Patents
Literature
1533results about How to "Improve survival" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
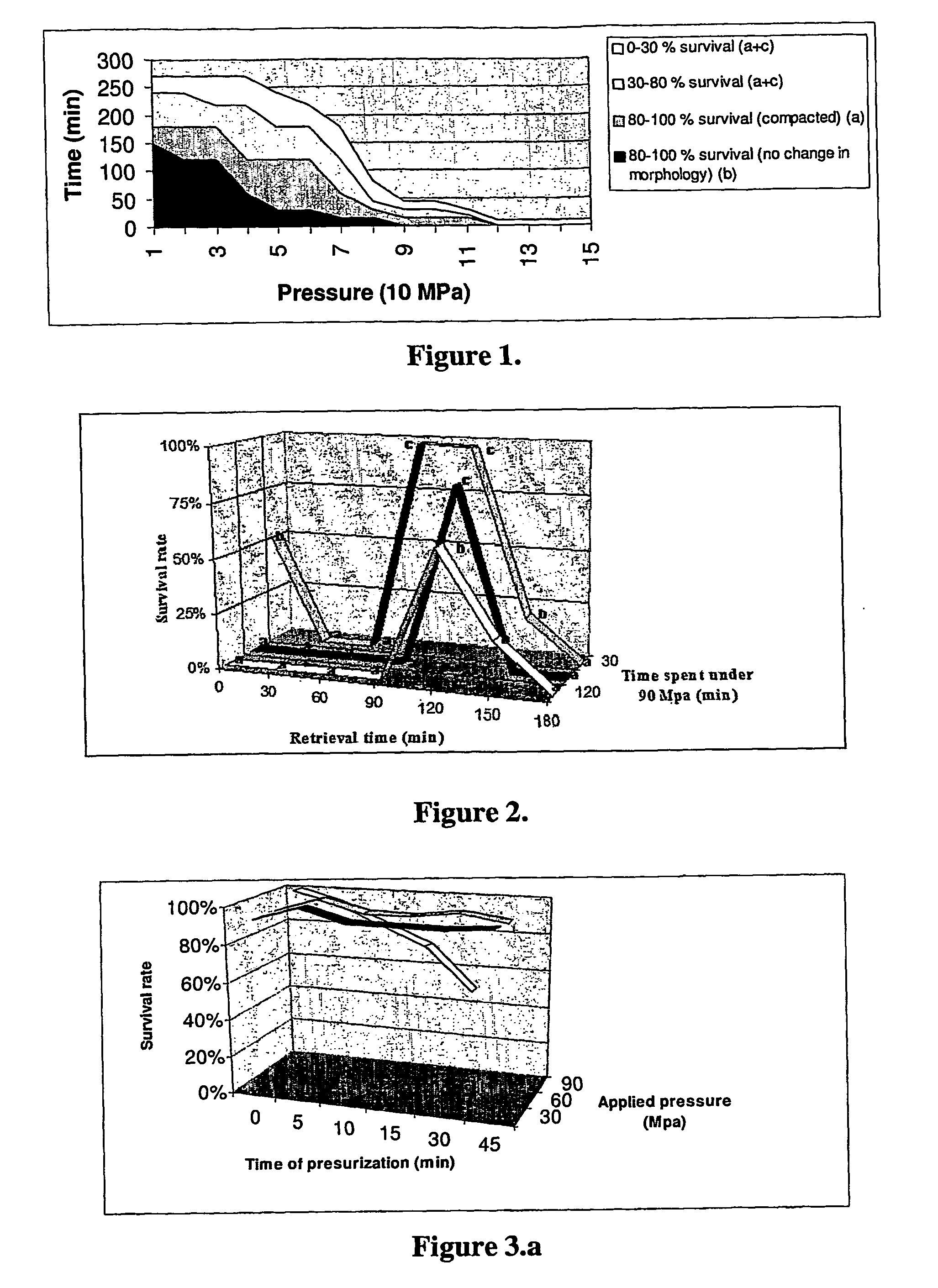
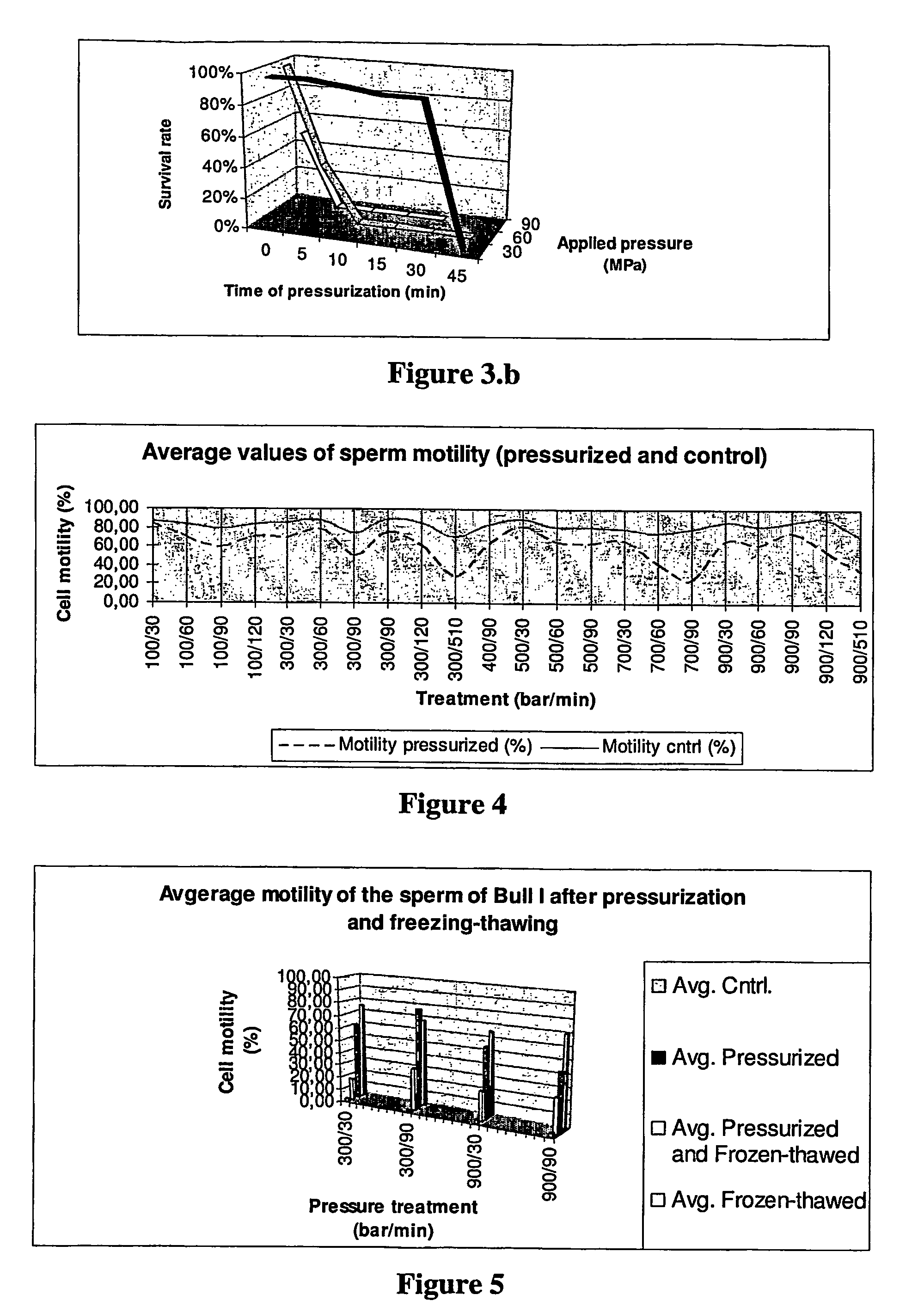
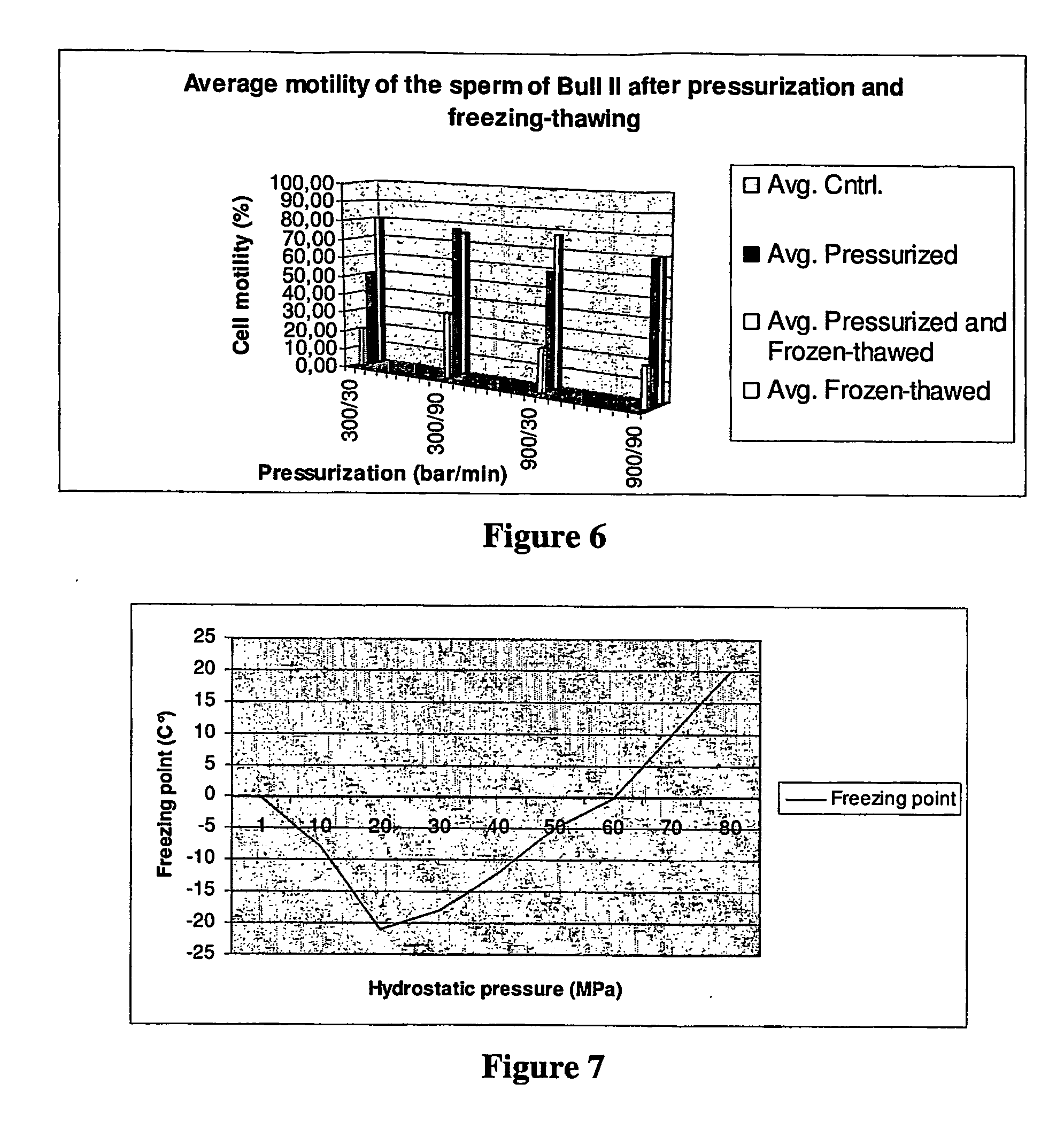
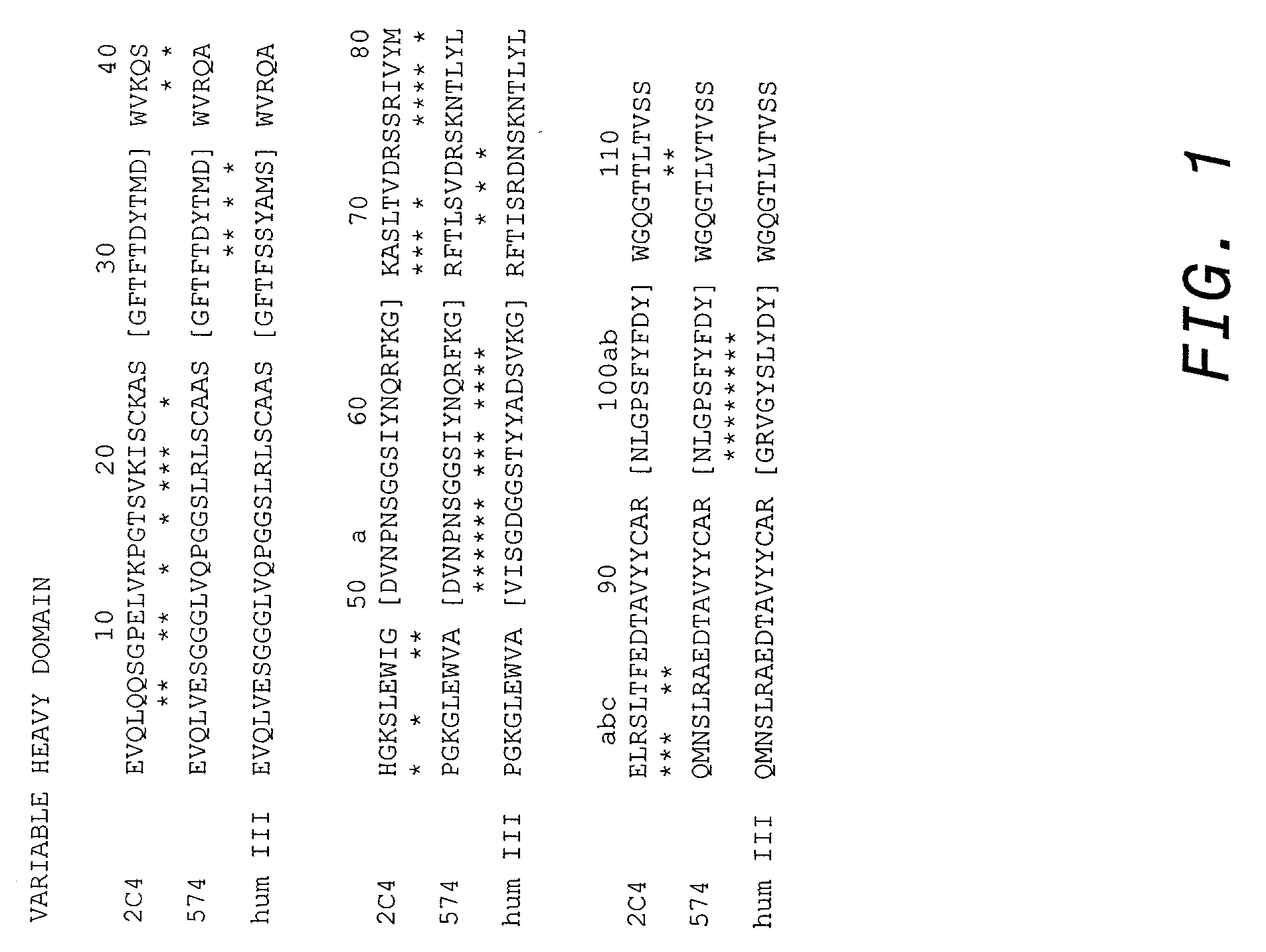
Post-thaw survival of chryopreserved biological material by hydrostatic pressure challenge
InactiveUS20070087321A1Improve survivalSimple methodDead animal preservationHydrostatic pressurePre treatment
The present invention relates to a method for improving post-thaw survival of cryopreserved biological material comprising applying hydrostatic pressure to said biological material; keeping the said biological material at the hydrostatic pressure for a predetermined time period; releasing the hydrostatic pressure; and freezing the said biological material using any protocol applicable thereto. The invention also relates to the use of a pressurizing device for the pretreatment of a biological material that is to be cryopreserved, as well as to a pressurizing device for the pretreatment of a biological material that is to be cryopreserved, said device comprising a pressure chamber for receiving biological material, means to produce said pressure, and means to maintain said pressure in said chamber.
Owner:APPL CELL TECH KORLATOLT FELELOSSEGU TARSASAG
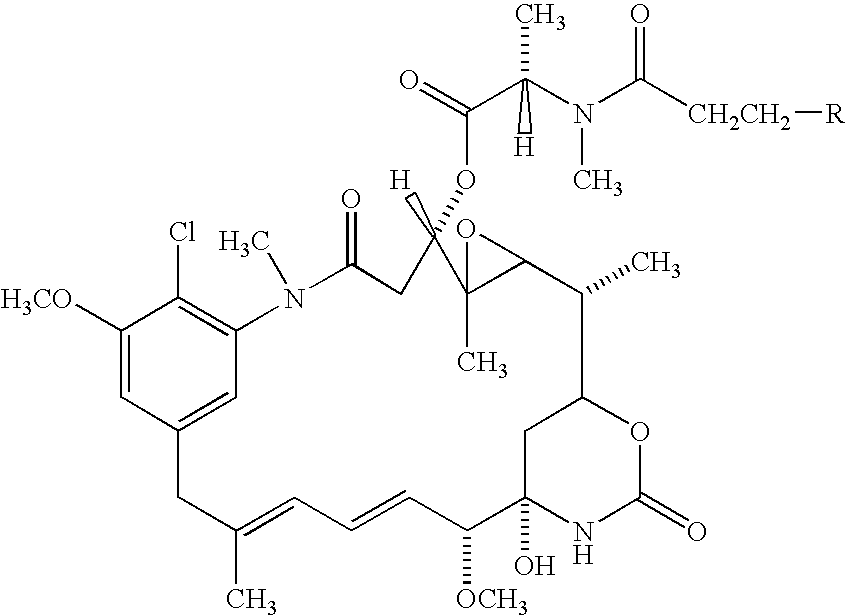
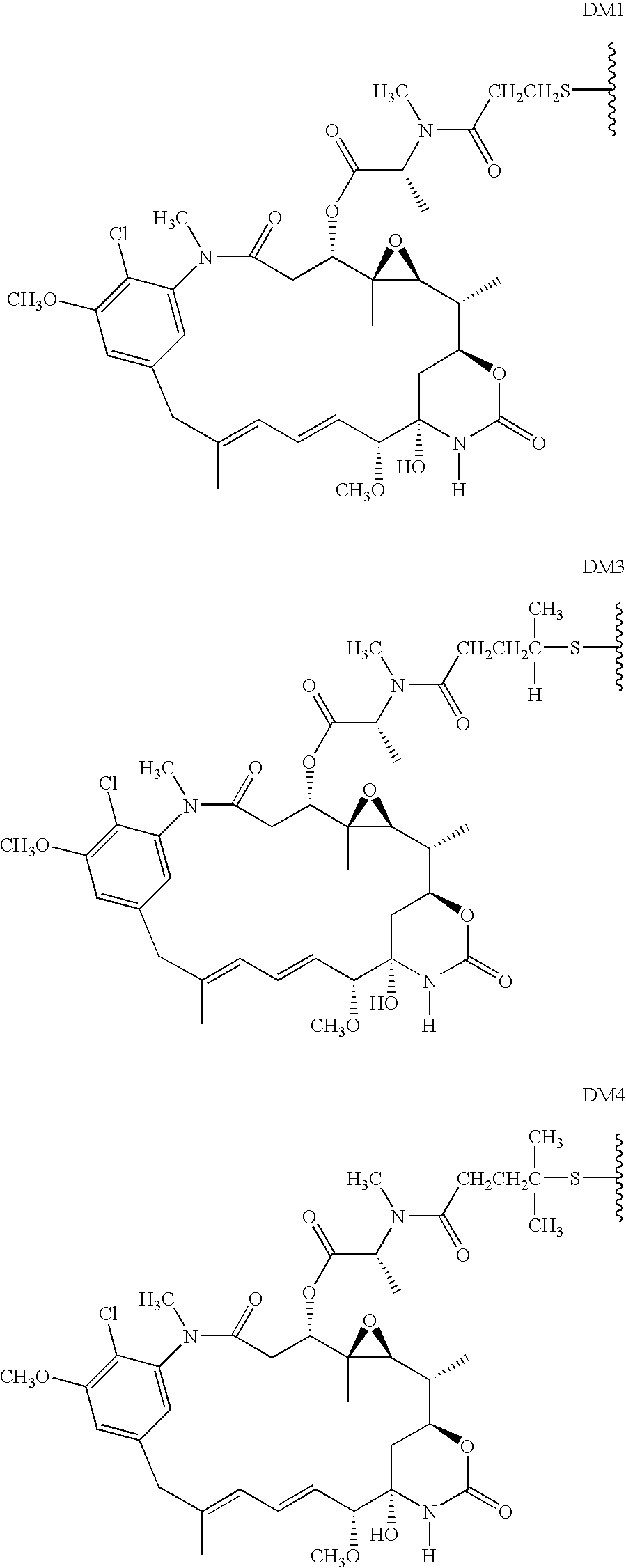
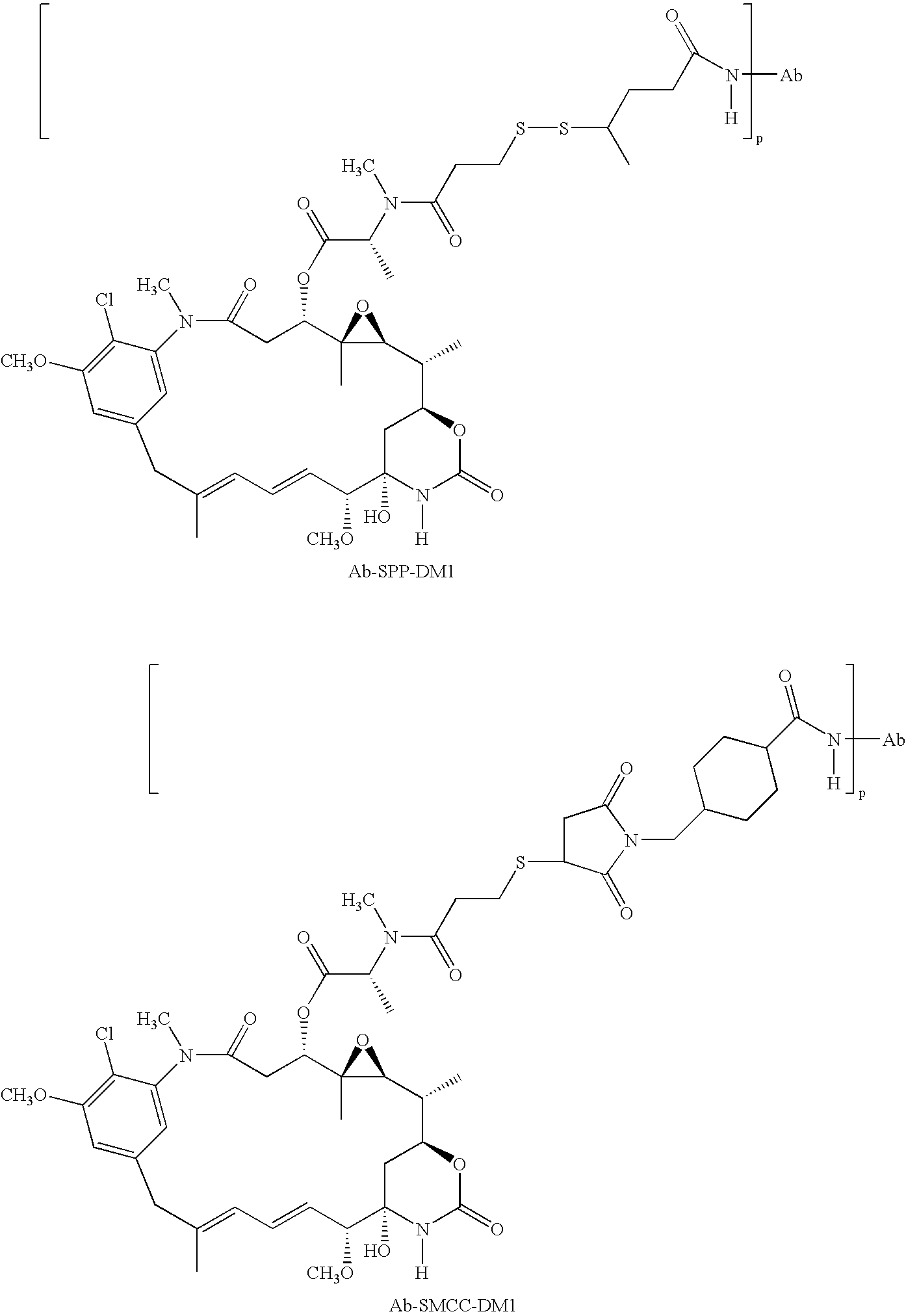
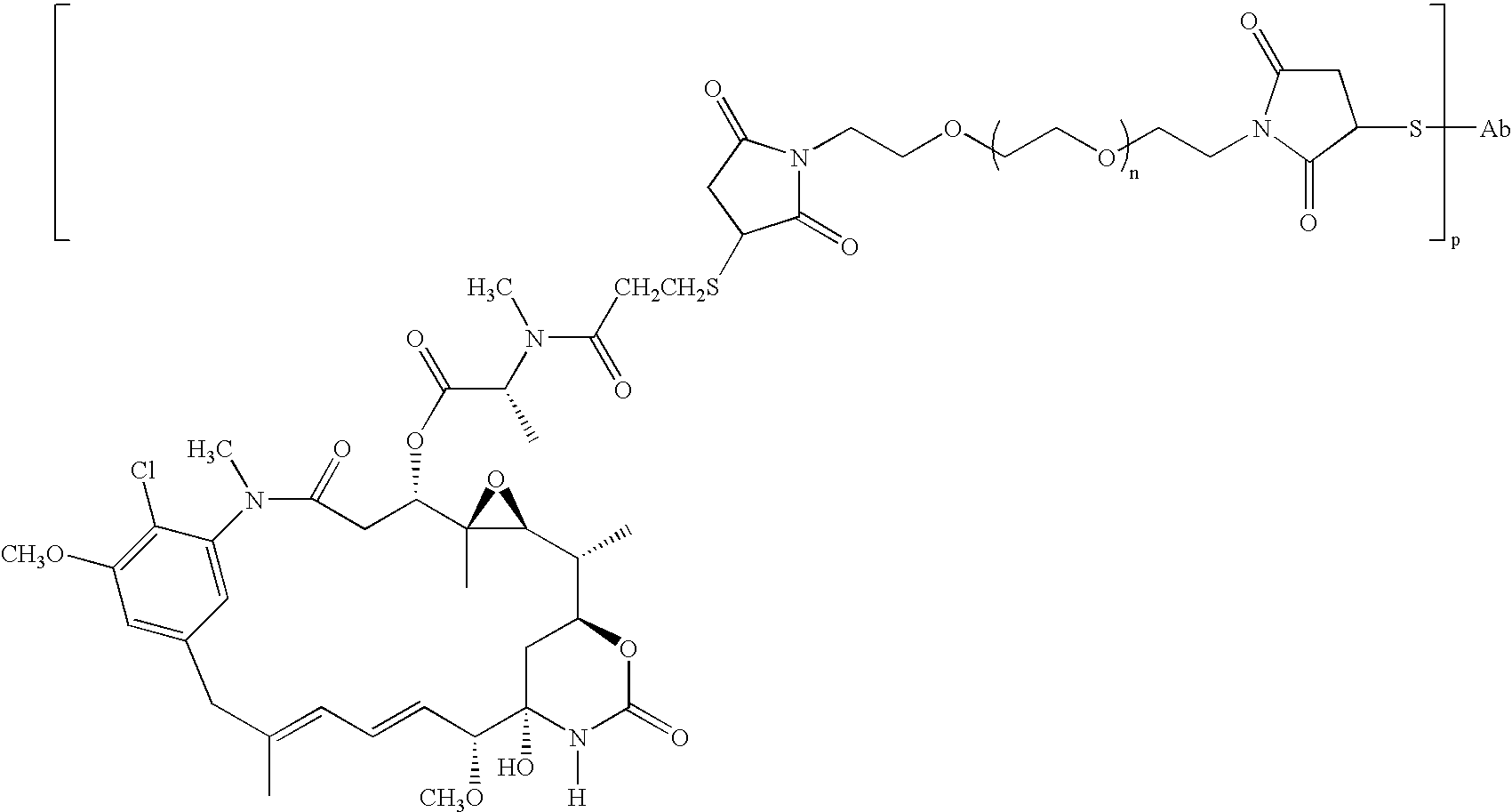
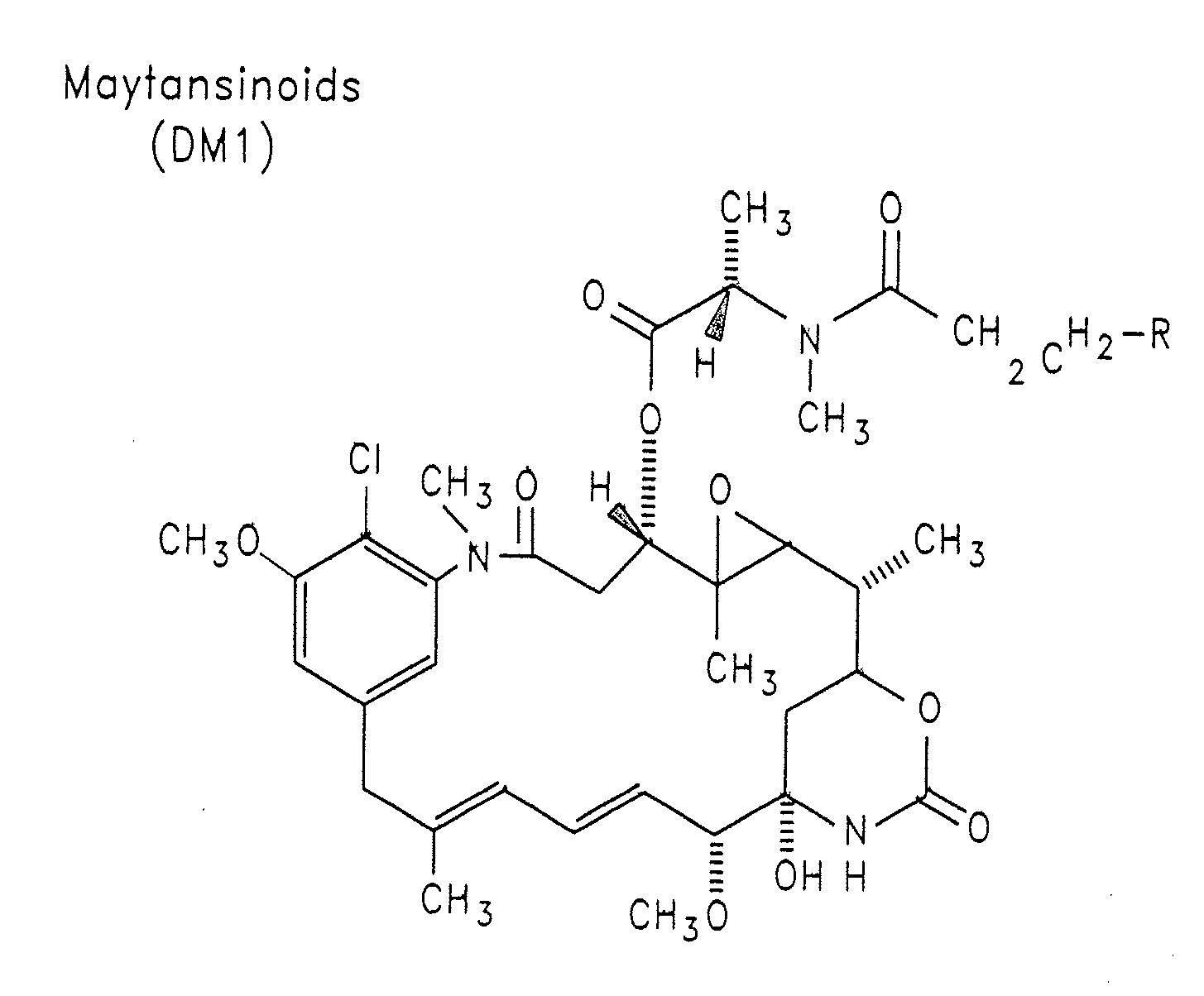
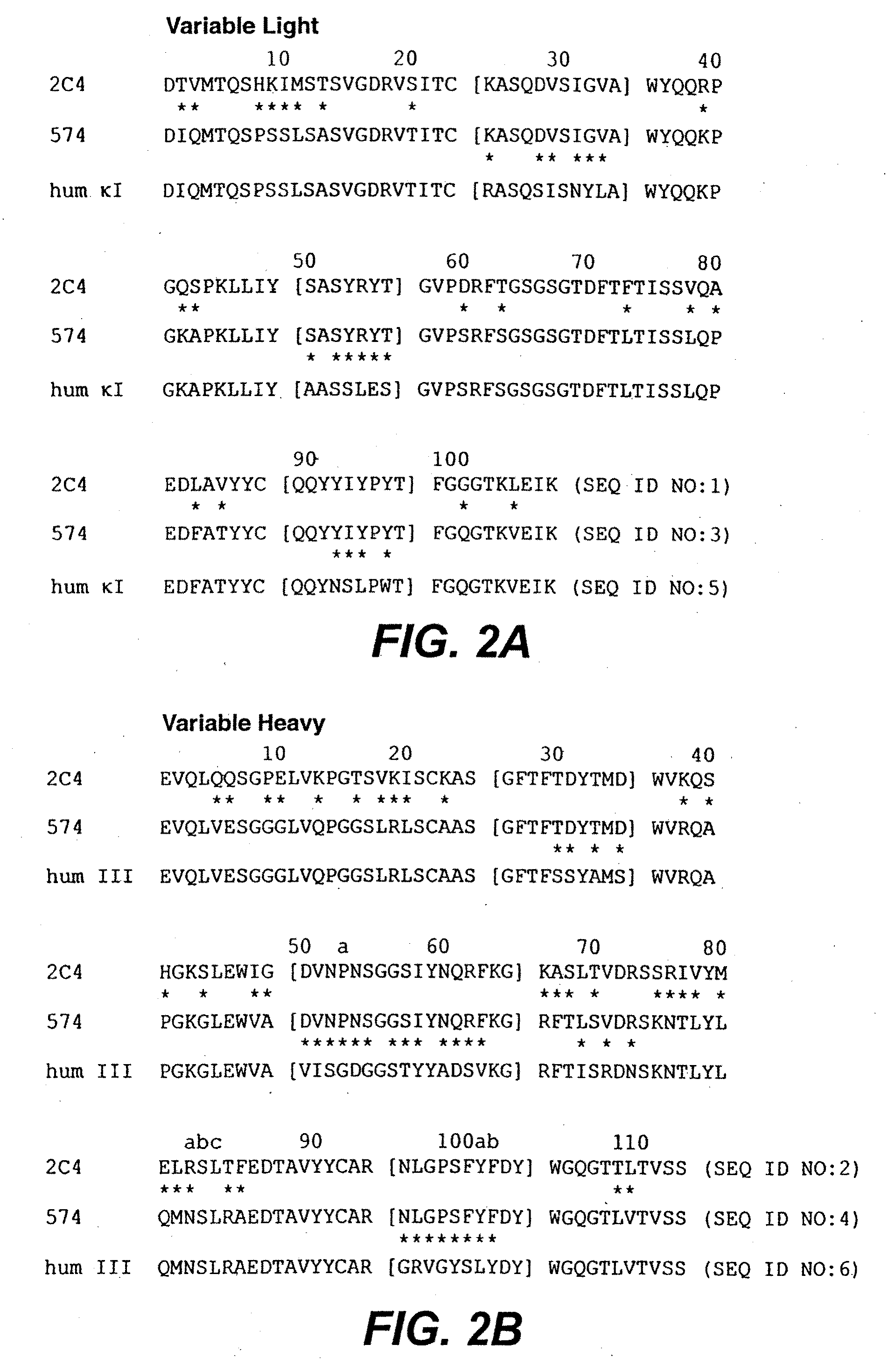
Methods of treatment using anti-ErbB antibody-maytansinoid conjugates
ActiveUS7097840B2Superior clinical activityBetter objective response rateOrganic active ingredientsPharmaceutical delivery mechanismMedicineCancer therapy
The application concerns methods of treatment using anti-ErbB receptor antibody-maytansinoid conjugates, and articles of manufacture suitable for use in such methods. In particular, the invention concerns ErbB receptor-directed cancer therapies, using anri-ErbB receptor antibody-maytansinoid conjugates.
