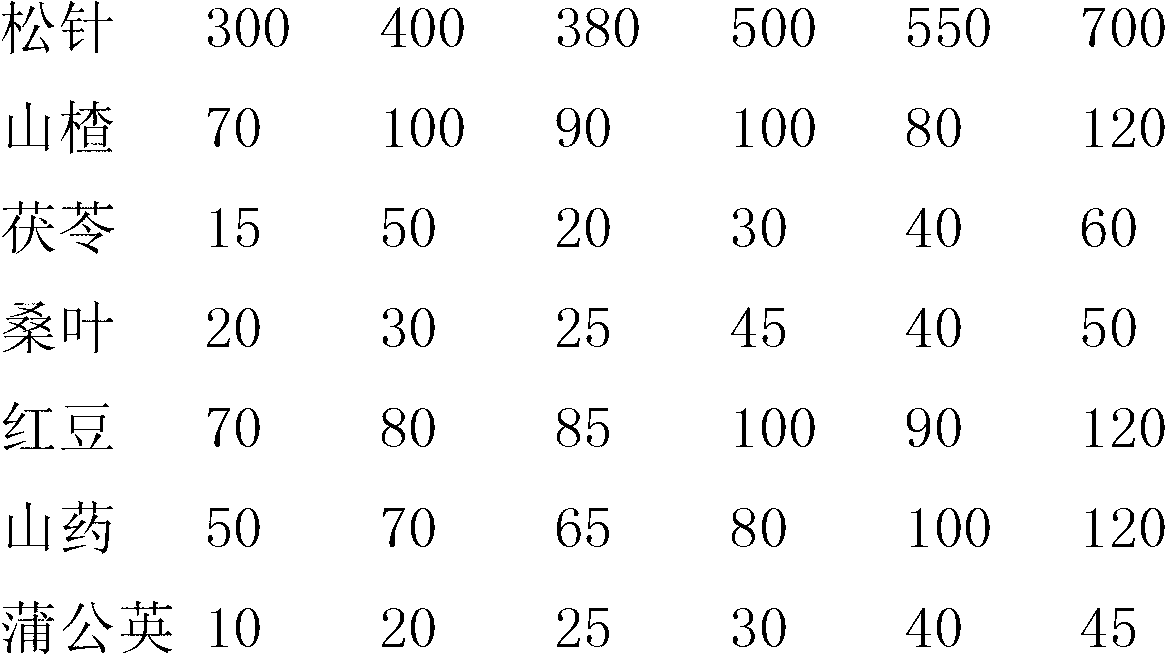Patents
Literature
196results about How to "Reduce thrombosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
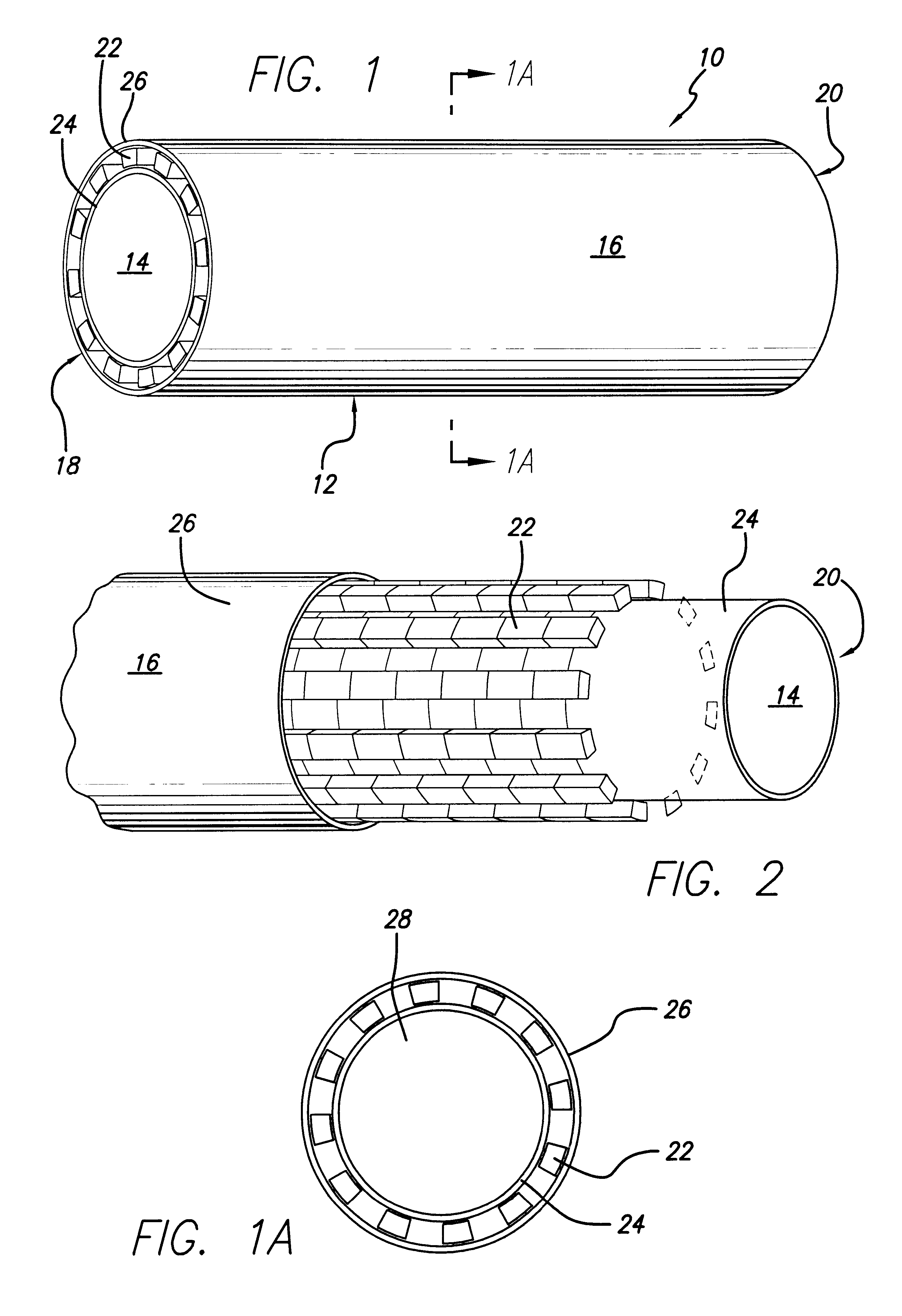
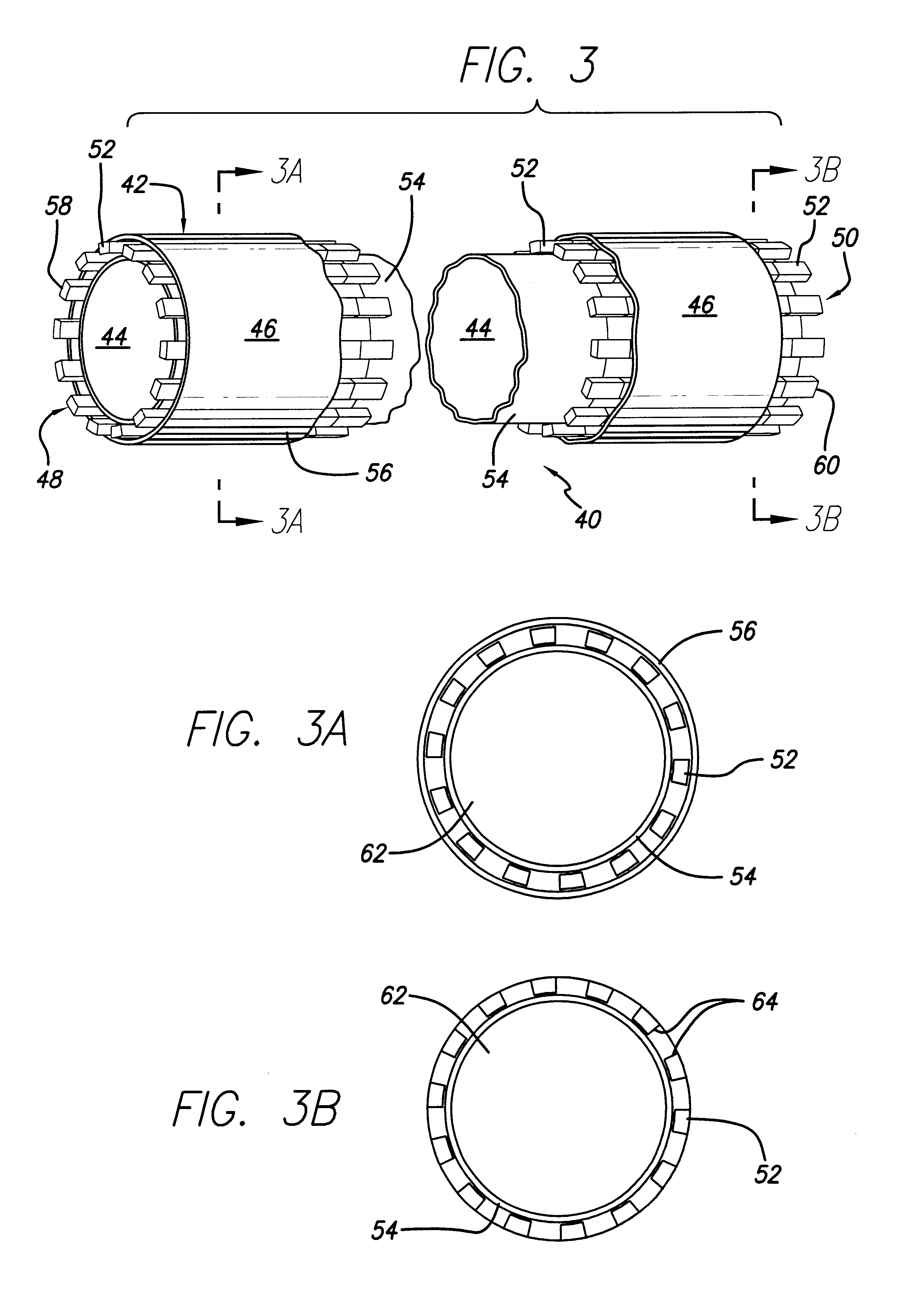
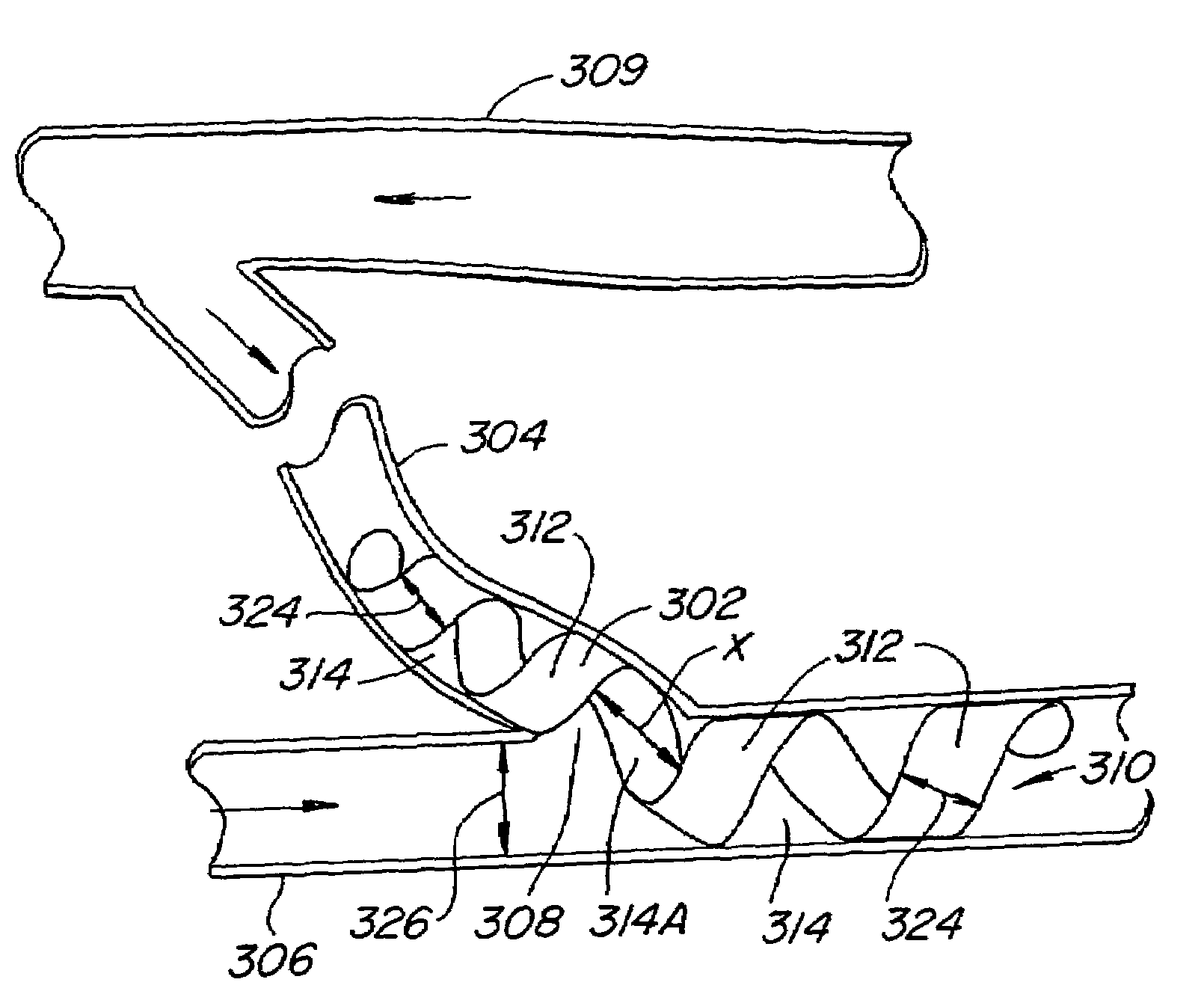
Encapsulated stent
InactiveUS6383214B1Reduce thrombosisEasy to separateStentsBlood vesselsStent graftingPolytetrafluoroethylene
An encapsulated stent having a stent or structural support layer sandwiched between two biocompatible flexible layers. One preferred embodiment has a stent cover which includes a tubular shaped stent that is concentrically retained between two tubular shaped grafts comprised of expanded polytetrafluoroethylene. Another preferred embodiment has a stent graft which includes at least one stent sandwiched between the ends of two tubular shaped grafts wherein at least a portion of the grafts are unsupported by the stent. Still another embodiment includes an articulating stented graft which includes a plurality of stents spaced apart from one another at a predetermined distance wherein each stent is contained between two elongated biocompatible tubular members. The graft / stent / graft assemblies all have inseparable layers.
