Patents
Literature
695 results about "Prophylactic treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A prophylactic treatment is a medical treatment used to prevent the appearance of a disease or other medical problem in a patient who is healthy at the time of treatment.
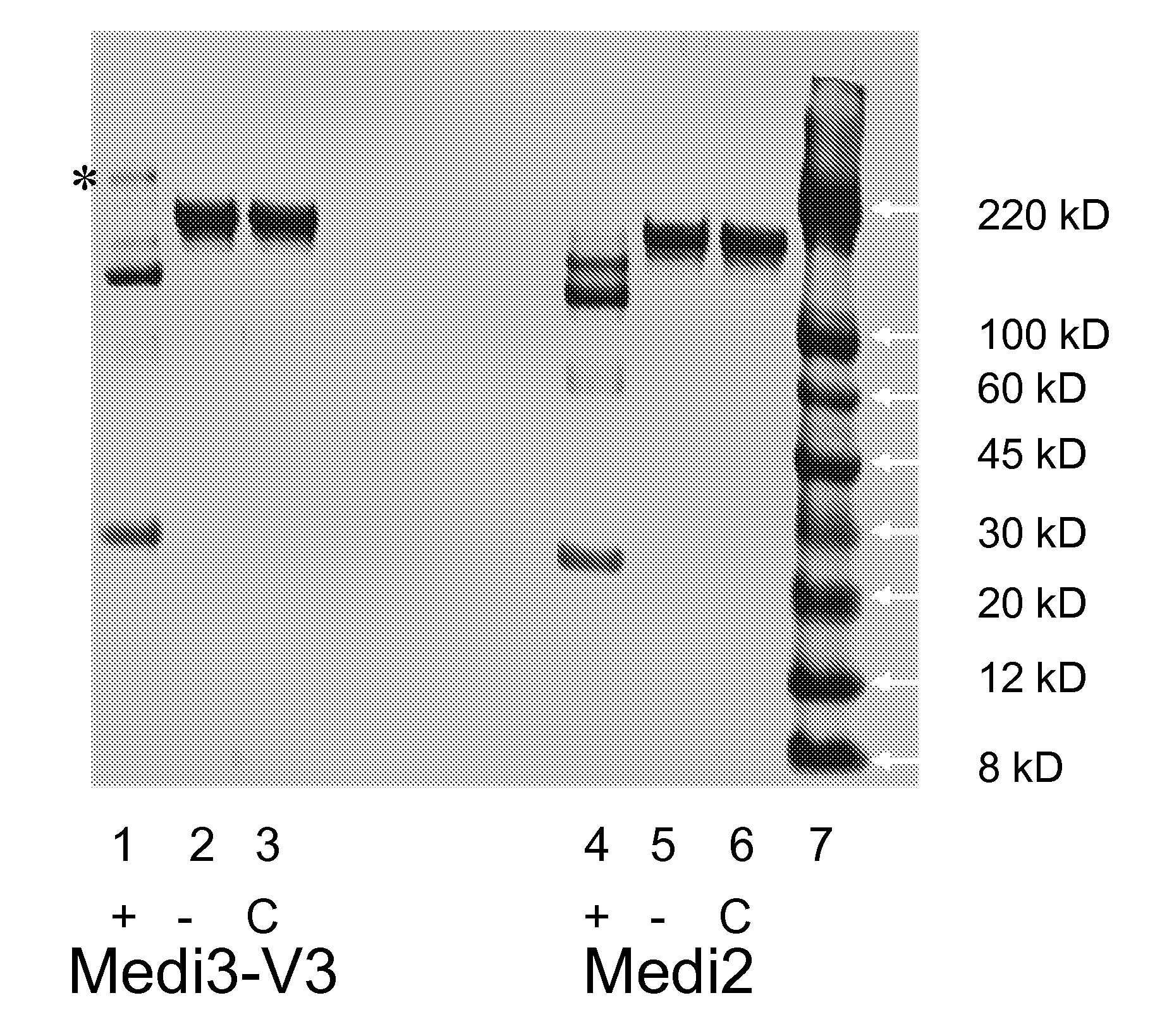
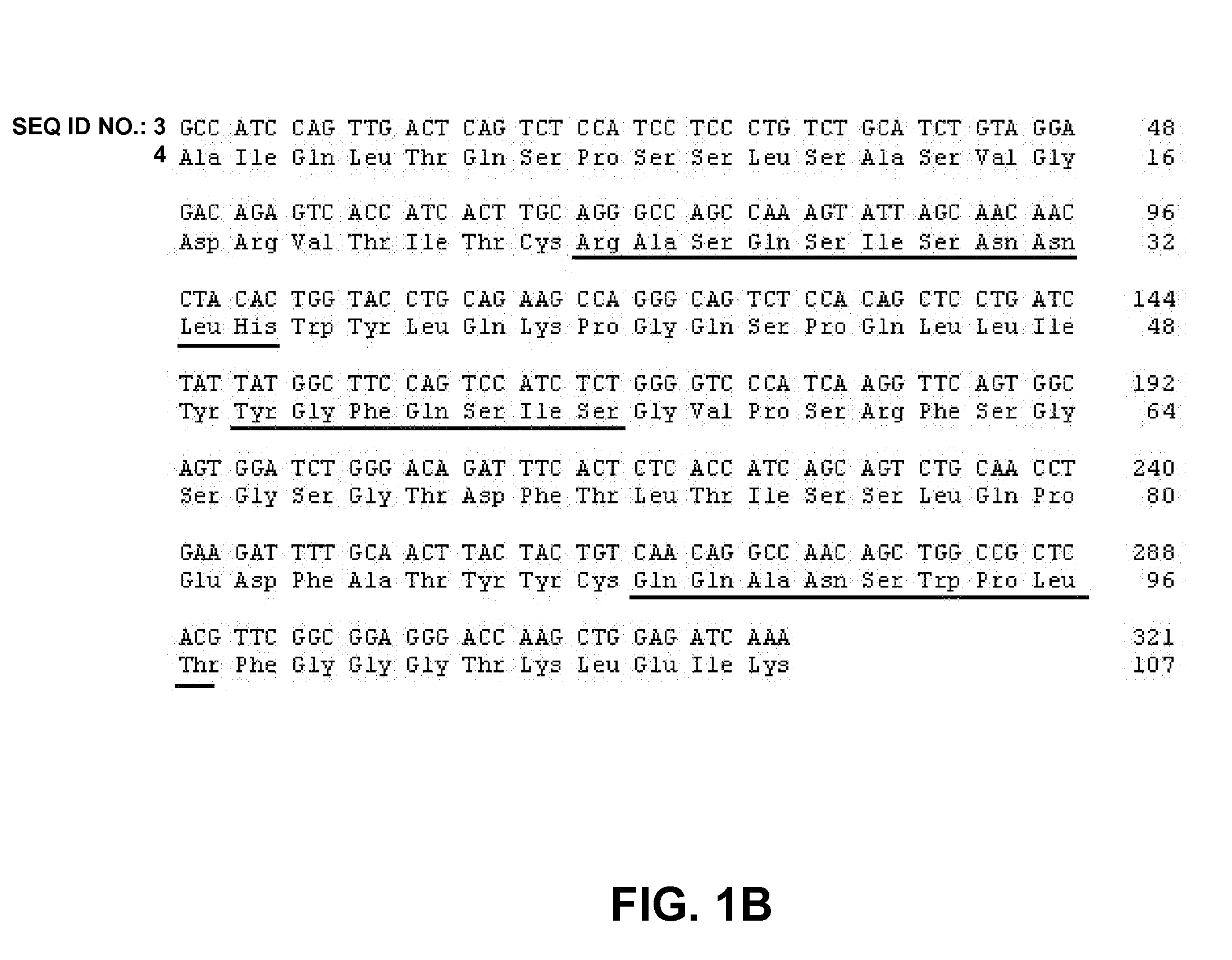
Protein Formulations
InactiveUS20080071063A1Improve stabilityLess restrictive temperature requirementAntipyreticAnalgesicsDiseaseProphylactic treatment
The present invention provides formulations of proteins comprising a variant Fc region that improve the stability in part by reducing the propensisty of such molecules to rapidly aggregate. The invention provides both liquid and lyophilized formulations either of which can be utilized to generate a high protein concentration liquid suitable for administration to a subject. The invention further provides methods of utilizing the formulations of the present invention for therapeutic or prophylactic treatment of diseases and disorders or for diagnostic purposes.
Owner:MEDIMMUNE LLC
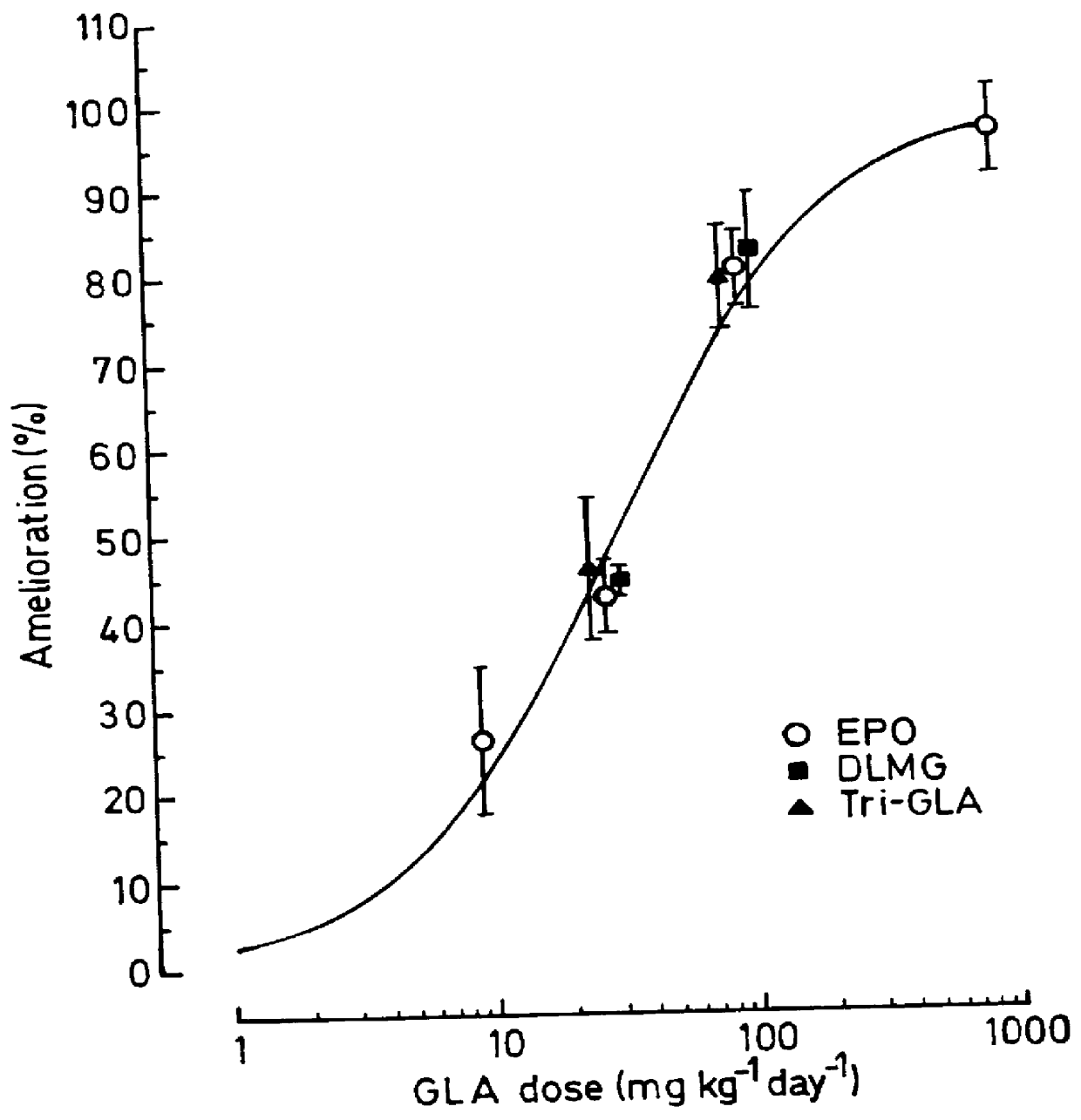
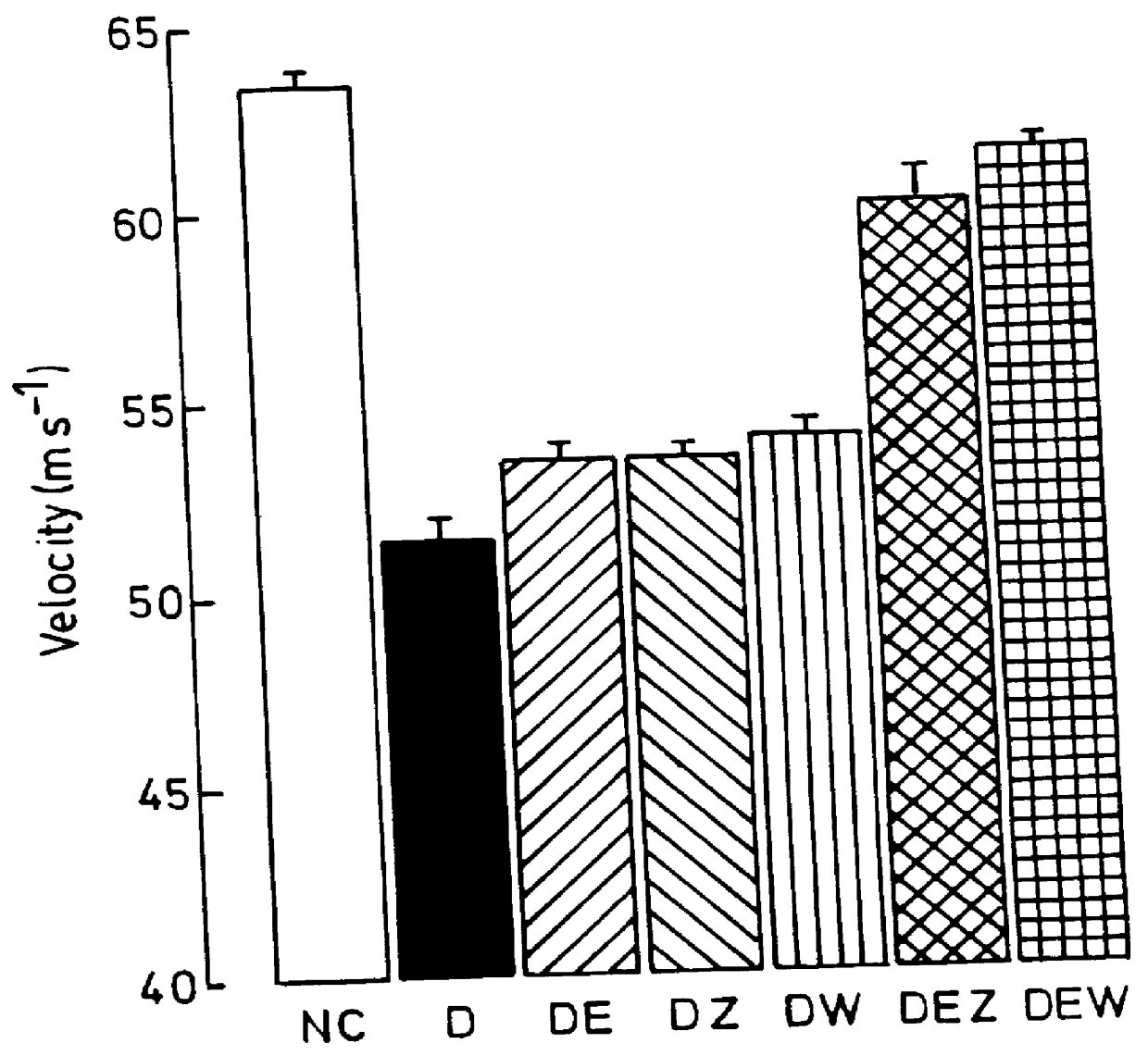
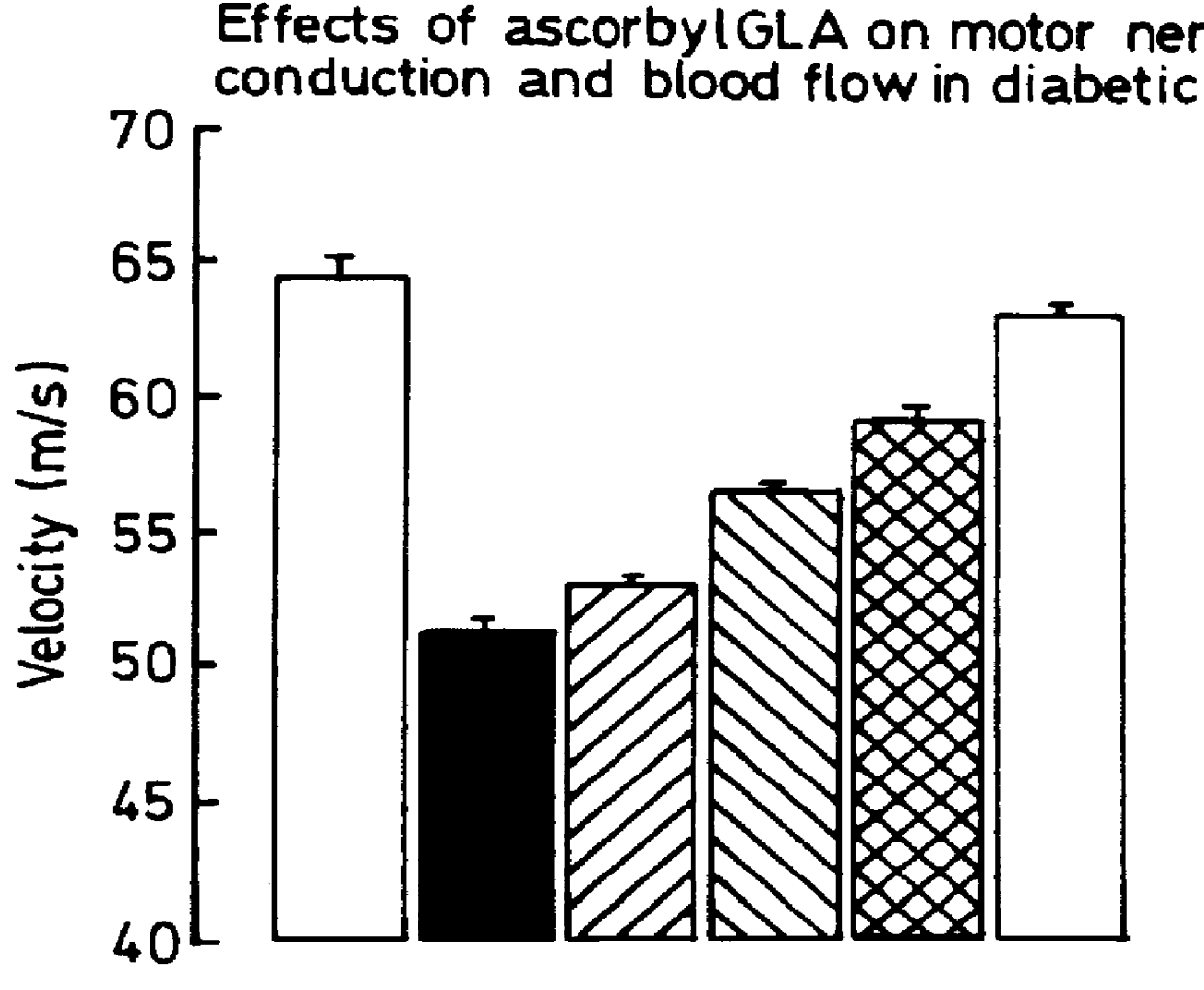
Compositions for treatment of diabetic complications
Use of 6-desaturated n-6 fatty acids, especially gammalinolenic acid (GLA), dihomogammalinolenic acid (DGLA) or arachidonic acid (AA), together with a pharmaceutically acceptable material reducing intracellular levels of sorbitol in the body, particularly an aldose reductase inhibitor, in the treatment of (including prophylactic treatment), and in the preparation of medicaments for the treatment of (including prophylactic treatment), the long-term complications of diabetes mellitus. Pharmaceutical compositions of said materials. The ascorbate esters of 6-desaturated n-6 fatty acids (other than GLA or DGLA) per se.
