Non-tumorigenic expansion of pluripotent stem cells
A technology of pluripotent stem cells and stem cells, applied in the field of non-tumorigenic proliferation, can solve the problems of insufficient maintenance of cell stability and inability to demonstrate long-term guarantee
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0022] RT-PCR and quantitative RT-PCR of this gene and other differentiation marker genes
[0023]Undifferentiated or differentiated hES cells cultured on HUCMSCs or MEF feeder layers were mechanically removed and treated with RLT lysis buffer (Qiagen). The first cDNA was synthesized using the SuperScript III One-Step RT-PCR kit (invitrogen) according to the manufacturer's instructions. Table 1 shows the sequence, binding temperature and product size of each pair of primers. All PCR samples were analyzed by electrophoresis on a 2% agarose gel containing 0.5 μg / ml ethidium bromide (Sigma). For quantitative RT-PCR (qRT-PCR), the FastStart universal SYBR green master (ROX, Roche, USA) gene expression assay was used in the ABI StepOne Plus system (Applied Biosystems) with GAPDH as an internal control. The sequences and binding temperatures of the primers are shown in Table 1.
[0024] Table 1
[0025]
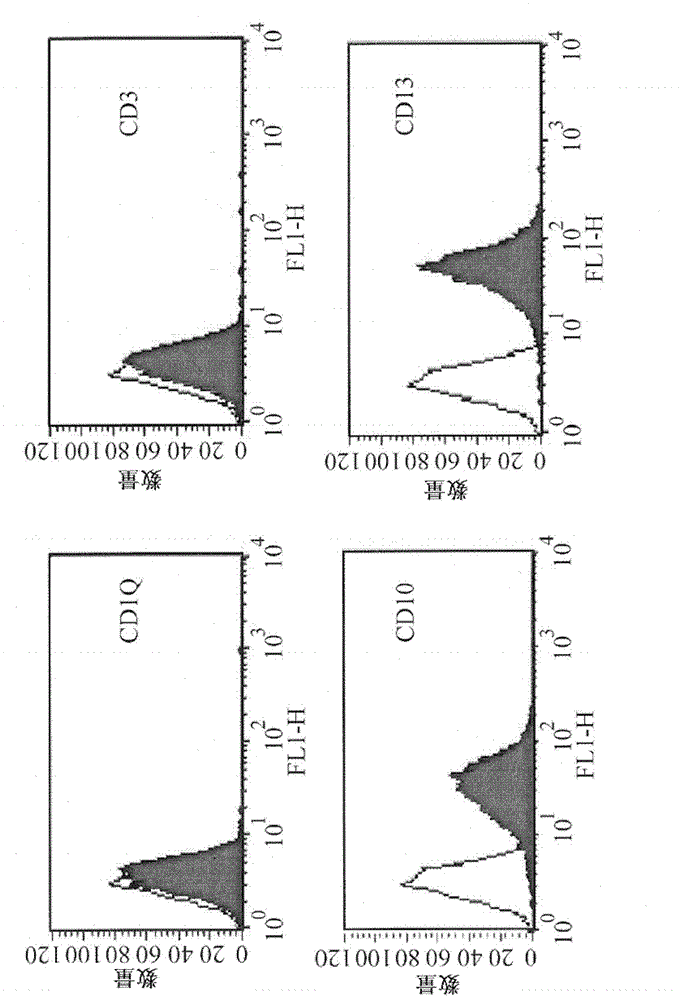
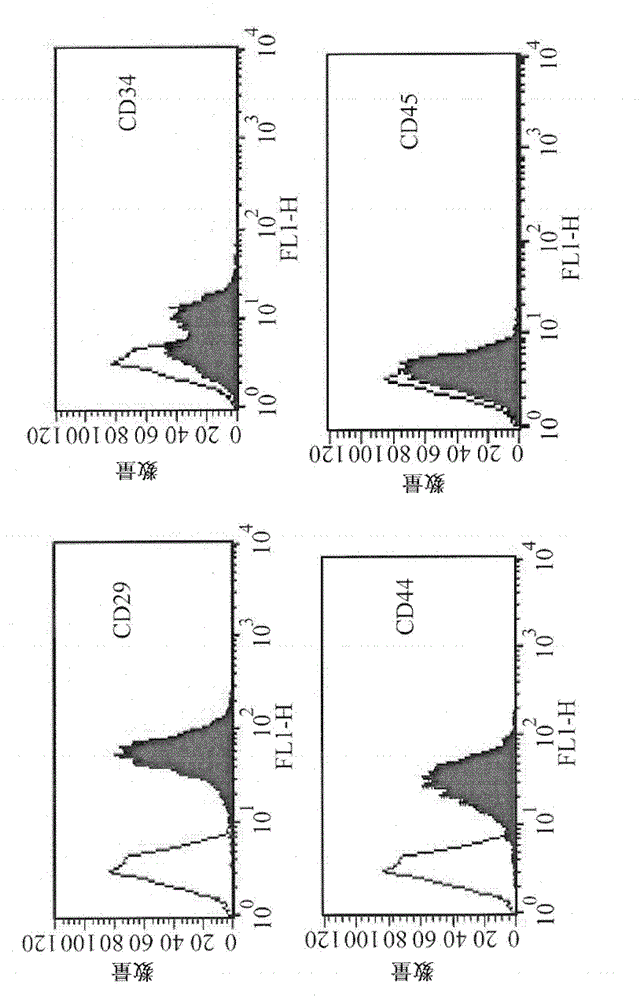
[0026] Isolation and proliferation of HUCMSCs
[0027] Human umbilical...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com