Owner:BARD PERIPHERAL VASCULAR
Device for Treating Mitral Valve Regurgitation
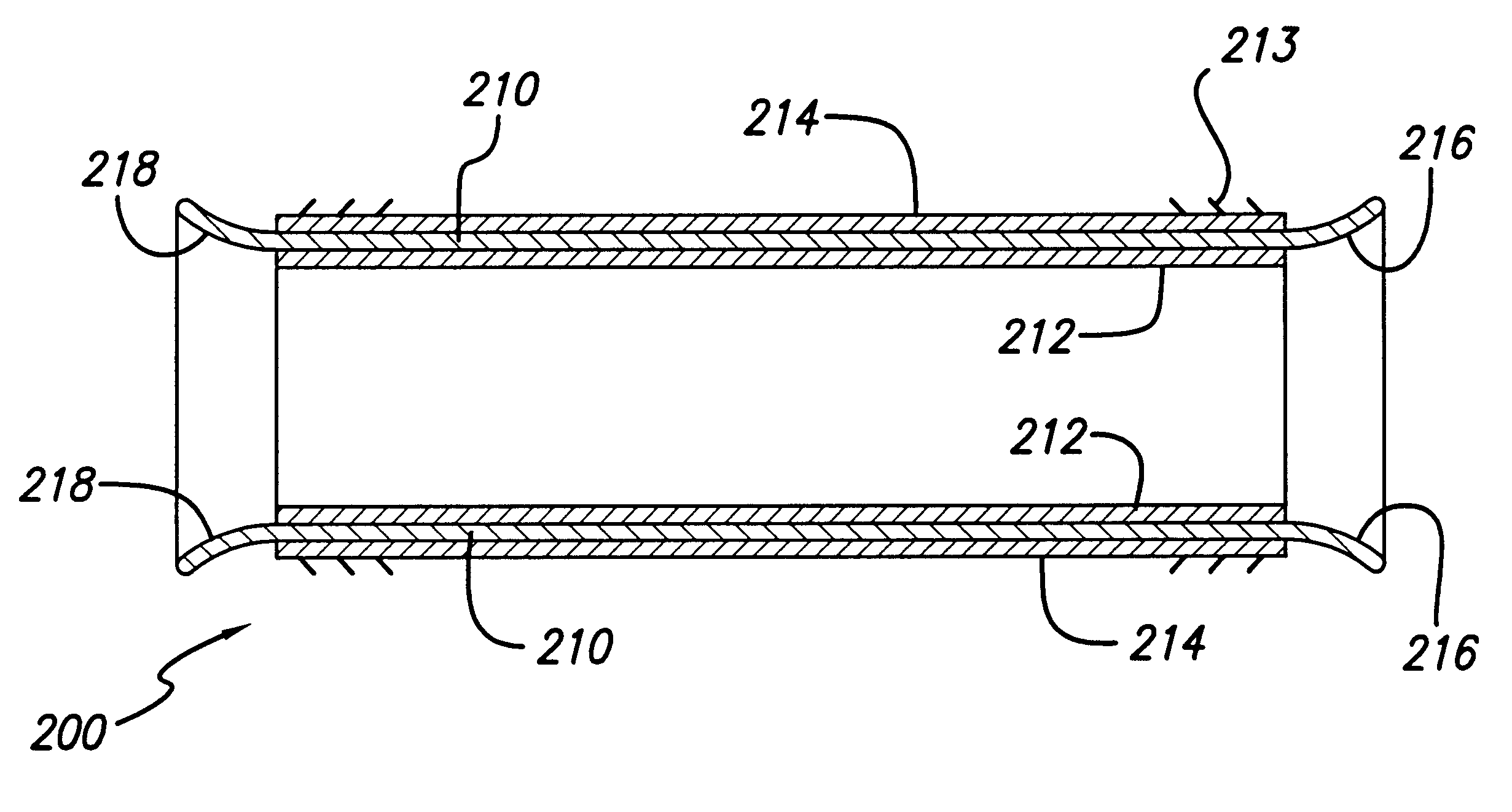
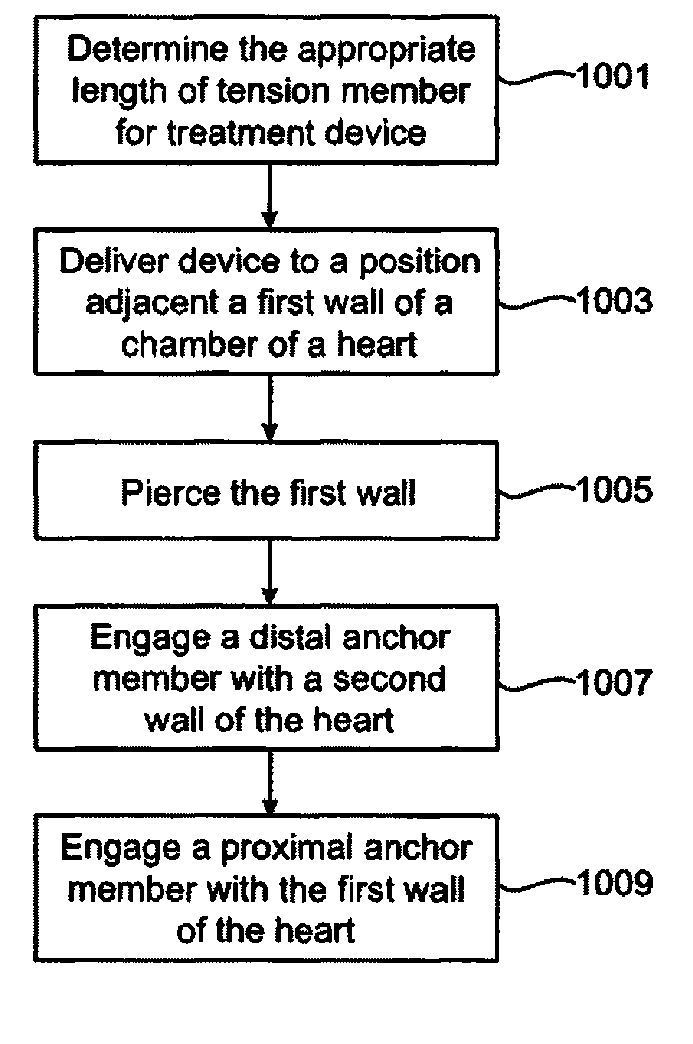
InactiveUS20070078297A1Reduce lateral distanceEasy to deploySuture equipmentsHeart valvesHeart chamberTension member
Owner:MEDTRONIC VASCULAR INC
Reduction of recirculation in catheters
InactiveUS20060004316A1Decrease outflow velocity of fluidPrevent sprayingMedical devicesCatheterDistal portionNose
A catheter tip designed to reduce the outflow velocity and / or directional momentum of fluid being infused by a catheter having such a tip. In one variation, a plurality of channels is provided at the distal portion of the catheter to increase the outflow cross-sectional area. In another variation, the diameter of the catheter at its distal portion where the fluid exits is increased. In yet another variation, a bullet-shaped nose is implemented which may decrease turbulence at the distal end of catheter tip. The low velocity outflow catheter tip may also be implemented on a dual lumen catheter, such as a hemodialysis catheter, to reduce recirculation rate. Various device configurations and methods for such implementations are also disclosed.