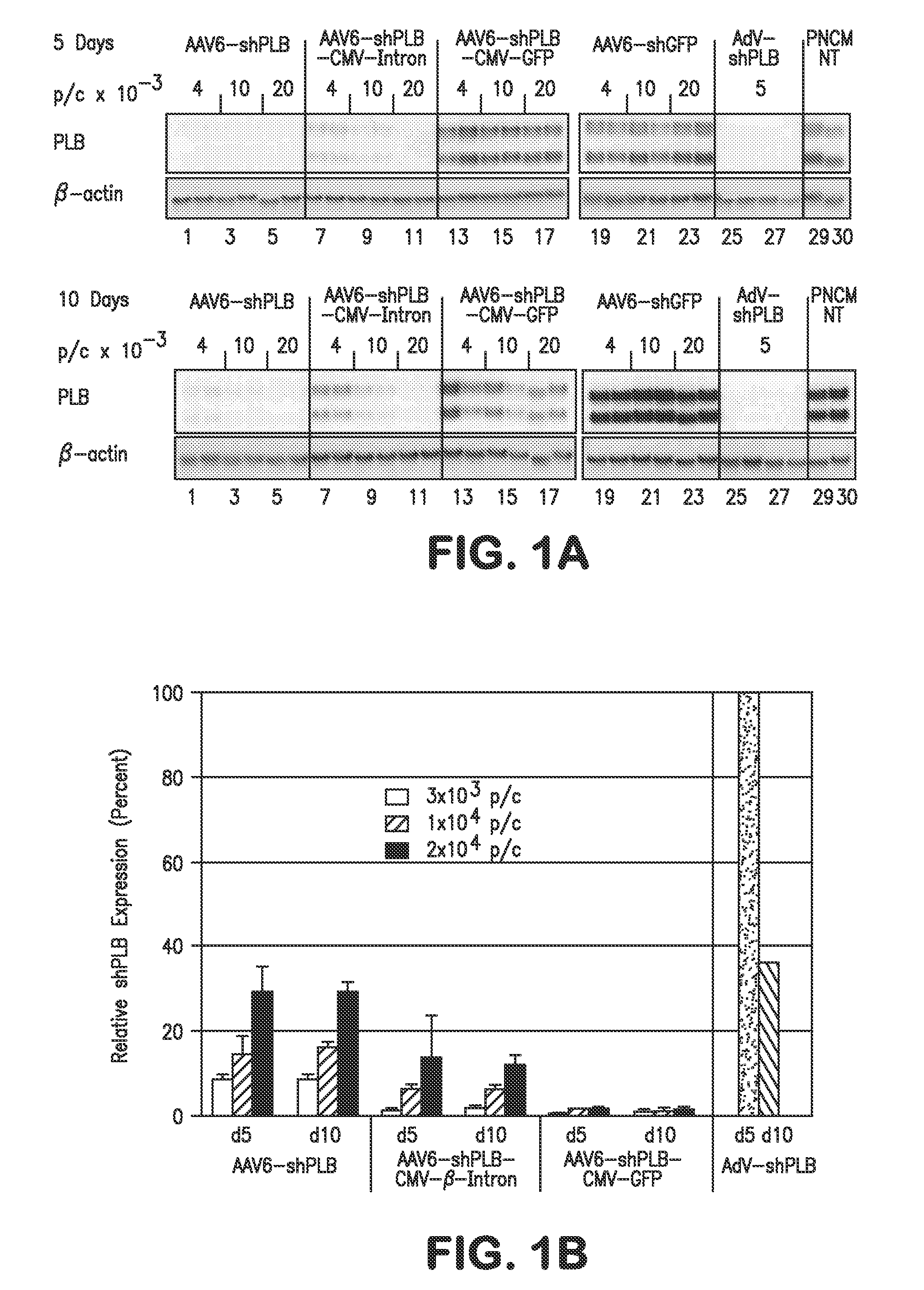
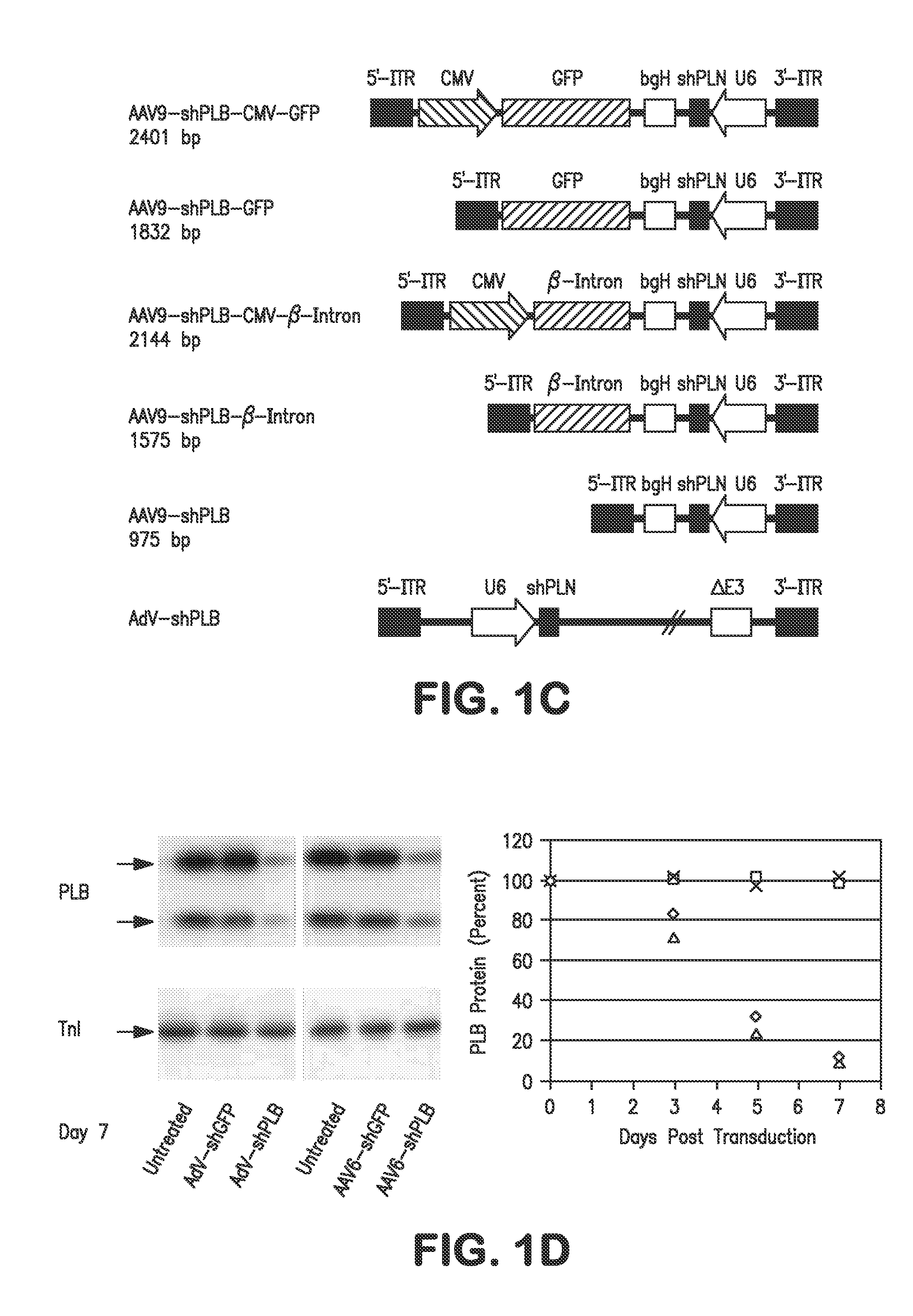
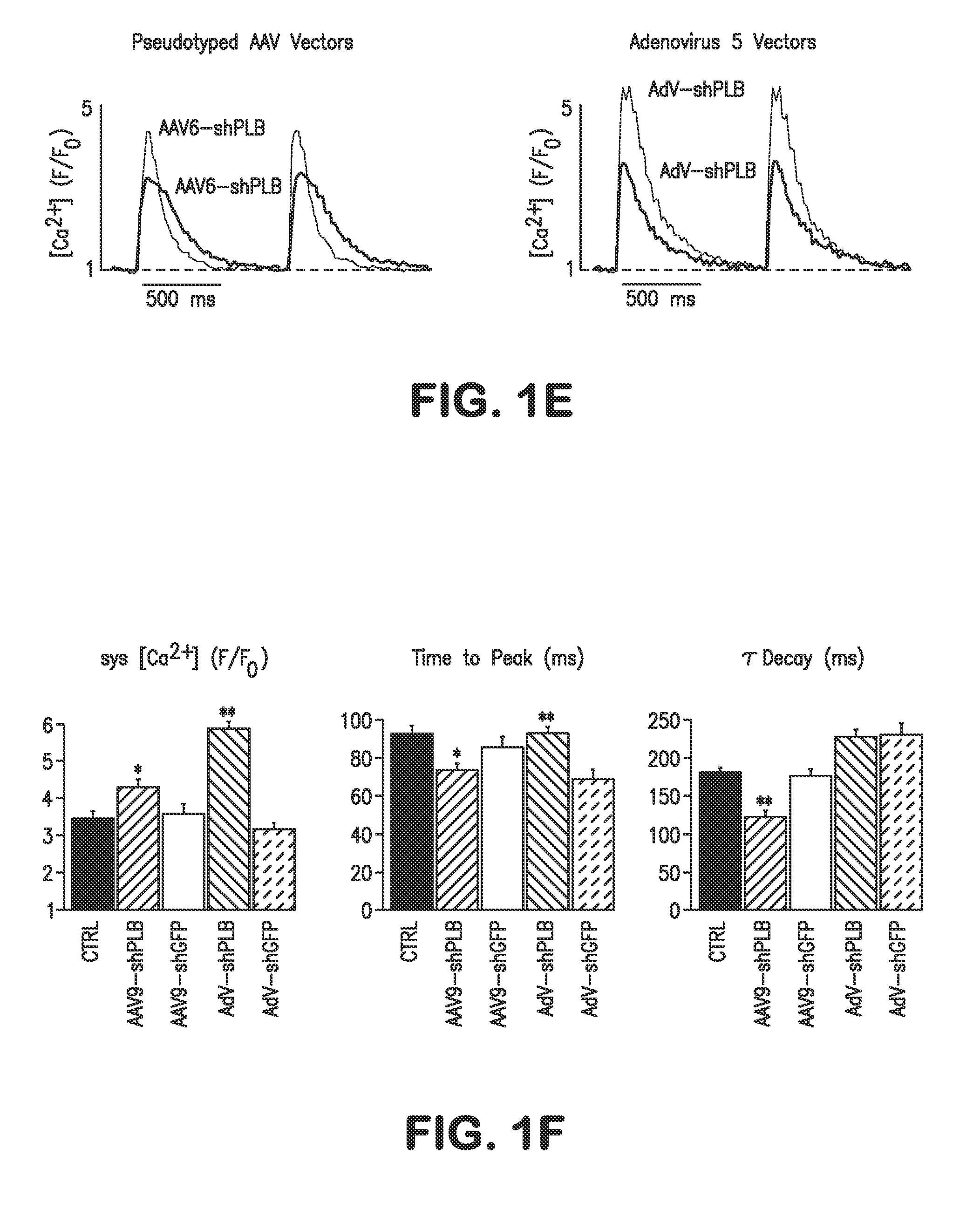
RNA interference for the treatment of heart failure
InactiveUS8404658B2Reduce expressionDecreasing ventricular arrhythmiasOrganic active ingredientsTissue culturePhospholambanTransfection
The present invention relates to targeted RNAi for the treatment of heart failure by modulating defective cardiac Ca2+ homeostasis via decreasing expression or activity of phospholamban (PLB) using adeno-associated virus (AAV) transfection of cardiomyocytes. Methods for decreasing ventricular arrhythmias, as well as methods for overall improvement of survival from heart failure in subjects are also disclosed. Further, the present invention provides methods which can be used to diagnose susceptibility to treatment by RNAi, and includes pharmaceutical compositions, kits and vectors including an RNAi sequence.
Owner:NANOCOR THERAPEUTICS +1
HERCEPTIN adjuvant therapy
ActiveUS20060275305A1Decrease subject ' likelihood of cancer recurrenceIncrease subject ' likelihood of survivalBiocideOrganic active ingredientsAdjuvantAdjuvant therapy
Owner:GENENTECH INC
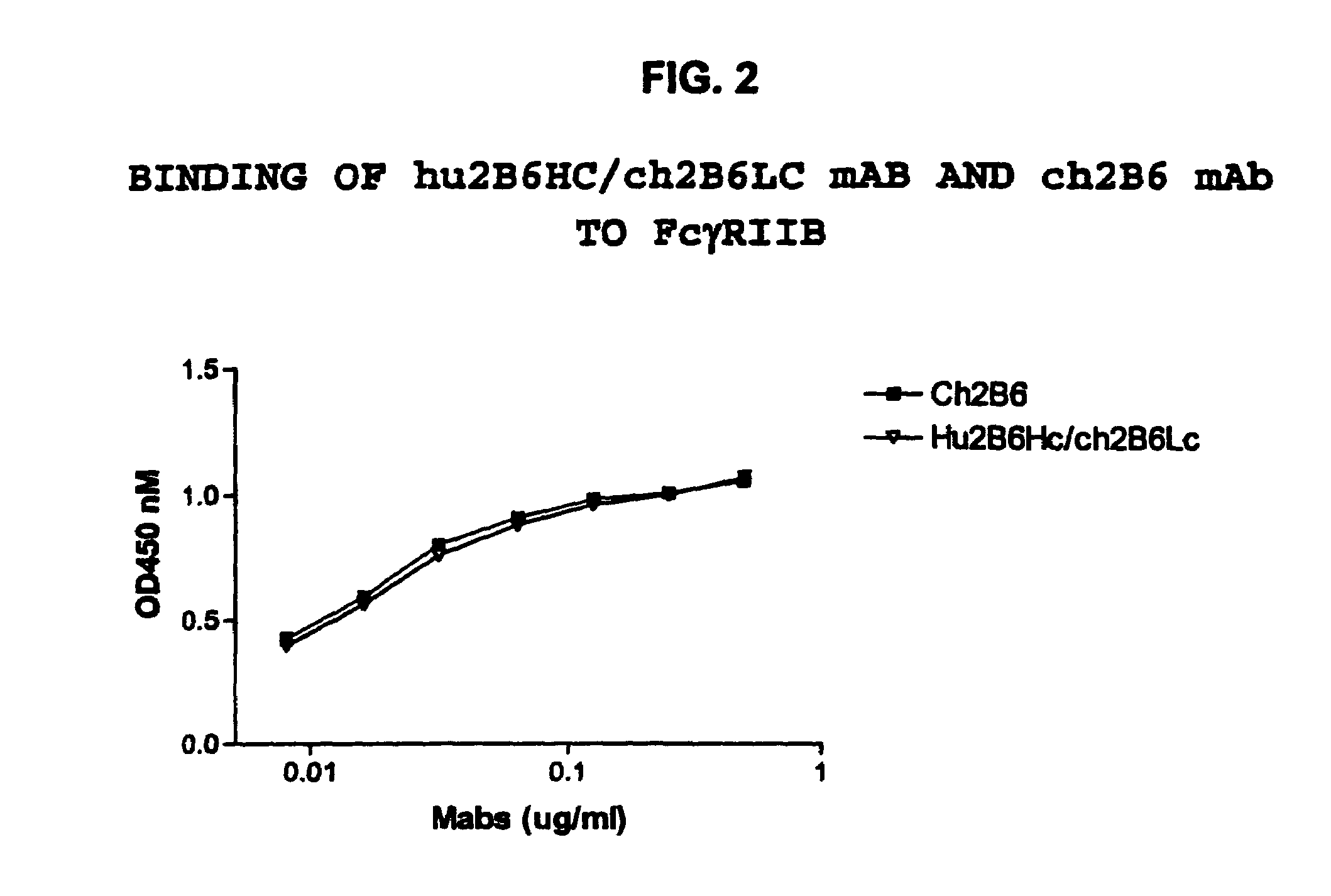
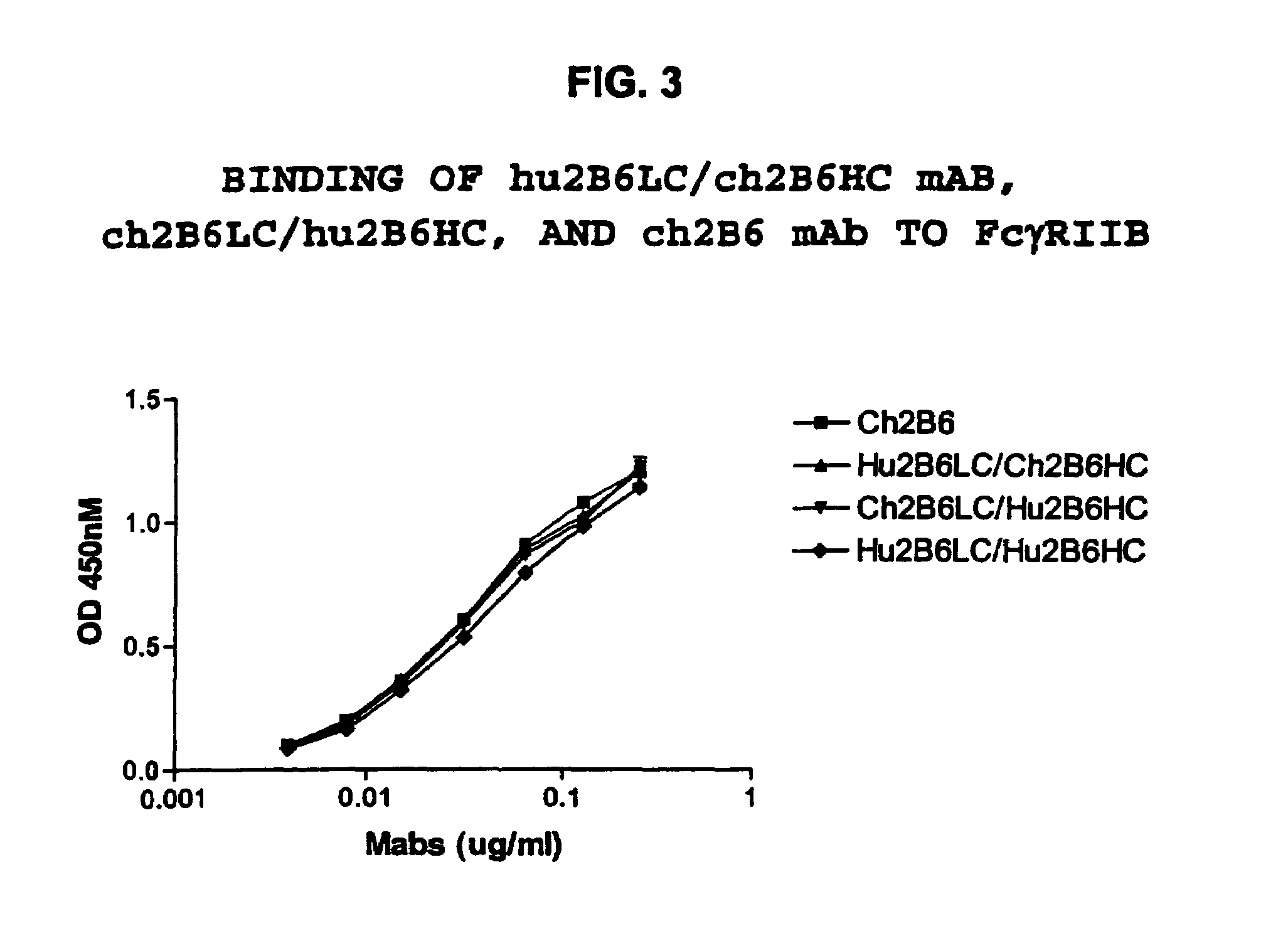
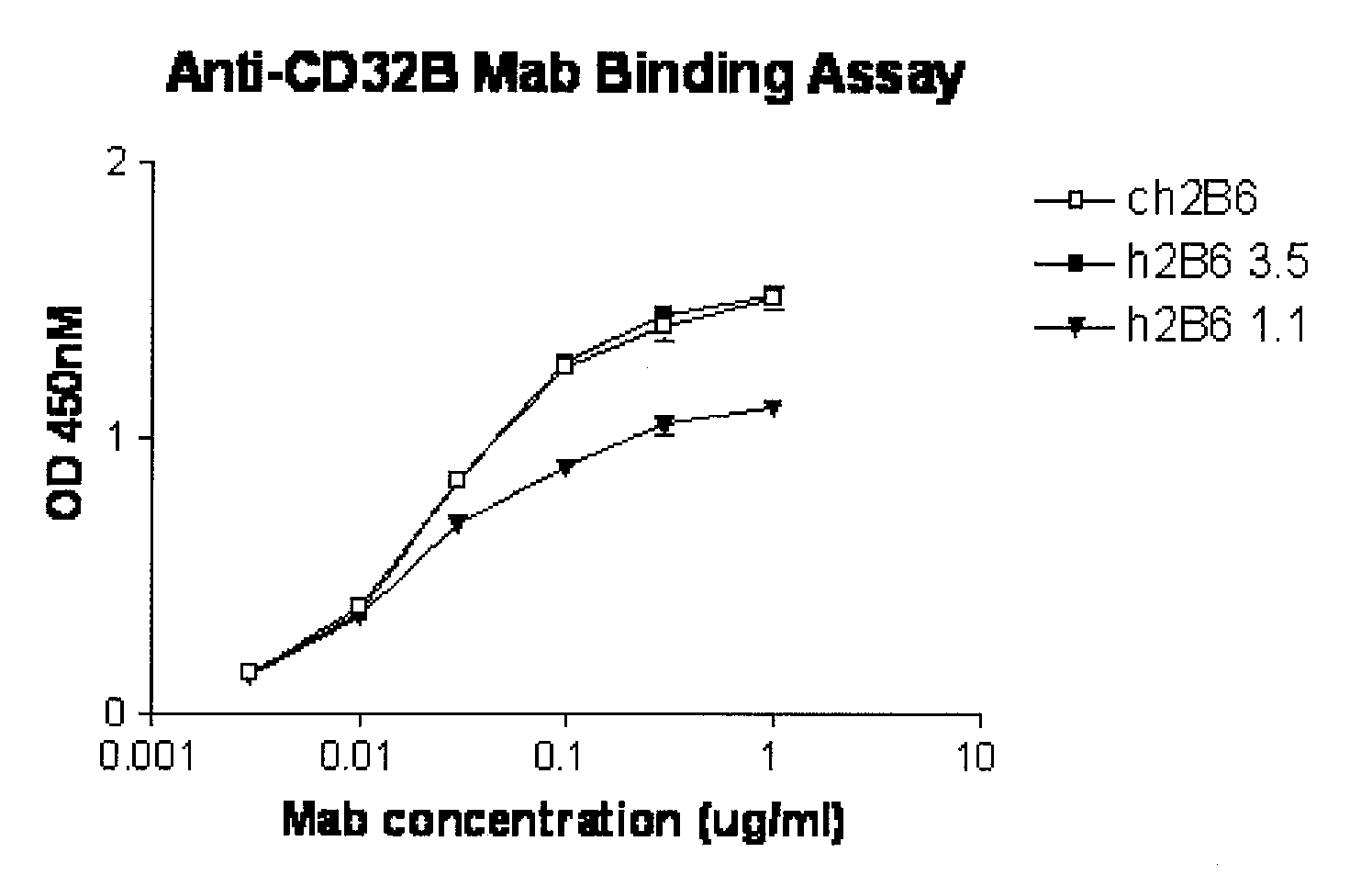
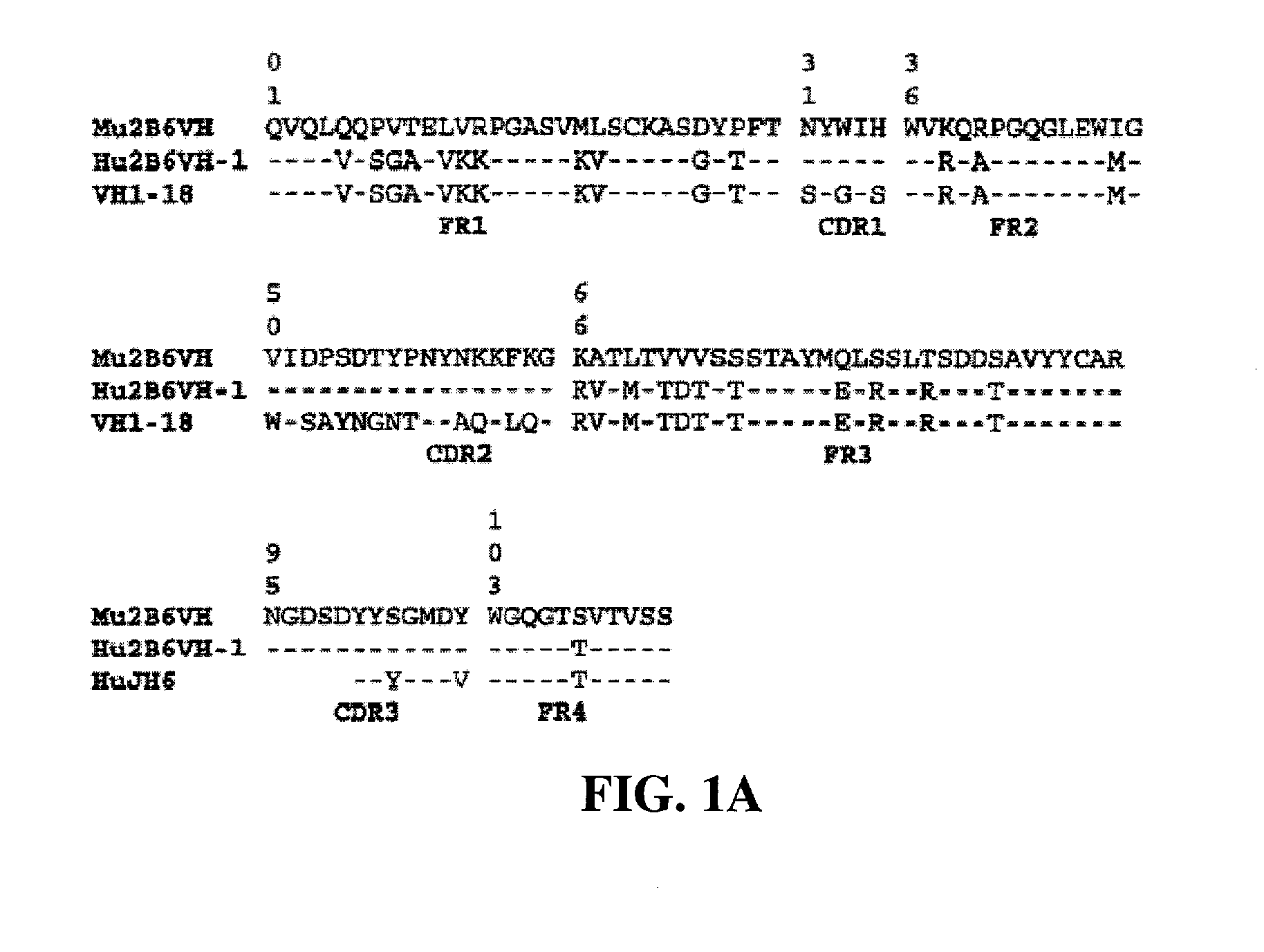
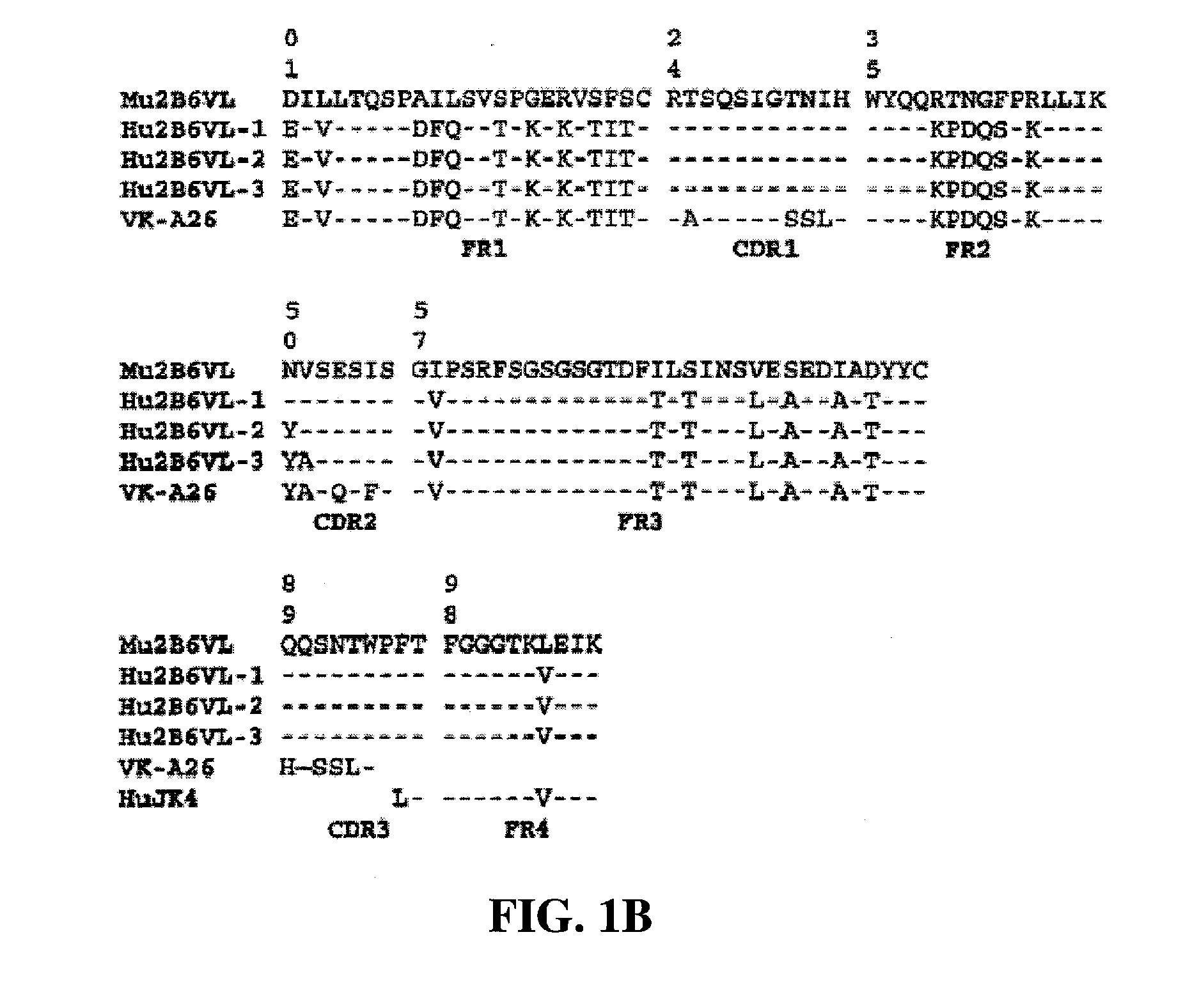
Humanized FcgammaRIIB-specific antibodies and methods of use thereof
ActiveUS7521542B2Immune responseAvoid immune responseSenses disorderNervous disorderFc(alpha) receptorTherapeutic antibody
The present invention relates to humanized FcγRIIB antibodies, fragments, and variants thereof that bind human FcγRIIB with a greater affinity than said antibody binds FcγRIIA. The invention encompasses the use of the humanized antibodies of the invention for the treatment of any disease related to loss of balance of Fc receptor mediated signaling, such as cancer, autoimmune and inflammatory disease. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the humanized antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing the efficacy of a vaccine composition by administering the humanized antibodies of the invention. The invention encompasses methods for treating an autoimmune disease and methods for elimination of cancer cells that express FcγRIIB.
