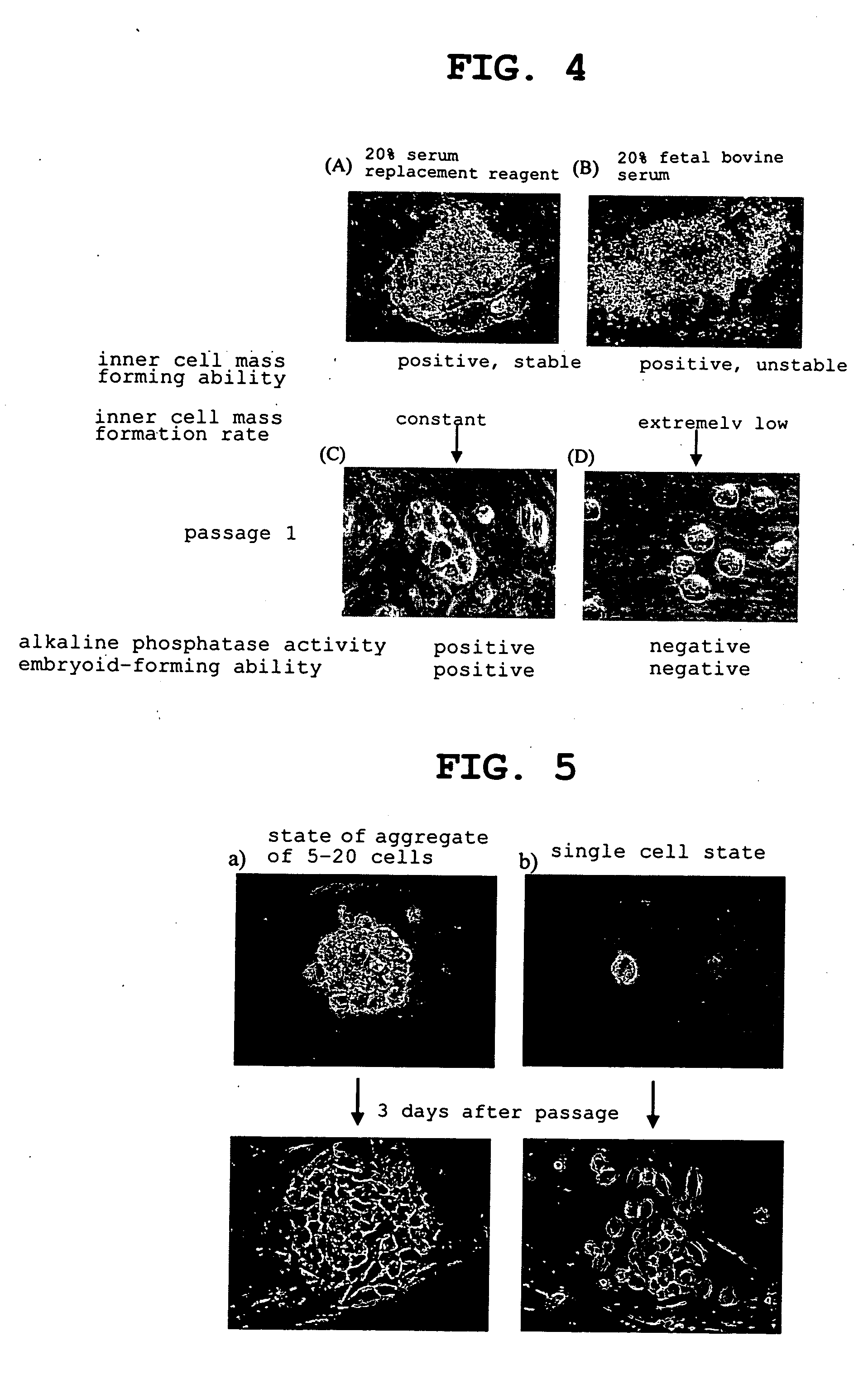
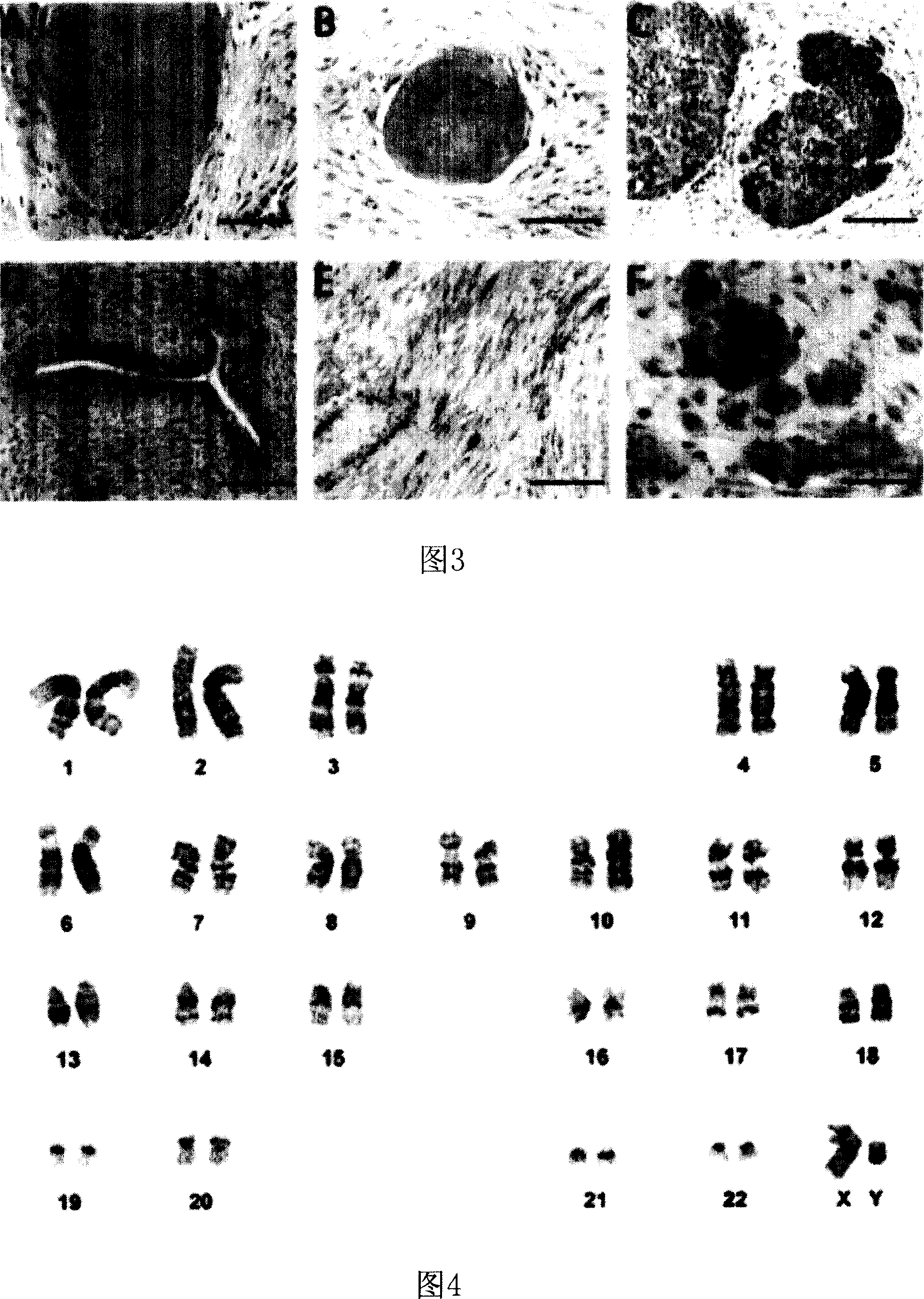
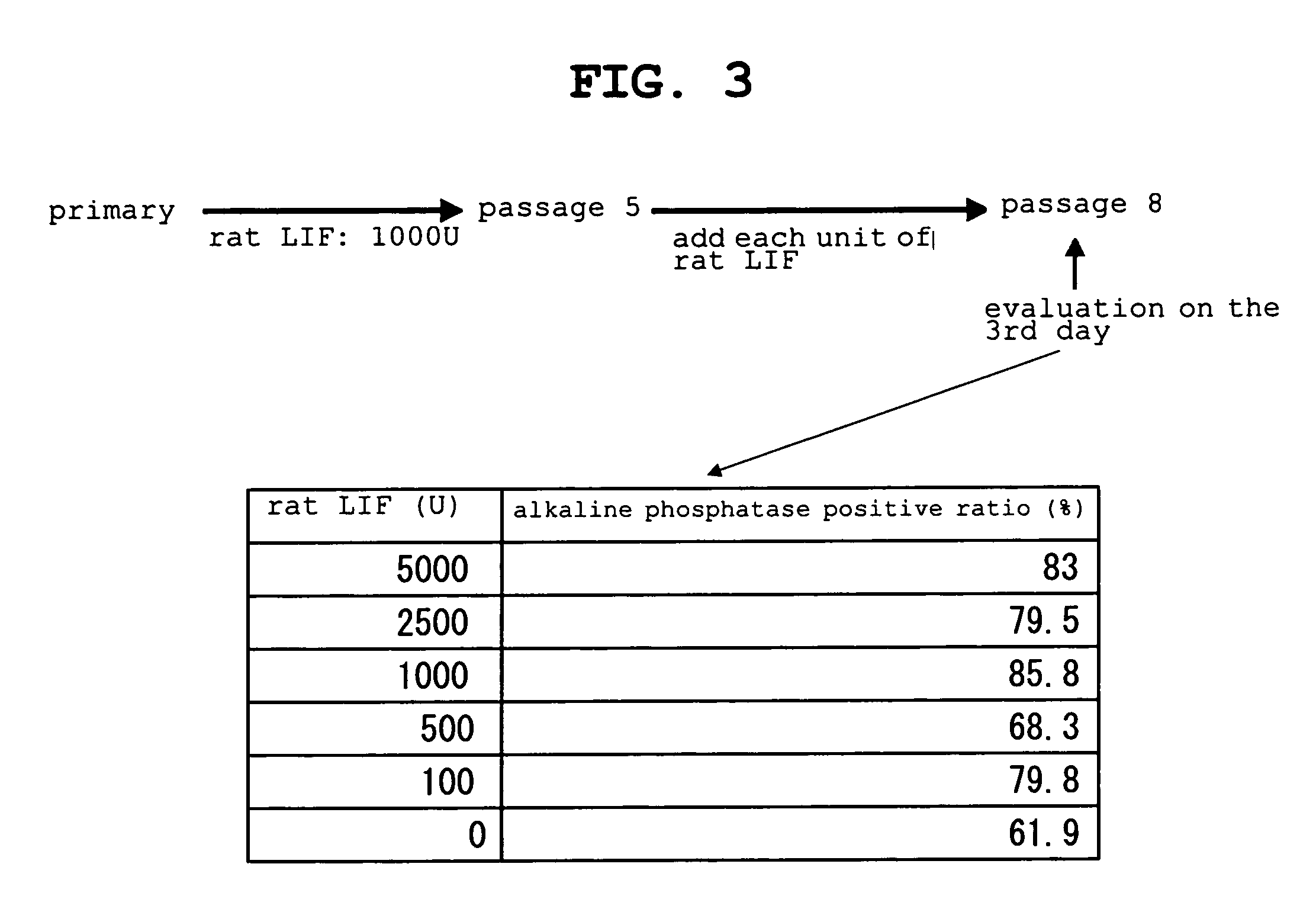
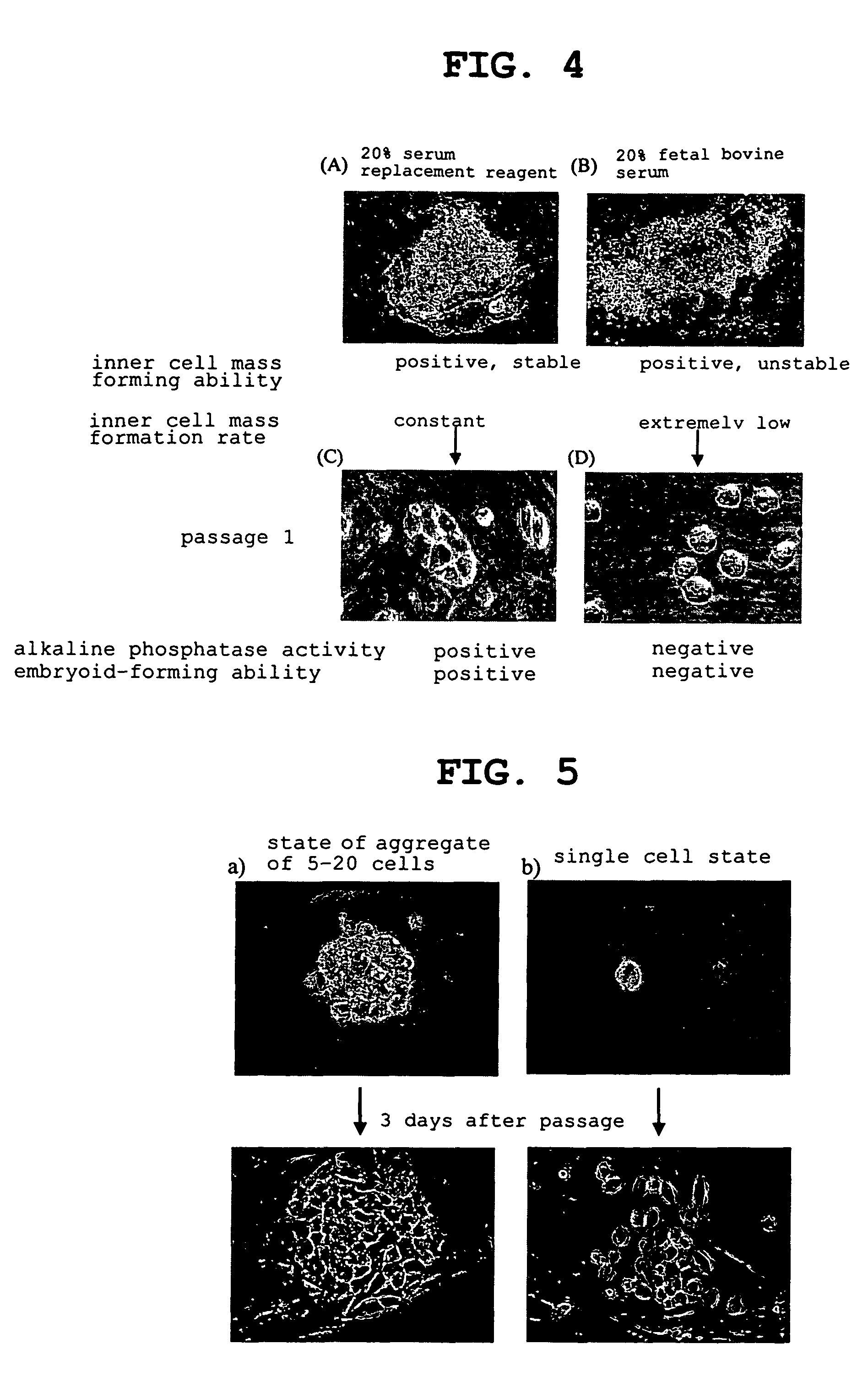
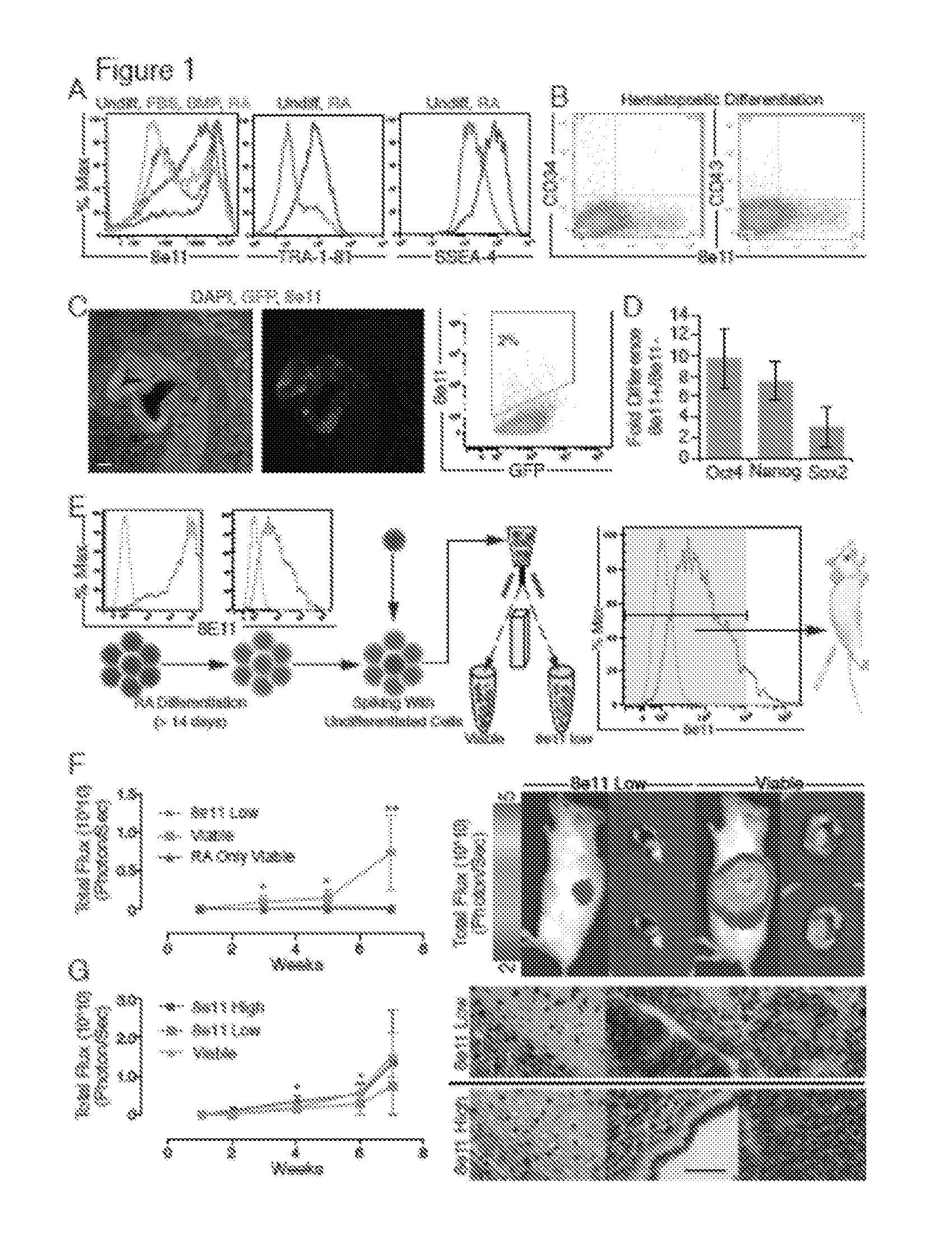
The present invention provides a rat embryonic stem cell characterized by having the following properties of (a) expressing Oct3 / 4 gene and Nanog gene, (b) positive for alkaline phosphatase activity, (c) having an embryoid body forming ability, (d) expressing SSEA (Stage-Specific Embryonic Antigen)-1 and SSEA-4, (e) having the same number of chromosomes as does a normal rat cell, (f) capable of being subcultured and holding the undifferentiated state, (g) having in vitro pluripotency, (h) having a potential to differentiate for cells of three embryonic germ lineages, (i) having teratoma formation ability, and (j) having an ability to produce a chimeric rat, a method of establishing the aforementioned rat embryonic stem cell and the like.
Owner:SUMITOMO CHEM CO LTD
Sub totipotential stem cell and preparation method and application thereof
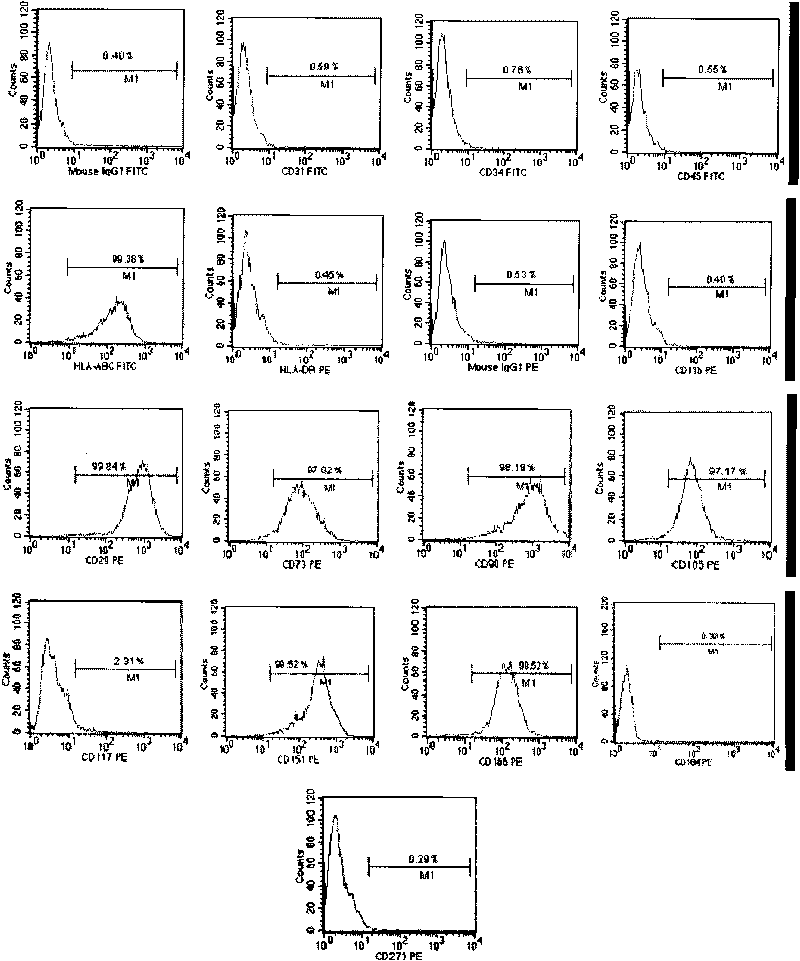
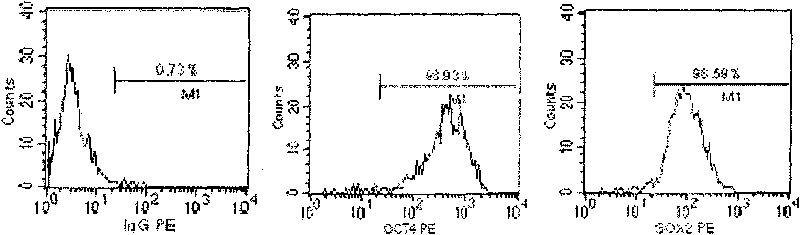
The invention discloses a method for preparing a population of?human pluripotent stem cells and the application thereof. The preparation of stem cells is characterized by comprising the following steps: CD151<+>, CD31<->, Sox<2+> pluripotent stem cells are separated and collected from human umbilical cord and or placenta tissues; the cells adhere to grow in a culture vessel under a predetermined condition and expand through passage 20 or above to be still stable in gene expression. The population of cells of this invention do not form teratoma after injection into animals. The human pluripotent stem cells highly express CD151, OCT4 and Sox-2 as specific markers of embryonic stem cells, as well as specific markers of epidermic cells, endothelial cells, thrombocytes, dendritic cells, while lack expression of CD31, CD34, CD45 and HLA-II. The pluripotent stem cells are also characterized as being able to adhere to tissue culture plastic and having the potential to differentiate into three germ layers: endoderm, mesoderm and ectoderm. These pluripotent stem cells are able to be used as carrier cells of gene therapy and for the treatment of diseases caused by cell damage or cell aging. The present invention provides a method of isolating, purifying and culturally expanding of a population of human pluripotent stem cell for preparing the high purity injection preparation. The preparation of stem cells has a good therapeutic effect on the treatment of diseases caused by cell damage or cell aging in animal and human clinical trials. The preparation also has no toxic side effect and no immune rejection.
