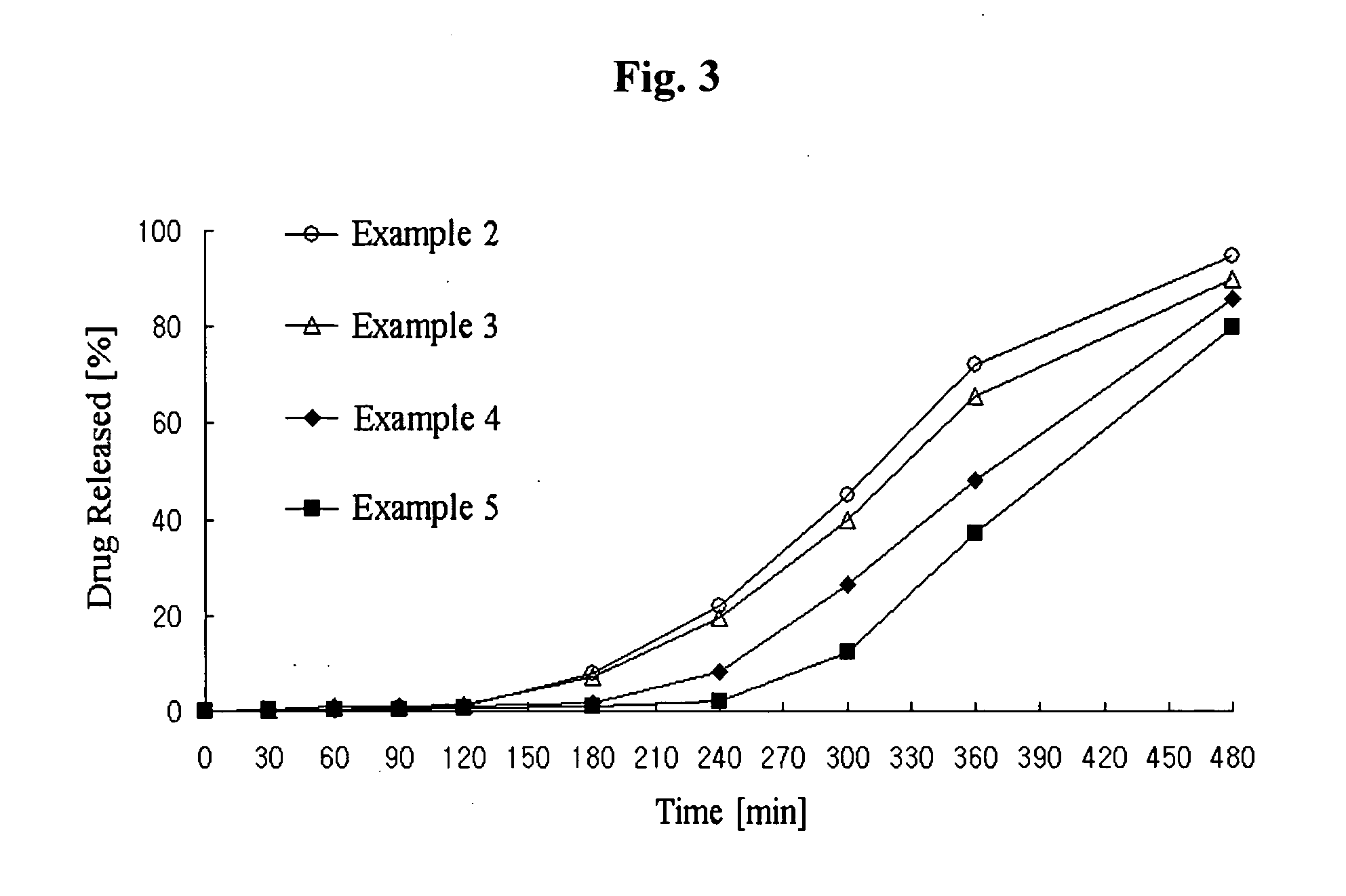
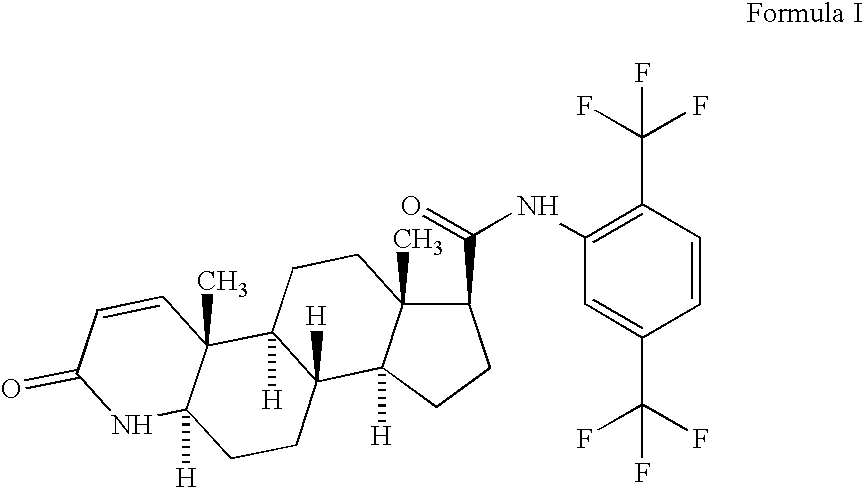
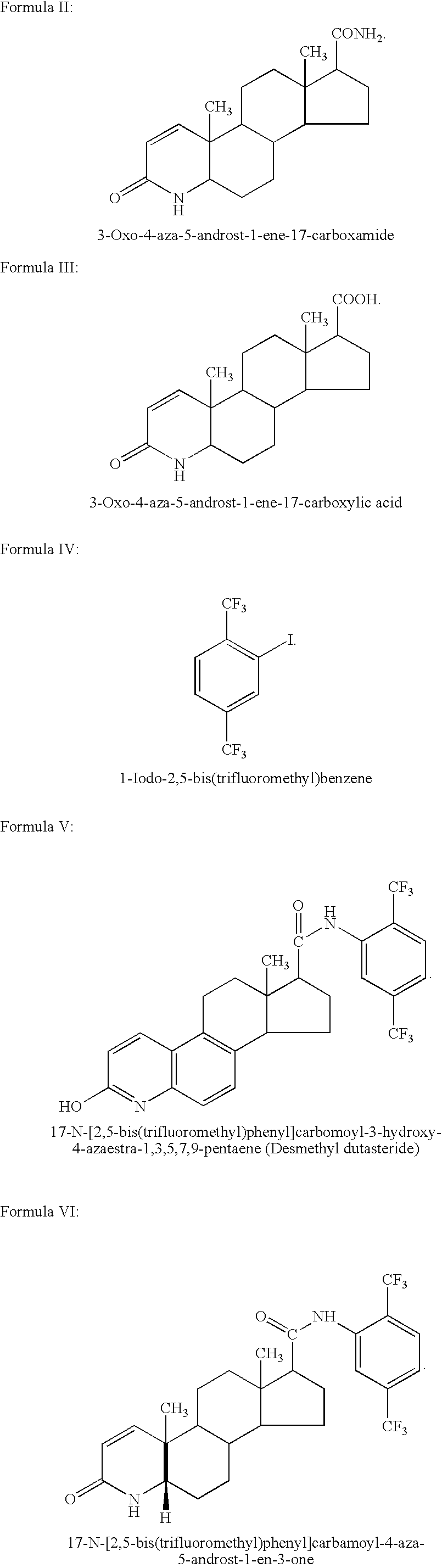
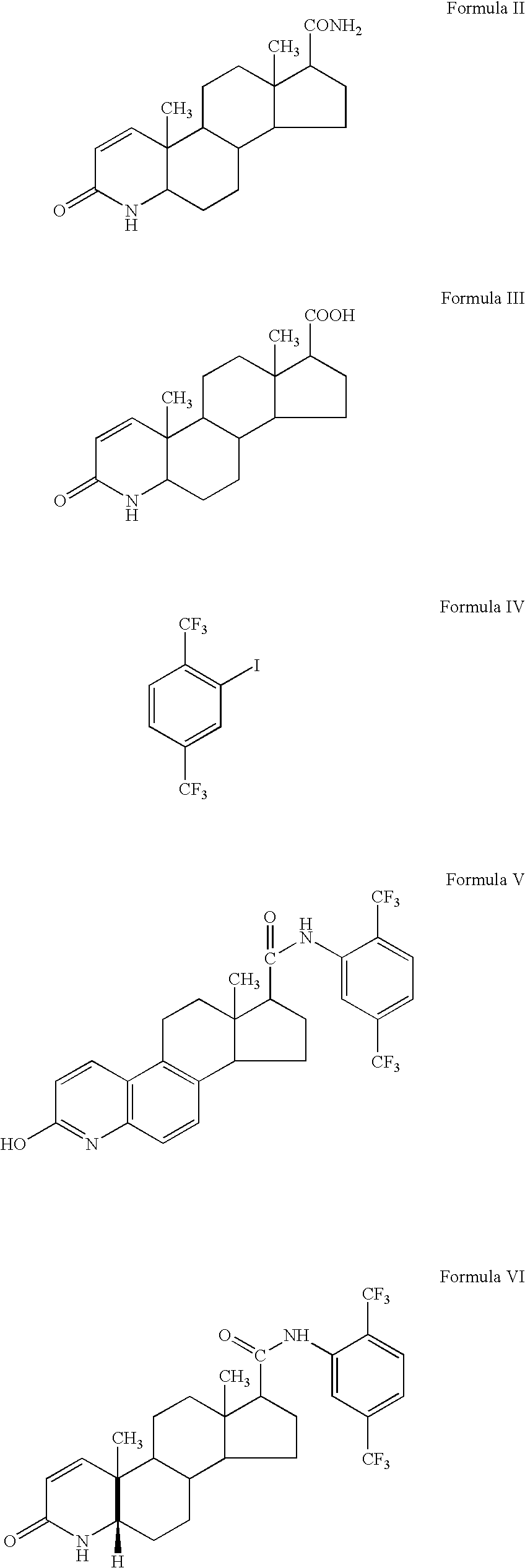
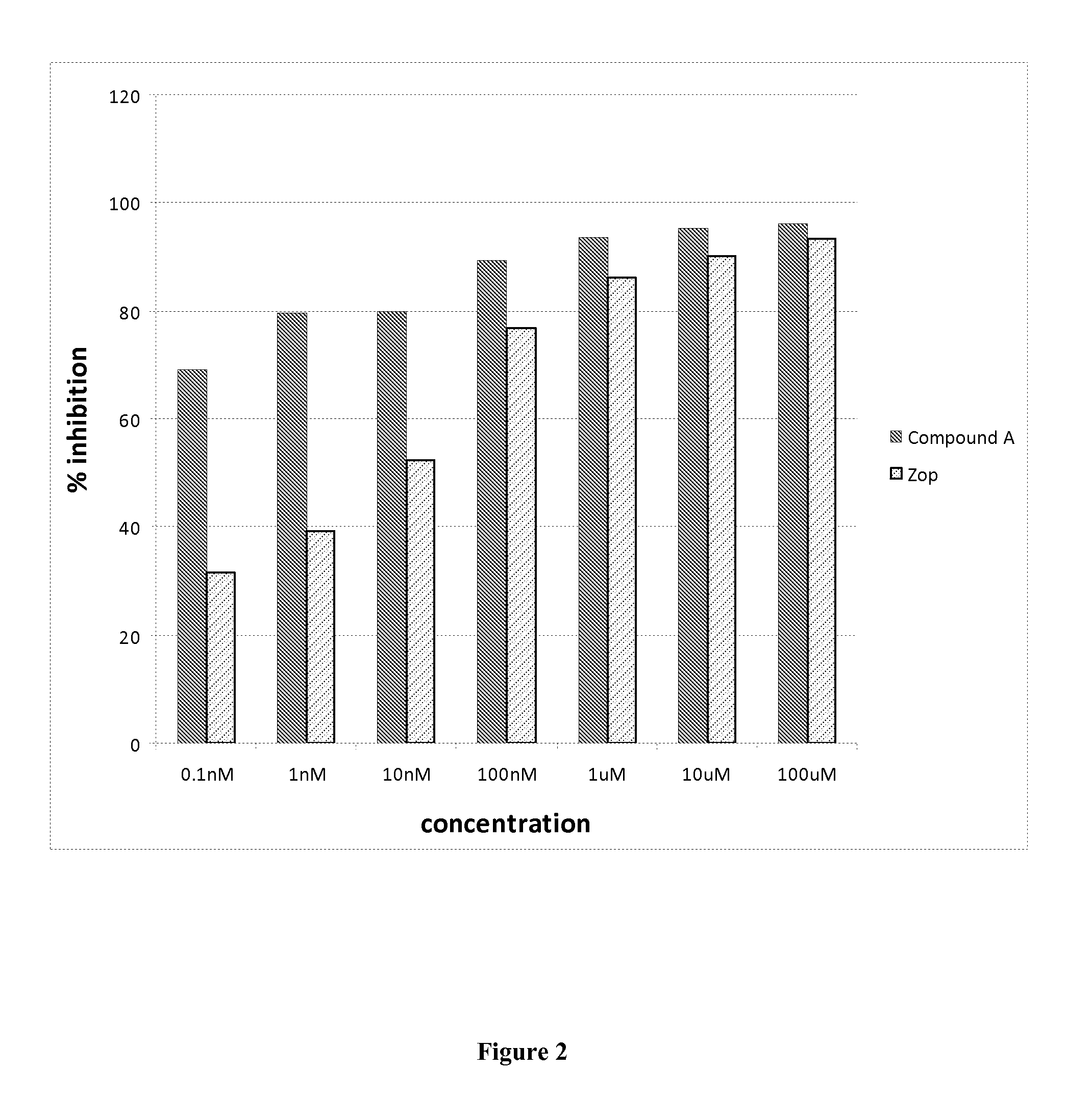
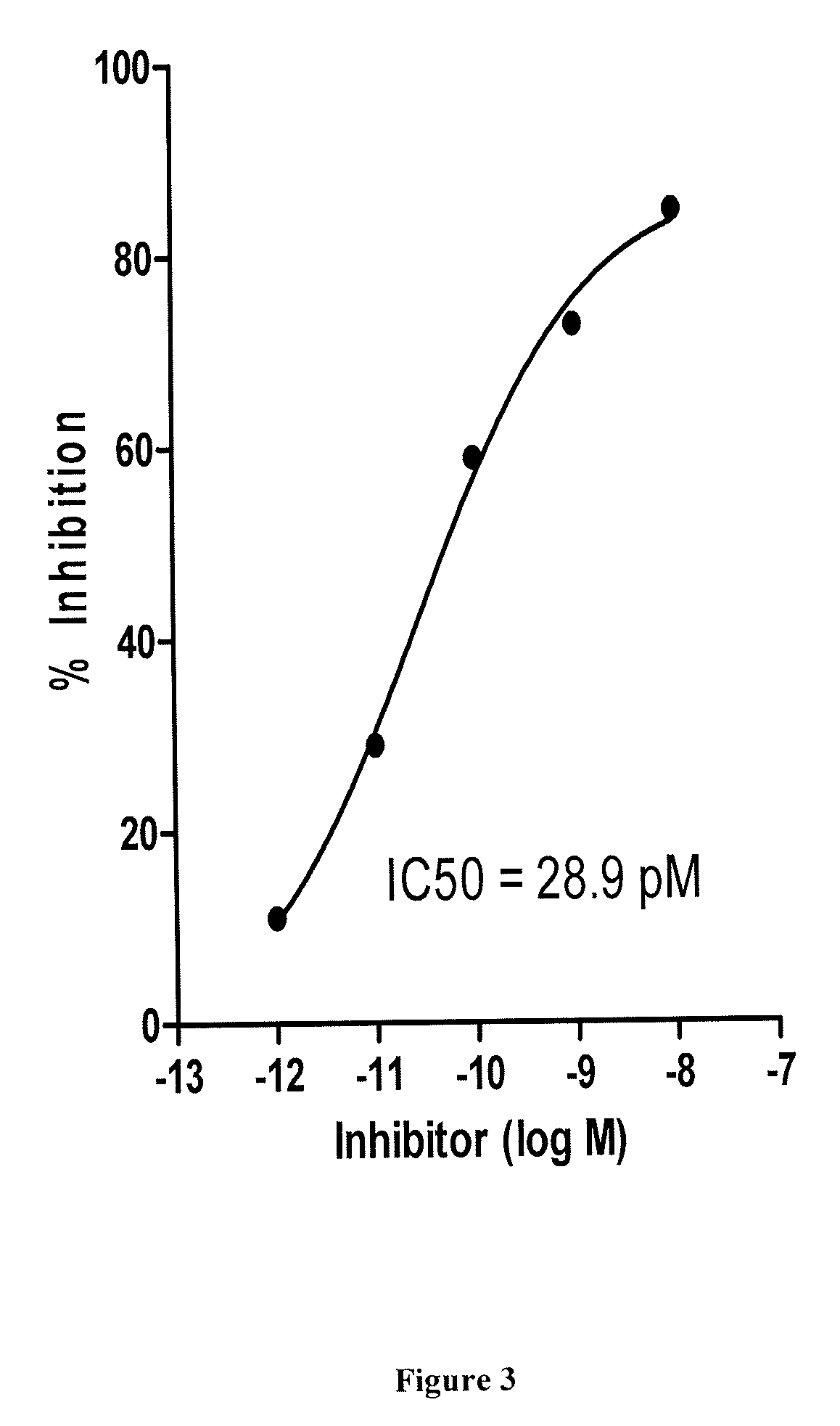
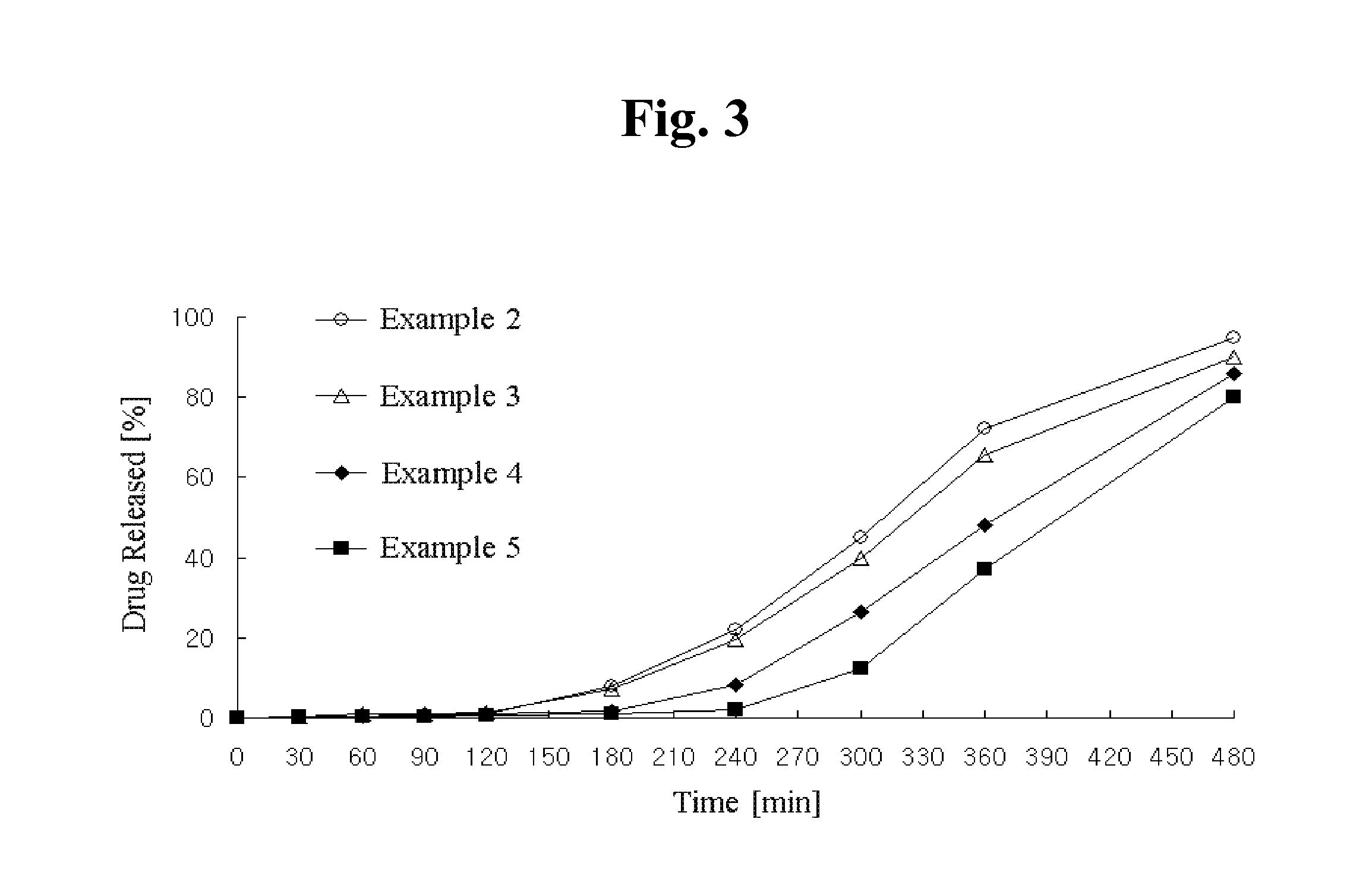
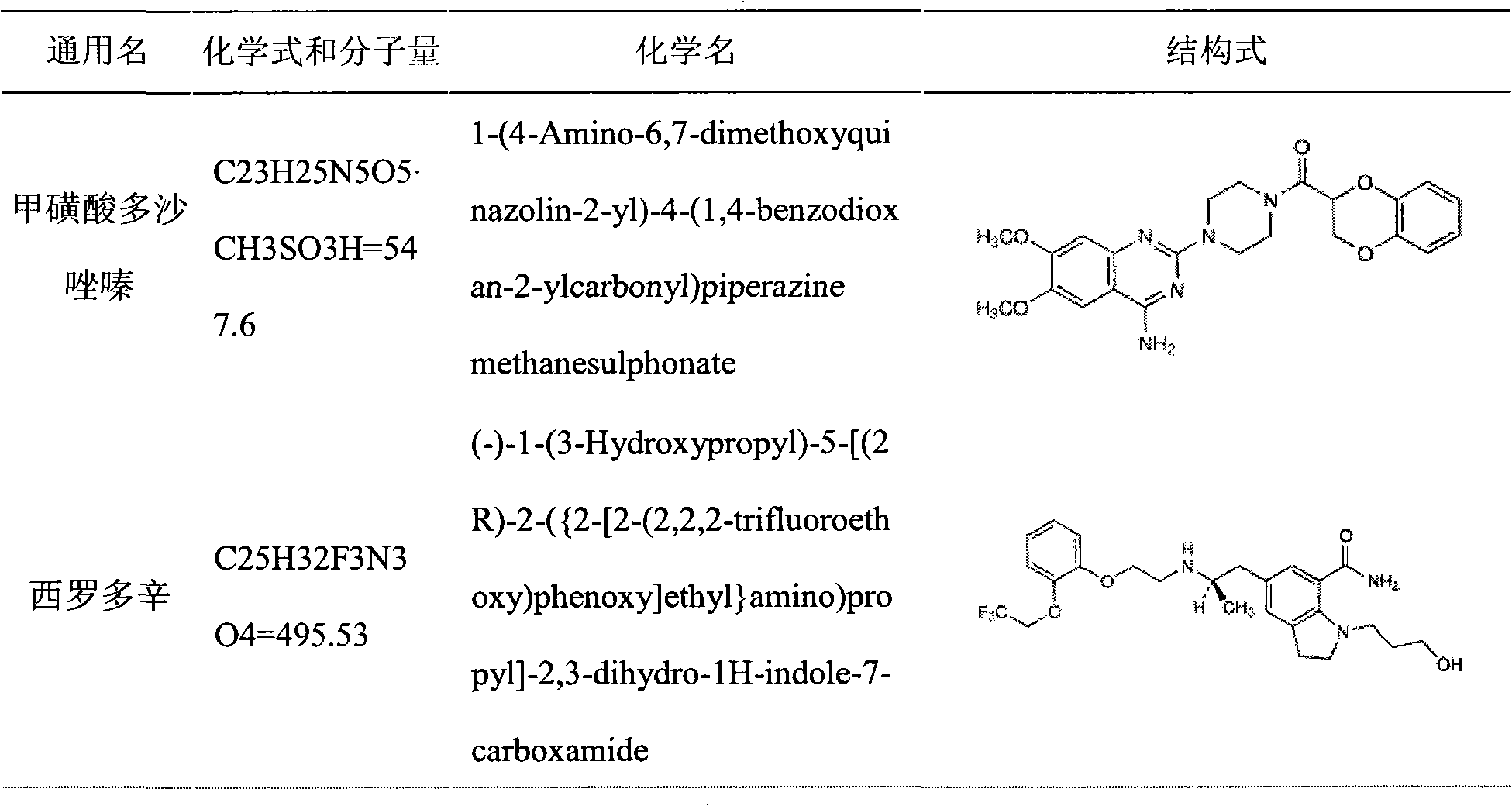
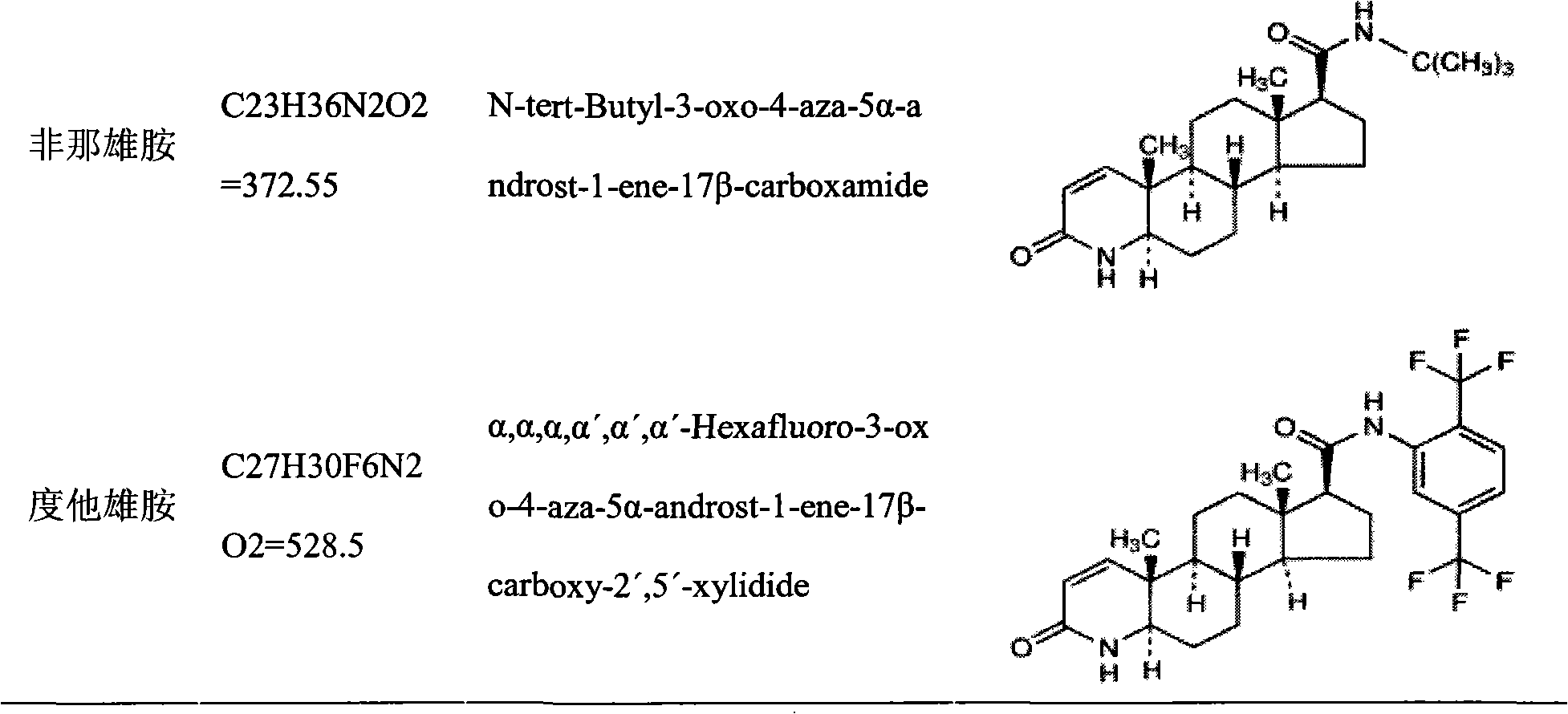
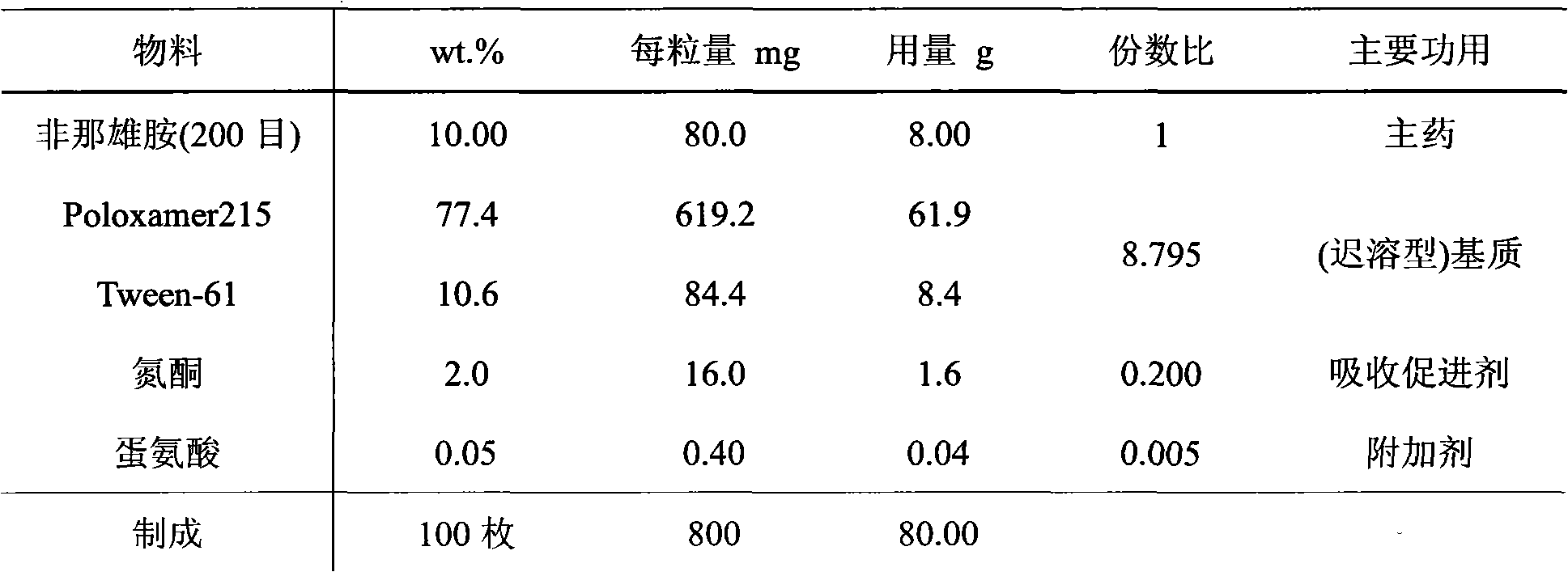
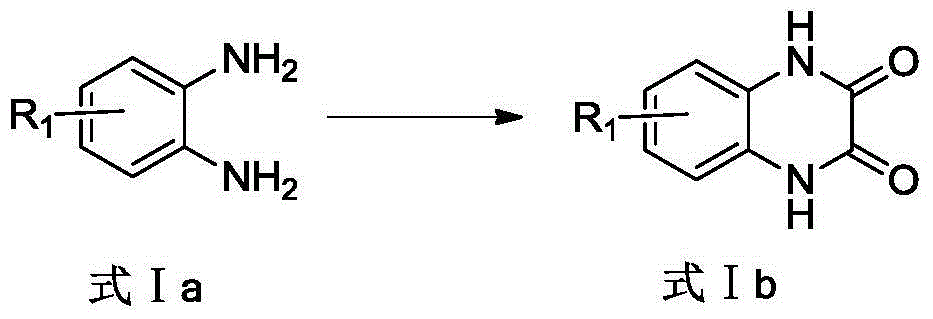
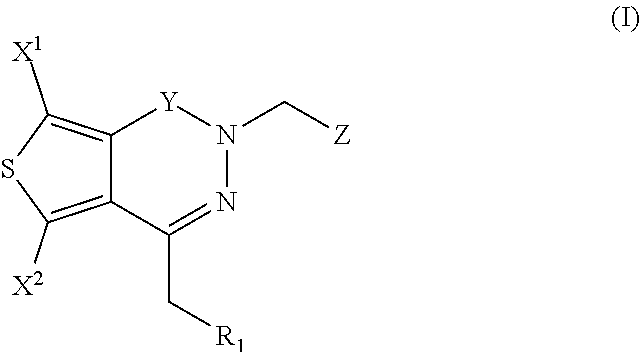
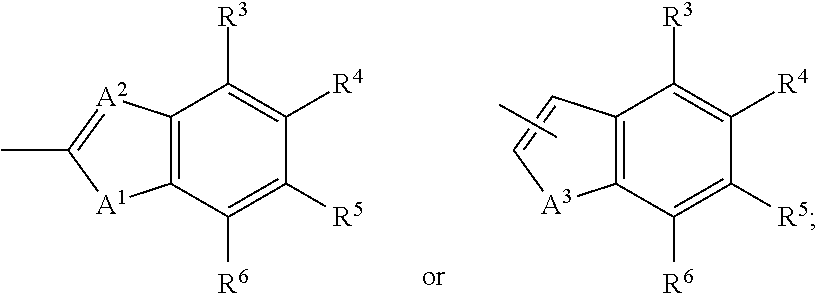