Owner:MACROGENICS INC
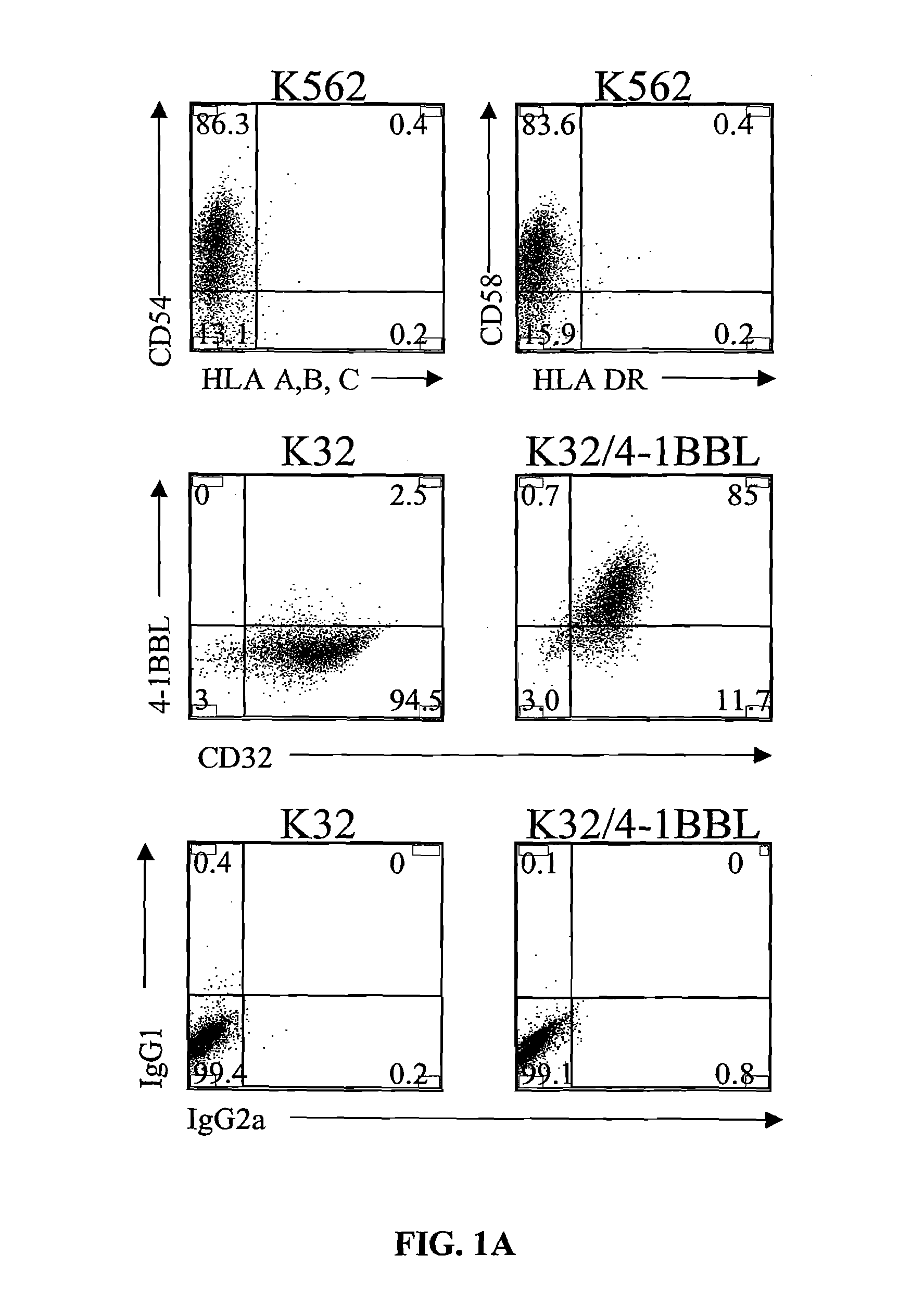
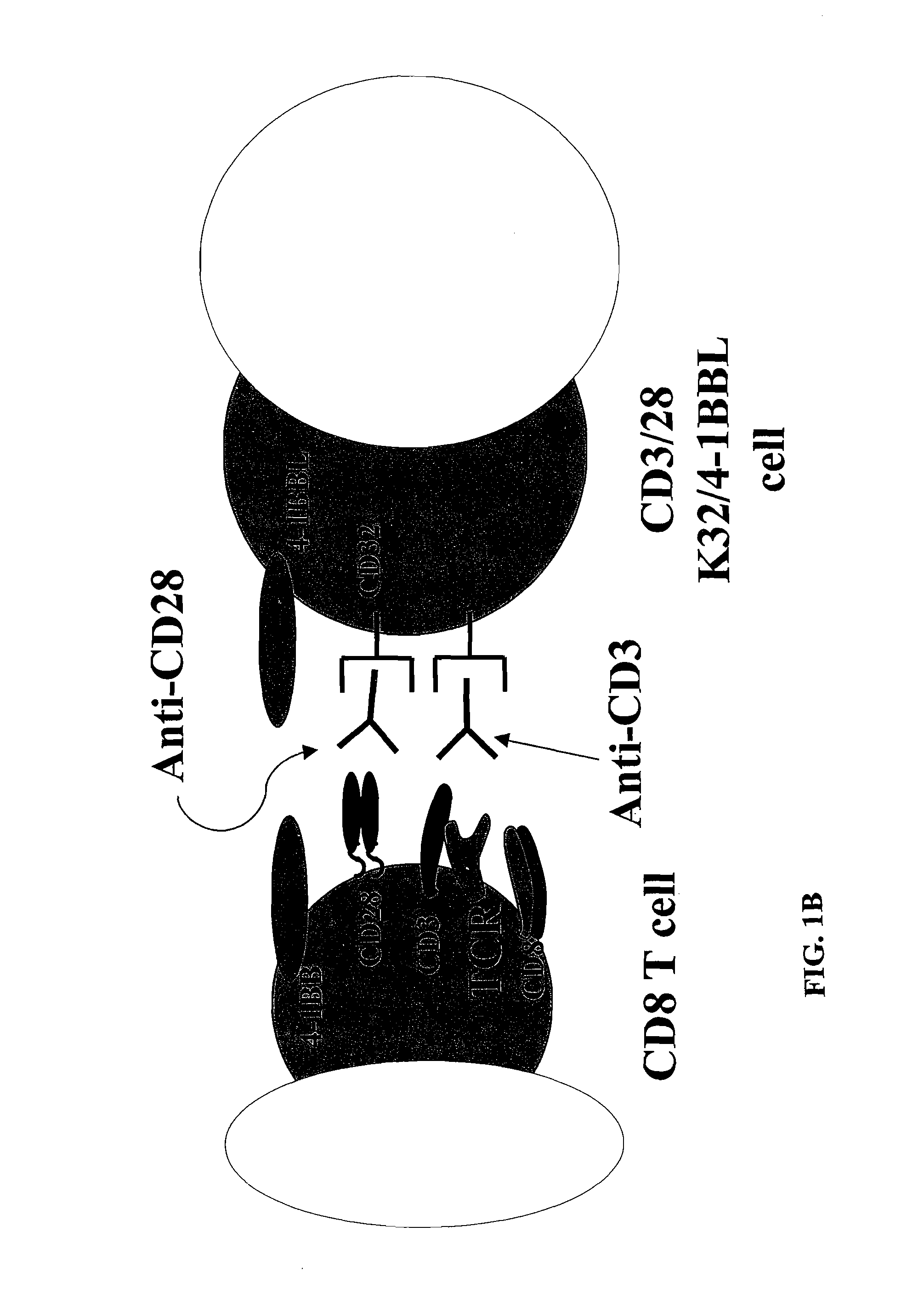
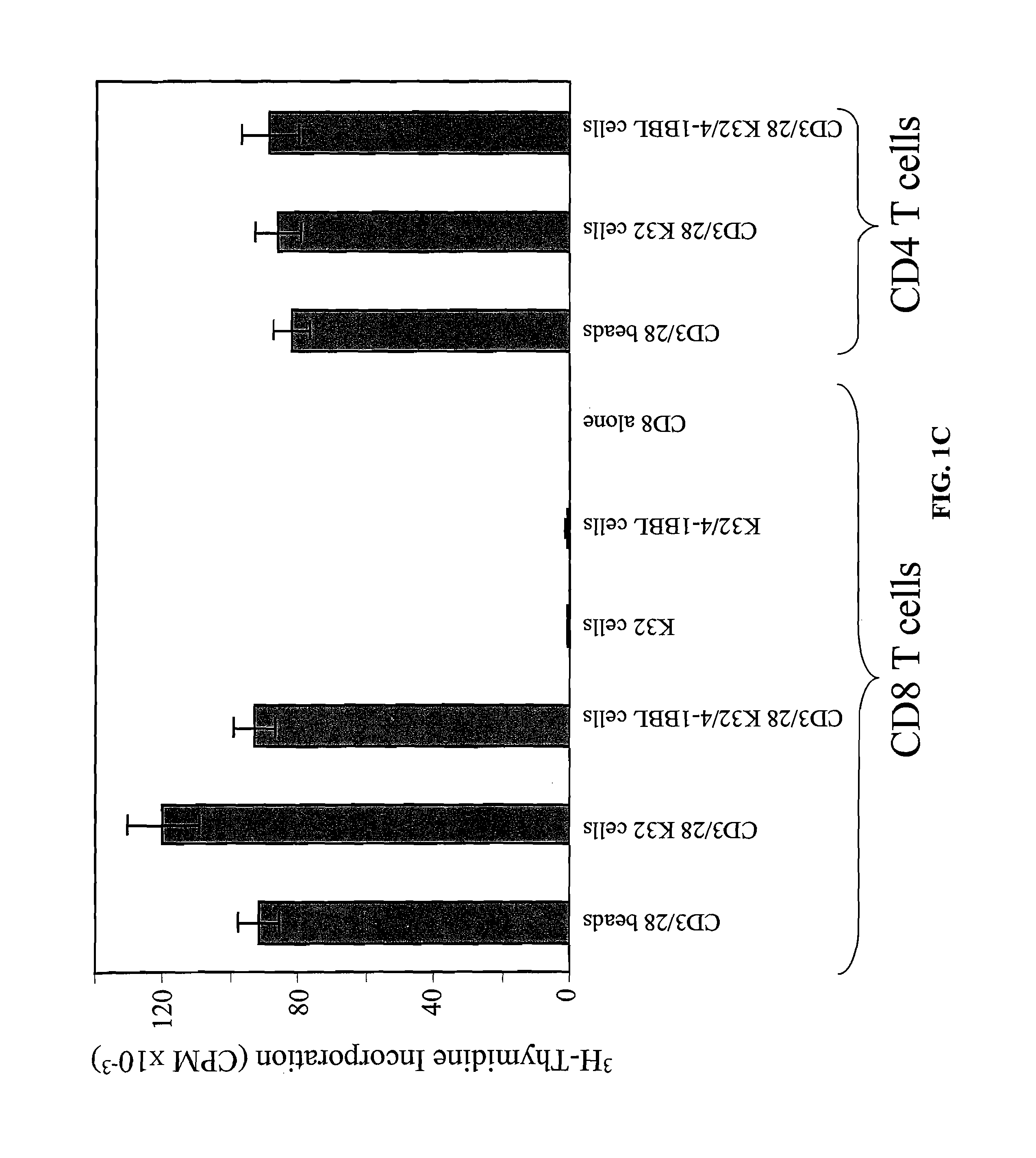
Activation and expansion of T-cells using an engineered multivalent signaling platform as a research tool
InactiveUS8637307B2Maintain their viabilityLower Level RequirementsGenetically modified cellsMammal material medical ingredientsT cellExogenous growth
Provided are a system and methods for selectively inducing expansion of a population of T cells in the absence of exogenous growth factors, such as lymphokines, and accessory cells for research purposes. The cell based expansion system and methods permit the long-term growth of CTLs, preferably human CTLs. In addition, T cell proliferation can be induced without the need for antigen, thus providing an expanded T cell population that is polyclonal with respect to antigen reactivity. Further provided are methods for using the system and methods to screen and identify antigens related to specific diseases or conditions, tumors, autoimmune disorders, or an infectious disease or pathogen, and to identify target molecule for research purposes, or for developing a vaccine based thereon.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Humanized Fc.gamma.RIIB-Specific Antibodies and Methods of Use Thereof
InactiveUS20080044417A1Good curative effectEnhanced effector functionDisease diagnosisTissue cultureFc(alpha) receptorFc receptor
The present invention relates to humanized FcγRIIB antibodies, fragments, and variants thereof that bind human FcγRIIB with a greater affinity than said antibody binds FcγRIIA. The invention encompasses the use of the humanized antibodies of the invention for the treatment of any disease related to loss of balance of Fc receptor mediated signaling, such as cancer, autoimmune and inflammatory disease. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the humanized antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing the efficacy of a vaccine composition by administering the humanized antibodies of the invention. The invention encompasses methods for treating an autoimmune disease and methods for elimination of cancer cells that express FcγRIIB.
Owner:MACROGENICS INC
Recombinant tissue protective cytokines and encoding nucleic acids thereof for protection, restoration, and enhancement of responsive cells, tissues, and organs
InactiveUS20040122216A1Increase in hematocritEnhance cell viabilityAntibacterial agentsSenses disorderMammalWhole body
Methods and compositions are provided for protecting or enhancing a responsive cell, tissue, organ or body part function or viability in vivo, in situ or ex vivo in mammals, including human beings, by systemic or local administration of an erythropoietin receptor activity modulator, such as an recombinant tissue protective cytokine.
Owner:H LUNDBECK AS +1
RNA containing composition for treatment of tumor diseases
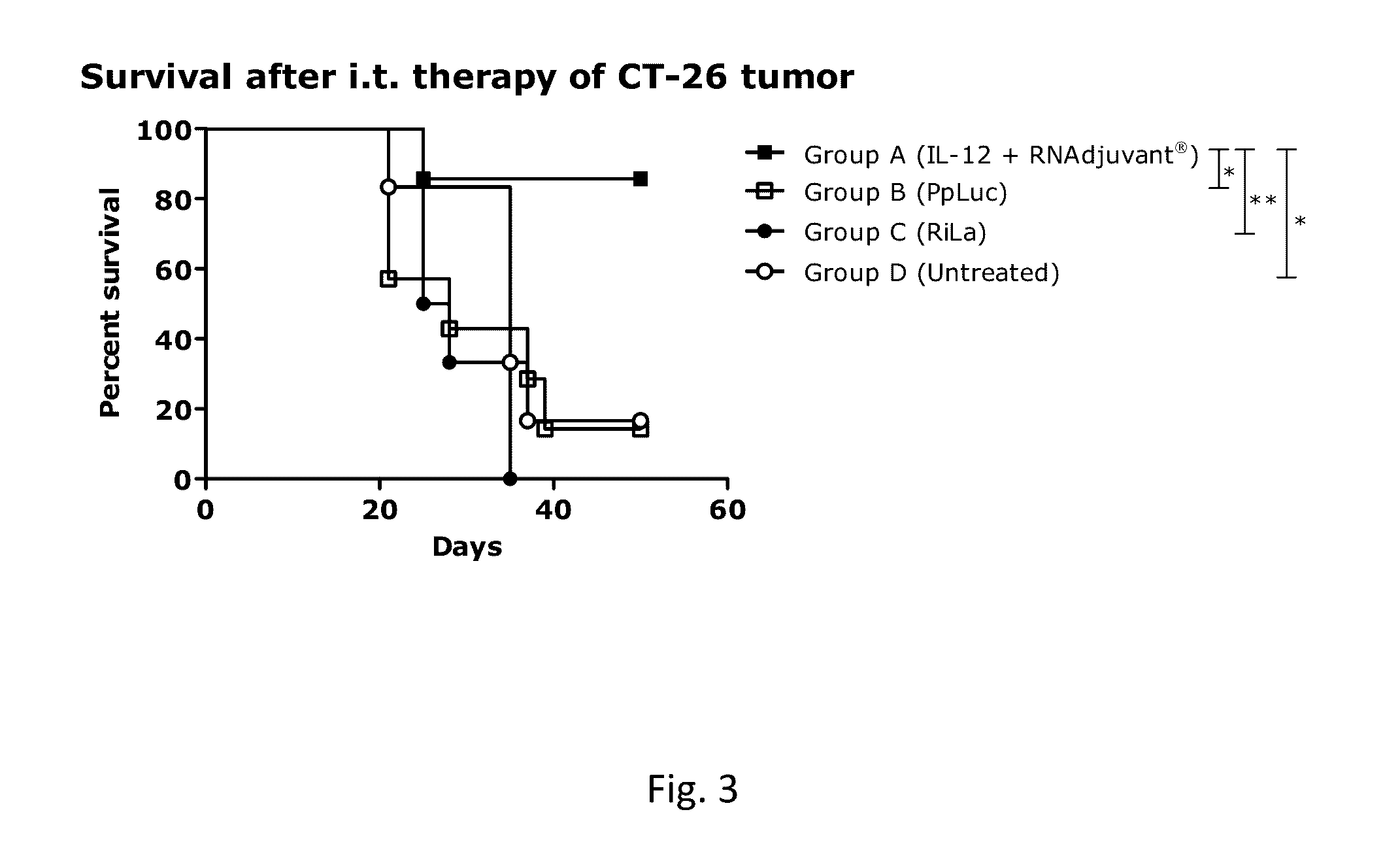
ActiveUS20160331844A1Improve survivalEffectively treating tumor and/or cancer diseasesSsRNA viruses negative-senseOrganic active ingredientsDiseasePharmaceutical drug
Owner:CUREVAC SE
Combination treatment of cd38-expressing tumors
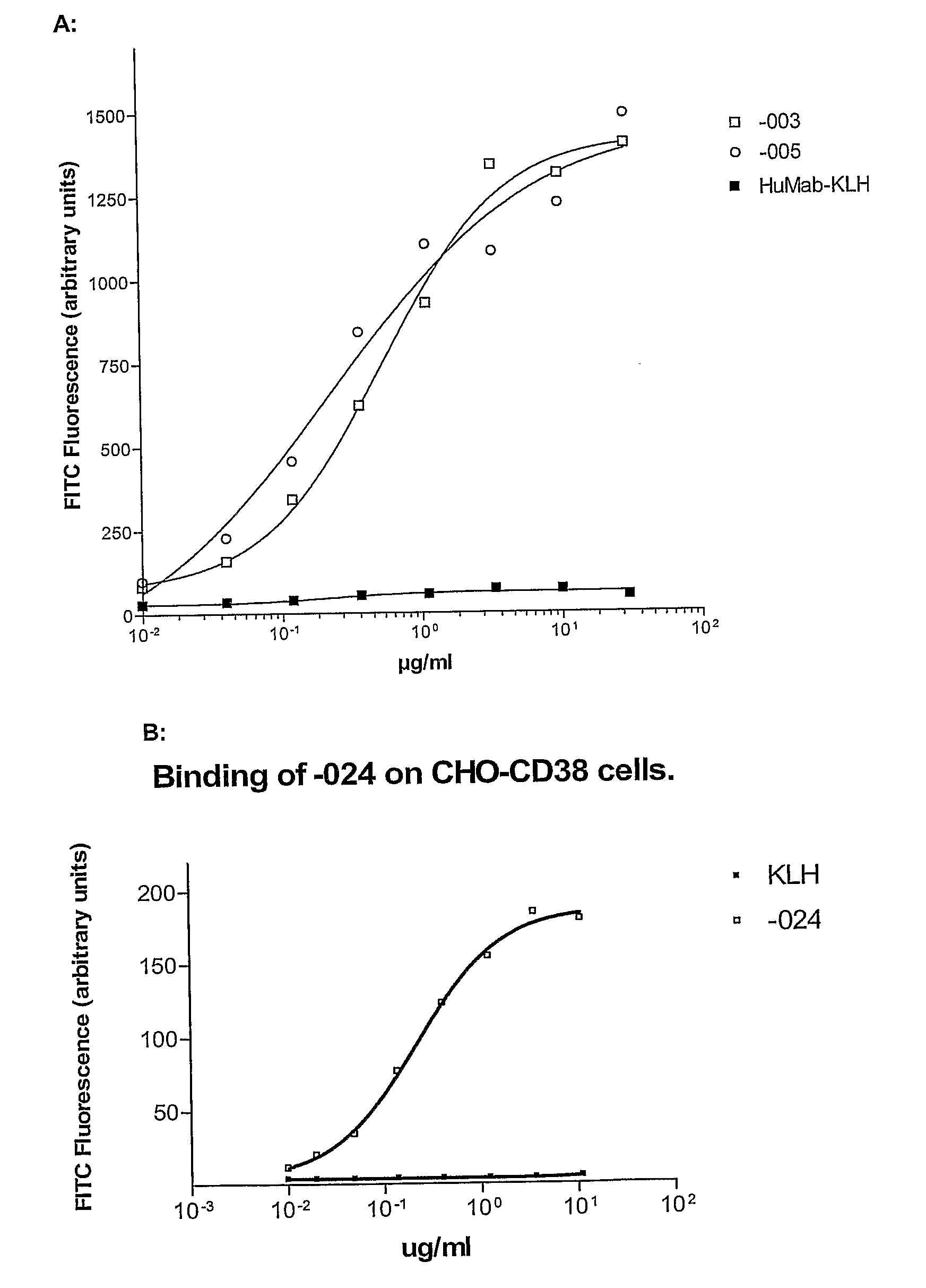
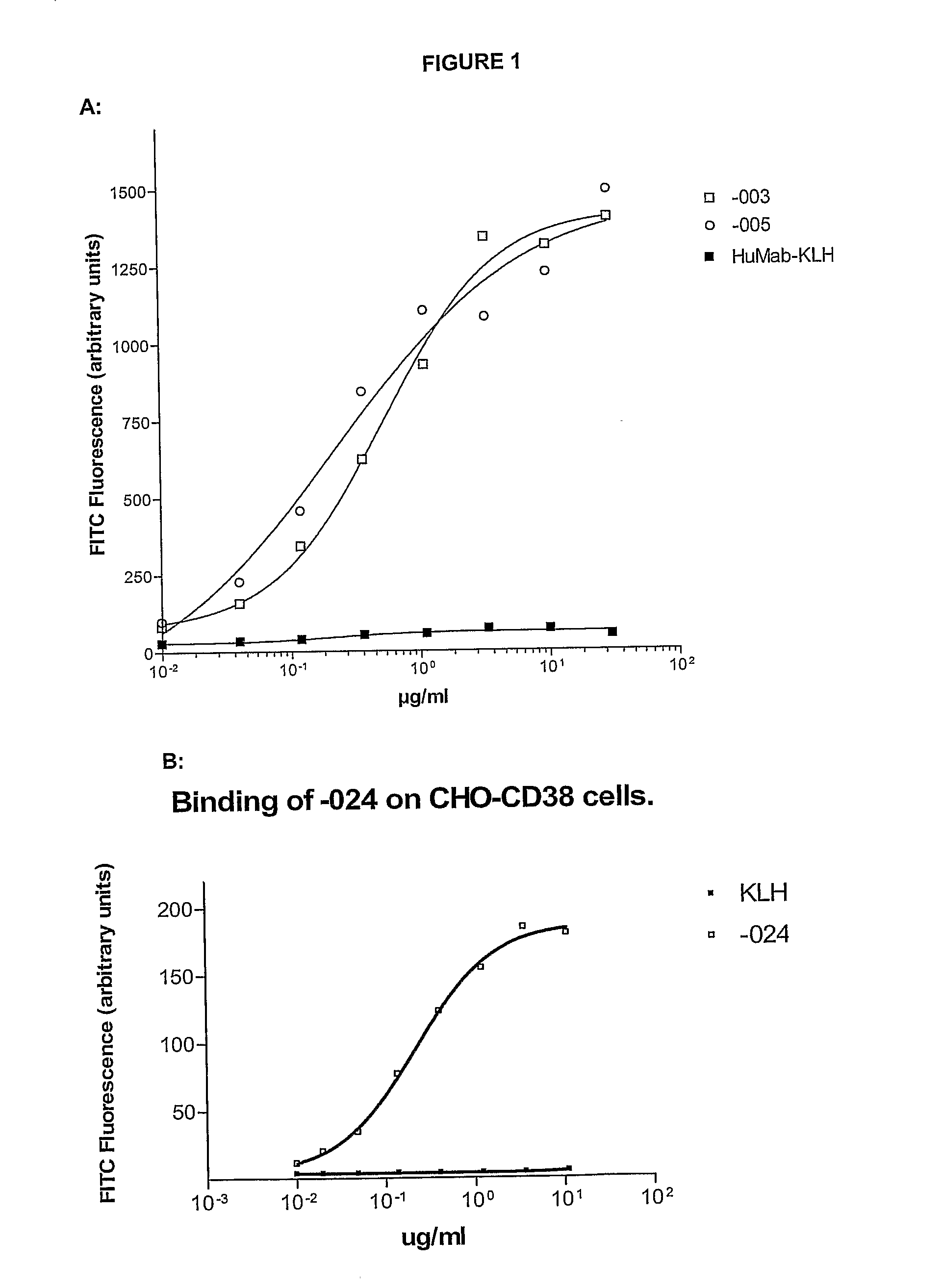
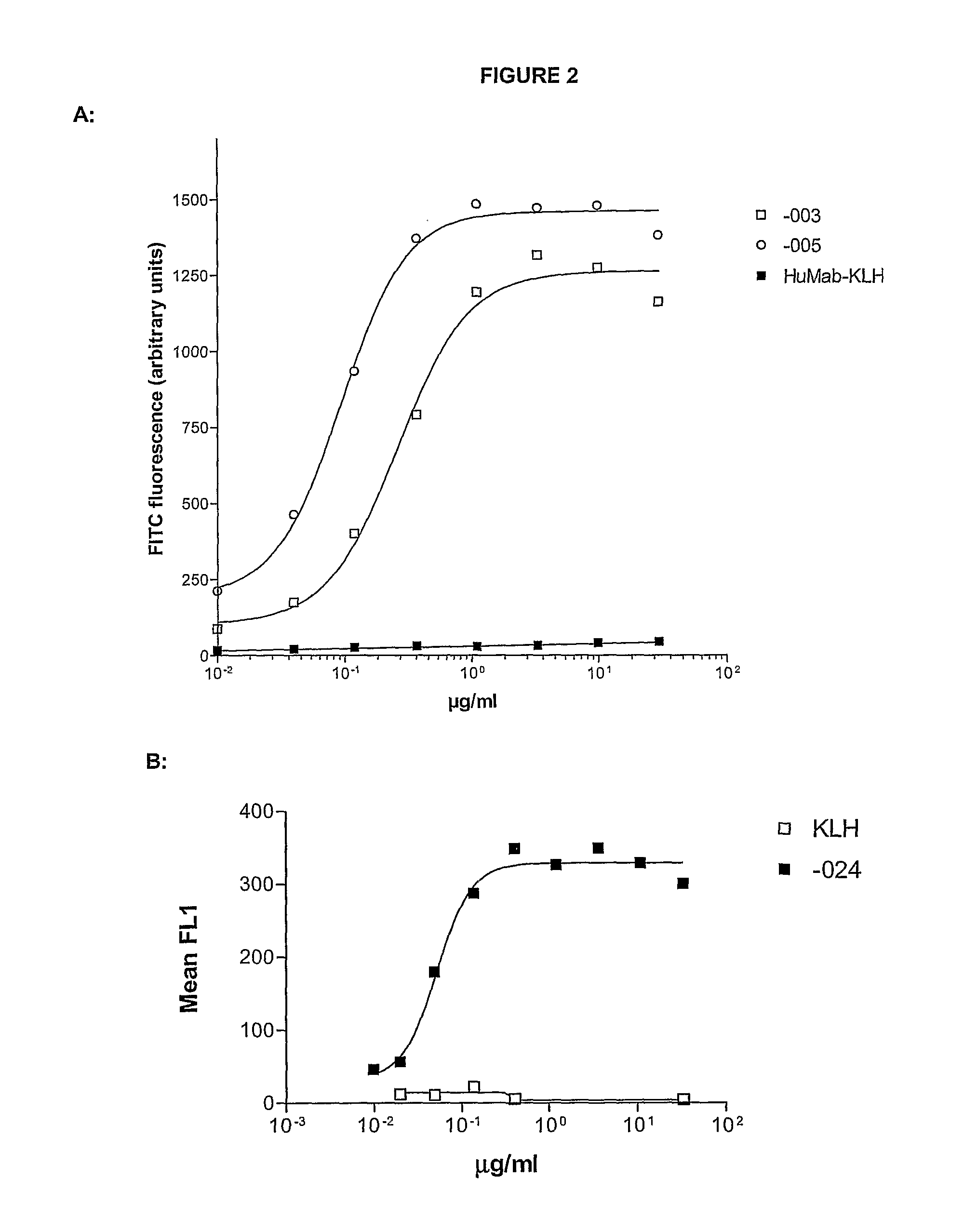
ActiveUS20100092489A1Good curative effectImprove survivalBoron compound active ingredientsSkeletal disorderAntiendomysial antibodiesOncology
The invention relates to novel method for the treatment of cancer using a combination therapy comprising an antibody that binds CD38, a corticosteroid and a non- corticosteroid chemotherapeutic agent.