Owner:SCOTIA HLDG
Microneedles, Microneedle Arrays, Methods for Making, and Transdermal and/or Intradermal Applications
InactiveUS20100121307A1Reduce and even eliminate painMinimize drug deliveryMicroneedlesSurgeryMulti materialProphylactic treatment
Embodiments are directed to microneedle array devices for intradermal and / or transdermal interaction with the body of patient to provide therapeutic, diagnostic or preventative treatment wherein portions of the devices may be formed by multi-layer, multi-material electrochemical fabrication methods and wherein individual microneedles may include valve elements or other elements for controlling interaction (e.g. fluid flow). In some embodiments needles are retractable and extendable from a surface of the device. In some embodiments, interaction occurs automatically with movement across the skin of the patient while in other embodiments interaction is controlled by an operator (e.g. doctor, nurse, technician, or patient).
Owner:MICROFAB
Hybrid dynamic stabilization
InactiveUS20090248077A1Preventing and slowing down effectAvoid relative motionSuture equipmentsInternal osteosythesisDiseaseVertebral level
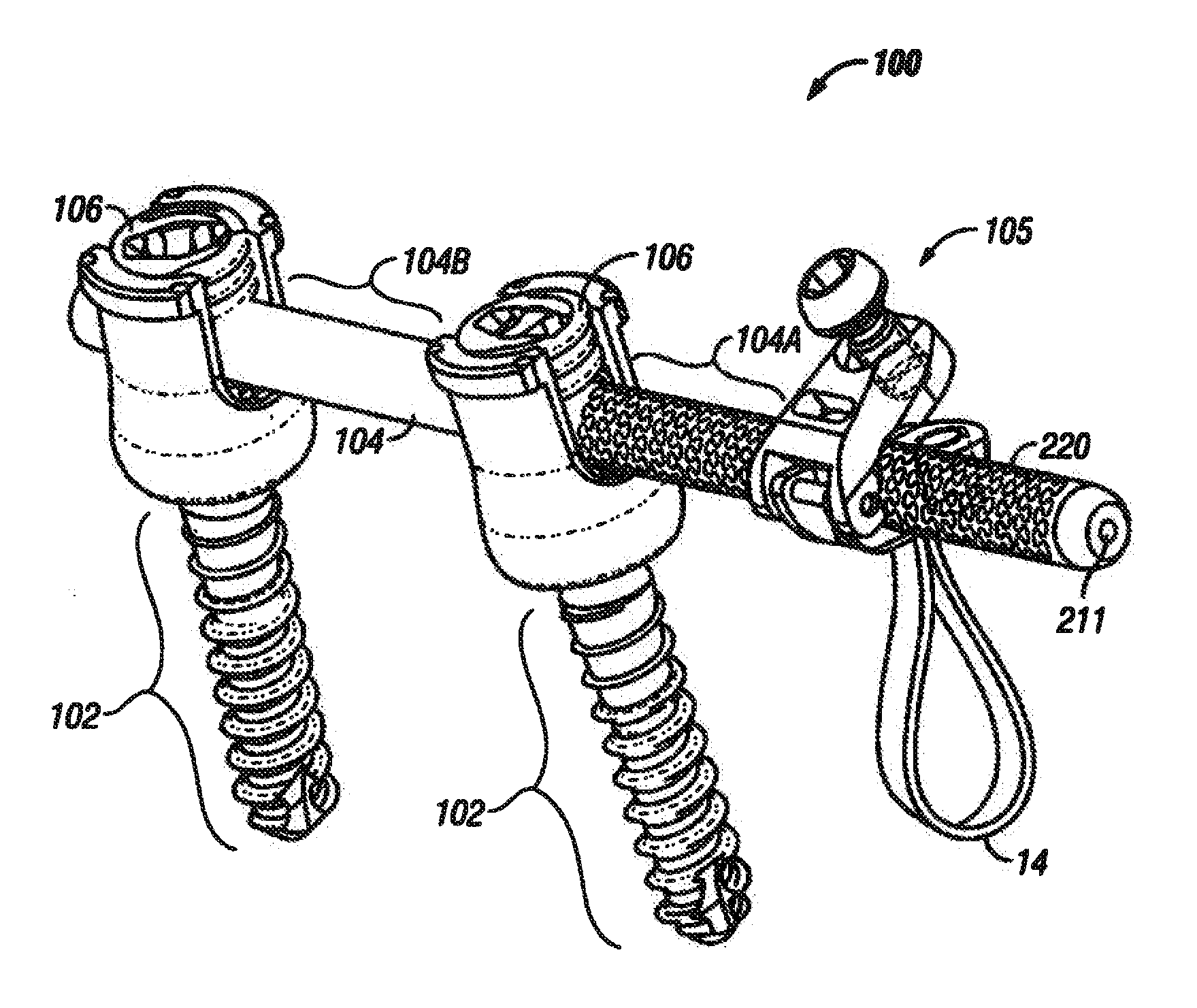
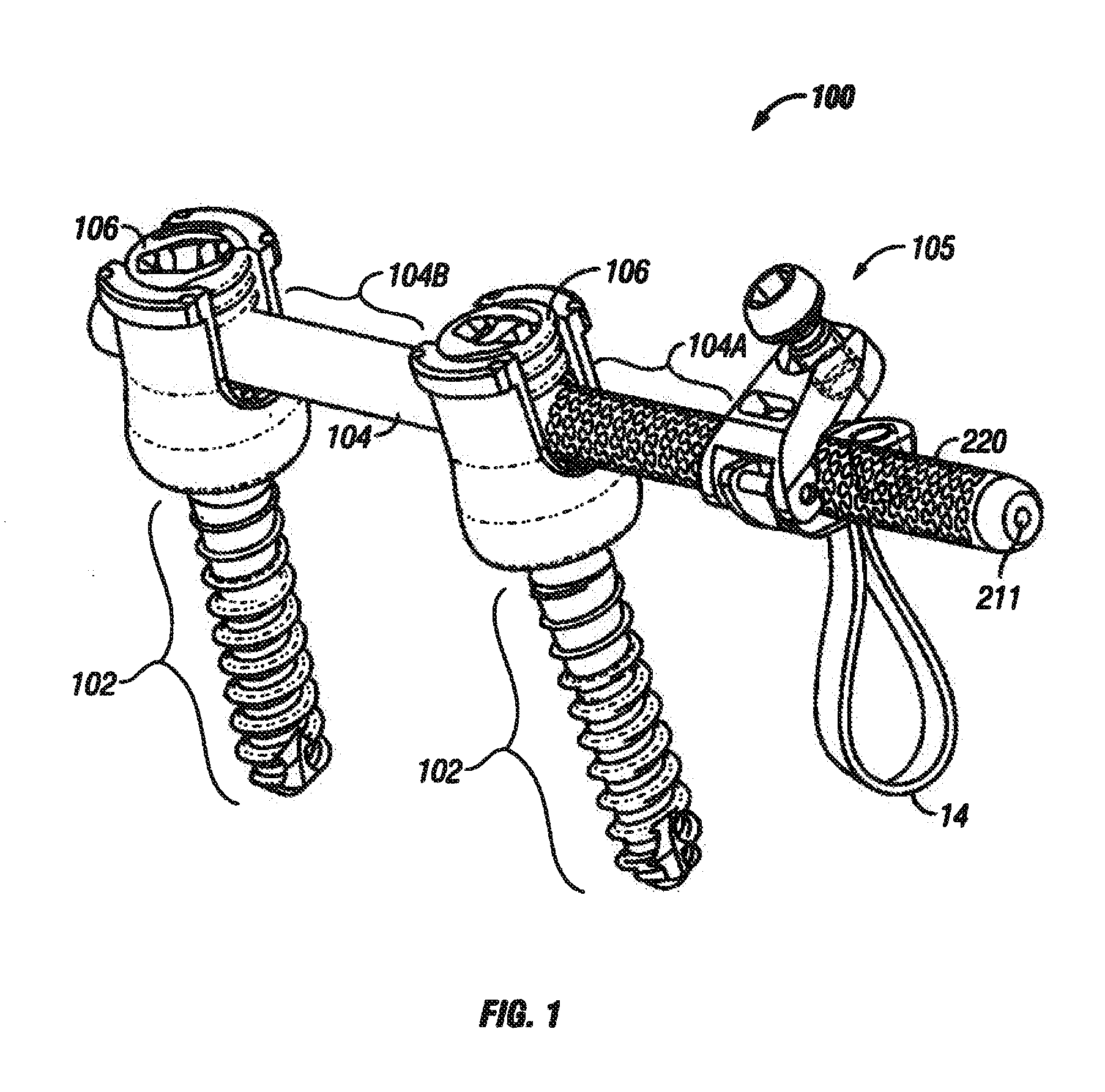
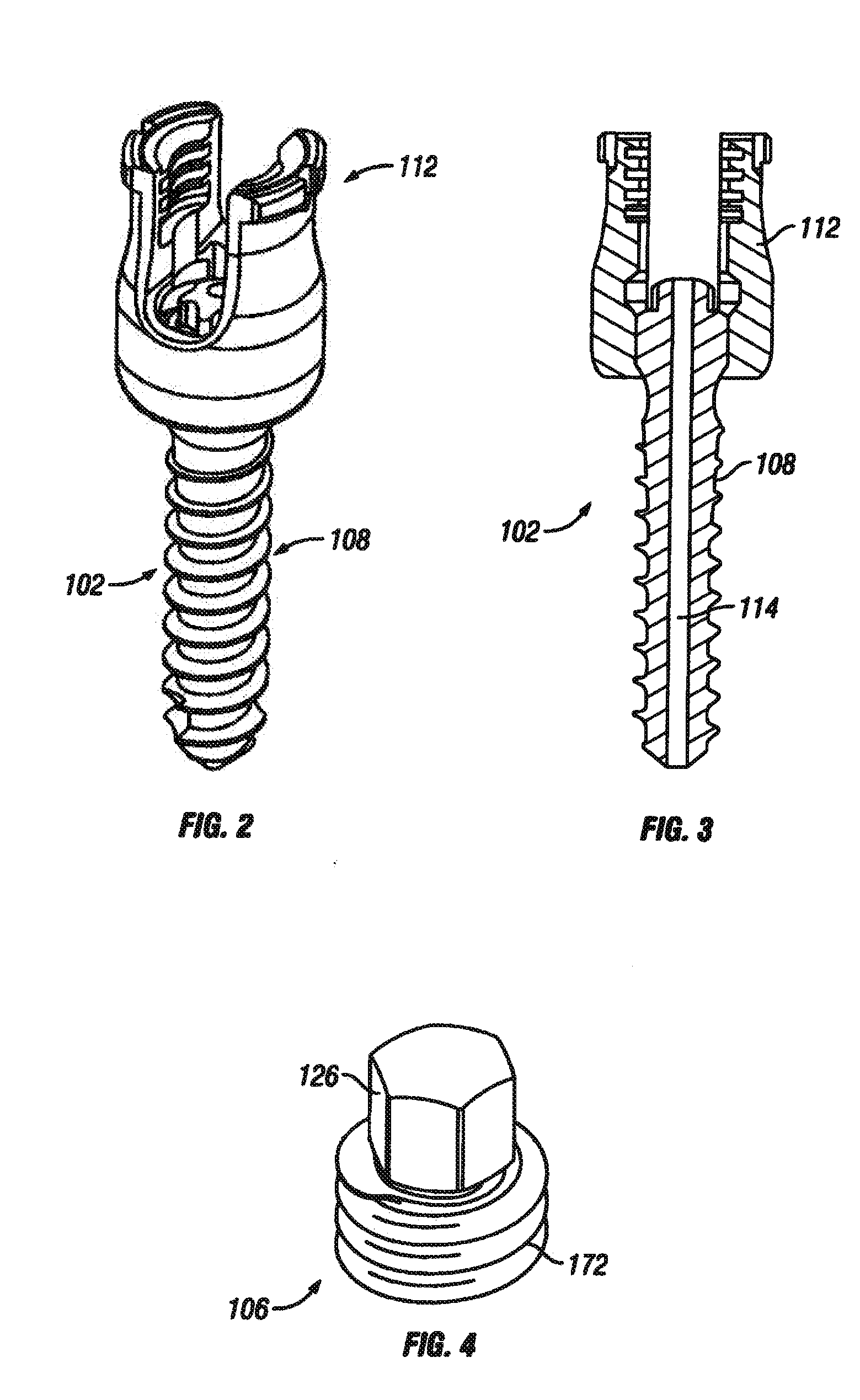
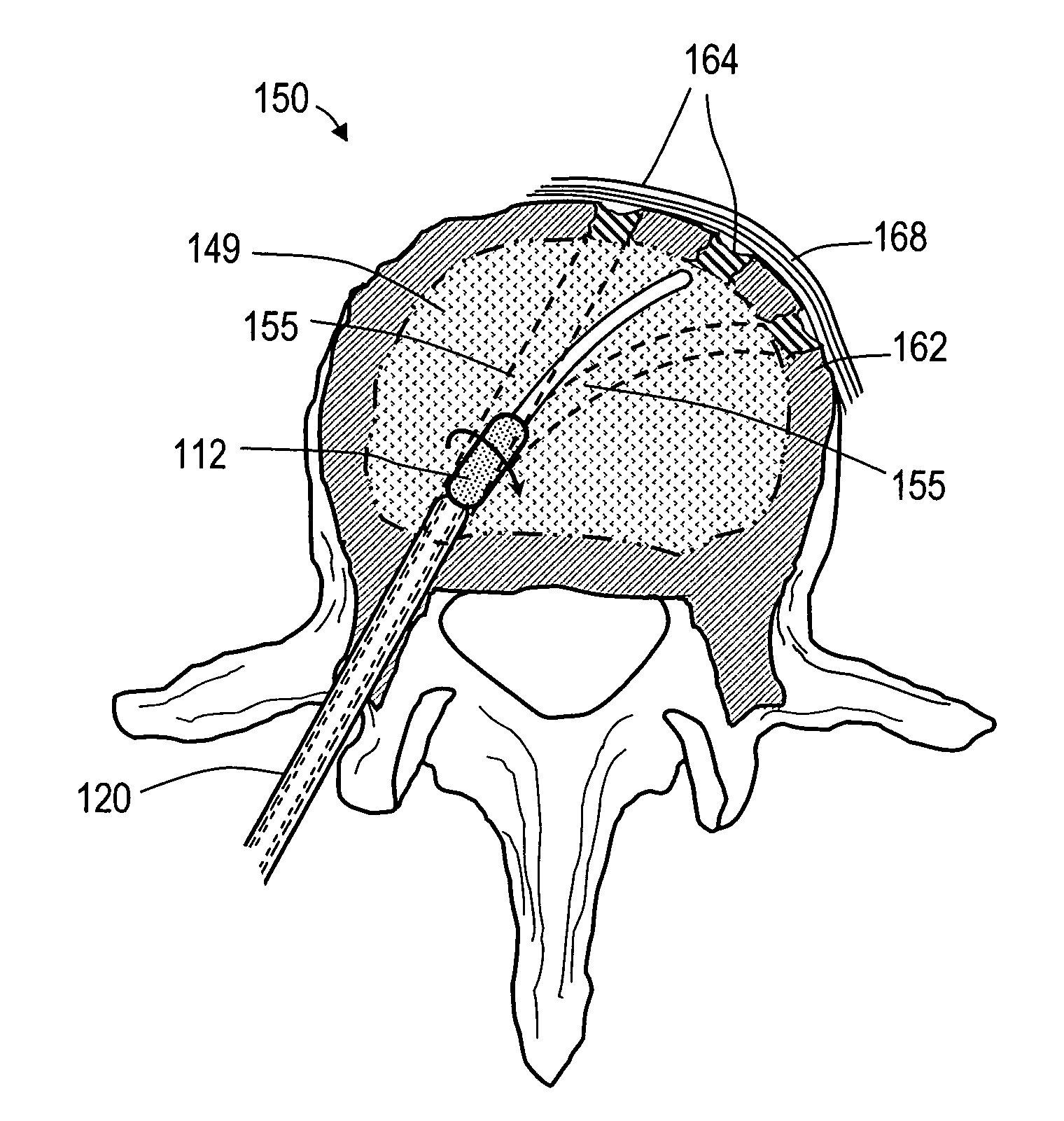
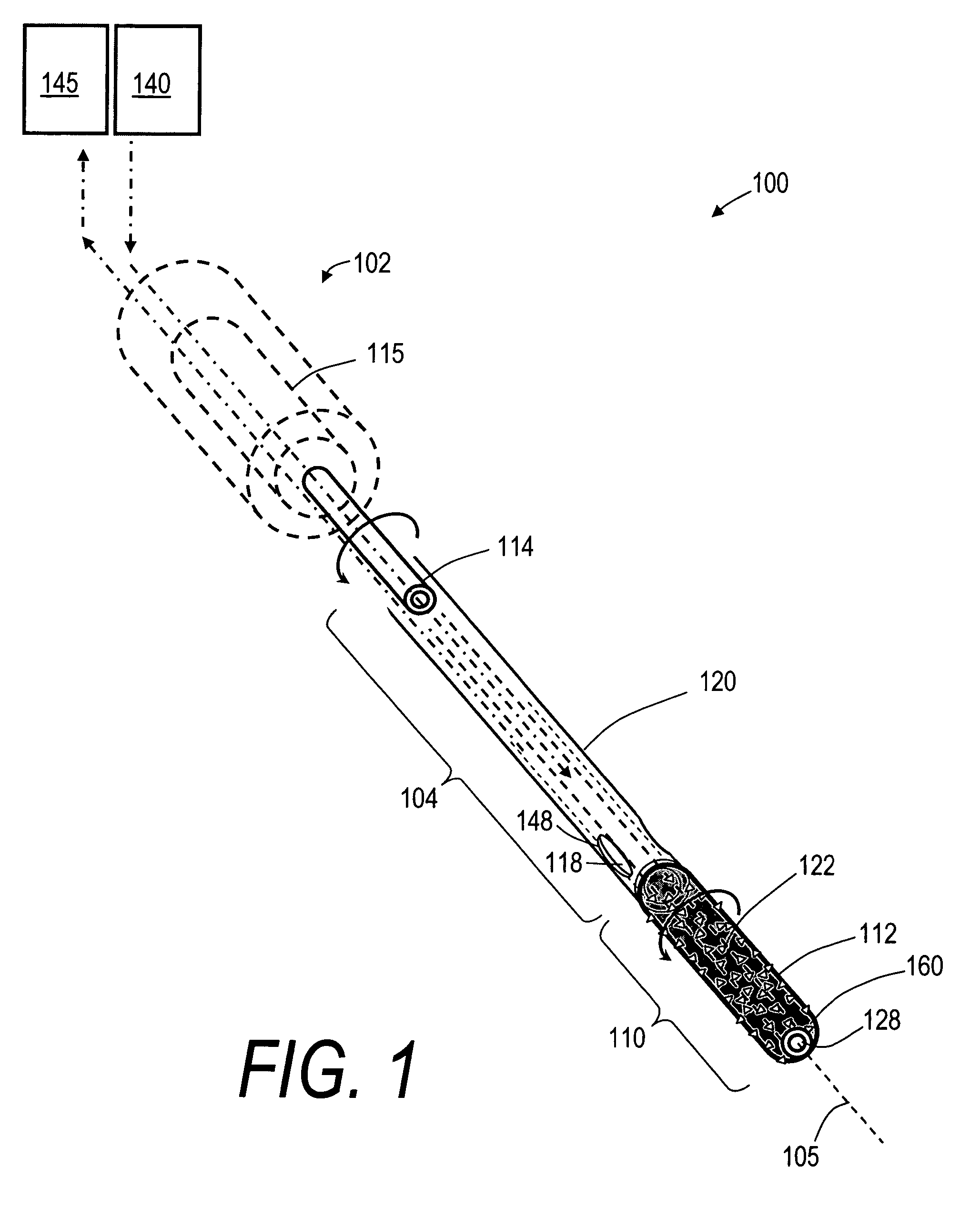
A spine stabilization for the prophylactic treatment of adjacent level disease. A first vertebral level may be fused by advancing a pedicle screw in the first and second vertebrae and coupling the bone screws to a rigid portion of a rod. A conformable ligature may be passed around a non-pedicle portion of a third vertebra and coupled to a dynamic portion of the rod using a blocking body. The dynamic properties of the dynamic portion of the rod allow movement of the third vertebra relative to the first and second vertebrae to slow down or prevent Adjacent Level Disease in the third vertebra.