Owner:BEIJING HEALTH & BIOTECH (H&B) CO LTD
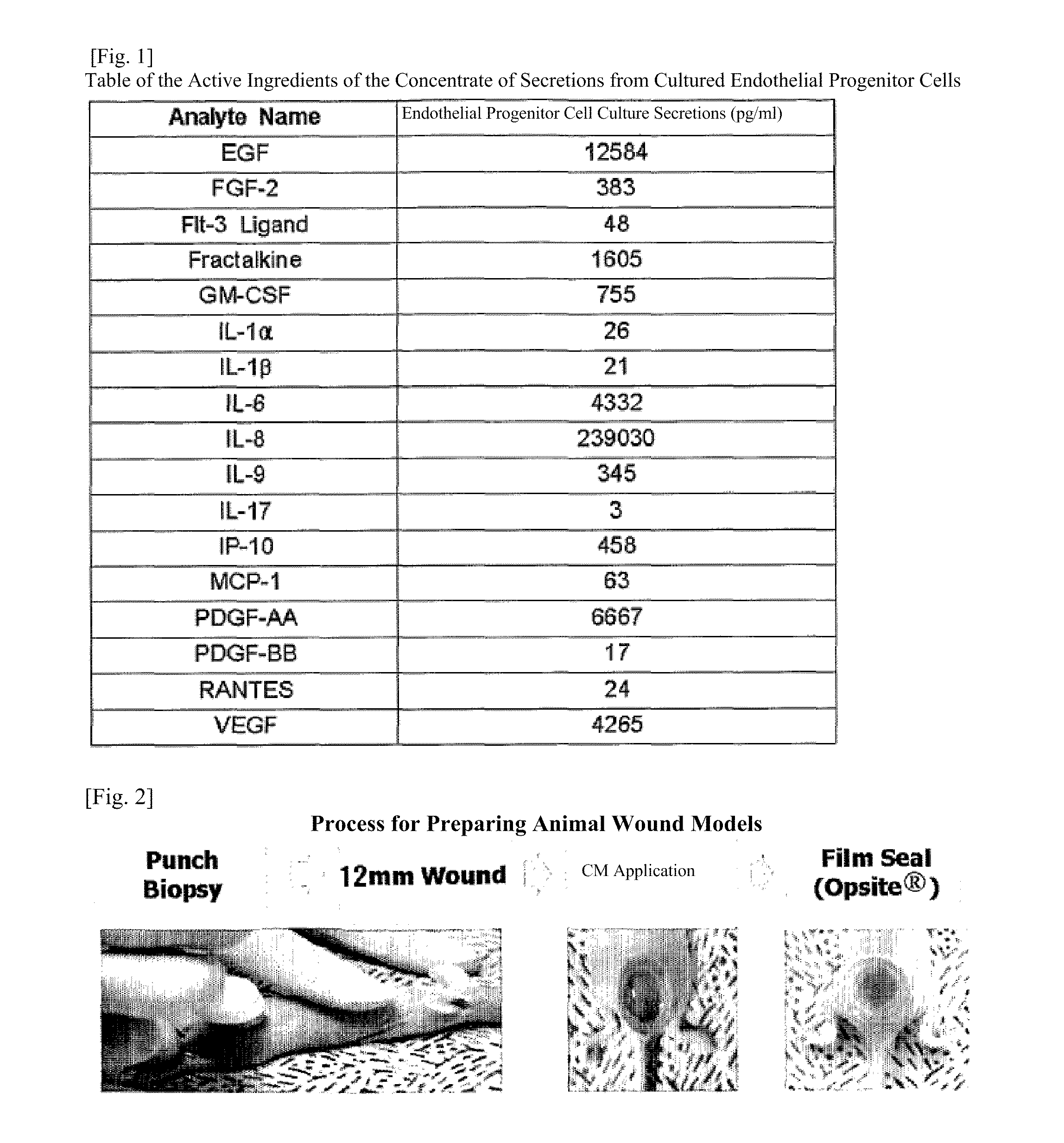
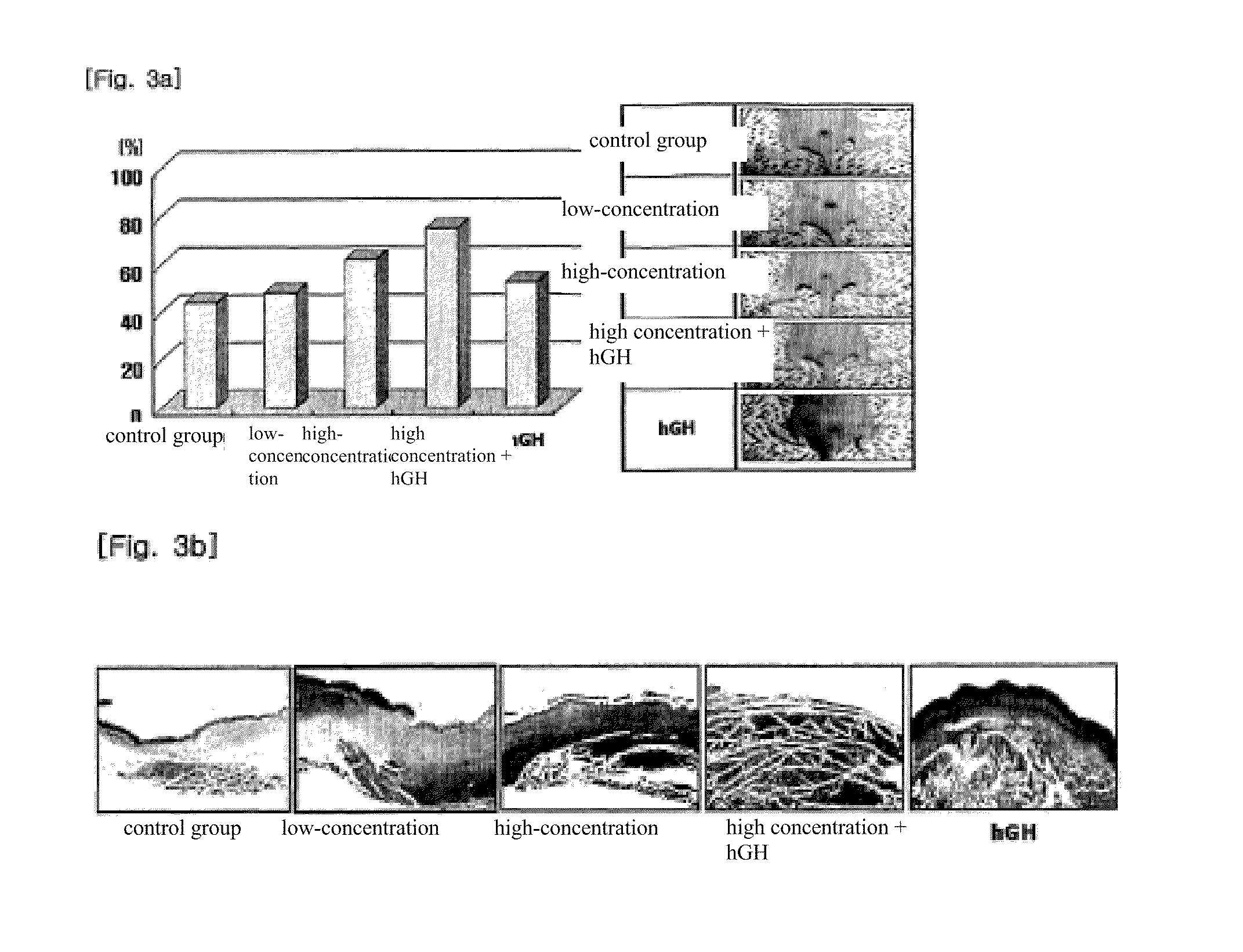
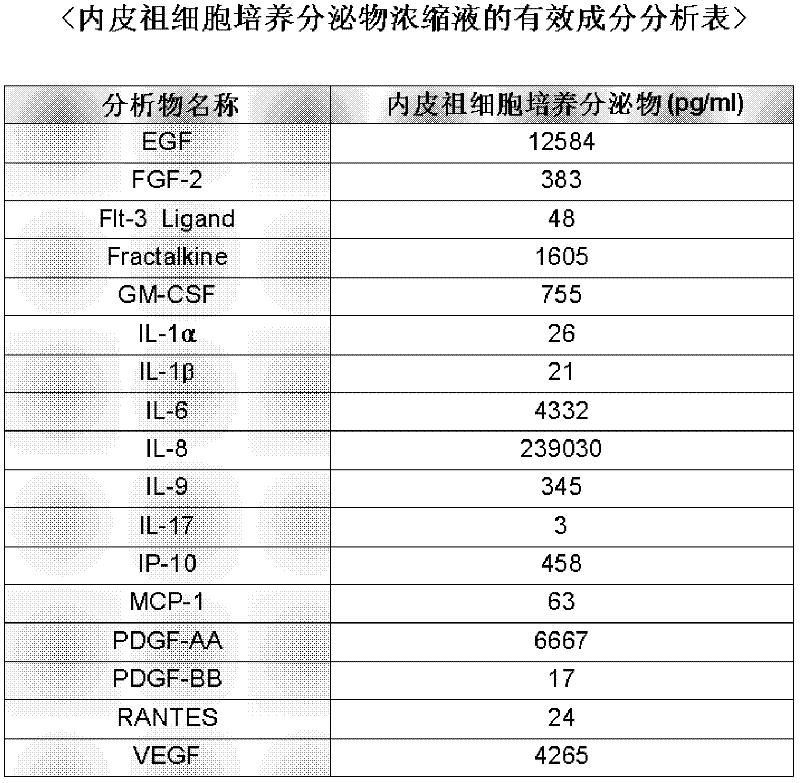
Composition for skin regeneration, containing a secretion in the culture of an embryonic stem cell-derived endothelial progenitor cell or fractions thereof, and use thereof
InactiveUS20110300102A1Improve wrinklesPrevent skin agingCosmetic preparationsPeptide/protein ingredientsHigh concentrationCollagen VI
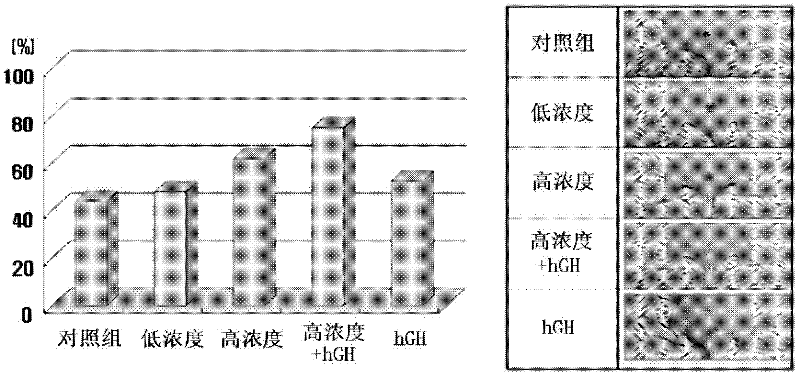
The present invention relates to a composition for skin regeneration, using a culture medium or a secretion in the culture of an embryonic stem cell-derived endothelial progenitor cell, and to the use thereof. As the present invention uses the secretion in the culture as a medicine and not the embryonic stem cell-derived endothelial progenitor cell itself, the risk of teratoma formation is prevented, and angiogenic activity is promoted to achieve improved effectiveness in healing wounds and burn wounds. The composition of the present invention, when used as a material in cosmetics, increases collagen synthesis and thus prevents skin aging. Particularly, the composition of the present invention in which the secretion in the culture is concentrated into a high concentration provides superior wound-healing effects as compared to a conventional human growth hormone (hGH).