Owner:GENMAB AS
Methods of treatment using Anti-erbb antibody-maytansinoid conjugates
InactiveUS20080226659A1High activityIncrease ratingsOrganic active ingredientsPharmaceutical delivery mechanismMedicineTreatment use
The application concerns methods of treatment using anti-ErbB receptor antibody-maytansinoid conjugates, and articles of manufacture suitable for use in such methods. In particular, the invention concerns ErbB receptor-directed cancer therapies, using anri-ErbB receptor antibody-maytansinoid conjugates.
Owner:IMMUNOGEN INC
Implantable Prosthetic Valve
InactiveUS20090276039A1Reduce thrombosisImprove survivalJoint implantsVenous valvesProsthetic valveThrombus
An implantable prosthetic valve for regulating fluid flow through a body vessel is provided. The prosthetic valve comprises an anchoring member, at least one leaflet, and a restraining member capable of temporarily preventing substantial movement of the leaflet between and open and closed position so as to allow fluid flow in the antegrade and retrograde directions. In various embodiments, the prosthetic valve reduces the risk of thrombosis. In various embodiments, the prosthetic valve reduces the appearance of potentially thrombogenic abnormal flow patterns at the site of implantation immediately following the implantation, allows cell deposition making the valve more biocompatible, less thrombogenic before flow changes resulting from valving action set in and allows tissue growth so that a partially or completely biological functioning valve may form on the scaffold provided by the implant.
Owner:CLINASYS
Chemotherapy treatment
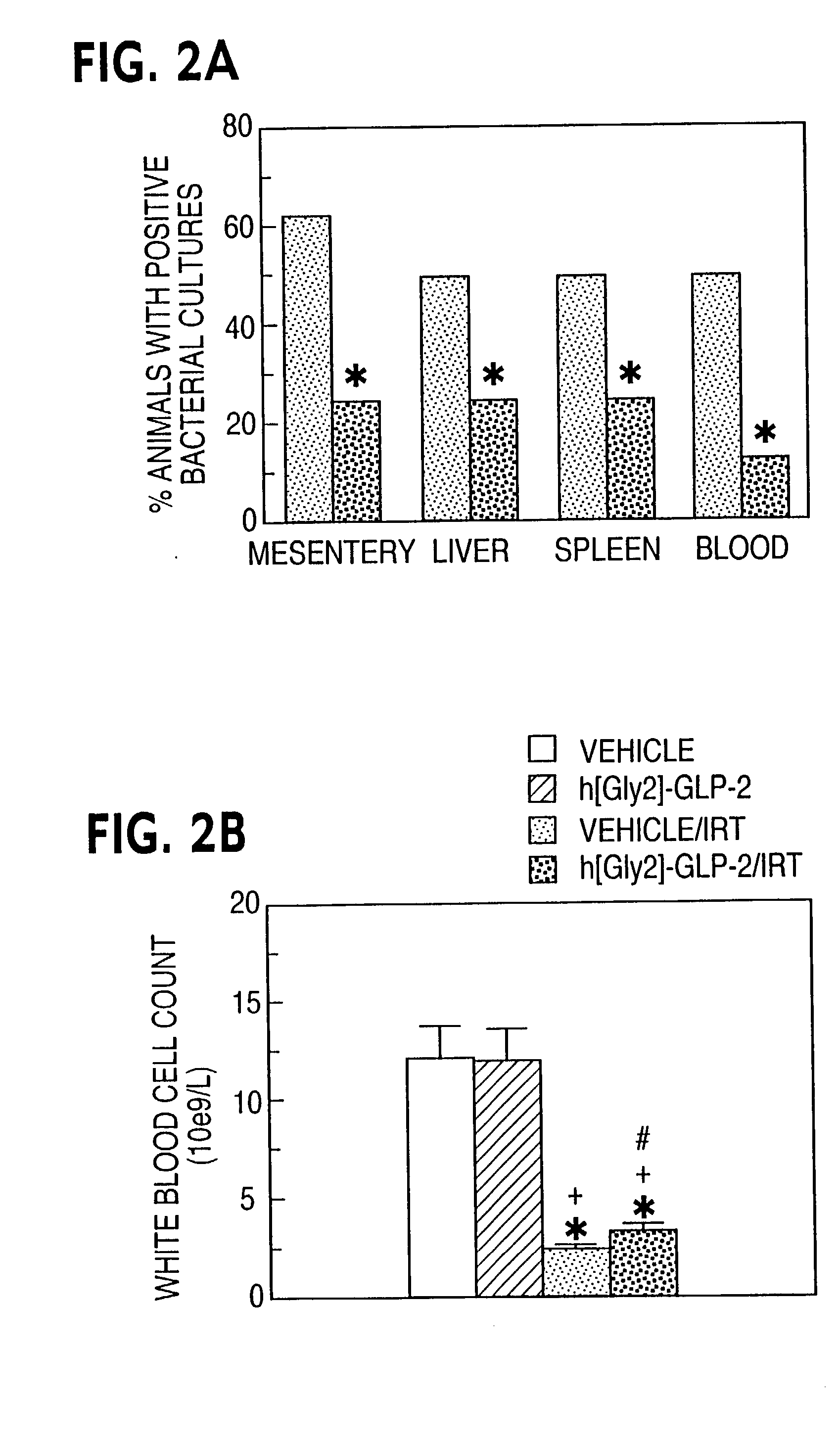
InactiveUS20030040478A1Improve welfareImprove survivalBiocideOrganic active ingredientsRegimenApoptosis
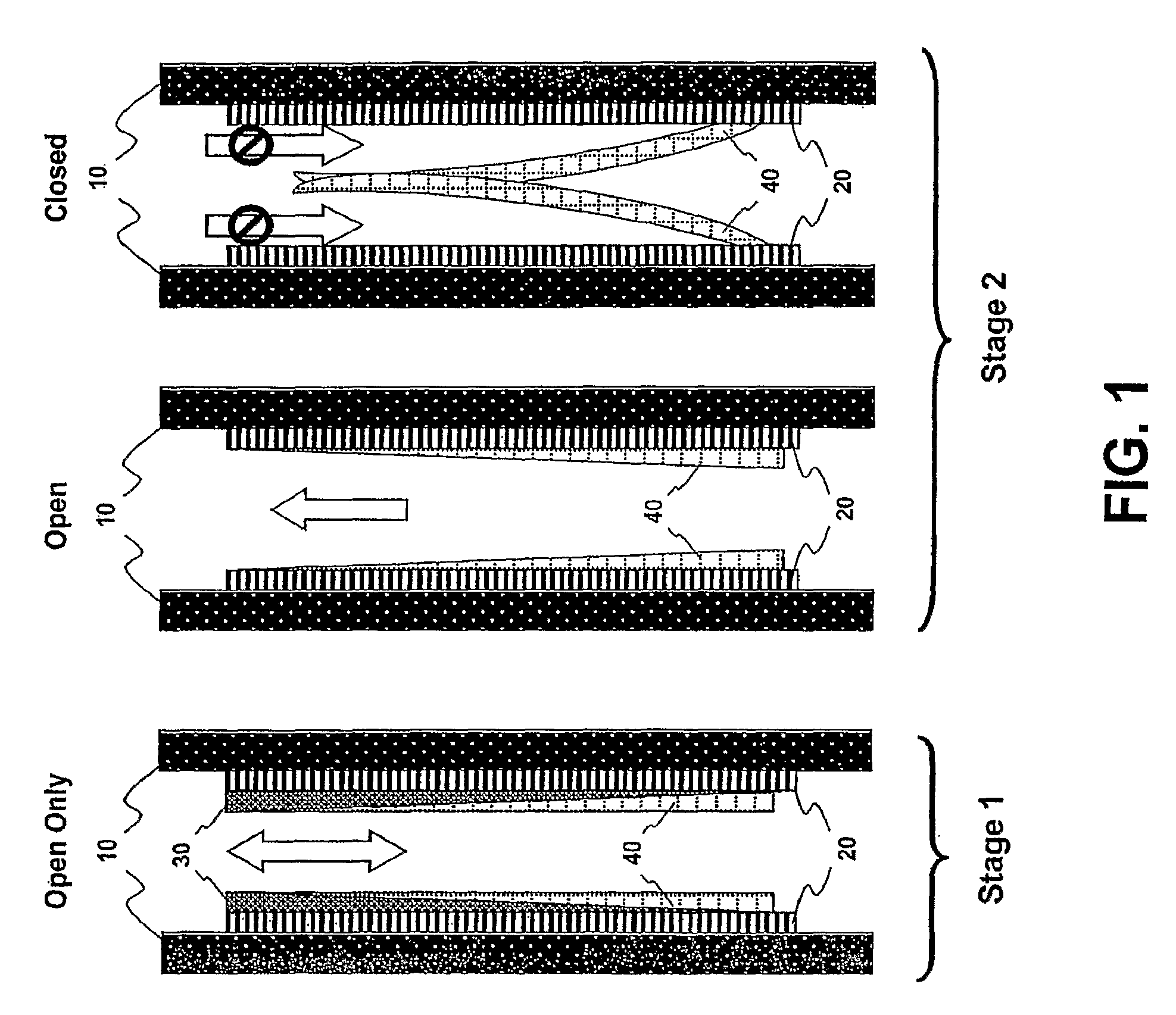
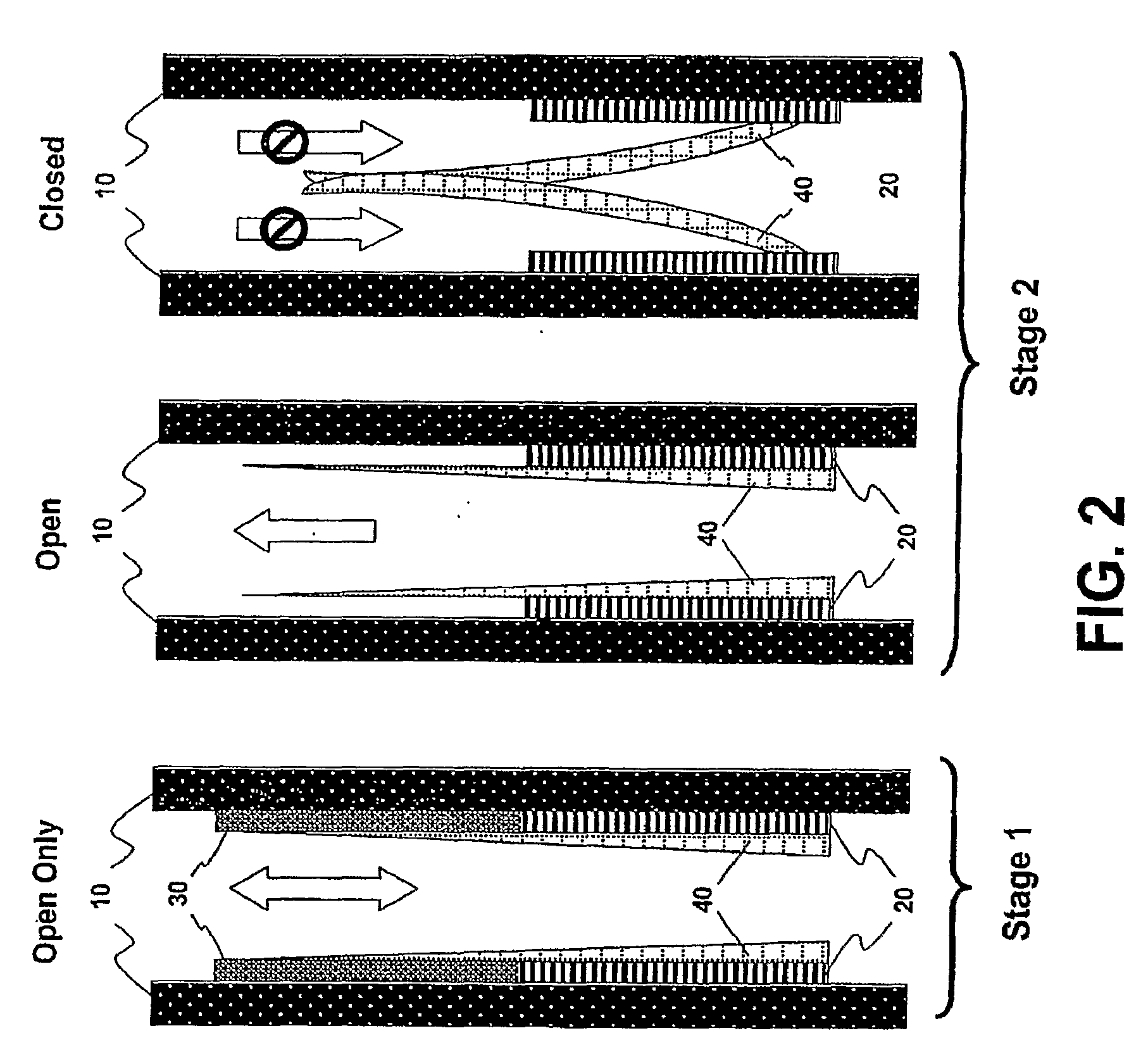
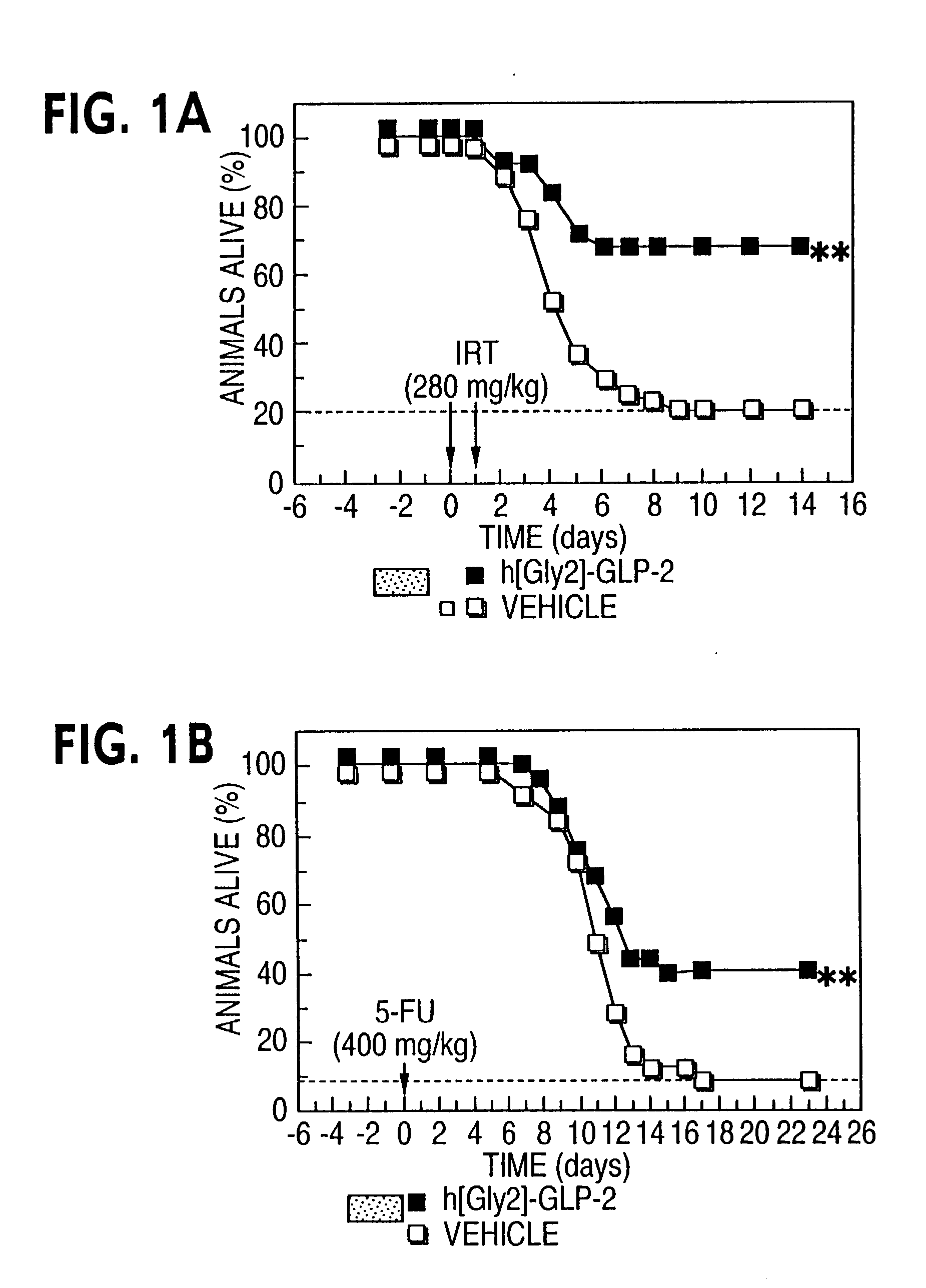
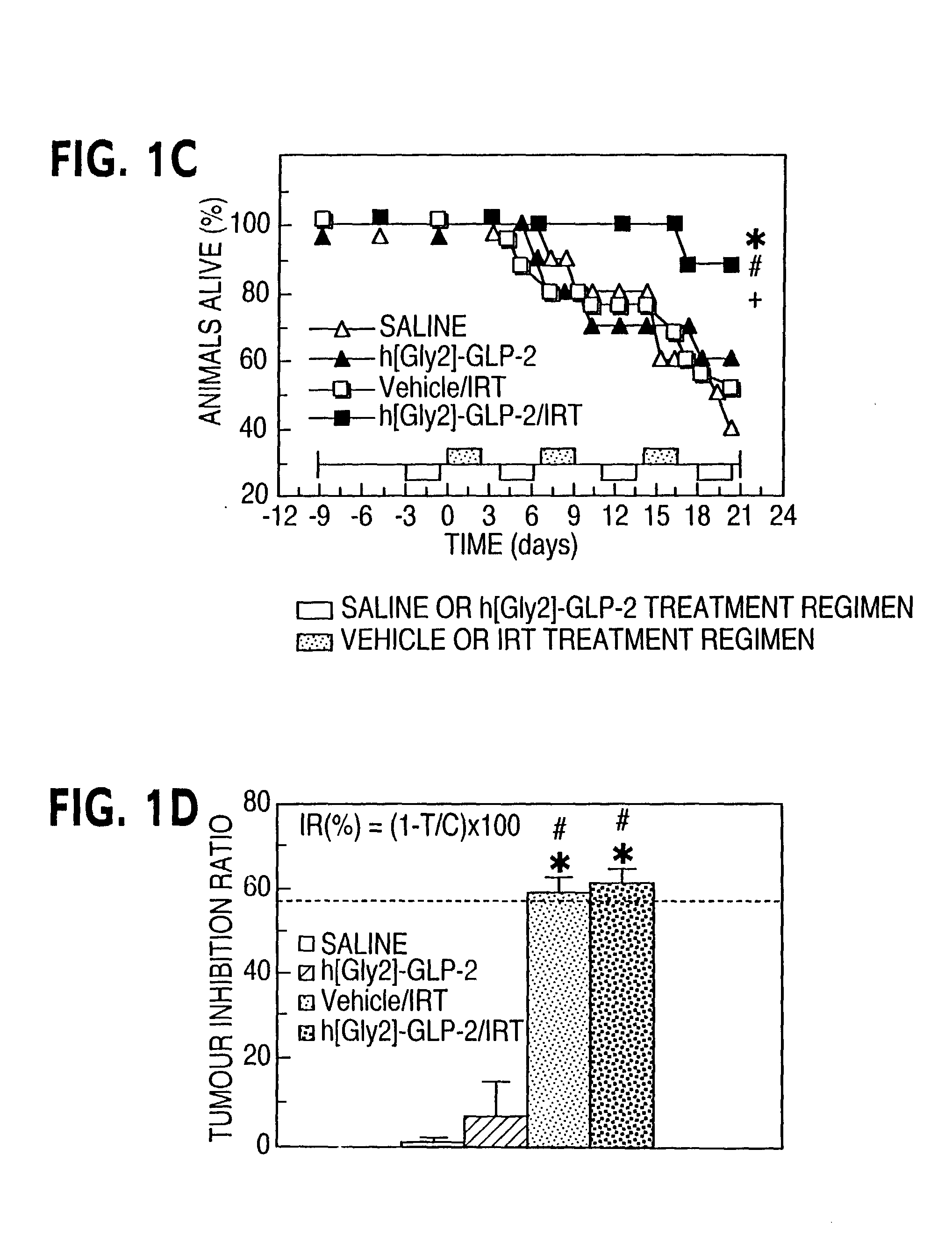
This invention provides a treatment regimen that is effective in inhibiting chemotherapy-induced apoptosis and promoting cell survival. The invention also relates to a treatment regimen that confers resistance to caspase activation, thereby inhibiting caspase-mediated, proteolytic cleavage of functional cellular enzymes. Specifically, subjects undergoing chemotherapy are first exposed to a pretreatment regimen. Under this regimen, a GLP-2 receptor activator, such as h[GLY2]-GLP2, is administered each day for a predetermined beneficial period, e.g., three consecutive days. Approximately about 1 week following pretreatment, the subjects are exposed to an appropriate chemotherapy treatment regimen. Pretreatment with a GLP-2 receptor activator followed by administration of chemotherapeutic agents improves cell survival, reduces bacteremia, attenuates epithelial injury, and inhibits cellular apoptosis. Moreover, it does not impair the effectiveness of chemotherapy nor result in weight loss. The anti-apoptotic effects of GLP-2 may be useful in the reduction of cytoxicity and bacterial infection induced by chemotherapeutic agents.
Owner:1149336 ONTARIO
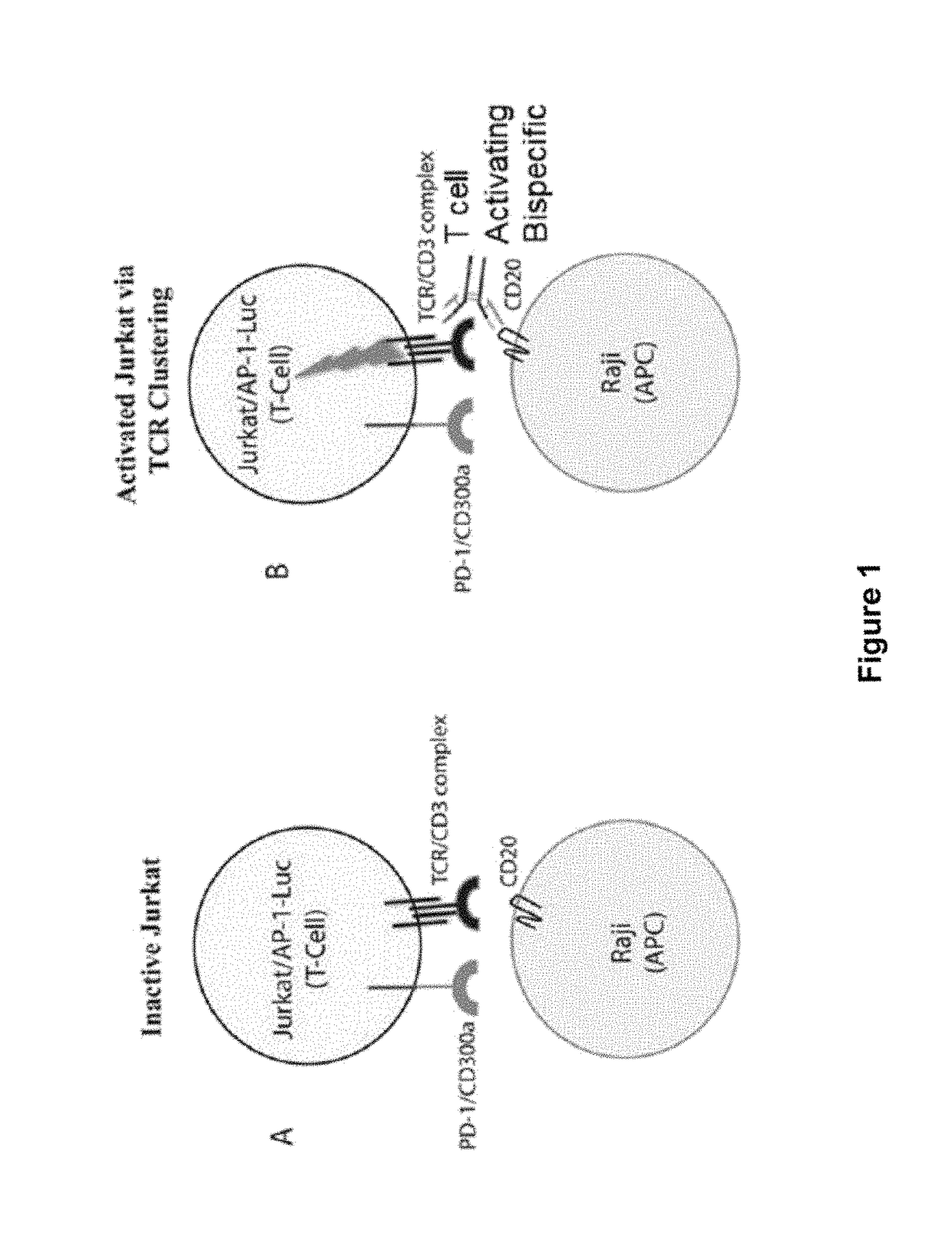
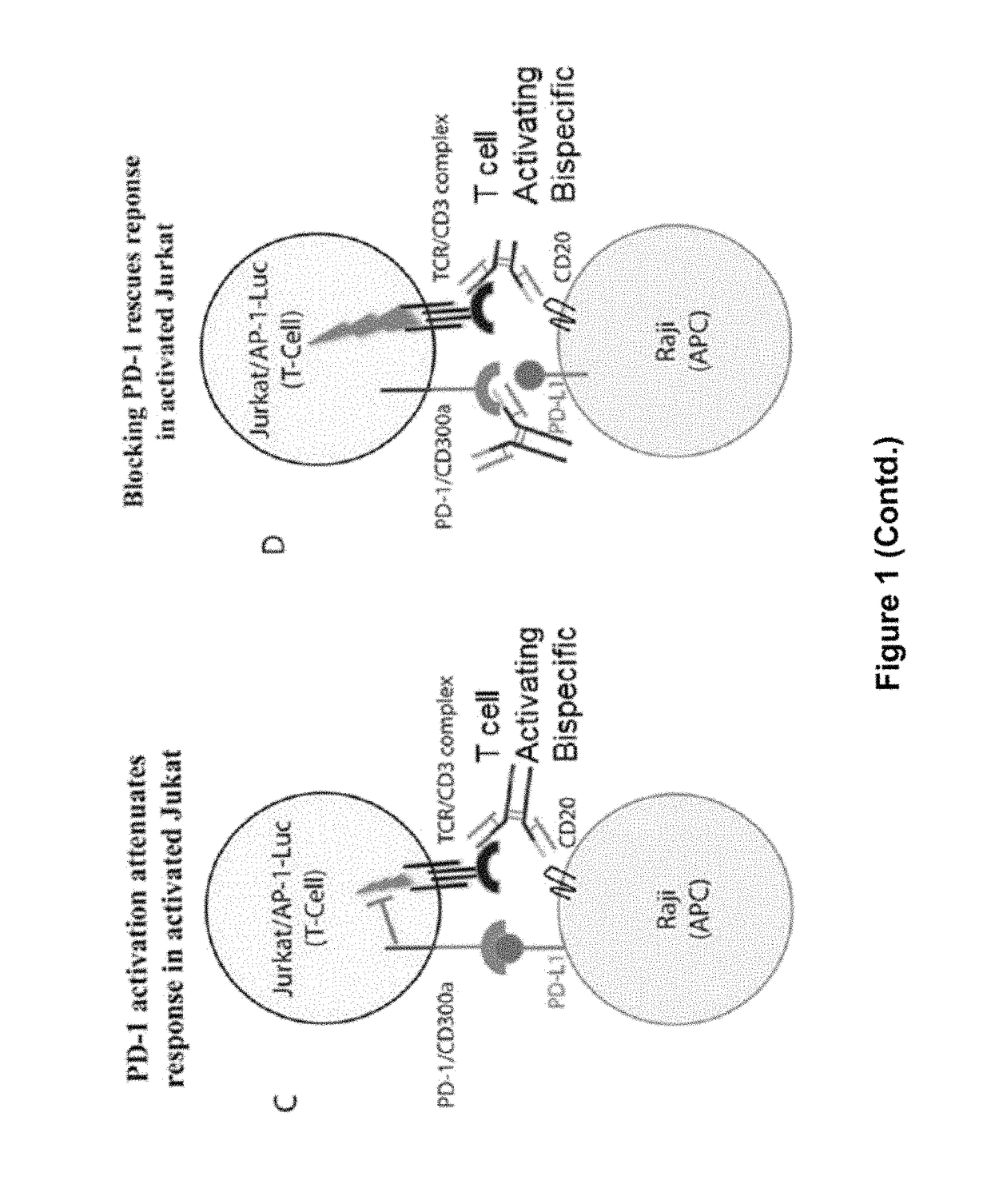
Human antibodies to PD-1
ActiveUS9987500B2Rescues T-cell signalingInhibit tumor growthNervous disorderAntipyreticFc(alpha) receptorDisease
The present invention provides antibodies that bind to the T-cell co-inhibitor programmed death-1 (PD-1) protein, and methods of use. In various embodiments of the invention, the antibodies are fully human antibodies that bind to PD-1. In certain embodiments, the present invention provides multi-specific antigen-binding molecules comprising a first binding specificity that binds to PD-1 and a second binding specificity that binds to an autoimmune tissue antigen, another T-cell co-inhibitor, an Fc receptor, or a T-cell receptor. In some embodiments, the antibodies of the invention are useful for inhibiting or neutralizing PD-1 activity, thus providing a means of treating a disease or disorder such as cancer or a chronic viral infection. In other embodiments, the antibodies are useful for enhancing or stimulating PD-1 activity, thus providing a means of treating, for example, an autoimmune disease or disorder.