Owner:CR BARD INC
Polymeric drug formulations
InactiveUS7005454B2Reduce the formation of blood clotsPrevent thrombosisPowder deliveryBiocideWater insolubleMedicine
Polymeric drug formulations containing a non-releasing single-phase dispersion of a water-soluble drug in a water-insoluble tissue-compatible polymer matrix. Polymeric drug formulations are also disclosed containing a single-phase dispersion of a water-soluble drug and a water-insoluble tissue-compatible polymer matrix, and a second, phase-disrupting polymer that is non-miscible with the tissue-compatible polymer and is present in an amount sufficient to form phase-separated microdomains of the second polymer in the tissue-compatible polymer matrix, so that the release rate of the water-soluble drug from the tissue-compatible polymer matrix is related to the amount of the second polymer. Methods of preparing the polymeric drug formulations are also described, as well as methods for site-specific drug delivery utilizing the polymeric drug formulations.
Owner:EMORY UNIVERSITY +1
Implantable Prosthetic Valve
InactiveUS20090276039A1Reduce thrombosisImprove survivalJoint implantsVenous valvesProsthetic valveThrombus
An implantable prosthetic valve for regulating fluid flow through a body vessel is provided. The prosthetic valve comprises an anchoring member, at least one leaflet, and a restraining member capable of temporarily preventing substantial movement of the leaflet between and open and closed position so as to allow fluid flow in the antegrade and retrograde directions. In various embodiments, the prosthetic valve reduces the risk of thrombosis. In various embodiments, the prosthetic valve reduces the appearance of potentially thrombogenic abnormal flow patterns at the site of implantation immediately following the implantation, allows cell deposition making the valve more biocompatible, less thrombogenic before flow changes resulting from valving action set in and allows tissue growth so that a partially or completely biological functioning valve may form on the scaffold provided by the implant.
Owner:CLINASYS
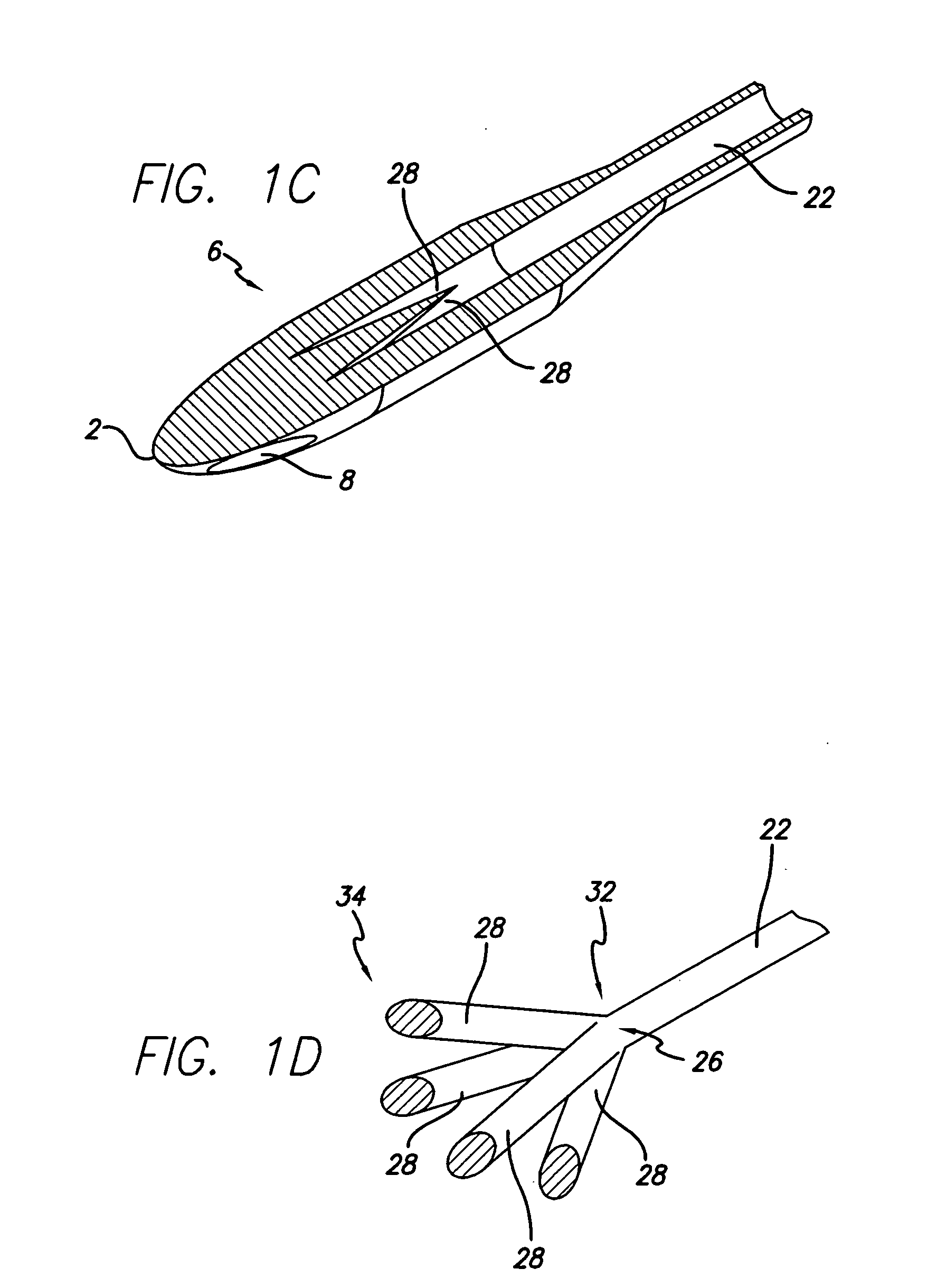
Function-enhanced thrombolytic AV fistula and method
A coiled stent graft, including a thrombolytic agent, is positionable within an AV fistula and optionally into one or both of the artery and the vein (6) to help reduce or eliminate blockages within the blood vessel at the junction between the AV fistula and the blood vessel.
Owner:LEMAITRE VASCULAR
Left atrial appendage closure device
This invention relates to an occlusion device for the closure of a physical lumen. More specifically, this invention relates to an occlusion device for the left atrial appendage of the heart, comprising a center post, a plurality of ribs extending along the center post and sheet which is attached to the ribs.
Owner:ATRIAL SOLUTIONS INC
Glycosaminoglycan and Synthetic Polymer Material for Blood-Contacting Applications
ActiveUS20150196688A1Improve surface chemistryFeasible at commercial productionSuture equipmentsOrganic active ingredientsLow-density polyethyleneLinear low-density polyethylene
Provided herein is a composite, comprising: a polymer host selected from the group consisting of low-density polyethylene (LDPE), linear low-density polyethylene (LLDPE), polyethylene terephthalate (PET), polytetrafluoroethylene (PTFE), and polypropylene (PP), polyurethane, polycaprolactone (PCL), polydimethylsiloxane (PDMS), polymethylmethacrylate (PMMA), and polyoxymethylene (POM); and a guest molecule comprising hyaluronic acid; wherein the guest molecule is disposed within the polymer host, and wherein the guest molecule is covalently bonded to at least one other guest molecule. Also provided herein are methods for forming the composite, and blood-contracting devices made from the composite, such as heart valves and vascular grafts.