Owner:CHABIO&DIOSTECH
Rat embryonic stem cell
The present invention provides a rat embryonic stem cell characterized by having the following properties of (a) expressing Oct3 / 4 gene and Nanog gene, (b) positive for alkaline phosphatase activity, (c) having an embryoid body forming ability, (d) expressing SSEA (Stage-Specific Embryonic Antigen)-1 and SSEA-4, (e) having the same number of chromosomes as does a normal rat cell, (f) capable of being subcultured and holding the undifferentiated state, (g) having in vitro pluripotency, (h) having a potential to differentiate for cells of three embryonic germ lineages, (i) having teratoma formation ability, and (j) having an ability to produce a chimeric rat, a method of establishing the aforementioned rat embryonic stem cell and the like.
Owner:SUMITOMO CHEM CO LTD +1
Medium and device for proliferation of stem cells and treatment of cancer-related stem cell with resveratrol
InactiveUS20100010099A1Improve survival rateStrong drug resistanceBiocideBioreactor/fermenter combinationsDiseaseSerum free media
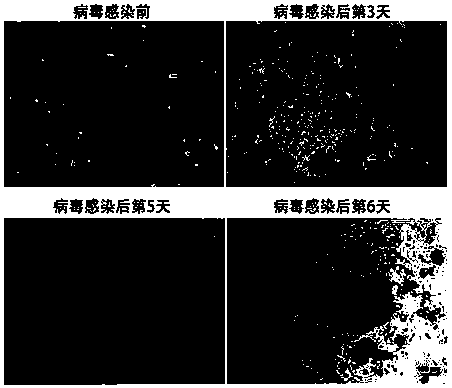
The invention relates to a device for selecting stem cells with a serum free medium for amplification of stem cells. The invention also relates to a method of treating or preventing diseases caused by cancer-related stem cells. The invention further provides a method of enhancing radiosensitivity of cancer-related stem cells comprising radiotherapy with resveratrol, and the cancer-related stem cell has stronger drug resistance. The present invention further provides that resveratrol promotes differentiation and inhibits teratoma / tumor formation in induced pluripotent stem cells (iPS) and embryonic stem cells
Owner:VETERANS GEN HOSPITAL TAIPEI
Non-tumorigenic expansion of pluripotent stem cells
InactiveUS20120270314A1Artificial cell constructsSkeletal/connective tissue cellsPluripotential stem cellInduced pluripotent stem cell
A method for expansion of human embryonic stem (hES) cells in a medium including human umbilical cord-derived mesenchymal stem cells (HUCMSCs) as a feeder is provided. The human embryonic stem cells (hES) maintain the features of embryonic stem cells in the medium, such as pluripotency, unlimited undifferentiated proliferation and normal karyotypes. Also provided is a method for non-tumorigenic expansion of the human embryonic stem cells (hES) that is free from forming teratoma.
Owner:MICROSOFT CORP +1
Use of Activin A in human embryo stem cell trophoblast-free cell culture
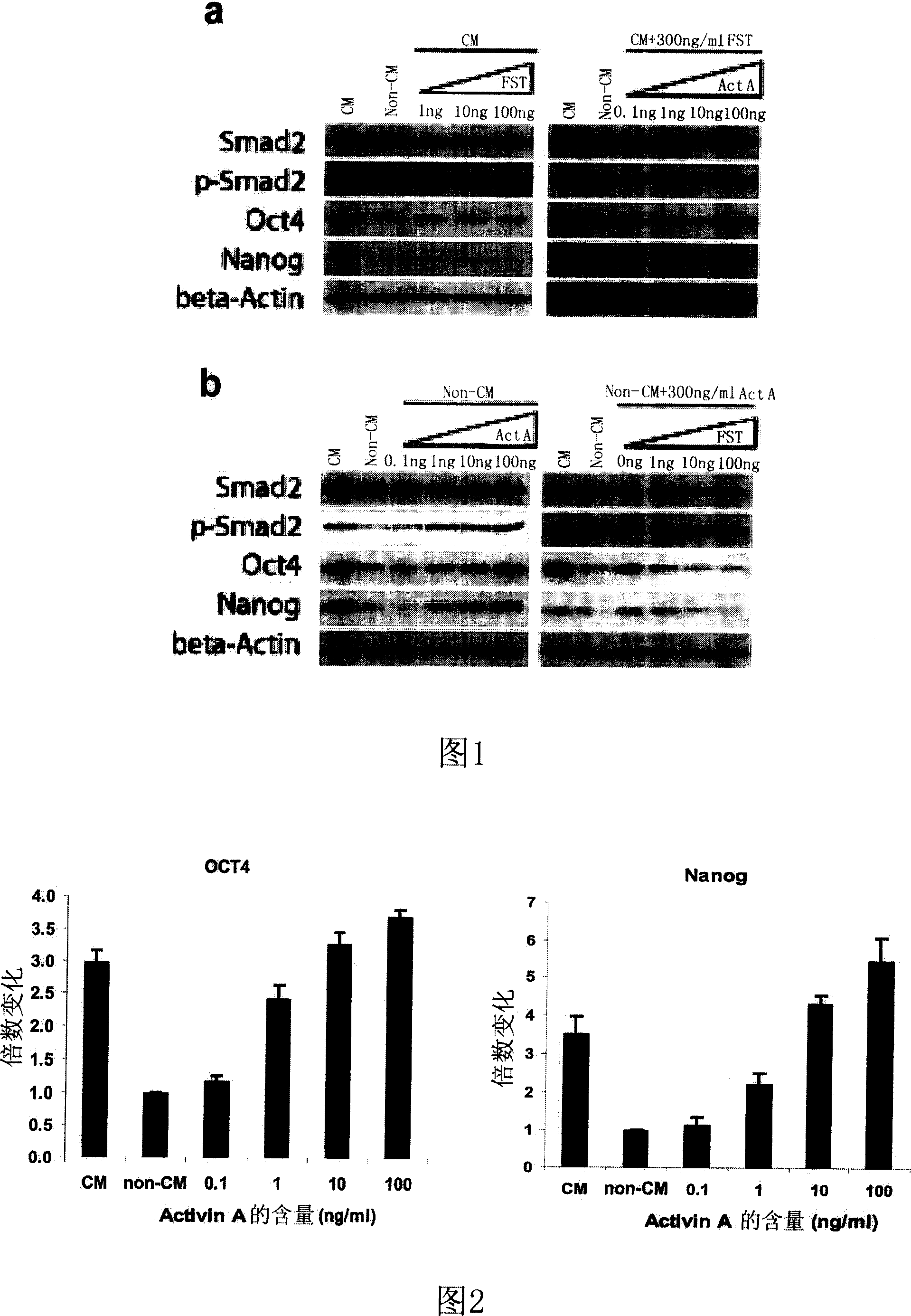
The invention discloses the application of Activin A for cell culture in human blast stem cell panoistic layer, belonging to biomedical technology field. It is necessary and adequate for Activin A to maintain self- refresh and multi-function for human blast stem cell, it can induce expression of transcription factor Oct4 and Nanog. The stem cell still possess differentiation potence in mouse body for teratoma after 10- generation culture with panoistic cell culture medium containing 5 ng / ml Activin A, and it still maintains normal karyotype after 150- day culture and more than 20- generation.
Owner:浙江煦顼技术有限公司
Composition for skin regeneration, containing a secretion in the culture of an embryonic stem cell-derived endothelial progenitor cell or fractions thereof, and use thereof
InactiveCN102333523APrevent agingElasticCosmetic preparationsToilet preparationsHigh concentrationCulture mediums
Owner:车比奥及戴奥斯泰有限公司
Serum-free feed layer-free mouse induced pluripotent stem cell (iPSC) induction medium and culture method using the same
InactiveCN107760654AShorten the timeImprove induction efficiencyCulture processCell culture active agentsGerm layerSerum free
The invention provides a serum-free feed layer-free mouse induced pluripotent stem cell (iPSC) induction medium and a culture method using the same. The medium is a serum-free feed layer-free medium,can be used as an induction medium for mouse somatic cell-induced formation of iPSCs, improves iPS cell induction efficiency, shortens the induction time and can be used for culturing mouse iPSCs. Inthe culture system, after 20 passages of mouse iPSCs, the mouse iPSCs retain stem cell pluripotency and the normal cell karyotype. An internal teratoma experiment result shows that after culture for 20 passages, the mouse iPSCs in the culture system retain a trilaminar differentiation potential of PSCs.
Owner:GUANGDONG XTEM BIOTECH CO LTD
Personalized production of biologics and method for reprogramming somatic cells
ActiveUS20120171722A1Improve efficiencyHydrolasesNew breed animal cellsSomatic cellBiologic Products
The invention provides a method for producing polypeptide protein products and nucleic acid products having reduced levels of antigenicity in an animal being treated with a biologic product. Somatic cells are isolated from an animal, transformed into pluripotent stem cells, transfected with a nucleic acid(s) of interest, and re-differentiated towards somatic cells known to be high level producers of the desired nucleic acid product. The invention can be used to derive a general cell line to treat populations, racial specific cell lines to treat ethnic groups, or patient specific cell lines to treat individuals. Additionally, the invention provides a method to allow induced pluripotent stem cells to be re-differentiated towards their somatic cell of origin so that the cells can be used to therapeutically treat an animal without resulting teratoma formation.
Owner:AVM BIOTECH
Method for inducing bovine induced pluripotent stem cells
InactiveCN102229909AColony growth state is stableNormal karyotypeViruses/bacteriophagesEmbryonic cellsGerm layerIn vivo
The invention discloses a method for inducing bovine induced pluripotent stem cells, which comprises: constructing a fusion protein lentivirus expression vector for inducing bovine induced pluripotent stem cells by using an exogenous defined factor and enhanced green fluorescent protein (EGPF) reporter gene; cultivating and passing bovine fetus fibroblast cells in which the fusion protein of the exogenous defined factor is expressed; gradually separating and cultivating cell colonies with clearly defined colony borders; and obtaining the bovine induced pluripotent stem cells, if the growth states of the cell colonies are stable, the karyotype is normal, the cell colonies are negative in alkaline phosphatase test, the expression of proteins Oct4, Nanog and SSEA-1 is positive in immunocyte chemical tests, teratoma having three embryonic layers can be differentiated in vivo, and the result proves the separated cell conolnies have the characteristics of embryonic stem cells.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Porcine somatic cell mutagensis method
InactiveCN102174466AColony growth state is stableStable growthVertebrate cellsArtificial cell constructsGerm layerStem cell culture
The invention discloses a porcine somatic cell mutagensis method. Porcine embryonic fibroblasts are infected and acted a plurality of times by using slow viruses carrying enhanced green fluorescent protein markers; the original fibrous growth forms of the porcine embryonic fibroblasts are changed step by step under a growing environment condition of a stem cell culture solution to form nest-like cell colonies; separated, cultured and sub-cultured cell colonies have clear edges and boundaries, stable growth states and normal karyotypes; alkaline phosphatase is expressed to be positive; immunocytochemical detection shows that Oct4, Nanog and SSEA-1 proteins are expressed to be positive; teratoma comprising three embryonic layers is formed by differentiation in vivo; and the result proves that the mutagenic porcine somatic cells have the stem cell properties under the action of the slow viruses.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Rat embryonic stem cell
The present invention provides a rat embryonic stem cell characterized by having the following properties of (a) expressing Oct3 / 4 gene and Nanog gene, (b) positive for alkaline phosphatase activity, (c) having an embryoid body forming ability, (d) expressing SSEA (Stage-Specific Embryonic Antigen)-1 and SSEA-4, (e) having the same number of chromosomes as does a normal rat cell, (f) capable of being subcultured and holding the undifferentiated state, (g) having in vitro pluripotency, (h) having a potential to differentiate for cells of three embryonic germ lineages, (i) having teratoma formation ability, and (j) having an ability to produce a chimeric rat, a method of establishing the aforementioned rat embryonic stem cell and the like.