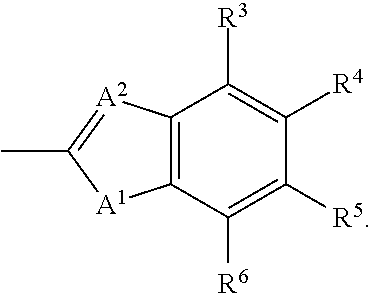
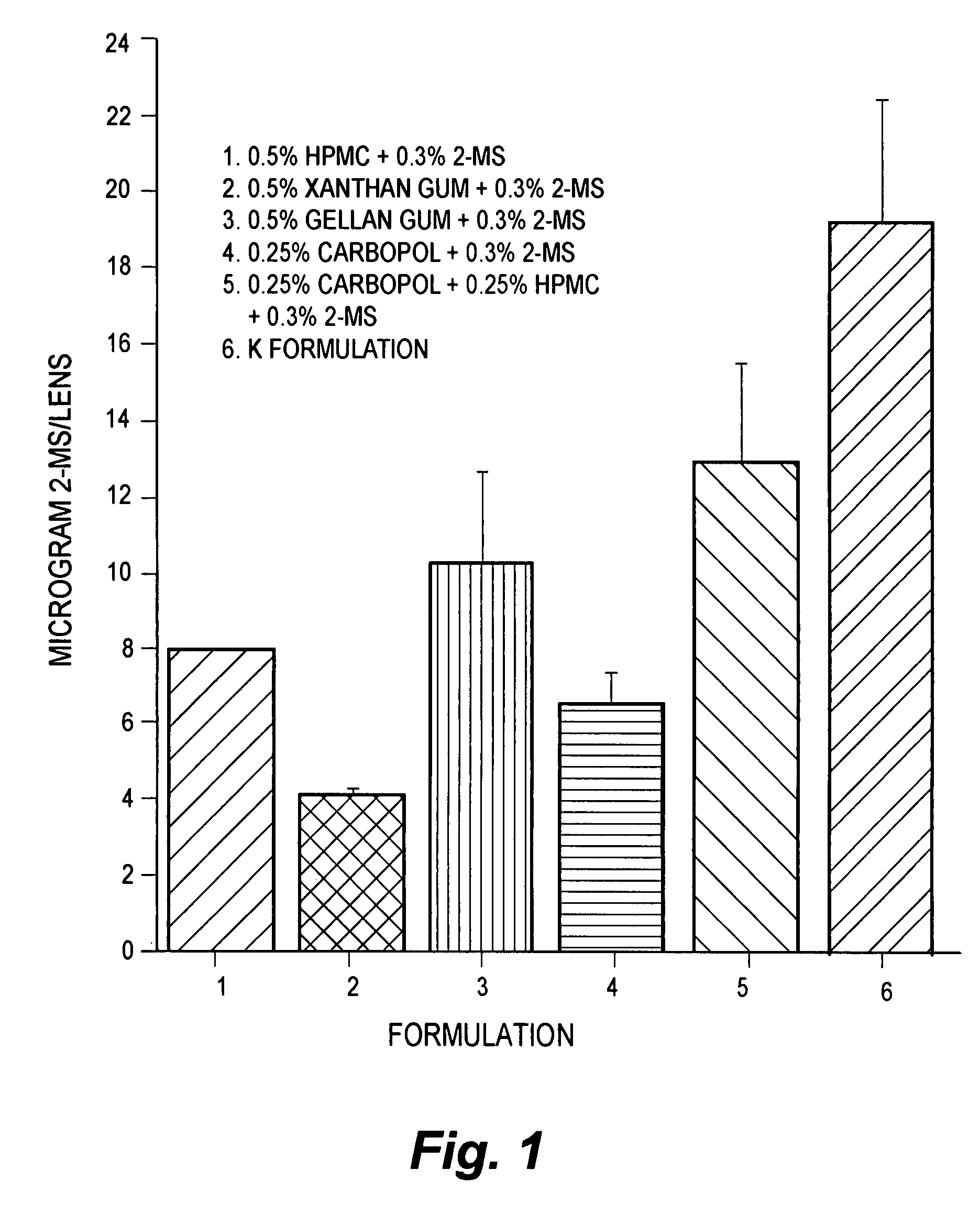
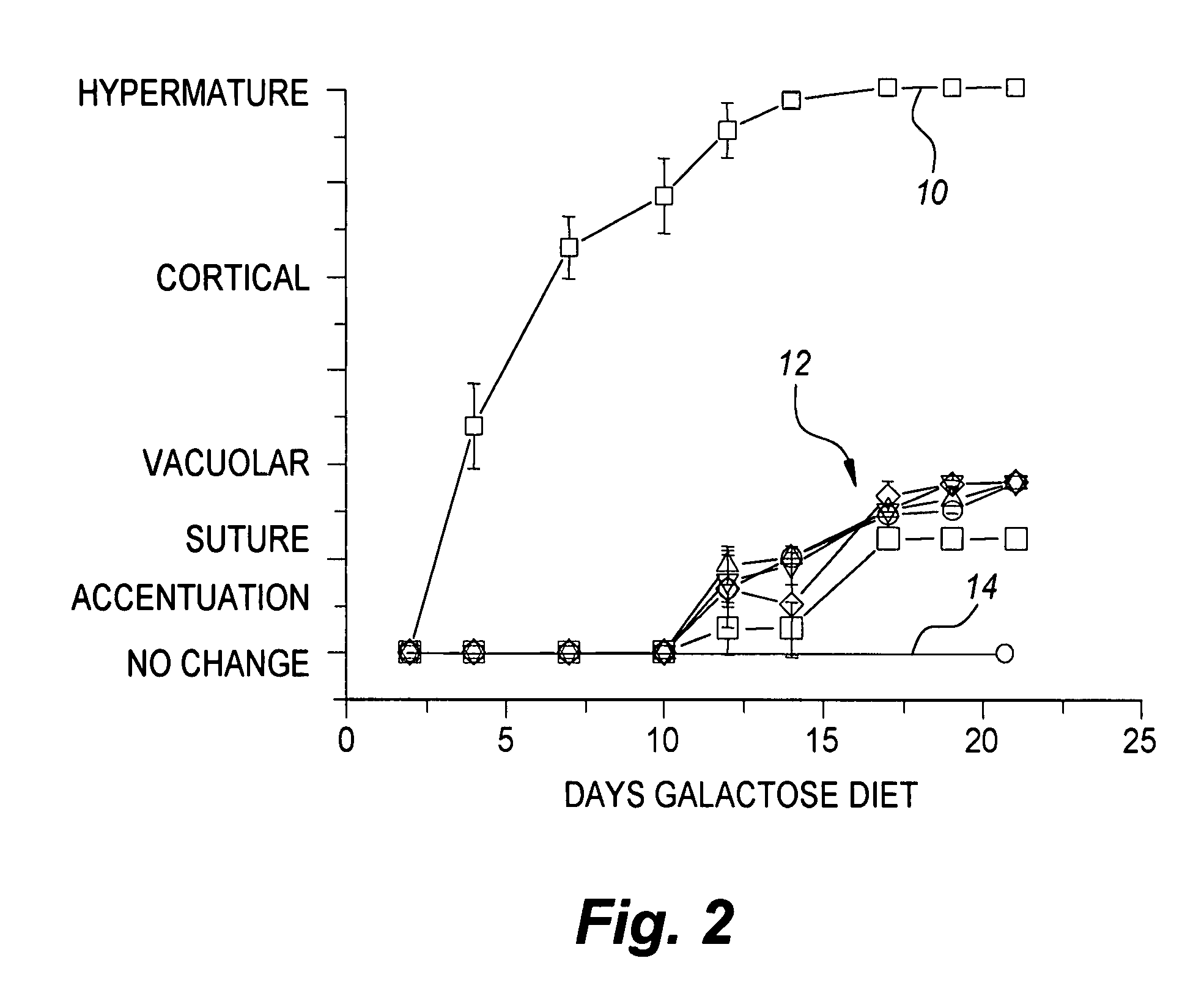
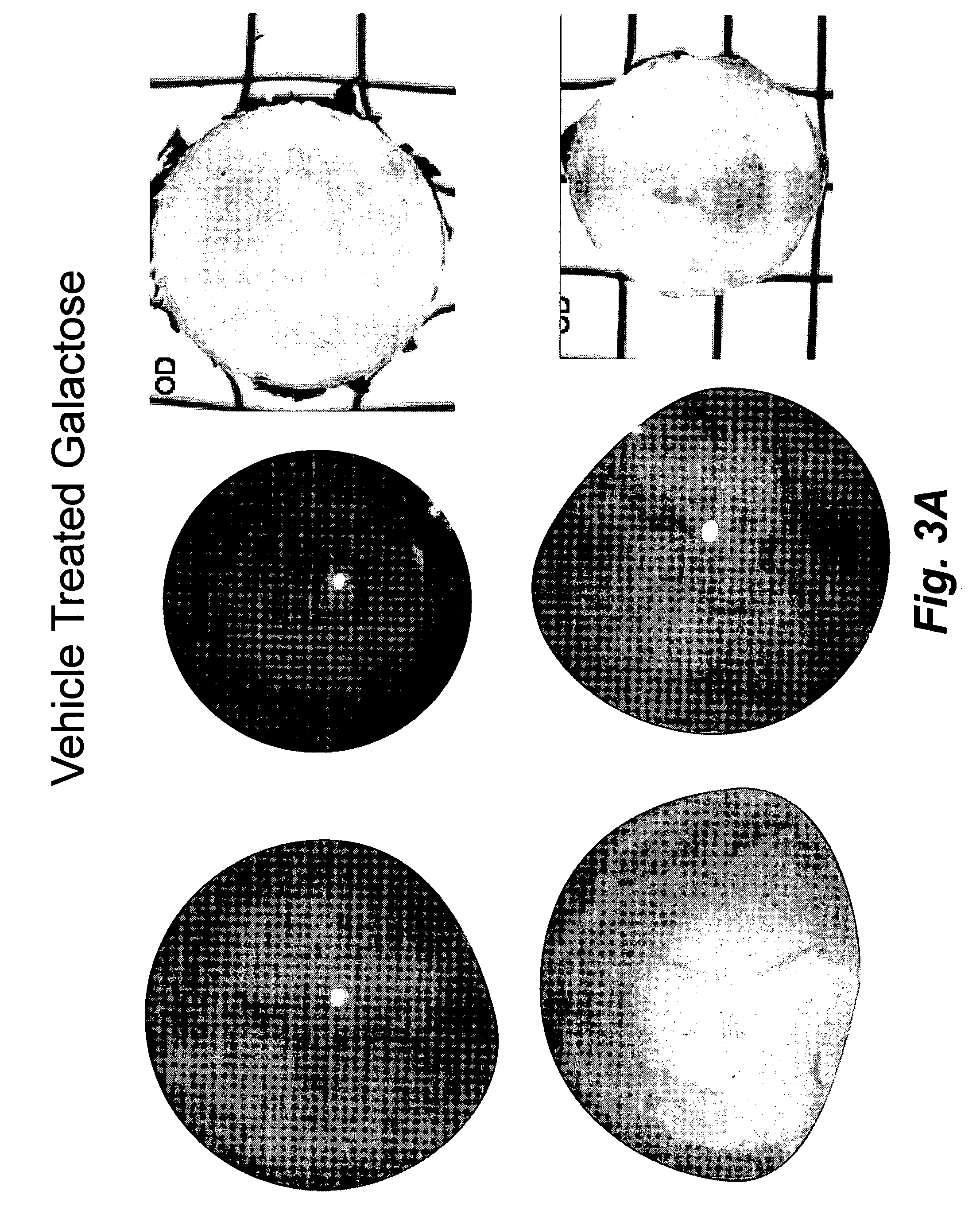
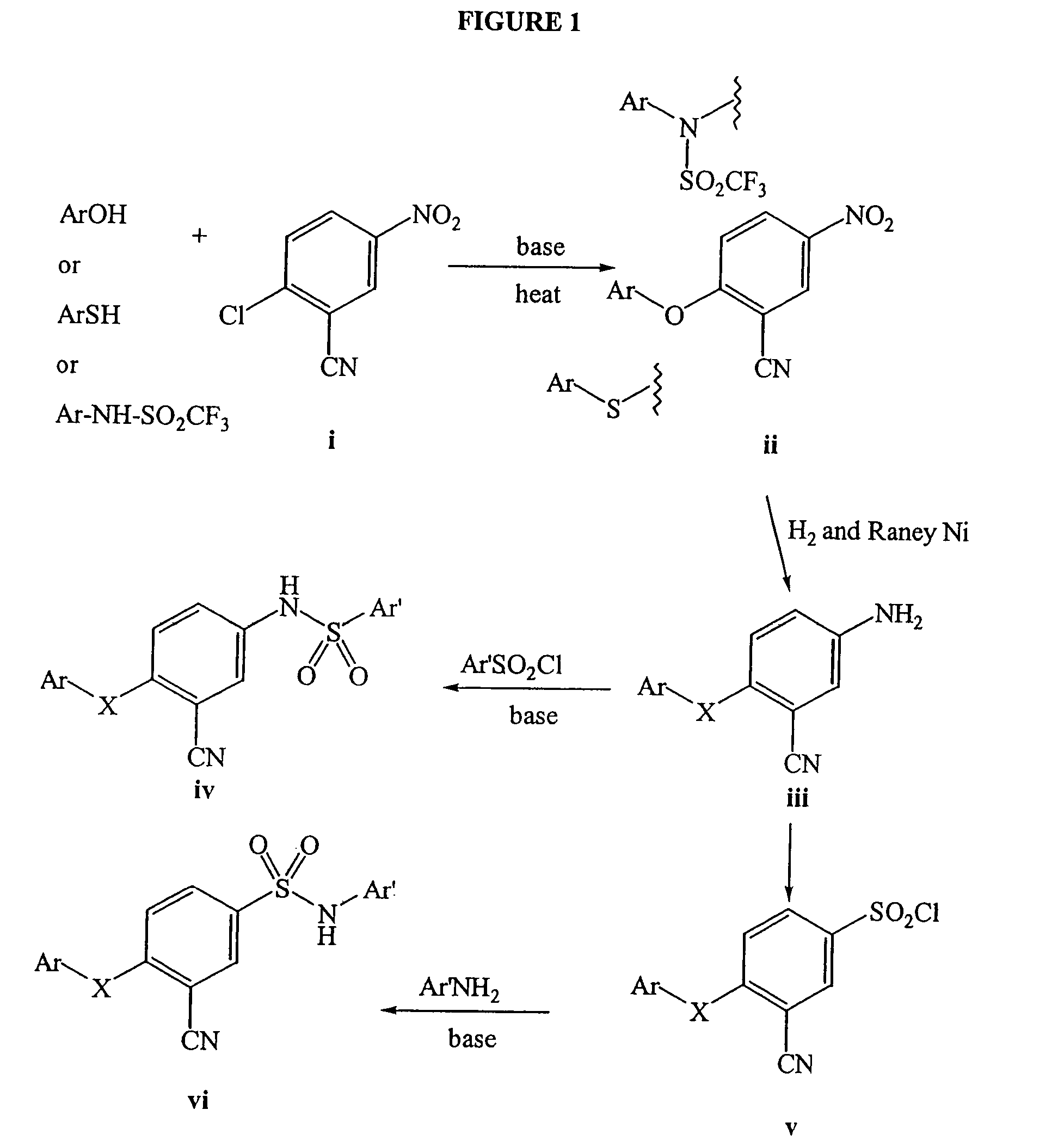
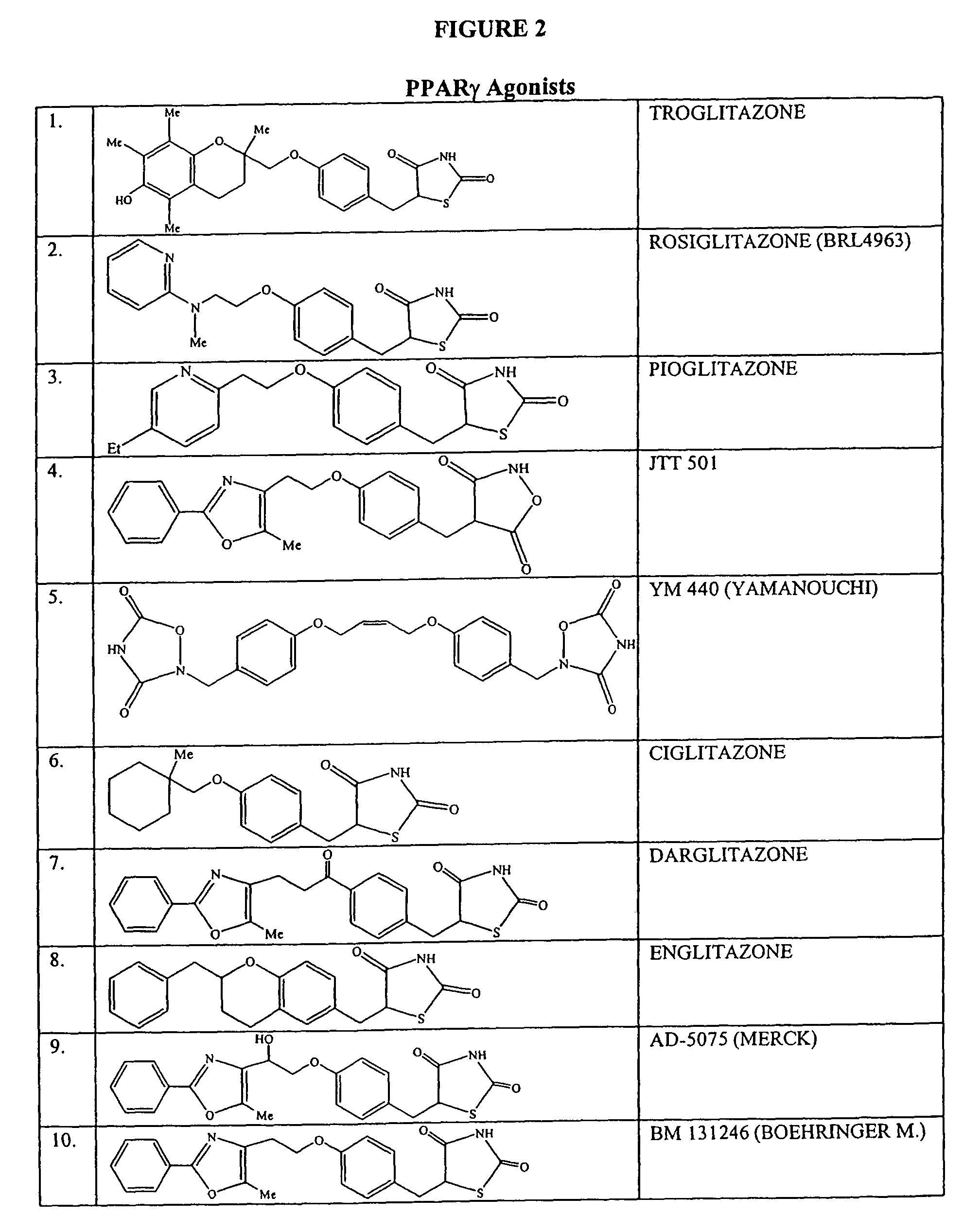
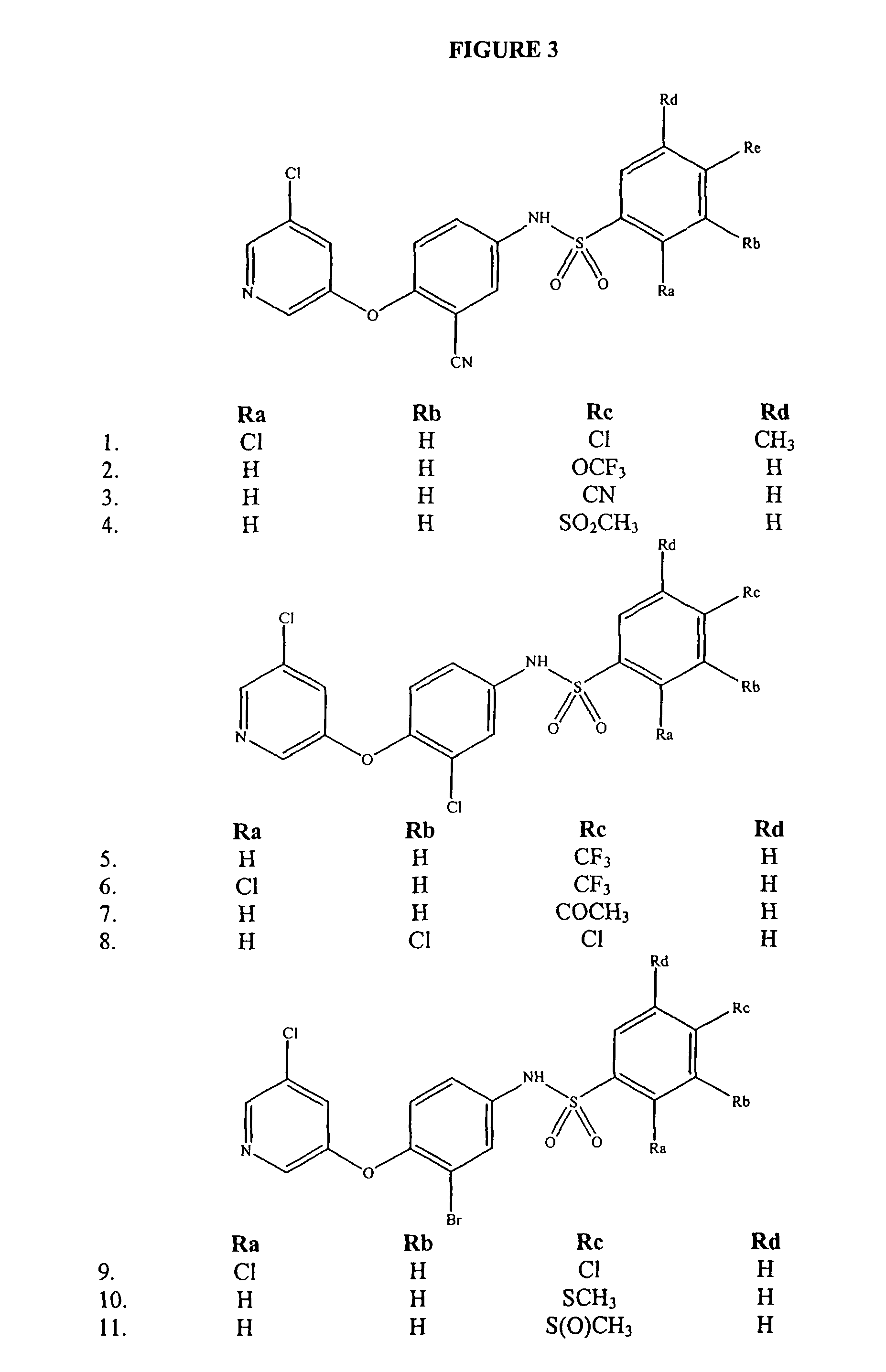
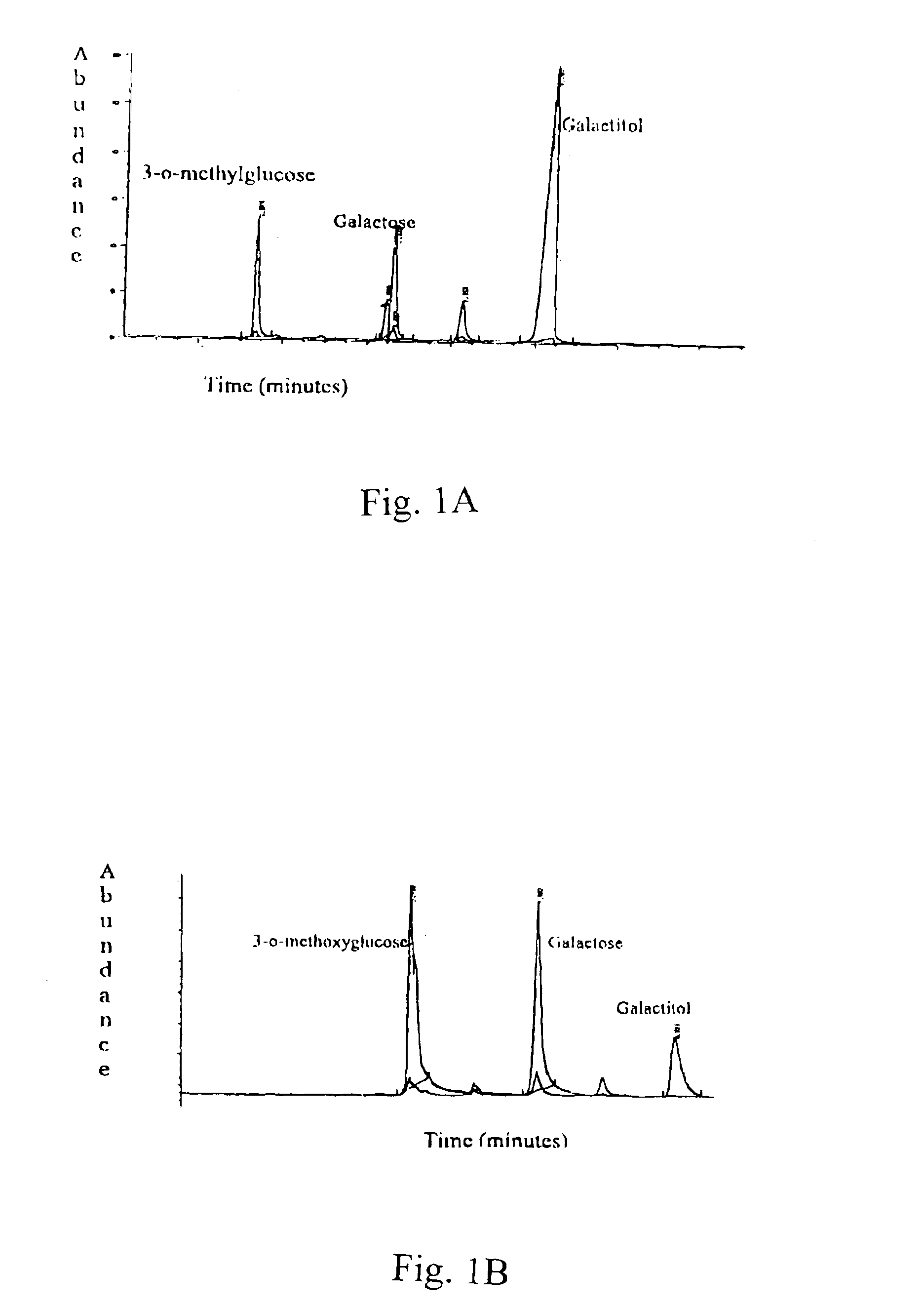
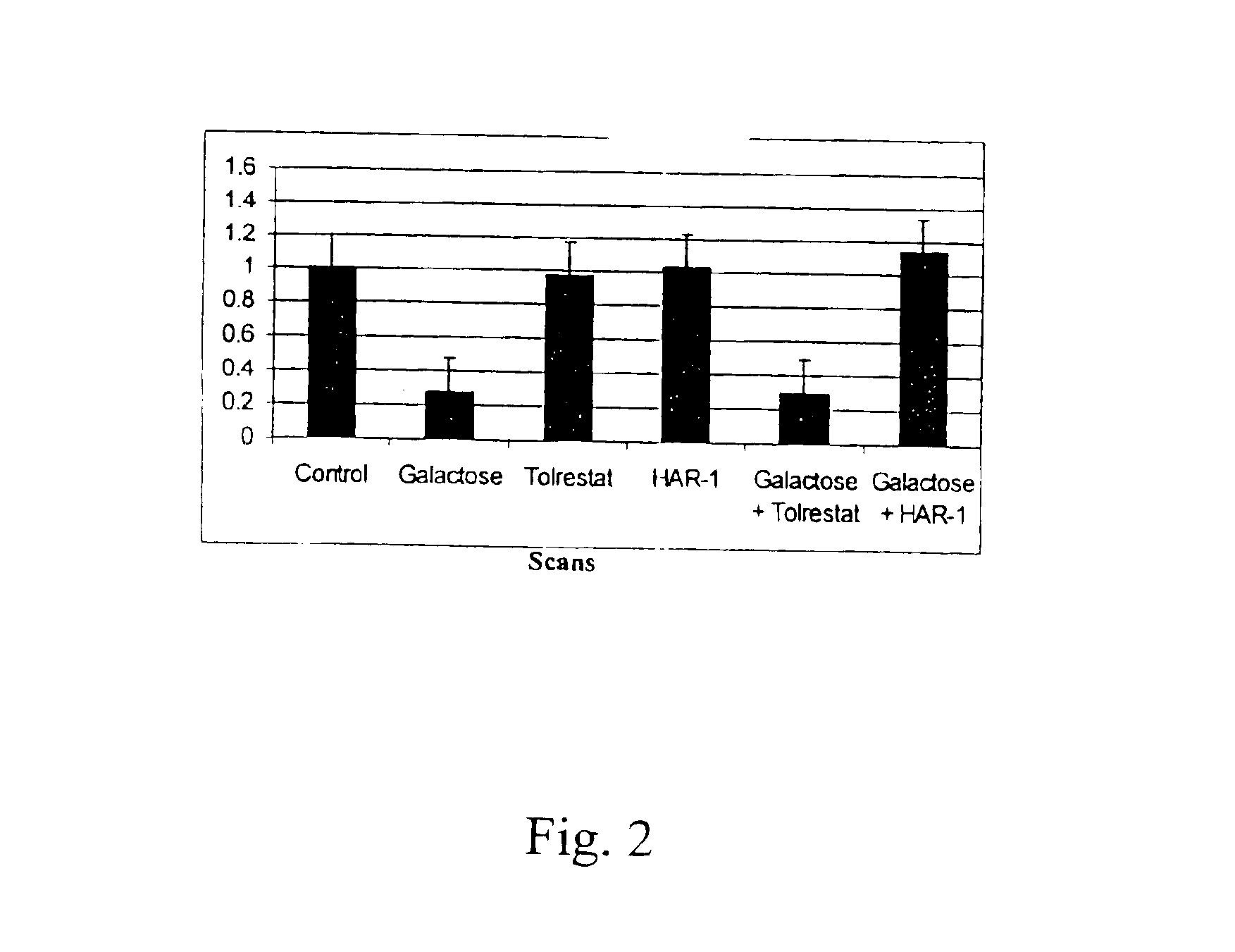
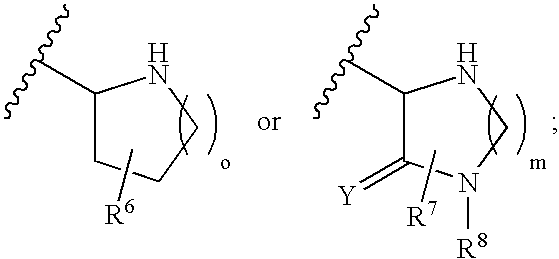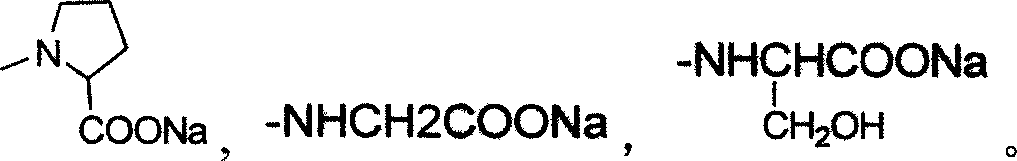Patents
Literature
155 results about "Aldose reductase inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