Owner:COLORADO STATE UNIVERSITY
Non-contact electrode basket catheters with irrigation
ActiveUS20100168647A1Reduce riskPrevent blood coagulationElectrotherapyElectrocardiographyMedicineBasket catheter
Catheter systems and methods are disclosed. An exemplary catheter includes an outer tubing housing and an inner fluid delivery tubing, the inner fluid delivery tubing having at least one fluid delivery port. The catheter also includes a deployment member movable axially within the inner fluid delivery tubing. A plurality of splines are each connected at a proximal end to the outer tubing and at a distal end to deployment member. A seal is provided between the outer tubing and the inner fluid delivery tubing. A gasket is provided between the deployment member and the inner fluid delivery tubing. Both the seal and the gasket are configured to prevent blood or other fluid from ingressing into the outer tubing.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
Biological component comprising artificial membrane
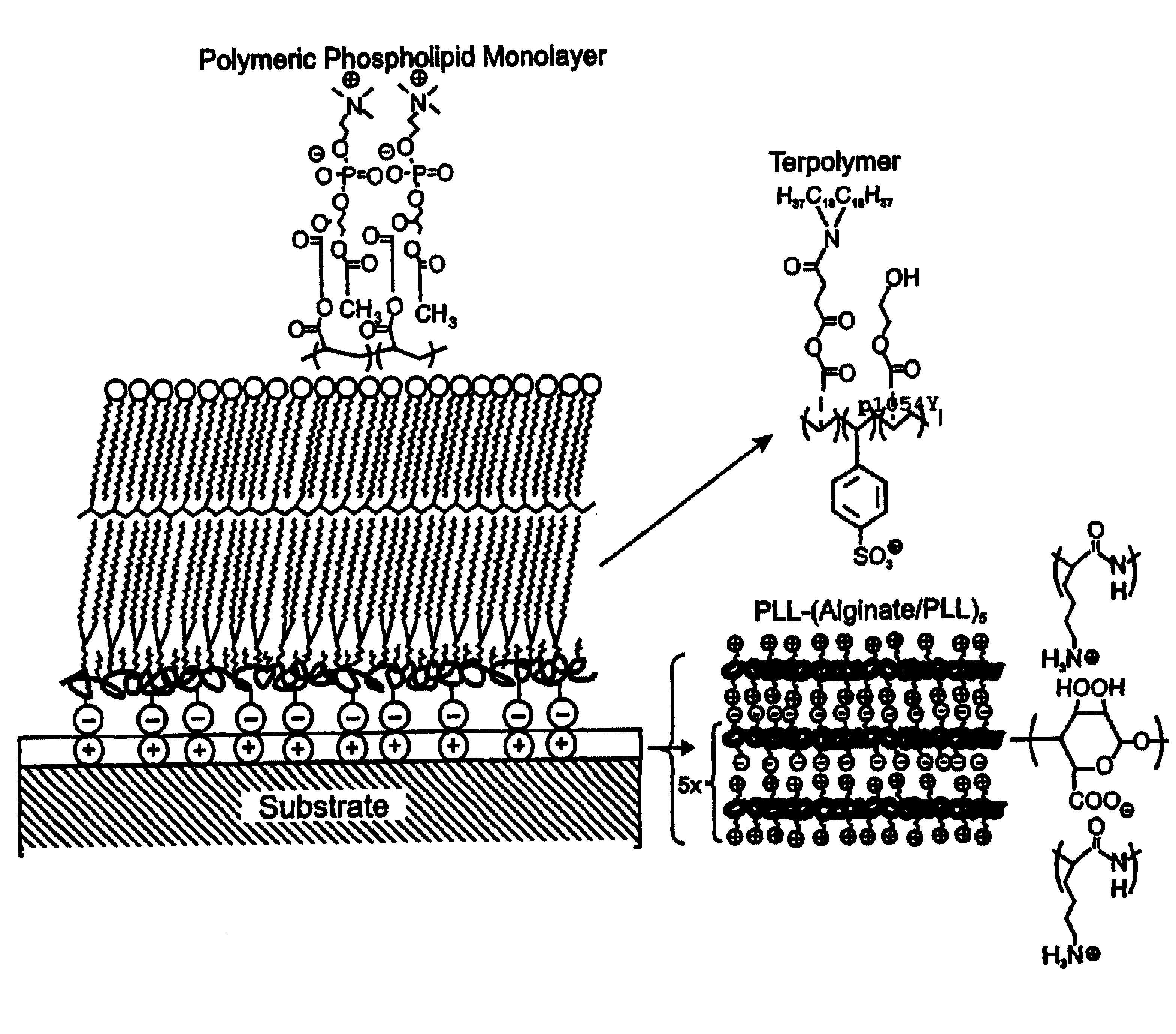
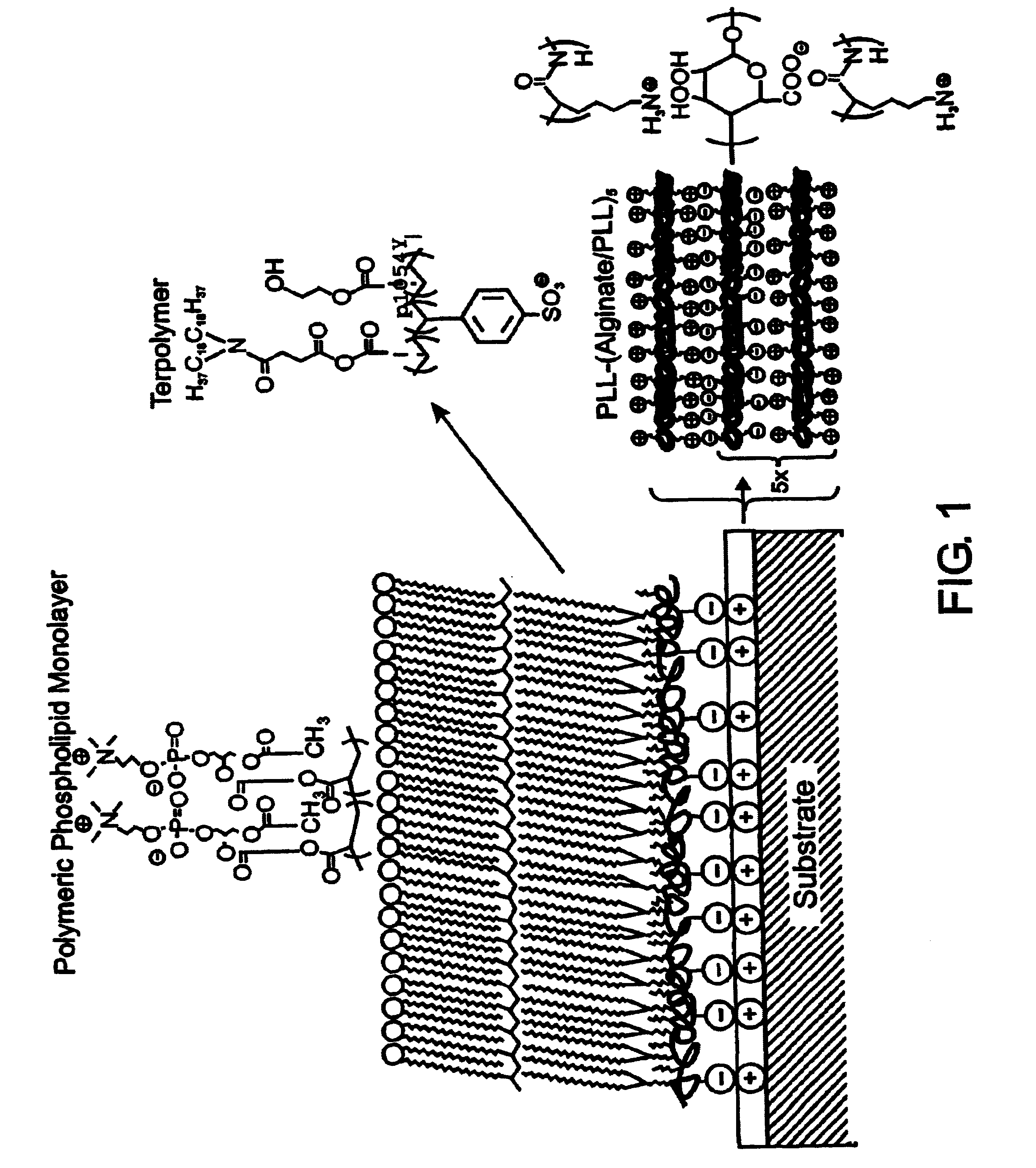
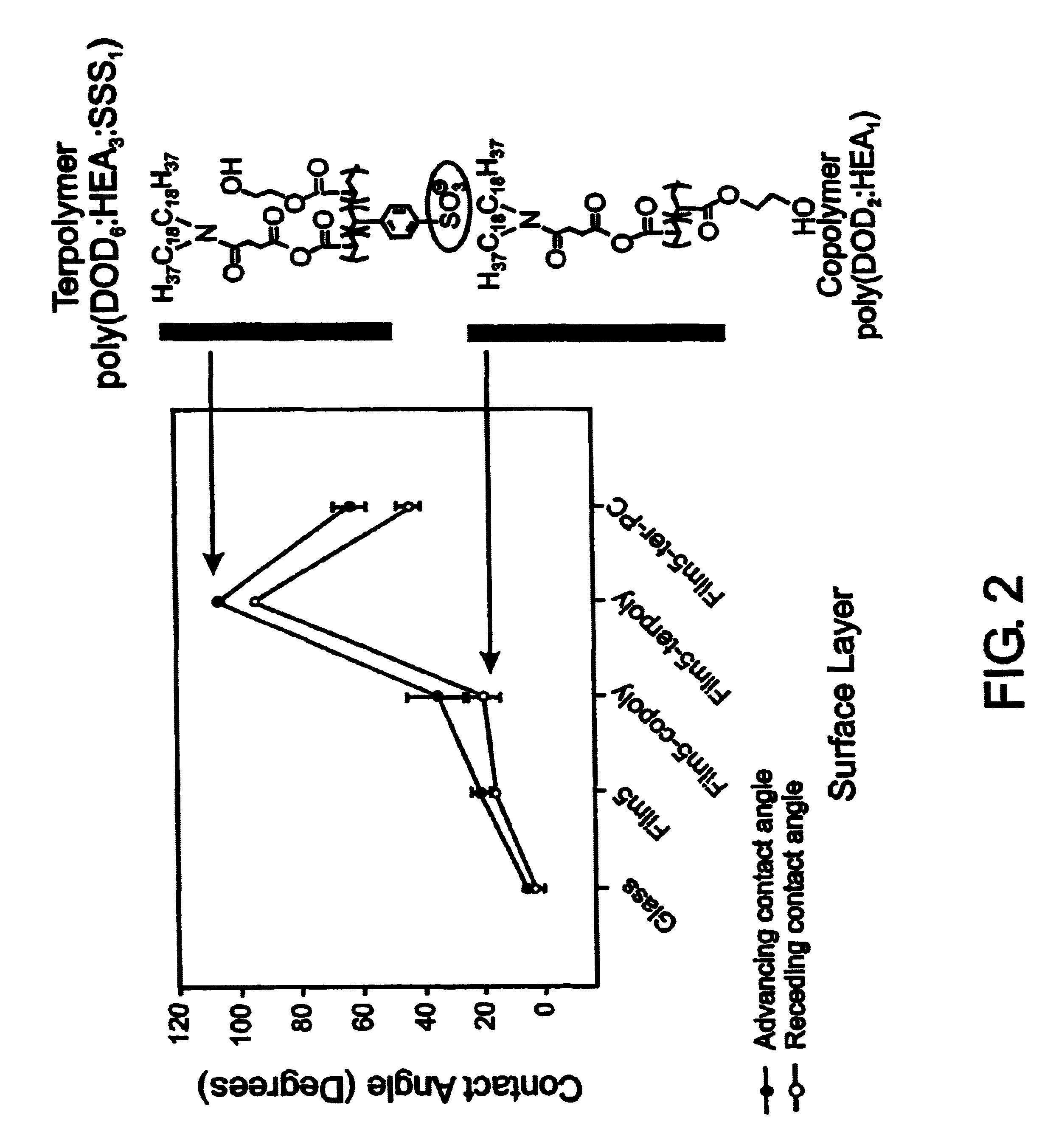
InactiveUS7713544B2Improved performance characteristicsMinimize adhesionMaterial nanotechnologyMicroorganismsAmount of substanceMembrane mimetic
A biocompatible biological component is provided comprising a membrane-mimetic surface film covering a substrate. Suitable substrates include hydrated substrates, e.g. hydrogels which may contain drugs for delivery to a patient through the membrane-mimetic film, or may be made up of cells, such as islet cells, for transplantation. The surface may present exposed bioactive molecules or moieties for binding to target molecules in vivo, for modulating host response when implanted into a patient (e.g. the surface may be antithrombogenic or antiinflammatory) and the surface may have pores of selected sizes to facilitate transport of substances therethrough. An optional hydrophilic cushion or spacer between the substrate and the membrane-mimetic surface allows transmembrane proteins to extend from the surface through the hydrophilic cushion, mimicking the structure of naturally-occurring cells. An alkylated layer directly beneath the membrane-mimetic surface facilates bonding of the surface to the remainder of the biological component. Alkyl chains may extend entirely through the hydrophilic cushion when present. To facilitate binding, the substrate may optionally be treated with a polyelectrolyte or alternating layers of oppositely-charged polyelectrolytes to facilitate charged binding of the membrane-mimetic film or alkylated layer beneath the membrane-mimetic film to the substrate. The membrane-mimetic film is preferably made by in situ polymerization of phospholipid vesicles.