Owner:SUMITOMO CHEM CO LTD +1
Preparation method of cold tablet containing Artemisia carvifolia and holly root, and application of cold tablet in teratoma cell F9 cell proliferation inhibition medicines.
InactiveCN103751424ATake a lot of medicineSmall dosePill deliveryAntineoplastic agentsLicorice rootsArtemisia carvifolia
The invention belongs to the technical field of traditional Chinese medicines, and concretely relates to a preparation method of a cold tablet containing Artemisia carvifolia and holly root, and an application of the cold tablet. The preparation method of the cold tablet containing Artemisia carvifolia and holly root is characterized in that the cold tablet is prepared by using 200g of Artemisia carvifolia, 267g of Fructus Citri Sarcodactylis, 134g of Herba Moslae, 200g of Dicliptera chinensis, 134g of licorice root, 134g of Centella asiatica and 267g of holly root as raw medicines through supercritical extraction and microwave extraction, so the glycyrrhizic acid content is greatly improved. The invention also provides an application of the cold tablet containing Artemisia carvifolia and holly root in the preparation of teratoma cell F9 cell proliferation inhibition medicines.
Owner:JINAN XINQIDIAN MEDICAL TECH
A serum-free, feeder-free pluripotent stem cell culture method
ActiveCN103555661BMaintain pluripotencyMaintain normal stateEmbryonic cellsGerm cellsFeeder LayerConceptus
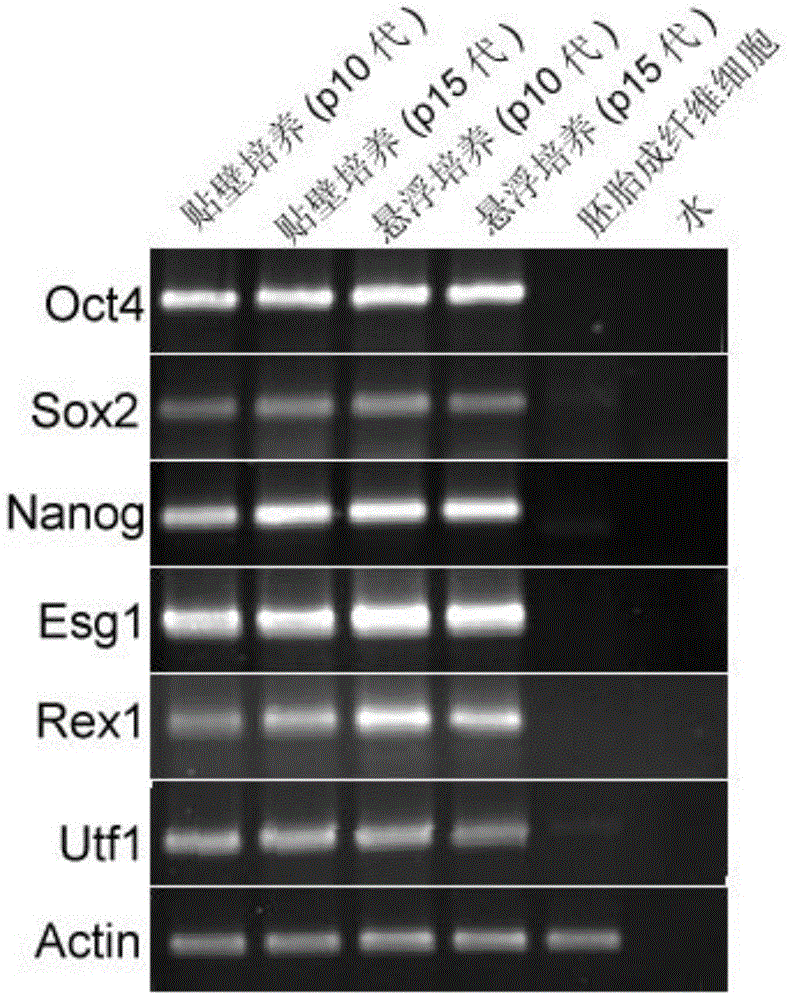
The invention relates to a novel method for culturing a multipotential stem cell without serum and a feeder layer. The method is stable, reliable, and can culture the multipotential stem cell for a long time and also can maintain the self-renewing of the multipotential stem cell. The method is characterized in that a culture system with clear chemical components and without serum and the feeder layer is adopted to perform suspension culture on the multipotential stem cell. According to the morphology of cultured stem cells after 15 generations, mRNA (Messenger Ribonucleic Acid) and protein expression of multipotential marker molecule, and analysis on cell chromatin karyotype, such novel generation system can maintain the properties of the multipotential stem cell during long-term culture. In addition, the differentiative potential of the multipotentail stem cell is further verified by 15-generation suspension culture in an in-vitro embryoid body formation experiment and an in-vivo teratoma formation experiment.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Depletion of teratoma-forming pluripotent stem cells
ActiveUS9175079B2Animal cellsPeptide/protein ingredientsInduced pluripotent stem cellMonoclonal antibody
Compositions and methods are provided for depletion of pluripotent cells. In one embodiment of the invention, methods are provided for depletion of pluripotent cells from a mixed population of differentiated cells and stem cells, to provide a population of cells substantially free of pluripotent stem cells. Monoclonal antibodies useful in depletion and in identification of pluripotent stem cells are also provided.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Preparation method of fomes officinalis cough and asthma treating tablets and application of fomes officinalis cough and asthma treating tablets in preparation of medicine for inhibiting cell proliferation of mouse teratoma cell P19
InactiveCN104288657AEasy to acceptReduce dosageFungi medical ingredientsPill deliveryOfficinalisActive ingredient
The invention belongs to the technical field of traditional Chinese medicines and particularly relates to a preparation method and application of fomes officinalis cough and asthma treating tablets. According to the preparation method of the fomes officinalis cough and asthma treating tablets, disclosed by the invention, the fomes officinalis cough and asthma treating tablets are prepared from the raw pharmaceutical materials by weight: 20g of fomes officinalis, 100g of plums, 80g of reed rhizomes, 60g of glycyrrhiza uralensis, 50g of herba ephedrae, 30g of elecampane, 120g of aniseed, 30g of nutagrass flatsedge rhizome and 20g of cardamom, through supercritical extraction and microwave extraction, so that the content of glycyrrhizic acid is greatly increased. The invention further provides the application of the fomes officinalis cough and asthma treating tablets in preparation of a medicine for inhibiting the cell proliferation of a mouse teratoma cell P19.
Owner:JINAN XINQIDIAN MEDICAL TECH
Method for extracting plant composition containing echinacea and application of plant composition
InactiveCN105194002ARich in active ingredientsReduce dosageAntineoplastic agentsPlant ingredientsDesmodium triflorumMicrowave
The invention provides a method for extracting a plant composition containing echinacea. The method is prepared from 100 parts of echinacea, 20 parts of desmodium triflorum, 50 parts of kalanchoe pinnatum, 40 parts of tubeflower viburnum herb and 50 parts of large-flowered gardenia leaves. A microwave extraction method is used for preparation, so that the content is greatly increased, and the using amount is reduced. The invention further provides an application of the plant composition containing the echinacea to preparation of P19 cell proliferation drugs for inhibiting mouse teratoma.
Owner:NANJING ZHENGLIANG MEDICAL TECH
Application of dizziness-relieving and calming tablet in preparing medicine for inhibiting cell proliferation of embryonal carcinoma cell F9
InactiveCN103800871ATake a lot of medicineSmall dosePill deliveryAntineoplastic agentsEmbryonal Carcinoma CellsEpimedium
The invention belongs to the technical field of traditional Chinese medicines, and in particular relates to application of a dizziness-relieving and calming tablet in preparing a medicine for inhibiting cell proliferation of an embryonal carcinoma cell F9 and a preparation method of the dizziness-relieving and calming tablet. According to the application of the dizziness-relieving and calming tablet in preparing the medicine for inhibiting cell proliferation of the embryonal carcinoma cell F9, the dizziness-relieving and calming tablet is prepared from the following bulk drugs: 400g of pyrola, 160g of epimedium, 160g of astragalus membranaceus, 140g of Chinese angelica, 200g of ligusticum wallichii, 240g of kudzu vine root, 120g of fried bighead atractylodes rhizome, 140g of spina date seed, 120g of prepared pinellia ternata, 120g of rhizoma alismatis, 100g of dried ginger, and 100g of liquorice. The dizziness-relieving and calming tablet is prepared by adopting supercritical extraction and microwave extraction, so that the content of icariin can be greatly improved.