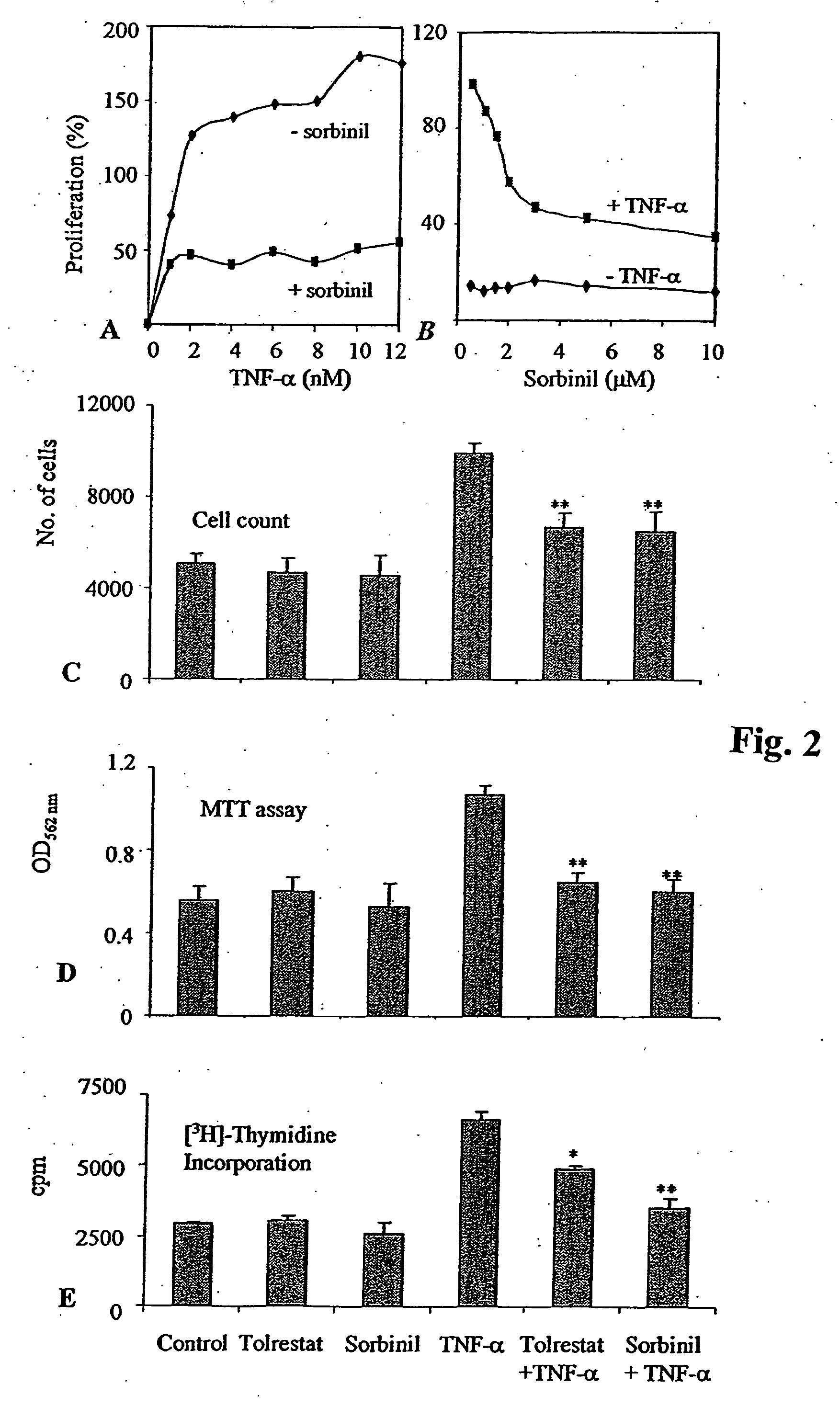
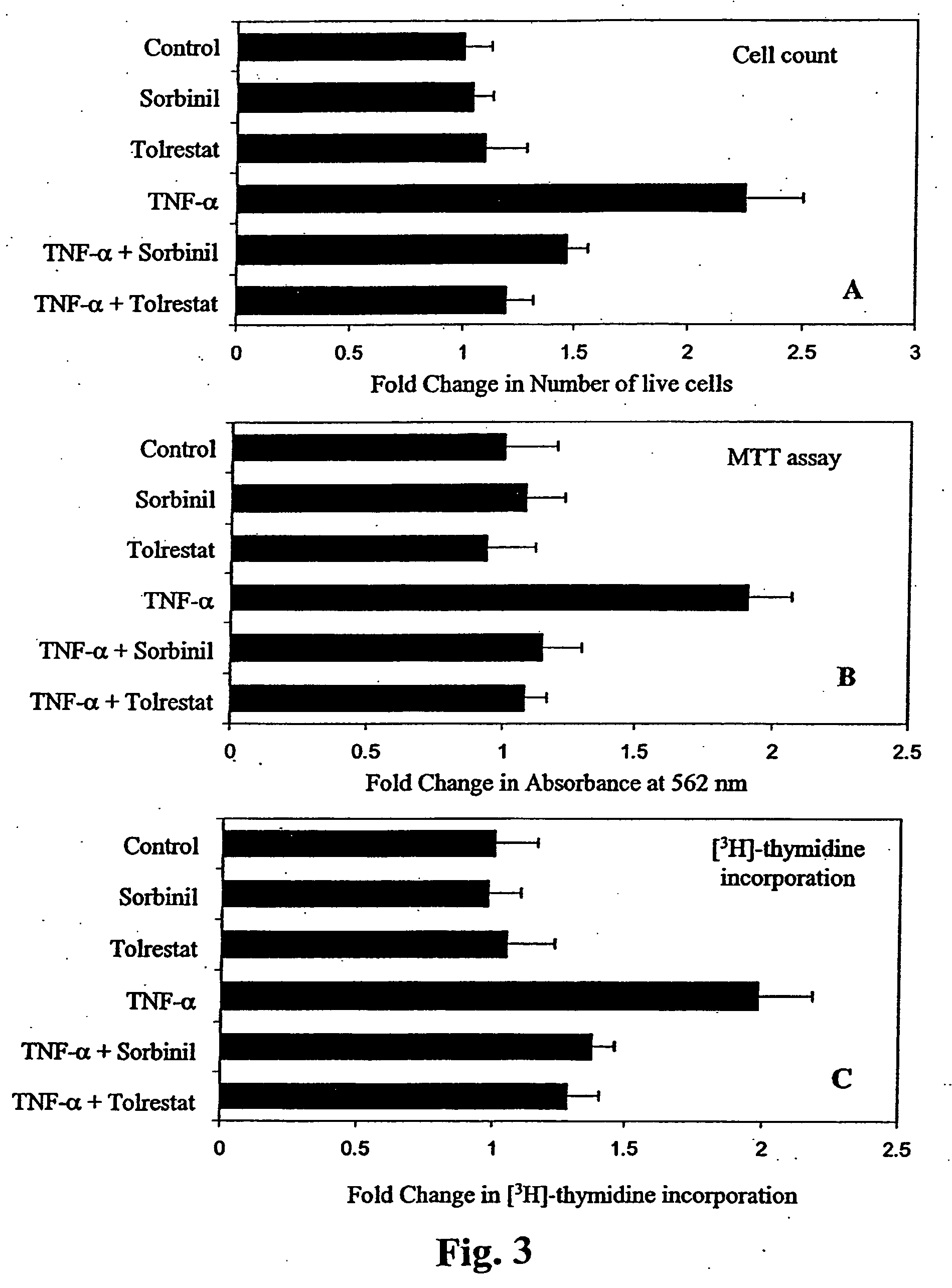
Aldose reductase inhibitors are a class of drugs being studied as a way to prevent eye and nerve damage in people with diabetes.
Compositions for treatment of diabetic complications
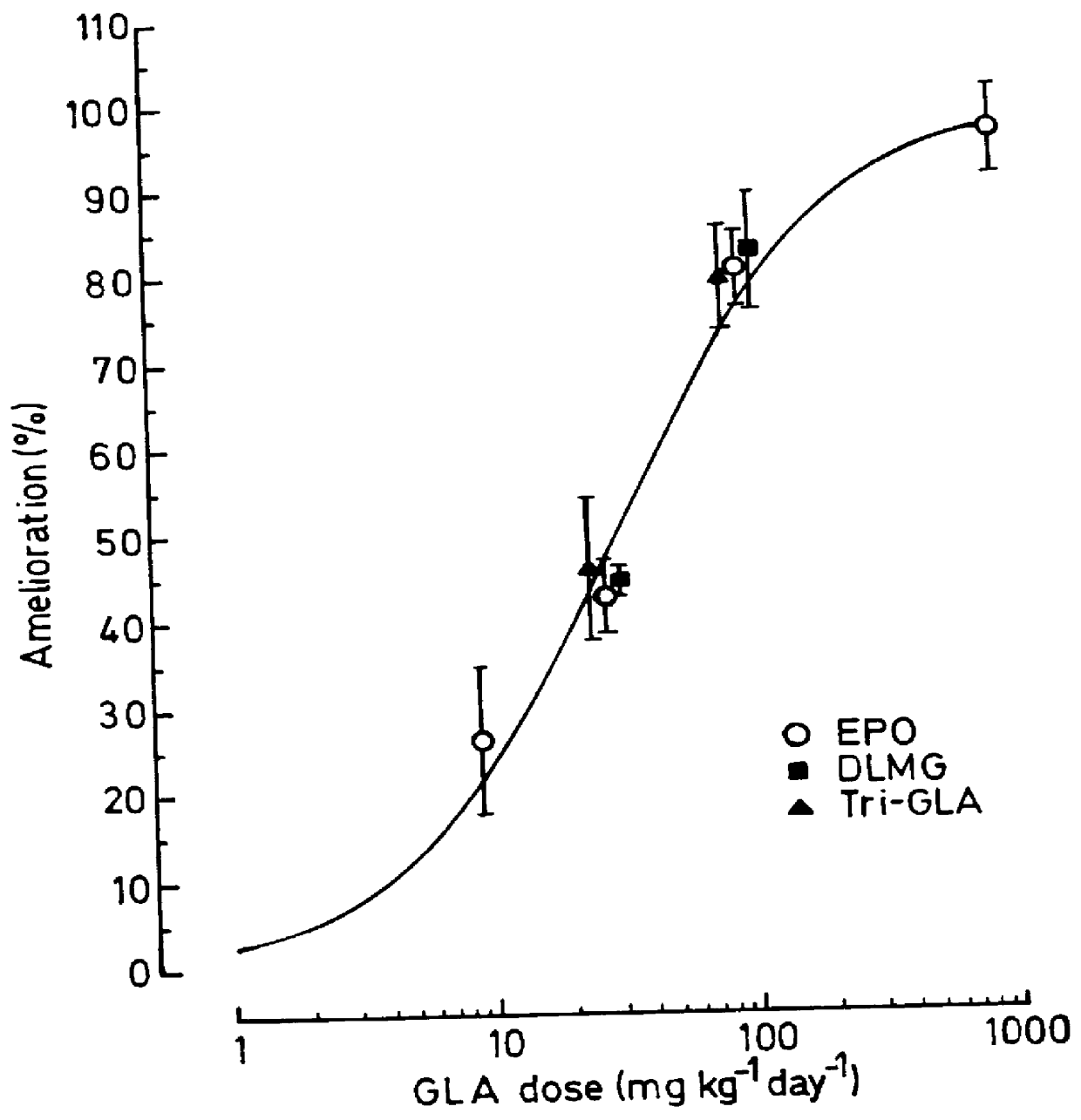
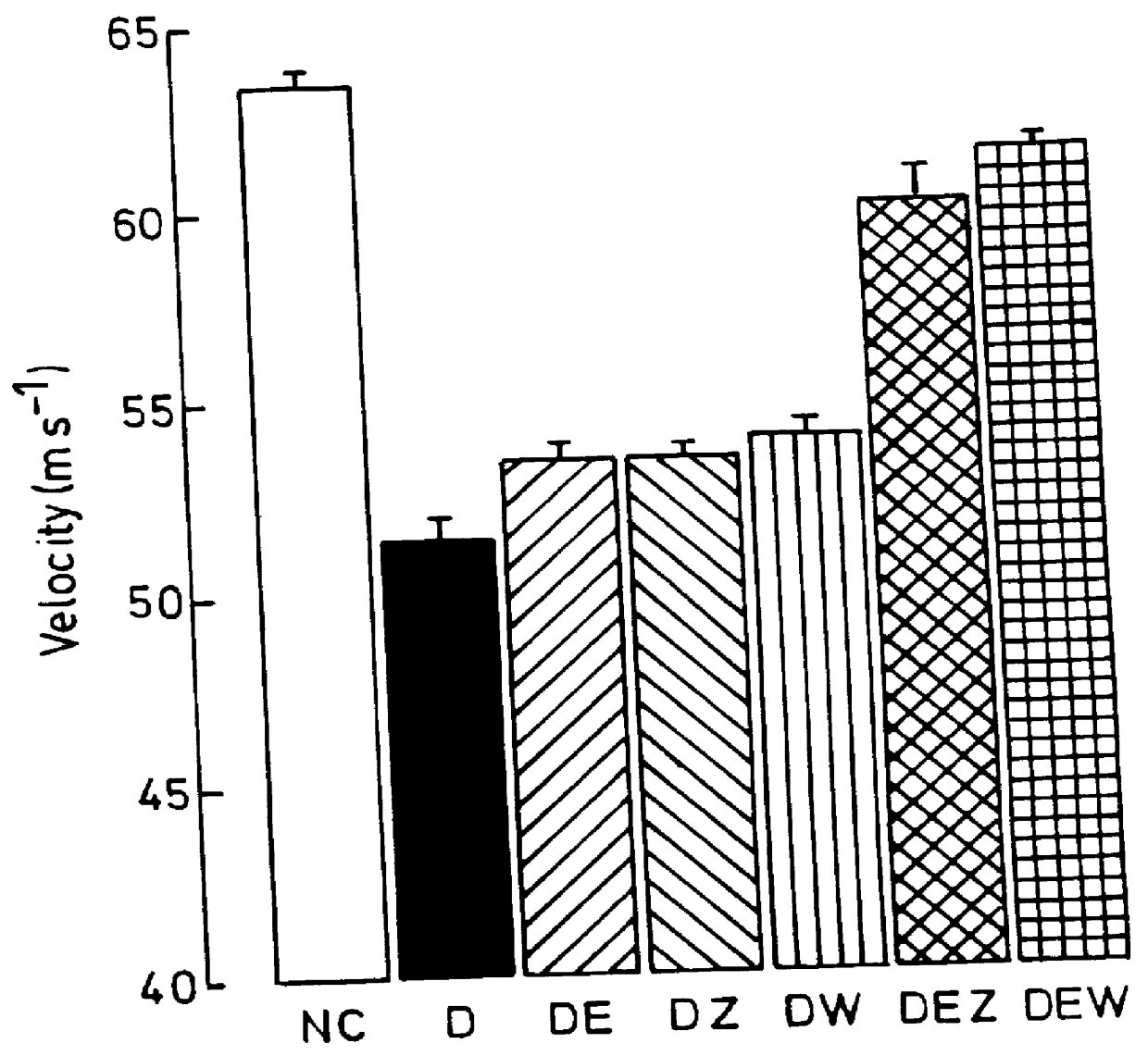
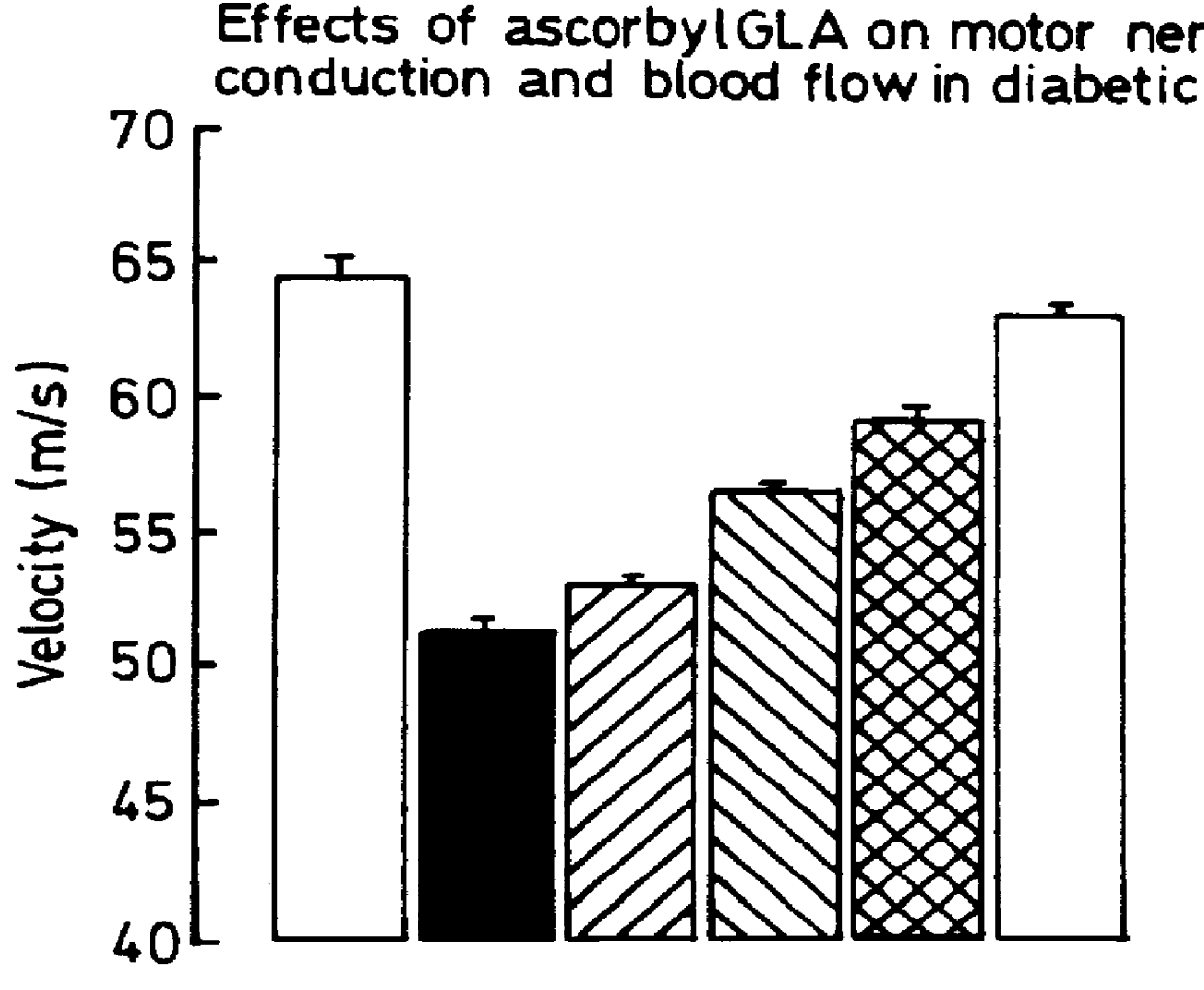
Use of 6-desaturated n-6 fatty acids, especially gammalinolenic acid (GLA), dihomogammalinolenic acid (DGLA) or arachidonic acid (AA), together with a pharmaceutically acceptable material reducing intracellular levels of sorbitol in the body, particularly an aldose reductase inhibitor, in the treatment of (including prophylactic treatment), and in the preparation of medicaments for the treatment of (including prophylactic treatment), the long-term complications of diabetes mellitus. Pharmaceutical compositions of said materials. The ascorbate esters of 6-desaturated n-6 fatty acids (other than GLA or DGLA) per se.