Owner:EMORY UNIVERSITY
Pine needle tea beverage
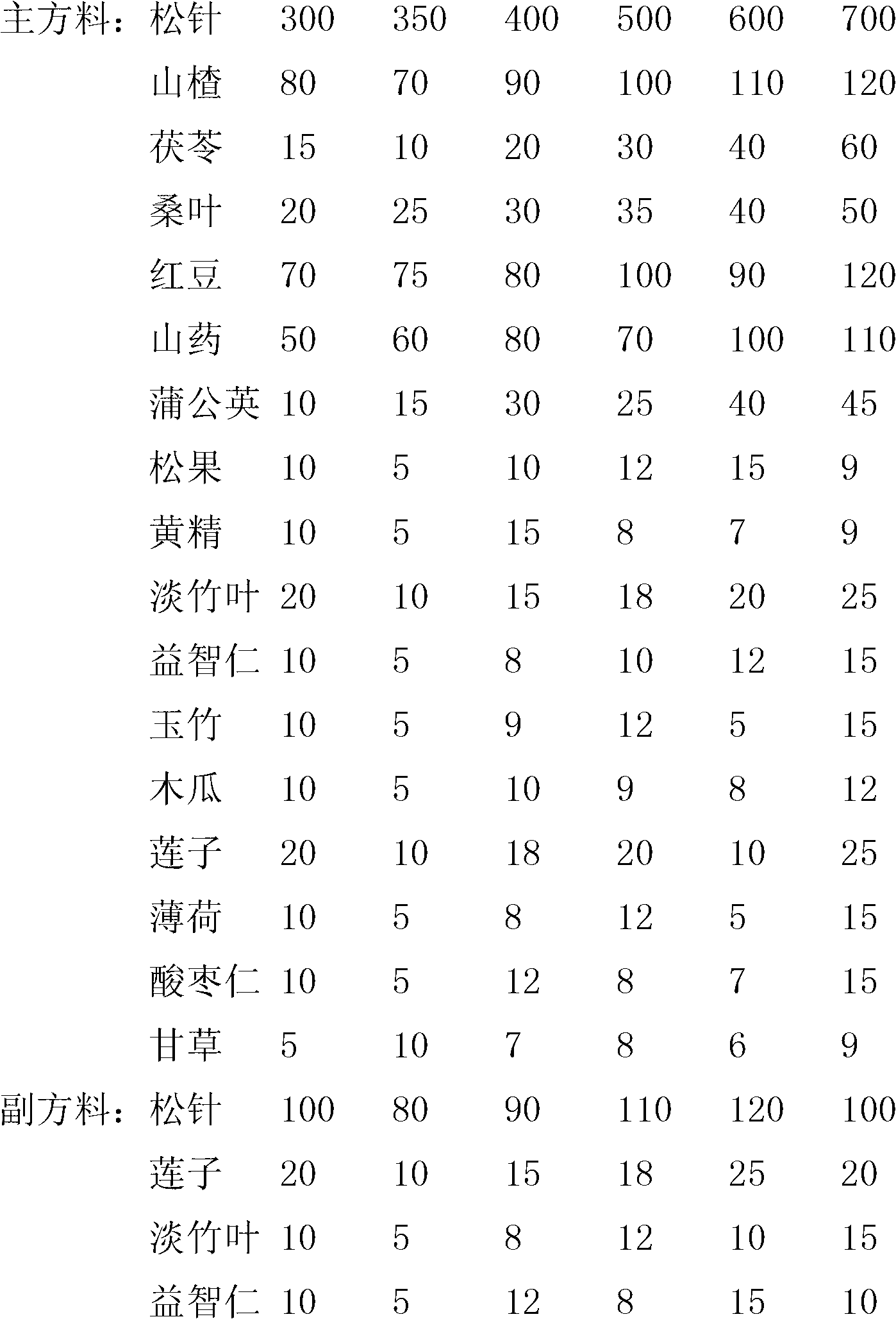
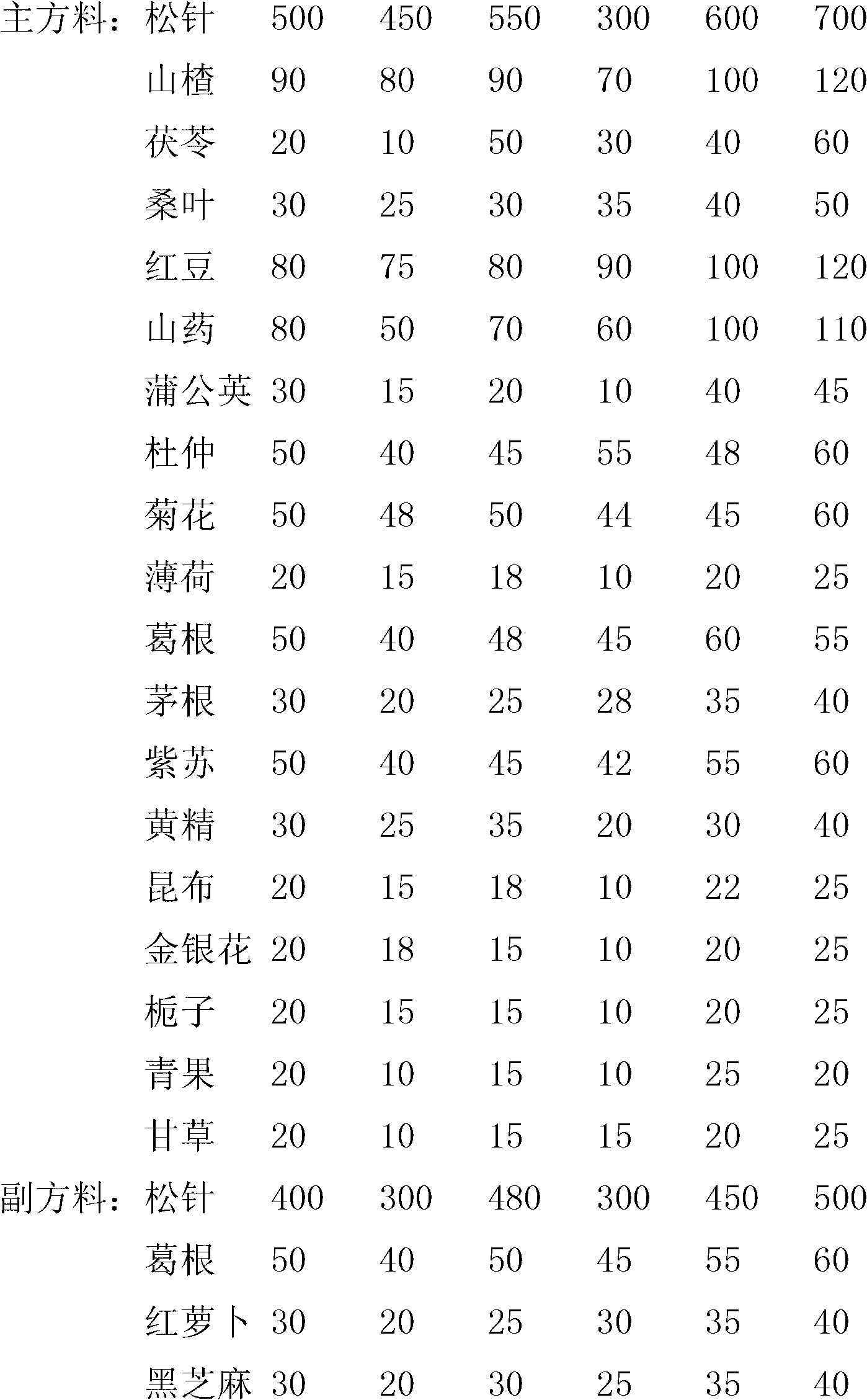
ActiveCN102698039AEasy to eatImprove eating effectMetabolism disorderTea substituesRed beanDandelion
The invention relates to a pine needle tea beverage. According to the technical scheme, the pine needle tea beverage comprises the following substances in parts by weight: 300-700 parts of pine needle, 70-120 parts of fructus crataegi, 10-60 parts of indian buead, 20-50 parts of mulberry leaf, 70-120 parts of red beans, 50-120 parts of Chinese yam and 5-50 parts of dandelion. The invention aims to provide a pine needle tea beverage which has a good effect.