Owner:REGENERON PHARM INC
Combination of immuno gene therapy and chemotherapy for treatment of cancer and hyperproliferative diseases
ActiveUS7964571B2Inhibiting growth and metastasisImprove survivalHeavy metal active ingredientsPeptide/protein ingredientsGene deliveryWhole body
Owner:CLSN LAB
Methods, compositions and articles of manufacture for enhancing survivability of cells, tissues, organs, and organisms
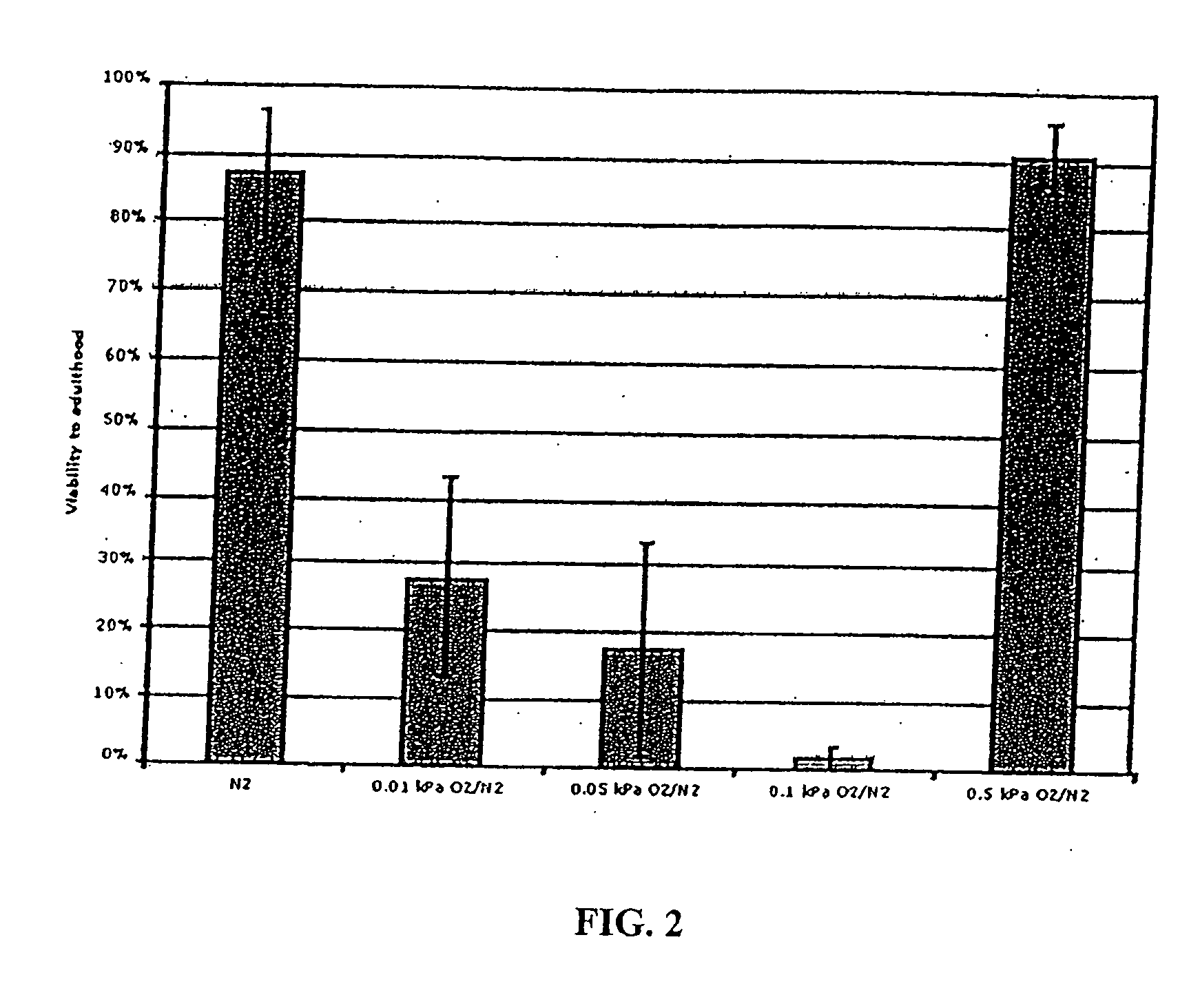
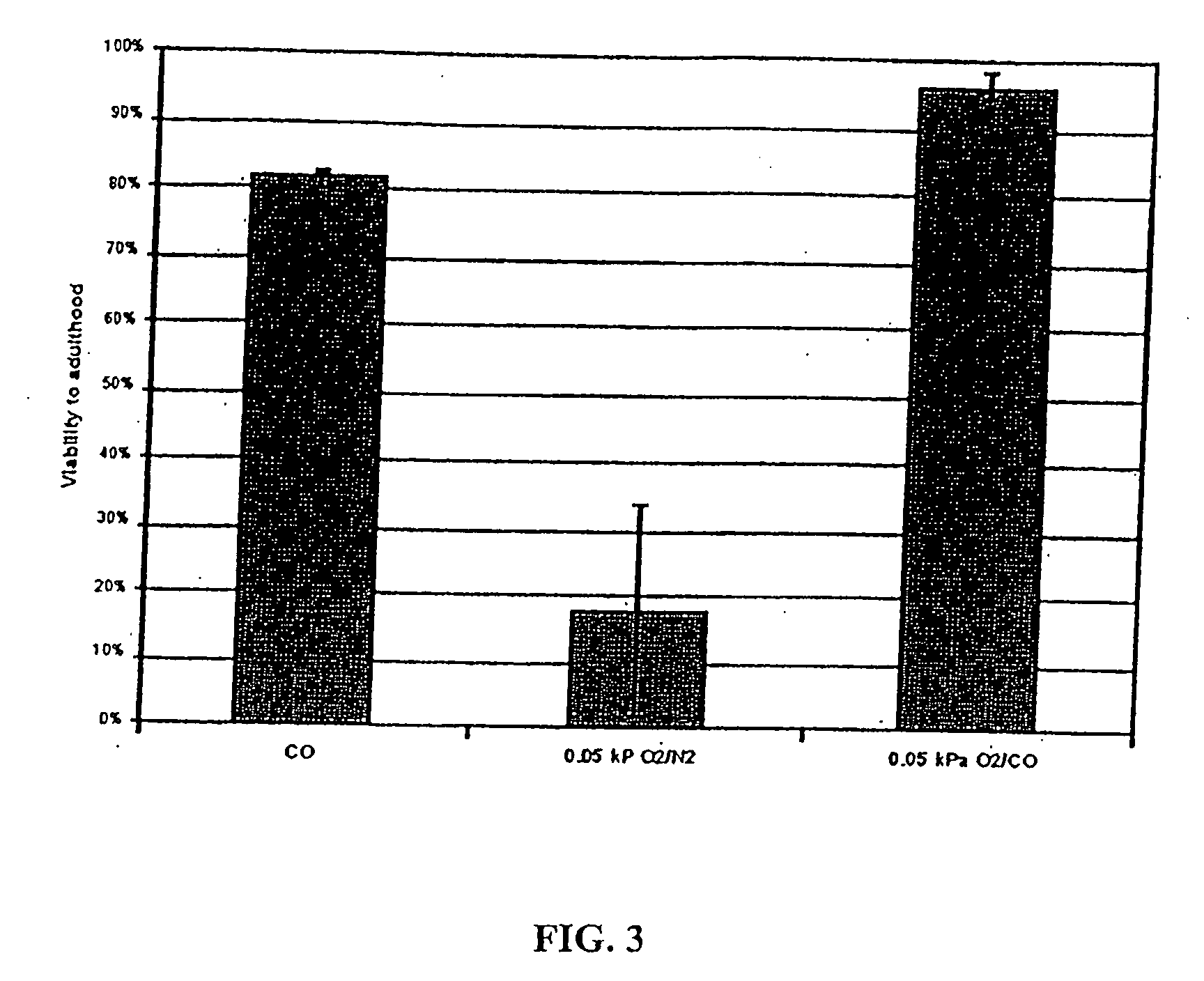
InactiveUS20070078113A1Easily damagedImprove survivabilityBiocideSulfur/selenium/tellurium active ingredientsSurvivabilityOxygen
The present invention concerns the use of oxygen antagonists and other active compounds for inducing stasis or pre-stasis in cells, tissues, and / or organs in vivo or in an organism overall, in addition to enhancing their survivability. It includes compositions, methods, articles of manufacture and apparatuses for enhancing survivability and for achieving stasis or pre-stasis in any of these biological materials, so as to preserve and / or protect them. In specific embodiments, there are also therapeutic methods and apparatuses for organ transplantation, hyperthermia, wound healing, hemorrhagic shock, cardioplegia for bypass surgery, neurodegeneration, hypothermia, and cancer using the active compounds described.
Owner:FRED HUTCHINSON CANCER RES CENT
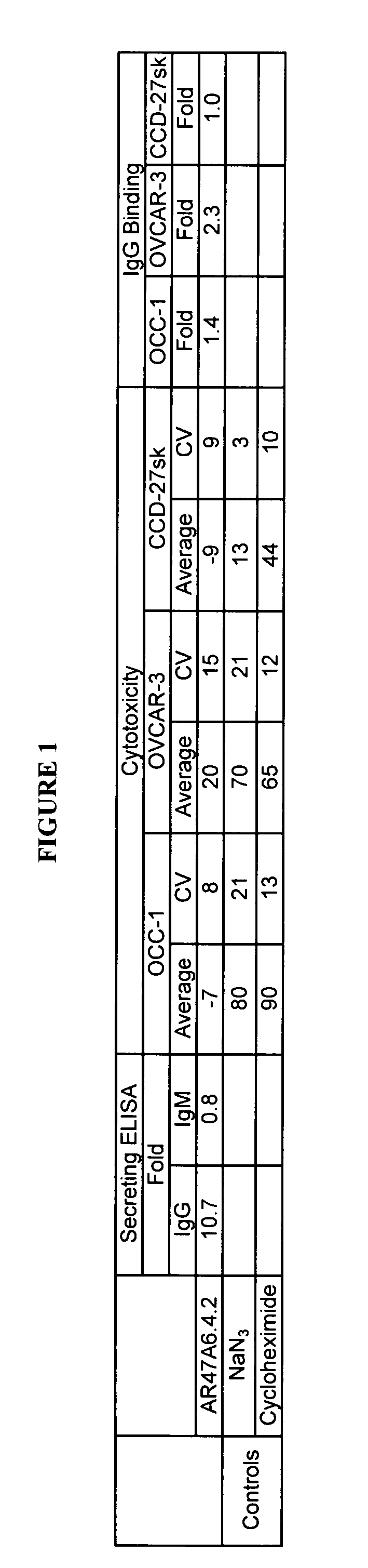
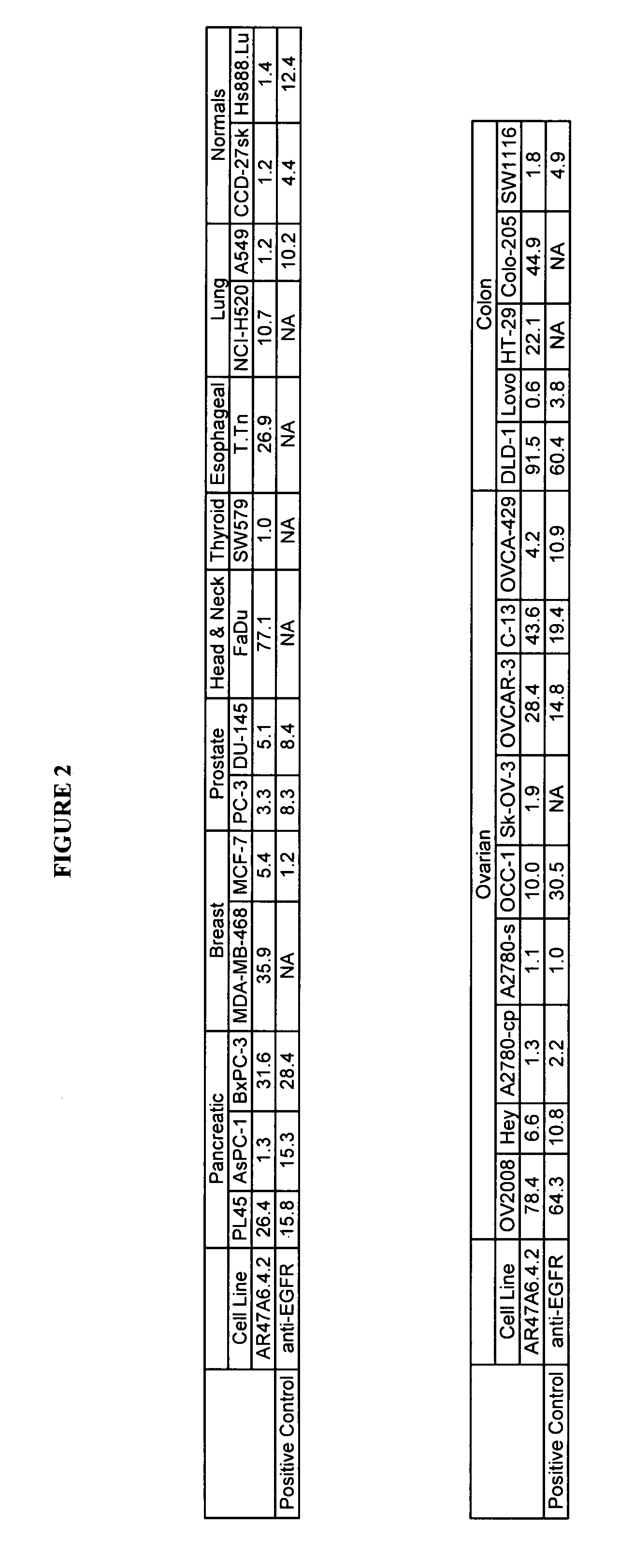
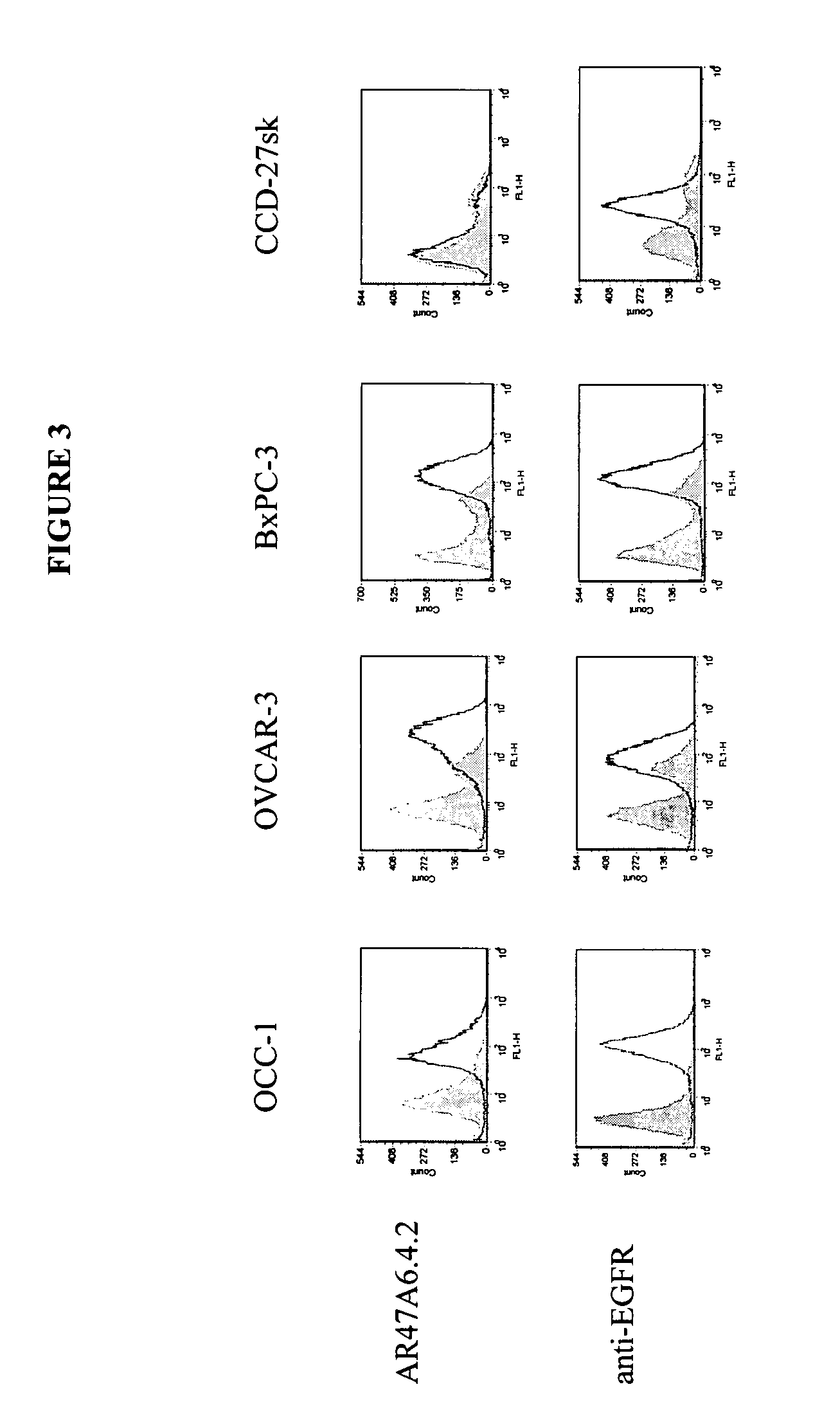
Cytotoxicity mediation of cells evidencing surface expression of TROP-2
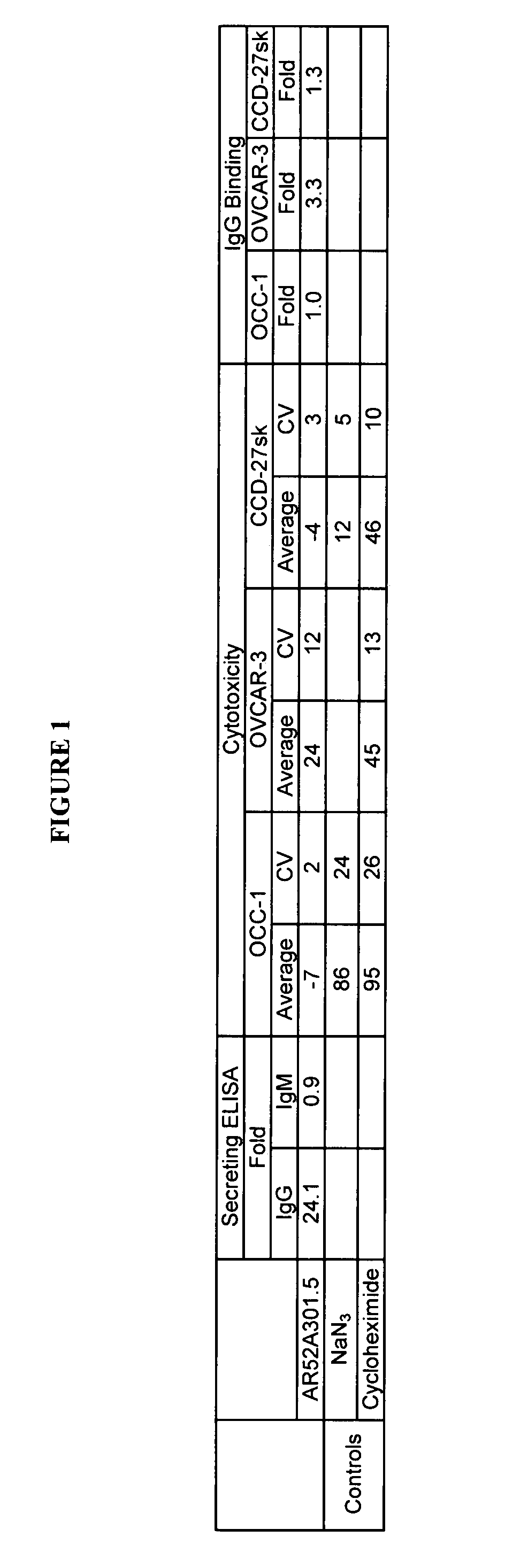
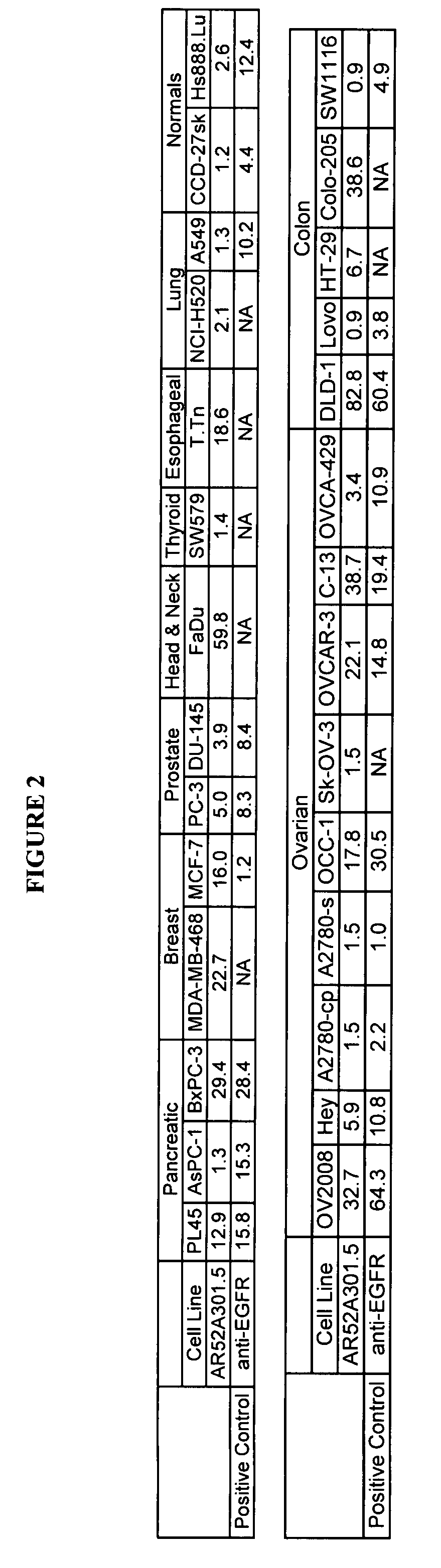
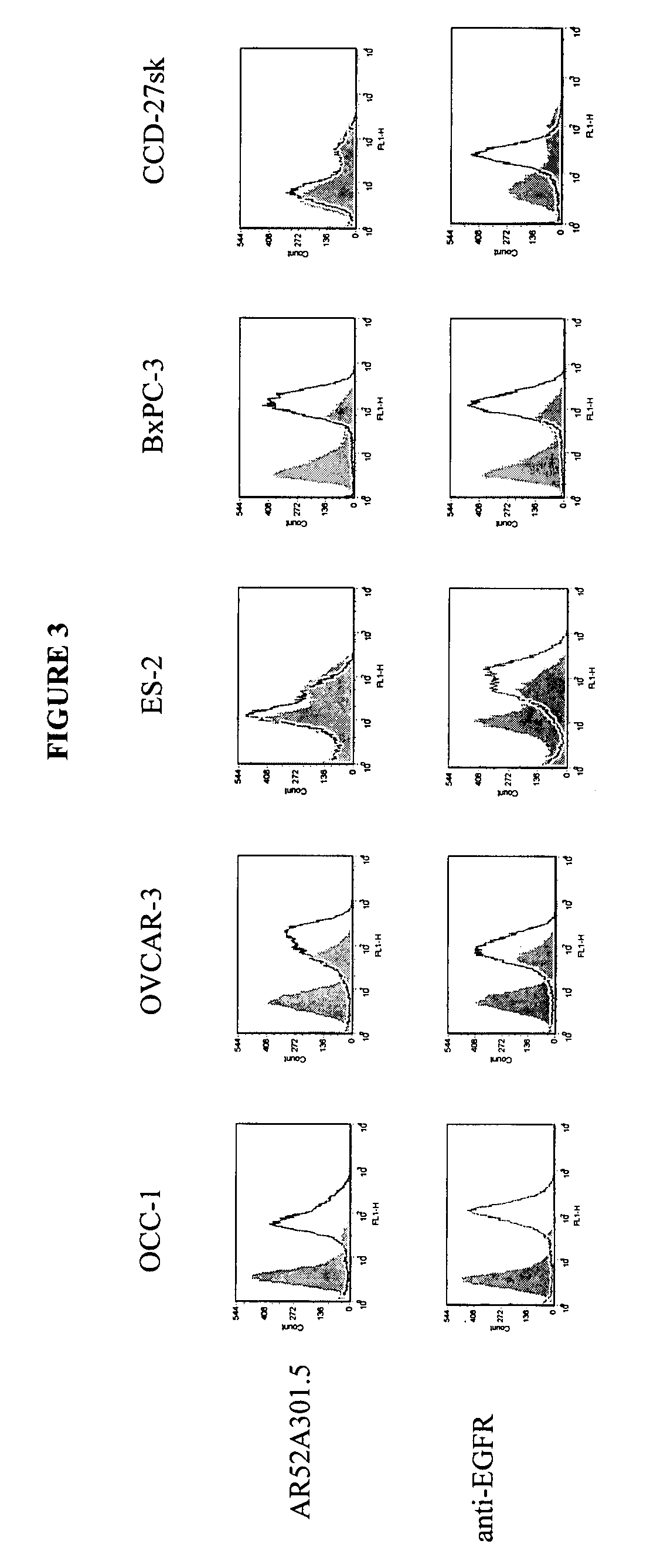
InactiveUS7420040B2Reduce the likelihood of problemsProlong survival timeImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationDiseaseHematopoietic cell
The present invention relates to a method for producing cancerous disease modifying antibodies using a novel paradigm of screening. By segregating the anti-cancer antibodies using cancer cell cytotoxicity as an end point, the process makes possible the production of anti-cancer antibodies for therapeutic and diagnostic purposes. The antibodies can be used in aid of staging and diagnosis of a cancer, and can be used to treat primary tumors and tumor metastases. The anti-cancer antibodies can be conjugated to toxins, enzymes, radioactive compounds, cytokines, interferons, target or reporter moieties and hematogenous cells.