Owner:SCOTIA HLDG
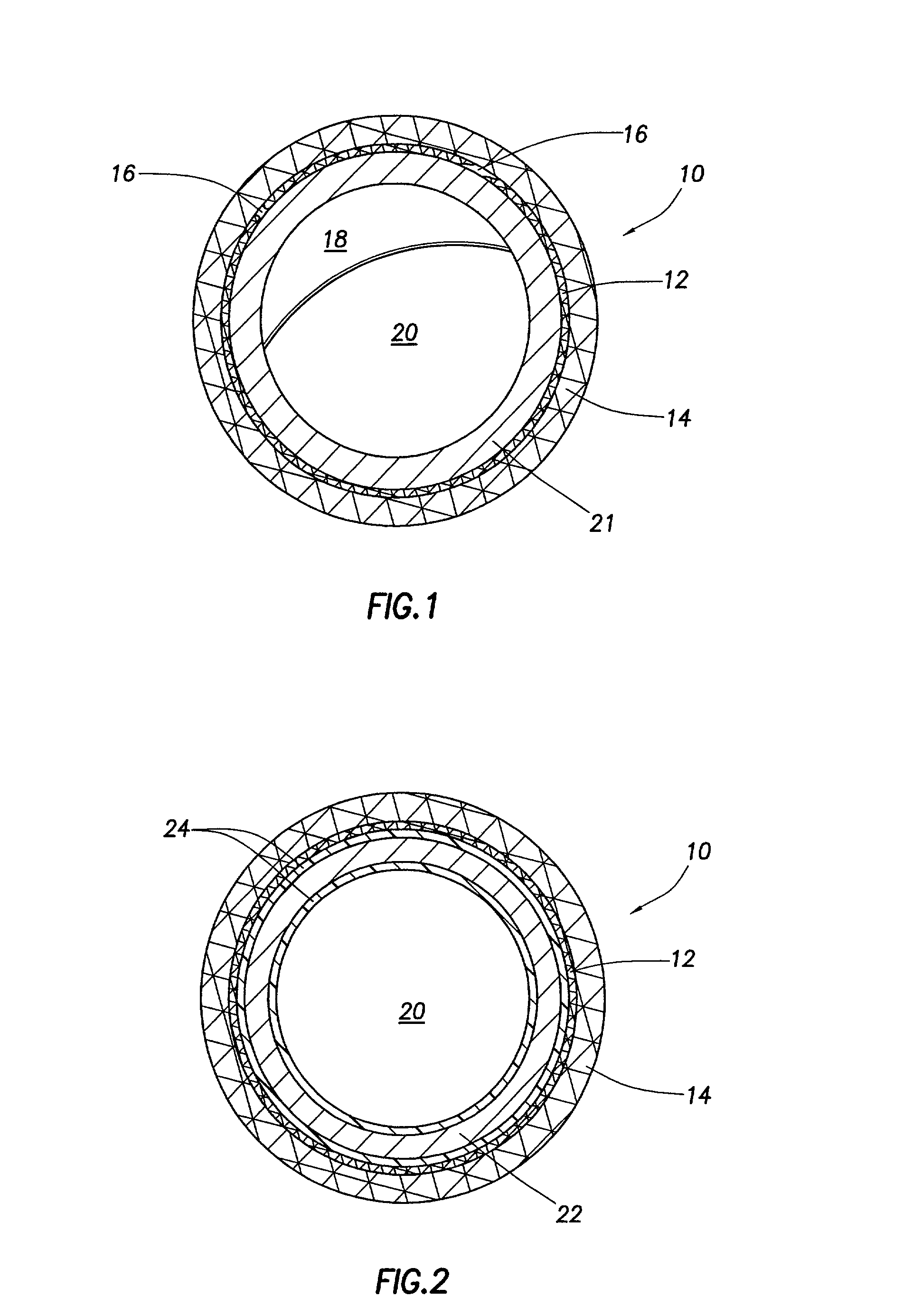
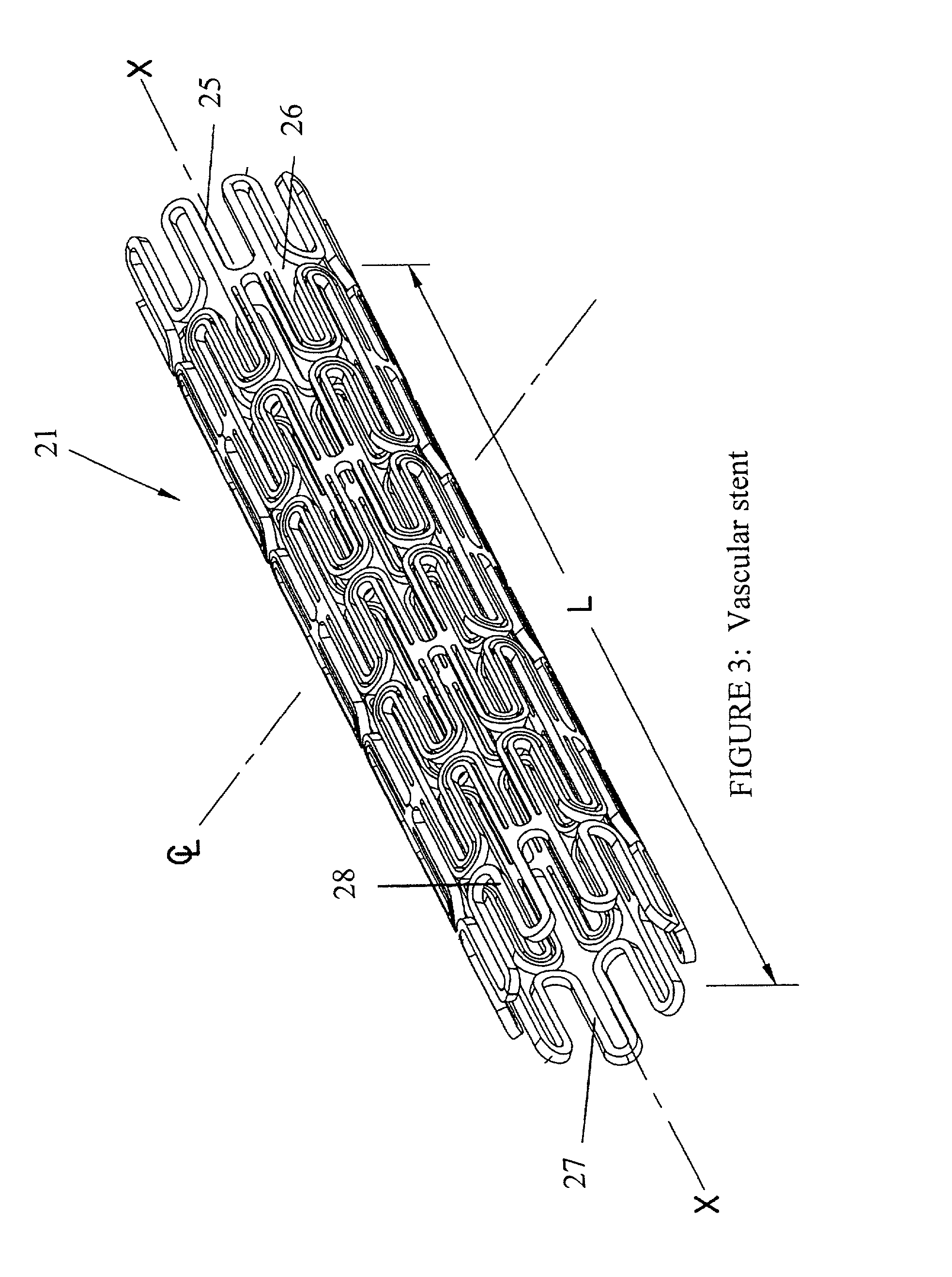
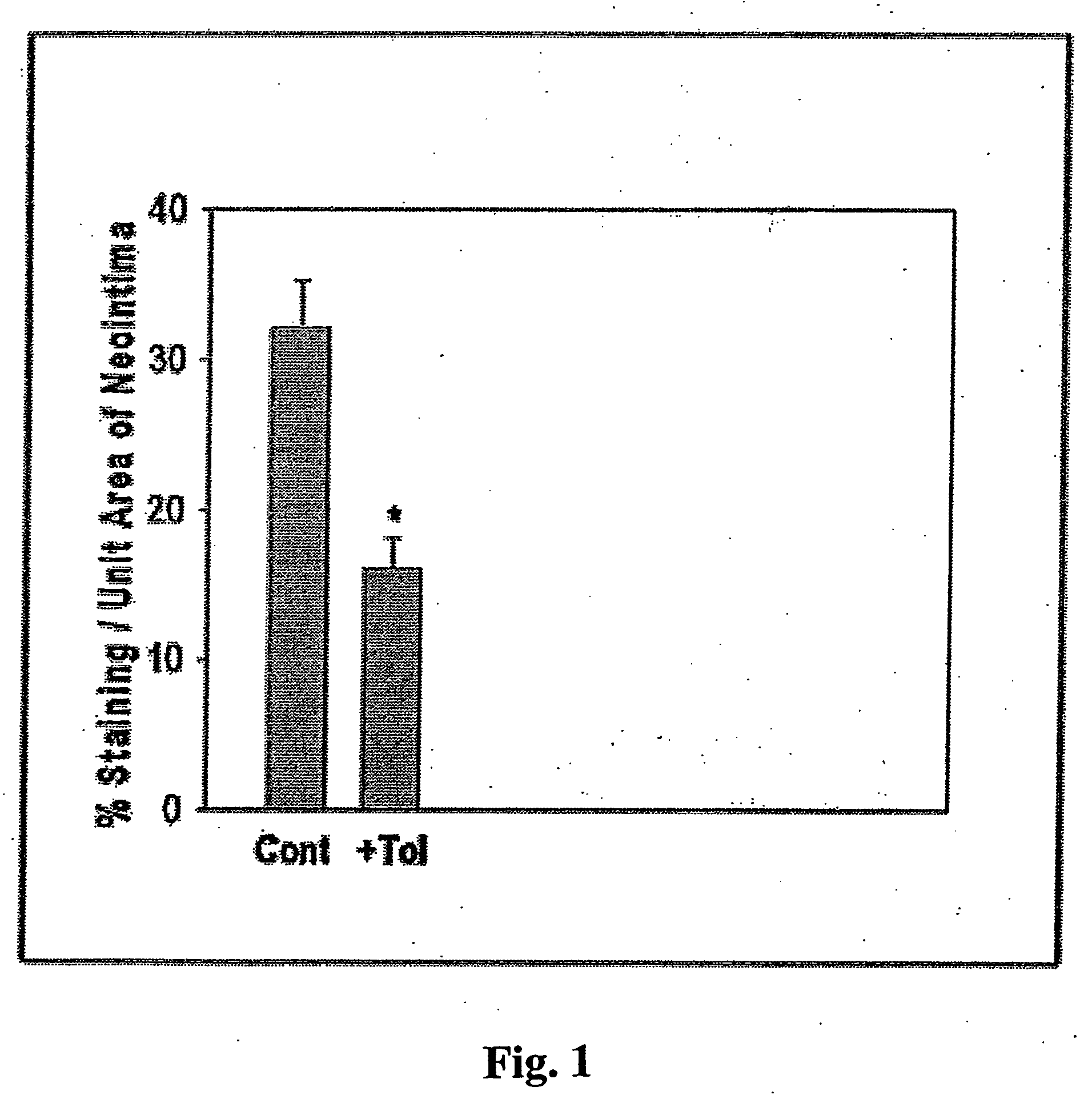
Stent coatings containing HMG-CoA reductase inhibitors
InactiveUS20030077310A1Hydrolysis can be preventedAvoid accessStentsSurgeryHMG-CoA reductaseDepressant
Stents with coatings comprising a combination of a restenosis inhibitor comprising an HMG-CoA reductase inhibitor and a carrier. Also provided are methods of coating stents with a combination of an HMG-CoA reductase inhibitor and a carrier. A preferred example of a restenosis inhibitor is cerivastatin. The stent coatings have been shown to release restenosis inhibitors in their active forms.
Owner:ZIMMER ORTHOBIOLIGICS
Combination therapy employing ileal bile acid transport inhibiting benzothiepines and HMG CO-A reductase inhibitors
Provided are novel benzothiepines, derivatives, and analogs thereof; pharmaceutical compositions containing them; and methods of using these compounds and compositions in medicine, particularly in the prophylaxis and treatment of hyperlipidemic conditions such as those associated with atherosclerosis or hypercholesterolemia, in mammals. Also provided are compositions and methods for combination therapy employing ileal bile acid transport inhibitors and HMG Co-A reductase inhibitors for the treatment of hyperlipidemic conditions.
Owner:GD SEARLE & CO
Methods involving aldose reductase inhibitors
InactiveUS20060293265A1Generating large amountPrevent and reduce damageHydroxy compound active ingredientsInorganic active ingredientsAutoimmune conditionCardiac Muscle Contraction
Embodiments of the invention include methods and compositions involving aldose reductase inhibitors for the treatment of sepsis and autoimmune diseases, including Type I diabetes and rheumatoid arthritis. The invention also pertains to preventing the loss of cardiac muscle contractibility.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Combination therapy employing ileal bile acid transport inhibiting benzothiepines and HMG Co-A reductase inhibitors
Provided are novel benzothiepines, derivatives, and analogs thereof; pharmaceutical compositions containing them; and methods of using these compounds and compositions in medicine, particularly in the prophylaxis and treatment of hyperlipidemic conditions such as those associated with atherosclerosis or hypercholesterolemia, in mammals. Also provided are compositions and methods for combination therapy employing ileal bile acid transport inhibitors and EG Co-A reductase inhibitors for the treatment of hyperlipidemic conditions.