Owner:ZIMMER SPINE INC +1
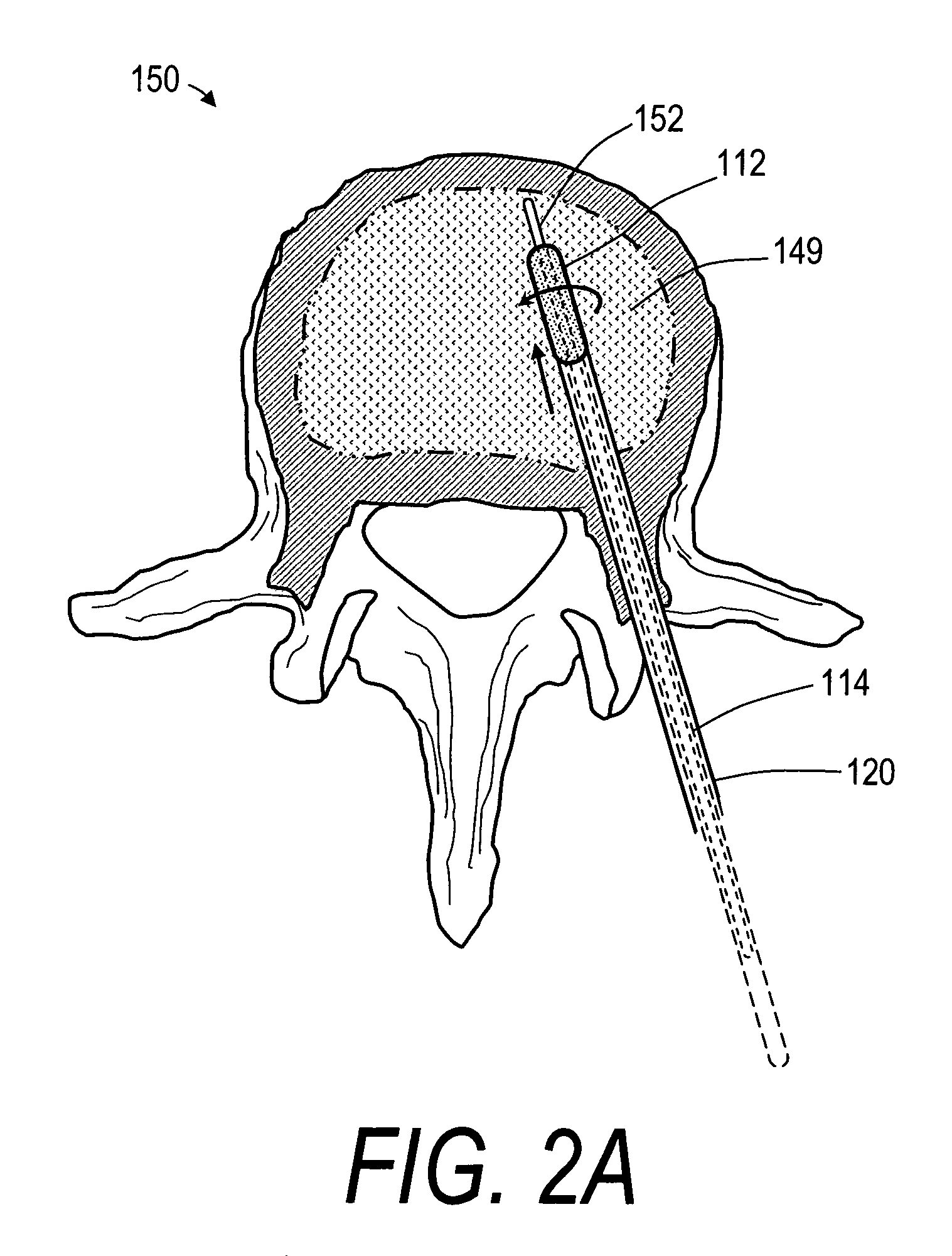
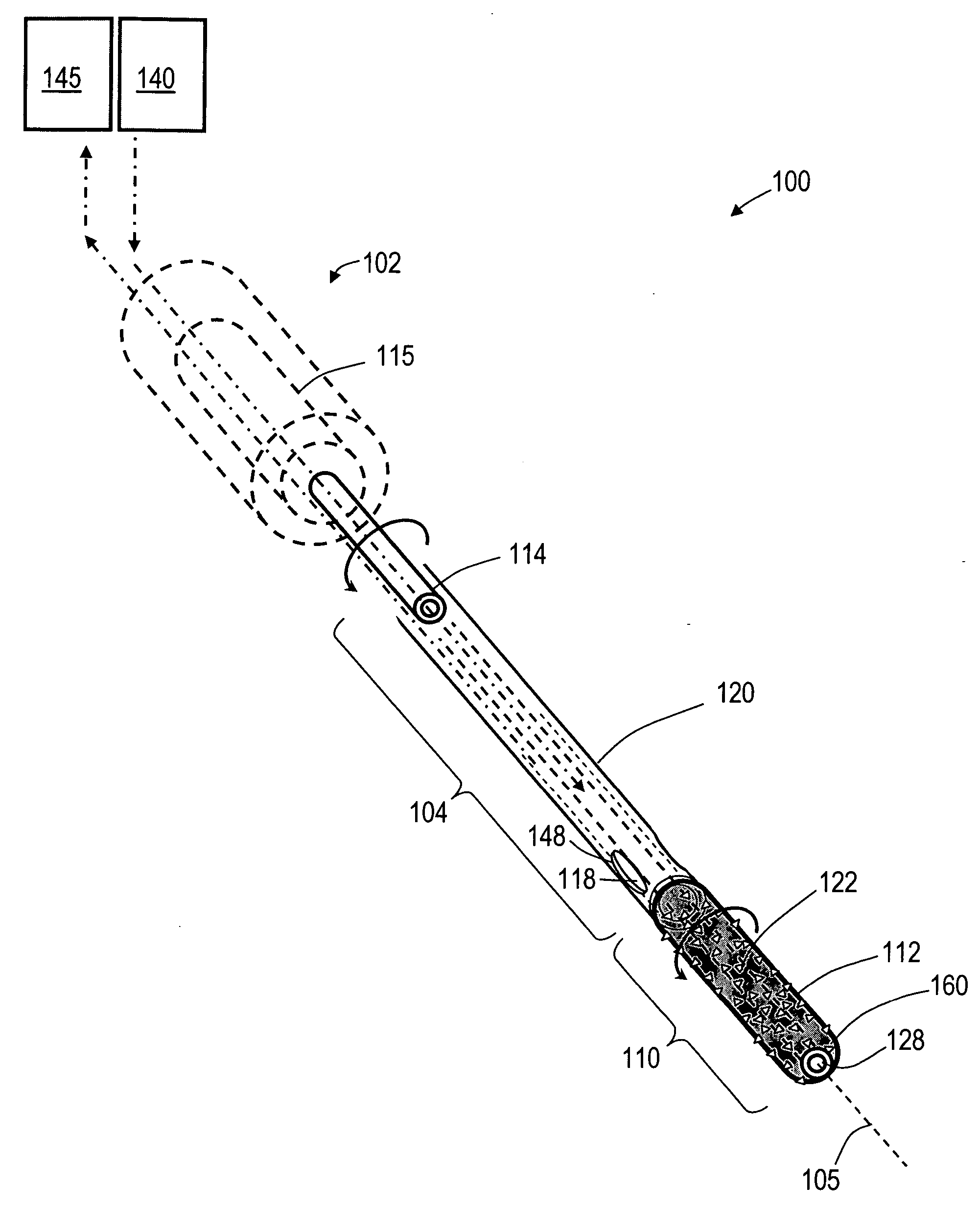
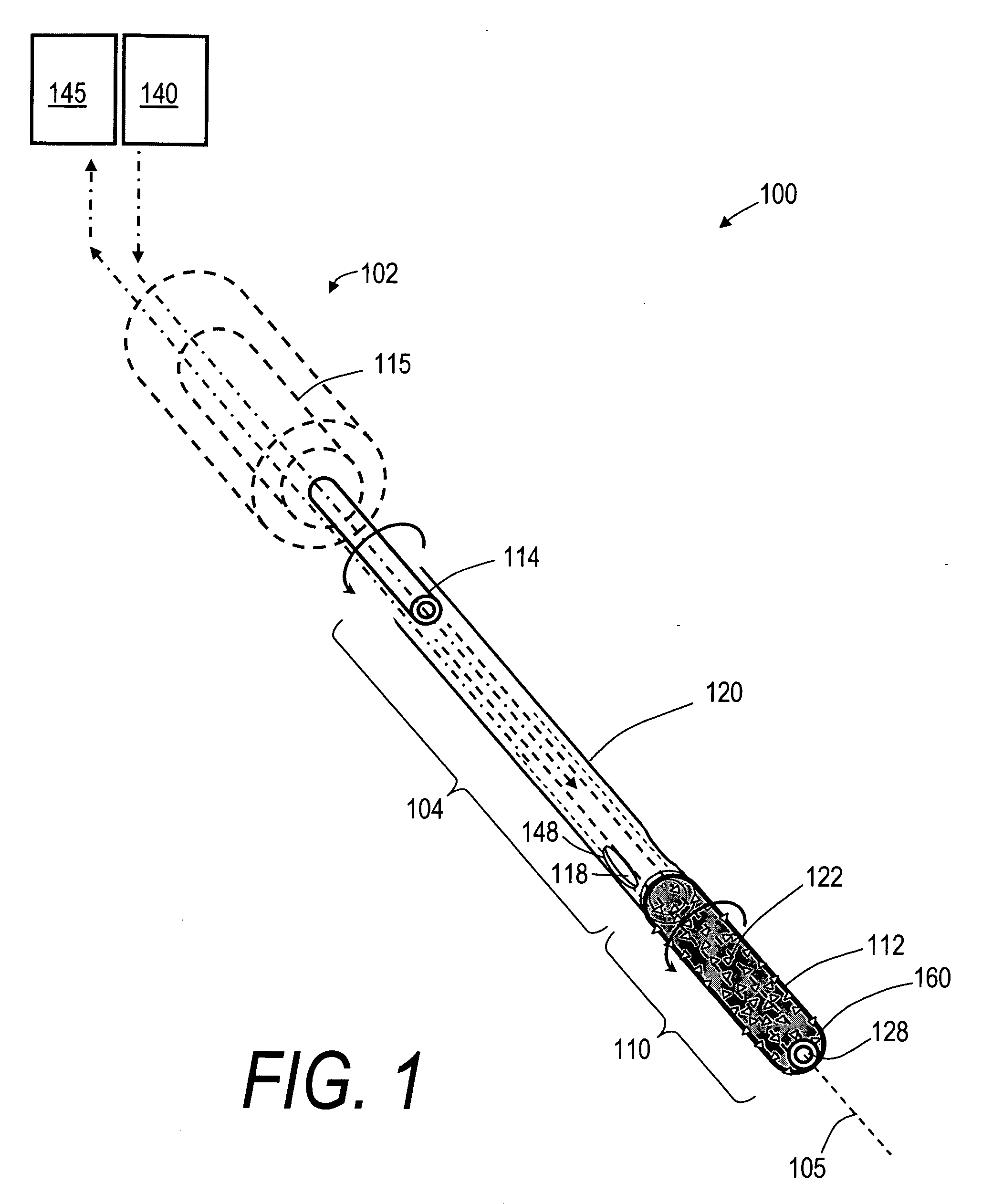
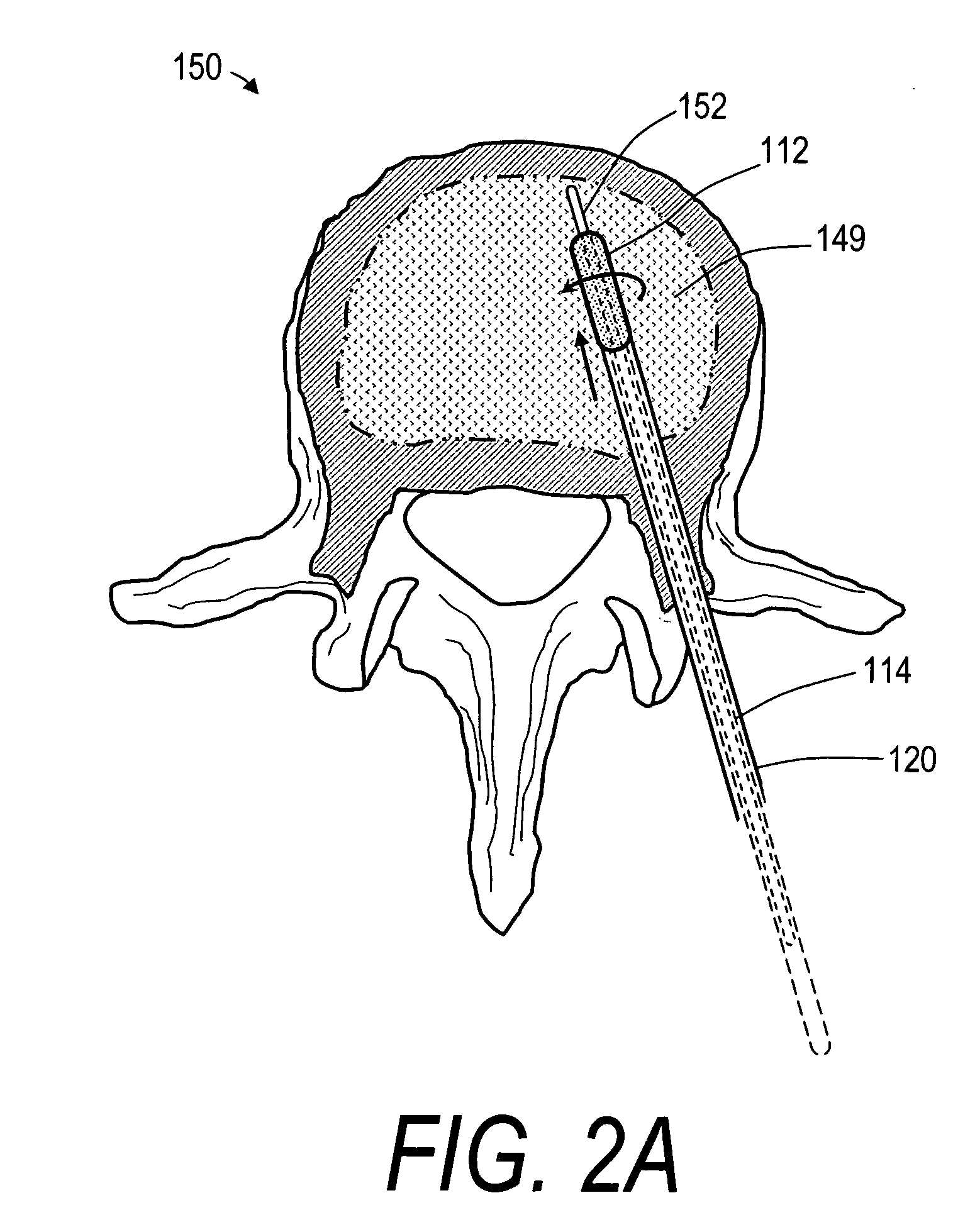
Bone treatment systems and methods for introducing an abrading structure to abrade bone
ActiveUS7682378B2Increase the sectionIncrease spacingJoint implantsExcision instrumentsMedicineProphylactic treatment
The invention provides instruments and methods for prophylactic treatment of an osteoporotic vertebral body or for treating a vertebral compression fracture (VCF). In one exemplary method, a probe system uses a high speed rotational elastomeric cutter having an optional expandable abrasive surface for abrading or cutting at least one path or region within vertebral cancellous bone. Irrigation and aspiration sources are included in the probe system for removing abraded bone debris. In one embodiment, the high speed rotational abrader uses a tissue-selective abrading surface that abrades or cuts bone but does not cut soft tissue. In another embodiment, an expandable abrading surface allows the treatment of bone with low pressures to create paths or spaces without explosive expansion forces known in prior art balloon procedures that are designed to crush and compact cancellous bone in a vertebra. After the creation of a path or space, an in-situ hardenable bone cement volume is introduced into each path or space to support the vertebra.