Owner:福州万丰和生物科技有限公司
Chimeric factor vii molecules
ActiveUS20100330059A1Reduce riskLow affinityPeptide/protein ingredientsSemiconductor/solid-state device detailsFactor VIIImmunology
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Reduction of recirculation in catheters
ActiveUS8920404B2Reduce the average velocityPrevent sprayingMedical devicesCatheterDistal portionNose
A catheter tip designed to reduce the outflow velocity and / or directional momentum of fluid being infused by a catheter having such a tip. In one variation, a plurality of channels is provided at the distal portion of the catheter to increase the outflow cross-sectional area. In another variation, the diameter of the catheter at its distal portion where the fluid exits is increased. In yet another variation, a bullet-shaped nose is implemented which may decrease turbulence at the distal end of catheter tip. The low velocity outflow catheter tip may also be implemented on a dual lumen catheter, such as a hemodialysis catheter, to reduce recirculation rate. Various device configurations and methods for such implementations are also disclosed.
Owner:CR BARD INC
Compositions and methods for treating pain
ActiveUS20060280718A1Reduce severityReduce thrombosisBiocideNervous disorderNervous systemPost injury
In the present invention, Applicants demonstrate the effect of a biomembrane sealing agent on the development of chronic pain following tissue injury as well as acute pain in a model of acute inflammation. Applicants demonstrate the ability of this class of agents referred to as “biomembrane sealing agents” to reduce the severity of hyperalgesia and allodynia following mechanical insult to the nervous system as well as their ability to reduce acute pain in a model of acute inflammation. Applicant describes the use of injectable or depot formulations of biomembrane sealing agent(s) for prophylactic treatment such as they could be administered after the insult (i.e. post-injury or post-surgery) but before the onset of acute or chronic pain. Alternatively, biomembrane sealing agents could be used to reduce the severity of acute or chronic pain after onset.
Owner:WARSAW ORTHOPEDIC INC
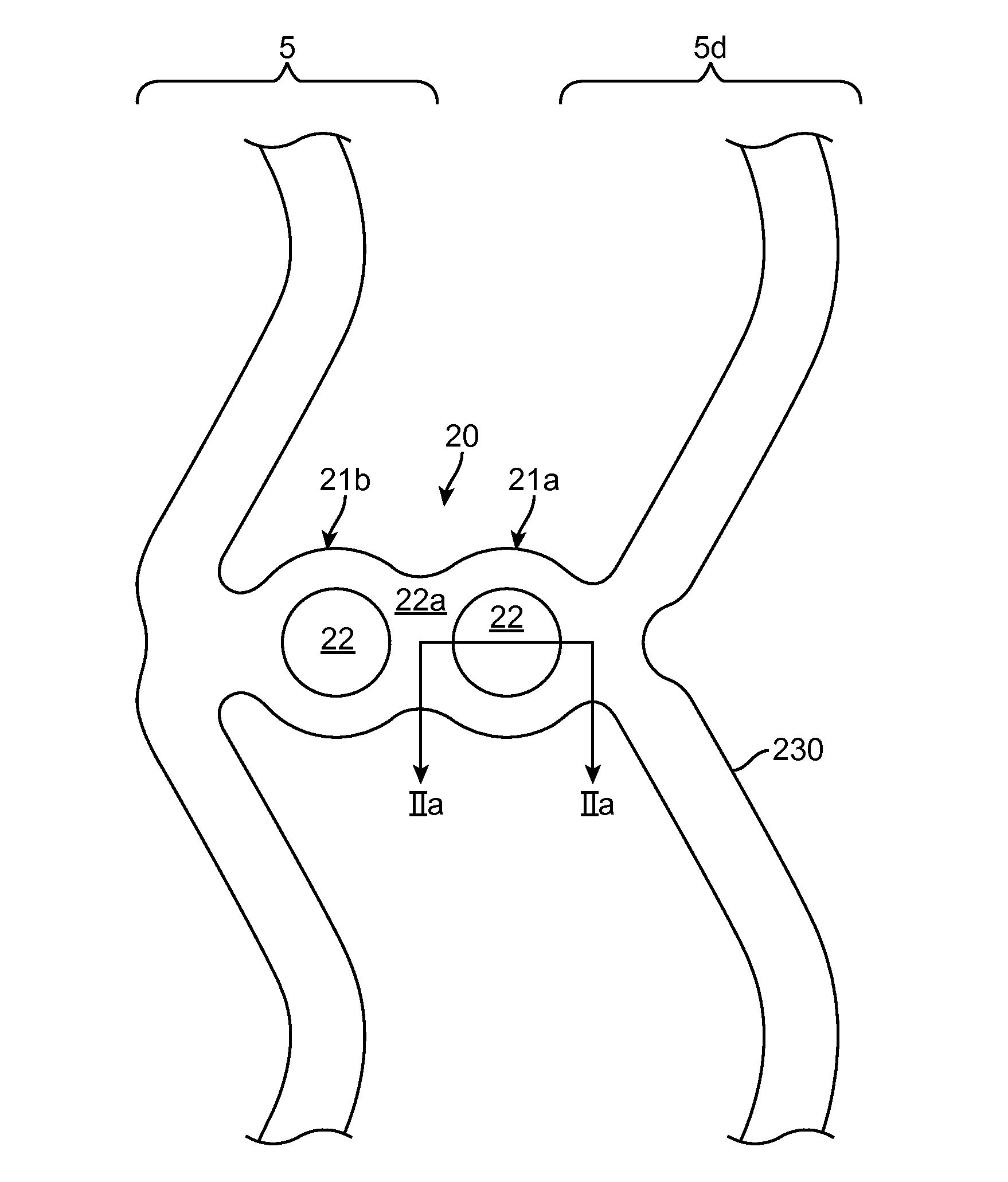
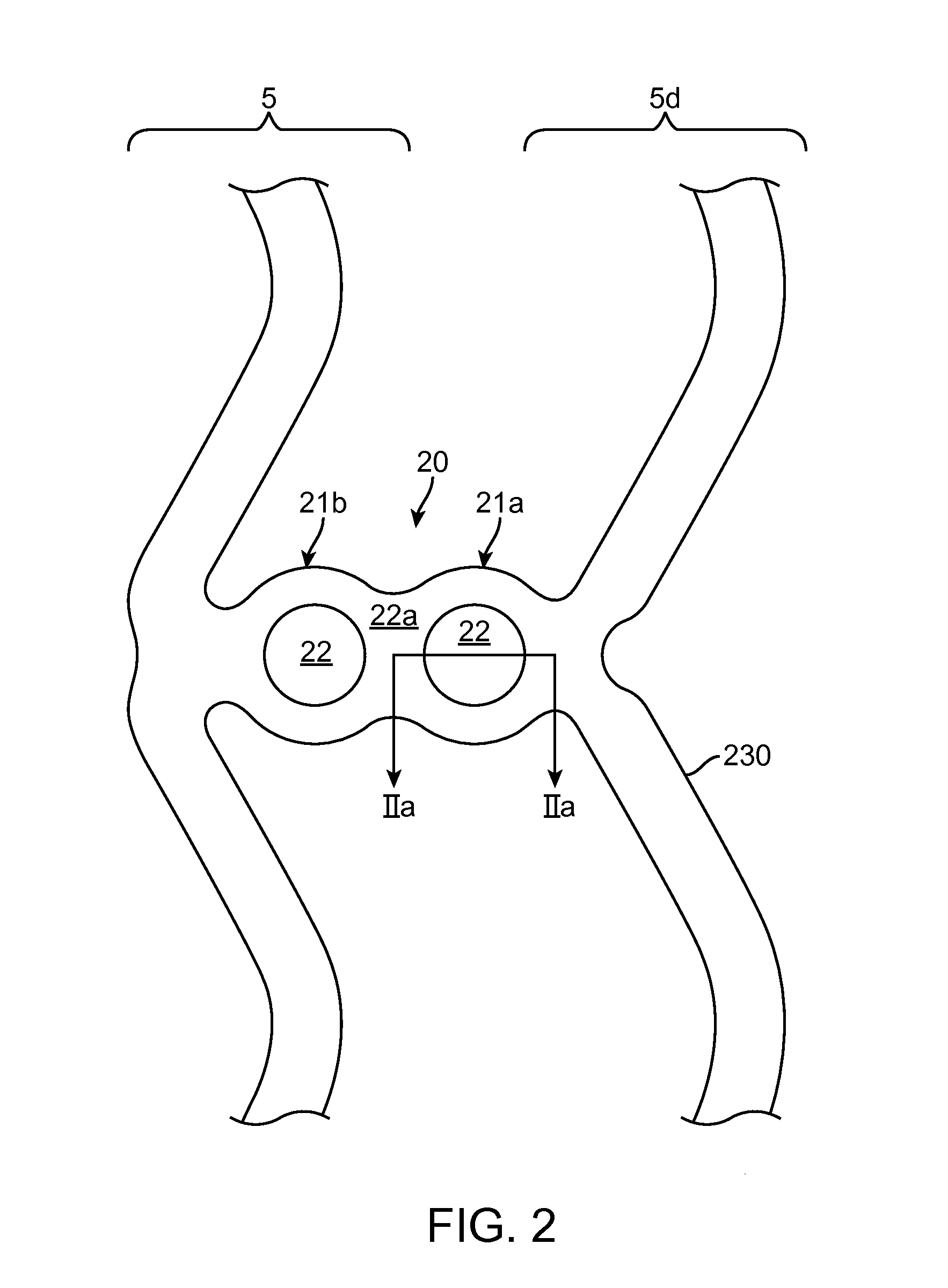
Scaffolds having radiopaque markers
A scaffold includes a radiopaque marker connected to a strut. The marker is retained within the strut by one or more of a mechanical interference fit, a polymer coating or melt, and / or by friction. The marker can take the form of a bead, rivet or snap-in marker, or a tube deformed when attached to the strut. The strut is made from a tube. The strut has a thickness of about 100 microns.