Owner:GD SEARLE & CO
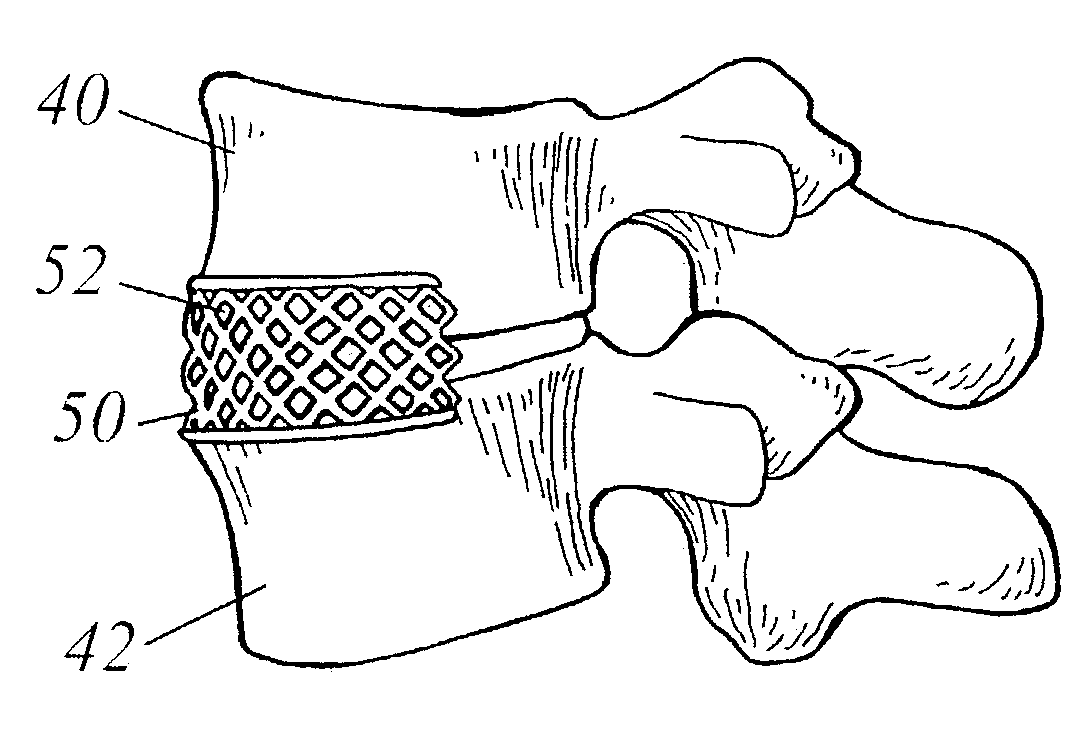
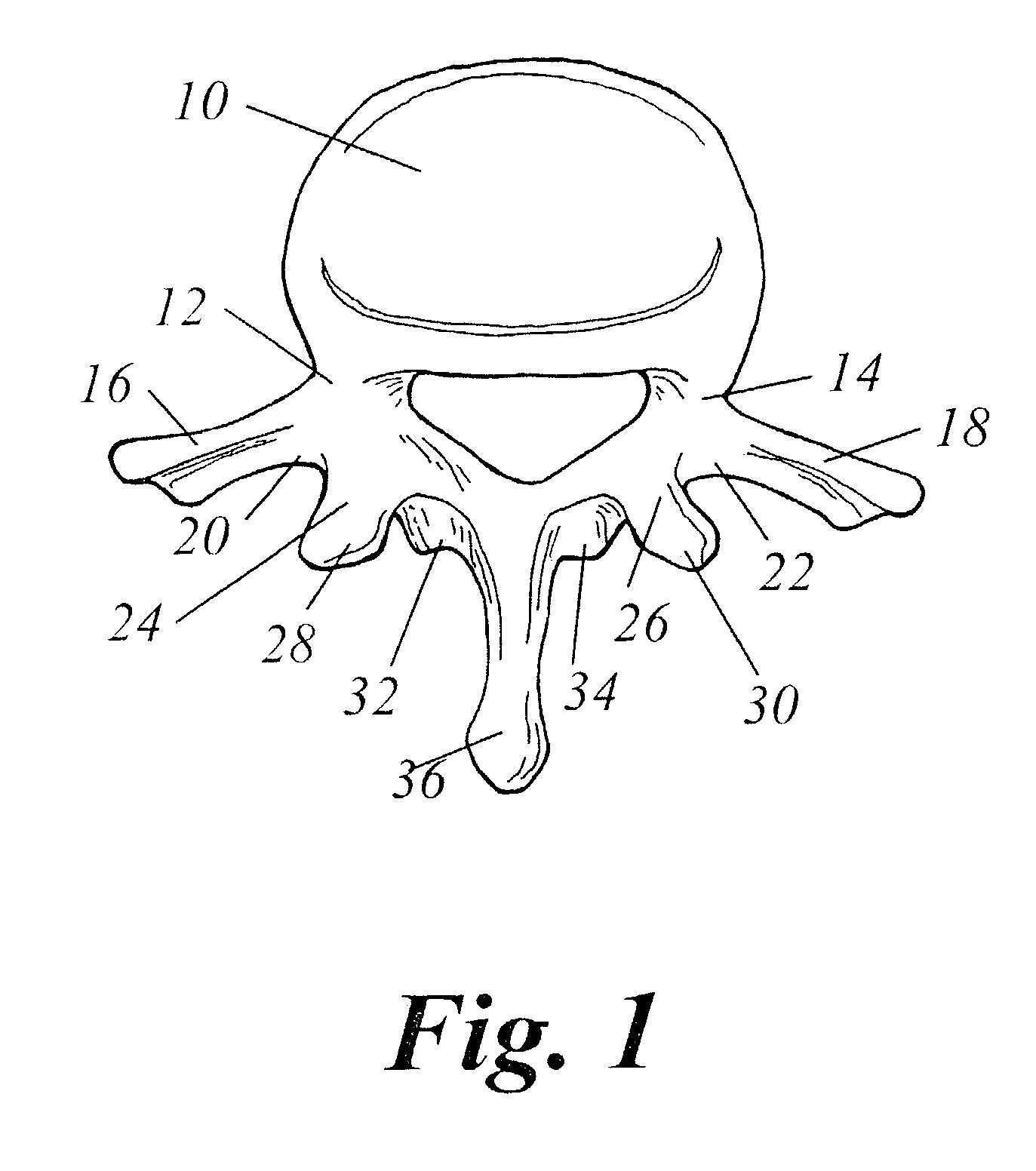
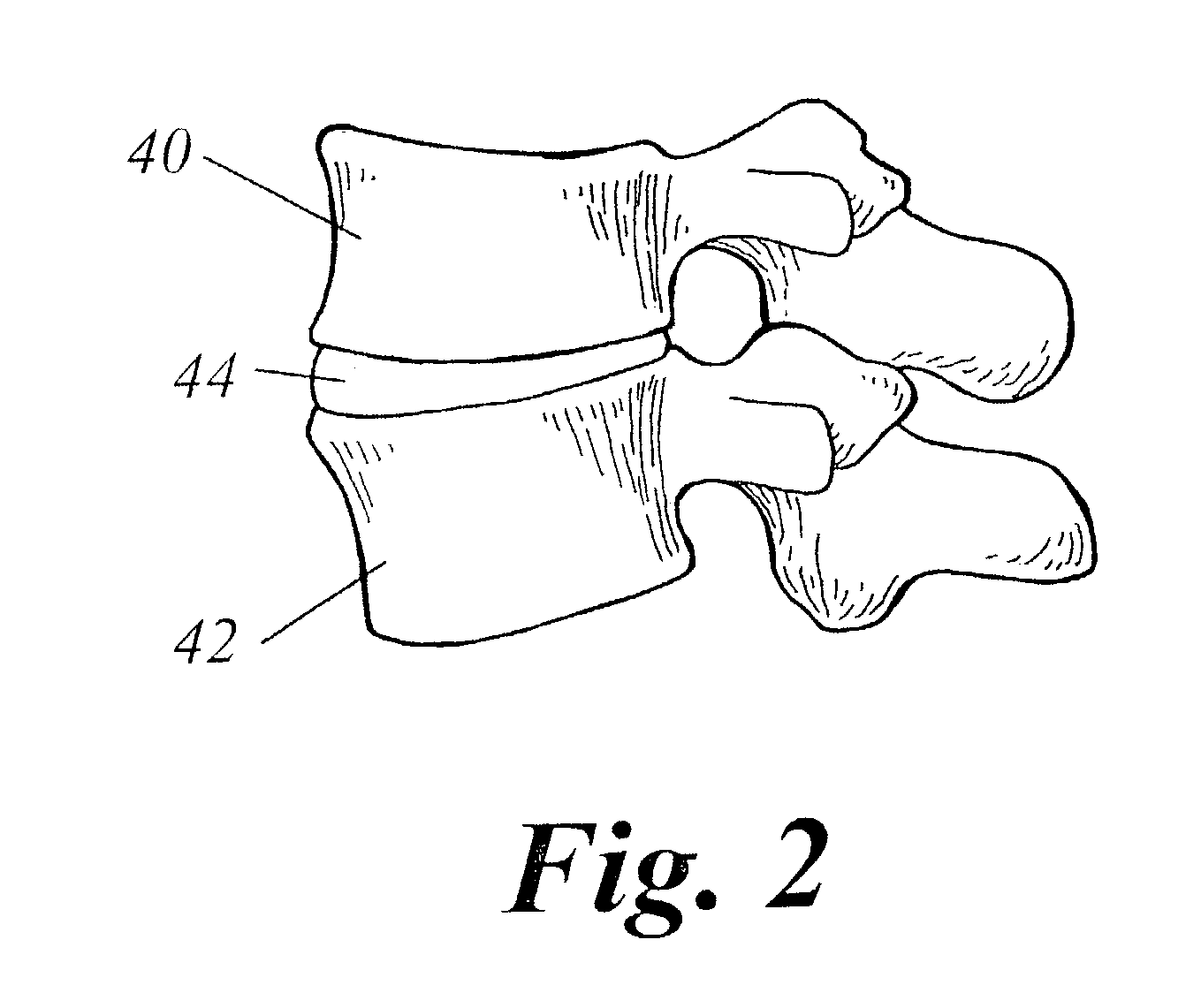
Spinal fusion using an HMG-CoA reductase inhibitor
ActiveUS7041309B2Promote bone growthImprove responseBiocideImpression capsHMG-CoA reductaseHeterotopic bone
An improved technique for spinal fusion including administration of an HMG-CoA reductase inhibitor to the fusion site. The HMG-CoA reductase inhibitor is preferably delivered to the site by a carrier. More preferably, the HMG-CoA reductase inhibitor is delivered to the site by a noncompressible delivery vehicle. The invention is suitable for promoting non-anatomic or heterotopic bone growth between any bony surfaces where bone growth is desired but does not naturally occur.
Owner:NEUROPRO TECH
Pharmaceutical combination comprising an ibat inhibitor and a bile acid binder
InactiveUS20130236541A1Maximal bile acid binding capacityDecrease needed doseOrganic active ingredientsBiocideDipeptidyl peptidaseAldose reductase inhibitor
The present invention relates to a combination comprising a substance with inhibiting effect on the ileal bile acid transport system (I BAT) and at least one other active substance selected from an IBAT inhibitor; an enteroendocrine peptide or enhancer thereof; a dipeptidyl peptidase-IV inhibitor; a biguanidine; an incretin mimetic; a thiazolidinone; a PPAR agonist; a HMG Co-A reductase inhibitor; a bile acid binder; and a TGR5 receptor modulator; wherein the IBAT inhibitor compound and the at least one other active substance are administered simultaneously, sequentially or separately.
Owner:ALBIREO
Macrocyclic beta-secretase inhibitors
Disclosed are novel compounds of the formula or a pharmaceutically acceptable salt or solvate thereof, wherein R1, R2, R3, n and X are as defined in the specification. Also disclosed are pharmaceutical compositions comprising the compounds of formula I. Also disclosed are methods of treating cognitive or neurodegenerative diseases such as Alzheimer's disease. Also disclosed are methods of treating a cognitive or neurodegenerative disease comprising administering to a patient I need of such treatment a combination of at least one compound of formula I and at least one compound selected from the group consisting of β-secretase inhibitors other than those of formula I, HMG-CoA reductase inhibitors, gamma-secretase inhibitors, non-steroidal anti-inflammatory agents, N-methyl-D-aspartate receptor antagonists, cholinesterase inhibitors and anti-amyloid antibodies.
Owner:MERCK SHARP & DOHME LLC
Combination therapy employing ileal bile acid transport inhibiting benzothiepines and HMG Co-A reductase inhibitors
Provided are novel benzothiepines, derivatives, and analogs thereof; pharmaceutical compositions containing them; and methods of using these compounds and compositions in medicine, particularly in the prophylaxis and treatment of hyperlipidemic conditions such as those associated with atherosclerosis or hypercholesterolemia, in mammals. Also provided are compositions and methods for combination therapy employing ileal bile acid transport inhibitors and HMG Co-A reductase inhibitors for the treatment of hyperlipidemic conditions.
Owner:GD SEARLE & CO
Controlled release complex composition comprising angiotensin-ii-receptor blockers and hmg-coa reductase inhibitors
ActiveUS20100074951A1Maximize the effect of treatmentPreventing and reducing side effectBiocideMetabolism disorderHMG-CoA reductaseCoronary artery disease
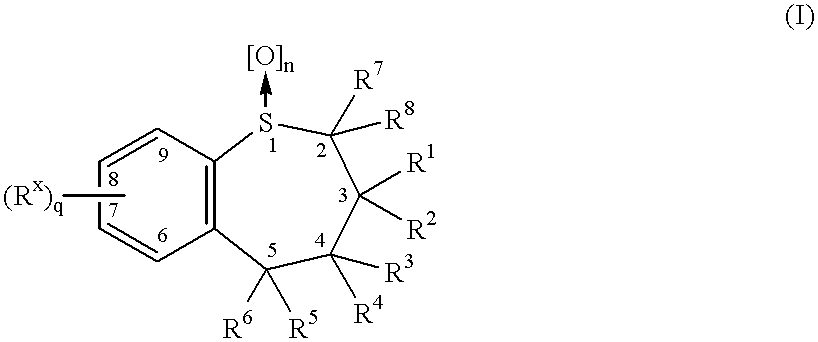
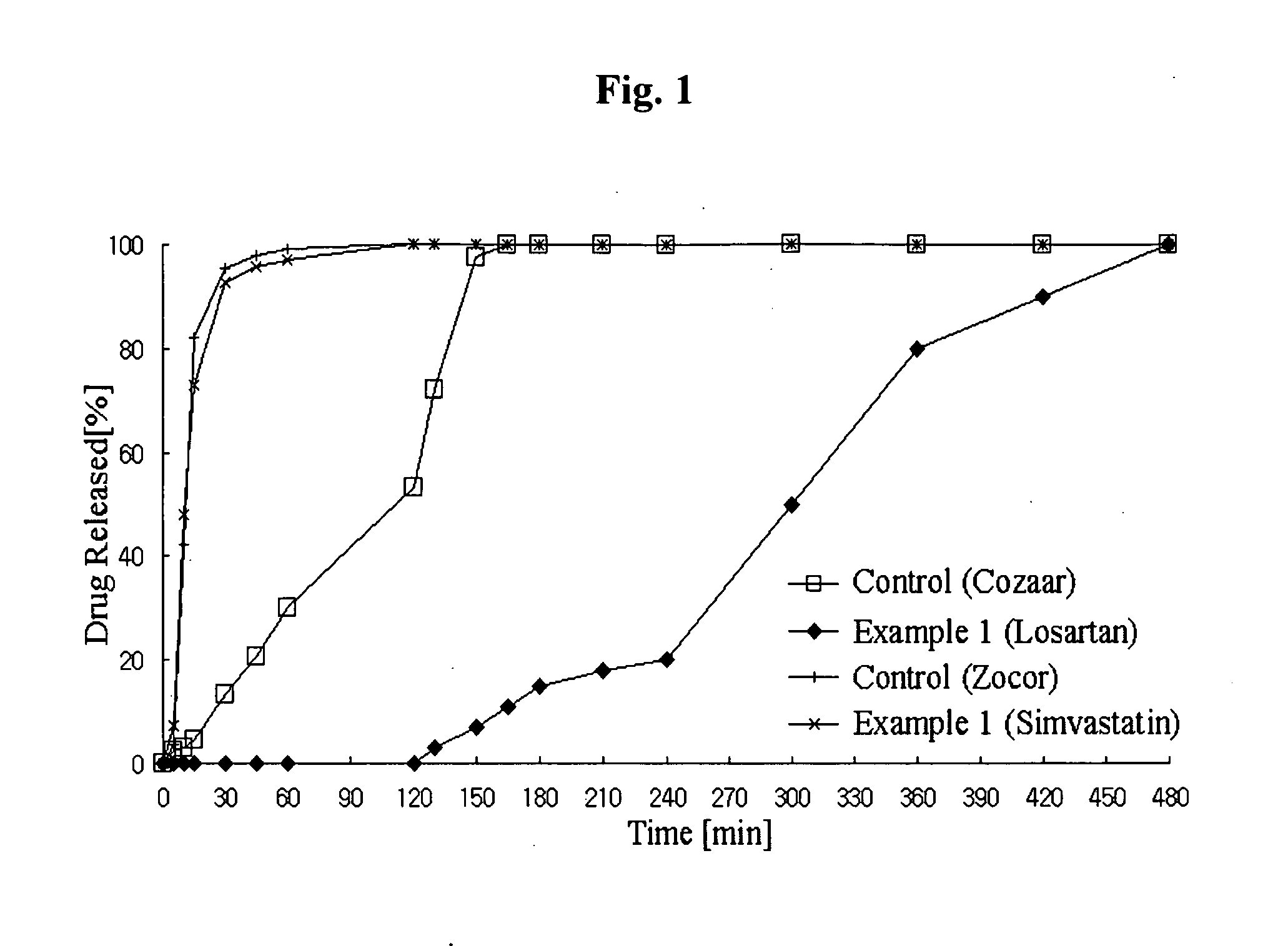
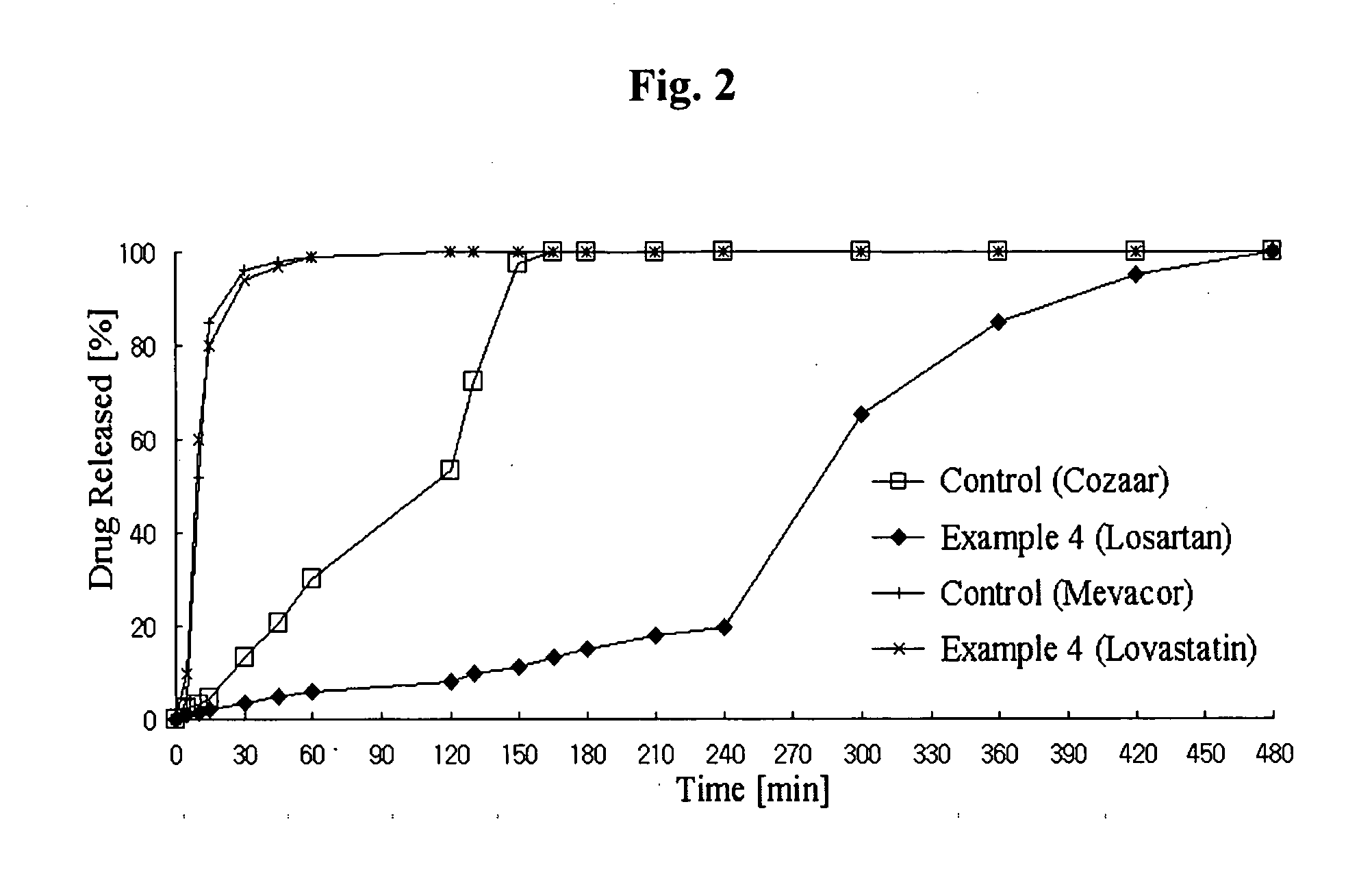
Disclosed herein is a lag time delayed-release combination pharmaceutical composition comprising of an angiotensin-II-receptor blocker and an HMG-CoA reductase inhibitor, as well as a preparation method thereof. The composition is designed based on chronotherapy in which active ingredients are administered to have different onset times, such that the release of each active ingredient of the composition in body can be lag time delayed to a specific rate. Also, the composition is very effective for the treatment of hypertension and the prevention of complications in patients having metabolic syndromes which show diabetes, obesity, hyperlipidemia, coronary artery diseases and the like. More specifically, the composition is a drug delivery system designed such that the release of each drug is controlled to a specific rate, and it can show the most ideal effect, when it is absorbed in body.
Owner:HANALL PHARMA CO LTD
Topical compositions comprising 5-alpha reductase inhibitors
InactiveUS20100048598A1Enhanced topical deliveryShorten the progressBiocideAerosol delivery5 Alpha-Reductase InhibitorChemical composition
The present invention relates to topical compositions comprising 5α-reductase inhibitors. The present invention also includes processes for preparation of such topical compositions and methods of using them.
Owner:DR REDDYS LAB LTD +1
Compositions of choleseteryl ester transfer protein inhibitors and HMG-CoA reductase inhibitors
InactiveUS20040132771A1Overcomes drawbackImprove concentrationPowder deliveryBiocideHMG-CoA reductaseBioavailability
A composition comprises (1) a solid amorphous adsorbate comprising a cholesteryl ester transfer protein (CETP) inhibitor and a substrate; and (2) an HMG-CoA reductase inhibitor. The solid amorphous adsorbate provides concentration enhancement of the CETP inhibitor relative to a control composition consisting essentially of the unadsorbed CETP inhibitor alone, resulting in improved bioavailability.
Owner:BEND RES
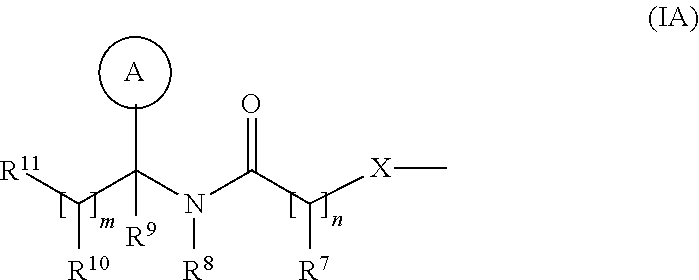
Combination therapeutic compositions and method of use
The present invention provides pharmaceutical compositions and methods for the treatment of diabetes mellitus using combination therapy. The compositions relate to a compound of Formula I selected from one or more of betaines, lipidic betaines, betaine lipids and an antidiabetic agent such as sulfonylureas, biguanides, glitazones, .alpha.-glucosidase inhibitors, potassium channel antagonists, aldose reductase inhibitors, glucagon antagonists, activators of RXR, insulin therapy or other anti-obesity agent. The methods include the administration of the combination of compound of Formula I with antidiabetic agent where the two components are delivered in a simultaneous manner, where the compound of Formula I is administered first, followed by the antidiabetic agent, as well as wherein the antidiabetic agent is delivered first followed by the compound of Formula I.