Owner:DFINE INC
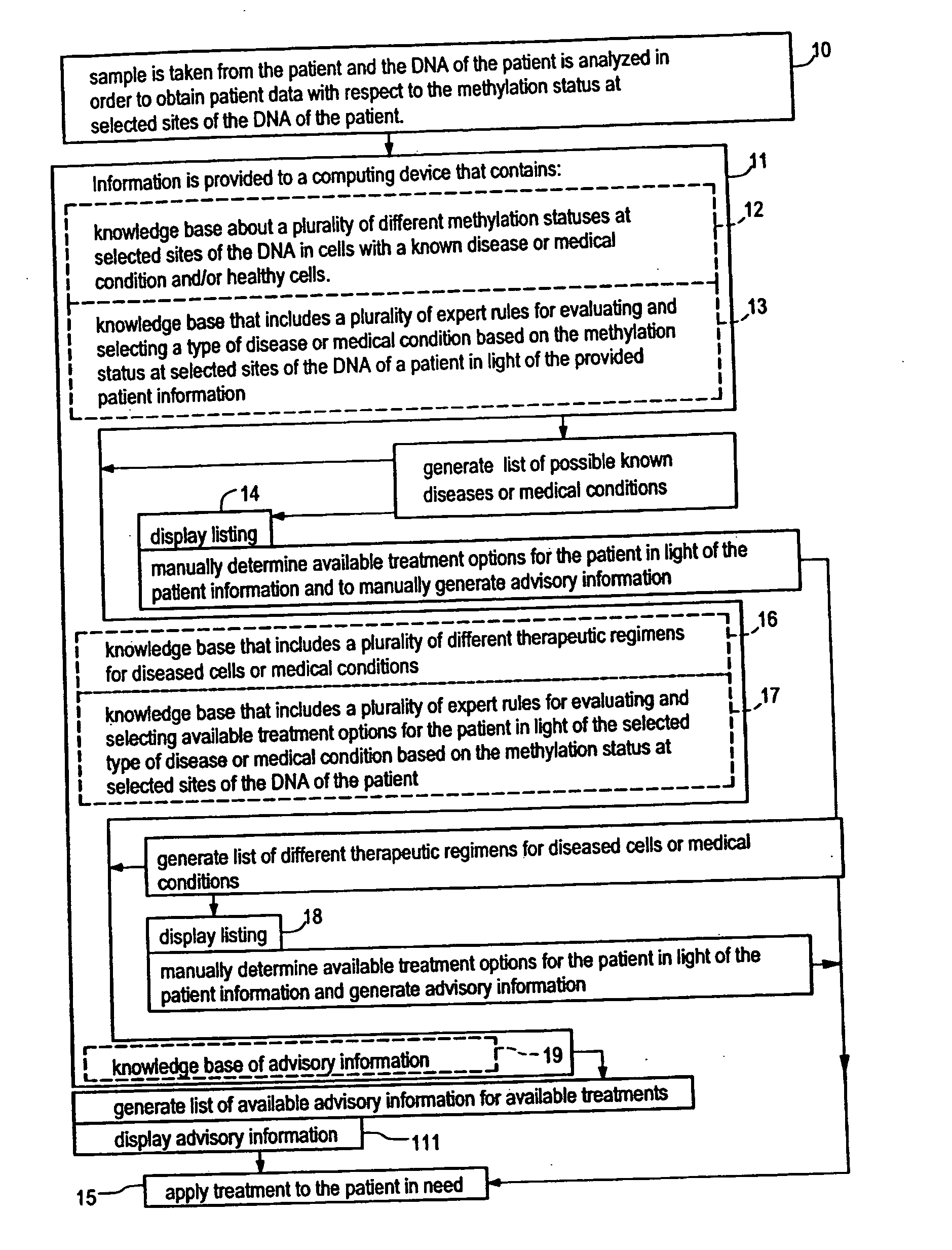
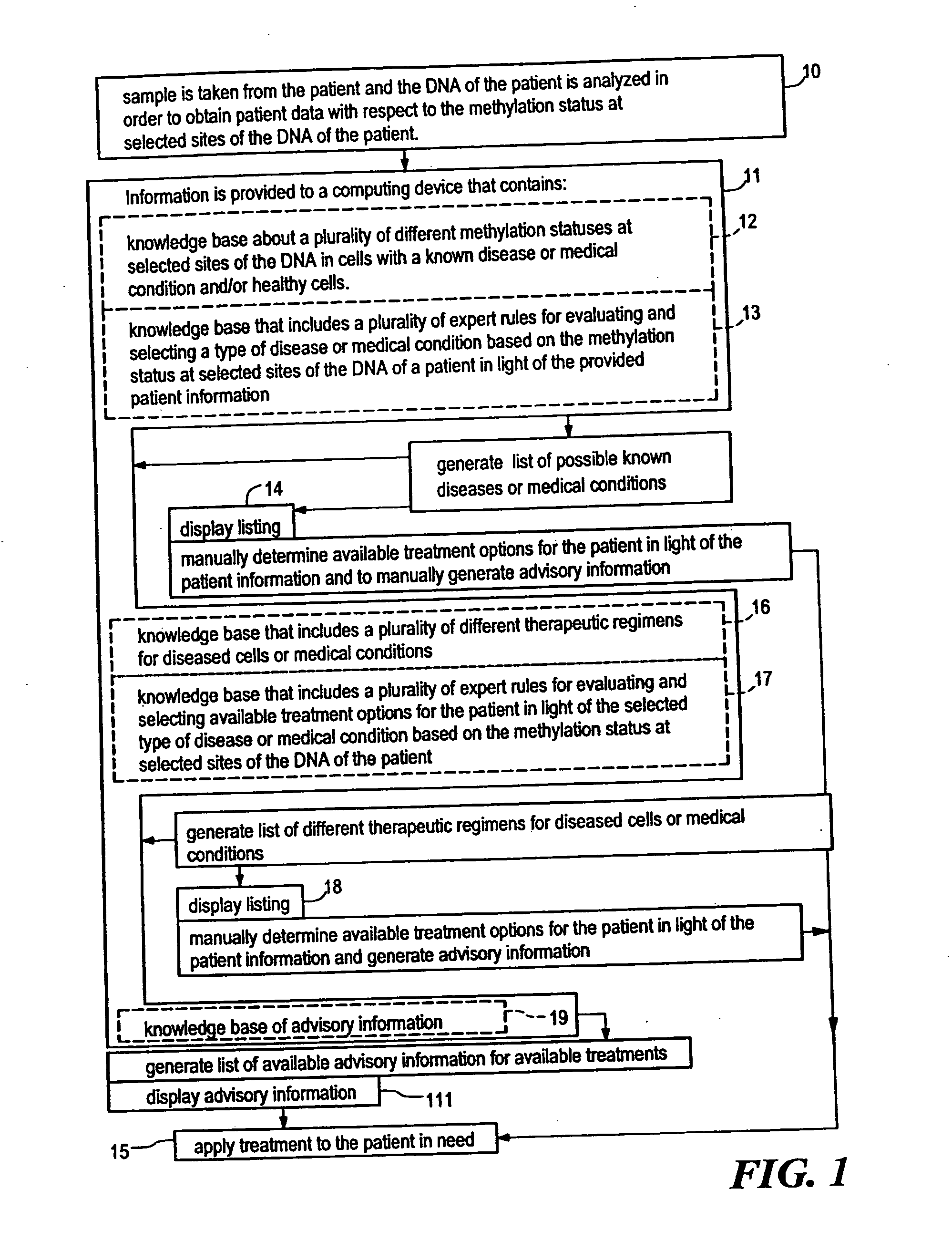
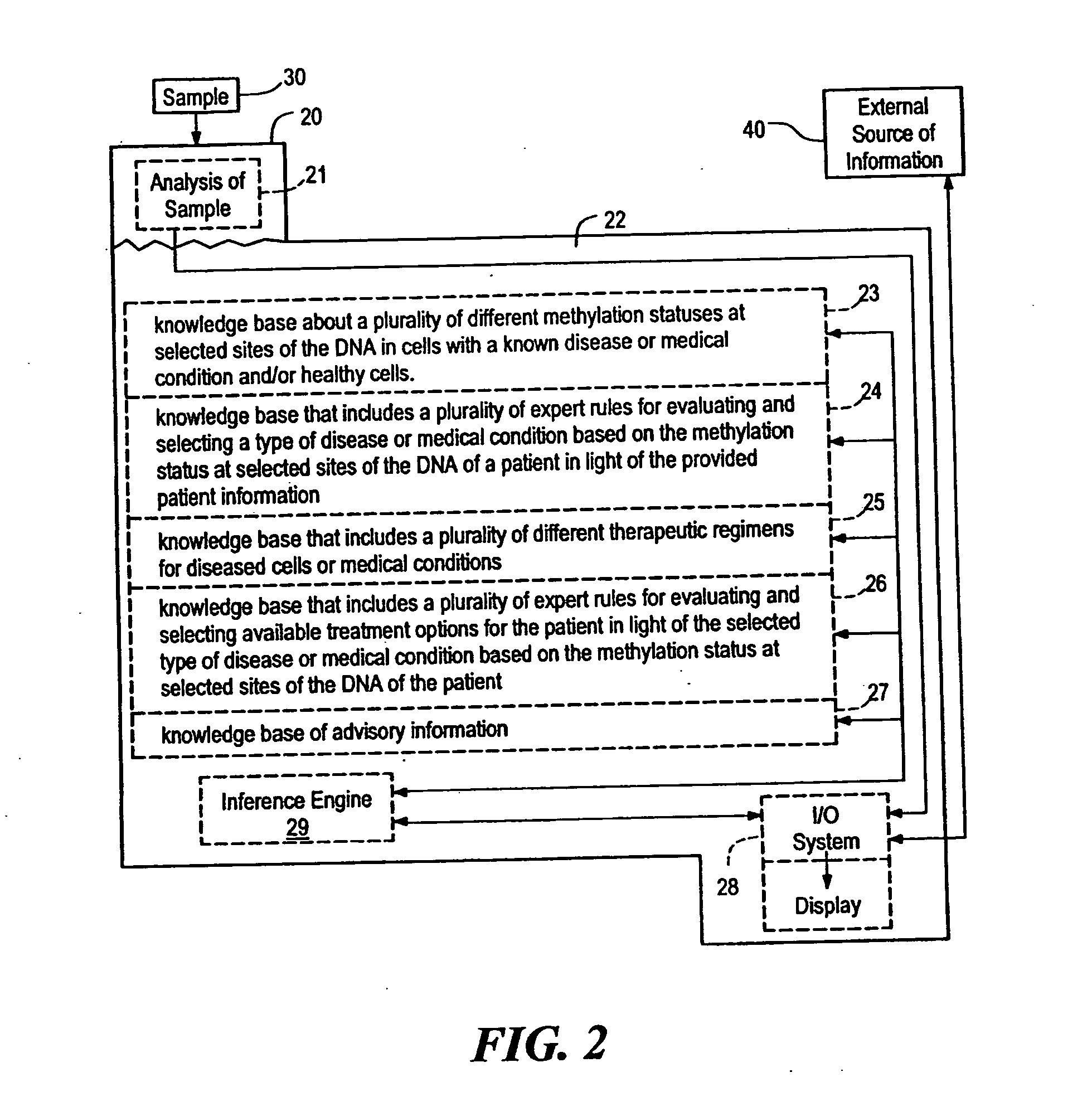
Systems, methods and computer program products for guiding selection of a therapeutic treatment regimen based on the methylation status of the DNA
InactiveUS20050021240A1Data processing applicationsMicrobiological testing/measurementRegimenProphylactic treatment
Systems, methods and computer program products for guiding selection of a therapeutic treatment regimen or a preventive therapeutic treatment regimen are disclosed. The method comprises (A) providing to a computing device comprising a first knowledge base comprising information about a plurality of different methylation statuses at selected sites of the DNA in cells with a known disease or medical condition and / or healthy cells, a second knowledge base comprising a plurality of expert rules for evaluating and selecting a type of disease or medical condition based on the methylation status at selected sites of the DNA of a patient, (B) generating in said computing device a ranked listing of diseases or medical conditions based on the information about the methylation status at selected sites of the DNA of the patient, the first knowledge base and the second knowledge base.
Owner:EPIGENOMICS AG
Bone treatment systems and methods
ActiveUS20060149268A1Increase the cross sectionIncrease spacingSurgeryIntravenous devicesProphylactic treatmentVertebra compression fracture
The invention provides instruments and methods for prophylactic treatment of an osteoporotic vertebral body or for treating a vertebral compression fracture (VCF). In one exemplary method, a probe system uses a high speed rotational cutter for abrading or cutting a plane within vertebral cancellous bone. Optional irrigation and aspiration sources are included in the probe system for removing abraded bone debris. In one embodiment, the high speed cutter uses a tissue-selective abrading surface that abrades or cuts bone but does not cut soft tissue. After the creation of a weakened cut plane in the bone, reduction of the fracture requires reduced forces.
Owner:DFINE INC
Methods of reducing risk of infection from pathogens
InactiveUS20050080093A1Reduce the risk of infectionAntibacterial agentsBiocideNatural sourceMedicine
Prophylactic treatment methods are provided for protection of individuals and / or populations against infection from airborne pathogens. In particular, prophylactic treatment methods are provided comprising administering a sodium channel blocker or pharmaceutically acceptable salts thereof to one or more members of a population at risk of exposure to or already exposed to one or more airborne pathogens, either from natural sources or from intentional release of pathogens into the environment.
Owner:PARION SCI DURHAM NC
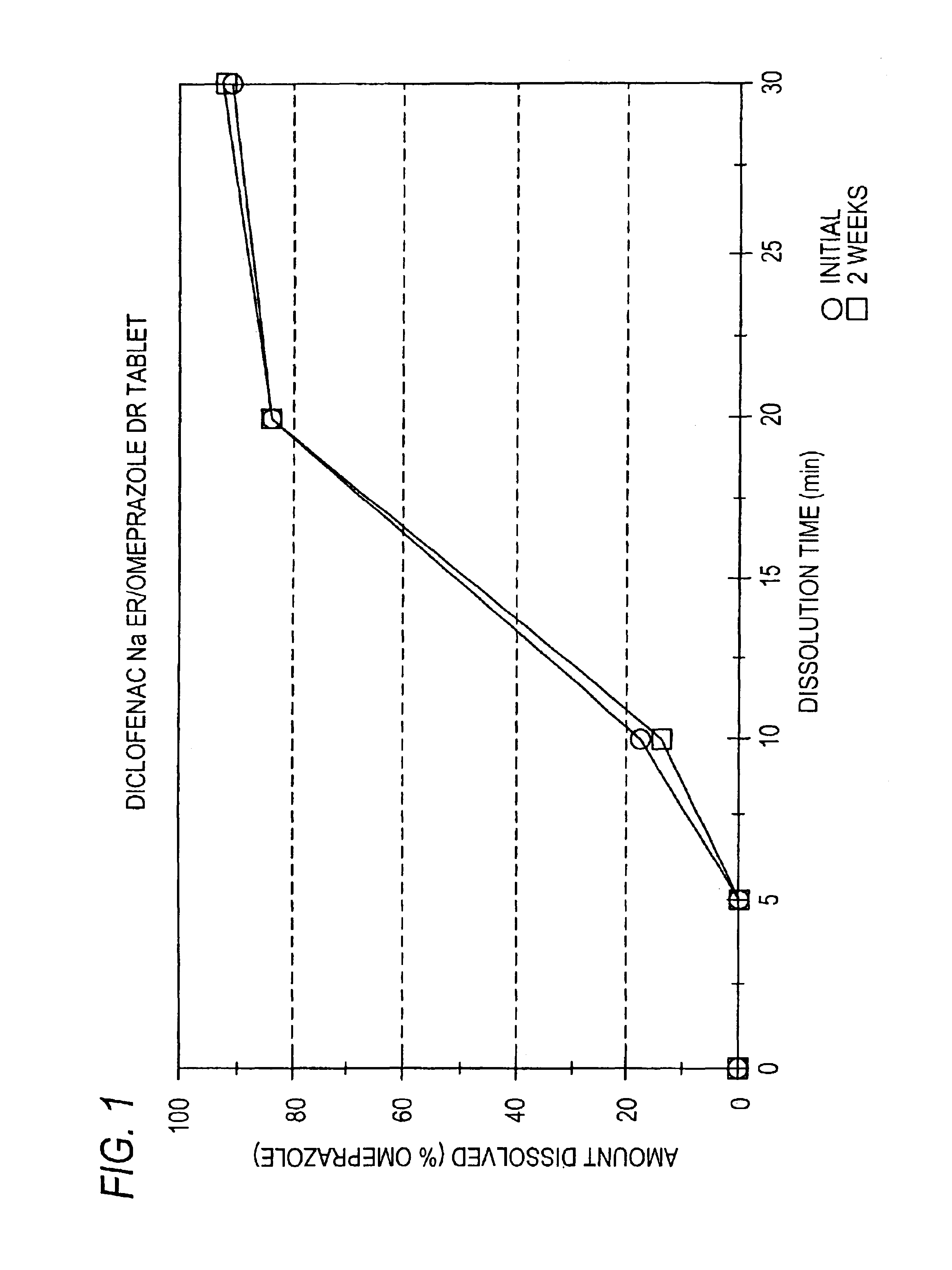
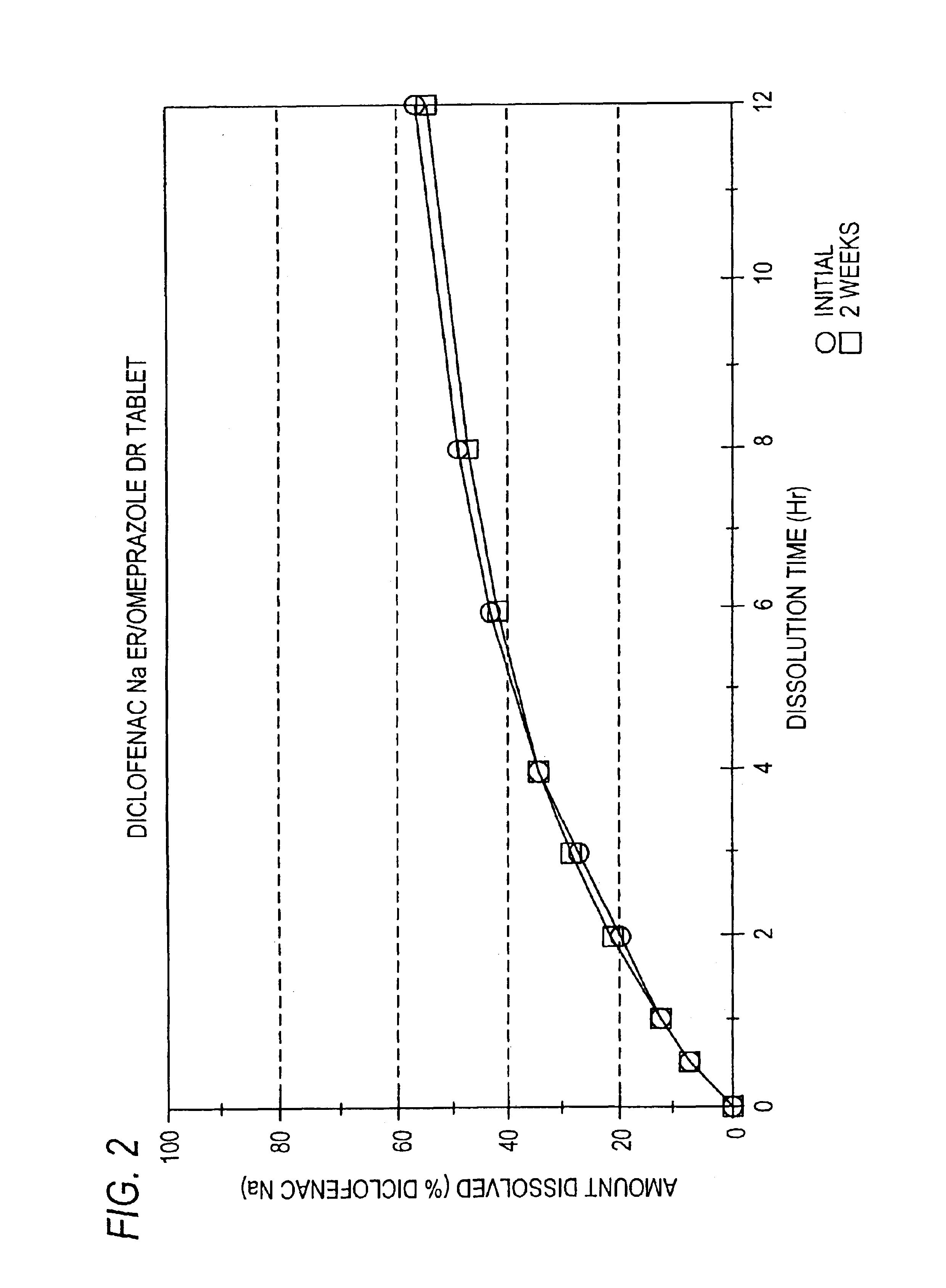
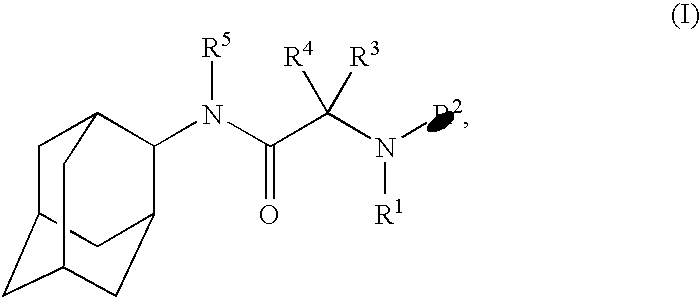
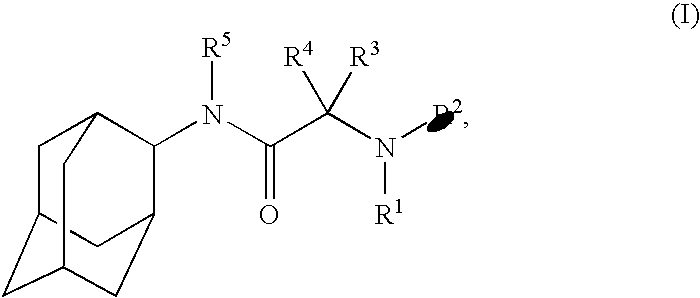
Pharmaceutical formulations containing a non-steroidal antiinflammatory drug and a proton pump inhibitor
InactiveUS6869615B2Decrease risk of development and exacerbationGood curative effectPowder deliveryAntipyreticSide effectDepressant
An oral solid dosage form includes a therapeutically effective amount of an NSAID and a proton pump inhibitor in an amount effective to inhibit or prevent gastrointestinal side effects normally associated with the NSAID. Also disclosed is a method of treating a human patient in need of antiinflammatory, analgesic and / or antipyretic therapy, comprising orally administering to the patient an oral pharmaceutical dosage form which includes a therapeutically effective amount of an NSAID and an amount of a proton pump inhibitor effective to substantially inhibit gastrointestinal side effects of the NSAID. The invention is further related to a method of prophylactically treating a human patient who is on a therapy known to have significant gastrointestinal side effects or is about to begin such a therapy, via concurrent administration of an NSAID and a proton pump inhibitor in a combination (single) oral dosage form.