Owner:F HOFFMANN LA ROCHE & CO AG
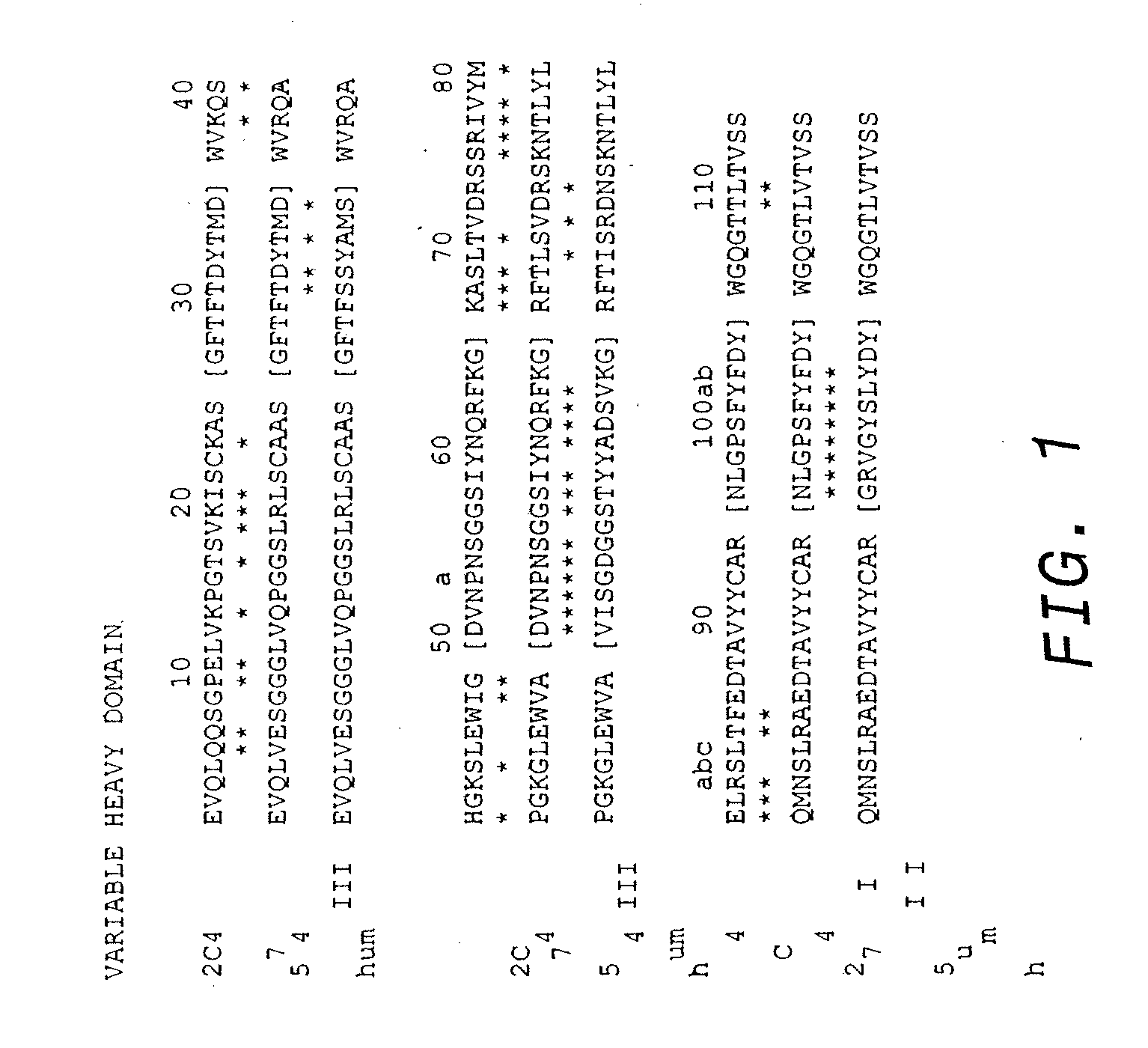
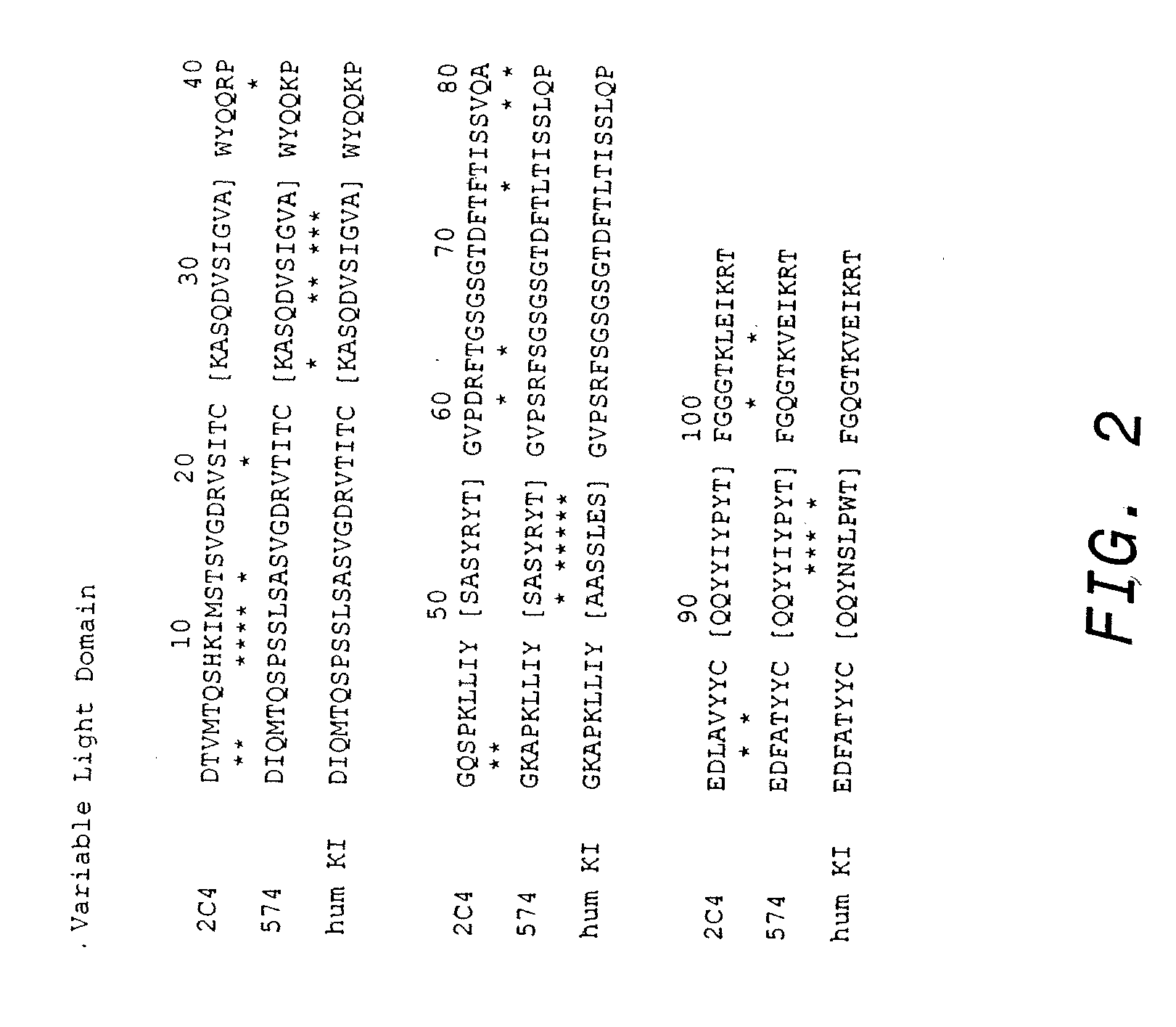
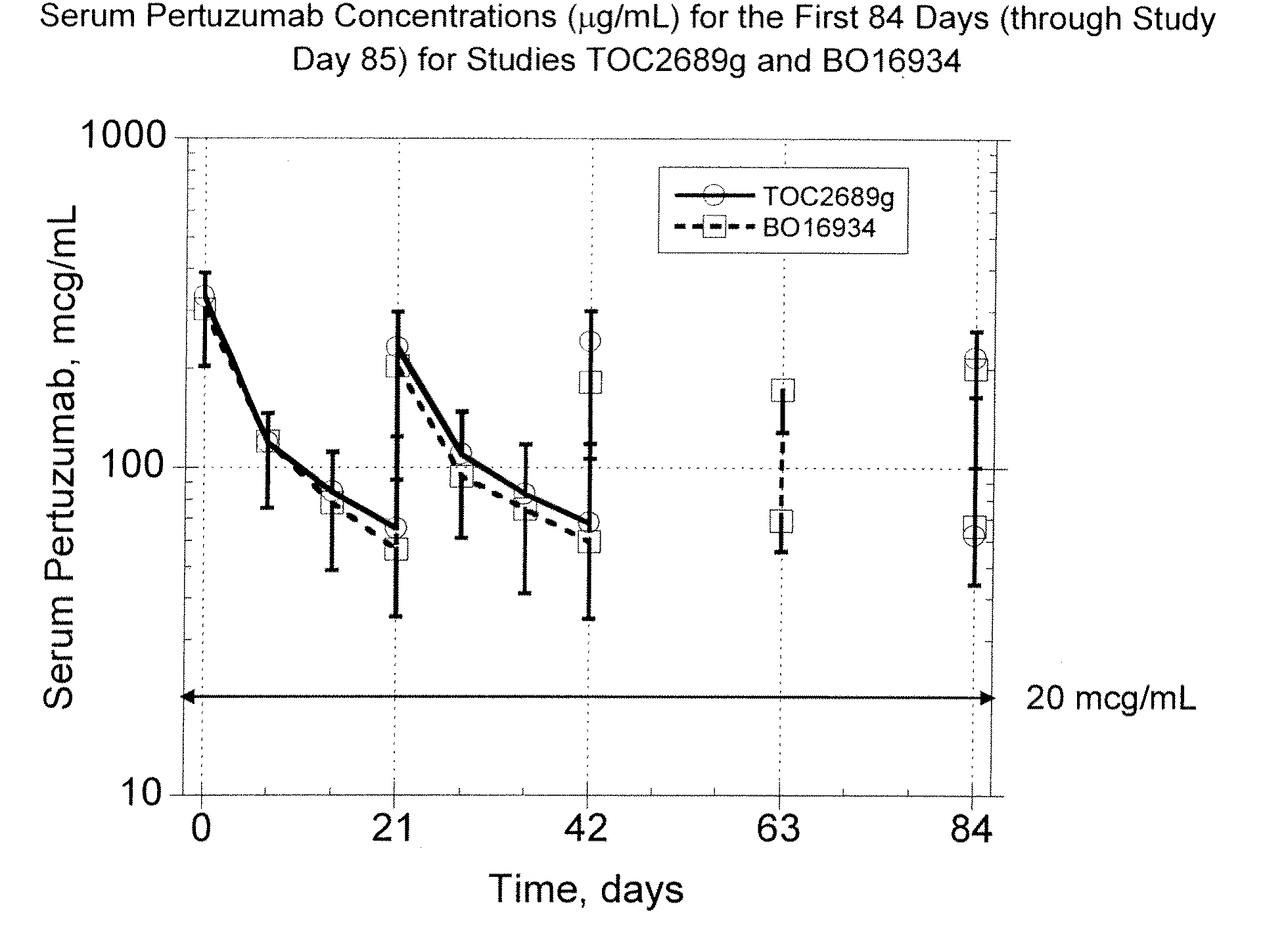
Treatment of metastatic breast cancer
InactiveUS20090317387A1Improve survivalOrganic active ingredientsAntibody ingredientsBiologic therapiesDocetaxel-PNP
The present invention concerns treatment of previously untreated HER2-positive metastatic breast cancer with a combination of a growth inhibitory HER2 antibody, a HER2 dimerization inhibitor antibody and a taxane. In particular, the invention concerns the treatment of HER2-positive metastatic breast cancer in patients who did not receive prior chemotherapy or biologic therapy with a HER2 antibody binding essentially to epitope 2C4, a HER2 antibody binding essentially to epitope 4D5, and a taxane. The invention further comprises extending survival of such patients by the combination therapy of the present invention. In a preferred embodiment, the treatment involves administration of trastuzumab, pertuzumab and docetaxel.
Owner:GENENTECH INC
Engineered listeria and methods of use thereof
InactiveUS20070207171A1Enhance survivalImprove survivalBacterial antigen ingredientsCell receptors/surface-antigens/surface-determinantsNucleotideAntibiotics
The invention provides a bacterium containing a polynucleotide comprising a nucleic acid encoding a heterologous antigen, as well as fusion protein partners. Also provided are vectors for mediating site-specific recombination and vectors comprising removable antibiotic resistance genes.
Owner:ANZA THERAPEUTICS INC
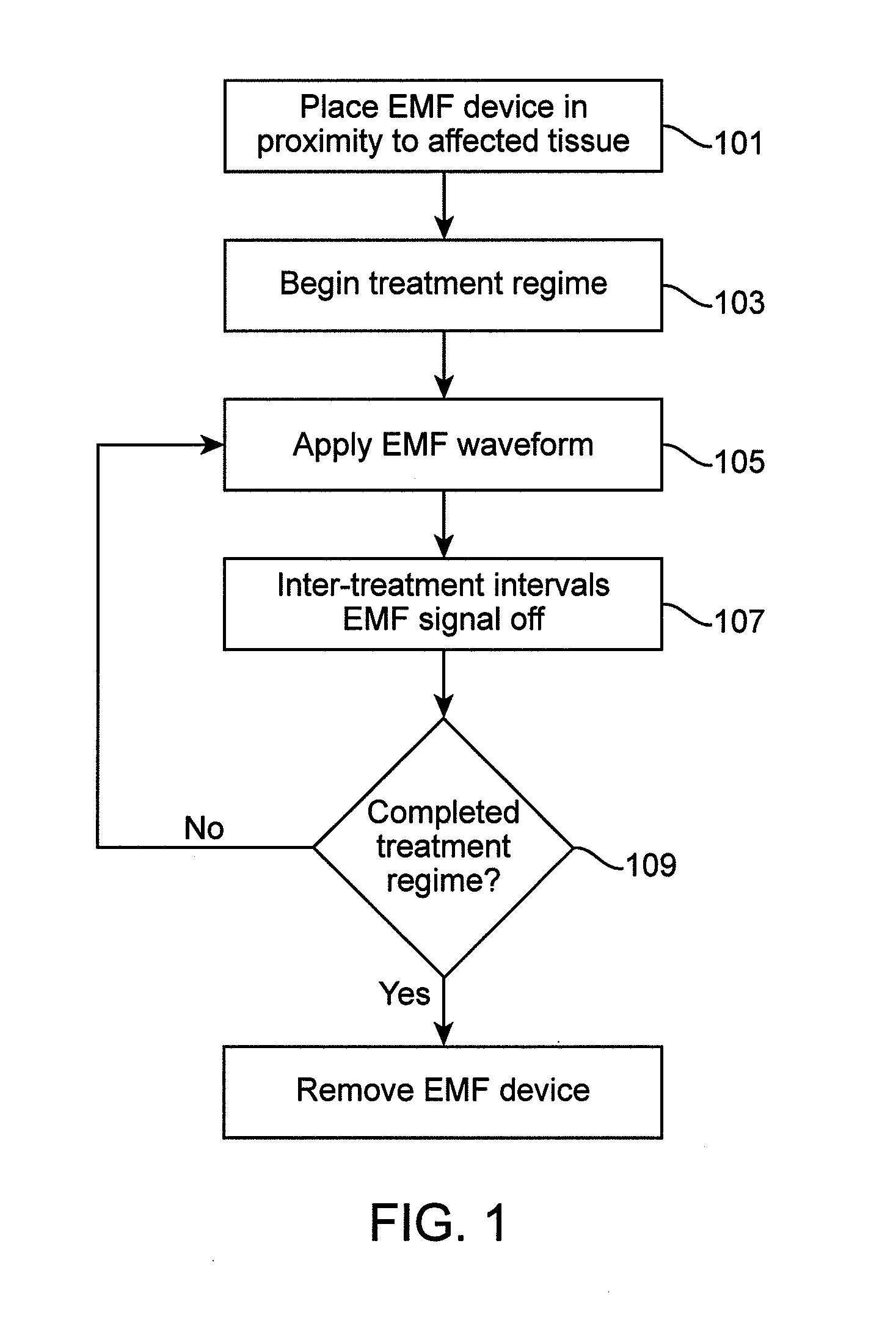
Method and apparatus for electromagnetic treatment of cognition and neurological injury
InactiveUS20140303425A1Accelerate and decelerate productionPrevent and reverse neurodegenerationElectroencephalographyElectrotherapyDiseaseTreatment delivery
Methods and devices for providing therapeutic electromagnetic field treatment to a subject having a cognitive or neurological condition or injury. Treatment devices can include headwear incorporating electromagnetic treatment delivery devices providing electromagnetic treatment to a user's head area. Such devices include protective headwear such as helmets with electromagnetic delivery devices. Additionally, embodiments of the invention provide for wearable and adjustable electromagnetic treatment devices that can be used to provide electromagnetic treatment to multiple areas of the user's head. Embodiments of the invention provide for sequential electromagnetic treatment with a single or a plurality of treatment applicators which target a single or multiple cerebral regions as determined by imaging, non-imaging and physiological monitoring before, during and after electromagnetic treatment.
Owner:RIO GRANDE NEUROSCI
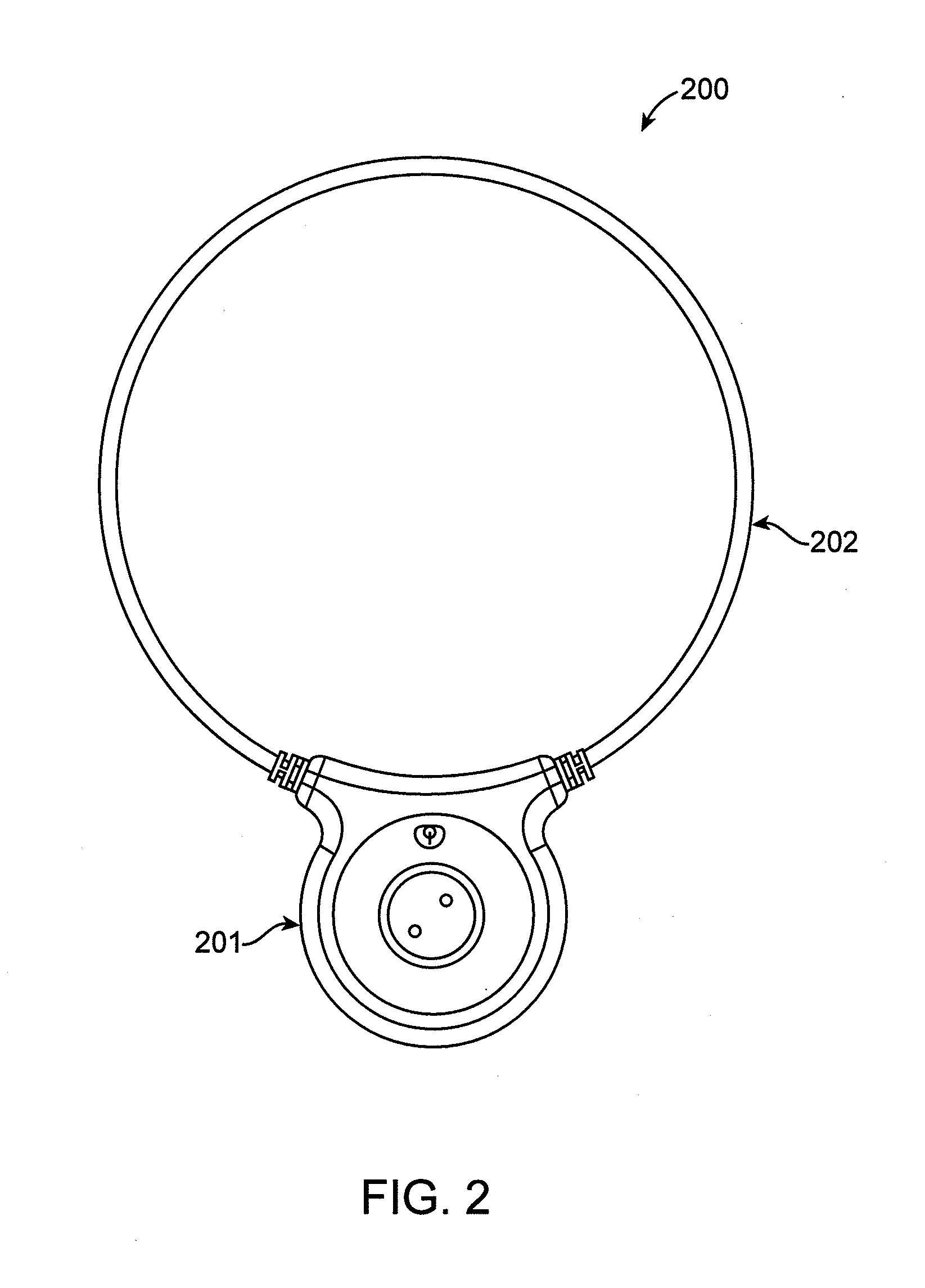
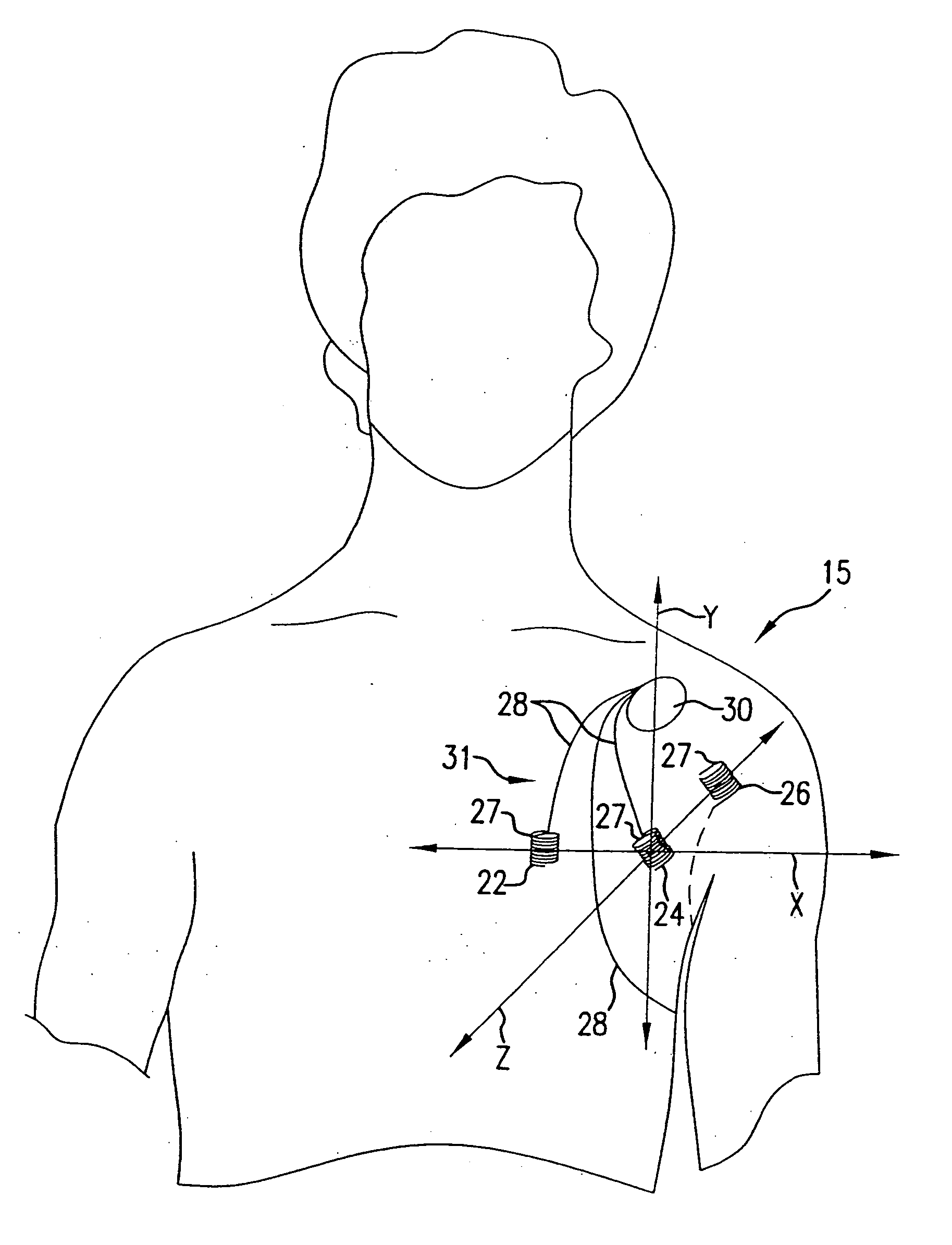
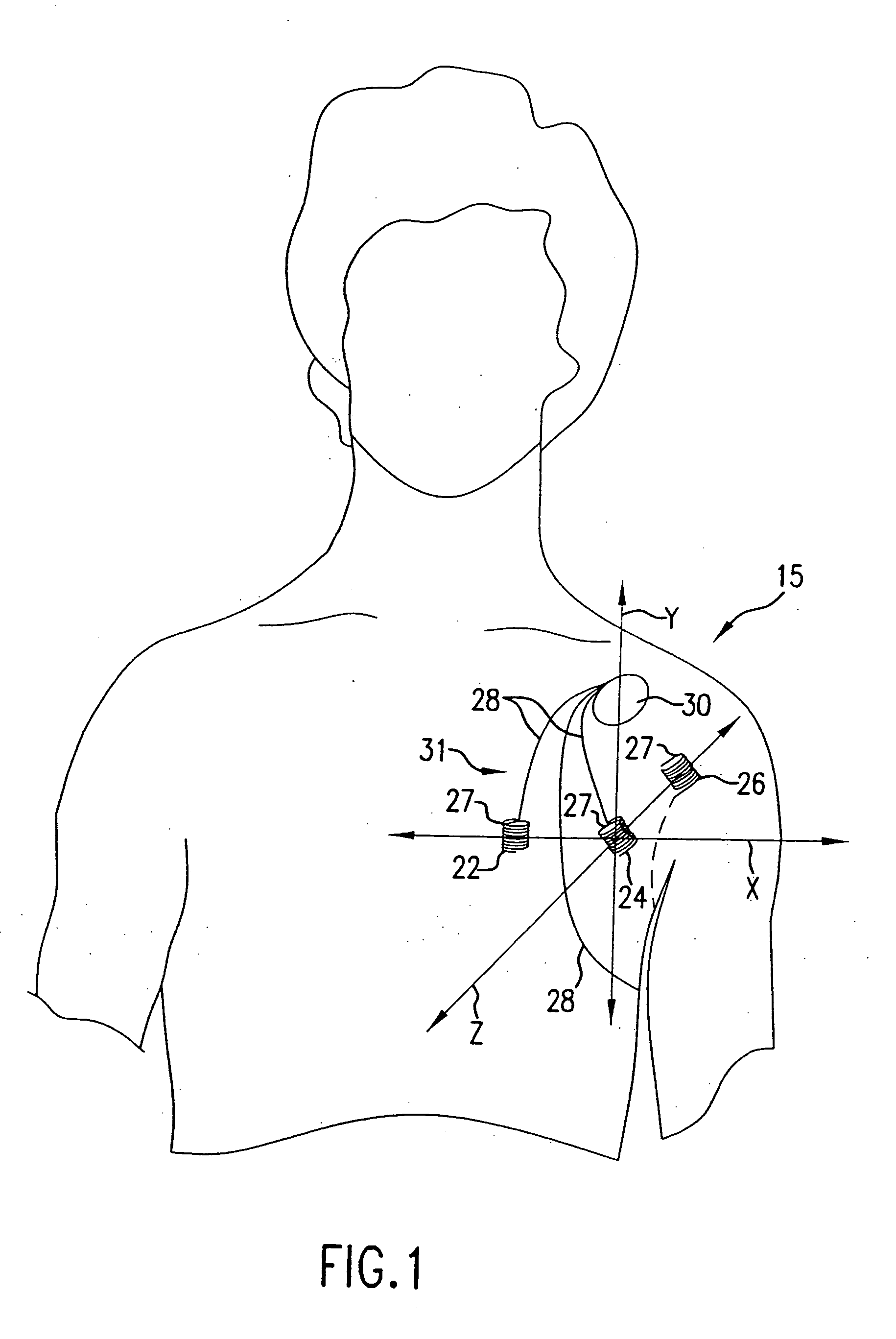
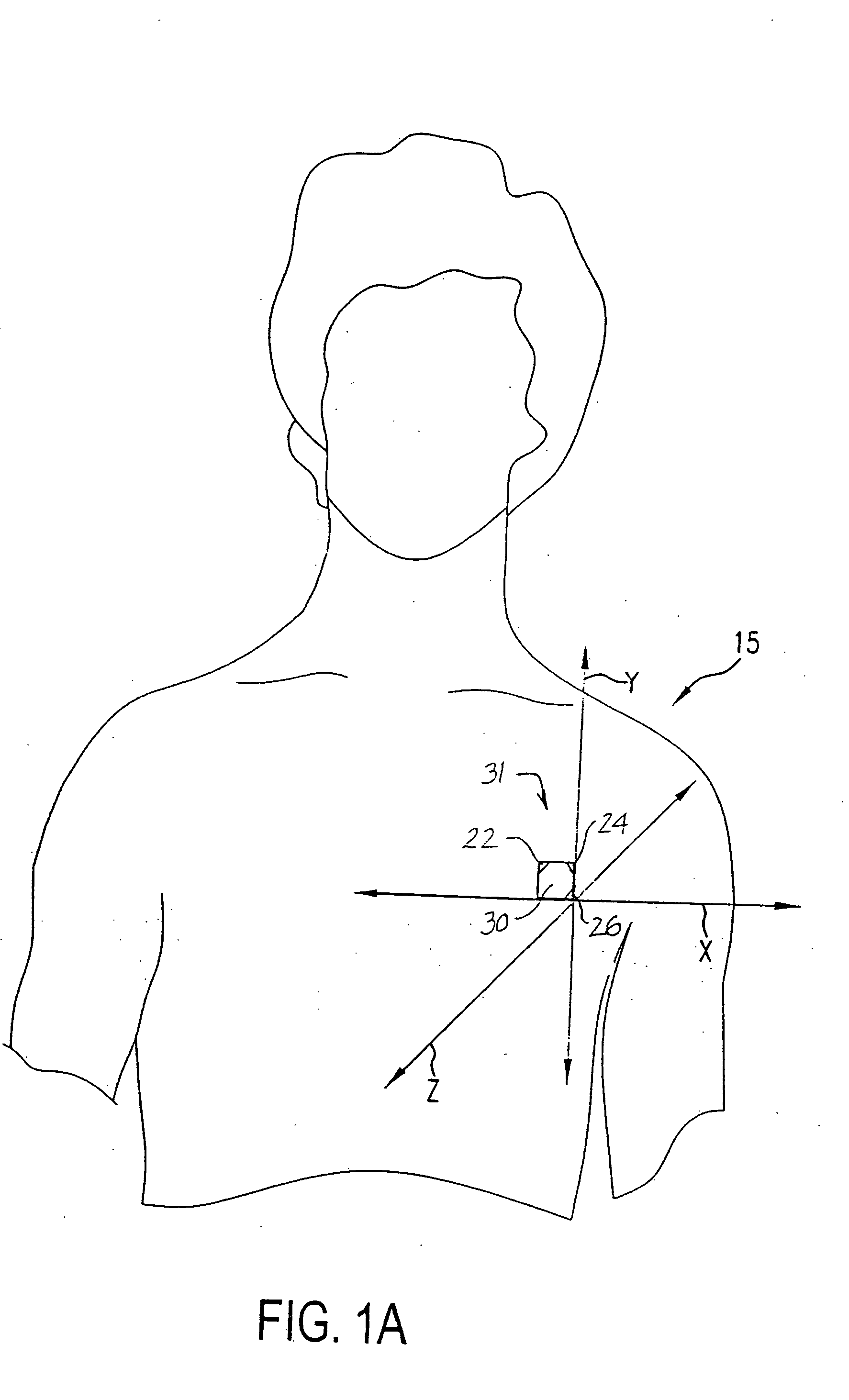
Cardiac arrest monitor and alarm system
InactiveUS20050065445A1Reduce materialImprove survivalElectrocardiographySensorsNormal heartTelecommunications link
A cardiac arrest monitor and alarm system including an implantable medical device having at least three electrodes, preferably but not necessarily subcutaneous, positioned with respect to a heart organ and forming an orthogonal lead configuration to continuously monitor an electrocardiographic signal of the heart organ. A microdevice, preferably but not necessarily operatively connected to the medical device, detects a deviation from a normal heart electrical activity and emits a signal to an external receiver. Upon verification of the signal from the microdevice, the external receiver activates a programmed annunciator circuit to alert bystanders to deploy an AED and / or activate a communication link automatically transmitting an alarm and the electrocardiographic signal to a remote transceiver.