Owner:MESSADEK JALLAL
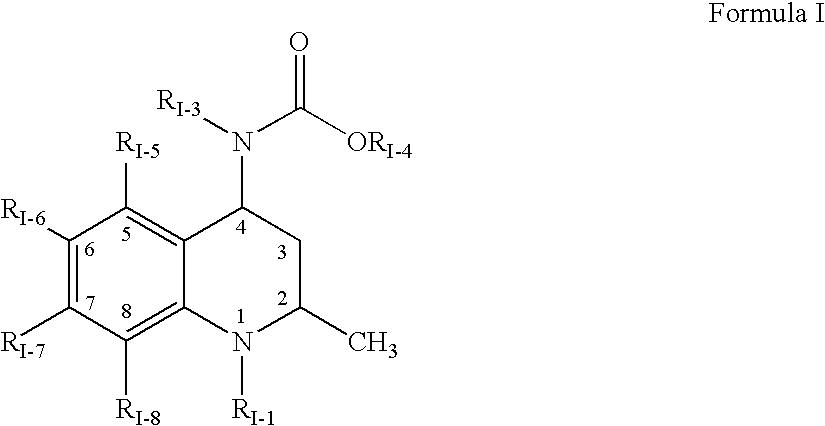
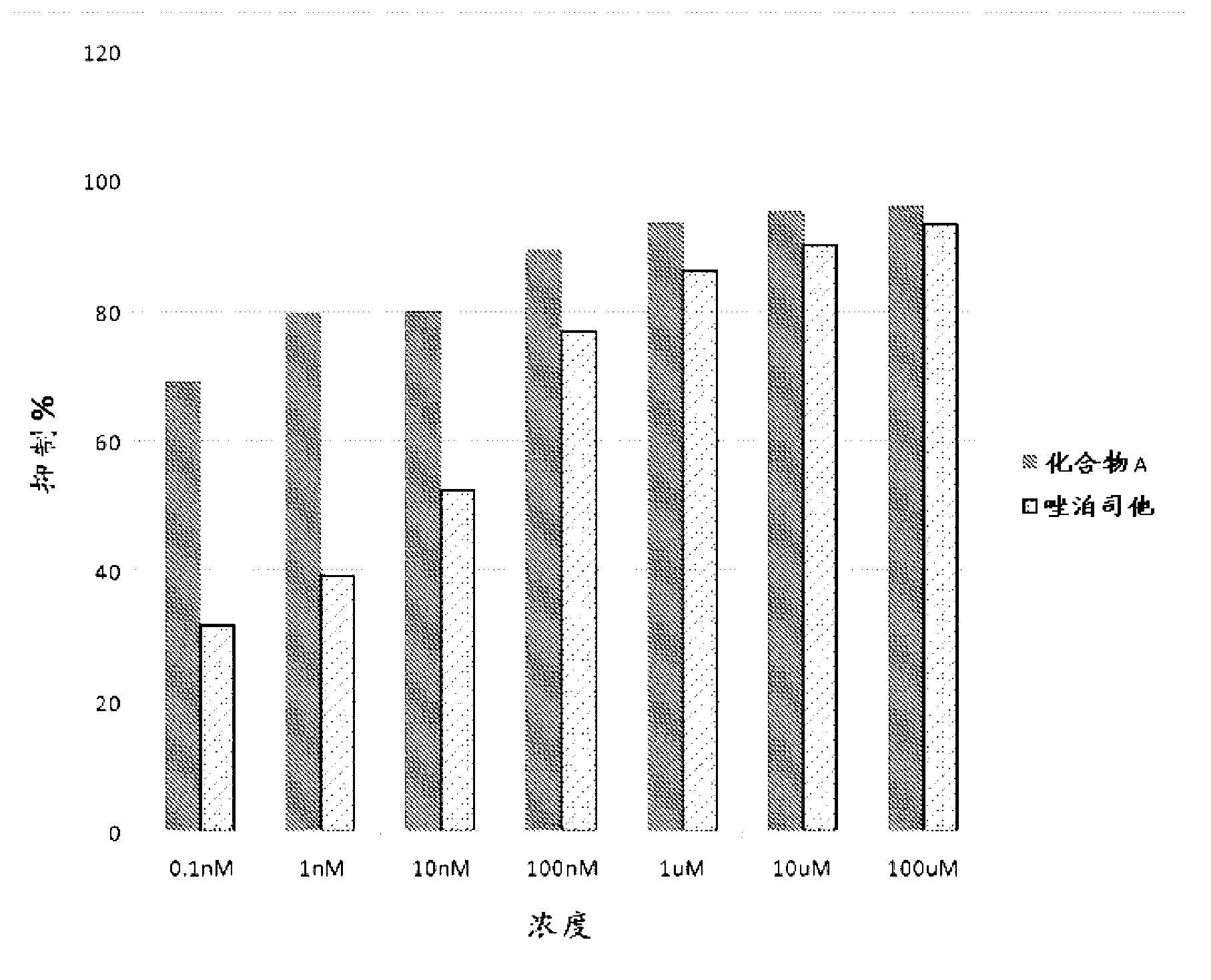
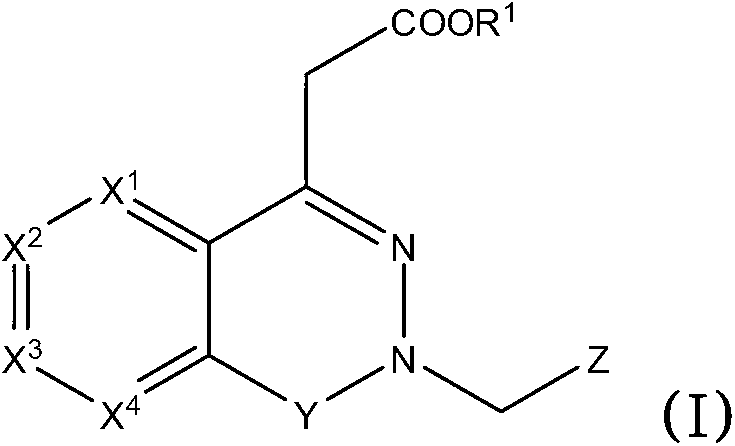
Aldose reductase inhibitors and uses thereof
The present invention relates to novel compounds and pharmaceutical compositions thereof, and methods for promoting healthy aging of skin, the treatment of skin disorders, the treatment of cardiovascular disorders, the treatment of renal disorders, the treatment of angiogenesis disorders, such as cancer, treatment of tissue damage, such as non-cardiac tissue damage, the treatment of evolving myocardial infarction, and the treatment of various other disorders, such as complications arising from diabetes with the compounds and compositions of the invention. Other disorders can include, but are not limited to, atherosclerosis, coronary artery disease, diabetic nephropathy, diabetic neuropathy, diabetic retinopathy, infections of the skin, peripheral vascular disease, stroke, and the like.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
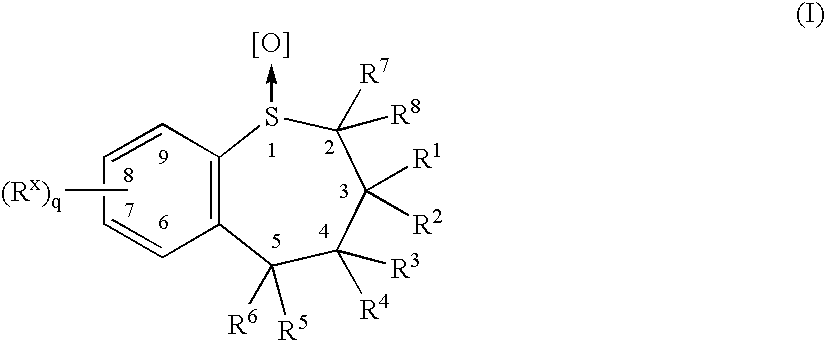
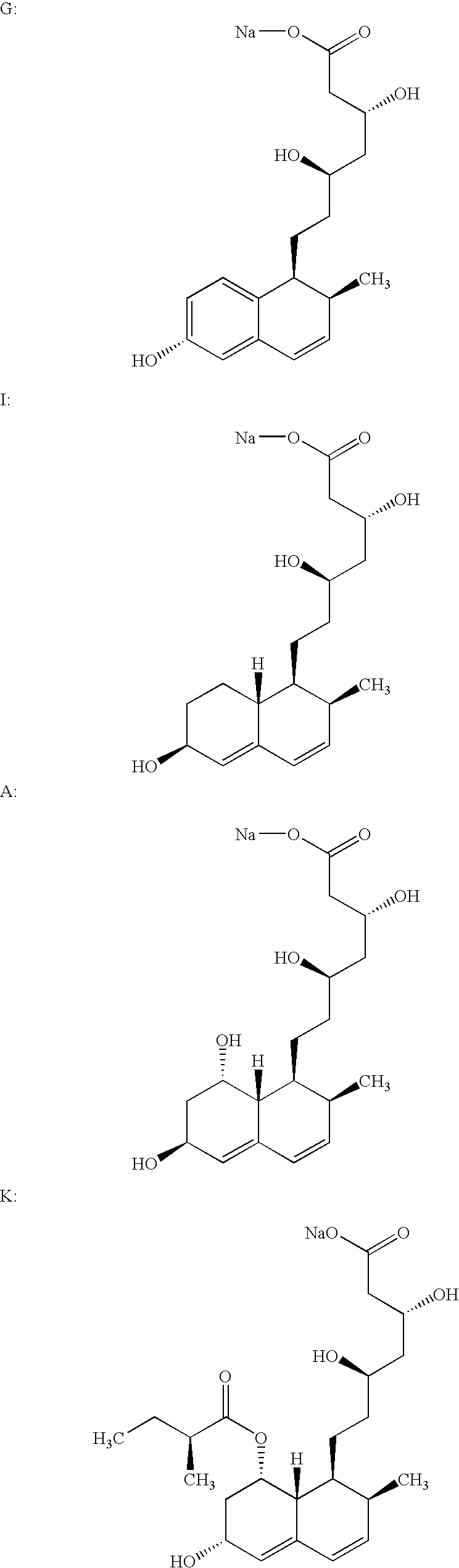
Tanshinone derivative and its application in preparing aldose reduction enzyme inhibitor pharmaceutical
InactiveCN101012270AInhibitory activityEnhanced inhibitory effectOrganic active ingredientsMetabolism disorderAldose reductase inhibitorInhibitory effect
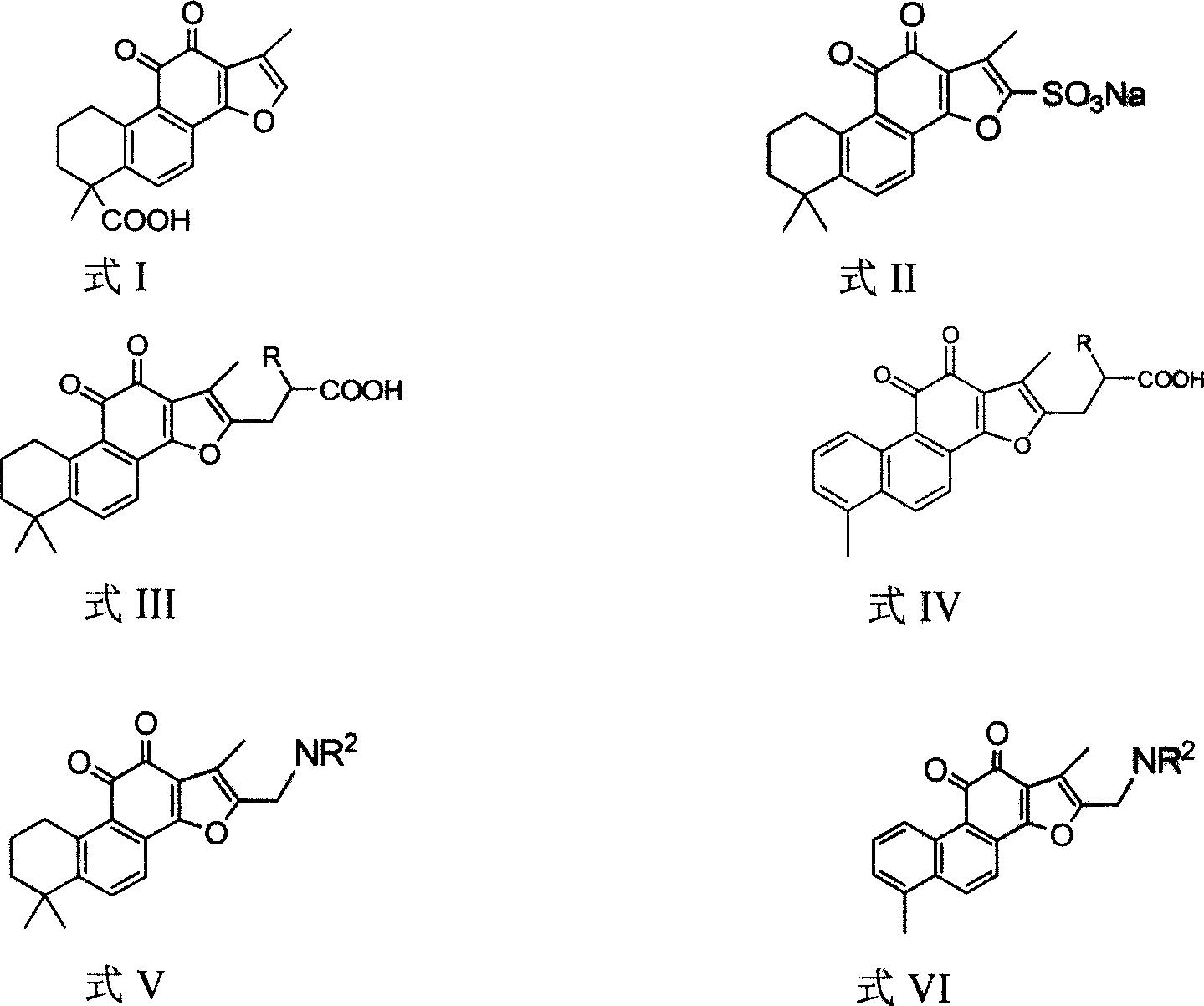
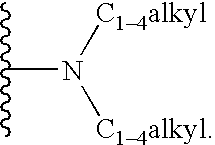
The invention discloses a tanshinone derivant and application to make aldose reductase inhibitor drug, which possesses chemical formula as formula I-IV, wherein R in the formula II and IV represents -COOH, -NO2, -CN; NR2 in the formula V and VI represents molecular formula (A).
Owner:GUANGDONG UNIV OF TECH
Stable pharmaceutical formulation comprising HMC-CoA reductase inhibitor
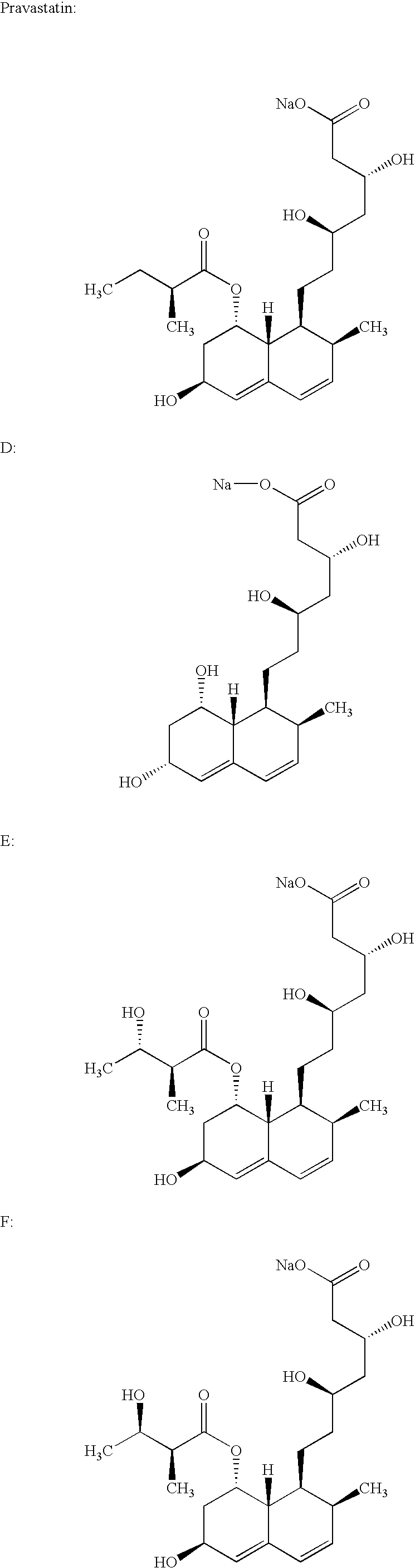
Lovastatin, pravastatin, simvastatin, mevastatin, atorvastatin, and derivatives and analogs thereof are known as HMG-CoA reductase inhibitors and are used as antihypercholesterolemic agents. The majority of them are produced by fermentation using microorganisms of different species identified as species belonging to Aspergillus, Monascus, Nocardia, Amycolatopsis, Mucor or Penicillium genus, and some are obtained by treating the fermentation products using the methods of chemical synthesis or they are the products of total chemical synthesis. The aforementioned active substances may be destabilised by the environmental factors, their degradation may also be accelerated by interactions with other pharmaceutical ingredients, such as fillers, binders, lubricants, glidants and disintegrating agents, therefore the pharmaceutical ingredients and the process for preparation of the pharmaceutical formulation should be meticulously chosen to avoid the aforementioned undesired interactions and reactions. The present invention relates to a stable solid pharmaceutical formulation for the treatment of hypercholesterolemia and hyperlipidemia. More precisely, the present invention relates to the new stable solid pharmaceutical formulation containing as an active ingredient a HMG-CoA reductase inhibitor, such as atorvastatin, pravastatin, fluvastatin and cerivastatin or pharmaceutically acceptable salts thereof.
Owner:LEK PHARMA & CHEM
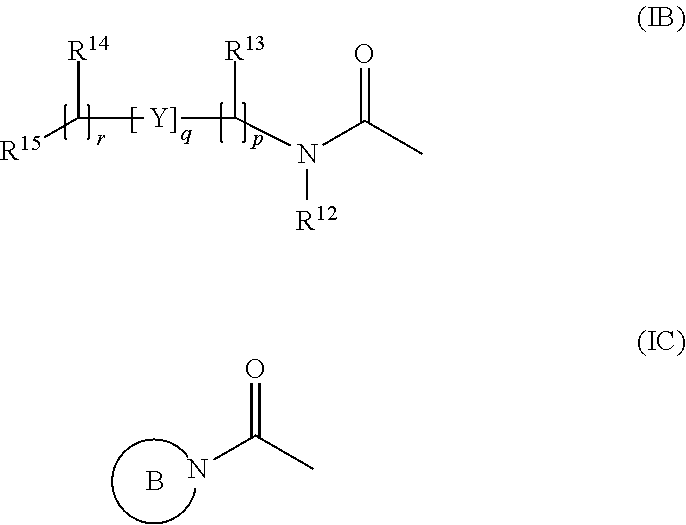
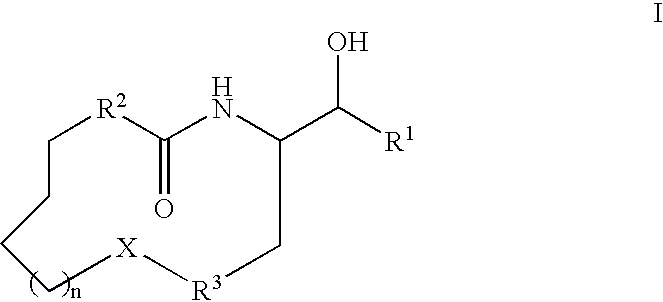
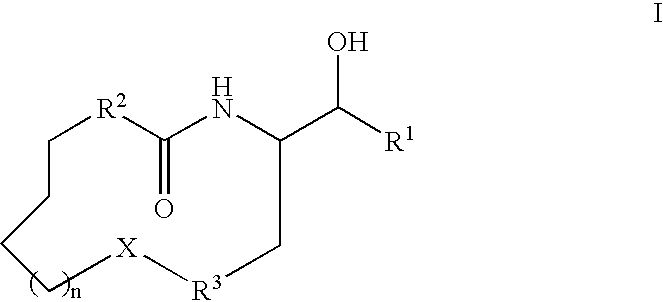
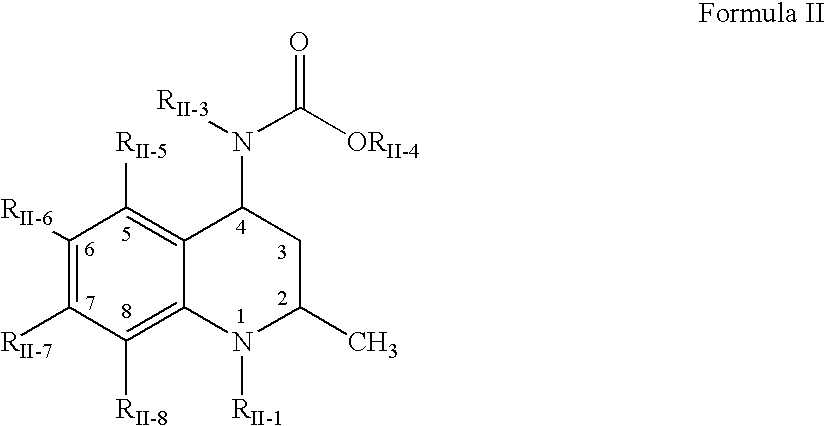
Aldose reductase inhibitors and uses thereof
The present invention relates to novel compounds and pharmaceutical compositions thereof, and methods for promoting healthy aging of skin, the treatment of skin disorders, the treatment of cardiovascular disorders, the treatment of renal disorders, the treatment of angiogenesis disorders, such as cancer, treatment of tissue damage, such as non-cardiac tissue damage, the treatment of evolving myocardial infarction, and the treatment of various other disorders, such as complications arising from diabetes with the compounds and compositions of the invention. Other disorders can include, but are not limited to, atherosclerosis, coronary artery disease, diabetic nephropathy, diabetic neuropathy, diabetic retinopathy, infections of the skin, peripheral vascular disease, stroke, and the like.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Compositions containing HMG Co-A reductase inhibitors and policosanol
InactiveUS6890941B1Lowering of total serum cholesterol levelInhibiting cholesterol synthesisBiocideHydroxy compound active ingredientsLipid storageElevated serum cholesterol
The present invention provides pharmaceutical compositions, methods, combinations, and kits for treating a disorder related to elevated serum cholesterol concentration, for example, hypercholesterolemia, atherosclerosis, elevated LDL plasma levels, low HDL plasma levels, hypertriglyceridemia, hyperlipidemia, hypertension, cholesterol gallstones, and lipid storage diseases. The compositions, methods, combinations, and kits of the present invention are pharmaceutical compositions comprising atherapeutically effective amount of a lipid regulating agent, such as a HMG-CoA reductase inhibitor, and compound that inhibits cholesterol synthesis at a point between the formation of acetate and mevalonate. A typical pharmaceutical composition of the invention contains and effective amount of atorvastatin and an effective amount of policosanol.