Owner:ANDRX LABS
Inhibitors of the 11-beta-hydroxysteroid dehydrogenase Type 1 enzyme and their therapeutic application
The present invention relates to the use of inhibitors of the 11-beta-hydroxysteroid dehydrogenase Type 1 enzyme. The present invention further relates to the use of inhibitors of 11-beta-hydroxysteroid dehydrogenase Type 1 enzyme for the treatment or prophylactically treatment of non-insulin dependent type 2 diabetes, insulin resistance, obesity, lipid disorders, metabolic syndrome, and other diseases and conditions mediated by excessive glucocorticoid action.
Owner:ABBOTT LAB INC
Compositions and methods for treatment of tumors and metastatic diseases
InactiveUS6406689B1Stimulate immune responseSimple and reliable to useBiocideSnake antigen ingredientsDiseaseActive immunization
Owner:FALKENBERG FR W
Dosage unit for sublingual, buccal or oral administration of water-insoluble pharmaceutically active substances
ActiveUS20100008985A1Disperse fastEfficient packagingBiocidePowder deliveryWater insolubleProphylactic treatment
One aspect of the invention relates to a pharmaceutical dosage unit for sublingual, buccal, pulmonary or oral administration, said dosage unit having a weight of 20-500 mg and comprising 1-80 Wt. % of a microgranulate that is distributed throughout a solid hydrophilic matrix; said microgranulate being characterised in that it: has a volume weighted average diameter of 5-100 m; contains at least 0.01 wt. %, preferably at least 0.1 wt. % of one or more water-insoluble pharmaceutically active substances; contains at least 10 wt. %, preferably at least 20 wt. % of an emulsifier component; and is capable of forming a micro-emulsion upon contact with saliva or water. The dosage units of the present invention achieve the inherent benefits of oral delivery whilst at the same time realising a high transmucosal absorption rate of the cannabinoids contained therein. Other aspects of the present invention relate to the use of the aforementioned dosage units in the therapeutic or prophylactic treatment and to a process for the manufacture of said dosage units.
Owner:ECHO PHARM BV (NL)
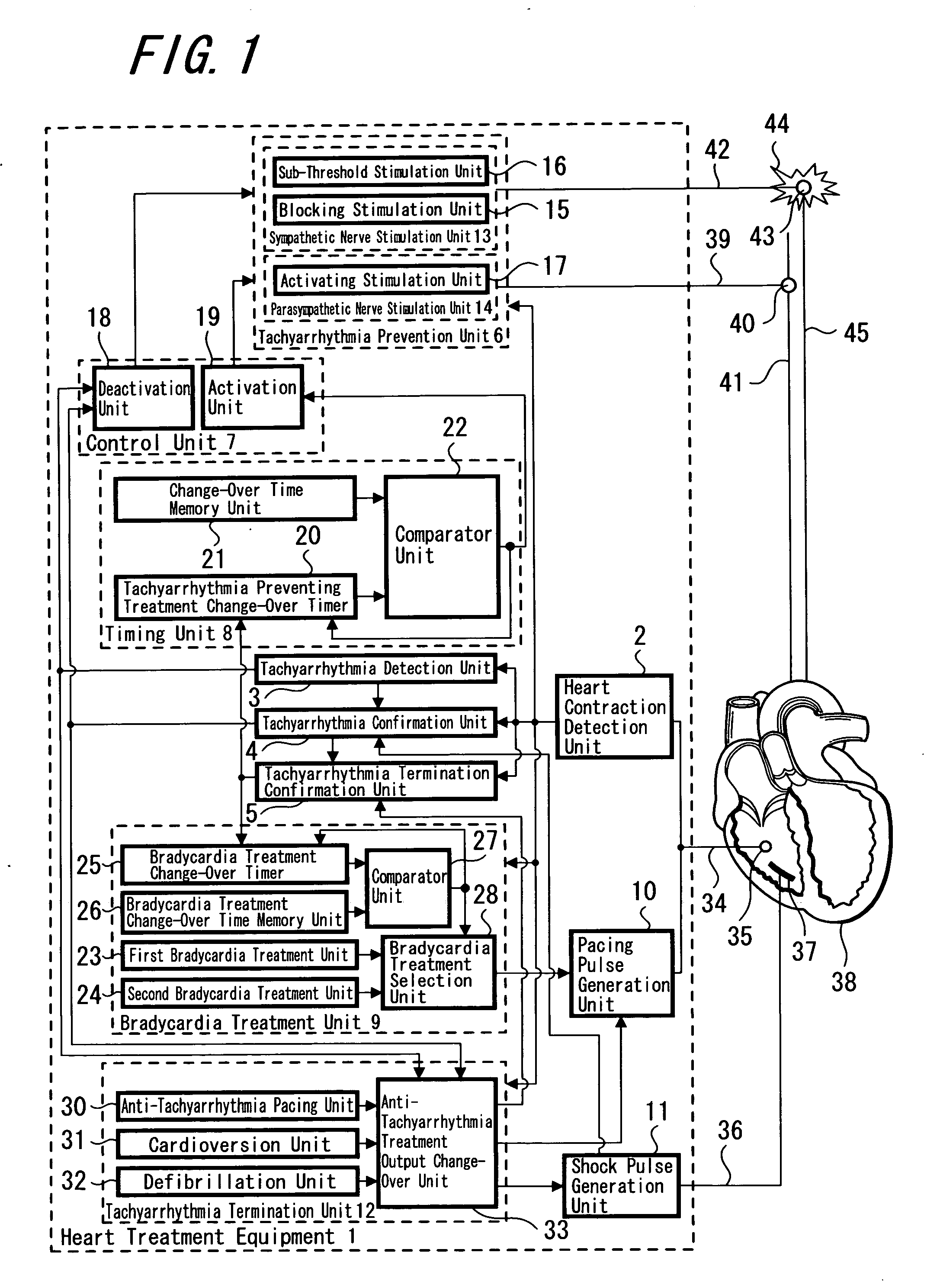
Heart treatment equipment and heart treatment method
InactiveUS20070100380A1Prevent deterioration of hemodynamicsReducing and stopping currentHeart defibrillatorsHeart stimulatorsTherapeutic DevicesSympathetic nerve
There is disclosed heart treatment equipment in which it is possible to carry out controlling of a preventive treatment after an anti-tachyarrhthmia treatment in order to execute prevention and treatment of a fatal arrhythmia and the anti-tachyarrhythmia treatment is carried out by controlling tachyarrhythmia prevention means and tachyarrhythmia treatment means (tachyarrhythmia termination means) according to the result of detecting occurrence of tachyarrhythmia. In the anti-tachyarrhythmia treatment, there is provided with a structure for reducing or stopping the activation current with respect to the vagus nerve or a repression current with respect to the sympathetic nerve after a supply of an electroshock to the heart such as cardioversion, defibrillation or the like, so that it is possible to prevent deterioration of hemodynamics, recurrence of fatal arrhythmia and supraventicular arrhythmia such as atrial fibrillation or the like.
Owner:TERUMO KK
Low-oil pharmaceutical emulsion compositions comprising progestogen
ActiveUS20110262494A1High progesterone solubilityElevation in histamine releaseOrganic active ingredientsNervous disorderIUD with progestogenTG - Triglyceride
Described are sterile, ready-to-use, pharmaceutical oil-in-water emulsion compositions for parenteral administration comprising:0.015 to 0.5% wt / vol progesterone;0.5 to 10% wt / vol oil, wherein the oil comprises at least 85% wt. / wt. triglyceride;0.0425 to 4.1% wt / vol phospholipid;80-99.4% wt / vol aqueous medium;wherein the composition has an osmolality in the range of 200-1000 mOsm / kg.Also described are methods of making such compositions and method of using such compositions in therapeutic or prophylactic treatment, such as treatments comprising intravenous administration of the pharmaceutical composition.
Owner:BESINS HEALTHCARE LUXEMBOURG (LU)
Use of rotigotine for treatment or prevention of dopaminergic neurone loss
The invention relates to the use of rotigotine or salts thereof and prodrugs for the production of a medicament for the treatment or prevention of dopaminergic cell destruction in diseases which are connected to increased dopaminergic cell destruction. The invention also relates to the use of rotigotine as a medicament for the preventive treatment of Parkinson's disease.
Owner:UCB SA
Methods for prevention and treatment of gastrointestinal disorders
InactiveUS20050004222A1High activityPrevent and reduce severity of and symptomBiocidePeptide/protein ingredientsGastrointestinal disorderHuman patient
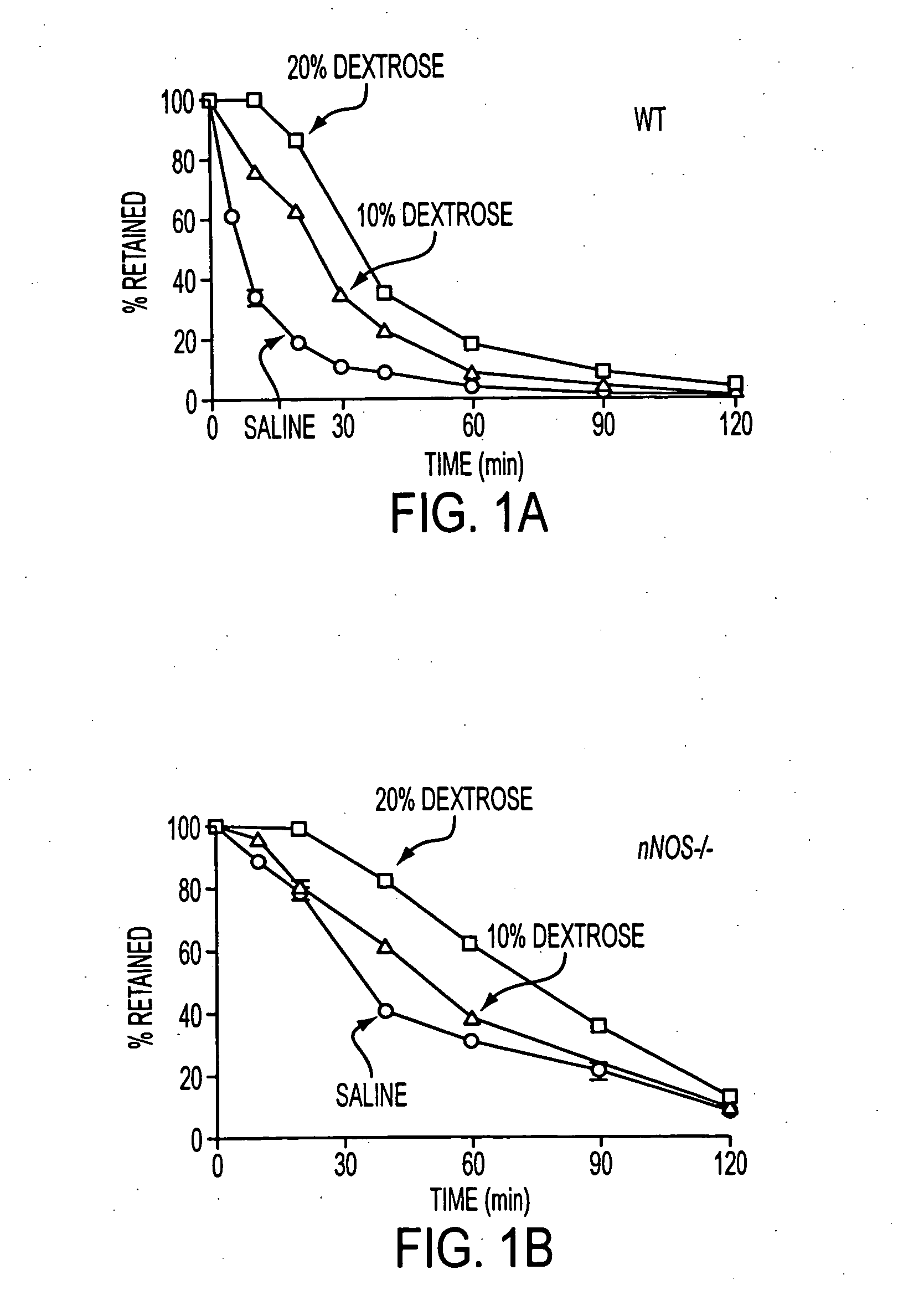
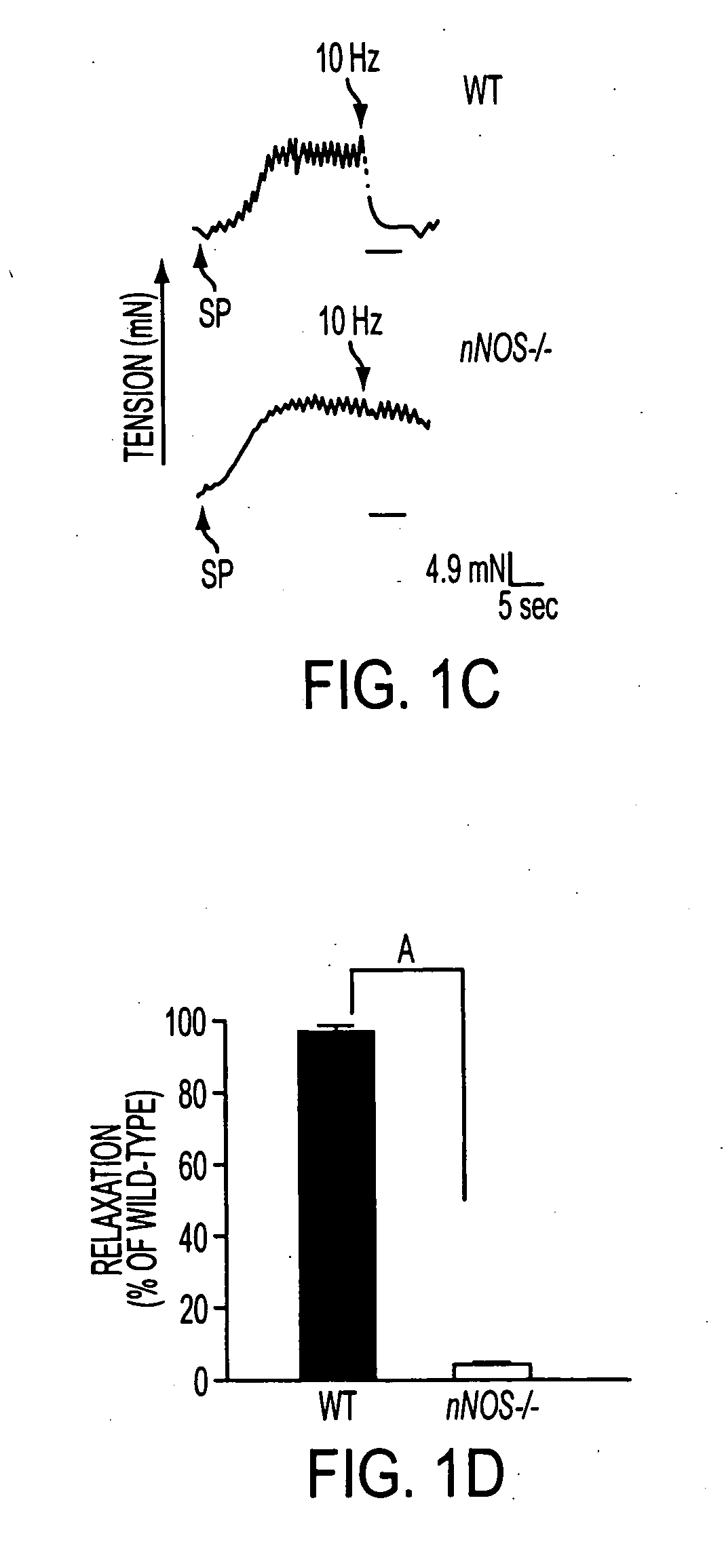
Disclosed are methods for preventing or treating a gastrointestinal (GI) disorder in a mammal such as a human patient. In one embodiment, the methods include administering to the mammal a therapeutically effective amount of a compound that modulates a nitric oxide (NO) signaling pathway, particularly in GI neurons. Methods of the invention are particularly useful for the treatment (including prophylactic treatment) of diabetic gastropathies and other GI disorders.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Retrograde transport of sirna and therapeutic uses to treat neurologic disorders
InactiveUS20080039415A1Enhanced uptake into neural cellsSpecial deliveryGenetic material ingredientsDiseaseNervous system
Methods of treating disorders affecting the central nervous system (CNS) are disclosed. More particularly, methods of treating neurological disorders are disclosed which show therapeutic or prophylactic treatment of a mammalian CNS disorder by effecting local administration of an iRNA agent, followed by retrograde transport of the iRNA agent away from the administration site and onto multiple regions within the CNS. This retrograde transport of iRNA results in an improved therapeutic involvement for the respective iRNA agent.