Owner:JINAN XINQIDIAN MEDICAL TECH
Methods for reprogramming cells and uses thereof
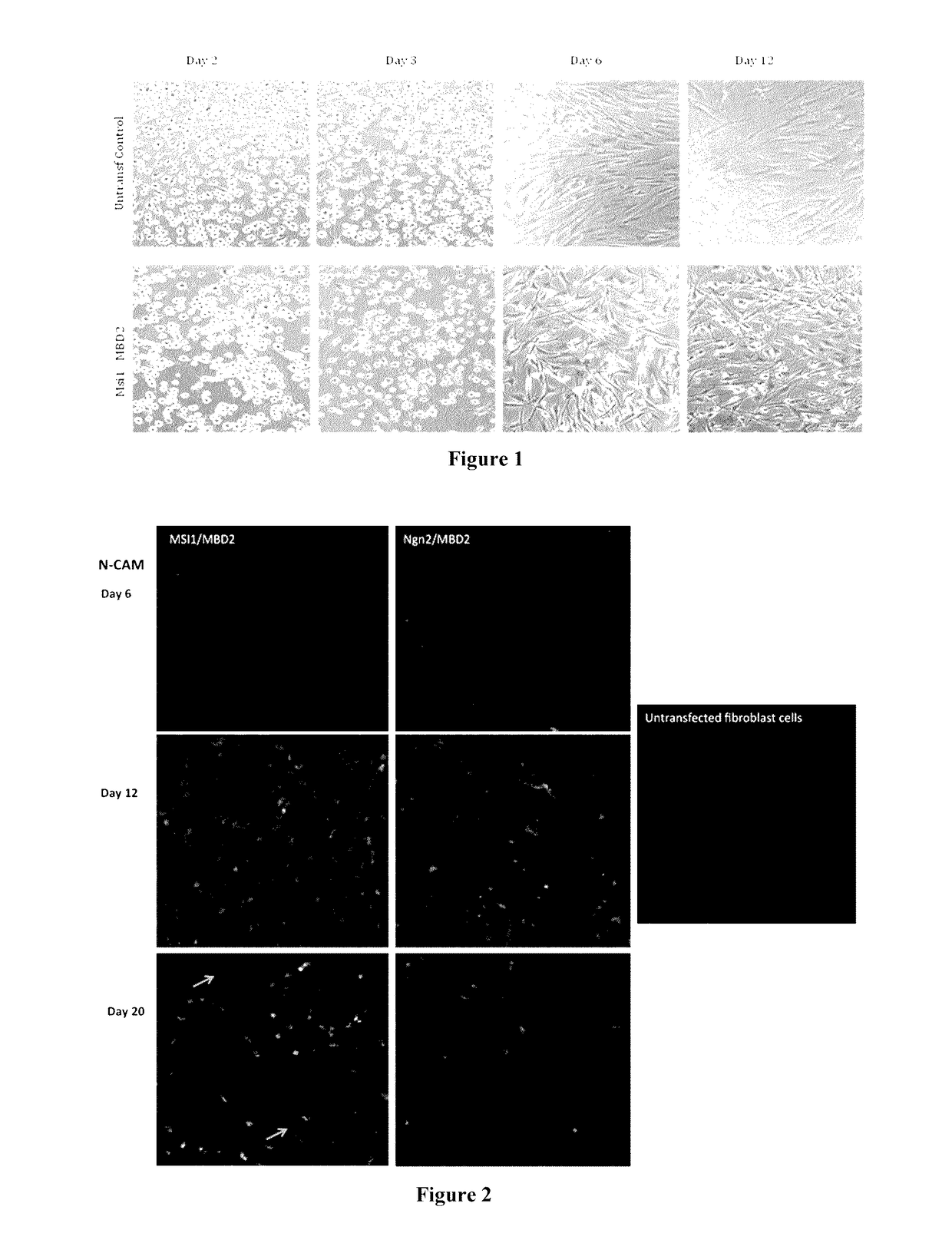
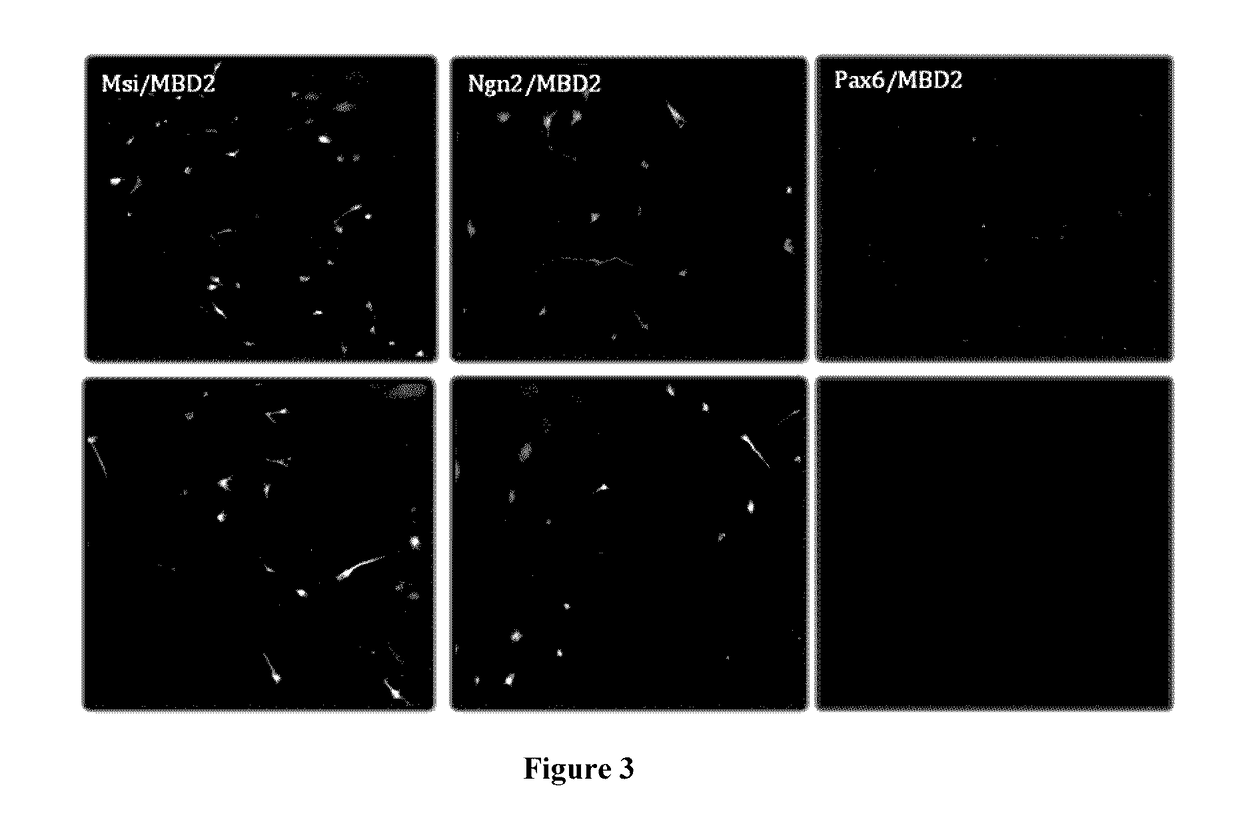
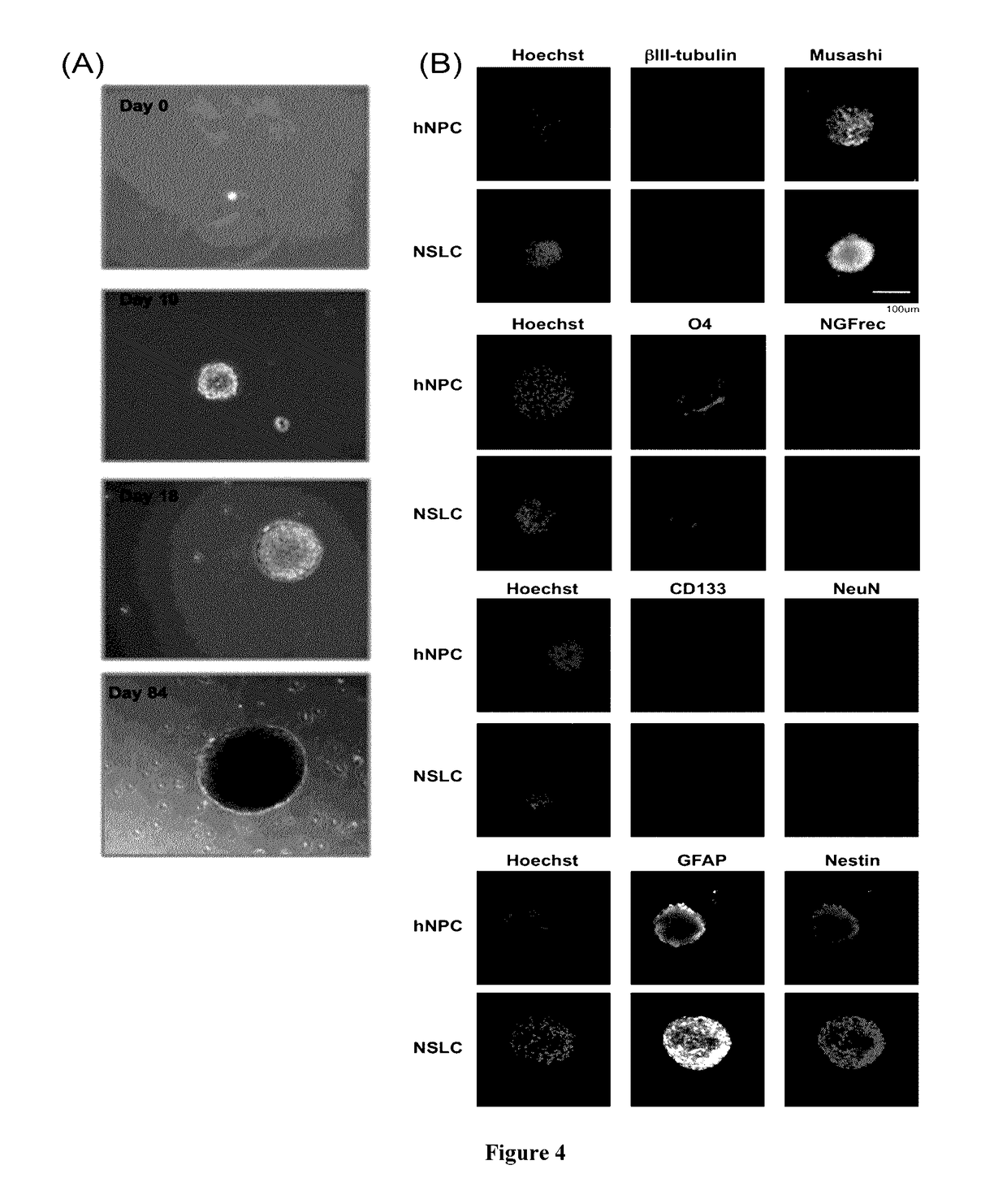
An in vitro human neural multipotent, unipotent, or somatic cell possessing all of the following characteristics: is derived from the reprogramming of a somatic cell, a progenitor cell or a stem cell that exhibits at least a transient increase in intracellular levels of at least one reprogramming agent; is not differentiated from a pluripotent cell; expresses one or more markers of a multipotent, unipotent or somatic cell not characteristic of a neural stem cell, neural precursor cell, neural progenitor cell, neuroblast, or neuron; is not a cancerous cell; is stable and not artificially maintained by forced gene expression and may be maintained in standard neural stem cell media or neural media; and does not exhibit uncontrolled growth, teratoma formation, and tumor formation in vivo; wherein the cell comprises at least one polypeptide or an expression vector encoding at least one polypeptide selected from the group consisting of: Musashi1 (Msi1); Ngn2; Msi1 and Ngn2; Msi1 and methyl-CpG binding domain protein 2 (MBD2); Ngn2 and MBD2; Msi1, Ngn2 and MBD2; Achaete-Scute Homolog 1 (Ascl1); Msi1, Ngn2 and Ascl1; Msi1, Ngn2, MBD2 and Ascl1; Sox2; Msi1, Ngn2 and Sox2; and Msi1, Ngn2, MBD2 and Sox2; wherein the expression vector is transiently expressed.