Owner:PROCAPS
Method of using combination preparation comprising angiotensin-ii-receptor blocker and hmg-coa reductase inhibitor
ActiveUS20110213004A1Reduce the impactIncreased riskBiocideMetabolism disorderHMG-CoA reductaseSide effect
Disclosed herein is a combination therapy and a combination preparation of an angiotensin-II-receptor blocker and an HMG-CoA reductase inhibitor characterized in that the angiotensin-II-receptor blocker is absorbed substantially later than the HMG-CoA reductase inhibitor. As the angiotensin-II-receptor blocker and the HMG-CoA reductase inhibitor are released at different times, the present combination therapy prevents competitive inhibition between the two drugs and side effects, as well as simultaneously provides synergistic effects for each active ingredient and convenience of taking the drugs.
Owner:HANALL PHARMA CO LTD
Medicinal composition containing insoluble medicament
InactiveCN102145003AAvoid degradationAvoid Absorption IrregularitiesUrinary disorderAmide active ingredientsPoor complianceSide effect
The invention discloses a medicinal composition containing an insoluble alpha-receptor retardant and / or 5alpha-reductase inhibitor with an effective dose, which comprises the following components of: principal ingredients, a substrate, a solubilizer, a sorbefacient and an additive in a weight ratio of 1:(8-7,000):(0-460):(0-150):(0-150), wherein the medicinal composition at least contains one of the solubilizer and the sorbefacient. The effective dose of the insoluble alpha-receptor retardant and / or the 5alpha-reductase inhibitor is in 0.05 to 80 milligrams of parent compounds of the insoluble alpha-receptor retardant and / or the 5alpha-reductase inhibitor, the weight of a preparation per unit is between 0.8 and 4.2 grams. The medicinal composition can be subjected to oral administration or rectum administration, so the defects of poor curative effect and large toxic and side effect in the conventional oral administration and systemic administration of injection and large side effect and poor compliance of patients in local injection administration can be overcome, the lasting time of the medicinal effect can be increased, and better treatment means can be provided for medical care personnel and patients; and a product process is simple and is suitable for industrial batch production.
Owner:张立英
Composition for treating chromic complications of diabetes mellitus
The invention relates to an aldose reductase inhibitor for treating chromic complications of diabetes mellitus after being taken orally. The composition comprises natural medicines with aldose reductase inhibition action and at least one medicine with alpha-glycosidase inhibition action and has double actions containing aldose reductase inhibition action and alpha-glycosidase inhibition action. Accidental clinical and pharmacological discovery proves that the composition has the effect of reducing postprandial blood sugar by glycosidase inhibition action and is capable of protecting the retina of a patient with diabetes mellitus, delaying the progress of diabetes mellitus caused senile dementia, and relieving the symptoms of the diabetes mellitus caused senile dementia by virtue of aldose reductase inhibition action.
Owner:JINZHOU BOZE PHARMA TECH DEV
Process for obtaining HMG-CoA reductase inhibitors of high purity
Lovastatin, pravastatin, simvastatin, mevastatin, atorvastatin, and derivatives and analogs thereof are known as HMG-CoA reductase inhibitors and are used as antihypercholesterolemic agents. The majority of them are produced by fermentation using microorganisms of different species identified as species belonging to Aspergillus, Monascus, Nocardia, Amycolatopsis, Mucor or Penicillium genus, Streptomyces, Actinomadura, Micromonospora, some are obtained by treating the fermentation products using the method of chemical synthesis or they are the products of total chemical synthesis. The purity of the active ingredient is an important factor for manufacturing the safe and effective pharmaceutical, especially if the pharmaceutical product must be taken on a longer term basis in the treatment or prevention of high plasma cholesterol. The accumulation of the impurities from the pharmaceuticals of lower purity may cause many side effects during the medical treatment. The present invention relates to a new industrial process for the isolation of HMG-CoA reductase inhibitors using so-called displacement chromatography. Use of the invention enables one to obtain HMG-CoA reductase inhibitors of high purity, with high yields, lower production costs and suitable ecological balance.
Owner:LEK PHARMA D D
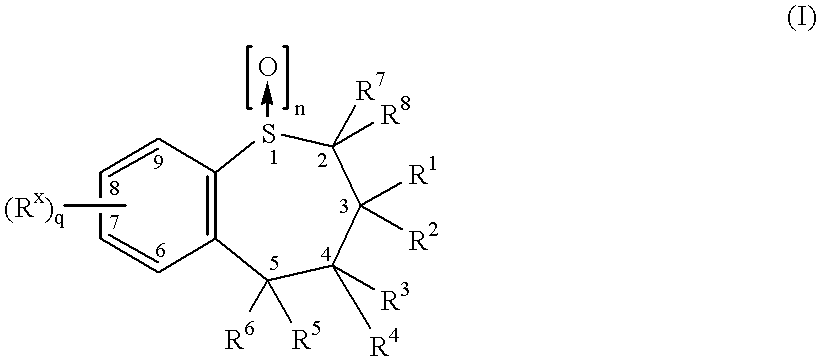
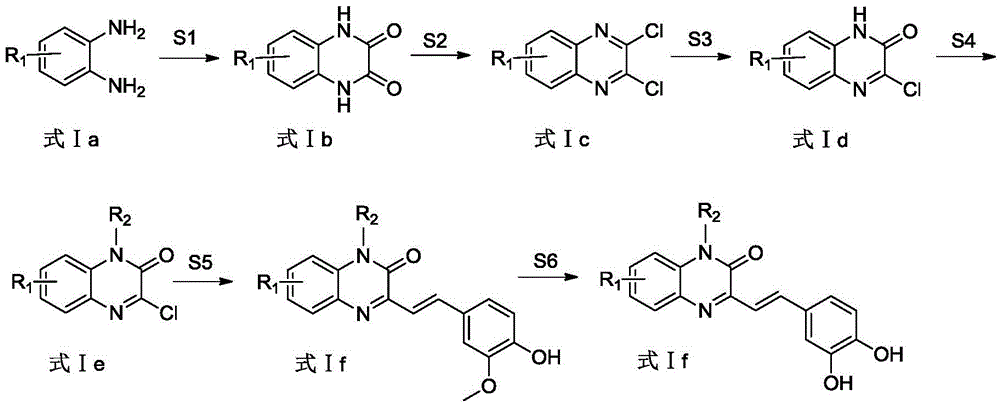
Structure of quinoxalinone derivatives as aldose reductase inhibitor, preparation method and use
InactiveCN104628661AHigh selectivityEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryQuinoxalineAntioxidant
The invention provides a structure of compounds shown in a formula I, a preparation method, and use of pharmaceutically acceptable salts or a mixture thereof in preparation of medicines for preventing and / or treating diabetic complication. The compounds as an aldose reductase inhibitor and an antioxidant effectively eliminate free radicals and inhibit generation of lipid peroxide by inhibiting the activity of aldose reductase so as to play a role of preventing and / or treating diabetic complication. The invention further provides pharmaceutical compositions which comprise the compounds and play a role of preventing and / or treating diabetic complication. The formulae are shown in the description.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Aldose reductase inhibitors and methods of use thereof
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Topical treatment of cataracts in dogs
ActiveUS8158667B2Improved stereospecific synthesis of R-methylReduces number of stageBiocideOrganic active ingredientsDiabetic retinopathyGlycerol
Owner:BOARD OF RGT UNIV OF NEBRASKA
Combination therapeutic compositions
InactiveUS7939551B2Good curative effectReduce doseBiocideMetabolism disorderSulfonylureaAldose reductase inhibitor
The present invention provides pharmaceutical compositions and methods for the treatment of diabetes mellitus using combination therapy. The compositions relate to a compound of Formula I and an antidiabetic agent such as sulfonylureas, biguanides, glitazones, α-glucosidase inhibitors, potassium channel antagonists, aldose reductase inhibitors, glucagon antagonists, activators of RXR, insulin therapy or other anti-obesity agent. The methods include the administration of the combination of compound of Formula I with antidiabetic agent where the two components are delivered in a simultaneous manner, where the compound of Formula I is administered first, followed by the antidiabetic agent, as well as wherein the antidiabetic agent is delivered first followed by the compound of Formula I.
Owner:AMGEN INC
Methods of treating cataracts and diabetic retinopathy with tricyclic pyrones
InactiveUS6916824B1Improve solubilityFacilitate slow-releaseBiocidePharmaceutical delivery mechanismDiabetic retinopathyWater soluble
Owner:KANSAS STATE UNIV RES FOUND
Sustained release formulation for oral administration of hmg-coa reductase inhibitor and method for the preparation thereof
The sustained release formulation for oral administration of an HMG-CoA reductase inhibitor of the present invention can be easily and economically prepared and is capable of maintaining a constant drug level in blood by slowly releasing the HMG-CoA reductase inhibitor at a uniform rate for 24 hrs. Accordingly, the sustained release formulation of the present invention can be effectively used for lowering blood cholesterol and triglyceride levels.
Owner:HANMI SCI CO LTD
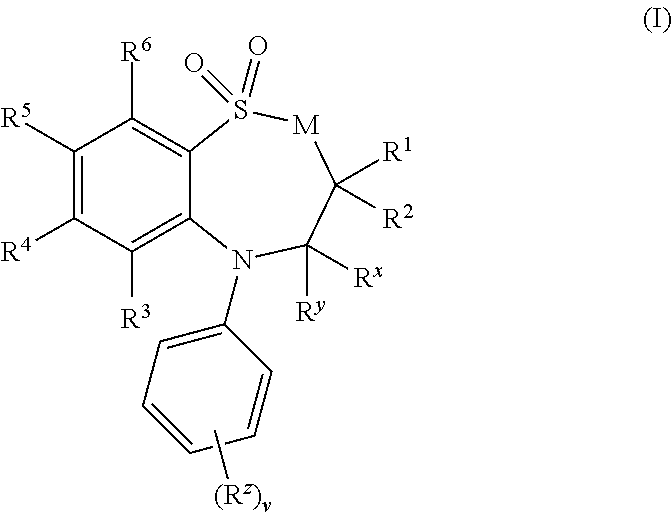
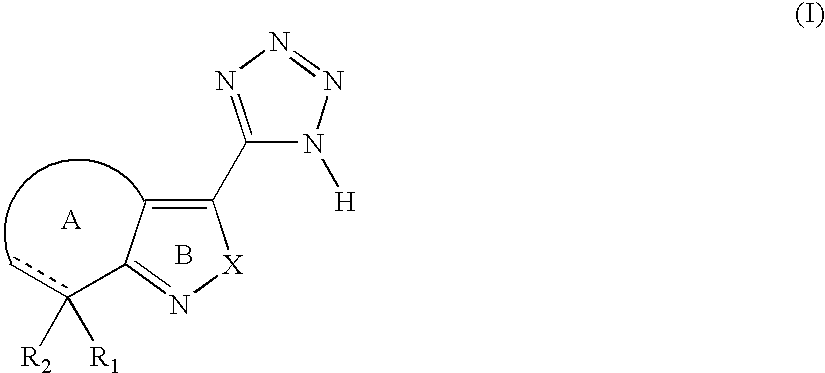
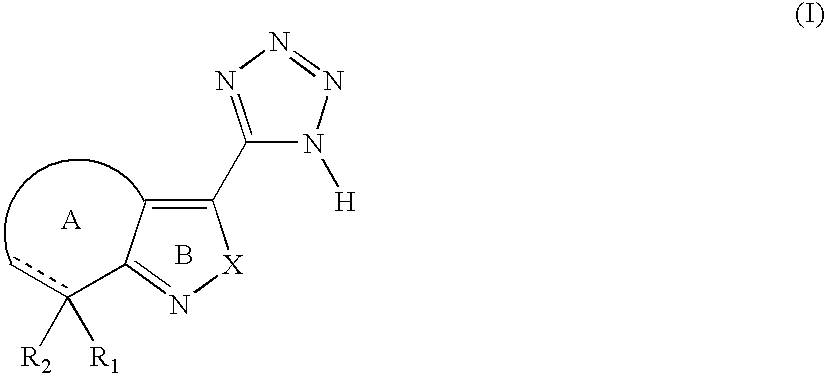
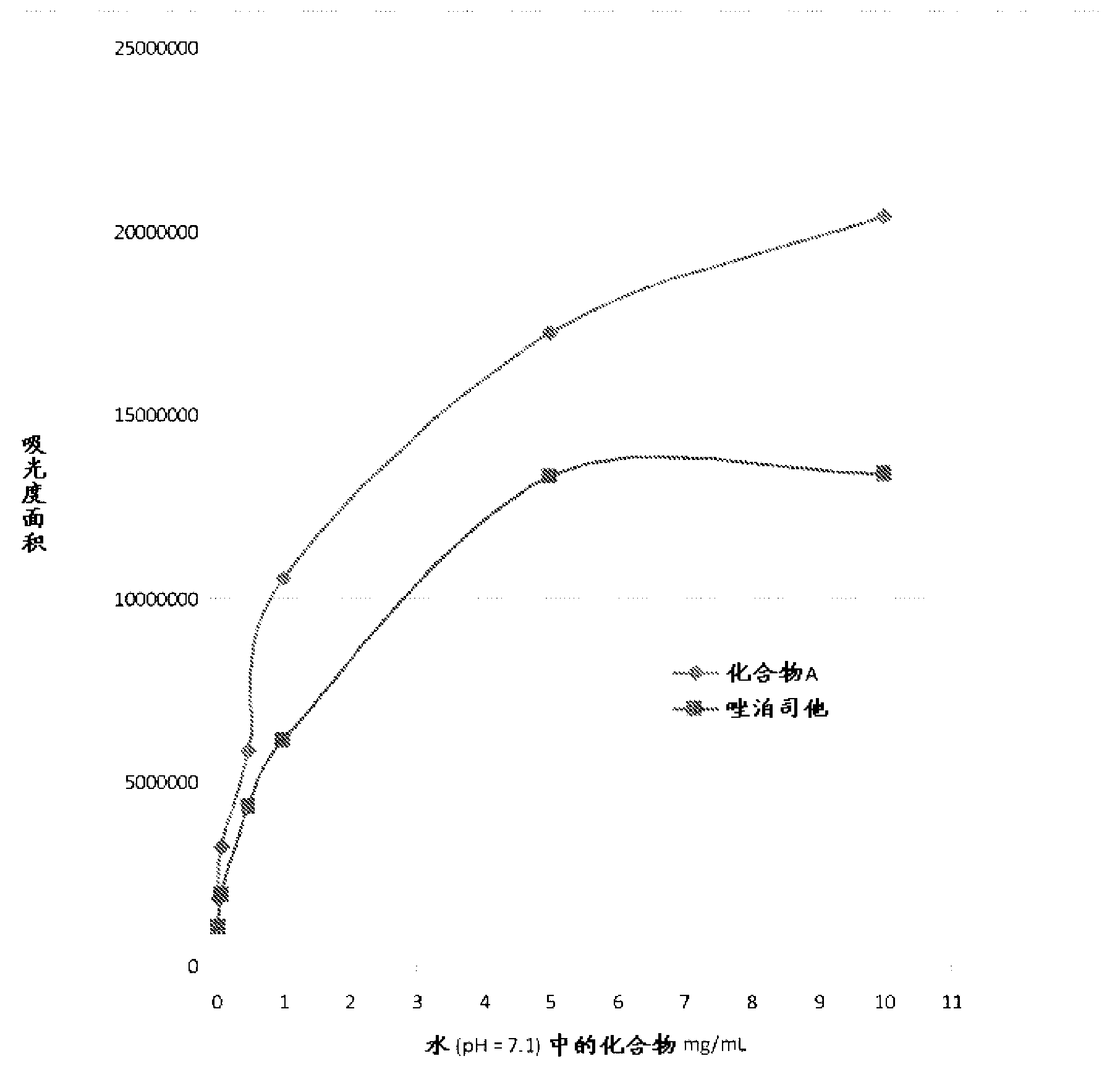
Tetrazole derivatives and methods of treatment of metabolic-related disorders thereof
The present invention relates to certain tetrazole derivatives of Formula (I), and pharmaceutically acceptable salts thereof, which exhibit useful pharmacological properties, for example, as agonists for the RUP25 receptor. Also provided by the present invention are pharmaceutical compositions containing compounds of the invention, and methods of using the compounds and compositions of the invention in the treatment of metabolic-related disorders, including dyslipidemia, atherosclerosis, coronary heart disease, insulin resistance, type 2 diabetes, Syndrome-X and the like. In addition, the present invention also provides for the use of the compounds of the invention in combination with other active agents such as those belonging to the class of α-glucosidase inhibitors, aldose reductase inhibitors, biguanides, HMG-CoA reductase inhibitors, squalene synthesis inhibitors, fibrates, LDL catabolism enhancers, angiotensin converting enzyme (ACE) inhibitors, insulin secretion enhancers and the like.
Owner:ARENA PHARMA +1
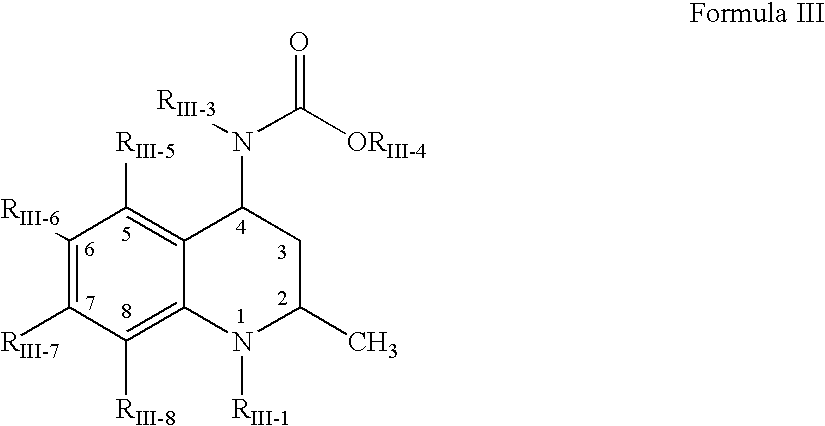
Aldose reductase inhibitors and uses thereof
The present invention relates to novel compounds and pharmaceutical compositions thereof, and methods for promoting healthy aging of skin, the treatment of skin disorders, the treatment of cardiovascular disorders, the treatment of renal disorders, the treatment of angiogenesis disorders, such as cancer, treatment of tissue damage, such as non-cardiac tissue damage, the treatment of evolving myocardial infarction, and the treatment of various other disorders, such as complications arising from diabetes with the compounds and compositions of the invention. Other disorders can include, but are not limited to, atherosclerosis, coronary artery disease, diabetic nephropathy, diabetic neuropathy, diabetic retinopathy, infections of the skin, peripheral vascular disease, stroke, and the like.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com