Owner:ABBOTT CARDIOVASCULAR
Synthetic non-fouling amino acids
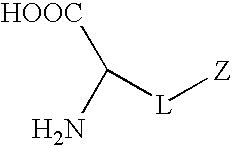
InactiveUS20090149673A1Good biocompatibilityReduce thrombosisOrganic chemistryPhosphorylcholineBiocompatibility Testing
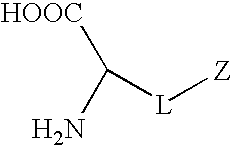
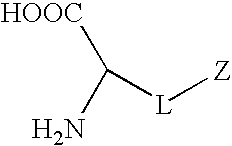
Synthetic amino acids containing one or more non-fouling groups or moieties are described herein. In one embodiment, the amino acid has the following chemical formula:where L is a linker group and Z is a non-fouling group including, but not limited to, polyethylene glycol (PEG); oligoethylene glycol (OEG); zwitterionic group, such as phosphorycholine, carboxybetaine, and sulfobetaine; groups that are hydrogen bond acceptors but not hydrogen bond donors. The non-fouling amino acids can be incorporated into a bioactive peptide as single amino acid residues, multiples amino acid residues, or as blocks of amino acids. The non-fouling amino acids, or peptides containing one or more non-fouling amino acids, can be applied to surfaces in order to improve biocompatibility, reduce thrombogenesis, and / or reduce fouling by proteins or bacteria present in solution.
Owner:ARROW INT INC
Composition comprising a combined thromboxane receptor antagonist and thromboxane synthase inhibitor and a COX-2 inhibitor
InactiveUS20060217431A1Inhibit platelet activationPrevents vasoconstrictive actionBiocideNervous disorderDiseaseCOX-2 inhibitor
The invention relates to a pharmaceutical composition comprising a combined thromboxane receptor antagonist and thromboxane synthase inhibitor and a COX-2 inhibitor. In addition a method of treating cyclooxygenase dependent disorders, including inflammation, pain and / or rheumatic diseases, and / or neoplasia is described.
Owner:BOEHRINGER INGELHEIM INT GMBH
Modulation platelet adhesion based on the surface-exposed beta-switch loop of platelet glycoprotein IB-alpha
ActiveUS20050192224A1Trend downReduce decreaseFactor VIICell receptors/surface-antigens/surface-determinantsGlycoprotein IbChemistry
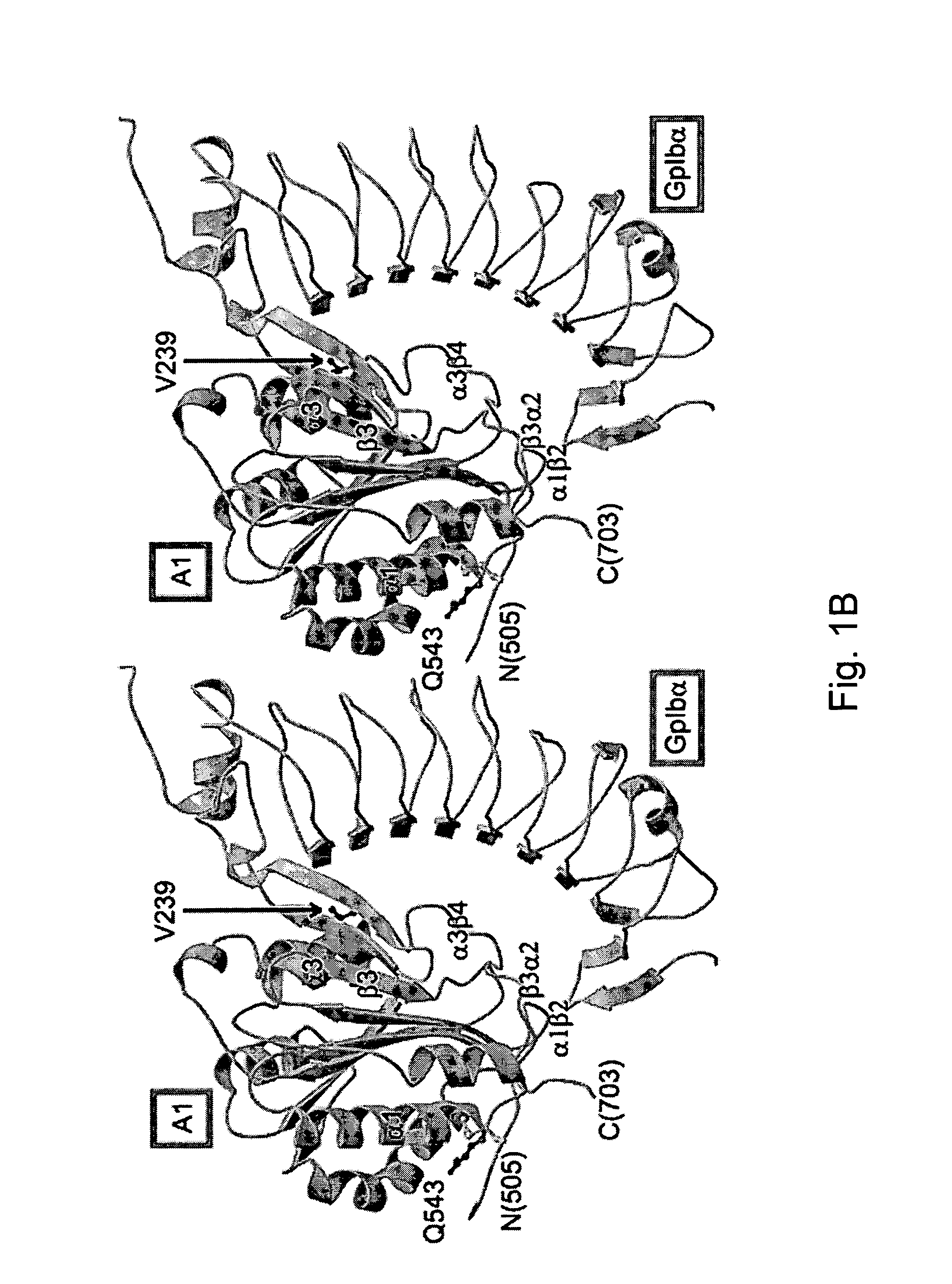
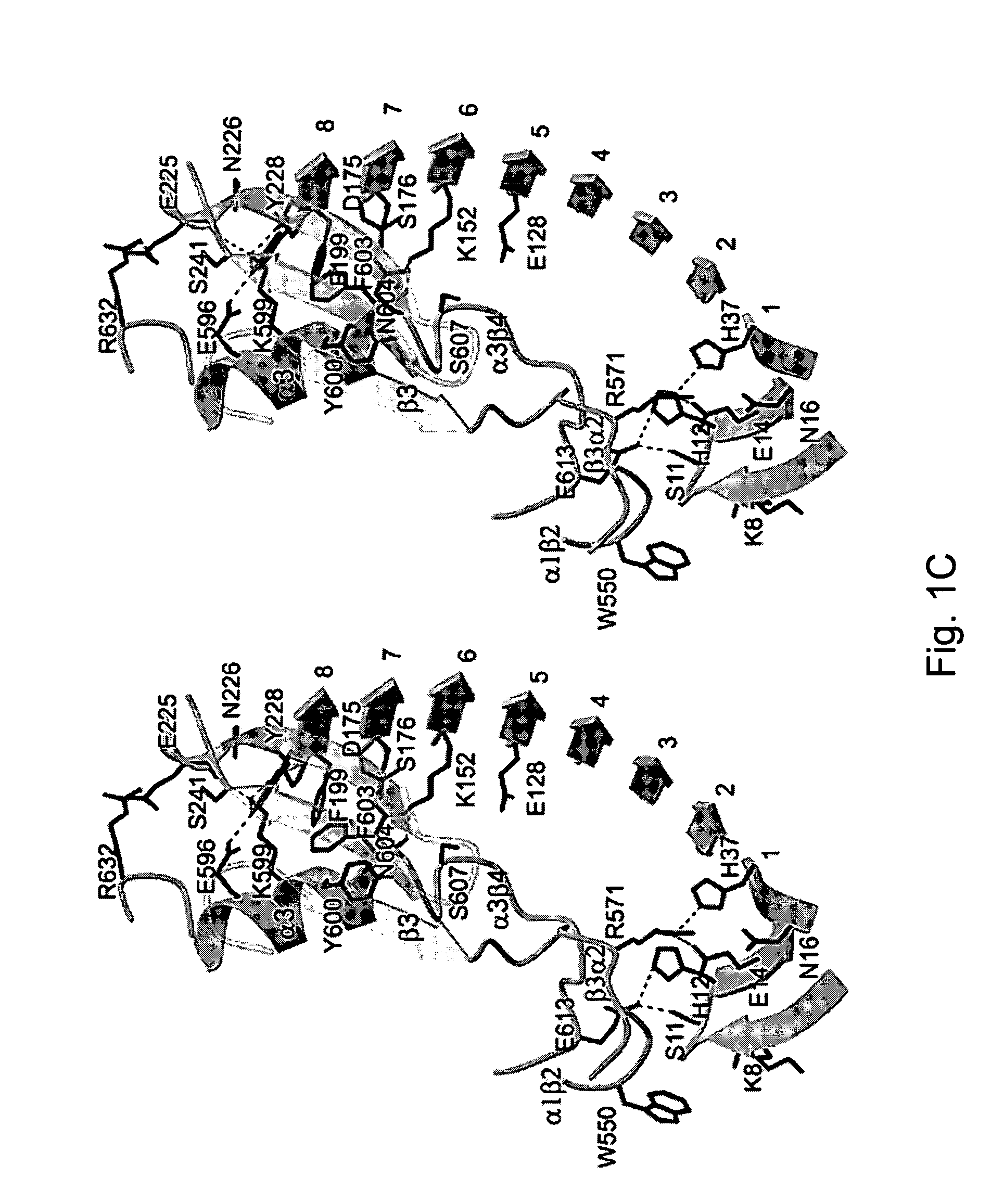
The invention relates to the adhesion of platelet GpIbα to strand β3 of domain A1 of von Willebrand factor (vWF), the strand β3 comprising amino acid residues at amino acid position 560-566 and / or a functional part or equivalent thereof, the platelet GpIbα, the GpIbα region comprising an amino acid sequence corresponding to a beta-switch loop of platelet GpIbα, comprising amino acid residues at amino acid position 227-242 and / or a functional part or equivalent thereof. The invention provides a method of interfering with adhesion of blood platelets to vWF that includes modulating adhesion. The invention further provides proteinaceous compounds, antibodies, medicaments and pharmaceutical compositions to that end. The invention also provides means and methods to increase platelet adhesion by topical application of a compound increasing platelet adhesion.