Owner:AJ MEDICAL DEVICES
Cytotoxicity mediation of cells evidencing surface expression of TROP-2
InactiveUS7420041B2Reduce the likelihood of problemsProlong survival timePeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphatic SpreadCancer cell
The present invention relates to a method for producing cancerous disease modifying antibodies using a novel paradigm of screening. By segregating the anti-cancer antibodies using cancer cell cytotoxicity as an end point, the process makes possible the production of anti-cancer antibodies for therapeutic and diagnostic purposes. The antibodies can be used in aid of staging and diagnosis of a cancer, and can be used to treat primary tumors and tumor metastases. The anti-cancer antibodies can be conjugated to toxins, enzymes, radioactive compounds, cytokines, interferons, target or reporter moieties and hematogenous cells.
Owner:F HOFFMANN LA ROCHE & CO AG
Influenza virus vaccines and uses thereof
ActiveUS20100297174A1Reduce severityImprove survivalSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininInfluenza virus vaccine
Owner:MT SINAI SCHOOL OF MEDICINE
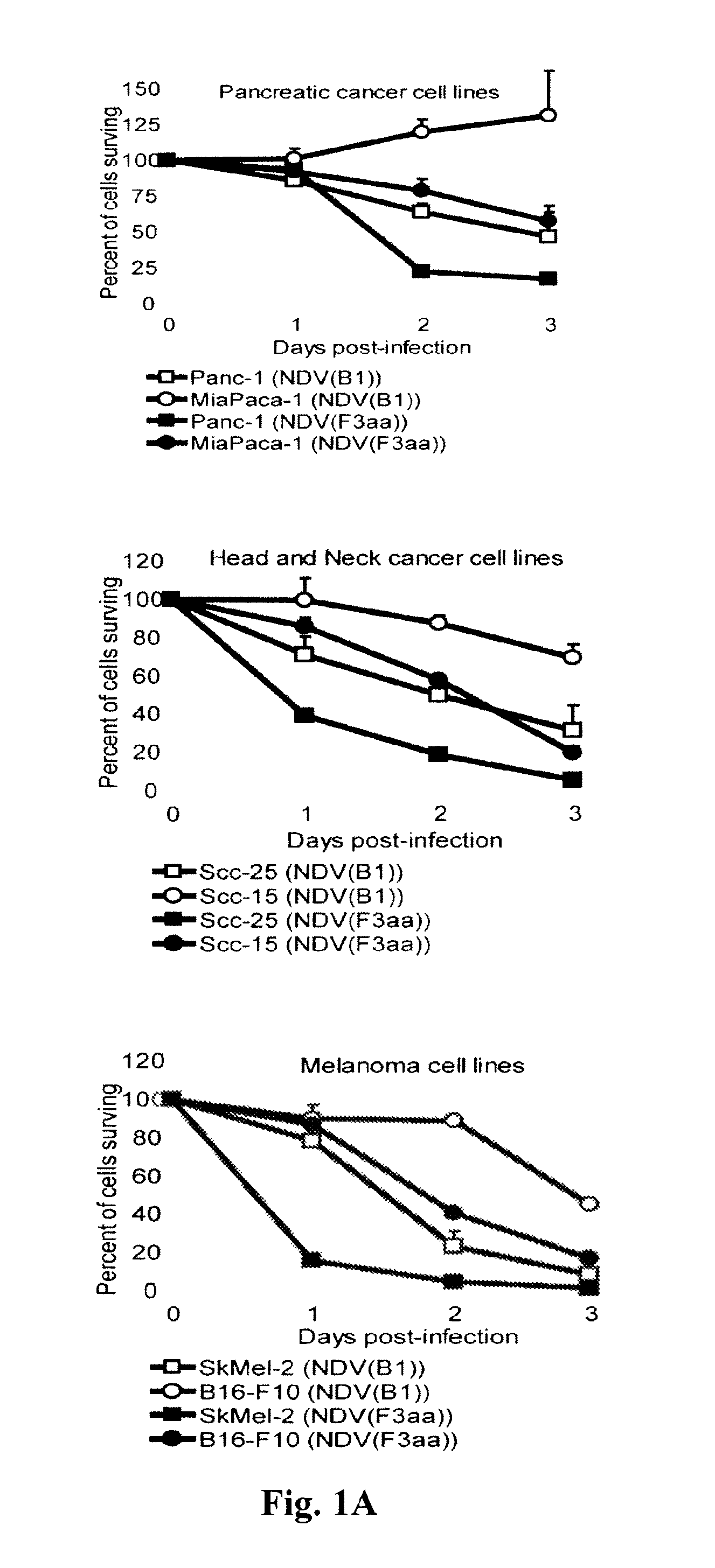
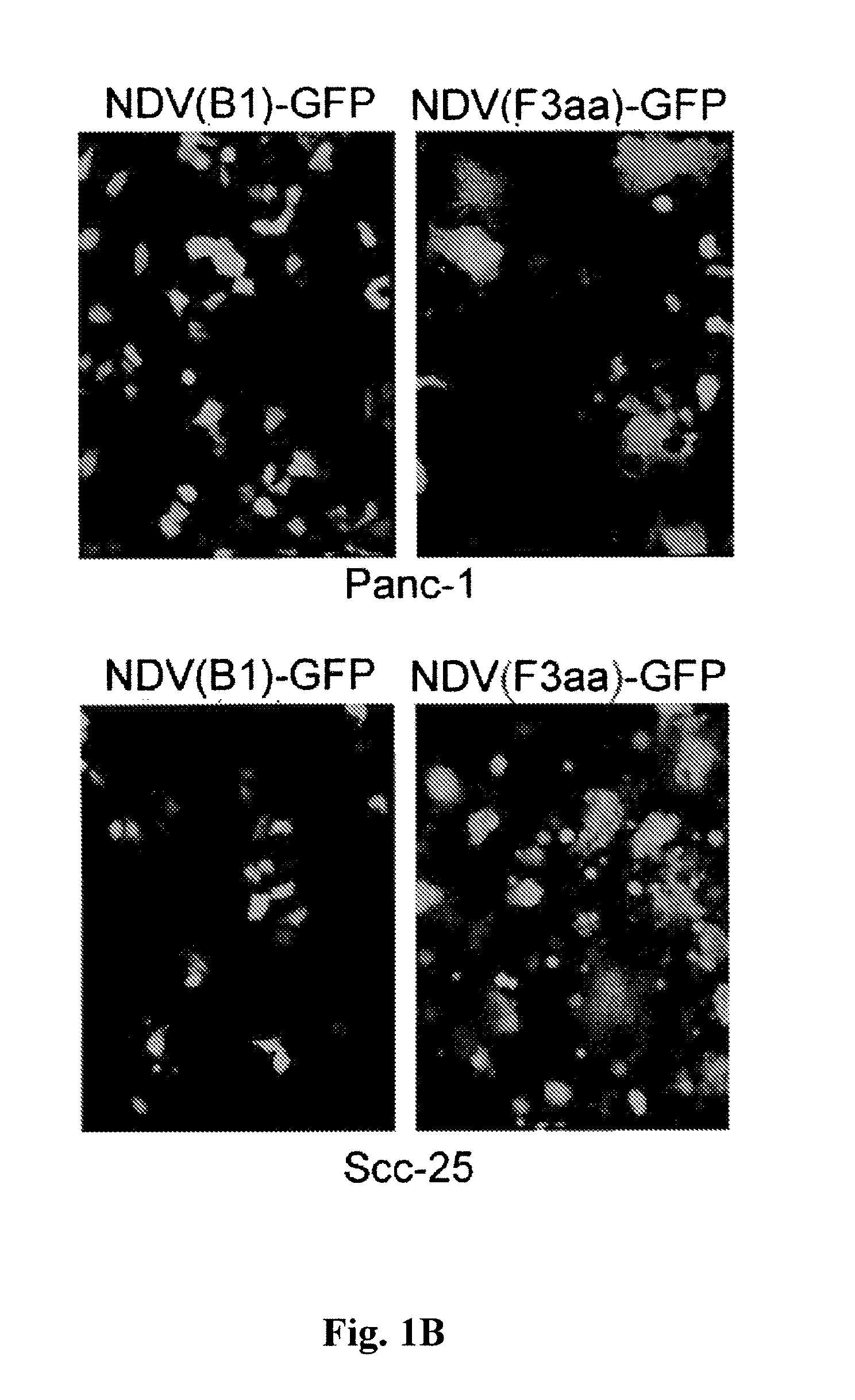
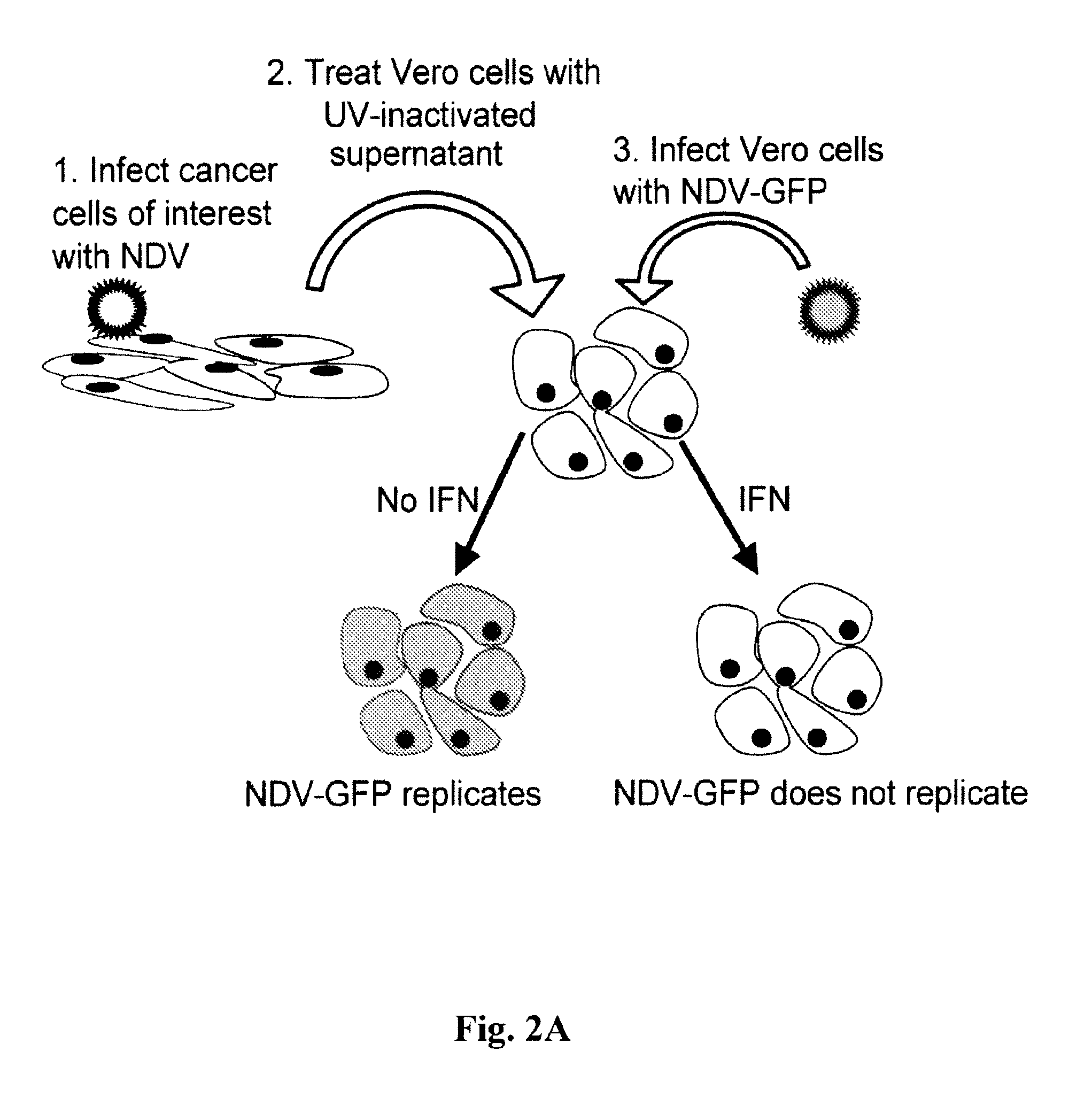
Chimeric Newcastle disease viruses and uses thereof
ActiveUS8591881B2Prevents progression and worseningReduce severitySsRNA viruses negative-senseBiocideNewcastle disease virus NDVAntagonist
Described herein are chimeric Newcastle disease viruses engineered to express a heterologous interferon antagonist and compositions comprising such viruses. The chimeric Newcastle disease viruses and compositions are useful in the treatment of cancer.
Owner:MT SINAI SCHOOL OF MEDICINE +1
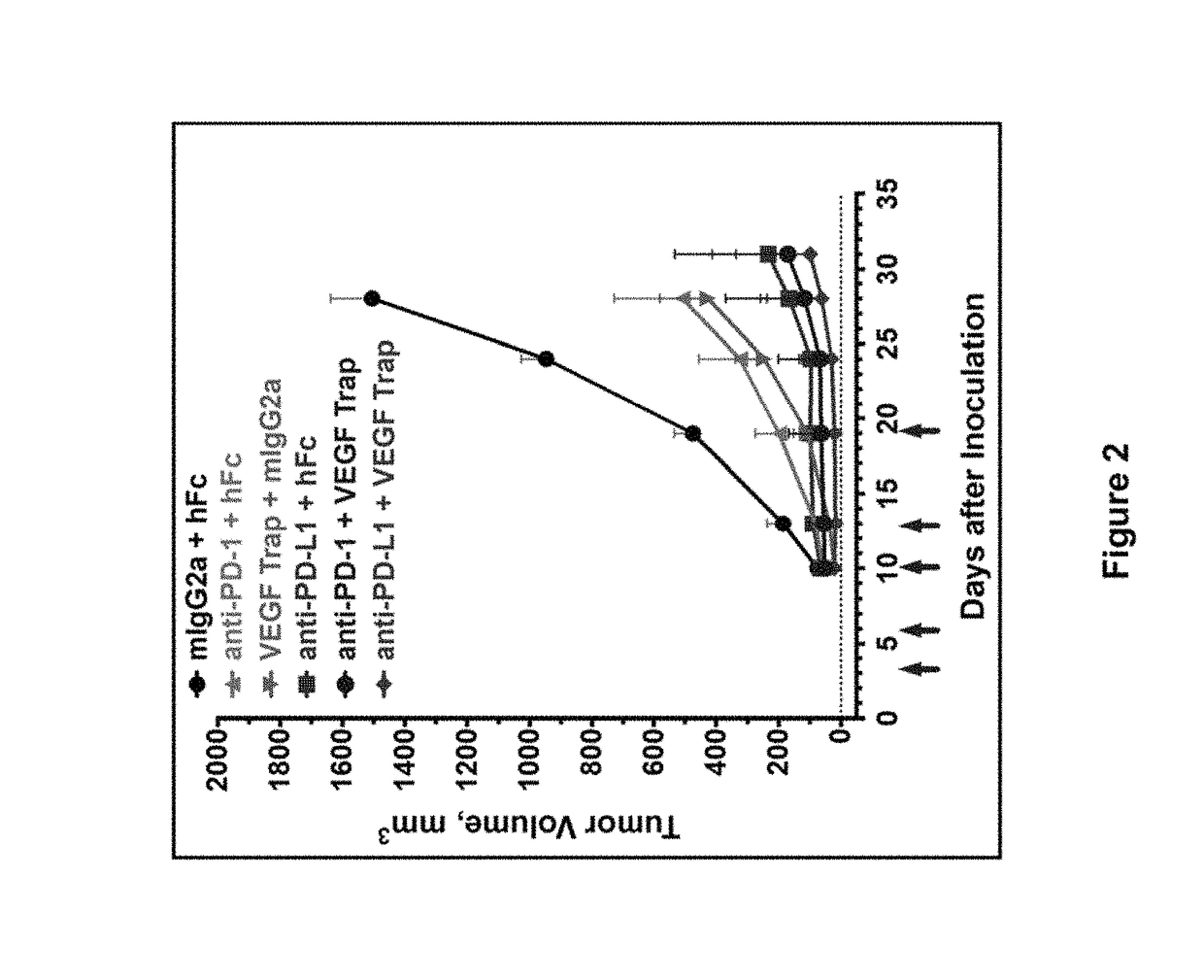
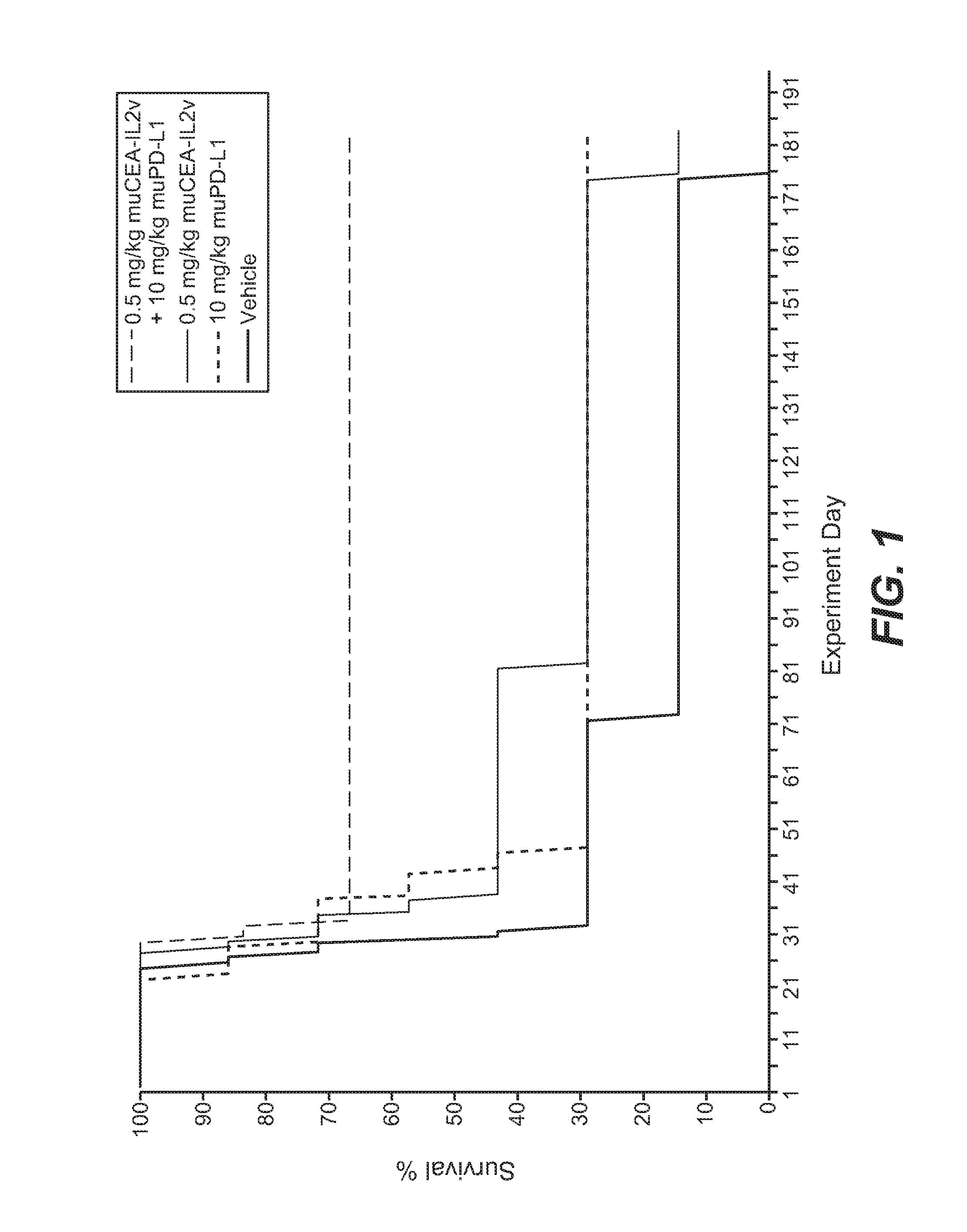
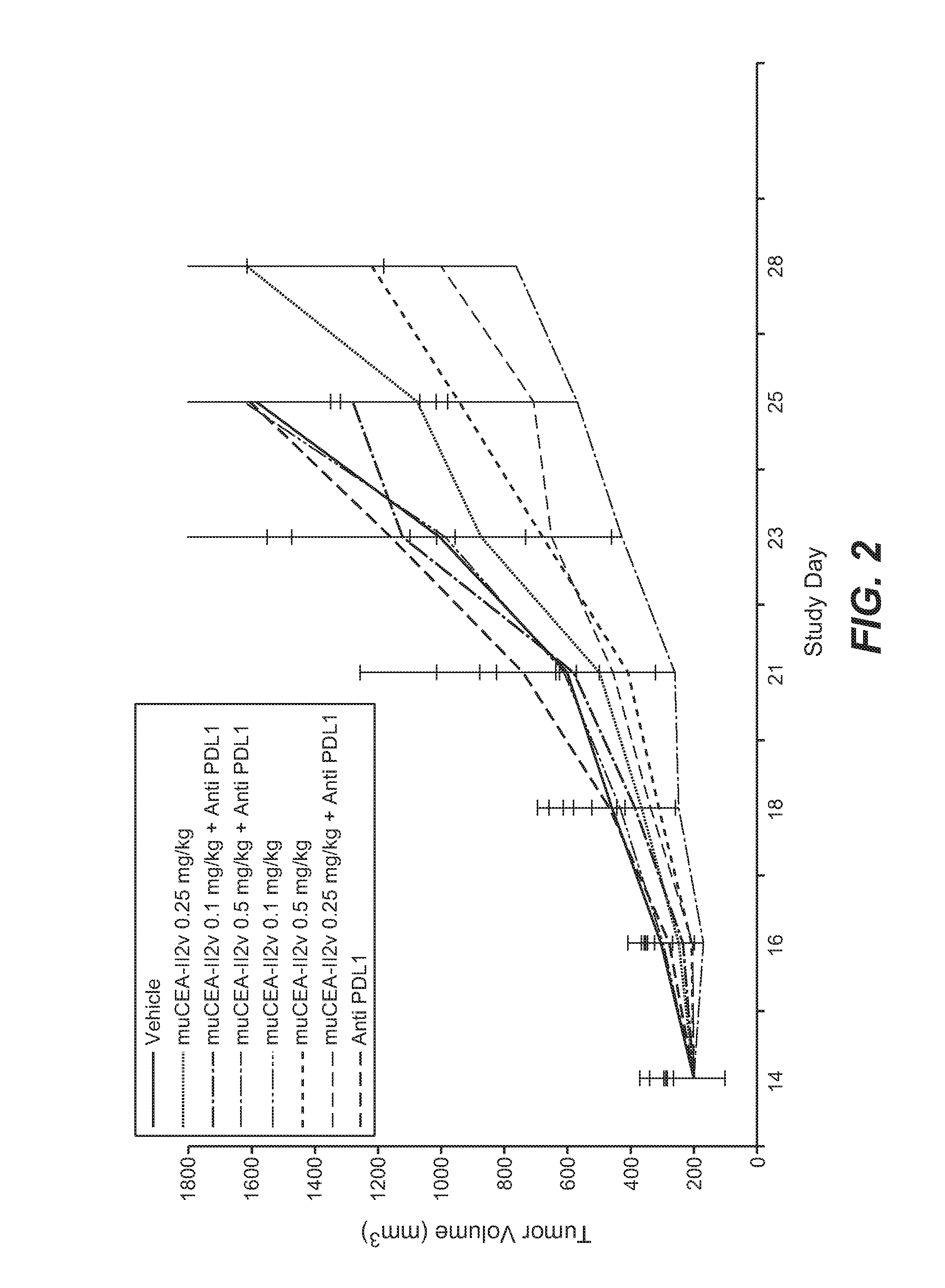
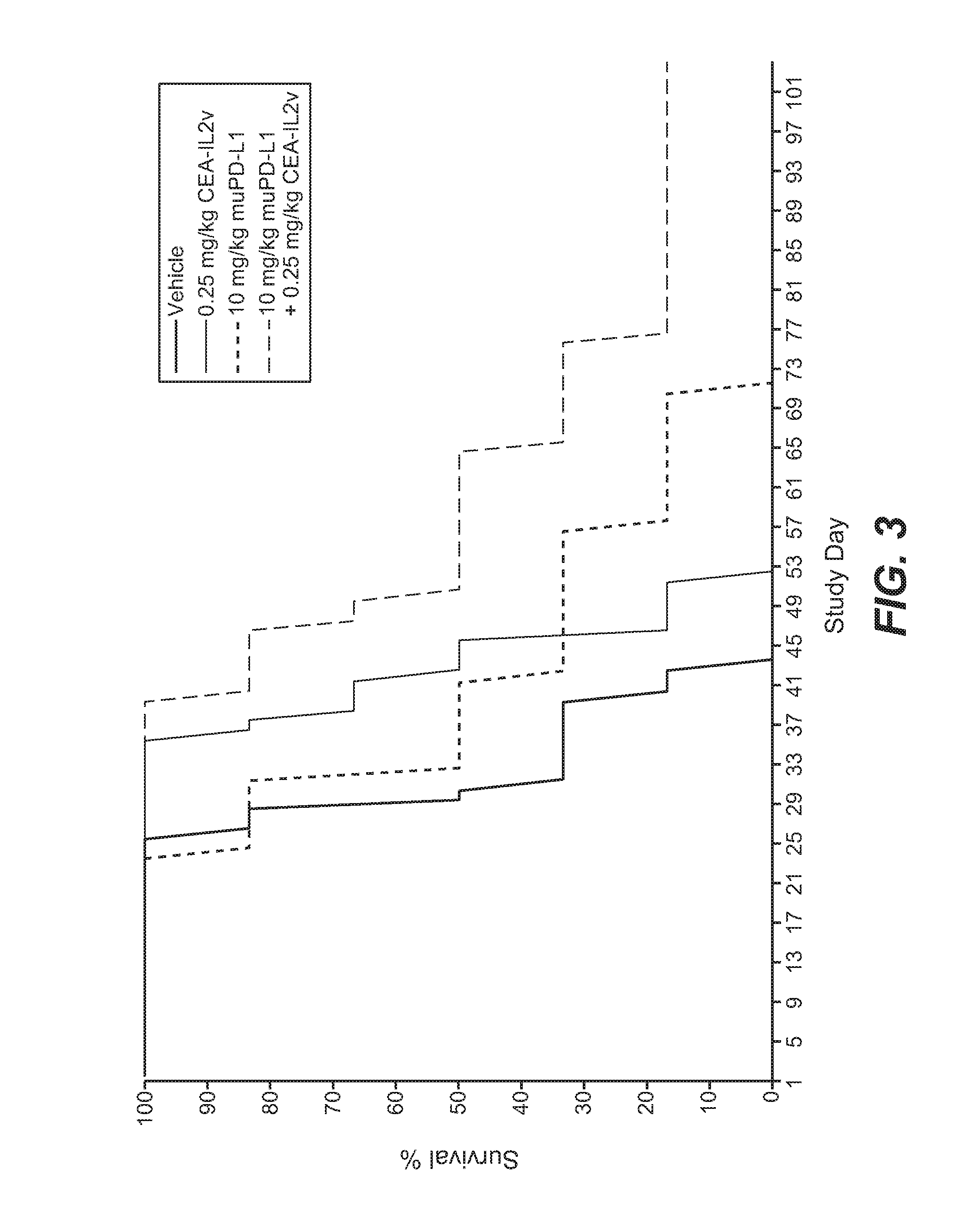
Combination therapy of tumor-targeted il-2 variant immunocytokines and antibodies against human pd-l1
InactiveUS20160175397A1Enhancing median and overall survivalImprove survivalNervous disorderPeptide/protein ingredientsTumor targetPD-L1
The present invention relates to the combination therapy of specific tumor-targeted IL-2 variant immunocytokines with specific antibodies which bind human PD-L1.