Owner:ALNYLAM PHARMA INC +1
Electromagnetic apparatus for prophylaxis and repair of ophthalmic tissue and method for using same
InactiveUS20080058793A1Increase blood flowPromote neovascularizationElectrotherapySurgical instruments for heatingOptic nerveProphylactic treatment
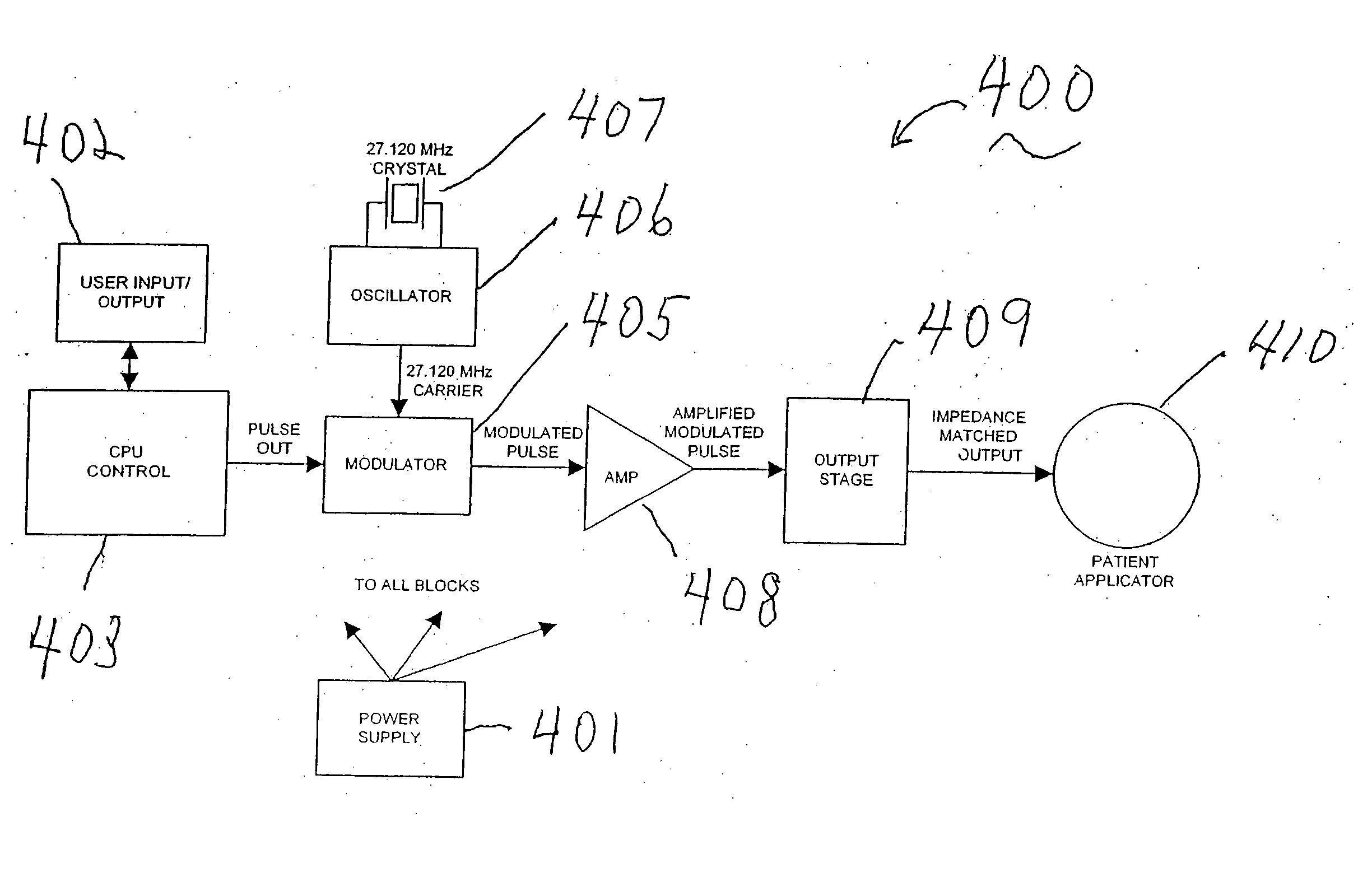
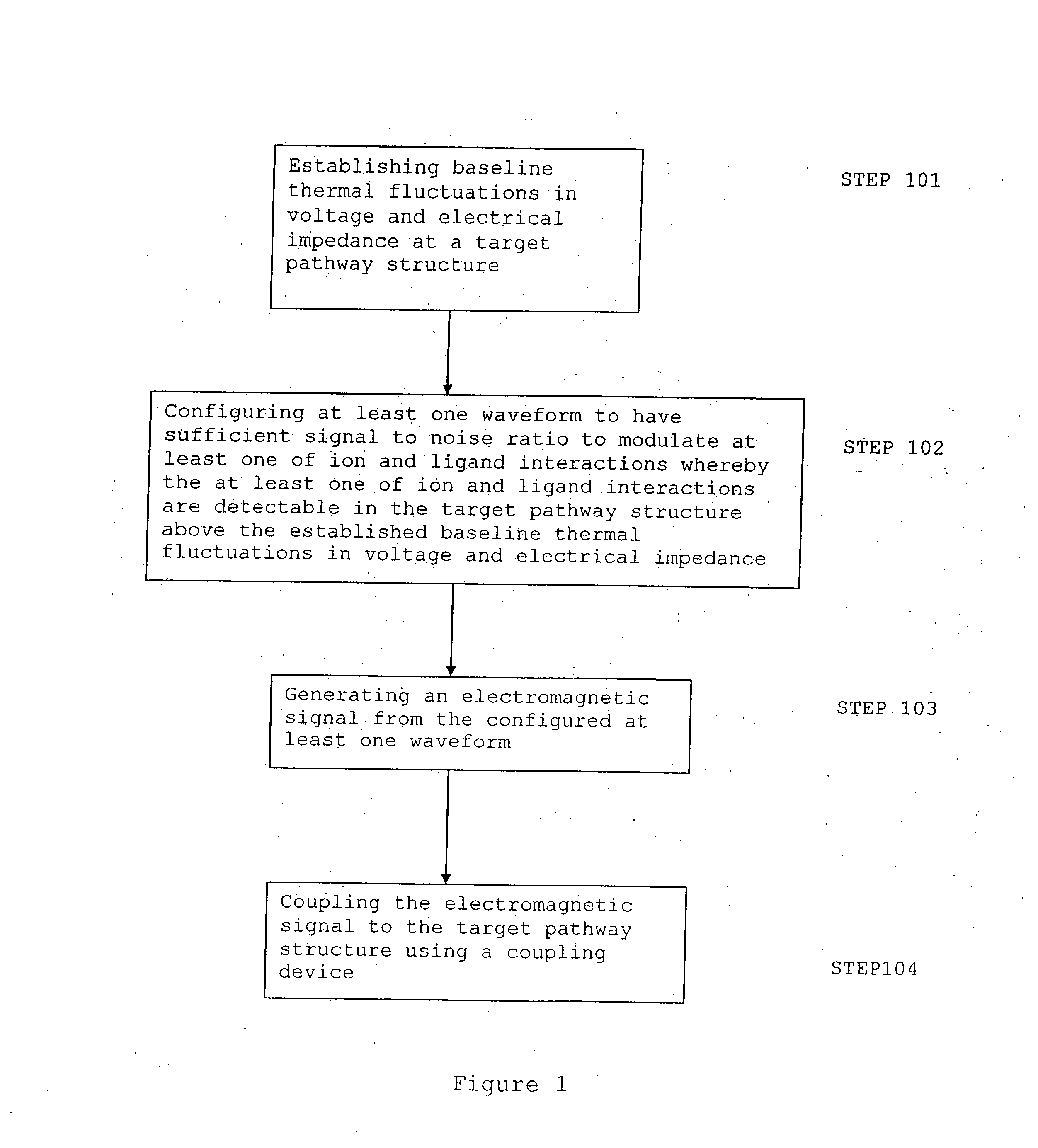
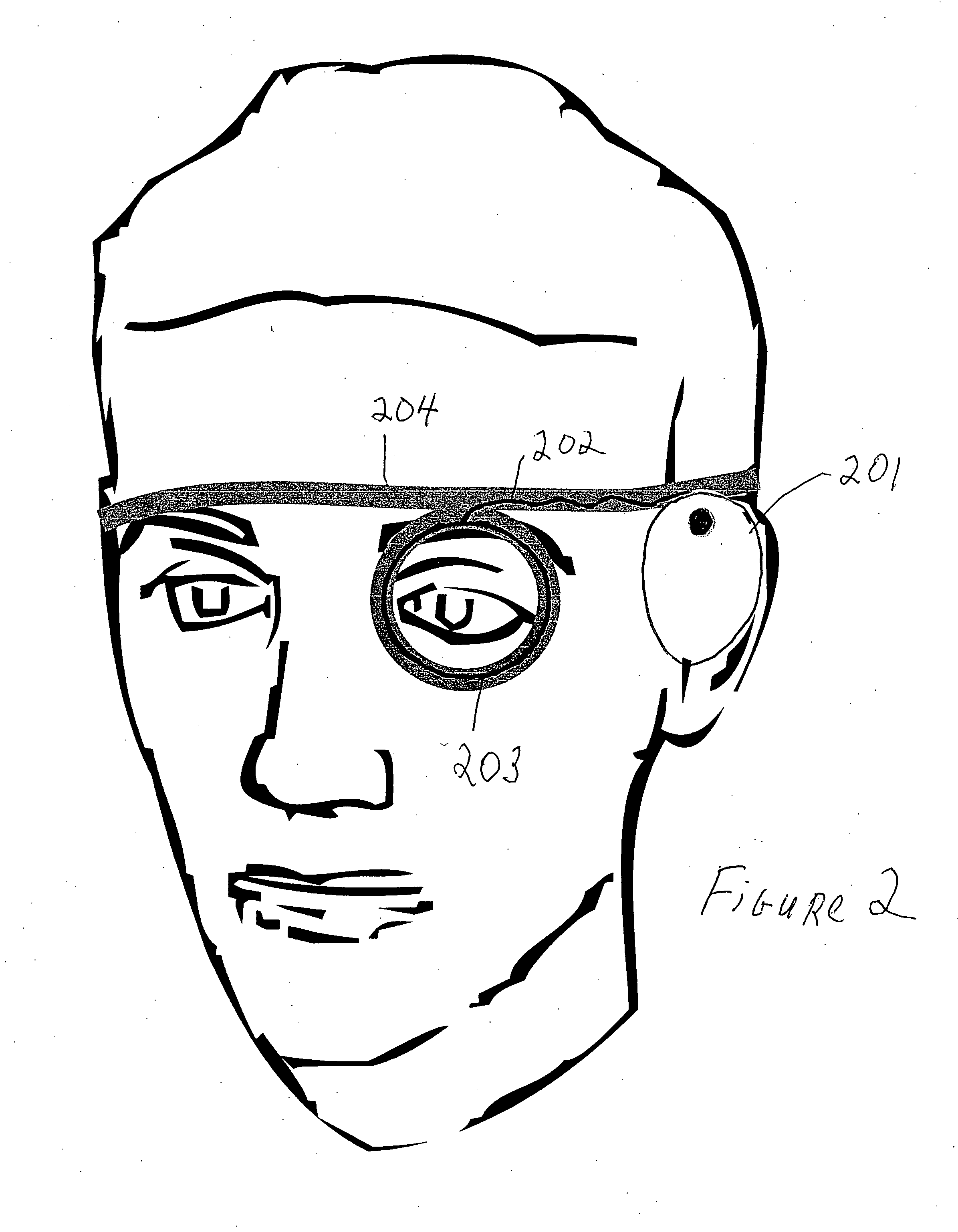
An apparatus and method for altering the electromagnetic environment of ophthalmic tissues, cells, and molecules comprising, establishing baseline thermal fluctuations in voltage and electrical impedance at a target pathway structure depending on a state of at least one of the ophthalmic tissue components, configuring at least one waveform to have sufficient signal to noise ratio to modulate at least one of ion and ligand interactions whereby the at least one of ion and ligand interactions are detectable in the target pathway structure above the established baseline thermal fluctuations in voltage and electrical impedance, generating an electromagnetic signal from the configured at least one waveform, and coupling the electromagnetic signal to the target pathway structure using a coupling device whereby enhancing the release of second messengers, such as NO, growth factors and cytokines at the target pathway structure. The use of the within specified electromagnetic waveforms can have particular utility in the prophylactic treatment of ophthalmic tissue and the treatment of ophthalmic diseases such as macular degeneration, glaucoma, retinosa pigmentosa, repair and regeneration of optic nerve prophylaxis and other related diseases that respond positively to the physiological effects of these waveforms.
Owner:RIO GRANDE NEUROSCI
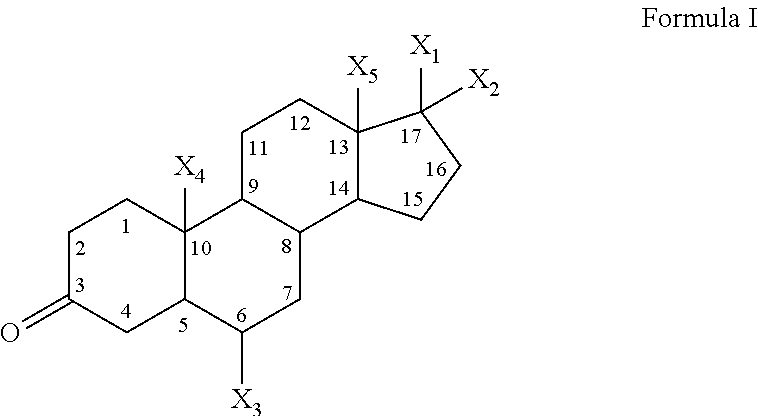
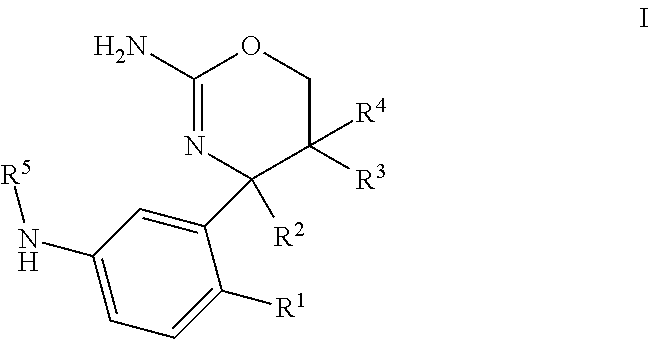
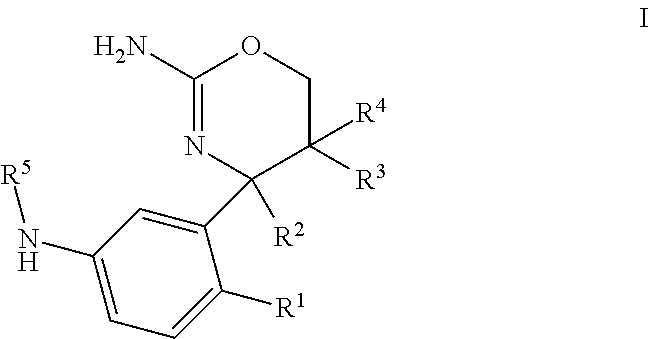
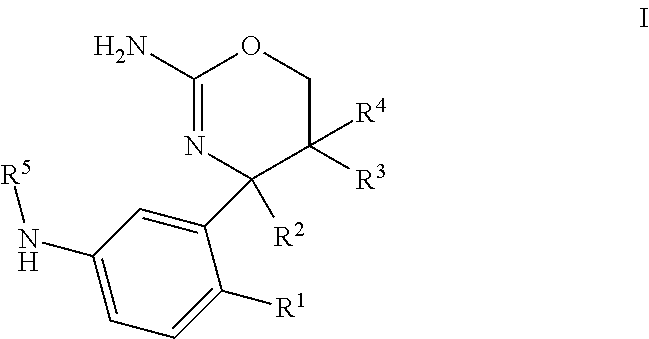
1,3-oxazines as bace1 and/or bace2 inhibitors
InactiveUS20120258962A1Improve propertiesOrganic active ingredientsNervous disorderDiseaseProphylactic treatment
The present invention provides 4-(3-Amino-phenyl)-5,6-dihydro-4H-[1,3]oxazin-2-ylamines of formula Ihaving BACE1 and / or BACE2 inhibitory activity, their manufacture, pharmaceutical compositions containing them and their use as therapeutically active substances. The active compounds of the present invention are useful in the therapeutic and / or prophylactic treatment of e.g. Alzheimer's disease and type 2 diabetes.