Owner:ABLYNX NV
Single nucleotide polymorphisms sensitively predicting adverse drug reactions (adr) and drug efficacy
InactiveUS20070128597A1Reducing primaryReducing secondary riskGenetic material ingredientsDisease diagnosisNucleotideEfficacy
Single Nucleotide Polymorphisms sensitively predicting Advserse Drug Reactions (ADR) and Drug Efficacy Abs tract. The invention provides diagnostic methods and kits including oligo and / or polynucleotides or derivatives, including as well antibodies determining whether a human subject is at risk of getting adverse drug reaction after statin therapy or whether the human subject is a high or low responder or a good a or bad metabolizer of statins. The invention provides further diagnostic methods and kits including antibodies determining whether a human subject is at risk for a cardiovascular disease. Still further the invention provides polymorphic sequences and other genes. The present invention further relates to isolated polynucleotides encoding a phenotype associated (PA) gene polypeptide useful in methods to identify therapeutic agents and useful for preparation of a medicament to treat cardiovascular disease or influence drug response, the polynucleotide is selected from the group comprising: SEQ ID 1-168 with allelic variation as indicated in the sequences section contained in a functional surrounding like full length cDNA for PA gene polypeptide and with or without the PA gene promoter sequence.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS GMBH
Non-contact electrode basket catheters with irrigation
ActiveUS9339331B2Reduce riskPrevent coagulationCatheterDiagnostic recording/measuringBasket catheterContact electrode
Catheter systems and methods are disclosed. An exemplary catheter includes an outer tubing housing and an inner fluid delivery tubing, the inner fluid delivery tubing having at least one fluid delivery port. The catheter also includes a deployment member movable axially within the inner fluid delivery tubing. A plurality of splines are each connected at a proximal end to the outer tubing and at a distal end to deployment member. A seal is provided between the outer tubing and the inner fluid delivery tubing. A gasket is provided between the deployment member and the inner fluid delivery tubing. Both the seal and the gasket are configured to prevent blood or other fluid from ingressing into the outer tubing.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
Adhesive composition for carrying therapeutic agents as delivery vehicle on coatings applied to vascular grafts
ActiveUS20060134218A1Decrease neointimal hyperplasiaDelayed healingPowder deliveryOrganic active ingredientsAdhesiveDelivery vehicle
Water-soluble polymeric adhesive compositions and their use as delivery vehicles for carrying therapeutic agents on implantable devices, such as vascular grafts, are disclosed. Use of drug-coated vascular grafts is demonstrated for delivery of the therapeutic agents in vivo, thereby inhibiting restenosis or neointimal hyperplasia of the vascular graft and inhibiting infection at the vascular graft site. Methods of forming the adhesive and making the coated vascular grafts are also disclosed.
Owner:UNIV OF TENNESSEE RES FOUND
Medical device coating with a biocompatible layer
ActiveUS10525170B2Reduce thrombosisReduced responseSurgeryPharmaceutical delivery mechanismPolyethylene glycolMedical device
Medical devices with a hydrogel layer covalently attached to a portion of the outer surface of the medical device are provided along with methods for applying the coating. The hydrogel layer can include a first polymer species comprising polyethylene glycol (PEG) and a second polymer species. Examples of the second polymer species include PEG and polyacrylamide (PAM). The first and second species can be at least partially cross-linked. Methods for forming the hydrogel coatings on the medical devices are provided including nucleophilic conjugate reactions, such as Click reactions.
Owner:TANGIBLE SCI LLC
Implantable article, method of forming same and method for reducing thrombogenicity
InactiveUS20080097620A1Reduce thrombosisPromoting and enhancing endothelializationPharmaceutical delivery mechanismAntithrombogenic treatmentThrombogenicityThrombus
Endothelialization of a bodily fluid or tissue-contacting, particularly blood-contacting, surface may be accomplished to render that surface substantially non-thrombogenic. Thrombosis may also be mitigated or eliminated by providing an eroding layer on the surface that results in the removal of any thrombus formation as the layer erodes. An implantable device may utilize at least one surface having a plurality of nano-craters thereon that enhance or promote endothelialization. Additionally, an implantable device may have at least one first degradable layer for contacting bodily fluid or tissue and disposed about a central core, and at least one second degradable layer between the first degradable layer and the central core. The first degradable layer has a first degradation rate and the second degradable layer has a second degradation rate which degrades more slowly than the first degradable layer on contact with bodily fluid or tissue.
Owner:NANYANG TECH UNIV
Treatment of pulmonary arterial hypertension with mesenchymal stem cells
ActiveUS10071123B2Reduce thrombosisPromotes proper endothelial morphologyOrganic active ingredientsAntipyreticProstacyclinMedicine
The application is directed to a method for treating or preventing vasculopathy comprising administrating to a subject in need thereof a pharmaceutical composition comprising mesenchymal precursor cells (MPCs) and a prostacyclin. Also provided a method for treating or preventing vasculopathy in a subject in need thereof, comprising administering to the subject a prostacyclin and a mesenchymal stem cell (MSC) or a MSC-conditioned culture medium or administering to the subject a MSC or a MSC-conditioned culture medium that has treated with prostacyclin. Pharmaceutical compositions suitable for such treatments are also provided.
Owner:UNITED THERAPEUTICS CORP
Thromboresistant coatings for aneurysm treatment devices
ActiveUS10653426B2Good biological propertiesReduce thrombosisStentsSurgical needlesRadiologyVascular device
Disclosed are coating compositions, processes, and designs for endowing vascular devices with thromboresistant and endothelializing properties. Also disclosed are designs of vascular devices used as aneurysm treating devices for assisting in the delivery, packing, and maintenance of embolization coils within an aneurysm, particularly a neurovascular aneurysm.
Owner:INCEPT LLC
Implantable prosthetic valve
InactiveUS7947074B2Reduce thrombosisImprove survivalVenous valvesBlood vesselsProsthetic valveMedicine
An implantable prosthetic valve for regulating fluid flow through a body vessel is provided. The prosthetic valve comprises an anchoring member, at least one leaflet, and a restraining member capable of temporarily preventing substantial movement of the leaflet between and open and closed position so as to allow fluid flow in the antegrade and retrograde directions. In various embodiments, the prosthetic valve reduces the risk of thrombosis. In various embodiments, the prosthetic valve reduces the appearance of potentially thrombogenic abnormal flow patterns at the site of implantation immediately following the implantation, allows cell deposition making the valve more biocompatible, less thrombogenic before flow changes resulting from valving action set in and allows tissue growth so that a partially or completely biological functioning valve may form on the scaffold provided by the implant.
Owner:CLINASYS
Health Chinese herbal medicine additive capable of promoting growth of crucian
InactiveCN105076699AIncrease food intakeImprove meat qualityMetabolism disorderAnimal feeding stuffSalvia miltiorrhizaGLYCYRRHIZA EXTRACT
The invention designs a health Chinese herbal medicine additive capable of promoting the growth of crucian. The additive is characterized by comprising the following components in parts by weight: 10-14 parts of astragalus membranaceus, 8-12 parts of liquorice root, 4-6 parts of dried tangerine or orange peel, 3-5 parts of kudzu root, 2-4 parts of hawthorn fruits, 8-12 parts of wolfberry fruits, 3-5 parts of eucommia ulmoides, 14-18 parts of Chinese yams, 3-5 parts of angelica sinensis, 2-4 parts of salvia miltiorrhiza, 2-4 parts of dahurian angelica root, 7-9 parts of isatis root, 3-5 parts of purslane, 4-6 parts of sophora flowers, 2-4 parts of cyrtomium fortunei and 3-5 parts of mint. According to the health Chinese herbal medicine additive capable of promoting the growth of the crucian, properties of various Chinese herbal medicines are sufficiently utilized, and by virtue of the synergistic effect of the Chinese herbal medicines, the functions of improving the immunity of fish bodies, promoting the growth of fishes and killing pathogenic bacteria and parasites in feed of the Chinese herbal medicines are achieved; the raised crucian has good body forms, scales with bright color and luster and flesh with rich umami, and the cooked crucian meat has a delicious flavor and is chewy; and the Chinese herbal medicines are readily available, the production method is simple and convenient, the cost is low and the use effect is good.
Owner:杨成胜
Methods and compositions for the delivery of biologically active agents
ActiveUS8574604B2Offset inflammatory responseReduce thrombosisAntibacterial agentsBiocideActive agentMedicinal chemistry
The invention features polymers noncovalently complexed with a biologically active agent. The polymer complexes include at least one shielding moiety covalently tethered to at least one complexing moiety, which is complexed with at least one biologically active agent.
Owner:INTERFACE BIOLOGICS INC
Grafted polymers and uses thereof
ActiveUS8445016B2Control migration and releaseEasy to controlBiocidePowder deliveryActive agentBackbone chain
Owner:INTERFACE BIOLOGICS INC +2
Stent with sheath and metal wire and methods
Owner:INSPIRE M D LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com