Owner:F HOFFMANN LA ROCHE INC
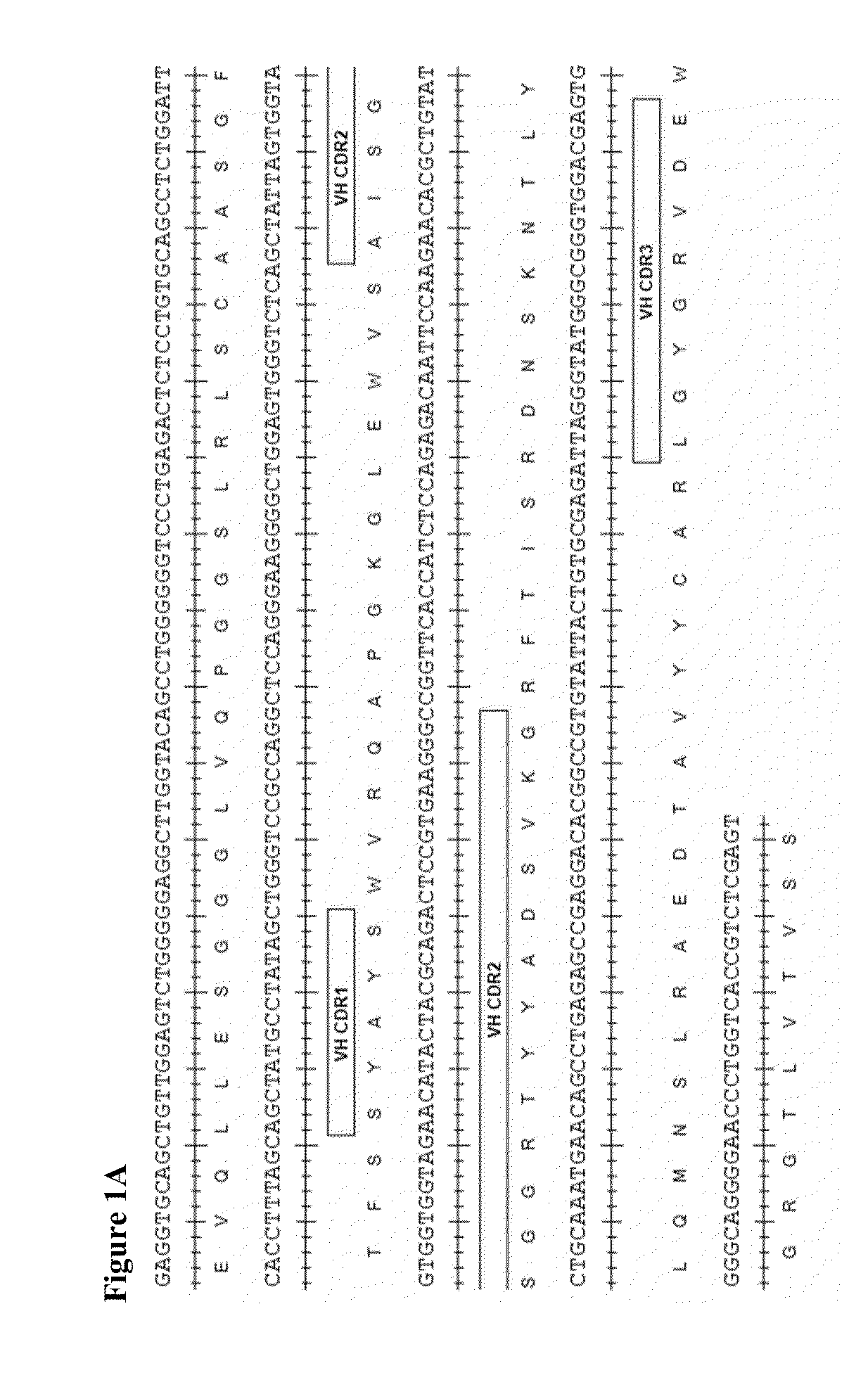
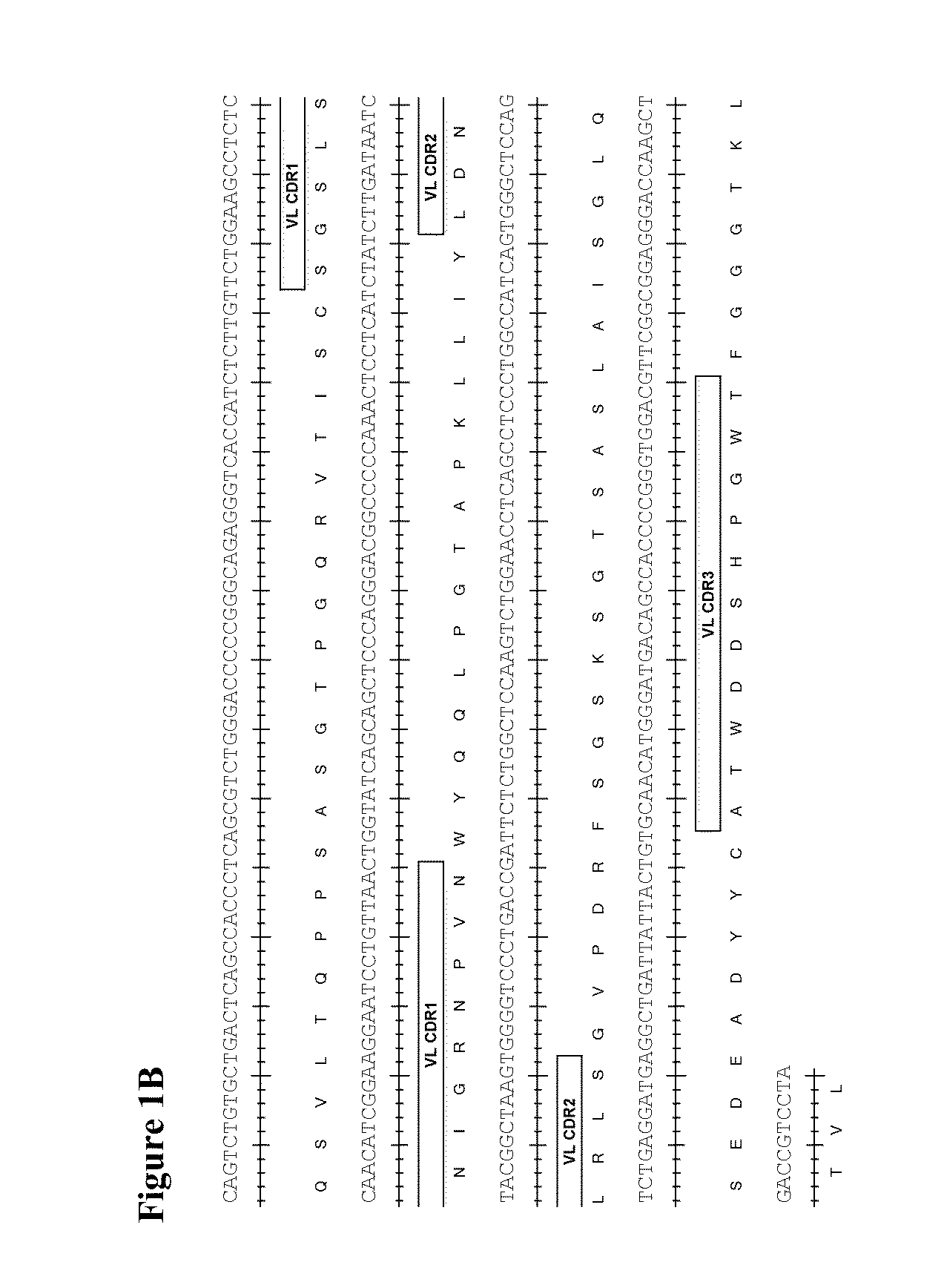
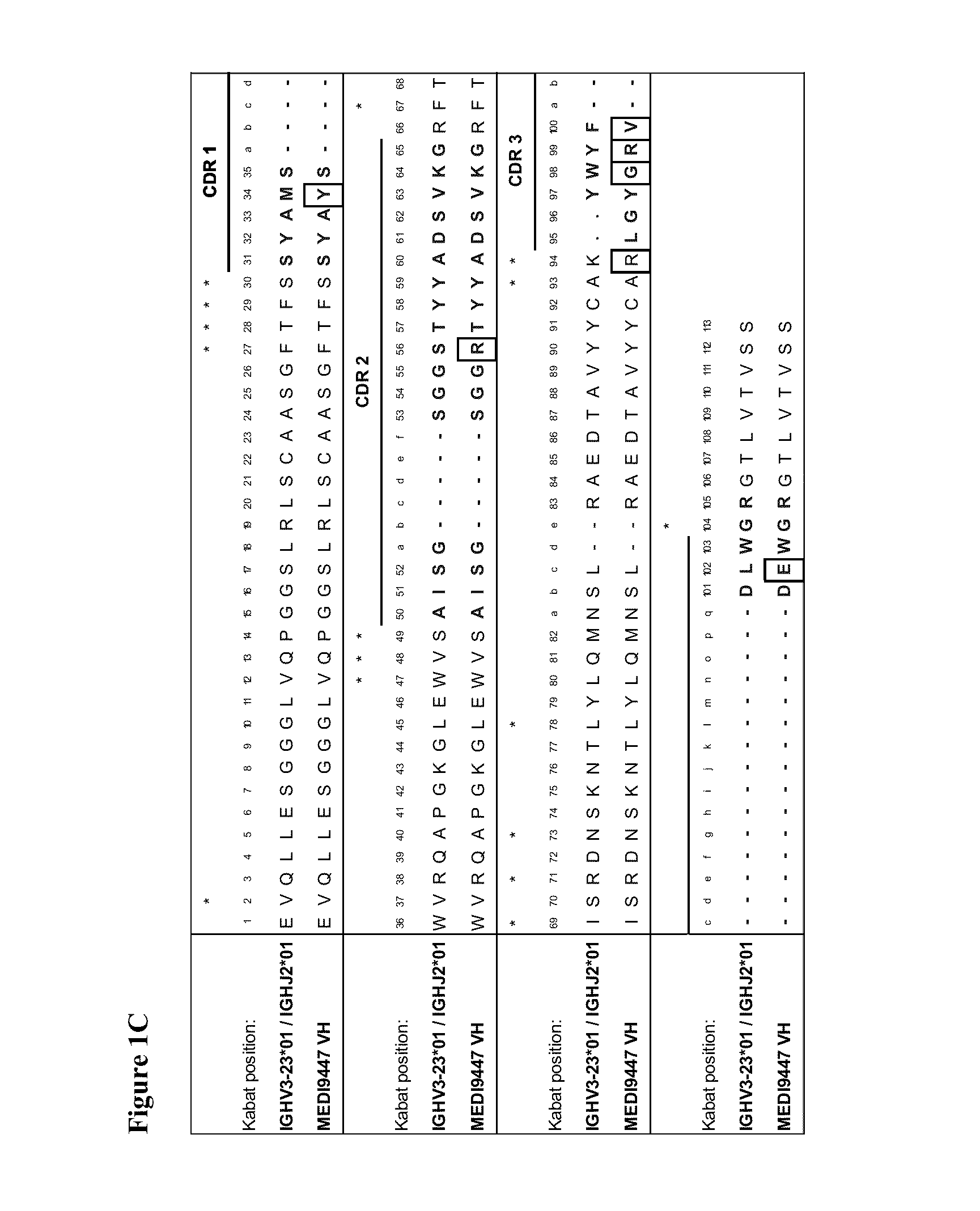
Therapeutic combinations comprising Anti-cd73 antibodies and uses thereof
InactiveUS20160129108A1Increase survivalIncrease immune responseOrganic active ingredientsAntibody ingredientsWilms' tumorAntibody
The present invention provides therapeutic combinations featuring anti-CD73 antibodies (e.g., MEDI9447) and A2A receptor inhibitors and methods of using such combinations for reducing tumor-mediated immunosuppression.
Owner:MEDIMMUNE LTD
Recombinant adeno-associated virus delivery of alpha-sarcoglycan polynucleotides
ActiveUS9434928B2High transduction efficiencyIncrease volumePeptide/protein ingredientsOther blood circulation devicesFhit genePolynucleotide
Owner:NATIONWIDE CHILDRENS HOSPITAL
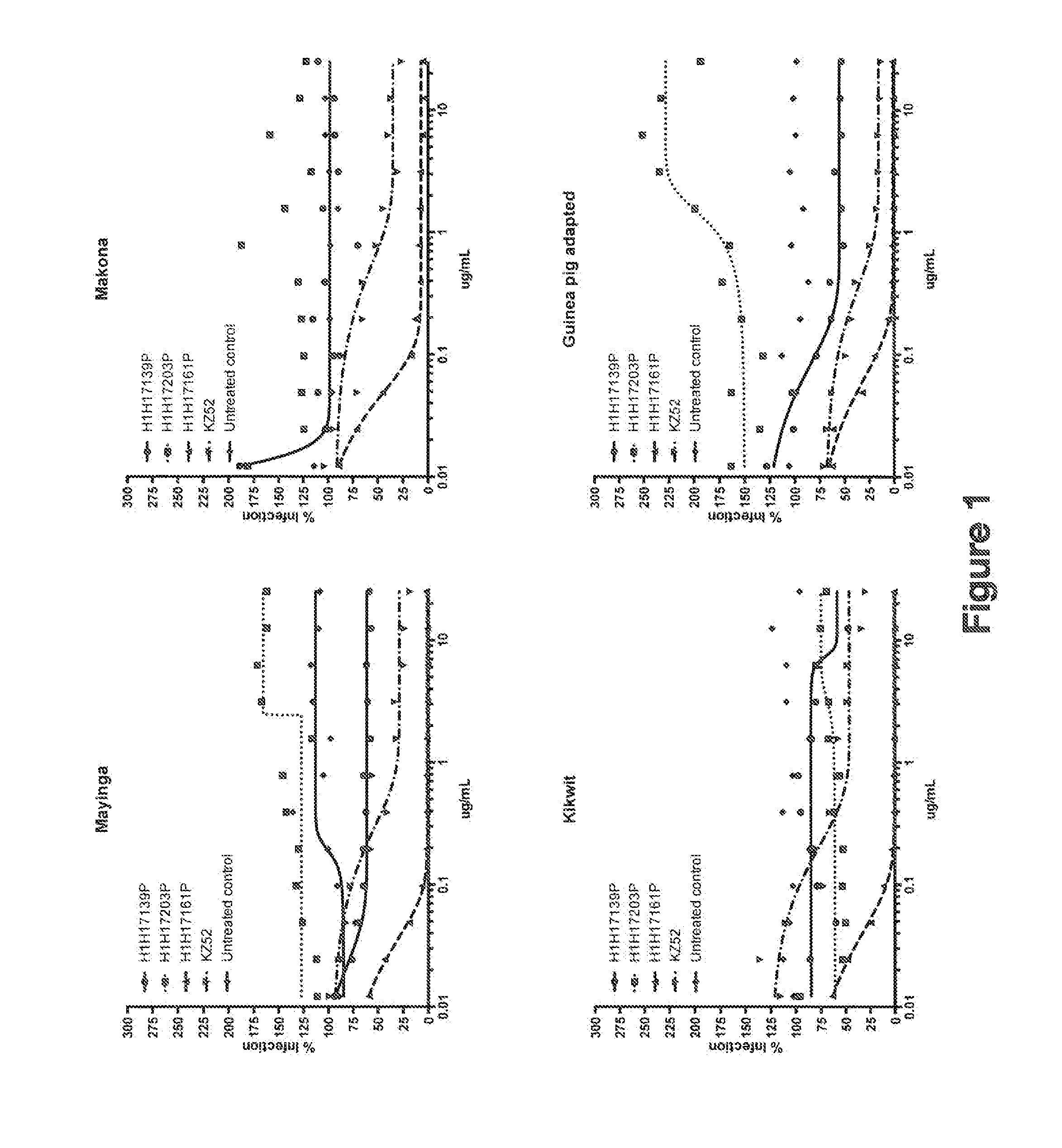
Human Antibodies to Ebola Virus Glycoprotein
ActiveUS20160215040A1Inhibiting and neutralizing activityAvoid enteringImmunoglobulins against virusesAntiviralsViral glycoproteinAntigen Binding Fragment
The present invention provides monoclonal antibodies, or antigen-binding fragments thereof, that bind to Ebola virus glycoproteins, pharmaceutical compositions comprising the antibodies and methods of use. The antibodies of the invention are useful for inhibiting or neutralizing Ebola virus activity, thus providing a means of treating or preventing Ebola virus infection in humans. In some embodiments, the invention provides for use of one or more antibodies that bind to the Ebola virus for preventing viral attachment and / or entry into host cells. The antibodies of the invention may be used prophylactically or therapeutically and may be used alone or in combination with one or more other anti-viral agents or vaccines.
Owner:REGENERON PHARM INC
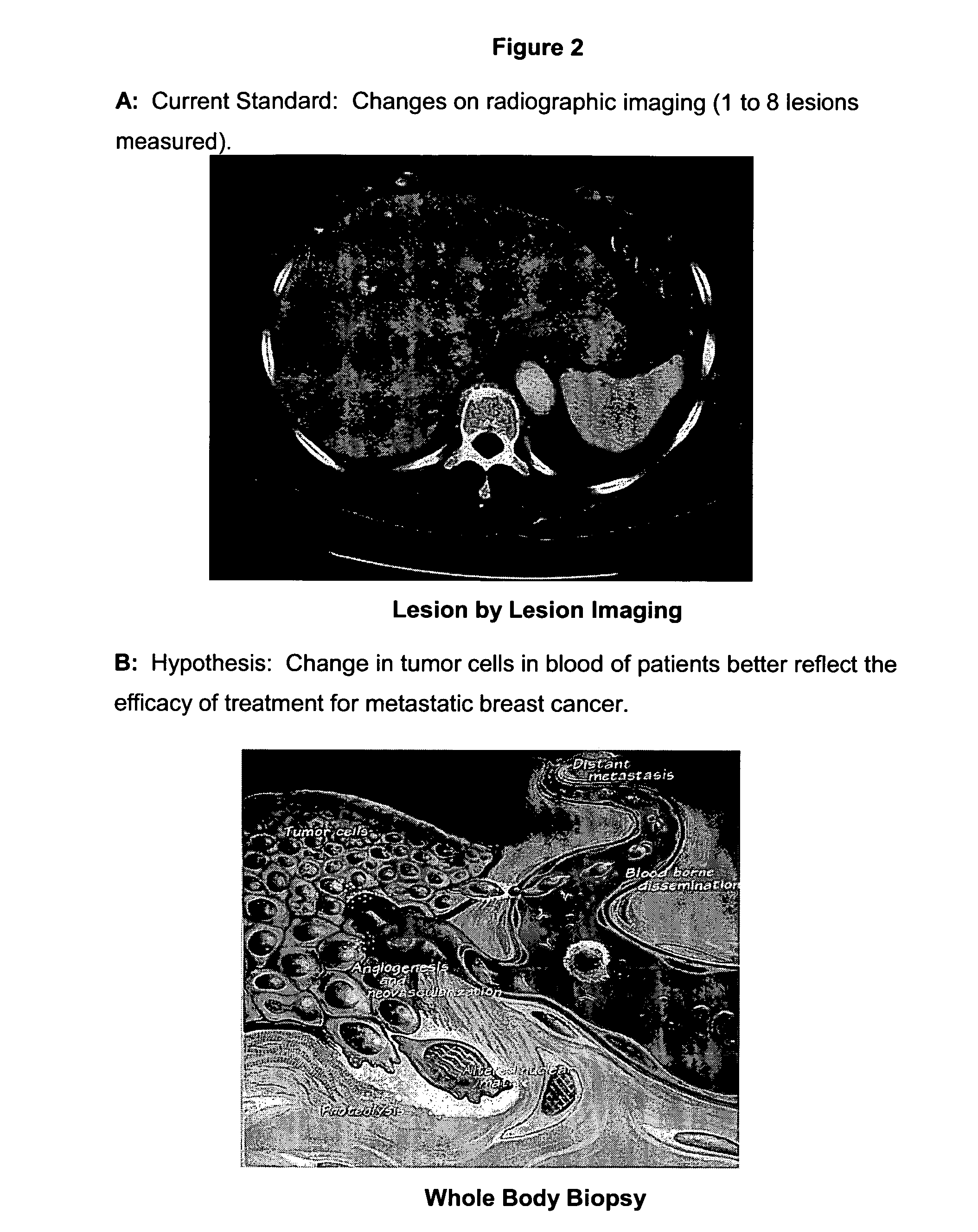
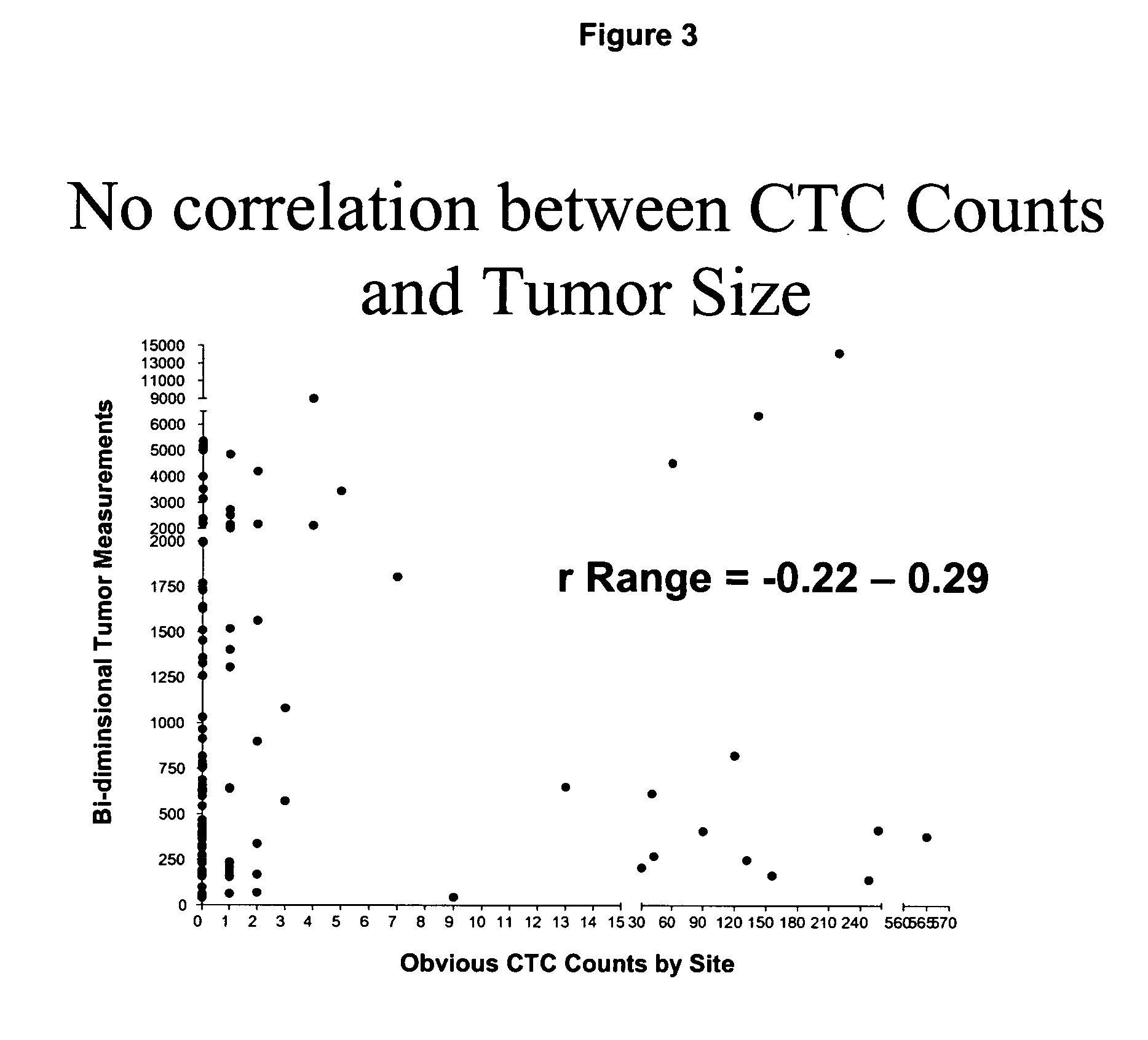
Circulating tumor cells (CTC's): early assessment of time to progression, survival and response to therapy in metastatic cancer patients
InactiveUS20070037173A1Less side effectsImprove the quality of lifeMicrobiological testing/measurementBiological testingClinical trialOncology
A cancer test having prognostic utility in predicting time to disease progression, overall survival, and response to therapy in patients with MBC based upon the presence and number of CTC's. The Cell Spotter® System is used to enumerate CTC's in blood. The system immunomagnetically concentrates epithelial cells, fluorescently labels the cells and identifies and quantifies CTC's. The absolute number of CTC's detected in the peripheral blood tumor load is, in part, a factor in prediction of survival, time to progression, and response to therapy. The mean time to survival of patients depended upon a threshold number of 5 CTC's per 7.5 ml of blood. Detection of CTC's in metastatic cancer represents a novel prognostic factor in patients with metastatic cancers, suggests a biological role for the presence of tumor cells in the blood, and indicates that the detection of CTC's could be considered an appropriate surrogate marker for prospective therapeutic clinical trials.
Owner:JANSSEN DIAGNOSTICS LLC
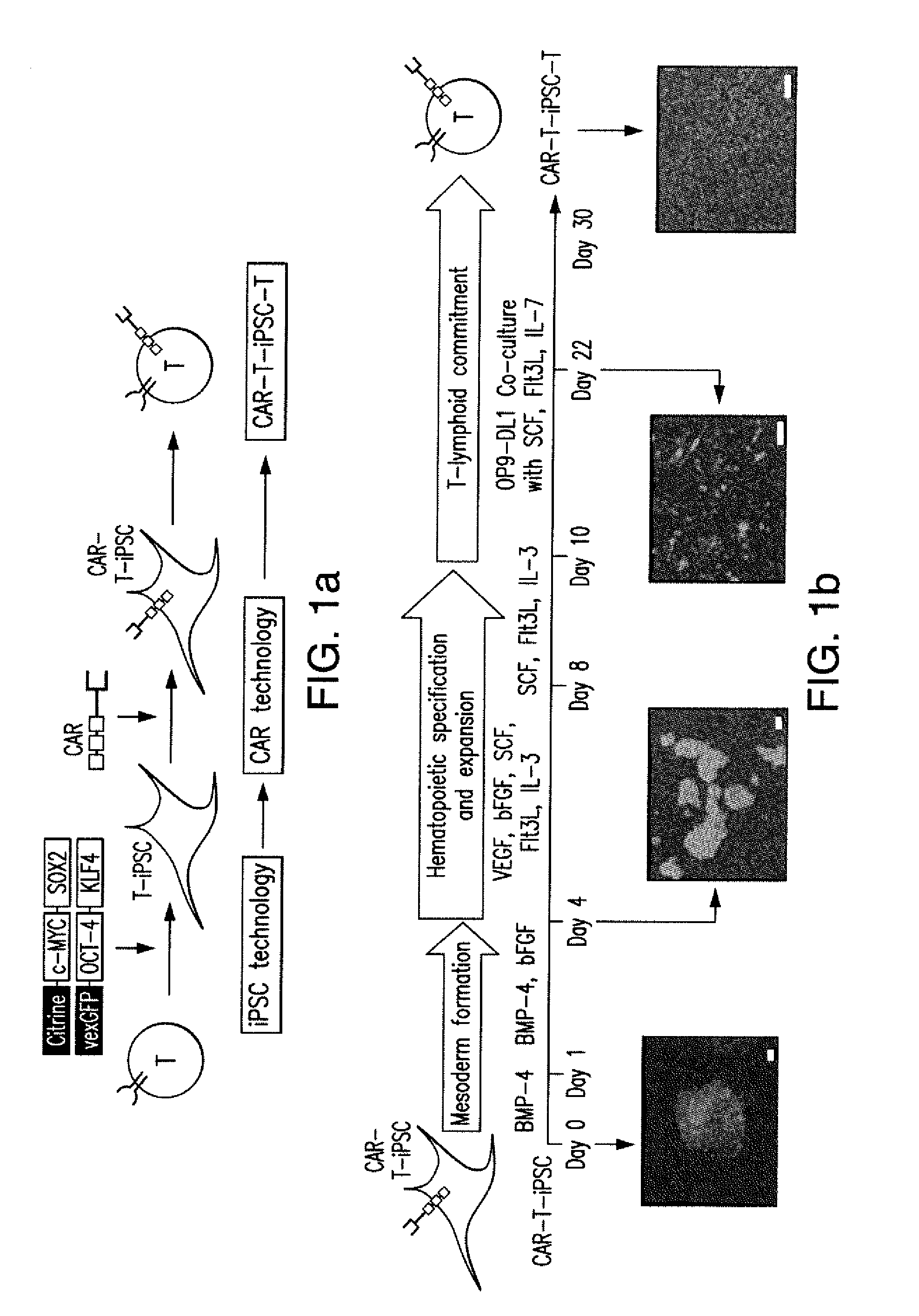
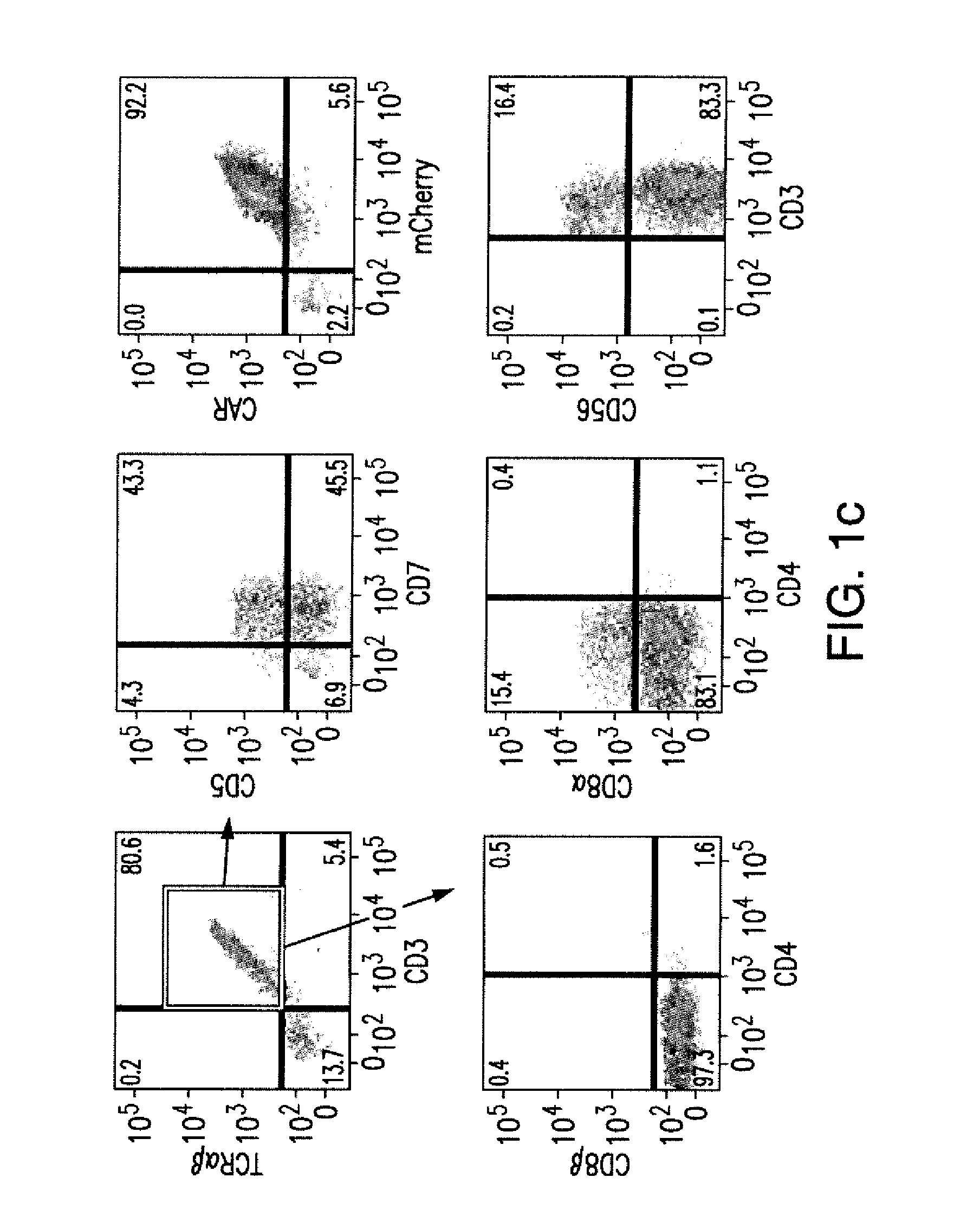
Effective generation of tumor-targeted t cells derived from pluripotent stem cells
ActiveUS20160009813A1Improve survivalGood antitumor activityAntibody mimetics/scaffoldsMammal material medical ingredientsAntigenPluripotential stem cell
The present invention relates to the field of adoptive immunotherapy. The invention provides methods for generating phenotypically defined, functional, and / or expandable T cells from pluripotent stem cells engineered through safe genetic modifications. The engineered cells may provide one or more of: 1) targeting a specific predetermined antigen expressed on the cell surface of a target cell in an HLA independent manner, 2) enhanced survival and functional potential 3) “off-the-shelf” T cells for administration to multiple recipients, eventually across immunogenic barriers, and / or 4) cytotoxic potential and anti-tumor activity.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com