Owner:F HOFFMANN LA ROCHE & CO AG
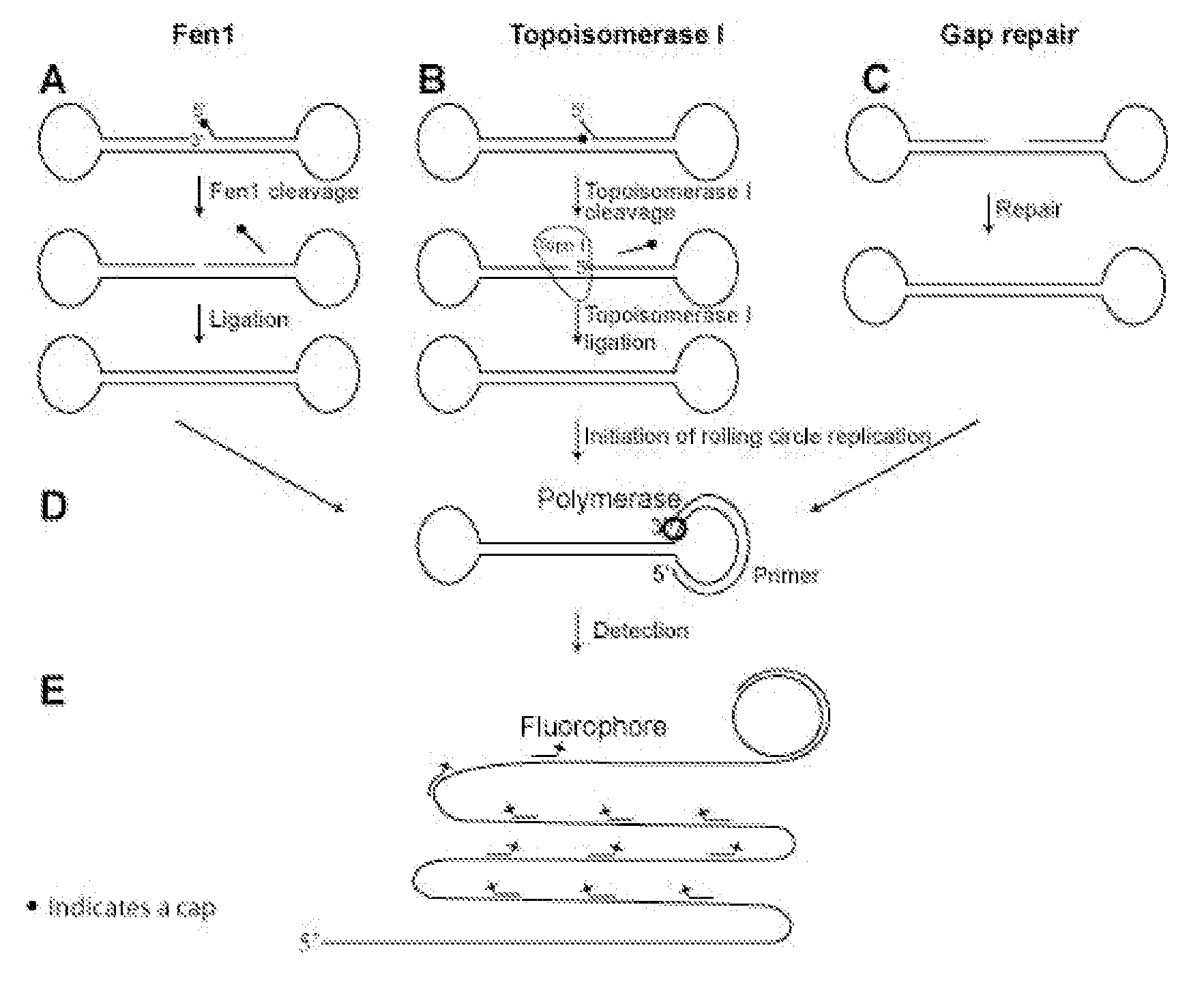
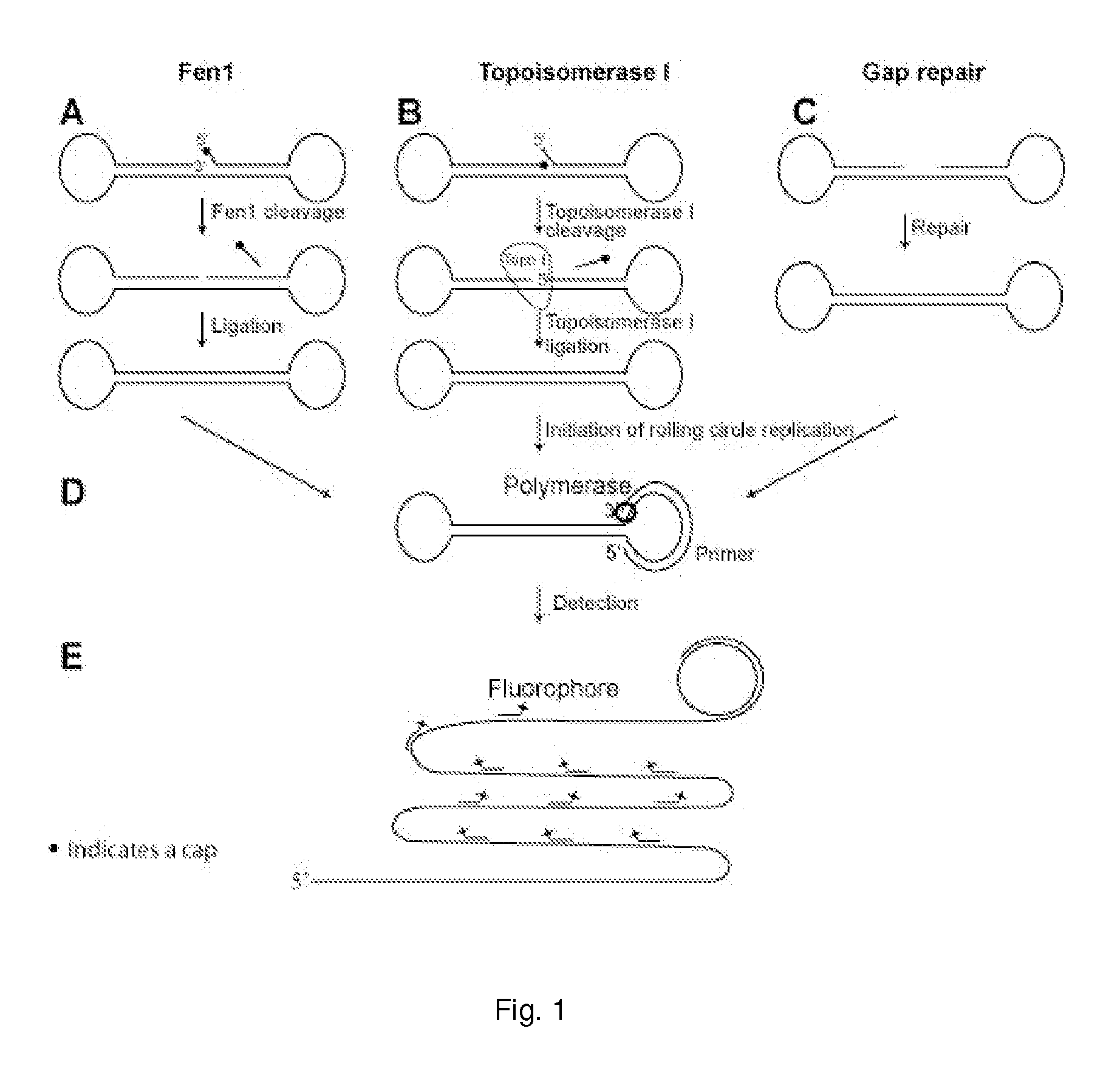
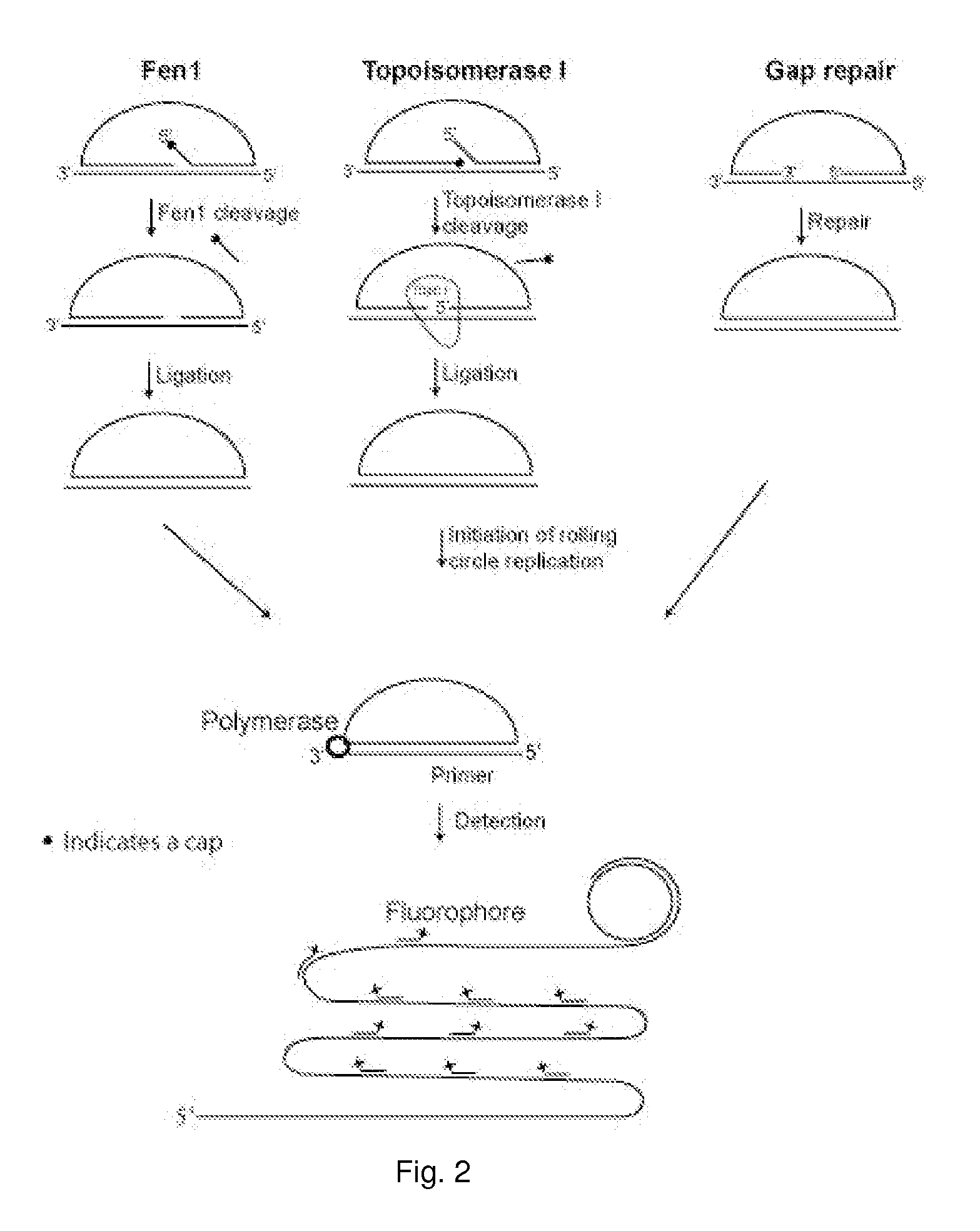
Enzyme activity assay using rolling circle amplification
The present invention relates to an enzyme activity assay using rolling circle amplification for verifying that a sample contains the enzyme activity in question. Thus, the present invention pertains to a method for determining the presence or absence of one or more enzyme activities involved in circularising a non-circular oligonucleotide probe in a biological sample. Furthermore, the present invention concerns liquid compositions comprising one or more oligonucleotide probes. Within the scope of the present invention is also a composition comprising a liquid composition and a tissue sample, and solid support of one or more oligonucleotides of the present invention. Disclosed is also a microfluidic device with one or more compartments for performing rolling circle amplification events, and a method for correlating one or more rolling circle amplification events. Methods for testing the efficacy of a drug, for diagnosing or prognosing a disease, for treating a disease, or for treating prophylactically a disease is furthermore disclosed.
Owner:IN SITU RCP
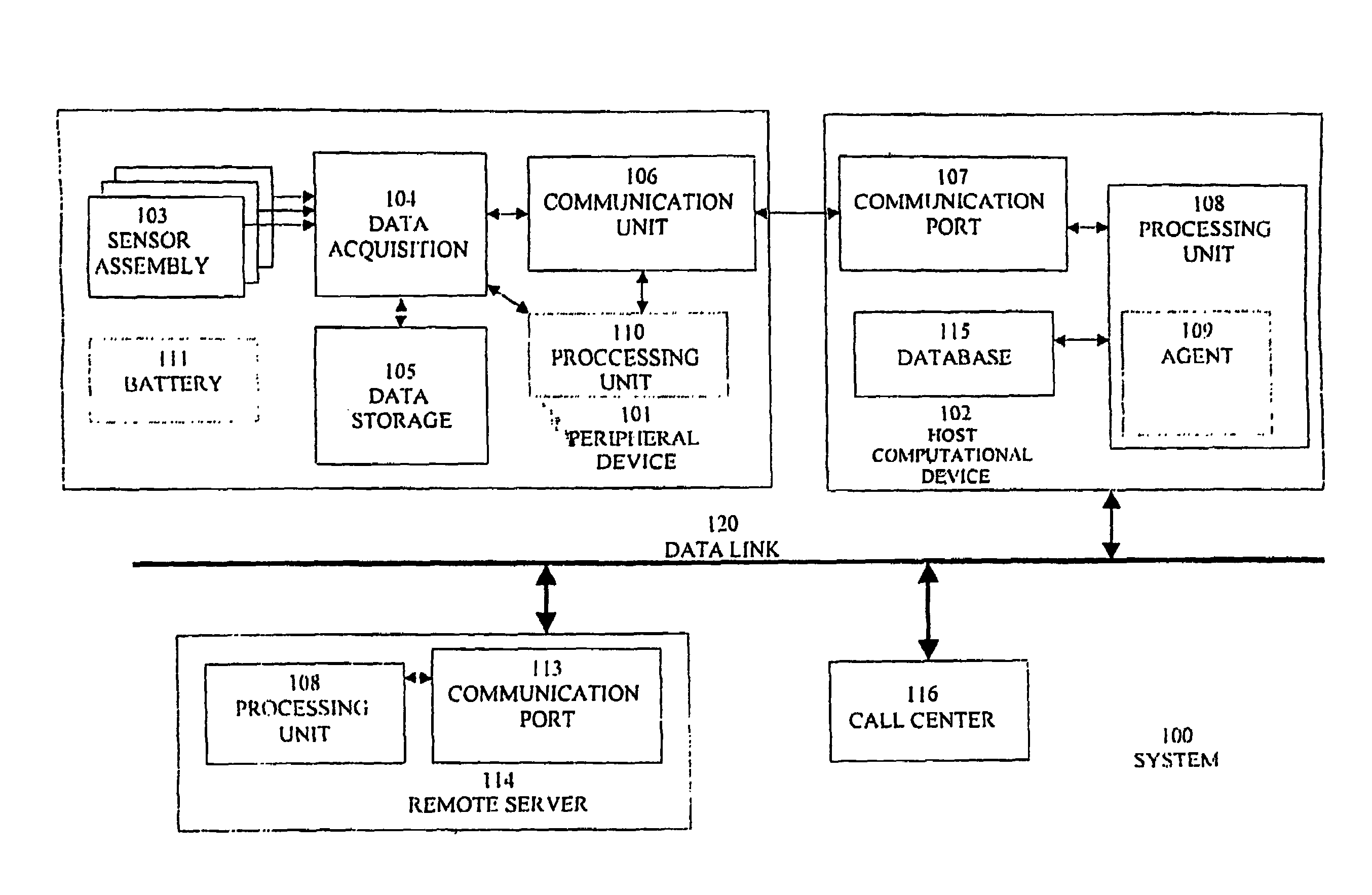
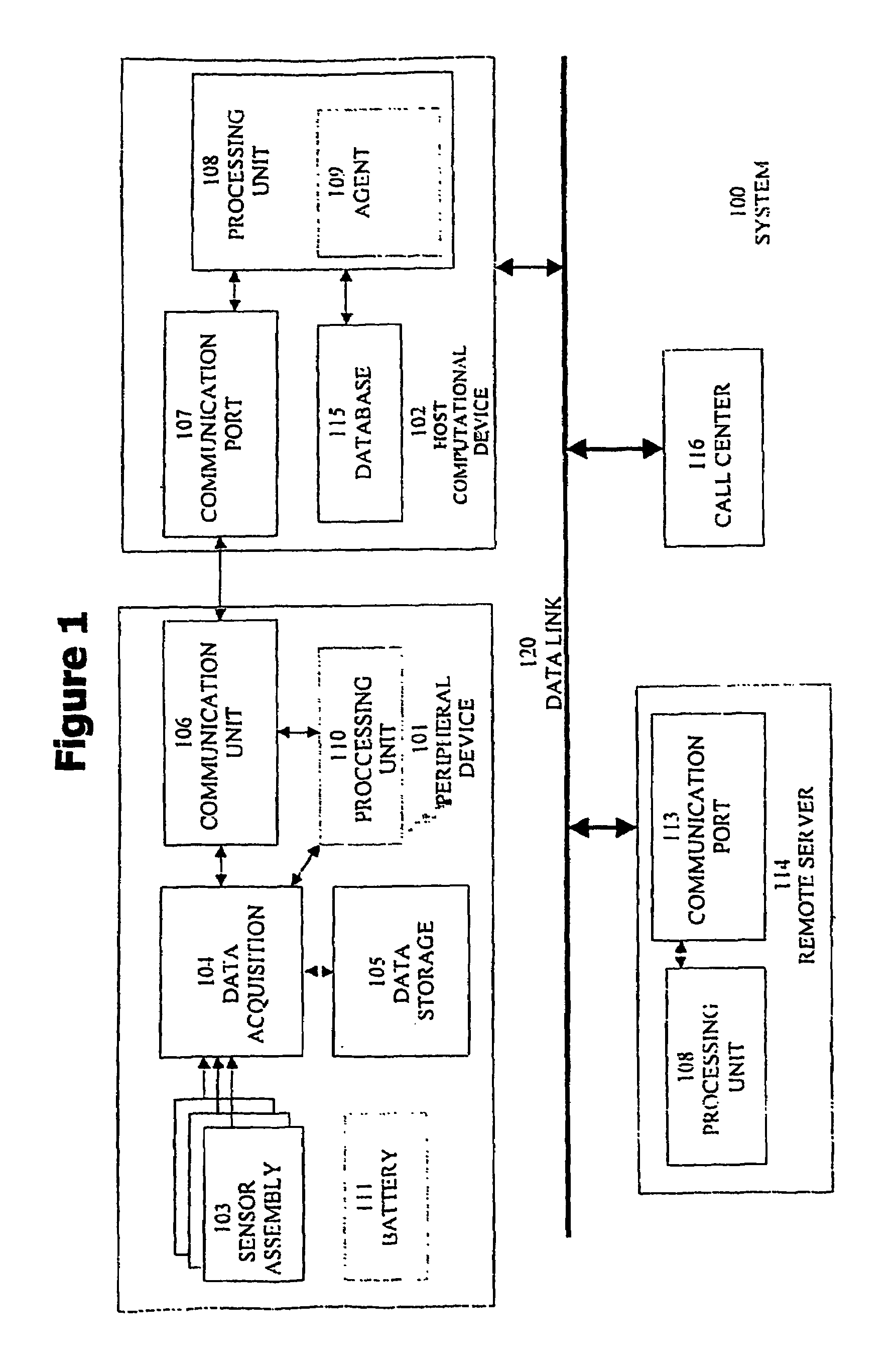
Physiological monitoring system for a computational device of a human subject
InactiveUS7407484B2Efficient use ofIncrease probabilityData processing applicationsCatheterPhysiological monitoringProphylactic treatment
A system for monitoring at least one physiological parameter of a human subject. The system of the present invention features a device with which the human subject regularly interacts, and which is connected to the computational device of the human subject for automatic collection of at least one physiological parameter which is also of medical interest. The device features at least one physiological sensor for collecting the measurement of the physiological parameter. The computational device of the human subject then preferably operates a software program to analyze the data which is collected, in order for the human subject to receive an alert when necessary. Alternatively or additionally, the collected data is sent to a remote computational device which is in communication with the computational device of the human subject for analysis. Optionally, the present invention enables the human subject to receive an alert if a deterioration in the physiological condition of the human subject is detected, thereby enabling the human subject to start preventive medical treatment with trained medical personnel as soon as possible. Thus, the awareness of the human subject about any incipient medical problem is immediately improved, which may result in an increased probability of being able to successfully treat and / or otherwise ameliorate those problems.