Owner:GENESIS TECH LTD
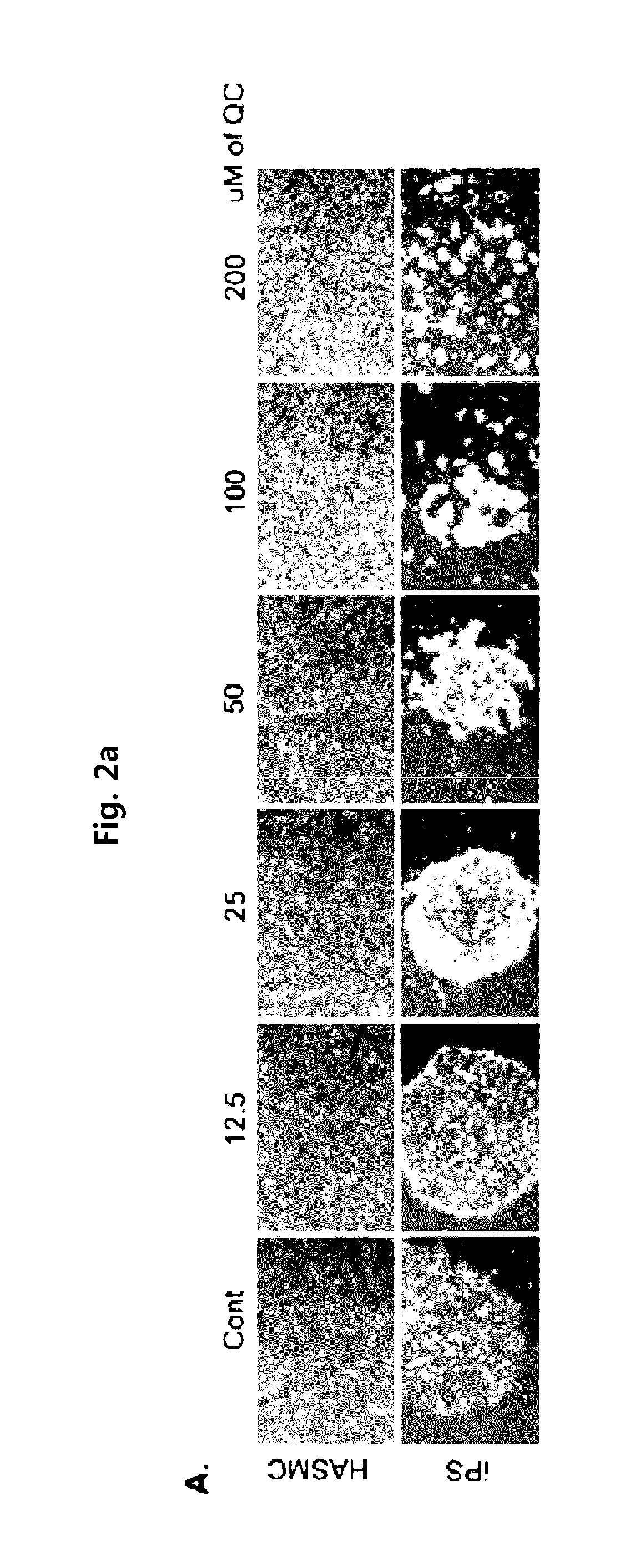
Method for suppressing teratoma formation via selective cell death induction in undifferentiated human-induced pluripotent stem cells
ActiveUS9657273B2Valid choiceEnsure safetyOrganic chemistryCulture processApoptosisCell Death Induction
The present invention relates to a method for preparing differentiated cells derived from induced pluripotent stem cell, wherein undifferentiated induced pluripotent stem cells (iPS) are removed, the method comprising steps of: (a) preparing a cell sample including undifferentiated induced pluripotent stem cells and differentiated cells by differentiating induced pluripotent stem cells; and (b) causing selective apoptosis of the undifferentiated induced pluripotent stem cells by treating the resultant in step (a) with quercetin of Formula 1 below or with YM-155 of Formula 2 below.According to the present invention, the present invention makes it possible to effectively selectively cause apoptosis only of undifferentiated induced pluripotent stem cells by causing induced pluripotent stem cells to differentiate into specific differentiated cells and then carrying out culturing in a differentiating culture medium comprising quercetin or YM-155, and, in the induced pluripotent stem cell differentiation method according to the present invention, only undifferentiated induced pluripotent stem cells that are a cause of teratoma formation are selectively caused to die, and thus differentiated differentiating cells are completely unaffected. In other words, the invention can be expected to ensure safety as the possibility of tumor formation during clinical use as a cell therapeutic agent is eliminated since the survival and functioning of the differentiated cells is maintained unchanged.
Owner:THE MCLEAN HOSPITAL CORP
Application of lespedeza cuneata turbidity removing tablet in preparation of medicine for inhibiting cell proliferation of embryonal carcinoma F9
InactiveCN103705825ATake a lot of medicineSmall doseAnthropod material medical ingredientsPill deliveryEmbryonal carcinomaGLYCYRRHIZA EXTRACT
The invention belongs to the technical field of traditional Chinese medicines, and specifically relates to an application of a lespedeza cuneata turbidity removing tablet in preparation of a medicine for inhibiting cell proliferation of embryonal carcinoma F9 as well as a preparation method of the lespedeza cuneata turbidity removing tablet. According to the application of the lespedeza cuneata turbidity removing tablet in preparation of the medicine for inhibiting cell proliferation of embryonal carcinoma F9, the lespedeza cuneata turbidity removing tablet is prepared from the following raw material medicines: 417g of cleavers, 417g of lespedeza cuneata, 333g of rhizoma dioscoreae hypoglaucae, 417g of houttuynia cordata, 417g of dandelion, 250g of silky ants, 417g of fructus akebiae, 250g of semen plantaginis, 250g of poria cocos, 250g of Chinese yam, 167g of alpinia oxyphylla, 250g of semen cuscutae, 250g of flatstem milkvetch seeds, 333g of cherokee rose roots, 125g of polygala tenuifolia and 50g of liquorice. The tablet is prepared by supercritical extraction and microwave extraction, so that the content of diosgenin is greatly improved.
Owner:滨州市人民医院
Medium and device for proliferation of stem cells and treatment of cancer-related stem cell with resveratrol
InactiveUS8183297B2Improve survival rateStrong drug resistanceBioreactor/fermenter combinationsBiocideSerum free mediaDisease
The invention relates to a device for selecting stem cells with a serum free medium for amplification of stem cells. The invention also relates to a method of treating or preventing diseases caused by cancer-related stem cells. The invention further provides a method of enhancing radiosensitivity of cancer-related stem cells comprising radiotherapy with resveratrol, and the cancer-related stem cell has stronger drug resistance. The present invention further provides that resveratrol promotes differentiation and inhibits teratoma / tumor formation in induced pluripotent stem cells (iPS) and embryonic stem cells.
Owner:VETERANS GEN HOSPITAL TAIPEI
Patient-specific stem cell lines derived from human parthenogenetic blastocysts
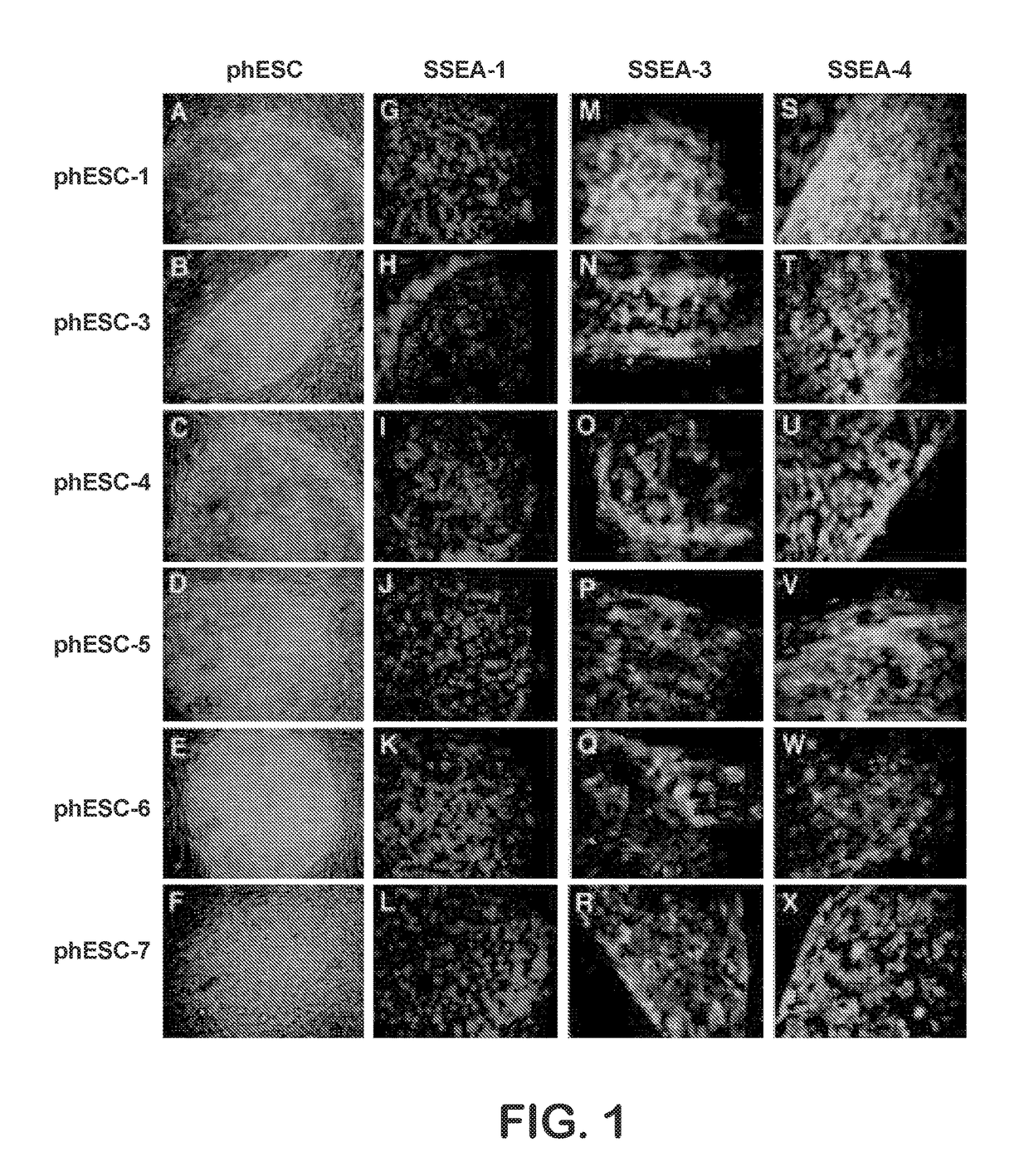
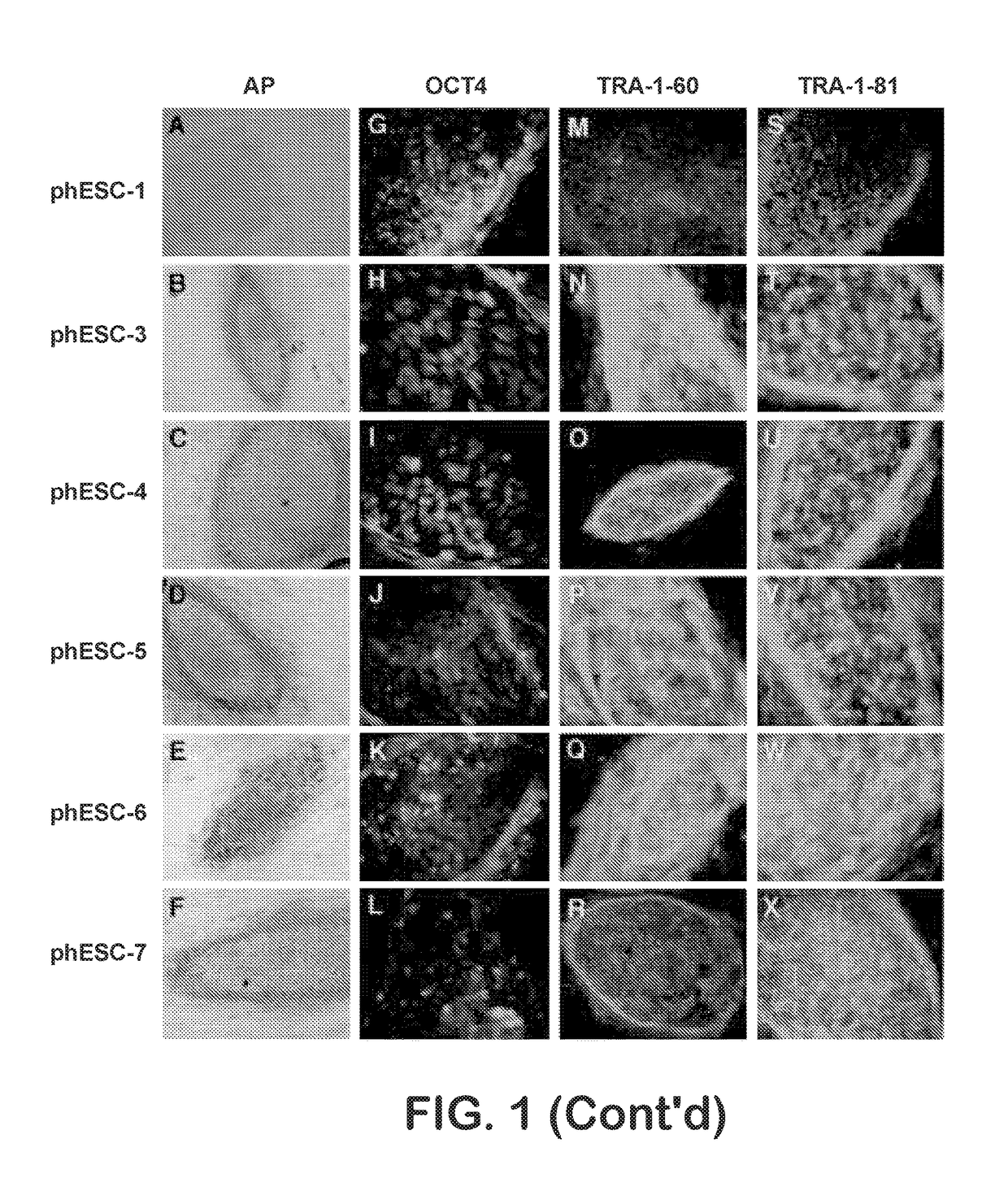
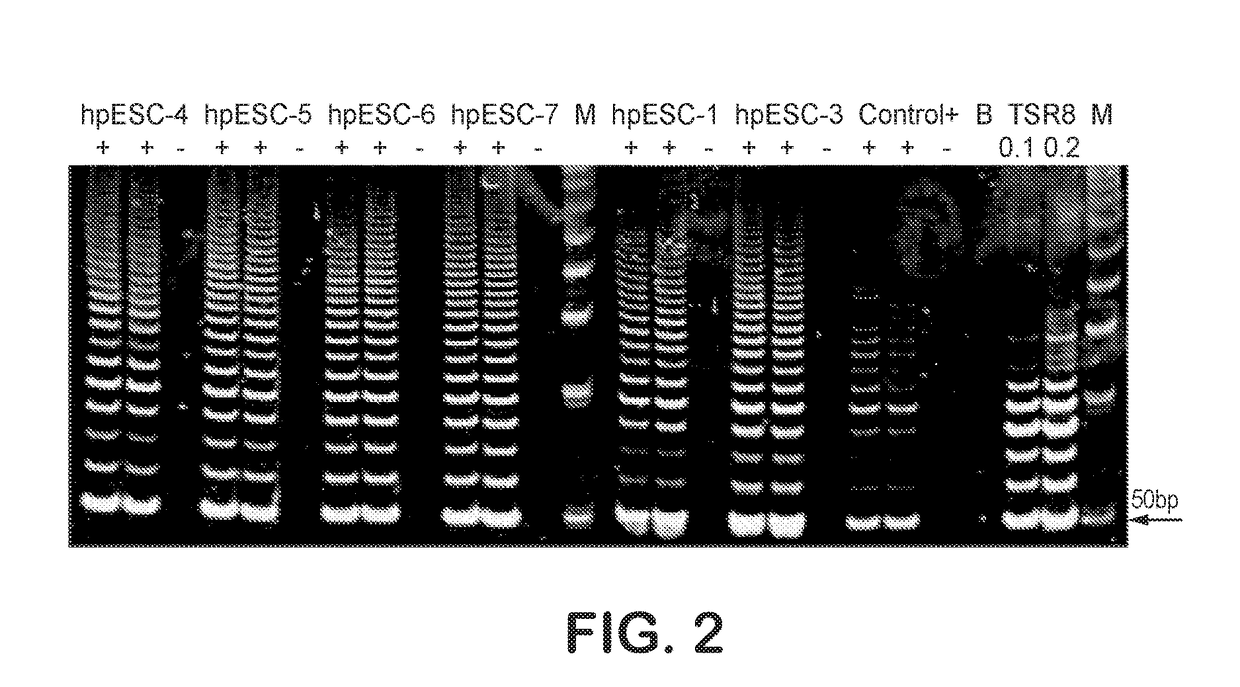