Owner:MEDIC4ALL INC
Methods of reducing risk of infection from pathogens
Prophylactic treatment methods are provided for protection of individuals and / or populations against infection from airborne pathogens. In particular, prophylactic treatment methods are provided including administering a sodium channel blocker or pharmaceutically acceptable salts thereof to one or more members of a population at risk of exposure to or already exposed to one or more airborne pathogens, either from natural sources or from intentional release of pathogens into the environment.
Owner:PARION SCI DURHAM NC
Targeted gastrointestinal tract delivery of probiotic organisms and/or therapeutic agents
ActiveUS20160022592A1Improve imbalanceAntibacterial agentsBiocideAntibiotic-associated diarrhoeaClostridium difficile infections
The present invention relates to the development of a targeted delivery system for the oral delivery of probiotics or therapeutic agent for various indications, including and not limited to active and prophylaxis treatment of Clostridium difficile infection, antibiotic associated diarrhea, irritable bowel syndrome, Crohn's disease, intestinal flora replacement, supplemental flora treatments for patients taking antibiotics, and for restoration of balance and signaling between the intestinal microbiome and the intestinal cells in patients under treatment of metabolic syndrome manifestations, specifically diabetes, insulin resistance, obesity, hyperlipidemia and hypertension.
Owner:THERABIOME
Inhibitors of the 11-beta-hydroxysteroid dehydrogenaseType 1 enzyme and their therapeutic application
The present invention relates to the use of inhibitors of the 11-beta-hydroxysteroid dehydrogenase Type 1 enzyme. The present invention further relates to the use of inhibitors of 11-beta-hydroxysteroid dehydrogenase Type 1 enzyme for the treatment or prophylactically treatment of non-insulin dependent type 2 diabetes, insulin resistance, obesity, lipid disorders, metabolic syndrome, and other diseases and conditions mediated by excessive glucocorticoid action.
Owner:ABBOTT LAB INC
Methods of reducing risk of infection from pathogens
InactiveUS20090253714A1Reduce the risk of infectionAntibacterial agentsOrganic active ingredientsNatural sourceMedicine
Prophylactic treatment methods are provided for protection of individuals and / or populations against infection from airborne pathogens. In particular, prophylactic treatment methods are provided comprising administering amiloride, benzamil, phenamil or pharmaceutically acceptable salts thereof to one or more members of a population at risk of exposure to or already exposed to one or more airborne pathogens, either from natural sources or from intentional release of pathogens into the environment.
Owner:JOHNSON MICHAEL R +1
Compositions comprising probiotic and sweetener components
Disclosed herein are compositions that may be sufficiently stable such that probiotic microorganisms are present in the compositions at the time of ingestion by a mammal. The compositions comprise:(a) a probiotic component; and(b) a sweetener component;wherein the composition is substantially free of a chewing gum base.Further disclosed are methods of prophylactic, therapeutic treatment or non-therapeutic treatment to alleviate diseases or conditions, or enhance overall health, that affect a mammal comprising administration of a composition as described herein.
Owner:STOJANOVIC MARKO
Controlled modulation of amino acid side chain length of peptide antigens
ActiveUS20050169934A1Extend and shortenReduce decreaseBacterial antigen ingredientsPeptide/protein ingredientsPeptide antigenEpitope
The invention provides a method for the creation of peptide antigens comprising epitopes with at least a first modification comprising a shortened or lengthened amino acid side chain. By extension or shortening of the side chain with CH3 / CH2 groups, for example, made by computer assisted modeling of the tumor antigen (peptide) bound in the MHC-I-groove, immunogenicity can be improved with minimal modification of adjacent tertiary structure, thereby avoiding cross-reactivity. Provided by the invention are methods of creating such antigens, as well as methods for therapeutic or prophylactic treatment of various conditions comprising administration of the antigens.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
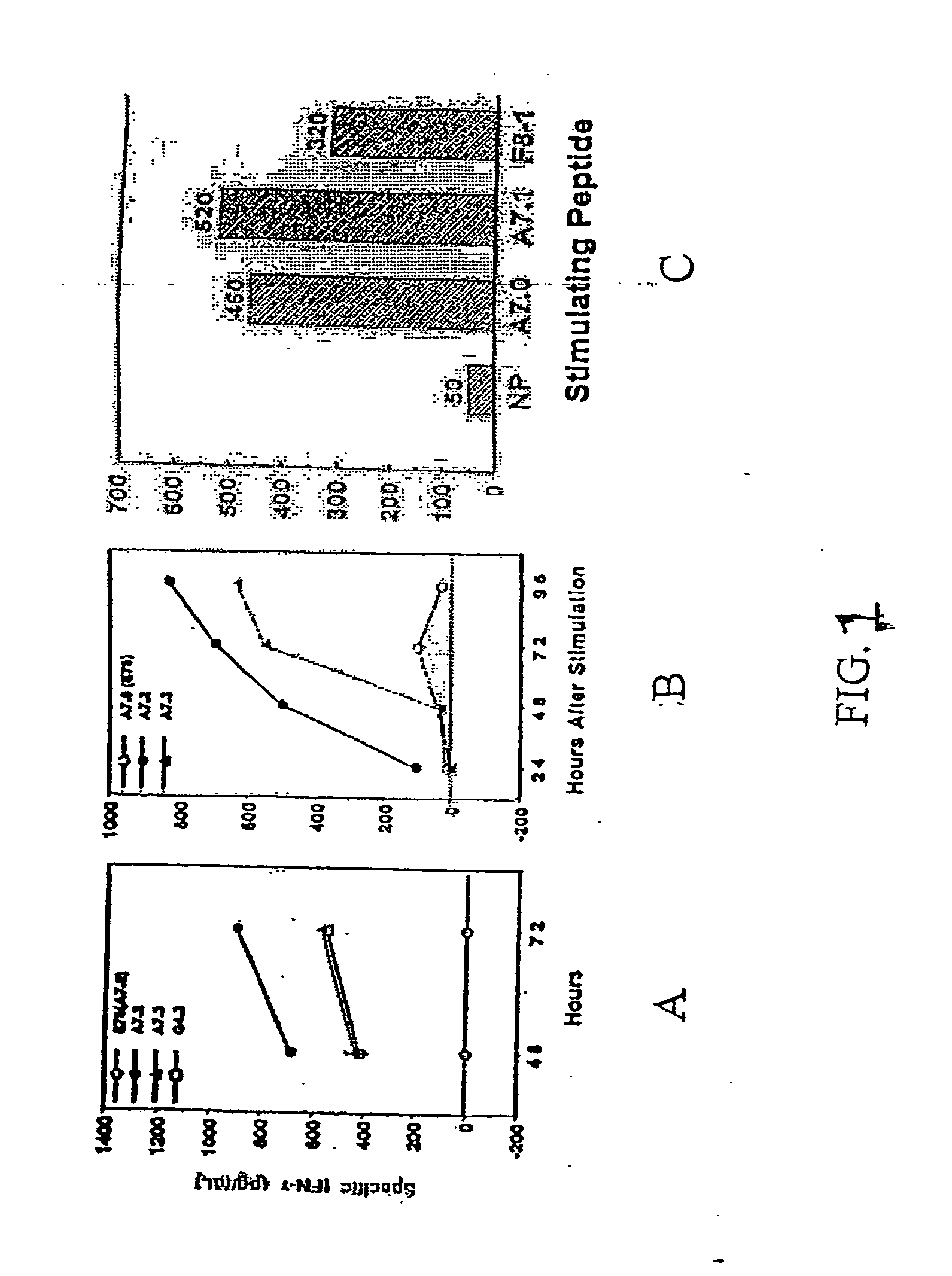
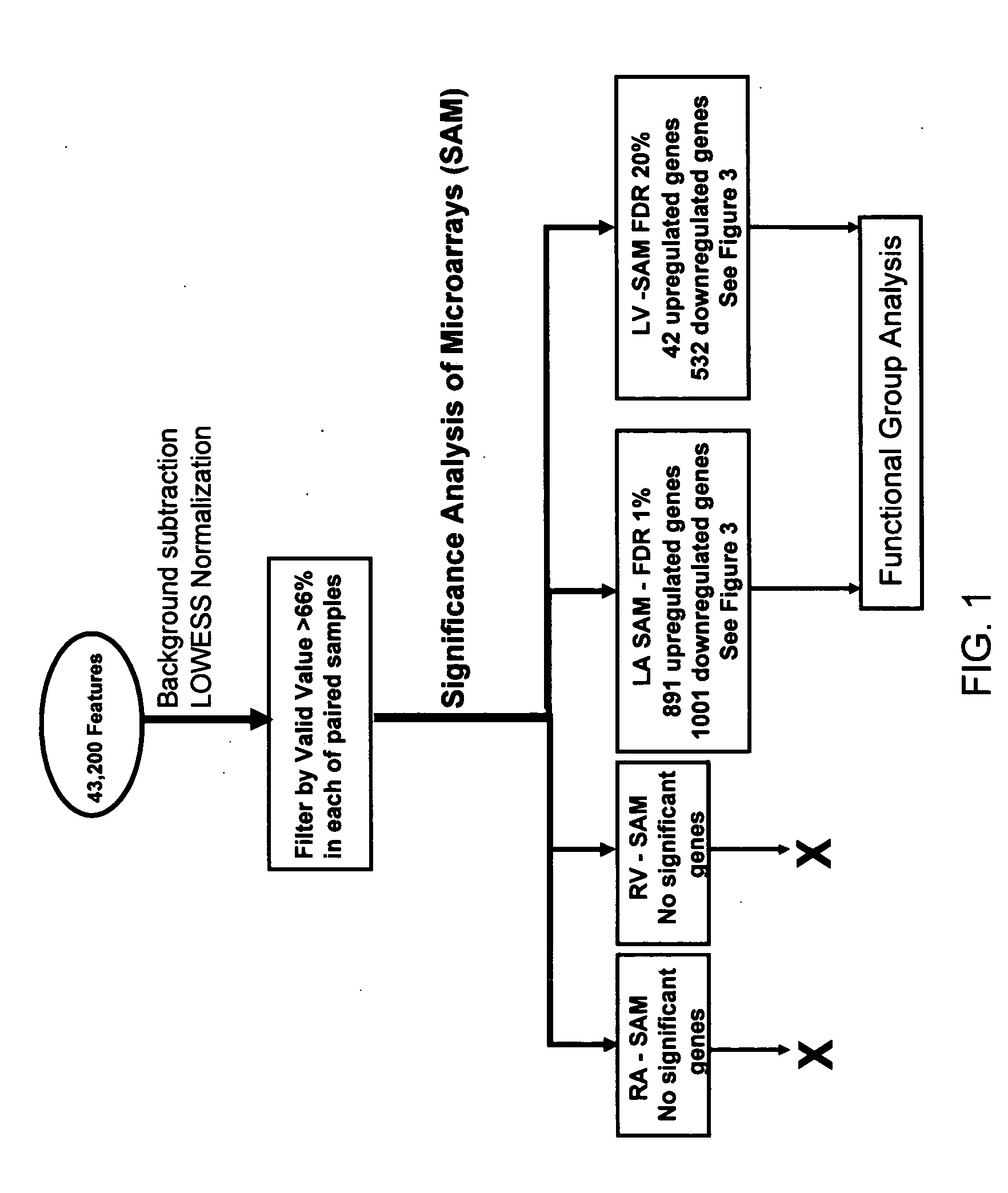
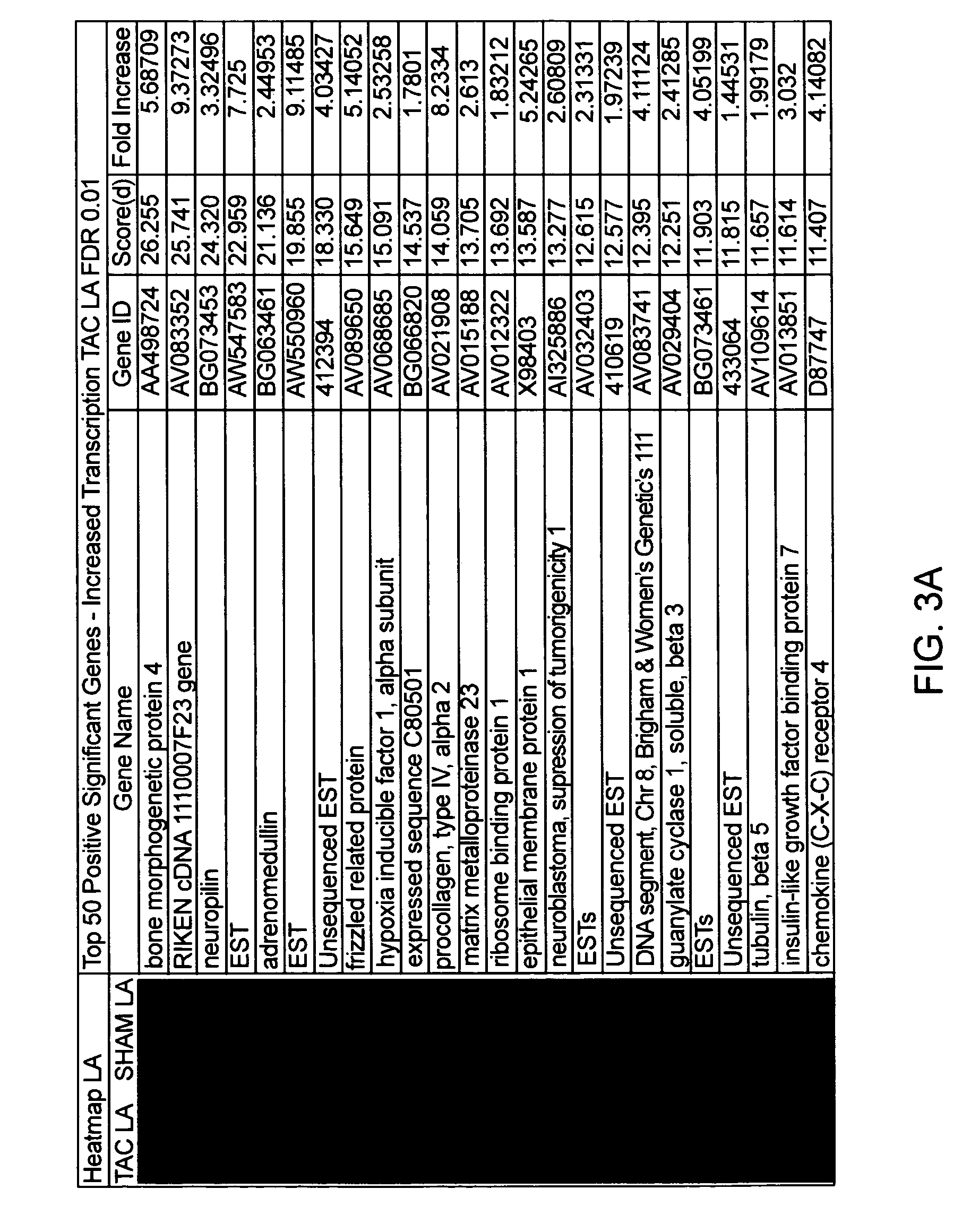
Cardiac pressure overload associated genes
InactiveUS20060094038A1Easy to changeBioreactor/fermenter combinationsBiological substance pretreatmentsEtiologyEccentric hypertrophy
The present invention identifies genes whose gene products are differentially expressed pressure overload of the heart. The invention provides methods for diagnosing or assessing an individual's susceptibility to heart failure from many etiologies, as well as the presence and severity of hypertrophy, chamber enlargement, or systolic heat failure. Also provided are therapeutic methods for treating a heart patient or methods for prophylactically treating an individual susceptible to heart failure. Additionally, the invention describes screening methods for identifying agents that can be administered to treat individuals that have suffered a heart attack or are at risk of heart failure.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Prophylactic therapeutic and industrial antioxidant compositions enhanced with stabilized atomic hydrogen/free electrons and methods to prepare and use such compositions
InactiveUS6649193B1Gain is not constantProtects against oxidative damageBiocideHydroxy compound active ingredientsAutoimmune conditionAutoimmune disease
The invention is directed to therapeutic antioxidant compositions which are enhanced by the stabilized atomic hydrogen; one of the most potent antioxidants. Such products can be used for prophylactic and therapeutic purposes in treatment of cancer, diabetes, autoimmune diseases, neurodegenerative diseases, cardiovascular diseases, skin diseases etc. The products described can be used independently or in combination with other drugs and treatment modalities. The products can also be used as dietetic products to aid in desired weigh loss. The described products can also be used to prevent oxidative and free radical damage to food and oxidation-prone industrial products. The invention also describes the methods to produce and stabilize atomic hydrogen and prepare and use such stabilized / encaged atomic hydrogen enhanced antioxidant compositions.
Owner:MOLECUTEC LICENSING
Protein Formulations
Owner:MEDIMMUNE LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com