Methods are disclosed for generating HLA homozygous parthenogenetic human stem cell (hpSC-Hhom) lines from both HLA homozygous and HLA heterozygous donors. These hpSC-Hhom lines demonstrate typical human embryonic stem cell morphology, expressing appropriate stem cell markers and possessing high levels of alkaline phosphatase and telomerase activity. Additionally, injection of these cell lines into immunodeficient animals leads to teratoma formation. Furthermore, in the case of HLA heterozygous donors, the hpSC-Hhom lines inherit the haplotype from only one of the donor's parents. SNP data analysis suggests that hpSC-Hhom lines derived from HLA heterozygous oocyte donors are homozygous throughout the genome as assessed by single-nucleotide polymorphism (SNP) analysis. The protocol as disclosed minimizes the use of animal-derived components, which makes the stem cells more practical for clinical application.
Owner:INT STEM CELL CORP
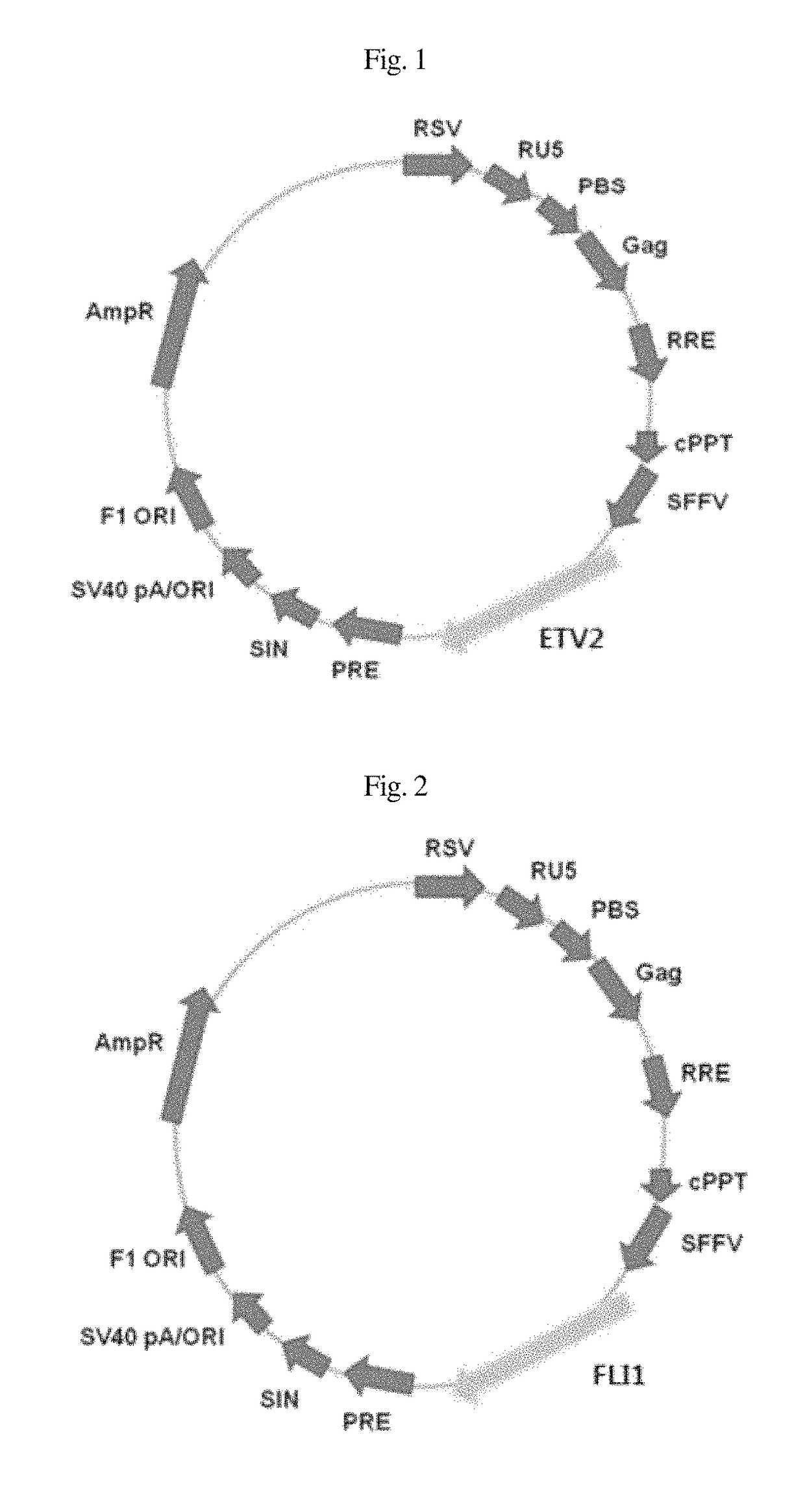
Composition for inducing direct transdifferentiation of somatic cell into vascular progenitor cell, and use thereof
ActiveUS10174287B2Minimize side effectsShorten the time periodGenetic material ingredientsGenetically modified cellsTransdifferentiationSide effect
Owner:UNIST (ULSAN NAT INST OF SCI & TECH)
Non-tumorigenic expansion of pluripotent stem cells
InactiveCN102747032ASkeletal/connective tissue cellsBlood/immune system cellsInduced pluripotent stem cellKaryotype
A method for expansion of human embryonic stem (hES) cells in a medium including human umbilical cord-derived mesenchymal stem cells (HUCMSCs) as a feeder is provided. The human embryonic stem cells (hES) maintain the features of embryonic stem cells in the medium, such as pluripotency, unlimited undifferentiated proliferation and normal karyotypes. Also provided is a method for non-tumorigenic expansion of the human embryonic stem cells (hES) that is free from forming teratoma.
Owner:BUDDHIST TZU CHI GEN HOSPITAL
Personalized production of biologics and method for reprogramming somatic cells
The invention provides a method for producing polypeptide protein products and nucleic acid products having reduced levels of antigenicity in an animal being treated with a biologic product. Somatic cells are isolated from an animal, transformed into pluripotent stem cells, transfected with a nucleic acid(s) of interest, and re-differentiated towards somatic cells known to be high level producers of the desired nucleic acid product. The invention can be used to derive a general cell line to treat populations, racial specific cell lines to treat ethnic groups, or patient specific cell lines to treat individuals. Additionally, the invention provides a method to allow induced pluripotent stem cells to be re-differentiated towards their somatic cell of origin so that the cells can be used to therapeutically treat an animal without resulting teratoma formation.
Owner:AVM BIOTECH
Method for Preparation of Hepatocyte Using Es Cell
InactiveUS20100055762A1High differentiationReduce riskPeptide/protein ingredientsMicrobiological testing/measurementCell biologyHepatocyte
This invention relates to an agent for promoting differentiation of an ES cell, preferably an agent for promoting differentiation of an ES cell into a hepatocyte or a prophylactic agent for teratoma, comprising uPA.Furthermore this invention relates to a method of promoting differentiation of an ES cell, preferably a method of promoting differentiation of an ES cell into a hepatocyte, comprising the step of contacting uPA with the ES cell, or a method of preparing a hepatocyte comprising the step of contacting uPA with an ES cell to differentiate the ES cell into a hepatocyte.
Owner:NIHON UNIVERSITY
Preparation method of fukean tablet and application of fukean tablet in preparation of drugs for inhibition of cell proliferation of mouse embryonic carcinoma cell P19
ActiveCN103655742ATake a lot of medicineReduce dosageDigestive systemPill deliveryChinese knotweedActive ingredient
The invention belongs to the technical field of traditional Chinese medicines, and particularly relates to a preparation method and application of a fukean tablet. The preparation method of the fukean tablet is as follows: the fukean tablet is prepared from 1000 g of jasminum elongatum, 1000 g of Chinese knotweed, 670 g of ovate leaf holly bark, 330 g of plantain herb and 330 g of pomegranate peel as raw materials by use of microwave extraction and macroporous resin adsorption, and the total flavonoid content is greatly improved. The invention also provides application of the fukean tablet in preparation of drugs for inhibition of cell proliferation of mouse embryonic carcinoma cell P19.
Owner:北京缘顺福咨询有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com